Abstract
Background
Trimethoprim-sulfamethoxazole (TMP-SMX) is considered first-line therapy for Stenotrophomonas maltophilia infections based on observational data from small studies. Levofloxacin has emerged as a popular alternative due to tolerability concerns related to TMP-SMX. Data comparing levofloxacin to TMP-SMX as targeted therapy are lacking.
Methods
Adult inpatient encounters January 2005 through December 2017 with growth of S maltophilia in blood and/or lower respiratory cultures were identified in the Cerner Healthfacts database. Patients included received targeted therapy with either levofloxacin or TMP-SMX. Overlap weighting was used followed by downstream weighted regression. The primary outcome was adjusted odds ratio (aOR) for in-hospital mortality or discharge to hospice. The secondary outcome was number of days from index S maltophilia culture to hospital discharge.
Results
Among 1581 patients with S maltophilia infections, levofloxacin (n = 823) displayed statistically similar mortality risk (aOR, 0.76 [95% confidence interval {CI}, .58–1.01]; P = .06) compared to TMP-SMX (n = 758). Levofloxacin (vs TMP-SMX) use was associated with a lower aOR of death in patients with lower respiratory tract infection (n = 1452) (aOR, 0.73 [95% CI, .54–.98]; P = .03) and if initiated empirically (n = 89) (aOR, 0.16 [95% CI, .03–.95]; P = .04). The levofloxacin cohort had fewer hospital days between index culture collection and discharge (weighted median [interquartile range], 7 [4–13] vs 9 [6–16] days; P < .0001).
Conclusions
Based on observational evidence, levofloxacin is a reasonable alternative to TMP-SMX for the treatment of bloodstream and lower respiratory tract infections caused by S maltophilia.
Keywords: levofloxacin, Stenotrophomonas maltophilia, TMP-SMX, trimethoprim-sulfamethoxazole
Our findings suggest that mortality outcomes are similar comparing levofloxacin and trimethoprim-sulfamethoxazole (TMP-SMX) treatment of Stenotrophomonas maltophilialower respiratory tract and bloodstream infections. Until trial data are available, levofloxacin appears to be a reasonable alternative to TMP-SMX for these infections.
Stenotrophomonas maltophilia is a ubiquitous, gram-negative organism increasingly recognized as an antibiotic-resistant pathogen that threatens hospitalized patients globally [1]. Though not particularly virulent, S maltophilia has proven itself a formidable pathogen in the setting of intensive healthcare contact, immunosuppression, and other comorbid conditions [2, 3]. It is of particular concern in the hospital setting resulting from its propensity to form biofilms and its survival in nutrient-poor environments [4]. Infections owing to this organism have increased significantly in recent years due in part to growing populations with these risk factors [5]. Infections caused by S maltophilia are often difficult to treat due to a variety of intrinsic and acquired resistance traits that evade traditional empiric antibiotic regimens [2, 3, 6, 7]. Stenotrophomonas maltophilia can cause a variety of serious infections in the human host; most notably, it is associated with lower respiratory tract infections (LRTIs) and bloodstream infections (BSIs). With limited targeted therapy options, this organism continues to pose a substantial mortality burden [4, 6, 8]. Thus, a better understanding of the optimal treatment of S maltophilia is needed.
Traditionally, the treatment of choice for S maltophilia infection is trimethoprim-sulfamethoxazole (TMP-SMX), based predominantly on in vitro studies and case series [4, 7, 9, 10]. However, TMP-SMX is often associated with treatment-limiting toxicities including renal and hepatic injury, fluid and electrolyte derangements, hemolysis (ie, glucose 6–phosphate dehydrogenase deficiency), bone marrow suppression, and hypersensitivity reactions [11, 12]. Additionally, S maltophilia isolates exhibiting resistance to TMP-SMX are increasingly reported [13–16]. Levofloxacin has emerged as a popular alternative for S maltophilia infections based on in vitro susceptibility data, available breakpoint recommendations, and clinician familiarity [17–21]. However, levofloxacin also has a variety of adverse effects, including cardiac conduction abnormalities, tendinopathy, gastrointestinal intolerance, and increased risk of Clostridioides difficile infection (CDI) [22, 23]. More S maltophilia isolates tend to be intrinsically resistant to levofloxacin than TMP-SMX, making it less often a targeted treatment option compared to TMP-SMX [10, 24]. Furthermore, prior fluoroquinolone use has been associated with resistance to levofloxacin [25]. Despite the clinical need, there is no randomized controlled trial (RCT) to date that compares TMP-SMX and levofloxacin for S maltophilia infections. Thus, the optimal treatment of serious S maltophilia infection is unknown. In the current study, we used a large electronic health record database to conduct a retrospective comparative effectiveness study of levofloxacin vs TMP-SMX for BSIs and LRTIs due to S maltophilia.
METHODS
Study Design and Case Selection
The Cerner Healthfacts database was queried for unique adult (≥18 years) inpatient encounters admitted between 1 January 2005 and 31 December 2017 that recorded growth of S maltophilia in ≥1 blood or lower respiratory tract culture. The latter included sputum, tracheal aspirate, bronchoalveolar lavage, and protected bronchial brush washings. One initial encounter per patient was included for analysis. Patients had to have been treated with either TMP-SMX or levofloxacin and were excluded if they received any other antimicrobial with known in vitro activity against S maltophilia. Antimicrobials that fit this exclusion criteria were erythromycin, moxifloxacin, ciprofloxacin, minocycline, tigecycline, doxycycline, eravacycline, ceftazidime, cefepime, ticarcillin-clavulanate, cefiderocol, colistin, and chloramphenicol (Supplementary Table 1). Patients who received both levofloxacin and TMP-SMX (either concomitantly or sequentially as empiric vs targeted therapy and vice versa) were excluded, as were patients with cystic fibrosis (given their unique epidemiology and risk profile) [26] and where the isolate was resistant to the antibiotic initially selected upon speciation (eg, if TMP-SMX was given during treatment window and isolate speciated resistant to TMP-SMX, patient was excluded; Supplementary Table 2). Henceforth, assuming day 0 as the day index culture resulted positive, empiric therapy was defined as occurring on day –2 or day –1, and targeted therapy was defined as occurring on or between day 0 to day +7 (Supplementary Table 3).
Patient Consent Statement
Given that the study exclusively used deidentified data, it was deemed not to require patient consent and was deemed exempt from ethics board review at the National Institutes of Health (NIH) Clinical Center, based on the policy of the NIH Office of Human Subjects Research Protections, under the revised Common Rule.
Statistical Analysis
Analyses were prespecified unless explicitly reported as post hoc. The study protocol and statistical analysis plan were published online on ClinicalTrials.gov (identifier NCT04639817) prior to conducting analyses. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for reporting observational studies were followed [27]. Baseline characteristics were compared between the 2 groups using χ2 tests for categorical variables and t test or Wilcoxon rank-sum test for continuous variables.
A propensity score was generated using clinically relevant patient, infection, treatment, and center-related variables. These include age, sex, immunocompromised status (Supplementary Table 4), baseline Sequential Organ Failure Assessment (SOFA) score (on day index culture was collected; Supplementary Table 5), Elixhauser comorbidity index, culture site, polymicrobial infection (Supplementary Methods and Supplementary Tables 6 and 7), intensive care unit (ICU) stay (between 1 day before and up to 2 days following the day of culture sampling), mechanical ventilation, vasopressor use, and year range, as well as hospital factors: academic status, urban/rural qualification, geographic region, and bed capacity. To ensure balance between the 2 groups, we used overlap weighting as implemented in R (version 4.0.3) package PSweight (version 1.1.2) [28].
Overlap Weighting
Overlap weighting is a relatively new statistical technique first described by Li et al in 2018 that allows for exact balance between groups and attempts to simulate randomization [29]. Unlike propensity matching, which invariably results in excluding some individuals by assigning an artificial cutoff for trimming, and inverse probability weighting, which tends to assign considerable weight to outliers, overlap weighting uses the propensity score to assign weights based on the probability that a given patient in 1 treatment group belongs to the other treatment group. This results in conscious underweighting of outliers, such that the results are based predominantly on those in whom the decision to use levofloxacin vs TMP-SMX may have been more discretionary than confounded by indication [30].
The primary outcome for analysis was in-hospital mortality or discharge to hospice. This was analyzed using weighted logistic regression and presented as an adjusted odds ratio (aOR). To capture morbidity potentially attributable to treatment choice, the number of days from index S maltophilia culture to discharge was selected as the secondary outcome, where mortality was censored at the longest length of stay and analyzed using weighted Cox regression.
Predefined subgroups for analysis include site of infection (LRTI, BSI), SOFA score (high ≥2 or low <2) and mechanical ventilation restricted to within ±3 days of culture collection. Sensitivity analyses were performed with and without polymicrobial culture growth, polymicrobial growth additionally treated with non–S maltophilia–active antibiotic(s), receipt of empiric therapy, empiric therapy not received, non–present-on-admission diagnosis coding for pneumonia, and imputed susceptibility.
Upon discovery that the median time from admission to culture was significantly different between study groups (weighted median, 4 [interquartile range {IQR}, 1–11] days for TMP-SMX vs 2 [IQR, 0–9] days for the levofloxacin group; P < .0001) and could possibly introduce immortal time bias, a post hoc analysis was performed including this variable in risk adjustment using weighted logistic regression. All statistical analyses were performed using R (version 4.0.3), package PSweight (version 1.1.2) or SAS (version 9.4) software.
RESULTS
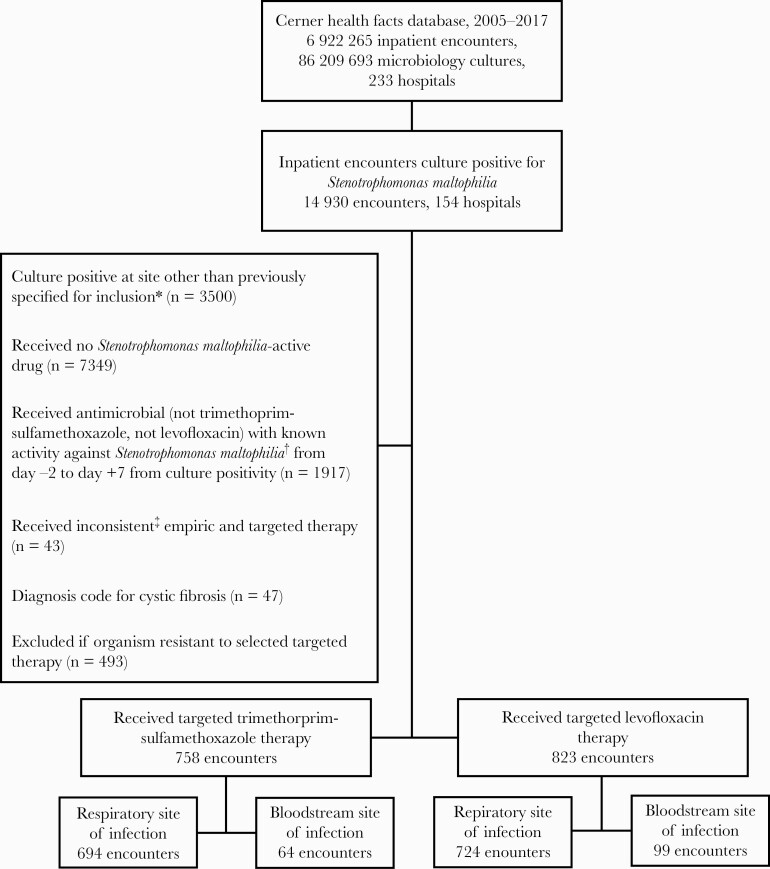
Between 2005 and 2017, there were 14 930 unique inpatients at 154 hospitals in the United States with any culture positive for S maltophilia in the database. After applying selection criteria (Figure 1), 1581 assessable inpatients were identified: 823 (52%) inpatients in the levofloxacin cohort and 758 (48%) in the TMP-SMX cohort.
Figure 1.
Case selection flowchart. Depiction of case selection process, including cases filtered in the data for exclusions. ∗Included clinical culture sites: blood culture, bronchoalveolar lavage, protected bronchial brush washing, sputum culture, and tracheal aspirate. Patients with bloodstream and respiratory infection due to Stenotrophomonas maltophilia were counted in the bloodstream site of infection category. †Antimicrobials with activity against Stenotrophomonas 2 days prior to and 7 days after culture positivity were excluded. Known Stenotrophomonas-active agents that were excluded are erythromycin, moxifloxacin, ciprofloxacin, minocycline, tigecycline, doxycycline, eravacycline, ceftazidime, cefepime, ticarcillin-clavulanate, cefiderocol, colistin, and chloramphenicol. ‡Defined as receiving levofloxacin as empiric therapy (day –2 to –1 from culture positivity) and trimethoprim-sulfamethoxazole as targeted therapy and vice versa.
Baseline Characteristics
Baseline characteristics are presented in Table 1. Patients in the TMP-SMX (vs levofloxacin) cohort were younger (median age, 60 [IQR, 31–72] years vs 66 [IQR, 53–76] years; P < .0001), more likely to be mechanically ventilated at any time during their admission (38.7% [293/758] vs 31.2% [257/823]; P = .002), and more likely to have an ICU stay (33.5% [254/758] vs 28.8% [237/823]; P = .04) during their hospitalization. Proportions of mechanically ventilated patients were similar, at 10.3% (78/758; P = .86) in the TMP-SMX group and 10.6% (87/823) in the levofloxacin group within the predefined window of ±3 days around culture sampling (Supplementary Table 8). Patients in the levofloxacin cohort, compared with TMP-SMX, displayed a greater frequency of S maltophilia bacteremia (12.0% [99/823] vs 8.4% [64/758], respectively; P = .02) and were more likely to have received concordant empiric therapy (10.0% [82/823] vs 0.9% [7/758]; P < .0001). In our study, 41.6% (658/1581) of overall patients (41.7% [343/823] in the levofloxacin cohort vs 41.6% [315/758] in the TMP-SMX cohort) had a polymicrobial index culture. Notably, 39.9% (65/163) with BSIs had a polymicrobial index culture that included S maltophilia. Other characteristics were comparable in the levofloxacin vs TMP-SMX cohorts, including SOFA score (median, 1 [IQR, 0–4] vs 1 [IQR, 0–4]) and Elixhauser comorbidity index (median, 2 [IQR, 0–4] vs 1 [IQR, 0–4]). After overlap weighting, the mean standard difference at each variable assessed was zero (Supplementary Figure 1).
Table 1.
Baseline Characteristics for Patients With Stenotrophomonas maltophilia Infection
| Characteristic | Levofloxacin | TMP -SMX |
|---|---|---|
| (n = 823) | (n = 758) | |
| Patient-level factors | ||
| Admission year | ||
| 2005–2010 | 204 (24.8) | 197 (26.0) |
| 2011–2013 | 208 (25.3) | 208 (27.4) |
| 2014–2015 | 234 (28.4) | 192 (25.3) |
| 2016–2017 | 177 (21.5) | 161 (21.2) |
| Age, y | ||
| 18–44 | 138 (16.8) | 239 (31.5) |
| 45–64 | 247 (30.0) | 203 (26.8) |
| 65–74 | 215 (26.1) | 174 (23.0) |
| ≥75 | 223 (27.1) | 142 (18.7) |
| Sex | ||
| Female | 362 (44.0) | 327 (43.1) |
| Male | 461 (56.0) | 431 (56.9) |
| Race | ||
| Black | 116 (14.1) | 143 (18.9) |
| White | 637 (77.4) | 505 (66.6) |
| Othera | 70 (8.5) | 110 (14.5) |
| Infection site | ||
| Bloodb | 99 (12.0) | 64 (8.4) |
| Respiratory | 724 (88.0) | 694 (91.6) |
| SOFA scorec, median (IQR) | 1.0 (0.0–4.0) | 2.0 (0.0–4.0) |
| Mechanical ventilationd | 87 (10.6) | 78 (10.3) |
| Polymicrobial infection | 343 (41.7) | 315 (41.6) |
| Polymicrobial infection receiving treatment | 165 (20.0) | 178 (23.5) |
| ICU admissione | 215 (26.1) | 237 (31.3) |
| Therapy initiated empirically | 82 (10.0) | 7 (0.9) |
| Pneumonia diagnosisf | 166 (20.2) | 141 (18.6) |
| Immunocompromised | 8 (1.0) | 13 (1.7) |
| Vasopressor administrationg | 89 (10.8) | 87 (11.5) |
| Elixhauser score, median (IQR) | 2.0 (0.0–4.0) | 2.0 (0.0–5.0) |
| Length of stay, median (IQR) | 10.0 (5.0–21.0) | 17.0 (9.0–31.8) |
| Discharge to hospice | 38 (4.6) | 26 (3.4) |
| In-hospital mortality | 87 (10.6) | 108 (14.2) |
| Total mortality | 125 (15.2) | 134 (17.7) |
| Hospital-level factors | ||
| Teaching facility | 506 (63.8) | 473 (67.4) |
| Bed capacity | ||
| >100 | 81 (9.8) | 90 (11.9) |
| 100–199 | 142 (17.3) | 81 (10.7) |
| 200–299 | 145 (17.6) | 129 (17.0) |
| 300–499 | 292 (35.5) | 262 (34.6) |
| ≥500 | 163 (19.8) | 196 (25.9) |
| Urban location | 718 (87.2) | 605 (79.8) |
| Census region | ||
| Midwest | 181 (22.0) | 196 (25.9) |
| Northeast | 156 (19.0) | 146 (19.3) |
| South | 350 (42.5) | 278 (36.7) |
| West | 136 (16.5) | 138 (18.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment; TMP-SMX, trimethoprim-sulfamethoxazole.
Includes Asian, Pacific Islander, biracial, Hispanic, Native American, other, and unknown.
Encounters with both blood and respiratory sites of infection were categorized as blood infections.
On day index culture was collected.
Day –1 to +2 where culture is collected.
Culture was drawn in ICU setting.
Presence of the International Classification of Diseases (ICD), Ninth Revision diagnosis codes 481, 485, 486, 514; or ICD, Tenth Revision diagnosis codes J18.0, J18.1, J18.2, J18.8, J18.9.
Day –1 to day +2 day index culture was collected.
Primary Outcome
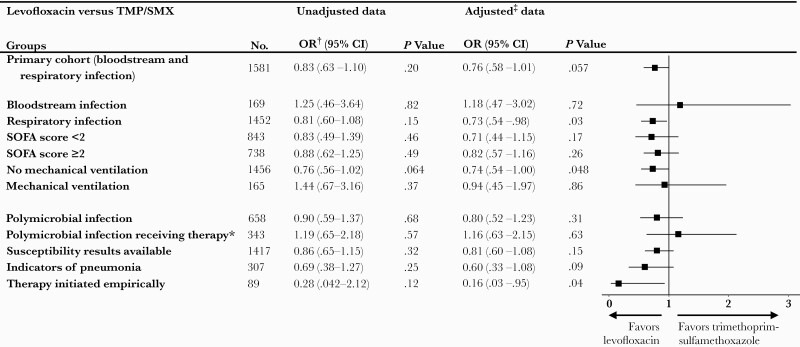
Unadjusted mortality for the study cohort was 16.4% (259/1581) overall, 14.1% (23/163) for patients with BSI, and 19.5% (60/307) for patients with LRTI due to S maltophilia. Approximately 15% (125/823) of patients in the levofloxacin group and 17.7% (134/758) of those in the TMP-SMX group died. Levofloxacin use was associated with a nonsignificant trend toward lower aOR of death as compared with TMP-SMX (aOR, 0.76 [95% CI, .58–1.01]; P = .057) (Figure 2). A statistically significant lower aOR of death associated with levofloxacin (vs TMP-SMX) was noted in the prespecified subgroups of patients who had an LRTI (n = 1452) (aOR, 0.73 [95% CI, .54–.98]; P = .03), nonventilated patients (n = 1456) (aOR, 0.74 [95% CI, .54–.997]; P = .048), and those in whom the agent was initiated empirically (n = 89) (aOR, 0.16 [95% CI, .03–.95]; P = .04). All other subgroup and sensitivity analyses yielded similar impacts of both agents on mortality risk (Figure 2).
Figure 2.
Odds ratios of mortality for levofloxacin vs trimethoprim-sulfamethoxazole targeted therapy among primary cohort and subpopulations of interest. Forest plot of boxes depicting adjusted odds ratio with 95% confidence intervals shown as horizontal lines. ∗Excluding drugs with known activity against S maltophilia other than Trimethoprim-sulfamethoxazole and Levofloxacin (Appendix Table 4). †Unadjusted data calculated using Fisher\'s exact test. ‡Adjusted values were calculated using logistic regression after controlling for baseline patient and hospital level factors. Clinically relevant sub-populations were also analyzed for potential disparate impacts on mortality. Abbreviations: CI, confidence interval; OR, odds ratio; SOFA, Sequential Organ Failure Assessment; TMP-SMX, trimethoprim-sulfamethoxazole.
Secondary Outcome
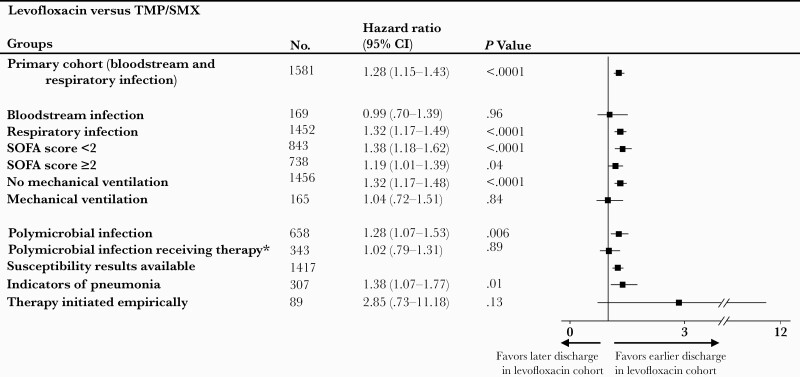
Compared to the TMP-SMX cohort, the levofloxacin cohort had fewer hospital days between index culture draw and discharge (weighted median, 7 [IQR, 4–13] days vs 9 [IQR, 6–16] days; P < .0001) (Figure 3). The hazard ratio (HR) of discharge alive in the levofloxacin group compared with the TMP-SMX group was 1.28 (95% CI, 1.15–1.43]; P < .0001), suggesting a statistically significant shorter length of stay. Secondary outcome findings were also fairly consistent across subgroups and sensitivity analyses (Figure 3), except for patients with BSI, those on mechanical ventilation, and those with polymicrobial infection having received therapy, in whom there was no statistically significant difference in HR for discharge (HR, 0.99 [95% CI, .70–1.39], P = .96; HR, 1.04 [95% CI, .72–1.51], P = .84; and HR, 1.02 [95% CI, .79–1.31], P = .89, respectively).
Figure 3.
Hazard ratios of discharge for levofloxacin vs trimethoprim-sulfamethoxazole (TMP-SMX) targeted therapy among primary cohort and subpopulations of interest. Forest plot of boxes depicting hazard ratios with 95% confidence intervals shown as horizontal lines. A higher hazard ratio of discharge for the levofloxacin cohort vs TMP-SMX cohort correlates with a shorter length of stay for the levofloxacin cohort, since this information is presented as a risk of being discharged. Abbreviations: CI, confidence interval; SOFA, Sequential Organ Failure Assessment; TMP-SMX, trimethoprim-sulfamethoxazole. ∗Excluding drugs with known activity against S maltophilia other than Trimethoprim-sulfamethoxazole and Levofloxacin (Appendix Table 4).
Post Hoc Analysis
After adjusting for time to culture as a continuous variable to mitigate immortal time bias, the OR of death for levofloxacin vs TMP-SMX was found to be 0.82 ([95% CI, .62–1.09]; P = .17). The HR for discharge alive in the levofloxacin group compared with the TMP-SMX group was 1.22 (95% CI, 1.09–1.37]; P = .0004). These results are generally consistent with findings from the initial planned analysis with 2 exceptions: The OR of death for levofloxacin vs TMP-SMX was 0.79 (95% CI, .58–1.06; P = .12) for the subgroup of patients with LRTI and 0.17 (95% CI, .02–1.36; P = .09) for those having received concordant therapy. Although the findings of these 2 subgroups were consistent in directionality with the corresponding prespecified analyses, adjustment for time to culture rendered estimates as not statistically significant. Post hoc analyses are presented in Supplementary Figure 2.
DISCUSSION
Our study of 1581 patients from 154 US hospitals represents the largest retrospective cohort analysis thus far comparing the effectiveness of levofloxacin vs TMP-SMX as targeted therapy for S maltophilia bloodstream and lower respiratory tract infections. Overall, we found comparable mortality risk with the use of either levofloxacin or TMP-SMX for these serious S maltophilia infections. Furthermore, patients treated with levofloxacin in the prespecified subgroup of those with pneumonia appeared to display greater survival compared to TMP-SMX and were discharged sooner. In vitro–active empiric therapy appeared to be associated with a decreased risk of death overall.
TMP-SMX is considered the first-line agent for S maltophilia largely due to its in vitro susceptibility profile [10, 24, 31] and observational studies supporting its use [17, 20, 21, 32, 33]. In the absence of RCT data, clinicians seek alternative agents for managing S maltophilia due to tolerability limitations of TMP-SMX, especially among patient populations uniquely susceptible to this pathogen (eg, neutropenic, critically ill). Our findings complement a previous meta-analysis [32] of 7 retrospective cohort and 7 case-control studies (pooled n = 663 patients) that also suggested comparable survival rates for both agents. However, the meta-analysis pooled unadjusted ORs from relatively small studies (range, 1–35 patients for levofloxacin and 2–68 patients for TMP-SMX) that did not account for confounding by indication—a major obstacle in nonrandomized comparisons of the effectiveness of alternatives to an established treatment standard—thus impeding inferences of comparative effectiveness.
Our study displays several strengths over previous studies and provides incremental evidence on the topic. First, our study provides greater statistical power than previous studies in inferring that the 2 agents are likely comparably effective against S maltophilia overall. Second, our study applied overlap weighting that resulted in excellent covariate balance and applied weighted regression to mitigate residual confounding. Third, our findings are robust to several sensitivity analyses. We used both monomicrobial and polymicrobial S maltophilia infections in our primary analysis; S maltophilia is often part of a polymicrobial infection, as demonstrated in our study (41.5%) and other studies (range, 33%–70%) [19, 34, 35]. Our findings were similar upon limiting the analysis to polymicrobial infections treated with agents not active against S maltophilia, further enhancing internal and external validity of our findings. We imputed susceptibility when these data were not reported for study agents (in approximately 10% of cases) and results were similar with or without inclusion of imputed susceptibility results.
Our study indicates a growing comfort level among clinicians in the use of levofloxacin to treat S maltophilia infection in the real-world setting. Despite demonstration of comparable efficacy, there was a nonsignificant trend toward favoring levofloxacin in the primary analysis. This signal was likely driven by the potential superiority of levofloxacin over TMP-SMX for pneumonia. The latter is hypothesis generating and prospective studies are needed to confirm these findings. However, pharmacokinetic/pharmacodynamic properties benefiting levofloxacin over TMP-SMX, such as a higher concentration in epithelial lining fluid, quicker time-to-peak serum concentration, bactericidality, and greater bioavailability of the oral formulation, add biologic plausibility to our observations [11, 36–39]. The estimate for the effect of TMP-SMX (vs levofloxacin) in BSIs is uninterpretable in the context of a wide CI. Furthermore, we found a statistically significant decrease in mortality risk when S maltophilia–active therapy was initiated empirically. Given the 10-fold greater empiric use of levofloxacin (vs TMP-SMX), this finding does not so much as indicate which agent is more effective for empiric use, but rather that earlier initiation of active therapy improves outcomes in S maltophilia infections, as previously suggested [33, 40]. No difference in mortality was found in the subgroup that did not receive empiric therapy, further corroborating the comparable effectiveness of levofloxacin and TMP-SMX for S maltophilia infections. As the nationwide uptake and reliability of rapid molecular diagnostics grows over time, an earlier diagnosis is likely to improve survival rates for S maltophilia infection. The overall shorter length of stay observed in the levofloxacin group, although statistically significant, is perhaps less clinically relevant, as there are many social and clinical explanations that could account for a 2-day difference in length of stay. Nevertheless, the results support the use of levofloxacin as an alternative standard of care for S maltophilia infections.
Our study has important limitations. Unmeasured confounding may still exist, and adequacy of source control could not be gauged; however, this bias is likely to be nondifferential. Despite use of a comprehensive definition for immunosuppression, billing codes may have limited our ability to capture the sum of immunosuppressed hosts. Our study does not address combination therapy, a treatment modality commonly used for multidrug-resistant gram-negative pathogens. Notably, levofloxacin is not the only alternative to TMP-SMX. Minocycline and the recently Food and Drug Administration–approved cefiderocol both appear to have in vitro activity against >99% of S maltophilia isolates [24, 41]. As such, the treatment paradigm may shift with additional efficacy data. Development of resistance under treatment, a concern with levofloxacin use in particular, was not addressed. Information required to adequately assess key tolerability outcomes (eg, urine output for acute kidney injury, stool characteristics for CDI) precluded their assessment and warrant further study. Notwithstanding, non–present-on-admission diagnosis codes for CDI were relatively infrequent in both study groups (<2%) (Supplementary Table 9). The crude mortality rate in our study of 16.3% is lower than has been previously described [4, 6, 8]. However, our study also included a large proportion of non–critically ill patients, which likely contributed this difference. Stenotrophomonas maltophilia is a known colonizer of the respiratory tract, making discrimination between infection vs colonization difficult. This is true in our study, but also true in the real world for clinicians at the bedside. Our study highlights real-world use; thus, primary analysis was limited to patients actively treated for S maltophilia infection once the clinician made the decision to treat. If a cohort of patients was colonized and not infected with S maltophilia, this is likely nondifferential across both treatment groups. Additionally, this study demonstrates corroborative findings from mechanically ventilated patients, patients with vasopressor use, patients with SOFA score >2, and those with administrative indicators of hospital-onset pneumonia, which collectively mitigate this concern by enriching the data for likelihood of infection.
Our study suggests there is equipoise for an RCT comparing levofloxacin and TMP-SMX among hospitalized patients with S maltophilia infection. Sample size calculations extrapolated from our study findings (weighted mortality difference, 15.3% vs 19.1% between groups) suggest that 1548 patients per arm would be required to achieve an 80% power using a 2-sided α = .05. However, protracted recruitment given relatively low incidence of S maltophilia and the potential lack of financial incentives for comparing 2 generic antibiotics represent potential hurdles.
CONCLUSIONS
Our large study of overlap-weighted cohorts of patients treated with levofloxacin vs TMP-SMX for S maltophilia LRTIs and BSIs suggest that levofloxacin might be a reasonable alternative to the current accepted standard TMP-SMX for these infections. An RCT comparing levofloxacin and TMP-SMX head-to-head is yet to be performed. In the interim, large, rigorous observational studies may offer a valuable stopgap in evidence.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. S. K. conceived the study. S. H. S. and S. S. K. designed the study, conducted the literature search, and wrote the draft of the manuscript. S. H. S., S. W., and S. S. K. collected and analyzed the data. S. H. S., S. W., S. S. K., R. M., and V. G. F. interpreted the data. All authors reviewed and critically revised the manuscript for important intellectual content. The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments. This study represents S. H. S.’s thesis for the National Institutes of Health (NIH)–Duke Master’s Program in Clinical Research. The authors thank David Fram and Huai Chun (Commonwealth Informatics, Waltham, Massachusetts) for their assistance with data curation; and Drs Sumathi Nambiar, John Farley, and Thushi Amini (Office of Antimicrobial Products, Center for Drug Evaluation and Research, Food and Drug Administration) for their feedback on the study design and regulatory input. The authors also thank Kelly Byrne (Critical Care Medicine Department, NIH Clinical Center, Bethesda, Maryland) for assistance with formatting the manuscript.
Disclaimer. The opinions expressed in this article are those of the authors and do not represent any position or policy of the NIH, the US Department of Health and Human Services, or the US government. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Financial support. This work was funded in part by the Intramural Research Program of the National Institutes of Health Clinical Center and the National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. V. G. F. reports personal fees from Novartis, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, The Medicines Co, Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Basilea, Affinergy, Janssen, xBiotech, Contrafect, Regeneron, Basilea, Destiny, Amphliphi Biosciences, Integrated Biotherapeutics, C3J, Armata, Valanbio, Akagera, and Aridis; grants from the NIH, MedImmune, Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Merck, Medical Biosurfaces, Locus, Affinergy, Contrafect, Karius, Genentech, Regeneron, Basilea, Janssen, and Akagera; royalties from UpToDate; stock options from Valanbio and ArcBio; a patent for sepsis diagnostics pending; and a stipend from the Infectious Diseases Society of America for service as Associate Editor on Clinical Infectious Diseases. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Annual Meeting of the Infectious Diseases Society of America, San Diego, California, 5 October 2017.
References
- 1. Brooke JS. New strategies against Stenotrophomonas maltophilia: a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert Rev Anti Infect Ther 2014; 12:1–4. [DOI] [PubMed] [Google Scholar]
- 2. Safdar A, Rolston KV.. Stenotrophomonas maltophilia: changing spectrum of a serious bacterial pathogen in patients with cancer. Clin Infect Dis 2007; 45:1602–9. [DOI] [PubMed] [Google Scholar]
- 3. Mojica MF, Rutter JD, Taracila M, et al. Population structure, molecular epidemiology, and beta-lactamase diversity among Stenotrophomonas maltophilia isolates in the United States. mBio 2019; 10:e00405-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 2012; 25:2–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denton M, Kerr KG.. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev 1998; 11:57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falagas ME, Kastoris AC, Vouloumanou EK, et al. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol 2009; 4:1103–9. [DOI] [PubMed] [Google Scholar]
- 7. Andelkovic MV, Jankovic SM, Kostic MJ, et al. Antimicrobial treatment of Stenotrophomonas maltophilia invasive infections: systematic review. J Chemother 2019; 31:297–306. [DOI] [PubMed] [Google Scholar]
- 8. Looney WJ, Narita M, Muhlemann K.. Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect Dis 2009; 9:312–23. [DOI] [PubMed] [Google Scholar]
- 9. Nicodemo AC, Paez JI.. Antimicrobial therapy for Stenotrophomonas maltophilia infections. Eur J Clin Microbiol Infect Dis 2007; 26:229–37. [DOI] [PubMed] [Google Scholar]
- 10. Sader HS, Farrell DJ, Flamm RK, Jones RN.. Antimicrobial susceptibility of gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: results from the SENTRY Antimicrobial Surveillance Program, 2009-2012. Int J Antimicrob Agents 2014; 43:328–34. [DOI] [PubMed] [Google Scholar]
- 11. AR Scientific. Sulfamethoxazole and trimethoprim injection [package insert]. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/073303s030lbl.pdf. Accessed 1 August 2021.
- 12. Giles A, Foushee J, Lantz E, Gumina G.. Sulfonamide allergies. Pharmacy (Basel) 2019; 7:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang YT, Lin CY, Chen YH, Hsueh PR.. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 2015; 6:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vidigal PG, Dittmer S, Steinmann E, Buer J, Rath PM, Steinmann J.. Adaptation of Stenotrophomonas maltophilia in cystic fibrosis: molecular diversity, mutation frequency and antibiotic resistance. Int J Med Microbiol 2014; 304:613–9. [DOI] [PubMed] [Google Scholar]
- 15. Toleman MA, Bennett PM, Bennett DM, Jones RN, Walsh TR.. Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg Infect Dis 2007; 13:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho SY, Lee DG, Choi SM, et al. Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations. BMC Infect Dis 2015; 15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho SY, Kang CI, Kim J, et al. Can levofloxacin be a useful alternative to trimethoprim-sulfamethoxazole for treating Stenotrophomonas maltophilia bacteremia? Antimicrob Agents Chemother 2014; 58:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grillon A, Schramm F, Kleinberg M, Jehl F.. Comparative activity of ciprofloxacin, levofloxacin and moxifloxacin against Klebsiella pneumoniae, Pseudomonas aeruginosa and Stenotrophomonas maltophilia assessed by minimum inhibitory concentrations and time-kill studies. PLoS One 2016; 11:e0156690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Juhasz E, Pongracz J, Ivan M, Kristof K.. Antibiotic susceptibility of sulfamethoxazole-trimethoprim resistant Stenotrophomonas maltophilia strains isolated at a tertiary care centre in Hungary. Acta Microbiol Immunol Hung 2015; 62:295–305. [DOI] [PubMed] [Google Scholar]
- 20. Watson L, Esterly J, Jensen AO, Postelnick M, Aguirre A, McLaughlin M.. Sulfamethoxazole/trimethoprim versus fluoroquinolones for the treatment of Stenotrophomonas maltophilia bloodstream infections. J Glob Antimicrob Resist 2018; 12:104–6. [DOI] [PubMed] [Google Scholar]
- 21. Nys C, Cherabuddi K, Venugopalan V, Klinker KP.. Clinical and microbiologic outcomes in patients with monomicrobial Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother 2019; 63:e00788–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rubinstein E, Camm J.. Cardiotoxicity of fluoroquinolones. J Antimicrob Chemother 2002; 49:593–6. [DOI] [PubMed] [Google Scholar]
- 23. Tandan M, Cormican M, Vellinga A.. Adverse events of fluoroquinolones vs. other antimicrobials prescribed in primary care: a systematic review and meta-analysis of randomized controlled trials. Int J Antimicrob Agents 2018; 52:529–40. [DOI] [PubMed] [Google Scholar]
- 24. Flamm RK, Shortridge D, Castanheira M, Sader HS, Pfaller MA.. In vitro activity of minocycline against U.S. isolates of Acinetobacter baumannii-Acinetobacter calcoaceticus species complex, Stenotrophomonas maltophilia, and Burkholderia cepacia complex: results from the SENTRY antimicrobial surveillance program, 2014 to 2018. Antimicrob Agents Chemother 2019; 63:e01154-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang CH, Yu CM, Hsu ST, Wu RX.. Levofloxacin-resistant Stenotrophomonas maltophilia: risk factors and antibiotic susceptibility patterns in hospitalized patients. J Hosp Infect 2020; 104:46–52. [DOI] [PubMed] [Google Scholar]
- 26. Goss CH, Otto K, Aitken ML, Rubenfeld GD.. Detecting Stenotrophomonas maltophilia does not reduce survival of patients with cystic fibrosis. Am J Respir Crit Care Med 2002; 166:356–61. [DOI] [PubMed] [Google Scholar]
- 27. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 2007; 4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. R Project. PSweight: propensity score weighting for causal inference with observational studies and randomized trials. Available at: https://CRAN.R-project.org/package=PSweight. Accessed 1 August 2021.
- 29. Li F, Morgan KL, Zaslavsky AM.. Balancing covariates via propensity score weighting. J Am Stat Assoc 2018; 113:390–400. [Google Scholar]
- 30. Thomas LE, Li F, Pencina MJ.. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA 2020; 323:2417–8. [DOI] [PubMed] [Google Scholar]
- 31. Gales AC, Jones RN, Forward KR, et al. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997-1999). Clin Infect Dis 2001; 32:S104–13. [DOI] [PubMed] [Google Scholar]
- 32. Ko JH, Kang CI, Cornejo-Juarez P, et al. Fluoroquinolones versus trimethoprim-sulfamethoxazole for the treatment of Stenotrophomonas maltophilia infections: a systematic review and meta-analysis. Clin Microbiol Infect 2019; 25:546–54. [DOI] [PubMed] [Google Scholar]
- 33. Wang YL, Scipione MR, Dubrovskaya Y, Papadopoulos J.. Monotherapy with fluoroquinolone or trimethoprim-sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother 2014; 58:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hamdi AM, Fida M, Abu Saleh OM, Beam E.. Stenotrophomonas bacteremia antibiotic susceptibility and prognostic determinants: Mayo Clinic 10-year experience. Open Forum Infect Dis 2020; 7:ofaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Samonis G, Karageorgopoulos DE, Maraki S, et al. Stenotrophomonas maltophilia infections in a general hospital: patient characteristics, antimicrobial susceptibility, and treatment outcome. PLoS One 2012; 7:e37375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonfiglio G, Cascone C, Azzarelli C, Cafiso V, Marchetti F, Stefani S.. Levofloxacin in vitro activity and time-kill evaluation of Stenotrophomonas maltophilia clinical isolates. J Antimicrob Chemother 2000; 45:115–7. [DOI] [PubMed] [Google Scholar]
- 37. Zelenitsky SA, Iacovides H, Ariano RE, Harding GK.. Antibiotic combinations significantly more active than monotherapy in an in vitro infection model of Stenotrophomonas maltophilia. Diagn Microbiol Infect Dis 2005; 51:39–43. [DOI] [PubMed] [Google Scholar]
- 38. Ortho-McNeil-Janssen Pharmaceuticals. Levofloxacin [package insert]. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020634s062s064,020635s068s070,021721s029s031lbl.pdf. Accessed 1 August 2021.
- 39. Rodvold KA, George JM, Yoo L.. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 2011; 50:637–64. [DOI] [PubMed] [Google Scholar]
- 40. Metan G, Uzun O.. Impact of initial antimicrobial therapy in patients with bloodstream infections caused by Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2005; 49:3980–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21:226–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.