Abstract
Bone mineral mass, geometry and microstructure, hence determinants of fracture risk, result bone accrual during growth and bone loss later in life. Peak bone mass, which is reached by the end of the second decade of life, is mainly determined by genetic factors. Among other factors influencing bone capital, dietary intakes, particularly calcium and protein, play a significant role in peak bone mass attainment. Both nutrients are provided in dairy products, which accounts for 50–60% and 20–30% of the daily calcium and protein intakes, respectively. Children avoiding dairy products are at higher risk of fracture, as are adults or older individuals following a diet devoid of dairy products, like vegans. Various intervention trials have shown some beneficial effects of dairy products on bone capital accumulation during growth and on bone turnover in adults. In observational studies, dairy products intake, particularly the fermented ones, which also provide probiotics in addition to calcium, phosphorus and protein, appear to be associated with a lower risk of hip fracture.
Keywords: Growth, Bone mineral density, Osteoporosis, Fracture, Nutrition, Probiotics, Protein, Calcium, Fermented dairy products
Introduction
Skeleton functions include body support, internal organ protection, mineral homeostasis and a role in acid–base regulation. Bone strength depends on bone mass, geometry, microstructure and material level properties. Maximal bone capital, i.e. peak bone mass, is reached by the end of the second decade of life, and, under usual conditions, allows us to successfully resist to a mechanical overload [1]. Between 60 and 80% peak bone mass variance are explained by genetic factors. Environmental factors can modify the influence of genetic factors, impair bone mass accrual, alter bone turnover and/or bone strength, and increase thereby fracture risk. During childhood and adolescence, height gain and bone mineral mass accrual are following a track which is genetically determined [1, 2]. Any nutritional insufficiency can alter bone growth and move the trajectory towards a less favorable track, and lead thereby to a lower peak bone mass. Nutrients such as calcium, phosphorus and protein are major nutritional determinants of bone mass accrual. These nutrients are combined in dairy products. Indeed, one liter of cow milk provides 1200 mg/l, 1150 mg/l phosphorus, 32–35 g/l protein, i.e. casein and whey protein, which also contains a series of cellular growth factors, together with calories, trace elements and vitamins (Table 1). Whey proteins are faster digested and absorbed than casein. Despite variations of milk composition according to cow breed, seasons and food, commercially available milk is usually standardized, and sometimes fortified with vitamin D in a few countries. Depending on the species, the nutrients content can considerably vary (Table 2). The macronutrient concentrations of some plant drinks may be similar to that of cow milk, such as for instance protein content. However, plant-based alternatives require the addition of mineral salts and of carbohydrates to reach concentration of calcium and of calories similar to cow milk [3] (Table 3). However, the nutritional quality of most plant drinks is markedly different. If cow milk is replaced by non-fortified and non-supplemented plant drinks, consumers may risk various deficiencies, thus children and adolescents receiving exclusively such plant drinks may be at risk of severe metabolic disturbances [4]. Indeed, the supplemental carbohydrate content cannot be considered as part of a healthy diet. Except for soy drink, the other preparations cannot bear the name of milk. Animal sources of protein tend to be more easily digested, and the distribution of essential amino acids is considered to better fit human requirements, particularly for muscle and bone formation [5].
Table 1.
Bone nutrient content per 100 g of selected dairy foods
| Dairy food | Calcium (mg) | Potassium (mg) | Phosphorus (mg) | Protein (g) |
|---|---|---|---|---|
| Milk, full-fat 3.7% | 119 | 151 | 93 | 3.3 |
| Milk, skimmed | 122 | 156 | 101 | 3.4 |
| Yoghurt, plain low-fat* | 183 | 234 | 144 | 5.3 |
| Yoghurt, fruit low-fat | 169 | 216 | 133 | 4.9 |
| Cheddar cheese | 721 | 98 | 512 | 24.9 |
| Cottage cheese, non-fat | 86 | 137 | 190 | 10.3 |
| Ice cream, soft serve, chocolate | 131 | 177 | 116 | 4.1 |
Data are from the USDA National Nutrient Database for Standard Reference, release 26. 2013
Available at: http://ndb.nal.usda.gov/ndb/nutrients/index
Table 2.
Chemical constituents of human, cow, goat, sheep, camel and buffalo milk
| Milk | Protein g/100 g | Lactose g/100 g | Fat g/100 g | Calcium mg/100 ml | pH |
|---|---|---|---|---|---|
| Human | 1.25 | 6.95 | 3.20 | 32.00 | 7.20 |
| Cow | 3.40 | 4.80 | 3.75 | 112.00 | 6.60 |
| Goat | 3.30 | 4.40 | 3.90 | 130.00 | 6.60 |
| Sheep | 6.35 | 5.00 | 6.90 | 197.50 | 6.60 |
| Camel | 2.95 | 4.30 | 3.60 | 94.40 | 6.50 |
| Buffalo | 4.52 | 4.80 | 7.94 | 173.4 | 6.77 |
From [80]
Table 3.
The nutritional profile of cow´s milk and plant-based alternatives, with and without fortification
| Nutritional content per 100 ml of beverage | ||||||||
|---|---|---|---|---|---|---|---|---|
| Milk | Soy drink | Soy drink, fortified | Almond drink | Rice drink | Rice drink, fortified | Oat drink | Oat drink, fortified | |
| Energy, kcal | 64 | 55 | 45 | 47 | 50 | 54 | 50 | 45 |
| Energy, kJ | 268 | 230 | 188 | 197 | 209 | 226 | 209 | 188 |
| Protein, g | 3.4 | 3.1 | 3.3 | 0.7 | 0.1 | 0.1 | 0.6 | 0.5 |
| Total lipid, g | 3.5 | 2.3 | 1.9 | 2.2 | 0.9 | 0.9 | 1 | 1.6 |
| Carbohydrate, g | 4.9 | 5.3 | 3.6 | 5.9 | 10.3 | 11.4 | 9.7 | 7.2 |
| Vitamin A, RE | 35.3 | 0 | 0 | 0 | 0 | 0 | 0.34 | 0 |
| Vitamin B2, mg | 0.18 | 0.01 | 0.02 | 0.02 | 0 | 0 | 0.01 | 0.01 |
| Vitamin B12, µg | 0.39 | 0 | NA | NA | NA | NA | NA | NA |
| Calcium, mg | 119 | 9.86 | 74.5 | 8.8 | 1.85 | 84.3 | 6.56 | 126 |
| Zinc, mg | 0.36 | 0.25 | 0.28 | 0.11 | 0.03 | 0.05 | 0.41 | 0.08 |
| Iron, mg | 0.02 | 0.45 | 0.5 | 0.12 | 0.01 | 0.23 | 0.03 | 0.44 |
| Iodine, µg | 16.5 | 1.3 | 9.35 | 0.89 | 1.04 | 2.5 | 0.418 | 5.9 |
| Phosphorus, mg | 91 | 44.1 | 41.5 | 14.3 | 7.39 | 28 | 13.2 | 16.9 |
Data from the Danish National Food Institute, food D Frida food data. DTU Fødevareinstituttet. https://frida.fooddata.dk/
RE retinol equivalents, NA not assessed
Dairy products are consumed by humans since millennia, as indicated by processed dairy residues detected in pottery vessels found in Dalmatian cost or in Anatolia, and going back to 6000 BC [6, 7]. Consumption of cow, sheep or goat milk is confirmed by finding dairy protein in dental calculus from northeastern Africa at least 6 millennia ago [8]. The first mention in the literature of cheese making is likely in the Odissey of Homer (chant IX), written around 750 BC. Fermented dairy products like cheese and yoghurts have allowed the preservation, the transport and an easier digestion of milk.
The role of the consumption of dairy products, which are a complex combination of macronutrients and micronutrients, in adult bone homeostasis is still debated. Indeed, whilst the natural function of milk and dairies is to ensure normal growth of young mammals, the importance for bone health of its nutrients content and of dairy products as foods in young adults and in the oldest old to meet calcium and protein requirements is still not fully appreciated. In this narrative review, the effects on bone health of the main nutrients present in dairy products and of whole dairy foods are summarized and discussed.
Literature search strategy
A literature search was conducted using MEDLINE database. Relevant observational studies and randomized controlled trials were selected using a combination of keywords including bone growth, osteoporosis, bone remodeling, BMD, BMC and fracture as outcomes; and dietary calcium and protein, milk, cheese and dairy products as explanatory variables. Additional studies were identified by an extensive manual search of bibliographic references in original papers and reviews. Abstracts and non-English papers were not included. Particular attention was given to finding randomized controlled trials. However, part of the information collected and presented is derived from observational studies.
Dietary calcium intake and bone growth
In controlled intervention trials, milk ultrafiltrate calcium supplements increased peripheral skeleton bone mineral content in both prepubertal girls and boys [1]. These effects are attributed to lower bone turnover thus reduced resorption cavities. A meta-analysis including intervention trials comparing calcium supplements to a placebo has concluded to a favorable effect of calcium on peripheral skeleton, persisting at least 18 months after calcium discontinuation [9]. During a long-term follow-up up to adulthood of a cohort of healthy girls, having participated at the age of 8 years to a trial with 850 mg of calcium supplement per day of milk origin, leading to a doubling of the spontaneous intake, a persistent effect was observed in those girls with a menarcheal age below the group median [10]. However, there is poor evidence that calcium intake during childhood and adolescence would be associated with fracture risk later in life.
Dietary phosphate
Adequate phosphate supply is required for cartilage and osteoid tissue mineralization [11]. Phosphate wasting syndromes are associated with impaired growth and fragility fractures [12]. A normal usual diet provides sufficient amounts of phosphate in most circumstances, so that phosphate deficiency from dietary origin is unlikely. Phosphate is found in high amounts in protein containing foods such as dairy products (1150 mg/l of milk and 500 mg/100 g of Swiss cheese), meat, and also in grains, beans, lentils and nuts. Recommended Dietary Allowance is 1250 mg/day for adolescents during growth and 700 mg/day for adults. Under normal conditions, 60–70% of dietary phosphate is absorbed. The low BMD in older women associated with colas beverages seems to be rather mediated by milk displacement, since 1 l cola contains 170 mg of phosphorus, thus far less than 1 l milk [13].
Dietary protein intake and bone growth
In a prospective longitudinal observational study over 4 years, with an annual record of nutritional intakes [14], bone size, bone mass and an estimation of bone strength were positively correlated to dietary protein intake. However, there is presently no randomized intervention trial assessing whether this correlation is an association only or reflects a causal relationship. Liver IGF-I production is stimulated by dietary protein, particularly by aromatic amino acids [15]. IGF-I increases longitudinal and radial bone growth [16]. By enhancing renal tubular reabsorption of phosphate and the renal synthesis of calcitriol, hence stimulating intestinal absorption of calcium and phosphate, IGF-I contributes to warranting an optimal mineral concentration for mineralization of newly deposited cartilage and osteoid matrix [16].
Dairy products and bone growth (for review see [17, 18])
Dairy products provide about 50–60% of calcium intake and 20–30% of protein intake during growth. Dairy products avoidance during childhood is a risk factor for fracture [19]. A diet devoid of dairies is associated with a 4.6-fold increase in fracture risk in girls between the age of 2 and 20 years [20]. Dairy products influence may intervene even before birth. Indeed, BMD of 6-year-old children was positively correlated with milk and calcium-rich foods consumed by the mother during pregnancy [21]. The first milk intervention trials took place in the 1920ies. Providing around 0.5 l milk to school children for 7 months increased height gain [22, 23]. Numerous trials have confirmed some benefits, even of small magnitude, of dairy products on bone mass accrual (Table 4). For instance, in a randomized controlled trial in 12-year-old girls, a pint of milk, corresponding to 568 ml, increased whole body mineral content, particularly in the lower limb, and IGF-I levels [24]. Compared to calcium supplements, cheese increased cortical bone mass [25, 26]. In 10–12-year old girls, calcium provided as cheese led to a higher bone gain as compared with calcium as pills [27]. An effect on bone modeling is likely since metacarpal bone diameter was higher in Chinese children receiving milk supplements than in controls [27]. Dairy products may thus influence bone mineral accrual through a remodeling process mediated by calcium and a modeling process through protein stimulated IGF-I production, favoring periosteal apposition.
Table 4.
Effects of dairy products on bone in children and adolescents (controlled trials)
| Study | Year | Number | Sex | Mean age (years) |
Duration (months) |
Intervention | Outcome | Main results Intervention—placebo changes |
|---|---|---|---|---|---|---|---|---|
| Baker et al. [81] | 1980 | 581 | F/M | 8.0 | 21 | Milk 190 ml/day | Height | Height: + 3% or + 2.93 mm |
| Cadogan et al. [24] | 1997 | 82 | F | 12.2 | 18 | Milk 568 ml/day | WB BMC; IGF-I | WB BMC: + 2.9% or + 37 g; IGF-I: + 10% |
| Chan et al. [82] | 1995 | 42 | F | 11 | 12 | Dairies | WB BMC; LS BMD | WB BMC: + 9.9%; LS BMD: + 6.6% |
| Cheng et al. [26] | 2005 | 195 | F | 11.2 | 24 | Cheese equivalent 1000 mg Ca | Tibia CTh; WB BMD | Tibia CTh: + 6%; WB BMD: + 2% |
| Du et al. [83] | 2004 | 757 | F | 10.1 | 24 | Calcium-fortified milk 330 ml/day | Height; size- adjusted WB BMC; WB BMD | Height: + 0.6%; size- adjusted WB BMC: + 1.2%; |
| WB BMD: + 3.2% | ||||||||
| Lau et al. [84] | 2004 | 344 | F/M | 10.0 | 18 | Milk powder equivalent to 1300 mg Ca | LS BMD; Hip BMD | LS BMD: + 1.4%; Hip BMD: + 1.1% |
| Leighton & Clark [23] | 1929 | 1425 | F/M | 6–13 | 7 | Milk 568 ml/day (426 ml if ≤ 7 yrs) | Height | Height: + 23.5% |
| Lu et al. [85] | 2019 | 232 | F/M | 13.1 | 18 | Milk powder fortified in Ca, equivalent to 20 g protein | IGF-I; WB, LS, Hip BMD | IGF-I: + 21%; BMD: no difference |
| Merrilees et al. [86] | 2000 | 91 | F | 16 | 24 | Milk equivalent to 1160 mg Ca | LS, FN, Trochanter BMD | Statistically significant differences in BMD changes from baseline |
| Orr [22] | 1928 | NR | M | 5–14 | 7 | Milk 568 ml/day (426 ml if ≤ 6 yrs) | Height | Height: + 21.3% |
| Vogel et al. [87] | 2017 | 240 | M/F | 11.8 | 18 | 3 servings dairies/day | LS, Hip BMD; 4% tibia BMC | LS, Hip BMD: no difference in BMD changes from baseline; 4% tibia BMC: higher gain |
| Volek et al. [88] | 2003 | 28 | M | 14 | 3 | 3 servings dairies/day with resistance training | Height; WB BMC; WB BMD | Height: + 0.8 cm; WB BMC: no difference; |
| Zhu et al. [27] | 2005 | 606 | F | 10.1 | 24 | Calcium-fortified milk 330 ml/day | Metacarpal outer diameter, CTh | Metacarpal outer diameter: + 1.2%; CTh: + 5.7% |
| Metacarpal medullary diameter | Metacarpal medullary diameter:—6.7% | |||||||
| Zhu et al. [89] | 2008 | 345 | F | 10.1 | 24 | Calcium-fortified milk 330 ml/day | Size-corrected WB BMD | Size-corrected WB BMD: + 3.6–5.8% |
+ Statistically significant greater change in the intervention group. − Statistically significant smaller change in the intervention group
WB BMC/BMD whole body BMC/BMD, CTh cortical thickness, LS lumbar spine. Hip total hip, FN femoral neck, NR: not reported
Dairy products consumption during childhood and adolescence leads to a higher peak bone mass, but data on statural height are less consistent [28]. In a recent systematic review, 8 out of 11 randomized trials performed during childhood and adolescence have revealed a 8% greater gain of BMD by 16 months of dairy products in various quantity [18]. A higher gain in lean mass with dairy products was reported in another meta-analysis [28].
One serving of dairies (30 g hard cheese, 2 dl milk or 1 yoghurt) represents 250 mg of calcium. Two servings are recommended below the age of 9 and 3 above, by various bodies in regions with Western style food habits [29, 30]. Three servings of dairies provide approximately 20 g of protein.
Long-term effects of dairy products intake during childhood and adolescence
A high peak bone mass at the end of growth could theoretically contribute to a lower risk of fracture later in life [1]. It is estimated that a 10% higher bone mass could be equivalent to a menopause occurring 13 years later or to a 50% lower fracture risk [31]. However, though dairy products have been shown to increase bone mineral mass during growth, attempts to relate fracture risk in adulthood and aging, with dairy products consumption during childhood and adolescence, have not provided consistent results, likely in relation with the large inaccuracy of food intake recorded more than 40 years later. A frequent consumption of milk before the age of 25 years was associated with a higher proximal femur BMD between the age of 44 and 74 years [32]. A history of more than 1 glass of milk during childhood, but not during adolescence, compared to less than 1 glass per week, was associated with a higher trochanter BMD in postmenopausal women [33]. Less than one serving a day of dairy products during childhood was accompanied by a twofold higher risk of fracture in 50-year old women [34, 35]. In contrast, in the Health Care Professional study, no association was found between milk consumption during adolescence and hip fracture risk in women, with even a higher risk in men (+ 9% per additional glass of milk daily) [36]. This has been partially attributed to a greater height in dairy products consumers, higher height being a risk factor for hip fracture.
Dairy products and bone mineral density and/or fracture risk in adults
Calcium intake and fracture
The influence of calcium intake on bone remodeling and particularly on fracture risk has raised numerous debates for both antifracture efficacy and safety. Without entering the debate, the present evidence can be summarized as follows [37]. The combination calcium and vitamin D is associated with a modest decrease in fracture risk, particularly in the oldest old living in nursing homes [38]. Calcium alone does not appear to significantly influence fracture risk. Among the adverse events associated with calcium supplements, gastro-intestinal discomfort, more frequent with calcium carbonate preparations, and a slightly increased risk of renal lithiasis should be mentioned. A higher risk of myocardial infarction is not consistently confirmed, and is not present when calcium is from dietary origin, such as provided by dairy products [37, 39]. Similarly, accelerated vascular calcification, which can result from high pharmacological calcium supplementation, is not observed with calcium of dietary origin [11, 37, 39].
Dietary protein and fracture risk (for review, see [40])
Numerous observational studies have addressed the issue of fracture risk in relation to dietary protein intake. The results of these studies are sometimes divergent. Positive associations, i.e. a higher fracture risk at high protein intake are rare, and are mostly observed with a low calcium intake [40]. In several systematic reviews and meta-analyses, hip fracture risk was lower with higher dietary protein (for review, see [40]), provided calcium intake is sufficient. It should be noted that there is no evidence of osteoporosis, changes in bone strength or in fracture risk in relation with dietary protein-derived acid load in a balanced diet [40, 41].
Dairy products and bone remodeling
In short-term intervention trials (usually less than 4 months), dairy products reduced bone turnover markers by 6–40% together with a lowering of PTH levels in younger adults (Table 5) as well as in older individuals (Table 6). In a 12-week trial in overweighed adolescent girls, who were following a physical exercise program for weight loss, four servings of dairy products per day compared to two or less, decreased serum CTX [42]. This decrease was proportional to the number of servings. In 85-year-old institutionalized people, 2 servings/day of soft white cheese fortified with vitamin D and calcium during 6 weeks reduced PTH and bone resorption markers [92].
Table 5.
Effects of dairy products on bone in younger adults (controlled trials)
| Study | Year | Population | N | Mean age (years) | Intervention | Duration | Outcomes | Main results | Conclusions: effects of dairies |
|---|---|---|---|---|---|---|---|---|---|
| Baran et al. [90] | 1990 | Premenopausal women | 37 | ~ 36 | Dairy products equivalent to + 610 mg/day of Ca | 3 year | PTH, LS BMD | PTH: no change; LS BMD:—0.4 vs—2.9% in controls | Prevention LS BMD loss |
| Bonjour et al. [91] | 2008 | Postmenopausal women | 30 | 59.5 | Semi-skimmed milk 500 ml/day | 6 weeks | BTM, PTH | PTH:—3.2 pg/ml; CTX:—624 pmol/l; P1NP:—5.5 ng/ml; Osteocalcin:—2.8 ng/ml | ↘ PTH, ↘ CTX, ↘ P1NP, ↘ Oc |
| Bonjour et al. [93] | 2012 | Postmenopausal women with low spontaneous supply of Ca and Vit D | 71 | 56.6 | 2 servings of skimmed-milk and soft white cheese fortified with Vit D (2.5 μg/d) and Ca (400 mg/d) | 6 weeks | IGF-I, BTM | IGF-I: + 18 µg/l; TRAP 5b:—0.3 U/l; CTX: NS | Greater ↗IGF-I and ↘ TRAP5b |
| Chee et al. [94] | 2003 | Postmenopausal (> 5 years) women (55–65 years) | 173 | 59 | Milk powder with 1200 mg/d Ca | 24 months | BMD | LS BMD:—13 vs—90%; Hip:—0.50 vs—2.17%; FN BMD: + 0.51 vs—1.21% in controls | ↗Vit D, ↘ spine and hip BMD loss, benefit still evident 21 months after the study end |
| Ting et al. [95] | 2007 | Postmenopausal (> 5 years) women (55–65 years) | 173 | 61 | Milk powder with 1200 mg/d Ca | 24 months | BMD | Some difference still detectable 18 months after intervention end | |
| Chen et al. [96] | 2015 | Postmenopausal women | 141 | 55.9 | Milk powder with 900 mg/d Ca | 24 months | BMD | LS:—013 T-score difference in favour of intervention group | ↘ LS BMD loss |
| Gui et al. [49] | 2012 | Postmenopausal women without osteoporosis (45–65 years) | 141 | 56.5 | Milk/Soymilk with 250 mg/d Ca | 18 months | BMD | Milk: Hip: + 2.5%; FN: + 2.8%. Soymilk: not different from controls | Prevention FN and Hip BMD loss |
| Josse et al. [102] | 2010 | Young women | 20 | 23.2 | 500 ml skimmed milk before and 1 h after exercise | 12 weeks | PTH, BTM | PTH:—1.2 pmol/l | ↘ PTH |
| Josse et al. [103] | 2012 | Young overweight women | 90 | ~ 31.5 | 6–7 servings/day dairy | 16 weeks | PTH, BTM | PTH:—1.2 vs + 0.8 pmol/l; P1NP: + 16 vs + 1 µg/l; CTX: + 0.01 vs + 0.12 nmol/l in controls | Prevention of ↗ bone resorption |
| Kristensen et al. [104] | 2005 | Healthy young men (22–29 years) | 11 | 24 | 2.5 l/day of Cola + low-Ca diet vs 2.5 l/day of semi-skimmed milk + low-Ca diet | 10 days | BTM | CTX: 0.8—> 0.6 with milk vs—> 0.9 with cola | ↗ BTM with cola diet, not milk diet |
| Kruger et al. [105] | 2006 | Premenopausal women 20–35 years | 82 | 27 | High Ca skimmed milk (1000 mg/d of extra Ca) | 16 weeks | BTM | sCTX: 0.49—> 0.30 ng/ml; P1NP: 55.9—> 42.1 ng/ml | ↘ CTX, ↘ osteocalcin, ↘ P1NP |
| Kruger et al. [106] | 2010 | Postmenopausal women | 120 | 57.5 | Milk powder fortified with 1200 mg Ca, 96 mg magnesium, 2.4 mg zinc and 9.6 μg Vit D /d | 16 weeks | Vit D, PTH, BTM | CTX:—40%; osteocalcin:—30%; P1NP:—30% | ↘ BTM |
| Lau et al. [109] | 2001 | Postmenopausal women | 200 | 57 | Milk powder providing 800 mg/day Ca and 18.8 g protein | 24 months | BMD | Hip:—0.06 vs—0.88%; LS:—0.56 vs—1.5%; FN:—0.70 vs—1.1% in controls | lower ↘ BMD, ↗ Vit D, ↘ PTH |
| Lau et al. [110] | 2002 | Postmenopausal women | 187 | 57 | Milk powder containing 800 mg/d Ca | 36 months | BMD | Lower BMD loss; Hip 81%; LS: 65%; FN: 73% | lower ↘ BMD |
| Liu et al. [111] | 2011 | Pregnant women (24–31 years) with habitual low Ca intake | 36 | 27 | Milk powder (containing 350 mg Ca); milk powder (containing 350 mg Ca) + 600 mg Ca/d | 20 weeks gestational age to 6 weeks post-partum | BMD, BTM | Higher WB and LS BMD in the milk high calcium group | ↗ BMD |
| Moschonis et al. [113] | 2010 | Postmenopausal women (55–65 years) | 66 | 60 | Milk and yogurt fortified with 1200 mg Ca and 7.5/22.5 μg Vit D + counselling | 30 months | BMD | WB BMD: + 0.003 vs—0.020 g/cm2 in controls; spine: + 0.118 vs + 0.049 g/cm2 in controls | ↗ WB BMD whole body and spine |
| Recker et al. [116] | 1985 | Postmenopausal women | 22 | NR | 192 ml/day milk | 24 months | Ca balance | Ca balance:—0.061—>—0.017 g/day | Better Ca balance |
| Rosado et al. [117] | 2011 | Young obese women | 139 | 34 | 3 × 250 ml/day low-fat milk | 16 weeks | BMC | WB BMC: + 28 vs—2 mg in controls | ↗ WB BMC |
| Tenta et al. [119] | 2011 | Postmenopausal women | 40 | 55–65 | Milk and yogurt fortified with Ca (1200 mg/day) and Vit D (7.5–30 μg/day) | 30 months | BTM, BMD | RANKL:—0.08 vs + 0.01 pg/ml; CTX:—0.11 ng/ml by 12 months | Prevention ↘ Vit D in winter. ↘ CTX and RANKL; ↗ WB BMD |
| Thorpe et al. [120] | 2008 | Overweight men and women (30–65 years) | 130 | 46 | 1.4 g/kg BW protein through 3 servings/day of dairies | 12 months | BMD | BW at 12 months:—10.5% in both groups. WB, LS and Hip BMD 1.6, 2.1 and 1.4% higher | ↘ BMD decrease |
| Woo et al. [122] | 2007 | Women (20–35 years) | 408 | 28 | Milk powder with 1000 mg Ca, 80 μg Vit K | 24 months | BMD, BTM | Overall, small BMD increases | No difference between groups |
NR not reported, BMD bone mineral density, BMC bone mineral content, WB whole body, LS lumbar spine, FN femoral neck, Ca Calcium, BTM bone turnover markers, Oc osteocalcin
Table 6.
Effects of dairy products on bone in older adults (controlled trials)
| Study | Year | Population | N | Mean age (years) | Intervention | Duration | Outcomes | Main results | Conclusions: effects of dairies |
|---|---|---|---|---|---|---|---|---|---|
| Bonjour et al. [92] | 2009 | Institutionalized women ≥ 65 years old with low Vit D status and Ca intake < 700 mg/d | 37 | 84.8 | 2 servings of soft white cheese fortified with Vit D (+ 1.25 µg/100 g) and calcium (total Ca 151 mg/100 g) | 6 weeks | PTH, BTM | CTX:—7.5%; TRAP 5b:—9.9%; P1NP: + 19.3%; PTH:—12.3%; IGF-I: + 16.9% | ↗ Vit D, ↗ IGF-I, ↘ PTH, |
| Daly et al. [97] | 2005 | Men (50–79 years) without Vit D deficiency | 167 | 62 | 400 ml/day fortified with 1000 mg calcium and 800 IU vit D | 24 months | BMD, PTH, vit D | 2 years: FN:—0.7 vs- 2.22%; UD radius:—0.71 vs—2.28%; first year: 25OHD: + 31%; PTH:—18% | ↗Vit D, ↘ FN and UD radius BMD loss |
| Daly et al. [98] | 2008 | Community living men (50–79 years) | 111 | 63 | Fortified milk with Ca (1000 mg/d) and Vit D (800 IU/d) | 18 months | BMD | Some difference still detectable 18 months after intervention end | |
| Green et al. [99] | 2002 | Postmenopausal women | 50 | 67.5 | Milk powder fortified with 1200 mg calcium | 4 weeks | PTH, BTM | sCTX: 0.43—> 0.28 ng/ml | ↘ CTX |
| Heaney et al. [100] | 1999 | Men and women, 55–85 years, less than 1.5 serving/day dairy | 204 | 65.1 | 3 servings/day of low–fat milk | 12 weeks | IIGF-I, urine NTX | uNTX:—13%; IGF-I: + 10%; IGFBP4: stable whilst + 7.9% in controls | ↗ IGF-I, ↘ uNTX |
| Heaney et al. [101] | 2002 | Postmenopausal white women with Ca intake < 600 mg/d | 29 | 61.4 | 3 servings/day of yogurts | 7–11 days | Urine NTX | uNTX:—22% | ↘ Urine NTX |
| Kruger et al. [107] | 2012 | Postmenopausal women | 63 | 62 | Milk fortified with 900 mg Ca, 96 mg magnesium, 2.4 mg zinc and 6.4 μg Vit D /d | 12 weeks | Vit D, PTH, BTM | PTH:—14%; CTX:—29%; P1NP:—18% | ↗ Vit D, ↘ PTH, CTX, Oc, P1NP |
| Kukuljan et al. [108] | 2009 | Men (50–79 years) without Vit D deficiency | 180 | 61 | 400 ml/day milk fortified with 1000 mg/d Ca and 800 IU/d Vit D ± exercise | 12 months | BMD | LS: + 1.5% vs controls; Hip: + 0.7% vs controls | No interaction with exercise |
| Manios et al. [112] | 2007 | Post-menopausal women | 101 | 61 | Milk and yogurt fortified with 1200 mg Ca and 7.5 μg Vit D + counselling | 12 months | IGF-I, BTM | IGF-I: + 38%; CTX: -23.2%; WB BMD: + 1.5 vs—0.7% in controls | ↗ WB BMD whole body and spine; ↘ CTX; ↗ IGF-I |
| Moschonis et al. [114] | 2011 | Postmenopausal women | 63 | 62 | Milk and yogurt fortified with 800 mg Ca + 10 μg Vit D & Vit K | 12 months | BMD | WB BMD: + 0.013 vs—0.001 g/cm2 in controls; LS: + 0.006 vs—0.032 g/cm2 in controls | ↗ WB and spine BMD |
| Prince et al. [115] | 1995 | Postmenopausal women | 84 | 63 | 208 ml/day milk with 1000 Ca | 24 months | BMD | Trochanter: + 0.2 vs—0.6% per year, distal tibia:—1.5 vs—2.5% in controls | lower ↘ BMD |
| Storm et al. [118] | 1998 | Postmenopausal women | 60 | 71 | Milk 4 × 240 ml /day | 24 months | BMD, BTM | Trochanter:—0.009 vs—0.022 g/cm2 in controls | ↘ BMD decrease |
| Tu et al. [121] | 2015 | Men and women | 65 | 66 | 1.6 l/day Kefir fortified with 1500 mg Ca | 6 months | BTM, BMD | No difference between groups | No difference between groups |
BMD bone mineral density, BMC bone mineral content, WB whole body, LS lumbar spine, FN femoral neck, Ca Calcium, BTM bone turnover markers, Oc osteocalcin
Dairy products and bone mineral density
In a meta-analysis including 20 studies and 37,174 subjects, lumbar spine and femoral neck BMD was lower in subjects avoiding any dairy product, like vegans, than in vegetarians, thus a diet without meat and fish but including dairy products, as well as in omnivores [43]. In a meta-analysis evaluating the role of dietary patterns on prevalence of low BMD, a diet rich in dairies was associated with a 41% lower prevalence of low BMD [44].
In a randomized trial assessing the effects of a calcium–vitamin D supplement on BMD in men and women older than 65 years, a positive association with dietary protein intake was observed, but only in the calcium–vitamin D-treated group [45]. This suggests a possible interaction between dietary calcium and protein [3]. Various intervention trials with milk powder, dairy products fortified in calcium or vitamin D, lasting between 5 and 30 months, have shown an attenuation of the age-related bone loss (Tables 5 and 6). As a possible mechanism of action of dairy products on bone strength, a tibia diaphysis cross-sectional area proportional to the number of serving of dairies has been reported [46]. Integrating values of bone microstructure to estimate bone strength, finite element analysis has revealed higher values of distal radius and tibia failure load in relation with dietary protein of dairy origin in both 65-year-old women [47] and in 84-year-old men [48]. In both studies, carried out in different populations of different sex and age, there was no significant association of bone failure load with protein of vegetable origin. A randomized controlled trial in 141 postmenopausal women has concluded that the consumption of cow milk was superior in preventing BMD loss at the hip and femoral neck over an 18 months period compared to soy drink [49] (Table 5). Although the calcium intake was similar in both groups, the observed skeletal differences were attributed to a potentially higher bioavailability of calcium from milk. In a meta-analysis including 618 participants from 6 trials, there was a significant effect of dairy products on BMD, with effect size of 0.21, 0.36 and 0.37 for lumbar spine, femoral neck and total hip, respectively [50].
Dairy products and fracture
In the same meta-analysis quoted above [43], the risk of any fracture was 44% higher in subjects avoiding any dairy product, like a vegan diet, as compared with a omnivore diet. The 25% higher fracture risk observed in vegetarians did not reach a level of statistical significance. These results suggest that a diet devoid of dairies could be associated with a higher fracture risk.
In the absence of controlled intervention trials with fracture as outcome, one should rely on observational studies, which have sometimes not provided consistent conclusions. In a 32-year follow-up of 123,906 subjects of both sexes, 1 serving of 240 ml of milk was associated with a 8% reduction of hip fracture risk. The reduction amounted to 6% per serving of any dairy products [51]. In two cohorts in Norway, a country with an usual high dietary calcium intake, which included 613,018 and 252,996 person-year, there was no association between hip fracture risk and milk consumption, with hazard ratio varying between 0.97 and 1.02 [52]. Not too far away, in Sweden, milk consumption up to six glasses of milk (200 ml glasses) was associated with a higher risk of hip fracture, but not of all fractures together, in a cohort of 61,433 women followed over 20 years, but not in men 45,339 men over 11 years [53]. Interestingly, in the same study, any serving of fermented dairy products, i.e. 200 g of yoghurt or 20 g of cheese, led to a 10–15% lower hip fracture risk, in women and in men [53]. Several recent meta-analyses have included various cohort or case–control observational studies assessing the relationship between hip fracture risk and dairy products consumption [3, 54–57]. Not only according to the number of studies included, but also according to the subjects origin and the type of dairy products, the results may vary, with differences in hip fracture risk reaching or not a level of statistical significance (Table 7). Overall, a lower hip fracture risk varying between 13 and 32% was found in dairy products consumers in some analyses, particularly with fermented dairy products. Thus, while the association between hip fracture risk and milk consumption is not fully consistent, the inverse relationship with fermented dairy products, particularly yoghurts, is more often reported (Table 7). However, during the menopause transition, fracture risk was not influenced by dairy products, probably in relation with the low number of events [58].
Table 7.
Hip fracture risk in relation with dairy products consumption in recent meta-analyses
| Meta-analyses | Studies | Hip Fracture | |||
|---|---|---|---|---|---|
| Milk | Yoghurt | Cheese | All dairies | ||
| Bian et al. 2018 [54] | Cohorts (10) | 0.91 | 0.75* | 0.68* | 0.87* |
| Case–control (8) | 0.71* | 0.77 | 0.77 | 0.75* | |
| Matia-Martin et al. 2019 [55] | Cohorts (5) | 0.91 | 0.87 | 0.80 | 0.87 |
| Malmir et al. 2020 [57] | Cohorts (14) | 0.93 | 0.90 | ||
| Case–control (9) | 0.75* | 0.86 | |||
| Hidayat et al. 2020 [56] | Cohorts (9) | 0.86 | 0.78* | 0.85 | |
| In USA | 0.75* | ||||
| In Scandinavian countries | 1.00 | ||||
| Ong et al. 2020§ [65] | Cohorts (3) | 0.76* | 0.89 |
*Bold value indicates statistically significant
§Fermented products only
Fermented dairy products (for review see [59])
The highest number of cells and particles in the human body are located in the digestive tract, as commensal organisms, collectively called gut microbiota [60]. The latter varies with age, living conditions, diet and some drugs, including calcium and vitamin D. Agents produced and released by the gut microbiota influence intestinal endocrine function, epithelial permeability and the immune system. Variations in gut microbiota composition and function are implicated in a large series of various disorders such as intestinal, tumor, metabolic, auto-immune, inflammatory and neurologic diseases. Gut microbiota is also modified by prebiotics, which are non-digestible food components, such as fibers or oligosaccharides, which are fermented in the large intestine. Galacto-oligosaccharides contained in mother milk help to child growth and to the development of the immune system [61]. Probiotics are organisms which, when ingested in sufficient amount, can influence intestinal content metabolisms. In human, one of the sources of probiotics is fermented dairy products, like yoghurts, fermented milk and cheese. One yoghurt serving contains about 10 million bacteriae (Lactobacillus bulgaricus et Streptococcus thermophilus). Dietary calcium could modify gut microbiota by favoring the proliferation of lactobacilli [62].
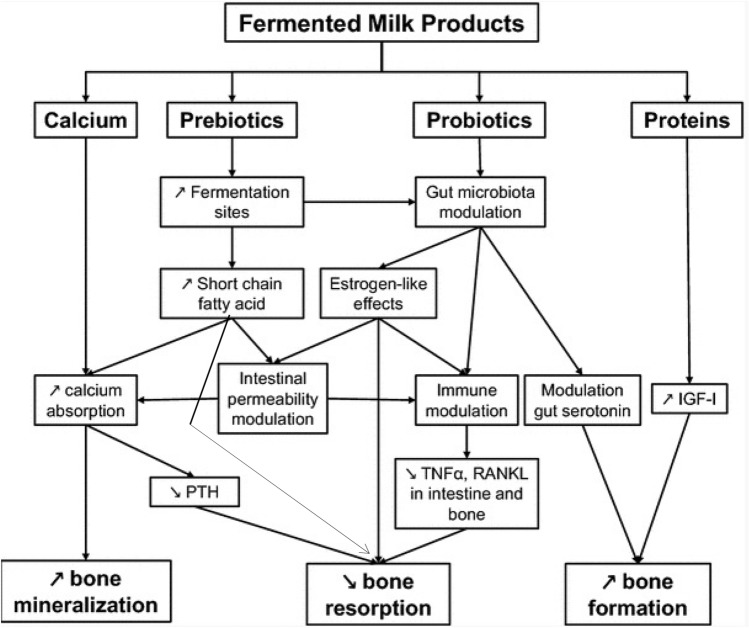
In adults, consumption of fermented dairy products attenuates age-related bone loss [59]. In a cross-sectional study in home dwelling subjects older than 60 years, yoghurts ingestion was associated with better bone mineral mass and muscle function [63]. For one serving of yoghurt, the risk of osteoporosis was 40 and 50% lower in women and men, respectively. In 65-year-old healthy women, peripheral skeleton cortical bone loss was inversely correlated to yoghurt intake frequency [64]. Short-term intervention trials have shown that yoghurt or cheese consumption reduced PTH and biochemical markers of bone resorption, without affecting bone formation markers [59, 65] (Tables 5 and 6). The effect of fermented dairy products on bone metabolism are summarized in Fig. 1. In a meta-analysis of 3 cohorts including 102,819 subjects, yoghurt consumption was associated with a 26% reduction in hip fracture risk [65] (Table 7).
Fig. 1.
Influence of fermented dairy products on bone metabolism. Adapted from [59] with permission from the publisher. Fermented dairy products provide calcium, protein, prebiotics and probiotics, which could favorably influence bone remodeling by acting through different pathways
Tolerance to dairy products and safety
The milk of ruminants contains around 5% lactose, a disaccharide composed of glucose and galactose (Table 2). To be absorbed, lactose has to be hydrolyzed by the enzyme lactase. Individuals homozygous for CC alleles in the lactase gene are not able to digest lactose, and tend therefore to consume less milk as compared with lactase persistent subjects, because of symptoms of lactose intolerance like flatulence, abdominal pain and diarrhea, resulting from the fermentation of undigested lactose in the large intestine. A meta-analysis comparing lactose absorbers to lactase-deficient subjects, as determined by genetic testing or breath hydrogen test, in five case–control studies, has not found a difference in areal BMD [66]. However, when expressed in Z score, i.e., age-adjusted, lumbar spine and total hip displayed lower BMD values in lactase-deficient subjects. Lactase persistence and lactase non persistence did not differ in terms of hip fracture risk [67].
Another cause of intolerance to cow milk is the presence of A1 beta-casein, produced by some cow breeds, particularly those of European origin, instead of A2 beta-casein, found in Asian or African cattle [68]. Both beta-casein proteins, which represent 30% of total protein content in cow milk, differ by only one nucleotide changing the codon in position 67 of the 209 amino acid protein, with a change of histidine to proline. A1 but not A2 beta-casein digestion produces beta-casomorphin-7, which activates µ-opioid receptors located along the gastro-intestinal tract, and may account for an increase in gastro-intestinal transit time and abdominal pain. In a randomized, double-blind, cross-over trial, A1 beta-casein was associated with worst post-dairy abdominal discomfort, higher concentrations of inflammation-related biomarkers and lower levels of short chain fatty acids, as compared to A2 beta-casein [69]. Digestion of A2 beta-casein is easier. Beta-casomorphin-7 may be hydrolyzed by bacteria present in yoghurts during the fermenting process [70]. Whether casomorphins are implicated in the modified brain activity in regions that controls the processing of emotion and sensation in healthy women with a 4-week intake of fermented dairy products, is not known [71].
Dairy is a major source of saturated fatty acids. Previous meta-analyses, on which many dietary guidelines are based, have considered saturated fatty acids as associated with increased risk of cardiovascular diseases [72]. However, recent studies have indicated that all saturated fatty acids do not exert the adverse effect on cardiovascular disease as previously believed, and that the various saturated fatty acids exert very different biological effects, which are dependent on the food matrix [3, 73, 74]. For instance, cheese could be expected to increase cardiovascular disease risk because of its high content of saturated fatty acids and sodium, but observational studies indicate in fact a reduction in blood pressure and lower risk of cardiovascular disease and stroke with increased cheese consumption [3, 75]. Dairy fat eaten in the form of cheese affected blood lipids differently from when the same constituents were ingested in different matrices [76]. Total cholesterol levels were even lower when all fat nutrients were eaten in cheese matrix. An updated meta-analysis including 29 cohort studies found inverse associations between total intake of fermented milk products, including soured products, yoghurt and cheese, with mortality and risk of cardiovascular disease (relative risk for both: 0.98) [77]. Neither plain milk nor low-fat milk were related to any increased risk of cardiovascular events. Risk of cardiovascular disease decreased by 2% per 10 g of cheese consumed per day. In a large cohort study of individuals aged 35–70 years enrolled from 21 countries in 5 continents, higher intake of total dairy (> 2 servings per day compared with no intake) was associated with a lower risk of total mortality, non-cardiovascular mortality, cardiovascular mortality and stroke. Higher intake (> 1 serving vs no intake) of milk and yoghurt was associated with lower risk of a composite outcome of the above events [78]. Finally, intake of whole fat yoghurt or cheese in place of milk was associated with a lower risk of myocardial infarction during a median 15.9-year follow-up [79].
Conclusions
Among various nutrients, calcium and protein are of major importance for bone health. These nutrients are provided by dairy products. The latter contribute to meet nutrients needs. Intervention studies have shown beneficial effects of dairy products on bone mass accrual in children and adolescents, and on bone turnover in young and older adults. In observational studies, dairy products, particularly those fermented appear to be associated with a lower hip fracture risk.
Funding
Open Access funding provided by Université de Genève. No funding.
Declarations
Conflict of interest
Fees for lecture or consultation from Abiogen, Amgen, Danone, Echolight, European Milk Forum, Nestlé, ObsEva, Pfizer Consumer Health, Radius Health and Theramex.
Ethical approval
No need for a review of published data.
Statement of human and animal rights
This article does not contain any studies with human participants or animals not previously published. All procedures performed in the previously published by the author were in accordance with ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
No need for a review of published data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rizzoli R, Bianchi ML, Garabedian M, et al. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari S, Rizzoli R, Slosman D, et al. Familial resemblance for bone mineral mass is expressed before puberty. J Clin Endocrinol Metab. 1998;83:358–361. doi: 10.1210/jcem.83.2.4583. [DOI] [PubMed] [Google Scholar]
- 3.Geiker NRW, Mølgaard C, Iuliano S, et al. Impact of whole dairy matrix on musculoskeletal health and aging-current knowledge and research gaps. Osteoporos Int. 2020;31:601–615. doi: 10.1007/s00198-019-05229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholz-Ahrens KE, Ahrens F, Barth CA. Nutritional and health attributes of milk and milk imitations. Eur J Nutr. 2020;59:19–34. doi: 10.1007/s00394-019-01936-3. [DOI] [PubMed] [Google Scholar]
- 5.Gorissen SHM, Crombag JJR, Senden JMG, et al. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50:1685–1695. doi: 10.1007/s00726-018-2640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salque M, Bogucki PI, Pyzel J, et al. Earliest evidence for cheese making in the sixth millennium BC in northern Europe. Nature. 2013;493:522–525. doi: 10.1038/nature11698. [DOI] [PubMed] [Google Scholar]
- 7.McClure SB, Magill C, Podrug E, et al. Fatty acid specific δ13C values reveal earliest Mediterranean cheese production 7,200 years ago. PLOS ONE. 2018;13:e0202807. doi: 10.1371/journal.pone.0202807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleasdale M, Richter KK, Janzen A, et al. Ancient proteins provide evidence of dairy consumption in eastern Africa. Nat Commun. 2021;12:632. doi: 10.1038/s41467-020-20682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winzenberg T, Shaw K, Fryer J, et al. Effects of calcium supplementation on bone density in healthy children: meta-analysis of randomised controlled trials. BMJ. 2006;333:775–778. doi: 10.1136/bmj.38950.561400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevalley T, Rizzoli R, Hans D, et al. Interaction between calcium intake and menarcheal age on bone mass gain: an eight-year follow-up study from prepuberty to postmenarche. J Clin Endocrinol Metab. 2005;90:44–51. doi: 10.1210/jc.2004-1043. [DOI] [PubMed] [Google Scholar]
- 11.Arnold A, Dennison E, Kovacs CS, et al. Hormonal regulation of biomineralization. Nat Rev Endocrinol. 2021;17:261–275. doi: 10.1038/s41574-021-00477-2. [DOI] [PubMed] [Google Scholar]
- 12.Marcucci G, Masi L, Ferrarì S, et al. Phosphate wasting disorders in adults. Osteoporos Int. 2018;29:2369–2387. doi: 10.1007/s00198-018-4618-2. [DOI] [PubMed] [Google Scholar]
- 13.Tucker KL, Morita K, Qiao N, et al. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: the Framingham osteoporosis study. Am J Clin Nutr. 2006;84:936–942. doi: 10.1093/ajcn/84.4.936. [DOI] [PubMed] [Google Scholar]
- 14.Alexy U, Remer T, Manz F, et al. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr. 2005;82:1107–1114. doi: 10.1093/ajcn/82.5.1107. [DOI] [PubMed] [Google Scholar]
- 15.Dawson-Hughes B, Harris SS, Rasmussen HM, et al. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos Int. 2007;18:955–961. doi: 10.1007/s00198-006-0320-x. [DOI] [PubMed] [Google Scholar]
- 16.Rizzoli R, Bonjour JP. Physiology of calcium and phosphate homeostases. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of bone and cartilage metabolism. 2. San Diego: Academic Press; 2006. pp. 345–360. [Google Scholar]
- 17.Rizzoli R. Dairy products, yogurts, and bone health. Am J Clin Nutr. 2014;99:1256s–1262s. doi: 10.3945/ajcn.113.073056. [DOI] [PubMed] [Google Scholar]
- 18.Kouvelioti R, Josse AR, Klentrou P. Effects of dairy consumption on body composition and bone properties in youth: a systematic review. Curr Dev Nutr. 2017;1:e001214. doi: 10.3945/cdn.117.001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulding A, Rockell JE, Black RE, et al. Children who avoid drinking cow's milk are at increased risk for prepubertal bone fractures. J Am Diet Assoc. 2004;104:250–253. doi: 10.1016/j.jada.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Konstantynowicz J, Nguyen TV, Kaczmarski M, et al. Fractures during growth: potential role of a milk-free diet. Osteoporos Int. 2007;18:1601–1607. doi: 10.1007/s00198-007-0397-x. [DOI] [PubMed] [Google Scholar]
- 21.Ganpule A, Yajnik CS, Fall CH, et al. Bone mass in Indian children–relationships to maternal nutritional status and diet during pregnancy: the Pune maternal nutrition study. J Clin Endocrinol Metab. 2006;91:2994–3001. doi: 10.1210/jc.2005-2431. [DOI] [PubMed] [Google Scholar]
- 22.Orr JB. Influence of amount of milk consumption on the rate of growth of school children. Br Med J. 1928;1:140–141. doi: 10.1136/bmj.1.3499.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leighton G, Clark ML. Milk consumption and the growth of school children: second preliminary report on tests to the scottish board of health. Br Med J. 1929;1:23–25. doi: 10.1136/bmj.1.3548.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cadogan J, Eastell R, Jones N, et al. Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ. 1997;315:1255–1260. doi: 10.1136/bmj.315.7118.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matkovic V, Landoll JD, Badenhop-Stevens NE, et al. Nutrition influences skeletal development from childhood to adulthood: a study of hip, spine, and forearm in adolescent females. J Nutr. 2004;134:701s–705s. doi: 10.1093/jn/134.3.701S. [DOI] [PubMed] [Google Scholar]
- 26.Cheng S, Lyytikainen A, Kroger H, et al. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10–12-y-old girls: a 2-y randomized trial. Am J Clin Nutr. 2005;82:1115–1126. doi: 10.1093/ajcn/82.5.1115. [DOI] [PubMed] [Google Scholar]
- 27.Zhu K, Du X, Cowell CT, et al. Effects of school milk intervention on cortical bone accretion and indicators relevant to bone metabolism in Chinese girls aged 10–12 y in Beijing. Am J Clin Nutr. 2005;81:1168–1175. doi: 10.1093/ajcn/81.5.1168. [DOI] [PubMed] [Google Scholar]
- 28.Kang K, Sotunde OF, Weiler HA. Effects of milk and milk-product consumption on growth among children and adolescents aged 6–18 years: a meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:250–261. doi: 10.1093/advances/nmy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States Dept of Health and Human Services DoA (2010) Dietary Guidelines Advisory Committee Dietary
- 30.Rozenberg S, Body JJ, Bruyère O, et al. Effects of dairy products consumption on health: benefits and beliefs–a commentary from the Belgian bone club and the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases. Calcif Tissue Int. 2016;98:1–17. doi: 10.1007/s00223-015-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez CJ, Beaupré GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 32.Murphy S, Khaw KT, May H, et al. Milk consumption and bone mineral density in middle aged and elderly women. BMJ. 1994;308:939–941. doi: 10.1136/bmj.308.6934.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Opotowsky AR, Bilezikian JP. Racial differences in the effect of early milk consumption on peak and postmenopausal bone mineral density. J Bone Miner Res. 2003;18:1978–1988. doi: 10.1359/jbmr.2003.18.11.1978. [DOI] [PubMed] [Google Scholar]
- 34.Kalkwarf HJ, Khoury JC, Lanphear BP. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am J Clin Nutr. 2003;77:257–265. doi: 10.1093/ajcn/77.1.257. [DOI] [PubMed] [Google Scholar]
- 35.Kalkwarf HJ. Childhood and adolescent milk intake and adult bone health. In: Burckhardt P, Dawson-Hughes B, Heaney R, editors. Nutritional aspects of osteoporosis 2006. Amsterdam: Elsevier B.V; 2007. pp. 39–49. [Google Scholar]
- 36.Feskanich D, Bischoff-Ferrari HA, Frazier AL, et al. Milk consumption during teenage years and risk of hip fractures in older adults. JAMA Pediatr. 2014;168:54–60. doi: 10.1001/jamapediatrics.2013.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey NC, Biver E, Kaufman JM, et al. The role of calcium supplementation in healthy musculoskeletal ageing: an expert consensus meeting of the european society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO) and the international foundation for osteoporosis (IOF) Osteoporos Int. 2017;28:447–462. doi: 10.1007/s00198-016-3773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao P, Bennett D, Mafham M, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw Open. 2019;2:e1917789. doi: 10.1001/jamanetworkopen.2019.17789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung M, Tang AM, Fu Z, et al. Calcium intake and cardiovascular disease risk: an updated systematic review and meta-analysis. Ann Intern Med. 2016;165:856–866. doi: 10.7326/M16-1165. [DOI] [PubMed] [Google Scholar]
- 40.Rizzoli R, Biver E, Bonjour JP, et al. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European society for clinical and economical aspects of osteopororosis, osteoarthritis, and musculoskeletal diseases and by the international osteoporosis foundation. Osteoporos Int. 2018;29:1933–1948. doi: 10.1007/s00198-018-4534-5. [DOI] [PubMed] [Google Scholar]
- 41.Papageorgiou M, Merminod F, Chevalley T, et al. Associations between age-related changes in bone microstructure and strength and dietary acid load in a cohort of community-dwelling, healthy men and postmenopausal women. Am J Clin Nutr. 2020;112:1120–1131. doi: 10.1093/ajcn/nqaa191. [DOI] [PubMed] [Google Scholar]
- 42.Josse AR, Ludwa IA, Kouvelioti R, et al. Dairy product intake decreases bone resorption following a 12-week diet and exercise intervention in overweight and obese adolescent girls. Pediatr Res. 2020;88:910. doi: 10.1038/s41390-020-0834-5. [DOI] [PubMed] [Google Scholar]
- 43.Iguacel I, Miguel-Berges ML, Gómez-Bruton A, et al. Veganism, vegetarianism, bone mineral density, and fracture risk: a systematic review and meta-analysis. Nutr Rev. 2019;77:1–18. doi: 10.1093/nutrit/nuy045. [DOI] [PubMed] [Google Scholar]
- 44.Fabiani R, Naldini G, Chiavarini M. Dietary patterns in relation to low bone mineral density and fracture risk: a systematic review and meta-analysis. Adv Nutr. 2019;10:219–236. doi: 10.1093/advances/nmy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. 2002;75:773–779. doi: 10.1093/ajcn/75.4.773. [DOI] [PubMed] [Google Scholar]
- 46.Hallkvist OM, Johansson J, Nordström A, et al. Dairy product intake and bone properties in 70-year-old men and women. Arch Osteoporos. 2018;13:9. doi: 10.1007/s11657-018-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durosier-Izart C, Biver E, Merminod F, et al. Peripheral skeleton bone strength is positively correlated with total and dairy protein intakes in healthy postmenopausal women. Am J Clin Nutr. 2017;105:513–525. doi: 10.3945/ajcn.116.134676. [DOI] [PubMed] [Google Scholar]
- 48.Langsetmo L, Shikany JM, Burghardt AJ, et al. High dairy protein intake is associated with greater bone strength parameters at the distal radius and tibia in older men: a cross-sectional study. Osteoporos Int. 2018;29:69–77. doi: 10.1007/s00198-017-4261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gui JC, Brašić JR, Liu XD, et al. Bone mineral density in postmenopausal Chinese women treated with calcium fortification in soymilk and cow's milk. Osteoporos Int. 2012;23:1563–1570. doi: 10.1007/s00198-012-1895-z. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, Zhan Y, Chen Y, et al. Effects of dairy products on bone mineral density in healthy postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Arch Osteoporos. 2020;15:48. doi: 10.1007/s11657-020-0694-y. [DOI] [PubMed] [Google Scholar]
- 51.Feskanich D, Meyer HE, Fung TT, et al. Milk and other dairy foods and risk of hip fracture in men and women. Osteoporos Int. 2018;29:385–396. doi: 10.1007/s00198-017-4285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holvik K, Meyer HE, Laake I, et al. Milk drinking and risk of hip fracture. The Norwegian epidemiologic osteoporosis studies (NOREPOS) Br J Nutr. 2018;121:709. doi: 10.1017/S0007114518003823. [DOI] [PubMed] [Google Scholar]
- 53.Michaëlsson K, Wolk A, Langenskiöld S, et al. Milk intake and risk of mortality and fractures in women and men: cohort studies. Bmj. 2014;349:g6015. doi: 10.1136/bmj.g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bian S, Hu J, Zhang K, et al. Dairy product consumption and risk of hip fracture: a systematic review and meta-analysis. BMC Public Health. 2018;18:165. doi: 10.1186/s12889-018-5041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matía-Martín P, Torrego-Ellacuría M, Larrad-Sainz A, et al. Effects of milk and dairy products on the prevention of osteoporosis and osteoporotic fractures in Europeans and non-hispanic whites from north America: a systematic review and updated meta-analysis. Adv Nutr. 2019;10:S120–s143. doi: 10.1093/advances/nmy097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hidayat K, Du X, Shi BM, et al. Systematic review and meta-analysis of the association between dairy consumption and the risk of hip fracture: critical interpretation of the currently available evidence. Osteoporos Int. 2020;31:1411. doi: 10.1007/s00198-020-05383-3. [DOI] [PubMed] [Google Scholar]
- 57.Malmir H, Larijani B, Esmaillzadeh A. Consumption of milk and dairy products and risk of osteoporosis and hip fracture: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;60:1722–1737. doi: 10.1080/10408398.2019.1590800. [DOI] [PubMed] [Google Scholar]
- 58.Wallace TC, Jun S, Zou P, et al. Dairy intake is not associated with improvements in bone mineral density or risk of fractures across the menopause transition: data from the study of women's health across the nation. Menopause. 2020;27:879. doi: 10.1097/GME.0000000000001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rizzoli R, Biver E. Effects of fermented milk products on bone. Calcif Tissue Int. 2018;102:489–500. doi: 10.1007/s00223-017-0317-9. [DOI] [PubMed] [Google Scholar]
- 60.Rizzoli R. Nutritional influence on bone: role of gut microbiota. Aging Clin Exp Res. 2019;31:743–751. doi: 10.1007/s40520-019-01131-8. [DOI] [PubMed] [Google Scholar]
- 61.Donovan SM, Comstock SS. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann Nutr Metab. 2016;69(Suppl 2):42–51. doi: 10.1159/000452818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes JM, Costa JA, Alfenas RC. Could the beneficial effects of dietary calcium on obesity and diabetes control be mediated by changes in intestinal microbiota and integrity? Br J Nutr. 2015;114:1756–1765. doi: 10.1017/S0007114515003608. [DOI] [PubMed] [Google Scholar]
- 63.Laird E, Molloy AM, McNulty H, et al. Greater yogurt consumption is associated with increased bone mineral density and physical function in older adults. Osteoporos Int. 2017;28:2409–2419. doi: 10.1007/s00198-017-4049-5. [DOI] [PubMed] [Google Scholar]
- 64.Biver E, Durosier-Izart C, Merminod F, et al. Fermented dairy products consumption is associated with attenuated cortical bone loss independently of total calcium, protein, and energy intakes in healthy postmenopausal women. Osteoporos Int. 2018;29:1771–1782. doi: 10.1007/s00198-018-4535-4. [DOI] [PubMed] [Google Scholar]
- 65.Ong AM, Kang K, Weiler HA, et al. Fermented milk products and bone health in postmenopausal women: a systematic review of randomized controlled trials, prospective cohorts, and case-control studies. Adv Nutr. 2020;11:251–265. doi: 10.1093/advances/nmz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Treister-Goltzman Y, Friger M, Peleg R. Does primary lactase deficiency reduce bone mineral density in postmenopausal women? A systematic review and meta-analysis. Osteoporos Int. 2018;29:2399–2407. doi: 10.1007/s00198-018-4635-1. [DOI] [PubMed] [Google Scholar]
- 67.Bergholdt HKM, Larsen MK, Varbo A, et al. Lactase persistence, milk intake, hip fracture and bone mineral density: a study of 97 811 Danish individuals and a meta-analysis. J Intern Med. 2018;284:254–269. doi: 10.1111/joim.12753. [DOI] [PubMed] [Google Scholar]
- 68.Kay SS, Delgado S, Mittal J, et al. Beneficial effects of milk having a2 β-casein protein: myth or reality? J Nutr. 2021;151:1061–1072. doi: 10.1093/jn/nxaa454. [DOI] [PubMed] [Google Scholar]
- 69.Jianqin S, Leiming X, Lu X, et al. Effects of milk containing only A2 beta-casein versus milk containing both A1 and A2 beta-casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows' milk. Nutr J. 2016;15:35. doi: 10.1186/s12937-016-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen DD, Busetti F, Johnson SK, et al. Degradation of β-casomorphins and identification of degradation products during yoghurt processing using liquid chromatography coupled with high resolution mass spectrometry. Food Res Int. 2018;106:98–104. doi: 10.1016/j.foodres.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 71.Tillisch K, Labus J, Kilpatrick L et al (2013) Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144:1394–1401, 1401.e1391–1394 [DOI] [PMC free article] [PubMed]
- 72.de Oliveira-Otto MC, Mozaffarian D, Kromhout D, et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Am J Clin Nutr. 2012;96:397–404. doi: 10.3945/ajcn.112.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 74.de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. Bmj. 2015;351:h3978. doi: 10.1136/bmj.h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pichler G, Amigo N, Tellez-Plaza M, et al. LDL particle size and composition and incident cardiovascular disease in a south-European population: the hortega-liposcale follow-up study. Int J Cardiol. 2018;264:172–178. doi: 10.1016/j.ijcard.2018.03.128. [DOI] [PubMed] [Google Scholar]
- 76.Feeney EL, Barron R, Dible V, et al. Dairy matrix effects: response to consumption of dairy fat differs when eaten within the cheese matrix-a randomized controlled trial. Am J Clin Nutr. 2018;108:667–674. doi: 10.1093/ajcn/nqy146. [DOI] [PubMed] [Google Scholar]
- 77.Guo J, Astrup A, Lovegrove JA, et al. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2017;32:269–287. doi: 10.1007/s10654-017-0243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dehghan M, Mente A, Rangarajan S, et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2018;392:2288–2297. doi: 10.1016/S0140-6736(18)31812-9. [DOI] [PubMed] [Google Scholar]
- 79.Kvist K, Laursen ASD, Overvad K, et al. Substitution of milk with whole-fat yogurt products or cheese is associated with a lower risk of myocardial infarction: the danish diet, cancer and health cohort. J Nutr. 2020;150:1252–1258. doi: 10.1093/jn/nxz337. [DOI] [PubMed] [Google Scholar]
- 80.Sabahelkhier MK, Faten MM, Omer FJ. Comparative determination of biochemical constituents between animals (goat, sheep, cow and camel) milk with human milk. Res J Recent Sci. 2012;1:69–71. [Google Scholar]
- 81.Baker IA, Elwood PC, Hughes J, et al. A randomised controlled trial of the effect of the provision of free school milk on the growth of children. J Epidemiol Community Health. 1980;34:31–34. doi: 10.1136/jech.34.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan GM, Hoffman K, McMurry M. Effects of dairy products on bone and body composition in pubertal girls. J Pediatr. 1995;126:551–556. doi: 10.1016/s0022-3476(95)70348-9. [DOI] [PubMed] [Google Scholar]
- 83.Du X, Zhu K, Trube A, et al. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10–12 years in Beijing. Br J Nutr. 2004;92:159–168. doi: 10.1079/BJN20041118. [DOI] [PubMed] [Google Scholar]
- 84.Lau EM, Lynn H, Chan YH, et al. Benefits of milk powder supplementation on bone accretion in Chinese children. Osteoporos Int. 2004;15:654–658. doi: 10.1007/s00198-004-1593-6. [DOI] [PubMed] [Google Scholar]
- 85.Lu JX, Pan H, Hu XQ, et al. Effects of milk powder intervention on bone mineral density and indicators related to bone metabolism in Chinese adolescents. Osteoporos Int. 2019;30:2231–2239. doi: 10.1007/s00198-019-05105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merrilees MJ, Smart EJ, Gilchrist NL, et al. Effects of diary food supplements on bone mineral density in teenage girls. Eur J Nutr. 2000;39:256–262. doi: 10.1007/s003940070004. [DOI] [PubMed] [Google Scholar]
- 87.Vogel KA, Martin BR, McCabe LD, et al. The effect of dairy intake on bone mass and body composition in early pubertal girls and boys: a randomized controlled trial. Am J Clin Nutr. 2017;105:1214–1229. doi: 10.3945/ajcn.116.140418. [DOI] [PubMed] [Google Scholar]
- 88.Volek JS, Gómez AL, Scheett TP, et al. Increasing fluid milk favorably affects bone mineral density responses to resistance training in adolescent boys. J Am Diet Assoc. 2003;103:1353–1356. doi: 10.1016/s0002-8223(03)01073-3. [DOI] [PubMed] [Google Scholar]
- 89.Zhu K, Greenfield H, Du X, et al. Effects of two years' milk supplementation on size-corrected bone mineral density of Chinese girls. Asia Pac J Clin Nutr. 2008;17(Suppl 1):147–150. [PubMed] [Google Scholar]
- 90.Baran D, Sorensen A, Grimes J, et al. Dietary modification with dairy products for preventing vertebral bone loss in premenopausal women: a three-year prospective study. J Clin Endocrinol Metab. 1990;70:264–270. doi: 10.1210/jcem-70-1-264. [DOI] [PubMed] [Google Scholar]
- 91.Bonjour JP, Brandolini-Bunlon M, Boirie Y, et al. Inhibition of bone turnover by milk intake in postmenopausal women. Br J Nutr. 2008;100:866–874. doi: 10.1017/S0007114508937429. [DOI] [PubMed] [Google Scholar]
- 92.Bonjour JP, Benoit V, Pourchaire O, et al. Inhibition of markers of bone resorption by consumption of vitamin D and calcium-fortified soft plain cheese by institutionalised elderly women. Br J Nutr. 2009;102:962–966. doi: 10.1017/S0007114509371743. [DOI] [PubMed] [Google Scholar]
- 93.Bonjour JP, Benoit V, Rousseau B, et al. Consumption of vitamin D-and calcium-fortified soft white cheese lowers the biochemical marker of bone resorption TRAP 5b in postmenopausal women at moderate risk of osteoporosis fracture. J Nutr. 2012;142:698–703. doi: 10.3945/jn.111.153692. [DOI] [PubMed] [Google Scholar]
- 94.Chee WS, Suriah AR, Chan SP, et al. The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporos Int. 2003;14:828–834. doi: 10.1007/s00198-003-1448-6. [DOI] [PubMed] [Google Scholar]
- 95.Ting GP, Tan SY, Chan SP, et al. A follow-up study on the effects of a milk supplement on bone mineral density of postmenopausal Chinese women in Malaysia. J Nutr Health Aging. 2007;11:69–73. [PubMed] [Google Scholar]
- 96.Chen Y, Zhang Q, Wang Y, et al. Estimating the causal effect of milk powder supplementation on bone mineral density: a randomized controlled trial with both non-compliance and loss to follow-up. Eur J Clin Nutr. 2015;69:824–830. doi: 10.1038/ejcn.2015.3. [DOI] [PubMed] [Google Scholar]
- 97.Daly RM, Brown M, Bass S, et al. Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2-year randomized controlled trial. J Bone Miner Res. 2006;21:397–405. doi: 10.1359/JBMR.051206. [DOI] [PubMed] [Google Scholar]
- 98.Daly RM, Petrass N, Bass S, et al. The skeletal benefits of calcium- and vitamin D3-fortified milk are sustained in older men after withdrawal of supplementation: an 18-mo follow-up study. Am J Clin Nutr. 2008;87:771–777. doi: 10.1093/ajcn/87.3.771. [DOI] [PubMed] [Google Scholar]
- 99.Green JH, Booth C, Bunning R. Impact of supplementary high calcium milk with additional magnesium on parathyroid hormone and biochemical markers of bone turnover in postmenopausal women. Asia Pac J Clin Nutr. 2002;11:268–273. doi: 10.1046/j.1440-6047.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- 100.Heaney RP, McCarron DA, Dawson-Hughes B, et al. Dietary changes favorably affect bone remodeling in older adults. J Am Diet Assoc. 1999;99:1228–1233. doi: 10.1016/S0002-8223(99)00302-8. [DOI] [PubMed] [Google Scholar]
- 101.Heaney RP, Rafferty K, Dowell MS. Effect of yogurt on a urinary marker of bone resorption in postmenopausal women. J Am Diet Assoc. 2002;102:1672–1674. doi: 10.1016/s0002-8223(02)90356-1. [DOI] [PubMed] [Google Scholar]
- 102.Josse AR, Tang JE, Tarnopolsky MA, et al. Body composition and strength changes in women with milk and resistance exercise. Med Sci Sports Exerc. 2010;42:1122–1130. doi: 10.1249/MSS.0b013e3181c854f6. [DOI] [PubMed] [Google Scholar]
- 103.Josse AR, Atkinson SA, Tarnopolsky MA, et al. Diets higher in dairy foods and dietary protein support bone health during diet- and exercise-induced weight loss in overweight and obese premenopausal women. J Clin Endocrinol Metab. 2012;97:251–260. doi: 10.1210/jc.2011-2165. [DOI] [PubMed] [Google Scholar]
- 104.Kristensen M, Jensen M, Kudsk J, et al. Short-term effects on bone turnover of replacing milk with cola beverages: a 10-day interventional study in young men. Osteoporos Int. 2005;16:1803–1808. doi: 10.1007/s00198-005-1935-z. [DOI] [PubMed] [Google Scholar]
- 105.Kruger MC, Booth CL, Coad J, et al. Effect of calcium fortified milk supplementation with or without vitamin K on biochemical markers of bone turnover in premenopausal women. Nutrition. 2006;22:1120–1128. doi: 10.1016/j.nut.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 106.Kruger MC, Schollum LM, Kuhn-Sherlock B, et al. The effect of a fortified milk drink on vitamin D status and bone turnover in post-menopausal women from South East Asia. Bone. 2010;46:759–767. doi: 10.1016/j.bone.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 107.Kruger MC, Ha PC, Todd JM, et al. High-calcium, vitamin D fortified milk is effective in improving bone turnover markers and vitamin D status in healthy postmenopausal Chinese women. Eur J Clin Nutr. 2012;66:856–861. doi: 10.1038/ejcn.2012.54. [DOI] [PubMed] [Google Scholar]
- 108.Kukuljan S, Nowson CA, Bass SL, et al. Effects of a multi-component exercise program and calcium-vitamin-D3-fortified milk on bone mineral density in older men: a randomised controlled trial. Osteoporos Int. 2009;20:1241–1251. doi: 10.1007/s00198-008-0776-y. [DOI] [PubMed] [Google Scholar]
- 109.Lau EM, Woo J, Lam V, et al. Milk supplementation of the diet of postmenopausal Chinese women on a low calcium intake retards bone loss. J Bone Miner Res. 2001;16:1704–1709. doi: 10.1359/jbmr.2001.16.9.1704. [DOI] [PubMed] [Google Scholar]
- 110.Lau EM, Lynn H, Chan YH, et al. Milk supplementation prevents bone loss in postmenopausal Chinese women over 3 years. Bone. 2002;31:536–540. doi: 10.1016/s8756-3282(02)00853-0. [DOI] [PubMed] [Google Scholar]
- 111.Liu Z, Qiu L, Chen YM, et al. Effect of milk and calcium supplementation on bone density and bone turnover in pregnant Chinese women: a randomized controlled trail. Arch Gynecol Obstet. 2011;283:205–211. doi: 10.1007/s00404-009-1345-0. [DOI] [PubMed] [Google Scholar]
- 112.Manios Y, Moschonis G, Trovas G, et al. Changes in biochemical indexes of bone metabolism and bone mineral density after a 12-mo dietary intervention program: the postmenopausal health study. Am J Clin Nutr. 2007;86:781–789. doi: 10.1093/ajcn/86.3.781. [DOI] [PubMed] [Google Scholar]
- 113.Moschonis G, Katsaroli I, Lyritis GP, et al. The effects of a 30-month dietary intervention on bone mineral density: the postmenopausal health study. Br J Nutr. 2010;104:100–107. doi: 10.1017/S000711451000019X. [DOI] [PubMed] [Google Scholar]
- 114.Moschonis G, Kanellakis S, Papaioannou N, et al. Possible site-specific effect of an intervention combining nutrition and lifestyle counselling with consumption of fortified dairy products on bone mass: the postmenopausal health study II. J Bone Miner Metab. 2011;29:501–506. doi: 10.1007/s00774-010-0256-2. [DOI] [PubMed] [Google Scholar]
- 115.Prince R, Devine A, Dick I, et al. The effects of calcium supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. J Bone Miner Res. 1995;10:1068–1075. doi: 10.1002/jbmr.5650100711. [DOI] [PubMed] [Google Scholar]
- 116.Recker RR, Heaney RP. The effect of milk supplements on calcium metabolism, bone metabolism and calcium balance. Am J Clin Nutr. 1985;41:254–263. doi: 10.1093/ajcn/41.2.254. [DOI] [PubMed] [Google Scholar]
- 117.Rosado JL, Garcia OP, Ronquillo D, et al. Intake of milk with added micronutrients increases the effectiveness of an energy-restricted diet to reduce body weight: a randomized controlled clinical trial in Mexican women. J Am Diet Assoc. 2011;111:1507–1516. doi: 10.1016/j.jada.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 118.Storm D, Eslin R, Porter ES, et al. Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 1998;83:3817–3825. doi: 10.1210/jcem.83.11.5289. [DOI] [PubMed] [Google Scholar]
- 119.Tenta R, Moschonis G, Koutsilieris M, et al. Calcium and vitamin D supplementation through fortified dairy products counterbalances seasonal variations of bone metabolism indices: the postmenopausal health study. Eur J Nutr. 2011;50:341–349. doi: 10.1007/s00394-010-0142-7. [DOI] [PubMed] [Google Scholar]
- 120.Thorpe MP, Jacobson EH, Layman DK, et al. A diet high in protein, dairy, and calcium attenuates bone loss over twelve months of weight loss and maintenance relative to a conventional high-carbohydrate diet in adults. J Nutr. 2008;138:1096–1100. doi: 10.1093/jn/138.6.1096. [DOI] [PubMed] [Google Scholar]
- 121.Tu MY, Chen HL, Tung YT, et al. Short-term effects of kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLOS ONE. 2015;10:e0144231. doi: 10.1371/journal.pone.0144231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Woo J, Lau W, Xu L, et al. Milk supplementation and bone health in young adult chinese women. J Womens Health (Larchmt) 2007;16:692–702. doi: 10.1089/jwh.2006.0222. [DOI] [PubMed] [Google Scholar]