Abstract
Objectives:
We characterised differences in BP control and use of antihypertensive medications in European (EA), South Asian (SA) and African-Caribbean (AC) people with hypertension and investigated the potential role of type 2 diabetes (T2DM), reduced arterial compliance (Ca), and antihypertensive medication use in any differences.
Methods:
Analysis was restricted to individuals with hypertension [age range 59–85 years; N = 852 (EA = 328, SA = 356, and AC =168)]. Questionnaires, anthropometry, BP measurements, echocardiography, and fasting blood assays were performed. BP control was classified according to UK guidelines operating at the time of the study. Data were analysed using generalised structural equation models, multivariable regression and treatment effect models.
Results:
SA and AC people were more likely to receive treatment for high BP and received a greater average number of antihypertensive agents, but despite this a smaller proportion of SA and AC achieved control of BP to target [age and sex adjusted odds ratio (95% confidence interval) = 0.52 (0.38, 0.72) and 0.64 (0.43, 0.96), respectively]. Differences in BP control were partially attenuated by controlling for the higher prevalence of T2DM and reduced Ca in SA and AC. There was little difference in choice of antihypertensive agent by ethnicity and no evidence that differences in efficacy of antihypertensive regimens contributed to ethnic differences in BP control.
Conclusions:
T2DM and more adverse arterial stiffness are important factors in the poorer BP control in SA and AC people. More effort is required to achieve better control of BP, particularly in UK ethnic minorities.
Keywords: hypertension, anti-hypertensive agents, ethnicity, hemodynamic/drug effects, diabetes, hemodynamic, arterial compliance
Introduction
Elevated blood pressure (BP) is a major global public health problem and a leading preventable cause of cardiovascular morbidity and mortality. Levels of BP and prevalence of hypertension differ quite markedly between countries and between ethnic groups within populations for reasons that are poorly understood (1).
In the UK and Europe, BP and prevalence of hypertension has generally been found to be higher in people of African/African-Caribbean (AC) ethnicity compared with people of European ancestry (EA) (2), findings in South Asian (SA) people are less consistent (3, 4). BP control in people with hypertension is also poorer in people of African heritage (5, 6), with data in SA being more equivocal (4, 5).
The extent to which differences in efficacy of antihypertensive medication contribute to ethnic differences in BP control in hypertension remains uncertain. Differences in levels of BP control may reflect several factors, not only drug effectiveness. There is evidence that some BP-lowering agents, notably beta-blockers and inhibitors of the renin-angiotensin system (RAS) are comparatively less effective in people of African ancestry (7, 8), while calcium channel blockers (CCB) and diuretics may be more effective (7–9). Although subsequent data examining the achieved differences in BP in a ‘real world setting' suggests differences may be minimal (10). There is limited comparable data in SA, but what exists suggests little or no difference in efficacy of antihypertensive agents (11, 12). On the basis of evidence from trials and mechanistic considerations, the UK National Institute for Health and Care Excellence (NICE) in 2006 recommended that patients of Black African or Black Caribbean ethnicity, should receive either a calcium-channel blocker or a thiazide-type diuretic as first-line therapy (13).
Type 2 diabetes mellitus (T2DM) often accompanies high BP and people with T2DM have a ~2-fold increased risk of hypertension. Rates of T2DM are 3–4 fold higher, respectively, in people of AC and SA ancestry compared with EA in the UK, and this may contribute to differences in hypertension prevalence (14). The greater prevalence of T2DM may also influence the efficacy of antihypertensive medication, since smaller reductions in systolic BP in response to antihypertensive therapy have been observed in T2DM and more therapies may be required to achieve equivalent BP control in T2DM (15, 16). In contrast, current evidence suggests other common risk factors, such as sex, cholesterol and smoking have little or no influence on the response to antihypertensive treatment (15).
Elevated systolic BP and wider pulse pressure in older age is largely attributable to an increase in arterial stiffness (i.e., a reduction in arterial compliance, Ca) (17), and increased arterial stiffness may account for the high rates of uncontrolled hypertension in diabetes (18). Previous studies have generally but not invariably found higher arterial stiffness (or lower Ca) in older people of AC or SA ethnicity (19–21). These differences may be at least partially due to differences in prevalence ofT2DM and hyperglycaemia (19–21).
The aims of this study were to characterise ethnic differences in BP control and use of antihypertensive medications in people with hypertension from a multi-ethnic population sample of older people in the UK. We hypothesised that BP control would be poorer in ethnic minority groups and that this would be due to factors associated with ethnicity, principally, the greater prevalence T2DM and greater arterial stiffness making BP harder to control. We also investigated whether responses to major classes of antihypertensive agents differed by ethnicity.
Methods
Study Population
Southall and Brent Revisited (SABRE) is a UK population-based cohort of European Ancestry (EA), South Asian (SA), and African Caribbean (AC) adults. Details of the cohort have been published (22, 23). In brief, participants, aged 40–69 years, were recruited largely from primary care in West London, EA and SA recruits were predominantly male by design and baseline measurements were performed between 1988 and 1991 (22). Surviving participants (N = 3,433) were invited to attend the 20-year follow-up clinic for investigation between 2008 and 2011 and a total 1,438 participants attended. The current analysis was limited to all participants with hypertension (71% of total participants–EA 61%, SA 79%, AC 82%). Participants were excluded from the analysis if they had evidence of heart failure, atrial fibrillation or dysrhythmias, or invalid blood pressure data. A flow diagram of participant numbers and exclusions is shown in the Supplementary Figure S1. The study was approved by the St Mary's Research Ethics Committee, and all participants gave written informed consent.
Investigations
Participants fasted and refrained from alcohol, smoking, and caffeine for ≥12 h before attendance and were asked not to take their medication on the morning of the clinic visit. A questionnaire was completed to obtain health behaviours, medical history, and medication. Height and weight were measured using a standardised protocol and body composition was measured using a Tanita BC 418 body composition analyzer. Seated brachial blood pressure was measured after 5–10 min rest using an automatic Omron 705IT according to ESH guidelines (24). An appropriately sized cuff was placed on to the left upper arm, 3 recordings were taken 2 min apart, and the second and third recordings were averaged. Blood pressure waveforms were acquired using a SphygmoCor device; measurements were acquired over at least six cardiac cycles and calibrated to brachial SBP and DBP according to the manufacturer's instructions. The commonest blood pressure targets in the UK for hypertension during this period were 140/90 or 130/80 mmHg in people with diabetes [reviewed in (25)], so achievement of BP control was therefore categorised on this basis. Since it was possible that differences in levels of BP control were explained by differences in prevalence of T2DM we also examined achievement of BP control in people without diabetes across the three ethnic groups.
Echocardiography was performed by two experienced cardiovascular physiologists using a Philips iE33 ultrasound machine. Data sets were acquired over 4 cardiac cycles during held respiration and analysed offline using Philips Qlab 7.0. Estimation of stroke volume, aortic flow velocity and left ventricular outflow tract diameter (LVOT) and calculation of left ventricular mass were performed according to American Society of Echocardiography (ASE) recommendations (26). Left ventricular mass was indexed to body surface area (LVMI) and left ventricular hypertrophy was categorised as >125 g/m2 for men and >110 g/m2 for women (27). Reproducibility of BP and echocardiography measures has been reported previously (28, 29).
Fasting blood samples were taken and stored at −80°C prior to analysis. Diabetes mellitus (T2DM) was defined according to the 1999 WHO guidelines (30), or physician diagnosis or receipt of anti-diabetes medications. Hypertension was defined as physician-diagnosed hypertension or participant-reported hypertension or receipt of BP-lowering medication. Study visit BP was not used to diagnose hypertension. Estimated glomerular filtration rate (eGFR) was calculated using the CKD Epi creatine/cystatin C method (31). Medications were based on participant self-report and general practitioner records. Smoking was classified into current or non-smoker based on self-report. Self-reported alcohol consumption was categorised according to UK guidelines into none, ≤ 14 units per week, or >14 units per week. Physical activity was summarised as the estimate of weekly energy expenditure in daily activities plus sport, walking, cycling, and strenuous activities.
Additional calculations were performed on BP waveforms using custom-written software in MATLAB (32). (https://github.com/adh30/Sphygmocor-Reservoir). Calculation of arterial compliance (Ca) and systemic vascular resistance (SVR) was performed using the area method described by Liu et al. (33), since Ca and SVR are expected to be related to height, statistical models including Ca and SVR also included height.
Statistics
All analyses were performed using Stata/MP 17.0 for Windows. Continuous sample data are summarised as means (SD) and categorical data as frequency (%). Comparison of sample characteristics by ethnicity was performed using analysis of variance for continuous variables followed by Tukey's test and categorical data were compared using a Chi2 test, followed by a logistic or ordered logistic regression for individual comparisons as appropriate. Comparisons between individual ethnic groups were made only if there was evidence against the null hypothesis from the omnibus test and Europeans were used as the reference group for all comparisons. Given the known differences in T2DM prevalence by ethnicity and the different BP targets in diabetes, stratified analyses by T2DM status were planned a priori. Global differences in antihypertensive medication use between ethnic groups were assessed by the normalised mutual information using the dseg user contributed program in Stata, which has previously been used to assess racial segregation and has advantages for non-dichotomous classes with multilevel structure (34).
Multivariable regression was performed using full-information maximum likelihood, or generalised structural equation modelling with equation-wise deletion, as appropriate, to minimise bias due to missingness under the assumption of missing at random. Choice of potential explanatory variables of differences in BP control was based on a priori knowledge (35). Treatment effects (TE) were estimated using augmented inverse probability weighting with conditioning on age, sex, T2DM, years of education, physical activity, % fat and alcohol consumption. % fat was included in model in preference to body mass index (BMI) since BMI is an inappropriate measure of adiposity across different ethnic groups (36). The TE method is a “doubly robust” method that models both the treatment and the outcome and is consistent even if one of the models is mis-specified (37). Potential outcomes and average treatment effects (ATE) were estimated using this approach. Results of regression models are presented as means [95% confidence intervals (95%CI)]. Inference was based on a combination of p-values, effect sizes and 95%CI, no adjustment was made for multiple comparisons.
Results
Participants from the three ethnic groups were of similar age but there were slightly more males amongst the SAs than EA. By design there were more women in the sample of AC people (Table 1).
Table 1.
Characteristics of participants by ethnicity.
| Variable | Ethnicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EA | SA | AC | ||||||||
| N | Mean/% | (SD) | N | Mean/% | (SD) | N | Mean/% | (SD) | χ2 and F-test p-values | |
| Age, years | 328 | 70.4 | (6.1) | 356 | 69.4 | (6.3) | 168 | 70.5 | (5.6) | 0.051 |
| Male sex | 259 | 79.0% | 303 | 85.1% | 82 | 48.8% | <0.001 | |||
| Peripheral systolic pressure, mmHg | 317 | 145.0 | (16.4) | 335 | 144.1 | (15.9) | 166 | 145.5 | (15.7) | 0.605 |
| Peripheral diastolic pressure, mmHg | 317 | 85.6 | (10.4) | 335 | 83.7 | (10.7) | 166 | 86.9 | (10.2) | 0.003 |
| Central systolic pressure, mmHg | 317 | 135.1 | (16.1) | 335 | 135.0 | (15.9) | 166 | 136.6 | (16.1) | 0.547 |
| Arterial compliance, ml/mmHg | 292 | 1.7 | (0.6) | 311 | 1.5 | (0.5) | 152 | 1.5 | (0.4) | <0.001 |
| Systemic vascular resistance, mmHg/L | 311 | 1.3 | (0.4) | 326 | 1.4 | (0.4) | 161 | 1.5 | (0.4) | <0.001 |
| Heart rate, bpm | 317 | 64.8 | (11.0) | 335 | 62.9 | (11.4) | 166 | 63.4 | (12.3) | 0.095 |
| Stroke index | 321 | 41.5 | (9.0) | 347 | 42.4 | (9.3) | 163 | 39.5 | (7.7) | 0.003 |
| Height, cm | 328 | 170.2 | (8.6) | 356 | 166.0 | (8.6) | 168 | 165.0 | (8.5) | <0.001 |
| BMI, kg/m2 | 327 | 29.0 | (4.9) | 356 | 26.7 | (3.9) | 168 | 29.2 | (5.3) | <0.001 |
| Fat percent, bioimpedance | 323 | 30.6 | (7.8) | 354 | 27.5 | (7.3) | 165 | 32.3 | (8.8) | <0.001 |
| HbA1c, mmol/mol | 327 | 43.3 | (8.0) | 354 | 50.0 | (13.4) | 165 | 49.0 | (12.8) | <0.001 |
| eGFR, ml/min/1.73 m2 | 322 | 65.0 | (16.6) | 348 | 61.4 | (16.3) | 163 | 79.6 | (20.7) | <0.001 |
| Energy expenditure in physical activity, MJ/wk | 310 | 9.7 | (4.6) | 344 | 9.2 | (4.3) | 152 | 8.8 | (3.7) | 0.081 |
| Years of education | 326 | 10.9 | (2.5) | 338 | 12.8 | (3.7) | 166 | 11.2 | (2.9) | <0.001 |
| Number of antihypertensive agents | 328 | 2.1 | (1.4) | 356 | 2.7 | (1.4) | 168 | 2.6 | (1.5) | <0.001 |
| Receiving antihypertensive medication | 290 | 88.4% | 343 | 96.3% | 157 | 93.5% | <0.001 | |||
| BP controlled, N % | 142 | 43.3% | 102 | 28.7% | 59 | 35.1% | <0.001 | |||
| Diabetes mellitus, N % | 84 | 25.6% | 177 | 49.7% | 77 | 45.8% | <0.001 | |||
| Coronary heart disease, N % | 84 | 25.6% | 145 | 40.7% | 23 | 13.7% | <0.001 | |||
| Stroke, N % | 16 | 4.9% | 17 | 4.8% | 18 | 10.7% | 0.016 | |||
| Left ventricular hypertrophy, N % | 62 | 18.9% | 47 | 13.2% | 39 | 23.2% | 0.012 | |||
| Alcohol consumption category | <0.001 | |||||||||
| None | 54 | 16.5% | 45 | 12.6% | 50 | 29.8% | ||||
| ≤ 14 units per week | 214 | 65.2% | 279 | 78.4% | 115 | 68.5% | ||||
| >14 units per week | 60 | 18.3% | 32 | 9.0% | 3 | 1.8% | ||||
| Current smoker | 18 | 7.7% | 17 | 10.2% | 7 | 7.7% | 0.634 | |||
AC, African Caribbean ethnicity; BP, blood pressure; eGFR, estimated glomerular filtration rate; EA, European ethnicity; LVH, left ventricular hypertrophy; SA South Asian ethnicity. Comparisons of groups were made using one-way analysis of variance.
Compared with EA, SA, and AC were shorter; BMI was lower in SA; % fat was lower in SA and higher in AC; eGFR was lower in SA and higher in AC; T2DM was more prevalent in both SA and AC (and HbA1c was higher); years of education was higher in SA and alcohol consumption was lower in AC. Prevalence of CHD was higher in SA and lower in AC, while a history of stroke was commoner in AC and LVH was less frequent in SA. Smoking patterns and physical activity levels did not differ by ethnicity. Brachial and central systolic BP were similar by ethnicity, but diastolic BP and heart rate was lower in SA, while stroke index was slightly lower in AC.
Antihypertensive Treatment and BP by Ethnicity
SA and AC people were more likely to receive treatment for high BP and received a greater average number of antihypertensive agents (Table 1), but despite this a smaller proportion of SA and AC achieved control of BP to target (Table 1). When, only people without T2DM were included in analyses, levels of control remained slightly poorer in ethnic minority individuals [number (%) with BP controlled in people without diabetes: EA 124 (51%), SA 75 (42%), AC 41 (45%)], although differences were substantially attenuated.
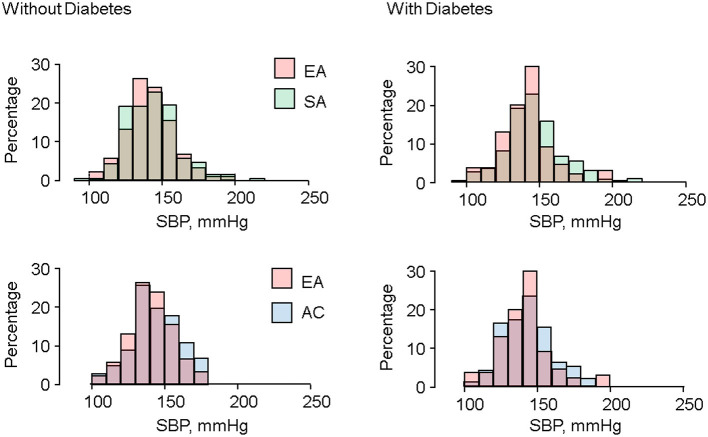
Distributions of systolic BP in SA and AC tended to be less peaked and more skewed to the right, particularly in the presence of T2DM (Figure 1). Distributions of cSBP and DBP are shown in Supplementary Figures 2, 3, respectively. Distributions of cSBP were similar to SBP, and there were no marked differences in distribution of DBP by ethnicity.
Figure 1.
Ethnic differences in distributions of systolic BP in people with and without diabetes.
Adjustment for risk factors that might act as potential mediators of poorer control in ethnic minority groups (age, sex, T2DM, % fat, eGFR, LVH, physical activity, years of education, alcohol intake, prior CHD, or stroke) reduced, but did not abolish differences in the proportion of people with controlled BP (Table 2); most of the attenuation in this adjusted model was attributable to T2DM, which decreased the odds of achieving BP targets. However, this was not the sole explanation of differences as restricting the analysis to people without T2DM still resulted in unfavourable odds ratios (OR) of achieving BP control for both ethnic minorities, albeit less unfavourable than in the complete sample (Table 2).
Table 2.
Blood pressure control in South Asian and African Caribbean people compared with Europeans with and without adjustment.
| Model | South Asian | African Caribbean | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Unadjusted | 0.53 (0.38, 0.72) | <0.001 | 0.71 (0.48, 1.04) | 0.08 |
| Model 1 | 0.52 (0.38, 0.72) | <0.001 | 0.64 (0.43, 0.96) | 0.03 |
| Model 2 | 0.55 (0.36, 0.80) | 0.002 | 0.75 (0.46, 1.21) | 0.24 |
| Model 3 | 0.70 (0.47, 1.04) | 0.08 | 0.70 (0.43, 1.16) | 0.17 |
| Model 4 | 0.52 (0.35, 0.78) | 0.001 | 0.74 (0.44, 1.23) | 0.25 |
| Model 5 | 0.65 (0.42, 1.00) | 0.05 | 0.84 (0.49, 1.43) | 0.52 |
Odds ratios (OR) with 95% confidence intervals (95% CI) are shown for Model 1, age, sex; Model 2, Model 1 plus T2DM, % fat, eGFR, LVH, physical activity, years of education, alcohol intake, prior CHD or stroke; Model 3, Model 1 plus restricted to people without T2DM; Model 4 model 1 plus restricted to people receiving antihypertensive medication; Model 5, Model 2 plus arterial compliance.
Restricting analysis to only people receiving antihypertensive treatment had little effect on estimates (Table 2). Additional adjustment for Ca, which strongly increased the odds of achieving BP control (i.e., higher Ca was associated with greater probability of control), further attenuated differences (Table 2), although it did not completely abolish them. Addition of SVR to the model or substituting HbA1c for T2DM had minimal effects. A fully adjusted model results including haemodynamic variables is shown in Supplementary Table 1.
Patterns of Antihypertensive Use by Ethnicity
Figure 2 shows the pattern of major classes and combinations of antihypertensive medication use by ethnicity. Overall patterns of antihypertensive therapy use appeared broadly similar by ethnicity, and the normalised mutual information was 0.051 indicative of very weak evidence of difference due to ethnicity. AC received more diuretic-based treatment regimens and both ethnic minority groups tended to receive more combination therapy, particularly 4 or more drugs (2%EA vs. 8%SA vs. 5%AC). A RAS inhibitor (A), either as monotherapy or in combination with another agent, was the most used therapy, with 59% of Europeans, 77% of SA, and 56% of AC receiving this class of antihypertensive agents. No participants in any ethnic group received a calcium channel blocker (C) as monotherapy, and no-one received the combinations of A + C, beta blocker (B) + C, C+ diuretic (D), B + C + D or A + C + D.
Figure 2.
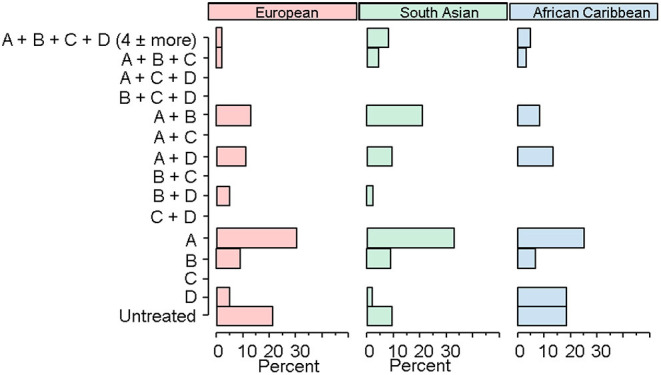
Ethnic patterns of antihypertensive medication.
Treatment Effects of Antihypertensives on Blood Pressure by Ethnicity
Treatment effects of the major classes of antihypertensives alone or in combination on BP, Ca, and SVR were compared across ethnicities. Potential outcome systolic and diastolic BP are shown in Figure 3. Results for most of the treatments were broadly similar, although for many drugs or combinations, the estimates were too imprecise to draw firm conclusions; there was some evidence that B was more effective for lowering systolic BP in SA.
Figure 3.
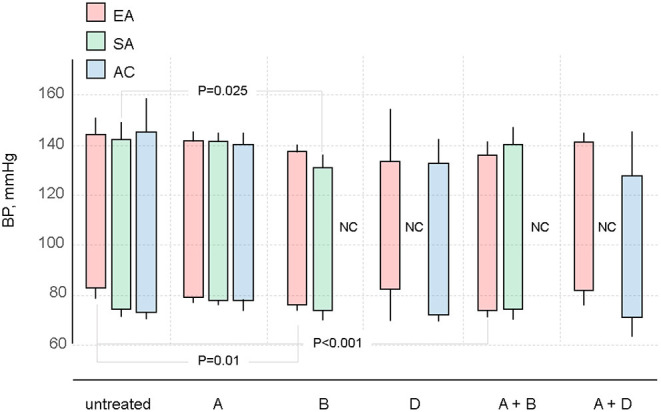
Potential outcome estimates for systolic and diastolic BP in different ethnic groups by antihypertensive treatment regimen. Abbreviations as in Table 3.
There was no convincing evidence that any of the evaluated regimens influenced Ca in any ethnic group (Table 3). There was some weak evidence that B reduced SVR in EA, but otherwise there was little evidence that any regimen affected SVR, although all estimates had wide confidence intervals.
Table 3.
Treatment effects of antihypertensive regimens on arterial compliance and systemic vascular resistance by ethnicity.
| EA | SA | AC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATE | 95% CI | p | ATE | 95% CI | p | ATE | 95% CI | p | ||||
| Arterial compliance | ||||||||||||
| A | 0.08 | (−0.20, 0.36) | 0.57 | −0.05 | (−0.37, 0.26) | 0.73 | −0.01 | (−0.20, 0.17) | 0.88 | |||
| B | 0.34 | (0.01, 0.68) | 0.06 | 0.02 | (−0.33, 0.36) | 0.93 | NC | |||||
| D | NC | NC | −0.03 | (−0.36, 0.30) | 0.85 | |||||||
| A + B | 0.05 | (−0.26, 0.36) | 0.76 | −0.17 | (−0.48, 0.14) | 0.28 | NC | |||||
| A + D | 0.02 | (−0.40, 0.44) | 0.93 | NC | NC | |||||||
| PO | 95% CI | PO | 95% CI | PO | 95% CI | |||||||
| Untreated | 1.62 | (1.38, 1.86) | 1.63 | (1.35, 1.91) | 1.42 | (1.29, 1.54) | ||||||
| ATE | 95% CI | p | ATE | 95% CI | p | ATE | 95% CI | p | ||||
| Systemic vascular resistance | ||||||||||||
| A | −0.11 | −0.26 | 0.03 | 0.14 | 0.03 | −0.10 | 0.16 | 0.67 | −0.07 | −0.41 | 0.28 | 0.70 |
| B | −0.20 | −0.37 | −0.04 | 0.01 | 0.07 | −0.09 | 0.22 | 0.38 | NC | |||
| D | NC | NC | 0.09 | −0.34 | 0.51 | 0.69 | ||||||
| A + B | −0.09 | −0.26 | 0.07 | 0.27 | 0.25 | −0.05 | 0.56 | 0.1 | NC | |||
| A + D | −0.06 | −0.30 | 0.18 | 0.64 | NC | NC | ||||||
| PO | 95% CI | PO | 95% CI | PO | 95% CI | |||||||
| Untreated | 1.39 | 1.28 | 1.50 | 1.39 | 1.29 | 1.49 | 1.57 | 1.25 | 1.89 | |||
A, renin-angiotensin system blocker; B, beta-blocker; C, calcium channel blocker; D, diuretic; NC, not calculated due to small numbers; ATE, average treatment effect; PO, potential outcome.
Discussion
Older people with hypertension from a UK tri-ethnic population-based sample in the UK had universally poor BP control. While average systolic pressures did not differ by ethnicity, attainment of BP targets in both SA and AC people was worse than in EA individuals, despite people in minority ethnic groups receiving more antihypertensive agents on average. Much, but not all, of this difference in BP control may be attributable to a higher burden of T2DM in the minority ethnic groups, but lower Ca (higher arterial stiffness) may also make an important contribution. Higher SVR was, if at all, associated with better likelihood of achievement of target BP.
At the time of the study contemporary guidelines in the UK required lower targets to be achieved in T2DM (38); this is likely to have contributed to the poorer levels of control as lower targets will naturally be more difficult to achieve, but there is also evidence that T2DM may reduce the efficacy of antihypertensive agents (15, 16). Antihypertensive agents can reduce arterial stiffness (39), but much of this effect may depend on BP reduction, as the elastic behaviour of arteries shows a non-linear incremental relationship with BP (40), and evidence for a direct effect of antihypertensive agents on intrinsic stiffness of arteries is limited (39, 40). Poorer arterial stiffness has previously been reported in these ethnic groups (19–21). Reduced Ca in SA and AC may be linked to the greater prevalence of T2DM or dysglycaemia in these ethnic groups, particularly since there is evidence that SA and AC experience a greater chronic glycaemic burden from childhood onwards (41), as well as having an earlier of onset T2DM (42, 43). Reasons for these ethnic differences in glycaemic burden, T2DM and Ca remain to be fully established, but there is evidence that obesity (particularly truncal obesity), low physical activity, psychosocial variables (e.g., racism) and socioeconomic disadvantage make important contributions (42, 44, 45).
Our observations are in keeping with earlier studies in UK which have found poorer rates of BP control in ethnic minorities (4–6). By design, SABRE recruited fewer women in the EA and SA samples which is a limitation (22). It should also be noted that the majority of SA recruits to SABRE were of Punjabi Sikh origin (46) and while migrants from the Indian subcontinent are generally at increased risk of T2DM compared with Europeans, the prevalence of T2DM varies between South Asian subgroups (47); so this may limit the generalizability of our findings. Our findings are consistent with a more recent European-wide study that found that African origin people were less likely to have their BP controlled, despite greater awareness of hypertension (4). We found little evidence that poorer control of BP in ethnic minorities in UK was attributable to marked differences in efficacy of antihypertensive regimens by ethnicity. This conclusion is in accord with some previous studies (10, 12); however, it should be stressed this part of our study had limited power and is vulnerable to uncontrolled confounding and bias by indication, consequently these findings should be viewed with considerable caution. We also note that BP was measured at trough in keeping with standard practise (48), but this will tend to under-estimate BP lowering for short-acting antihypertensive agents. Another limitation of our study is that there was a high rate of attrition in the overall sample and while there were no major differences in measured variables between baseline and clinic follow-up (22), this could have introduced bias. Previous data from randomised clinical trials (7–9) have indicated small differences in response to antihypertensive agents between people of European ancestry and African ancestry, but it is not clear how important these differences in efficacy are at a population level (10).
It is possible that therapeutic inertia on the physician's part or poor adherence on the patient's part contributed to the differences in BP control we observed, but our study lacks information on these questions. It was notable, however that utilisation of antihypertensive agents differed little by ethnicity and that patterns of utilisation seemed relatively uninfluenced by UK guidelines operating at the time of the study which made recommendations regarding preferred antihypertensive agents in ethnic minorities (38). This is particularly evident for calcium channel blockers which at the time of the study were advocated as first line agents for people of African or Caribbean ethnicity but were only used infrequently and not at all as monotherapy in any ethnic group. More recent data from more than 40,000 Medicare beneficiaries in the US are also consistent with the observation of poor physician concordance with guidelines (49).
Our observations of poorer BP control in ethnic minorities in the UK are important since both groups are at elevated risk of CVD: CHD and stroke for SA, and stroke for AC. While the issue of lower targets in T2DM remains under debate (50), our findings add to recent data suggesting that more aggressive antihypertensive treatment to achieve lower BP targets may be warranted (51). However, the poor levels of control reported in this study also underscore the difficulty of achieving such lower targets in a real-world setting. We speculate that initiation of antihypertensive therapy via a combination polypill, which has been reported to achieve better levels of BP control (52) might be a better strategy.
Conclusion
In a tri-ethic population-based sample of older people control of hypertension was generally poor, but worse in people of AC and SA ethnicity. This poorer BP control occurred despite greater use of antihypertensive agents. Poorer BP control was partially accounted for by statistical adjustment for the greater prevalence of T2DM and more adverse arterial stiffness in these groups. Lowering blood pressure remains an important public health goal, and more effort is required to achieve better control of BP, particularly in ethnic minorities in UK.
Data Availability Statement
Publicly available datasets were analysed in this study. This data can be found at: https://www.sabrestudy.org/home-2/data-sharing/.
Ethics Statement
The studies involving human participants were reviewed and approved by St Mary's Research Ethics Committee. The participants provided their written informed consent to participate in this study.
Author Contributions
AH contributed to conception and design of the study, performed the statistical analysis, and wrote the first draft of the manuscript. TT and AH contributed to data curation. NC and AH contributed to supervision and funding acquisition. SE, TT, and NC contributed to sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The SABRE study was funded at baseline by the Medical Research Council, Diabetes UK, and the British Heart Foundation. At follow-up the study was funded by the Wellcome Trust (067100, 37055891, and 086676/7/08/Z), the British Heart Foundation (PG/06/145, PG/08/103/26133, PG/12/29/29497, and CS/13/1/30327), and Diabetes UK (13/0004774). AH receives support from the British Heart Foundation, the Horizon 2020 Framework Programme of the European Union, the National Institute for Health Research University College London Hospitals Biomedical Research Centre, the UK Medical Research Council, the Wellcome Trust, and works in a unit that receives support from the UK Medical Research Council. SE was funded by a Diabetes UK Sir George Alberti Research Training Fellowship (Grant No. 17/0005588, https://www.diabetes.org.uk/).
Conflict of Interest
NC receives funding from AstraZeneca to serve on data safety and monitoring committees for clinical trials. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are extremely grateful to all the people who took part in the study, and past and present members of the SABRE team who helped to collect the data. The study team also acknowledges the support of the National Institute of Health Research Clinical Research Network (NIHR CRN).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.795267/full#supplementary-material
References
- 1.Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. (2021) 18:785–802. 10.1038/s41569-021-00559-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Primatesta P, Bost L, Poulter NR. Blood pressure levels and hypertension status among ethnic groups in England. J Hum Hypertens. (2000) 14:143–8. 10.1038/sj.jhh.1000960 [DOI] [PubMed] [Google Scholar]
- 3.Agyemang C, Bhopal RS. Is the blood pressure of South Asian adults in the UK higher or lower than that in European white adults? A review of cross-sectional data. J Hum Hypertens. (2002) 16:739–51. 10.1038/sj.jhh.1001488 [DOI] [PubMed] [Google Scholar]
- 4.van der Linden EL, Couwenhoven BN, Beune E, Daams JG, van den Born BH, Agyemang C. Hypertension awareness, treatment and control among ethnic minority populations in Europe: a systematic review and meta-analysis. J Hypertens. (2021) 39:202–13. 10.1097/HJH.0000000000002651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappuccio FP, Cook DG, Atkinson RW, Strazzullo P. Prevalence, detection, and management of cardiovascular risk factors in different ethnic groups in south London. Heart. (1997) 78:555–63. 10.1136/hrt.78.6.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan BM. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. (2010) 303:2043. 10.1001/jama.2010.650 [DOI] [PubMed] [Google Scholar]
- 7.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The department of veterans affairs cooperative study group on antihypertensive agents. N Engl J Med. (1993) 328:914–21. 10.1056/NEJM199304013281303 [DOI] [PubMed] [Google Scholar]
- 8.Wright JT Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, et al. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. (2005) 293:1595–608. 10.1001/jama.293.13.1595 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TT, Kaufman JS, Whitsel EA, Cooper RS. Racial differences in blood pressure response to calcium channel blocker monotherapy: a meta-analysis. Am J Hypertens. (2009) 22:911–7. 10.1038/ajh.2009.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinnott SJ, Douglas IJ, Smeeth L, Williamson E, Tomlinson LA. First line drug treatment for hypertension and reductions in blood pressure according to age and ethnicity: cohort study in UK primary care. Br Med J. (2020) 371:m4080. 10.1136/bmj.m4080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta AK, Poulter NR, Dobson J, Eldridge S, Cappuccio FP, Caulfield M, et al. Ethnic differences in blood pressure response to first and second-line antihypertensive therapies in patients randomized in the ASCOT trial. Am J Hypertens. (2010) 23:1023–30. 10.1038/ajh.2010.105 [DOI] [PubMed] [Google Scholar]
- 12.Brewster LM, van Montfrans GA, Oehlers GP, Seedat YK. Systematic review: antihypertensive drug therapy in patients of African and South Asian ethnicity. Intern Emerg Med. (2016) 11:355–74. 10.1007/s11739-016-1422-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Collaborating Centre for Chronic Conditions (UK) . Hypertension: Management in Adults in Primary Care: Pharmacological Update. London: Royal College of Physicians; (2006). [PubMed] [Google Scholar]
- 14.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N. The role of diabetes and components of the metabolic syndrome in stroke and coronary heart disease mortality in U.K. white and African-Caribbean populations. Diabetes Care. (2006) 29:2127–9. 10.2337/dc06-0779 [DOI] [PubMed] [Google Scholar]
- 15.Brown MJ, Castaigne A, de Leeuw PW, Mancia G, Palmer CR, Rosenthal T, et al. Influence of diabetes and type of hypertension on response to antihypertensive treatment. Hypertension. (2000) 35:1038–42. 10.1161/01.HYP.35.5.1038 [DOI] [PubMed] [Google Scholar]
- 16.Grossman A, Grossman E. Blood pressure control in type 2 diabetic patients. Cardiovasc Diabetol. (2017) 16:3. 10.1186/s12933-016-0485-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham heart study. Circulation. (2010) 122:1379–86. 10.1161/CIRCULATIONAHA.109.914507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung C-M, Chang J-J, Lin Y-S, Hsiao J-F, Chang S-T, Hsu J-T, et al. Relationship between resistant hypertension and arterial stiffness assessed by brachial-ankle pulse wave velocity in the older patient. Clin Interv Aging. (2014) 9:1495–502. 10.2147/CIA.S68544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schutte AE, Kruger R, Gafane-Matemane LF, Breet Y, Strauss-Kruger M, Cruickshank JK. Ethnicity and arterial stiffness. Arterioscler Thromb Vasc Biol. (2020) 40:1044–54. 10.1161/ATVBAHA.120.313133 [DOI] [PubMed] [Google Scholar]
- 20.Park CM, Tillin T, March K, Jones S, Whincup PH, Mayet J, et al. Adverse effect of diabetes and hyperglycaemia on arterial stiffness in Europeans, South Asians, and African Caribbeans in the SABRE study. J Hypertens. (2016) 34:282–9. 10.1097/HJH.0000000000000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance. Circulation. (2002) 106:2085–90. 10.1161/01.CIR.0000033824.02722.F7 [DOI] [PubMed] [Google Scholar]
- 22.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N. Southall and brent revisited: cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. Int J Epidemiol. (2012) 41:33–42. 10.1093/ije/dyq175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones S, Tillin T, Park C, Williams S, Rapala A, Al Saikhan L, et al. Cohort profile update: southall and brent revisited (SABRE) study: a UK population-based comparison of cardiovascular disease and diabetes in people of European, South Asian and African Caribbean heritage. Int J Epidemiol. (2020) 49:1441–2. 10.1093/ije/dyaa135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, et al. Practice guidelines of the European society of hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. (2005) 23:697–701. 10.1097/01.hjh.0000163132.84890.c4 [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulou E, Angeloudi E, Karras S, Sarafidis P. The optimal blood pressure target in diabetes mellitus: a quest coming to an end? J Hum Hypertens. (2018) 32:641–50. 10.1038/s41371-018-0079-5 [DOI] [PubMed] [Google Scholar]
- 26.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Doppler Doppler Quantification Task Force of the N. Recommendations for quantification of doppler echocardiography: a report from the doppler quantification task force of the nomenclature and standards committee of the American society of echocardiography. J Am Soc Echocardiogr. (2002) 15:167–84. 10.1067/mje.2002.120202 [DOI] [PubMed] [Google Scholar]
- 27.Marcus R, Krause L, Weder AB, Dominguez-Meja A, Schork NJ, Julius S. Sex-specific determinants of increased left ventricular mass in the tecumseh blood pressure study. Circulation. (1994) 90:928–36. 10.1161/01.CIR.90.2.928 [DOI] [PubMed] [Google Scholar]
- 28.Park CM, March K, Ghosh AK, Jones S, Coady E, Tuson C, et al. Left-ventricular structure in the southall and Brent REvisited (SABRE) study: explaining ethnic differences. Hypertension. (2013) 61:1014–20. 10.1161/HYPERTENSIONAHA.111.00610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park CM, Korolkova O, Davies JE, Parker KH, Siggers JH, March K, et al. Arterial pressure: agreement between a brachial cuff-based device and radial tonometry. J Hypertens. (2014) 32:865–72. 10.1097/HJH.0000000000000082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization . Definition, Diagnosis and Classification of diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; (1999). [Google Scholar]
- 31.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes AD, Park C, Ramakrishnan A, Mayet J, Chaturvedi N, Parker KH. Feasibility of estimation of aortic wave intensity using non-invasive pressure recordings in the absence of flow velocity in man. Front Physiol. (2020) 11:550. 10.3389/fphys.2020.00550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol. (1986) 251 (3 Pt. 2):H588–600. 10.1152/ajpheart.1986.251.3.H588 [DOI] [PubMed] [Google Scholar]
- 34.Mora R, Ruiz-Castillo J. Entropy-Based segregation indices. Sociol Methodol. (2011) 41:159–94. 10.1111/j.1467-9531.2011.01237.x [DOI] [Google Scholar]
- 35.He J, Muntner P, Chen J, Roccella EJ, Streiffer RH, Whelton PK. Factors associated with hypertension control in the general population of the United States. Arch Intern Med. (2002) 162:1051. 10.1001/archinte.162.9.1051 [DOI] [PubMed] [Google Scholar]
- 36.Deurenberg P, Yap M, Van Staveren W. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obesity. (1998) 22:1164–171. 10.1038/sj.ijo.0800741 [DOI] [PubMed] [Google Scholar]
- 37.Bang H, Robins JM. Doubly robust estimation in missing data and causal inference models. Biometrics. (2005) 61:962–73. 10.1111/j.1541-0420.2005.00377.x [DOI] [PubMed] [Google Scholar]
- 38.Williams B. British hypertension society guidelines for hypertension management 2004 (BHS-IV): summary. Br Med J. (2004) 328:634–40. 10.1136/bmj.328.7440.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boutouyrie P, Lacolley P, Briet M, Regnault V, Stanton A, Laurent S, et al. Pharmacological modulation of arterial stiffness. Drugs. (2011) 71:1689–701. 10.2165/11593790-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 40.Reesink KD, Spronck B. Constitutive interpretation of arterial stiffness in clinical studies: a methodological review. Am J Physiol Heart Circ Physiol. (2019) 316:H693–709. 10.1152/ajpheart.00388.2018 [DOI] [PubMed] [Google Scholar]
- 41.Whincup PH, Nightingale CM, Owen CG, Rudnicka AR, Gibb I, McKay CM, et al. Early emergence of ethnic differences in type 2 diabetes precursors in the UK: the child heart and health study in England (CHASE study). PLoS Med. (2010) 7:e1000263. 10.1371/journal.pmed.1000263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tillin T, Hughes AD, Godsland IF, Whincup P, Forouhi NG, Welsh P, et al. Insulin resistance and truncal obesity as important determinants of the greater incidence of diabetes in Indian Asians and African Caribbeans compared with Europeans: the Southall and Brent REvisited (SABRE) cohort. Diabetes Care. (2013) 36:383–393. 10.2337/dc12-0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang MC, Shah NS, Carnethon MR, O'Brien MJ, Khan SS. Age at diagnosis of diabetes by race and ethnicity in the United States From 2011 to 2018. JAMA Intern Med. (2021) 181:1537–9. 10.1001/jamainternmed.2021.4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruickshank JK, Silva MJ, Molaodi OR, Enayat ZE, Cassidy A, Karamanos A, et al. Ethnic differences in and childhood influences on early adult pulse wave velocity: the determinants of adolescent, now young adult, social wellbeing, and health longitudinal study. Hypertension. (2016) 67:1133–41. 10.1161/HYPERTENSIONAHA.115.07079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farmaki AE, Garfield V, Eastwood SV, Farmer RE, Mathur R, Giannakopoulou O, et al. Type 2 diabetes risks and determinants in second-generation migrants and mixed ethnicity people of South Asian and African Caribbean descent in the UK. Diabetologia. (2021) 65:113–27. 10.1007/s00125-021-05580-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tillin T, Hughes AD, Mayet J, Whincup P, Sattar N, Forouhi NG, et al. The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans: SABRE (Southall and brent revisited)—a prospective population-based study. J Am Coll Cardiol. (2013) 61:1777–86. 10.1016/j.jacc.2012.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agyemang C, van der Linden EL, Bennet L. Type 2 diabetes burden among migrants in Europe: unravelling the causal pathways. Diabetologia. (2021) 64:2665–75. 10.1007/s00125-021-05586-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zannad F. Practical relevance of the 24-hour trough: peak ratio of antihypertensive drugs. J Hypertens Suppl. (1995) 13:S109–12. 10.1097/00004872-199508001-00018 [DOI] [PubMed] [Google Scholar]
- 49.Colvin CL, King JB, Oparil S, Wright JT, Ogedegbe G, Mohanty A, et al. Association of race/ethnicity-specific changes in antihypertensive medication classes initiated among medicare beneficiaries with the eighth joint national committee panel member report. JAMA Netw Open. (2020) 3:e2025127. 10.1001/jamanetworkopen.2020.25127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American Diabetes Association . 10 cardiovascular disease and risk management: standards of medical care in diabetes-−2021. Diabetes Care. (2021) 44:S125–50. 10.2337/dc21-S010 [DOI] [PubMed] [Google Scholar]
- 51.Sprint Research Group, Lewis CE, Fine LJ, Beddhu S, Cheung AK, Cushman WC, et al. Final report of a trial of intensive versus standard blood-pressure control. N Engl J Med. (2021) 384:1921–30. 10.1056/NEJMoa1901281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webster R, Salam A, de Silva HA, Selak V, Stepien S, Rajapakse S, et al. Fixed Low-Dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. JAMA. (2018) 320:566–9. 10.1001/jama.2018.10359 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analysed in this study. This data can be found at: https://www.sabrestudy.org/home-2/data-sharing/.