Abstract
The kinetics of amyloid beta turnover within human brain is still poorly understood. We previously found a dramatic decline in the turnover of Aβ peptides in normal aging. It was not known if brain interstitial fluid/cerebrospinal fluid (ISF/CSF) fluid exchange, CSF turnover, blood-brain barrier function or proteolysis were affected by aging or the presence of β amyloid plaques. Here, we describe a non-steady state physiological model developed to decouple CSF fluid transport from other processes. Kinetic parameters were estimated using: (1) MRI-derived brain volumes, (2) stable isotope labeling kinetics (SILK) of amyloid-β peptide (Aβ), and (3) lumbar CSF Aβ concentration during SILK. Here we show that changes in blood-brain barrier transport and/or proteolysis were largely responsible for the age-related decline in Aβ turnover rates. CSF-based clearance declined modestly in normal aging but became increasingly important due to the slowing of other processes. The magnitude of CSF-based clearance was also lower than that due to blood-brain barrier function plus proteolysis. These results suggest important roles for blood-brain barrier transport and proteolytic degradation of Aβ in the development Alzheimer’s Disease in humans.
Subject terms: Computational models, Alzheimer's disease, Ageing
To understand if brain interstitial fluid/cerebrospinal fluid (ISF/CSF) exchange, CSF turnover, blood-brain barrier function or proteolysis were affected by aging or the presence of β amyloid plaques, Elbert et al. develop a non-steady state physiological model using MRI-derived brain volumes, stable isotope labeling kinetics of Aβ, and lumbar CSF Aβ concentration. Their model suggests an important role for blood-brain barrier transport and proteolytic degradation of Aβ in the development Alzheimer’s Disease in humans.
Introduction
Stable isotope-labeling kinetics (SILK) in humans have previously revealed that: (1) the turnover rate of Aβ42 (FTR42) relative to Aβ40 (FTR40) increases in both sporadic Alzheimer’s disease (AD) and autosomal dominant Alzheimer’s Disease (ADAD), likely due to active deposition of Aβ42 into plaques; (2) the production rate of Aβ42 relative to Aβ40 increases in ADAD; and (3) the turnover rates of Aβ peptides decrease with age1–3. One weakness of the previous analysis was the assumption that the system was at a steady state. As such, the kinetic models could not account for the effects of secretase inhibitors4–6, or explain the rise in the lumbar cerebrospinal fluid (CSF) concentration of Aβ peptides observed during the 36–48 h SILK studies7–10. Other existing non-steady-state models do not address the concentration rise observed in SILK studies11–17.
The goal of this approach is to more accurately model Aβ peptide kinetics in the central nervous system (CNS). Myriad processes affect Aβ turnover in the brain, including transport across the blood–brain barrier, enzymatic/lysosomal degradation, interaction and deposition into plaques, and the complex flow and exchange of CSF and interstitial fluid (ISF)1,18–20. Ideally, brain tissue or ISF would be sampled. However, such procedures are highly invasive21. Our approach was to use subject-specific MRI-derived CNS compartment volumes to better model transport of CSF from the cranial subarachnoid space (SAS) to the lumbar SAS. Although we do not directly measure or model “glymphatic” fluxes (i.e., flow within the perivascular space and brain interstitium resulting in mixing of CSF and ISF), we can infer these fluxes via the model, because the flux of Aβ transferred from ISF to CSF will equal the flux of Aβ lost from the CSF. CSF is lost from the CNS via arachnoid granulations, dural lymphatics, at the cribriform plate, and down cranial or spinal nerves20. These exit processes, involving bulk fluid flow of CSF, affect the Aβ concentration throughout the SAS, including at the lumbar sampling site. Via the combination of kinetic measurements, MRI volumes and CSF concentration rises upon CSF withdrawal in humans, we can estimate the turnover of Aβ in CSF and thus infer the flux of Aβ from ISF to CSF and the rate of irreversible loss of Aβ from within the ISF.
Glymphatics is defined by Nedergaard et al. as “astrocyte-mediated transport of CSF and ISF that clears metabolic waste from the interstitial space of the brain parenchyma primarily during non-REM (nonrapid eye movement) sleep and states of high slow-wave activity. This process serves a pseudolymphatic function in the CNS”22. Others argue that aquaporin-4 deletion does not affect dye penetration into the paravascular space and surrounding parenchyma23. Some have observed that transport through brain parenchyma is molecular-weight dependent suggesting a dominant role for molecular diffusion versus convective flow17,24–27. Correctly modeling the tortuous flow in the presence of cardiac-driven pressure fluctuations through the highly cellular cortex and dense extracellular matrix is daunting, and the current approach is desirable as it is largely if not entirely agnostic to the exact mechanism of the “glymphatic” process.
CSF flow in the CNS is generally directed from the choroid plexus to the superior sagittal sinus, although this is a simplification28,29. Each heartbeat and breath also induces substantial oscillatory CSF flow30–32. The complicated flow patterns of the CSF resulting from these pulsations are observable by MRI velocimetry and enhance mass transport in the CNS33–35. The presence of trabeculations and nerve roots passing through the SAS result in complicated, time-dependent recirculation patterns15,36. The trabeculations and nerve roots are similar to baffles, which are known in the field of reactor engineering to enhance fluid mixing37. Accurate flow analysis by computational fluid dynamics is impractical without higher-resolution imaging, so the model presented here assumes that oscillatory CSF flow results in the perfect mixing of fluids within distinct compartments in the SAS. This simplification is consistent with our previous compartmental model that fit the SILK data exceptionally well1,2. The model accounts for changes in lumbar space volume due to the hourly withdrawal of CSF from the lumbar space and potential CSF leaks due to the indwelling catheter, which is perhaps the most important feature of the model.
It is believed that the rate of nucleation and growth of amyloid plaques depends upon the concentration of Aβ42 in the cortical interstitial fluid12,38. Thus, changes in the rate of clearance of Aβ42 from the cortical interstitial fluid may be a critical factor in the onset of plaque formation, just as differences in the rate of production of Aβ42 affect the age of onset of Alzheimer’s disease1. In this study, we estimate the rates of clearance of Aβ in cortical interstitial fluid due to different processes, such as transport across the blood–brain barrier, proteolysis, and deposition into plaques. Transport of Aβ42 across the blood–brain barrier is mediated by lipoprotein-related protein 1 (LRP-1)39, leading to the transfer of Aβ42 from the brain interstitial fluid to the blood40. Binding of Aβ40 and Aβ42 to LRP-1 is saturable and clearance across mouse brain capillaries is blocked by antibodies against LRP-141. Expression of LRP-1 declines in aging rats and is negatively correlated with the concentration of Aβ40 and Aβ42 in rat brain cortex homogenates42. Multiple enzymes contribute to the proteolysis of Aβ42 within the CNS, including secreted and membrane-bound enzymes such as an angiotensin-converting enzyme, neprilysin, insulin-degrading enzyme and various MMPs, in addition to degradation downstream of endocytosis or phagocytosis by cells, particularly microglia and monocyte-derived macrophages43. Transport across the BBB, enzymatic degradation and deposition into plaques within the brain will result in similar kinetics for isotope-labeled Aβ, as all processes likely exhibit close to first-order kinetics and occur within the same compartment. In contrast, transfer of Aβ from ISF to CSF will result in elimination of Aβ by processes governed by CSF turnover kinetics. CSF-based processes will affect the shape of the SILK curves differently from processes that occur within the ISF, and this provides the rationale for the current study.
Results and discussion
Correlation of MRI-derived volumes with age and steady-state model parameters
CNS volumes were recorded by MRI for 100 study subjects who also completed SILK kinetic studies, with four subjects excluded due to poor fits of the model to SILK or lumbar concentration data. All of the subjects were part of earlier published studies1,2. Subject demographics, lumbar CSF tau and Aβ concentrations, and results from the previous steady-state model are shown in Supplementary Tables 1 and 2. Correlations of MRI-measured CNS volumes with amyloid status were consistent with findings in the literature (Supplementary Table 3)44–47. Amygdala, hippocampus, and precuneus volumes declined most dramatically due to β-amyloidosis. Total ventricle volume was increased in amyloid-positive subjects, while the total gray volume decreased. The effects of normal aging on CNS volumes and thicknesses were assessed in amyloid-negative subjects to eliminate effects of β-amyloidosis (Supplementary Table 4). Putamen, accumbens-area, thalamus, and hippocampus volumes declined most dramatically with age, as expected.
Development of a subject-specific physiological model
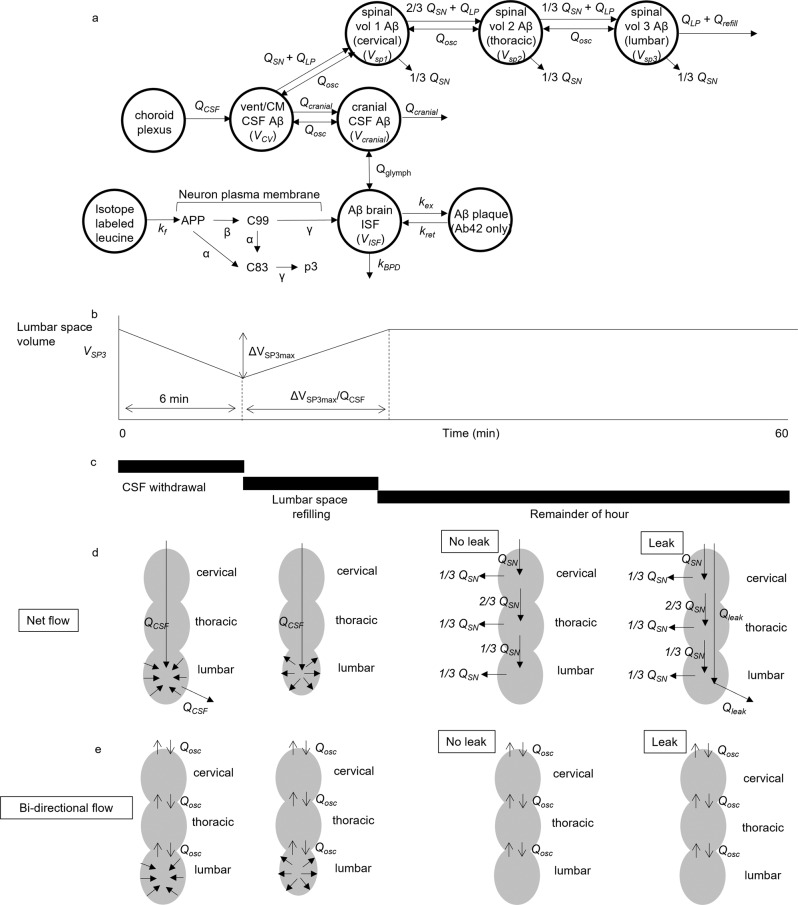
A new physiological model was developed to decouple transport of Aβ peptides within CSF from (1) transport across the blood–brain barrier, (2) changes in proteolytic degradation, and (3) deposition into plaques. The new physiological model (Fig. 1a) assumed that the CNS consists of the following compartments: (1) cells producing Aβ via enzymatic reactions at their plasma membranes following Michaelis–Menten kinetics, (2) brain ISF, (3) cranial subarachnoid space (SAS), (4) cisternal and ventricular SAS, and (5) spinal SAS divided into cervical, thoracic and lumbar regions. The model was written as a nonlinear system of 51 ordinary differential equations (Supplementary Methods 1). Parameter optimization and differential equation solving were performed in Julia48–50. The volumes of compartments were estimated from Freesurfer analysis of MRI scans (Supplementary Methods 2). The spinal volumes were not measured in this study and literature values were assumed for the spinal SAS volumes (VSP1 ≈ cervical SAS, VSP2 ≈ thoracic SAS, VSP3 ≈ lumbar SAS)51–53. In addition, a range of CSF volumes was examined to discover the best fit to the observed change in lumbar concentration of Aβ during the course of the 36–48 h SILK experiment. Transfer between compartments was modeled as volumetric flows (mL/h), which were divided by the volumes of the compartments to obtain first-order rate constants for mass transfer between compartments.
Fig. 1. Model of Aβ production, transport, and clearance.
Physiological model of CNS during SILK study, incorporating fluid flows, ISF and CSF compartment volumes, and Michaelis–Menten kinetics for Aβ production. a Model structure. b Flows in the spinal SAS due to hourly CSF withdrawal. The volume of the lumbar SAS decreases when the CSF withdrawal rate QLP exceeds the CSF production rate (QCSF) and subsequently refills with CSF to its original volume. c Timeline of hourly CSF withdrawal. d Net flow in the spinal SAS. e Bidirectional flow in the spinal SAS.
CSF production was assumed to entirely arise from the choroid plexus, specified as the CSF production rate QCSF. During the SILK study, 6 mL of CSF were withdrawn every hour from an indwelling lumbar catheter. The withdrawal occurred over 5–10 min. During the hourly CSF collection, the CSF withdrawal rate (QLP) was much greater than the model-predicted CSF production rate (QCSF) in all subjects. It was thus assumed that the volume of the lumbar space (VSP3) decreased during withdrawal (Fig. 1b–e), which has been observed in humans by MRI during lumbar puncture51. After CSF withdrawal, CSF production served to refill the lumbar space to its original volume. Although the majority of CSF was assumed to be reabsorbed within the cranial compartment, some amount of CSF was assumed to be lost down spinal nerves (QSN)54. Qosc and Qglymph are bidirectional continuous flows that approximate the oscillatory flows due to the cardiac and respiratory cycles. For some subjects with large lumbar CSF Aβ concentration rises, the data were better fit assuming a leak due to catheterization (Qleak). Further details of the model are provided in Supplementary Methods 1.
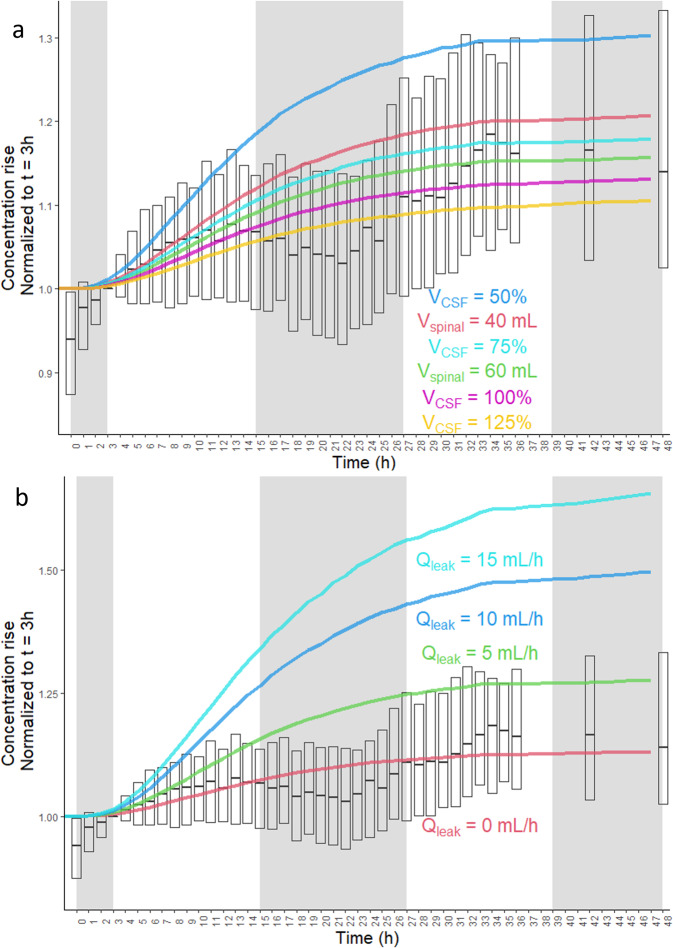
The lumbar CSF concentration rise of Aβ peptides was substantial (Fig. 2 and Supplementary Fig. 1), with a mean rise of 27% between 0 and 36 h. All experiments began at 8 a.m. (time = 0) and a dip in the lumbar concentration of Aβ peptides was seen during the nighttime and following morning, which has been previously associated with the effects of sleep9,10. However, sleep was not modeled in this study as the sleep-wake cycle was not controlled and was not apparent in 19 out of 96 subjects. In this study, the subjects arose early (how early was uncontrolled) to reach the hospital by 7 a.m. and the study was started at 8 a.m. They were instructed to remain mostly in bed for 36–48 h and could sleep/nap at will. Of the 77 subjects that showed a sleep effect, many showed abbreviated dips, double dips, or prolonged dips in the lumbar Aβ concentration. SILK and concentration-time courses for all 96 subjects are presented in Supplementary Data 3. To reduce the effect of sleep on the conclusions, concentration data was not fit to the model between 11 p.m. and 11 a.m., accounting for transport delays between ISF and the lumbar space. The sleep effect also overlapped with the first three hours of the experiment.
Fig. 2. Increasing lumbar Aβ CSF concentration due to hourly CSF withdrawal.
Normalized lumbar CSF concentrations of Aβ peptides averaged over all subjects (n = 96). Lumbar CSF Aβ concentration time courses for each subject were first normalized to time = 3 h to account for the sleep effect from the previous night (experiments started at 8 a.m.). The Aβ concentration was the average of Aβ38, Aβ40, and total Aβ for each subject (total Aβ was from an immunoprecipitation with an antibody recognizing a sequence common to Aβ38, Aβ40, and Aβ42). Some subjects (n = 17) only had concentration timecourse measured by ELISA for Aβ40 and Aβ42. The ELISA time courses were averaged for these two peptides at each time point. a The total volume of CSF (VCSF) was scaled between 50 and 125% in separate simulations, and the volume of spinal CSF was varied from 40 to 80 mL. b The presence of a CSF leak around the catheter was also simulated, ranging from 0 to 20 mL/h, or equal to the entire CSF production rate (Qleak = QCSF). Medians and means of measurements are shown, and bars represent the interquartile range. Shaded areas represent the sleep effect. Simulations of CSF leaks equal to 20 mL/h or QCSF overlapped with Qleak = 15 mL/h. For the full boxplot of measured concentrations, see Supplementary Fig. 1.
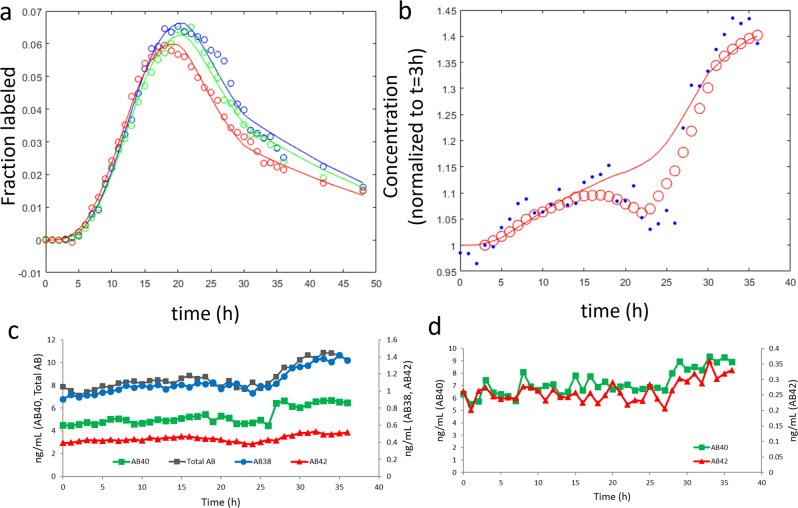
The physiological model predicted a lumbar Aβ concentration rise of 12% between 3 and 36 h (see VCSF 100% curve in Fig. 2a and Qleak = 0 mL/h curve in Fig. 2b). This was lower than both the mean and median concentration rises between 3 and 36 h (19% and 17%, respectively). The total CSF volume and/or the spinal CSF volume were thus systematically varied to improve the fit of the model to the observed concentration rise, selecting the simulation with the lowest sum-of-squares residual. For 20 out of 96 subjects, the concentration rise was greater than that predicted even if the total CSF volume was decreased by 50%. For these subjects, an additional parameter was added, Qleak, which represented continual leakage of CSF around the indwelling catheter. A leak was also assumed for the seven subjects with clinical records of a blood patch to seal a CSF leak. Qleak was not different by amyloid status but did significantly decrease with age (Table 1 and Supplementary Table 5). For ten of the subjects, the leak rate appeared to change at specific points during the 36–48 h experiment, and Qleak was allowed to change to better fit the data (for example, see Fig. 3). Fits to SILK and lumbar concentration data for all 96 subjects are included as Supplementary Data 3.
Table 1.
Model results.
| Predicted marginal means (S.E.) at age = 69.9 y | Correlation coefficient with age | |||||
|---|---|---|---|---|---|---|
| Amyloid negative (N = 58) | Amyloid positive (N = 38) | P valuea | Amyloid negative (N = 58) | P value | ||
| CSF leak | Qleak > 0? | 20/58 | 10/38 | 0.40* | ||
| Qleak (mL/h) | 2.6 (0.6) | 2.6 (0.8) | 0.92 | −0.36 | 0.0050 | |
| Aβ clearance | kBPD38 (h−1) | 0.31 (0.02) | 0.22 (0.03) | 0.015 | −0.68 | <0.0001 |
| kBPD40 (h−1) | 0.37 (0.03) | 0.24 (0.03) | 0.0024 | −0.59 | <0.0001 | |
| kBPD42 (h−1) | 0.53 (0.06) | 0.96 (0.07) | <0.0001 | −0.27 | 0.044 | |
| kBPD42/kBPD38 | 2.1 (0.4) | 5.3 (0.5) | <0.0001 | 0.24 | 0.069 | |
| kBPD42/kBPD40 | 1.7 (0.3) | 4.8 (0.4) | <0.0001 | 0.13 | 0.33 | |
| kBPD40/kBPD38 | 1.36 (0.08) | 1.1 (0.1) | 0.10 | 0.21 | 0.11 | |
| Aβ production (w/o mutation carriers) | Vmax,γ42/Vmax,γ38 | 1.02 (0.04)b | 1.07 (0.06)c | 0.52 | 0.28b | 0.039 |
| Vmax,γ42 (μg/(mL·h)) | 125 (5)b | 139 (8)c | 0.15 | 0.34b | 0.013 | |
| APP production (w/o mutation carriers) | kf (ng/h) | 3500 (300)b | 3400 (500)c | 0.79 | −0.58b | <0.0001 |
| Total gray volume (mL) | 590 (8)b | 570 (10)c | 0.15 | −0.42b | 0.0014 | |
| Exchange | kex42 (h−1) | 0.06 (0.02) | 0.17 (0.02) | <0.0001 | 0.087 | 0.52 |
| CSF fluid flow | QCSF = Qglymph (mL/h) | 23 (1) | 26 (2) | 0.31 | −0.0062 | 0.99 |
| Qosc (mL/h) | 8.8 (0.8) | 11 (1) | 0.16 | 0.47 | <0.0001 | |
| [Aβ40]ISF/[Aβ40]lumbar | 10.1 (0.8) | 10 (1) | 0.85 | −0.51 | <0.0001 | |
| Predicted cisternography half-life (h) | 20.7 (0.6) | 19.8 (0.7) | 0.32 | −0.30 | 0.021 | |
| VCSF (mL) | 300 (10) | 310 (10) | 0.47 | 0.38 | 0.0030 | |
| Flux Aβ38 | CSF-based (ng/min) | 3.1 (0.4) | 3.4 (0.5) | 0.54 | −0.24 | 0.070 |
| BBB + proteolysis (ng/min) | 4.9 (0.4) | 3.2 (0.5) | 0.0082 | −0.81 | <0.0001 | |
| % CSF-based | 43 (3) | 52 (3) | 0.037 | 0.47 | <0.0001 | |
| Flux Aβ40 | CSF-based (ng/min) | 14 (2) | 17 (2) | 0.29 | −0.33 | 0.011 |
| BBB + proteolysis (ng/min) | 28 (2) | 16 (3) | 0.0037 | −0.76 | <0.0001 | |
| % CSF-based | 39 (2) | 50 (3) | 0.0042 | 0.45 | <0.0001 | |
| Flux Aβ42 | CSF-based (ng/min) | 2.2 (0.2) | 1.7 (0.3) | 0.19 | −0.30 | 0.021 |
| BBB + proteolysis (ng/min) | 4.1 (0.4) | 1.6 (0.5) | <0.0001 | −0.70 | <0.0001 | |
| Deposition (ng/min) | 1.4 (0.4) | 4.6 (0.5) | <0.0001 | −0.21 | 0.11 | |
| % CSF-based | 33 (2) | 22 (3) | 0.0014 | 0.33 | 0.012 | |
*Chi-square statistic = 0.71.
at test of predicted marginal means (least-squares means) except where noted by *. See Supplementary Table 5 for interactions between age and amyloid status; italic values signify: P < 0.05; bold italic values signify: P < 0.01.
bN = 54.
cN = 33.
Fig. 3. Example of model fits to SILK and lumbar Aβ concentration.
The physiological model fits with step changes in Qleak at 3 h and 20 h for a single amyloid-positive subject. a Fit of the physiological model to SILK data. Blue = Aβ38, green = Aβ40, red = Aβ42. b Fit of lumbar CSF concentration data (0 < time < 3, Qleak = 8 mL/h; 3 < time ≤ 20 h, Qleak = 0 mL/h; 20 ≤ time < 30 h, Qleak = 13 mL/h; time ≥ 30 h, Qleak = 0 mL/h). Blue circles = mass spectrometric measurements normalized to time = 3 h. Red circles = moving mean smoothed data. c Lumbar CSF concentrations were measured by immunoprecipitation followed by quantitative mass spectrometry, showing the abrupt change in concentration at about 27 h for all four peptides measured. d Lumbar CSF concentrations measured by ELISA also showed an abrupt change at about 27 h for both peptides measured.
Kinetic results from the physiological model
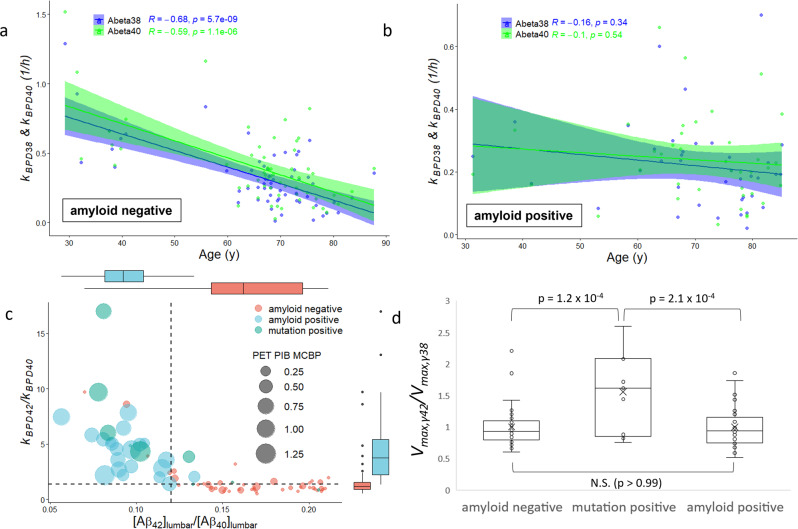
The parameter kBPD describes irreversible loss in the ISF compartment by processes that include: (1) transport across the blood–brain barrier (“B”), (2) proteolytic degradation (“P”), and (3) permanent deposition into plaques (“D”). kBPD is similar to FTR from the steady-state model, but the effects of CSF transport are modeled explicitly, which should allow kBPD to more accurately represent processes that occur in the brain ISF. Like FTR, both kBPD38 and kBPD40 were highly correlated with age in amyloid-negative subjects (Table 1 and Fig. 4a). Unlike FTR, kBPD38 and kBPD40 are not correlated with age in the amyloid-positive subject (Fig. 4b). However, the CSF production rate (QCSF) was negatively correlated with age in amyloid-positive subjects (r = −0.42, P = 0.0079, n = 38) but not in amyloid-negative subjects (r = −0.0062, P = 0.96, n = 58). This implies that changes in Aβ clearance in normal aging are driven by changes in blood–brain barrier transport or proteolysis (deposition should be minimal in normal aging). Aβ clearance in amyloid-positive subjects appears to be influenced by changes in CSF production, and potentially from the substantial decrease in brain parenchymal volumes and increase in ventricle volume (Supplementary Table 3).
Fig. 4. Derived Aβ production and clearance rate constants.
Aβ clearance and production rate constants. a The rate constant describing the irreversible loss of Aβ peptides from the brain ISF compartment (kBPD) significantly declined with age in amyloid-negative subjects for Aβ38 and Aβ40 (n = 58; also see Table 1). b The age effect on kBPD38 and kBPD40 was absent in amyloid-positive subjects (n = 38). c The rate of irreversible loss of Aβ42 (kBPD42) was elevated relative to Aβ40 (kBPD40) in the presence of amyloid plaques, as evidenced by elevated PET PIB MCBP (n = 77). This elevation likely represented active deposition of Aβ42 into plaques, and a cutoff of 1.3 (horizontal dashed line) distinguishes most plaque-bearing subjects from non-plaque-bearing subjects. Declining ratios of lumbar CSF Aβ42 to Aβ40 are a hallmark of AD, with a cutoff of 0.12 in common use (vertical dashed line). See statistical comparisons in Table 1. d The gamma-secretase production rate constant ratio Vmax,γ42/Vmax,γ38 reflects the kinetics of gamma-secretase toward the production of Aβ42 relative to Aβ38. The production rate ratio did not differ by amyloid status, but presenilin mutation carriers had elevated production of Aβ42 relative to Aβ38 as compared to both groups of non-mutation carriers. ANOVA with Tukey post hoc analysis.
kBPD38 and kBPD40 are significantly lower in amyloid-positive subjects after controlling for age (Table 1). However, kBPD42 is significantly higher in amyloid-positive subjects, as is the ratio kBPD42/kBPD40 (Table 1 and Fig. 4c). This mirrors the finding from the steady-state model that the turnover of Aβ42 was elevated in amyloid-positive subjects relative to Aβ40. Because transport and proteolysis rates of Aβ40 and Aβ42 are expected to be similar, the increased clearance of Aβ42 is believed to represent deposition of Aβ42 into plaques1,2. The ratio kBPD42/kBPD40 is not correlated with age in amyloid-negative subjects (Table 1) or in amyloid-positive subjects (r = −0.29, P = 0.073, n = 38).
The forward rate constant for reversible exchange in the ISF compartment (kex42) was significantly higher in amyloid-positive subjects (Table 1). It is hypothesized that soluble Aβ42 becomes temporarily insoluble sometime between production and clearance. The greater magnitude in subjects with AD implies a role for plaques in this process2. Isotope labeling of plaques has also been observed in SILK subjects postmortem55.
With certain presenilin mutations, the rate of production of Aβ42 is expected to be elevated relative to the rate of production of Aβ40, which was indeed observed with the steady-state model1. However, the steady-state model also exhibited a strong correlation between the production rate ratio of Aβ42 to Aβ40 and the lumbar CSF concentration ratio [Aβ42]lumbar /[Aβ40]lumbar (Supplementary Fig. 2a). This would require a highly specific change in the kinetics of secretases in AD, or changes in intracellular trafficking that selectively decreased the rate of production of Aβ42 relative to Aβ40, which both seem unlikely. In the physiological model, the Vmax of gamma-secretase for the production of Aβ42 (Vmax,γ42) was determined relative to Vmax,γ40, with Vmax,γ40 fixed for all subjects to a literature-derived value56,57. Vmax,γ42 was thus normalized by Vmax,γ38 instead of Vmax,γ40. The production rate ratio of Aβ42 relative to Aβ38 by gamma-secretase was elevated in presenilin mutation carriers compared to amyloid negatives and amyloid positives (Fig. 4d). There was no difference in the relative production rate based on amyloid status, as expected, and in contrast to the steady-state model (Supplementary Fig. 2b). Without normalization, Vmax,γ42 was higher in mutation positives versus amyloid negatives as expected, but the comparison with amyloid positives did not reach significance (Supplementary Fig. 2c).
The production rate of APP (kf) declined with age, perhaps related to the decline in total gray volume (Table 1) and a loss of synaptic activity58–60. The production rate of APP was also compared between presenilin mutation carriers and sibling non-mutation-carrier controls (Table 2). In these age-matched subjects, the production rate of Aβ42 increased due to the presence of presenilin mutations, but kf was not different.
Table 2.
Production rates in presenilin mutation carriers and age-matched non-carriers.
| Mutation-carrier mean (S.E.) N = 9 | Sibling non-carriers mean (S.E.) N = 10 | P valuea | |
|---|---|---|---|
| Age | 45 (4) | 49 (5) | 0.61 |
| kf (ng/h) | 8000 (1000) | 8000 (1000) | 0.99 |
| Vmax,γ42 (μg/(mL h)) | 1.6 (0.2) | 0.80 (0.05) | 0.0079 |
| Vmax,γ42/ Vmax,γ38 | 160 (20) | 85 (3) | 0.0014 |
| Total gray volume (mL) | 600 (20) | 640 (10) | 0.14 |
at test; values in italics signify P < 0.05; values in bold italic signify: P < 0.01.
The CSF production rate QCSF should be identical to the CSF production rate typically measured by, for example, the modified Masserman method61. QCSF did not differ by amyloid status (Table 1). The mean values were similar to those found in a carefully performed study in which the CSF production rate was found to be 24.0 ± 5.00 mL/h (95% CI: 16.2–33.8 mL/h)62. The CSF production rate has been found to decline with age61. QCSF did not vary significantly with age in amyloid-negative subjects (Table 1). CSF pressure transients and thus CSF production rate were not measured during the data collection and the CSF production rate is inferred from the data. Our mode of measurement is not as precise and the decrease is not detected. However, on average, the CSF production rates are reasonable and arise from the model structure and data without being fit. In addition, amyloid subjects under 60 years in age show a greater CSF production rate than those over 60, but it is not significant (P = 0.2; Supplementary Table 6). However, QCSF did decline with age in amyloid-positive subjects, as mentioned previously.
The physiological model accounts for the oscillatory flow of CSF driven by circulatory and respiratory pulsations. The oscillatory flow was modeled as a continuous bidirectional flow with magnitude Qosc. Qosc did not differ between AD and non-AD subjects (Table 1). Qosc increased with age, while the ISF-to-lumbar concentration “gradient” [Aβ40]ISF/[Aβ40]lumbar decreased with age (Table 1), consistent with previous observations in humans61. The concentration gradient is highly positively correlated with 1/Qosc1/2 (r = 0.82, P = 1.3 × 10−24, n = 96). Qosc is mathematically similar to a diffusion coefficient and higher values will tend to decrease the ISF/lumbar concentration gradient. It is known that the total cerebral blood flow decreases in aging, as does the cervical CSF stroke volume63. This suggests that the enhanced mixing of CSF that decreases the ISF/lumbar gradient in aging is due to biomechanical factors other than CSF pulsatility. The increase in Qosc with age may be similar to the increase in pulse wave velocity with age due to stiffening of the arteries64. Although ISF-to-lumbar gradients can be inferred from experiments, there is scant evidence of a ventriculo-lumbar gradient for Aβ21,65, in contrast to the negative gradient for brain proteins such as tau and the positive gradient for blood proteins such as albumin66.
Allowing bidirectional flow results in mass transfer down concentration gradients. For example, within the model, the introduction of a labeled species in the lumbar space results in eventual transport of the label to the brain, similar to cisternograpy and related MRI-based CNS tracer studies54,67,68. Although the study protocol did not include cisternography, the model makes specific predictions about the distribution and disappearance of label within the CNS. This includes a prediction of a “cisternography half-life”, the half-life in the CNS of a labeled compound introduced into the lumbar space. Moriyama et al. studied the effects of age on cisternography half-life and developed a linear regression model67. The Moriyama et al. model predicted a mean cisternography half-life for the subjects in our study of 20 ± 2.4 h, compared to 21 ± 4.5 h predicted by the physiological model. The predicted cisternography half-life was strongly influenced by the exit of CSF from the CNS down spinal nerves and this was used to fix QSN at 10% of QCSF for all subjects. Higher or lower values of QSN resulted in unreasonable values for the predicted cisternography half-life (Supplementary Fig. 3). The chosen value for QSN was slightly lower than previous estimates (12–25% of QCSF)69. Although the observed increase in Qosc with age would tend to decrease the cisternography half-life, the increase in the total volume of CSF (VCSF) with age seems to negate this effect (Table 1). While cisternography data is useful for imaging tracer in the entire CNS simultaneously, modern methods such as glymphatic MRI can contribute more refined spatial information that will be useful in the development of future versions of the model with more accurate geometric modeling of the CNS70–72.
Flux of Aβ through distinct pathways
The concentrations and fluxes of Aβ peptides within ISF are difficult to measure in humans. The measured Aβ40 concentration in homogenized cortex varies widely but tends to be in the range of 1–10 ng/g wet cortical tissue40,73–75, although some studies report much higher values76–78. APP is an integral membrane protein and Aβ peptides are predominantly extracellular79 (particularly when considering endosomes and lysosomes to be topologically extracellular). Assuming a cellularity of the cortex of ~90%80, this suggests that cortical ISF concentrations are about 10× higher than concentrations measured in the homogenized cortex. The physiological model specifically incorporates the volume of gray matter ISF, and the mean rise in Aβ40 concentration from lumbar SAS to brain ISF ([Aβ40]ISF/[Aβ40]lumbar) was about tenfold and did not differ by amyloid status (Table 1). However, the concentration of Aβ in homogenized cortex should be about tenfold lower than the concentration in brain ISF. Using this assumption, the physiological model predicted a concentration of Aβ40 in the homogenized cortex of 6.6 ± 3.5 ng/g wet tissue, within the range of typical values (assuming a density of the cortex tissue of 1.03 g/mL81). However, using microdialysis, the concentration of Aβ in the brain ISF is about the same as in ventricular and lumbar CSF, and this is more than an order of magnitude lower than the value predicted by the physiological model21,82. Given the dramatic concentration rise observed during sustained withdrawal of lumbar CSF, some fluid within the CNS must have a higher concentration of Aβ and this is likely the ISF. This suggests that microdialysis measurements underreport the ISF concentration of Aβ.
The flux of Aβ40 peptides from the CNS into blood was previously reported as 9.7 ng/min, which was found by measuring the venous–arterial Aβ concentration difference during inferior petrosal sinus sampling40. This flux should include Aβ transported to the venous system by CSF absorption at the superior sagittal sinus and Aβ transported across the blood–brain barrier. It should not include Aβ transported to the lymphatic system via CSF absorption at the dura, cribriform plate, or via perivascular transport20. The physiological model predicted a mean Aβ40 flux of 15 ± 13 ng/min due to CSF absorption in all subjects according to Eq. (1).
| 1 |
A key feature of glymphatics is the entrance of CSF along periarterial pathways and ISF exit along perivenous pathways83. The flux of Aβ40 peptides from the brain ISF into the CSF is given by Eq. (2). Due to mass balance, Eqs. (1) and (2) must be equal at steady state or pseudo-steady state.
| 2 |
The mean rate of irreversible loss of Aβ40 peptides by transport across the BBB or via proteolysis is predicted by the physiological model to be 25 ± 28 ng/min (Eq. (3)). This is greater than the experimentally measured value from inferior petrosal sinus sampling, suggesting that at least 60% of Aβ40 is cleared from the brain by proteolysis. The amount of CSF that is absorbed at arachnoid granulations is controversial and may be quite low84. In that case, the vast majority of Aβ measured during inferior petrosal sinus sampling would represent transport across the blood–brain barrier.
| 3 |
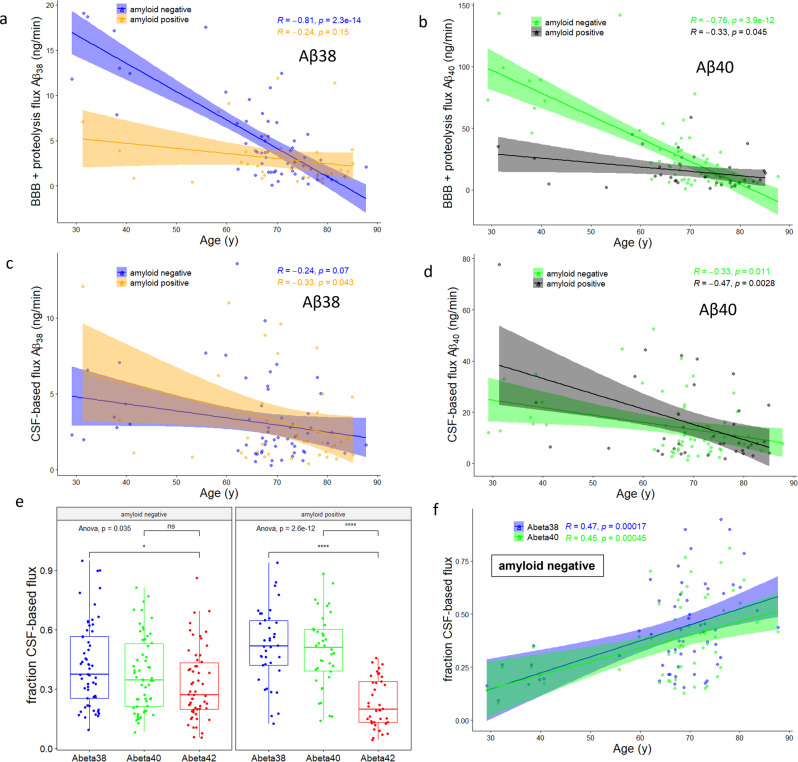
The model predicts that the fluxes of Aβ peptides across the BBB and via proteolysis decrease dramatically in normal aging (Fig. 5a, b). However, the presence of amyloid plaques depresses BBB and proteolysis fluxes (Table 1) and mutes the age effect (Fig. 5a, b). In stark contrast, the CSF-based flux and thus the ISF-to-CSF flux was only weakly correlated with age (Fig. 5c, d). However, the CSF-based and ISF-to-CSF flux became more important with age, mostly because of the dramatic decline in BBB and proteolysis fluxes (Fig. 5e). By amyloid status, the fraction of Aβ clearance that was due to ISF-to-CSF transfer increased for Aβ38 and Aβ40 but decreased for Aβ42 (Table 1). The decrease of Aβ42 in amyloid-positive subjects (Fig. 5f) is likely related to the large deposition flux of Aβ42 in the presence of amyloid plaques (Table 1). The magnitudes of the pathways also differed in amyloid-negative subjects. CSF-based flux of Aβ38 was 3.1 (0.3) ng/h, while BBB + proteolysis flux was 5.5 (0.7) ng/h (n = 58; P = 0.003). CSF-based flux of Aβ40 was 14 (2) ng/h, while BBB + proteolysis flux was 31 (4) ng/h (n = 58; P = 0.0005). CSF-based flux of Aβ42 was 2.2 (0.2) ng/h, while BBB + proteolysis flux was 4.5 (0.6) ng/h (n = 58; P = 0.0006).
Fig. 5. Derived Aβ mass fluxes.
Importance of CSF-based clearance increases with age largely due to changes in clearance within the ISF. Clearance of: a Aβ38 and b Aβ40 from brain ISF declined with age in amyloid-negative subjects, but not in amyloid-positive subjects. In contrast, clearance of: c Aβ38 and d Aβ40 from CSF (and thus also by transfer from ISF to CSF) was weakly correlated with age. e The fraction of Aβ42 cleared by CSF-based processes was significantly lower than that of Aβ38 and Aβ40 in amyloid-positive subjects. This may be due to increased Aβ42 deposition into plaques in amyloid-positive subjects (Table 1), which may lower the brain ISF/cranial CSF concentration gradient that contributes to ISF-to-CSF clearance. *P < 0.05, ****P < 0.0001. f The fraction of CSF-based clearance significantly increased with age for Aβ38 and Aβ40 in amyloid-negative subjects due to the significant decline in clearance across the blood–brain barrier or by proteolysis (also see Table 1).
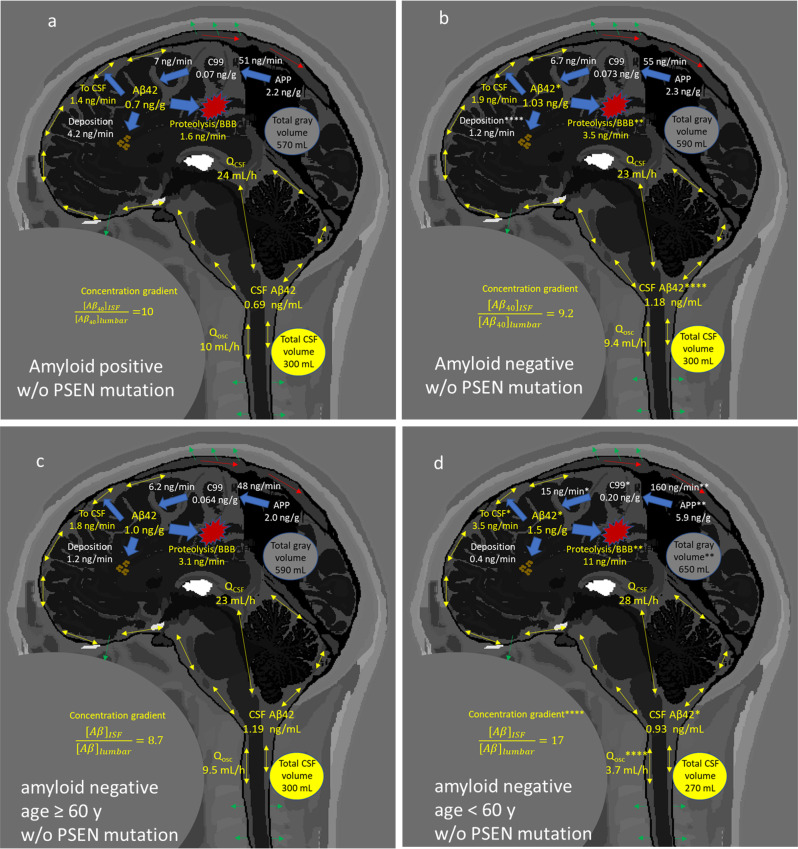
The major results of the study are summarized in Fig. 6, with full results presented in Supplementary Table 6. The presence of amyloid-β plaques is associated with a decrease in the ISF and CSF concentrations of Aβ42, a decrease in the BBB/proteolysis fluxes of Aβ42, and an increase in deposition flux of Aβ42 (Fig. 6a, b). In normal aging (Fig. 6c, d), a decrease in the cortical concentration of Aβ is predicted by the model, which has been previously observed85. The ISF-to-lumbar CSF concentration gradient is also predicted to be greater in younger subjects, associated with a lower Qosc. Much greater clearance of Aβ42 via BBB/proteolysis is found in younger subjects, with a smaller increase in CSF-based clearance. These balance the greater rate of production of APP- > C99- > Aβ42, perhaps due to greater neuron number reflected in the larger volume of gray matter or due to greater neuronal activity.
Fig. 6. Graphical summary of major results by amyloid status and age.
Summary of major findings. a, b In non-mutation carriers, the presence of amyloid plaques decreases the CSF and ISF concentration of Aβ42, due to enhanced deposition. This is despite the decreased mass flux across the blood–brain barrier or due to proteolysis, and a decrease in transfer from ISF to CSF. t test of predicted marginal means at age = 69.9 y: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. c, d In older amyloid-negative, non-mutation carriers, total gray volume and production of APP are reduced relative to younger subjects. This decreases the flux toward Aβ peptides, including Aβ42. However, the ISF concentration of Aβ42 is only slightly elevated in younger subjects, because of greater fluxes across the BBB or due to proteolysis and greater ISF-to-CSF transfer. In younger subjects, the concentration gradient of Aβ42 from the ISF to lumbar space is larger, due to a smaller Qosc. t test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. See Supplementary Table 6 for complete results.
Conclusion
The age-related decline in Aβ peptide turnover has now been ascribed largely to changes in proteolysis and/or blood–brain barrier transport. While the current model and dataset cannot distinguish proteolysis from BBB transport, both should receive enhanced focus as targets for early AD interventions.
Methods
Human studies
The human studies were performed at Washington University in St. Louis. The studies were approved by the Washington University Human Studies Committee and the General Clinical Research Center Advisory Committee. Participants completed informed written consent. The current results are from a subset of 100 subjects who completed the SILK study and MRI imaging scans. Subject demographics are summarized in Supplementary Table 1.
SILK, MRI, and PET
SILK data collection procedures and PET PIB measurements were previously described1,2. Briefly, subjects received intravenous and intrathecal lumbar catheters between 7 a.m. and 9 a.m. The collection of samples began between 8 a.m. and 10 a.m. Time zero CSF and plasma baseline samples were withdrawn, then 3 mg/kg l-[U-13C6]leucine was given as a bolus for 10 min, followed by a constant infusion of 2 mg/kg/h for the remainder of the first 9 h. Blood samples (12 mL) were obtained hourly for the first 16 h and every other hour for the rest of the experiment (36 h). CSF (6 mL) was withdrawn hourly. The deposition of amyloid plaques was quantified by [11C]PIB-PET in 77 subjects. Subjects with mean cortical binding potential (MCBP) score >0.18 were considered to be amyloid positive. If PET PIB was not available, a lumbar CSF concentration ratio Aβ42/Aβ40 < 0.12 was considered to be amyloid positive. PET PIB scans were performed within 3 years before or after the SILK tracer study date. MRI scans (3 T volumetric T1-weighted) were processed using FreeSurfer version 5.3 as previously described86. FDA, Center for Devices and Radiological Health, and IT’IS Foundation collected the MRI data used to generate the images in Fig. 687.
Mass spectrometry
Sample processing and mass spectrometric measurements were previously described1,2. Briefly, Aβ peptides were immunoprecipitated with a mid-domain antibody, in the presence of isotope-labeled internal standard peptides. Concentrations of peptides and fractional isotope labeling were quantified by LC-MS/MS analysis on a Xevo TQ-S (Waters) or TSQ Vantage (ThermoScientific) mass spectrometer. Isotope-labeled leucine was captured from the blood by cation exchange chromatography. The N-heptafluorobutyryl n-propyl ester derivative was quantified by GCMS (Agilent 6890N GC and Agilent 5973N Mass Selective Detector).
Mathematical model
Model equations were solved numerically in Julia as described in Supplementary Methods 1. Parameter meaning, values, and sources are shown in Supplementary Table 7.
The parameters that were fit to the model were: kBPD38, kBPD40, kBPD42, SF38, SF40, SF42, Qosc, QCSF, kex42. Other parameters were estimated as follows:
The volumetric rate of CSF production was QCSF, which was assumed to arise solely from the choroid plexus. Extravasation of fluid from the capillaries of the brain parenchyma was not considered in the model. It was assumed that the concentration of Aβ peptides in newly formed CSF was zero.
VISF represents the volume of brain gray matter ISF, which was 10% of MRI-derived total gray volume. Vcranial is the volume of the cranial SAS. VCV is the volume of the cisterns and ventricles, which are assumed to be in intimate communication due to oscillatory flow. Nominally, the cervical spinal volume is VSP1, the thoracic spinal volume is VSP2, and the lumbar spinal volume is VSP3.
CSF was withdrawn from the lumbar catheter at the start of each hour and the model assumptions are summarized in Fig. 1b–e. The volume of CSF removed (6 mL) was divided by the time required for withdrawal (assumed to be 6 min for all subjects, actual range was stated by the practitioners to be 5–10 min). This yielded the volumetric flow rate of VLP/tCSF draw = 60 mL/h, where VLP is 6 mL and tCSF draw is 0.1 h. It was assumed that the net caudal flow could not exceed QCSF during CSF withdrawal. If VLP/tCSF draw exceeded QCSF, which it did for all subjects, it was assumed that the excess flow came exclusively from a decrease in volume of the lumbar space (VSP3), which has been observed in humans during lumbar puncture51. The parameter QLP represented net caudal flow during withdrawal and refill, while Qrefill was used to account for volumetric changes in the lumbar compartment.
Following CSF withdrawal, it was assumed that the entirety of QCSF was used to refill the lumbar volume to its natural dimensions. The time required to refill the lumbar space was ΔVSP3/QCSF.
After the lumbar space was fully refilled, QLP = 0 for the rest of the hour. However, if a CSF leak was suggested by the lumbar Aβ concentration data, then QLP = Qleak, where Qleak is a constant during the entire 36–48 h SILK experiment, unless otherwise modified to better fit the concentration rise data. The maximum value for Qleak was assumed to be QCSF. During CSF withdrawal and lumbar space refilling, it was assumed that the drop in pressure halted the leak, i.e., Qleak = 0 during withdrawal and refilling.
Some concentration-time courses suggested step changes in Qleak during the study. This might have occurred if the catheter shifted due to a change in the subject position. In these cases, an initial constant value of Qleak was changed to another constant value at a certain time to better fit the lumbar Aβ concentration data.
The loss of CSF down spinal nerves, QSN, was modeled as a net flow of CSF out of each spinal SAS compartment. It was assumed that QSN was divided equally between cervical, thoracic and lumbar compartments. The full flow entered the cervical space, but 1/3 QSN left down spinal nerves within the cervical space, while 2/3 QSN continued into the thoracic compartment. In the thoracic space, 1/3 QSN left down spinal nerves within the thoracic space, while 1/3 QSN continued into the lumbar space. The final 1/3 QSN left down spinal nerves in the lumbar space. During removal of CSF and refilling of the lumbar space, it was assumed that the drop in pressure completely halted loss of CSF down spinal nerves (QSN = 0). The model-predicted cisternography half-life was sensitive to QSN. QSN was varied between 5 and 20% of QCSF. The Moriyama et al. model predicted a mean half-life for all subjects of 20 ± 2.4 h67. The mean cisternography half-life predicted by the current model best agreed with the Moriyama et al. prediction when QSN equaled 10% of QCSF (Supplementary Table 8). In a study in sheep, the average of three methods indicated that 20.6% of CSF was absorbed by spinal nerves69. Other studies have indicated higher values, e.g., 38% in resting humans54.
The loss of CSF from the cranial space (e.g., at arachnoid granulations, dural lymphatics, cribriform plate, etc.) was Qcranial = QCSF − QSN − QLP. This reflected that the outflow from the cranial space was only a part of the total CSF produced by the choroid plexus. During CSF withdrawal and refilling, QSN = 0 and QLP = QCSF, so Qcranial = 0.
Qosc and Qglymph represent the oscillatory CSF flow generated by the cardiac and respiratory cycles but are modeled as steady bidirectional flows instead of pulsatile flows due to computational and MRI data constraints. In both cases, mass transport would only occur in the presence of a concentration difference between compartments.
The exchange compartment was only relevant to Aβ42 and was implemented as in the previous steady-state model3.
The SILK isotope-labeling data were also scaled by three additional free parameters, the scaling factors (SF38, SF40, and SF42). These are believed to account for instrument calibration errors and were previously described1–3.
The Michaelis–Menten rate constants for conversion of APP to C99 + C83 and C99 to Aβ38–42 were determined by Ortega et al56. Because the rate constants were given in relative units, units for the rate constants were derived by comparison with the carefully performed study of APP conversion to C99 (see Supplementary Table 9)57. The production rate of APP (kf), Vmax,gamma38 and Vmax,gamma42 were determined from solution of the steady-state equations. An example of labeling kinetics from plasma leucine to the lumbar SAS is shown in Supplementary Fig. 4.
APP is a transmembrane protein largely expressed by neurons in the brain. It appears to react with beta-secretase on the cell exterior and gamma-secretase within endosomes79, which are both topologically extracellular. Because the Michaelis–Menten rate constants were determined in cultured cells in a relative manner56, and for beta-secretase kinetics in an absolute manner in human temporal cortex preparations57, it was assumed that all of the rate constant values were applicable to the neuronal plasma membrane found in cortical tissue. The volume of ISF was assumed to be 10% of the total gray volume80, and the total gray volume was determined for each subject by MRI (Supplementary Fig. 5). Aβ peptides were assumed to be directly released into the ISF and all other species were transmembrane spanning. The intracellular concentration of Aβ peptides was assumed to be zero and the volume of endosome/lysosomes to be negligible.
The parameter Qglymph accounts for the exchange of Aβ between CSF and ISF but was not well defined by the SILK data when set as a free parameter. The concentration “gradient” [Aβ40]ISF/[Aβ40]lumbar was sensitive to the glymphatic exchange flow rate parameter (Qglymph). In pilot studies, when Qglymph was decreased from QCSF to QCSF/2, the lumbar-to-ISF concentration rise increased from 9.6 ± 6.9 to 14 ± 11. Further decreasing Qglymph by setting it equal to Qosc (slightly less than QCSF/2) resulted in a lumbar-to-ISF concentration rise of 71 ± 130. Given the similarity between Aβ concentrations measured in homogenized cortex and CSF, lower values of Qglymph were ruled out and Qglymph was set equal to QCSF for all subjects. The governing assumption was that soluble Aβ exists entirely in ISF and that homogenization of cortical tissue dilutes the ISF about tenfold with intracellular fluids, thus decreasing the measured concentration by a factor of 10. With this assumption, cortical ISF concentrations are predicted to be tenfold higher than those measured in the homogenized cortex. In the final model with Qglymph = QCSF, the mean lumbar-to-ISF concentration rise was 10 ± 6.6.
For each equation shown (except for dVSP3/dt), isotopically labeled and unlabeled peptides were modeled separately. The APP production rate (kf) was scaled by fLeu (the fraction of labeled leucine) for production of labeled APP, and scaled by (1 − fLeu) for production of unlabeled APP. It was assumed that the fraction of labeled leucine in ISF was the same as the fraction of labeled leucine in plasma, which was measured hourly for the first 13 h, then at 17 and 35 h. The tail of the plasma leucine labeling curve was fit to an exponential model2.
Statistics and reproducibility
Statistical analyses were performed in R and comparisons were by two-sided t test with unequal variances or ANOVA with Tukey post hoc analysis. Errors are standard deviations except where noted. Box plots show: center line = median; box limits = upper and lower quartiles; whiskers = 1.5× interquartile range; points = outliers. Confidence interval region = 95%. Measurements were taken from distinct samples. Replicates are biological replicates.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This study was supported by the National Institute on Aging (NIA) grant NIH R01NS065667 (R.J.B.), R01AG054567-01A1 (T.L.S.B.), P01AG003991 (T.L.S.B.), Washington University Nutrition Obesity Research Center (NIH DK 056341; B.W.P.), NIH K76 AG054863 (B.P.L.), and the Cockrell School of Engineering at the University of Texas at Austin.
Author contributions
All authors contributed to the conception of the project. D.L.E developed the current model, wrote the software, and performed statistical analysis on the results. B.W.P. developed the steady-state model and assisted with the development of the current model and data/statistical analysis. B.P.L. and R.J.B. aided in improving the physiological relevance of the model. T.L.S.B. was responsible for MRI data acquisition and analysis. R.J.B. was responsible for SILK data acquisition and mass spectrometric analysis. D.L.E. wrote the manuscript and all authors edited the manuscript.
Peer review
Peer review information
Communications Biology thanks Ian Harrison and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editors: Karli Montague-Cardoso.
Data availability
De-identified source data (Supplementary Data 1) and model results (Supplementary Data 2 and 3) are included in the Supplementary Materials.
Code availability
Code deposited at https://zenodo.org/badge/latestdoi/437570053.
Competing interests
The authors declare the following competing interests: D.L.E consults for DIAN-TU, which did not support this work. Washington University and R.J.B. have an equity ownership interest in C2N Diagnostics and received royalty income based on SILK technology licensed by Washington University to C2N Diagnostics. R.J.B. receives income from C2N Diagnostics for serving on the scientific advisory board. Washington University, with R.J.B. as co-inventor, has submitted the US nonprovisional patent application “Methods for Measuring the Metabolism of CNS Derived Biomolecules In Vivo” and provisional patent application “Plasma Based Methods for Detecting C.N.S. Amyloid Deposition.” C2N Diagnostics did not support this work. T.B. has served on advisory boards for Eli Lilly, Eisai and Biogen, is on the Biogen Speakers Bureau, and has received research funding from Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly). B.P.L. consults for Merck. These relationships are not related to the content in the manuscript. The remaining author declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-03037-0.
References
- 1.Potter R, et al. Increased in vivo amyloid-β42 production, exchange, and loss in presenilin mutation carriers. Sci. Transl. Med. 2013;5:189ra77–189ra77. doi: 10.1126/scitranslmed.3005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson BW, et al. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann. Neurol. 2015;78:439–453. doi: 10.1002/ana.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbert DL, Patterson BW, Bateman RJ. Analysis of a compartmental model of amyloid beta production, irreversible loss and exchange in humans. Math. Biosci. 2015;261:48–61. doi: 10.1016/j.mbs.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman RJ, et al. A γ-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann. Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobrowolska JA, et al. CNS amyloid-β, soluble APP-α and -β kinetics during BACE inhibition. J. Neurosci. 2014;34:8336–8346. doi: 10.1523/JNEUROSCI.0540-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook JJ, et al. Neurobiology of disease acute γ-secretase inhibition of nonhuman primate CNS shifts amyloid precursor protein (APP) metabolism from amyloid-β production to alternative APP fragments without amyloid-β rebound. J. Neurosci. 2010;30:6743–6750. doi: 10.1523/JNEUROSCI.1381-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-β levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68:666–669. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- 8.Li J, et al. Effect of human cerebrospinal fluid sampling frequency on amyloid-β levels. Alzheimer’s Dement. 2012;8:295–303. doi: 10.1016/j.jalz.2011.05.900. [DOI] [PubMed] [Google Scholar]
- 9.Lucey BP, et al. Associations between β-amyloid kinetics and the β-amyloid diurnal pattern in the central nervous system. JAMA Neurol. 2017;74:207–215. doi: 10.1001/jamaneurol.2016.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucey BP, et al. Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann. Neurol. 2018;83:197–204. doi: 10.1002/ana.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karelina T, et al. A translational systems pharmacology model for Aβ kinetics in mouse, monkey, and human. CPT Pharmacometrics Syst. Pharmacol. 2017;6:666–675. doi: 10.1002/psp4.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoore M, Khailaie S, Montaseri G, Mitra T, Meyer-Hermann M. Mathematical model shows how sleep may affect amyloid-β fibrillization. Biophys. J. 2020;119:862–872. doi: 10.1016/j.bpj.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertsch M, Franchi B, Marcello N, Tesi MC, Tosin A. Alzheimer’s disease: a mathematical model for onset and progression. Math. Med. Biol. 2017;34:193–214. doi: 10.1093/imammb/dqw003. [DOI] [PubMed] [Google Scholar]
- 14.Buishas J, Gould IG, Linninger AA. A computational model of cerebrospinal fluid production and reabsorption driven by Starling forces. Croat Med J. 2014;55:481–497. doi: 10.3325/cmj.2014.55.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez AL, et al. On the bulk motion of the cerebrospinal fluid in the spinal canal. J. Fluid Mech. 2018;841:203–227. [Google Scholar]
- 16.Bottan S, Poulikakos D, Kurtcuoglu V. Phantom model of physiologic intracranial pressure and cerebrospinal fluid dynamics. IEEE Trans. Biomed. Eng. 2012;59:1532–1538. doi: 10.1109/TBME.2012.2187448. [DOI] [PubMed] [Google Scholar]
- 17.Asgari, M., Zélicourt, D. De & Kurtcuoglu, V. Glymphatic solute transport does not require bulk flow. Nat. Publ. Gr. 1–11 10.1038/srep38635 (2016). [DOI] [PMC free article] [PubMed]
- 18.Deane R, Bell R, Sagare A, Zlokovic B. Clearance of amyloid-β peptide across the blood-brain barrier: implication for therapies in Alzheimers disease. CNS Neurol. Disord. Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemere, C. A. et al. Sequence of deposition of heterogeneous amyloid β-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol. Dis.32, 16–32 (1996). [DOI] [PubMed]
- 20.Louveau A, et al. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Investig. 2017;127:3210–3219. doi: 10.1172/JCI90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herukka SK, et al. Amyloid-β and tau dynamics in human brain interstitial fluid in patients with suspected normal pressure hydrocephalus. J. Alzheimer’s Dis. 2015;46:261–269. doi: 10.3233/JAD-142862. [DOI] [PubMed] [Google Scholar]
- 22.Mestre H, Mori Y, Nedergaard M. The brain’s glymphatic system: current controversies. Trends Neurosci. 2020;43:458–466. doi: 10.1016/j.tins.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the’glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife. 2017;6:1–16. doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith AJ, Verkman AS. The ‘glymphatic’ mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB J. 2018;32:543–551. doi: 10.1096/fj.201700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holter KE, et al. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc. Natl Acad. Sci. USA. 2017;114:9894–9899. doi: 10.1073/pnas.1706942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem. Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Faghih, M. M. & Sharp, M. K. Is bulk flow plausible in perivascular, paravascular and paravenous channels? Fluids Barriers CNS 1–10 10.1186/s12987-018-0103-8 (2018). [DOI] [PMC free article] [PubMed]
- 28.Fishman, R. A. Cerebrospinal Fluid in Diseases of the Nervous System, 2nd edn. (W.B. Saunders, 1992).
- 29.Orešković D, Klarica M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res. Rev. 2010;64:241–262. doi: 10.1016/j.brainresrev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Schroth G, Klose U. Cerebrospinal fluid flow - I. Physiology of cardiac-related pulsation. Neuroradiology. 1992;35:1–9. doi: 10.1007/BF00588270. [DOI] [PubMed] [Google Scholar]
- 31.Howden L, et al. Three-dimensional cerebrospinal fluid flow within the human ventricular system. Comput. Methods Biomech. Biomed. Engin. 2008;11:123–133. doi: 10.1080/10255840701492118. [DOI] [PubMed] [Google Scholar]
- 32.Schroth G, Klose U. Cerebrospinal fluid flow II. Physiology of respiration-related pulsations. Neuroradiology. 1992;35:10–15. doi: 10.1007/BF00588271. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Soellinger M, Boesiger P, Poulikakos D, Kurtcuoglu V. Three-dimensional computational modeling of subject-specific cerebrospinal fluid flow in the subarachnoid space. J. Biomech. Eng. 2009;131:1–11. doi: 10.1115/1.3005171. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, et al. Cerebrospinal fluid dynamics in the human cranial subarachnoid space: an overlooked mediator of cerebral disease. I. Computational model. J. R. Soc. Interface. 2010;7:1195–1204. doi: 10.1098/rsif.2010.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hettiarachchi HDM, Hsu Y, Harris TJ, Linninger AA. The effect of pulsatile flow on intrathecal drug delivery in the spinal canal. Ann. Biomed. Eng. 2011;39:2592–2602. doi: 10.1007/s10439-011-0346-x. [DOI] [PubMed] [Google Scholar]
- 36.Khani M, et al. Anthropomorphic model of intrathecal cerebrospinal fluid dynamics within the spinal subarachnoid space: spinal cord nerve roots increase steady-streaming. J. Biomech. Eng. 2018;140:081012. doi: 10.1115/1.4040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stonestreet P, Van Der Veeken PMJ. The effects of oscillatory flow and bulk flow components on residence time distribution in baffled tube reactors. Chem. Eng. Res. Des. 1999;77:671–684. [Google Scholar]
- 38.Cohen SIA, et al. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. USA. 2013;110:9758–9763. doi: 10.1073/pnas.1218402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storck SE, et al. Endothelial LRP1 transports amyloid-β 1–42 across the blood-brain barrier. J. Clin. Investig. 2016;126:123–136. doi: 10.1172/JCI81108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts KF, et al. Amyloid-β efflux from the central nervous system into the plasma. Ann. Neurol. 2014;76:837–844. doi: 10.1002/ana.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deane R, et al. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Silverberg GD, et al. Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. J. Neuropathol. Exp. Neurol. 2010;69:1034–1043. doi: 10.1097/NEN.0b013e3181f46e25. [DOI] [PubMed] [Google Scholar]
- 43.Zuroff L, Daley D, Black KL, Koronyo-Hamaoui M. Clearance of cerebral Aβ in Alzheimer’s disease: reassessing the role of microglia and monocytes. Cell. Mol. Life Sci. 2017;74:2167–2201. doi: 10.1007/s00018-017-2463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dukart J, et al. Relationship between imaging biomarkers, age, progression and symptom severity in Alzheimer’s disease. NeuroImage Clin. 2013;3:84–94. doi: 10.1016/j.nicl.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daianu M, et al. An advanced white matter tract analysis in frontotemporal dementia and early-onset Alzheimer’s disease. Brain Imaging Behav. 2016;10:1038–1053. doi: 10.1007/s11682-015-9458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landin-Romero R, et al. Disease-specific patterns of cortical and subcortical degeneration in a longitudinal study of Alzheimer’s disease and behavioural-variant frontotemporal dementia. Neuroimage. 2017;151:72–80. doi: 10.1016/j.neuroimage.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 47.Cash DM, et al. The pattern of atrophy in familial Alzheimer disease Volumetric MRI results from the DIAN study. Neurology. 2013;81:1425–1433. doi: 10.1212/WNL.0b013e3182a841c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bezanson J, Edelman A, Karpinski S, Shah VB. Julia: A fresh approach to numerical computing. SIAM Rev. 2017;59:65–98. [Google Scholar]
- 49.Rackauckas, C. & Nie, Q. Differentialequations. jl–a performant and feature-rich ecosystem for solving differential equations in julia. J. Open Res. Softw. 5, 15 https://openresearchsoftware.metajnl.com/articles/10.5334/jors.151/# (2017).
- 50.Mogensen PK, Riseth AN. Optim: a mathematical optimization package for Julia. J. Open Source Softw. 2018;3:615. [Google Scholar]
- 51.Alperin N, Bagci AM, Lee SH, Lam BL. Automated quantitation of spinal CSF volume and measurement of craniospinal CSF redistribution following lumbar withdrawal in idiopathic intracranial hypertension. Am. J. Neuroradiol. 2016;37:1957–1963. doi: 10.3174/ajnr.A4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chazen JL, et al. Automated segmentation of MR imaging to determine normative central nervous system cerebrospinal fluid volumes in healthy volunteers. Clin. Imaging. 2017;43:132–135. doi: 10.1016/j.clinimag.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Sass LR, et al. A 3D subject-specific model of the spinal subarachnoid space with anatomically realistic ventral and dorsal spinal cord nerve rootlets. Fluids Barriers CNS. 2017;14:1–16. doi: 10.1186/s12987-017-0085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edsbagge M, Tisell M, Jacobsson L, Wikkelso C. Spinal CSF absorption in healthy individuals. AJP Regul. Integr. Comp. Physiol. 2004;287:R1450–R1455. doi: 10.1152/ajpregu.00215.2004. [DOI] [PubMed] [Google Scholar]
- 55.Wildburger, N. C. et al. Amyloid-β Plaques in clinical Alzheimer’s disease brain incorporate stable isotope tracer in vivo and exhibit nanoscale heterogeneity. Front. Neurol. 9, 169 (2018). [DOI] [PMC free article] [PubMed]
- 56.Ortega F, Stott J, Visser SAG, Bendtsen C. Interplay between α-, β-, and γ-secretases determines biphasic amyloid-β protein level in the presence of a γ-secretase inhibitor. J. Biol. Chem. 2013;288:785–792. doi: 10.1074/jbc.M112.419135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stockley JH, Ravid R, Neill CO. Altered β-secretase enzyme kinetics and levels of both BACE1 and BACE2 in the Alzheimer’s disease brain. FEBS Lett. 2006;580:6550–6560. doi: 10.1016/j.febslet.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 58.Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 59.Terry RD, Katzman R. Life span and synapses: will there be a primary senile dementia? Neurobiol. Aging. 2001;22:347–348. doi: 10.1016/s0197-4580(00)00250-5. [DOI] [PubMed] [Google Scholar]
- 60.Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- 61.May C, et al. Cerebrospinal fluid production is reduced in healthy aging. Neurology. 1990;40:500–503. doi: 10.1212/wnl.40.3_part_1.500. [DOI] [PubMed] [Google Scholar]
- 62.Ekstedt J. CSF hydrodynamic studies in man. J. Neurol. Neurosurg. Psychiatry. 1977;40:105–119. doi: 10.1136/jnnp.40.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoquart-ElSankari S, et al. Aging effects on cerebral blood and cerebrospinal fluid flows. J. Cereb. Blood Flow Metab. 2007;27:1563–1572. doi: 10.1038/sj.jcbfm.9600462. [DOI] [PubMed] [Google Scholar]
- 64.Rogers WJ, et al. Age-associated changes in regional aortic pulse wave velocity. J. Am. Coll. Cardiol. 2001;38:1123–1129. doi: 10.1016/s0735-1097(01)01504-2. [DOI] [PubMed] [Google Scholar]
- 65.Pyykkö OT, et al. Cerebrospinal fluid biomarker and brain biopsy findings in idiopathic normal pressure hydrocephalus. PLoS ONE. 2014;9:e91974. doi: 10.1371/journal.pone.0091974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reiber H. Blood-cerebrospinal fluid (CSF) barrier dysfunction means reduced CSF flow not barrier leakage—conclusions from CSF protein data. Arq. Neuropsiquiatr. 2021;79:56–67. doi: 10.1590/0004-282X-anp-2020-0094. [DOI] [PubMed] [Google Scholar]
- 67.Moriyama E, Ogawa T, Nishida A, Ishikawa S, Beck H. Quantitative analysis of radioisotope cisternography in the diagnosis of intracranial hypotension. J. Neurosurg. 2004;101:421–426. doi: 10.3171/jns.2004.101.3.0421. [DOI] [PubMed] [Google Scholar]
- 68.Eide PK, Vatnehol SAS, Emblem KE, Ringstad G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci. Rep. 2018;8:7194. doi: 10.1038/s41598-018-25666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bozanovic-Sosic R, Mollanji R, Johnston MG. Spinal and cranial contributions to total cerebrospinal fluid transport. Am. J. Physiol. Integr. Comp. Physiol. 2017;281:R909–R916. doi: 10.1152/ajpregu.2001.281.3.R909. [DOI] [PubMed] [Google Scholar]
- 70.Ringstad, G., Are, S., Vatnehol, S. & Eide, P. K. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain140, 2691–2705 (2017). [DOI] [PMC free article] [PubMed]
- 71.Lindstrøm EK, Ringstad G, Mardal KA, Eide PK. Cerebrospinal fluid volumetric net flow rate and direction in idiopathic normal pressure hydrocephalus. NeuroImage Clin. 2018;20:731–741. doi: 10.1016/j.nicl.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magnus, L. et al. Apparent diffusion coefficient estimates based on 24 h tracer movement support glymphatic transport in human cerebral cortex. 1–12 10.1038/s41598-020-66042-5 (2020). [DOI] [PMC free article] [PubMed]
- 73.Wang J, Dickson DW, Trojanowski JQ, Lee VM-Y. The levels of soluble versus insoluble brain Aβ distinguish Alzheimer’s disease from normal and pathologic aging. Exp. Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 74.Freeman SH, Raju S, Hyman BT, Frosch MP, Irizarry MC. Plasma Aβ levels do not reflect brain Aβ levels. J. Neuropathol. Exp. Neurol. 2007;66:264–271. doi: 10.1097/NEN.0b013e31803d3ae4. [DOI] [PubMed] [Google Scholar]
- 75.Lue L, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am. J. Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naslund J, et al. Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. J. Am. Med. Assoc. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 77.McDonald JM, Cairns NJ, Taylor-Reinwald L, Holtzman D, Walsh DM. The levels of water-soluble and triton-soluble Aβ are increased in Alzheimer’s disease brain. Brain Res. 2012;1450:138–147. doi: 10.1016/j.brainres.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewis H, et al. Quantification of Alzheimer pathology in ageing and dementia: Age-related accumulation of amyloid-β(42) peptide in vascular dementia. Neuropathol. Appl. Neurobiol. 2006;32:103–118. doi: 10.1111/j.1365-2990.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 79.Israel MA, et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bender B, Kiose U. Cerebrospinal fluid and interstitial fluid volume measurements in the human brain at 3T with EPI. Magn. Reson. Med. 2009;61:834–841. doi: 10.1002/mrm.21915. [DOI] [PubMed] [Google Scholar]
- 81.Lescot T, et al. A quantitative computed tomography assessment of brain weight, volume, and specific gravity in severe head trauma. Intensive Care Med. 2005;31:1042–1050. doi: 10.1007/s00134-005-2709-y. [DOI] [PubMed] [Google Scholar]
- 82.Brody DL, et al. Amyloid-β dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4:147ra111–147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Da Mesquita SDA, Fu Z, Kipnis J. Perspective the meningeal lymphatic system: a new player in neurophysiology. Neuron. 2018;100:375–388. doi: 10.1016/j.neuron.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Helmond Z, Miners JS, Kehoe PG, Love S. Higher soluble amyloid β concentration in frontal cortex of young adults than in normal elderly or Alzheimer’s disease. Brain Pathol. 2010;20:787–793. doi: 10.1111/j.1750-3639.2010.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang G, et al. Staging biomarkers in preclinical autosomal dominant Alzheimer’s disease by estimated years to symptom onset. Alzheimer’s Dement. 2019;15:506–514. doi: 10.1016/j.jalz.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iacono MI, et al. MIDA: a multimodal imaging-based detailed anatomical model of the human head and neck. PLoS ONE. 2015;10:e0124126. doi: 10.1371/journal.pone.0124126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
De-identified source data (Supplementary Data 1) and model results (Supplementary Data 2 and 3) are included in the Supplementary Materials.
Code deposited at https://zenodo.org/badge/latestdoi/437570053.