Abstract
This study was done to improve the medicinal properties of Syzygium Aromaticum L by processing S. Aromaticum L. bud essential oil (SABE) to the Nanoemulsion drug delivery system (SABE-NE) and investigating its anti-tumor and apoptotic impacts against the human HT-29 colon cancer cells. Applying the ultra-sonication method and characterization by DLS and FESEM analysis facilitates the nanoemulsification procedure. Human cancer (HT-29) and normal (HFF) cell lines were then evaluated based on the SABE-NE apoptotic and cytotoxic effects. In an in vitro section, flow cytometry method, Cas3 gene profile, AO/PI cell staining, and MTT assays are used to analyze the apoptotic and cytotoxic activities. In further analysis, liver lipid peroxidation and antioxidant genes expression (SOD, CAT, and GPx) investigate alterations in mice organs. As a result, produced 131.2 nm SABE-NE induces apoptosis response and cellular death (Cas3 up-regulation and enhanced SubG1 peaks). Subsequently, the HT-29 cells' viability can reduce significantly, while HFF cells indicate confined cytotoxic impacts. Moreover, in vivo test results on mice livers demonstrate the cytoprotective properties of SABE-NE (reduced lipid peroxidation and increased antioxidant enzymes gene expression and nondetectable cytotoxic impacts). We produced a novel nanoemulsion drug delivery system called SABE-NE, a cell-specific apoptotic inducer. We thus can be utilized as an efficient anti-cancer compound for human colon cancer treatment. However, further supplementary studies are required to verify and approve its cell-specific anti-tumor activity.
Keywords: Anti-cancer, Apoptotic activity, Syzygium aromaticum L. bud essential oil nanoemulsion (SABE-NE)
Introduction
Colon cancer is the third most common malignancy and the fourth leading cause of cancer-associated death worldwide. There are estimated 700,000 deaths from colorectal cancer among approximately 1.4 million patients worldwide (Arnold et al. 2017). Previous studies have used several therapies, such as radiotherapy, surgery, and chemotherapy for colon cancer therapy.
Despite the numerous adverse side effects of chemotherapy on the liver and kidney organs, unfortunately, the method is widely applied as the only tumor-suppressive tool for colon cancer therapy. Based on studies, chemotherapy affects the liver by inducing lipid peroxidation and reducing renal functionality in the kidney (Assayag et al. 2017). Different kinds of bio-compatible phytochemicals have been used considerably as an alternative remedy instead of harmful chemical drugs in cancer therapy (Hosseini and Ghorbani 2015). Due to the ample content of phenolic structures in Syzygium Aromaticum L. (clove) essential oil, it can be used as an edible antioxidant and antibacterial compound (Donsì et al. 2011). Previous approaches have been reported its medicinal role against bacterial and fungal disease, likewise, lung and breast cancers (Banerjee et al. 2006). Another study showed the significant anti-tumor potential of clove essential oil in the in vivo and in vitro model due to bioactive components, like phenylpropanoids and terpenoids (Lesgards et al. 2014). A similar study carried out by Dwivedi et al. illustrated that clove oil (300 μl/ml) exhibited strong cytotoxicity against TE-13 esophageal cancer, MDA-MB-231, and HeLa cervical cancer (Dwivedi et al. 2011). Meanwhile, Chaieb manifested that eugenol and dehydrodieugenol isolated from Syzygium aromaticum L. have been shown to stimulate human cancer cell death (Chaieb et al. 2007). Researchers have successfully applied a variety of anti-cancer compounds and drug delivery systems in cancer therapy by strategies that increase drugs’ efficiency and specificity, such as nanoemulsionalization techniques. Nanoemulsion systems improve the anti-cancer compound’s safety and efficiency by encapsulating toxic or therapeutic agents, making them highly bio-compatible.
These bio-compatible nanodroplets have been utilized to enhance phytocompounds (plants’ essential oil substances) solubility and bioavailability, increasing the efficiency and safety of therapeutic compounds (Rao et al. 2016). Nanoemulsions are 20–200 nm transparent/opaque nanodroplets consisting of polar/nonpolar bioactive compounds and emulsifiers (Solans et al. 2005). Regarding the nanoemulsion formation principles, ultrasonic-based emulsification is a typical high energy-consuming strategy [~ 108–1010 Watt per kilogram (W.kg−1)[. However, high-pressure homogenization and spontaneous emulsification are two distinct methods applied in nanoemulsification (Date et al. 2010). Ultrasonication is a simple, fast, clean, and cost-effective procedure for nanoemulsification (Mahdi Jafari et al. 2006). The emulsions disintegration is usually done in two ways: (1) Erupting oil into the water, which makes interfacial waves in an acoustic field; (2) Suspension agitation by low-energy waves. A generalized principle of nanoemulsion systems formulation is hydrophobic encapsulation in two stages of suspension liquid Dispersed Nonpolar Phase (DNP) and Polar-Continuous Phase (PCP) (Rossi et al. 2006). These two widespread steps are triggered in the pharmaceutical industry and the bio-compatible efficient delivery systems (Jaiswal et al. 2015). Augmentable stability and quick disruption are properties of nanoemulsions that can be programmed to release their constituents around cells (Constantinides et al. 2008). Therefore, they have been used prevalently to deliver therapeutics into cancer cells and induce apoptotic death in lung and breast cancers (Mahato 2017). The apoptotic Cas-3 gene is a well-known marker of apoptosis and is down-regulated in cancer cells. Moreover, several kinds of research support its central role in inducing apoptotic death. In this regard, Syzygium Aromaticum L. (Rutaceae) essential oil nanoemulsion (SABE-NE) is produced to investigate their anti-cancer and cytotoxic effects on human colon cancer cells (HT-29) and to compare their histopathologic effects in the mice liver, kidney, and jejunum tissues.
Material and methods
Chemical material
The nanoemulsification and cell culture material, including the polysorbate 80 and 20, Fetal Bovine Serum (FBS), MTT [3-(4, 5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide], trypsinized DMEM culture medium, polyethylene glycol (PEG), antibiotic, and acridine orange dye were purchased from Merck (Germany). Cancer (HT-29) and normal (HFF) cell lines were prepared from the Pasteur Institute of Iran (Tehran, Iran). All reagents and solvents not mentioned in this paragraph were bought from Sigma (Germany).
SABE oil extraction and purification
The Syzygium Aromaticum L. bud (SAB) was dried and powdered. 100 g of SAB was added in 500 ml double distilled water. The solution was boiled 120 min to collect the volatile oil using the Clevenger apparatus. Following that, CaCl2 was used to dry the extracted oil (Jaradat et al. 2016). SAB essential oil (SABE) was collected and weighed. Finally, the oil phytochemical contents were analyzed using gas chromatography-mass spectrometry (GC–MS).
Preparation of SABE-NE
To prepare CABE-NE, we mixed water (97 g) with CAE oil (3 g). The ratio of essential oil to nonionic surfactant was 1:3 (v/v). The ultrasonication was then applied to homogenate emulsions at 20 kHz ultrasonic frequency and 750 watts for 30 min of sonication (Navaei Shoorvarzi et al. 2020).
Nanoemulsions characterization
The polydispersity index (PDI) and the size of nanodroplets were estimated using the dynamic light scattering method (DLS) (Malvern Instruments Ltd., UK). The mean droplet diameter (Z-average) value was calculated based on the distributions' intensity. A Field Emission Scanning Electron Microscopy (FESEM) was used to define the morphology of SABE-NE. Moreover, the 1/100 diluted samples were prepared to analyze nanoemulsion Zeta potentials which were analyzed by a Zetasizer (nanoparticle SZ-100) at 25 °C. All measurements were performed in triplicates.
Cell culture
The cancerous (HT-29) and normal (HFF) cell lines were cultured at 37 °C in DMEM cell culture media supplemented with FBS (10%), penicillin (100 U/mL), and streptomycin (100 mg/mL).
MTT assay
The cytotoxic potential of SABE-NE on both cancer (HT-29) and normal (HFF) cells was checked in triplicate culture plates for 48 h at different concentrations of SABE-NE (125, 62.5, 32, and 15 µg/ml). The previous medium was removed and renewed with MTT (0.5 mg/mL)-supplemented media. The mediums passed 3 h incubation at 37 °C and were renewed by media containing dimethylsulfoxide (DMSO). The last medium passed 10 min of stirring. In the end, the absorbance at 570 nm was read for all samples utilizing a plate reader spectrophotometer (Navaei Shoorvarzi et al. 2020). The following equation calculated the viability of the cells: Cell viability (%) = (OD sample/OD control) × 100.
Gene expression analyzing
In triplet samples, both cancerous and normal cell lines passed 10 h of treatment at four different concentrations of SABE-NE (125, 62.5, 32, and 15 µg/ml). The RNeasy Mini kit (Qiagen, Hilden, Germany) was then utilized to extract their transcriptome. Their specific cDNA library was synthesized by the Quantitect Reverse Transcription kit (Qiagen, Hilden, Germany). The Cas-3 primer sets were designed for cDNA amplification as the best-known apoptotic gene and GAPDH as a famous control housekeeping gene (Table 1). Finally, we applied an SYBR green PCR Master Mix (Qiagen, Hilden, Germany) to amplify cDNA. We performed a comparative real-time PCR using a Stratagene Mx-3000P real-time thermocycler (Stratagene, La Jolla, CA). Moreover, we performed standard curves to verify the gene amplification process (Navaei Shoorvarzi et al. 2020).
Table 1.
The primer sets characteristics used in this study
| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) | |
|---|---|---|---|
| A549 cell lines | Cas-3 | gctggatgccgtctagagtc | atgtgtggatgatgctgcca |
| GAPDH | gaaggtgaaggtcggagtc | gaagatggtgatgggatttc | |
| Mice tissues | SOD | gagacctgggcaatgtgact | gtttactgcgcaatcccaat |
| CAT | acatggtctgggacttctgg | caagtttttgatgccctggt | |
| GPx | caagtttttgatgccctggt | tcggacgtacttgagggaat | |
| GAPDH | gacttcaacagcaactcccac | tccaccaccctgttgctgta |
GAPDH glyceraldehyde 3-phosphate dehydrogenase, Cas-3 Caspase 3, SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase
Flow cytometry measurement and fluorescent staining (AO/PI)
The cells cultured for 48 h were treated using different concentrations of SABE-NE (15, 32, 62.5, and 125 µg/ml). After washing and mixing the cells with triton X100 (0.2%), Propidium Iodide (PI), and sodium citrate (0.1%), the cells were incubated for 5 min at 37 °C in dark conditions. Finally, the cell cycle status was defined by a FACScan laser flow cytometer (FACSCalibur, Becton Dickinson, USA). The fluorescent staining was performed by mixing the fluorescent dyes (AO/PI) with washed-harvested cells. The cell status comparison was evaluated by a fluorescence microscope.
In vivo study
The in vivo study was designed by employing 15 female Balb C mice (20 ± 4 g) purchased from the BuAli research center of Mashhad, Iran. The animals' maintenance condition was as following instruments: (1) Animals were kept in cages; (2) the maintenance temperature and humidity were at 24 ± 1 °C and 50 ± 10%, respectively; and (3) Animals had 12‐h dark period intervals and standard Ad libitum feeding pellet diet and water. They were divided into three groups (n = 5). To adopt mice with a lab environment, a 7-day gap was considered before the mice's dietary treatment plane. A group receiving free SABE-NE enriched diet was defined as the control group, and the other two groups received two different doses of SABE-NE dietary treatment (10 and 20 mg/kg body weight). The experiment lasts for 30 days. The ethical committee approved all the research protocols used in this experiment of the Azad university of Mashhad, and the laws, norms, and regulations dealing with international animal ethics were followed (22nd September 2019).
Histopathological study, biopsy preparation, and samples staining
To study the histopathological effects of SABE-NE, a portion of the mice's liver, kidney, and Jejunum were sliced and washed twice by NaCl (0.9%) serum. After weighting tissue biopsies, the samples were fixed by formalin solution (10%) and settled into the tubes. The paraffinized samples were then sliced into 5 μm thin layer. Hematoxylin and eosin were utilized to stain the prepared samples as Cardiff et al. have described (Sarker 2005). Finally, an inverted microscope was used to study the morphology of the cells. An expert pathologist interpreted the morphology of the samples.
Antioxidant genes expression pattern of mice liver samples
The expression pattern of liver cell antioxidant genes (SOD, CAT, and GPx) was studied in the mice liver feeding with three different concentrations of SABE-NE (20, 10, and 0 mg/Kg body weight). The mentioned genes were analyzed by designing their specific forward and reverse primer (Table. 1) according to the protocol described in the previous section.
Lipid peroxidation measurements
This protocol was performed based on Beyrami et al. (2020). Malondialdehyde (MDA) reacted with thiobarbituric acid (TBA) as a lipid peroxidation marker to produce a visible reddish color at 532 nm. The intensity of color shows the concentration of MDA.
Statistics
All statistical measurements were calculated by SPSS 21 statistical software. ANOVA analysis was performed, and p values less than 0.001 were defined as meaningful results.
Results
In vitro section
SABE oil properties
The SABE oil extraction analysis shows 5.1 ± 0.26 percent oil content among the bud part. Based on GC–MS analysis, SABE oil has various phytochemical contents, including Eugenol (69.8%), Caryophyllene (9.1%), Eugenyl acetate (5.2%), and Humulene (7.3%), which have been shown to have anti-cancer activity (31–34) (Table 2A, B).
Table 2.
The Syzygium aromaticum L. bud essential oil properties and processing data
| (A) The SABE content | ||
| Plant material | Essential oil content (%) | |
| Syzygium aromaticum L. bud | 5.1 ± 0.26 | |
| (B) The phytochemical composition of SABE | ||
| Peak | Compound | % of total |
| 1 | Eugenol | 69.8 |
| 2 | Caryophyllene | 9.1 |
| 3 | Humulene | 7.3 |
| 4 | Eugenyl acetate | 5.2 |
| (c) The nanoemulsion characteristic of SABE | ||
| Particle size (nm) | Polydispersity Index | Zeta potential (mV) |
| 131.2 | 0.248 | − 42.1 ± 0.68 |
(A) The Syzygium aromaticum L. bud essential oil content; (B) The phytochemical composition of Syzygium aromaticum L. bud essential oil; (C) The nanoemulsion characteristic of Syzygium aromaticum L. bud essential oil
SABE Syzygium aromaticum L. bud essential oil
SABE-NE characterization
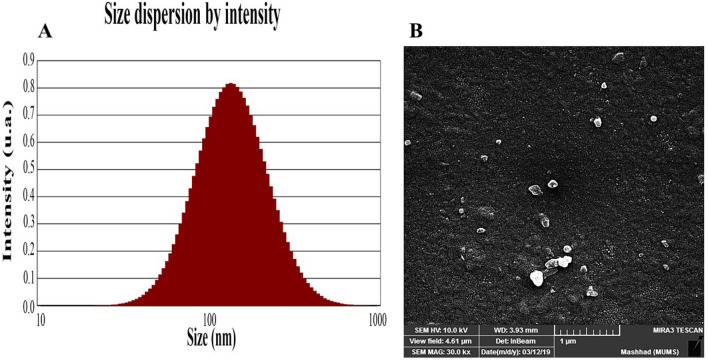
Figure 1A indicates the SABE-NE size dispersion by distributions intensity. Table 2C and Fig. 1B show the DLS indices (DPI and Z-average), stability index (Zeta potential), and morphology features. According to the results, 30 min of ultrasonication with a power of 750 W produces 131.2 nm emulsions with a sufficient value of 0.248 DPI (Table 1C) (35). The emulsion zeta potential was measured at − 42.1 ± 0.68 mV, which means a good degree of stability for SABE-NE regarding B. Salopek et al. (36). Also, the morphology results of FESEM microscopy indicate the pseudo-spherical shape for SABE-NE nanoemulsions (Fig. 1B).
Fig. 1.
Particle size properties. A the size dispersion of Syzygium aromaticum L. bud essential oil nanoemulsion analyzed by DLS (Size dispersion by intensity and volume); B Morphocharacteristic of Syzygium aromaticum L. bud essential oil nanoemulsion analyzed using FESEM. FESEM field emission scanning electron microscopy
Cytotoxic impacts of SABE-NE on HT-29 colon cancer cells
A meaningful dose-dependent correlation among cancer HT-29 cell line and SABE-NE treatment concentrations is observable (Fig. 2) (p value ≤ 0.001). At the same time, there is no significant relationship between normal HFF cells and SABE-NE. The 48-h-IC50 value of SABE-NE was calculated at 74.8 µg/mL. It can be explained that the SABE-NE has a cell-specific cytotoxic effect on HT-29 colon cancer cells.
Fig. 2.
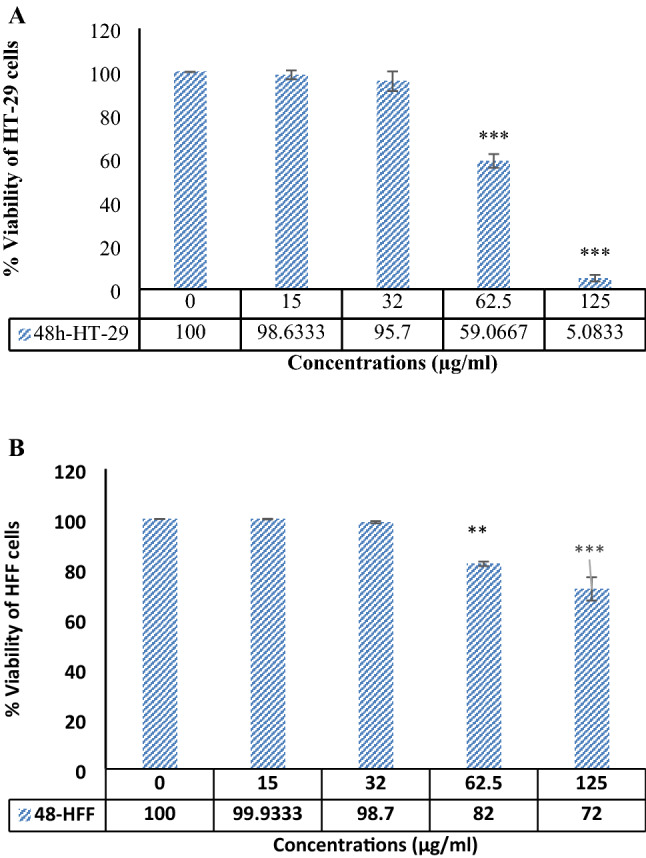
MTT assay data. A and B HFF and HT-29 cell lines viability, respectively. The cells were under different SABE-NE treatment doses. SABE-NE: Syzygium aromaticum L. essential oil nanoemulsion. “***” indicates the p < 0.001
Cas-3 gene expression in HT-29 colon cancer cells
A significant Cas-3 up-regulation following the increasing doses of SABE-NE in treated cells shows a dose-dependent effect on HT-29 cells [Fig. 3 (p < 0.001)]. The results indicate a significant apoptotic activity of SABE-NE in human HT-29 colon cancer cells.
Fig. 3.
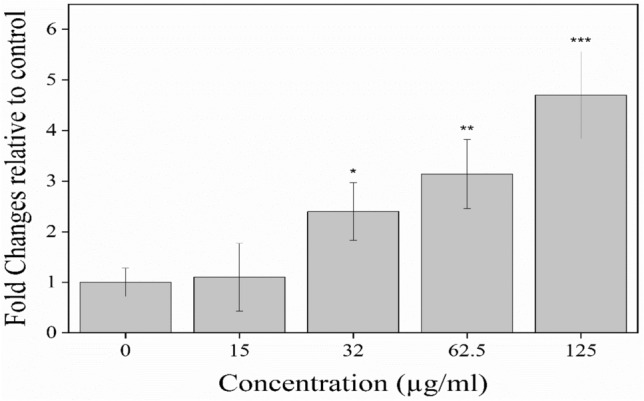
The expression analysis of caspase 3 gene in HT-29 cell line during 10 h incubation with different concentrations of SABE-NE; SABE-NE Syzygium aromaticum L. essential oil nanoemulsion; “***” indicates the p value at less than < 0.001 levels
Flow cytometry data and AO/PI–fluorescent imaging of HT-29 Cells
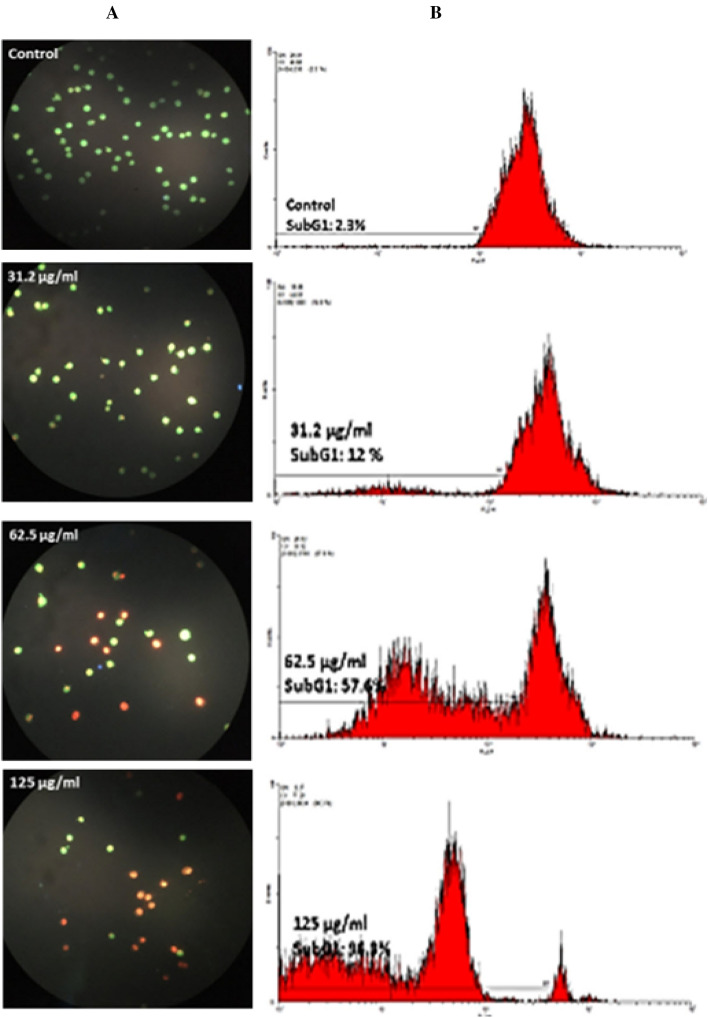
According to the results (Fig. 4), the cellular death in HT-29 cells appears to be a type of apoptosis. There is a significant relationship between the increasing SABE-NE treatment concentrations and Sub G1 peaks (p < 0.001).
Fig. 4.
The HT-29 colon cancer cells death type and cell cycle status after treatment with different doses of SABE-NE. A The AO/PI staining of HT-29 cell line treated using different concentrations of SABE-NE (125, 62.5, and 31.2 µg/mg) using fluorescence microscopy. B Flow cytometry data indicating the subG1values in A549 cell line treated with different concentrations of SABE-NE; SABE-NE Syzygium aromaticum L. essential oil nanoemulsion
In vivo section
Histopathology study in treated mice
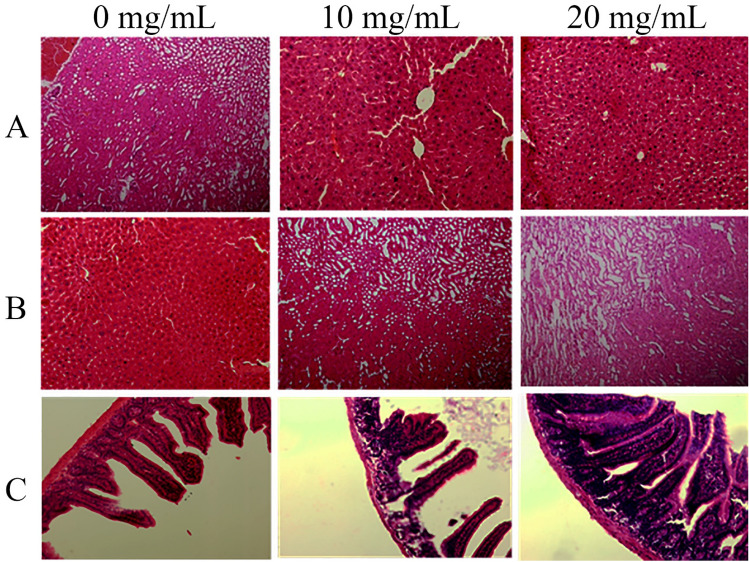
Both Fig. 5 and pathologist report indicate no major cellular death in mice liver, kidney, and jejunum tissues. However, Table 3 shows a significant jejunum villus, crypts, and goblet cell enlargement. Therefore, it can be described that SABE-NE has no cytotoxic effect on normal jejunum cells.
Fig. 5.
The histopathological analysis of liver, kidney, and jejunum of the mice feed by different concentrations of SABE-NE (20, 10, and 0 mg/Kg). A–C the mice liver, kidney, and jejunum tissues alterations; SABE-NE Syzygium aromaticum L. essential oil nanoemulsion
Table 3.
Morphocharacteristic of mice received different concentrations of nanoemulsion from Syzygium aromaticum L. bud essential oil
| Jejunum morphocharacteristic | 0 mg/kg body weight | 10 mg/kg body weight | 20 mg/kg body weight | SEM |
|---|---|---|---|---|
| Villus height (µm) | 284.4c | 316b | 346a | 3.23 |
| Villus width (µm) | 72c | 80b | 87a | 2.16 |
| Crypt depth (µm) | 75c | 99b | 110a | 4.81 |
| Goblet cells (µm) | 2.1c | 2.8b | 3.4a | 0.21 |
Means with different superscript letters differ significantly (p < 0.05)
SEM standard error of the mean
Liver peroxidation and antioxidant gene expression profile in treated mice
The SABE-NE significantly reduces lipid peroxidation in mice liver tissue (p value ≤ 0.001) (Fig. 6). Therefore, it can be interpreted as having cytoprotective effects on mice liver cells that are suitable in suppressing the side effects of chemical drugs.
Fig. 6.
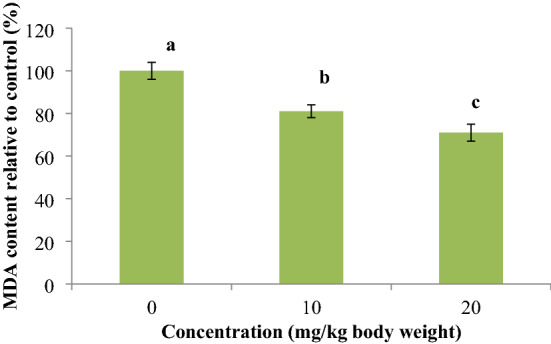
The lipid peroxidation percentage in the mice liver feeding with different concentrations of SABE-NE; SABE-NE Syzygium aromaticum L. essential oil nanoemulsion; Charts bearing different letters differ significantly (p < 0.05)
Expression of antioxidant genes (SOD, CAT, and GPx) in mice liver
The genes expression profile of mice liver cells indicates a significant overexpression in antioxidant enzymes (SOD, CAT, and GPx) (p < 0.001) (Table 4).
Table 4.
Antioxidant genes expression analysis in the liver of mice received different concentrations of nanoemulsion from Syzygium aromaticum L. bud essential oil
| Genes | 0 mg/kg body weight | 10 mg/kg body weight | 20 mg/kg body weight | SEM |
|---|---|---|---|---|
| SOD | 1c | 1.6b | 3.5a | 0.26 |
| CAT | 1c | 1.1b | 2.4a | 0.17 |
| GPx | 1c | 1.2b | 1.8a | 0.1 |
Means with different superscript letters differ significantly (p < 0.05)
SEM standard error of the mean
Discussion
We analyzed the phytochemical contents of extracted SABE oil and synthesized the stable 131.2 nm pseudo-spherical SABE-NE (Tables 1, 2—Fig. 1) and investigated their meaningful cell-specific cytotoxic effects on HT-29 human colon cancer cells compared to normal HFF cells (p ≤ 0.001) (Fig. 2). Moreover, we detected a significant Cas-3 up-regulation and increased SubG1 peaks by enhancing SABE-NE treatment concentration in HT-29 cells (p ≤ 0.001) (Figs. 3 and 4), which verify the apoptotic type of death. To the best of our knowledge, this is the first study producing the SABE-NE as a novel optimized therapeutic system and studying its apoptotic, antioxidant, and cytotoxic effects on HT-29 human colon cancer cells compared to mice liver, kidney, and jejunum cells.
Finally, the SABE-NE dietary treatment concentration effects on mice liver, kidney, and jejunum cells were studied. The results indicate that it has no significant negative effect on the cells of these mice. According to results, SABE-NE improves the antioxidant enzymes (SOD, CAT, and GPx) mission in normal liver cells and decreases lipid peroxidation. Moreover, it can enlarge the jejunum villus, crypt, and goblet cell size (Tables 3 and 4) (Figs. 5 and 6). Therefore, we suggest using it as an efficient natural cell-dependent anti-cancer compound.
Nanoemulsions are used as the drug carriers for safe-transporting therapeutics and phytochemical agents toward the cells. They improve their contents' efficiency and optimize their biological properties, such as antibacterial, antioxidant, and anti-cancer activities (Khan et al. 2018; Farshi et al. 2017). Nanoemulsions as safe bio-compatible efficient drug delivery systems have revealed a high degree of safety and have dramatically modified the cancer therapy strategies (Sharma et al. 2010). Several studies utilize the essential oils of various types of plants as therapeutic nanoemulsions, such as thyme, eucalyptus, neem, lemongrass, clove oil, and citronella (Khan et al. 2018; Farshi et al. 2017).
Antioxidant and apoptotic activities are considered the essential properties of anti-cancer compounds due to their potential of affecting reactive oxygen specious (ROS) cellular levels. It is well known that whenever ROS levels increase and do not refine, a normal to cancer transformation reveals and alters the gene expression profile under oxidative stress conditions (Dawane and Pandit 2012). In this regard, the two main cellular response systems, such as antioxidant defense systems and apoptosis response pathways, are programmed to be activated in stressed cells.
The accuracy of cell proliferation and growth is reliable because of the apoptosis pathways' responsibility, which is weakened in cancer cells (Sznarkowska et al. 2017). In other words, cancer cells try to neutralize reactive radicals by improving their antioxidant defense systems, including up-regulation of CAT, SOD, and GPx. Moreover, they can suppress apoptotic pathways and thus apoptotic death by decreasing the apoptotic gene expressions.
Cas-3, as the apoptosis response effector, is responsible for inducing death in response to increased amounts of ROS in stressed cells. Its activation reveals the morphology of apoptotic cells, including DNA fragmentation and cell vesiculation. Fortunately, the cytotoxic properties of various plant essential oils, such as Satureja khuzistanica Jamzad, S. zenkeri, Solanum spirale Roxb, Casearia sylvestris, A. lepidophyllus, and Cedrelopsis grevei, have been approved for the effect of apoptosis on breast cancer cells by up-regulating Cas3 gene (Yousefzadi et al. 2014; Bou et al. 2013).
Syzygium aromaticum L. (clove) has a high degree due to its components, including tannins, flavonol glycosides, and volatile phenolic oils (eugenol, acetyl eugenol) of antioxidant activity among the other plants. The clove phytochemical compounds act as a strong antioxidant, antiproliferative, antibacterial, antiseptic, and anti-inflammatory agent, making it an appropriate cancer chemopreventive compound (Shan et al. 2005), which can be improved by nanoemulsion drug delivery systems (Jaiswal et al. 2015). Due to their individual ability to improve the solubility and effectiveness of therapeutic compounds, such as dicumarol, tetanus toxoid, insulin, and anti-cancer agents in the drug delivery process, nanoemulsions have widely attracted researchers’ attention in pharmaceutical industries and the field of cancer therapy (Paim et al. 2018; Ahmad et al. 2018).
Moreover, plant bioactive compounds, like phenolic and flavonoids as therapeutic compounds, have low solubility in polar solutions. Therefore, they are not efficient natural anti-cancer compounds due to their low solubility and intestinal absorption (Erlejman et al. 2004). To solve this problem, loading plant bioactive compounds into an appropriate carrier, such as nanoemulsions, could improve their solubility and intestinal absorption. Nanoemulsions-based drugs can benefit from the increased solubility of the cargo, the longer half-life, and the ability to overcome the resistance of cancer cells to chemotherapy. As a result of the pharmacokinetic changes that follow, adverse effects can be reduced, and the therapeutic index of the encapsulated drugs can be improved. (Bozzuto and Molinari 2015).
Eugenol (4-allyl(-2-methoxy phenol)) is approved as a natural phenolic compound in honey and Syzygium aromaticum, Cinnamomum verum, and Pimenta racemosa, which is used as a pain reliever, antiseptic, antibacterial, antiviral, antioxidant, and anti-inflammatory compound. Also, eugenol has apoptotic activity on diverse cancer cell lines, including mast cells, melanoma cells, and HL-60 leukemia cells (Ghosh et al. 2005; Atsumi et al. 2005).
Overexpression of the antioxidant enzymes (SOD, CAT, and GPx) in mice liver tissue after SABE-NE treatment doses approves its cytoprotective role in normal liver cells. Saravana Kumar Jaganathan et al. demonstrated that eugenol induces apoptotic death in human HT-29 colon cancer cells by up-regulating the apoptotic genes, such as caspase 3 and P53. They suggested eugenol as a natural chemopreventive compound in colon cancer treatment (Jaganathan et al. 2011). Also, Kim et al. investigated the apoptotic activity of eugenol on G361 melanoma cells by detecting Caspase 3 protein after the cells' treatment with eugenol (Kim et al. 2006). In this regard, we investigated that SABE-NE can reveal apoptotic activity and thus induce cellular death in HT-29 colon cancer cells. Up-regulation of Cas3, SubG1 peaks enhancement, and fluorescent AO/PI imaging indicate apoptotic death in HT-29 cells. Moreover, its effects on the status of antioxidant enzymes (SOD, CAT, and GPx) in the treated mice liver approve its cytoprotective effects on normal mice liver cells, which supports the cause of lipid peroxidation reduction in such cells.
Conclusion
According to results, the novel, safe, natural SABE-NE significantly induced cell-depended apoptotic death by up-regulating caspase 3 in the HT-29 colon cancer cell line. However, further cancerous and normal cell lines are required to confirm the specificity of the nanoemulsions. Consequently, the nanoemulsion developed in this study could be administrated as an oral solution and could be considered as an oral solution and considered a promising option in colon cancer treatment and progression. We suggest SABE-NE as a novel efficient drug delivery system containing eugenol and additional bioactive agents in clove essential oil for use in colon cancer treatment approaches.
Funding
There has been no financial support for this work.
Data availability
The datasets applied during the current study are available on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
References
- Ahmad N, Alam MA, Ahmad FJ, Sarafroz M, Ansari K, Sharma S, Amir M. Ultrasonication techniques used for the preparation of novel eugenol-nanoemulsion in the treatment of wounds healings and anti-inflammatory. J Drug Deliv Sci Technol. 2018;46:461–473. doi: 10.1016/j.jddst.2018.06.003. [DOI] [Google Scholar]
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691. 10.1136/gutjnl-2015-310912 [DOI] [PubMed]
- Assayag M, Rouvier P, Gauthier M, Costel G, Cluzel P, Mercadal L, Deray G, Bagnis CI. Renal failure during chemotherapy: renal biopsy for assessing subacute nephrotoxicity of pemetrexed. BMC Cancer. 2017;17(1):1–8. doi: 10.1186/s12885-017-3705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi T, Murakami Y, Shibuya K, Tonosaki K, Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, α-diisoeugenol. Anticancer Res. 2005;25(6B):4029–4036. [PubMed] [Google Scholar]
- Banerjee S, Panda CK, Das S. Clove (Syzygium aromaticum L), a potential chemopreventive agent for lung cancer. Carcinogenesis. 2006;27(8):1645–1654. doi: 10.1093/carcin/bgi372. [DOI] [PubMed] [Google Scholar]
- Beyrami M, Karimi E, Oskoueian E. Synthesized chrysin-loaded nanoliposomes improves cadmium-induced toxicity in mice. Environ Sci Pollut Res. 2020;27(32):40643–40651. doi: 10.1007/s11356-020-10113-7. [DOI] [PubMed] [Google Scholar]
- Bou DD, Lago JHG, Figueiredo CR, Matsuo AL, Guadagnin RC, Soares MG, Sartorelli P. Chemical composition and cytotoxicity evaluation of essential oil from leaves of Casearia sylvestris, its main compound α-zingiberene and derivatives. Molecules. 2013;18(8):9477–9487. doi: 10.3390/molecules18089477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomed. 2015;10:975–999. doi: 10.2147/ijn.s68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaieb K, Hajlaoui H, Zmantar T, Kahla-Nakbi AB, Rouabhia M, Mahdouani K, Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L Myrtaceae): a short review. Phytother Res. 2007;21(6):501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- Constantinides PP, Chaubal MV, Shorr R. Advances in lipid nanodispersions for parenteral drug delivery and targeting. Adv Drug Deliv Rev. 2008;60(6):757–767. doi: 10.1016/j.addr.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine. 2010;5(10):1595–1616. doi: 10.2217/nnm.10.126. [DOI] [PubMed] [Google Scholar]
- Dawane JS, Pandit VA. Understanding redox homeostasis and its role in cancer. J Clin Diagn Res JCDR. 2012;6(10):1796. doi: 10.7860/JCDR/2012/4947.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsì F, Annunziata M, Sessa M, Ferrari G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT-Food Sci Technol. 2011;44(9):1908–1914. doi: 10.1016/j.lwt.2011.03.003. [DOI] [Google Scholar]
- Dwivedi V, Shrivastava R, Hussain S, Ganguly C, Bharadwaj M. Comparative anticancer potential of clove (Syzygium aromaticum)—an Indian spice—against cancer cell lines of various anatomical origin. Asian Pac J Cancer Prev. 2011;12(8):1989–1993. [PubMed] [Google Scholar]
- Erlejman A, Verstraeten S, Fraga C, Oteiza P. The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radical Res. 2004;38(12):1311–1320. doi: 10.1080/10715760400016105. [DOI] [PubMed] [Google Scholar]
- Farshi P, Tabibiazar M, Ghorbani M, Hamishehkar H. Evaluation of antioxidant activity and cytotoxicity of cumin seed oil nanoemulsion stabilized by sodium caseinate-guar gum. Pharmaceutical Sci. 2017;23(4):293–300. doi: 10.15171/PS.2017.43. [DOI] [Google Scholar]
- Ghosh R, Nadiminty N, Fitzpatrick JE, Alworth WL, Slaga TJ, Kumar AP. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chem. 2005;280(7):5812–5819. doi: 10.1074/jbc.M411429200. [DOI] [PubMed] [Google Scholar]
- Hosseini A, Ghorbani A. Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomed. 2015;5(2):84. [PMC free article] [PubMed] [Google Scholar]
- Jaganathan SK, Mazumdar A, Mondhe D, Mandal M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol Int. 2011;35(6):607–615. doi: 10.1042/CBI20100118. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, Dudhe R, Sharma P. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaradat NA, Zaid AN, Abuzant A, Shawahna R. Investigation the efficiency of various methods of volatile oil extraction from Trichodesma africanum and their impact on the antioxidant and antimicrobial activities. J Intercult Ethnopharmacol. 2016;5(3):250. doi: 10.5455/jice.20160421065949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Bahuguna A, Kumar P, Bajpai VK, Kang SC. In vitro and in vivo antitumor potential of carvacrol nanoemulsion against human lung adenocarcinoma A549 cells via mitochondrial mediated apoptosis. Sci Rep. 2018;8(1):1–15. doi: 10.1038/s41598-017-18644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GC, Choi DS, Lim JS, Jeong HC, Kim IR, Lee MH, Park BS. Caspases-dependent apoptosis in human melanoma cell by eugenol. Korean J Anat. 2006;39(3):245–253. [Google Scholar]
- Lesgards JF, Baldovini N, Vidal N, Pietri S. Anticancer activities of essential oils constituents and synergy with conventional therapies: a review. Phytother Res. 2014;28(10):1423–1446. doi: 10.1002/ptr.5165. [DOI] [PubMed] [Google Scholar]
- Mahato R. Nanoemulsion as targeted drug delivery system for cancer therapeutics. J Pharm Sci Pharmacol. 2017;3(2):83–97. doi: 10.1166/jpsp.2017.1082. [DOI] [Google Scholar]
- Mahdi Jafari S, He Y, Bhandari B. Nano-emulsion production by sonication and microfluidization—a comparison. Int J Food Prop. 2006;9(3):475–485. doi: 10.1080/10942910600596464. [DOI] [Google Scholar]
- Navaei Shoorvarzi S, Shahraki F, Shafaei N, Karimi E, Oskoueian E. Citrus aurantium L bloom essential oil nanoemulsion: synthesis, characterization, cytotoxicity, and its potential health impacts on mice. J Food Biochem. 2020;44(5):e13181. doi: 10.1111/jfbc.13181. [DOI] [PubMed] [Google Scholar]
- Paim LFNA, Dalla Lana DF, Giaretta M, Danielli LJ, Fuentefria AM, Apel MA, Külkamp-Guerreiro IC. Poiretia latifolia essential oil as a promising antifungal and anti-inflammatory agent: chemical composition, biological screening, and development of a nanoemulsion formulation. Ind Crops Prod. 2018;126:280–286. doi: 10.1016/j.indcrop.2018.10.016. [DOI] [Google Scholar]
- Rao PV, Nallappan D, Madhavi K, Rahman S, Jun Wei L, Gan SH. Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxid Med Cell Longev. 2016;2016:1–15. doi: 10.1155/2016/3685671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Leroux J, Wasan K. Role of lipid excipients in modifying oral and parenteral drug delivery. Hoboken: Wiley; 2006. [Google Scholar]
- Sarker DK. Engineering of nanoemulsions for drug delivery. Curr Drug Deliv. 2005;2(4):297–310. doi: 10.2174/156720105774370267. [DOI] [PubMed] [Google Scholar]
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53(20):7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Sharma N, Bansal M, Visht S, Sharma P, Kulkarni G. Nanoemulsion: a new concept of delivery system. Chron Young Sci. 2010;1(2):2. [Google Scholar]
- Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3–4):102–110. doi: 10.1016/j.cocis.2005.06.004. [DOI] [Google Scholar]
- Sznarkowska A, Kostecka A, Meller K, Bielawski KP. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget. 2017;8(9):15996. doi: 10.18632/oncotarget.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Zagreb. MEASUREMENT AND APPLICATION OF ZETA-POTENTIAL. https://hrcak.srce.hr/24757 (1992)
- Yousefzadi M, Riahi-Madvar A, Hadian J, Rezaee F, Rafiee R, Biniaz M. Toxicity of essential oil of Satureja khuzistanica: in vitro cytotoxicity and anti-microbial activity. J Immunotoxicol. 2014;11(1):50–55. doi: 10.3109/1547691X.2013.789939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets applied during the current study are available on reasonable request.