Abstract
Great progress has been made in cancer therapeutics. However, metastasis remains the predominant cause of death from cancer. Importantly, metastasis can manifest many years after initial treatment of the primary cancer. This is because cancer cells can remain dormant before forming symptomatic metastasis. An important question is whether metastasis research should focus on the early treatment of metastases, before they are clinically evident (“overt”), or on developing treatments to stop overt metastasis (stage IV cancer). In this commentary we want to clarify why it is important that all avenues of treatment for stage IV patients are developed. Indeed, future treatments are expected to go beyond the mere shrinkage of overt metastases and will include strategies that prevent disseminated tumor cells from emerging from dormancy.
Keywords: stage IV cancer, minimal residual disease, metastasis, cancer dormancy, liquid biopsy
Introduction
Despite great progress that has been made in cancer therapeutics in recent years, metastasis remains the predominant cause of death from cancer. For stage IV patients who already have metastases, the development of treatments that effectively kill their metastatic cancer is therefore the major primary focus. However, as patients live longer with their metastatic disease it has become increasingly evident that preventing metastasis will also play an important role in the treatment of stage IV patients who are at risk of developing additional metastases. The Metastasis Research Society (MRS) receives numerous questions regarding the nature of the metastatic process and how to best treat metastasis. A frequent and perhaps challenging question for cancer patients and advocates is whether metastasis research should focus on the early treatment of metastases, before they are clinically evident (“overt”), or more on developing new treatments for overt metastasis (stage IV cancer). In this short perspective we want to clarify why it is important that all avenues of treatment for stage IV patients are developed. Indeed, future treatments are expected to go beyond the mere killing of tumor cells, and will include strategies that prevent disseminated tumor cells from emerging from dormancy.
To begin the discussion about the best strategy to target metastasis, it is important to define the steps of metastatic disease, and to understand the windows of opportunity that are available to counter it (Table I and Figure 1).
Table I:
terms and definitions of the steps of the metastatic process required to understand the windows of opportunity that are available to counter it.
| Term | Definition |
|---|---|
| Primary tumor | The original cancer within the tissue of origin. |
| Dissemination | The process by which cancer cells spread from the primary tumor to other sites. |
| Disseminated cancer | Cancer that has spread from the primary tumor to other sites in the body. It may or may not be detectable and it may be proliferative (active) or dormant (see below) |
| Metastasis | The formation of secondary cancer growth(s) in other sites in the body. This process involves dissemination, entry into distant sites and growth in these locations. |
| Dormant disseminated cancer | Disseminated tumor cells located at sites that are separate from the primary tumor, and which are not increasing in numbers to form an overt metastasis. They exist as solitary cancer cells or very small groups of cells over long periods of time. Although not detectable by traditional clinical methods, they can sometimes be detected by more sensitive experimental methods such as liquid biopsies. |
| Overt Metastases | Disseminated cancer that has grown sufficiently to be detectable by conventional imaging methods (e.g. MRI), or that can be tracked by established biomarkers (e.g. PSA in prostate cancer) and confirmed by imaging. These metastases cause clinical symptoms and can be lethal. |
| Liquid Biopsy | A sample obtained from blood, a bone marrow aspirate or other bodily fluids. In these biopsies, cells that are in the blood (circulating tumor cells) or that have lodged in the bone marrow can be detected and analyzed at a single cell resolution. The non-cellular component from both samples can be tested for protein biomarkers, exosomes, or cell-free RNA and DNA, which can be used for patient monitoring. |
Figure 1: Side-by-side depictions of treatment scenarios for advanced cancer to highlight the benefit of stopping the emergence of overt metastasis from dormant disseminated cancer.
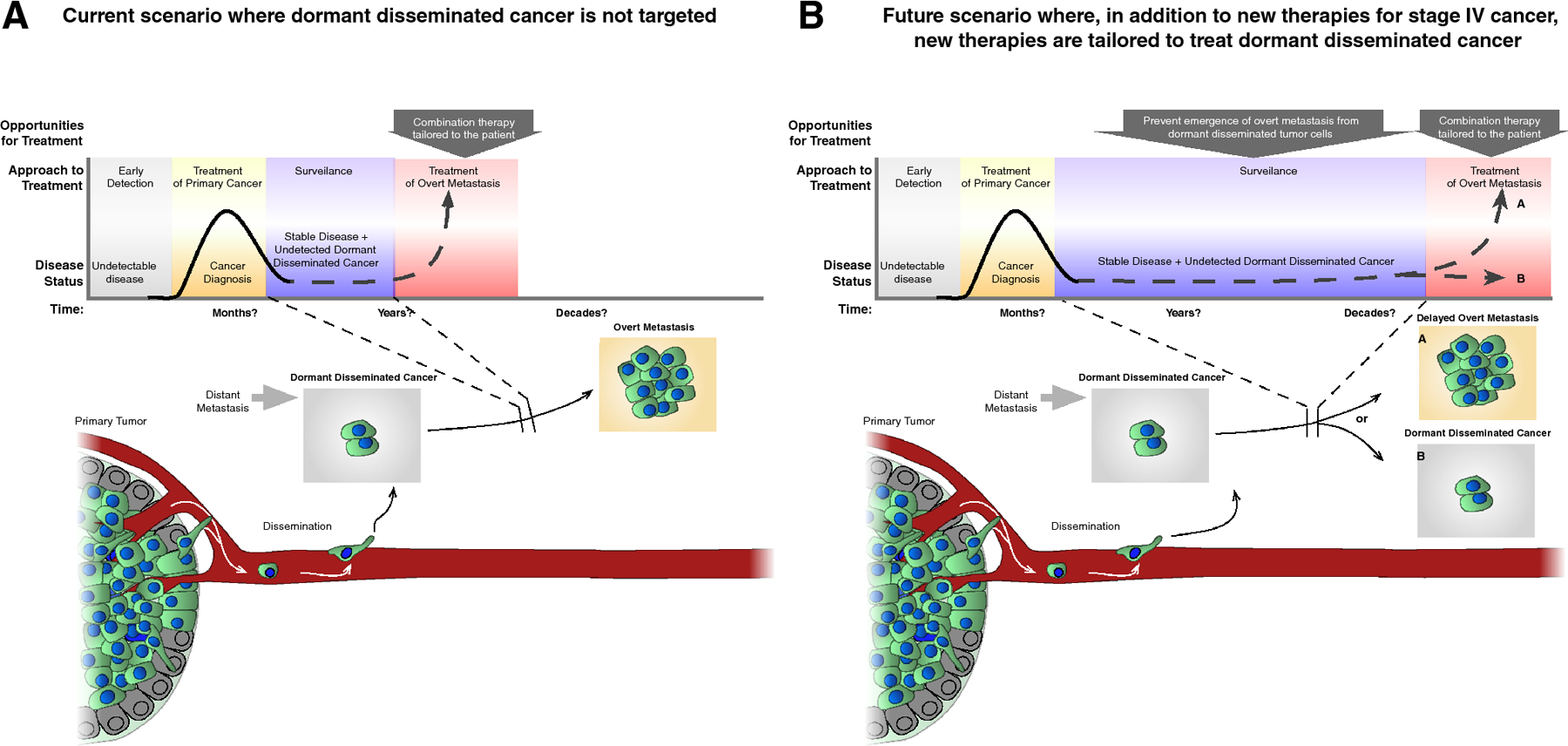
A) A treatment scenario in which the dormant disseminated cancer is not considered. B) A treatment scenario where, in conjunction with conventional therapy, additional treatment targeting dormant disease significantly delays (a) or prevents entirely (b) the emergence of overt metastasis years and even decades after treatment for the primary tumor was deemed successful; scenario b could be achieve by maintaining dormancy or by eradicating the dormant disseminated tumor cells. Also treatment of overt metastasis could include therapies to kill dormant disseminated tumor cells and thus stop not only proliferative, but also non-proliferative cells in metastatic organs.
Definitions
We define dissemination as the process by which cells exit the primary lesion, disseminate through the body (often through, but not necessarily, e.g. ovarian cancer, through the circulatory system) and then lodge in target organs (See also Table I and Figure 1). In some patients, dissemination not only occurs from the primary tumor lesion but also from secondary tumors or regional metastases in the lymph nodes (LN) that drain the tissue harboring the original lesion1,2. We define overt metastasis as the formation of lesions in organs such as the liver, bone, lung, brain, skin and LN that are detectable in the clinic by conventional imaging methods (e.g. MRI), and that can be tracked by established biomarkers (e.g. PSA in prostate cancer). In other words, overt metastasis is what we can “see” and/or detect, and it usually manifests with clinical symptoms3. The term disseminated cancer includes not only overt metastases, but also solitary cancer cells or very small lesions at secondary sites that are not yet detectable using the above methods, which may however be possible to detect using more sensitive and often experimental methods. While tumors and overt metastases are traditionally identified using a tissue biopsy, minimally invasive methods called liquid biopsies that sample bodily fluids (urine, blood, saliva, etc. Table I) are increasingly used to test for disseminated cancer that is not detectable through conventional tests4,5. Unfortunately, we do not currently have routine methods to determine whether disseminated cancer cells that are detected in an individual will or will not grow into overt metastasis, but recent progress suggests it may be possible to develop such tests6. Consequently, parallel methods for treating stage IV patients must be pursued, including therapies that kill tumor cells in overt metastases, as well as those that prevent the emergence of disseminated tumor cells from dormancy.
The concept of “preventing metastasis”
Long-term clinical studies suggest that in many patients, dissemination has already taken place by the time of primary cancer diagnosis7. Based on the evidence that dissemination occurs early, a patient diagnosed with a primary tumor may have disseminated cancer, which can manifest as dormant disseminated cancer, active disseminated cancer (proliferative but not yet symptomatic) and overt metastasis (Table I – Figure 1)8. However, even if dissemination has occurred, much of the disseminated cancer may never grow into overt metastasis. Even when it does, for many cancer types a delay of years to decades can occur between dissemination from the primary lesion and the development of overt metastasis (Figure 1)5,7. Most treatments for metastatic cancer are very hard on patients, have a big impact on the quality of life, and may possibly induce additional mutations that foster cancer and metastasis. Such treatments may therefore be withheld until there is evidence of overt metastasis through imaging or blood-based biomarkers. However, the success of existing cancer treatments has allowed patients to live longer. Among these long-term survivors, metastatic disease can emerge years after treatment of the primary tumor5,7. These observations have motivated scientists in the field of metastasis to work on prevention strategies that not only limit dissemination but also eliminate or suppress disseminated cancer cells before they develop into overt metastases.
Scientists have shared findings that support the idea that disseminated cancer can remain dormant for years at a number of recent meetings, including the AACR Cancer Dormancy and Residual Disease Meeting in Montreal (June 2018), and at the Metastasis Research Society Meeting (August 2018). New strategies, such as liquid biopsies, are being developed to detect the presence of dormant disseminated cancer in patients in whom there is no evidence of active disease4. This has further supported the hope that, although dissemination may not always be prevented, we can stop or delay disseminated cancer from progressing and emerging as overt metastasis (Figure 1)8,9.
The concept of preventing dissemination and the emergence of overt metastasis is not new. Adjuvant therapies are routinely given in an effort to prevent relapse, be that at the primary site, or through the growth of metastases. In the adjuvant setting, chemotherapy, as well as targeted therapies such as tamoxifen and aromatase inhibitors, have been shown to prevent or delay relapse, even when no disease is detectable. The goal of these adjuvant therapies is to kill or arrest disseminated cancer cells7. A similar paradigm has been applied to target androgen receptor signaling in prostate cancer, and new adjuvant trials using anti-HER2 are also ongoing10. Several new strategies are being developed to prevent the emergence of disseminated cancer cells as overt metastasis. These include stimulating the immune system to keep disseminated cells at bay11, and using new treatment regimens (Clinical trial identifier NCT03572387) to keep the dormant disseminated cancer cells from reactivating12 (Figure 1). Recent studies have also shown that dormant disseminated cancer cells can be rendered sensitive to chemotherapy, which resulted in reduced bone metastasis in breast cancer models13,14. Thus, for a patient with dormant disseminated cancer, preventing the emergence of overt metastasis (in other words, treatment of disseminated cancer) is widely seen as a necessary component of future therapies (Figure 1)8. These types of therapy are aimed at eliminating the chance that disseminated cancer cells will grow into an overt metastasis, which is often referred to as “preventing metastasis.” This use of the term “prevention” in the context of treating metastasis should not be confused with “cancer prevention”, which is intended to block the development of the initial primary cancer.
Experimental studies have revealed that in some patients and mouse models, inflammation caused by chemotherapy may fuel cancer dissemination15–19. This is due to specific immune cells that foster dissemination after chemotherapy treatment. Fortunately, through this work, scientists have discovered a new drug that can stop immune cells from stimulating dissemination, which leads to greatly reduced dissemination and overt metastatic disease17. Thus, serendipitously, by studying a stage of metastasis that was thought to be impossible to stop because it can occur so early (dissemination), it has been possible to discover a new potential strategy to improve conventional chemotherapy.
Approaches to “prevent” or treat metastasis at various stages are not necessarily mutually exclusive. Recent studies in patients and mouse models have revealed that dormant disseminated cancer cells can persist during therapy for overt metastasis (stage IV disease) because dormant disseminated cancer cells tend to be chemo-resistant and/or immune evasive20–22. Thus, while a therapy may kill overt metastases, the dormant disseminated cancer cells that co-exist with the overt lesions can survive, and may emerge later in patients that were thought to be cancer-free after treatment21. Simultaneous targeting of both dormant disseminated cancer cells and overt metastasis is therefore an important new strategy for the management of metastatic disease. This approach was identified because scientists considered all the stages of metastasis. While therapies that stop the emergence of dormant metastases are currently experimental, we expect that it will be possible to use them in combination with existing therapies for stage IV disease, and thereby accelerate their deployment in the clinic.
A positive outlook on metastasis research
Metastasis is a complex disease process. Therapies that target metastatic cancer, whether at early or late stages of the disease, will need to be equally sophisticated in order to cure the patient, regardless of how this is achieved. Research in recent years has revealed that metastasis is not simply an aggressive extension of the original tumor, but that dormant disease is often present in patients who seem otherwise cured. Stopping the emergence of overt metastases from dormant disseminated disease will become increasingly important, given that many cancer survivors who have benefitted from the success of current cancer treatments may carry dormant disease that can emerge years, if not decades later. The MRS will continue to support dedicated scientists, physicians and, importantly, highly committed advocates to improve metastasis treatment across the entire metastatic spectrum. Unfortunately, cancer research is complex and can be frustratingly slow. Those at MRS are acutely aware of the impact of metastatic cancer and are thankful to all the physicians, scientists and patients who show an indomitable support for the MRS mission. We hope that this brief commentary, which articulates a common vocabulary for metastasis research and highlights the importance that we see in studying all stages of the metastatic process, will motivate an important dialogue that emphasizes the impact of multidisciplinary research, with the ultimate goal of developing new strategies that will provide comprehensive prevention, treatment, and cures for metastatic cancer.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Brown M et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359, 1408–1411, doi: 10.1126/science.aal3662 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Pereira ER et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359, 1403–1407, doi: 10.1126/science.aal3622 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pantel K, Brakenhoff RH & Brandt B Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nature reviews. Cancer 8, 329–340, doi: 10.1038/nrc2375 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Wan JCM et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nature reviews. Cancer 17, 223–238, doi: 10.1038/nrc.2017.7 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Sosa MS, Bragado P & Aguirre-Ghiso JA Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature reviews. Cancer 14, 611–622, doi: 10.1038/nrc3793 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgen E et al. NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients. Breast cancer research : BCR 20, 120, doi: 10.1186/s13058-018-1049-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goss PE & Chambers AF Does tumour dormancy offer a therapeutic target? Nature reviews. Cancer 10, 871–877 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Aguirre-Ghiso JA, Bragado P & Sosa MS Metastasis awakening: targeting dormant cancer. Nat Med 19, 276–277, doi: 10.1038/nm.3120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polzer B & Klein CA Metastasis awakening: the challenges of targeting minimal residual cancer. Nat Med 19, 274–275, doi: 10.1038/nm.3121 (2013). [DOI] [PubMed] [Google Scholar]
- 10.von Minckwitz G et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. The New England journal of medicine 377, 122–131, doi: 10.1056/NEJMoa1703643 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok J Putting the Immunologic Brakes on Cancer. Cell 175, 1452–1454, doi: 10.1016/j.cell.2018.11.006 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Sosa MS Dormancy programs as emerging antimetastasis therapeutic alternatives. Molecular & cellular oncology 3, e1029062, doi: 10.1080/23723556.2015.1029062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson P et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat Cell Biol, doi: 10.1038/s41556-018-0267-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghajar CM Metastasis prevention by targeting the dormant niche. Nature reviews. Cancer 15, 238–247, doi: 10.1038/nrc3910 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastoriza JM et al. Black race and distant recurrence after neoadjuvant or adjuvant chemotherapy in breast cancer. Clinical & experimental metastasis, doi: 10.1007/s10585-018-9932-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karagiannis GS, Condeelis JS & Oktay MH Chemotherapy-induced metastasis: mechanisms and translational opportunities. Clinical & experimental metastasis 35, 269–284, doi: 10.1007/s10585-017-9870-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karagiannis GS et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Science translational medicine 9, doi: 10.1126/scitranslmed.aan0026 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Rayes T et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci U S A 112, 16000–16005, doi: 10.1073/pnas.1507294112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Cock JM et al. Inflammation Triggers Zeb1-Dependent Escape from Tumor Latency. Cancer Res 76, 6778–6784, doi: 10.1158/0008-5472.CAN-16-0608 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pommier A et al. Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science 360, doi: 10.1126/science.aao4908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fluegen G et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat Cell Biol 19, 120–132, doi: 10.1038/ncb3465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chery L et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 5, 9939–9951 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


