Significance
Interactions between the nervous and immune systems control the generation and maintenance of inflammatory pain. However, the immune cells and mediators controlling this response remain poorly characterized. We identified the cytokines CCL22 and CCL17 as secreted mediators that act directly on sensory neurons to mediate postoperative pain via their shared receptor, CCR4. We also show that skin-resident dendritic cells are key contributors to the inflammatory pain response. Blocking the interaction between these dendritic cell–derived ligands and their receptor can abrogate the pain response, highlighting CCR4 antagonists as potentially effective therapies for postoperative pain. Our findings identify functions for these tissue-resident myeloid cells and uncover mechanisms underlying pain pathophysiology.
Keywords: pain, inflammation, cytokines, myeloid cells, electrophysiology
Abstract
Inflammatory pain, such as hypersensitivity resulting from surgical tissue injury, occurs as a result of interactions between the immune and nervous systems with the orchestrated recruitment and activation of tissue-resident and circulating immune cells to the site of injury. Our previous studies identified a central role for Ly6Clow myeloid cells in the pathogenesis of postoperative pain. We now show that the chemokines CCL17 and CCL22, with their cognate receptor CCR4, are key mediators of this response. Both chemokines are up-regulated early after tissue injury by skin-resident dendritic and Langerhans cells to act on peripheral sensory neurons that express CCR4. CCL22, and to a lesser extent CCL17, elicit acute mechanical and thermal hypersensitivity when administered subcutaneously; this response abrogated by pharmacological blockade or genetic silencing of CCR4. Electrophysiological assessment of dissociated sensory neurons from naïve and postoperative mice showed that CCL22 was able to directly activate neurons and enhance their excitability after injury. These responses were blocked using C 021 and small interfering RNA (siRNA)-targeting CCR4. Finally, our data show that acute postoperative pain is significantly reduced in mice lacking CCR4, wild-type animals treated with CCR4 antagonist/siRNA, as well as transgenic mice depleted of dendritic cells. Together, these results suggest an essential role for the peripheral CCL17/22:CCR4 axis in the genesis of inflammatory pain via direct communication between skin-resident dendritic cells and sensory neurons, opening therapeutic avenues for its control.
Surgical procedures are common, and the resulting pain is often insufficiently managed and can become chronic if poorly controlled (1, 2). The most-prominent cause of tissue injury–induced pain is inflammation, through its complex and dynamic interactions with the nervous system. Mechanisms governing neuro–immune interactions in pain states are not completely understood, with most studies focusing on the role of circulating immune cells (e.g., neutrophils, monocytes, and T cells) (3–6) or central nervous system (CNS)-resident glia (7, 8). Immune cells contribute to inflammatory pain by secreting mediators that act directly on their cognate receptors expressed by peripheral sensory neurons, modulating neuronal excitation and pain transduction (9–12).
We previously identified a subset of nonneutrophil myeloid (CD11b+Ly6Clow) cells that control mechanical hypersensitivity in both complete Freund’s adjuvant (CFA) and postoperative models of inflammatory pain (13). Our finding that CCR2+ circulating monocytes (which express high levels of Ly6C) do not contribute to this inflammatory pain response suggests that a population of tissue-resident myeloid immune cells likely mediate these effects. Tissue-resident myeloid cells in the skin include macrophages, dendritic (DC), Langerhans (LC), and mast cells. During peripheral inflammation, these resident immune cells are activated as a first line of defense, including enhanced nociception as a protective mechanism, and as such may contribute contributing to mechanical and thermal hypersensitivity (14). Thus, the exact immune cells and mediators that mediate these effects to develop and maintain pain behaviors remain unknown.
Previously, we have shown that expression of CCL17 and CCL22 are significantly up-regulated in a subset of immune cells that control pain outcomes during inflammation (13). These chemokines are expressed by LCs and dermal DCs (DDCs), which are present in the skin and act as a first line of defense in case of injury or infection (15). Work from others suggests that sensory neurons can stimulate skin-resident DC activation and inflammation (16, 17). Furthermore, it has been shown that CCL17 mediates pain and inflammation in a murine model of arthritis; inhibition of the chemokine ameliorated both pain and disease outcomes (18–20). Currently, there is no evidence of a role for CCL22 in the pain response. However, it has been demonstrated that serum levels of this chemokine were associated with pain in patients with osteoarthritis (21). Moreover, it has been suggested that CCR4, the cognate receptor for CCL17 and CCL22, is associated with neuropathic pain responses (22).
Using a robust and validated model of surgical wound, we provide evidence that CCL17 and CCL22, produced by DCs infiltrating the lesion, act through CCR4 on peripheral sensory neurons to elicit the development of inflammatory pain. We report a direct activation of nociceptors by CCL22 and show that loss of DCs abrogates the postoperative pain response. These findings may provide therapeutic avenues for the treatment of postoperative pain.
Results
CCL17 and CCL22 Are Up-Regulated after Incisional Wound by Skin-Resident DCs.
Transcriptomic analysis was carried out on three subsets of nonneutrophil myeloid cells (CD11b+Ly6G−) sorted using flow cytometry from the hindpaw 24 h after incisional wound (13), using expression of the cell-surface antigen Ly6C (i.e., Ly6Chi/med/lo), to obtain mechanistic insight into their functional states. The Ly6Clo population, which we previously showed mediate to mechanical hypersensitivity in inflammatory pain, displayed high expression of CCL17 and CCL22 relative to Ly6Chi and Ly6Cmed cells (Fig. 1A). We subsequently evaluated the temporal expression of these two chemokines in the inflamed hindpaw following postoperative wound. CCL17 expression increased at 3 and 6 h postinjury, returning to basal levels by 24 h (Fig. 1B). CCL22 expression appears to occur in a later phase, with an increase 1 h after injury and again at 6, 24, and 72 h (Fig. 1C). Collectively, these results confirm that CCL17 and CCL22 are expressed in the skin after injury.
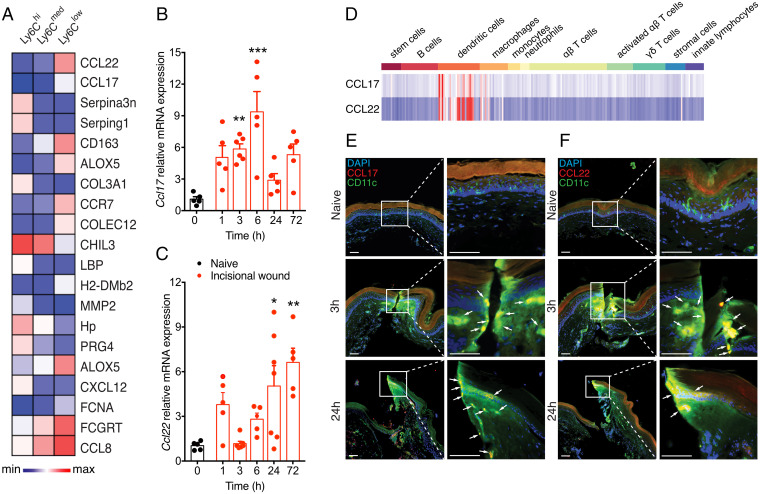
Fig. 1.
CCL17 and CCL22 are up-regulated by skin-resident DCs after incisional wound. (A) Expression patterns of the 20 most-variable immune/inflammatory genes from all expressed transcripts across CD11b+Ly6G− myeloid cells were ranked by coefficient of variability. (B and C) CCL17 mRNA showed elevated expression in the hindpaw early (3 to 6 h) after injury, while CCL22 mRNA was highest at later stages (24 to 72 h) (n = 5/timepoint). (D) Transcriptomic analysis of immune cells shows preferential expression of both CCL17 and CCL22 among DC subsets. (E and F) Immunohistochemical analysis revealed colocalization of both CCL17 and CCL22 in CD11c+ DCs at the site of injury at both 3 and 24 h after incision; Insets show high-magnification images at the site of incision. Arrows point to immunopositive cells. (Scale bar, 50 μm.) Data are represented as mean ± SEM; all data were analyzed using one-way ANOVA with post hoc Bonferroni test (*P < 0.05, **P < 0.01, and ***P < 0.001 versus sham controls). Data are representative of at least n = 2 experiments.
CCL17 and CCL22 are expressed transiently in peripheral immune cells, such as myeloid DCs, endothelial cells, keratinocytes, and fibroblasts (23). LCs produce high levels of CCL22 and CCL17 constitutively during culture, even without exogenous stimuli (15). Thus, we hypothesized that myeloid DCs, which include LCs and DDCs, would be the major cell types involved in CCL17 and CCL22 production. Indeed, analysis of naïve and activated immune cell subsets from the Immunological Genome database (24) found these two chemokines to be preferentially expressed in DCs (Fig. 1D). We then used immunofluorescence to assess coexpression of CD11c (a pan-DC marker) and CD207 (a LC-specific marker) with CCL17 and CCL22 in the skin after incisional wound. We observed that CD11c+ cells expressed both CCL17 (Fig. 1E and SI Appendix, Fig. S1A) and CCL22 (Fig. 1F and SI Appendix, Fig. S1B) after incisional wound. Similarly, we detected CCL17 and CCL22 expression on LCs after injury (SI Appendix, Fig. S2 A and B). Interestingly, the expression of both chemokines was restricted to the injury site, with little expression detected at sites away from the incision (SI Appendix, Fig. S3 A and B). Thus, the results obtained corroborate our initial assumption that DCs (including LCs) are the main source of these chemokines after injury.
CCL17 and CCL22 Elicit Acute Pain Behaviors.
Since an up-regulation of CCL17 and CCL22 was observed after injury, we evaluated whether these chemokines may be involved in the development of pain. Standard pain behavior assays were used to assess changes in mechanical, thermal heat, and cold responses before and after mice received an intraplantar injection of either recombinant CCL17 or CCL22 or a combination of both. While neither chemokine was able to elicit a nociceptive response in the first 5 min after injection (e.g., licking/biting or flinching of the affected hindpaw), both caused significant reductions in mechanical threshold as early as 1 h after treatment, reaching peak hypersensitivity at 3 h postinjection; thresholds returned to baseline levels by 24 h (Fig. 2 A and D). Injection with CCL17 resulted in a less-intense mechanical pain response than with CCL22, with thresholds returning to basal levels 6 h after injection at all three doses used (Fig. 2A). Meanwhile, injection with 300 nM CCL22 resulted in a more-robust response, with significantly reduced mechanical thresholds observed up to 6 h after treatment (Fig. 2D). Significant differences in mechanical sensitivity were not observed between the 30- and 300-nM injections of either CCL17 or CCL22. The combination of both CCL17 and CCL22 did not show an additive effect in mechanical pain responses (SI Appendix, Fig. S4A).
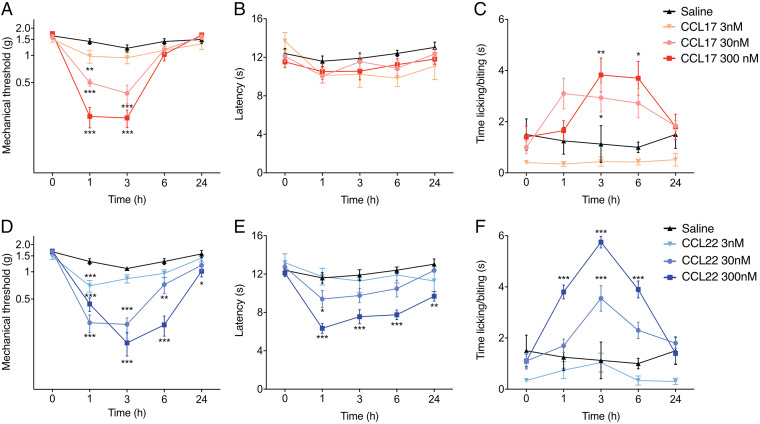
Fig. 2.
CCL17 and CCL22 elicit pain behaviors in naïve animals. Male mice received an intraplantar injection of either CCL17 (n = 15/group) (A–C) or CCL22 (n = 10/group) (D–F) at 3, 30, and 300 nM; saline (n = 10/group) was used as a control. (A and D) Changes in mechanical hypersensitivity, measured using von Frey monofilaments, show that both CCL17 and CCL22 cause dose-dependent reductions in threshold over the first 6 h after injection. (B and E) Thermal heat hypersensitivity shows that CCL17 did not alter thermal thresholds at any dose examined, while CCL22 exhibited a dose-dependent response. (C and F) CCL17 primarily evoked a cold response at 3 and 6 h, while CCL22 exhibited a dose-dependent response early after injection. Data are represented as mean ± SEM; all data were analyzed using two-way ANOVA with post hoc Bonferroni test (*P < 0.05, **P < 0.01, and ***P < 0.001 versus saline-treated controls). Data are representative of at least n = 2 experiments.
Changes in heat hypersensitivity were only observed following treatment with CCL22 and only with the highest dose used (300 nM; Fig. 2E). Lastly, CCL17 and CCL22 were both capable of inducing dose-dependent cold hypersensitivity, though only at 30- and 300-nM concentrations (Fig. 2 C and F). Combinatorial treatment with these chemokines resulted in the development of cold hypersensitivity from 1 to 6 h after intraplantar injection (SI Appendix, Fig. S4C). Altogether, these results indicate that CCL17 and CCL22 induce dose-dependent pain behavior responses in naïve mice.
Pharmacological Blockade of CCR4 Attenuates Pain Elicited by CCL17 and CCL22.
Preliminary dose–response experiments using the CCR4 antagonist C 021 identified 2 mM as the minimum effective dose required to block the pain response elicited by intraplantar injection of CCL22 (SI Appendix, Fig. S5). Localized injection of 2 mM C 021 to the hindpaw did not alter mechanical or thermal hypersensitivity, nor were any signs of inflammation observed. Thus, 2 mM C 021 was coinjected with either CCL17 or CCL22 and pain responses observed over the first 6 h. Blocking the interaction of CCL17 with CCR4 resulted in significantly reduced mechanical hypersensitivity at 1 and 3 h postinjection (Fig. 3A). As expected, there was no effect on heat hypersensitivity induced by CCL17 (Fig. 3B), while the response to cold was significantly reduced at 3 h after injection (Fig. 3C). Coinjection of the CCR4 antagonist with CCL22 resulted in significantly reduced mechanical and heat thresholds (Fig. 3 D and E), while cold responses were significantly reduced at 3 h (Fig. 3F). These results suggest that the mechanical response is dependent on the interaction of these chemokines with their receptor CCR4.
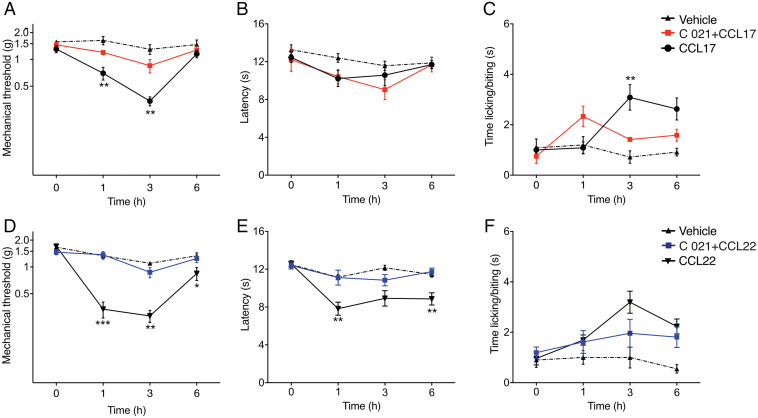
Fig. 3.
CCL17 and CCL22 elicit pain through CCR4. Male mice received intraplantar injections of either 30 nM CCL17 (n = 5 to 10/group) or CCL22 (n = 10/group) with or without 2 mM of CCR4 antagonist C 021. Injection of the two chemokines caused hypersensitivity, as previously observed. Addition of the C 021 antagonist was able to significantly reduce mechanical (A and D), thermal heat (B and E), and cold (C and F) hypersensitivity. Data are represented as mean ± SEM; all data were analyzed using two-way ANOVA with post hoc Bonferroni test (*P < 0.05, **P < 0.01, and ***P < 0.001 versus saline-treated controls). Data are representative of at least n = 2 experiments.
CCR4 Regulates Inflammatory Hypersensitivity but Not via Immune Cells.
Whether CCR4-signaling contributes to the inflammatory pain response associated with tissue injury remained unknown. We therefore assessed whether loss of CCR4 expression could affect the inflammatory pain response associated with tissue lesion, using the incisional wound model of postoperative pain. Naïve mice received an intraplantar injection of either C 021 or saline 1 h prior to incisional wound and were assessed for changes in mechanical and thermal hypersensitivity at 30 min prior to incisional wound and after surgery. No changes in mechanical thresholds were observed in animals treated with C 021 30 min after injection. Treatment with C 021 resulted in a significant attenuation of mechanical but not thermal hypersensitivity at 1 h after surgery (Fig. 4 A and B). We then assessed whether blocking CCR4 activation therapeutically, during the peak inflammatory response, influences pain outcomes. Mice received intraplantar injections of C 021 at 24 h after surgery, and pain outcomes were assessed 1 h after treatment. C 021 significantly increased both mechanical thresholds and latency to a heat stimulus (Fig. 4 C and D). Incisional wound was also carried out in CCR4−/− and littermate controls, and a significant attenuation of mechanical pain hypersensitivity was observed from 24 to 72 h after injury in CCR4−/− mice (SI Appendix, Fig. S6A). Absence of the CCR4 receptor did not alter thermal hypersensitivity (SI Appendix, Fig. S6B). Given that incisional wound results in tissue damage, we asked whether central/peripheral alterations may contribute to these pain responses. We found a lack of change in expression of genes associated with injury-induced neuronal regeneration (ATF3) or glial activation (P2X 2/4/7, CCL2, and GFAP) centrally, with only a transient increase in ATF3 in the dorsal root ganglia (DRG), suggesting that neither microglia nor astroglia are activated after incisional wound (SI Appendix, Fig. S7).
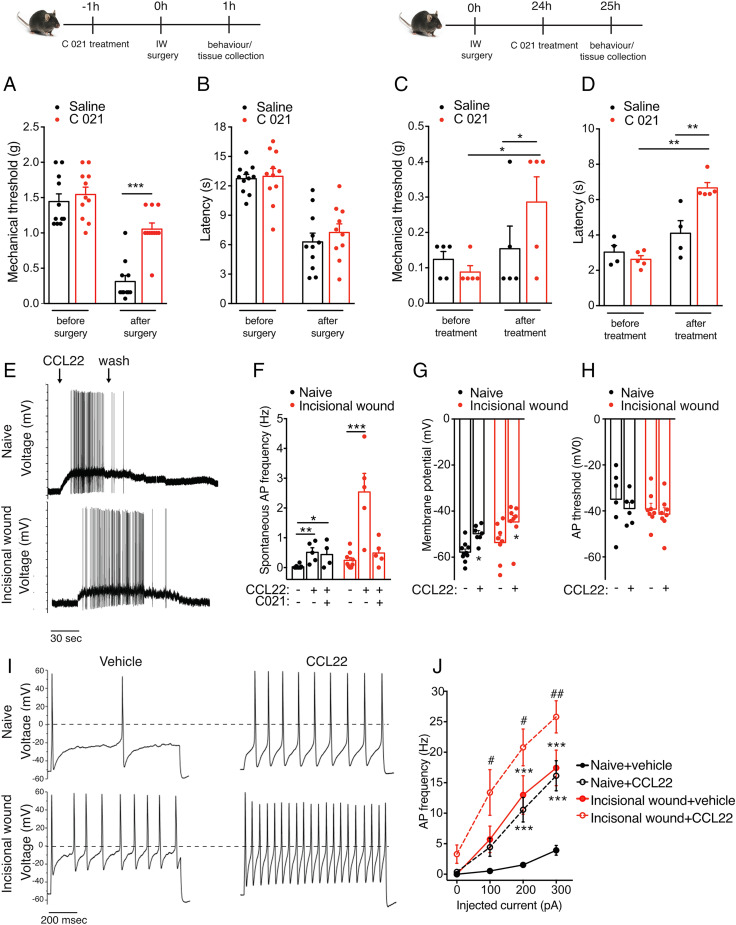
Fig. 4.
CCR4 activation modulates postoperative pain and results in altered membrane properties and increased AP firing. (A and C) Mechanical hypersensitivity was significantly reduced in animals in which CCR4 is inhibited 1 h before incision (n = 10/group). (B and D) Thermal hypersensitivity was significantly reduced in animals treated with C 021 24 h after incision. (E and F) CCL22 induces an increase in AP generation in cultured DRG neurons isolated from both naïve and 24 h postoperative mice, and this effect is partially reversed by pretreatment with CCR4 antagonist (1 µM) (naïve: 0.025 ± 0.01 Hz, 0.51 ± 0.15 Hz after CCL22, and 0.44 ± 0.22 Hz after CCR4 antagonist and CCL22; n = 5, 5, and 4 neurons recorded; incisional wound: 0.24 ± 0.09 Hz, 2.54 ± 0.6 Hz after CCL22, and 0.049 ± 0.18 Hz after CCR4 antagonist and CCL22; n = 9, 5, and 5 neurons recorded). (G and H) The increase in AP frequency is associated with a membrane depolarization (from −57.82 ± 1.35 mV to −49.8 ± 1.38 mV, from −53.63 ± 2.99 mV to −44.68 ± 2.79 mV, and from −55.2 ± 1.72 mV to −46.4 ± 2.1 mV after CCL22 treatment in naïve and incisional wound neurons, respectively; n = 10, 7, 8, 8, 8, and 8). However, the AP threshold remained unchanged. (I and J) CCL22 treatment increased the evoked neuronal activity as shown by these representative data and mean values on the right (n = 7 and 5 for naïve and incisional wound, respectively). The AP discharge was evoked by a 200-pA current injection (1 s) in control (Upper) and 24 h after surgery (Lower). n = 4 mice/group in E–J. Data are represented as mean ± SEM; all data were analyzed using two-way ANOVA with post hoc Bonferroni test (*P < 0.05, **P < 0.01, and ***P < 0.001; # <0.05 and ## <0.01). Data are representative of at least n = 2 experiments.
We next sought to identify the cells expressing CCR4 that might regulate these nociceptive responses. We used the Immunological Genome Project dataset (24) to evaluate its expression across immune cell subsets, which are known to play an important role in postoperative pain. We found that the receptor is expressed primarily among αβ T cells and tissue-resident γδ T cells (SI Appendix, Fig. S8A). Our previous work has shown that αβ T cells do not contribute to inflammatory pain in postoperative wounds and are recruited to the skin late after tissue injury (13). While our previous work found that γδ T cells do not contribute to incisional pain (25), we sought to identify whether this was true for the direct effects of CCL17 and CCL22. We carried out intraplantar injection with a combination of CCL17 and CCL22 in TCRδ−/− mice, using their respective littermate controls. Significant changes in mechanical (SI Appendix, Fig. S8B), thermal (SI Appendix, Fig. S8C), or cold (SI Appendix, Fig. S8D) hypersensitivity were not observed between the groups. Collectively, these results suggest that CCR4 activation contributes to inflammatory pain and its expression in T cells does not contribute to this response but instead uses another cellular mechanism to modulate hypersensitivity.
Activation of CCR4 Alters Sensory Neuron Excitability.
We then investigated whether CCR4 was expressed by peripheral sensory neurons. Transcriptomic analysis of four peripheral neuron subsets (26) shows that CCR4 is weakly expressed among sensory neurons in naïve mice (SI Appendix, Fig. S9A); this expression does not change after incisional wound (SI Appendix, Fig. S9B). Moreover, CCR4 messenger RNA (mRNA) expression is lost in the DRGs of CCR4−/− mice, relative to wild-type littermates (SI Appendix, Fig. S9C). Because CCL22 is known to have higher affinity for CCR4 (27, 28) and was able to elicit a more-robust response in our mice, we decided to focus our efforts on this interaction. Genetic deletion of CCR4 (using CCR4−/− mice) resulted in attenuated thermal (heat and cold) and mechanical responses to CCL22 injection compared to littermate controls (SI Appendix, Fig. S10).
Acutely dissociated small-diameter DRG neurons from both naïve and postoperative mice generated action potentials (AP) in response to CCL22, but the AP firing was greater after incisional wound (Fig. 4E). This effect in response to the chemokine was reversible and blocked by a pretreatment with CCR4 antagonist (1 µM) (Fig. 4F). The increase in AP frequency was associated with a slight rise in membrane depolarization (Fig. 4G) but the AP threshold remained unchanged (Fig. 4H). To assess whether CCR4-signaling was affecting voltage-gated ionic conductance, we measured the neuronal activity evoked by injected current. Neurons from naïve mice exhibited an increase in the frequency of AP upon exposure to CCL22 compared to vehicle (Fig. 4I). Interestingly, neurons from the incisional wound model showed a more-pronounced activity, to a level similar to naïve neurons exposed to CCL22. Additional application of CCL22 on neurons postoperatively caused an increased activity to a slighter higher level (Fig. 4 I and J).
Inhibition of Neuronal CCR4 Attenuates Pain Responses.
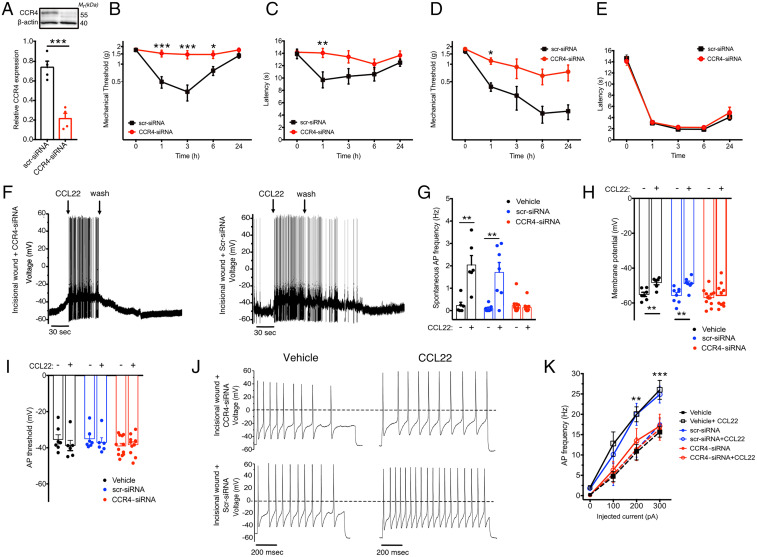
To further confirm that CCR4 is expressed in neurons, we specifically targeted CCR4 expression in sensory neurons with small interfering RNA (siRNA) using a well-described method (29). CCR4-siRNA treatment resulted in significantly reduced CCR4 levels in the DRGs of mice relative to those injected with control scrambled siRNA (scr-siRNA; Fig. 5A). We then performed intraplantar injections of CCL22 and observed attenuated thermal and mechanical hypersensitivity in CCR4-siRNA–treated mice (Fig. 5 B and C). Similarly, CCR4 knockdown significantly reduced mechanical hypersensitivity induced by tissue lesion but did not alter heat hypersensitivity responses (Fig. 5 D and E).
Fig. 5.
CCR4 knockdown results in attenuated pain responses and reduced neuronal activity. (A) CCR4 expression in DRGs (normalized to β-actin) of mice treated with scr-siRNA or CCR4-siRNA (n = 5/group). (B–E) Mechanical and thermal pain hypersensitivity were assessed in mice treated with CCR4- or scr-siRNA. Mechanical (B) and thermal (C) hypersensitivity are reduced in mice treated with intraplantar CCL22 (n = X-X per group). Following surgical injury, mechanical (D) but not thermal (heat) hypersensitivity (E) is reduced in mice treated with CCR4-siRNA relative to scr-siRNA (n = X-X per group). (F and G) CCL22 induces an increase in AP generation in cultured DRG neurons isolated from both naïve and 24 h postoperative mice, and this effect is completely reversed by pretreatment with CCR4-siRNA but not with scr-siRNA (vehicle baseline: 0.23 ± 0.14 Hz, 2.03 ± 1.03 Hz after CCL22; scr-siRNA: 0.1 ± 0.14 Hz, 1.71 ± 1.16 after CCL22; CCR4-siRNA: 0.21 ± 0.36, 0.13 ± 0.07 after CCL22; n = 6, 6, 7, 7, 10, and 10 neurons recorded, respectively). (H) The increase in AP frequency is associated with a membrane depolarization, blunted by CCR4-siRNA treatment (from −55.07 ± 1.21 mV to −48.28 ± 1.29 mV, from −55.65 ± 1.55 mV to −48.79 ± 1.37 mV, and from −56.92 ± 1.34 mV to −55.64 ± 1.55 mV after CCL22 treatment in vehicle, scr-siRNA, and CCR4-siRNA incisional wound neurons, respectively; n = 7, 6, 8, 7, 12, and 10). (I) However, the AP threshold remained unchanged. (J and K) CCL22 treatment increased the evoked neuronal activity as shown by these representative data and mean values on the right (n = 10 and 7 for CCR4-siRNA and scr-siRNA, respectively). The AP discharge was evoked by a 200-pA current injection (1 s) in CCR4-siRNA (Upper) and scr-siRNA (Lower). n = 4 mice/group for vehicle and n = 6 mice/group for CCR4 and scr-siRNA in F–K. Data are represented as mean ± SEM; all data were analyzed using two-way ANOVA with post hoc Bonferroni test (*P < 0.05, **P < 0.01, and ***P < 0.001; # <0.05 and ## <0.01). Data are representative of at least n = 2 experiments.
We next tested the effectiveness of CCL22 in altering neuronal excitability following siRNA-mediated knockdown of CCR4. Small-diameter DRG neurons were collected from mice intrathecally injected with CCR4- or scr-siRNA, or vehicle control, 24 h after injury. APs generated in response to CCL22 were significantly reduced in neurons from mice treated with CCR4-siRNA (Fig. 5 F and G). The increased AP frequency was associated with a slight rise in membrane depolarization for control neurons (scr-siRNA and vehicle), while CCR4-siRNA neurons were not affected (Fig. 5H); the AP threshold remained unchanged for all groups investigated (Fig. 5I). To assess whether CCR4-signaling affected voltage-gated ion conductance, we measured the neuronal activity evoked by injected current. Neurons from scr-siRNA mice showed a more-pronounced activity after exposure to CCL22, while neurons from CCR4-siRNA–treated mice were unaffected (Fig. 5 J and K). Collectively, these results suggest that peripheral sensory neuron hyperexcitability is at least partially dependent on CCR4 signaling, leading to an alteration in voltage-gated ion channel function in DRG neurons that contributes to hypersensitivity induced by activation of CCR4.
Skin-Resident DCs Control Postoperative Pain.
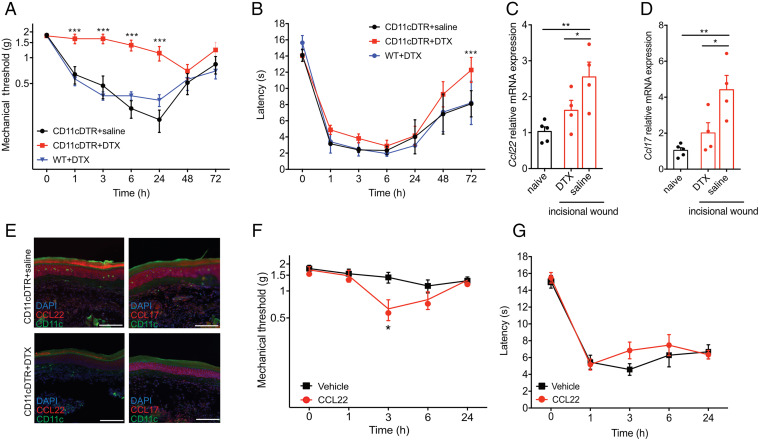
Finally, we investigated the cellular source contributing to postoperative pain via CCR4. We focused our efforts on CD11c+ DCs that we have shown express CCL17 and CCL22 after tissue injury (Fig. 1). These cells are locally depleted in CD11c-DTR mice (30) following intraperitoneal injection of diphtheria toxin (DTX) (SI Appendix, Fig. S11 A and B). Significantly reduced mechanical hypersensitivity was observed in mice depleted of DCs, compared to transgenic CD11c-DTR mice treated with saline or wild-type littermates treated with DTX (Fig. 6A). Heat hypersensitivity, however, was not affected by loss of these cells (Fig. 6B). Moreover, DC-depleted mice showed a reduction of both CCL22 and CCL17 mRNA and protein expression at 24 h after incision (Fig. 6 C–E and SI Appendix, Fig. 12 A–D), further suggesting that CD11c+ DCs are the main producers of these chemokines after tissue injury. Finally, we carried out intraplantar injection of CCL22 in CD11c-DTR mice and identified a partial recovery of the hypersensitivity phenotype (Fig. 6 F and G). These results collectively suggest that CCL17 and CCL22 are produced by CD11c+ DCs after tissue injury and contribute to the generation of inflammatory pain responses.
Fig. 6.
DCs regulate the development of mechanical hypersensitivity in postoperative pain. Incisional wound was carried out in CD11c-DTR+/− mice treated with injections of DTX to deplete DCs or saline as control; wild-type littermates were treated with DTX as an additional control (n = 10/group). (A) Mechanical hypersensitivity was abrogated in mice lacking DCs. (B) Thermal hypersensitivity was not altered between the groups. (C and D) CCL22 (C) and CCL17 (D) mRNA expression 24 h after incisional wound are significantly increased in the hindpaw of CD11c-DTR+/− mice treated with saline, but not in those treated with DTX, relative to naïve CD11c-DTR+/− mice (n = 7/group). (E) Protein levels of CCL22 and CCL17, measured by histology, are similarly reduced in the hindpaw of CD11c-DTR+/− mice treated with DTX but not in those treated with saline. (F) Mechanical hypersensitivity is partially recovered in surgically injured CD11c-DTR+/− mice treated with DTX when injected with CCL22 in the hindpaw compared to animals that received saline injections (n = 5/group). (G) Thermal (heat) hypersensitivity is not affected by this CCL22 treatment. Data are represented as mean ± SEM; data are analyzed using two-way ANOVA with post hoc Bonferroni test (A and B) or one-way ANOVA with post hoc Bonferroni test (*P < 0.05, **P < 0.01, and ***P < 0.001). Data are representative of at least n = 2 experiments.
Discussion
Understanding the physiological mechanisms underlying inflammatory pain, such as in postoperative wounds, may help identify new therapeutic strategies. Recent work has highlighted the contribution of circulating immune cells, such as monocytes, neutrophils, and T cells, to the development and maintenance of pain (13, 31–35). However, what role tissue-resident immune cells may play in this pain response remains unclear. We therefore used the incisional wound model of postoperative pain to evaluate the role of CCL17 and CCL22, two chemokines preferentially expressed by skin-resident DCs, to the development of inflammatory pain. Our results show that 1) CCL17 and CCL22 cause acute hypersensitivity in naïve mice, acting through their cognate receptor CCR4, 2) blocking CCR4 activity using an antagonist results in reduced hypersensitivity after tissue injury, 3) activation of CCR4 results in changes in the electrophysiological properties of sensory neurons, and 4) DCs control this inflammatory pain response via their secretion of these two chemokines. While further studies are needed to understand the consequences of this neuro–immune interaction, our work identifies the critical role of these cells and mediators in the pain response. We used male mice to assess inflammatory pain outcomes, but there is no suggestion in the literature that behavioral outcomes are different between the sexes, and no publications have addressed sexual dimorphism in the incisional wound model. CFA injection resulted in similar changes to mechanical threshold in both male and female mice (36). Furthermore, there is extensive literature showing that a sexual dimorphism exists for central inflammatory responses in pain (37). However, our work here shows that peripheral inflammation is responsible for the development of pain, which our previous studies suggest is similar between male and female mice (25).
CCL17 and CCL22 are known mediators of T-cell development in the thymus, play a role in the trafficking and activation of mature T cells, and are involved in the development of the Th2 response (38). As such, both chemokines have been implicated in Th2 inflammatory diseases including atopic dermatitis, allergic asthma, and urticaria (23, 39–41). However, their role in pain response is poorly understood. One of the first studies identifying a role for these chemokines in the activation of sensory neurons demonstrated that CCL17 and CCL22 increased calcium influx in cultured rat DRGs neurons and that intraplantar injection of a high-dose of CCL22 was able to produce mechanical hypersensitivity (42). More-recent studies linking CCL17 to pain outcomes have focused on its up-regulation following intraplantar GM-CSF injection, in which CCL17 is necessary for GM-CSF-driven inflammatory pain (18). Interestingly, GM-CSF administration was also able to up-regulate the expression of TNF-α and IL-1β, known mediators of inflammatory pain and sensory neuron activation/sensitization (43–45). On the other hand, our study characterizes the direct contribution of both CCL17 and CCL22 to acute pain. Potential interference by other cytokines/immune mediators was avoided by measuring changes in hypersensitivity early after the injection of the two chemokines into the footpad. Thus, CCL17 and CCL22, without the presence of other inflammatory molecules, are sufficient to induce hypersensitivity via the CCR4 receptor.
CCR4-expressing cells candidates can be categorized in two main groups: skin-resident immune cells or sensory neurons. Among immune cells, several studies show that the receptor is expressed predominantly on conventional T lymphocytes (46–48) and γδ T cells (49). Our data show that intraplantar injection of C 021 results in effective antagonism of pain over the first 3 h after incision. There are no T lymphocytes detected in the mouse skin at this time point (13). Therefore, it is likely that some other cell(s) expressing CCR4 is activated in the early stages of tissue injury. We hypothesized that γδ T lymphocytes would be the major cell population expressing CCR4 and, thus, activated by CCL17 and CCL22. However, our data showed that these cells do not participate in the development or maintenance of pain in response to CCL17 and CCL22. Other cells in the skin known to express CCR4 include CD56+CD16− NK cells, which are activated by CCL22 (50, 51), and a subset of skin-resident T cells (CLA+ memory T cells) (52). Whether these two cell types play a role in the CCL17/22:CCR4 pain axis remains unknown and suggests that CCL17 and CCL22 likely mediate their pronociceptive effects via peripheral sensory neurons.
Expression of CCR4 in peripheral sensory neurons is supported by our analysis of transcriptomic data from isolated DRG neurons. Others have shown similar results in mouse sensory neuron datasets across all both myelinated and unmyelinated subsets (53) and across sexes in mice (54); human and macaque DRG neurons have also been shown to express CCR4 (55–58). Our electrophysiological assessment of CCR4 activation by CCL22 in primary sensory neurons provides further proof that the receptor is indeed functional and mediates hyperexcitability of small-size sensory neurons. Though we did not determine which conductance was altered in these neurons, the evoked excitability suggests a change in the function and/or expression of the voltage-gated ion channels in response to CCL22. Interestingly, the more-depolarized, resting membrane potential observed postsurgery could also account for a decrease in the threshold of AP (59), thus triggering spontaneous pain that arises as a result of ectopic AP firing. It remains to determine what conductance among voltage-gated K+ currents (M channels, K2P, and 4-aminopiridine-sensitive KV channels), or even hyperpolarization-activated, cyclic, nucleotide-gated, voltage-gated Na+, and T-type Ca2+ channels that, to a lesser extent, also contribute to adjust the resting membrane potential (60–64) are affected by CCL22-CCR4 signaling. Overall, our results suggest that activation of peripheral sensory neuron-expressed CCR4 contributes to pain-enhancing effects of these chemokines.
The differential behavioral responses observed after injection of CCL17 and CCL22 bring about the possibility that their activation of CCR4 results in differential activation of the receptor. Indeed, there is evidence in the literature that while CCL22 and CCL17 presented similar affinity to CCR4, they are conformationally selective ligands for the receptor (28). CCL22 showed dominance over CCL17 in receptor endocytosis assays (27) and competitive binding assays despite the chemokines having similar potency in eliciting calcium flux and inducing chemotaxis (28). This disparity may be due to the differential response observed in the internalization of CCR4 by the two chemokines. CCL22 is a potent and rapid inducer of CCR4 internalization through coupling with β-arrestin, while CCL17 is not capable of mediating these effects (27, 65). Further, there are two distinct conformations of CCR4, in which the predominant conformation is activated by both CCL17 and CCL22 and the minor conformation is only activated by CCL22 (28). These previous findings are in line with our work here, in which only CCL22 elicited thermal (heat) responses. It is possible that higher concentrations of CCL17 may also cause increased thermal responses, though this also increases the possibility of off-target effects.
Finally, our DC-depletion experiments, using CD11c-DTR mice, provides evidence of a critical role for these skin-resident immune cells in the development of inflammatory pain. Our initial study showing that depletion of CD11b+ myeloid cells results in a loss of mechanical hypersensitivity (13) provided the impetus for this work, in which we had shown that depletion of Ly6Clow myeloid cells in the skin resulted in the loss of mechanical hypersensitivity in the incisional model of pain. This data suggested that a tissue-resident myeloid cell mediated the altered pain outcomes observed. DCs are antigen-presenting cells and can be divided into two main populations in the skin—epidermal LCs and DDCs (66). The CD11c-DTR mice used in our study depletes both LCs and DDCs (67). While little is known of the role of DCs in inflammatory pain, their contribution to neuroimmune responses in the skin has been well documented. LCs have been shown to play an important role in contact hypersensitivity (68–70), while DDCs have been shown to modulate Th17 responses (16, 71). Further elucidating the contribution of DCs to inflammatory and neurogenic responses will be critical to understanding their role in acute pain.
The discovery of a role for the CCL17/CCL22:CCR4 axis in postoperative pain provides opportunities for the development of therapeutics. Small-molecule antagonists of CCR4 have been generated by others and evaluated in animal models of allergic diseases, with promising results (72, 73). However, most compounds are still only available preclinically. A promising candidate, GSK2239633, was discontinued after a phase I trial in healthy male subjects, because the compound did not reach the minimum target level of ≥90% CCR4 inhibition in whole blood (74). Moreover, several CCR4 antibodies are being developed for cancer treatment. Mogamulizumab, a fully humanized monoclonal antibody specific for CCR4, promotes antibody-dependent cellular cytotoxicity of CCR4+ T cells and is potentially effective in the treatment of adult T cell leukemia/lymphoma (75). These antibodies were not tested in our study given their cytotoxic properties; the doses provided in humans may be effective at targeting CCR4+ T cells with high receptor expression but not the sensory neurons that likely express lower levels of CCR4. Development of antibodies or small molecules specifically targeting CCR4 in sensory neurons may provide an effective treatment of postoperative pain.
Materials and Methods
For a detailed description of the materials and methods, please refer to SI Appendix.
Experimental Animals.
All work was performed in 6- to 12-wk-old C57BL/6J male mice. CD11c-DTR/GFP, Lang-DTREGFP, and CCR4−/− mice were used for some of the experiments. The institutional animal care and use committees of Queen’s University approved all animal care and procedures (Protocols # 2015–1562 and 2019–1963) under guidelines of the Canadian Council on Animal Care.
Drugs.
The following drugs were used: recombinant CCL17 and CCL22 were purchased from BioLegend. The CCR4 antagonist C 021 (CAS 864289–85-0) was purchased from MilliporeSigma.
CD11c+ DC Depletion.
The depletion of CD11c+ cells was performed as described elsewhere (30). Briefly, heterozygous CD11c-DTR/GFP were treated with DTX and used for the experiments 24 h after the injections.
Incisional Wound Surgery.
Sterile tissue injury-based peripheral inflammation was induced by using a deep plantar incision of the left hind paw using a modification of a technique previously described (76).
CCR4 Knockdown in DRGs.
The CCR4 knockdown in DRGs was performed as described elsewhere (29, 44). Briefly, CCR4 and control siRNA (Thermo Fisher Scientific) were mixed to in vivo jetPEI® (Polyplus). A total of 3 µg of siRNA were intrathecally injected in the space between L4 and L5 for 3 consecutive d. Incisional wound or CCL22 injections were performed 24 h after the last siRNA treatment.
Thermal and Mechanical Behavioral Analysis.
Mice were assessed for thermal and mechanical hypersensitivity as previously described (13). Mechanical threshold was measured using von Frey monofilaments (Ugo Basile). The Plantar Analgesia Meter (Hargreaves’s test; IITC Life Science) was used to assess response latency to a radiant heat stimulus. Cold hypersensitivity was measured using the acetone test (77).
RNA Extraction and Real-Time PCR.
RNA extraction of the skin and transcription of total RNA to complementary DNA (cDNA) were performed. and real-time PCR using primers specific for the mouse genes Ccl17, Ccl22, and Ccr4 and Gapdh was performed. Reactions were conducted using the SYBR green fluorescence system (Thermo Fisher Scientific). The data were analyzed with the 2−ΔΔCt method and expressed relative to control samples.
Paw Immunofluorescence.
The skin of the hindpaw from CD11c-DTR/GFP and Lang-DTREGFP was removed and frozen in Tissue-Tek Optimal Cutting Temperature (OCT) compound (Thermo Fisher Scientific). Serial 14-μm sections were collected, blocked with 1% bovine serum albumin (BSA), and incubated with anti-CCL17 or CCL22 antibodies. After washing, secondary antibodies were added. Images were captured using and analyzed.
Western Blot Analysis.
DRGs were homogenized, and total protein concentrations were determined. The proteins were loaded and separated on 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% BSA and incubated with primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Immunodetection was performed, and the density of bands was quantified.
Electrophysiological Measurements.
DRG neurons were excised from mice at 24 h postincision enzymatically dissociated collagenase/dispase solution and resuspended in culture medium. Individual neurons were dispersed by trituration through a fire-polished glass Pasteur pipette and cultured overnight. Current clamp experiments were performed using small-diameter mouse DRG neurons on the stage of an inverted epi-fluorescence microscope (Olympus IX51). Borosilicate glass (Harvard Apparatus Ltd.) pipettes were pulled and polished to 3 to 5 MΩ resistance with a DMZ-Universal Puller (Zeitz-Instruments GmbH). Recordings were performed using an Axopatch 200B amplifier (Axon Instruments). Current clamp protocols were applied using pClamp 10.5 software (Axon Instruments). Data were filtered at 1 kHz (8-pole Bessel) and digitized at 10 kHz with a Digidata 1440 A converter (Axon Instruments).
Statistics.
Data are reported as mean ± SEM. Two-way repeated measures-ANOVA was used to compare the groups and doses at the different times. The normality of data was analyzed by D’Agostino and Pearson tests. If the responses were measured only once, the differences were evaluated by one-way ANOVA followed by Tukey’s t test. P values less than 0.05 were considered significant. Statistical analysis was performed with Prism 8 (GraphPad). For electrophysiology, the data analysis was completed using Clampfit 10.4 (Axon Instruments), and all electrophysiology curves were fitted using Origin 7.0 analysis software (OriginLab).
Supplementary Material
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (Grants PJT173288 and PJT148980 to N.G. and PJT156418 to C.A.), the J.P. Bickell Foundation (to N.G.), and the São Paulo Research Foundation (Grant 2013/08216-2—Center for Research in Inflammatory Diseases) (to T.M.C.). J.R.S. was a recipient of a Queen’s University Research Leaders’ Fund Postdoctoral Fellowship. M.D. holds a fellowship from the Alberta Children’s Hospital Research Institute. C.A. holds a Canada Research Chair in inflammatory pain.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2118238119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix. Microarray data analyzed was previously deposited in PubMed Gene Expression Omnibus (GSE73667).
References
- 1.Kehlet H., Jensen T. S., Woolf C. J., Persistent postsurgical pain: Risk factors and prevention. Lancet 367, 1618–1625 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Pogatzki-Zahn E. M., Segelcke D., Schug S. A., Postoperative pain-from mechanisms to treatment. Pain Rep. 2, e588 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao L., DeLeo J. A., CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur. J. Immunol. 38, 448–458 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunha T. M., et al. , Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J. Leukoc. Biol. 83, 824–832 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Hu P., Bembrick A. L., Keay K. A., McLachlan E. M., Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav. Immun. 21, 599–616 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Hu P., McLachlan E. M., Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience 112, 23–38 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Milligan E. D., Watkins L. R., Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 10, 23–36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghavendra V., Tanga F. Y., DeLeo J. A., Complete freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 20, 467–473 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Pinho-Ribeiro F. A., Verri W. A. Jr., Chiu I. M., Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 38, 5–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji R. R., Xu Z. Z., Gao Y. J., Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 13, 533–548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baral P., Udit S., Chiu I. M., Pain and immunity: Implications for host defence. Nat. Rev. Immunol. 19, 433–447 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grace P. M., et al. , The neuroimmunology of chronic pain: From rodents to humans. J. Neurosci. 41, 855–865 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghasemlou N., Chiu I. M., Julien J. P., Woolf C. J., CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc. Natl. Acad. Sci. U.S.A. 112, E6808–E6817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi T., Naik S., Nagao K., Choreographing immunity in the skin epithelial barrier. Immunity 50, 552–565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita H., et al. , Differential production of Th1- and Th2-type chemokines by mouse Langerhans cells and splenic dendritic cells. J. Invest. Dermatol. 124, 343–350 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Kashem S. W., et al. , Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43, 515–526 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh T. P., et al. , Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nat. Commun. 7, 13581 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achuthan A., et al. , Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J. Clin. Invest. 126, 3453–3466 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook A. D., et al. , TNF and granulocyte macrophage-colony stimulating factor interdependence mediates inflammation via CCL17. JCI Insight 3, e99249 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M. C., et al. , CCL17 blockade as a therapy for osteoarthritis pain and disease. Arthritis Res. Ther. 20, 62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren G., et al. , CCL22 is a biomarker of cartilage injury and plays a functional role in chondrocyte apoptosis. Cytokine 115, 32–44 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Bogacka J., et al. , CCR4 antagonist (C021) administration diminishes hypersensitivity and enhances the analgesic potency of morphine and buprenorphine in a mouse model of neuropathic pain. Front. Immunol. 11, 1241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeki H., Tamaki K., Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J. Dermatol. Sci. 43, 75–84 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Heng T. S., Painter M. W.; Immunological Genome Project Consortium, The immunological genome project: Networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Petrović J., et al. , γδ T cells modulate myeloid cell recruitment but not pain during peripheral inflammation. Front. Immunol. 10, 473 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu I. M., et al. , Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife 3, e04660 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariani M., Lang R., Binda E., Panina-Bordignon P., D’Ambrosio D., Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur. J. Immunol. 34, 231–240 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Viney J. M., et al. , Distinct conformations of the chemokine receptor CCR4 with implications for its targeting in allergy. J. Immunol. 192, 3419–3427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berta T., Liu T., Liu Y. C., Xu Z. Z., Ji R. R., Acute morphine activates satellite glial cells and up-regulates IL-1β in dorsal root ganglia in mice via matrix metalloprotease-9. Mol. Pain 8, 18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hochweller K., Striegler J., Hämmerling G. J., Garbi N., A novel CD11c.DTR transgenic mouse for depletion of dendritic cells reveals their requirement for homeostatic proliferation of natural killer cells. Eur. J. Immunol. 38, 2776–2783 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Brack A., et al. , Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain 112, 229–238 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Sahbaie P., Li X., Shi X., Clark J. D., Roles of Gr-1+ leukocytes in postincisional nociceptive sensitization and inflammation. Anesthesiology 117, 602–612 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakobsson P. J., Pain: How macrophages mediate inflammatory pain via ATP signaling. Nat. Rev. Rheumatol. 6, 679–681 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Silva J. R., et al. , Neuroimmune-glia interactions in the sensory ganglia account for the development of acute herpetic neuralgia. J. Neurosci. 37, 6408–6422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moalem G., Xu K., Yu L., T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 129, 767–777 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Cardenas A., Papadogiannis A., Dimitrov E., The role of medial prefrontal cortex projections to locus ceruleus in mediating the sex differences in behavior in mice with inflammatory pain. FASEB J. 35, e21747 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosen S., Ham B., Mogil J. S., Sex differences in neuroimmunity and pain. J. Neurosci. Res. 95, 500–508 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Crane I. J., Forrester J. V., Th1 and Th2 lymphocytes in autoimmune disease. Crit. Rev. Immunol. 25, 75–102 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Machura E., et al. , Serum TARC and CTACK concentrations in children with atopic dermatitis, allergic asthma, and urticaria. Pediatr. Allergy Immunol. 23, 278–284 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Perros F., Hoogsteden H. C., Coyle A. J., Lambrecht B. N., Hammad H., Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy 64, 995–1002 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Kataoka Y., Thymus and activation-regulated chemokine as a clinical biomarker in atopic dermatitis. J. Dermatol. 41, 221–229 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Oh S. B., et al. , Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J. Neurosci. 21, 5027–5035 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Junger H., Sorkin L. S., Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain 85, 145–151 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Gustafson-Vickers S. L., et al. , Long-term actions of interleukin-1beta on delay and tonic firing neurons in rat superficial dorsal horn and their relevance to central sensitization. Mol. Pain 4, 63 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sachs D., Cunha F. Q., Poole S., Ferreira S. H., Tumour necrosis factor-alpha, interleukin-1beta and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain 96, 89–97 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Anderson C. A., Solari R., Pease J. E., Biased agonism at chemokine receptors: Obstacles or opportunities for drug discovery? J. Leukoc. Biol. 99, 901–909 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Bonecchi R., et al. , Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187, 129–134 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iellem A., et al. , Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 194, 847–853 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura K., et al. , Differential requirement for CCR4 in the maintenance but not establishment of the invariant Vγ5(+) dendritic epidermal T-cell pool. PLoS One 8, e74019 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebert L. M., Meuter S., Moser B., Homing and function of human skin gammadelta T cells and NK cells: Relevance for tumor surveillance. J. Immunol. 176, 4331–4336 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Inngjerdingen M., Damaj B., Maghazachi A. A., Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J. Immunol. 164, 4048–4054 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Clark R. A., et al. , The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Usoskin D., et al. , Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Mecklenburg J., et al. , Transcriptomic sex differences in sensory neuronal populations of mice. Sci. Rep. 10, 15278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray P., et al. , Comparative transcriptome profiling of the human and mouse dorsal root ganglia: An RNA-seq-based resource for pain and sensory neuroscience research. Pain 159, 1325–1345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kupari J., et al. , Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat. Commun. 12, 1510 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wangzhou A., et al. , Pharmacological target-focused transcriptomic analysis of native vs cultured human and mouse dorsal root ganglia. Pain 161, 1497–1517 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.North R. Y., et al. , Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 142, 1215–1226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du X., et al. , Control of somatic membrane potential in nociceptive neurons and its implications for peripheral nociceptive transmission. Pain 155, 2306–2322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu D. L., et al. , Upregulation of Ih expressed in IB4-negative Aδ nociceptive DRG neurons contributes to mechanical hypersensitivity associated with cervical radiculopathic pain. Sci. Rep. 5, 16713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan N., Sikand P., Donnelly D. F., Ma C., Lamotte R. H., Increased Na+ and K+ currents in small mouse dorsal root ganglion neurons after ganglion compression. J. Neurophysiol. 106, 211–218 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao H., Donnelly D. F., Ma C., LaMotte R. H., Upregulation of the hyperpolarization-activated cation current after chronic compression of the dorsal root ganglion. J. Neurosci. 23, 2069–2074 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cummins T. R., Dib-Hajj S. D., Black J. A., Waxman S. G., Sodium channels and the molecular pathophysiology of pain. Prog. Brain Res. 129, 3–19 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Abdulla F. A., Smith P. A., Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J. Neurophysiol. 85, 644–658 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Ajram L., et al. , Internalization of the chemokine receptor CCR4 can be evoked by orthosteric and allosteric receptor antagonists. Eur. J. Pharmacol. 729, 75–85 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malissen B., Tamoutounour S., Henri S., The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 14, 417–428 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Bar-On L., Jung S., Defining in vivo dendritic cell functions using CD11c-DTR transgenic mice. Methods Mol. Biol. 595, 429–442 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Schwarz A., et al. , Langerhans cells are required for UVR-induced immunosuppression. J. Invest. Dermatol. 130, 1419–1427 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Kaplan D. H., Jenison M. C., Saeland S., Shlomchik W. D., Shlomchik M. J., Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23, 611–620 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Bobr A., et al. , Acute ablation of Langerhans cells enhances skin immune responses. J. Immunol. 185, 4724–4728 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riol-Blanco L., et al. , Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pease J. E., Horuk R., Chemokine receptor antagonists: Part 1. Expert Opin. Ther. Pat. 19, 39–58 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Pease J. E., Horuk R., Recent progress in the development of antagonists to the chemokine receptors CCR3 and CCR4. Expert Opin. Drug Discov. 9, 467–483 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Cahn A., et al. , Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: Results from an open-label and from a randomised study. BMC Pharmacol. Toxicol. 14, 14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogura M., et al. , Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol. 32, 1157–1163 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Brennan T. J., Vandermeulen E. P., Gebhart G. F., Characterization of a rat model of incisional pain. Pain 64, 493–502 (1996). [DOI] [PubMed] [Google Scholar]
- 77.Vissers K., Meert T., A behavioral and pharmacological validation of the acetone spray test in gerbils with a chronic constriction injury. Anesth. Analg. 101, 457–464 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix. Microarray data analyzed was previously deposited in PubMed Gene Expression Omnibus (GSE73667).