Abstract
One of the most common pathogens in infection of hydrogel contact lens wearers is Pseudomonas aeruginosa, which can gain access to the eye via contamination of the lens, lens case, and lens care solutions. Only one strain per species is used in current regulatory testing for the marketing of chemical contact lens disinfectants. The aim of this study was to determine whether P. aeruginosa strains vary in their susceptibility to hydrogel contact lens disinfectants. A method for rapidly screening bacterial susceptibility to contact lens disinfectants was developed, based on measurement of the MIC. The susceptibility of 35 P. aeruginosa isolates to two chemical disinfectants was found to vary among strains. MICs ranged from 6.25 to 100% for both disinfectants at 37°C, and a number of strains were not inhibited by a 100% disinfectant concentration in the lens case environment at room temperature (22°C). Resistance to disinfection appeared to be an inherent rather than acquired trait, since some resistant strains had been isolated prior to the introduction of the disinfectants and some susceptible P. aeruginosa strains could not be made more resistant by repeated disinfectant exposure. A number of P. aeruginosa strains which were comparatively more resistant to short-term disinfectant exposure also demonstrated the ability to grow to levels above the initial inoculum in one chemical disinfectant after long-term (24 to 48 h) disinfectant exposure. Resistance was correlated with acute cytotoxic activity toward corneal epithelial cells and with exsA, which encodes a protein that regulates cytotoxicity via a complex type III secretion system. These results suggest that chemical disinfection solutions may select for contamination with cytotoxic strains. Further investigation of the mechanisms and factors responsible for resistance may also lead to strategies for reducing adverse responses to contact lens wear.
A number of infectious and inflammatory complications of microbial origin have been linked to the wear of contact lenses. Ulcerative keratitis is the most serious complication of contact lens wear, sometimes causing scarring of the cornea and resulting in permanent vision loss (35).
One of the most common pathogens in corneal infection of hydrogel contact lens wearers is Pseudomonas aeruginosa (1, 4). Two different genotypes of P. aeruginosa have been identified from corneal infections (16); invasive and acutely cytotoxic strains differ in certain genes that are regulated by ExsA (a transcriptional activator of genes encoding several secreted proteins) (15). Acutely cytotoxic P. aeruginosa strains have been shown to possess the ExsA-regulated genes exoU and exoT but to lack exoS (15). Acute cytotoxicity is not affected by mutation of exoT; however, exoU is required for cytotoxicity (10). Invasive P. aeruginosa strains have been shown to possess exoS and exoT but to lack exoU, which probably explains their lack of acute cytotoxic activity (10).
Acutely cytotoxic P. aeruginosa strains can kill corneal epithelial cells within 3 h of exposure; invasive strains enter epithelial cells and lack acute cytotoxic activity. Although the cytotoxic genotype is thought to be unusual among P. aeruginosa strains, the majority of strains isolated from corneal infections are of this type (16).
While the pathogenesis of contact lens-related infectious and inflammatory complications remains unclear, bacterial contamination of the eye appears to play a significant role (8, 22). During contact lens wear, bacteria can gain access to the eye from the environment via contamination of the lens, lens case, and lens care solutions (9, 32, 41). A high incidence of microbial contamination is observed in both contact lens cases and lens care solutions used during normal contact lens wear (12, 25, 47, 49), even in the presence of good compliance with lens care and maintenance procedures (5, 25, 42).
The development of bacterial resistance to antibiotics is well recognized in medical microbiology (6, 20, 27, 28, 34). Other types of disinfectants, such as the compound triclosan (commonly used in soaps, toothpaste, plastics, etc.), also have the potential to promote resistance by selecting for mutants that are equipped to survive in those environments (29). It is estimated that there are 80 million contact lens wearers worldwide, with 33 million in the United States alone (2).
Hydrogel lenses constitute nearly 90% of lens sales in the United States, and chemically preserved hydrogel disinfecting solutions are currently the most common solutions used for contact lens care (33). In most cases, these solutions are used daily. Since the introduction of these high-molecular-weight chemical disinfectants in the late 1980s, there has been little change in disinfectant formulations. Repeated usage of these disinfectants may increase the probability of bacterial resistance to the preservatives used. Strains of Serratia marcescens have been shown to become adapted to certain chlorhexidine- and benzalkonium chloride-based disinfecting solutions recommended for the care of rigid gas-permeable contact lenses (19, 48). Studies investigating resistance to hydrogel disinfecting solutions have not yet been performed.
In this study, the susceptibilities of multiple strains of P. aeruginosa to two commonly used hydrogel disinfectants were investigated. The hypothesis tested was that long-term worldwide usage of these systems has caused the selection of resistant P. aeruginosa strains. An investigation of whether susceptible strains could become resistant to chemical contact lens disinfectants following repeated disinfectant exposure was conducted, and the relationship between cytotoxic activity and resistance of P. aeruginosa strains to contact lens disinfection was assessed.
MATERIALS AND METHODS
Bacteria.
Thirty-five P. aeruginosa strains were collected from various sources in the United States, Australia, and the United Kingdom. These included 15 strains (recent clinical isolates) which were cultured from January 1994 onward and 12 strains (older clinical isolates) which were isolated prior to 1988 (prior to the introduction of the new generation of chemical contact lens disinfectants). The strains included isolates from eyes, contact lenses, lens cases, and lens care solutions and are listed in Table 1. Details of the laboratory strains used in the experiments are also included in this table. In addition, the wild-type strain PA103 (functional exsA allele; cytotoxic), an exsA mutant (PA103 exsA::Ω; invasive), an exoT mutant (PA103 exoT::Tc; cytotoxic), and an exoU mutant (PA103 exoU::Tn5Tc; neither cytotoxic nor invasive) were used to assess the role of exsA in resistance of P. aeruginosa to disinfection.
TABLE 1.
P. aeruginosa strains used in this studya
| Strain | Isolation source | Phenotype | Isolation dateb | Country | Source |
|---|---|---|---|---|---|
| Recent clinical isolates | |||||
| CL79 | CL case (AOSept) | NA | 3/94 | Aus | C. Lakkis |
| CL197 | CL solution (in AOSept case) | NA | 2/94 | Aus | C. Lakkis |
| K555 | CL—blepharitis | NA | 9/95 | U.S. | R. P. Kowalski |
| K648 | CL—keratitis | NA | 3/96 | U.S. | R. P. Kowalski |
| K735 | CL—keratitis | NA | 3/96 | U.S. | R. P. Kowalski |
| K406 | CL—keratitis | NA | 10/94 | U.S. | R. P. Kowalski |
| K331 | CL—keratitis | NA | 1/94 | U.S. | R. P. Kowalski |
| KEI1025 | Cornea—keratitis (CL wearer) | NA | 10/96 | U.S. | J. Hobden |
| 5 | CL | NA | 4/94 | Aus | M. D. P. Willcox |
| 8 | CL | NA | 9/94 | Aus | M. D. P. Willcox |
| D1 | Cornea—keratitis (CL wearer) | NA | 1995–1997c | UK | J. K. G. Dart |
| D2 | Cornea—keratitis (CL wearer) | NA | 1995–1997 | UK | J. K. G. Dart |
| D3 | Cornea—keratitis (CL wearer) | NA | 1995–1997 | UK | J. K. G. Dart |
| D4 | Cornea—keratitis (CL wearer) | NA | 1995–1997 | UK | J. K. G. Dart |
| B1 | Keratitis—CL wearer | NA | 3/97 | U.S. | E. J. Bottone |
| Older clinical isolates | |||||
| GW | CL case | NA | 10/86 | U.S. | M. F. Mowry-McKee |
| AJ | CL solution (Boston) | NA | 10/86 | U.S. | M. F. Mowry-McKee |
| 6294 | Cornea—keratitis | Invasive | Pre-1988 | U.S. | S. M. J. Fleiszig |
| 6206 | Cornea—keratitis | Cytotoxic | Pre-1988 | U.S. | S. M. J. Fleiszig |
| 6077 | Cornea—keratitis | Cytotoxic | Pre-1988 | U.S. | S. M. J. Fleiszig |
| 6389 | Cornea—keratitis | Cytotoxic | Pre-1988 | U.S. | S. M. J. Fleiszig |
| 6354 | Cornea—keratitis | Both | Pre-1988 | U.S. | S. M. J. Fleiszig |
| 6436 | Cornea—keratitis | Both | Pre-1988 | U.S. | S. M. J. Fleiszig |
| 6382 | Cornea—keratitis | Both | Pre-1988 | U.S. | S. M. J. Fleiszig |
| 6073 | Cornea—keratitis | Cytotoxic | Pre-1988 | U.S. | S. M. J. Fleiszig |
| 6452 | Cornea—keratitis | Both | Pre-1988 | U.S. | S. M. J. Fleiszig |
| 6487 | Cornea—keratitis | Invasive | Pre-1988 | U.S. | S. M. J. Fleiszig |
| Laboratory strains | |||||
| PA01 | Infected wound | Invasive | 1955 | Aus | NA |
| PAK | NA | Invasive | Pre-1988 | NA | NA |
| 1244 | Human burn | Invasive | Early 1970s | U.S. | NA |
| PA103 | NA | Cytotoxic | Pre-1988 | NA | NA |
| ATCC 19660 | Human septicemia | Cytotoxic | 1957 | U.S. | NA |
| ATCC 27853 | Blood | NA | Pre-1971 | U.S. | NA |
| ATCC 9027 | Outer ear infection | NA | Pre-1985 | U.S. | NA |
| ATCC 19582 | Urine | NA | 1969 | NA | NA |
NA, information not available or not applicable. CL, contact lens. UK, United Kingdom. Aus, Australia. Both, shows properties of both phenotypes. AOSept, CIBA Vision AOSept disinfection system.
Values with shills are given as month/year.
1995–1997, between 1995 and 1997.
All cultures received were checked for purity, with impure cultures excluded from experimentation. Species identification was confirmed through growth on centrimide agar (Difco Laboratories, Detroit, Mich.). Bacteria were stored in tryptic soy broth (Difco Laboratories) at −80°C with 10% glycerol to prevent frost injury. Working stocks were kept in tryptic soy broth and 10% glycerol at −20°C and renewed every 2 months. For use in the experiments, bacteria were grown overnight on tryptic soy agar at 37°C and resuspended in 0.9% sodium chloride (Sigma, St. Louis, Mo.). Resuspension to an optical density of 1.0 at 650 nm resulted in approximately 109 CFU/ml, which was confirmed by viable counts in each experiment.
Cell cultures.
Rabbit corneal epithelial cells were maintained in supplemented hormonal epithelial medium and passaged as previously described (16). For use in the experiments, freshly passaged cells were seeded into wells (125 μl per well) of 96-well tissue culture plates (Becton Dickinson Labware, Franklin Lakes, N.J.) and incubated at 37°C with 5% CO2 for a minimum of 3 days until confluent.
Quantification of acute cytotoxic activity.
Acute cytotoxicity assays were performed as previously described (14). Briefly, corneal epithelial cells were washed once with 100 μl of buffered minimal essential medium (MEM), and then cells were inoculated with 106 CFU of each test strain/ml. Control samples were inoculated with MEM without bacteria. Following 3 h of incubation at 37°C, the medium was removed and the cells were washed once with MEM to remove most nonassociated bacteria. To halt cytotoxicity at the 3-h time point, cells were incubated with 200 μg of gentamicin (BioWhittaker, Walkersville, Md.)/ml at 37°C for 1 h. All strains were found to be susceptible to this concentration of gentamicin (data not shown). After washing once with 200 μl of MEM to remove the antibiotic, 100 μl of 0.4% trypan blue (Sigma, St. Louis, Mo.) was added to stain dead or dying epithelial cells. After incubation at 37°C for 15 min, the extent of cytotoxicity per well was scored on a scale of 0 to 10, using an Olympus IX 70 microscope (×10 eyepiece; ×10 objective). Negative control samples incubated with MEM alone (i.e., no bacteria) were given a score of 0, and positive control samples infected with the known highly cytotoxic strain P. aeruginosa 6206 were assigned a score of 10. The invasive, noncytotoxic strain P. aeruginosa 6294 was also included as a negative control for cytotoxicity. Three wells were used for each strain during each experiment, and experiments were repeated at least once. The median cytotoxicity score was determined for each strain. Masking was achieved by coding each test strain.
Quantification of invasive capacity.
Using the same infected cells, the capacity for invasion was also determined. Following assessment of cytotoxicity, cells were washed with 100 μl of Ham's F-12 medium and lysed with 0.25% Triton X-100 (Lab Chem Inc., Pittsburgh, Pa.) (37°C for 15 min). Viable counts of the lysate were performed to quantify the number of intracellular bacteria (extracellular bacteria had been eliminated by the earlier gentamicin treatment). The median level of invasion was determined for each strain.
Characterization of the production of ExsA-regulated proteins.
ExsA-regulated protein production was qualitatively assessed by Western blot analysis of culture supernatants from each P. aeruginosa strain grown under inducing conditions described previously (17). Supernatants were harvested after 12 h of growth, at 32°C in a shaking water bath, and concentrated 20-fold by the addition of saturated ammonium sulfate (Fisher Scientific, Raleigh, N.C.) to a final concentration of 55%. Three microliters of the concentrated supernatant was loaded per lane of a 10% polyacrylamide–sodium dodecyl sulfate gel (Bio-Rad Laboratories, Hercules, Calif.). Gels were subjected to Western blot analysis using a mixture of rabbit antisera that recognized both the ExoS and ExoT (R726 immunoglobulin G), and ExoU (R16616 immunoglobulin G) proteins (provided by Dara W. Frank). Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (H+L) (Bio-Rad Laboratories) and a horseradish peroxidase conjugate substrate kit (Bio-Rad Laboratories) were used to detect antibodies bound to each of the three exoproteins.
Comparison of susceptibility to disinfection.
Susceptibilities of the various strains were compared for two chemical disinfectants: solution A (boric acid, edetate disodium, poloxamine, sodium borate, sodium chloride, polyaminopropyl biguanide [0.00005%]) and solution B (sodium citrate, sodium chloride, disodium edetate [0.5%], and polyquaternium-1 [0.001%]).
Variation in susceptibility was assessed via measurement of the MICs for both chemical disinfectants, using 96-well, flat-bottom plates (Corning Costar Co., Cambridge, Mass.). The full-concentration disinfectants were diluted to give the following concentrations: 0, 6.25, 12.5, 25, 50, and 100%. Disinfectants were diluted with 0.9% sodium chloride. Minimal medium for growth (per liter: 7.5 mmol of NaH2PO4, 16.8 mmol of K2HPO4, 10 mmol of MgSO4, 0.2% NaNO3, 10 mmol of CH3COONa) (13) was added to the disinfectants to supply the bacteria with essential nutrients for growth (nutrients are plentiful in lens cases during normal use and are readily available to bacteria). Addition of minimal medium ingredients did not result in disinfectant dilution.
Each dilution was inoculated with 107 CFU of bacteria/ml, which is within the range of bacterial levels isolated from hydrogel contact lens storage cases (26, 43). Plates were incubated for 18 h at 37°C on an orbital mixer (RotoMix; Barnstead/Thermolyne, Dubuque, Iowa). Wells containing 0.9% saline and minimal medium served as controls. MIC measurements were made using an enzyme-linked immunosorbent assay reader (Titertek Instruments Inc., Huntsville, Ala.), and each plate was read twice at 620 nm. The variability in absorbance readings for wells containing 0.9% saline and minimal medium was determined using 24 samples. Growth in test wells was considered positive if the absorbance measures differed from the saline control measures by more than the previously determined amount of variability. Three wells were used for each strain, and experiments were repeated at least once. The median MIC was then determined for each strain. In addition, to determine the ability of each strain to grow in a full concentration of disinfectant over time, separate plates containing samples of 100% disinfecting solution were inoculated with bacteria and read at 24 and 48 h after incubation.
Growth in disinfectants in contact lens cases at room temperature (22°C).
Contact lens wearers usually perform contact lens disinfection in lens cases at room temperature (22°C). To determine whether measurement of the MIC at 37°C was a valid predictor of susceptibility differences that might occur during actual use, 11 of the P. aeruginosa strains were used to challenge the disinfectants in their respective lens cases. Lens case wells containing 2.5 ml of disinfectant were inoculated with 107 CFU of bacteria/ml and nutrients as described above. Cases were stored at 22°C, and viable counts were performed by sampling the solutions at 4, 18, 24, 48, and 168 (7 days) h postinoculation. Four wells were used for each strain, and four wells containing saline (i.e., no disinfectant) served as controls.
Repeated disinfectant exposure.
The ability of P. aeruginosa to develop resistance to hydrogel chemical contact lens disinfectants through repeated disinfectant exposure was investigated. For this purpose, two of the most susceptible strains were used (see Table 3) (recent clinical isolate K555 and laboratory strain ATCC 9027). After determination of the MIC, 10-μl aliquots of survivors from wells containing the highest concentration of disinfectant where visible growth had been detected (12.5%) were used to inoculate new test plates containing the full range of solution B concentrations. At 24 to 48 h after incubation at 37°C on an orbital mixer, the procedure was repeated. After five serial repetitions, 1-μl aliquots from wells containing 12.5% disinfectant were used to inoculate plates containing the full range of solution B concentrations, and the MIC at 18 h was determined. Plates inoculated with fresh bacteria (that had not been preexposed to solution B) served as controls for each strain.
TABLE 3.
Median MIC (percent disinfecting solution) for the various P. aeruginosa strains at 18 h and growth in 100% disinfecting solution at 24 and 48 ha
| Strain | Phenotype | Median MIC (%)
|
Growth
|
||||
|---|---|---|---|---|---|---|---|
|
t = 18 h
|
t = 24 h
|
t = 48 h
|
|||||
| A | B | 100% A | 100% B | 100% A | 100% B | ||
| Recent clinical isolates | |||||||
| CL79 | Invasive | 25 | 12.5 | − | − | − | − |
| CL197 | Invasive | 25 | 25 | − | − | − | − |
| K555 | Cytotoxic | 12.5 | 25 | − | − | − | − |
| K648 | Cytotoxic | 100 | 50 | − | − | − | − |
| K735 | Invasive | 12.5 | 12.5 | − | − | − | − |
| K406 | Invasive | 25 | 12.5 | − | − | − | − |
| K331 | Both | 12.5 | 12.5 | − | − | − | − |
| KEI1025 | Invasive | 25 | 12.5 | − | − | − | − |
| 5 | Invasive | 6.25 | 25 | − | − | − | − |
| 8 | Invasive | 6.25 | 12.5 | − | − | − | − |
| D1 | Invasive | 25 | 25 | − | − | − | − |
| D2 | Invasive | 12.5 | 25 | − | − | − | − |
| D3 | Invasive | 25 | 25 | − | − | − | − |
| D4 | Invasive | 12.5 | 25 | − | − | − | − |
| B1 | Invasive | 6.25 | 25 | − | − | − | − |
| Older clinical isolates | |||||||
| GW | Cytotoxic | 25 | 25 | − | − | − | − |
| AJ | Invasive | 25 | 25 | − | + | − | + |
| 6294 | Invasive | 25 | 12.5 | − | − | − | − |
| 6206 | Cytotoxic | 12.5 | 50 | − | − | − | − |
| 6077 | Cytotoxic | 6.25 | 50 | − | − | − | − |
| 6389 | Cytotoxic | 6.25 | 50 | − | − | − | − |
| 6354 | Both | 12.5 | 12.5 | − | − | − | − |
| 6436 | Both | 25 | 12.5 | − | − | + | − |
| 6382 | Both | 12.5 | 50 | − | − | − | − |
| 6073 | Cytotoxic | 12.5 | 50 | − | + | − | + |
| 6452 | Both | 6.25 | 12.5 | − | − | − | − |
| 6487 | Invasive | 12.5 | 12.5 | − | − | − | − |
| Laboratory strains | |||||||
| PA01 | Invasive | 25 | 25 | − | − | − | − |
| PAK | Invasive | 12.5 | 25 | − | − | − | − |
| 1244 | Invasive | 50 | 25 | − | − | − | − |
| PA103 | Cytotoxic | 100 | 100 | − | − | − | − |
| ATCC 19660 | Cytotoxic | 12.5 | 12.5 | − | − | − | − |
| ATCC 27853 | Invasive | 25 | 25 | − | − | − | − |
| ATCC 9027 | Invasive | 25 | 25 | − | − | − | − |
| ATCC 19582 | Invasive | 6.25 | 6.25 | − | − | − | − |
A, solution A; B, solution B; both, shows properties of both phenotypes.
Statistical analyses.
Statistical comparisons between the MICs of the recent clinical isolates and older clinical and laboratory isolates were made using the χ2 test of association. Excellent repeatability of the MIC measures was observed, with 100% of MIC values varying by no more than one dilution step for each strain. MIC results were compared with median viable counts from the lens cases at the various time points via determination of the Spearman's rank correlation coefficient. The Mann-Whitney U test was used to determine whether there was a significant difference in resistance between cytotoxic and invasive strains, using the MIC data. A P value of less than 0.05 was considered to be significant for these tests.
For statistical comparisons using the lens case viable count data, a log transformation was applied to the CFU measures to produce data appropriate for parametric analysis. Repeated measures analysis of variance was performed, using SuperANOVA (Abacus Concepts Inc., Berkeley, Calif.), to assess the interaction between the bacterial strain and the disinfectant exposure time (0 to 168 h). Post-hoc testing, via one-way analysis of variance and Fisher's pairwise comparison, was performed to assess the differences between strains at each time point. The P value for statistical significance was adjusted to account for the multiple comparisons by dividing 0.05 by the total number of comparisons made.
RESULTS
Determination of phenotype.
Two methods were used to classify the P. aeruginosa strains under study, where the phenotype had not been established previously. Acute cytotoxic activity and the ability to invade corneal epithelial cells were determined through in vitro assays of cytotoxicity and invasion using rabbit corneal epithelial cells. ExsA-regulated protein profiles of the strains were characterized via Western blotting.
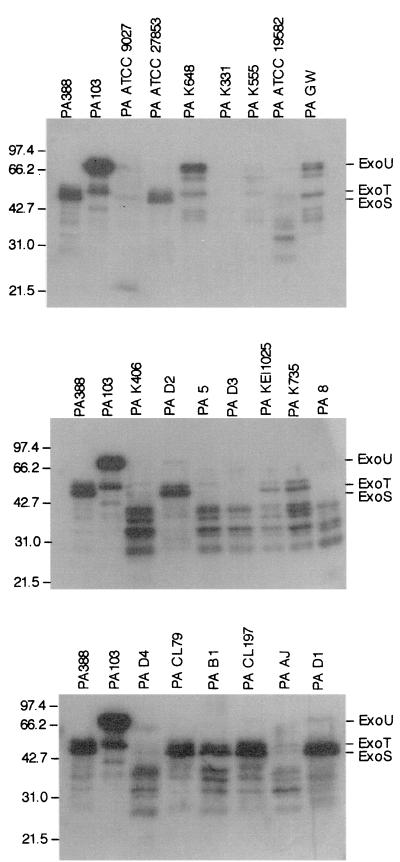
Median acute cytotoxicity scores ranged from 0 to 8 for the test strains, and median invasion (the number of intracellular bacteria) ranged from 0 to 1.55 × 104 CFU. Acute cytotoxicity and invasion results were inversely correlated (Spearman rank correlation test; R = −2.54; P = 0.01), as has been previously reported for other strains (16). The Western blot results are presented in Fig. 1.
FIG. 1.
Western blot analysis of P. aeruginosa strains. Molecular weight markers are shown on the left-hand side of each panel and are reported in thousands. Strains 388 (PA388) (invasive; ExoS+ ExoT+ ExoU−) and PA103 (cytotoxic; ExoS− ExoT+ ExoU+) were used as controls.
For the purposes of this study, cytotoxic strains were defined as having a median acute cytotoxicity score of 7 or higher and/or a clear signal for ExoU with no signal for ExoS. Invasive strains were defined as having a median cytotoxicity score of 3 or less and/or a clear signal for ExoS with no signal for ExoU. Strains which did not fit into either category were considered to have characteristics of both phenotypes. A summary of the median acute cytotoxicity scores and invasion levels, Western blotting results, and subsequent phenotypic classification of each strain under study is provided in Table 2.
TABLE 2.
Median cytotoxicity scores, invasion levels, Western blotting results, and classification of phenotype for each of the strains under study
| Strain | Median cytotoxicityc | Median invasion (no. of CFU) | Western blotting resultsd
|
Phenotype | ||
|---|---|---|---|---|---|---|
| ExoS | ExoT | ExoU | ||||
| Recent clinical isolates | ||||||
| CL79 | 0 | 114,500 | + | + | − | Invasive |
| CL197 | 0 | 80,500 | + | + | − | Invasive |
| K555 | 7 | 0 | − | + | + | Cytotoxic |
| K648 | 6 | 850 | − | + | + | Cytotoxic |
| K735 | 0 | 15,000 | + | + | − | Invasive |
| K406 | 0 | 83,000 | + | + | − | Invasive |
| K331 | 4 | 200 | −e | −e | −e | Both |
| KEI1025 | 3.5 | 47,000 | + | + | − | Invasive |
| 5 | 3 | 28,500 | + | + | − | Invasive |
| 8 | 1 | 8,750 | + | + | − | Invasive |
| D1 | 2 | 44,500 | + | + | −e | Invasive |
| D2 | 3 | 33,000 | + | + | −e | Invasive |
| D3 | 2 | 31,000 | + | + | − | Invasive |
| D4 | 2 | 155,000 | + | + | −e | Invasive |
| B1 | 3 | 66,000 | + | + | − | Invasive |
| Older clinical isolates | ||||||
| GW | 8 | 300 | − | + | + | Cytotoxic |
| AJ | 2.5 | 84,500 | + | + | − | Invasive |
| 6294a | 0 | 59,500 | ||||
| 6206b | 10 | 900 | ||||
| Laboratory strains | ||||||
| ATCC 27853 | 2 | 3,250 | + | + | − | Invasive |
| ATCC 9027 | 2.5 | 10,500 | − | − | −e | Invasive |
| ATCC 19582 | 0 | 3,650 | —d | —d | − | Invasive |
Invasion control strain.
Cytotoxicity control strain.
For an explanation of median cytotoxicity scores, see Materials and Methods.
For PA ATCC 19582, breakdown products were observed.
No clear signal.
Signals for the presence of ExoS, ExoT, or ExoU were not noted for strain K331, despite repeated attempts and variations in exposure times. Due to its median acute cytotoxicity score of 4 and very low levels of invasion, K331 was therefore classified as having properties of both phenotypes. A very faint signal for ExoU was detected for the D1, D2, and D4 strains, despite their being positive for ExoS and ExoT. These strains were classified as invasive due to the results of the in vitro tissue culture assays but may potentially show properties of both phenotypes. ATCC 9027, the Food and Drug Administration (FDA) and International Organization for Standardization (ISO) challenge microorganism (23, 46), was classified as invasive based on the cytotoxicity-invasion assay results; however, it was negative for both ExoS and ExoT and produced a faint signal for ExoU.
Using the phenotype classification system described above, three of the P. aeruginosa strains were classified as acutely cytotoxic, 16 as invasive, and one as having properties of both phenotypes.
Variation in susceptibility to disinfection at 37°C.
At 18 h, large differences were found among the P. aeruginosa strains, with MICs ranging from 6.25 to 100% for both solutions (Table 3). Resistance was not related to the date of isolation for either solution A (χ2 = 0.01; df = 1; P > 0.1) or solution B (χ2 = 0.61; df = 1; P > 0.1).
By 24 h, all strains were susceptible to full-concentration solution A, and 2 of 35 strains showed growth in a 100% concentration of solution B. By 48 h, 1 of the 35 strains had begun to grow in 100% solution A; no additional strains showed growth in 100% solution B.
Growth in disinfectants in contact lens cases at room temperature (22°C).
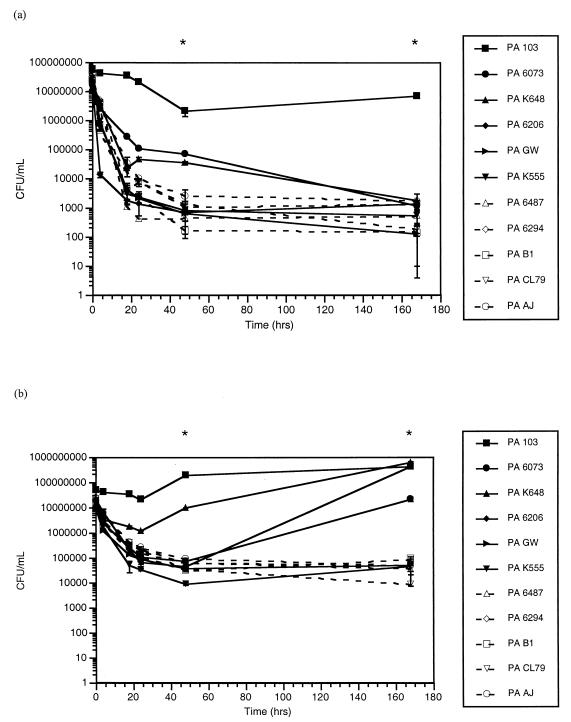
The P. aeruginosa strains also varied in their susceptibilities to both disinfection solutions when tested at 22°C. For both solutions A (Fig. 2a) and B (Fig. 2b), variation on the order of 4 log units was observed at all time points for all of the strains. For solution B (Fig. 2b), four out of six of the cytotoxic strains grew to levels equivalent to or significantly higher than the initial inoculum after 24 to 48 h of exposure to the disinfectant (P < 0.001). None of the strains grew to levels at or above the initial inoculum with solution A.
FIG. 2.
Bacteria (median no. of CFU/ml ± standard error) recovered from the lens cases over the 7-day (168 h) test period for solution A (a) and solution B (b) at 22°C. Cytotoxic strains are represented by filled symbols and solid lines, and invasive strains are represented by open symbols and dotted lines. Asterisk, P < 0.008.
The results obtained using the lens cases incubated at 22°C were compared and contrasted to the MIC test results using 96-well plates at 37°C. When compared to the MIC screening test results for both solutions, the susceptibility differences observed in the lens cases were significantly correlated (P < 0.05) for solution A at all time points except at 168 h (7 days) and only at 168 h for solution B (P < 0.01).
The relationship between acute cytotoxic activity and resistance to disinfection.
Analysis of the MIC data (measured at 18 h, after incubation at 37°C) showed a statistically significant relationship between acute cytotoxic activity and resistance for solution B, with cytotoxic strains tending to be more resistant than invasive strains (Mann-Whitney U test; P < 0.01). A similar relationship was not evident for susceptibility to solution A (Mann-Whitney U test; P > 0.1).
For the data collected at 22°C (Fig. 2), there was a significant interaction between cytotoxic activity and disinfectant exposure time for both solution A (F5,210 = 9.27; P < 0.001) and solution B (F5,210 = 28.45; P < 0.001). Cytotoxic strains were significantly more resistant to full concentrations of both disinfectants than invasive strains at 48 (P < 0.008) and 168 h (P < 0.008).
Repeated disinfectant exposure.
Repeated disinfectant exposure did not reduce susceptibility to killing. For both of the susceptible strains tested (K555 and ATCC 9027), the MIC was 25% before exposure to solution B and remained at 25% after five passages through solution B.
The role of exsA in resistance of P. aeruginosa to contact lens disinfection.
Since acutely cytotoxic strains tended to be more resistant to disinfection than invasive strains, the role of ExsA in resistance was explored. Susceptibilities of the acutely cytotoxic strain PA103 and three isogenic mutants of PA103 were assessed. These were an exsA mutant (noncytotoxic) and mutants in two genes downstream of exsA: exoT (cytotoxic) and exoU (noncytotoxic).
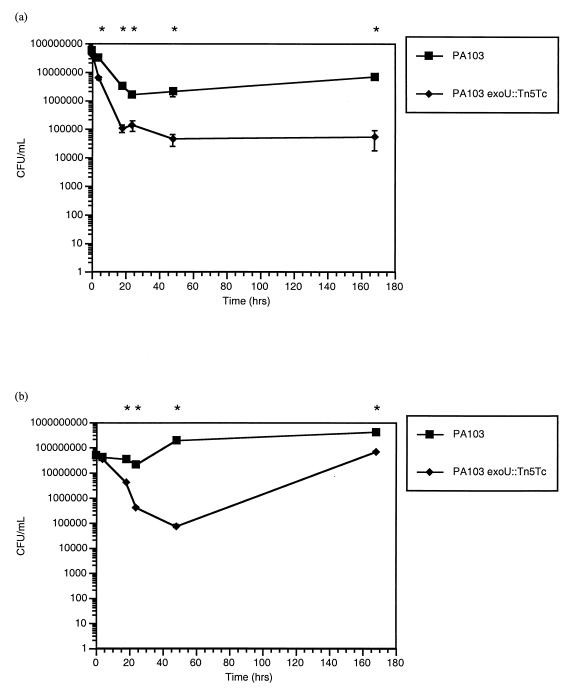
A pilot study was conducted in which PA103 and the three mutants were used to challenge full concentrations of solutions A and B in their respective lens cases at 22°C. Cases containing saline (i.e., no disinfectant) served as controls. Results of this pilot study showed less resistance to both solutions A and B by the exsA mutant and the exoU mutant compared to results for the wild-type strain and exoT mutant. These results suggested that exoU was involved in resistance.
Subsequent experiments focused on directly comparing wild-type PA103 with the exoU mutant in the contact lens case at 22°C (Fig. 3a and b). Repeated measures analysis of variance revealed a significant interaction between the bacterial strain and the disinfectant exposure time for both solution A (F5,30 = 38.82; P < 0.001) and solution B (F5,30 = 371.52; P < 0.001). Statistically significant differences between PA103 and the exoU mutant were observed at all time points from 4 h for solution A (P < 0.008) (Fig. 2a) and all time points from 18 h for solution B (P < 0.008) (Fig. 2b). Growth of the exoU mutant was eventually observed after long-term exposure to solution B (>48 h), but the numbers of bacteria in the solution remained significantly lower than with PA103 even at 168 h (P < 0.008).
FIG. 3.
PA103 and PA103 exoU::Tn5Tc (median no. of CFU/ml ± standard error) recovered from contact lens cases containing solution A (a) and solution B (b) over the 7-day (168 h) test period. Asterisk, P < 0.008.
Adaptation of acutely cytotoxic P. aeruginosa to solution B in the lens case environment at 22°C.
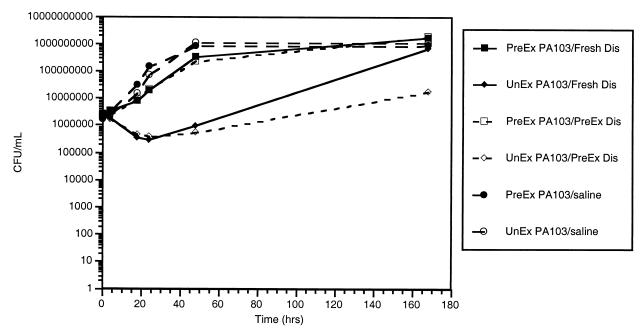
Growth of some of the initially more resistant cytotoxic strains after long-term (24 to 48 h) exposure to solution B (Fig. 2) may be due to further adaptation to the disinfectant and/or the low nutrient environment of the contact lens case or to decreased efficacy of the disinfectant over time. To explore why this growth occurred, the susceptibility of preexposed (exposed to solution B for 1 week in the lens case at 22°C) bacteria was compared to the susceptibility of bacteria without previous exposure to solution B in contact lens cases containing fresh solution B. To assess the potential effect of decreased disinfection solution efficacy, susceptibilities of preexposed and unexposed bacteria were also assessed using solution B which had been inoculated with bacteria for 1 week and then filter sterilized (Millex-GP; pore size, 0.22 μm; Millipore, Bedford, Mass.).
A summary of the observations over the 7-day (168 h) test period, from one representative experiment, is presented in Fig. 4. When the growth rates of preexposed and unexposed PA103 in fresh solution B were compared, the preexposed bacteria showed statistically significantly higher levels of growth at all time points from 4 h. The magnitude of the effect was greatest at 18, 24, and 48 h (approximately 1, 2, and 3 log units of difference, respectively). By 168 h, the unexposed PA103 bacteria had grown to levels above the initial inoculum (as demonstrated previously); however, they were still recovered at levels significantly lower than for the preexposed bacteria (P < 0.001).
FIG. 4.
Bacteria (median no. of CFU/ml ± standard error) recovered from the lens cases over the 7-day (168 h) test period. PreEx PA103, preexposed (to solution B) PA103; UnEx PA103, unexposed PA103; Fresh Dis, fresh solution B; PreEx Dis, preexposed (to bacteria and then filtered) solution B.
Unexposed PA103 grew better in fresh solution than in preexposed solution (P < 0.001) at 168 h. These results suggest that the growth of PA103 in solution B after long-term exposure is not due to decreased efficacy of the solution over time. Otherwise, enhanced (rather than reduced) growth of unexposed PA103 in preexposed solution would have been observed.
DISCUSSION
The MIC test showed differences in susceptibility to two hydrogel contact lens disinfectants among 35 P. aeruginosa strains, including clinical isolates and laboratory strains. One of the hypotheses tested was that recent clinical isolates would be more resistant than older clinical and laboratory strains; however, resistance was not related to the date of isolation of the strains. Repeated disinfectant exposure did not result in increased resistance of two of the most susceptible strains, suggesting that P. aeruginosa strains do not acquire resistance after repeated disinfectant exposure, at least in these experimental circumstances. While this does not rule out the possibility that other relatively susceptible P. aeruginosa strains may become more resistant with repeated disinfectant exposure, these results suggest that resistance appears to be an inherent rather than an acquired trait.
The FDA Premarket Notification [510(k)] Guidance Document for Contact Lens Care Products sets out the safety and efficacy requirements for marketing approval of contact lens care products (46). It is clear that the FDA testing procedures do not ensure disinfection efficacy during normal use, as demonstrated by the numerous reports of microbial contamination in patients' cases and solutions (12, 25, 47, 49), even in the presence of good compliance with lens care and maintenance procedures (5, 25, 42). A number of potential inadequacies in the testing procedures can be identified. For example, FDA disinfectant testing is performed without nutrients being made available to the microorganisms, which does not reflect the situation during normal patient use. Also, a limited panel of microorganisms is used to challenge the disinfectants. The assumption that the behavior of one strain will be representative of that of the rest of the species is questionable. In addition, the challenge microorganisms are all ATCC laboratory strains, which have been grown under ideal conditions. The disinfection susceptibility of clinical isolates, which typically grow in low-nutrient environments, is likely to be quite different from that of laboratory strains (3).
The most common approach to testing bacterial susceptibility to contact lens disinfectants is by performing viable counts from inoculated solutions. This method has been approved by the FDA (46) and is recommended by ISO (23). Many studies in the literature have evaluated efficacy in this manner (30, 37, 38). In this study, the development of a quantitative screening method to assess bacterial susceptibility to disinfection enabled comparisons between different strains of the same species to be made in a less time-consuming manner. By adaptation of the MIC procedure, a standardized approach to assessing bacterial resistance was achieved, and use of an enzyme-linked immunosorbent assay reader for assessment of visible growth in solution eliminated subjectivity in the interpretation of the results.
The addition of nutrients and organic soil to disinfectants during testing remains a controversial area. The presence of organic material has been reported to affect microbial activity of some disinfection products (7, 39). To reflect patient use situations, organic soil, consisting of heat-killed yeast cells and heat-inactivated bovine serum, is added to lenses during FDA regimen testing (46). Addition of such soil is not required by ISO on the basis that a standardized model which accurately reflects the patient situation is not available (23). Since nutrients are plentiful in lens cases during normal use, minimal medium (containing only the five essential elements for growth) was added to the disinfectants in the present study. Organic material was not added to the disinfectants.
Conflicting reports regarding the susceptibilities of two clinical P. aeruginosa isolates to hydrogel disinfection have recently appeared in the professional literature (31, 45). A difference in susceptibility between the strains for one disinfectant was reported using methods similar to those required by the FDA (45). No difference was reported in another study which adhered to the FDA protocol (31). Another recently published study, which used a selection of P. aeruginosa isolates but also adhered to the FDA protocol, showed minimal variation between strains (36). Clearly, testing methodology will influence the results of these types of studies. In all of the reports, nutrients were not made available to the bacteria during testing.
Resistance was linked to acute cytotoxicity for both disinfectants when experiments were performed at room temperature (22°C) in the contact lens case (Fig. 1). As might be expected from this result, resistance of PA103 to chemical disinfection was found to involve ExsA. At 22°C, exoU, which is regulated by ExsA, was shown to be involved, suggesting that at least part of the role of ExsA in resistance is indirect. Secretion of ExsA-regulated proteins by P. aeruginosa has been shown to occur via a type III secretion system (18, 50). Type III secretion systems are complex secretion pathways utilized by many gram-negative bacteria for the transfer of toxic proteins to host cells from the bacterial cytoplasm (24, 40). There are no obvious structural features of exoU that indicate it might affect the ability of P. aeruginosa strains to grow in chemical contact lens disinfectants, and little is known about the biochemistry of the molecule (11, 21). Clearly, further research will be required to determine the exact mechanisms for ExsA-regulated resistance to disinfectants.
When experiments were performed at 37°C, a significant association between acute cytotoxic activity and resistance to disinfection was shown for solution B but not for solution A. The variation in results at different temperatures might relate to ExsA-regulated protein secretion. In the laboratory, modification of growth media and environmental conditions can influence toxin expression and secretion through the ExsA-regulated system (17, 44). One such example is growth temperature, which has been shown to affect the secretion of ExsA-regulated proteins and influence cytotoxic activity (A. Zarimani, A. Hauser, J. Comolli, and J. Engel, Abstr. 98th Gen. Meet. Am. Soc. Microbiol. 1998, abstr. B-148, 1998). Whether or not the discrepancy in findings reflects a real difference in susceptibility at the different temperatures, the results from the experiments involving growth in lens cases at room temperature are the most relevant to the normal contact lens wear situation. The finding that resistance to both contact lens disinfectants was correlated with acute cytotoxicity in P. aeruginosa at room temperature is of great concern because cytotoxic P. aeruginosa strains do not require corneal compromise to cause damage (14).
While resistance to disinfection appeared to be an inherent factor of some P. aeruginosa strains, four out of six cytotoxic strains, which were more resistant to disinfection initially, grew to levels equivalent to or significantly higher than the initial inoculum after long-term exposure to solution B (Fig. 2). This result showed that some strains were not only resistant to killing after short-term disinfectant exposure but also were able to adapt to the disinfection solution and subsequently increase in number. Inhibition of the growth of unexposed PA103 in preexposed solution compared to results with fresh solution (Fig. 4) suggested that the growth of certain cytotoxic strains in solution B after long-term exposure in the contact lens case was not due to decreased efficacy of the solution over time. Instead, growth of the bacteria in the disinfectant appeared to represent an adaptation to the disinfectant and/or to the low-nutrient environment of the contact lens case. It appears that this adaptation was not related to the production of ExoU, since adaptation was also observed with an exoU mutant (Fig. 3b). The mechanism by which this adaptation occurs requires further investigation.
Although acute cytotoxic activity was implicated in resistance to chemical disinfection, other factors are also likely to be involved. Not all acutely cytotoxic strains were resistant. In addition, a small percentage of the invasive strains also showed resistance to disinfection; invasive strains produce ExsA but not ExoU. Whether ExsA plays a role in resistance of invasive strains is yet to be determined.
In conclusion, resistance to hydrogel chemical contact lens disinfection varies between P. aeruginosa isolates. Nutrient levels and differences among strains should be considered during the manufacture and testing of hydrogel chemical contact lens disinfectants, since bacterial resistance and adaptation to disinfection are likely to be contributing factors to contamination of lens care systems. Resistance is linked to acute cytotoxic activity; the ExsA-regulated pathway appears to be involved, and a type III secretion system may play a role. These results suggest that current contact lens disinfection systems may select for contamination with cytotoxic strains, which could explain the high prevalence of cytotoxic isolates in contact lens-related corneal infections. Further understanding of the mechanisms of resistance and the bacterial factors responsible for resistance may lead to rational strategies for reducing adverse responses to contact lens wear.
ACKNOWLEDGMENTS
This work was supported by the following grants: NIH RO1-EY11221 (SMJF), Melbourne Research Scholarship, University of Melbourne, Australia (CL), and Contact Lens Society of Australia Research Award (CL).
We thank Dara Frank of the Medical College of Wisconsin for her assistance with methodology development and the Western blot analyses. Thanks also to Florence Choo of the Statistical Consulting Centre, University of Melbourne, Australia, for advice regarding statistical analysis and to the investigators who donated strains for use in this study.
REFERENCES
- 1.Alfonso E, Mandelbaum S, Fox M, Forster R. Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol. 1986;101:429–433. doi: 10.1016/0002-9394(86)90641-0. [DOI] [PubMed] [Google Scholar]
- 2.Barr J T. The contact lens spectrum millennium report. CL Spectrum. 2000;15:24–30. [Google Scholar]
- 3.Caroline P, Campbell R. Strategies of microbial cell survival in contact lens cases. Contact Lens Forum. 1990;15:27–36. [Google Scholar]
- 4.Chatterjee A, Kwartz J, Ridgway A E A, Storey J K. Disposable soft contact lens ulcers: a study of 43 cases seen at Manchester Royal Eye Hospital. Cornea. 1995;14:138–141. [PubMed] [Google Scholar]
- 5.Claydon B E, Efron N, Woods C. A prospective study of non-compliance in contact lens wear. J Br Contact Lens Assoc. 1996;19:133–140. [Google Scholar]
- 6.Collignon P J, Bell J M. Drug-resistant Streptococcus pneumoniae: the beginning of the end for many antibiotics? Med J Aust. 1996;164:64–67. [PubMed] [Google Scholar]
- 7.Copley C A. Chlorine disinfection of soft contact lenses. Clin Exp Optom. 1989;72:3–7. [Google Scholar]
- 8.Dart J K G, Stapleton F, Minassian D. Contact lenses and other risk factors in microbial keratitis. Lancet. 1991;338:650–653. doi: 10.1016/0140-6736(91)91231-i. [DOI] [PubMed] [Google Scholar]
- 9.Donzis P, Mondino B, Weissman B, Bruckner D. Microbial contamination of contact lens care systems. Am J Ophthalmol. 1987;104:325–333. doi: 10.1016/0002-9394(87)90219-4. [DOI] [PubMed] [Google Scholar]
- 10.Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M J, Wu C, Mende-Muller L, Frank D W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial cell injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 11.Finck-Barbançon V, Yahr T L, Frank D W. Identification and characterization of SpcU, a chaperone required for efficient secretion of the ExoU cytotoxin. Infect Immun. 1998;180:6224–6231. doi: 10.1128/jb.180.23.6224-6231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleiszig S M, Efron N. Microbial flora in eyes of current and former contact lens wearers. J Clin Microbiol. 1992;30:1156–1161. doi: 10.1128/jcm.30.5.1156-1161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiszig S M J, Efron N, Pier G B. Extended contact lens wear enhances Pseudomonas Aeruginosa adherence to human corneal epithelium. Investig Ophthalmol Vis Sci. 1992;33:2908–2916. [PubMed] [Google Scholar]
- 14.Fleiszig S M J, Lee E J, Wu C, Andika R C, Vallas V, Portoles M, Frank D W. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. CLAO J. 1998;24:41–47. [PubMed] [Google Scholar]
- 15.Fleiszig S M J, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K E, Kanada D, Sawa T, Yen T S B, Frank D W. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleiszig S M J, Zaidi T, Preston M J, Grout M, Evans D J, Pier G B. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank D W, Iglewski B H. Cloning and sequence analysis of a trans-regulatory locus required for exoenzyme S synthesis in Pseudomonas aeruginosa. J Bacteriol. 1991;173:6460–6468. doi: 10.1128/jb.173.20.6460-6468.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank D W, Nair G, Schweizer H P. Construction and characterization of chromosomal insertional mutations of the Pseudomonas aeruginosa exoenzyme S trans-regulatory locus. Infect Immun. 1994;62:554–563. doi: 10.1128/iai.62.2.554-563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi P A, Sawant A D, Wilson L A, Ahearn D G. Adaptation and growth of Serratia marcescens in contact lens disinfectant solutions containing chlorhexidine gluconate. Appl Environ Microbiol. 1993;59:183–188. doi: 10.1128/aem.59.1.183-188.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb T. Drug-resistant Streptococcus pneumoniae: the beginning of the end for many antibiotics? Med J Aust. 1996;165:233–234. [PubMed] [Google Scholar]
- 21.Hauser A R, Kang P J, Engel J N. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- 22.Holden B A, La Hood D, Grant T, Newton-Howes J, Baleriola-Lucas C, Willcox M D P, Sweeney D F. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J. 1996;22:47–52. [PubMed] [Google Scholar]
- 23.International Organization for Standardization. ISO/CD 14729: Ophthalmic optics—contact lens care products—microbiological requirements for products and regimens for hygienic management of contact lenses. Geneva, Switzerland: International Organization for Standardization; 1999. [Google Scholar]
- 24.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galán J E, Aizawa S-I. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–604. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 25.Lakkis C, Harding A S, Brennan N A. Hydrogel lens case contamination. Investig Ophthalmol Vis Sci. 1995;36(Suppl.):313. [Google Scholar]
- 26.Larkin D, Kilvington S, Easty D. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br J Ophthalmol. 1990;74:133–135. doi: 10.1136/bjo.74.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leclercq R, Courvalin P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob Agents Chemother. 1991;35:1273–1276. doi: 10.1128/aac.35.7.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy S B. The challenge of antibiotic resistance. Sci Am. 1998;278:32–39. doi: 10.1038/scientificamerican0398-46. [DOI] [PubMed] [Google Scholar]
- 30.Lowe R, Vallas V, Brennan N A. Comparative efficacy of contact lens disinfecting solutions. CLAO J. 1992;18:34–40. [PubMed] [Google Scholar]
- 31.Miller M J, Manchester R J, Callahan D. Antimicrobial activity of ReNu MultiPlus against Pseudomonas. CL Spectrum. 2000;15:23–30. [Google Scholar]
- 32.Mowrey-McKee M F, Monnat K, Sampson H J, Smith C M, Davies G A, Mandt L, Proskin H M. Microbial contamination of hydrophilic contact lenses. Part I: Quantitation of microbes on patient worn-and-handled lenses. CLAO J. 1992;18:87–91. [PubMed] [Google Scholar]
- 33.Mummert R. Trends in lens care. Spectrum. 1998;13(Suppl.):4S–8S. [Google Scholar]
- 34.Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer M L, Hyndiuk R A. Contact lens-related infectious keratitis. Int Ophthalmol Clin. 1993;33:23–49. doi: 10.1097/00004397-199303310-00005. [DOI] [PubMed] [Google Scholar]
- 36.Rosenthal R A, McAnally C L, McNamee L S, Buck S L, Schlitzer R L, Stone R P. Broad spectrum antimicrobial activity of a new multi-purpose disinfecting solution. CLAO J. 2000;26:120–126. [PubMed] [Google Scholar]
- 37.Rosenthal R A, Stein J M, McNally C L, Schlech B A. A comparative study of microbiologic effectiveness of chemical disinfectants and peroxide-neutralizer systems. CLAO J. 1995;21:99–110. [PubMed] [Google Scholar]
- 38.Sakuma S, Reeh B, Dang D, Harris M G. Comparative efficacies of four soft contact lens disinfection solutions. Int Contact Lens Clin. 1997;23:234–241. [Google Scholar]
- 39.Schunk T, Schweisfurth R. Disinfectant performance of oxidizing contact lens solutions: quantitative suspension tests with organic soil contaminants. Contactologica. 1989;11:84–89. [Google Scholar]
- 40.Silhavy T J. Death by lethal injection. Science. 1997;278:1085–1086. doi: 10.1126/science.278.5340.1085. [DOI] [PubMed] [Google Scholar]
- 41.Stapleton F, Dart J. Pseudomonas keratitis associated with biofilm formation on a disposable soft contact lens. Br J Ophthalmol. 1995;79:864–865. doi: 10.1136/bjo.79.9.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stapleton F, Dart J K G, Seal D V, Matheson M. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 1995;114:395–402. doi: 10.1017/s0950268800052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stehr-Green J K, Bailey T M, Brandt F H, Carr J H, Bond W W, Visvesvara G S. Acanthamoeba keratitis in soft contact lens wearers. A case control study. JAMA. 1987;258:57–60. [PubMed] [Google Scholar]
- 44.Thompson M R, Bjorn M J, Sokol P A, Lile J D, Iglewski B H. Exoenzyme S: an ADP-ribosyl transferase produced by Pseudomonas aeruginosa. In: Smulson M E, Sugimura T, editors. Novel ADP-ribosylations of regulatory enzymes and proteins. Amsterdam, The Netherlands: Elsevier/North-Holland; 1980. pp. 425–433. [Google Scholar]
- 45.Tsai T, Dannelly H K. How dangerous is noncompliance with multipurpose solutions. Spectrum. 2000;15:48–50. [Google Scholar]
- 46.U.S. Department of Health and Human Services. Premarket notification (510(k)) guidance document for contact lens care products. Washington, D.C.: Food and Drug Administration, Center for Devices and Radiological Health; 1997. [Google Scholar]
- 47.Velasco J, Bermudez J. Comparative study of the microbial flora on contact lenses, in lens cases, and in maintenance liquids. Int Contact Lens Clin. 1996;23:55–58. [Google Scholar]
- 48.Wilson L, Sawant A, Ahearn D. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch Ophthalmol. 1991;109:1155–1157. doi: 10.1001/archopht.1991.01080080115043. [DOI] [PubMed] [Google Scholar]
- 49.Wilson L, Sawant A, Simmons R, Ahearn D. Microbial contamination of contact lens storage cases and solutions. Am J Ophthalmol. 1990;110:193–198. doi: 10.1016/s0002-9394(14)76991-0. [DOI] [PubMed] [Google Scholar]
- 50.Yahr T L, Goranson J, Frank D W. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III secretion pathway. Mol Microbiol. 1996;22:991–1003. doi: 10.1046/j.1365-2958.1996.01554.x. [DOI] [PubMed] [Google Scholar]