Abstract
Background:
Oral cladribine has been approved for the treatment of relapsing multiple sclerosis (MS) yet real-world evidence regarding its effectiveness and safety remains scarce.
Objective:
To evaluate efficacy and safety outcomes of MS patients following induction of cladribine.
Methods:
We evaluated our prospective cohort of cladribine-treated MS patients from two tertiary centres in Germany. Relapses, disability worsening and occurrence of new or enlarging T2-hyperintense magnetic resonance imaging (MRI) lesions were assessed as well as lymphocyte counts and herpes virus infections.
Results:
Among 270 patients treated with cladribine, we observed a profound reduction of both relapses and new or enlarging MRI lesions. Treatment appeared more efficacious, especially in patients without previous therapy or following platform substances. Patients switching from natalizumab were prone to re-emerging disease activity. Among patients following dimethyl fumarate pre-treatment, severe lymphopenia was common and associated with increased rates of herpes virus manifestations.
Conclusion:
Overall, we observed an efficacy and safety profile of cladribine consistent with data from the phase 3 clinical trial. However, patients switching from natalizumab experienced suboptimal disease control beyond rebound activity following cessation of natalizumab. Furthermore, dimethyl fumarate pre-treatment was associated with a profound risk of developing severe lymphopenia and subsequent herpes virus infections.
Keywords: Cladribine, treatment response, real-world evidence
Introduction
Cladribine is a synthetic purine analogue and induces lymphocyte depletion by accumulation of intracellular chloro-deoxyadenosine triphosphate resulting in apoptosis of B and T lymphocytes.1,2
Initially established for the treatment of haematologic malignancies such as hairy cell leukaemia, histiocytosis or acute myeloid leukaemia, cladribine was first approved for the treatment of active relapsing multiple sclerosis (RMS) in 2017 in Europe and subsequently in 2019 in the United States after having been positively evaluated in placebo-controlled randomized clinical trials.3,4
As so-called immune reconstitution therapy, 5 it offers the advantage of only few treatment days per year yet providing durably efficacy in absence of treatment. It is usually administered in two courses of 5 days being 4 weeks apart in year 1 and 2, respectively.
Although finally proven safe within the highly controlled pivotal trial and its extension, little is known about the safety and efficacy of cladribine under diversified real-world conditions, especially when used following immunomodulatory treatment different from injectable substances.
Thus, we here analysed our large dual-centre cohort of cladribine-treated RMS patients with a focus on the impact of previous immunomodulatory treatments on safety and efficacy outcomes of cladribine treatment.
Methods
Adult patients diagnosed with RMS according to 2017 revised McDonald criteria 6 who underwent treatment with cladribine were longitudinally evaluated at our two tertiary referral centres from November 2017 to March 2021. Patients with a minimum follow-up of 6 months were included. Exclusion criteria were any progressive form of MS, inability to undergo magnetic resonance imaging (MRI) examination, presence of other autoimmune disorders than MS and a history of malignant disorders or previous exposition to cytostatic substances. Patients having delayed their second course of cladribine treatment beyond month 13 due to the ongoing coronavirus disease 2019 (COVID-19) pandemic were only evaluated until then.
Administration of cladribine was performed according to national and international guidelines as well as to the most recent summary of product characteristics. Following treatment induction, patients were evaluated every 3 months involving standardized neurologic examination. During the second peak of COVID-19 pandemic in Germany since autumn 2020, the follow-up interval in clinically stable patients was eventually expanded to 6 months. Relapses were evaluated either at unscheduled visits or at least within the next scheduled visit. Baseline MRI was performed no earlier than 3 months prior to treatment induction. Follow-up MRI was conducted every 6 months. MRI data were independently evaluated regarding abundance of new or enlarging T2-hyperintense lesions since gadolinium was not administered regularly during follow-up (but was administered at baseline MRI in all cases). ‘No evidence of disease activity-3’ (NEDA3) status was assumed in patients without clinical relapses, 6-month confirmed worsening of disability and new or enlarging T2-hyperintense MRI lesions.
Epidemiological data at baseline were evaluated using descriptive statistics. Kaplan–Meier plots were generated for efficacy outcomes, and multivariate analysis was conducted using the Cox proportional hazards model. ‘Sex’, ‘age at baseline’, ‘last previous disease-modifying treatment (DMT)’, ‘baseline expanded disability status scale (EDSS) score’, ‘baseline annualized relapse rate’ (refers to the patient’s relapse rate during the last 12 months prior to cladribine induction) and ‘disease duration since MS onset’ were used as covariates in an enter method. Lymphocyte levels were transformed into lymphopenia severity grades according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Binary logistic regression for evaluation of meaningful covariates of development of severe lymphopenia was conducted using an enter method with the abovementioned covariates excluding ‘annualized relapse rate at baseline’.
Worsening of disability was considered clinically relevant if two independent clinical assessments 6 months apart indicated an increase of the EDSS as follows: +1.5 points (baseline = 0.0), +1.0 point (baseline = 1.0–4.0) and +0.5 points (baseline ⩾4.5). To determine progression to secondary progressive multiple sclerosis (SPMS), patient datasets were analysed according to Lorscheider criteria. 7 In addition, the fraction of patients having undergone confirmed worsening of disability in absence of a clinical relapse during the last 3 months (‘progession independent of relapse activity’ (PIRA)) was evaluated.
Further analyses were carried out using Fisher’s exact test for categorical variables and Kruskal–Wallis test including Dunn’s post-test for continuous variables where appropriate. A value of p < 0.05 was considered significant. All analyses were considered exploratory. Statistical analysis was conducted using SPSS Statistics 27 (IBM, NY, USA). All patients gave consent to data conduction, and ethical approval was given by local authorities (Ethical board of the Medical Council Westphalia-Lippe and the University of Münster; 2020-459-f-S).
Data availability statement
Anonymized patient data will be shared with qualified investigators upon reasonable request.
Results
Patients
During observation period, 313 patients were treated at both centres. Of those, 43 patients were excluded: 39 had a follow-up shorter than 6 months, 2 patients retrospectively fulfilled Lorscheider criteria for SPMS conversion at baseline and 1 patient was exposed to mitoxantrone earlier in their disease course.
In total, 270 patients were included. Notably, we lost two patients to follow-up and hence only evaluated their datasets until month 12. Ninety-seven (36%) patients were treatment-naïve, whereas the other patients had previously been treated with different DMTs. Among those pre-treated patients, 74 patients received one previous DMT (27%), 49 received two previous DMTs (18%) and 50 patients received three or more previous DMTs (19%).
Our patients had a median age of 39 years and a median disease course of 6 years since RMS onset, and the median EDSS score was 2.0 indicating a low disability burden. None of the patients fulfilled the Lorscheider criteria for SPMS conversion at baseline. Table 1 shows baseline epidemiological data of our cohort. Median follow-up duration was 25 months. Follow-up duration did not differ significantly between patient subgroups stratified according to the last previous DMT (p = 0.253). A total of 234 patients passed month 12 (and hence received the dose of 3.5 mg/kg) and 142 patients passed month 24. In total, data are representative of 6496 patient-months. The recommended treatment interval between first and second course was usually maintained with the exception of 17 patients who received their second course 14–17 months following induction as a consequence of the COVID-19 pandemic. Notably, 11 patients decided to postpone their second course in the hopes of upcoming vaccination at the time of data analysis.
Table 1.
Baseline characteristics of our cohort.
| Whole cohort | Naïve | ‘Platform’ | DMF | FTY | NTZ | DAC | ||
|---|---|---|---|---|---|---|---|---|
| IFN/GLAT | TRF | |||||||
| Patients | 270 | 97 | 59 | 21 | 42 | 18 | 23 | 10 |
| Age, years, median (IQR) | 39 (32–44) | 38 (29–44.5) | 39 (32–44) | 37 (29–48) | 40 (34–48) | 37 (31–48) | 40 (35–44) | 43 (37–50) |
| Male sex, no. (%) | 104 (39) | 38 (39) | 19 (32) | 8 (38) | 17 (41) | 8 (44) | 9 (39) | 5 (50) |
| Disease duration, years, median (IQR) | ||||||||
| Since onset | 6 (2–12) | 1 (1–6.5) | 8 (4–13) | 7 (3.5–11) | 5.5 (4–13.5) | 10 (7–16.5) | 8 (6–14) | 14 (10–15) |
| Since diagnosis | 4 (1–9) | 0 (0–1) | 5 (2–9) | 4 (2–9) | 4.5 (2–12) | 7.5 (6–11.5) | 6 (4–9) | 12 (9–15) |
| EDSS at baseline, median (IQR) | 2.0 (1.5–3.0) | 2.0 (1.0–3.0) | 2.0 (1.5–2.5) | 2.0 (1.5–3.0) | 2.0 (1.0–3.0) | 3.0 (2.0–3.5) | 2.5 (1.5–3.0) | 3.5 (2.0–4.0) |
| ARR at baseline, median (IQR) | 1 (0–2) | 1 (1–2) | 1 (0–2) | 1 (1–2) | 1 (0–1) | 1 (0–1.5) | 0 (0–1) | 0 (0–1) |
| Number of previous DMT, median (IQR) | 1 (0–2) | x | 1 (1–2) | 1 (1–2) | 2 (1–3) | 3 (2–4) | 2 (1–3) | 3 (2–4) |
| Number of last previous DMT, no. (%) | ||||||||
| 0 | 97 (36) | 97 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 | 74 (27) | 0 (0) | 39 (66) | 11 (52) | 16 (38) | 0 (0) | 7 (30) | 1 (10) |
| 2 | 49 (18) | 0 (0) | 11(19) | 6 (29) | 14 (33) | 8 (44) | 7 (30) | 3 (30) |
| ⩾3 | 50 (19) | 0 (0) | 9 (15) | 4 (19) | 12 (29) | 10 (56) | 9 (40) | 6 (60) |
| Washout duration of last previous DMT, days, median (IQR) | 39 (13.5–72) | x | 11 (3–23) | 65 (44–92) | 32.5 (13–56) | 82 (56–117) | 66 (49–81) | 115 (75–190) |
| Number of baseline T2 MRI lesions, median (IQR) | 15 (11–20) | 13 (9–18) | 14 (11–18) | 14 (12–17) | 15 (11–19) | 21 (13.5–24) | 25 (19–28) | 20 (13–25) |
IQR: interquartile range; EDSS: expanded disability status scale; ARR: annualized relapse rate; DMT: disease-modifying treatment; IFN: beta-interferon; GLAT: glatiramer acetate; DMF: dimethyl fumarate; TRF: teriflunomide; FTY: fingolimod; NTZ: natalizumab; DAC: daclizumab.
Of 142 patients having passed month 24, 5 patients received additional courses of cladribine (months 24, 25, 28, 34 and 36, respectively) within the observation period due to ongoing disease activity. In addition, three patients were switched to treatment with ocrelizumab due to ongoing disease activity in month 12 instead of undergoing the second course of cladribine.
Clinical efficacy
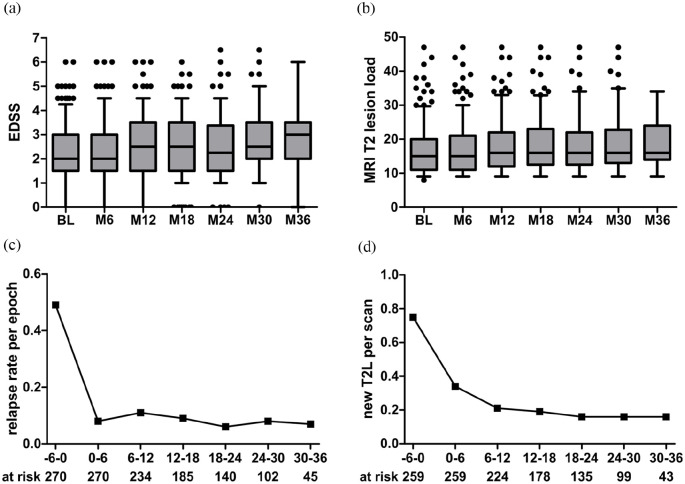
We observed 85 relapses in 69 patients in our cohort following cladribine induction in contrast to 279 relapses in 191 patients in the year prior to induction (132 relapses within 6 months prior to cladribine induction). Median time to first relapse was 9 months and 40 patients had a relapse within the first year of treatment. Sixty-five patients experienced confirmed worsening of disability during the observation period. Conversely, EDSS scores as well as the total cranial T2-hyperintense lesion load remained stable in the majority of patients (Figure 1(a) and (b)). Evaluation of cranial MRI data (1194 follow-up scans available in 259 patients (96%)) showed 218 new or enlarging T2-hyperintense lesions in 104 patients. Overall, the relapse rate per treatment epoch substantially declined compared to the last 6-month epoch prior to induction and similar findings were made for detection of new or enlarging T2-hyperintense MRI lesions per epoch compared to baseline (Figure 1(c) and (d)).
Figure 1.
Efficacy outcomes of our cladribine cohort. (a) Expanded disability status scale (EDSS) scores at baseline and within follow-up. Boxes indicate 25%–75% interquartile ranges; lines indicate the median. Whiskers include 5%–95% of patients. (b) Total T2-hyperintense MRI lesion load at baseline and within follow-up. (c) Development of mean relapse rates per 6-month treatment epoch. (d) Development of MRI T2-hyperintense lesion load calculated as new T2-hyperintense lesion per scan conducted within end of the indicated treatment epoch. Numbers at risk are listed below (c) and (d) and also refer to (a) and (b), respectively.
MRI: magnetic resonance imaging.
Baseline T2 lesion load was equally distributed among patients except for patients having previously been treated with natalizumab (p < 0.001 for all comparisons to other DMT). All patients coming from natalizumab (n = 23) were switched due to increased risk for development of progressive multifocal leukoencephalopathy while having been clinically stable before. Thus, only four patients following natalizumab exposed new MRI lesions in their baseline MRI (all lesions were contrast-enhancing attributing their development to natalizumab cessation). One of these patients developed a clinical relapse during the washout period. Among other patients who stopped their previous immunomodulatory treatment, three further relapses were identified within the washout period. All these relapses resolved quickly following the administration of intravenous methylprednisolone, and none of the patients experienced confirmed worsening of disability because of these relapses. Of those, two relapses were observed in patients having stopped treatment with daclizumab due to market withdrawal, whereas one relapse was documented in a patient who stopped fingolimod treatment due to ongoing disease activity (indicated by multiple contrast-enhancing lesions in their last previous MRI).
We next aimed for investigation of potential risk factors for suboptimal disease control by cladribine treatment and stratified patients according to their last previous DMT:
Since no relevant differences in both baseline and follow-up parameters were observed between patients previously exposed to beta-interferons, glatiramer acetate or teriflunomide, these patients were combined to a group termed ‘platform’ treatment.
We excluded the subgroup of previously daclizumab-treated patients since patient numbers were low and this substance is no longer approved for treatment of RMS patients. However, we did not observe notable differences in efficacy or – eventually more important – safety outcomes in patients coming from daclizumab (data not shown).
Generally, follow-up duration was equally distributed among treatment groups (Supplemental Figure S1).
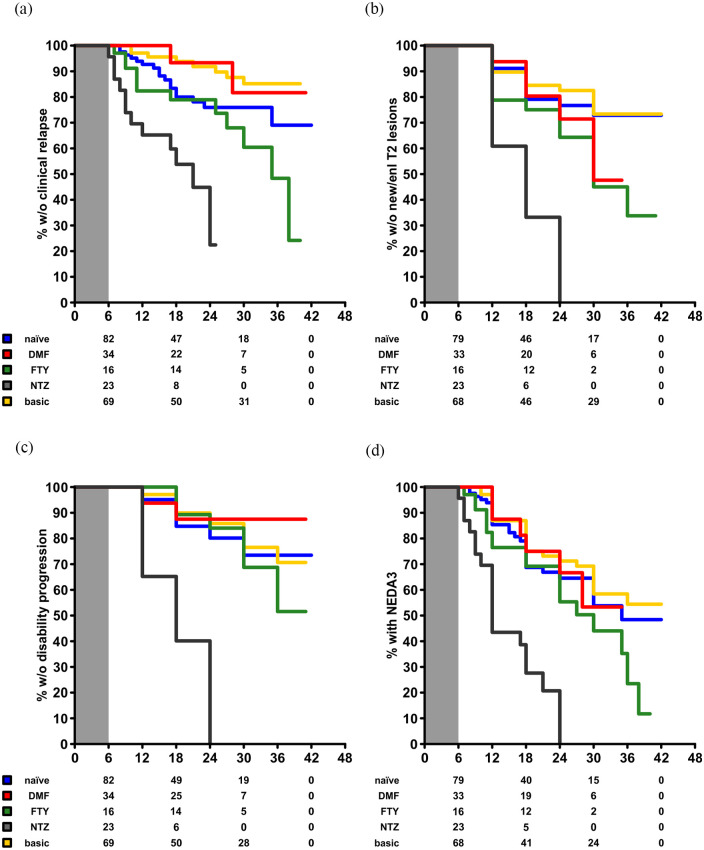
Univariate analysis using the Kaplan–Meier method suggested an impact of the previous immunotherapy (Supplemental Figure S2). However, since we aimed to rule out disease activity predominantly driven by rebound following cessation of the last previous DMT, we performed re-baselining to month 6 parameters following cladribine induction (Figure 2).
Figure 2.
Efficacy outcomes of our cohort stratified according to the last previous DMT using the Kaplan–Meier method. Data are re-baselined towards month 6. Numbers at risk are displayed below the respective graphs. (a) Proportion of patients without a clinical relapse over time. (b) Proportion of patients without confirmed worsening of disability over time. (c) Proportion of patients without new or enlarging T2-hyperintense MRI lesions. (d) Proportion of patients with persistent NEDA3 status over time.
DMF: dimethyl fumarate; FTY: fingolimod; NTZ: natalizumab; NEDA3: no evidence of disease activity-3; MRI: magnetic resonance imaging; DMT: disease-modifying treatment.
Here, patients following natalizumab appeared to be substantially prone to clinical and paraclinical disease activity following induction of cladribine. Accordingly, 18 of 23 patients exhibited disease activity following cladribine induction. Of those, 12 experienced this already within 6 months following treatment induction, which of course could have also been driven by rebound activity following natalizumab cessation.
Multivariate regression analyses were performed using the Cox-proportional hazards model following re-baselining of patient data to month 6. A first model confirmed natalizumab pre-treatment as a relevant risk factor for relapses following cladribine induction (Table 2A). Adjusted hazard ratio (HR) was 4.771 (95% confidence interval (CI): 2.074–10.972; p < 0.001) against natalizumab (reference: naïve patients). Notably, male patients were less prone to relapses (adjusted HR: 0.473 (95% CI: 0.251–0.889; p = 0.020)).
Table 2.
Cox proportional hazards models using the outcome parameters depicted in Figure 1 as dependent variables.
| HR | 95% CI | p value | |
|---|---|---|---|
| A (time to first clinical relapse) | |||
| Last previous DMT (naïve = ref.) | |||
| Platform (IFN/GLAT/TRF) | 0.588 | 0.161–1.338 | 0.436 |
| Dimethyl fumarate | 1.825 | 0.833–3.997 | 0.133 |
| Fingolimod | 0.476 | 0.105–2.155 | 0.335 |
| Natalizumab | 4.771 | 2.074–10.972 | <0.001 |
| Male vs. female sex (ref.) | 0.473 | 0.251–0.889 | 0.020 |
| Age at baseline (years) | 0.999 | 0.965–1.034 | 0.961 |
| MS duration since onset (years) | 0.998 | 0.953–1.045 | 0.919 |
| ARR at baseline | 1.042 | 0.741–1.464 | 0.815 |
| EDSS at baseline (<3.0 = ref.) | 0.870 | 0.417–1.816 | 0.710 |
| B (time to first confirmed worsening of disability) | |||
| Last previous DMT (naïve = ref.) | |||
| Platform (IFN/GLAT/TRF) | 1.075 | 0.484–2.386 | 0.859 |
| Dimethyl fumarate | 1.293 | 0.503–3.320 | 0.594 |
| Fingolimod | 0.725 | 0.153–3.432 | 0.685 |
| Natalizumab | 8.582 | 3.583–20.555 | <0.001 |
| Male vs. female sex (ref.) | 1.009 | 0.571–1.781 | 0.976 |
| Age at baseline (years) | 0.996 | 0.963–1.031 | 0.824 |
| MS duration since onset (years) | 0.940 | 0.819–1.064 | 0.129 |
| ARR at baseline | 0.864 | 0.655–1.168 | 0.233 |
| EDSS at baseline (<3.0 = ref.) | 1.085 | 0.539–2.186 | 0.819 |
| C (time to first new/enlarging T2 MRI lesion) | |||
| Last previous DMT (naïve = ref.) | |||
| Platform (IFN/GLAT/TRF) | 0.939 | 0.455–1.939 | 0.866 |
| Dimethyl fumarate | 2.011 | 0.933–4.331 | 0.074 |
| Fingolimod | 1.432 | 0.489–4.189 | 0.512 |
| Natalizumab | 5.168 | 2.406–11.102 | <0.001 |
| Male vs. female sex (ref.) | 0.809 | 0.487–1.344 | 0.413 |
| Age at baseline (years) | 0.987 | 0.958–1.016 | 0.369 |
| MS duration since onset (years) | 1.009 | 0.968–1.050 | 0.680 |
| ARR at baseline | 0.928 | 0.684–1.258 | 0.629 |
| EDSS at baseline (<3.0 = ref.) | 0.836 | 0.442–1.578 | 0.580 |
| D (time to first loss of NEDA3) | |||
| Last previous DMT (naïve = ref.) | |||
| Platform (IFN/GLAT/TRF) | 0.889 | 0.508–1.554 | 0.679 |
| Dimethyl fumarate | 1.655 | 0.885–3.093 | 0.114 |
| Fingolimod | 1.069 | 0.423–2.699 | 0.888 |
| Natalizumab | 5.162 | 2.646–10.070 | <0.001 |
| Male vs. female sex (ref.) | 0.720 | 0.468–1.108 | 0.135 |
| Age at baseline (years) | 0.987 | 0.963–1.011 | 0.287 |
| MS duration since onset (years) | 0.982 | 0.947–1.018 | 0.314 |
| ARR at baseline | 0.898 | 0.693–1.163 | 0.415 |
| EDSS at baseline (<3.0 = ref.) | 1.281 | 0.781–2.101 | 0.326 |
HR: hazard ratio; CI: confidence interval; DMT: disease-modifying treatment; IFN: beta-interferon; GLAT: glatiramer acetate; TRF: teriflunomide; MS: multiple sclerosis; ARR: annualized relapse rate; EDSS: expanded disability status scale; MRI: magnetic resonance imaging; NEDA3: no evidence of disease activity-3.
A: Proportion of patients without a clinical relapse over time. B: Proportion of patients without confirmed worsening of disability over time. C: Proportion of patients without new or enlarging T2-hyperintense MRI lesions. D: Proportion of patients without loss of NEDA3 over time. Bold values represent significant covariates.
Previous exposition to natalizumab was confirmed as a risk factor in a model using ‘time to confirmed worsening of disability’ as a dependent variable with an adjusted HR of 8.582 (95% CI: 3.583–20.555; p < 0.001)) with none of the further covariates being selected (Table 2B).
Regarding abundance of new or enlarging T2-hyperintense MRI lesions following cladribine induction, natalizumab was again identified as a relevant risk factor for development of new or enlarging T2-lesions with an adjusted HR of 5.168 (95% CI: 2.406–11.102; p < 0.001; Table 2C).
Consequently, patients switching from natalizumab to cladribine were also more prone to lose their status of NEDA3 with an adjusted HR of 5.162 (95% CI: 2.646–10.070; p < 0.001; Table 2D).
Progression independent of relapse activity (PIRA) was evaluated in 21 patients. Of those, two patients fulfilled Lorscheider criteria for SPMS progression. Among patients having experienced PIRA, no significant difference among last previously administered DMT became visible (p = 0.572).
Lymphopenia and herpes virus infections
Complete longitudinal data on blood lymphocyte levels were available in 226 of 243 (93%) patients. Baseline lymphocyte counts were >1200/mm3 in all patients prior to first treatment course and >800/mm3 prior to re-exposition. Datasets were censored beyond month 24 since the number of available datasets decreased substantially afterwards. Patients in whom the second course of cladribine was delayed due to COVID-19 pandemic were also excluded from this analysis (17 patients).
Overall, our patients largely reflected the well-known pattern of lymphocyte kinetics following cladribine exposition with two peaks of lymphopenia in months 3 and 14, respectively.
Stratified according to CTCAE v5.0, 2 patients (0.7%) were spared from this phenomenon, 216 patients (80.0%) developed grade I–II lymphopenia, whereas 48 patients (17.8%) suffered from grade III and 3 patients (1.1%) from grade IV lymphopenia.
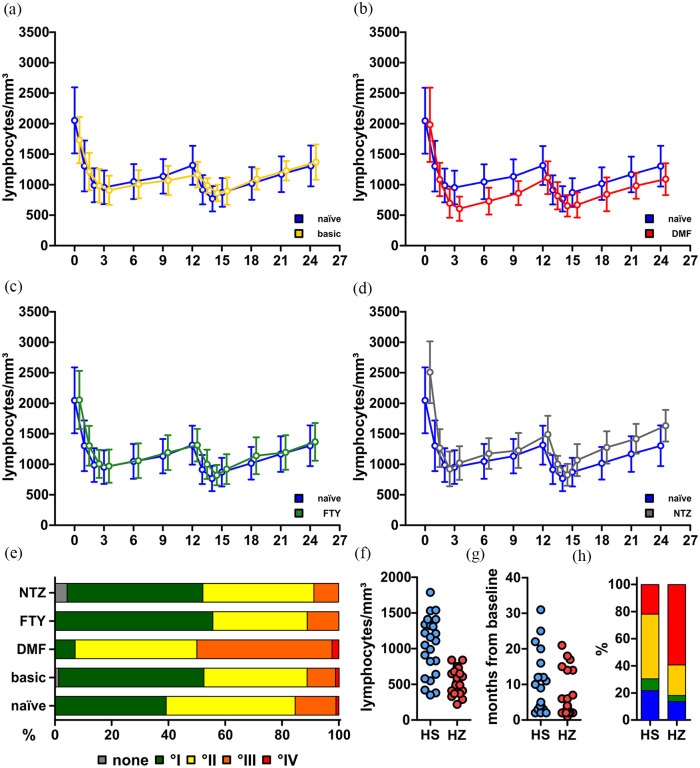
Compared to previously naïve patients, individuals having previously received DMT experienced slightly pronounced decreases of lymphocyte counts. However, this did not lead to an increase of the respective CTCAE severity grade in the majority of patients. Patients following glatiramer acetate, beta-interferons or teriflunomide again showed similar trends and were again merged to one group (Figure 3(a)–(c)).
Figure 3.
Lymphocyte levels and safety outcomes of our cohort. (a–d) Absolute lymphocyte counts over time since baseline in different treatment groups compared to naïve patients. (a) Naïve (n = 73) vs. platform (IFN/GLAT/TRF; n = 69); (b) naïve vs. DMF (n = 35); (c) naïve vs. FTY (n = 17); (d) naïve vs. NTZ (n = 23). Data are expressed as mean ± standard deviance. Data are censored at month 24. (e) Proportions of patients with the respective lymphopenia severity grade according to the CTCAE at their nadir. (f) Absolute lymphocyte counts at disease manifestation in patients with herpes simplex (HS) and herpes zoster (HZ). (g) Treatment months since baseline at disease manifestation in patients with HS and HZ. (h) Patients with HS and HZ according to their last previous DMT (blue: naïve; green: FTY; yellow: IFN/TRF/GLAT; red: DMF).
IFN: beta-interferon; GLAT: glatiramer acetate; TRF: teriflunomide; DMF: dimethyl fumarate; FTY: fingolimod; NTZ: natalizumab; CTCAE: Common Terminology Criteria for Adverse Events; DMT: disease-modifying treatment.
Contrasting this, patients following dimethyl fumarate (DMF) were substantially prone to development of severe lymphopenia already in year 1, although their baseline lymphocyte counts were not lower compared to other treatment groups (DMF: median 1930 (interquartile range (IQR): 1540–2270) vs. other: median 2000 (IQR: 1650–2390); p = 0.2695; Figure 3(d)). Even more surprisingly, baseline lymphocyte counts between previously DMF-exposed patients who developed grade III–IV lymphopenia were not lower compared to DMF-exposed patients without this phenomenon (I–II: median 1970 (IQR: 1590–2325) vs. III–IV: median 1850 (IQR: 1475–2210); p = 0.3651). In total, 21 patients coming from DMF (50%) were affected with grade III–IV compared to 29 patients with a different last previous DMT (13%) (Figure 3(e)).
Nine patients (21%) required a delay of cladribine re-exposition in month 12 (range: 2- to 8-month delay) due to ongoing lymphopenia compared to one previously beta-interferon-exposed and one naïve patient (4- and 7-month delay).
Multivariate binary logistic regression confirmed DMF pre-treatment as a risk factor for development of grade III–IV lymphopenia with an adjusted odds ratio of 5.037 (95% CI: 2.108–12.034; p < 0.001). Of note, patients with an EDSS score above 3.0 at cladribine induction were also more likely to experience severe lymphopenia (adjusted OR: 2.761 (95% CI: 1.255–6.075; p = 0.007)). None of the further covariates were selected (Table 3).
Table 3.
Binary logistic regression model with ‘development of grade III/IV lymphopenia’ as a dependent variable.
| OR | 95% CI | p value | |
|---|---|---|---|
| Last previous DMT (naïve = ref.) | |||
| Platform (IFN/GLAT/TRF) | 0.621 | 0.244–1.578 | 0.317 |
| Dimethyl fumarate | 5.037 | 2.108–12.034 | <0.001 |
| Fingolimod | 0.500 | 0.098–2.547 | 0.404 |
| Natalizumab | 0.406 | 0.082–2.016 | 0.270 |
| Male vs. female sex (ref.) | 0.508 | 0.238–1.082 | 0.079 |
| Age at baseline (years) | 1.009 | 0.970–1.050 | 0.653 |
| MS duration since onset (years) | 1.032 | 0.983–1.084 | 0.209 |
| EDSS at baseline (<3.0 = ref.) | 2.761 | 1.255–6.075 | 0.007 |
DMT: disease modifying treatment; IFN: beta-interferon; GLAT: glatiramer acetate; TRF: teriflunomide; EDSS: expanded disability status scale; OR: odds ratio; CI: confidence interval; MS: multiple sclerosis.
Bold values indicate significant covariates.
We also evaluated the occurrence of herpes infections in our cohort and identified 33 patients who suffered thereof at least once. Of those, 23 patients suffered from herpes simplex infections, whereas 22 developed herpes zoster manifestations (two cases of cranial nerve involvement with one case of zoster ophthalmicus and one case of zoster oticus were noted). Whereas herpes simplex infections usually resolved following local treatment (apart from three cases that received intravenous acyclovir due to symptom persistence and concomitant lymphopenia), all herpes zoster patients received intravenous acyclovir treatment. Despite this, nine cases of post-herpetic neuralgia were observed and this involves the patient with previous zoster ophthalmicus who continues to suffer from trigeminal neuropathy. A transient Ramsay Hunt syndrome in the patient with zoster oticus resolved completely within 2 months. No case of herpes virus-associated encephalitis was observed.
Notably, all patients presented with sufficient anti-varicella-zoster virus titres at baseline. Lymphopenia was present in all cases of herpes zoster, and in 11 patients, lymphocyte counts were <500/mm3 at zoster manifestation. Herpes simplex manifested also in 13 patients with lymphocyte counts >1000/mm3 (Figure 3(f)). Remarkably, herpes infections mostly occurred in year 1 (34 of 45 patients, Figure 3(g)), although 14 patients experienced further episodes of herpes simplex manifestation in year 2. There were no cases of recurrent zoster manifestation. Since lymphopenia appears as major risk factor for development of zoster manifestation, it was not surprising to see the majority of patients coming from the previously DMF-exposed group, whereas most herpes simplex cases were observed in patients following platform treatment (as this was simply the largest subgroup; Figure 3(h)).
Unfortunately, data on conduction of oral acyclovir prophylaxis within lymphopenia are only incomplete in our cohort (although there is documentation of such prescription in 21 of 50 patients). Therefore, no assessment of efficacy of this measure can be deducted from our cohort.
Discussion
Here, we present a large longitudinal real-world dataset on 243 RMS patients following cladribine treatment from two tertiary centres. Overall, our cohort closely resembled those in the pivotal CLARITY trial as median age at baseline, sex distribution and the proportion of previously treatment-naïve patients are fairly comparable. However, disease duration (mean: 7.9 vs. 8.9 years among the CLARITY study population) and median EDSS score at baseline (mean: 2.3 vs. 2.9) were lower in our cohort. 4 Our data underline that cladribine treatment results in a profound reduction of relapse rates per treatment epoch as well as abundance of new or enlarging T2-hyperintense MRI lesions.
Since the amount of DMT approved for RMS has tremendously increased since completion of the clinical development programme, the exact position of cladribine within the therapeutic armamentarium is of interest. Two previous studies suggested that cladribine was more efficacious than beta-interferons, comparable to fingolimod and less efficacious to natalizumab.8,9 Generally, our data corroborate these findings. Patients coming from platform therapies and previously naïve patients experienced sufficient control of disease activity in the majority of cases. Patients following fingolimod pre-treatment experienced predominantly paraclinical disease activity following treatment switch yet most patients experienced disease stability after having passed month 6. However, patients following natalizumab pre-treatment were not only prone to natalizumab cessation-related disease reactivation but also experienced disease activity throughout the whole follow-up time contrasting data from previous case series. 10 Disease reactivation following natalizumab is a well-known phenomenon and is observed in patients switching to fingolimod 11 but can be controlled by subsequent use of high-efficacy treatment including alemtuzumab or rituximab.12,13
We were unable to demonstrate superiority or inferiority of cladribine or fingolimod to each other since disease activity in this group mostly restricted to the early period following switch.
We also evaluated lymphocyte counts in our cohort and were again able to reproduce kinetics known from previous clinical trials.4,14 Yet, relevant lymphopenia was more common compared to data from clinical trials. Furthermore, we found that patients with DMF as last previous DMT were susceptible to development of severe lymphopenia. DMF exerts profound and long-lasting changes of the lymphocyte repertoire and mainly targets T cells.15,16 Certain risk factors for development of lymphopenia among DMF-treated patients are described involving increased age and low baseline lymphocyte counts.17,18 Among our patients, only few patients developed this phenomenon within DMF treatment and baseline lymphocyte counts were normal prior to cladribine induction. We can only speculate about the synergistic effect of previous DMF exposure and cladribine induction on lymphocyte counts. A previous hypothesis was that circulating T cells following DMF exposure represent DMF-insensitive cells as they were not more susceptible to induction of apoptosis than untreated cells in vitro. 19 Our clinical observations however contradict this hypothesis since pronounced lymphopenia in year 1 compared to year 2 indeed indicates prolonged susceptibility of lymphocytes. We also observed several relapses in cladribine patients previously treated with DMF, even in patients with lymphopenia. However, relapses during DMF-related lymphopenia have been observed before. 20
Notably, we did observe neither prolonged lymphopenia nor increased relapse rates compared to naïve patients following teriflunomide as last previous DMT, despite the known property to interfere with T cell proliferation via alteration of T cell metabolic properties. 21
Switching from DMF to cladribine seems to be problematic and requires a closer clinical monitoring since lymphopenia affected the majority of patients and subsequent zoster manifestations were substantially more common than was expected from previous studies. 14 The question arises whether patients should definitely be immunized with the newly available herpes zoster vaccine before switching from DMF to cladribine. 22
Our study faces some limitations. These include of course the non-controlled real-world setting and unknown existence of confounders in our patient subgroups. In addition, we can currently finally comment neither on long-term outcomes nor on the proportion of patients requiring additional treatment courses. Nonetheless, this cohort represents a high number of patient years considering the time passed since approval of cladribine. Furthermore, follow-up density is high including thorough follow-up of MRI and lymphocyte count data.
Taken together, the efficacy and safety profile of cladribine appears consistent with previously published data. However, lymphopenia and subsequent herpes virus infections appear more abundant than has been suggested from the clinical development programme. Our data furthermore indicate that DMF might represent a risk factor for development of lymphopenia, and therefore, a decision towards cladribine as escalation treatment should be weighted carefully in those patients.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_13524585211012227 for Effectiveness and safety of cladribine in MS: Real-world experience from two tertiary centres by Steffen Pfeuffer, Leoni Rolfes, Jana Hackert, Konstanze Kleinschnitz, Tobias Ruck, Heinz Wiendl, Luisa Klotz, Christoph Kleinschnitz, Sven G Meuth and Refik Pul in Multiple Sclerosis Journal
Footnotes
Authors’ Note: All authors have read and agreed to the submitted version of the manuscript. This manuscript is not submitted to or under revision at another journal. The submitting author hereby declares that he takes responsibility for conduction of the study and analysis of the data and that he had full access to all study data. The submitting author furthermore declares that there are no competing interests concerning these data and that the authors have all rights to publish the data. The submitted manuscript does not contain data that have been published in any other journal. The authors have no related articles under submission.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.P. received travel grants from Sanofi Genzyme and Merck Serono; lecturing honoraria from Sanofi Genzyme, Mylan Healthcare and Biogen; and research support from Diamed, Merck Serono and the German Multiple Sclerosis Society Northrhine-Westphalia. L.R. received travel grants from Merck Serono and Sanofi Genzyme. J.H. received travel grants and honoraria for lecturing from Celgene, Merck, Novartis, Roche and Sanofi Genzyme. K.K. declares no conflicts of interest T.R. received travel grants and financial research support from Genzyme and Novartis and received honoraria for lecturing from Roche, Merck, Genzyme, Biogen and Teva. H.W. received compensation for serving on Scientific Advisory Boards/Steering Committees for Bayer Healthcare, Biogen Idec, Sanofi Genzyme, Merck Serono and Novartis. He received speaker honoraria and travel support from Bayer Vital GmbH, Bayer Schering AG, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, Genzyme, Merck Serono, Omniamed, Novartis and Sanofi-Aventis. He received compensation as a consultant from Biogen Idec, Merck Serono, Novartis, Roche and Sanofi Genzyme. He also received research support from Bayer Healthcare, Bayer Vital, Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, Sanofi US and Teva. L.K. received compensation for serving on Scientific Advisory Boards for Genzyme, Janssen, Novartis and Roche. She received speaker honoraria and travel support from Biogen, Genzyme, Merck Serono, Novartis, Roche and Teva. She receives research support from the German Ministry for Education and Research, the German Research Foundation, the IZKF Münster, IMF Münster, Biogen, Novartis and Merck Serono. C.K. received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus, Merck Serono, Desitin, Sanofi Genzyme, Biogen, Teva, Bayer, Novartis, Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, Daiichi Sankyo, Siemens, Eisai, Biotronik, Roche, Stago, Ever Pharma, CSL Behring, Mylan, MedDay Pharmaceuticals, Celegene, Amgen and Stada. S.G.M. received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Centre for Clinical Studies (IZKF) Muenster, German Foundation Neurology and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche and Teva. R.P. received honoraria for lecturing and travel expenses for attending meetings from Alexion, Bayer Health Care, Biogen, Merck Serono, Mylan, Novartis, Roche, Sanofi Genzyme and Teva and has received research funding from Novartis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was conducted outside of third-party funding.
Ethical Approval: The local institutional review board (IRB) has approved the conduction of this trial. Further details are listed in the ‘Methods’ chapter.
ORCID iDs: Steffen Pfeuffer  https://orcid.org/0000-0001-5171-4845
https://orcid.org/0000-0001-5171-4845
Heinz Wiendl  https://orcid.org/0000-0003-4310-3432
https://orcid.org/0000-0003-4310-3432
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Steffen Pfeuffer, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Münster, Germany.
Leoni Rolfes, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Münster, Germany.
Jana Hackert, Department of Neurology and Center for Translational and Behavioral Neurosciences (C-TNBS), University Medicine Essen, Essen, Germany.
Konstanze Kleinschnitz, Department of Neurology and Center for Translational and Behavioral Neurosciences (C-TNBS), University Medicine Essen, Essen, Germany.
Tobias Ruck, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Münster, Germany/Department of Neurology, Heinrich Heine University Düsseldorf, Düsseldorf, Germany.
Heinz Wiendl, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Münster, Germany.
Luisa Klotz, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Münster, Germany.
Christoph Kleinschnitz, Department of Neurology and Center for Translational and Behavioral Neurosciences (C-TNBS), University Medicine Essen, Essen, Germany.
Sven G Meuth, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Münster, Germany/Department of Neurology, Heinrich Heine University Düsseldorf, Düsseldorf, Germany.
Refik Pul, Department of Neurology and Center for Translational and Behavioral Neurosciences (C-TNBS), University Medicine Essen, Essen, Germany.
References
- 1. Jacobs BM, Ammoscato F, Giovannoni G, et al. Cladribine: Mechanisms and mysteries in multiple sclerosis. J Neurol Neurosurg Psychiatry 2018; 89(12): 1266–1271. [DOI] [PubMed] [Google Scholar]
- 2. Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord 2019; 29: 168–174. [DOI] [PubMed] [Google Scholar]
- 3. Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult Scler 2018; 24(12): 1594–1604. [DOI] [PubMed] [Google Scholar]
- 4. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 5. Lunemann JD, Ruck T, Muraro PA, et al. Immune reconstitution therapies: Concepts for durable remission in multiple sclerosis. Nat Rev Neurol 2020; 16: 56–62. [DOI] [PubMed] [Google Scholar]
- 6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 7. Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis. Brain 2016; 139: 2395–2405. [DOI] [PubMed] [Google Scholar]
- 8. Kalincik T, Jokubaitis V, Spelman T, et al. Cladribine versus fingolimod, natalizumab and interferon beta for multiple sclerosis. Mult Scler 2018; 24: 1617–1626. [DOI] [PubMed] [Google Scholar]
- 9. Signori A, Sacca F, Lanzillo R, et al. Cladribine vs other drugs in MS: Merging randomized trial with real-life data. Neurol Neuroimmunol Neuroinflamm 2020; 7(6): e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohn N, Skripuletz T, Suhs KW, et al. Therapy with cladribine is efficient and safe in patients previously treated with natalizumab. Ther Adv Neurol Disord 2019; 12: 1756286419887596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kappos L, Radue EW, Comi G, et al. Switching from natalizumab to fingolimod: A randomized, placebo-controlled study in RRMS. Neurology 2015; 85: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pfeuffer S, Schmidt R, Straeten FA, et al. Efficacy and safety of alemtuzumab versus fingolimod in RRMS after natalizumab cessation. J Neurol 2019; 266(1): 165–173. [DOI] [PubMed] [Google Scholar]
- 13. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol 2016; 79(6): 950–958. [DOI] [PubMed] [Google Scholar]
- 14. Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis. Mult Scler Relat Disord 2019; 29: 157–167. [DOI] [PubMed] [Google Scholar]
- 15. Gross CC, Schulte-Mecklenbeck A, Klinsing S, et al. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016; 3(1): e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diebold M, Sievers C, Bantug G, et al. Dimethyl fumarate influences innate and adaptive immunity in multiple sclerosis. J Autoimmun 2018; 86: 39–50. [DOI] [PubMed] [Google Scholar]
- 17. Morales FS, Koralnik IJ, Gautam S, et al. Risk factors for lymphopenia in patients with relapsing-remitting multiple sclerosis treated with dimethyl fumarate. J Neurol 2020; 267: 125–131. [DOI] [PubMed] [Google Scholar]
- 18. Longbrake EE, Naismith RT, Parks BJ, et al. Dimethyl fumarate-associated lymphopenia: Risk factors and clinical significance. Mult Scler J Exp Transl Clin 2015; 1: 2055217315596994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghadiri M, Rezk A, Li R, et al. Dimethyl fumarate-induced lymphopenia in MS due to differential T-cell subset apoptosis. Neurol Neuroimmunol Neuroinflamm 2017; 4(3): e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zecca C, Antozzi CG, Torri Clerici V, et al. Severe multiple sclerosis reactivation during prolonged lymphopenia after dimethyl fumarate discontinuation. Acta Neurol Scand 2018; 137(6): 623–625. [DOI] [PubMed] [Google Scholar]
- 21. Klotz L, Eschborn M, Lindner M, et al. Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects. Sci Transl Med 2019; 11: eaao5563. [DOI] [PubMed] [Google Scholar]
- 22. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372: 2087–2096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_13524585211012227 for Effectiveness and safety of cladribine in MS: Real-world experience from two tertiary centres by Steffen Pfeuffer, Leoni Rolfes, Jana Hackert, Konstanze Kleinschnitz, Tobias Ruck, Heinz Wiendl, Luisa Klotz, Christoph Kleinschnitz, Sven G Meuth and Refik Pul in Multiple Sclerosis Journal
Data Availability Statement
Anonymized patient data will be shared with qualified investigators upon reasonable request.