Abstract
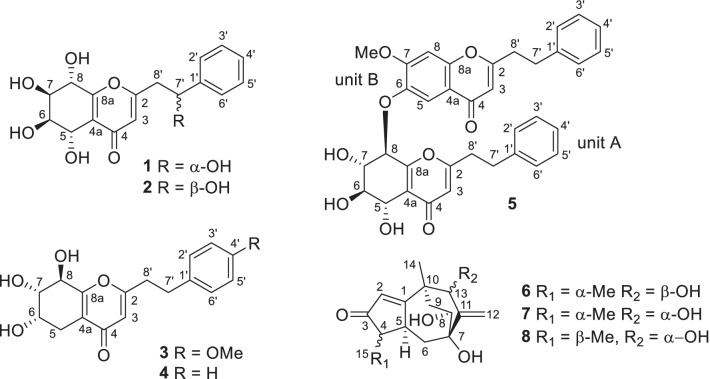
Five new 2-(2-phenylethyl)chromone derivatives, (5S,6R,7R,8S,7ʹR)-7ʹ-hydroxyagarotetrol (1), (5S,6R,7R,8S,7ʹS)-7ʹ-hydroxyagarotetrol (2), (6S,7S,8R)-2‑[2‑(4-methoxyphenyl)ethyl]‑6,7,8‑trihydroxy‑5,6,7,8‑tetrahydrochromone (3), (6S,7S,8R)-2‑(2-phenylethyl)‑6,7,8‑trihydroxy‑5,6,7,8‑tetrahydrochromone (4), (5S,6R,7S,8R)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)-7-methoxychromonyl-6-oxy]chromone (5), three new sesquiterpenoids, (4S,5S,7S,8S,10S,13R)-7,8,13-trihydroxyrotunda-1,11-dien-3-one (6), (4S,5S,7S,8S,10S,13S)-7,8,13-trihydroxyrotunda-1,11-dien-3-one (7), and (4R,5S,7S,8S,10S,13S)-7,8,13-trihydroxyrotunda-1,11-dien-3-one (8), along with 14 known compounds were isolated from the resinous heartwood of Aquilaria sinensis (Thymelaeaceae). The chemical structures of these new compounds were elucidated by 1D and 2D NMR and MS data, single-crystal X-ray diffraction analysis, and electronic circular dichroism (ECD) calculations. The neuroprotective activities of these isolates were evaluated using an in vitro model of rat adrenal pheochromocytoma (PC12) cell injury induced by corticosterone. At concentrations from 5 to 40 µM, compounds 4 and 6, agarotetrol (9), and 6-hydroxy-2-(2-phenylethyl)chromone (17) showed significant protective activities against corticosterone-induced PC12 cell injury (P < 0.001).
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s13659-022-00326-3.
Keywords: Thymelaeaceae, Aquilaria sinensis, Sesquiterpenoids, 2-(2-Phenylethyl)chromones, Neuroprotective
Introduction
Chen-xiang (Aquilariae Lignum Resinatum), resinous heartwoods of the Thymelaeaceous plant Aquilaria sinensis (Lour.) Spreng., is one of the most well-known aromatic medicines in China [1, 2]. More than 240 compounds, mainly sesquiterpenoids, diterpenoids, steroids, benzyl acetones, chromones, phenolic acids, and aliphatic compounds, have been found in chen-xiang. Some compounds showed antibacterial, anticancer, acetylcholinesterase inhibitory, and other pharmacological activities [3]. Aromatic plants are thought to be a source of chemical constituents with neuroprotective effects [4]. In our continuing efforts to search for neuroprotective compounds from chen-xiang [5, 6], five new 2-(2-phenylethyl)chromone derivatives (1–5, Fig. 1) and three new sesquiterpenoids (6–8, Fig. 1), along with 14 known compounds (9–22, Additional file 1: Fig. S1), were isolated. In the present paper, structural elucidation of these new compounds and bioassay results for the neuroprotective activity of these isolates are reported.
Fig. 1.
Chemical structures of compounds 1–8 from Aquilaria sinensis
Results and discussion
Structure elucidation
Compound 1 was obtained as colorless needles (MeOH). Based on the HRESIMS at m/z 357.0942 [M + Na]+ (calcd for C17H18NaO7, 357.0950) and 13C NMR data (Table 1), its molecular formula was deduced to be C17H18O7 with nine indices of hydrogen deficiency. Its IR spectrum indicated the presence of hydroxy groups (3406 cm−1), an α,β-unsaturated carbonyl (1658 cm−1), and a monosubstituted phenyl ring (1601, 1448, and 701 cm−1). According to the 1H NMR data of compound 1 (Table 1), a trisubstituted double bond [δH 6.19 (1H, s)] and a monosubstituted phenyl ring [δH 7.26–7.41 (5H, m)] were deduced. Its NMR data (Table 1) were very similar to those of agarotetrol (9) [7, 8], a common 2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone found in Aquilaria plants. The main difference was that signals for a methylene in agarotetrol were replaced by signals [δH 5.10 (dd, J = 8.0, 5.8 Hz); δC 72.4] for an oxygenated methine in compound 1.
Table 1.
1H (500 MHz) and 13C (126 MHz) NMR data of 1 and 2 in methanol-d4 (δ in ppm, J in Hz)
| No | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | 169.1 | 169.2 | ||
| 3 | 6.19 (s) | 115.5 | 6.22 (s) | 115.5 |
| 4 | 181.9 | 181.9 | ||
| 4a | 121.8 | 121.9 | ||
| 5 | 4.75 (d, 4.0) | 66.7 | 4.75 (d, 4.0) | 66.7 |
| 6 | 4.02 (dd, 4.0, 2.4) | 74.0 | 4.02 (dd, 4.0, 2.3) | 74.0 |
| 7 | 4.04 (dd, 7.5, 2.4) | 72.4 | 4.04 (dd, 7.5, 2.3) | 72.4 |
| 8 | 4.56 (d, 7.5) | 70.1 | 4.56 (d, 7.5) | 70.1 |
| 8a | 165.4 | 165.4 | ||
| 1′ | 144.8 | 145.0 | ||
| 2′,6′ | 7.41 (m) | 126.9 | 7.41 (m) | 126.9 |
| 3′,5′ | 7.34 (m) | 129.5 | 7.35 (m) | 129.5 |
| 4′ | 7.26 (m) | 128.8 | 7.27 (m) | 128.8 |
| 7′ | 5.10 (dd, 8.0, 5.8) | 72.4 | 5.11 (dd, 8.0, 5.4) | 72.3 |
|
8′α 8′β |
3.01 (dd, 14.6, 8.0) 2.97 (dd, 14.6, 5.8) |
44.4 |
3.00 (dd, 14.6, 5.4) 2.96 (dd, 14.6, 8.0) |
44.4 |
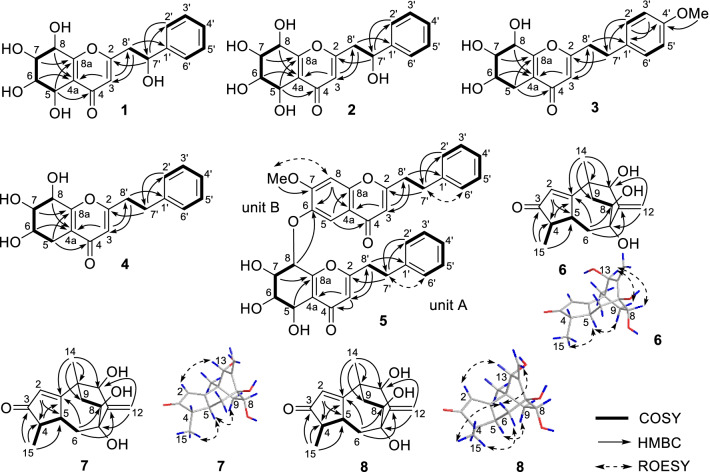
Based on the 1H‒1H COSY correlations of 1 (Fig. 2), fragments of C-5 to C-8, C-2ʹ to C-6ʹ, and C-7ʹ to C-8ʹ were deduced. HMBC correlations (Fig. 2) from δH 7.41 (H-2′, H-6′) to δC 72.3 (C-7′) and from δH 2.97 (H-8′β) to δC 144.8 (C-1′) indicated the presence of a 2-hydroxy-2-phenylethyl fragment in 1. HMBC correlations from δH 2.97 (H-8′β) to δC 115.5 (C-3), from δH 6.19 (H-3) to δC 44.4 (C-8′), and from δH 5.11 (H-7ʹ) to δC 169.1 (C-2) implied that the 2-hydroxy-2-phenylethyl fragment was located at C-2. HMBC correlations from δH 4.75 (H-5) to δC 181.9 (C-4) and δC 165.4 (C-8a), from δH 4.56 (H-8) to δC 121.8 (C-4a), and from δH 6.19 (H-3) to δC 121.8 (C-4a), a planar structure 2‑(2‑phenylethyl)‑5,6,7,8,7ʹ‑pentahydroxy‑5,6,7,8‑tetrahydrochromone was deduced.
Fig. 2.
Key 2D NMR correlations of compounds 1–8
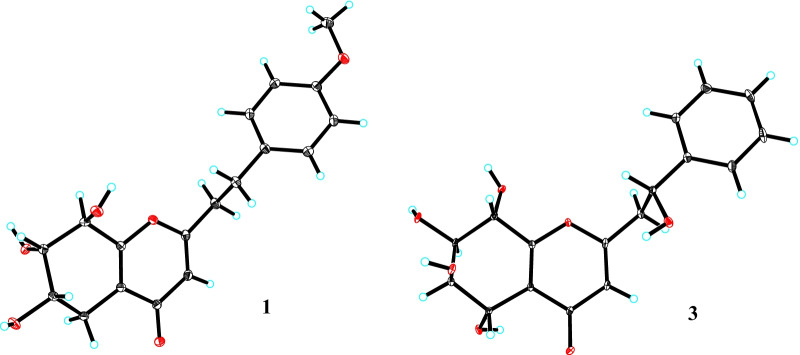
It is difficult to determine the relative configuration of 5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromones by ROESY correlations, while the coupling constants of H-5 to H-8 are helpful. J5,6 (4.0 Hz), J6,7 (2.4 Hz), and J7,8 (7.5 Hz) values of compound 1 were close to those of 5α,6β,7β,8α-tetrahydroxy-5,6,7,8-tetrahydrochromone analogs [9], implying the 5α,6β,7β,8α-tetrahydroxy configuration in 1. The relative configuration of H-7ʹ was also determined by comparing its coupling constant with that of known compounds. In isomers (5S,6R,7S,8R,7ʹR)-7ʹ-hydroxyisoagarotetrol (15) [10] and (5S,6R,7S,8R,7′S)-7′-hydroxyisoagarotetrol (16) [10], the main differences in 1H NMR spectra were J7ʹ,8ʹ values. Because J7ʹ,8ʹα (8.0 Hz) and J7ʹ,8ʹβ (5.8 Hz) values of compound 1 were close to those of the 7ʹα-OH isomer (15; J7ʹ,8ʹα = 8.0 Hz and J7ʹ,8ʹβ = 5.5 Hz), 7ʹ-OH of 1 was deduced to be α-oriented. The absolute configuration of 1 was determined to be (5S,6R,7R,8S,7ʹR)-7ʹ-hydroxyagarotetrol (Fig. 3) by single-crystal X-ray diffraction using graphite monochromated CuKα radiation with a Flack parameter of 0.11 (10).
Fig. 3.
X-ray crystallographic structures of 1 and 3
The molecular formula of 2 was deduced to be the same as that of compound 1, C17H18O7, by 13C NMR (Table 1) and HRESIMS at m/z 357.0945 [M + Na]+ (calcd for C17H18NaO7, 357.0950). Detailed comparison of its NMR data with those of (5S,6R,7S,8R,7ʹR)-7ʹ-hydroxyisoagarotetrol (1), (5S,6R,7S,8R,7ʹR)-7ʹ-hydroxyisoagarotetrol (15), and (5S,6R,7S,8R,7′S)-7′-hydroxyisoagarotetrol (16) [10], compound 2 was elucidated to be 7ʹ-epimer of 1, which was confirmed by 2D NMR correlations of 2 (Fig. 2). Because J7ʹ,8ʹα (5.4 Hz) and J7ʹ,8ʹβ (8.0 Hz) values of compound 2 were close to those of the 7ʹβ-OH isomer (16; J7ʹ,8ʹα = 5.0 Hz and J7ʹ,8ʹβ = 8.5 Hz) [10], the 7ʹ-OH of 2 was deduced to be β-oriented. Thus, compound 2 was elucidated to be (5S,6R,7S,8R,7ʹS)-7ʹ-hydroxyisoagarotetrol.
Compound 3 had the molecular formula C18H20O6 based on 13C NMR data (Table 2) and the positive ion at m/z 355.1150 [M + Na]+ (calcd for C18H20NaO6, 355.1158) in the HRESIMS. The 1H and 13C NMR spectra showed resonances for one p-disubstituted phenyl ring [δH 7.12 (2H, br d, J = 8.6 Hz) and 6.82 (2H, br d, J = 8.6 Hz); δC 159.8, 133.2, 130.4 × 2, and 115.0 × 2], one trisubstituted 4H-pyran-4-one [δH 6.07 (s); δC 182.0, 171.2, 163.0, 120.9, and 113.1], one methoxy group [δH 3.75 (3H, s); δC 55.6], three sp3 methylenes (δC 36.6, 33.0, and 26.5), and three oxygenated methine [δH 4.50 (d, J = 5.0 Hz), 4.11 (ddd, J = 7.4, 5.1, 2.2 Hz), and 3.90 (dd, J = 5.0, 2.2 Hz); δC 75.0, 71.2, and 67.3], implying that it might also be a 5,6,7,8-tetrahydrochromone with the substituted mode of three hydroxy groups at ring B rather than the usual mode of four hydroxy groups at ring B. Four fragments, C-5 to C-8, C-2ʹ to C-3ʹ, C-5ʹ to C-6ʹ, and C-7ʹ to C-8ʹ, were deduced by 1H‒1H COSY correlations (Fig. 2). Its planar structure was deduced to be 2‑[2‑(4-methoxyphenyl)ethyl]‑6,7,8‑trihydroxy‑5,6,7,8‑tetrahydrochromone by key HMBC correlations (Fig. 2) from H-3 to C-4a and C-8ʹ, from H-5 to C-4 and C-8a, from H-6 and H-8 to C-4a, from H-7 to C-8a, from H-2ʹ and H-6ʹ to C-7ʹ, H-3ʹ and H-5ʹ to C-1ʹ, 4ʹ-OMe to C-4ʹ, H2-7ʹ to C-2, C-2ʹ, and C-6ʹ, and H2-8ʹ to C-1ʹ and C-3. Finally, the absolute configuration of 3 was determined to be (6S,7S,8R)-2‑[2‑(4-methoxyphenyl)ethyl]‑6,7,8‑trihydroxy‑5,6,7,8‑tetrahydrochromone (Fig. 3) by single-crystal X-ray diffraction using graphite monochromated CuKα radiation with a Flack parameter of 0.09 (4).
Table 2.
1H and 13C NMR data of 3 and 4 in methanol-d4 (δ in ppm, J in Hz)
| No | 3 | 4 | ||
|---|---|---|---|---|
| δH (600 MHz) | δC (151 MHz) | δH (500 MHz) | δC (126 MHz) | |
| 2 | 171.2 | 171.1 | ||
| 3 | 6.07 (s) | 113.1 | 6.09 (s) | 113.0 |
| 4 | 182.0 | 181.9 | ||
| 4a | 120.9 | 120.9 | ||
|
5β 5α |
2.66 (dd, 16.9, 5.1) 2.51 (dd, 16.9, 7.4) |
26.5 |
2.67 (dd, 17.2, 5.0) 2.51 (dd, 17.2, 7.5) |
26.5 |
| 6 | 4.11 (ddd, 7.4, 5.1, 2.2) | 67.3 | 4.11 (ddd, 7.5, 5.0, 2.1) | 67.3 |
| 7 | 3.90 (dd, 5.0, 2.2) | 75.0 | 3.90 (dd, 5.0, 2.1) | 75.0 |
| 8 | 4.50 (d, 5.0) | 71.2 | 4.50 (d, 5.0) | 71.2 |
| 8a | 163.0 | 163.0 | ||
| 1′ | 133.2 | 141.2 | ||
| 2′,6′ | 7.12 (br d, 8.6) | 130.4 | 7.26 (m) | 129.6 |
| 3′,5′ | 6.82 (br d, 8.6) | 115.0 | 7.22 (m) | 129.5 |
| 4′ | 159.8 | 7.18 (m) | 127.4 | |
| 7′ | 2.96 (m) | 33.0 | 3.02 (m) | 33.8 |
| 8′ | 2.89 (m) | 36.6 | 2.92 (m) | 36.3 |
| 4′-OMe | 3.75 (s) | 55.6 | ||
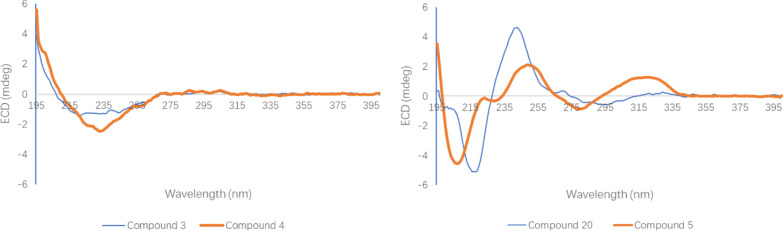
Compound 4 was assigned the molecular formula C17H18O5 based on 13C NMR data (Table 2) and the positive ion at m/z 325.1046 [M + Na]+ (calcd for C17H18NaO5, 325.1052) in the HRESIMS. By extensively comparing the NMR data (Table 2) of compounds 3 and 4, signals for one monosubstituted phenyl ring were found in 4 rather than the disubstituted phenyl ring in 3, and signals for a methoxy group disappeared in 4. Otherwise, the NMR data of these two compounds were very close to each other, implying that compound 4 is the 4ʹ-demethoxy derivative of 3, which was confirmed by the 1H‒1H COSY and HMBC correlations of 4 (Fig. 2). The chemical shifts and coupling constants of H-6 [δH 4.11 (ddd, J = 7.5, 5.0, 2.1 Hz)], H-7 [δH 3.90 (dd, J = 5.0, 2.1 Hz)], and H-8 [δH 4.50 (d, J = 5.0 Hz)] of compound 4 were very close to those of H-6 [δH 4.11 (ddd, J = 7.4, 5.1, 2.2 Hz)], H-7 [δH 3.90 (dd, J = 5.0, 2.2 Hz)], and H-8 [δH 4.50 (d, J = 5.0 Hz)] of compound 3, implying 6α-OH, 7α-OH, and 8β-OH configurations in 4. The absolute configuration of 4 was suggested to be (6S,7S,8R)-2‑(2-phenylethyl)‑6,7,8‑trihydroxy‑5,6,7,8‑tetrahydrochromone in view of its ECD spectrum similar to that of compound 3 (Fig. 4).
Fig. 4.
ECD spectra of compounds 3–5 and 20
A molecular formula, C35H32O9, was assigned to compound 5 by positive HRESIMS with an ion at m/z 619.1939 [M + Na]+ (calcd C35H32NaO9, 619.1944) and 13C NMR data (Table 3). According to its NMR data (Table 3), signals for two sets of phenylethyl moieties (δC 141.3, 140.9, 129.2 × 2, 129.6 × 2, 129.5 × 4, 127.5, 127.4, 37.0, 36.2, 34.1, and 33.4), one 5,6,7,8-tretrahydroxy-5,6,7,8-tetrahydrochromone moiety [δH 6.13 (s), 5.45 (dd, J = 7.3, 1.3 Hz), 4.75 (dd, J = 6.9, 1.3 Hz), 4.00 (dd, J = 9.6, 7.3 Hz), and 3.83 (dd, J = 9.6, 6.9 Hz); δC182.0, 171.2, 160.7, 122.5, 114.4, 79.5, 74.9, 73.6, and 70.3], one trisubstituted chromone [δH 7.90 (s), 7.22 (s), and 6.11 (s); δC 179.7, 170.9, 157.3, 155.0, 148.8, 117.4, 110.4, 110.1, and 101.7], and one methoxy group [δH 3.98 (s); δC 57.2], were observed, which implied that this compound might be a dimer of a 2-(2-phenylethyl)chromone and a 5,6,7,8-tretrahydroxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromone. In unit A of 5 (Fig. 2), three 1H‒1H COSY fragments, H-5/H-6/H-7/H-8, H-2ʹ/H-3ʹ/H-4ʹ/H-5ʹ/C-6ʹ, and H2-7ʹ/H2-8ʹ, along with HMBC correlations from H-3 to C-4a and C-8ʹ, from H-5 and H-7 to C-8a, from H-2ʹ and H-6ʹ to C-7ʹ, from H2-7ʹ to C-2, C-2ʹ, and C-6ʹ, and from H2-8ʹ to C-1ʹ and C-3, were observed, which implied the presence of a 5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromone moiety in 5. In unit B of 5 (Fig. 2), two 1H‒1H COSY fragments, H-2ʹ/H-3ʹ/H-4ʹ/H-5ʹ/C-6ʹ and H2-7ʹ/H2-8ʹ, along with HMBC correlations from H-3 to C-4a and C-8ʹ, from H-5 to C-4 and C-8a, from 7-OMe to C-7, from H-8 to C-4a and C-6, from H-2ʹ and H-6ʹ to C-7ʹ, from H2-7ʹ to C-2, C-2ʹ, and C-6ʹ, and from H2-8ʹ to C-1ʹ and C-3, were observed, which implied the presence of a 6-hydroxy-7-methoxy-2-(2-phenylethyl)chromone moiety in 5. Units A and B were connected through an ether bond by the HMBC correlation from H-8 of unit A to C-6 of unit B (Fig. 2). The relative configuration of unit A was deduced to be the same as that of a structural analog (5S,6R,7S,8R)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone (20) [11] by comparing the coupling constants of H-5 to H-8 (J5,6 = 6.9 Hz, J6.7 = 9.6 Hz, and J7.8 = 7.3 Hz) in 5 with those (J5,6 = 7.0 Hz, J6.7 = 9.8 Hz, and J7.8 = 7.5 Hz) of H-5 to H-8 in the known analog [11]. The absolute configuration of 5 was suggested to be (5S,6R,7S,8R)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)-7-methoxychromonyl-6-oxy]chromone because the ECD spectrum of 5 was similar to that of (5S,6R,7S,8R)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone (20) (Fig. 4).
Table 3.
1H (600 MHz) and 13C (151 MHz) NMR data of 5 in methanol-d4 (δ in ppm, J in Hz)
| No | δH | δC |
|---|---|---|
| Unit A | ||
| 2 | 171.2 | |
| 3 | 6.13 (s) | 114.4 |
| 4 | 182.0 | |
| 4a | 122.5 | |
| 5 | 4.75 (dd, 6.9, 1.3) | 70.3 |
| 6 | 3.83 (dd, 9.6, 6.9) | 74.9 |
| 7 | 4.00 (dd, 9.6, 7.3) | 73.6 |
| 8 | 5.45 (dd, 7.3, 1.3) | 79.5 |
| 8a | 160.7 | |
| 1′ | 140.9 | |
| 2′,6′ | 6.95 (m) | 129.2 |
| 3′,5′ | 7.16 (m) | 129.5 |
| 4′ | 7.11 (m) | 127.4 |
| 7′ | 2.62 (m) | 33.4 |
| 8′ |
2.75 (m) 2.65 (m) |
36.2 |
| Unit B | ||
| 2 | 170.9 | |
| 3 | 6.11 (s) | 110.1 |
| 4 | 179.7 | |
| 4a | 117.4 | |
| 5 | 7.90 (s) | 110.4 |
| 6 | 148.8 | |
| 7 | 157.3 | |
| 8 | 7.22 (s) | 101.7 |
| 8a | 155.0 | |
| 1′ | 141.3 | |
| 2′,6′ | 7.22 (m) | 129.5 |
| 3′,5′ | 7.24 (m) | 129.6 |
| 4′ | 7.17 (m) | 127.5 |
| 7′ | 3.08 (t, 7.5) | 34.1 |
| 8′ | 3.02 (t, 7.5) | 37.0 |
| 7-OMe | 3.98 (s) | 57.2 |
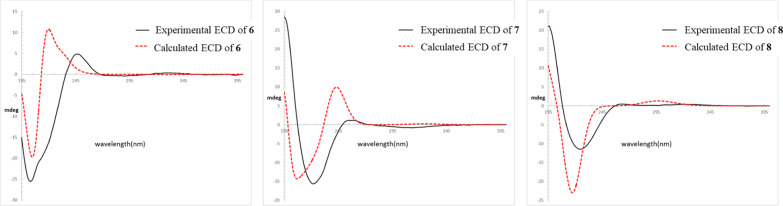
Compound 6 was assigned the molecular formula C15H20O4, as determined by 13C NMR data (Table 4) and the positive ion at m/z 287.1255 [M + Na]+ (calcd for C15H20NaO4, 287.1259) in the HRESIMS. The 1H and 13C NMR data (Table 4) indicated the presence of one α,β-unsaturated ketone [δH 5.91 (br s); δC 214.1, 188.3, and 130.4], one exocyclic double bond [δH 5.55 (br s) and 5.36 (br s); δC 156.3 and 112.3], two methyl groups [δH 1.29 (s) and 1.14 (d, J = 7.4 Hz); δC 25.2 and 15.4], one methylene, four methines including two oxygenated groups [δH 4.12 (t, J = 2.2 Hz) and 3.88 (dd, J = 10.7, 5.3 Hz); δC 75.4 and 71.8], and two quaternary carbon atoms (δC 77.3 and 42.8). Its NMR data were similar to those of (4R,5S,7R,8S,10S,13R)-8,13-dihydroxyrotunda-1,11-dien-3-one with a rare tricyclic rotundane skeleton [5]. According to the 1H‒1H COSY correlations (Fig. 2), two fragments, C-15-C-4-C-5-C-6 and C-8-C-9, were deduced. According to HMBC correlations (Fig. 2) from H-2 to C-4 and C-5, from H3-15 to C-3, C-4, and C-5, from H2-6 to C-1, C-8, and C-11, from H2-12 to C-7 and C-13, and from H3-14 to C-1, C-9, C-10, and C-13, the planar structure of 6 was elucidated to be 7,8,13-trihydroxyrotunda-1,11-dien-3-one. The ROESY correlations (Fig. 2) of H3-15/H-5 and H-5/H-9α indicated that these protons should be cofacial; the ROESY correlations of H-9β/H-13 and H-8/H-13 indicated that these protons should also be cofacial. Thus, the relative configuration of 6 was determined, as shown in Fig. 2. By comparing its experimental and calculated ECD spectra (Fig. 5), the structure of compound 6 was finally elucidated to be (4S,5S,7S,8S,10S,13R)-7,8,13-trihydroxyrotunda-1,11-dien-3-one.
Table 4.
1H and 13C NMR data of 6–8 in methanol-d4 (δ in ppm, J in Hz)
| No | 6 | 7 | 8 | |||
|---|---|---|---|---|---|---|
| δHa | δCb | δHc | δCd | δHc | δCd | |
| 1 | 188.3 | 191.5 | 191.7 | |||
| 2 | 5.91 (br s) | 130.4 | 5.95 (d, 1.6) | 128.5 | 5.88 (d, 1.4) | 128.5 |
| 3 | 214.1 | 214.1 | 214.5 | |||
| 4 | 1.87 (m) | 51.1 | 1.89 (qd, 7.5, 2.1) | 51.5 | 2.60 (m) | 45.5 |
| 5 | 2.98 (m) | 47.0 | 3.02 (m) | 47.3 | 3.62 (m) | 41.6 |
|
6α 6β |
2.75 (dd, 12.5, 8.9) 1.35 (t, 12.5) |
45.9 |
2.83 (dd, 12.3, 9.3) 1.26 (ddd, 12.3, 10.2, 1.2) |
46.8 |
2.46 (dd, 12.1, 8.8) 1.22 (ddd, 12.1, 11.1, 1.1) |
43.2 |
| 7 | 77.3 | 77.1 | 77.4 | |||
| 8 | 3.88 (dd, 10.7, 5.3) | 71.8 | 4.11 (ddd, 10.5, 6.0, 1.2) | 71.7 | 4.09 (ddd, 10.6, 5.6, 0.9) | 71.8 |
|
9α 9β |
1.88 (m) 2.05 (dd, 15.0, 10.7) |
41.5 |
1.73 (ddd, 14.5, 6.0, 1.0) 2.31 (dd, 14.5, 10.5) |
38.6 |
1.81 (ddd, 14.5, 5.6, 0.9) 2.33 (dd, 14.5, 10.7) |
38.4 |
| 10 | 44.8 | 43.7 | 43.6 | |||
| 11 | 156.3 | 156.3 | 156.3 | |||
| 12 |
5.55 (br s) 5.36 (br s) |
112.3 |
5.54 (d, 0.8) 5.19 (br s) |
116.5 |
5.53 (s) 5.17 (s) |
116.5 |
| 13 | 4.12 (t, 2.2) | 75.4 | 3.88 (s) | 78.5 | 3.89 (br s) | 78.6 |
| 14 | 1.29 (s) | 25.2 | 1.30 (s) | 24.5 | 1.29 (s) | 24.5 |
| 15 | 1.14 (d, 7.4) | 15.4 | 1.15 (d, 7.5) | 15.9 | 1.02 (d, 7.6) | 10.5 |
aMeasured at 500 MHz; bmeasured at 126 MHz; cmeasured at 800 MHz; dmeasured at 201 MHz
Fig. 5.
Experimental and calculated ECD spectra of compounds 6–8
Compound 7 has the molecular formula C15H20O4 according to its 13C NMR data (Table 4) and HRESIMS at 287.1253 [M + Na]+ (calcd for C15H20NaO4, 287.1259). Its 13C NMR data exhibited 15 signals for one α,β-unsaturated ketone functionality (δC 214.1, 191.5, and 128.5), one exocyclic double bond (δC 156.3 and 116.5), two quaternary carbon atoms (δC 77.1 and 43.7), one methine, four methylenes including two oxygenated groups (δC 78.5 and 71.7), and two methyl groups (δC 24.5 and 15.9). The NMR data of compounds 6 and 7 were very close to each other, and both of these compounds have the same molecular formula, which implied that compound 7 might also be a rotundane-type sesquiterpenoid.
The 1H‒1H COSY fragments H3-15/H-4/H-5/H2-6 and H-8/H2-9 were determined from the 1H‒1H COSY correlations of 7 (Fig. 2). Based on the HMBC correlations (Fig. 2) from H-2 to C-4 and C-5, from H3-15 to C-3, C-4, and C-5, from H2-6 to C-1, C-8, and C-11, from H2-12 to C-7 and C-13, and from H3-14 to C-1, C-9, C-10, and C-13, the planar structure of 7 was elucidated to be the same as that of compound 6, namely, 7,8,13-trihydroxyrotunda-1,11-dien-3-one. Because J8,9α (6.0 Hz) and J8,9β (10.5 Hz) values in the 1H NMR data of compound 7 were similar to those (J8,9α = 5.3 Hz and J8,9β = 10.8 Hz) of compound 6, H-8 in compound 7 was elucidated to be β-oriented. Correlations of H3-15/H-5, H-5/H-9α, and H-2/H-13 were observed in the ROESY spectrum (Fig. 2), indicating that compound 7 is a C-13 epimer of compound 6. Finally, the absolute configuration of 7 was elucidated to be (4S,5S,7S,8S,10S,13S)-7,8,13-trihydroxyrotunda-1,11-dien-3-one based on the ECD calculations (Fig. 5).
Compound 8 was assigned the molecular formula C15H20O4, the same as that of 6 and 7, by 13C NMR data (Table 4) and the ion peak at m/z 264.1359 [M]+ (calcd for C15H20O4, 264.1362) in the HREIMS. The 1H and 13C NMR data (Table 4) indicated that this compound might also be a rotundane-type sesquiterpenoid with one α,β-unsaturated ketone functionality (δC 214.5, 191.7, and 128.5), one exocyclic double bond (δC 156.3 and 116.5), two quaternary carbon atoms (δC 77.4 and 43.6), one methine, four methylenes including two oxygenated groups (δC 78.6 and 71.8), and two methyl groups (δC 24.5 and 10.5). Based on its 1H‒1H COSY and HMBC correlations (Fig. 2), the planar structure of 8, namely, 7,8,13-trihydroxyrotunda-1,11-dien-3-one, was deduced to be the same as that of compounds 6 and 7. The H-4α, H-5α, H-8β, and H-13β configurations were determined by the key ROESY correlations of H3-15/H-6α, H3-15/H-6β, H-5/H-9α, and H-2/H-13 (Fig. 2) and by comparing J values in its 1H NMR spectrum with those of compounds 6 and 7. Finally, the absolute configuration of 8 was elucidated to be (4R,5S,7S,8S,10S,13S)-7,8,13-trihydroxyrotunda-1,11-dien-3-one, a C-4 epimer of 7, based on the ECD calculations (Fig. 5).
NMR data of C-5 to C-8 in agarotetrol (9) were not correctly assigned before [7, 8], which were revised by 2D NMR correlations (Additional file 1: Fig. S2). NMR data of 4ʹ-methoxyagarotetrol (11) in DMSO-d6 [12], 2ʹ-hydroxyagarotetrol (13) in DMSO-d6 [13], (5S,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone (19) in DMSO-d6 [14], and (−)-aquisinenone G (21) in CDCl3 [15] have been reported in the literature. Their NMR data in methanol-d4 are presented in this paper. Other known compounds, isoagarotetrol (10) [8], 4ʹ-methoxyisoagarotetrol (12) [16], (5S,6R,7S,8R,7ʹR)-7ʹ-hydroxyisoagarotetrol (15) [10], (5S,6R,7S,8R,7′S)-7′-hydroxyisoagarotetrol (16) [10], (5S,6S,7S,8R)‑8‑chloro‑2‑(2‑phenylethyl)‑5,6,7‑trihydroxy‑5,6,7,8‑tetrahydrochromone (14) [5, 17], 6-hydroxy-2-(2-phenylethyl)chromone (17) [18], 2,2ʹ-di-(2-phenylethyl)-8,6ʹ-dihydroxy-5,5ʹ-bichromone (18) [19], (5R,6R,7R,8S)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone (20) [11], and syringin (22) [20], were identified by comparing their spectroscopic data with those in literature.
Rat adrenal pheochromocytoma (PC12) cell injury induced by corticosterone is an in vitro model for screening neuroprotective and antidepressant compounds [6]. All isolates except for compound 3 were evaluated for their protective activities against corticosterone-induced damage in PC12 cells. After testing these compounds at a single concentration of 20 µM (Table 5), several compounds were selected for testing at gradient concentrations of 2.5, 5, 10, 20, and 40 µM. Among them, (6S,7S,8R)-2‑(2-phenylethyl)‑6,7,8‑trihydroxy‑5,6,7,8‑tetrahydrochromone (4), (4S,5S,7S,8S,10S,13R)-7,8,13-trihydroxyrotunda-1,11-dien-3-one (6), agarotetrol (9), and 6-hydroxy-2-(2-phenylethyl)chromone (17) showed the most protective activities against corticosterone-induced PC12 cell injury at concentrations from 5 to 40 µM (P < 0.001) (Table 6). Among the chromone derivatives (1–5 and 9–21), the types and positions of substituent groups seem to have effects on the activity, although no obvious patterns of structure-activity relationships (SAR) were observed. Nevertheless, a hydroxy substituent at C-2ʹ or C-7ʹ would reduce the activity by comparing the bioassay data of agarotetrol (9) and their derivatives (1, 2, 11, and 13) (Tables 5 and 6).
Table 5.
The effects of compounds at a single concentration on PC12 cell injury induced by corticosteronea
| Compound | Concentration (µM) | Survival rate ± SD (%)b |
|---|---|---|
| Blank | – | 100.00 ± 0.22*** |
| Negative control | – | 59.92 ± 0.33 |
| Desipramine (positive control) | 10 | 89.66 ± 0.78*** |
| 1 | 20 | 60.24 ± 0.51 |
| 2 | 20 | 59.27 ± 1.01 |
| 4 | 20 | 72.14 ± 1.35*** |
| 6 | 20 | 74.79 ± 0.73*** |
| 7 | 20 | 63.10 ± 0.82** |
| 9 | 20 | 79.50 ± 1.79*** |
| 10 | 20 | 68.50 ± 1.43*** |
| 11 | 20 | 65.28 ± 1.54** |
| 12 | 20 | 71.64 ± 1.08*** |
| 17 | 20 | 76.24 ± 1.10*** |
| Blank | – | 100.00 ± 0.73*** |
| Negative control | – | 60.83 ± 0.93 |
| Desipramine (positive control) | 10 | 90.07 ± 0.45*** |
| 5 | 20 | 60.26 ± 1.14 |
| 8 | 20 | 60.18 ± 1.84 |
| 13 | 20 | 54.29 ± 1.04 |
| 14 | 20 | 60.16 ± 1.20 |
| 15 | 20 | 67.07 ± 1.27** |
| 16 | 20 | 59.47 ± 0.81 |
| 18 | 20 | 60.27 ± 1.24 |
| 19 | 20 | 60.27 ± 1.55 |
| 20 | 20 | 65.69 ± 0.57** |
| 21 | 20 | 71.34 ± 1.01*** |
| 22 | 20 | 60.55 ± 1.21 |
aThe concentration of corticosterone was 150 μM
bCompared with the negative control, **P < 0.01, ***P < 0.001
Table 6.
The effects of compounds at gradient concentrations on PC12 cell injury induced by corticosteronea
| Compound | Concentration (µM) | Survival rate ± SD (%)b |
|---|---|---|
| Blank | – | 100.01 ± 0.77*** |
| Negative control | – | 60.29 ± 0.44 |
| Desipramine (positive control) | 10 | 89.74 ± 0.58*** |
| 4 | 40 | 70.30 ± 0.12*** |
| 20 | 72.18 ± 0.33*** | |
| 10 | 67.46 ± 0.61*** | |
| 5 | 64.05 ± 0.50*** | |
| 2.5 | 60.39 ± 0.50 | |
| 6 | 40 | 71.77 ± 0.39*** |
| 20 | 74.22 ± 0.54*** | |
| 10 | 69.90 ± 0.26*** | |
| 5 | 66.76 ± 0.79*** | |
| 2.5 | 60.18 ± 0.53 | |
| 7 | 40 | 68.02 ± 0.44*** |
| 20 | 63.67 ± 0.27*** | |
| 10 | 60.79 ± 0.42 | |
| 5 | 60.16 ± 0.25 | |
| 2.5 | 59.97 ± 0.39 | |
| 9 | 40 | 72.00 ± 0.98*** |
| 20 | 79.11 ± 0.80*** | |
| 10 | 73.53 ± 0.39*** | |
| 5 | 64.87 ± 0.49*** | |
| 2.5 | 60.17 ± 0.41 | |
| 10 | 40 | 66.05 ± 0.21*** |
| 20 | 68.63 ± 1.05*** | |
| 10 | 64.60 ± 0.27*** | |
| 5 | 60.06 ± 0.34 | |
| 2.5 | 60.21 ± 0.30 | |
| 11 | 40 | 70.06 ± 0.15*** |
| 20 | 65.87 ± 0.37*** | |
| 10 | 63.91 ± 0.19*** | |
| 5 | 59.97 ± 0.36 | |
| 2.5 | 60.13 ± 0.38 | |
| 12 | 40 | 71.66 ± 0.22*** |
| 20 | 70.86 ± 0.54*** | |
| 10 | 63.50 ± 0.85** | |
| 5 | 62.01 ± 0.54* | |
| 2.5 | 59.98 ± 0.39 | |
| 17 | 40 | 77.87 ± 0.70*** |
| 20 | 76.06 ± 0.40*** | |
| 10 | 71.62 ± 0.40*** | |
| 5 | 66.26 ± 1.07*** | |
| 2.5 | 60.39 ± 0.50 |
aThe concentration of corticosterone was 150 μM
bCompared with the negative control, *P < 0.05, **P < 0.01, ***P < 0.001
Experimental section
General experimental procedures
The material and instruments used for isolating compounds and measuring spectroscopic data are provided in the Additional file 1.
Plant material
The plant material was purchased from the Flagship Store of Jiabaohua Pharmacy, Zhuhai, Guangdong, China (order number: 172979790097330735) in June 2018, produced by Guangdong Huiqun Chinese Traditional Medicine Co., Ltd., Shantou, Guangdong, China (lot number: 20171101), and identified as the resinous heartwood of Aquilaria sinensis (Lour.) Spreng. by Professor Shu-De Yang at Yunnan University of Traditional Chinese Medicine, China. The voucher specimen (No. GD171101) was kept in the Key Laboratory of Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences.
Extraction and isolation
The dried resinous heartwood of A. sinensis (2.9 kg) was ultrasonically extracted with 90% EtOH (10 L × 5) at 60 °C for half an hour each time. The crude extract (459.3 g) was suspended in 1 L of water, followed by extraction with petroleum ether (1 L × 5), EtOAc (1 L × 5), and n-BuOH (1 L × 5). After removing the solvent, the petroleum ether-soluble fraction (0.7 g), EtOAc-soluble fraction (374.8 g), and n-BuOH-soluble fraction (55.9 g) were obtained.
The n-BuOH-soluble fraction (55.9 g) was separated by a silica gel column with EtOAc/MeOH (100:0 → 0:1, v/v) as the eluent to yield five further fractions (Fr. 1 to Fr. 5). Fr. 1 (786.8 mg) was subjected to a reversed-phase (RP) C18 silica gel column (MeOH/H2O, 5% → 100%) to yield 10 further fractions (Fr. 1-1 to Fr. 1-10). Compound 17 (2.1 mg) is obtained from Fr. 1–6 by recrystallization (MeOH).
Fr. 2 (16.7 g) was purified by an RP C18 column (MeOH/H2O, 5% → 100%) to yield 13 further fractions (Fr. 2-1 to Fr. 2-13). Fr. 2-6 was recrystallized from MeOH to yield 9 (1.1 g). Fr. 2-3 (456.6 mg) was applied to a silica gel column via elution by CH2Cl2/MeOH (50:1 → 1:1) to yield five further fractions (Fr. 2-3-1 to Fr. 2-3-5). Fr. 2-3-2 (114.7 mg) was purified by semipreparative HPLC (Welch Ultimate AQ-C18, ϕ 7.8 × 250 mm; MeOH/H2O, 20:80, v = 2 mL/min) to yield 6 (10.6 mg, tR = 15.072 min) and 7 (11.9 mg, tR = 22.831 min). Fr. 2-3-5 (58.3 mg) was further purified by semipreparative HPLC (Welch Ultimate AQ-C18, ϕ 7.8 × 250 mm; MeOH/H2O, 40:60, v = 2 mL/min) to yield 1 (14.1 mg, tR = 9.282 min) and 2 (7.1 mg, tR = 10.161 min). Fr. 2-3-4 (41.3 mg) was separated by a silica gel column (CH2Cl2/MeOH, 30:1 → 1:1) and was then purified by semipreparative HPLC (Welch Ultimate AQ-C18, ϕ 7.8 × 250 mm; MeCN/H2O, 10:90, v = 2 mL/min) to yield 15 (1.5 mg, tR = 22.158 min). Fr. 2-8 (524.3 mg) was separated by a silica gel column (CH2Cl2/MeOH, 50:1) to yield 11 further fractions (Fr. 2-8-1 to Fr. 2-8-11). Fr. 2-8-6 was recrystallized from MeOH to yield 12 (204.0 mg). Fr. 2-8-9 (51.3 mg) was purified by semipreparative HPLC (Welch Ultimate AQ-C18, ϕ 7.8 × 250 mm; MeOH/H2O, 30:70, v = 2 mL/min) to yield 4 (4.2 mg, tR = 37.011 min) and 3 (2.0 mg, tR = 41.898 min). Fr. 2-8-10 (49.3 mg) was applied to a silica gel column via elution by CH2Cl2/MeOH (30:1 → 1:1) and was further purified by semipreparative HPLC (Welch Ultimate AQ-C18, ϕ 7.8 × 250 mm; MeOH/H2O, 33:67, v = 2 mL/min) to yield 11 (1.4 mg, tR = 21.190 min). Fr. 2-9 (489.4 mg) was applied to a silica gel column and eluted with CH2Cl2/MeOH (100:1 → 1:1) to yield five further fractions (Fr. 2–9-1 to Fr. 2-9-5). Fr. 2-9-2 (118.8 mg) was separated by Sephadex LH-20 (MeOH) and was further purified by semipreparative HPLC (YMC-Pack ODS-A, ϕ 10 × 250 mm; MeOH/H2O, 40:60, v = 2 mL/min) to yield 14 (2.9 mg, tR = 14.865 min). Fr 2-9-4 (46.9 mg) was purified by Sephadex LH-20 (MeOH) to yield 10 (4.0 mg). Fr 2-12 (599.0 mg) was subjected to a silica gel column via elution by CH2Cl2/MeOH (100:1 → 1:1) to yield five further fractions (Fr. 2-12-1 to Fr. 2-12-5). Fr. 2-12-1 was recrystallized from MeOH to yield 18 (1.2 mg). Fr. 2-12-2 (164.1 mg) was applied to a silica gel column (CH2Cl2/MeOH, 100:1 → 1:1) to yield three further fractions (Fr. 2-12-2-1 to Fr. 2-12-2-3). Fr. 2-12-2-1 (80.0 mg) was purified by semipreparative HPLC (YMC-Pack ODS-A, ϕ 10 × 250 mm; MeCN/H2O, 48:52, v = 2 mL/min) to yield 21 (4.0 mg, tR = 27.886 min). Fr. 2-12-2-3 (60.9 mg) was purified by semipreparative HPLC (Welch Ultimate AQ-C18, ϕ 7.8 × 250 mm; MeOH/H2O, 65:35, v = 2 mL/min) to yield 5 (2.3 mg, tR = 26.957 min). Fr. 2-12-4 (29.0 mg) was separated by Sephadex LH-20 gel column chromatography (MeOH) and then by semipreparative HPLC (Welch Ultimate AQ-C18, ϕ 7.8 × 250 mm; MeOH/H2O, 65:35, v = 2 mL/min) to yield 19 (3.7 mg, tR = 25.515 min) and 20 (3.9 mg, tR = 30.107 min).
Fr. 3 (6.0 g) was separated by an RP C18 silica gel column (MeOH/H2O, 5% → 100%) to yield 11 further fractions (Fr. 3-1 to Fr. 3-11). Fr. 3–3 (158.0 mg) was separated by a silica gel column (CH2Cl2/MeOH, 50:1) and then purified by semipreparative HPLC (Welch Ultimate AQ-C18, ϕ 7.8 × 250 mm; MeOH/H2O, 30:70, v = 2 mL/min) to yield 8 (0.8 mg, tR = 10.757 min). Fr. 3-4 (195.5 mg) was separated by a silica gel column (CH2Cl2/MeOH, 50:1) and was then purified by semipreparative HPLC (YMC-Pack ODS-A, ϕ 10 × 250 mm; MeOH/H2O, 35:65, v = 2 mL/min) to yield 22 (7.1 mg, tR = 11.198 min) and 16 (0.9 mg, tR = 15.727 min). Fr. 3-5 (39.0 mg) was purified by semipreparative HPLC (YMC-Pack ODS-A, ϕ 10 × 250 mm; MeCN/H2O, 20:80, v = 2 mL/min) to yield 13 (7.5 mg, tR = 31.475 min).
Spectroscopic data of compounds 1–9, 11, 13, and 19–21
(5S,6R,7R,8S,7′R)-7′-hydroxyagarotetrol (1)
Colorless needle crystal (MeOH); [α]21D − 29.1 (c = 0.13, MeOH); UV (MeOH) λmax (logε) 252 (4.03), 207 (4.17) nm; ECD (c 0.013, MeOH) λmax (Δε) 298 (+ 0.68), 261 (− 0.14), 245 (+ 0.13), 222 (− 1.02), 212 (+ 1.35), 194 (+ 3.92) nm; IR vmax (KBr) 3406, 1658, 1601, 1448, 1089, 1057, 1039, 1018, 701 cm−1; 1H NMR and 13C NMR data see Table 1; ESIMS (positive) m/z 357 [M + Na]+, 691 [2 M + Na]+; HRESIMS (positive) m/z 357.0942 [M + Na]+ (calcd for C17H18NaO7, 357.0950).
Crystal data of compound 1: C17H18O7·2(H2O), M = 370.34, a = 5.5739(3) Å, b = 8.0353(5) Å, c = 19.5345(12) Å, α = 90°, β = 97.596(2)°, γ = 90°, V = 867.23(9) Å3, T = 100(2) K, space group P1211, Z = 2, μ(Cu Kα) = 0.987 mm−1, 7517 reflections measured, 2687 independent reflections (Rint = 0.0345). The final R1 values were 0.0287 [I > 2σ(I)]. The final wR(F2) values were 0.0916 [I > 2σ(I)]. The final R1 values were 0.0292 (all data). The final wR(F2) values were 0.0929 (all data). The goodness of fit on F2 was 0.837. Flack parameter = 0.11(10). The crystallographic data for the structure of 1 have been deposited in the Cambridge Crystallographic Data Centre (deposition number CCDC 2,118,605). Copies of the data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk.
(5S,6R,7R,8S,7′S)-7′-hydroxyagarotetrol (2)
Light yellow powder; [α]21D − 28.3 (c = 0.35, MeOH); UV (MeOH) λmax (logε) 252 (3.85), 207 (4.00) nm; ECD (c 0.018, MeOH) λmax (Δε) 301 (+ 0.14), 254 (− 0.93), 223 (+ 1.69) nm; IR vmax (KBr) 3423, 1658, 1601, 1447, 1384, 1044, 703 cm−1; 1H NMR and 13C NMR data see Table 1; ESIMS (positive) m/z 357 [M + Na]+; HRESIMS (positive) m/z 357.0945 [M + Na]+ (calcd for C17H18NaO7, 357.0950).
(6S,7S,8R)-2‑[2‑(4-methoxyphenyl)ethyl]‑6,7,8‑trihydroxy‑5,6,7,8‑tetrahydrochromone (3)
Colorless plate crystal (MeOH); [α]27D + 7.4 (c = 0.23, MeOH); UV (MeOH) λmax (logε) 435 (1.81), 365 (2.05), 254 (3.88), 220 (3.99), 201(4.04) nm; ECD (c 0.0099, MeOH) λmax (Δε) 221 (− 1.43) nm; 1H NMR and 13C NMR data see Table 2; ESIMS (positive) m/z 302 [M + Na]+, 627 [2 M + Na]+; HRESIMS (positive) m/z 355.1150 [M + Na]+ (calcd for C18H20NaO6, 355.1158).
Crystal data of compound 3: C18H20O6, M = 332.34, a = 4.9601(2) Å, b = 7.0550(2) Å, c = 23.2769(7) Å, α = 90°, β = 93.5950(10)°, γ = 90°, V = 812.94(5) Å3, T = 100.(2) K, space group P1211, Z = 2, μ(Cu Kα) = 0.850 mm−1, 12,448 reflections measured, 3180 independent reflections (Rint = 0.0352). The final R1 values were 0.0271 [I > 2σ(I)]. The final wR(F2) values were 0.0704 [I > 2σ(I)]. The final R1 values were 0.0277 (all data). The final wR(F2) values were 0.0710 (all data). The goodness of fit on F2 was 1.031. Flack parameter = 0.09(4). The crystallographic data for the structure of 3 have been deposited in the Cambridge Crystallographic Data Centre (deposition number CCDC 2118606). Copies of the data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk.
(6S,7S,8R)-2‑(2-phenylethyl)‑6,7,8‑trihydroxy‑5,6,7,8‑tetrahydrochromone (4)
Yellow powder; [α]27D + 14.2 (c = 0.50, MeOH); UV (MeOH) λmax (logε) 372 (2.22), 254 (4.07), 209 (4.19) nm; ECD (c 0.0072, MeOH) λmax (Δε) 233 (− 3.14) nm; 1H NMR and 13C NMR data see Table 2; ESIMS (positive) m/z 302 [M + Na]+, 627 [2 M + Na]+; HRESIMS (positive) m/z 325.1046 [M + Na]+ (calcd for C17H18NaO5, 325.1052).
(5S,6R,7S,8R)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8- [2-(2-phenylethyl)-7-methoxychromonyl-6-oxy]chromone (5)
Yellow powder; [α]20D + 98.6 (c = 0.10, MeOH); UV (MeOH) λmax (logε) 314 (4.29), 237 (4.85), 204 (5.02) nm; ECD (c 0.0041, MeOH) λmax (Δε) 320 (+ 5.57), 280 (− 3.87), 249 (+ 9.33), 229 (− 1.47), 207 (− 20.23) nm; IR vmax (KBr) 3387, 1657, 1603, 1504, 1453, 1384, 1271, 1216, 1197, 1101, 1080, 998, 957, 847, 749, 700 cm−1; 1H NMR and 13C NMR data see Table 3; ESIMS (positive) m/z 619 [M + Na]+; HRESIMS (positive) m/z 619.1939 [M + Na]+ (calcd C35H32NaO9, 619.1944).
(4S,5S,7S,8S,10S,13R)-7,8,13-trihydroxyrotunda-1,11-dien-3-one (6)
Pale yellow oil; [α]27D − 95.4 (c = 0.50, MeOH); UV (MeOH) λmax (logε) 234 (4.12) (3.81) nm; ECD (c 0.015, MeOH) λmax (Δε) 246 (+ 2.59), 203 (− 13.63) nm; IR νmax (KBr) 3416, 2962, 2932, 2872, 1686, 1683, 1600, 1456, 1384, 1281, 1196, 1082, 1044, 1025, 997, 930 cm−1; 1H NMR and 13C NMR data see Table 4; ESIMS (negative) m/z 299 [M + Cl]−, 309 [M + HCOO−]+; ESIMS (positive) m/z 287 [M + Na]+, 551 [2 M + Na]+; HRESIMS (positive) m/z 287.1255 [M + Na]+ (calcd for C15H20NaO4, 287.1259).
(4S,5S,7S,8S,10S,13S)-7,8,13-trihydroxyrotunda-1,11-dien-3-one (7)
Pale yellow oil; [α]27D − 87.1 (c = 0.09, MeOH); UV (MeOH) λmax (logε) 236 (4.02) nm; ECD (c 0.018, MeOH) λmax (Δε) 258 (+ 0.52), 222 (− 6.96) nm; IR νmax (KBr) 3425, 2964, 2927, 2874, 2852, 1688, 1633, 1597, 1456, 1384, 1284, 1188, 1073, 1055, 1028, 982, 928 cm−1; 1H NMR and 13C NMR data see Table 4; ESIMS (positive) m/z 287 [M + Na]+, 551 [2 M + Na]+; HRESIMS (positive) m/z 287.1253 [M + Na]+ (calcd for C15H20NaO4, 287.1259).
(4R,5S,7S,8S,10S,13S)-7,8,13-trihydroxyrotunda-1,11-dien-3-one (8)
Pale yellow oil; [α]25D − 13.3 (c = 0.05, MeOH); UV (MeOH) λmax (logε) 235 (4.01) nm; ECD (c 0.015, MeOH) λmax (Δε) 223 (− 6.08) nm; 1H NMR and 13C NMR data see Table 4; ESIMS (positive) m/z 287 [M + Na]+, 551 [2 M + Na]+; EIMS m/z (rel. int.) 264 [M]+ (30), 246 (15), 179 (47), 136 (77), 91 (68), 55 (90), 43 (100); HREIMS m/z 264.1359 [M]+ (calcd for C15H20O4, 264.1362).
Agarotetrol (9)
White solid; [α]18D − 17.9 (c = 0.20, MeOH); 1H NMR (methanol-d4, 500 MHz) δ 7.26 (2H, m, H-3ʹ,5ʹ), 7.22 (2H, m, H-2ʹ,6ʹ), 7.18 (1H, m, H-4ʹ), 6.12 (1H, s, H-3), 4.74 (1H, d, J = 4.0 Hz, H-5), 4.57 (1H, d, J = 7.5 Hz, H-8), 4.04 (1H, dd, J = 7.5, 2.3 Hz, H-7), 4.02 (1H, dd, J = 4.0, 2.3 Hz, H-6), 3.03 (2H, dd, J = 7.7, 7.3 Hz, H2-7ʹ), 2.93 (2H, m, H2-8ʹ); 13C NMR (methanol-d4, 126 MHz) δ 182.0 (C-4), 171.2 (C-2), 165.4 (C-8a), 141.2 (C-1ʹ), 129.6 (C-3ʹ,5ʹ), 129.5 (C-2ʹ,6ʹ), 127.5 (C-4ʹ), 121.8 (C-4a), 114.1 (C-3), 74.0 (C-6), 72.4 (C-7), 70.1 (C-8), 66.7 (C-5), 36.3 (C-8ʹ), 33.7 (C-7ʹ); ESIMS (positive) m/z 341 [M + Na]+, 659 [2 M + Na]+.
4′-Methoxyagarotetrol (11)
Light yellow solid; [α]24D − 18.3 (c = 0.10, MeOH); 1H NMR (methanol-d4, 500 MHz) δ 7.13 (2H, br d, J = 8.6 Hz, H-2ʹ,6ʹ), 6.82 (2H, br d, J = 8.6 Hz, H-3ʹ,5ʹ), 6.10 (1H, s, H-3), 4.74 (1H, d, J = 4.0 Hz, H-5), 4.56 (1H, d, J = 7.5 Hz, H-8), 4.04 (1H, dd, J = 7.5, 2.3 Hz, H-7), 4.01 (1H, dd, J = 4.0, 2.3 Hz, H-6), 3.74 (3H, s, 4ʹ-OMe), 2.89 (2H, m, H2-8ʹ), 2.97 (2H, m, H2-7ʹ); 13C NMR (methanol-d4, 126 MHz) δ 182.0 (C-4), 171.4 (C-2), 165.4 (C-8a), 159.8 (C-4ʹ), 133.1 (C-1ʹ), 130.4 (C-2ʹ,6ʹ), 121.7 (C-4a), 115.0 (C-3ʹ,5ʹ), 114.1 (C-3), 74.0 (C-6), 72.4 (C-7), 70.1 (C-8), 66.7 (C-5), 55.6 (4ʹ-OMe), 36.5 (C-8ʹ), 32.9 (C-7ʹ); ESIMS (positive) m/z 371 [M + Na]+, 719 [2 M + Na]+; HRESIMS (positive) m/z 371.1100 [M + Na]+ (calcd for C18H20NaO7, 371.1107).
2′-Hydroxyagarotetrol (13)
Light yellow solid; [α]28D − 8.3 (c = 0.18, MeOH); 1H NMR (methanol-d4, 500 MHz) δ 7.05 (1H, dd, J = 7.4, 1.6 Hz, H-6ʹ), 7.01 (1H, ddd, J = 8.0, 7.4, 1.6 Hz, H-4ʹ), 6.74 (1H, br d, J = 8.0 Hz, H-3ʹ), 6.72 (1H, ddd, J = 7.4, 7.4, 1.2 Hz, H-5ʹ), 6.10 (1H, s, H-3), 4.74 (1H, d, J = 4.0 Hz, H-5), 4.56 (1H, d, J = 7.4 Hz, H-8), 4.04 (1H, dd, J = 7.4, 2.3 Hz, H-7), 4.02 (1H, dd, J = 4.0, 2.3 Hz, H-6), 3.00 (2H, m, H2-7ʹ), 2.92 (2H, m, H2-8ʹ); 13C NMR (methanol-d4, 126 MHz) δ 182.1 (C-4), 172.0 (C-2), 165.4 (C-8a), 156.5 (C-2ʹ), 131.2 (C-6ʹ), 128.7 (C-4ʹ), 127.3 (C-1ʹ), 121.6 (C-4a), 120.6 (C-5ʹ), 115.9 (C-3ʹ), 113.8 (C-3), 74.0 (C-6), 72.5 (C-7), 70.1 (C-8), 66.7 (C-5), 34.7 (C-8ʹ), 29.0 (C-7ʹ); ESIMS (positive) m/z 357 [M + Na]+, 691 [2 M + Na]+.
(5S,6R,7R,8S)-2-(2-Phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8- [2-(2-phenylethyl)chromonyl-6-oxy]chromone (19)
Yellow powder; [α]24D − 75.8 (c = 0.38, MeOH); 1H NMR (methanol-d4, 500 MHz) Unit A δ 7.15 (2H, m, H-3ʹ,5ʹ), 7.10 (1H, m, H-4ʹ), 6.92 (2H, m, H-2ʹ,6ʹ), 6.15 (1H, s, H-3), 5.46 (1H, d, J = 7.7 Hz, H-8), 4.80 (1H, d, J = 3.7 Hz, H-5), 4.38 (1H, dd, J = 7.7, 2.3 Hz, H-7), 4.10 (1H, dd, J = 3.7, 2.3 Hz, H-6), 2.71 (2H, m, H2-8ʹ), 2.62 (2H, m, H2-7ʹ); Unit B δ 7.89 (1H, d, J = 2.5 Hz, H-5), 7.62 (1H, dd, J = 9.2, 2.5 Hz, H-7), 7.60 (1H, d, J = 9.2 Hz, H-8), 7.24 (2H, m, H-3ʹ,5ʹ), 7.22 (2H, m, H-2ʹ,6ʹ), 7.16 (1H, m, H-4ʹ), 6.16 (1H, s, H-3), 3.08 (2H, m, H2-7ʹ), 3.03 (2H, m, H2-8ʹ); 13C NMR (methanol-d4, 126 MHz) Unit A δ 181.5 (C-4), 170.9 (C-2), 162.8 (C-8a), 140.9 (C-1ʹ), 129.4 (C-3ʹ,5ʹ), 129.2 (C-2ʹ,6ʹ), 127.4 (C-4ʹ), 122.7 (C-4a), 114.5 (C-3), 78.2 (C-8), 74.5 (C-6), 70.8 (C-7), 66.3 (C-5), 36.2 (C-8ʹ), 33.5 (C-7ʹ); Unit B δ 180.2 (C-4), 171.6 (C-2), 158.6 (C-6), 153.4 (C-8a), 141.2 (C-1ʹ), 129.6 (C-3ʹ,5ʹ), 129.5 (C-2ʹ,6ʹ), 127.5 (C-4ʹ), 126.2 (C-7), 125.0 (C-4a), 120.8 (C-8), 110.4 (C-5), 110.1 (C-3), 37.0 (C-8ʹ), 34.0 (C-7ʹ); ESIMS (positive) m/z 589 [M + Na]+; HRESIMS (positive) m/z 589.1831 [M + Na]+ (calcd for C34H30NaO8, 589.1838).
(5S,6R,7S,8R)-2-(2-phenylethyl)-5,6,7-trihydroxy-5,6,7,8-tetrahydro-8-[2-(2-phenylethyl)chromonyl-6-oxy]chromone (20)
Yellow powder; [α]23D + 22.5 (c = 0.23, MeOH); ECD (c 0.01, MeOH) λmax (Δε) 243 (+ 8.07), 216 (− 8.85) nm; ESIMS (positive) m/z 567 [M + H]+; HRESIMS (positive) m/z 589.1833 [M + Na]+ (calcd C34H30NaO8, 589.1838).
(−)-Aquisinenone G (21)
Yellow powder; [α]20D − 26.8 (c = 0.22, MeOH); UV (MeOH) λmax (logε) 314 (3.99), 229 (4.58) nm; ECD (c 0.007, MeOH) λmax (Δε) 313 (− 1.36), 277 (+ 4.38), 249 (− 5.58), 221 (− 7.71), 205 (+ 8.69) nm; 1H NMR (methanol-d4, 500 MHz) Unit A δ 7.16 (1H, m, H-4ʹ), 7.08 (2H, m, H-3ʹ,5ʹ), 6.95 (2H, m, H-2ʹ,6ʹ), 6.03 (1H, s, H-3), 5.52 (1H, dd, J = 3.0, 2.0 Hz, H-5), 4.77 (1H, m, H-7), 4.59 (1H, dd, J = 3.3, 3.0 Hz, H-6), 4.45 (1H, br s, H-8), 2.86 (2H, m, H2-7ʹ), 2.80 (2H, m, H2-8ʹ); Unit B δ 7.67 (1H, s, H-5), 7.23 (2H, m, H-3ʹ,5ʹ), 7.20 (2H, m, H-2ʹ,6ʹ), 7.06 (1H, m, H-4ʹ), 6.89 (1H, s, H-8), 6.12 (1H, s, H-3), 3.02 (2H, m, H2-7ʹ), 2.96 (2H, m, H2-8ʹ); 13C NMR (methanol-d4, 126 MHz) Unit A δ 179.8 (C-4), 171.5 (C-2), 166.0 (C-8a), 140.8 (C-1ʹ), 129.4 (C-2ʹ,3ʹ,5ʹ,6ʹ), 127.5 (C-4ʹ), 117.4 (C-4a), 114.5 (C-3), 79.3 (C-7), 72.6 (C-5), 69.7 (C-6), 66.8 (C-8), 36.1 (C-8ʹ), 33.6 (C-7ʹ); Unit B δ 179.5 (C-4), 171.6 (C-2), 155.0 (C-8a), 154.5 (C-7), 147.0 (C-6), 141.2 (C-1ʹ), 129.6 (C-3ʹ,5ʹ), 129.3 (C-2ʹ,6ʹ), 127.3 (C-4ʹ), 120.9 (C-4a), 119.1 (C-5), 112.5 (C-8), 110.2 (C-3), 36.9 (C-8ʹ), 33.9 (C-7ʹ); ESIMS (positive) m/z 587 [M + Na]+.
Computational methods
Theoretical calculations of ECD spectra for compounds 6–8 were performed with the Gaussian 16 program package [21]. The preliminary conformational distribution search was performed by Tripos sybyl- × 2 software [22]. Selected conformers with distributions higher than 1% were further optimized by the DFT method at the B3LYP/6-311 g (d) level in the Gaussian 16 program package. The ECD of the conformer of selected conformers was then calculated by the TD-DFT method at the CAM-B3LYP/tzvp levels with the PCM model in methanol solution. The overall calculated ECD curves were weighted by Boltzmann distribution. The calculated ECD spectra were produced by SpecDis 1.71 [23]. Detailed calculated parameters are provided in the Additional file 1.
Corticosterone‑induced damage in PC12 cellular assay
The method for bioassay testing was carried out according to our previously published papers [5, 6].
Conclusion
Five new 2-(2-phenylethyl)chromone derivatives, three new sesquiterpenoids, and 14 known compounds were isolated from the resinous heartwood of Aquilaria sinensis. The neuroprotective activities of these isolates were evaluated using an in vitro model of PC12 cell injury induced by corticosterone. (6S,7S,8R)-2‑(2-Phenylethyl)‑6,7,8‑trihydroxy‑5,6,7,8‑tetrahydrochromone (4), (4S,5S,7S,8S,10S,13R)-7,8,13-trihydroxyrotunda-1,11-dien-3-one (6), agarotetrol (9), and 6-hydroxy-2-(2-phenylethyl)chromone (17) showed the most protective activities against corticosterone-induced PC12 cell injury at concentrations from 5 to 40 µM (P < 0.001).
Supplementary Information
Additional file 1. Chemical structures of known compounds (9–22), key 2D NMR correlations of agarotetrol (9), general experimental procedures, computational methods for ECD of compounds 6–8, and NMR, HRMS, and ECD spectra of compounds 1–8.
Acknowledgements
This study was supported by Beijing Sino-Science Aquilaria Technology Co., Ltd., Beijing, China (no. KET202101).
Authors’ contributions
All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare that there are no conflicts of interest associated with this work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lu Zhang and Ping Yi contributed equally to this work
Contributor Information
Yue-Qiu He, Email: ynfh2007@163.com.
Yue-Hu Wang, Email: wangyuehu@mail.kib.ac.cn.
References
- 1.Editorial Board of Chinese Pharmacopoeia . Chinese Pharmacopoeia. Beijing: China Medical Science Press; 2020. pp. 192–193. [Google Scholar]
- 2.Su J, Liu Z, Li R-C, Yang X-J. Literature research of traditional Chinese medicine prescription containing agilawood. China J Tradit Chin Med Pharm. 2017;32:1853–1855. [Google Scholar]
- 3.Peng D, Wang C, Liu Y, Wei J. Research progress on the chemical constituents of Aquilariae Lignum Resinatum and their pharmacological activities. Chin J Mod Appl Pharm. 2021;38:358–365. [Google Scholar]
- 4.Gonçalves S, Mansinhos I, Romano A. Aromatic plants: a source of compounds with antioxidant and neuroprotective effects. In: Martin CR, Preedy VR, editors. Oxidative stress and dietary antioxidants in neurological diseases. Amsterdam: Elsevier; 2020. pp. 155–173. [Google Scholar]
- 5.Wei S-Y, Hu D-B, Xia M-Y, Luo J-F, Yan H, Yang J-H, Wang Y-S, Wang Y-H. Sesquiterpenoids and 2-(2-phenylethyl)chromone derivatives from the resinous heartwood of Aquilaria sinensis. Nat Prod Bioprosp. 2021;11:545–555. doi: 10.1007/s13659-021-00313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Q, Hu D-B, Zhang L, Xia M-Y, Yan H, Li X-N, Luo J-F, Wang Y-S, Yang J-H, Wang Y-H. Neuroprotective compounds from the resinous heartwood of Aquilaria sinensis. Phytochemistry. 2021;181:112554. doi: 10.1016/j.phytochem.2020.112554. [DOI] [PubMed] [Google Scholar]
- 7.Yoshii E, Koizumi T, Oribe T, Takeuchi F, Kubo K. The structure of agarotetrol, a novel highly oxygenated chromone from agarwood (jinko) Tetrahedron Lett. 1978;19:3921–3924. doi: 10.1016/S0040-4039(01)95098-1. [DOI] [Google Scholar]
- 8.Shimada Y, Konishi T, Kiyosawa S, Nishi M, Miyahara K, Kawasaki T. Studies on the agalwood (Jinko). IV. Structures of 2-(2-phenylethyl)chromone derivatives, agarotetrol and isoagarotetrol. Chem Pharm Bull. 1986;34:2766–2773. doi: 10.1248/cpb.34.2766. [DOI] [Google Scholar]
- 9.Sugiyama T, Narukawa Y, Shibata S, Masui R, Kiuchi F. Three new 5,6,7,8-tetrahydroxy-5,6,7,8-tetrahydrochromone derivatives enantiomeric to agarotetrol from agarwood. J Nat Med. 2018;72:667–674. doi: 10.1007/s11418-018-1201-2. [DOI] [PubMed] [Google Scholar]
- 10.Konishi T, Sugimoto A, Kiyosawa S, Fujiwara Y. Studies on the agalwood “Jinko”. XII. Structures of pentahydroxy-2-(2-phenylethy)chromone derivatives. Chem Pharm Bull. 1992;40:778–779. doi: 10.1248/cpb.40.778. [DOI] [Google Scholar]
- 11.Xiang P, Mei W, Chen H, Kong F, Wang H, Liao G, Zhou L, Dai H. Four new bi-phenylethylchromones from artificial agarwood. Fitoterapia. 2017;120:61–66. doi: 10.1016/j.fitote.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Zheng K-M, Zhang J-Q, Shen P-N, Zhuo C. Preparative isolation and purification of agarotetrol and 4′-methoxyagarotetrol from Aquilaria sinensis (Lour.) Gilg by high-speed counter-current chromatography. Chin Tradit Pat Med. 2011;33:96–99. [Google Scholar]
- 13.Konishi T, Iwagoe K, Sugimoto A, Kiyosawa S, Fujiwara Y, Shimada Y. Studies on agalwood (Jinko). X. Structures of 2-(2-phenylethyl)chromone derivatives. Chem Pharm Bull. 1991;39:207–209. doi: 10.1248/cpb.39.207. [DOI] [Google Scholar]
- 14.Iwagoe K, Kakae T, Konishi T, Kiyosawa S, Fujiwara Y, Shimada Y, Miyahara K, Kawasaki T. Studies on the agalwood (Jinko). VIII. Structures of bi-phenylethylchromone derivatives. Chem Pharm Bull. 1989;37:124–128. doi: 10.1248/cpb.37.124. [DOI] [Google Scholar]
- 15.Huo H-X, Zhu Z-X, Song Y-L, Shi S-P, Sun J, Sun H, Zhao Y-F, Zheng J, Ferreira D, Zjawiony JK, Tu P-F, Li J. Anti-inflammatory dimeric 2-(2-phenylethyl)chromones from the resinous wood of Aquilaria sinensis. J Nat Prod. 2017;81:543–553. doi: 10.1021/acs.jnatprod.7b00919. [DOI] [PubMed] [Google Scholar]
- 16.Shimada Y, Konishi T, Kiyosawa S. Studies on the agalwood (Jinko). VI. Structures of three 2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone derivatives, AH1A, AH2a and AH2b. Chem Pharm Bull. 1986;34:3033–3037. doi: 10.1248/cpb.34.3033. [DOI] [Google Scholar]
- 17.Yagura T, Ito M, Kiuchi F, Honda G, Shimada Y. Four new 2-(2-phenylethyl)chromone derivatives from withered wood of Aquilaria sinensis. Chem Pharm Bull. 2003;51:560–564. doi: 10.1248/cpb.51.560. [DOI] [PubMed] [Google Scholar]
- 18.Yang JS, Wang YL, Su YL. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. V.: isolation and characterization of three 2-(2-phenylethyl)chromone derivatives. Acta Pharm Sin. 1989;24:678–683. [PubMed] [Google Scholar]
- 19.Iwagoe K, Konishi T, Kiyosawa S, Shimada Y, Miyahara K, Kawasaki T. The structures of AH10 and AH11, nobel bi-phenylethylchromones from agalwood. Chem Pharm Bull. 1986;34:4889–4891. doi: 10.1248/cpb.34.4889. [DOI] [Google Scholar]
- 20.Kitajima J, Ishikawa T, Tanaka Y, Ono M, Ito Y, Nohara T. Water-soluble constituents of fennel. V. Glycosides of aromatic compounds. Chem Pharm Bull. 1998;46:1587–1590. doi: 10.1248/cpb.46.1587. [DOI] [Google Scholar]
- 21.Gaussian 16 Citation, http://gaussian.com/citation/ (accessed on December 17, 2021).
- 22.SYBYL-X 2.1.1, Tripos International, 1699 South Hanley Rd., St. Louis, Missouri, 63144, USA.
- 23.Bruhn T, Schaumlöffel A, Hemberger Y, Pecitelli G. SpecDis version 1.71, Berlin, Germany, 2017. http://specdis-software.jimdo.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Chemical structures of known compounds (9–22), key 2D NMR correlations of agarotetrol (9), general experimental procedures, computational methods for ECD of compounds 6–8, and NMR, HRMS, and ECD spectra of compounds 1–8.