Significance
Immunotherapy is a promising approach to treat cancers, infectious diseases, and autoimmunity by harnessing the power of immune cells, especially T cells. To improve the precision and efficacy of immunotherapy, the ability to genetically modify antigen-specific T cells is needed but cannot be accomplished using current methods. Here, we present a method, V-CARMA (Viral ChimAeric Receptor MHC-Antigen), to generate lentiviruses displaying peptide-MHC complex to specifically target T cells that express cognate TCRs and subsequently deliver genes into target T cells for genetic modification. Our results demonstrate that V-CARMA is a versatile tool to detect and modify antigen-specific T cells.
Keywords: pseudotyped lentivirus, T cell modification, peptide-MHC complex
Abstract
T cells promote our body’s ability to battle cancers and infectious diseases but can act pathologically in autoimmunity. The recognition of peptides presented by major histocompatibility complex (pMHC) molecules by T cell receptors (TCRs) enables T cell–mediated responses. To modify disease-relevant T cells, new tools to genetically modify T cells and decode their antigen recognition are needed. Here, we present an approach using viruses pseudotyped with peptides loaded on MHC called V-CARMA (Viral ChimAeric Receptor MHC-Antigen) to specifically target T cells expressing cognate TCRs for antigen discovery and T cell engineering. We show that lentiviruses displaying antigens on human leukocyte antigen (HLA) class I and class II molecules can robustly infect CD8+ and CD4+ T cells expressing cognate TCRs, respectively. The infection rates of the pseudotyped lentiviruses (PLVs) are correlated with the binding affinity of the TCR to its cognate antigen. Furthermore, peptide-HLA pseudotyped lentivirus V-CARMA constructs can identify target cells from a mixed T cell population, suppress PD-1 expression on CD8+ T cells via PDCD1 shRNA delivery, and induce apoptosis in autoreactive CD4+ T cells. Thus, V-CARMA is a versatile tool for TCR ligand identification and selective T cell manipulation.
T cells play critical roles in fighting cancer and infectious diseases but can also become dysregulated, leading to autoimmunity. Understanding the specificity of T cell receptors (TCRs) to certain antigens is central to studying basic immunology and developing treatments for diseases. To achieve this, we and others have recently developed approaches to interrogate T cell specificities. These technologies can systematically identify the antigens presented by major histocompatibility complex (MHC) class I molecules, which are recognized by TCRs of CD8+ T cells utilizing different methods: TSCAN (1), yeast display (2, 3), or trogocytosis (4), and MHC class II antigen discovery using TCRs from CD4+ T cells (5, 6). Although these strategies are useful for identifying the antigens recognized by TCRs, they are limited by their inability to further engineer the T cell subsets of interest, which is essential for some T cell–mediated immunotherapies.
Delivery of cargo genes into specific target T cells is important for improving the potency of antigen-specific T cells to kill cancer or infected cells and to eliminate autoreactive T cells in T cell–mediated autoimmune diseases. It is well established that lentivirus pseudotyped with vesicular stomatitis virus-glycoprotein (VSV-G) can deliver target genes into T cells, mediated through binding of the low-density lipid receptor (LDLR) surface protein, which resides on all T cells as well as many other cell types. Hence, pseudotyping lentiviruses with VSV-G does not fulfill the requirement of delivering genes of interest specifically into T cells, not to mention those with a desired TCR specificity. To improve the ability of lentiviruses to specifically infect T cells, others have reported the use of lentivirus pseudotyped with antibodies to achieve cell type–specific targeting (7–9), for example, by displaying the OKT3 antibody on viral particles to target total T cells (9). However, since those antibodies bind to CD3, this approach lacks the ability to modify T cell subsets based on their TCR specificity. Thus, a strategy with such applications remains to be developed.
Here, we present an approach, V-CARMA (Viral ChimAeric Receptor MHC-Antigen), to generate peptide-human leukocyte antigen (pHLA)-pseudotyped lentiviruses (pHLA-PLVs) to specifically infect T cells that express the cognate TCRs and deliver genes into these T cells of interest. Utilizing these pHLA-PLVs, we show that both primary and immortalized human CD8+ or CD4+ T cells expressing cognate TCRs can be specifically infected by the virus displaying class I HLA presenting a viral antigen or cancer antigen or class II HLA presenting a self-antigen. The infection rate of PLVs is correlated to the binding affinity of the TCR to its cognate antigen. In addition, pHLA-PLVs can robustly identify a minor population of T cells with cognate TCRs from a heterogenous T cell population, with low infection rate to the cells with irrelevant TCRs. Furthermore, after infection with pHLA-PLVs carrying PD-1 shRNAs or dimerizable Caspase-8, antigen-specific T cell populations can be engineered to suppress PD-1 expression in tumor-reactive CD8+ T cells or undergo inducible apoptosis in autoreactive CD4+ T cells, respectively. Thus, we demonstrate that pHLA-PLVs are not only versatile tools with which to study the basic immunology of TCR specificity, but also provide an immunotherapy strategy to implement its use for genetic engineering of disease-relevant T cells.
Results
TCR-Specific Infection of T Cells Using V-CARMA Technology.
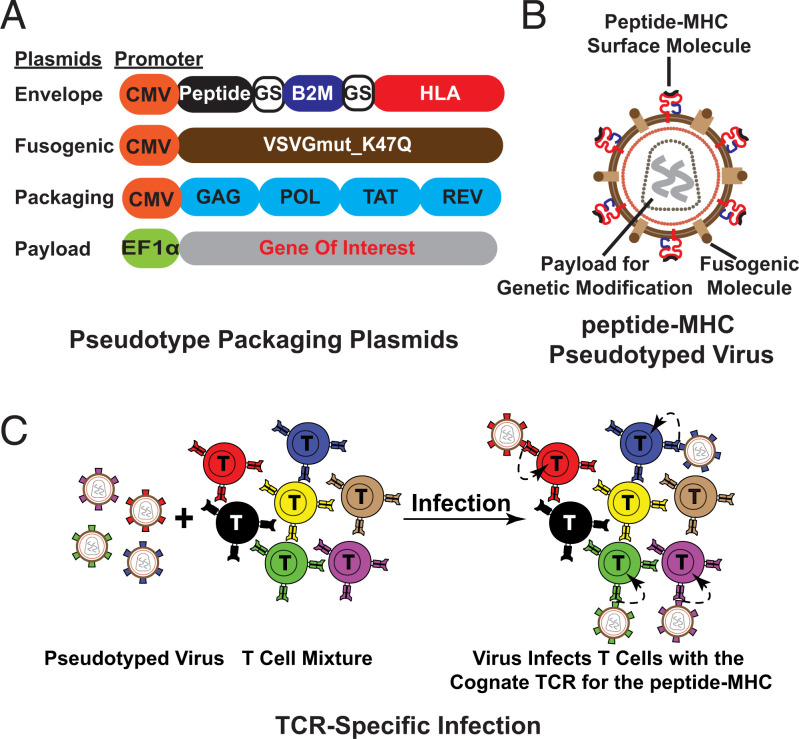
We sought to develop a method to genetically modify subsets of T cells. The most specific aspect of a T cell’s functional identity is its TCR, which recognizes a specific peptide bound to MHC. While an antibody specific to one TCR could be used for pseudotyping, for a given antigen, multiple TCRs are likely to exist that detect that particular peptide and all of these could not be recognized by a given antibody. Thus, we chose to use the peptide-MHC itself as the guiding molecule for the virus and a pseudotyping strategy to target subsets of T cells expressing TCRs of interest. To generate TCR-specific PLVs, we employed a four-plasmid system consisting of an envelope plasmid (peptide-HLA single chain trimer separated by a glycine-serine linker [GS]), a fusogenic molecule (VSV-G with K47Q mutation), a packaging plasmid (psPAX2, carrying genes required for lentiviral packaging), and a cargo plasmid (the genes to be packaged and delivered into target cells) (Fig. 1 A and B). Upon infection, the peptide-MHC on the surface of PLVs binds to the cognate TCR on T cells and the virus is subsequently endocytosed (Fig. 1C). The VSV-G mutation, K47Q, abolishes the ability of VSV-G to bind to the LDLR on the cell surface of T cells, while maintaining the ability of VSV-G–mediated viral fusion (10).
Fig. 1.
Overview of V-CARMA. (A) Four plasmids used for packaging pHLA-PLVs (class I MHC). (B) Schematic of the peptide -MHC(red) PLVs (class I MHC). (C) Schematic of antigen-specific T cell infection by pHLA-PLVs. Identical colors indicate cognate antigen-TCR pairs.
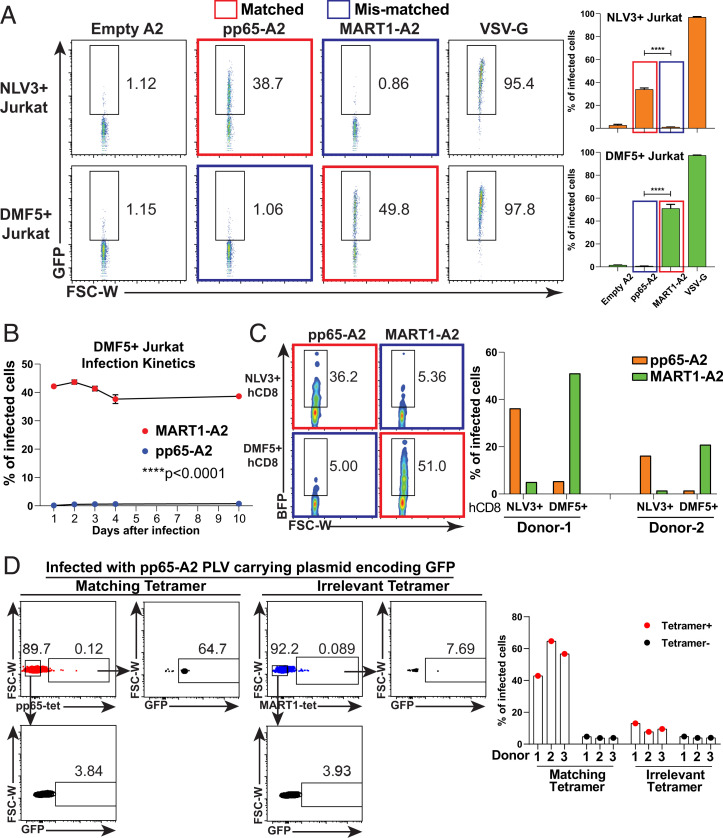
Following this design strategy, we generated pHLA-PLVs pseudotyped with empty-HLA-A2, pp65-HLA-A2 (viral antigen), and MART1-HLA-A2 (cancer antigen). Each pseudotyped virus carries a lentiviral vector expressing a GFP expression cassette, separately from the envelope plasmid, to label successfully infected CD8+ Jurkat cells expressing either the NLV3 TCR, which recognizes the cytomegalovirus (CMV)/pp65 antigen or the DMF5 TCR, which recognizes Melan-A/MART126–35(A27L) antigen. Forty-eight hours after infection, ∼40% of NLV3-expressing Jurkat cells infected by pp65-A2 PLVs expressed GFP, compared to only ∼1% when infected with the empty-HLA or the mismatched MART1-A2 PLVs (Fig. 2A). Conversely, ∼50% of DMF5-expressing Jurkat cells can be infected by the MART1-A2 PLVs. As a positive control, PLVs pseudotyped with wild-type VSV-G infected more than 95% of target cells. To further determine whether gene delivery by pHLA-PLVs is stable, the infected Jurkat cells were examined for GFP expression at days 1, 2, 3, 4, and 10 after infection. GFP expression was stably maintained for 10 d for both experimental and control groups (Fig. 2B). In addition to infecting immortalized human T cells, the pp65-A2 and MART1-A2 PLVs can specifically infect primary human CD8+ T cells from two blood donors engineered to express NLV3 and DMF5 TCRs (Fig. 2C).
Fig. 2.
The infection of V-CARMA with antigens presented by class I MHC is TCR specific. (A, Left) Fluorescence activated cell sorting (FACS) plots showing the infections of pHLA-PLVs displaying empty A2, pp65-A2, MART1-A2, and VSV-G to CD8+ Jurkat cells expressing the NLV3 TCR (Top) and the DMF5 TCR (Bottom). The matched pair of antigen and TCR is labeled with a red box, and the mismatched pair of antigen and TCR is labeled with a blue box. (A, Right) Statistical analysis of the FACS results on the Left (n = 3 per group). (B) Time-course analysis of pHLA-PLVs displaying MART1-A2 (red dots) and pp65-A2 (blue dots) infection to CD8+ Jurkat cells expressing DMF5 TCR at days 1, 2, 3, 4, and 10 after infection by flow cytometry (n = 2 per group). (C, Left) FACS plots of pHLA-PLVs displaying empty-A2, pp65-A2, and MART1-A2 infection to primary human CD8 T cells expressing NLV3 TCR (Top) and DMF5 TCR (Bottom). (C, Right) Quantitation graph of the flow cytometry results on the Left. Primary CD8 T cells from two individual donors were tested. (D, Left) FACS plots of pHLA-PLVs displaying pp65-A2 infection to primary human CD8 T cells and tetramer staining with matching tetramer (Left) and irrelevant tetramer (Right). (D, Right) Quantitation graph of the flow cytometry results on the Left. Primary CD8 T cells from three individual donors were tested. Data are presented as mean ± SEM and are representative of two independent experiments. ****P < 0.0001.
A key question is the extent to which this method can also demonstrate specificity with authentic T cells from patients. To examine this, we looked for the ability of appropriately pseudotyped PLVs to infect TCRs that recognize epitopes commonly found in humans that are easily detectible by tetramer staining, the CMV/ pp65, and the MART1-A2Melan-A/MART1 epitopes. Therefore, total human CD8 T cells from three blood donors (HLA-A2+) were isolated and infected with pp65-A2 PLVs (11). Forty-eight hours after infection, the infected cells were stained with either pp65 tetramer (matching tetramer) or MART1 tetramer for flow cytometric analysis (irrelevant control tetramer) (Fig. 2D). The results show that more than 50% of the pp65 tetramer positive cells were infected with pp65-A2 PLVs, while less than 10% of irrelevant tetramer positive cells were infected, which suggests the pMHC-PLVs can infect T cells with cognate TCRs specifically in a pool of polyclonal T cell population. Thus, pHLA-PLVs can specifically infect and deliver target genes into T cells for stable expression, with a 40- to 50-fold increase over background level of specificity.
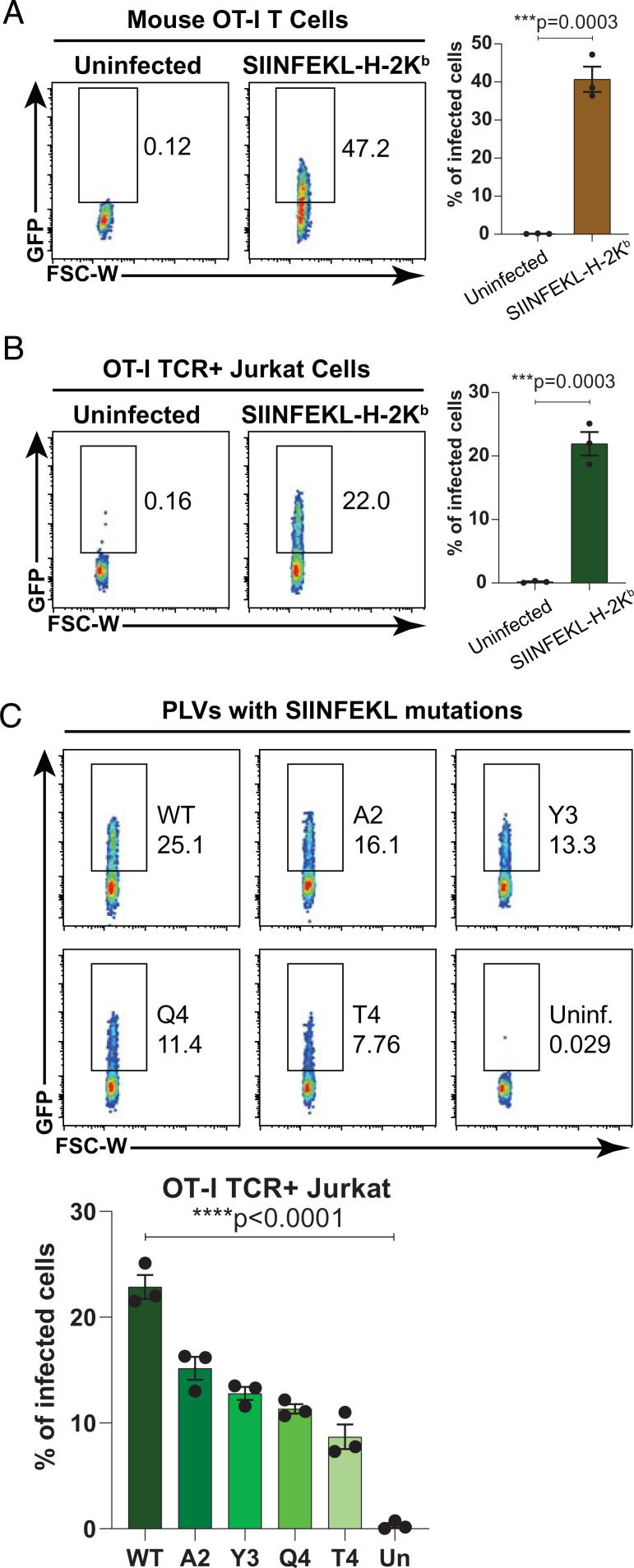
In order to test whether the affinity of the TCR for pMHC has influence on the pMHC-PLV infection, we pseudotyped the lentivirus with ovalbumin peptide SIINFEKL presented by the mouse MHC class I H-2Kb, which can be recognized by mouse OT-I TCR. Mouse SIINFEKL-H-2Kb PLVs can robustly infect primary CD8 T cells isolated from OT-I mice (Fig. 3A) and Jurkat cells expressing OT-I TCR (Fig. 3B), which suggests the pMHC-PLVs can be used to infect mouse T cells as well. A previous report has shown that several single mutations in the SIINFEKL peptide can curtail its binding affinity to OT-I TCR (12). Hence, we pseudotyped the lentiviruses with SIINFEKL and its mutants, containing I2A (A2), I3Y (Y3), N4Q (Q4), and N4T (T4), and infected Jurkat cells expressing OT-I TCR. The results reveal a decreased infection rate of the PLVs with SIINFEKL mutants, relative to wild-type SIINFEKL. The infection rate changes directly correlate with their previously determined binding affinity to the OT-I TCR (12). Therefore, our results indicate that the pMHC-PLVs can be used to infect T cells expressing human and mouse TCRs and the efficacy of infection is correlated with the binding affinity of the TCR to the pMHC.
Fig. 3.
PLVs with SIINFEKL-H-2Kb and its mutants can infect T cells expressing OT-I TCR by their binding affinity. (A and B, Left) FACS plots showing the infections of pHLA-PLVs displaying SIINFEKL-H-2Kb to primary mouse OT-I CD8 T cells (A) and Jurkat cells expressing OT-I TCR (B). (A and B, Right) Statistical analysis of the FACS results on the Left (n = 3 per group). (C, Top) FACS plots showing the infections of pHLA-PLVs displaying SIINFEKL-H-2Kb and mutants (I2A, I3Y, N4Q, and N4T) to Jurkat cells expressing OT-I TCR. (C, Bottom) Quantitation graph of the flow cytometry results on the Top (n = 3 per group). Data are presented as mean ± SEM and are representative of two independent experiments.
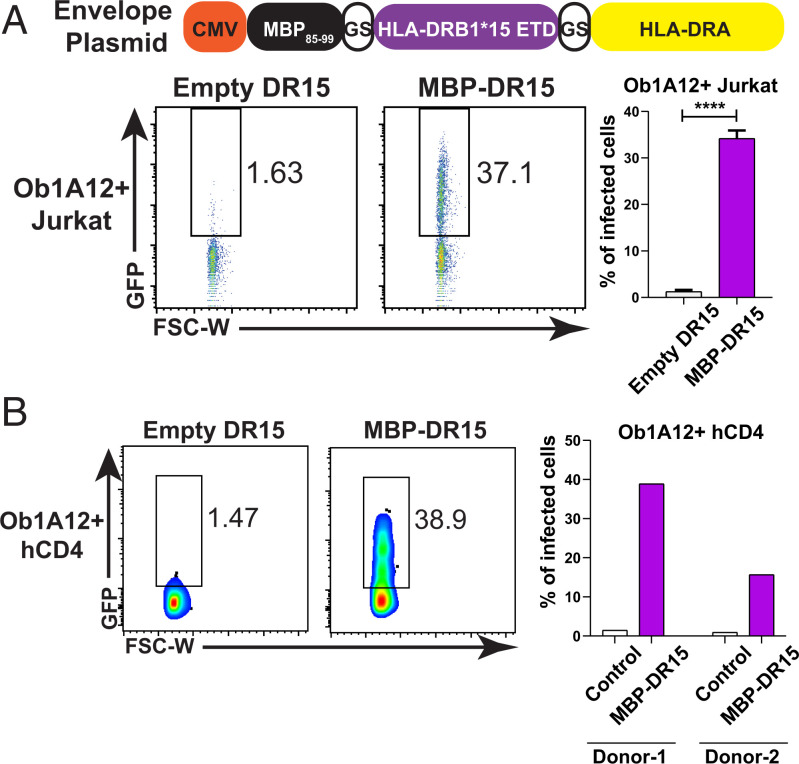
Next, we determined whether class II-restricted CD4+ T cells could also be targeted using pHLA-PLVs. To test this, we pseudotyped lentivirus with a well-known MHC class II antigen from myelin basic protein (MBP) and loaded the antigenic peptide on human HLA-DRB1*15/DRA*01 (DR15) using a similar single-chain trimer design as for class I pMHC complexes (13). The MBP-DR15 PLVs were packaged as described for class I PLVs and used to infect Ob1A12 TCR-expressing CD4+ Jurkat cells, which specifically recognize the MBP peptide on DR15. Compared to the empty DR15 PLVs, the MBP-DR15 PLVs significantly and specifically infected the target CD4+ T cells (Fig. 4A). Similarly, the MBP-DR15 PLVs can specifically infect primary human CD4+ T cells from two blood donors engineered to express Ob1A12 TCR (Fig. 4B). Together our data show that peptide-HLA PLVs can infect target CD4+ T cells expressing cognate TCRs and deliver a genetically encoded payload into specific T cells of interest.
Fig. 4.
V-CARMA can infect CD4 T cells expressing cognate TCR via displaying peptide presented by class II MHC. (A, Top) Schematic of envelope plasmid design for MBP85–99-DR15. (A, Bottom) FACS plots and quantitation graph of pHLA-PLVs displaying empty and MBP-DR15 infection to CD4+ Jurkat cells expressing Ob1A12 TCR (n = 3 per group). (B, Left) FACS plots of pHLA-PLVs displaying empty and MBP-DR15 infection to human primary CD4 T cells expressing Ob1A12 TCR. (B, Right) Quantitation graph of the flow cytometry results on the Left. Primary CD4 T cells from two individual donors were tested. Data are presented as mean ± SEM and are representative of two independent experiments. ****P < 0.0001.
Applications of V-CARMA.
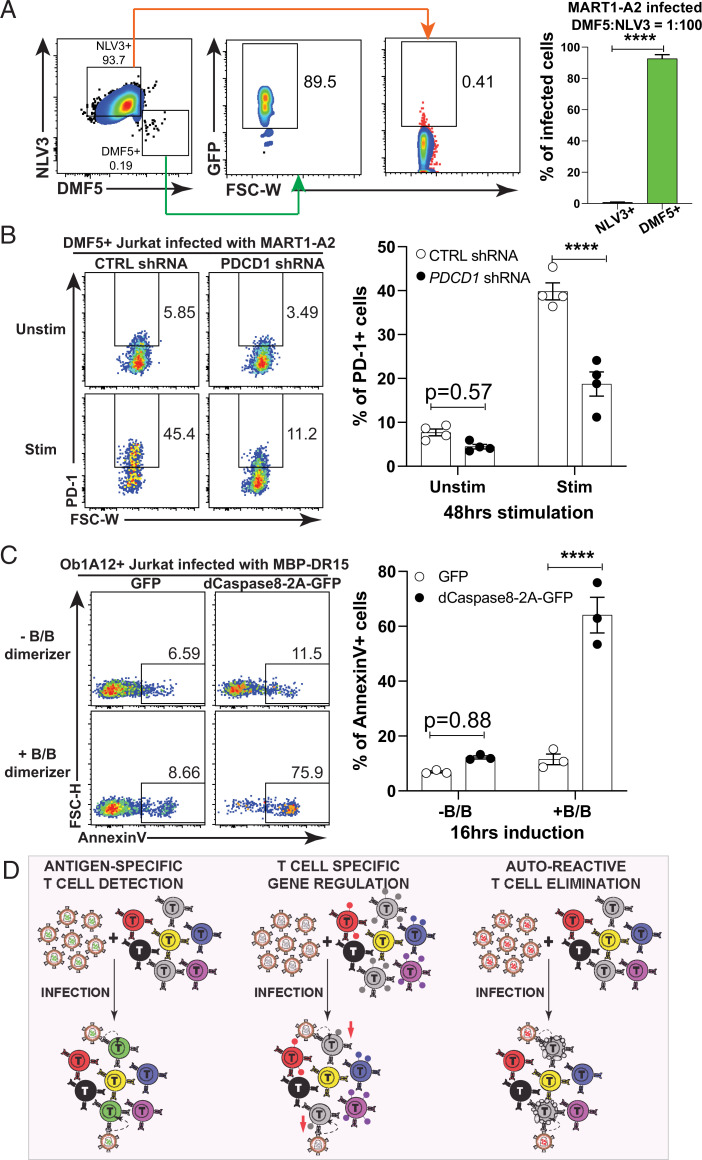
After establishing that pHLA-PLVs can specifically infect target TCR-expressing T cells, we tested potential applications for this technology. Initially, we examined the sensitivity of pHLA-PLVs to identify T cells expressing cognate TCRs from a mixed population with DMF5 TCR-expressing T cells and NLV3 TCR-expressing T cells at a ratio of 1:100. We then infected with MART1-A2 PLVs and analyzed selective infection by checking the GFP expression (Fig. 5A). The MART1-A2 PLVs were found to infect ∼90% of DMF5 TCR-expressing cells while only 0.4% of NLV3 TCR-expressing cells were infected. Thus, the peptide-HLA PLVs can identify the target cells from a mixed cell population, which can potentially be applied to screening purposes for TCR identification.
Fig. 5.
Applications of V-CARMA. (A, Left) FACS plots of pHLA-PLVs displaying MART1-A2 infection to a mixed population of CD8+ Jurkat cells expressing DMF5 TCR and NLV3 TCR at a ratio of 1:100, respectively. (A, Right) Quantitation of the flow cytometry results on the Left (n = 3 per group). (B, Left) FACS plots of CD8+ Jurkat cells expressing DMF5 TCR that presorted on the PD1+ population with PMA/ionomycin stimulation infected with pHLA-PLVs displaying MART1-A2 carrying control or PDCD1 shRNAs with or without PMA/ionomycin restimulation and tested for surface PD-1 expression. (B, Right) Quantitation graph of the flow cytometry results on the Left (n = 4 per group). (C, Left) FACS plots of CD4+ Jurkat cells expressing Ob1A12 TCR infected with pHLA-PLVs displaying MBP-DR15 carrying a lentiviral plasmid with GFP or dimerizable Caspase8-2A-GFP and tested for AnnexinV staining. (C, Right) Quantitation graph of the flow cytometry results on the Left (n = 3 per group). (D) Schematic of potential applications of V-CARMA. (D, Left) Detection and infection of T cells bearing the cognate TCR for the specific antigen-MHC complex. (D, Center) Infection of cognate T cells to regulate gene expression illustrated as colored dots on the surface of the T cell down-regulated by the V-CARMA construct. (D, Right) Elimination of autoreactive cognate T cells using an inducible caspase gene. Data are presented as mean ± SEM and are representative of two independent experiments. ****P < 0.0001.
Not only can these peptide-MHC PLVs identify T cells expressing specific TCRs, but they also have the ability to deliver genes into the target cells with the potential for therapy. Based on this notion, we packed human PD-1 shRNA vectors into MART1-A2 PLVs and examined their ability to decrease surface PD-1 levels on CD8+ T cells. Following viral infection, DMF5 TCR-expressing Jurkat cells were stimulated by PMA/ionomycin for 48 h and analyzed by flow cytometry for their PD-1 expression. Compared to the cells infected by the PLVs packed with control shRNAs, the ones infected by the PLVs with PD-1 shRNAs had significantly lower expression of PD-1 than those infected by the PLVs with control shRNAs (Fig. 5B). These results indicate the potential application of pHLA-PLVs for promoting T cell potency in the context of immunotherapy.
In addition to genetically altering the phenotype of specific T cell populations, we explored the feasibility of using PLVs to kill autoreactive T cells that are responsible for autoimmune diseases. To test this, we generated PLVs with a lentiviral vector expressing dimerizable human Caspase 8 (dCaspase8), where dimerization can be induced by addition of a B/B homodimerizer to potently induce apoptosis in cells (14). Using MBP-DR15 pseutodypted viral particles, PLVs carrying dCaspase8 induced significant levels of apoptosis 16 h after dimerization induction, while control T cells that received a GFP control vector did not (Fig. 5C). Therefore, the results suggest that the PLVs can be applied to inducibly kill autoreactive T cells, which provides an approach for the treatment of T cell–mediated autoimmune diseases.
In summary, our results indicate that V-CARMA has multiple applications, including the ability to identify specific TCRs from heterogeneous pools, down-regulate immune checkpoint–related genes, and provide a mechanism for suicide gene delivery to inducibly kill autoreactive T cells (Fig. 5D). These applications can not only contribute to basic immunology studies on TCR specificity, but also to T cell immunotherapy to cancers and autoimmunity.
Discussion
Immunotherapy is a promising method to treat cancers, infectious diseases, and autoimmune diseases by harnessing the power of T cells. Targeting specific T cell subsets is an important step for precision treatment of T cells for improved therapies. Here, we report V-CARMA that employs pseudotyped lentiviruses expressing peptide-MHC complexes on the surface of virions to specifically bind to cognate TCRs and infect T cells expressing the cognate TCRs. We have demonstrated that the pHLA-PLVs displaying antigens in either MHC class I or class II molecules can successfully infect target T cells, including primary and immortalized human and mouse CD8+ and CD4+ T cells, and the infection efficacy reflects the binding affinity of the TCR to the pMHC.
Through the establishment of the V-CARMA platform, we have shown that pHLA-PLVs can be used to identify TCR ligands, in a sense acting like tetramers but with the additional potential of either modifying T cell functions or killing specific subsets of autoreactive T cells. The advantage of pHLA-PLVs over previous technologies is their ability to deliver genes into target T cells specifically due to the requirement for TCR and peptide/MHC interactions. This approach increases both the specificity and stability of cell labeling in the process of TCR ligand discovery and enables T cell engineering and modification. A future challenge here is the generation of higher titer V-CARMA to push infectivity toward 100% if we seek to achieve in vivo efficacy for eliminating T cell subsets. However, for certain applications, full subset infection is not essential. For example, inactivating PD-1 even in a subset of tumor infiltrating lymphocytes (TILs) holds the promise of impacting TIL function in a dominant manner. Importantly, V-CARMA is not limited to lentiviruses, particularly for the applications of therapeutics and in vivo studies. In principle, it can be applied to a number of viral backbones like adenovirus to provide transient gene therapies such as CRISPR modification or base editors in cancers and to eliminate autoreactive T cells in autoimmune diseases. Ultimately, this approach has broad potential to help improve the efficiency for immunotherapies to treat cancers and fight infectious diseases and autoimmunity.
Materials and Methods
Cell Lines and Primary Cells.
HEK-293T cells were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco's Modified Eagle Medium (DMEM) (Gibco) with 10% (vol/vol) fetal bovine serum (FBS) (HyClone), 100 units/mL penicillin (Gibco), and 0.1 mg/mL streptomycin (Gibco). Jurkat J.RT3-T3.5 cells were obtained from ATCC and cultured in Roswell Park Memorial Institute (RPMI) (Gibco) with 10% (vol/vol) FBS (HyClone), 100 units/mL penicillin, and 0.1 mg/mL streptomycin.
Deidentified apheresis collars were obtained from the Brigham and Women’s Hospital specimen bank under protocol T0276. Primary human CD8 T cells were isolated from donor blood using RosetteSep Human CD8+ T Cell Enrichment Mixture (StemCell), per manufacturer’s instructions. Primary CD8 T cells were cultured in RPMI with 10% (vol/vol) FBS (HyClone), 100 units/mL penicillin, 0.1 mg/mL streptomycin, and 50 IU/mL human interleukin-2 (Peprotech).
DNA Constructs.
For the envelope plasmid, peptide-MHC single-chain trimer (SCT) sequences, composed of signal sequence, antigenic peptide and human B2M-HLA-A2 or DR15 linked by (GGGGS)3 linkers, were cloned into a mammalian expression vector driven by cytomegalovirus (CMV) promoter.
The pMD2.G plasmid was obtained from Addgene (#12259) and the K47Q mutation was introduced by PCR mutagenesis and used as the fusogenic plasmid. psPAX2 plasmid was obtained from Addgene (#12260) and used as packaging plasmid.
The gBlocks gene fragments (IDT) encoding TCRs (NLV3, DMF5, OT-I, and Ob1A12) and human CD8ɑβ and CD4 were synthesized and cloned into pHAGE_EF1a_DEST_ GFP/BFP/puromycin/blasticidin lentiviral destination vectors.
The sequences of GFP and dCaspase8-2A-GFP were amplified from Fv-hCaspase 8-2A-GFP plasmid obtained from Addgene (#82712) and cloned into a pLVX lentiviral destination vector.
Lentivirus Production.
Lentivirus was produced by transfecting HEK-293T cells with the envelope plasmid (pMHC) or pMD2.G, the fusogenic plasmid (VSVGmut_K47Q), the lentiviral transfer vector, and the packaging plasmid (psPAX2) at a ratio of 1:1:2:1 in mass. Transfection was performed using the PolyJet In Vitro DNA Transfection Reagent (SignaGen) according to the manufacturer’s protocol. Lentiviral supernatants were collected at 24 and 48 h posttransfection, filtered through a 0.45-μm filter, and concentrated using Lenti-X Concentrator (Clontech).
Primary CD8 T Cell Transduction.
One million primary CD8 T cells were seeded per well of a 24-well plate and stimulated with a 1:1 ratio of Dynabeads Human T-Activator CD3/CD28 (Gibco) and simultaneously transduced with 100 μL of concentrated lentivirus encoding the TCR of interest. After 3 d, transduced T cells were sorted based on the GFP expression.
T Cell Infection.
The 5 × 105 Jurkat cells or primary CD8 T cells expressing TCRs of interest were seeded per well of a 24-well plate and 100 μL of concentrated lentivirus was added to cells in the presence of 8 μg/mL Polybrene. The plate was sealed with parafilm and centrifuged at 2,000 rpm for 1 h at 30 °C. Following the centrifugation, the supernatant was replaced with fresh medium.
Tetramer Staining.
Forty-eight hours after pp65-A2 PLV infection, 1 × 106 infected human CD8 T cells were incubated with 100 nM dasatinib for 30 min and stained with tetramers for 30 min. The pp65 and MART1 tetramers were synthesized by QuickSwitch Quant HLA-A*02:01 Tetramer Kit per manufacturer’s instruction. Subsequently, the cells were washed twice by phosphate-buffered saline (PBS) and stained with Ghost Dye Violet 510 (Tonbo Biosciences). The cells were washed twice with PBS and analyzed by flow cytometry.
PD-1 Induction and shRNA Knockdown.
The lentiviral vectors carrying PDCD1 or control shRNAs were picked from the GIPZ shRNA library (Open Biosystems) and packaged into MART1-A2 pHLA-PLV. DMF5-expressing Jurkat cells were stimulated with cell activation mixture without Brefeldin A (BioLegend) at 1:500 dilution for 48 h and PD-1+ cells were sorted, expanded, and infected with MART1-A2 pHLA-PLV carrying PDCD1 or control shRNAs. After 24 h of infection, the cells were restimulated with cell activation mixture without Brefeldin A at 1:100 dilution for 48 h and examined for PD-1 induction by flow cytometry.
Apoptosis Induction and AnnexinV Detection.
The lentiviral vectors carrying GFP or dCaspase8-2A-GFP were packaged into MBP-DR15 pHLA-PLV and infected Ob1A12-expressing Jurkat cells. After 48 h, B/B homodimerizer (Takara) was added to the culture and the cells were incubated for 16 h and stained for AnnexinV with Allophycocyanin (APC)-conjugated AnnexinV Apoptosis Detection Kit with Propidium Iodide (PI) (BioLegend) per manufacturer’s instruction.
Quantification and Statistical Analysis.
Statistical details of experiments can be found in the figure legends. Data analysis was performed in Flowjo, Excel, and GraphPad Prism. All error bars in figures indicate SEM.
Acknowledgments
We thank A. Kohkgruber, T. Martin, and M. Dezfullian for helpful discussions and/or reading this manuscript. X.-z.J.G. is a Cancer Research Institute/Dr. Keith Landesman Memorial Fellow (CRI Award #3439). This research was supported by the Department of Defense Distinguished Investigator Award W81XWH-18-1-0469 to S.J.E. and the Ludwig Center at Harvard Medical School. S.J.E. is an investigator with the Howard Hughes Medical Institute.
Footnotes
Reviewers: N.L.G., Monash University; and M.N., Massachusetts General Hospital.
Competing interest statement: S.J.E. is a founder of TScan Therapeutics, Maze Therapeutics, ImmuneID and Mirimus; a scientific advisory board member for Homology Medicines, TScan Therapeutics, Maze Therapeutics, and ImmuneID; and an advisor for MPM Capital.
Data Availability
All study data are included in this article.
References
- 1.Kula T., et al. , T-Scan: A genome-wide method for the systematic discovery of T cell epitopes. Cell 178, 1016–1028.e13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnbaum M. E., et al. , Deconstructing the peptide-MHC specificity of T cell recognition. Cell 157, 1073–1087 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gee M. H., et al. , Antigen identification for orphan T cell receptors expressed on tumor-infiltrating lymphocytes. Cell 172, 549–563.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li G., et al. , T cell antigen discovery via trogocytosis. Nat. Methods 16, 183–190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisielow J., Obermair F.-J., Kopf M., Deciphering CD4+ T cell specificity using novel MHC-TCR chimeric receptors. Nat. Immunol. 20, 652–662 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Rappazzo C. G., Huisman B. D., Birnbaum M. E., Repertoire-scale determination of class II MHC peptide binding via yeast display improves antigen prediction. Nat. Commun. 11, 4414 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taube R., et al. , Lentivirus display: Stable expression of human antibodies on the surface of human cells and virus particles. PLoS One 3, e3181 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank A. M., Buchholz C. J., Surface-engineered lentiviral vectors for selective gene transfer into subtypes of lymphocytes. Mol. Ther. Methods Clin. Dev. 12, 19–31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H., Joo K.-I., Ziegler L., Wang P., Cell type-specific targeting with surface-engineered lentiviral vectors co-displaying OKT3 antibody and fusogenic molecule. Pharm. Res. 26, 1432–1445 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolic J., et al. , Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat. Commun. 9, 1029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeth N., et al. , Humoral and cellular CMV responses in healthy donors; identification of a frequent population of CMV-specific, CD4+ T cells in seronegative donors. PLoS One 7, e31420 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zehn D., Lee S. Y., Bevan M. J., Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J., et al. , HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in multiple sclerosis. Cell 183, 1264–1281.e20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberst A., et al. , Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J. Biol. Chem. 285, 16632–16642 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in this article.