Abstract
Thirty-two isolates of the dimorphic fungus Penicillium marneffei were studied for their biochemical properties. All isolates possessed the enzyme urease and were inhibited by 500 mg of cycloheximide per liter. No strain fermented glucose, and thus no strain fermented any of the other five sugars tested. All assimilated glucose, maltose, and cellobiose; only one of the isolates did not assimilate salicin. Totals of 65.6, 84.4, and 71.9% of the isolates assimilated trehalose, xylose, and nitrate, respectively. Twelve strains possessed the enzyme β-galactosidase. Overall, 17 different biotypes were recognized, but no association was found between the human immunodeficiency virus status of the patients and the biotype. A novel finding of concentration-dependent growth inhibition of P. marneffei by galactose is described. Inhibition of growth occurred at a low concentration of galactose (0.015 to 0.25%) when galactose was the sole carbon source in the medium. Morphological changes of the fungal cells were observed in the presence of galactose.
First discovered in Vietnam in 1956, Penicillium marneffei has changed from being a fungus of obscure clinical significance to being a prominent opportunistic endemic pathogen. The pathogenic potential of the fungus remained elusive until 1973, when the organism was recovered from the spleen of an American missionary suffering from Hodgkin's disease who had a history of travel to Southeast Asia (8). Clinical cases of infection remained rare until the AIDS pandemic began to emerge in Southeast Asia. P. marneffei causes diverse syndromes ranging from fungemia to molluscum contagiosum-like skin lesions, pulmonary infiltrates, and disseminated abscess formation, including osteomyelitis, primarily in human immunodeficiency virus (HIV)-infected patients. Infections in non-HIV-infected patients have also been observed, primarily among immunocompromised patients and less frequently in patients without any known underlying diseases (4, 9, 13, 16).
Laboratory diagnosis of penicilliosis still depends on isolation of the fungus from clinical specimens, with its identification based on morphological features and demonstration of a diffusible red pigment as well as thermal dimorphism (14). Several serological tests have been described in recent years, including indirect immunofluorescent-antibody testing and enzyme immunoassays based on a variety of antigens (5, 7, 11, 12, 24). A number of genotypic studies on the fungus have been published, including the description of the first gene cloned (MP1, encoding a P. marneffei-specific cell wall mannoprotein, Mp1p) (2, 3). There is, however, relatively little description of the biochemical properties of P. marneffei in the literature. Methodology for typing of P. marneffei also is not well described, except for one recent report on the molecular typing of the fungus (10). We report an evaluation of the biochemical properties of 32 strains of P. marneffei and their possible use in strain biotyping. A unique finding of concentration-dependent growth inhibition by galactose in the assimilation test is described.
MATERIALS AND METHODS
Thirty-two strains of P. marneffei were tested. They were all clinical isolates from patients admitted to six major tertiary-care hospitals in Hong Kong (Queen Mary Hospital, Queen Elizabeth Hospital, Princess Margaret Hospital, Tuen Mun Hospital, Pamela Youde Hospital, and Yan Chai Hospital) from 1996 to 1999. All patients had documented penicilliosis marneffei as evidenced by a compatible clinical picture (22) and isolation of P. marneffei from a sterile body site. Each isolate was recovered from a different patient. P. marneffei was identified by the following criteria: (i) demonstration of thermal dimorphism by showing a conversion from the yeast form at 37°C to the mold form at 25°C, (ii) production of a diffusible red pigment from the mold form when cultured at 25°C on Sabouraud dextrose agar, and (iii) microscopic morphology of the mycelia, including the presence of conidiophore-bearing biverticillate penicilli and each penicillus being composed of four or five metulae with smooth-walled conidia (14, 20). Twenty strains (62.5%) were isolated from HIV-positive patients, and nine (28.1%) were isolated from HIV-negative patients. For three patients (9.4%), the HIV status was not known. Strains were stored in sterile distilled water at room temperature until use and then recovered by subculture on Sabouraud dextrose agar without cycloheximide at 25°C and on brain heart infusion agar (Oxoid, Basingstoke, United Kingdom) at 37°C.
Positive and negative control strains were included for each biochemical test (15, 17). A 3-day-old pure culture of the control strains and yeast forms of P. marneffei were used in all reactions. All biochemical tests were performed in duplicate by standard protocols (6, 17, 21). The tests were repeated three times to ensure reproducibility of the results. The urea hydrolysis test was performed by using 2% Christensen urease broth (Oxoid) incubated at 37°C for 72 h. A change of color of the broth to deep pink indicates the production of urease. Cycloheximide resistance was determined by the ability of the fungus to grow on Sabouraud dextrose agar with 500 mg of cycloheximide per ml (bioMerieux, Marcy l'Etoile, France). Culture plates were incubated at 37°C for 14 days. The test for the presence of β-galactosidase was performed by utilization of o-nitrophenyl-β-d-galactopyranoside (ONPG) (Sigma, St. Louis, Mo.). The ONPG solution was prepared by adding one part of 0.6% ONPG in phosphate buffer (pH 7.5) to three parts of 1% peptone water. Two-milliliter aliquots of the ONPG solution were prepared, and one yeast colony of P. marneffei was used to inoculate the medium. The medium was incubated at 37°C for 48 h, and a positive reaction was indicated by a change from colorless to yellow.
Biochemical tests.
Yeast colonies of P. marneffei were suspended in 25 ml of sterile distilled water and washed twice to avoid carryover of carbohydrates from the culture medium. The yeast cells were centrifuged, and the pellet was resuspended in 5 ml of distilled water to make a homogenous suspension with turbidity equivalent to McFarland no. 1 standard. One hundred microliters of the suspension was used as the inoculum. Carbohydrates at a final concentration of 2% were added to the fermentation broth containing yeast extract-peptone and phenol red indicator (Oxoid) in test tubes with an inverted Durham tube for the detection of gas formation. The tubes were incubated at 32°C for 14 days with daily shaking and examined for acid and gas production. For carbohydrate assimilation and fermentation tests, the incubation temperature was 32 rather than 30°C, which is generally recommended in order to ensure that the P. marneffei isolates are maintained in the yeast form. An incubation temperature of 37°C was not used because it may result in the degradation of some disaccharides. Assimilation tests for 17 carbohydrates were performed using the auxanographic technique (17). A drop of 5% carbohydrate solution was pipetted onto a sterilized filter paper disk of 6 mm in diameter placed in a petri dish. Any excess solution was removed, and the disks were dried before use. Control disks were made by using sterile distilled water in place of carbohydrate solutions.
One milliliter of the yeast suspension was mixed thoroughly with 20 ml of molten agar (yeast nitrogen base medium and Noble agar; Difco, Detroit, Mich.) and then poured into a 90-mm-diameter petri dish. After the agar was set, carbohydrate and distilled water (control) paper disks were placed on the agar surface. Only one disk was used for each plate. The plates were incubated at 32°C for 4 days. A positive assimilation reaction was indicated by growth of the yeast around the paper disk.
The nitrate assimilation test was performed in the same way as the carbohydrate assimilation test except that paper disks were prepared with 10% KNO3 (Sigma) and the agar medium was yeast carbon base medium (Difco). A positive assimilation reaction was indicated by growth of the yeast around the paper disk.
Effect of galactose concentration on growth inhibition and determination of MACgal.
The galactose assimilation test was repeated using paper disks prepared with different concentrations of galactose solution (1 to 5%) to examine the effects on growth inhibition. The diameter of the zone of growth inhibition at each concentration was noted for each strain. The maximal assimilable concentration of galactose (MACgal) is defined as the lowest concentration of galactose at which there is no inhibition of growth of P. marneffei yeasts in the yeast nitrogen base assimilation medium. To determine the MACgal, the procedure for the carbohydrate assimilation test was used except that serial concentrations (0.0075 to 1%) of galactose were incorporated into the agar medium instead of onto disks. The MACgal is read as the concentration of galactose at which visible growth occurs in the medium after 4 days of incubation.
The microscopic appearances of yeast cells in the presence of glucose and galactose were compared. Yeast nitrogen base broth (Difco) was supplemented with either 1% glucose or 1% galactose. The broth media were inoculated with P. marneffei yeast suspension and incubated at 32°C as for carbohydrate fermentation tests. Ten microliters of the broth culture was examined at a magnification of ×400 after staining with lactophenol cotton blue (Becton Dickinson, Cockeysville, Md.).
RESULTS
All patients with culture-documented penicilliosis marneffei were residents of Hong Kong. One strain was recovered from a patient of Filipino origin. All of the remaining 31 patients were ethnic Chinese; five of them were born in mainland China, and 26 were born in Hong Kong.
The 32 isolates of P. marneffei were grouped into different biotypes according to the biochemical profile of the reactions tested. Overall, 17 different biotypes were recognized, as shown in Table 1. Two biotypes (biotypes 1 and 2) predominated, with six isolates (18.8%) each. These biotypes differ only in the β-galactosidase reaction.
TABLE 1.
Biochemical reactions of 32 strains of P. marneffeia
| Biotype | Strain | HIVb | CYCc | UREc | ONPGc | Assimilationc
|
Fermentationd
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KNO3 | Glucose | Lactose | Galactosee | Maltose | Sucrose | Trehalose | Xylose | Glycerol | Raffinose | Cellobiose | Melibiose | Inositol | Rhamnose | Arabinose | Ribose | Mannitol | Salicin | Glucose | Lactose | Maltose | Sucrose | Galactose | Trehalose | ||||||
| 1 | PM1 | − | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − |
| PM22 | − | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM27 | + | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM30 | + | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM31 | + | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM32 | + | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| 2 | PM6 | + | − | + | + | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − |
| PM12 | ? | − | + | + | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM13 | − | − | + | + | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM16 | ? | − | + | + | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM26 | + | − | + | + | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM28 | + | − | + | + | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| 3 | PM4 | − | − | + | − | + | + | − | + | + | − | − | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − |
| PM14 | + | − | + | − | + | + | − | + | + | − | − | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM15 | ? | − | + | − | + | + | − | + | + | − | − | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| 4 | PM20 | + | − | + | − | − | + | − | + | + | − | − | − | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − |
| PM21 | + | − | + | − | − | + | − | + | + | − | − | − | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| PM24 | + | − | + | − | − | + | − | + | + | − | − | − | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| 5 | PM19 | + | − | + | + | + | + | − | + | + | − | − | − | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − |
| PM25 | + | − | + | + | + | + | − | + | + | − | − | − | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − | |
| 6 | PM2 | + | − | + | + | − | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | A | − | − |
| 7 | PM3 | + | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | A | A |
| 8 | PM5 | − | − | + | − | − | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − |
| 9 | PM7 | + | − | + | + | − | + | − | + | + | − | − | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − |
| 10 | PM8 | + | − | + | + | − | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | A |
| 11 | PM9 | − | − | + | − | − | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | − | A | − | A | − | A | A |
| 12 | PM10 | − | − | + | + | − | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | − |
| 13 | PM11 | − | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | − | A |
| 14 | PM17 | − | − | + | − | + | + | − | + | + | − | − | + | − | − | + | − | − | − | − | − | − | + | A | − | − | A | − | − |
| 15 | PM18 | + | − | + | − | + | + | − | + | + | − | − | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | A | − |
| 16 | PM23 | + | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | A | − | − |
| 17 | PM29 | + | − | + | − | + | + | − | + | + | − | + | + | − | − | + | − | − | − | − | − | − | + | A | − | − | − | A | − |
HIV, HIV status of patient; CYC, resistance to cycloheximide; URE, urease; ONPG, β-galactosidase.
+, positive; −, negative; ?, unknown.
+, positive; −, negative.
−, no change in acidity of medium; A, acid formation but no gas formation.
See text for discussion.
The results of the biochemical tests are shown in Table 1. All isolates were urease positive and were inhibited by 500 mg of cycloheximide per liter. Twenty-three (71.9%) of the isolates assimilated nitrate, and nine (28.1%) did not. For the β-galactosidase test, 12 (37.5%) gave a positive reaction. None of the isolates fermented any of the six sugars with acid and gas production, but all produced acid from glucose. None produced acid from lactose. One (3.1%), three (9.4%), four (12.5%), and four (12.5%) of the isolates produced acid from maltose, sucrose, galactose, and trehalose, respectively. All strains assimilated glucose, maltose, and cellobiose, and most assimilated salicin (96.9% positive). None assimilated lactose, sucrose, glycerol, raffinose, melibiose, inositol, rhamnose, arabinose, ribose, or mannitol. For trehalose and xylose, 21 strains (65.6%) and 27 strains (84.4%), respectively, gave a positive assimilation reaction.
An unusual phenomenon was observed in the galactose assimilation test. There was no growth within the vicinity of the galactose disk, giving rise to a large zone of inhibition of growth, while macroscopically visible growth of the yeast was observed beyond that zone of inhibition (Fig. 1). Despite the presence of a zone of growth inhibition, galactose assimilation was considered positive in view of the presence of growth at the periphery of the zone. The diameter of the zone of inhibition around the galactose disk increases with increasing concentrations of the galactose solution used to prepare the disks (data not shown). The MACgal values for the 32 strains of P. marneffei ranged from 0.015 to 0.25%, and the diameter of inhibition ranged from 10 to 56 mm. There was, however, no correlation between the MACgal and the diameter of the zone of inhibition.
FIG. 1.
Zone of inhibition of growth around a galactose disk in the carbohydrate assimilation test.
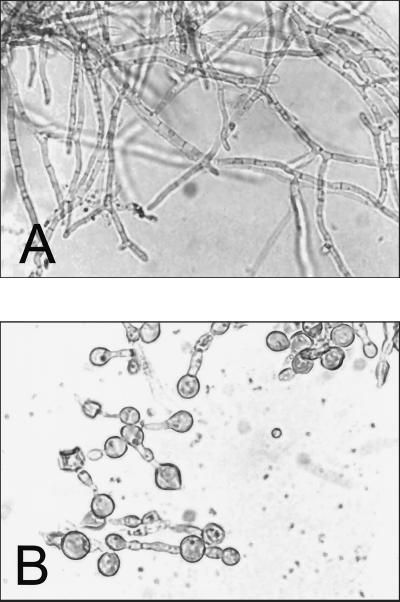
The morphology of yeast cells grown in 1% glucose- or galactose-supplemented yeast nitrogen base broth is shown in Fig. 2. Cells were found to be rounded up in the presence of galactose but not in the presence of glucose or other sugars (pictures of yeast cells in the presence of other sugars are not shown). Similar appearances of yeast cells are observed in broth cultures with concentrations of galactose at and above the MACgal.
FIG. 2.
Effect of galactose on the morphology of P. marneffei cells. (A) Growth in yeast nitrogen base broth at 37°C with 1% glucose; (B) growth in the same medium with 1% galactose. Magnification, ×240.
DISCUSSION
P. marneffei is a well-recognized opportunistic dimorphic fungus among HIV-infected patients in Southeast Asia. A recent report described the use of chromosomal DNA restriction fragment length polymorphism and randomly amplified polymorphic DNA patterns for typing of P. marneffei (10). A small number of physiological and biochemical properties of P. marneffei have been described in mycology texts (20), and a recent article reported the cell-associated and extracellular enzymatic activities of 10 strains of P. marneffei (23). The present article presents a major study on the biochemical properties of a large number of P. marneffei isolates. Although less discriminative than molecular typing methods, biotyping has the virtue of being easily performed. Most laboratories carrying out routine mycological culture and identification should be able to perform biotyping. This is especially important in developing countries (including most of Southeast Asia, where penicilliosis marneffei is endemic), where resources for sophisticated procedures such as molecular techniques are often limited. Although the ethnicities of the 32 patients from whom the isolates were recovered are known, the geographical origins of the isolates are difficult if not impossible to determine. Despite the fact that all patients are residents of Hong Kong, they might have acquired the infection from neighboring countries, since the infection is likely to have a long latency in the hosts prior to clinical manifestation of the disease.
Some of the published biochemical reactions (20) are confirmed by our study. Growth inhibition by cycloheximide and utilization of glucose or maltose as the sole carbon source are in accordance with previous studies. Sucrose is considered to be weakly utilized, but we failed to demonstrate its assimilation by any of the 32 strains. Xylose assimilation is observed in the majority (84.4%) of the isolates. In contrast, previous studies showed that lactose and mannitol are utilized but that nitrate is not utilized by P. marneffei; however, in the present series, none of the isolates assimilated lactose and mannitol, and 71.9% of the isolates were able to assimilate potassium nitrate.
In a study involving 10 P. marneffei isolates, Youngchim et al. demonstrated that β-galactosidase activity was present in whole-cell mycelia for 50% of the strains and in cell-free supernatants for 10% of the strains (23). Using ONPG to detect the presence of β-galactosidase, 37.5% of our isolates were found to possess this enzyme. Although β-galactosidase is one of the important enzymes involved in the metabolism of lactose, none of our isolates produced acid or assimilated lactose. This could be due to the fact that the transport of lactose in yeasts requires a membrane transport protein permease, and hence the presence of β-galactosidase alone is not sufficient for the fungus to utilize lactose.
The following reactions have not been previously documented for P. marneffei: presence of urease, lack of a positive fermentation reaction but presence of acid production from glucose, and utilization of a number of carbohydrates, such as cellobiose, glycerol, raffinose, melibiose, inositol, rhamnose, arabinose, and ribose. The stability and consistency of the biochemical properties have been confirmed by repeating the tests on three separate occasions.
The most significant finding of this study is the inhibition of growth by galactose. This phenomenon occurs only when galactose is the sole carbon source, and the inhibitory effect is negated by the presence of other sugars such as glucose (data not shown). This is manifested by the presence of a zone of inhibition of growth around the galactose disk (Fig. 1). Since there is fungal growth at the periphery of the zone, galactose assimilation is considered to be positive, signifying that the inhibition disappears at a lower concentration of galactose. As seen in Fig. 1, fungal colonies in the agar medium become progressively smaller toward the perimeter of the zone of inhibition. Taken together with the fact that the size of the zone of inhibition increases with the concentration of galactose solution used in preparing the disk, this phenomenon appears to be a concentration-dependent inhibition of growth. It is unlikely to be due to a high osmolarity of the sugar being used, since such inhibition of growth is not observed with other sugars, such as glucose, used in the same concentration. The MACgal represents the maximal concentration of galactose at which assimilation is possible (and hence the inhibitory effect disappears), and this ranges from 0.015 to 0.25%. Galactose also produces morphological changes microscopically, as shown in Fig. 2. When grown in 1% glucose broth at 37°C, P. marneffei produces elongated hyphae after 72 h (Fig. 2A), which is also the morphology seen in laboratory cultures in liquid media. This is in contrast to the oval cells with central septation seen in histological sections of tissues in natural infections. However, in the presence of galactose, the fungal cells lose their elongated morphology and a large number of cells are rounded up (Fig. 2B). The ballooning induced by galactose is not a lethal process, though, as shown by the failure to take up the vital stain trypan blue and the ability to revert to the normal morphology and macroscopic growth after addition of another carbon source such as glucose (data not shown).
Galactose is normally present in humans at a concentration in serum of less than 5 mg/dl (equivalent to <0.28 mmol/liter or <0.5%) (1). In vivo, galactose is unlikely to have any inhibitory effect on the growth of P. marneffei, either because of the low concentration of galactose in blood and body fluids or because of the presence of other carbon sources for metabolism, predominantly glucose, which negates the inhibitory effects of galactose. The latter possibility is suggested by the fact that concomitant or subsequent addition of glucose to the galactose assimilation medium allows growth or regrowth of the fungus, respectively. In addition, as shown in Table 1, four of the isolates produced acid in the galactose fermentation test. The most likely explanation for the lack of inhibition by galactose in this test is that the base medium used contained peptone, in contrast to the yeast nitrogen base medium used in assimilation tests. Hence, the presence of an alternative carbon source apparently bypasses the pathways accounting for galactose inhibition.
The metabolic pathways of galactose are remarkably similar among eukaryotes (18). Galactose is converted primarily to galactose-1-phosphate, which is further metabolized to UDP galactose before being used for the synthesis of glycoproteins and glycolipids. Galactose is also oxidized to galactitol and galactonate. The key enzymes involved in galactose metabolism, such as galactokinase, galactose-1-phosphate uridyltransferase, UDP galactose 4-epimerase, and UDP galactose pyrophosphorylase, can be found in mammalian cells, yeasts, and a number of bacteria. In tissue cultures with a high concentration of galactose and in clinical cases of galactosemia, cellular toxicity has been demonstrated. Various metabolites of galactose have been implicated as the cause of toxicity, including galactitol and galactose-1-phosphate. Toxicity due to galactose metabolites has also been described for bacteria, including mutants of Escherichia coli and Salmonella, with an important example of the latter being the galE mutant Ty21a, the strain used as the oral Salmonella enterica serovar Typhi vaccine. The mechanism of galactose toxicity in Ty21a is attributable to the synthesis of incomplete cell wall lipopolysaccharide and accumulation of galactose-1-phosphate and UDP galactose. The metabolic pathways of galactose in P. marneffei have not been described, although we conjecture that pathways similar to those of yeasts such as Saccharomyces cerevisiae could be present. Growth inhibition of P. marneffei in the presence of a high galactose concentration could be due to accumulation of one or more of the galactose metabolites, such as galactose-1-phosphate. On the other hand, the ballooning effect of galactose on the cellular morphology could be related to the synthesis of abnormal cell wall components, since galactomannan has been shown to be present in the cell wall of P. marneffei. The elucidation of the galactose metabolic pathways in P. marneffei could allow the construction of avirulent mutants to be used as vaccines for protection of susceptible subjects. Alternatively, this phenomenon could be used for prophylaxis. Xylitol, for example, has been shown to possess inhibitory effects on the adhesion of otopathogenic bacteria (e.g., Streptococcus pneumoniae) to epithelial cells, and xylitol has been shown to have a preventive effect against acute otitis media in a double-blind randomized trial (19). On the other hand, once the biochemical basis for growth inhibition by galactose is elucidated, a P. marneffei-specific antifungal target could theoretically be identified, which would be highly desirable in the management of patients with penicilliosis marneffei.
P. marneffei is a well known opportunistic pathogen among HIV-infected patients. However, when isolates were grouped according to the HIV status of the patients from whom they were isolated, no significant association was found between HIV status and physiological biotypes. This could mean that most of the P. marneffei strains are potentially pathogenic or that the biochemical profiles tested failed to reveal an existing relationship.
Further studies of the biochemical properties of P. marneffei could yield important information on the pathogenesis of the organism. This is the first study that documents the presence of the enzyme urease in all strains of P. marneffei tested. Although the in vivo biological function of the enzyme for the fungus is unknown, this could be further investigated in terms of its role in virulence, since urease has been found to be a virulence factor in pathogens such as Helicobacter pylori and Cryptococcus neoformans. Different biotypes of the same species of microorganism are, in some instances, associated with significant differences in their pathogenicity. Examples include Cryptococcus neoformans var. neoformans and Cryptococcus neoformans var. gattii, of which the former biotype is associated with the majority of human infections. Another example is Burkholderia pseudomallei, in which the avirulent biotype (recently classified as a new species, B. thailandensis) is associated with the ability to assimilate arabinose. Unfortunately, owing to the difficulty in recovering the fungus from the environment, a direct correlation between physiological behavior and virulence is not presently possible.
ACKNOWLEDGMENTS
We thank W. H. Seto of Queen Mary Hospital, Dominic N. C. Tsang of Queen Elizabeth Hospital, T. K. Ng of Princess Margaret Hospital, Raymond W. H. Yung of Pamela Youde Eastern Hospital, and W. K. To of Yan Chai Hospital for supplying the strains used in this study. We are also most grateful to all of the staff of the clinical microbiology laboratories of the hospitals involved and especially to W. T. Hui for his excellent technical support and expertise.
This study was supported by grants from the Research Grants Council (grant number HKU498/96 M) and the Committee on Research and Conference Grants, Hong Kong.
REFERENCES
- 1.Burtis C A, Ashwood E R. Tietz textbook of clinical chemistry. W. B. Philadelphia, Pa: Saunders; 1999. [Google Scholar]
- 2.Cao L, Chan C M, Lee C, Wong S S, Yuen K Y. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect Immun. 1998;66:966–973. doi: 10.1128/iai.66.3.966-973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao L, Chan K M, Chen D, Vanittanakom N, Lee C, Chan C M, Sirisanthana T, Tsang D N, Yuen K Y. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J Clin Microbiol. 1999;37:981–986. doi: 10.1128/jcm.37.4.981-986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chim C S, Fong C Y, Ma S K, Wong S S, Yuen K Y. Reactive hemophagocytic syndrome associated with Penicillium marneffei infection. Am J Med. 1998;104:196–197. doi: 10.1016/s0002-9343(97)00253-2. [DOI] [PubMed] [Google Scholar]
- 5.Chongtrakool P, Chaiyaroj S C, Vithayasai V, Trawatcharegon S, Teanpaisan R, Kalnawakul S, Sirisinha S. Immunoreactivity of a 38-kilodalton Penicillium marneffei antigen with human immunodeficiency virus-positive sera. J Clin Microbiol. 1997;35:2220–2223. doi: 10.1128/jcm.35.9.2220-2223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collee J G, Duguid J P, Fraser A G, Marmion B P. Mackie & McCartney practical medical microbiology. Edinburgh, United Kingdom: Churchill Livingstone; 1989. [Google Scholar]
- 7.Desakorn V, Smith M D, Walsh A L, Simpson A J H, Sahassananda D, Rajanuwong A, Wuthiekanun V, Howe P, Angus B J, Suntharasamai P, White N J. Diagnosis of Penicillium marneffei infection by quantitation of urinary antigen by using an enzyme immunoassay. J Clin Microbiol. 1999;37:117–121. doi: 10.1128/jcm.37.1.117-121.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiSalvo A F, Fickling A M, Ajello L. Infection caused by Penicillium marneffei: description of first natural infection in man. Am J Clin Pathol. 1973;60:259–263. doi: 10.1093/ajcp/60.2.259. [DOI] [PubMed] [Google Scholar]
- 9.Duong T A. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh P R, Teng L J, Hung C C, Hsu J H, Yang P C, Ho S W, Luh K T. Molecular evidence for strain dissemination of Penicillium marneffei: an emerging pathogen in Taiwan. J Infect Dis. 2000;181:1706–1712. doi: 10.1086/315432. [DOI] [PubMed] [Google Scholar]
- 11.Jeavons L, Hamilton A J, Vanittanakom N, Ungpakorn R, Evans E G V, Sirisanthana T, Hay R J. Identification and purification of specific Penicillium marneffei antigens and their recognition by human immune sera. J Clin Microbiol. 1998;36:949–954. doi: 10.1128/jcm.36.4.949-954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman L, Standard P G, Jalbert M, Kantipong P, Limpakarnjanarat K, Mastro T D. Diagnostic antigenemia tests for penicilliosis marneffei. J Clin Microbiol. 1996;34:2503–2505. doi: 10.1128/jcm.34.10.2503-2505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwan E Y, Lau Y L, Yuen K Y, Jones B M, Low L C. Penicillium marneffei infection in a non-HIV infected child. J Paediatr Child Health. 1997;33:267–271. doi: 10.1111/j.1440-1754.1997.tb01596.x. [DOI] [PubMed] [Google Scholar]
- 14.Kwon-Chung K J, Bennett J E. Medical mycology. Malvern, Pa: Lea & Febiger; 1992. [Google Scholar]
- 15.Larone D H. Medically important fungi: a guide to identification. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 16.Lo C Y, Chan D T, Yuen K Y, Li F K, Cheng K P. Penicillium marneffei infection in a patient with SLE. Lupus. 1995;4:229–231. doi: 10.1177/096120339500400313. [DOI] [PubMed] [Google Scholar]
- 17.McGinnis M R. Laboratory handbook of medical mycology. New York, N.Y: Academic Press; 1980. [Google Scholar]
- 18.Segal S, Berry G T. Disorders of galactose metabolism. In: Scriver C R, Beaudet A L, Sly W S, Valle D, editors. The metabolic and molecular basis of inherited disease. 7th ed. New York, N.Y: McGraw Hill, Inc.; 1995. pp. 967–1000. [Google Scholar]
- 19.Uhari M, Kontiokari T, Koskela M, Niemela M. Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. Br Med J. 1996;313:1180–1184. doi: 10.1136/bmj.313.7066.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viviani A M, Tortorano A M. Penicillium marneffei. In: Ajello L, Hay R J, editors. Topley and Wilson's microbiology and microbial infections. 9th ed. 4. Medical mycology. London, United Kingdom: Arnold; 1998. pp. 409–419. [Google Scholar]
- 21.Warren N G, Hazen K C. Candida, Cryptococcus, and other yeasts of medical importance. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: ASM Press; 1999. pp. 1184–1199. [Google Scholar]
- 22.Wong S S Y, Cao L, Yuen K Y. Management of penicilliosis marneffei. JAMA Southeast Asia. 1998;14:7–9. [Google Scholar]
- 23.Youngchim S, Vanittanakom N, Hamilton A J. Analysis of the enzymatic activity of mycelial and yeast phases of Penicillium marneffei. Med Mycol. 1999;37:445–450. doi: 10.1046/j.1365-280x.1999.00235.x. [DOI] [PubMed] [Google Scholar]
- 24.Yuen K Y, Wong S S, Tsang D N, Chau P Y. Serodiagnosis of Penicillium marneffei infection. Lancet. 1994;344:444–445. doi: 10.1016/s0140-6736(94)91771-x. [DOI] [PubMed] [Google Scholar]