Abstract
Glycophytic plants are susceptible to salinity and their growth is hampered in more than 40 mM of salt. Salinity not only affects crop yield but also limits available land for farming by decreasing its fertility. Presence of distinct traits in response to environmental conditions might result in evolutionary adaptations. A better understanding of salinity tolerance through a comprehensive study of how Na+ is transported will help in the development of plants with improved salinity tolerance and might lead to increased yield of crops growing in strenuous environment. Ion transporters play pivotal role in salt homeostasis and maintain low cytotoxic effect in the cell. High-affinity potassium transporters are the critical class of integral membrane proteins found in plants. It mainly functions to remove excess Na+ from the transpiration stream to prevent sodium toxicity in the salt-sensitive shoot and leaf tissues. However, there are large number of HKT proteins expressed in plants, and it is possible that these members perform in a wide range of functions. Understanding their mechanism and functions will aid in further manipulation and genetic transformation of different crops. This review focuses on current knowledge of ion selectivity and molecular mechanisms controlling HKT gene expression. The current review highlights the mechanism of different HKT transporters from different plant sources and how this knowledge could prove as a valuable tool to improve crop productivity.
Keywords: High-affinity potassium transporter (HKT), Ion homeostasis, Salinity stress, Sodium transport
Introduction:
Salinity is one of the major abiotic stress factors for agricultural crops. Sodium chloride (NaCl) is the most abundant salt found in saline soil. Approximately 10% of the cultivated and half of the irrigated lands are highly salt affected (Shabala 2013; Dang et al. 2013). At global level, 952.2 million ha of land is affected by soil salinity (Arora et al. 2016) and in India alone, approximately 6.74 million ha area is salt-affected (Kumar and Sharma 2020). Na+ is not essential for plant growth, and in higher concentration it can disrupt cellular processes by interfering with some enzymes such as those involved in starch degradation in chloroplast, antioxidative enzymes such as CAT (catalase), SOD (superoxide dismutase), GR (glutathione reductase), APX (ascorbate peroxidase), and enzymes involved in Calvin cycle (Acosta-Motos et al. 2017). Salt tolerant plants alleviate Na+ toxicity from sensitive shoot tissues by limiting absorption of Na+ from the soil, reducing the transport of Na+ to xylem of shoot, exclusion of Na+ from young leaves by storing it in lower part of leaf sheath or in old leaves.
Membrane embedded transporter proteins are important for improving the efficiency of plants to uptake and use ions. P-type H+-ATPases which hydrolyse ATP to pump H+ into the cell wall is responsible for active Na+ uptake into cells across the plasma membrane (Reinhold and Guy 2002), whereas active ion transport into the vacuole is driven by V-type H+-ATPases, which hydrolyse ATP to pump H+ into the vacuole (Binzel and Ratajczak 2002). In addition, there is an H+-PPiase on the tonoplast, which hydrolyses pyrophosphate to pump H+ into the vacuole (Binzel and Ratajczak 2002; Gaxiola et al. 2001). No exquisite Na+ pumps have been reported in higher plants. However, in fungi and mosses there is a Na+ pump, ENA1, which hydrolyses ATP to pump Na+ out of the cell (Benito and Rodríguez-Navarro 2003). Na+ that enters a cell will have one of the three fates—Na+ can enter into an adjacent cell via plasmodesmata; it can be effluxed back into the cell wall; or it can be ‘compartmentalized’ (i.e. transported into the vacuole). Cyclic-nucleotide gated channels (CNGCs), glutamate receptors (GLRs), non-selective cation channels (NSCCs) and HKT transporters (AtHKT1;1, OsHKT1;4, OsHKT1;5 and OsHKT2;1) are responsible for influx of Na+ into cells. Na+ efflux occurs through Na+/H+ antiporters on the plasma membrane by SOS1 (Zhu 2003), while compartmentalization occurs through vacuolar Na+/H+ antiporters such as NHX1 (Blumwald 2000). Intracellular NHX transporters are included into the IC-NHE/NHX family (Pardo et al. 2006), which is a part of the large cation/proton antiporter 1 (CPA1) family (Saier et al. 2006). The IC clades are further subdivided into two major groups Class-I and Class II. It is reported that Class-I type contains AtNHX1-4 and Class-II type has AtNHX5-6 in Arabidopsis (Yokoi et al. 2002). Class-I NHX proteins are localized on vacuolar membrane, whereas Class-II members are found on endosomal vesicles (Pardo et al. 2006). NHX proteins act as Na+/H+ and K+/H+ antiporters and are driven by proton gradient generated by vacuolar H+-ATPase and H+-PPase (Apse et al. 2003; Barragán et al. 2012; Bassil et al. 2011; Rodríguez-Rosales et al. 2008). Under salt stress condition, the Na+ toxicity in plant cells is also reduced by exclusion of Na+ from cytosol via plasma membrane. This is accomplished through one of the most widely characterized transporters SOS1 in Arabidopsis (Blumwald 2000; Shi et al. 2000; Wu et al. 1996). The SOS1 gene help in extruding Na+ from the cytoplasm and accumulate in the apoplast region. Similar to NHX, SOS1 also belongs to CPA1 family and functions as Na+/H+ antiporter. SOS1 interacts with the serine/threonine protein kinase (SOS2), which further interacts with the calcium binding protein (SOS3), which is activated by a higher concentration of Ca2+ in the cytosol (Brett et al. 2005; Shi et al. 2000; Wu et al. 1996).
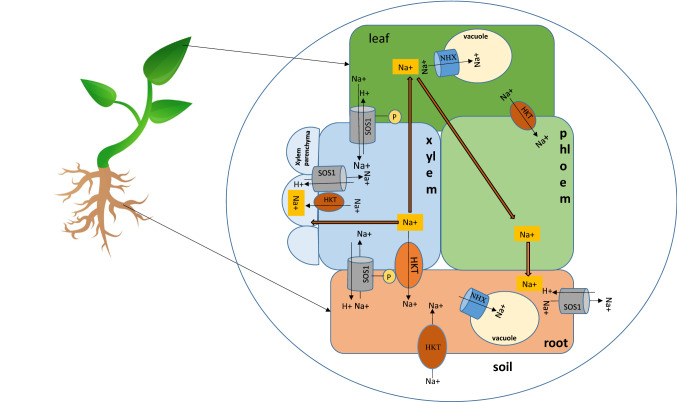
The potassium transporters in plants such as the K+ channels (Shaker family) and transporters (HKT, HAK) may regulate Na+ transport—either directly, because they may be incompletely selective for K+ and transport Na+ when it is present in higher concentration or cells have a high Na+/K+ ratio; or indirectly, by buffering the cells against Na+ uptake by maintaining rigorous K+ homeostasis. Amongst these potassium transporters, HKTs are an essential class of important membrane protein (IMPs) that transport cations across plasma membranes mainly from shoot tissues. An Internationally agreed nomenclature divides the HKT family into subfamily 1 and subfamily 2 (Platten et al. 2006). The two HKT subfamilies can be distinguished on the basis of gene organization. HKT Gene organization (from start to stop codon) of some plants with known genomic DNA sequences was studied and it was observed that all HKT genes contains two introns. However, the size of the introns were observed to be larger in the subfamily 1 as compared to subfamily 2, while no difference was seen in the size of exons in the two subfamilies (Platten et al. 2006). The members of subfamily 1 contains serine residue at the first pore-loop domain, and they show higher selectivity for Na+ than K+, whereas the subfamily 2 has a glycine residue in place of serine and function as Na+/ K+ co-transporters (Horie et al. 2001; Platten et al. 2006). HKT transporters express mostly in vascular bundles, xylem, phloem, root cortex and epidermis. While type 1 are located exclusively in the vasculature (very rarely in other places as exceptions), the type 2 transporters are found in the root epidermis and tissues surrounding the vasculature (Jabnoune et al. 2009). HKT transporters are involved in preventing Na+ accumulation in shoots and leaves by redirecting it into roots (Fig. 1).
Fig. 1.
Schematic representation of Na+ uptake and translocation in glycophytes via Na+ and/or K+ transporters and channels
Manipulating HKT gene expression through genetic engineering and conventional plant breeding methods proved as a great tool to improve the salinity tolerance of commercially important crop plants (Table 1). However, Na+–K+ transport mechanism still presents several loopholes, and the practical understanding of HKT transporters requires further input. The present review provides a detailed discussion of the fundamental research carried out to study HKT transporters in different plant species, emphasizing on their functional mechanism, localization and potential role in improving stress-tolerance and yield in major crops.
Table 1.
The detailed functional characterization of different HKT types
| S no | Gene/size (bp)/accession no | Plant species | Functional characterization | Localization | Functions | References |
|---|---|---|---|---|---|---|
| 1 | AtHKT1;1/1521 bp/AF237672 | Arabidopsis thaliana | Xenopus laevis oocyte and saccharomyces cerevisiae | Prominently in roots | Selective Na+ uptake | Uozumi et al. (2000) |
| atHKT1/sos3 double mutants | Rus et al. (2001) | |||||
| athkt1 null mutants | Root stele, leaf vasculature | Controls root/shoot Na+ distribution and counteracts salt stress in leaves by reducing leaf Na+ accumulation | Mäser et al. (2002) | |||
| sas2 mutants | Recirculates Na+ from shoots to roots via phloem | Berthomieu et al. (2003) | ||||
| Control both root accumulation of Na+ and retrieval of Na+ from the xylem, but is not involved in recirculation in the phloem | Davenport et al. (2007) | |||||
| Nicotiana tabacum | Xylem of stem, root and leaf vein | Improved activities of antioxidant enzymes, enhanced physiochemical traits and plant productivity | Wang et al. (2018) | |||
| Solanum tuberosum | Promotes the K+/Na+ homeostasis that minimizes osmotic imbalance, maintains photosynthesis and stomatal conductance, and increases plant productivity | Wang et al. (2019) | ||||
| 2 | CmHKT1;1/1461 bp/Ch10G003830.1 | Cucurbita moschata | Ectopic expression in Cucumis sativus and transient expression in N. tabcum | Root stele | Decreases the Na+ content in shoots | Sun et al. (2018) |
| 3 | CmHKT1;1/1491 bp/MK986658 | Cucumis melo | X. laevis oocyte, S. cerevisiae and A. thaliana | Abundant in roots than stem and leaves | UNLOAD NA+ FROM THE XYLEM AND MAINTAIN HIGH K+/NA+ RATIOS DURING SALT STRESS | Gao et al. (2020) |
| 4 | EcHKT1;1, /1986 bp/AF176035 EcHKT1;2/1909 bp/AF176036 | Eucalyptus camaldulensis | E.coli, X. laevis, | More in shoots than roots | K+ Na+ symporter. Limit the transport of Na+ around the plant by excluding Na+ from the transpiration stream | Fairbairn et al. (2000) |
| X. laevis | K+ uptake over Na+ | Liu et al. (2001) | ||||
| 5 | EpHKT1;1, EpHKT1;2 | Eutrema parvula | X. laevis oocyte, S. cerevisiae & A. thaliana | EpHKT1;2 acts as K+ transporter with low affinity to Na+ | Ali et al. 2018) | |
| 6 | GmHKT1;1/138 bp | Glycine max | N. tabacum | Root and shoot | Na+ and K+ transport in roots and shoots | Chen et al. (2010) |
| 7 | HvHKT1;1/1925 bp/AM000056 | Hordeum vulgare | X. laevis oocyte, S. cerevisiae, Knockout in H. vulgare and overexpression in Arabidopsis | Root stele and epidermis | Radial root Na+ transport, which eventually reduces shoot Na+ accumulation | Han et al. (2018) |
| 8 | McHKT1/1600 bp/AF367366 | Mesembry-anthemum crystallinum | X. laevis oocyte, S. cerevisiae | Highly expressed in plasma membrane of leaves, present in stem flowers and seed pods but absent in roots | – | Su et al. (2003) |
| 9 | OsHKT1;1/1669 bp/AJ491816 | Oryza sativa | X. laevis oocyte and S. cerevisiae | – | Na+ uptake in roots during K+ deficit condition | Jabnoune et al. (2009) |
| 10 | OsHKT1;3/1679 bp/AJ491818 | O. sativa | X. laevis and loss of function mutants in O. sativa | Vascular tissues, root periphery and osmocontractile leaf, bulliform cells | Accumulation of Na+ in old leaves | Jabnoune et al. (2009), Abdulhussein et al. (2018) |
| 11 | PeHKT1;1 | (Populus davidiana x Populus bolleana) | Populus | Improved physiology, increased expression of CAT, SOD, and POD genes related with oxidative stress tolerance | Xu et al. (2018) | |
| 12 | PhaHKT1/1906 bp/AB234305 | Phragmites australis | S. cerevisiae | Better salt tolerance and acquisition of K+ | Takahashi et al. (2007) | |
| 13 | PpHKT1/3000 bp/FN401372 | Physcomitrella patens | S. cerevisiae and knockout P. patens | High affinity Na+ uptake | Haro et al. (2010) | |
| 14 | ZmHKT1;5/1404 bp | Zea mays | N. tabacum | Improves salt tolerance by maintaining lower Na+/K+ ratio in plants and regulating ROS metabolism | Jiang et al. (2018) | |
| 15 | ReHKT1;1/1857 bp/ LC350016 | Reaumuria trigyna | X. laevis and A. thaliana | Improved Na+ and K+ uptake in roots, promote K+ accumulation and prevent Na+ transport from roots to leaves | Li et al. (2018) | |
| 16 | SlHKT1;2/1512 bp/HE962484 | Solanum lycopersicum | S. cerevisiae | Asins et al. (2013) | ||
| 17 | HvHKT2;1/531 bp/CAJ01326 | H. vulgare | X. laevis oocyte, S. cerevisiae and H. vulgare | Root cortex | Na+/K+ symporter in heterologous system. In barley, Na+ is concentrated in xylem sap and leaves | Mian et al. (2011) |
| 18 | PutHKT2;1/1778 bp/FJ716169 | Pucinellia tenuiflora | S. cerevisiae and P. tenuiflora | High affinity K+–Na+ symport in yeast. Na+ uptake at higher external K+ concentration and increased sensitivity to Na+, K+ and Li+ | Ardie et al. (2009) | |
| 19 | OsHKT2;1/1875 bp/AB061311 | O. sativa | X. laevis Transposon insertion rice lines and Tobacco BY2 cells | Root cortex and vascular bundle of leaves | Na+ uptake, even at low concentrations of Na+ and in absence of K+ | Golldack et al. (2002); Horie et al. (2007); Jabnoune et al. (2009); Yao et al. (2010) |
| 20 | OsHKT2;2/1593 bp/EF373552 | O. sativa | X. laevis oocyte, S. cerevisiae and Tobacco BY2 cells | Na+ uptake in presence of K+ and Na+ stimulated K+ uptake | Horie et al. (2001); Yao et al. (2010) | |
| 21 | OsHKT2;4/3414 bp/KT796353 | O. sativa | X. laevis oocyte, S. cerevisiae and O. sativa transgenics | Root hair and vascular parenchyma, bulliform cells | Mainly K+ transporter. Unique function as Ca2+ and Mg2+ permeable non selective ion channels | Lan et al. (2010); Horie et al. (2011) |
| 22 | PutHKT2;1/1778 bp/FJ716169 | Pucinellia tenuiflora | S. cerevisiae and P. tenuiflora | High affinity K+–Na+ symport in yeast. Na+ uptake at higher external K+ concentration and increased sensitivity to Na+, K+ and Li+ | Ardie et al. (2009) |
Role of subfamily HKT1 in asserting salt tolerance
The HKT protein structure was elucidated for the first time by comparing sequences of HKTs and the K+ channel KcsA from Streptomyces lividans (Durell et al. 1999). The HKT polypeptide comprises of a hydrophobic core made up of four tandemly arranged protomeric “MPM” (Membrane Pore Membrane) domains (a MPM domain comprises of one transmembrane segment, one P loop and one transmembrane segment), and a short N- and C-terminal cytosolic tail. The “MPM” domains successively arranged in a single polypeptide chain (M1PAM2A–M1PBM2B–M1PCM2C–M1PDM2D), and organized in a fourfold radial symmetry. The P loops aggregate at the centre of the hydrophobic core forming a central permeation pathway. The MPM domain topology of HKTs proved by biochemical (Kato et al. 2001) and crystal structure analysis of bacterial Trk/Ktr homologues (Cao et al. 2013; Vieira-Pires et al. 2013). On the basis of structural differences and selectivity of the pore forming region, the HKT genes are divided into the subfamily I (SGGG-type) and II (GGGG-type) (Platten et al. 2006).
Initially, the functional characterization of different HKTs was achieved by studying its expression in a heterologous system such as yeast, E. coli and oocytes. AtHKT1;1 transporter protein from Arabidopis was first identified (Uozumi et al. 2000) as a homolog of wheat TaHKT1 (now referred to as TaHKT2;1) and functions as Na+ selective transporter when expressed in yeast in high Na+ containing medium. AtHKT1;1 did not complement growth in yeast mutants defective in K+ uptake, when grown in K+ deficient medium. However, in the E. coli expression system, K+ uptake was observed in AtHKT1;1 expressing cells in K+ limiting medium (Uozumi et al. 2000). These observations conclude that AtHKT1; 1 functions as Na+ transporter and to a lesser extent, a K+ transporter in heterologous expression systems.
Another interesting observation was that extracellular Ca2+ concentration is known to activate high affinity K+ uptake system (Rains and Epstein 1967; Maathuis et al. 1996) which in turn aids to overcome Na+ mediated inhibition of K+. During stress, Ca2+ acts as a signal for activating a high-affinity K+ uptake via SOS3 intermediate (in SOS signal transduction pathway). At the same time, SOS3 also activates transcription of a plasma membrane Na+/H+ antiporter—SOS1. Two extragenic mutations in Arabidopsis that suppresses NaCl sensitivity of the sos3–1 mutant were identified. sos3–1 hkt1–1 and sos3–1 hkt1–2 plants showed allelic mutations in AtHKT1;1, leading to its inactivation, were selected for the study. The hkt1–1 and hkt1–2 mutations showed equal suppression of Na+ hypersensitivity in sos3-1 mutant seedlings and reduced the accumulation of intracellular Na+. It was also observed that sos3–1 hkt1–1 and sos3–1 hkt1–2 seedlings are better in maintaining K+ concentration intracellularly in NaCl supplemented medium and also exhibits a substantially higher intracellular ratio of K+/ Na+ than the sos3–1 mutant alone. The hkt1 mutations nullifies the growth inhibition in the sos3–1 mutant even at low K+ and Ca2+ in the culture medium. This result conclude that AtHKT1;1 functions as a high-affinity K+ uptake transporter and controls Na+ entry into the cell (Rus et al. 2001). The hkt1 mutant study also revealed that there must be another Na+ transport system that reduces Na+ uptake in the presence of high Ca2+ (external). AtHKT1;1 was found to be localized in root stele and leaf vasculature but not in the root tip (Mäser et al. 2001).
Arabidopsis lines with disrupted AtHKT1;1 led to lower Na+ content in roots, and therefore, root growth became less sensitive to the presence of Na+. This result correlates with athkt1/sos3 double mutant analysis. The athkt1 null mutant plants showed a higher Na+ content in shoots than in wild-type, which indicates presence of a pathway for Na+ translocation from root to shoot that works independent of AtHKT1;1. During salt stress, the AtHKT1;1 negates the effect of such transporters (Mäser et al. 2001). Using an antibody raised to AtHKT1;1, Sunarpi et al. (2005) demonstrated localization of the AtHKT1;1 protein to the plasma membrane of xylem parenchyma cells in the shoot, in accordance with a direct role for AtHKT1;1 in withdrawal of Na+ from the xylem in the shoot. To conclude, it can be said that AtHKT1;1 functions to prevent Na+ accumulation in shoots and leaves by sequestering Na+ in roots, unloading Na+ from xylem (from xylem vessels to xylem parenchyma cells; (Sunarpi et al. 2005) and redirecting excess Na+ from leaves to root via the phloem. Two allelic mutations sas2-1 and sas2-2 (Sodium over accumulation in shoots on the basis of phenotype displayed by the mutants as compared to wild type) were identified as Na+ accumulator in shoots. Positional cloning and sequencing of the two mutant alleles indicated that the sas2 locus corresponds to the AtHKT1;1 gene (Berthomieu et al. 2003). The sas2-1 mutation led to over accumulation of Na+ in aerial parts, mainly leaves and decreased Na+ concentration in roots. In addition, these mutants were highly sensitive to salt stress and could not survive even in moderate salinity. These observations indicate that sas2 locus is involved in controlling Na+ accumulation in shoots and corresponds to the AtHKT1;1 gene.
Several hypotheses have been put forth in relation to AtHKT1;1 function in Arabidopsis. Rus et al. (2001) proposed that AtHKT1;1 was an influx pathway for Na+ uptake into the root in Arabidopsis. He observed that hkt1;1 mutations improved the sos phenotypes and reduced Na+ content in the seedlings of sos3 mutant lines. On the contrary, Berthomieu et al. (2003) and Davenport et al. (2007) showed that hkt1;1 mutants did not have lower root Na+ influx, and further proposed that AtHKT1;1 functioned in Na+ recirculation from shoots to roots by loading Na+ from the shoot into phloem and then unloading it into the roots for efflux. This hypothesis supported the observation of shoot Na+ hyper-accumulation and reduced root Na+ in hkt1;1 mutants, and reduced phloem Na+ content and apparent phloem localization of AtHKT1;1 transcripts (Berthomieu et al. 2003). Furthermore, Rus et al. (2004) demonstrated that AtHKT1;1 plays a role in regulation of K+ and Na+ homeostasis using knockout mutants. Until this time, hypotheses regarding the role of AtHKT1;1 in Na+ transport mostly relied on measurements of tissue ion contents, which are the net result of a number of different transport processes (Davenport et al. 2007). Many of the previous experiments were conducted in plants grown on agar plates (with limited transpiration) or in soil (where the effect of nutrients and toxic ions was not accounted for), and thus makes it difficult to compare the results of different experiments. However, Davenport et al. (2007) conducted the experiment in hydroponic system and minimal media under control conditions, so that reproducible results could be obtained. They used radioactive tracers to identify the individual transport processes separately contributing to Na+ and K+ accumulation in intact, transpiring plants to test whether AtHKT1;1 was involved in (1) root Na+ influx, (2) recirculation from shoot to root, (3) Na+ retrieval from the xylem and (4) K+ influx. By measuring unidirectional fluxes using radioactive tracers, they confirmed that AtHKT1;1 does not have any role in either Na+ recirculation via the phloem nor is responsible for Na+ influx into roots. On the other hand, AtHKT1;1 appears to control retrieval of Na+ from the xylem and loading in root vacuole.
For functional characterization in heterologous system, AtHKT1;1 gene was overexpressed in tobacco (Wang et al. 2018) and potato (Wang et al. 2019), and the transgenic lines showed constant K+ concentration, reduced Na+ content in leaf, and better physicochemical behavior under salt stress. In addition, the over-expression of AtHKT1;1 confers multiple functions, such as enhanced activity of antioxidants, higher chlorophyll content, soluble sugars, decreased proline and MDA contents and electrolyte leakage destruction. These minimize cell damage under high salt concentrations and subsequently increase plant productivity. Thus, overexpression of AtHKT1;1 in heterologous plants through a transgenic approach may help in improving crop productivity under salinity (Wang et al. 2019). Further studies were undertaken to understand the regulation of AtHKT1;1 transporters. Role of cytokinins in response to salt salinity via regulating AtHKT1;1 transporter was identified (Mäser et al. 2001). Cytokinins regulates salinity through TFs—ARR1 and ARR12 that modifies Na+ transport from roots to shoots by modulating the expression of AtHKT1;1 (Fig. 2) (Mason et al. 2010).
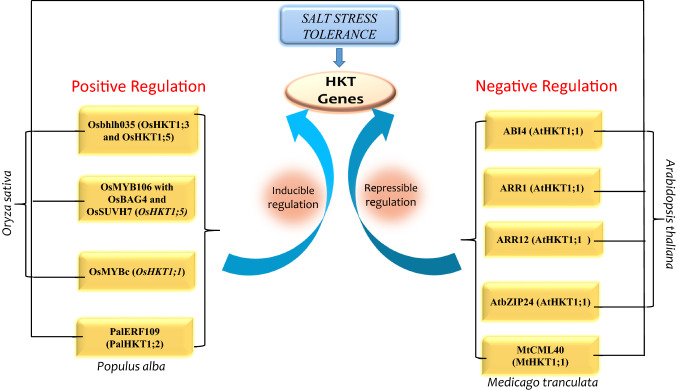
Fig. 2.
Regulatory mechanism of HKT genes. ABI4, ARR1, ARR12, AtbZIP24 (AtHKT1;1) and CML (MtHKT1;1) proteins negatively regulate expression of HKT genes. ARR1 and ARR12 in presence of cytokinin negatively regulates HKT gene. Osbhlh035, OsMYB106, MYBc and ERF are positive regulators of HKT gene. The TFs; ABI4 (abscisic acid insensitive 4); ARR (Arabidopsis response regulator); CML (calmodulin such as protein); ERF (ethylene response factor), control the respective HKT genes from different plant species, which are given in brackets
Genetic variation for improving the salt tolerance in durum wheat was extensively studied. Durum wheat (Triticum turgidum L. subsp. Durum, AABB, tetraploid) is more salt sensitive than bread wheat (Triticum aestivum, AABBDD, hexaploid) probably because of its poor ability to maintain low Na+ concentration in leaves (Gorham et al. 1990; Rawson et al. 1988). Different strategies were implemented to introduce salt tolerance into durum wheat-traditional breeding techniques using physiology-based phenotyping, marker-assisted selection, and transformation of genes known to improve Na+ exclusion or tissue tolerance (Lindsay et al. 2004). Hexaploid wheat (ABD genomes) showed Na+ accumulation and enhanced K+/Na+ ratio, a character identified to be located on chromosome 4D (Gorham et al. 1987) on the D genome (Shah et al. 1987). This had an important salinity tolerance locus (Kna1) on long arm of chromosome 4D (4DL) (Dubcovsky et al. 1996).
Tetraploid wheat (AB genomes) showed high Na+ accumulation and poor K+/Na+ ratio as they lack this trait. (Shah et al. 1987). Munns et al. (2000) identified a novel source of Na+ exclusion in a durum landrace. This landrace showed very low Na+ accumulation in the leaf blade, almost equivalent to bread wheat cultivars, and maintained a high rate of K+ accumulation, with consequent high K+/Na+ ratios. There may be a possibility that the low-Na+ durum landrace carries a homologue of the Kna1 locus.
Wide genetic variations in Na+ accumulation was found in durum wheat, and a particular landrace was named as Line 149 for breeding. A locus for the Na+ exclusion trait (Nax) in Line 149 was successfully mapped using a QTL approach. Line 149 was derived from a cross between Triticum monococcum (AA) accession C68–101 and the durum cv Marrocos. Here, T. monococcum (C68–101) was used as the source of the Nax1 and Nax2 genes in Line 149 (James et al. 2006). Genetic analysis of the populations developed by the cross between genotypes with low and high rates of Na+ uptake, indicated two dominant and interacting genes (Munns and James 2003). It is likely that one gene controls the loading of Na+ in the xylem in the roots, while the other controls the retrieval of Na+ from the xylem in the lower part of the leaves (Davenport et al. 2005). A locus for one gene, designated Nax1 (Na+ exclusion), was mapped to the long arm of chromosome 2A using a QTL approach with AFLP, RFLP, and microsatellite markers (Lindsay et al. 2004). Nax1 was mapped on chromosome 2AL (Lindsay et al. 2004) and Nax2 was mapped on chromosome 5AL (Byrt et al. 2007) that allowed the removal of excess Na+ from root xylem and leaf sheath, resulting in low ion concentration in leaf blade (James et al. 2006). Two putative sodium transporter genes (TmHKT7) related to OsHKT7 were mapped to chromosome 2AL. One TmHKT7 member (TmHKT7-A1) was polymorphic between the salt-tolerant and -sensitive lines, and co-segregated with Nax1 in the high-resolution mapping family. The other member (TmHKT7-A2) was situated approximately 145 bp from TmHKT7-A1. TmHKT7-A2 was found to be expressed in roots and leaf sheaths of the salt-tolerant durum wheat Line 149. The expression pattern of TmHKT7-A2 was consistent with the physiological role of Nax1 in reducing Na+ concentration in leaf blades by retaining Na+ in the sheaths (Huang et al. 2006). Sequence analyses of known Na+ transporter genes, mainly HKTs (Rubio et al. 1995; Schachtman and Schroeder 1994) within the rough mapping regions of Kna1, Nax1 and Nax2 have suggested that all three of these major salinity tolerance QTLs are attributed to polymorphisms in copies of wheat HKT genes, TmHKT1;4-A1 and TmHKT1;4-A2 (also named TmHKT7-A1 and -A2) for Nax1 and TmHKT1;5 and TaHKT1;5 for Nax2 and Kna1 (Byrt et al. 2007, 2014; James et al. 2011). Field trials showed improved salt tolerance as well as a 25% increase grain yield in NILs (Near isogenic lines) with Nax2 (TmHKT1;5 A) as compared to durum wheat NIL without Nax2. These findings could pave the way for developing T. aestivum (bread wheat) NIL with Nax1, Nax2 genes showing improved grain yield and salt tolerance. Interestingly, The Nax2 chromosomal region of a salt-tolerant durum wheat, Line149, was found to correspond to the Kna1 region of T. aestivum, suggesting that Kna1 and Nax2 are orthologous (James et al. 2006). A homologous gene of Nax2 (TmHKT1;5-A) was observed at the Kna1 locus (on chromosome 4D; TaHKT1;5-D). It was observed that TaHKT1;5-D retrieves Na+ from the xylem vessels in the root and has an important role in restricting the transport of Na+ from the root to the leaves. Thus, TaHKT1;5-D confers salinity tolerance in bread wheat through a mechanism associated with the Kna1 locus via shoot Na+ exclusion and maintaining a high K+/Na+ ratio in the leaves (Byrt et al. 2014).
Plants are classified as salt-includers and salt-excluders based on their salt stress adaptive strategies. Salt-includers uptake Na+ and translocate it to the shoot, where it is compartmentalized in the vacuole and used as alternative vacuolar osmoticum to maintain the water potential, whereas salt excluders avoid Na+ accumulation. Initial studies of HKT function focused the role of HKT transporters in salt-excluder species mostly in type-1 HKT. Two foremost conclusions that have been drawn so far are—first, Na+ is transported to the shoots and accumulated in leaves by Na+ unloading (term used is ‘desalinization’) from the ascending xylem sap and then loading back into the descending phloem sap, leading to recirculation of sodium from leaves to roots. The second is that the genetic manipulation of HKT transport activity leading to improved plant tolerance to salt stress, is always in conjunction with a decrease in leaf Na+ content (Berthomieu et al. 2003; Davenport et al. 2007; Møller et al. 2009; Rus et al. 2004; Sunarpi et al. 2005). HvHKT1;1, isolated from Tibetan barley accession was functionally characterized in heterologous systems (yeast and Xenopus) showed higher selectivity for Na+ over K+ and other monovalent cations (Han et al. 2018). Furthermore, the overexpression of HvHKT1;1 in Arabidopsis was found to be important in radial root Na+ transport which eventually led to Na+ accumulation in shoot. In addition, it was found that HvHKT1;1 may be indirectly involved in the homeostasis of K+ and Ca2+ in roots, which also improves plant salt tolerance (Han et al. 2018).
Cucumber is a common vegetable crop cultivated worldwide and is sensitive to salt stress (Mian et al. 2011). Cucumber germplasm does not have salt-tolerant genotypes (Tiwari et al. 2010). Not many papers are available on breeding techniques to increase the salt tolerance of cucumber (Kere et al. 2013). Other techniques to overcome salt stress in cucumber such as using pumpkin as rootstock have gained popularity (Huang et al. 2011, 2013; Lei et al. 2014). Pumpkin could restrict Na+ transport from root to shoot. This was experimentally proved by Lei et al. (2014), using atomic absorption spectroscopy (AAS), Scanning Ion-selective Electrode Technique (SIET), and X-ray microanalysis. Li et al. observed that Na+/H+ antiporter system in plasma membrane causes a high Na+ efflux from the root to the external solution. The root cortical cells of pumpkin have a higher capacity to sequester Na+ into the vacuoles that decreases the Na+ transport into the root stele and xylem. CmHKT1;1 from Cucurbita moschata play a vital role in the grafted plants with salt-tolerant pumpkin as the rootstock by improving the growth of cucumber under different salt conditions via restricting Na+ transport from the pumpkin rootstock to the cucumber scion (Sun et al. 2018). Thus, CmHKT1;1 plays an essential role in the Na+ homeostasis and salt tolerance when grafted with cucumber plants. The findings may prove useful for engineering salt tolerance in cucurbit crops.
Another plant species, the Poplars (Populus sp.), are economically important perennial woody plants and widely grown for its fast growth and high yield. A Class I type transporter of the HKT family was isolated and characterized from hybrid poplar (Xu et al. 2018). Overexpression of PeHKT1;1 in transgenic poplar resulted in improved growth of root and plant height and also showed higher transcript expression of CAT, SOD, and POD genes than non-transgenic poplar under salt stress. These observations indicated that PeHKT1;1 might boost the salt tolerance by improving the efficiency of antioxidant systems that influence plant growth and metabolism under salt stress conditions (Xu et al. 2018).
Extensive research has been done in various rice cultivars for understanding salt tolerance. Rice is sensitive to salt stress and its sensitivity varies among genotypes, indicating natural variation in regulatory mechanisms and genetic makeup. Rice cultivars, ‘Nona Bokra’ and ‘Pokkali’ are salt tolerant, whereas ‘Nipponbare’ is salt sensitive. In rice, as many as nine HKTs have been isolated and characterized. OsHKT2;1 showed the symport activity of both Na+ and K+, while OsHKT1;1 OsHKT1;3 and OsHKT1;5 were found to be permeable to Na+ only (Garciadeblás et al. 2003; Jabnoune et al. 2009; Ren et al. 2005). OsHKT1;3 removes excess Na+ from young leaves and redirects it into old leaves and roots. At the same time, it was found that the K+ concentration reduced in old leaves and roots (Abdulhussein et al. 2018; Jabnoune et al. 2009). Quantitative trait loci (QTLs) controls many complex agronomic traits including salt tolerance. Mapping and isolating these QTLs paved the way to study genetic control of complex tolerance mechanisms in plants. Using an F2 Oryza sativa population derived from a cross between a salt-tolerant indica variety, Nona Bokra and a susceptible elite japonica variety, Koshihikari, eight QTLs responsible for the variations in their K+ or Na+ content were mapped. The main QTLs were—shoot K+ concentration on chromosome 1 (qSKC-1) and shoot Na+ concentration on chromosome 7 (qSNC-7; Lin et al. 2004). The extent of salt tolerance in rice varieties was observed due to allelic variations (Thomson et al. 2010). SKC1 locus is mainly responsible for maintaining higher K+ concentration in shoots during salt stress. OsHKT1; 5 transporter is present in SKC1 locus, is a Na+ selective transporter. It was observed that SKC1 alleles do not show a difference in phloem Na+ content; therefore, the loading and unloading of phloem Na+ might be done by other members of the OsHKT family (Ren et al. 2005). Kobayashi et al. used immuno-staining to indicate robust expression of OsHKT1;5 in the phloem of some diffused vascular bundles of basal nodes connected to young leaves. They inferred that OsHKT1;5 also mediates Na+ exclusion in phloem parenchyma cells in the basal nodes to prevent Na+ transfer to phloem sieve and Na+ translocation to young leaf blades (Kobayashi et al. 2017). A major QTL, named Saltol was found to be associated with the Na+–K+ ratio and seedling-stage salinity tolerance in rice variety Pokkali, on chromosome 1. A comparison of background introgression location on Pokkali and Nona Bokra (Ren et al. 2005) allele found that Pokkali Saltol QTL might be controlled by the same gene as the SKC1 QTL (Thomson et al. 2010).
The association of amino acid substitutions, with salt tolerance was studied across rice genotypes. Analysis of 3D structure of HKT1;5 protein showed that aspartic acid (at position 332) and valine (at 395 position) are at the opposite ends of the embedded protein in the transmembrane region and close to the channel or pore for movement of either Na+ or K+, due to which substitution in this position has an immense effect on the functioning of the HKT1;5 protein. Strategic positions of these two amino acids might be playing a decisive role for the tolerant variety to survive but not the sensitive varieties. The selectivity filter of the HKT1;5 protein consist of 76Ser-264 Gly-391 Gly-496 Gly in both Nona Bokra (salt tolerant) and Nipponbare (salt sensitive), and all are present in the four P-loops (Horie et al. 2001). This filter selectively transports Na+ across the membrane. Simulation results showed that Val/Leu at the 395 position might be crucial to the selectivity filter, because 391 Gly residue is present in the same loop as 395 Val/Leu. 395 Leu forms an intricate residual network using Van der Waals forces with the nearby hydrophobic amino acids. This restricts the loop motion and would eventually reduce flexibility of 391 Gly. Therefore, the sensitive variety, i.e. Nipponbare has a narrow rigid pore. While, in the tolerant variety Nona Bokra 395 Val do not form rigid hydrophobic bonds and has a flexible loop motion and hence flexibility of 391 Gly. The flexibility of the selective filter allowed more favourable permeability of Na+ (Cotsaftis et al. 2012). Therefore, there will be a higher movement of Na+ from xylem sap into xylem parenchyma in tolerant variety, resulting in low concentration of Na+ in transpiration stream and lower toxicity. Another important substitution is at the 332 position at the opening of HKT1;5 protein to the xylem parenchyma part of plant root. This position has either a histidine (sensitive) or aspartic acid (tolerant) residue surrounded by polar residues in the large extra cellular loop. The histidine 395 showed lesser polar interactions with the surrounding amino acids, allowing greater loop flexibility. The free mobility of loop causes hindrance as it constricts the opening of pore due to polar interaction with the membrane polar head-groups. On the other hand, aspartic acid present in Nona Bokra (salt tolerant) at the same position, can get involved in polar interaction with the loop residues, affecting the loop dynamics in favour of shifting the loop away from the vicinity of the constriction pore and makes the efflux of Na+ easier. The introduction of aspartate in place of histidine and valine in place of leucine in HKT1;5 transporters proposes a model for an altered ion selectivity and uptake kinetics (Shohan et al. 2019).
During the study of regulation of OsHKT1;1 gene, a novel OsMYBc TF (MYB coil-coiled type) was found to bind to the OsHKT1;1 promoter. Mutation of the OsMYBc-binding site resulted in decreased promoter activity of OsHKT1;1 and knockout of OsMYBc resulted in a reduction in NaCl-induced expression of OsHKT1;1 (Wang et al. 2015).
Some plants are incredibly tolerant to salt stress and use specialized mechanisms to survive in high-salinity environments are coined as the halophytes and considered as a good resource for isolation and characterization of HKT genes in crop plants. Eutrema salsuginea and Eutrema parvula formerly known as Thellungiella halophila and Thellungiella parvula, respectively are close relatives to Arabidopsis (Ali et al. 2012; Inan et al. 2004; Oh et al. 2009). The Arabidopsis has only one HKT1 gene, while E. salsuginea has three—EsHKT1;1, EsHKT1;2, and EsHKT1;3; each coding for a subclass1 HKT transporter (Ali et al. 2016; Wu et al. 2012). The expression of EsHKT1;2 is significantly increased under high salinity, but the expression of EsHKT1;1 is down regulated under salt stress, similar to AtHKT1;1 in Arabidopsis (Ali et al. 2012; Oh et al. 2010; Wu et al. 2012). In yeast, EsHKT1;2- and EpHKT1;2 expressing cells could tolerate better salt stress, and the addition of K+ further improved their resistance. On the contrary, EpHKT1;1-expressing cells were equally sensitive of salinity as cells expressing EsHKT1;1 and EsHKT1;3. Interestingly, it was found that the affinity of transporter towards Na+and/K+ not only depends on serine/glycine in the second pore loop but also, aspartic acid/asparagine residues in the same domain (Ali et al. 2018).
Another interesting finding in the field of HKT research recently was high-affinity potassium transporter gene (RtHKT1) from Reaumuria trigyna, an endangered recretohalophyte, and a small archaic feral shrub, considered as a living fossil and are found to grow under harsh conditions (up to 0.7% salinity). The unique adaptations of R. trigyna such as the presence of succulent acicular leaves, salt excretion glands on leaf and stem surfaces, and a well-defined root system that confer salt-tolerance (Xue et al. 2012). RtHKT1 improves Na+ and K+ uptake in roots, assist K+ accumulation, and resist Na+ transport from roots to leaves, and thus maintain Na+/K+ homeostasis in stressed transgenic plants (Li et al. 2018).
Role of HKT subclass-2 members during salt stress
HKT gene was first identified in wheat, where the cDNA of HKT1 (now known as TaHKT2;1) from wheat roots was cloned in a yeast mutant that allowed to uptake K+ (Schachtman and Schroeder 1994). It was initially characterized as K+–H+ symporter that mediated the high-affinity K+ uptake in wheat roots, but it was later found to co-transport Na+–K+ when expressed in yeast or Xenopus oocytes (Rubio et al. 1995). Another group of scientists is of the opinion that the high-affinity K+ uptake occurs via HAK transporters (Banuelos et al. 2002; Santa-María et al. 1997), whereas TaHKT2;1 and orthologous transporters in other species are the high-affinity Na+ uptake systems in the roots (Garciadeblás et al. 2003). Consistent with this proposed idea, it was demonstrated that wheat TaHKT2;1 act as Na+/K+ symporter at micromolar concentrations of Na+ and a Na+ uniporter at millimolar concentrations of Na+, after more detailed analysis in Xenopus oocytes (Gassmann et al. 1996; Rubio et al. 1995).
To solve the contradictory theories related to Na+ uptake in roots, HKT1 barley homolog (HvHKT2;1) was studied and cDNA was expressed in yeast system and it behaved as either a Na+ (or K+) uniport or a Na+–K+ symport, depending on which expression vector construct used for inserting the HvHKT2;1 cDNA into the yeast. The symport function was exclusively expressed in K+-starved cells, while the uniport function was expressed in both, cells growing under normal and starved conditions. Since the transporter behaved as a uniporter in barley roots, it can be assumed that the symport function was merely an artifact in yeast expression system. The mutational analysis suggested that the translation initiation step in plants and yeast system were different and resulted in an alternative sequence or a different secondary mRNA structure in yeast and hence the artifact. It was proposed that these functional differences might be the result of the expression of transporters with different length N-termini (Haro et al. 2005). Nevertheless, Banuelos et al. (2008) disagreed with the findings of Haro et al. (2005), they proposed that the cause of transporters acting as Na+ or K+ uniporters or Na+–K+ symporter depends on the amount of the transporter. HvHKT2;1, when expressed in yeast cells, was found to act as a uniporter, if the promoter was directly upstream to the HvHKT2;l cDNA. However, it functions as a symporter, if a 59 nucleotide polylinker is inserted between the gene and promoter. The results further showed that three ATG triplets in the polylinker are responsible for the decreased synthesis of the transporter and that the lower amount of transporter caused the functional change.
The loss-of-function mutant alleles in oshkt2;1 resulted in an excessive decrease in Na+ influx in already K+ starved rice roots, inferring that OsHKT2;1 must be involved in Na+ uptake (Horie et al. 2007). These mutants showed improved growth as compared to WT in the presence of Na+ and under K+ starved environment. Earlier studies indicate that a moderate amount of Na+ may improve the growth of plant species, though no genetic pieces of evidence are available in this regard. The oshkt2;1 mutant showed no difference in growth under K+ rich condition and behaved similar to WT. This may indicate that K+ ion deficient environment triggers overexpression of OsHKT2;1 which uptakes more Na+ that works as growth nutrient and replaces K+ in some of its functions such as maintaining cell turgor pressure (Golldack et al. 2002; Garciadeblás et al. 2003). A multi-ion pore theory has been given for Na+–K+ transport via OsHKT2;1 and OsHKT2;2. Here, multiple ions can occupy the pore simultaneously and change the relative selectivity depending on external ionic content (Gassmann et al. 1996; Hille 2001). The OsHKT2;1 exhibit Na+ uptake in tobacco BY2 (bright yellow 2) cells in the absence of added K+, while OsHKT2;2 did not show any Na+ uptake in absence of added K+. OsHKT2;2-mediated Na+ uptake into BY2 cells was found to be external K+ dependent. OsHKT2;2, however, also functions as Na+-stimulated K+ transporter in BY2 cells. Taken together, the findings suggested that OsHKT2;2 is a Na+–K+ co-transporter, showing K+-stimulated Na+ influx and Na+-stimulated K+ influx. K+-independent Na+ influx was seen in OsHKT2;2 at a millimolar external concentration of Na+. While OsHKT2;1 shows Na+ uptake (predominantly Na+ selectivity) under low Na+ and K+ condition, no such activity was seen in OsHKT2;2 transporters under the same conditions (Yao et al. 2009). These findings concluded that plant HKT transporters can be divided into two different classes based on their Na+/K+ selectivities—a Na+–K+ co-transporter and a more Na+- over K+-selective transporter depending on external ionic conditions (Horie et al. 2001; Mäser et al. 2002; Rubio et al. 1995) with a few exceptions. One of the rice HKT protein, OsHKT2;4 also acts as a Ca2+ permeable ion transporter or non-specific cation channel (Lan et al. 2010). Since it regulates Ca2+ traffic in the cell, it may play a role in signal transduction, where Ca2+ acts as a secondary messenger. Ion selectivity of OsHKT2;4 was characterized in yeast and oocyte systems. OsHKT2;4-expressing oocytes exhibited strong K+ permeability even in the absence of extracellular Na+ concentration as against the other type 2 HKTs (Horie and Schroeder 2004). OsHKT2;4 was also found to be significantly permeable to Mg2+, Zn2+, Mn2+, Cu2+, Fe2+ and Cd2+ during electrophysiological analysis in oocytes. However, besides Ca2+ no other cation produced unique tail currents, indicating that the tail current was Ca2+ dependent exclusively (Lan et al. 2010). Comparative analyses of Ca2+ and Mg2+ permeabilities were studied in all type II HKTs and also AtHKT1;1 (Arabidopsis), and it was found that only OsHKT2;4 and to a lesser extent TaHKT2;1 favored Mg2+ transport. Importantly, K+ ions competitively inhibits Ca2+ and Mg2+ permeability through these transporters (Horie et al. 2011). Soon after this, Sassi et al. (2012) demonstrated that even at a very low K+ concentration, no Ca2+ permeability was seen in the oocyte system, and therefore, the previously attributed Ca2+ permeability could be due to activation of an endogenous oocyte conductance. So far, no clear evidence of Na+–K+ symporter or HKT as K+ transporter has been identified in planta (Haro et al. 2005). Nevertheless, it has come to light that HKT2;1 subgroup transporters, can also function as Na+–K+ symporter in heterologous systems such as yeast and Xenopus oocytes and can transport K+ in planta. OsHKT2;4 appears highly K+ permeable in all conducting modes and can even permeate pure K+ transport at a low external Na+ concentrations when expressed in Xenopus oocyte. Recently, OsHKT2;2 transporters when overexpressed in cultured tobacco (Nicotiana tabacum) cells have shown to mediate K+ transport in planta (Yao et al. 2009).
A type-II HKT transporter from barley (Hordeum vulgare L.), named HvHKT2;1 was studied to correlate salt—including behavior of barley and function of HKT2;1. Barley is described as a salt includers and is sometimes considered a halophyte (Glenn et al. 1999). As many as eight HKT isoforms are present in barley (Huang et al. 2008) but their roles are unknown. So far, HvHKT2;1 expression levels have been seen to increase in K+ starvation condition (Wang et al. 1998) and partial characterization of HvHKT2;1 protein in yeast deduced that it acts as Na+/K+ co-transporter over a broad range of external concentrations (Haro et al. 2005). Over-expression of HvHKT2;1 in barley improved Na+ uptake, increased Na+ concentrations in the xylem sap, and improved Na+ translocation to leaves. Transgenic barley plants over-expressing HvHKT2;1 showed improved relative growth rate than wild type plants. Hence overexpression of HvHKT2;1 resulted in improved salt tolerance (Mian et al. 2011). The result was surprisingly opposite to that of TaHKT2;1, where salt tolerance led to a reduction in expression of TaHKT2;1. The decreased expression of TaHKT2;1 in transgenic wheat resulted in lower root Na+ uptake and reduced translocation to xylem sap, which is consistent with the fact that hexaploid wheat is a salt-excluder species. While over-expression of HvHKT2;1 led to enhanced Na+ uptake, higher Na+ concentrations in the xylem sap, and enhanced translocation of Na+ to leaves when plants were grown in high salt conditions reinforcing the fact that barley act as salt includers (Laurie et al. 2002).
Regulation mechanism of HKT genes in controlling salt tolerance in plants
Gene expression and tissue localization
HKT transporters from different plant species have been found to express in all parts of a plant, from shoot to roots, leaves and even in flower parts (Table 1). They are mainly found in vascular tissues such as root stele and xylem parenchyma. All HKT transporters are localized on plasma membrane with the exception of OsHKT1;3, which is found in Golgi endomembrane (Rosas-Santiago et al. 2015). The Na+ exchangers present on the Golgi membrane accumulates the Na+ ions within Golgi to create a Na+ gradient necessary for the function of OsHKT1;3. It is proposed that OsHKT1;3 might act as an alternative conductance shunt to the proton transporters located on Golgi membrane, by transporting Na+ into the cytoplasm (Dettmer et al. 2005; Mitsuda et al. 2001; Shimaoka et al. 2004; Strompen et al. 2005). HKT gene is expressed in response to Na+ and K+ concentrations but they seem to follow no particular trend in relation to the concentration of these ions across the plant species. For instance, some HKT genes are up-regulated under high Na+ concentration, while some are down-regulated. In addition, the HKT gene expression increases in roots and decreases in shoots during stress in some species and vice versa in other species. Therefore, it can be concluded that HKT gene regulation and expression follows a complex pattern and it will be crucial to understand the versatile mechanisms of HKT regulation to improve salt tolerance in plants.
HKT gene expression has been studied in many plants such as Arabidopsis, rice and some other less popular plant species. The first studied transcription factor (TF) regulating HKT gene expression in Arabidopsis was basic leucine zipper AtbZIP24. This TF was repressed using RNAi (double-stranded RNA-mediated interference) and this led to improved salt tolerance. It was found that repression of AtbZIP24 led to increased activation of AtHKT1;1 and other genes involved in ion homeostasis (Yang et al. 2009). This indicates that AtbZIP24 acts as negative regulator of AtHKT1;1 gene. In another study, it has been reported that cytokinin plays a role in salt tolerance and mutation in the response regulators ARR type A and B (part of cytokinin signalling pathway) lead to an increased expression of AtHKT1;1 gene in roots. The expression of AtHKT1;1 was decreased due to cytokinin treatment, while AtHKT1;1 expression was increased in the arr1, arr12 double mutant background. These observations indicated that ARR1 and ARR12 are negative regulators of AtHKT1;1 (Mason et al. 2010).
Similarly, another plant TF ABA-INSENSITIVE 4 (ABI4) which was first identified in mutant plants that showed insensitivity to ABA during germination (Finkelstein 1994) was studied in relation to regulation of AtHKT1;1. Expression of numerous genes that are affected by ABI4 was studied by comparing gene expression in seedlings of wild type (WT) and those of abi4 mutants or ABI4 overexpressors. It was reported that AtHKT1;1 expression was higher in abi4 mutants and lower expression in ABI4 overexpressing plants. Study has suggested that ABI4 and AtHKT1;1 are expressed in the same cells, which suggests the possibility of direct binding of ABI4 to the AtHKT1;1 promoter site known as ABEs (ABI4-binding element), causing down regulation of this gene (Shkolnik-Inbar et al. 2013). Very few studies have linked the Ca2+ sensors to Na+ transporters under salt stress. Calmodulin (CaM) and calmodulin-like proteins (CMLs) are essential plant Ca2+ sensors involved in Ca2+ ion mediated signal transductions to execute downstream physiological responses. CML proteins are known to be involved in the modulation of many developmental processes, but very little is known about their roles in abiotic stresses in plants. MtCML40 protein from Medicago trunculata was found to be up regulated during salt, cold and osmotic stress as well as ABA treatment, suggesting its role in abiotic stress (Zhang et al. 2019). It was further identified that the overexpression of MtCML40 resulted in down-regulation of MtHKT1;1 and MtHKT1;2 that are involved in removal of Na+ from shoots (Fig. 2).
Few other TFs responsible for the up-regulation of HKT genes in different plant species during salinity stress were studied. Likewise, in rice OsHKT1;1, OsMYBc TF positively regulates its expression by binding to its promoter site (Wang et al. 2015). OsMYBc binds to AAANATNC(C/T) fragments of the promoter of OsHKT1;1. Mutation in the sequence of this fragment resulted in decline of activity of OsHKT1;1 promoter.
Another transcription factor OsbHLH035 regulates the expression of OsHKT1;3 and OsHKT1;5 gene. It was observed that osbhlh035 mutant seedlings were unable to recover from salt-stress treatment and the expression of the sodium transporters OsHKT1;3 and 1;5 was reduced compared to wild type. These results indicated that Osbhlh035 positively regulates OsHKT1;3 and OsHKT1;5 gene expression (Chen et al. 2018).
A study has revealed that a protein complex consisting of rice BCL-2-ASSOCIATED ATHANOGENE4 (OsBAG4, a chaperone regulator), OsMYB106 (a MYB TF), and OsSUVH7 (a DNA methylation reader) regulates OsHKT1;5 expression in response to salt stress (Wang et al. 2020). OsMYB106 and OsSUVH7 bind to the MYB binding cis-element (MYBE) and the miniature inverted repeat transposable element (MITE) which is upstream of the MYBE, respectively, on the OsHKT1;5 promoter sequence. OsBAG4 functions as a bridge between OsSUVH7 and OsMYB106 to facilitate OsMYB106 binding to the consensus MYBE in the OsHKT1;5 promoter, thereby activating the OsHKT1;5 expression (Wang et al. 2020).
The circadian clock also referred to as the endogenous time-keeping system in plants have also been known to integrate several abiotic stress signals including salinity in Arabidopsis (Greenham and McClung 2015; Sanchez and Kay 2016). In addition, a number of circadian-clock regulated genes were found to be involved in salinity tolerance in rice, such as cyclophilin 2 (OsCYP2) and Receptor for Activated C Kinase 1(OsRACK1) (Ruan et al. 2011; Zhang et al. 2018). Amongst members of OsPRR (Oryza sativa Pseudo-Response Regulator) gene family, OsPRR73 specifically confers salt tolerance in rice. Salt-induced expression of OsPRR73 confers salt tolerance by recruiting HDAC10 (a nuclear interactor and co-repressor) to transcriptionally repress OsHKT2;1, thus reducing cellular Na+ accumulation (Wei et al. 2021).
One of the TF, from Ethylene Responsive Factor (ERF) family, in poplar plant, PalERF109 was examined for expression under salinity stress. In Populus alba var. pyramidalis, overexpression of PalERF109 enhanced the salt tolerance by directly up regulating a high-affinity K+ transporter (HKT) gene, PalHKT1;2 (Chen et al. 2020) (Fig. 2).
Previous findings in plant HKT gene demonstrated splicing events, incomplete splicing (Golldack et al. 2002), intron retention and exon skipping events (Takahashi et al. 2007). The intron-retaining transcripts and different splice variants of HvHKT2;1 showed a link to ion homeostasis and their role in salt stress through expression profiling in yeast and plant systems (Shahzad et al. 2015). Relative expression analysis was carried out under salt stress in transgenic tobacco plants and heterologous expression in K+ uptake-deficient mutant yeast strains (trk1, trk2). Mixed proportions of three HKT transcripts, namely, HvHKT2;1-e (HvHKT2;1 with first exon), HvHKT2;1-i1 (HvHKT2;1 with first intron) and HvHKT2;1-i2 (HvHKT2;1 with second intron) in barley was observed. Salt treatment led to a drastic increase in HvHKT2;1-i1 transcript. In addition, better growth was observed in trk1, trk2 yeast cells expressing HvHKT2;1-i under low K+ conditions. Thus it can be said that the first intron retaining HKT variant play a role in salt tolerance in barley (Shahzad et al. 2015).
Conclusion and future perspective
The fundamental research into HKT ion transport mechanisms in plants has resulted in conception of new ideas that can be used for crops to improve their performance under different stress conditions. Our knowledge of the molecular identity and mechanism of regulation of HKT transporters has expanded immensely over the past 20 years or so. It has been observed that HKT proteins play a pivotal role in alleviation of excess Na+ from the transpiration stream hence mitigate sodium toxicity in the salt-sensitive shoot and leaf tissues. The implementation of genetic engineering as an effective means to enhance salt tolerance using the knowledge of HKT transporters has shown its potential for improving the growth of major crops and cereals under salinity stress. A major future challenge in agriculture is to combine genetic traits using a multigene transformation in crop plants without adversely affecting the yield. For instance, the characteristics traits of sodium ion removal from the xylem sap and accumulation into vacuoles, if combined in plants can give better salt tolerance. However, further studies are required to establish whether these traits will be compatible when combined.
Acknowledgements
CSIR-CSMCRI Communication No.49/2021. The authors are thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India. AD is supported by the CSIR-SRF fellowship. PA acknowledges the financial support from DST-WOS-A scheme. AD acknowledges AcSIR for enrollment in Ph.D.
Abbreviations
- HKT
High-affinity potassium transporter
- SOD
Superoxide dismutase
- POD
Peroxidase
- CAT
Catalase
- ROS
Reactive oxygen species
- NaCl
Sodium chloride
- KCl
Potassium chloride
- ABA
Abscisic acid
Author contributions
All authors have contributed to MS writing and approved the final manuscript.
Funding
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
References
- Abdulhussein F, Mutlag N, Sarheed A. Genotypic characterization and tissue localization of the mutant lines expression of HKT1; 3 gene in rice under salt stress. Plant Arch. 2018;18(1):489–495. [Google Scholar]
- Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA. Plant responses to salt stress: adaptive mechanisms. Agronomy. 2017;7(1):18. [Google Scholar]
- Ali Z, Park H, Ali A, Oh D, Aman R, Kropornicka A, Hong H, Choi W, Chung W, Kim W. TsHKT1; 2, a HKT1 homolog from the extremophile Arabidopsis relative Thellungiella salsuginea, shows K+ specificity in the presence of NaCl. Plant Physiol. 2012;158(3):1463–1474. doi: 10.1104/pp.111.193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Raddatz N, Aman R, Kim S, Park H, Jan M, Baek D, Khan I, Oh D, Lee S. A single amino-acid substitution in the sodium transporter HKT1 associated with plant salt tolerance. Plant Physiol. 2016;171(3):2112–2126. doi: 10.1104/pp.16.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Khan I, Jan M, Khan H, Hussain S, Nisar M, Chung W, Yun D. The high-affinity potassium transporter EpHKT1; 2 from the extremophile Eutrema parvula mediates salt tolerance. Front Plant Sci. 2018;9:1108. doi: 10.3389/fpls.2018.01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apse MP, Sottosanto JB, Blumwald E. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 2003;36(2):229–239. doi: 10.1046/j.1365-313x.2003.01871.x. [DOI] [PubMed] [Google Scholar]
- Arora S, Singh Y, Vanza M, Sahni DJJoS, Conservation W Bio-remediation of saline and sodic soils through halophilic bacteria to enhance agricultural production. J Soil Water Conserv. 2016;15(4):302–305. [Google Scholar]
- Banuelos MA, Garciadeblas B, Cubero B, Rodrıguez-Navarro A. Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol. 2002;130(2):784–795. doi: 10.1104/pp.007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernández J, Cubero B, Pardo J. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell. 2012;24(3):1127–1142. doi: 10.1105/tpc.111.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Ohto M-a, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell. 2011;23(1):224–239. doi: 10.1105/Tpc.110.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito B, Rodríguez-Navarro A. Molecular cloning and characterization of a sodium-pump ATPase of the moss Physcomitrella patens. Plant J. 2003;36(3):382–389. doi: 10.1046/j.1365-313X.2003.01883.x. [DOI] [PubMed] [Google Scholar]
- Berthomieu P, Conéjéro G, Nublat A, Brackenbury W, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003;22(9):2004–2014. doi: 10.1093/emboj/cdg207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M, Ratajczak R. Function of membrane transport systems under salinity environment-plants-molecules. In: Läuchli A, Lüttge U, editors. Salinity: environment—plants—molecules. Dordrecht: Springer; 2002. pp. 423–449. [Google Scholar]
- Blumwald E. Sodium transport and salt tolerance in plants. Curr Opin Cell Biol. 2000;12(4):431–434. doi: 10.1016/s0955-0674(00)00112-5. [DOI] [PubMed] [Google Scholar]
- Brett C, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288(2):C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- Byrt C, Platten J, Spielmeyer W, James R, Lagudah E, Dennis E, Tester M, Munns R. HKT1; 5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol. 2007;143(4):1918–1928. doi: 10.1104/pp.106.093476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrt C, Xu B, Krishnan M, Lightfoot D, Athman A, Jacobs A, Watson-Haigh N, Plett D, Munns R, Tester M. The Na+ transporter, TaHKT1;5-D, limits shoot Na+ accumulation in bread wheat. Plant J. 2014;80(3):516–526. doi: 10.1111/tpj.12651. [DOI] [PubMed] [Google Scholar]
- Cao Y, Pan Y, Huang H, Jin X, Levin E, Kloss B, Zhou M. Gating of the TrkH ion channel by its associated RCK protein, Trka. Nature. 2013;496:317–322. doi: 10.1038/nature12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cheng W, Hong C, Chang Y, Chang M. The transcription factor OsbHLH035 mediates seed germination and enables seedling recovery from salt stress through ABA-dependent and ABA-independent pathways, respectively. Rice. 2018;11(1):1–17. doi: 10.1186/s12284-018-0244-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Tong S, Tang H, Zhang Z, Liu B, Lou S, Liu J, Liu H, Ma T, Jiang Y. The PalERF109 transcription factor positively regulates salt tolerance via PalHKT1; 2 in Populus alba var. pyramidalis. Tree Physiol. 2020;40(6):717–730. doi: 10.1093/treephys/tpaa018. [DOI] [PubMed] [Google Scholar]
- Cotsaftis O, Plett D, Shirley N, Tester M, Hrmova M. A two-staged model of Na+ exclusion in rice explained by 3D modeling of HKT transporters and alternative splicing. PLoS ONE. 2012;7(7):e39865. doi: 10.1371/journal.pone.0039865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Z, Zheng L, Wang J, Gao Z, Wu S, Qi Z, Wang Y. Transcriptomic profiling of the salt-stress response in the wild recretohalophyte Reaumuria Trigyna. BMC Genomics. 2013;14(1):1–18. doi: 10.1186/1471-2164-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport R, James R, Zakrisson-Plogander A, Tester M, Munns R. Control of sodium transport in durum wheat. Plant Physiol. 2005;137(3):807–818. doi: 10.1104/pp.104.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RJ, Muñoz-Mayor A, Jha D, Essah PA, Rus A, Tester M. The Na+ transporter AtHKT1; 1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 2007;30(4):497–507. doi: 10.1111/j.1365-3040.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- Dettmer J, Schubert D, Calvo-Weimar O, Stierhof Y, Schmidt R, Schumacher K. Essential role of the V-ATPase in male gametophyte development. Plant J: Cell Mol Biol. 2005;41(1):117–124. doi: 10.1111/j.1365-313X.2004.02282.x. [DOI] [PubMed] [Google Scholar]
- Dubcovsky J, Santa Maria G, Epstein E, Luo M-C, Dvořák J. Mapping of the K+/Na+ discrimination locus Kna1 in wheat. Theor Appl Genet. 1996;92(3–4):448–454. doi: 10.1007/BF00223692. [DOI] [PubMed] [Google Scholar]
- Durell S, Hao Y, Nakamura T, Bakker E, Guy H. Evolutionary relationship between K(+) channels and symporters. Biophysics J. 1999;77(2):775–788. doi: 10.1016/S0006-3495(99)76931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. Mutations at two new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J. 1994;5(6):765–771. doi: 10.1046/j.1365-313X.1994.5060765.x. [DOI] [Google Scholar]
- Garciadeblás B, Senn ME, Bañuelos MA, Rodríguez-Navarro A. Sodium transport and HKT transporters: the rice model. Plant J. 2003;34(6):788–801. doi: 10.1046/j.1365-313x.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- Gassmann W, Rubio F, Schroeder J. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996;10(5):869–882. doi: 10.1046/j.1365-313x.1996.10050869.x. [DOI] [PubMed] [Google Scholar]
- Gaxiola R, Li J, Undurraga S, Dang L, Allen G, Alper S, Fink G. Drought-and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA. 2001;98(20):11444–11449. doi: 10.1073/pnas.191389398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn E, Brown J, Blumwald E. Salt tolerance and crop potential of halophytes. Crit Rev Plant Sci. 1999;18(2):227–255. doi: 10.1080/07352689991309207. [DOI] [Google Scholar]
- Golldack D, Su H, Quigley F, Kamasani UR, Muñoz-Garay C, Balderas E, Popova O, Bennett J, Bohnert H, Pantoja O. Characterization of a HKT-type transporter in rice as a general alkali cation transporter. Plant J. 2002;31(4):529–542. doi: 10.1046/j.1365-313x.2002.01374.x. [DOI] [PubMed] [Google Scholar]
- Gorham J, Hardy C, Jones R, Joppa L, Law C. Chromosomal location of a K/Na discrimination character in the D genome of wheat. Theor Appl Genet. 1987;74(5):584–588. doi: 10.1007/BF00288856. [DOI] [PubMed] [Google Scholar]
- Gorham J, Jones RW, Bristol A. Partial characterization of the trait for enhanced K+− Na+ discrimination in the D genome of wheat. Planta. 1990;180(4):590–597. doi: 10.1007/BF02411458. [DOI] [PubMed] [Google Scholar]
- Greenham K, McClung C. Integrating circadian dynamics with physiological processes in plants. Nat Rev Genet. 2015;16(10):598–610. doi: 10.1038/nrg3976. [DOI] [PubMed] [Google Scholar]
- Han Y, Yin S, Huang L, Wu X, Zeng J, Liu X, Qiu L, Munns R, Chen Z, Zhang G. A sodium transporter HvHKT1; 1 confers salt tolerance in barley via regulating tissue and cell ion homeostasis. Plant Cell Physiol. 2018;59(10):1976–1989. doi: 10.1093/pcp/pcy116. [DOI] [PubMed] [Google Scholar]
- Haro R, Bañuelos MA, Senn ME, Barrero-Gil J, Rodríguez-Navarro A. HKT1 mediates sodium uniport in roots. Pitfalls in the expression of HKT1 in yeast. Plant Physiol. 2005;139(3):1495–1506. doi: 10.1104/pp.105.067553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- Horie T, Schroeder J. Sodium transporters in plants. Diverse genes and physiological functions. Plant Physiol. 2004;136(1):2457–2462. doi: 10.1104/pp.104.046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J. 2001;27(2):129–138. doi: 10.1046/j.1365-313x.2001.01077.x. [DOI] [PubMed] [Google Scholar]
- Horie T, Costa A, Kim T, Han M, Horie R, Leung H, Miyao A, Hirochika H, Schroeder J. Rice OsHKT2; 1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 2007;26(12):3003–3014. doi: 10.1038/sj.emboj.7601732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Brodsky D, Costa A, Kaneko T, Schiavo F, Katsuhara M, Schroeder J. K+ transport by the OsHKT2; 4 transporter from rice with atypical Na+ transport properties and competition in permeation of K+ over Mg2+ and Ca2+ ions. Plant Physiol. 2011;156(3):1493–1507. doi: 10.1104/pp.110.168047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Spielmeyer W, Lagudah E, James R, Platten J, Dennis E, Munns R. A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol. 2006;142(4):1718–1727. doi: 10.1104/pp.106.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Spielmeyer W, Lagudah E, Munns R. Comparative mapping of HKT genes in wheat, barley, and rice, key determinants of Na+ transport, and salt tolerance. J Exp Bot. 2008;59(4):927–937. doi: 10.1093/jxb/ern033. [DOI] [PubMed] [Google Scholar]
- Huang Y, Bie Z, Liu Z, Zhen A, Jiao X. Improving cucumber photosynthetic capacity under NaCl stress by grafting onto two salt-tolerant pumpkin rootstocks. Biol Plant. 2011;55(2):285–290. [Google Scholar]
- Huang Y, Bie Z, Liu P, Niu M, Zhen A, Liu Z, Lei B, Gu D, Lu C, Wang B. Reciprocal grafting between cucumber and pumpkin demonstrates the roles of the rootstock in the determination of cucumber salt tolerance and sodium accumulation. Sci Hortic. 2013;149:47–54. doi: 10.1016/j.scienta.2012.04.018. [DOI] [Google Scholar]
- Inan G, Zhang Q, Li P, Wang Z, Cao Z, Zhang H, Zhang C, Quist T, Goodwin S, Zhu J. Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol. 2004;135(3):1718–1737. doi: 10.1104/pp.104.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabnoune M, Espeout S, Mieulet D, Fizames C, Verdeil J, Conéjéro G, Rodríguez-Navarro A, Sentenac H, Guiderdoni E, Abdelly C. Diversity in expression patterns and functional properties in the rice HKT transporter family. Plant Physiol. 2009;150(4):1955–1971. doi: 10.1104/pp.109.138008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RA, Davenport RJ, Munns R. Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol. 2006;142(4):1537–1547. doi: 10.1104/pp.106.086538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R, Blake C, Byrt C, Munns R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1; 4 and HKT1; 5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J Exp Bot. 2011;62(8):2939–2947. doi: 10.1093/jxb/err003. [DOI] [PubMed] [Google Scholar]
- Kato Y, Sakaguchi M, Mori Y, Saito K, Nakamura T, Bakker E, Sato Y, Goshima S, Uozumi N. Evidence in support of a four transmembrane-pore-transmembrane topology model for the Arabidopsis thaliana Na+/K+ translocating AtHKT1 protein, a member of the superfamily of K+ transporters. Proc Natl Acad Sci USA. 2001;98:6488–6493. doi: 10.1073/pnas.101556598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kere GM, Guo Q, Shen J, Xu J, Chen J. Heritability and gene effects for salinity tolerance in cucumber (Cucumis sativus L.) estimated by generation mean analysis. Sci Hortic. 2013;159:122–127. [Google Scholar]
- Kobayashi NI, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Fujii-Kashino M, Horiuchi T. OsHKT1; 5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J. 2017;91(4):657–670. doi: 10.1111/tpj.13595. [DOI] [PubMed] [Google Scholar]
- Kumar P, Sharma PK. Soil Salinity and Food Security in India. Front Sustain Food Syst. 2020;4:533781. doi: 10.3389/fsufs.2020.533781. [DOI] [Google Scholar]
- Lan W-Z, Wang W, Wang S, Li L, Buchanan B, Lin H, Gao J, Luan S. A rice high-affinity potassium transporter (HKT) conceals a calcium-permeable cation channel. Proc Natl Acad Sci USA. 2010;107(15):7089–7094. doi: 10.1073/pnas.1000698107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie S, Feeney K, Maathuis F, Heard P, Brown S, Leigh R. A role for HKT1 in sodium uptake by wheat roots. Plant J. 2002;32(2):139–149. doi: 10.1046/j.1365-313X.2002.01410.x. [DOI] [PubMed] [Google Scholar]
- Lei B, Huang Y, Xie J, Liu Z, Zhen A, Fan M, Bie Z. Increased cucumber salt tolerance by grafting on pumpkin rootstock and after application of calcium. Biol Plant. 2014;58(1):179–184. [Google Scholar]
- Li N, Du C, Ma B, Gao Z, Wu Z, Zheng L, Niu Y, Wang Y. Functional analysis of ion transport properties and salt tolerance mechanisms of RtHKT1 from the Recretohalophyte Reaumuria trigyna. Plant Cell Physiol. 2018;60(1):85–106. doi: 10.1093/pcp/pcy187. [DOI] [PubMed] [Google Scholar]
- Lin H, Zhu M, Yano M, Gao J, Liang Z, Su W, Hu X, Ren Z, Chao D. QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor Appl Genet. 2004;108(2):253–260. doi: 10.1007/s00122-003-1421-y. [DOI] [PubMed] [Google Scholar]
- Lindsay M, Lagudah E, Hare R, Munns R. A locus for sodium exclusion (Nax1), a trait for salt tolerance, mapped in durum wheat. Funct Plant Biol. 2004;31(11):1105–1114. doi: 10.1071/FP04111. [DOI] [PubMed] [Google Scholar]
- Maathuis F, Verlin D, Smith F, Sanders D, Fernandez J, Walker N. The physiological relevance of Na+-coupled K+-transport. Plant Physiol. 1996;112(4):1609–1616. doi: 10.1104/pp.112.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder J, Ward J, Hirschi K, Sze H, Talke I, Amtmann A, Maathuis F, Sanders D. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126(4):1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Gierth M, Schroeder J. Progress in plant nutrition: plenary lectures of the XIV international plant nutrition colloquium. Springer; 2002. Molecular mechanisms of potassium and sodium uptake in plants; pp. 43–54. [Google Scholar]
- Mason M, Jha D, Salt D, Tester M, Hill K, Kieber J, Schaller G. Type-B response regulators ARR1 and ARR12 regulate expression of AtHKT1; 1 and accumulation of sodium in Arabidopsis shoots. Plant J. 2010;64(5):753–763. doi: 10.1111/j.1365-313X.2010.04366.x. [DOI] [PubMed] [Google Scholar]
- Mian A, Oomen R, Isayenkov S, Sentenac H, Maathuis F, Véry A. Over-expression of an Na+-and K+-permeable HKT transporter in barley improves salt tolerance. Plant J. 2011;68(3):468–479. doi: 10.1111/j.1365-313X.2011.04701.x. [DOI] [PubMed] [Google Scholar]
- Mitsuda N, Enami K, Nakata M, Takeyasu K, Sato M. Novel type Arabidopsis thaliana H+-PPase is localized to the Golgi apparatus. FEBS Lett. 2001;488(1–2):29–33. doi: 10.1016/s0014-5793(00)02400-5. [DOI] [PubMed] [Google Scholar]
- Møller I, Gilliham M, Jha D, Mayo G, Roy S, Coates J, Haseloff J, Tester M. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type–specific alteration of Na+ transport in Arabidopsis. Plant Cell. 2009;21(7):2163–2178. doi: 10.1105/tpc.108.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, James RA. Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil. 2003;253(1):201–218. [Google Scholar]
- Oh D, Leidi E, Zhang Q, Hwang S, Li Y, Quintero F, Jiang X, D'Urzo M, Lee S, Zhao Y. Loss of halophytism by interference with SOS1 expression. Plant Physiol. 2009;151(1):210–222. doi: 10.1104/pp.109.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D, Lee S, Bressan R, Yun D, Bohnert H. Intracellular consequences of SOS1 deficiency during salt stress. J Exp Bot. 2010;61(4):1205–1213. doi: 10.1093/jxb/erp391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J, Cubero B, Leidi EO, Quintero F. Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot. 2006;57(5):1181–1199. doi: 10.1093/jxb/erj114. [DOI] [PubMed] [Google Scholar]
- Platten JD, Cotsaftis O, Berthomieu P, Bohnert H, Davenport RJ, Fairbairn D, Horie T, Leigh R, Lin H, Luan S. Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci. 2006;11(8):372–374. doi: 10.1016/j.tplants.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Rains D, Epstein E. Sodium absorption by barley roots: its mediation by mechanism 2 of alkali cation transport. Plant Physiol. 1967;42(3):319–323. doi: 10.1104/pp.42.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson H, Richards R, Munns R. An examination of selection criteria for salt tolerance in wheat, barley and triticale genotypes. Aust J Agric Res. 1988;39(5):759–772. [Google Scholar]
- Reinhold L, Guy M. Function of membrane transport systems under salinity: plasma membrane. In: Läuchli A, Lüttge U, editors. Salinity: environment-plants-molecules. Berlin: Springer; 2002. pp. 397–421. [Google Scholar]
- Ren Z, Gao J, Li L, Cai X, Huang W, Chao D, Zhu M, Wang Z, Luan S, Lin H. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet. 2005;37(10):1141. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rosales M, Jiang X, Gálvez F, Aranda M, Cubero B, Venema K. Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol. 2008;179(2):366–377. doi: 10.1111/j.1469-8137.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- Rosas-Santiago P, Lagunas-Gómez D, Barkla BJ, Vera-Estrella R, Lalonde S, Jones A, Frommer WB, Zimmermannova O, Sychrová H, Pantoja O. Identification of rice cornichon as a possible cargo receptor for the Golgi-localized sodium transporter OsHKT1; 3. J Exp Bot. 2015;66(9):2733–2748. doi: 10.1093/jxb/erv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan S-L, Ma H, Wang S, Fu Y, Xin Y, Liu W, Wang F, Tong J, Wang S, Chen H. Proteomic identification of OsCYP2, a rice cyclophilin that confers salt tolerance in rice (Oryza sativa L.) seedlings when overexpressed. BMC Plant Biol. 2011;11(1):1–15. doi: 10.1186/1471-2229-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder J. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270(5242):1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee B, Matsumoto T, Koiwa H, Zhu J, Bressan R, Hasegawa P. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci USA. 2001;98(24):14150–14155. doi: 10.1073/pnas.241501798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus A, Lee B, Muñoz-Mayor A, Sharkhuu A, Miura K, Zhu J, Bressan R, Hasegawa P. AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta. Plant Physiol. 2004;136(1):2500–2511. doi: 10.1104/pp.104.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr, Tran CV, Barabote R. TCDB: the transporter classification database for membrane transport protein analyses and information. Nucleic Acids Res. 2006;34(Database issue):D181–D186. doi: 10.1093/nar/gkj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez SE, Kay S. The plant circadian clock: from a simple timekeeper to a complex developmental manager. Cold Spring Harb Perspect Biol. 2016;8(12):a027748. doi: 10.1101/cshperspect.a027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9(12):2281. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman D, Schroeder J. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370(6491):655. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Shabala S. Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot. 2013;112(7):1209–1221. doi: 10.1093/aob/mct205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Gorham J, Forster B, Wyn Jones R. Salt tolerance in the Triticeae: the contribution of the D genome to cation selectivity in hexaploid wheat. J Exp Bot. 1987;38(2):254–269. [Google Scholar]
- Shahzad K, Rauf M, Ahmed M, Malik ZA, Habib I, Ahmed Z, Mahmood K, Ali R, Masmoudi K, Lemtiri-Chlieh F, Gehring C, Berkowitz G, Saeed NA. Functional characterisation of an intron retaining K+ transporter of barley reveals intron-mediated alternate splicing. Plant Biol J. 2015;17(4):840–851. doi: 10.1111/plb.12290. [DOI] [PubMed] [Google Scholar]
- Shi H, Ishitani M, Kim C, Zhu J. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA. 2000;97(12):6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka T, Ohnishi M, Sazuka T, Mitsuhashi N, Hara-Nishimura I, Shimazaki K, Maeshima M, Yokota A, Tomizawa K, Mimura T. Isolation of intact vacuoles and proteomic analysis of tonoplast from suspension-cultured cells of Arabidopsis thaliana. Plant Cell Physiol. 2004;45(6):672–683. doi: 10.1093/pcp/pch099. [DOI] [PubMed] [Google Scholar]
- Shkolnik-Inbar D, Adler G, Bar-Zvi D. ABI4 downregulates expression of the sodium transporter HKT1; 1 in Arabidopsis roots and affects salt tolerance. Plant J. 2013;73(6):993–1005. doi: 10.1111/tpj.12091. [DOI] [PubMed] [Google Scholar]
- Shohan M, Sinha S, Nabila FH, Dastidar SG, Seraj ZI. HKT1;5 transporter gene expression and association of amino acid substitutions with salt tolerance across rice genotypes. Front Plant Sci. 2019 doi: 10.3389/fpls.2019.01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strompen G, Dettmer J, Stierhof Y, Schumacher K, Jürgens G, Mayer U. Arabidopsis vacuolar H+-ATPase subunit E isoform 1 is required for Golgi organization and vacuole function in embryogenesis. Plant J. 2005;41(1):125–132. doi: 10.1111/j.1365-313X.2004.02283.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Cao H, Cheng J, He X, Sohail H, Niu M, Huang Y, Bie Z. Pumpkin CmHKT1;1 controls shoot Na+ accumulation via limiting Na+ transport from rootstock to scion in grafted cucumber. Int J Mol Sci. 2018;19(9):2648. doi: 10.3390/ijms19092648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunarpi HT, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan W-Y, Leung H, Hattori K, Konomi M, Osumi M, Yamagami M, Schroeder J, Uozumi N. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005;44(6):928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Liu S, Takano T. Cloning and functional comparison of a high-affinity K+ transporter gene PhaHKT1 of salt-tolerant and salt-sensitive reed plants. J Exp Bot. 2007;58:4387–4395. doi: 10.1093/jxb/erm306. [DOI] [PubMed] [Google Scholar]
- Thomson MJ, de Ocampo M, Egdane J, Rahman M, Sajise A, Adorada D, Tumimbang-Raiz BE, Seraj Z, Singh R. Characterizing the saltol quantitative trait locus for salinity tolerance in rice. Rice. 2010;3(2–3):148–160. doi: 10.1007/s12284-010-9053-8. [DOI] [Google Scholar]
- Tiwari J, Munshi A, Kumar R, Pandey RN, Arora A, Bhat J, Sureja A. Effect of salt stress on cucumber: Na+–K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol Plant. 2010;32(1):103–114. [Google Scholar]
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder J. The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Mol Biol. 2000;122(4):1249–1260. doi: 10.1023/A:1025445612244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira-Pires RS, Szollosi A, Morais-Cabral J. The structure of the KtrAB potassium transporter. Nature. 2013;496(7445):323–328. doi: 10.1038/nature12055. [DOI] [PubMed] [Google Scholar]
- Wang T-B, Gassmann W, Rubio SJ, Glass A. Rapid up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol. 1998;118(2):651–659. doi: 10.1104/pp.118.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Jing W, Xiao L, Jin Y, Shen L, Zhang W. The rice high-affinity potassium transporter1;1 is involved in salt tolerance and regulated by an MYB-type transcription factor. Plant Physiol. 2015;168(3):1076–1090. doi: 10.1104/pp.15.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu Y, Feng S, Wang Z, Zhang J, Zhang J, Wang D, Gan Y. AtHKT1 gene regulating K+ state in whole plant improves salt tolerance in transgenic tobacco plants. Sci Rep. 2018;8(1):16585. doi: 10.1038/s41598-018-34660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu Y, Li D, Feng S, Yang J, Zhang J, Zhang J, Wang D, Gan Y. Improving salt tolerance in potato through overexpression of AtHKT1 gene. BMC Plant Biol. 2019;19(1):357. doi: 10.1186/s12870-019-1963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Nan N, Li N, Liu Y, Wang T-J, Hwang I, Liu B, Xu Z. A DNA methylation reader–chaperone regulator–transcription factor complex activates OsHKT1; 5 expression during salinity stress. Plant Cell. 2020;32(11):3535–3558. doi: 10.1105/tpc.20.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Wang X, He Y, Xu H, Wang L. Clock component OsPRR73 positively regulates rice salt tolerance by modulating OsHKT2;1-mediated sodium homeostasis. EMBO J. 2021;40(3):e105086. doi: 10.15252/embj.2020105086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Ding L, Zhu J. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell. 1996;8(4):617–627. doi: 10.1105/tpc.8.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhang Z, Wang J, Oh D, Dassanayake M, Liu B, Huang Q, Sun H, Xia R, Wu Y. Insights into salt tolerance from the genome of Thellungiella salsuginea. Proc Natl Acad Sci USA. 2012;109(30):12219–12224. doi: 10.1073/pnas.1209954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Chen C, Cai H, Wu L. Overexpression of PeHKT1; 1 improves salt tolerance in populus. Genes. 2018;9(10):475. doi: 10.3390/genes9100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Wang Y, Wang T. Responses of antioxidant system of an endemic halophyte Reaumuria trigyna to NaCl stress. J Desert Res. 2012;32:1669–1673. [Google Scholar]
- Yang O, Popova O, Süthoff U, Lüking I, Dietz K, Golldack D. The Arabidopsis basic leucine zipper transcription factor AtbZIP24 regulates complex transcriptional networks involved in abiotic stress resistance. Gene. 2009;436(1–2):45–55. doi: 10.1016/j.gene.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Yao X, Horie T, Xue S, Leung H, Katsuhara M, Brodsky D, Wu Y, Schroeder J. Differential sodium and potassium transport selectivities of the rice OsHKT2;1 and OsHKT2;2 transporters in plant cells. Plant Physiol. 2009;152(1):341–355. doi: 10.1104/pp.109.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S, Quintero F, Cubero B, Ruiz M, Bressan R, Hasegawa P, Pardo J. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002;5:529–539. doi: 10.1046/j.1365-313X.2002.01309.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Wang Y, Shen J, Yin J, Li D, Gao Y, Xu W, Liang J. OsRACK1A, encodes a circadian clock-regulated WD40 protein, negatively affect salt tolerance in rice. Rice. 2018;11(1):1–15. doi: 10.1186/s12284-018-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang T, Liu M, Sun W, Zhang W. Calmodulin-like gene MtCML40 is involved in salt tolerance by regulating MtHKTs transporters in Medicago truncatula. Environ Exp Bot. 2019;157:79–90. doi: 10.1016/j.envexpbot.2018.09.022. [DOI] [Google Scholar]
- Zhu J. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003;6(5):441–445. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]