Abstract
The neonatal hemostatic system is different from that of adults. The differences in levels of procoagulant and anticoagulant factors and the evolving equilibrium in secondary hemostasis during the transition from fetal/neonatal life to infancy, childhood and adult life are known as “developmental hemostasis”. In regard to primary hemostasis, while the number (150,000–450,000/μl) and structure of platelets in healthy neonates closely resemble those of adults, there are significant functional differences between neonatal and adult platelets. Specifically, platelets derived from both cord blood and neonatal peripheral blood are less reactive than adult platelets to agonists such as adenosine diphosphate (ADP), epinephrine, collagen, thrombin, and thromboxane (TXA2) analogs. This platelet hyporeactivity is due to differences in expression levels of key surface receptors and/or in signaling pathways, and is more pronounced in preterm neonates. Despite these differences in platelet function, bleeding times and PFA-100 closure times (an in vitro test of whole blood primary hemostasis) are shorter in healthy full-term infants than in adults, reflecting enhanced primary hemostasis. This paradoxical finding is explained by the presence of factors in neonatal blood that increase the platelet-vessel wall interaction, such as high von Willebrand factor (vWF) levels, predominance of ultralong vWF multimers, high hematocrit and high red cell mean corpuscular volume. Thus, the hyporeactivity of neonatal platelets should not be viewed as a developmental deficiency, but rather as an integral part of a developmentally unique, but well balanced, primary hemostatic system. In clinical practice, due to the high incidence of bleeding (especially intraventricular hemorrhage, IVH) among preterm infants, neonatologists frequently transfuse platelets to non-bleeding neonates when platelet counts fall below an arbitrary limit, typically higher than that used in older children and adults. However, recent studies have shown that prophylactic platelet transfusions not only fail to decrease bleeding in preterm neonates, but are associated with increased neonatal morbidity and mortality. In this review we will describe the developmental differences in platelet function and primary hemostasis between neonates and adults, and will analyze the implications of these differences to platelet transfusion decisions.
Keywords: neonatal platelets, hemostasis, bleeding, neonates, transfusion, development
Although recent research has shown that platelets play roles in many diverse biological processes, their role in haemostasis and thrombosis is of unquestionable importance and it will be the focus of our review.
Functional differences between neonatal and adult platelets
While the number (150,000–450,000/μl), mean platelet volume (MPV and structure of platelets in healthy neonates closely resemble those of adults, there are significant functional differences between neonatal and adult platelets.
Upon damage to the vascular endothelium, sub-endothelial matrix (SEM) proteins such as collagen and von Willebrand Factor (vWF) are exposed, which provide docking sites for platelet receptors. These receptors bind SEM proteins and trigger platelet activation, shape change and degranulation, resulting in the recruitment and activation of more platelets from the circulation.
I. Initiation (platelet adhesion)
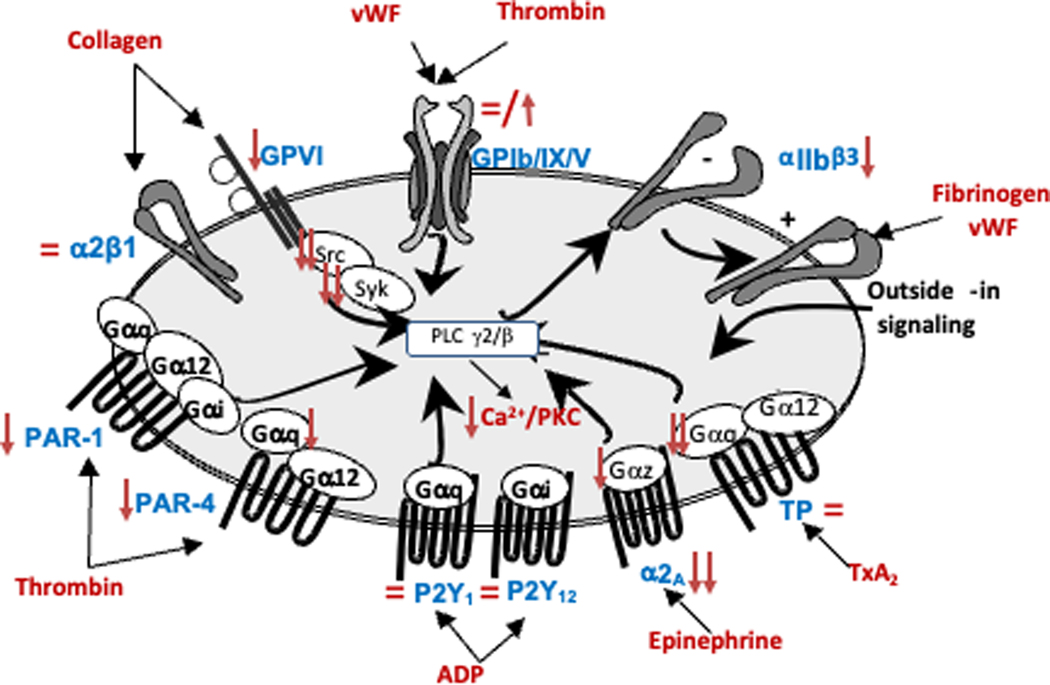
The adhesion mechanism is highly dependent on blood flow. Under high flow conditions (arteries and microcirculation), adhesion is mediated by the binding of the high molecular weight vWF to its platelet receptor (GPIb/IX/V). In this condition of blood flow, GPIIb/IIIa (αIIbβ3 integrin, essential for platelet aggregation) also participates. Under low flow conditions (venous territory), in contrast, platelet adhesion is mediated by the direct binding of GPIa/IIa (α2β1 integrin) to collagen.1 Expression levels of the main platelet adhesive receptors, including GPIb-IX-V (receptor for vWF), GPIa-IIa (receptor for collagen), and GPIIb-IIIa (receptor for fibrinogen, fibronectin and vWF) have been studied in neonatal (vs adult) platelets (Figure 1).
Figure 1.
Schematic representation of the main platelet signaling pathways, surface receptors, and their ligands. The red arrows indicate developmental differences (upregulation or downregulation) in expression level or function of the various receptors and signaling molecules in neonatal compared to adult platelets.
Overall, most studies have shown that the surface expression of αIIbβ3 is around 15–20% lower on neonatal platelets than on platelets of older children and adults,2 while levels of GPIb-IX-V2 and GPIa-IIa3 are similar in these three groups (Figure1). Applying quantitative flow cytometry (q-FC), our group recently evaluated the number of antigenic molecules of GPIIIa (the β3-integrin subunit from the GPIIb-IIIa complex), GPIbα (from the GPIb-IX-V), and GPIa (from the GPIa-IIa) per platelet in pre-term and full-term neonates and in adults.4 We also found a mild reduction in the expression of integrin αIIbβ3, but no significant differences in the levels of integrin GPIbα and α2β1 (Figure1). In contrast, other authors have found increased GPIbα levels in cord blood (CB)-derived platelets of full-term5 and preterm neonates,6 measured by immunoblotting5 or q-FC,6 respectively. In the latter study, preterm neonates exhibited higher GPIbα expression levels and an increased number of platelets interacting with vWF, with a positive correlation between both.6
Regarding adhesion assays, different results have been found depending on the sample (plasma-free vs. whole blood), flow conditions (static or dynamic) and developmental stage. Under static and plasma-free conditions, we observed delayed adhesion and spreading of washed full-term CB platelets, compared to washed adult platelets.7 In neonatal whole blood, however, where higher concentrations of ultra-large multimers of vWF are found,8, 9 adhesion of platelets from full-term neonates is similar to10 or higher11 than that of adult-platelets, both under static11 and flow conditions.10
II. Extension (platelet activation)
In parallel to the adhesion process, the coagulation system (secondary hemostasis), activated by endothelial damage, generates small amounts of thrombin. This, together with collagen and high local ADP concentrations, causes platelet activation and degranulation. Platelet activation begins with a ligand (such as ADP, epinephrine, TXA2, thrombin or collagen) binding to its receptor.
Hyporeactivity of neonatal platelets to TXA2, ADP and thrombin (Gαq protein- coupled receptors)
Multiple studies have consistently showed that in vitro stimulation of full-term and preterm neonatal platelets with thrombin, the TXA2 mimetic U46619, or ADP induces a poor response compared to that seen in adult platelets.2, 10, 12 The decreased expression of the protease-activated thrombin receptors (PAR1 and PAR 4) is one of the factors that may explain the hyporeactivity of neonatal platelets to thrombin,5 (Figure1). However, other factors could be involved. Indeed, although the differences between neonatal and adult platelets are more pronounced for PAR4 than for PAR1 expression,5 we found that platelets from preterm and full-term neonates exhibited a marked reduction in fibrinogen binding and P-selectin expression in response to PAR-1 (over 40–50%), but a minimal reduction in response to PAR-4.4 Regarding the platelet hyporeactivity in response to ADP and TXA2, developmental differences have not been found in the expression levels of P2Y1/P2Y12,13 (the ADP receptors) or in the number or affinity of TXA2 receptors.14
Importantly, the TXA2 receptors, P2Y1 (one of the two ADP receptors) and PAR1/PAR4 (both thrombin receptors) belong to the G-protein coupled receptors (GPCR) family, which couples through the Gαq-protein to phospholipase-Cβ (PLCβ).15 A previous study showed that the poor response of neonatal platelets to TXA2 is caused by decreased GTPase activity of the α-subunit of Gq,16 which is more pronounced in platelets of preterm infants.16, 17 Since the ADP and thrombin receptors also couple through the Gαq-protein, decreased GTPase activity of Gαq could contribute to the poor response of neonatal platelets to these three agonists.
Hyporeactivity of neonatal platelets to epinephrine (binding to Gαz-protein coupled receptors)
Although in vitro neonatal platelets show a relative hyporeactivity to most platelet agonists, the poorest responses are observed with epinephrine and collagen. Using ligand-binding studies, an early study in 1981 demonstrated that the number of α-adrenergic receptors on neonatal platelets is approximately half the number on adult platelets.18 Consistently, a more recent study showed a 50% reduction in ADRA2A mRNA expression levels in neonatal compared to adult platelets.19 Furthermore, epinephrine receptors couple to Gαz, and Gαz mRNA and protein expression levels were also recently found to be decreased in CB platelets,19 (Figure 1).
Hyporeactivity of neonatal platelets to collagen (binding to ITAM receptors)
The major collagen receptors on platelets are integrin α2β1 and GPVI, both of which support adhesion and activation in response to collagen. GPVI shares a common signal transduction pathway with the other hemi-ITAM receptor in platelets, CLEC-2.4 Both GPVI and CLEC-2 activate Src and Syk tyrosine kinases, culminating in the activation of PLCγ2 and Ca2+ mobilization4 (Figure 1). While there is no difference in α2β1 expression levels between neonatal and adult platelets,3, 20 two recent studies showed a significant hypo-responsiveness of preterm and full-term neonatal platelets to GPVI and CLEC-2 ligands (collagen-related peptide, CRP, and rhodocytin, respectively).4, 10 Same results were obtained in fetal and neonatal murine platelets.4 These findings are explained by a significant reduction in the expression levels of the GPVI-FcRγ complex and CLEC-2, accounted for at the transcriptional level (reduced GP6, FCER1G and CLEC1B mRNA levels), coupled with an intracellular signaling defect, as reflected by the reduced CRP- and rhodocytin-induced phosphorylation of PLCγ2 and Syk.4
Neonatal platelet signaling machinery.
Agonist–receptor interactions trigger a series of activation responses, including an increase in intracellular calcium (Ca2+) levels, degranulation, a conformational change in the GPIIb/IIIa complex toward a high-affinity conformation (Figure 1), and cytoskeletal reorganization allowing platelet spreading.
Intracellular calcium signaling.
Neonatal platelets exhibit impaired mobilization of calcium following agonist stimulation.17 Since the Ca2+ content of the dense tubular system, the major intracellular Ca2+ store, is similar in neonatal and adult platelets, the observed phenotype is most likely due to a reduction in the calcium signaling pathway. The main mechanism of Ca2+ influx from the extracellular space is store-operated Ca2+-entry (SOCE) upon activation by the Ca2+ sensor STIM1. Data from a mRNA expression array in ultrapure platelets obtained from adult blood and CB suggest that the altered Ca2+ mobilization in neonatal platelets may be due to differences in the expression levels of the genes involved in Ca2+ signaling.19
Degranulation and spreading.
P-selectin translocates from α-granules to the platelet surface membrane following platelet activation. Interestingly, although both human and murine neonatal platelets show decreased surface P-selectin expression upon activation compared to adult platelets, the total P-selectin content is developmentally reduced in platelets from newborn mice21, 22 but not in platelets from human neonates7. In regard to granule content, ultrastructural studies have shown that whereas the number of dense granules in human neonatal platelets is lower than in adult platelets,23 the number of α-granules, which are the most abundant, is similar7, 23–25. The reduced exocytosis of α-granules in CB-derived platelets is associated with a lower expression of β1-tubulin (required for granule centralization) and of Syntaxin-11/Munc18b complex and VAMP7 (SNARE proteins required for the fusion of vesicles to plasma membranes).7 As a result, neonatal platelet spreading is reduced and delayed both in humans (in plasma-free conditions)7 and in mice.21
III. Perpetuation (platelet aggregation)
Agonist–receptor interactions trigger a series of activation responses, including a conformational change in the platelet integrin αIIbβ3 (GPIIb/IIIa) toward a high-affinity conformation for its ligands, fibrinogen and vWF1 (Figure 1). Platelets from pre-term and full-term neonates display a mild reduction in the expression of integrin αIIbβ3,2, 4 which may contribute to the mild impairment in αIIbβ3 activation (i.e. fibrinogen binding) by all agonists.4, 26, 27 In mice, however, despite similar αIIbβ3 levels in neonates and adults,4 there is a marked impairment in fibrinogen binding in response to ITAM and some GPCR receptors due to the reduced levels of adaptor molecules that regulate integrin activation.27
Hypersensitivity of neonatal platelets to inhibitory signals
At the site of injury, platelets adhere, activate and aggregate to form a “plug”. To limit the uncontrolled growth of the hemostatic plug, the endothelium responds by reversing platelet reactivity (“endothelial thromboregulation”).1 Recently, development differences in platelet inhibitory signaling pathways were reported. Compared to adult platelets, neonatal platelets display a hyper-sensitivity to inhibition by PGE1, increased basal and PGE1-induced cAMP levels, higher Gαs protein expression and a trend to increased PKA-dependent protein phosphorylation28. Thus, the functional increase in the PGE1-cAMP-PKA axis may also contribute to neonatal platelet hypo-responsiveness.
Duration of the hyporeactivity platelet in fetal/neonatal life
Overall, the poor responses of neonatal platelets to both activating and inhibiting agonists appear to result from differences in the receptor expression level and/or in the signaling pathways downstream from the receptor15 (Figure 1). Although the decreased number of α-adrenergic receptors on neonatal platelets, seems to persist for as late as two months of age,15 both full-term2, 29 and preterm neonates12 improve their responses to most platelet agonist to near-adult levels by 10–14 postnatal days.
Developmental differences in the interactions between platelets and leucocytes, NET-formation and beyond
Beyond its role in hemostasis and thrombosis, platelets also play critical roles in other physiological and pathological processes including, among others, inflammation, host defense, vascular integrity, and development.30
Activated platelets interact with leukocytes (neutrophils and monocytes) modulating each other’s functions. Platelets enhance leukocyte activation through the release of CCL5 (RANTES) and platelet factor 4 (PF4), and in turn, neutrophils stimulate platelet activation by releasing elastase and cathepsin G.31 The two major receptor-ligand interactions in platelet-neutrophil communication are P-selectin/P-selectin glycoprotein ligand-1 (PSGL1) and GPIbα/Mac-1. Downstream effects of the platelet–neutrophil interaction include, among others, activation of tissue factor (TF), production of inflammatory cytokines by both platelets and leukocytes, and generation of neutrophil extracellular traps (NETs).31 NETs are extracellular structures composed by DNA and histones associated with antibacterial proteins that entrap, immobilize and kill pathogens, aiding against infection.32 Thus, activated platelets may facilitate pathogen clearance by activating neutrophils, promoting NET formation, and generating microthrombi (Immunothrombosis).33 Likely due to the lower expression of P-selectin in neonatal platelets upon activation, formation of platelet-neutrophil aggregates is lower in CB of premature newborns than in adult blood.34 In addition, human neonates exhibit significantly reduced NET formation due to the presence of a NET inhibitor produced by the placenta. 35 This effect disappears between 3 and 14 days after birth.35
Activated platelets also release extracellular vesicles, a heterogeneous pool of vesicles that share many functional features with platelets, such as high procoagulant capacity.36 The majority of extracellular vesicles in the blood (60 to 90%) are platelet-derived37 and they include apoptotic bodies (1–5 μm), microparticles (0.1–1 μm) and exosomes (~100 nm). Interestingly, isolated microparticles derived from CB have increased procoagulant activity compared to microparticles isolated from adult plasma, despite similar phosphatidylserine content.15 Recently, Peñas-Martinez et al. characterized plasma exosomes from CB and adult blood. A Mass Spectrometry-based proteomic analysis showed 104 differentially expressed proteins (FCh > 2), 64 of which were upregulated in neonatal (vs. adult) exosomes. Newborn plasma exosomes exhibited an abundance of proteins involved in platelet function and hemostasis, such as platelet activation and signaling proteins (integrins αIIb and β3; GNAI2, filamin-A; Talin-1, RAP1A, CD9); ligand receptor (CD36); platelet chemoattractant proteins (PF4); and proteins associated to coagulation, and thrombus formation (Factor V, Factor VIII, vWF, fibrinogen, α2-macroglobulin).38 Taken together, these studies suggest that platelet-derived exosomes, in particular, and extracellular vesicles, in general, could play an important role in the hemostatic balance during development.
Hyporeactivity of neonatal platelets is not synonymous with thrombocytopathy: Whole-blood hemostasis
In vitro platelet function studies in neonates have been hampered by difficulties in obtaining adequate blood samples from neonates, particularly those born preterm. For that reason, most studies so far have been conducted on full term CB samples. A few studies have compared CB to peripheral blood, and have found that platelet count, MPV, expresión of surface glycoproteins and surface activation markers, 2, 39, while platelet function and primary hemostasis are different. 39, 40.Last generation technologies have allowed the study of platelet function using smaller blood volumes and in the context of neonatal/cord whole blood instead of platelet-rich plasma (PRP). These studies have shown that, despite the poor reactivity of platelets during fetal/neonatal life, healthy full-term neonatal platelets show similar or even more extensive adhesion than adult platelets on SEM coated surfaces.11 In capillary blood obtained from healthy full-term neonates, neonatal platelet adhesion and aggregate formation, under both static and shear conditions, were similar to adult blood.10 Furthermore, bleeding times41 and closure times (CT) measured using the Platelet Function Analyzer (PFA-100), an in vitro test of primary hemostasis,42–44 are shorter in healthy full-term neonates than in adults. This seemingly paradoxical finding has been explained by the presence of factors in neonatal blood that enhance the platelet–vessel wall interaction, such as increased vWF levels and function, higher hematocrits, and higher mean corpuscular volumes of erythrocytes.45 Thus, in healthy full-term neonates under physiological conditions, the relative hyporeactivity of neonatal platelets is viewed not as a deficiency, but rather as an integral part of a unique and well balanced neonatal hemostatic system, which is otherwise titled toward a procoagulant phenotype.26
Importantly, bleeding times and PFA-100 closure times in response to collagen and ADP (CT-ADPs) are inversely correlated to gestational age.40, 46 Compared to full-term infants, preterm neonates have more pronounced platelet hyporeactivity in response to agonists and longer bleeding times and CT-ADPs, although at all gestational ages they remain shorter or similar to adult CT-ADPs.40 Taken together, these findings suggest that heathy preterm neonates have an “appropriate” or well-balanced primary hemostatic system. Under pathological conditions, however, the pro-adhesive mechanisms might be insufficient to compensate for the platelet hyporeactivity. Supporting this hypothesis, platelet adhesion to SEM is significantly reduced in sick47 and septic preterm infants,48 compared to healthy preterm infants or full-term newborns. However, although preterm infants are those at highest risk of bleeding, the majority of studies on neonatal platelet function have been conducted on full-term neonates. In fact, an important limitation of the in vitro studies published to date has been the reliance on full term cord blood samples for most studies (due to availability), and the paucity of data on how platelet function and primary hemostasis change over time after birth, particularly in “healthy” preterm neonates. Therefore, it is unclear whether the increased platelet hypofunction observed in preterm infants contributes in any way to their high bleeding risk.49
Bleeding risk in neonates and children
In a prospective multicenter study that included almost 170 infants with platelet counts <60 × 109/L, bleeding risk was significantly associated with a lower gestational age at birth (63% among infants <28 weekś gestational compared to 14% among those ≥ 28 weeks), but not with a lower platelet count.50 Bleeding in extremely preterm infants is most commonly intracranial. In fact, approximately 25–30% of neonates with very low birth weight (VLBW, <1500 g at birth) develop an intraventricular hemorrhage (IVH), usually in the first week of life.51 As with the risk of bleeding, the frequency of thrombocytopenia increases with decreasing gestational age, reaching ~70% among neonates born with a weight <1,000g.52
The pathogenesis of bleeding in preterm neonates is complex and multifactorial and results from the interaction of developmental stage-specific factors (such as vascular fragility, cardiovascular and respiratory instability, changes in vascular perfusion pressures),53 along with factors related to the underlying disease process (sepsis, necrotizing enterocolitis, need for mechanical ventilation, among others). Indeed, with the same degree of thrombocytopenia, infants with sepsis, necrotizing enterocolitis and alloimmune thrombocytopenia had a higher incidence of serious bleeding than infants with thrombocytopenia due to intrauterine growth restriction.50
Although within the infant population preterm infants have the highest risk of bleeding, infants in general have a higher risk of bleeding than adults. In a large randomized controlled trial to evaluate the impact of three different prophylactic platelet doses in children (n=198, 0–18 years) and adults (n=1044) with onco-hematologic neoplasms,54 the incidence of grade 2 or higher bleeding ranged from 43–79% in adults and up to 84% in children. Within the same range of platelet counts, children had a higher risk of bleeding than adults, suggesting that the excess risk of bleeding in children is not due to the degree of thrombocytopenia. Importantly, a direct correlation between the severity of the thrombocytopenia and the occurrence of major bleeding has not been found in any age group (neither in children nor in adults),50, 55, 56 suggesting that other clinical factors, and not only the platelet count, are important.
Platelet-transfusion thresholds
Over two-thirds of platelet transfusions in neonates are prescribed prophylactically. In the setting of bleeding, platelets are often transfused to keep platelet counts >50.000/μL,57 although this depends on the degree and underlying reason for bleeding.58–60 For example, the threshold increases above 50.000/μL-100.000/μL in neonates with bleeding on ECMO or during surgery.57, 59
Thresholds for prophylactic platelet-transfusion are much more controversial. Data from the PLADO study suggest that, in adults without associated risk factors, the risk of bleeding does not appear to change once the platelet count is above 5000/μL.54 The results of the PLADO study along with several randomized trial in stable onco-hematology patients showed that using the standard trigger level (10,000/μL) is associated with no increase in the risk of bleeding compared to higher trigger levels (20,000/μL or 30,000/μL).61 In this adult population, however, a therapeutic-only platelet transfusion policy was associated with increased risk of bleeding compared with a prophylactic platelet transfusion policy.62 These data are in concordance with a platelet kinetic study in adult patients with bone marrow hypoplasia, in which a platelet count of 7,000/μL was required to maintain endothelial integrity.63 However, the platelet count is not the only consideration when determining bleeding risk in individual patients. Adult patients with splenomegaly, fever, infections, graft-versus-host disease, anticoagulant therapy, etc. might need a higher prophylactic platelet-transfusion threshold to ensure that the nadir platelet count is always maintained above the critical 5000–7000/μL.61
As adults and older children, thrombocytopenic neonates may have clinical factors associated with an increased platelet consumption and/or an increased risk of bleeding (e.g. prematurity, fever, sepsis, infections, cardiovascular instability, mechanical ventilation, or coagulopathy). Because of their high risk of both thrombocytopenia and bleeding, preterm neonates are commonly transfused prophylactically when the platelet count falls below an arbitrary limit, which is usually higher than for older children or adults.49 Indeed, a recent multicenter study including 972 VLBW infants treated in 6 US NICUs showed that a large proportion (65%) of platelet transfusions were given to VLBW infants with platelet counts greater than 50,000/μL.64 In the same study, severity of illness influenced transfusion decisions. However, the severity of thrombocytopenia did not correlate with the risk for IVH, and platelet transfusions did not reduce this risk. Despite the questionable benefit of prophylactic platelet-transfusion in neonates, the majority of platelet transfusions in the NICU are given in the absence of bleeding50, 58, 65, 66 and with a large variability in platelet transfusion thresholds used67, 68. This high variability is in part due to lack of understanding of the platelet count below which a transfusion is beneficial. Three randomized controlled trials have tried to answer this question.
Platelet transfusions in neonates
The first randomized controlled trial of neonatal platelet transfusion thresholds was published in the early 1990s.69 Premature neonates (<1500 gr) with moderate thrombocytopenia (platelet count between 50,000–150,000/μL) were randomly assigned to receive a platelet transfusion during the first week of life when the platelet-count was <150,000/μL or <50,000/μL or with active bleeding. The authors found no differences in the incidence or severity of intracranial hemorrhages between the 2 arms of the study.69 It is important to emphasize that neonates with severe thrombocytopenia (<50,000/mm3), at the time of randomization, were excluded from this study. In the absence of new trials, neonatologists adopted a platelet count of 50,000/mm3 as the standard threshold for prophylactic platelet transfusion in preterm neonates. Whether, in the absence of active bleeding, platelet counts lower than 50,000/mm3 could be safely tolerated remained unresolved.
Twenty five years after the publication of this initial trial, an international multicenter randomized study (PlaNeT-2) was designed to answer this question. In this trial, 660 infants with a gestational age <34 weeks, without active bleeding and with a platelet count <50,000/mm3, were randomized to receive prophylactic platelet transfusions when the platelet count was lower than 50,000/mm3 (high threshold group) vs lower than 25,000/mm3 (low threshold group).70 In the high threshold group, 90% of the infants received at least one platelet transfusion, as compared with 53% in the low threshold group. Surprisingly, within 28 days after randomization, a significantly higher incidence of death or major bleeding was reported in the high threshold group compared to the low threshold group (26% vs 19%, 7% absolute-risk reduction). The incidence of bronchopulmonary dysplasia was also higher in the high threshold group. The rate of serious adverse events related to platelet transfusion, in contrast, was comparable in both groups.70
A later sub-analysis of the PlaNeT-2 trial investigated whether all preterm neonates benefit from the low threshold. For this, the authors developed a multivariate logistic regression model to predict the baseline risk of major bleeding and/or mortality for all neonates enrolled in PlaNeT-2.71 Based on their predicted baseline risk, neonates were ranked in 4 groups/quartiles (very low, low, moderate and high risk) and the absolute risk difference between the high threshold group (<50,000/ mm3) and low threshold group (<25 000/mm3) was assessed within each quartile. Importantly, the 25,000/mm3 threshold was associated with an absolute risk reduction in all risk groups (even in high risk neonates), suggesting that a 25,000/mm3 prophylactic platelet-transfusion threshold can be adopted in all preterm neonates, irrespective of predicted baseline outcome risk.71 However, it is important to note some limitations. First, only 37% of infants in PlaNeT-2 were randomized by day of life 5, the highest-risk period of bleeding (although this might reflect the time of onset of thrombocytopenia). Second, almost 40% of the infants received one or more transfusions before randomization, for unclear reasons and at non-specified platelet counts.72
The third trial explored the effects of a higher transfusion threshold on the time to closure of a patent ductus arteriosus in premature neonates, and found no differences between liberal (<100,000/mm3) and restrictive (<20,000/mm3) platelet-transfusion criteria.73 However, in the liberal transfusion group, 41% of infants had any grade of IVH compared with 4.5% in the restrictive group (P = .009).
Overall, these studies strongly supported previous observational studies describing that increasing the transfusion threshold is not effective in preventing bleeding and suggesting an association between platelet transfusions and increased neonatal morbidity and mortality 58, 60, 74, 75.
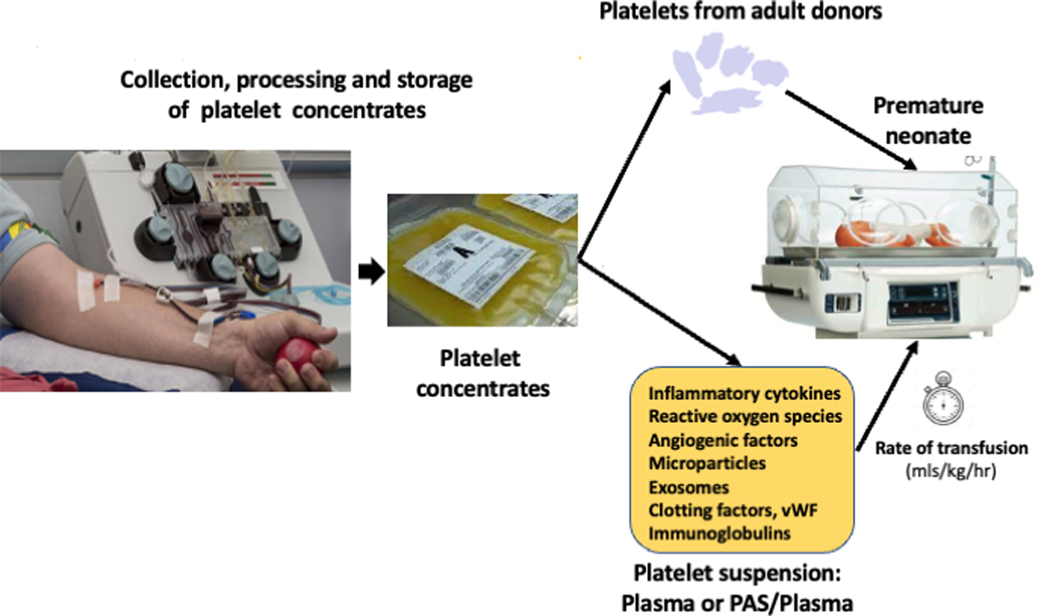
The mechanisms by which platelet transfusions in preterm neonates may be harmful are not well understood but this topic has been discussed in detail by Moore and Curley.76 Some mechanisms have been suggested (Figure 2): a) A biophysical mechanism or hemodynamic effect, based on rapid volume expansion in a population that is vulnerable due to vascular fragility and low intracranial pressures. Transfused preterm neonates receive platelet suspensions at a volume (~15 mL/kg) that is up to 3 times higher than that typically transfused to adults or older children (~5 mL/kg), but at the same infusion time (~30 min); b) Thromboinflammation. As discussed above, adult platelets are more reactive than neonatal platelets. In vitro, “transfusion” of adult platelets into thrombocytopenic CB resulted in shorter CTs-EPI (PFA-100) and higher clot strength and firmness than in vitro “transfusion” of neonatal autologous platelets.77 Whether this hypercoagulable profile promotes microthrombus formation in vivo is unknown. However, transfusion of adult platelets into the fetal murine circulation also led to rapid platelet aggregate formation,21 suggesting that this “developmental mismatch” may induce a prothrombotic phenotype. In this regard, prophylactic platelet transfusion in adults with HIV while on platelet antiaggregants has been associated with increased mortality.78 Moreover, in thrombotic microangiopathies such as thrombotic thrombocytopenic purpura or disseminated intravascular coagulation, where microvascular thrombi coexist with purpura and bleeding, prophylactic platelet-transfusions are not recommended. Whereas, in vivo, the formation of microaggregates or microthrombosis induced by transfusions of adult platelets on neonatal blood is currently only a hypothesis, there is increasing evidence that platelets play a key role in processes such as inflammation and immune responses.30 Platelet-derived proangiogenic factors, reactive oxygen species (ROS) and inflammatory mediators may exacerbate the bronchopulmonary dysplasia in this vulnerable population.70
Figure 2.
Schematic representation of the process of platelet collection and transfusion into neonates, and the potential factors mediating the deleterious effects of platelet transfusions in preterm infants.
Overall, changes during development affect not only platelet reactivity, but many other aspects of platelet biology.19, 38, 79 Whether and how these ontogenetic differences influence the “off target” effects (i.e. beyond hemostasis) of platelets and the interplay between thrombosis, inflammation and immunity during development is poorly understood.38, 80
Implications for transfusion practice
In the absence of bleeding, when to transfuse. The available evidence suggests that platelet counts alone are likely not a good marker to evaluate bleeding risk in neonates, and especially in preterm infants. Thus, new risk prediction biomarkers (such as the CT-ADP measured with a PFA-100)81 or dynamic risk prediction models (with time-dependent variables)82 are currently under investigation and awaiting evaluation in external cohorts for their value in supporting clinical decisions. In the meantime, however, results from randomized controlled trials strongly suggest that platelet transfusions might have deleterious effects in neonates, and that non-bleeding neonates in general benefit from a restrictive transfusion threshold of <25,000/μL regardless of their baseline risk. Whether this threshold can be further lowered will require additional studies.
What product to transfuse. Platelets used in neonatal transfusion are typically collected by apheresis from a single donor, instead of pooled platelets from multiple donors. International practice is different between countries. Some countries provide a specific product for neonatal transfusion, whereas others used the standard adult product and give less of it based on ml/kg dosing. Other countries produce smaller units split from one donation so that donor exposure (risk) can be limited by reserving multiple packs from the same donor for the same patient. In the UK, since January 2020, “neonatal” platelets packs manufactured by NHS Blood and Transplant in the UK include 20% of platelet additive solution (PAS).83 PAS was already used to prepare platelet products for adults and older children, and for neonates in other countries. In addition, the number of platelets per volume of component was reduced ~20% in these new “neonatal” platelet packs from the UK. .Moreover, platelets used in neonatal transfusion should be CMV-negative or leukoreduced (CMV-safe).57 ABO-identical or ABO-compatible platelets are always desirable. However, evidence on the effects of ABO-incompatible platelet transfusions in neonates is limited, and ABO compatibility of platelet products depends mainly on logistical reasons in the blood bank.76, 84 Regarding the use of pathogen reduction systems, such as INTERCEPT and MIRASOL, for reducing the risk of platelet transfusion-transmitted infection, studies in the pediatric population are rare, and data in adults suggest that these products increase platelet transfusion requirements in some populations, including the pediatric population.59
How to transfuse. A typical dose is 10–20 (~15) mL/kg of a platelet suspension, which is a substantially higher volume than that transfused to adult recipients. This volume represents 17% of the total circulating blood volume for a 1-Kg preterm infant,80 and is traditionally infused within 30–60 min. This rapid volume expansion may lead to changes in cerebral blood flow and intracranial pressure in vulnerable neonates, and ultimately, to an increased risk of developing or worsening IVH.80 For those reasons, since the publication of the PlaNeT-2 study, we recommend limiting the platelet transfusion volume to 10 mL/kg and extending the transfusion time to over 2–3 hours. This is consistent with the recommendations from the American Association of Blood Banks (AABB) to transfuse blood products at a rate not faster than 5 mL/kg/h. In support of this practice, a study comparing platelet transfusions given over 30 minutes vs. 2 hours to neonates found no differences in platelet recovery85. Thus, this might be an appropriate strategy to mitigate the risks of platelet transfusions in extremely preterm neonates, although further studies are needed to determine whether changes in the infusion rate indeed decrease the risk of bleeding.
Conclusions
Although the hyporeactivity of neonatal (vs. adult) platelets has been known for decades, the underlying molecular mechanisms are only recently being elucidated, including changes at the transcriptomic level, in the expression and/or functionality of receptors, and in signalling pathways. This platelet hyporeactivity is counterbalanced by other factors that promote hemostasis and clot formation. In fact, healthy term neonates have a low risk of bleeding. However, preterm and sick neonates are at increased risk of both thrombocytopenia and bleeding, and receive platelet transfusions from adult donors (in most cases prophylactically) when the platelet threshold falls below a limit generally higher than that established for adults. This liberal practice of transfusing platelets to this vulnerable population has not only failed to reduce the risk of bleeding, but, on the contrary, has been shown to increase bleeding and mortality. A major effort is underway to find new biomarkers and models to predict bleeding risk in this population. In addition, studies to determine the specific mechanisms that mediate the platelet transfusion-related increases in neonatal bleeding and mortality are critically important. Without this information, it is difficult to know what the main drivers of these adverse effects are, and what characteristics an ideal platelet product for neonates would have. In the meantime, the Hippocratic principle “primum non nocere” and “blood product not indicated is contraindicated” should be applied.
Acknowledgments
Financial Support:
This study was supported by research grants from Instituto de Salud Carlos III and Feder (PI14/01956 and PI18/00316). Dr. Sola-Visner is supported by NIH P01HL046925.
Footnotes
Conflict of interest disclosure
The authors declare no conflict of interest.
References
- 1.Michelson A, Cattaneo M, Frelinger A, Newman P. Platelets, 4th edn Academic Press: Boston (MA); US, 2019. [Google Scholar]
- 2.Sitaru AG, Holzhauer S, Speer CP, Singer D, Obergfell A, Walter U et al. Neonatal platelets from cord blood and peripheral blood. Platelets 2005; 16(3–4): 203–10. [DOI] [PubMed] [Google Scholar]
- 3.Israels SJ, Daniels M, McMillan EM. Deficient collagen-induced activation in the newborn platelet. Pediatr Res 1990; 27(4 Pt 1): 337–43. [DOI] [PubMed] [Google Scholar]
- 4.Hardy AT, Palma-Barqueros V, Watson SK, Malcor JD, Eble JA, Gardiner EE et al. Significant Hypo-Responsiveness to GPVI and CLEC-2 Agonists in Pre-Term and Full-Term Neonatal Platelets and following Immune Thrombocytopenia. Thromb Haemost 2018; 118(6): 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlagenhauf A, Schweintzger S, Birner-Gruenberger R, Leschnik B, Muntean W. Newborn platelets: lower levels of protease-activated receptors cause hypoaggregability to thrombin. Platelets 2010; 21(8): 641–7. [DOI] [PubMed] [Google Scholar]
- 6.Cowman J, Quinn N, Geoghegan S, Mullers S, Oglesby I, Byrne B et al. Dynamic platelet function on von Willebrand factor is different in preterm neonates and full-term neonates: changes in neonatal platelet function. J Thromb Haemost 2016; 14(10): 2027–2035. [DOI] [PubMed] [Google Scholar]
- 7.Caparros-Perez E, Teruel-Montoya R, Palma-Barquero V, Torregrosa JM, Blanco JE, Delgado JL et al. Down Regulation of the Munc18b-syntaxin-11 Complex and beta1-tubulin Impairs Secretion and Spreading in Neonatal Platelets. Thromb Haemost 2017; 117(11): 2079–91. [DOI] [PubMed] [Google Scholar]
- 8.Katz JA, Moake JL, McPherson PD, Weinstein MJ, Moise KJ, Carpenter RJ et al. Relationship between human development and disappearance of unusually large von Willebrand factor multimers from plasma. Blood 1989; 73(7): 1851–8. [PubMed] [Google Scholar]
- 9.Weinstein MJ, Blanchard R, Moake JL, Vosburgh E, Moise K. Fetal and neonatal von Willebrand factor (vWF) is unusually large and similar to the vWF in patients with thrombotic thrombocytopenic purpura. Br J Haematol 1989; 72(1): 68–72. [DOI] [PubMed] [Google Scholar]
- 10.Baker-Groberg SM, Lattimore S, Recht M, McCarty OJ, Haley KM. Assessment of neonatal platelet adhesion, activation, and aggregation. J Thromb Haemost 2016; 14(4): 815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenkman B, Linder N, Savion N, Tamarin I, Dardik R, Kennet G et al. Increased neonatal platelet deposition on subendothelium under flow conditions: the role of plasma von Willebrand factor. Pediatr Res 1999; 45(2): 270–5. [DOI] [PubMed] [Google Scholar]
- 12.Bednarek FJ, Bean S, Barnard MR, Frelinger AL, Michelson AD. The platelet hyporeactivity of extremely low birth weight neonates is age-dependent. Thrombosis research 2009; 124(1): 42–5. [DOI] [PubMed] [Google Scholar]
- 13.Ngo ATP, Sheriff J, Rocheleau AD, Bucher M, Jones KR, Sepp AI et al. Assessment of neonatal, cord, and adult platelet granule trafficking and secretion. Platelets 2020; 31(1): 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Israels SJ, Odaibo FS, McMillan E, Robertson C, McNicol A. The platelet Thromboxane A2 receptor of full term and preterm neonates. . Pediatric Research 1996; 39(4): 288. [Google Scholar]
- 15.Lorenz V, Ferrer-Marin F, Israels S, Sola-Visner M. Platelet Function in the Newborn. In: Michelson A, Cattaneo M, Frelinger A, Newman P (eds). Platelets, 4th edition. Academic Press: Boston (MA); USA, 2019, pp 443–458. [Google Scholar]
- 16.Israels SJ, Cheang T, Roberston C, McMillan-Ward EM, McNicol A. Impaired signal transduction in neonatal platelets. Pediatr Res 1999; 45(5 Pt 1): 687–91. [DOI] [PubMed] [Google Scholar]
- 17.Gelman B, Setty BN, Chen D, Amin-Hanjani S, Stuart MJ. Impaired mobilization of intracellular calcium in neonatal platelets. Pediatr Res 1996; 39(4 Pt 1): 692–6. [DOI] [PubMed] [Google Scholar]
- 18.Corby DG, O’Barr TP. Decreased alpha-adrenergic receptors in newborn platelets: cause of abnormal response to epinephrine. Dev Pharmacol Ther 1981; 2(4): 215–25. [PubMed] [Google Scholar]
- 19.Caparros-Perez E, Teruel-Montoya R, Lopez-Andreo MJ, Llanos MC, Rivera J, Palma-Barqueros V et al. Comprehensive comparison of neonate and adult human platelet transcriptomes. PLoS One 2017; 12(8): e0183042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Israels SJ, Rand ML, Michelson AD. Neonatal platelet function. Seminars in thrombosis and hemostasis 2003; 29(4): 363–72. [DOI] [PubMed] [Google Scholar]
- 21.Margraf A, Nussbaum C, Rohwedder I, Klapproth S, Kurz ARM, Florian A et al. Maturation of Platelet Function During Murine Fetal Development In Vivo. Arterioscler Thromb Vasc Biol 2017; 37(6): 1076–1086. [DOI] [PubMed] [Google Scholar]
- 22.Stolla MC, Catherman SC, Kingsley PD, Rowe RG, Koniski AD, Fegan K et al. Lin28b regulates age-dependent differences in murine platelet function. Blood advances 2019; 3(1): 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urban D, Pluthero FG, Christensen H, Baidya S, Rand ML, Das A et al. Decreased numbers of dense granules in fetal and neonatal platelets. Haematologica 2017; 102(2): e36–e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whaun JM. Platelet function in the neonate: including qualitative platelet abnormalities associated with bleeding. In: Stockman JA, Pochedly C, eds. Developmental and neonatal hematology. New York: Raven Press; 1988: 131–144. [Google Scholar]
- 25.Ts’ao CH, Green D, Schultz K. Function and ultrastructure of platelets of neonates: enhanced ristocetin aggregation of neonatal platelets. Br J Haematol 1976; 32(2): 225–33. [DOI] [PubMed] [Google Scholar]
- 26.Sola-Visner M. Platelets in the neonatal period: developmental differences in platelet production, function, and hemostasis and the potential impact of therapies. Hematology Am Soc Hematol Educ Program 2012; 2012: 506–11. [DOI] [PubMed] [Google Scholar]
- 27.Margraf A, Nussbaum C, Sperandio M. Ontogeny of platelet function. Blood advances 2019; 3(4): 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palma-Barqueros V, Torregrosa JM, Caparros-Perez E, Mota-Perez N, Bohdan N, Llanos MDC et al. Developmental Differences in Platelet Inhibition Response to Prostaglandin E1. Neonatology 2020; 117(1): 15–23. [DOI] [PubMed] [Google Scholar]
- 29.Gatti L, Guarneri D, Caccamo ML, Gianotti GA, Marini A. Platelet activation in newborns detected by flow-cytometry. Biol Neonate 1996; 70(6): 322–7. [DOI] [PubMed] [Google Scholar]
- 30.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015; 126(5): 582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lisman T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell and tissue research 2018; 371(3): 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews. Immunology 2013; 13(3): 159–75. [DOI] [PubMed] [Google Scholar]
- 33.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nature reviews. Immunology 2013; 13(1): 34–45. [DOI] [PubMed] [Google Scholar]
- 34.Esiaba I, Angeles DM, Milford TM, Salto LM, Payne KJ, Kidder MY et al. Platelet-Neutrophil Interactions Are Lower in Cord Blood of Premature Newborns. Neonatology 2019; 115(2): 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yost CC, Schwertz H, Cody MJ, Wallace JA, Campbell RA, Vieira-de-Abreu A et al. Neonatal NET-inhibitory factor and related peptides inhibit neutrophil extracellular trap formation. J Clin Invest 2016; 126(10): 3783–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matijevic N, Wang YW, Wade CE, Holcomb JB, Cotton BA, Schreiber MA et al. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thrombosis research 2014; 134(3): 652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brisson AR, Tan S, Linares R, Gounou C, Arraud N. Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets 2017; 28(3): 263–271. [DOI] [PubMed] [Google Scholar]
- 38.Penas-Martinez J, Barrachina MN, Cuenca-Zamora EJ, Luengo-Gil G, Bravo SB, Caparros-Perez E et al. Qualitative and Quantitative Comparison of Plasma Exosomes from Neonates and Adults. International journal of molecular sciences 2021; 22(4): 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grevsen AK, Hviid CVB, Hansen AK, Hvas AM. Platelet count and function in umbilical cord blood versus peripheral blood in term neonates. Platelets 2021; 32(5): 626–632. [DOI] [PubMed] [Google Scholar]
- 40.Saxonhouse MA, Garner R, Mammel L, Li Q, Muller KE, Greywoode J et al. Closure times measured by the platelet function analyzer PFA-100 are longer in neonatal blood compared to cord blood samples. Neonatology 2010; 97(3): 242–9. [DOI] [PubMed] [Google Scholar]
- 41.Andrew M, Paes B, Bowker J, Vegh P. Evaluation of an automated bleeding time device in the newborn. Am J Hematol 1990; 35(4): 275–7. [DOI] [PubMed] [Google Scholar]
- 42.Carcao MD, Blanchette VS, Dean JA, He L, Kern MA, Stain AM et al. The Platelet Function Analyzer (PFA-100): a novel in-vitro system for evaluation of primary haemostasis in children. Br J Haematol 1998; 101(1): 70–3. [DOI] [PubMed] [Google Scholar]
- 43.Israels SJ, Cheang T, McMillan-Ward EM, Cheang M. Evaluation of primary hemostasis in neonates with a new in vitro platelet function analyzer. J Pediatr 2001; 138(1): 116–9. [DOI] [PubMed] [Google Scholar]
- 44.Roschitz B, Sudi K, Kostenberger M, Muntean W. Shorter PFA-100 closure times in neonates than in adults: role of red cells, white cells, platelets and von Willebrand factor. Acta Paediatr 2001; 90(6): 664–70. [PubMed] [Google Scholar]
- 45.Gerrard JM, Docherty JC, Israels SJ, Cheang MS, Bishop AJ, Kobrinsky NL et al. A reassessment of the bleeding time: association of age, hematocrit, platelet function, von Willebrand factor, and bleeding time thromboxane B2 with the length of the bleeding time. Clinical and investigative medicine. Medecine clinique et experimentale 1989; 12(3): 165–71. [PubMed] [Google Scholar]
- 46.Del Vecchio A, Latini G, Henry E, Christensen RD. Template bleeding times of 240 neonates born at 24 to 41 weeks gestation. J Perinatol 2008; 28(6): 427–31. [DOI] [PubMed] [Google Scholar]
- 47.Linder N, Shenkman B, Levin E, Sirota L, Vishne TH, Tamarin I et al. Deposition of whole blood platelets on extracellular matrix under flow conditions in preterm infants. Arch Dis Child Fetal Neonatal Ed 2002; 86(2): F127–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finkelstein Y, Shenkman B, Sirota L, Vishne TH, Dardik R, Varon D et al. Whole blood platelet deposition on extracellular matrix under flow conditions in preterm neonatal sepsis. Eur J Pediatr 2002; 161(5): 270–4. [DOI] [PubMed] [Google Scholar]
- 49.Ferrer-Marin F SS, Josephson C, Sola-Visner M. Distinct Differences in Platelet Production and Function between Neonates and Adults: Implications for Platelet Transfusion Practice. Transfusion 2013; 53(11): 2814–2821. [DOI] [PubMed] [Google Scholar]
- 50.Stanworth SJ, Clarke P, Watts T, Ballard S, Choo L, Morris T et al. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics 2009; 124(5): e826–34. [DOI] [PubMed] [Google Scholar]
- 51.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126(3): 443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Sola-Visner MC, Lambert DK et al. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol 2006; 26(6): 348–53. [DOI] [PubMed] [Google Scholar]
- 53.Stanworth SJ. Thrombocytopenia, bleeding, and use of platelet transfusions in sick neonates. Hematology Am Soc Hematol Educ Program 2012; 2012: 512–6. [DOI] [PubMed] [Google Scholar]
- 54.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med 2010; 362(7): 600–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baer VL, Lambert DK, Henry E, Christensen RD. Severe Thrombocytopenia in the NICU. Pediatrics 2009; 124(6): e1095–100. [DOI] [PubMed] [Google Scholar]
- 56.Josephson CD, Granger S, Assmann SF, Castillejo MI, Strauss RG, Slichter SJ et al. Bleeding risks are higher in children versus adults given prophylactic platelet transfusions for treatment-induced hypoproliferative thrombocytopenia. Blood 2012; 120(4): 748–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kahn S, Chegondi M, Nellis ME, Karam O. Overview of Plasma and Platelet Transfusions in Critically Ill Children. Frontiers in pediatrics 2020; 8: 601659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Del Vecchio A, Sola MC, Theriaque DW, Hutson AD, Kao KJ, Wright D et al. Platelet transfusions in the neonatal intensive care unit:factors predicting which patients will require multiple transfusions. Transfusion 2001; 41(6): 803–8. [DOI] [PubMed] [Google Scholar]
- 59.Patel RM, Josephson C. Neonatal and pediatric platelet transfusions: current concepts and controversies. Curr Opin Hematol 2019; 26(6): 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kenton AB, Hegemier S, Smith EO, O’Donovan DJ, Brandt ML, Cass DL et al. Platelet transfusions in infants with necrotizing enterocolitis do not lower mortality but may increase morbidity. J Perinatol 2005; 25(3): 173–7. [DOI] [PubMed] [Google Scholar]
- 61.Triulzi DJ. How well do platelets prevent bleeding? Hematology Am Soc Hematol Educ Program 2020; 2020(1): 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crighton GL, Estcourt LJ, Wood EM, Trivella M, Doree C, Stanworth S. A therapeutic-only versus prophylactic platelet transfusion strategy for preventing bleeding in patients with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation. Cochrane Database Syst Rev 2015; (9): CD010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood 1985; 66(5): 1105–9. [PubMed] [Google Scholar]
- 64.Sparger KA, Assmann SF, Granger S, Winston A, Christensen RD, Widness JA et al. Platelet Transfusion Practices Among Very-Low-Birth-Weight Infants. JAMA pediatrics 2016; 170(7): 687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Vecchio A, Franco C, Petrillo F, D’Amato G. Neonatal Transfusion Practice: When do Neonates Need Red Blood Cells or Platelets? American journal of perinatology 2016; 33(11): 1079–84. [DOI] [PubMed] [Google Scholar]
- 66.Dohner ML, Wiedmeier SE, Stoddard RA, Null D Jr., Lambert DK, Burnett J et al. Very high users of platelet transfusions in the neonatal intensive care unit. Transfusion 2009; 49(5): 869–72. [DOI] [PubMed] [Google Scholar]
- 67.Cremer M, Sola-Visner M, Roll S, Josephson CD, Yilmaz Z, Buhrer C et al. Platelet transfusions in neonates: practices in the United States vary significantly from those in Austria, Germany, and Switzerland. Transfusion 2011; 51(12): 2634–41. [DOI] [PubMed] [Google Scholar]
- 68.Patel RM, Hendrickson JE, Nellis ME, Birch R, Goel R, Karam O et al. Variation in Neonatal Transfusion Practice. J Pediatr 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, Ohlsson A et al. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr 1993; 123(2): 285–91. [DOI] [PubMed] [Google Scholar]
- 70.Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C et al. Randomized Trial of Platelet-Transfusion Thresholds in Neonates. N Engl J Med 2019; 380(3): 242–251. [DOI] [PubMed] [Google Scholar]
- 71.Fustolo-Gunnink FK, van Klaveren D, et al. PlaNeT-2 MATISSE Collaborators. Preterm neonates benefit from low prophylactic platelet transfusion threshold despite varying risk of bleeding or death. Blood. 2019;134(26):2354–2360. Blood 2020; 135(24): 2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sola-Visner MC. Platelet Transfusions in Neonates - Less Is More. N Engl J Med 2019; 380(3): 287–288. [DOI] [PubMed] [Google Scholar]
- 73.Kumar J, Dutta S, Sundaram V, Saini SS, Sharma RR, Varma N. Platelet Transfusion for PDA Closure in Preterm Infants: A Randomized Controlled Trial. Pediatrics 2019; 143(5). [DOI] [PubMed] [Google Scholar]
- 74.Patel RM, Josephson CD, Shenvi N, Maheshwari A, Easley KA, Stowell S et al. Platelet transfusions and mortality in necrotizing enterocolitis. Transfusion 2019; 59(3): 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davenport P, Sola-Visner M. Hemostatic Challenges in Neonates. Frontiers in pediatrics 2021; 9: 627715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore CM, Curley A. Platelet transfusion thresholds in neonatal medicine. Early Hum Dev 2019; 138: 104845. [DOI] [PubMed] [Google Scholar]
- 77.Ferrer-Marin F, Chavda C, Lampa M, Michelson AD, Frelinger AL 3rd, Sola-Visner M Effects of in vitro adult platelet transfusions on neonatal hemostasis. J Thromb Haemost 2011; 9(5): 1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baharoglu MI, Cordonnier C, Al-Shahi Salman R, de Gans K, Koopman MM, Brand A et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 2016; 387(10038): 2605–2613. [DOI] [PubMed] [Google Scholar]
- 79.Liu ZJ, Hoffmeister KM, Hu Z, Mager DE, Ait-Oudhia S, Debrincat MA et al. Expansion of the neonatal platelet mass is achieved via an extension of platelet lifespan. Blood 2014; 123(22): 3381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fustolo-Gunnink SF, Roehr CC, Lieberman L, Christensen RD, Van Der Bom JG, Dame C et al. Platelet and red cell transfusions for neonates: lifesavers or Trojan horses? Expert review of hematology 2019; 12(10): 797–800. [DOI] [PubMed] [Google Scholar]
- 81.Deschmann E, Saxonhouse MA, Feldman HA, Norman M, Barbian M, Sola-Visner M. Association of Bleeding Scores and Platelet Transfusions With Platelet Counts and Closure Times in Response to Adenosine Diphosphate (CT-ADPs) Among Preterm Neonates With Thrombocytopenia. JAMA network open 2020; 3(4): e203394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fustolo-Gunnink SF, Fijnvandraat K, Putter H, Ree IM, Caram-Deelder C, Andriessen P et al. Dynamic prediction of bleeding risk in thrombocytopenic preterm neonates. Haematologica 2019; 104(11): 2300–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.New HV, Stanworth SJ, Gottstein R, Cantwell C, Berryman J, Chalmers EA et al. British Society for Haematology Guidelines on transfusion for fetuses, neonates and older children (Br J Haematol. 2016;175:784–828). Addendum August 2020. Br J Haematol 2020; 191(5): 725–727. [DOI] [PubMed] [Google Scholar]
- 84.Zerra PE, Josephson CD. Transfusion in Neonatal Patients: Review of Evidence-Based Guidelines. Clinics in laboratory medicine 2021; 41(1): 15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dannaway DC, Noori S. A randomized trial of platelet transfusions over 30 vs 120 minutes: is there an effect on post-transfusion platelet counts? J Perinatol 2013; 33(9): 703–6. [DOI] [PubMed] [Google Scholar]