Abstract
This study aims to investigate the multivariate relationship between different sociodemographic, clinical, and neurophysiological variables with resting-state, high-definition, EEG spectral power in subjects with chronic knee osteoarthritis (OA) pain. This was a cross-sectional study. Sociodemographic and clinical data were collected from 66 knee OA subjects. To identify associated factors, we performed independent univariate and multivariate regression models by frequency bands (delta, theta, alpha, beta, low-beta, and high-beta) and by pre-defined regions (frontal, central, and parietal). From adjusted multivariate models, we found that: (1) increased frontocentral high-beta power and reduced central theta activity are positively correlated with pain intensity (β = 0.012, 95% CI 0.004–0.020; and β = − 0.008; 95% CI 0.014 to − 0.003; respectively); (2) delta and alpha oscillations have a direct relationship with higher cortical inhibition; (3) diffuse increased power at low frequencies (delta and theta) are associated with poor cognition, aging, and depressive symptoms; and (4) higher alpha and beta power over sensorimotor areas seem to be a maladaptive compensatory mechanism to poor motor function and severe joint degeneration. Subjects with higher pain intensity and higher OA severity (likely subjects with maladaptive compensatory mechanisms to severe OA) have higher frontocentral beta power and lower theta activity. On the other hand, subjects with less OA severity and less pain have higher theta oscillations power. These associations showed the potential role of brain oscillations as a marker of pain intensity and clinical phenotypes in chronic knee OA patients. Besides, they suggest a potential compensatory mechanism of these two brain oscillators according to OA severity.
Subject terms: Neurology, Chronic pain, Rehabilitation, Biomarkers
Introduction
Chronic pain associated with osteoarthritis is a leading cause of disability, decreased quality of life, and represents a high economic burden worldwide1,2. The perpetuation of pain in knee osteoarthritis (OA) has been associated with maladaptive changes in peripheral and central nervous systems3,4. In addition to knee OA, other chronic pain conditions, such as fibromyalgia, orofacial pain, spinal cord injury, and phantom limb pain, also present central nervous system (CNS) alterations like central sensitization of nociceptive pathways, impairment in the descending pain modulation system, and altered emotional-motivational brain systems1,5–8.
The use of electroencephalography (EEG)—a noninvasive measure of electrical neuronal activity at different frequencies (delta, theta, alpha, beta, and gamma bands)—has been shown as a promising tool for assessing potential brain function changes in chronic pain patients. Recent EEG studies have shown that chronic pain populations compared to healthy controls have a distinct brain oscillatory signature, mainly an increase in theta and alpha power at rest9,10. These results are usually explained by the presence of thalamocortical dysrhythmia (TCD), that is characterized by an increased theta power with decreased dominant alpha activity8,11–13. However, these changes are predominately studied in neuropathic pain and not consistently found in musculoskeletal conditions14–17.
Besides, fewer studies have explicitly investigated the brain oscillations associated with pain intensity. These studies revealed that objective noxious stimulus intensity was inversely related to alpha and beta oscillations in sensorimotor areas, whereas subjective pain was positively related to oscillations at higher frequencies (high beta and gamma) in frontal areas17,18.
Modulating these pain-related brain oscillations can be used as new pain management approach19–21. However, most of the current findings are limited by its correlative nature, without considering demographic, clinical, and neurophysiological variables into the analysis as potential confounders or effect modifiers. Therefore, the clinical variables that could influence and predict brain oscillations behavior in chronic pain patients are not completely understood.
Our main hypothesis is that clinical features in patients with chronic pain such as pain intensity, motor function, cognitive-emotional status, quantitative sensory testing, and cortical excitability (indexed by transcranial magnetic stimulation) are associated with specific brain oscillatory patterns at resting EEG. Therefore, this study aims to investigate the multivariate associations of different sociodemographic and clinical variables and EEG brain oscillations of chronic knee OA pain patients. Furthermore, this knowledge may help us understand pain-related brain oscillations, their underlying brain functions, and its potential utility as biomarkers of chronic pain in chronic knee OA patients.
Results
Demographics and clinical characteristics
We included 66 patients with knee OA in this study. All participants reported chronic knee OA pain, most of them (96.97%) with bilateral symptoms. The majority of the participants were female, older adults, and overweight or obese. The average pain was moderate (VAS pain of 5.65 [SD = 1.83] from 0 to 10 scale; and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain of 11.03 [SD = 3.92] from 0 to 20 scale). We found no statistically significant correlation between the WOMAC pain intensity and the K–L grade (p = 0.10). Further baseline clinical and neurophysiological data are provided in Table 1.
Table 1.
Baseline clinical and sociodemographic characteristics of knee OA study participants.
| Variables | Knee OA subjects (N = 66) |
|---|---|
| Demographics | |
| Age (range) | 68.90 ± 9.53 (52–92) |
| Gender (%) | |
| Female | 61 (92.42) |
| Male | 5 (7.58) |
| Ethnicity | |
| White | 44 (66.67) |
| Black | 6 (9.09) |
| Mixed | 11 (16.67) |
| Asian | 5 (7.58) |
| Education level (%) | |
| Illiterate | 1 (1.52) |
| Elementary | 25 (37.88) |
| High-school | 20 (30.30) |
| Superior | 20 (30.30) |
| Weight (kg) | 78.62 ± 13.03 |
| Height (m) | 1.56 ± 0.08 |
| BMI | 31.88 ± 4.65 |
| Clinical assessments | |
| Bilateral knee OA (%) | 64 (96.97) |
| Time of ongoing pain (months) | 97.39 ± 103.89 |
| Total knee arthroplasty (%) | |
| Right | 1 (1.52) |
| Left | 2 (3.03) |
| Pain—visual analogue scale | |
| Right | 5.96 ± 2.72 |
| Left | 5.35 ± 2.76 |
| Average | 5.65 ± 1.83 |
| WOMAC total score | 51.26 ± 19.01 |
| WOMAC pain | 11.03 ± 3.92 |
| WOMAC stiffness | 4.64 ± 1.92 |
| WOMAC physical function | 35.58 ± 14.34 |
| Kellgren–Lawrence classification | |
| Right (%) | |
| 1 | 17 (26.15) |
| 2 | 15 (23.08) |
| 3 | 11 (16.92) |
| 4 | 22 (33.85) |
| Mean (SD) | 2.58 ± 1.21 |
| Left | |
| 1 | 21 (32.81) |
| 2 | 15 (23.44) |
| 3 | 12 (18.75) |
| 4 | 16 (25) |
| Mean (SD) | 2.36 ± 1.19 |
| Average between right and left | 2.50 ± 1.15 |
| Pain Catastrophizing Scale | 14.18 ± 10.98 |
| HAM-L Scale | 9.36 ± 5.60 |
| Hospital Anxiety and Depression Scale | |
| Anxiety | 5.68 ± 3.93 |
| Depression | 4.46 ± 3.56 |
| Montreal Cognitive Assessment | 20.67 ± 5.05 |
| Epworth sleepiness scale | 11.00 ± 6.19 |
| Quality of life (sf-36)—total score | 52.47 ± 20.40 |
| Quantitative sensory testing | |
| Pain pressure threshold | |
| Upper limb | |
| Right | 5.36 ± 1.96 |
| Left | 5.18 ± 2.06 |
| Average | 5.27 ± 1.95 |
| Knee | |
| Right | 4.39 ± 2.55 |
| Left | 4.39 ± 2.37 |
| Average | 4.39 ± 2.42 |
| Conditioned pain modulation | |
| Right | 1.22 ± 1.18 |
| Left | 1.01 ± 1.21 |
| Average | 1.16 ± 1.04 |
| Average (% of change from baseline) | 22.48 ± 22.29 |
| Transcranial magnetic stimulation | |
| Motor threshold | |
| Right | 51.94 ± 11.10 |
| Left | 50.30 ± 10.58 |
| Average | 51.12 ± 9.86 |
| Motor evoked potential | |
| Right | 1.64 ± 1.08 |
| Left | 1.72 ± 1.44 |
| Average | 1.68 ± 1.02 |
| Cortico-silent period | |
| Right | 93.20 ± 35.51 |
| Left | 82.32 ± 32.54 |
| Average | 87.76 ± 31.64 |
| Short intracortical inhibition | |
| Right | 0.46 ± 0.32 |
| Left | 0.47 ± 0.31 |
| Average | 0.47 ± 0.26 |
| Intracortical facilitation | |
| Right | 1.59 ± 0.71 |
| Left | 1.63 ± 0.82 |
| Average | 1.61 ± 0.60 |
Delta band oscillations models
Cognitive function (as indexed by the Montreal Cognitive Assessment [MoCA] scale) has been negatively associated with delta oscillation activity in the frontal (β = − 0.008, 95% CI − 0.013 to − 0.003; p = 0.002), central (β = − 0.009, 95% CI − 0.014 to − 0.004; p = 0.001), and parietal (β = − 0.006, 95% CI − 0.012 to − 0.001; p = 0.013) regions. Short interval intracortical inhibition (SICI) was negatively associated with delta band activity in the frontal (β = − 0.137, 95% CI − 0.232 to − 0.042; p = 0.005) and central (β = − 0.100, 95% CI − 0.194 to − 0.006; p = 0.037) areas, indicating a direct relationship between delta oscillation and intracortical inhibition. Furthermore, a significant, positive relationship between age and delta activity can be observed in the frontal (β = 0.004, 95% CI 0.002–0.007; p = 0.001) and parietal (β: 0.003, 95% CI 0.0006–0.006; p = 0.015) regions, suggesting an increase in delta activity with aging. Moreover, depression (as indexed by the Hamilton Depression Rating Scale [HAM-L]) was positively associated with delta activity in the frontal (β = 0.007, 95% CI 0.002–0.011; p = 0.001) and parietal (β = 0.007, 95% CI 0.002–0.012; p = 0.004) areas. Finally, social function (as indexed by the SF-36 questionnaire) was also identified as associated with delta band activity with a positive relationship, in all regions (frontal: β = 0.002, central: β = 0.001, and parietal: β = 0.007). Time of ongoing pain was the main confounder identified in delta band models (Table 2).
Table 2.
Baseline delta band multivariate analyses according to region of interest (ROI).
| Variables | Beta-coefficient | 95% CI | p-value | R2 |
|---|---|---|---|---|
| Frontal region | 0.440 | |||
| MoCA score | − 0.008 | − 0.013 to − 0.003 | 0.002 | |
| SF-36 social function subscale | 0.002 | 0.001 to 0.003 | < 0.001 | |
| SICI average | − 0.137 | − 0.232 to − 0.042 | 0.005 | |
| HAM-L depression scale | 0.007 | 0.002 to 0.011 | 0.004 | |
| Age | 0.004 | 0.002 to 0.007 | 0.001 | |
| Time of ongoing pain | − 0.0001 | − 0.0003 to 0.0001 | 0.312 | |
| Central region | 0.291 | |||
| MoCA score | − 0.009 | − 0.014 to − 0.004 | 0.001 | |
| SF-36 social function subscale | 0.001 | 0.0004 to 0.002 | 0.005 | |
| SICI average | − 0.100 | − 0.194 to − 0.006 | 0.037 | |
| Time of ongoing pain | − 0.0001 | − 0.0003 to 0.0001 | 0.339 | |
| Age | 0.002 | − 0.0005 to 0.004 | 0.114 | |
| Parietal region | 0.344 | |||
| MoCA score | − 0.006 | − 0.012 to − 0.001 | 0.013 | |
| HAM-L depression scale | 0.007 | 0.002 to 0.012 | 0.004 | |
| SF-36 social function subscale | 0.002 | 0.0009 to 0.003 | < 0.001 | |
| Age | 0.003 | 0.0006 to 0.006 | 0.015 | |
| Time of ongoing pain | − 0.0002 | − 0.0004 to 0.00004 | 0.109 |
Theta band oscillations models
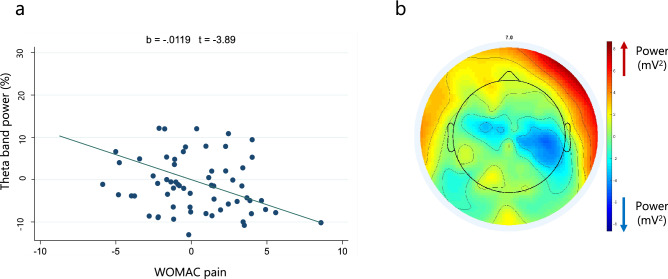
In relation to theta oscillations, pain intensity (as indexed by the WOMAC pain scale) is conveyed as having a statistically significant, negative association with theta activity in the frontal (β = − 0.012; 95% CI − 0.018 to − 0.006; p < 0.001), central (β = − 0.008; 95% CI 0.014 to − 0.003; p = 0.001), and parietal (β = − 0.008; 95% CI − 0.013 to − 0.002; p = 0.006) areas. This negative correlation is presented in the Fig. 1a and corroborated by the topographical map from representative patients with high pain intensity (Fig. 1b). In contrast, positive associations between motor function (indexed by timed up and go test) and theta activity was seen in the frontal (β = 0.003; 95% CI 0.001–0.005; p = 0.003), central (β = 0.005, 95% CI 0.002–0.007; p < 0.001), and parietal (β = 0.005, 95% CI 0.002–0.008; p < 0.001) regions. Besides, we found female participants have statistically significant higher theta band power in all three areas (frontal: β = 0.083; central β = 0.104; and parietal: β = 0.114). Moreover, OA severity was shown to be negatively correlated to theta oscillations in the central (β = − 0.023; 95% CI − 0.042 to − 0.004; p = 0.019) and parietal (β = − 0.025; 95% CI − 0.046 to − 0.004; p = 0.019) regions. Depression was only significantly correlated with theta bands in the frontal (β = 0.008; 95% CI 0.0004–0.017; p = 0.039) region, conveying a positive correlation (Table 3).
Figure 1.
(a) Theta band power and pain intensity adjusted correlation (from multivariate model of central region). (b) Topographical plot of spectral power from representative subjects (n = 15) with high pain intensity (higher than ten in WOMAC pain scale).
Table 3.
Baseline theta band multivariate analyses according to ROI.
| Variables | Beta-coefficient | 95% CI | p-value | R2 |
|---|---|---|---|---|
| Frontal region | 0.330 | |||
| WOMAC pain scale | − 0.012 | − 0.018 to − 0.006 | < 0.001 | |
| Timed up and go score | 0.003 | 0.001 to 0.005 | 0.003 | |
| HAD-depression scale | 0.008 | 0.0004 to 0.017 | 0.039 | |
| Gender (female) | 0.083 | 0.006 to 0.159 | 0.034 | |
| HAD-Anxiety scale | − 0.004 | − 0.011 to 0.003 | 0.238 | |
| Central region | 0.374 | |||
| WOMAC pain scale | − 0.008 | − 0.014 to − 0.003 | 0.001 | |
| Timed up and go score | 0.005 | 0.002 to 0.007 | < 0.001 | |
| Gender (female) | 0.104 | 0.031 to 0.177 | 0.006 | |
| Kellgren–Lawrence scale | − 0.023 | − 0.042 to − 0.004 | 0.019 | |
| Age | 0.0007 | − 0.002 to 0.003 | 0.556 | |
| Parietal region | 0.353 | |||
| Timed up and go total | 0.005 | 0.002 to 0.008 | < 0.001 | |
| WOMAC pain scale | − 0.008 | − 0.013 to − 0.002 | 0.006 | |
| Gender (female) | 0.114 | 0.034 to 0.193 | 0.006 | |
| Kellgren–Lawrence scale | − 0.025 | − 0.046 to − 0.004 | 0.019 | |
| Age | 0.001 | − 0.001 to 0.004 | 0.429 |
Alpha band oscillations models
We found that equivalent set of variables were associated with alpha band oscillations in the frontal, central, and parietal regions. We found a statistically significant positive relationship with motor evoked potentials (MEP) (b = 0.040, b = 0.032, and b = 0.045 in frontal, central, and parietal regions, respectively); with cortical silent period (CSP) (b = 0.001, b = 0.001, b = 0.001, respectively), indicating a direct relationship between alpha oscillation and cortical inhibition; and with WOMAC stiffness score (b = 0.020, b = 0.019, b = 0.022, respectively). Additionally, an association with gender was found, namely female participants have lower alpha band power in all areas (b = − 0.123, b = − 0.137, b = − 0.164, respectively). Depression scale was found as the main confounder of alpha band oscillations (Table 4).
Table 4.
Baseline alpha band multivariate analyses according to ROI.
| Variables | Beta-coefficient | 95% CI | p-value | R2 |
|---|---|---|---|---|
| Frontal region | 0.280 | |||
| MEP average | 0.040 | 0.010 to 0.071 | 0.011 | |
| CSP average | 0.001 | 0.0002 to 0.002 | 0.014 | |
| Gender (female) | − 0.123 | − 0.241 to − 0.005 | 0.042 | |
| WOMAC stiffness score | 0.020 | 0.003 to 0.037 | 0.021 | |
| HAM-L depression scale | − 0.005 | − 0.011 to 0.001 | 0.122 | |
| Central region | 0.271 | |||
| Gender (female) | − 0.137 | − 0.251 to − 0.023 | 0.019 | |
| MEP average | 0.032 | 0.002 to 0061 | 0.036 | |
| CSP average | 0.001 | 0.0002 to 0.002 | 0.014 | |
| WOMAC stiffness score | 0.019 | 0.002 to 0.035 | 0.028 | |
| HAM-L depression scale | − 0.004 | − 0.010 to 0.001 | 0.153 | |
| Parietal region | 0.247 | |||
| MEP average | 0.045 | 0.006 to 0.083 | 0.023 | |
| Gender (female) | − 0.164 | − 0.312 to − 0.016 | 0.030 | |
| CSP average | 0.001 | 0.0001 to 0.002 | 0.032 | |
| WOMAC stiffness score | 0.022 | 0.0003 to 0.043 | 0.047 | |
| HAM-L depression scale | − 0.006 | − 0.013 to 0.002 | 0.138 |
Beta band oscillations models
In the beta band we found distinct set of associated variables in each ROI. Regarding the frontal region, we found a positive association of WOMAC pain (b = 0.013, 95% CI 0.003–0.024, p = 0.010), upper limb PPT (b = 0.031, 95% CI 0.012–0.050, p = 0.002), and OA severity (K–L scale) (b = 0.033, 95% CI 0.004–0.062, p = 0.027). The bilateral affectation status (i.e., whether the subject have bilateral symptoms or not) and depression scale were maintained in the models as confounders. In the central region, similar positive relationship was found regarding OA severity (Kellgren–Lawrence Scale) (b = 0.044, 95% CI 0.012–0.075, p = 0.007). Additionally, a negative association was found with Timed Up and Go score (b = − 0.004, 95% CI − 0.008 to − 0.0003, p = 0.037). CSP and WOMAC stiffness score were kept in the model as confounders. At last, in the parietal region, a similar positive association was found with OA severity (K–L scale) (b = 0.064, 95 CI 0.029–0.099, p = 0.001). We also found a positive relationship with Berg Balance Scale (b = 0.006, 95% CI 0.002–0.011, p = 0.006) and a negative one with CSP (b = − 0.001, 95% CI − 0.002 to − 8.520, p = 0.048). The bilateral affectation was the main confounder in the model (Table 5).
Table 5.
Baseline beta band multivariate analyses according to ROI.
| Variables | Beta-coefficient | 95% CI | p-value | R2 |
|---|---|---|---|---|
| Frontal region | 0.240 | |||
| WOMAC pain scale | 0.013 | 0.003 to 0.024 | 0.010 | |
| Upper limb PPT | 0.031 | 0.012 to 0.050 | 0.002 | |
| Kellgren–Lawrence scale | 0.033 | 0.004 to 0.062 | 0.027 | |
| Bilateral affectation | 0.024 | − 0.026 to 0.074 | 0.343 | |
| HAD-depression scale | − 0.008 | − 0.020 to 0.003 | 0.133 | |
| Central region | 0.161 | |||
| Kellgren–Lawrence scale | 0.044 | 0.012 to 0.075 | 0.007 | |
| Timed up and go score | − 0.004 | − 0.008 to − 0.0003 | 0.037 | |
| CSP average | − 0.001 | − 0.002 to 0.00005 | 0.063 | |
| WOMAC stiffness scale | 0.006 | − 0.010 to 0.022 | 0.471 | |
| Parietal region | 0.259 | |||
| CSP average | − 0.001 | − 0.002 to − 8.520 | 0.048 | |
| Kellgren–Lawrence scale | 0.064 | 0.029 to 0.099 | 0.001 | |
| Berg balance scale | 0.006 | 0.002 to 0.011 | 0.006 | |
| Bilateral affectation | 0.027 | − 0.025 to 0.080 | 0.299 |
Sensitivity analysis by beta sub-bands
Low-beta band oscillations models
In this sub-band, we confirmed the positive relationship with WOMAC pain in the frontal (b = 0.006, 95% CI 0.002–0.010, p = 0.006) and central regions (b = 0.005, 95% CI 0.0007–0.010, p = 0.023). Also, the direct association with OA severity in frontal (b = 0.013, 95% CI 0.0007–0.025, p = 0.038), central (b = 0.014, 95% CI 0.0007–0.028, p = 0.039), and parietal regions (b = 0.026, 95% CI 0.009–0.044, p = 0.003). Similarly, to the beta band model, we found a positive correlation with PPT, only present in the frontal region but neither in central nor parietal areas; and a direct relationship with Berg balance score, but only in the parietal region. Moreover, we found a differential association in this sub-band, a positive correlation with SF-36 emotion subscale for frontal and central, but not for parietal areas. In all the low-beta band models, the bilateral affectation status was the only identified confounder (Table 6).
Table 6.
Baseline low beta band multivariate analyses according to ROI.
| Variables | Beta-coefficient | 95% CI | p-value | R2 |
|---|---|---|---|---|
| Frontal region | 0.271 | |||
| WOMAC pain scale | 0.006 | 0.002 to 0.010 | 0.006 | |
| Upper limb PPT | 0.011 | 0.003 to 0.012 | 0.005 | |
| SF-36 emotion subscale | 0.0004 | 0.00008 to 0.0007 | 0.014 | |
| Kellgren–Lawrence scale | 0.013 | 0.0007 to 0.025 | 0.038 | |
| Bilateral affectation | 0.008 | − 0.012 to 0.029 | 0.417 | |
| Central region | 0.284 | |||
| SF-36 emotion subscale | 0.0006 | 0.0002 to 0.0009 | 0.001 | |
| Kellgren–Lawrence scale | 0.014 | 0.0007 to 0.028 | 0.039 | |
| Upper limb PPT | 0.007 | − 0.0009 to 0.016 | 0.079 | |
| WOMAC pain scale | 0.005 | 0.0007 to 0.010 | 0.023 | |
| Bilateral affectation | 0.017 | − 0.006 to 0.040 | 0.138 | |
| Parietal region | 0.186 | |||
| Berg balance scale | 0.003 | 0.0007 to 0.005 | 0.001 | |
| Kellgren–Lawrence scale | 0.026 | 0.009 to 0.044 | 0.003 | |
| Bilateral affectation | 0.018 | − 0.008 to 0.044 | 0.169 |
High-beta band oscillations models
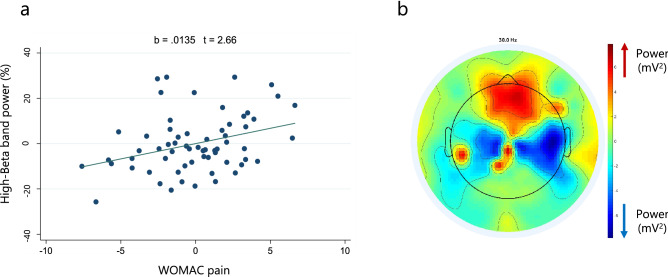
Regarding this sub-band, equally to beta and low-beta band models, we confirmed the robust positive association with WOMAC pain in the frontal region (b = 0.012, 95% CI 0.004–0.020, p = 0.004). This correlation is presented in the Fig. 2a and corroborated by the topographical map from representative patients with high pain intensity (Fig. 2b). Likewise, we verified the direct relationship with OA severity in frontal (b = 0.039, 95% CI 0.005–0.029, p < 0.001), central (b = 0.027, 95% CI − 0.008 to 0.047, p = 0.007), and parietal areas (b = 0.035, 95% CI 0.014–0.055, p = 0.001), as well as with upper limb PPT in the frontal region (b = 0.017, 95% 0.005–0.029, p = 0.005). Additionally, we confirmed the negative association with Timed Up and Go score (b = − 0.002, 95% CI − 0.005 to − 0.00006, p = 0.045) and 10-m walking test score (b = − 0.004, 95% CI − 0.007 to − 0.0003, p = 0.034) in the central and parietal regions, respectively. Moreover, we corroborated the negative association with CSP in the central (b = − 0.0008, 95% CI − 0.001 to − 0.0002, p = 0.010) and parietal (b = − 0.0009, 95% CI − 0.001 to − 0.0002, p = 0.010) areas. Finally, we found a differential association in this sub-band, a positive correlation with SF-36 physical function subscale for frontal and parietal, but not for central regions. The bilateral affectation, Hospital Anxiety and Depression (HAD)-Depression scale and WOMAC stiffness were the identified confounders in those models (Table 7).
Figure 2.
(a) High-beta band power and pain intensity adjusted correlation (from multivariate model of frontal region). (b) Topographical plot of spectral power from representative subjects (n = 15) with high pain intensity (higher than 10 in WOMAC pain scale).
Table 7.
Baseline high beta band multivariate analyses according to ROI.
| Variables | Beta-coefficient | 95% CI | p-value | R2 |
|---|---|---|---|---|
| Frontal region | 0.320 | |||
| WOMAC pain scale | 0.012 | 0.004 to 0.020 | 0.004 | |
| Upper limb PPT | 0.017 | 0.005 to 0.029 | 0.005 | |
| Kellgren–Lawrence scale | 0.039 | 0.018 to 0.060 | < 0.001 | |
| SF-36 Physical function scale | 0.002 | 0.0008 to 0.003 | 0.002 | |
| Bilateral affectation | 0.012 | − 0.019 to 0.044 | 0.435 | |
| HAD-depression scale | − 0.001 | − 0.008 to 0.006 | 0.696 | |
| Central region | 0.196 | |||
| CSP average | − 0.0008 | − 0.001 to − 0.0002 | 0.010 | |
| Kellgren–Lawrence scale | 0.027 | − 0.008 to 0.047 | 0.007 | |
| Timed Up and Go score | − 0.002 | − 0.005 to − 0.00006 | 0.045 | |
| WOMAC stiffness scale | 0.005 | − 0.005 to 0.015 | 0.343 | |
| Parietal region | 0.271 | |||
| CSP average | − 0.0009 | − 0.001 to − 0.0002 | 0.010 | |
| SF-36 physical function scale | 0.0006 | 0.00005 to 0.001 | 0.034 | |
| Kellgren–Lawrence scale | 0.035 | 0.014 to 0.055 | 0.001 | |
| 10-m walking test score | − 0.004 | − 0.007 to − 0.0003 | 0.034 |
The summary of all significant predictors of brain oscillations in chronic knee osteoarthritis pain is presented in Table 8.
Table 8.
Findings summary from multivariate models by brain oscillation and ROI.
| Relative power | Frontal | Central | Parietal |
|---|---|---|---|
| ↑ Delta | ↑ SF-36 social functioning | ↑ SF-36 social functioning | ↑ SF-36 social functioning |
| ↑ Depression (Hamilton) | ↑ Depression (Hamilton) | ||
| ↑ Age | ↑ Age | ||
| ↓ MOCA test | ↓ MOCA test | ↓ MOCA test | |
| ↓ SICI (higher inhibition) | ↓ SICI (higher inhibition) | ||
| ↑ Theta | ↑ Timed up and go test | ↑ Timed up and go test | ↑ Timed up and go test |
| ↑ in women | ↑ in women | ↑ in women | |
| ↑ Depression (HAD) | |||
| ↓ Pain (WOMAC) | ↓ Pain (WOMAC) | ↓ Pain (WOMAC) | |
| ↓ KL severity | ↓ KL severity | ||
| ↑ Alpha | ↑ MEP | ↑ MEP | ↑ MEP |
| ↑ Cortical silent period | ↑ Cortical silent period | ↑ Cortical silent period | |
| ↑ WOMAC stiffness | ↑ WOMAC stiffness | ↑ WOMAC stiffness | |
| ↓ in women | ↓ in women | ↓ in women | |
| ↑ Beta | ↑ WOMAC pain | ↑ KL severity | ↑ KL severity |
| ↑ Pain threshold (hand) | ↑ Balance test (EEB) | ||
| ↑ KL severity | |||
| ↓ Timed up and go test | ↓ Cortical silent period | ||
| ↑ Low beta | ↑ SF-36 emotional functioning | ↑ SF-36 emotional functioning | ↑ Balance test (EEB) |
| ↑ KL severity | ↑ KL severity | ↑ KL severity | |
| ↑ Pain (WOMAC) | ↑ Pain (WOMAC) | ||
| ↑ Pain threshold (Hand) | |||
| ↑ High beta | ↑ KL severity | ↑ KL severity | ↑ KL severity |
| ↑ SF-36 emotional functioning | ↑ SF-36 physical functioning | ||
| ↑ Pain threshold (Hand) | |||
| ↑ Pain (WOMAC) | |||
| ↓ Cortical silent period | ↓ Cortical silent period | ||
| ↓ Timed up and go test | ↓ Walking test speed (10 MWT) |
Discussion
Main findings
This study aimed to explore the association of different sociodemographic, clinical, and neurophysiological variables and the resting-state EEG spectral power in subjects with chronic pain due to knee OA. Based on previous systematic review10, this is one of the largest studies exploring the brain oscillations correlates in chronic knee OA pain using a multivariate approach, looking to understand better pain-related oscillatory activity, their underlying brain processes, and its potential utility as biomarkers of chronic pain. Our main findings showed important relationships between clinical and demographic variables and EEG power: (1) multivariate analyses showed that higher pain intensity and higher OA severity (indexed by K–L scale) is associated with higher frontocentral beta and high-beta power and a reduction of diffuse theta activity; (2) delta and alpha oscillations have a direct relationship with higher cortical inhibition (SICI and CSP, respectively); (3) increased power at low frequencies (delta and theta) are associated with poor cognition, aging, and depressive symptoms; (4) higher alpha and beta power seems to be a maladaptive compensatory mechanism to poor motor function and severe joint degeneration; and (5) gender seems to be an important biological variable, acting as confounder in pain-related brain oscillations assessment.
Brain oscillations and pain intensity
Theta oscillations and pain
Increased theta band activity is shown to be positively related to pain in different chronic pain conditions such as fibromyalgia, spinal cord injury (SCI), and other forms of neuropathic pain22,23. This relationship has been justified by the theoretical framework of thalamocortical dysrhythmia (TCD), which is thought to originate from abnormal oscillatory activity and interference to increase pain24,25. Interestingly, our findings convey a negative correlation between theta band oscillation and pain in individuals with knee OA, similar to a previous study in hip OA26. It is worth mentioning that OA consists of a mixed phenotype of pain mechanisms, completely distinct from the mechanisms observed in fibromyalgia and in neuropathic pain27,28.
One interesting aspect of theta rhythm is that it seems to be correlated with emotional control. In fact, a group of investigators, in a previous study with 30 healthy subjects, showed that subjects with high theta power especially in midline structures had low anxiety scores29. Thus, interestingly our results showing that high theta is associated with less pain but at the same time with less OA severity and also being more frequent in women, likely points out to a potential affective control of pain. Studies have also shown that increased theta is associated with higher metabolic activity in the anterior cingulate cortex (ACC). Therefore, we can hypothesize that in musculoskeletal disorders, theta may be a modulator of affective networks associated with pain control and does not support the TCD framework.
Beta oscillations and pain
Our results also show that higher beta power, in frontocentral areas are associated with higher self-reported pain intensity during functional activities as measured by the WOMAC pain subscale. It is also important to underscore that beta increase was also correlated with a greater OA severity, although WOMAC pain and K–L grade were not correlated in our sample In this context, increased beta seems to be related to a compensatory mechanism of greater neuronal injury and representing a subgroup of patients with less adaptative response and potentially higher central sensitization in response to the chronic joint degeneration. Such finding can be seen also in other examples of neural injury such as in stroke30,31 and spinal cord injury8,32,33. In fact, beta oscillations seem to be related to increased local metabolic activity34,35, thus likely in the case of OA generating additional electric activity to compensate for the OA indirect neural lesion. When looking at studies on musculoskeletal pain conditions, our results agree with previous reports on chronic hip OA17 and low back pain36 where higher frequency brain oscillations in frontal areas are associated with higher pain intensity. A potential explanation relies on the evidence that supports the link between the presence of beta oscillations and cortical dysfunction in motor impairment conditions37,38, as well as its association with cortex activation during motor tasks39,40.
Cortical inhibition and brain oscillations
Studies have found strong correlations between cortical silent period (CSP) and alpha band oscillations, indicating its potential as an indicator of inhibitory processes41. Moreover, our finding, of this relationship in other studied thus indicate alpha oscillation's inhibitory race between brain regions, not only within them, implying a state of lowered excitability and heightened inhibition in patients with high alpha band oscillations42. Our results also found a positive correlation between motor evoked potential (MEP) and alpha bands. This finding can be associated with the positive correlation between alpha band and CSP given MEP modulates cortical-spinal and the cortical-spinal pathway requires cortical inhibition. Moreover, studies evaluating markers of cortical excitability have found that markers such as intracortical facilitation and short-interval intracortical inhibition affect changes in MEP, suggesting that MEP is an unreliable biomarker for cortical excitability43.
Regarding delta bands, we found a negative relationship with short intracortical inhibition (SICI), that means that higher delta is associated with higher intracortical inhibition in this sample. Delta band activity is thought of influencing cortical facilitation in different brain pathways44. A negative relationship between SICI and delta waves observed in this study convey that, individuals with high delta activity have more inhibitory functions in the frontal and central cortical regions. This finding is consistent with studies that report delta is shown to be significantly involved in cognitive processes throughout the brain. Studies have found that inhibition of specific pathways in the cortex contribute to increase focus and attention required to perform certain cognitive tasks44. Therefore, our findings suggest that delta might be a good marker for cortical inhibitory activity.
Emotional-cognitive systems and low-frequency bands
A consistent finding within the signature of EEG low-frequency bands is their association with depression45. Our model depicts theta activity directly associated with depression scores in the frontal and parietal regions, supporting the increase in neuro physiologic connectivity depicted by delta, theta, and beta bands in other studies46. It is thought that high theta activity in individuals with major depressive disorder (MDD) functions as a compensatory mechanism in response to cortical deficits cause by MDD. In return, a negative relationship between delta activity is observed in individuals with MDD due to this cortical deficit47. Although a positive relationship between delta oscillations and depression in our results may be seem as a contradictory finding at the first glance, we see the opposite. It does confirm these previous studies. Given that our individuals in our sample do not have MDD, and only exhibit sub-clinical symptoms of depression, subjects with increased depression scores are those with active delta likely as a compensatory marker compared to individual with no depression symptoms. It is the issue of correlational tests. In this case we believe here that subjects with depression elicit higher delta as a compensatory mechanism and not the opposite. For that reason, we do not expect the classic conclusions of MDD brain oscillation activity. Moreover, delta waves have been shown to affect motivational and reward areas of the cortex, suggesting its influence on mood brain functions48.
Moreover, an inverse relationship was found between cognition and delta oscillations. This finding is consistent with studies that convey associations between increased delta and dementia in individuals with Parkinson Disease (PD)49. Although this result may seem conflicting with the relationship of delta and SICI, cognitive decline associated with increased delta activity may be related to different causal pathways than those that relate delta inhibitory function with attention. This hypothesis can be supported by the positive relationship between delta waves and age also found in our study. Cognitive decline is associated with aging and delta activity is increased in both or these processes50. Thus, age and cognition could be possible predictors of delta activity in patients w/one in the chronic pain condition.
Brain oscillations, poor motor function, and severe OA
Consistent with the association between pain and theta bands, a positive correlation between theta bands and motor function was observed in our models. Increased theta band activity is required to trigger fine initiation of lower-limb movement in individuals with PD51. In the context of OA, more movement is associated with less pain, therefore individuals with high theta activity display higher motor function and motor control, which relates to the inverse relationship between theta activity and pain; individuals with better motor function have less pain, observed in individuals with increased theta oscillations, according to this study’s results45.
The theta band signature in OA patients is further strengthened by the negative association found with disease severity. Disease severity is reportedly colinear to pain intensity, thus, the inverse relationships observed between theta, pain, and disease severity suggest theta band activity as a potential biomarker for individuals with OA pain.
Besides, our results also showed that higher beta power in centroparietal areas associated with poor balance and motor function. This finding concurs with previous research that has found that high-beta EEG oscillations power can predict motor recovery in spinal cord injury patients33. Thus, we hypothesize that pain processing in knee OA requires a balanced and harmonized cortex activation of which the high beta frequency over frontal areas can potentially serve as a signature of a compensatory pattern of high frequencies oscillatory activity in response to a dysfunctional cortical-subcortical pain regulation caused by chronic inflammation and movement impairment associated with the OA condition.
An unexpected finding in this study was the positive association between alpha bands and stiffness (as indexed by the WOMAC Stiffness scale). Individuals with osteoarthritis commonly report stiffness and rigidity in the affected joints in the mornings or after long periods of being still52. Considering stiffness has some degree of collinearity with disease severity in OA and the role of alpha band oscillations in cortical inhibition, this association might indicate a compensatory inhibitory mechanism in which peripheral signals of disease severity, such as cartilage destruction and osteophyte formation, might trigger cortical inhibition causing an increase in alpha oscillations, and thus increasing stiffness and restricting movement.
Gender differences and brain oscillations
Reported gender differences regarding high alpha and theta relative power was found in our study. Not many studies have accounted for gender when evaluating alpha wave oscillation differences in individuals with chronic pain. However, given that different gender exhibit different pain mechanisms in the context of chronic pain, it is reasonable to hypothesize that those same differences might be present in EEG band oscillation changes, particularly those related to pain, such as theta bands53. Moreover, a study evaluating resting-brain differences in male and female individuals have suggested differences in cortical excitability in different genders, thus, given the relationship between CSP and alpha band, it is likely that the association between alpha band activity and females in this study supports this finding54. Further studies are needed to explore the differences between genders on pain-related brain oscillations, but also a carefully inclusion of gender as mandatory covariate in classic EEG analysis plan.
Future perspectives
One of our main results was the suggestion of two potential EEG-based pain phenotypes in chronic pain due to knee OA. Patients with higher pain intensity and OA severity (K–L grade) have higher beta band power in the frontocentral regions. On the other hand, patients with low pain intensity and less OA severity have higher diffuse theta band power. As reported by previous studies55, chronic pain appears to be associated with abnormal oscillations at theta and beta frequencies. One potential use is the validation of a brain-based biomarker of pain severity and central sensitization56–58, which is highly needed considering the subjective metrics we are using to assess this condition in the clinic. Another potential application is using these EEG signatures to guide and stratify pain treatments among patients with chronic pain due to knee OA59,60. Due to the potential difference in central maladaptive mechanisms between these two subgroups of patients, likely more neuroplasticity-oriented treatments (such as noninvasive brain stimulation) could have better clinical effects. Finally, these main EEG findings can be used as targets for special neuromodulatory techniques such as transcranial alternating current stimulation (tACS) and neurofeedback61. These techniques can be used to revert the high frontocentral beta oscillations associated with higher pain or to induce higher theta band power associated with less pain intensity. However, the potential applicability of these results warrants future confirmatory explorations before its clinical use in chronic pain.
Limitations
The main limitation of our study is its exploratory nature; thus, no adjustment for multiple comparisons was performed. Future confirmatory research is needed to test and validated our findings as markers of different pain phenotypes in chronic OA pain. Furthermore, the lack of control group could be considered a limitation; however, since our main objective was to describe the associations of EEG and chronic OA clinical variables, the use of healthy controls or other rheumatological disease would be inappropriate. Finally, as chronic pain is affected by a wide range of factors, as medications and comorbidities that could affect also the neurophysiological and pain-related measurements (such as mental disorders, diabetes, and peripheral neuropathies), it is a challenge to control all of them and some influences in the pain perception and EEG findings could be overlooked in our study.
Conclusions
In summary, our study could identify clear associations of demographic, clinical, and neurophysiological variables, and resting-state EEG spectral power in patients with chronic knee osteoarthritis pain. These associations showed the potential role of brain oscillations as a marker of pain intensity and clinical phenotypes in chronic pain patients. Subjects with higher pain intensity (likely subjects with maladaptive compensatory mechanisms to poor motor function and severe joint degeneration) have higher frontocentral beta power and lower central theta activity. Also, it is important to note that brain oscillation at low frequencies are significantly affected by cognitive and emotional factors, suggesting its potential use for phenotyping clinical profiles of chronic knee osteoarthritis patients. However, our study has some limitations regarding our methodology and the generalizability of our results. Finally, the suggested cortical inhibitory nature (indexed by SICI and CSP) of frontal delta and alpha oscillations underscore the opportunity of modulating pain-related oscillations as new pain management approach. More research is needed with broader and more general samples to bring more consistency for the role of EEG as pain biomarker.
Methods
Study design
We performed a cross sectional analysis of patients with knee OA from an ongoing, prospective cohort study titled “Deficit of Inhibition as a Marker of Neuroplasticity (DEFINE study) in rehabilitation” (protocol paper under review). The DEFINE protocol and this study were approved by the Research and Ethical Comitee of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC FMUSP) (Registration number: 86832518.7.0000.0068). All the proceedings and methods of this study are in accordance with Brazilian research ethics regulations and the Declaration of Helsinki.
Participants
Inclusion criteria
Adults (over 18 years old), male and female, clinical and radiological diagnosis (magnetic resonance imaging or computerized tomography; or bilateral knee radiography) of knee OA, clinical stability verified by medical evaluation, informed consent form signed by the subject, and meet the eligibility criteria for the Instituto de Medicina Fisica e Reabilitacao (IMREA) rehabilitation program (protocol paper under review).
Exclusion criteria
Subjects were excluded if they were pregnant, have active OA with clinical manifestations in joints other than the knee, or if they had any other clinical or social conditions that interfere with the patient’s participation in the rehabilitation program62. We did not exclude patients with adequate functionality but with specific comorbidities (such as hypertension or diabetes).
Study procedures
Patients admitted to the IMREA’s conventional rehabilitation program with knee OA were invited to participate in the study and included after signing the informed consent form. During one visit, a qualified researcher performed a series of clinical and neurophysiological assessments. Instruments were selected to enable a global assessment of patients. Evaluations were carried out by the same examiner. The evaluators were trained to standardize the assessments.
Demographic and clinical assessments
Information regarding the participants’ age, gender, time of ongoing pain, height, weight, and body mass index were collected from a standardized medical interview. We assessed pain intensity using visual analog scale and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scale. The OA severity was assessed by Kellgren Lawrence radiographic classification. Besides, in order to characterize the study’s sample, we performed a multidimensional assessment including quality of life (SF-36), functional status (WOMAC), motor (Timed up and Go [TUG], 6-min and 10-m walk test), cognitive (MOCA test), sleep (Epworth sleepiness scale), and emotional (Hamilton depression scale, Hospital anxiety and depression scale) functions using standardized scales. A summary of all assessments can be seen in the Supplementary material S1.
Static and dynamic quantitative sensory testing (QST)
Pressure pain threshold (PPT)
We used an algometer to define the minimum amount of pressure that triggers pain in pre-established regions (thenar region, and region located one inch above the knee)63. We performed three algometry measurements (15-s intervals) and calculated the average.
Conditioned pain modulation (CPM)
We used the CPM response as measurement of changes in pain processing. This test assessed, through intense heterotopic stimulation, the response of the descending pain inhibitory system64,65. Producing a “pain inhibits pain” phenomenon66. Based on previous studies67,68, subjects immersed one of their hands into a recipient containing cold water (10–12 °C) for one minute. After 30 s of immersion, the Visual Analogue Scale (VAS) was presented to patients to indicate their pain level, referring to the submerged hand. Subsequently, three algometric measures (PPTs) were taken (spaced between 15 s) for the contralateral hand. After an interval of approximately 10 min (time for hand to return to normal body temperature), the other hand was immersed in the recipient, and follow the previously stated protocol68. CPM response was calculated as the difference between the average PPTs minus the average PPTs during the conditioned stimulus.
Transcranial magnetic stimulation (TMS)
The Magstim Rapid® stimulator (The Magstim Company Limited, UK). We placed a 70 mm coil in figure-of-eight at 45° of the scalp, to send a perpendicular pulse over the right and left motor cortex (for all assessments), the coil stability and direction was managed by the assessor without neuronavigation. The muscular response to the stimulus was recorded using surface electromyography (EMG) with Ag/AgCl electrodes positioned on first dorsal interosseous (FDI) muscle of the hand and the grounding electrode positioned on the wrist69.
We performed a bilateral upper limb assessment. We used anatomical references for motor cortex localization. Initially, we identified the vertex (intersection between the nasion-inion lines and zygomatic arches); then, a mark was made 5 cm from the vertex towards the ear tragus in the coronal plane. The hotspot was determined as the location with the highest and most stable motor evoked potential (MEP) amplitudes over the FDI. The resting motor threshold (rMT) was defined as the minimum intensity necessary for a single TMS pulse on the hot spot to generate an MEP, with at least 50 μV peak to peak amplitude, in 50% of attempts70. We performed the following measures: MEP (intensity at 120% of rMT, we calculated the peak-to-peak amplitude), cortical silent period (CSP), which represents the temporary suppression of electromyographic activity during a sustained voluntary contraction. Moreover, we performed paired-pulse protocols of intracortical inhibition (SICI), which was assessed by interstimulus intervals of 2 ms; and intracortical facilitation (ICF) assessed by 10 ms interim stimulus intervals70. Ten randomized stimuli were applied at each interval and the average were calculated.
For the measurement of neurophysiological markers through TMS, we pooled the rMT, CSP, SICI, ICF, and MEP results from each hemisphere to obtain a bi-hemispheric average. This approach can be justified due to the bi-hemispheric nature of pain perception71; besides, most of our sample includes patients with bilateral knee OA. We then analyzed the relationship between the bi-hemispheric average of these neuro markers with possible associated variables to their behavior (markers magnitude and direction), including clinical and sociodemographic subject characteristics. TMS data was recorded and stored in a computer for off-line analysis.
Resting-state electroencephalography (EEG)
EEG acquisition
We recorded the EEG following a standardized approach72. Recordings were performed in a quiet room. Patients were asked to sit in a comfortable position, have their sight directed naturally below the horizon line, not to move or talk, and relax as much as possible. The investigator made sure they did not fall asleep by observing the patient and verbally calling his attention if drowsiness was noticed. Resting-state EEG was recorded for 5 min with eyes closed using a 128-channel EGI system (Electrical Geodesics, Inc) (EGI, Eugene, USA). The EEG was recorded with a band-pass filter of 0.3–200 Hz and digitized at the sampling rate of 250 Hz.
Resting-state spectral power analysis
The data were exported for offline analysis with EEGLab73 and MATLAB (MATLAB R2012a, The MathWorks Inc. Natick, MA, 2000). EEG was re-referenced to the average, we used finite impulse response filters, one high-pass filter of 1 Hz and a low-pass filter of 40 Hz, followed by manual artifact detection and rejection by a blinded assessor to exclude the existence of any signal of drowsiness (attenuation of the alpha rhythm), epileptiform or any abnormal discharges prior to admission into full study (no epileptiform or abnormal discharges were found). This analysis was followed by a manual artifact detection and rejection and Independent Component Analysis (ICA); finally, we removed the ICs associated to artifacts and reconstructed the signal74. The artifact-free data was processed using pop_spectopo EEGLab function with Fast Fourier Transformation with 5 s windows with 50% overlap. Absolute power (μV2) and relative power (power in a specific frequency range/total power from 1 to 40 Hz) were calculated for the following frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and the sub-bands: low beta (13–20 Hz) and high beta (20–30 Hz). All the EEG-related measurements were calculated from three regions of interests (ROIs): the central, parietal, and frontal areas, since they are important cortical regions involved in pain perception75. Electrodes representing these regions were selected and averaged (the electrode placement is presented in the Supplementary Material S2).
Statistical analysis
We used descriptive statistics to report baseline characteristics. Continuous data were expressed as mean and standard deviation (SD) or as median and interquartile ranges dependent on their distribution. Dichotomous and categorical data were described in frequency and respective percentages. Histogram and Shapiro–Wilk test assessed data distribution for normality. Values greater than 3 SDs away from the mean scores of the dependent or independent variables were labeled as outliers. After determining that data had a sufficiently normal distribution, we conducted exploratory multivariate linear regression models to identify relationships between resting EEG spectral power values (dependent variables) and clinical, QST, and TMS variables (independent variables). The models were conducted independently by frequency bands (delta, theta, alpha, beta, low-beta, and high-beta) and by region (frontal, central, and parietal). First, to select the best explanatory covariates, univariate linear models were created with each independent variable to detect significant covariates for an alpha level of 0.2. Variables that were not significant at the univariate models were eliminated. As a second step, a model was created with all variables that were significant (p < 0.2) in the univariate models. Thirdly, the regression coefficients were then checked for significance and those with a p-value > 0.05 were excluded from the model leaving only the significant variables (p < 0.05). Finally, to select our final multivariate modes, we search for confounders using a multicriteria approach: (1) based on previous literature supporting physiological plausibility, (2) considering changes of β coefficients more than 10%, and (3) using the Akaike’s information criteria to select the variables that would result in the best fit76,77. We also tested the interaction of demographic and clinical variables with the main predictors’ variables which was included in the final models if significant. Age and gender were explored as biological variables that could potentially confound all final models. Once the final model was determined, we added these variables as covariates, and if not significant, they were excluded from the models.
The assumption of linearity was assessed by visually comparing the scatterplot of each independent variable and a superimposed regression line. The assumption of homoscedasticity was checked by visual inspection of the scatterplot of the standardized predicted values and standardized residuals78. Residuals were tested for normality using histograms and the Shapiro–Wilk normality test79. Durbin Watson estimates and Cook’s distances were used for analysis of regression diagnostics such as multicollinearity and influential cases.
We used Stata Statistical Software 15 (Stata Corp LLC) for the statistical analyses. Because this was an exploratory study and to minimize the risk of type II errors, no correction for multiple comparisons was done.
Supplementary Information
Acknowledgements
This study is supported by a Grant from FAPESP (SPEC project, fund number 2017/12943-8). We acknowledge Alba Navarro-Flores, MD, Universidad Nacional Federico Villarreal, for her help on the data cleaning and formatting. She was not compensated for her contributions.
Author contributions
All authors designed the study. M.S. and M.I. collected the data. A.J.M. and K.P.-B. cleaned the EEG data. K.P.-B. performed the EEG analysis. K.P.-B., A.M., and P.S.M. performed statistical analyses. K.P.-B., A.M., and P.S.M. interpreted the data. Further, K.P.-B., A.M., and P.S.M. completed the first draft of the article. All authors participated in the interpretation of the results, the writing of the manuscript, and approved of its final version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Marcel Simis, Marta Imamura and Kevin Pacheco-Barrios.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-04957-x.
References
- 1.Courtney CA, O'Hearn MA, Hornby TG. Neuromuscular function in painful knee osteoarthritis. Curr. Pain Headache Rep. 2012;16:518–524. doi: 10.1007/s11916-012-0299-2. [DOI] [PubMed] [Google Scholar]
- 2.Blyth FM, Briggs AM, Schneider CH, Hoy DG, March LM. The global burden of musculoskeletal pain-where to from here? Am. J. Public Health. 2019;109:35–40. doi: 10.2105/ajph.2018.304747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willett MJ, Siebertz M, Petzke F, Erlenwein J, Rushton A, Soldini E, Barbero M, Falla D. The extent of pain is associated with signs of central sensitization in patients with hip osteoarthritis. Pain Pract. 2020;20:277–288. doi: 10.1111/papr.12851. [DOI] [PubMed] [Google Scholar]
- 4.Guler MA, Celik OF, Ayhan FF. The important role of central sensitization in chronic musculoskeletal pain seen in different rheumatic diseases. Clin. Rheumatol. 2020;39:269–274. doi: 10.1007/s10067-019-04749-1. [DOI] [PubMed] [Google Scholar]
- 5.Uygur-Kucukseymen E, Castelo-Branco L, Pacheco-Barrios K, Luna-Cuadros MA, Cardenas-Rojas A, Giannoni-Luza S, Zeng H, Gianlorenco AC, Gnoatto-Medeiros M, Shaikh ES, Caumo W, Fregni F. Decreased neural inhibitory state in fibromyalgia pain: A cross-sectional study. Neurophysiol. Clin. 2020;50:279–288. doi: 10.1016/j.neucli.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teixeira PEP, Pacheco-Barrios K, Gunduz ME, Gianlorenço AC, Castelo-Branco L, Fregni F. Understanding intracortical excitability in phantom limb pain: A multivariate analysis from a multicenter randomized clinical trial. Neurophysiol. Clin. 2021;51:161–173. doi: 10.1016/j.neucli.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baroni, A., Severini, G., Straudi, S., Buja, S., Borsato, S. & Basaglia, N. Hyperalgesia and central sensitization in subjects with chronic orofacial pain: Analysis of pain thresholds and EEG biomarkers. Front. Neurosci.14, 115–128. 10.3389/fnins.2020.552650 (2020). [DOI] [PMC free article] [PubMed]
- 8.Simis, M., Pacheco-Barrios, K., Uygur-Kucukseymen, E., Castelo-Branco, L., Battistella, L. R. & Fregni, F. Specific electroencephalographic signatures for pain and descending pain inhibitory system in spinal cord injury. Pain Med. 10.1093/pm/pnab124 (2021). [DOI] [PubMed]
- 9.Prichep LS, John ER, Howard B, Merkin H, Hiesiger EM. Evaluation of the pain matrix using EEG source localization: A feasibility study. Pain Med. 2011;12:1241–1248. doi: 10.1111/j.1526-4637.2011.01191.x. [DOI] [PubMed] [Google Scholar]
- 10.Pinheiro ESDS, Queirós FCD, Montoya P, Santos CL, Nascimento MAD, Ito CH, Silva M, NunesSantos DB, Benevides S, Miranda JGV. Electroencephalographic patterns in chronic pain: A systematic review of the literature. PLoS ONE. 2016;11:e0149085. doi: 10.1371/journal.pone.0149085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129:55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- 12.Olesen SS, Hansen TM, Graversen C, Steimle K, Wilder-Smith OHG, Drewes AM. Slowed EEG rhythmicity in patients with chronic pancreatitis: Evidence of abnormal cerebral pain processing? Eur. J. Gastroenterol. Hepatol. 2011;23:418–424. doi: 10.1097/MEG.0b013e3283457b09. [DOI] [PubMed] [Google Scholar]
- 13.Lim M, Kim JS, Kim DJ, Chung CK. Increased low-and high-frequency oscillatory activity in the prefrontal cortex of fibromyalgia patients. Front. Hum. Neurosci. 2016;10:111. doi: 10.3389/fnhum.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt S, Naranjo JR, Brenneisen C, Gundlach J, Schultz C, Kaube H, Hinterberger T, Jeanmonod D. Pain ratings, psychological functioning and quantitative EEG in a controlled study of chronic back pain patients. PLoS ONE. 2012;7:e31138. doi: 10.1371/journal.pone.0031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanneste S, Ost J, Van Havenbergh T, De Ridder D. Resting state electrical brain activity and connectivity in fibromyalgia. PLoS ONE. 2017;12:e0178516. doi: 10.1371/journal.pone.0178516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinh ST, Nickel MM, Tiemann L, May ES, Heitmann H, Hohn VD, Edenharter G, Utpadel-Fischler D, Tölle TR, Sauseng P. Brain dysfunction in chronic pain patients assessed by resting-state electroencephalography. Pain. 2019;160:2751. doi: 10.1097/j.pain.0000000000001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gram M, Erlenwein J, Petzke F, Falla D, Przemeck M, Emons MI, Reuster M, Olesen SS, Drewes AM. The cortical responses to evoked clinical pain in patients with hip osteoarthritis. PLoS ONE. 2017;12:e0186400. doi: 10.1371/journal.pone.0186400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May ES, Nickel MM, Ta Dinh S, Tiemann L, Heitmann H, Voth I, Tölle TR, Gross J, Ploner M. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum. Brain Mapp. 2019;40:293–305. doi: 10.1002/hbm.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen MP, Day MA, Miró J. Neuromodulatory treatments for chronic pain: Efficacy and mechanisms. Nat. Rev. Neurol. 2014;10:167. doi: 10.1038/nrneurol.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J, Weiskopf N, Blefari ML, Rana M, Oblak E. Closed-loop brain training: The science of neurofeedback. Nat. Rev. Neurosci. 2017;18:86–100. doi: 10.1038/nrn.2016.164. [DOI] [PubMed] [Google Scholar]
- 21.Thut G, Bergmann TO, Fröhlich F, Soekadar SR, Brittain J-S, Valero-Cabré A, Sack AT, Miniussi C, Antal A, Siebner HR. Guiding transcranial brain stimulation by EEG/MEG to interact with ongoing brain activity and associated functions: A position paper. Clin. Neurophysiol. 2017;128:843–857. doi: 10.1016/j.clinph.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallon, N., Chiu, Y., Nurmikko, T. & Stancak, A. Altered theta oscillations in resting EEG of fibromyalgia syndrome patients. 22, 49–57. 10.1002/ejp.1076 (2018). [DOI] [PMC free article] [PubMed]
- 23.Vuckovic A, Hasan MA, Fraser M, Conway BA, Nasseroleslami B, Allan DB. Dynamic oscillatory signatures of central neuropathic pain in spinal cord injury. J. Pain. 2014;15:645–655. doi: 10.1016/j.jpain.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Ridder D, Vanneste S, Langguth B, Llinas R. Thalamocortical dysrhythmia: A theoretical update in tinnitus. Front. Neurol. 2015;6:124. doi: 10.3389/fneur.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim M, Kim JS, Kim DJ, Chung CK. Increased low- and high-frequency oscillatory activity in the prefrontal cortex of fibromyalgia patients. Front. Hum. Neurosci. 2016;10:111–111. doi: 10.3389/fnhum.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: An EEG/PET study of normal and depressed subjects. Psychophysiology. 2003;40:939–949. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- 27.Dell'Isola A, Allan R, Smith SL, Marreiros SSP, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: A systematic review of the literature. BMC Musculoskelet. Disord. 2016;17:425–425. doi: 10.1186/s12891-016-1286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nijs, J., George, S. Z., Clauw, D. J., Fernández-de-las-Peñas, C., Kosek, E., Ickmans, K., Fernández-Carnero, J., Polli, A., Kapreli, E. & Huysmans, E. J. T. L. R. Central sensitisation in chronic pain conditions: Latest discoveries and their potential for precision medicine. 10.1016/S2665-9913(21)00032-1 (2021). [DOI] [PubMed]
- 29.Inanaga K. Frontal midline theta rhythm and mental activity. Psychiatry Clin. Neurosci. 1998;52:555–566. doi: 10.1046/j.1440-1819.1998.00452.x. [DOI] [PubMed] [Google Scholar]
- 30.Simis M, Doruk D, Imamura M, Anghinah R, Morales-Quezada L, Fregni F, Battistella LR. Neurophysiologic predictors of motor function in stroke. Restor. Neurol. Neurosci. 2016;34:45–54. doi: 10.3233/rnn-150550. [DOI] [PubMed] [Google Scholar]
- 31.Thibaut A, Simis M, Battistella LR, Fanciullacci C, Bertolucci F, Huerta-Gutierrez R, Chisari C, Fregni F. Using brain oscillations and corticospinal excitability to understand and predict post-stroke motor function. Front. Neurol. 2017;8:187. doi: 10.3389/fneur.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simis M, Pacheco-Barrios K, Uygur-Kucukseymen E, Castelo-Branco L, Battistella LR, Fregni F. Specific electroencephalographic signatures for pain and descending pain inhibitory system in spinal cord injury. Pain Med. 2021 doi: 10.1093/pm/pnab124. [DOI] [PubMed] [Google Scholar]
- 33.Simis M, Uygur-Kucukseymen E, Pacheco-Barrios K, Battistella LR, Fregni F. Beta-band oscillations as a biomarker of gait recovery in spinal cord injury patients: A quantitative electroencephalography analysis. Clin. Neurophysiol. 2020;131:1806–1814. doi: 10.1016/j.clinph.2020.04.166. [DOI] [PubMed] [Google Scholar]
- 34.Cook IA, O'Hara R, Uijtdehaage SH, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr. Clin. Neurophysiol. 1998;107:408–414. doi: 10.1016/s0013-4694(98)00092-3. [DOI] [PubMed] [Google Scholar]
- 35.Kropotov, J. Quantitative EEG, Event-Related Potentials and Neurotherapy. 11–58 (Academic Press, San Diego, USA. 2009).
- 36.May ES, Nickel MM, Ta Dinh S, Tiemann L, Heitmann H, Voth I, Tölle TR, Gross J, Ploner MJHBM. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum. Brain Mapp. 2019;40:293–305. doi: 10.1002/hbm.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quandt F, Bönstrup M, Schulz R, Timmermann JE, Mund M, Wessel MJ, Hummel FC. The functional role of beta-oscillations in the supplementary motor area during reaching and grasping after stroke: A question of structural damage to the corticospinal tract. Hum. Brain Mapp. 2019;40:3091–3101. doi: 10.1002/hbm.24582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossiter HE, Davis EM, Clark EV, Boudrias MH, Ward NS. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage. 2014;91:360–365. doi: 10.1016/j.neuroimage.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khanna P, Carmena JM. Neural oscillations: Beta band activity across motor networks. Curr. Opin. Neurobiol. 2015;32:60–67. doi: 10.1016/j.conb.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Kilavik BE, Zaepffel M, Brovelli A, MacKay WA, Riehle A. The ups and downs of β oscillations in sensorimotor cortex. Exp. Neurol. 2013;245:15–26. doi: 10.1016/j.expneurol.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Farzan F, Barr MS, Hoppenbrouwers SS, Fitzgerald PB, Chen R, Pascual-Leone A, Daskalakis ZJ. The EEG correlates of the TMS-induced EMG silent period in humans. Neuroimage. 2013;83:120–134. doi: 10.1016/j.neuroimage.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brignani D, Manganotti P, Rossini PM, Miniussi C. Modulation of cortical oscillatory activity during transcranial magnetic stimulation. Hum. Brain Mapp. 2008;29:603–612. doi: 10.1002/hbm.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Candido Santos L, Gushken F, Gadotti GM, Dias BDF, Marinelli Pedrini S, Barreto MESF, Zippo E, Pinto CB, Piza PVDT, Fregni F. Intracortical inhibition in the affected hemisphere in limb amputation. Front. Neurol. 2020;11:720–720. doi: 10.3389/fneur.2020.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harmony T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 2013;7:83–83. doi: 10.3389/fnint.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Susko AM, Fitzgerald GK. The pain-relieving qualities of exercise in knee osteoarthritis. Open Access Rheumatol. 2013;5:81–91. doi: 10.2147/OARRR.S53974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Kang C, Qu X, Zhou Y, Wang W, Hu Y. Depression-related brain connectivity analyzed by EEG event-related phase synchrony measure. Front. Hum. Neurosci. 2016;10:477–477. doi: 10.3389/fnhum.2016.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammadzadeh, B., Sattari, K. & Lotfizadeh, M. J. I. J. O. E. R. Determining the relationship between depression and brain waves in depressed subjects using Pearson correlation and regression. Int. J. Epidemiol. Res.3, 376–385 (2016).
- 48.Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 2012;36:677–695. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Pal A, Pegwal N, Behari M, Sharma R. High delta and gamma EEG power in resting state characterise dementia in Parkinson’s patients. Biomark. Neuropsychiatry. 2020;3:100027. doi: 10.1016/j.bionps.2020.100027. [DOI] [Google Scholar]
- 50.Legdeur N, Heymans MW, Comijs HC, Huisman M, Maier AB, Visser PJ. Age dependency of risk factors for cognitive decline. BMC Geriatr. 2018;18:187. doi: 10.1186/s12877-018-0876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh A, Cole RC, Espinoza AI, Brown D, Cavanagh JF, Narayanan NS. Frontal theta and beta oscillations during lower-limb movement in Parkinson’s disease. Clin. Neurophysiol. 2020;131:694–702. doi: 10.1016/j.clinph.2019.12.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lespasio MJ, Piuzzi NS, Husni ME, Muschler GF, Guarino A, Mont MA. Knee osteoarthritis: A primer. Perm. J. 2017;21:16–183. doi: 10.7812/TPP/16-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kisler LB, Kim JA, Hemington KS, Rogachov A, Cheng JC, Bosma RL, Osborne NR, Dunkley BT, Inman RD, Davis KD. Abnormal alpha band power in the dynamic pain connectome is a marker of chronic pain with a neuropathic component. Neuroimage Clin. 2020;26:102241–102241. doi: 10.1016/j.nicl.2020.102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jausovec N, Jausovec K. Resting brain activity: Differences between genders. Neuropsychologia. 2010;48:3918–3925. doi: 10.1016/j.neuropsychologia.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Ploner M, Sorg C, Gross J. Brain rhythms of pain. Trends Cogn. Sci. 2017;21:100–110. doi: 10.1016/j.tics.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulz E, Zherdin A, Tiemann L, Plant C, Ploner M. Decoding an individual's sensitivity to pain from the multivariate analysis of EEG data. Cereb. Cortex. 2012;22:1118–1123. doi: 10.1093/cercor/bhr186. [DOI] [PubMed] [Google Scholar]
- 57.Kuo PC, Chen YT, Chen YS, Chen LF. Decoding the perception of endogenous pain from resting-state MEG. Neuroimage. 2017;144:1–11. doi: 10.1016/j.neuroimage.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 58.Ploner M, Bingel U, Wiech K. Towards a taxonomy of pain modulations. Trends Cogn. Sci. 2015;19:180–182. doi: 10.1016/j.tics.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Nijs J, George SZ, Clauw DJ, Fernández-de-las-Peñas C, Kosek E, Ickmans K, Fernández-Carnero J, Polli A, Kapreli E, Huysmans E, Cuesta-Vargas AI, Mani R, Lundberg M, Leysen L, Rice D, Sterling M, Curatolo M. Central sensitisation in chronic pain conditions: Latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021;3:e383–e392. doi: 10.1016/S2665-9913(21)00032-1. [DOI] [PubMed] [Google Scholar]
- 60.Romei V, Thut G, Silvanto J. Information-based approaches of noninvasive transcranial brain stimulation. Trends Neurosci. 2016;39:782–795. doi: 10.1016/j.tins.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Hohn VD, May ES, Ploner M. From correlation towards causality: Modulating brain rhythms of pain using transcranial alternating current stimulation. Pain Rep. 2019;4:e723. doi: 10.1097/PR9.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simis M, Imamura M, Sampaio de Melo P, Marduy A, Battistella L, Fregni F. Deficit of Inhibition as a Marker of Neuroplasticity (DEFINE Study) in rehabilitation: A longitudinal cohort study protocol. Front. Neurol. 2021;12:695406. doi: 10.3389/fneur.2021.695406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reidler JS, Mendonca ME, Santana MB, Wang X, Lenkinski R, Motta AF, Marchand S, Latif L, Fregni F. Effects of motor cortex modulation and descending inhibitory systems on pain thresholds in healthy subjects. J. Pain. 2012;13:450–458. doi: 10.1016/j.jpain.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Mackey, I. G., Dixon, E. A., Johnson, K. & Kong, J.-T. Dynamic quantitative sensory testing to characterize central pain processing. JoVE (J. Visual. Exp), e54452. 10.3791/54452 (2017). [DOI] [PMC free article] [PubMed]
- 65.den Bandt HL, Paulis WD, Beckwée D, Ickmans K, Nijs J, Voogt L. Pain mechanisms in low back pain: A systematic review with meta-analysis of mechanical quantitative sensory testing outcomes in people with nonspecific low back pain. J. Orthop. Sports Phys. Therapy. 2019;49:698–715. doi: 10.2519/jospt.2019.8876. [DOI] [PubMed] [Google Scholar]
- 66.Nir R-R, Yarnitsky D. Conditioned pain modulation. Curr. Opin. Support. Palliat. Care. 2015;9:131–137. doi: 10.1097/SPC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 67.Lautenbacher S, Kunz M, Burkhardt S. The effects of DNIC-type inhibition on temporal summation compared to single pulse processing: does sex matter? Pain. 2008;140:429–435. doi: 10.1016/j.pain.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 68.Streff A, Michaux G, Anton F. Internal validity of inter-digital web pinching as a model for perceptual diffuse noxious inhibitory controls-induced hypoalgesia in healthy humans. Eur. J. Pain. 2011;15:45–52. doi: 10.1016/j.ejpain.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Malcolm MP, Triggs WJ, Light KE, Shechtman O, Khandekar G, Rothi LJG. Reliability of motor cortex transcranial magnetic stimulation in four muscle representations. Clin. Neurophysiol. 2006;117:1037–1046. doi: 10.1016/j.clinph.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwenkreis P, Janssen F, Rommel O, Pleger B, Völker B, Hosbach I, Dertwinkel R, Maier C, Tegenthoff M. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology. 2003;61:515–519. doi: 10.1212/wnl.61.4.515. [DOI] [PubMed] [Google Scholar]
- 72.Nuwer MR, Lehmann D, da Silva FL, Matsuoka S, Sutherling W, Vibert JF. IFCN guidelines for topographic and frequency analysis of EEGs and EPs. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999;52:15–20. [PubMed] [Google Scholar]
- 73.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jensen KB, Regenbogen C, Ohse MC, Frasnelli J, Freiherr J, Lundström JN. Brain activations during pain: A neuroimaging meta-analysis of patients with pain and healthy controls. Pain. 2016;157:1279–1286. doi: 10.1097/j.pain.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 76.Faraway JJ. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models. CRC Press; 2016. [Google Scholar]
- 77.Shtatland, E. S., Cain, E. & Barton, M. B. 22–25 (Citeseer).
- 78.Osborne JW, Waters E. Four assumptions of multiple regression that researchers should always test. Pract. Assess. Res. Eval. 2002;8:2. [Google Scholar]
- 79.Yap BW, Sim CH. Comparisons of various types of normality tests. J. Stat. Comput. Simul. 2011;81:2141–2155. doi: 10.1080/00949655.2010.520163. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.