Abstract
Objective:
To project the future age distribution of people with HIV using antiretroviral therapy (ART) in the US, under expected trends in HIV diagnosis and survival (baseline scenario) and achieving the Ending the HIV Epidemic (EHE) goals of a 75% reduction in HIV diagnoses from 2020–25 and sustaining levels to 2030 (EHE75% scenario).
Design:
An agent-based simulation model with mathematical functions estimated from North American AIDS Cohort Collaboration on Research and Design data and parameters from the US Centers for Disease Control and Prevention’s annual HIV surveillance reports.
Methods:
The PEARL (ProjEcting Age, multimoRbidity, and poLypharmacy in adults with HIV) model simulated individuals in 15 subgroups of sex-and-HIV acquisition risk and race/ethnicity. Simulation outcomes from the baseline scenario are compared with outcomes from the EHE75% scenario.
Results:
Under the baseline scenario, PEARL projects a substantial increase in number of ART-users over time, reaching a population of 909,638 [95% uncertainty range (UR): 878,449 – 946,513] by 2030. The overall median age increased from 50 years (y) in 2020 to 52y in 2030, with 23% of ART-users age ≥65y in 2030. Under the EHE75% scenario, the projected number of ART-users was 718,348 [703,044 – 737,817] (median age=56y) in 2030, with a 70% relative reduction in ART-users <30y and a 4% relative reduction in ART-users age ≥65y compared to baseline, and persistent heterogeneities in projected numbers by sex-and-HIV acquisition risk group and race/ethnicity.
Conclusions:
It is critical to prepare healthcare systems to meet the impending demand of the US population aging with HIV.
Keywords: HIV, age distribution, race, ethnicity, HIV acquisition risk, simulation model, Ending the HIV Epidemic
INTRODUCTION
Since the introduction of effective antiretroviral therapy (ART) in 1996, life expectancy of people with HIV (PWH) in the US has improved substantially.1 In 2012, HIV treatment guidelines recommended immediate ART initiation among all people diagnosed with HIV regardless of their CD4 cell count.2 Early treatment and improved survival on ART has led to an increasing prevalence of HIV in the United States (US) with more than 1 million PWH in 2019, among whom 52% were age ≥50 years (≥50y) and 11% were age ≥65y.3 Yet, significant disparities in life expectancy of PWH persist, affecting the age distribution of PWH by sex-and-HIV acquisition risk groups and race and ethnicity.4
The Ending the HIV Epidemic: A Plan for America (EHE) initiative was announced in early 2019 with the overarching goals of achieving 75% reduction in new HIV diagnoses by 2025 (EHE75%) and at least 90% reduction by 2030.5 Without a cure, however, the HIV epidemic will persist among populations infected with HIV for decades to come. As such, it is critical to prepare and strengthen HIV care systems to meet the needs of people aging with HIV, including strategies for long-term viral suppression, ensuring uninterrupted access to ART and prevention and care for comorbid conditions. At present, the future age distribution and heath care needs of PWH in the US among sex-and-HIV acquisition risk groups, and by race and ethnicity within these groups remains uncertain. The objective of this study was to project the age distribution of PWH using ART from 2020 to 2030, and to assess the impact of achieving short-term EHE goals on future age distributions.
METHODS
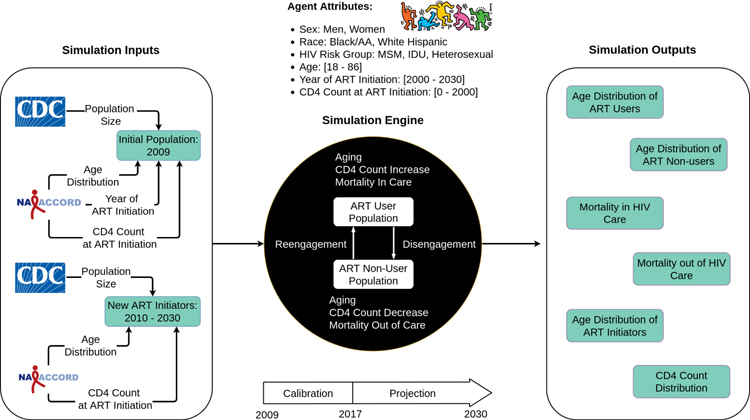
The Projecting Age, multimoRbidity, and poLypharmacy (PEARL) model is an agent-based simulation of PWH who have ever initiated ART in the US, including those who disengage from HIV care and are off ART after initiation (Figure 1). The simulated population is composed of 15 subgroups: (1 & 2) men and women with history of injection drug use (IDU) as their HIV acquisition risk factor (including those who have IDU and any additional HIV acquisition risk category specified); (3) men who have sex with men (MSM) who do not have IDU as an HIV acquisition risk factor; and (4 & 5) heterosexual men and women who do not have IDU as an HIV acquisition risk factor. These five groups were further stratified into White, Black/African American (Black/AA), and Hispanic. The model is parameterized via mathematical functions modeling the probability of simulated events by calendar year and individual-level attributes (e.g., estimated probability of mortality on ART as a function of calendar year, patient’s age, race/ethnicity, CD4 count, etc.). These functions are informed by the observed data from North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) and Centers for Disease Control and Prevention’s (CDC) HIV surveillance reports. All parameters and mathematical functions in PEARL are estimated separately in each of the 15 subgroups, except for the rate of decline in CD4 count among those disengaged from care and off-ART which was estimated as a single function across all subgroups to ensure adequate sample size. Furthermore, due to the small sample size of Hispanic IDU women in the NA-ACCORD, the age distribution at ART initiation was estimated by combining NA-ACCORD data from 2010 to 2015 and the distribution was fixed during simulated years 2010 to 2030. Further methodological details regarding the model structure, parameterizations, and estimated functions and values can be found Supplement Section 1, including tables S1–S24 and figures S1–S10, in a prior publication6 with updated functions at PEARLHIVMODEL.org/method_details.html, and in previous CDC and NA-ACCORD publications.
Figure 1:
Schematic presentation of the Projecting Age, multimoRbidity, and poLypharmacy (PEARL) model, an agent-based simulation model of people with HIV (PWH) who have initiated ART in the United States. The model is initialized with a simulated population of PWH receiving ART in year 2009, with population size estimated from the CDC’s HIV surveillance data, and age and CD4 count distributions estimated from the NA-ACCORD. ART-initiators enter the model every calendar year. We estimated the number of ART-initiators from 2010–2017 and projected the observed trends into the future (2018–2030). Simulated agents can disengage from ART, reengage with ART, or die; the probabilities of these events were estimated via mathematical functions that included calendar year, age, ART initiation date, CD4 at ART initiation, and other individual-level characteristics. Simulation projections are made from 2018–2030 in terms of population size, age-distribution and mortality in 15 subgroups of PWH who have initiated ART in the US (for an interactive map of the PEARL model that provides details on the functions that comprise the model, see PEARLHIVmodel.org)
Simulation analysis
The PEARL model was used to project the age distribution of PWH using ART in each of the 15 subgroups from 2020–2030. The baseline scenario represents the expected trends in future number of new HIV diagnoses in the absence of additional interventions. To project the future number of people diagnosed with HIV under the baseline scenario, we experimented with several candidate models to fit the CDC’s reported number of new HIV diagnoses in each of the 15 subgroups from their annual HIV surveillance reports from 2009 to 2018.7–15 After removing inferior models, predictions from the remaining models were combined to generate a range for the number of new diagnosis in each year. We modeled a gradual increase in percentage of people linking to HIV care and initiating ART over time.16,17
To estimate the impact of achieving short-term EHE75% goals, we simulated a 75% decrease in projected number of HIV diagnoses in 2020 over the course of 5 years (to 2025) as a uniform linear decline in each of the 15 subgroups (Supplement Section 2, including figure S11). Simulated scenarios were replicated for 200 runs to achieve a precision level of <5% around primary outcomes. Additional information on simulation validation to ensure the fidelity of estimated parameters and mathematical functions in PEARL under the baseline and EHE75% scenarios can be found in the Supplement Section 3, including table S25 and figures S12–S18. A description of the characteristics of projected population of ART users with and without achieving short-term EHE goals, as well as the differences in the age distributions in these two scenarios can be found in Supplement Section 4, including tables S26–S29.
Sensitivity analysis
One-way sensitivity analysis was performed by varying each of the 15 simulation parameter groups between 90% and 110% of their original value. These changes were modeled in year 2009 for parameters describing the initial population and from 2020 to 2030 for all other dynamic parameters (see Supplement Section 5, including table S30 and figures S19–21). We assessed the impact of these changes on the proportion of ART-users aged ≥65y, number of deaths in care, and number of ART users by 2030. Simulated scenarios were replicated for 200 runs, and results were compared against the baseline scenario simulations.
RESULTS
In 2020, we simulated a median population of 670,770 (95% uncertainty range [665,063 – 678,021]) ART-users using PEARL, of whom 32% were White, 45% were Black/AA, 23% were Hispanic, and included 60% MSM, 19% heterosexual women, and 9% IDU men (see Table 1 for point estimates and Table S26 for uncertainty ranges).
Table 1:
Characteristics of the projected population of people with HIV using ART in the United States. The projected characteristics of PWH using ART in PEARL are shown at selected years. Values represents the projected size and percentage of individuals in each category. These projections are compared against available estimates from CDC in year 2010 and 2018, suggesting considerable similarity across all age, race and HIV risk acquisition risk groups (detecting <5 percentage point difference in the proportion of individuals in all categories.)
| 2010 | 2018 | 2020 | 2030 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDC surveillance (all PWH)a | PEARL (PWH using ARTb) | CDC surveillance (all PWH)c | PEARL (PWH using ARTb) | PEARL (PWH using ARTb) | PEARL (PWH using ARTb) | |||||||
| Characteristics | N = 855,977 | N = 395,033 | N = 1,025,744 | N = 618,578 | N = 670,770 | N = 909,638 | ||||||
|
| ||||||||||||
| Age (in years) | ||||||||||||
| <13 | 2,971 | 0% | n/a | -- | 1,912 | 0% | n/a | -- | n/a | -- | n/a | -- |
| 13–14 | 1,177 | 0% | n/a | -- | 664 | 0% | n/a | -- | n/a | -- | n/a | -- |
| 15–19d | 7,293 | 1% | 696 | 0% | 4,322 | 0% | 648 | 0% | 696 | 0% | 1179 | 0% |
| 20–24 | 29,400 | 3% | 8,447 | 2% | 26,914 | 3% | 11,223 | 2% | 11,438 | 2% | 12,001 | 1% |
| 25–29 | 49,774 | 6% | 20,064 | 5% | 70,839 | 7% | 39,821 | 6% | 40,385 | 6% | 40,455 | 4% |
| 30–34 | 68,482 | 8% | 30,866 | 8% | 87,843 | 9% | 58,915 | 10% | 66,879 | 10% | 78,078 | 9% |
| 35–39 | 89,707 | 10% | 47,939 | 12% | 93,817 | 9% | 54,294 | 9% | 63,324 | 9% | 100,995 | 11% |
| 40–44 | 136,593 | 16% | 67,560 | 17% | 96,965 | 9% | 62,629 | 10% | 61,786 | 9% | 102,485 | 11% |
| 45–49 | 168,608 | 20% | 78,977 | 20% | 123,900 | 12% | 83,901 | 14% | 80,627 | 12% | 86,341 | 9% |
| 50–54 | 135,106 | 16% | 66,251 | 17% | 158,278 | 15% | 99,404 | 16% | 98,786 | 15% | 82,619 | 9% |
| 55–59 | 86,743 | 10% | 42,387 | 11% | 153,473 | 15% | 92,261 | 15% | 100,436 | 15% | 95,938 | 11% |
| 60–64 | 46,106 | 5% | 20,630 | 5% | 104,620 | 10% | 63,134 | 10% | 75,456 | 11% | 103,721 | 11% |
| ≥65 | 34,017 | 4% | 11,216 | 3% | 102,197 | 10% | 52,467 | 8% | 70,988 | 11% | 205,447 | 23% |
| Male sex | 640,239 | 75% | 290,003 | 73% | 774,422 | 75% | 472,306 | 76% | 515,496 | 77% | 714,112 | 79% |
| Race | ||||||||||||
| White | 278,081 | 32% | 145,705 | 37% | 304,131 | 30% | 202,832 | 33% | 215,576 | 32% | 257,936 | 28% |
| Black/AA | 364,116 | 43% | 167,444 | 42% | 422,994 | 41% | 275,564 | 45% | 298,888 | 45% | 398,817 | 44% |
| Hispanic | 170,378 | 20% | 81,919 | 21% | 231,317 | 23% | 140,611 | 23% | 156,937 | 23% | 255,411 | 28% |
| Other | 43,401 | 5% | n/a | -- | 66,538 | 6% | n/a | -- | n/a | -- | n/a | -- |
| Subgroups | ||||||||||||
| MSM | 431,076 | 50% | 210,618 | 53% | 571,934 | 56% | 366,232 | 59% | 403,855 | 60% | 581,243 | 64% |
| White MSM | 102,908 | 26% | 215,956 | 21% | 146,996 | 24% | 155,946 | 23% | 178,440 | 20% | ||
| Black/AA MSM | 63,076 | 16% | 178,685 | 17% | 127,938 | 21% | 143,342 | 21% | 215,384 | 24% | ||
| Hispanic MSM | 44,628 | 11% | 138,023 | 13% | 91,340 | 15% | 104,668 | 16% | 187,474 | 21% | ||
| IDU mene | 133,714 | 16% | 49,864 | 13% | 124,577 | 12% | 56,363 | 9% | 57,752 | 9% | 64,336 | 7% |
| White IDU men | 17,542 | 4% | 36,254 | 4% | 21,235 | 3% | 22,411 | 3% | 28,677 | 3% | ||
| Black/AA IDU men | 20,538 | 5% | 48,151 | 5% | 21,312 | 3% | 20,834 | 3% | 17,618 | 2% | ||
| Hispanic IDU men | 11,783 | 3% | 31,935 | 3% | 13,814 | 2% | 14,464 | 2% | 17,926 | 2% | ||
| IDU women | 53,226 | 6% | 25,819 | 7% | 48,916 | 5% | 26,613 | 4% | 26,714 | 4% | 26,198 | 3% |
| White IDU women | 7,464 | 2% | 12,495 | 1% | 8,248 | 1% | 8,462 | 1% | 9,288 | 1% | ||
| Black/AA IDU women | 14,572 | 4% | 23,664 | 2% | 14,931 | 2% | 14,888 | 2% | 14,140 | 2% | ||
| Hispanic IDU women | 3,786 | 1% | 9,389 | 1% | 3,458 | 1% | 3,385 | 1% | 2,952 | 0% | ||
| Heterosexual men | 37,751 | 4% | 29,508 | 7% | 79,401 | 8% | 49,504 | 8% | 53,416 | 8% | 67,536 | 7% |
| White heterosexual men | 3,487 | 1% | 26,263 | 3% | 6,336 | 1% | 7,032 | 1% | 10,342 | 1% | ||
| Black/AA heterosexual men | 19,168 | 5% | 49,661 | 5% | 32,670 | 5% | 35,096 | 5% | 42,544 | 5% | ||
| Hispanic heterosexual men | 6,853 | 2% | 15,719 | 2% | 10,520 | 2% | 11,317 | 2% | 14,644 | 2% | ||
| Heterosexual women | 153,479 | 18% | 79,229 | 20% | 183,954 | 18% | 119,830 | 19% | 128,970 | 19% | 169,990 | 19% |
| White heterosexual women | 14,290 | 4% | 9,895 | 1% | 19,874 | 3% | 21,454 | 3% | 29,712 | 3% | ||
| Black/AA heterosexual women | 50,082 | 13% | 113,952 | 11% | 78,626 | 13% | 84,585 | 13% | 108,412 | 12% | ||
| Hispanic heterosexual women | 14,856 | 4% | 32,848 | 3% | 21,296 | 3% | 23,012 | 3% | 31,766 | 3% | ||
| ART-initiators | n/a | -- | 25,087 | -- | n/a | -- | 33,068 | -- | 32,934 | -- | 33,044 | -- |
| ART-users | n/a | -- | 395,033 | -- | n/a | -- | 618,578 | -- | 670,770 | -- | 909,638 | -- |
CDC’s HIV surveillance data capture all persons with diagnosed HIV, regardless of whether they have initiated ART. Data are publicly available. Centers for Disease Control and Prevention. HIV Surveillance Report, 2014; vol 26. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2014-vol-26.pdf. Table 14b, estimated numbers. Published November 2015. Accessed June 22, 2020.
Values represent the median for each simulated outcome across 200 random simulation replications (see Supplement Table S26 for 95% uncertainty ranges). PEARL projections of PWH using ART do not include the “42,216”, “43,134”, “41,728” and “32,988” people who initiated ART but were not using ART treatment in 2010, 2018, 2020, and 2030 (respectively) and people of “other” races/ethnicities.
CDC’s HIV surveillance data capture all persons with diagnosed HIV, regardless of whether they have initiated ART. Data are publicly available. Centers for Disease Control and Prevention. Diagnosis of HIV Infection in the United States and Dependent Areas, 2018; vol31. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.Tables 14a and 14b. Published May 2020. Accessed September 9, 2020.
This category includes only ages 18–20 for PEARL model.
MSM who also have IDU as an HIV acquisition risk group were include in the IDU HIV acquisition risk group.
Validity of the model was confirmed by comparing the observed age distributions of PWH using ART from 2010–2017 in NA-ACCORD with the simulated outcomes, suggesting no significant pattern of bias in projections from the model among 15 subgroups over time (Supplement Section 3.2, table S25 and figure S18). Furthermore, we detected no relevant differences (>5%) in characteristics of the simulated populations of ART-users and the CDC’s report of PWH (not restricted to PWH using ART) in years 2010 and 2018 in the US (Table 1).
Projections under the baseline scenario
A relatively stable number of ART-initiators was projected over time using PEARL, from 32,934 [31,890 – 34,141] in 2020 to 33,044 [28,696 – 38,085] in 2030. From 2020 to 2030, White MSM experience the largest reduction of 49% in number of ART initiators (from 5,738 [5,231 – 6,251] to 2,907 [1,062 – 4,904]), while Hispanic MSM experience the largest increase of 43% (from 7,498 [6,980 – 8,001] to 10,743 [7,857 – 13,624]), followed by Hispanic IDU women with a 43% increase (136 [93 – 184] to 194 [49 – 345]) and a 41% increase in White IDU women (from 464 [432 – 494] to 654 [545 – 758] (Supplement Section 4.2, table S27). Projected median ages at ART initiation in 2030 will range from 30y in Black/AA MSM to 55y in White heterosexual men (Figure 2). The projected distribution of age at ART initiation shifts toward younger ages from 2020–2030 among White MSM, White and Hispanic IDU men, and Black/AA heterosexual men, and Hispanic heterosexual women, and remains similar (+/− 2 years) from 2020 to 2030 among Black/AA and Hispanic MSM and Black/AA heterosexual women.
Figure 2:
Distributions of age at ART initiation among subgroups of people with HIV using ART in the United States in 2010, 2020, and 2030. Median age in 2010, 2020 and 2030 is shown in blue, purple and red, respectively. The area under 2030’s age distribution is shaded in red for better visibility.
Figure 2 footnotes: Due to the small sample size of Hispanic IDU women in the NA-ACCORD, the age distribution at ART initiation was estimated by combining NA-ACCORD data from 2010 to 2015 and this distribution was kept fixed during simulated years (2010–2030).
The population size of ART-users projected by PEARL increased over time, reaching a total of 909,638 [878,449 – 946,513] ART-users by 2030, with 23% of this population age ≥65y. The projected proportion of ART-users who are Black/AA remains stable at 45% in 2020 and 44% in 2030. Areduction in the proportion of White ART-users (32% in 2020 to 28% in 2030) was projected, as well as an increase in the proportion of Hispanic ART-users (23% in 2020 to 28% in 2030) over the next decade (Table 1). In 2030, MSM will account for the largest subgroup of ART-users at 64%, followed by heterosexual women at 19%. The proportion of IDU men and women using ART is projected to decline from 9% to 7%, and from 4% to 3% from 2020–2030, respectively.
Despite projected reductions in median age at ART initiation among most subgroups, the overall median age of ART-users is projected to increase from 50y [50y – 50y] in 2020 to 52y [51y – 52y] in 2030 (Figure 3a). This is accompanied by a shift in structure of the age distribution, evolving from a unimodal distribution in 2010 to a bimodal distribution in 2020 (with peaks at 33 and 55 years) and in 2030 (with peaks at 40 and 63 years). After stratifying by subgroup, the projected median age of ART-users decreased by 1 year from 2020 to 2030 among Hispanic MSM and Hispanic IDU men, increased by 1 year among Black/AA MSM and white IDU men and women, by and by 3 years among Black/AA IDU men and Hispanic IDU women; all other subgroups experienced ≥5y increase in median age from 2020–2030 (Figure 3b). In 2030, all but two subgroups (Black/AA and Hispanic MSM) had a median age that was greater than the overall median age of 52 years.
Figure 3a-d:
Projected age distributions, overall and among subgroups, of people with HIV using ART in the United States in 2010, 2020, and 2030 in the baseline scenario (panels a & b) and after simulating a 75% reduction in new HIV diagnoses from 2020–2025 (EHE75% - panels c & d). Median age in 2010, 2020 and 2030 is shown in blue, purple and red respectively. The area under 2030’s age distribution is shaded in red for better visibility. Black dots reflect observed data from the NA-ACCORD in year 2010, and colored lines reflect projections from the PEARL model.
3a) Overall age distribution, baseline scenario
3b) Age distributions among subgroups, baseline scenario
3c) Overall age distribution, EHE75% scenario
3d) Age distributions among subgroups, EHE75% scenario
Projections under the EHE75% scenario
Under EHE75% scenario, a gradual reduction in number of ART-initiators over time is projected using PEARL, from 33,058 [32,082 – 34,268] in 2020 to 8,580 [7,503 – 9,849] in 2030. These reductions are modeled similarily among all subgroups. Projected median ages at ART initiation remained stable at 33 [32 – 33] in 2020 to 33 [31 – 34] in 2030.
Achieving EHE75% goals results in subsequent reductions in the number of ART-users in 2030, from 909,638 [878,449 – 946,513] at baseline to 718,348 [703,044 – 737,817] under EHE75% scenario (Figure 4a). The projected reductions are primarily observed among younger individuals (e.g., the number of ART-users age <30y decreases by 70% from 54,206 [46,751 – 61,422] under baseline scenario to 16,263 [13,589 – 18,799] under EHE75% scenario), and were not consistent in magnitude across all 15-subgroups (Table S28). For example, White MSM ART-users experienced a 10% relative decline (from 178,440 [164,452 – 193,405] under baseline scenario to 159,940 [151,954 – 167,447] under EHE75% scenario, Figure 4b), while Black/AA heterosexual women experienced a 16% relative decline (108,412 [100,915 – 116,887] at baseline to 91,240 [86,453 – 95,960] under EHE75% scenario, Figure 4c).
Figure 4a-c:
Projected number of people with HIV using ART in the United States in the baseline scenario and after simulating a 75% reduction in ART-initiators from 2020–2025 (EHE75%) a) overall, b) among White MSM, and c) among heterosexual Black/AA women
4a) Projected number of people with HIV using ART in the baseline scenario (left) and in the EHE75% scenario (right), 2010–2030
4b) Projected number of White MSM using ART in the baseline scenario (left) and in the EHE75% scenario (right), 2010–2030
4c) Projected number of Black/AA heterosexual women using ART in the baseline scenario (left) and in the EHE75% scenario(right), 2010–2030
Despite projected changes in size of ART-user subgroups under the EHE75% scenario, we did not project significant differences in the demographic distribution of ART-users in 2030 compared to the baseline scenario (Supplement Section 4.3, table S28). The overall median age of PWH using ART in 2030 is projected to increase from 52y [51y – 52y] at baseline to 56y [55y – 56y] under EHE75% scenario (Figures 3a, 3c). Similar to the baseline scenario, the EHE75% scenario suggests the emergence of a bimodal age distribution (peaks at 42y and 64y) in 2030. The simulated decline in number of new diagnoses and ART-initiators from 2020 to 2030 under the EHE75% scenario accelerated an increase in median age among all subgroups, corresponding to an increase in projected median age of 5 years among Black/AA and Hispanic MSM, Black/AA and Hispanic IDU men, and White IDU women, 6 years among White IDU men and ≥7y among all other subgroups(Figure 3d). The projected median age among all subgroups except for Black/AA MSM and Hispanic MSM was be greater than the overall median age of 56y in 2030. Detailed comparison of the projected age distributions of ART-users under the baseline and EHE75% scenarios can be found in Supplement Section 4.4, table S29.
Sensitivity Analyses
Using a threshold of 10% to identify clinically-relevant changes relative to the baseline scenario, the proportion of ART-users age ≥65y and number of deaths among ART users in year 2030 were sensitive to variation in: a) mean age of ART-users in year 2009 and b) mean age of ART initiators as a dynamic parameter from 2020 to 2030 (Supplement Section 5, figures S19–S20), while the projected number of ART-users in 2030 was robust to variation in value of all parameters (Supplement Section 5, figure S21).
DISCUSSION
We projected a population of 909,638 ART-users (and an additional 32,988 individuals who initiated ART but were subsequently disengaged from ART) in the US by year 2030 using the PEARL model. Our results suggest a gradual increase in median age among those on ART, from 46y in 2010, to 50y in 2020, and 52y in 2030. Achieving short-term EHE’s goals for a 75% reduction in new HIV diagnoses from 2020–2025 will reduce the number of ART-users to 718,348 by 2030 and will accelerate the increase in median age among ART-users. Significant heterogeneities in size and age distributions of ART-users were projected in 15 subgroups defined by sex, HIV acquisition risk groups and race/ethnicity.
Our results suggest an increase in proportion of older ART-users over time, with 54% of the population age ≥50y and 23% age ≥65y by 2030. CDC reports that 52% of all PWH in the US were age ≥50y in 2019; this proportion has been increasing by appproximately 2 percentage points annually over the last decade, and the increase has begun to slow down in recent years.3 The reduction in the median age at ART initiation in the Treat All era can potentially contribute to the slowed increase in proportion of older adults with HIV in recent years. Despite a projected increase in the overall median age, two subgroups (Hispanic MSM and Hispanic IDU men) experienced small (1-year) reductions in median age from 2020–2030 which can be attributed to the young age at ART initiation (Hispanic MSM) and/or high sensitivity of these subgroups to variability in the mean age of ART users in 2009 and dynamicially from 2020 to 2030 (as shown in the sensitivity analysis), driven by the small sample size observed in the NA-ACCORD of these subgroups.
Projections of size and age distribution of PWH from the PEARL model align with previous findings/projections in high income countries.18,19 In comparison to HIV surveillance reports, the projected population of 618,578 ART-users in 2018 is 21% lower than the CDC’s estimate of 779K individuals living with HIV and receiving medical care (as defined by having ≥1 CD4 or HIV RNA measurement) in 2018.15 Even after accounting for the 43,134 agents who were disengaged from ART in 2018 (but are not necessarily out of care) and the 6.6% of PWH3 in racial/ethnic and HIV acquisition subgroups not currently included in the PEARL model, the adjusted estimate of ART-users in 2018 from the PEARL model (~7050K) is lower than the CDC’s estimate of 779K receiving medical care – as such our projections of the ART-user population are conservative estimates.
Adults aging with HIV experience a high burden of age-related comorbidities, which may be reduced with comorbidity prevention and treatment interventions.20–26 Existing heterogeneities in burden of type-specific comorbidities by race/ethnicity suggest the need for tailoring of existing interventions. For example, preventing hypertension in Black/AA men with HIV, who have high rates of hypertension, may result in a greater reduction in overall comorbidity burden as compared to the same interventions in non-Black women, who have lower rates of hypertension.27 The difference in our projected age distributions of ART-users by sex, HIV acquisition risk and race/ethnicity over the next decade further suggests the need for tailoring future comorbidity prevention programs to the needs of specific subgroups, ensuring the most effective intervention meets the most appropriate subgroup at the right time.28 Significant challenges exist to bolster the current HIV workforce to meet the growing needs of populations aging with HIV who require further expertise to manage multimorbidity.29,30 In terms of cost, the projected increase in number of ART-users to >900K by 2030 will likely result in a substantial increase in national spending on ART (estimated at $420,285 life time discounted costs in 2019 or $22.5 billion annual undiscounted in 2018) and additional costs associated with treating HIV comorbidities and preventing falls and frailty.31–33 New interventions aiming to increase the effectiveness of comorbidity prevention among PWH are likely to be cost-effective (and even cost-saving) over the next decade.
Our projections are subject to specific limitations. First, given that successful long-term aging with HIV can only occur in the context of ART (with the rare exception of the long-term non-progressors), the PEARL model does not include individuals who do not initiate ART.34 Second, due to low numbers, the PEARL model does not currently include transgender people, American Native, Native Hawaiian/Native Other Pacific Islander, Asian, those reporting multiple races/ethnicities, and perinatally-infected individuals; accounting for 6.6% of all PWH in the US in 2019.3 Third, the PEARL model does not model HIV transmission. Our approach to estimating the number of new ART-initiators relies on CDC estimates of new HIV diagnoses, receipt of HIV care in 1 to 3 months after diagnosis, and estimates from the ART guidelines that suggest 97% of those presenting for care immediately initiate ART.[15,34,35] Fourth, the assumption that the current HIV guidelines and present trends in ART initiation persist to 2030 is inherent in the projections. Fifth, the estimated functions in of the PEARL model may be affected by the sample size considerations in the underling NA-ACCORD dataset, as suggested by higher sensitivity of specific subgroups with small sample size to variation of model parameters. Finally, the experimental simulation of achieving the EHE75% goal is a simplified representation with a uniform linear 75% decline over five years in all subgroups; it does not account for non-linear relationships or anticipated heterogeneities in the decline by subgroups and the impact on other outcomes like engagement in ART use. While modeling all of these intricacies is important for an in-depth evaluation of the impact of achieving EHE75%, our goal with our experimental simulation was to represent an “optimistic” scenario for achieving short-term goals of HIV control in the US in order to demonstrate that even under such ambitious targets, the US HIV epidemic will still sustain over several decades.
The Ending the HIV Epidemic goal to reduce the number of new HIV diagnoses by 75% to 2025 is an ambitious, but a much-needed target to reduce the long-term burden of HIV in the US. However, without a cure, the HIV epidemic will sustain among populations infected with HIV for decades to come. Our modeling results suggest that without a substantial decrease in HIV incidence, a gradual increase in number of ART-users to >900K by 2030. Along with projected increase in median age of ART-users, these individuals are likely to experience an increased burden of comorbidities. It is critical to prepare and strengthen HIV care systems to meet the needs of the changing epidemic, including strategies for long-term viral suppression, ensuring uninterrupted access to ART and prevention and care for comorbid conditions that will likely grow in burden among this aging population.
Supplementary Material
Key points:
The age distribution of people with HIV using ART in the US will shift to older ages in the next decade, with heterogeneity by sex-and-HIV acquisition risk group and race/ethnicity. Even if EHE goals are achieved, healthcare systems must be readied to meet the needs of a large population of people aging with HIV.
ACKNOWELDGEMENTS
This research was supported by the National Institute on Aging, National Institutes of Health [grant number R01 AG053100] (PI: KN Althoff). PK was supported through the National Institution of Allergy and Infectious Diseases of the National Institutes of Health (Career development award [grant number K01AI138853]).
The NA-ACCORD is supported by National Institutes of Health grants U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01AG053100, R24AI067039, R34DA045592, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794, U54GM133807, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR002378, Z01CP010214 and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; the Grady Health System; grants CBR-86906, CBR-94036, HCP-97105 and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care, and the Government of Alberta, Canada. Additional support was provided by the National Institute Of Allergy And Infectious Diseases (NIAID), National Cancer Institute (NCI), National Heart, Lung, and Blood Institute (NHLBI), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute for Mental Health (NIMH) and National Institute on Drug Abuse (NIDA), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Nursing Research (NINR), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Footnotes
NA-ACCORD Collaborating Cohorts and Representatives:
AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch
AIDS Link to the IntraVenous Experience: Gregory D. Kirk
Emory-Grady HIV Clinical Cohort: Vincent Marconi and Jonathan Colasanti
Fenway Health HIV Cohort: Kenneth H. Mayer and Chris Grasso
HAART Observational Medical Evaluation and Research: Robert S. Hogg, Viviane Lima, Zabrinna Brummer, Julio SG Montaner, Paul Sereda, and Kate Salters
HIV Outpatient Study: Kate Buchacz and Jun Li
HIV Research Network: Kelly A. Gebo and Richard D. Moore
Johns Hopkins HIV Clinical Cohort: Richard D. Moore
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Jeffrey Jacobson
Kaiser Permanente Mid-Atlantic States: Michael A. Horberg
Kaiser Permanente Northern California: Michael J. Silverberg
Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne
MACS/WIHS Combined Cohort Study: Todd Brown, Phyllis Tien, and Gypsyamber D’Souza
Maple Leaf Medical Clinic: Graham Smith, Mona Loutfy, and Meenakshi Gupta
The McGill University Health Centre, Chronic Viral Illness Service Cohort: Marina B. Klein
Multicenter Hemophilia Cohort Study–II: Charles Rabkin
Ontario HIV Treatment Network Cohort Study: Abigail Kroch, Ann Burchell, Adrian Betts, and Joanne Lindsay
Parkland/UT Southwestern Cohort: Ank Nijhawan
Retrovirus Research Center, Universidad Central del Caribe, Bayamon Puerto Rico: Angel M. Mayor
Southern Alberta Clinic Cohort: M. John Gill
Study of the Consequences of the Protease Inhibitor Era: Jeffrey N. Martin
Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Jun Li and John T. Brooks
University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig
University of California at San Diego: Laura Bamford and Maile Karris
University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik
University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane
Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner
Veterans Aging Cohort Study: Lesley Park and Amy Justice
NA-ACCORD Study Administration:
Executive Committee: Richard D. Moore, Keri N. Althoff, Stephen J. Gange, Mari M. Kitahata, Jennifer S. Lee, Michael S. Saag, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman
Administrative Core: Richard D. Moore, Keri N. Althoff, and Aimee M. Freeman
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Liz Morton, Justin McReynolds, and William B. Lober
Epidemiology and Biostatistics Core: Stephen J. Gange, Jennifer S. Lee, Brenna Hogan, Bin You, Elizabeth Humes, Lucas Gerace, Cameron Stewart, and Sally Coburn
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Center for Disease Control and Prevention.
CW is currently employed by Regeneron Pharmaceuticals Inc and contributed to this article as a prior trainee of Johns Hopkins University. The views expressed do not necessarily represent the views of Regeneron Pharmaceuticals Inc.
REFERENCES
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents.; 2012. https://aidsinfo.nih.gov/contentfiles/adultandadolescentgl003093.pdf. [Google Scholar]
- 3.US Centers for Disease Control and Prevention. HIV Surveillance Report, 2019. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published 2019. Accessed August 4, 2021.
- 4.Althoff KN, Chandran A, Zhang J, et al. Life-expectancy disparities among adults with HIV in the United States and Canada: The impact of a reduction in drug- and alcohol-related deaths using the lives saved simulation model. Am J Epidemiol. 2019;188(12):2097–2109. doi: 10.1093/aje/kwz232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: A plan for the United States. JAMA. 2019;321(9):844–845. doi: 10.1001/jama.2019.1343 [DOI] [PubMed] [Google Scholar]
- 6.Kasaie P, Stewart C, Humes E, et al. Projecting the age-distribution of men who have sex with men receiving HIV treatment in the United States. Ann Epidemiol. 2021;S1047–2797. Online ahead of print. doi: 10.1016/j.annepidem.2021.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data — United States and 6 dependent areas, 2010. HIV Surveillance Supplemental Report 2013;18(No. 2, part B). http://www.cdc.gov/hiv/topics/surveillance/resources/reports/#supplemental. Published January 2013. Accessed August 4, 2021. [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data - United States and 6 dependent areas, 2011. HIV Surveillance Supplemental Report; 2013;18(No. 5). http://www.cdc.gov/hiv/library/reports/surveillance. Published October 2013. Accessed August 4, 2021. [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data — United States and 6 dependent areas, 2014. HIV Surveillance Supplemental Report 2014;19(No. 3) t. http://www.cdc.gov/hiv/library/reports/surveillance. Published November 2014. Accessed August 4, 2021. [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data — United States and 6 dependent areas, 2013. HIV Surveillance Supplemental Report 2015;20(No. 2). http://www.cdc.gov/hiv/library/reports/surveillance. Published July 2015. Accessed August 4, 2021. [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data — United States and 6 dependent areas, 2014. HIV Surveillance Supplemental Report 2016;21(No. 4). http://www.cdc.gov/hiv/library/reports/surveillance. Published July 2016. Accessed August 4, 2021. [Google Scholar]
- 12.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data — United States and 6 Dependent Areas, 2015. HIV Surveillance Supplemental Report 2017;22(No. 2). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published July 2017. Accessed August 4, 2021. [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data — United States and 6 dependent areas, 2016. HIV Surveillance Supplemental Report 2018;23(No. 4). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published June 2018. Accessed August 4, 2021. [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data — United States and 6 dependent areas, 2017. HIV Surveillance Supplemental Report 2019;24(No. 3). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published June 2019. Accessed August 4, 2021. [Google Scholar]
- 15.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data — United States and 6 dependent areas, 2018. HIV Surveillance Supplemental Report 2020;2 (No. 2). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2020. Accessed August 4, 2021. [Google Scholar]
- 16.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Behavioral and Clinical Characteristics of Persons Receiving Medical Care for HIV Infection—Medical Monitoring Project, United States, 2014 Cycle (June 2014-May 2015). HIV Surveillance Special Report 17. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published December 2016. Accessed August 4, 2021. [Google Scholar]
- 18.Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: A modelling study. Lancet Infect Dis. 2015;15(7):810–818. doi: 10.1016/S1473-3099(15)00056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Klein PW, Matosky MM, Mills R, Redwood RC, Cheever LW. Projected growth and needs of PLWH in HRSA’S Ryan White HIV/AIDS Program. 2019 Conference on Retroviruses and Opportunistic Infections. Seattle, WA, USA. Abstract 1065. Available at https://www.croiconference.org/abstract/projected-growth-and-needs-aging-plwh-hrsas-ryan-white-hivaids-program/. [Google Scholar]
- 20.Wong C, Gange SJ, Moore RD, et al. Multimorbidity among persons living with Human Immunodeficiency Virus in the United States. Clin Infect Dis. 2018;66(8):1230–1238. doi: 10.1093/cid/cix998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015;6(941–949). doi: 10.1093/cid/ciu919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverberg MJMJ, Lau B, Achenbach CJCJ, et al. Cumulative incidence of cancer among persons With HIV in North America: A cohort study. Ann Intern Med. 2015;163(7):507–518. doi: 10.7326/M14-2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein MB, Althoff KN, Jing Y, et al. Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis. 2016;63(9):1160–1167. doi: 10.1093/cid/ciw531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drozd DR, Kitahata MM, Althoff KN, et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr. 2017;75(5):568–576. doi: 10.1097/QAI.0000000000001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open. 2020;3(6):e207954. doi: 10.1001/jamanetworkopen.2020.7954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Althoff KN, Gebo KA, Moore RD, et al. Contributions of traditional and HIV-related risk factors on non-AIDS-defining cancer, myocardial infarction, and end-stage liver and renal diseases in adults with HIV in the USA and Canada: A collaboration of cohort studies. Lancet HIV. 2019;6(2):e93–e104. doi: 10.1016/S2352-3018(18)30295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong C, Gange SJ, Buchacz K, et al. First occurrence of diabetes, chronic kidney disease, and hypertension among north American HIV-infected adults, 2000–2013. Clin Infect Dis. 2017;64:45–67. doi: 10.1093/cid/ciw804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoury MJ, Iademarco MF, Riley WT. Precision Public Health for the Era of Precision Medicine. Am J Prev Med. 2016;50(3):398–401. doi: 10.1016/j.amepre.2015.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walensky RP, Del Rio C, Armstrong WS. Charting the future of infectious disease: Anticipating and addressing the supply and demand mismatch. Clin Infect Dis. 2017;64(10):1299–1301. doi: 10.1093/cid/cix173 [DOI] [PubMed] [Google Scholar]
- 30.Armstrong WS. The Human Immunodeficiency Virus Workforce in Crisis: An Urgent Need to Build the Foundation Required to End the Epidemic. Clin Infect Dis. 2021;72(9):1627–1630. doi: 10.1093/cid/ciaa302 [DOI] [PubMed] [Google Scholar]
- 31.Aitken M, Kleinrock M, Lyle J, Nass D, Cskey L. Medicines Use and Spending Shifts: A Review of the Use of Medicines in the US in 2014. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/medicines-use-and-spending-shifts-in-the-us-in-2014.pdf?la=en&hash=34E4E2AD15D82812DD3FAA229854A0E9. Published April 2015. Accessed March 31, 2021.
- 32.IQVIA Institute for Human Data Science. Medicine Use and Spending in the US: A Review of 2018 and Outlook to 2023. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 2019. Accessed March 31, 2021.
- 33.Bingham A, Shrestha RK, Khurana N, Jacobson E, Farnham PG. Estimated Lifetime HIV-related Medical Costs in the United States. Sex Transm Dis. 2021;48(4):299–304. doi: 10.1097/olq.0000000000001366 [DOI] [PubMed] [Google Scholar]
- 34.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed July 24, 2021. [Google Scholar]
- 35.US Centers for Disease Control and Prevention. HIV Surveillance Report, 2018. (Updated); vol 31. http://www.cdc.gov/hiv/library/reports/surveillance/. Published May 2020. Accessed August 4, 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.