Abstract
Mature megakaryocytes, the platelet precursors, originate from hematopoietic stem cell progenitors, which once committed to this lineage, undergo endomitosis leading to polyploidization. The process entails repeated rounds of DNA replication without cell division, yielding polyploid cells. Supporting the cell’s developmental process and various cellular functions are integrin receptors, a conduit of communication between the extracellular environment and the cell actin cytoskeleton. Integrins are heterodimers of α and β subunits, where different combinations of the known 18 α and 8 β subunits confer specificity to the receptor. Integrin ligands range from extracellular matrices through soluble ligands, infectious agents, and counterreceptors, to cells. In this review, we describe the different integrins expressed on bone marrow megakaryocytes and their attributed roles in lineage development and cellular functions, including adhesion, spreading, proplatelet formation, and functional interaction with other cells. Pathologies associated with dysregulated megakaryocyte integrin expression are also reviewed.
Keywords: megakaryocyte, integrins, megakaryopoiesis, platelet biogenesis
Graphical Abstract
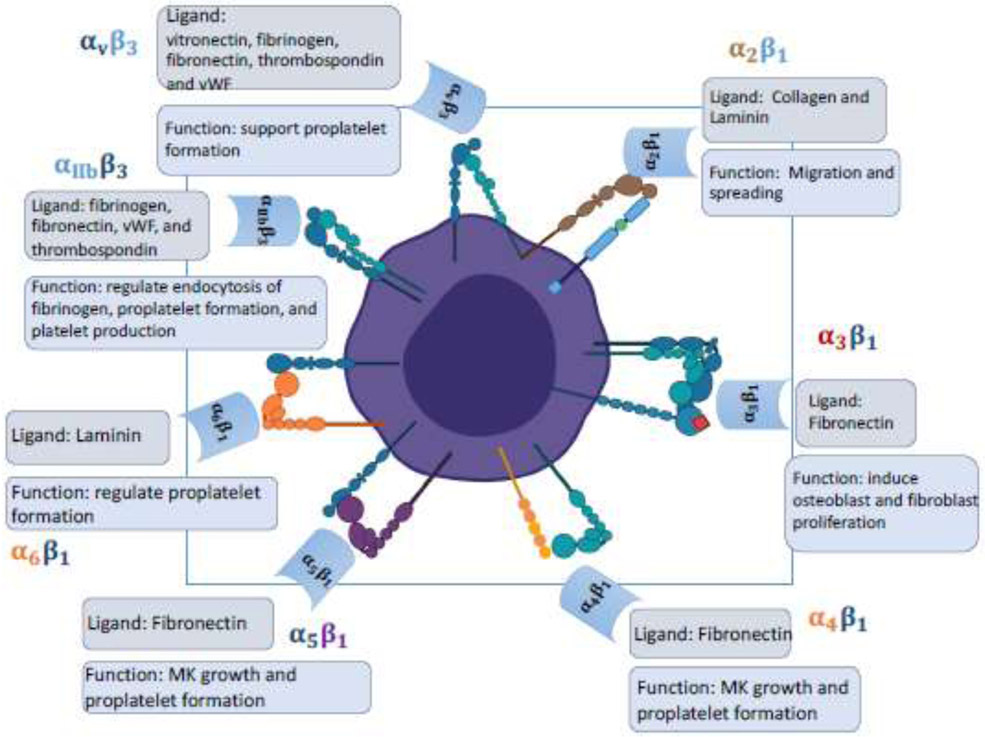
Major integrins expressed on megakaryocytes
Historical background:
First discovered by Richard Hynes in the late 1980s, integrins are transmembrane heterodimer proteins consisting of two subunits called α and β, and a total of 18 α and 8 β subunits have been identified on mammalian cells, the combination of which forms 24 distinct types [1]. Integrins contain an extracellular domain interacting with extracellular matrix (ECM) ligands such as collagen, fibronectin, vitronectin, and laminin as well as a cytoplasmic domain connecting to the actin cytoskeleton via an adhesion complex . Such complex consists of multiple other factors, and the ECM-integrin-actin cytoskeleton axis serves as a mechanical signal that can activate a variety of downstream signaling pathways, regulating cell survival, cell proliferation, cell motility, transcriptional control and cytoskeletal organization [2-4].
Megakaryocytes (MKs) are large polyploid platelet-producing cells, primarily residing in the bone marrow (BM). The importance of integrins in MK/platelet function was first appreciated through seminal studies of the severe congenital bleeding disorder, Glanzmann’s thrombasthenia, characterized by absence or deficiency of the platelet glycoprotein (GP)IIb-IIIa, or integrin αIIbβ3 [5]. Early studies on MK integrins were performed by Leven et al. on guinea pigs, showing their important role in maintaining normal MK functions [6, 7]. In this review, we will provide an overview of major types of integrins expressed on MKs, their respective ECM ligands as well as their effects on MK development and specific functions.
The β1 integrins and ECM ligand:
The β1 integrins, also called the Very Late Antigens (VLA), refer to a group of integrins that share a common β1 subunit [8]. The mature MKs express specific integrins, such as α2β1 (VLA-2), α3β1 (VLA-3), α5β1 (VLA-5), and α6β1 (VLA-6). Small immature MKs express α4β1 (VLA-4) integrins [9]. α2β1 integrin adheres to collagen and laminin in bone marrow, which is important for their spreading and migration [10]. α3β1 integrin adhere primarily to fibronectin, and in contrast to a homogenous distribution pattern on MKs exhibited by other integrins, α3β1 integrin exhibits a heterogeneous focal distribution in MKs [11], and it plays a key role in MK-induced osteoblast and fibroblast proliferation [11, 12]. Both α4β1 and α5β1 integrins bind to fibronectin, but at different sites, with α4β1 binding to the spliced V region and α5β1 to the RGD tripeptide motif [8, 13]. α4β1 and α5β1 integrins are involved with MK growth and proplatelet formation [14-16]. Finally, α6β1 integrin binds to laminin [17], but the specific effects of α6β1 integrin on MK functions are not well documented, with it being reported to have a slight regulatory effect on proplatelet formation [18].
αVβ3 integrin and ECM ligands:
αVβ3 integrin mediates binding to vitronectin, but it can also bind to fibrinogen, fibronectin, thrombospondin, and von Willebrand factor (vWF) [6, 19-21]. The presence of αβ3 integrin was first reported on guinea pig MKs and shown to support proplatelet formation [6]. αVβ3 integrin is also involved in endocytosis into MKs of fibrinogen, an essential factor for blood clot formation, and fibrinogen is stored in α granules for later secretion [19].
αIIbβ3 integrin and ECM ligands:
αIIbβ3 integrins, also known as glycoprotein GPIIb/IIIa complex, are required for platelet aggregation, and found to be a MK/platelet lineage-specific integrin [22]. αIIbβ3 mainly serves as a receptor for fibrinogen, but it can also bind to the RGD motif-containing ligands such as fibronectin, vWF, and thrombospondin [23]. αIIbβ3 plays multiple important roles in regulating MK function such as endocytosis of fibrinogen, proplatelet formation, and platelet production [24].
Figure 1 illustrates the main integrins expressed on bone marrow MKs, the functions of which is further reviewed in ensuing texts.
Figure 1:
Megakaryocyte integrins play a central role in various cellular functions to maintain homeostasis.
Integrins expression and role during megakaryopoiesis
Megakaryopoiesis, the process of hematopoietic stem cells (HSCs) differentiating into mature MKs, is complex and involves multiple progenitors. Early research to investigate integrin expression during megakaryocyte differentiation was performed on K562 cell line derived from human chronic myeloid leukemia cells. K562 cells induced to differentiate into MKs under phorbol 12,13-dibutyrate treatment showed increased expression of integrin αIIbβ3 and α2β1 [25, 26]. Studies with primary MKs differentiated from purified CD34+ progenitors corroborated the data obtained with K562 cells. Namely, α2β1 integrin is expressed on mature polyploid MKs, and the MK lineage-specific αIIbβ3 integrin is expressed early in MK progenitors, and keeps increasing as MKs mature and grow larger in size [9, 27, 28].
α4β1 integrin is expressed on MK progenitors and small immatures MKs, but not on large mature human MKs. In contrast, α5β1 integrin is highly expressed on large mature MKs and poorly in immature ones [27]. The expression of the laminin receptor, α6β1integrin, appears early in immature MKs and remains relatively constant throughout MK maturation [9]. The observations that early immature MKs mainly adhere to fibronectin and laminin but less to collagen, and the capacity of mature MKs to acquire the ability to bind collagen while retaining the ability to bind fibronectin and laminin are consistent with the expression levels of those β1 integrins during MK maturation [27]. As for the vitronectin receptor, αvβ3 integrin expression is detected in early MKs, and as a MK grows in size and becomes more mature, αvβ3 expression decreases [28].
Blocking studies using antibodies against integrins during megakaryopoiesis can provide valuable insights to assess how integrins affect MK differentiation and development. Studies by Zeng et al. showed that incubation of cord-blood CD34+ in vitro with LM609, the anti-αVβ3 antibody, does not affect megakaryocyte differentiation [29]. Using human cord blood CD34+ cells, Han. et al. showed that blocking of β1 integrins significantly reduced the generation of MK progenitors assessed by CFU-MK assay [30]. Similar results were obtained by Fox et al. using an integrin pan-blockade molecule, kistrin, which abolished thrombopoietin (TPO)-induced megakaryopoiesis in vitro, but a more specific inhibitor of α5β1 integrin, EMF10, surprisingly did not affect megakaryopoiesis [14]. In contrast, a recent study showed that a neutralizing antibody to α5 subunit caused a reduction in MK cultured from murine CD34+ cells [16]. On the other hand, a neutralizing anti-α4β1 monoclonal antibody blocked megakaryopoiesis. Conversely, supplementing the medium with an α4β1 integrin ligand, the H296 fragment of fibronectin, stimulated MK growth at all concentrations of added TPO [14]. This result is in agreement with a study of Han and colleagues in which they found that the addition of fibronectin stimulated expression of CD41d (α4 subunit) and enhanced the generation of MK progenitors [30]. β3 integrin was also proven to be essential for megakaryopoiesis, as anti-β3 antibodies drastically inhibited MK proliferation and the formation of CFU-MK [31].
Collectively, the above studies underscore the tight regulation of integrins during MK differentiation and maturation and highlight the importance of MK binding to cognate matrix components during their development, as also summarized in Table 1.
Table 1:
Integrins expressed on megakaryocytes (MKs), their major extracellular matrix (ECM) ligands, and regulatory functions
| Integrin Type |
ECM ligands | Regulatory functions in MKs |
|---|---|---|
| α 2 β 1 | Collagen, laminin | MK spreading, migration, and proplatelet formation [10, 48] |
| α 3 β 1 | Fibronectin | MK-induced osteoblast and fibroblast proliferation [12] |
| α 4 β 1 | Fibronectin | Only exists on MK progenitors and immature MKs, affecting MK growth [14, 27] |
| α 5 β 1 | Fibronectin | MK growth, proplatelet formation [15] |
| α 6 β 1 | Laminin | Proplatelet formation [17, 18] |
| αIIbβ 3 | Fibrinogen, fibronectin, vWF | Endocytosis of fibrinogen, MK spreading, migration, and proplatelet formation [7, 18, 19] |
| αvβ 3 | Vitronectin | Proplatelet formation, MK migration [6, 7] |
Effect of specific integrins on megakaryocyte functional properties: a focus on adhesion, spreading and migration
Effects of different megakaryocyte integrins on adhesion and spreading:
Adhesion, spreading, and migration are interconnected processes that happen in sequential order. Both intracellular and extracellular signals can cause integrins to be activated, cluster together, and bind to ECM ligands, thereby forming focal adhesion complexes [32]. Upon binding to the ECM ligand, cells become flattened out and exhibit protrusion structures, the filopodia and lamellipodia, which are formed by polymerized actin stress fiber assembled by the focal adhesion complex [33]. The assembly of actin stress fibers and the formation of cell protrusions play central roles in cell motility and migration along the ECM [34]. MK migration from within the bone marrow environment to the capillary-rich vascular niche is an essential step for platelet production. The stromal cell-derived factor 1α (SDF1α), also known as CXCL12 is a central chemokine in this process, mediated by the SDF1α receptor CXCR4 [35]. Activation of integrins on MKs, adhesion to ECM ligands and spreading have been extensively studied and proved to be involved in controlling several cellular pathways.
Adhesion of mature MKs to fibronectin is mediated mainly by β1 integrin complexes. Anti-β3 and anti-αIIbβ3 antibodies did not affect binding to fibronectin, whereas anti-α5 subunit antibody reduced this property by 40%, and anti-β1 antibody totally abolished adhesion to fibronectin [36]. αIIbβ3 integrin does not contribute to adhesion to fibronectin. However, αIIbβ3 can be activated under TPO stimulation, and gain the ability to bind both fibronectin and fibrinogen [24]. Interestingly, Schick et. al found that resting MKs could not bind fibrinogen, and EMF-10, a disintegrin highly specific for α5β1 [37] blocked adhesion to fibronectin whereas antibodies against αIIbβ3, αvβ3, α4β1 did not.
As to MK spreading and migration, αIIbβ3 integrin seemed to play a role in these properties. An early study by Leven et al. showed that MK spreading was stimulated by thrombin, but an antibody to αIIbβ3 inhibited spreading of guinea pig MKs [7]. Mazharian et al. showed that MK spreading and migration on fibronectin upon activation was abolished by an αIIbβ3 integrin antagonist, lotrafiban [38]. Although the collagen receptor, α2β1 integrin allows mature MKs to spread on collagen [10], MKs were not observed to migrate on a collagen matrix [38]. The effect of other MK integrins on MK spreading and migration are not well documented, only with one study showing that anti-αvβ3 antibodies inhibit MK migration via suppressing Protein kinase B (AKT) activation [29].
Effectors and signaling involved in megakaryocyte integrin-mediated adhesion and spreading:
Once MKs are activated by thrombin, they adhere to fibrinogen primarily via αIIbβ3 integrin, and both α5β1 and αIIbβ3 integrins mediate fibronectin adhesion, as evidenced by the requirement for inhibitors of both integrins to abolish fibronectin binding [39]. Besides thrombin and TPO, typical platelet agonists, ADP and epinephrine, as well as thrombin receptor agonists increased αIIbβ3 adhesion to fibrinogen [40]. SDF-1α, an agonist to α-chemokine receptor CXCR4, also stimulated fibrinogen binding [41]. Adhesion to fibrinogen was shown to be mediated by the Rap signaling pathway. The increased expression of CalDAG-GEFI, a Rap1 exchange factor, activated Rap1b and enhanced MK adhesion to fibrinogen through inside-out signaling to αIIbβ3 integrin [42]. Inhibition of Rasa3, a GTPase activating protein targeting Rap1, activated Rap1 and resulted in a constitutive binding of fibrinogen to αIIbβ3 integrin [43]. αIIbβ3 integrin stimulates MK motility and migration via SFKs (Src Family Kinase)-Syk-PLCγ2 signaling. αIIbβ3 signaling phosphorylates Src kinases, Syk, as well as PLCγ2, and inhibitors against SFKs and Syk kinase block MK spreading and migration, consistent with the finding that these properties are inhibited in MKs derived from mice deficient in PLCγ2 [38].
The cytoplasmic tail of αIIb subunit also serves as a regulatory domain for αIIbβ3 activation. Talin, a factor involved in focal adhesion complex, activates αIIbβ3 integrin by interacting with the cytoplasmic tail of αIIb subunit [44], whereas calcium and integrin-binding protein 1 (CIB1) binds to the same site and serves as a competitive inhibitor that blocks αIIbβ3 integrin activation [45]. The αIIbβ3 pathway is also regulated by the WAVE/Abi pathway associated with the Wiskott-Aldrich syndrome, MKs in WAVE2 knock-out mice exhibit reduced spreading on fibrinogen due to defects in the formation of lamellipodia [46].
Roles of megakaryocyte integrins during proplatelet and platelet development
Complexes with ß1 integrin:
Proplatelet formation was reported to be reduced by 3-fold when MKs were attached via α2β1 integrin to collagen I-coated slides [10]. The process involved the Rho/ROCK signaling pathway as evidenced by restoration of proplatelet formation by inhibitors of Rho/ROCK [47]. The mechanical tensile strength exerted by collagen has been suggested to regulate α2β1 integrin signaling. In one study, collagen was modified to be less stiff via N-acetylation, and proplatelet formation was not inhibited when MKs were attached to the modified collagen [48]. In contrast to in-vitro studies, the in vivo effects of α2β1 integrin on proplatelet formation and platelet production seem to be controversial. Total knockout of α2 subunit in mice resulted in no difference in platelet number, platelet volume, MK count, and MK maturation, compared to wildtype mice. MKs were only observed to have reduced adhesion to collagen type I filaments at sinusoids compared to wildtype mice [49]. In another study using a Cre/α2β1 model in which the gene encoding α2 subunit is conditionally knocked out only in MKs, platelet count was normal, with a modest reduction if mean platelet volume [50]. The normality observed in systematic knockout mice suggests α2β1 integrin signaling is not essential for proplatelet formation and platelet production. On the other hand, a recent study focused on the role of ß1 in regulating MK maturation and thrombopoiesis, with emphasis on ß1 modification by glycosylation [51], concluded that while activation of β1 is important for MK maturation, ß1 inactivation by its galactosylation is important for MK migration to the vascular niche for eventual thrombopoiesis.
The role of α5β1 or α4β1 integrins in proplatelet formation was not well studied. Some conclusions were derived based on using a MK cell line and adhesion to fibronectin. Using the human megakaryoblastic CHRF-288 cell line treated with phorbol 12-myristate 13-acetate (PMA) and co-stimulated with fibronectin, the cells underwent megakaryocytic differentiation, maturation, and proplatelet formation, whereas stimulation with other ECM ligands such as collagen, vitronectin, and laminin led to minimal proplatelet formation. Fibronectin adhesion, together with PMA-induced activation of protein kinase C (PKC) were concluded to be essential for proplatelet formation in these cells [52]. Experiments with primary cells and in vivo studies are needed to further examine the direct role of α4β1 and α5β1 integrins in proplatelet formation.
Finally, the role of α6β1 integrin in controlling platelet biogenesis was also suggested. Murine MKs plated on laminin were reported to have a moderate level of proplatelets, which, albeit much less than on fibrinogen, was greater than on other ECM ligands such as vitronectin, collagen, and vWF [18], suggesting α6β1 signaling may serve as complementary but inefficient controller of proplatelet formation.
Complexes with ß3 integrin:
Early research to investigate the effect of αvβ3 integrin on proplatelet formation was done on guinea pig MKs. Leven et al. showed that LM609, an antibody specific for αvβ3 integrin, blocked proplatelet formation, whereas antibodies to β1, α5, α6 subunits as well as a fibrinogen gamma chain peptide designed to neutralize αIIbβ3 had no effect on proplatelet formation. The authors also showed that adding vitronectin to a MK culture depleted of serum stimulated proplatelet formation to a level equivalent to that of a serum MK culture, suggesting vitronectin/αvβ3 signaling pathway is the major player in proplatelet formation [6]. However, in another study performed using human MKs, antibody to αvβ3 had no significant effect on proplatelet formation [53]. Those contradictory results may be partly due to the difference in expression levels of αVβ3 integrin in the two species. αVβ3 integrin is present more in α-granules and less on the plasma membrane in mature human MKs, and the cellular expression of αVβ3 decreases as human MKs mature [28]. On the other hand, guinea pig MKs express much more αVβ3 integrin compared to human MKs [7].
αIIbβ3 integrin was too studied in the context of platelet biogenesis. Murine MKs plated on fibrinogen showed the greatest proportion of proplatelet formation compared to MKs plated on laminin, vitronectin, vWF, or collagen, and the introduction of lotrafiban, an αIIbβ3 integrin antagonist, abolished proplatelet formation on fibrinogen [18]. As previously introduced, αIIbβ3 integrin activates downstream SFKs (Src Family Kinase)-Syk-PLCγ2 signaling [38]. This signaling pathway, although having a positive effect on MK spreading and migration, seems to negatively regulate proplatelet formation as Larson et al. also showed that treating MKs with a Src family kinase inhibitor led to increased percentage of MKs with proplatelets [18]. Chinese Hamster Ovary (CHO) cells expressing mutant versions of αIIbβ3 integrin, αIIbβ3D723H and αIIbβ3D723 that are partially active formed cytoplasmic protrusions after adhesion to fibrinogen, mimicking proplatelets [54]. Interesting to note, small Rho GTPase RhoA activity was strongly inhibited in these cells. As previously mentioned, Rho/ROCK pathway was activated by α2β1 integrin binding to collagen, which inhibits proplatelet formation in human MKs [10]. After adhesion of αIIbβ3 to fibrinogen, filamin A (FLNa) connects the cytoplasmic tail of αIIbβ3 to RhoA, keeping RhoA in a GDP - bound inactivated state. Inactivated RhoA inhibits downstream ROCK protein, and the inhibition stimulates proplatelet formation. However, the absence of FLNa results in RhoA in an GTP-bound activated state, which in turn activates downstream ROCK protein causing defective proplatelet formation [55]. Taken together, these findings suggest integrin-dependent Rho/ROCK pathway as regulator of proplatelet biogenesis.
Integrin-dependent interactions of megakaryocytes with other cells
Megakaryocytes interact with osteoblasts:
MK to osteoblast interaction was first characterized in mice deficient in GATA-1 or NF-E2, two essential transcription factors for MK differentiation. Those mice were observed with elevated osteoblast number and highly increased bone density. Further in vitro experiments showed that mutant MKs derived from female mice enhance osteoblast proliferation through direct cell contact [56]. MK-induced osteoblast proliferation was also observed in mice treated with total body irradiation. Surviving MKs were observed migrating from their normal para-sinusoidal site toward the endosteal surface of trabecular bone, and inducing a twofold expansion of osteoblasts [57].
Further studies revealed the role of MK integrins and their signaling pathway in regulating osteoblast proliferation. Lemieux et al. showed that EDTA and tetrapeptide RGDS treatment of cultured MKs and osteoblasts diminished MK-induced osteoblast proliferation, strongly suggesting the involvement of RGD-binding integrins [12]. Neutralizing antibodies against RGD-binding integrins (α3β1, α5β1, and αIIbβ3 integrins) were applied to test their respective effect. All antibodies had an inhibitory effect on osteoblast proliferation, suggesting that fibronectin-binding integrins on MKs played a role in osteoblast proliferation [12]. The integrin-dependent proliferation of osteoblasts has been suggested to be mediated by proline-rich tyrosine kinase 2 (Pyk2). Pyk2 level in osteoblasts was elevated when cocultured with MKs, and Pyk2 inhibited downstream p53-Mdm2 pathway, leading osteoblasts to be more permissive of cell cycle entry [58]. Since Pyk2 is known to be activated after integrin engagement in other cell types [59], it is likely to be an essential mediator in MK-induced osteoblast proliferation.
Megakaryocytes interact with fibroblasts:
Platelet derived growth factor (PDGF), and transforming growth factor β1 (TGF-β1) secreted by MKs play essential roles in stimulating proliferation of fibroblasts as well as secretion of matrix products such as collagen and fibronectin [60, 61]. In addition, a direct contact between MKs and fibroblasts is necessary for stimulation of fibroblast proliferation. Tissue culture inserts were employed to inhibit direct cell contact in human MK-fibroblast co-cultures, leading to significant impairment of fibroblast proliferation [62]. Further, this effect was significantly ablated with the addition of anti-α3 and anti-α5 antibodies to the human MK-fibroblast co-culture, and the adhesion of MKs to fibroblasts was also reduced. On the other hand, anti-α2 and anti-α6 antibodies did not cause significant decrease in adhesion and fibroblast growth, suggesting that MK interaction with fibroblasts was mediated by fibronectin-binding α3β1 and α5β1 integrins [11]. In addition, other cell adhesion molecules such as selectins were reported to facilitate MK attachment to fibroblasts, thereby stimulating fibroblast growth [63].
Megakaryocytes interact with endothelial cells:
Stimulation of endothelial cell line HUVECs or bone marrow MKs with inflammatory cytokines, such as IL-1 beta, GM-CSF, IL-6, IL-3 promotes adhesion of MKs to the endothelial cells via lymphocyte function-associated antigen-1 (LFA-1) or αL integrin subunit of the leukocyte adherence complex CD18 and VLA-4/VCAM-1. Adhesion blocking experiments indicated that this function was important for MK maturation as assessed by increased ploidy and upregulation of glycoproteins GpIb and GpIIb/IIIa [64]. Interestingly, using a combination of fluorescence, electron, and three-dimensional microscopy, a more recent study nicely illustrated MK podosomes interaction with endothelial cells to initiate pore formation as part of promoting thrombopoiesis [65]. As reviewed before, activation of β1 integrin controls the interaction of MKs with the endosteal niche, as part of MK maturation [66]. Other MK integrins involved in this interaction are yet to be explored.
Pathologies associated with dysregulated MK integrin expression
Glanzmann’s thrombasthenia (GT):
GT is an autosomal recessive bleeding disorder associated with mutations in αIIbβ3 integrin, classically characterized by prolonged bleeding time and failure of platelets binding to fibrinogen in response to ADP, collagen, thrombin and epinephrine [5]. In rare cases, GT can also present as an acquired autoimmune disorder caused by an autoantibody targeting αIIbβ3 integrin [67]. Although platelet count is usually normal in GT patients, certain mutations in some cases can cause impaired platelet production and function, leading to macrothrombocytopenia characterized by low platelet counts with enlarged platelet volume [68-70]. Those cases have been suggested to involve proplatelet formation in MKs.
MKs of patients with mutations associated with αIIb R995/β3 D723 salt bridge exhibit defective proplatelet formation, leading to macrothrombocytopenia and GT-like syndromes [71]. Similar findings were reported in another study where a β3 subunit deletion (del647-686) also led to macrothrombocytopenia resulted by lowered proplatelet number and enlarged proplatelet tips [68]. Additional mutations associated with αIIbβ3 integrins were reported in patients with congenital macrothrombocytopenia, including αIIb Gly991Cys, αIIb Phe993del, and β3 (del621-660) [69]. All these mutations were reported to result in constitutively active αIIbβ3 integrins and reduced αIIbβ3 expression on surface of MKs and platelets, suggesting the regulation of αIIbβ3 activation plays central roles in proplatelet formation and morphology.
Primary Myelofibrosis (PMF):
Primary Myelofibrosis (PMF) is marked by increased number of MKS, and accumulation of ECM fibers in the BM [72]. The role of MK integrins in promoting PMF Phenotype, including increased MK number, has been recently studied in a transgenic mouse model of the disease; one bearing the human JAK2V617F mutation. MKs in those mice had increased cell surface expression of α5 integrin and enhanced adhesion to fibronectin, and neutralizing antibody to the α5 subunit lowered adhesion to fibronectin and reduced the number of MKs in vivo [16]. Taken together with the fact that fibronectin content is elevated in BM of JAK2V617F mice [16], these finding suggest a positive feedback in which MK proliferation is stimulated by an active α5β1 integrin complex.
Immune thrombocytopenic purpura (ITP):
ITP is an autoimmune disease resulted by platelet autoantibodies which lead to platelet destruction; however, the cause of platelet autoantibodies formation is still largely unknown [73]. Autoantibodies against MK integrins such as αIIbβ3 and αvβ3 were detected on chronic ITP patients [29, 74]. Elevated anti-αIIbβ3 and/or anti-αvβ3 autoantibodies is associated with low platelet count [29]. As previously discussed, an antibody to αvβ3 had no significant effect on proplatelet formation by human MKs, however αvβ3 neutralizing antibody reduced MK adhesion and spreading. [29, 53].
Conclusions and future studies
As illustrated in Figure 1 and summarized in Table 1, MK integrins play a central role in various MK functions to maintain homeostasis. The deregulation and malfunction of MK integrins results in systemic diseases. Despite well-established roles of bone marrow MK integrins in cell adhesion, spreading and interaction with neighboring cells, there are several areas warranting further exploration. For instance, researching the role of specific integrins in MK development and biology within distant organs, such as lung (reviewed in [75]), will shed light on this lineage function and development outside the bone marrow. Although the extracellular ligands have been identified for integrins, the intracellular signaling cascade, molecular mediators triggered by ligand binding to integrins and, importantly, factors such as ECM mechanical attributes altering this biology remain to be further elucidated. Further research is also required to generate potential new integrin-based therapeutic targets to treat some MK pathologies. In this context, there is much promise also in gene editing in MKs derived from human induced pluripotent stem cells for molecular investigations of different roles of integrins, normal or mutated, as described in the study of αIIbβ3 integrin [76].
Bullet points for the article 21-218 submitted to Experimental Hematology.
Integrins are heterodimers of α and β subunits
Major integrin complexes expressed in megakaryocytes include αVβ3, α3β1 and α5β1
Megakaryocyte integrins play key roles in this lineage development and function
Some megakaryocyte and platelet pathologies are associated with dysregulated/mutated integrins
Modulators of megakaryocyte integrin functions have therapeutic potential
Acknowledgements of Funding Sources:
KR is an established investigator with the American Heart Association and funded by NHLBI grant R01HL136363.
Footnotes
Conflict of interest: None of the authors have relevant conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hynes RO, Integrins: a family of cell surface receptors. Cell, 1987. 48(4): p. 549–54. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO, Integrins: versatility, modulation, and signaling in cell adhesion. Cell, 1992. 69(1): p. 11–25. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO, Integrins: bidirectional, allosteric signaling machines. Cell, 2002. 110(6): p. 673–87. [DOI] [PubMed] [Google Scholar]
- 4.Zamir E and Geiger B, Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci, 2001. 114(Pt 20): p. 3583–90. [DOI] [PubMed] [Google Scholar]
- 5.Seligsohn U, Glanzmann thrombasthenia: a model disease which paved the way to powerful therapeutic agents. Pathophysiol Haemost Thromb, 2002. 32(5-6): p. 216–7. [DOI] [PubMed] [Google Scholar]
- 6.Leven RM and Tablin F, Extracellular matrix stimulation of guinea pig megakaryocyte proplatelet formation in vitro is mediated through the vitronectin receptor. Exp Hematol, 1992. 20(11): p. 1316–22. [PubMed] [Google Scholar]
- 7.Leven RM, Differential regulation of integrin-mediated proplatelet formation and megakaryocyte spreading. J Cell Physiol, 1995. 163(3): p. 597–607. [DOI] [PubMed] [Google Scholar]
- 8.Hemler ME, VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol, 1990. 8: p. 365–400. [DOI] [PubMed] [Google Scholar]
- 9.Mossuz P, et al. , Expression and function of receptors for extracellular matrix molecules in the differentiation of human megakaryocytes in vitro. Br J Haematol, 1997. 98(4): p. 819–27. [DOI] [PubMed] [Google Scholar]
- 10.Sabri S, et al. , Differential regulation of actin stress fiber assembly and proplatelet formation by alpha2beta1 integrin and GPVI in human megakaryocytes. Blood, 2004. 104(10): p. 3117–25. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz B, et al. , Evidence for integrin receptor involvement in megakaryocyte-fibroblast interaction: a possible pathomechanism for the evolution of myelofibrosis. J Cell Physiol, 1998. 176(3): p. 445–55. [DOI] [PubMed] [Google Scholar]
- 12.Lemieux JM, Horowitz MC, and Kacena MA, Involvement of integrins alpha(3)beta(1) and alpha(5)beta(1) and glycoprotein IIb in megakaryocyte-induced osteoblast proliferation. J Cell Biochem, 2010. 109(5): p. 927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan JL and Hynes RO, Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell, 1990. 60(1): p. 53–61. [DOI] [PubMed] [Google Scholar]
- 14.Fox NE and Kaushansky K, Engagement of integrin alpha4beta1 enhances thrombopoietin-induced megakaryopoiesis. Exp Hematol, 2005. 33(1): p. 94–9. [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga T, et al. , Potentiated activation of VLA-4 and VLA-5 accelerates proplatelet-like formation. Ann Hematol, 2012. 91(10): p. 1633–43. [DOI] [PubMed] [Google Scholar]
- 16.Matsuura S, et al. , Adhesion to fibronectin via alpha5beta1 integrin supports expansion of the megakaryocyte lineage in primary myelofibrosis. Blood, 2020. 135(25): p. 2286–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnenberg A, Modderman PW, and Hogervorst F, Laminin receptor on platelets is the integrin VLA-6. Nature, 1988. 336(6198): p. 487–9. [DOI] [PubMed] [Google Scholar]
- 18.Larson MK and Watson SP, Regulation of proplatelet formation and platelet release by integrin alpha IIb beta3. Blood, 2006. 108(5): p. 1509–14. [DOI] [PubMed] [Google Scholar]
- 19.Handagama P, et al. , Kistrin, an integrin antagonist, blocks endocytosis of fibrinogen into guinea pig megakaryocyte and platelet alpha-granules. J Clin Invest, 1993. 91(1): p. 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao AG, et al. , Thrombospondin modulates alpha v beta 3 function through integrin-associated protein. J Cell Biol, 1996. 135(2): p. 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, et al. , CD41-YFP mice allow in vivo labeling of megakaryocytic cells and reveal a subset of platelets hyperreactive to thrombin stimulation. Exp Hematol, 2007. 35(3): p. 490–499. [DOI] [PubMed] [Google Scholar]
- 22.Bennett JS, Structure and function of the platelet integrin alphaIIbbeta3. J Clin Invest, 2005. 115(12): p. 3363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perutelli P and Mori PG, The human platelet membrane glycoprotein IIb/IIIa complex: a multi functional adhesion receptor. Haematologica, 1992. 77(2): p. 162–8. [PubMed] [Google Scholar]
- 24.Zauli G, et al. , Thrombopoietin enhances the alpha IIb beta 3-dependent adhesion of megakaryocytic cells to fibrinogen or fibronectin through PI 3 kinase. Blood, 1997. 89(3): p. 883–95. [PubMed] [Google Scholar]
- 25.Burger SR, et al. , Induced cell surface expression of functional alpha 2 beta 1 integrin during megakaryocytic differentiation of K562 leukemic cells. Exp Cell Res, 1992. 202(1): p. 28–35. [DOI] [PubMed] [Google Scholar]
- 26.Lozzio BB and Lozzio CB, Properties of the K562 cell line derived from a patient with chronic myeloid leukemia. Int J Cancer, 1977. 19(1): p. 136. [DOI] [PubMed] [Google Scholar]
- 27.Molla A, Mossuz P, and Berthier R, Extracellular matrix receptors and the differentiation of human megakaryocytes in vitro. Leuk Lymphoma, 1999. 33(1-2): p. 15–23. [DOI] [PubMed] [Google Scholar]
- 28.Poujol C, Nurden AT, and Nurden P, Ultrastructural analysis of the distribution of the vitronectin receptor (alpha v beta 3) in human platelets and megakaryocytes reveals an intracellular pool and labelling of the alpha-granule membrane. Br J Haematol, 1997. 96(4): p. 823–35. [DOI] [PubMed] [Google Scholar]
- 29.Zeng DF, et al. , Autoantibody against integrin alphav beta3 contributes to thrombocytopenia by blocking the migration and adhesion of megakaryocytes. J Thromb Haemost, 2018. 16(9): p. 1843–1856. [DOI] [PubMed] [Google Scholar]
- 30.Han P, Guo X, and Story C, Role of beta(1)-integrins and their associated tetraspanin molecules in fibronectin-enhanced megakaryopoiesis. Cytotherapy, 2004. 6(5): p. 465–75. [DOI] [PubMed] [Google Scholar]
- 31.Pan R, et al. , The inhibition effect of anti-GPIIIa49-66 antibody on megakaryocyte differentiation. Thromb Haemost, 2011. 106(3): p. 484–90. [DOI] [PubMed] [Google Scholar]
- 32.Giancotti FG, A structural view of integrin activation and signaling. Dev Cell, 2003. 4(2): p. 149–51. [DOI] [PubMed] [Google Scholar]
- 33.Fardin MA, et al. , Cell spreading as a hydrodynamic process. Soft Matter, 2010. 6: p. 4788–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridley AJ, et al. , Cell migration: integrating signals from front to back. Science, 2003. 302(5651): p. 1704–9. [DOI] [PubMed] [Google Scholar]
- 35.Mazharian A, Assessment of megakaryocyte migration and chemotaxis. Methods Mol Biol, 2012. 788: p. 275–88. [DOI] [PubMed] [Google Scholar]
- 36.Berthier R, et al. , Adhesion of mature polyploid megakaryocytes to fibronectin is mediated by beta 1 integrins and leads to cell damage. Exp Cell Res, 1998. 242(1): p. 315–27. [DOI] [PubMed] [Google Scholar]
- 37.Marcinkiewicz C, et al. , Structural and functional characterization of EMF10, a heterodimeric disintegrin from Eristocophis macmahoni venom that selectively inhibits alpha 5 beta 1 integrin. Biochemistry, 1999. 38(40): p. 13302–9. [DOI] [PubMed] [Google Scholar]
- 38.Mazharian A, et al. , Critical role of Src-Syk-PLC{gamma}2 signaling in megakaryocyte migration and thrombopoiesis. Blood, 2010. 116(5): p. 793–800. [DOI] [PubMed] [Google Scholar]
- 39.Schick PK, et al. , Integrins involved in the adhesion of megakaryocytes to fibronectin and fibrinogen. Blood, 1998. 92(8): p. 2650–6. [PubMed] [Google Scholar]
- 40.Shiraga M, et al. , Primary megakaryocytes reveal a role for transcription factor NF-E2 in integrin alpha IIb beta 3 signaling. J Cell Biol, 1999. 147(7): p. 1419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang JF, Liu ZY, and Groopman JE, The alpha-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood, 1998. 92(3): p. 756–64. [PubMed] [Google Scholar]
- 42.Eto K, et al. , Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc Natl Acad Sci U S A, 2002. 99(20): p. 12819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molina-Ortiz P, et al. , Rasa3 controls megakaryocyte Rap1 activation, integrin signaling and differentiation into proplatelet. PLoS Genet, 2014. 10(6): p. e1004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrich BG, et al. , Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med, 2007. 204(13): p. 3103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan W, et al. , CIB1 is an endogenous inhibitor of agonist-induced integrin alphaIIbbeta3 activation. J Cell Biol, 2006. 172(2): p. 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eto K, et al. , The WAVE2/Abi1 complex differentially regulates megakaryocyte development and spreading: implications for platelet biogenesis and spreading machinery. Blood, 2007. 110(10): p. 3637–47. [DOI] [PubMed] [Google Scholar]
- 47.Chen Z, et al. , The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood, 2007. 110(1): p. 171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malara A, et al. , Extracellular matrix structure and nano-mechanics determine megakaryocyte function. Blood, 2011. 118(16): p. 4449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Semeniak D, et al. , Impact of Itga2-Gp6-double collagen receptor deficient mice for bone marrow megakaryocytes and platelets. PLoS One, 2019. 14(8): p. e0216839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habart D, et al. , Conditional knockout of integrin alpha2beta1 in murine megakaryocytes leads to reduced mean platelet volume. PLoS One, 2013. 8(1): p. e55094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giannini S, et al. , beta4GALT1 controls beta1 integrin function to govern thrombopoiesis and hematopoietic stem cell homeostasis. Nat Commun, 2020. 11(1): p. 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang F, Jia Y, and Cohen I, Fibronectin- and protein kinase C-mediated activation of ERK/MAPK are essential for proplateletlike formation. Blood, 2002. 99(10): p. 3579–84. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi R, Sekine N, and Nakatake T, Influence of monoclonal antiplatelet glycoprotein antibodies on in vitro human megakaryocyte colony formation and proplatelet formation. Blood, 1999. 93(6): p. 1951–8. [PubMed] [Google Scholar]
- 54.Schaffner-Reckinger E, et al. , Overexpression of the partially activated alpha(IIb)beta3D723H integrin salt bridge mutant downregulates RhoA activity and induces microtubule-dependent proplatelet-like extensions in Chinese hamster ovary cells. J Thromb Haemost, 2009. 7(7): p. 1207–17. [DOI] [PubMed] [Google Scholar]
- 55.Donada A, et al. , Disrupted filamin A/alphaIIbbeta3 interaction induces macrothrombocytopenia by increasing RhoA activity. Blood, 2019. 133(16): p. 1778–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kacena MA, et al. , Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res, 2004. 19(4): p. 652–60. [DOI] [PubMed] [Google Scholar]
- 57.Dominici M, et al. , Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood, 2009. 114(11): p. 2333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng YH, et al. , Pyk2 regulates megakaryocyte-induced increases in osteoblast number and bone formation. J Bone Miner Res, 2013. 28(6): p. 1434–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avraham H, et al. , RAFTK/Pyk2-mediated cellular signalling. Cell Signal, 2000. 12(3): p. 123–33. [DOI] [PubMed] [Google Scholar]
- 60.Kimura A, et al. , Transforming growth factor-beta regulates growth as well as collagen and fibronectin synthesis of human marrow fibroblasts. Br J Haematol, 1989. 72(4): p. 486–91. [DOI] [PubMed] [Google Scholar]
- 61.Kimura A, Katoh O, and Kuramoto A, Effects of platelet derived growth factor, epidermal growth factor and transforming growth factor-beta on the growth of human marrow fibroblasts. Br J Haematol, 1988. 69(1): p. 9–12. [DOI] [PubMed] [Google Scholar]
- 62.Schmitz B, et al. , Megakaryocytes and fibroblasts--interactions as determined in normal human bone marrow specimens. Leuk Res, 1995. 19(9): p. 629–37. [DOI] [PubMed] [Google Scholar]
- 63.Wickenhauser C, et al. , Selectins (CD62L, CD62P) and megakaryocytic glycoproteins (CD41a, CD42b) mediate megakaryocyte-fibroblast interactions in human bone marrow. Leuk Res, 2000. 24(12): p. 1013–21. [DOI] [PubMed] [Google Scholar]
- 64.Avraham H, et al. , Characterization of adhesive interactions between human endothelial cells and megakaryocytes. J Clin Invest, 1993. 91(6): p. 2378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eckly A, et al. , Megakaryocytes use in vivo podosome-like structures working collectively to penetrate the endothelial barrier of bone marrow sinusoids. J Thromb Haemost, 2020. 18(11): p. 2987–3001. [DOI] [PubMed] [Google Scholar]
- 66.Mazzarini M, et al. , Role of beta1 integrin in thrombocytopoiesis. Fac Rev, 2021. 10: p. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tholouli E, et al. , Acquired Glanzmann's thrombasthenia without thrombocytopenia: a severe acquired autoimmune bleeding disorder. Br J Haematol, 2004. 127(2): p. 209–13. [DOI] [PubMed] [Google Scholar]
- 68.Bury L, et al. , Outside-in signalling generated by a constitutively activated integrin alphaIIbbeta3 impairs proplatelet formation in human megakaryocytes. PLoS One, 2012. 7(4): p. e34449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kashiwagi H, et al. , Demonstration of novel gain-of-function mutations of alphaIIbbeta3: association with macrothrombocytopenia and glanzmann thrombasthenia-like phenotype. Mol Genet Genomic Med, 2013. 1(2): p. 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nurden AT, et al. , Glanzmann thrombasthenia-like syndromes associated with Macrothrombocytopenias and mutations in the genes encoding the alphaIIbbeta3 integrin. Semin Thromb Hemost, 2011. 37(6): p. 698–706. [DOI] [PubMed] [Google Scholar]
- 71.Favier M, et al. , Mutations of the integrin alphaIIb/beta3 intracytoplasmic salt bridge cause macrothrombocytopenia and enlarged platelet alpha-granules. Am J Hematol, 2018. 93(2): p. 195–204. [DOI] [PubMed] [Google Scholar]
- 72.Malara A, et al. , Megakaryocyte Contribution to Bone Marrow Fibrosis: many Arrows in the Quiver. Mediterr J Hematol Infect Dis, 2018. 10(1): p. e2018068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cines DB, et al. , The ITP syndrome: pathogenic and clinical diversity. Blood, 2009. 113(26): p. 6511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Najaoui A, et al. , Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur J Haematol, 2012. 88(2): p. 167–74. [DOI] [PubMed] [Google Scholar]
- 75.Tilburg J, Becker IC, and Italiano JE, Don't you forget about me(gakaryocytes). Blood, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kasirer-Friede A and Shattil SJ, Genetic Instruction of Megakaryocytes and Platelets Derived from Human Induced Pluripotent Stem Cells for Studies of Integrin Regulation. Methods Mol Biol, 2021. 2217: p. 237–249. [DOI] [PubMed] [Google Scholar]