Significance
Sheep and goats (caprines) were domesticated in Southwest Asia, but how and in how many places remain open questions. Our analysis of caprine age and sex structures and related data reveal a local (endemic) domestication process at Aşıklı Höyük in Central Anatolia. Beginning ca. 10,400 y ago, caprine management segued through a series of viable systems over the next 1,000 y. The earliest stage simply involved capturing wild lambs and kids and growing them on site to supplement a broad-spectrum forager diet. Soon, low-level breeding began within the settlement along with catching and raising wild infants. By the end of the archaeological sequence, large numbers of animals were produced from captive herds, which gave rise to early domesticated forms.
Keywords: pre-Pottery Neolithic, sheep and goat management, mortality patterns, zooarchaeology, forager–producer transition
Abstract
Sheep and goats (caprines) were domesticated in Southwest Asia in the early Holocene, but how and in how many places remain open questions. This study investigates the initial conditions and trajectory of caprine domestication at Aşıklı Höyük, which preserves an unusually high-resolution record of the first 1,000 y of Neolithic existence in Central Anatolia. Our comparative analysis of caprine age and sex structures and related evidence reveals a local domestication process that began around 8400 cal BC. Caprine management at Aşıklı segued through three viable systems. The earliest mode was embedded within a broad-spectrum foraging economy and directed to live meat storage on a small scale. This was essentially a “catch-and-grow” strategy that involved seasonal capture of wild lambs and kids from the surrounding highlands and raising them several months prior to slaughter within the settlement. The second mode paired modest levels of caprine reproduction on site with continued recruitment of wild infants. The third mode shows the hallmarks of a large-scale herding economy based on a large, reproductively viable captive population but oddly directed to harvesting adult animals, contra to most later Neolithic practices. Wild infant capture likely continued at a low level. The transitions were gradual but, with time, gave rise to early domesticated forms and monumental differences in human labor organization, settlement layout, and waste accumulation. Aşıklı was an independent center of caprine domestication and thus supports the multiple origins evolutionary model.
Animal domestication is a coevolutionary mutualism that, in rare cases and with sufficient time, may develop between humans and another species (1–3). Many of the domesticated ungulates that we know today evolved within the Neolithic period between about 10,000 and 5,000 y ago. Accumulating evidence has revealed that, as with many plants, domestication of hoofed animal species occurred in multiple loci and over longer periods than previously recognized. Most of what we know about animal domestication pertains to the later outcomes of the process. We know far less about the formative conditions of domestication because the physical traits commonly studied only diverge from wild progenitors after many generations. Reliable contextual data about the timing and nature of the early phases of animal management are harder to come by, yet they are utterly essential to understanding early domestication processes. These contextual data concern the physical and social aspects of the human-altered environment to which the partner species adapts and the human behaviors that repeatedly biased survivorship in the partner population. Most of this contextual information is provided by archaeological science.
Three common pathways to animal domestication are widely recognized—the commensalism pathway, the prey pathway, and the directed pathway (2, 4). They differ in the conditions of selection but nonetheless describe a continuum between free-ranging populations that colonize and adapt in specific ways to human-altered environments (commensal pathway) and highly orchestrated manipulation by humans of survivorship and mate choice within a controlled environment (directed pathway). Prey domestication pathways lie within these extremes, variously involving partial captivity, periodic admixture, and subtraction of the most undesirable individuals from the managed population. Continuous admixture with wild counterparts is bound to slow the overall pace of morphological change in a managed population but may have been common in early periods. Such admixture can be critical for preserving genetic diversity over the long term by allowing a relatively small subpopulation to accumulate a wide range of neutral traits (1, 3, 5–7). Under these conditions, significant behavioral and physiological shifts can occur in the managed population well in advance of obvious skeletal changes (the purview of zooarchaeologists) if body size reduction is not the focus of strong selection.
The prey pathway model applies well to sheep and goat (caprine) domestication. Mortality patterns by age and sex are particularly sensitive indicators of caprine management by humans and of variation in humans’ strategies for obtaining meat, fat, and, in some cases, milk and/or hair (8–14). Some mortality patterns are subject to equifinality, as they can arise from more than one causal factor. When coupled with independent lines of archaeological evidence, however, mortality patterns are central to understanding the evolutionary process of domestication and its outcomes.
This study investigates the early conditions and trajectory of caprine management at the Pre-Pottery Neolithic (PPN) site of Aşıklı Höyük (AH) and the question of whether early domestic forms arose within this context. Located in the Central Anatolian region of Cappadocia (Fig. 1), AH preserves an unusually high-resolution stratigraphy and rich zooarchaeological record (15–17) that spans 1,000 y of continuous occupation (ca. 8400 to 7350 cal BC) (18). AH lies within the natural ranges of both mouflon sheep (Ovis gmelinii, a.k.a. O. orientalis anatolica) and bezoar goat (Capra aegagrus). These wild species came under human management around the time that the settlement was first established by broad-spectrum foragers (17, 19, 20).
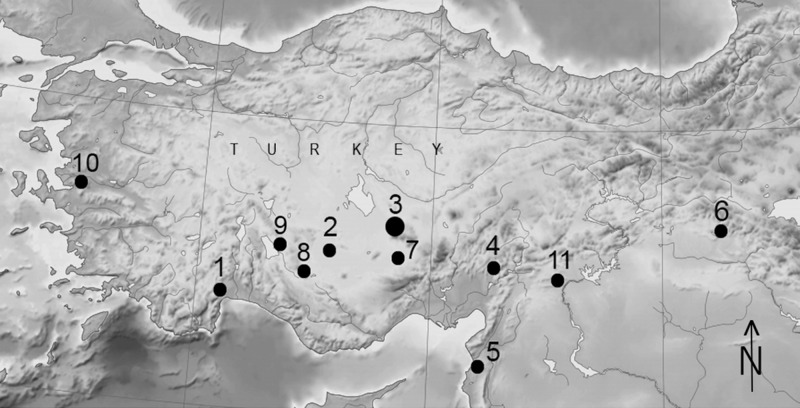
Fig. 1.
Location of Aşıklı Höyük and other Anatolian sites discussed: 1) Öküzini and Karain B caves, 2) Çatalhöyük, 3) Aşıklı Höyük, 4) Direkli cave, 5) Üçağızlı caves I and II, 6) Hallan Çemi, 7) Köşk Höyük, 8) Suberde, 9) Erbaba, 10) Ulucak, and 11) Gritille.
Core questions persist about how the management relationship evolved at AH, the pace and scale of change, and whether domestic forms emerged within the 1,000 y of the community’s existence. To address these questions, we use measures of 1) caprine importance relative to other prey, 2) the ratio of sheep to goats, 3) caprine sex ratios, and 4) caprine mortality patterns. A comparison of the AH caprine mortality patterns to Epipaleolithic and Paleolithic cases provides an independent “yardstick” for interpreting the extent of change within a larger human–caprine interaction continuum. The zooarchaeological trends at AH are then linked to independent contextual data on phytoliths, sediment micromorphology and chemistry, caprine bone pathologies, stable isotopes, and genetic evidence. A synthesis of these data offers much clarification on the selective forces experienced by the AH caprines, the extent of evolutionary change, and the probable mechanisms of genetic isolation.
Archaeological Background
AH is the earliest known aggregated PPN settlement in Central Anatolia (15, 21, 22). A variety of mobile and semimobile foragers resided in Cappadocia and the Konya Plain at this time, but the sites created by other groups were smaller and their occupation histories shorter (compare refs. 22–25). AH nonetheless was part of a regional patchwork of complex hunter-gatherers. The faunal and botanical records of AH document a socioeconomic transition from broad-spectrum foraging to food production.
The AH settlement was founded ca. 10400 cal B.P. along a wide stretch of the Melendiz River floodplain, where rich soils had developed from late Pleistocene marsh deposits (26). By the time of its abandonment, the site had expanded into a 4-ha mound roughly 16 m high (15). The earliest cultural deposit, Level 5, represents a small and probably semisedentary settlement with round wattle-and-daub structures set deep into the ground. Levels 4 to 3 were permanent occupations with semisubterranean oval mudbrick residential structures. Level 2 is distinguished by rectangular mudbrick structures built entirely aboveground. The settlement became increasingly dense through structural infilling as its total area expanded. Levels 5 to 4 span ca. 8400 to 8000 cal BC, Level 3 spans 8000 to 7700 cal BC, and Level 2 spans 7700 to 7300 cal BC (18).
Gradual change was the rule at AH over the millennium of its existence, but the major facets of the archaeological record changed at very different rates. Lithic technology shifted very slowly until the final phases of occupation, whereas architecture and human–caprine interactions changed greatly throughout the sequence (15, 27–29). AH preserves the earliest known occurrence of the new glume wheat type and free-threshing wheat (in Level 5, Triticum aestivum/durum) in Anatolia and perhaps elsewhere in the Middle East (28). However, the ratio of cereals and pulses to other plant foods increased only mildly from Level 5 through Level 2, and people continued to rely heavily on wild plants (28). Zooarchaeological and geoarchaeological data indicate that small numbers of caprines were held captive inside the settlement by 10350 cal B.P., if not earlier (17).
Rare exotic materials such as shells from the Mediterranean Sea and nonnative cultigens such as chickpea occur in Levels 5 to 4. This situation was followed by a prolonged interval of limited or no long-distance exchanges between AH and other communities. Strangely, the Aşıklans did not participate in the obsidian exchange networks that targeted high-quality obsidian sources only 20 to 30 km east of the settlement (27, 29) (see also refs. 30–32); these sources were used heavily by the locals and nonlocal prospectors alike (29, 33). The tendency for community insularity persisted until the final phases of Level 2 (2D to C), when a revival of interregional exchange is marked by the influx in exotic decorative objects (34, 35). The gradual nature of change at AH and limited evidence of outside cultural influences for much of the settlement’s history help to explain many of the unique properties of this community (15, 36).
Results
Trends in Caprine Importance at Aşıklı.
The early meat diet at AH included a wide range of large and small wild prey (SI Appendix, Fig. S1). Caprines were a minor component at the outset (27% in Level 5) but gradually became the dominant meat source (81% in Level 2J to D) (SI Appendix, Table S1). Changes in the ratio of sheep to goats with time are also of interest because the two species have somewhat different food and habitat tolerances. Sheep were the dominant caprine species in all levels, but dung phytolith evidence demonstrates that both species were held captive within the settlement from an early date (37). We used eight diagnostic skeletal elements that are common and similarly represented across levels—scapula, humerus, radius, tibia, astragalus, calcaneum, third phalanx, and lower third molar—to determine the relative representation of Ovis and Capra (38, 39) (SI Appendix, Fig. S2). The aggregate means show that a) goats were always fewer than sheep, but b) the proportion of goats dropped from 26 to 29% in Levels 5 and 4 to 14 to 10% in Levels 3 and 2. The ratio in AH Level 2 matches that for the later Neolithic at Çatalhöyük on the Konya Plain (40).
Trends in Caprine Survivorship at Aşıklı.
Several kinds of mortality data were cross-referenced to test for human manipulation of caprine survivorship at AH. Considered first was the relative abundance of preterm (fetal) and neonate remains from multiple ungulate species in the assemblages, followed by culling patterns for young caprines by sex as determined from the pelvis, and, finally, caprine mortality patterns over the full potential lifespan based on dental remains. The faunal samples are from the post-2009 excavations, spanning Level 5 through Level 2D, where sediments were systematically fine screened and floated to ensure complete recovery of small and immature specimens (SI Appendix, Fig. S3 and Table S2).
Fetus versus neonate deaths.
Preterm and neonate remains are noted in small numbers in a variety of prehistoric contexts, but the two age groups co-occur more consistently in situations that involved management (e.g., ref. 40). Table 1 presents a comparison of such remains for caprines and other ungulate species by level at AH. The immature bones were separated into preterm and neonate cohorts using photographs of modern newborn skeletons as the central standard (see also ref. 41) but excluding individuals that appeared to straddle the boundary between the two developmental states. The high proportion of fetal remains (12 to 35%) clearly sets the caprines apart from the other (wild) ungulate species, and the differences are statistically significant (17). Preterm remains are absent or rare for the other ungulate species, although pigs may be a minor exception. The distinct pattern for caprines is manifest early in the AH chronology and persists through Level 2.
Table 1.
Relative representation of ungulate preterm fetus and neonate remains by level from the post-2009 excavations at AH
| Ungulate type | Level 5 | Level 4 | Level 3 | Level 2J to D | ||||
| Fetus | Neonate | Fetus | Neonate | Fetus | Neonate | Fetus | Neonate | |
| NISP | NISP | NISP | NISP | NISP | NISP | NISP | NISP | |
| Caprines (sheep, goat) | 8 | 39 | 21 | 72 | 28 | 51 | 6 | 46 |
| Bos (aurochs) | — | 27 | — | 15 | — | 3 | — | 5 |
| Cervus (red deer) | — | — | — | — | — | — | — | — |
| Sus (pig) | 1 | 27 | — | 29 | — | 4 | 2 | 9 |
| Equidae (onager, horse) | — | 3 | — | 10 | — | — | — | 3 |
| Capreolus (roe deer) | — | — | — | 1 | — | — | — | — |
Data are presented as specimen counts rather than percentages due to small sample sizes for the noncaprine ungulates. NISP: number of identified specimens. Dashes indicate none present.
Sex-biased early culling.
Preferential culling of very young males is another common signature of human manipulation and not typical of predator–prey interactions between Pleistocene humans and wild artiodactyl ungulates. Mild male biases do occur in some Paleolithic archaeofaunas, depending on the species and its social adaptations, but mainly in adult individuals (e.g., refs. 42 and 43). The biological logic behind culling young males in managed populations is simply that females are the limiting reproductive resource, and male behavior can be disruptive. A common solution is to harvest many (but not all) males at a young age. Evidence of sex-biased culling in the caprines at AH is based on visual observations of the morphology and fusion state of the acetabulum–pubis region of the innominate. As the acetabular region develops in young individuals, the pubis and the adjacent part of the ilium take on different shapes and thicknesses in males versus females (44) (see SI Appendix, SI Background on pelvic morphology and fusion). Fusion tends to occur before 7 mo of age in caprines, although the timing can vary (45).
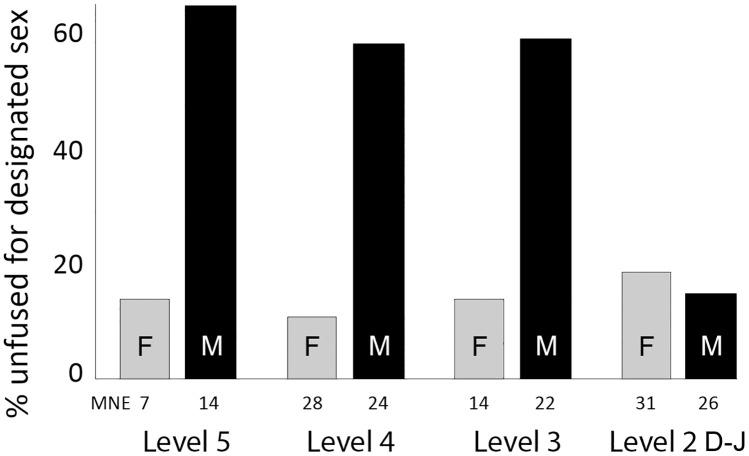
Culling was indeed biased to young males throughout Levels 5 to 3 at AH, with about 60% of all males killed before 7 mo of age (Fig. 2, Table 2, and SI Appendix, Table S3). Some females also died before 7 mo, but very few. The situation changed markedly in Level 2, when the majority (ca. 80%) of both males and females were allowed to live beyond the age of 7 mo.
Fig. 2.
Percent unfused caprine innominates that could be assigned to sex in each level at AH. Male and female fusion percentages are calculated separately with the minimum number of elements (MNE) shown below each bar.
Table 2.
Percentage of culled male specimens among all fully fused (adult) innominates by caprine species from uppermost Level 3 through Level 2 in the old excavations at AH
| Total MNE |
% Male | Total MNE |
% Male | Total MNE |
% Male | |
| Level | 3B to 2H | 3B to 2H | 2G to D | 2G to D | 2C to A | 2C to A |
| Capra | 16 | 62.5 | 24 | 54.2 | 10 | 80.6 |
| Ovis | 112 | 53.6 | 66 | 59.1 | 45 | 57.6 |
Age-biased culling over the full potential lifespan.
Mortality patterns are fundamental to investigations of the ungulate management practices of the Neolithic period, but these data are seldom compared to hunted assemblages from earlier periods. Because the first PPN economies in Southwest Asia emerged from local Epipaleolithic forager systems, such a comparison can be helpful for clarifying thresholds in the transformation from hunting to management and/or domestication, and, more importantly, for gauging the scale of difference between the economic systems. We know that prey age selection continued to evolve from the Paleolithic through the Epipaleolithic and that some Epipaleolithic death assemblages contain relatively more young animals (43, 46–48). Neolithic systems are expected to have diverged even further as management practices developed. However, there are few clear expectations for how mortality patterns generated by nascent caprine management practices would differ from ancestral Epipaleolithic ones. Some late Epipaleolithic cultures are known to have intensified their offtake from wild prey populations enough to drive down the mean age of reproduction (43). This does not qualify as evidence of deliberate management, but the resulting mortality patterns could, in principle, overlap with those generated by the earliest management practices. We would expect a true herding system to optimize growth and enhance bioproduct outputs by sparing the reproductive core of the population as much as possible. Did early management of wild captives fit the latter expectation, or did other strategies exist that have since gone extinct?
Other expectations may also apply to the early PPN, depending on the character of exploitation: 1) if meat was the main product to be gained from exploited captives, then a somatic (S-shaped) growth curve should predict culling decisions to a great degree, with many individuals killed before or as the upper developmental asymptote is reached; 2) if people sought (and could afford) to increase the size of the captive population to enhance future production, the culling of reproductive females should be limited; and 3) if secondary (nonmeat) products were important, whether as live units of wealth or sources of milk and/or hair, then many animals of one or both sexes should be allowed to live relatively long adult lives until biological senescence is reached (e.g., refs. 9, 12, 13, 49, and 50).
We used simple, easily replicated criteria for age-scoring caprine teeth to construct the mortality patterns, with no adjustments to estimated age in years. Our choice of procedure limits the number of published cases suitable for comparison, but it ensures primary data comparability in a field in which dental age scoring and compilation criteria vary greatly (50, 51). The age cohorts are based on eruption and wear states for the dP4 to P4 dental sequence, which collectively spans infancy to old age. Age scoring follows Grant’s (52) dental scheme. The scheme was also cross-referenced to Payne’s (13) system to accommodate other cases that used the dP4 to M3 dental sequence.
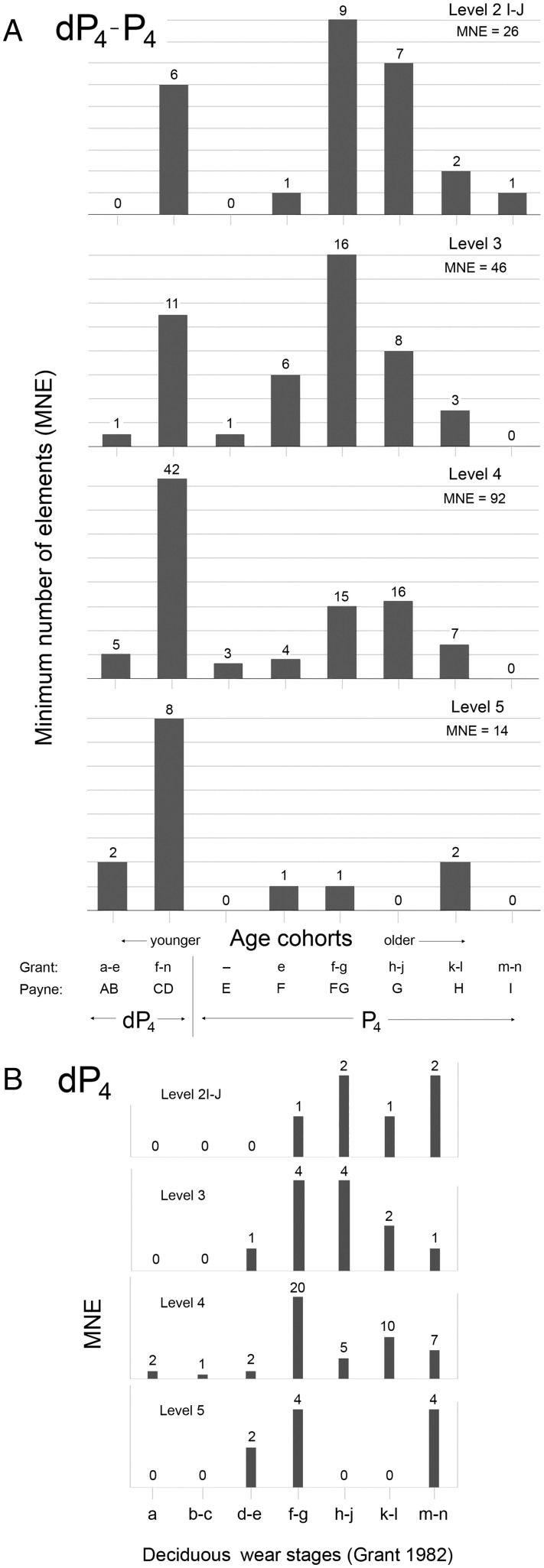
Dividing the maximum potential lifespan (MPL) into eight cohorts (SI Appendix, Table S4) reveals bimodal mortality patterns throughout the AH sequence (Fig. 3A). The first peak corresponds to animals ca. 6 to 18 mo old and is the dominant one in Levels 5 and 4. Though comprised overwhelmingly of juveniles, the Level 5 assemblage also contains just a few adults, including one old adult (Fig. 3A). These individuals may have been hunted from the wild, but this is not certain. The second peak spans the mid-adult years and becomes more dominant with time. Many more animals survived into productive adulthood during the later occupations. By Level 2I to J, some old adults (>6 y) are also present in the death assemblage.
Fig. 3.
AH caprine mortality patterns by level. (A) For the total potential lifespan based on the dP4 to P4 dental sequence in eight-cohort format. Stages represented by the deciduous tooth are collapsed into two cohorts to improve comparability with older age cohorts of longer duration. (B) Subdistribution within the juvenile group (dP4) using Grant’s categories.
Fig. 3B examines juvenile mortality in greater detail by subdividing the first 0 to 24 mo of life into multiple cohorts. Level 5 shows a major gap in the age distribution between Grant’s deciduous stages h to l. This gap may reflect a seasonal rhythm in the deaths of young caprines. The empty cohorts increasingly are filled in the later assemblages, and the mortality patterns appear less seasonal.
Diachronic Comparisons of Caprine Mortality Patterns.
Next, we compared the AH assemblages to those from a variety of Neolithic, Epipaleolithic, and Paleolithic sites in Anatolia and the eastern Mediterranean basin (SI Appendix, Table S5). The number of age cohorts was reduced to three so that many assemblages could be compared at once. The pre-Neolithic cases emphasize caprines from Anatolia (Fig. 1) (46, 53, 54) and the northern Levant (47, 55) along with a few ibex, chamois, and roe deer assemblages from Italy (56) and mountain gazelle (bovid) assemblages from the southern Levant (43, 57). The Neolithic assemblages are either dominated by sheep or contain relatively even mixtures of sheep and goats. The Anatolian comparison cases are from Erbaba (58), Köşk Höyük (59), Ulucak (60), Gritille (61), and Çatalhöyük (40). The cases outside Anatolia are Shillourokambos on Cyprus (62, 63) and Franchthi Cave in southern Greece (64). Shillourokambos provides PPN samples, whereas the other Neolithic cases date to the Pottery Neolithic (SI Appendix, Table S6) and represent fully domesticated stock.
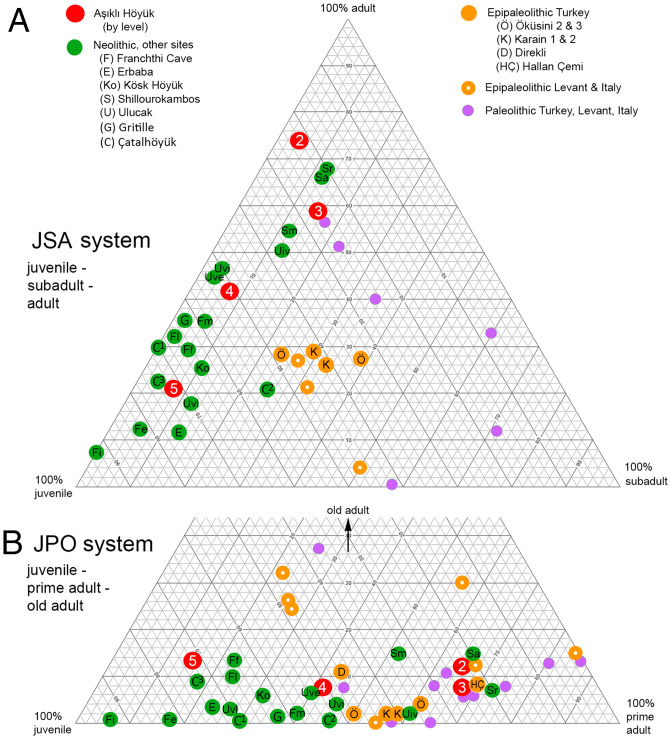
Two different three-cohort systems were used for subdividing the MPL in order to expose possible thresholds in the mortality pattern distributions. Fundamental life history transitions provide the most reliable points of reference. Specifically, the replacement of the deciduous tooth with the permanent one generally coincides with the onset of female reproductive maturity. Biological senescence begins in artiodactyl ungulates at roughly 67% of the MPL (42). The first three-cohort system divides the MPL into juvenile, subadult, and adult cohorts—thus named the JSA system—and was developed for this study. The second three-cohort system uses juvenile, prime adult, and old adult cohorts—the JPO system; it was developed previously for research on Paleolithic hunting societies and is based on independent demographic models (42, 56). The mortality pattern results are presented in a pair of tripolar plots in Fig. 4.
Fig. 4.
Tripolar comparison of AH caprine mortality patterns to Paleolithic, Epipaleolithic, and Neolithic caprine and other medium-sized ungulate assemblages in Anatolia and the eastern Mediterranean area. (A) JSA system is comprised of juvenile, subadult, and adult cohorts. (B) JPO system is comprised of juvenile, prime adult, and old adult cohorts.
In the JSA plot (Fig. 4A), the Neolithic caprine assemblages do not overlap with the Paleolithic and Epipaleolithic distributions, mainly because they are consistently poor in subadults. Epipaleolithic assemblages tend to contain fewer adults and subadults than the Paleolithic ones, but the Neolithic cases are exceptional for their lack of subadults.
The Neolithic cases in the JPO plot vary greatly in the proportions of juveniles and prime adults (Fig. 4B). The gamut of variation for all the Neolithic cases is double that of the earlier periods combined. Remarkably, the four AH assemblages dating to pre-pottery phases span the entire distribution of the Neolithic comparison cases. Level 5 shows the greatest bias to juveniles at 71%. Level 2 is the least biased to juveniles at 23% and instead is rich in prime adults (65%, SI Appendix, Table S5). Strong juvenile biases also occur in many of the later Neolithic cases such as at Franchthi Cave, Erbaba, Çatalhöyük, and Ulucak vl. The total Neolithic distribution in the JPO plot (Fig. 4B) partly overlaps with those dating to the Epipaleolithic and Paleolithic. This overlap is due to the substantial presence of prime adult individuals in the Neolithic assemblages from AH Levels 3 to 2J to I, Shillourokambos, and Ulucak iv. The AH Level 4 assemblage instead overlaps with Epipaleolithic caprines from Öküzini, Karain, Direkli, and Hallan Çemi.
A strong temporal trend is apparent within the AH series. Oddly, there is no such ordering among the other Neolithic cases as a group nor within stratigraphic series with the minor exception of Franchthi. Because our post-2009 sample lacks the final phases of Level 2 (2C to A), we compared larger samples recovered during the old excavations of Level 2 (SI Appendix, Tables S7 and S8). These data are not entirely comparable to the post-2009 samples due to minor differences in recovery and quantification and so are plotted separately in SI Appendix, Fig. S4. The distribution of the cases by phase within Level 2 confirms an increasing proportion of adults from phases 2I to J to 2A to C. The special purpose building (SPB) assemblage is coeval with 2C to A but represents a major anomaly; this spatially restricted sample is strongly biased to young animals and associates with sacrificial contexts within the communal building sector (16). The sex ratio data based on innominates (Table 2) indicate a relatively even balance of adult male and female sheep culled in Level 2. Goats were kept in lower numbers in this period, but their treatment in the final phases (Level 2C to A) differed from sheep in that many more adult males were culled than adult females.
Other Evidence on Human–Caprine Interactions at Aşıklı
How do the mortality results square with other evidence of human–caprine interactions at AH? Small outdoor spaces in Levels 5 to 4 contain highly localized, primary dung concentrations that were trampled in place based on phytolith and micromorphology studies (65). These lenses testify to the presence of a small number of captive animals in Level 5 and somewhat more in Level 4. Phytolith assemblages from the dung lenses also testify to the localized presence of both grazers (sheep) and browsers (goats) within the settlement (37). Dung accumulations became much more massive and widespread toward the end of Level 4 and upwards (15, 65), indicating that many more animals were held in communal corrals as time went on.
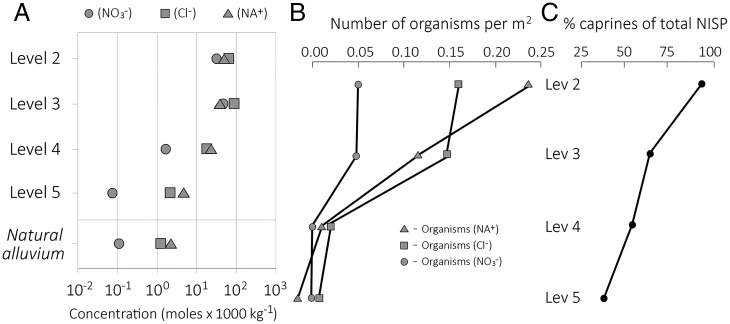
Abell et al. (66) have shown that urine salt inputs—sodium [Na+], chlorine [Cl−], and nitrate [NO3−]—to the AH sediments increased by orders of magnitude through the cultural sequence (Fig. 5). The urine salt inputs greatly exceed what the human inhabitants could have produced and thus illustrate the scale of growth in caprine management with time. Specifically, there is a 5 to 10× increase in [Na+], [Cl−], and [NO3−] from Levels 5 to 4 and a 10 to 1,000× increase from Levels 5 to 3. Level 2 inputs are similar to, or somewhat lower than, the inputs in Level 3, but humans’ dependence on caprines nonetheless continued to rise (Fig. 5C). The site structure evidence indicates that large animal enclosures were eventually moved off the mound top during Level 2 and replaced with residential buildings.
Fig. 5.
Summary of urine salt input trends in the sediments of AH (66). (A) Mean soluble salt concentrations in the archaeological levels and in the natural alluvium at the base of the mound. Sodium [Na+], chlorine [Cl−], and nitrate [NO3−] are given in moles × 1,000 kg−1. (B) Model-predicted densities of organisms (per m2) required to produce urine-related [Na+], [Cl−], and [NO3−] values, averaged by level using variable sedimentation rates based on 14C-dated level boundaries. (C) Changes in caprine number of identified specimens (NISP) as percent of total taxon-specific NISP.
Phytolith (37) and macrobotanical (28) evidence indicate that the captive animals were fed wild grasses in all periods. However, Peters et al. (67) detected a minor, positive shift in δ13C values in sheep bone collagen in Level 2, suggesting that caprine diets began to include C4 types. At the later Neolithic site of Çatalhöyük, δ13C and δ15N values in caprine bone collagen also indicate a varied diet with some C4 plants (68), possibly from feeding on pastures beyond the zone of active plant cultivation (69). Zimmermann et al.’s (70) comparative study of pathologies on the surfaces of sheep astragali at AH also points to increased livestock mobility by the times of Level 2. Circumscribed mesoscopic lesions can occur in any wild or captive ungulate population, but their severity in Levels 5 to 3 suggests excessive confinement followed by improvements in joint health (i.e., increased mobility) in Level 2.
Yet another dimension of the human–caprine relationship at AH is revealed by multi-isotope analyses of human collagen (71). The ratio of animal protein to plant foods in the Aşıklans’ diets did not decline from Level 4 through the end of Level 2 (no data were available for the Level 5 humans). This is a striking result given that the size of the human population grew roughly 10-fold by the final phases of Level 2. In fact, the plant diet changed only mildly with time, with slight increases in cereal and pulse production alongside heavy use of wild plant foods (28). The balance of hunted and managed meat sources meanwhile tilted strongly toward the latter. Importantly, this development was not accompanied by a reduction in animal protein consumption per capita. The situation at AH is distinctive in this regard, in contrast to later Neolithic diets at Çatalhöyük where a heavier reliance on plant carbohydrates took appreciable tolls on human health (72).
Despite clear indications of human-imposed selection and manipulation of caprines over many centuries at AH, there was little if any body size reduction in the sheep and goats from Levels 5 through 2 (16). A broader comparison puts these results in perspective. The application of a logarithmic size index to skeletal measurements (73, 74) shows that wild sheep from the 10th millennium cal BC at Hallan Çemi and early ninth millennium cal BC at Göbekli Tepe were larger on average than the AH sheep (SI Appendix, Fig. S5). Domesticated sheep were notably smaller by the later Neolithic at Çatalhöyük, Gürcütepe, Mezraa Teleilat, and Gritille. A similar situation is found for goats (16). The body sizes of the AH sheep and goats occupy intermediate positions to other earlier and later caprine assemblages. There is, however, no statistically significant diminution trend for either species within the millennium-long history of AH (SI Appendix, Tables S9 and S10).
Discussion
A diachronic comparison of caprine mortality patterns confirms that Neolithic harvesting practices, including those of the early PPN at AH, were largely distinct from Epipaleolithic and Paleolithic patterns. The Neolithic assemblages are most unique for the consistent underrepresentation of subadult individuals. They display great variation in the proportions of juveniles versus prime adults. Remarkably, AH is the only series that spans the entire gamut of Neolithic variation (Fig. 4). With the minor exception of the Franchthi Cave series, other multicomponent sites form atemporal clusters within the total Neolithic distribution.
Biases in culling by sex and age indicate a deliberate manipulation of caprine survivorship by the Aşıklans from the outset (Level 5). The modes of management transitioned through a series of apparently viable forms over the next 1,000 y, effectively reversing the relative contributions of wild versus captive meat sources. Three successive modes of human–caprine interaction are recognized. The earliest was embedded within a hunting economy and directed to small-scale live meat storage. It was a low-investment, quick-return strategy that involved capturing live lambs and kids after the birthing season and raising them for some months inside the settlement. This “catch-and-grow” strategy may have been the sole means of recruitment of captive stock for the Level 5 community, as there is little if any evidence for a reproductive core of captive adults. The motivations behind the catch-and-grow strategy were most likely about forestalling the next lean season and/or to supply special social events within the community. Such practices are known among some recent foraging and farming cultures (see refs. 75 and 76 on pigs) but are most feasible in semi- or fully permanent settlements. The archaeological record of Anatolia suggests that catch-and-grow methods may have been commonplace around the time of the Pleistocene–Holocene transition such as for piglets at the Epipaleolithic site of Hallan Çemi (77, 78).
While catch-and-grow traditions have the potential to shift into a domesticating relationship, they rarely did so. AH represents the exception within Central Anatolia, where the multigenerational propagation of captive caprines first emerged within the time frame of upper Level 5 through Level 4. Modest levels of captive reproduction on site are indicated by the presence of some young adults in the death assemblage. Another clue to the development of on-site reproduction is the increasing ratio of fetus to neonate remains (Table 1). A few preterm individuals are present in Level 5 (17% of lamb/kid specimens), but the percentage rises to 23% in Level 4 and to 35% in Level 3. These preterm individuals may have been aborted in pens because of congenital conditions and/or poor care.
The third mode of caprine management shows the hallmarks of a large-scale herding economy but one oddly directed to harvesting adult animals. At this point, many adult sheep of both sexes were allowed to live well into adulthood. Level 2 also associates with a sharp decline in preterm individuals (12%), but here the low percentage coincides with improvements in caprine joint health and dietary changes that imply greater herd mobility. Either the living conditions for pregnant livestock improved at this time, or preterm infants were more often lost off site and thus not recorded in settlement refuse.
In the framework of the Neolithic management models proposed by Payne (13), Halstead (11), and others (12, 50), the pattern in AH Levels 5 and 4 best fits the Meat-type A model with a focus on “yearlings” (killed at 6 to 12 mo of age). In Levels 3 and 2, something akin to Meat-type B production is evidenced (mainly for sheep), but here the analogy to AH departs from common patterns of the later Neolithic. The caprine mortality patterns in Levels 3 and 2 are peculiar in that they fall within the Paleolithic optimum of harvesting prime adults, especially the Level 2 assemblage. The only other Neolithic cases that display this pattern in the comparisons data are from PPN layers in Shillourokambos on Cyprus. The fact that the juvenile bias in the SPB assemblage in AH Level 2C to A bucks the larger tendency of the residential middens suggests that adult harvesting was motivated by general socioeconomics more than specific ritual uses in this period. Juveniles were reserved in small numbers for public sacrifice.
While the system of Level 2 remained focused on meat production in sheep, the management of goats shifted in Level 2C to A in a manner suggesting low-level milk production. Fewer goats than sheep were kept in the later periods as a rule, declining from one-third of all caprines in Level 5 to one-tenth in Level 2. Most Level 2 caprines of both species were allowed to live into adulthood, and some to an advanced age of 6 to 8 y, but the adult sex ratios differ between the two species in the final phases. The sex ratio in culled adult sheep is roughly even throughout Level 2, whereas that for adult goats in Level 2C to A is strongly biased to males (81%, up from 54% in Level 2G to D, Table 2). The ratio of potential milk producers (adult females) increased markedly among goats toward the end of the cultural sequence.
Most or all the management strategies at AH have gone extinct, perhaps as early as the late Neolithic. The mode indicated in AH Level 2 and at Shillourokambos may have become unsustainable as Pottery Neolithic (PN) farming economies expanded in size, and crop dependency increased. In fact, an emphasis on killing full adults, especially adult females, is an ecologically expensive use of livestock. Rare exceptions also exist in early PN Greece, where Halstead (79) notes unusually high culling of subadult–adult caprines at Tsoungiza and Knossos. He relates this mortality pattern along with an even sex ratio in the caprines to a demand for large meat carcasses to supply “collective commensality” social events (79). Cattle were rare at these sites, making caprines the largest common livestock. A need for large sharable carcasses also seems to apply for AH Level 2 and much of the Shillourokambos sequence.
The position of the AH series in the larger universe of Neolithic variation (Fig. 4) reveals other intriguing anomalies as well. The apparent overlap in a juvenile bias between the AH Levels 5 and 4 (early PPN) assemblages and many later PN assemblages is striking. The PN cases certainly involved domesticated stock, whereas those from AH Levels 5 and 4 involved small numbers of captive young animals with no or few reproductive-aged adults in residence. Clearly, strong juvenile biases can arise from more than one kind of management strategy (11, 76), conflating in this study an early catch-and-grow method at AH with fully developed herding practices of the PN oriented to building and maintaining adult stock. Supporting information on cultural contexts helps to sort this out.
The lack of body size diminution in both caprine species at AH over 1,000 y of occupation might seem to contraindicate local domestication, as this criterion is frequently called upon by domestication scholars. Body size reduction can and often does signal advanced levels of domestication in ungulate species. However, it is bound to be a tardy measure of the domestication process (7, 14) (on plants, refs. 80 and 81) unless a smaller body size is directly selected for from the beginning (7, 82). The lack of a diminution effect thus cannot exclude the possibility of an early domesticating process in early Neolithic contexts. It is interesting that the AH caprines were somewhat smaller bodied than wild comparison populations from eastern Anatolia, but this most probably is explained by regional size differences among wild sheep and goat populations in Anatolia around the Pleistocene–Holocene transition. The stability in caprine mean body size within the AH series is instead explained, we believe, by continued lateral admixture (introgression) between the captive population and small numbers of wild live individuals introduced into the flock as lambs and kids. Introgression did not prevent the domestication of sheep and goats in this situation but rather supported its success by maintaining genetic viability in the expanding captive population. The AH herds experienced intense selection for tolerance to captivity and food supplements while retaining/accumulating considerable genetic diversity in the form of neutral and beneficial traits. Conservatively speaking, the upper half of the AH sequence alone could have involved ca. 250 generations of captive livestock, ample time for major selective changes to occur.
There is the related question of whether the AH caprines were in any way ancestral to later domesticated populations in Anatolia and beyond. The genetic contribution of PPN Anatolian sheep to modern domesticates is still being worked out (83–85). However, the representation of mitochondrial DNA clades A, B, D, and E in the AH sheep (including from Level 2) testifies to their high mitochondrial diversity (67). Molecular phylogenetic analysis also indicates that the early domestication process in sheep retained high levels of diversity in general (86). The Anatolian sheep are genetically closest to modern European breeds, especially those from Central and Northern Europe (85). Y chromosome DNA traits in 10,000-y-old sheep remains from this part of the world persist in recent Northern European sheep that retain mouflon-like morphology (84). The AH sheep likely represent one of several formative nodes for modern sheep (67). Continued work is addressing the relationship between AH caprines and later domestic populations.
Conclusion
Virtually every line of evidence we have examined—species importance, caprine mortality patterns, diet, and pathologies—points to an endemic evolution of a management-cum-domestication relationship at AH. Several independent centers for sheep and goat domestication are thought to have emerged in Southwest Asia during the early Neolithic (62, 87, 88). AH certainly was one of these, although it is located well to the west of the “Fertile Crescent” as originally defined (36). When considered against the larger universe of variation in caprine mortality patterns, the 1,000-y trajectory in human–caprine interactions at AH is exceptional in its scope and without precedent in Central Anatolia. The balance of meat and plant food sources changed little with time, yet each management strategy in the succession required more infrastructure and labor than the last. The changes in human–caprine interactions with time amounted to monumental differences in many dimensions of human existence. Much of this process unfolded during a long interval of community isolation as indicated by the cessation of interregional trade. We conclude that these trends were driven primarily by dynamics internal to the AH community (36). Humans’ motivations within the larger dynamic were likely twofold: 1) live meat storage alongside investments in plant cultivation and 2) mounting social incentives for commensal meat eating and, later, also for building wealth. Some juvenile caprines were also reserved for ritual sacrifice but not in high volumes. By the times of Level 2, adult cattle had become the most important objects of sacrifice within the SPB area of Level 2C to A at AH (16) and at the sister complex of Musular just across the river (89).
Methods and Materials
Our analyses prioritized faunal samples from the “new” (post-2009) excavations at AH because these sediments were fine sieved and floated systematically (22) (SI Appendix, Fig. S1 and Table S2) and so yield more reliable results on caprine age and sex structure, the main focus of this study. The samples from Levels 2 and 3 are from Area 2JK on the western part of the mound, the Level 5 sample from Area 4GH, and the Level 4 sample from both areas. The assemblage from each level represents a wide range of units and contexts across the site. The primary quantitative unit is the number of identified specimens and is used to investigate variation in species importance. The minimum number of elements is used for the analyses of bone epiphyseal fusion, sex ratios, and dental age (90). Mandibular teeth and pelves are consistently well represented relative to other skeletal parts throughout the AH levels.
The mortality pattern analyses of teeth are based on dental development and occlusal wear. The data were not adjusted to accommodate estimates of age in real years, a practice which would interfere with efforts to isolate real but unexpected patterns in the raw data (91). Our approach is comparative and seeks evidence of divergence from preexisting tendencies (distributions) of earlier cultures and the directionality of divergence. The dental sample includes articulated (still seated in the mandible) and nonarticulated (isolated) teeth. To prevent the double counting of individuals, the deciduous premolar should not have been shed, as indicated by the state of the root, and the permanent tooth must show signs of wear of its occlusal surface (42, 43).
In the JSA cohort system, the juvenile cohort is represented entirely by the development–wear stages of the dP4 and spans the first two years of life. The subadult group is narrowly defined as the onset of occlusal wear of the P4 through Grant’s (52) wear stage e or Payne’s (13) stage E; this interval spans roughly 2 to 3 y of age. The adult cohort includes P4 wear stages f through n or Payne’s F through I. For the JPO cohort system, juveniles are defined as in the JSA system, but prime adult animals are defined by the onset of occlusal wear of the P4 through Grant’s stage j, corresponding to Payne’s stages E through G; this interval spans roughly 2 to 6 y of age. Old adults are defined as Grant’s stages k through n or Payne’s stages H through I.
Supplementary Material
Acknowledgments
We owe many thanks to Ben Arbuckle, Jean-Denis Vigne, and Angelos Hadjikoumis for generously sharing information with us and to two anonymous reviewers and the handling PNAS editor for their critical comments, which have helped us greatly improve this paper. Our research was funded by Archaeology Program grants from the NSF (BCS-0912148 and BCS-1354138 to M.C.S.) and the Istanbul University Research Fund (Project Nos. 24030 and 25754 to M.Ö.). We also wish to thank our colleagues and students for their unfailing support to the AH project as well as the community of Kızılkaya Village and the Turkish Ministry of Culture and Tourism, General Directorate of Cultural Assets and Museums.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2110930119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Clutton-Brock J., The process of domestication. Mammal Rev. 22, 79–85 (2008). [Google Scholar]
- 2.Larson G., Fuller D. Q., The evolution of animal domestication. Annu. Rev. Ecol. Evol. Syst. 45, 115–136 (2014). [Google Scholar]
- 3.Larson G., et al. , Current perspectives and the future of domestication studies. Proc. Natl. Acad. Sci. U.S.A. 111, 6139–6146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeder M. A., “Pathways to animal domestication” in Biodiversity in Agriculture: Domestication, Evolution, and Sustainability, Gepts P. et al., Eds. (Cambridge University Press, 2012), pp. 227–259. [Google Scholar]
- 5.Larson G., Burger J., A population genetics view of animal domestication. Trends Genet. 29, 197–205 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Marshall F. B., Dobney K., Denham T., Capriles J. M., Evaluating the roles of directed breeding and gene flow in animal domestication. Proc. Natl. Acad. Sci. U.S.A. 111, 6153–6158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price E. O., Behavioral aspects of animal domestication. Q. Rev. Biol. 59, 1–32 (1984). [Google Scholar]
- 8.Ducos P., “Domestication defined and methodological approaches to its recognition in faunal assemblages” in Approaches to Faunal Analysis in the Middle East, Meadow R. H., Zeder M. A., Eds. (Peabody Museum Press, 1978), pp. 53–56. [Google Scholar]
- 9.Greenfield H. J., The origins of milk and wool production in the Old World: A zooarchaeological perspective from the Central Balkans. Curr. Anthropol. 29, 573–593 (1988). [Google Scholar]
- 10.Halstead P., Pastoralism or household herding? Problems of scale and specialization in early Greek animal husbandry. World Archaeol. 28, 20–42 (1996). [Google Scholar]
- 11.Halstead P., Mortality models and milking: Problems of uniformitarianism, optimality and equifinality reconsidered. Anthropozoologica 27, 3–20 (1998). [Google Scholar]
- 12.Helmer D., Gourichon L., Vila E., The development of the exploitation of products from Capra and Ovis (meat, milk, and fleece) from the PPNB to the Early Bronze Age in the northern Near East (8700-9200 cal BP). Anthropozoologica 42, 41–69 (2007). [Google Scholar]
- 13.Payne S., Kill-off patterns in sheep and goats: The mandibles from Aşvan Kale. Anatolian Studies 23, 281–303 (1973). [Google Scholar]
- 14.Zeder M. A., Hesse B., The initial domestication of goats (Capra hircus) in the Zagros mountains 10,000 years ago. Science 287, 2254–2257 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Özbaşaran M., Duru G., Stiner M. C., Eds., The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin (Ege Yayınları, Istanbul, 2018). [Google Scholar]
- 16.Buitenhuis H., et al. , “The faunal remains from Level 3 and 2 of Aşıklı Höyük: Evidence for early management practices” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 281–323. [Google Scholar]
- 17.Stiner M. C., et al. , A forager-herder trade-off, from broad-spectrum hunting to sheep management at Aşıklı Höyük, Turkey. Proc. Natl. Acad. Sci. U.S.A. 111, 8404–8409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quade J., Stiner M. C., Copeland A., Clark A. E., “Summary of carbon-14 dating of the cultural levels of Aşıklı Höyük” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 43–56. [Google Scholar]
- 19.Arbuckle B. S., et al. , Data sharing reveals complexity in the westward spread of domestic animals across Neolithic Turkey. PLoS One 9, e99845 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buitenhuis H., Aşıklı Höyük: A protodomestication site. Anthropozoologica 25-26, 655–662 (1997). [Google Scholar]
- 21.Esin U., Harmankaya S., “Aşıklı” in Neolithic in Turkey, The Cradle of Civilization, Özdoğan M., Başgelen N., Eds. (Arkeoloji ve Sanat, Istanbul, vol. 1, 1999), pp. 115–132. [Google Scholar]
- 22.Özbaşaran M., Duru G., “Introduction to the Aşıklı Höyük project” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 1–14. [Google Scholar]
- 23.Baird D., et al. , Agricultural origins on the Anatolian plateau. Proc. Natl. Acad. Sci. U.S.A. 115, E3077–E3086 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Özdoğan M., Archaeological evidence on the westward expansion of farming communities from Eastern Anatolia to the Aegean and the Balkans. Curr. Anthropol. 52, 415–430 (2011). [Google Scholar]
- 25.Duru G., Kayacan N., In the footsteps of Epipalaeolithic communities in volcanic Cappadocia: Initial evaluations. Süleyman Demirel Üniversitesi Fen-Edebiyat Fakültesi Sosyal Bilimler Dergisi 45, 91–104 (2018). [Google Scholar]
- 26.Kuzucuoğlu C., Dumoulin J.-P., Saulnier-Copard S., “Geomorphological and paleoenvironmental setting of Aşıklı Höyük” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 15–42. [Google Scholar]
- 27.Astruc L., Grenet M., “Obsidian use during the Level 4 occupations at Aşıklı Höyük” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 345–362. [Google Scholar]
- 28.Ergun M., Tengberg M., Willcox G., Douché C., “Plants of Aşıklı Höyük and changes through time: First archaeobotanical results from the 2010-2014 excavation seasons” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 191–217. [Google Scholar]
- 29.Kayacan N., Altınbilek-Algül C., “Aşıklı Höyük obsidian studies: Production, use and diachronic changes” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 363–382. [Google Scholar]
- 30.Balkan-Atlı N., Binder D., Gratuze B., Göllü Dağ (Central Anatolia): Obsidian sources, workshops and trade. Anatolian Metal IV, pp. 203–210 (2008). [Google Scholar]
- 31.Binder D., “Stones making sense: What obsidian could tell about the origins of the Central Anatolian Neolithic” in The Neolithic of Central Anatolia: Internal Developments and External Relations during the 9th–6th Millennia cal BC, Gérard F., Thissen L., Ed. (Ege Yayınları, Istanbul, 2002), pp. 79–90. [Google Scholar]
- 32.Ibáñez J. J., Ortega D., Campos D., Khalidi L., Méndez V., Testing complex networks of interaction at the onset of the Near Eastern Neolithic using modelling of obsidian exchange. J. R. Soc. Interface 12, 20150210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balkan-Atlı N., Binder D., Cauvin M.-C., “Obsidian sources, workshops and trade in Central Anatolia” in Neolithic in Turkey: The Cradle of Civilization. New Discoveries, Özdoğan M., Başgelen N., Eds. (Arkeoloji ve Sanat Yayınları, Istanbul, 1999), pp. 133–145. [Google Scholar]
- 34.Sönmez D., “Interaction of the Aşıklı Höyük community with contemporary communities based on small finds,” Master’s thesis, Department of Prehistory, Istanbul University, Istanbul (2018).
- 35.Yelözer S., “The beads from Aşıklı Höyük” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 383–404. [Google Scholar]
- 36.Stiner M. C., Özbaşaran M., Duru G., Aşıklı Höyük: The generative evolution of a central Anatolian PPN settlement in regional context. J. Archaeol. Res. 10.1007/s10814-021-09167-z (2021). [DOI] [Google Scholar]
- 37.Tsartsidou G., “The microscopic record of Aşıklı Höyük: Phytolith analysis of material from the 2012-2016 field seasons” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 147–189. [Google Scholar]
- 38.Boessneck J., Müller H.-H., Teichert M., Osteologische Unterscheidungmerkmale zwischen Schaf (Ovis aries Linné) und Ziege (Capra hircus Linné). Kühn-Archiv 78, 5–129 (1964). [Google Scholar]
- 39.Zeder M. A., Lapham H. A., Assessing the reliability of criteria used to identify postcranial bones in sheep, Ovis, and goats, Capra. J. Archaeol. Sci. 37, 2887–2905 (2010). [Google Scholar]
- 40.Russell N., Martin L., “The Çatalhöyük mammal remains” in Inhabiting Çatalhöyük: Reports from the 1995-99 Seasons, Hodder I., Ed. (McDonald Institute for Archaeological Research, Cambridge, 2005), pp. 33–98. [Google Scholar]
- 41.Pöllath N., et al. , A non-linear prediction model for ageing foetal and neonatal sheep reveals basic issues in early neolithic husbandry. J. Archaeol. Sci. 10.1016/j.jas.2021.105344 (2021). [DOI] [Google Scholar]
- 42.Stiner M. C., The use of mortality patterns in archaeological studies of hominid predatory adaptations. J. Anthropol. Archaeol. 9, 305–351 (1990). [Google Scholar]
- 43.Stiner M. C., The Faunas of Hayonim Cave, Israel: A 200,000-Year Record of Paleolithic Diet, Demography, and Society (Peabody Museum Press, Cambridge, 2005). [Google Scholar]
- 44.Greenfield H. J., “Sexing fragmentary ungulate acetabulae” in Recent Advances in Ageing and Sexing Animal Bones, Ruscillo D., Ed. (Oxbow Books, Oxford, 2006), pp. 68–86. [Google Scholar]
- 45.Popkin P. R., Baker P., Worley F., Payne S., Hammon A., The Sheep Project (1): Determining skeletal growth, timing of epiphyseal fusion and morphometric variation in unimproved Shetland sheep of known age, sex, castration status and nutrition. J. Archaeol. Sci. 39, 1775–1792 (2012). [Google Scholar]
- 46.Atıcı L., Implications of age structures for Epipaleolithic hunting strategies in the Western Taurus Mountains, southwest Turkey. Anthropozoologica 44, 13–39 (2009). [Google Scholar]
- 47.Kersten A. M. P., Age and sex composition of Epipalaeolithic fallow deer and wild goat from Ksar ‘Akil. Palaeohistoria 29, 119–131 (1987). [Google Scholar]
- 48.Munro N. D., Zooarchaeological measures of hunting pressure and occupation intensity in the Natufian: Implications for agricultural origins. Curr. Anthropol. 45, S5–S34 (2004). [Google Scholar]
- 49.Halstead P., Collins P., Isaakidou V., Sorting the sheep from the goats: Morphological distinctions between the mandibles and mandibular teeth of adult Ovis and Capra. J. Archaeol. Sci. 29, 545–553 (2002). [Google Scholar]
- 50.Vigne J.-D., Helmer D., Was milk a “secondary product” in the Old World Neolithisation process? Its role in the domestication of cattle, sheep and goats. Anthropozoologica 42, 9–40 (2007). [Google Scholar]
- 51.Twiss K. C., An assessment of the archaeological applicability of faunal ageing methods based on dental wear. Int. J. Osteoarchaeol. 18, 329–351 (2008). [Google Scholar]
- 52.Grant A., “The use of tooth wear as a guide to the age of domestic animals” in Aging and Sexing Animal Bones from Archaeological Sites, Wilson B., Grigson C., Payne S., Eds. (British Archaeological Reports, Oxford, 1982), pp. 91–108. [Google Scholar]
- 53.Arbuckle B. S., Erek C. M., Late Epipaleolithic hunters of the central Taurus: Faunal remains from Direkli Cave, Kahramanmaraş, Turkey. Int. J. Osteoarchaeol. 22, 694–707 (2010). [Google Scholar]
- 54.Starkovich B. M., Stiner M. C., Hallan Çemi Tepesi: High-ranked game exploitation alongside intensive seed processing at the Epipaleolithic–Neolithic transition in Southeastern Turkey. Anthropozoologica 44, 41–61 (2009). [Google Scholar]
- 55.Stiner M. C., An unshakeable Middle Paleolithic? Trends versus conservatism in the predatory niche and their social ramifications. Curr. Anthropol. 54, S288–S304 (2013). [Google Scholar]
- 56.Stiner M. C., Honor Among Thieves: A Zooarchaeological Study of Neandertal Ecology (Princeton University Press, 1994). [Google Scholar]
- 57.Speth J. D., Tchernov E., “The Middle Paleolithic occupations at Kebara Cave: A faunal perspective” in The Middle Paleolithic Archaeology of Kebara Cave, Mount Carmel, Israel, Part 1, Bar-Yosef O., Meignen L., Eds. (Peabody Museum Press, Cambridge, 2007), pp. 165–260. [Google Scholar]
- 58.Arbuckle B. S., “Caprine exploitation at Erbaba Höyük: A Pottery Neolithic village in Central Anatolia” in Archéozoologie de l'Asie du Sud-Ouest et des Régions Adjacentes (Maison de l'Orient et de la Méditerranée, Lyon, 2008), pp. 345–365. [Google Scholar]
- 59.Arbuckle B. S., Zooarchaeology at Köşk Höyük. Kazı Sonuçları Toplantısı 27, 124–136 (2008a). [Google Scholar]
- 60.Çakırlar C., The evolution of animal husbandry in Neolithic central-west Anatolia: The zooarchaeological record from Ulucak Höyük (c. 7040-5660 cal. BC, Izmir, Turkey). Anatolian Studies 62, 1–33 (2012). [Google Scholar]
- 61.Stein G. J., “Strategies of risk reduction in herding and hunting systems of Neolithic southeast Anatolia” in Early Animal Domestication and Its Cultural Context, Crabtree P., Campana D., Ryan K., Eds. (MASCA, University Museum of Archaeology and Anthropology, 1989), pp. 87–97. [Google Scholar]
- 62.Vigne J.-D., Carrère I., Briois F., Guilaine J., The early process of mammal domestication in the Near East: New evidence from the Pre-Neolithic and Pre-Pottery Neolithic in Cyprus. Curr. Anthropol. 52, S255–S271 (2011). [Google Scholar]
- 63.Guilaine J., Briois F., Vigne J.-D., Eds., Shillourokambos: Un établissement néolithique pré-céramique à Chypre les fouilles du secteur 3 (CNRS Éditions, 2021). [Google Scholar]
- 64.Munro N. D., Stiner M. C., A zooarchaeological history of the Neolithic occupations at Franchthi Cave and Paralia in southern Greece. J. Anthropol. Archaeol. 58, 101162 10.1016/j.jaa.2020.101162 (2020). [DOI] [Google Scholar]
- 65.Mentzer S. M., “Micromorphological analyses of anthropogenic materials and insights into tell formation processes at Aşıklı Höyük, 2008-2012 field seasons” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 105–128. [Google Scholar]
- 66.Abell J. T., et al. , Urine salts elucidate Early Neolithic animal management at Aşıklı Höyük, Turkey. Sci. Adv. 5, eaaw0038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peters J., Nueberger F., Wiechmann I., Zimmerman M., Balasse M., Pöllath N., “Shaping the sheep: Human management and decision-making at Aşıklı Höyük, Central Anatolia” in The Early Settlement of Aşıklı Höyük: Essays in Honor of Ufuk Esin, Özbaşaran M., Duru G., Stiner M. C., Eds. (Ege Yayınları, Istanbul, 2018), pp. 325–344. [Google Scholar]
- 68.Pearson J. A., et al. , New light on early caprine herding strategies from isotope analysis: A case study from Neolithic Anatolia. J. Archaeol. Sci. 34, 2170–2179 (2007). [Google Scholar]
- 69.Henton E., The combined use of oxygen isotopes and microwear in sheep teeth to elucidate seasonal management of domestic herds: The case study of Çatalhöyük, central Anatolia. J. Archaeol. Sci. 39, 3264–3276 (2012). [Google Scholar]
- 70.Zimmermann M., Pöllath N., Özbașaran M., Peters J., Joint health in free-ranging and confined small bovids: Implications for early stage caprine management. J. Archaeol. Sci. 92, 13–27 (2018). [Google Scholar]
- 71.Itahashi Y., et al. , The impact of the transition from broad-spectrum hunting to sheep herding on human meat consumption: Multi-isotopic analyses of human bone collagen at Aşıklı Höyük, Turkey. J. Archaeol. Sci. 136, 105505 (2021). [Google Scholar]
- 72.Larsen C. S., et al. , Bioarchaeology of Neolithic Çatalhöyük reveals fundamental transitions in health, mobility, and lifestyle in early farmers. Proc. Natl. Acad. Sci. U.S.A. 116, 12615–12623 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uerpmann H.-P., Animal bone finds and economic archaeology: A critical study of ‘osteo-archaeological’ method. World Archaeol. 4, 307–322 (1973). [DOI] [PubMed] [Google Scholar]
- 74.Meadow R. H., “The use of size index scaling techniques for research on archaeozoological collections from the Middle East” in Historia animalium ex ossibus: Beiträge zur Paläoanatomie, Archäologie, Ägyptologie, Ethnologie und Geschichte der Tiermedizin, Becker C., Manhart H., Peters J., Schibler J., Eds. (Verlag Marie Leidorf, Rahden, 1999), pp. 285–300. [Google Scholar]
- 75.Price M. D., Hongo H., The archaeology of pig domestication in Eurasia. J. Archaeol. Res. 28, 557–615 (2020). [Google Scholar]
- 76.Dwyer P. D., Minnegal M., “Person, place or pig: Animal attachments and human transactions in New Guinea” in Animals in Person: Cultural Perspectives on Human-Animal Relations, Knight J., Ed. (Berg, Oxford, 2005), pp. 37–60. [Google Scholar]
- 77.Rosenberg M., Nesbitt R. M., Redding R., Peasnall B. L., Hallan Çemi, pig husbandry, and post-Pleistocene adaptations along the Taurus-Zagros Arc (Turkey). Paéorient 24, 25–41 (1998). [Google Scholar]
- 78.Price M. D., Evin A., Long-term morphological changes and evolving human-pig relations in the northern Fertile Crescent from 11,000 to 2000 cal. BC. Archaeol. Anthropol. Sci. 11, 237–251 (2019). [Google Scholar]
- 79.Halstead P., Zooarchaeological evidence for animal exploitation at earlier Neolithic Tsoungiza, Ancient Nemea. Hesperia 89, 191–214 (2020). [Google Scholar]
- 80.Tanno K. I., Willcox G., Distinguishing wild and domestic wheat and barley spikelets from early Holocene sites in the Near East. Veg. Hist. Archaeobot. 21, 107–115 (2012). [Google Scholar]
- 81.Willcox G., Anthropology. The roots of cultivation in southwestern Asia. Science 341, 39–40 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Trut L., Oskina I., Kharlamova A., Animal evolution during domestication: The domesticated fox as a model. BioEssays 31, 349–360 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Demirci S., et al. , Mitochondrial DNA diversity of modern, ancient and wild sheep(Ovis gmelinii anatolica) from Turkey: New insights on the evolutionary history of sheep. PLoS One 8, e81952 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng J., et al. , Paternal origins and migratory episodes of domestic sheep. Curr. Biol. 30, 4085–4095.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Yurtman E., et al. , Archaeogenetic analysis of Neolithic sheep from Anatolia suggests a complex demographic history since domestication. Commun. Biol. 4, 1279 (2021). [DOI] [PMC free article] [PubMed]
- 86.Rezaei H., Phylogénie moléculaire du Genre Ovis (Mouton et Mouflons), Implications pour la Conservation du Genre et pour l’Origine de l’Espèce Domestique. (Université de Grenoble, France, 2007). [Google Scholar]
- 87.Arbuckle B. S., Pace and process in the origins of animal husbandry in Neolithic Southwest Asia. Bioarchaeol Near East 8, 53–81 (2014). [Google Scholar]
- 88.Peters J., von den Driesch A., Helmer D., “The Upper Euphrates-Tigris basin: Cradle of agro-pastoralism?” in The First Steps of Animal Domestication: New Archaeozoological Approaches, Vigne J.-D., Peters J., Helmer D., Eds. (Oxbow Books, Oxford, 2005), pp. 96–124. [Google Scholar]
- 89.Özbaşaran M., Duru G., Kayacan N., Erdoğdu B., Buitenhuis H., “Musular: The 8th mill. cal. BC sattelite site of Aşıklı” in The Neolithic in Turkey, Central Anatolia, Özdoğan M., Başgelen N., Kuniholm P., Eds. (Arkeoloji ve Sanat Yayınları, İstanbul, 2012), pp. 159–180. [Google Scholar]
- 90.Lyman R. L., Vertebrate Taphonomy (Cambridge University Press, Cambridge, 1994). [Google Scholar]
- 91.Marom N., Bar-Oz G., Culling profiles: The indeterminacy of archaeozoological data to survivorship curve modelling of sheep and goat herd maintenance strategies. J. Archaeol. Sci. 36, 1184–1187 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.