Significance
We develop temperature sensors on the basis of charges accumulated at the electrolyte/dielectric interface and dielectric/electrode interface. The accumulated charges make the temperature sensors self-powered, which simplifies circuit design and enables portable sensing. The sensors are stretchable, but deformation does not affect temperature sensing. The sensors have high sensitivity and fast response. They can be made small and transparent. Such temperature sensors open new possibilities to create human–machine interfaces and soft robots in healthcare and engineering.
Keywords: stretchable electronics, ionotronics, nonfaradaic interface, self-powered thermometer, hydrogel
Abstract
Sensing technology is under intense development to enable the Internet of everything and everyone in new and useful ways. Here we demonstrate a method of stretchable and self-powered temperature sensing. The basic sensing element consists of three layers: an electrolyte, a dielectric, and an electrode. The electrolyte/dielectric interface accumulates ions, and the dielectric/electrode interface accumulates electrons (in either excess or deficiency). The ions and electrons at the two interfaces are usually not charge-neutral, and this charge imbalance sets up an ionic cloud in the electrolyte. The design functions as a charged temperature-sensitive capacitor. When temperature changes, the ionic cloud changes thickness, and the electrode changes open-circuit voltage. We demonstrate high sensitivity (∼1 mV/K) and fast response (∼10 ms). Such temperature sensors can be made small, stable, and transparent. Depending on the arrangement of the electrolyte, dielectric, and electrode, we develop four designs for the temperature sensor. In addition, the temperature sensor has good linearity in the range of tens of Kelvin. We further show that the temperature sensors can be integrated into stretchable electronics and soft robots.
The art of thermometry, dating back at least to the time of Galileo, relies on a universal principle: Temperature affects all physical properties, and their measurements can function as thermometers (1, 2). The choice of physical property to monitor results from compromises among accuracy, speed, cost, and convenience (2, 3). Resistive temperature sensors and thermocouples are widely used, but they are rigid and do not conform to curved and dynamic surfaces. An infrared thermometer is noncontact but is unsuitable when the thermometer needs to be integrated within a structure. Stretchable temperature sensors have been developed using metallic serpentines and elastomeric substrates (4–8). They can conform to curved and dynamic surfaces and have high accuracy, but require external power sources for sensing.
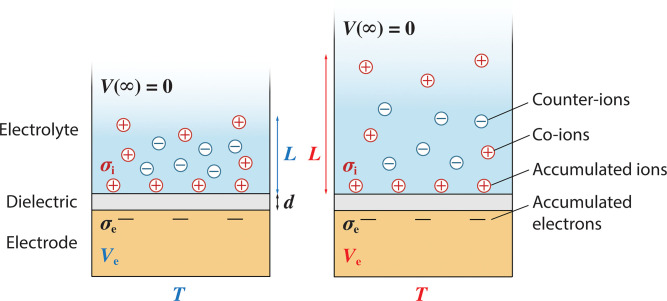
Here we demonstrate a type of self-powered, stretchable thermometry, which we call ionotronic thermometry (Fig. 1). A specific design involves three layers: an electrolyte, a dielectric, and an electrode. The dielectric blocks charge transfer between the electrolyte and the electrode. Ions move in the electrolyte and accumulate at the electrolyte/dielectric interface, whereas electrons move in the electrode and accumulate at the dielectric/electrode interface. Let be the accumulated ionic charge per unit area of the electrolyte/dielectric interface and be the accumulated electronic charge per unit area of the dielectric/electrode interface. In general, the ions and the electrons accumulated at the two interfaces are not charge-neutral, . The concentration of mobile ions in the interior of the electrolyte is low, and the concentration of mobile electrons in the interior of the electrode is high. Consequently, the interior of the electrolyte forms an ionic cloud and has an electric field, but the interior of the electrode is neutral and has no electric field. According to Gauss’s law, the electric field in the dielectric is uniform, given by , where is the permittivity of the dielectric. According to the linearized Poisson–Boltzmann equation, the electric field in the electrolyte is given by , where is the permittivity of the electrolyte, x is the distance from the electrolyte/dielectric interface, and L is the Debye length (9):
| [1] |
Fig. 1.
Ionotronic thermometry. A sensing element has three layers: an electrolyte, a dielectric, and an electrode. At the electrolyte/dielectric interface, the accumulated ionic charge per unit area is . At the dielectric/electrode interface, the accumulated electronic charge per unit area is . So long as the net interfacial charge , an ionic cloud forms in the electrolyte—that is, the concentrations of co-ions and counter-ions deviate from their bulk values. As the temperature T changes, the ionic cloud changes thickness L, which changes the voltage of the electrode, . The voltage in the interior of the electrolyte, far away from the interfaces, is set to be zero, .
Here kT is the thermal energy (Boltzmann’s constant times temperature), is the Avogadro constant, is the concentration of positive ions, e is the elementary charge, is the valence of the positive ions, and is the valence of the negative ions. The thickness of the electrolyte is taken to be much larger than the Debye length. The voltage in the interior of the electrolyte, far away from the interfaces, is set to be zero, . The voltage of the electrode is
| [2] |
where d is the thickness of the dielectric (SI Appendix, Supplementary Note 1). The first term on the right-hand side is the voltage built up by the competition between charge neutrality and entropy. When temperature is high, the ionic cloud expands, and the Debye length is large. When temperature is low, the ionic cloud collapses, and the Debye length is small. The second term on the right-hand side is the voltage drop across the dielectric. In an open circuit, changes negligibly. To estimate the order of magnitude of the sensitivity, , we further assume that , , , and are insensitive to temperature, but the Debye length L is temperature-sensitive. Consequently, the voltage Ve is sensitive to temperature. The sensitivity is given by
| [3] |
We are interested in measuring changes of temperature in the range of about tens of Kelvin. This range is much smaller than the room temperature, ∼300 K, so that the change in voltage is nearly linear in the change in temperature—that is, the sensitivity is insensitive to the absolute temperature. Taking representative values, , (10), , , and , as well as constants , , and , we estimate the sensitivity . The combination of the dielectric and the ionic cloud functions as a precharged, temperature-sensitive capacitor. In an open circuit, the charge stored by the capacitor is fixed. When the temperature changes, the Debye length changes, which is equivalent to a change in the thickness of the capacitor, so that the voltage across the capacitor changes. The dielectric layer should be made thin compared to the Debye length, so that the effective capacitance is dominated by the ionic cloud in the electrolyte.
In recent years, a versatile sensing platform has been under development using both mobile electrons and mobile ions. These devices often use stretchable, transparent, ionic conductors, such as hydrogels (11–14), ionogels (15, 16), and ionoelastomers (17). Ionic conductors have been used to construct temperature sensors using effects other than interfacial voltage. For example, resistance of an ionic conductor is temperature-sensitive and has been used to construct temperature sensors (18–21). Such a resistive temperature sensor, however, is not self-powered. Here we use this platform to demonstrate the method of temperature sensing.
Results and Discussion
Many designs of ionotronic thermometry are possible, depending on the arrangement of the electrode (electronic conductor), dielectric, and electrolyte (ionic conductor). For brevity, we denote the three substances by e, d, and i, and call the design in Fig. 1 an e-d-i junction. If an electrolyte/electrode interface is ideally polarizable and does not undergo electrochemical reaction, such an interface can enable ionotronic thermometry without the dielectric layer. The temperature sensitivity of electrolyte/electrode interfaces has been used to demonstrate energy harvesting (22, 23), but has so far not been developed for temperature sensing.
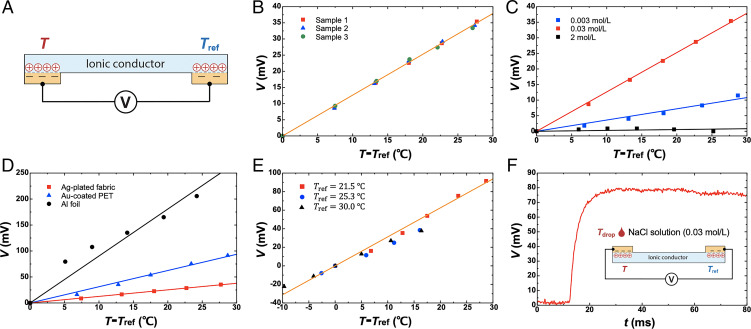
We begin our experiment with a design using two e-i junctions (Fig. 2A). Each junction involves an electronic conductor and an ionic conductor in contact. This design uses no dielectric between the electrolyte and the electrode, and it is assumed that no electrochemical reaction occurs at the electrolyte/electrode interface. One junction serves as a sensing end, and the other junction serves as a reference end. The two ends are connected by an ionic conductor. When temperature at the sensing end differs from that at the reference end, a change in the open-circuit voltage is generated between the two ends and is measured by a voltmeter. The change in voltage depends on the type and concentration of ions, as well as the type of electronic conductor at the sensing end.
Fig. 2.
Ionotronic thermometry using two e-i junctions. (A) Unless otherwise specified, the ionic conductor is polyacrylamide hydrogel containing 0.03 mol/L NaCl, the electronic conductor at the reference end is gold-coated PET, and the temperature at the reference end is at room temperature. (B) The voltage is measured as a function of the temperature difference between the two junctions. A silver-plated fabric is used as the sensing-end electrode. Sensitivity depends on (C) the concentration of ions and (D) the type of electronic conductor at the sensing end. (E) Sensitivity is independent of the temperature at the reference end, Tref. Electrodes at both ends are gold-coated PET. (F) Two e-i junctions connected by an ionic conductor and the voltage recorded as a function of time. (Inset) When a drop of 0.03 mol/L NaCl solution of temperature Tdrop is released onto one junction, the voltage between the two junctions changes. Electrodes at both ends are gold. Each solid line is a linear fit to the data.
To characterize the sensor, we change the temperature at the sensing end, T, keep the temperature at the reference end, Tref, at room temperature, and measure the change in the open-circuit voltage between the two electrodes. We measure the temperatures at the two ends separately using two commercial thermometers (SI Appendix, Fig. S1). For the sensing-end electrode, we choose a commercial silver-plated fabric as a stretchable electronic conductor (MedTex 130) and characterize its electrical and mechanical behavior (SI Appendix, Fig. S2). For the reference-end electrode, we sputter gold (30-nm thickness) on a polyester (PET) film (100-μm thickness; McMaster Carr). For the ionic conductor, we fabricate a stretchable and transparent polyacrylamide (PAAm) hydrogel containing NaCl. The hydrogel is sealed by a stretchable and transparent elastomer (VHB 4905; 3M).
We test three samples in which the concentration of ions in the hydrogel is 0.03 mol/L and measure voltage V as a function of temperature difference between the two ends, T − Tref (Fig. 2B). Within a temperature range of tens of degrees, the voltage is linear in temperature and the measured sensitivity is .
Any type of ions can be used as long as the net interfacial charge and the ions cause no electrochemical reactions. Both the net interfacial charge and the Debye length are affected by the type of ions and ionic concentration. We use three concentrations of NaCl to demonstrate the principle. The sensitivity depends on the concentration of ions and reaches a large value at an intermediate concentration of ions (Fig. 2C). When the electrolyte has a high concentration of ions, the Debye length is small, and the capacitance of the junction is weakly sensitive to temperature. When the electrolyte has a low concentration of ions, the Debye length is large, and the capacitance of the junction is temperature-sensitive. In general, varies with concentration, and the concentration dependence of sensitivity is difficult to predict quantitatively. The effect of ion type and ionic concentration on sensitivity deserves a future study.
In principle, any electronic conductor can be used, as long as it does not undergo any electrochemical reaction with the ionic conductor. We use three electronic conductors at the sensing end and measure the corresponding voltage–temperature relations (Fig. 2D). In all three cases, gold-coated PET is used as the electronic conductor at the reference end. The sensitivity depends on the type of electronic conductor at the sensing end, which affects the net interfacial charge. The sensitivity does not depend on the type of electronic conductor at the reference end, so long as the temperature at the reference end is unchanged during sensing.
This design involves two e-i junctions, one at the sensing end and the other at the reference end. Both e-i junctions are, of course, temperature-sensitive. The two ends are connected with an ionic conductor. As the length of ionic conductor (centimeter) is much larger than the Debye length (nanometer), the two e-i junctions can be considered thermally separated during the time of temperature sensing. The temperature change is much smaller than the absolute temperature. Our model above predicts that the measured open-circuit voltage is linear in the difference in the temperatures of the two ends, T − Tref. To confirm this prediction, we place the two ends on two hot plates and measure the V–T relation at various reference temperatures, Tref. We use gold-coated PET as the electronic conductors at both ends. Our data show that the sensitivity is independent of temperature at the reference end (Fig. 2E).
In the design of e-i junctions connected by an ionic conductor, gold (100-μm thickness) is used as the electronic conductor at both ends (Fig. 2F). The sensor is unsealed and placed in open air at room temperature (∼25 °C in this case). Upon releasing a drop of NaCl solution (0.03 mol/L, ∼50 °C) onto one junction, we record a voltage spike within a short time of ∼10 ms. The fast thermal response originates from the small thickness of the electronic conductor. In practice, however, the sensor needs to be sealed. As a result, the thermal response will be affected by the material and thickness of the seal (SI Appendix, Fig. S3).
As shown by Eq. 3, the sensitivity is independent of the area of the e-i junction. However, to ensure accurate measurement, the sensing area should be sufficiently large to produce a measurable electric current through the voltmeter. Thus, the minimum area is determined by the resolution of the voltmeter, as well as the sensitivity (SI Appendix, Supplementary Note 2).
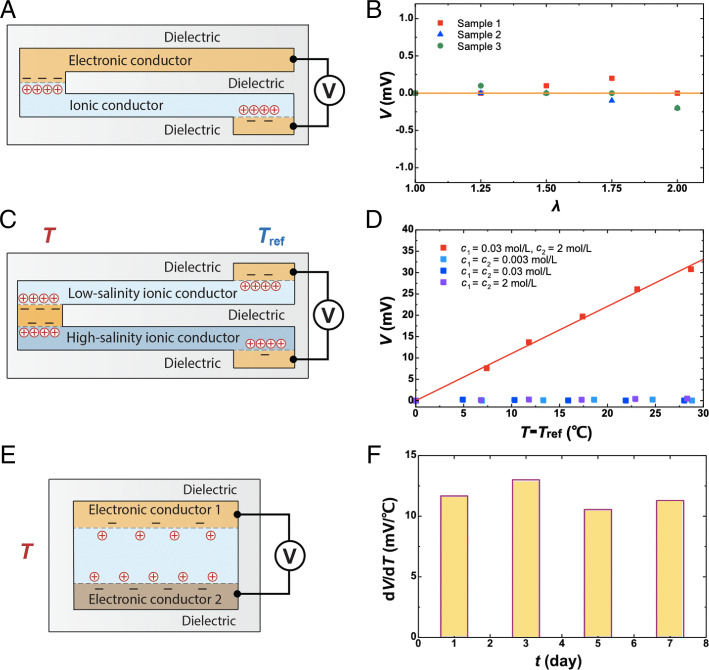
Next, we describe several designs of ionotronic thermometry and their properties. Unless otherwise stated, the ionic conductor is PAAm hydrogel containing 0.03 mol/L NaCl, and the electronic conductor is gold-coated PET. In a second design, an e-i junction is formed by a stretchable electronic conductor (silver-plated fabric) and a stretchable ionic conductor (hydrogel) (Fig. 3A and SI Appendix, Fig. S4A). A dielectric elastomer (VHB 4905; 3M) is used as a seal and an electrical insulator. This design functions as a stretchable thermometer. Its voltage is sensitive to temperature but insensitive to stretch (Fig. 3B). At the interface between stretchable electronic and ionic conductors, e-i junction forms between the individual silver particles and the hydrogel. Because the silver particles are rigid, the electrolyte/electrode interface is unaffected by stretch.
Fig. 3.
Ionotronic thermometry of several designs. (A) An electronic conductor and an ionic conductor are both elastomeric and stretchable. (B) At room temperature, stretch of the conductors negligibly affects voltage. A solid line is drawn to guide the eye. (C) The two ionic conductors have different ionic concentrations. (D) This difference causes a change in voltage when the temperature changes. The solid line is a linear fit to the data. (E) An ionic conductor is sandwiched between two dissimilar electronic conductors. The voltage between the two electronic conductors changes with temperature. (F) The sensitivity remains nearly constant over days.
In a third design, a small piece of electronic conductor connects to two stripes of ionic conductors (Fig. 3C). This design functions as a stretchable and transparent thermometer (SI Appendix, Fig. S4B). It is difficult to identify a stretchable and transparent electronic conductor, but the hydrogel is a stretchable and transparent ionic conductor. The two ionic conductors have different concentrations of ions, so that the two e-i junctions at the sensing end are dissimilar. Outside the sensing end, a transparent dielectric elastomer (VHB) separates the two stripes of ionic conductors. The three-layer structure forms an ionic cable, which serves as a stretchable and transparent interconnect between the sensing end and the reference end. In this design, the voltmeter measures the voltage between the two ionic conductors at the reference end. The two ionic conductors are in contact with two electronic conductors, forming two additional e-i junctions. Consequently, the four e-i junctions are in series. When temperature of the sensing end changes and temperature of the reference end is unchanged, a voltage will be generated. The electronic conductors at the sensing and reference ends are the silver-plated fabric and the gold-coated PET, respectively. The two ionic conductors are PAAm hydrogels containing 0.03 mol/L and 2 mol/L NaCl. When the concentrations of ions in the two ionic conductors are the same, the voltages across the four e-i junctions will cancel out and not respond to temperature (Fig. 3D). Therefore, the asymmetry is crucial for the ionotronic thermometry. Additional analyses and characterizations of this design can be found in SI Appendix, Supplementary Note 3 and Fig. S5.
In a fourth design, an ionic conductor is sandwiched between two different electronic conductors (an aluminum foil and a gold-coated PET) (Fig. 3E). The two interfaces form dissimilar junctions. This design does not require a reference end. When temperature changes over the sandwich, a voltage is generated (Fig. 3F). The sensitivity is stable over days, which indicates negligibly slow electrochemical reactions, if any. This stability is understood as follows. We have used VHB to seal the device, so that the hydrogel loses negligible amount of water during the experiment. Gold is inert in contact with the hydrogel. Aluminum itself is an active metal but forms a thin and stable oxide layer, which serves as a dielectric. The aluminum oxide retards further electrochemical reaction. When the hydrogel loses water to open air, the concentration of ions changes, which affects the sensitivity of a temperature sensor. As we have demonstrated, a sensor sealed by VHB is stable within a week (Fig. 3F). Other elastomers, such as butyl rubbers, have lower water permeability than VHB (24) and are expected to make a sensor stable over a longer period of time. In addition, ionic liquid has negligible vapor pressure and is expected to make long-lasting sensors.
We further note that the sensitivity of this design, , is about one order of magnitude higher than other designs without using aluminum. The aluminum oxide has hydroxyl groups covalently anchored on the surface (25, 26), which possibly increases the ionic charges on the hydrogel/dielectric interface, . Furthermore, dissimilar electronic conductors can anchor ionic charges of opposite signs. The design in Fig. 3E involves two thermal sensing junctions in series. When the temperature changes, the changes in the voltages of the two junctions can be either additive or subtractive, depending on the two electronic conductors (SI Appendix, Supplementary Note 4 and Fig. S6). These considerations suggest that chemistry greatly affects sensitivity, which we will pursue in a subsequent study.
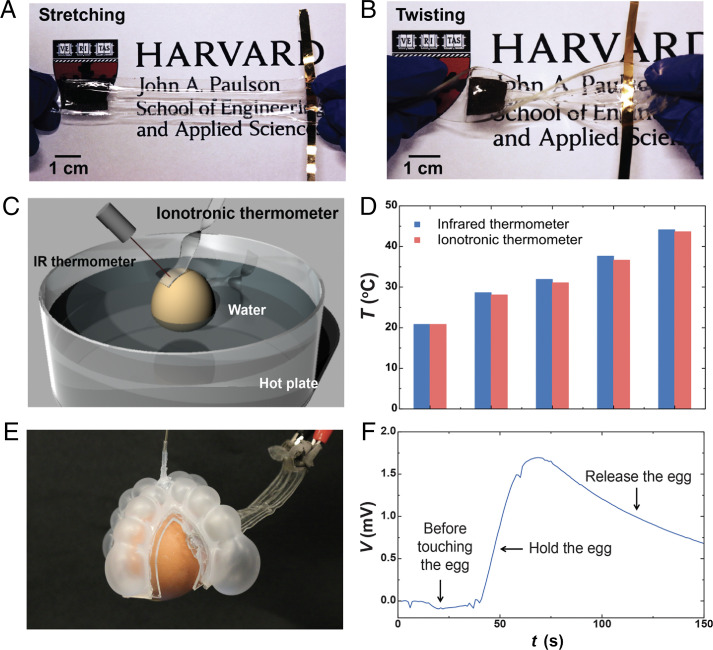
The ionotronic thermometer can be made stretchable, flexible, and transparent (Fig. 4 A and B). The stretch insensitivity makes the ionotronic thermometer convenient for sensing temperature for curved surfaces. We use a stretchable and transparent ionotronic thermometer (Fig. 3C) to measure the surface temperature of an egg partly immersed in hot water (Fig. 4C) and compare the ionotronic thermometer with a commercial infrared thermometer (ennoLogic eT650D) (Fig. 4D). Further, we fabricate a pneumatic soft gripper and glue an ionotronic thermometer, in which the silver-plated fabric is used as the electronic conductor at both ends, to one of the four arms. The gripper is used to grip an egg and to measure the temperature (Fig. 4E). The voltage changes negligibly before the gripper touches the egg, rises sharply when the gripper touches the egg, and decreases after the gripper releases the egg (Fig. 4F and Movie S1). This experiment corroborates that the voltage is sensitive to temperature, but not to stretch.
Fig. 4.
Stretchable and transparent ionotronic thermometers for curved surfaces. Stretchable and transparent ionotronic thermometer in a stretched state (A) and a twisted state (B). (C) A schematic of experimental setup to monitor temperature on a curved surface. An egg is partly immersed in hot water. (D) The ionotronic thermometer is compared with a commercial infrared thermometer. (E) The stretchable and transparent ionotronic thermometer integrated with a pneumatic soft gripper to measure surface temperature of a hot egg. (F) The voltage changes negligibly before the gripper touches the egg, rises sharply when the gripper holds the egg, and decreases after the gripper releases the egg.
We compare a thermocouple and an ionotronic thermometer. The former relies on an irreversible thermodynamic phenomenon and the latter on an equilibrium thermodynamic phenomenon. The junction of two metals in the thermocouple conducts electricity, but the junction of a metal and a hydrogel behaves like a capacitor. A change in temperature causes a steady current in a thermocouple but not in an ionotronic thermometer (SI Appendix, Fig. S7). A thermocouple and an ionotronic thermometer are both self-powered: A change in temperature causes a change in voltage (SI Appendix, Fig. S8). A thermocouple relies on a bulk phenomenon and requires temperature difference between two ends. By contrast, an ionotronic thermometer relies on an interfacial phenomenon and can be used in a design without temperature difference (e.g., the e-i-e junction; Fig. 3E). A thermocouple maintains a constant voltage when the temperature difference between two ends is fixed. On the other hand, an e-i junction behaves like a capacitor and discharging the capacitor results in a voltage drop over time. In an open circuit, taking the internal resistance of voltmeter and the capacitance of e-i junction , we estimate the decay time . That is to say, the voltage will drop when the sensor is constantly connected to a voltmeter for hours. It is confirmed by experimental measurement (SI Appendix, Fig. S8D). Furthermore, thermocouples are rigid and opaque, but ionotronic thermometers can be stretchable and transparent. We have demonstrated several designs of ionotronic thermometry. In principle, still other designs are possible (SI Appendix, Fig. S9). The detection range of temperature of the ionotronic thermometer is mainly dependent on the ionic conductor. In this work, hydrogel is used as the ionic conductor, so the detection range is within 0 to 100 °C. The detection range can be expanded by using ionogel or organogel as ionic conductors. The ionotronic thermometer can be miniaturized by making arrays of electrolyte/dielectric/electrode interfaces. To make the ionotronic thermometer fully stretchable and transparent, stretchable and transparent electronic conductors are needed, such as those described in refs. 27 and 28. We also study the electrical response of the ionic cable and show that the electrical response time of the stretchable interconnect is much shorter than the thermal response time (SI Appendix, Supplementary Note 5).
Conclusion
In summary, we have shown that junctions of ionic conductor, electronic conductor, and dielectric can be used to measure temperature. The ionotronic thermometry self-energizes, is highly sensitive, responds rapidly, has a small size, and can be made stretchable and transparent. Such soft, stretchable, transparent thermometry has potential for broad applications in healthcare, engineering, and entertainment.
Materials and Methods
All chemicals were purchased, including acrylamide (AAm; Sigma-Aldrich, A8887), N,N’-methylenebis(acrylamide) (MBAA; Sigma-Aldrich, M7279), N,N,N′,N′-tetramethylenediamine (TEMED; Sigma-Aldrich, T7024), ammonium persulfate (APS; Sigma-Aldrich, 248614), and sodium chloride (NaCl; Sigma-Aldrich, 746398).
Fabrication of NaCl-Containing PAAm Hydrogel.
The precursor of a PAAm hydrogel was an aqueous solution of AAm (1.916 mol/L) and NaCl (0.003 mol/L, 0.03 mol/L, or 2 mol/L). Also added were MBAA (0.058% the weight of AAm) as the cross-linker, TEMED (0.05% the weight of AAm) as the catalyst, and APS (0.17% the weight of AAm) as the initiator. The solutions were poured into a mold. A glass sheet was used to seal the mold. The solutions were cured in air overnight. The voltage across the electrode/dielectric/electrolyte interfaces is measured using a voltmeter of much larger internal resistance (∼1 GΩ) than that of the ionic conductor. Consequently, the resistance of the ionic conductor negligibly affects the measured voltage. The PAAm hydrogels were made in a 3-mm-thick acrylic mold or 0.5-mm-thick rubber mold. The length of the PAAm hydrogel was ∼10 cm in the designs shown in Figs. 2A and 3 A and C. The length of the PAAm hydrogels used in Fig. 2E was 25 cm. The thickness of the PAAm hydrogel was 3 mm in the designs shown in Figs. 2A and 3 A and E and was 0.5 mm in the design shown in Fig. 3C. A representative value of the resistivity of hydrogel is 1 Ωm. A hydrogel of length 10 cm, thickness 1 mm, and width 1 cm has a resistance of 104 Ω.
Fabrication of Pneumatic Soft Gripper (SI Appendix, Fig. S10).
We cut rectangular acrylic parts using a laser cutter (Universal) and used the cyanoacrylate to assemble them to form a cruciform mold and glued it on an acrylic substrate. We cut an acrylic layer with grooves using a laser cutter and aligned the acrylic layer in the middle of the mold. Then we prepared the precursor of Ecoflex by mixing the base and the curing agent of Ecoflex 0030 (Smooth-On) at weight ratio 1:1 and filled the mold with the Ecoflex precursor. The Ecoflex was cured at ambient condition for 3 h. After curing, the Ecoflex was peeled out of the mold and the acrylic layer was removed from the mold. Next, we prepared the precursor of polydimethylsiloxane (PDMS) by mixing the base and the curing agent of Sylgard 184 (Dow Corning) at weight ratio 10:1 and poured it into the cruciform mold. The PDMS was cured at 65 in an oven (Jeio Tech Co., Inc. OF11E) for 12 h. The PDMS was then peeled out of the mold and used to seal the fabricated Ecoflex using silicone glue, forming a gripper. A needle was used to poke a hole into the gripper from the Ecoflex side and a rubber tube was inserted into the hole. The other end of the tube was connected to a syringe, which was used to pump air into the gripper.
Characterization of Ionotronic Thermometer.
For the designs shown in Figs. 2A and 3 A and C, the sensing end was placed on a hot plate (VWR hotplate/stirrer), while the reference end was placed in open air and connected to a voltmeter (Fluke 8846A) (SI Appendix, Fig. S1). A commercial resistive temperature sensor with a sampling unit (Supco LT2 Logit Temperature Data Logger) was placed on top of the sensing end to monitor temperature. When temperature reached a stable level, the corresponding voltage was recorded by the voltmeter. For the design shown in Fig. 3E, the sandwich was placed on the hot plate. Temperature was recorded by the commercial resistive temperature sensor placed on top of the sandwich.
Measurement of Thermal Response Time.
A hot drop of NaCl solution (0.03 mol/L) was released onto an e-i junction between a gold and a hydrogel placed on an acrylic substrate. Before use, the gold was first soaked in NaOH solution (2 mol/L) for 10 min, flushed by deionized water, and then placed in a Falcon tube filled with ethanol for ultrasonic cleaning for 5 min. After that, the gold was flushed by deionized water and dried by blowing air. Voltage as a function of time was recorded by a sampling module (TiePie HANDYSCOPE HS6D). The sampling rate was 10 kHz.
Scanning Electron Microscope (SEM) Observation.
The silver-plated fabric was observed in the SEM (Zeiss Ultra 55).
X-Ray Photoelectron Spectroscopy (XPS) Observation.
The XPS analysis was conducted using K-Alpha XPS system (Thermal Scientific).
Mechanical Test of the Fabric.
A silver-plated fabric sample with dimensions of 85 mm × 10 mm × 0.45 mm was loaded uniaxially to rupture using a tensile tester (Instron 5966, 500 N load cell). The loading rate was 20 mm/min.
Electrical Test of the Fabric.
A silver-plated fabric sample with dimensions of 20 mm × 13 mm × 0.45 mm was loaded cyclically to a maximum stretch λmax = 2 using the tensile tester. Resistances at stretches of 1, 1.25, 1.5, 1.75, and 2 were recorded during each loading-unloading cycle by a multimeter (Fluke 8846A).
Supplementary Material
Acknowledgments
The work was supported by NSF through the Harvard University Materials Research Science and Engineering Center DMR2011754. K.J. was supported by the National Natural Science Foundation of China (NSFC) (11872292). S.Z. was supported by NSFC (12002259) and Post-doctoral Innovation Talent Program of China (BX20200271). H.J.K. and R.C.H. were supported by the NSF through Grants DMR-1609972 and DMR-2105825. We thank Dr. Xi Yao for assistance in SEM and XPS observations.
Footnotes
Reviewers: M.D., North Carolina State University; and M.M., University of Minnesota.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2117962119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Pearce J. M., A brief history of the clinical thermometer. QJM 95, 251–252 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Childs P. R. N., Greenwood J. R., Long C. A., Review of temperature measurement. Rev. Sci. Instrum. 71, 2959–2978 (2000). [Google Scholar]
- 3.Li Q., Zhang L.-N., Tao X.-M., Ding X., Review of flexible temperature sensing networks for wearable physiological monitoring. Adv. Healthc. Mater. 6, 1601371 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Webb R. C., et al. , Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 12, 938–944 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim D.-H., et al. , Epidermal electronics. Science 333, 838–843 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Yokota T., et al. , Ultraflexible, large-area, physiological temperature sensors for multipoint measurements. Proc. Natl. Acad. Sci. U.S.A. 112, 14533–14538 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trung T. Q., Ramasundaram S., Hwang B.-U., Lee N.-E., An all-elastomeric transparent and stretchable temperature sensor for body-attachable wearable electronics. Adv. Mater. 28, 502–509 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Yu C., Wang Z., Yu H., Jiang H., A stretchable temperature sensor based on elastically buckled thin film devices on elastomeric substrates. Appl. Phys. Lett. 95, 141912 (2009). [Google Scholar]
- 9.Bard A. J., Faulkner L. R., Leddy J., Zoski C. G., Electrochemical Methods: Fundamentals and Applications (Wiley, New York, 1980), vol. 2. [Google Scholar]
- 10.Grahame D. C., The electrical double layer and the theory of electrocapillarity. Chem. Rev. 41, 441–501 (1947). [DOI] [PubMed] [Google Scholar]
- 11.Keplinger C., et al. , Stretchable, transparent, ionic conductors. Science 341, 984–987 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Jia K., Li X., Wang Y., Electrochemical breakdown in hydrogel ionotronic devices. Soft Matter 17, 834–839 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Xie S., Bai Y., Suo Z., Jia K., Transduction between magnets and ions. Mater. Horiz. 8, 1959–1965 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Zhu Z., Park H. S., McAlpine M. C., 3D printed deformable sensors. Sci. Adv. 6, eaba5575 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen B., et al. , Highly stretchable and transparent ionogels as nonvolatile conductors for dielectric elastomer transducers. ACS Appl. Mater. Interfaces 6, 7840–7845 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Vallem V., Sargolzaeiaval Y., Ozturk M., Lai Y.-C., Dickey M. D., Energy harvesting and storage with soft and stretchable materials. Adv. Mater. 33, e2004832 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Kim H. J., Chen B., Suo Z., Hayward R. C., Ionoelastomer junctions between polymer networks of fixed anions and cations. Science 367, 773–776 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Yamada S., Toshiyoshi H., Temperature sensor with a water-dissolvable ionic gel for ionic skin. ACS Appl. Mater. Interfaces 12, 36449–36457 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Yang B., Yuan W., Highly stretchable and transparent double-network hydrogel ionic conductors as flexible thermal–mechanical dual sensors and electroluminescent devices. ACS Appl. Mater. Interfaces 11, 16765–16775 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Wu Z., et al. , Ultrasensitive, stretchable, and fast-response temperature sensors based on hydrogel films for wearable applications. ACS Appl. Mater. Interfaces 13, 21854–21864 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Wu J., et al. , Highly stretchable and transparent thermistor based on self-healing double network hydrogel. ACS Appl. Mater. Interfaces 10, 19097–19105 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Janssen M., Härtel A., van Roij R., Boosting capacitive blue-energy and desalination devices with waste heat. Phys. Rev. Lett. 113, 268501 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Härtel A., Janssen M., Weingarth D., Presser V., van Roij R., Heat-to-current conversion of low-grade heat from a thermocapacitive cycle by supercapacitors. Energy Environ. Sci. 8, 2396–2401 (2015). [Google Scholar]
- 24.Le Floch P., et al. , Wearable and washable conductors for active textiles. ACS Appl. Mater. Interfaces 9, 25542–25552 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Peri J. B., Hannan R. B., Surface hydroxyl groups on γ-alumina. J. Phys. Chem. 64, 1526–1530 (1960). [Google Scholar]
- 26.Tamura H., Mita K., Tanaka A., Ito M., Mechanism of hydroxylation of metal oxide surfaces. J. Colloid Interface Sci. 243, 202–207 (2001). [Google Scholar]
- 27.McCoul D., Hu W., Gao M., Mehta V., Pei Q., Recent advances in stretchable and transparent electronic materials. Adv. Electron. Mater. 2, 1500407 (2016). [Google Scholar]
- 28.Guo C. F., et al. , Fatigue-free, superstretchable, transparent, and biocompatible metal electrodes. Proc. Natl. Acad. Sci. U.S.A. 112, 12332–12337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.