Abstract
Treatment of chronic hepatitis B virus (HBV) infection with lamivudine is associated with the appearance in the circulation of HBV variants with mutations in the YMDD (tyrosine, methionine, aspartate, aspartate) motif of the polymerase gene. Fluorometric real-time PCR with the LightCycler assay was used for the detection of resistant variants. Differences in the hybridization melting curve kinetics of probes bound to the sequences encoding the wild-type or the mutant YMDD motifs (YIDD or YVDD in which the methionine residue is altered to an isoleucine or a valine, respectively) distinguished the single-base changes responsible for the resistance phenotype. The LightCycler probe hybridization assay was applied to 40 serum specimens from 19 patients, and the results were correlated with the nucleotide sequences determined for the corresponding PCR products. All three variants could be identified in the specimens. PCR clones obtained from four patients early in the course and prior to lamivudine therapy were investigated for the appearance of YIDD and YVDD variants with the LightCycler assay. In one patient, a transient appearance of the YIDD variant was observed 6 weeks into therapy. Subsequently, after 11 months of lamivudine therapy, the YVDD variant emerged in that patient.
Hepatitis B virus (HBV) infection is a major health problem, with about 5% of the world's population being chronically infected. Of these, about 1 million die each year due to progression to cirrhosis or hepatocellular carcinoma (12). The nucleoside analogue lamivudine (2′,3′-dideoxy-3′-thiacytidine) is effective in inhibiting HBV replication (7) by inhibiting viral DNA synthesis through chain termination (18). After 1 year of lamivudine therapy, HBV DNA remains undetectable by solution hybridization in up to 60% of patients (15). Unfortunately, by this time resistance has emerged in about 14% of patients, and a rise in serum HBV DNA concentrations occurs (10).
Lamivudine resistance is associated with the appearance of HBV variants with mutations in the highly conserved tyrosine, methionine, aspartate, aspartate (YMDD) motif of the HBV polymerase gene (3, 11, 19). Two types of polymerase gene mutations are most frequently observed: group 1 contains two mutations at amino acids 528 and 552 (L528M and M552V), which invariably occur together; the mutation at codon 552 specifies the YVDD variant. Group 2 contains a mutation only at amino acid 552 (M552I), which specifies the YIDD variant. The YIDD and YVDD variants are equally resistant to lamivudine (1).
Fluorometric real-time PCR has been reported to detect single nucleotide (nt) mutations (21). Here we describe rapid PCR amplification across the YMDD-encoding gene locus and analysis of the hybridization kinetics of an integrated probe to infer its sequence. The detection of mutations that result in the lamivudine-resistant phenotype by the LightCycler assay can be completed in 20 to 30 min for up to 25 samples. Preparation of the polymerase gene PCR product requires a further 3 to 5 h.
MATERIALS AND METHODS
Patients.
We first validated the fluorometric hybridization assay by analyzing serum HBV DNA of known sequence that belonged to three groups: (i) YVDD (13 samples from 5 patients), (ii) YIDD (six samples from 2 patients), and (iii) wild type (wt) YMDD (21 samples from 12 patients).
Longitudinal samples collected from four patients (patients 1, 2, 3, and 4) with chronic HBV infection who were treated with lamivudine (100 mg/day) were then studied.
Extraction and quantification of HBV DNA.
HBV DNA was extracted from serum using the QIAamp DNA mini kit (Qiagen Ltd., Crawley, United Kingdom). The Roche Amplicor HBV monitor assay (Roche Diagnostics, Basel, Switzerland) was used to quantitate the level of HBV DNA in serum (sensitivity, 400 copies/ml).
Conventional PCR and cloning.
Heminested PCR was used to amplify a 399-bp region of the polymerase gene, between nt 395 and nt 794. Reaction mixes contained the Expand High Fidelity PCR system (Boehringer Mannheim Biochemicals, Mannheim, Germany) buffer and enzyme mix (0.69 U), MgCl2 (5 mM), deoxynucleoside triphoshates (0.125 mM each) and 12 pmol of each primer. The first-round primers were 5′-GGA TGT GTC TGC GGC GTT T-3′ (nts 377 to 395) and 5′-ACC CCA TCT TTT TGT TTT GTT AGG-3′ (nts 840 to 863). The second-round primers were 5′-GGA TGT GTC TGC GGC GTT T-3′ (nts 377 to 395) and 5′-CAA AAG AAA ATT GGT AAC AGC GGT A-3′ (nts 794 to 818). The final volume of this PCR reaction was 25 μl, which included 2 μl of DNA extract (first round) or 1 μl of first-round product. Thermal cycling was performed using the following conditions: initial incubation at 94°C for 60 s and then 25 cycles of 94°C for 30 s, 45°C for 30 s (first round) or 50°C for 30 s (second round), and 72°C for 90 s.
Amplified PCR products derived from patient samples were cloned using the TOPO TA cloning kit (Invitrogen, Groningen, The Netherlands).
PCR and biprobe hybridization assay.
The hybridization assay is based on the Bi-probe system, which uses Cy5-labeled probes in conjunction with SYBR Green I (Bio/Gene, Kimbolton, United Kingdom; 9). The LightCycler (Idaho Technology, Salt Lake City, Utah) performs real-time product detection by measuring fluorescence changes in spectral windows with wavelengths around 540 or 675 nm. Fluorescence emitted by SYBR Green I bound to double-stranded DNA PCR products is predominantly detected in the 540-nm window. In addition, fluorescent resonance energy transfer occurs between the bound SYBR Green I and Cy5 attached to an oligonucleotide probe. Photons emitted by the Cy5 fluor are at longer wavelengths than those from SYBR Green I and are detected in the 675-nm window. The LightCycler was used for amplification of PCR clones and to determine the melting characteristics of the probe-amplicon hybrid.
Two 5′ Cy5-labeled oligonucleotide probes were designed—one matching the (wt) at nts 738 to 759 (5′-TAT ATG GAT GAT GTG GTA TTG G-3′) and the other matching a sequence variant (5′-TAT ATG GAT GAT GTG GTT TTG G-3′ [underline indicates altered nt]). The probes were biotinylated at their 3′ ends to prevent them from acting as PCR primers. The PCR reaction was performed in glass capillaries. Reaction mixes contained buffer Tris-HCI (pH 8.3; 50 mM), MgCl2 (5 mM), 0.2 mM each deoxynucleoside triphoshate (dATP, dCTP, dTTP, and dGTP), 0.4 U of Platinum Taq (Life Technologies, Paisley, Scotland), 0.1 μM sense primer (5′-CAT TTG TTC AGT GGT TCG TA 3′; nts 689 to 708), and 0.5 μM antisense primer (5′-CAA AAG AAA ATT GGT AAC AGC GGT A-3′; nts 794 to 818), 0.25 μM probe, bovine serum albumin (0.5 μg/μl), and 0.5 μl of 1:1,000 SYBR Green I. The final volume of this PCR reaction mixture was 10 μl, which included 1 μl of each 1:10 dilution of the product from conventional PCR. Thermal cycling was performed using the following conditions: 20 cycles of 94°C for 1 s, 50°C for 2 s, and 72°C for 5 s. Excess antisense primer was used to favor synthesis of the complementary strand and so enhance the annealing of probe by reducing competitive binding of the sense strand.
After real-time PCR, the amplified product was denatured by heating to 94°C, cooling rapidly to 40°C, and then heating gradually to 94°C at 0.1°C per s with constant measurement of the changing level of fluorescence. This analysis gave the melting temperature (Tm) at which 50% of the hybridizing probe was annealed to the PCR product.
DNA sequence analysis.
Amplified PCR products were purified using the Qiagen DNA purification kit (Qiagen Ltd.). DNA fragments derived from conventional PCR of serum-derived HBV DNA were sequenced on both strands with sense primer 5′-GGA TGT GTC TGC GGC GTT T-3′ (nts 377 to 395) and antisense primer 5′-CAA AAG AAA ATT GGT AAC AGC GGT A-3′ (nts 794 to 818). Cloned DNA fragments were sequenced using the sense primer 5′-CAT TTG TTC AGT GGT TCG TA-3′ (nts 689 to 708) and antisense primer 5′-CAA AAG AAA ATT GGT AAC AGC GGT A-3′ (nts 794 to 818). Sequencing was performed using an ABI 377 automated sequencer (Perkin-Elmer Ltd., Applied Biosystems Division, Warrington, United Kingdom).
RESULTS
During the Bi-probe hybridization assay, the PCR amplicon was heat denatured in the presence of probe and then rapidly cooled. Cooling of the mixture allowed the probe to hybridize to its target strand and form a double-stranded DNA zone. Some renaturation of the full-length amplicons also occurred on cooling. Photons emitted by SYBR Green I bound to zones of probe-amplicon binding excited probe-bound Cy5 by fluorescent resonant energy transfer. The fluorescence at both 540 (SYBR Green I) and 675 nm (Cy5) was measured as the temperature in the reaction capillary was slowly increased. With increasing temperature the probe-amplicon duplex and then the double-stranded amplicons were denatured, resulting in decreases in fluorescence in both detection windows.
The probes were designed to hybridize with a DNA sequence between nts 738 and 759, specifying the YMDD motif. The sequence of the Bi-probe corresponded to that of patient 1 but differed from the wt sequence by substitution of T for A at position 755. This sequence was empirically determined to be better than the wt sequence in differentiating between the Tms of wt and variant sequences.
Plots of the rate of change of fluorescence (−dF/dT) in relation to temperature gave melting curves for each variant with the peak corresponding to the Tm. Except for samples from patients 1 and 3, those carrying the YVDD variant (codon 552; valine encoded by GTG) gave a Tm of 51°C, those carrying the YIDD variant (codon 552; isoleucine encoded by ATT) gave a Tm of 49.5°C, and wt strains (codon 552; methionine encoded by ATG) gave a Tm of 55.5°C (melting curves not shown). The three sequence variants (YMDD, YVDD, and YIDD) of the strain extracted from patient 1 each gave higher Tms than the corresponding variants from other strains tested due to the exact match to the probe at nt position 755 (Fig. 1). The pretreatment strains from patient 3 also gave a different melting curve (Fig. 2).
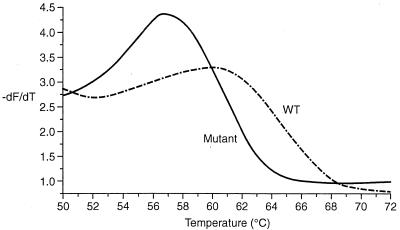
FIG. 1.
Melting curves from clones at 6 weeks into lamivudine therapy showing the wt (YMDD) and detection of a mutant (YIDD) clone for patient 1.
FIG. 2.
Melting curve analysis of the wt clones from patient 3 showing a unique pattern due to a silent mutation downstream of the YMDD motif.
For all samples containing mutations in the YMDD-specifying nt sequence, the probe disassociated from the mutant DNA template at a lower temperature than for the pretreatment (wt) DNA template. Mutations detected in this way were confirmed by DNA sequencing. The change in melting characteristics was therefore a clear and consistent marker of mutation.
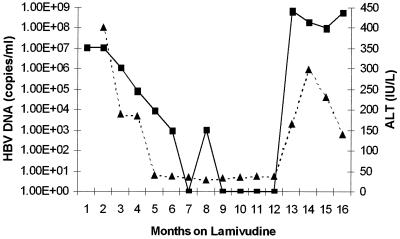
DNA sequencing across the YMDD locus showed that patients 1 and 2 had developed lamivudine-resistant viruses, YVDD mutants, by 11 and 13 months, respectively. The viruses from patients 3 and 4 developed the YIDD mutation by 13 and 28 months, respectively. Patient 1 was started on lamivudine therapy for decompensated chronic hepatitis B while awaiting liver transplantation. Serum alanine aminotransferase (ALT) became normalized although HBV DNA remained detectable in serum albeit at low levels (Fig. 3). LightCycler analysis of the 15 clones from patient 1 derived 6 weeks into lamivudine therapy demonstrated that 14 of 15 clones gave melting curves that were indistinguishable (−dF/dT maxima to within 0.5°C variation) from that of the YMDD-encoding sequence from this patient. However, the remaining clone from patient 1 gave a different melting pattern (Fig. 1). The variant clone gave a peak Tm at 57°C compared with 60°C for the remaining clones. DNA sequencing confirmed the presence of the M552I mutation in this sequence. After 11 months of lamivudine therapy, there was a rise in serum ALT and HBV DNA (Fig. 3). At this stage of lamivudine resistance, the only variant detected carried the double mutation M552V plus L526M.
FIG. 3.
Serial serum HBV DNA (copies per milliliter) (solid line) and ALT (international units per liter) (dotted line) profiles over time of lamivudine therapy for patient 1.
All 15 clones derived from serum samples taken from patients 2 and 4 prior to lamivudine therapy showed melting characteristics identical to those of the wt; i.e., no variants were identified before treatment. The 15 clones of the strain isolated before treatment of patient 3 all gave a unique probe melting curve. A “double peak” was seen with melting occurring at both 49°C and 56°C (Fig. 2). Sequence analysis of these clones revealed the wt YMDD motif and a silent T-to-C substitution at nt 756. The failure to detect the YIDD or YVDD mutants in patients 2 through 4 prior to therapy suggests that, if these mutants preexist, they do so at <1:15, i.e., <7% of the total population.
DISCUSSION
The emergence of HBV strains carrying mutations associated with lamivudine resistance is probably a result of the nature of the replication process that occurs via an RNA intermediate requiring the activity of a reverse transcriptase (6). This replicating process lacks 3′ exonuclease proofreading activity, leading to misincorporation of bases. Mutation of HBV occurs at a relatively high frequency, estimated to be 2 × 10−5 nts per year (14), compared to 10−9 nts per year for most other DNA viruses (13). The high mutation and replication rate results in a viral population containing many variants with single mutations, many of which are capable of producing their own progeny. Thus, lamivudine-resistant variants probably exist in the viral population even prior to the start of antiviral therapy, albeit at a very low level.
In this study we have shown the utility of an assay that evaluates the melting characteristics of an oligonucleotide probe hybridized to the YMDD-specifying region of the HBV polymerase gene. This assay depends on real-time PCR and could be used to rapidly identify mutations associated with lamivudine resistance. The precision of measurement of the Tm of probe-product hybrids in the LightCycler assay was sufficient to enable us to distinguish samples differing by a single nt base. Since many nt sequence variations occurred at the site of probe binding, it was not always possible to predict the phenotype of any particular virus from its melting characteristics with the probe alone. This was the case with the pretreatment virus from patient 3, which gave an unusual melting profile (Fig. 2). The method was most valuable for monitoring the emergence of YMDD locus mutations under lamivudine selection. Analysis of the probe melting curves also allowed the detection of viral subpopulations with different sequences encoding the YMDD motif. However, for unequivocal detection we estimate that the subpopulation would need to comprise at least 25% of the total. The detection of smaller subpopulations relies on prior cloning of the sequences, before rapid analysis using the LightCycler, as described here.
As in HBV carriers, patients with HIV infection treated with lamivudine can develop resistance, although this occurs rapidly within 2 weeks of therapy (17, 20). Resistance is caused by mutations in the YMDD motif (codon 184) of the HIV RT gene and, similar to mutations in HBV, the methionine residue is replaced by either an isoleucine residue (M1841) or a valine residue (M184V) (4, 8, 16, 17, 20). It has been reported that the M1841 mutation in the YMDD region of HIV is a precursor to M184V in the development of resistance to lamivudine (2, 17). It is unknown whether the appearance of M5521 (YIDD) mutations in lamivudine-resistant HBV harbingers the eventual emergence of the L528M-M552V (YVDD) mutant (1). Our study with patient 1, in whom a transient appearance of YIDD was noted as early as 6 weeks posttherapy, suggested that this might be the case. HBV variants carrying the GTG or ATT mutations are likely to be present in the host viral population from a very early stage and probably exist prior to lamivudine therapy. During lamivudine therapy, replication of the wt becomes suppressed so that strains of the YVDD and YIDD variant types become dominant components of the viral population. Subsequently the YVDD variant emerges as the major type in the HBV population upon acquisition of an additional L528M mutation. In HIV-infected patients treated with lamivudine, a similar outgrowth of the YVDD variant at the expense of YIDD has been described. Indeed, in HIV the YVDD variant has been shown to have a superior RT RNA-dependent DNA-polymerase function (2, 5).
The real-time PCR-hybridization technique described here is a rapid, reliable and accurate method to detect mutants arising early during lamivudine therapy. Its further application should allow improved management of patients with chronic hepatitis.
ACKNOWLEDGMENTS
We thank G. Colluci of Roche Diagnostics, Basel, Switzerland, for provision of the Roche Amplicor Monitor HBV Kits.
S.A.W. gratefully acknowledges support provided by The Peter Samuel's Royal Free Grant.
REFERENCES
- 1.Allen M I, Deslauriers M, Andrews C W, Tipples G A, Walters K A, Tyrrell D L, Brown N, Condreay L D. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine: Lamivudine Clinical Investigation Group. Hepatology. 1998;27:1670–1677. doi: 10.1002/hep.510270628. [DOI] [PubMed] [Google Scholar]
- 2.Back N K, Nijhuis M, Keulen W, Boucher C A, Oude Essink B O, van Kuilenburg A B, van Gennip A H, Berkhout B. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 1996;15:4040–4049. [PMC free article] [PubMed] [Google Scholar]
- 3.Bartholomew M M, Jansen R W, Jeffers L J, Reddy K R, Johnson L C, Bunzendahl H, Condreay L D, Tzakis A G, Schiff E R, Brown N A. Hepatitis-B-virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet. 1997;349:20–22. doi: 10.1016/S0140-6736(96)02266-0. [DOI] [PubMed] [Google Scholar]
- 4.Boucher C A, Cammack N, Schipper P, Schuurman R, Rouse P, Wainberg M A, Cameron J M. High-level resistance to (−) enantiomeric 2′-deoxy-3′-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1993;37:2231–2234. doi: 10.1128/aac.37.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer P L, Hughes S H. Analysis of mutations at position 184 in reverse transcriptase of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1995;39:1624–1628. doi: 10.1128/aac.39.7.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffin J M. Genetic diversity and evolution of retroviruses. Curr Top Microbiol Immunol. 1992;176:143–164. doi: 10.1007/978-3-642-77011-1_10. [DOI] [PubMed] [Google Scholar]
- 7.Dienstag J L, Perrillo R P, Schiff E R, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- 8.Gao Q, Gu Z, Parniak M A, Cameron J, Cammack N, Boucher C, Wainberg M A. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1993;37:1390–1392. doi: 10.1128/aac.37.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson J R, Saunders N A, Burke B, Owen R J. Novel method for rapid determination of clarithromycin sensitivity in Helicobacter pylori. J Clin Microbiol. 1999;37:3746–3748. doi: 10.1128/jcm.37.11.3746-3748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai C L, Chien R N, Leung N W, Chang T T, Guan R, Tai D I, Ng K Y, Wu P C, Dent J C, Barber J, Stephenson S L, Gray D F. A one-year trial of lamivudine for chronic hepatitis B: Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 11.Ling R, Mutimer D, Ahmed M, Boxall E H, Elias E, Dusheiko G M, Harrison T J. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996;24:711–713. doi: 10.1002/hep.510240339. [DOI] [PubMed] [Google Scholar]
- 12.Mahoney F J. Update on diagnosis, management, and prevention of hepatitis B virus infection. Clin Microbiol Rev. 1999;12:351–366. doi: 10.1128/cmr.12.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngui S L, Hallet R, Teo C G. Natural and iatrogenic variation in hepatitis B virus. Rev Med Virol. 1999;9:183–209. doi: 10.1002/(sici)1099-1654(199907/09)9:3<183::aid-rmv248>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto H, Imai M, Kametani M, Nakamura T, Mayumi M. Genomic heterogeneity of hepatitis B virus in a 54-year-old woman who contracted the infection through materno-fetal transmission. Jpn J Exp Med. 1987;57:231–236. [PubMed] [Google Scholar]
- 15.Perrillo R, Rakela J, Dienstag J, Levy G, Martin P, Wright T, Caldwell S, Schiff E, Gish R, Villeneuve J P, Farr G, Anschuetz G, Crowther L, Brown N. Multicenter study of lamivudine theapy for hepatitis B after liver transplantation: Lamivudine Transplant Group. Hepatology. 1999;29:1581–1586. doi: 10.1002/hep.510290507. [DOI] [PubMed] [Google Scholar]
- 16.Schinazi R F, Lloyd R M, Jr, Nguyen M H, Cannon D L, McMillan A, Ilksoy N, Chu C K, Liotta D C, Bazmi H Z, Mellors J W. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993;37:875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuurman R, Nijhuis M, van Leeuwen R, Schipper P, de Jong D, Collis P, Danner S A, Mulder J, Loveday C, Christopherson C, et al. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC) J Infect Dis. 1995;171:1411–1419. doi: 10.1093/infdis/171.6.1411. [DOI] [PubMed] [Google Scholar]
- 18.Severini A, Liu X Y, Wilson J S, Tyrrell D L. Mechanism of inhibition of duck hepatitis B virus polymerase by (−)-beta-l-2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1995;39:1430–1435. doi: 10.1128/aac.39.7.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tipples G A, Ma M M, Fischer K P, Bain V G, Kneteman N M, Tyrrell D L. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996;24:714–717. doi: 10.1002/hep.510240340. [DOI] [PubMed] [Google Scholar]
- 20.Tisdale M, Kemp S D, Parry N R, Larder B A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo T H, Patel B K, Smythe L D, Symonds M L, Norris M A, Dohnt M F. Identification of pathogenic Leptospira genospecies by continuous monitoring of fluorogenic hybridization probes during rapid-cycle PCR. J Clin Microbiol. 1997;35:3140–3146. doi: 10.1128/jcm.35.12.3140-3146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]