Abstract
Studies of the ethyl acetate extract bark extract of Olea ferruginea led to the isolation of one new compound Ferruginan A (1) in addition to two known compounds, Ferruginan (2) and cycloolivil (3). Structures of the isolated compounds were confirmed by mass spectrometry (MS) and NMR spectral data. The ethyl acetate fraction and compounds (1–3) were evaluated against breast cancer cell line (MCF-7) and as antioxidants using the free radical scavenging assay. Results revealed that compound 2 exhibits significant antioxidant activity with an IC50 value of 21.74 μg/mL. In addition, the ethyl acetate fraction showed good cytotoxic activity (79.31% inhibition at 250 μg/mL), whereas compounds 1–3 exerted mild cytotoxic activity (IC50 = 8.03–12.01 μg/mL) as compared to the standard (IC50 = 4.41 μg/mL) against MCF-7. Docking studies suggested that antioxidant activity is due to the chelation of compounds with copper present in the active site of tyrosinase. These results suggest that the extract exhibits considerable antioxidant activity, and the isolated compounds exert moderate anticancer activity.
1. Introduction
Natural products obtained from terrestrial and marine plants have been used as a source of traditional medicines since ancient times. According to the World Health Organization (WHO), people of developing countries rely on natural products for their health care. In the last century, natural products such as Taxol, docetaxel, morphine, cyclosporin A, quinine, simvastain, and lovastatin, among others, have been successfully marketed as safe drugs for the treatment of various diseases. In this respect, around 40% of recent drugs have been developed from natural products [1]. Phenolic compounds, commonly present in fruits and vegetables, are highly bioactive. Currently, these compounds are gaining much attention due to their broad application in the health care systems of several countries. These compounds significantly cure diseases related to free radical-induced stress, cancer, renal, and cardiovascular diseases and act as anti-inflammatory and hepatoprotective [2]. Due to their outstanding benefits, there is an increasing demand for these compounds in the pharmaceutical industry. Additionally, the scientific community is paying much attention to natural products [3].
Olea ferruginea is a 10- to 50-feet tall tree belonging to the genus Olea and family Oleaceae. This evergreen plant grows in the mountainous areas of Afghanistan, Kashmir, and Pakistan [4]. In Pakistan, the plant is predominantly found in the hilly regions of Azad Kashmir, Khyber Pakhtunkhwa, and Rawalpindi [5]. Traditionally, the stem bark of Olea ferruginea has been prescribed for the treatment of fever [6], whereas fresh leaf extracts of this plant were used by people suffering from bleeding gums, toothache, skin diseases, skeletal muscular, and dental problems [7, 8]. In addition, the fruit of O. ferruginea is a rich source of oil, which can be applied as massage ointment to relieve pain during dislocated bones and rheumatism [9]. Furthermore, research findings indicated that various parts of this plant have shown significant antimalarial, anti-inflammatory, antiperiodic, antileprosy, astringent, antidiabetic, and antiasthmatic properties [10].
The genus Olea is represented by nearly 35 species, and Olea europaea L. is the most cultivated around the globe for its oil production [11]. Within this context, extracts of Olea plant contain numerous phenolic compounds, flavonoids and lignin, and secoiridoid. Due to the significant phenolic content (17–23%), this plant exhibits antioxidant, anticancer, and antibacterial properties [12, 13]. Biophenols isolated from other Olea species reduce cancer growth by various mechanisms. These compounds can either cause a cell cycle arrest in a specific phase or inhibit signaling pathways, which promote proliferation of cells [14]. Furthermore, the increasing demand of olive oil encouraged cultivation of Olea europaea in several regions of the world and/or finding alternative sources of olive oil production [15]. For this purpose, Olea ferruginea, also known as wild-type olives, has been investigated as a potent source of ample amounts of olive oil. Olive oil production from wild-type olives will not only help in maintaining balance between demand and supply but also improve the national economy and human health [16]. In this respect, studies are required to authenticate the health-promoting benefits and oil quality and production of Olea ferruginea (wild olive) [17]. Although numerous studies have dealt with the local cultivars, less attention has been given to the phytochemistry and health-promoting benefits of the plant [15, 18]. Recently, we have reported on the ethyl acetate extract and the isolation of a new compound, Ferruginan, having antibacterial and antileishmanial activity [19]. Based on the preceding discussion, and in view of the importance of Olea ferruginea and lack of studies pertaining to its health benefits, the current study was designed to evaluate the antioxidant and anticancer activity of ethyl acetate extract of O. ferruginea and to search for new natural compounds present in it having potent antioxidant and chemopreventive properties.
2. Material and Methods
2.1. General Experimental Procedures
UV-Vis and IR records of isolated compounds were obtained using a spectrophotometer and a Bruker FT-IR spectrophotometer (Japan). Mass spectral data were acquired by employing a FAB-MS spectrometer (Thermo Scientific, United State). Nuclear magnetic resonance spectroscopic data were obtained with the aid of a Bruker Avance (Germany): 1H (400 MHz) and 13C NMR (100 MHz). Isolated compounds were purified using polar silica gel purchased from Merck-Germany (mesh size of 70–230), and purity was checked by precoated TLC and visualized under UV light.
2.2. Plant Material
The O. ferruginea plant was collected from the Agriculture Research Institute Tarnab, Peshawar division. Steam, twigs, and leaves were separated and dried in a dark room.
2.3. Extraction and Isolation
Crude extract and various fractions were obtained according to our previously reported method [20]. For the isolation of compounds 1–3, 200 g of ethyl acetate extract was loaded on a silica gel column 60 (0.0062-0.200 mm; Merck), using a mixture of n-hexane : EtoAC (100 : 0-0 : 100) as a mobile phase. Compound 1 was obtained using n-hexane : EtoAC (70 : 30), while compounds 2 and 3 were obtained using n-hexane: ethyl acetate(80 : 20-30 : 70).
2.3.1. Ferruginan A (1)
Compound 1 was isolated as a white amorphous powder (80.23 mg): yield = 15%; mp = 124°C; [α]D25 = +210 (c = 2.0, CH3OH); IR (KBr) = 1027 cm–1 for C = O stretching, 1260 cm–1, 1462 cm–1 for aromatic C = C stretching, and 2900 cm–1 for C − H stretching; UV (λmax) = 283 nm, 230 nm; 1H and 13CNMR data (see Table 1); and FAB − MS = m/z 417.1 [M + H]+.
Table 1.
1H and 13C NMR data of Ferruginan A (1) (δ in ppm) in CD3OH.
| C. No. | 1H-δ (J, Hz) | 13C (δ) observed | 13C (δ) Ferruginan | Multiplicity |
|---|---|---|---|---|
| 1 | 6.57, s | 112.0 | 113.8 | CH |
| 2 | — | 145.0 | 145.3 | C |
| 3 | — | 145.2 | 146.3 | C |
| 4 | 6.57, s | 110.0 | 116.7 | CH |
| 5 | 3.86, d, J3,4 = 12.0 Hz | 45.4 | 45.4 | CH |
| 6 | 2.51-2.59, m | 49.1 | 49.2 | CH |
| 7 | — | 79.2 | 79.4 | C |
| 8 |
H
a = 2.82, d, Ja,b = 16.4 Hz Hb = 3.36, d, Ja,b = 16.8 |
36.9 | 36.7 | CH2 |
| 9 | — | 133.1 | C | |
| 10 | — | 130.9 | C | |
| 11 | H a = 3.72 − 3.75, m; Hb = 3.75 − 3.78, m | 71.3 | 71.3 | CH2 |
| 12 | 4.92, s | 109.5 | 109.5 | CH |
| 13 | 3.48-3.51, m; 3.78-3.85, m | 64.0 | 64.1 | CH2 |
| 14 | 1.17, t, J13,14 = 7.2 Hz | 15.4 | 15.5 | CH3 |
| 15 | 3.75, s | 56.3 | 56.3 | CH3 |
| 16 | 3.75, s | 56.3 | — | — |
| 1' | — | 137.0 | 137.0 | C |
| 2' | 6.60, br s | 113.2 | 113.1 | CH |
| 3' | — | 149.2 | 149.2 | C |
| 4' | — | 147.3 | 147.3 | C |
| 5' | 6.80, dd, J5′,6,2″ = 8.2 Hz | 116.7 | 116.7 | CH |
| 6' | 6.64, d, J5′,6′ = 8.2, 1.2 Hz | 122.3 | 122.3 | CH |
| 7' | 3.78, s | 56.5 | 56.5 | CH3 |
2.4. Bioactivity
The antioxidant activity of ethyl acetate fractions and compounds 1–3 was determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay [21]. According to this assay, 7% of DPPH stock solution having a violet color was prepared in methanol. A mixture of 7% DPPH solution (1 mL), extracts and compounds with a concentration of 15–30 μg/mL, and ascorbic acid (positive control) was placed in a labeled test tube for 30 min at room temperature. The optical density (λmax = 517 nm) was measured against a standard by means of a UV/visible spectrophotometer; experiments were carried out in triplicate. The relative radical scavenging activity (%) was calculated as [1–absorbance of solution with sample and DPPH/absorbance of solution with DPPH] × 100.
The cytotoxicity study of ethyl acetate extract and compounds 1–3 against MCF-7 was carried out by using the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT assay according to a procedure described by Shah et al. [20]. Reduction of the salt to formazan insoluble crystals is the conformation of inhibitory effect of the extract/compounds. Briefly, the MCF-7 cell line at a concentration of 3 × 104 was seeded in a 96-well plate and incubated at 37°C overnight under 5% CO2. On the next day, the original medium was substituted with fresh medium containing 15–30 μg/mL of test samples and subjected to incubation under similar conditions for 48 h. After 48 h incubation, the MTT and fresh medium was added to achieve 0.5 mg/mL, and the plates were further incubated for 4 h. The formazan crystals were dissolved in DMSO, and absorbance was measured at 550 nm using a spectrophotometer (Thermo Fisher Scientific). The percent MCF-7 cell inhibition was calculated using the following formula:
| (1) |
2.5. Docking Studies
For docking simulations, we employed the Molecular Operating Environment-2016 (MOE) software. Three-dimensional (3-D) structure of tyrosinase in complex with the antioxidant ligand, kojic acid, having accession code No. 5I38 was obtained from the Protein Data Bank. Preparation of downloaded enzymes for binding site determination, 3-D protonation, and energy minimization was conducted as per previously reported methods [22, 23]. Structures of the compounds were built using the builder option in the MOE software. The built structures were then energy minimized using the MMFF94X force field in addition to 0.0001 gradient and database. The docking study was carried out using validated parameters including placement/refinement stage and scoring/rescoring functions. Interpretation of docking results was achieved using MOE and discovery studio visualizer.
3. Results and Discussion
3.1. 1H and 13C NMR
Bioassay-guided fractionation of O. ferruginea crude extract and isolation of compounds from the most active fraction, ethyl acetate fraction, resulted in the separation of Ferruginan A (1), a new lignin, having [M + H]+ peak at m/z 417.1 in the FAB-MS spectrum. The 1H-NMR (400 MHz) spectrum showed that compound 1 has five aromatic protons at δH 6.57 (2H, s, H-1, 4), 6.60 (1H, s, H-2′), 6.64 (1H, dd, J = 8.2, 1.2 Hz, H-6′), and 6.80 (1H, d, J = 8.2 Hz, H-5′) and three methine protons at δH 2.51–2.59 (m, H-6), 3.86 (d, J = 12.0 Hz, H-5), and 4.92 (1H, s, H-12). The spectrum also revealed that the compound contains twelve methyl protons at δH 1.17 (3H, t, J = 7.2 Hz, H-14), 3.75 (6H, s, H-15, 16), and 3.78 (3H, s, H-7′), along with six methylene protons at δH 2.82 (1H, d, J = 16.4 Hz, H-8a), 3.36 (1H, d, J = 16.8 Hz, H-8b), 3.72–3.75 (1H, m, H-11a), 3.75–3.78 (1H, m, H-11b), 3.48–3.51(1H, m, H13a), and 3.82–3.85 (1H, m, H-13b). The positions of protons were confirmed by using the heteronuclear single-quantum coherence spectroscopy (HSQC), which showed the aromatic protons at C-4 (δc 110.0), C-1 (δc 112.0), C-2′ (δc 113.2), C-5′ (δc 116.7), and C-6′ (δc 122.3), methine protons at C-5 (δc 45.4), C-6 (δc 49.1), and C-12 (δc 109.5), methylene protons at C-8 (δc 37.0), C-13 (δc 64.0), and C-11 (δc 71.3), and methyl protons at C-14 (δc 15.4), C-15 (δc 56.3), C-16 (δc 56.3), and C-7′ (δc 56.5). The broadband decoupled 13C-NMR spectrum of 1 revealed that besides the six aromatic carbons, three methine carbons, six methylene carbons, and four methyl carbons, the compounds contains eight quaternary carbons at δc 79.2 (C-7), 137.0 (C-1′), 130.9 (C-10), 133.1 (C-9), 145.2 (C-3), 145.0 (C-2), 149.2 (C-3′), and 147.3 (C-4′). Based on FAB-MS, and on 1H and 13C-NMR spectral data, the molecular formula (C23H28O7) for compound 1 was established. The 1H- and 13C-NMR data revealed one extra methoxy group that appeared as singlet at δH 6.57, and the up-field shifting of C-4 signal, from δc 116 to 110 (Figure 1).
Figure 1.
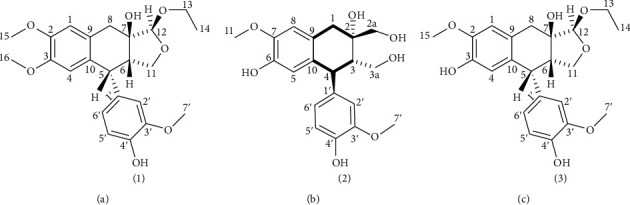
Structures of compounds 1–3.
In the HMBC spectrum, H-12 of tetrahydrofuran showed correlation with the tertiary alcoholic carbon C-7 (δc 79.2) and H-6 of the tetrahydronaphthalene moiety with C-11 (δc 71.3) of tetrahydrofuran, which supported the linkage of tetrahydrofuran ring with tetrahydronaphthalene ring. Similarly, correlations of methoxy protons H-15, 16, and 7′ with C-2 (δc 145.0), C-3 (145.2), and C-3′ (δc 149.1), respectively, confirmed their positions. Connection of the ortho-methoxy phenol with tetrahydronaphthalene moiety was verified by the connection between H-2′ and C-5 (δc 45.4) (Figure 2). The three-spin system was detected in the COSY 45° spectrum (Figure 2). The correlation of H-5, H-6, and H-11 with each other was noted in the spin system I, while the correlation between H-5′ and H-6′ was noticed in the spin system II. The connection between H-13 and H-14 was noticed in the spin system III. 1H- and 13C-NMR data for 1 are presented in Table 1.
Figure 2.
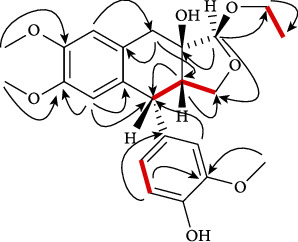
Key 2-D NMR correlations of compound 1.
3.2. Bioactivity
Ethyl acetate fraction and isolated compounds 1–3 were tested for free radical scavenging activity using the DPPH assay. Results showed that the ethyl acetate fraction and compound 2 exhibit potent antioxidant potential with IC50 values of 79.03 and 21.74 μg/mL, respectively, while compounds 1 and 3 exerted good scavenging activity (Table 2). The antioxidant potential data of compound 2 matched those reported [24]. The potent antioxidant activity may be due to the presence of phenolic compounds in ethyl acetate (polar) fraction, and five hydroxyl groups (-OH) in compound 2, which support the concept that compounds having higher number of hydroxyl groups (-OH) or other H-donating groups (-NH; -SH) have display antioxidant activity [21].
Table 2.
In vitro antioxidant and cytotoxic activity of ethyl acetate extract and compounds (1–3) of O. ferruginea.
| Compound No. | Antioxidant activity IC50 value (μg/mL) | MCF-7 assay IC50 value (μg/mL) |
|---|---|---|
| Ethyl acetate extract | 79.03 | >100 |
| Ferruginan A (1) | 28.74 | 12.01 |
| Cycloolivil (2) | 21.74 | 8.03 |
| Ferruginan (3) | 25.74 | 10.41 |
| Ascorbic acid | 22.80 | N/A |
| Doxorubicin | N/A | 4.41 |
N/A: not applicable.
The anticancer potential of ethyl acetate fraction and compounds 1–3 against breast cancer cell line (MCF-7) was also investigated. Results listed in Table 2 revealed that the percentage inhibition of MCF-7 cell line increases as the concentration (50–250 μg/mL) of ethyl acetate extract and doxorubicin (std) increase. Results also showed that compounds 1–3 exhibit good potential against MCF-7 cell lines (Table 3). These findings support the notion that natural products of Olea species have been shown to exhibit cytotoxic activity toward human cancer cells with little or no effect on normal cells [25].
Table 3.
In vitro cytotoxic activity of ethyl acetate extract of O. ferruginea against MCF-7 cell lines at different concentrations.
| Concentration (μg/mL) | % inhibition on MCF-7 cell line |
|---|---|
| Ethyl acetate extract | |
| 50 μg/mL | 24.30 |
| 100 μg/mL | 42.34 |
| 150 μg/mL | 57.02 |
3.3. Docking Studies
We performed docking studies on tyrosinase, which is a widely dispersed copper containing enzyme. This enzyme has a fundamental role in catalyzing the hydroxylation of mono- and diphenol oxidation reactions [26]. Three-dimensional (3-D) structure of tyrosinase in complex with the antioxidant ligand kojic acid having accession code No. 5I38 was obtained from the Protein Data Bank. We employed the MOE (Molecular Operating Environment-2016) software for docking simulations. After validation of the docking protocol by redock method (RMSD = 0.85 Å), we docked all three isolated compounds into the binding site of 5I38. Two-dimensional (2-D) interaction plots of the ligand-enzyme complexes are shown in Figures 3 and 4. The native compound, kojic acid, is an inhibitor of tyrosinases from various origins such as bacteria and mushrooms [27]. Additionally, 2-D interaction plots indicate that kojic acid chelates with copper ions in a bidentate manner via hydroxyl and carbonyl groups. Moreover, it forms hydrogen bond interactions with Asn205 (active site entrance residue) and His60 (Figure 3(a)). Isolated compound 1 chelates with Cu ions and forms hydrogen bond interactions with Val218 present on a loop adjacent to Cu301 [27]. It also displayed a conventional hydrogen bond interaction with Arg209 positioned near the entrance of the active site (Figure 3(b)).
Figure 3.
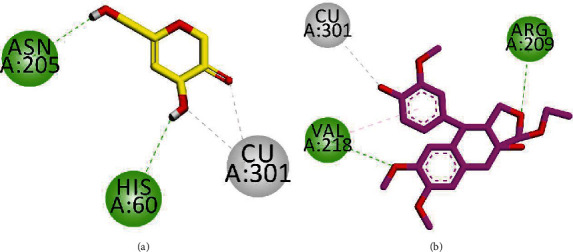
2-D interaction plot of kojic acid ((a) yellow) and isolated compound 1 ((b) pink).
Figure 4.
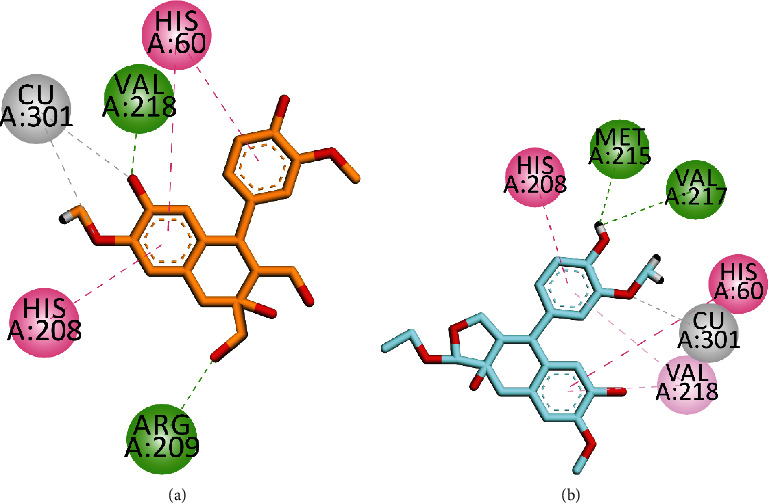
2-D interaction plot of compound 2 ((a) orange) and isolated compound 1 ((b) turquoise).
As for compound 2, the 2-D interaction plot showed that it chelates with copper ions in a bidentate manner via hydroxyl and methoxy oxygen atoms. Conventional hydrogen bond interactions were also observed with Arg209 and Val218 via hydroxyl groups. His60 and His208 displayed π − π interactions with phenyl groups (Figure 4(a)). Compound 3 is oriented toward Cu ion via the methoxy group and forms hydrogen bond interactions with Met215 and Val217. His60 and His208 displayed π − π interactions with phenyl groups, while val218 displayed π-alkyl interactions (Figure 4(b)).
4. Conclusions
In addition, findings from this investigation reveal that the isolated compounds and BEPS exhibit significant anti-inflammatory effects in both in vitro and in vivo anti-inflammatory models, which justifies the use of this plant in Algerian folk medicine. In the study, we have succeeded in isolating a new compound (1) and two known compounds (2 and 3) from O. ferruginea. In addition, findings from this investigation reveal that the ethyl acetate fraction and compound 2 exhibit potent antioxidant activity. Moreover, the ethyl acetate fraction and compounds 1–3 also showed significant anticancer activity against the breast cancer cell line (MCF-7). Docking studies suggest that the isolated compounds show tyrosinase inhibition by chelating with copper present in the active site and thus exhibited antioxidant activity.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the Research Group Project (under grant number R.G.P. 1/185/42).
Data Availability
The supporting information is available online (see supplementary file).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Spectroscopic data related to isolated compounds 1–3 are available online.
References
- 1.Dias D. A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites . 2012;2(2):303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., Qi Y., Chen X., et al. Phenolic compounds and antioxidant activity in red- and in green-fleshed kiwifruits. Food Research International . 2019;116:291–301. doi: 10.1016/j.foodres.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Wojdyło A., Nowicka P., Grimalt M., et al. Polyphenol compounds and biological activity of caper (Capparis spinosa L.) flowers buds. Plants . 2019;8(12):p. 539. doi: 10.3390/plants8120539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashmi M. A., Khan A., Ayub K., Farooq U. Spectroscopic and density functional theory studies of 5,7,3′,5′-tetrahydroxyflavanone from the leaves of _Olea ferruginea_. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy . 2014;128:225–230. doi: 10.1016/j.saa.2014.02.163. [DOI] [PubMed] [Google Scholar]
- 5.Ginai M. A. Bureau of Agriculture Information . Publication Division, Department of Agriculture, Government of West Pakistan, Lahore; 1968. [Google Scholar]
- 6.Haq F., Ahmad H., Alam M. Traditional uses of medicinal plants of Nandiar Khuwarr catchment (District Battagram), Pakistan. Journal of Medicinal Plants Research . 2011;5(1):39–48. [Google Scholar]
- 7.Murad W., Ahmad A., Gilani S. A., Khan M. A. Indigenous knowledge and folk use of medicinal plants by the tribal communities of Hazar Nao Forest, Malakand District, North Pakistan. Journal of Medicinal Plants Research . 2011;5(7):1072–1086. [Google Scholar]
- 8.Yousaf Z., Shinwari Z. K., Ali S. M. Medicinally important flora of Dhibbia Karsal Village (Mianwali district Punjab) Asian Journal of Plant Sciences . 2004;3(6):757–762. doi: 10.3923/ajps.2004.757.762. [DOI] [Google Scholar]
- 9.Haq I., Hussain Z. Medicinal plants of Palandri district Poonch (AJK) Pakistan Journal of Plant Sciences . 1995;1(1):115–126. [Google Scholar]
- 10.Abbasi A. M., Khan M., Ahmad M., Zafar M., Jahan S., Sultana S. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. Journal of Ethnopharmacology . 2010;128(2):322–335. doi: 10.1016/j.jep.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 11.Bendini A., Valli E., Cerretani L., Chiavaro E., Lercker G. Study on the effects of heating of virgin olive oil blended with mildly deodorized olive oil: focus on the hydrolytic and oxidative state. Journal of Agricultural and Food Chemistry . 2009;57(21):10055–10062. doi: 10.1021/jf901813s. [DOI] [PubMed] [Google Scholar]
- 12.Ray N. B., Hilsabeck K. D., Karagiannis T. C., McCord D. E. The Role of Functional Food Security in Global Health . Elsevier; 2019. Bioactive olive oil polyphenols in the promotion of health; pp. 623–637. [DOI] [Google Scholar]
- 13.Zoric N., Kopjar N., Kraljić K., Oršolić N., Tomić S., Kosalec I. Olive leaf extract activity against Candida albicans and C. dubliniensis – the in vitro viability study. Acta Pharmaceutica . 2016;66(3):411–421. doi: 10.1515/acph-2016-0033. [DOI] [PubMed] [Google Scholar]
- 14.Gorzynik-Debicka M., Przychodzen P., Cappello F., et al. Potential health benefits of olive oil and plant polyphenols. International Journal of Molecular Sciences . 2018;19(3):p. 686. doi: 10.3390/ijms19030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anwar P., Bendini A., Gulfraz M., et al. Characterization of olive oils obtained from wild olive trees ( _Olea ferruginea_ Royle) in Pakistan. Food Research International . 2013;54(2):1965–1971. doi: 10.1016/j.foodres.2013.09.029. [DOI] [Google Scholar]
- 16.Knoops K. T., de Groot L. C. P. G. M., Kromhout D., et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and Women. JAMA . 2004;292(12):1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 17.Abaza I., Taamalli W., Ben Temime S., Daoud D., Gutiérrez F., Zarrouk M. Natural antioxidant composition as correlated to stability of some Tunisian virgin olive oils. Rivista italiana delle sostanze grasse . 2005;82(1):12–18. [Google Scholar]
- 18.Joshi S. Olea ferrugineaRoyle, Indian olive: an underutilised fruit tree crop of north-west Himalaya. Fruits . 2012;67(2):121–126. doi: 10.1051/fruits/2012003. [DOI] [Google Scholar]
- 19.Zafar S., Ur-Rehman F., Shah Z. A., et al. Potent leishmanicidal and antibacterial metabolites fromOlea ferruginea. Journal of Asian Natural Products Research . 2019;21(7):679–687. doi: 10.1080/10286020.2018.1467894. [DOI] [PubMed] [Google Scholar]
- 20.Shah Z. A., Hameed A., Ahmed A., et al. Cytotoxic and anti-inflammatory salicin glycosides from leaves of Salix acmophylla. Phytochemistry Letters . 2016;17:107–113. doi: 10.1016/j.phytol.2016.07.013. [DOI] [Google Scholar]
- 21.Amarowicz R., Estrella I., Hernández T., et al. Free radical-scavenging capacity, antioxidant activity, and phenolic composition of green lentil (Lens culinaris) Food Chemistry . 2010;121(3):705–711. doi: 10.1016/j.foodchem.2010.01.009. [DOI] [Google Scholar]
- 22.Jan M. S., Ahmad S., Hussain F., et al. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2,5-dione derivatives as multitarget anti-inflammatory agents. European Journal of Medicinal Chemistry . 2020;186, article 111863 doi: 10.1016/j.ejmech.2019.111863. [DOI] [PubMed] [Google Scholar]
- 23.Sadiq A., Mahnashi M. H., Alyami B. A., Alqahtani Y. S., Alqarni A. O., Rashid U. Tailoring the substitution pattern of Pyrrolidine-2,5-dione for discovery of new structural template for dual COX/LOX inhibition. Bioorganic Chemistry . 2021;112, article 104969 doi: 10.1016/j.bioorg.2021.104969. [DOI] [PubMed] [Google Scholar]
- 24.Rao J. M., Tiwari A. K., Kumar U. S., Yadav J. S., Raghavan K. V. (+) Cycloolivil as an antioxidant from a new source namely Stereospurmum personatum . Washington, DC, USA: U.S. Patent and Trademark Office; 2003. US Patent 6,562,381. [Google Scholar]
- 25.Elamin M. H., Daghestani M. H., Omer S. A., et al. Olive oil oleuropein has anti-breast cancer properties with higher efficiency on ER-negative cells. Food and Cosmetics Toxicology . 2013;53:310–316. doi: 10.1016/j.fct.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 26.al-Salahi R., Taie H., Bakheit A. H., et al. Antioxidant activities and molecular docking of 2-thioxobenzo[g ]quinazoline derivatives. Pharmacological Reports . 2019;71(4):695–700. doi: 10.1016/j.pharep.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Sendovski M., Kanteev M., Ben-Yosef V. S., Adir N., Fishman A. First structures of an active bacterial tyrosinase reveal copper plasticity. Journal of Molecular Biology . 2011;405(1):227–237. doi: 10.1016/j.jmb.2010.10.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spectroscopic data related to isolated compounds 1–3 are available online.
Data Availability Statement
The supporting information is available online (see supplementary file).


