Abstract
Objective.
B cells can become activated in germinal center (GC) reactions in secondary lymphoid tissue and ectopic GCs in synovium in RA that may be TNF and lymphotoxin (LT) dependent. Here we characterized the peripheral B cell compartment longitudinally during anti-TNF therapy in RA.
Methods.
Participants were randomized in a 2:1 ratio to receive standard dosing regimens of etanercept (n=43) or adalimumab (n=20) for 24 weeks. Eligible participants met the 1987 ACR criteria for RA, clinically active (DAS28>4.4), and on stable doses of methotrexate. The primary mechanistic endpoint was the change in switched memory B cell fraction from baseline to week 12 in each treatment group.
Results.
B cell subsets remained surprisingly stable over the course of the study regardless of treatment group, with no significant change in memory B cells. Blockade of TNF and LT with etanercept compared to TNF blockade alone with adalimumab did not translate into significant differences in clinical response. Multiple activated B cell populations including CD21- double negative memory and activated naive were higher in RA non-responders (NR) at all time points, and CD95+ activated B cells increased with anti-TNF in the NR group. In contrast, transitional B cells- a putative regulatory subset- were lower in the NRs.
Conclusions.
Overall, our results support that peripheral blood B cell subsets are remarkably stable in RA and not differentially impacted by dual blockade of TNF and LT with etanercept or single blockade of TNF with adalimumab. Activated B cells do associate with a less robust response.
The trial is registered at ClinicalTrials.gov, number NCT00837434
Keywords: Rheumatoid arthritis, B cells, anti-TNF, biomarkers
INTRODUCTION
Rheumatoid arthritis (RA) is an inflammatory joint disease affecting 1.5 million people in the United States and associated with substantial morbidity and mortality (1). Although multiple cell types play a role in the pathogenesis of RA, the key participation of B cells has long been appreciated since the discovery of rheumatoid factor (RF) and has been re-highlighted over the past several years. Thus, RF and anti-cyclic-citrullinated peptide (anti-CCP) autoantibodies are well-established indicators of disease and disease severity and may precede the onset of disease by many years (2–4). The efficacy of B cell depletion therapy further highlights the pathogenic significance of B cells in RA (5–7). B cells may provide a critical link between the development of tertiary lymphoid structure within the inflamed synovium and the propagation of the autoimmune process. This connection is supported by the finding of germinal center (GC)-like structures within the inflamed RA synovium and the observation that T cell activation in the RA synovium is dependent on the presence of B cells within these active GCs (8, 9).
TNF α is also clearly established as a central player in the pathogenesis of RA. The current paradigm regarding the mechanism of action of TNF blockade in RA focuses on the pro-inflammatory effects of TNF. Indeed, TNF is a sentinel pro-inflammatory cytokine in normal immune responses and pathologically in the RA synovium. Along with IL1 it orchestrates many of the pathophysiological abnormalities that characterize RA including the local effects of inflammation and the development of joint damage (10). Inhibiting TNF interrupts the disease process by blocking the activation of T cells, macrophages, and synovial fibroblasts. In addition to TNF, Lymphotoxin alpha (LTα) and beta (LTβ) are two related TNF superfamily members that have been shown to be increased in RA serum and synovial tissue (11–13). Notably, in mice, LT signaling is particularly critical for the development and maintenance of normal spleen and lymph node microarchitecture (14). LT signaling has also been associated with lymphoid aggregates in the synovium of RA patient (11, 12). Signaling by TNF and LT is required for the development of follicular dendritic cells (FDCs), the cells that are responsible for the initiation of secondary lymphoid GC structures (15, 16). Despite the potential for TNF and LT to directly signal through TNFR1 and TNFR2 expressed on B cells and indirectly impact B cell activation via promotion of tissue lymphoid aggregates, the effect of TNF blockade on B cells in RA is not well characterized.
We have previously reported in an observational cross-sectional study that RA patients on anti-TNF (etanercept) display a paucity of tonsil FDC networks and GC structures accompanied by a reduction in peripheral blood memory B cells compared with healthy controls and RA patients on methotrexate, suggesting that the combination of TNF and LT blockade may disrupt GC reactions at least in part via effects on FDCs (17). Despite these findings, the precise in vivo effects of blockade of TNF and LTα signaling pathways on human B cells remain unclear as careful longitudinal studies after anti-TNF initiation are few (18). Moreover, the potential relationship between B cell changes and the efficacy of TNF blockade requires better elucidation. This study of active RA patients on concurrent methotrexate was undertaken to evaluate the hypothesis that, due to etanercept’s ability to block both TNF and LTα, it would have more profound effects on B cell populations relative to adalimumab which only blocks TNF.
METHODS
Study design.
The study was a phase IV, randomized, partially blinded, multi-center trial. Eligible participants were randomized in a 2:1 ratio to receive standard dosing regimens of etanercept or adalimumab for 24 weeks. Participants were randomized to receive either one injection of etanercept 50 mg (or 2 injections of etanercept 25 mg on the same day), SQ every week for 24 weeks or one injection of adalimumab 40 mg, SQ every other week. The drug was either self-administered or administered by a trained designated caregiver and administered at approximately the same time of the same day every week for etanercept or every other week for adalimumab, in the form of one or two injections (per dosage regimen). Randomization was conducted through a web-based system, RhoRAND, and was stratified based on the presence or absence of antibodies to RF and/or CCP with a block size of 3. Participants met ACR 1987 revised criteria for the classification of RA for at least 3 months, had active disease with DAS28>4.4, and were on stable doses of MTX for at least 8 weeks (19). Participants were clinically evaluated and blood was drawn for processing at baseline, 12 weeks, and 24 weeks.
Disease endpoints were evaluated by assessors blinded to the treatment status. The DAS response was determined as follows: a decrease in the DAS response of ≥ 1.2 and a DAS ≤ 3.2 was considered a good responder, a decrease in DAS of < 0.6 or 0.6 to 1.2 with a DAS > 5.1 was considered a non-responder, and a moderate responder was between the two groups. ACR 20, 50, and 70 responder status was also defined (20, 21).
This trial was conducted between July 2009 and January 2014. Participants were recruited at the University of Rochester Medical Center (URMC) and other Autoimmunity of Excellence (ACE) participating sites (MUSC in Charleston, University of Alabama at Birmingham, Feinstein Institute for Medical Research, University of California San Francisco, and University of Chicago). Detailed written informed consent was obtained from all participants. Age-matched healthy donors were included from a separate URMC protocol in accordance with protocols specifically approved by the respective Human Subjects Institutional Review Board (IRB). Full detail of the trial protocol can be found in the Supplementary Appendix, available with the full text of this article.
PBMC isolation and Flow Cytometry.
Peripheral blood (PB) samples were collected in heparin tubes and shipped overnight to URMC. Peripheral blood mononuclear cells (PBMCs) were then immediately isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation (Pharmacia Biotech, Uppsala, Sweden) and frozen in freezing media until analysis. Immunofluorescence staining for flow cytometric analysis was performed. We used 2 different core B cell panels of 12-color/14-parameter flow cytometry with well-validated Standard Operating Procedures (SOPs) that incorporate extensive Quality Assessment and Quality Controls as previously described (22, 23).
B cells were identified based on CD19 expression, exclusion of CD3, and gating out cell aggregates and dead cells. Naïve B cells were distinguished from transitional cells and memory B cells by the expression of ABCB1 transporter activity and Mitotracker dye extrusion as described (24). CD21 and CD95 were incorporated to evaluate activation status (25, 26). B cells in PBMCs were additionally classified by multi-parameter flow cytometry along a developmental pathway based on the expression of defined surface markers as defined in Supplementary Figure 1. T cell subsets were gated using a separate panel from B cells with the focus on CD4 T cells. CD45RA separates T cells into memory (CD45RA-) and naïve T cell (CD45RA+). CCR6, CXCR3 and CCR4 are important markers for classifying Th1 (CD4+CD45RA-CXCR3+CCR6+), Th2 (CD4+CD45RA-CCR6-CCR4+) and Th17 (CD4+CD45RA-CCR4+CCR6+) (25, 26). Circulating T follicular helper cells (TFH) were classified as CD4+CD45RA-CXCR5+ICOS+PD1+ (27). In addition, we used CD25 and CD127 to identify natural occurring regulatory T cells in blood as CD4+CD25+CD127low/- (28).
Cytokine assays and Ki67 expression.
PBMCs were aliquoted into 1 million per 100 μl of RPMI and stimulated with 200 ng/ml PMA and 2 μg/ml Ionomycin with the addition of 1 μl/ml Golgi Plug and 0.68μl/ml Golgi Stop at 37°C for 4 hours. The PBMCs were surfaced stained for CD19 and followed with live dead staining. Cells were fixed and internally stained for TNF, IL-17, IFNγ, IL2, IL6, IL-10, CD3, CD4, and Foxp3 (eBioscience). Cells were analyzed on a three-laser 12-color LSRII (BD Biosciences).
In a subset of participants where enough cells were available, unstimulated PBMCs were surface stained for IgD, IgG, IgA, IgM, CD24, CD21, CD38, CD19, CD20, CD27, CD95, CD86, and CD3, followed by live/dead stain, fixation (0.1% Formalin), and antibody against Ki-67. Cells were analyzed on a three-laser 12-color LSRII (BD Biosciences).
Statistical analysis.
The study was powered for the primary analysis to test for a treatment group difference in change in the percentage of memory B cells in the peripheral blood at Week 12. Assumptions for the sample size calculation were based on data collected from individuals treated in Rochester. It was assumed that the change in the mean percentage of peripheral memory B cells would differ between the two treatments by between 8% and 12% and the within-treatment standard deviation (pooled across arms) would be around 10%. Sixty participants, randomized 2:1 with α=0.05, were needed for power between 81% and 95%. Participants who withdrew prior to week 12 were to be replaced.
The primary endpoint was the change from day 0 to week 12 in CD27+ switched memory B cells expressed as a percent of B cells. Primary mechanistic analyses were conducted on the Per-Protocol population (PP), which consists of participants with day 0 and week 12 assessments, who completed at least 75% of planned injections prior to week 12, and had no serious protocol deviations. Missing data were not imputed. An analysis of covariance (ANCOVA) model was used for the primary analysis comparing CD27+ switched memory B cells between treatment groups at week 12, adjusted for the day 0 value. Because we hypothesized that the percentage of CD27+ switched memory would not be impacted by adalimumab but would decline after treatment with etanercept, a key secondary analysis was to compare slopes for the regression lines describing the relationship between CD27+ switched memory B cells at week 12 and day 0 between treatment arms. To test for the equivalence of slopes, the day 0-by-treatment interaction term was added to the ANCOVA model from the primary analysis. These analyses were repeated for the subset of moderate and good DAS responders at week 12.
Secondary analyses to support the primary and secondary objectives were considered exploratory and included all randomized participants regardless of treatment group and analysis population for whom blood samples were available. p-values are presented without adjustment for multiple comparisons. A t-test was performed to test for differences in cell subsets between treatment groups, DAS response groups and to compare healthy controls to study participants at each visit. Three group comparisons were conducted using ordinary one-way ANOVA and Tukey’s multiple comparisons test. Statistical analyses were performed using Prism or SAS software.
RESULTS
Participant clinical characteristics and response.
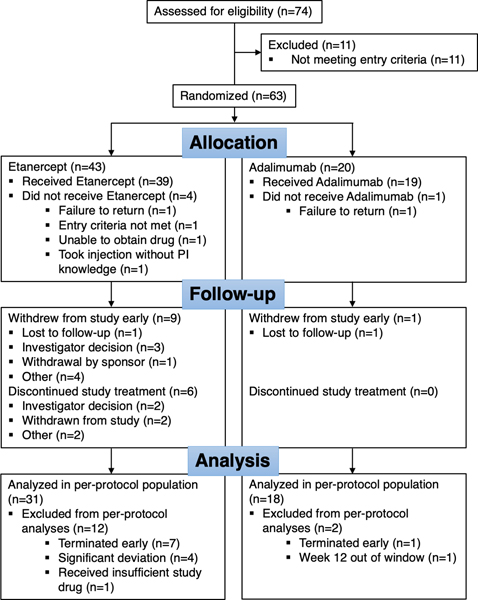
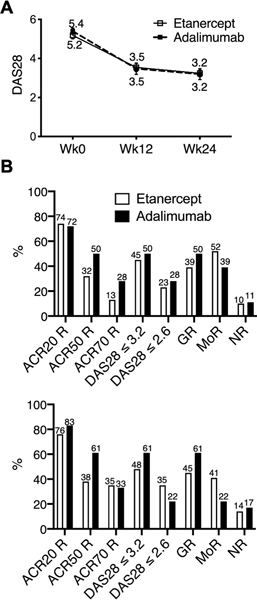
Sixty-three participants were randomized in the trial, 43 to etanercept and 20 to adalimumab. There were 49 participants in the per-protocol population. Fourteen randomized participants were excluded from the per-protocol population: 8 terminated the study early, 9 received less than 75% of the planned injections, 5 reported significant deviations, and 2 participants had the week 12 visit more than 1 week from the expected visit date (Figure 1). Some participants were excluded from the per-protocol population for more than one criterion. Baseline participant characteristics are summarized in Table 1. The treatment groups were similar with respect to CCP status and disease activity. Overall, disease activity was high at entry with a mean DAS28 of 5.3, 10 swollen joints, and 13 tender joints, typical of an RA cohort clinically failing MTX and starting biologic therapy. As shown in Figure 2 clinical responses were high with 90% of etanercept and 89% of adalimumab treated participants achieving a good or moderate DAS28 response by 12 weeks and ~45% of participants achieving low disease activity (DAS28 ≤ 3.2) by 12 weeks with numbers increasing further by 24 weeks. There were 7 DAS28 non-responders at week 12. DAS28 results over time were similar between the two treatment arms (Figure 2A). Although the study was not designed to test for a difference between treatment arms based on disease activity endpoints, responder indexes appeared modestly higher in the adalimumab group at 12 weeks with further increases at 24 weeks (Figure 2B).
Figure 1.
Flow chart showing deposition of the study participants
Table 1.
Participant baseline clinical characteristics (PP)
| Baseline characteristics | Etanercept (n=31) | Adalimumab (n=18) |
|---|---|---|
| Age Mean (SD) | 52 (10.0) | 52 (13.8) |
| Gender, Female n (%) | 25 (81) | 14 (78) |
| Caucasian n (%) | 26 (84) | 17 (94) |
| Disease duration (years) Mean (SD) | 4.5 (6.5) | 4.4 (5.4) |
| CCP+, n (%) | 19 (61) | 10 (56) |
| RF positive, n (%) | 17 (55) | 8 (44) |
| DAS Mean (SD) | 5.2 (1.1) | 5.4 (0.7) |
| MTX dose (mg) Mean (SD) | 16.8 (4.0) | 18.4 (3.5) |
| Swollen joints (of 28) Mean (SD) | 9.5 (5.9) | 9.6 (6.0) |
| Tender joints (of 28) Mean (SD) | 12.6 (6.8) | 12.9 (7.2) |
| CRP (mg/L) Mean (SD) | 11.0 (25.9) | 13.6 (25.0) |
Figure 2. Clinical response at 12 and 24 weeks.
(A) Mean DAS28 at baseline (Wk0), week 12 (Wk12) and week 24 (Wk24) by treatment group included only per-protocol participants, etanercept (n=29 Wk0, n=31 Wk12, n=29 Wk24), adalimumab (n=17 Wk0, n=18 Wk12, n=18 Wk24). Error bars represent standard error of the mean. (B) Clinical responses at 12 weeks (top) and 24 weeks (bottom) by treatment group (etanercept: n=31 Wk12, n=26 Wk24; adalimumab n=18 Wk12 and 24). GR=good responders, MoR=moderate responders, NR=non-responders. The values on the graph represent the mean.
Memory B cells in the peripheral blood are stable over 24 weeks of anti-TNF treatment.
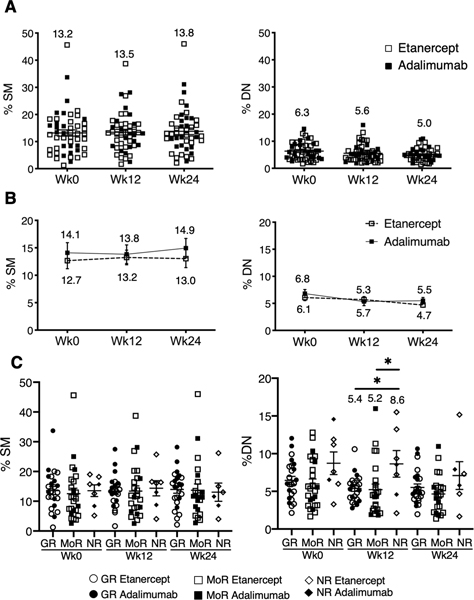
The primary endpoint, the change in CD27+ switched memory B cells between day 0 and week 12, was not significantly different between treatment groups (p=0.301) (Supplementary Table 1). The slopes describing the relationship between CD27+ switched memory B cells at week 12 versus day 0 were also not significantly different between treatment groups (p=0.996). Results were analogous for the subset of moderate and good DAS responders (p=0.286; data not shown) and also regardless of autoantibody status. As a further analysis of the B cell compartment, we examined the other canonical B cell subsets revealed by CD27 and IgD expression (Supplementary Figure 1 for gating strategy and supplementary Figure 2A) and further refined B cell populations. Overall, B cell subsets did not change over the course of the study regardless of treatment arm (Supplementary Table 1; Figures 3A and B; Supplementary Figure 2B and C) or responder status (Supplementary Table 2 and 3; Figure 3C; Supplementary Figure 3D). Non-responders did have higher CD27- DN memory (IgD-CD27-) subsets at baseline and week 12 compared to good and moderate responders (Figure 3C). Given the putative differences in drug mechanism of action, we also analyzed the B cell populations by responder status within each treatment arm. Analysis comparing responder status within the adalimumab arm over the course of the study did not reveal significant differences in frequency of SM (p=0.876), USM (p=0.318), DN (p=0.251) or naïve (p=0.686) B cells. Within the etanercept group, the frequency of DN was significantly different between non-responder and responder (higher in NR) (ANOVA p=0.0082). In contrast, there was no difference for SM (p=0.952), USM (p=0.913) or naïve (p=0.851) within etanercept arm.
Figure 3. Core B cell subsets over time.
(A) Scatter plots of frequencies of switched memory (SM), and double negative (DN) of total CD19+ B cells over time, n=49. (B) Line plot of SM, and DN frequencies over time (mean±SEM) in each treatment group. n=18 adalimumab, n=31 etanercept. (C) Scatter plots represent frequencies of SM, and DN over time for non-responders (NR: n=7 Wk0, n=7 Wk12, n=6 Wk24), good responders (GR: n=24 Wk0, n=23 Wk12, n=24 Wk24) and moderate responders (MoR: n=25 Wk0, n=24 Wk12, n=22 Wk24). Error bars depict mean±SEM. All longitudinal comparisons resulted in p-value > 0.05. In 4D, * denote p<0.05 for comparison between responder groups at the noted time points. The values on the graph represent the mean.
We also examined the frequency of plasmablasts (PB) in both treatment groups over time, but these did not change significantly regardless of treatment arm (Supplementary Figure 2B and C) or responder status (Supplementary Table 3; Supplementary Figure 2D).
Activated B cells are higher in non-responders.
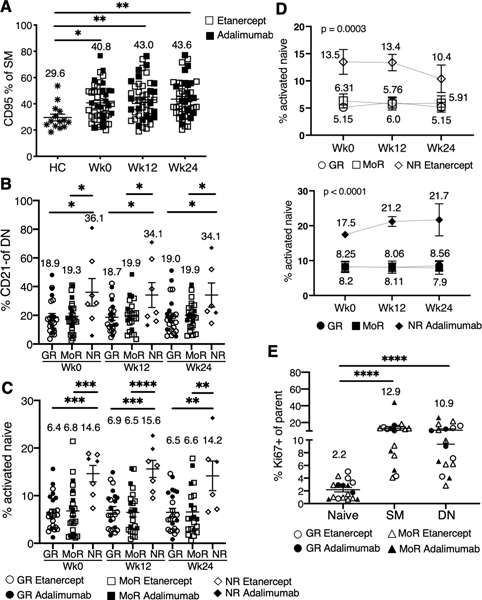
When pre-treatment RA participants were compared to age-matched healthy controls, the total naïve (IgD+CD27-) (includes transitional B cells), unswitched memory (IgD+CD27+), CD27+ switched memory (IgD-CD27+) (includes plasmablasts), and CD27- DN memory (IgD-CD27-) subsets were similar (data not shown). However, notably there were more activated B cell populations in RA as revealed by up-regulation of CD95 in both the SM and DN memory (Figure 4A; Supplementary Figure 3A-C). The baseline expansion in activated B cell populations persisted after anti-TNF treatment (Figure 4A and B; Supplementary Figure 3B). Additionally, activated CD21 negative DN memory B cells were higher at baseline and follow-up time points in the non-responder group (Figure 4B). Additionally, we observed significant differences in percentage of activated CD21- DN memory within the etanercept group (ANOVA, p= 0.0023) but not in the adalimumab group (p=0.116).
Figure 4. RA responders have lower frequencies of activated memory B cells and more proliferating B cells.
(A) Frequencies of CD95+ SM at baseline (Wk0, n=49), 12 week (Wk12, n=48) and 24 week (Wk24, n=46) compared to healthy controls (HC, n=14). *p<0.05, **p<0.01. (B) Means (±SEM) for % CD21- DN at different time points for good (GR), moderate (MoR) and non-responders (NR). *p<0.05. (C) Scatter plots showing frequencies of activated naïve B cells in GR: (n=24 Wk0, n=23 Wk12, n=23 Wk24), MoR (n=24 Wk0, n=23 Wk12, n=21 Wk24) and NR (n=7 Wk0, n=7 Wk12, n=6 Wk24). Error bars represent Mean+ SEM. **p<0.01,*** p<0.001, **** p<0.0001 by Tukey’s multiple comparison test. (D) Line plots represent frequencies of activated naïve B cells in etanercept (top) or adalimumab (bottom) group. Each line represents GR, MoR or NR over time (etanercept: n=14 GR, n=18 MoR, n=5 NR; adalimumab: n=10 GR, n=7 MoR, n=2 NR). The values on the graph represent the mean. (E) The plot is a scatter plot of frequencies of Ki67+ in naïve, SM and DN B cell from GR (n=7) or MoR (n=10) (no NR B cells available for analysis) at Wk0. Mean± SEM, ****p<0.0001 as indicated comparisons.
We also examined a recently identified population of activated naïve B cells characterized as IgD+CD27-MTG+CD38+CD24- (29). Activated naïve B cells were significantly higher in the non-responders and remained elevated throughout the course of treatment (Figure 4C) but did not change with treatment. The significant differences in percentage of activated naïve were also observed when each treatment group was analyzed separately (ANOVA: adalimumab (p<0.0001); etanercept (p=0.0003)) (Figure 4D).
Impact of anti-TNF on distinct class-switched memory or recently activated B cell subsets.
The lack of change in global memory B cell populations after anti-TNF treatment was surprising but may be explained if the majority of peripheral blood memory B cells are a relatively long-lived and stable cell pool. Previous work has demonstrated that there are multiple memory B cell subsets originating from distinct GC dependent and independent immune pathways that can be distinguished based on class-switch profile (isotype) (30). We reasoned that anti-TNF may have a greater impact on certain memory B cell populations, depending on immune pathway of origin. From a subset of participants where samples are available (n=7 good responders, n=10 moderate responders, no non-responders available) we performed additional flow cytometry analysis to address this possibility. However, none of the class-switch memory B cells examined (IgM, IgG or IgA) decreased with anti-TNF, including the CD27-IgA+ memory B cell fraction, proposed to originate from GC independent reactions possibly of mucosal origin (30) (data not shown). Ki67 is a proliferation antigen which may mark B cells recently activated in ongoing immune reactions. There were interesting differences in Ki67 expression depending on the B cell subset examined, with higher proliferation in the memory B cell compartment particularly activated memory (CD21-, CD95+, CD86+) (Figure 4E; Supplementary Figure 3D). We also examined the expression of Ki67 longitudinally with anti-TNF initiation. Although, there were changes in individual patients, overall Ki67 B cells did not change with anti-TNF (Supplementary Figure 3E, data not shown for CD95+ and CD86+)
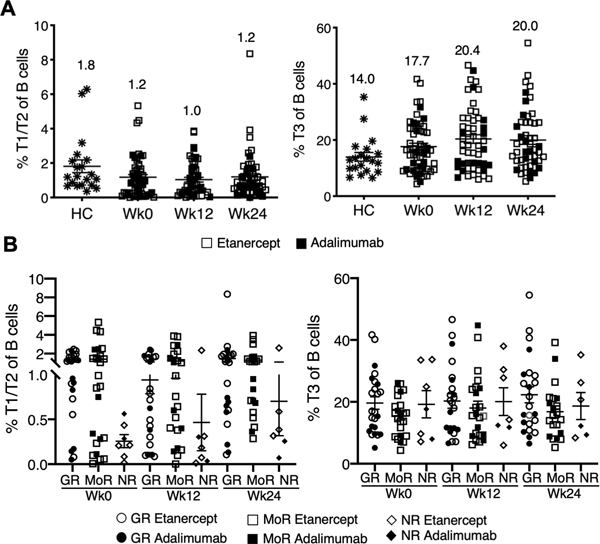
Transitional B cells vary depending on responder status.
We examined changes in transitional B cells. The naïve/transitional subset is composed of T1, T2, T3, and mature naïve B cells (31). The frequency of early transitional T1/T2 cells, a putative regulatory subset (32, 33), was higher in the RA responder group compared to the non-responders (Figure 5). However, transitional B cells did not change over the 24 weeks of treatment with anti-TNF regardless of treatment response. In a small number of participants (n=8) cytokine production by B cells was examined after short-term stimulation for 4 hours but no differences were observed in IL10, TNF, or IFN-g production (data not shown).
Figure 5. Frequency of T1/T2 B cells is higher in RA responders at all time points.
(A) Scatter plot showing frequencies of T1/T2 B cells in all participants (n= 56 Wk0, n=54 Wk12, n=52 Wk24) regardless of treatment over time in comparison to healthy controls (HC, n=20). Frequencies of T3 B cells are shown in the right scatter plot comparing HC (n=20) to all participants (n= 49 Wk0, n=48 Wk12, n=46 Wk24) over time. (B) Scatter plots demonstrate frequencies of T1/T2 B cells time in Good responder (GR: n=24 Wk0, n=24 Wk12, n=23 Wk24), moderate responder (MoR: n=24 Wk0, n=23 Wk12, n=21 Wk24) and non-responders (NR: n=7 Wk0, n=7 Wk12, n=6 Wk24) and T3 B cells over time in Good responder (GR: n=24 Wk0, n=23 Wk12, n=23 Wk24), moderate responder (MoR: n=24 Wk0, n=23 Wk12, n=21 Wk24) and non-responders (NR: n=7 Wk0, n=7 Wk12, n=6 Wk24). Mean±SEM. All comparisons resulted in p-values > 0.05. The values on the graph represent the mean.
T cell effects of TNF blockade.
We also examined T cell subsets including Th1, Th2, TFH, and T regulatory cells. They did not change over the course of the study (data not shown for Th1 and Th2, Supplementary Figure 4A and B), though Tregs were noted to be higher in the good and moderate responders at week 12 compared to the non-responders (Supplementary Figure 4C).
DISCUSSION
In this study of active RA, we found no difference in the effects of dual blockade of TNF and LT with etanercept compared to single blockade of TNF with adalimumab on peripheral blood B cell subsets. Surprisingly, peripheral blood B cell subsets remained remarkably stable after initiation of anti-TNF treatment. We suggest that once generated the bulk of memory B cells may be long-lived and not altered by TNF blockade, whereas ongoing generation of memory B cells in lymphoid tissue or ectopic locations may be more amenable to interruption. Although most B cell subsets did not change with treatment, we did observe differences depending on treatment response, with higher frequency of activated memory and activated naïve B cells in non-responders at baseline and follow-up time points. In contrast, transitional B cells, a putative regulatory subset, as well as T regulatory cells were lower in non-responders. Overall, this suggests an imbalance in the B cell compartment in RA that is heterogeneous and predictive of anti-TNF response. However, these results should be interpreted with caution as there were few non-responders in the current study.
Our results also suggest that agents which block both TNF and LT are not necessarily more efficacious than TNF blockade alone. This is in accord with clinical trial data suggesting that TNF blockade is the dominant clinical effect. Thus, a monospecific anti- LT α antibody pateleclizumab failed to show efficacy in a head-to-head phase 2 randomized controlled study (34).
Our results confirm prior findings that peripheral blood B cells are abnormal in RA. However, results in the literature have not been entirely consistent, perhaps because of variability in disease phenotypes, duration, activity, and therapy. For example, we have previously reported that in contrast to systemic lupus a disease that is characterized by profound alterations in peripheral blood B cells, RA patients have similar core peripheral blood B cell subsets to healthy controls (based on CD27 and IgD expression). However, RA patients had a significant expansion of activated memory B cell populations expressing high levels of CD95 and low levels of CD21 in the peripheral blood (23), results recapitulated here. In addition, we also observed increased frequency of IgD-CD27- DN memory B cells in non-responders compared to responders, a result consistent with data reporting increased DN B cells in early and established RA. In contrast to our data, others have described the DN expansion restored by anti-TNF therapy (35). Souto-Carneiro and colleagues described an increased frequency of post-switch CD27+IgD- peripheral blood memory B cells in RA patients with longer disease duration compared to shorter disease duration or normal controls (36), results recapitulated in another study (37, 38). They also noted an increase in the frequency of pre-switch memory B cells in the peripheral blood of RA patients after anti-TNF. These studies differed from ours as the disease duration was significantly longer (12–13 years in duration), suggesting that global peripheral blood B cell abnormalities may accumulate over time related to ongoing activation in target tissue. In accord with this hypothesis, another study reported an expansion of autoreactive B cell and plasma cell clones in the synovium in early RA (<6 months) and established RA (>20 months) that was absent from the peripheral blood (39). This suggests that the inflamed synovium forms a niche where class switched activated memory B cells and PCs accumulate, in accord with our own published data that CD27+ memory B cells dominate in the RA synovium (40).
Although total peripheral blood memory B cells did not decrease with TNF blockade over the time course of the study, the majority of peripheral blood memory B cells are likely long-lived (30, 41, 42) and may not be altered over a short time of treatment. In a smaller subset of subjects, we examined more discrete memory B cell populations including IgA and IgG memory and Ki67 expressing cells. Although we did not observe clear changes with anti-TNF, future studies with a larger sample size may be more informative. For example, CD27-IgA+ memory B cells may be a particularly interesting population to further examine as it has been proposed to be generated in T independent mucosal immune reactions (30) but may also develop in GC reactions including in ectopic locations (43, 44). Additionally highlighting the importance of IgA immune reactions, there are recent reports of IgA plasmablast dominance in CCP+ individuals at risk for developing RA (45). We previously reported that RA patients on anti-TNF (etanercept: TNF receptor-Ig p75 decoy that binds both TNF and LTα) display a paucity of follicular dendritic cell networks and germinal center (GC) structures in lymphoid tissue accompanied by a peripheral blood memory B cell lymphopenia (17). Treatment with either etanercept or anti-TNF monoclonal antibodies (adalimumab or infliximab) has also been associated with a decrease in ectopic lymphoid structures in the RA synovium that correlated with good clinical response (46). It is likely that global peripheral blood B cells do not adequately reflect the effects of TNF blockade on immune reactions in synovium or lymphoid tissue, as suggested by the finding of impaired generation of influenza vaccine specific antibody secreting and memory B cells in RA patients on anti-TNF (47).
Several candidate markers to predict response to anti-TNF therapy have been investigated, including genetic and protein markers, but their predictive power has been poor (48, 49). A recent study combined high-throughput RNA sequencing, DNA genotyping, and proteomics measurements in 185 RA patients including 59 starting anti-TNF therapy and found 2 proteins, 2 SNPs and 8 mRNA biomarkers that could be replicated from the literature and in combination explained 51% of the variation in DAS response (50). There is also interest now in RNA sequencing biomarkers within discrete cell populations as has recently been described for neutrophils (51). It has also been suggested that analysis of immune cells in target tissue may provide critical additional information (44, 52), as has been demonstrated by a myeloid phenotype predicting the most robust response to TNF blockade (53). Here we observed several peripheral B cell flow cytometry based biomarkers that associate with inadequate response to TNF blockade including CD21 low DN and switched memory B cells and activated naïve B cells. The CD21 low B cell population is particularly interesting given the recent description of age or autoimmunity associated B cells (ABCs) that encompasses this phenotype. This B cell population, dependent on T-bet for generation and expressing CD11c, was first reported in aging mice (54); subsequently it was seen to be expanded in autoimmune mice and SLE patient peripheral blood and enriched for autoreactive specificities (55). We have also recently reported the presence of ABCs in the RA synovium correlating with disease activity (44). Results from the current study overall suggest that an activated B cell compartment in RA is associated with inadequate response to TNF blockade. However, it is important to note that these biomarkers are likely not specific to anti-TNF therapy given that they are also predictive of inadequate response to B cell depletion (23). Although there are abnormalities in the RA B cell compartment detectable in peripheral blood and associated with response to anti-TNF, we suggest that analysis of cells in joint target tissue likely by a multiple-OMICs data approach has the greatest potential reveal new biomarkers of treatment response and elucidate disease pathogenesis.
Supplementary Material
ACKNOWLEDGMENT
This study was done on behalf of the Autoimmunity Centers of Excellence supported by a grant from NIAID U19 AI563262.
Funding: on behalf of the Autoimmunity Centers of Excellence supported by a grant from NIAID U19 AI563262.
The funder was involved in the design and conduct of the study. The funder assisted with preparation, review, and approval of this manuscript. Employee of Rho Federal System Division, Inc. worked with the investigators to prepare the statistical analysis plan and performed an independent statistical analysis to verify the results presented in this article.
REFERENCES
- 1.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis & Rheumatism. 2010;62(6):1576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machold KP, Stamm TA, Nell VP, Pflugbeil S, Aletaha D, Steiner G, et al. Very recent onset rheumatoid arthritis: clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology (Oxford). 2007;46(2):342–9. [DOI] [PubMed] [Google Scholar]
- 3.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis and rheumatism. 2004;50(2):380–6. [DOI] [PubMed] [Google Scholar]
- 4.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis and rheumatism. 2003;48(10):2741–9. [DOI] [PubMed] [Google Scholar]
- 5.Anolik J, Barnard J, Cappione A, Pugh-Bernard A, Felgar R, Looney J, et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis & Rheumatism. 2004;50:3580–90. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JC, Cambridge G. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford). 2001;40(2):205–11. [DOI] [PubMed] [Google Scholar]
- 7.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. The New England journal of medicine. 2004;350(25):2572–81. [DOI] [PubMed] [Google Scholar]
- 8.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167(8):4710–8. [DOI] [PubMed] [Google Scholar]
- 9.Manzo A, Bombardieri M, Humby F, Pitzalis C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol Rev. 2010;233(1):267–85. [DOI] [PubMed] [Google Scholar]
- 10.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. [DOI] [PubMed] [Google Scholar]
- 11.Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167(2):1072–80. [DOI] [PubMed] [Google Scholar]
- 13.O’Rourke KP, O’Donoghue G, Adams C, Mulcahy H, Molloy C, Silke C, et al. High levels of Lymphotoxin-Beta (LT-Beta) gene expression in rheumatoid arthritis synovium: clinical and cytokine correlations. Rheumatol Int. 2008;28(10):979–86. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y-X, Huang G, Wang Y, Chaplin DD. B Lymphocytes Induce the Formation of Follicular Dendritic Cell Clusters in a Lymphotoxin alpha -dependent Fashion. J Exp Med. 1998;187:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto M, Fu YX, Molina H, Chaplin DD. Lymphotoxin-alpha-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers. Immunological reviews. 1997;156:137–44. [DOI] [PubMed] [Google Scholar]
- 16.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. The Journal of experimental medicine. 1996;184(4):1397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anolik JH, Ravikumar R, Barnard J, Owen T, Almudevar A, Milner EC, et al. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008;180(2):688–92. [DOI] [PubMed] [Google Scholar]
- 18.Pala O, Diaz A, Blomberg BB, Frasca D. B Lymphocytes in Rheumatoid Arthritis and the Effects of Anti-TNF-alpha Agents on B Lymphocytes: A Review of the Literature. Clin Ther. 2018;40(6):1034–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and rheumatism. 1988;31(3):315–24. [DOI] [PubMed] [Google Scholar]
- 20.Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis and rheumatism. 1993;36(6):729–40. [DOI] [PubMed] [Google Scholar]
- 21.van Gestel AM, Prevoo ML, van ‘t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis and rheumatism. 1996;39(1):34–40. [DOI] [PubMed] [Google Scholar]
- 22.Wei C, Jung J, Sanz I. OMIP-003: Phenotypic analysis of human memory B cells. Cytometry A. 2011;79(11):894–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adlowitz DG, Barnard J, Biear JN, Cistrone C, Owen T, Wang W, et al. Expansion of Activated Peripheral Blood Memory B Cells in Rheumatoid Arthritis, Impact of B Cell Depletion Therapy, and Biomarkers of Response. PLoS One. 2015;10(6):e0128269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirth S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol. 2005;35:3433–41. [DOI] [PubMed] [Google Scholar]
- 25.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–46. [DOI] [PubMed] [Google Scholar]
- 26.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. The Journal of experimental medicine. 2004;200(6):725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35(9):436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu N, Li X, Song W, Li D, Yu D, Zeng X, et al. CD4(+)CD25 (+)CD127 (low/-) T cells: a more specific Treg population in human peripheral blood. Inflammation. 2012;35(6):1773–80. [DOI] [PubMed] [Google Scholar]
- 29.Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16(7):755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118(8):2150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, et al. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182(10):5982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–40. [DOI] [PubMed] [Google Scholar]
- 33.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5(173):173ra23. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy WP, Simon JA, Offutt C, Horn P, Herman A, Townsend MJ, et al. Efficacy and safety of pateclizumab (anti-lymphotoxin-alpha) compared to adalimumab in rheumatoid arthritis: a head-to-head phase 2 randomized controlled study (The ALTARA Study). Arthritis Res Ther. 2014;16(5):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moura RA, Quaresma C, Vieira AR, Goncalves MJ, Polido-Pereira J, Romao VC, et al. B-cell phenotype and IgD-CD27- memory B cells are affected by TNF-inhibitors and tocilizumab treatment in rheumatoid arthritis. PloS one. 2017;12(9):e0182927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souto-Carneiro MM, Mahadevan V, Takada K, Fritsch-Stork R, Nanki T, Brown M, et al. Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor. Arthritis research & therapy. 2009;11(3):R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fekete A, Soos L, Szekanecz Z, Szabo Z, Szodoray P, Barath S, et al. Disturbances in B- and T-cell homeostasis in rheumatoid arthritis: suggested relationships with antigen-driven immune responses. J Autoimmun. 2007;29(2–3):154–63. [DOI] [PubMed] [Google Scholar]
- 38.Fedele AL, Tolusso B, Gremese E, Bosello SL, Carbonella A, Canestri S, et al. Memory B cell subsets and plasmablasts are lower in early than in long-standing rheumatoid arthritis. BMC Immunol. 2014;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doorenspleet ME, Klarenbeek PL, de Hair MJ, van Schaik BD, Esveldt RE, van Kampen AH, et al. Rheumatoid arthritis synovial tissue harbours dominant B-cell and plasma-cell clones associated with autoreactivity. Ann Rheum Dis. 2014;73(4):756–62. [DOI] [PubMed] [Google Scholar]
- 40.Meednu N, Zhang H, Owen T, Sun W, Wang V, Cistrone C, et al. Production of RANKL by Memory B Cells: A Link Between B Cells and Bone Erosion in Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(4):805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macallan DC, Wallace DL, Zhang Y, Ghattas H, Asquith B, de Lara C, et al. B-cell kinetics in humans: rapid turnover of peripheral blood memory cells. Blood. 2005;105(9):3633–40. [DOI] [PubMed] [Google Scholar]
- 42.van Zelm MC, Szczepanski T, van der Burg M, van Dongen JJ. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. The Journal of experimental medicine. 2007;204(3):645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkowska MA, Schickel JN, Grosserichter-Wagener C, de Ridder D, Ng YS, van Dongen JJ, et al. Circulating Human CD27-IgA+ Memory B Cells Recognize Bacteria with Polyreactive Igs. J Immunol. 2015;195(4):1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. 2019;20(7):928–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinslow JD, Blum LK, Deane KD, Demoruelle MK, Okamoto Y, Parish MC, et al. Elevated IgA Plasmablast Levels in Subjects at Risk of Developing Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(10):2372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canete JD, Celis R, Moll C, Izquierdo E, Marsal S, Sanmarti R, et al. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68(5):751–6. [DOI] [PubMed] [Google Scholar]
- 47.Kobie JJ, Zheng B, Bryk P, Barnes M, Ritchlin CT, Tabechian DA, et al. Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor. Arthritis research & therapy. 2011;13(6):R209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hueber W, Tomooka BH, Batliwalla F, Li W, Monach PA, Tibshirani RJ, et al. Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis research & therapy. 2009;11(3):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu C, Batliwalla F, Li W, Lee A, Roubenoff R, Beckman E, et al. Genome-wide association scan identifies candidate polymorphisms associated with differential response to anti-TNF treatment in rheumatoid arthritis. Mol Med. 2008;14(9–10):575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Folkersen L, Brynedal B, Diaz-Gallo LM, Ramskold D, Shchetynsky K, Westerlind H, et al. Integration of known DNA, RNA and protein biomarkers provides prediction of anti-TNF response in rheumatoid arthritis: results from the COMBINE study. Mol Med. 2016;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright HL, Cox T, Moots RJ, Edwards SW. Neutrophil biomarkers predict response to therapy with tumor necrosis factor inhibitors in rheumatoid arthritis. J Leukoc Biol. 2016. [DOI] [PubMed] [Google Scholar]
- 52.Donlin LT, Rao DA, Wei K, Slowikowski K, McGeachy MJ, Turner JD, et al. Methods for high-dimensonal analysis of cells dissociated from cyropreserved synovial tissue. Arthritis Res Ther. 2018;20(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dennis G Jr., Holweg CT, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA, et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis research & therapy. 2014;16(2):R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubtsova K, Rubtsov AV, Cancro MP, Marrack P. Age-Associated B Cells: A T-bet-Dependent Effector with Roles in Protective and Pathogenic Immunity. J Immunol. 2015;195(5):1933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. 2018;49(4):725–39 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.