Abstract
Background:
Estimates indicate that nearly eight percent of the over 500,000 survivors of childhood cancer living in the United States are frail in their fourth and fifth decades of life, a phenotype typically seen in geriatric populations. Participation in regular physical activity to improve physical fitness in healthy and diseased populations reduces risk for frail health by increasing physiologic reserve. However, physical activity may not have the same effects on fitness in childhood cancer survivors as it does among their peers with no cancer history.
Aims:
This scoping review seeks to describe associations between physical activity, physical fitness, chronic disease, and mortality in childhood cancer survivors.
Methods:
Relevant literature was identified through a comprehensive search in the PubMed, Web of Science, CINAHL, and Cochrane databases. A narrative synthesis was performed on observational studies that had physical activity or physical fitness clearly defined and compared with chronic disease outcomes.
Results:
A total of 595 studies were screened, and results from 11 studies are presented. Childhood cancer survivors who participate in regular physical activity have improved markers of cardiovascular health, decreased risk of overt cardiovascular disease, and decreased risk of all-cause mortality compared to survivors who are not physically active. Childhood cancer survivors who are physically fit have increased neurocognition, and decreased risk of all-cause mortality compared to survivor’s who are not fit. The differential effects of physical activity on fitness and health among childhood cancer survivors when compared to peers is potentially related to treatment exposures that damage cardiovascular tissue and impact regenerative potential.
Conclusion:
Research is needed to determine the optimal timing, frequency, intensity, and duration of physical activity necessary to optimize fitness in childhood cancer survivors.
Keywords: survivorship, physical activity, physical fitness, chronic disease
Introduction
Advancements in biology and cancer therapy for pediatric malignancies have resulted in dramatic improvement in survival of children with cancer. Current 5-year survival rates now exceed 80%, and the number of long-term survivors residing in the United States is estimated to reach 500,000 by 2020.1,2 Unfortunately, some childhood cancer survivors are at an increased risk of early onset chronic disease, and mortality compared to peers.3
Frailty, a phenotype characterized by reduced physiologic reserve, is not typically seen in young populations. However, frailty is prevalent among 7.9% of young adult childhood cancer survivors (mean age: 33.6 ± 8.1 years).4 This percentage is comparable to that of older adults (70 years of age) in the general population, where it is reported as 9.9%.5 Frailty increases the relative risk for chronic disease by 2.2 (95% confidence interval [CI]: 1.2, 4.2) among survivors of childhood cancer, who by 30 years of age, experience an average of 7.72 (95% CI: 7.26 – 8.18) chronic health conditions, 2.13 (95% CI: 2.03 – 2.22) of which are serious or life-threatening. This burden is not seen among community controls with no childhood cancer history, until they are at least 45 years of age, where the average number of any, and serious and/or life threatening chronic conditions per individual are estimated at 7.27 (95% CI: 6.39 – 8.15) and 1.96 (95% CI: 1.68 – 2.24), respectively.4,6–8 Frailty also increases risk for mortality among childhood cancer survivors (hazard ratio [HR]:) 2.6; 95% CI: 1.2 – 6.2),4 whose excess risk of death, primarily due to new primary cancers, cardiovascular disease (CVD), and pulmonary conditions, remains substantially elevated 30 years from primary cancer diagnosis (standardized mortality ratio: 6.9; 95% CI: 4.7, 9.8).9,10
Although recent advances in care for aging adults and other frail populations include promising pharmaceutical and/or nutraceutical interventions with biological or cellular targets,11–14 robust health is best preserved and often restored through engagement in health optimizing behaviors, including physical activity (PA).15,16 Regular PA improves physical fitness, a cardinal sign of physiologic reserve.17,18 While PA and physical fitness are related, they are separate constructs with two distinct definitions. PA is defined as repetitive bodily movements produced by muscle contractions and is usually viewed has a health behavior.19 Physical fitness is defined as the ability to carry out daily tasks without undue fatigue, and is mostly viewed as a physiological adaptation.20 Engaging in regular PA and optimizing physical fitness prevents and mitigates frailty, disease, and mortality in healthy members of the general population and among adults with chronic disease, including CVD, metabolic syndrome, and asthma.21–26 Adult survivors of childhood cancer who are more active are also less likely to be frail.27
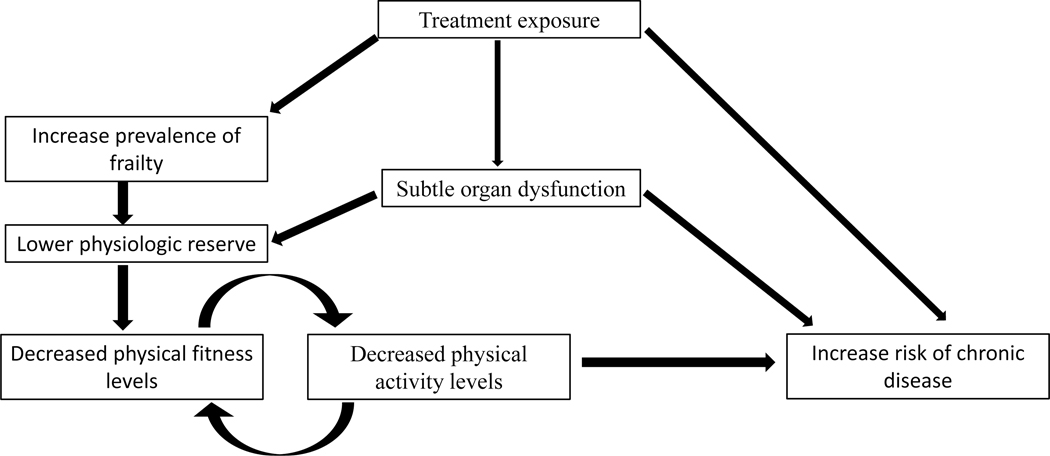
Due in part to disease and treatment exposures, children with cancer and young cancer survivors are less likely than siblings or peers to engage in PA.28,29 In fact, young survivors of childhood cancer are less physically fit than siblings, even after accounting for PA levels.30 This suggests that there may be other factors, such as subtle organ dysfunction, as a result of treatment, that impact the way childhood cancer survivors respond to PA.30,31 Lack of engagement in PA over time among childhood cancer survivors likely exacerbates initial compromises in physical fitness and physiologic reserve, eventually leading to increased risk of chronic disease (Figure 1).32–36
Figure 1.
Hypothesized model of the association between physical activity, physical fitness, and chronic disease
In the general population, participation in PA increases physical fitness, including metabolic health, muscular strength, and cardiopulmonary function.37–39 Increasing PA, and consequently physical fitness, also reduces risk for chronic disease, and may be a tool that childhood cancer survivors can use to optimize health.40,41 Therefore, research examining the effects of PA and physical fitness on chronic disease in childhood cancer survivors has recently become a topic of interest. While this topic is not novel in the general population, it is relatively new in childhood cancer survivors. Many reviews on this topic in childhood cancer survivors focus on PA or physical fitness levels as outcomes,42,43 rather than on associations between PA and physical fitness and chronic disease. Thus, this scoping review seeks to describe the impact of PA, and physical fitness on chronic disease, and mortality in childhood cancer survivors.
Methods
Search strategy
A search was conducted for studies published in PubMed, Web of Science, CINAHL, and Cochrane database of systematic reviews via OVID. The search was performed on March 28th, 2020 and included the following medical subject headings and text words: “survivor” and “childhood cancer” or “survivor” and “neoplasms” and “child”, in combination with each of the following terms: “physical activity” or “exercise” or “exercise therapy” or “exercise tolerance” or “physical exertion” or “physical fitness” and “chronic disease” or “organ dysfunction” or “late effect” or “mortality”. Additionally, each identified manuscript’s reference section was searched for relevant papers.
Inclusion and exclusion criteria
Manuscripts were retained if the study included childhood cancer survivors, and if the study design was cross-sectional, case-control, retrospective- or prospective-cohort. The exposure must have included either PA, physical fitness or both and the outcomes either chronic disease or markers of chronic disease. We excluded randomized control trials, studies that were not peer reviewed, and studies that were not written in English.
Screening data extraction
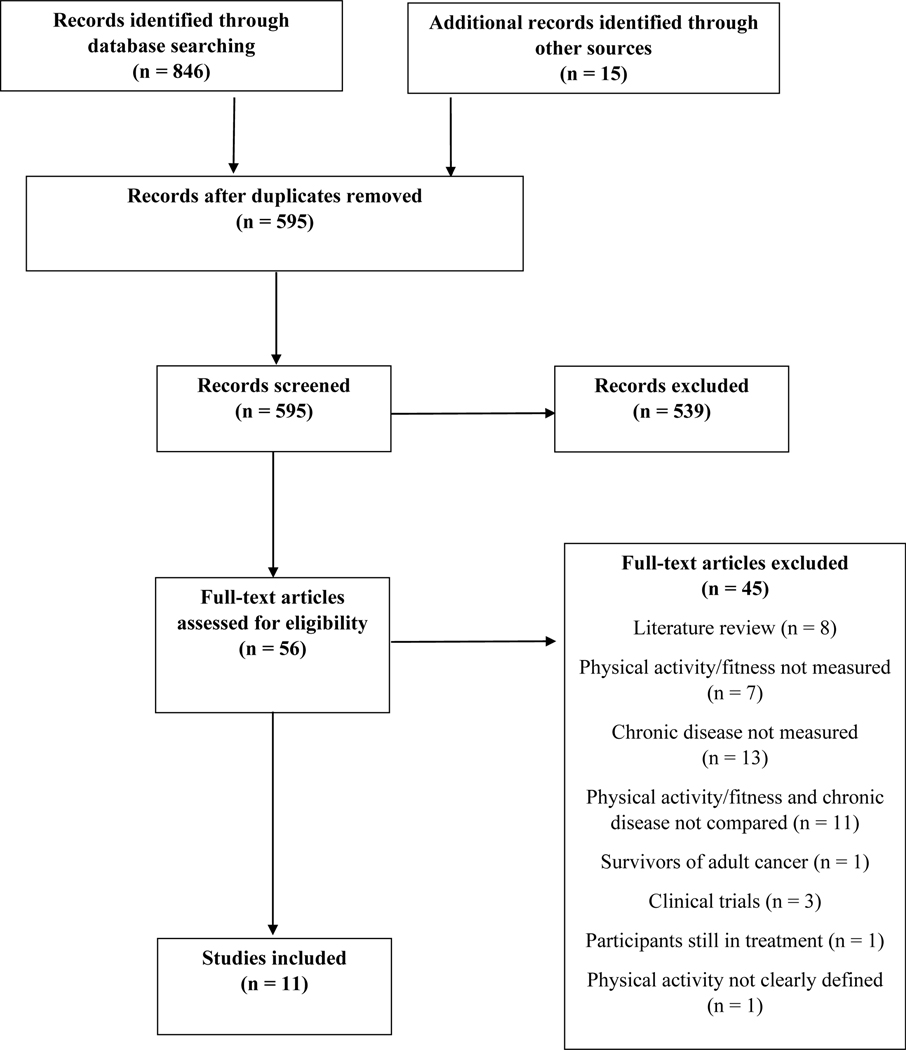
In total, the search yielded 846 records and 15 additional records identified through other sources. After duplicates were removed, a total of 595 records remained for screening. One reviewer (MDW) initially screened the study titles and abstracts and reviewed the full texts to determine if the studies were still eligible. During the full-text screening, reference lists of the articles were screened for additional papers. If the reviewer was unsure about a paper during any point of the screening process, it was brought to a second reviewer (KKN) to decide. Finally, a narrative synthesis was conducted to summarize the articles that were retained, relevant to the topics outlined in this scoping review (Figure 2). The results from 11 primary research studies are discussed in this review (Table 1).
Figure 2.
Manuscript selection flowchart
Table 1:
The effects of physical activity or physical fitness on chronic disease in childhood cancer survivor
| Author Country | Year published | Population | Study design | N | Age | Measure of physical activity / fitness | Measure of chronic disease | Results |
|---|---|---|---|---|---|---|---|---|
| Physical activity and risk factors for, or intermediate markers of, cardiovascular disease among childhood cancer survivors | ||||||||
| Meacham56 United States | 2010 | Adult survivors of childhood cancer 51.5% male 85.3% white | Cross-Sectional | 8,599 survivors | <19- >50y | Single question from the Youth risk Behavior Surveillance Survey Classified as sedentary if they answered “no” to the following question: “During the past month, did you participate in any physical activities or exercises, such as running, calisthenics, gold, bicycling, swimming, wheelchair basketball, or walking for exercise.” | Cardiovascular risk factors: Obesity Hypertension Dyslipidemia | In sedentary survivors: ↑ risk of obesity ↑ risk of hypertension ↑ risk of dyslipidemia ↑ risk of any three risk factors |
| Slater55 United States | 2015 | Young adult survivors of childhood cancer 46.4% male 85.9% white Sibling controls 53.9% male 93.3% white | Cross-sectional | 319 survivors; 208 controls | Survivors: mean 14.6y; Controls: mean 13.6y | Modifiable Activity Questionnaire for Adolescents, past year leisure time physical activity Low physical activity: self-reported ≤60 minutes of moderate to vigorous activity per day. High physical activity: self-reported >60 minutes of moderate to vigorous activity per day. | Cardiovascular risk factors: Waist circumference Percent fat mass Abdominal subcutaneous fat Visceral fat Lean body mass Triglycerides HDL-C LDL-C Blood pressure Insulin Sensitivity HOMA-IR cCSC cIMT | In low PA survivors: ↑percent body mass ↑Subcutaneous fat High PA survivors had sharper reductions in waist circumference, percent fat mass, abdominal subcutaneous fat and abdominal visceral fat than high PA controls. |
| Howell57 United States | 2017 | Adult survivors of childhood acute lymphoblastic leukemia; 87.6% white 52.1% male Community controls 86.7% white 50.1% male |
Prospective | 330 survivors; 331 controls | Survivors: mean 28.9y Controls: Mean 29.2y | Sedentary behavior measured by triaxial accelerometry Sedentary: ≥ the population mean of 60% time spent sedentary Active: < the population mean of 60% time spent sedentary |
Hypertension; High cholesterol; Hypertriglyceridemia; Obesity; Abnormal glucose; Graded with the Criteria for Adverse Events v. 4.03 | Survivors spend more time in sedentary activity compared to controls In sedentary survivors: ↑ risk of onset high cholesterol ↑ risk of any cardiovascular risk factor |
| Physical activity and physical fitness reduce chronic disease risk among childhood cancer survivors | ||||||||
| Jones59 United States | 2014 | Adult survivors of childhood Hodgkin lymphoma 53.2% male 91.0% white | Prospective | 1,187 survivors | Median: 31.2y | Single question from the Youth risk Behavior Surveillance Survey Categories of fitness: 0 MET hours/week (referent) 3 MET hours/week 6 MET hours/week 9 MET hours/week 12 MET hours/week 15–21 MET hours/week | Serious cardiovascular disease graded by the Common Terminology Criteria for Adverse Events v. 4.03 | Significant inverse relationship between time spent in vigorous activity and incident cardiovascular disease |
| Wolfe60 United States | 2012 | Young survivors of posterior fossa tumors 85.7% male 85.7% white | Cross-sectional | 14 survivors | Mean: 14.4y | Maximal cardiopulmonary exercise stress test performed on a leg ergometer Peak VO2 in ml/kg/min VO2 was multiplied by 1.11 to reflect treadmill testing | Late effects severity score for neurological, endocrinological and visual/auditory outcomes | Significant positive correlation between physical fitness and improved neurological outcomes |
| Phillips61 United States | 2019 | Survivors of childhood acute lymphoblastic leukemia 51.0% male 86.5% white Community Controls 48.2% male 89.2% white | Cross-sectional | 341 survivors 288 controls | Survivors Median: 28.5y Controls Median: 32.2 | Submaximal cardiopulmonary exercise test Peak VO2 converted to METs. 1 MET = VO2 of 3.5 ml/kg/min | Neurocognitive testing | A one MET increase resulted in significant increases in Verbal ability Focused attention Verbal Fluency Working memory Dominate motor speed Non-dominate motor speed Memory Academics |
| Physical activity and physical fitness reduce mortality risk in childhood cancer survivors | ||||||||
| Cox63 United States | 2013 | Adult survivors of childhood cancer deceased 49% male 86% white Adult survivors of childhood cancer alive 51% male 89% white | Matched case-control | 445 cases 7,162 controls | Cases Mean: 30.5 Controls Mean: 28.8 | Single question from the Youth risk Behavior Surveillance Survey Categories of activity: 0 days per week 1–2 days per week 3+ days per week (referent) | All-cause mortality Cause-specific mortality | Increased odds of all-cause mortality in those performing less days of PA. Increased odds of cardiovascular or pulmonary morality in survivors who perform less days of PA |
| Scott64 United States | 2018 | Adult survivors of childhood cancer 81.0% white 52.8% male | Prospective | 15,450 survivors | Median 25.9y | Single question from the Youth risk Behavior Surveillance Survey | All-cause mortality Cause-specific mortality | Vigorous Exercise >0 MET/H significantly reduced cumulative incidence of all-cause and cause specific mortality Change in exercise habits decreased risk of all-cause mortality |
| Ness65 United States | 2019 | Survivors of childhood cancer 51.1% male 84.1% white Community Controls 48.8% male 90.2% white | Prospective | 1,260 survivors 285 Controls | Mean 35.7y in survivors exposed to anthracycline/chest radiation Mean 35.3y in survivors not exposed Controls: 34.1y | Peak oxygen consumption (VO2 peak) Exercise intolerance: ≤85% sex- age-predicted VO2 | Organ system impairment measured with: cardiac imaging; autonomic response; pulmonary function; muscle strength; neurosensory integrity Mortality measured with national death index search | ↓Fitness in all survivors compared to control group ↑All-cause mortality in those who have exercise intolerance. ↑odds of exercise intolerance in those who had impaired global longitudinal strain; chronotropic intolerance; impaired strength; impaired pulmonary function |
| Comparing physical fitness and physical activity as separate constructs, and examining their ability to reduce cardiovascular disease risk factors | ||||||||
| Slater69 United States | 2015 | Adult survivors of childhood cancers who underwent hematopoietic cell transplantation; 56.3% male 91.6% white Sibling controls 54.6% male 92.4% white | Cross-sectional | 119 survivors 66 controls | Survivors: mean 27.4y Controls: Mean 25.1y | Physical activity: Modifiable Activity Questionnaire Low: <2.5 hours/week of moderate to vigorous physical activity High: ≥2.5 hours/week of moderate to vigorous physical activity Physical fitness: Six-minute walk test Low ≤588.9 meters High >588.9 meters | Cardiovascular risk factors: Waist circumference Percent fat mass Abdominal subcutaneous fat Visceral fat Lean body mass Triglycerides HDL-C LDL-C Blood pressure Insulin Sensitivity HOMA-IR cCSC cIMT | In low PA survivors: ↑Waist circumference In low physically fit survivors: ↑ Waist circumference ↑ percent fat mass ↓ insulin sensitivity |
| Lemay70 Canada | 2019 | Adult survivors of childhood ALL 49.6% male | Cross-sectional | 246 survivors | 22.2y | Physical activity: Minnesota Leisure Time Physical Activity Questionnaire Low activity: <150 minutes of moderate to vigorous leisure-time physical activity High activity: ≥150 minutes of moderate to vigorous leisure-time physical activity Physical Fitness: Submaximal cardiopulmonary exercise test Examined in increments of 10% | BMI Total body fat % Waist Circumference Dyslipidemia Insulin resistance Metabolic syndrome Reduced ejection fraction Hypertension Cognitive health Depression Bone mineral density | Preventative fraction of PA: ↑Percentage body fat ↑depression ↑bone mineral density Preventative fraction of physical fitness: ↑BMI ↑Percent body fat ↑Waist Circumference ↑Dyslipidemia ↑Depression |
=percent, y=years,
=increased,
=decreased HDL-C= high density lipoprotein cholesterol, LDL-C= low density lipoprotein cholesterol, PA=physical activity, HOMA-IR= homeostasis model assessment of insulin resistance, cCSC= carotid cross-sectional compliance, cIMT= carotid intima-media thickness, MET= metabolic equivalent,
=more than, MET/H=Metabolic equivalent hours
Synthesis
We grouped the studies by the outcomes the study examined. The groups included: risk factors of CVD, clinically evident CVD and neurological outcomes, mortality, and a section examining the differential effects of PA and fitness. The studies in each group were then summarized. Additional information such as study design, demographics of the population, measurements of the exposure and outcomes can be found in Table 1.44
Results
Summary of the characteristics of the studies
We included 11 studies in our scoping review. Of the 11 studies, 8 studies focused on the associations between PA, physical fitness and either clinically evident CVD, or CVD risk factors. Thus, most of this review will be focused on CVD as the outcome. The other three studies reported on the associations between PA, physical fitness, neurological outcomes, and mortality. Two studies examined the differential impact of PA on CVD risk factors and physical fitness on CVD risk factors.
Of the 11 studies, six were cross-sectional, four studies used a prospective cohort design, and one study used matched case-control design. Nearly all studies were conducted in the United States, with one study being conducted in Canada. All studies were published in between 2010 and 2019, indicating a recent interest in this topic. Results from the review are described in the sections below with additional information provided in Table 1.
Physical activity, and risk factors for, or intermediate markers of, cardiovascular disease among childhood cancer survivors
Clinically evident CVD is often defined as a distant end point. Therefore, investigators examining the cardiovascular health of the general population have identified a set of markers that either indicate early damage or predict future disease (Table 2).45–47 These indices are used as surrogates or intermediate markers of disease in both cross sectional studies and intervention studies to identify persons most at risk for adverse outcomes and/or to select those who might be responsive to intervention.48–54 These indices have also been used to examine early cardiovascular health in childhood cancer survivors, discussed below.55–57
Table 2:
Markers of early cardiovascular disease and how they are measured
| Markers | Measured outcomes |
|---|---|
| Obesity | Body mass index Waist circumference Percent body fat Abdominal subcutaneous fat Abdominal visceral fat Lean body mass |
| Hypertension | Systolic blood pressure Diastolic blood pressure |
| Hyperlipidemia | Triglycerides High density lipoprotein cholesterol Low density lipoprotein cholesterol |
| Insulin homeostasis dysfunction | Insulin sensitivity Homeostasis model assessment of insulin resistance |
| Structural or functional heart defects | Carotid cross-sectional distensibility Carotid cross-sectional compliance Carotid intima-media thickness |
Associations between PA, measured by questionnaires of habitual activity or with activity monitors, and CVD risk factors are reported in three key papers among childhood cancer survivors with mean ages ranging from 14 to 50 years of age.55–57 Slater et al. reported that young childhood cancer survivors who were physically active ≥60 minutes per day (min/day) had lower percent fat mass (24.4 ± 1.3 vs. 29.8 ± 0.9%, p<0.01) and higher lean body mass (41.3 ± 0.7 vs. 39.5 ± 0.5 kg, p<0.01) compared to less active (<60 min/day) survivors.55 The effects of high PA on body composition were more pronounced in cancer survivors than in controls, with greater differences in waist circumference (2.1 cm vs. 1.0 cm, pinteraction = 0.04), percent body fat (5.4 vs. 2.7%, pinteraction = 0.04), abdominal subcutaneous fat (29.8 vs. 9.2 cm3 pinteraction = 0.02), and abdominal visceral fat (4.9 vs. 0.2 cm3 pinteraction <0.01). PA was not associated with lipid values, glucose homeostasis, blood pressure, or carotid artery measures. Fortunately, as survivors age, PA can positively impact not only body composition, like the younger survivors, but also blood pressure, lipid values, and glucose homeostasis.56 In an older cohort of childhood cancer survivors, Meacham et al. reported sedentary survivors had increased odds of obesity (odds ratio: 1.3; 95% CI: 1.1 – 1.5), hypertension (OR: 1.5; 95% CI: 1.2 – 1.8), dyslipidemia (OR: 1.3; 95% CI: 1.0 −1.6), diabetes (OR: 1.7; 95% CI: 1.2 – 2.3), and having a combination of any three cardiovascular risk factors (OR: 1.7; 95% CI: 1.1 – 2.6).56 Measuring PA with accelerometers, which is more objective than self-report, confirm the previous results.57 Howell et al. reported longitudinal (mean follow-up 5.2 ± 1.5 years) associations between PA (assessed via accelerometry) and CVD risk factors among acute lymphoblastic leukemia survivors and community controls.57 Survivors completed, on average, fewer min/day of moderate to vigorous PA compared to controls (17.3, 95% CI: 15.6 – 19.1 vs. 21.2, 95% CI: 19.5 – 22.9 min/day, p <0.01). Importantly, survivors who spent >60% per day of their time being sedentary had increased risk of developing high total cholesterol (HR: 2.52, 95% CI: 1.12, 5.64), or any cardiovascular risk factor (HR: 1.96, 95% CI: 1.16 – 3.30) when compared to those not sedentary. These data, while observational, suggest that participating in PA can help reduce CVD risk factors in survivors, even after they transition from early to long-term survivorship.
Physical activity and physical fitness reduce chronic disease risk among childhood cancer survivors
While previous studies indicate that PA is an important tool in the management of CVD risk factors, eventual development of CVD may be unavoidable in some childhood cancer survivors because of previous exposure to cardiotoxic agents as part of their cancer therapy.58 Nevertheless, data from a prospective observational study shows that, even among individuals with these exposures, childhood cancer survivors who report more PA have lower risk for clinically evident CVD.59 In a study that included adult survivors of Hodgkin Lymphoma, Jones et al., ascertained PA by self-report, and reported participation in vigorous PA ≥9 metabolic equivalent [MET] hours/week reduced risk for coronary artery disease (relative risk [RR]: 0.53; 95% CI: 0.32 – 0.89), valve replacement (RR: 0.36; 95% CI: 0.14 – 0.95), or any grade 3–5 cardiovascular event (RR: 0.49; 95% CI: 0.31 – 0.76) when compared to reported participation in PA <9 MET hours/week.59 In this study, duration of self-reported vigorous exercise also had a graded, inverse relationship with any serious, life threatening, disabling or fatal cardiovascular event, with risk ratios of 0.87 (95% CI: 0.56 – 1.34) in those who performed 3–6 MET hours/week, 0.45 (95% CI: 0.26 – 0.80) for 9–12 MET hours/week and 0.47 (95% CI: 0.23 – 0.95) for 15 to 21 MET hours/week, compared to those who performed 0 MET hours/week (ptrend = 0.002).59
While most physical fitness and/or PA studies among childhood cancer survivors have focused on associations between PA and CVD, increasing physical fitness has potential benefit for other organ systems. While this research is well established in the general population, it is still novel in childhood cancer survivors.21 Despite the novelty, observational research indicates increasing physical fitness among childhood cancer survivors may also provide neurological benefit.60,61 Two relevant studies report associations between cardiorespiratory fitness and neurological function in both adolescent (mean age 14.4 years) and young adult (mean age 28.5 years) childhood cancer survivors.60,61 Among adolescent survivors of medulloblastoma, cardiorespiratory fitness was measured using a maximal cardiopulmonary exercise test (CPET) on a stationary cycle to ascertain aerobic capacity (VO2peak), and late effects (including neurologic outcomes) with a system that assigns a score from 0 (no impairment) to 2 (impairment requiring medical intervention).60,62 Aerobic capacity was associated with lower neurological scores (partial r:−0.69, p <0.05). In a cohort of young adult survivors of childhood cancer, a one MET higher performance on CPET was associated with better verbal ability (beta [β]: 0.10; 95% CI: 0.024 – 0.18; p <0.01), focus (β: 0.096; 95% CI: 0.0045 – 0.19; p=0.04), verbal fluency (β: 0.13; 95% CI: 0.053 – 0.20; p <0.01), working memory (β : 0.07; 95% CI: 0.0062 – 0.13; p=0.03), dominant hand motor speed (β: 0.15; 95% CI: 0.0062 – 0.24; p <0.01), non-dominant hand motor speed (0.15; 95% CI: 0.058 – 0.25; p<0.01), visual-motor speed (β: 0.010; 95% CI: 0.031 – 0.16; p <0.01), memory (β: 0.11; 95% CI: 0.035 – 0.18;p <0.01), reading (β: 0.050; 95% CI: 0.0050 – 0.095; p=0.03), and math (β: 0.098; 95% CI: 0.039 – 0.16; p <0.01).61
Physical activity and physical fitness reduce mortality risk in childhood cancer survivors
There is observational evidence that childhood cancer survivors who are more active or more physically fit have lower risk for mortality when compared to those less active or physically fit, even among survivors with clinically evident organ dysfunction and exposure to cardiotoxic therapies.63–65 Cox et al. used a matched case-control design to examine associations between PA, all-cause and cause-specific mortality (determined via National Death Index).63 Cases (survivors who died) and controls (survivors who did not die) self-reported PA at baseline, and were matched by primary diagnosis, age at baseline questionnaire, and survival time. Length of follow up time from baseline to death was categorized into tertiles, <5.7 years (n = 148), 5.7 – 8.9 years (n=148), and >8.9 years (n=149). Among survivors who were <10.7 years from diagnosis, those who reported 0 days per week of PA had increased odds of all-cause mortality compared to those who reported 3+ days per week of PA (OR: 2.06, 95% CI: 1.18 – 3.61).63 In survivors who were 10.7 – 18.9 years from diagnosis, those who reported only 1–2 days per week of PA had increased odds of dying compared to survivors who reported 3+ days per week of PA (OR: 2.61, 95% CI: 1.50 – 4.53).63 In survivors who were >18.9 years from diagnosis who reported 0 days per week of PA had increased odds of dying compared to those who reported 3+ days per week of PA (OR: 2.13, 95% CI: 1.27 – 3.56).63 Finally, survivors who reported 1–2 days per week of PA had increased odds of dying from cardiac or pulmonary reasons compared to survivors who performed 3+ days per week of PA (OR: 2.07, 95% CI: 1.00 – 4.30).63 More recently, Scott et al. examined associations between self-reported vigorous PA and both all-cause mortality and non-accidental causes of death among adult childhood cancer survivors.64 Survivors who reported 15–18 MET hours/week of vigorous PA (compared to those who reported 0 MET hours/week) had reduced risk for all-cause (RR: 0.58; 95% CI: 0.42 – 0.80) and non-accidental death (RR: 0.63; 95% CI: 0.43 – 0.92).64 Additionally, in survivors who completed a follow-up questionnaire (n=2,279), a reported increase in PA (6 MET hours/week) over 7.9 (6.4 – 9.4) years was associated with 13% reduction of all-cause mortality.64 While both studies indicated strong associations between PA and mortality, independent of baseline chronic disease, they relied on self-report to ascertain both PA and baseline chronic disease.
The results of the prior studies were further validated in childhood cancer survivors whose physical fitness and baseline chronic disease were measured objectively.65 The authors examined the associations between physical fitness (using CPET to ascertain VO2peak), and mortality in childhood cancer survivors. Survivors were categorized into two groups: those who were exposed to cardiotoxic therapies (chest radiation/anthracyclines), and those not exposed to cardiotoxic therapies. Cancer-free community controls were also examined. Organ system impairment was accounted for, and defined as impaired ejection fraction (ejection fraction <53%), impaired global longitudinal strain (global longitudinal strain >1.5 standard deviations above sex-, age-, and vendor-specific means), chronotropic incompetence (<80% of age- sex-predicted HR reserve on the stress test), impaired pulmonary function (forced expiratory volume <80%), impaired muscular strength (peak torque knee extension z-score <1.5 standard deviations of control group), and impaired neurosensory integrity (modified total neuropathy score >5).65 Both exposed (VO2peak = 25.7 ± 8.6 ml/kg/min) and non-exposed (VO2peak = 26.8 ± 8.4 ml/kg/min) survivors had significantly lower aerobic capacity compared to community controls (VO2peak = 32.7 ± 7.8, p<0.01). After accounting for organ system dysfunction, with a median of 4 years of follow-up, the HR for National Death Index determined all-cause mortality in both exposed and non-exposed survivors was 3.93 (95% CI: 1.09 – 14.14) in those with exercise intolerance compared to those who were normal.65
Comparing physical fitness and physical activity as separate constructs, and examining their ability to reduce cardiovascular disease risk factors
Most studies evaluating associations between PA and chronic disease or mortality among childhood cancer survivors use PA as a surrogate for physical fitness. This is because there is observational evidence that suggest both participating in more PA and better physical fitness are associated with better health outcomes in this population.57,59,65 Thus, PA and physical fitness are highly correlated. However, PA and physical fitness are not equivalent. This is an important distinction, because there is some indication that childhood cancer survivors may not respond to regular PA when compared to peers. Even when survivors and peers report similar levels of PA, survivors’ performance on physical fitness measures is less robust.66 The differences between PA and physical fitness may be methodological due known biases with self-reported PA.67,68 However, several observational studies have evaluated both PA and physical fitness in childhood cancer survivors and demonstrate that physical fitness is negatively associated with more adverse health outcomes than is PA.69,70
The differential association between physical fitness (measured by submaximal or maximal exercise tests), PA (measured by questionnaires of habitual activity), and CVD risk factors among young adult childhood cancer survivors a mean age of 22 to 25 years is reported in two studies.69,70 Slater et al. found only one outcome associated with PA: waist circumference.67 Survivors who reported ≥2.5 hours/week of PA had lower values (81.9 ± 2.5 centimeters (cm)) compared to survivors who reported <2.5 hours/week of PA (88.6 ± 3.1 cm, p=0.009).69 However, in a subset of the sample (82 survivors, 33 controls) who completed a six-minute walk test to measure physical fitness, and who were categorized with low or high physical fitness using the median walk distance (588.9 meters) as the cut point, physical fitness was associated with a greater number of outcomes. Survivors with high physical fitness had, on average, lower waist circumference (77.8 ± 2.6 vs. 87.8 ± 2.5 cm, p<0.01), lower percent fat mass (33.6 ± 1.8% vs. 39.4 ± 1.7%, p<0.01), and higher insulin sensitivity (10.9 ± 1.0 mg/kg/min vs. 7.4 ± 1.1 mg/kg/min, p<0.01) compared to survivors with low physical fitness.69 Similar results are reported in a study by Lemay et al., who found that ≥150 minutes/week of moderate or vigorous PA was associated with three significant preventive fractions (PF): body fat >25% males/ >35% females ([PF]: 0.55, 95% CI: 0.10 – 0.78), depression (Brief Symptom Inventory T-score ≥63, PF: 0.81, 95% CI: 0.39 – 0.94), and low bone mineral density (≤−1 z-score on dual-energy x-ray absorptiometry [PF: 0.60, 95% CI: 0.20, 0.80]).70 However, every 10% increase in physical fitness (measured by a maximal CPET using a leg ergometer) was associated with five: BMI ≥30 kg/m2 (PF: 0.24, 95% CI: 0.11 – 0.36), body fat (PF: 0.22, 95% CI: 0.03 – 0.46), waist circumference (males: ≥102 cm females: ≥88 cm [PF: 0.25, 95% CI: 0.08 – 0.38]), HDL-C (males: <1.03/females: <1.3 mmol/L [PF: 0.21, 95% CI: 0.03 – 0.38]), and depression (Brief Symptom Inventory T-score ≥63 [PF: 0.26, 95% CI: 0.02 – 0.43]).70
Discussion
With more than 500,000 childhood cancer survivors living in the United States today,1,2 and 62.3% of them at risk for chronic disease,3 interventions to ameliorate chronic disease are of interest and relevance. Our review shows that modifiable lifestyle choices, including PA, are likely effective in improving physical fitness and reducing the risk for future organ dysfunction. Most research has been focused on improving physical fitness to CVD risk, due to its association with early mortality in childhood cancer survivors.71 However, because childhood cancer survivors are also at increased risk for early onset endocrine,72,73 musculoskeletal,74 and severe neurological disorders,75,76 and because PA and associated gains in physical fitness mitigates risk for these diseases in other populations,77–79 observational and interventional work is needed to determine if increasing PA and improving physical fitness decreases the risk of future chronic disease in childhood cancer survivors.
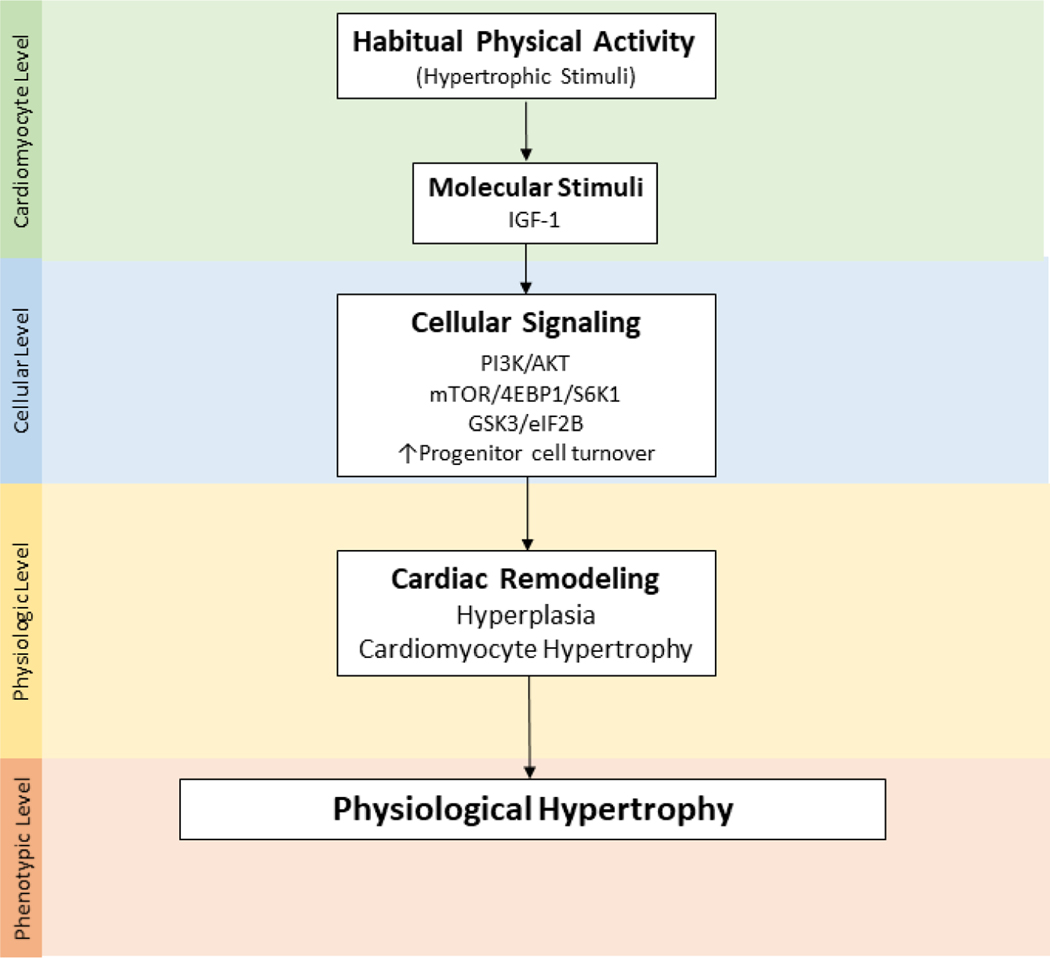
The robust literature describing biological mechanisms responsible for associations between PA, physical fitness, and CVD in non-cancer populations provides some insight into why PA and/or better physical fitness may reduce, but not completely eliminate CVD risk among childhood cancer survivors.80–82 Acute, and consistent bouts of moderate and/or vigorous PA influence cardiovascular homeostasis by stimulating specific cellular signaling pathways that initiate cellular adaptations of cardiac tissue, eventually resulting in beneficial or physiologic hypertrophy (Figure 3).
Figure 3.
Key molecular and cellular cardiac outcome from habitual physical activity
Phosphatidylinositol-3-kinase (PI3K), protein kinase B (AKT), mammalian target of rapamycin (mTOR), ribosomal protein S6 kinase beta-1 (S6K1), eukaryotic translation initiation factor 4E binding protein 1 (4EBP1), glycogen synthase kinase-3 beta (GSK3β), eukaryotic translation initiation factor 2B (eIF2B)
During childhood, adolescence and young adulthood, in order to respond to the changing needs of the body, cardiac tissue has robust growth and regenerative capacity.83 Cardiac hyperplasia (new cell formation) exceeds cell death, significantly influencing cardiac growth.84 Physiologic hypertrophy is normal, and typically characterized by a modest increase in ventricular volume with a coordinated growth in wall and septal thicknesses, and/or a reduction in left ventricular chamber dimension, with an increase in free wall and septal thicknesses. In childhood cancer survivors, physiologic cardiac hypertrophy is blunted.85 Survivors who were unexposed and exposed to cardiotoxic therapies (anthracyclines and chest radiation) have decreased left ventricular mass and wall thickness compared to sibling controls.85
An abnormal hypertrophic response to exercise among childhood cancer survivors may be due, in part, to abnormal molecular responses to exercise. In non-cancer populations, molecular responses to exercise contribute to cardiomyocyte growth by influencing intracellular signaling pathways, gene expression and ribonucleic acid transcription/translation, resulting in contractile protein accumulation within the cell and increased cell mass.86–91 There is also evidence that resident endogenous cardiac stem and progenitor cells (eCSC) are activated under increased cardiac workloads and contribute to cellular hypertrophy.92,93 Unfortunately, anthracycline treatment, often included as part of therapy for childhood cancer interferes with mechanisms of cardiovascular adaptation by increasing oxidative stress, causing premature myocyte apoptosis,94 and targeting and destroying myocyte eCSC.95 Young heart tissue in children with cancer is also sensitive to radiation induced deoxyribonucleic acid damage, resulting in activation of the p53 pathway and further inducing cellular apoptosis.96 It is possible that chemotherapy induced dysregulation of eCSC turnover and radiation induced apoptosis during childhood reduce the number of healthy adult myocytes, impairing adaptive growth when young survivors transition into adulthood.
Past cancer treatment also interferes with other cardiac and vascular adaptative mechanisms, including endothelial cell response. Endothelial cells line the walls of the heart chambers, coronary vessels, and peripheral vasculature, and are mechanosensitive.97,98 In healthy individuals, these cells respond to sheer forces and increase venous return by increasing production of nitric oxide, a potent vasodilator.97,98 Vasodilation improves luminol blood flow, tissue elasticity, vascular diameter, and blood flow to all of the body’s organs.99–103 However, in childhood cancer survivors, treatment exposures reduce system wide nitric oxide reserves (causing endothelial dysfunction).95
The relationship between PA, physical fitness, and health is interdependent.79,104,105 As the heart and the rest of the body adapt, physical fitness increases, enabling increased frequency, duration, and intensity of PA. However, childhood cancer survivors do not appear to reap the same rewards from PA as their peers,30 and have lower physiological reserve than the cancer-free population.106–108 For survivors of childhood cancer, low physiological reserve may limit healthy, physiological adaptation from repeated bouts of PA, thus, increasing the risk of age-related chronic diseases. However, this does not mean that childhood cancer survivors should not engage in exercise. The current literature demonstrates that survivors who engage in PA, even if they have past exposure to cardiotoxic therapies, have reduced risk of adverse cardiac outcomes. More studies are needed to determine the most effective interventions (intervention timing, behavioral strategies, delivery mechanisms, exercise dose) to improve physical fitness in survivors to a level that there is an impact on chronic disease risk.
While this review gives insight into how PA and physical fitness impact chronic disease among childhood cancer survivors, there are limitations that should be discussed. First, this review only examined observational studies. This was an intentional choice given other systematic reviews have examined exercise interventions in childhood cancer survivors.109,110 Furthermore, most of the interventions focus on improving physical fitness as an outcome, with some including early CVD risk factors. Synthesizing the observational research allowed us to examine more late effects, such as clinically evident CVD, and mortality, in an older cohort. Thus, this review does not address if changing PA or physical fitness through an intervention will influence cardiovascular outcomes or mortality among childhood cancer survivors Also, most of the studies examining the association between PA or physical fitness and chronic disease were cross-sectional, limiting an evaluation of the temporal association between PA/fitness and chronic disease. Second, we made no attempt to identify unpublished studies such as dissertations, raising the possibility that some studies were missed. Third, the majority of studies reviewed used self-reported PA.55,59 Measurement error, resulting from seasonal, response, and recall bias are common when persons are asked about their PA, impacting accuracy when used as a substitute for cardiopulmonary fitness.67,68,111 Fourth, when comparing the differences between PA and physical fitness and their impact on chronic disease, one of the two studies used the six-minute walk test as the measure for physical fitness. In childhood cancer survivors, the six-minute walk test has been associated with decreased muscular strength and neuropathy.112,113 Therefore, the six-minute walk test may predict overall physical function rather than purely cardiopulmonary fitness. Fortunately, the six-minute walk test is valid when predicting childhood cancer survivors’ VO2.114 Finally, only 11 studies were discussed in this review. Thus, the association we describe between PA, physical fitness and chronic disease is preliminary. The conclusions may change as more research is done. Systematic reviews and meta-analysis will be needed as future studies are published on this topic.
Childhood cancer survivors who perform PA or who are physically fit have improved markers of CVD, decreased risk for clinically evident CVD, and mortality compared to less active/fit survivors. The literature indicates that physical fitness is more strongly associated with future health than self-reported or measured PA. Unfortunately, childhood cancer survivors do not appear to benefit as much as their peers from regular PA. Cytotoxic cancer therapies are known to impact organ function, reducing physiologic reserve and associated exercise responses. Thus, research is needed to determine if and which specific exercise interventions (with documented duration, intensity, frequency, and timing) best perturb cardiovascular adaptation and maximize health benefits in childhood cancer survivors. Mechanistic work is also needed to better understand why survivors respond differently than their peers to exercise interventions and to identify supplement or pharmaceutical mechanisms that might enhance survivors exercise response. Nevertheless, while the research community awaits new insights into how PA and physical fitness play a role in chronic disease risk, inactive childhood cancer survivors should be encouraged to be more physically active, and be referred to exercise specialists with expertise adapting programs to address the unique physiological characteristics of this population.
Acknowledgments
Financial support for this work provided by a Cancer Center Core Grant, P30CA021765 Charles Roberts, from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC). The authors would like to acknowledge Tracie Gatewood for her assistance with article preparation.
Footnotes
Data sharing
Data sharing not applicable to this article as no new data was created or analyzed.
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 2.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. NEJM. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 4.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St. Jude Lifetime cohort study. J Clin Oncol. 2013;31(36):4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community‐dwelling older persons: a systematic review. J Am Geri Soc. 2012;60(8):1487–1492. [DOI] [PubMed] [Google Scholar]
- 6.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St. Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St. Jude Lifetime Cohort Study. Lancet Oncol. 2016;17(9):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19(12):1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. Jnci-J Natl Cancer I. 2008;100(19):1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado-Cruzata L, Zhang W, McDonald JA, et al. Dietary modifications, weight loss, and changes in metabolic markers affect global DNA methylation in Hispanic, African American, and Afro-Caribbean breast cancer survivors. J Nutr. 2015;145(4):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112–1120. [DOI] [PubMed] [Google Scholar]
- 13.Kraig E, Linehan LA, Liang H, et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp Gerontol. 2018;105:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23(6):1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daum C, Cochrane S, Fitzgerald J, Johnson L, Buford T. Exercise Interventions for Preserving Physical Function Among Cancer Survivors in Middle to Late Life. J Frailty Aging. 2016;5(4):214–224. [DOI] [PubMed] [Google Scholar]
- 16.Negm AM, Kennedy CC, Thabane L, et al. Management of frailty: a systematic review and network meta-analysis of randomized controlled trials. J Am Med Dir Assoc. 2019;20(10):1190–1198. [DOI] [PubMed] [Google Scholar]
- 17.Furtado G, Patrício M, Loureiro M, Teixeira AM, Ferreira JP. Physical fitness and frailty syndrome in institutionalized older women. Percept Mot Skills. 2017;124(4):754–776. [DOI] [PubMed] [Google Scholar]
- 18.Ness KK, Partin M, Robyn, Howell CR, et al. Progression of frailty in young adult survivors of childhood cancer: St. Jude Lifetime Cohort. J Clin Oncol. 2019;37(15_suppl):10057. [Google Scholar]
- 19.ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer; 2018. [Google Scholar]
- 20.ACSM’s Guidelines for Exercise Testing and Prescription. 9th ed. Philadelphia, PA: Wolters Kluwer; 2013. [Google Scholar]
- 21.Willis BL, Gao A, Leonard D, DeFina LF, Berry JD. Midlife fitness and the development of chronic conditions in later life. Arch Intern Med. 2012;172(17):1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. NEJM. 2002;346(11):793–801. [DOI] [PubMed] [Google Scholar]
- 23.Martin B-J, Arena R, Haykowsky M, et al. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc. 2013;88(5):455–463. [DOI] [PubMed] [Google Scholar]
- 24.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med. 2004;164(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 25.Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Cardiorespiratory fitness and the risk for stroke in men. Arch Intern Med. 2003;163(14):1682–1688. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen F, Lambrechtsen J, Siersted H, Hansen H, Hansen N. Low physical fitness in childhood is associated with the development of asthma in young adulthood: the Odense schoolchild study. Eur Respir J. 2000;16(5):866–870. [DOI] [PubMed] [Google Scholar]
- 27.Hayek S, Gibson TM, Leisenring WM, et al. Prevalence and predictors of frailty in childhood cancer survivors and siblings: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2020;38(3):232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hocking MC, Schwartz LA, Hobbie WL, et al. Prospectively examining physical activity in young adult survivors of childhood cancer and healthy controls. Pediatr Blood Cancer. 2013;60(2):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung OJ, Li HCW, Chiu SY, Ho KYE, Lopez V. The impact of cancer and its treatment on physical activity levels and behavior in Hong Kong Chinese childhood cancer survivors. Cancer Nurs. 2014;37(3):E43–E51. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31(22):2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wogksch MD, Howell CR, Wilson CL, et al. Physical fitness in survivors of childhood Hodgkin lymphoma: A report from the St. Jude Lifetime Cohort. Pediatr Blood Cancer. 2019;66(3):e27506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AM, Lopez‐Mitnik G, Somarriba G, et al. Exercise capacity in long‐term survivors of pediatric cancer: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Pediatr Blood Cancer. 2013;60(4):663–668. [DOI] [PubMed] [Google Scholar]
- 33.Antwi GO, Jayawardene W, Lohrmann DK, Mueller EL. Physical activity and fitness among pediatric cancer survivors: A meta-analysis of observational studies. Support Care Cancer. 2019;27(9):1–12. [DOI] [PubMed] [Google Scholar]
- 34.Van Leeuwen P, Van Der Net J, Helders P, Takken T. Exercise parameters in healthy Dutch children. Geneeskd Sport. 2004;37(5):126–132. [Google Scholar]
- 35.Tonorezos ES, Snell PG, Moskowitz CS, et al. Reduced cardiorespiratory fitness in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60(8):1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartman A, Pluijm SM, Wijnen M, et al. Health‐related fitness in very long‐term survivors of childhood cancer: A cross‐sectional study. Pediatr Blood Cancer. 2018;65(4). [DOI] [PubMed] [Google Scholar]
- 37.Lefai E, Blanc S, Momken I, et al. Exercise training improves fat metabolism independent of total energy expenditure in sedentary overweight men, but does not restore lean metabolic phenotype. Int J Obesity. 2017;41(12):1728–1736. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira ML, Sherrington C, Smith K, et al. Physical activity improves strength, balance and endurance in adults aged 40–65 years: a systematic review. J Physiother. 2012;58(3):145–156. [DOI] [PubMed] [Google Scholar]
- 39.Dunn AL, Garcia ME, Marcus BH, Kampert JB, Kohl H, Blair SN. Six-month physical activity and fitness changes in Project Active, a randomized trial. Med Sci Sports Exerc. 1998;30(7):1076–1083. [DOI] [PubMed] [Google Scholar]
- 40.Tikkanen E, Gustafsson S, Ingelsson E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK Biobank Study. Circulation. 2018;137(24):2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers J, Kokkinos P, Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. 2019;11(7):1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stolley MR, Restrepo J, Sharp LK. Diet and physical activity in childhood cancer survivors: a review of the literature. Ann Behav Med. 2010;39(3):232–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizrahi D, Wakefield CE, Fardell JE, et al. Distance-delivered physical activity interventions for childhood cancer survivors: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;118:27–41. [DOI] [PubMed] [Google Scholar]
- 44.Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2013;13(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di EA, Gao P, Pennells L, et al. Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307(23):2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PloS One. 2012;7(12):e52036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–1313. [DOI] [PubMed] [Google Scholar]
- 48.Dalton M, Cameron AJ, Zimmet PZ, et al. Waist circumference, waist–hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. 2003;254(6):555–563. [DOI] [PubMed] [Google Scholar]
- 49.Van Pelt R, Evans E, Schechtman K, Ehsani A, Kohrt W. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab. 2002;282(5):E1023-E1028. [DOI] [PubMed] [Google Scholar]
- 50.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383(9932):1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25(7):1177–1184. [DOI] [PubMed] [Google Scholar]
- 52.Barenbrock M, Kosch M, Jöster E, Kisters K, Rahn K-H, Hausberg M. Reduced arterial distensibility is a predictor of cardiovascular disease in patients after renal transplantation. J Hypertens. 2002;20(1):79–84. [DOI] [PubMed] [Google Scholar]
- 53.Ozaki H, Miyachi M, Nakajima T, Abe T. Effects of 10 weeks walk training with leg blood flow reduction on carotid arterial compliance and muscle size in the elderly adults. Angiology. 2011;62(1):81–86. [DOI] [PubMed] [Google Scholar]
- 54.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168(12):1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slater ME, Ross JA, Kelly AS, et al. Physical activity and cardiovascular risk factors in childhood cancer survivors. Pediatr Blood Cancer. 2015;62(2):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer—a report from the childhood cancer survivor study. Cancer Epidemiol Biomark Prev. 2010;19(1):170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howell CR, Wilson CL, Ehrhardt MJ, et al. Clinical impact of sedentary behaviors in adult survivors of acute lymphoblastic leukemia: a report from the St. Jude Lifetime Cohort study. Cancer. 2018;124(5):1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bates JE, Howell RM, Liu Q, et al. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the childhood cancer survivor study. J Clin Oncol. 2019;37(13):1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones LW, Liu Q, Armstrong GT, et al. Exercise and Risk of Major Cardiovascular Events in Adult Survivors of Childhood Hodgkin Lymphoma: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2014;32(32):3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolfe KR, Hunter GR, Madan-Swain A, Reddy AT, Baños J, Kana RK. Cardiorespiratory fitness in survivors of pediatric posterior fossa tumor. Pediatr Hematol Oncol J. 2012;34(6):e222–e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phillips NS, Howell CR, Lanctot JQ, et al. Physical fitness and neurocognitive outcomes in adult survivors of childhood acute lymphoblastic leukemia: A report from the St. Jude Lifetime cohort. Cancer. 2020;126(3):640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patton J, Vogel J, Mello R. Evaluation of a maximal predictive cycle ergometer test of aerobic power. Eur J Appl Physiol. 1982;49(1):131–140. [DOI] [PubMed] [Google Scholar]
- 63.Cox CL, Nolan VG, Leisenring W, et al. Noncancer-related mortality risks in adult survivors of pediatric malignancies: the childhood cancer survivor study. J Cancer Surviv. 2014;8(3):460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scott JM, Li N, Liu Q, et al. Association of Exercise With Mortality in Adult Survivors of Childhood Cancer. JAMA Oncol. 2018;4(10):1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ness KK, Plana JC, Joshi VM, et al. Exercise Intolerance, Mortality, and Organ System Impairment in Adult Survivors of Childhood Cancer. J Clin Oncol. 2020;38(1):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31(22):2799–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pivarnik JM, Reeves MJ, Rafferty AP. Seasonal variation in adult leisure-time physical activity. Med Sci Sports Exerc. 2003;35(6):1004–1008. [DOI] [PubMed] [Google Scholar]
- 68.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr. 2008;5(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slater ME, Steinberger J, Ross JA, et al. Physical Activity, Fitness, and Cardiometabolic Risk Factors in Adult Survivors of Childhood Cancer with a History of Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21(7):1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lemay V, Caru M, Samoilenko M, et al. Prevention of long-term adverse health outcomes with cardiorespiratory fitness and physical activity in childhood acute lymphoblastic leukemia survivors. Pediatr Hematol Oncol J. 2019;41(7):e450–e458. [DOI] [PubMed] [Google Scholar]
- 71.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27(14):2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gurney JG, Kadan‐Lottick NS, Packer RJ, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer-Am Cancer Soc. 2003;97(3):663–673. [DOI] [PubMed] [Google Scholar]
- 73.Chemaitilly W, Sklar CA. Endocrine complications in long-term survivors of childhood cancers. Endocr Relat Cancer. 2010;17(3):R141–R159. [DOI] [PubMed] [Google Scholar]
- 74.P L Gawade, M M Hudson, S C Kaste, et al. A systematic review of selected musculoskeletal late effects in survivors of childhood cancer. Curr Pediatr Rev. 2014;10(4):249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21(17):3255–3261. [DOI] [PubMed] [Google Scholar]
- 76.Kandula T, Farrar MA, Cohn RJ, et al. Chemotherapy-induced peripheral neuropathy in long-term survivors of childhood cancer: clinical, neurophysiological, functional, and patient-reported outcomes. JAMA Neurol. 2018;75(8):980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2018;26(2):615–624. [DOI] [PubMed] [Google Scholar]
- 78.Katzmarzyk P, Craig C, Gauvin L. Adiposity, physical fitness and incident diabetes: the physical activity longitudinal study. Diabetologia. 2007;50(3):538–544. [DOI] [PubMed] [Google Scholar]
- 79.Pareja-Galeano H, Garatachea N, Lucia A. Exercise as a polypill for chronic diseases. Progress in molecular biology and translational science. Vol 135: Elsevier; 2015:497–526. [DOI] [PubMed] [Google Scholar]
- 80.Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993;88(1):116–126. [DOI] [PubMed] [Google Scholar]
- 81.Ehsani AA, Biello DR, Schultz J, Sobel BE, Holloszy J. Improvement of left ventricular contractile function by exercise training in patients with coronary artery disease. Circulation. 1986;74(2):350–358. [DOI] [PubMed] [Google Scholar]
- 82.Schrauwen-Hinderling VB, Hesselink MK, Meex R, et al. Improved ejection fraction after exercise training in obesity is accompanied by reduced cardiac lipid content. J Clin Endocr Metab. 2010;95(4):1932–1938. [DOI] [PubMed] [Google Scholar]
- 83.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92(2):139–150. [DOI] [PubMed] [Google Scholar]
- 85.Lipshultz SE, Landy DC, Lopez-Mitnik G, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30(10):1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sutton J, Lazarus L. Growth hormone in exercise: comparison of physiological and pharmacological stimuli. J Appl Physiol. 1976;41(4):523–527. [DOI] [PubMed] [Google Scholar]
- 87.Neri Serneri GG, Boddi M, Modesti PA, et al. Increased cardiac sympathetic activity and insulin-like growth factor-I formation are associated with physiological hypertrophy in athletes. Circ Res. 2001;89(11):977–982. [DOI] [PubMed] [Google Scholar]
- 88.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. [DOI] [PubMed] [Google Scholar]
- 89.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(20):3589–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol. 2008;153 Suppl 1(Suppl 1):S137–S153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bogorad AM, Lin KY, Marintchev A. eIF2B Mechanisms of Action and Regulation: A Thermodynamic View. Biochemistry. 2018;57(9):1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torella D, Ellison GM, Méndez-Ferrer S, Ibanez B, Nadal-Ginard B. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S8–S13. [DOI] [PubMed] [Google Scholar]
- 93.Urbanek K, Quaini F, Tasca G, et al. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100(18):10440–10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen B, Peng X, Pentassuglia L, Lim CC, Sawyer DB. Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7(2):114–121. [DOI] [PubMed] [Google Scholar]
- 95.Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of Anthracyclines. Front Cardiovasc Med. 2020;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spetz J, Moslehi J, Sarosiek K. Radiation-induced cardiovascular toxicity: mechanisms, prevention, and treatment. Curr Treat Options Cardiovasc Med. 2018;20(4):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Casey DP, Hart EC. Cardiovascular function in humans during exercise: role of the muscle pump. J Physiol. 2008;586(21):5045–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88(3):1009–1086. [DOI] [PubMed] [Google Scholar]
- 100.White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol. 1998;85(3):1160–1168. [DOI] [PubMed] [Google Scholar]
- 101.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297(3):H1109-H1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miyazaki H, Oh-ishi S, Ookawara T, et al. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol. 2001;84(1–2):1–6. [DOI] [PubMed] [Google Scholar]
- 103.Gielen S, Sandri M, Erbs S, Adams V. Exercise-induced modulation of endothelial nitric oxide production. Curr Pharm Biotechnol. 2011;12(9):1375–1384. [DOI] [PubMed] [Google Scholar]
- 104.Ruegsegger GN, Booth FW. Health benefits of exercise. Cold Spring Harb Perspect Med. 2018;8(7):a029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L. Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget. 2017;8(27):45008–45019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaneko S, Tham EB, Haykowsky MJ, et al. Impaired left ventricular reserve in childhood cancer survivors treated with anthracycline therapy. Pediatr Blood Cancer. 2016;63(6):1086–1090. [DOI] [PubMed] [Google Scholar]
- 107.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. NEJM. 1991;324(12):808–815. [DOI] [PubMed] [Google Scholar]
- 108.Mulrooney DA, Ness KK, Huang S, et al. Endothelial dysfunction in adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort study. J Clin Oncol. 2017;35(15_suppl):10564. [Google Scholar]
- 109.Morales JS, Valenzuela PL, Herrera-Olivares AM, et al. Exercise interventions and cardiovascular health in childhood cancer: A meta-analysis. Int J Sports Med. 2020;41(03):141–153. [DOI] [PubMed] [Google Scholar]
- 110.Morales JS, Valenzuela PL, Herrera-Olivares AM, et al. What are the effects of exercise training in childhood cancer survivors? A systematic review. Cancer Metastasis Rev. 2020:1–11. [DOI] [PubMed] [Google Scholar]
- 111.Adams SA, Matthews CE, Ebbeling CB, et al. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol. 2005;161(4):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCrary JM, Goldstein D, Wyld D, Henderson R, Lewis CR, Park SB. Mobility in survivors with chemotherapy-induced peripheral neuropathy and utility of the 6-min walk test. J Cancer Surviv. 2019;13(4):495–502. [DOI] [PubMed] [Google Scholar]
- 113.Ramari C, Moraes AG, Tauil CB, von Glehn F, Motl R, de David AC. Knee flexor strength and balance control impairment may explain declines during prolonged walking in women with mild multiple sclerosis. Mult Scler Relat Disord. 2018;20:181–185. [DOI] [PubMed] [Google Scholar]
- 114.Mizrahi D, Fardell JE, Cohn RJ, et al. The 6-minute walk test is a good predictor of cardiorespiratory fitness in childhood cancer survivors when access to comprehensive testing is limited. Int J Cancer. 2020;147(3):847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]