Abstract
Introduction
Over-prescription of opioids following surgical procedures is recognized as an important contributor to opioid misuse. Dialysis access procedures are commonly performed outpatient operations with little data or guidelines to inform prescription pain management practices. We sought to characterize opioid pain medication use following dialysis access surgery to promote a conservative approach to postoperative opioid prescriptions.
Methods
We performed a retrospective review of patients who underwent surgical dialysis access procedures from July 2018 through January 2019. Patient-reported opioid use information was captured in a brief questionnaire administered during routine follow-up appointments or phone calls and recorded in the electronic medical record. The procedure, type of intraoperative anesthesia/analgesia, postoperative prescription provided, and patient factors including age, sex, dialysis type, history of chronic pain, and preoperative opioid or benzodiazepine use were recorded. All procedures were classified by type (AVF-S: short incision arteriovenous fistula or graft, AVF-L: long incision arteriovenous fistula or graft, or PD: peritoneal dialysis catheter), and descriptive statistics were performed using R.
Results
Eighty-six patients underwent dialysis access procedures in the study time-frame, of which 63 were administered the pain questionnaire and 58 quantified opioid use. Eighty-five percent of patients received a prescription, however 31% took no opioids and 71% used opioids for 2 days or less. Inter-quartile ranges (IQR, 25th–75th percentile) of prescription and consumption quantities for patients who underwent AVF-L procedures were 10–28 and 2.5–20 pills, AVF-S quantities were 4.0–8.4 and 0–4.3, and PD quantities were 10 and 3.3–9, respectively. Thirty-one patients (53%) reported receiving more pain medication than they used, which resulted in a median of 8 excess pills per patient with an unused pill IQR of 0–22 for AVF-L procedures, 0–4.2 for AVF-S procedures, and 1.3–6.7 for PD procedures. Patients who were prescribed oxycodone or had a repeat operation had significantly increased opioid use.
Conclusions
This investigation of opioid use following surgical dialysis access procedures suggests that most patients use relatively few opioid pills following surgery, which translates into over-prescription and leftover medication for over 50% of patients. A conservative approach to postoperative prescription guidelines using lower prescription quantities would encourage opioid-related risk reduction while providing adequate postoperative analgesia. Recommended quantities for postoperative prescriptions were generated using the 80th percentile consumed and were 0–6 pills for brachiobasilic/brachiocephalic fistulas, 0–5 for basilic vein transposition, 0–5 for radiocephalic AVF, 0–15 for upper arm grafts, and 0–10 for peritoneal dialysis catheter placement.
Keywords: opioid, dialysis access, vascular surgery, fistula, peritoneal dialysis
Table of Contents Summary
This single-center retrospective study characterized post-operative opioid use following common dialysis access procedures. The authors suggest that most patients use few pills and for a limited duration of time, and advocate for conservative prescription practices.
Introduction
Accidental and intentional opioid overdose has emerged as a nationwide crisis after widespread prescription by the medical community, misinformation regarding the addictive nature of these medications, and susceptibility to misuse and diversion1. The National Safety Council estimates on data from the National Center for Health Statistics now lists opioid overdose as the fifth leading cause of death among Americans in 2017, surpassing motor vehicle collisions for the first time, and is also the number one cause of preventable death2. Synthetic opioids have contributed to the increase in opioid related deaths, and there is a strong component of prescription drugs with an estimated 35% of overdose deaths involving legally obtained opioids3. Prescription drug misuse following surgery has been associated with greater dose and duration of the postoperative prescription, highlighting the need for prescription recommendations following routine surgical procedures to reduce this risk by standardizing a reduced quantity and duration of pills provided4. Oxycodone and hydrocodone, two of the most common formulations for postoperative acute pain management, are consistently among the most commonly abused medications with the highest risk of adverse events5.
The incidence of new diagnoses of ESRD is nearly 120,000 patients per year, and the majority of patients undergo a surgical dialysis access procedure within one year of diagnosis. This is most commonly an arteriovenous fistula or graft, and although this is the initial dialysis access for only 17% of patients, 79% are dialyzed by this method at the end of the first year6. Peritoneal dialysis catheters are placed in 9.7% of patients, accounting for 12,000 patients who started dialysis in 2016 6. Despite careful dialysis planning for near-ESRD patients, the failure rate after fistula creation is as high as 38.9% in the first year, which can lead to additional surgeries for revision, attempts at other sites, or multi-stage procedures with larger incisions in the upper arm that could contribute to multiple prescriptions or pain-sensitization at these sites6.
Patients with ESRD are particularly vulnerable to the risks associated with opioid use. The prevalence of ESRD patients using opioid pain medication for a variety of chronic pain disorders is unusually high compared to other chronic illnesses and estimated to be between 40 and 60%7. Patients with severe kidney dysfunction also have the potential for greater susceptibility to life-threatening opioid-related complications since many opioids and metabolites are renally excreted8.
Investigations aimed at optimization of opioid and non-opioid pain management strategies in dialysis patients would be valuable for establishing prescription guidelines, however, have yet to be performed. Given the absence of formal recommendations, we hypothesized that there is wide variation in opioid prescribing practices following surgical dialysis access procedures, and that many patients use fewer opioid medications than they are provided. We sought to investigate and characterize opioid consumption following five commonly performed procedures to inform future studies on reduction strategies and promote a conservative approach to current prescription practices.
Methods
This study was determined to be exempt from institutional review board procedures by the University of Wisconsin Health Sciences Institutional Review Board following principles outlined in the Declaration of Helsinki. Waivers of informed consent and HIPAA authorization were granted by the IRB prior to the initiation of study analyses.
All patients who underwent dialysis access surgery, including arteriovenous fistula (AVF), arteriovenous graft (AVG), or laparoscopic peritoneal dialysis catheter (PD) procedures from August 2018 through January 2019 at UW-affiliated hospitals were identified and reviewed. The patient’s medical record was used to determine the age, sex, dialysis type, history of chronic opioid use, preoperative opioid or benzodiazepine prescription, procedure type, postoperative opioid prescription name and quantity, and complications. Routine postoperative follow-up included either routine telephone or office-based questions typically one to four weeks following surgery. At this follow up, clinic staff asked patients the number of opioid pills used, duration used, current pain on a scale of 0–10, if they were taking non-opioid adjuncts, and whether they knew of safe disposal strategies for leftover opioid pills. If they did not know safe disposal strategies, they were educated by the provider or office staff. This information was then recorded in the electronic medical record.
To characterize postoperative pain in a procedure-specific manner, operations were classified into three different categories defined by the procedure performed. The category of long incision arteriovenous fistulas or grafts (AVF-L) included procedures which typically require an extended incision length or subcutaneous tunneling and included basilic vein transposition (stage II BVT or single-stage BVT), loop graft, or upper arm straight graft. The category of arteriovenous fistulas or grafts with a short incision (AVF-S) included brachiocephalic, brachiobasilic (stage 1 BVT), radiocephalic fistula, and non-tunneled jump graft procedures. Peritoneal dialysis catheters (PD) were categorized separately due to the need for laparoscopy.
Opioid dose was standardized to one 5 mg hydrocodone pill as this was the most frequently prescribed medication, therefore, each oxycodone tablet was given an equivalent dose of 1.5, hydromorphone 4, and codeine 0.15. Patients who were unable to quantify opioid use were excluded from quantitative statistical analysis. Unused opioid quantities were calculated by subtracting the number of pills used from the number of pills prescribed in the original prescription. Patients who reported taking a greater number of pills than the original prescription, either leftover from a previous prescription or had a refill before follow-up, were recorded with a negative excess quantity. Data was stored on Microsoft Excel, descriptive statistics and univariate analysis was performed using R and R Studio9 and figures were made using GraphPad Prism (Version 6.0 for Mac, GraphPad Software, La Jolla California USA). The median and interquartile range (IQR, 25th-75th percentile) were reported due to non-normal distribution.
Results
From August 2018 through January 2019, there were 86 identified dialysis access operations which were performed at two hospitals associated with the University of Wisconsin, of which 63 were administered the postoperative pain questionnaire and 58 had self-reported quantity of opioids consumed which were used for analysis. Baseline demographics of patients who reported opioid use including age, sex, race, type of dialysis, and BMI are presented in Table I. The quantity of pills prescribed, quantity of pills used, the duration of opioid use, and leftover amounts are provided in Table II.
Table I:
Demographics of patients who underwent dialysis access procedures August 2018-January 2019. AVF-L=arteriovenous fistula/graft long incision, AVF-S=arteriovenous fistula/graft short incision, PD=peritoneal dialysis
| Median (Range) | |
|---|---|
| Age | 59 (30–79) |
| BMI | 30 (17–62) |
| Sex |
Number (%)
Total n=58 |
| Male | 41 (71) |
| Female | 17 (29) |
| |Pain History | |
| History of chronic pain | 18 (31) |
| Pre-op opioid | 8 (14) |
| Pre-op benzodiazepine | 6 (10) |
| Procedure classification | |
| AVF-L | 10 (17.2) |
| AVF-S | 42 (72.4) |
| PD | 6 (10.4) |
| Race/Ethnicity | |
| White | 34 (59) |
| Black | 18 (31) |
| Asian | 2 (3.3) |
| Hispanic | 3 (5) |
| Other | 1 (1.7) |
| Pre-op Dialysis | |
| Catheter | 27 (46) |
| Fistula | 8 (14) |
| Graft | 2 (3.5) |
| PD | 2 (3.5) |
| None | 19 (33) |
| Received Opioid Rx | 51 (88) |
| Opioid Rx Type | |
| Hydrocodone/acetaminophen | 36 (62) |
| Oxycodone | 12 (21) |
| Oxycodone/acetaminophen | 1 (2) |
| Tramadol | 1 (2) |
| Codeine | 0 (0) |
| Hydromorphone | 0 (0) |
Table II:
Opioid prescription quantity, number of pills taken, duration, and number of unused opioid pills by procedure. Each pill was standardized to a dose of 5 mg of hydrocodone. IQR=interquartile range.
| Procedure | Opioid questions (n) | Pills prescribed Median (IQR) | Pills taken Median (IQR) | Unused pills Median (IQR) | Duration (Days) Median (IQR) |
|---|---|---|---|---|---|
| AFV - L | 13 | 17.5 (10–28.1) | 5.5 (2.5–20.25) | 6.5 (0–20.2) | 2 (1.5–3.5) |
| Basilic Vein Transposition | 5 | 12.5 (10–16.3) | 3.5 (1.5–7.5) | 6.5 (3.8–11)) | 2 (1.5–2.8) |
| Upper arm graft | 5 | 15 (10–22.5) | 5 (0–22.5) | 0 (0–10) | 2 (0–5) |
| Forearm graft | 2 | 22.5, 33 | 22.5, 33 | 0 (0) | 10 |
| Axillary graft | 1 | 30 | 42 | −12 | unknown |
| AVF - S | 41 | 4.0 (4.0–8.4) | 1.75 (0–4.3) | 1.75 (0–4.2) | 1 (0–2) |
| Brachiocephalic/basilic fistula | 36 | 5 (4–10) | 2 (0–5) | 1.5 (0–8) | 1 (0–2) |
| Radiocephalic fistula | 5 | 4 (4–7.5) | 0 (0–4) | 0 (0–4) | 1 (0–2) |
| PD | 7 | 10 (10) | 5 (3.3–9) | 5.5 (1.3–6.7) | 2 (1.5–2.5) |
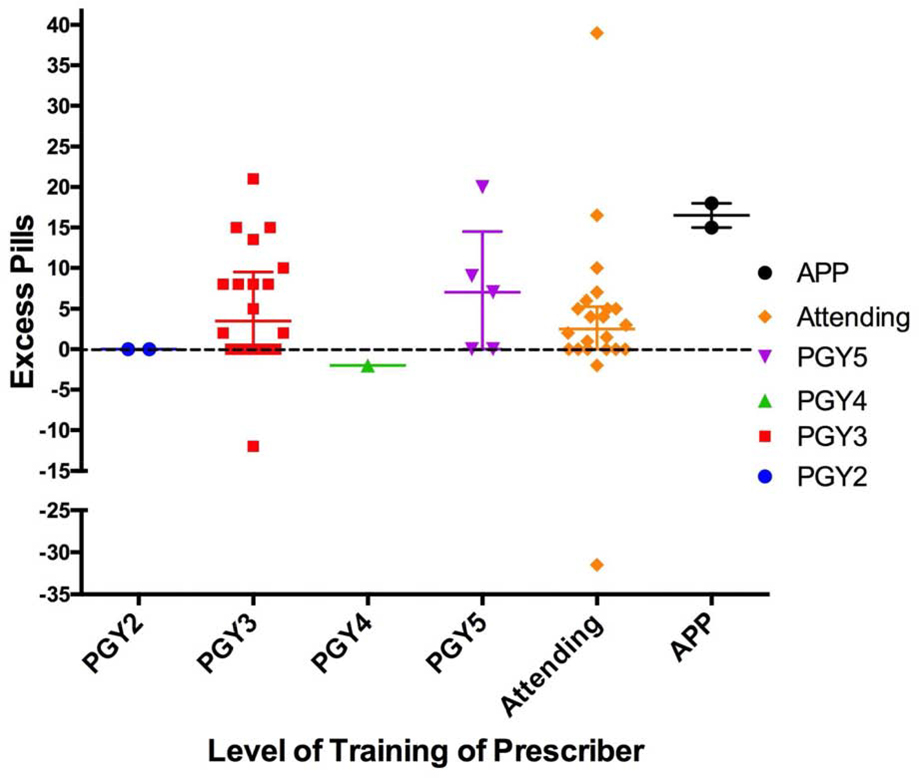
The mean follow-up time was 15 days with a standard deviation of 16 days (range 4–88). Overall, 85% of patients who underwent a surgical dialysis access procedure in this study received a new prescription for opioid pain medication. Of the 58 patients that quantified opioid consumption, 53.4% reported using a quantity that was less than what was provided, and 71% used opioids for 2 days or less. Eighteen patients (31%) reported taking no opioids postoperatively. The IQR of the number of pills consumed for patients who underwent AVF-L procedures was 2.5–20 (median 5.5), AVF-S procedures 0–4.3 (median 1.75), and PD catheter procedures 3.3–9 (median 5). For all procedure types, the median duration was 2 days or fewer. Most patients reported tolerable pain at the time of follow-up, with 80% of patients with a pain score of 4 or less out of 10 at the post-op phone call or office visit. Five patients requested a refill; two of these patients had a complication (bleeding, hematoma), three had a history of chronic pain disorder, and one had concurrent use of an opioid for a non-surgical indication. Postoperative prescriptions were written by 22 prescribers, including staff surgeons, residents, and advanced practitioners. The unused pill quantity (excess) was determined for each level of training of prescriber and was highly variable, ranging from −33 (indicating the patient took much more than the original prescription) to +39 with nearly all practitioners prescribing excess pills (Figure 1).
Figure 1:
Excess pills by level of training of provider were determined by subtracting the quantity used from the quantity originally prescribed. Negative numbers represent more pills used than original prescription. Bars indicate mean and IQR (25th–75th percentile).
For each of the three procedure categories, we show the number of pills used for each of the independent variables listed (Table III). Patients with chronic pain typically had more median opioid use in all categories (AVF-L 15 vs 5 pills, AVF-S 5 vs 1.3, and PD 6 vs 4), as did patients with a complication (AVF-L 30 vs 3.5, AVF-S 5 vs 1.2). The median quantity of opioid consumption was also increased in patients who were prescribed oxycodone vs hydrocodone (AVF-L 42 vs 5, AVF-S 6.8 vs 2, PD 6 vs 4) and those who rated their pain as greater than 4/10 (AVF-L 6 vs 2, AVF-S 2.5 vs 2, PD 10 vs 4).
Table III:
Comparative table of the number of opioid pills taken by procedure type. Each pill was standardized to the dose of one 5 mg dose of hydrocodone. IQR = interquartile range.
| Variable | AVF Long (number of subjects) Median # pills used (IQR) |
AVF Short (n) Median (IQR) |
Intra-peritoneal (n) Median (IQR) |
|||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | |
| Chronic Pain | (5) 5 (2–6) |
(5) 15 (0–42) |
(32) 1.3 (0–3) |
(10) 5 (1.3–7.9) |
(3) 4 (3.5–7) |
(3) 6 (4.5–8) |
| Current Opioid | (7) 6 (3.5–10.5) |
(3) 0 (0–22.5) |
(39) 2 (0–5) |
(3) 5 (2.5–25) |
(4) 5 (3.8–7) |
(2) 6.5 (4.8–8.3) |
| Current Benzo | (10) 5.5 (1.6–12.7) |
(0) | (37) 2 (0–5) |
(5) 0 (0–0) |
(5) 4 (3–10) |
(1) 6 (N/A) |
| Regional Block | (4) 10.5 (5.8–22.5) |
(6) 1.8 (0.4–5) |
(8) 1 (0–4.3) |
(34) 2 (0–5) |
N/A | N/A |
| Complication | (8) 3.5 (1.1–6) |
(2) 30 (22.5–37.5) |
(34) 1.2 (0–3.8) |
(8) 5 (2.5–5.6) |
(6) 5 (3.3–9) |
(0) |
| Pain score > or = to 4 | (5) 2 (1.5–6) |
(5) 6 (5–15) |
(33) 2 (0–5) |
(8) 2.5 (0.8–9.4) |
(5) 4 (3–6) |
(1) 10 |
| Re-operation | (5) 1.5 (0–6) |
(5) 15 (5–42) |
(29) 2 (0–5) |
(13) 1 (0–6) |
(3) 4 (3.5–5) |
(3) 10 (6.5–10) |
| # Pills Given >10 | (3) 6 (6–6) |
(8) 5.5 (1.9–21.8) |
(27) 3 (1–5) |
(15) 2 (0–7) |
(1) 3 (N/A) |
(5) 6 (4–10) |
| Name of Rx | Oxycodone | Hydrocodone | Oxycodone | Hydrocodone | Oxycodone | Hydrocodone |
| (3) 42 (24–43.5) |
(5) 5 (2–6) |
(8) 6.8 (1.1–26) |
(25) 2 (0–4) |
(1) 6 (N/A) |
(5) 4 (3–10) |
|
Statistical comparisons of independent variables were limited by sample size and variability but were performed when possible to identify risk factors for increased opioid use using Welch’s t test with unequal variance using a two-tailed hypothesis. Patients who underwent long incision procedures had non-significant increase in opioid consumption compared to short incision procedures (mean 12.3 vs 5.1, p=0.23). Regional blocks in upper extremity procedures had a non-significant reduction in opioid use (mean 5.2 vs 10.6, p value = 0.31). Patients who had a repeat operation in the same or similar location had a significant increase in opioid usage (11.8 vs 3.3, p=.03). There was no difference in the number of pills taken by gender (males 6.7 vs females 5.7, p=0.75). Patients who were prescribed oxycodone took more MME-equivalents pills (18.3 vs 3.5, p=0.02) and number of un-adjusted pills (12.1 vs 3.5, p=0.03) than those who were prescribed hydrocodone. There was a trend for decreasing opioid consumption after the age of 60 but this was non-significant, limited by the numbers of patients in each age group. Self-reported pain control was not associated with a difference in the number of opioid pills consumed. The mean number of pills taken by patients with good pain control (pain score=<4/10, n=50) was 5.0 pills vs poor pain control (pain score>4/10) of 12.6, p=0.15.
After identifying that many patients used little to no pain medication, we sought to quantify the number of pills taken by the 80th percentile of patients, considering that the high consumers may have confounding factors such as chronic pain, pre-operative opioid use, or a postoperative complication that could explain a skewed distribution (Table IV).
Table IV:
Quantity of opioid pills utilized for management of acute, post-operative pain by 80th percentile and number of patients per procedure who used no opioids. Recommended quantities were determined by a range including up to the 80th percentile of all patients and an upper limit of the highest amount consumed (excluding outliers) by procedure.
| Procedure | 80th percentile pills used | Zero opioids used n (%) | Recommended Quantity (Maximum) |
|---|---|---|---|
| Brachiobasilic, Brachiocephalic (n=33) | 6 | 12 (36) | 0–6 (12) |
| PD catheter (n=6) | 10 | 0 (0) | 0–10 |
| Single Stage / Stage II BVT (n=5) | 5 | 1 (20) | 0–5 (15) |
| Upper arm graft (n=5) | 15 | 2 (40) | 0–15 |
| Radiocephalic AVF (n=5) | 4 | 3 (60) | 0–5 |
Discussion
Over-prescription of opioid pain medication for surgical pain has been common throughout the United States. This is likely due to several factors, including a desire to provide adequate postoperative pain control, difficulty in refilling an opioid prescription remotely, and the lack of evidence-based procedure-specific opioid guidelines. Opioid reduction strategies consisting of patient education, healthcare provider education, intraoperative and postoperative multimodal analgesia have shown promise to reduce opioid use in a group of general surgery procedures10.
The quantities of opioid pills consumed after dialysis access surgery is consistent with previous reports in both upper extremity soft tissue and vascular surgery, where most patients took less than 5 pills, used opioids for fewer than 2 days, and in which many did not require opioid pills at all11,12. The variability of provider-dependent prescription practices in these reports based on the interquartile range of prescription (Table II) highlights the need for procedure-specific recommendations as a way to standardize and reduce risk of addiction and adverse events. Prescribers of all levels of experience would likely benefit from education about evidence-based strategies to reduce excess opioid prescription.
In this study, patients who had a diagnosis of chronic pain disorder used more opioids in all procedure types, however there were insufficient numbers to compare by procedure (Table III). Not surprisingly, complications such as bleeding or infection also typically increased pain medication usage; patients who have difficulty with pain control should be evaluated to exclude a complication that would require intervention. Patients who were prescribed oxycodone had significantly higher consumption. This could be due to likelihood of preselection bias as experienced providers may identify patients who are more likely to require higher opioid requirements due to uncaptured intraoperative or patient factors and prescribed oxycodone as a stronger pain medication. Also, patients who were prescribed oxycodone typically received a greater quantity than patients who were prescribed hydrocodone, and in at least one study, a greater quantity of opioids prescribed was associated with higher amount consumed after controlling for patient factors13. It is unlikely to be explained by complication as only one patient who had oxycodone had a complication and the rest had hydrocodone. Repeat operation was also associated with greater opioid consumption, which could be partially attributable to patients who had revisions performed for the indication of pain.
Although the majority of our patients (83%) stated they knew of safe disposal practices, there is often little to no accountability or assurance that these are disposed in a responsible manner. Current recommendations for disposal include safe drop zones (can be located online on websites such as https://nabp.pharmacy/initiatives/awarxe/drug-disposal-locator/) or national take-back events. However, if these are not feasible options, current FDA and DEA recommendations include flushing pills down the toilet or mixing with unpalatable substances such as dirt, cat litter, or used coffee grounds.
Patients who have a history of chronic pain disorder or are currently taking opioids are likely to require additional pain medication in the postoperative period, and may require a higher, individualized regimen. Non-opioid pain medication such as acetaminophen should be recommended to most patients, however NSAIDs are typically contraindicated in this population. Older patients undergoing upper extremity surgical procedures have been shown to have decreased pain medication requirements, and recommendations from pharmacodynamic and observational studies include initiating opioids at a lower dose for the elderly14,15.
The Opioid Prescribing Engagement Network has published both investigative and validation studies on postoperative opioid consumption following several outpatient surgical procedures16,17. Due to the size limitations in our study, we utilized the 80th percentile to generate recommendations for prescription quantities following vascular access procedures by expanding the upper limit to the highest amount consumed after excluding outliers (Table IV). In the future, these recommendations will be used to design a prospective randomized study comparing a strict versus liberal opioid regimen to evaluate effect of prescribing strategies on opioid utilization and quantity of excess medication that will provide stronger procedure-specific guidelines.
There are several limitations of our study. The study was completed at one academic hospital system, and largely reflects the combined suburban and rural patient population in and around Madison, Wisconsin. The study was performed in a retrospective manner and does not control for the variability of provider practice including setting pain expectations. Two of the categories (upper arm graft and forearm loop graft) had a low number of patients due to the infrequency of procedures performed, and we believe that the forearm loop graft opioid use was affected by this as both had a complication which greatly increased the quantity used. Additionally, there are a number of limitations from the patient-reported method of quantification. Patient recall accuracy may diminish after several weeks (5 patients were unable to recall the number of pills taken and duration), and patients that take daily opioids for chronic conditions may have a difficult time separating the number of pills used to treat surgical pain from their normal use. Also, preoperative opioid use was determined by an active prescription in the chart and may inflate this number by including patients who had an active prescription but not active use. Self-reported quantification has a potential for dishonesty, and two patients who had a concurrent opioid prescription reported no opioid use after surgery. Patients may also use pills intended for surgical pain to treat non-surgical ailments such as back pain that have gone untreated. People who used all of their prescription may be hesitant to reveal additional pain medication used that may have been left-over from other procedures or obtained in other ways and cannot be controlled for by the study design. Interestingly, although 23 patients (40%) patients used the entire prescription, only 5 (8.6%) requested a refill, which could suggest adequate pain control despite limited opioids and possible benefit to further reduction.
Conclusions
Opioid reduction strategies in post-surgical dialysis access procedures are important to minimize the risk of adverse events and include minimization of over-prescribing. We characterized the quantity and duration of opioid use following five common dialysis access procedures and found that 31% of patients used no opioids postoperatively, 53% had leftover pills, and 71% used opioids for 2 days or less. Patients who had a large incision or tunneling (loop graft, basilic vein transposition, etc) tended to take more pain medication than patients with a smaller incision, as well as patients who had a repeat operation in the same location. Recommended quantities for postoperative prescriptions were generated using the 80th percentile consumed and were 0–6 pills for brachiobasilic/brachiocephalic fistulas, 0–5 for basilic vein transposition, 0–5 for radiocephalic AVF, 0–15 for upper arm grafts, and 0–10 for peritoneal dialysis catheter placement. Further studies are needed to determine ideal prescription practices with opioid-reduction strategies and establish procedure-specific guidelines.
ARTICLE HIGHLIGHTS.
Type of Research
Single center retrospective cohort study
Key Findings
Nearly all patients who underwent dialysis access surgery received a prescription for opioid pain medication, however 31% report taking no opioid pills and 53% had leftover medication. 71% used opioids for 2 days or less.
Take home Message
Following dialysis access surgery, most patients consume few opioids for a brief period of time. Prescription practices should be tailored to reduce over-prescription.
Acknowledgments
Funding source: This work is supported by the NIH T32 Training Grant NIAID AI125231.
Footnotes
Conflict of interest: There are no conflicts of interest from any authors related to the study to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Zee A The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health [Internet]. 2009. Feb [cited 2019 Feb 28];99(2):221–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18799767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odds of Dying - Injury Facts [Internet]. [cited 2019 Jun 17]. Available from: https://injuryfacts.nsc.org/all-injuries/preventable-death-overview/odds-of-dying/
- 3.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G, Release E. Drug and Opioid-Involved Overdose Deaths — United States, 2013 – 2017. Morb Mortal Wkly Rep. 2019;67:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brat GA, Agniel D, Beam A, Yorkgitis B, Bicket M, Homer M, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: Retrospective cohort study. BMJ. 2018;360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedegaard H, Bastian BA, Trinidad JP, Food US, Administration D, Spencer M, et al. National Vital Statistics Reports Volume 67, Number 9 December 12, 2018. Natl Vital Stat Reports [Internet]. 2018;67(9):1–13. Available from: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Program_ [Google Scholar]
- 6.Pisoni Ronald; Saran Rajiv; Schaubel Douglas E; Kenneth J Woodside M. Chapter 3: Vascular Access. Am J Kidney Dis. 2018;71(3):S303–20. [Google Scholar]
- 7.Chapter 10: Prescription Drug Coverage in Patients With ESRD. In: American Journal of Kidney Diseases. 2018. p. S441–60. [Google Scholar]
- 8.Pham PC, Khaing K, Sievers TM, Pham PM, Miller JM, Pham S V., et al. 2017 Update on Pain Management in Patients With Chronic Kidney Disease. Clin Kidney J. 2017;10(5):688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R Core Team. R: A language and environment for statistical computing. [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2018. Available from: https://www.r-project.org/ [Google Scholar]
- 10.Hartford LB, Van Koughnett JAM, Murphy PB, Vogt KN, Hilsden RJ, Clarke CF, et al. Standardization of Outpatient Procedure (STOP) Narcotics: A Prospective Non-Inferiority Study to Reduce Opioid Use in Outpatient General Surgical Procedures. J Am Coll Surg [Internet]. 2019;228(1):81–88.e1. Available from: 10.1016/j.jamcollsurg.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 11.Colton IB, Fujii MH, Ahern TP, MacLean CD, Lahiri JE, Alef M, et al. Postoperative opioid prescribing patterns and use after vascular surgery. Vasc Med (United Kingdom). 2018; [DOI] [PubMed] [Google Scholar]
- 12.Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am [Internet]. 2012;37(4):645–50. Available from: 10.1016/j.jhsa.2012.01.035 [DOI] [PubMed] [Google Scholar]
- 13.Howard R, Fry B, Gunaseelan V, Lee J, Waljee J, et al. , Association of Opioid Prescribing With Opioid Consumption After Surgery in Michigan, JAMA Surgery. 2019;154(1):e184234. doi: 10.1001/jamasurg.2018.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard R, Waljee J, Brummett C, Englesbe M, Lee J. Reduction in Opioid Prescribing Through Evidence-Basec Prescribing Guidelines. JAMA Surgery. Published online December 06, 2017153(3):285–287. doi: 10.1001/jamasurg.2017.4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard R, Alameddine M, Klueh Englesbe M, Brummet C, Waljee J, Lee J. Spillover Effect of Evidence-Based Postoperative Opioid Prescribing. Journal of the American College of Surgeons. 2018; 227, 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim N, Matzon JL, Abboudi J, Jones C, Kirkpatrick W, Leinberry CF, et al. A Prospective Evaluation of Opioid Utilization After Upper-Extremity Surgical Procedures: Identifying Consumption Patterns and Determining Prescribing Guidelines. J Bone Joint Surg Am [Internet]. 2016. Oct 19 [cited 2019 Jun 28];98(20):e89. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27869630 [DOI] [PubMed] [Google Scholar]
- 17.Gupta DK, Avram MJ. Rational Opioid Dosing in the Elderly: Dose and Dosing Interval When Initiating Opioid Therapy. Clin Pharmacol Ther [Internet]. 2012. Feb 28 [cited 2019 Jun 28];91(2):339–43. Available from: http://doi.wiley.com/10.1038/clpt.2011.307 [DOI] [PubMed] [Google Scholar]