Abstract
Objective:
To determine whether intra-articular corticosteroid injections are associated with increased knee osteoarthritis progression compared to hyaluronic acid injections which has been reported to delay OA progression and knee replacement.
Methods:
We identified participants from two large cohort studies, the Osteoarthritis Initiative and the Multicenter Osteoarthritis Study. Study visits were performed at regular intervals and included questionnaires about intraarticular corticosteroid or hyaluronic acid injection use in the previous 6 months, incident total knee replacement and knee radiographs, which were obtained and interpreted in similar fashion. Outcomes were radiographic progression based on Kellgren and Lawrence grade and joint space narrowing for both cohorts; medial joint space width for Osteoarthritis Initiative participants; and incident total knee replacement. We compared pre- and post-injection x-rays to generate rate ratios of progression comparing corticosteroid injection with hyaluronic acid users. A Cox proportional hazards model was used to estimate rate of total knee replacement for both groups.
Results:
We studied 791 participants (980 knees) with knee osteoarthritis, of whom 629 reported CSI use and 162 HAI use. Rate ratios of progression were similar between corticosteroid and hyaluronic acid injection users for Joint Space Narrowing (1.00 [95% CI 0.83–1.21]), Kellgren and Lawrence grade (1.03 [95% CI: 0.83 – 1.29]) and medial joint space width (1.03 [95% CI 0.72 – 1.48]). Hazard of total knee replacement was slightly lower for intraarticular corticosteroid compared to hyaluronic acid users (HR 0.75, 95% CI 0.51 – 1.09).
Conclusions:
Intraarticular corticosteroid injections are not associated with increased risk of progression compared to hyaluronic acid.
INTRODUCTION
Knee osteoarthritis (OA) affects 1 in 8 Americans over the age of 50 (1) and is associated with reduced quality of life and increased mortality (2). Intra-articular corticosteroid injections (CSI) and hyaluronic acid injections (HAI) are popular treatments for this disease.
Recent studies have raised the concern that knees treated with CSI are at high risk of OA progression. A randomized controlled trial showed a small but statistically significant increase in cartilage loss in knees treated with CSI (3) and a large cohort study reported a three-fold increased risk of knee OA progression with repeated CSI compared with nonusers (4). A limitation of observational studies is that subjects receiving CSI are not compared to those receiving comparable treatment. Patients receiving CSI have more advanced knee OA, itself a risk factor for disease progression (5).
A natural comparator for knees receiving CSI are those receiving HAI. Both injections are used in similar patients, and HAI has not been associated with increased radiographic progression(6) and may even delay knee replacement.(7, 8) The purpose of this study was to compare radiographic knee OA progression and knee replacement risk in CSI compared to HAI.
METHODS
Study population
We utilized 2 observational prospective cohort studies of knee OA risks in persons with or at risk of disease which collected data on OA treatments and outcomes. In the Osteoarthritis Initiative (OAI) (for detail see https://nda.nih.gov/oai/). Study visits were performed every 12 months; we utilized data from baseline through the 8th annual visit. In the Multicenter Osteoarthritis Study (MOST) (for detail see https://most.ucsf.edu/) study visits are performed approximately every 30 months, and we utilized data from baseline through 8-year visits.
Assessment of CSI and HAI Use
At baseline and each follow-up visit for both MOST and OAI, participants were questioned as to whether they had received CSI or HAI in their knees in the preceding 6 months and, if yes, which knees received injection(s).
Assessment of radiographic progression and total knee replacement
In both studies, knee x-rays were obtained at baseline and follow-up visits using similar acquisition and reading protocols. The same readers read x-rays from both studies. Kellgren and Lawrence grades (KL) (0–4) for the knee, and joint space narrowing (JSN) (0–3) were scored separately in medial and lateral tibiofemoral compartments) (9). We used partial grades when JSN progression did not reach a full grade of narrowing (e.g. 1 to 1.5) and considered any increase in JSN score in either medial or lateral joint as progression (10). Disagreements were resolved by a 3-reader adjudication panel. In OAI, per recommendations (11), the JSW250 site in the medial joint was used for analysis of progression which provided a continuous quantitative measure for progression assessment.
Presence of TKR was evaluated by history and x-rays at each visit for both studies. Incident TKR was reported by participants at the time of occurrence and confirmed on radiographs.
Statistical analysis
For radiographic progression, we excluded knees with baseline KL 4 and CSI or HAI reported at baseline visit as we could not evaluate progression from before to after treatment. Knees from participants reporting CSI and HAI at the same examination were also excluded. We compared x-rays from the visit before the first reported injection to x-rays at the visit after the last report (see Figure 1 for injections at single and consecutive exams; note that all radiographic follow-ups were at least one year after the injections). If multiple injections were reported at nonconsecutive exams, we analyzed only the first post-injection visit. For those reporting one treatment at one exam and the other treatment later, we examined progression from the first treatment, censoring them when they reported the second. If a knee had undergone a TKR, we assigned it KL grade 4 and JSN 3 at the first TKR visit and, because x-rays with knee replacement do not permit assessment of joint space loss, JSW250 was not calculated. Because those with prior injections may be closer to needing TKRs, we also carried out a secondary analysis which we limited to knees where participants had not reported prior injections of the comparator drug.
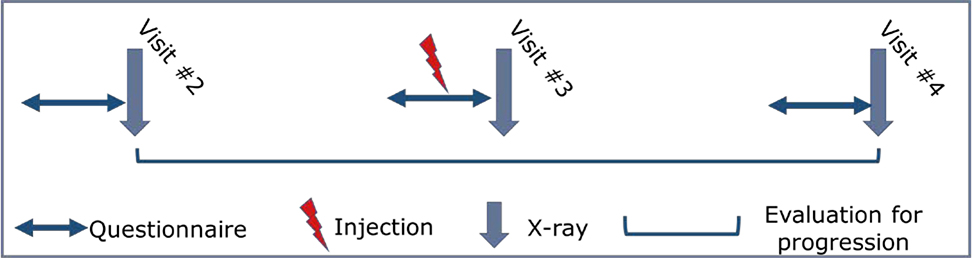
Figure 1. Sample analysis of participant reporting single injection.
Example analysis for participant reporting single knee injection at visit #3 questionnaire. Radiographic progression would be evaluated from the examination before (visit #2) to the examination after (visit #4) the reported injection.
Negative binomial regression estimated progression rates based on the number of exams with progression with an offset to account for the duration of time under observation. In the OAI where joint space was quantitatively assessed at each examination, we calculated an annualized rate of change in JSW250. We also carried out a sensitivity analysis excluding knees with lateral joint space narrowing at the pre-injection examination. Because some subjects had both knees injected, we used generalized estimating equations to adjust for the correlation between knees.
For incident TKR analysis, we included all knees from the progression analysis in addition to knees reporting treatment with CSI or HAI at the baseline visit and knees with baseline KL ≤4. Starting with the pre-injection visit, we carried out Cox Proportional Hazards Models where injections were time dependent covariates. For knees with repeated consecutive injections, we increased the covariate value from 1 to 2 to examine whether repeated injections increase risk. We censored events occurring after 7 years from baseline. All regression analyses were adjusted for age, sex, BMI, study of origin and baseline KL grade of knee using SAS version 9.4.
RESULTS
For radiographic progression, we assessed 980 knees in 791 subjects (65.61% female, mean age 66.2 years) with mean BMI 30.7 kg/m2 and mean baseline KL grade 1.91 (see Table 1). 773 knees were treated with CSI and 207 with HAI.
Table 1.
Pre-injection clinical and radiographic status
| Variable (MOST&OAI) | CSI (single) N=553 people | HAI (single) N=142 people | CSI (consecutive) N=76 people | HAI (consecutive) N=20 people |
|---|---|---|---|---|
| Mean Age in years (s.d.) | 66.3 (9.0) | 64.8 (8.3) | 66.2 (9.1) | 62.6 (7.3) |
| % Women | 69% | 55% | 58% | 65% |
| Mean BMI (s.d.) | 30.6 (5.3) | 31.4 (6.2) | 29.2 (4.6) | 31.8 (7.4) |
| (MOST&OAI) | n=651 knees | n=178 knees | n=122 knees | n=29 knees |
| Mean WOMAC pain (s.d.) | 4.3 (3.3) | 5.0 (3.8) | 4.7 (3.7) | 5.5 (2.6) |
| Mean JSN (0–3) (s.d.) | 1.0 (0.9) | 1.3 (0.9) | 1.0 (0.88) | 1.3 (1.0) |
| Mean Kellgren and Lawrence Grade (0–4) (s.d.) | 1.9 (1.1) | 2.1 (1.1) | 1.9 (1.03) | 2.0 (1.1) |
| (OAI only) | n=476 knees | n=111 knees | n=110 knees | n=18 knees |
| Mean JSW250 in mm (s.d.) | 5.2 (1.8) | 4.7 (1.6) | 5.2 (1.9) | 5.3 (2.1) |
Rates of radiographic progression were similar for knees reporting CSI compared to HAI (see Table 2). The rate ratio for JSN progression for CSI compared to HAI was 1.00 (95% CI: 0.83 – 1.21). The rate ratio for KL progression for CSI compared to HAI was 1.03 (95% CI: 0.83 – 1.29) and for progression of JSW250 for CSI compared to HAI was 1.03 (95% CI: 0.72 – 1.48).
Table 2.
Rate of radiographic progression
| Rate Ratio* of Progression: CSI vs HAI (95% CI) | ||
|---|---|---|
|
| ||
| Joint space narrowing (JSN) | 1.00 | (0.83 – 1.21) |
| Kellgren and Lawrence grade (KL) | 1.03 | (0.83 – 1.29) |
| Medial joint space width (JSW250)¶ | 1.03 | (0.72 – 1.48) |
Reported as difference in rates, positive value denotes higher value for progression of corticosteroid injection. Analyses adjusted for age, sex, BMI, study of origin and baseline KL grade.
JSW250 calculated using only OAI data, progression defined as difference > 0.5 mm.
For incident TKR, we assessed 1513 knees (63% female, mean age 63.1 years) with mean BMI 30.8 kg/m2. 1235 knees were treated with CSI and 278 with HAI. CSI showed a slightly lower risk of later TKR than HAI (HR 0.75, 95% CI: 0.51 – 1.09).
In secondary analysis of knees in which prior injections of the comparator drug was not reported (775 with CSI and 244 with HA), we found that the risk of TKR for those getting steroid injections was 0.74 (95% CI 0.37 – 1.47).
DISCUSSION
Our findings suggest similar rates of disease progression and TKR in those receiving CSI and HAI.
HAI has been proposed as a treatment that may delay time to TKR. Delbarre et al. found that knees treated with HAI had a prolonged TKR-free survival time compared to HAI nonusers (7). While this has not been demonstrated consistently (8), no studies have suggested that HAI accelerates disease progression. While we found that knees receiving CSI has a slightly lower rate of subsequent TKR than those treated with HA; this difference was modest, not statistically significant and of uncertain meaning.
Using data from OAI, Zeng et al. reported that those receiving CSI had a greater risk of radiographic progression and TKR than untreated persons (4). Patients often receive CSI in an attempt to delay surgery. Our findings suggest there may be fundamental differences between patients getting injections and those who do not, differences not eliminated by statistical adjustments.
While current insurance coverage for HA injections requires that persons have failed steroid injections, that was not true when our study was in the field. For Medicare, insurance coverage policy changed in October, 2015 and our baseline examinations were in 2004 and 2005.
Our study has limitations. In both cohorts, participants only reported injections received 6 months prior to the study visit and earlier injections were not recorded. Participants may also not have correctly recalled the type of injection received.
In conclusion, in two large prospective cohorts, the rate of disease progression among knees receiving CSI was not different from those receiving HAI. Our data should provide reassurance to clinicians and patients. The risk of OA progression attributed to CSI in earlier studies may reflect more advanced disease in those receiving injections.
Acknowledgments
Supported by NIH grants U01-AG018820, U01AG18832; U01AG18947; U01AG19069 and P30 AR072571 and support from the NIHR Manchester Biomedical Research Centre
REFERENCES
- 1.Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci USA. 2017;114:9332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Nguyen UDT, Lane NE, Lu N, Wei J, Lei G, et al. Knee osteoarthritis, potential mediators, and risk of all-cause mortality: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2021;73:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A randomized clinical trial. JAMA. 2017;317:1967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng C, Lane NE, Hunter DJ, Wei J, Choi HK, McAlindon TE, et al. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: results from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2019;27:855–62. [DOI] [PubMed] [Google Scholar]
- 5.Bastick AN, Runhaar J, Belo JN, Bierma-Zeinstra SM. Prognostic factors for progression of clinical osteoarthritis of the knee: a systematic review of observational studies. Arthritis Res Ther. 2015;17:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jubb RW, Piva S, Beinat L, Dacre J, Gishen P. A one-year, randomised, placebo (saline) controlled clinical trial of 500–730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract. 2003;57:467–74. [PubMed] [Google Scholar]
- 7.Delbarre A, Amor B, Bardoulat I, Tetafort A, Pelletier-Fleury N. Do intra-articular hyaluronic acid injections delay total knee replacement in patients with osteoarthritis - A Cox model analysis. PLoS One. 2017;12:e0187227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shewale AR, Barnes CL, Fischbach LA, Ounpraseuth ST, Painter JT, Martin BC. Comparative effectiveness of intra-articular hyaluronic acid and corticosteroid injections on the time to surgical knee procedures. J Arthroplasty. 2017;32:3591–7 e24. [DOI] [PubMed] [Google Scholar]
- 9.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15 Suppl A:A1–56. [DOI] [PubMed] [Google Scholar]
- 10.Cranney A, Tugwell P, Cummings S, Sambrook P, Adachi J, Silman AJ, et al. Osteoporosis clinical trials endpoints: candidate variables and clinimetric properties. J Rheumatol. 1997;24:1222–9. [PubMed] [Google Scholar]
- 11.Neumann G, Hunter D, Nevitt M, Chibnik LB, Kwoh K, Chen H, et al. Location specific radiographic joint space width for osteoarthritis progression. Osteoarthritis Cartilage. 2009;17:761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]