Abstract
Solid-organ transplant recipients are at risk for development of lymphoproliferative diseases. The purpose of this study was to examine the distribution of Epstein-Barr virus (EBV) load in the peripheral blood of pediatric transplant recipients who had become chronic viral load carriers (>8 copies/105 lymphocytes for >2 months). A total of 19 patients with viral loads ranging from 20 to 5,000 viral genome copies/105 lymphocytes were studied. Ten patients had no previous diagnosis of posttransplant lymphoproliferative disease (PT-LPD), while nine had recovered from a diagnosed case of PT-LPD. No portion of the peripheral blood viral load was detected in the cell-free plasma fraction. Viral DNA was found in a population of cells characterized as CD19hi and immunoglobulin D negative, a phenotype that is consistent with the virus being carried exclusively in the memory B-cell compartment of the peripheral blood. There was no difference in the compartmentalization based upon either the level of the viral load or the past diagnosis of an episode of PT-LPD. These results have implications for the design of tests to detect EBV infection and for the interpretation and use of positive EBV PCR assays in the management of transplant recipients.
Most primary infections with Epstein-Barr virus (EBV), a ubiquitous B-lymphotropic herpesvirus, occur in childhood and have a clinically asymptomatic presentation. Symptomatic infection in adolescents and adults is infrequent and associated with a clinically recognizable entity (infectious mononucleosis) (9). In immunocompetent hosts, infections are controlled by cell-mediated immunity that leads to a lifelong carrier state characterized by episodic shedding of virus into saliva, persistent low antibody titers to EBNA1, and a corps of latently infected B cells that can represent as many as 1 to 10 per million of the circulating B-cell population (1, 2, 13, 17, 25). An even higher frequency of circulating virus-specific cytotoxic-T-cell precursors appears to be required to prevent significant reactivation of virus from this latent state. Recent estimates from HLA tetramer staining suggest that more than 5% of the circulating CD8+-T-cell population in healthy long-term carriers are specific for EBV epitopes (24).
In immunosuppressed individuals EBV-driven B-cell proliferation is not regulated by such powerfully focused immune responses, and infection can thus produce a lymphoproliferative disease which has varied clinical presentations and histopathologic features and may progress to an immunoblastic lymphoma (4, 17). One group at very high risk is transplant recipients undergoing primary EBV infection while receiving an immunosuppressive drug regimen. A number of studies have recently shown that posttransplant lymphoproliferative disease (PT-LPD) is associated with a very high viral load of EBV circulating in the peripheral blood (6, 7, 8, 12, 15, 19, 22, 23,). Many of these reports have suggested that tests aimed at detection of this viral load could be used as a diagnostic marker for the presence of PT-LPD. Tests, such as quantitative load measurement, that provide early detection of infection and lymphoproliferation hold the possibility of allowing preemptive therapeutic intervention early in the course of EBV infection before symptomatic disease and progression to a lymphoma. However, recent cross-sectional and longitudinal analyses suggest that clinical resolution of primary EBV infection in a significant proportion of transplant recipients results in a chronic viral load carrier state in which high copy numbers of EBV persist asymptomatically in the peripheral blood for months or, in some cases, years (references 5, 6, 12, and 27 and our unpublished observations). Persistently elevated viral load in asymptomatic transplant recipients represents a newly recognized phenomenon revealed by the advent of PCR-based load measurement as a diagnostic tool. The utility of single isolated viral load measurements as a diagnostic marker of EBV-driven disease needs to be assessed in view of these findings.
Viral load monitoring in immunosuppressed individuals following solid-organ transplantation has provided a unique opportunity to study the virus interacting with a host where there is a high risk of the development of a PT-LPD or where that risk has been realized and a PT-LPD has already occurred. The purpose of this study was to examine the relative distribution of viral DNA load among plasma, the naive B-cell compartment, and the memory B-cell compartment in the chronic carrier state that has developed in immunosuppressed pediatric transplant recipients.
MATERIALS AND METHODS
Subjects.
Since 1995, transplant recipients attending Children's Hospital of Pittsburgh and University of Pittsburgh Medical Center have been regularly monitored for EBV viral load using a quantitative-competitive PCR (QC-PCR) protocol developed in our laboratory (22). We have monitored bowel, liver, lung, renal, and heart transplant recipients for EBV DNA copy number in peripheral blood lymphocytes. We have focused on the pediatric transplant population because within this group there are more than 190 patients for whom we have multiple prospectively collected specimens. In only 65 of these patients (34.2%) has there never been a detectable viral load. A chronic low load (defined as detectable but <200 genome copies/105 lymphocytes for >2 months) has been detected in 69 patients (36.3%), and a chronic high load (defined as >200 genome copies/105 lymphocytes for >2 months) has been detected in 35 (18.4%). The remaining 21 patients have had viral loads that have fluctuated. We developed lists of patients who met the criteria for the chronic low-load and chronic high-load carrier states. From these patient pools, we randomly selected 10 chronic low-load carriers and 9 chronic high-load carriers equally split with respect to whether their clinical history included a recognized episode of PT-LPD (Table 1). When blood specimens in excess of the requirements for viral DNA load testing were available, they were used to prepare the plasma and B-cell subfractions for load distribution measurements.
TABLE 1.
Characterization of patient population categorized into two groups
| Viral loada | Patient | PT-LPD historyb | Sexc | Age (mo) | Mo posttransplant | EBV status pretransplantd | Type of transplant | EBV load
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Per 105 PBMCe | Per 105 sIgD− cells | Per 104 sIgD+ cells | In plasma equivalent to 105 cells | ||||||||
| Chronic low | 1 | Y | M | 89 | Neg | Second heart | 8 | 8 | fNDf | ND | |
| 2 | Y | F | 101 | 29 | Neg | Liver | 20 | 20 | ND | ND | |
| 3 | Y | M | 87 | 25 | Liver | 40 | 40 | <8 | ND | ||
| 4 | Y | M | 64 | 56 | Liver | 80 | 100 | ND | ND | ||
| 5 | Y | F | 115 | 17 | Neg | Heart | 100 | 100 | ND | ND | |
| 6 | N | F | 45 | Renal | 20 | 20 | ND | ND | |||
| 7 | N | M | 146 | 29 | Small bowel | 80 | 80 | ND | ND | ||
| 8 | N | F | 16 | 9 | Neg | Heart-lung | 100 | 100 | ND | ND | |
| 9 | N | F | 109 | 77 | Pos | Liver-bowel | 100 | 200 | ND | ND | |
| 10 | N | M | 65 | 42 | Pos | Liver-small bowel | 200 | 400 | ND | ND | |
| Chronic high | 11 | Y | M | 28 | 22 | Neg | Heart | 400 | 200 | ND | ND |
| 12 | Y | 26 | 16 | Neg | Liver | 500 | 500 | ND | ND | ||
| 13 | Y | M | Renal | 800 | 800 | ND | ND | ||||
| 14 | Y | F | 112 | 100 | Liver | 1000 | 1000 | ND | ND | ||
| 15 | Y | M | 220 | 62 | Neg | Heart-lung | 5000 | >1000 | ND | ND | |
| 16 | N | F | 26 | 20 | Neg | Heart | 200 | 400 | ND | ND | |
| 17 | N | M | 33 | Pos | Liver-small bowel | 400 | 500 | ND | ND | ||
| 18 | N | F | 86 | 61 | Renal | 400 | 500 | ND | ND | ||
| 19 | N | M | 38 | 25 | Neg | Liver-small bowel | 2000 | 2000 | ND | ND | |
Chronic low load, EBV load of 8 to 200 genomes/105 peripheral blood mononuclear cells; chronic high load, EBV load of 7200 genomes/105 peripheral blood mononuclear cells.
Y, yes; N, no.
M, male; F, female.
Neg, negative; Pos, positive.
PBMC, peripheral blood mononuclear cells.
ND, not detected.
Cell lines.
The X50-7 and B95-8 cell lines (gifts of G. Miller, Yale University, New Haven, Conn.) are widely used cell lines for studies on immortalization and viral production. BJAB is an EBV-negative B-cell lymphoma used as a virus-negative control and as carrier cells. Namalwa is a BL cell line that contains two copies of the EBV genome integrated into the host genome and is a source of easily measured cell-associated wild-type EBV genome copy number. All cell lines were maintained in 5% CO2 with 10% fetal calf serum–RPMI 1640 with penicillin and streptomycin.
QC-PCR of EBV load in lymphocytes and plasma.
Lymphocytes were prepared from whole-blood samples by centrifugation onto a Histopaque (Sigma) cushion. The cells were washed in phosphate-buffered saline and counted. Cell pellets were stored at −20°C until ready for PCR. A plasma volume equivalent to 4 × 105 cells for each patient was ultracentrifuged at 14,000 rpm for 90 min (Eppendorf 5417R) in order to pellet cell-free virus. To make lymphocyte and plasma lysates, 20 μl of PCR lysis buffer (50 mM KCl, 10 mM Tris [pH 7.6], 2.5 mM MgCl2, 1% Tween 20, and 100 μg of proteinase K per ml) was added for every 105 lymphocytes or plasma volume equivalents. The lysates were incubated at 55°C for 1 h, boiled for 10 min to inactivate the proteinase K, and chilled on ice. Primers for the PCR target sequence in the EBV genome were designed with OLIGO software (National Biosciences). TP1Q5′ (AGGAACGTGAATCTAATGAAGA) and TP1Q3′ (GAGTCATCCCGTGGAGAGTA) amplify a 177-bp EBV sequence (exon 1) in the Lmp2a gene. A competitor target was made by deleting 42 bp from a 177-bp EBV amplicon derived from the viral LMP2a exon 1 sequence. For each sample, four tubes containing 8, 40, 200, or 1,000 copies of the viral LMP2a competitor sequence along with lymphocyte or plasma lysates equivalent to 105 cells were subjected to 30 cycles of amplification (94°C for 1 min, 54°C for 1 min, and 72°C for 1 min). Each PCR mixture (50 μl) contained 20 pmol of 5′ and 3′ primers, 50 mM KCl, 2.5 mM MgCl2, 10 mM Tris [pH 9.0], 0.1% Triton X-100, and 0.25 mM deoxynucleotides (Pharmacia). One unit of Amplitaq Gold DNA polymerase (Perkin-Elmer) was used in each reaction. The PCR products were analyzed on 3% agarose gels containing 0.5× Tris-borate-EDTA electrophoresis buffer and 0.5 μg of ethidium bromide per ml.
The QC-PCR assay for EBV is used to quantitate viral loads in the range of 8 to 5,000 copies of viral DNA in 105 lymphocytes. Normal latent infection (0.01 to 0.1 copies/105 lymphocytes) is not detected by this protocol (22), and detectable levels of viral DNA reflect a viral genome burden at least 2 to 3 orders of magnitude above normal latency.
Cell sorting with magnetic beads.
Lymphocytes were positively sorted for the CD15+ (granulocytes), CD19+ (B cells), or surface immunoglobulin D-positive (sIgD+) (naive B cells) phenotype by using Dynabeads 450 CD15 or CD19 (pan B) or a CELLection Pan Mouse IgG kit conjugated with anti-IgD (PharMingen). Ficoll-Hypaque lymphocyte preparations from patient blood samples were mixed with Dynabeads at a concentration of 107 beads/ml and incubated for 30 min at 4°C. The positively selected cells were isolated using a magnet (Dynal MPC) and detached from the Dynabeads using DETACHaBEAD CD19 or a releasing buffer (CELLection Pan Mouse IgG kit). The detachment of positively selected CD15 cells was not feasible. The positive and negative sorted cell compartments were stored at −20°C as dried pellets for QC-PCR.
Flow cytometric analysis.
Ficoll-Hypaque-prepared lymphocytes (106) were stained for three-color or two-color analysis with CD45-allophycocyanin (Caltag) and with CD3-fluorescein isothiocyanate (CD3-FITC) (Coulter) plus CD19-phycoerythrin (CD19-PE) (Coulter) or CD14-PE (Becton Dickinson) plus CD15-FITC (Coulter). Naive and memory B cells were stained with anti-IgD–FITC and anti-IgG–PE, respectively. Cells were stained with the appropriate antibodies at 4°C for 30 min and fixed in 1% paraformaldehyde. As negative controls, MsIgG2b-RD1 (IgG2 isotype control; Coulter), IgM-FITC (IgM isotype control; Coulter), and IgG1-PE and IgG1-FITC (IgG1 isotype control; Coulter) were used, as well as unstained cells. Flow cytometry measurements were analyzed using the Win MDI version 2.7 flow cytometry application.
Wright staining and microscopy.
Cytospun cells were fixed on slides for 15 s in absolute methanol and air dried. Each sample was stained with Wright's stain (Shannon) for 1 min, after which an equal volume of a pH-balanced buffer was added and left for 8 to 10 min. The stained slides were rinsed thoroughly in distilled water. Cell morphology was assessed by bright-field microscopy using a Nikon E600 microscope.
RESULTS
EBV viral DNA load is in CD19+ lymphocytes.
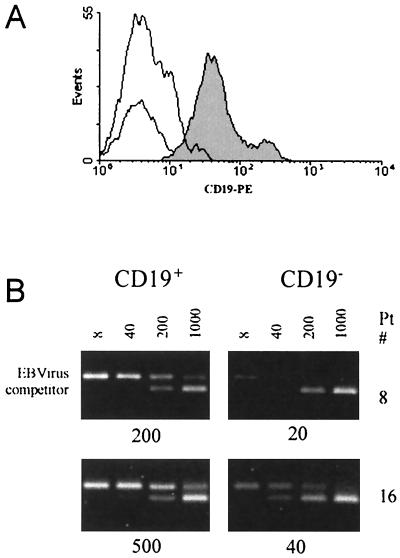
After 5 years of observation of the dynamics of viral load in transplant recipients, we have obtained quantitative PCR results for EBV load for over 800 patients. One striking observation from the data (to be analyzed and published separately) is the development and persistence of asymptomatic chronic viral loads in over 50% of the subjects. The first question to be addressed was whether the chronic viral load that had been measured in the unsorted peripheral blood lymphocytes was indeed associated with the B-cell fraction of the lymphocyte population. Fresh peripheral blood lymphocytes from three chronic low-load carriers (patients 1, 5, and 8) and three chronic high-load carriers (patients 13, 16, and 19) were fractionated into B cells (CD19+) and T cells (CD19−) using anti-CD19 magnetic beads. Flow cytometric analysis of the fluorescent-antibody-tagged lymphocytes revealed that the CD19+ cells consisted of distinct CD19hi and CD19lo subpopulations (Fig. 1). More than 90% of both CD19 subpopulations were recovered from the magnetic bead separation of the CD19-positive cells from purified peripheral blood lymphocytes. QC-PCR assays were then performed on 104 CD19+ or 105 CD19− cells. These numbers of cells were used in the PCRs to approximate the numbers of each cell type that are normally present in QC-PCRs conducted on unsorted lymphocytes. For the three low-load carriers and the three high-load carriers analyzed, PCRs using the CD19+ cells were positive for viral DNA. Detectable EBV DNA was sometimes present in the CD19− populations, but this positivity was always less than 10% and correlated with the degree to which the CD19− population contained residual CD19+ cells. Complete removal of all CD19+ cells eliminated PCR positivity from the CD19− populations. These results are consistent with the viral load detected in chronic load carriers being carried by the B-cell population.
FIG. 1.
Quantitation of viral load in cells fractionated on the basis of CD19 expression. Viral load is restricted to the CD19+ fraction of peripheral blood lymphocytes. (A) Flow cytometric profile of CD19 fluorescence in a typical lymphocyte preparation from a chronic viral load carrier. The CD19+ fraction is composed of a CD19hi and a CD19lo peak of fluorescence. (B) Ethidium bromide-stained DNA products of QC-PCRs using 104 CD19+ or 105 CD19− cells and 8, 40, 200, or 1,000 copies of an identical competitor target sequence (lower of the two PCR product bands) were analyzed by agarose gel electrophoresis. Results for patient 8 (a low-load carrier) and patient 16 (a high-load carrier) are shown. The interpolated DNA concentration in copy numbers of EBV genomes detected is shown below each panel.
One explanation for the persistence of viral loads in the peripheral lymphocyte pool at levels 3 to 4 orders of magnitude greater than has been observed in normal latently infected immunocompetent carriers would be that there is a continuously ongoing viral replication process. An analysis of viral load in the plasma was conducted to determine if the product of such an active replication process could be detected in the form of circulating cell-free virus. For all 19 patients in the study, a volume of plasma equivalent to the volume of blood containing 105 lymphocytes was analyzed by QC-PCR for the presence of free virus. Free virus was not detected from either the chronic high-load carriers or the chronic low-load carriers (Table 1). If a chronic load carrier state requires an active virus-producing state of infection, it was not manifested by the presence of free virus in the plasma.
EBV viral DNA load is in the CD19hi subpopulation.
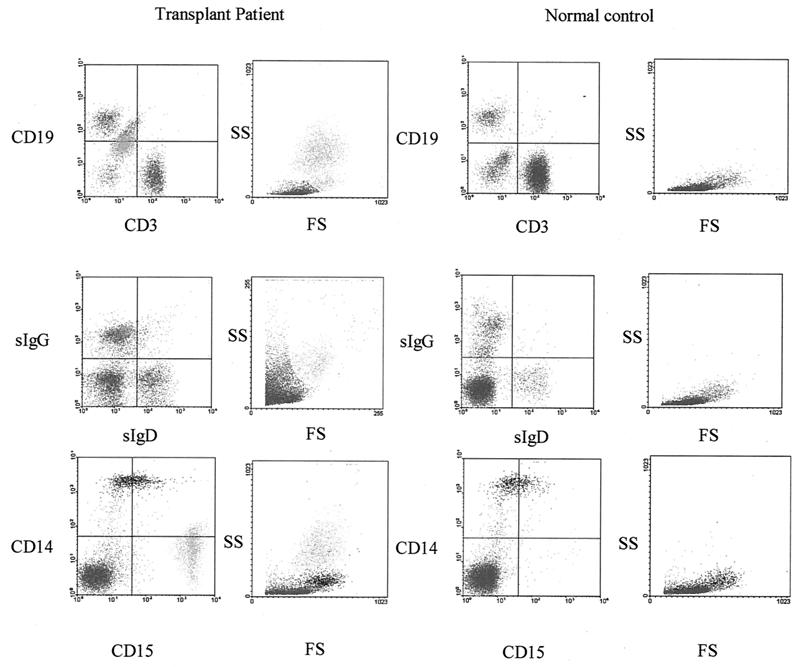
The EBV viral load in normal latently infected virus carriers is not distributed equally among all B-cell compartments but is carried exclusively in the memory B-cell subpopulation (17, 18). The presence of two distinct subpopulations of CD19+ cells in the patient blood specimens seemed unusual and immediately suggested that there could be a relationship to the chronic load carrier state detected by QC-PCR. This notion was reinforced by the observation that normal control lymphocyte preparations did not contain a CD19lo subpopulation. In addition, the CD19lo population was positive for sIgG and not for sIgD, a characteristic of memory B cells (Fig. 2).
FIG. 2.
Characterization of the CD19hi and CD19lo cell populations detected in the peripheral blood lymphocyte fraction. (Top row) Flow cytometric analysis of B-cell (CD19) and T-cell (CD3) markers after gating on the population of high-FS and -SS cells unique to lymphocyte preparations of transplant recipients compared to a normal healthy EBV-positive control donor. (Center panels) Analysis of the sIgG and sIgD expression. (Bottom panels) Analysis of monocyte (CD14) and granulocyte (CD15) markers. High-FS and -SS cells appear to be CD19lo, IgG+, and CD15+.
Another feature of the patient specimens was the presence of an uncharacteristic forward light scatter (FS)-to-side light scatter (SS) profile. Gating on the unusual cells in the FS-SS window revealed that these cells exclusively constituted the CD19lo subpopulation. Further analysis of the lymphocyte preparation using a monocyte marker (CD14) and a granulocyte marker (CD15) revealed that the CD19lo population was strongly CD15+ (Fig. 2, lower panels). The CD15hi CD19lo sIgG+ phenotype and the high FS-SS light-scattering profile are consistent with these cells being mature granulocytes. Wright staining of the patient Ficoll-Hypaque lymphocyte preparations confirmed the presence of granulocytes in the patients' specimens but not in normal control lymphocyte preparations.
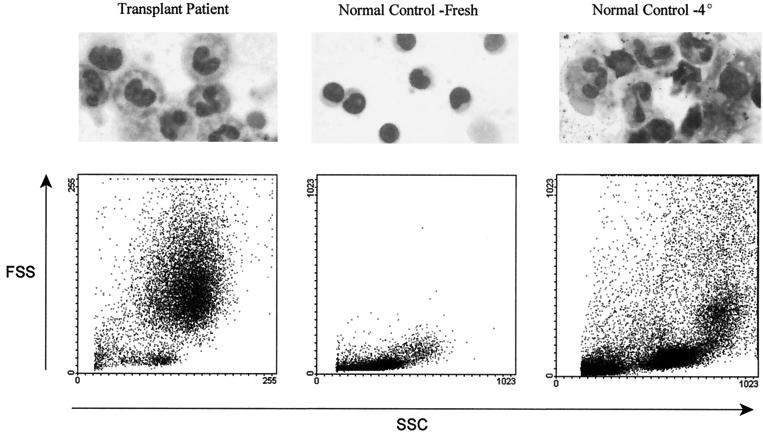
The principal difference between the patient specimens and the control blood, as far as handling in the laboratory, was that patient specimens were often 24 to 48 h old owing to the individual's location, with specimens being either from the hospital ward or from remote sites such as outpatient clinics or doctors' offices. Control blood was always drawn immediately prior to lymphocyte preparation. Instructions for preserving patient blood samples specify that refrigeration at 40C be used to prevent changes in viral load caused by postvenipuncture replication of the virus during transport. The effects of refrigeration during transport were duplicated by incubating an aliquot of a control blood draw overnight at 4°C prior to lymphocyte separation. When this was done, the Wright staining and FS-SS flow cytometric profile showed the appearance of granulocytes in a specimen that had shown no evidence of granulocyte contamination when it had been processed immediately (Fig. 3).
FIG. 3.
Wright staining of lymphocyte preparations from a pediatric transplant recipient (left panel) reveals the presence of polymorphonuclear cells, and the light-scattering profile contains large numbers of high-FS and -SS cells. A normal control donor lymphocyte preparation (center panel) has no polymorphonuclear cells and no high-FS and -SS cells. Incubation of a blood specimen at 4°C overnight prior to Ficoll-Hypaque gradient preparation shows the appearance of polymorphonuclear cells and high-FS and -SS cells (right panel)
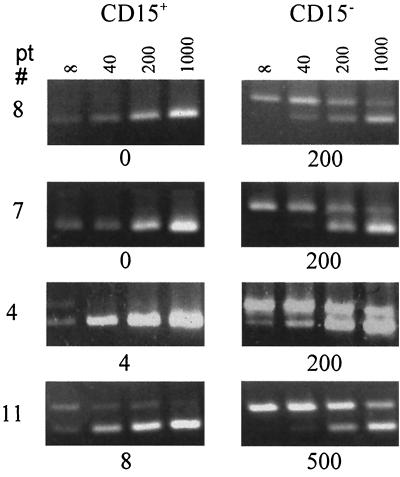
The presence of granulocytes in the patient specimens was most probably the consequence of 4°C storage prior to processing (26). Although EBV infection of granulocytes has not been reported, the possibility that the chronically elevated viral load detected in unsorted patient lymphocyte specimens was carried in these contaminating granulocytes still formally remained. To address this, we depleted CD15+ cells from the lymphocyte preparations of four load carriers (patients 4, 7, 8, and 11) by incubation with anti-CD15 beads. More than 90% removal of granulocytes was achieved. The CD15+-cell-depleted lymphocytes and the CD15+ cells were then used for QC-PCR (Fig. 4). All of the viral DNA from two of the carriers (patients 1 and 4) was present in the CD15+-cell-depleted cells, while approximately 1% of the viral load was detected in the CD15+ cells for the other two load carriers (patients 5 and 7). The detection of small amounts of viral DNA in the CD15+ cells is most likely due to impurity of the population caused by the presence of adherent lymphocytes including B cells. The presence of viral DNA in the CD15+-cell-depleted lymphocytes was consistent with the viral DNA load being carried in the CD19hi (B-cell) fraction of the peripheral blood lymphocytes.
FIG. 4.
Ethidium bromide-stained DNA products of QC-PCRs using 105 CD15+ or 105 CD15− cells and 8, 40, 200, or 1,000 copies of an identical competitor target sequence were analyzed by agarose gel electrophoresis. Results for patients (pt) 1, 4, 5, and 8 are shown. The concentration in copy numbers of EBV genomes detected is shown below each panel.
EBV viral DNA load is in the sIgD− B-cell subpopulation.
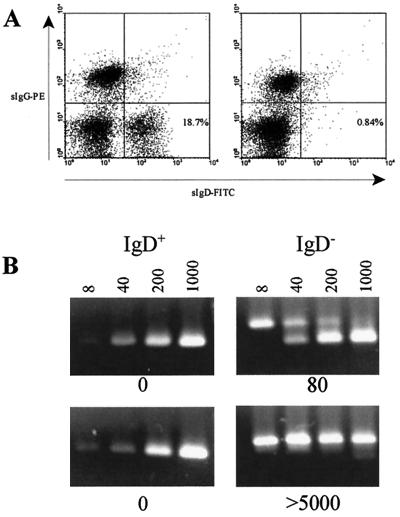
Normal immunocompetent virus carriers have loads equivalent to 0.2 to 0.5 copies/105 lymphocytes and carry the load in the memory B-cell compartment of the B-cell population (2, 22). This has been demonstrated by sorting cells on the basis of whether they express sIgD, a marker for mature, naive B cells. Therefore, the key issue concerning the chronic load in immunocompromised carriers is whether the sIgD+ B cells contain a significant proportion of the circulating viral load. For this analysis, we analyzed 10 chronic low-load and 9 chronic high-load carriers. Five of the chronic low-load carriers and four of the chronic high-load carriers had no history of PT-LPD, while five of each of the low- and high-load carriers had a previous diagnosis of PT-LPD. Anti-IgD magnetic beads were used to fractionate these cells from the lymphocyte preparations. More than 90% depletion of sIgD+ cells was obtained with a single incubation with magnetic beads (Fig. 5A). The sIgD− and sIgD+ cell populations were then analyzed by QC-PCR for the presence of viral DNA. An example of the QC-PCR analysis for a low-load carrier and a high-load carrier is shown in Fig. 5B. The high-load carrier had a viral load of 5,000 copies/105 lymphocytes, which was the highest load of any patient in this study. QC-PCR analysis of this specimen clearly indicates that no portion of the load was detected in the positively selected sIgD+ cells.
FIG. 5.
EBV viral load in naive B cells fractionated from lymphocyte preparations from chronic viral load carriers. (A) Flow cytometric profiles of sIgG- and sIgD-stained cells before (left panel) and after (right panel) anti-IgD magnetic bead separation of the naive B cells. (B) Ethidium bromide-stained DNA products of QC-PCRs using 104 IgD+ or 105 IgD− cells and 8, 40, 200, or 1,000 copies of an identical competitor target sequence were analyzed by agarose gel electrophoresis. Results for patient 7, a low-load carrier, and patient 15 (a high-load carrier) are shown. The interpolated DNA concentration in copy numbers of EBV genomes detected is shown below each panel. A complete analysis of all 19 patients in the study is summarized in Table 1.
Specimens from the 10 chronic low-load carriers and 9 chronic high-load carriers were similarly analyzed after anti-IgD magnetic bead fractionation. Nine of the 10 low-load carriers and all nine of the high-load carriers showed no detectable viral DNA in the sIgD+ population (Table 1). The only low-load carrier to have detectable viral DNA in the quantitative assay had a value reported as <8. The standard QC-PCR assay interpolates viral load values between 8 and 5,000 (22). A value of <8 means that viral DNA was detected but that there were fewer than eight copies present in the PCR. This is consistent with the presence of a single virus-infected cell in the reaction mixture, and it is possible that a virus-infected IgD− cell contaminated the sIgD+ population. Overall, the results indicate that there is no difference between high- and low-load carriers with respect to the B-cell compartment carrying the viral load. There was also no difference between patients who had a prior diagnosis of PT-LPD and patients who had no history of PT-LPD with respect to the B-cell compartment in which viral load was carried.
Relative proportions of B cells in lymphocyte populations.
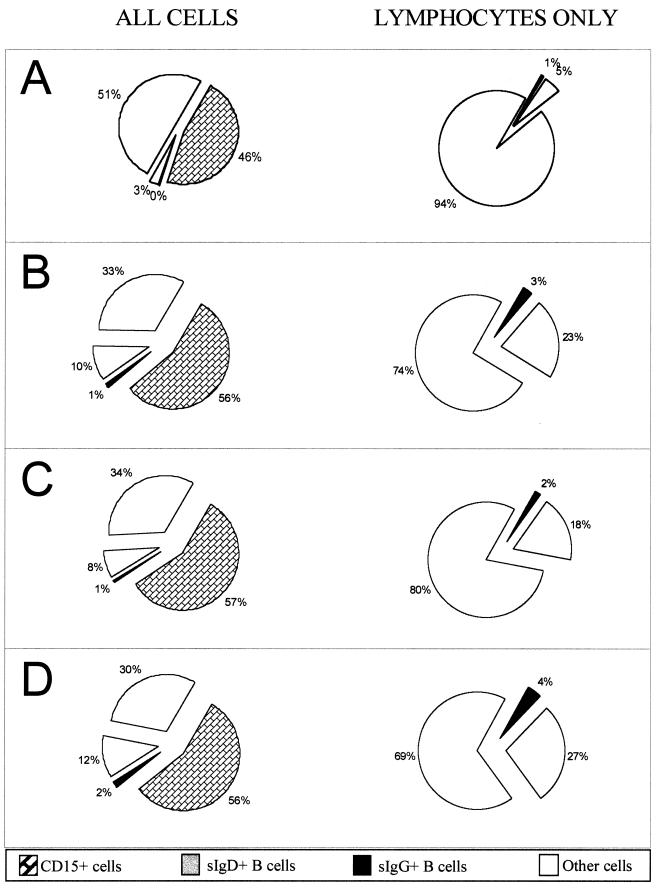
The chronic viral loads of immunosuppressed transplant recipients are 100- to 10,000-fold higher than the loads that have been measured in latently infected immunocompetent individuals (6). For both patients and controls the viral loads are carried in the same subfraction of the B-cell population. It was possible from the studies we have presented thus far to conclude that the greater viral loads were due, at least in part, to an expansion of the IgD− B-cell compartment. We therefore conducted an analysis of the relative proportions of B cells carrying sIgD (naive B cells), B cells carrying sIgG (memory B cells), CD15+ cells (granulocytes), and other cells (T cells, NK cells, monocytes, and granulocytes) in Ficoll-Hypaque lymphocyte preparations (Fig. 6). Blood samples from pediatric transplant recipients with no detected EBV infection incubated overnight at 40C were used as controls. The 4°C storage produced a similar level of contaminating granulocytes in the control population as in the chronic carriers, and there was no difference between the chronic low- and high-load carriers in the numbers of granulocytes present, indicating that the level of the viral load was not a factor in determining the presence of granulocytes. When only lymphocytes were considered, the proportion of B cells to total lymphocytes was 6% for the controls and 25% for the chronic viral load carriers. There was no significant difference between the high- and low-load carriers. The larger proportion of B cells in the viral load-carrying patient population is at most 4 times greater than normal. This modest increase in the fraction of B cells in lymphocyte preparations includes a relative increase in the uninfected IgD+ population and is within the range expected for pediatric blood specimens (9, 13).
FIG. 6.
Pictographs showing the relative proportions of B cells in the population of cells purified from the blood of immunosuppressed transplant recipients. On the left are the percentages of B cells in the total population, while on the right the CD15+ cells have been removed and only lymphocytes have been considered. (A) Control EBV-negative pediatric transplant recipients; (B) mean of all 19 chronic load carriers in the study group; (C) the 10 chronic low-load carriers; (D) the 9 chronic high-load carriers.
DISCUSSION
We have determined that a large proportion (104 of 190 [54.7%]) of immunocompromised pediatric transplant recipients whom we have prospectively monitored become chronic viral load carriers carrying loads several orders of magnitude greater than normally observed in immunocompetent individuals. The load appears to be largely or entirely cell associated, as no viral DNA was detected in plasma specimens from even the highest-load carriers. Plasma-based PCR assays for EBV have been suggested for diagnostic purposes to detect EBV infection and lymphoproliferative disease (15). We have found that even during EBV productive disease the plasma fraction contains only a small fraction (<10%) of the total load in an equivalent volume of blood (data not shown) The inability to detect virus in the plasma of chronic viral load carriers indicates that plasma-based assays will probably miss the detection of virus infection in most cases.
We have identified the cells that are carrying the viral DNA in the lymphocyte populations obtained upon Ficoll-Hypaque separation of blood from chronic viral load carriers. To do this, it was necessary to adopt a strategy limited by the use of single incubations with antibody-coated magnetic beads. This was because (i) there were restricted numbers of available cells from patient specimens, which were subject to the losses inherent to magnetic bead sorts, and (ii) the sorted cells were often unable to survive secondary or tertiary bead incubations. A strategy for obtaining pure IgG+ B cells would involve an anti-CD15 bead negative sort to remove granulocytes followed by an anti-CD19 bead positive sort followed by an sIg positive sort. This type of strategy was not practical for most patients' specimens. We therefore set out to confirm that the EBV load was in memory B cells by a series of experiments using single bead sorts. We confirmed that the load was in the CD19+ cell fraction and then eliminated the CD19lo cells from consideration by demonstrating that (i) they were granulocytes and (ii) they did not account for any significant portion of the viral load. The small amounts of viral DNA detected in the granulocyte preparations from the highest-load carriers were most likely the result of adherent B cells. We then assayed a large number of specimens using an anti-IgD bead separation to subfractionate the naive B cells from the total lymphocyte pool and determined that these cells did not harbor a viral load. The sum of the observations is that the viral load appears to be carried by CD19high CD15− sIgD− cells, a phenotype which is consistent with EBV being carried in memory B cells. The same B-cell compartment carries the latently infected cells of immunocompetent adults (2). Thus, these results suggest that the chronic viral load of immunosuppressed pediatric transplant recipients is most likely carried in a latent state.
In related work, preliminary results from our laboratory with reverse transcriptase PCR analysis of sentinel viral gene expression confirms the presence of RNA for the LMP2a latency-associated gene and the absence of expression of genes associated with immortalization and lytic virus production in the peripheral blood of the chronic low-load carriers (20). The pattern of viral gene expression in the high-load carrier state is not as simple. It is characterized by expression of LMP1 and LMP2a. The results reported here indicate that the different viral gene expression patterns seen in low- and high-load carriers occur within the memory B-cell fraction. The proportion of memory B cells to naive B cells and other lymphocytes in the peripheral circulation of chronic viral load carriers is not greatly skewed in favor of memory B cells and actually falls within the range of expected values for children. Since this relatively small fraction of cells harbors all the elevated viral load in immunocompromised transplant recipients, then within the memory B-cell compartment a high proportion of the cells is likely to be infected. If the estimate of 2 to 5 genomes per cell for latently infected cells in adults (17) is assumed to be correct for this patient population as well, then approximately 1 of 10 memory B cells (for a load of 1,000/105 lymphocytes) to 1 of 1,000 memory B cells (for a load of 10/105 lymphocytes) carries viral DNA. These frequencies for viral DNA-positive cells make in situ studies feasible for further defining and characterizing this population.
Although intuitively a high viral load cannot be viewed as desirable, it is not yet clear exactly what risks are associated with the persistently elevated-load carrier state of immunocompromised patients. If the state of the virus infection in the peripheral blood is indicative of the state of infection in lymphoid tissues and other organs, then the present studies reveal an essentially quiescent, but potentially dangerous, situation that conventional antiviral therapies cannot affect. Experimental therapies involving intravenous administration of anti-CD20 antibodies to abolish the B-cell compartment do diminish the circulating viral load but in many cases may unnecessarily affect the large and uninfected naive segment of the B-cell population. Our data suggest that it might be more logical to use therapeutic agents that target only the memory B-cell population not only as therapy for disease, but also in preemptive therapeutic strategies aimed at reducing high-load carrier states.
Our preliminary analyses of chronic load carriers and patients with PT-LPD suggest that there are shifts in the distribution of the load in the peripheral blood between naive and memory compartments (not shown) and in the patterns of viral gene expression that are associated with the PT-LPD disease state 20). We are developing new diagnostic tools based on these observations that will define the state of the infection more clearly than is presently possible with measurements of a viral load alone.
ACKNOWLEDGMENTS
We thank Martin Cottrill and Monica Bailey for their dedicated technical support.
This work was supported by a grant from the Cancer Treatment Research Foundation, Chicago, Ill., and an unrestricted research and educational grant from Medimmune Inc.
REFERENCES
- 1.Babcock G J, Decker L L, Freeman R B, Thorley-Lawson D A. Epstein-Barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med. 1999;190:567–576. doi: 10.1084/jem.190.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babcock G J, Decker L L, Volk M, Thorley-Lawson D. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 3.Barkholt L, Dahl H, Endom M, Lindé A. Epstein-Barr virus DNA in serum after liver transplantation—surveillance of viral activity during treatment with different immunosuppressive agents. Transpl Int. 1996;9:439–445. doi: 10.1007/BF00336820. [DOI] [PubMed] [Google Scholar]
- 4.Finn L, Reyes J, Bueno J, Yunis E. Epstein-Barr virus infections in children after transplantation of the small intestine. Am J Surg Pathol. 1998;22:299–309. doi: 10.1097/00000478-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Green M, Cacciarelli T V, Mazariegos G V, Sigurdsson L, Qu L, Rowe D T, Reyes J. Serial measurement of Epstein-Barr viral load in peripheral blood in pediatric liver transplant recipients during treatment for posttransplant lymphoproliferative diseases. Transplantation. 1998;66:1641–1644. doi: 10.1097/00007890-199812270-00012. [DOI] [PubMed] [Google Scholar]
- 6.Green M, Cacciarelli T V, Mazariegos G V, Sigurdsson L, Rowe D, Reyes J. Natural history of Epstein-Barr virus load in pediatric thoracic recipients with post transplant lymphoproliferative disorders and other primary EBV infections. Transplantation. 1998;67:S215. [Google Scholar]
- 7.Green M, Michaels M G, Webber S A, Rowe D, Reyes J. The management of Epstein-Barr virus associated post-transplant lymphoproliferative disorders in pediatric solid-organ transplant recipients. Pediatr Transplant. 1999;3:271–281. doi: 10.1034/j.1399-3046.1999.00066.x. [DOI] [PubMed] [Google Scholar]
- 8.Green M, Reyes J, Webber S, Michaels M G, Rowe D. The role of viral load in the diagnosis, management and possible prevention of Epstein-Barr virus-associated post-transplant lymphoproliferative disease following solid organ transplantation. Curr Opin Org Transplant. 1999;4:292–296. [Google Scholar]
- 9.Hicks M J, Jones J, Minnich L, Weigle K, Thies A C, Layton J. Age-related changes in T- and B-lymphocyte subpopulations in the peripheral blood. Arch Pathol Lab Med. 1983;107:518–523. [PubMed] [Google Scholar]
- 10.Khan G, Miyashita E, Yang B, Babcock G, Thorley-Lawson D. Is EBV persistence in vivo a model for B cell homeostasis? Immunity. 1996;5:173–179. doi: 10.1016/s1074-7613(00)80493-8. [DOI] [PubMed] [Google Scholar]
- 11.Kieff E. Herpesviridae. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 2343–2396. [Google Scholar]
- 12.Kogan D L, Burroughs M, Emre S, Fishbein T, Moscona A, Ramson C, Schneider B L. Prospective longitudinal analysis of quantitative Epstein-Barr virus polymerase chain reaction in pediatric liver transplant recipients. Transplantation. 1999;67:1068–1070. doi: 10.1097/00007890-199904150-00023. [DOI] [PubMed] [Google Scholar]
- 13.Kotylo P, Fineberg N, Freeman K, Redmond N, Charland C. Reference ranges for lymphocyte subsets in pediatric patients. Am J Clin Pathol. 1993;100:111–115. doi: 10.1093/ajcp/100.2.111. [DOI] [PubMed] [Google Scholar]
- 14.Lewin N, Aman P, Masucci M G, Klein E, Klein G, Oberg B, Strander H, Henle W, Henle G. Characterization of EBV carrying B cell populations in healthy seropositive individuals with regard to density, release of transforming virus and spontaneous outgrowth. Int J Cancer. 1987;39:472–476. doi: 10.1002/ijc.2910390411. [DOI] [PubMed] [Google Scholar]
- 15.Limaye A P, Huang M-L, Atienza E E, Ferrenberg J M, Corey L. Detection of Epstein-Barr virus DNA in sera from transplant recipients with lymphoproliferative disorders. J Clin Microbiol. 1999;37:1113–1116. doi: 10.1128/jcm.37.4.1113-1116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas K G, Burton R L, Zimmerman S E, Wang J, Cornetta K G, Robertson K A, Lee C H, Emanuel D J. Semiquantitative Epstein-Barr virus (EBV) polymerase chain reaction for the determination of patients at risk for EBV-induced lymphoproliferative disease after stem cell transplantation. Blood. 1998;91:3654–3661. [PubMed] [Google Scholar]
- 17.Miyashita E, Yang B, Babcock G, Thorley-Lawson D. Identification of the site of Epstein-Barr persistence in vivo as a resting B cell. J Virol. 1997;71:4882–4891. doi: 10.1128/jvi.71.7.4882-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyashita E, Yang B, Lam K, Crawford D, Thorley-Lawson D. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell. 1995;80:593–601. doi: 10.1016/0092-8674(95)90513-8. [DOI] [PubMed] [Google Scholar]
- 19.Nalesnik M. Clinical and pathological features of post transplant lymphoproliferative disorders. Springer Semin Immunopathol. 1998;20:325–342. doi: 10.1007/BF00838047. [DOI] [PubMed] [Google Scholar]
- 20.Qu L, Green M, Webber S, Reyes J, Ellis D, Rowe D. Epstein-Barr virus gene expression in the peripheral blood of transplant recipients with chronic circulating viral loads. J Infect Dis. 2000;182:1013–1021. doi: 10.1086/315828. [DOI] [PubMed] [Google Scholar]
- 21.Riddler S A, Breining B K, McKnight J C. Increased levels of circulating Epstein-Barr virus (EBV)-infected lymphocytes and decreased EBV nuclear antigen antibody responses are associated with the development of post-transplant lymphoproliferative disease in solid-organ transplant recipients. Blood. 1994;84:1972–1984. [PubMed] [Google Scholar]
- 22.Rowe D T, Qu L, Reyes J, Jabbour N, Yunis E, Putnam P, Todo S, Green M. Use of quantitative competitive PCR to measure Epstein-Barr virus genome load in the peripheral blood of pediatric transplant patients with lymphoproliferative disorders. J Clin Microbol. 1997;35:1612–1615. doi: 10.1128/jcm.35.6.1612-1615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shapiro R, Nalesnik M, McCauley J, Fedorek S, Jordan M L, Scantlebury V P, Jain A, Vivas C, Ellis D, Lombardozzi-Lane S, Randhawa P, Johnston J, Hakala T R, Simmons R L, Fung J J, Starzl T E. Posttransplant lymphoproliferative disorders in adult and pediatric renal transplant patients receiving Tacrolimus-based immunosuppression. Transplantation. 1999;68:1851–1854. doi: 10.1097/00007890-199912270-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan L C, Gudgeon N, Annels N E, Hansasuta P, O'Callaghan C A, Rowland-Jones S, McMichael A J, Rickinson A B, Callan M F. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 25.Thorley-Lawson D, Babcock G. A model for persistent infection with Epstein-Barr virus: the stealth virus of human B cells. Life Sci. 1999;65:1433–1453. doi: 10.1016/s0024-3205(99)00214-3. [DOI] [PubMed] [Google Scholar]
- 26.Van Lambalgen R, Van Meurs G. Lymphocyte subpopulations do not alter during blood storage at 4°C. J Immunol Methods. 1985;80:39–43. doi: 10.1016/0022-1759(85)90162-0. [DOI] [PubMed] [Google Scholar]
- 27.Zangwill S D, Hsu D T, Kichuk M P, Garvin J H, Stolar C J, Haddad J, Jr, Stylianos S, Michler R E, Chadburn A, Knowles D M, Addonizio L J. Incidence and outcome of primary Epstein-Barr virus infection and lymphoproliferative disease in pediatric heart transplant recipients. J Heart Lung Transplant. 1998;17:1161–1166. [PubMed] [Google Scholar]