Abstract
Background
Debates on effective and safe diets for managing obesity in adults are ongoing. Low‐carbohydrate weight‐reducing diets (also known as 'low‐carb diets') continue to be widely promoted, marketed and commercialised as being more effective for weight loss, and healthier, than 'balanced'‐carbohydrate weight‐reducing diets.
Objectives
To compare the effects of low‐carbohydrate weight‐reducing diets to weight‐reducing diets with balanced ranges of carbohydrates, in relation to changes in weight and cardiovascular risk, in overweight and obese adults without and with type 2 diabetes mellitus (T2DM).
Search methods
We searched MEDLINE (PubMed), Embase (Ovid), the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science Core Collection (Clarivate Analytics), ClinicalTrials.gov and WHO International Clinical Trials Registry Platform (ICTRP) up to 25 June 2021, and screened reference lists of included trials and relevant systematic reviews. Language or publication restrictions were not applied.
Selection criteria
We included randomised controlled trials (RCTs) in adults (18 years+) who were overweight or living with obesity, without or with T2DM, and without or with cardiovascular conditions or risk factors. Trials had to compare low‐carbohydrate weight‐reducing diets to balanced‐carbohydrate (45% to 65% of total energy (TE)) weight‐reducing diets, have a weight‐reducing phase of 2 weeks or longer and be explicitly implemented for the primary purpose of reducing weight, with or without advice to restrict energy intake.
Data collection and analysis
Two review authors independently screened titles and abstracts and full‐text articles to determine eligibility; and independently extracted data, assessed risk of bias using RoB 2 and assessed the certainty of the evidence using GRADE. We stratified analyses by participants without and with T2DM, and by diets with weight‐reducing phases only and those with weight‐reducing phases followed by weight‐maintenance phases. Primary outcomes were change in body weight (kg) and the number of participants per group with weight loss of at least 5%, assessed at short‐ (three months to < 12 months) and long‐term (≥ 12 months) follow‐up.
Main results
We included 61 parallel‐arm RCTs that randomised 6925 participants to either low‐carbohydrate or balanced‐carbohydrate weight‐reducing diets. All trials were conducted in high‐income countries except for one in China. Most participants (n = 5118 randomised) did not have T2DM. Mean baseline weight across trials was 95 kg (range 66 to 132 kg). Participants with T2DM were older (mean 57 years, range 50 to 65) than those without T2DM (mean 45 years, range 22 to 62). Most trials included men and women (42/61; 3/19 men only; 16/19 women only), and people without baseline cardiovascular conditions, risk factors or events (36/61). Mean baseline diastolic blood pressure (DBP) and low‐density lipoprotein (LDL) cholesterol across trials were within normal ranges. The longest weight‐reducing phase of diets was two years in participants without and with T2DM. Evidence from studies with weight‐reducing phases followed by weight‐maintenance phases was limited.
Most trials investigated low‐carbohydrate diets (> 50 g to 150 g per day or < 45% of TE; n = 42), followed by very low (≤ 50 g per day or < 10% of TE; n = 14), and then incremental increases from very low to low (n = 5). The most common diets compared were low‐carbohydrate, balanced‐fat (20 to 35% of TE) and high‐protein (> 20% of TE) treatment diets versus control diets balanced for the three macronutrients (24/61). In most trials (45/61) the energy prescription or approach used to restrict energy intake was similar in both groups. We assessed the overall risk of bias of outcomes across trials as predominantly high, mostly from bias due to missing outcome data. Using GRADE, we assessed the certainty of evidence as moderate to very low across outcomes.
Participants without and with T2DM lost weight when following weight‐reducing phases of both diets at the short (range: 12.2 to 0.33 kg) and long term (range: 13.1 to 1.7 kg).
In overweight and obese participants without T2DM: low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets (weight‐reducing phases only) probably result in little to no difference in change in body weight over three to 8.5 months (mean difference (MD) −1.07 kg, (95% confidence interval (CI) −1.55 to −0.59, I2 = 51%, 3286 participants, 37 RCTs, moderate‐certainty evidence) and over one to two years (MD −0.93 kg, 95% CI −1.81 to −0.04, I2 = 40%, 1805 participants, 14 RCTs, moderate‐certainty evidence); as well as change in DBP and LDL cholesterol over one to two years. The evidence is very uncertain about whether there is a difference in the number of participants per group with weight loss of at least 5% at one year (risk ratio (RR) 1.11, 95% CI 0.94 to 1.31, I2 = 17%, 137 participants, 2 RCTs, very low‐certainty evidence).
In overweight and obese participants with T2DM: low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets (weight‐reducing phases only) probably result in little to no difference in change in body weight over three to six months (MD −1.26 kg, 95% CI −2.44 to −0.09, I2 = 47%, 1114 participants, 14 RCTs, moderate‐certainty evidence) and over one to two years (MD −0.33 kg, 95% CI −2.13 to 1.46, I2 = 10%, 813 participants, 7 RCTs, moderate‐certainty evidence); as well in change in DBP, HbA1c and LDL cholesterol over 1 to 2 years. The evidence is very uncertain about whether there is a difference in the number of participants per group with weight loss of at least 5% at one to two years (RR 0.90, 95% CI 0.68 to 1.20, I2 = 0%, 106 participants, 2 RCTs, very low‐certainty evidence).
Evidence on participant‐reported adverse effects was limited, and we could not draw any conclusions about these.
Authors' conclusions
There is probably little to no difference in weight reduction and changes in cardiovascular risk factors up to two years' follow‐up, when overweight and obese participants without and with T2DM are randomised to either low‐carbohydrate or balanced‐carbohydrate weight‐reducing diets.
Plain language summary
Low‐carbohydrate diets or balanced‐carbohydrate diets: which works better for weight loss and heart disease risks?
Key messages
• There is probably little to no difference in the weight lost by people following low‐carbohydrate weight‐reducing diets (also known as 'low‐carb diets') compared to the weight lost by people following balanced‐carbohydrate weight‐reducing diets, for up to two years.
• Similarly, there is probably little to no difference between the diets for changes in heart disease risks, like diastolic blood pressure, glycosylated haemoglobin (HbA1c, a measure of blood sugar levels over 2‐3 months) and LDL cholesterol (‘unhealthy’ cholesterol) up to two years.
• This was the case in people with and without type 2 diabetes.
What are low‐carbohydrate and balanced‐carbohydrate weight‐reducing diets?
People spend lots of money on trying to lose weight using diets, products, foods and books, and continue to debate about which diets are effective and safe. So, examining the scientific evidence behind claims made is important. Low‐carbohydrate diets are a broad category of weight‐reducing diets that manipulate and restrict carbohydrates, protein and fat in diets. There are no consistent, widely‐accepted definitions of these diets and different descriptions are used (such as, 'low‐carbohydrate, high‐protein’, 'low‐carbohydrate, high‐fat', or ‘very low‐carbohydrate’).
Low‐carbohydrate diets are implemented in different ways, but they restrict grains, cereals and legumes, and other carbohydrate‐containing foods; such as dairy, most fruit and certain vegetables. These foods are then typically replaced with foods higher in fat and protein; such as meats, eggs, cheese, butter, cream, oils. Some low‐carbohydrate diets recommend eating as desired, while others recommend restricting the amount of energy eaten.
Balanced‐carbohydrate diets contain more moderate amounts of carbohydrates, protein and fats, in line with current healthy eating advice from health authorities. When used for weight reduction, balanced diets recommend restricting the amount of energy eaten by guiding people to reduce their portion sizes and choose healthier foods (e.g. lean instead of fatty meat).
Low‐carbohydrate weight‐reducing diets are widely promoted, marketed and commercialised as being more effective for weight loss, and healthier, than 'balanced'‐carbohydrate weight‐reducing diets.
What did we want to find out?
We wanted to find out if low‐carbohydrate weight‐reducing diets were better for weight loss and heart disease risk factors than balanced‐carbohydrate weight‐reducing diets in adults who were overweight or living with obesity.
We wanted to find this out for people with and without type 2 diabetes.
What did we do? We searched six electronic databases and trial registries for all trials* that compared low‐carbohydrate weight‐reducing diets with balanced‐carbohydrate weight‐reducing diets in adults who were overweight or living with obesity. The trials had to last for at least three months. We compared and summarised the results of the trials and rated our confidence in the combined evidence, based on factors such as study methods and sizes.
*A trial is a type of study in which participants are assigned randomly to two or more treatment groups. This is the best way to ensure similar groups of participants.
What did we find?
We found 61 trials involving 6925 people who were overweight or living with obesity. The biggest trial was in 419 people and the smallest was in 20 people. All except one of the trials were conducted in high‐income countries worldwide, and nearly half were undertaken in the USA (26). Most trials (36) were undertaken in people who did not have heart disease or risk factors. Most people (5118 people) did not have type 2 diabetes. The average starting weight of people across the trials was 95 kg. Most studies (37) lasted for six months or less; and the longest studies (6) lasted for two years.
Main results
Low‐carbohydrate weight‐reducing diets probably result in little to no difference in weight loss over the short term (trials lasting 3 to 8.5 months) and long term (trials lasting one to two years) compared to balanced‐carbohydrate weight‐reducing diets, in people with and without type 2 diabetes. In the short term, the average difference in weight loss was about 1 kg and in the long term, the average difference was less than 1 kg.
People lost weight on both diets in some trials. The amount of weight lost on average varied greatly with both diets across the trials from less than 1 kg in some trials and up to about 12 kg in others in the short term and long term.
Similarly, low‐carbohydrate weight‐reducing diets probably result in little to no difference in diastolic blood pressure, glycosylated haemoglobin (HbA1c) and LDL cholesterol (‘unhealthy’ cholesterol) for up to two years.
We could not draw any conclusions about unwanted effects reported by participants because very few trials reported these.
What are the limitations of the evidence?
We are moderately confident in the evidence. Our confidence was lowered mainly because of concerns about how some the trials were conducted, which included that many trials did not report all their results. Further research may change these results.
How up to date is this evidence?
The evidence is up‐to‐date to June 2021.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only).
| Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only) | ||||||
| Patient or population: overweight and obese participants without T2DM (weight‐reducing phase only) Setting: outpatient clinics, medical/research centres in high‐income countries Intervention: low‐carbohydrate weight‐reducing diets Comparison: balanced‐carbohydrate weight‐reducing diets | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with balanced‐carbohydrate weight‐reducing diets | Risk with low‐carbohydrate weight‐reducing diets | |||||
| Change in body weight (kg) at 3 to < 12 months | The mean change in body weight (kg) at 3 to < 12 months ranged from ‐11.34 to ‐2.3 kg | MD 1.07 kg lower (1.55 lower to 0.59 lower) | ‐ | 3286 (37 RCTs) | ⊕⊕⊕⊝ Moderatea | Low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in weight at 3 to 8.5 months. |
| Change in body weight (kg) at ≥ 12 months | The mean change in body weight (kg) at ≥ 12 months ranged from ‐11.6 to ‐1.7 kg | MD 0.93 kg lower (1.81 lower to 0.04 lower) | ‐ | 1805 (14 RCTs) | ⊕⊕⊕⊝ Moderatea | Low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in weight (kg) at 1 to 2 years. |
| Number of participants per group with weight loss of at least 5% at ≥ 12 months | 789 per 1000 | 875 per 1000 (741 to 1000) | RR 1.11 (0.94 to 1.31) | 137 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c | The evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets on number of participants per group with weight loss of at least 5% at 1 year. |
| Cardiovascular mortality ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Change in DBP (mmHg) at ≥ 12 months | The mean change in DBP (mmHg) at ≥ 12 months ranged from ‐11 to 2.9 mmHg | MD 0.09 mmHg lower (1.29 lower to 1.12 higher) | ‐ | 1419 (11 RCTs) | ⊕⊕⊕⊝ Moderatea | Low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in DBP at 1 to 2 years. |
| Change in LDL cholesterol (mmol/L) at ≥ 12 months | The mean change in LDL cholesterol (mmol/L) at ≥ 12 months ranged from ‐0.31 to 0.1 mmol/L | MD 0.04 mmol/L higher (0.05 lower to 0.12 higher) | ‐ | 1494 (13 RCTs) | ⊕⊕⊕⊝ Moderatea | Low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in LDL cholesterol at 1 to 2 years. |
| Constipation at 3 to < 12 months | 267 per 1000 | 283 per 1000 (216 to 368) | RR 1.06 (0.81 to 1.38) | 564 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,d | The evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets on constipation at 3 to 6 months. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_420942855893718659. | ||||||
a Serious risk of bias: The majority of information is from studies at high overall risk of bias b Serious risk of bias: All information is from a study at high overall risk of bias c Very serious imprecision: OIS not met; 95% CI for the pooled estimate is consistent with the possibility for important benefit (245 per 1000 absolute increase) and unimportant harm (47 per 1000 absolute reduction) d Very serious imprecision: OIS not met; 95% CI for the pooled estimate is consistent with the possibility for unimportant benefit (51 per 1000 absolute reduction) and for important harm (101 per 1000 absolute increase)
Summary of findings 2. Summary of findings table ‐ Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase).
| Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase) | ||||||
| Patient or population: overweight and obese participants without T2DM (weight‐reducing phase followed by weight maintenance phase) Setting: outpatient clinics, medical/research centres in high‐income countries Intervention: low‐carbohydrate weight‐reducing diets Comparison: balanced‐carbohydrate weight‐reducing diets | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with balanced‐carbohydrate weight‐reducing diets | Risk with low‐carbohydrate weight‐reducing diets | |||||
| Change in body weight (kg) at 3 to < 12 months ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Change in body weight (kg) at ≥ 12 months | The mean change in body weight (kg) at ≥ 12 months ranged from ‐5.5 to ‐1.5 kg | MD 0.3 kg lower (2.77 lower to 2.16 higher) | ‐ | 73 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | Low‐carbohydrate weight‐reducing diets may result in little to no difference in change in weight at 1 to 2 years. |
| Weight loss of at least 5% at ≥ 12 months ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Cardiovascular mortality ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Change in DBP (mmHg) at ≥ 12 months | The mean change in DBP (mmHg) at ≥ 12 months was ‐6 mmHg | MD 6 mmHg lower (17.55 lower to 5.55 higher) | ‐ | 13 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d | The evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets on change in DBP at 1 year. |
| Change in LDL cholesterol (mmol/L) at ≥ 12 months | The mean change in LDL cholesterol (mmol/L) at ≥ 12 months was ‐1.15 to ‐0.14 mmol/L | MD 0.08 mmol/L lower (0.44 lower to 0.28 higher) | ‐ | 56 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,d | The evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets on change in LDL cholesterol at 1 to 2 years. |
| Constipation at 3 to < 12 months ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_420946341206467227. | ||||||
a Serious risk of bias: All information is from studies at high overall risk of bias b Serious imprecision: OIS not met c Serious risk of bias: All information is from a study at high overall risk of bias d Very serious imprecision: OIS not met; 95% CI for the pooled estimate is consistent with the possibility for important harm and for important benefit
Summary of findings 3. Summary of findings table ‐ Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only).
| Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only) | ||||||
| Patient or population: overweight and obese participants with T2DM (weight‐reducing phase only) Setting: outpatient clinics, medical/research centres in high‐income countries Intervention: low‐carbohydrate weight‐reducing diets Comparison: balanced‐carbohydrate weight‐reducing diets | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with balanced‐carbohydrate weight‐reducing diets | Risk with low‐carbohydrate weight‐reducing diets | |||||
| Change in body weight (kg) at 3 to < 12 months | The mean change in body weight (kg) at 3 to < 12 months ranged from ‐11.5 to ‐0.33 kg | MD 1.26 kg lower (2.44 lower to 0.09 lower) | ‐ | 1114 (14 RCTs) | ⊕⊕⊕⊝ Moderatea | Low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in weight at 3 to 6 months. |
| Change in body weight (kg) at ≥ 12 months | The mean change in body weight (kg) at ≥ 12 months ranged from ‐7.4 to ‐1.7 | MD 0.33 lower (2.13 lower to 1.46 higher) | ‐ | 813 (7 RCTs) | ⊕⊕⊕⊝ Moderatea | Low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in weight at 1 to 2 years. |
| Weight loss of at least 5% at ≥ 12 months | 654 per 1000 | 588 per 1000 (445 to 785) | RR 0.90 (0.68 to 1.20) | 106 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c | The evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets on weight loss of at least 5% at 1 to 2 years. |
| Cardiovascular mortality ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Change in DBP (mmHg) at ≥ 12 months | The mean change in DBP (mmHg) at ≥ 12 months ranged from ‐6 to 4.3 | MD 0.28 lower (1.84 lower to 1.28 higher) | ‐ | 631 (6 RCTs) | ⊕⊕⊕⊝ Moderated | Low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in DBP at 1 to 2 years. |
| Change in HbA1c (%) at ≥ 12 months | The mean change in HbA1c (%) at ≥ 12 months ranged from ‐1.8 to 0.2 | MD 0.14 lower (0.38 lower to 0.1 higher) | ‐ | 668 (6 RCTs) | ⊕⊕⊕⊝ Moderatea | Low carbohydrate weight‐reducing diets probably result in little to no difference in change in HbA1c at 1 to 2 years. |
| Change in LDL cholesterol (mmol/L) at ≥ 12 months | The mean change in LDL cholesterol (mmol/L) at ≥ 12 months ranged from ‐0.55 to 0.1 | MD 0.12 higher (0.03 lower to 0.26 higher) | ‐ | 753 (7 RCTs) | ⊕⊕⊕⊝ Moderatea | Low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in LDL cholesterol at 1 to 2 years. |
| Constipation at 3 to < 12 months | 194 per 1000 | 265 per 1000 (166 to 422) | RR 1.37 (0.86 to 2.18) | 177 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,e | The evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets on constipation at 6 months. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_420965823167913797. | ||||||
a Serious risk of bias: The majority of information is from studies at high overall risk of bias b Serious risk of bias: All information is from a study at high overall risk of bias c Very serious imprecision: OIS not met; 95% CI for the pooled estimate is consistent with the possibility for important harm (222 per 1000 absolute reduction) and for unimportant benefit (124 per 1000 absolute increase) d Serious risk of bias: All the information is from studies at high risk or have some concerns for overall risk of bias e Very serious imprecision: OIS not met; 95% CI for the pooled estimate is consistent with the possibility for no effect (27 per 1000 absolute reduction) and for important harm (228 per 1000 absolute increase)
Summary of findings 4. Summary of findings table ‐ Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase).
| Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase) | ||||||
| Patient or population: overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase) Setting: outpatient clinics, medical/research centres in high‐income countries Intervention: Low‐carbohydrate Comparison: Balanced | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Balanced | Risk with Low‐carbohydrate | |||||
| Change in body weight (kg) at 3 to < 12 months | The mean change in body weight (kg) at 3 to < 12 months was ‐0.2 kg | MD 0.8 kg lower (2.46 lower to 0.86 higher) | ‐ | 61 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Low‐carbohydrate weight‐reducing diets may result in little to no difference in change in weight at 6 months. |
| Change in body weight (kg) at ≥ 12 months | The mean change in body weight (kg) at ≥ 12 months ranged from ‐2.17 to ‐1.5 kg | MD 0.07 kg lower (1.31 lower to 1.17 higher) | ‐ | 158 (3 RCTs) | ⊕⊕⊝⊝ Lowb,c | Low‐carbohydrate weight‐reducing diets may result in little to no difference in change in weight at 1 to 2 years. |
| Weight loss of at least 5% at ≥ 12 months ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Cardiovascular mortality ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Change in DBP (mmHg) at ≥ 12 months | The mean change in DBP (mmHg) at ≥ 12 months was 0.65 mmHg | MD 0.44 mmHg lower (4.89 lower to 4.01 higher) | ‐ | 99 (1 RCT) | ⊕⊝⊝⊝ Very lowd,e | The evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets on change in DBP at 1 year. |
| Change in HbA1c (%) at ≥ 12 months | The mean change in HbA1c (%) at ≥ 12 months ranged from ‐0.37 to 0.4 % | MD 0.02 % higher (0.3 lower to 0.33 higher) | ‐ | 160 (3 RCTs) | ⊕⊕⊝⊝ Lowb,f | Low‐carbohydrate weight‐reducing diets may result in little to no difference in change in HbA1c at 1 to 2 years. |
| Change in LDL cholesterol (mmol/L) at ≥ 12 months | The mean change in LDL cholesterol (mmol/L) at ≥ 12 months ranged from ‐0.17 to 0.04 mmol/L | MD 0.06 mmol/L lower (0.26 lower to 0.13 higher) | ‐ | 145 (2 RCTs) | ⊕⊕⊝⊝ Lowb,f | Low‐carbohydrate weight‐reducing diets may result in little to no difference in change in LDL cholesterol at 1 to 1.5 years. |
| Constipation at 3 to < 12 months ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_421058088671156171. | ||||||
a Serious risk of bias: All information is from a study at high overall risk of bias b Serious imprecision: OIS not met c Serious risk of bias: All information is from studies at high overall risk of bias d Serious risk of bias: All information is from a study that has some concerns for overall risk of bias e Very serious imprecision: OIS not met; 95% CI for the pooled estimate is consistent with the possibility for important harm and for important benefit f Serious risk of bias: All information is from studies at high risk or that have some concerns for overall risk of bias
Background
Description of the condition
The 2017 Global Nutrition Report estimates that two billion adults worldwide are overweight or obese, and 41 million children are overweight (Development Initiatives 2017). For the first time in history, more people globally are dying from the consequences of overeating than starvation and malnutrition (Forouzanfar 2015). This shift has happened in the last 20 to 30 years and is thought to be mainly due to diets that are of poor nutritional quality, high in energy density and often ultra‐processed. Such diets often result in cumulative weight gain over time and consequently increase the risk of cardiovascular disease, diabetes, stroke and some cancers (Forouzanfar 2015). Globalised food systems promote over‐consumption of these foods (Moubarac 2014; Swinburn 2011).
High body mass index (BMI) is an important modifiable risk factor for cardiovascular disease, diabetes, kidney diseases, certain cancers and musculoskeletal conditions (Berrington 2010; ERFC 2011; GBMRF for CD Collaboration 2014; Singh 2013; Whitlock 2009; Zheng 2011). The disease burdens related to obesity are particularly evident in low‐ and middle‐income countries. Cardiovascular disease deaths rose by 12.5% from 15.9 million in 2005 to 17.9 million in 2015, and low‐ and middle‐income countries accounted for over three‐quarters of these deaths (GBD MCDC 2016). In Africa, between 1980 and 2014, age‐standardised prevalence of type 2 diabetes mellitus (T2DM) increased from 4.8% to 9.7% in men and from 7.7% to 12.6% in women (NCD‐RisC 2017). These shifting disease patterns have a major impact on individual and family well‐being, and on economies, with large direct and indirect costs being associated with illness.
Description of the intervention
Pharmacotherapy, bariatric surgery and counselling that target diet, physical activity, and behaviour change are used to treat adult obesity (Dietz 2015). Due to the chronic and relapsing nature of obesity and its related conditions, current guidelines for the treatment of obesity recommend comprehensive management approaches that aim to achieve long‐term weight reduction. This includes intensive lifestyle intervention characterised by dietary restriction, increased physical activity, and behavioural management as first‐line treatment (Dietz 2015; Jensen 2014). Importantly, there is no 'one‐size‐fits‐all' weight‐reducing diet and different diets work for different people, based on preferences and ease of adherence (Jensen 2014; Johnston 2014).
The public, families and health professionals face an often dizzying array of weight‐reducing diets, many of which have been commercialised as books, seminars, diet food products, supplements and other related products. These include, but are not limited to, various versions of low‐carbohydrate diets (for example, Atkins diet (Atkins 1999)), low‐fat or so‐called 'balanced diets', very low‐fat diets (for example, Ornish diet (Ornish 2001)), and low‐glycaemic diets (for example, South Beach diet (Agatston 2003)).
Nutrients are needed by the body in small (i.e. micronutrients such as vitamins and minerals) or large amounts (i.e. macronutrients) for growth, repair and optimal functioning (Lichtenstein 2005). Total daily energy intake is made up of the sum of the energy provided by the three macronutrients (i.e. carbohydrate, protein and fat). Per gram of macronutrient, carbohydrates and protein each provides about 17 kilojoules (approximately 4 kilocalories), while fat provides about 37 kilojoules (approximately 9 kilocalories) (Carreiro 2016). Carbohydrates are primarily contained in grains, cereals and sugar, and in the digestive tract are broken down into glucose. Carbohydrates are the largest nutrient class, and traditionally, the greatest energy source. If energy intake exceeds energy requirements, excess carbohydrates will be mainly stored in the liver as glycogen for later use or be converted to fatty acids when glycogen stores are saturated. In contrast, if the diet contains limited amounts of carbohydrate, the liver converts fat into fatty acids and ketones to replace glucose as an energy source (Paoli 2013).
Low‐carbohydrate diets are a broad category of weight‐reducing diets and programmes that manipulate and restrict macronutrient (carbohydrate, protein, fat) intake (Astrup 2004; Bazzano 2012; Campbell 2012; Hession 2009). There are no consistent and widely accepted definitions of these diets and different descriptions are used, such as 'low‐carbohydrate, high‐protein' or 'low‐carbohydrate, high‐fat', depending on the macronutrient manipulation and focus. In practice, low‐carbohydrate diets are applied in different ways, but generally restrict grains, cereals and legumes, and other foods that contain carbohydrates, such as dairy, most fruit and certain vegetables. The energy required is then typically replaced with food higher in fat and protein, such as meats, eggs, cheese, butter, cream and oils ‐ many of which are animal source foods. Some low‐carbohydrate diets recommend eating as desired, while others apply restrictions to total energy intake (Atkins 2011; Campbell 2012).
Conventional authorities such as the European Food Safety Authority (EFSA 2017), American Institute of Medicine Food and Nutrition Board (IOM 2005), Australian National Health and Medical Research Council together with the New Zealand Ministry of Health (NHMRC 2006), and the Nordic Council of Ministers, Nordic Committee of Senior Officials for Food Issues (NNR 2012), as well as the UK's Scientific Advisory Committee on Nutrition (SACN 2015), have recommended 45% to 65% of total energy as the appropriate carbohydrate intake for adults. Thus, some people regard low‐carbohydrate diets to be those with carbohydrate intakes below 45% of total energy. Some published definitions of low‐carbohydrate diets disregard the official recommendations, and use an upper limit of 40% of total energy from carbohydrates as indicative of a low‐carbohydrate diet (Frigolet 2011; Wylie‐Rosett 2013). In absolute, rather than proportional terms, low‐carbohydrate diets have been defined as having less than 200 g of carbohydrate (Frigolet 2011), while some disagree with this liberal definition, preferring to distinguish between 'non‐ketogenic low‐carbohydrate diets' as containing 50 g to 150 g of carbohydrates, and 'ketogenic low‐carbohydrate diets' (or very low‐carbohydrate diets) as having a maximum of 50 g of carbohydrates, with this latter variant seen by some as being more effective for weight loss (Westman 2007; Yancy 2004). Ketogenic diets are characterised by a high production of ketones in the liver as an alternative energy source, as well as high levels of ketones in the blood (ketonaemia) and urine (ketonuria) when fat or protein intake is very high and carbohydrate intake is very low (less than 50 g/day) (Paoli 2013).
Weight‐reducing diets aligned with current dietary recommendations are often referred to as 'low‐fat diets' or 'balanced, weight‐reducing diets', and will be referred to as 'balanced diets' in this review (British Dietetic Association 2013). Globally, current dietary recommendations ‐ in terms of macronutrients, micronutrients, food choices and dietary patterns ‐ are generally consistent, and governmental bodies from Europe, the USA, Australia and Nordic countries recommend that 45% to 65% of total energy intake should be provided by carbohydrates, between 10% and 35% by protein and between 20% and 35% by fat (EFSA 2017; IOM 2005; NHMRC 2006; NNR 2012). There is room for flexibility within these ranges from lower to higher intakes of carbohydrate, fat and protein. These dietary recommendations are accompanied by information on 'better food' choices; improving the quality of carbohydrates (e.g. whole grains versus refined grains), protein (e.g. fish versus processed meat) and fat (e.g. olive oil versus butter); as well as on maintaining a healthy body weight by aiming to keep energy intake and energy expenditure balanced.
Nutrition recommendations are moving away from macronutrient‐focused, single food and single nutrient messages towards recommendations about dietary patterns and ‘total diets’ (NICE 2014; NNR 2012; USDA 2014). This has been driven mainly by the lack of clear and consistent associations between individual nutrients (micronutrients and macronutrients) and disease risk, limitations of single nutrient trials, and greater successes of 'total diet' or dietary pattern interventions.
Dietary patterns can be defined as "the quantities, proportions, variety, or combination of different foods, drinks, and nutrients (when available) in diets, and the frequency with which they are habitually consumed" (USDA 2014). Current evidence supports associations between some dietary patterns and lower risk of obesity and chronic diseases, especially T2DM, cardiovascular disease, hypertension, and certain cancers (DGAC 2015; USDA 2014). For example, systematic reviews of large long‐term studies show that several dietary patterns are consistently and equally associated with a lower risk of developing T2DM in the future (Alhazmi 2014; Esposito 2014; Koloverou 2014). These dietary patterns have different macronutrient compositions, but share several common components, including fruit, vegetables, whole grains, legumes, nuts, healthy oils, adequate proteins (such as seafood and lean meat), reduced intake of red and processed meats and sugar‐sweetened beverages, and little or moderate alcohol (Ndanuko 2016; NNR 2012; USDA 2014). It has been suggested that carbohydrate avoidance and the resultant food restrictions typical of low‐carbohydrate diets make it difficult to achieve a dietary pattern that is nutrient‐ and fibre‐rich, diverse, and that promotes good health (USDA 2014).
How the intervention might work
Energy balance and body‐weight regulation is complex and interactive, and questions on certain components of energy balance and their interactions ‐ especially over the longer term ‐ are yet to be answered (Hall 2012). The first law of thermodynamics and evidence from various types of studies over the past 50 years, including randomised controlled trials (RCTs), support the view that weight loss occurs when the amount of kilojoules consumed during eating and drinking is less than the amount of kilojoules expended over weeks or months (ACC/AHA 2013 Full Report; Hall 2011; Hall 2012; Hall 2015; Jensen 2014). Thus, a plausible mechanism whereby low‐carbohydrate diets enable weight loss is by achieving a sustained energy deficit over time even when advice to explicitly restrict energy intake is not provided. When people eliminate and restrict carbohydrate‐rich foods, they are more likely to reduce energy intake because they eat less food (Brehm 2003; Sondike 2003). Related mechanisms reported in the literature include a reduction in appetite with low‐carbohydrate diets, possibly related to the increased intake of fat and production of ketones (Boden 2005; Nordmann 2006; Westman 2007). Since total energy intake is known to drive changes in body weight, its role must be considered when examining the effect on any diet on weight changes.
Some literature indicates that certain macronutrients may have metabolic advantages over others, more specifically that lower carbohydrate intake is more effective for weight loss, independent of energy intake (Atkins 2011; Westman 2007). However, it has been proposed that when proportions of macronutrients in the diet are changed, rapid physiological adaptations occur that aim to match metabolic fuel selection to the diet. Changes in body composition and energy expenditure may be minimised by these adaptations. In this scenario, in the shorter term, all reduced energy diets would have a similar effect on loss of body fat (Hall 2011; Noakes 2006; Tay 2008).
Literature on low‐carbohydrate diets also suggest that the reduced insulin secretion resulting from a low‐carbohydrate diet causes greater release of adipose tissue free fatty acids, fat oxidation and energy expenditure, and increased loss of body fat compared to restricting fat intake (Ludwig 2014; Taubes 2007; Westman 2007). However, a study in 19 obese adults confined to a metabolic ward demonstrated that an equal kilojoule‐selective reduction in dietary fat resulted in no changes in insulin secretion, fat oxidation or energy expenditure and a greater net fat loss when compared to restricting carbohydrates by the same amount, which resulted in decreased insulin secretion, increased fat oxidation and decreased energy expenditure (Hall 2015).
According to recent clinical guidelines for obesity, a high‐quality systematic review, a scientific report to inform dietary guidelines and a recent six‐month randomised controlled feeding trial, a number of different diets may lead to weight loss over the short term if they achieve a sustained energy deficit, but some of these diets may be more advantageous than others for maintaining longer‐term cardiovascular and metabolic health (DGAC 2015; Jensen 2014; Johnston 2014; Wan 2017). There is evidence that weight loss of 5% and more, or BMI reduction of at least 5%, may result in clinically meaningful improvements in cardio metabolic health following dietary regimens (Brown 2016; Truby 2006; Wald 2012; Wing 2010).
Adherence and weight maintenance
Poor dietary adherence has been regarded as one of the reasons popular and traditional dieting strategies are unsuccessful, and it is well known that adherence to nutrition counselling by study participants varies widely. Evidence from quality RCTs, systematic reviews and other study designs suggest that adherence to diets is a primary driver of weight‐loss success, regardless of the macronutrient composition, and may explain a considerable part of whether dieters are able to achieve energy deficit for weight loss (Alhassan 2008; Dansinger 2005; Hall 2011; Johnston 2014; Sacks 2009). Dietary intake is difficult to measure accurately, and fidelity of application of dietary assessment methods varies widely across studies (Shim 2014), which may introduce a lot of variation into the assessment of adherence. Consequently, data on adherence to assigned diets are often lacking in weight‐loss trials. Also, keeping the weight off once lost is also a considerable challenge in treating obesity, with most people tending to relapse (Dietz 2015). Indeed, a systematic review including 56 RCTs reported that dieting to lose weight is most often over weeks, months or years, and it is challenging for most people to maintain the weight lost over the long term (Collins 2013). Thus, ease of adherence to weight‐reducing diets is a key factor to consider. It is also necessary to consider the time‐dependant nature of the relationship between diet and weight change when examining the effect of diets on weight change. Trials typically have different periods of follow‐up, and different frequencies or intervals of study contacts and measurement. Tay 2015 indicated that changes sustained over a 12‐month period reflects durability of effects over the long term.
Why it is important to do this review
The public spends considerable amounts of money and time on trying to diet, on books about diet, and on products and foods to enhance weight loss. It is therefore important to examine scientific evidence behind the claims made.
Some advocates claim low‐carbohydrate diets decrease cardiovascular disease and diabetes risk profiles: reducing triglycerides, increasing high‐density lipoprotein (HDL) cholesterol and improving glycaemic control over one year (Stern 2004); improving triglycerides, HDL cholesterol and glycaemic control over four years (Wing 2010); and improving aortic stiffness over four weeks (Syed‐Abdul 2018). However, the diets are not without potential side effects. These include gastrointestinal disturbances, such as constipation, flatulence, indigestion or diarrhoea over the short term (Bhardwaj 2017; Brinkworth 2009a; Saslow 2014); and increasing low‐density lipoprotein (LDL) cholesterol and non‐HDL cholesterol over 12 months (Brinkworth 2009b; Wan 2017). Some participants report mood disturbance and impaired ability to concentrate (Brinkworth 2009c; Halyburton 2007). Other side effects of low‐carbohydrate diets include lack of appetite, bad breath, headaches, muscle cramps, general weakness and hair loss (Foster 2010; Rio 2001; Yancy 2004).
A prospective cohort study and meta‐analysis that combined 25‐year follow‐up of the Atherosclerosis Risk in Community (ARIC) data (USA) and seven other cohort studies (USA, Europe, Asia and multinational) assessed the association between carbohydrate intake and mortality (Seidelmann 2018). Findings indicated that both high‐ and low‐carbohydrate diets increased mortality, with the lowest risk observed among those who consumed a diet containing 50% to 55% carbohydrates. The low‐carbohydrate dietary patterns that favoured animal fat and protein sources were associated with higher mortality, while those that favoured plant‐based foods were associated with lower mortality. Additionally, diets very high in animal source foods could pose a significant threat to environmental sustainability (Sabate 2014; Soret 2014).
The debate on effective and safe diets for treating obesity continues. Many trials and systematic reviews involving obese people (with and without comorbidities), of varying methodological quality, have assessed the effects of low‐carbohydrate diets on weight and other risk factors. A number of these studies show little or no clinically important difference in weight loss of up to two years' follow‐up (Chawla 2020; Dong 2020; Van Zuuren 2018). A systematic review of eight RCTs found that improvements in psychosocial outcomes occur in participants on short‐ and longer‐term weight‐loss programmes, regardless of the macronutrient composition of the diet (Ghoch 2016). However, low‐carbohydrate weight‐reducing diets continue to be widely promoted, marketed and commercialised as being more effective for weight loss, and healthier, than weight‐reducing diets that have 'balanced' or macronutrient compositions in line with current global dietary recommendations. Healthcare officials argue that very restrictive carbohydrate diets do not promote behaviour changes that foster varied, nutrient‐ and fibre‐rich dietary patterns (USDA 2014), known to reduce risks of cardiovascular disease, hypertension, T2DM and certain cancers. Extreme restriction or excess of macronutrients, as advised with very low‐carbohydrate diets, instead promote a way of eating that is likely to result in an imbalance of macronutrient intake, suboptimal micronutrient intake and increased disease risk over time.
Previously, we took stock of existing systematic reviews on low‐carbohydrate diets for adults wanting to lose weight. We found 50 existing reviews (last search date: 3 March 2014), with a number of shortcomings as reported in Naude 2014. This exercise helped inform the protocol for our earlier systematic review and meta‐analysis of RCTs (Naude 2014). This Cochrane Review will be a fresh edition to Naude 2014, by taking into account new eligible trials, and comments and criticisms generated by the earlier work.
Objectives
To compare the effects of low‐carbohydrate weight‐reducing diets to weight‐reducing diets with balanced ranges of carbohydrates, in relation to changes in weight and cardiovascular risk, in overweight and obese adults without T2DM (comparisons 1 and 2) and with T2DM (comparisons 3 and 4)
Methods
Criteria for considering studies for this review
Types of studies
As per our protocol (Naude 2019), we included parallel‐arm individual‐ and cluster‐randomised controlled trials (RCTs) that had an active weight‐reducing intervention phase of at least 12 weeks. According to Jensen 2014, obese individuals on a moderately restricted energy intake can potentially lose between six and eight kilograms, or five to ten per cent of initial body weight (clinically meaningful) over a six‐month period. From this, we inferred that it would be plausible to lose five per cent of initial weight over a three‐month period. According to the UK National Institute for Health and Care Excellence (NICE), 12 weeks is the standard length for most commissioned dietary interventions (Ahern 2017; NICE 2014). Follow‐up of participants after the intervention ended could be of any duration.
We excluded quasi‐randomised trials (that is, trials that used an inadequate method of randomisation, such as alternation or date of birth). We included cross‐over trials where the first phase was 12 weeks or longer, and where data for the first phase per group were available. We excluded cross‐over trials not meeting these criteria due to the possible period and carry‐over effects that would arise with the eligible dietary interventions, condition (overweight and obesity) and outcomes in this review, with these not being easily reversible as required for a valid cross‐over design (Younge 2015).
We only included studies with a weight‐maintenance phase if the preceding weight‐reducing phase was for 12 weeks or longer, and relevant data from this phase were available. We separated analyses for weight‐reducing and weight‐maintenance phases.
Types of participants
We included adults (18 years and older) who were overweight or living with obesity (as defined by trial authors), with or without T2DM, and with or without cardiovascular conditions or risk factors such as hypertension or dyslipidaemia (as defined by trial authors).
We excluded studies where pregnant and lactating women were included, as well as studies in people with specific medical conditions such as bipolar disorder, polycystic ovary syndrome, chronic renal disease, and so on.
We included studies involving a subset of eligible participants (for example, adults and children, as defined by trial authors) if results were reported separately for the eligible subset (for example, those ≥ 18 years). If not, we only included such studies if 80% or more of the baseline sample were eligible for our review (for example, aged ≥ 18 years). We excluded data from such studies in sensitivity analyses to test the robustness of the primary meta‐analyses.
Types of interventions
Treatment diet
We included RCTs investigating low‐carbohydrate weight‐reducing diets where the diets were explicitly implemented for the primary purpose of reducing weight, with or without explicit advice to restrict total energy intake.
Control diet
We included RCTs where the control diets had a carbohydrate content within the balanced range of 45% to 65% of total energy, and where the diets were explicitly implemented for the primary purpose of reducing weight, with or without advice to restrict total energy intake.
We included studies where diets were implemented by provision of advice, food or both. However, we excluded studies with the following.
Treatment diet had carbohydrate content 45% or more of total energy or more than 150 g per day.
Treatment and control diets were different in some other respect that may influence the predefined outcomes, except for total energy intake.
Treatment or control diets were not adequately defined (and could not be obtained from study authors) or where the control diet was ‘no dietary intervention'.
Diets were combined with any other co‐interventions (e.g. exercise, pharmacological, surgical) where these differed by group.
Dietary interventions had an exclusive focus on energy restriction (i.e. no macronutrient manipulation was explicitly instituted).
Interventions focused solely on specific foods (e.g. oats), food groups (e.g. dairy) or food components (e.g. plant sterols), or where meal replacements or supplements were part of the diets and were different in the diets being compared.
Participants were selected based on a possible prognostic variable (for example, genotype).
Types of outcome measures
We did not exclude studies on the basis of outcomes measured. However, we did exclude studies measuring only immediate meal responses (e.g. postprandial changes in blood sugar) and not longer‐term physiological responses to diet.
Primary outcomes
Change in body weight (kg) from baseline
Number of participants per group with weight loss of at least 5% from baseline
We assessed the primary outcomes at short‐term (3 months to < 12 months) and long‐term (≥ 12 months) follow‐up.
Secondary outcomes
Clinical
Change in body mass index (BMI; kg/m2) from baseline
Number of participants per group with reduction in BMI of at least 5% from baseline
We assessed the above clinical outcomes at short‐term (three months to < 12 months) and long‐term (≥ 12 months) follow‐up.
Change in diastolic blood pressure (mmHg)
Change in systolic blood pressure (mmHg)
All‐cause mortality
Cardiovascular mortality
Non‐fatal myocardial infarction
Non‐fatal stroke
Diagnosis of T2DM (as reported by study authors)
We assessed these clinical outcomes at long‐term (≥ 12 months) follow‐up only.
Laboratory
Change in glycosylated haemoglobin (HbA1c) (%)
Change in serum low‐density lipoprotein (LDL) cholesterol (mmol/L)
Change in serum high‐density lipoprotein (HDL) cholesterol (mmol/L)
Change in serum non‐HDL cholesterol (mmol/L)
Change in serum total cholesterol (mmol/L)
Change in serum triglycerides (mmol/L)
We assessed these laboratory outcomes at long‐term (≥ 12 months) follow‐up only.
Participant‐reported adverse effects
Participant‐reported adverse effects, specifically in regard to lack of appetite, bad breath, weakness, headaches, gastrointestinal problems (constipation, diarrhoea, flatulence, indigestion) and psychosocial problems (mood disturbances) at any time point, limited to those described in included studies. We regarded constipation as a patient‐important outcome since it is frequently reported by people following low‐carbohydrate diets.
Search methods for identification of studies
We used a comprehensive search strategy aiming to identify all eligible studies regardless of language or publication status.
Electronic searches
We identified RCTs through systematic searches of the following bibliographic databases:
MEDLINE (PubMed, from 1946 to 25 June 2021)
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 6), in the Cochrane Library
Embase (Ovid, from 1947 to 28 June 2021)
Web of Science Core Collection with Indexes SCI‐Expanded, SSCi, CPCI‐S (Clarivate Analytics, from 1970 to 25 June 2021)
We also on 25 June 2021 searched ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) for ongoing and unpublished trials (apps.who.int/trialsearch).
We first designed the search strategy for MEDLINE (PubMed) and then adapted it for use in the other sources (Appendix 1).
We did not do a separate search for adverse effects of interventions; we only considered adverse effects reported in included studies.
Searching other resources
We checked reference lists of all included studies and relevant systematic reviews identified for additional references to trials. We set out to examine any relevant retraction statements and errata for included studies; however, we did not come across any.
Data collection and analysis
Selection of studies
We imported all de‐duplicated search records into Covidence. Two review authors independently screened the titles and abstracts of these records to identify all potential eligible studies. Discrepancies in first‐line screening choices were resolved by discussion among at least two review authors. We retrieved the full‐text report for each record that the screeners considered potentially eligible, and two review authors (CN, KN, AS, AB) independently screened all full texts, identifying studies for inclusion, and identifying and recording reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, consultation with a third review author (TY, JV). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and 'Characteristics of excluded studies' table. Studies where full reports (published or unpublished) were not available (e.g. conference abstracts), or where there was unclear or missing information (that could not be obtained from study authors) such that we could not ascertain study eligibility, were placed, with a reason, in the 'Characteristics of studies awaiting classification' table.
Data extraction and management
To extract study characteristics and outcome data, we created a data collection form in DistillerSR (DistillerSR), and piloted it on two included studies. Data from each included study were extracted independently by two review authors (AB, KN, AS, CN). We contacted the study authors when reported information was unclear or contradictory, or when important data were missing. It was not necessary to seek translations. We extracted the following:
Methods: authors' contact details, type of record (e.g. journal article, thesis), study design, study population, study dates, total duration of the intervention and follow‐up duration after the intervention where relevant, details of 'run‐in' periods where relevant, number of study centres and location, study setting, method of recruitment, number of study arms, description of eligible study arms, outcome used for sample size calculation, unit of allocation, number randomised per study arm (for individually‐randomised trials), number of clusters and number of participants per cluster who consented (for cluster‐randomised trials), number of withdrawals and those lost to follow‐up, number completed and analysed, other relevant notes on the methods.
Participants: inclusion and exclusion criteria, age, gender (number of male and female participants per group), baseline body weight status, other baseline cardiovascular disease risk factors and potential confounders, any group differences.
Interventions: treatment diet, control diet, implementation or delivery of diets, dietary intake assessments (e.g. what, how frequent, by whom), concomitant interventions.
Outcomes: primary and secondary outcomes specified and collected at relevant time points, data on adherence to the interventions, and whether primary study authors analysed results separately according to gender.
Notes: study funding, conflicts of interest declarations of study authors, and other relevant notes.
For outcomes, we extracted change data (i.e. change from baseline to outcome assessment per group) where possible, with relevant data on variance for treatment and control arms and numbers of participants per arm at that time point. Where change data were not available, we extracted and used data at study end (i.e. end values), or other relevant time points, along with variance and number of participants per arm at that time point.
Where possible, we converted variables to comparable units to allow pooling of data, if appropriate. Calleja‐Fernández 2012 stratified by homeostatic model assessment (insulin resistant (IR) and insulin sensitive (IS)) before randomisation. We combined IR and IS group data using Cochrane methods to combine subgroups (Higgins 2021). Saslow 2017a reported appetite change as hunger change scores using means and Cohen's d values for each diet group. We converted Cohen's d values to standard deviations (SD) using the formula SD = mean/Cohen's d.
We stratified included trials into those in participants without T2DM (comparisons 1 and 2) and those in participants with T2DM (comparisons 3 and 4).
We resolved disagreements by consensus or by involving a third review author (MC, TY, JV). We exported data from DistillerSR and imported it into the latest version of Review Manager Web (RevMan Web 2020) via Microsoft Excel. We completed the 'Characteristics of included studies' table for all included studies. We used key items from the TIDieR checklist (Hoffman 2014) to aid description, interpretation and discussion of the results. Brief details of ongoing studies are reported in the 'Characteristics of ongoing studies' table, and these studies will be considered for inclusion in a future update of the review.
Assessment of risk of bias in included studies
Three reviewers (CN, AB and AS) assessed risk of bias for each study using the Cochrane Risk of Bias 2 tool (RoB2) for randomised controlled trials (Sterne 2019). This tool was used to assess the effect of the assignment to the intervention, for the following domains:
bias arising from the randomisation process;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
We did not have to carry out the planned assessments of risk of bias for cross‐over and cluster‐randomised controlled trials, as none of the eligible trials had these designs.
We judged each domain as either 'low risk of bias', 'some concerns' or 'high risk of bias' for the following outcomes reported in the summary of findings tables:
change in body weight (kg) measured from baseline to between three and < 12 months;
change in body weight (kg) measured from baseline to ≥ 12 months;
number of participants per group with weight loss of at least 5% measured from baseline to ≥ 12 months;
cardiovascular mortality measured from baseline to ≥ 12 months;
change in diastolic blood pressure (mmHg) measured from baseline to ≥ 12 months;
change in HbA1c (%) (for comparisons in participants with T2DM) measured from baseline to ≥ 12 months;
change in serum LDL cholesterol (mmol/L) measured from baseline to ≥ 12 months;
participant‐reported constipation at any time point from baseline to three and < 12 months.
Overall, per included study, we made judgements according to the following criteria:
low risk of bias: when all domains were at low risk;
some concerns: when one or more domains had some concerns, but none were at a high risk of bias;
high risk of bias: when one or more domains were at high risk of bias, or multiple domains had some concerns to the extent that these reduced confidence in the results.
We resolved any disagreements by discussion or by involving another review author(MC or AS).
Measures of treatment effect
For dichotomous data, we used the number of events as the numerator and the total sample size per outcome as the denominator in each relevant comparison group and computed the risk ratio (RR) (available case data). For continuous data, we reported results per outcome as the difference in the mean change (and, if not available, the difference in end values) between the treatment and control groups, and computed the mean difference (MD) (available case data). We entered data presented as a scale with a consistent direction of effect. We used Review Manager Web to conduct meta‐analyses for each outcome, where appropriate, to determine a pooled effect of low‐carbohydrate diets compared to balanced‐carbohydrate diets. We narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
In the case of multiple intervention groups, we selected one pair of interventions (treatment and control) most relevant to this systematic review question. We did not need to apply the planned approach for unit of analysis issues related to clustering as no cluster‐randomised trials were eligible. Where a study reported outcome data for more than one time point within our time point categories (3 months to < 12 months; and ≥ 12 months), we used the longest time point (for example, where results were available at three months and five months, we only used the five‐month data).
Dealing with missing data
We contacted study authors or sponsors to clarify key study characteristics and obtain missing numerical outcome data, where needed. Where study authors had not reported all relevant statistics per outcome (for example, sample size, mean change and standard deviation of change per group), we calculated or estimated the required data from other reported statistics using formulas specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), if possible. Where we could not calculate or estimate these statistics with reasonable confidence, we contacted the study authors by email. When we did not receive a response, we did not impute the missing values, but reported the available results narratively or in a table, as appropriate. For interventions in which there was substantial attrition (15% or more for at least one of the groups) of study participants, we reported the attrition rate. We also performed sensitivity analyses excluding studies with some concerns or a high risk of bias for the missing outcome data domain in RoB 2.
Assessment of heterogeneity
We examined heterogeneity per outcome firstly by visual inspection of the forest plots (i.e. we looked at physical overlap of confidence intervals across the included studies). Secondly, we assessed statistical heterogeneity among the intervention effects across the included studies in each meta‐analysis as follows:
Chi2 test for heterogeneity
I2 statistic to quantify heterogeneity
Tau2 statistic to measure the extent of heterogeneity
In our meta‐analyses, we considered substantial heterogeneity as an I2 statistic value of greater than 50% and either a Chi2 test of less than 0.1 or Tau2 statistic greater than 0. In meta‐analyses with substantial heterogeneity, we performed prespecified subgroup analyses on the outcomes in the summary of findings tables. Where we identified unexplained substantial heterogeneity, we did not pool results into an overall effect estimate but rather presented the individual effect sizes per study for the specific outcome.
Assessment of reporting biases
Where data per comparison and outcome allowed us to pool more than 10 studies, we explored the possibility of small study biases for the primary outcomes using funnel plots. In the case of asymmetry, we considered various explanations such as publication bias, poor study design and the effect of study size.
Data synthesis
We used a random‐effects model for meta‐analyses since we anticipated heterogeneity between included studies due to variations in the composition of weight‐loss diets, adherence to diets, intervention duration and dietary assessment methodology. For dichotomous outcomes, we used the Mantel‐Haenszel method, unless the number of events were not available but estimates of effect measure and their standard errors were, in which case we used the inverse variance method. For continuous outcomes, we used the inverse variance method.
We analysed trials in overweight and obese participants without T2DM following diets with a weight‐reducing phase only (comparison 1), or a weight‐reducing phase followed by a weight‐maintenance phase (comparison 2); as well as overweight and obese participants with T2DM following diets with a weight‐reducing phase only (comparison 3), or a weight‐reducing phase followed by a weight‐maintenance phase (comparison 4). These analyses were done separately, where possible, as the presence of diabetes is likely to influence the effects of the diets. When not explicitly stated, we regarded weight‐maintenance phases as periods where initial diet prescriptions or advice changed to indicate less focus on active weight reduction, for example, changes in energy prescriptions or no restrictions on carbohydrate intake.
We analysed outcome data at the time point ≥ 12 months, because it captures sustainability of effects on weight loss, clinical, as well as laboratory outcomes. However, for the weight and BMI outcomes, we also analysed data at the time point three months to < 12 months, as many people going on weight‐loss diets are especially interested to know how fast they would be losing weight, and experienced weight loss over the short term also serves as motivation to keep going longer term.
We preferentially extracted and used data from the intention‐to‐treat (ITT) analyses of trials (as reported by trial authors) in all our meta‐analyses. By ITT, we mean that randomised participants were analysed according to the group to which they were randomised; however, if there were missing data we did not perform any imputations.
Subgroup analysis and investigation of heterogeneity
Where data allowed, we carried out the following subgroup analyses for the outcomes in the summary of findings tables, to explore substantial heterogeneity and the stability of findings in different study subgroups, as follows.
By extent of carbohydrate restriction (i.e. very low‐carbohydrate or ketogenic diets: carbohydrate prescription of ≤ 50 g per day or < 10% of total daily energy intake from a nominal 8400 kilojoule (approximately 2000 kilocalories) diet; and non‐ketogenic low‐carbohydrate diets: > 50 g to 150 g per day or < 45% of total energy intake)
By similarity of total energy prescription (i.e. studies with substantial differences in daily total energy prescription (> 500 kilojoules or approximately 120 kilocalories) in treatment and control groups, for example, ad libitum energy prescription in the treatment diet and restricted energy prescription in the control diet, studies with similar energy prescription or similar approach used to restrict energy intake in treatment and control groups, studies with no energy prescription reported or unrestricted energy intake prescribed in both treatment and control groups)
By diagnosed cardiovascular event or disease (i.e. studies in people with no events or disease, studies in people with events or disease, and studies in people with and without events or disease)
By gender
Sensitivity analysis
Where data allowed, we carried out sensitivity analyses for primary outcomes, assessing the effect of:
overall low risk and 'some concerns' of bias (i.e. we first pooled all relevant studies per outcome, and then pooled only studies with overall low risk and 'some concerns' of bias);
attrition bias (i.e. we first pooled all relevant studies per outcome, and then pooled only studies at low risk of bias for the missing outcome data domain);
studies including only a subset of eligible participants for this review (i.e. we first pooled all relevant studies per outcome, and then pooled only studies that included only participants eligible for inclusion in this review);
clustering (i.e. we first pooled all relevant studies per outcome, and then pooled only studies that randomised individual participants); and
source of funding (i.e. we first pooled studies with all funding sources, and then pooled only studies without diet/food industry funding).
Summary of findings and assessment of the certainty of the evidence
Based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we prepared four summary of findings tables, one for each comparison in participants without and with T2DM. In these tables, we included change in body weight (kg) measured from baseline to between three and < 12 months, and the following outcomes measured from baseline to ≥ 12 months: change in body weight (kg); number of participants per group with weight loss of at least 5%; cardiovascular mortality; change in diastolic blood pressure (mmHg); change in HbA1c (%) (for comparisons 3 and 4 in participants with T2DM); change in serum LDL cholesterol (mmol/L), as well as participant‐reported constipation at 3 to < 12 months. We used the GRADE approach to rank the certainty of the evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes, using GRADEpro GDT software (www.gradepro.org). The GRADE tool includes five considerations (study limitations, inconsistencies of results, imprecision, indirectness and publication bias) to assess the certainty of the evidence from RCTs.
We justified all decisions to downgrade the quality of evidence using footnotes, and made comments to aid readers' understanding where necessary.
Three review authors (CN, AS, AB) made judgements about evidence certainty, with disagreements resolved by discussion, and involving a third review author where needed.
We extracted study data, formatted our comparisons in data tables and prepared a summary of findings table for each of the four comparisons before writing the results and conclusions of our review.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; Characteristics of studies awaiting classification.
Results of the search
Our electronic and manual searches yielded 5563 references, which included screening the reference lists of included trials and existing systematic reviews, with 2882 remaining after duplicate removal. We screened the titles and abstracts of these records and identified 475 as potentially eligible and obtained their full texts. We included 61 trials from 113 references (Figure 1), of which three did not provide data that could be used in the quantitative syntheses (meta‐analyses) (Foster 2003; Pittas 2005; Racette 1995).
1.
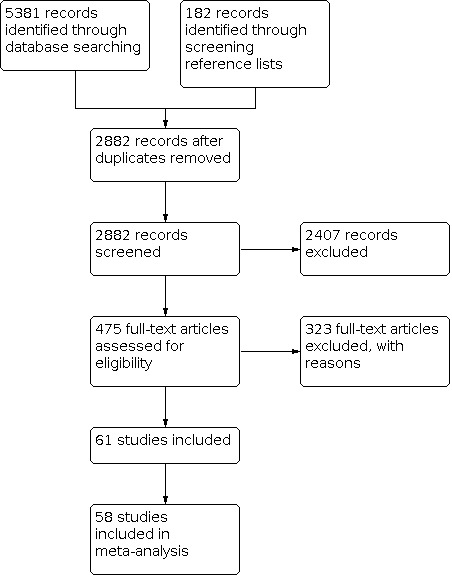
Included studies
Study locations and participants
We included 61 parallel‐arm individual RCTs that randomised 6925 participants to either low‐carbohydrate or balanced‐carbohydrate weight‐reducing diets, and were published between 1978 and 2021. All trials were done in high‐income countries, except for one in China (Liu 2013); with 26 in the USA (Aude 2004; Bales 2017; Bazzano 2014; Brehm 2003; Brehm 2005; Cornier 2005; Ebbeling 2007;Evangelista 2021 Foraker 2014; Foster 2003; Foster 2010; Gardner 2007; Kitabchi 2013; Landers 2002; Lasker 2008; Layman 2005; Layman 2009; Pittas 2005; Racette 1995; Ruth 2013; Sacks 2009; Samaha 2003; Saslow 2017a; Stentz 2016; Volek 2009; Westman 2008); 14 in Australia (Benassi‐Evans 2009; Farnsworth 2003; Griffin 2013; Jesudason 2013; Keogh 2007; Larsen 2011; Lim 2010; Parr 2016; Pedersen 2014; Tay 2008; Tay 2014; Watson 2016; Wycherley 2010; Wycherley 2012); five in Spain (Calleja‐Fernández 2012; Goni 2018; Juanola‐Falgarona 2014; Marco‐Benedi 2019; Mateo‐Gallego 2017); three in the UK (Dyson 2007; Hockaday 1978; Lean 1997), two in Sweden (Guldbrand 2012; Mellberg 2014), Norway (Klemsdal 2010; Veum 2017), Germany (Frisch 2009; Haufe 2013) and Japan (Sato 2017; Yamada 2014); and one each in Canada (Josse 2011), Israel (Elhayany 2010), Singapore (Ooi 2021) and New Zealand (Krebs 2012). The large majority of trials were undertaken in outpatient clinics, medical centres and research centres (50/61 trials), with the remaining 11 not reporting a specific study setting. See the Characteristics of included studies for additional details per study.
Type 2 diabetes mellitus status of participants
We stratified trials into those in participants without T2DM (comparisons 1 and 2) and those in participants with T2DM (comparisons 3 and 4), and also separated findings where people implemented only an active weight‐reducing phase of the diets, from findings where people implemented a weight‐reducing phase followed by a weight‐maintenance phase of the diets, as follows:
comparison 1: trials in participants without T2DM including only weight‐reducing phases of diets;
comparison 2: trials in participants without T2DM including weight‐reducing phases of diets followed by weight‐maintenance phases;
comparison 3: trials in participants with T2DM including only weight‐reducing phases of diets;
comparison 4: trials in participants with T2DM including weight‐reducing phases of diets followed by weight‐maintenance phases.
We included 45/61 trials (n = 5118 randomised) in the two comparisons in participants without T2DM (comparisons 1 and 2). Of these, 36 trials explicitly excluded participants with T2DM. For the remaining trials with mixed participants or where this was unclear (n = 9), the participants' baseline characteristics (e.g. HbA1c, fasting insulin, use of diabetes medication) were not indicative of diabetes (Bales 2017; Foraker 2014; Liu 2013; Marco‐Benedi 2019), more than 90% of the sample did not have T2DM (Aude 2004; Frisch 2009), participants with unstable metabolic disease were excluded (Jesudason 2013), or no relevant information was reported or could be obtained (Layman 2005; Layman 2009).
We included 14/61 trials (n = 1604 participants randomised) in the two comparisons in participants with T2DM (comparisons 3 and 4). Thirteen of these trials were conducted in participants with diagnosed T2DM (Elhayany 2010; Evangelista 2021; Guldbrand 2012; Hockaday 1978; Krebs 2012; Larsen 2011; Pedersen 2014; Sato 2017; Tay 2014; Watson 2016; Westman 2008; Wycherley 2010; Yamada 2014). The other trial had mixed participants with eligibility criteria being diabetes or prediabetes and more than 70% of participants were on diabetes medication at baseline (Saslow 2017a).
Two trials (n = 158 participants randomised) were conducted in participants without and with T2DM and reported data separately for all (Dyson 2007), or some outcomes (Samaha 2003), which we included in the appropriate comparisons, where possible. For the descriptions of participants, treatment and control diets that follow in this section, these two trials (Dyson 2007; Samaha 2003), are included with both strata of participants (without and with T2DM).
Participants without T2DM: weight‐reducing phase only (comparison 1) and weight‐reducing phase followed by a weight‐maintenance phase (comparison 2)
Sample size: The total numbers of participants randomised to the treatment and control diet arms ranged widely across these trials. The smallest trial randomised 20 participants (Racette 1995). Other small trials with 40 or fewer participants randomised included Cornier 2005; Dyson 2007; Kitabchi 2013 and Layman 2005. The largest trial in participants without T2DM was Sacks 2009 (n = 405 randomised), with other trials including > 150 participants being Foster 2010; Frisch 2009; Haufe 2013; Jesudason 2013; and Klemsdal 2010.
Gender and age: In three of these trials, only men were eligible (Benassi‐Evans 2009; Veum 2017; Wycherley 2012), in 16 only women (Bales 2017; Brehm 2003; Brehm 2005; Cornier 2005; Foraker 2014; Gardner 2007; Griffin 2013; Jesudason 2013; Josse 2011; Kitabchi 2013; Layman 2005; Lean 1997; Liu 2013; Mateo‐Gallego 2017; Mellberg 2014; Racette 1995), and in the rest, both men and women were eligible. Mean baseline age of participants across these trials was 45 years (range: 22 to 62 years).
Weight status: At baseline, mean body weight and BMI of participants without T2DM was 93.9 kg (range: 65 to 132 kg) and 33.6 kg/m2 (range: 30 to 43 kg/m2), respectively.
Cardiovascular health and risk factors: The majority of these trials included participants without cardiovascular conditions, risk factors or events at baseline (n = 30), one included participants with and without these (Aude 2004), and for the remaining trials this was unclear (Bales 2017; Calleja‐Fernández 2012; Dyson 2007; Foraker 2014; Juanola‐Falgarona 2014; Keogh 2007; Kitabchi 2013; Lasker 2008; Layman 2005; Marco‐Benedi 2019; Mateo‐Gallego 2017; Samaha 2003; Stentz 2016; Veum 2017; Volek 2009). Mean baseline LDL cholesterol concentration across these trials was 3.28 mmol/L (range: 2.58 to 4.47 mmol/L) and mean baseline DBP was 79 mmHg (range: 62 to 92 mmHg).
Participants with T2DM: weight‐reducing phase only (comparison 3) and weight‐reducing phase followed by a weight‐maintenance phase (comparison 4)
Sample size: The smallest trial in participants with T2DM randomised a total of 24 participants to the treatment and control diet arms (Yamada 2014), with other small trials (40 or fewer participants randomised) being Dyson 2007; Saslow 2017a; and Wycherley 2010. The largest trial in participants with T2DM randomised 419 participants (Krebs 2012). Other trials including more than 130 participants were Elhayany 2010; Samaha 2003; and Tay 2014.
Gender and age: In all trials in participants with T2DM, both men and women were eligible for inclusion. Mean age of participants at baseline was 57 years (range: 50 to 65 years).
Weight status: Mean baseline body weight of participants across these trials was 96.7 kg (range: 67 to 132 kg) and mean BMI was 34.5 kg/m2 (range 25 to 42.9 kg/m2).
HbA1c: Mean baseline HbA1c of participants across these trials was 7.64% (range: 6.6% to 8.8%).
Cardiovascular health: Six of these trials included participants without cardiovascular conditions, risk factors or events at baseline (Hockaday 1978; Krebs 2012; Larsen 2011; Tay 2014; Watson 2016; Wycherley 2010), one included participants with heart failure (Evangelista 2021), and, for the rest of the trials, this was unclear. Mean baseline LDL cholesterol across these trials was 2.63 mmol/L (range: 1.7 to 3.2 mmol/L) and mean baseline DBP was 78 mmHg (range: 70 to 84 mmHg).
Treatment and control diets
Trials in participants without T2DM: weight‐reducing phase only (comparison 1) and weight‐reducing phase followed by a weight‐maintenance phase (comparison 2)
Duration: The duration of the weight‐reducing phases of diets tested in these trials ranged from 12 weeks to two years, as follows: 10 trials for 12 weeks (Aude 2004; Dyson 2007; Farnsworth 2003; Keogh 2007; Landers 2002; Liu 2013; Racette 1995; Ruth 2013; Veum 2017; Volek 2009), eight trials for 16 weeks (Brehm 2005; Cornier 2005; Goni 2018; Josse 2011; Lasker 2008; Layman 2005; Ooi 2021; Parr 2016), 12 trials for six months (Bales 2017; Brehm 2003; Foster 2003; Foster 2010; Haufe 2013; Juanola‐Falgarona 2014; Kitabchi 2013; Lean 1997; Marco‐Benedi 2019; Mateo‐Gallego 2017; Pittas 2005; Stentz 2016), 12 trials for one year (Bazzano 2014; Benassi‐Evans 2009; Calleja‐Fernández 2012; Foraker 2014; Frisch 2009; Gardner 2007; Griffin 2013; Klemsdal 2010; Layman 2009; Samaha 2003; Tay 2008; Wycherley 2012), one trial for 15 months (Lim 2010), one trial for 18 months (Ebbeling 2007), and three trials for two years (Jesudason 2013; Mellberg 2014; Sacks 2009). We used only the data from the first six months in two trials with total durations of one year (Foster 2003) and two years (Foster 2010) since the treatment diets were ineligible from six months onwards due to the prescribed incremental carbohydrate increases. The results for only the active weight‐reducing phases of the diets in participants without T2DM were reported in comparison 1.
Five of these trials also implemented a weight‐maintenance phase following the weight‐reducing phase for six weeks (Racette 1995), 40 weeks (Keogh 2007), 56 weeks (Farnsworth 2003), a variable time period with an average of 18 months (Haufe 2013) and 21 months (Dyson 2007), and these results were reported in comparison 2.
Macronutrient prescription: Table 5 contains the thresholds and ranges used to characterise the macronutrient prescriptions of the treatment and control diets in this review. In Table 6, we used the characterisations in Table 5 to summarise the macronutrient compositions of the treatment and control diets tested in each trial in participants without T2DM.
1. Thresholds and ranges used to characterise the macronutrient compositions of the treatment and control diets.
| "Very low" | "Low" | "Balanced" | "High" | |
| Carbohydrate | < 10% of TE or < 50 g per day |
< 45% of TE | 45 to 65% of TE | > 65% of TE |
| Fat | < 20% of TE | 20‐35% of TE | > 35% of TE | |
| Protein | < 10% of TE | 10‐20% of TE | > 20% of TE |
TE: total energy intake per day
2. Macronutrient compositions of the treatment diets (rows) and control diets (columns) per trial in participants without T2DM.
C: carbohydrates, F: fat, P: protein; See Table 1 for characterisations of "very low", "low", "balanced" and "high" for treatment and control diets
The extent of carbohydrate restriction in the treatment diets ranged from very low (≤ 50 g per day or < 10% of total daily energy intake) (n = 11) to low (> 50 g to 150 g per day or < 45% of total energy intake) (n = 31), with five trials examining the effects of treatment diets with incremental increases in carbohydrates from very low to low (Table 6). Implementation of these incremental increases varied widely across the five trials, with the prescribed time for following the very low‐carbohydrate diets ranging from one week to three months. Different prescriptions for incremental increases in carbohydrate intake in the later phases included increases from 10% to 27% of total energy as carbohydrates, by 5 g/day/week, by 10 g weekly, and from 20 g to 50 g per day. As expected, the protein and fat proportions of treatment diets also varied concordantly. The control diets in the majority of trials in participants without T2DM were balanced for carbohydrates, fat and protein (n = 38) (Table 6).
The most common diets compared across the trials in participants without T2DM were low‐carbohydrate, balanced‐fat and high‐protein treatment diets compared to control diets balanced for all three macronutrients (n = 19), followed by very low‐carbohydrate treatment diets with unspecified fat and protein contents compared to control diets with balanced proportions of carbohydrates, fat and protein (n = 7) (Table 6).
Total energy prescription: In most of these trials, the total energy prescription was similar, or the approach used to restrict energy intake was similar in treatment and control groups (n = 34). In nine other trials, the energy prescription was ad libitum in the treatment diets and restricted in the control diets (Aude 2004; Brehm 2003; Brehm 2005; Foster 2003; Foster 2010; Gardner 2007; Landers 2002; Liu 2013; Samaha 2003). In the remaining trials, no energy prescription was reported, or unrestricted energy intake was prescribed for both treatment and control diets (Bazzano 2014; Ebbeling 2007; Mellberg 2014; Volek 2009).
Trials in participants with T2DM: weight‐reducing phase only (comparison 3) and weight‐reducing phase followed by a weight‐maintenance phase (comparison 4)
Duration: The duration of the weight‐reducing phases of diets tested in these trials ranged from 12 weeks to two years, as follows: four trials for 12 weeks (Dyson 2007; Evangelista 2021; Larsen 2011; Watson 2016), one trial for 16 weeks (Wycherley 2010), three trials for six months (Sato 2017; Westman 2008; Yamada 2014), five trials for one year (Elhayany 2010; Hockaday 1978; Pedersen 2014; Samaha 2003; Saslow 2017a), and three trials for two years (Guldbrand 2012; Krebs 2012; Tay 2014). The results for only the active weight‐reducing phases of diets in participants with T2DM were reported in comparison 3.
Four of these trials also implemented a weight‐maintenance phase following their weight‐reducing phase for 12 weeks (Watson 2016), nine months (Larsen 2011), 12 months (Sato 2017), and 21 months (Dyson 2007), and we reported these results in comparison 4.
Macronutrient prescriptions: Table 7 summarises the macronutrient prescriptions of the treatment and control diets tested in each trial in participants with T2DM using the characterisations in Table 5.
3. Macronutrient compositions of the treatment diets (rows) and control diets (columns) per trial in participants with T2DM.
| CONTROL DIETS | ||||
| TREATMENT DIETS |
C: balanced F: balanced P: balanced |
C: balanced F: balanced P: high |
C: balanced F: low P: high |
C: balanced F: unclear P: unclear |
|
C: very low F: high P: high |
Tay 2014 | |||
|
C: very low F: unclear P: unclear |
Dyson 2007; Samaha 2003 | Saslow 2017a; Westman 2008 | ||
|
C: low F: balanced P: high |
Evangelista 2021; Krebs 2012; Larsen 2011; Pedersen 2014; Wycherley 2010 | Watson 2016 | ||
|
C: low F: high P: balanced |
Elhayany 2010; Hockaday 1978 | |||
|
C: low F: high P: high |
Guldbrand 2012 | |||
|
C: low F: unclear P: unclear |
Sato 2017; Yamada 2014 | |||
C: carbohydrates, F: fat, P: protein; See Table 1 for characterisations of "very low", "low", "balanced" and "high" for treatment and control diets
The extent of carbohydrate restriction in the treatment diets ranged from very low (≤ 50 g per day) (n = 5) to low (> 50 g to 150 g per day or < 45% of total energy intake) (n = 11). No trials in participants with T2DM tested treatment diets with incremental increases in carbohydrates. Protein and fat proportions of treatment diets varied concordantly or were not reported. The control diets in the majority of trials in participants with T2DM were balanced for carbohydrates, fat and protein (n = 13) (Table 7).
As seen in the trials in participants without T2DM, the most common treatment diets compared across the trials in participants with T2DM were 'low'‐carbohydrate, balanced‐fat and high‐protein treatment diets compared to control diets balanced for all three macronutrients (n = 5). This was followed by 'very low'‐carbohydrate treatment diets with unspecified fat and protein contents compared to control diets with balanced‐carbohydrates and unspecified fat and protein contents (n = 2) (Table 7).
Total energy prescription: In most of these trials, the total energy prescription was similar, or the approach used to restrict energy intake was similar in treatment and control groups (n = 11). In the remaining trials, the energy prescription was ad libitum in the treatment diets and restricted in the control diets (Samaha 2003; Saslow 2017a; Sato 2017; Westman 2008; Yamada 2014).
The Characteristics of included studies table contains key items from the template for intervention description and replication (TIDieR) checklist and guide (Hoffman 2014), including materials and procedures used to implement the diets; and who, how, where, and when the diets were implemented in each trial.
Funding of included trials
About 60% (n = 28) of the trials including participants without T2DM reported being funded only by non‐commercial funders, including government and public research institutes, while 36% (n = 17) reported some or all funding by food/diet industry and related private companies, such as food and nutrition product manufacturers, organisations representing the interests of food producers (e.g. Pork Council, Dairy Farmers Association) and diet book and diet product organisations (Bales 2017; Benassi‐Evans 2009; Dyson 2007; Farnsworth 2003; Frisch 2009; Griffin 2013; Josse 2011; Lasker 2008; Layman 2005; Layman 2009; Lean 1997; Liu 2013; Parr 2016; Racette 1995; Ruth 2013; Volek 2009; Wycherley 2012). Sources of funding were not reported by two trials in participants without T2DM (Aude 2004; Landers 2002).
About a third of trials including participants with T2DM (38%; n = 6) were funded solely by non‐commercial funders, with seven trials (41%) reporting some or all funding by food/diet industry and related private companies (Dyson 2007; Hockaday 1978; Larsen 2011; Sato 2017; Watson 2016; Westman 2008; Wycherley 2010), and three trials not reporting funding source (Elhayany 2010; Pedersen 2014; Yamada 2014).
Excluded studies
We contacted 64 corresponding authors for further information to assist with study inclusion. We excluded 323 full‐text articles with reasons, as follows.
Linked to an included trial but no data used: 82
No defined carbohydrate prescription used by study authors for the intervention and/or control diets: 28
Duplicate: 27
Ineligible study design: 26
Not low‐carbohydrate intervention diet: 27
Carbohydrate contents of diets not adequately defined and could not be obtained from study authors: 22
Focus of diets are on specific foods/replacements, food groups/components: 20
Both diets are not low in carbohydrates: 18
Ineligible duration: 17
Ineligible participants: 16
Diets not for intended for weight loss: 9
Both diets are low in carbohydrates: 7
Diets differ by group in other ways that may influence outcomes: 7
Control diet not balanced in carbohydrates: 4
Co‐interventions differ by group: 4
Data from the weight‐reducing phase of diets not available: 2
Record of an additional study using participants from an included trial: 4
Control diet not for weight loss: 1
Other: 2
The Characteristics of excluded studies table contains the key studies excluded with reasons. The remaining references where we could not reach the authors or information provided was not sufficient to make a clear judgement (n = 33), were included as Studies awaiting classification. The nine ongoing studies are detailed in Characteristics of ongoing studies.
Risk of bias in included studies
Risk of bias assessments for each outcome included in the summary of findings tables, along with all domain judgements, are included in the forest plots. Figure 2 provides our judgements about each risk of bias item presented as percentages across all included trials for bias arising from the randomisation process, and across all outcomes in the summary of findings tables for all other items using RoB 2. Additional information, such as support for judgements, is located in the risk of bias section (located after the Characteristics of ongoing studies).
2.

Risk of bias figure: systematic review authors’ judgements about each risk of bias item presented as percentages across all included trials for bias arising from the randomisation process, and across all outcomes in the Summary of Findings tables for all other items using the Cochrane risk of bias 2 tool
For most trials in participants without T2DM, the risk of bias arising from the randomisation process was judged as having 'some concerns' (n = 25), 10 trials were judged as low risk (Bales 2017; Dyson 2007; Ebbeling 2007; Gardner 2007; Griffin 2013; Haufe 2013; Jesudason 2013; Liu 2013; Marco‐Benedi 2019; Sacks 2009), and four trials as high risk for this domain (Keogh 2007; Parr 2016; Tay 2008; Wycherley 2012). The overall risk of bias of outcomes across trials in participants without T2DM was similar and predominantly high. High overall risk of bias judgements were mostly as a result of bias due to missing outcome data, with high proportions of missing outcome data in many trials and this not being adequately handled by recommended statistical approaches. Bias due to deviations from intended interventions also contributed to high overall risk of bias. Problems with the measurement of outcomes did not contribute to high overall risk as most outcomes were not subjective or observer‐reported; the exception was constipation, which was participant‐reported and judged as high risk for most of the trials. None of the trials for any outcome in participants without T2DM reported prespecified analysis intentions in sufficient detail to enable assessment of selection of the reported result. Three trials did not contribute any numeric data to meta‐analyses and risk of bias for these trials was not assessed, in line with guidance on implementing RoB 2 (Foster 2003; Pittas 2005; Racette 1995). Five trials did not report any outcomes included in the summary of findings tables, and we did not assess risk of bias of these (Juanola‐Falgarona 2014; Kitabchi 2013; Ruth 2013; Samaha 2003; Stentz 2016).
Bias arising from the randomisation process was judged as low risk for approximately half of the trials in participants with T2DM (n = 8) (Dyson 2007; Evangelista 2021; Krebs 2012; Larsen 2011; Pedersen 2014; Saslow 2017a; Sato 2017; Watson 2016), with the remaining trials judged as having 'some concerns' (Elhayany 2010; Guldbrand 2012; Samaha 2003; Tay 2014; Westman 2008; Wycherley 2010; Yamada 2014). Across trials in participants with T2DM, the overall risk of bias of outcomes was similar and mostly high. As in the trials in participants without T2DM, the high overall risk of bias judgements were largely driven by bias due to missing outcome data, with high proportions of missing data not being adequately handled, and to a lesser extent by bias due to deviations from intended interventions. None of the trials in participants with T2DM reported prespecified analysis intentions in sufficient detail to enable us to assess the selection of the reported result. We did not assess risk of bias for one older study as it did not contribute usable data for an outcome included in the summary of findings tables (Hockaday 1978).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
See: Table 1; Table 2; Table 3; Table 4.
We analysed results from trials in overweight and obese participants without T2DM separately from those in participants with T2DM. We also separated analyses for weight‐reducing phases of diets, and for weight‐reducing phases followed by weight‐maintenance phases. We report here the effects on the prespecified outcomes according to the four comparisons resulting from these stratifications.
We assessed the follow‐up periods for the various outcomes as short term (three to < 12 months) or long term (≥ 12 months). The exact author‐reported follow‐up periods per outcome per trial appear as footnotes in each forest plot (i.e. using weeks, months or years). To summarise the follow‐up period applicable per outcome, we also reported the specific follow‐up range across all the trials that reported on that outcome.
None of the included trials reported on the following outcomes for any of the four comparisons: all‐cause mortality, cardiovascular mortality, non‐fatal myocardial infarction, non‐fatal stroke and diagnosis of T2DM. Trials without usable data for outcomes in the summary of findings tables are summarised in Table 8 for comparisons 1 and 2 and in Table 9 for comparisons 3 and 4.
4. Trials without usable data for outcomes in Summary of findings tables in Comparisons 1 and 2 .
| Outcomes included in Summary of Findings tables | Included studies that are believed to have measured the outcome, but did not report it in a usable format |
| Change in body weight (kg) at three to < 12 months |
Foster 2003; Kitabchi 2013; Ruth 2013; Stentz 2016: reported as percentage change in weight, data not provided when authors contacted Mellberg 2014; Pittas 2005; Racette 1995; Samaha 2003: reported in a figure, data not provided when authors contacted Juanola‐Falgarona 2014: BMI reported as per the published protocol; change in weight data not provided when authors contacted Ooi 2021: did not report data from baseline to the end of the weight‐maintenance phase, only from the start to the end of the weight‐maintenance phase of 8 weeks |
| Change in body weight (kg) at ≥ 12 months |
Calleja‐Fernández 2012: reported as percentage change in weight; data not provided when authors contacted Mellberg 2014: reported in a figure; data not provided when authors contacted |
| Number of participants per group with weight loss of at least 5% at ≥ 12 months | Sacks 2009: narrative range reported across all groups randomised and not per group; data not provided when authors contacted |
| Cardiovascular mortality at ≥ 12 months | None |
| Change in DBP (mmHg) at ≥ 12 months | None |
| Change in LDL cholesterol (mmol/L) at ≥ 12 months | Layman 2009: unreadable values reported in a figure only; data not provided when authors contacted |
| Constipation at three to < 12 months | None |
5. Trials without usable data for outcomes in Summary of findings tables in Comparisons 3 and 4.
| Outcomes included in Summary of Findings tables | Included studies that are believed to have measured the outcome, but did not report it in a usable format |
| Change in body weight (kg) at three to < 12 months | Hockaday 1978: mean change and range reported, but no variance or exact P values reported; authors not contacted due to publication date of study |
| Change in body weight (kg) at ≥ 12 months | Hockaday 1978: mean change and range reported, but no variance or exact P values reported; authors not contacted due to publication date of study |
| Number of participants per group with weight loss of at least 5% at ≥ 12 months | None |
| Cardiovascular mortality at ≥ 12 months | None |
| Change in HbA1c (%) at ≥ 12 months | Samaha 2003: mean change and standard deviation reported, but numbers of participants per trial arm not reported; authors unresponsive |
| Change in DBP (mmHg) at ≥ 12 months | None |
| Change in LDL cholesterol (mmol/L) at ≥ 12 months | None |
| Constipation at three to < 12 months | Saslow 2017a: Data captured using an adaptation of the Health Symptom Checklist at 3 months; reported that participants in low‐carbohydrate diet group experienced increased constipation (mean change of 0.4, P = 0.03); no data reported for balanced‐carbohydrate diet group; data not provided when authors contacted |
The assumed risk in comparison groups was expressed as the range of change values reported in the balanced‐carbohydrate weight‐reducing diet groups across the studies that reported results for a given outcome. If only end values were reported for a given outcome, a mean change value was calculated by using the mean baseline value and subtracting it from the reported mean end value.
Comparison 1. Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only)
Table 1 presents the effects of low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets (weight‐reducing phase only) in overweight and obese participants without T2DM on short‐term (three to <12 months) and long‐term (≥ 12 months) changes in body weight, as well as on the number of participants per group with weight loss of at least 5% over the long term, long‐term change in DBP and LDL cholesterol, and short‐term constipation.
Key details about studies in this comparison, including characteristics of the intervention, population, outcomes and method of synthesis, are included in the Overview of Synthesis and Included Studies (OSIS) table (Table 10).
6. Overview of Synthesis and Included Studies (OSIS) .
| Study name (year); country of conduct | Other key detail of intervention (trial duration; extent of carbohydrate restriction; energy prescription between groups; exercise component included; what materials, when and how much was provided) | Population (sample size: intervention/ control numbers randomised) | Outcome domains with available data | Specific outcomes measured and reported | Time point of measurement | Method of synthesis |
| Comparison 1 ‐ Population and diet phase: People without T2DM; weight‐reducing phase only | ||||||
| Aude 2004 | 12 weeks; incremental carbohydrate restriction; different energy prescription; no exercise component; written diet guidelines and counselling every two weeks (1 hour initially and 30 minute sessions thereafter) provided | Women & men (n = 30/30) | Change in body weight | 1. Short‐term change in body weight | 1. 12 weeks | 1. MA |
| Bales 2017 | 6 months; low‐carbohydrate; similar energy prescription; no exercise component; individualised calorie prescription and meal plans, two individual counselling sessions and weekly group sessions thereafter provided to both groups ‐ additionally, preportioned lean meat provided to the intervention group | Women (n = 51/29) | Change in body weight | 1. Short‐term change in body weight | 1. 6 months | 1. MA |
| Bazzano 2014 | 52 weeks; very low‐carbohydrate; ad libitum energy prescription; no exercise component; recipes, sample menus, food and shopping lists, meal planners and guides, one meal replacement per day, weekly individual counselling sessions (1 hour) for the first four weeks, biweekly group sessions for five months thereafter and monthly sessions for the last six months, provided | Women & men (n = 75/73) | Change in body weight Change in blood pressure Change in blood lipids Participant‐reported adverse events |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term change in DBP 4. Long‐term change in SBP 5. Long‐term change in LDL‐c 6. Long‐term change in HDL‐c 7. Long‐term change in TC 8. Long‐term change in TG 9. Short‐term constipation 10. Long‐term constipation 11. Short‐term diarrhoea 12. Long‐term diarrhoea 13. Short‐term nausea 14. Long‐term nausea 15. Short‐term flatulence 16. Long‐term flatulence 17. Short‐term heartburn 18. Long‐term heartburn 19. Short‐term appetite change 20. Long‐term appetite change 21. Short‐term fatigue 22. Long‐term fatigue 23. Short‐term headaches 24. Long‐term headaches |
1. 6 months 2. 12 months 3. 12 months 4. 12 months 5. 12 months 6. 12 months 7. 12 months 8. 12 months 9. 6 months 10. 12 months 11. 6 months 12. 12 months 13. 6 months 14. 12 months 15. 6 months 16. 12 months 17. 6 months 18. 12 months 19. 6 months 20. 12 months 21. 6 months 22. 12 months 23. 6 months 24. 12 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA 11. MA 12. MA 13. MA 14. MA 15. MA 16. MA 17. MA 18. MA 19. MA 20. MA 21. MA 22. MA 23. MA 24. MA |
| Benassi‐Evans 2009 | 52 weeks; low carbohydrate; similar energy prescription; no exercise component; food checklists and individual fortnightly clinic visits in the weight‐loss phase (12 weeks) provided | Men (n = 16/17) | Change in body weight | 1. Short‐term change in body weight 2. Long‐term change in body weight |
1. 12 weeks 2. 52 weeks |
1. MA 2. MA |
| Brehm 2003 | 6 months; very low‐carbohydrate; different energy prescription; no exercise component; test kits for ketosis, cooking tips, food diaries, pedometers and biweekly group meetings, alternated with individual meetings, were provided | Women (n = 26/27) | Change in body weight | 1. Short‐term change in body weight | 1. 6 months | 1. MA |
| Brehm 2005 | 4 months; very low‐carbohydrate; different energy prescription; no exercise compoment; food records, pedometers and weekly counselling sessions, alternating between group (1 hour) and individual (30 minute) sessions each week, for the first half of the study were provided | Women (n = 25/25) | Change in body weight | 1. Short‐term change in body weight | 1. 4 months | 1. MA |
| Calleja‐Fernández 2012 | 1 year; low‐carbohydrate; similar energy prescription; no exercise component; written materials, daily food diary and counselling visits every two weeks for 16 weeks, and every third month thereafter, provided | Women & men (n = 21/19) | Change in body weight Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Short‐term change in BMI 4. Long‐term change in BMI 5. Long‐term change in LDL‐c 6. Long‐term change in HDL‐c 7. Long‐term change in TC 8. Long‐term change in TG |
1. 16 weeks 2. See Table 8 3. 16 weeks 4. 12 months 5. 12 months 6. 12 months 7. 12 months 8. 12 months |
1. MA 2. NR 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA |
| Cornier 2005 | 16 weeks; low‐carbohydrate; similar energy prescription; no exercise component; prepared foods for all meals and weekly meetings provided | Women (n = 11/10) | Change in body weight | 1. Short‐term change in body weight | 1. 16 weeks | 1. MA |
| Dyson 2007 | 24 months (3 months active weight loss); very low‐carbohydrate; similar energy prescription; exercise component included; recipe booklet and sessions for three months provided | Women & men (n = 6/7) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 3 months 2. 3 months |
1. MA 2. MA |
| Ebbeling 2007 | 18 months; low‐carbohydrate; ad libitum energy prescription; exercise component included; food choice lists, cooking demonstrations, 23 group workshops (1 hour each, six in the first two months and monthly thereafter), one initial private counselling (1 hour) followed by five monthly motivational phone calls (30 minutes each) provided | Women & men (n = 36/37) | Change in body weight Change in blood pressure Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term change in DBP 4. Long‐term change in SBP 5. Long‐term change in LDL‐c 6. Long‐term change in HDL‐c 7. Long‐term change in TG |
1. 26 weeks 2. 74 weeks 3. 18 months 4. 18 months 5. 18 months 6. 18 months 7. 18 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA |
| Farnsworth 2003 | 68 weeks (12 weeks active weight loss); low‐carbohydrate; similar energy prescription; no exercise component; fixed‐menu plans, key foods making up 60% of the diet and sessions in two week intervals over 16 weeks provided to both groups ‐ additionally, low‐fat cheese and skim milk powder provided to the intervention group | Women & men (n = 33/33) | Change in body weight | 1. Short‐term change in body weight | 1. 12 weeks | 1. MA |
| Foraker 2014 | 52 weeks; low‐carbohydrate; similar energy prescription; exercise component included; pedometer and weekly counselling sessions (individual or telephonic) for the first month, then every three weeks until the fourth month and every six weeks thereafter, provided | Women (n = 38/41) | Change in body weight Change in blood pressure Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term change in DBP 4. Long‐term change in SBP 5. Long‐term change in LDL‐c 6. Long‐term change in HDL‐c 7. Long‐term change in TC |
1. 34 weeks 2. 52 weeks 3. 52 weeks 4. 52 weeks 5. 52 weeks 6. 52 weeks 7. 52 weeks |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA |
| Foster 2003 | 12 months (only first 6 months eligible as low‐carbohydrate); incremental carbohydrate restriction; different energy prescription; no exercise component; books of the relevant diet, an initial individual meeting and follow‐up meetings at three and six months (15 to 30 minutes each) provided | Women & men (n = 33/30) | Change in body weight | 1. Short‐term change in body weight | 1. See Table 8 | 1. NR |
| Foster 2010 | 2 years (only first 6 months eligible as low‐carbohydrate); incremental carbohydrate restriction; different energy prescription; exercise component included; diet guidelines and group sessions (75 to 90 minutes) every other week for 20 weeks, and monthly thereafter, provided | Women & men (n = 153/154) | Change in body weight Participant‐reported adverse events |
1. Short‐term change in body weight 2. Short‐term constipation 3. Short‐term halitosis |
1. 6 months 2. 6 months 3. 6 months |
1. MA 2. MA 3. MA |
| Frisch 2009 | 12 months; low‐carbohydrate; similar energy prescription; no exercise component; ambulatory training, diet books, electronic scale and weekly individual (telephonic) counselling sessions provided | Women & men (n = 100/100) | Change in body weight Change in blood pressure Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Short‐term change in BMI 4. Long‐term change in BMI 5. Long‐term change in DBP 6. Long‐term change in SBP 7. Long‐term change in LDL‐c 8. Long‐term change in HDL‐c 9. Long‐term change in TC 10. Long‐term change in TG |
1. 6 months 2. 12 months 3. 6 months 4. 12 months 5. 12 months 6. 12 months 7. 12 months 8. 12 months 9. 12 months 10. 12 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA |
| Gardner 2007 | 12 months; incremental carbohydrate restriction; different energy prescription; no exercise component; books of the relevant diet and weekly group sessions (1 hour each) for eight weeks provided | Women (n = 77/79) | Change in body weight Change in blood pressure Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Short‐term change in BMI 4. Long‐term change in BMI 5. Long‐term change in DBP 6. Long‐term change in SBP 7. Long‐term change in LDL‐c 8. Long‐term change in HDL‐c 9. Long‐term change in non‐HDL‐c 10. Long‐term change in TG |
1. 6 months 2. 12 months 3. 6 months 4. 12 months 5. 12 months 6. 12 months 7. 12 months 8. 12 months 9. 12 months 10. 12 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA |
| Goni 2018 | 4 months; low‐carbohydrate; similar energy prescription; no exercise component; information on feeding schedules, portion sizes and cooking methods as well as counselling sessions for 16 weeks provided | Women & men (n = 72/75) | Change in body weight Participant‐reported adverse events |
1. Short‐term change in body weight 2. Short‐term change in BMI 3. Short‐term depressive symptoms 4. Short‐term anxiety symptoms |
1. 16 weeks 2. 16 weeks 3. 16 weeks 4. 16 weeks |
1. MA 2. MA 3. MA 4. MA |
| Griffin 2013 | 12 months; low‐carbohydrate; similar energy prescription; exercise component included; kitchen scales, checklists, recipe ideas, vouchers (at baseline, three, six, nine and 12 months) and weekly counselling sessions for three months, fortnightly sessions from three to six months and monthly sessions thereafter, provided | Women (n = 36/35) | Change in body weight Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term change in LDL‐c 4. Long‐term change in HDL‐c 5. Long‐term change in TC 6. Long‐term change in TG |
1. 6 months 2. 12 months 3. 12 months 4. 12 months 5. 12 months 6. 12 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA |
| Haufe 2013 | 23 to 42 months (6 months active weight loss); low‐carbohydrate; similar energy prescription; no exercise component; weekly group sessions and individual sessions every two months provided | Women & men (n = 33/33) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 6 months 2. 6 months |
1. MA 2. MA |
| Jesudason 2013 | 24 months; low‐carbohydrate; similar energy prescription; no exercise component; sample food packs or monetary vouchers (baseline, 12 and 26 weeks), protein counter, checklist, monthly group education and support sessions for the first six months, and every three months thereafter, and monthly individual or telephonic counselling provided | Women (n = 164/159) | Change in body weight | 1. Long‐term change in body weight | 1. 24 months | 1. MA |
| Josse 2011 | 16 weeks; low‐carbohydrate; similar energy prescription; exercise component included; individualised eating plans, two study drinks per day, all dairy products, measuring cups and spoons, energy expenditure device, and biweekly private (individual or small‐group) counselling sessions provided | Women (n = 31/31) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 16 weeks 2. 16 weeks |
1. MA 2. MA |
| Juanola‐Falgarona 2014 | 6 months; low‐carbohydrate; similar energy prescription; no exercise component; leaflet with general dietary recommendations, menus, seasonal receipts, access to an informative website, and individual counselling sessions at baseline and two weeks, monthly thereafter, provided | Women & men (n = 41/40) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. See Table 8 2. 6 months |
1. NR 2. MA |
| Keogh 2007 | 52 weeks (12 weeks active weight loss); low‐carbohydrate; similar energy prescription; no exercise component; counselling sessions every two weeks provided | Women & men (n = 36 in total, per group NR) | Change in body weight | 1. Short‐term change in body weight | 1. 12 weeks | 1. MA |
| Kitabchi 2013 | 6 months; low‐carbohydrate; similar energy prescription; no exercise component; individualised weekly food diary as well as fresh, prepackaged and frozen foods, shakes and meal bars, and weekly visits provided | Women (n = 14/18) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. See Table 8 2. 6 months |
1. NR 2. MA |
| Klemsdal 2010 | 12 months; low‐carbohydrate; similar energy prescription; no exercise component; dietary suggestions and recipes, as well as individual (baseline, week two, months one and nine) and group (at month two, three, four five and six) counselling sessions provided | Women & men (n = 100/102) | Change in body weight Change in blood pressure Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term change in DBP 4. Long‐term change in SBP 5. Long‐term change in LDL‐c 6. Long‐term change in HDL‐c 7. Long‐term change in TC 8. Long‐term change in TG |
1. 6 months 2. 12 months 3. 12 months 4. 12 months 5. 12 months 6. 12 months 7. 12 months 8. 12 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA |
| Landers 2002 | 12 weeks; very low‐carbohydrate; different energy prescription; no exercise component; diet materials, sample menus, carbohydrate counting booklets, exchange lists, food diaries and individual weekly counselling meetings provided | Women & men (n = 28/33) | Change in body weight | 1. Short‐term change in body weight | 1. 12 weeks | 1. MA |
| Lasker 2008 | 4 months; low‐carbohydrate; similar energy prescription; exercise component included; menu plan with meals, electronic food scales, accelerometers and weekly group meetings (1 hour) provided | Women & men (n = 32/33) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 4 months 2. 4 months |
1. MA 2. MA |
| Layman 2005 | 4 months; low‐carbohydrate; similar energy prescription; no exercise component; two‐week menu plans, food scales and weekly group sessions (1 hour) provided | Women (n = 12/12) | Change in body weight | 1. Short‐term change in body weight | 1. 16 weeks | 1. MA |
| Layman 2009 | 12 months; low‐carbohydrate; similar energy prescription; exercise component included; menu plans, electronic food scales, armband accelerometers, and weekly counselling sessions (1 hour) provided | Women & men (n = 64/66) | Change in body weight Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term change in LDL‐c 4. Long‐term change in TG |
1. 4 months 2. 12 months 3. See Table 8 4. 12 months |
1. MA 2. MA 3. NR 4. MA |
| Lean 1997 | 6 months; low‐carbohydrate; similar energy prescription; no exercise component; written materials on the relevant diet, recipes, certain foods to support compliance, six‐weekly sessions, and telephone contact to reduce attrition provided | Women (n = 53/57) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 6 months 2. 6 months |
1. MA 2. MA |
| Lim 2010 | 15 months; very low‐carbohydrate; similar energy prescription; no exercise component; meal plans, recipe information as well as uncooked, pre‐weighed key foods and dietary counselling, every two weeks for the first three months and at month three, six, nine, twelve and 15 thereafter, provided | Women & men (n = 30/30) | Change in body weight Change in blood pressure Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term change in DBP 4. Long‐term change in SBP 5. Long‐term change in LDL‐c 6. Long‐term change in HDL‐c 7. Long‐term change in TC 8. Long‐term change in TG |
1. 3 months 2. 15 months 3. 15 months 4. 15 months 5. 15 months 6. 15 months 7. 15 months 8. 15 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA |
| Liu 2013 | 12 weeks; incremental carbohydrate restriction; different energy prescription; no exercise component; all meals, and daily monitoring of intake provided | Women (n = 25/25) | Change in body weight Participant‐reported adverse events |
1. Short‐term change in body weight 2. Short‐term change in BMI 3. Short‐term constipation 4. Short‐term diarrhoea 5. Short‐term nausea 6. Short‐term stomach upset 7. Short‐term headaches |
1. 12 weeks 2. 12 weeks 3. 12 weeks 4. 12 weeks 5. 12 weeks 6. 12 weeks 7. 12 weeks |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA |
| Marco‐Benedi 2019 | 6 months; low‐carbohydrate; similar energy prescription; exercise component included; daily menus and individual counselling sessions every two weeks provided | Women & men (n = 40/40) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 6 months 2. 6 months |
1. MA 2. MA |
| Mateo‐Gallego 2017 | 6 months; low‐carbohydrate; similar energy prescription; exercise component included; recipes and individual meetings every two weeks provided | Women (n = 30/30) | Change in body weight Participant‐reported adverse events |
1. Short‐term change in body weight 2. Short‐term constipation 3. Short‐term anxiety |
1. 3 months 2. 6 months 3. 3 months |
1. MA 2. MA 3. MA |
| Mellberg 2014 | 24 months; low‐carbohydrate; ad libitum energy prescription; no exercise component; recipes, written instructions to prepare meals, and group sessions (12 within 24 months) provided | Women (n = 35/35) | Change in body weight Change in blood pressure Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Short‐term change in BMI 4. Long‐term change in BMI 5. Long‐term change in DBP 6. Long‐term change in SBP 7. Long‐term change in LDL‐c 8. Long‐term change in HDL‐c 9. Long‐term change in TC 10. Long‐term change in TG |
1. See Table 8 2. See Table 8 3. 6 months 4. 24 months 5. 24 months 6. 24 months 7. 24 months 8. 24 months 9. 24 months 10. 24 months |
1. NR 2. NR 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA |
| Ooi 2021 | 24 weeks (16 weeks active weight loss); low‐carbohydrate; similar energy prescription; no exercise component; weekly food delivery for a six‐day food supply in the first four weeks as well as lunch, dinner and snacks for six days in the subsequent 12 weeks, food checklists, accelerometers, counselling sessions every four weeks and phone calls every fortnight provided | Women & men (n = 43/44) | Change in body weight | 1. Short‐term change in body weight | 1. 16 weeks | 1. MA |
| Parr 2016 | 16 weeks; low‐carbohydrate; similar energy prescription; exercise component included; prescribed menus, 'dairy snack baskets' with two servings of dairy intended for post‐exercise, educational resources, measuring cups and spoons, fortnightly sessions and supervised resistance exercise three days per week provided | Women & men (n = 39/40) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 16 weeks 2. 16 weeks |
1. MA 2. MA |
| Pittas 2005 | 24 weeks; low‐carbohydrate; similar energy prescription; no exercise component; all food and group meetings provided | Women & men (n = 34 in total, per group NR) | Change in body weight | 1. Short‐term change in body weight | 1. See Table 8 | 1. NR |
| Racette 1995 | 18 weeks (12 weeks active weight loss); low‐carbohydrate; similar energy prescription; no exercise component; food scales, measuring cups and spoons, meal plans and recipes, and weekly group sessions, including nutrition eduction, provided | Women (n = 41 in total, per group NR) | Change in body weight | 1. Short‐term change in body weight | 1. See Table 8 | 1. NR |
| Ruth 2013 | 12 weeks; very low‐carbohydrate; similar energy prescription; no exercise component; individual meetings provided on a bi‐weekly basis | Women & men (n = 29/26) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term weight loss of ≥ 5% 3. Short‐term change in BMI 4. Short‐term BMI reduction of ≥ 5% |
1. See Table 8 2. 12 weeks 3. 12 weeks 4. 12 weeks |
1. NR 2. MA 3. MA 4. MA |
| Sacks 2009 | 2 years; low‐carbohydrate; similar energy prescription; exercise component included; structured meal plans, food diaries, food shopping lists, recipes, group sessions (1 hour) three out of every four weeks in the first six months, two out of every four weeks thereafter, and individual sessions (30 minutes) every eight weeks provided | Women & men (n = 201/204) | Change in body weight Change in blood pressure Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term weight loss of ≥ 5% 4. Long‐term change in DBP 5. Long‐term change in SBP 6. Long‐term change in LDL‐c 7. Long‐term change in HDL‐c 8. Long‐term change in TC 9. Long‐term change in TG |
1. 6 months 2. 2 years 3. See Table 8 4. 2 years 5. 2 years 6. 2 years 7. 2 years 8. 2 years 9. 2 years |
1. MA 2. MA 3. NR 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA |
| Samaha 2003 | 36 months (12 months active weight loss); very low‐carbohydrate; different energy prescription; no exercise component; diet overviews, instructional nutrition labels, sample menus and recipes, a book on counting calories and carbohydrates, dietary guidelines (control group only), and weekly sessions (2 hours) for the first month, monthly sessions (1 hour) thereafter, provided | Women & men (n = 64/68 in total, people without T2DM NR) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. See Table 8 2. 6 months |
1. NR 2. MA |
| Stentz 2016 | 6 months; low‐carbohydrate; similar energy prescription; no exercise component; daily food menus, prepackaged frozen food, weekly consultations, and phone/email consultations, as needed, provided | Women & men (n = 18/20) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. See Table 8 2. 6 months |
1. NR 2. MA |
| Tay 2008 | 52 weeks; very low‐carbohydrate; similar energy prescription; no exercise component; key foods for the relevant macronutrient profile, food vouchers, scales, and fortnightly sessions for the first eight weeks, monthly thereafter, provided | Women & men (n = 57/61) | Change in body weight Change in blood pressure Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Short‐term change in BMI 4. Long‐term change in BMI 5. Short‐term weight loss of ≥ 5% 6. Long‐term weight loss of ≥ 5% 7. Long‐term change in DBP 8. Long‐term change in SBP 9. Long‐term change in LDL‐c 10. Long‐term change in HDL‐c 11. Long‐term change in non‐HDL‐c 12. Long‐term change in TC 13. Long‐term change in TG |
1. 24 weeks 2. 12 months 3. 24 weeks 4. 12 months 5. 24 weeks 6. 12 months 7. 12 months 8. 12 months 9. 12 months 10. 12 months 11. 12 months 12. 12 months 13. 12 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA 11. MA 12. MA 13. MA |
| Veum 2017 | 12 weeks; very low‐carbohydrate; similar energy prescription; no exercise component; food products, recipe booklets and meetings (15 minutes) at four, eight and twelve weeks provided | Men (n = 24/22) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 12 weeks 2. 12 weeks |
1. MA 2. MA |
| Volek 2009 | 12 weeks; very low‐carbohydrate; ad libitum energy prescription; no exercise component; booklets outlining dietary goals, food lists, recipes, meal plans, multivitamin and ‐mineral complex, urine reagent strips to test for ketosis, and weekly counselling sessions provided | Women & men (n = 20/20) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term weight loss of ≥ 5% 3. Short‐term change in BMI |
1. 12 weeks 2. 12 weeks 3. 12 weeks |
1. MA 2. MA 3. MA |
| Wycherley 2012 | 52 weeks; low‐carbohydrate; similar energy prescription; no exercise component; diet‐specific key foods, digital weighing scales, individual sessions at baseline and every two weeks during the first 12 weeks, monthly thereafter, provided | Men (n = 59/64) | Change in body weight Change in blood pressure Change in blood lipids Participant‐reported adverse events |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term weight loss of ≥ 5% 4. Long‐term change in DBP 5. Long‐term change in SBP 6. Long‐term change in LDL‐c 7. Long‐term change in HDL‐c 8. Long‐term change in TC 9. Long‐term change in TG 10. Short‐term total mood disturbances 11. Long‐term total mood disturbances |
1. 12 weeks 2. 52 weeks 3. 52 weeks 4. 52 weeks 5. 52 weeks 6. 52 weeks 7. 52 weeks 8. 52 weeks 9. 52 weeks 10. 12 weeks 11. 52 weeks |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA 11. MA |
| Comparison 2 ‐ Population and diet phase: People without T2DM; weight‐reducing phase followed by maintenance phase | ||||||
| Dyson 2007 | 24 months (21 months of weight maintenance); very low‐carbohydrate; similar energy prescription; exercise component included; no further intervention components provided in this phase | Women & men (n = 6/7) | Change in body weight | 1. Long‐term change in body weight | 1. 24 months | 1. MA |
| Haufe 2013 | 23 to 42 months (17 to 36 months of weight maintenance); low‐carbohydrate; similar energy prescription; no exercise component; no further intervention components provided in this phase | Women & men (n = 33/33) | Change in body weight Change in blood lipids |
1. Long‐term change in body weight 2. Long‐term change in LDL‐c 3. Long‐term change in HDL‐c 4. Long‐term change in TC 5. Long‐term change in TG |
1. 24 months (average) 2. 24 months (average) 3. 24 months (average) 4. 24 months (average) 5. 24 months (average) |
1. MA 2. MA 3. MA 4. MA 5. MA |
| Keogh 2007 | 52 weeks (40 weeks of weight maintenance); low‐carbohydrate; similar energy prescription; no exercise component; monthly counselling sessions provided | Women & men (n = 36 in total, per group NR) | Change in body weight Change in blood pressure Change in blood lipids |
1. Long‐term change in body weight 2. Long‐term change in DBP 3. Long‐term change in SBP 4. Long‐term change in LDL‐c 5. Long‐term change in HDL‐c 6. Long‐term change in TC 7. Long‐term change in TG |
1. 52 weeks 2. 52 weeks 3. 52 weeks 4. 52 weeks 5. 52 weeks 6. 52 weeks 7. 52 weeks |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA |
| Comparison 3 ‐ Population and diet phase: People with T2DM; weight‐reducing phase only | ||||||
| Dyson 2007 | 24 months (3 months active weight loss); very low‐carbohydrate; similar energy prescription; exercise component included; recipe booklet and sessions for three months provided | Women & men (n = 6/7) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 3 months 2. 3 months |
1. MA 2. MA |
| Elhayany 2010 | 12 months; low‐carbohydrate; similar energy prescription; exercise component included; meetings in a community setting every two weeks provided | Women & men (n = 85/89) | Change in body weight Change in blood glucose Change in blood lipids |
1. Long‐term change in body weight 2. Long‐term change in BMI 3. Long‐term change in HbA1c 4. Long‐term change in LDL‐c 5. Long‐term change in HDL‐c 6. Long‐term change in TC 7. Long‐term change in TG |
1. 12 months 2. 12 months 3. 12 months 4. 12 months 5. 12 months 6. 12 months 7. 12 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA |
| Evangelista 2021 | 15 months (3 months active weight loss); low‐carbohydrate; similar energy prescription; exercise component included; participant handbook with resources and tools, pedometer, and counselling sessions (45 to 60 minutes) at baseline, two, four eight and twelve weeks provided | Women & men (n = 45/45) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 3 months 2. 3 months |
1. MA 2. MA |
| Guldbrand 2012 | 2 years; low‐carbohydrate; similar energy prescription; no exercise component; menus for meal suggestions, weighing scales, notebooks, and group sessions (60 minutes) at baseline, two, six and twelve months provided | Women & men (n = 30/31) | Change in body weight Change in blood pressure Change in blood glucose Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Short‐term change in BMI 4. Long‐term change in BMI 5. Long‐term change in DBP 6. Long‐term change in SBP 7. Long‐term change in HbA1c 8. Long‐term change in LDL‐c 9. Long‐term change in HDL‐c 10. Long‐term change in TC 11. Long‐term change in TG |
1. 6 months 2. 24 months 3. 6 months 4. 24 months 5. 24 months 6. 24 months 7. 24 months 8. 24 months 9. 24 months 10. 24 months 11. 24 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA 11. MA |
| Hockaday 1978 | 12 months; low‐carbohydrate; similar energy prescription; no exercise component; counselling sessions, one month from baseline and every three months thereafter, provided | Women & men (n = 54/39) | Change in body weight Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term change in TC 4. Long‐term change in TG |
1. See Table 9 2. See Table 9 3. 12 months 4. 12 months |
1. NR 2. NR 3. MA 4. MA |
| Krebs 2012 | 24 months; low‐carbohydrate; similar energy prescription; no exercise component; portion charts, sample diet plans, culturally appropriate recipes, individual session at baseline, and group sessions (1 hour), every two weeks for the first six months and every month for the next six months, provided ‐ no further instructions were offered in the second 12 months | Women & men (n = 207/212) | Change in body weight Change in blood pressure Change in blood glucose Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term change in DBP 4. Long‐term change in SBP 5. Long‐term change in HbA1c 6. Long‐term change in LDL‐c 7. Long‐term change in HDL‐c 8. Long‐term change in TC |
1. 6 months 2. 24 months 3. 24 months 4. 24 months 5. 24 months 6. 24 months 7. 24 months 8. 24 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA |
| Larsen 2011 | 12 months (3 months active weight loss); low‐carbohydrate; similar energy prescription; exercise component included; information on key messages, menu plans, food choice lists, four individual sessions (2.5 hours), and one group session (3.3 hours) provided | Women & men (n = 57/51) | Change in body weight | 1. Short‐term change in body weight | 1. 3 months | 1. MA |
| Pedersen 2014 | 52 weeks; low‐carbohydrate; similar energy prescription; no exercise component; Continuous Glucose Measuring System 48 hours prior to assessment, daily food checklists, food frequency questionnnaires, and contact sessions (frequency NR) provided | Women & men (n = 76 in total, per group NR) | Change in body weight Change in blood pressure Change in blood glucose Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term weight loss of ≥ 5% 4. Long‐term change in DBP 5. Long‐term change in SBP 6. Long‐term change in HbA1c 7. Long‐term change in LDL‐c 8. Long‐term change in HDL‐c 9. Long‐term change in non‐HDL‐c 10. Long‐term change in TC 11. Long‐term change in TG |
1. 6 months 2. 12 months 3. 12 months 4. 12 months 5. 12 months 6. 12 months 7. 12 months 8. 12 months 9. 12 months 10. 12 months 11. 12 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA 11. MA |
| Samaha 2003 | 36 months (12 months active weight loss); very low‐carbohydrate; different energy prescription; no exercise component; diet overviews, instructional nutrition labels, sample menus and recipes, a book on counting calories and carbohydrates, dietary guidelines (control group only), and weekly sessions (2 hours) for the first month, monthly sessions (1 hour) thereafter, provided | Women & men (n = 64/68 in total, people with T2DM NR) | Change in body weight Change in blood pressure Change in blood glucose Change in blood lipids |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Short‐term change in BMI 4. Long‐term change in DBP 5. Long‐term change in SBP 6. Long‐term change in HbA1c 7. Long‐term change in LDL‐c 8. Long‐term change in HDL‐c 9. Long‐term change in TC 10. Long‐term change in TG |
1. 6 months 2. 1 year 3. 6 months 4. 1 year 5. 1 year 6. See Table 9 7. 1 year 8. 1 year 9. 1 year 10. 1 year |
1. MA 2. MA 3. MA 4. MA 5. MA 6. NR 7. MA 8. MA 9. MA 10. MA |
| Saslow 2017a | 12 months; very low‐carbohydrate; different energy prescription; no exercise component; Abbott Precision Xtra Monitoring System, blood ketone test strips, home glucose meter (for participants on diabetes medication other than metformin), audio CDs for meditation, twelve weekly classes (2 hours), three classes every two weeks (2 hours), and four classes every two months (1.5 hours) provided | Women & men (n = 16/18) | Change in body weight Change in blood pressure Change in blood glucose Change in blood lipids Participant‐reported adverse events |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Short‐term change in BMI 4. Long‐term change in BMI 5. Long‐term change in DBP 6. Long‐term change in SBP 7. Long‐term change in HbA1c 8. Long‐term change in LDL‐c 9. Long‐term change in HDL‐c 10. Long‐term change in TG 11. Short‐term constipation 12. Short‐term heartburn symptoms 13. Short‐term appetite change 14. Short‐term depressive symptoms |
1. 6 months 2. 12 months 3. 6 months 4. 12 months 5. 12 months 6. 12 months 7. 12 months 8. 12 months 9. 12 months 10. 12 months 11. See Table 9 12. 3 months 13. 3 months 14. 3 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA 11. NR 12. MA 13. MA 14. MA |
| Sato 2017 | 18 months (6 months active weight loss); low‐carbohydrate; different energy prescription; no exercise component; dietary recommendations and other written materials, and individual sessions, a detailed initial meeting and nutrition meetings (30 minutes) at one, two four and six months thereafter, provided | Women & men (n = 33/33) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 6 months 2. 6 months |
1. MA 2. MA |
| Tay 2014 | 2 years; very low‐carbohydrate; similar energy prescription; exercise component included; key foods or food vouchers on alternate months, and biweekly individual sessions for twelve weeks, monthly sessions thereafter, provided | Women & men (n = 64/67) | Change in body weight Change in blood pressure Change in blood glucose Change in blood lipids Participant‐reported adverse events |
1. Short‐term change in body weight 2. Long‐term change in body weight 3. Long‐term weight loss of ≥ 5% 4. Short‐term change in BMI 5. Long‐term change in BMI 6. Long‐term change in DBP 7. Long‐term change in SBP 8. Long‐term change in HbA1c 9. Long‐term change in LDL‐c 10. Long‐term change in HDL‐c 11. Long‐term change in non‐HDL‐c 12. Long‐term change in TC 13. Long‐term change in TG 14. Short‐term constipation 15. Long‐term constipation |
1. 24 weeks 2. 2 years 3. 2 years 4. 24 weeks 5. 2 years 6. 2 years 7. 2 years 8. 52 weeks 9. 2 years 10. 2 years 11. 2 years 12. 2 years 13. 2 years 14. 24 weeks 15. 52 weeks |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA 9. MA 10. MA 11. MA 12. MA 13. MA 14. MA 15. MA |
| Watson 2016 | 24 weeks (12 weeks active weight loss); low‐carbohydrate; similar energy prescription; exercise component included; core study foods, and dietary advice sessions every two weeks provided | Women & men (n = 32/31) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term weight loss of ≥ 5% 3. Short‐term change in BMI |
1. 12 weeks 2. 12 weeks 3. 12 weeks |
1. MA 2. MA 3. MA |
| Westman 2008 | 24 weeks; very low‐carbohydrate; different energy prescription; exercise component included; lay‐press diet book, additional handouts, individual sessions (duration and frequency NR), and group sessions every week for three months, every other week thereafter, provided | Women & men (n = 48/49) | Change in body weight Participant‐reported adverse events |
1. Short‐term change in body weight 2. Short‐term change in BMI 3. Short‐term constipation 4. Short‐term diarrhoea 5. Short‐term headaches |
1. 24 weeks 2. 24 weeks 3. 24 weeks 4. 24 weeks 5. 24 weeks |
1. MA 2. MA 3. MA 4. MA 5. MA |
| Wycherley 2010 | 16 weeks; low‐carbohydrate; similar energy prescription; no exercise component; key foods representative of the relevant diet, scale to weigh food, and counselling sessions every two weeks provided | Women & men (n = 21/19) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 16 weeks 2. 16 weeks |
1. MA 2. MA |
| Yamada 2014 | 6 months; low‐carbohydrate; different energy prescription; no exercise component; written dietary recommendations and two monthly follow‐up visits provided | Women & men (n = 12/12) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 6 months 2. 6 months |
1. MA 2. MA |
| Comparison 4 ‐ Population and diet phase: People with T2DM; weight‐reducing phase followed by maintenance phase | ||||||
| Dyson 2007 | 24 months (21 months of weight maintenance); very low‐carbohydrate; similar energy prescription; exercise component included; no further intervention components provided in this phase | Women & men (n = 6/7) | Change in body weight Change in blood glucose |
1. Long‐term change in body weight 2. Long‐term change in HbA1c |
1. 24 months 2. 24 months |
1. MA 2. MA |
| Larsen 2011 | 12 months (9 months of weight maintenance); low‐carbohydrate; similar energy prescription; exercise component included; five individual sessions (2.5 hours), and group sessions (3.3 hours) every three months provided | Women & men (n = 57/51) | Change in body weight Change in blood pressure Change in blood glucose Change in blood lipids |
1. Long‐term change in body weight 2. Long‐term change in DBP 3. Long‐term change in SBP 4. Long‐term change in HbA1c 5. Long‐term change in LDL‐c 6. Long‐term change in HDL‐c 7. Long‐term change in TC 8. Long‐term change in TG |
1. 12 months 2. 12 months 3. 12 months 4. 12 months 5. 12 months 6. 12 months 7. 12 months 8. 12 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA 7. MA 8. MA |
| Sato 2017 | 18 months (12 months of weight maintenance); low‐carbohydrate; different energy prescription; no exercise component; one individual session provided | Women & men (n = 33/33) | Change in body weight Change in blood glucose Change in blood lipids |
1. Long‐term change in body weight 2. Long‐term change in BMI 3. Long‐term change in HbA1c 4. Long‐term change in LDL‐c 5. Long‐term change in HDL‐c 6. Long‐term change in TG |
1. 18 months 2. 18 months 3. 18 months 4. 18 months 5. 18 months 6. 18 months |
1. MA 2. MA 3. MA 4. MA 5. MA 6. MA |
| Watson 2016 | 24 weeks (12 weeks of weight maintenance); low‐carbohydrate; similar energy prescription; exercise component included; core study foods, and dietary advice sessions every two weeks provided | Women & men (n = 32/31) | Change in body weight | 1. Short‐term change in body weight 2. Short‐term change in BMI |
1. 24 weeks 2. 24 weeks |
1. MA 2. MA |
BMI, DBP, diastolic blood pressure HbA1c, HDL‐c, high density lipoprotein‐cholesterol LDL‐c, low density lipoprotein‐cholesterol MA, meta‐analysis NR, not reported SBP, systolic blood pressure T2DM, type 2 diabetes mellitus TC, total cholesterol TG, triglycerides
Primary outcomes
Change in body weight (kg) at three to < 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in body weight over three to 8.5 months compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (moderate‐certainty evidence, downgraded once for risk of bias).
Average weight reductions from baseline over the short term ranged from 12.18 kg to 4.45 kg with low‐carbohydrate weight‐reducing diets and from 11.34 kg to 2.3 kg with balanced‐carbohydrate weight‐reducing diets in the 37 trials that reported this outcome. The meta‐analysis showed little to no difference in weight reduction on average between low‐carbohydrate and balanced‐carbohydrate diet groups (MD ‐1.07 kg, (95% confidence interval (CI) ‐1.55 to ‐0.59, I2 = 51%, 3286 participants, 37 RCTs, moderate‐certainty evidence, Analysis 1.1) over three to 8.5 months. This lack of a difference in effect was confirmed by sensitivity analyses, including only trials with 'some concerns' of bias overall (Analysis 1.6; no trials at overall low risk of bias), those at low risk of attrition bias (Analysis 1.7) and those without reported diet/food industry funding (Analysis 1.8).
1.1. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 1: Change in body weight (kg) at 3 to < 12 months
1.6. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 6: Change in body weight (kg) at 3 to < 12 months: sensitivity analysis overall risk of bias
1.7. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 7: Change in body weight (kg) at 3 to < 12 months: sensitivity analysis attrition domain
1.8. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 8: Change in body weight (kg) at 3 to < 12 months: sensitivity analysis source of funding
Subgrouping by similarity of energy prescription (Analysis 1.2), extent of carbohydrate restriction (Analysis 1.3), diagnosed cardiovascular event or disease at baseline (Analysis 1.4) or gender (Analysis 1.5) did not suggest important clinical differences in average effects between subgroups, with mean differences in weight reduction in overweight and obese participants at three to < 12 months ranging between 0.25 kg and 2.71 kg across the various subgroups. Subgrouping by similarity of energy prescription and extent of carbohydrate restriction explained some heterogeneity. Heterogeneity was reduced (I2 = 0%) in the subgroup including only trials with similar energy prescriptions/approaches to energy restriction in both diets, and in the subgroup including only trials investigating low‐carbohydrate diets (> 50 g to 150 g per day or < 45% of total energy intake). We did not downgrade for inconsistency. The subgroup including only trials with no energy prescriptions or unrestricted energy prescriptions in both diets had small total numbers of participants (n < 400). This was also the case for the subgroup of trials only including men.
1.2. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 2: Change in body weight (kg) at 3 to < 12 months: subgroup similarity of energy prescription
1.3. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 3: Change in body weight (kg) at 3 to < 12 months: subgroup extent of carbohydrate restriction
1.4. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 4: Change in body weight (kg) at 3 to < 12 months: subgroup diagnosed cardiovascular event or disease
1.5. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 5: Change in body weight (kg) at 3 to < 12 months: subgroup gender
Nine trials reported this outcome in an unusable format (e.g. in figures or as percentage change in weight from baseline) and usable data were not provided when authors were contacted (Table 8). The funnel plot (Figure 3) shows most trials had similar standard errors of intervention effect estimates limiting the ability to assess asymmetry. The mean difference in fixed‐effect analysis, which gives less weight to small studies (‐0.91 kg, 95% CI ‐1.21 to ‐0.62), is similar to the mean difference in random‐effects meta‐analysis (‐1.07 kg, 95% CI ‐1.55 to ‐0.59, Analysis 1.1), which gives more weight to smaller studies, suggesting that any small‐study effects have little effect on the intervention effect estimate.
3.

Funnel plot for change in weight at 3 to < 12 months (Analysis 1.1) in comparison 1
Thirteen of the 37 trials had follow‐up at three months from baseline, nine at four months, 13 at six months, one at 26 weeks (about 6.5 months), and one trial at 34 weeks (about 8.5 months)
Change in body weight (kg) at ≥ 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets probably result in little to no difference to change in body weight over one to two years compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (moderate‐certainty evidence, downgraded once for risk of bias).
Long‐term average weight reductions in the 14 trials that reported this outcome ranged from 13.1 kg to 2.46 kg in low‐carbohydrate diet groups and from 11.6 kg to 1.7 kg in balanced‐carbohydrate diet groups. The meta‐analysis of the mean difference in weight reduction between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one to two years (MD ‐0.93 kg, 95% CI ‐1.81 to ‐0.04, I2 = 40%, 1805 participants, 14 RCTs, moderate‐certainty evidence, Analysis 1.9). Sensitivity analyses including only trials at low risk of attrition bias (Analysis 1.10) and trials without diet/food industry funding confirmed this lack of a difference in effect (Analysis 1.11). Other planned sensitivity analyses included fewer than two studies and thus were not performed.
1.9. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 9: Change in body weight (kg) at ≥ 12 months
1.10. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 10: Change in body weight (kg) at ≥ 12 months: sensitivity analysis attrition domain
1.11. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 11: Change in body weight (kg) at ≥ 12 months: sensitivity analysis source of funding
Two trials reported this outcome in an unusable format (e.g. in figures or as percentage change in weight from baseline) and usable data were not provided when authors were contacted (Table 8). The funnel plot (Figure 4) suggests that smaller studies may be missing. The mean difference in fixed‐effect analysis, which gave less weight to small studies (‐0.81 kg, 95% CI ‐1.43 to ‐0.19), was similar to the mean difference in the random‐effects meta‐analysis (‐0.93 kg, 95% CI ‐1.81 to ‐0.04, Analysis 1.9), which gave more weight to smaller studies, suggesting that any small‐study effects had little effect on the intervention effect estimate.
4.

Funnel plot for change in weight at ≥ 12 months (Analysis 1.9) in comparison 1
Ten of the 14 trials had follow‐up at one year, one at 15 months, one at 74 weeks (about 18.5 months), with the two longest trials having follow‐up at two years.
Number of participants per group with weight loss of at least 5% at three to < 12 months
The number of participants achieving at least 5% weight loss over the short term in the three trials reporting this outcome ranged from 14 to 41 with low‐carbohydrate weight‐reducing diets and from eight to 35 with balanced‐carbohydrate weight‐reducing diets. The meta‐analysis of the risk ratio (RR) of the number of participants per group with weight loss of at least 5% at three to six months was 1.30 (95% CI 1.00 to 1.68, I2 = 47%, 161 participants, 3 RCTs, Analysis 1.12) when comparing low‐carbohydrate and balanced‐carbohydrate diet groups. Two of the three trials that reported this outcome had follow‐up at three months and the other at six months.
1.12. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 12: Number of participants per group with weight loss of at least 5% at 3 to < 12 months
Number of participants per group with weight loss of at least 5% at ≥ 12 months
GRADE assessment suggests that the evidence is very uncertain about whether there is a difference in the number of participants per group with weight loss of at least 5% at one year, when comparing low‐carbohydrate weight‐reducing diets with balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (very low‐certainty evidence; downgraded once for risk of bias and twice for imprecision).
Two studies reported on this outcome at one year (RR 1.11, 95% CI 0.94 to 1.31; I² = 17%; 137 participants, 2 RCTs, very low‐certainty evidence, Analysis 1.13), with the number of participants achieving at least 5% weight loss ranging from 28 to 30 in the low‐carbohydrate weight‐reducing group and from 27 to 29 in the balanced‐carbohydrate weight‐reducing group. Another included trial also measured this outcome at two years but it was not reported in a usable format (Table 8).
1.13. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 13: Number of participants per group with weight loss of at least 5% at ≥ 12 months
Secondary outcomes
For comparison 1, no trials reported on the number of participants per group with reduction in BMI (kg/m2) of at least 5% at ≥ 12 months.
Change in BMI (kg/m2) at three to < 12 months
Twenty‐one trials (n = 1517 participants) reported on short‐term BMI change for comparison 1, with five of the 20 trials having follow‐up at three months, five at four months and 11 trials at six months. Across the trials, average reduction in BMI from baseline ranged from 4.7 kg/m2 to 1.8 kg/m2 with low‐carbohydrate diets and from 3.7 kg/m2 to 0.98 kg/m2 with balanced‐carbohydrate diet groups. We did not pool these results into an overall effect estimate due to substantial heterogeneity (I² = 53%, Tau² = 0.18; Chi² = 42.52, df = 20 (P = 0.002)) but rather presented the individual effect sizes between the low‐carbohydrate and balanced‐carbohydrate diets per study (Analysis 1.14).
1.14. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 14: Change in BMI (kg/m2) at 3 to < 12 months
Change in BMI (kg/m2) at ≥ 12 months
Average reduction in BMI from baseline over the long term in the five trials reporting this outcome ranged from 5.2 kg/m2 to 1.65 kg/m2 in low‐carbohydrate diet groups and from 4.1 kg/m2 to 0.92 kg/m2 in balanced‐carbohydrate diet groups. When low‐carbohydrate weight‐reducing diets were compared to balanced‐carbohydrate weight‐reducing diets, the difference in BMI change on average at one to two years was ‐0.61 kg/m2 (95% CI ‐0.99 to ‐0.23, I2 = 0%, 490 participants, 5 RCTs, Analysis 1.15). Four trials had follow‐up at one year and the remaining trial at two years.
1.15. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 15: Change in BMI (kg/m2) at ≥ 12 months
Number of participants per group with reduction in BMI (kg/m2) of at least 5% at three to < 12 months
A single small study reported on this outcome at three months and the risk ratio was 1.57 (95% CI 0.79 to 3.12, 55 participants, 1 RCT, Analysis 1.16) with 14 participants in the low‐carbohydrate weight‐reducing group and eight participants in the balanced‐carbohydrate weight‐reducing group achieving at least 5% reduction in BMI.
1.16. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 16: Number of participants per group with reduction in BMI of at least 5% at 3 to < 12 months
Change in DBP (mmHg) at ≥ 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets probably result in little to no difference to change in DBP compared to balanced‐carbohydrate weight‐reducing diets over one to two years in overweight and obese participants without T2DM (moderate‐certainty evidence, downgraded once for risk of bias).
Over one to two years, average change in DBP from baseline ranged from a 9 mmHg reduction to a 4 mmHg increase with low‐carbohydrate diets and from an 11 mmHg reduction to 2.9 mmHg increase with balanced‐carbohydrate diets across the 11 trials that reported this. The meta‐analysis of the mean difference in change in DBP between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one to two years (MD ‐0.09 mmHg, 95% CI ‐1.29 to 1.12, I2 = 44%, 1419 participants, 11 RCTs, moderate‐certainty evidence, Analysis 1.17). Seven of the 11 trials had follow‐up at one year, one at 15 months, one at 18 months and the other two trials at two years.
1.17. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 17: Change in DBP (mmHg) at ≥ 12 months
Change in SBP (mmHg) at ≥ 12 months
Average change in SBP from baseline in the 11 trials reporting this outcome ranged from reductions of 13.8 mmHg to 0.2 mmHg in low‐carbohydrate diet groups and from a reduction of 14.6 mmHg to an increase of 1.7 mmHg in balanced‐carbohydrate diet groups over one to two years of follow‐up. For this comparison, the mean difference in change in SBP at one to two years was ‐1.37 mmHg (95% CI ‐2.99 to 0.24, I2 = 34%, 1397 participants, 11 RCTs, Analysis 1.18). Seven of the 11 trials had follow‐up at one year, one at 15 months, one at 18 months and the other two trials at two years.
1.18. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 18: Change in SBP (mmHg) at ≥ 12 months
Change in LDL cholesterol (mmol/L) at ≥ 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in LDL cholesterol over one to two years compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (moderate‐certainty evidence, downgraded once for risk of bias).
Over one to two years, average change in LDL cholesterol from baseline across the 13 trials that reported this outcome ranged from reduction of 0.3 mmol/L to an increase of 0.6 mmol/L with low‐carbohydrate diets and from a reduction of 0.31 mmol/L to an increase of 0.1 mmol/L with balanced‐carbohydrate diets. The meta‐analysis of the mean difference in change in LDL cholesterol between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one to two years (MD 0.04 mmol/L 95% CI ‐0.05 to 0.12, I2 = 33%, 1494 participants, 13 RCTs, moderate‐certainty evidence, Analysis 1.19). Nine of the 13 trials had follow‐up at one year, one at 15 months, one at 18 months and the other two trials at two years. One of the included trials measured this outcome but it was not reported in a usable format (Table 8).
1.19. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 19: Change in LDL cholesterol (mmol/L) at ≥ 12 months
Change in HDL cholesterol (mmol/L) at ≥ 12 months
Average change in HDL cholesterol from baseline over one to two years ranged from a reduction of 0.1 mmol/L to an increase of 0.3 mmol/L in low‐carbohydrate diet groups and from a reduction of 0.21 mmol/L to an increase of 0.18 mmol/L in balanced‐carbohydrate diet groups in the 13 trials that reported this outcome. When low‐carbohydrate weight‐reducing diets were compared to balanced‐carbohydrate weight‐reducing diets, the mean difference in change in HDL cholesterol at one to two years was 0.06 mmol/L (95% CI 0.02 to 0.10, I2 = 48%, 1519 participants, 13 RCTs, Analysis 1.20). Nine of the 13 trials had follow‐up at one year, one at 15 months, one at 18 months and the other two trials at two years.
1.20. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 20: Change in HDL cholesterol (mmol/L) at ≥ 12 months
Change in non‐HDL cholesterol (mmol/L) at ≥ 12 months
Two small studies reported on this outcome at one year and the mean difference in non‐HDL cholesterol was 0.06 mmol/L (95% CI ‐0.2 to 0.33, I2 = 47%, 225 participants, 2 RCTs, Analysis 1.21) with average change in non‐HDL ranging from a reduction of 0.13 mmol/L to an increase of 0.24 mmol/L in low‐carbohydrate weight‐reducing groups and average reductions ranging from 0.1 mmol/L to 0.02 mmol/L in balanced‐carbohydrate weight‐reducing groups.
1.21. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 21: Change in non‐HDL cholesterol (mmol/L) at ≥ 12 months
Change in total cholesterol (mmol/L) at ≥ 12 months
Over one to two years, average change in total cholesterol from baseline ranged from a reduction of 0.4 mmol/L to an increase of 0.7 mmol/L with low‐carbohydrate diets and from a reduction of 0.42 mmol/L to an increase of 0.13 mmol/L with balanced‐carbohydrate diets across the 11 trials that reported this. On average, there was no difference in change in total cholesterol at one to two years between the two types of diet (MD 0.01 mmol/L, 95% CI ‐0.1 to 0.12, I2 = 41%, 1291 participants, 11 RCTs, Analysis 1.22). Eight of the 11 trials had follow‐up at one year, one at 15 months and the other two trials at two years.
1.22. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 22: Change in total cholesterol (mmol/L) at ≥ 12 months
Change in triglycerides (mmol/L) at ≥ 12 months
Across the 13 trials that reported on triglycerides over one to two years, average change from baseline ranged from reductions of 0.58 mmol/L to 0.1 mmol/L with low‐carbohydrate diets and from a reduction of 0.44 mmol/L to an increase of 0.02 mmol/L with balanced‐carbohydrate diets. For this comparison, the mean difference in change in triglycerides at one to two years was ‐0.11 mmol/L (95% CI ‐0.16 to ‐0.06, I2 = 0%, 1559 participants, 13 RCTs, Analysis 1.23). Nine of the 13 trials had follow‐up at one year, one at 15 months, one at 18 months and the other two trials at two years.
1.23. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 23: Change in triglycerides (mmol/L) at ≥ 12 months
Participant‐reported adverse effects
Participant‐reported adverse effects were scantily reported by six included trials for comparison 1, with all outcomes except short‐term constipation being reported only by single trials, and one of the trials reporting on most of these outcomes (Bazzano 2014). All 19 dichotomous outcomes reported very few events, had small total sample sizes and had wide 95% CIs. The four continuous outcomes all had small total samples sizes, mean differences close to 0 and wide 95% CIs.
Gastrointestinal
GRADE assessment suggests that the evidence is very uncertain about whether there is a difference in the number of participants per group with constipation at three to six months when comparing low‐carbohydrate weight‐reducing diets with balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (very low‐certainty evidence, downgraded once for risk of bias and twice for imprecision).
The meta‐analysis of the risk ratio of the number of participants per group reporting constipation at three to six months was 1.06 (95% CI 0.81 to 1.38, I2 = 0%, 564 participants, 4 RCTs, very low‐certainty evidence, Analysis 1.24) when comparing the two diet groups.
1.24. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 24: Constipation at 3 to < 12 months
One trial reported constipation at one year (RR 0.63, 95% CI 0.32 to 1.25, 148 participants, 1 RCT, Analysis 1.25).
1.25. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 25: Constipation at ≥ 12 months
Two trials reported on events of diarrhoea finding no difference over the shorter term (at three months and at six months) (RR 0.98, 95% CI 0.33 to 2.93, 244 participants, 2 RCTs, Analysis 1.26) or at one year (RR 0.32, 95% CI 0.03 to 3.05, 148 participants, 1 RCT, Analysis 1.27). This was also the case for nausea over the shorter term (RR 0.73, 95% CI 0.05 to 10.68, 244 participants, 2 RCTs, Analysis 1.28) and at one year (RR 0.14, 95% CI 0.01 to 2.65, 148 participants, 1 RCT, Analysis 1.29).
1.26. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 26: Diarrhoea at 3 to < 12 months
1.27. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 27: Diarrhoea at ≥ 12 months
1.28. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 28: Nausea at 3 to < 12 months
1.29. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 29: Nausea at ≥ 12 months
One trial reported on events offlatulence at six months (RR 0.75, 95% CI 0.44 to 1.30, 148 participants, 1 RCT, Analysis 1.30) and again at one year (RR 0.68 95% CI 0.39 to 1.17, 148 participants, 1 RCT, Analysis 1.31), as well on events of heartburn at six months (RR 0.53, 95% CI 0.21 to 1.36, 148 participants, 1 RCT, Analysis 1.32) and at one year (RR 0.49, 95% CI 0.23 to 1.01, 148 participants, 1 RCT, Analysis 1.33).
1.30. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 30: Flatulence at 3 to < 12 months
1.31. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 31: Flatulence at ≥ 12 months
1.32. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 32: Heartburn at 3 to < 12 months
1.33. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 33: Heartburn at ≥ 12 months
One trial reported on halitosis at six months (RR 1.99, 95% CI 1.32 to 2.99, 263 participants, 1 RCT, Analysis 1.34) and another trial reported stomach upsets at three months (RR 1.00, 95% CI 0.41 to 2.45, 96 participants, 1 RCT, Analysis 1.35).
1.34. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 34: Halitosis at 3 to < 12 months
1.35. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 35: Stomach upset at 3 to < 12 months
Appetite and hunger
Appetite change was reported by one trial at six months follow‐up (RR 0.19, 95% CI 0.01 to 3.99, 148 participants, 1 RCT, Analysis 1.36) and at one year (RR 0.49, 95% CI 0.05 to 5.25, 148 participants, 1 RCT, Analysis 1.37).
1.36. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 36: Appetite change at 3 to < 12 months
1.37. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 37: Appetite change at ≥ 12 months
In another trial, hunger and desire to eat were gauged using a visual analog score as change from baseline at one year for this comparison. The mean score for hunger was ‐1.05 (SEM 0.56; n = 21) in the low‐carbohydrate diet group and ‐0.18 (SEM 0.66; n = 15) in the balanced‐carbohydrate diet group. Mean scores for desire to eat were ‐1.02 (SEM 0.59; n = 21) in the low‐carbohydrate diet group and ‐0.16 (SEM 0.76; n = 15) in the balanced‐carbohydrate diet group (Griffin 2013).
Fatigue
One trial reported on events offatigue at six months (RR 0.80, 95% CI 0.47 to 1.36, 148 participants, 1 RCT, Analysis 1.38) and at one year (RR 0.91, 95% CI 0.49 to 1.71, 148 participants, 1 RCT, Analysis 1.39).
1.38. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 38: Fatigue at 3 to < 12 months
1.39. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 39: Fatigue at ≥ 12 months
Headaches
Two trials reported on headaches with no difference over the shorter term (at three months and at six months) (RR 1.25, 95% CI 0.26 to 6.02, I2 = 55%, 244 participants, 2 RCTs, Analysis 1.40) or at one year (RR 0.49, 95% CI 0.25 to 0.93, 148 participants, 1 RCT, Analysis 1.41).
1.40. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 40: Headaches at 3 to < 12 months
1.41. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 41: Headaches at ≥ 12 months
Psychosocial problems (mood disturbances)
A single trial measured events of reported anxiety at three months follow‐up for comparison 1 (RR 1.0, 95% CI 0.07 to 15.26, 60 participants, 1 RCT, Analysis 1.42).
1.42. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 42: Reported anxiety at 3 to < 12 months
One trial reportedtotal mood disturbance scores according to the Profile of Mood States (POMS) at three months (MD 1.91, 95% CI ‐8.06 to 11.88, 118 participants, 1 RCT, Analysis 1.43) and one year (MD 2.87, 95% CI ‐8.48 to 14.22, 118 participants, 1 RCT, Analysis 1.44).
1.43. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 43: Total mood disturbances (participant‐reported) at 3 to < 12 months
1.44. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 44: Total mood disturbances (participant‐reported) at ≥ 12 months
At four months follow‐up, one trial reported depressive symptoms according to the Beck Depression Inventory (MD ‐0.30, 95% CI ‐1.36 to 0.76, 217 participants, 1 RCT, Analysis 1.45) and anxiety symptoms according to the State‐Trait Anxiety Inventory (MD ‐0.20, 95% CI ‐1.44 to 1.04, 217 participants, 1 RCT, Analysis 1.46).
1.45. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 45: Depressive symptoms (participant‐reported) at 3 to <12 months
1.46. Analysis.

Comparison 1: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only), Outcome 46: Anxiety symptoms (participant‐reported) at 3 to < 12 months
Comparison 2. Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase)
Table 2 presents the effects of low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets (weight‐reducing phase followed by weight‐maintenance phase) in overweight and obese participants without T2DM on long‐term (≥ 12 months) changes in body weight, DBP and LDL cholesterol.
Key details about studies in this comparison, including characteristics of the intervention, population, outcomes and method of synthesis are detailed in the OSIS table (Table 10).
Primary outcomes
The only primary outcome reported by the included trials for comparison 2 was change in body weight at ≥ 12 months.
Change in body weight (kg) at ≥ 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase may result in little to no difference in change in body weight over one to two years compared to balanced‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase in overweight and obese participants without T2DM (low‐certainty evidence, downgraded once for risk of bias and once for imprecision).
Over the long term, average weight change from baseline ranged from a reduction of 5.4 kg to an increase of 0.86 kg with low‐carbohydrate diets and from reductions of 5.5 kg to 1.5 kg with balanced‐carbohydrate diets across the three trials that reported this outcome. The meta‐analysis of the mean difference in weight reduction between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one to two years (MD ‐0.30 kg, 95% CI ‐2.77 to 2.16, I2 = 0%, 73 participants, 3 RCTs, low‐certainty evidence, Analysis 2.1). One of the trials had a weight‐reducing phase of 12 weeks followed by a weight‐maintenance phase of 40 weeks, measuring change in weight at one year. The other two trials measured change in weight at two years, with one trial testing a three‐month weight‐reducing phase followed by a 21‐month maintenance phase, and the other trial testing a 6‐month weight‐reducing phase followed by a maintenance‐phase that averaged 18 months in duration. This lack of a difference in effect was confirmed by a sensitivity analysis including only the two trials without reported diet/food industry funding (MD ‐0.80 kg, 95% CI ‐3.48 to 1.88, I2 = 0%, 63 participants, 2 RCTs, analysis not shown).
2.1. Analysis.

Comparison 2: Low‐carbohydrate weight‐reducing diets versus balanced carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 1: Change in body weight (kg) at ≥ 12 months
Secondary outcomes
The only secondary outcomes reported by the included trials for comparison 2 were changes in DBP, SBP, LDL and HDL cholesterol, total cholesterol and triglycerides.
Changes in DBP and SBP (mmHg) at ≥ 12 months
GRADE assessment suggests that the evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase compared to balanced‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase on change in DBP at one year in overweight and obese participants without T2DM (very low‐certainty evidence, downgraded once for risk of bias and twice for imprecision).
One very small trial compared these diets using a weight‐reducing phase of 12 weeks followed by a maintenance phase of 40 weeks, and reported on changes in DBP (MD ‐6.0 mmHg, 95% CI ‐17.55 to 5.55, 13 participants, 1 RCT, very low‐certainty evidence, Analysis 2.2) and SBP (MD ‐15.0 mmHg, 95% CI ‐32.36 to 2.36, 13 participants, 1 RCT, Analysis 2.3) at one year. The study reported average DBP reductions from baseline of 4 mmHg in the low‐carbohydrate diet group and of 6 mmHg in the balanced‐carbohydrate diet group. For SBP, the study reported an average decrease from baseline of 2 mmHg in the low‐carbohydrate group and an increase of 2 mmHg in the balanced‐carbohydrate group.
2.2. Analysis.

Comparison 2: Low‐carbohydrate weight‐reducing diets versus balanced carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 2: Change in DBP (mmHg) at ≥ 12 months
2.3. Analysis.

Comparison 2: Low‐carbohydrate weight‐reducing diets versus balanced carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 3: Change in SBP (mmHg) at ≥ 12 months
Changes in LDL, HDL and total cholesterol and triglycerides (mmol/L) at ≥ 12 months
Two small trials reported changes in these blood lipids at one to two years. GRADE assessment suggests that the evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase compared to balanced‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase on change in LDL at one to two years in overweight and obese participants without T2DM (very low‐certainty evidence, downgraded once for risk of bias and twice for imprecision).
One trial used a weight‐reducing phase of 12 weeks followed by a maintenance phase of 40 weeks, and the other trial used a weight‐reducing phase of six months followed by a maintenance phase of 18 months with changes reported at one and two years, respectively for LDL cholesterol (MD ‐0.08 mmol/L, 95% CI ‐0.44 to 0.28, I2 = 0%, 56 participants, 2 RCTs, very low‐certainty evidence, Analysis 2.4), HDL cholesterol (MD ‐0.05 mmol/L, 95% CI ‐0.37 to 0.28, I2 = 5%, 56 participants, 2 RCTs, Analysis 2.5), total cholesterol (MD ‐0.08 mmol/L, 95% CI ‐0.43 to 0.27, I2 = 0%, 56 participants, 2 RCTs, Analysis 2.6) and triglycerides (MD ‐0.08 mmol/L, 95% CI ‐0.34 to 0.17, I2 = 0%, 56 participants, 2 RCTs, Analysis 2.7).
2.4. Analysis.

Comparison 2: Low‐carbohydrate weight‐reducing diets versus balanced carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 4: Change in LDL cholesterol (mmol/L) at ≥ 12 months
2.5. Analysis.

Comparison 2: Low‐carbohydrate weight‐reducing diets versus balanced carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 5: Change in HDL cholesterol (mmol/L) at ≥ 12 months
2.6. Analysis.

Comparison 2: Low‐carbohydrate weight‐reducing diets versus balanced carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 6: Change in total cholesterol (mmol/L) at ≥ 12 months
2.7. Analysis.

Comparison 2: Low‐carbohydrate weight‐reducing diets versus balanced carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 7: Change in triglycerides (mmol/L) at ≥ 12 months
Average LDL cholesterol reductions from baseline ranged from 0.79 mmol/L to 0.2 mmol/L with low‐carbohydrate diets and from 1.15 mmol/L to 0.14 mmol/L with balanced‐carbohydrate diets. For HDL‐cholesterol, average changes ranged from a decrease of 0.2 mmol/L to an increase of 0.11 mmol/L with low‐carbohydrate diets and from increases of 0.03 mmol/L to 0.04 mmol/L with balanced‐carbohydrate diets. Average reductions in total cholesterol ranged from 0.24 mmol/L to 0.79 mmol/L with low‐carbohydrate diets and from 0.17 mmol/L to 1.14 mmol/L with balanced‐carbohydrate diets. For triglycerides, average reductions ranged from 0.19 mmol/L to 0.28 mmol/L with low‐carbohydrate diets and from 0.04 mmol/L to 0.13 mmol/L with balanced‐carbohydrate diets.
Comparison 3. Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐loss phase only)
Table 3 presents the effects of low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets (weight‐reducing phase only) in overweight and obese participants with T2DM on short‐term (three to < 12 months) and long‐term (≥ 12 months) changes in body weight, as well as on the number of participants per group with weight loss of at least 5% over the long term, long‐term change in DBP, HbA1c and LDL cholesterol, and short‐term constipation.
Key details about studies in this comparison, including characteristics of the intervention, population, outcomes and method of synthesis can be found in the OSIS table (Table 10).
Primary outcomes
Change in body weight (kg) at three to < 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in body weight over three to six months compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (moderate‐certainty evidence, downgraded once for risk of bias).
Average weight reductions from baseline over three to six months ranged from 12 kg to 2.41 kg with low‐carbohydrate weight‐reducing diets and from 11.5 kg to 0.33 kg with balanced‐carbohydrate weight‐reducing diets in the 14 trials reporting this outcome. The meta‐analysis showed little to no difference in weight reduction on average between low‐carbohydrate and balanced‐carbohydrate diet groups over three to six months (MD ‐1.26 kg, 95% CI ‐2.44 to ‐0.09, I2 = 47%, 1114 participants, 14 RCTs, moderate‐certainty evidence, Analysis 3.1). This lack of a difference in effect was also seen with sensitivity analyses including only trials with 'some concerns' of bias overall (Analysis 3.2, no trials at overall low risk of bias), at low risk of attrition bias (Analysis 3.3) and without reported diet/food industry funding (Analysis 3.4).
3.1. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 1: Change in body weight (kg) at 3 to < 12 months
3.2. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 2: Change in body weight (kg) at 3 to < 12 months: sensitivity analysis overall risk of bias
3.3. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 3: Change in body weight (kg) at 3 to < 12 months: sensitivity analysis attrition domain
3.4. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 4: Change in body weight (kg) at 3 to < 12 months: sensitivity analysis source of funding
One included trial also measured change in body weight at three to < 12 months but reported it in an unusable format (Table 9). The funnel plot (Figure 5) suggests that smaller studies may be missing. The mean difference in fixed‐effect analysis, which gives less weight to small studies (‐0.92 kg, 95% CI ‐1.65 to ‐0.19), is similar to the mean difference in random‐effects meta‐analysis (‐1.26 kg, 95% CI ‐2.44 to ‐0.09, Analysis 3.1), which gives more weight to smaller studies, suggesting that any small‐study effects have little effect on the intervention effect estimate.
5.

Funnel plot for change in weight at 3 to < 12 months (Analysis 3.1) in comparison 3
Four of the 14 trials had follow‐up at three months from baseline, one trial at four months and nine at six months.
Change in body weight (kg) at ≥ 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in body weight over one to two years compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (moderate‐certainty evidence, downgraded once for risk of bias).
Average weight reductions from baseline over one to two years in the seven trials reporting this outcome ranged from 8.9 kg to 2 kg with low‐carbohydrate weight‐reducing diets and from 7.4 kg to 1.7 kg with balanced‐carbohydrate weight‐reducing diets. The meta‐analysis showed little to no difference in weight reduction on average between low‐carbohydrate and balanced‐carbohydrate diet groups over one to two years (MD ‐0.33 kg, 95% CI ‐2.13 to 1.46, I2 = 10%, 813 participants, 7 RCTs, moderate‐certainty evidence, Analysis 3.5). This lack of a difference in effect was also seen with the sensitivity analysis including only trials without diet/food industry funding (Analysis 3.6). Other planned sensitivity analyses included only one or two studies and thus were not performed.
3.5. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 5: Change in body weight (kg) at ≥ 12 months
3.6. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 6: Change in body weight (kg) at ≥ 12 months: sensitivity analysis source of funding
Of the seven trials, four had follow‐up at one year and three had follow‐up at two years. The oldest included trial also measured change in body weight at ≥ 12 months but reported it in an unusable format (Table 9).
Number of participants per group with weight loss of at least 5% at three to < 12 months
A single small study reported on this outcome at three months and the risk ratio was 1.01 (95% CI 0.77 to 1.33, 45 participants, 1 RCT, Analysis 3.7) with 19 participants in the low‐carbohydrate group and 18 participants in the balanced‐carbohydrate group achieving at least 5% weight loss from baseline.
3.7. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 7: Weight loss of at least 5% at 3 to < 12 months
Number of participants per group with weight loss of at least 5% at ≥ 12 months
GRADE assessment suggests that the evidence is very uncertain about whether there is a difference in the number of participants per group with weight loss of at least 5% at one to two years, when comparing low‐carbohydrate weight‐reducing diets with balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (very low‐certainty evidence, downgraded once for risk of bias, once for indirectness and twice for imprecision).
Two studies reported on this outcome at one and two years (RR 0.90, 95% CI 0.68 to 1.20; I² = 0; 106 participants, 2 RCTs, very low‐certainty evidence, ) with the number of participants achieving at least 5% weight loss from baseline ranging from 10 to 22 in the low‐carbohydrate weight‐reducing group and 14 to 20 in the balanced‐carbohydrate weight‐reducing group.
Secondary outcomes
For comparison 3, no studies reported on the number of participants per group with reduction in BMI (kg/m2) of at least 5%.
Change in BMI (kg/m2) at three to < 12 months
Eleven trials (n = 515 participants) reported on short‐term BMI change. Across these trials, average reductions in BMI from baseline ranged from 4 kg/m2 to 0.87 kg/m2 with low‐carbohydrate diets and from 4 kg/m2 to 0.1 kg/m2 with balanced‐carbohydrate diets. We did not pool these results into an overall effect estimate due to substantial heterogeneity (I² = 57%, Tau² = 0.56; Chi² = 23.11, df = 10 (P = 0.01)) but rather presented the individual effect sizes between the two types of diet per study (Analysis 3.9). Three of the 10 trials had follow‐up at three months, one at four months and seven at six months.
3.9. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 9: Change in BMI (kg/m2) at 3 to < 12 months
Change in BMI (kg/m2) at ≥ 12 months
Average reductions in BMI from baseline over the long term in the four trials reporting this outcome ranged from 3.3 kg/m2 to 0.8 kg/m2 with low‐carbohydrate diets and from 2.6 kg/m2 to 0.9 kg/m2 with balanced‐carbohydrate diets. When low‐carbohydrate weight‐reducing diets were compared to balanced‐carbohydrate weight‐reducing diets, the difference in BMI change on average at one to two years was ‐0.29 kg/m2 (95% CI ‐1.06 to 0.48, I2 = 13%, 329 participants, 4 RCTs, Analysis 3.10). Two trials had follow‐up at one year and the remaining two at two years.
3.10. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 10: Change in BMI (kg/m2) at ≥ 12 months
Change in DBP (mmHg) at ≥ 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets probably result in little to no difference to change in DBP compared to balanced‐carbohydrate weight‐reducing diets over one to two years in overweight and obese participants with T2DM (moderate‐certainty evidence, downgraded once for risk of bias).
Over one to two years, average changes from baseline in DBP ranged from a reduction of 5 mmHg to an increase 3 mmHg in low‐carbohydrate diet groups and from a reduction of 6 mmHg to an increase of 4.3 mmHg in balanced‐carbohydrate diet groups across the six trials reporting on this outcome. The meta‐analysis of the mean difference in change in DBP between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one to two years (MD ‐0.28 mmHg 95% CI ‐1.84 to 1.28, I2 = 0%, 631 participants, 6 RCTs, moderate‐certainty evidence, Analysis 3.11). Three of the six trials had follow‐up at one year and the other three at two years.
3.11. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 11: Change in DBP (mmHg) at ≥ 12 months
Change in SBP (mmHg) at ≥ 12 months
Average change in SBP from baseline in the six trials reporting this outcome ranged from a reduction of 9 mmHg to an increase of 3.2 mmHg with low‐carbohydrate diets and from a reduction of 11 mmHg to an increase of 2.4 mmHg with balanced‐carbohydrate diets over one to two years of follow‐up. The mean difference in change in SBP at one to two years was 0.32 mmHg (95% CI ‐2.30 to 2.93, I2 = 0%, 629 participants, 6 RCTs, Analysis 3.12). Three of the six trials had follow‐up at one year and the other three at two years.
3.12. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 12: Change in SBP (mmHg) at ≥ 12 months
Change in HbA1c (%) at 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets probably result in little to no difference to change in HbA1c compared to balanced‐carbohydrate weight‐reducing diets over one to two years in overweight and obese participants with T2DM (moderate‐certainty evidence, downgraded once for risk of bias).
Average change in HbA1c from baseline over one to two years ranged from a reduction of 2% to an increase of 0.1% with low‐carbohydrate diets and from a reduction of 1.8% to an increase of 0.2% with balanced‐carbohydrate diets across the six trials that reported this outcome. The meta‐analysis of the mean difference in change in HbA1c between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one to two years (MD ‐0.14% 95% CI ‐0.38 to 0.10, I2 = 31%, 668 participants, 6 RCTs, moderate‐certainty evidence, Analysis 3.13). Four of the six trials had follow‐up at one year and the other two had follow‐up at two years. One included trial also measured change in HbA1c in diabetic participants only at one year but reported it in an unusable format (Table 9).
3.13. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 13: Change in HbA1c (%) at ≥ 12 months
Change in LDL cholesterol (mmol/L) at ≥ 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets probably result in little to no difference in change in LDL‐cholesterol over one to two years compared to balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (moderate‐certainty evidence, downgraded once for risk of bias).
Average change from baseline over one to two years across the seven trials reporting on change in LDL cholesterol ranged from a reduction of 0.61 mmol/L to an increase of 0.2 mmol/L in low‐carbohydrate diet groups and from a reduction of 0.55 mmol/L to an increase of 0.1 mmol/L in balanced‐carbohydrate diet groups. The meta‐analysis of the mean difference in change in LDL cholesterol between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one to two years (MD 0.12 mmol/L, 95% CI ‐0.03 to 0.26, I2 = 34%, 753 participants, 7 RCTs, moderate‐certainty evidence, Analysis 3.14). Four of the seven trials had follow‐up at one year; the other three had follow‐up at two years.
3.14. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 14: Change in LDL cholesterol (mmol/L) at ≥ 12 months
Change in HDL cholesterol (mmol/L) at ≥ 12 months
Over one to two years, average change from baseline across the seven trials reporting on change in HDL cholesterol ranged from a reduction of 0.03 mmol/L to an increase of 0.23 mmol/L in low‐carbohydrate diet groups and from a reduction of 0.13 mmol/L to an increase of 0.11 mmol/L in balanced‐carbohydrate diet groups. We did not pool these results into an overall effect estimate due to substantial heterogeneity (I² = 67%, Tau² = 0.01; Chi² = 18.34, df = 6 (P = 0.005)) but rather presented the individual effect sizes between the low‐carbohydrate and balanced‐carbohydrate diets per study (Analysis 3.15). Four of the seven trials had follow‐up at one year and the other three had follow‐up at two years.
3.15. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 15: Change in HDL cholesterol (mmol/L) at ≥ 12 months
Change in non‐HDL cholesterol (mmol/L) at ≥ 12 months
Two studies reported on this outcome at one and two years and the mean difference in non‐HDL cholesterol was 0.09 mmol/L (95% CI ‐0.39 to 0.58, I2 = 0%, 159 participants, 2 RCTs, Analysis 3.16) with average increases in non‐HDL ranging from 0.13 mmol/L to 0.2 mmol/L in the low‐carbohydrate weight‐reducing group and from 0.1 mmol/L to 0.17 mmol/L in the balanced‐carbohydrate weight‐reducing group.
3.16. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 16: Change in non‐HDL cholesterol (mmol/L) at ≥ 12 months
Change in total cholesterol (mmol/L) at ≥ 12 months
Average change from baseline over one to two years across the seven trials reporting on change in total cholesterol ranged from a reduction of 0.88 mmol/L to an increase of 0.2 mmol/L with low‐carbohydrate diets and from reductions of 0.96 mmol/L to 0.1 mmol/L with balanced‐carbohydrate diets. The mean difference in change in total cholesterol at one to two years was 0.21 mmol/L (95% CI 0.06 to 0.37, I2 = 25%, 838 participants, 7 RCTs, Analysis 3.17). Four of the seven trials had follow‐up at one year and the other three trials at two years.
3.17. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 17: Change in total cholesterol (mmol/L) at ≥ 12 months
Change in triglycerides (mmol/L) at ≥ 12 months
Over one to two years, average change in triglycerides from baseline triglycerides ranged from reductions of 1.52 mmol/L to 0.1 mmol/L in low‐carbohydrate diet groups and from a reduction of 1.46 mmol/L to an increase of 0.16 mmol/L in balanced‐carbohydrate diet groups across the seven trials reporting on this outcome. We did not pool these results into an overall effect estimate due to substantial heterogeneity (I² = 75%, Tau² = 0.07; Chi² = 23.97, df = 6 (P = 0.0005) but rather presented the individual effect sizes between the low‐carbohydrate and balanced‐carbohydrate diets per study (Analysis 3.18). Five of the seven trials had follow‐up at one year and the remaining two trials at two years. Another trial reported change in triglycerides at two years as medians and interquartile ranges as the data were not normally distributed (Krebs 2012).
3.18. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 18: Change in triglycerides (mmol/L) at ≥ 12 months
Participant‐reported adverse effects
Participant‐reported adverse effects were scantily reported by three included trials for comparison 3, with all outcomes except short‐term constipation being reported only by single trials. All adverse effect outcomes reported in these trials had very few events or mean differences close to 0 and small total sample sizes.
Gastrointestinal
GRADE assessment suggests that the evidence is very uncertain about whether there is a difference in the number of participants per group with constipation at three to six months when comparing low‐carbohydrate weight‐reducing diets with balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (very low‐certainty evidence, downgraded once for risk of bias and twice for imprecision).
The meta‐analysis of the risk ratio of the number of participants per group reporting constipation at six months was 1.37 (95% CI 0.86 to 2.18, I2 = 0%, 177 participants, 2 RCTs, very low‐certainty evidence, Analysis 3.19) when comparing low‐carbohydrate and balanced‐carbohydrate diet groups. One included trial also measured constipation at three months but reported data in an unusable format (Table 9).
3.19. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 19: Constipation at 3 to < 12 months
One trial reported constipation at one year (RR 2.71, 95% CI 0.11 to 64.65, 78 participants, 1 RCT, Analysis 3.20).
3.20. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 20: Constipation at ≥ 12 months
One trial reported on events of diarrhoea at six months (RR 1.07, 95% CI 0.62 to 1.84, 84 participants, 1 RCT, Analysis 3.21). Another trial (using an adaption of the Health Symptom Checklist) (Saslow 2017a) reported "no statistically significant changes" for diarrhoea at three months (data were not reported or provided when authors were contacted), and also reported heartburn symptoms at three months (MD ‐0.30, 95% CI ‐0.66 to 0.06, 33 participants, 1 RCT, Analysis 3.22).
3.21. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 21: Diarrhoea 3 to < 12 months
3.22. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 22: Heartburn symptoms (participant‐reported) at 3 to < 12 months
Appetite
One trial reported on appetite change from baseline at three months using the three‐factor eating questionnaire (MD ‐0.70, 95% CI ‐3.03 to 1.63, 33 participants, 1 RCT, Analysis 3.23).
3.23. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 23: Appetite change (participant‐reported) at 3 to < 12 months
Headaches
One trial reported on events of headaches at six months (RR 1.15, 95% CI 0.75 to 1.78, 84 participants, 1 RCT, Analysis 3.24). Another trial reported no statistical difference between participants in the low‐carbohydrate diet group and the balanced‐diet group for headaches at three months (used adaptation of the Health Symptom Checklist), but data were not reported or provided when authors were contacted (Saslow 2017a).
3.24. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 24: Headaches at 3 to < 12 months
Psychosocial problems (mood disturbances)
At three months follow‐up, one trial reported depressive symptoms according to the 20‐item Center for Epidemiologic Studies Depression Scale (CES‐D) (MD ‐0.20, 95% CI ‐9.20 to 8.80, 33 participants, 1 RCT, Analysis 3.25).
3.25. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 25: Depressive symptoms (participant‐reported) at 3 to <12 months
Comparison 4. Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase)
Table 4 presents the effects of low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets (weight‐reducing phase followed by weight‐maintenance phase) in overweight and obese participants with T2DM on short‐term (three to <12 months) and long‐term (≥ 12 months) changes in body weight, DBP, HbA1c and LDL cholesterol, and short‐term constipation.
Key details about studies in this comparison, including characteristics of the intervention, population, outcomes and method of synthesis are detailed in the OSIS table (Table 10).
Primary outcomes
The only primary outcomes reported by the included trials for comparison 4 were change in body weight at three to < 12 months and change in body weight at ≥ 12 months.
Change in body weight (kg) at three to < 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase may result in little to no difference in change in body weight at six months compared to balanced‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase in overweight and obese participants with T2DM (low‐certainty evidence, downgraded once for risk of bias and once for imprecision).
The single study reporting change in weight at six months found average weight reductions of 1 kg with the low‐carbohydrate diet and 0.2 kg with the balanced‐carbohydrate diet. The mean difference in weight reduction between the two diets in this study demonstrated little to no difference at six months (MD ‐0.80 kg, 95% CI ‐2.46 to 0.86, 61 participants, 1 RCT, low‐certainty evidence, Analysis 4.1). This trial tested diets with a three‐month weight‐reducing phase followed by a three‐month weight‐maintenance phase.
4.1. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 1: Change in body weight (kg) at 3 to < 12 months
Change in body weight (kg) at ≥ 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase may result in little to no difference in change in body weight over one to two years compared to balanced‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase in overweight and obese participants with T2DM (low‐certainty evidence, downgraded once for risk of bias and once for imprecision).
Average changes in weight from baseline over one to two years in the three trials reporting this outcome ranged from a reduction of 2.23 kg to an increase 1.3 kg with low‐carbohydrate diets and from reductions of 2.17 kg to 1.5 kg with balanced‐carbohydrate diets. The meta‐analysis of the mean difference in weight reduction between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one to two years (MD ‐0.07 kg, 95% CI ‐1.31 to 1.17, I² = 0%, 158 participants, 3 RCTs, low‐certainty evidence, Analysis 4.2). The shortest of the trials measured change in weight at one year with the diets having a three‐month weight‐loss phase followed by a nine‐month weight‐reducing phase. The second trial had follow‐up at 18 months and tested diets with a six‐month weight‐reducing phase followed by a 12‐month maintenance phase. The third trial had follow‐up at two years, and tested diets with a three‐month weight‐reducing followed by a 21‐month weight‐maintenance phase. All trials had high risk of attrition bias and overall bias, and had diet/food industry funding, precluding sensitivity analyses.
4.2. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 2: Change in body weight (kg) at ≥ 12 months
Secondary outcomes
The secondary outcomes reported by the included trials for comparison 4 were change in BMI at three to < 12 months, change in BMI at ≥ 12 months, change in HbA1c, DBP, SBP, LDL and HDL cholesterol, total cholesterol and triglycerides.
Change in BMI (kg/m2) at three to < 12 months
One trial compared these diets using a three‐month weight‐reducing phase followed by a three‐month weight‐maintenance phase and reported average BMI reductions from baseline of 0.3 kg/m2 in the low‐carbohydrate group and 0.01 kg/m2 in the balanced‐carbohydrate group after six months. The meta‐analysis of the mean difference in BMI change at six months was ‐0.29 kg/m2 (95% CI ‐0.83 to 0.25, 61 participants, 1 RCT, Analysis 4.3).
4.3. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 3: Change in BMI (kg/m2) at 3 to < 12 months
Change in BMI (kg/m2) at ≥ 12 months
The single trial reporting on this outcome in comparison 4 found average BMI reductions from baseline of 0.75 kg/m2 with the low‐carbohydrate diet and 0.63 kg/m2 with the balanced diet after 18 months (six‐month weight‐reducing phase followed by 12‐month weight‐maintenance phase). The mean difference in BMI change at 18 months in this trial was ‐0.12 kg/m2 (95% CI ‐0.86 to 0.62, 47 participants, 1 RCT, Analysis 4.4).
4.4. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 4: Change in BMI (kg/m2) at > 12 months
Changes in DBP and SBP (mmHg) at ≥ 12 months
GRADE assessment suggests that the evidence is very uncertain about the effect of low‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase compared to balanced‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase on change in DBP at one year in overweight and obese participants with T2DM (very low‐certainty evidence, downgraded once for risk of bias and twice for imprecision).
One trial compared these diets in comparison 4 using a three‐month weight‐reducing phase followed by a nine‐month maintenance phase, and reported on changes in DBP (MD ‐0.44 mmHg, 95% CI ‐4.89 to 4.01, 99 participants, 1 RCT, very low‐certainty evidence, Analysis 4.5) and SBP (MD ‐4.27 mmHg, 95% CI ‐8.74 to 0.20, 99 participants, 1 RCT, Analysis 4.6) at one year. The study reported average DBP increases from a baseline of 0.21 mmHg in the low‐carbohydrate diet group and of 0.65 mmHg in the balanced‐carbohydrate diet group at one year. For SBP, the study reported an average decrease from baseline of 5.03 mmHg in the low‐carbohydrate group and 0.76 mmHg in the balanced‐carbohydrate group.
4.5. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 5: Change in DBP (mmHg) at ≥ 12 months
4.6. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 6: Change in SBP (mmHg) at ≥ 12 months
Change in HbA1c (%) at 12 months
GRADE assessment suggests that low‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase may result in little to no difference in change in HbA1c over one to two years compared to balanced‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase in overweight and obese participants with T2DM (low‐certainty evidence, downgraded once for risk of bias and once for imprecision).
Average changes in HbA1c from baseline over one to two years in the three trials reporting this outcome ranged from a reduction of 0.39% to an increase of 0.3% with low‐carbohydrate diets and from a reduction of 0.37% to an increase of 0.4% with balanced‐carbohydrate diets. The meta‐analysis of the mean difference in change in HbA1c between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one to two years (MD 0.02%, 95% CI ‐0.30 to 0.33, I² = 0%, 160 participants, 3 RCTs, low‐certainty evidence, Analysis 4.7). The shortest of the trials measured change in HbA1c at one year with diets having a three‐month weight‐loss phase followed by a nine‐month weight‐reducing phase. The second trial had follow‐up at 18 months and tested diets with a six‐month weight‐reducing phase followed by a 12‐month maintenance phase. The third trial had follow‐up at two years, and tested diets with a three‐month weight‐reducing followed by a 21‐month weight‐maintenance phase.
4.7. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 7: Change in HbA1c (%) at ≥ 12 months
Change in LDL, HDL and total cholesterol and triglycerides (mmol/L) at ≥ 12 months
Two trials reported changes in these blood lipids at one to two years. GRADE assessment suggests that low‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase may result in little to no difference in change in LDL over one year to 18 months compared to balanced‐carbohydrate weight‐reducing diets that include a weight‐maintenance phase in overweight and obese participants with T2DM (low‐certainty evidence, downgraded once for risk of bias and once for imprecision).
One trial had follow‐up at one year with diets having a three‐month weight‐loss phase followed by a nine‐month weight‐reducing phase. The other trial assessed follow‐up at 18 months and tested diets with a six‐month weight‐reducing phase followed by a 12‐month maintenance phase. The meta‐analysis of the mean difference in change in LDL cholesterol between the low‐carbohydrate and balanced‐carbohydrate diets demonstrated little to no difference at one year to 18 months (MD ‐0.06 mmol/L, 95% CI ‐0.26 to 0.13, I² = 0%, 145 participants, 2 RCTs, low‐certainty evidence, Analysis 4.8). The two trials reported on HDL cholesterol (MD 0.0 mmol/L, 95% CI ‐0.07 to 0.08, I² = 0%, 148 participants, 2 RCTs, Analysis 4.9) and triglycerides (MD ‐0.06 mmol/L, 95% CI ‐0.45 to 0.32, I² = 0%, 148 participants, 2 RCTs, Analysis 4.11), while only one of the trials reported on total cholesterol at one year (MD ‐0.16 mmol/L, 95% CI ‐0.51 to 0.19, 99 participants, 1 RCT, Analysis 4.10).
4.8. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 8: Change in LDL cholesterol (mmol/L) at ≥ 12 months
4.9. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 9: Change in HDL cholesterol (mmol/L) at ≥ 12 months
4.11. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 11: Change in triglycerides (mmol/L) at ≥ 12 months
4.10. Analysis.

Comparison 4: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase), Outcome 10: Change in total cholesterol (mmol/L) at ≥ 12 months
Average changes in LDL cholesterol over one year to 18 months in the two trials ranged from reductions of 0.2 mmol/L to 0.05 mmol/L with low‐carbohydrate diets and from a reduction of 0.17 mmol/L to an increase of 0.04 mmol/L with balanced‐carbohydrate diets. For HDL cholesterol, average increases over this period ranged from 0.04 mmol/L to 0.08 mmol/L with low‐carbohydrate weight‐reducing diets and from 0.03 mmol/L to 0.08 mmol/L with balanced‐carbohydrate weight‐reducing diets. Average changes in total cholesterol at one year reported by the single study ranged from a reduction of 0.15 mmol/L in the low‐carbohydrate group to an increase of 0.01 mmol/L in the balanced‐carbohydrate group. For triglycerides, average changes over one year to 18 months in the two trials ranged from a reduction of 0.47 mmol/L to an increase of 0.07 mmol/L with low‐carbohydrate diets and from reductions of 0.3 mmol/L to 0.05 mmol/L with balanced‐carbohydrate diets.
Participant‐reported adverse effects
Two adverse effects were reported by a single trial in comparison 4 (Watson 2016). This trial reported on anxiety at six months using the 'Anxiety and worry' subscale of the D‐39 diabetes QoL questionnaire, but did not report sample sizes per group. The mean score in the low‐carbohydrate diet group was 33.91 (SE 3.55) and in the balanced diet group was 31.43 (SE 3.71). Fatigue at six months was also reported on by this trial using the 'Energy and mobility' subscale of the D‐39 diabetes QoL questionnaire. The mean score in the low‐carbohydrate diet group was 22.04 (SE 2.49) and in the balanced diet group was 23.28 (SE 2.61), with no sample sizes being reported per group.
Discussion
Summary of main results
This review examined the effects of low‐carbohydrate weight‐reducing diets compared to 'balanced'‐carbohydrate weight‐reducing diets on weight and cardiovascular risk factors. We used approaches aimed at addressing factors that may limit interpretation in other similar reviews. These included explicit criteria for the compositions and intentions of the treatment and control diets, stratifying findings in participants without and with T2DM, separating findings from weight‐reducing and weight‐maintenance phases of diets, meaningful and comparable follow‐up periods, comprehensive searches, and multiple efforts to obtain and include all relevant and necessary data and information not reported in published papers; as well as assessing the certainty of this evidence using GRADE. To our knowledge, this review includes the largest number of trials that have examined this question to date.
Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets (weight‐reducing phases only)
Participants without and with T2DM in both low‐carbohydrate and balanced‐carbohydrate diet groups reduced their weight to varying degrees in the short term (range of weight reduction across both diets: 12.2 to 0.33 kg) and long term (range of weight reduction across both diets: 13.1 to 1.7 kg). We considered a loss of at least 5% of initial weight to be clinically meaningful, and a weight change that could reasonably be expected in our minimum diet duration of 12 weeks. Thus, reductions from around the middle of these ranges (about 4 to 6 kg in most trials) would start to become clinically meaningful and translate to improvements in cardio metabolic health (Brown 2016; Franz 2015; Jensen 2014; Truby 2006; Wald 2012; Wing 2010).
Notably, in overweight and obese participants without and with T2DM, the meta‐analyses showed little or no difference between the effects of low‐carbohydrate weight‐reducing diets and balanced‐carbohydrate weight‐reducing diets on weight reduction over three to 8.5 months (short term), with mean differences in weight reduction of about one kilogram. This was also the case over one to two years (long term), with mean differences in weight reduction of less than one kilogram. The 95% CIs of pooled mean differences in short‐ and long‐term weight change did not include appreciable harm or benefit (ranging from 2.44 kg lower to 1.46 kg higher), and we therefore did not downgrade for imprecision. The lack of differences found in effects on weight reduction were confirmed by sensitivity analyses. There was, however, some heterogeneity in effects in participants without T2DM over the short term. Subgrouping by similarity of energy prescription, extent of carbohydrate restriction, diagnosed cardiovascular event or disease at baseline or gender did not suggest important clinical differences in average effects between the various subgroups. Mean differences in weight reduction between diet groups across the various subgroups at the short term ranged between 0.25 kg and 2.71 kg. Some heterogeneity could be explained by subgrouping studies according to similarity of energy prescription and extent of carbohydrate restriction.
We do not know whether there is a difference in the number of participants per group with weight loss of at least 5% at the long term since only one trial (n = 68) in men, judged as being at high risk of bias, reported on this outcome.
For mean changes in DBP at one to two years, some trials reported reductions and other trials reported increases in both diet groups in participants without and with T2DM (range of mean change across diet groups: ‐11 to 4.3 mmHg). In all participants, meta‐analyses showed little or no difference between the effects of the two diets on changes in DBP at one to two years, with mean differences of less than 0.5 mmHg. The 95% CIs of these pooled mean differences did not include appreciable harm or benefit (ranging from 1.84 mmHg lower to 1.28 mmHg higher) and we did not downgrade for imprecision. We considered changes in DBP of greater than 2 mmHg to be clinically meaningful (Whelton 2002).
At one to two years in both diet groups, some trials reported reductions in LDL cholesterol and others reported increases in participants without and with T2DM (range of mean change across diet groups: ‐0.61 to 0.6 mmol/L). Meta‐analyses showed little or no difference between the effects of the two diets on changes in LDL cholesterol at one to two years in all participants, with mean differences of up to 0.1 mmol/L. The 95% CIs of these pooled mean differences did not include appreciable harm or benefit (ranging from 0.05 mmol/L lower to 0.26 mmol/L higher) and we did not downgrade for imprecision. We considered changes in LDL cholesterol of greater than 0.26 mmol/L to be clinically meaningful (Fernandez‐Friera 2017; Howard 2000).
At one to two years, some trials in participants with T2DM reported reductions in HbA1c and others reported small increases in HbA1c in both diet groups (range of change across diet groups: ‐2% to 0.2%). Pooling these mean differences found little or no difference between the effects of low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets on change in HbA1c over one to two years in overweight and obese participants with T2DM, with a mean difference of 0.14%. The 95% CI of the pooled mean difference did not include appreciable harm or benefit (0.38% lower to 0.1% higher) and we did not downgrade for imprecision. We considered changes in HbA1c of greater than 0.5% to be clinically meaningful (NICE 2015).
Participant‐reported adverse effects were poorly reported. In the few trials where these were reported, the effects included constipation, which we regarded as a patient‐important adverse effect since it is frequently reported by people following low‐carbohydrate diets. These limited data mean that we are very uncertain about whether there is a difference in the number of participants per diet group with constipation at three to six months in all participants.
Low‐carbohydrate weight‐reducing diets compared to balanced‐carbohydrate weight‐reducing diets (weight‐reducing phase followed by weight‐maintenance phase)
Evidence about the comparative effects of diets from studies using weight‐reducing phases followed by weight‐maintenance phases was limited. Only eight trials reported these data for some outcomes, and one of these trials did not contribute any numeric data to meta‐analyses. All the trials applied different approaches and time frames to the weight‐maintenance phases of the diets, with the shortest weight‐maintenance phase being six weeks and the longest being 21 months. Findings were similar to the comparisons that included only weight‐reducing phases of the two diets, but our certainty in these findings was lower, largely due to imprecision, with small total sample sizes and wide 95% CIs around the pooled estimates.
In overweight and obese participants without T2DM, meta‐analyses showed that low‐carbohydrate weight‐reducing diets including a weight‐maintenance phase may result in little or no difference in change in weight over one to two years compared to balanced‐carbohydrate weight‐reducing diets including a weight‐maintenance phase. We are very uncertain about whether there are differences in effects on DBP and LDL cholesterol between the two diets in these participants. None of the eight trials reported usable data for the other outcomes in our summary of findings table.
In overweight and obese participants with T2DM, meta‐analyses showed that low‐carbohydrate weight‐reducing diets including a weight‐maintenance phase may result in little or no difference in change in weight at six months, and at one to two years, compared to balanced‐carbohydrate weight‐reducing diets including a weight‐maintenance phase. This was also the case for change in HbA1c and LDL cholesterol. We are very uncertain about whether there are differences in effects on DBP between the two diets in these participants. None of the eight trials reported usable data for the other outcomes in our summary of findings table.
Overall completeness and applicability of evidence
Our review employed a comprehensive search strategy with no language restrictions to identify all RCTs assessing the effects of low‐carbohydrate weight‐reducing diets on weight and cardiovascular risk in overweight and obese adults without or with T2DM. We tapped multiple sources of information for all trials and contacted study authors multiple times in all cases where we needed additional data or information. This included requesting information from 30 author teams to enable clear judgements and reporting related to eligibility, stratification, risk of bias, as well as numeric data to enable the inclusion of as much usable data in meta‐analyses as we could obtain.
We prespecified outcomes at defined lengths of follow‐up to examine possible differences in effects over time. No trials reported on outcomes after two years, and 60% of trials had a duration of six months or less. Change in body weight was reported by most trials, but few studies trials reported on the number of participants per group with weight loss of at least 5% from baseline. Despite multiple attempts, we were not unable to obtain and include weight change data from 11 trials, as they reported data in unusable formats.
None of the trials reported on all‐cause mortality, cardiovascular mortality, non‐fatal myocardial infarction, non‐fatal stroke and diagnosis of T2DM. This is to be expected to some degree in view of the practical challenges posed by long durations of follow‐up in weight‐reducing diet trials. Pooled data from long‐term prospective cohort studies on dietary carbohydrate intake and mortality have demonstrated harm at the extremes of intake (Seidelmann 2018). Moreover, a range of potential shorter‐ and longer‐term safety concerns of low‐carbohydrate diets have been discussed in recent papers, such as reduced dietary intake of fibre, minerals, vitamins, trace elements and polyunsaturated fats, unknown effects of long‐term ketosis, harmful environmental impacts and dysbiosis (Barber 2021; Sievenpiper 2020).
The effect of weight reduction per se on cardiovascular risk factors is also important for interpreting this evidence, as it is known that a weight reduction of 5% or more is associated with improvements in cardio metabolic risks, including blood lipids, blood pressure and HbA1c (Brown 2016; Franz 2015). As we found little to no difference in weight reduction between low‐carbohydrate and balanced‐carbohydrate diets up to two years, one would reasonably not expect to find meaningful differences in effects on blood lipids, blood pressure and HbA1c between the two diets, which was the case in our review.
Our review did not seek to address effects of differences in the quality of dietary carbohydrates, or the quality of the replacement macronutrients (e.g. fat). Both quality and quantity or proportions are vital components of understanding the effects of macronutrients on health outcomes (Abdelhamid 2018; Barber 2021; Hooper 2020; Reynolds 2019; Sievenpiper 2020).
A criticism of examining mean differences in weight between diet groups is that they do not fully account for true different individual responses, where ‘true’ refers to individual response differences that are not a result of random trial‐to‐trial variability. In the context of 'personalised' nutrition, there is interest in ascertaining whether there are true individual differences in the responses to weight‐reducing diets large enough to be clinically relevant. A recent systematic review, using a response variance comparison approach, examined whether the weight responses to low‐carbohydrate diets or low‐fat diets (typically, balanced‐carbohydrate diets) differ between individuals, and was unable to detect clinically relevant differences in individual response heterogeneity between the two diets (Smith 2020).
All except one of the 61 trials were conducted in high‐income countries. There are, however, no obvious reasons to expect the effects of low‐carbohydrate weight‐reducing diets on body weight to be different in people from low‐ and middle‐income countries. High costs of low‐carbohydrate diets and disproportionate financial effects on lower socioeconomic groups, which are beyond the scope of our review, have been covered elsewhere (Barber 2021; Raffensperger 2008).
Nearly 40% of included trials reported some or all funding by food/diet industry and related private companies, such as food and nutrition product manufacturers, organisations representing the interests of food producers (e.g. Pork Council, Dairy Farmers Association) and diet book and diet product organisations.
There are many individual and environmental factors that influence a person's ability to adhere to weight‐reducing diets, and studies have shown that adherence to weight‐reducing diets is one of the major factors that determine success over the short and long term (Gibson 2017). Systematic reviews and RCTs of low‐ and balanced‐carbohydrate weight‐reducing diets have demonstrated that adherence is a problem, and reduces over time with both diets, with some of these specifically reporting difficulty with adhering to severely carbohydrate‐restricted diets over the long term (Foster 2003; Gibson 2017; Ross 2021; Sacks 2009). We did not investigate the possible influence of adherence on the effects of the dietary interventions. However, we did extract information on fidelity for both diets (Characteristics of included studies), i.e. the degree to which an intervention happened in the way the investigators intended it to (Hoffman 2014).
Quality of the evidence
The interpretation of most weight reduction trials is constrained by small samples, a lack of blinding, and large loss to follow‐up (Simons‐Morton 2006), which were also observed across the trials in our review. None of the trials in the review were judged as having low risk of bias overall. The overall risk of bias of outcomes across trials in participants without and with T2DM was predominantly high, largely due to high proportions of missing outcome data in many trials.
Potential biases in the review process
The review may be affected by non‐reporting bias. For the 'known unknowns' (particular results from a trial not reported in a usable format), we contacted trial authors multiple times to obtain the data in a usable format. Failure to obtain this and consequent exclusion thereof is a limitation of the review process, as we cannot be absolutely certain if and how the inclusion of these data would have affected the pooled estimates. However, given the agreement in pooled mean differences in weight reduction between our review and other similar reviews, we think it is unlikely that these data would have meaningfully changed our pooled estimates. It was harder to make a judgement about the 'unknown unknowns' (entire eligible trials not reported or picked up by our comprehensive searching), but it is possible that small unpublished trials are missing. Interpreting funnel plot asymmetry was challenging since many trials had similar standard errors.
We excluded RCTs of less than 12 weeks' duration which may have been relevant to the review question. The reason for choosing only trials with at least 12 weeks follow‐up was to allow sufficient time to detect clinically meaningful weight and cardiovascular risk factor changes.
Agreements and disagreements with other studies or reviews
Pooled mean differences in weight reduction and cardiovascular risk factors in our review are in line with a number of recent systematic reviews with pairwise meta‐analyses (Chawla 2020; Dong 2020; Smith 2020; Yang 2021), and an older review with network meta‐analyses (Johnston 2014), in participants without T2DM. This is also the case for recent similar reviews in participants with T2DM (Meng 2017; Silverii 2020; Snorgaard 2017; Van Zuuren 2018). Systematic reviews with varying definitions of low‐carbohydrate diets and differing eligibility criteria have shown that low‐carbohydrate diets do not result in superior long‐term clinically meaningful weight reduction. An overview of systematic reviews of RCTs comparing low‐carbohydrate to control diets (low‐fat or energy‐restricted) in adults with overweight and obesity included 12 reviews (10 with meta‐analyses) and reported an inverse relationship between number of trial participants and the size of differences in weight loss (Churuangsuk 2018).
Authors' conclusions
Implications for practice.
The small pooled mean differences in weight reduction between the diets (about 1 to 2 kg), while statistically significant in the short term, were not clinically important in the short or long term in an overweight and obese adult, without or with T2DM (low‐to moderate‐certainty evidence). These differences are similar to typical ranges of biological weight fluctuations over time (Liska 2019; Vuorinen 2021), and can at least in part be ascribed to biological weight fluctuations, which are influenced by various factors including level of activity, hydration status, season and medications. When interpreting the sizes of the mean differences of weight reduction between the two diets, one should also consider the total body water loss (2 to 3 kg) that follows dietary carbohydrate restriction due to diet‐induced diuresis from glycogen depletion and production of ketone bodies, which is restored when carbohydrates are eaten again (Denke 2001; Yang 1976).
Our systematic review failed to show that low‐carbohydrate weight‐reducing diets are superior to balanced‐carbohydrate weight‐reducing diets, with little or no difference in weight reduction and cardiovascular risk factors over the short (three to 8.5 months) and long term (one to two years). Trials did not report on our prespecified outcomes after two years of follow‐up. The range of potential safety concerns of low‐carbohydrate diets not addressed in this review over the short and longer term should also be considered.
Most trials included participants without diagnosed cardiovascular disease or events at baseline, and average baseline LDL cholesterol concentrations and DBP across the trials were within the normal ranges defined for people without coronary artery disease. In people with lipid disorders and variability with atherogenic lipoprotein response, caution in recommending low‐carbohydrate and consequent high‐fat diets is warranted. Evidence on participant‐reported adverse effects over two years was limited and we could not draw any conclusions about these.
Dietary modification is central to weight reduction, however, in view of the complex aetiology of obesity, it is important to consider the demonstrated value of combining diet and other positive lifestyle and behavioural interventions to reduce weight and cardiovascular risk. Current dietary guidance allows for flexibility in the proportion of macronutrients, including a wide range of carbohydrate intakes, with greater emphasis on quality over quantity and on total dietary patterns over single nutrients.
Implications for research.
In view of the challenges associated with weight‐reducing diet trials and the similar findings from this and other comparable reviews, it could reasonably be argued that additional trials on this question are not needed. However, the effects of low‐carbohydrate diets compared to balanced‐carbohydrate diets beyond two years is unknown. If conducted, future trials on this question in participants without and with T2DM, should be longer than two years, adequately powered with greater attention to methodological quality, and specifically the use of more appropriate and rigorous statistical approaches to address loss to follow‐up, where relevant. Prospective registration, publication of protocols and of detailed data analysis plans, as well as use of appropriate reporting guidelines (e.g. CONSORT) by future trials, would also be beneficial to the quality and utility of any future research. Other key research areas related to dietary interventions for weight‐reduction and cardiovascular health include improving dietary adherence, which is critical to both short‐ and long‐term weight loss, total dietary patterns and macronutrient quality.
History
Protocol first published: Issue 5, 2019
Risk of bias
Risk of bias for analysis 1.1 Change in body weight (kg) at 3 to < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Aude 2004 | Some concerns | Randomisation was done using a block design by sex to achieve an equal sex distribution. No information on allocation concealment. "At baseline, there were no significant differences in age, weight, BMI, waist‐to‐hip ratio, and any of the lipid variables between the NCEP and MLC groups" (only for completers). | High risk of bias | No information on blinding of participants and people delivering these dietary interventions, but blinding is unlikely. No reasons or details about deviations from intended interventions reported. A quarter of the participants were not included in the analysis: 8/30 participants in the intervention group and 7/30 in the control group were excluded from analysis; 6/60 did not complete the trial (missing), but no information about remaining 9/60 who were not included. | High risk of bias | 8/30 (27%) participants in the intervention and 7/30 (23%) participants in the control arm did not have data available. No reasons for loss to follow‐up or withdrawal provided, so difficult to judge if related to participants' health status, but attrition was high in both groups; thus missingness could and is likely to depend on the true value. | Low risk of bias | Weight was measured with indoor clothing and without shoes on a single scale. No evidence that a different method was used across intervention groups to measure weight. "All anthropometric measurements were obtained by an observer blinded to the treatment assignment." | Some concerns | No trial registry reported and no mention of a prespecified analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. A quarter of participants were not included in the analysis, with withdrawal reported for 10% of participants. No reasons were provided for the remaining missingness. Attrition was high in both groups with no reasons provided, therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Bales 2017 | Low risk of bias | We used a computerised centralised randomisation scheme blocking by race and marital or partner status. "...also blocked by functional status..." "The 1:2 allocation to the C‐WL and HP‐WL groups was chosen...to make within‐group comparisons in the protein group in subsequent analyses. This oversampling of the protein group did not cause a substantial loss of power for the between‐group comparisons; the relative power for a 2:1 split is 0.94(23)." No statements or statistical tests about differences between groups, no problematic imbalance suspected. | Low risk of bias | Not blinded (open‐label trial) (trial registry). Deviations reported are expected to arise in usual care: "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study." Number analysed unclear, but seemed to be a modified ITT: "The study was conducted under “intent to treat” criteria." | High risk of bias | 22/51 (43%) participants in the intervention and 16/29 (55%) participants in the control arm did not have data available. "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study. In particular, the tendency for more dropouts among minorities and those with less education..." Very high attrition, thus missingness could and is likely to depend on the true value. | Low risk of bias | Weekly weigh‐ins. No evidence that a different method was used across intervention groups to measure weight. Open‐label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | ...treatment codes revealed only after the study statistician locked the database at the end of the trial. No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. All time points mentioned in trial registry reported on in publication. Analysis intentions were not reported in sufficient detail prior to study publication to enable an assessment. | High risk of bias | Very high attrition with a variety of reasons relating to health, therefore some missingness could be and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | No information about participant blinding, however, blinding of participants is highly unlikely. "...dietitians were not blinded to the study hypothesis." No reasons or details about deviations from intended interventions reported. "All analyses were conducted using the intention‐to‐treat principle." n = 148 randomised, and n = 148 analysed. | Low risk of bias | 13/75 (17%) participants in the intervention and 9/73 (12%) participants in the control arm did not have data available. Imputed missing values, and performed sensitivity analyses to determine whether missing at random (MAR) assumptions were met. "We performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption." "We used Markovchain Monte Carlo techniques to impute missing values, including additional covariates..." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses." | Low risk of bias | Body weight was measured to the nearest 0.1 kg using a single calibrated scale. No evidence that a different method was used across intervention groups to measure weight. "all outcome assessors were blinded to the diet group assignment..." | Some concerns | No mention of a prespecified analysis plan. "Study protocol and data set: Not available." Trial registry outcomes amended after study publication; but trial registry entry at date prior to study publication mentioned the outcome and time points. All time points mentioned in trial registry at date prior to study publication, were reported on in publication. Analysis intentions were not reported in sufficient detail prior to study publication to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Benassi‐Evans 2009 | Some concerns | "Subjects were randomised to one of two dietary interventions…" No further information about allocation sequence or concealment. "Subject characteristics were not significantly different between groups at baseline." | Low risk of bias | No information, but blinding of participants and people delivering the interventions was highly unlikely. No reasons or details about deviations from intended interventions reported. No details about analysis were reported, but it looked to be intention‐to‐treat (number randomised per group = number analysed per group). | High risk of bias | No details about attrition reported, but some attrition was implied: "...in the subjects who completed the 12 weeks intensive weight loss and 9 months weight maintenance..." No further information or evidence that the result was not biased due to missing data were provided. | Low risk of bias | Volunteers fasted overnight and were weighed (model AMZ14; Mercury Digital Scales, Tokyo, Japan) wearing light clothing and no shoes at weeks. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a prespecified analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition was implied, with no reasons or evidence to show that results were not biased by this missingness. No pre‐registered document available to assess analysis intentions. |
| Brehm 2003 | Some concerns | "... after each block of subjects was assessed, the principal investigator used a random number table to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences at baseline. | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. dislike diet, could not make scheduled visit. Modified ITT: excluded only missing patients | High risk of bias | 7/26 (27%) in the intervention and 14/27 (52%) in the control group did not have data. "In this intention to treat analysis, the initial weights for the subjects who withdrew from the study were used as their follow‐up weights at 3 and 6 months..." Lost to follow‐up and withdrawal reasons were stated for all participants, but very high attrition in the control group and differential attrition, thus missingness could and is likely to depend on the true value. | Some concerns | Subjects were weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan. Analysis intentions were not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Differential attrition and high attrition in the control group, therefore missingness could and is likely to be dependent on the true value of the outcome. It was not clear whether outcome assessors were blinded to allocation. No pre‐registered document available to assess analysis intentions. |
| Brehm 2005 | Some concerns | "After each block of subjects was enrolled, the principal investigator used a computerized randomization program to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences between groups at baseline (completers only). | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. pregnancy, disliked diet. Modified ITT: all those who completed the study were analysed. | High risk of bias | 5/25 (20%) in the intervention and 5/25 (20%) in the control group did not have data available. Difficult to judge whether it was likely that missingness was related to the true value for weight, as reasons were not explicit enough. "The most common reason for discontinuing the study was the inability to commit to weekly counseling sessions. Other reasons included pregnancy, new out‐of‐state job, dislike of the low‐carbohydrate diet, and advice against following the low carbohydrate diet by family physician." | Low risk of bias | Weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan. Analysis intentions were not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Reasons for attrition were vague and it was difficult to judge whether it was as a result of the true value of the outcome, but with 20% attrition in both groups it is likely that some missingness was dependent on true value. No pre‐registered document available to assess analysis intentions. |
| Calleja‐Fernández 2012 | Some concerns | Random allocation to each dietary group was performed by the main investigator (MDBP) by using the Web site Randomization.com (http://www.randomization.com) upon the request of the study dietitian (ACF) after the eligibility of a participant was confirmed and assignment to either of the insulin resistance or insulin sensitivity groups had been performed. No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected. | Low risk of bias | No information on blinding of participants, however, blinding was highly unlikely. People delivering these dietary interventions were unblinded. No reasons or details about deviations from intended interventions reported. ITT analysis conducted. | Low risk of bias | It seemed data were available for all participants randomised for 16‐week study, but we could not be sure (flowcharts in Fernandez et al 2012 and Ballesteros‐Pomar 2009 incongruent). | Low risk of bias | "Body weight was measured to the nearest 0.5 kg and then taken using calibrated digital scales…" No evidence that a different method was used across intervention groups to measure weight. "The medical assessments were blinded to the type of diet being followed." | Some concerns | No mention of a prespecified analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions. |
| Cornier 2005 | Some concerns | No information on how randomisation was done or allocation concealment. "Subject characteristics are summarized in Table 1. BW, BMI, percentage body fat, RMR, and RQ were similar in all groups." | Low risk of bias | No information, however, blinding of participants and people delivering these interventions was highly unlikely. "Forty‐four obese, healthy normoglycemic women 23 to 53 years old with BMI of 30 to 35 kg/m2 were screened, and 21 of them were enrolled and completed the 16‐week intervention." ITT analysis conducted: number randomised equalled number analysed; no subjects lost to follow‐up. | Low risk of bias | Number randomised equalled number analysed; no subjects lost to follow‐up. | Low risk of bias | Once a week, subjects were weighed. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a prespecified analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions. |
| Dyson 2007 | Low risk of bias | "Randomization was undertaken by means of sealed envelopes equivalent to the number of subjects and filled fifty‐fifty with an indicator of either a low‐carbohydrate diet or healthy‐eating advice. Two separate sets of envelopes were prepared for both diabetic and non‐diabetic subjects. An independent observer witnessed randomization." Author reported in correspondence that no formal test of hypothesis was done for difference at baseline for non‐diabetic participants. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions was highly unlikely. Deviations reported are expected to arise in usual care: n = 3 of randomised participants in balanced group refused follow‐up as not allocated to low‐carbohydrate diet. Modified ITT conducted. | High risk of bias | No participants in the intervention group and 3/7 (43%) in the control group did not have data. Modified ITT with last‐observation‐carried‐forward. High attrition in control group, and differential attrition. Refusal of follow‐up due to not being allocated to low carbohydrate may be influenced by expected change in weight, therefore missingness could and was likely to be influenced by the true value. | Low risk of bias | Body weight recorded monthly. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a prespecified analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition in the control group, with disappoinment at not being assigned to the low‐carbohydrate diet cited as a reason; therefore, missingness could be and was likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Ebbeling 2007 | Low risk of bias | "Enrolled participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, with stratification by sex and ethnicity/race..." "To avoid any bias in assigning participants to diet groups, staff conducting recruitment and enrollment were masked to sequence. The study director assigned participants to groups." No problematic differences in baseline characteristics between the two groups | Low risk of bias | No masking (open‐label trial). No reasons or details about deviations from intended interventions reported. ITT with imputation for missing values | High risk of bias | 4/36 (11%) in the intervention and 3/37 (8%) in the control group did not have outcome data. "For body weight after dropout, an increase of 1 kg per year was imputed, starting from the last available measurement." No reasons for loss to follow‐up or withdrawal provided; could not judge if related to participants' health status and if missingness depended on its true value | Low risk of bias | Dietitians measured at each visit using electronic scale. No evidence that a different method was used across intervention groups to measure weight. "Data were collected by personnel who were masked to group assignment." | Some concerns | No mention of a prespecified analysis plan; trial registry did not mention the outcome and time point in any versions. Analysis intentions were not reported in sufficient detail to enable assessment. | High risk of bias | Attrition reported in both groups, with no reasons provided. Missingness was addressed through last‐observation‐carried‐forward. It was not possible to determine whether missingness could or was likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions |
| Farnsworth 2003 | Some concerns | No information on how the randomisation sequence was generated. No information on allocation concealment. "There were no significant differences in any of the variables between the diet groups." | Some concerns | No information on blinding of participants and people delivering these dietary interventions; however, blinding of these dietary interventions was highly unlikely. Deviations reported are expected to arise in usual care, e.g. work commitments, pregnancy. 66 randomised, 57 completed and 56 analysed. Per protocol analysis. "...data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." 1/57 participant data excluded | Low risk of bias | 5/33 (15%) in the intervention and 5/33 (15%) in the control group did not have data available. Data from n = 1 participant where missingness possibly influenced by true value. "Six subjects withdrew before study commencement because of family and work commitments; in addition, one subject withdrew because of pregnancy during the course of the study, the clinic lost contact with one subject, and data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." "... one subject declined to undergo a DXA scan for personal reasons." | Low risk of bias | Body weight measured after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a prespecified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Data from one subject was excluded due to 'noncompliance' with the diet, but other reasons for loss to follow‐up were expected to arise in usual care. No pre‐registered document available to assess analysis intentions |
| Foraker 2014 | Some concerns | "Women were randomized using stratified randomization, based on BMI (< 30 kg/m2 vs. ≥ 30 kg/m2) to ensure equal distribution of overweight and obese participants into each intervention group." No information on allocation concealment. No problematic imbalance in baseline characteristics between groups | Low risk of bias | Double‐masked: investigator and outcomes assessor. Deviations reported were expected to arise in usual care, e.g. personal/family medical issues, time constraints. Modified ITT: completers analysed "number still under follow‐up". | High risk of bias | 13/38 (34%) in the intervention and 15/41 (37%) in the control group did not have data available. High attrition in both groups with limited reporting of reasons, but likely that such high attrition missingness was possible and likely to depend on true value of outcome. | Low risk of bias | Anthropometric tests at each visit included body‐weight measurements. No evidence that a different method was used across intervention groups to measure weight. Double‐masked: investigator and outcomes assessor | Some concerns | No mention of prespecified data analysis plan, and trial registry at date prior to study publication did not mention the outcome and time point (mentioned only number of women who lost weight as an outcome). Analysis intentions were not reported in sufficient detail to enable an assessment; weight findings were emphasised as part of the primary aim in the paper's methods and trial registry; with limited reporting of these findings in the paper and none reported in the abstract. | High risk of bias | No specific information on allocation concealment. High attrition in both groups with limited reasons reported; therefore, missingness could and was likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Foster 2010 | Some concerns | "Using a random‐number generator, we randomly assigned participants within each site to treatment with either a low‐carbohydrate or low‐fat, calorie‐restricted diet for 2 years." No information on allocation concealment. No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | Masking: none; open‐label. Deviations reported were expected to arise in usual care, e.g. dissatisfied with the programme, pregnancy, life stressors, relocated. ITT analysis with n = 307; all randomised. "We used a random‐effects linear model that was fitted to all observed data for each variable on each of the 307 participants for the primary analysis." | Low risk of bias | 25/153 (16%) in the intervention and 19/154 (12%) in the control group did not have data available. "..longitudinal models implemented for this study relax this missing‐completely‐at‐random assumption in various ways." "Under the sensitivity analysis based on imputing missing outcomes with the highest (13.795) and lowest (‐18.355) random‐effects slopes (that is, change in weight per month) under the mixed‐effects model for weight, our qualitative findings were not sensitive to either imputation approach." Results of sensitivity analyses not provided | Low risk of bias | Body weight was measured at each treatment visit on calibrated scales while participants wore light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "Assessors were not blinded to treatment condition." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan; trial registry at date prior to study publication mentioned outcome, but did not mention this time point. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods |
| Frisch 2009 | Some concerns | "Participants (n = 200) were randomly assigned by computer‐generated random number lists in two equal groups…" No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | No masking (open‐label trial). Deviations reported were expected to arise in usual care, e.g. non‐compliance. ITT analysis conducted | High risk of bias | 5/100 (5%) in the intervention and 11/100 (11%) in the control group did not have data available. Data were evaluated by ITT and per protocol method. ITT done with baseline values carried forward. "Results did not differ when the per protocol method was used (data not shown)." 4/100 (4%) in the intervention and 10/100 (10%) in the control group withdrew due to non‐compliance, which may be related to change in weight (missingness possibly influenced by true value). | Low risk of bias | Participants were weighed using a calibrated electronic scale. No evidence that a different method was used across intervention groups to measure weight. Open‐label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan; trial registry at date prior to study publication mentioned outcome, but did not mention this time point. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported with reasons such as 'non‐compliance', therefore missingness could have and likely depended on the true value of the outcome. Both per protocol and baseline values carried forward approaches were used for missing data. Lack of information in the pre‐registered methods |
| Gardner 2007 | Low risk of bias | "Randomization was conducted in blocks of 24 (6 per treatment group) and occurred by having a blinded research technician select folded pieces of paper with group assignments from an opaque envelope." No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking: single ("Clinic and laboratory staff members were blinded to treatment assignment"). Deviations reported were expected to arise in usual care, e.g. moved away, disliked diet, pregnancy. ITT analysis conducted | High risk of bias | 6/77 (8%) in the intervention and 15/79 (19%) in the control did not have data available. ITT with baseline values carried forward. "Also for exploratory purposes, all analyses of weight and secondary outcome measures were tested using only available data, without using baseline values carried forward for missing data or other imputation methods. There were no substantive differences in any of these findings compared with the analyses with baseline values carried forward and, therefore, only the primary analyses are presented." 5/9 in the intervention and 5/18 in the control group withdrew due to health and "other" reasons (vague); but attrition was differential and thus missingness could and was likely to depend on the true value. | Low risk of bias | "Body weight was measured to the nearest 0.1 kg on a calibrated clinical scale." No evidence that a different method was used across intervention groups to measure weight. "Clinic and laboratory staff members were blinded to treatment assignment." | Some concerns | No mention of a prespecified analysis plan, trial registry at date prior to study publication mentioned outcome and time point. The time point mentioned in the trial registry at date prior to publication was reported on in the publication. Analysis intentions were not reported in sufficient detail to enable assessment. | High risk of bias | Differential attrition reported, with vague reasons; therefore, missingness could and was likely to depend on the true value of the outcome. Per protocol and baseline values carried forward approaches were used to account for missingness. Lack of information in the pre‐registered methods |
| Goni 2018 | Some concerns | "Subjects were randomly assigned to one of the two diets by a specific logarithm design for the study by MATLAB using stratified block randomization..." No information about allocation concealment. No significant differences between groups at baseline | Low risk of bias | Masking: none; open‐label. The only deviations reported were dropouts. Modified ITT: looked as if only missing outcome data excluded | High risk of bias | 21/75 (28%) in the intervention 19/72 (26%) in the control group did not have data available for this outcome. No reasons for dropouts provided but, with this high attrition in both groups, missingness was possibly and likely to depend on true value of outcome. | Low risk of bias | Assessed using a Tanita BC418 scale. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open‐label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication was reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. High attrition reported in both groups with no reasons provided, therefore missingness could and was likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods |
| Griffin 2013 | Low risk of bias | "The study consisted of a random block design per every four participants. Random allocation was prepared by a researcher not involved in participant enrolment or assignment." "random number table"; "sealed opaque envelopes" (trial registry). No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | "Participants were blind to diet macronutrient composition and were informed eating plans differed only in the type of meat (i.e. red or white)." "...inability to double‐blind the intervention were limitations." Deviations reported were expected to arise in usual care, e.g. did not like diet. ITT analysis conducted | High risk of bias | 12/36 (33%) participants in the intervention and 15/35 (43%) in the control group did not have data available. ITT with last‐observation‐carried‐forward, and sensitivity analysis comparing completers with ITT. 5/6 (83%) in the intervention and 5/9 (56%) in the control discontinued intervention due to illness, too difficult and not losing weight. Very high attrition in both groups; likely that, with such high attrition, missingness was possible and likely to depend on the true value. | Low risk of bias | Taken at every visit on a digital scale with light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "...inability to double‐blind the intervention were limitations." Single‐blind with blinded participants. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication is reported on in the publication. Analysis intentions were not reported in sufficient detail to enable assessment. | High risk of bias | Very high attrition reported in both groups, with reasons such as 'illness', diets being 'too difficult' and 'not losing weight'; missingness could be and was likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods |
| Haufe 2013 | Low risk of bias | "For allocation of the subjects, a computer‐generated list of random numbers was used. The randomization sequence was created using SPSS 18 (Chicago, IL) statistical software and subjects were assigned to reduced carbohydrate or reduced fat diet with a 1:1 allocation using random block sizes of 2, 4, and 6. Study nurses and physicians screening and enrolling volunteers were blinded for the randomization sequence." "Both groups were well matched for gender, age, body weight, body mass index, blood lipid profiles, glucose metabolism, and cardiorespiratory fitness." | High risk of bias | "Blinding of participants for the allocated dietary intervention was impossible." "Except for the dieticians, study nurses and physicians were blinded for the treatment assignment." Deviations reported were expected to arise in usual care, e.g. lost to follow‐up, withdrew consent. Per protocol analysis conducted: 24/28 (86%) in the intervention and 30/33 (91%) in the control group excluded from the analysis including 'protocol violations' | High risk of bias | 25/80 (31%) in the intervention and 29/83 (35%) in the control group did not have data available. ITT with last‐observation‐carried‐forward. High attrition in both groups with limited reporting of reasons, but likely that, with such high attrition, missingness was possibly and likely to depend on true value of outcome. | Low risk of bias | Measured body weight in a standardised manner after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. "....study nurses and physicians were blinded for the treatment assignment." | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in trial registry at the date prior to publication is reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | The majority of data not included in the analyses were due to exclusions based on 'protocol violations'. High overall attrition was reported in both groups, with no reasons (aside from exclusions); missingness could be and was likely dependent on the true value of the outcome. Last‐observation‐carried‐forward approach used for dealing with missingness. Lack of information in the pre‐registered methods |
| Josse 2011 | Some concerns | No information about how the randomisation sequence was generated, or allocation concealment. "Our stratification scheme was adequate, because there were no between‐group differences in any baseline variable." | Low risk of bias | Masking: none; open‐label. Deviations reported were expected to arise in usual care, e.g. moving house, exam time at school, sickness of a relative. Modified ITT "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model‐based multiple imputation." | Low risk of bias | 4/30 (13%) participants in the intervention and 1/30 (3%) in the control group did not have data available for this outcome. The only information reported was "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model based multiple imputation." Based on the reasons provided, it was unlikely that missingness could be dependent on the true value of the outcome. | Low risk of bias | Digital physician's scale used to determine weight. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open‐label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan, but trial registry at the date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication is reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Lack of information in the pre‐registered methods |
| Keogh 2007 | High risk of bias | Clinstat used to generate sequence; "allocation concealment: No" (trial registry). From Table 1 there appeared to be no large differences between groups at baseline. | Low risk of bias | Open (masking not used). Deviations reported were expected to arise in usual care, e.g. personal reasons, unable to comply. Intention‐to‐treat: all randomised were analysed. | Low risk of bias | All participants, 13/13 in intervention and 12/12 in control, had data available. | Low risk of bias | Weight was measured using a Mercury digital scale in light clothing at baseline, and every 2 weeks. No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication is reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Lack of information in the pre‐registered methods |
| Klemsdal 2010 | Some concerns | No information about randomisation or allocation concealment. Footnote to Table 1: "There were no significant differences between groups." | Low risk of bias | Masking: None (open‐label). Deviations reported were vague, but those reported were expected to arise in usual care, e.g. non‐attendance of study visits. ITT analysis: "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." All randomised participants were analysed. | High risk of bias | Short‐term outcomes: 16% of participants overall did not have data available (not reported per trial arm). "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." No mention of sensitivity analysis. No reasons for lost to follow‐up or withdrawal provided and therefore we could not make a judgement about whether missingness was dependent on the true value of the outcome. | Low risk of bias | "Body weight...measured with standard methods." No evidence that a different method was used across intervention groups to measure weight. Masking: None (open ‐label). Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to the study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication was reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition with no reasons reported for the overall group; it was therefore not possible to determine whether missingness could be or was likely dependent on the true value of the outcome. Missingness was addressed by using last‐observation‐carried‐forward. Lack of information in the pre‐registered methods |
| Landers 2002 | Some concerns | No information on randomisation or allocation concealment. Families were assigned to the same dietary intervention. Baseline characteristics were not reported, with the exception of body weight, fat mass and lean mass; all of which did not appear to be significantly different. | Low risk of bias | No information about blinding, however, blinding of participants and people delivering these dietary interventions was highly unlikely. Deviations reported were expected to arise in usual care, e.g. did not attend follow‐up visits. Modified ITT excluding participants with missing outcome data | High risk of bias | Data were not available for 12/28 (43%) participants in the intervention and 12/33 (36%) in the control group. High attrition in both groups with limited reporting of reasons, but likely that, with such high attrition, missingness was possibly and likely to depend on true value of outcome. | Low risk of bias | Measured in light clothing without shoes at the initial visit. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified data analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and was likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions |
| Lasker 2008 | Some concerns | "Subjects were blocked on gender, matched on age...and fasting glucose..." No information on allocation concealment. Significant baseline imbalance for insulin; no significant differences for 13 other characteristics at baseline | High risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions was highly unlikely. Deviations reported were expected to arise in usual care, e.g. training for marathon. Per protocol analysis (participants excluded for lack of adherence to protocol: 23% excluded for various reasons, including lack of adherence, but breakdown by trial arm and reason not reported) | High risk of bias | Data were not available for 7/32 (22%) participants in the intervention and 8/33 (24%) in the control group. High attrition with reasons including non‐compliance, which may be related to change in weight, so likely that with such high attrition and this reason, some missingness was possibly and likely to depend on true value of outcome | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified data analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Some participants were excluded from the analysis due to a lack of adherence, with high attrition reported in both trial arms. Based on these numbers and known exclusions for lack of adherence, missingness could be and was likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions |
| Layman 2005 | Some concerns | "Note that the randomization was blocked on age and BMI for each wave of recruitment." No information on allocation concealment. "Baseline characteristics did not differ among subjects in each of the treatment groups..." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions was highly unlikely. No deviations or dropouts reported. Appeared to be a full ITT with number randomised equal to number analysed | Low risk of bias | All randomised participants appeared to have been analysed. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified data analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions |
| Layman 2009 | Some concerns | No information on how the randomisation sequence was generated. No information about allocation concealment. "Randomization after stratifying participants for gender, age, BMI, and TC was successful with no significant differences between treatment groups in baseline characteristics." | Low risk of bias | "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Deviations were expected to arise in usual care, e.g. personal reasons, unable to comply. "Prior to the 4‐mo data collection, 15 participants withdrew from the CHO group (9 stated personal reasons and 6 stated unable to comply with diet protocol) and 12 participants withdrew from the PRO group (11 stated personal reasons and 1 stated inability to comply with diet protocol)." Modified ITT conducted | High risk of bias | 12/64 (19%) in the intervention and 15/66 (23%) in the control group did not have data available. No methods used to correct for bias, or sensitivity analyses conducted. 6/15 (40%) withdrawals in the intervention and 1/12 (8%) withdrawals in the control group were due to inability to comply with diet. Inability to comply may have been related to change in weight, and high attrition in the control group, thus missingness could and was likely to depend on the true value. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified data analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition reported in both groups, with high attrition in the control group, and some attrition in both groups due to an 'inability to comply with diet'; therefore missingness could and was likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions |
| Lean 1997 | Some concerns | No information on randomisation or allocation concealment. "All variables were not significantly different between the two diet treatment groups." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions was highly unlikely. No reasons or details about deviations from intended interventions reported. ITT with last‐observation‐carried‐forward conducted | High risk of bias | 13/53 (25%) in the intervention and 15/57 (26%) in the control group did not contribute data to this outcome. ITT with last‐observation‐carried‐forward conducted. High attrition in both groups with no reasons reported but, with this high attrition, some missingness was possibly and likely to depend on true value of outcome. | Low risk of bias | "Measurements were made with subjects wearing light clothes using regularly calibrated scales..." Reference to WHO procedures (classification and description of anthropometric data). No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified data analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition in both groups, with no reasons reported; therefore missingness could and was likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions |
| Lim 2010 | Some concerns | No information about randomisation or allocation concealment. Baseline characteristics table showed small differences, consistent with differences resulting by chance with small sample sizes. | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions was highly unlikely. Deviations reported were expected to arise in usual care, e.g. travel, work commitment, did not like diet. Modified ITT with all partial data contributed by non‐completers included in linear mixed effects model | High risk of bias | 6/30 (20%) in the intervention and 8/30 (27%) in the control group did not have data available. ITT with all partial data contributed by non‐completers included in linear mixed effects model. Results of ITT not presented and no sensitivity analyses performed (author correspondence). High attrition in control group and some missingness could possibly and was likely to depend on true value of outcome. | Low risk of bias | Recorded in light clothing on Mercury digital scales. No evidence that a different method was used across intervention groups to measure weight. No information about blinding. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified data analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and was likely to depend on the true value of the outcome. Partial data from missing participants included in linear mixed effects model, but results of this ITT analysis not reported. No pre‐registered document available to assess analysis intentions |
| Liu 2013 | Low risk of bias | No information about sequence generation. "Randomisation was conducted by a statistician who was not involved in any other aspects of the study by using block randomisation, stratified by 10‐year age categories." "Although the participants were randomised by age, baseline BMI levels of the two groups were similar after randomisation." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions was highly unlikely. Deviations reported were expected to arise in usual care, e.g. personal reasons, busy schedule. Modified ITT: one dropout due to busy schedule in control group excluded | Low risk of bias | 1/25 (4%) in the intervention and 1/25 (4%) in the control group did not have data available for this outcome. | Low risk of bias | "Body weight was measured to the nearest 0.1 kg at the beginning and then biweekly during the trial using an electronic scale." No evidence that a different method was used across intervention groups to measure weight. Masking: single (outcomes assessor) | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication is reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods |
| Marco‐Benedi 2019 | Low risk of bias | "Randomization was performed by using an online software..." Paper reported "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding (trial registry reported 'Open‐label'). Only one out of numerous outcomes was significantly different between groups at baseline. | Low risk of bias | Trial registry stated "Masking: None (open‐label)", but paper reported "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding. Deviations reported were expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 5/40 (12.5%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. Reason for withdrawal was only available for 1/13 excluded participants. Difficult to judge whether it was likely that missingness was related to true value for 12/13 participants, based on reasons provided (unknown, personal reasons) | Low risk of bias | "Body weight was measured...with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. Trial registry stated "Masking: None (open‐label)". Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan, but trial registry at the date prior to the study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication was reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with vague reasons for loss to follow‐up and one exclusion; therefore, it was not possible to judge whether missingness could be or was likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods |
| Mateo‐Gallego 2017 | Some concerns | "We carried out the randomization process by generating a number sequence (including 93 correlative numbers because this was the number of subjects we planned to randomize) in the following webpage: https://www.random.org. The number for each participant was correlatively assigned (e.g; the first randomized women was assigned with number 1)" (from author correspondence). No information about allocation concealment. "There were no significant between‐group differences at baseline." | Low risk of bias | "Participants were blinded to their assigned macronutrient composition." "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." Study was reported as single‐blind (masking: single (participant)). Deviations reported were expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 3/30 (10%) in the intervention and 6/30 (20%) in the control group did not have data available for this outcome. Limited reporting of reasons for missingness, therefore difficult to judge whether missingness could or was likely to be dependent on the true value of the outcome | Low risk of bias | "Body weight was measured in subjects without shoes to the nearest 0.1 kg with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." | Some concerns | No mention of prespecified analysis plan, but trial registry at the date prior to the study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication was reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported in both groups with limited reporting of reasons, therefore, it is not possible to judge if missingness could be or was likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods |
| Ooi 2021 | Some concerns | "This was a randomized single‐blinded, placebo‐controlled study where subjects were block randomly assigned by gender and BMI groups…" Allocation concealment: no trial registry available and no information in paper. No significant differences between groups at baseline | High risk of bias | Subjects blinded only to supplements. No information about blinding of diets, however, blinding of participants highly unlikely, the study was stated as single‐blind, thus people delivering these dietary interventions were unblinded. Deviations reported were expected to arise in usual care e.g family, work or personal commitments. Per protocol analysis, with exclusion of available data for reasons such as non‐compliance and inadequate weight loss. Vague reasons for exclusions and specific numbers excluded per trial arm were unclear, with 8% of subjects being excluded by the principal investigator. | High risk of bias | 3/43 (7%) in the intervention and 7/44 (16%) in the control group did not have data available. "Study subjects with available information for each analysis were included and missing data were not imputed." Study subjects that did not comply with the diets or did not lose a specific amount of weight were excluded from analyses by the investigators; thus missingness in the outcome could and was likely to depend on its true value. | Low risk of bias | "Height and weight were taken using a SECA 763 digital scale…" No evidence that a different method was used across intervention groups to measure weight. The study was single‐blinded for participants to supplements. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan, no trial registry. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Subjects were likely not blinded to dietary allocation. Per protocol analysis conducted with non‐compliant participants excluded. Missing data were not imputed. Exclusions of available data were done based on non‐compliance with diet or inadequate weight loss, thus missingness in the outcome could and was likely to depend on its true value. No pre‐registered document available to assess analysis intentions |
| Parr 2016 | High risk of bias | "The randomization will be initially stratified by baseline BMI values (27–29, 30–34, and 35–40 kg/m2) and sex. Subsequently, simple randomisation by roll of dice within the stratified segregation will be used to allocate individuals to each of the 3 groups." "Allocation is not concealed" "No significant differences were observed among groups at baseline for any variable (P > 0.05)." | High risk of bias | "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment." From registry: open (masking not used). Deviations reported were expected to arise in usual care, e.g. time commitment, ankle injury. Per protocol (participants excluded from analysis due to compliance). 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance and 1/8 (12.5%) due to being an outlier | High risk of bias | 10/39 (14%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance, which may have been related to change in weight; thus, missingness could and was likely dependent on the true value. | Low risk of bias | Measured with DEXA. No evidence that a different method was used across intervention groups to measure weight. "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment.", but from the registry: "open (masking not used)". | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication was reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition was reported in both groups, with nearly half of all missing data due to participants being excluded from the per protocol analysis due to 'non‐compliance' and being an 'outlier'. Missingness could and was likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods |
| Sacks 2009 | Low risk of bias | "Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian, after eligibility of a participant was confirmed." Independent trial unit, fully funded by public funds and large trial; it was likely the sequence was random. " Baseline characteristics were similar among participants assigned to the four diets..." | Low risk of bias | "Blinding was maintained by the use of similar foods for each diet. Staff and participants were taught that each diet adhered to principles of a healthful diet..." True blinding is a known challenge with dietary interventions. Deviations reported were expected to arise in usual care, e.g. moved away, did not appear at scheduled appointments. Full ITT: "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." | High risk of bias | Linked paper reported 39/201 (19%) in the intervention and 47/204 (23%) in the control group did not have data available for this outcome at 6 months (De Jonge et al 2012); 2‐year attrition (Sacks 2009) reported less attrition per group. Single imputation "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." No sensitivity analyses (author correspondence). No detailed reasons for loss to follow‐up or withdrawals provided; high attrition in the control group thus missingness could and was likely to depend on the true value. | Low risk of bias | "Body weight, the primary outcome variable, was measured by calibrated hospital scales, in the morning before breakfast and after urinating, clothed in a hospital gown, on two nonconsecutive days at baseline, and at 6 and 24 months; and on a single day at 12 and 18 months." No evidence that a different method was used across intervention groups to measure weight. "Investigators and staff who measured outcomes were unaware of the diet assignment of the participants." | Some concerns | No mention of prespecified analysis plan; trial registry at date prior to study publication mentioned outcome, but did not mention this time point. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with high attrition in the control group, and limited reporting of reasons; therefore missingness could be and was likely dependent on the true value of the outcome. Simple imputation was performed, but no sensitivity analyses. Lack of information in the pre‐registered methods |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | High risk of bias | From registry: "Open (masking not used)". Deviations reported were expected to arise in usual care, e.g. work commitments, personal reasons. Per protocol analysis, ITT with last‐observation‐carried‐forward also performed but data not reported. 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons. | High risk of bias | 10/55 (18%) in the intervention and 11/54 (20%) in the control group did not have data available for this outcome. ITT with last‐observation‐carried‐forward: "The ITT with either baseline values carried forward or the last follow‐up visit carried forward for those who did not complete the study also showed no difference in weight loss between the diets (P <= 0.23)." 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons, which may be related to change in weight; thus missingness could or was likely dependent on the true value of the outcome. | Low risk of bias | "Body mass was measured using calibrated electronic digital scales...to the nearest 0.05 kg, with subjects wearing light clothing and no footwear." No evidence that a different method was used across intervention groups to measure weight. From registry: "Open (masking not used)". Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan; trial registry at date prior to study publication mentioned outcome, but did not mention this time point. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition was reported in both trial arms, with high attrition in the control group due to unclear reasons and 'non‐compliance'; therefore, missingness could and was likely to be related to the true value of the outcome. ITT analyses with baseline values and last‐observations‐carried‐forward were conducted, but neither were considered appropriate to address potential bias due to missingness. Lack of information in the pre‐registered methods |
| Veum 2017 | Some concerns | "The list of prescreening appointments was used to randomly assign the participants by drawing ballots and blocking with block sizes of 2." No information about allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | From registry: "Masking: None (open‐label)" "All participants were informed of group allocation after the baseline measurements and sampling. The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Deviations reported were expected to arise in usual care, e.g. busy job, fracture. "We also performed an intention‐to‐treat (ITT) analysis that included all randomly assigned participants except 2 cases that were lost before baseline (44 eligible participants in total). These 2 were unaware of the group allocation and did not provide any dietary or clinical data." | Low risk of bias | 4/24 (17%) in the intervention and 4/22 (18%) in the control group did not have data available for this outcome. "Notably, the results from the PP and ITT analyses did not differ in nominal significance for any of the primary or secondary outcomes. Our missing data analysis (data not shown), including Little’s missing completely at random test, indicated that the dropouts were missing (completely) at random, and we found no significant differences between dropouts and completers in baseline characteristics, except for significant higher diastolic blood pressure, total cholesterol (TC), and LDL cholesterol in completers. In the reported PP and ITT analyses, we used mixed models without imputation to handle missing data. However, we also conducted an ITT analysis based on a full dataset in which missing values in the original data were replaced by values from multiple imputation (data not shown)." | Low risk of bias | Taken barefoot and wearing light clothing and asked to use restroom shortly before. No evidence that a different method was used across intervention groups to measure weight. "The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan; trial registry at date prior to study publication mentioned outcome, but did not mention this time point. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods |
| Volek 2009 | Some concerns | No information about randomisation or allocation concealment. No apparent imbalances between two groups at baseline | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions was highly unlikely. No deviations or dropouts reported. Appeared to be a full ITT with number randomised equal to number analysed | Low risk of bias | Number randomised equalled number analysed. | Low risk of bias | "Body mass was measured in the morning after an overnight fast to the nearest 100 g on a calibrated digital scale." No evidence that a different method was used across intervention groups to measure weight. "Analyses were performed by the same blinded technician." | Some concerns | No mention of prespecified data analysis plan. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | Some concerns | Open (masking not used). Deviations reported were expected to arise in usual care, e.g. relocated overseas, unable to commit to study protocol/time requirements. Per protocol analysis: 3/59 and 1/64 participants excluded due to being unable to commit to study protocol or did not receive intervention. 3% of total not included (4/123) | High risk of bias | 7/59 (12%) in the intervention and 6/64 (9%) in the control group did not have data available. Modified ITT only done at 52 weeks using maximal likelihood mixed model analysis. "Overall, the participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks..."; missingness could and was likely to depend on the true value. | Low risk of bias | "... body weight was measured using calibrated electronic digital scales (Mercury; AMZ 14, Tokyo, Japan)." No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication was reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition was reported in both groups, and authors remarked that "...participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks…; missingness was therefore likely dependent on the true value of the outcome. Furthermore, participants were excluded for an inability to commit to the study protocol or not receiving the intervention. Lack of information in the pre‐registered methods |
Risk of bias for analysis 1.2 Change in body weight (kg) at 3 to < 12 months: subgroup similarity of energy prescription.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.2.1 Similar energy prescriptions or approaches to restriction in both diets | ||||||||||||
| Bales 2017 | Low risk of bias | We used a computerised centralised randomisation scheme blocking by race and marital or partner status. "...also blocked by functional status..." "The 1:2 allocation to the C‐WL and HP‐WL groups was chosen...to make within‐group comparisons in the protein group in subsequent analyses. This oversampling of the protein group did not cause a substantial loss of power for the between‐group comparisons; the relative power for a 2:1 split is 0.94(23)." No statements or statistical tests about differences between groups, no problematic imbalance suspected. | Low risk of bias | Not blinded (open label trial) (trial registry). Deviations reported are expected to arise in usual care: "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study." Number analysed unclear, but seems to be a modified ITT: "The study was conducted under “intent to treat” criteria." | High risk of bias | 22/51 (43%) participants in the intervention and 16/29 (55%) participants in the control arm did not have data available. "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study. In particular, the tendency for more dropouts among minorities and those with less education..." Very high attrition, thus missingness could and is likely to depend on the true value. | Low risk of bias | Weekly weigh‐ins. No evidence that a different method was used across intervention groups to measure weight. Open‐label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | ...treatment codes revealed only after the study statistician locked the database at the end of the trial. No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. All time points mentioned in trial registry reported on in publication. Analysis intentions are not reported in sufficient detail prior to study publication to enable an assessment. | High risk of bias | Very high attrition with a variety of reasons relating to health, therefore some missingness could be and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Benassi‐Evans 2009 | Some concerns | "Subjects were randomised to one of two dietary interventions…" No further information about allocation sequence or concealment. "Subject characteristics were not significantly different between groups at baseline." | Low risk of bias | No information, but blinding of participants and people delivering the interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. No details about analysis are reported, but looks to be intention to treat (number randomised per group = number analysed per group). | High risk of bias | No details about attrition reported, but some attrition is implied: "...in the subjects who completed the 12 weeks intensive weight loss and 9 months weight maintenance..." No further information or evidence that the result was not biased due to missing data are provided. | Low risk of bias | Volunteers fasted overnight and were weighed (model AMZ14; Mercury Digital Scales, Tokyo, Japan) wearing light clothing and no shoes at weeks. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition is implied, with no reasons or evidence to show that results were not biased by this missingness. No pre‐registered document available to assess analysis intentions. |
| Calleja‐Fernández 2012 | Some concerns | Random allocation to each dietary group was performed by the main investigator (MDBP) by using the Web site Randomization.com (http://www.randomization.com) upon the request of the study dietitian (ACF) after the eligibility of a participant was confirmed and assignment to either of the insulin resistance or insulin sensitivity groups had been performed. No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected. | Low risk of bias | No information on blinding of participants, however, blinding is highly unlikely. People delivering these dietary interventions were unblinded. No reasons or details about deviations from intended interventions reported. ITT analysis conducted. | Low risk of bias | It seems data were available for all participants randomised for 16 week study, but cannot be sure (flowcharts in Fernandez et al 2012 and Ballesteros‐Pomar 2009 incongruent). | Low risk of bias | "Body weight was measured to the nearest 0.5 kg and then taken using calibrated digital scales…" No evidence that a different method was used across intervention groups to measure weight. "The medical assessments were blinded to the type of diet being followed." | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions. |
| Cornier 2005 | Some concerns | No information on how randomisation was done or allocation concealment. "Subject characteristics are summarized in Table 1. BW, BMI, percentage body fat, RMR, and RQ were similar in all groups." | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. "Forty‐four obese, healthy normoglycemic women 23 to 53 years old with BMI of 30 to 35 kg/m2 were screened, and 21 of them were enrolled and completed the 16‐week intervention." ITT analysis conducted: number randomised equals number analysed; no subjects lost to follow‐up. | Low risk of bias | Number randomised equals number analysed; no subjects lost to follow‐up. | Low risk of bias | Once a week, subjects were weighed. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions. |
| Dyson 2007 | Low risk of bias | "Randomization was undertaken by means of sealed envelopes equivalent to the number of subjects and filled fifty‐fifty with an indicator of either a low‐carbohydrate diet or healthy‐eating advice. Two separate sets of envelopes were prepared for both diabetic and non‐diabetic subjects. An independent observer witnessed randomization." Author reported in correspondence that no formal test of hypothesis was done for difference at baseline for non‐diabetic participants. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care: n=3 of randomised participants in balanced group refused follow‐up as not allocated to low carbohydrate diet. Modified ITT conducted. | High risk of bias | No participants in the intervention group and 3/7 (43%) in the control group did not have data. Modified ITT with last observation carried forward. High attrition in control group, and differential attrition. Refusal of follow‐up due to not being allocated to low carbohydrate may be influenced by expected change in weight, therefore missingness could and likely influenced by the true value. | Low risk of bias | Body weight recorded monthly. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition in the control group, with disappoinment at not being assigned to the low carbohydrate diet cited as reason; therefore missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Farnsworth 2003 | Some concerns | No information on how the randomisation sequence was generated. No information on allocation concealment. "There were no significant differences in any of the variables between the diet groups." | Some concerns | No information on blinding of participants and people delivering these dietary interventions, however blinding of these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. work commitments, pregnancy. 66 randomised, 57 completed and 56 analysed. Per protocol analysis. "...data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." 1/57 participant data excluded. | Low risk of bias | 5/33 (15%) in the intervention and 5/33 (15%) in the control group did not have data available. Data from n=1 participant where missingness possibly influenced by true value. "Six subjects withdrew before study commencement because of family and work commitments; in addition, one subject withdrew because of pregnancy during the course of the study, the clinic lost contact with one subject, and data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." "... one subject declined to undergo a DXA scan for personal reasons." | Low risk of bias | Body weight measured after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Data from one subject was excluded due to 'noncompliance' with the diet, but other reasons for loss to follow‐up were expected to arise in usual care. No pre‐registered document available to assess analysis intentions. |
| Foraker 2014 | Some concerns | "Women were randomized using stratified randomization, based on BMI (< 30 kg/m2 vs. ≥ 30 kg/m2) to ensure equal distribution of overweight and obese participants into each intervention group." No information on allocation concealment. No problematic imbalance in baseline characteristics between groups | Low risk of bias | Double masked: investigator and outcomes assessor. Deviations reported are expected to arise in usual care, e.g. personal/family medical issues, time constraints. Modified ITT: completers analysed "number still under follow‐up". | High risk of bias | 13/38 (34%) in the intervention and 15/41 (37%) in the control group did not have data available. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Anthropometric tests at each visit included body weight measurements. No evidence that a different method was used across intervention groups to measure weight. Double masked: investigator and outcomes assessor. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint (mentions only number of women who lose weight as outcome). Analysis intentions are not reported in sufficient detail to enable an assessment; weight findings are emphasised as part of the primary aim in the paper's methods and trial registry; with limited reporting of these findings in the paper and none reported in the abstract. | High risk of bias | No specific information on allocation concealment. High attrition in both groups with limited reasons reported, therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Frisch 2009 | Some concerns | "Participants (n = 200) were randomly assigned by computer‐generated random number lists in two equal groups…" No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | No masking (open label trial). Deviations reported are expected to arise in usual care, e.g. non‐compliance. ITT analysis conducted. | High risk of bias | 5/100 (5%) in the intervention and 11/100 (11%) in the control group did not have data available. Data were evaluated by ITT and per protocol method. ITT done with baseline values carried forward. "Results did not differ when the per protocol method was used (data not shown)." 4/100 (4%) in the intervention and 10/100 (10%) in the control group withdrew due to non‐compliance, which may be related to change in weight (missingness possibly influenced by true value). | Low risk of bias | Participants were weighed using a calibrated electronic scale. No evidence that a different method was used across intervention groups to measure weight. Open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported with reasons such as 'non‐compliance', therefore missingness could have and likely depended on the true value of the outcome. Both per protocol and baseline values carried forward approaches were used for missing data. Lack of information in the pre‐registered methods. |
| Goni 2018 | Some concerns | "Subjects were randomly assigned to one of the two diets by a specific logarithm design for the study by MATLAB using stratified block randomization..." No information about allocation concealment. No significant differences between groups at baseline | Low risk of bias | Masking: none; open label. The only deviations reported are drop‐outs. Modified ITT: looks as if only missing outcome data excluded. | High risk of bias | 21/75 (28%) in the intervention 19/72 (26%) in the control group did not have data available for this outcome. No reasons for drop‐out provided, but with this high attrition in both groups missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Assessed using a Tanita BC418 scale. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. High attrition reported in both groups with no reasons provided, therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Griffin 2013 | Low risk of bias | "The study consisted of a random block design per every four participants. Random allocation was prepared by a researcher not involved in participant enrolment or assignment." "random number table"; "sealed opaque envelopes" (trial registry). No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | "Participants were blind to diet macronutrient composition and were informed eating plans differed only in the type of meat (i.e. red or white)." "...inability to double‐blind the intervention were limitations." Deviations reported are expected to arise in usual care, e.g. did not like diet. ITT analysis conducted. | High risk of bias | 12/36 (33%) participants in the intervention and 15/35 (43%) in the control group did not have data available. ITT with last observation carried forward, and senstivity analysis comparing completers with ITT. 5/6 (83%) in the intervention and 5/9 (56%) in the control discontinued intervention due to illness, too difficult and not losing weight. Very high attrition in both groups; likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | Taken at every visit on a digital scale with light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "...inability to double‐blind the intervention were limitations." Single‐blind with blinded participants. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Very high attrition reported in both groups, with reasons such as 'illness', diets being 'too difficult' and 'not losing weight'; missingness could be and is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Haufe 2013 | Low risk of bias | "For allocation of the subjects, a computer‐generated list of random numbers was used. The randomization sequence was created using SPSS 18 (Chicago, IL) statistical software and subjects were assigned to reduced carbohydrate or reduced fat diet with a 1:1 allocation using random block sizes of 2, 4, and 6. Study nurses and physicians screening and enrolling volunteers were blinded for the randomization sequence." "Both groups were well matched for gender, age, body weight, body mass index, blood lipid profiles, glucose metabolism, and cardiorespiratory fitness." | High risk of bias | "Blinding of participants for the allocated dietary intervention was impossible." "Except for the dieticians, study nurses and physicians were blinded for the treatment assignment." Deviations reported are expected to arise in usual care, e.g. lost to follow‐up, withdrew consent. Per protocol analysis conducted: 24/28 (86%) in the intervention and 30/33 (91%) in the control group excluded from the analysis including 'protocol violations'. | High risk of bias | 25/80 (31%) in the intervention and 29/83 (35%) in the control group did not have data available. ITT with last observation carried forward. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured body weight in a standardised manner after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. "....study nurses and physicians were blinded for the treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | The majority of data not included in the analyses were due to exclusions based on 'protocol violations'. High overall attrition was reported in both groups, with no reasons (aside from exclusions); missingness could be and is likely dependent on the true value of the outcome. Last observation carried forward approach used for dealing with missingness. Lack of information in the pre‐registered methods. |
| Josse 2011 | Some concerns | No information about how the randomisation sequence was generated, or allocation concealment. "Our stratification scheme was adequate, because there were no between‐group differences in any baseline variable." | Low risk of bias | Masking: none; open label. Deviations reported are expected to arise in usual care, e.g. moving house, exam time at school, sickness of a relative. Modified ITT "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model‐based multiple imputation." | Low risk of bias | 4/30 (13%) participants in the intervention and 1/30 (3%) in the control group did not have data available for this outcome. The only information reported is "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model based multiple imputation." Based on the reasons provided, it is unlikely that missingness could be dependent on the true value of the outcome. | Low risk of bias | Digital physician's scale used to determine weight. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Lack of information in the pre‐registered methods. |
| Keogh 2007 | High risk of bias | Clinstat used to generate sequence; "allocation concealment: No" (trial registry). From Table 1 there appeared to be no large differences between groups at baseline. | Low risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. personal reasons, unable to comply. Intention to treat: all randomised were analysed. | Low risk of bias | All participants, 13/13 in intervention and 12/12 in control, had data available. | Low risk of bias | Weight was measured using a Mercury digital scale in light clothing at baseline, and every 2 weeks. No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Lack of information in the pre‐registered methods. |
| Klemsdal 2010 | Some concerns | No information about randomisation or allocation concealment. Footnote to Table 1: "There were no significant differences between groups." | Low risk of bias | Masking: None (Open Label). Deviations reported are vague, but those reported are expected to arise in usual care, e.g. non‐attendance of study visits. ITT analysis: "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." All randomised participants were analysed. | High risk of bias | Short‐term outcomes: 16% of participants overall did not have data available (not reported per trial arm). "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." No mention of sensitivity analysis. No reasons for lost to follow‐up or withdrawal provided and therefore cannot make a judgment about whether missingness was dependent on the true value of the outcome. | Low risk of bias | "Body weight...measured with standard methods." No evidence that a different method was used across intervention groups to measure weight. Masking: None (Open Label). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition with no reasons reported for the overall group, it is therefore not possible to determine whether missingness could be or is likely dependent on the true value of the outcome. Missingness was addressed by using last observation carried forward. Lack of information in the pre‐registered methods. |
| Lasker 2008 | Some concerns | "Subjects were blocked on gender, matched on age...and fasting glucose..." No information on allocation concealment. Significant baseline imbalance for insulin; no significant differences for 13 other characteristics at baseline | High risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. training for marathon. Per protocol analysis (participants excluded for lack of adherence to protocol: 23% excluded for various reasons, including lack of adherence, but breakdown by trial arm and reason not reported). | High risk of bias | Data were not available for 7/32 (22%) participants in the intervention and 8/33 (24%) in the control group. High attrition with reasons including non‐compliance, which may be related to change in weight, so likely that with such high attrition and this reason some missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Some participants were excluded from the analysis due to a lack of adherence, with high attrition reported in both trial arms. Based on these numbers and known exclusions for lack of adherence, missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Layman 2005 | Some concerns | "Note that the randomization was blocked on age and BMI for each wave of recruitment." No information on allocation concealment. "Baseline characteristics did not differ among subjects in each of the treatment groups..." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No deviations or drop‐outs reported. Appears to be a full ITT with number randomised equal to number analysed. | Low risk of bias | All randomised participants appear to have been analysed. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions. |
| Layman 2009 | Some concerns | No information on how the randomisation sequence was generated. No information about allocation concealment. "Randomization after stratifying participants for gender, age, BMI, and TC was successful with no significant differences between treatment groups in baseline characteristics." | Low risk of bias | "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Deviations are expected to arise in usual care, e.g. personal reasons, unable to comply. "Prior to the 4‐mo data collection, 15 participants withdrew from the CHO group (9 stated personal reasons and 6 stated unable to comply with diet protocol) and 12 participants withdrew from the PRO group (11 stated personal reasons and 1 stated inability to comply with diet protocol)." Modified ITT conducted. | High risk of bias | 12/64 (19%) in the intervention and 15/66 (23%) in the control group did not have data available. No methods used to correct for bias, or sensitivity analyses conducted. 6/15 (40%) withdrawals in the intervention and 1/12 (8%) withdrawals in the control group were due to inability to comply with diet. Inability to comply may be related to change in weight, and high attrition in the control group, thus missingness could and is likely to depend on the true value. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition reported in both groups, with high attrition in the control group, and some attrition in both groups due to an 'inability to comply with diet'; therefore missingness could and is likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Lean 1997 | Some concerns | No information on randomisation or allocation concealment. "All variables were not significantly different between the two diet treatment groups." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. ITT with last observation carried forward conducted. | High risk of bias | 13/53 (25%) in the intervention and 15/57 (26%) in the control group did not contribute data to this outcome. ITT with last observation carried forward conducted. High attrition in both groups with no reasons reported, but with this high attrition some missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | "Measurements were made with subjects wearing light clothes using regularly calibrated scales..." Reference to WHO procedures (classification and description of anthropometric data). No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition in both groups, with no reasons reported; therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Lim 2010 | Some concerns | No information about randomisation or allocation concealment. Baseline characteristics table showed small differences, consistent with differences resulting by chance with small sample sizes. | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. travel, work commitment, did not like diet. Modified ITT with all partial data contributed by non‐completers included in linear mixed effects model. | High risk of bias | 6/30 (20%) in the intervention and 8/30 (27%) in the control group did not have data available. ITT with all partial data contributed by non‐completers included in linear mixed effects model. Results of ITT not presented and no sensitivity analyses performed (author correspondence). High attrition in control group and some missingness could possibly and is likely to depend on true value of outcome. | Low risk of bias | Recorded in light clothing on Mercury digital scales. No evidence that a different method was used across intervention groups to measure weight. No information about blinding. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and is likely to depend on the true value of the outcome. Partial data from missing participants included in linear mixed effects model, but results of this ITT analysis not reported. No pre‐registered document available to assess analysis intentions. |
| Marco‐Benedi 2019 | Low risk of bias | "Randomization was performed by using an online software..." Paper reported "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding (trial registry reported 'Open‐label'). Only one out of numerous outcomes was significantly different between groups at baseline. | Low risk of bias | Trial registry states "Masking: None (Open Label)", but paper reports "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding. Deviations reported are expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 5/40 (12.5%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. Reason for withdrawal is only available for 1/13 excluded participants. Difficult to judge whether it is likely that missingness is related to true value for 12/13 participants, based on reasons provided (unknown, personal reasons). | Low risk of bias | "Body weight was measured...with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. Trial registry states "Masking: None (Open Label)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with vague reasons for loss to follow‐up and one exclusion; therefore it is not possible to judge whether missingness could be or is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Mateo‐Gallego 2017 | Some concerns | "We carried out the randomization process by generating a number sequence (including 93 correlative numbers because this was the number of subjects we planned to randomize) in the following webpage: https://www.random.org. The number for each participant was correlatively assigned (e.g; the first randomized women was assigned with number 1)" (from author correspondence). No information about allocation concealment. "There were no significant between‐group differences at baseline." | Low risk of bias | "Participants were blinded to their assigned macronutrient composition." "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." Study is reported as single blind (Masking: Single (Participant)). Deviations reported are expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 3/30 (10%) in the intervention and 6/30 (20%) in the control group did not have data available for this outcome. Limited reporting of reasons for missingness, therefore difficult to judge whether missingness could or is likely to be dependent on the true value of the outcome. | Low risk of bias | "Body weight was measured in subjects without shoes to the nearest 0.1 kg with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported in both groups with limited reporting of reasons, therefore it is not possible to judge if missingness could be or is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Ooi 2021 | Some concerns | "This was a randomized single‐blinded, placebo‐controlled study where subjects were block randomly assigned by gender and BMI groups…" Allocation concealment: no trial registry available and no information in paper. No significant differences between groups at baseline | High risk of bias | Subjects blinded only to supplements. No information about blinding of diets, however blinding of participants highly unlikely, the study is stated as single‐blind, thus people delivering these dietary interventions were unblinded. Deviations reported are expected to arise in usual care e.g family, work or personal commitments. Per protocol analysis, with exclusion of available data for reasons such as non‐compliance and inadequate weight loss. Vague reasons for exclusions and specific numbers excluded per trial arm are unclear, with 8% of subjects being excluded by the principal investigator. | High risk of bias | 3/43 (7%) in the intervention and 7/44 (16%) in the control group did not have data available. "Study subjects with available information for each analysis were included and missing data were not imputed." Study subjects that did not comply with the diets or did not lose a specific amount of weight were excluded from analyses by the investigators; thus missingness in the outcome could and is likely to depend on its true value. | Low risk of bias | "Height and weight were taken using a SECA 763 digital scale…" No evidence that a different method was used across intervention groups to measure weight. The study was single blinded for participants to supplements. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, no trial registry. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Subjects were likely not blinded to dietary allocation. Per protocol analysis conducted with non‐compliant participants excluded. Missing data were not imputed. Exclusions of available data were done based on non‐compliance with diet or inadequate weight loss, thus missingness in the outcome could and is likely to depend on its true value. No pre‐registered document available to assess analysis intentions. |
| Parr 2016 | High risk of bias | "The randomization will be initially stratified by baseline BMI values (27–29, 30–34, and 35–40 kg/m2) and sex. Subsequently, simple randomisation by roll of dice within the stratified segregation will be used to allocate individuals to each of the 3 groups." "Allocation is not concealed" "No significant differences were observed among groups at baseline for any variable (P > 0.05)." | High risk of bias | "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment." From registry: Open (masking not used). Deviations reported are expected to arise in usual care, e.g. time commitment, ankle injury. Per protocol (participants excluded from analysis due to compliance). 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance and 1/8 (12.5%) due to being an outlier. | High risk of bias | 10/39 (14%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance, which may be related to change in weight; thus missingness could and is likely dependent on the true value. | Low risk of bias | Measured with DEXA. No evidence that a different method was used across intervention groups to measure weight. "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment.", but from the registry: "Open (masking not used)" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition is reported in both groups, with nearly half of all missing data due to participants being excluded from the per protocol analysis due to 'non‐compliance' and being an 'outlier'. Missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Sacks 2009 | Low risk of bias | "Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian, after eligibility of a participant was confirmed." Independent trial unit, fully funded by public funds and large trial; it was likely the sequence was random. " Baseline characteristics were similar among participants assigned to the four diets..." | Low risk of bias | "Blinding was maintained by the use of similar foods for each diet. Staff and participants were taught that each diet adhered to principles of a healthful diet..." True blinding is a known challenge with dietary interventions. Deviations reported are expected to arise in usual care, e.g. moved away, did not appear at scheduled appointments. Full ITT "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." | High risk of bias | Linked paper reported 39/201 (19%) in the intervention and 47/204 (23%) in the control group did not have data available for this outcome at 6 months (De Jonge et al 2012); 2‐year attrition (Sacks et al 2009) reports less attrition per group. Single imputation "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." No sensitivity analyses (author correspondence). No detailed reasons for loss to follow‐up or withdrawals provided, high attrition in the control group thus missingness could and is likely to depend on the true value. | Low risk of bias | "Body weight, the primary outcome variable, was measured by calibrated hospital scales, in the morning before breakfast and after urinating, clothed in a hospital gown, on two nonconsecutive days at baseline, and at 6 and 24 months; and on a single day at 12 and 18 months." No evidence that a different method was used across intervention groups to measure weight. "Investigators and staff who measured outcomes were unaware of the diet assignment of the participants." | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with high attrition in the control group, and limited reporting of reasons; therefore missingness could be and is likely dependent on the true value of the outcome. Simple imputation was performed, but no sensitivity analyses. Lack of information in the pre‐registered methods. |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | High risk of bias | From registry: "Open (masking not used)". Deviations reported are expected to arise in usual care, e.g. work commitments, personal reasons. Per protocol analysis, ITT with last observation carried forward also performed but data not reported. 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons. | High risk of bias | 10/55 (18%) in the intervention and 11/54 (20%) in the control group did not have data available for this outcome. ITT with last observation carried forward: "The ITT with either baseline values carried forward or the last follow‐up visit carried forward for those who did not complete the study also showed no difference in weight loss between the diets (p<=0.23)." 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons, which may be related to change in weight; thus missingness could or is likely dependent on the true value of the outcome. | Low risk of bias | "Body mass was measured using calibrated electronic digital scales...to the nearest 0.05 kg, with subjects wearing light clothing and no footwear." No evidence that a different method was used across intervention groups to measure weight. From registry: "Open (masking not used)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition is reported in both trial arms, with high attrition in the control group due to unclear reasons and 'non‐compliance'; therefore missingness could and is likely to be related to the true value of the outcome. ITT analyses with baseline values and last observations carried forward were conducted, but neither were considered appropriate to address potential bias due to missingness. Lack of information in the pre‐registered methods. |
| Veum 2017 | Some concerns | "The list of prescreening appointments was used to randomly assign the participants by drawing ballots and blocking with block sizes of 2." No information about allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | From registry: "Masking: None (Open Label)" "All participants were informed of group allocation after the baseline measurements and sampling. The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Deviations reported are expected to arise in usual care, e.g. busy job, fracture. "We also performed an intention‐to‐treat (ITT) analysis that included all randomly assigned participants except 2 cases that were lost before baseline (44 eligible participants in total). These 2 were unaware of the group allocation and did not provide any dietary or clinical data." | Low risk of bias | 4/24 (17%) in the intervention and 4/22 (18%) in the control group did not have data available for this outcome. "Notably, the results from the PP and ITT analyses did not differ in nominal significance for any of the primary or secondary outcomes. Our missing data analysis (data not shown), including Little’s missing completely at random test, indicated that the dropouts were missing (completely) at random, and we found no significant differences between dropouts and completers in baseline characteristics, except for significant higher diastolic blood pressure, total cholesterol (TC), and LDL cholesterol in completers. In the reported PP and ITT analyses, we used mixed models without imputation to handle missing data. However, we also conducted an ITT analysis based on a full dataset in which missing values in the original data were replaced by values from multiple imputation (data not shown)." | Low risk of bias | Taken barefoot and wearing light clothing and asked to use restroom shortly before. No evidence that a different method was used across intervention groups to measure weight. "The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | Some concerns | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. relocated overseas, unable to commit to study protocol/time requirements. Per protocol analysis: 3/59 and 1/64 participants excluded due to being unable to commit to study protocol or did not receive intervention. 3% of total not included (4/123). | High risk of bias | 7/59 (12%) in the intervention and 6/64 (9%) in the control group did not have data available. Modified ITT only done at 52 weeks using maximal likelihood mixed model analysis. "Overall, the participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks..." missingness could and is likely to depend on the true value. | Low risk of bias | "... body weight was measured using calibrated electronic digital scales (Mercury; AMZ 14, Tokyo, Japan)." No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition was reported in both groups, and authors remark that "...participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks…; missingness is therefore likely dependent on the true value of the outcome. Furthermore, participants were excluded for an inability to commit to the study protocol or not receiving the intervention. Lack of information in the pre‐registered methods. |
| Subgroup 1.2.2 Different, ad libitum energy in treatment diet and restricted in control diet | ||||||||||||
| Aude 2004 | Some concerns | Randomisation was done using a block design by sex to achieve an equal sex distribution. No information on allocation concealment. "At baseline, there were no significant differences in age, weight, BMI, waist‐to‐hip ratio, and any of the lipid variables between the NCEP and MLC groups" (only for completers). | High risk of bias | No information on blinding of participants and people delivering these dietary interventions, but blinding is unlikely. No reasons or details about deviations from intended interventions reported. A quarter of the participants were not included in the analysis: 8/30 participants in the intervention group and 7/30 in the control group were excluded from analysis; 6/60 did not complete the trial (missing), but no information about remaining 9/60 who were not included. | High risk of bias | 8/30 (27%) participants in the intervention and 7/30 (23%) participants in the control arm did not have data available. No reasons for loss to follow‐up or withdrawal provided, so difficult to judge if related to participants' health status, but attrition is high in both groups; thus missingness could and is likely to depend on the true value. | Low risk of bias | Weight was measured with indoor clothing and without shoes on a single scale. No evidence that a different method was used across intervention groups to measure weight. "All anthropometric measurements were obtained by an observer blinded to the treatment assignment." | Some concerns | No trial registry reported and no mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. A quarter of participants were not included in the analysis, with withdrawal reported for 10% of participants. No reasons were provided for the remaining missingness. Attrition is high in both groups with no reasons provided, therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Brehm 2003 | Some concerns | "... after each block of subjects was assessed, the principal investigator used a random number table to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences at baseline. | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. dislike diet, could not make scheduled visit. Modified ITT: excluded only missing patients. | High risk of bias | 7/26 (27%) in the intervention and 14/27 (52%) in the control group did not have data. "In this intention to treat analysis, the initial weights for the subjects who withdrew from the study were used as their follow‐up weights at 3 and 6 months..." Lost to follow‐up and withdrawal reasons are stated for all participants, but very high attrition in the control group and differential attrition, thus thus missingness could and is likely to depend on the true value. | Some concerns | Subjects were weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Differential attrition and high attrition in the control group, therefore missingness could and is likely to be dependent on the true value of the outcome. It is not clear whether outcome assessors were blinded to allocation. No pre‐registered document available to assess analysis intentions. |
| Brehm 2005 | Some concerns | "After each block of subjects was enrolled, the principal investigator used a computerized randomization program to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences between groups at baseline (completers only). | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. pregnancy, disliked diet. Modified ITT: all those who completed the study were analysed. | High risk of bias | 5/25 (20%) in the intervention and 5/25 (20%) in the control group did not have data available. Difficult to judge whether it is likely that missingness is related to the true value for weight, as reasons are not explicit enough. "The most common reason for discontinuing the study was the inability to commit to weekly counseling sessions. Other reasons included pregnancy, new out‐of‐state job, dislike of the low‐carbohydrate diet, and advice against following the low carbohydrate diet by family physician." | Low risk of bias | Weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Reasons for attrition are vague and it is difficult to judge whether it was as a result of the true value of the outcome, but wit 20% attrition in both groups it is likely that some missingness was dependent on true value. No pre‐registered document available to assess analysis intentions. |
| Foster 2010 | Some concerns | "Using a random‐number generator, we randomly assigned participants within each site to treatment with either a low‐carbohydrate or low‐fat, calorie‐restricted diet for 2 years." No information on allocation concealment. No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | Masking: none; open label. Deviations reported are expected to arise in usual care, e.g. dissatisfied with the program, pregnancy, life stessors, relocated. ITT analysis with n=307; all randomised. "We used a random‐effects linear model that was fitted to all observed data for each variable on each of the 307 participants for the primary analysis." | Low risk of bias | 25/153 (16%) in the intervention and 19/154 (12%) in the control group did not have data available. "..longitudinal models implemented for this study relax this missing‐completely‐at‐random assumption in various ways." "Under the sensitivity analysis based on imputing missing outcomes with the highest (13.795) and lowest (‐18.355) random‐effects slopes (that is, change in weight per month) under the mixed‐effects model for weight, our qualitative findings were not sensitive to either imputation approach." Results of sensitivity analyses not provided. | Low risk of bias | Body weight was measured at each treatment visit on calibrated scales while participants wore light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "Assessors were not blinded to treatment condition." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Gardner 2007 | Low risk of bias | "Randomization was conducted in blocks of 24 (6 per treatment group) and occurred by having a blinded research technician select folded pieces of paper with group assignments from an opaque envelope." No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking: single ("Clinic and laboratory staff members were blinded to treatment assignment"). Deviations reported are expected to arise in usual care, e.g. moved away, disliked diet, pregnancy. ITT analysis conducted. | High risk of bias | 6/77 (8%) in the intervention and 15/79 (19%) in the control did not have data available. ITT with baseline values carried forward. "Also for exploratory purposes, all analyses of weight and secondary outcome measures were tested using only available data, without using baseline values carried forward for missing data or other imputation methods. There were no substantive differences in any of these findings compared with the analyses with baseline values carried forward and, therefore, only the primary analyses are presented." 5/9 in the intervention and 5/18 in the control group withdrew due to health and "other" reasons (vague); but attrition is differential and thus missingness could and is likely to depend on the true value. | Low risk of bias | "Body weight was measured to the nearest 0.1 kg on a calibrated clinical scale." No evidence that a different method was used across intervention groups to measure weight. "Clinic and laboratory staff members were blinded to treatment assignment." | Some concerns | No mention of a pre‐specified analysis plan, trial registry at date prior to study publication mentions outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Differential attrition reported, with vague reasons; therefore missingness could and is likely to depend on the true value of the outcome. Per protocol and baseline values carried forward approaches were used to account for missingness. Lack of information in the pre‐registered methods. |
| Landers 2002 | Some concerns | No information on randomisation or allocation concealment. Families were assigned to the same dietary intervention. Baseline characteristics were not reported, with the exception of body weight, fat mass and lean mass; all of which did not appear to be significantly different. | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. did not attend follow‐up visits. Modified ITT excluding participants with missing outcome data. | High risk of bias | Data were not available for 12/28 (43%) participants in the intervention and 12/33 (36%) in the control group. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured in light clothing without shoes at the initial visit. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and is likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Liu 2013 | Low risk of bias | No information about sequence generation. "Randomisation was conducted by a statistician who was not involved in any other aspects of the study by using block randomisation, stratified by 10‐year age categories." "Although the participants were randomised by age, baseline BMI levels of the two groups were similar after randomisation." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. personal reasons, busy schedule. Modified ITT: one drop‐out due to busy schedule in control group excluded. | Low risk of bias | 1/25 (4%) in the intervention and 1/25 (4%) in the control group did not have data available for this outcome. | Low risk of bias | "Body weight was measured to the nearest 0.1kg at the beginning and then biweekly during the trial using an electronic scale." No evidence that a different method was used across intervention groups to measure weight. Masking: Single (Outcomes Assessor). | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Subgroup 1.2.3 No prescriptions reported or unrestricted for both diets | ||||||||||||
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | No information about participant blinding, however, blinding of participants is highly unlikely. "...dietitians were not blinded to the study hypothesis." No reasons or details about deviations from intended interventions reported. "All analyses were conducted using the intention‐to‐treat principle." n=148 randomised, and n=148 analysed. | Low risk of bias | 13/75 (17%) participants in the intervention and 9/73 (12%) participants in the control arm did not have data available. Imputed missing values, and performed sensitivity analyses to determine whether missing at random (MAR) assumptions were met. "We performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption." "We used Markovchain Monte Carlo techniques to impute missing values, including additional covariates..." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses." | Low risk of bias | Body weight was measured to the nearest 0.1 kg using a single calibrated scale. No evidence that a different method was used across intervention groups to measure weight. "all outcome assessors were blinded to the diet group assignment..." | Some concerns | No mention of a pre‐specified analysis plan. "Study protocol and data set: Not available." Trial registry outcomes amended after study publication; but trial registry entry at date prior to study publication mentions the outcome and timepoints. All time points mentioned in trial registry at date prior to study publication, were reported on in publication. Analysis intentions are not reported in sufficient detail prior to study publication to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Ebbeling 2007 | Low risk of bias | "Enrolled participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, with stratification by sex and ethnicity/race..." "To avoid any bias in assigning participants to diet groups, staff conducting recruitment and enrollment were masked to sequence. The study director assigned participants to groups." No problematic differences in baseline characteristics between the two groups | Low risk of bias | No masking (open label trial). No reasons or details about deviations from intended interventions reported. ITT with imputation for missing values. | High risk of bias | 4/36 (11%) in the intervention and 3/37 (8%) in the control group did not have outcome data. "For body weight after dropout, an increase of 1 kg per year was imputed, starting from the last available measurement." No reasons for loss to follow‐up or withdrawal provided, cannot judge if related to participants' health status and if missingness depends on its true value. | Low risk of bias | Dietitians measured at each visit using electronic scale. No evidence that a different method was used across intervention groups to measure weight. "Data were collected by personnel who were masked to group assignment." | Some concerns | No mention of a pre‐specified analysis plan; trial registry does not mention the outcome and timepoint in any versions. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Attrition reported in both groups, with no reasons provided. Missingness was addressed through last observation carried forward. It is not possible to determine whether missingness could or is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Volek 2009 | Some concerns | No information about randomisation or allocation concealment. No apparent imbalances between two groups at baseline | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No deviations or drop‐outs reported. Appears to be a full ITT with number randomised equal to number analysed. | Low risk of bias | Number randomised equals number analysed. | Low risk of bias | "Body mass was measured in the morning after an overnight fast to the nearest 100 g on a calibrated digital scale." No evidence that a different method was used across intervention groups to measure weight. "Analyses were performed by the same blinded technician." | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions. |
Risk of bias for analysis 1.3 Change in body weight (kg) at 3 to < 12 months: subgroup extent of carbohydrate restriction.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.3.1 Very low carbohydrate/ketogenic | ||||||||||||
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | No information about participant blinding, however, blinding of participants is highly unlikely. "...dietitians were not blinded to the study hypothesis." No reasons or details about deviations from intended interventions reported. "All analyses were conducted using the intention‐to‐treat principle." n=148 randomised, and n=148 analysed. | Low risk of bias | 13/75 (17%) participants in the intervention and 9/73 (12%) participants in the control arm did not have data available. Imputed missing values, and performed sensitivity analyses to determine whether missing at random (MAR) assumptions were met. "We performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption." "We used Markovchain Monte Carlo techniques to impute missing values, including additional covariates..." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses." | Low risk of bias | Body weight was measured to the nearest 0.1 kg using a single calibrated scale. No evidence that a different method was used across intervention groups to measure weight. "all outcome assessors were blinded to the diet group assignment..." | Some concerns | No mention of a pre‐specified analysis plan. "Study protocol and data set: Not available." Trial registry outcomes amended after study publication; but trial registry entry at date prior to study publication mentions the outcome and timepoints. All time points mentioned in trial registry at date prior to study publication, were reported on in publication. Analysis intentions are not reported in sufficient detail prior to study publication to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Brehm 2003 | Some concerns | "... after each block of subjects was assessed, the principal investigator used a random number table to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences at baseline. | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. dislike diet, could not make scheduled visit. Modified ITT: excluded only missing patients. | High risk of bias | 7/26 (27%) in the intervention and 14/27 (52%) in the control group did not have data. "In this intention to treat analysis, the initial weights for the subjects who withdrew from the study were used as their follow‐up weights at 3 and 6 months..." Lost to follow‐up and withdrawal reasons are stated for all participants, but very high attrition in the control group and differential attrition, thus thus missingness could and is likely to depend on the true value. | Some concerns | Subjects were weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Differential attrition and high attrition in the control group, therefore missingness could and is likely to be dependent on the true value of the outcome. It is not clear whether outcome assessors were blinded to allocation. No pre‐registered document available to assess analysis intentions. |
| Brehm 2005 | Some concerns | "After each block of subjects was enrolled, the principal investigator used a computerized randomization program to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences between groups at baseline (completers only). | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. pregnancy, disliked diet. Modified ITT: all those who completed the study were analysed. | High risk of bias | 5/25 (20%) in the intervention and 5/25 (20%) in the control group did not have data available. Difficult to judge whether it is likely that missingness is related to the true value for weight, as reasons are not explicit enough. "The most common reason for discontinuing the study was the inability to commit to weekly counseling sessions. Other reasons included pregnancy, new out‐of‐state job, dislike of the low‐carbohydrate diet, and advice against following the low carbohydrate diet by family physician." | Low risk of bias | Weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Reasons for attrition are vague and it is difficult to judge whether it was as a result of the true value of the outcome, but wit 20% attrition in both groups it is likely that some missingness was dependent on true value. No pre‐registered document available to assess analysis intentions. |
| Dyson 2007 | Low risk of bias | "Randomization was undertaken by means of sealed envelopes equivalent to the number of subjects and filled fifty‐fifty with an indicator of either a low‐carbohydrate diet or healthy‐eating advice. Two separate sets of envelopes were prepared for both diabetic and non‐diabetic subjects. An independent observer witnessed randomization." Author reported in correspondence that no formal test of hypothesis was done for difference at baseline for non‐diabetic participants. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care: n=3 of randomised participants in balanced group refused follow‐up as not allocated to low carbohydrate diet. Modified ITT conducted. | High risk of bias | No participants in the intervention group and 3/7 (43%) in the control group did not have data. Modified ITT with last observation carried forward. High attrition in control group, and differential attrition. Refusal of follow‐up due to not being allocated to low carbohydrate may be influenced by expected change in weight, therefore missingness could and likely influenced by the true value. | Low risk of bias | Body weight recorded monthly. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition in the control group, with disappoinment at not being assigned to the low carbohydrate diet cited as reason; therefore missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Landers 2002 | Some concerns | No information on randomisation or allocation concealment. Families were assigned to the same dietary intervention. Baseline characteristics were not reported, with the exception of body weight, fat mass and lean mass; all of which did not appear to be significantly different. | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. did not attend follow‐up visits. Modified ITT excluding participants with missing outcome data. | High risk of bias | Data were not available for 12/28 (43%) participants in the intervention and 12/33 (36%) in the control group. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured in light clothing without shoes at the initial visit. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and is likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Lim 2010 | Some concerns | No information about randomisation or allocation concealment. Baseline characteristics table showed small differences, consistent with differences resulting by chance with small sample sizes. | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. travel, work commitment, did not like diet. Modified ITT with all partial data contributed by non‐completers included in linear mixed effects model. | High risk of bias | 6/30 (20%) in the intervention and 8/30 (27%) in the control group did not have data available. ITT with all partial data contributed by non‐completers included in linear mixed effects model. Results of ITT not presented and no sensitivity analyses performed (author correspondence). High attrition in control group and some missingness could possibly and is likely to depend on true value of outcome. | Low risk of bias | Recorded in light clothing on Mercury digital scales. No evidence that a different method was used across intervention groups to measure weight. No information about blinding. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and is likely to depend on the true value of the outcome. Partial data from missing participants included in linear mixed effects model, but results of this ITT analysis not reported. No pre‐registered document available to assess analysis intentions. |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | High risk of bias | From registry: "Open (masking not used)". Deviations reported are expected to arise in usual care, e.g. work commitments, personal reasons. Per protocol analysis, ITT with last observation carried forward also performed but data not reported. 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons. | High risk of bias | 10/55 (18%) in the intervention and 11/54 (20%) in the control group did not have data available for this outcome. ITT with last observation carried forward: "The ITT with either baseline values carried forward or the last follow‐up visit carried forward for those who did not complete the study also showed no difference in weight loss between the diets (p<=0.23)." 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons, which may be related to change in weight; thus missingness could or is likely dependent on the true value of the outcome. | Low risk of bias | "Body mass was measured using calibrated electronic digital scales...to the nearest 0.05 kg, with subjects wearing light clothing and no footwear." No evidence that a different method was used across intervention groups to measure weight. From registry: "Open (masking not used)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition is reported in both trial arms, with high attrition in the control group due to unclear reasons and 'non‐compliance'; therefore missingness could and is likely to be related to the true value of the outcome. ITT analyses with baseline values and last observations carried forward were conducted, but neither were considered appropriate to address potential bias due to missingness. Lack of information in the pre‐registered methods. |
| Veum 2017 | Some concerns | "The list of prescreening appointments was used to randomly assign the participants by drawing ballots and blocking with block sizes of 2." No information about allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | From registry: "Masking: None (Open Label)" "All participants were informed of group allocation after the baseline measurements and sampling. The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Deviations reported are expected to arise in usual care, e.g. busy job, fracture. "We also performed an intention‐to‐treat (ITT) analysis that included all randomly assigned participants except 2 cases that were lost before baseline (44 eligible participants in total). These 2 were unaware of the group allocation and did not provide any dietary or clinical data." | Low risk of bias | 4/24 (17%) in the intervention and 4/22 (18%) in the control group did not have data available for this outcome. "Notably, the results from the PP and ITT analyses did not differ in nominal significance for any of the primary or secondary outcomes. Our missing data analysis (data not shown), including Little’s missing completely at random test, indicated that the dropouts were missing (completely) at random, and we found no significant differences between dropouts and completers in baseline characteristics, except for significant higher diastolic blood pressure, total cholesterol (TC), and LDL cholesterol in completers. In the reported PP and ITT analyses, we used mixed models without imputation to handle missing data. However, we also conducted an ITT analysis based on a full dataset in which missing values in the original data were replaced by values from multiple imputation (data not shown)." | Low risk of bias | Taken barefoot and wearing light clothing and asked to use restroom shortly before. No evidence that a different method was used across intervention groups to measure weight. "The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Volek 2009 | Some concerns | No information about randomisation or allocation concealment. No apparent imbalances between two groups at baseline | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No deviations or drop‐outs reported. Appears to be a full ITT with number randomised equal to number analysed. | Low risk of bias | Number randomised equals number analysed. | Low risk of bias | "Body mass was measured in the morning after an overnight fast to the nearest 100 g on a calibrated digital scale." No evidence that a different method was used across intervention groups to measure weight. "Analyses were performed by the same blinded technician." | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions. |
| Subgroup 1.3.2 Low carbohydrate/non‐ketogenic | ||||||||||||
| Bales 2017 | Low risk of bias | We used a computerised centralised randomisation scheme blocking by race and marital or partner status. "...also blocked by functional status..." "The 1:2 allocation to the C‐WL and HP‐WL groups was chosen...to make within‐group comparisons in the protein group in subsequent analyses. This oversampling of the protein group did not cause a substantial loss of power for the between‐group comparisons; the relative power for a 2:1 split is 0.94(23)." No statements or statistical tests about differences between groups, no problematic imbalance suspected. | Low risk of bias | Not blinded (open label trial) (trial registry). Deviations reported are expected to arise in usual care: "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study." Number analysed unclear, but seems to be a modified ITT: "The study was conducted under “intent to treat” criteria." | High risk of bias | 22/51 (43%) participants in the intervention and 16/29 (55%) participants in the control arm did not have data available. "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study. In particular, the tendency for more dropouts among minorities and those with less education..." Very high attrition, thus missingness could and is likely to depend on the true value. | Low risk of bias | Weekly weigh‐ins. No evidence that a different method was used across intervention groups to measure weight. Open‐label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | ...treatment codes revealed only after the study statistician locked the database at the end of the trial. No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. All time points mentioned in trial registry reported on in publication. Analysis intentions are not reported in sufficient detail prior to study publication to enable an assessment. | High risk of bias | Very high attrition with a variety of reasons relating to health, therefore some missingness could be and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Benassi‐Evans 2009 | Some concerns | "Subjects were randomised to one of two dietary interventions…" No further information about allocation sequence or concealment. "Subject characteristics were not significantly different between groups at baseline." | Low risk of bias | No information, but blinding of participants and people delivering the interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. No details about analysis are reported, but looks to be intention to treat (number randomised per group = number analysed per group). | High risk of bias | No details about attrition reported, but some attrition is implied: "...in the subjects who completed the 12 weeks intensive weight loss and 9 months weight maintenance..." No further information or evidence that the result was not biased due to missing data are provided. | Low risk of bias | Volunteers fasted overnight and were weighed (model AMZ14; Mercury Digital Scales, Tokyo, Japan) wearing light clothing and no shoes at weeks. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition is implied, with no reasons or evidence to show that results were not biased by this missingness. No pre‐registered document available to assess analysis intentions. |
| Calleja‐Fernández 2012 | Some concerns | Random allocation to each dietary group was performed by the main investigator (MDBP) by using the Web site Randomization.com (http://www.randomization.com) upon the request of the study dietitian (ACF) after the eligibility of a participant was confirmed and assignment to either of the insulin resistance or insulin sensitivity groups had been performed. No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected. | Low risk of bias | No information on blinding of participants, however, blinding is highly unlikely. People delivering these dietary interventions were unblinded. No reasons or details about deviations from intended interventions reported. ITT analysis conducted. | Low risk of bias | It seems data were available for all participants randomised for 16 week study, but cannot be sure (flowcharts in Fernandez et al 2012 and Ballesteros‐Pomar 2009 incongruent). | Low risk of bias | "Body weight was measured to the nearest 0.5 kg and then taken using calibrated digital scales…" No evidence that a different method was used across intervention groups to measure weight. "The medical assessments were blinded to the type of diet being followed." | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions. |
| Cornier 2005 | Some concerns | No information on how randomisation was done or allocation concealment. "Subject characteristics are summarized in Table 1. BW, BMI, percentage body fat, RMR, and RQ were similar in all groups." | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. "Forty‐four obese, healthy normoglycemic women 23 to 53 years old with BMI of 30 to 35 kg/m2 were screened, and 21 of them were enrolled and completed the 16‐week intervention." ITT analysis conducted: number randomised equals number analysed; no subjects lost to follow‐up. | Low risk of bias | Number randomised equals number analysed; no subjects lost to follow‐up. | Low risk of bias | Once a week, subjects were weighed. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions. |
| Ebbeling 2007 | Low risk of bias | "Enrolled participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, with stratification by sex and ethnicity/race..." "To avoid any bias in assigning participants to diet groups, staff conducting recruitment and enrollment were masked to sequence. The study director assigned participants to groups." No problematic differences in baseline characteristics between the two groups | Low risk of bias | No masking (open label trial). No reasons or details about deviations from intended interventions reported. ITT with imputation for missing values. | High risk of bias | 4/36 (11%) in the intervention and 3/37 (8%) in the control group did not have outcome data. "For body weight after dropout, an increase of 1 kg per year was imputed, starting from the last available measurement." No reasons for loss to follow‐up or withdrawal provided, cannot judge if related to participants' health status and if missingness depends on its true value. | Low risk of bias | Dietitians measured at each visit using electronic scale. No evidence that a different method was used across intervention groups to measure weight. "Data were collected by personnel who were masked to group assignment." | Some concerns | No mention of a pre‐specified analysis plan; trial registry does not mention the outcome and timepoint in any versions. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Attrition reported in both groups, with no reasons provided. Missingness was addressed through last observation carried forward. It is not possible to determine whether missingness could or is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Farnsworth 2003 | Some concerns | No information on how the randomisation sequence was generated. No information on allocation concealment. "There were no significant differences in any of the variables between the diet groups." | Some concerns | No information on blinding of participants and people delivering these dietary interventions, however blinding of these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. work commitments, pregnancy. 66 randomised, 57 completed and 56 analysed. Per protocol analysis. "...data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." 1/57 participant data excluded. | Low risk of bias | 5/33 (15%) in the intervention and 5/33 (15%) in the control group did not have data available. Data from n=1 participant where missingness possibly influenced by true value. "Six subjects withdrew before study commencement because of family and work commitments; in addition, one subject withdrew because of pregnancy during the course of the study, the clinic lost contact with one subject, and data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." "... one subject declined to undergo a DXA scan for personal reasons." | Low risk of bias | Body weight measured after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Data from one subject was excluded due to 'noncompliance' with the diet, but other reasons for loss to follow‐up were expected to arise in usual care. No pre‐registered document available to assess analysis intentions. |
| Foraker 2014 | Some concerns | "Women were randomized using stratified randomization, based on BMI (< 30 kg/m2 vs. ≥ 30 kg/m2) to ensure equal distribution of overweight and obese participants into each intervention group." No information on allocation concealment. No problematic imbalance in baseline characteristics between groups | Low risk of bias | Double masked: investigator and outcomes assessor. Deviations reported are expected to arise in usual care, e.g. personal/family medical issues, time constraints. Modified ITT: completers analysed "number still under follow‐up". | High risk of bias | 13/38 (34%) in the intervention and 15/41 (37%) in the control group did not have data available. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Anthropometric tests at each visit included body weight measurements. No evidence that a different method was used across intervention groups to measure weight. Double masked: investigator and outcomes assessor. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint (mentions only number of women who lose weight as outcome). Analysis intentions are not reported in sufficient detail to enable an assessment; weight findings are emphasised as part of the primary aim in the paper's methods and trial registry; with limited reporting of these findings in the paper and none reported in the abstract. | High risk of bias | No specific information on allocation concealment. High attrition in both groups with limited reasons reported, therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Frisch 2009 | Some concerns | "Participants (n = 200) were randomly assigned by computer‐generated random number lists in two equal groups…" No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | No masking (open label trial). Deviations reported are expected to arise in usual care, e.g. non‐compliance. ITT analysis conducted. | High risk of bias | 5/100 (5%) in the intervention and 11/100 (11%) in the control group did not have data available. Data were evaluated by ITT and per protocol method. ITT done with baseline values carried forward. "Results did not differ when the per protocol method was used (data not shown)." 4/100 (4%) in the intervention and 10/100 (10%) in the control group withdrew due to non‐compliance, which may be related to change in weight (missingness possibly influenced by true value). | Low risk of bias | Participants were weighed using a calibrated electronic scale. No evidence that a different method was used across intervention groups to measure weight. Open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported with reasons such as 'non‐compliance', therefore missingness could have and likely depended on the true value of the outcome. Both per protocol and baseline values carried forward approaches were used for missing data. Lack of information in the pre‐registered methods. |
| Goni 2018 | Some concerns | "Subjects were randomly assigned to one of the two diets by a specific logarithm design for the study by MATLAB using stratified block randomization..." No information about allocation concealment. No significant differences between groups at baseline | Low risk of bias | Masking: none; open label. The only deviations reported are drop‐outs. Modified ITT: looks as if only missing outcome data excluded. | High risk of bias | 21/75 (28%) in the intervention 19/72 (26%) in the control group did not have data available for this outcome. No reasons for drop‐out provided, but with this high attrition in both groups missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Assessed using a Tanita BC418 scale. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. High attrition reported in both groups with no reasons provided, therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Griffin 2013 | Low risk of bias | "The study consisted of a random block design per every four participants. Random allocation was prepared by a researcher not involved in participant enrolment or assignment." "random number table"; "sealed opaque envelopes" (trial registry). No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | "Participants were blind to diet macronutrient composition and were informed eating plans differed only in the type of meat (i.e. red or white)." "...inability to double‐blind the intervention were limitations." Deviations reported are expected to arise in usual care, e.g. did not like diet. ITT analysis conducted. | High risk of bias | 12/36 (33%) participants in the intervention and 15/35 (43%) in the control group did not have data available. ITT with last observation carried forward, and senstivity analysis comparing completers with ITT. 5/6 (83%) in the intervention and 5/9 (56%) in the control discontinued intervention due to illness, too difficult and not losing weight. Very high attrition in both groups; likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | Taken at every visit on a digital scale with light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "...inability to double‐blind the intervention were limitations." Single‐blind with blinded participants. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Very high attrition reported in both groups, with reasons such as 'illness', diets being 'too difficult' and 'not losing weight'; missingness could be and is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Haufe 2013 | Low risk of bias | "For allocation of the subjects, a computer‐generated list of random numbers was used. The randomization sequence was created using SPSS 18 (Chicago, IL) statistical software and subjects were assigned to reduced carbohydrate or reduced fat diet with a 1:1 allocation using random block sizes of 2, 4, and 6. Study nurses and physicians screening and enrolling volunteers were blinded for the randomization sequence." "Both groups were well matched for gender, age, body weight, body mass index, blood lipid profiles, glucose metabolism, and cardiorespiratory fitness." | High risk of bias | "Blinding of participants for the allocated dietary intervention was impossible." "Except for the dieticians, study nurses and physicians were blinded for the treatment assignment." Deviations reported are expected to arise in usual care, e.g. lost to follow‐up, withdrew consent. Per protocol analysis conducted: 24/28 (86%) in the intervention and 30/33 (91%) in the control group excluded from the analysis including 'protocol violations'. | High risk of bias | 25/80 (31%) in the intervention and 29/83 (35%) in the control group did not have data available. ITT with last observation carried forward. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured body weight in a standardised manner after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. "....study nurses and physicians were blinded for the treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | The majority of data not included in the analyses were due to exclusions based on 'protocol violations'. High overall attrition was reported in both groups, with no reasons (aside from exclusions); missingness could be and is likely dependent on the true value of the outcome. Last observation carried forward approach used for dealing with missingness. Lack of information in the pre‐registered methods. |
| Josse 2011 | Some concerns | No information about how the randomisation sequence was generated, or allocation concealment. "Our stratification scheme was adequate, because there were no between‐group differences in any baseline variable." | Low risk of bias | Masking: none; open label. Deviations reported are expected to arise in usual care, e.g. moving house, exam time at school, sickness of a relative. Modified ITT "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model‐based multiple imputation." | Low risk of bias | 4/30 (13%) participants in the intervention and 1/30 (3%) in the control group did not have data available for this outcome. The only information reported is "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model based multiple imputation." Based on the reasons provided, it is unlikely that missingness could be dependent on the true value of the outcome. | Low risk of bias | Digital physician's scale used to determine weight. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Lack of information in the pre‐registered methods. |
| Keogh 2007 | High risk of bias | Clinstat used to generate sequence; "allocation concealment: No" (trial registry). From Table 1 there appeared to be no large differences between groups at baseline. | Low risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. personal reasons, unable to comply. Intention to treat: all randomised were analysed. | Low risk of bias | All participants, 13/13 in intervention and 12/12 in control, had data available. | Low risk of bias | Weight was measured using a Mercury digital scale in light clothing at baseline, and every 2 weeks. No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Lack of information in the pre‐registered methods. |
| Klemsdal 2010 | Some concerns | No information about randomisation or allocation concealment. Footnote to Table 1: "There were no significant differences between groups." | Low risk of bias | Masking: None (Open Label). Deviations reported are vague, but those reported are expected to arise in usual care, e.g. non‐attendance of study visits. ITT analysis: "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." All randomised participants were analysed. | High risk of bias | Short‐term outcomes: 16% of participants overall did not have data available (not reported per trial arm). "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." No mention of sensitivity analysis. No reasons for lost to follow‐up or withdrawal provided and therefore cannot make a judgment about whether missingness was dependent on the true value of the outcome. | Low risk of bias | "Body weight...measured with standard methods." No evidence that a different method was used across intervention groups to measure weight. Masking: None (Open Label). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition with no reasons reported for the overall group, it is therefore not possible to determine whether missingness could be or is likely dependent on the true value of the outcome. Missingness was addressed by using last observation carried forward. Lack of information in the pre‐registered methods. |
| Lasker 2008 | Some concerns | "Subjects were blocked on gender, matched on age...and fasting glucose..." No information on allocation concealment. Significant baseline imbalance for insulin; no significant differences for 13 other characteristics at baseline | High risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. training for marathon. Per protocol analysis (participants excluded for lack of adherence to protocol: 23% excluded for various reasons, including lack of adherence, but breakdown by trial arm and reason not reported). | High risk of bias | Data were not available for 7/32 (22%) participants in the intervention and 8/33 (24%) in the control group. High attrition with reasons including non‐compliance, which may be related to change in weight, so likely that with such high attrition and this reason some missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Some participants were excluded from the analysis due to a lack of adherence, with high attrition reported in both trial arms. Based on these numbers and known exclusions for lack of adherence, missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Layman 2005 | Some concerns | "Note that the randomization was blocked on age and BMI for each wave of recruitment." No information on allocation concealment. "Baseline characteristics did not differ among subjects in each of the treatment groups..." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No deviations or drop‐outs reported. Appears to be a full ITT with number randomised equal to number analysed. | Low risk of bias | All randomised participants appear to have been analysed. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions. |
| Layman 2009 | Some concerns | No information on how the randomisation sequence was generated. No information about allocation concealment. "Randomization after stratifying participants for gender, age, BMI, and TC was successful with no significant differences between treatment groups in baseline characteristics." | Low risk of bias | "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Deviations are expected to arise in usual care, e.g. personal reasons, unable to comply. "Prior to the 4‐mo data collection, 15 participants withdrew from the CHO group (9 stated personal reasons and 6 stated unable to comply with diet protocol) and 12 participants withdrew from the PRO group (11 stated personal reasons and 1 stated inability to comply with diet protocol)." Modified ITT conducted. | High risk of bias | 12/64 (19%) in the intervention and 15/66 (23%) in the control group did not have data available. No methods used to correct for bias, or sensitivity analyses conducted. 6/15 (40%) withdrawals in the intervention and 1/12 (8%) withdrawals in the control group were due to inability to comply with diet. Inability to comply may be related to change in weight, and high attrition in the control group, thus missingness could and is likely to depend on the true value. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition reported in both groups, with high attrition in the control group, and some attrition in both groups due to an 'inability to comply with diet'; therefore missingness could and is likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Lean 1997 | Some concerns | No information on randomisation or allocation concealment. "All variables were not significantly different between the two diet treatment groups." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. ITT with last observation carried forward conducted. | High risk of bias | 13/53 (25%) in the intervention and 15/57 (26%) in the control group did not contribute data to this outcome. ITT with last observation carried forward conducted. High attrition in both groups with no reasons reported, but with this high attrition some missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | "Measurements were made with subjects wearing light clothes using regularly calibrated scales..." Reference to WHO procedures (classification and description of anthropometric data). No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition in both groups, with no reasons reported; therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Marco‐Benedi 2019 | Low risk of bias | "Randomization was performed by using an online software..." Paper reported "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding (trial registry reported 'Open‐label'). Only one out of numerous outcomes was significantly different between groups at baseline. | Low risk of bias | Trial registry states "Masking: None (Open Label)", but paper reports "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding. Deviations reported are expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 5/40 (12.5%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. Reason for withdrawal is only available for 1/13 excluded participants. Difficult to judge whether it is likely that missingness is related to true value for 12/13 participants, based on reasons provided (unknown, personal reasons). | Low risk of bias | "Body weight was measured...with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. Trial registry states "Masking: None (Open Label)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with vague reasons for loss to follow‐up and one exclusion; therefore it is not possible to judge whether missingness could be or is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Mateo‐Gallego 2017 | Some concerns | "We carried out the randomization process by generating a number sequence (including 93 correlative numbers because this was the number of subjects we planned to randomize) in the following webpage: https://www.random.org. The number for each participant was correlatively assigned (e.g; the first randomized women was assigned with number 1)" (from author correspondence). No information about allocation concealment. "There were no significant between‐group differences at baseline." | Low risk of bias | "Participants were blinded to their assigned macronutrient composition." "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." Study is reported as single blind (Masking: Single (Participant)). Deviations reported are expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 3/30 (10%) in the intervention and 6/30 (20%) in the control group did not have data available for this outcome. Limited reporting of reasons for missingness, therefore difficult to judge whether missingness could or is likely to be dependent on the true value of the outcome. | Low risk of bias | "Body weight was measured in subjects without shoes to the nearest 0.1 kg with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported in both groups with limited reporting of reasons, therefore it is not possible to judge if missingness could be or is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Ooi 2021 | Some concerns | "This was a randomized single‐blinded, placebo‐controlled study where subjects were block randomly assigned by gender and BMI groups…" Allocation concealment: no trial registry available and no information in paper. No significant differences between groups at baseline | High risk of bias | Subjects blinded only to supplements. No information about blinding of diets, however blinding of participants highly unlikely, the study is stated as single‐blind, thus people delivering these dietary interventions were unblinded. Deviations reported are expected to arise in usual care e.g family, work or personal commitments. Per protocol analysis, with exclusion of available data for reasons such as non‐compliance and inadequate weight loss. Vague reasons for exclusions and specific numbers excluded per trial arm are unclear, with 8% of subjects being excluded by the principal investigator. | High risk of bias | 3/43 (7%) in the intervention and 7/44 (16%) in the control group did not have data available. "Study subjects with available information for each analysis were included and missing data were not imputed." Study subjects that did not comply with the diets or did not lose a specific amount of weight were excluded from analyses by the investigators; thus missingness in the outcome could and is likely to depend on its true value. | Low risk of bias | "Height and weight were taken using a SECA 763 digital scale…" No evidence that a different method was used across intervention groups to measure weight. The study was single blinded for participants to supplements. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, no trial registry. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Subjects were likely not blinded to dietary allocation. Per protocol analysis conducted with non‐compliant participants excluded. Missing data were not imputed. Exclusions of available data were done based on non‐compliance with diet or inadequate weight loss, thus missingness in the outcome could and is likely to depend on its true value. No pre‐registered document available to assess analysis intentions. |
| Parr 2016 | High risk of bias | "The randomization will be initially stratified by baseline BMI values (27–29, 30–34, and 35–40 kg/m2) and sex. Subsequently, simple randomisation by roll of dice within the stratified segregation will be used to allocate individuals to each of the 3 groups." "Allocation is not concealed" "No significant differences were observed among groups at baseline for any variable (P > 0.05)." | High risk of bias | "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment." From registry: Open (masking not used). Deviations reported are expected to arise in usual care, e.g. time commitment, ankle injury. Per protocol (participants excluded from analysis due to compliance). 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance and 1/8 (12.5%) due to being an outlier. | High risk of bias | 10/39 (14%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance, which may be related to change in weight; thus missingness could and is likely dependent on the true value. | Low risk of bias | Measured with DEXA. No evidence that a different method was used across intervention groups to measure weight. "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment.", but from the registry: "Open (masking not used)" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition is reported in both groups, with nearly half of all missing data due to participants being excluded from the per protocol analysis due to 'non‐compliance' and being an 'outlier'. Missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Sacks 2009 | Low risk of bias | "Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian, after eligibility of a participant was confirmed." Independent trial unit, fully funded by public funds and large trial; it was likely the sequence was random. " Baseline characteristics were similar among participants assigned to the four diets..." | Low risk of bias | "Blinding was maintained by the use of similar foods for each diet. Staff and participants were taught that each diet adhered to principles of a healthful diet..." True blinding is a known challenge with dietary interventions. Deviations reported are expected to arise in usual care, e.g. moved away, did not appear at scheduled appointments. Full ITT "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." | High risk of bias | Linked paper reported 39/201 (19%) in the intervention and 47/204 (23%) in the control group did not have data available for this outcome at 6 months (De Jonge et al 2012); 2‐year attrition (Sacks et al 2009) reports less attrition per group. Single imputation "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." No sensitivity analyses (author correspondence). No detailed reasons for loss to follow‐up or withdrawals provided, high attrition in the control group thus missingness could and is likely to depend on the true value. | Low risk of bias | "Body weight, the primary outcome variable, was measured by calibrated hospital scales, in the morning before breakfast and after urinating, clothed in a hospital gown, on two nonconsecutive days at baseline, and at 6 and 24 months; and on a single day at 12 and 18 months." No evidence that a different method was used across intervention groups to measure weight. "Investigators and staff who measured outcomes were unaware of the diet assignment of the participants." | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with high attrition in the control group, and limited reporting of reasons; therefore missingness could be and is likely dependent on the true value of the outcome. Simple imputation was performed, but no sensitivity analyses. Lack of information in the pre‐registered methods. |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | Some concerns | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. relocated overseas, unable to commit to study protocol/time requirements. Per protocol analysis: 3/59 and 1/64 participants excluded due to being unable to commit to study protocol or did not receive intervention. 3% of total not included (4/123). | High risk of bias | 7/59 (12%) in the intervention and 6/64 (9%) in the control group did not have data available. Modified ITT only done at 52 weeks using maximal likelihood mixed model analysis. "Overall, the participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks..." missingness could and is likely to depend on the true value. | Low risk of bias | "... body weight was measured using calibrated electronic digital scales (Mercury; AMZ 14, Tokyo, Japan)." No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition was reported in both groups, and authors remark that "...participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks…; missingness is therefore likely dependent on the true value of the outcome. Furthermore, participants were excluded for an inability to commit to the study protocol or not receiving the intervention. Lack of information in the pre‐registered methods. |
| Subgroup 1.3.3 Incremental increase from very low to low carbohydrate | ||||||||||||
| Aude 2004 | Some concerns | Randomisation was done using a block design by sex to achieve an equal sex distribution. No information on allocation concealment. "At baseline, there were no significant differences in age, weight, BMI, waist‐to‐hip ratio, and any of the lipid variables between the NCEP and MLC groups" (only for completers). | High risk of bias | No information on blinding of participants and people delivering these dietary interventions, but blinding is unlikely. No reasons or details about deviations from intended interventions reported. A quarter of the participants were not included in the analysis: 8/30 participants in the intervention group and 7/30 in the control group were excluded from analysis; 6/60 did not complete the trial (missing), but no information about remaining 9/60 who were not included. | High risk of bias | 8/30 (27%) participants in the intervention and 7/30 (23%) participants in the control arm did not have data available. No reasons for loss to follow‐up or withdrawal provided, so difficult to judge if related to participants' health status, but attrition is high in both groups; thus missingness could and is likely to depend on the true value. | Low risk of bias | Weight was measured with indoor clothing and without shoes on a single scale. No evidence that a different method was used across intervention groups to measure weight. "All anthropometric measurements were obtained by an observer blinded to the treatment assignment." | Some concerns | No trial registry reported and no mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. A quarter of participants were not included in the analysis, with withdrawal reported for 10% of participants. No reasons were provided for the remaining missingness. Attrition is high in both groups with no reasons provided, therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Foster 2010 | Some concerns | "Using a random‐number generator, we randomly assigned participants within each site to treatment with either a low‐carbohydrate or low‐fat, calorie‐restricted diet for 2 years." No information on allocation concealment. No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | Masking: none; open label. Deviations reported are expected to arise in usual care, e.g. dissatisfied with the program, pregnancy, life stessors, relocated. ITT analysis with n=307; all randomised. "We used a random‐effects linear model that was fitted to all observed data for each variable on each of the 307 participants for the primary analysis." | Low risk of bias | 25/153 (16%) in the intervention and 19/154 (12%) in the control group did not have data available. "..longitudinal models implemented for this study relax this missing‐completely‐at‐random assumption in various ways." "Under the sensitivity analysis based on imputing missing outcomes with the highest (13.795) and lowest (‐18.355) random‐effects slopes (that is, change in weight per month) under the mixed‐effects model for weight, our qualitative findings were not sensitive to either imputation approach." Results of sensitivity analyses not provided. | Low risk of bias | Body weight was measured at each treatment visit on calibrated scales while participants wore light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "Assessors were not blinded to treatment condition." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Gardner 2007 | Low risk of bias | "Randomization was conducted in blocks of 24 (6 per treatment group) and occurred by having a blinded research technician select folded pieces of paper with group assignments from an opaque envelope." No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking: single ("Clinic and laboratory staff members were blinded to treatment assignment"). Deviations reported are expected to arise in usual care, e.g. moved away, disliked diet, pregnancy. ITT analysis conducted. | High risk of bias | 6/77 (8%) in the intervention and 15/79 (19%) in the control did not have data available. ITT with baseline values carried forward. "Also for exploratory purposes, all analyses of weight and secondary outcome measures were tested using only available data, without using baseline values carried forward for missing data or other imputation methods. There were no substantive differences in any of these findings compared with the analyses with baseline values carried forward and, therefore, only the primary analyses are presented." 5/9 in the intervention and 5/18 in the control group withdrew due to health and "other" reasons (vague); but attrition is differential and thus missingness could and is likely to depend on the true value. | Low risk of bias | "Body weight was measured to the nearest 0.1 kg on a calibrated clinical scale." No evidence that a different method was used across intervention groups to measure weight. "Clinic and laboratory staff members were blinded to treatment assignment." | Some concerns | No mention of a pre‐specified analysis plan, trial registry at date prior to study publication mentions outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Differential attrition reported, with vague reasons; therefore missingness could and is likely to depend on the true value of the outcome. Per protocol and baseline values carried forward approaches were used to account for missingness. Lack of information in the pre‐registered methods. |
| Liu 2013 | Low risk of bias | No information about sequence generation. "Randomisation was conducted by a statistician who was not involved in any other aspects of the study by using block randomisation, stratified by 10‐year age categories." "Although the participants were randomised by age, baseline BMI levels of the two groups were similar after randomisation." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. personal reasons, busy schedule. Modified ITT: one drop‐out due to busy schedule in control group excluded. | Low risk of bias | 1/25 (4%) in the intervention and 1/25 (4%) in the control group did not have data available for this outcome. | Low risk of bias | "Body weight was measured to the nearest 0.1kg at the beginning and then biweekly during the trial using an electronic scale." No evidence that a different method was used across intervention groups to measure weight. Masking: Single (Outcomes Assessor). | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
Risk of bias for analysis 1.4 Change in body weight (kg) at 3 to < 12 months: subgroup diagnosed cardiovascular event or disease.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.4.1 None | ||||||||||||
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | No information about participant blinding, however, blinding of participants is highly unlikely. "...dietitians were not blinded to the study hypothesis." No reasons or details about deviations from intended interventions reported. "All analyses were conducted using the intention‐to‐treat principle." n=148 randomised, and n=148 analysed. | Low risk of bias | 13/75 (17%) participants in the intervention and 9/73 (12%) participants in the control arm did not have data available. Imputed missing values, and performed sensitivity analyses to determine whether missing at random (MAR) assumptions were met. "We performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption." "We used Markovchain Monte Carlo techniques to impute missing values, including additional covariates..." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses." | Low risk of bias | Body weight was measured to the nearest 0.1 kg using a single calibrated scale. No evidence that a different method was used across intervention groups to measure weight. "all outcome assessors were blinded to the diet group assignment..." | Some concerns | No mention of a pre‐specified analysis plan. "Study protocol and data set: Not available." Trial registry outcomes amended after study publication; but trial registry entry at date prior to study publication mentions the outcome and timepoints. All time points mentioned in trial registry at date prior to study publication, were reported on in publication. Analysis intentions are not reported in sufficient detail prior to study publication to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Benassi‐Evans 2009 | Some concerns | "Subjects were randomised to one of two dietary interventions…" No further information about allocation sequence or concealment. "Subject characteristics were not significantly different between groups at baseline." | Low risk of bias | No information, but blinding of participants and people delivering the interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. No details about analysis are reported, but looks to be intention to treat (number randomised per group = number analysed per group). | High risk of bias | No details about attrition reported, but some attrition is implied: "...in the subjects who completed the 12 weeks intensive weight loss and 9 months weight maintenance..." No further information or evidence that the result was not biased due to missing data are provided. | Low risk of bias | Volunteers fasted overnight and were weighed (model AMZ14; Mercury Digital Scales, Tokyo, Japan) wearing light clothing and no shoes at weeks. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition is implied, with no reasons or evidence to show that results were not biased by this missingness. No pre‐registered document available to assess analysis intentions. |
| Brehm 2003 | Some concerns | "... after each block of subjects was assessed, the principal investigator used a random number table to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences at baseline. | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. dislike diet, could not make scheduled visit. Modified ITT: excluded only missing patients. | High risk of bias | 7/26 (27%) in the intervention and 14/27 (52%) in the control group did not have data. "In this intention to treat analysis, the initial weights for the subjects who withdrew from the study were used as their follow‐up weights at 3 and 6 months..." Lost to follow‐up and withdrawal reasons are stated for all participants, but very high attrition in the control group and differential attrition, thus thus missingness could and is likely to depend on the true value. | Some concerns | Subjects were weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Differential attrition and high attrition in the control group, therefore missingness could and is likely to be dependent on the true value of the outcome. It is not clear whether outcome assessors were blinded to allocation. No pre‐registered document available to assess analysis intentions. |
| Brehm 2005 | Some concerns | "After each block of subjects was enrolled, the principal investigator used a computerized randomization program to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences between groups at baseline (completers only). | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. pregnancy, disliked diet. Modified ITT: all those who completed the study were analysed. | High risk of bias | 5/25 (20%) in the intervention and 5/25 (20%) in the control group did not have data available. Difficult to judge whether it is likely that missingness is related to the true value for weight, as reasons are not explicit enough. "The most common reason for discontinuing the study was the inability to commit to weekly counseling sessions. Other reasons included pregnancy, new out‐of‐state job, dislike of the low‐carbohydrate diet, and advice against following the low carbohydrate diet by family physician." | Low risk of bias | Weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Reasons for attrition are vague and it is difficult to judge whether it was as a result of the true value of the outcome, but wit 20% attrition in both groups it is likely that some missingness was dependent on true value. No pre‐registered document available to assess analysis intentions. |
| Cornier 2005 | Some concerns | No information on how randomisation was done or allocation concealment. "Subject characteristics are summarized in Table 1. BW, BMI, percentage body fat, RMR, and RQ were similar in all groups." | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. "Forty‐four obese, healthy normoglycemic women 23 to 53 years old with BMI of 30 to 35 kg/m2 were screened, and 21 of them were enrolled and completed the 16‐week intervention." ITT analysis conducted: number randomised equals number analysed; no subjects lost to follow‐up. | Low risk of bias | Number randomised equals number analysed; no subjects lost to follow‐up. | Low risk of bias | Once a week, subjects were weighed. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions. |
| Ebbeling 2007 | Low risk of bias | "Enrolled participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, with stratification by sex and ethnicity/race..." "To avoid any bias in assigning participants to diet groups, staff conducting recruitment and enrollment were masked to sequence. The study director assigned participants to groups." No problematic differences in baseline characteristics between the two groups | Low risk of bias | No masking (open label trial). No reasons or details about deviations from intended interventions reported. ITT with imputation for missing values. | High risk of bias | 4/36 (11%) in the intervention and 3/37 (8%) in the control group did not have outcome data. "For body weight after dropout, an increase of 1 kg per year was imputed, starting from the last available measurement." No reasons for loss to follow‐up or withdrawal provided, cannot judge if related to participants' health status and if missingness depends on its true value. | Low risk of bias | Dietitians measured at each visit using electronic scale. No evidence that a different method was used across intervention groups to measure weight. "Data were collected by personnel who were masked to group assignment." | Some concerns | No mention of a pre‐specified analysis plan; trial registry does not mention the outcome and timepoint in any versions. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Attrition reported in both groups, with no reasons provided. Missingness was addressed through last observation carried forward. It is not possible to determine whether missingness could or is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Farnsworth 2003 | Some concerns | No information on how the randomisation sequence was generated. No information on allocation concealment. "There were no significant differences in any of the variables between the diet groups." | Some concerns | No information on blinding of participants and people delivering these dietary interventions, however blinding of these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. work commitments, pregnancy. 66 randomised, 57 completed and 56 analysed. Per protocol analysis. "...data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." 1/57 participant data excluded. | Low risk of bias | 5/33 (15%) in the intervention and 5/33 (15%) in the control group did not have data available. Data from n=1 participant where missingness possibly influenced by true value. "Six subjects withdrew before study commencement because of family and work commitments; in addition, one subject withdrew because of pregnancy during the course of the study, the clinic lost contact with one subject, and data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." "... one subject declined to undergo a DXA scan for personal reasons." | Low risk of bias | Body weight measured after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Data from one subject was excluded due to 'noncompliance' with the diet, but other reasons for loss to follow‐up were expected to arise in usual care. No pre‐registered document available to assess analysis intentions. |
| Foster 2010 | Some concerns | "Using a random‐number generator, we randomly assigned participants within each site to treatment with either a low‐carbohydrate or low‐fat, calorie‐restricted diet for 2 years." No information on allocation concealment. No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | Masking: none; open label. Deviations reported are expected to arise in usual care, e.g. dissatisfied with the program, pregnancy, life stessors, relocated. ITT analysis with n=307; all randomised. "We used a random‐effects linear model that was fitted to all observed data for each variable on each of the 307 participants for the primary analysis." | Low risk of bias | 25/153 (16%) in the intervention and 19/154 (12%) in the control group did not have data available. "..longitudinal models implemented for this study relax this missing‐completely‐at‐random assumption in various ways." "Under the sensitivity analysis based on imputing missing outcomes with the highest (13.795) and lowest (‐18.355) random‐effects slopes (that is, change in weight per month) under the mixed‐effects model for weight, our qualitative findings were not sensitive to either imputation approach." Results of sensitivity analyses not provided. | Low risk of bias | Body weight was measured at each treatment visit on calibrated scales while participants wore light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "Assessors were not blinded to treatment condition." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Frisch 2009 | Some concerns | "Participants (n = 200) were randomly assigned by computer‐generated random number lists in two equal groups…" No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | No masking (open label trial). Deviations reported are expected to arise in usual care, e.g. non‐compliance. ITT analysis conducted. | High risk of bias | 5/100 (5%) in the intervention and 11/100 (11%) in the control group did not have data available. Data were evaluated by ITT and per protocol method. ITT done with baseline values carried forward. "Results did not differ when the per protocol method was used (data not shown)." 4/100 (4%) in the intervention and 10/100 (10%) in the control group withdrew due to non‐compliance, which may be related to change in weight (missingness possibly influenced by true value). | Low risk of bias | Participants were weighed using a calibrated electronic scale. No evidence that a different method was used across intervention groups to measure weight. Open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported with reasons such as 'non‐compliance', therefore missingness could have and likely depended on the true value of the outcome. Both per protocol and baseline values carried forward approaches were used for missing data. Lack of information in the pre‐registered methods. |
| Gardner 2007 | Low risk of bias | "Randomization was conducted in blocks of 24 (6 per treatment group) and occurred by having a blinded research technician select folded pieces of paper with group assignments from an opaque envelope." No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking: single ("Clinic and laboratory staff members were blinded to treatment assignment"). Deviations reported are expected to arise in usual care, e.g. moved away, disliked diet, pregnancy. ITT analysis conducted. | High risk of bias | 6/77 (8%) in the intervention and 15/79 (19%) in the control did not have data available. ITT with baseline values carried forward. "Also for exploratory purposes, all analyses of weight and secondary outcome measures were tested using only available data, without using baseline values carried forward for missing data or other imputation methods. There were no substantive differences in any of these findings compared with the analyses with baseline values carried forward and, therefore, only the primary analyses are presented." 5/9 in the intervention and 5/18 in the control group withdrew due to health and "other" reasons (vague); but attrition is differential and thus missingness could and is likely to depend on the true value. | Low risk of bias | "Body weight was measured to the nearest 0.1 kg on a calibrated clinical scale." No evidence that a different method was used across intervention groups to measure weight. "Clinic and laboratory staff members were blinded to treatment assignment." | Some concerns | No mention of a pre‐specified analysis plan, trial registry at date prior to study publication mentions outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Differential attrition reported, with vague reasons; therefore missingness could and is likely to depend on the true value of the outcome. Per protocol and baseline values carried forward approaches were used to account for missingness. Lack of information in the pre‐registered methods. |
| Goni 2018 | Some concerns | "Subjects were randomly assigned to one of the two diets by a specific logarithm design for the study by MATLAB using stratified block randomization..." No information about allocation concealment. No significant differences between groups at baseline | Low risk of bias | Masking: none; open label. The only deviations reported are drop‐outs. Modified ITT: looks as if only missing outcome data excluded. | High risk of bias | 21/75 (28%) in the intervention 19/72 (26%) in the control group did not have data available for this outcome. No reasons for drop‐out provided, but with this high attrition in both groups missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Assessed using a Tanita BC418 scale. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. High attrition reported in both groups with no reasons provided, therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Griffin 2013 | Low risk of bias | "The study consisted of a random block design per every four participants. Random allocation was prepared by a researcher not involved in participant enrolment or assignment." "random number table"; "sealed opaque envelopes" (trial registry). No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | "Participants were blind to diet macronutrient composition and were informed eating plans differed only in the type of meat (i.e. red or white)." "...inability to double‐blind the intervention were limitations." Deviations reported are expected to arise in usual care, e.g. did not like diet. ITT analysis conducted. | High risk of bias | 12/36 (33%) participants in the intervention and 15/35 (43%) in the control group did not have data available. ITT with last observation carried forward, and senstivity analysis comparing completers with ITT. 5/6 (83%) in the intervention and 5/9 (56%) in the control discontinued intervention due to illness, too difficult and not losing weight. Very high attrition in both groups; likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | Taken at every visit on a digital scale with light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "...inability to double‐blind the intervention were limitations." Single‐blind with blinded participants. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Very high attrition reported in both groups, with reasons such as 'illness', diets being 'too difficult' and 'not losing weight'; missingness could be and is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Haufe 2013 | Low risk of bias | "For allocation of the subjects, a computer‐generated list of random numbers was used. The randomization sequence was created using SPSS 18 (Chicago, IL) statistical software and subjects were assigned to reduced carbohydrate or reduced fat diet with a 1:1 allocation using random block sizes of 2, 4, and 6. Study nurses and physicians screening and enrolling volunteers were blinded for the randomization sequence." "Both groups were well matched for gender, age, body weight, body mass index, blood lipid profiles, glucose metabolism, and cardiorespiratory fitness." | High risk of bias | "Blinding of participants for the allocated dietary intervention was impossible." "Except for the dieticians, study nurses and physicians were blinded for the treatment assignment." Deviations reported are expected to arise in usual care, e.g. lost to follow‐up, withdrew consent. Per protocol analysis conducted: 24/28 (86%) in the intervention and 30/33 (91%) in the control group excluded from the analysis including 'protocol violations'. | High risk of bias | 25/80 (31%) in the intervention and 29/83 (35%) in the control group did not have data available. ITT with last observation carried forward. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured body weight in a standardised manner after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. "....study nurses and physicians were blinded for the treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | The majority of data not included in the analyses were due to exclusions based on 'protocol violations'. High overall attrition was reported in both groups, with no reasons (aside from exclusions); missingness could be and is likely dependent on the true value of the outcome. Last observation carried forward approach used for dealing with missingness. Lack of information in the pre‐registered methods. |
| Josse 2011 | Some concerns | No information about how the randomisation sequence was generated, or allocation concealment. "Our stratification scheme was adequate, because there were no between‐group differences in any baseline variable." | Low risk of bias | Masking: none; open label. Deviations reported are expected to arise in usual care, e.g. moving house, exam time at school, sickness of a relative. Modified ITT "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model‐based multiple imputation." | Low risk of bias | 4/30 (13%) participants in the intervention and 1/30 (3%) in the control group did not have data available for this outcome. The only information reported is "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model based multiple imputation." Based on the reasons provided, it is unlikely that missingness could be dependent on the true value of the outcome. | Low risk of bias | Digital physician's scale used to determine weight. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Lack of information in the pre‐registered methods. |
| Klemsdal 2010 | Some concerns | No information about randomisation or allocation concealment. Footnote to Table 1: "There were no significant differences between groups." | Low risk of bias | Masking: None (Open Label). Deviations reported are vague, but those reported are expected to arise in usual care, e.g. non‐attendance of study visits. ITT analysis: "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." All randomised participants were analysed. | High risk of bias | Short‐term outcomes: 16% of participants overall did not have data available (not reported per trial arm). "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." No mention of sensitivity analysis. No reasons for lost to follow‐up or withdrawal provided and therefore cannot make a judgment about whether missingness was dependent on the true value of the outcome. | Low risk of bias | "Body weight...measured with standard methods." No evidence that a different method was used across intervention groups to measure weight. Masking: None (Open Label). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition with no reasons reported for the overall group, it is therefore not possible to determine whether missingness could be or is likely dependent on the true value of the outcome. Missingness was addressed by using last observation carried forward. Lack of information in the pre‐registered methods. |
| Landers 2002 | Some concerns | No information on randomisation or allocation concealment. Families were assigned to the same dietary intervention. Baseline characteristics were not reported, with the exception of body weight, fat mass and lean mass; all of which did not appear to be significantly different. | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. did not attend follow‐up visits. Modified ITT excluding participants with missing outcome data. | High risk of bias | Data were not available for 12/28 (43%) participants in the intervention and 12/33 (36%) in the control group. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured in light clothing without shoes at the initial visit. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and is likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Layman 2009 | Some concerns | No information on how the randomisation sequence was generated. No information about allocation concealment. "Randomization after stratifying participants for gender, age, BMI, and TC was successful with no significant differences between treatment groups in baseline characteristics." | Low risk of bias | "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Deviations are expected to arise in usual care, e.g. personal reasons, unable to comply. "Prior to the 4‐mo data collection, 15 participants withdrew from the CHO group (9 stated personal reasons and 6 stated unable to comply with diet protocol) and 12 participants withdrew from the PRO group (11 stated personal reasons and 1 stated inability to comply with diet protocol)." Modified ITT conducted. | High risk of bias | 12/64 (19%) in the intervention and 15/66 (23%) in the control group did not have data available. No methods used to correct for bias, or sensitivity analyses conducted. 6/15 (40%) withdrawals in the intervention and 1/12 (8%) withdrawals in the control group were due to inability to comply with diet. Inability to comply may be related to change in weight, and high attrition in the control group, thus missingness could and is likely to depend on the true value. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition reported in both groups, with high attrition in the control group, and some attrition in both groups due to an 'inability to comply with diet'; therefore missingness could and is likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Lean 1997 | Some concerns | No information on randomisation or allocation concealment. "All variables were not significantly different between the two diet treatment groups." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. ITT with last observation carried forward conducted. | High risk of bias | 13/53 (25%) in the intervention and 15/57 (26%) in the control group did not contribute data to this outcome. ITT with last observation carried forward conducted. High attrition in both groups with no reasons reported, but with this high attrition some missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | "Measurements were made with subjects wearing light clothes using regularly calibrated scales..." Reference to WHO procedures (classification and description of anthropometric data). No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition in both groups, with no reasons reported; therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Lim 2010 | Some concerns | No information about randomisation or allocation concealment. Baseline characteristics table showed small differences, consistent with differences resulting by chance with small sample sizes. | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. travel, work commitment, did not like diet. Modified ITT with all partial data contributed by non‐completers included in linear mixed effects model. | High risk of bias | 6/30 (20%) in the intervention and 8/30 (27%) in the control group did not have data available. ITT with all partial data contributed by non‐completers included in linear mixed effects model. Results of ITT not presented and no sensitivity analyses performed (author correspondence). High attrition in control group and some missingness could possibly and is likely to depend on true value of outcome. | Low risk of bias | Recorded in light clothing on Mercury digital scales. No evidence that a different method was used across intervention groups to measure weight. No information about blinding. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and is likely to depend on the true value of the outcome. Partial data from missing participants included in linear mixed effects model, but results of this ITT analysis not reported. No pre‐registered document available to assess analysis intentions. |
| Liu 2013 | Low risk of bias | No information about sequence generation. "Randomisation was conducted by a statistician who was not involved in any other aspects of the study by using block randomisation, stratified by 10‐year age categories." "Although the participants were randomised by age, baseline BMI levels of the two groups were similar after randomisation." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. personal reasons, busy schedule. Modified ITT: one drop‐out due to busy schedule in control group excluded. | Low risk of bias | 1/25 (4%) in the intervention and 1/25 (4%) in the control group did not have data available for this outcome. | Low risk of bias | "Body weight was measured to the nearest 0.1kg at the beginning and then biweekly during the trial using an electronic scale." No evidence that a different method was used across intervention groups to measure weight. Masking: Single (Outcomes Assessor). | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Ooi 2021 | Some concerns | "This was a randomized single‐blinded, placebo‐controlled study where subjects were block randomly assigned by gender and BMI groups…" Allocation concealment: no trial registry available and no information in paper. No significant differences between groups at baseline | High risk of bias | Subjects blinded only to supplements. No information about blinding of diets, however blinding of participants highly unlikely, the study is stated as single‐blind, thus people delivering these dietary interventions were unblinded. Deviations reported are expected to arise in usual care e.g family, work or personal commitments. Per protocol analysis, with exclusion of available data for reasons such as non‐compliance and inadequate weight loss. Vague reasons for exclusions and specific numbers excluded per trial arm are unclear, with 8% of subjects being excluded by the principal investigator. | High risk of bias | 3/43 (7%) in the intervention and 7/44 (16%) in the control group did not have data available. "Study subjects with available information for each analysis were included and missing data were not imputed." Study subjects that did not comply with the diets or did not lose a specific amount of weight were excluded from analyses by the investigators; thus missingness in the outcome could and is likely to depend on its true value. | Low risk of bias | "Height and weight were taken using a SECA 763 digital scale…" No evidence that a different method was used across intervention groups to measure weight. The study was single blinded for participants to supplements. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, no trial registry. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Subjects were likely not blinded to dietary allocation. Per protocol analysis conducted with non‐compliant participants excluded. Missing data were not imputed. Exclusions of available data were done based on non‐compliance with diet or inadequate weight loss, thus missingness in the outcome could and is likely to depend on its true value. No pre‐registered document available to assess analysis intentions. |
| Parr 2016 | High risk of bias | "The randomization will be initially stratified by baseline BMI values (27–29, 30–34, and 35–40 kg/m2) and sex. Subsequently, simple randomisation by roll of dice within the stratified segregation will be used to allocate individuals to each of the 3 groups." "Allocation is not concealed" "No significant differences were observed among groups at baseline for any variable (P > 0.05)." | High risk of bias | "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment." From registry: Open (masking not used). Deviations reported are expected to arise in usual care, e.g. time commitment, ankle injury. Per protocol (participants excluded from analysis due to compliance). 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance and 1/8 (12.5%) due to being an outlier. | High risk of bias | 10/39 (14%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance, which may be related to change in weight; thus missingness could and is likely dependent on the true value. | Low risk of bias | Measured with DEXA. No evidence that a different method was used across intervention groups to measure weight. "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment.", but from the registry: "Open (masking not used)" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition is reported in both groups, with nearly half of all missing data due to participants being excluded from the per protocol analysis due to 'non‐compliance' and being an 'outlier'. Missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Sacks 2009 | Low risk of bias | "Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian, after eligibility of a participant was confirmed." Independent trial unit, fully funded by public funds and large trial; it was likely the sequence was random. " Baseline characteristics were similar among participants assigned to the four diets..." | Low risk of bias | "Blinding was maintained by the use of similar foods for each diet. Staff and participants were taught that each diet adhered to principles of a healthful diet..." True blinding is a known challenge with dietary interventions. Deviations reported are expected to arise in usual care, e.g. moved away, did not appear at scheduled appointments. Full ITT "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." | High risk of bias | Linked paper reported 39/201 (19%) in the intervention and 47/204 (23%) in the control group did not have data available for this outcome at 6 months (De Jonge et al 2012); 2‐year attrition (Sacks et al 2009) reports less attrition per group. Single imputation "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." No sensitivity analyses (author correspondence). No detailed reasons for loss to follow‐up or withdrawals provided, high attrition in the control group thus missingness could and is likely to depend on the true value. | Low risk of bias | "Body weight, the primary outcome variable, was measured by calibrated hospital scales, in the morning before breakfast and after urinating, clothed in a hospital gown, on two nonconsecutive days at baseline, and at 6 and 24 months; and on a single day at 12 and 18 months." No evidence that a different method was used across intervention groups to measure weight. "Investigators and staff who measured outcomes were unaware of the diet assignment of the participants." | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with high attrition in the control group, and limited reporting of reasons; therefore missingness could be and is likely dependent on the true value of the outcome. Simple imputation was performed, but no sensitivity analyses. Lack of information in the pre‐registered methods. |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | High risk of bias | From registry: "Open (masking not used)". Deviations reported are expected to arise in usual care, e.g. work commitments, personal reasons. Per protocol analysis, ITT with last observation carried forward also performed but data not reported. 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons. | High risk of bias | 10/55 (18%) in the intervention and 11/54 (20%) in the control group did not have data available for this outcome. ITT with last observation carried forward: "The ITT with either baseline values carried forward or the last follow‐up visit carried forward for those who did not complete the study also showed no difference in weight loss between the diets (p<=0.23)." 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons, which may be related to change in weight; thus missingness could or is likely dependent on the true value of the outcome. | Low risk of bias | "Body mass was measured using calibrated electronic digital scales...to the nearest 0.05 kg, with subjects wearing light clothing and no footwear." No evidence that a different method was used across intervention groups to measure weight. From registry: "Open (masking not used)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition is reported in both trial arms, with high attrition in the control group due to unclear reasons and 'non‐compliance'; therefore missingness could and is likely to be related to the true value of the outcome. ITT analyses with baseline values and last observations carried forward were conducted, but neither were considered appropriate to address potential bias due to missingness. Lack of information in the pre‐registered methods. |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | Some concerns | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. relocated overseas, unable to commit to study protocol/time requirements. Per protocol analysis: 3/59 and 1/64 participants excluded due to being unable to commit to study protocol or did not receive intervention. 3% of total not included (4/123). | High risk of bias | 7/59 (12%) in the intervention and 6/64 (9%) in the control group did not have data available. Modified ITT only done at 52 weeks using maximal likelihood mixed model analysis. "Overall, the participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks..." missingness could and is likely to depend on the true value. | Low risk of bias | "... body weight was measured using calibrated electronic digital scales (Mercury; AMZ 14, Tokyo, Japan)." No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition was reported in both groups, and authors remark that "...participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks…; missingness is therefore likely dependent on the true value of the outcome. Furthermore, participants were excluded for an inability to commit to the study protocol or not receiving the intervention. Lack of information in the pre‐registered methods. |
| Subgroup 1.4.2 Mixed or unclear | ||||||||||||
| Aude 2004 | Some concerns | Randomisation was done using a block design by sex to achieve an equal sex distribution. No information on allocation concealment. "At baseline, there were no significant differences in age, weight, BMI, waist‐to‐hip ratio, and any of the lipid variables between the NCEP and MLC groups" (only for completers). | High risk of bias | No information on blinding of participants and people delivering these dietary interventions, but blinding is unlikely. No reasons or details about deviations from intended interventions reported. A quarter of the participants were not included in the analysis: 8/30 participants in the intervention group and 7/30 in the control group were excluded from analysis; 6/60 did not complete the trial (missing), but no information about remaining 9/60 who were not included. | High risk of bias | 8/30 (27%) participants in the intervention and 7/30 (23%) participants in the control arm did not have data available. No reasons for loss to follow‐up or withdrawal provided, so difficult to judge if related to participants' health status, but attrition is high in both groups; thus missingness could and is likely to depend on the true value. | Low risk of bias | Weight was measured with indoor clothing and without shoes on a single scale. No evidence that a different method was used across intervention groups to measure weight. "All anthropometric measurements were obtained by an observer blinded to the treatment assignment." | Some concerns | No trial registry reported and no mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. A quarter of participants were not included in the analysis, with withdrawal reported for 10% of participants. No reasons were provided for the remaining missingness. Attrition is high in both groups with no reasons provided, therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Bales 2017 | Low risk of bias | We used a computerised centralised randomisation scheme blocking by race and marital or partner status. "...also blocked by functional status..." "The 1:2 allocation to the C‐WL and HP‐WL groups was chosen...to make within‐group comparisons in the protein group in subsequent analyses. This oversampling of the protein group did not cause a substantial loss of power for the between‐group comparisons; the relative power for a 2:1 split is 0.94(23)." No statements or statistical tests about differences between groups, no problematic imbalance suspected. | Low risk of bias | Not blinded (open label trial) (trial registry). Deviations reported are expected to arise in usual care: "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study." Number analysed unclear, but seems to be a modified ITT: "The study was conducted under “intent to treat” criteria." | High risk of bias | 22/51 (43%) participants in the intervention and 16/29 (55%) participants in the control arm did not have data available. "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study. In particular, the tendency for more dropouts among minorities and those with less education..." Very high attrition, thus missingness could and is likely to depend on the true value. | Low risk of bias | Weekly weigh‐ins. No evidence that a different method was used across intervention groups to measure weight. Open‐label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | ...treatment codes revealed only after the study statistician locked the database at the end of the trial. No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. All time points mentioned in trial registry reported on in publication. Analysis intentions are not reported in sufficient detail prior to study publication to enable an assessment. | High risk of bias | Very high attrition with a variety of reasons relating to health, therefore some missingness could be and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Calleja‐Fernández 2012 | Some concerns | Random allocation to each dietary group was performed by the main investigator (MDBP) by using the Web site Randomization.com (http://www.randomization.com) upon the request of the study dietitian (ACF) after the eligibility of a participant was confirmed and assignment to either of the insulin resistance or insulin sensitivity groups had been performed. No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected. | Low risk of bias | No information on blinding of participants, however, blinding is highly unlikely. People delivering these dietary interventions were unblinded. No reasons or details about deviations from intended interventions reported. ITT analysis conducted. | Low risk of bias | It seems data were available for all participants randomised for 16 week study, but cannot be sure (flowcharts in Fernandez et al 2012 and Ballesteros‐Pomar 2009 incongruent). | Low risk of bias | "Body weight was measured to the nearest 0.5 kg and then taken using calibrated digital scales…" No evidence that a different method was used across intervention groups to measure weight. "The medical assessments were blinded to the type of diet being followed." | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions. |
| Dyson 2007 | Low risk of bias | "Randomization was undertaken by means of sealed envelopes equivalent to the number of subjects and filled fifty‐fifty with an indicator of either a low‐carbohydrate diet or healthy‐eating advice. Two separate sets of envelopes were prepared for both diabetic and non‐diabetic subjects. An independent observer witnessed randomization." Author reported in correspondence that no formal test of hypothesis was done for difference at baseline for non‐diabetic participants. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care: n=3 of randomised participants in balanced group refused follow‐up as not allocated to low carbohydrate diet. Modified ITT conducted. | High risk of bias | No participants in the intervention group and 3/7 (43%) in the control group did not have data. Modified ITT with last observation carried forward. High attrition in control group, and differential attrition. Refusal of follow‐up due to not being allocated to low carbohydrate may be influenced by expected change in weight, therefore missingness could and likely influenced by the true value. | Low risk of bias | Body weight recorded monthly. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition in the control group, with disappoinment at not being assigned to the low carbohydrate diet cited as reason; therefore missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Foraker 2014 | Some concerns | "Women were randomized using stratified randomization, based on BMI (< 30 kg/m2 vs. ≥ 30 kg/m2) to ensure equal distribution of overweight and obese participants into each intervention group." No information on allocation concealment. No problematic imbalance in baseline characteristics between groups | Low risk of bias | Double masked: investigator and outcomes assessor. Deviations reported are expected to arise in usual care, e.g. personal/family medical issues, time constraints. Modified ITT: completers analysed "number still under follow‐up". | High risk of bias | 13/38 (34%) in the intervention and 15/41 (37%) in the control group did not have data available. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Anthropometric tests at each visit included body weight measurements. No evidence that a different method was used across intervention groups to measure weight. Double masked: investigator and outcomes assessor. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint (mentions only number of women who lose weight as outcome). Analysis intentions are not reported in sufficient detail to enable an assessment; weight findings are emphasised as part of the primary aim in the paper's methods and trial registry; with limited reporting of these findings in the paper and none reported in the abstract. | High risk of bias | No specific information on allocation concealment. High attrition in both groups with limited reasons reported, therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Keogh 2007 | High risk of bias | Clinstat used to generate sequence; "allocation concealment: No" (trial registry). From Table 1 there appeared to be no large differences between groups at baseline. | Low risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. personal reasons, unable to comply. Intention to treat: all randomised were analysed. | Low risk of bias | All participants, 13/13 in intervention and 12/12 in control, had data available. | Low risk of bias | Weight was measured using a Mercury digital scale in light clothing at baseline, and every 2 weeks. No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Lack of information in the pre‐registered methods. |
| Lasker 2008 | Some concerns | "Subjects were blocked on gender, matched on age...and fasting glucose..." No information on allocation concealment. Significant baseline imbalance for insulin; no significant differences for 13 other characteristics at baseline | High risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. training for marathon. Per protocol analysis (participants excluded for lack of adherence to protocol: 23% excluded for various reasons, including lack of adherence, but breakdown by trial arm and reason not reported). | High risk of bias | Data were not available for 7/32 (22%) participants in the intervention and 8/33 (24%) in the control group. High attrition with reasons including non‐compliance, which may be related to change in weight, so likely that with such high attrition and this reason some missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Some participants were excluded from the analysis due to a lack of adherence, with high attrition reported in both trial arms. Based on these numbers and known exclusions for lack of adherence, missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Layman 2005 | Some concerns | "Note that the randomization was blocked on age and BMI for each wave of recruitment." No information on allocation concealment. "Baseline characteristics did not differ among subjects in each of the treatment groups..." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No deviations or drop‐outs reported. Appears to be a full ITT with number randomised equal to number analysed. | Low risk of bias | All randomised participants appear to have been analysed. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions. |
| Marco‐Benedi 2019 | Low risk of bias | "Randomization was performed by using an online software..." Paper reported "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding (trial registry reported 'Open‐label'). Only one out of numerous outcomes was significantly different between groups at baseline. | Low risk of bias | Trial registry states "Masking: None (Open Label)", but paper reports "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding. Deviations reported are expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 5/40 (12.5%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. Reason for withdrawal is only available for 1/13 excluded participants. Difficult to judge whether it is likely that missingness is related to true value for 12/13 participants, based on reasons provided (unknown, personal reasons). | Low risk of bias | "Body weight was measured...with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. Trial registry states "Masking: None (Open Label)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with vague reasons for loss to follow‐up and one exclusion; therefore it is not possible to judge whether missingness could be or is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Mateo‐Gallego 2017 | Some concerns | "We carried out the randomization process by generating a number sequence (including 93 correlative numbers because this was the number of subjects we planned to randomize) in the following webpage: https://www.random.org. The number for each participant was correlatively assigned (e.g; the first randomized women was assigned with number 1)" (from author correspondence). No information about allocation concealment. "There were no significant between‐group differences at baseline." | Low risk of bias | "Participants were blinded to their assigned macronutrient composition." "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." Study is reported as single blind (Masking: Single (Participant)). Deviations reported are expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 3/30 (10%) in the intervention and 6/30 (20%) in the control group did not have data available for this outcome. Limited reporting of reasons for missingness, therefore difficult to judge whether missingness could or is likely to be dependent on the true value of the outcome. | Low risk of bias | "Body weight was measured in subjects without shoes to the nearest 0.1 kg with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported in both groups with limited reporting of reasons, therefore it is not possible to judge if missingness could be or is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Veum 2017 | Some concerns | "The list of prescreening appointments was used to randomly assign the participants by drawing ballots and blocking with block sizes of 2." No information about allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | From registry: "Masking: None (Open Label)" "All participants were informed of group allocation after the baseline measurements and sampling. The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Deviations reported are expected to arise in usual care, e.g. busy job, fracture. "We also performed an intention‐to‐treat (ITT) analysis that included all randomly assigned participants except 2 cases that were lost before baseline (44 eligible participants in total). These 2 were unaware of the group allocation and did not provide any dietary or clinical data." | Low risk of bias | 4/24 (17%) in the intervention and 4/22 (18%) in the control group did not have data available for this outcome. "Notably, the results from the PP and ITT analyses did not differ in nominal significance for any of the primary or secondary outcomes. Our missing data analysis (data not shown), including Little’s missing completely at random test, indicated that the dropouts were missing (completely) at random, and we found no significant differences between dropouts and completers in baseline characteristics, except for significant higher diastolic blood pressure, total cholesterol (TC), and LDL cholesterol in completers. In the reported PP and ITT analyses, we used mixed models without imputation to handle missing data. However, we also conducted an ITT analysis based on a full dataset in which missing values in the original data were replaced by values from multiple imputation (data not shown)." | Low risk of bias | Taken barefoot and wearing light clothing and asked to use restroom shortly before. No evidence that a different method was used across intervention groups to measure weight. "The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Volek 2009 | Some concerns | No information about randomisation or allocation concealment. No apparent imbalances between two groups at baseline | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No deviations or drop‐outs reported. Appears to be a full ITT with number randomised equal to number analysed. | Low risk of bias | Number randomised equals number analysed. | Low risk of bias | "Body mass was measured in the morning after an overnight fast to the nearest 100 g on a calibrated digital scale." No evidence that a different method was used across intervention groups to measure weight. "Analyses were performed by the same blinded technician." | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions. |
Risk of bias for analysis 1.5 Change in body weight (kg) at 3 to < 12 months: subgroup gender.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.5.1 Mixed | ||||||||||||
| Aude 2004 | Some concerns | Randomisation was done using a block design by sex to achieve an equal sex distribution. No information on allocation concealment. "At baseline, there were no significant differences in age, weight, BMI, waist‐to‐hip ratio, and any of the lipid variables between the NCEP and MLC groups" (only for completers). | High risk of bias | No information on blinding of participants and people delivering these dietary interventions, but blinding is unlikely. No reasons or details about deviations from intended interventions reported. A quarter of the participants were not included in the analysis: 8/30 participants in the intervention group and 7/30 in the control group were excluded from analysis; 6/60 did not complete the trial (missing), but no information about remaining 9/60 who were not included. | High risk of bias | 8/30 (27%) participants in the intervention and 7/30 (23%) participants in the control arm did not have data available. No reasons for loss to follow‐up or withdrawal provided, so difficult to judge if related to participants' health status, but attrition is high in both groups; thus missingness could and is likely to depend on the true value. | Low risk of bias | Weight was measured with indoor clothing and without shoes on a single scale. No evidence that a different method was used across intervention groups to measure weight. "All anthropometric measurements were obtained by an observer blinded to the treatment assignment." | Some concerns | No trial registry reported and no mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. A quarter of participants were not included in the analysis, with withdrawal reported for 10% of participants. No reasons were provided for the remaining missingness. Attrition is high in both groups with no reasons provided, therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | No information about participant blinding, however, blinding of participants is highly unlikely. "...dietitians were not blinded to the study hypothesis." No reasons or details about deviations from intended interventions reported. "All analyses were conducted using the intention‐to‐treat principle." n=148 randomised, and n=148 analysed. | Low risk of bias | 13/75 (17%) participants in the intervention and 9/73 (12%) participants in the control arm did not have data available. Imputed missing values, and performed sensitivity analyses to determine whether missing at random (MAR) assumptions were met. "We performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption." "We used Markovchain Monte Carlo techniques to impute missing values, including additional covariates..." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses." | Low risk of bias | Body weight was measured to the nearest 0.1 kg using a single calibrated scale. No evidence that a different method was used across intervention groups to measure weight. "all outcome assessors were blinded to the diet group assignment..." | Some concerns | No mention of a pre‐specified analysis plan. "Study protocol and data set: Not available." Trial registry outcomes amended after study publication; but trial registry entry at date prior to study publication mentions the outcome and timepoints. All time points mentioned in trial registry at date prior to study publication, were reported on in publication. Analysis intentions are not reported in sufficient detail prior to study publication to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Calleja‐Fernández 2012 | Some concerns | Random allocation to each dietary group was performed by the main investigator (MDBP) by using the Web site Randomization.com (http://www.randomization.com) upon the request of the study dietitian (ACF) after the eligibility of a participant was confirmed and assignment to either of the insulin resistance or insulin sensitivity groups had been performed. No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected. | Low risk of bias | No information on blinding of participants, however, blinding is highly unlikely. People delivering these dietary interventions were unblinded. No reasons or details about deviations from intended interventions reported. ITT analysis conducted. | Low risk of bias | It seems data were available for all participants randomised for 16 week study, but cannot be sure (flowcharts in Fernandez et al 2012 and Ballesteros‐Pomar 2009 incongruent). | Low risk of bias | "Body weight was measured to the nearest 0.5 kg and then taken using calibrated digital scales…" No evidence that a different method was used across intervention groups to measure weight. "The medical assessments were blinded to the type of diet being followed." | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions. |
| Dyson 2007 | Low risk of bias | "Randomization was undertaken by means of sealed envelopes equivalent to the number of subjects and filled fifty‐fifty with an indicator of either a low‐carbohydrate diet or healthy‐eating advice. Two separate sets of envelopes were prepared for both diabetic and non‐diabetic subjects. An independent observer witnessed randomization." Author reported in correspondence that no formal test of hypothesis was done for difference at baseline for non‐diabetic participants. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care: n=3 of randomised participants in balanced group refused follow‐up as not allocated to low carbohydrate diet. Modified ITT conducted. | High risk of bias | No participants in the intervention group and 3/7 (43%) in the control group did not have data. Modified ITT with last observation carried forward. High attrition in control group, and differential attrition. Refusal of follow‐up due to not being allocated to low carbohydrate may be influenced by expected change in weight, therefore missingness could and likely influenced by the true value. | Low risk of bias | Body weight recorded monthly. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition in the control group, with disappoinment at not being assigned to the low carbohydrate diet cited as reason; therefore missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Ebbeling 2007 | Low risk of bias | "Enrolled participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, with stratification by sex and ethnicity/race..." "To avoid any bias in assigning participants to diet groups, staff conducting recruitment and enrollment were masked to sequence. The study director assigned participants to groups." No problematic differences in baseline characteristics between the two groups | Low risk of bias | No masking (open label trial). No reasons or details about deviations from intended interventions reported. ITT with imputation for missing values. | High risk of bias | 4/36 (11%) in the intervention and 3/37 (8%) in the control group did not have outcome data. "For body weight after dropout, an increase of 1 kg per year was imputed, starting from the last available measurement." No reasons for loss to follow‐up or withdrawal provided, cannot judge if related to participants' health status and if missingness depends on its true value. | Low risk of bias | Dietitians measured at each visit using electronic scale. No evidence that a different method was used across intervention groups to measure weight. "Data were collected by personnel who were masked to group assignment." | Some concerns | No mention of a pre‐specified analysis plan; trial registry does not mention the outcome and timepoint in any versions. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Attrition reported in both groups, with no reasons provided. Missingness was addressed through last observation carried forward. It is not possible to determine whether missingness could or is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Farnsworth 2003 | Some concerns | No information on how the randomisation sequence was generated. No information on allocation concealment. "There were no significant differences in any of the variables between the diet groups." | Some concerns | No information on blinding of participants and people delivering these dietary interventions, however blinding of these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. work commitments, pregnancy. 66 randomised, 57 completed and 56 analysed. Per protocol analysis. "...data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." 1/57 participant data excluded. | Low risk of bias | 5/33 (15%) in the intervention and 5/33 (15%) in the control group did not have data available. Data from n=1 participant where missingness possibly influenced by true value. "Six subjects withdrew before study commencement because of family and work commitments; in addition, one subject withdrew because of pregnancy during the course of the study, the clinic lost contact with one subject, and data from one subject were excluded from the analysis because of the subject's noncompliance with the diet." "... one subject declined to undergo a DXA scan for personal reasons." | Low risk of bias | Body weight measured after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Data from one subject was excluded due to 'noncompliance' with the diet, but other reasons for loss to follow‐up were expected to arise in usual care. No pre‐registered document available to assess analysis intentions. |
| Foster 2010 | Some concerns | "Using a random‐number generator, we randomly assigned participants within each site to treatment with either a low‐carbohydrate or low‐fat, calorie‐restricted diet for 2 years." No information on allocation concealment. No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | Masking: none; open label. Deviations reported are expected to arise in usual care, e.g. dissatisfied with the program, pregnancy, life stessors, relocated. ITT analysis with n=307; all randomised. "We used a random‐effects linear model that was fitted to all observed data for each variable on each of the 307 participants for the primary analysis." | Low risk of bias | 25/153 (16%) in the intervention and 19/154 (12%) in the control group did not have data available. "..longitudinal models implemented for this study relax this missing‐completely‐at‐random assumption in various ways." "Under the sensitivity analysis based on imputing missing outcomes with the highest (13.795) and lowest (‐18.355) random‐effects slopes (that is, change in weight per month) under the mixed‐effects model for weight, our qualitative findings were not sensitive to either imputation approach." Results of sensitivity analyses not provided. | Low risk of bias | Body weight was measured at each treatment visit on calibrated scales while participants wore light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "Assessors were not blinded to treatment condition." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Frisch 2009 | Some concerns | "Participants (n = 200) were randomly assigned by computer‐generated random number lists in two equal groups…" No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | No masking (open label trial). Deviations reported are expected to arise in usual care, e.g. non‐compliance. ITT analysis conducted. | High risk of bias | 5/100 (5%) in the intervention and 11/100 (11%) in the control group did not have data available. Data were evaluated by ITT and per protocol method. ITT done with baseline values carried forward. "Results did not differ when the per protocol method was used (data not shown)." 4/100 (4%) in the intervention and 10/100 (10%) in the control group withdrew due to non‐compliance, which may be related to change in weight (missingness possibly influenced by true value). | Low risk of bias | Participants were weighed using a calibrated electronic scale. No evidence that a different method was used across intervention groups to measure weight. Open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported with reasons such as 'non‐compliance', therefore missingness could have and likely depended on the true value of the outcome. Both per protocol and baseline values carried forward approaches were used for missing data. Lack of information in the pre‐registered methods. |
| Goni 2018 | Some concerns | "Subjects were randomly assigned to one of the two diets by a specific logarithm design for the study by MATLAB using stratified block randomization..." No information about allocation concealment. No significant differences between groups at baseline | Low risk of bias | Masking: none; open label. The only deviations reported are drop‐outs. Modified ITT: looks as if only missing outcome data excluded. | High risk of bias | 21/75 (28%) in the intervention 19/72 (26%) in the control group did not have data available for this outcome. No reasons for drop‐out provided, but with this high attrition in both groups missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Assessed using a Tanita BC418 scale. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. High attrition reported in both groups with no reasons provided, therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Haufe 2013 | Low risk of bias | "For allocation of the subjects, a computer‐generated list of random numbers was used. The randomization sequence was created using SPSS 18 (Chicago, IL) statistical software and subjects were assigned to reduced carbohydrate or reduced fat diet with a 1:1 allocation using random block sizes of 2, 4, and 6. Study nurses and physicians screening and enrolling volunteers were blinded for the randomization sequence." "Both groups were well matched for gender, age, body weight, body mass index, blood lipid profiles, glucose metabolism, and cardiorespiratory fitness." | High risk of bias | "Blinding of participants for the allocated dietary intervention was impossible." "Except for the dieticians, study nurses and physicians were blinded for the treatment assignment." Deviations reported are expected to arise in usual care, e.g. lost to follow‐up, withdrew consent. Per protocol analysis conducted: 24/28 (86%) in the intervention and 30/33 (91%) in the control group excluded from the analysis including 'protocol violations'. | High risk of bias | 25/80 (31%) in the intervention and 29/83 (35%) in the control group did not have data available. ITT with last observation carried forward. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured body weight in a standardised manner after an overnight fast. No evidence that a different method was used across intervention groups to measure weight. "....study nurses and physicians were blinded for the treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | The majority of data not included in the analyses were due to exclusions based on 'protocol violations'. High overall attrition was reported in both groups, with no reasons (aside from exclusions); missingness could be and is likely dependent on the true value of the outcome. Last observation carried forward approach used for dealing with missingness. Lack of information in the pre‐registered methods. |
| Keogh 2007 | High risk of bias | Clinstat used to generate sequence; "allocation concealment: No" (trial registry). From Table 1 there appeared to be no large differences between groups at baseline. | Low risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. personal reasons, unable to comply. Intention to treat: all randomised were analysed. | Low risk of bias | All participants, 13/13 in intervention and 12/12 in control, had data available. | Low risk of bias | Weight was measured using a Mercury digital scale in light clothing at baseline, and every 2 weeks. No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Lack of information in the pre‐registered methods. |
| Klemsdal 2010 | Some concerns | No information about randomisation or allocation concealment. Footnote to Table 1: "There were no significant differences between groups." | Low risk of bias | Masking: None (Open Label). Deviations reported are vague, but those reported are expected to arise in usual care, e.g. non‐attendance of study visits. ITT analysis: "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." All randomised participants were analysed. | High risk of bias | Short‐term outcomes: 16% of participants overall did not have data available (not reported per trial arm). "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." No mention of sensitivity analysis. No reasons for lost to follow‐up or withdrawal provided and therefore cannot make a judgment about whether missingness was dependent on the true value of the outcome. | Low risk of bias | "Body weight...measured with standard methods." No evidence that a different method was used across intervention groups to measure weight. Masking: None (Open Label). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition with no reasons reported for the overall group, it is therefore not possible to determine whether missingness could be or is likely dependent on the true value of the outcome. Missingness was addressed by using last observation carried forward. Lack of information in the pre‐registered methods. |
| Landers 2002 | Some concerns | No information on randomisation or allocation concealment. Families were assigned to the same dietary intervention. Baseline characteristics were not reported, with the exception of body weight, fat mass and lean mass; all of which did not appear to be significantly different. | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. did not attend follow‐up visits. Modified ITT excluding participants with missing outcome data. | High risk of bias | Data were not available for 12/28 (43%) participants in the intervention and 12/33 (36%) in the control group. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured in light clothing without shoes at the initial visit. No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and is likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Lasker 2008 | Some concerns | "Subjects were blocked on gender, matched on age...and fasting glucose..." No information on allocation concealment. Significant baseline imbalance for insulin; no significant differences for 13 other characteristics at baseline | High risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. training for marathon. Per protocol analysis (participants excluded for lack of adherence to protocol: 23% excluded for various reasons, including lack of adherence, but breakdown by trial arm and reason not reported). | High risk of bias | Data were not available for 7/32 (22%) participants in the intervention and 8/33 (24%) in the control group. High attrition with reasons including non‐compliance, which may be related to change in weight, so likely that with such high attrition and this reason some missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Some participants were excluded from the analysis due to a lack of adherence, with high attrition reported in both trial arms. Based on these numbers and known exclusions for lack of adherence, missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Layman 2009 | Some concerns | No information on how the randomisation sequence was generated. No information about allocation concealment. "Randomization after stratifying participants for gender, age, BMI, and TC was successful with no significant differences between treatment groups in baseline characteristics." | Low risk of bias | "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Deviations are expected to arise in usual care, e.g. personal reasons, unable to comply. "Prior to the 4‐mo data collection, 15 participants withdrew from the CHO group (9 stated personal reasons and 6 stated unable to comply with diet protocol) and 12 participants withdrew from the PRO group (11 stated personal reasons and 1 stated inability to comply with diet protocol)." Modified ITT conducted. | High risk of bias | 12/64 (19%) in the intervention and 15/66 (23%) in the control group did not have data available. No methods used to correct for bias, or sensitivity analyses conducted. 6/15 (40%) withdrawals in the intervention and 1/12 (8%) withdrawals in the control group were due to inability to comply with diet. Inability to comply may be related to change in weight, and high attrition in the control group, thus missingness could and is likely to depend on the true value. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. Attrition reported in both groups, with high attrition in the control group, and some attrition in both groups due to an 'inability to comply with diet'; therefore missingness could and is likely to depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Lim 2010 | Some concerns | No information about randomisation or allocation concealment. Baseline characteristics table showed small differences, consistent with differences resulting by chance with small sample sizes. | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. travel, work commitment, did not like diet. Modified ITT with all partial data contributed by non‐completers included in linear mixed effects model. | High risk of bias | 6/30 (20%) in the intervention and 8/30 (27%) in the control group did not have data available. ITT with all partial data contributed by non‐completers included in linear mixed effects model. Results of ITT not presented and no sensitivity analyses performed (author correspondence). High attrition in control group and some missingness could possibly and is likely to depend on true value of outcome. | Low risk of bias | Recorded in light clothing on Mercury digital scales. No evidence that a different method was used across intervention groups to measure weight. No information about blinding. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition reported in both groups, therefore missingness could be and is likely to depend on the true value of the outcome. Partial data from missing participants included in linear mixed effects model, but results of this ITT analysis not reported. No pre‐registered document available to assess analysis intentions. |
| Marco‐Benedi 2019 | Low risk of bias | "Randomization was performed by using an online software..." Paper reported "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding (trial registry reported 'Open‐label'). Only one out of numerous outcomes was significantly different between groups at baseline. | Low risk of bias | Trial registry states "Masking: None (Open Label)", but paper reports "...participants and the staff, except for the nutritionists, were blinded to the type of diet individuals were assigned." Likely referring to allocation concealment, not blinding. Deviations reported are expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 5/40 (12.5%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. Reason for withdrawal is only available for 1/13 excluded participants. Difficult to judge whether it is likely that missingness is related to true value for 12/13 participants, based on reasons provided (unknown, personal reasons). | Low risk of bias | "Body weight was measured...with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. Trial registry states "Masking: None (Open Label)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with vague reasons for loss to follow‐up and one exclusion; therefore it is not possible to judge whether missingness could be or is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Ooi 2021 | Some concerns | "This was a randomized single‐blinded, placebo‐controlled study where subjects were block randomly assigned by gender and BMI groups…" Allocation concealment: no trial registry available and no information in paper. No significant differences between groups at baseline | High risk of bias | Subjects blinded only to supplements. No information about blinding of diets, however blinding of participants highly unlikely, the study is stated as single‐blind, thus people delivering these dietary interventions were unblinded. Deviations reported are expected to arise in usual care e.g family, work or personal commitments. Per protocol analysis, with exclusion of available data for reasons such as non‐compliance and inadequate weight loss. Vague reasons for exclusions and specific numbers excluded per trial arm are unclear, with 8% of subjects being excluded by the principal investigator. | High risk of bias | 3/43 (7%) in the intervention and 7/44 (16%) in the control group did not have data available. "Study subjects with available information for each analysis were included and missing data were not imputed." Study subjects that did not comply with the diets or did not lose a specific amount of weight were excluded from analyses by the investigators; thus missingness in the outcome could and is likely to depend on its true value. | Low risk of bias | "Height and weight were taken using a SECA 763 digital scale…" No evidence that a different method was used across intervention groups to measure weight. The study was single blinded for participants to supplements. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, no trial registry. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Subjects were likely not blinded to dietary allocation. Per protocol analysis conducted with non‐compliant participants excluded. Missing data were not imputed. Exclusions of available data were done based on non‐compliance with diet or inadequate weight loss, thus missingness in the outcome could and is likely to depend on its true value. No pre‐registered document available to assess analysis intentions. |
| Parr 2016 | High risk of bias | "The randomization will be initially stratified by baseline BMI values (27–29, 30–34, and 35–40 kg/m2) and sex. Subsequently, simple randomisation by roll of dice within the stratified segregation will be used to allocate individuals to each of the 3 groups." "Allocation is not concealed" "No significant differences were observed among groups at baseline for any variable (P > 0.05)." | High risk of bias | "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment." From registry: Open (masking not used). Deviations reported are expected to arise in usual care, e.g. time commitment, ankle injury. Per protocol (participants excluded from analysis due to compliance). 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance and 1/8 (12.5%) due to being an outlier. | High risk of bias | 10/39 (14%) in the intervention and 8/40 (20%) in the control group did not have data available for this outcome. 5/10 (50%) excluded due to non‐compliance in the intervention group and 3/8 (43%) in the control group due to non‐compliance, which may be related to change in weight; thus missingness could and is likely dependent on the true value. | Low risk of bias | Measured with DEXA. No evidence that a different method was used across intervention groups to measure weight. "From then on, apart from the study dietitian, all investigators were blinded to the assigned dietary group by using participant codes unrelated to their dietary assignment.", but from the registry: "Open (masking not used)" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition is reported in both groups, with nearly half of all missing data due to participants being excluded from the per protocol analysis due to 'non‐compliance' and being an 'outlier'. Missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Sacks 2009 | Low risk of bias | "Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian, after eligibility of a participant was confirmed." Independent trial unit, fully funded by public funds and large trial; it was likely the sequence was random. " Baseline characteristics were similar among participants assigned to the four diets..." | Low risk of bias | "Blinding was maintained by the use of similar foods for each diet. Staff and participants were taught that each diet adhered to principles of a healthful diet..." True blinding is a known challenge with dietary interventions. Deviations reported are expected to arise in usual care, e.g. moved away, did not appear at scheduled appointments. Full ITT "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." | High risk of bias | Linked paper reported 39/201 (19%) in the intervention and 47/204 (23%) in the control group did not have data available for this outcome at 6 months (De Jonge et al 2012); 2‐year attrition (Sacks et al 2009) reports less attrition per group. Single imputation "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." No sensitivity analyses (author correspondence). No detailed reasons for loss to follow‐up or withdrawals provided, high attrition in the control group thus missingness could and is likely to depend on the true value. | Low risk of bias | "Body weight, the primary outcome variable, was measured by calibrated hospital scales, in the morning before breakfast and after urinating, clothed in a hospital gown, on two nonconsecutive days at baseline, and at 6 and 24 months; and on a single day at 12 and 18 months." No evidence that a different method was used across intervention groups to measure weight. "Investigators and staff who measured outcomes were unaware of the diet assignment of the participants." | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with high attrition in the control group, and limited reporting of reasons; therefore missingness could be and is likely dependent on the true value of the outcome. Simple imputation was performed, but no sensitivity analyses. Lack of information in the pre‐registered methods. |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | High risk of bias | From registry: "Open (masking not used)". Deviations reported are expected to arise in usual care, e.g. work commitments, personal reasons. Per protocol analysis, ITT with last observation carried forward also performed but data not reported. 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons. | High risk of bias | 10/55 (18%) in the intervention and 11/54 (20%) in the control group did not have data available for this outcome. ITT with last observation carried forward: "The ITT with either baseline values carried forward or the last follow‐up visit carried forward for those who did not complete the study also showed no difference in weight loss between the diets (p<=0.23)." 4/10 (40%) participants in the intervention group and 2/11 (18%) in the control group were excluded from analysis due to non‐compliance or unclear reasons, which may be related to change in weight; thus missingness could or is likely dependent on the true value of the outcome. | Low risk of bias | "Body mass was measured using calibrated electronic digital scales...to the nearest 0.05 kg, with subjects wearing light clothing and no footwear." No evidence that a different method was used across intervention groups to measure weight. From registry: "Open (masking not used)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition is reported in both trial arms, with high attrition in the control group due to unclear reasons and 'non‐compliance'; therefore missingness could and is likely to be related to the true value of the outcome. ITT analyses with baseline values and last observations carried forward were conducted, but neither were considered appropriate to address potential bias due to missingness. Lack of information in the pre‐registered methods. |
| Volek 2009 | Some concerns | No information about randomisation or allocation concealment. No apparent imbalances between two groups at baseline | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No deviations or drop‐outs reported. Appears to be a full ITT with number randomised equal to number analysed. | Low risk of bias | Number randomised equals number analysed. | Low risk of bias | "Body mass was measured in the morning after an overnight fast to the nearest 100 g on a calibrated digital scale." No evidence that a different method was used across intervention groups to measure weight. "Analyses were performed by the same blinded technician." | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions. |
| Subgroup 1.5.2 Males | ||||||||||||
| Benassi‐Evans 2009 | Some concerns | "Subjects were randomised to one of two dietary interventions…" No further information about allocation sequence or concealment. "Subject characteristics were not significantly different between groups at baseline." | Low risk of bias | No information, but blinding of participants and people delivering the interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. No details about analysis are reported, but looks to be intention to treat (number randomised per group = number analysed per group). | High risk of bias | No details about attrition reported, but some attrition is implied: "...in the subjects who completed the 12 weeks intensive weight loss and 9 months weight maintenance..." No further information or evidence that the result was not biased due to missing data are provided. | Low risk of bias | Volunteers fasted overnight and were weighed (model AMZ14; Mercury Digital Scales, Tokyo, Japan) wearing light clothing and no shoes at weeks. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition is implied, with no reasons or evidence to show that results were not biased by this missingness. No pre‐registered document available to assess analysis intentions. |
| Veum 2017 | Some concerns | "The list of prescreening appointments was used to randomly assign the participants by drawing ballots and blocking with block sizes of 2." No information about allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | From registry: "Masking: None (Open Label)" "All participants were informed of group allocation after the baseline measurements and sampling. The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Deviations reported are expected to arise in usual care, e.g. busy job, fracture. "We also performed an intention‐to‐treat (ITT) analysis that included all randomly assigned participants except 2 cases that were lost before baseline (44 eligible participants in total). These 2 were unaware of the group allocation and did not provide any dietary or clinical data." | Low risk of bias | 4/24 (17%) in the intervention and 4/22 (18%) in the control group did not have data available for this outcome. "Notably, the results from the PP and ITT analyses did not differ in nominal significance for any of the primary or secondary outcomes. Our missing data analysis (data not shown), including Little’s missing completely at random test, indicated that the dropouts were missing (completely) at random, and we found no significant differences between dropouts and completers in baseline characteristics, except for significant higher diastolic blood pressure, total cholesterol (TC), and LDL cholesterol in completers. In the reported PP and ITT analyses, we used mixed models without imputation to handle missing data. However, we also conducted an ITT analysis based on a full dataset in which missing values in the original data were replaced by values from multiple imputation (data not shown)." | Low risk of bias | Taken barefoot and wearing light clothing and asked to use restroom shortly before. No evidence that a different method was used across intervention groups to measure weight. "The nature of the trial required an open intervention with no blinding of the trial participants or the investigators." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome, but does not mention this timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | Some concerns | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. relocated overseas, unable to commit to study protocol/time requirements. Per protocol analysis: 3/59 and 1/64 participants excluded due to being unable to commit to study protocol or did not receive intervention. 3% of total not included (4/123). | High risk of bias | 7/59 (12%) in the intervention and 6/64 (9%) in the control group did not have data available. Modified ITT only done at 52 weeks using maximal likelihood mixed model analysis. "Overall, the participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks..." missingness could and is likely to depend on the true value. | Low risk of bias | "... body weight was measured using calibrated electronic digital scales (Mercury; AMZ 14, Tokyo, Japan)." No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Attrition was reported in both groups, and authors remark that "...participants who withdrew after week 12 were those who lost less weight during weeks 0–12 than those who completed the full 52 weeks…; missingness is therefore likely dependent on the true value of the outcome. Furthermore, participants were excluded for an inability to commit to the study protocol or not receiving the intervention. Lack of information in the pre‐registered methods. |
| Subgroup 1.5.3 Females | ||||||||||||
| Bales 2017 | Low risk of bias | We used a computerised centralised randomisation scheme blocking by race and marital or partner status. "...also blocked by functional status..." "The 1:2 allocation to the C‐WL and HP‐WL groups was chosen...to make within‐group comparisons in the protein group in subsequent analyses. This oversampling of the protein group did not cause a substantial loss of power for the between‐group comparisons; the relative power for a 2:1 split is 0.94(23)." No statements or statistical tests about differences between groups, no problematic imbalance suspected. | Low risk of bias | Not blinded (open label trial) (trial registry). Deviations reported are expected to arise in usual care: "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study." Number analysed unclear, but seems to be a modified ITT: "The study was conducted under “intent to treat” criteria." | High risk of bias | 22/51 (43%) participants in the intervention and 16/29 (55%) participants in the control arm did not have data available. "We examined the reasons for dropout to inform future trials and found that 56% of dropouts had difficulty meeting study requirements due to family commitments or health issues; another 11% developed new health problems unrelated to the study. In particular, the tendency for more dropouts among minorities and those with less education..." Very high attrition, thus missingness could and is likely to depend on the true value. | Low risk of bias | Weekly weigh‐ins. No evidence that a different method was used across intervention groups to measure weight. Open‐label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | ...treatment codes revealed only after the study statistician locked the database at the end of the trial. No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. All time points mentioned in trial registry reported on in publication. Analysis intentions are not reported in sufficient detail prior to study publication to enable an assessment. | High risk of bias | Very high attrition with a variety of reasons relating to health, therefore some missingness could be and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Brehm 2003 | Some concerns | "... after each block of subjects was assessed, the principal investigator used a random number table to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences at baseline. | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. dislike diet, could not make scheduled visit. Modified ITT: excluded only missing patients. | High risk of bias | 7/26 (27%) in the intervention and 14/27 (52%) in the control group did not have data. "In this intention to treat analysis, the initial weights for the subjects who withdrew from the study were used as their follow‐up weights at 3 and 6 months..." Lost to follow‐up and withdrawal reasons are stated for all participants, but very high attrition in the control group and differential attrition, thus thus missingness could and is likely to depend on the true value. | Some concerns | Subjects were weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Differential attrition and high attrition in the control group, therefore missingness could and is likely to be dependent on the true value of the outcome. It is not clear whether outcome assessors were blinded to allocation. No pre‐registered document available to assess analysis intentions. |
| Brehm 2005 | Some concerns | "After each block of subjects was enrolled, the principal investigator used a computerized randomization program to randomly assign those subjects to one of two diets." No information on allocation concealment. No significant differences between groups at baseline (completers only). | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. pregnancy, disliked diet. Modified ITT: all those who completed the study were analysed. | High risk of bias | 5/25 (20%) in the intervention and 5/25 (20%) in the control group did not have data available. Difficult to judge whether it is likely that missingness is related to the true value for weight, as reasons are not explicit enough. "The most common reason for discontinuing the study was the inability to commit to weekly counseling sessions. Other reasons included pregnancy, new out‐of‐state job, dislike of the low‐carbohydrate diet, and advice against following the low carbohydrate diet by family physician." | Low risk of bias | Weighed on a single electronic scale. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors measuring weight. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | No specific information on allocation concealment. Reasons for attrition are vague and it is difficult to judge whether it was as a result of the true value of the outcome, but wit 20% attrition in both groups it is likely that some missingness was dependent on true value. No pre‐registered document available to assess analysis intentions. |
| Cornier 2005 | Some concerns | No information on how randomisation was done or allocation concealment. "Subject characteristics are summarized in Table 1. BW, BMI, percentage body fat, RMR, and RQ were similar in all groups." | Low risk of bias | No information, however, blinding of participants and people delivering these interventions is highly unlikely. "Forty‐four obese, healthy normoglycemic women 23 to 53 years old with BMI of 30 to 35 kg/m2 were screened, and 21 of them were enrolled and completed the 16‐week intervention." ITT analysis conducted: number randomised equals number analysed; no subjects lost to follow‐up. | Low risk of bias | Number randomised equals number analysed; no subjects lost to follow‐up. | Low risk of bias | Once a week, subjects were weighed. No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. No pre‐registered document available to assess analysis intentions. |
| Foraker 2014 | Some concerns | "Women were randomized using stratified randomization, based on BMI (< 30 kg/m2 vs. ≥ 30 kg/m2) to ensure equal distribution of overweight and obese participants into each intervention group." No information on allocation concealment. No problematic imbalance in baseline characteristics between groups | Low risk of bias | Double masked: investigator and outcomes assessor. Deviations reported are expected to arise in usual care, e.g. personal/family medical issues, time constraints. Modified ITT: completers analysed "number still under follow‐up". | High risk of bias | 13/38 (34%) in the intervention and 15/41 (37%) in the control group did not have data available. High attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | Anthropometric tests at each visit included body weight measurements. No evidence that a different method was used across intervention groups to measure weight. Double masked: investigator and outcomes assessor. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint (mentions only number of women who lose weight as outcome). Analysis intentions are not reported in sufficient detail to enable an assessment; weight findings are emphasised as part of the primary aim in the paper's methods and trial registry; with limited reporting of these findings in the paper and none reported in the abstract. | High risk of bias | No specific information on allocation concealment. High attrition in both groups with limited reasons reported, therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Gardner 2007 | Low risk of bias | "Randomization was conducted in blocks of 24 (6 per treatment group) and occurred by having a blinded research technician select folded pieces of paper with group assignments from an opaque envelope." No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking: single ("Clinic and laboratory staff members were blinded to treatment assignment"). Deviations reported are expected to arise in usual care, e.g. moved away, disliked diet, pregnancy. ITT analysis conducted. | High risk of bias | 6/77 (8%) in the intervention and 15/79 (19%) in the control did not have data available. ITT with baseline values carried forward. "Also for exploratory purposes, all analyses of weight and secondary outcome measures were tested using only available data, without using baseline values carried forward for missing data or other imputation methods. There were no substantive differences in any of these findings compared with the analyses with baseline values carried forward and, therefore, only the primary analyses are presented." 5/9 in the intervention and 5/18 in the control group withdrew due to health and "other" reasons (vague); but attrition is differential and thus missingness could and is likely to depend on the true value. | Low risk of bias | "Body weight was measured to the nearest 0.1 kg on a calibrated clinical scale." No evidence that a different method was used across intervention groups to measure weight. "Clinic and laboratory staff members were blinded to treatment assignment." | Some concerns | No mention of a pre‐specified analysis plan, trial registry at date prior to study publication mentions outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Differential attrition reported, with vague reasons; therefore missingness could and is likely to depend on the true value of the outcome. Per protocol and baseline values carried forward approaches were used to account for missingness. Lack of information in the pre‐registered methods. |
| Griffin 2013 | Low risk of bias | "The study consisted of a random block design per every four participants. Random allocation was prepared by a researcher not involved in participant enrolment or assignment." "random number table"; "sealed opaque envelopes" (trial registry). No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | "Participants were blind to diet macronutrient composition and were informed eating plans differed only in the type of meat (i.e. red or white)." "...inability to double‐blind the intervention were limitations." Deviations reported are expected to arise in usual care, e.g. did not like diet. ITT analysis conducted. | High risk of bias | 12/36 (33%) participants in the intervention and 15/35 (43%) in the control group did not have data available. ITT with last observation carried forward, and senstivity analysis comparing completers with ITT. 5/6 (83%) in the intervention and 5/9 (56%) in the control discontinued intervention due to illness, too difficult and not losing weight. Very high attrition in both groups; likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | Taken at every visit on a digital scale with light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. "...inability to double‐blind the intervention were limitations." Single‐blind with blinded participants. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable assessment. | High risk of bias | Very high attrition reported in both groups, with reasons such as 'illness', diets being 'too difficult' and 'not losing weight'; missingness could be and is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Josse 2011 | Some concerns | No information about how the randomisation sequence was generated, or allocation concealment. "Our stratification scheme was adequate, because there were no between‐group differences in any baseline variable." | Low risk of bias | Masking: none; open label. Deviations reported are expected to arise in usual care, e.g. moving house, exam time at school, sickness of a relative. Modified ITT "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model‐based multiple imputation." | Low risk of bias | 4/30 (13%) participants in the intervention and 1/30 (3%) in the control group did not have data available for this outcome. The only information reported is "...we conducted an intent‐to‐treat analysis where missing data values were imputed using model based multiple imputation." Based on the reasons provided, it is unlikely that missingness could be dependent on the true value of the outcome. | Low risk of bias | Digital physician's scale used to determine weight. No evidence that a different method was used across intervention groups to measure weight. Masking: none; open label. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information on how randomisation was done and whether, or how, allocation concealment was done. Lack of information in the pre‐registered methods. |
| Layman 2005 | Some concerns | "Note that the randomization was blocked on age and BMI for each wave of recruitment." No information on allocation concealment. "Baseline characteristics did not differ among subjects in each of the treatment groups..." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No deviations or drop‐outs reported. Appears to be a full ITT with number randomised equal to number analysed. | Low risk of bias | All randomised participants appear to have been analysed. | Low risk of bias | Measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. No information about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. No pre‐registered document available to assess analysis intentions. |
| Lean 1997 | Some concerns | No information on randomisation or allocation concealment. "All variables were not significantly different between the two diet treatment groups." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. ITT with last observation carried forward conducted. | High risk of bias | 13/53 (25%) in the intervention and 15/57 (26%) in the control group did not contribute data to this outcome. ITT with last observation carried forward conducted. High attrition in both groups with no reasons reported, but with this high attrition some missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | "Measurements were made with subjects wearing light clothes using regularly calibrated scales..." Reference to WHO procedures (classification and description of anthropometric data). No evidence that a different method was used across intervention groups to measure weight. No information on blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was done. High attrition in both groups, with no reasons reported; therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Liu 2013 | Low risk of bias | No information about sequence generation. "Randomisation was conducted by a statistician who was not involved in any other aspects of the study by using block randomisation, stratified by 10‐year age categories." "Although the participants were randomised by age, baseline BMI levels of the two groups were similar after randomisation." | Low risk of bias | No information about blinding, however blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. personal reasons, busy schedule. Modified ITT: one drop‐out due to busy schedule in control group excluded. | Low risk of bias | 1/25 (4%) in the intervention and 1/25 (4%) in the control group did not have data available for this outcome. | Low risk of bias | "Body weight was measured to the nearest 0.1kg at the beginning and then biweekly during the trial using an electronic scale." No evidence that a different method was used across intervention groups to measure weight. Masking: Single (Outcomes Assessor). | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Mateo‐Gallego 2017 | Some concerns | "We carried out the randomization process by generating a number sequence (including 93 correlative numbers because this was the number of subjects we planned to randomize) in the following webpage: https://www.random.org. The number for each participant was correlatively assigned (e.g; the first randomized women was assigned with number 1)" (from author correspondence). No information about allocation concealment. "There were no significant between‐group differences at baseline." | Low risk of bias | "Participants were blinded to their assigned macronutrient composition." "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." Study is reported as single blind (Masking: Single (Participant)). Deviations reported are expected to arise in usual care, e.g. change of residence, personal reasons. Modified ITT conducted: "All subjects who completed the study were included in the data analysis, independent of reported dietary compliance, as indicated by food records, or weight loss according to intention‐to‐treat analysis." | High risk of bias | 3/30 (10%) in the intervention and 6/30 (20%) in the control group did not have data available for this outcome. Limited reporting of reasons for missingness, therefore difficult to judge whether missingness could or is likely to be dependent on the true value of the outcome. | Low risk of bias | "Body weight was measured in subjects without shoes to the nearest 0.1 kg with a calibrated scale." No evidence that a different method was used across intervention groups to measure weight. "The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported in both groups with limited reporting of reasons, therefore it is not possible to judge if missingness could be or is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 1.9 Change in body weight (kg) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | "...dietitians were not blinded to the study hypothesis" No information about participant blinding, however, blinding of participants for these dietary interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. "All analyses were conducted using the intention‐to‐treat principle" n=148 randomised and n=148 analysed. | Low risk of bias | 16/75 (21.3%) in the intervention and 13/73 (17.8%) in the control group did not have data. "...performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses." | Low risk of bias | "Body weight ..... were measured to the nearest 0.1 kg and 0.1 cm, respectively, using a single calibrated scale" No evidence that a different method was used across intervention groups to measure the outcome. "....all outcome assessors were blinded to the diet group assignment." | Some concerns | "Study protocol and data set: Not available." No mention of pre‐specified data analysis plan. Trial registry outcomes amended after study publication; but trial registry entry at date prior to study publication mentions the outcome and timepoints. All time points mentioned in trial registry at date prior to study publication, were reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Benassi‐Evans 2009 | Some concerns | "Subjects were randomised to one of two dietary interventions…" No further information about allocation sequence or concealment. "Subject characteristics were not significantly different between groups at baseline." | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. No details about analysis reported, but looks to be ITT (number randomised per group = number analysed per group). | High risk of bias | No details about attrition reported, but some attrition is likely after 52 weeks and implied: "In the subjects who completed the 12 weeks intensive weight loss and 9 months weight maintenance..." | Low risk of bias | "Volunteers fasted overnight and were weighed (model AMZ14; Mercury Digital Scales, Tokyo, Japan) wearing light clothing and no shoes at weeks 0, 12 and 52." No evidence that a different method was used across intervention groups to measure weight. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done or whether, and how, allocation concealment was ensured. Attrition is implied, but no numbers are reported; some missingness could have and likely did depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Ebbeling 2007 | Low risk of bias | "Enrolled participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, with stratification by sex and ethnicity/race..." "To avoid any bias in assigning participants to diet groups, staff conducting recruitment and enrollment were masked to sequence. The study director assigned participants to groups." No problematic differences in baseline characteristics between the two groups | Low risk of bias | Masking: none (open‐label). No reasons or details about deviations from intended interventions reported. ITT with imputation for missing values. | High risk of bias | 8/36 (22%) participants in the intervention and 14/37 (38%) in the control group did not have data for this outcome. "For body weight after dropout, an increase of 1 kg per year was imputed starting from the last available measurement." Differential attrition and high attrition in both groups, with no reporting of reasons, but it is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | Dieticians measured at each visit using an electronic scale. No evidence that a different method was used across intervention groups to measure weight. "Data were collected by personnel who were masked to group assignment". | Some concerns | No mention of a pre‐specified analysis plan; trial registry does not mention the outcome and timepoint in any versions. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition in both trial arms, with no reporting of reasons; therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Foraker 2014 | Some concerns | "Women were randomized using stratified randomization, based on BMI (< 30 kg/m2 vs. ≥ 30 kg/m2) to ensure equal distribution of overweight and obese participants into each intervention group." No information on allocation concealment. No problematic imbalance in baseline characteristics between groups | Low risk of bias | Double‐masked (investigator and outcome assessor). Deviations reported are expected to arise in usual care e.g. personal/family medical issues, time constraints. Modified ITT (completers analysed "numbers still under follow‐up"). | High risk of bias | 31/38 (82%) participants in the intervention group and 27/41 (66%) in the control group did not have data for this outcome. Very high attrition in both groups, with limited reporting of reasons, but it is likely that with such high attrition, missingness is possibly and likely to depend on its true value. | Low risk of bias | Anthropometric tests at each visit included measurement of body weight. No evidence that a different method was used across intervention groups to measure weight. Double‐masked (investigator and outcome assessor). | Some concerns | No mention of pre‐specified data analysis plan; trial registry at date prior to study publication does not mention the outcome and timepoint (mentions only number of women who lose weight as outcome). Analysis intentions are not reported in sufficient detail to enable an assessment, but weight findings are emphasised as part of the primary aim in the paper and trial registry, with limited reporting of these findings in the paper and none reported in the abstract. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Differential and very high attrition in both groups, with limited reporting of reasons; therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Frisch 2009 | Some concerns | "Participants (n = 200) were randomly assigned by computer‐generated random number lists in two equal groups…" No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking none (open‐label). Deviations reported are expected to arise in usual care e.g. non‐compliance. ITT analysis conducted. | High risk of bias | 15/100 (15%) participants in the intervention group and 20/100 (20%) in the control group did not have data for this outcome. "Data were evaluated following the intention‐to‐treat and the per‐protocol method. Missing data were replaced with baseline data in the intention‐to‐treat analysis." "Results did not differ when the per protocol method was used (data not shown)." 13/100 in intervention group and 16/100 in control group withdrew due to non‐compliance, which may be related to change in weight (missingness possibly influenced by true value). | Low risk of bias | Calibrated electronic scale. No evidence that a different method was used across intervention groups to measure weight. Masking: None (open label) from trial registry. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions the outcome and the timepoint. The time point mentioned in trial registry at date prior to publication, is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported in both groups, with high attrition in the control group and the majority of loss to follow up in both groups due to withdrawals for non‐compliance; therefore missingness is likely to be related to the true value of the outcome. Analyses were done per protocol and using ITT with baseline values carried forward, neither of which are considered appropriate to address bias due to missing data. Lack of information in the pre‐registered methods. |
| Gardner 2007 | Low risk of bias | "Randomization was conducted in blocks of 24 (6 per treatment group) and occurred by having a blinded research technician select folded pieces of paper with group assignments from an opaque envelope." No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking: single ("Clinic and laboratorystaff members were blinded to treatment assignment.") Deviations reported are expected to arise in usual care e.g. moved away, disliked diet, pregnancy. ITT analysis conducted. | High risk of bias | 9/77 (12%) participants in the intervention group and 18/79 (23%) in the control group did not have data for this outcome. ITT with baseline values carried forward. "Also for exploratory purposes, all analyses of weight and secondary outcome measures were tested using only available data, without using baseline values carried forward for missing data or other imputation methods. There were no substantive differences in any of these findings compared with the analyses with baseline values carried forward and, therefore, only the primary analyses are presented." 5/9 participants in the intervention group and 5/18 in the control group withdrew due to health or "other" reasons; resulting in differential attrition and high attrition in the control group. With such high and differential attrition as well as vague reasons missingness could and is likely to depend on the true value of the outcome. | Low risk of bias | "Body weight was measured to the nearest 0.1 kg on a calibrated clinical scale." No evidence that a different method was used across intervention groups to measure weight. "Clinic and laboratory staff members were blinded to treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication me ntions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition reported in the control group, with the some attrition in both groups due to vague reasons such as 'health' or 'other'; therefore missingness could and is likely to be dependent on the true value of the outcome. Analyses were done per protocol and using ITT with baseline values carried forward, neither of which are considered appropriate to address bias due to missing data. Lack of information in the pre‐registered methods. |
| Griffin 2013 | Low risk of bias | "The study consisted of a random block design per every four participants. Random allocation was prepared by a researcher not involved in participant enrolment or assignment." "random number table"; "sealed opaque envelopes" (trial registry). No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | "Participants were blind to diet macronutrient composition and were informed eating plans differed only in the type of meat (i.e. red or white). "....an inability to double‐blind the intervention were limitations". Deviations reported are expected to arise in usual care e.g. did not like diet. ITT analyses conducted. | High risk of bias | 15/36 (42%) participants in the intervention group and 20/35 (57%) in the control group did not have data. ITT with last observation carried forward, and sensitivity analysis comparing completers with ITT with completers. "HP completers demonstrated significantly greater weight loss than HC at 6 (p=0.034) but not 12 months (p=0.07)". "In all participants (intention‐to‐treat), no significant between diet differences were observed (Table 2; figure S2)". 5/7 participants in the intervention group and 5/11 in the control group discontinued due to illness, too difficult and not losing weight, resulting in differential and very high attrition. With such high and differential attrition as well as vague reasons missingness could and is likely to depend on the true value of the outcome. | Low risk of bias | Weight was measured to the nearest 0.1 kg on a digital platform scale at every visit, with participants wearing light clothing and no shoes. No evidence that a different method was used across intervention groups to measure weight. Single blind (participants only). Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential and very high attrition in both groups, for reasons such as 'illness', the diets being 'too difficult' and for 'not losing weight'; therefore missingness could and is likely to be dependent on the true value of the outcome. Completers analyses and ITT with last observation carried forward conducted, neither of which are considered appropriate to address bias due to missing data. Lack of information in the pre‐registered methods. |
| Jesudason 2013 | Low risk of bias | From registry: "Random allocation using computer software "Clinstat"" and "Allocation was concealed when it was done by central randomisation by computer." No significant differences between groups at baseline for four tested variables | Low risk of bias | "Although the investigators were blinded until the end of the study, subjects and dietitians were unblinded." Deviations reported were expected to arise in usual care, e.g. unwilling/unable to follow protocol. Appeared to be an modified ITT; completers analysed; however n = 1 participant excluded from control group analysis for some outcomes with no explanation provided | High risk of bias | 95/164 (58%) participants in the intervention and 92/159 (58%) in the control group did not have data available for this outcome. "As shown in Table 7, baseline characteristics of subjects who dropped out before the end of the trial were matched between HP and HNP groups and were similar to the whole cohort, which suggested dropouts did not bias the results. The last recorded measurements of participants who did not complete the 24‐mo trial..." Very high attrition in both groups with missing data due to non‐compliance/unwilling or unable to follow trial protocol and diet being unpalatable; 68/95 (72%) in the intervention and 59/91 (65%) in the control group, which may be related to change in weight thus missingness possibly and likely influenced by true value of the outcome | Low risk of bias | Weight measured at baseline, 3, 6, 12, 24 months (trial registry). No evidence that a different method was used across intervention groups to measure weight. "Although the investigators were blinded until the end of the study, subjects and dietitians were unblinded." | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication is reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Very high attrition reported in both groups for reasons such as 'non‐compliance', 'unwilling or unable to follow protocol' and diets 'being unpalatable'; therefore missingness could and was likely to be related to the true value of the outcome. Lack of information in the pre‐registered methods |
| Klemsdal 2010 | Some concerns | No information about randomisation or allocation concealment. Footnote to Table 1: "There were no significant differences between groups." | Low risk of bias | Masking none (open‐label). Deviations reported are vague, but those reported are expected to arise in usual care e.g. non‐attendance of study visits. ITT analysis: "Analyses were done on an intention to treat basis, with the last value carried forward for non‐completers". | High risk of bias | 22/100 (22%) participants in the intervention group and 16/102 (16%) in the control group did not have data. "Analyses were done on an intention to treat basis, with the last value carried forward for non‐completers". No mention of sensitivity analyses. No reasons for loss to follow‐up or withdrawal provided, so cannot judge if related to participants’ health status. High attrition in the intervention group. With such high attrition, missingness could and is likely to depend on the true value of the outcome. | Low risk of bias | Body weight ...were measured with standard methods. No evidence that a different method was used across intervention groups to measure weight. Open label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done or whether, and how, allocation concealment was ensured. Attrition reported in both groups, with high attrition in the intervention group, and no reasons provided; therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Layman 2009 | Some concerns | No information on how the randomisation sequence was generated. No information about allocation concealment. "Randomization after stratifying participants for gender, age, BMI, and TC was successful with no significant differences between treatment groups in baseline characteristics." | Low risk of bias | "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Deviations reported are expected to arise in usual care. "Between the 4‐ and 12‐mo data collection points, 21 participants withdrew from the CHO group (16 stated personal reasons and 5 stated unable to comply with diet protocol) and 11 participants withdrew from the PRO group (8 stated personal reasons and 1 stated unable to comply with diet protocol). Modified ITT conducted. | High risk of bias | 23/64 (36%) participants in the intervetion group and 31/61 (52%) in the control group did not have data for this outcome. Modified ITT with last observation carried forward and sensitivity analysis including only completers, comparative data not shown. "Assessing long‐term weight loss using ITT or completers, weight loss did not differ between treatments at 12 mo. However, the PRO group had a greater number of participants (64%) complete the 12‐mo study than the CHO group (45%; P0.05). For completers, the mean weight loss was 23% greater for the PRO group, but individual weight loss ranged from 0.6 to 30.8 kg within the PRO group and 1.7 to 23.2 kg for the CHO group..." Very high attrition in the control group and differential attrition, and differential attrition by inability to comply with diet: 11 participants withdrew from the PRO group (8 stated personal reasons and 1 stated unable to comply with diet protocol); 21 participants withdrew from the CHO group (16 stated personal reasons and 5 stated unable to comply with diet protocol). Inability to comply may be related to change in weight (missingness possibly influenced by true value). | Low risk of bias | Body weight was measured using an electronic scale (Tanita). No evidence that a different method was used across intervention groups to measure weight. "Due to study design of the diet treatment, it was not possible to blind subjects and research staff to group assignment." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition reported in both groups, with differential attrition and very high attrition in the control groups. Differential attrition also detected for inability to comply with diet. As a result, missingness is considered likely to be dependent on the true value of the outcome. Completers analyses and ITT with last observation carried forward conducted, neither of which are considered appropriate to address bias due to missing data. No pre‐registered document available to assess analysis intentions. |
| Lim 2010 | Some concerns | No information about randomisation or allocation concealment. Baseline characteristics table showed small differences, consistent with differences resulting by chance with small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care e.g. travel, work commitment, did not like diet. Modified ITT, included all partial data contributed by non‐completers. | High risk of bias | 13/30 (43%) participants in the intevention group and 15/30 (50%) in the control group did not have data. ITT with all partial data contributed by non‐completers included in linear mixed effects model. Results of ITT not presented and no sensitivity analyses performed (author correspondence). Very high attrition in both groups, with limited reporting of reasons ("unspecified"), but it is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | Body weights recorded in light clothing (Mercury digital scales). No evidence that a different method was used across intervention groups to measure weight. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done or whether, and how, allocation concealment was ensured. Very high attrition reported in both groups, with limited reporting of reasons; therefore missingness could and is likely to be related to the true value of the outcome. ITT with partial data from non‐completers using linear mixed effects model was conducted, but results are not available. No pre‐registered document available to assess analysis intentions. |
| Sacks 2009 | Low risk of bias | "Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian, after eligibility of a participant was confirmed." Independent trial unit, fully funded by public funds and large trial; it was likely the sequence was random. " Baseline characteristics were similar among participants assigned to the four diets..." | Low risk of bias | "Blinding was maintained by the use of similar foods for each diet. Staff and participants were taught that each diet adhered to principles of a healthful diet and that each had been recommended for long‐term weight loss, thereby establishing equipoise". True blinding is known to be a challenge in these diet trials. Deviations reported are expected to arise in usual care e.g. moved away, did not appear at scheduled appointments. ITT analysis conducted: "We performed an intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight..." | High risk of bias | 33/201 (16%) participants in the intervention group and 35/204 (17%) did not have data for this outcome. Single imputation "intention‐to‐treat analysis in which long‐term weight loss for persons who withdrew from the study early (after at least 6 months of participation) was imputed on the basis of a rate of 0.3 kg per month of regained weight." No sensitivity analysis conducted (author correspondence). No reasons for loss to follow‐up or withdrawal provided, so not able to judge if missingness is possibly and likely to depend on the true value. | Low risk of bias | Calibrated hospital scale. No evidence that a different method was used across intervention groups to measure weight. "Investigators and staff who measured outcomes were unaware of the diet assignment of the participants". | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with no reasons provided; therefore it is not possible to judge how likely it is that missingness was dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | Low risk of bias | From registry: "Open (masking not used)". Most deviations reported are expected to arise in usual care, e.g. work commitments, personal reasons. Maximum likelihood mixed‐model analysis, including all participants who started in the study. | Low risk of bias | 22/55 (40%) in the intervention and 16/52 (31%) in the control group did not have data available for this outcome. Results of the maximum likelihood mixed‐model analysis, including all participants who started the study, correspondend with that of the completers analysis (no statistically significant difference between groups). Independent t tests conducted on baseline characteristics of study dropouts and completers showed that data were missing at random. | Low risk of bias | Weight was measured using calibrated electronic digital scales (AMZ14; Mercury, Tokyo, Japan). No evidence that a different method was used across intervention groups to measure weight. From registry: "Open (masking not used)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Lack of information in the pre‐registered methods. |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | Low risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care e.g. time, unable to commit to study protocol. Modified ITT conducted. | Low risk of bias | 26/59 (44%) participants in the intervention group and 29/64 (45%) in the control group did not have data. "...intention to treat evaluation was conducted using maximal likelihood mixed model analysis with fixed and random effects to analyse expected mean changes over time. The secondary analysis was based on the 120 participants that had outcomes assessed at week 0 and commenced the dietary programme." "The secondary maximal likelihood mixed model analysis showed a similar pattern of results to the primary completer’s analysis". Results of a sensitivity analysis not reported, but MD reported at 52 weeks is the same with completers analysis (Table 3 in Wycherley et al 2012), as with ITT analysis (section 3.2 in Lutze et al 2013), but variances differ. | Low risk of bias | "…body weight was measured using calibrated electronic digital scales..." No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 1.10 Change in body weight (kg) at ≥ 12 months: sensitivity analysis attrition domain.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | "...dietitians were not blinded to the study hypothesis" No information about participant blinding, however, blinding of participants for these dietary interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. "All analyses were conducted using the intention‐to‐treat principle" n=148 randomised and n=148 analysed. | Low risk of bias | 16/75 (21.3%) in the intervention and 13/73 (17.8%) in the control group did not have data. "...performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses." | Low risk of bias | "Body weight ..... were measured to the nearest 0.1 kg and 0.1 cm, respectively, using a single calibrated scale" No evidence that a different method was used across intervention groups to measure the outcome. "....all outcome assessors were blinded to the diet group assignment." | Some concerns | "Study protocol and data set: Not available." No mention of pre‐specified data analysis plan. Trial registry outcomes amended after study publication; but trial registry entry at date prior to study publication mentions the outcome and timepoints. All time points mentioned in trial registry at date prior to study publication, were reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | Low risk of bias | From registry: "Open (masking not used)". Most deviations reported are expected to arise in usual care, e.g. work commitments, personal reasons. Maximum likelihood mixed‐model analysis, including all participants who started in the study. | Low risk of bias | 22/55 (40%) in the intervention and 16/52 (31%) in the control group did not have data available for this outcome. Results of the maximum likelihood mixed‐model analysis, including all participants who started the study, correspondend with that of the completers analysis (no statistically significant difference between groups). Independent t tests conducted on baseline characteristics of study dropouts and completers showed that data were missing at random. | Low risk of bias | Weight was measured using calibrated electronic digital scales (AMZ14; Mercury, Tokyo, Japan). No evidence that a different method was used across intervention groups to measure weight. From registry: "Open (masking not used)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Lack of information in the pre‐registered methods. |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | Low risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care e.g. time, unable to commit to study protocol. Modified ITT conducted. | Low risk of bias | 26/59 (44%) participants in the intervention group and 29/64 (45%) in the control group did not have data. "...intention to treat evaluation was conducted using maximal likelihood mixed model analysis with fixed and random effects to analyse expected mean changes over time. The secondary analysis was based on the 120 participants that had outcomes assessed at week 0 and commenced the dietary programme." "The secondary maximal likelihood mixed model analysis showed a similar pattern of results to the primary completer’s analysis". Results of a sensitivity analysis not reported, but MD reported at 52 weeks is the same with completers analysis (Table 3 in Wycherley et al 2012), as with ITT analysis (section 3.2 in Lutze et al 2013), but variances differ. | Low risk of bias | "…body weight was measured using calibrated electronic digital scales..." No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 1.13 Number of participants per group with weight loss of at least 5% at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | High risk of bias | From registry: "Open (masking not used)". Most deviations reported are expected to arise in usual care, e.g. work commitments, personal reasons. Per protocol completers analysis. 13/24 (54%) withdrawals in the intervention group and 16/25 (64%) in the control group were excluded from analysis due to unclear reasons; 2/24 (8%) withdrawals in the intervention and 2/25 (8%) in the control group were due to participants being 'unable to continue diet'. | High risk of bias | 24/57 (42%) in the intervention and 25/61 (41%) in the control group did not have data available for this outcome. Completers analysis was conducted, including data only from participants who finished the study to week 52. Independent t tests conducted on baseline characteristics of study dropouts and completers showed that data were missing at random. Very high attrition in both groups, with limited reporting of reasons, but it is likely that with such high attrition missingness is possibly and likely dependent on the true value of the outcome. | Low risk of bias | Weight was measured using calibrated electronic digital scales (AMZ14; Mercury, Tokyo, Japan). No evidence that a different method was used across intervention groups to measure weight. From registry: "Open (masking not used)". Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions weight. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. A per protocol completers analysis was conducted, with some participants withdrawn due to an inability to continue with their assigned diet. Very high attrition in both groups, with limited reporting of reasons; therefore missingness could and is likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | High risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. relocated overseas, unable to commit to study protocol/time requirements. Per protocol analysis: 26/59 and 29/64 participants excluded due to being unable to commit to study protocol or did not receive intervention. Over 5% of total participants excluded with 9/26 in the intervention and 8/29 in the control group not analysed for reasons other than 'lost to contact'. | High risk of bias | 26/59 (44%) participants in the intervention and 29/64 (45%) in the control group did not have data available. Modified ITT only done for change in weight at 52 weeks. Very high attrition in both groups, with limited reporting of reasons, but it is likely that with such high attrition missingness is possibly and likely dependent on the true value of the outcome. | Low risk of bias | "...body weight was measured using calibrated electronic digital scales (Mercury; AMZ 14, Tokyo, Japan)." No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Some participants were excluded from the analysis due to an inability to commit to the study protocol. Very high attrition in both groups, with limited reporting of reasons; therefore missingness could and is likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 1.17 Change in DBP (mmHg) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | No information about partipant blinding, however, blinding of participants is highly unlikely. "...dietitians were not blinded to study hypothesis." No reasons or details about deviations from intended interventions reported. "All analyses were conducted using the intention‐to‐treat principle." n‐148 randomised, and n=148 analysed. | Low risk of bias | 16/75 (21.3%) participants in the intervention and 13/73 (17.8%) participants in the control arm did not have data available. Imputed missing values, and performed sensitivity analyses to determine whether missing at random assumptions were met. "We performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption. We used Markovchain Monte Carlo techniques to impute missing values, including additional covariates (age, sex, race, marital status, education, and employment status), in the model to make the MAR [missing at random] assumption more plausible." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses." | Low risk of bias | "We measured blood pressure 3 times with a mercury sphygmomanometer using procedures recommended by the American Heart Association. The systolic and diastolic blood pressures were recorded as the first and fifth Korotkoff sounds, respectively." No evidence that a different method was used across intervention groups to measure blood pressure. "...all outcome assessors were blinded to the diet group assignment." | Some concerns | "Study protocol and data set: Not available." No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Ebbeling 2007 | Low risk of bias | "Enrolled participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, with stratification by sex and ethnicity/race..." "To avoid any bias in assigning participants to diet groups, staff conducting recruitment and enrollment were masked to sequence. The study director assigned participants to groups." No problematic differences in baseline characteristics between the two groups | Low risk of bias | Open label trial. No reasons or details about deviations from intended interventions reported. ITT with imputations for missing values. | High risk of bias | 8/36 (22%) participants for the intervention and 14/37 (38%) for the control diet did not have outcome data for diastolic blood pressure. "For the cardiovascular disease risk factors after dropout, the last measurement obtained or the baseline value was imputed, whichever was least favorable (eg, higher for blood pressure, lower for HDL cholesterol)." No reasons for lost to follow up or withdraw provided, but high attrition and differential attrition thus missingness could and is likely to depend on its true value. | Low risk of bias | "Blood pressure was determined using an automated system (Model Pro 400, Dinamap, Tampa, Fla) with the participant sitting quietly." No evidence that a different method was used across intervention groups to measure blood pressure. "Data were collected by personnel who were masked to group assignment." | Some concerns | No mention of a pre‐specified analysis plan; trial registry does not mention the outcome and timepoint in any versions. Analysis intensions are not reported in sufficient detail to enable assesssment. | High risk of bias | Differential attrition and high attrition in both groups, with no reasons reported; therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Foraker 2014 | Some concerns | "Women were randomized using stratified randomization, based on BMI (< 30 kg/m2 vs. ≥ 30 kg/m2) to ensure equal distribution of overweight and obese participants into each intervention group." No information on allocation concealment. No problematic imbalance in baseline characteristics between groups | Low risk of bias | Double masked: investigator and outcomes assessor. Deviations reported are expected to arise in usual care, e.g. personal/family medical issues, time constraints. Modified ITT, completers analysed "numbers still under follow‐up". | High risk of bias | 27/38 (71%) participants in the intervention and 32/41 (78%) in the control group did not have data available. "In studies with incomplete follow‐up (such as LEAF), mixed models provide unbiased estimates of treatment effects under the assumption of missing at random (MAR). In the first step of the model building process, we evaluated the functional form of time for each outcome of interest (TC, LDL, HDL, SBP, and DBP). We compared linear, quadratic, and cubic trends and selected the model with the smallest Bayesian Information Criterion." Very high attrition in both groups with limited reporting of reasons, but likely that with such high attrition missingness is possibly and likely to depend on true value of outcome. | Low risk of bias | The authors report a sphygomanometer "SBP and DBP were measured via sphygmomanometer and documented to the nearest 2mm Hg." No evidence that a different method was used across intervention groups to measure weight. Double masked: investigator and outcomes assessor. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition in both groups with limited reporting of reasons for loss to follow‐up; therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Frisch 2009 | Some concerns | "Participants (n = 200) were randomly assigned by computer‐generated random number lists in two equal groups…" No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking none (open‐label). Deviations reported are expected to arise in usual care e.g. non‐compliance. ITT analyses conducted. | Low risk of bias | 15/100 (15%) participants in the intervention group and 20/100 (20%) in the control group had outcome data for blood pressure. "Data were evaluated following the intention‐to‐treat and the per‐protocol method. Missing data were replaced with baseline data in the intention‐to‐treat analysis." "Results did not differ when the per protocol method was used (data not shown)." 13/100 in intervention group and 16/100 in control group withdrew due to non‐compliance, but it is unlikely that missingness in data for blood pressure was influenced by its true value. | Low risk of bias | "Blood pressure was manually determined using a cuff and stethoscope." No evidence that a different method was used across intervention groups to measure weight. Open‐label. BP measurement is not observer‐reported and does not involve judgement. No information on reasons to believe that knowledge of assignment influenced blood pressure measurement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. No pre‐specified detailed analysis plan or detailed description in trial register. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Gardner 2007 | Low risk of bias | "Randomization was conducted in blocks of 24 (6 per treatment group) and occurred by having a blinded research technician select folded pieces of paper with group assignments from an opaque envelope." No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking: single ("Clinic and laboratory staff members were blinded to treatment assignment."). Deviations reported are expected to arise in usual care e.g. moved away, disliked diet, pregnancy. ITT analyses conducted. | High risk of bias | 9/77 (12%) participants in the intervention group and 18/79 (23%) in the control group did not have data for this outcome. ITT with baseline values carried forward. "Also for exploratory purposes, all analyses of weight and secondary outcome measures were tested using only available data, without using baseline values carried forward for missing data or other imputation methods. There were no substantive differences in any of these findings compared with the analyses with baseline values carried forward and, therefore, only the primary analyses are presented." 5/9 participants of the dropoupts in the intervention group and 5/18 in the control group withdrew due to "health" or "other" reasons (vague); Differential attrition and high attrition in the control group; thus missingness could and is likely to depend on the true value. | Low risk of bias | "Resting blood pressure was measured 3 times at 2‐minute intervals as described elsewhere[30]; the initial reading was discarded and the last 2 readings were averaged." However, because it is not reported what type of device was used, we can't judge whether or not blood pressure measurement could have differed between groups. No evidence that a different method was used across intervention groups to measure blood pressure. "Clinic and laboratory staff members were blinded to treatment assignment." | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition in the control group, with reasons for some loss to follow‐up in both groups cited as 'health' or 'other' reasons; therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Klemsdal 2010 | Some concerns | No information about randomisation or allocation concealment. Footnote to Table 1: "There were no significant differences between groups." | Low risk of bias | Masking: None (Open label). Deviations reported are vague, but those reported are expected to arise in usual care, e.g. non‐attendance of study visits. ITT analysis conducted: "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." All randomised participants were analysed. | High risk of bias | 2/100 (22%) participants in the intervention group and 16/102 (16%) in the control group did not have data. "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." No mention of sensitivity analysis." No reasons for lost to follow‐up or withdrawal provided, high attrition in the intervention group, thus missingness is possibly and likely to depend on the true value. | Low risk of bias | "Blood pressure and heart rate were measured using an automatic device. These measurements were performed after the subject had rested in the sitting position for 5 min." No evidence that a different method was used across intervention groups to measure weight. Open label. Automatic BP measurements are unlikely to be influenced by observer judgement. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was ensured. Attrition in both groups, with high attrition in the intervention group, and no reasons reported; therefore missingness is possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Lim 2010 | Some concerns | No information about randomisation or allocation concealment. Baseline characteristics table showed small differences, consistent with differences resulting by chance with small sample sizes. | Low risk of bias | No information about blinding, howevere, blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. travel, work commitment, did not like diet. Modified ITT with all partial data contributed by non‐completers included in linear mixed effects model. | High risk of bias | 13/30 (43%) participants in the intervention group and 15/30 (50%) in the control group did not have data. ITT with all partial data contributed by non‐completers included in linear mixed effects model. Results of ITT not presented and no sensitivity analyses performed (author correspondence). Very high attition with limited reporting of reasons (unspecified) in both groups. It is possible that with such high attrition missingness in data for blood pressure was influenced by its true value. | Low risk of bias | "Blood pressure was measured using an automated oscillometry (Dinamap, 845XT/XT‐IEC, Tampa, Florida), with subjects in a seated position." No evidence that a different method was used across intervention groups to measure diastolic blood pressure. No information about blinding. Automatic blood pressure measurements are unlikely to be influenced by observer judgement. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was ensured. Very high attrition in both groups with limited reporting of reasons; therefore missingness could and is likely to be dependent on the true value of the outcome. ITT analysis, with partial data from non‐completers included in a linear mixed effects model, conducted; but results are not available and sensitivity analyses were not conducted. No pre‐registered document available to assess analysis intentions. |
| Mellberg 2014 | Some concerns | "Block randomization, with a block size of four and an allocation ratio of 1:1, was done by a statistician, blinded to the study" No information about allocation concealment. "No differences were found in the baseline characteristics between the diet groups, except for higher HDL cholesterol in the PD group" | Low risk of bias | Masking: None (open label) (from trial registry). "All study personnel (except the dieticians) were blinded to the dietary allocation of the participants." Deviations reported are expected to arise in usual care, e.g. problems with transport, disappointment with allocation, family reason, lack of time. Modified ITT, excluding only participants lost to follow‐up "The primary analysis in this trial was an intention‐to‐treat analysis". | High risk of bias | 8/35 (23%) participants in the intervention 13/35 (37%) in the control arm did not have data available for this outcome. "We used generalized estimating equations (GEEs) to evaluate repeated measurements over time." High and differential attition with limited reporting of reasons in both groups. It is possible that with such high attrition missingness in data for blood pressure was influenced by its true value. | Low risk of bias | "Systolic and diastolic blood pressure was measured twice at 2‐minute intervals in the sitting position after five minutes rest, using an automatic blood pressure meter (Boso‐medicus, Bosch, Germany)." No evidence that a different method was used across intervention groups to measure diastolic blood pressure. "All study personnel (except the dieticians) were blinded to the dietary allocation of the participants." | Some concerns | No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was ensured. High and differential attrition between groups, with limited reporting of reasons; therefore missingness could and is likely to have been influenced by the true value of the outcome. Lack of information in the pre‐registered methods. |
| Sacks 2009 | Low risk of bias | "Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian, after eligibility of a participant was confirmed." Independent trial unit, fully funded by public funds and large trial; it was likely the sequence was random. " Baseline characteristics were similar among participants assigned to the four diets..." | Low risk of bias | "Blinding was maintained by the use of similar foods for each diet. Staff and participants were taught that each diet adhered to principles of a healthful diet..." True blinding is a known challenge with dietary interventions. Deviations reported are expected to arise in usual care, e.g. moved away, did not appear at scheduled appointments. Full ITT "...with zero change from baseline imputed for missing data." | High risk of bias | 33/201 (16%) participants in the intervention and 35/204 (17%) in the control group did not have data available for this outcome (Sacks et al 2009). Single imputation "...with zero change from baseline imputed for missing data." No detailed reasons for lost to follow‐up or withdrawals provided, so cannot judge if missingness is possbly or likely dependent on the true value of the outcome. | Low risk of bias | "Blood pressure was measured on two days during screening and at 6, 12, and 24 months, by automated device (Omron HeathCare, IntelliSense Professional Digital Blood Pressure Monitor, HEM907XL), by methods established in other large NIH trials. The calibration was evaluated at regular intervals using a mercury manometer." No evidence that a different method was used across intervention groups to measure weight. "Investigators and staff who measured outcomes were unaware of the diet assignment of the participants." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with no detailed reasons reported; therefore it is not possible to judge whether missingness was influenced by the true value of the outcome. Lack of information in the pre‐registered methods. |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | High risk of bias | From registry: "Open (masking not used)". Most deviations reported are expected to arise in usual care, e.g. work commitments, personal reasons. Per protocol completers analysis. 13/24 (54%) withdrawals in the intervention group and 16/25 (64%) in the control group were excluded from analysis due to unclear reasons; 2/24 (8%) withdrawals in the intervention and 2/25 (8%) in the control group were due to participants being 'unable to continue diet'. | High risk of bias | 24/57 (42%) in the intervention and 25/61 (41%) in the control group did not have data available for this outcome. Completers analysis was conducted, including data only from participants who finished the study to week 52. Independent t tests conducted on baseline characteristics of study dropouts and completers showed that data were missing at random. Very high attrition in both groups, with limited reporting of reasons, but it is likely that with such high attrition missingness is possibly and likely dependent on the true value of the outcome. | Low risk of bias | Seated blood pressure was measured with an automated sphygmomanometer (DYNAMAP 8100; Criticon, Tampa, FL). No evidence that a different method was used across intervention groups to measure blood pressure. From registry: "Open (masking not used)". Automatic BP measurements are unlikely to be influenced by observer judgement. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. A per protocol completers analysis was conducted, with some participants withdrawn due to an inability to continue with their assigned diet. Very high attrition in both groups, with limited reporting of reasons; therefore missingness could and is likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | High risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. relocat ed overseas, unable to commit to study protocol/time requirements. Per protocol analysis: 28/59 and 30/64 participants excluded from analysis. Over 5% of participants' data excluded, with 9/28 and 8/30 being participants not analyzed for reasons other than "lost to contact". | High risk of bias | 28/59 (47%) participants in the intervention and 30/64 (47%) in the control group did not have data available. Modified ITT only done on weight at 52 weeks using maximal likelihood mixed model analyses. Very high attrition in both groups, with limited reporting of reasons, but with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | Seated blood pressure was measured using an automated sphygmomanometer. No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Automatic blood pressure measurements are unlikely to be influenced by observer judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Some participants were exlcuded from analyses for reasons other than 'lost to contact'. Very high attrition in both groups, with limited reporting of reasons; therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 1.19 Change in LDL cholesterol (mmol/L) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | No information about partipant blinding, however, blinding of participants is highlly unlikely. "...dietitians were not blinded to study hypothesis." No reasons or details about deviations from intended interventions reported. All analyses were conducted using hte intention‐to‐treat principle. n=148 randomised, and n=148 analysed. | Low risk of bias | 16/75 (21.3%) participants in the intervention and 13/73 (17.8%) participants in the control arm did not have data available. Imputed missing values, and performed sensitivity analyses to determine whether missing at random assumptions were met. "We performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption. We used Markovchain Monte Carlo techniques to impute missing values, including additional covariates (age, sex, race, marital status, education, and employment status), in the model to make the MAR [missing at random] assumption more plausible." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses." | Low risk of bias | We assayed serum total cholesterol, high‐density lipoprotein (HDL) cholesterol, and triglyceride levels according to procedures recommended by the National Heart, Lung, and Blood Institute and the Centers for Disease Control and Prevention. Low‐density lipoprotein (LDL) cholesterol level was calculated using the Friedewald formula. No evidence that a different method was used across intervention groups to measure LDL. "...all outcome assessors were blinded to the diet group assignment." | Some concerns | Study protocol and data set: Not available. No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Calleja‐Fernández 2012 | Some concerns | Random allocation to each dietary group was performed by the main investigator (MDBP) by using the Web site Randomization.com (http://www.randomization.com) upon the request of the study dietitian (ACF) after the eligibility of a participant was confirmed and assignment to either of the insulin resistance or insulin sensitivity groups had been performed. No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected. | Low risk of bias | No information on blinding of participants but blinding is of these dietary interventions is highly unlikely; People delivering these dietary interventions were unblinded. No reasons or details about deviations from intended interventions reported. ITT analysis conducted. | High risk of bias | 13/21 (62%) participants in the intervention group and 11/19 (58%) in the control group did not have data for this outcome for 12 month study (Fernandez et al 2012). ITT with last observation carried forward (Fernandez et al 2012). Very high attrition in both groups, with no reporting of reasons, but it is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | The analyses were performed by the Clinical Biochemistry Department at the Complejo Asistencial Universitario de León. No evidence that a different method was used across intervention groups to measure weight. "The medical assessments were blinded to the type of diet being followed." | Some concerns | No mention of a pre‐specified analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition in both groups with no reporting of reasons; therefore, missingness could and is likely to be related to the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Ebbeling 2007 | Low risk of bias | "Enrolled participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, with stratification by sex and ethnicity/race..." "To avoid any bias in assigning participants to diet groups, staff conducting recruitment and enrollment were masked to sequence. The study director assigned participants to groups." No problematic differences in baseline characteristics between the two groups | Low risk of bias | Open label trial. No reasons or details about deviations from intended interventions reported. ITT with imputation of missing values. | High risk of bias | 8/36 (22%) participants for the intervention and 14/37 (38%) for the control diet did not have outcome data for LDL cholesterol. "For the cardiovascular disease risk factors after dropout, the last measurement obtained or the baseline value was imputed, whichever was least favorable (eg, higher for blood pressure, lower for HDL cholesterol)." High attrition in both groups and differential attrition, with no reporting of reasons, but it is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | "Plasma lipid concentrations were determined in a laboratory certified by the Lipid Standardization Program of the Centers for Disease Control and Prevention and the National Heart, Lung, and Blo od Institute." "Low‐density lipoprotein (LDL) cholesterol level was measured by a homogeneous enzymatic assay (Genzyme Corp, Cambridge, Mass)..." No evidence that a different method was used across intervention groups to measure LDL. "Data were collected by personnel who were masked to group assignment." | Some concerns | No mention of a pre‐specified analysis plan; trial registry does not mention the outcome and timepoint in any versions. Analysis intensions are not reported in sufficient detail to enable assesssment. | High risk of bias | High attrition in both groups as well as differential attrition, with no reporting of reasons; therefore, missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Foraker 2014 | Some concerns | "Women were randomized using stratified randomization, based on BMI (< 30 kg/m2 vs. ≥ 30 kg/m2) to ensure equal distribution of overweight and obese participants into each intervention group." No information on allocation concealment. No problematic imbalance in baseline characteristics between groups | Low risk of bias | Double‐masked (investigator and outcome assessor). Deviations reported are expected to arise in usual care e.g. personal/family medical issues, time constraints. Modified ITT conducted (completers analysed "numbers still under follow‐up"). | High risk of bias | 34/38 (89%) participants in the intervention group and 37/41 (90%) in the control group did not have data for this outcome. In studies with incomplete follow‐up (such as LEAF), mixed models provide unbiased estimates of treatment effects under the assumption of missing at random (MAR). In the first step of the model building process, we evaluated the functional form of time for each outcome of interest (TC, LDL, HDL, SBP, and DBP). We compared linear, quadratic, and cubic trends and selected the model with the smallest Bayesian Information Criterion." Very high attrition in both groups, with limited reporting of reasons, but it is likely that with such high attrition, missingness is possibly and likely to depend on its true value. | Low risk of bias | "Blood lipids (TC, LDL, and HDL)...were measured at each in‐person clinic visit." "Fasting TC was analyzed using Beckman Coulter LX20 (Indianapolis, IN) instrumentation and the timed‐endpoint method." No evidence that a different method was used across intervention groups to measure weight. Double‐masked (investigator and outcome assessor). | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition in both groups with limited reporting of reasons; therefore missingness could and is likely to be related to the true value of the outcome. Lack of information in the pre‐registered methods. |
| Frisch 2009 | Some concerns | "Participants (n = 200) were randomly assigned by computer‐generated random number lists in two equal groups…" No information on allocation concealment. No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking none (open‐label). Deviations reported are expected to arise in usual care e.g. non‐compliance. ITT analysis conducted. | Low risk of bias | 15/100 (15%) participants in the intervention group and 20/100 (20%) in the control group had outcome data for LDL cholesterol. "Data were evaluated following the intention‐to‐treat and the per‐protocol method. Missing data were replaced with baseline data in the intention‐to‐treat analysis." "Results did not differ when the per protocol method was used (data not shown)." 13/100 in intervention group and 16/100 in control group withdrew due to non‐compliance. It is unlikely that missingness in data for LDL cholesterol was influenced by its true value. | Low risk of bias | "...Architect autoanalyzer (Abbott, Wiesbaden, Germany)..." "Blood samples were drawn from the antecubital vein after a 12‐h overnight fast. After centrifugation at room temperature for 20 minutes (1500 × g), aliquots of serum and EDTA‐plasma samples were frozen consecutively and stored at ‐80°C until analyzed." No evidence that a different method was used across intervention groups to measure weight. Masking none (open‐label). LDL cholesterol is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. No pre‐specified detailed analysis plan or detailed description in trial register. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Gardner 2007 | Low risk of bias | "Randomization was conducted in blocks of 24 (6 per treatment group) and occurred by having a blinded research technician select folded pieces of paper with group assignments from an opaque envelope." No problematic imbalance in baseline characteristics between groups suspected | Low risk of bias | Masking: single ("Clinic and laboratory staff members were blinded to treatment assignment.") Deviations reported are expected to arise in usual care e.g. moved away, disliked diet, pregnancy. ITT analysis conducted. | High risk of bias | 9/77 (12%) participants in the intervention group and 18/79 (23%) in the control group did not have data for this outcome. ITT with baseline values carried forward. "Also for exploratory purposes, all analyses of weight and secondary outcome measures were tested using only available data, without using baseline values carried forward for missing data or other imputation methods. There were no substantive differences in any of these findings compared with the analyses with baseline values carried forward and, therefore, only the primary analyses are presented." 5/9 participants in the intervention group and 5/18 in the control group withdrew due to health or "other" reasons; resulting in differential attrition and high attrition in the control group. With such high and differential attrition as well as vague reasons missingness could and is likely to depend on the true value of the outcome. | Low risk of bias | "Low‐density lipoprotein cholesterol (LDL‐C) was calculated according to the methods described by Friedewald et al. Lipid assays were monitored by the Lipid Standardization Program of the Centers for Disease Control and Prevention and were consistently within specified limits (monthly coefficients of variation were all 3.1%)." No evidence that a different method was used across intervention groups to measure weight. "Clinic and laboratory staff members were blinded to treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition in the control group with vague reasons, therefore missingness could and is likely to be related to the true value of the outcome. Lack of information in the pre‐registered methods. |
| Griffin 2013 | Low risk of bias | "The study consisted of a random block design per every four participants. Random allocation was prepared by a researcher not involved in participant enrolment or assignment." "random number table"; "sealed opaque envelopes" (trial registry). No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | "Participants were blind to diet macronutrient composition and were informed eating plans differed only in the type of meat (i.e. red or white). "....an inability to double‐blind the intervention were limitations". Deviations reported are expected to arise in usual care e.g. did not like diet. ITT analysis conducted. | High risk of bias | 15/36 (42%) participants in the intervention group and 20/35 (57%) in the control group did not have data. ITT with last observation carried forward, and sensitivity analysis comparing completers with ITT with completers. "HP completers demonstrated significantly greater weight loss than HC at 6 (p=0.034) but not 12 months (p=0.07)" "In all participants (intention‐to‐treat), no significant between diet differences were observed (Table 2; figure S2)." 5/7 participants in the intervention group and 5/11 in the control group discontinued due to illness, too difficult and not losing weight. Very high attrition in both groups, and it is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | "Lipids, glucose, urine urea and creatinine were measured using the Roche Modular instrument with Roche kits" No evidence that a different method was used across intervention groups to measure LDL. Single‐blinded (participants). Laboratory determined outcomes are not observer‐reported involving judgement. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Very high attrition reported in both trial arms, therefore missingness could and is likely to be dependent on the true value of the outcome. Completers' analysis and ITT with last observation carried forward conducted, neither of which is considered appropriate to account for bias relating to missing data. Lack of information in the pre‐registered methods. |
| Klemsdal 2010 | Some concerns | No information about randomisation or allocation concealment. Footnote to Table 1: "There were no significant differences between groups." | Low risk of bias | Masking: None (Open label). Deviations reported are vague, but those reported are expected to arise in usual care, e.g. non‐attendance of study visits. ITT analysis: "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." All randomised participants were analysed. | High risk of bias | 2/100 (22%) participants in the intervention group and 16/102 (16%) in the control group did not have data. "All analyses were done on an intention to treat basis, with the last value carried forward for non‐completers." No mention of sensitivity analysis." No reasons for loss to follow‐up or withdrawal provided, so cannot judge if related to participants’ health status. High attrition in the intervention group. With such high attrition, missingness could and is likely to depend on the true value of the outcome. | Low risk of bias | Lipid analysis performed with standardised methods at the Department of Clinical Chemistry, Ullevaal University Hospital. No evidence that a different method was used across intervention groups to measure weight. Open label. Laboratory measurements are unlikely to be influenced by observer judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was ensured. High attrition in the intervention group with no reasons reported; therefore missingness could be and is likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Lim 2010 | Some concerns | No information about randomisation or allocation concealment. Baseline characteristics table showed small differences, consistent with differences resulting by chance with small sample sizes. | Low risk of bias | No information about blinding, howevere, blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care, e.g. travel, work commitment, did not like diet. Modified ITT with all partial data contributed by non‐completers included in linear mixed effects model. | High risk of bias | 13/30 (43%) participants in the intervention group and 15/30 (50%) in the control group did not have data. ITT with all partial data contributed by non‐completers included in linear mixed effects model. Results of ITT not presented and no sensitivity analyses performed (author correspondence). Very high attition with limited reporting of reasons (unspecified) in both groups. It is possible that with such high attrition missingness in data for LDL was influenced by its true value. | Low risk of bias | Determined by a single assay on a Cobas‐Bio centrifugal analyser using standard kits. No evidence that a different method was used across intervention groups to measure weight. No information about blinding. Laboratory measurements are unlikely to be influenced by observer judgement. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was ensured. Very high attrition reported in both groups, with limited reporting of reasons; therefore it is judged that missingness could and is likely to be related to the true value of the outcome. ITT with partial data from non‐completers included in linear mixed effects model, but the results are not available and no sensitivity analyses were conducted. No pre‐registered document available to assess analysis intentions. |
| Mellberg 2014 | Some concerns | "Block randomization, with a block size of four and an allocation ratio of 1:1, was done by a statistician, blinded to the study" No information about allocation concealment. "No differences were found in the baseline characteristics between the diet groups, except for higher HDL cholesterol in the PD group" | Low risk of bias | "All study personnel (except the dieticians) were blinded to the dietary allocation of the participants" Masking none (open label) (trial registry). Deviations reported are expected to arise in usual care e.g. problems with transport, disappointment with allocation, family reason, lack of time. Modified ITT, excluding only participants lost to follow‐up "The primary analysis in this trial was an intention‐to‐treat analysis". | High risk of bias | 8/35 (23%) participants in the intervention group and 13/35 (37%) in the control group did not have data available. We used generalized estimating equations (GEEs) to evaluate repeated measurements over time; no mention of sensitivity analysis. High attrition in both groups and differential attrition, and limited reporting of reasons; with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | Fasting blood samples were drawn from the antecubital vein after a resting period. Aliquots were frozen immediately at −20°C until analysed for plasma glucose, plasma insulin, serum cholesterol, triglycerides, and HDL cholesterol, tissue plasminogen activator (tPA) activity, plasminogen activator inhibitor type 1 (PAI‐1) activity and high sensitivity C‐reactive protein (hsCRP). Serum LDL was calculated as (serum cholesterol ‐ serum HDL ‐ serum triglycerides). No evidence that a different method was used across intervention groups to measure lab outcomes. "All study personnel (except the dieticians) were blinded to the dietary allocation of the participants" | Some concerns | No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No information on how randomisation was done and whether, or how, allocation concealment was ensured. Differential attrition and high attrition in both groups with limited reporting of reasons; therefore missingness could and is likely to be related to the true value of the outcome. Lack of information in the pre‐registered methods. |
| Sacks 2009 | Low risk of bias | "Random assignments to one of four diet groups were generated by the data manager at the coordinating center on request of a study dietitian, after eligibility of a participant was confirmed." Independent trial unit, fully funded by public funds and large trial; it was likely the sequence was random. " Baseline characteristics were similar among participants assigned to the four diets..." | Low risk of bias | "Blinding was maintained by the use of similar foods for each diet. Staff and participants were taught that each diet adhered to principles of a healthful diet and that each had been recommended for long‐term weight loss, thereby establishing equipoise". True blinding is known to be a challenge in these diet trials. Deviations reported are expected to arise in usual care e.g. moved away, did not appear at scheduled appointments. ITT analysis conducted: "Risk factors for cardiovascular disease and diabetes were also analyzed according to the intention‐to‐treat principle, with zero change from baseline imputed for missing data". | High risk of bias | 33/201 (16%) participants in the intervention group and 35/204 (17%) in the control group did not have data (Sacks et al 2009). "Risk factors for cardiovascular disease and diabetes were also analyzed according to the intention‐to‐treat principle, with zero change from baseline imputed for missing data". No reasons for loss to follow‐up or withdrawal provided, so not able to judge if missingness is possibly and likely to depend on the true value. | Low risk of bias | "Levels of serum lipids... were measured at the clinical laboratory at the Pennington Biomedical Research Center." No evidence that a different method was used across intervention groups to measure weight. "Investigators and staff who measured outcomes were unaware of the diet assignment of the participants" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups with no reasons provided, therefore it is not possible to judge whether missingness could or is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Tay 2008 | High risk of bias | From registry: "random allocation using computer software clinstat" "allocation is not concealed". "Baseline characteristics between diet groups were not different". | High risk of bias | From registry: "Open (masking not used)". Most deviations reported are expected to arise in usual care, e.g. work commitments, personal reasons. Per protocol completers analysis. 13/24 (54%) withdrawals in the intervention group and 16/25 (64%) in the control group were excluded from analysis due to unclear reasons; 2/24 (8%) withdrawals in the intervention and 2/25 (8%) in the control group were due to participants being 'unable to continue diet'. Two participants were additionally excluded from the control group due to their triglyceride concentrations exceeding 4.5 mmol/L. | High risk of bias | 24/57 (42%) in the intervention and 27/61 (44%) in the control group did not have data available for this outcome. Completers analysis was conducted, including data only from participants who finished the study to week 52. Independent t tests conducted on baseline characteristics of study dropouts and completers showed that data were missing at random. Very high attrition in both groups, with limited reporting of reasons, but it is likely that with such high attrition missingness is possibly and likely dependent on the true value of the outcome. | Low risk of bias | Serum lipids were measured using standard methods (Roche Hitachi 902 auto‐analyzer (Roche Diagnostics, Indianapolis, Indiana) using standard Roche enzymatic kits (Roche Diagnostics, Basel, Switzerland) compared to control sera. LDL‐C was calculated according to the Friedewald method. No evidence that a different method was used across intervention groups to measure lipids. From registry: "Open (masking not used)". Laboratory measurements are unlikely to be influenced by observer judgement. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication mentions outcome. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. A per protocol completers analysis was conducted, with some participants withdrawn due to an inability to continue with their assigned diet and some excluded based on triglyceride values. Very high attrition in both groups, with limited reporting of reasons; therefore missingness could and is likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Wycherley 2012 | High risk of bias | "...participants were blocked, matched for age and body mass index, then randomised by the trial coordinators using computer‐generated random number allocation..." Allocation concealment: No (trial registry). From Table 3, only triglycerides were significantly different between groups at baseline (P < 0.05), of 17 characteristics measured. | High risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care, e.g. relocated overseas, unable to commit to study protocol/time requirements. Per protocol analysis: 27/59 and 30/64 participants excluded from analysis. Well over 5% of participants' data excluded, with 8/27 in the intervention 8/30 in the control group not analysed for reasons other than 'lost to contact'. | High risk of bias | 27/59 (46%) participants and in the intervention and 30/64 (47%) in the control group did not have data available. Modified ITT only done at change in weight at 52 weeks. Very high attition with limited reporting of reasons (unspecified) in both groups. It is possible that with such high attrition missingness in data for LDL was influenced by its true value. | Low risk of bias | Serum lipids (total cholesterol, high‐density lipoprotein (HDL) cholesterol), triglycerides, plasma glucose, C‐reactive protein and creatinine were measured using commercial enzymatic kits on a Hitachi 902 autoanalyzer. No evidence that a different method was used across intervention groups to measure weight. Open (masking not used). Laboratory measurements are unlikely to be influenced by observer judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Some participants were excluded from the analysis for reasons other than 'lost to contact'. Very high attrition reported in both groups, therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 1.24 Constipation at 3 to < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Bazzano 2014 | Some concerns | "We used a computer‐generated, blocked randomization, stratified by sex, to allocate participants to 1 of the 2 diet groups." No information on allocation concealment. "Demographic characteristics and cardiovascular risk factors were similar between groups." No formal hypothesis tests were reported, but baseline values in Table 1 showed no apparent imbalance. | Low risk of bias | "...dietitians were not blinded to the study hypothesis" No information about participant blinding, however, blinding of participants for these dietary interventions is highly unlikely. No reasons or details about deviations from intended interventions reported. "All analyses were conducted using the intention‐to‐treat principle" n=148 randomised and n=148 analysed. | Low risk of bias | 16/75 (21.3%) participants in the intervention and 13/73 (17.8%) in the control group did not have data. "...performed sensitivity analyses to assess the robustness of our conclusions and departures from the MAR assumption." "Results of sensitivity analyses using multiple imputation techniques to impute missing values were consistent with those presented in our primary analyses" | High risk of bias | Reported under adverse events. "A range of adverse effects was assessed using closed‐ended questions at each counseling session." Constipation is subjective and participant‐reported, however, use of closed‐ended questions less likely to be applied differently across groups. "....all outcome assessors were blinded to the diet group assignment." Constipation is subjective and participant‐reported, and participants were likely unblinded. Participant‐reported outcome involving judgement; “mainstream”, overt and often distressing symptom; well‐known with low carb diets, thus likely to be influenced by knowledge of the intervention received. | Some concerns | "Study protocol and data set: Not available." No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Constipation is a subjective outcome and was participant‐reported, likely by unblinded participants.The outcome involves judgement; is a “mainstream”, overt and often distressing symptom; well‐known with low carb diets, thus its reporting is likely to be influenced by knowledge of the intervention received. Lack of information in the pre‐registered methods. |
| Foster 2010 | Some concerns | "Using a random‐number generator, we randomly assigned participants within each site to treatment with either a low‐carbohydrate or low‐fat, calorie‐restricted diet for 2 years." No information on allocation concealment. No problematic differences observed between the two groups for baseline characteristics | Low risk of bias | Masking none; open‐label. Deviations reported are expected to arise in usual care e.g. relocated, dissatisfied with the program, pregnancy. Modified ITT; participants with missing data excluded. | Low risk of bias | 25/153 (16%) participants in the intervention group and 19/154 (12%) in the control group did not have data. Analysis approach for constipation specifically not well reported, but assume similar approach as with other outcomes ("..longitudinal models implemented for this study relax this missing‐completely‐at‐random assumption in various ways." "Under the sensitivity analysis based on imputing missing outcomes with the highest (13.795) and lowest (‐18.355) random‐effects slopes (that is, change in weight per month) under the mixed‐effects model for weight, our qualitative findings were not sensitive to either imputation approach."). | High risk of bias | "We assessed general symptoms with a symptom checklist used in previous weight‐loss studies (17). The checklist contains 26 symptoms rated as none, mild, moderate, or severe. Symptoms were categorized as either absent (none) or present (mild, moderate, or severe because the symptom data were not normally distributed (most symptoms were listed as none or mild)." No evidence that a different method was used across intervention groups to measure weight. Use of a checklist and Likert scale less likely to be applied differently across groups, however, constipation is subjective and participant‐reported. Masking none; open‐label. Participant‐reported outcome involving judgement; “mainstream”, overt and often distressing symptom; well‐known with low carb diets, thus likely to be influenced by knowledge of the intervention received. | Some concerns | No mention of pre‐specified analysis plan; trial registry at date prior to study publication does not mention outcome or timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Constipation is a subjective outcome and was participant‐reported, by unblinded participants.The outcome involves judgement; is a “mainstream”, overt and often distressing symptom; well‐known with low carb diets, thus its reporting is likely to be influenced by knowledge of the intervention received. Lack of information in the pre‐registered methods. |
| Liu 2013 | Low risk of bias | No information about sequence generation. "Randomisation was conducted by a statistician who was not involved in any other aspects of the study by using block randomisation, stratified by 10‐year age categories." "Although the participants were randomised by age, baseline BMI levels of the two groups were similar after randomisation." | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care e.g. busy schedule or for personal reasons. Modified ITT, one drop‐out due to busy schedule excluded in control group. | Low risk of bias | 1/25 (4%) in the intervention group and 1/25 (4%) in the control group did not have data. | High risk of bias | Reported under adverse events; "...participants were asked to report any side‐effects or discomfort to the dietitian". Subjective assessment by unblinded participants; passive collection with open‐ended approach that could be applied differently across groups. | Some concerns | No mention of pre‐specified data analysis plan; and trial registry at date prior to study publication does not mention the outcome and timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Outcome is subjective and was assessed by unblinded participants through passive collection with an open‐ended approach. Lack of information in the pre‐registered methods. |
| Mateo‐Gallego 2017 | Some concerns | "We carried out the randomization process by generating a number sequence (including 93 correlative numbers because this was the number of subjects we planned to randomize) in the following webpage: https://www.random.org. The number for each participant was correlatively assigned (e.g; the first randomized women was assigned with number 1)" (from author correspondence). No information about allocation concealment. "There were no significant between‐group differences at baseline." | Low risk of bias | "Participants were blinded to their assigned macronutrient composition. The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." "...single‐blind study". Deviations reported are expected to arise in usual care e.g. lost to follow‐up, personal reasons. ITT (appears that number randomised = number analysed). | Low risk of bias | The number of participants who were randomised were also analysed. | Some concerns | Reported under adverse events; no information about procedures used for reporting of adverse events. "Participants were blinded to their assigned macronutrient composition. The dietician who formulated the diets and carried out the individual consultations was aware of each participant's group assignment, but the rest of the staff was blinded to that information." | Some concerns | No mention of pre‐specified data analysis plan, and trial registry at date prior to study publication does not mention the outcome and timepoint. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Very little information on how the outcome was assessed or by whom, but participants and all staff other than the dietician (who formulated the diets and conducted the individual consultations) were reportedly blinded. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 2.1 Change in body weight (kg) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Dyson 2007 | Low risk of bias | "Randomization was undertaken by means of sealed envelopes equivalent to the number of subjects and filled fifty‐fifty with an indicator of either a low‐carbohydrate diet or healthy‐eating advice. Two separate sets of envelopes were prepared for both diabetic and non‐diabetic subjects. An independent observer witnessed randomization." Author reported in correspondence that no formal test of hypothesis was done for difference at baseline for non‐diabetic participants. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. Deviations reported are expected to arise in usual care. n=3 of randomised participants in balanced group refused follow‐up as not allocated to low carbohydrate diet (missingness possibly influenced by true value). Modified ITT analysis conducted. | High risk of bias | In low carbohydrate diet group, 0% and in balanced diet group, 43% (n=3/7) of randomised participants did not have data available. Modified ITT with last observation carried forward conducted. High attrition in the control group, and differential attrition; refusal of follow‐up as not allocated to low carbohydrate diet may be influenced by expected change in weight, therefore missingness could be and was likely influenced by the true value. | Low risk of bias | Body weight recorded at each monthly interval up to 3 months and at 3 months intervals for the next 21 months. No evidence that a different method was used across intervention group to measure weight. No details about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition and high attrition in the control group due to withdrawals related to disappointment with not being assigned to the low carbohydrate diet; therefore missingness could and is likely related to the true value of the outcome. Lack of information in the pre‐registered methods. |
| Haufe 2013 | Low risk of bias | "For allocation of the subjects, a computer‐generated list of random numbers was used. The randomization sequence was created using SPSS 18 (Chicago, IL) statistical software and subjects were assigned to reduced carbohydrate or reduced fat diet with a 1:1 allocation using random block sizes of 2, 4, and 6. Study nurses and physicians screening and enrolling volunteers were blinded for the randomization sequence." "Both groups were well matched for gender, age, body weight, body mass index, blood lipid profiles, glucose metabolism, and cardiorespiratory fitness." | High risk of bias | "Blinding of participants for the allocated dietary intervention was impossible." "Except for the dieticians, study nurses and physicians were blinded for the treatment assignment". Deviations reported are expected to arise in usual care e.g. lost to follow up, withdrew consent. Per protocol analysis conducted. 24/28 participants in the intervention group and 30/33 in the control group excluded from the analysis for reasons including protocol violations and "other". | High risk of bias | 57/80 (71%) participants in the intervention group and 56/83 (67%) in the control group did not have data. Very high attrition in both groups, with limited reporting of reasons, but it is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | "We measured body weight, waist circumference,and height in a standardized manner after an overnight fast." No evidence that a different method was used across intervention groups to measure weight. "...study nurses and physicians were blinded for the treatment assignment". | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Some participants in both groups were excluded form analyses due to protocol violations. Very high attrition in both groups, with limited reporting of reasons; therefore missingness could and is likely to be related to the true value of the outcome. Lack of information in the pre‐registered methods. |
| Keogh 2007 | High risk of bias | Clinstat used to generate sequence; "allocation concealment: No" (trial registry). From Table 1 there appeared to be no large differences between groups at baseline. | High risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care e.g. personal reasons, unable to comply. Per protocol, 8% participants excluded from analysis as they did not comply with the diet protocol. | High risk of bias | 6/13 (46%) participants in the intervention group and 6/12 (50%) in the control group did not have data. 2/25 participants withdrew as unable to comply with diet, which may be related to change in weight (missingness possibly influenced by true value). Attrition is very high, therefore missingness could and is likely to depend on the true value of the outcome. | Low risk of bias | Weight was measured in light clothing and no shoes on a Mercury digital scale at baseline and every 2 weeks. No evidence that a different method was used across intervention groups to measure weight. Open label. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Some participants were excluded from the analysis as they did not comply with the diet protocol. Very high attrition in both groups, therefore missingness could and is likely to be dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
Risk of bias for analysis 2.2 Change in DBP (mmHg) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Keogh 2007 | High risk of bias | Clinstat used to generate sequence; "allocation concealment: No" (trial registry). From Table 1 there appeared to be no large differences between groups at baseline. | High risk of bias | Open (masking not used). Deviations reported are expexted to arise in usual care, e.g. personal reasons, unable to comply. Per‐protocol analysis: participants who did not comply with the diet protocol were excluded from the analysis. 8% (2/25) who did not comply were excluded from the analysis. | High risk of bias | Only 6/13 (46%) participants in intervention and 6/12 (50%) in control group were available for this outcome. Very high attrition in both groups, thus likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | "Resting blood pressure (mean of three measurements) was measured by automated oscillometry (845XT/XT‐IEC, Dinamape, Tampa, FL, USA) with subjects in a seated position for 5 min at baseline and every 4 weeks and also at 6 weeks after the start of weight loss when FMD was measured." No evidence that a different method was used across intervention groups to measure weight. Open (masking not done). Automated blood pressure measurements are unlikely to be influenced by judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Participants who did not comply with the diet protocol were excluded from analysis. Very high attrition in both groups; therefore missingness could and is likely to be related to the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 2.4 Change in LDL cholesterol (mmol/L) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Haufe 2013 | Low risk of bias | "For allocation of the subjects, a computer‐generated list of random numbers was used. The randomization sequence was created using SPSS 18 (Chicago, IL) statistical software and subjects were assigned to reduced carbohydrate or reduced fat diet with a 1:1 allocation using random block sizes of 2, 4, and 6. Study nurses and physicians screening and enrolling volunteers were blinded for the randomization sequence." "Both groups were well matched for gender, age, body weight, body mass index, blood lipid profiles, glucose metabolism, and cardiorespiratory fitness." | High risk of bias | "Blinding of participants for the allocated dietary intervention was impossible." "Except for the dieticians, study nurses and physicians were blinded for the treatment assignment." Deviations reported are expected to arise in usual care, e.g. lost to follow‐up, withdrew consent. Per‐protocol analysis (did not analyse those that violated the protocol). 24/28 participants in the intervention group and 30/33 in the control group were excluded from the analysis, for reasons including protocol violations and "other". | High risk of bias | 62/80 (78%) participants in the intervention and 58/83 (70%) in the control group did not have data for this outcome. Very high attrition in both groups with limited reasons given. It is likely that with such high attrition the missingness is likely related to its true value. | Low risk of bias | "...lipoproteins... were determined by standard methods in a certified clinical chemistry laboratory." No evidence that a different method was used across intervention groups to measure weight. "...study nurses and physicians were blinded for the treatment assignment." | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to permit judgement. | High risk of bias | Some participants were excluded from analysis due to protocol violations. Very high attrition in both groups with limited reasons reported; therefore missingness could and is likely to be related to the true value of the outcome. Lack of information in the pre‐registered methods. |
| Keogh 2007 | High risk of bias | Clinstat used to generate sequence; "allocation concealment: No" (trial registry). From Table 1 there appeared to be no large differences between groups at baseline. | High risk of bias | Open (masking not used). Deviations reported are expected to arise in usual care e.g. personal reasons, unable to comply. Per protocol, 8% of participants excluded as they did not comply. | High risk of bias | 6/13 (46%) participants in the intervention group and 6/12 (50%) in the control group did not have data. With very high attrition in both groups missingness could and is likely to depend on the true value of the outcome. | Low risk of bias | Lipids measured using Boehringer Mannheim/Hitachi 902 automatic analyser. No evidence that a different method was used across intervention groups to measure weight. Open label. Lipid measurement is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Allocation was not concealed. Participants were excluded from analysis if they did not comply with the diet protocol. Very high attrition in both groups; therefore missingness could and is likely to be dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 3.1 Change in body weight (kg) at 3 to < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Dyson 2007 | Low risk of bias | "Randomization was undertaken by means of sealed envelopes equivalent to the number of subjects and filled fifty‐fifty with an indicator of either a low‐carbohydrate diet or healthy‐eating advice. Two separate sets of envelopes were prepared for both diabetic and non‐diabetic subjects. An independent observer witnessed randomization." Author reported in correspondence that no formal test of hypothesis was done for difference at baseline for non‐diabetic participants. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. n=1 of randomised participants in balanced group refused follow‐up as not allocated to low carbohydrate diet. Modified ITT conducted; n=1 from control group refused follow‐up, thus data not included as missing. | High risk of bias | In low carbohydrate diet group, 0% and in balanced diet group, 14% (n=1) of randomised participants were not included in the analyses. Refusal of follow‐up due to not being allocated to low carbohydrate diet may be influenced by expected change in weight, therefore missingness possibly influenced by the true value. | Low risk of bias | Body weight recorded at each monthly interval. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. No information about blinding of outcome data. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition, with all attrition coming from the control group and disappointment cited as reason; missingness is possibly due to the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Evangelista 2021 | Low risk of bias | "Treatment assignments to high protein or standard protein diets were implemented by the study coordinator. A randomization algorithm provided by the study biostatistician was used to determine treatment assignments. Participants were equally allocated between treatment arms (by site) and permuted blocks with unequal block sizes on patient‐level factors ..." "Baseline demographic and clinical characteristics between the two groups were comparable..." | Some concerns | "Masking: None (open‐label)" reported in trial registry. No reasons for deviations from intended interventions reported. Analysis appeared to be modified ITT, but without reasons for loss to follow‐up reported, it was difficult to judge whether analysis was appropriate. | High risk of bias | 12/45 (27%) in intervention group and 2/45 (4%) in control group did not have data for this outcome. High attrition in the intervention group and differential attrition with no reasons provided. It is likely that with such high and differential attrition, missingness was possibly dependent, and likely to depend, on the true value. | Low risk of bias | "Participants were weighed in clothing without shoes (to the nearest 0.1 kg) using a professional beam scale (model 402KLS; Health‐o‐Meter, Bridgeview, IL)." No evidence that a different method was used across intervention groups to measure weight. "Masking: None (open‐label" reported in trial registry. Weight is not an observer‐reported outcome involving judgement. | High risk of bias | No mention of prespecified data analysis plan; protocol and trial registry mentioned weight at 12 weeks. Protocol (published in 2013) and trial registry (2016 last updated) mentioned weight at 12 weeks and 60 weeks/15 months, however, only 12 week data was reported. Analysis intentions were reported as multivariate imputation techniques in the protocol published in 2013, but no mention of these analyses in the published paper in 2021 | High risk of bias | Reasons for loss to follow‐up were not reported, making it difficult to judge whether analyses were appropriate. Attrition was inferred from both trial arms, with high attrition in the intervention group and no reasons provided; therefore, missingness could and was likely to be related to the true value of the outcome. A protocol and trial registry were available, reporting intended time points and analytical approaches not included in the published paper. |
| Guldbrand 2012 | Some concerns | "Randomisation was not stratified and was based on drawing blinded ballots." No information describing allocation concealment. No significant differences were present at baseline (P > 0.1 for all characteristics). | Low risk of bias | "Non‐blinded study". Deviations reported are expected to arise in usual care. Discontinued interventions (n = 4 in low‐carbohydrate diet group and n = 3 in balanced diet group did not attend group meetings). Primary intention‐to‐treat analysis, with secondary completers analysis also done | High risk of bias | 4/30 (13%) participants in the intervention group and 3/31 (10%) in the control group did not have data for this outcome. No details about methods for ITT. Participants discontinued diets in both groups as they had "severe difficulty" in following the diets, which may be related to change in weight (missingness possibly and likely influenced by true value). | Some concerns | No information about method used for body weight measurements. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified data analysis plan, and trial registry did not mention the outcome and time point in any versions thereof. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition in both groups due to "severe difficulty" following diets, therefore missingness was possibly and likely related to the true value of the outcome. Lack of information in the pre‐registered methods |
| Krebs 2012 | Low risk of bias | "Baseline measures completed prior to randomisation. Computer randomised sequence undertaken by an independent biostatistician at a distant site and random allocation of each individual emailed to personnel independent of those assessing outcome measures. These personnel organise allocation of the individuals into the two groups according to random allocation." No statements or statistical tests about differences between groups, large sample size and baseline characteristics table showed similar weight and BMI. | Low risk of bias | "Allocation of randomisation was concealed from the participant until after written informed consent and baseline assessment." "Blinded randomised controlled trial" "If two members from the same household were enrolled, they were both allocated to the same intervention group." "Dieticians delivering the intervention were notified of the dietary allocation after baseline assessment and before the first group session..." Deviations reported were expected to arise in usual care, e.g. disliked diet. A modified ITT analysis was done regardless of intervention adherence (missing outcome data excluded). | Low risk of bias | 34/207 (16%) participants in the intervention and 37/211 (18%) participants in the control arm did not have data available. "Generalized mixed linear models were used... including baseline as a co‐variate." "Both the ‘intention‐to‐treat’ analysis and the analysis that only included those who completed the study found no difference between the groups in any outcome." (data not shown). | Low risk of bias | Tanita electronic scales were used to measure weight. No evidence that a different method was used across intervention groups to measure weight. "...research assessors remained blind to intervention allocation at all assessment points and until after database lock." | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication is reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods |
| Larsen 2011 | Low risk of bias | " Randomisation was carried out by a third party using computer‐generated random numbers (using block randomisation and random block sizes) and stratified according to diabetes treatment..." "Dietary assignment was performed by a third party on the day of the initial dietary counselling visit." "The baseline characteristics for the intention‐to‐treat population were well‐matched at baseline...although non‐significant differences were apparent in calcium excretion rate, systolic blood pressure and the proportion of men." | Low risk of bias | "...we were not able to blind participants or the study personnel involved in dietary counselling." Deviations reported were expected to arise in usual care, e.g. lost interest, dissatisfied with weight loss. Modified ITT condicted: "This study was carried out using an intention‐to‐treat model following delivery of dietary advice." "...study staff encouraged all participants to return for follow‐up assessments of primary and secondary outcomes, regardless of dietary adherence." | High risk of bias | 9/57 (16%) participants in the intervention and 6/51 (12%) participants in the control arm did not have data available. This included 4 participants in intervention and 5 in control arm that changed their mind after randomisation but before starting the diets. Data imputed for ITT for 5/57 (intervention) and 1/51 (control) participants with missing data. "This study used the single imputation method of last measurement carried forward for missing data for primary and secondary outcomes." Reasons are vague and include 'changing their mind' and inconvenient'; thus missingness could and was likely to depend on the true value. | Low risk of bias | Weight was measured in light clothes. No evidence that a different method was used across intervention groups to measure weight. "...a trained assessor, who was blinded to the dietary assignment of the individual, measured and recorded weight..." | Some concerns | No mention of prespecified analysis plan; trial registry at date prior to study publication mentioned outcome, but did not mention this time point. "Study endpoints were assessed blinded to the diet group, but the statistical analysis was performed unblinded." Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported, with vague reasons such as participants "changing their mind" or finding the trial "inconvenient"; therefore, missingness could and was likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods |
| Pedersen 2014 | Low risk of bias | "Volunteers were blocked on sex, BMI and HbA1c before randomization. The randomization was performed by a trial manager not directly involved in the intervention..." "Allocation concealment has been followed in that the person responsible for the allocation schedule will be a trial manager who does not decide who is eligible for the trial and has no input into medical or dietetic decision making in the trial" (trial registry). No significant differences between groups for 16 out of 18 variables assessed at baseline | Low risk of bias | Participants and people delivering these dietary interventions were not blinded (trial registry). No reasons or details about deviations from intended interventions reported. Modified ITT analysis done: "All randomized volunteers who completed the baseline visit and had all measurements at this visit done were included in the analysis." | High risk of bias | No information about how many participants were randomised to each arm (total of 76 randomised). A total of 10/31 (32%) participants from the intervention and 9/33 (27%) from the control group did not have data available. Therefore, 76 ‐ 31 ‐ 33 = 12 participants (16%) were missing with no reasons provided. Modified ITT with last‐observation‐carried‐forward used: volunteers who dropped out of the study were not contacted at the end of the 12‐month intervention. n = 12 missing with no reasons provided | Low risk of bias | "Weight was measured at all visits; with participants wearing light clothing and no shoes." No evidence that a different method was used across intervention groups to measure weight. Trial registry implied that outcome assessors were blinded: "The analysis was conducted with the investigator blinded to the dietary allocation." | Some concerns | No mention of prespecified data analysis plan, and trial registry did not mention the outcome and time point in any versions thereof. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | High attrition with no reasons provided, modified ITT with last value carried forward used. Lack of information in pre‐registered methods |
| Samaha 2003 | Some concerns | "...randomly assigned to either the low‐carbohydrate diet or the low‐fat diet, with use of a pre‐established algorithm generated from a random set of numbers." No information describing allocation concealment. "Subjects in the two groups were well matched with regard to base‐line characteristics (Table 1)." No formal hypothesis test, but no apparent significant differences in Table 1 | Some concerns | "The study was not blinded." Total attrition was 40%. No reasons or details about deviations from intended interventions reported. ITT analysis was conducted: "The primary analysis included all 132 subjects: the 79 subjects who completed the study, the 29 subjects who dropped out but had six‐month data available from records of routine office visits, and the 24 subjects for whom the weight recorded at the last follow‐up visit was carried forward." | High risk of bias | 21/64 (33%) participants in the intervention and 32/68 (47%) participants in the control arm did not have data available. ITT with last‐observation‐carried‐forward. n = 53 missing with no reasons provided; very high attrition in both groups, and it was likely that with such high attrition, missingness was possibly and likely to depend on the true value. | Low risk of bias | "The subjects’ weights were measured monthly on a single calibrated scale..." No evidence that a different method was used across intervention groups to measure weight. "The study was not blinded." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan. Outcome data were never blinded. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Very high attrition with no reasons provided, modified ITT with last value carried forward used. Outcome assessors were not blinded to allocation. No pre‐registered document available to assess analysis intentions |
| Saslow 2017a | Low risk of bias | "Eligible participants provided written, informed consent and were randomly assigned following block randomization procedures (computerized random numbers in blocks of four) to either the MCCR or LCK diet..." "An assistant, not associated with any other aspect of the research, concealed the sequence in opaque envelopes, which were opened by other research assistants unaware of the sequence." No statements of statistical tests or differences between groups. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Deviations reported were expected to arise in usual care, e.g. moved away, family responsibilities. Modified ITT conducted: "This primary analysis was conducted on an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." | High risk of bias | No participants (0/16) in the intervention and 3/18 (17%) participants in the control arm did not have data available. Modified ITT analysis was done, "including all participants who were randomly assigned and had data at both baseline and 3 months." Differential attrition (> 10%), reasons vague and citing work schedules and family responsibilities; missingness could depend on its true value for weight, but difficult to say if this was likely. | Low risk of bias | "Subjects were weighed with no jackets or shoes using the same calibrated scale at each time." No evidence that a different method was used across intervention groups to measure weight. "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified data analysis plan; protocol and trial registry did not mention the outcome and time point in any versions thereof. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition with all attrition in the control arm, and vague reasons; therefore difficult to determine whether missingness was dependent on the true value of the outcome. Lack of information in the pre‐registered methods |
| Sato 2017 | Low risk of bias | "Randomization was achieved by the minimization and biased coin method." "Allocation sequence conducted by third party organization (Soiken, Inc., Osaka, Japan) was concealed until interventions were assigned." No problematic differences in baseline characteristics of participants | Low risk of bias | "...they [patients] could not be blinded due to characteristics of dietary therapy." "Open ‐ no one is blinded" (trial registry). Deviations reported were expected to arise in usual care, e.g. lacked motivation, disappointed with assigned diet, moved to a different hospital. All participants randomised received their interventions and all were analysed except for those that had missing outcome data, thus a modified ITT was done. | Low risk of bias | 3/33 (9%) participants in the intervention and 1/33 (3%) participants in the control arm did not have data available. Modified ITT done. Reasons for loss to follow‐up and withdrawal were stated for all particpants and included 3 transferring to other hospitals and 1 admission for different diseases. | Low risk of bias | Body weight was measured by the staff. No evidence that a different method was used across intervention groups to measure weight. Open ‐ no one was blinded (trial registry). Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a prespecified analysis plan. Could not obtain trial registry record. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | No pre‐registered document available to assess analysis intentions |
| Tay 2014 | Some concerns | Randomization procedures (sequence generation and allocation concealment) were performed by research associates independent of outcome assessments and intervention delivery and was done "...using random varying block sizes before random computer‐generated assignment to either an LC or HC diet in a 1:1 ratio." Baseline characteristics were "...not significantly different between diet groups (p>0.05) in independent‐samples t‐test...or Chi2 test" | Low risk of bias | "...diet assignment was discernible by participants and interventionists..." Deviations reported are expected to arise in usual care, e.g. work commitments, time constraints, unable to comply with diet. Modified ITT conducted, all participants except those who dropped out or were lost to follow‐up, were analysed in group to which they were assigned. | High risk of bias | 18/64 (28%) participants in the intervention and 20/67 (30%) participants in the control arm did not have data available. High attrition in both groups, with reasons including unable to comply to diet and health issue external to study. It is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | "Body mass was measured using calibrated electronic scales..." No evidence that a different method was used across intervention groups to measure weight. "...blinding was maintained for outcome assessment and data analysis." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. High attrition reported, with missingness possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Watson 2016 | Low risk of bias | Participants were randomly allocated by an investigator, who had no participant contact, using the process of minimisation." "An independent holder of the randomisation schedule will perform treatment allocation without contact with the volunteers." (trial registry). "All p values [for baseline characteristics] are > 0.05 for between group differences based on independent t‐tests." | Low risk of bias | Open (masking not used) (trial registry). Deviations reported were expected to arise in usual care e.g. work commitments, unable to continue with diet. Modified ITT conducted ‐ including participants who commenced and completed the study irrespective of compliance. Two participants were not included as they withdrew after randomisation but before the start of the study. | High risk of bias | 9/32 (28%) in the intervention and 10/31 (32%) participants in the control arm did not have data available. "Sensitivity analysis was conducted for the BMI, fat mass (kg), and DBP variables using two methods: all participants who commenced the study and completers only. Both analyses showed the same pattern of outcomes with a time effect (P < 0.001) but no differences between the groups (P >= 0.51) or group by time interactions (P >= 0.07)." High attrition in both groups, with reasons including personal reasons, unable to comply with the intervention. It was likely that with such high attrition, missingness was possibly and likely to depend on the true value. | Low risk of bias | "Body mass was measured twice using calibrated electronic scales (Tanita Ultimate Scale 2000; Tokyo)". No evidence that a different method was used across intervention groups to measure. "The investigator was trained in ISAK International Standards for Anthropometric Assessment". Open (masking not used). Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of prespecified analysis plan, but trial registry at date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication is reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment and protocol published in 2015: "Study Status: Data collection has been completed." | High risk of bias | High attrition reported, with missingness possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods |
| Westman 2008 | Some concerns | "...participants were randomized to one of two treatment groups stratified upon BMI greater or less than 32 kg/m2 using a computer‐generated list, and invited to attend the baseline visit." No information describing allocation concealment. "There were no clinically significant differences between the treatment groups." | High risk of bias | Participants received instructions, books and handouts about the diet they were to follow; "The delivery of the intervention and the assessment of outcomes were not blinded to the treatment assignment." Low‐carbohydrate diet group: 21/48 (44%) randomised completed; reasons for discontinuation were: 3 refused assigned diet, 2 were unsatisfied with the diet, 2 were lost to follow‐up, 2 were too busy, 1 relocated, and 7 cited no reason; balanced diet group: 29/49 (59%) randomised completed; reasons for discontinuation were: 1 refused assigned diet, 1 was unsatisfied with the diet, 2 were lost to follow‐up, 3 were too busy, 1 relocated, 1 had difficulty adhering to the diet and 9 cited no reason. Per protocol analysis. Very high proportion of participants excluded | High risk of bias | Outcome data were not available for 27/48 (56%) in the intervention group and for 20/49 (41%) in balanced diet group. Per protocol, no sensitivity analyses. Very high attrition in both groups, with reasons including dissatisfied with diet, as well as vague reasons. It is likely that with such high attrition, missingness was possibly and likely to depend on the true value. | Low risk of bias | "Wearing light clothing and no shoes, participants were weighed at each visit on the same calibrated scale." No evidence that a different method was used across intervention groups to measure weight. "The delivery of the intervention and the assessment of outcomes were not blinded to the treatment assignment." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a prespecified analysis plan. Outcome data never blinded. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition with some due to participants being unsatisfied with, or having trouble following, their allocated diet; it was therefore likely that at least some missingness was dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions |
| Wycherley 2010 | Some concerns | Central randomisation by computer; "Random allocation using computer software 'Clinstat'". No information about allocation concealment. For completers per group: "At baseline, there were no significant differences in age, weight, BMI, A1C, and sex distribution between groups (P=>0.12)."; "These characteristics were also similar for participants who completed or did not complete the study (P>=0.09), except for age in which completers were on average five years younger." | Low risk of bias | "Open (masking not used)". In the low carb diet group, 3/21 were excluded due to non‐compliance with the diet program and 2/21 withdrew as they were unable to continue with the diet. In the balanced diet group, 1/19 were excluded due to non‐compliance with the diet program and 1/19 withdrew as they were unable to continue with the diet. Modified ITT used. "Primary analysis was conducted on participants who completed the study per protocol. Secondary intention‐ to‐treat (ITT) analysis was also conducted for the primary outcome measures (body weight and composition, cardiometabolic and glycemic control), including participants who completed the study irrespective to protocol adherence." | High risk of bias | 6/21 (29%) participants in the intervention group and 2/19 (11%) in the control group did not contribute data to the analyses as they withdrew from the study. Results of the sensitivity analysis narratively reported for the 4 arms of the trial, but no data shown or evidence provided for the 2 arms of interest. Effect sizes for per protocol: MD ‐0.40 (‐3.93; 3.13) and for mITT: MD 1.40 (‐2.53; 5.33). Reasons are vague and include 'unable to continue diet', with differential attrition and high attrition in the intervention group; thus missingness could and is likely to depend on the true value. | Low risk of bias | "Body mass was measured using calibrated electronic digital scales." No evidence that a different method was used across intervention groups. "Open (masking not used)." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Differential attrition and high attrition in the intervention arm with vague reasons reported, therefore missingness could be and is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Yamada 2014 | Some concerns | "Enrolled patients were randomly allocated ....... using a permuted randomised block of four patients per block". No information describing allocation concealment. "There were no statistically significant differences in any of the parameters between the two groups." | Low risk of bias | "The patients and investigators were not masked to group assignment." No attrition reported in the trial. No participants with missing outcome data | Low risk of bias | No participants with missing outcome data | Some concerns | No information about method used for body weight measurements. No details about procedures used for body weight measurement to assess if good practice anthropometric guidelines were used in both groups. No mention of training. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a prespecified analysis plan. Outcome data never blinded. Analysis intentions were not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information on how outcome was assessed. No pre‐registered document available to assess analysis intentions |
Risk of bias for analysis 3.4 Change in body weight (kg) at 3 to < 12 months: sensitivity analysis source of funding.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.4.1 Without food/diet industry funding | ||||||||||||
| Evangelista 2021 | Low risk of bias | "Treatment assignments to high protein or standard protein diets were implemented by the study coordinator. A randomization algorithm provided by the study biostatistician was used to determine treatment assignments. Participants were equally allocated between treatment arms (by site) and permuted blocks with unequal block sizes on patient‐level factors ..." "Baseline demographic and clinical characteristics between the two groups were comparable..." | Some concerns | "Masking: None (open label)" reported in trial registry. No reasons for deviations from intended interventions reported. Analysis appears to be modified ITT, but without reasons for loss to follow‐up reported, it is difficult to judge whether analysis was appropriate. | High risk of bias | 12/45 (27%) in intervention group and 2/45 (4%) in control group did not have data for this outcome. High attrition in the intervention group and differential attrition with no reasons provided. It is likely that with such high and differential attrition, missingness is possibly dependent, and likely to depend, on the true value. | Low risk of bias | "Participants were weighed in clothing without shoes (to the nearest 0.1 kg) using a professional beam scale (model 402KLS; Health‐o‐Meter, Bridgeview, IL)." No evidence that a different method was used across intervention groups to measure weight. "Masking: None (open label" reported in trial registry. Weight is not an observer‐reported outcome involving judgement. | High risk of bias | No mention of pre‐specified data analysis plan; protocol and trial registry mentions weight at 12 weeks. Protocol (published in 2013) and trial registry (2016 last updated) mention weight at 12 weeks and 60 weeks/15 months, however, only 12 week data is reported. Analysis intentions are reported as multivariate imputation techniques in the protocol published in 2013, but no mention of these analyses in the published paper in 2021. | High risk of bias | Reasons for loss to follow‐up were not reported, making it difficult to judge whether analyses were appropriate. Attrition is inferred from both trial arms, with high attrition in the intervention group and no reasons provided; therefore missingness could and is likely to be related to the true value of the outcome. A protocol and trial registry are available, reporting intended timepoints and analytical approaches not included in the published paper. |
| Guldbrand 2012 | Some concerns | "Randomisation was not stratified and was based on drawing blinded ballots." No information describing allocation concealment. No significant differences were present at baseline (P > 0.1 for all characteristics). | Low risk of bias | "Non‐blinded study". Deviations reported are expected to arise in usual care. Discontinued interventions (n=4 in low carbohydrate diet group and n=3 in balanced diet group did not attend group meetings). Primary intention‐to‐treat analysis, with secondary completers analysis also done. | High risk of bias | 4/30 (13%) participants in the intervention group and 3/31 (10%) in the control group did not have data for this outcome. No details about methods for ITT. Participants discontinued diets in both groups as they had "severe difficulty" in following the diets, which may be related to change in weight (missingness possibly and likely influenced by true value). | Some concerns | No information about method used for body weight measurements.Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition in both groups due to "severe difficulty" following diets, therefore missingness is possibly and likely related to the true value of the outcome. Lack of information in the pre‐registered methods. |
| Krebs 2012 | Low risk of bias | "Baseline measures completed prior to randomisation. Computer randomised sequence undertaken by an independent biostatistician at a distant site and random allocation of each individual emailed to personnel independent of those assessing outcome measures. These personnel organise allocation of the individuals into the two groups according to random allocation." No statements or statistical tests about differences between groups, large sample size and baseline characteristics table showed similar weight and BMI. | Low risk of bias | "Allocation of randomisation was concealed from the participant until after written informed consent and baseline assessment." "Blinded randomised controlled trial" "If two members from the same household were enrolled, they were both allocated to the same intervention group." "Dieticians delivering the intervention were notified of the dietary allocation after baseline assessment and before the first group session..." Deviations reported are expected to arise in usual care, e.g. disliked diet. A modified ITT analysis was done regardless of intervention adherence (missing outcome data excluded). | Low risk of bias | 34/207 (16%) participants in the intervention and 37/211 (18%) participants in the control arm did not have data available. "Generalized mixed linear models were used... including baseline as a co‐variate." "Both the ‘intention‐to‐treat’ analysis and the analysis that only included those who completed the study found no difference between the groups in any outcome." (data not shown). | Low risk of bias | Tanita electronic scales were used to measure weight. No evidence that a different method was used across intervention groups to measure weight. "...research assessors remained blind to intervention allocation at all assessment points and until after database lock." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Samaha 2003 | Some concerns | "...randomly assigned to either the low‐carbohydrate diet or the low‐fat diet, with use of a pre‐established algorithm generated from a random set of numbers." No information describing allocation concealment. "Subjects in the two groups were well matched with regard to base‐line characteristics (Table 1)." No formal hypothesis test, but no apparent significant differences in Table 1 | Some concerns | "The study was not blinded." Total attrition is 40%. No reasons or details about deviations from intended interventions reported. ITT analysis was conducted: "The primary analysis included all 132 subjects: the 79 subjects who completed the study, the 29 subjects who dropped out but had six‐month data available from records of routine office visits, and the 24 subjects for whom the weight recorded at the last follow‐up visit was carried forward." | High risk of bias | 21/64 (33%) participants in the intervention and 32/68 (47%) participants in the control arm did not have data available. ITT with last observation carried forward. n=53 missing with no reasons provided; very high attrition in both groups, and it is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Some concerns | "The subjects’ weights were measured monthly on a single calibrated scale..." No evidence that a different method was used across intervention groups to measure weight. "The study was not blinded." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan. Outcome data were never blinded. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Very high attrition with no reasons provided, modified ITT with last value carried forward used. Outcome assessors were not blinded to allocation. No pre‐registered document available to assess analysis intentions. |
| Saslow 2017a | Low risk of bias | "Eligible participants provided written, informed consent and were randomly assigned following block randomization procedures (computerized random numbers in blocks of four) to either the MCCR or LCK diet..." "An assistant, not associated with any other aspect of the research, concealed the sequence in opaque envelopes, which were opened by other research assistants unaware of the sequence." No statements of statistical tests or differences between groups. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Deviations reported are expected to arise in usual care, e.g. moved away, family responsibilities. Modified ITT conducted: "This primary analysis was conducted on an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." | High risk of bias | No participants (0/16) in the intervention and 3/18 (17%) participants in the control arm did not have data available. Modified ITT analysis was done, "including all participants who were randomly assigned and had data at both baseline and 3 months." Differential attrition (>10%), reasons vague and citing work schedules and family responsibilities; missingness could depend on its true value for weight, but difficult to say if this is likely. | Low risk of bias | "Subjects were weighed with no jackets or shoes using the same calibrated scale at each time." No evidence that a different method was used across intervention groups to measure weight. "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Weight is not an observer‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified data analysis plan; Protocol and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential attrition with all attrition in the control arm, and vague reasons; therefore difficult to determine whether missingness is dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Tay 2014 | Some concerns | Randomization procedures (sequence generation and allocation concealment) were performed by research associates independent of outcome assessments and intervention delivery and was done "...using random varying block sizes before random computer‐generated assignment to either an LC or HC diet in a 1:1 ratio." Baseline characteristics were "...not significantly different between diet groups (p>0.05) in independent‐samples t‐test...or Chi2 test" | Low risk of bias | "...diet assignment was discernible by participants and interventionists..." Deviations reported are expected to arise in usual care, e.g. work commitments, time constraints, unable to comply with diet. Modified ITT conducted, all participants except those who dropped out or were lost to follow‐up, were analysed in group to which they were assigned. | High risk of bias | 18/64 (28%) participants in the intervention and 20/67 (30%) participants in the control arm did not have data available. High attrition in both groups, with reasons including unable to comply to diet and health issue external to study. It is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | "Body mass was measured using calibrated electronic scales..." No evidence that a different method was used across intervention groups to measure weight. "...blinding was maintained for outcome assessment and data analysis." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. High attrition reported, with missingness possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 3.5 Change in body weight (kg) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Elhayany 2010 | Some concerns | No information about randomisation or allocation concealment. No significant differences in baseline values between groups for all characteristics | High risk of bias | Masking single (outcome assessor) (trial registry). Deviations reported were expected to arise in usual care, e.g. noncompliance, moved away. Appeared as if 13/85 in the intervention and 11/89 in the control (14% in total) were not included due to "noncompliance" | High risk of bias | 24/85 (28%) in intervention group and 26/89 (29%) did not have data for this outcome. "Additional analysis was performed including the 80 individuals without 12‐month data using the latest available of 3‐, 6‐ or 9‐month follow‐up data. The results were comparable to the analysis of those who completed the study." Attrition was high and reasons for some of the missing participants were vague and differed between groups; missingness could and was likely to depend on its true value. | Low risk of bias | No evidence that a different method was used across intervention groups to measure weight. Masking (single) outcome assessors | Some concerns | No mention of prespecified analysis plan, but the trial registry at the date prior to study publication mentioned the outcome and time point. The time point mentioned in the trial registry at the date prior to publication is reported on in the publication. Analysis intentions were not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on how randomisation was done, and how (or whether) allocation concealment was done. High attrition and vague reasons, therefore, missingness could be and was likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods |
| Guldbrand 2012 | Some concerns | "Randomisation was not stratified and was based on drawing blinded ballots." No information describing allocation concealment. No significant differences were present at baseline (P > 0.1 for all characteristics). | Low risk of bias | "Non‐blinded study". n=4 in low carbohydrate diet group and n=3 in balanced diet group discontinued interventions (did not attend group meetings). Primary intention‐to‐treat analysis, with secondary completers analysis also done. | High risk of bias | 4/30 (13%) participants in the intervention group and 3/31 (10%) in the control group did not have data for this outcome. Discontinued diets in both groups as had "severe difficulty" in following the diets, which may be related to change in weight (missingness possibly and likely influenced by true value). | Some concerns | No information about method used for body weight measurements. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition in both groups due to "severe difficulty" following diets, possible and likely that the missingness is dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Krebs 2012 | Low risk of bias | "Baseline measures completed prior to randomisation. Computer randomised sequence undertaken by an independent biostatistician at a distant site and random allocation of each individual emailed to personnel independent of those assessing outcome measures. These personnel organise allocation of the individuals into the two groups according to random allocation." No statements or statistical tests about differences between groups, large sample size and baseline characteristics table showed similar weight and BMI. | Low risk of bias | "Allocation of randomisation was concealed from the participant until after written informed consent and baseline assessment." "If two members of the same household were enrolled they were both allocated to the same intervention group." "Dieticians delivering the intervention were notified of the dietary allocation after baseline assessment and before the first group session" Modified intention‐to‐treat analyses were undertaken, regardless of intervention adherence (missing outcome data excluded). Deviations reported are expected to arise in usual care e.g. disliked diet, missed visit. | Low risk of bias | 63/207 (30%) participants in the intervention group and 61/211 (29%) in the control group did not have data available for this outcome. "Generalized mixed linear models were used... including baseline as a co‐variate." "Both the ‘intention‐to‐treat’ analysis and the analysis that only included those who completed the study found no difference between the groups in any outcome." (data not shown). | Low risk of bias | Tanita electronic scale was used to measure weight. No evidence that a different method was used across intervention groups to measure weight. "...research assessors remained blind to intervention allocation at all assessment points and until after database lock" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Pedersen 2014 | Low risk of bias | "Volunteers were blocked on sex, BMI and HbA1c before randomization. The randomization was performed by a trial manager not directly involved in the intervention..." "Allocation concealment has been followed in that the person responsible for the allocation schedule will be a trial manager who does not decide who is eligible for the trial and has no input into medical or dietetic decision making in the trial" (trial registry). No significant differences between groups for 16 out of 18 variables assessed at baseline | Low risk of bias | Participants and people delivering these dietary interventions were not blinded (trial registry). No reasons or details about deviations from intended interventions reported. Modified ITT analysis done. "All randomized volunteers who completed the baseline visit and had all measurements at this visit done were included in the analysis." | High risk of bias | No information about how many participants were randomized to each arm (n=76 total randomized); 10/31 (32%) in intervention and 9/33 (27%) in control group did not have data available, thus n=12 missing (16%) with no reasons provided. Modified ITT with last observation carried forward used; volunteers who dropped out of the study were not contacted at the end of the 12 month intervention. | Low risk of bias | "Weight was measured...wearing light clothing and no shoes." No evidence that a different method was used across intervention groups to measure weight. Trial registry implies that outcome assessors were blinded. "The analysis was conducted with the investigator blinded to the dietary allocation" | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment.” | High risk of bias | High attrition with no reasons provided, and ITT with last value carried forward conducted. Lack of information in the pre‐registered methods. |
| Samaha 2003 | Some concerns | "...randomly assigned to either the low‐carbohydrate diet or the low‐fat diet, with use of a pre‐established algorithm generated from a random set of numbers." No information describing allocation concealment. "Subjects in the two groups were well matched with regard to base‐line characteristics (Table 1)." No formal hypothesis test, but no apparent significant differences in Table 1 | Some concerns | "The study was not blinded." Total attrition is 34%. No reasons or details about deviations from intended interventions reported. A modified intention to treat analysis was conducted, including weight data for most participants, though some of this information was obtained from medical records. | Low risk of bias | Total attrition was 34%, but weights from missing participants were obtained from medical records. Consequently, weight data were only missing for 6/132 (4.5%) of the total group. | Low risk of bias | Weight data for participants remaining in the study were obtained using a single calibrated scale. Weight data for missing participants were obtained from medical records, with authors reporting that these were obtained using different scales and were likely 'obtained in a nonuniform manner with regard to clothing'. No evidence that a different method was used across intervention groups to measure weight. "The study was not blinded." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan. Outcome data were never blinded. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. The study was not blinded, and it is not clear whether deviations from intended interventions may have been influenced by this. Though weight data were available for nearly all participants randomised, some of these were obtained from medical records and may have been measured in a non‐uniform manner. No pre‐registered document available to assess analysis intentions. |
| Saslow 2017a | Low risk of bias | "Eligible participants provided written, informed consent and were randomly assigned following block randomization procedures (computerized random numbers in blocks of four) to either the MCCR or LCK diet..." "An assistant, not associated with any other aspect of the research, concealed the sequence in opaque envelopes, which were opened by other research assistants unaware of the sequence." No statements of statistical tests or differences between groups. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Deviations reported are expected to arise in usual care e.g. moved away, work responsibilities. Modified ITT was conducted: "This primary analysis was conducted on an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." | High risk of bias | 1/16 (6%) in the intervention group and 4/18 (22%) in the control group did not have data. Modified ITT conducted: "This primary analysis was conducted on an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." Flowchart for longer timepoint also indicates a modified ITT. Differential attrition and very high attrition in the control group, reasons vague and citing work schedules and/or family responsibilities; thus missingness could depend on its true value for weight but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "Subjects were weighed with no jackets or shoes using the same calibrated scale at each time." No evidence that a different method was used across intervention groups to measure weight. "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry and protocol does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential and high attrition in the control group with vague reasons, missingness could be and is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Tay 2014 | Some concerns | Randomization procedures (sequence generation and allocation concealment) were performed by research associates independent of outcome assessments and intervention delivery and was done "...using random varying block sizes before random computer‐generated assignment to either an LC or HC diet in a 1:1 ratio." Baseline characteristics were "...not significantly different between diet groups (p>0.05) in independent‐samples t‐test...or Chi2 test" | Low risk of bias | "...diet assignment was discernible by participants and interventionists..." Deviations reported are expected to arise in usual care e.g. moved away, work responsibilities. Modified ITT, all participants except those who withdrew or were LTFU, were analysed in group to which they were assigned. | High risk of bias | 31/64 (48%) participants in the intervention and 39/67 (58%) participants in the control arm did not have data available. Very high attrition in both groups; reasons vague and include work commitments, time constraints and change in life situation; thus missingness could depend on its true value for weight but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "Body mass was measured using calibrated electronic scales..." No evidence that a different method was used across intervention groups to measure weight. "The researchers involved in outcome assessment and data analysis were blinded to treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition reported, with missingness possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 3.6 Change in body weight (kg) at ≥ 12 months: sensitivity analysis source of funding.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.6.1 Without diet/food industry funding | ||||||||||||
| Guldbrand 2012 | Some concerns | "Randomisation was not stratified and was based on drawing blinded ballots." No information describing allocation concealment. No significant differences were present at baseline (P > 0.1 for all characteristics). | Low risk of bias | "Non‐blinded study". n=4 in low carbohydrate diet group and n=3 in balanced diet group discontinued interventions (did not attend group meetings). Primary intention‐to‐treat analysis, with secondary completers analysis also done. | High risk of bias | 4/30 (13%) participants in the intervention group and 3/31 (10%) in the control group did not have data for this outcome. Discontinued diets in both groups as had "severe difficulty" in following the diets, which may be related to change in weight (missingness possibly and likely influenced by true value). | Some concerns | No information about method used for body weight measurements. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Attrition in both groups due to "severe difficulty" following diets, possible and likely that the missingness is dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Krebs 2012 | Low risk of bias | "Baseline measures completed prior to randomisation. Computer randomised sequence undertaken by an independent biostatistician at a distant site and random allocation of each individual emailed to personnel independent of those assessing outcome measures. These personnel organise allocation of the individuals into the two groups according to random allocation." No statements or statistical tests about differences between groups, large sample size and baseline characteristics table showed similar weight and BMI. | Low risk of bias | "Allocation of randomisation was concealed from the participant until after written informed consent and baseline assessment." "If two members of the same household were enrolled they were both allocated to the same intervention group." "Dieticians delivering the intervention were notified of the dietary allocation after baseline assessment and before the first group session" Modified intention‐to‐treat analyses were undertaken, regardless of intervention adherence (missing outcome data excluded). Deviations reported are expected to arise in usual care e.g. disliked diet, missed visit. | Low risk of bias | 63/207 (30%) participants in the intervention group and 61/211 (29%) in the control group did not have data available for this outcome. "Generalized mixed linear models were used... including baseline as a co‐variate." "Both the ‘intention‐to‐treat’ analysis and the analysis that only included those who completed the study found no difference between the groups in any outcome." (data not shown). | Low risk of bias | Tanita electronic scale was used to measure weight. No evidence that a different method was used across intervention groups to measure weight. "...research assessors remained blind to intervention allocation at all assessment points and until after database lock" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Samaha 2003 | Some concerns | "...randomly assigned to either the low‐carbohydrate diet or the low‐fat diet, with use of a pre‐established algorithm generated from a random set of numbers." No information describing allocation concealment. "Subjects in the two groups were well matched with regard to base‐line characteristics (Table 1)." No formal hypothesis test, but no apparent significant differences in Table 1 | Some concerns | "The study was not blinded." Total attrition is 34%. No reasons or details about deviations from intended interventions reported. A modified intention to treat analysis was conducted, including weight data for most participants, though some of this information was obtained from medical records. | Low risk of bias | Total attrition was 34%, but weights from missing participants were obtained from medical records. Consequently, weight data were only missing for 6/132 (4.5%) of the total group. | Low risk of bias | Weight data for participants remaining in the study were obtained using a single calibrated scale. Weight data for missing participants were obtained from medical records, with authors reporting that these were obtained using different scales and were likely 'obtained in a nonuniform manner with regard to clothing'. No evidence that a different method was used across intervention groups to measure weight. "The study was not blinded." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan. Outcome data were never blinded. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. The study was not blinded, and it is not clear whether deviations from intended interventions may have been influenced by this. Though weight data were available for nearly all participants randomised, some of these were obtained from medical records and may have been measured in a non‐uniform manner. No pre‐registered document available to assess analysis intentions. |
| Saslow 2017a | Low risk of bias | "Eligible participants provided written, informed consent and were randomly assigned following block randomization procedures (computerized random numbers in blocks of four) to either the MCCR or LCK diet..." "An assistant, not associated with any other aspect of the research, concealed the sequence in opaque envelopes, which were opened by other research assistants unaware of the sequence." No statements of statistical tests or differences between groups. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Deviations reported are expected to arise in usual care e.g. moved away, work responsibilities. Modified ITT was conducted: "This primary analysis was conducted on an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." | High risk of bias | 1/16 (6%) in the intervention group and 4/18 (22%) in the control group did not have data. Modified ITT conducted: "This primary analysis was conducted on an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." Flowchart for longer timepoint also indicates a modified ITT. Differential attrition and very high attrition in the control group, reasons vague and citing work schedules and/or family responsibilities; thus missingness could depend on its true value for weight but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "Subjects were weighed with no jackets or shoes using the same calibrated scale at each time." No evidence that a different method was used across intervention groups to measure weight. "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry and protocol does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential and high attrition in the control group with vague reasons, missingness could be and is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Tay 2014 | Some concerns | Randomization procedures (sequence generation and allocation concealment) were performed by research associates independent of outcome assessments and intervention delivery and was done "...using random varying block sizes before random computer‐generated assignment to either an LC or HC diet in a 1:1 ratio." Baseline characteristics were "...not significantly different between diet groups (p>0.05) in independent‐samples t‐test...or Chi2 test" | Low risk of bias | "...diet assignment was discernible by participants and interventionists..." Deviations reported are expected to arise in usual care e.g. moved away, work responsibilities. Modified ITT, all participants except those who withdrew or were LTFU, were analysed in group to which they were assigned. | High risk of bias | 31/64 (48%) participants in the intervention and 39/67 (58%) participants in the control arm did not have data available. Very high attrition in both groups; reasons vague and include work commitments, time constraints and change in life situation; thus missingness could depend on its true value for weight but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "Body mass was measured using calibrated electronic scales..." No evidence that a different method was used across intervention groups to measure weight. "The researchers involved in outcome assessment and data analysis were blinded to treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition reported, with missingness possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 3.8 Weight loss of at least 5% at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Pedersen 2014 | Low risk of bias | "Volunteers were blocked on sex, BMI and HbA1c before randomization. The randomization was performed by a trial manager not directly involved in the intervention..." "Allocation concealment has been followed in that the person responsible for the allocation schedule will be a trial manager who does not decide who is eligible for the trial and has no input into medical or dietetic decision making in the trial" (trial registry). No significant differences between groups for 16 out of 18 variables assessed at baseline | Low risk of bias | Participants and people delivering these dietary interventions were not blinded (trial registry). No reasons or details about deviations from intended interventions reported. Modified ITT analysis was done: "Alll randomized volunteers who completed the baseline visit and had all measurements at this visit done were includd in the analysis." | High risk of bias | No information how many participants were randomised to each arm (total of 76 randomised). A total of 10/31 (32%) participants from the intervention and 9/33 (27%) from the control arm did not have data available. Therefore, 76‐31‐33=12 participants appear to be missing (16%) with no reasons provided. Modified ITT with last observation carried forward used. Volunteers who dropped out of the study were not contacted at the end of the 12 month intervention. | Low risk of bias | "Weight was measured...wearing light clothing and no shoes." No evidence that a different method was used across intervention groups to measure weight. Trial registry implies that outcome assessors were blinded: "...the analysis was conducted with the investigator blinded to the dietary allocation". | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis interventions are not reported in sufficient detail to enable an assessment. | High risk of bias | High attrition with no reasons provided, and ITT with last value carried forward conducted. Lack of information in the pre‐registered methods. |
| Tay 2014 | Some concerns | Randomization procedures (sequence generation and allocation concealment) were performed by research associates independent of outcome assessments and intervention delivery and was done "...using random varying block sizes before random computer‐generated assignment to either an LC or HC diet in a 1:1 ratio." Baseline characteristics were "...not significantly different between diet groups (p>0.05) in independent‐samples t‐test...or Chi2 test" | Low risk of bias | "...diet assignment was discernible by participants and interventionists..." Deviations reported are expected to arise in usual care e.g. moved away, work responsibilities. Modified ITT, all participants except those who withdrew or were LTFU, were analysed in group to which they were assigned. | High risk of bias | 31/64 (48%) participants in the intervention and 39/67 (58%) participants in the control arm did not have data available. Very high attrition in both groups; reasons vague and include work commitments, time constraints and change in life situation; thus missingness could depend on its true value for weight but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "Body mass was measured using calibrated electronic scales..." No evidence that a different method was used across intervention groups to measure weight. "The researchers involved in outcome assessment and data analysis were blinded to treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition reported, with missingness possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 3.11 Change in DBP (mmHg) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Guldbrand 2012 | Some concerns | "Randomisation was not stratified and was based on drawing blinded ballots." No information describing allocation concealment. No significant differences were present at baseline (P > 0.1 for all characteristics). | Low risk of bias | "Non‐blinded study". Discontinued interventions (n=4 in low carbohydrate diet group and n=3 in balanced diet group did not attend group meetings).Primary intention‐to‐treat analysis, with secondary completers analysis also done. | Low risk of bias | 4/30 (13%) in the intervention group and 3/31 (10%) in the control group did not have data for this outcome. No details about ITT. It is improbable that discontinuation (missingness) and "severe difficulty" in following the diets is related to change in BP values. | Some concerns | No information about method used for blood pressure measurements. BP is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment; time frame specified only as 2 years. | Some concerns | No specific information about allocation concealment, or the method used to measure blood pressure. Lack of information in pre‐registered methods. |
| Krebs 2012 | Low risk of bias | "Baseline measures completed prior to randomisation. Computer randomised sequence undertaken by an independent biostatistician at a distant site and random allocation of each individual emailed to personnel independent of those assessing outcome measures. These personnel organise allocation of the individuals into the two groups according to random allocation." No statements or statistical tests about differences between groups, large sample size and baseline characteristics table showed similar weight and BMI. | Low risk of bias | "Allocation of randomisation was concealed from the participant until after written informed consent and baseline assessment." "If two members from the same household were enrolled, they were both allocated to the same intervention group." "Dieticians delivering the intervention were notified of the dietary allocation after baseline assessment and before the first group session..." Deviations reported are expected to arise in usual care, e.g. dislike diet, missed visit. A modified ITT analysis was done regardless of intervention adherence (missing outcome data excluded). | Low risk of bias | 63/207 (30%) participants in the intervention and 62/212 (29%) participants in the control arm did not have data available. "Generalized mixed linear models were used... including baseline as a co‐variate." "Both the ‘intention‐to‐treat’ analysis and the analysis that only included those who completed the study found no difference between the groups in any outcome." (data not shown). | Some concerns | No details provided on blood pressure measurements, but authors state that "research assessors remained blind to intervention allocation at all assessment points and until after database lock." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No details on how blood pressure measurements were taken. Lack of information in pre‐registered methods. |
| Pedersen 2014 | Low risk of bias | "Volunteers were blocked on sex, BMI and HbA1c before randomization. The randomization was performed by a trial manager not directly involved in the intervention..." "Allocation concealment has been followed in that the person responsible for the allocation schedule will be a trial manager who does not decide who is eligible for the trial and has no input into medical or dietetic decision making in the trial" (trial registry). No significant differences between groups for 16 out of 18 variables assessed at baseline | Low risk of bias | Participants and people delivering these dietary interventions were not blinded (trial registry). No reasons or details about deviations from intended interventions reported. Modified ITT analysis done: "All randomized volunteers who completed the baseline visit and had all measurements at this visit done were included in the analysis." | High risk of bias | No information about how many participants were randomised to each arm (total of 76 randomised). A total of 10/31 (32%) participants from the intervention and 9/33 (27%) from the control group did not have data available. Therefore, 76 ‐ 31 ‐ 33 = 12 participants (16%) are missing with no reasons provided. Modified ITT with last observation carried forward used: volunteers who dropped out of the study were not contacted at the end of the 12 month intervention. | Low risk of bias | Blood pressure was measured at baseline and at 4 month intervals. No additional information provided, but no evidence that a different method was used across intervention groups to measure BP. Trial registry implies that outcome assessors were blinded "The analysis was conducted with the investigator blinded to the dietary allocation." | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | High attrition with no reasons provided, modified ITT with last value carried forward used. Lack of information in pre‐registered methods. |
| Samaha 2003 | Some concerns | "...randomly assigned to either the low‐carbohydrate diet or the low‐fat diet, with use of a pre‐established algorithm generated from a random set of numbers." No information describing allocation concealment. "Subjects in the two groups were well matched with regard to base‐line characteristics (Table 1)." No formal hypothesis test, but no apparent significant differences in Table 1 | Some concerns | "The study was not blinded." Total attrition is 34%. No reasons or details about deviations from intended interventions reported. A modified ITT was done, including all 87 participants who returned for follow‐up at 1 year. | High risk of bias | 20/64 (31%) participants in the intervention and 25/68 (37%) participants in the control arm did not have data available. Completers and non‐completers of the study did not differ significantly for blood pressure, though participants who dropped out were younger than those who remained. No sensitivity analyses were reported for the blood pressure results. High attrition in both groups with no reasons for attrition provided; thus missingness could depend on the true value of the outcome, but difficult to say if this is likely based on a lack of reasons for attrition. | Low risk of bias | No mention of the methods or device used in measuring blood pressure. No evidence that a different method was used across intervention groups to measure DBP. "The study was not blinded." Blood pressure is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified analysis plan. Outcome data were never blinded. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. The study was not blinded, and it is not clear whether deviations from intended interventions may have been influenced by this. High attrition with no reasons provided, and no sensitivity analyses to assess the robustness of the findings reported, therefore the missingness could depend on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Saslow 2017a | Low risk of bias | "Eligible participants provided written, informed consent and were randomly assigned following block randomization procedures (computerized random numbers in blocks of four) to either the MCCR or LCK diet..." "An assistant, not associated with any other aspect of the research, concealed the sequence in opaque envelopes, which were opened by other research assistants unaware of the sequence." No statements of statistical tests or differences between groups. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Deviations reproted are expected to arise in usual care, e.g. moved away, family responsibilities. Modified ITT: "This primary analysis was conducted in an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." | High risk of bias | 1 out of 16 (6%) participants in the intervention group and 4 out of 18 (22%) in the control group did not have data available. Modified ITT: "This primary analysis was conducted on an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." In the flowchart for outcomes at 12 months, it is also indicated that authors performed a modified ITT. Differential attrition and very high attrition in the control group, reasons vague and citing work schedules and/or family responsibilities; thus missingness could depend on its true value, but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "Blood pressure and heart rate will be measured using an automated blood pressure and heart rate monitor." No evidence that a different method was used across intervention groups to measure blood pressure. "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Blood pressure is not an observer‐reported outcome involving judgement. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry and protocol does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | High attrition with vague reasons, missingness could depend on the true value of the outcome. Lack of information in pre‐registered methods. |
| Tay 2014 | Some concerns | Randomization procedures (sequence generation and allocation concealment) were performed by research associates independent of outcome assessments and intervention delivery and was done "...using random varying block sizes before random computer‐generated assignment to either an LC or HC diet in a 1:1 ratio." Baseline characteristics were "...not significantly different between diet groups (p>0.05) in independent‐samples t‐test...or Chi2 test" | Low risk of bias | "...diet assignment was discernible by participants and interventionists…" Deviations reported are expected to arise in usual care e.g. moved away, work responsibilities. Modified ITT, all participants except those who withdrew or were lost to follow‐up, were analysed in group to which they were assigned. | High risk of bias | 31/64 (48%) participants in the intervention and 39/67 (58%) participants in the control arm did not have data available. Very high attrition in both groups; reasons vague and include work commitments, time constraints and change in life situation; thus missingness could depend on its true value for weight but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "Resting blood pressure was measured by automated sphygmomanometry (SureSigns VS3; Phillips, Andover, Massachusetts)..." No evidence that a different method was used across intervention groups to measure DBP. "The researchers involved in outcome assessment and data analysis were blinded to treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition reported, with missingness possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 3.13 Change in HbA1c (%) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Elhayany 2010 | Some concerns | No information about randomisation or allocation concealment. No significant differences in baseline values between groups for all characteristics | Low risk of bias | Masking single (outcome assessor) (trial registry). Deviations reported are expected to arise in usual care, e.g. noncompliance, moved away. Appears as if 13/85 in the intervention and 11/89 in the control (14% in total) were not included due to "noncompliance". | High risk of bias | 24/85 (28%) participants in intervention group and 26/89 (29%) in control group did not have data for this outcome. "Additional analysis was performed including the 80 individuals without 12‐month data using the latest available of 3‐,6‐ or 9‐month follow‐up data. The results were comparable to the analysis of those who completed the study." Attrition is high and reasons for some of the missing participants are vague and differ between groups; missingness could and is likely to depend on its true value. | Low risk of bias | "All tests were performed in the Clalit Health Services Central District laboratory." No evidence that a different method was used across intervention groups to measure. Masking single (outcome assessor) (trial registry). | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on how randomisation was done, and how (or whether) allocation concealment was done. High attrition, therefore missingness could be and is likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Guldbrand 2012 | Some concerns | "Randomisation was not stratified and was based on drawing blinded ballots." No information describing allocation concealment. No significant differences were present at baseline (P > 0.1 for all characteristics). | Low risk of bias | "Non‐blinded study". n=4 participants in low carbohydrate diet group and n=3 in balanced diet group discontinued interventions (did not attend group meetings). Primary intention‐to‐treat analysis, with secondary completers analysis also done. | Low risk of bias | 4/30 (13%) in the intervention group and 3/31 (10%) in the control group did not have data for this outcome. No detail about the ITT, not possible to judge whether the result was biased due to missing data. It is improbable that discontinuation (missingness) and "severe difficulty" in following the diets is related to change in lab values. | Low risk of bias | "laboratory tests were analysed at the Department of Clinical Chemistry ....as part of clinical routine analyses" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. Lack of information in the pre‐registered methods. |
| Krebs 2012 | Low risk of bias | "Baseline measures completed prior to randomisation. Computer randomised sequence undertaken by an independent biostatistician at a distant site and random allocation of each individual emailed to personnel independent of those assessing outcome measures. These personnel organise allocation of the individuals into the two groups according to random allocation." No statements or statistical tests about differences between groups, large sample size and baseline characteristics table showed similar weight and BMI. | Low risk of bias | "Allocation of randomisation was concealed from the participant until after written informed consent and baseline assessment." "If two members from the same household were enrolled, they were both allocated to the same intervention group." "Dieticians delivering the intervention were notified of the dietary allocation after baseline assessment and before the first group session..." Deviations reported are expected to arise in usual care, e.g. dislike diet, missed visit. A modified ITT analysis was done regardless of intervention adherence (missing outcome data excluded). | Low risk of bias | 63/207 (30%) participants in the intervention and 62/212 (29%) participants in the control arm did not have data available. Generalized mixed linear models were used... including baseline as a co‐variate. "Both the ‘intention‐to‐treat’ analysis and the analysis that only included those who completed the study found no difference between the groups in any outcome." (data not shown) | Low risk of bias | Centralised accredited laboratory performed analyses. No evidence that a different method was used across intervention groups to measure HbA1C. "research assessors remained blind to intervention allocation at all assessment points and until after database lock." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Pedersen 2014 | Low risk of bias | "Volunteers were blocked on sex, BMI and HbA1c before randomization. The randomization was performed by a trial manager not directly involved in the intervention..." "Allocation concealment has been followed in that the person responsible for the allocation schedule will be a trial manager who does not decide who is eligible for the trial and has no input into medical or dietetic decision making in the trial" (trial registry). No significant differences between groups for 16 out of 18 variables assessed at baseline | Low risk of bias | Participants and people delivering these dietary interventions were not blinded (trial registry). No reasons or details about deviations from intended interventions reported. Modified ITT analysis was done: "Alll randomized volunteers who completed the baseline visit and had all measurements at this visit done were includd in the analysis." | High risk of bias | No information how many participants were randomised to each arm (total of 76 randomised). A total of 10/31 (32%) participants from the intervention and 9/33 (27%) from the control arm did not have data available. Therefore, 76‐31‐33=12 participants appear to be missing (16%) with no reasons provided. Modified ITT with last observation carried forward used. Volunteers who dropped out of the study were not contacted at the end of the 12 month intervention. | Low risk of bias | "All samples were analysed at a commercial laboratory in Adelaide (IMVS)." No evidence that a different method was used across intervention groups to measure HbA1c. Trial registry implies that outcome assessors were blinded: "...the analysis was conducted with the investigator blinded to the dietary allocation" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis interventions are not reported in sufficient detail to enable an assessment. | High risk of bias | High attrition with no reasons provided, and ITT with last value carried forward conducted. Lack of information in the pre‐registered methods. |
| Saslow 2017a | Low risk of bias | "Eligible participants provided written, informed consent and were randomly assigned following block randomization procedures (computerized random numbers in blocks of four) to either the MCCR or LCK diet..." "An assistant, not associated with any other aspect of the research, concealed the sequence in opaque envelopes, which were opened by other research assistants unaware of the sequence." No statements of statistical tests or differences between groups. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Deviations reported are expected to arise in usual care, e.g. moved away, family responsibilities. Modified ITT analysis: "This primary analysis was conducted in an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." | High risk of bias | 1 out of 16 (6%) participants in the intervention group and 4 out of 18 (22%) in the control group did not have data available. Modified ITT conducted: "This primary analysis was conducted on an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." In the flowchart for outcomes at 12 months, it is also indicated that authors performed a modified ITT. Differential attrition and high attrition in the control group, reasons vague and citing work schedules and/or family responsibilities; thus missingness could depend on its true value but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "HbA1c ... were assessed from a fasting blood specimen at a commercial CLIA‐certified laboratory (Quest Diagnostics, Madison, NJ)." No evidence that a different method was used across intervention groups to measure weight. "Specimens will be identified by participant ID only." | Some concerns | No mention of pre‐specified data analysis plan, and trial registry and mentions the outcome but not the timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Differential and high attrition in the control group with vague reasons, missingness could be and is likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Tay 2014 | Some concerns | Randomization procedures (sequence generation and allocation concealment) were performed by research associates independent of outcome assessments and intervention delivery and was done "...using random varying block sizes before random computer‐generated assignment to either an LC or HC diet in a 1:1 ratio." Baseline characteristics were "...not significantly different between diet groups (p>0.05) in independent‐samples t‐test...or Chi2 test" | Low risk of bias | "...diet assignment was discernible by participants and interventionists..." Deviations reported are expected to arise in usual care e.g. moved away, work responsibilities. Modified ITT, all participants except those who withdrew or were lost to follow‐up, were analysed in group to which they were assigned. | High risk of bias | 23/64 (34%) participants in the intervention and 30/67 (45%) participants in the control arm did not have data available. Very high attrition in the control group and high attrition in the intervention group; reasons vague and include work commitments, time constraints and change in life situation; thus missingness could depend on its true value for weight but difficult to say if this is likely based on the reasons provided. | Low risk of bias | Laboratory measurement of HbA1c. No evidence that a different method was used across intervention groups to measure the outcome. "...blinding was maintained for researchers involved in the outcome assessment and data analysis until study completion." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. Time point of 52 weeks for this outcome not mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition reported in the control group and high attrition in the intervention group, with missingness possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 3.14 Change in LDL cholesterol (mmol/L) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Elhayany 2010 | Some concerns | No information about randomisation or allocation concealment. No significant differences in baseline values between groups for all characteristics | High risk of bias | Masking: single (outcomes assessor) from trial registry. Deviations reported are expected to arise in usual care, e.g. noncompliance, moved away. Appears as though 13/85 (15%) participants in the intervention group and 11/89 (12%) participants in the control group were not included due to 'noncompliance'. | High risk of bias | 24/85 (28%) participants in intervention group and 26/89 (29%) in control group did not have data for this outcome. "Additional analysis was performed including the 80 individuals without 12‐month data using the latest available of 3‐,6‐ or 9‐month follow‐up data. The results were comparable to the analysis of those who completed the study." Attrition is high and reasons for some of the missing participants are vague and differ between groups. With such high attrition, missingness could and is likely to depend on the true value. | Low risk of bias | "All tests were performed in the Clalit Health Services Central District laboratory." No evidence that a different method was used across intervention groups. Masking: single (outcomes assessor) from trial registry. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on how randomisation was done, and how (or whether) allocation concealment was done. High attrition, therefore missingness could be and is likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Guldbrand 2012 | Some concerns | "Randomisation was not stratified and was based on drawing blinded ballots." No information describing allocation concealment. No significant differences were present at baseline (P > 0.1 for all characteristics). | Low risk of bias | "Non‐blinded study". n=4 in low carbohydrate diet group and n=3 in balanced diet group discontinued interventions (did not attend group meetings). Primary intention‐to‐treat analysis, with secondary completers analysis also done. | Low risk of bias | 4/30 (13%) participants in the intervention group and 3/31 (10%) in the control group did not have data for this outcome. No detail about ITT, therefore not possible to say if the result was biased by missing data. It is improbable that discontinuation (missingness) and "severe difficulty" in following the diets is related to change in lab values. | Low risk of bias | "laboratory tests were analysed at the Department of Clinical Chemistry ....as part of clinical routine analyses". No evidence that a different method was used across intervention groups. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on how, or whether, allocation concealment was done. Lack of information in the pre‐registered methods. |
| Krebs 2012 | Low risk of bias | "Baseline measures completed prior to randomisation. Computer randomised sequence undertaken by an independent biostatistician at a distant site and random allocation of each individual emailed to personnel independent of those assessing outcome measures. These personnel organise allocation of the individuals into the two groups according to random allocation." No statements or statistical tests about differences between groups, large sample size and baseline characteristics table showed similar weight and BMI. | Low risk of bias | "Allocation of randomisation was concealed from the participant until after written informed consent and baseline assessment." "Blinded randomised controlled trial" "If two members from the same household were enrolled, they were both allocated to the same intervention group." "Dieticians delivering the intervention were notified of the dietary allocation after baseline assessment and before the first group session..." Deviations are expected to arise in usual care, e.g. disliked diet. A modified ITT analysis was done regardless of intervention adherence (missing outcome data excluded). | Low risk of bias | 63/207 (30%) in the intervention group and 61/211 (29%) in the control group did not have data available for this outcome. "Generalized mixed linear models were used... including baseline as a co‐variate." "Both the ‘intention‐to‐treat’ analysis and the analysis that only included those who completed the study found no difference between the groups in any outcome." (data not shown). | Low risk of bias | Centralized accredited laboratory. No evidence that a different method was used across intervention groups to measure LDL‐cholesterol. ..."research assessors remained blind to intervention allocation at all assessment points and until after database lock". | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Pedersen 2014 | Low risk of bias | "Volunteers were blocked on sex, BMI and HbA1c before randomization. The randomization was performed by a trial manager not directly involved in the intervention..." "Allocation concealment has been followed in that the person responsible for the allocation schedule will be a trial manager who does not decide who is eligible for the trial and has no input into medical or dietetic decision making in the trial" (trial registry). No significant differences between groups for 16 out of 18 variables assessed at baseline | Low risk of bias | Participants and people delivering these dietary interventions were not blinded (trial registry). No reasons or details about deviations from intended interventions reported. Modified ITT analysis done. "All randomized volunteers who completed the baseline visit and had all measurements at this visit done were included in the analysis." | High risk of bias | No information about how many participants were randomized to each arm (n=76 total randomized); 10/31 (32%) in intervention and 9/33 (27%) in control group did not have data available, thus n=12 missing (16%) with no reasons provided. Modified ITT with last observation carried forward used; volunteers who dropped out of the study were not contacted at the end of the 12 month intervention. | Low risk of bias | "All samples were analysed at a commercial laboratory in Adelaide (IMVS)". No evidence that a different method was used across intervention groups to measure LDL. Trial registry implies that outcome assessors were blinded: "the analysis was conducted with the investigator blinded to the dietary allocation" | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint". The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment.” | High risk of bias | High attrition with no reasons provided, modified ITT with last value carried forward used. Lack of information in pre‐registered methods. |
| Samaha 2003 | Some concerns | "...randomly assigned to either the low‐carbohydrate diet or the low‐fat diet, with use of a pre‐established algorithm generated from a random set of numbers." No information describing allocation concealment. "Subjects in the two groups were well matched with regard to base‐line characteristics (Table 1)." No formal hypothesis test, but no apparent significant differences in Table 1 | Some concerns | "The study was not blinded." Total attrition is 34%. No reasons or details about deviations from intended interventions reported. A modified ITT was done, including all 87 participants who returned for follow‐up at 1 year. | Low risk of bias | 20/64 (31%) participants in the intervention and 25/68 (37%) participants in the control arm did not have data available. Baseline lipid values did not differ between completers and non‐completers of the study, though participants who dropped out were younger. Sensitivity analyses indicated that these findings remain significant under a missing at random assumption as well as with baseline value carried forward. | Low risk of bias | "Fasting blood specimens were obtained." LDL calculated from Friedewald equation. No evidence that a different method was used across intervention groups to measure LDL. "The study was not blinded." Laboratory determined outcomes are not observer‐reported involving judgement. | Some concerns | No mention of pre‐specified analysis plan. Outcome data were never blinded. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No specific information on allocation concealment. The study was not blinded, and it is not clear whether deviations from intended interventions may have been influenced by this. No pre‐registered document available to assess analysis intentions. |
| Saslow 2017a | Low risk of bias | "Eligible participants provided written, informed consent and were randomly assigned following block randomization procedures (computerized random numbers in blocks of four) to either the MCCR or LCK diet..." "An assistant, not associated with any other aspect of the research, concealed the sequence in opaque envelopes, which were opened by other research assistants unaware of the sequence." No statements of statistical tests or differences between groups. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | "For this trial, it was not possible for the participants and staff to be blinded to group allocation." Deviations reproted are expected to arise in usual care, e.g. moved away, family responsibilities. Modified ITT: "This primary analysis was conducted in an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." | High risk of bias | 1/16 (6%) in the intervention group and 4/18 (22%) in the control group did not have data. Modified ITT. "This primary analysis was conducted on an intention‐to‐treat basis, including all participants who were randomly assigned and had data at both baseline and 3 months." Flowchart for longer timepoint also indicates a modified ITT. Differential attrition and very high attrition in the control group, reasons vague and citing work schedules and/or family responsibilities; thus missingness could depend on its true value, but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "...assessed from a fasting blood specimen at a commercial CLIA‐certified laboratory (Quest Diagnostics, Madison, NJ)". No evidence that a different analysis method was used across intervention groups. "Specimens will be identified by participant ID only" | Some concerns | No mention of pre‐specified data analysis plan, and trial registry and protocol does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | High attrition with vague reasons, missingness could depend on the true value of the outcome. Lack of information in pre‐registered methods. |
| Tay 2014 | Some concerns | Randomization procedures (sequence generation and allocation concealment) were performed by research associates independent of outcome assessments and intervention delivery and was done "...using random varying block sizes before random computer‐generated assignment to either an LC or HC diet in a 1:1 ratio." Baseline characteristics were "...not significantly different between diet groups (p>0.05) in independent‐samples t‐test...or Chi2 test" | Low risk of bias | "...diet assignment was discernible by participants and interventionists..." Deviations reported are expected to arise in usual care e.g. moved away, work responsibilities. Modified ITT, all participants except those who withdrew or were lost to follow‐up, were analysed in group to which they were assigned. | High risk of bias | 31/64 (48%) participants in the intervention and 39/67 (58%) participants in the control arm did not have data available. Very high attrition in both groups; reasons vague and include work commitments, time constraints and change in life situation; thus missingness could depend on its true value for weight but difficult to say if this is likely based on the reasons provided. | Low risk of bias | "Fasting blood samples were collected from forearm vein... LDL calculated from Friedewald equation." No evidence that a different method was used across intervention groups to measure LDL. "The researchers involved in outcome assessment and data analysis were blinded to treatment assignment." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment. Very high attrition reported, with missingness possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 3.19 Constipation at 3 to < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Tay 2014 | Some concerns | Randomization procedures (sequence generation and allocation concealment) were performed by research associates independent of outcome assessments and intervention delivery and was done "...using random varying block sizes before random computer‐generated assignment to either an LC or HC diet in a 1:1 ratio." Baseline characteristics were "...not significantly different between diet groups (p>0.05) in independent‐samples t‐test...or Chi2 test" | Low risk of bias | "....diet assignment was discernible by participants and interventionists". Deviations reported are expected to arise in usual care e.g. work commitments, unable to comply with diet, time constraints. A modified ITT analysis was conducted, all participants except those who dropped out or were lost to follow up were analysed in group to which they were assigned. | High risk of bias | 18/64 (28%) participants in intervention group and 20/67 (30%) in the control group did not have data available.High attrition in both groups with reasons including inabilty to comply with diet, work commitments, health issues external to study and personal reasons. It is likely that with such high attrition, missingness is possible and likely to depend on the true value. | High risk of bias | Outcome reported under adverse events, no information about procedures used for ascertaining/reporting of adverse events. Constipation is subjective and participant‐reported, and participants were unblinded. The outcome, being participant‐reported, involves judgement, but there is no detailed information about how this adverse event was assessed. | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment provided. High attrition, with missingness possibly and likely related to true value. Subjective, participant‐reported outcome involving judgment, with no information on how the outcome was ascertained. Lack of information on pre‐registered methods. |
| Westman 2008 | Some concerns | "...participants were randomized to one of two treatment groups stratified upon BMI greater or less than 32 kg/m2 using a computer‐generated list, and invited to attend the baseline visit." No information describing allocation concealment. "There were no clinically significant differences between the treatment groups." | Low risk of bias | Participants received instructions, books and handouts about the diet they were to follow; "The delivery of the intervention and the assessment of outcomes were not blinded to the treatment assignment." Unclear whether an appropriate analysis was done; 10/48 (21%) in the intervention group and 3/49 (6%) in the control group discontinued the study, with no reasons for dropout or details about deviations from intended interventions provided (only that participants discontinued the diets). | High risk of bias | Outcome data not available for non‐completers in low carbohydrate diet group: 10/48 (21%) and in balanced diet group: 3/49 (6%). Differential attrition between groups, and high attrition in the intervention group; thus missingness could and is likely to depend on the true value. | High risk of bias | "participants completed an open‐ended side effects questionnaire. To enhance the description of side effects, participants completed a checklist of side effects commonly mentioned during weight loss studies at both the 20 and 24‐week visit. These two measures were combined to report the proportion in each group who experienced an adverse effect at any time during the study". Subjective assessment by unblinded participants; passive collection, however, use of same checklist and questionnaire in both groups. Constipation is subjective and participant‐reported, and participants were likely unblinded. The outcome, being participant‐reported, involves judgement This could be influenced by beliefs about the diet, but it is difficult to judge how likely this is. | Some concerns | No mention of a pre‐specified analysis plan. Outcome data never blinded. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | No specific information on allocation concealment provided. Differential and high attrition in the intervention arm, with no reasons. Subjective, participant‐reported outcome involving judgment, with no information on how the outcome was ascertained. Lack of information on pre‐registered methods. |
Risk of bias for analysis 4.1 Change in body weight (kg) at 3 to < 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Watson 2016 | Low risk of bias | Participants were randomly allocated by an investigator, who had no participant contact, using the process of minimisation." "An independent holder of the randomisation schedule will perform treatment allocation without contact with the volunteers." (trial registry). "All p values [for baseline characteristics] are > 0.05 for between group differences based on independent t‐tests." | Low risk of bias | Trial registry: Open (masking not used). Deviations reported are expected to arise in usual care e.g. work commitments, unable to continue with diet. Modified intention to treat analysis; including participants who commenced and completed the study irrespective of compliance, only two participants were not included as they withdrew after randomisation but before the start of the study. | High risk of bias | 9/32 (28%) participants in the intervention and 10/31 (32%) participants in the control arm did not have data available. "Sensitivity analysis was conducted for the BMI, fat mass (kg), and DBP variables using two methods: all participants who commenced the study and completers only. Both analyses showed the same pattern of outcomes with a time effect (p<0.001) but no differences between the groups (>=0.51)or group by time interactions (>=0.07). This implies that our linear mixed effects analysis has accurately modelled the missing data and the baseline differences have not had an adverse influence on our conclusions." High attrition in both groups, with reasons including personal reasons, unable to comply with the intervention. It is likely that with such high attrition, missingness is possibly and likely to depend on the true value. | Low risk of bias | "Body mass was measured twice using calibrated electronic scales (Tanita Ultimate Scale 2000; Tokyo)." No evidence that different methods were used across intervention groups to measure weight. "The investigator was trained in ISAK International Standards for Anthropometric Assessment". Trial registry: Open (masking not used). Weight is not an observed‐reported outcome involving judgment. | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | High attrition reported in both groups, including 'personal reasons' and 'inability to comply with interventions'; therefore missingness is possibly and likely dependent on the true value of the outcome. Lack of information in the pre‐registered methods. |
Risk of bias for analysis 4.2 Change in body weight (kg) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Dyson 2007 | Low risk of bias | "Randomization was undertaken by means of sealed envelopes equivalent to the number of subjects and filled fifty‐fifty with an indicator of either a low‐carbohydrate diet or healthy‐eating advice. Two separate sets of envelopes were prepared for both diabetic and non‐diabetic subjects. An independent observer witnessed randomization." Author reported in correspondence that no formal test of hypothesis was done for difference at baseline for non‐diabetic participants. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. n=1 of randomised participants in balanced group refused follow‐up as not allocated to low carbohydrate diet. Modified intention to treat, n=1 refused follow‐up (missing data) and not included. | High risk of bias | In low carbohydrate diet group, 0% and in balanced diet group, 14% (n=1) of randomised participants were not included in the analyses. Refusal of follow‐up as not allocated to low carbohydrate diet may be influenced by expected change in weight (missingness possibly influenced by true value). | Low risk of bias | Body weight recorded every 3 months. No evidence that a different method was used across intervention groups to measure weight. No details about blinding of outcome assessors. Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. No information about blinding of outcome data. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition in the control group due to disappointment at not being assigned to the low carbohydrate diet, missingness possibly influenced by the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
| Larsen 2011 | Low risk of bias | " Randomisation was carried out by a third party using computer‐generated random numbers (using block randomisation and random block sizes) and stratified according to diabetes treatment..." "Dietary assignment was performed by a third party on the day of the initial dietary counselling visit." "The baseline characteristics for the intention‐to‐treat population were well‐matched at baseline...although non‐significant differences were apparent in calcium excretion rate, systolic blood pressure and the proportion of men." | Low risk of bias | "we were not able to blind participants or the study personnel involved in dietary counselling" Deviations reported are expected to arise in usual care e.g. lost interest, dissatisfied with weight loss. Modified ITT analysis done: "This study used an intention‐to‐treat model for all participants who received dietary advice..... study staff encouraged all participants to return for follow‐up assessments of primary and secondary outcomes, regardless of dietary adherence" | High risk of bias | 9/57 (16%) participants in the intervention group and 6/51 (12%) in the control group did not have data available. Data imputed for ITT for 5/57 (intervention) and 1/51 (control) participants with missing data." This study used the single imputation method of last measurement carried forward for missing data for primary and secondary outcomes" Reasons are vague and include 'changing their mind' and 'inconvenient' thus missingness could and is likely to depend on the true value. | Low risk of bias | Weight was measured in light clothes. No evidence that a different method was used across intervention groups to measure weight. "a trained assessor, who was blinded to the dietary assignment of the individual, measured and recorded weight..." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint. "the statistical analysis was performed unblinded to the treatment allocation." The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | Attrition reported in both groups, with vague reasons; therefore missingness could and is likely to depend on the true value of the outcome. Lack of information in the pre‐registered methods. |
| Sato 2017 | Low risk of bias | "Randomization was achieved by the minimization and biased coin method." "Allocation sequence conducted by third party organization (Soiken, Inc., Osaka, Japan) was concealed until interventions were assigned." No problematic differences in baseline characteristics of participants | Low risk of bias | "...the number of patients was limited, and they could not be blinded due to the characteristics of the dietary therapy." "Open ‐ no one is blinded" (trial registry). Deviations reported are expected to arise in usual care e.g. moved to another hospital, lacked motivation, disappointed with assigned diet. All participants randomised received their assigned interventions and all were analysed except for participants with missing outcome data, thus modified ITT. | High risk of bias | 8/33 (24%) in the intervention and 6/33 (17%) in the control group did not have data. Modified ITT conducted, but high attrition in the intervention group. Reasons for loss to follow up and withdrawal are clearly stated for all participants and included transferring to other hospitals and admission for different diseases, including diabetes. Missingness could be and is likely to be dependent on the true value. | Low risk of bias | No details on method used for weight measurements. No evidence that different methods were used across intervention groups to measure weight. "Open ‐ no one is blinded" (trial registry). Weight is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. Could not obtain trial registry record. Analysis intentions are not reported in sufficient detail to enable an assessment. | High risk of bias | High attrition reported in the intervention group, with clear reasons for withdrawal which indicate that missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
Risk of bias for analysis 4.5 Change in DBP (mmHg) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Larsen 2011 | Low risk of bias | " Randomisation was carried out by a third party using computer‐generated random numbers (using block randomisation and random block sizes) and stratified according to diabetes treatment..." "Dietary assignment was performed by a third party on the day of the initial dietary counselling visit." "The baseline characteristics for the intention‐to‐treat population were well‐matched at baseline...although non‐significant differences were apparent in calcium excretion rate, systolic blood pressure and the proportion of men." | Low risk of bias | "...we were not able to blind participants or the study personnel involved in dietary counselling." Deviations reported are expected to arise in usual care, e.g. lost interest, dissatisfied with weight loss. Modified ITT analyses conducted: "...study staff encouraged all participants to return for follow‐up assessments of primary and secondary outcomes, regardless of dietary adherence." | Low risk of bias | 9/57 (16%) participants in the intervention group and 6/51 (12%) in the control group did not have data available. Data imputed for ITT for 5/57 (intervention) and 1/51 (control) participants with missing data. "This study used the single imputation method of last measurement carried forward for missing data for primary and secondary outcomes." Based on reasons provided, it is unlikely that missingness in data for DBP was influenced by its true value. | Low risk of bias | Study authors reported that a mercury sphygmomanometer was used. No evidence that a different method was used across intervention groups to measure DBP. "...(anthropometrics and laboratory variables) were measured by personnel who were blinded to group allocation..." | Some concerns | No mention of pre‐specified data analysis plan, and trial registry does not mention the outcome and timepoint in any versions thereof "Study endpoints were assessed blinded to the diet group, but the statistical analysis was performed unblinded." Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
Risk of bias for analysis 4.7 Change in HbA1c (%) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Dyson 2007 | Low risk of bias | "Randomization was undertaken by means of sealed envelopes equivalent to the number of subjects and filled fifty‐fifty with an indicator of either a low‐carbohydrate diet or healthy‐eating advice. Two separate sets of envelopes were prepared for both diabetic and non‐diabetic subjects. An independent observer witnessed randomization." Author reported in correspondence that no formal test of hypothesis was done for difference at baseline for non‐diabetic participants. Baseline characteristics table showed differences which were consistent with differences resulting by chance with very small sample sizes. | Low risk of bias | No information, however, blinding of participants and people delivering these dietary interventions is highly unlikely. n=1 of randomised participants in balanced group refused follow‐up as not allocated to low carbohydrate diet. Modified intention to treat, n=1 from control group refused follow‐up, thus data missing and not included. | Low risk of bias | In low carbohydrate diet group, 0% and in balanced diet group, 14% (n=1) of randomised participants were not included in the analyses. Based on reason provided, it is unlikely that missingness in data for HbA1c was influenced by its true value. | Some concerns | No information about method used for HbA1c measurements. No details about blinding of outcome assessors. Laboratory determined outcomes are not observer‐reported involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. No information about blinding of outcome data. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | No information regarding the analytical method used to measure the outcome, or whether assessors were blinded. No pre‐registered document available to assess analysis intentions. |
| Larsen 2011 | Low risk of bias | " Randomisation was carried out by a third party using computer‐generated random numbers (using block randomisation and random block sizes) and stratified according to diabetes treatment..." "Dietary assignment was performed by a third party on the day of the initial dietary counselling visit." "The baseline characteristics for the intention‐to‐treat population were well‐matched at baseline...although non‐significant differences were apparent in calcium excretion rate, systolic blood pressure and the proportion of men." | Low risk of bias | "...we were not able to blind participants or the study personnel involved in dietary counselling." Deviations reported are expected to arise in usual care, e.g. lost interest, dissatisfied with weight loss. Modified ITT conducted: "...study staff encouraged all participants to return for follow‐up assessments of primary and secondary outcomes, regardless of dietary adherence." | Low risk of bias | 9/57 (16%) participants in the intervention and 6/51 (12%) participants in the control arm did not have data available. This includes 4 participants in intervention and 5 in control arm that changed their mind after randomisation but before starting the diets. Data imputed for ITT for 5/57 (intervention) and 1/51 (control) participants with missing data. "This study used the single imputation method of last measurement carried forward for missing data for primary and secondary outcomes." Based on reasons provided, it is unlikely that missingness in data for HbA1c was influenced by its true value. | Low risk of bias | "HbA1c was determined using immunoturbidimetric spectrophotometry with the Roche Integra 800 analyser (Roche Diagnostics, Castle Hill, NSW, Australia)." No evidence that a different method was used across intervention groups to measure HbA1c. "However, key study endpoints (anthropometrics and laboratory variables) were measured by personnel who were blinded to group allocation." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint "Study endpoints were assessed blinded to the diet group, but the statistical analysis was performed unblinded." The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Sato 2017 | Low risk of bias | "Randomization was achieved by the minimization and biased coin method." "Allocation sequence conducted by third party organization (Soiken, Inc., Osaka, Japan) was concealed until interventions were assigned." No problematic differences in baseline characteristics of participants | Low risk of bias | "....and they could not be blinded due to the characteristics of dietary therapy." "Open ‐ no one is blinded" (trial registry). Deviations are expected to arise in usual care, e.g. dissapointed with assigned diet, lacked motivation, moved to a different hospital. "All statistical analyses were conducted per‐protocol analysis..." However, all participants randomised received their assigned interventions and all were analysed except for participants with missing outcome data. Thus, a modified ITT was done." | High risk of bias | 8/33 (24%) intervention participants and 6/33 (17%) control participants did not have data. Modified ITT was done. High attrition in the intervention group. Reasons for lost to follow up and withdrawal are clearly stated for all participants and included transferring to other hospitals and admission for different diseases, including diabetes. Missingness could be and is likely to be dependent on the true value. | Low risk of bias | "...HbA1c ... were measured with standard techniques" No evidence that different methods were used across intervention groups to measure HbA1c. "Open ‐ no one is blinded" (trial registry). HbA1c is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. Could not obtain trial registry record. Analysis intentions not reported in sufficient detail to enable judgement. | High risk of bias | High attrition in the intervention group, and reasons for withdrawal indicate that missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
Risk of bias for analysis 4.8 Change in LDL cholesterol (mmol/L) at ≥ 12 months.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Larsen 2011 | Low risk of bias | " Randomisation was carried out by a third party using computer‐generated random numbers (using block randomisation and random block sizes) and stratified according to diabetes treatment..." "Dietary assignment was performed by a third party on the day of the initial dietary counselling visit." "The baseline characteristics for the intention‐to‐treat population were well‐matched at baseline...although non‐significant differences were apparent in calcium excretion rate, systolic blood pressure and the proportion of men." | Low risk of bias | "we were not able to blind participants or the study personnel involved in dietary counselling." Deviations reported are expected to arise in usual care e.g. lost interest, dissatisfied with weight loss. Modified ITT conducted: "This study used an intention‐to‐treat model for all participants who received dietary advice..... study staff encouraged all participants to return for follow‐up assessments of primary and secondary outcomes, regardless of dietary adherence." | Low risk of bias | 9/57 (16%) participants in the intervention and 6/51 (12%) participants in the control arm did not have data available. This includes 4 participants in intervention and 5 in control arm that changed their mind after randomisation but before starting the diets. Data imputed for ITT for 5/57 (intervention) and 1/51 (control) participants with missing data. "This study used the single imputation method of last measurement carried forward for missing data for primary and secondary outcomes." Based on reasons provided, it is unlikely that missingness in data for LDL was influenced by its true value. | Low risk of bias | Lipids were measured by spectrophotometric methods on the Roche Modular Analyser. All biochemical analyses were performed by an independent laboratory. No evidence that a different method was used across intervention groups to measure LDL. "However, key study endpoints (anthropometrics and laboratory variables) were measured by personnel who were blinded to group allocation." | Some concerns | No mention of pre‐specified analysis plan, but trial registry at date prior to study publication mentions the outcome and timepoint "the statistical analysis was performed unblinded to the treatment allocation." The time point mentioned in trial registry at date prior to publication is reported on in publication. Analysis intentions are not reported in sufficient detail to enable an assessment. | Some concerns | Lack of information in the pre‐registered methods. |
| Sato 2017 | Low risk of bias | "Randomization was achieved by the minimization and biased coin method." "Allocation sequence conducted by third party organization (Soiken, Inc., Osaka, Japan) was concealed until interventions were assigned." No problematic differences in baseline characteristics of participants | Low risk of bias | "....and they could not be blinded due to the characteristics of dietary therapy.""All statistical analyses were conducted per‐protocol analysis..." However, all participants randomised received their assigned interventions and all were analysed except for participants with missing outcome data. Thus, a modified ITT was done. "Open‐ no one is blinded" (trial registry). Deviations are expected to arise in usual care, e.g. dissapointed with assigned diet, lacked motivation, moved to a different hospital. | High risk of bias | 8/33 (24%) intervention participants and 6/33 (17%) control participants did not have data. Modified ITT was done. High attrition in the intervention group. Reasons for lost to follow up and withdrawal are clearly stated for all participants and included transferring to other hospitals and admission for different diseases, including diabetes. Missingness could be and is likely to be dependent on the true value. | Low risk of bias | "...Low density lipoprotein cholesterol [LDL‐C] ... were measured with standard techniques" No evidence that a different method was used across intervention groups to measure LDL‐C. "Open‐ no one is blinded" (trial registry). LDL cholesterol is not an observer‐reported outcome involving judgement. | Some concerns | No mention of a pre‐specified analysis plan. Could not obtain trial registry record. Analysis intentions not reported in sufficient detail to enable judgement. | High risk of bias | High attrition in the intervention group, and reasons for withdrawal indicate that missingness could be and is likely dependent on the true value of the outcome. No pre‐registered document available to assess analysis intentions. |
Acknowledgements
CN, AB and AS are partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
We thank the following people:
Staff from Cochrane Public Health and Cochrane Heart for editorial assistance and support with the protocol and review process. This review is co‐registered with both editorial groups.
Vittoria Lutje, Information Specialist for Cochrane Infectious Diseases Group, for the Embase searches.
Marianne Visser, Nina Robertson, Sarah Gordon, Sadiyya Sheik for support with screening and data extraction.
Authors of included studies that provided additional data and information when requested.
External peer reviewers for their time and inputs to help us improve the protocol. Professor Dulce Estêvão, Dr Lee Hooper and an anonymous external referee are gratefully acknowledged for their peer review of the full manuscript.
Appendices
Appendix 1. Search strategies
MEDLINE (PubMed)
Searched: 1946 to 25 June 2021
#1 Search randomized controlled trial [pt]
#2 Search controlled clinical trial [pt]
#3 Search randomized [tiab]
#4 Search placebo [tiab]
#5 Search clinical trials as topic [mesh: noexp]
#6 Search randomly [tiab]
#7 Search trial [ti]
#8 Search (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
#9 Search (animals [mh] NOT humans [mh])
#10 Search (#8 NOT #9)
#11 Search ("low carbohydrate" OR "lower carbohydrate" OR "high fat" OR "higher fat" OR "high protein" OR "higher protein")
#12 Search "ketogenic diet"
#13 Search ("carbohydrate restricted" OR "carbohydrates restricted" OR "fat restricted" OR "protein restricted")
#14 Search ("low carbohydrates" OR "lower carbohydrates")
#15 Search ("balanced diet" OR "recommended diet")
#16 Search ("reduced carbohydrate" OR "reduced carbohydrates" OR "reduced fat" OR "reduced protein")
#17 Search ("macronutrient manipulation" OR "macro nutrient manipulation" OR "macronutrients manipulation" OR "macro nutrients manipulation")
#18 Search "ketogenic diets"
#19 Search (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18)
#20 Search "Diet, Carbohydrate‐Restricted"[Mesh]
#21 Search "Diet, High‐Fat"[Mesh]
#22 Search "Diet, High‐Protein Low‐Carbohydrate"[Mesh]
#23 Search "Diet, High‐Protein"[Mesh]
#24 Search "Diet, Ketogenic"[Mesh]
#25 Search "Diet, Fat‐Restricted"[Mesh]
#26 Search "Diet, Mediterranean"[Mesh]
#27 Search (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26)
#28 Search (#19 OR #27)
#29 Search ("weight reducing diet" OR "weight reducing diets" OR "weight loss diet" OR "weight loss diets" OR "calorie restricted diet" OR "calorie restricted diets" OR "energy restricted diet" OR "energy restricted diets" OR "kilojoule restricted diet" OR "kilojoule restricted diets")
#30 Search ("isocaloric diet" OR "hypocaloric diet" OR "isoenergetic diet" OR "hypoenergetic diet")
#31 Search ("isocaloric diets" OR "hypocaloric diets" OR "isoenergetic diets" OR "hypoenergetic diets")
#32 Search ("iso caloric diet" OR "hypo caloric diet" OR "iso energetic diet" OR "hypo energetic diet")
#33 Search ("iso caloric diets" OR "hypo caloric diets" OR "iso energetic diets" OR "hypo energetic diets")
#34 Search "Diet, Reducing"[Mesh]
#35 Search "Caloric Restriction"[Mesh]
#36 Search (#29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35)
#37 Search (#10 AND #28 AND #36)
CENTRAL, Cochrane Library
Searched: Issue 6 of 12, 2021
#1 ("low carbohydrate" OR "lower carbohydrate" OR "high fat" OR "higher fat" OR "high protein" OR "higher protein")
#2 "ketogenic diet"
#3 ("carbohydrate restricted" OR "carbohydrates restricted" OR "fat restricted" OR "protein restricted")
#4 ("low carbohydrates" OR "lower carbohydrates")
#5 ("balanced diet" OR "recommended diet")
#6 ("reduced carbohydrate" OR "reduced carbohydrates" OR "reduced fat" OR "reduced protein")
#7 ("macronutrient manipulation" OR "macro nutrient manipulation" OR "macronutrients manipulation" OR "macro nutrients manipulation")
#8 "ketogenic diets"
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10 MeSH descriptor: [Diet, Carbohydrate‐Restricted] explode all trees
#11 MeSH descriptor: [Diet, High‐Protein Low‐Carbohydrate] explode all trees
#12 MeSH descriptor: [Diet, High‐Fat] explode all trees
#13 MeSH descriptor: [Diet, High‐Protein Low‐Carbohydrate] explode all trees
#14 MeSH descriptor: [Diet, High‐Protein] explode all trees
#15 MeSH descriptor: [Diet, High‐Protein Low‐Carbohydrate] explode all trees
#16 MeSH descriptor: [Diet, Ketogenic] explode all trees
#17 MeSH descriptor: [Diet, Fat‐Restricted] explode all trees
#18 MeSH descriptor: [Diet, High‐Protein Low‐Carbohydrate] explode all trees
#19 MeSH descriptor: [Diet, Mediterranean] explode all trees
#20 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
#21 #9 OR #20
#22 ("weight reducing diet" OR "weight reducing diets" OR "weight loss diet" OR "weight loss diets" OR "calorie restricted diet" OR "calorie restricted diets" OR "energy restricted diet" OR "energy restricted diets" OR "kilojoule restricted diet" OR "kilojoule restricted diets")
#23 ("isocaloric diet" OR "hypocaloric diet" OR "isoenergetic diet" OR "hypoenergetic diet")
#24 ("isocaloric diets" OR "hypocaloric diets" OR "isoenergetic diets" OR "hypoenergetic diets")
#25 ("iso caloric diet" OR "hypo caloric diet" OR "iso energetic diet" OR "hypo energetic diet")
#26 ("iso caloric diets" OR "hypo caloric diets" OR "iso energetic diets" OR "hypo energetic diets")
#27 MeSH descriptor: [Diet, Reducing] explode all trees
#28 MeSH descriptor: [Caloric Restriction] explode all trees
#29 #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28
#30 #21 AND #29 in Trials
Embase (Ovid)
Searched: 1947 to 28 June 2021
1 ("low carbohydrate" or "lower carbohydrate" or "high fat" or "higher fat" or "high protein" or "higher protein").ab. or ("low carbohydrate" or "lower carbohydrate" or "high fat" or "higher fat" or "high protein" or "higher protein").ti.
2 ketogenic diet.mp. or ketogenic diet/
3 ("carbohydrate restricted" or "carbohydrates restricted" or "fat restricted" or "protein restricted").ab. or ("carbohydrate restricted" or "carbohydrates restricted" or "fat restricted" or "protein restricted").ti.
4 ("low carbohydrates" or "lower carbohydrates").ab. or ("low carbohydrates" or "lower carbohydrates").ti.
5 ("low carbohydrate*" or "lower carbohydrate*").ab. or ("low carbohydrate*" or "lower carbohydrate*").ti.
6 ("balanced diet" or "recommended diet").ab. or ("balanced diet" or "recommended diet").ti.
7 ("balanced diet*" or "recommended diet*").ab. or ("balanced diet*" or "recommended diet*").ti.
8 ("reduced carbohydrate" or "reduced carbohydrates" or "reduced fat" or "reduced protein").ab. or ("reduced carbohydrate" or "reduced carbohydrates" or "reduced fat" or "reduced protein").ti.
9 (macronutrient* adj2 manipulation).mp.
10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
11 Mediterranean diet/ or low carbohydrate diet/ or protein diet/ or Atkins diet/ or high‐protein low‐carbohydrate diet/ or carbohydrate diet/
12 ("weight reducing diet" or "weight reducing diets" or "weight loss diet" or "weight loss diets" or "calorie restricted diet" or "calorie restricted diets" or "energy restricted diet" or "energy restricted diets" or "kilojoule restricted diet" or "kilojoule restricted diets").mp.
13 ("isocaloric diet" or "hypocaloric diet" or "isoenergetic diet" or "hypoenergetic diet").mp.
14 ("isocaloric diet*" or "hypocaloric diet*" or "isoenergetic diet*" or "hypoenergetic diet*").mp.
15 ("iso caloric diet" or "hypo caloric diet" or "iso energetic diet" or "hypo energetic diet").mp.
16 ("iso caloric diet*" or "hypo caloric diet*" or "iso energetic diet*" or "hypo energetic diet*").mp.
17 caloric restriction.mp. or caloric restriction/
18 caloric restricted.mp.
19 10 or 11
20 12 or 13 or 14 or 15 or 16 or 17 or 18
21 19 and 20
22 crossover‐procedure/ or double‐blind procedure/ or randomized controlled trial/ or single‐blind procedure/ or (random* or factorial* or crossover* or cross over* or placebo* or (doubl* adj blind*) or (singl* adj blind*) or assign* or allocat* or volunteer*).mp.
23 21 and 22
Web of Science Core Collection with Indexes SCI‐Expanded, SSCi, CPCI‐S (Clarivate Analytics)
Searched: 1970 to 25 June 2021
#1 ALL = (("randomized controlled trial" OR "randomised controlled trial" OR "randomised control trial" OR "randomized control trial" OR "controlled clinical trial" OR random* OR double‐blind OR "double blind" OR single‐blind OR "single blind") )
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#2 ALL = ((("low carbohydrate" OR "lower carbohydrate" OR "high fat" OR "higher fat" OR "high protein" OR "higher protein") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S ALL = (("ketogenic diet") )
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#4 ALL = ((("carbohydrate restricted" OR "carbohydrates restricted" OR "fat restricted" OR "protein restricted") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#5 ALL = ((("low carbohydrates" OR "lower carbohydrates") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#6 ALL = ((("balanced diet" OR "recommended diet" OR Mediterranean) ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#7 ALL = ((("reduced carbohydrate" OR "reduced carbohydrates" OR "reduced fat" OR "reduced protein") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#8 ALL = ((("macronutrient manipulation" OR "macro nutrient manipulation" OR "macronutrients manipulation" OR "macro nutrients manipulation") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#9 ALL = (("ketogenic diets") )
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#10 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#11 ALL = ((("weight reducing diet" OR "weight reducing diets" OR "weight loss diet" OR "weight loss diets" OR "calorie restricted diet" OR "calorie restricted diets" OR "calorie restriction" OR "calorie restrictions" OR "energy restricted diet" OR "energy restricted diets" OR "kilojoule restricted diet" OR "kilojoule restricted diets") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#12 ALL = ((("isocaloric diet" OR "hypocaloric diet" OR "isoenergetic diet" OR "hypoenergetic diet") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#13 ALL = ((("isocaloric diets" OR "hypocaloric diets" OR "isoenergetic diets" OR "hypoenergetic diets") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#14 ALL = ((("iso caloric diet" OR "hypo caloric diet" OR "iso energetic diet" OR "hypo energetic diet") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#15 ALL = ((("iso caloric diets" OR "hypo caloric diets" OR "iso energetic diets" OR "hypo energetic diets") ))
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#16 #15 OR #14 OR #13 OR #12 OR #11
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
#17 #16 AND #10 AND #1
Indexes=SCI‐EXPANDED, SSCI, CPCI‐S
ClinicalTrials.gov (https://clinicaltrials.gov/)
Searched: 25 June 2021
Condition or disease: (weight loss OR weight lost OR reduced weight OR weight reduction OR calorie restriction OR calorie restricted OR hypoenergetic OR hypocaloric OR isocaloric OR isoenergetic OR energy restricted OR energy restriction OR kilojoule restriction)
Other terms: (low carbohydrate OR lower carbohydrate OR carbohydrate restricted OR carbohydrate restriction OR high fat OR higher fat OR high protein OR higher protein OR ketogenic OR Mediterranean)
Study type: Interventional studies (Clinical Trials)
WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/)
Searched: 25 June 2021
In the Condition: weight loss OR weight lost OR reduced weight OR weight reduction OR calorie restriction OR calorie restricted OR hypoenergetic OR hypocaloric OR isocaloric OR isoenergetic OR energy restricted OR energy restriction OR kilojoule restriction
In the Intervention: low carbohydrate OR lower carbohydrate OR carbohydrate restricted OR carbohydrate restriction OR high fat OR higher fat OR high protein OR higher protein OR ketogenic OR Mediterranean
Recruitment Status is ALL
Data and analyses
Comparison 1. Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase only).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change in body weight (kg) at 3 to < 12 months | 37 | 3286 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.55, ‐0.59] |
| 1.2 Change in body weight (kg) at 3 to < 12 months: subgroup similarity of energy prescription | 37 | 3286 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.55, ‐0.59] |
| 1.2.1 Similar energy prescriptions or approaches to restriction in both diets | 27 | 2370 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.85, ‐0.11] |
| 1.2.2 Different, ad libitum energy in treatment diet and restricted in control diet | 7 | 655 | Mean Difference (IV, Random, 95% CI) | ‐1.84 [‐3.07, ‐0.62] |
| 1.2.3 No prescriptions reported or unrestricted for both diets | 3 | 261 | Mean Difference (IV, Random, 95% CI) | ‐2.71 [‐4.20, ‐1.22] |
| 1.3 Change in body weight (kg) at 3 to < 12 months: subgroup extent of carbohydrate restriction | 37 | 3286 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.55, ‐0.59] |
| 1.3.1 Very low carbohydrate/ketogenic | 9 | 494 | Mean Difference (IV, Random, 95% CI) | ‐2.29 [‐3.45, ‐1.13] |
| 1.3.2 Low carbohydrate/non‐ketogenic | 24 | 2256 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.75, 0.03] |
| 1.3.3 Incremental increase from very low to low carbohydrate | 4 | 536 | Mean Difference (IV, Random, 95% CI) | ‐1.51 [‐2.89, ‐0.13] |
| 1.4 Change in body weight (kg) at 3 to < 12 months: subgroup diagnosed cardiovascular event or disease | 37 | 3286 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.55, ‐0.59] |
| 1.4.1 None | 25 | 2799 | Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.40, ‐0.37] |
| 1.4.2 Mixed or unclear | 12 | 487 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐3.03, ‐1.45] |
| 1.5 Change in body weight (kg) at 3 to < 12 months: subgroup gender | 37 | 3286 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.55, ‐0.59] |
| 1.5.1 Mixed | 22 | 2401 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.64, ‐0.46] |
| 1.5.2 Males | 3 | 187 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.70, 1.21] |
| 1.5.3 Females | 12 | 698 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐2.37, ‐0.33] |
| 1.6 Change in body weight (kg) at 3 to < 12 months: sensitivity analysis overall risk of bias | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.6.1 Some concerns | 10 | 785 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.78, 0.33] |
| 1.7 Change in body weight (kg) at 3 to < 12 months: sensitivity analysis attrition domain | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 Low attrition | 11 | 810 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.75, 0.30] |
| 1.8 Change in body weight (kg) at 3 to < 12 months: sensitivity analysis source of funding | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 Without diet/food industry funding | 20 | 2183 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.97, ‐0.43] |
| 1.9 Change in body weight (kg) at ≥ 12 months | 14 | 1805 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.81, ‐0.04] |
| 1.10 Change in body weight (kg) at ≥ 12 months: sensitivity analysis attrition domain | 3 | 373 | Mean Difference (IV, Random, 95% CI) | ‐2.81 [‐4.50, ‐1.11] |
| 1.11 Change in body weight (kg) at ≥ 12 months: sensitivity analysis source of funding | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.11.1 Without food/diet industry funding | 9 | 1280 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.81, 0.56] |
| 1.12 Number of participants per group with weight loss of at least 5% at 3 to < 12 months | 3 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.00, 1.68] |
| 1.13 Number of participants per group with weight loss of at least 5% at ≥ 12 months | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.94, 1.31] |
| 1.14 Change in BMI (kg/m2) at 3 to < 12 months | 21 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.15 Change in BMI (kg/m2) at ≥ 12 months | 5 | 490 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.99, ‐0.23] |
| 1.16 Number of participants per group with reduction in BMI of at least 5% at 3 to < 12 months | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.79, 3.12] |
| 1.17 Change in DBP (mmHg) at ≥ 12 months | 11 | 1419 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐1.29, 1.12] |
| 1.18 Change in SBP (mmHg) at ≥ 12 months | 11 | 1397 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐2.99, 0.24] |
| 1.19 Change in LDL cholesterol (mmol/L) at ≥ 12 months | 13 | 1494 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.05, 0.12] |
| 1.20 Change in HDL cholesterol (mmol/L) at ≥ 12 months | 13 | 1519 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 1.21 Change in non‐HDL cholesterol (mmol/L) at ≥ 12 months | 2 | 225 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.20, 0.33] |
| 1.22 Change in total cholesterol (mmol/L) at ≥ 12 months | 11 | 1291 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.10, 0.12] |
| 1.23 Change in triglycerides (mmol/L) at ≥ 12 months | 13 | 1559 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.16, ‐0.06] |
| 1.24 Constipation at 3 to < 12 months | 4 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.81, 1.38] |
| 1.25 Constipation at ≥ 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.32, 1.25] |
| 1.26 Diarrhoea at 3 to < 12 months | 2 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.33, 2.93] |
| 1.27 Diarrhoea at ≥ 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.05] |
| 1.28 Nausea at 3 to < 12 months | 2 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.05, 10.68] |
| 1.29 Nausea at ≥ 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.65] |
| 1.30 Flatulence at 3 to < 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.44, 1.30] |
| 1.31 Flatulence at ≥ 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.39, 1.17] |
| 1.32 Heartburn at 3 to < 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.21, 1.36] |
| 1.33 Heartburn at ≥ 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.23, 1.01] |
| 1.34 Halitosis at 3 to < 12 months | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.32, 2.99] |
| 1.35 Stomach upset at 3 to < 12 months | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.41, 2.45] |
| 1.36 Appetite change at 3 to < 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.99] |
| 1.37 Appetite change at ≥ 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.25] |
| 1.38 Fatigue at 3 to < 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.47, 1.36] |
| 1.39 Fatigue at ≥ 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.49, 1.71] |
| 1.40 Headaches at 3 to < 12 months | 2 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.26, 6.02] |
| 1.41 Headaches at ≥ 12 months | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.25, 0.93] |
| 1.42 Reported anxiety at 3 to < 12 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.07, 15.26] |
| 1.43 Total mood disturbances (participant‐reported) at 3 to < 12 months | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [‐8.06, 11.88] |
| 1.44 Total mood disturbances (participant‐reported) at ≥ 12 months | 1 | 118 | Mean Difference (IV, Fixed, 95% CI) | 2.87 [‐8.48, 14.22] |
| 1.45 Depressive symptoms (participant‐reported) at 3 to <12 months | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.36, 0.76] |
| 1.46 Anxiety symptoms (participant‐reported) at 3 to < 12 months | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.44, 1.04] |
Comparison 2. Low‐carbohydrate weight‐reducing diets versus balanced carbohydrate weight‐reducing diets in overweight and obese participants without T2DM (weight‐reducing phase followed by weight‐maintenance phase).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Change in body weight (kg) at ≥ 12 months | 3 | 73 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.77, 2.16] |
| 2.2 Change in DBP (mmHg) at ≥ 12 months | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐6.00 [‐17.55, 5.55] |
| 2.3 Change in SBP (mmHg) at ≥ 12 months | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐15.00 [‐32.36, 2.36] |
| 2.4 Change in LDL cholesterol (mmol/L) at ≥ 12 months | 2 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.44, 0.28] |
| 2.5 Change in HDL cholesterol (mmol/L) at ≥ 12 months | 2 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.37, 0.28] |
| 2.6 Change in total cholesterol (mmol/L) at ≥ 12 months | 2 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.43, 0.27] |
| 2.7 Change in triglycerides (mmol/L) at ≥ 12 months | 2 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.17] |
Comparison 3. Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Change in body weight (kg) at 3 to < 12 months | 14 | 1114 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.44, ‐0.09] |
| 3.2 Change in body weight (kg) at 3 to < 12 months: sensitivity analysis overall risk of bias | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 Some concerns | 3 | 420 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐3.45, 1.22] |
| 3.3 Change in body weight (kg) at 3 to < 12 months: sensitivity analysis attrition domain | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3.1 Low attrition | 3 | 420 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐3.45, 1.22] |
| 3.4 Change in body weight (kg) at 3 to < 12 months: sensitivity analysis source of funding | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.4.1 Without food/diet industry funding | 6 | 740 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐3.04, 0.60] |
| 3.5 Change in body weight (kg) at ≥ 12 months | 7 | 813 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐2.13, 1.46] |
| 3.6 Change in body weight (kg) at ≥ 12 months: sensitivity analysis source of funding | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.6.1 Without diet/food industry funding | 5 | 625 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐2.92, 2.38] |
| 3.7 Weight loss of at least 5% at 3 to < 12 months | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.77, 1.33] |
| 3.8 Weight loss of at least 5% at ≥ 12 months | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.68, 1.20] |
| 3.9 Change in BMI (kg/m2) at 3 to < 12 months | 11 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.10 Change in BMI (kg/m2) at ≥ 12 months | 4 | 329 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐1.06, 0.48] |
| 3.11 Change in DBP (mmHg) at ≥ 12 months | 6 | 631 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.84, 1.28] |
| 3.12 Change in SBP (mmHg) at ≥ 12 months | 6 | 629 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐2.30, 2.93] |
| 3.13 Change in HbA1c (%) at ≥ 12 months | 6 | 668 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.38, 0.10] |
| 3.14 Change in LDL cholesterol (mmol/L) at ≥ 12 months | 7 | 753 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.03, 0.26] |
| 3.15 Change in HDL cholesterol (mmol/L) at ≥ 12 months | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.16 Change in non‐HDL cholesterol (mmol/L) at ≥ 12 months | 2 | 159 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.39, 0.58] |
| 3.17 Change in total cholesterol (mmol/L) at ≥ 12 months | 7 | 838 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.06, 0.37] |
| 3.18 Change in triglycerides (mmol/L) at ≥ 12 months | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.19 Constipation at 3 to < 12 months | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.86, 2.18] |
| 3.20 Constipation at ≥ 12 months | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.11, 64.65] |
| 3.21 Diarrhoea 3 to < 12 months | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.62, 1.84] |
| 3.22 Heartburn symptoms (participant‐reported) at 3 to < 12 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.66, 0.06] |
| 3.23 Appetite change (participant‐reported) at 3 to < 12 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.03, 1.63] |
| 3.24 Headaches at 3 to < 12 months | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.75, 1.78] |
| 3.25 Depressive symptoms (participant‐reported) at 3 to <12 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐9.20, 8.80] |
3.8. Analysis.

Comparison 3: Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase only), Outcome 8: Weight loss of at least 5% at ≥ 12 months
Comparison 4. Low‐carbohydrate weight‐reducing diets versus balanced‐carbohydrate weight‐reducing diets in overweight and obese participants with T2DM (weight‐reducing phase followed by weight‐maintenance phase).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Change in body weight (kg) at 3 to < 12 months | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐2.46, 0.86] |
| 4.2 Change in body weight (kg) at ≥ 12 months | 3 | 158 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐1.31, 1.17] |
| 4.3 Change in BMI (kg/m2) at 3 to < 12 months | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.83, 0.25] |
| 4.4 Change in BMI (kg/m2) at > 12 months | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.86, 0.62] |
| 4.5 Change in DBP (mmHg) at ≥ 12 months | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐4.89, 4.01] |
| 4.6 Change in SBP (mmHg) at ≥ 12 months | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐4.27 [‐8.74, 0.20] |
| 4.7 Change in HbA1c (%) at ≥ 12 months | 3 | 160 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.30, 0.33] |
| 4.8 Change in LDL cholesterol (mmol/L) at ≥ 12 months | 2 | 145 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.26, 0.13] |
| 4.9 Change in HDL cholesterol (mmol/L) at ≥ 12 months | 2 | 148 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.07, 0.08] |
| 4.10 Change in total cholesterol (mmol/L) at ≥ 12 months | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.51, 0.19] |
| 4.11 Change in triglycerides (mmol/L) at ≥ 12 months | 2 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.45, 0.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aude 2004.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: 1998 Sample size calculation: yes Outcome(s) used for sample size calculation: weight loss Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient medical centre/heart institute in Miami Eligibility criteria: Participants older than 18 years with a BMI of 27 kg/m2 or higher; willing to adhere to a diet for three months. Participants were excluded if they had a history of thyroid disease or insulin‐dependent diabetes; were pregnant; had unstable medical conditions or used corticosteroids (regardless of route of administration), testosterone, appetite suppressants or other medication altering weight or appetite. Lipid‐lowering agents were permitted if it had remained unchanged for at least three months. Type 2 diabetes at baseline: Mixed; stratified with non‐T2DM since at baseline 93% of treatment and 100% of control group did not have non‐insulin dependent diabetes mellitus; insulin‐dependent diabetes mellitus excluded Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Mixed Gender: Mixed Total number randomised: 60 Total attrition in trial: 6 Treatment diet Participants randomised: 30 Participants withdrawn (voluntary): NR Total attrition: 1 Control diet: Participants randomised: 30 Participants withdrawn (voluntary): NR Total attrition: 5 Baseline data treatment diet: Randomised participants not included: 1/30 Age (years): mean (SD) 46 (10) Gender distribution (as reported): female 14/29 (48%), male 15/29 (52%) Weight (kg): mean (SD) 99.1 (31.9) BMI (kg/m2): mean (SD) 34.9 (4.0) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.32 (1.16) HDL (mmol/L): mean (SD) 1.30 (0.37) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.51 (1.20) TG (mmol/L): mean (SD) 2.05 (1.51) Baseline data control diet: Randomised participants not included: 5/30 Age (years): mean (SD) 44 (10) Gender distribution (as reported): female 14/25 (56%), male 11/25 (44%) Weight (kg): mean (SD) 99.9 (21.1) BMI (kg/m2): mean (SD) 35.5 (6.0) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.17 (0.69) HDL (mmol/L): mean (SD) 1.41 (0.48) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.33 (0.95) TG (mmol/L): mean (SD) 1.65 (0.90) Group differences at baseline: no Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet, restricted in control diet Treatment diet: Name (as reported) and brief description: Modified low‐carbohydrate diet, first phase (two weeks) higher intake of fat (62%), very low intake of carbohydrates (10%) and protein intake of 28%. In the second phase (two weeks) intakes were adjusted to 43% fat, 27% carbohydrate and 30% protein. In the third phase (eight weeks) 39% fat, 28% carbohydrates and 33% protein. In the maintenance phase, approximate calories consumed based on the recommended guidelines were also about 1300 for women and 1600 for men. Treatment diet type (carbohydrate‐fat‐protein): Very low2w to low10w‐high‐high Exercise component? No Recipients: N = 14 women and N = 15 men with mean (SD) age 46 (10) years, mean (SD) weight 99.1 (31.9) kg, mean (SD) BMI 34.9 ( 4.0) kg/m2, mean(SD) total cholesterol 212.9 (46.2) mg/dL, mean (SD) LDL 128.3 (44.8) mg/dL, mean (SD) HDL 50.3 (14.3) mg/dL, mean (SD) triglycerides 181.3 (134.0) mg/dL, N = 13 (44.8%) had hypertension, N = 2 (6.9%) had had a myocardial infarction, N = 0 had congestive heart failure, N = 0 had had a stroke, N = 2 (6.9%) had diabetes and N = 2 (6.9%) were smokers. Why? Isocaloric diet lower in carbohydrates and higher in protein and monounsaturated fat would have a more favorable effect on weight loss and reduce cardiovascular risk factors. What (materials)? Written diet guidelines What (procedures)? The MLC diet consisted of 2 phases of 2 weeks’ duration and of a third or maintenance phase of 8 weeks. The first phase was characterised by higher intake of fat (62%), very low intake of carbohydrates (10%), and an intake of protein of 28%. In the second phase, fat intake was decreased to 43% whereas carbohydrate intake was increased to 27% and protein intake to 30%. In the third phase, the percentages of calories were 39% from fat, 28% from carbohydrates, and 33% from protein. Sessions with the dietitian/nurse for 1 hr at baseline and 30 mins at subsequent visits with 24‐hr food recall being asked. Lipid profiles were done at baseline and final visit. Who provided? "A single specially trained dietitian and nurse practitioner instructed participants about their assigned diet..." How and where? Face‐to‐face at the Mount Sinai Medical Center–Miami Heart Institute When and how much? "All participants, regardless of their assignment, met with the dietitian or the nurse practitioner for 1 hour at the first visit and 30 minutes at subsequent visits, every two weeks for 12 weeks." Strategies to improve or maintain fidelity; tailoring and modification: "To assess the appropriateness of the foods consumed, 24‐hour food recalls were obtained. Because the goal of the 24‐hour food recalls was to provide feedback to the participants, the information was insufficient to perform any quantitative analysis." Extent of intervention fidelity: NR Concomitant interventions: N = 2 (6.9%) were on lipid lowering drugs. Control diet: Name (as reported) and brief description: US National Cholesterol Education Program (NCEP) diet, 30% of calories from fat, 55% from carbohydrates and 15% from protein. Saturated fat < 7% of total fat, MUFA between 10 and 15%. Diet tailored to provide approximately 1300 calories for women and 1600 calories for men. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 14 women and N = 11 men with mean (SD) age 44 (10) years, mean (SD) weight 99.9 (21.1) kg, mean (SD) BMI 35.5 (6.0) kg/m2, mean(SD) total cholesterol 205.8 (36.5) mg/dL, mean (SD) LDL 122.4 (26.7) mg/dL, mean (SD) HDL 54.3 (18.6) mg/dL, mean (SD) triglycerides 145.6 ( 80) mg/dL, N = 7 (28.0%) had hypertension, N = 0 had had a myocardial infarction, N = 0 had congestive heart failure, N = 0 had had a stroke, N = 0 had diabetes and N = 2 (8.0%) were smokers. Why? NCEP diets increase the risk of cardiovascular events due to rise in triglycerides and reduction in HDL. What (materials)? Written diet guidelines What (procedures)? In the NCEP diet the percentages of calories from fat (30%), carbohydrate (55%), and protein (15%) were unchanged throughout the trial. Saturated fat comprised less than 7% of the total fat intake, and monounsaturated fat between 10% and 15%. The diet was tailored to provide approximately 1300 calories for women and 1600 calories for men. Who provided? "A single specially trained dietitian and nurse practitioner instructed participants about their assigned diet..." How and where? Face‐to‐face at the Mount Sinai Medical Center–Miami Heart Institute When and how much? "All participants, regardless of their assignment, met with the dietitian or the nurse practitioner for 1 hour at the first visit and 30 minutes at subsequent visits, every two weeks for 12 weeks." Strategies to improve or maintain fidelity; tailoring and modification: "To assess the appropriateness of the foods consumed, 24‐hour food recalls were obtained. Because the goal of the 24‐hour food recalls was to provide feedback to the participants, the information was insufficient to perform any quantitative analysis." Extent of intervention fidelity: NR Concomitant interventions: N = 0 were on lipid lowering drugs. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: NR Declaration of interest: "Dr Hennekens is funded by the Agatston Research Institute, a nonprofit foundation, as Director of Research." |
|
Bales 2017.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT02033655 Total number of trial arms: 2 Year trial started: 2014 Sample size calculation: no Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient university medical centre in Durham Eligibility criteria: Participants were women aged ≥ 45 years with BMI ≥ 30 kg/m2. Participants were excluded if they suffered from dementia; had a functional limitation caused by a neurological condition; unstable or terminal medical conditions or a glomerular filtration rate decrease of ≥ 10% or < 45 mL/min/1.73 m². Type 2 diabetes at baseline: Unclear Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Female Total number randomised: 80 Total attrition in trial: 38 Treatment diet Participants randomised: 51 Participants withdrawn (voluntary): NR Total attrition: 22 Control diet: Participants randomised: 29 Participants withdrawn (voluntary): NR Total attrition: 16 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 58.9 (8.4), range 45.0 to 78.0 Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 98.6 (18.6) BMI (kg/m2): mean (SD) 37.5 (6.1) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 61.8 (7.6), range 46.0 to 73.0 Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 103.0 (15.6) BMI (kg/m2): mean (SD) 38.3 (5.8) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Group differences at baseline: Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein weight loss (HP‐WL) diet prescribing 1.2 g/kg body weight of protein with a target of 30 g protein/meal and a distribution of ~30% protein, 30% fat and 40% carbohydrates. Energy intake ~500 kcal below calculated requirement Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: N = 51 women, mean (SD) age 58.9 (8.4) and range 45.0–78.0 years, mean (SD) weight 98.6 (18.6) kg, mean (SD) BMI 37.5 (6.1) kg/m2 and mean (SD) fasting blood glucose 111.7 (40.9) mg/dL Why? To evaluate the effects of a high protein diet with balanced protein distribution on lean muscle mass for weight loss and preserving physical function What (materials)? "Participants received individualized kilocalorie prescription and meal plans. To promote achievement of the protein intake target, participants were supplied with preportioned frozen or chilled lean meats (lean ground pork, pork tenderloins, pork chops, and low‐sodium ham) sufficient to provide $420 g protein/wk ($30 g for 2 meals/d) for the duration of the trial." What (procedures)? HP‐WL participants were prescribed a protein intake of 1.2 g/kg body weight, with a target of 30 g protein/meal and a distribution of 30% protein, 30% fat, and 40% carbohydrates. Because high‐quality protein is superior for promoting anabolism (29, 30), the HP‐WL meal plan emphasised protein from animal sources, primarily lean meats and poultry, low‐fat dairy, fish, and eggs. To avoid monotony and allow flexibility, participants consumed other complete proteins (e.g. other lean meats, poultry and fish, low‐fat dairy foods, and eggs) at the third meal of each day according to their prescribed meal plan. All of the participants were prescribed an energy intake; 500 kcal below their calculated requirement, as derived from calculations of estimated total energy expenditure on the basis of weight, height, sex, age, and activity level with the use of published equations. Meetings with the dietitian (individual and group) with diet plans provided and assessments of nutrient intakes made per protocol Who provided? "Registered dietitians who were experienced in obesity treatment..." How and where? Face‐to‐face at Duke University Medical Center facilities When and how much? "After the 2 individual sessions, participants attended weekly group meetings (specific to study group but equivalent in structure and duration) for diet and health‐related counseling, peer support, and weekly weigh‐ins." Strategies to improve or maintain fidelity; tailoring and modification: "Interventionists reviewed participants’ daily food journals each week and adjusted their menus to ensure that the target kilocalorie intake and, for the HP‐WL group, 30 g protein/meal for breakfast, lunch, and dinner were regularly achieved, as previously described." Weigh‐ins and group meetings were also conducted. Extent of intervention fidelity: Weekly sessions and assessments. To promote achievement of the protein intake target, participants were supplied with preportioned frozen or chilled lean meats during the whole trial. Concomitant interventions: "All participants were supplied with a low‐dose multivitamin supplement (GNC Teen Multivitamin), along with 400 mg Ca and 600 IU vitamin D (Bayer Citracal Calcium Supplement +D3) to ensure adequate nutrient intake and to standardize supplement use (participants were instructed to discontinue all other nutritional supplements)." Control diet: Name (as reported) and brief description: Control weight loss (C‐WL) diet prescribing RDA for protein of 0.8 g/kg body weight with a distribution of calories of ~15% protein, 30% fat and 55% carbohydrates. Energy intake ~500 kcal below calculated requirement Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 29 women, mean (SD) age 61.8 (7.6) and range 46.0–73.0 years, mean (SD) weight 103.0 (15.6) kg, mean (SD) BMI 38.3 (5.8) kg/m2 and mean (SD) fasting blood glucose 106.9 (31.2) mg/dL Why? NR What (materials)? "Participants received individualized kilocalorie prescription and meal plans." What (procedures)? The C‐WL group was prescribed the RDA for protein of 0.8 g/kg body weight, with a distribution of calories of 15% protein, 30% fat, and 55% carbohydrates. All of the participants were prescribed an energy intake 500 kcal below their calculated requirement, as derived from calculations of estimated total energy expenditure on the basis of weight, height, sex, age, and activity level with the use of published equations. Meetings with the dietitian (individual and group) with diet plans provided and assessments of nutrient intakes made per protocol Who provided? "Registered dietitians who were experienced in obesity treatment..." How and where? Face‐to‐face at Duke University Medical Center facilities When and how much? "After the 2 individual sessions, participants attended weekly group meetings (specific to study group but equivalent in structure and duration) for diet and health‐related counseling, peer support, and weekly weigh‐ins." Strategies to improve or maintain fidelity; tailoring and modification: "Interventionists reviewed participants’ daily food journals each week and adjusted their menus to ensure that the target kilocalorie intake and, for the HP‐WL group, 30 g protein/meal for breakfast, lunch, and dinner were regularly achieved, as previously described." Weigh‐ins and group meetings were also conducted. Extent of intervention fidelity: Weekly sessions and assessments. To promote achievement of the protein intake target, participants were supplied with preportioned frozen or chilled lean meats during the whole trial. Concomitant interventions: "All participants were supplied with a low‐dose multivitamin supplement (GNC Teen Multivitamin), along with 400 mg Ca and 600 IU vitamin D (Bayer Citracal Calcium Supplement +D3) to ensure adequate nutrient intake and to standardize supplement use (participants were instructed to discontinue all other nutritional supplements)." |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: POWR‐UP Trial funded by: Pork Checkoff Program, North Carolina Pork Council, Smithfield Foods and the NIH (grants 5T32 AG000029, 1P30 AG028716 and K12 HD043446) Declaration of interest: "Author disclosures: CW Bales, KN Porter Starr, MC Orenduff, SR McDonald, K Molnar, AK Jarman, A Onyenwoke, H Mulder, ME Payne, and CF Pieper, no conflicts of interest. The sponsors had no influence on the protocol design, conduct of the trial, or data analysis." |
|
Bazzano 2014.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT00609271 Total number of trial arms: 2 Year trial started: 2008 Sample size calculation: yes Outcome(s) used for sample size calculation: weight change Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 52 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient university health sciences centre in New Orleans. One low‐carbohdyrate or low‐fat meal replacement (bar or shake) per day was provided. Eligibility criteria: Participants aged 22 to 75 years with a BMI 30 to 45 kg/m2. Participants were excluded if they had self‐reported cardiovascular disease, type 2 diabetes or kidney disease; used prescription medication for weight loss; had surgery or weight loss of more than 6.8 kg in the past six months. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 148 Total attrition in trial: 29 Treatment diet Participants randomised: 75 Participants withdrawn (voluntary): NR Total attrition: 16 Control diet: Participants randomised: 73 Participants withdrawn (voluntary): NR Total attrition: 13 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 45.8 (9.9) Gender distribution (as reported): female 66/75 (88%), male 9/75 (12%) Weight (kg): mean (SD) 96.3 (12.7) BMI (kg/m2): mean (SD) 35.2 (3.8) DBP (mmHg): mean (SD) 77.5 (9.0) SBP (mmHg): mean (SD) 120.3 (12.8) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.2 (0.9) HDL (mmol/L): mean (SD) 1.4 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.1 (1.1) TG (mmol/L): mean (SD) 1.3 (0.6) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 47.8 (10.4) Gender distribution (as reported): female 65/73 (89%), male 8/73 (11%) Weight (kg): mean (SD) 97.9 (13.5) BMI (kg/m2): mean (SD) 35.6 (4.5) DBP (mmHg): mean (SD) 79.4 (8.3) SBP (mmHg): mean (SD) 124.9 (13.8) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.2 (1.0) HDL (mmol/L): mean (SD) 1.5 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.3 (1.1) TG (mmol/L): mean (SD) 1.4 (0.9) Group differences at baseline: no Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Unrestricted/ad libitum prescription in both diets Treatment diet: Name (as reported) and brief description: Low‐carbohydrate diet. Participants assigned to the low‐carbohydrate diet were instructed to maintain intake of digestible carbohydrate (total carbohydrate minus total fiber) of less than 40 g/d. Diet did not include a specific calorie or energy goal. Treatment diet type (carbohydrate‐fat‐protein): Very low‐unclear‐unclear Exercise component? No Recipients: N = 75 of which N = 66 (88%) female, mean (SD) age 45.8 (9.9) years, mean (SD) weight 96.3 (12.7) kg, mean (SD) BMI 35.2 (3.8) kg/m2, mean (SD) waist circumference 108.4 (9.3) cm, mean (SD) systolic BP 120.3 (12.8) mmHg, mean (SD) diastolic BP 77.5 (9.0) mmHg, mean (SD) total cholesterol 5.1 (1.1) mmol/L, mean (SD) LDL 3.2(0.9) mmol/L, mean (SD) HDL 1.4 (0.3) mmol/L, mean (SD) triglycerides 1.3 (0.6) mmol/L, mean (SD) plasma glucose 5.2 (0.6) mmol/L. Why? "Few randomized, controlled trials thus far have examined the effects of carbohydrate restriction on CVD risk factors in a diverse population with a significant proportion of black persons." What (materials)? "A handbook was given to participants that contained recipes, sample menus for 1 week of food intake at various energy levels, food lists, shopping lists, meal planners, and guides on counting macronutrients and reading nutrition labels. We also provided 1 low‐carbohydrate or low‐fat meal replacement (bar or shake) per day to participants in each group for the duration of the study." What (procedures)? Participants assigned to the low‐carbohydrate diet were instructed to maintain an intake of digestible carbohydrate (total carbohydrate minus total fiber) of less than 40 g/d. Dietary advice as per the "National Cholesterol Education Program guidelines". Neither diet included a specific calorie or energy goal. Participants in each group were asked to refrain from changing their physical activity levels during the intervention. Meetings were conducted with the dietitian weekly then monthly for the study period; these meetings were initially individualised and then group wise. Dietary recalls were also conducted to reflection on consumption over weekday and weekend. Who provided? Dietitian conducted the counselling sessions and all dietary recalls were obtained by a trained and certified staff member. How and where? Face‐to‐face at the Tulane University Health Sciences Center in New Orleans, Louisiana When and how much? "Participants met with a dietitian in weekly individual counseling sessions for the first 4 weeks, followed by small group counseling sessions every other week for the next 5 months (a total of 10 sessions) and monthly for the last 6 months of the intervention period. Individual sessions generally lasted about 1 hour and included dietary instruction and supportive counseling. Group counseling sessions were held separately for participants in the low‐fat and low‐carbohydrate groups but followed a common behavioral curriculum." Strategies to improve or maintain fidelity; tailoring and modification: Trained and certified staff, single calibrated scales, standard procedures for all Extent of intervention fidelity: NR Concomitant interventions: N = 21 (28%) on antihypertensive meds, N = 12 (16%) were on lipid‐lowering meds. Control diet: Name (as reported) and brief description: Low‐fat diet. Those assigned to the low‐fat diet were instructed to maintain less than 30% of their daily energy intake from total fat (with < 7% saturated fat) and 55% from carbohydrate. Diet did not include a specific calorie or energy goal. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 73 of which N = 65 (89%) female, mean (SD) age 47.8 (10.4) years, mean (SD) weight 97.9 (13.4) kg, mean (SD) BMI 35.6 (4.5) kg/m2, mean (SD) waist circumference 111.0 (10.7) cm, mean (SD) systolic BP 124.9 (13.8) mmHg, mean (SD) diastolic BP 79.4 (8.3) mmHg, mean (SD) total cholesterol 5.3 (1.1) mmol/L, mean (SD) LDL 3.2 (1.0) mmol/L, mean (SD) HDL 1.5 (0.3) mmol/L, mean (SD) triglycerides 1.4 (0.9) mmol/L, mean (SD) plasma glucose 5.2 (0.5). mmol/L Why? NR What (materials)? "A handbook was given to participants that contained recipes, sample menus for 1 week of food intake at various energy levels, food lists, shopping lists, meal planners, and guides on counting macronutrients and reading nutrition labels. We also provided 1 low‐carbohydrate or low‐fat meal replacement (bar or shake) per day to participants in each group for the duration of the study." What (procedures)? Those assigned to the low‐fat diet were instructed to maintain less than 30% of their daily energy intake from total fat (with < 7% from saturated fat) and 55% from carbohydrate, based on National Cholesterol Education Program guidelines. Neither diet included a specific calorie or energy goal. Participants in each group were asked to refrain from changing their physical activity levels during the intervention. Who provided? Dietitian conducted the counselling sessions and all dietary recalls were obtained by a trained and certified staff member. How and where? Face‐to‐face at the Tulane University Health Sciences Center in New Orleans, Louisiana When and how much? "Participants met with a dietitian in weekly individual counseling sessions for the first 4 weeks, followed by small group counseling sessions every other week for the next 5 months (a total of 10 sessions) and monthly for the last 6 months of the intervention period. Individual sessions generally lasted about 1 hour and included dietary instruction and supportive counseling. Group counseling sessions were held separately for participants in the low‐fat and low‐carbohydrate groups but followed a common behavioral curriculum." Strategies to improve or maintain fidelity; tailoring and modification: Trained and certified staff, single calibrated scales, standard procedures for all Extent of intervention fidelity: NR Concomitant interventions: N = 24 (34.9%) on antihypertensive meds, N = 9 (12.3%) were on lipid lowering meds. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: MACRO Trial funded by: NIH/NCRR P20‐RR017659 to the Tulane University Hypertension and Renal Center of Excellence Declaration of interest: Drs He, Chen, Yao, Reynolds, Klag, Bunol, Hu and Liu has nothing to disclose. "Dr. Whelton reports grants from the NIH, during the conduct of the study." "Dr. Bazzano reports grants from NIH, P20 RR017659 and K08 HL091108, during the conduct of the study." |
|
Benassi‐Evans 2009.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 52 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Australia, outpatient clinic in Adelaide Eligibility criteria: Participants were males aged 20 to 65 with a BMI of 27 to 40 kg/m2 and at least one additional risk factor for cardiovascular disease; as well as no history of metabolic or coronary disease or type 1 or 2 diabetes. Exclusion criteria NR Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Male Total number randomised: 33 Total attrition in trial: NR Treatment diet Participants randomised: 16 Participants withdrawn (voluntary): NR Total attrition: NR Control diet: Participants randomised: 17 Participants withdrawn (voluntary): NR Total attrition: NR Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SE) 54.94 (1.17) Gender distribution (as reported): female 0%, male 100% Weight (kg): mean (SE) 99.84 (2.45) BMI (kg/m2): mean (SE) 32.42 (0.79) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Baseline data control diet: Randomised participants not included: None Age (years): mean (SE) 52.94 (1.50) Gender distribution (as reported): female 0%, male 100% Weight (kg): mean (SE) 99.58 (3.61) BMI (kg/m2): mean (SE) 31.47 (0.96) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein high red meat (HP) diet. Consisted of 35% protein, 40% carbohydrate and 25% fat. Total energy content was 7000 kJ. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: Men only N = 16, mean (SE) age 54.94 (1.17) years, mean (SE) weight 99.84 (2.45) kg, mean (SE) BMI 32.42 (0.79) kg/m2. Why? "...we anticipated that the expected increased intake in the HP diet of folate, vitamin B12, niacin, vitamin E and calcium might result in lower peripheral blood lymphocytes (PBL), MN frequency given that higher intake of these vitamins is associated with lower MN frequency in PBLs in previous studies." What (materials)? Food checklists What (procedures)? Participants were prescribed a high protein–high red meat (HP) diet (35% protein, 40% carbohydrate, 25% fat). The total energy content of both diets was 7000 kJ, with some adjustments in energy (while maintaining protein to energy ratios) necessary for individuals to achieve an approximate weight loss of 1 kg per week. Twelve‐week intensive weight loss phase and 1 year maintenance phase continuing with the same diet strategy that the participant consumed in the 1st 12 weeks. Dietitian consultations were provided over the whole 1 year period with checklists being issued every 4 weeks. Who provided? Dietitian How and where? Face‐to‐face individual consultations, location NR When and how much? Fortnightly clinic visits during weight loss phase (12 weeks), thereafter monthly during weight maintenance phase (until 1 year) Strategies to improve or maintain fidelity; tailoring and modification: Dietary intake for 6 days/month was analysed from the checklists using FoodWorks dietary analysis package. Extent of intervention fidelity: The HP‐diet group recorded a significantly greater intake of protein, folate and iron at both weeks 12 and 52. Fibre and calcium intake was significantly higher in the HP group at week 12 only, while total fat intake was recorded as significantly higher in the HP group for week 52 only. Concomitant interventions: NR Control diet: Name (as reported) and brief description: High‐carbohydrate low red meat (HC) diet. Consisted of 17% protein, 58% carbohydrate and 25% fat. Total energy content was 7000 kJ. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Men only N = 17, mean (SE) age 52.94 (1.50) years, mean (SE) weight 99.58 (3.61) kg, mean (SE) BMI 31.47 (0.96) kg/m2. Why? NR What (materials)? Food checklists What (procedures)? Participants were prescribed a high carbohydrate–low red meat (HC) diet (17% protein, 58% carbohydrate, 25% fat). The total energy content of both diets was 7000 kJ, with some adjustments in energy (while maintaining protein to energy ratios) necessary for individuals to achieve an approximate weight loss of 1 kg per week. Who provided? Dietitian How and where? Face‐to‐face individual consultations, location NR When and how much? Fortnightly clinic visits during weight loss phase (12 weeks), thereafter monthly during weight maintenance phase (until 1 year) Strategies to improve or maintain fidelity; tailoring and modification: Dietary intake for 6 days/month was analysed from the checklists using FoodWorks dietary analysis package. Extent of intervention fidelity: The HP‐diet group recorded a significantly greater intake of protein, folate and iron at both weeks 12 and 52. Fibre and calcium intake was significantly higher in the HP group at week 12 only, while total fat intake was recorded as significantly higher in the HP group for week 52 only. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Meat and Livestock Australia (Medical Research Grant) Declaration of interest: "None declared." |
|
Brehm 2003.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: 2000 Sample size calculation: Yes Outcome(s) used for sample size calculation: Difference in weight loss and low density lipoprotein (LDL) cholesterol levels Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient general clinical research centre at a hospital in Cincinnati Eligibility criteria: Participants were women aged at least 18 years old who were moderately obese (BMI of 30 to 35 kg/m2) with stable weight over the past six months (defined as no weight loss or gain exceeding 10%). Participants were excluded if they had cardiovascular disease, untreated hypertension, diabetes, hyperthyroidism; abused substances; were pregnant or lactating. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 53 Total attrition in trial: 21 Treatment diet Participants randomised: 26 Participants withdrawn (voluntary): 4 Total attrition: 7 Control diet: Participants randomised: 27 Participants withdrawn (voluntary): 7 Total attrition: 14 Baseline data treatment diet: Randomised participants not included: 4/26 Age (years): mean (SD) 44.22 (6.84), range 29.01 to 53.49 Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 91.20 (8.4), range 76.9 to 113.7 BMI (kg/m2): mean (SD) 33.17 (1.83), range 30.87 to 37.03 DBP (mmHg): mean (SE) 79 (2.69) SBP (mmHg): mean (SE) 116 (3.23) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.23 (0.14) HDL (mmol/L): mean (SE) 1.34 (0.07) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.34 (0.17) TG (mmol/L): mean (SE) 1.68 (0.15) Baseline data control diet: Randomised participants not included: 7/27 Age (years): mean (SD) 43.10 (8.56), range 31.08 to 58.55 Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 92.31 (6.0), range 83.4 to 105.2 BMI (kg/m2): mean (SD) 34.04 (1.83), range 29.57 to 36.05 DBP (mmHg): mean (SE) 75 (1.99) SBP (mmHg): mean (SE) 115 (2.47) HbA1c (%): NR LDL (mmol/L): mean (SE) 2.95 (0.16) HDL (mmol/L): mean (SE) 1.26 (0.06) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 4.78 (0.16) TG (mmol/L): mean (SE) 1.23 (0.11) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: triglycerides (P < 0.01); intervention vs control Other notes about participants: Persons who withdrew from the study were excluded from analysis. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Very low carbohydrate diet, dieters instructed to follow an ad libitum diet with a maximum intake of 20 g/d carbohydrate to induce ketosis. After 2 weeks subjects were permitted to increase their intake of carbohydrates to 40‐60 g/d only if testing of urinary ketones continued to indicate ketosis. Treatment diet type (carbohydrate‐fat‐protein): Very low‐unclear‐unclear Exercise component? No Recipients: 22 women with mean (SD) age 44.22 (6.84) years, mean (SD) weight 91.20 (8.4) kg's, mean (SD) BMI 33.17 (1.83) kg/m2, mean (SD) systolic BP 116 (3.23) mmHg, mean (SD) diastolic BP 79 (2.69) mmHg, mean (SD) total cholesterol 206.32 (6.63) mg/dL, mean (SD) triglycerides 148.73 (13.41) mg/dL, mean (SD) LDL 124.86 (5.39) mg/dL, mean (SD) HDL 51.77 (2.82) mg/dL, mean (SD) glucose 99.1 (2.6) mg/dL Why? "Increased consumption of fat, particularly saturated fat, has been linked to increased plasma concentrations of lipids, insulin resistance, glucose intolerance, and obesity. Therefore, it is possible that many Americans could actually suffer adverse health effects by using very low carbohydrate diets in an attempt to lose weight." What (materials)? Self‐testing kits for ketosis, cooking tips, food diaries and pedometers (worn for 3 days a week). No specific exercise routine What (procedures)? Participants were instructed to follow an ad libitum diet with a maximum intake of 20 g carbohydrate. It was anticipated that this diet would induce ketosis. After 2 wk of dieting, subjects were permitted to increase their intake of carbohydrate to 40–60 g/d only if self‐testing of urinary ketones continued to indicate ketosis. Group meetings addressed cooking tips, stress management, behaviour modification, and relapse prevention. On alternating weeks, individual counselling sessions took place where the dietitian reviewed their 3‐d food records from the previous week and provided dietary recommendations and positive reinforcement. Subjects were advised to continue their baseline level of activity. At the end of the 3‐month intervention, subjects were instructed to continue with their weight loss efforts, but without scheduled contact with the dietitians until the 6‐month assessment. Who provided? Dietitians provided the intervention and the research nurses did the assessments. How and where? Face‐to‐face on the University of Cincinnati campus When and how much? Two registered dietitians delivered a 3‐month intervention aimed at promoting dietary compliance. Group meetings with subjects on the same diet were held biweekly. On alternating weeks, subjects met for individual counselling session. Strategies to improve or maintain fidelity; tailoring and modification: "On alternating weeks, subjects met for individual counseling session during which their assigned dietitian reviewed their 3‐d food records from the previous week, analyzed by Nutritionist V (First Data Bank, San Bruno, CA), and provided dietary recommendations and positive reinforcement." Extent of intervention fidelity: At 3 months the caloric intake in the very low carbohydrate diet group was distributed as 15% carbohydrate, 28% protein, and 57% fat. At 3 months, the very low carbohydrate diet group consumed significantly less carbohydrate, vitamin C, and fiber and significantly more protein, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, and cholesterol than the low fat diet group (P < 0.01 for all comparisons). Concomitant interventions: NR Control diet: Name (as reported) and brief description: Calorie‐restricted, moderately low‐fat diet with recommended macronutrient distribution of 55% carbohydrate, 15% protein and 30% fat. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: 20 women with mean (SD) age 43.10 (8.56) years, mean (SD) weight 92.31 (6.0) kg's, mean (SD) BMI 34.04 (1.83) kg/m2, mean (SD) systolic BP 115 (2.47) mmHg, mean (SD) diastolic BP 75 (1.99) mmHg, mean (SD) total cholesterol 184.45 (6.07) mg/dL, mean (SD) triglycerides 109.25 (9.49) mg/dL, mean (SD) LDL 113.80 (6.36) mg/dL, mean (SD) HDL 48.75 (2.23) mg/dL, mean (SD) glucose 91.1 (2.1) mg/dL Why? NR What (materials)? Self‐testing kits for ketosis, cooking tips, food diaries and pedometers (worn for 3 days a week). No specific exercise routine What (procedures)? Participants were instructed on a calorie‐restricted, moderately low‐fat diet with a recommended macronutrient distribution of 55% carbohydrate, 15% protein, and 30% fat. Calorie prescriptions were based on body size and calculated using the Harris‐Benedict equation. Group meetings addressed cooking tips, stress management, behaviour modification, and relapse prevention. Subjects were advised to continue their baseline level of activity. At the end of the 3‐month intervention, subjects were instructed to continue with their weight loss efforts, but without scheduled contact with the dietitians until the 6‐month assessment. Registered dietitians delivered a 3‐month intervention via biweekly group meetings (addressing cooking tips, stress management, behaviour modification, and relapse prevention) and alternating individual meetings with the assigned dietitian. Who provided? Dietitians provided the intervention and the research nurses did the assessments. How and where? Face‐to‐face group sessions, location University of Cincinnati campus When and how much? Two registered dietitians delivered a 3‐month intervention aimed at promoting dietary compliance. Group meetings with subjects on the same diet were held biweekly. On alternating weeks, subjects met for individual counselling session. Strategies to improve or maintain fidelity; tailoring and modification: "On alternating weeks, subjects met for individual counseling session during which their assigned dietitian reviewed their 3‐d food records from the previous week, analyzed by Nutritionist V (First Data Bank, San Bruno, CA), and provided dietary recommendations and positive reinforcement." Extent of intervention fidelity: The low‐fat diet group had daily calories distributed as 54% carbohydrate, 18% protein, and 28% fat. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: American Heart Association, University of Cincinnati Obesity Research Center, University of Cincinnati Research Council, Children's Hospital Medical Center Clinical Research Center (supported in part by USPHS grant M01‐RR‐08084 from the General Clinical Research Center Program, National Center for Research Resources, NIH) and NIH grants DK‐54263 and DK‐56863 Declaration of interest: NR for primary reference. Summer et al 2011: "The authors declared no conflict of interest." |
|
Brehm 2005.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase (as reported): 4 months What was the duration of the weight maintenance phase (as reported): NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient medical centre at a university in Cincinnati Eligibility criteria: Participants were obese women aged at least 18 years with moderate obesity (defined as BMI of 30 to 35 kg/m2) and a stable weight (within ± 10%) over the past six months. Participants were excluded if they had cardiovascular disease or abnormal electrocardiogram; diabetes or fasting plasma glucose above 100 mg/dL; untreated hypertension, or hypothyroidism; were pregnant or lactating or abused substances. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 39 Total attrition in trial: 10 Treatment diet Participants randomised: 25 Participants withdrawn (voluntary): NR Total attrition: 5 Control diet: Participants randomised: 25 Participants withdrawn (voluntary): NR Total attrition: 5 Baseline data treatment diet: Randomised participants not included: 5/25 Age (years): mean (SE) 44.8 (2.4) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SE) 90.6 (2.4) BMI (kg/m2): mean (SE) 32.8 (0.5) DBP (mmHg): mean (SE) 76 (1.7) SBP (mmHg): mean (SE) 119 (3.5) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.49 (0.21) HDL (mmol/L): mean (SE) 1.15 (0.05) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.31 (0.25) TG (mmol/L): mean (SE) 1.46 (0.15) Baseline data control diet: Randomised participants not included: 5/25 Age (years): mean (SE) 41.4 (3.2) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SE) 90.9 (2.1) BMI (kg/m2): mean (SE) 33.5 (0.5) DBP (mmHg): mean (SE) 77 (1.7) SBP (mmHg): mean (SE) 119 (2.9) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.24 (0.15) HDL (mmol/L): mean (SE) 1.15 (0.04) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.08 (0.21) TG (mmol/L): mean (SE) 1.65 (0.23) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Primary reference had blood pressure values (baseline), but for 20 participants only. Values not extracted |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Very low carbohydrate diet, dieters instructed to follow an ad libitum diet with a maximum intake of 20 g/d carbohydrate to induce ketosis. After 2 weeks subjects were permitted to increase their intake of carbohydrates to 40‐60 g/d only if testing of urinary ketones continued to indicate ketosis. Treatment diet type (carbohydrate‐fat‐protein): Very low‐unclear‐unclear Exercise component? No Recipients: N = 42 females only, mean (SE) age 44.5 (1.4) years, mean (SE) weight 90.9 (1.4) kg, mean (SE) BMI 33.2 (0.4) kg/m2. Why? Low carbohydrate diets increase energy expenditure leading to weight loss. What (materials)? Food records and pedometer What (procedures)? Participants were instructed to follow an ad libitum diet with a maximum intake of 20 g of carbohydrate per day, with the intent of producing ketosis. After 2 wk of dieting, subjects were permitted to increase their intake of carbohydrate to 40–60 g/d only if self‐testing of urinary ketones continued to indicate ketosis. Energy prescriptions were based on body size and calculated using the Harris‐Benedict equation plus an activity factor. All subjects were advised to continue their baseline level of physical activity. Group meetings with subjects on the same diet were held biweekly and addressed cooking tips, stress management, behavior modification, and relapse prevention. On alternating weeks, subjects met for individual counselling sessions during which their assigned dietitian reviewed their 3‐d food records and pedometer records from the previous week and provided dietary recommendations and positive reinforcement. Who provided? "Two counseling dietitians were trained to provide instruction on both diets. Each dietitian counseled half of the LC group and half of the LF group. This was done in order to minimize bias resulting from any differences in instruction by the dietitians." How and where? Face‐to‐face, individual and group sessions; General Clinical Research Center (GCRC) of Cincinnati Children’s Hospital Medical Center When and how much? Weekly counselling with a registered dietitian during the first half of the study with alternating group and individual sessions each week. Group sessions lasted 1h and individual counselling sessions were 30 min each. After the study’s midpoint, the subjects followed their assigned diets on their own. Strategies to improve or maintain fidelity; tailoring and modification: Subjects kept 3‐day diet records in which they recorded their entire food and beverage intake for 3 consecutive days each week. The study dietitian assigned to each subject reviewed the diet records for completeness, and provided ongoing instruction to the subjects on how to accurately record dietary intake. Extent of intervention fidelity: Though only the LF dieters were instructed to restrict energy intake, both groups significantly reduced their daily energy intake from baseline. As expected, intakes of dietary fat and protein by the LF group at the end of the intervention were significantly lower than baseline (though protein increased as a percent of total energy intake). Concomitant interventions: NR Control diet: Name (as reported) and brief description: Calorie‐restricted, moderately low‐fat diet with recommended macronutrient distribution of 55% carbohydrate, 15% protein and 30% fat. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 39 females only, mean (SE) age 41.9 (1.8) years, mean (SE) weight 91.3 (1.2) kg, mean (SE) BMI 33.8 (0.4) kg/m2. Why? NR What (materials)? Food records and pedometer What (procedures)? The other group of dieters was instructed to follow an energy‐restricted, moderately low‐fat diet with a recommended macronutrient distribution of 55% carbohydrate, 15% protein, and 30% fat. Energy prescriptions were based on body size and calculated using the Harris‐Benedict equation plus an activity factor. All subjects were advised to continue their baseline level of physical activity. Group meetings with subjects on the same diet were held biweekly and addressed cooking tips, stress management, behaviour modification, and relapse prevention. On alternating weeks, subjects met for individual counselling sessions during which their assigned dietitian reviewed their 3‐d food records and pedometer records from the previous week and provided dietary recommendations and positive reinforcement. Who provided? "Two counseling dietitians were trained to provide instruction on both diets. Each dietitian counseled half of the LC group and half of the LF group. This was done in order to minimize bias resulting from any differences in instruction by the dietitians." How and where? Face‐to‐face, individual and group sessions; General Clinical Research Center (GCRC) of Cincinnati Children’s Hospital Medical Center When and how much? Weekly counselling with a registered dietitian during the first half of the study with alternating group and individual sessions each week. Group sessions lasted 1h and individual counselling sessions were 30 min each. After the study’s midpoint, the subjects followed their assigned diets on their own. Strategies to improve or maintain fidelity; tailoring and modification: Subjects kept 3‐day diet records in which they recorded their entire food and beverage intake for 3 consecutive days each week. The study dietitian assigned to each subject reviewed the diet records for completeness, and provided ongoing instruction to the subjects on how to accurately record dietary intake. Extent of intervention fidelity: Not surprisingly, the LC diet group consumed significantly less carbohydrate by the end of the intervention, as compared to their baseline intake. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: American Heart Association, University of Cincinnati Research Council, University of Cincinnati Obesity Research Center (supported in part by National Institutes of Health grants DK54263 and DK56863) and Children's Hospital Medical Center Clinical Research Center (supported in part by the United States Public Health Service General Clinical Research Center grant M01 RR 08084 from the National Center for Research Resources, National Institutes of Health) Declaration of interest: NR for primary reference. Summer et al 2011: "The authors declared no conflict of interest." |
|
Calleja‐Fernández 2012.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: Yes Outcome(s) used for sample size calculation: Difference in weight Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 1 year What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Spain, outpatient university endocrinology and nutrition complex in León Eligibility criteria: Participants were overweight or obese (BMI between 28 and 35 kg/m2) and aged between 18 and 70 years. Participants were excluded if they participated in a weight loss treatment in the past six months; had a severe psychiatric illness; were pregnant or diabetic (defined as fasting plasma glucose > 126 mg/dL or > 200 mg/dL following an oral 75 g glucose tolerance test); had previous bariatric surgery or an eating disorder. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 40 Total attrition in trial: 24 Treatment diet Participants randomised: 21 Participants withdrawn (voluntary): NR Total attrition: 13 Control diet: Participants randomised: 19 Participants withdrawn (voluntary): NR Total attrition: 11 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 40.19 (13.16) Gender distribution (as reported): female 14/21 (66.7%), male 7/21 (33.3%) Weight (kg): mean (SD) 88.72 (13.43) BMI (kg/m2): mean (SD) 31.87 (2.71) DBP (mmHg): mean (SD) 79.62 (13.27) SBP (mmHg): mean (SD) 124.88 (18.08) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.06 (0.69) HDL (mmol/L): mean (SD) 1.35 (0.47) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.26 (1.05) TG (mmol/L): mean (SD) 1.75 (1.15) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 41.58 (14.50) Gender distribution (as reported): female 13/19 (68.4%), male 6/19 (31.6%) Weight (kg): mean (SD) 87.16 (10.38) BMI (kg/m2): mean (SD) 31.81 (2.04) DBP (mmHg): mean (SD) 77.24 (12.15) SBP (mmHg): mean (SD) 124.61 (17.20) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.09 (0.73) HDL (mmol/L): mean (SD) 1.37 (0.38) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.25 (0.83) TG (mmol/L): mean (SD) 1.66 (0.68) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1: waist circumference (P = 0.026); intervention vs control Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Diet A: 40% carbohydrate, 30% protein and 30% fat. The daily caloric intake for weight loss was calculated as the total caloric requirement minus 1000 kcal. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: 40% carb ‐ participants were a mix of insulin sensitive and insulin resistant. Mean age 40.19 years, and mean BMI 31.87 kg/m2 Why? To evaluate the long‐term effects on weight loss and insulin resistance of the diet in obese people either with insulin resistance (IR) or without insulin resistance (IS) What (materials)? Written materials and daily food diary What (procedures)? Daily energy requirements were estimated using the resting metabolic rate calculated by the Harris‐Benedict equation, 10 and an activity factor of 1.5 was added in order to estimate the total caloric requirement. The daily caloric intake for weight loss was calculated as the total caloric requirement minus 1000 kcal. The subjects were free living and were asked to maintain their usual patterns of activity. Participants were prescribed a 40% carbohydrate, 30% protein and 30% fat diet. The dietitian was responsible for providing individual counselling and written material to the participants on the initial visit and then the patients were instructed to record their food intake in a daily food diary and to discuss it with the dietitian on each visit. Nutritional intake history, 24‐hr dietary recall and written food records were also assessed at all visits. Who provided? Dietitian How and where? face‐to‐face, location NR When and how much? "...visit every 2 weeks for 16 weeks at the beginning of the trial and on each 3 monthly visit for 1 year." Strategies to improve or maintain fidelity; tailoring and modification: A 72‐h dietary recall was performed at every visit. Same dietitian and personnel making assessments Extent of intervention fidelity: NR Concomitant interventions: NR Control diet: Name (as reported) and brief description: Diet B: 55% carbohydrate, 15% protein and 30% fat. The daily caloric intake for weight loss was calculated as the total caloric requirement minus 1000 kcal. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: 55% carb ‐ participants were a mix of insulin sensitive and insulin resistant. Mean age 41.58 years, and mean BMI 31.81 kg/m2 Why? To evaluate the long‐term effects on weight loss and insulin resistance of the diet in obese people either with insulin resistance (IR) or without insulin resistance (IS) What (materials)? Written materials and daily food diary What (procedures)? Daily energy requirements were estimated using the resting metabolic rate calculated by the Harris‐Benedict equation 10 and an activity factor of 1.5 was added in order to estimate the total caloric requirement. The daily caloric intake for weight loss was calculated as the total caloric requirement minus 1000 kcal. The subjects were free living and were asked to maintain their usual patterns of activity. Participants were prescribed a 55% carbohydrate, 15% protein and 30% fat diet.The dietitian was responsible for providing individual counselling and written material to the participants on the initial visit and then the patients were instructed to record their food intake in a daily food diary and to discuss it with the dietitian on each visit. Nutritional intake history, 24‐hr dietary recall and written food records were also assessed at all visits. Who provided? Dietitian How and where? Face‐to‐face, location NR When and how much? "...visit every 2 weeks for 16 weeks at the beginning of the trial and on each 3 monthly visit for 1 year." Strategies to improve or maintain fidelity; tailoring and modification: A 72‐h dietary recall was performed at every visit. Same dietitian and personnel making assessments Extent of intervention fidelity: NR Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: None Declaration of interest: NR for primary reference. Ballesteros‐Pomar et al 2009: "No conflict of interests needs to be reported." Author contacted, but requested information not provided |
|
Cornier 2005.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: Yes Outcome(s) used for sample size calculation: Weight difference Duration of run‐in period (weeks): 0.43 What was the duration of the weight loss phase: 16 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient general clinical research centre of a university hospital in Denver. Food was prepared at the research centre, but the majority was consumed at participants' homes as they came to pick meals up every three days. Eligibility criteria: Participants were obese females who were insulin sensitive (defined by a fasting insulin level of < 10 μU/mL) or insulin resistant (defined as fasting insulin level of > 15 μU/mL). Participants were excluded if they had intermediate levels of fasting insulin. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 21 Total attrition in trial: 0 Treatment diet Participants randomised: 11 Participants withdrawn (voluntary): 0 Total attrition: 0 Control diet: Participants randomised: 10 Participants withdrawn (voluntary): 0 Total attrition: 0 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 42.35 (8.53) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 87.79 (11.28) BMI (kg/m2): mean (SD) 32.69 (1.72) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 2.98 (0.78) HDL (mmol/L): mean (SD) 1.16 (0.28) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.93 (0.97) TG (mmol/L): mean (SD) 1.70 (0.76) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 40.82 (9.08) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 84.72 (5.74) BMI (kg/m2): mean (SD) 31.68 (2.29) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 2.94 (0.80) HDL (mmol/L): mean (SD) 1.25 (0.41) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.84 (1.41) TG (mmol/L): mean (SD) 1.48 (0.89) Group differences at baseline: no Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Meals were prepared and provided to participants. Baseline characteristics reported per trial arm and IR/IS subgroup. These data were combined. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐carbohydrate high‐fat (LC/HF) diet, comprising 40% carbohydrate, 40% fat and 20% protein. Energy deficit of 400 kcal/day Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐balanced Exercise component? No Recipients: N = 11 women with a mean (SD) age of 42.35 (8.53), mean (SD) weight 87.79 (11.28) kg, mean (SD) BMI 32.69 (1.72) kg/m2, mean (SD) total cholesterol 190.36 (37.62) mg/dL, mean (SD) HDL 44.73 (10.66) mg/dL, mean (SD) LDL 115 (30.04) mg/dL, mean (SD) triglycerides 150.64 (67.59) mg/dL who were either insulin sensitive or insulin resistant as per fasting insulin levels Why? "We hypothesized that if in the insulin‐sensitive (IS) individuals, insulin promotes better use of dietary CHOs, perhaps through increased dietary induced and/or cellular thermogenesis compared with the IR individuals, we might observe a greater weight loss in the IS group on a high‐CHO (HC) hypocaloric diet." What (materials)? All food was prepared and provided by the hospital kitchen. Participants picked up their diet every 3 days but ate the majority of the food at home. What (procedures)? Participants were prescribed a hypocaloric diet (400 kcal deficit/d) comprised of 40% CHO, 40% fat, and 20% protein for 16 weeks. The polyunsaturated to monounsaturated to saturated fatty acid ratio (1:1:1) and fiber and cholesterol content of the diets were identical in both diets. The subjects were otherwise free‐living and were expected not to consume food outside of the diet but could have eaten food in addition to or other than the diet. Subjects were asked to maintain their usual activity pattern and were regularly questioned regarding activity. Estimates of daily energy intake were made using 3‐day food diary, 3‐day control diet, and baseline RMR plus an activity factor. Who provided? Dietitian How and where? Face‐to‐face at the General Clinical Research Center (GCRC) at the University of Colorado Hospital When and how much? Daily food was provided which was made in the kitchen and collected by participants for 16 weeks. Subjects were asked to maintain their usual activity pattern and were regularly questioned regarding activity. Once a week, subjects were weighed and met with a dietitian to determine compliance. Strategies to improve or maintain fidelity; tailoring and modification: Weekly assessments by dietitian, food prepared by the trial kitchen Extent of intervention fidelity: NR Concomitant interventions: NR Control diet: Name (as reported) and brief description: High‐carbohydrate low‐fat (HC/LF) diet, comprising 60% carbohydrate, 20% fat and 20% protein. Energy deficit of 400 kcal/day Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 10 women with a mean (SD) age of 40.82 (9.08), mean (SD) weight 84.72 (5.74) kg, mean (SD) BMI 31.68 (2.29) kg/m2, mean (SD) total cholesterol 186.8(54.31) mg/dL, mean (SD) HDL 48.2 (15.81) mg/dL, mean (SD) LDL 113.6 (30.99) mg/dL, mean (SD) triglycerides 131.2 (78.89) mg/dL who were either insulin sensitive or insulin resistant as per fasting insulin levels Why? "...IR individuals might display a lesser response to an HC diet and respond better to an LC hypocaloric diet." What (materials)? All food was prepared and provided by the hospital kitchen. Participants picked up their diet every 3 days but ate the majority of the food at home. What (procedures)? Participants received a hypocaloric diet (400 kcal deficit/d) comprised of 60% CHO, 20% fat, and 20% protein for 16 weeks. The polyunsaturated to monounsaturated to saturated fatty acid ratio (1:1:1) and fiber and cholesterol content of the diets were identical in both diets. The subjects were otherwise free‐living and were expected not to consume food outside of the diet but could have eaten food in addition to or other than the diet. Subjects were asked to maintain their usual activity pattern and were regularly questioned regarding activity. Estimates of daily energy intake were made using 3‐day food diary, 3‐day control diet, and baseline RMR plus an activity factor. Who provided? Dietitian How and where? Face‐to‐face at the General Clinical Research Center (GCRC) at the University of Colorado Hospital When and how much? Daily food was provided which was made in the kitchen and collected by participants for 16 weeks. Subjects were asked to maintain their usual activity pattern and were regularly questioned regarding activity. Once a week, subjects were weighed and met with a dietitian to determine compliance. Strategies to improve or maintain fidelity; tailoring and modification: Weekly assessments by dietitian, food prepared by the trial kitchen Extent of intervention fidelity: NR Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: CGRC grant M01 RR00051 and Clinical Nutrition Research Unit grant DK48520; indirect support from the Veterans Administration Research Service, American Diabetes Association, American Heart Association and the National Center for Research Resources (grant RR16185) Declaration of interest: NR |
|
Dyson 2007.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1, 2, 3, 4 Study design: RCT, parallel‐group, number of centres NR Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: Yes Outcome(s) used for sample size calculation: Weight change Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 3 months What was the duration of the weight maintenance phase: 21 months Other notes about methods: NA |
|
| Participants |
Country and setting: UK, outpatient diabetes, endocrinology and metabolism research unit in Oxford Eligibility criteria: Participants over 18 years of age with BMI > 25 kg/m2 who do not have type 2 diabetes, or have type 2 diabetes treated by diet or metformin monotherapy. Participants were excluded if they had type 1 or type 2 diabetes treated with insulin, sulphonylurea or thiazolidinedione; were pregnant, of childbearing age without contraception or were breastfeeding; had major psychiatric disease including eating disorders; had a history of alcohol or drug abuse, abnormal liver function, known malignancy or serum creatinine level above 150 μmol/L. Type 2 diabetes at baseline: Mixed; included persons with or without T2DM; at baseline 13/26 included did not have T2DM and 13/26 had T2DM; eligible outcome data was reported separately for participants with and without T2DM and was included in the appropriate comparisons where possible. Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 26 Total attrition in trial: 4 Treatment diet Participants randomised: 12 Participants withdrawn (voluntary): 0 Total attrition: 0 Control diet: Participants randomised: 14 Participants withdrawn (voluntary): 4 Total attrition: 4 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 55 (5) Gender distribution (as reported): female 83%, male 17% Weight (kg): mean (SD) 95.6 (16.7) BMI (kg/m2): mean (SD) 35.1 (6.8) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): mean (SD) 6.7 (1.3) LDL (mmol/L): mean (SD) 3.1 (1.1) HDL (mmol/L): mean (SD) 1.28 (0.44) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.1 (1.3) TG (mmol/L): mean (SD) 1.55 (1.01, 2.35) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 50 (12) Gender distribution (as reported): female 64%, male 36% Weight (kg): mean (SD) 97.0 (17.2) BMI (kg/m2): mean (SD) 35.0 (7.4) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): mean (SD) 6.6 (1.0) LDL (mmol/L): mean (SD) 3.1 (0.8) HDL (mmol/L): mean (SD) 1.37 (0.33) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.1 (0.8) TG (mmol/L): mean (SD) 1.12 (0.74, 1.72) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Triglycerides are geometric means. Discrepancy in reported percentage of male participants for the total group (25% in Dyson et al 2010, but 23% in Dyson et al 2007, the latter did not appear correct when looking at percentage per trial arm). |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low carbohydrate diet: carbohydrates 40 g⁄day, with a deficit of 500 kcal/d and physical activity for 30 min at least 5 days per week. Treatment diet type (carbohydrate‐fat‐protein): Very low‐unclear‐unclear Exercise component? Yes Recipients: Subjects were 17% male, aged mean (SD) 55 (5), with BMI mean (SD) 35.1 (6.8) and weight 95.6 (16.7) kgs Why? Low‐carbohydrate diets are effective for weight reduction in people without diabetes, but there is limited evidence for people with Type 2 diabetes. What (materials)? Recipe booklet What (procedures)? Low‐carbohydrate diet (40 g ⁄ day). All subjects were encouraged to increase physical activity and advised to exercise at moderate intensity for 30 min at least 5 and preferably 7 days per week. Who provided? Dietitian How and where? NR When and how much? 3 months of dietary intervention Strategies to improve or maintain fidelity; tailoring and modification: NR Extent of intervention fidelity: Significant reduction in energy intake in both groups, and a greater reduction in calories in the low‐carbohydrate group (949 vs. 515 kcal/day, P = 0.036). There were no differences in changes in absolute protein and fat intakes between the two groups, but there was a highly significant reduction in carbohydrate intake in the low‐carbohydrate group to 56.8 g/day. This reduction in carbohydrate intake was reflected in significant changes in %energy from macronutrients. Concomitant interventions: Hypoglycemics Control diet: Name (as reported) and brief description: Low‐fat diet following the nutritional recommendations of Diabetes UK, with a deficit of 500 kcal/d and physical activity for 30 min at least 5 days per week Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: Subjects were 36% male, aged mean (SD) 50 (12), with BMI mean (SD) 35.0 (7.4) and weight 97.0 (17.2) kgs Why? NR What (materials)? NR What (procedures)? Low‐fat diet (healthy eating advice). All subjects were encouraged to increase physical activity and advised to exercise at moderate intensity for 30 min at least 5 and preferably 7 days per week. Who provided? Dietitian How and where? NR When and how much? 3 month of dietary intervention Strategies to improve or maintain fidelity; tailoring and modification: NR Extent of intervention fidelity: Significant reduction in energy intake in both groups, and a greater reduction in calories in the low‐carbohydrate group (949 vs. 515 kcal/day, P = 0.036). There were no differences in changes in absolute protein and fat intakes between the two groups, but there was a highly significant reduction in carbohydrate intake in the low‐carbohydrate group to 56.8 g/day. This reduction in carbohydrate intake was reflected in significant changes in %energy from macronutrients. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: Yes Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): letter Trial acronym/name: None Trial funded by: Medisense UK and Abbott Laboratories Declaration of interest: "DRM and PAD have received research funding from the Sugar Bureau. SB has no competing interests to declare." |
|
Ebbeling 2007.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel‐group, single‐centre (1) Trial registry number: NCT00130299 Total number of trial arms: 2 Year trial started: 2004 Sample size calculation: Yes Outcome(s) used for sample size calculation: Body fat percentage Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 18 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient hospital in Boston Eligibility criteria: Participants aged between 18 and 35 years with BMI ≥ 30 kg/m2 with medical clearance from a primary care provider. Participants were excluded if they weighed more than 140 kg; were current smokers; had recently followed a weight loss diet; used medications which could affect outcomes; had diabetes (defined as fasting plasma glucose ≥ 126 mg/dL) or any other major illness as assessed by medical history or laboratory screening tests. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 73 Total attrition in trial: 22 Treatment diet Participants randomised: 36 Participants withdrawn (voluntary): NR Total attrition: 8 Control diet: Participants randomised: 37 Participants withdrawn (voluntary): NR Total attrition: 14 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 28.2 (3.8) Gender distribution (as reported): female 29/36 (81%), male 7/36 (19%) Weight (kg): mean (SD) 103.5 (17.3) BMI (kg/m2): NR DBP (mmHg): mean (SD) 63 (8) SBP (mmHg): mean (SD) 105 (12) HbA1c (%): NR LDL (mmol/L): mean (SD) 2.64 (0.91) HDL (mmol/L): mean (SD) 1.48 (0.52) Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): mean (SD) 1.27 (1.08) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 26.9 (4.2) Gender distribution (as reported): female 29/37 (78%), male 8/37 (22%) Weight (kg): mean (SD) 103.3 (15.1) BMI (kg/m2): NR DBP (mmHg): mean (SD) 62 (9) SBP (mmHg): mean (SD) 108 (11) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.26 (0.88) HDL (mmol/L): mean (SD) 1.40 (0.34) Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): mean (SD) 1.42 (0.92) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: LDL (P = 0.005); intervention vs control Other notes about participants: Triglyceride concentrations were skewed and assessed with Mann‐Whitney‐Wilcoxon test. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Unrestricted/ad libitum prescription in both diets Treatment diet: Name (as reported) and brief description: Low‐glycemic load diet. The target macronutrient composition was 40% of energy from carbohydrate, emphasizing low–glycemic index sources, 35% from fat, and 25% from protein. Physical activity recommendations based on public health guidelines. Diet was prescribed using an ad libitum approach. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: N = 7 (19%) male, N = 29 (81%) female with overall mean (SD) age 28.2 (3.8) years, mean (SD) weight 103.5 (17.3) kg, mean (SD) LDL 102 (35) mg/dL, mean (SD) HDL 57 (20) mg/dL, mean (SD) triglycerides 112 (96) mg/dL, mean (SD) systolic BP 105 (12) mmHg and mean (SD) diastolic BP 63 (8) mmHg Why? "Diets with a high glycemic load (the mathematical product of the glycemic index and the carbohydrate amount) result in higher postprandial insulin concentration, calorie for calorie, than those with a low glycemic load." Individuals with a high insulin response to glucose may be most sensitive to the effects of glycemic load. The purpose of this study was to determine whether insulin secretion affects body fat loss among obese individuals consuming self‐prepared diets. What (materials)? "Participants were equipped with food‐choice lists that delineated products into low–,moderate–, and high–glycemic load categories. Registered dietitians provided information during cooking demonstrations to encourage consumption of low–glycemic load foods and led interactive activities using food models to define appropriate serving sizes of high–glycemic load foods (e.g. refined grain products, sweets)." What (procedures)? Participants were counselled to consume low–glycemic load foods (particularly nonstarchy vegetables, legumes, and temperate fruits) and to limit intake of high–glycemic load foods (such as refined grains, starchy vegetables, fruit juices, and sweets). Attention also was directed toward consuming sources of healthful fat including nuts, seeds, and oils. The target macronutrient composition was 40% of energy from carbohydrate, emphasising low–glycemic index sources, 35% from fat, and 25% from protein. Nutrition education and dietary counselling were provided to participants. Registered dietitians provided information during cooking demonstrations to encourage consumption of low‐glycemic load foods and led interactive activities using food models to define appropriate serving sizes. 23 group workshops and 1 private counselling session each one for an hour as well as 5 motivational telephone calls for 30 mins each were conducted. Six of the group workshops were scheduled during the first 2 months of the intervention period, and the remaining workshops were held on a monthly basis thereafter. The private session occurred during the initial month, and a telephone call was scheduled for each of the subsequent 5 months of the intensive intervention period. Participants were asked to keep one 3‐day food diary prior to each workshop, particularly during the intensive intervention period. Three unannounced telephone‐administered 24‐hour recall interviews were conducted at baseline and again at 6, 12, and 18 months to assess diet and physical activity. Who provided? Registered dietitian How and where? Face‐to‐face group sessions as well as private sessions, location NR When and how much? "There were 23 group workshops (1 hour each), 1 private counseling session (1 hour), and 5 motivational telephone calls (30 minutes each). Six of the group workshops were scheduled during the first 2 months of the intervention period, and the remaining workshops were held on a monthly basis thereafter. The private session occurred during the initial month, and a telephone call was scheduled for each of the subsequent 5 months of the intensive intervention period." Strategies to improve or maintain fidelity; tailoring and modification: "Principles of nonformal adult education and participant centered counseling were applied to promote adherence to the diets. Respectful consideration of participant perspectives, core values, life experiences, current circumstances, and available resources formed a foundation for education and counseling. Participants were asked to keep a food diary (prior to each workshop). Dietitians prepared written feedback on submitted diaries, highlighting successes and providing advice for correcting deviations from diet prescriptions. Several strategies were used to maximize treatment fidelity. First, group workshops were scripted and written educational materials were developed to ensure delivery of well‐defined nutrition messages for each diet group; otherwise, the format of the workshops and quality of the materials were completely parallel to maintain equal treatment intensity. Second, flowcharts provided structure for the private session and motivational telephone calls and were used to foster dietitian adherence to a participant centered counseling model, with adequate flexibility for addressing situations unique to each individual. Prompts for open‐ended questions were included in the flowcharts to enhance dialogue. The private session and telephone calls were digitally recorded, such that the study director and lead dietitian could monitor deviations from the protocol and provide feedback to dietitians as necessary. The duration of the private session and each telephone call also was monitored as an indicator of integrity with regard to treatment intensity. Third, weekly staff meetings provided an opportunity for continued discussion on intervention delivery, particularly strategies for assisting individual participants without compromising differentiation between diets. Fourth, dietitians were given detailed guidelines for providing written feedback on food diaries to avoid unintended overlap in dietary advice between groups. Participant adherence was evaluated based on attendance at group workshops and private sessions, completion of motivational telephone called and self‐reported dietary intake. In addition, data were obtained in regard to physical activity and participant satisfaction with program." Extent of intervention fidelity: For the low–glycemic load group, glycemic index and carbohydrate intake decreased, producing a significant mean (SE) decrease in glycemic load (–19.8 [2.5] g/1000 kcal; P = 0.001). Total dietary fat increased (mean [SE], 3.0% [1.3%] of energy; P = 0.02) whereas saturated fat did not change (mean [SE], 0.5% [0.6%] of energy; P = 0.36). Treatment intensity did not differ between diet groups (P > 0.40). On average, participants attended a mean (SE) of 13.4 (0.7) of the 23 scheduled workshops and completed 5.4 (0.2) of the 6 planned individual contacts with a registered dietitian. They provided food diaries on a mean (SE) of 5.6 (0.3) occasions during the 6‐month intensive intervention period. Satisfaction with the programme also did not differ between groups. Concomitant interventions: NR Control diet: Name (as reported) and brief description: Low‐fat diet. The target macronutrient composition was 55% of energy from carbohydrate, 20% from fat, and 25% from protein. Physical activity recommendations based on public health guidelines. Diet was prescribed using an ad libitum approach Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐high Exercise component? Yes Recipients: N = 8 (22%) male, N = 29 (78%) female with overall mean (SD) age 26.9 (4.2) years, mean (SD) weight 103.3 (15.1) kg, mean (SD) LDL 126 (34) mg/dL, mean (SD) HDL 54 (13) mg/dL, mean (SD) triglycerides 126 (81) mg/dL, mean (SD) systolic BP 108 (11) mmHg and mean (SD) diastolic BP 62 (9) mmHg Why? NR What (materials)? "Participants were equipped with food‐choice lists that delineated products into low–,moderate–, and high–glycemic load categories. Registered dietitians provided information during cooking demonstrations to encourage consumption of low–glycemic load foods and led interactive activities using food models to define appropriate serving sizes of high–glycemic load foods (e.g. refined grain products, sweets)." What (procedures)? Nutrition education and dietary counselling were provided to participants. Control participants were counselled to consume low‐fat grains, vegetables, fruits and legumes and to limit intake of added fats, sweets and high‐fat snacks. Registered dietitians provided information during cooking demonstrations to encourage consumption of low‐glycemic load foods and led interactive activities using food models to define appropriate serving sizes. The target macronutrient composition was 55% of energy from carbohydrate, 20% from fat, and 25% from protein. Counselling, cooking demonstrations and interactive activities using food models to define appropriate serving sizes of low‐fat foods and led interactive activities using food models to define appropriate serving sizes of high‐fat foods (e.g. butter) and sweets. 23 group workshops and 1 private counselling session each one for an hour as well as 5 motivational telephone calls for 30 mins each were conducted. Six of the group workshops were scheduled during the first 2 months of the intervention period, and the remaining workshops were held on a monthly basis thereafter. The private session occurred during the initial month, and a telephone call was scheduled for each of the subsequent 5 months of the intensive intervention period. Participants were asked to keep one 3‐day food diary prior to each workshop, particularly during the intensive intervention period. Three unannounced telephone administered 24‐hour recall interviews were conducted at baseline and again at 6, 12, and 18 months to assess diet and physical activity. Who provided? Registered dietitian How and where? Face‐to‐face group sessions as well as private sessions, location NR When and how much? "There were 23 group workshops (1 hour each), 1 private counseling session (1 hour), and 5 motivational telephone calls (30 minutes each). Six of the group workshops were scheduled during the first 2 months of the intervention period, and the remaining workshops were held on a monthly basis thereafter. The private session occurred during the initial month, and a telephone call was scheduled for each of the subsequent 5 months of the intensive intervention period." Strategies to improve or maintain fidelity; tailoring and modification: "Principles of nonformal adult education and participant centered counseling were applied to promote adherence to the diets. Respectful consideration of participant perspectives, core values, life experiences, current circumstances, and available resources formed a foundation for education and counseling. Participants were asked to keep a food diary (prior to each workshop). Dietitians prepared written feedback on submitted diaries, highlighting successes and providing advice for correcting deviations from diet prescriptions. Several strategies were used to maximize treatment fidelity. First, group workshops were scripted and written educational materials were developed to ensure delivery of well‐defined nutrition messages for each diet group; otherwise, the format of the workshops and quality of the materials were completely parallel to maintain equal treatment intensity. Second, flowcharts provided structure for the private session and motivational telephone calls and were used to foster dietitian adherence to a participant centered counseling model, with adequate flexibility for addressing situations unique to each individual. Prompts for open‐ended questions were included in the flowcharts to enhance dialogue. The private session and telephone calls were digitally recorded, such that the study director and lead dietitian could monitor deviations from the protocol and provide feedback to dietitians as necessary. The duration of the private session and each telephone call also was monitored as an indicator of integrity with regard to treatment intensity. Third, weekly staff meetings provided an opportunity for continued discussion on intervention delivery, particularly strategies for assisting individual participants without compromising differentiation between diets. Fourth, dietitians were given detailed guidelines for providing written feedback on food diaries to avoid unintended overlap in dietary advice between groups. Participant adherence was evaluated based on attendance at group workshops and private sessions, completion of motivational telephone called and self‐reported dietary intake. In addition, data were obtained in regard to physical activity and participant satisfaction with program." Extent of intervention fidelity: For the low‐fat group, total fat intake decreased (mean [SE], –10.8% [1.3%] of energy; P = 0.001), as did saturated fat intake (mean [SE],–4.5% [0.6%] of energy; P = 0.001). Carbohydrate intake increased, producing an increase in glycemic load (mean [SE], 5.0 [2.5] g/1000 kcal; P = 0.05) even though glycemic index decreased slightly. Treatment intensity did not differ between diet groups (P > 0.40). On average, participants attended a mean (SE) of 13.4 (0.7) of the 23 scheduled workshops and completed 5.4 (0.2) of the 6 planned individual contacts with a registered dietitian. They provided food diaries on a mean (SE) of 5.6 (0.3) occasions during the 6‐month intensive intervention period. Satisfaction with the program also did not differ between groups. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: National Institute of Diabetes and Digestive and Kidney Diseases (grant R01 DK59240), the Charles H. Hood Foundation and support for the General Clinical Research Center at Children's Hospital from the National Center for Research Resources (grant M01 RR02172) Declaration of interest: "Dr Ludwig reported being the author of a book on childhood obesity (Ending the Food Fight: Guide Your Child to a Healthy Weight in a Fast Food/Fake Food World). No other authors reported disclosures." |
|
Elhayany 2010.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, multicentre (10) Trial registry number: NCT00520182 Total number of trial arms: 3 Year trial started: 2003 Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): 2 What was the duration of the weight loss phase: 12 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Israel, urban outpatient primary care clinics in the central district Eligibility criteria: Participants aged 30 to 65 years with BMI 27 to 34 kg/m2, diagnosis of type 2 diabetes within the past one to 10 years, last values of 7 to 10% for HbA1c, 1.8 to 4.5 mmol/L for plasma triglycerides, < 123.2 μmol/L for serum creatinine and no change in diabetes medication in the three months prior. Participants were excluded if they had proliferative diabetic retinopathy, active cancer or psychiatric disease, current insulin treatment or uncontrolled hypo‐ or hyperthyroidism. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 259 Total attrition in trial: 80 Treatment diet Participants randomised: 85 Participants withdrawn (voluntary): 6 Total attrition: 24 Control diet: Participants randomised: 89 Participants withdrawn (voluntary): 1 Total attrition: 26 Baseline data treatment diet: Randomised participants not included: 24/85 Age (years): mean (SD) 55.5 (6.5) Gender distribution (as reported): female 30/61 (49.2%), male 31/61 (50.8%) Weight (kg): mean (SD) 86.7 (14.3) BMI (kg/m2): mean (SD) 31.4 (2.8) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): mean (SD) 8.3 (1.0) LDL (mmol/L): mean (SD) 3.1 (0.8) HDL (mmol/L): mean (SD) 1.1 (0.2) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.4 (0.9) TG (mmol/L): mean (SD) 3.2 (0.8) Baseline data control diet: Randomised participants not included: 26/89 Age (years): mean (SD) 57.4 (6.1) Gender distribution (as reported): female 28/63 (44.4%), male 35/63 (55.6%) Weight (kg): mean (SD) 85.5 (10.6) BMI (kg/m2): mean (SD) 31.1 (2.8) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): mean (SD) 8.3 (1.0) LDL (mmol/L): mean (SD) 3.2 (0.8) HDL (mmol/L): mean (SD) 1.1 (0.2) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.5 (0.8) TG (mmol/L): mean (SD) 3.0 (0.7) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐carbohydrate Mediterranean (LCM); 35% carbohdyrates (30 g fibre), 45% fat (23% MUFA, 15% PUFA and 7% SFA) and 20% protein. Physical activity three times a week for 30 to 45 min. Calories prescribed as 20/kg body weight Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐balanced Exercise component? Yes Recipients: 30 (49.2%) females and 31 (50.8%) males with mean (SD) age of 55.5 (6.5) years, mean (SD) years since DM diagnosis 5.5 (3.8), mean (SD) weight 86.7 (14.3) kg, mean (SD) BMI 31.4 (2.8) kg/m2, mean (SD) HbA1c 8.3 (1.0) %, mean (SD) fasting plasma glucose 10.5 (2.0) mmol/L, mean (SD) total cholesterol 5.4 (0.9) mmol/L, mean (SD) HDL 1.1 (0.2) mmol/L, mean (SD) LDL 3.1 (0.8) mmol/L, mean (SD) triglycerides 3.2 (0.8) mmol/L Why? The relative importance of glycaemic index and of glycaemic load as well as the relative advantages of higher fat diets and the type of dietary fat vs. diets with high carbohydrate content are unclear. What (materials)? NR What (procedures)? All dietitians followed a structured protocol for the scheduled meetings. The LCM diet included only LGI carbohydrates. The diet provided 35% carbohydrate and 45% fat from total energy. Participants were counselled to eat 4–6 meals/day according to their lifestyle. All patients were advised to engage in 30–45 min of aerobic activity at least 3 days a week. Who provided? Dietitians. All the dietitians participated in a training workshop to ensure standardisation in questionnaire administration. How and where? Face‐to‐face meetings in community setting When and how much? Every two weeks for one year (24 meetings) Strategies to improve or maintain fidelity; tailoring and modification: Participants completed a 24‐h food recall questionnaire, a validated food frequency questionnaire (FFQ) and a physical activity questionnaire that included quality of life measures, at baseline, 3 and 6 months. Dietary adherence only assessed from the dietary data collected at 6 months Extent of intervention fidelity: 13 participants excluded during follow‐up due to non‐compliance. The mean reported energy intake was similar in the three diets (2221.6 calories ± 1086.6). Concomitant interventions: NR Control diet: Name (as reported) and brief description: Traditional Mediterranean (TM); 50% carbohdyrates (30 g fibre), 30% fat (10% MUFA, 12% PUFA and 7% SFA) and 20% protein. Physical activity three times a week for 30 to 45 min. Calories prescribed as 20/kg body weight Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: 28 (44.4%) females and 35 (55.6%) males with mean (SD) age 57.4 (6.1) years, mean (SD) years since DM diagnosis 6.2 (9.9), mean (SD) weight 85.5 (10.6) kg, mean (SD) BMI 31.1 (2.8) kg/m2, mean (SD) HbA1c 8.3 (1.0) %, mean (SD) fasting plasma glucose 10.1 (1.8) mmol/L, mean (SD) total cholesterol 5.5 (0.8) mmol/L, mean (SD) HDL 1.1 (0.2) mmol/L, mean (SD) LDL 3.2 (0.8) mmol/L, mean (SD) triglycerides 3.0 (0.7) mmol/L Why? The relative importance of glycaemic index and of glycaemic load as well as the relative advantages of higher fat diets and the type of dietary fat vs. diets with high carbohydrate content are unclear. What (materials)? NR What (procedures)? All dietitians followed a structured protocol for the scheduled meetings. The TM diet included only LGI carbohydrates. Prescribed TM diet with carbohydrates (50–55%), fat (30%) and protein (15–20%) where carbohydrate sources come from low‐glycemic index foods. Participants were counselled to eat 4–6 meals/day according to their lifestyle. All patients were advised to engage in 30–45 min of aerobic activity at least 3 days a week. Who provided? Dietitians. All the dietitians participated in a training workshop to ensure standardisation in questionnaire administration. How and where? Face‐to‐face meetings in community setting When and how much? Every two weeks for one year (24 meetings) Strategies to improve or maintain fidelity; tailoring and modification: Participants completed a 24‐h food recall questionnaire, a validated food frequency questionnaire (FFQ) and a physical activity questionnaire that included quality of life measures, at baseline, 3 and 6 months. Dietary adherence only assessed from the dietary data collected at 6 months Extent of intervention fidelity: 11 participants excluded during follow‐up due to non‐compliance. The mean reported energy intake was similar in the three diets (2221.6 calories ± 1086.6). Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: No Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: Yes Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: DIPAC Trial funded by: NR Declaration of interest: NR Author contacted, but requested information not provided. |
|
Evangelista 2021.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, multicentre (2) Trial registry number: NCT01423266 Total number of trial arms: 2 Year trial started: 2009 Sample size calculation: Yes Outcome(s) used for sample size calculation: Fourteen variables, including body mass index Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 3 months What was the duration of the weight maintenance phase: 1 year Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient university‐affiliated medical centres in California Eligibility criteria: Participants with chronic heart failure, a BMI ≥ 27 kg/m2, and a history of diabetes mellitus or documented impaired fasting glucose (IFG) of 100 to 125 mg/dL; alternatively, a history of metabolic syndrome (at least three of the four criteria) and optimisation of medical therapy. Participants were excluded if they were 18 years or younger; had a histroy of clinically significant illness including acute myocardial infarction or sustained ventricular arrhythmia in the last three months, or current liver, respiratory or gastrointestinal disease or malignancy; were pregnant or lactating; had a serum creatinine level above 1.5 mg/dL; were currently participating in a weight loss program; their physician refused to permit participation; experienced weight loss greater than 6% in the past 6 months; or had gout, or a history of gout Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: Mixed Cardiovascular conditions/risk factors/events at baseline: Yes Gender: Mixed Total number randomised: 90 Total attrition in trial: 14 Treatment diet Participants randomised: 45 Participants withdrawn (voluntary): NR Total attrition: 12 Control diet: Participants randomised: 45 Participants withdrawn (voluntary): NR Total attrition: 2 Baseline data treatment diet: Randomised participants not included: 12 Age (years): mean (SD) 57.3 (10.1) Gender distribution (as reported): female 7/33 (21%), male 26/33 (79%) Weight (kg): mean (SD) 105.5 (22.4) BMI (kg/m2): mean (SD) 36.2 (7.1) DBP (mmHg): mean (SD) 72.8 (9.6) SBP (mmHg): mean (SD) 123.3 (12.8) HbA1c (%): mean (SD) 7.2 (1.3) LDL (mmol/L): mean (SD) 2.21 (0.83) HDL (mmol/L): mean (SD) 0.98 (0.27) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.15 (1.09) TG (mmol/L): mean (SD) 1.84 (0.67) Baseline data control diet: Randomised participants not included: 2 Age (years): mean (SD) 58.0 (9.6) Gender distribution (as reported): female 14/43 (33%), male 29/43 (67%) Weight (kg): mean (SD) 109.9 (19.3) BMI (kg/m2): mean (SD) 37.3 (5.4) DBP (mmHg): mean (SD) 73.30 (1.0) SBP (mmHg): mean (SD) 116.8 (19.5) HbA1c (%): mean (SD) 7.3 (1.8) LDL (mmol/L): mean (SD) 2.43 (0.84) HDL (mmol/L): mean (SD) 1.04 (0.24) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.24 (1.02) TG (mmol/L): mean (SD) 1.74 (0.74) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein diet prescribing 30% protein (~110 g/day), 40% carbohydrates (150 g/day) and 30% fat (~50 g/day). Energy intake decreased by 500 and 800 kcal from daily energy requirements Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: N = 7 women and N = 26 men with mean (SD) age 57.3 (10.1) years, mean (SD) weight 105.5 (22.4) kg, mean (SD) BMI 36.2 (7.1) kg/m2, mean (SD) total cholesterol 160.2 (41.9) mg/dL, mean (SD) LDL‐cholesterol 85.5 (32.2) mg/dL, mean (SD) HDL‐cholesterol 37.9 (10.6) mg/dL, mean (SD) triglycerides 163.2 (59.2) mg/dL, mean (SD) diastolic blood pressure 72.8 (9.6) mmHg, mean (SD) systolic blood pressure 123.3 (12.8) mmHg, N = 33 (100%) had heart failure and diabetes mellitus/impaired fasting glucose, N = 15 (46%) had hypertension, N = 9 (27%) had coronary artery disease, and N = 12 (36%) were previous smokers. Why? Examining the role of a high‐protein diet consumed over 12 weeks in slowing the progression heart failure and diabetes mellitus What (materials)? Participants were prescribed a hypocaloric diet incorporating a 500 to 800 kcal deficit per day into their individualised energy requirements. This was done through the provision of a standard structured energy‐restricted meal plan. Participants were provided with a 'Participant Handbook' containing resources (such as lists of food items and serving sizes) and tools (such as weight charts, food checklists and three‐day food records). Participants were also provided with a pedometer. What (procedures)? The high‐protein diet consisted of 12 weeks following a diet with a macronutrient composition of 30% fat (~50 g/day), 40% carbohydrate (150 g/day) and 30% protein (~110 g/day). Participants were also encouraged to exercise regularly to promote weight loss, by engaging in 20 to 30 minutes of physical activity. Diet counselling sessions were provided. Who provided? A licenced dietitian provided the counselling sessions. How and where? Face‐to‐face counselling sessions were provided; the location of sessions was not reported. Sessions were on a one‐to‐one basis. Spouses, significant family members or friends were asked to participate in the sessions, if appropriate. When and how much? Counselling sessions were provided at visits at baseline, 2, 4, 8 and 12 weeks. The duration of sessions was between 45 to 60 minutes. The dietician provided detailed information on the meal plan and keeping daily food logs. Strategies to improve or maintain fidelity; tailoring and modification: Participants received a list of counselling appointments at baseline, and were reminded of sessions by phone call, text or email before each visit. The body weight, waist circumference and dietary intake of participants were reviewed at each visit; dietary adjustments were made if necessary. The dietitian reviewed completed dietary checklists and three‐day food records. The latter was done to clarify captured information, verify portions and obtain data that were incomplete. Pedometers were used by participants to monitor their own physical activity, changes in activity were also captured at each follow‐up visit. The authors also reported the following strategies to improve adherence: establishing a trusting relationship with participants; expressing appreciation for participation with cards and phone calls; providing easy access to investigators and research staff; providing culturally sensitive meal plans; paying for parking during visits; recognising potential barriers and helping to identify solutions; and enlisting family support. Early recognition of non‐adherence, identified through three‐day food records, prompted additonal education of participants and removal of obstacles. Extent of intervention fidelity: Three‐day food records reviewed at each visit were used to assess adherence. The authors reported "high adherence" as a strength of the study. Concomitant interventions: Of intervention participants; 82% were treated with ACE inhibitors, 21% with angiotensin receptor blockers, 88% with beta‐blockers, 70% with diuretics, 36% with digoxin, 33% with pain medication and 18% with antidepressants. Control diet: Name (as reported) and brief description: Standard‐protein diet prescribing 15% protein (~55 g/day), 55% carbohydrates (~200 g/day) and 30% fat (~50 g/day). Energy intake decreased by 500 and 800 kcal from daily energy requirements Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: N = 14 women and N = 29 men with mean (SD) age 58.0 (9.6) years, mean (SD) weight 109.9 (19.3) kg, mean (SD) BMI 37.3 (5.4) kg/m2, mean (SD) total cholesterol 163.7 (39.9) mg/dL, mean (SD) LDL‐cholesterol 94.0 (32.4) mg/dL, mean (SD) HDL‐cholesterol 40.0 (9.2) mg/dL, mean (SD) triglycerides 154.0 (65.1) mg/dL, mean (SD) diastolic blood pressure 73.30 (1.0) mmHg, mean (SD) systolic blood pressure 116.8 (19.5) mmHg, N = 43 (100%) had heart failure and diabetes mellitus/impaired fasting glucose, N = 20 (47%) had hypertension, N = 13 (30%) had coronary artery disease, and N = 19 (44%) were previous smokers. Why? Examining the role of a standard‐protein diet consumed over 12 weeks in the progression of heart failure and diabetes mellitus, compared to a diet higher in protein What (materials)? Participants were prescribed a hypocaloric diet incorporating a 500 to 800 kcal deficit per day into their individualised energy requirements. This was done through the provision of a standard structured energy‐restricted meal plan. Participants were provided with a 'Participant Handbook' containing resources (such as lists of food items and serving sizes) and tools (such as weight charts, food checklists and three‐day food records). Participants were also provided with a pedometer. What (procedures)? The standard‐protein diet consisted of 12 weeks following a diet with a macronutrient composition of 30% fat (~50 g/day), 55% carbohydrate (~200 g/day) and 15% protein (~55 g/day). Participants were also encouraged to exercise regularly to promote weight loss, by engaging in 20 to 30 minutes of physical activity. Personalised nutritional counselling sessions were provided. Who provided? A licenced dietitian provided the counselling sessions. How and where? Face‐to‐face counselling sessions were provided; the location of sessions was not reported. Sessions were on a one‐to‐one basis. Spouses, significant family members or friends were asked to participate in the sessions, if appropriate. When and how much? Counselling sessions were provided at visits at baseline, 2, 4, 8 and 12 weeks. The duration of sessions was between 45 to 60 minutes. The dietician provided detailed information on the meal plan and keeping daily food logs. Strategies to improve or maintain fidelity; tailoring and modification: Participants received a list of counselling appointments at baseline, and were reminded of sessions by phone call, text or email before each visit. The body weight, waist circumference and dietary intake of participants were reviewed at each visit; dietary adjustments were made if necessary. The dietitian reviewed completed dietary checklists and three‐day food records. The latter was done to clarify captured information, verify portions and obtain data that were incomplete. Pedometers were used by participants to monitor their own physical activity, changes in activity were also captured at each follow‐up visit. The authors also reported the following strategies to improve adherence: establishing a trusting relationship with participants; expressing appreciation for participation with cards and phone calls; providing easy access to investigators and research staff; providing culturally sensitive meal plans; paying for parking during visits; recognising potential barriers and helping to identify solutions; and enlisting family support. Early recognition of non‐adherence, identified through three‐day food records, prompted additonal education of participants and removal of obstacles. Extent of intervention fidelity: Three‐day food records reviewed at each visit were used to assess adherence. The authors reported "high adherence" as a strength of the study. Concomitant interventions: Of control participants; 63% were treated with ACE inhibitors, 37% with angiotensin receptor blockers, 95% with beta‐blockers, 72% with diuretics, 42% with digoxin, 26% with pain medication and 19% with antidepressants. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: Pro‐HEART trial Trial funded by: National Heart, Lung, and Blood Institute (1R01HL093466), NIH/NCRR/NCATS through the University of California Irvine Institute for Clinical and Translational Science (grant number UL1TR000153) and NIH/NCRR/NCATS through the Universityof California, Los Angeles Clinical and Translational Science Institute (grant number UL1TR000124) Declaration of interest: "None declared." |
|
Farnsworth 2003.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1, 2 Study design: RCT, parallel, number of centres NR Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: 56 weeks Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely Australia based on authors and food suppliers Eligibility criteria: Participants were overweight or obese (BMI of 27 to 43 kg/m2) and aged 20 to 65 years, and had to have a fasting serum insulin concentration of > 12 mU/L. Participants were excluded if they had diabetes or proteinuria; a history of liver, unstable cardiovascular, respiratory or gastrointestinal disease or had a malignancy (or history thereof). Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 66 Total attrition in trial: 9 Treatment diet Participants randomised: 33 Participants withdrawn (voluntary): NR Total attrition: NR Control diet: Participants randomised: 33 Participants withdrawn (voluntary): NR Total attrition: NR Baseline data treatment diet: Randomised participants not included: 5/33 Age (years): mean (SD) 50.93 (11.43) Gender distribution (as reported): female 21/28 (75%), male 7/28 (25%) Weight (kg): mean (SD) 93.98 (13.54) BMI (kg/m2): mean (SD) 34.30 (3.82) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.77 (0.94) HDL (mmol/L): mean (SD) 0.98 (0.22) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.55 (1.12) TG (mmol/L): mean (SD) 1.88 (0.72) Baseline data control diet: Randomised participants not included: 4/33 Age (years): mean (SD) 50.12 (9.43) Gender distribution (as reported): female 22/29 (75.9%), male 7/29 (24.1%) Weight (kg): mean (SD) 93.39 (14.31) BMI (kg/m2): mean (SD) 33.82 (3.75) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.76 (0.82) HDL (mmol/L): mean (SD) 0.97 (0.25) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.59 (0.85) TG (mmol/L): mean (SD) 1.88 (0.60) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Number of participants not included in baseline characteristics for total cholesterol, LDL, HDL and triglycerides: intervention 5/33; control 5/33 |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High protein diet: 30% of energy as protein, 40% as carbohydrate, and 30% as fat 8% of energy as saturated, 12% as monounsaturated, and 5% as polyunsaturated fatty acids. Energy restriction of 6400 kJ per day on average Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: N = 7 men and N = 21 women, mean (SD) age 50.93 (11.43) years, mean (SD) weight 93.98 (13.54) kg, mean (SD) BMI 34.3 (3.82) kg/m2, with fasting insulin levels greater than 12 mU/L (mean 16 mU/L). Why? "There are no data on the effects of high‐protein diets on changes in bone resorption and blood pressure after moderate weight loss. HP diet would spare lean mass and result in greater decreases in fasting and postprandial insulin concentrations." What (materials)? The diets were prescriptive fixed‐menu plans, and subjects were supplied with key foods that made up 60% of their energy intake. The key foods supplied to both diet groups were preweighed meat and poultry, shortbread biscuits, canola margarine (Canola Lite; Meadow Lea Foods Ltd, Mascot, Australia), and high‐oleic acid sunflower oil (Sunola; Meadow Lea Foods Ltd). The HP diet group also received low‐fat (3% fat) cheese (Kraft Free; Kraft Foods Ltd, Melbourne, Australia) and skim milk powder. What (procedures)? Participants were prescribed a HP diet: 30% of energy as protein (= 110 g/d), 40% as carbohydrate, and 30% as fat for 16 weeks. The fatty acid profiles for each diet were matched (8% of energy as saturated, 12% as monounsaturated, and 5% as polyunsaturated fatty acids). Each subject completed weighed daily checklists of all foods consumed. Group training in the use of scales and in keeping food records was provided. Both dietary groups underwent 12 wks of energy restriction (roughly 30% restriction of total energy, or 6.4 MJ on average), followed by 4 wks of energy balance with the same macronutrient composition. Participants also recorded physical activity. Who provided? Dietitian How and where? The study was conducted on an outpatient basis, location NR. When and how much? 2‐wk intervals over 16 weeks according to the method described by Parker et al (25) Strategies to improve or maintain fidelity; tailoring and modification: Each subject completed weighed daily checklists of all foods consumed and was assessed by the same dietitian at 2‐wk intervals according to the method described by Parker et al Extent of intervention fidelity: As prescribed protein was higher and the carbohydrate was lower with the HP diet. Concomitant interventions: Anti‐hypertensive or lipid lowering medication and supplements Control diet: Name (as reported) and brief description: Standard protein diet: 15% of energy as protein, 55% as carbohydrate, and 30% as fat with 8% of energy as saturated, 12% as monounsaturated, and 5% as polyunsaturated fatty acids. Energy restriction of 6400 kJ per day on average Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 7 men and N = 22 women, mean (SD) age 50.12 (9.43) years, mean (SD) weight 93.39 (14.31) kg, mean (SD) BMI 33.82 (3.75) kg/m2, with fasting insulin levels greater than 12 mU/L ( mean 16 mU/L) Why? NR What (materials)? The diets were prescriptive fixed‐menu plans, and subjects were supplied with key foods that made up 60% of their energy intake. The key foods supplied to both diet groups were preweighed meat and poultry, shortbread biscuits, canola margarine (Canola Lite; Meadow Lea Foods Ltd, Mascot, Australia), and high‐oleic acid sunflower oil (Sunola; Meadow Lea Foods Ltd). The SP diet group received rice and rice noodles. What (procedures)? Participants were prescribed a SP diet: 15% of energy as protein (roughly 60 g/d), 55% as carbohydrate, and 30% as fat. The fatty acid profiles for each diet were matched (8% of energy as saturated, 12% as monounsaturated, and 5% as polyunsaturated fatty acids). Both dietary groups underwent 12 wks of energy restriction (roughly 30% restriction of total energy, or 6.4 MJ on average), followed by 4 wks of energy balance with the same macronutrient composition. A weighed daily checklist of all foods consumed was required and a dietitian assessed the diet individually every 2 weeks. Also, group training in the use of scales and in keeping food records was provided. Who provided? Dietitian How and where? The study was conducted on an outpatient basis, location NR. When and how much? 2‐wk intervals over 16 weeks according to the method described by Parker et al (25) Strategies to improve or maintain fidelity; tailoring and modification: Each subject completed weighed daily checklists of all foods consumed and was assessed by the same dietitian at 2‐wk intervals according to the method described by Parker et al Extent of intervention fidelity: NR Concomitant interventions: Anti‐hypertensive or lipid lowering medication and supplements |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: National Health and Medical Research grant 158012 and Dairy Research and Development Corporation grant CSHN10003 Declaration of interest: "None of the authors had financial or personal conflicts of interest." |
|
Foraker 2014.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT01559194 Total number of trial arms: 2 Year trial started: 2005 Sample size calculation: Yes Outcome(s) used for sample size calculation: Difference in weight loss Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 52 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient general clinical research centre in Columbus Eligibility criteria: Participants were overweight and obese premenopausal women aged 30 and above with BMI of 25 to 34 kg/m2; with no prior diagnosis of cancer (except nonmelanoma skin cancer) and medical clearance from their primary physician. Participants were excluded if they were pregnant or planning to become pregnant; were currently participating in a weight loss program; had medical conditions which preclude adherence or had uncontrolled existing medical conditions. Type 2 diabetes at baseline: Unclear; stratified with non‐T2DM since fasting insulin levels provided by author were mostly not indicative of diabetes. Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Female Total number randomised: 79 Total attrition in trial: 33 Treatment diet Participants randomised: 38 Participants withdrawn (voluntary): NR Total attrition: 19 Control diet: Participants randomised: 41 Participants withdrawn (voluntary): NR Total attrition: 14 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 41.9 (5.4) Gender distribution (as reported): female 100%, male 0% Weight (kg): NR BMI (kg/m2): mean (SD) 30.1 (2.6) DBP (mmHg): mean (SD) 74.0 (8.7) SBP (mmHg): mean (SD) 121.3 (11.0) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.09 (0.75) HDL (mmol/L): mean (SD) 1.42 (0.41) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.93 (0.87) TG (mmol/L): NR Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 40.9 (5.1) Gender distribution (as reported): female 100%, male 0% Weight (kg): NR BMI (kg/m2): mean (SD) 30.5 (2.9) DBP (mmHg): mean (SD) 75.1 (9.0) SBP (mmHg): mean (SD) 122.4 (14.7) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.28 (0.84) HDL (mmol/L): mean (SD) 1.33 (0.29) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.12 (0.93) TG (mmol/L): NR Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: DBP and SBP baseline data not available for all randomised participants: intervention 2/38; control 1/41 |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐carbohydrate diet comprising 40% carbohydrates, 30% protein and 30% fat with exercise (10,000 steps a day). Each participant provided with a calorie goal based on the Harris‐Benedict equation Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: Subjects were 100% female, aged mean (SD) 41.9 (5.4) years, BMI (SD) 30.1 (2.6). Why? We evaluated the effects of low‐fat and low‐carbohydrate weight‐loss diets on longitudinal measures of blood pressure and blood lipids among overweight or obese premenopausal women who were also advised to increase their level of physical activity. We hypothesised that we would observe favourable changes over time in blood pressure and blood lipids in both diet groups but that results would differ by level of adherence to the respective diet protocol and baseline presence of hypertension or hyperlipidemia. What (materials)? Digiwalker pedometer to count daily steps What (procedures)? LCD: 40% of total calories from carbohydrates, 30% from protein and 30% from fat plus physical activity. All participants were educated on colairic restriction and given personalised PA prescriptions. Each participant met with an RD at the university’s Clinic Research Centre (CRC) once per week for the first month of the study period, every three weeks during the second, third and fourth months of the study, and every 6 weeks for the remainder of the study, except weeks 34 and 52, which were clinic visits. All women received counselling regarding their respective calorie‐restricted diet, along with an individualised physical‐activity prescription to promote weight loss. If a participant couldn't make it, telephonic counselling sessions were arranged. Each participant was advised to walk 10,000 steps per day. Who provided? Dietitian How and where? Face‐to‐face individually or telephonically at the Clinical Research Centre When and how much? Weekly sessions for the first month, every 3 weeks for until month 4 then every 6 weeks Strategies to improve or maintain fidelity; tailoring and modification: If a participant was unable to attend a meeting with the RD, sessions were conducted by telephone. Adherence to the dietary interventions was assessed through 7‐day dietary recalls. Extent of intervention fidelity: Adherence to the dietary interventions was low for both arms (22% and 29% for LFD and LCD, respectively). Overall, participants were more compliant with the PA component of the intervention (66% and 61% among those randomised to the LFD and LCD arms, respectively). Concomitant interventions: NR Control diet: Name (as reported) and brief description: Low‐fat diet comprising 60% carbohydrates, 20% protein and 20% fat with exercise (10,000 steps a day). Each participant provided with a calorie goal based on the Harris‐Benedict equation Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: Subjects were 100% female, aged mean (SD) 40.9 (5.1) years, BMI (SD) 30.5 (2.9) Why? We evaluated the effects of low‐fat and low‐carbohydrate weight‐loss diets on longitudinal measures of blood pressure and blood lipids among overweight or obese premenopausal women who were also advised to increase their level of physical activity. We hypothesised that we would observe favourable changes over time in blood pressure and blood lipids in both diet groups but that results would differ by level of adherence to the respective diet protocol and baseline presence of hypertension or hyperlipidemia. What (materials)? Digiwalker pedometer to count daily steps. What (procedures)? LFD: 20% of total calories from fat, 20% from protein and 60% from carbohydrates plus physical activity. All participants were educated on colairic restriction and given personalised PA prescriptions. Each participant met with an RD at the university’s Clinic Research Centre (CRC) once per week for the first month of the study period, every three weeks during the second, third and fourth months of the study, and every 6 weeks for the remainder of the study, except weeks 34 and 52, which were clinic visits. All women received counselling regarding their respective calorie‐restricted diet, along with an individualised physical‐activity prescription to promote weight loss. If a participant couldn't make it, telephonic counselling sessions were arranged. Each participant was advised to walk 10,000 steps per day. Who provided? Dietitian How and where? Face‐to‐face individually or telephonically at the Clinical Research Centre When and how much? Weekly sessions for the first month, every 3 weeks for until month 4 then every 6 weeks Strategies to improve or maintain fidelity; tailoring and modification: If a participant was unable to attend a meeting with the RD, sessions were conducted by telephone. Adherence to the dietary interventions was assessed through 7‐day dietary recalls. Extent of intervention fidelity: Adherence to the dietary interventions was low for both arms (22% and 29% for LFD and LCD, respectively). Overall, participants were more compliant with the PA component of the intervention (66% and 61% among those randomised to the LFD and LCD arms, respectively). Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: LEAF Trial funded by: Breast Cancer Research Foundation and the National Center for Advancing Translational Sciences (grant 8UL1TR000090‐05) Declaration of interest: "No competing financial interests exist." |
|
Foster 2003.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, multicentre (number NR) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 months (only first 6 months eligible as low‐carbohydrate diet) What was the duration of the weight maintenance phase (as reported): NA Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely USA based on authors, funders and trial registry Eligibility criteria: Participants who were obese were included. Participants were excluded if they suffered from clinically significant illness (including diabetes); took lipid‐lowering medication or drugs that affect body weight; were pregnant or lactating. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 63 Total attrition in trial: 21 Treatment diet Participants randomised: 33 Participants withdrawn (voluntary): NR Total attrition: 9 Control diet: Participants randomised: 30 Participants withdrawn (voluntary): NR Total attrition: 12 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 44.0 (9.4) Gender distribution (as reported): female 21/33, male 12/33 Weight (kg): mean (SD) 98.7 (19.5) BMI (kg/m2): mean (SD) 33.9 (3.8) DBP (mmHg): mean (SD) 74.6 (8.5) SBP (mmHg): mean (SD) 120.5 (11.0) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.35 (0.78) HDL (mmol/L): mean (SD) 1.21 (0.29) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.19 (0.87) TG (mmol/L): mean (SD) 1.48 (1.29) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 44.2 (7.0) Gender distribution (as reported): female 22/30, male 8/30 Weight (kg): mean (SD) 98.3 (16.4) BMI (kg/m2): mean (SD) 34.4 (3.1) DBP (mmHg): mean (SD) 77.6 (10.8) SBP (mmHg): mean (SD) 123.3 (14.1) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.10 (0.78) HDL (mmol/L): mean (SD) 1.28 (0.32) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.02 (0.83) TG (mmol/L): mean (SD) 1.39 (0.93) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Carbohydrate prescription in intervention group was incremental; data only extracted up to 6 months. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Low‐carbohydrate, high‐protein, high‐fat (Atkins) ad libitum diet; carbohydrates limited to 20 g/d for the first two weeks and then gradually increased by 5 g/d per week until participants were within 2.2 to 4.5 kg from their goal weight [only low‐carbohydrate for the first 6 months]. Treatment diet type (carbohydrate‐fat‐protein): Very low8w to low20w‐unclear‐unclear Exercise component? No Recipients: Participants were 63.6% female, aged 44.0 (9.4), with BMI 33.9 (3.8) and weight 98.7 (19.5) kg. Why? Low‐carbohydrate, high‐protein and high‐fat diets are increasingly popular, and are promoted by many best‐selling books to promote weight loss and prevent risk factors for coronary heart disease in obese persons. What (materials)? 'Dr. Atkins' New Diet Revolution' book (Atkins 1998). What (procedures)? Participants were prescribed a limited carbohydrate diet with no restriction of fat and protein, with gradual increase of carbohydrate over time [only the first six months was eligible]. Participants receiving the LCD met individually with a registered dietitian before beginning the programme to review the central features of the diet. Who provided? Dietitian How and where? Individual face‐to‐face meetings, location NR When and how much? The duration of [eligible] intervention comparison was six months. An initial meeting at baseline with the dietitian reviewed the central features of the diet; follow‐up meetings with dietitians, to review dietary issues, were between 15 and 30 minutes long and occurred at three, six and 12 [ineligible] months. Strategies to improve or maintain fidelity; tailoring and modification: Face‐to‐face meetings with dietitians to review dietary issues, weigh‐ins at follow‐up visits Extent of intervention fidelity: Adherence was reported as poor, lack of statistical significance at 12 months was interpreted as a lack of adherence to the Atkins diet, but the comparison at 12 months was ineligible. Concomitant interventions: Daily multi‐vitamins Control diet: Name (as reported) and brief description: LEARN program for weight management, approximately 60% of calories from carbohydrates, 25% from fat and 15% from protein. Low‐calorie, 1200 to 1500 kcal per day for women and 1500 to 1800 kcal per day for men Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Participants were 73.3% female, aged 44.2 (7.0), with BMI 34.4 (3.1) and weight 98.3 (16.4) kg. Why? To assess whether a conventional diet is better in promoting weight loss and preventing risk factors for coronary heart disease in obese persons What (materials)? A copy of The LEARN Program for Weight Management 17 which provided 16 lessons covering various aspects of weight control What (procedures)? Participants were prescribed a high‐carbohydrate, low‐fat, low‐calorie diet and were instructed how to count calories. A 15‐ to 30‐minute session with a registered dietitian at 3, 6, and 12 months to review dietary issues. Participants receiving the HCD met individually with a registered dietitian before beginning the programme to review the components of a HCD and to receive instructions about calorie counting. Who provided? Dietitian How and where? Individual face‐to‐face meetings, location NR When and how much? The duration of [eligible] intervention comparison was six months. An initial meeting at baseline with the dietitian reviewed the components of the diet; follow‐up meetings with dietitians, to review dietary issues, were between 15 and 30 minutes long and occurred at three, six and 12 [ineligible] months. Strategies to improve or maintain fidelity; tailoring and modification: Face‐to‐face meetings with dietitians to review dietary issues, weigh‐ins at follow‐up visits Extent of intervention fidelity: Adherence was reported as poor. Concomitant interventions: Daily multi‐vitamins |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Grants number RR00036, RR00040, RR00051, AT1103, DK 37948, DK 56341, DK 48520, DK 42549, DK02703 and AT00058 from the National Institutes of Health Declaration of interest: "Dr. Foster reports having received consulting fees from Abbott Laboratories and HealtheTech and lecture fees from Abbott Laboratories and Roche Laboratories. Dr. Wyatt reports having received consulting fees from Ortho‐McNeil, USANA, and GlaxoSmithKline and lecture fees from Roche Laboratories, Abbott Laboratories, Slim‐Fast, and Ortho‐McNeil. Dr. Hill reports having received consulting fees from HealtheTech, Johnson & Johnson, Procter & Gamble, Coca‐Cola, and the International Life Sciences Institute; lecture fees from Abbott Laboratories, Roche Laboratories, and Kraft Foods; and grant support from M&M Mars, Procter & Gamble, and Abbott Laboratories. Dr. Szapary reports having received lecture fees from AstraZeneca and Kos Pharmaceuticals and grant support from AstraZeneca. Dr. Klein reports having received consulting fees from Roche Laboratories and HealtheTech, lecture fees from Ortho‐McNeil, and grants from GlaxoSmithKline and Regeneron." Author contacted, but requested information not provided. |
|
Foster 2010.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, multicentre (3) Trial registry number: NCT00143936 Total number of trial arms: 2 Year trial started: 2003 Sample size calculation: Yes Outcome(s) used for sample size calculation: Body weight, LDL Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 2 years (only first 6 months eligible as low‐carbohydrate diet) What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient academic medical centres in Denver, St. Louis and Philadelphia Eligibility criteria: Participants aged 18 to 65 years with a BMI of 30 to 40 kg/m2 and body weight < 136 kg. Participants were excluded if they had serious medical illness, e.g. diabetes; if they took lipid‐lowering medication or drugs that affect body weight; were pregnant or lactating or had blood pressures ≥ 140/90 mmHg. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 307 Total attrition in trial: 44 Treatment diet Participants randomised: 153 Participants withdrawn (voluntary): 5 Total attrition: 25 Control diet: Participants randomised: 154 Participants withdrawn (voluntary): 7 Total attrition: 19 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 46.2 (9.2) Gender distribution (as reported): female 103/153 (67%), male 50/153 (33%) Weight (kg): mean (SD) 103.3 (15.5) BMI (kg/m2): mean (SD) 36.1 (3.59) DBP (mmHg): mean (SD) 73.9 (9.4) SBP (mmHg): mean (SD) 124.3 (14.1) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.11 (0.67) HDL (mmol/L): mean (SD) 1.20 (0.35) Non‐HDL (mmol/L): mean (SD) 3.68 (0.75) TC (mmol/L): mean (SD) 4.88 (0.78) TG (mmol/L): mean (SD) 1.28 (0.62) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 44.9 (10.2) Gender distribution (as reported): female 105/154 (68%), male 49/154 (32%) Weight (kg): mean (SD) 103.5 (14.4) BMI (kg/m2): mean (SD) 36.1 (3.46) DBP (mmHg): mean (SD) 76 (9.7) SBP (mmHg): mean (SD) 124.6 (15.8) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.21 (0.76) HDL (mmol/L): mean (SD) 1.18 (0.30) Non‐HDL (mmol/L): mean (SD) 3.80 (0.82) TC (mmol/L): mean (SD) 4.98 (0.85) TG (mmol/L): mean (SD) 1.40 (0.83) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Low‐carbohydrate ad libitum diet, participants were instructed to limit carbohydrates to 20 g/d in the form of low GI vegetables. After 12 weeks participants gradually increased carbohydrate intake by 5 g/d/week by consuming more vegetables, limited amounts of fruits and eventually small quantities of whole grains and dairy products. Physical activity prescribed. Treatment diet type (carbohydrate‐fat‐protein): Very low18w to low20w‐unclear‐unclear Exercise component? Yes Recipients: N = 50 (33%) males and N = 103 (67%) females with overall mean (SD) age 46.2 (9.2) years, mean (SD) BMI 36.1 (3.59) kg/m2, mean (SD) weight 103.3 (15.5) kg, mean (SD) systolic BP 124.3 (14.1) mmHg, mean (SD) 73.9 (9.4) mmHg, mean (SD) triglycerides 1.28 (0.62) mmol/L, mean (SD) total cholesterol 4.88 (0.78) mmol/L, mean (SD) LDL 3.11 (0.67) mmol/L and mean (SD) HDL 1.20 (0.35) mmol/L Why? "... a low‐carbohydrate diet would produce greater weight loss at 2 years than a low‐calorie, low fat diet." What (materials)? "They followed guidelines described in Dr. Atkins’ New Diet Revolution but were not provided with a copy of the book." What (procedures)? Participants were assigned to a low‐carbohydrate diet, which limited carbohydrate intake but allowed unrestricted consumption of fat and protein. For the first 12 weeks, carb intake was limited to 20g per day. After the first 12 weeks, participants gradually increased carbohydrate intake (5 g/d per week) by consuming more vegetables, a limited amount of fruits, and eventually small quantities of whole grains and dairy products, until a stable and desired weight was achieved which was in keeping with guidelines from Dr. Atkins’ New Diet Revolution book. Topics in the contact sessions included self‐monitoring, stimulus control, and relapse management. All participants were prescribed the same level of physical activity (principally walking), beginning at week 4, with 4 sessions of 20 minutes each and progressing by week 19 to 4 sessions of 50 minutes each. Group sessions reviewed participants’ completion of their eating and activity records, as well as other skill builders. Who provided? NR How and where? "Face‐to‐face at the University of Colorado Denver, Colorado; Washington University, St. Louis, Missouri; and the University of Pennsylvania, Philadelphia, Pennsylvania." When and how much? "All participants received comprehensive, in‐person group behavioral treatment weekly for 20 weeks, every other week for 20 weeks, and then every other month for the remainder of the 2‐year study period. Each treatment session lasted 75 to 90 minutes." Strategies to improve or maintain fidelity; tailoring and modification: Overnight fasting samples, weight and height measured on calibrated scales with light clothing and no shoes, standard methods for measuring blood pressure Extent of intervention fidelity: Group sessions reviewed participants’ completion of their eating and activity records, as well as other skill builders. Concomitant interventions: Participants in both groups were instructed to take a daily multivitamin supplement (provided by the study). Control diet: Name (as reported) and brief description: Low‐fat diet, participants were instructed to limit energy intake to 1200 to 1500 kcal/d for women and 1500 to 1800 kcal/day for men, with approximately 55% of calories from carbohydrates, 30% from fat and 15% from protein. Physical activity prescribed Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: N = 49 (32%) males and N = 105 (68%) females with overall mean (SD) age 44.9 (10.2) years, mean (SD) BMI 36.1 (3.46) kg/m2, mean (SD) weight 103.5 (14.4) kg, mean (SD) systolic BP 124.6 (15.8) mmHg, mean (SD) 76 (9.7) mmHg, mean (SD) triglycerides 1.40 (0.83) mmol/L, mean (SD) total cholesterol 4.98 (0.85) mmol/L, mean (SD) LDL 3.21 (0.76) mmol/L and mean (SD) HDL 1.18 (0.30) mmol/L Why? To assess whether a low‐fat diet produced greater weight loss at 2 years compared to a low‐carb diet What (materials)? NR What (procedures)? Participants were assigned to consume a low‐fat diet, which consisted of limiting energy intake to 1200 to 1500 kcal/d for women and 1500 to1800 kcal/d for men, with approximately 55% of calories from carbohydrate, 30% from fat, and 15% from protein. Participants were instructed to limit calorie intake, with a focus on decreasing fat intake. However, limiting overall energy intake (kcal/d) was the primary behavioural target. Who provided? NR How and where? "Face‐to‐face at the University of Colorado Denver, Colorado; Washington University, St. Louis, Missouri; and the University of Pennsylvania, Philadelphia, Pennsylvania." When and how much? "All participants received comprehensive, in‐person group behavioral treatment weekly for 20 weeks, every other week for 20 weeks, and then every other month for the remainder of the 2‐year study period. Each treatment session lasted 75 to 90 minutes." Strategies to improve or maintain fidelity; tailoring and modification: Overnight fasting samples, weight and height measured on calibrated scales with light clothing and no shoes, standard methods for measuring blood pressure Extent of intervention fidelity: Group sessions reviewed participants’ completion of their eating and activity records, as well as other skill builders. Concomitant interventions: Participants in both groups were instructed to take a daily multivitamin supplement (provided by the study). |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Grants from Washington University (UL1 RR024992), Temple University (grant R01 AT1103), University of Pennsylvania (UL1RR024134), University of Colorado (UL1 RR000051) and the National Center for Research Resources, a component of the National Institutes of Health (DK 56341), to Washington University Clinical Nutrition Research Unit Declaration of interest: Potential conflicts of interest were listed as accessible through www.acponline.org, but could not be obtained from the web address provided. Author contacted, but requested information not provided. |
|
Frisch 2009.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT00868387 Total number of trial arms: 2 Year trial started: 2005 Sample size calculation: Yes Outcome(s) used for sample size calculation: Difference in body weight Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Germany, outpatient heart centre in Bad Oeynhausen Eligibility criteria: Participants were aged 18 to 70 with a BMI > 27 kg/m2. Participants were excluded if they had a history of cardiovascular symptomatology; ischaemia, cholelithiasis, urolithiasis, insulin‐dependent diabetes or a pacemaker; were pregnant or lactating; vegetarian; participated in another weight loss programme or received medical treatment for weight loss. Type 2 diabetes at baseline: Mixed; stratified with non‐T2DM since at baseline 96% in treatment and 99% in control group were not on diabetes medication and HbA1c levels were not indicative of diabetes; insulin‐dependent diabetes mellitus excluded Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 200 Total attrition in trial: 35 Treatment diet Participants randomised: 100 Participants withdrawn (voluntary): 0 Total attrition: 15 Control diet: Participants randomised: 100 Participants withdrawn (voluntary): 0 Total attrition: 20 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 47 (10.3) Gender distribution (as reported): female 62/100 (62%), male 38/100 (38%) Weight (kg): mean (SD) 100.3 (15.9) BMI (kg/m2): mean (SD) 33.5 (3.9) DBP (mmHg): mean (SD) 86 (8) SBP (mmHg): mean (SD) 126 (13) HbA1c (%): mean (SD) 5.6 (0.5) LDL (mmol/L): mean (SD) 3.54 (0.80) HDL (mmol/L): mean (SD) 1.49 (0.37) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.50 (0.93) TG (mmol/L): mean (SD) 1.31 (0.56) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 47 (10.8) Gender distribution (as reported): female 76/100 (76%), male 24/100 (24%) Weight (kg): mean (SD) 98.8 (16.9) BMI (kg/m2): mean (SD) 33.8 (4.8) DBP (mmHg): mean (SD) 86 (8) SBP (mmHg): mean (SD) 128 (14) HbA1c (%): mean (SD) 5.6 (0.5) LDL (mmol/L): mean (SD) 3.56 (0.91) HDL (mmol/L): mean (SD) 1.46 (0.37) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.54 (1.10) TG (mmol/L): mean (SD) 1.39 (0.65) Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: LOGI (low‐carbohydrate) diet. Less than 40% of total energy from carbohydrates, more than 35% from fat and 25% from protein. All participants were advised to reduce their daily energy intake by at least 500 kcal/day. Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐high Exercise component? No Recipients: Subjects were 62% female and aged mean (SD) 47 (10.3) years, with BMI mean (SD) 33.5 (3.9) and weight 100.3 (15.9) kgs. 45% had a metabolic syndrome. Why? To assess whether a carbohydrate‐restricted telemedically‐guided weight‐loss programme results in a more pronounced weight loss and influences metabolic risk markers more beneficial than a fat‐restricted diet What (materials)? Ambulatory training, diet books and an electronic scale with Bluetooth technology What (procedures)? Intervention diet consisted of less than 40% of total energy intake (% energy) from carbohydrates, more than 35% energy from fat, and 25% energy from protein. Weekly nutrition education programme and dietary counselling by telephone, and had to transfer actual body weight data to our clinic weekly with added Bluetooth® technology by mobile phone. Who provided? Dietitian How and where? Telephonic (individual) sessions When and how much? Weekly actual body weight was sent through mobile phones together with weekly education and dietary counselling by phone. Strategies to improve or maintain fidelity; tailoring and modification: Regular weekly calls with the dietitian. Dietary compliance was assessed using a 3‐day validated food record. Weight sent via Bluetooth Extent of intervention fidelity: NR Concomitant interventions: Of the intervention participants 24% were taking anti‐hypertensive drugs, 6% lipid‐lowering drugs and 4% anti‐diabetic drugs. Control diet: Name (as reported) and brief description: DGE (high‐carbohydrate) diet. More than 55% of total energy from carbohydrates, less than 30% from fat and 15% from protein. All participants were advised to reduce their daily energy intake by at least 500 kcal/day. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Subjects were 76% female and aged mean (SD) 47 (10.8) years, with BMI mean (SD) 33.8 (4.8) and weight 98.8 (16.9) kgs; 31% had a metabolic syndrome. Why? NR What (materials)? Ambulatory training, diet books and an electronic scale with Bluetooth technology What (procedures)? The control diet consisted of more than 55% energy from carbohydrates, less than 30% energy from fat, and 15% energy from protein together with weekly nutrition education programme and dietary counselling by telephone, and had to transfer actual body weight data to our clinic weekly with added Bluetooth® technology by mobile phone. Who provided? Dietitian How and where? Telephonic (individual) sessions When and how much? Weekly actual body weight was sent through mobile phones together with weekly education and dietary counselling by phone. Strategies to improve or maintain fidelity; tailoring and modification: Regular weekly calls with the dietitian. Dietary compliance was assessed using a 3‐day validated food record. Weight sent via Bluetooth Extent of intervention fidelity: NR Concomitant interventions: Of control participants 31% were taking anti‐hypertensive drugs, 4% lipid‐lowering drugs and 1% anti‐diabetic drugs. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: Yes Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: SMART study Trial funded by: Various German health insurances and the Institute for Applied Telemedicine at Ruhr University Bochum Declaration of interest: "The authors declare that they have no competing interests." |
|
Gardner 2007.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT00079573 Total number of trial arms: 4 Year trial started: 2003 Sample size calculation: Yes Outcome(s) used for sample size calculation: Weight loss Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient research centre in Stanford Eligibility criteria: Participants were women aged 25 to 50 with a BMI of 27 to 40 kg/m2, with stable body weight for the past two months and stable medication for the past three months. Participants were excluded if they self‐reported hypertension which was not controlled with antihypertensive medication; had diabetes or heart, liver or renal disease; cancer or active neoplasms; uncontrolled or untreated hyperthyroidism; using medications which affect weight; consume three or more alcoholic drinks a day; were pregnant, lactating or had not menstruated in the past 12 months or if they planned to become pregnant in the next year. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 311 Total attrition in trial: 62 Treatment diet Participants randomised: 77 Participants withdrawn (voluntary): 6 Total attrition: 9 Control diet: Participants randomised: 79 Participants withdrawn (voluntary): 12 Total attrition: 18 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 42 (5) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 86 (13) BMI (kg/m2): mean (SD) 32 (4) DBP (mmHg): mean (SD) 75 (8) SBP (mmHg): mean (SD) 118 (11) HbA1c (%): NR LDL (mmol/L): mean (SD) 2.82 (0.75) HDL (mmol/L): mean (SD) 1.37 (0.36) Non‐HDL (mmol/L): mean (SD) 3.47 (0.85) TC (mmol/L): NR TG (mmol/L): mean (SD) 1.41 (0.88) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 40 (7) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 85 (14) BMI (kg/m2): mean (SD) 31 (4) DBP (mmHg): mean (SD) 75 (9) SBP (mmHg): mean (SD) 116 (12) HbA1c (%): NR LDL (mmol/L): mean (SD) 2.69 (0.75) HDL (mmol/L): mean (SD) 1.32 (0.28) Non‐HDL (mmol/L): mean (SD) 3.29 (0.88) TC (mmol/L): NR TG (mmol/L): mean (SD) 1.34 (0.82) Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Atkins diet. Aimed for 20 g/d or less of carbohydrates for induction of 2 to 3 months and 50 g/d for the 'ongoing weight loss' phase, ad libitum energy prescription Treatment diet type (carbohydrate‐fat‐protein): Very low8‐12w to low40‐44w‐unclear‐unclear Exercise component? No Recipients: Overweight or obese premenopausal women, aged mean (SD) 42 (5) years with BMI mean (SD) 32 (4) and weight mean (SD) 86 (13) kg, with or without metabolic syndrome, n (%) 22/77 (29%) Why? Low‐carbohydrate diets ad libitum diets may be at least as effective as traditional low‐fat, high‐carbohydrate diets for weight loss up to one year. The evidence to date is limited. What (materials)? Dr Atkins' New Diet Revolution (Atkins 2002). What (procedures)? Participants in this diet group aimed for the consumption of 20 g/d or less of carbohydrate for 'induction' (usually 2‐3 months) and 50 g/d or less of carbohydrate for the subsequent 'ongoing weight loss' phase. Participants were assigned their diet book, attended regular classes covering the contents of the book and were administered unannounced dietary intake and energy expenditure questionnaires. Who provided? A registered dietitian led the classes and covered a portion of the book's contents per session so it would be complete at the end of the contact period. Unannounced dietary and exercise data were collected by persons trained and certified by the Nutrition Coordinating Centre. How and where? Class sessions were held face‐to‐face and in groups, location NR. Unannounced dietary and exercise data were collected by telephone. When and how much? Class sessions were one hour each and occurred every week for eight weeks. Unannounced dietary and exercise data were collected at baseline and at 2, 6 and 12 months. This entailed recall of three days (two weekdays and one weekend day, preferably non‐consecutive) of dietary intake as well as seven days of physical activity. Strategies to improve or maintain fidelity; tailoring and modification: Strategies included email and telephone reminders for appointments, email or telephone contact from staff between the 2‐ and 6‐month and between the 6‐ and 12‐month data collection points, and incentive payments of $25, $50, and $75 for completing the 2‐, 6‐, and 12‐month data collection, respectively. A ‘Food Amounts Booklet’ was used to assist participants with portion size estimation during dietary recalls. Unannounced dietary and exercise recalls, group sessions, measurement of body weight as part of study outcomes Extent of intervention fidelity: Dietary adherence scores calculated as the difference between reported and recommended daily carbohydrate intake (< = 20 g carbohydrate per day during the first two months; and <= 50 g carbohydrate per day for the subsequent 10 months), using the average of three non‐consecutive 24‐h dietary recalls at each time point. A lower score reflects better adherence. Adherence score, means (SD): at 2 months, 35.2 (32.7); at 6 months, 62.6 (64.7); at 12 months, 85.8 (70.5). In all 4 diet groups, 85% to 89% of participants attended at least 75% of their assigned classes (>= 6 of 8). Attendance was not different by diet group (P = 0.68). Concomitant interventions: Participants on antihypertensive medications whose blood pressure was stable, as well as those on treatment for hyperthyroidism vs "were not taking medications for cardiac risk factors"? Control diet: Name (as reported) and brief description: LEARN diet. Prudent diet that included 55 to 60% of energy from carbohydrates and less than 10% energy from saturated fat, unspecified caloric restriction, increased exercise Control diet type (carbohydrate‐fat‐protein): Balanced‐unclear‐unclear Exercise component? Yes Recipients: Overweight or obese premenopausal women, aged mean (SD) 40 (7) years with BMI mean (SD) 31 (4) and weight mean (SD) 85 (14) kg, with or without metabolic syndrome, n (%) 29/79 (37) Why? This diet is based on national American guidelines. Such national guidelines have been challenged by proponents of low‐carbohydrate diets, but limited evidence is currently available. What (materials)? Participants were instructed to follow a prudent diet that included 55% to 60% energy from carbohydrate and less than 10%energy from saturated fat, caloric restriction, increased exercise, and behaviour modification strategies. The LEARN program is intended to be a 16‐week programme and, therefore, the 8 weeks of guidance in this study. Participants were assigned to a diet book (the LEARN Manual for Weight Management). What (procedures)? Participants were assigned their diet book, attended regular classes covering the contents of the book and were administered unannounced dietary intake and energy expenditure questionnaires. Participants were instructed to follow a prudent diet that included 55% to 60% energy from carbohydrate and less than 10% energy from saturated fat, caloric restriction, increased exercise, and behaviour modification strategies. Who provided? A registered dietitian led the classes and covered a portion of the book's contents per session so it would be complete at the end of the contact period. Unannounced dietary and exercise data were collected by persons trained and certified by the Nutrition Coordinating Centre. How and where? Class sessions were held face‐to‐face and in groups, location NR. Unannounced dietary and exercise data were collected by telephone. When and how much? Class sessions were one hour each and occurred every week for eight weeks. Since the LEARN program spans 16 weeks, therefore these sessions reflected an accelerated timespan. Unannounced dietary and exercise data were collected at baseline and at 2, 6 and 12 months. This entailed recall of three days (two weekdays and one weekend day, preferably non‐consecutive) of dietary intake as well as seven days of physical activity. Strategies to improve or maintain fidelity; tailoring and modification: Strategies included email and telephone reminders for appointments, email or telephone contact from staff between the 2‐ and 6‐month and between the 6‐ and 12‐month data collection points, and incentive payments of $25, $50, and $75 for completing the 2‐, 6‐, and 12‐month data collection, respectively. A ‘Food Amounts Booklet’ was used to assist participants with portion size estimation during dietary recalls. Unannounced dietary and exercise recalls, group sessions, measurement of body weight as part of study outcomes Extent of intervention fidelity: Dietary adherence scores could not be calculated due to the multiple dimensions of the intervention programme's recommended goals. In all 4 diet groups, 85% to 89% of participants attended at least 75% of their assigned classes (>= 6 of 8). Attendance was not different by diet group (P = 0.68). Concomitant interventions: Participants on antihypertensive medications whose blood pressure was stable, as well as those on treatment for hyperthyroidism vs "were not taking medications for cardiac risk factors"? |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: Yes Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: British Diabetic Association and the International Sugar Research Foundation Inc. Declaration of interest: "None reported." |
|
Goni 2018.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT02737267 Total number of trial arms: 2 Year trial started: 2015 Sample size calculation: Yes Outcome(s) used for sample size calculation: Weight loss Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 4 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Spain, outpatient university centre for nutrition research in Navarra Eligibility criteria: Participants were overweight or obese with BMI 25 to 40 kg/m2. Participants were excluded if they were suffering from cardiovascular disease, type 1 or type 2 insulin‐dependent diabetes; were pregnant or lactating; used medication which could affect body weight or an unstable dose of drugs for hyperlipidemia in type 2 diabetics; had experienced weight change of more than 3 kg in the past three months; were treated with hypoglycemic or for hypertension. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 147 Total attrition in trial: 40 Treatment diet Participants randomised: 72 Participants withdrawn (voluntary): NR Total attrition: 21 Control diet: Participants randomised: 75 Participants withdrawn (voluntary): NR Total attrition: 19 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 45.6 (11.2) Gender distribution (as reported): female 48/72 (66.7%), male 24/72 (33.3%) Weight (kg): mean (SD) 88.5 (13.2) BMI (kg/m2): mean (SD) 31.7 (3.6) DBP (mmHg): mean (SD) 76.3 (10.6) SBP (mmHg): mean (SD) 131.3 (23.5) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.78 (0.80) HDL (mmol/L): mean (SD) 1.36 (0.32) Non‐HDL (mmol/L): mean (SD) 4.35 (0.93) TC (mmol/L): mean (SD) 5.71 (0.94) TG (mmol/L): mean (SD) 1.23 (0.70) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 47.5 (8.6) Gender distribution (as reported): female 51/75 (68.0%), male 24/75 (32.0%) Weight (kg): mean (SD) 88.8 (13.3) BMI (kg/m2): mean (SD) 32.1 (3.8) DBP (mmHg): mean (SD) 81.0 (11.9) SBP (mmHg): mean (SD) 132.2 (17.8) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.63 (0.78) HDL (mmol/L): mean (SD) 1.36 (0.32) Non‐HDL (mmol/L): mean (SD) 4.19 (0.87) TC (mmol/L): mean (SD) 5.56 (0.93) TG (mmol/L): mean (SD) 1.23 (0.66) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: DBP, SBP, LDL, HDL, non‐HDL, total cholesterol and triglycerides baseline values are not for all randomised (missing: intervention 19/72; control 21/75). Last phrase of exclusion criteria in Goni et al 2018 reads strangely. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Moderately high protein diet, 40% carbohydrates, 30% protein and 30% fat. Restriction of 30% of total energy. Energy requirements were individually evaluated from resting energy expenditure. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: Subjects were 66.7% female and aged mean (SD) 45.6 (11.2) years, with BMI mean (SD) 31.7 (3.6) and weight of 88.5 (13.2) kgs. Why? The hypothesis of the present study is that part of the interindividual variability in relation to the success of certain weight loss treatments is based on gene‐diet interactions. Depending on the composition of the diet and the genotype of each individual, it is more or less easy to reduce and maintenance body weight. What (materials)? Participants received detailed information from trained nutritionists regarding feeding schedules, portion sizes, and cooking methods. What (procedures)? Moderately‐high protein diet: 40% of total energy from carbohydrates, 30% from protein, and 30% from fat for 16 weeks. Habitual dietary intake at baseline was evaluated by a previously validated semi‐quantitative food frequency questionnaire. Detailed information from trained nutritionists regarding feeding schedules, portion sizes, and cooking methods Who provided? Dietitian How and where? Face‐to‐face sessions, location NR When and how much? 16 weeks Strategies to improve or maintain fidelity; tailoring and modification: Compliance analysis to the recommended diet of the participants was conducted taking into account a three‐day weighed food record (two weekdays and one weekend day) at two times: the eighth week and at the end of the intervention period (the 16th week). The adherence to the diet was graded by trained dietitians during each visit of the intervention according to the following scale: 0: failure to follow the diet at any time (poor adherence); 1: follow‐up across weekdays but not during weekends (regular adherence); 2: occasionally exceeded from recommendations (good adherence); and 3: continuous follow‐up (very good adherence). Also, during the 4 months of nutritional intervention, nutritionists conducted motivational telephone calls with each participant in order to increase the adherence to the dietary advice based on previous trials. Extent of intervention fidelity: The adherence to the diets was graded by trained dietitians during each visit of the intervention according to a scale. In the moderately‐high protein diet group, the targets of macronutrient intakes during the intervention were achieved. Concomitant interventions: NR Control diet: Name (as reported) and brief description: Low‐fat diet, 60% carbohydrates, 18% protein and 22% fat. Restriction of 30% of total energy. Energy requirements were individually evaluated from resting energy expenditure. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Subjects were 68% female and aged mean (SD) 47.5 (8.6) years, with BMI mean (SD) 32.1 (3.8) and weight of 88.8 (13.3) kgs. Why? NR What (materials)? Participants received detailed information from trained nutritionists regarding feeding schedules, portion sizes, and cooking methods. What (procedures)? low‐fat diet: 60% of total energy from carbohydrates, 18% from protein, and 22% from fat for 16 weeks Who provided? Dietitian How and where? Face‐to‐face sessions, location NR When and how much? 16 weeks Strategies to improve or maintain fidelity; tailoring and modification: Compliance analysis to the recommended diet of the participants was conducted taking into account a three‐day weighed food record (two weekdays and one weekend day) at two times: the eighth week and at the end of the intervention period (the 16th week). The adherence to the diet was graded by trained dietitians during each visit of the intervention according to the following scale: 0: failure to follow the diet at any time (poor adherence); 1: follow‐up across weekdays but not during weekends (regular adherence); 2: occasionally exceeded from recommendations (good adherence); and 3: continuous follow‐up (very good adherence). Also, during the 4 months of nutritional intervention, nutritionists conducted motivational telephone calls with each participant in order to increase the adherence to the dietary advice based on previous trials. Extent of intervention fidelity: The targets of macronutrient intake were not fully achieved among subjects of the low‐fat diet. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: Obekit Trial funded by: Government of Navarra (ADITECH; Obekit project), Linea Especial (University of Navarra; LE/97) and CIBERobn/RETICS schedules (Insituto de Salud Carlos III) Declaration of interest: "The authors declare no conflict of interest." |
|
Griffin 2013.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, number of centres NR Trial registry number: ACTRN12609000307202 Total number of trial arms: 2 Year trial started: NR Sample size calculation: Outcome(s) used for sample size calculation: Yes Duration of run‐in period (weeks): Weight difference What was the duration of the weight loss phase: 12 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely Australia based on authors and funder Eligibility criteria: Participants were otherwise healthy women aged 18 to 25 years with BMI > 27.5 kg/m2. Participants were excluded if they were vegetarians; were pregnant or lactating; had secondary causes of obesity, previous bariatric surgery, diagnosed psychiatric disorders, severe and untreated obstructive sleep apnoea; used medication which could affect their appetite, weight or metabolic rate; were smokers; had anaemia (defined by haemoglobin levels < 115 g/L), abnormal fasting glucose or thyroid function. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 71 Total attrition in trial: 35 Treatment diet Participants randomised: 36 Participants withdrawn (voluntary): 8 Total attrition: 15 Control diet: Participants randomised: 35 Participants withdrawn (voluntary): 11 Total attrition: 20 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 22.4 (2.45) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 95.2 (12.70) BMI (kg/m2): mean (SD) 34.1 (4.29) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 2.9 (0.61) HDL (mmol/L): mean (SD) 1.4 (0.46) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.9 (0.77) TG (mmol/L): mean (SD) 1.2 (0.61) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 22.5 (2.41) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 94.3 (15.09) BMI (kg/m2): mean (SD) 33.8 (5.13) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 2.7 (0.91) HDL (mmol/L): mean (SD) 1.5 (0.30) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.7 (0.91) TG (mmol/L): mean (SD) 1.0 (0.60) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Values reported as mean (95% CI) were converted to mean (SD). |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein (HP) diet, providing 32% protein, 41% carbohydrate and 25% fat. Low‐GI foods were recommended, saturated fat was < 10% TE. Walking (30 min most days) prescribed. Energy prescription of 5600 kJ/day Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: N = 36 women with mean (95%CI) age 22.4 (21.6 to 23.2) years, mean (95% CI) weight 95.2 (91.0 to 99.3) kg, mean (95% CI) BMI 34.1 (32.7 to 35.5) kg/m2 Why? Higher‐protein (HP) diets have been reported as effective for weight loss in middle‐aged obese adults. Enhanced satiety with higher protein diets may be helpful for young women as hunger and disinhibition are reported to be problematic in this population. These diets tend to be more nutrient‐dense and provide greater iron, a nutrient often under‐consumed in young women and for which requirements are higher due to menstrual losses. What (materials)? Participants were provided with kitchen scales, daily checklists, recipe ideas and money vouchers (AUD $20) at baseline, three, six, nine and 12 months to assist with food measurement, cooking healthy meals and dietary compliance. Participants also received a validated activity diary. What (procedures)? The HP diet provided 32% protein (107 g/day), 41% carbohydrate (138 g/day) and 25% fat (38 g/day) Low‐glycaemic index (< 55) carbohydrate foods were recommended. Diets were matched for energy (5600 kJ/day), saturated fat (< 10% daily energy), dietary fibre and calcium. Total fat was similar (HP: 37.9; HC: 31.9 g/day). Limited alcohol intake (≤ 20 g/week; ∼1% of daily energy) was permitted. HP and HC participants consumed 300 and 80 g (raw weight) of meat/day, respectively, with set amounts of red and white meats prescribed. 27 dietetic and behaviour modification sessions: weekly from 0 to 3 months, fortnightly from 3 to 6 months, then monthly from 6 to 12 months. 10‐module weight‐management programme (Bodylines). Participants were prescribed an identical exercise programme (30 min walking most days) with expenditure estimated using a validated activity diary. Who provided? NR How and where? Face‐to‐face sessions, location, NR When and how much? Weekly until 3 months, fortnightly between 3 and 6 months then monthly until 12 months Strategies to improve or maintain fidelity; tailoring and modification: Dietary intake was estimated using 3‐day food records. Twenty‐four hour urine collections provided urea/creatinine ratio (UCR) to monitor recent protein intake. Extent of intervention fidelity: Reported intakes were close to the dietary prescription but HC tended to report lower energy intake (1148 kJ less in 12‐month completers). Iron intake was significantly higher on HP [HP: 12.9 (11.0 to 14.9); HC: 8.5 (7.4 to 9.6) mg/day; P < 0.001 in 6‐month completers]. Concomitant interventions: NR Control diet: Name (as reported) and brief description: High‐carbohydrate (HC) diet, providing 20% protein, 58% carbohydrate and 21% fat. Low‐GI foods were recommended, saturated fat was < 10% TE. Walking (30 min most days) prescribed. Energy prescription of 5600 kJ/day Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: N = 36 women with mean (95% CI) age 22.5 (21.7 to 23.3) years, mean (95% CI) weight 94.3 (89.3 to 99.3) kg, mean (95% CI) BMI 33.8 (32.1 to 35.5) kg/m2 Why? NR What (materials)? Participants were provided with kitchen scales, daily checklists, recipe ideas and money vouchers (AUD $20) at baseline, three, six, nine and 12 months to assist with food measurement, cooking healthy meals and dietary compliance. Participants also received a validated activity diary. What (procedures)? Participants were prescribed a HC diet providing 20% protein (67 g/day), 58% carbohydrate (191 g/day) and 21% fat (32 g/day). Low‐glycaemic index (< 55) carbohydrate foods were recommended. Diets were matched for energy (5600 kJ/day), saturated fat (< 10% daily energy), dietary fibre and calcium. Total fat was similar (HP: 37.9; HC: 31.9 g/day). Limited alcohol intake (≤ 20 g/week; ∼1% of daily energy) was permitted. HP and HC participants consumed 300 and 80 g (raw weight) of meat/day, respectively, with set amounts of red and white meats prescribed. Participants were prescribed an identical exercise programme (30‐min walking most days) with expenditure estimated using a validated activity diary. 27 dietetic and behaviour modification sessions: weekly from 0 to 3 months, fortnightly from 3 to 6 months, then monthly from 6 to 12 months. HC: red meat 1 time/week; white meat 4 times/week. 10‐module weight‐management programme (Bodylines). A standard recommendation of walking 30 min on most days was prescribed to all. Who provided? NR How and where? Face‐to‐face sessions, location, NR When and how much? Weekly until 3 months, fortnightly between 3 and 6 months then monthly until 12 months Strategies to improve or maintain fidelity; tailoring and modification: Dietary intake was estimated using 3‐day food records. Twenty‐four hour urine collections provided urea/creatinine ratio (UCR) to monitor recent protein intake. Extent of intervention fidelity: NR Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): research letter Trial acronym/name: WOW study Trial funded by: Meat and Livestock Australia Declaration of interest: "H. J. G., H. T. O., K. B. R. and K. S. S. designed the research. H. J. G. and H. L. C. conducted the research. K. B. R., H. J. G., H. L. C. and P. P. analysed the biochemistry and data. H. J. G., H. T. O. and H. L. C. drafted the manuscript. All authors approved the final manuscript." No further declaration reported. |
|
Guldbrand 2012.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, multicentre (2) Trial registry number: NCT01005498 Total number of trial arms: 2 Year trial started: 2008 Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 2 years What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Sweden, outpatient primary healthcare centres in Motala and Borensberg Eligibility criteria: Participants had type 2 diabetes which was treated with diet, with or without orally administered glucose‐lowering medication, incretin or insulin. Participants were excluded if they were suffering from a severe mental disease; had a malignancy or were abusing drugs. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 61 Total attrition in trial: 21 Treatment diet Participants randomised: 30 Participants withdrawn (voluntary): 4 Total attrition: 14 Control diet: Participants randomised: 31 Participants withdrawn (voluntary): 3 Total attrition: 7 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 61.2 (9.5) Gender distribution (as reported): female 16/30 (53.3%), male 14/30 (46.7%) Weight (kg): mean (SD) 91.4 (19) BMI (kg/m2): mean (SD) 31.6 (5.0) DBP (mmHg): mean (SD) 76 (11) SBP (mmHg): mean (SD) 135 (15) HbA1c (%): mean (SD) 7.5 (3.1) LDL (mmol/L): mean (SD) 2.7 (0.9) HDL (mmol/L): mean (SD) 1.13 (0.33) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.5 (1.0) TG (mmol/L): mean (SD) 1.7 (1.4) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 62.7 (11) Gender distribution (as reported): female 18/31 (58.1%), male 13/31 (41.9%) Weight (kg): mean (SD) 98.8 (21) BMI (kg/m2): mean (SD) 33.8 (5.7) DBP (mmHg): mean (SD) 77 (9) SBP (mmHg): mean (SD) 136 (13) HbA1c (%): mean (SD) 7.2 (2.9) LDL (mmol/L): mean (SD) 2.4 (0.7) HDL (mmol/L): mean (SD) 1.09 (0.29) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.3 (1.0) TG (mmol/L): mean (SD) 1.8 (0.8) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: HbA1c mmol/mol baseline values also reported: intervention mean (SD) 58.5 (10.2) mmol/mol; control mean (SD) 55.6 (8.0) mmol/mol |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐carbohydrate diet, energy content with 50% from fat, 20% from carbohydrates and 30% from protein. Energy content of 6694 kJ/day for women or 7531 kJ/day for men Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐high Exercise component? No Recipients: N = 14 men and N = 16 women, mean (SD) age 61.2 (9.5) years, mean (SD) weight 91.4 (19) kg, mean (SD) BMI 31.6(5.0) kg/m2, mean (SD) duration of known diabetes of 9.8 (5.5) years Why? High‐fat diet (i.e. with low carbs) would improve glycaemic control more efficiently than the traditional low‐fat diet (LFD). What (materials)? Menus for 1 week were provided to the participants as meal suggestions by the dietitian. Weighing scales and notebooks from the organisers with which to weigh and record all food items that were consumed during these periods What (procedures)? The LCD had an energy content where 50 energy per cent (E%) was from fat, 20 E% was from carbohydrate and 30 E% was from protein. Both diets had an energy content of 6694 kJ/day (1600 kcal/day) for women or 7531 kJ/day (1800 kcal/day) for men. 4 group meetings of 60 mins each for the first year. Group sessions on which food items to choose from and suitable recipes Who provided? Dietitian provided recipes and a menu for one week. Two different physicians conducted the group session where participants learnt which foods to chose from. How and where? Face‐to‐face at two primary healthcare centres in the cities of Motala and Borensberg, located in southeast Sweden When and how much? Four group meetings with a duration of 60 min each for the first year; no further group meetings during the remaining 12 months were held. Group sessions were given at baseline, and 2, 6 and 12 months by two different physicians. A dietitian was available consecutively during the trial for questions from the participants. Strategies to improve or maintain fidelity; tailoring and modification: Diet records were also performed at these four visits, with one additional recording at 3 months. The diet records were conducted during 3 consecutive days, of which 1 day was a Saturday or a Sunday. Extent of intervention fidelity: NR Concomitant interventions: Of the intervention participants the total insulin dose was 42 (65), metformin (mg) 1375 (950), glibenclamide (mg) 1.1 (2.6), simvastatin (mg) 19 (18) and atorvastatin (mg) 2 (5). Control diet: Name (as reported) and brief description: Low‐fat diet, energy content with 30% from fat (< 10% from saturated fat), 55 to 60% from carbohydrates and 10 to 15% from protein. Energy content of 6694 kJ/day for women or 7531 kJ/day for men Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: NR Why? NR What (materials)? Menus for 1 week were provided to the participants as meal suggestions by the dietitian. Weighing scales and notebooks from the organisers with which to weigh and record all food items that were consumed during these periods What (procedures)? The LFD had a nutrient composition that was similar to that traditionally recommended for the treatment of type 2 diabetes in Sweden, with 30 E% from fat (less than 10 E% from saturated fat), 55–60 E% from carbohydrate and 10–15 E% from protein. 4 group meetings of 60 mins each for the first year Who provided? Dietitian provided recipes and a menu for one week. Two different physicians conducted the group session where participants learnt which foods to chose from. How and where? Face‐to‐face at two primary healthcare centres in the cities of Motala and Borensberg, located in southeast Sweden When and how much? Four group meetings with a duration of 60 min each for the first year; no further group meetings during the remaining 12 months were held. Group sessions were given at baseline, and 2, 6 and 12 months by two different physicians. A dietitian was available consecutively during the trial for questions from the participants. Strategies to improve or maintain fidelity; tailoring and modification: Diet records were also performed at these four visits, with one additional recording at 3 months. The diet records were conducted during 3 consecutive days, of which 1 day was a Saturday or a Sunday. Extent of intervention fidelity: NR Concomitant interventions: Of the control participants the total insulin dose was 39 (51), metformin (mg) 1435 (946), glibenclamide (mg) 0.4 (1.9), simvastatin (mg) 19 (17) and atorvastatin (mg) 2 (5). |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: Yes Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: VÄSTKOST Trial funded by: University Hospital of Linköping Research Funds, Linköping University, County Council of Östergötland and the Diabetes Research Centre of Linköping University Declaration of interest: "The authors declare that there is no duality of interest associated with this manuscript." |
|
Haufe 2013.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1, 2 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT00956566 Total number of trial arms: 2 Year trial started: 2007 Sample size calculation: Yes Outcome(s) used for sample size calculation: Weight loss Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: 17 to 36 months (average 24 months) Other notes about methods: NA |
|
| Participants |
Country and setting: Germany, outpatient academic clinical research centre in Berlin Eligibility criteria: Participants who were overweight or obese, but otherwise healthy. Participants were excluded if they did more than two hours of physical activity per week; consumed more than 20 g of alcohol per day; had type 2 diabetes, acute or chronic infections; were pregnant or nursing. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Mixed Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 170 Total attrition in trial: 68 Treatment diet Participants randomised: 84 Participants withdrawn (voluntary): 20 Total attrition: 32 Control diet: Participants randomised: 86 Participants withdrawn (voluntary): 24 Total attrition: 36 Baseline data treatment diet: Randomised participants not included: 32/84 (except for age, weight and BMI 29/84) Age (years): mean (SD) 43 (9) Gender distribution (as reported): female 44/52, male 8/52 Weight (kg): mean (SD) 94 (15) BMI (kg/m2): mean (SD) 33.4 (4.2) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 2.92 (0.64) HDL (mmol/L): mean (SD) 1.52 (0.73) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.85 (0.66) TG (mmol/L): mean (SD) 1.14 (0.49) Baseline data control diet: Randomised participants not included: 36/86 (except for age, weight and BMI 32/86) Age (years): mean (SD) 45 (9) Gender distribution (as reported): female 40/50, male 10/50 Weight (kg): mean (SD) 93 (19) BMI (kg/m2): mean (SD) 33.5 (4.3) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.15 (0.89) HDL (mmol/L): mean (SD) 1.38 (0.56) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.9 (0.95) TG (mmol/L): mean (SD) 1.20 (0.63) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: All baseline characteristics are reported by trial arm and intrahepatic lipids; these were combined to give characteristics by trial arm only. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Reduced‐carbohydrate diet. Aimed at achieving ≤ 90 g/d of carbohydrates, 0.8 g protein per kg body weight and a minimum of 30% fat. Total energy prescription of 30% less than baseline intake (to a minimum of 1200 kcal/day) Treatment diet type (carbohydrate‐fat‐protein): Low‐unclear‐high Exercise component? No Recipients: Participants were 84.6% female, aged 43.15 (8.67) years, with BMI 33.38 (4.24) and weight 92.85 (14.81) kg Why? Weight reduction through caloric restriction decreases hepatic fat up to 12 months and improves insulin sensitivity while preventing type 2 diabetes. What (materials)? NR What (procedures)? Participants followed a reduced energy diet with nutritional counselling to a reduced‐carbohydrate diet, with regular group sessions and analysis of seven‐day food protocols. Who provided? Group sessions and individual counselling, with food protocol analysis, were run by nutritionists. How and where? Face‐to‐face group sessions and individual face‐to‐face sessions, location NR. Telephonic check‐ins on weight changes were done in the weight maintenance phase. When and how much? The active intervention duration was six months, with follow‐up after weight maintenance of between 17 and 36 months [from 18 Haufe 2011]. Group sessions occurred on a weekly basis throughout the active weight loss phase, with individual counselling occurring every two months in this phase. An analysis of seven‐day food protocol was conducted at every individual counselling. No active intervention occurred in the weight maintenance phase. Strategies to improve or maintain fidelity; tailoring and modification: Group sessions, follow‐up measurement of body weight, keeping of food protocols, adherence monitoring in individual sessions Extent of intervention fidelity: The authors reported that participants adhered to their assigned intervention. Concomitant interventions: "They [participants] ingested no medications." Control diet: Name (as reported) and brief description: Reduced‐fat diet. Aimed at achieving a fat content of ≤ 20% of total energy intake, 0.8 g protein per kg body weight and the remaining energy content provided by carbohydrates. Author reported via email that this group ingested 53.7% carbohydrates. Total energy prescription of 30% less than baseline intake (to a minimum of 1200 kcal/day) Control diet type (carbohydrate‐fat‐protein): Balanced‐low‐high Exercise component? No Recipients: Participants were 80.0% female, aged 45.08 (8.96) years, with BMI 32.98 (3.55) and weight 92.32 (17.2) kg. Why? Low‐fat caloric restriction diets reduce the hepatic fat content in humans. What (materials)? NR What (procedures)? Participants followed a reduced energy diet with nutritional counselling to a reduced‐fat diet, with regular group sessions and analysis of seven‐day food protocols. Who provided? Group sessions and individual counselling, with food protocol analysis, were run by nutritionists. How and where? Face‐to‐face group sessions and individual face‐to‐face sessions, location NR. Telephonic check‐ins on weight changes were done in the weight maintenance phase. When and how much? The active intervention duration was six months, with follow‐up after weight maintenance of between 17 and 36 months [from 18 Haufe 2011]. Group sessions occurred on a weekly basis throughout the active weight loss phase, with individual counselling occurring every two months in this phase. An analysis of seven‐day food protocol was conducted at every individual counselling. No active intervention occurred in the weight maintenance phase. Strategies to improve or maintain fidelity; tailoring and modification: Group sessions, follow‐up measurement of body weight, keeping of food protocols, adherence monitoring in individual sessions Extent of intervention fidelity: The authors reported that participants adhered to their assigned intervention. Concomitant interventions: "They [participants] ingested no medications." |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: B‐SMART Trial funded by: Federal Ministry of Education and Research (BMBF‐0313868) Declaration of interest: "No potential conflicts of interest relevant to this article were reported." |
|
Hockaday 1978.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: UK, outpatient hospital diabetic clinic in Oxford Eligibility criteria: Participants were newly‐diagnosed diabetics under 65 years who did not suffer from co‐existing major illness and did not need insulin therapy immediately. Participants were excluded if they had a history of, or current, endocrine disease, myocardial infarction or neurological deficit after a cerebrovascular accident; or were currently suffering from liver disease, however, those with a past history of liver disease were not excluded. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 93 Total attrition in trial: NR Treatment diet Participants randomised: 54 Participants withdrawn (voluntary): NR Total attrition: NR Control diet: Participants randomised: 39 Participants withdrawn (voluntary): NR Total attrition: NR Baseline data treatment diet: Randomised participants not included: None Age (years): mean (range) 53 (22 to 65) Gender distribution (as reported): female 22/54, male 32/54 Weight (kg): mean (range) 76.4 (51 to 99) BMI (kg/m2): NR DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 6.5 (0.21) TG (mmol/L): mean (SE) 1.69 (0.12) Baseline data control diet: Randomised participants not included: None Age (years): mean (range) 50 (24 to 65) Gender distribution (as reported): female 19/39, male 20/39 Weight (kg): mean (range) 82.2 (56 to 114) BMI (kg/m2): NR DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 6.2 (0.20) TG (mmol/L): mean (SE) 1.59 (0.12) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1: number of overweight participants (P < 0.02) Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐carbohydrate (LC) diet, 20% protein, 40% carbohydrate, 40% total fat (28% saturated and monounsaturated and 12% PUFA). Energy prescription of 6300 kJ a day Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐balanced Exercise component? No Recipients: Newly‐diagnosed diabetics, 40.7% female, aged mean 53 (range 22 to 65) years with weight mean 76.4 (range 51 to 99) kg. Having raised baseline levels of cholesterol, triglycerides, glucose and insulin Why? To investigate the effects of a low‐carb diet compared to low‐fat diet for development of cardiovascular risk factors. Low‐carbohydrate diets are normally prescribed to diabetic patients in the Western world. What (materials)? NR What (procedures)? Participants were instructed to consume a low‐carbohydrate diet, and were seen regularly to reinforce dietary advice. Dietary counselling at each visit Who provided? Dietitians met with participants to reinforce dietary advice at follow‐up visits How and where? Meeting with dieticians occurred face‐to‐face at the diabetic clinic from which participants were recruited. When and how much? The duration of the intervention was one year. Patients were seen by dietitians for the reinforcement of dietary advice after one month, and then every three months. Strategies to improve or maintain fidelity; tailoring and modification: Meetings at one month and at three‐month intervals thereafter to repeat dietary advice; weigh‐in at one month and one year Extent of intervention fidelity: Authors reported only that participants varied in their cooperation. Concomitant interventions: Participants did not require treatment with insulin or another oral hypoglycemic medication during the study. Control diet: Name (as reported) and brief description: Modified‐fat high‐carbohydrate (MF) diet, 20% protein, 54% carbohydrate, 26% total fat (10% saturated and monounsaturated and 16% PUFA). Energy prescription of 6300 kJ a day Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Newly‐diagnosed diabetics, 48.7% female, aged mean 50 (range 24 to 65) years with weight mean 82.2 (range 56 to 114) kg. Having raised baseline levels of cholesterol, triglycerides, glucose and insulin Why? The Japanese, Trappists and Yemenite Jews consume diets high in carbohydrates; these populations tend to have lower serum lipids and lower prevalence of ischaemic heart disease ‐ both in diabetics as well as the general population. What (materials)? NR What (procedures)? Participants were instructed to consume a modified‐fat diet, and were seen regularly to reinforce dietary advice. Who provided? Dietitians met with participants to reinforce dietary advice at follow‐up visits. How and where? Meeting with dieticians occurred face‐to‐face at the diabetic clinic from which participants were recruited. When and how much? The duration of the intervention was one year. Patients were seen by dietitians for the reinforcement of dietary advice after one month, and then every three months. Strategies to improve or maintain fidelity; tailoring and modification: Meetings at one month and at three‐month intervals thereafter to repeat dietary advice; weigh‐in at one month and one year Extent of intervention fidelity: Authors reported only that participants varied in their cooperation. Concomitant interventions: Participants did not require treatment with insulin or another oral hypoglycemic medication during the study. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: British Diabetic Association and the International Sugar Research Foundation Inc. Declaration of interest: NR |
|
Jesudason 2013.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, number of centres NR Trial registry number: ACTRN12608000229370 Total number of trial arms: 2 Year trial started: 2008 Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 24 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Australia, outpatient research organisation in Adelaide. Sample food packs or 20 AUD vouchers were given to participants at inception, 12 and 26 weeks. Eligibility criteria: Participants were women aged 40 to 70 years with a BMI > 27 kg/m2; women were postmenopausal (absence of periods for 12 months and follicular‐stimulating hormone concentration > 20 U/L). Participants were excluded if their body weight was > 140 kg; had a total hip BMD t‐score less than ‐2.0 or a t‐score less than ‐1.0 with a history of low‐trauma peripheral or spine fracture; took hormone‐replacement therapy, bisphosphonates, steroids, diuretics, calcium or vitamin D; had parathyroid disease, a vitamin D concentration < 60 nmol/L with secondary hyperparathyroidism; significant diseases including unstable metabolic, cardiac, gastrointestinal or renal conditions or malignancies. Type 2 diabetes at baseline: Unclear; stratified with non‐T2DM since unstable metabolic disease was an exclusion criterion. Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 323 Total attrition in trial: 186 Treatment diet Participants randomised: 164 Participants withdrawn (voluntary): 68 Total attrition: 95 Control diet: Participants randomised: 159 Participants withdrawn (voluntary): 59 Total attrition: 91 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SE) 59.5 (0.4) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SE) 88.6 (1.1) BMI (kg/m2): mean (SE) 34.0 (0.4) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Baseline data control diet: Randomised participants not included: None Age (years): mean (SE) 59.4 (0.4) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SE) 88.6 (1.1) BMI (kg/m2): mean (SE) 33.4 (0.4) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1: 24‐hour urea excretion (P < 0.02); intervention vs control Other notes about participants: Baseline BMI value in intervention group was for n = 163, instead of n = 164. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein diet. Protein/fat/carbohydrate recommended at 32/24/44% of total energy. The diet provided ~5500 kJ/d. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: N = 164 women with a mean (SE) age 59.5 (0.42) years, mean (SE) BMI 34.0 (0.43) kg/m2; N = 12 of 164 (7.3%) were smokers and consumed mean (SE) 355 (36) grams of alcohol per week. Why? To assess if a high‐protein (HP) weight‐loss diet was detrimental to bone health compared with a high‐normal–protein (HNP) diet matched for energy. What (materials)? Sample food packs or $20 vouchers were provided to participants at study commencement and 12 and 26 wks. Participants were instructed to record their dietary intakes by using a protein counter and checklist. What (procedures)? The HP diet plan contained less or equal to 32% of calories as protein (> 90 g/d) with low saturated and total fat (< 10% and < 30%, respectively). The diets were isocaloric and provided roughly 5500 kJ/d. During group sessions, participants received general information regarding diet and lifestyle self‐management. Subjects were made aware of the benefits of exercise although there was no formal exercise programme. Participants were instructed to record their dietary intakes by using a protein counter and checklist which were collected at each group session. Who provided? Dietitian How and where? "Participants were offered phone support with a dietitian, individual consultations, and monthly phone or email contact support as well as monthly face‐to‐face groups sessions." When and how much? Participants received monthly group dietetic education and support for the first 6 mo and then every 3 mo for the next 18 mo. Individual or telephonic consultations were also offered monthly. Change in bone mineral density (BMD) of the total hip was assessed at 12 and 24 mo. Strategies to improve or maintain fidelity; tailoring and modification: "Compliance to the HP diet was assessed by reference to the blood urea nitrogen and 24‐h urine for urea nitrogen excretion. Protein‐compliance checklists were collected from each participant at each group session." Extent of intervention fidelity: mean (SE) blood urea nitrogen at 12 months 17.9 (0.3) mg/dL and at 24 months 17.(0.6) mg/dL; mean (SE) urine urea nitrogen at 12 months 11.8 (0.3) g/24 h and at 24 months 12.0 (0.3) g/24 h Concomitant interventions: Women were ineligible if they were taking hormone‐replacement therapy, bisphosphonates, steroids, diuretics, calcium, or vitamin D. Selective serotonin reuptake inhibitor drugs were allowed. Control diet: Name (as reported) and brief description: High‐normal protein diet. Protein/fat/carbohydrate recommended at 22/23/55. The diet provided ~5500 kJ/d. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐high Exercise component? No Recipients: N = 159 women with a mean (SE) age 59.4 (0.4) years, mean (SE) BMI 33.4 (0.4) kg/m2, N = 8 of 159 (5.0%) were smokers and consumed mean (SE) 449 (56) grams of alcohol per week. Why? To evaluate whether a lower protein diet has fewer detrimental effects on bone health What (materials)? Sample food packs or $20 vouchers were provided to participants at study commencement and 12 and 26 wk. Participants were instructed to record their dietary intakes by using a protein counter and checklist. What (procedures)? The normal protein (HNP) diet plan had less or equal to 22% of calories as protein (< 80 g/d), similar fat, and 55% of energy from low–glycemic index carbohydrates. The diets were isocaloric and provided roughly 5500 kJ/d. During group sessions, participants received general information regarding diet and lifestyle self‐management. Subjects were made aware of the benefits of exercise although there was no formal exercise programme. Participants were instructed to record their dietary intakes by using a protein counter and checklist which were collected at each group session. Who provided? Dietitian How and where? Participants were offered phone support with a dietitian, individual consultations, and monthly phone or email contact support as well as monthly face‐to‐face group sessions. When and how much? Participants received monthly group dietetic education and support for the first 6 mo and then every 3 mo for the next 18 mo. Individual or telephonic consultations were also offered monthly. Change in bone mineral density (BMD) of the total hip was assessed at 12 and 24 mo. Strategies to improve or maintain fidelity; tailoring and modification: "Compliance to the HP diet was assessed by reference to the blood urea nitrogen and 24‐h urine for urea nitrogen excretion. Protein‐compliance checklists were collected from each participant at each group session." Extent of intervention fidelity: mean (SE) blood urea nitrogen at 12 months 14.8 (0.1) mg/dL and at 24 months 14.8 (0.6) mg/dL; mean (SE) urine urea nitrogen at 12 months 9.3 (0.3) g/24 h and at 24 months 9.9 (0.3) g/24 h Concomitant interventions: Women were ineligible if they were taking hormone‐replacement therapy, bisphosphonates, steroids, diuretics, calcium, or vitamin D. Selective serotonin reuptake inhibitor drugs were allowed. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: No Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: BD Trial funded by: Commonwealth Scientific and Industrial Research Organisation, a research organisation supported by the Australian government Declaration of interest: "None of the authors had a conflict of interest." |
|
Josse 2011.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT00710398 Total number of trial arms: 3 Year trial started: 2008 Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 16 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Canada, outpatient university centre. Participants were provided with a study drink, appropriate for their diet, before exercise and immediately following exercise on a daily basis. Eligibility criteria: Participants were premenopausal women who were overweight and obese (defined as BMI 27 to 40 kg/m2) aged between 19 and 45 years; who consumed low levels of dairy, had a sedentary lifestyle, regular menstrual cycle, did not take vitamin or mineral supplements and were otherwise healthy as confirmed by short medical screening questionnaire. Participants were excluded if they had any of the conditions comprising the short medical screening questionnaire: the presence of metabolic risk factors; heart or other organ disease; orthopaedic injury which would affect exercise; gastrointestinal disease; diagnosed diary protein allergy; diagnosed lactose intolerance or use of prescription medication. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 90 Total attrition in trial: NR Treatment diet Participants randomised: 31 Participants withdrawn (voluntary): 3 Total attrition: NR Control diet: Participants randomised: 31 Participants withdrawn (voluntary): 0 Total attrition: NR Baseline data treatment diet: Randomised participants not included: 1/31 Age (years): mean (SE) 30 (1) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SE) 86.7 (2.2) BMI (kg/m2): mean (SE) 31.4 (0.6) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): mean (SE) 5.4 (0.1) LDL (mmol/L): mean (SE) 2.77 (0.13) HDL (mmol/L): mean (SE) 1.43 (0.05) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 4.67 (0.15) TG (mmol/L): mean (SE) 1.00 (0.07) Baseline data control diet: Randomised participants not included: 1/31 Age (years): mean (SE) 26 (1) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SE) 85.2 (2.0) BMI (kg/m2): mean (SE) 31.8 (0.6) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): mean (SE) 5.4 (0.1) LDL (mmol/L): mean (SE) 2.58 (0.09) HDL (mmol/L): mean (SE) 1.39 (0.05) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 4.48 (0.11) TG (mmol/L): mean (SE) 1.11 (0.07) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Baseline data for intervention 14/30, control 13/30 and third arm 12/30 for HbA1c, total cholesterol, LDL, HDL and TG. Of the intervention arm 3 additional participants withdrew, and from the third trial arm 6 additional participants withdrew. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein high‐dairy (HPHD) diet. Consisting of 40% carbohydrate, 30% fat and 30% protein. Aerobic exercise 7 days a week to expend 250 kcal/day. Prescribed energy was maintenance energy requirements reduced by 500 kcal/d. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: NR Why? Higher‐protein, lower‐carbohydrate, energy‐restricted diets have been shown to help offset the lean mass loss observed with conventions (‐55% energy intake carbohydrate diets). The study was designed such that the HPDH group consumed twice the amount of protein (and lower carbohydrate) than the other 2 groups and twice the amount of dairy. What (materials)? Individualised plan outlining their required macronutrients intake in grams (corresponding to their new energy requirements); 2 study drinks/d (one immediately post‐exercise and another drink at least 5 hours before or after exercise); Splenda‐sweetened 1% chocolate milk). All dairy products required during the study period (1% artificially sweetened chocolate milk, white milk, yoghurt, cheddar cheese); set of measuring cups and spoon. Participants also received a SenseWear Pro energy expenditure device (BodyMedia). What (procedures)? HPHD group consumed a diet consisting of 40:30:30. HPHD group to consume 15% of energy/d from dairy protein at 6–7 servings/d of dairy products. All dairy products needed to control dairy and calcium intakes were provided to participants in the HPHD groups and were generously donated by Agropur Dairy Cooperative. All participants were prescribed the same exercise regimen and underwent individual biweekly dietary counselling during the 16 wks. Individual diet counselling led by study dietitian and research nutrition. Participants engaged in various modes of aerobic exercise every day, 5 d/wk with us and 2 d on their own on the weekend. They were required to consume twice daily drinks (375 mL) of 1% artificially sweetened chocolate milk, directly after exercise and another at least 5 h before or after exercise. Who provided? Diet: registered dietitians, research nutritionists. Exercise training: personal trainer, kinesiologist How and where? Private nutrition counselling on a biweekly basis, location NR. Exercise in main fitness centre at McMaster University (exercise sessions were mainly individual or in small groups (i.e. 1 trainer to 2‐3 participants). When and how much? Diet: Biweekly session exercise training: supervised aerobic exercise 5 days per week and 2 days unsupervised exercise on the weekend. At each workout participants were to expend 250 kcal. Supervised progressive resistance training regimen 2 days per week (upper body, lower body split). Weight lifted by each participant recorded every session and increased once they could complete 3 sets of 10 repetitions or more at any given weight. Strategies to improve or maintain fidelity; tailoring and modification: Every 2 wk thereafter, participants provided a 3‐d food record to track compliance with the nutrition protocol. All 3‐d food records were analysed with ESHA and participants were provided with feedback in their next biweekly private counselling session. During the week (Monday–Friday), they reported to our study office daily and were often given a SenseWear Pro energy expenditure device (BodyMedia) to wear, programmed for them, so they could track their energy expenditure during their workout. Compliance with weekend workouts was assessed by having women take home and wear the SenseWear Pro device on random occasions. Resistance exercise logs were completed by the trainer and aerobic exercise logs were completed by the participant and checked frequently by study personnel to ensure compliance. Extent of intervention fidelity: HPHD group consumed ‐1.3 g/(kg × d) protein for 16 wks, which could have contributed to the lean mass gain in this group. In addition, the protein and carbohydrate intakes of the HPHD group (30 and 40% daily energy intake, respectively) were not as excessive as those employed in more extreme protocols. Dietary protein consumption increased in the HPHD group, and dietary calcium and vitamin D intake increased in the HPHD over 16 wks. Concomitant interventions: NR Control diet: Name (as reported) and brief description: Adequate‐protein medium‐dairy (APMD) diet. Consisting of 55% carbohydrates, 30% fat and 15% protein. Aerobic exercise 7 days a week to expend 250 kcal/day. Prescribed energy was maintenance energy requirements reduced by 500 kcal/d. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: Healthy premenopausal women with a mean (SE) age of 30 (5.5) years, with a mean (SE) BMI of 31.4 (3.3) kg/m2, with a low diary consumption (~< 500 mg/d Ca2+) and a sedentary lifestyle (exercise less than once/week) Why? An adequate protein intake, along with medium dairy consumption, during a period of energy restriction, combined with daily exercise, may provide adequate levels of bone‐supporting nutrients to positively affect markers of bone health. What (materials)? Individualised plan outlining their required macronutrients intake in grams (corresponding to their new energy requirements); 2 study drinks/d (one immediately post‐exercise and another dink at least 5 hours before or after exercise); Splenda‐sweetened 1% chocolate milk). All dairy products required during the study period (1% artificially sweetened chocolate milk, white milk, yoghurt, cheddar cheese); set of measuring cups and spoon. Participants also received a SenseWear Pro energy expenditure device (BodyMedia). What (procedures)? Participants completed a FFQ for calcium and dairy foods to verify low baseline consumption. Thereafter, they were instructed to complete a 7‐day food record which provided a starting point on which dietary counselling was based. Maintenance energy requirements were calculated per participant, and reduced by 500 kcal/d in order to calculate each participant’s targeted total energy intake throughout the study. Participants were provided with an individualised plan according to a high‐protein, medium‐dairy diet that included 3 to 4 servings of dairy products (of total energy: 55% carbohydrate; 30% fat and 15% protein, of which 7.5% from dairy protein). They were required to consume twice daily drinks (375 mL) of 1% chocolate milk, directly after exercise and another at least 5 h before or after exercise. Who provided? Diet: registered dietitians, research nutritionists. Exercise training: personal trainer, kinesiologist How and where? Private nutrition counselling on a biweekly basis, location NR. Exercise in main fitness centre at McMaster University (exercise sessions were mainly individual or in small groups (i.e. 1 trainer to 2‐3 participants). When and how much? Diet: Biweekly sessions exercise training: supervised aerobic exercise 5 days per week and 2 days unsupervised exercise on the weekend. At each workout participants were to expend 250 kcal. Supervised progressive resistance training regimen 2 days per week (upper body, lower body split). Weight lifted by each participant recorded every session and increased once they could complete 3 sets of 10 repetitions or more at any given weight Strategies to improve or maintain fidelity; tailoring and modification: Every 2 wk thereafter, participants provided a 3‐d food record to track compliance with the nutrition protocol. All 3‐d food records were analysed with ESHA and participants were provided with feedback in their next biweekly private counseling session. During the week (Monday–Friday), they reported to our study office daily and were often given a SenseWear Pro energy expenditure device (BodyMedia) to wear, programmed for them, so they could track their energy expenditure during their workout. Compliance with weekend workouts was assessed by having women take home and wear the SenseWear Pro device on random occasions. Resistance exercise logs were completed by the trainer and aerobic exercise logs were completed by the participant and checked frequently by study personnel to ensure compliance. Extent of intervention fidelity: Dietary calcium and vitamin D intake increased in the APMD over 16 wk (r234). Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: IDEAL Trial funded by: Dairy Farmers of Canada, The Dairy Research Institute and the Canadian Institutes of Health Research Declaration of interest: "A. R. Josse, S. A. Atkinson, M. A. Tarnopolsky, and S. M. Phillips, no conflicts of interest." |
|
Juanola‐Falgarona 2014.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: ISRCTN54971867 Total number of trial arms: 3 Year trial started: 2010 Sample size calculation: Yes Outcome(s) used for sample size calculation: Weight loss Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Spain, outpatient university hospital in Reus Eligibility criteria: Participants were aged between 30 and 60 years with BMI between 27 and 35 kg/m2. Participants were excluded if they had uncontrolled type 2 diabetes (defined as HbA1c above 8%), systolic blood pressure or diastolic blood pressure above 159 or 99 mmHg, respectively, plasma LDL concentration above 160 mg/dL, plasma triglyceride concentration above 400 mg/dL; suspicion of secondary cause of obesity; presence of an inflammatory or chronic obstructive pulmonary disease, infection or active neoplasm; active endocrine or haematologic disease at the time of the study; blood leukocyte count ≥ 11 x 10^6 cells; were using anti‐inflammatory drugs, steroids, hormones or antibiotics which could affect study outcomes; had changes in medication for lipids, diabetes or hypertension in the past three months; were alcoholic or drug‐dependent (excluding tobacco); had a restrictive diet or more than 5 kg weight loss in the past three months; any medical condition which advised against inclusion; problems understanding the study, or reservations about adhering to the study diet. Type 2 diabetes at baseline: No ‐ confirmed by author: "T2D was not an exclusion criteria to participate in the study but, at the end, none of the participants had T2D". Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 122 Total attrition in trial: 18 Treatment diet Participants randomised: 41 Participants withdrawn (voluntary): 1 Total attrition: 5 Control diet: Participants randomised: 40 Participants withdrawn (voluntary): 0 Total attrition: 9 Baseline data treatment diet: Randomised participants not included: 1/41 Age (years): mean (SE) 44.0 (1.3) Gender distribution (as reported): female 33/40 (83%), male 7/40 (17%) Weight (kg): mean (SE) 82.7 (1.6) BMI (kg/m2): mean (SE) 30.8 (0.3) DBP (mmHg): mean (SE) 81.2 (1.5) SBP (mmHg): mean (SE) 128.0 (2.4) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.15 (0.10) HDL (mmol/L): mean (SE) 1.47 (0.05) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.13 (0.13) TG (mmol/L): mean (SE) 1.03 (0.70) Baseline data control diet: Randomised participants not included: 0/40 Age (years): mean (SE) 44.1 (1.3) Gender distribution (as reported): female 31/40 (78%), male 9/40 (22%) Weight (kg): mean (SE) 83.5 (1.7) BMI (kg/m2): mean (SE) 30.8 (0.3) DBP (mmHg): mean (SE) 82.8 (1.4) SBP (mmHg): mean (SE) 131.3 (2.2) HbA1c (%): NR LDL (mmol/L): mean (SE) 2.95 (0.10) HDL (mmol/L): mean (SE) 1.37 (0.05) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 4.82 (0.13) TG (mmol/L): mean (SE) 0.98 (0.85) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1: HOMA‐BCF (P = 0.037) and adiponectin (P = 0.020); NR between which arms Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐GI diet. Advised to consume 42% of energy from high‐GI carbohydrates, 18% from protein and 40% from fat. GI = 62. Energy prescription was determined by subtracting 500 kcal/d from the total estimated baseline energy intake. Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐balanced Exercise component? No Recipients: Healthy adults (83% female, 17% male), with a mean age of 44.0 (8.2) years and a mean BMI of 30.8 (1.9) kg/m2, with or without hypertension (5/40; 13%), hypercholesterlaemia (2/40; 5%) Why? To determine whether an isocaloric diet, rich in high‐glycaemic index carbohydrates has a smaller effect on weight loss, compared to a diet, rich in low‐glycaemic index carbohydrates. "...low‐carbohydrate diets were related to better improvements in the lipid profile. Nonetheless, in a pooled analysis that was based on observational studies, low‐carbohydrate diets seemed to be associated with increased risk of all‐cause mortality." What (materials)? "...subjects received a dossier containing a leaflet with written general dietary recommendations, biweekly menus, and seasonal receipts. An informative website was available for all participants (http://www.glyndiet.org/)." What (procedures)? "Participants were encouraged to eat whole grain cereals and pulses as the base of their diet, avoid the rice and potatoes, and were also recommended to select specific types of fruit (apple, orange, peach) and vegetables (courgette, tomato, onion) with low GI, avoiding the ripe pieces. They were advised to reduce the time cooking of carbohydrate rich‐foods in order to maintain the low GI of the foods. The principal animal protein sources of the diet were white fish and white meat. In order to obtain the desired weight loss, a 500 kcal restriction in diet was applied to each participant. Total daily energy expenditure for each participant was estimated using the WHO (2001) equations corrected by the physical activity degree. Diets were designed at 1500, 1700, 2000, and 2500 kcal/d, and all participants were categorised as having one of the 4 categories of dietary energy content after subtracting 500 kcal/d of the total estimated energy intake to achieve a desired weight loss." Who provided? Dietitian How and where? Face‐to‐face personalised advice was given to each patient. When and how much? Individual examination visits at baseline, after 2 weeks and thereafter monthly. Unclear at which of these visits the intervention was reinforced. The study authors reported: "Across the visits, different evaluations and questionnaires were conducted to assess changes on anthropometry and the adherence to the intervention". Strategies to improve or maintain fidelity; tailoring and modification: "Dietary intake was estimated at baseline and at the 1st, 3th and 6th month of intervention by mean of 3‐day dietary records including two workdays and a weekend day. Subjects were encouraged to weigh the food that they eat; otherwise trained dietitians estimated weight using an illustrated book of food portions. Energy and nutrient intake were calculated from Spanish food composition tables. Values of GI for each food were extracted from the International Glycemic Index and Glycemic Load Values using glucose as the reference scale." Extent of intervention fidelity: NR Concomitant interventions: NR Control diet: Name (as reported) and brief description: Low‐fat diet. Advised to consume 52% of energy from high‐GI carbohydrates, 18% from protein and 30% from fat. GI = 65. Energy prescription was determined by subtracting 500 kcal/d from the total estimated baseline energy intake. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Healthy adults (78% female, 22% male), with a mean age of 44.1 (8.2) years and a mean BMI of 30.8 (1.9) kg/m2, with or without hypertension (5/40; 13%), hypercholesterlaemia (5/40; 13%) Why? To determine whether an isocaloric low‐fat diet, rich in high‐glycaemic index carbohydrates, has a smaller effect on weight loss, compared to a diet, rich in low‐glycaemic index carbohydrates What (materials)? "...subjects received a dossier containing a leaflet with written general dietary recommendations, biweekly menus, and seasonal receipts. An informative website was available for all participants (http://www.glyndiet.org/)." What (procedures)? Subjects randomised to a low‐fat diet were also advised to maintain a high‐GI diet but with lower fat content. Participants had to follow a low‐fat diet fulfilling the criteria defined by the American Heart Association (30% of energy from fat, 52% of energy from high‐glycaemic index carbohydrates, 18% of energy from proteins). Participants were encouraged to eat refined grain cereals, fruits (banana, kiwi, melon) and vegetables (carrot, green bean, cabbage) with high GI, and avoid pulses. Additionally, daily sugar was substituted by glucose in order to rise GI of this intervention. In this case, they were recommended to avoid red meat and blue fish due its high fat content and also recommended to eat low‐fat dairy products. In order to obtain the desired weight loss, a 500 kcal restriction in diet was applied to each participant. Total daily energy expenditure for each participant was estimated using the WHO (2001) equations corrected by the physical activity degree. Who provided? Dietitian How and where? Face‐to‐face personalised advice was given to each patient. When and how much? Individual examination visits at baseline, after 2 weeks and thereafter monthly. Unclear at which of these visits the intervention was reinforced. The study authors reported: " Across the visits, different evaluations and questionnaires were conducted to assess changes on anthropometry and the adherence to the intervention". Strategies to improve or maintain fidelity; tailoring and modification: "Dietary intake was estimated at baseline and at the 1st, 3th and 6th month of intervention by mean of 3‐day dietary records including two workdays and a weekend day. Subjects were encouraged to weigh the food that they eat; otherwise trained dieticians estimated weight using an illustrated book of food portions. Energy and nutrient intake were calculated from Spanish food composition tables. Values of GI for each food were extracted from theInternational Glycemic Index and Glycemic Load Values using glucose as the reference scale." Extent of intervention fidelity: NR Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: No Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: GLYNDIET Trial funded by: Institut d'Investigació Sanitaria Pere Virgili (PV11059S) and the Fondo de Investigación Sanitaria (PI120153) Declaration of interest: "None of the authors had a personal or financial conflict of interest." |
|
Keogh 2007.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1, 2 Study design: RCT, parallel, single‐centre (1) Trial registry number: ACTRN12605000614695 Total number of trial arms: 2 Year trial started: NR Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in flow‐mediated dilatation (FMD) Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: 40 weeks Other notes about methods: NA |
|
| Participants |
Country and setting: Australia, outpatient research clinic; exact location NR Eligibility criteria: Participants were 20 to 65 years old with a BMI of 27 to 40 kg/m2. Participants were excluded if they had diabetes or a fasting glucose of > 7.0 mmol/L; resting blood pressure above 150/95 mmHg; used medication which could affect study measurements; had active liver or kidney disease, malignancy, current gastrointestinal disease; were pregnant or lactating or consumed > 50 g alcohol/day. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 36 Total attrition in trial: 31 Treatment diet Participants randomised: NR Participants withdrawn (voluntary): NR Total attrition: NR Control diet: Participants randomised: NR Participants withdrawn (voluntary): NR Total attrition: NR Baseline data treatment diet: Randomised participants not included: NR Age (years): mean (SE) 50.1 (1.4) Gender distribution (as reported): NR Weight (kg): mean (SE) 91.5 (4.1) BMI (kg/m2): mean (SE) 32.6 (1.0) DBP (mmHg): mean (SE) 74 (2) SBP (mmHg): mean (SE) 122 (4) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.5 (0.2) HDL (mmol/L): mean (SE) 1.3 (0.1) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.3 (0.2) TG (mmol/L): mean (SE) 1.7 (0.2) Baseline data control diet: Randomised participants not included: NR Age (years): mean (SE) 46.9 (1.6) Gender distribution (as reported): NR Weight (kg): mean (SE) 97.6 (2.4) BMI (kg/m2): mean (SE) 33.2 (0.8) DBP (mmHg): mean (SE) 76 (2) SBP (mmHg): mean (SE) 123 (4) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.8 (0.3) HDL (mmol/L): mean (SE) 1.3 (0.1) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.7 (0.3) TG (mmol/L): mean (SE) 1.4 (0.1) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1: SBP for completers (P value not given); intervention vs control Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low carbohydrate (LC) diet. Approximately 33% energy as carbohydrate, 27% as fat and 40% as protein (7% saturated fat, 6% PUFA and 13% MUFA) with 26 g fibre. Energy prescription of ~ 6000 kJ per day Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: N = 13 (gender NR) with mean (SE) age 50.1 (1.4) years, mean (SE) BMI 32.6 (1.0) kg/m2, mean (SE) weight 91.5 (4.1) kg Why? "Weight loss per se is usually associated with reductions in LDL‐C and reducing saturated fat also decreases LDL‐C. We therefore developed a diet low in saturated fat and low in carbohydrate to maximise LDL‐C reduction and reduce glucose concentrations designed to achieve moderate weight loss in order to test whether the combined effects with weight loss would improve FMD." What (materials)? NR What (procedures)? Participants were prescribed a weight‐loss diet that was low carbohydrate: 33% energy as carbohydrate, 27% energy as fat, 40% energy as protein, 7% energy from saturated fat, 6% energy from PUFA and 13% energy from MUFA and 26 g fibre. Both diets were designed to be 6000 kJ so that weight loss would approximate 0·5–1 kg per week. Subjects saw the dietitian individually at the start of weight loss and every 2 weeks and also attended monthly group meetings for follow‐up consultations. Who provided? Dietitian How and where? Face‐to‐face at the Commonwealth Scientific Industrial Research Organisation clinic When and how much? Every two weeks during the weight loss phase and monthly during the follow‐up phase Strategies to improve or maintain fidelity; tailoring and modification: "Records of daily food intake were maintained in order to monitor food intake. The dietary data were analysed using FoodWorks version 3.1 dietary analysis software (Xyris Software, Highgate Hill, Australia). In order to ensure that the energy restriction was approximately 30% below estimated usual intake a FFQ was completed at baseline to assess usual energy and nutrient intake." Extent of intervention fidelity: NR Concomitant interventions: NR Control diet: Name (as reported) and brief description: High‐carbohydrate (HC) diet. Approximately 20% energy as fat, 20% as protein and 60% from carbohydrate (4% saturated fat, 5% PUFA and 7% MUFA) with 40 g fibre. Energy prescription of ~6000 kJ per day Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 12 (gender NR) with mean (SE) age 469 (1.6) years, mean (SE) BMI 33.2 (0.8) kg/m2, mean (SE) weight 97.6 (2.4) kg Why? NR What (materials)? NR What (procedures)? Participants were prescribed a high‐carbohydrate, low‐fat (20% energy as fat, 20% energy as protein and 60% energy as carbohydrate, 4% energy from saturated fat, 5% from PUFA and 7% from MUFA and 40 g fibre). Both diets were designed to be 6000 kJ so that weight loss would approximate 0·5–1 kg per week. Subjects saw the dietitian individually at the start of weight loss and every 2 weeks and also attended monthly group meetings for follow‐up consultations. Who provided? Dietitian How and where? Face‐to‐face at the Commonwealth Scientific Industrial Research Organisation clinic When and how much? Every two weeks during the weight loss phase and monthly during the follow‐up phase Strategies to improve or maintain fidelity; tailoring and modification: "Records of daily food intake were maintained in order to monitor food intake. The dietary data were analysed using FoodWorks version 3.1 dietary analysis software (Xyris Software, Highgate Hill, Australia). In order to ensure that the energy restriction was approximately 30% below estimated usual intake a FFQ was completed at baseline to assess usual energy and nutrient intake." Extent of intervention fidelity: NR Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Partial funding by Diabetes Australia Research Trust (DART) Declaration of interest: NR |
|
Kitabchi 2013.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT01642849 Total number of trial arms: 2 Year trial started: NR Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in weight Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: NA Other notes about methods: ITT approach was not used. |
|
| Participants |
Country and setting: USA, outpatient clinical research centre at a university in Knoxville. All food required to complete the respective diets were provided, predominantly as frozen foods. Eligibility criteria: Premenopausal women aged 20–50 years with a BMI > 30 to < 55 kg/m2. fasting glucose < 110 mg/dL and 2‐h glucose level < 170 mg/dL. Participants were excluded if they had proteinuria or elevated serum creatinine (> 1.5 mg/dL), surgical or premature menopause, history of liver disease or abnormal liver function tests, diabetes, thyroid disease with abnormal thyrotropin, weight > 350 lbs, triglycerides > 400 mg/dL or LDL cholesterol > 160 mg/dL, systolic blood pressure > 145 mmHg or diastolic blood pressure > 100 mmHg, used medications known to affect lipid or glucose metabolism (niacin, steroids, and statins), were pregnant or wanted to become pregnant in the next six months, had weight loss of > 5% of body weight in the last six months, or had a history of cancer undergoing active treatment. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Female Total number randomised: 32 Total attrition in trial: 8 Treatment diet Participants randomised: 14 Participants withdrawn (voluntary): 2 Total attrition: 2 Control diet: Participants randomised: 18 Participants withdrawn (voluntary): 6 Total attrition: 6 Baseline data treatment diet: Randomised participants not included: 2/14 Age (years): mean (SE) 35.9 (2.1) Gender distribution (as reported): female 100%, male 0% Weight (kg): NR BMI (kg/m2): mean (SD) 41.3 (6.24) DBP (mmHg): mean (SD) 83 (1.3) SBP (mmHg): mean (SD) 129 (1.5) HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Baseline data control diet: Randomised participants not included: 6/18 Age (years): mean (SE) 35.4 (2.0) Gender distribution (as reported): female 100%, male 0% Weight (kg): NR BMI (kg/m2): mean (SD) 37.0 (5.2) DBP (mmHg): mean (SD) 82 (1.4) SBP (mmHg): mean (SD) 128 (1.7) HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Group differences at baseline: no Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Only the baseline characteristics for completers (12 in intervention and 12 in control group) were reported. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein low‐carbohydrate (HP) diet, 40% carbohydrates, 30% fat and 30% protein. Energy prescription 500 kcal/day from calculated maintenance needs established by resting energy expenditure Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: N = 12 women with a mean (SE) age of 35.9 (2.1) years and mean (SE) BMI of 41.3 (1.8) kg/m2 with no history of diabetes or prediabetes Why? A hypocaloric high‐protein diet may result in the suppression of hunger and induction of satiety, resulting in weight loss. It may also result in a positive nitrogen balance, therefore assisting in the maintenance of lean body mass, compared to a hypocaloric high‐carbohydrate diet. Furthermore, it may have positive effects on metabolic parameters, such as b‐cell function, cardiovascular risk factors, oxidative stress, and lipid peroxidation. What (materials)? At baseline, an individualised weekly food diary (which included the participant’s preferences) was printed and given to each subject with instructions for recording all food intake on a daily basis. Weekly provision of fresh, prepackaged, and frozen foods for the duration of the study. Most of the entrees for the diet were frozen entrees purchased directly from the manufacturer. The diet included 1 cup of frozen vegetables at both lunch and dinner. Snacks in between meals included shakes and meal bars. Participants were given all food needed to meet their dietary assignment for the duration of the study. All food was dispensed by the dietitian at the CRC on a weekly basis to each participant. An individualised weekly food diary (which included the participant’s preferences) was printed and given to each subject with instructions for recording all food intake on a daily basis. What (procedures)? High‐protein (40% carbohydrates, 30% fat, and 30% protein) hypocaloric diet for 6 months. Weight reduction caloric needs were established for each individual by subtracting 500 kcal/day from their calculated maintenance needs established by REE. Weight loss of 1–2 lbs weekly was targeted. On average, an 1800 kcal/day diet for a 70‐kg individual was used to achieve adequate weight loss. If a subject reached a plateau and did not lose weight for two consecutive weeks, calories were reduced by an additional 200 kcal or to a minimum of 1200 kcal/day. Caloric adjustments were made by altering the amount of supplement bars and/or shakes. Since these were very similar to the nutrient composition of each diet, they could be easily taken out of the diet to reduce calories but maintain adequate nutrient composition. The study diets provided more than the recommended amount of calcium (1000 mg/day) for women 20–50 years of age by providing an average of 1725 mg for HC and 1684 mg for HP diets. Both HP and HC diets were designed to minimise participant health risks. Dietary fat sources focussed on monounsaturated and polyunsaturated fats, i.e. plant oils, semi‐liquid margarine, and nuts; dietary carbohydrate sources emphasised whole grains, fruits, vegetables, and legumes; and dietary protein sources included lean meats, fish, chicken, eggs, and nonfat dairy foods, i.e. fat‐free milk and low‐fat cheese, consistent with American Diabetes Association and Institute of Medicine guidelines. Who provided? Dietitian How and where? "All participants were seen in the Clinical Research Center (CRC) at the University of Tennessee Health Science Center for all their visits." When and how much? Every week for 24 weeks. If a participant did not lose weight for two consecutive weeks, calories were reduced by an additional 200 kcal or to a minimum of 1200 kcal/day altering the amount of supplement bars and/or shakes provided. Strategies to improve or maintain fidelity; tailoring and modification: Subjects were asked to turn in completed food diaries each week in order to pick up the next week’s food. At times, the dietitian adjusted the participant’s meal choices if issues arose regarding compliance. Studies have shown increased dietary compliance with frequent interaction, an individualised diet with food variety, and some form of food recording system. The study dietitian assessed compliance by both subjective and objective parameters including weekly individual contact with patients that included a detailed review of their food diaries. Extent of intervention fidelity: Compliance mean (SE) 94 (1.5)% Concomitant interventions: NR Control diet: Name (as reported) and brief description: High‐carbohydrate low‐protein (HC) diet, 55% carbohydrates, 30% fat and 15% protein. Energy prescription 500 kcal/day from calculated maintenance needs established by resting energy expenditure Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 12 women with a mean (SE) age of 35.4 (2.0) years and mean (SE) BMI of 37.0 (1.5) kg/m2 with no history of diabetes or prediabetes Why? A hypocaloric high‐protein diet may result in the suppression of hunger and induction of satiety, resulting in weight loss. It may also result in a positive nitrogen balance, therefore assisting in the maintenance of lean body mass, compared to a hypocaloric high‐carbohydrate diet. Furthermore, it may have positive effects on metabolic parameters, such as b‐cell function, cardiovascular risk factors, oxidative stress, and lipid peroxidation. What (materials)? At baseline, an individualised weekly food diary (which included the participant’s preferences) was printed and given to each subject with instructions for recording all food intake on a daily basis. Weekly provision of fresh, prepackaged, and frozen foods for the duration of the study. Most of the entrees for the diet were frozen entrees purchased directly from the manufacturer. The diet included 1 cup of frozen vegetables at both lunch and dinner. Snacks in between meals included shakes and meal bars. Participants were given all food needed to meet their dietary assignment for the duration of the study. All food was dispensed by the dietitian at the CRC on a weekly basis to each participant. An individualised weekly food diary (which included the participant’s preferences) was printed and given to each subject with instructions for recording all food intake on a daily basis. What (procedures)? "High‐carb (55% carbohydrates, 30% fat, and 15% protein) hypocaloric diet for 6 months. Weight reduction caloric needs were established for each individual by subtracting 500 kcal/day from their calculated maintenance needs established by REE. Weight loss of 1–2 lbs weekly was targeted. On average, an 1800 kcal/day diet for a 70‐kg individual was used to achieve adequate weight loss. If a subject reached a plateau and did not lose weight for two consecutive weeks, calories were reduced by an additional 200 kcal or to a minimum of 1200 kcal/day. Caloric adjustments were made by altering the amount of supplement bars and/or shakes. Since these were very similar to the nutrient composition of each diet, they could be easily taken out of the diet to reduce calories but maintain adequate nutrient composition. The study diets provided more than the recommended amount of calcium (1000 mg/day) for women 20–50 years of age by providing an average of 1725 mg for HC and 1684 mg for HP diets. Both HP and HC diets were designed to minimise participant health risks. Dietary fat sources focussed on monounsaturated and polyunsaturated fats, i.e. plant oils, semi‐liquid margarine, and nuts; dietary carbohydrate sources emphasised whole grains, fruits, vegetables, and legumes; and dietary protein sources included lean meats, fish, chicken, eggs, and nonfat dairy foods, i.e. fat‐free milk and low‐fat cheese, consistent with American Diabetes Association and Institute of Medicine guidelines." Who provided? Dietitian How and where? "All participants were seen in the Clinical Research Center (CRC) at the University of Tennessee Health Science Center for all their visits." When and how much? Every week for 24 weeks. If a participant did not lose weight for two consecutive weeks, calories were reduced by an additional 200 kcal or to a minimum of 1200 kcal/day altering the amount of supplement bars and/or shakes provided. Strategies to improve or maintain fidelity; tailoring and modification: Subjects were asked to turn in completed food diaries each week in order to pick up the next week’s food. At times, the dietitian adjusted the participant’s meal choices if issues arose regarding compliance. Studies have shown increased dietary compliance with frequent interaction, an individualised diet with food variety, and some form of food recording system. The study dietitian assessed compliance by both subjective and objective parameters including weekly individual contact with patients that included a detailed review of their food diaries. Extent of intervention fidelity: Compliance mean (SE) 9 (4.8)% Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: No Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: American Diabetes Association (grant 1‐09‐CR‐32) Declaration of interest: "No potential conflicts of interest relevant to this article were reported." |
|
Klemsdal 2010.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT00230919 Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Norway, outpatient hospital clinic in Oslo Eligibility criteria: Participants aged 30 to 65 with a BMI between 28 and 40 kg/m2 (for men) and 28 to 35 kg/m2 (for women), and at least one criterion of metabolic syndrome as defined by the National Cholesterol Education Program. Participants were excluded if they had symptomatic cardiovascular disease, except for those on stable antihypertensives, or diabetes requiring medication; were taking lipid lowering or weight‐reducing drugs in the 12 weeks prior; or had a history of eating disorder. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 202 Total attrition in trial: 38 Treatment diet Participants randomised: 100 Participants withdrawn (voluntary): NR Total attrition: 22 Control diet: Participants randomised: 102 Participants withdrawn (voluntary): NR Total attrition: 16 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 50.1 (9.3) Gender distribution (as reported): female 54%, male 46% Weight (kg): mean (SD) 100.0 (16.4) BMI (kg/m2): NR DBP (mmHg): mean (SD) 91 (8.5) SBP (mmHg): mean (SD) 130 (12.7) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.76 (0.94) HDL (mmol/L): mean (SD) 1.28 (0.37) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.8 (0.97) TG (mmol/L): mean (SD) 1.93 (1.21) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 49.9 (8.4) Gender distribution (as reported): female 62%, male 38% Weight (kg): mean (SD) 99.9 (14.7) BMI (kg/m2): NR DBP (mmHg): mean (SD) 92 (9.9) SBP (mmHg): mean (SD) 129 (15.8) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.84 (1.01) HDL (mmol/L): mean (SD) 1.29 (0.37) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 6.0 (1.04) TG (mmol/L): mean (SD) 1.91 (1.13) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐glycaemic load (LGL) diet, aimed to achieve intake of 35 to 40% of energy from fat, 25 to 30% from protein and 30 to 35% from carbohydrates and up to 3% from alcohol. A 500 kcal/d deficit relative to the estimated daily energy requirements was recommended. Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐high Exercise component? No Recipients: Participants with varying degrees of the metabolic syndrome (mean 2.8) , 54% female, aged 50.1 (9.3) with weight 100.0 (16.4) kg. Why? Recent evidence shows that low‐carbohydrate diets are as effective as low‐fat diets in reducing body weight for up to one year, with some variation in the effect on lipids. Low‐carbohydrate diets are an alternative which are still being assessed for long‐term safety. What (materials)? Dietary suggestions and recipes were made available. What (procedures)? After a 48‐hour dietary recall at screening, subjects were instructed to follow a low‐glycaemic load diet and asked to fill each plate of food with one‐third protein. For two meals per day the remaining two‐thirds were vegetables, salads and legumes; for the third meal the balance of the plate was made up of low‐glycaemic index bread and other carbohydrates. Individual and group dietary counselling sessions were offered over the course of the study, as well as a seven‐day dietary recall during the intervention period. Who provided? A dietitian provided individual and group counselling sessions. How and where? Face‐to‐face counselling sessions with dietitian, in individual as well as group format. Location of sessions NR When and how much? Individual counselling sessions with the dietitian occurred at baseline, week 2, month 1 and month 9. Group sessions led by the dietitians occurred at months 2, 3, 4, 5 and 6. Duration of sessions NR Strategies to improve or maintain fidelity; tailoring and modification: After the first month, sessions were held in groups until month 6; seven‐day dietary recall at month 3, regular weigh‐in as part of study outcomes. A 48‐h dietary recall was obtained at the screening visit to help the dietitian evaluate the diet prior to intervention. A 7‐day dietary questionnaire was obtained at 3 months. Extent of intervention fidelity: NR Concomitant interventions: antihypertensives Control diet: Name (as reported) and brief description: Low‐fat diet, aimed to achieve intake of < 30% energy from fat, about 15% from protein and 55 to 60% from carbohydrates and up to 3% from alcohol. A 500 kcal/d deficit relative to the estimated daily energy requirements was recommended. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Participants with varying degrees of the metabolic syndrome (mean 2.9), 62% female, aged 49.9 (8.4) with weight 99.9 (14.7) kg Why? Epidemiological studies report mixed findings on the role of total carbohydrate intake, as well as proportion of high‐glycaemic index carbohydrates, as a cause of obesity and diabetes, therefore the European Society of Cardiology and American Heart Association have not thus far recommended reducing total carbohydrates or using glycaemic index to guide diets. What (materials)? Dietary recommendations were based on the 2004 Nordic guidelines (Becker 2005) as well as the 2007 lifestyle recommendations of the European Society of Cardiology (Graham 2007). What (procedures)? After a 48‐hour dietary recall at screening, subjects were asked to follow a low‐fat diet with a focus on unrefined, high‐fibre carbohydrates; subjects were not instructed to consume low‐glycaemic index carbohydrates. Individual and group dietary counselling sessions were offered over the course of the study, as well as a seven‐day dietary recall during the intervention period. Who provided? A dietitian provided individual and group counselling sessions. How and where? Face‐to‐face counselling sessions with dietitian, in individual as well as group format. Location of sessions NR When and how much? Individual counselling sessions with the dietitian occurred at baseline, week 2, month 1 and month 9. Group sessions led by the dietitians occurred at months 2, 3, 4, 5 and 6. Duration of sessions NR Strategies to improve or maintain fidelity; tailoring and modification: After the first month, sessions were held in groups until month 6; seven‐day dietary recall at month 3, regular weigh‐in as part of study outcomes. A 48‐h dietary recall was obtained at the screening visit to help the dietitian evaluate the diet prior to intervention. A 7‐day dietary questionnaire was obtained at 3 months. Extent of intervention fidelity: NR Concomitant interventions: antihypertensives |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Norwegian National Research Council grant Declaration of interest: NR |
|
Krebs 2012.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, multi‐centre (3) Trial registry number: ACTRN12606000490572 Total number of trial arms: 2 Year trial started: 2007 Sample size calculation: Yes Outcome(s) used for sample size calculation: Difference in weight and waist circumference Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 24 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: New Zealand, outpatient clinical centres in Wellington, Auckland and Christchurch Eligibility criteria: Participants with type 2 diabetes, according to WHO criteria, aged between 30 and 76 years with a BMI of ≥ 27 kg/m2. Participants were excluded if they were currently taking weight‐reducing medication; had lost > 5% weight in the past three months; had a psychiatric or eating disorder, HbA1c above 9.5% (80 mmol/mol); or suffered from renal disease (defined as glomerular filtration rate < 60 mL/min or urine albumin:creatinine ratio above 30 mg/mmol), abnormal liver enzymes, heart failure. active malignancies or myocardial infarction in the six months prior. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 419 Total attrition in trial: 141 Treatment diet Participants randomised: 207 Participants withdrawn (voluntary): 48 Total attrition: 71 Control diet: Participants randomised: 212 Participants withdrawn (voluntary): 50 Total attrition: 70 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 57.7 (9.9) Gender distribution (as reported): female 112/207 (54%), male 95/207 (46%) Weight (kg): mean (SD) 103.4 (19.7) BMI (kg/m2): mean (SD) 36.6 (6.7) DBP (mmHg): mean (SD) 76.8 (10.3) SBP (mmHg): mean (SD) 131.1 (14.8) HbA1c (%): mean (SD) 8.1 (1.2) LDL (mmol/L): mean (SD) 2.74 (0.91) HDL (mmol/L): mean (SD) 1.09 (0.32) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.77 (0.98) TG (mmol/L): median (IQR) 1.74 (1.32 to 2.29) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 58.0 (9.2) Gender distribution (as reported): female 139/212 (66%), male 73/212 (34%) Weight (kg): mean (SD) 101.9 (20.1) BMI (kg/m2): mean (SD) 36.7 (6.4) DBP (mmHg): mean (SD) 76.6 (11.0) SBP (mmHg): mean (SD) 130.6 (17.0) HbA1c (%): mean (SD) 8.0 (1.2) LDL (mmol/L): mean (SD) 2.67 (0.92) HDL (mmol/L): mean (SD) 1.11 (0.28) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.61 (1.03) TG (mmol/L): median (IQR) 1.61 (1.18 to 2.33) Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐fat high‐protein diet. Prescribed 30% of energy as protein, 40% as carbohydrate and 30% as fat. Energy prescription to reduce total energy intake by 2000 kJ/day using an individualised dietary prescription based on estimation of energy requirements Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: Subjects were 54% female, aged mean (SD) 57.7 (9.9), BMI mean (SD) 36.6 (6.7). 61.8% on lipid‐lowering drugs, 77.3% on BP‐lowering drugs and years on average with the diagnosis of diabetes of over 7 years. Why? Substituting protein for carbohydrate while maintaining reduced total fat may have particular benefits in type 2 diabetes. High‐protein diets promote weight loss, maintain lean body mass and improve lipid and glycaemic profiles in obese non‐diabetic individuals. Studies specifically in type 2 diabetes are limited, are generally short, often using very intensive interventions and/or providing a significant proportion of participants’ food, making the translation of the findings to a general population difficult. Therefore the specific long‐term effect of increasing protein intake in individuals with type 2 diabetes in a free‐living situation, using an intervention that can be realistically implemented, requires further investigation. What (materials)? Portion charts, sample diet plans and culturally appropriate recipes were made available for specific ethnic groups. What (procedures)? low‐fat high‐protein (30% of energy as protein, 40% as carbohydrate, 30% as fat). Group sessions every 2 weeks with an education component and time for discussing and concluded with goal‐setting. Dietary counselling included information on appropriate intakes and physical activity advice. Who provided? Dietitian How and where? Face‐to‐face, individual and group sessions When and how much? At the beginning of the study, individualised dietary prescription based on estimation of energy requirements were discussed on a one‐to‐one basis with each participant. Participants mainly had group sessions thereafter which were 1 hour long consisting of diet‐specific information and education and were conducted every 2 weeks for the first 6 months, then every month for the second 6 months. No further dietary advice was offered by the dietitians after 12 months. Participants were then asked to continue following their prescribed diets on their own in the second year. Outcome measures were assessed at 6, 12 and 24 months. Strategies to improve or maintain fidelity; tailoring and modification: The programmes were specifically designed for delivery in a ‘real‐world’ setting, keeping the time commitment for both participants and staff to levels that could be readily achieved in most healthcare systems. Ongoing self‐recording of food intake. Weekly text or email reminders and motivational messages were also offered to participants to enhance adherence to the diets. Extent of intervention fidelity: NR Concomitant interventions: Hypoglycemics, lipid‐lowering and antihypertensives Control diet: Name (as reported) and brief description: Low‐fat high‐carbohydrate diet. Prescribed 15% of energy as protein, 55% as carbohydrate and 30% as fat. Energy prescription to reduce total energy intake by 2000 kJ/day using an individualised dietary prescription based on estimation of energy requirements Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Subjects were 66% female, aged mean (SD) 58.0 (9.2), BMI mean (SD) 36.7 (6.4). 69.3% on lipid‐lowering drugs, 74.5% on BP‐lowering drugs and on average with the diagnosis of diabetes of over 7 years. Why? NR What (materials)? Portion charts, sample diet plans and culturally appropriate recipes were made available for specific ethnic groups. What (procedures)? a low‐fat high‐carbohydrate (15% of energy as protein, 55% as carbohydrate, 30% as fat) diet. Group sessions every 2 weeks with an education component and time for discussing and concluded with goal‐setting. Dietary counselling included information on appropriate intakes and physical activity advice. Who provided? Dietitians How and where? Face‐to‐face, individual and group sessions When and how much? At the beginning of the study, individualised dietary prescription based on estimation of energy requirements were discussed on a one‐to‐one basis with each participant. Participants mainly had group sessions thereafter which were 1 hour long consisting of diet‐specific information and education and were conducted every 2 weeks for the first 6 months, then every month for the second 6 months. No further dietary advice was offered by the dietitians after 12 months. Participants were then asked to continue following their prescribed diets on their own in the second year. Outcome measures were assessed at 6, 12 and 24 months. Strategies to improve or maintain fidelity; tailoring and modification: The programmes were specifically designed for delivery in a ‘real‐world’ setting, keeping the time commitment for both participants and staff to levels that could be readily achieved in most healthcare systems. Ongoing self‐recording of food intake. Weekly text or email reminders and motivational messages were also offered to participants to enhance adherence to the diets. Extent of intervention fidelity: NR Concomitant interventions: Hypoglycemics, lipid‐lowering and antihypertensives |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: Yes Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: DEWL Trial funded by: Health Research Council of New Zealand (grant 06/337) Declaration of interest: "The authors declare that there is no duality of interest associated with this manuscript." |
|
Landers 2002.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, number of centres NR Trial registry number: NR Total number of trial arms: 3 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely USA based on authors and journal Eligibility criteria: Participants aged 18 to 55 who were obese with BMI > 27 kg/m2 and had certification from a primary care physician. Participants were excluded if they had a history of kidney or liver disease; were pregnant or lactating, medicated for hypertension; had diabetes or hypercholesterolemia. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 91 Total attrition in trial: 42 Treatment diet Participants randomised: 28 Participants withdrawn (voluntary): NR Total attrition: 12 Control diet: Participants randomised: 33 Participants withdrawn (voluntary): NR Total attrition: 12 Baseline data treatment diet: Randomised participants not included: NR Age (years): NR Gender distribution (as reported): NR Weight (kg): NR BMI (kg/m2): NR DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Baseline data control diet: Randomised participants not included: NR Age (years): NR Gender distribution (as reported): NR Weight (kg): NR BMI (kg/m2): NR DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Low‐carbohydrate high‐protein (LCHP) ad libitum diet; participants were instructed to maintain carbohydrate intake of less than 30 g/day and dietitian calculated the minimum grams of high biological value (HBV) protein to be eaten daily. Treatment diet type (carbohydrate‐fat‐protein): Very low‐unclear‐unclear Exercise component? No Recipients: Healthy adults aged 18 to 55 years, with a BMI > 27 kg/m2 Why? To determine changes in lean body mass and fat mass using a DEXA scan on ketogenic diets What (materials)? Diet materials (not specified), sample menus and carbohydrate counting booklets What (procedures)? Based on the measurement of lean body mass (DEXA), the minimum daily amount of high biological value protein (HBV) was calculated. Participants were instructed on HBV sources. A carbohydrate counter booklet assisted them in maintaining their carbohydrate intake at < 30 grams per day. They were encouraged to use salt and Morton Lite Salt ad libitum to supplement duiretic losses of sodium and potassium. Participants were instructed to continue their current physical activity, to drink at 64 oz of non‐caffeinated calorie‐free fluid per day and take a multiple vitamin and mineral supplement. No specific exercise routine was recommended. Who provided? Dietitian How and where? Individualised face‐to‐face counselling at the clinic When and how much? Weekly (12 visits). At each follow‐up visit the diet was reinforced. Strategies to improve or maintain fidelity; tailoring and modification: Participants recorded their food intake in a diary; this diary was reviewed weekly. They were weighed and also provided a weekly urine specimen to check for ketones. Extent of intervention fidelity: The extent of dietary non‐compliance was NR for this intervention group. However, the study authors reported the following:" ... it was evident that some subjects were non‐compliant to the diet." ; "LCHP dieters did not consistently spill ketones in the urine"; "There were subjects on LCHP diets who continued to eat beans, cornbread, and other starchy foods on a regular basis despite weekly education". Concomitant interventions: NR Control diet: Name (as reported) and brief description: Conventional diet; macronutrient distribution of 50% carbohydrate, 20% protein and 30% fat. Energy prescription was based on adjusted body weight and the Harris‐Benedict equation to estimate energy needed to promote 0.45 kg weight loss per week. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Healthy adults aged 18 to 55 years, with a BMI > 27 kg/m2 Why? To provide data on body composition changes on a conventional weight loss diet, such as the amount of lean and fat mass) during periods of weight loss What (materials)? Diet plans were based on the diabetic exchange lists and each subject received a customised sample meal plan as well as a copy of Exchange lists for meal planning. A food diary was also provided. What (procedures)? Using adjusted body weight (actual weight ‐ ideal weight) * 0.25 + ideal body weight) and the Harris Benedict equation estimated energy needed to promote weight loss of 0.45kg. No subject was given a diet less than 1200 kCal. All subjects were instructed to continue their physical activity, drink at least 64 oz of non‐caffeinated calorie‐free fluid per day, take a multiple mineral and vitamin supplements. Who provided? Dietitian How and where? Individualised face‐to‐face counselling at the clinic When and how much? Weekly (12 visits). At each follow‐up visit the diet was reinforced. Strategies to improve or maintain fidelity; tailoring and modification: Participants recorded their food intake in a diary; this diary was reviewed weekly. They were also weighed. Extent of intervention fidelity: The extent of dietary non‐compliance was NR for this intervention group. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: NR Declaration of interest: NR |
|
Larsen 2011.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3, 4 Study design: RCT, parallel, single‐centre (1) Trial registry number: ACTRN12605000063617 Total number of trial arms: 2 Year trial started: 2005 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in HbA1c Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 3 months What was the duration of the weight maintenance phase: 9 months Other notes about methods: "This study used the single imputation method of last measurement carried forward for missing data for primary and secondary outcomes." |
|
| Participants |
Country and setting: Australia, outpatient heart and diabetes institute in Melbourne Eligibility criteria: Participants aged 30–75 years, with a BMI of 27–40 kg/m2 and type 2 diabetes (HbA1c levels of 6.5–10%). Participants were excluded if they had significant heart disease (unstable angina, cardiac failure, or recent myocardial infarction or coronary intervention), stroke within the previous three months, renal disease (proteinuria or serum creatinine > 0.13 mmol/L), liver disease, or malignancy. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 108 Total attrition in trial: 34 Treatment diet Participants randomised: 57 Participants withdrawn (voluntary): 16 Total attrition: 19 Control diet: Participants randomised: 51 Participants withdrawn (voluntary): 15 Total attrition: 15 Baseline data treatment diet: Randomised participants not included: 4/57 Age (years): mean (SD) 59.6 (7.99) Gender distribution (as reported): female 23/53 (43%), male 30/53 (57%) Weight (kg): mean (SD) 94.6 (15.41) BMI (kg/m2): NR DBP (mmHg): mean (SD) 81.5 (7.61) SBP (mmHg): mean (SD) 131.8 (11.14) HbA1c (%): mean (SD) 7.89 (0.97) LDL (mmol/L): mean (SD) 2.49 (0.78) HDL (mmol/L): mean (SD) 1.19 (0.24) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.73 (0.85) TG (mmol/L): mean (SD) 2.39 (1.39) Baseline data control diet: Randomised participants not included: 5/51 Age (years): mean (SD) 58.8 (10.21) Gender distribution (as reported): female 28/46 (61%), male 18/46 (39%) Weight (kg): mean (SD) 95.5 (14.01) BMI (kg/m2): NR DBP (mmHg): mean (SD) 81.5 (9.34) SBP (mmHg): mean (SD) 127.4 (9.86) HbA1c (%): mean (SD) 7.78 (0.95) LDL (mmol/L): mean (SD) 2.42 (0.90) HDL (mmol/L): mean (SD) 1.20 (0.28) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.71 (1.06) TG (mmol/L): mean (SD) 2.37 (1.82) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Baseline characteristics were reported for ITT population. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein (HP) diet consisting of 30% of energy from protein (lean meat, chicken and fish), 40% from carbohdyrate and 30% from fat (7% saturated, 10% PUFA and 13% MUFA). Energy prescription of ∼6400 kJ/day or 30% energy restriction for three months, followed by energy balance for nine months Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: Participants were n = 30 (57% ) male with a mean (95% CI) age of 59.6 (57.5, 61.8) years, mean (95% CI) duration of DM 8.7 (6.8, 10.5) years, mean (95% CI) weight 94.6 (90.5, 98.8) kg, mean (95% CI) HbA1C 7.89 (7.63, 8.15) %, mean (95% CI) total cholesterol 4.73 (4.50, 4.96) mmol/L, mean (95% CI) LDL 2.49 (2.28, 2.70) mmol/L, mean (95% CI) HDL 1.19 (1.11, 1.24) mmol/L, mean (95% CI) triglycerides 2.39 (2.01, 2.76) mmol/L, mean (95% CI) systolic BP 131.8 (128.8, 134.8) mmHg, mean (95% CI) diastolic BP 81.5 (79.5, 83.6) mmHg. Why? A high‐protein low‐carbohydrate diet may improve glycaemic control and insulin sensitivity in people with type 2 diabetes mellitus; however the long term health effects of such a diet is poorly understood. What (materials)? "written materials were supplied to both groups containing information on the key nutrition intervention messages, prescriptive fixed menu plans and food choice lists." What (procedures)? Dietary intakes of each participant were calculated from weighed/measured food records collected at baseline (5 days). Diet‐specific advice was administered to each study participant. The diet consisted of 30% energy intake from protein (a combination of lean meat, chicken and fish) and 40% energy intake from carbohydrate; low‐glycaemic index carbohydrates were recommended. The diet provided 30% of total energy from fat (7% saturated fat, 10% polyunsaturated fat, 13% monounsaturated fat). The dietary intervention consisted of an initial 3‐month energy restriction (6400 kJ/day or 30% energy restriction), followed by 9 months of energy balance. Topics at group meetings included healthy cooking, goal‐setting and problem‐solving, physical activity, and supportive counselling. Physical activity was encouraged as a strategy to increase energy expenditure for those without limitations or complications, and the recommendations were consistent with public health guidelines. Who provided? Dietitian How and where? Face to‐face dietary counselling appointments conducted at the Baker IDI Heart and Diabetes Institute (Melbourne, Australia) When and how much? Individual sessions; four visits during the 3‐month energy restrictive period (total of 2.5 hours), and five visits during the 9 months of energy balance (total of 2.5 hours). Group meetings: every 3 months (total of 3.3 hours) Strategies to improve or maintain fidelity; tailoring and modification: "Dietary intakes were calculated from weighed/measured food records collected at baseline (5 days) and every 3 months during the intervention period (1 day/month) using Australia‐specific dietary analysis software (Foodworks; Xyris Software, Highgate Hill, QLD, Australia). Dietary compliance was monitored by self‐reported food intakes and 24‐h urine samples for an assessment of urea excretion as a marker of protein intake." Self‐reported physical activity was measured using the validated Active Australia survey questionnaire. Extent of intervention fidelity: Attendance at individual sessions: 25% of participants attended 9 individual sessions; while 21% missed one session. Attendance at group sessions: ranged from 26% to 37% per session. Ability to self‐manage the diet (score): median 4 (IQR 3 to 4). Urinary nitrogen excretion: study authors reported higher levels at 3 months, and at 12 months; however they stated the following at 12 months: "significance was lost following conservative statistical correction". Concomitant interventions: Insulin, n (%): 10/53 (19); oral glycaemic tablets, n (%): 38/53 (72) Control diet: Name (as reported) and brief description: High‐carbohydrate (HC) diet consisting of 15% of energy from protein, 55% from carbohydrate and 30% from fat (7% saturated, 10% PUFA and 13% MUFA). Energy prescription of ∼6400 kJ/day or 30% energy restriction for three months, followed by energy balance for nine months Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: Participants were n = 18 (39) male with a mean (95% CI) age of 58.8 (55.8, 61.7) years, mean (95% CI) duration of DM 8.6 (6.6, 10.6) years, mean (95% CI) weight 95.5 (91.5, 99.6) kg, mean (95% CI) HbA1C 7.78 (7.50, 8.05) %, mean (95% CI) total cholesterol 4.71 (4.40, 5.01) mmol/L, mean (95% CI) LDL 2.42 (2.16, 2.68) mmol/L, mean (95% CI) HDL 1.20 (1.12, 1.28) mmol/L, mean (95% CI) triglycerides 2.37 (1.85, 2.90) mmol/L, mean (95% CI) systolic BP 127.4 (124.5, 130.2)mmHg, mean (95% CI) diastolic BP 81.5 (78.8, 84.2) mmHg. Why? Consensus‐based nutritional guidelines currently recommend diets that are high in carbohydrates (45–65% of total daily energy intake) and low in fat (< 30% of energy intake) for people with Type 2 diabetes mellitus. What (materials)? Written materials containing information on key nutrition intervention messages, prescriptive fixed menu plans and food choice lists What (procedures)? Dietary intakes of each participant were calculated from weighed/measured food records collected at baseline (5 days). Diet‐specific advice was administered to each study participant. The diet consisted of 15% energy intake from protein (a combination of lean meat, chicken and fish) and 55% energy intake from carbohydrate; low‐glycaemic index carbohydrates were recommended. The diet provided 30% of total energy from fat (7% saturated fat, 10% polyunsaturated fat, 13% monounsaturated fat). The dietary intervention consisted of an initial 3‐month energy restriction (6400 kJ/day or 30% energy restriction), followed by 9 months of energy balance. Topics at group meetings included healthy cooking, goal‐setting and problem‐solving, physical activity, and supportive counselling. Physical activity was encouraged as a strategy to increase energy expenditure for those without limitations or complications, and the recommendations were consistent with public health guidelines. Who provided? Dietitian How and where? Face to‐face dietary counselling appointments conducted at the Baker IDI Heart and Diabetes Institute (Melbourne, Australia) When and how much? Individual sessions; four visits during the 3‐month energy restrictive period (total of 2.5 hours), and five visits during the 9 months of energy balance (total of 2.5 hours). Group meetings: every 3 months (total of 3.3 hours) Strategies to improve or maintain fidelity; tailoring and modification: "Dietary intakes were calculated from weighed/measured food records collected at baseline (5 days) and every 3 months during the intervention period (1 day/month) using Australia‐specific dietary analysis software (Foodworks; Xyris Software, Highgate Hill, QLD, Australia). Dietary compliance was monitored by self‐reported food intakes and 24‐h urine samples for an assessment of urea excretion as a marker of protein intake." Self‐reported physical activity was measured using the validated Active Australia survey questionnaire. Extent of intervention fidelity: Attendance at individual sessions: 37% of participants attended 9 individual sessions; while 26% missed one session. Attendance at group sessions: ranged from 26% to 37% per session. Ability to self‐manage the diet (score): median 4 (IQR 3 to 4) Concomitant interventions: Insulin, n (%): 7/46 (15); oral glycaemic tablets, n (%): 34/46 (74) |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: Yes Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Meat and Livestock Australia nutritional reseach grant Declaration of interest: "J. E. Shaw has received grants, honoraria and speakers’ fees from: GlaxoSmithKline, Lilly Pharmaceuticals, Bristol Myers Squibb, Astra Zeneca, Pfizer, Merck Sharp and Dolme, and Novo Nordisk. N. Mann has received two nutrition grants from MLA in the last 5 years. The protocol and execution of the study was the responsibility of the investigators. MLA had no role in the study design, data collection, data analysis, data interpretation or the decision to submit this paper for publication." |
|
Lasker 2008.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in 2‐hour post‐prandial insulin sensitivity (INS) Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 4 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient research laboratory in Urbana‐Champaign Eligibility criteria: Participants were adults. They were excluded if their BMI was below 26 kg/m2 or weight was above 140 kg; were smokers; had any existing medical condition requiring medication which may impact outcomes of the study; or used steroids or anti‐depressants. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 65 Total attrition in trial: 15 Treatment diet Participants randomised: 32 Participants withdrawn (voluntary): NR Total attrition: 7 Control diet: Participants randomised: 33 Participants withdrawn (voluntary): NR Total attrition: 8 Baseline data treatment diet: Randomised participants not included: 7/32 Age (years): NR Gender distribution (as reported): NR Weight (kg): mean (SE) 96.6 (3.9) BMI (kg/m2): mean (SE) 33.8 (1.1) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SE) 3.41 (0.16) HDL (mmol/L): mean (SE) 1.14 (0.07) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.33 (0.19) TG (mmol/L): mean (SE) 1.72 (0.18) Baseline data control diet: Randomised participants not included: 8/33 Age (years): NR Gender distribution (as reported): NR Weight (kg): mean (SE) 94.3 (2.1) BMI (kg/m2): mean (SE) 33.4 (0.7) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SE) 3.49 (0.13) HDL (mmol/L): mean (SE) 1.27 (0.07) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.52 (0.17) TG (mmol/L): mean (SE) 1.66 (0.17) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1: insulin (P < 0.05); intervention vs control Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: PRO diet. Prescribed dietary protein at 1.6 g/kg/d (~30% of energy intake) and carbohydrate < 170 g/d (~40% of energy) and dietary lipids ~30% energy intake. Recommendation for 30 min of walking 5 d/week. Energy prescription of 7100 kJ/d Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: N = 25 (gender NR) with mean (SEM) weight 96.6 (3.9) kg, mean (SEM) BMI 33.8 (1.1) kg/m2, mean (SEM) total cholesterol 5.33 (0.19) mmol/L, mean (SEM) LDL 3.41 (0.16), mean (SEM) HDL 1.14 (0.07) mmol/L, mean (SEM) triglycerides 1.72 (0.18) mmol/L Why? "Reducing carbohydrate intake with replacement of either fat or protein has been shown to reduce TAG and increase HDL‐C even under weight stable conditions. Substitution with protein may be more beneficial than fat for lipid changes and improvement in INS action. Indeed, independent effects of protein on glycemic regulation suggests protein may be a more effective dietary change than increases in fat intake for reducing risk for metabolic disease." What (materials)? "Each group received a menu plan with meals for each day meeting established nutritional requirements and dietary lipid guidelines. Subjects were provided electronic food scales and instructed to weigh foods at all meals." Armband accelerometers also provided What (procedures)? The prescribed PRO diet provided dietary protein at 1.6 g/kg‐1/d‐1 and < 170 g/d carbohydrate (~30% and ~40% of energy intake, respectively). Dietary lipids were constant between diets (~30% energy intake). For the PRO group, education guidelines emphasised use of high‐quality proteins including lean meats, dairy and eggs. Both diets included 5 servings/d of vegetables and 2 to 3 servings/d of fruit. After baseline data collection, subjects received instructions from a research dietitian about their specific diet including menus, food substitutions, portion sizes, and procedures for maintaining weighed diet records. Activity guidelines emphasised lifestyle recommendations for physical activity based on NIH Guidelines for Weight Management. These guidelines recommend a minimum of 30 min of walking 5 d/wk. Participation in physical activity for the groups was voluntary. Physical activity was monitored using daily activity logs and 3 d/mo subjects wore armband accelerometers (BodyMedia, Cincinnati, OH). Activity logs were collected each week. Subjects also attended 1‐hour meetings for 4 months. Who provided? Dietitian How and where? Face‐to‐face at the nutrition research facility When and how much? Diet intervention lasted for 4 months. At baseline instructions on diet including menus, food substitutions, portion sizes, and procedures for maintaining weight records. Over 4 months, weekly 1‐hr group meetings with dietitian who provided diet and exercise information and reviewed diet records for treatment compliance. Weekly weight checks were done. Physical activity was monitored using daily activity logs and armband accelerometers. At the end of 4 months blood tests were conducted fasting and 1‐ and 2‐hr post‐prandial as a meal challenge. Strategies to improve or maintain fidelity; tailoring and modification: "Nutrient intakes were evaluated as mean daily intakes from 3‐d weighed records using Nutritionist Pro software (First DataBank Inc. 2003, San Bruno, CA) to improve compliance." Extent of intervention fidelity: NR Concomitant interventions: NR Control diet: Name (as reported) and brief description: CHO diet. Provided dietary protein equal to 0.8 g/kg/d (~15% of energy intake) and carbohydrate 220 g/d (~55% of energy) and total fat ~30% of energy intake. Recommendation for 30 min of walking 5 d/week. Energy prescription of 7100 kJ/d Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: N = 25 (gender NR) with mean (SEM) weight 94.3 (2.1) kg, mean (SEM) BMI 33.4 (0.7) kg/m2, mean (SEM) total cholesterol 5.52 (0.17) mmol/L, mean (SEM) LDL 3.49 (0.13), mean (SEM) HDL 1.27 (0.07) mmol/L, mean (SEM) triglycerides 1.66 (0.17) mmol/L Why? To assess whether conventionally accepted carbohydrate is better then moderate‐carbohydrate diet at achieving greater fat mass loss and more favourable changes in post‐prandial insulin response and features of dyslipidaemia What (materials)? "Each group received a menu plan with meals for each day meeting established nutritional requirements and dietary lipid guidelines. Subjects were provided electronic food scales and instructed to weigh foods at all meals." Armband accelerometers also provided What (procedures)? The prescribed CHO diet provided dietary protein at 0.8 g/kg‐1/d‐1 and > 220 g/d carbohydrate (~15% and ~55% of energy intake respectively). Education guidelines for the CHO group followed USDA MyPyramid and emphasised restricting dietary fat and cholesterol with use of whole grain breads, rice, cereals and pasta. Both diets included 5 servings/d of vegetables and 2 to 3 servings/d of fruit. After baseline data collection, subjects received instructions from a research dietitian about their specific diet including menus, food substitutions, portion sizes, and procedures for maintaining weighed diet records. Activity guidelines emphasised lifestyle recommendations for physical activity based on NIH Guidelines for Weight Management. These guidelines recommend a minimum of 30 min of walking 5 d/wk. Participation in physical activity for the groups was voluntary. Physical activity was monitored using daily activity logs and 3 d/mo subjects wore armband accelerometers (BodyMedia, Cincinnati, OH). Activity logs were collected each week. Who provided? Dietitian How and where? Face‐to‐face at the nutrition research facility When and how much? Diet intervention lasted for 4 months. At baseline instructions on diet including menus, food substitutions, portion sizes, and procedures for maintaining weight records. Over 4 months, weekly 1‐hr group meetings with dietitian who provided diet and exercise information and reviewed diet records for treatment compliance. Weekly weight checks were done. Physical activity was monitored using daily activity logs and armband accelerometers. At the end of 4 months blood tests were conducted fasting and 1‐ and 2‐hr post‐prandial as a meal challenge Strategies to improve or maintain fidelity; tailoring and modification: "Nutrient intakes were evaluated as mean daily intakes from 3‐d weighed records using Nutritionist Pro software (First DataBank Inc. 2003, San Bruno, CA) to improve compliance." Extent of intervention fidelity: NR Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: National Cattleman's Beef Association, The Beef Board and Kraft Foods Declaration of interest: "DKL received grant/research support from the funding agencies for this research: the National Cattleman's Beef Association, The Beef Board and Kraft Foods." |
|
Layman 2005.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, number of centres NR Trial registry number: NR Total number of trial arms: 4 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 4 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely USA based on authors and funders Eligibility criteria: Women who were 40 to 56 years old were included in the study. Participants were excluded if they had a BMI < 26 kg/m2; weighed more than 140 kg; smoked; had any existing medical conditions requiring medications which could affect study outcomes; or used oral steroids or antidepressants. Type 2 diabetes at baseline: Unclear; stratified with non‐T2DM since existing medical conditions requiring medications which may affect study outcomes was an exclusion criterion. Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Female Total number randomised: 48 Total attrition in trial: 6 Treatment diet Participants randomised: 12 Participants withdrawn (voluntary): NR Total attrition: 3 Control diet: Participants randomised: 12 Participants withdrawn (voluntary): NR Total attrition: 3 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 47.0 (5.89) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 91.1 (17.67) BMI (kg/m2): mean (SD) 34.8 (6.24) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.61 (0.66) HDL (mmol/L): mean (SD) 1.33 (0.31) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.59 (0.90) TG (mmol/L): mean (SD) 1.42 (0.52) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 45.2 (4.85) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 93.7 (12.12) BMI (kg/m2): mean (SD) 35.4 (3.81) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.52 (0.66) HDL (mmol/L): mean (SD) 1.3 (0.21) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.46 (0.83) TG (mmol/L): mean (SD) 1.4 (0.48) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1: BMI (P = 0.047); NR between which arms Other notes about participants: Four trial arms in the study. There were group differences, but these were between the 4 groups. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: The PRO diet provided 1.6 g/kg.day protein (~30%), ~30% fat and ~40% carbohydrates. Energy prescription of 7100 kJ/d. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: Women only N = 12 with mean (SE) age 47.0 (1.7) years, mean (SE) weight 91.1 (5.1) kg, mean (SE) BMI 34.8 (1.8) kg/m2 Why? NR What (materials)? "We developed a 2‐wk menu plan for each group with meals for each day meeting established nutritional requirements and lipid guidelines." Participants also received food scales. What (procedures)? The PRO diet provided dietary protein at 1.6 g/kg/d (30% of energy intake) with a carbohydrate:protein ratio > 1.5 and dietary lipids at roughly 30% energy intake. These diets were designed to fall within the Acceptable Macronutrient Distribution Range established by the Institute of Medicine with minimum Recommended Dietary Allowance (RDA) intakes for carbohydrates = 130 g/d and protein = 0.8 g/kg and with upper limits for carbohydrates < 65% and protein < 35% of total energy intake. The 2 diets were formulated to be equal in energy (7100 kJ/d; 1700 kcal/d), total fat intake (57 g/d) and fibre (17 g/d). For the PRO group, the education guidelines emphasised use of high‐quality proteins including meats, dairy, and eggs. Both diets included 5 servings/d of vegetables and 2–3 servings of fruits. Who provided? Dietitian How and where? Face‐to‐face group sessions at the weight management research facility When and how much? During the 16‐wk weight loss programme, subjects were required to attend a 1‐h meeting each week at the weight management research facility. Strategies to improve or maintain fidelity; tailoring and modification: Reductions in energy intake determined from weekly 3‐d weighed food records. Extent of intervention fidelity: Subjects in the PRO groups maintained protein intakes of 107 g/d and 30% of energy intake. The ratio of carbohydrate:protein was 1.24. Total dietary lipid intake decreased to 49 g/d (32% of dietary energy) and SFA decreased to 19.1 g/d (12.4% of energy). Concomitant interventions: NR Control diet: Name (as reported) and brief description: The CHO diet provided 0.8 g/kg.day protein (~15%), ~30% fat and ~55% carbohydrates. Energy prescription of 7100 kJ/d Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Women only N = 12 with mean age (SE) 45.2 (1.4) years, mean (SE) weight 93.7 (3.5) kg, mean (SE) BMI 35.4 (1.1) kg/m2 Why? NR What (materials)? "We developed a 2‐wk menu plan for each group with meals for each day meeting established nutritional requirements and lipid guidelines." Participants also received electronic food scales. What (procedures)? The CHO diet provided dietary protein at 0.8g/kg/d (15% of energy intake) with a carbohydrates:protein ratio > 3.5 and dietary lipids at roughly 30% energy intake. These diets were designed to fall within the Acceptable Macronutrient Distribution Range established by the Institute of Medicine with minimum Recommended Dietary Allowance (RDA) intakes for carbohydrates = 130 g/d and protein = 0.8 g/kg and with upper limits for carbohydrates < 65% and protein < 35% of total energy intake. The 2 diets were formulated to be equal in energy (7100 kJ/d; 1700 kcal/d), total fat intake (57 g/d) and fibre (17 g/d). The education guidelines for the CHO group followed the USDA Food Guide Pyramid and emphasised restricting dietary fat and cholesterol with use of breads, rice, cereals, and pasta. Both diets included 5 servings/d of vegetables and 2–3 servings of fruits. Who provided? Dietitian How and where? Face‐to‐face group sessions at the weight management research facility When and how much? During the 16‐wk weight loss programme, subjects were required to attend a 1‐h meeting each week at the weight management research facility. Strategies to improve or maintain fidelity; tailoring and modification: Reductions in energy intake determined from weekly 3‐d weighed food records Extent of intervention fidelity: Subjects in the CHO groups maintained carbohydrate intake at 198 g/d (61% of energy) and reduced total lipid intake to 37g/d (25.5% of energy) and SFA to 11.0 g/d (7.5% of energy). Protein intake in the CHO group was 57g/d (18% of energy intake) and the ratio of carbohydrates was ‐ 3.5. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Illinois Council on Food and Agricultural Research, National Cattlemen's Beef Association, The Beef Board and Kraft Foods Declaration of interest: NR |
|
Layman 2009.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, multi‐centre (2) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in fat mass (FM) Duration of run‐in period (weeks): 2.86 What was the duration of the weight loss phase: 12 months What was the duration of the weight maintenance phase: NA Other notes about methods: Run‐in period was 10‐20 days and participants were instructed to eat as they had been the 6 months prior. |
|
| Participants |
Country and setting: USA, outpatient research at universities in Urbana‐Champaign and Pennsylvania Eligibility criteria: Inclusion criteria NR. Participants were excluded if they had a BMI < 26 kg/m2 or weight > 140 kg; were smokers; had any existing medical condition requiring medication which may affect outcomes, such as lipid‐lowering medications, oral steroids or antidepressants. Type 2 diabetes at baseline: Unclear; stratified with non‐T2DM since no relevant information was reported or could be obtained. Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 130 Total attrition in trial: 59 Treatment diet Participants randomised: 64 Participants withdrawn (voluntary): 23 Total attrition: 23 Control diet: Participants randomised: 66 Participants withdrawn (voluntary): 36 Total attrition: 36 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SE) 45.2 (1.2) Gender distribution (as reported): female 36/64 (56.3%), male 28/64 (43.7%) Weight (kg): mean (SE) 91.7 (2.0) BMI (kg/m2): mean (SE) 32.2 (0.5) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Baseline data control diet: Randomised participants not included: None Age (years): mean (SE) 46.0 (1.0) Gender distribution (as reported): female 35/66 (53.0%), male 31/66 (47.0%) Weight (kg): mean (SE) 93.8 (1.6) BMI (kg/m2): mean (SE) 32.7 (0.5) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: PRO diet. Prescribed dietary protein at 1.6 g/kg/d (~30% of energy intake) with a carbohydrate/protein ratio < 1.5 and dietary lipids ~30% energy intake. Recommendation for 30 min of walking 5 d/week. Energy prescription of 7100 kJ/d for women and 7940 kJ/d for men Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: N = 64 in total of which N = 28 males (44%) and N = 36 females (56%), mean (SE) age 45.2 (1.2) years, mean (SE) weight 91.7 (2.0) kg, mean (SE) BMI 32.2 (0.5) kg/m2 Why? Increased protein and reduced carbohydrate diets are often effective, for short‐term weight loss. This study investigated the longer‐term benefits of a high‐protein diet while adding a comprehensive nutrition education and a high level of monitoring. We anticipated that regardless of the compliance definition, the PRO diet would invoke more favourable body composition and blood lipid changes both after active weight loss and through the 12‐mo weight loss and maintenance periods. What (materials)? "Each diet group received a menu plan with meals for each day meeting established nutritional requirements and dietary fat guidelines. Participants were provided with electronic food scales and were instructed to weigh all food servings at all meals. Participants received specific diet programme instructions from a research dietitian, including the menus, food substitutions, and portion sizes. Physical activity was monitored using daily activity logs and armband accelerometers (BodyMedia) worn 3 d/mo. Activity logs were collected each week." What (procedures)? High‐protein, low‐carbohydrate diet with comprehensive nutrition education and high levels of monitoring. The PRO diet provided dietary protein at 1.6 g/kg/day (30% of energy intake) with a carbohydrate:protein ratio < 1.5 and dietary lipids roughly 30% energy intake. These diets were designed to fall within the Acceptable Macronutrient Distribution Range (AMDR) of the DRI as established by the Institute of Medicine with minimum Recommended Daily Allowance (RDA) intakes for carbohydrates > 130 g/d and protein > 0.8 g/kg/d and with upper ranges for carbohydrates, 65% and protein, 35% of total energy intake. The 2 diets were formulated to be equal in energy [7.10 MJ/d (1700 kcal/d for females; 7.94 MJ/d (1900/d kcal) for males], total fat intake (30% of energy), and fibre (17 g/4.18 MJ). For the PRO group, the education guidelines emphasised use of high‐quality, low‐fat proteins including lean meats, reduced‐fat dairy, and eggs or egg substitutes. Both diets included 5 vegetable servings/d and 2–3 fruit servings/d. With weekly sessions (60 mins) led by a dietitian. The exercise guidelines emphasised physical activity lifestyle recommendations based on the NIH Guidelines for Weight Management. These guidelines recommend a minimum of 30 min of walking 5 d/wk; however, participation was voluntary. Who provided? Dietitian How and where? Face‐to‐face at the weight management research facility When and how much? Throughout the 12‐mo study, participants were required to attend a 1‐h meeting each week. Strategies to improve or maintain fidelity; tailoring and modification: Group meeting with the research dietitians who provided diet information, answered questions, and reviewed diet records for treatment compliance. Participants were required to report 2 3‐d weighed food records during the baseline period prior to assignment to diet groups. Nutrient intakes were evaluated as mean daily intakes from the 3‐d weighed records using Nutritionist Pro software (First DataBank) for treatment compliance. Compliance with dietary protocols was also monitored with plasma TAG at 0, 4,and 12 mo as a marker of carbohydrate intake and 24‐h urinary urea at 0, 4, and 8 mo as a marker of protein intake. High degree of nutrition monitoring throughout the 12 months with weekly meetings that included monitoring body weight, weighed food records, and diet instruction. 3‐d weighed food records, which were reviewed each week with feedback to participants about diet compliance. Any participant with an unexcused absence at a weekly meeting was called by a staff dietitian to promote attendance and any participant with 3 consecutive absences was asked to define their participation status in the study. Extent of intervention fidelity: All participants completing data collection participated in < 75% of meetings, including weigh‐ins and diet records. Compliance with dietary protocols was also monitored with plasma TAG at 0, 4, and 12 mo as a marker of carbohydrate intake and 24‐h urinary urea at 0, 4, and 8 mo as a marker of protein intake. Concomitant interventions: Participants were excluded if they were on lipid‐lowering medications, oral steroids or antidepression medication. Control diet: Name (as reported) and brief description: CARB diet. Provided dietary protein equal to 0.8 g/kg/d (~15% of energy intake) with a carbohydrate/protein ratio > 3.5 and total fat ~30% of energy intake. Recommendation for 30 min of walking 5 d/week. Energy prescription of 7100 kJ/d for women and 7940 kJ/d for men Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: N = 66 in total of which N = 31 males (47%) and N = 35 females (53%), mean (SE) age 46.0 (1.0) years, mean (SE) weight 93.8 (1.6) kg, mean (SE) BMI 32.7 (0.5) kg/m2. Why? NR What (materials)? "Each diet group received a menu plan with meals for each day meeting established nutritional requirements and dietary fat guidelines. Participants were provided with electronic food scales and were instructed to weigh all food servings at all meals. Participants received specific diet program instructions from a research dietitian, including the menus, food substitutions, and portion sizes. Physical activity was monitored using daily activity logs and armband accelerometers (BodyMedia) worn 3 d/mo. Activity logs were collected each week." What (procedures)? The CHO diet provided dietary protein equal to 0.8 g/kg/d (15% of energy intake) with a carbohydrate:protein ratio > 3.2 and total fat roughly 30% of energy intake. High‐carbohydrate, low‐protein, low‐fat diet with comprehensive nutrition education and high levels of monitoring with weekly sessions (60 mins) led by a dietitian. These diets were designed to fall within the Acceptable Macronutrient Distribution Range (AMDR) of the DRI as established by the Institute of Medicine with minimum Recommended Daily Allowance (RDA) intakes for carbohydrates > 130 g/d and protein > 0.8 g/kg/d and with upper ranges for carbohydrates, 65% and protein, 35% of total energy intake. The 2 diets were formulated to be equal in energy [7.10 MJ/d (1700 kcal/d for females; 7.94 MJ/d (1900/d kcal) for males], total fat intake (30% of energy), and fibre (17 g/4.18 MJ). The education guidelines for the CHO group followed the USDA Food Guide Pyramid (19) and emphasised restricting dietary fat and cholesterol with use of whole‐grain breads, rice, cereals, and pasta. Both diets included 5 vegetable servings/d and 2–3 fruit servings/d. The exercise guidelines emphasised physical activity lifestyle recommendations based on the NIH Guidelines for Weight Management. These guidelines recommend a minimum of 30 min of walking 5 d/wk; however, participation was voluntary. Who provided? Dietitian How and where? Face‐to‐face at the weight management research facility When and how much? Throughout the 12‐mo study, participants were required to attend a 1‐h meeting each week. Strategies to improve or maintain fidelity; tailoring and modification: Group meeting with the research dietitians who provided diet information, answered questions, and reviewed diet records for treatment compliance. Participants were required to report 2 3‐d weighed food records during the baseline period prior to assignment to diet groups. Nutrient intakes were evaluated as mean daily intakes from the 3‐d weighed records using Nutritionist Pro software (First DataBank) for treatment compliance. Compliance with dietary protocols was also monitored with plasma TAG at 0, 4,and 12 mo as a marker of carbohydrate intake and 24‐h urinary urea at 0, 4, and 8 mo as a marker of protein intake. High degree of nutrition monitoring throughout the 12 months with weekly meetings that included monitoring body weight, weighed food records, and diet instruction, 3‐d weighed food records, which were reviewed each week with feedback to participants about diet compliance. Any participant with an unexcused absence at a weekly meeting was called by a staff dietitian to promote attendance and any participant with 3 consecutive absences was asked to define their participation status in the study. Extent of intervention fidelity: All participants completing data collection participated in < 75% of meetings, including weigh‐ins and diet records. Compliance with dietary protocols was also monitored with plasma TAG at 0, 4, and 12 mo as a marker of carbohydrate intake and 24‐h urinary urea at 0, 4, and 8 mo as a marker of protein intake. Concomitant interventions: Participants were excluded if they were on lipid‐lowering medications, oral steroids or antidepression medication. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: National Cattlemen's Beef Association, Beef Checkoff and Kraft Foods Declaration of interest: "D. K. Layman serves on Speakers Bureau for National Cattlemen’s Beef Association. E. M. Evans, D. Erickson, J. Seyler, J. Weber, D. Bagshaw, A. Griel, T. Psota, and P. Kris‐Etherton, no conflicts of interest." Author contacted, but requested information not provided. |
|
Lean 1997.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Scotland, outpatient diabetic clinic in Aberdeen Eligibility criteria: Postmenopausal women with BMI > 25 kg/m2 and no active disease. Postmenopausal status was confirmed through medical questionnaire, or follicle‐stimulating hormone in those with hysterectomy; menopause was presumed at age 50. Exclusion criteria NR Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 110 Total attrition in trial: NR Treatment diet Participants randomised: 53 Participants withdrawn (voluntary): NR Total attrition: NR Control diet: Participants randomised: 57 Participants withdrawn (voluntary): NR Total attrition: NR Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 50.1 (14.0) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 84.8 (14.1) BMI (kg/m2): mean (SD) 32.8 (5.1) DBP (mmHg): mean (SD) 86.0 (11.6) SBP (mmHg): mean (SD) 135.7 (23.8) HbA1c (%): NR LDL (mmol/L): mean (SD) 4.46 (1.08) HDL (mmol/L): mean (SD) 1.48 (0.35) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 6.65 (1.34) TG (mmol/L): mean (SD) 1.47 (0.83) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 51.1 (13.6) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 83.9 (17.4) BMI (kg/m2): mean (SD) 32.3 (5.5) DBP (mmHg): mean (SD) 84.2 (12.7 ) SBP (mmHg): mean (SD) 136.7 (21.5) HbA1c (%): NR LDL (mmol/L): mean (SD) 4.47 (1.03) HDL (mmol/L): mean (SD) 1.43 (0.38 ) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 6.63 (1.38) TG (mmol/L): mean (SD) 1.49 (0.87) Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Numbers of participants missing for lipids and blood pressure at baseline: total cholesterol: intervention 2/53; control 4/57, LDL cholesterol: intervention 4/53; control 6/57, HDL cholesterol: intervention 4/53; control 6/57, triglycerides: intervention 4/53; control 5/57, systolic BP: intervention 5/53; 6/57, diastolic BP: intervention 5/53; control 6/57 |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐carbohydrate diet with 34.5% energy from carbohydrates, 35.0% from fat and 29.9% from protein. Energy restriction of 1200 kcal/d Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: Participants were female only with mean (SD) age 50.1 (14.0) years, mean (SD) weight 84.8 (14.1) kg, mean (SD) BMI 32.8 (5.1) kg/m2, mean (SD) total cholesterol 6.65 (1.34) mmol/L, mean (SD) LDL 4.46 (1.08) mmol/L, mean (SD) HDL 1.48 (0.35) mmol/L, mean (SD) triglycerides 1.47 (0.83) mmol/L, mean (SD) diastolic BP 86.0 (11.6) mmHg, mean (SD) systolic BP 135.7 (23.8). Why? Weight management should not focus on weight loss only. There is a need to assess the effects of a low‐carbohydrate diet on weight loss and medical outcomes, such as cardiovascular risk factors. What (materials)? "Written material described the diet in terms of exchanges (meat or alternative, bread, fruit, and allowances for milk and spreads) supported by specially written recipes. Certain foods such as jam or marmalade were included to help compliance." What (procedures)? 1200 kcal/d diet were designed after pilot studies conducted by negotiation with the research dietitian to determine the lowest and highest proportion of energy from carbohydrate which were acceptable to overweight women similar to those in the study population. A diet with 24% energy from carbohydrate was found unacceptable so the diet was modified to contain 35% carbohydrate. Subjects were asked to continue with 1200 kcal/d as long as possible, but if more was eaten, to try to use the same diet composition (low‐carbohydrate), and proportion of exchanges. Who provided? Dietitian How and where? Review at hospital outpatient clinic (mode of delivery not described); telephone contact by the dietitian was used to reduce dropouts. When and how much? Six weekly intervals for six months (4 sessions) Strategies to improve or maintain fidelity; tailoring and modification: The diet included certain foods such as jam or marmalade to assist compliance. Extent of intervention fidelity: NR Concomitant interventions: NR Control diet: Name (as reported) and brief description: High‐carbohydrate diet with 58.0% energy from carbohydrates, 20.5% from fat and 20.7% from protein. Energy restriction of 1200 kcal/d Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐high Exercise component? No Recipients: Participants were female only with mean (SD) age 51.1 (13.6) years, mean (SD) weight 83.9 (17.4) kg, mean (SD) BMI 32.3 (5.5) kg/m2, mean (SD) total cholesterol 6.63 (1.38) mmol/L, mean (SD) LDL 4.47 (1.03) mmol/L, mean (SD) HDL 1.43 (0.38) mmol/L, mean (SD) triglycerides 1.49 (0.87) mmol/L, mean (SD) diastolic BP 84.2 (12.7) mmHg, mean (SD) systolic BP 136.7 (21.5). Why? There is a need to assess the effects of a high‐ carbohydrate diet, as comparator, on weight loss and medical outcomes, such as cardiovascular risk factors. What (materials)? "Written material described the diet in terms of exchanges (meat or alternative, bread, fruit, and allowances for milk and spreads) supported by specially written recipes. Certain foods such as jam or marmalade were included to help compliance." What (procedures)? 1200 kcal/d diets were designed after pilot studies conducted by negotiation with the research dietitian to determine the lowest and highest proportion of energy from carbohydrate which were acceptable to overweight women similar to those in the study population. A high CHO diet of 58% energy was found to be acceptable. Subjects were asked to continue with 1200 kcal/d as long as possible, but if more was eaten, to try to use the same diet composition (high or carbohydrate), and proportion of exchanges. Who provided? Dietitian How and where? Review at hospital outpatient clinic (mode of delivery not described); telephone contact by the dietitian was used to reduce dropouts. When and how much? Six weekly intervals for six months (4 sessions) Strategies to improve or maintain fidelity; tailoring and modification: NR Extent of intervention fidelity: NR Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Department of Human Nutrition through discretionary funds, University of Glasgow, Diet and Health Food Company Limited (UK), Medical Research Council Nutrition Training Fellowship, Rank Foundation and Rank prize funds Declaration of interest: "Conflict of interest: None." |
|
Lim 2010.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 4 Year trial started: NR Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in weight Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 15 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Australia, outpatient clinic in Adelaide. Uncooked, pre‐weighed key foods representative of each diet's macronutrient profile, totalling approximately 65% of energy, were provided to participants every two weeks. Eligibility criteria: Participants aged between 20 and 65 years, with at least one risk factor for CVD (other than obesity) and BMI between 25 and 40 kg/m2. Participants were excluded if they used hypoglycemic medication affecting insulin sensitivity; had a history of heavy alcohol consumption, metabolic or coronary heart disease, type 1 or 2 diabetes, or exercise patterns with large fluctuations; or dined out frequently (more than twice a week) and could not stop. Subjects on beta‐blockers, fish oil supplements, cholesterol‐lowering medication, prednisolone, steroids and diuretics were instructed to maintain doses of these. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 113 Total attrition in trial: 44 Treatment diet Participants randomised: 30 Participants withdrawn (voluntary): 6 Total attrition: 13 Control diet: Participants randomised: 30 Participants withdrawn (voluntary): 6 Total attrition: 15 Baseline data treatment diet: Randomised participants not included: 3/30 Age (years): mean (SD) 48.3 (7.6) Gender distribution (as reported): female 24/30 (80%), male 6/30 (20%) Weight (kg): mean (SD) 87.6 (2.3) BMI (kg/m2): mean (SD) 32.3 (3.1) DBP (mmHg): mean (SD) 77.2 (13) SBP (mmHg): mean (SD) 129.8 (15.1) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.1 (1.7) HDL (mmol/L): mean (SD) 1.3 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.9 (1) TG (mmol/L): mean (SD) 1.8 (1) Baseline data control diet: Randomised participants not included: 3/30 Age (years): mean (SD) 47.2 (10.5) Gender distribution (as reported): female 24/30 (80%), male 6/30 (20%) Weight (kg): mean (SD) 93.0 (2.8) BMI (kg/m2): mean (SD) 34.6 (3.7) DBP (mmHg): mean (SD) 79.8 (8.3) SBP (mmHg): mean (SD) 130.9 (12.1) HbA1c (%): NR LDL (mmol/L): mean (SD) 3 (2.1) HDL (mmol/L): mean (SD) 1.3 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 6.1 (1.1) TG (mmol/L): mean (SD) 1.6 (0.6) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1 : total cholesterol (P < 0.001); characteristic 2 : apolipoprotein B48 (P < 0.001); characteristic 3 : systolic blood pressure (P = 0.001); characteristic 4: diastolic blood pressure (P = 0.026) Other notes about participants: Gender distribution was for all participants randomised. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Very low‐carbohydrate (VLC) diet prescribing 6500 kJ as 35% protein, 60% fat (20% saturated fat) and 4% carbohydrate Treatment diet type (carbohydrate‐fat‐protein): Very low‐high‐high Exercise component? No Recipients: Total n = 30 with n = 6 (20%) males and n = 24 (80%) females, mean (SD) age 48.3 (7.6) years, mean (SD) weight 87.6 (2.3) kg, mean (SD) BMI 32.3 (3.1) kg/m2, mean (SD) total cholesterol 5.9 (1) mmol/L, mean (SD) HDL 1.3 (0.3) mmol/L, mean (SD) LDL 3.1 (1.7) mmol/L, mean (SD) triglycerides 1.8 (1) mmol/L, mean (SD) glucose 5.4 (0.6) mmol/L, mean (SD) systolic BP 129.8 (15.1) mmHg, mean (SD) diastolic BP 77.2 (13) mmHg Why? Very low‐carbohydrate diets have been suggested as possibly having greater effectiveness in weight loss and improvements in metabolism than traditional weight loss. No trials have determined the long‐term effects of such diets in persons with cardiovascular risks. What (materials)? Participants were provided with prescriptive meal plans and recipe information. Uncooked, pre‐weighed key foods representative of the very low‐carbohydrate diet's macronutrient profile, and providing approximately 65% of energy, were provided to participants. What (procedures)? "The diets had the same energy content (6500 KJ). The planned macronutrient profiles for the diets were: VLC diet (35% of energy as protein, 60% fat, 20% saturated fat, 4% carbohydrate). Participants were provided with prescriptive meal plans and foods contributing to 65% energy of the meal plans. They also received individual dietary counselling every 2 weeks from a qualified dietitian to monitor compliance to their assigned diets. Following the intensive phase, subjects were advised to maintain their allocated energy‐restricted diet for an additional 12months." They were also instructed to keep three‐day weighed food records before five follow‐up visits. Who provided? Qualified dietitian provided consultations and analysed three‐day weighed food diaries using Diet/1 Nutrition Calculation software (Xyris Software 1998, Highgate Hill, Queensland, Australia). How and where? Dietary counselling, compliance monitoring and advice for adherence was individual and conducted at the CSIRO outpatient clinic. When and how much? Key foods were provided every two weeks. Dietary counselling and compliance monitoring was done every two weeks during the first three months; thereafter dietary advice and analysis of three‐day food diaries (two weekdays and one weekend day) were done at 3, 6, 9, 12 and 15 months from baseline. Duration of counselling sessions NR Strategies to improve or maintain fidelity; tailoring and modification: Provision of key foods representative of the diet, compliance monitoring on a fortnightly basis during the first three months of the study, individualised dietary advice on adherence and keeping of three‐day weighed food records at 3, 6, 9, 12 and 15 months Extent of intervention fidelity: NR Concomitant interventions: Subjects on beta‐blockers, fish oil supplements, cholesterol‐lowering agents, prednisolone, steroids and diuretics were asked to maintain the doses throughout the study. Control diet: Name (as reported) and brief description: High‐unsaturated fat (HUF) diet prescribing 6500 kJ as 20% protein, 30% fat (6% saturated fat and 8% polyunsaturated fat) and 50% carbohydrate Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Total n = 30 with n = 6 (20%) males and n = 24 (80%) females, mean (SD) age 47.2 (10.5) years, mean (SD) weight 93.0 (2.8) kg, mean (SD) BMI 34.6 (3.7) kg/m2, mean (SD) total cholesterol 6.1 (1.1) mmol/L, mean (SD) HDL 1.3 (0.3) mmol/L, mean (SD) LDL 3 (2.1) mmol/L, mean (SD) triglycerides 1.6 (0.6) mmol/L, mean (SD) glucose 5.4 (0.6) mmol/L, mean (SD) systolic BP 130.9 (12.1) mmHg, mean (SD) diastolic BP 79.8 (8.3) mmHg Why? Weight loss is associated with lowered cardiovascular risk, such as lowering of blood pressure and serum lipids. Traditional approaches have been to advise a high‐carbohydrate low‐fat diet. What (materials)? Participants were provided with prescriptive meal plans and recipe information. Uncooked, pre‐weighed key foods representative of the very low‐carbohydrate diet's macronutrient profile, and providing approximately 65% of energy, were provided to participants. What (procedures)? Participants were instructed to follow the high‐unsaturated fat diet, comprising 50% carbohydrate, 20% protein and 30% fat (6% saturated and 8% polyunsaturated), according to provided materials. They were also instructed to keep three‐day weighed food records before five follow‐up visits. Who provided? Qualified dietitian provided consultations and analysed three‐day weighed food diaries using Diet/1 Nutrition Calculation software (Xyris Software 1998, Highgate Hill, Queensland, Australia). How and where? Dietary counselling, compliance monitoring and advice for adherence was individual and conducted at the CSIRO outpatient clinic. When and how much? Key foods were provided every two weeks. Dietary counselling and compliance monitoring was done every two weeks during the first three months; thereafter dietary advice and analysis of three‐day food diaries (two weekdays and one weekend day) were done at 3, 6, 9, 12 and 15 months from baseline. Duration of counselling sessions NR Strategies to improve or maintain fidelity; tailoring and modification: Provision of key foods representative of the diet, compliance monitoring on a fortnightly basis during the first three months of the study, individualised dietary advice on adherence and keeping of three‐day weighed food records at 3, 6, 9, 12 and 15 months Extent of intervention fidelity: NR Concomitant interventions: Subjects on beta‐blockers, fish oil supplements, cholesterol‐lowering agents, prednisolone, steroids and diuretics were asked to maintain the doses throughout the study. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: National Heart Foundation, Australia and CSIRO Health Sciences and Nutrition, Adelaide, Australia Declaration of interest: "No conflict of interest exists for any of the authors." |
|
Liu 2013.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT01358890 Total number of trial arms: 2 Year trial started: 2011 Sample size calculation: Yes Outcome(s) used for sample size calculation: Difference in high‐density lipoprotein (HDL) cholesterol Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: Participants were randomised after stratification by age. |
|
| Participants |
Country and setting: China, outpatient institute for nutritional sciences at a hospital in Shanghai. Meals were prepared in a designated kitchen at the hospital; participants received these meals daily and had them in a designated dining room. Eligibility criteria: Participants were women aged 30 to 65 years with BMI ≥ 24 kg/m2. Participants were excluded if they were pregnant or lactating; had a history of cardiovascular disease, cancer, mental disorders or gastrointestinal disease which would prevent compliance; had gastrointestinal surgery previously, with the exception of hernia or appendicitis; currently used antidepressants; had plasma glutamic‐pyruvic transaminase, glutamic‐oxaloacetic transaminase, creatinine or urea N within specified ranges; were participating in other research studies in the past three months or had liver or renal biomarkers beyond reference levels. Type 2 diabetes at baseline: Unclear; stratified with non‐T2DM since baseline fasting blood glucose levels in both groups were not indicative of diabetes. Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 50 Total attrition in trial: 2 Treatment diet Participants randomised: 25 Participants withdrawn (voluntary): 1 Total attrition: 1 Control diet: Participants randomised: 25 Participants withdrawn (voluntary): 1 Total attrition: 1 Baseline data treatment diet: Randomised participants not included: None Age (years): NR Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SE) 64.8 (1.3) BMI (kg/m2): mean (SE) 26.6 (0.5) DBP (mmHg): mean (SE) 86.5 (1.6) SBP (mmHg): mean (SE) 134.0 (3.4) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.30 (0.16) HDL (mmol/L): mean (SE) 1.30 (0.07) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.01 (0.21) TG (mmol/L): mean (SE) 1.69 (0.26) Baseline data control diet: Randomised participants not included: None Age (years): NR Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SE) 67.0 (1.3) BMI (kg/m2): mean (SE) 26.9 (0.4) DBP (mmHg): mean (SE) 85.5 (2.0) SBP (mmHg): mean (SE) 131.4 (3.4) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.44 (0.20) HDL (mmol/L): mean (SE) 1.44 (0.08) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.19 (0.24) TG (mmol/L): mean (SE) 1.33 (0.12) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Participants were Chinese and BMI cut‐offs are lower for this population. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Low‐carbohydrate ad libitum diet. Designed to provide 20 g carbohydrate daily in the first week, with a gradual increase to 120 g by adding 10 g weekly until the 11th week. Treatment diet type (carbohydrate‐fat‐protein): Very low4w to low11w‐unclear‐unclear Exercise component? No Recipients: Women only N = 25 mean (SE) weight 64.8 ( 1.3) kg, mean (SE) BMI 26.6 (0.5). Why? Unclear if Chinese people, given their habitually high‐carbohydrate consumption, are able to adapt to a LC diet for weight control. This study investigated the adherence to a LC diet on weight loss and its effects on the improvement of cardiovascular risk factors among overweight or obese Chinese women, in comparison with an energy‐restricted (ER) diet. What (materials)? "All experimental meals were prepared in a designated kitchen at the hospital. All participants received their experimental meals every day including weekends." What (procedures)? The LC diet was designed to provide 20 g carbohydrate daily in the first week, with a gradual increase to 120 g, by adding 10 g weekly until the 11th week, since carbohydrate intake up to 120 g/d has been reported to be sufficient to benefit weight control and metabolic profiles. In addition, a ketogenic diet with no more than 50 g carbohydrate might be rather difficult for a population with a habitually high‐carbohydrate intake. Carbohydrate‐rich foods, such as white rice, steamed bread and tubers, were substituted with fish, poultry and plant oil. In addition to three meals, snacks, including boiled eggs (with or without yolk), cucumbers and tomatoes, were also provided ad libitum any time from 06.00 to 17.30 hours each day. Participants were encouraged to consume every meal in a designated dining room within the building where they worked. Sometimes the participants also consumed meals close to their working place in the hospital if they had a short lunch break. In this case, the participants were required to report to dietitians about any leftovers or intake of foods other than the experimental meals. At the beginning of the intervention, participants were instructed to maintain their usual physical activity levels throughout the study; those in the LC diet group were particularly recommended to drink plenty of plain water, to compensate for possible water loss in ketosis. Who provided? Dietitian How and where? Face‐to‐face at a hospital in Shanghai When and how much? Daily monitoring of intake Strategies to improve or maintain fidelity; tailoring and modification: "All meals were prepared and consumed on the hospital premises. Any leftovers were reported to the dietitian. Dietary compliance was evaluated by using food diaries and measuring urinary ketones. Hunger levels and overall diet acceptance were assessed through self‐reports. At weeks 6 and 12, intakes of energy and nutrients were evaluated through a combined analysis of meal menus and a 7‐d food diary recorded by a dietitian. Energy and nutrients were calculated using Nutrition Star Software (Zhending Company Limited), in accordance with the Chinese food composition table." Extent of intervention fidelity: At baseline, intakes of energy and nutrients were obtained through a 3‐d food diary. At the end of the intervention, a five‐point Likert scale (20,21) was used to evaluate the hunger level at each month of the intervention. Concomitant interventions: NR Control diet: Name (as reported) and brief description: Energy‐restricted diet. Traditional Chinese style with target total energy intake of 5021 kJ/d; energy from carbohydrate, protein and fat was 50 to 55%, 17 to 19% and 26 to 33%. Energy prescription with an initial target for a total energy intake of 5021 kJ/d and adjusted to 6276 kJ/d due to extreme hunger reported by some participants Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: NR Why? A higher‐carb energy‐restricted diet should be more acceptable in the Chinese population for weight loss. What (materials)? "All experimental meals were prepared in a designated kitchen at the hospital. All participants received their experimental meals every day including weekends." What (procedures)? The ER diet was designed in the traditional Chinese style with an initial target for a total energy intake of 5021 kJ/d (1200 kcal/d). Energy from carbohydrate, protein and fat in the ER diet was 50–55, 17–19 and 26–33 %, respectively. Participants were encouraged to consume every meal in a designated dining room within the building where they worked. Sometimes the participants also consumed meals close to their working place in the hospital if they had a short lunch break. In this case, the participants were required to report to dietitians about any leftovers or intake of foods other than the experimental meals. At the beginning of the intervention, participants were instructed to maintain their usual physical activity levels throughout the study. All the main meals and snacks were prepared/provided by the hospital kitchen daily including weekends over a period of 12 weeks. Who provided? Dietitian How and where? Face‐to‐face at a hospital in Shanghai When and how much? Daily monitoring of intake Strategies to improve or maintain fidelity; tailoring and modification: "All meals were prepared and consumed on the hospital premises. Any leftovers were reported to the dietitian. Dietary compliance was evaluated by using food diaries and measuring urinary ketones. Hunger levels and overall diet acceptance were assessed through self‐reports. At weeks 6 and 12, intakes of energy and nutrients were evaluated through a combined analysis of meal menus and a 7‐d food diary recorded by a dietitian. Energy and nutrients were calculated using Nutrition Star Software (Zhending Company Limited), in accordance with the Chinese food composition table." Extent of intervention fidelity: At baseline, intakes of energy and nutrients were obtained through a 3‐d food diary. At the end of the intervention, a five‐point Likert scale (20,21) was used to evaluate the hunger level at each month of the intervention. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Ministry of Science and Technology of China (2012CB524900 and 2011CB504002), National Natural Science Foundation of China (81021002), the Chinese Academy of Sciences (KSCX2‐EW‐R‐10) and the Dr. Robert C. and Veronica Atkins Research Foundation Declaration of interest: "The authors have no conflict of interest." Author contacted, but requested information not provided. |
|
Marco‐Benedi 2019.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT02559479 Total number of trial arms: 2 Year trial started: 2015 Sample size calculation: Yes Outcome(s) used for sample size calculation: HOMA‐IR Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Spain, outpatient university hospital unit in Zaragoza Eligibility criteria: Participants aged 18 to 70 with a BMI ranging from 27.5 to 40 kg/m2 and a steady weight in the previous two months, with a diagnosis of impaired fasting glucose or type 2 diabetes defined as fasting glucose concentration over 100 mg/dL and/or HbA1c over 5.7% and taking no antidiabetic drugs; or taking a stable dose of metformin for the past two months (regardless of glucose or HbA1c levels). Participants were excluded if their HbA1c level at baseline exceeded 7%; if they used lipid‐lowering medication and/or sterol supplements, omega 3 fatty acids, weight‐loss medication; had kidney disease (according to specified glomerular filtration rate), active liver disease or uncontrolled hypothyroidism; or any other condition which could threaten compliance. Type 2 diabetes at baseline: Mixed; stratified with non‐T2DM since even though inclusion criteria were diagnosis of impaired fasting glucose or diabetes, baseline HbA1c in both groups was not indicative of T2DM. Impaired glucose tolerance at baseline: Mixed Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 80 Total attrition in trial: 13 Treatment diet Participants randomised: 40 Participants withdrawn (voluntary): 5 Total attrition: 5 Control diet: Participants randomised: 40 Participants withdrawn (voluntary): 8 Total attrition: 8 Baseline data treatment diet: Randomised participants not included: 2/40 Age (years): mean (SD) 56.5 (8.59) Gender distribution (as reported): female 18/38 (47.4%), male 20/38 (52.6%) Weight (kg): mean (SD) 91.4 (12.7) BMI (kg/m2): mean (SD) 33.2 (3.63) DBP (mmHg): mean (SD) 86.9 (9.86) SBP (mmHg): mean (SD) 134 (14.5) HbA1c (%): mean (SD) 6.38 (1.00) LDL (mmol/L): mean (SD) 3.52 (0.98) HDL (mmol/L): mean (SD) 1.41 (0.31) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.80 (1.23) TG (mmol/L): mean (SD) 1.85 (1.22) Baseline data control diet: Randomised participants not included: 5/40 Age (years): mean (SD) 54.6 (8.11) Gender distribution (as reported): female 23/35 n/N (65.7%), male 12/35 n/N (34.3%) Weight (kg): mean (SD) 86.3 (11.8) BMI (kg/m2): mean (SD) 32.3 (3.70) DBP (mmHg): mean (SD) 82.9 (6.97) SBP (mmHg): mean (SD) 134 (13.9) HbA1c (%): mean (SD) 6.11 (1.00) LDL (mmol/L): mean (SD) 3.42 (0.76) HDL (mmol/L): mean (SD) 1.31 (0.25) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.57 (1.00) TG (mmol/L): mean (SD) 2.08 (0.99) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1 : visceral fat (P = 0.019); intervention vs control arm; characteristic 2: physical activity level (P = 0.046); intervention vs control arm Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Food typical of the Mediterranean diet with macronutrient distribution of 35% protein, 30% fat and 35% carbohydrates. 80% of protein came from lean animal sources. Heterogeneous physical activity advice based on participant condition. Total number of calories prescribed was calculated using the Harris‐Benedict equation and applying an activity factor and a daily 2510 kJ deficit. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: Persons with impaired fasting glucose or diabetes, 47.4% male, aged 56.5 (8.59) years with BMI 33.2 (3.63) and weight 91.4 (12.7) kg Why? An energy‐restricted diet with high protein content may improve insulin resistance in persons with type 2 diabetes, and achieve great weight loss than standard protein diets. What (materials)? Daily menus for high‐protein diet What (procedures)? Participants were instructed to follow a high‐protein diet, with dietitians providing follow‐up consultations to reinforce the intervention. When certain weight loss targets were achieved, calories were further reduced by 100 to 200 kcal to compensate for the change in basal metabolic rate. Three‐day weighed food records were kept to focus participants on the intervention. Dietitians performed individual consultations every 2 weeks to reinforce the intervention and to motivate weight loss. Who provided? Dietitians provided follow‐up consultations. How and where? Consultations were per individual. Location of these sessions NR When and how much? The duration of the intervention was 6 months, with follow‐up consultations every two weeks. Three‐day weighed food records were kept before study visits at three and six months. Strategies to improve or maintain fidelity; tailoring and modification: Consultations every two weeks to reinforce the intervention, measurement of body weight at three and six months, three‐day weighed food record before each of these visits. Diet compliance was assessed by self‐reported questionnaire and urea nitrogen concentrations were determined from urine sample. Extent of intervention fidelity: Urine urea/weight (kg) ratio was increased by 10.2 (99.6)% at three months and 33.3 (71.9)% at six months. Blood urea increased more in high‐protein dieters when compared to standard‐protein dieters (P = 0.007 at three months and 0.029 at six months). The authors inferred correct adherence from these data. Concomitant interventions: A total of 35.1% of participants were on metformin. Control diet: Name (as reported) and brief description: Food typical of the Mediterranean diet with macronutrient distribution of 18% protein, 30% fat and 52% carbohydrates. 80% of protein came from lean animal sources. Heterogeneous physical activity advice based on participant condition. Total number of calories prescribed was calculated using the Harris‐Benedict equation and applying an activity factor and a daily 2510 kJ deficit. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: Persons with impaired fasting glucose or diabetes, 34.3% male, aged 54.6 (8.11) years with BMI 32.3 (3.70) and weight 86.3 (11.8) kg. Why? Weight loss is the first‐order treatment for type 2 diabetes, with loss of adipose tissue most efficient in improving insulin resistance. What (materials)? Daily menus for standard‐protein diet What (procedures)? Participants were instructed to follow a standard‐protein diet, with dietitians providing follow‐up consultations to reinforce the intervention. When certain weight loss targets were achieved, calories were further reduced by 100 to 200 kcal to compensate for the change in basal metabolic rate. Three‐day weighed food records were kept to focus participants on the intervention. Dietitians performed individual consultations every 2 weeks to reinforce the intervention and to motivate weight loss. Who provided? Dietitians provided follow‐up consultations. How and where? Consultations were per individual. Location of these sessions NR When and how much? The duration of the intervention was 6 months, with follow‐up consultations every two weeks. Three‐day weighed food records were kept before study visits at three and six months. Strategies to improve or maintain fidelity; tailoring and modification: Consultations every two weeks to reinforce the intervention, measurement of body weight at three and six months, three‐day weighed food record before each of these visits. Diet compliance was assessed by self‐reported questionnaire and urea nitrogen concentrations were determined from urine sample. Extent of intervention fidelity: Urine urea/weight (kg) ratio was decreased by 23.6 (104)% at three months and 6.99 (66.7)% at six months. The authors inferred correct adherence from these data. Concomitant interventions: A total of 36.4% of participants were on metformin. |
|
| Outcomes |
Change in body weight (kg) at 3 to <12 months: Yes Change in body weight (kg) at ≥12 months: No Number of participants with 5% weight loss from baseline at 3 to <12 months: No Number of participants with 5% weight loss from baseline at ≥12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥12 months: No Change in DBP (mmHg) at ≥12 months: No Change in SBP (mmHg) at ≥12 months: No All‐cause mortality at ≥12 months: No Cardiovascular mortality at ≥12 months: No Non‐fatal myocardial infarction at ≥12 months: No Non‐fatal stroke at ≥12 months: No Diagnosis of type 2 diabetes mellitus at ≥12 months: No Change in HbA1c (%) at ≥12 months: No Change in LDL (mmol/L) at ≥12 months: No Change in HDL (mmol/L) at ≥12 months: No Change in non‐HDL (mmom/L) at ≥12 months: No Change in total cholesterol (TC) (mmol/L) at ≥12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Grants from the Carlos III Research Institute: CIBERCV (co‐supported by the European Regional Development Fund (ERDF) allocated by the European Union; IIS16/0114), PI13/02507 and PI15/01983; and a grant from INTEROVIC Declaration of interest: "Authors have no relevant conflict of interest to disclose." |
|
Mateo‐Gallego 2017.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT02160496 Total number of trial arms: 3 Year trial started: 2014 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in weight Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Spain, outpatient university hospital in Zaragoza Eligibility criteria: Participants aged 18 to 80 with BMI 27.5 to 45 kg/m2 and a steady weight (± 3 kg) in the past three months. Participants were excluded if they had uncontrolled hypothyroidism, type 2 diabetes (defined as glycated haemoglobin above 8%), another disease which could interfere with their ability to comply with protocol; were current users of lipid‐lowering or anti‐diabetic drugs; or were taking supplements of phytosterols or omega‐3 fatty acid or obesity drugs. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Female Total number randomised: 91 Total attrition in trial: 25 Treatment diet Participants randomised: 30 Participants withdrawn (voluntary): 3 Total attrition: 5 Control diet: Participants randomised: 30 Participants withdrawn (voluntary): 6 Total attrition: 14 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 43.2 (9.17) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 85.1 (8.39) BMI (kg/m2): mean (SD) 32.4 (2.96) DBP (mmHg): mean (SD) 79.3 (9.58) SBP (mmHg): mean (SD) 119 (12.6) HbA1c (%): mean (SD) 5.42 (0.28) LDL (mmol/L): mean (SD) 3.42 (0.77) HDL (mmol/L): mean (SD) 1.55 (0.37) Non‐HDL (mmol/L): mean (SD) 4.07 (0.78) TC (mmol/L): mean (SD) 5.62 (0.98) TG (mmol/L): mean (SD) 1.40 (0.56) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 43.7 (9.74) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 86.4 (8.35) BMI (kg/m2): mean (SD) 33.2 (3.31) DBP (mmHg): mean (SD) 77.3 (9.03) SBP (mmHg): mean (SD) 122 (12.1) HbA1c (%): mean (SD) 5.50 (0.29) LDL (mmol/L): mean (SD) 3.34 (1.08) HDL (mmol/L): mean (SD) 1.46 (0.35) Non‐HDL (mmol/L): mean (SD) 3.96 (0.90) TC (mmol/L): mean (SD) 5.44 (1.05) TG (mmol/L): mean (SD) 1.36 (0.47) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Diet comprised 35% protein, 30% fat and 35% carbohydrates with exercise recommendations. Each participant's caloric prescription represented a deficit of 600 kcal/day as calculated from energy intakes estimated by multiplying the activity factor by the resting energy expenditure calculated with the Harris‐Benedict equation. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: 30 females only with mean (SD) weight (kg) 85.1 (8.39), mean (SD) systolic BP 119 (12.6), mean (SD) diastolic BP 79.3 (9.58), mean (SD) total cholesterol 217 (37.8) mg/dL, mean (SD) LDL 132 (29.9) mg/dL, mean (SD) HDL 59.9 (14.3) mg/dL, mean (SD) triglycerides 124 (49.8) mg/dL, mean (SD) glucose 85.9 (8.26) mg/dL, mean (SD) HbA1c 5.42 (0.28)% Why? High‐protein energy‐restricted diets have demonstrated efficacy in promoting weight loss in overweight and obesity. However, the protein percentage that achieves optimal efficacy and acceptability remains unknown. We sought to assess the effects of three energy‐reduced diets with different percentages of calories from protein (20%, 27%, and 35%) on weight loss and lipids. What (materials)? Recipes What (procedures)? The diet had the following distribution of calories: protein, 35%; carbohydrates, 35%; fat, 30% in all diets. Who provided? Dietitian How and where? Individual, face‐to‐face, location NR When and how much? Every two weeks individual meetings with dietitian for 3 months Strategies to improve or maintain fidelity; tailoring and modification: The dietitian provided participants with recipes and shopping counselling to improve intervention compliance and to achieve weight‐loss goals. A single dietitian performed individual consultations every 2 weeks to reinforce the intervention and to motivate weight loss. Participants were asked to complete a 3‐day weighted food record before each visit. Extent of intervention fidelity: Dietary compliance and physical activity were assessed through self‐reported questionnaires. Compliance was defined as protein consumption within ±3% of the prescribed quantity. Twenty‐seven of the 27 (100.0%) women who completed the intervention at three months were defined as compliant. At the end of the intervention, mean macronutrient intakes were 37.9% (SD 5.38) carbohydrates, 31.8% (SD 5.19) fat and 30.4% (SD 6.58) protein; mean energy intake was 1172 kcal (range of 1107 to 1226 kcal), representing a 31.0% (SD 21.8) reduction from baseline. Concomitant interventions: The use of lipid‐lowering or anti‐diabetic medications, phytosterols, omega‐3 fatty acids or any obesity medication was an exclusion criterion. Control diet: Name (as reported) and brief description: Diet comprised 20% protein, 30% fat and 50% carbohydrates with exercise recommendations. Each participant's caloric prescription represented a deficit of 600 kcal/day as calculated from energy intakes estimated by multiplying the activity factor by the resting energy expenditure calculated with the Harris‐Benedict equation. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: Recipients were 100% female, aged 43.7 (9.74) years old, weighed mean (SD) 86.4 (8.35) kgs with a mean (SD) BMI 33.2 (3.31). Why? High‐protein energy‐restricted diets have demonstrated efficacy in promoting weight loss in overweight and obesity. However, the protein percentage that achieves optimal efficacy and acceptability remains unknown. We sought to assess the effects of three energy‐reduced diets with different percentages of calories from protein (20%, 27%, and 35%) on weight loss and lipids. What (materials)? Recipes What (procedures)? The diet had the following distribution of calories: protein, 20%; carbohydrates, 50%; fat, 30%. Who provided? Dietitian How and where? Individual, face‐to‐face, location NR When and how much? Every two weeks individual meetings with dietitian for 3 months Strategies to improve or maintain fidelity; tailoring and modification: The dietitian provided participants with recipes and shopping counselling to improve intervention compliance and to achieve weight‐loss goals. A single dietitian performed individual consultations every 2 weeks to reinforce the intervention and to motivate weight loss. Participants were asked to complete a 3‐day weighted food record before each visit. Extent of intervention fidelity: Dietary compliance and physical activity were assessed through self‐reported questionnaires. Compliance was defined as protein consumption within ±3% of the prescribed quantity. Twenty‐one of the 24 (87.5%) women who completed the intervention at three months were defined as compliant. At the end of the intervention, mean macronutrient intakes were 44.6% (SD 5.37) carbohydrates, 31.7% (SD 5.46) fat and 23.4% (SD 1.76) protein; mean energy intake was 1179 kcal (range of 1151 to 1241 kcal), representing a 34.8% (SD 20.9) reduction from baseline. Concomitant interventions: The use of lipid‐lowering or anti‐diabetic medications, phytosterols, omega‐3 fatty acids or any obesity medication was an exclusion criterion. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Teruel Investment Fund from Ministerio de Ciencia e Innovación, the Spanish Ministry of Health (grants number FIS PI12/01087 and RETIC RIC RD12/0042/0055 Declaration of interest: "No authors have conflicts of interest to declare." |
|
Mellberg 2014.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT00692536 Total number of trial arms: 2 Year trial started: 2007 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in fat mass Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 24 months What was the duration of the weight maintenance phase: NA Other notes about methods: ITT analysis was not used. |
|
| Participants |
Country and setting: Sweden, outpatient clinical research centre at a university hospital in Umeå Eligibility criteria: Postmenopausal, non‐smoking women with BMI ≥ 27 kg/m2. Participants were excluded if they followed a vegetarian diet; were allergic to key components of the intervention diets; had a history of heart disease, kidney disease, hyper‐ or hypothyreosis, osteoporosis or diabetes; had abnormal fasting plasma glucose levels (≥ 7 mmol/L), high blood pressure above 150/90 mmHg; or were on hormone replacement therapy, statins, beta‐blockers or any medication for psychiatric disorders. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 70 Total attrition in trial: 21 Treatment diet Participants randomised: 35 Participants withdrawn (voluntary): 5 Total attrition: 8 Control diet: Participants randomised: 35 Participants withdrawn (voluntary): 9 Total attrition: 13 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 59.5 (5.5) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 87.0 (10.6) BMI (kg/m2): mean (SD) 32.7 (3.6) DBP (mmHg): mean (SD) 83.0 (1.3) SBP (mmHg): mean (SD) 141 (2.2) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.87 (0.13) HDL (mmol/L): mean (SD) 1.49 (0.06) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.91 (0.14) TG (mmol/L): mean (SD) 1.22 (0.09) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 60.3 (5.9) Gender distribution (as reported): female 100%, male 0% Weight (kg): mean (SD) 86.8 (10.0) BMI (kg/m2): mean (SD) 32.6 (3.3) DBP (mmHg): mean (SD) 82.9 (1.5) SBP (mmHg): mean (SD) 138 (2.2) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.64 (0.21) HDL (mmol/L): mean (SD) 1.28 (0.05) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.52 (0.23) TG (mmol/L): mean (SD) 1.27 (0.10) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: HDL (P = 0.01); intervention and control arm Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Unrestricted/ad libitum prescription in both diets Treatment diet: Name (as reported) and brief description: Paleolithic diet (PD) providing 30% of energy from protein, 40% from fat (with high intake of MUFA and PUFA) and 30% from carbohydrates. Consumed ad libitum Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐high Exercise component? No Recipients: N = 35 (100%) women with mean (SD) age 59.5 (5.5) years, mean (SD) body weight 87.0 (10.6) kg, mean (SD) BMI 32.7 (3.6) kg/m2 Why? " PD would be more efficient than a conventional low‐fat/high‐fibre diet at reducing fat mass during a 2‐year randomized dietary intervention trial in obese postmenopausal women." What (materials)? "The subjects were given recipes and written instructions to facilitate the preparation of meals at home." What (procedures)? The PD provided 30% of energy intake (E%) from protein, 40 E% fat, and 30 E% carbohydrates and included a recommendation for a high intake of MUFA and polyunsaturated fatty acids (PUFA). The diet was based on lean meat, fish, eggs, vegetables, fruits, berries, and nuts. Dairy products, cereals, added salt, and refined fats and sugar were excluded. The group sessions consisted of information on and cooking of the intervention diets, dietary effects on health, behavioural changes, and group discussions. The subjects were given recipes and written instructions to facilitate the preparation of meals at home. Eight group sessions (four cooking classes and four follow‐up sessions) were held during the first 6 months of the intervention. Additional group meetings were held at 9, 12, 18, and 24 months. Who provided? One dietitian per study arm How and where? Face‐to‐face, location NR When and how much? 12 group sessions throughout 24‐month period Strategies to improve or maintain fidelity; tailoring and modification: Dietary intake was assessed using 4‐day estimated self‐reported food records conducted at baseline (2 × 4 days) and monthly until 6 months, thereafter at 9, 12, 18 and 24 months. Subjects were instructed to keep a record of all food items consumed over four consecutive days (three weekdays and one weekend day) and to describe and estimate the amount of food eaten by using coloured food‐portion photographs representing known weights and household measuring utensils (e.g. cup, spoon, and standard weight of food items). The reported food intake was converted to estimates of energy and nutrient intake using the nutritional analysis package Dietist XP (version 3.0) based on the food composition database of the Swedish National Food Administration (2008‐03‐06).Nitrogen excretion in urine (NU) was used as a biomarker for protein intake, with three 24‐h urine samples collected at baseline and after 6 and 24 months. The para‐aminobenzoic acid (PABA) method 14 was used to verify the completeness of the urine collections. NU was determined using the Kjeldahl technique with a Kjeltec analyser (model NMKL nr 6, Eurofins Food & Agro AB, Lidköping, Sweden). Extent of intervention fidelity: The PD group reported a significantly lower intake (E% and g/d) of carbohydrates, higher intake (E% and g/d) of protein, MUFA, PUFA, cholesterol, and higher total fat (E%), MUFA:SFA and PUFA:SFA ratios compared to the NNR group. The PD group reported a more pronounced change in the ratio (E%) protein:carbohydrates:total fat from baseline to 6 and 24 months (17:46:33, 23:29:44, 22:34:40; respectively) compared to the NNR group (17:45:35, 19:48:32, 17:43:34; respectively). Target intakes were not fully achieved; the PD did not reach the target amounts of percent energy of protein (30 E%) at 6 and 24 months, and the NNR group did not reach the target amounts of carbohydrates (55‐60%). Concomitant interventions: NR Control diet: Name (as reported) and brief description: Nordic Nutrition Recommendations (NNR) diet aimed for an energy intake comprising 15% protein, 25 to 30% fat and 55‐60% carbohydrates, with emphasis on low‐fat dairy products and high fibre. Consumed ad libitum Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 35 (100%) women with mean (SD) age 60.3 (5.9) years, mean (SD) body weight 86.8 (10.0) kg, mean (SD) BMI 32.6 ( 3.3 ) kg/m2 Why? NR What (materials)? "We developed a 2‐wk menu plan for each group with meals for each day meeting established nutritional requirements and lipid guidelines." Participants also received food scales. What (procedures)? The NNR diet was aiming at a daily intake of 15 E% protein, 25‐30 E% fat, and 55‐60 E% carbohydrates, with emphasis on low‐fat dairy products and high‐fibre products. The group sessions consisted of information on and cooking of the intervention diets, dietary effects on health, behavioural changes, and group discussions. The subjects were given recipes and written instructions to facilitate the preparation of meals at home. Eight group sessions (four cooking classes and four follow‐up sessions) were held during the first 6 months of the intervention. Additional group meetings were held at 9, 12, 18, and 24 months. Who provided? One dietitian per study arm How and where? Face‐to‐face, location NR When and how much? 12 group sessions throughout 24 month period Strategies to improve or maintain fidelity; tailoring and modification: Dietary intake was assessed using 4‐day estimated self‐reported food records conducted at baseline (2 × 4 days) and monthly until 6 months, thereafter at 9, 12, 18 and 24 months. Subjects were instructed to keep a record of all food items consumed over four consecutive days (three weekdays and one weekend day) and to describe and estimate the amount of food eaten by using coloured food‐portion photographs representing known weights and household measuring utensils (e.g. cup, spoon, and standard weight of food items). The reported food intake was converted to estimates of energy and nutrient intake using the nutritional analysis package Dietist XP (version 3.0) based on the food composition database of the Swedish National Food Administration (2008‐03‐06). Nitrogen excretion in urine (NU) was used as a biomarker for protein intake, with three 24‐h urine samples collected at baseline and after 6 and 24 months. The para‐aminobenzoic acid (PABA) method 14 was used to verify the completeness of the urine collections. NU was determined using the Kjeldahl technique with a Kjeltec analyser (model NMKL nr 6, Eurofins Food & Agro AB, Lidköping, Sweden). Extent of intervention fidelity: The PD group reported a significantly lower intake (E% and g/d) of carbohydrates, higher intake (E% and g/d) of protein, MUFA, PUFA, cholesterol, and higher total fat (E%), MUFA:SFA and PUFA:SFA ratios compared to the NNR group. The PD group reported a more pronounced change in the ratio (E%) protein:carbohydrates:total fat from baseline to 6 and 24 months (17:46:33, 23:29:44, 22:34:40; respectively) compared to the NNR group (17:45:35, 19:48:32, 17:43:34; respectively). Target intakes were not fully achieved; the PD did not reach the target amounts of percent energy of protein (30 E%) at 6 and 24 months, and the NNR group did not reach the target amounts of carbohydrates (55‐60%). Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: KNOTA Trial funded by: The Swedish Council for Working Life and Social Research (grants 2006‐0699 and 2010‐0398), the Swedish Research Council (grant K2011‐12237‐15‐6), the Swedish Heart and Lung Foundation, the County Council of Västerbotten and Umeå University, Sweden Declaration of interest: "The authors declare no conflict of interest." Author contacted, but requested information not provided. |
|
Ooi 2021.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 3 Year trial started: NR Sample size calculation: Yes Outcome(s) used for sample size calculation: Lean mass and HOMA‐IR Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 16 weeks What was the duration of the weight maintenance phase: 8 weeks Other notes about methods: NA |
|
| Participants |
Country and setting: Singapore, outpatient research centre. Prescribed meals were provided and delivered to the homes of participants during the weight loss phase. Eligibility criteria: Chinese men and women aged 21 to 45 years who are classed as overweight or obese (BMI 25 to 36 kg/m2) and with a body fat percentage of ≥ 25%. No explicit exclusion criteria are reported, but the authors reported that none of the participants had existing medical conditions and only engaged in low to moderate physical activity Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 132 Total attrition in trial: 24 Treatment diet Participants randomised: 43 Participants withdrawn (voluntary): 2 Total attrition: 4 Control diet: Participants randomised: 44 Participants withdrawn (voluntary): 4 Total attrition: 8 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 32.0 (6.82) Gender distribution (as reported): female 21/43 (49%), male 22/43 (51%) Weight (kg): mean (SD) 81.8 (12.59) BMI (kg/m2): mean (SD) 29.6 (3.02) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 33.5 (7.89) Gender distribution (as reported): female 24/44 (55%), male 20/44 (45%) Weight (kg): mean (SD) 80.1 (10.61) BMI (kg/m2): mean (SD) 29.3 (2.39) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein diet with placebo supplementation (HP) prescribing 27% protein, 43% carbohydrates and 30% fat. Energy deficit of 500 kcal per day Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: N = 21 women and N = 22 men with mean (SD) age 32.0 (6.82) years, mean (SD) weight 81.8 (12.59) kg, mean (SD) BMI 29.6 (3.02) kg/m2, none had existing diabetes or hypertension and all had normal renal, liver and thyroid function. Why? The diet was included as a comparator for a standard‐protein diet with branched‐chain amino acids (BCAAs) supplementation. High‐protein diets are stated as reducing the loss of lean mass during weight loss, but they are expensive. What (materials)? Participants were prescribed a hypocaloric diet with a deficit of 500 kcal per day. "To encourage compliance, subjects had prescribed meals provided and delivered to their homes weekly..." "Weekly food delivery for a 6‐d food supply was provided for the first 4 wk. Subjects had 1 “free day” each week of their own food. For the subsequent 12 wk, only lunch and dinner meals and snacks were provided for 6 d per wk." Participants were also provided with food checklists with their weekly food delivery, to check off food and record additional food and beverages they consumed. Accelerometers were provided to participants at week 0 and week 8 visits. What (procedures)? The high‐protein diet consisted of 16 weeks following a diet with a macronutrient composition of 30% fat, 43% carbohydrate and 27% protein; with placebo supplementation (as opposed to BCAAs). Diet counselling sessions were provided and participants were called to assess and encourage compliance. Who provided? A dietitian provided the counselling sessions and called participants. How and where? Face‐to‐face counselling sessions were provided; the location of sessions, and whether these were group or individual sessions, was not reported. Participants were called to improve compliance. When and how much? Counselling sessions were provided at visits at 0, 4, 8, 12 and 16 weeks; duration of sessions was not reported. Participants were called on a fortnightly basis; duration not reported. Strategies to improve or maintain fidelity; tailoring and modification: Prescribed meals were provided and delivered to encourage compliance, food checklists were used to document dietary compliance, counselling sessions and regular phone calls from the dietitian were used to assess and encourage compliance. Where certain foods could not be consumed by a participant, the dietitian would substitute with foods of a similar composition to adhere to prescribed energy and macronutrients. Extent of intervention fidelity: Urinary urea:creatinine ratio was used as a surrogate for protein intake and dietary compliance. This increased in the treatment group during the weight loss phase, indicating compliance. Food checklists showed modest deviations from dietary prescription, indicating acceptable compliance. Concomitant interventions: NR Control diet: Name (as reported) and brief description: Standard‐protein diet with placebo supplementation (control, CT) prescribing 14% protein, 56% carbohydrates and 30% fat. Energy deficit of 500 kcal per day Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: N = 24 women and N = 20 men with mean (SD) age 33.5 (7.89) years, mean (SD) weight 80.1 (10.61) kg, mean (SD) BMI 29.3 (2.39) kg/m2, none had existing diabetes or hypertension and all had normal renal, liver and thyroid function. Why? The diet was included as a control for an identical diet with branched‐chain amino acids (BCAAs) supplementation. The latter is hypothesised to improve lean mass preservation over a standard‐protein diet without supplementation. What (materials)? Participants were prescribed a hypocaloric diet with a deficit of 500 kcal per day. "To encourage compliance, subjects had prescribed meals provided and delivered to their homes weekly..." "Weekly food delivery for a 6‐d food supply was provided for the first 4 wk. Subjects had 1 “free day” each week of their own food. For the subsequent 12 wk, only lunch and dinner meals and snacks were provided for 6 d per wk." Participants were also provided with food checklists with their weekly food delivery. What (procedures)? The standard‐protein diet consisted of 16 weeks following a diet with a macronutrient composition of 30% fat, 56% carbohydrate and 14% protein; with placebo supplementation (as opposed to BCAAs). Diet counselling sessions were provided and participants were called to assess and encourage compliance. Who provided? A dietitian provided the counselling sessions and called participants. How and where? Face‐to‐face counselling sessions were provided; the location of sessions, and whether these were group or individual sessions, was not reported. Participants were called to improve compliance. When and how much? Counselling sessions were provided at visits at 0, 4, 8, 12 and 16 weeks; duration of sessions was not reported. Participants were called on a fortnightly basis; duration not reported. Strategies to improve or maintain fidelity; tailoring and modification: Prescribed meals were provided and delivered to encourage compliance, food checklists were used to document dietary compliance, counselling sessions and regular phone calls from the dietitian were used to assess and encourage compliance. Where certain foods could not be consumed by a participant, the dietitian would substitute with foods of a similar composition to adhere to prescribed energy and macronutrients. Extent of intervention fidelity: Urinary urea:creatinine ratio was used as a surrogate for protein intake and dietary compliance. This decreased in the control group during the weight loss phase, indicating compliance. Food checklists showed modest deviations from dietary prescription, indicating acceptable compliance. Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Clinical Scientist‐Individual Research Grant administered by Ministry of Health’s National Medical Research Council (NMRC), Singapore‐ NMRC/CIRG/1375/2013, and the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A∗STAR), Singapore Declaration of interest: "The authors report no conflicts of interest." |
|
Parr 2016.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: ACTRN12612000021875 Total number of trial arms: 3 Year trial started: 2011 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in fat mass Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 16 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Australia, outpatient university institute in Victoria Eligibility criteria: Participants aged 35 to 59 who were overweight or obese (BMI 27 to 40 kg/m2), and who completed medical clearance if required (> 45 years for males and > 55 years for females; or > 2 cardiac risk factors). Taking medication for blood pressure, cholesterol, depression, anxiety or arthritis was allowed if participants had been taking them for three months or longer. Exclusion criteria NR Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 111 Total attrition in trial: 22 Treatment diet Participants randomised: 39 Participants withdrawn (voluntary): 6 Total attrition: 10 Control diet: Participants randomised: 40 Participants withdrawn (voluntary): 6 Total attrition: 8 Baseline data treatment diet: Randomised participants not included: 10/39 Age (years): mean (SD) 47.0 (5.9) Gender distribution (as reported): female 21/29, male 8/29 Weight (kg): mean (SD) 91.7 (12.6) BMI (kg/m2): mean (SD) 32.6 (3.9) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.1 (0.7) HDL (mmol/L): mean (SD) 1.3 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.1 (1.0) TG (mmol/L): mean (SD) 1.7 (1.9) Baseline data control diet: Randomised participants not included: 8/40 Age (years): mean (SD) 47.1 (5.7) Gender distribution (as reported): female 22/32, male 10/32 Weight (kg): mean (SD) 90.3 (9.9) BMI (kg/m2): mean (SD) 32.1 (3.6) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.3 (0.9) HDL (mmol/L): mean (SD) 1.3 (0.4) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.2 (1.1) TG (mmol/L): mean (SD) 1.4 (0.8) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High dairy protein, moderate CHO (HDPMC). ~30% protein, 40% carbohydrate, 30% fat; 4+ dairy servings/day of unsweetened/artificially sweetened, full‐fat products. Resistance exercise 3 days per week. Dietary energy restriction of ~250 kcal/day from estimated maintenance energy requirements Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: Overweight and obese participants, 72.4% female, aged mean (SD) 47.0 (5.9), with mean (SD) BMI 32.6 (3.9), mean (SD) weight of 91.7 (6.12) kg Why? When compared to higher‐carbohydrate diets, higher‐protein reduced‐carbohydrate diets minimise the loss of lean mass during weight loss in the absence of exercise. The inclusion of dairy protein has been associated with a greater decrease in fat mass than those who do not incorporate dairy. What (materials)? A prescribed menu consisting of three meals per day and a “Dairy/Snack Basket”. Two dairy servings of unsweetened/ artificially sweetened full‐fat products from the basket were to be consumed post‐exercise training. Educational resources were provided to partcipants by the dietitian at follow‐up visits (not described). Participants received measuring cups and spoons to use throughout the study to assist with recording of portion sizes of their food and drink intake. What (procedures)? Free‐living energy restricted eating plan according to a restriction of 2250 kcal/day from estimated maintenance energy requirements, and required macronutrient composition (30% protein, 40% CHO, 30% fat). Dietary intervention followed a transition from a prescribed menu plan (weeks 0 to 8) to a flexible self‐chosen plan (weeks 9 to 16) based on a points system. Participants were prescribed a high dairy protein and moderate carbohydrate diet, as well as supervised resistance exercise, and were provided with dietary advice, educational resources, menus, meal plans and individualised training programmes. Participants were instructed, verbally and in writing, how to complete 7‐day food diaries. Daily checklists were were conducted; not clear whether this was self‐reported. Who provided? Dietitians met with participants to discuss menus and food choices as well as assess daily checklists. Resistance exercise was supervised by study trainers. How and where? Meetings with dietitian and exercise supervisor were face‐to‐face. Location and and mode of delivery (group or individual) NR When and how much? Food checklists were performed daily, meetings with dietitian were fortnightly, duration NR. Seven‐day food records were assessed at baseline and weeks 1, 4, 8, 12 and 16. Supervised resistance exercise was undertaken 3 days per week, with participants completing aerobic exercise (walking, cycling or swimming) on other days. Resistance exercise was increased every four weeks. Duration of exercise sessions NR Strategies to improve or maintain fidelity; tailoring and modification: Dietary non‐compliance defined as exceeding prescribed energy restriction by > 200 kcal/day or demonstrating an inability or unwillingness to adhere to the “Dairy/Snack Baskets” and the desired macronutrient manipulation. Compliance to each dietary intervention was assessed by the study dietitian via daily checklists and completion of 7‐day food diaries (at weeks 1, 4, 8, 12 and 16). Being non‐compliant to the REX was defined as missing > 2 consecutive training sessions. REXs were increased every 4 wks after further 1RM testing. Exercise diaries were kept by study trainers. Extent of intervention fidelity: Five of 39 participants did not comply with diet and/or exercise. Non‐compliance was defined as exceeding restriction by > 200 kcal per day, not adhering to dairy/snack 'baskets' or macronutrient manipulation, or missing > 2 consecutive exercise sessions. Concomitant interventions: Participants using prescription medication for blood pressure and/or cholesterol, depression, anxiety and arthritis for ≥ 3 months were included. Nutritional supplements were discontinued before the commencement of the trial. Control diet: Name (as reported) and brief description: High‐dairy protein, high CHO (HDPHC). ~30% protein, 55% carbohydrate, 15% fat; 4+ dairy servings/day of sweetened, low‐fat products. Resistance exercise 3 days per week. Dietary energy restriction of ~250 kcal/day from estimated maintenance energy requirements Control diet type (carbohydrate‐fat‐protein): Balanced‐low‐high Exercise component? Yes Recipients: Overweight and obese participants, 68.8% female, aged mean (SD) 47.1 (5.7), with mean (SD) BMI 32.1 (3.6), mean (SD) weight 90.3 (9.9) kg Why? It is not clear how changes in the carbohydrate to fat ratio within an energy‐restricted diet affect loss of lean mass. What (materials)? A prescribed menu consisting of three meals per day and a “Dairy/Snack Basket”. Two dairy servings of unsweetened/artificially sweetened full‐fat products from the basket were to be consumed post‐exercise training. Educational resources were provided to participants by the dietitian at follow‐up visits (not described). Participants received measuring cups and spoons to use throughout the study to assist with recording of portion sizes of their food and drink intake. What (procedures)? Participants were prescribed a high‐dairy protein and high‐carbohydrate diet (30% protein, 55% CHO, 15% fat), as well as supervised resistance exercise, and were provided with dietary advice, educational resources, menus, meal plans and individualised training programmes. Participants were instructed, verbally and in writing, how to complete 7‐day food diaries. Daily checklists were were conducted; not clear whether this was self‐reported. Who provided? Dietitians met with participants to discuss menus and food choices as well as assess daily checklists. Resistance exercise was supervised by study trainers. How and where? Meetings with dietician and exercise supervisor were face‐to‐face. Location and and mode of delivery (group or individual) NR When and how much? Food checklists were performed daily, meetings with dietitian were fortnightly, duration NR. Seven‐day food records were assessed at baseline and weeks 1, 4, 8, 12 and 16. Supervised resistance exercise was undertaken 3 days per week, with participants completing aerobic exercise (walking, cycling or swimming) on other days. Resistance exercise was increased every four weeks. Duration of exercise sessions NR Strategies to improve or maintain fidelity; tailoring and modification: Dietary non‐compliance defined as exceeding prescribed energy restriction by > 200 kcal/day or demonstrating an inability or unwillingness to adhere to the “Dairy/Snack Baskets” and the desired macronutrient manipulation. Compliance to each dietary intervention was assessed by the study dietitian via daily checklists and completion of 7‐day food diaries (at weeks 1, 4, 8, 12 and 16). Being non‐compliant to the REX was defined as missing > 2 consecutive training sessions. REXs were increased every 4 wks after further 1RM testing. Exercise diaries were kept by study trainers. Extent of intervention fidelity: Three of 40 participants did not comply with diet and/or exercise. Non‐compliance was defined as exceeding restriction by > 200 kcal per day, not adhering to dairy/snack 'baskets' or macronutrient manipulation, or missing > 2 consecutive exercise sessions. Concomitant interventions: Participants using prescription medication for blood pressure and/or cholesterol, depression, anxiety and arthritis for ≥ 3 months were included. Nutritional supplements were discontinued before the commencement of the trial. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: DAIRY‐FIT Trial funded by: Dairy Health and Nutrition Consortium, Dairy Innovation Australia Ltd grant (ID 201134D) Declaration of interest: "The authors declared no conflict of interest." |
|
Pedersen 2014.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, number of centres NR Trial registry number: ACTRN12608000045314 Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 52 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely Australia based on authors and trial registry Eligibility criteria: Participants who were overweight or obese, with BMI ≥ 27 kg/m2 and albuminuria (specified ranges of albumin in urine over 24 h or albumin to creatinine ratio, and glomerular filtration rate). Participants were excluded if they had impaired kidney function unrelated to diabetes. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 76 Total attrition in trial: 31 Treatment diet Participants randomised: NR Participants withdrawn (voluntary): NR Total attrition: NR Control diet: Participants randomised: NR Participants withdrawn (voluntary): NR Total attrition: NR Baseline data treatment diet: Randomised participants not included: NR Age (years): mean (SE) 59.4 (2.2) Gender distribution (as reported): female 6/21 (28.6%), male: 15/21 (71.4%) Weight (kg): mean (SE) 108.1 (5.0) BMI (kg/m2): mean (SE) 36.7 (NR) DBP (mmHg): mean (SE) 74.7 (1.6) SBP (mmHg): mean (SE) 127.2 (3) HbA1c (%): mean (SE) 7.5 (0.2) LDL (mmol/L): mean (SE) 1.8 (0.2) HDL (mmol/L): mean (SE) 1.1 (0.0) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 3.9 (0.2) TG (mmol/L): mean (SE) 2.4 (0.3) Baseline data control diet: Randomised participants not included: NR Age (years): mean (SE) 62.4 (1.7) Gender distribution (as reported): female 4/24 n/N (16.7%), male 20/24 n/N (83.3%) Weight (kg): mean (SE) 104.7 (3.8) BMI (kg/m2): mean (SE) 35.4 (NR) DBP (mmHg): mean (SE) 70.1 (1.93) SBP (mmHg): mean (SE) 125.5 (2) HbA1c (%): mean (SE) 7.1 (0.2) LDL (mmol/L): mean (SE) 1.7 (0.1) HDL (mmol/L): mean (SE) 1.1 (0.1) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 3.6 (0.1) TG (mmol/L): mean (SE) 2.0 (0.2) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: iGFR (P = 0.03); intervention vs control arm as well as albumin excretion rate (P = 0.02); intervention vs control arm Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein diet. Planned protein:fat:carbohydrate ratio was 30:30:40 total energy with a range of protein intake of 90‐120 g/d. Saturated fat intake was 10% TE; energy limited to 6000 kJ/day. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: Participants were 28.6% female, aged 59.4 (2.2) years with BMI of 36.7 and weight 108.1 (5.0) kg. Why? Short‐term diets which replace refined carbohydrates with protein improve cardiovascular risk factors, weight loss and lipid profile. Studies show an inverse relationship between dietary protein and hypertension, and in persons with diabetes high‐protein, low‐carbohydrate diets have a beneficial effect on postprandial blood glucose and HbA1c. What (materials)? Continuous Glucose Measuring System (CGMS) to be worn for 48 hours prior to assessments at baseline, 4 months and 12 months, daily food checklist, food frequency questionnaire What (procedures)? Participants were instructed to consume a high‐protein diet. Who provided? NR. Endocrinologists and own doctors monitored glycaemic control, blood pressure and lipids. How and where? Face‐to‐face When and how much? The intervention duration was 12 months. Further information on contact sessions, their frequency and duration NR. Assessments were done at baseline and at 4‐monthly intervals. Strategies to improve or maintain fidelity; tailoring and modification: Compliance with the protein prescription was monitored by a daily food checklist and a food frequency questionnaire (at baseline, 4 and 12 months) and was also assessed by 24‐h urine urea excretion. Extent of intervention fidelity: Adjusted urea excretion at 12 months was significantly different between groups 519 (39) in high‐protein and 456 (25) in standard‐protein group; P = 0.04). Self‐reported diet data confirmed this with a highly significant (P < 0.01) difference in change in protein intake between groups. Concomitant interventions: All but four participants from the total group treated their diabetes with oral blood glucose medication and/or insulin. All were treated with blood pressure medication, and all but two were on statins. Control diet: Name (as reported) and brief description: Standard protein diet. Planned protein:fat:carbohydrate ratio was 20:30:50 total energy with a range of protein intake of 55 to 70 g/d. Saturated fat intake was 10% TE; energy limited to 6000 kJ/day. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Participants were 16.7% female, aged 62.4 (1.7) years with BMI of 35.4 and weight 104.7 (3.8) kg. Why? High‐saturated fat and high‐protein diets reportedly increase LDL cholesterol, raising some concerns with high‐protein low‐carbohydrate diets. What (materials)? Continuous Glucose Measuring System (CGMS) to be worn for 48 hours prior to assessments at baseline, 4 months and 12 months, daily food checklist, food frequency questionnaire What (procedures)? Participants were instructed to consume a standard‐protein diet. Who provided? NR. Endocrinologists and own doctors monitored glycaemic control, blood pressure and lipids. How and where? Face‐to‐face When and how much? The intervention duration was 12 months. Further information on contact sessions, their frequency and duration NR. Assessments were done at baseline and at 4‐monthly intervals. Strategies to improve or maintain fidelity; tailoring and modification: Compliance with the protein prescription was monitored by a daily food checklist and a food frequency questionnaire (at baseline, 4 and 12 months) and was also assessed by 24‐h urine urea excretion. Extent of intervention fidelity: Adjusted urea excretion at 12 months was significantly different between groups 519 (39) in high‐protein and 456 (25) in standard‐protein group; P = 0.04). Self‐reported diet data confirmed this with a highly significant (P < 0.01) difference in change in protein intake between groups. Concomitant interventions: All but four participants from the total group treated their diabetes with oral blood glucose medication and/or insulin. All were treated with blood pressure medication, and all but two were on statins. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: Yes Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: Yes Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: Yes Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: NR Declaration of interest: "PMC is the co‐author of The CSIRO Total Wellbeing Diet Book. No other conflict of interest reported." |
|
Pittas 2005.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 24 weeks. What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient human nutrition research centre at a university in Boston. Food was provided by the research centre and consumed at the participants' homes. Eligibility criteria: Healthy women and men aged 24 to 42 years, with a BMI of between 25 and 29.9 kg/m2 and fasting plasma glucose < 100 mg/dL were recruited. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 34 Total attrition in trial: 2 Treatment diet Participants randomised: NR Participants withdrawn (voluntary): NR Total attrition: NR Control diet: Participants randomised: NR Participants withdrawn (voluntary): NR Total attrition: NR Baseline data treatment diet: Randomised participants not included: NR (2/34 from total group randomised) Age (years): NR Gender distribution (as reported): NR Weight (kg): NR BMI (kg/m2): NR DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Baseline data control diet: Randomised participants not included: NR (2/34 from total group randomised) Age (years): NR Gender distribution (as reported): NR Weight (kg): NR BMI (kg/m2): NR DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐glycaemic load diet of 40% carbohydrates, 30% protein and 30% fat. Fibre content was 15g/1000 kcal, daily GI 53 and GL 45g/1000 kcal. Energy restriction of 30% compared with baseline individual energy needs Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: NR Why? "...individuals with higher insulin secretion lose more weight when randomized to a low–glycemic load diet compared with a high–glycemic load diet." What (materials)? "During the 6‐month intervention period, all food was provided by the research center, and participants were requested to consume only this food and report additional foods if they were eaten." What (procedures)? "Participants were prescribed a low– glycemic load diet (40% carbohydrate, 30% protein, 30% fat, 15 g fibre/1,000 kcal, mean estimated daily glycemic index of 53, and glycemic load of 45 g/1,000 kcals) at 30% calorie restriction. To maximize adherence to the study diet, regular behavioral group meetings and individual sessions with a dietitian were held. From participants’ reports of leftover food and extra items, actual daily nutrient intake during the intervention period was calculated." Who provided? Dietitian How and where? Group meetings, location NR When and how much? 24 weeks Strategies to improve or maintain fidelity; tailoring and modification: Participants were required to only eat food provided and report on any additional food intake. To maximise adherence to the study diet, regular behavioural group meetings and individual sessions with a dietitian were held. Extent of intervention fidelity: NR Concomitant interventions: NR Control diet: Name (as reported) and brief description: High‐glycaemic load diet of 60% carbohydrates, 20% protein and 20% fat. Fibre content was 15g/1000 kcal, daily GI 86 and GL 116g/1000 kcal. Energy restriction of 30% compared with baseline individual energy needs Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: NR Why? NR What (materials)? "During the 6‐month intervention period, all food was provided by the research center, and participants were requested to consume only this food and report additional foods if they were eaten." What (procedures)? Participants were prescribed a high– glycaemic load diet (60% carbohydrate, 20% protein, 20% fat, 15 g fibre/1,000 kcal, mean estimated daily glycaemic index of 86, and glycaemic load of 116 g/1000 kcal). To maximise adherence to the study diet, regular behavioural group meetings and individual sessions with a dietitian were held. From participants’ reports of leftover food and extra items, actual daily nutrient intake during the intervention period was calculated. Who provided? Dietitian How and where? Group meetings, location NR When and how much? 24 weeks Strategies to improve or maintain fidelity; tailoring and modification: Participants were required to only eat food provided and report on any additional food intake. To maximise adherence to the study diet, regular behavioural group meetings and individual sessions with a dietitian were held. Extent of intervention fidelity: NR Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: CALERIE trial Trial funded by: National Institutes of Health grants (K23‐DK61506 and U01‐AG20480) and the US Department of Agriculture (cooperative agreement number 58‐1950‐4‐401) Declaration of interest: NR Author contacted, but requested information not provided. |
|
Racette 1995.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1, 2 Study design: RCT, parallel, number of centres NR Trial registry number: NR Total number of trial arms: 4 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): 5 What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: 6 weeks Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient medical centre at a university in Chicago Eligibility criteria: Healthy, premenopausal women aged between 21 and 47 years, who are non‐smokers, were included. These women were included if they had a body weight between 140 and 180% of the midpoint for height (according to the 1959 Metropolitan Life Insurance Company table of desirable weights); had a body fat mass of more than 35% of total body weight; were weight‐stable; and had not engaged in formal exercise for more than three months prior to screening. Exclusion criteria NR Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Female Total number randomised: 41 Total attrition in trial: 10 Treatment diet Participants randomised: NR Participants withdrawn (voluntary): NR Total attrition: NR Control diet: Participants randomised: NR Participants withdrawn (voluntary): NR Total attrition: NR Baseline data treatment diet: Randomised participants not included: NR (18/41 from total group randomised) Age (years): mean (SD) 41 (6) Gender distribution (as reported): NR Weight (kg): mean (SD) 94.0 (10.9) BMI (kg/m2): NR DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Baseline data control diet: Randomised participants not included: NR (18/41 from total group randomised) Age (years): mean (SD) 37 (4) Gender distribution (as reported): NR Weight (kg): mean (SD) 92.7 (9.0) BMI (kg/m2): NR DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): NR HDL (mmol/L): NR Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): NR Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐carbohydrate diet prescribing 25% carbohydrate, 25% protein and 50% fat. Energy prescription of 75% of each participant's resting metabolic rate Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐high Exercise component? No Recipients: N = 6 women only with mean (SD) age 41 (6) years, mean (SD) weight 94 (10.9) kg Why? Increasing carbohydrate when total energy intakes exceed 2.5 MJ/d is proposed to mitigate the adverse effects of a low‐energy diet by preventing the decreases in thyroid hormone activity and resting metabolism that are frequently observed. What (materials)? Participants were given food scales, measuring cups and spoons, and a notebook of meal plans and recipes to assist in their meal preparation. What (procedures)? The prescribed compositions of the weight reducing low‐carbohydrate diet was 25% carbohydrate, 25% protein, 50% fat. The reducing diets were designed to promote a weight loss of 1 kg/wk, with energy levels individually prescribed to approximate 75% of each subject’s measured RMR. All participants were required to attend weekly meetings, which included nutrition education classes, group discussions, collection of food diary booklets. Subjects were taught the exchange meal‐planning system and were instructed to consume a specific number of exchanges based on their prescribed energy level and diet composition. Subjects recorded all food and beverages they consumed, along with the corresponding exchanges, in daily food diaries. Booklets containing 7 d of daily diaries were collected weekly and analysed by the study dietitian for energy content and distribution by using the exchange system. Each subject received weekly feedback regarding both her dietary compliance and her accuracy in using the exchange system, and the dietitian met individually with subjects as necessary to improve these areas. Who provided? Dietitian How and where? Participants received weekly feedback and met individually with dietitian as required, location NR When and how much? Weekly meetings which included nutrition education classes, group discussions, collection of food diary booklets and weigh‐ins Strategies to improve or maintain fidelity; tailoring and modification: Subjects recorded all food and beverages they consumed, along with the corresponding exchanges, in daily food diaries. Booklets containing 7 d of daily diaries were collected weekly. Each subject received weekly feedback regarding both her dietary compliance and her accuracy in using the exchange system and the dietitian met individually with subjects as necessary to improve these areas. Extent of intervention fidelity: NR Concomitant interventions: NR Control diet: Name (as reported) and brief description: Low‐fat diet prescribing 60% carbohydrate, 25% protein and 15% fat. Energy prescription of 75% of each participant's resting metabolic rate Control diet type (carbohydrate‐fat‐protein): Balanced‐low‐high Exercise component? No Recipients: N = 7 women only with mean (SD) age 37 (4) years, mean (SD) weight 92.7 (9.0) kg Why? The maintenance diet was designed to maintain energy balance, with energy levels individually prescribed based on each subject’s RMR and an individualised activity factor of 1.6‐1.9. RMR values were calculated from the Harris‐Benedict formula during MI, whereas the measured RMR values were used during MII. Adjustments were made in the prescribed energy levels as necessary to minimise weight changes. The prescribed composition of the maintenance diet was 45% carbohydrate, 20% protein, and 35% fat. What (materials)? Participants were given food scales, measuring cups and spoons, and a notebook of meal plans and recipes to assist in their meal preparation. What (procedures)? The prescribed compositions of the weight‐reducing low‐fat diet was 60% carbohydrate, 25% protein, 15% fat. The reducing diets were designed to promote a weight loss of 1 kg/wk, with energy levels individually prescribed to approximate 75% of each subject’s measured RMR. All participants were required to attend weekly meetings, which included nutrition education classes, group discussions, collection of food diary booklets. Subjects were taught the exchange meal‐planning system and were instructed to consume a specific number of exchanges based on their prescribed energy level and diet composition. Subjects recorded all food and beverages they consumed, along with the corresponding exchanges, in daily food diaries. Booklets containing 7 d of daily diaries were collected weekly and analysed by the study dietitian for energy content and distribution by using the exchange system. Each subject received weekly feedback regarding both her dietary compliance and her accuracy in using the exchange system, and the dietitian met individually with subjects as necessary to improve these areas. Who provided? Dietitian How and where? Participants received weekly feedback and met individually with dietitian as required, location NR When and how much? Weekly meetings which included nutrition education classes, group discussions, collection of food diary booklets and weigh‐ins Strategies to improve or maintain fidelity; tailoring and modification: Subjects recorded all food and beverages they consumed, along with the corresponding exchanges, in daily food diaries. Booklets containing 7 d of daily diaries were collected weekly. Each subject received weekly feedback regarding both her dietary compliance and her accuracy in using the exchange system and the dietitian met individually with subjects as necessary to improve these areas. Extent of intervention fidelity: NR Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: NIH (grant DK 30031), the Clinical Nutrition Research Unit (grant DK 26678), the Clinical Research Center (grant RR 00055) and Quaker Oats Co. Declaration of interest: NR Author contacted, but requested information not provided. |
|
Ruth 2013.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: 2009 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in flow mediated dilatation (FMD) Duration of run‐in period (weeks): 1 What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient nutrition and weight management centre in Boston Eligibility criteria: Participants were included if they were obese. Participants were excluded if they had cardiovascular disease, type 2 diabetes with HbA1c above 8.0% and taking anti‐diabetes medication; had recent weight loss of 3% or more in the past three months; used weight‐loss medication in the four weeks prior; had an eating disorder, renal or hepatic disease; undergone bariatric surgery; were pregnant; used tobacco; had a thyroid disorder or were currently using angiotensin receptor blockers. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 55 Total attrition in trial: 22 Treatment diet Participants randomised: 29 Participants withdrawn (voluntary): 6 Total attrition: 11 Control diet: Participants randomised: 26 Participants withdrawn (voluntary): 9 Total attrition: 11 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 43.5 (11.5) Gender distribution (as reported): female 90%, male 10% Weight (kg): mean (SD) 100.5 (14.8) BMI (kg/m2): mean (SD) 37.1 (4.6) DBP (mmHg): mean (SD) 76.8 (9.3) SBP (mmHg): mean (SD) 122.3 (14.2) HbA1c (%): mean (SD) 5.70 (0.49) LDL (mmol/L): mean (SD) 2.87 (0.89) HDL (mmol/L): mean (SD) 1.32 (0.38) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.81 (1.00) TG (mmol/L): mean (SD) 1.37 (0.75) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 41.5 (12.8) Gender distribution (as reported): female 88%, male 12% Weight (kg): mean (SD) 99.3 (14.5) BMI (kg/m2): mean (SD) 35.9 (4.8) DBP (mmHg): mean (SD) 76.4 (9.4) SBP (mmHg): mean (SD) 117.2 (15.0) HbA1c (%): mean (SD) 5.65 (0.25) LDL (mmol/L): mean (SD) 2.79 (0.66) HDL (mmol/L): mean (SD) 1.36 (0.37) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.62 (0.78) TG (mmol/L): mean (SD) 1.02 (0.38) Group differences at baseline: Yes Characteristic(s) with significant group difference and relevant statistic: characteristic 1: fasting insulin (P = 0.029); intervention vs control arm; characteristic 2: triglycerides (P = 0.036); intervention vs control arm Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐fat low‐carbohydrate (HFLC) diet, participants counselled to consume ≤ 40 g/d carbohydrates and 60% of calories as fat (< 7% calories from saturated fat), and the remaining ~35% from protein. Energy prescription was a 500 kcal deficit from daily caloric needs. Treatment diet type (carbohydrate‐fat‐protein): Very low‐high‐high Exercise component? No Recipients: Participants were 90% female, aged 43.5 (11.5) years, with BMI 37.1 (4.6) and weight 100.5 (14.8) kg. Why? High‐fat, low‐carbohydrate diets have shown beneficial effect on markers of cardiovascular risk as well as improvements in systemic inflammation. What (materials)? NR What (procedures)? Participants were instructed to consume a high‐fat, low‐carbohydrate diet through regular consultations. They were also required to complete food records. A run‐in period 1 week before starting the diet. Individual diet counselling biweekly. Daily caloric needs were estimated by the Mifflin‐St Jeor equation. All subjects completed take home 3‐day food records every two weeks. Who provided? Study dietitian provided counselling and analysed three‐day food records. How and where? Individual face‐to‐face meetings with dietitian, Boston Medical Center’s General Clinical Research Unit When and how much? The duration of the intervention was 12 weeks. During this time, participants were counselled by a study dietician on a biweekly basis. Participants had to complete take‐home three‐day food records (comprising two weekdays and a weekend day) every two weeks. Strategies to improve or maintain fidelity; tailoring and modification: Completing of food records, regular meetings with dietitian, measurement of weight at the end of the study. Same nurse took all the measurements. Extent of intervention fidelity: The proportion of participants in the intervention group who lost more than 5% of their body weight was greater than in the control group, which the authors interpreted as greater adherence. Carbohydrate intake was significantly lower in the intervention group when compared to control group (P < 0.0001), as was protein and fat (P < 0.0001 for both). This group consumed the prescribed amounts of carbohydrate and fat. Concomitant interventions: No medications were permitted, with participants being asked to stop taking NSAIDs seven days before baseline and follow‐up measures. Control diet: Name (as reported) and brief description: Low‐fat high‐carbohydrate (LFHC) diet, participants were counselled to consume ~60% of calories from complex carbohydrates, 25% from fat (< 7% from saturated fat) and 15% from protein. Energy prescription was a 500 kcal deficit from daily caloric needs. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Participants were 88% female, aged 41.5 (12.8) years, with BMI 35.9 (4.8) and weight 99.3 (14.5) kg. Why? Low‐fat, high‐carbohydrate diets have shown improvements in systemic inflammation. What (materials)? NR What (procedures)? Participants were instructed to consume a low‐fat, high‐carbohydrate diet through regular consultations. They were also required to complete food records. Individual diet counselling biweekly. Who provided? Study dietitian provided counselling and analysed three‐day food records. How and where? Individual face‐to‐face meetings with dietician, Boston Medical Center’s General Clinical Research Unit When and how much? The duration of the intervention was 12 weeks. During this time, participants were counselled by a study dietician on a biweekly basis. Participants had to complete take‐home three‐day food records (comprising two weekdays and a weekend day) every two weeks. Strategies to improve or maintain fidelity; tailoring and modification: Completing of food records, regular meetings with dietitian, measurement of weight at the end of the study. Same nurse took all the measurements. Extent of intervention fidelity: Carbohydrate intake was somewhat lower than prescribed (56% versus 60%) and protein intake slightly higher (22% versus 15%). Saturated fat intake in this group was higher than was recommended. Concomitant interventions: No medications were permitted, with participants being asked to stop taking NSAIDs seven days before baseline and follow‐up measures. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: No Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: Yes Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Dr. Robert C. and Veronica Atkins Foundation research grant and M01 RR000533 and U54 RR025771 to the Boston University Clinical and Translational Science Institute Declaration of interest: "M.R. Ruth, M. Shah, A.M. Port, A.C. Bourland, N.W. Istfan, K. Nelson and N. Gokce have no competing interests. C.M. Apovian has served on the advisory boards for Allergan, Amylin, Orexigen, Merck, Johnson and Johnson, Abbott, Arena, Zafgen, Novo Nordisk and Sanofi‐Aventis, and has received research funding from Lilly, Amylin, Pfizer, Sanofi‐Aventis, Orexigen, MetaProteomics, and the Dr. Robert C. and Veronica Atkins Foundation." Author contacted, but requested information not provided. |
|
Sacks 2009.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, multi‐centre (2) Trial registry number: NCT00072995 Total number of trial arms: 4 Year trial started: 2004 Sample size calculation: Yes Outcome(s) used for sample size calculation: Weight loss Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 2 years What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient university hospital in Boston and outpatient biomedical research centre in Baton Rouge Eligibility criteria: Participants aged between 30 and 70 years with a BMI between 25 and 40 kg/m2. Participants were excluded if they had diabetes or unstable cardiovascular conditions; used medication which affected weight or had insufficient motivation as judged by questionnaire. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 811 Total attrition in trial: 166 Treatment diet Participants randomised: 201 Participants withdrawn (voluntary): 6 Total attrition: 33 Control diet: Participants randomised: 204 Participants withdrawn (voluntary): 7 Total attrition: 35 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 51 (9) Gender distribution (as reported): female 129/201 (64%), male 72/201 (36%) Weight (kg): mean (SD) 94 (16) BMI (kg/m2): mean (SD) 33 (4) DBP (mmHg): mean (SD) 76 (10) SBP (mmHg): mean (SD) 120 (15) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.26 (0.80) HDL (mmol/L): mean (SD) 1.32 (0.41) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.28 (0.91) TG (mmol/L): mean (SD) 1.59 (0.96) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 51 (9) Gender distribution (as reported): female 126/204 (62%), male 78/204 (38%) Weight (kg): mean (SD) 94 (16) BMI (kg/m2): mean (SD) 33 (4) DBP (mmHg): mean (SD) 75 (9) SBP (mmHg): mean (SD) 118 (13) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.21 (0.85) HDL (mmol/L): mean (SD) 1.27 (0.39) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.15 (0.98) TG (mmol/L): mean (SD) 1.53 (0.93) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐fat high‐protein, 40% fat, 25% protein and 35% carbohydrates; 90 min of moderate physical exercise. Each participant’s caloric prescription represented a deficit of 750 kcal per day from baseline resting energy expenditure. Treatment diet type (carbohydrate‐fat‐protein): Low‐high‐high Exercise component? Yes Recipients: Participants were 64% female, aged 51 (9) years, with BMI 33(4) and weight 94 (16) kg. A total of 29% of participants had a BMI between 25 and 29.9; 71% had a BMI ≥ 30, and 40% were hypertensive. Why? To determine the effects of a high‐fat high‐protein diet, together with regular exercise, on long‐term weight loss (2 years). To facilitate a dose‐response test of carbohydrate content What (materials)? Structured meal plans, food diaries, food shopping lists and easy‐to‐prepare recipes, based on the American Dietetic Association (ADA) exchange system (booklet) What (procedures)? Participants were instructed to consume a high‐fat, high‐protein diet with regular group and individual sessions over the course of the intervention. Participants were asked to maintain food and beverage diaries and self‐monitor their intake goals. Group sessions consisted of nutrition education, behavioural methods and social support. Each participant’s caloric prescription represented a deficit of 750 kcal per day from baseline, as calculated from the person’s resting energy expenditure and activity level. Structured meal plans were provided based on the American Dietetic Association (ADA) exchange system.The goal for physical activity was 90 minutes of moderate exercise per week. Who provided? Behavioural counsellors, registered dietitians and 'other study staff members'. How and where? Face‐to‐face group and individual sessions attended regularly, location NR. Web‐based self‐monitoring was completed by participants. When and how much? The duration of the intervention was two years. During the first six months, group sessions lasting one hour were provided three out of every four weeks; and thereafter two our of every four weeks for the remainder of the intervention. Individual sessions with dietitians, lasting 30 minutes each, were scheduled every eight weeks for the duration of the intervention. Food and beverage diaries and web‐based self‐monitoring of food intake was done daily. Strategies to improve or maintain fidelity; tailoring and modification: Participants were instructed to record their food and beverage intake in a daily food diary and in a Web based self‐monitoring tool that provided information on how closely their daily food intake met the goals for macronutrients and energy. Behavioural counselling was integrated into the group and individual sessions to promote adherence to the assigned diets (e.g. behavioural strategies such as goal‐setting, problem‐solving). Self‐monitoring indicated how well food intake corresponded with the prescribed macronutrient and energy intakes, regular weight monitoring; team members praised participants for success. Dietary intake of participants was assessed by 24‐hour recalls at 6 months and 2 years (three telephone interviews within a 3‐week period, 2 weekdays and a weekend day) in a 50% random sample selected. Participation in exercise was monitored by questionnaire (at 12 and 24 months follow‐up) and by the online self‐monitoring tool. Extent of intervention fidelity: Attendance at individual counselling was higher than for group counselling (64.3 versus 53.8% overall, 69.5 versus 55.3% in intervention arm). Adherence data from subsample (n = 177): % Individual sessions attended, means (SD): 69.5 (30.7); % group sessions attended, means (SD): 55.3 (20.0); % of days with completed food entries, means (SD): 60.1 (31.9); % of days with physical activity entries, means (SD): 29.8 (22.4); % discrepancy (kcal goal), means (SD): ‐9.5 (11.4); % discrepancy (protein goal), means (SD): ‐14.5 (13.2); % discrepancy (fat goal), means (SD): ‐13.8 (14.8); % discrepancy (carbohydrate goal), means (SD): ‐1.4 (13.2) Concomitant interventions: Use of medicines at baseline: Antihypertensive medication, n/N (%) : 61/201 (30%); lipid‐lowering medication, n/N (%): 31/201 (15%) Control diet: Name (as reported) and brief description: Low‐fat average‐protein, 20% fat, 15% protein and 65% carbohydrates; 90 min of moderate physical exercise. Each participant’s caloric prescription represented a deficit of 750 kcal per day from baseline resting energy expenditure. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: Participants were 62% female, aged 51 (9) years, with BMI 33(4) and weight 94 (16) kg. A total of 25% of participants had a BMI between 25 and 29.9; 75% had a BMI ≥ 30, and 34% were hypertensive. Why? Studies which followed up participants on different diets found that low‐carbohydrate, high‐protein were not superior to high‐carbohydrate, low‐fat diets. What (materials)? Structured meal plans, food diaries, food shopping lists and easy‐to‐prepare recipes, based on the American Dietetic Association (ADA) exchange system (booklet) What (procedures)? Participants were instructed to consume a low‐fat, average‐protein diet with regular group and individual sessions over the course of the intervention. Participants were asked to maintain food and beverage diaries and self‐monitor their intake goals. Group sessions consisted of nutrition education, behavioural methods and social support [from 72 Williamson 2010]. The goal for physical activity was 90 minutes of moderate exercise per week. Who provided? Behavioural counsellors, registered dietitians and 'other study staff members'. How and where? Face‐to‐face group and individual sessions attended regularly, location NR. Web‐based self‐monitoring was completed by participants. When and how much? The duration of the intervention was two years. During the first six months, group sessions lasting one hour were provided three out of every four weeks; and thereafter two our of every four weeks for the remainder of the intervention. Individual sessions with dietitians, lasting 30 minutes each, were scheduled every eight weeks for the duration of the intervention. Food and beverage diaries and web‐based self‐monitoring of food intake was done daily. Strategies to improve or maintain fidelity; tailoring and modification: Participants were instructed to record their food and beverage intake in a daily food diary and in a Web‐based self‐monitoring tool that provided information on how closely their daily food intake met the goals for macronutrients and energy. Behavioural counselling was integrated into the group and individual sessions to promote adherence to the assigned diets (e.g. behavioural strategies such as goal‐setting, problem‐solving). Self‐monitoring indicated how well food intake corresponded with the prescribed macronutrient and energy intakes, regular weight monitoring; team members praised participants for success. Dietary intake of participants was assessed by 24‐hour recalls at 6 months and 2 years (three telephone interviews within a 3‐week period, 2 weekdays and a weekend day) in a 50% random sample selected. Participation in exercise was monitored by questionnaire (at 12 and 24 months follow‐up) and by the online self‐monitoring tool. Extent of intervention fidelity: Attendance at individual counselling was higher than for group counselling (64.3 versus 53.8% overall, 61.6 versus 55.2% in intervention arm). Adherence data from subsample (n = 170): % Individual sessions attended, means (SD): 61.6 (31.2); % group sessions attended, means (SD): 55.2 (21.8); % of days with completed food entries, means (SD): 56.7 (34.2); % of days with physical activity entries, means (SD): 27.9 (23.1); % discrepancy (kcal goal), means (SD): ‐13.3 (13.1); % discrepancy (protein goal), means (SD): ‐3.1 (14.9); % discrepancy (fat goal), means (SD): 0.4 (26.3); % discrepancy (carbohydrate goal), means (SD): ‐19.9 (14.5) Concomitant interventions: Use of medicines at baseline: Antihypertensive medication, n/N (%) : 54/204 (26%); lipid‐lowering medication, n/N (%): 32/204 (16%) |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: Yes Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: POUNDS LOST Trial funded by: Grants from the National Heart, Lung, and Blood Institute (HL073286) and the General Clinical Research Center, National Institutes of Health (RR‐02635) Declaration of interest: "Dr. Greenway reports receiving consulting fees from or serving on a paid advisory board for Anian, Bristol‐Myers Squibb, Clarus Health, Encore Pharmaceutical, Leptos Biomedical, MDRNA, Novo Nordisk, General Nutrition Corporation, Catalyst, Jenny Craig, Orexigen, Lithera, and Basic Research, receiving lecture fees from BAROnova, Lazard, and Biologene, and owning equity in Lithera. No other potential conflict of interest relevant to this article was reported. Dr. Ryan is chairperson of the Obesity Committee of the National Heart, Lung, and Blood Institute’s Clinical Guidelines for Cardiovascular Risk Reduction Expert Panel; Dr. Loria is a member of that committee; and Dr. Sacks is a member of the Lifestyle Working Group of the Expert Panel that interacts with the Obesity Committee. Dr. Sacks is also vice‐chair of the Nutrition Committee of the American Heart Association, which advises the Association on nutrition topics, including those related to overweight and obesity." |
|
Samaha 2003.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1, 3 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: 2001 Sample size calculation: Yes Outcome(s) used for sample size calculation: Weight loss Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 months What was the duration of the weight maintenance phase: 24 months Other notes about methods: NA |
|
| Participants |
Country and setting: USA, veteran affairs medical centre in Philadelphia Eligibility criteria: Participants aged 18 years or older with a BMI of at least 35 kg/m2. Participants were excluded if they had a serum creatinine level of more than 1.5 mg/dL (132.6 µmol/L); hepatic disease; severe, life‐limiting medical illness; an inability of diabetic subjects to monitor their own glucose levels; or if they had active participation in a dietary programme, or use of weight‐loss medications. Type 2 diabetes at baseline: Mixed; at baseline 64% in treatment and 56% in control group had diabetes ‐ stratified with T2DM for change in weight at 3 to < 12 months; change in BMI at at 3 to < 12 months was reported separately for participants without and with diabetes ‐ these data were included in the appropriate comparisons where possible. Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 132 Total attrition in trial: 53 Treatment diet Participants randomised: 64 Participants withdrawn (voluntary): NR Total attrition: 21 Control diet: Participants randomised: 68 Participants withdrawn (voluntary): NR Total attrition: 32 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 53 (9) Gender distribution (as reported): female 20.0%, male 80.0% Weight (kg): mean (SD) 130.0 (22.7) BMI (kg/m2): mean (SD) 42.9 (6.6) DBP (mmHg): mean (SD) 78 (11) SBP (mmHg): mean (SD) 133 (15) HbA1c (%): mean (SD) 7.8 (1.2) LDL (mmol/L): mean (SD) 2.95 (0.93) HDL (mmol/L): mean (SD) 1.06 (0.28) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.69 (1.35) TG (mmol/L): mean (SD) 2.12 (1.99) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 54 (9) Gender distribution (as reported): female 15%, male 85% Weight (kg): mean (SD) 131.8 (27.3) BMI (kg/m2): mean (SD) 42.9 (7.7) DBP (mmHg): mean (SD) 80 (9) SBP (mmHg): mean (SD) 135 (16) HbA1c (%): mean (SD) 7.4 (1.5) LDL (mmol/L): mean (SD) 3.06 (0.75) HDL (mmol/L): mean (SD) 1.06 (0.26) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.97 (0.78) TG (mmol/L): mean (SD) 1.99 (1.36) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: HbA1c and blood lipid baseline equivalence NR; could not assess statistical significance |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Low‐carbohydrate ad libitum diet, subjects were instructed to restrict carbohydrates to 30 g per day or less. No instructions on reducing total fat intake were provided. Vegetables and fruits with high ratios of fibre to carbohydrates were recommended. Treatment diet type (carbohydrate‐fat‐protein): Very low‐unclear‐unclear Exercise component? No Recipients: Subjects were 20% female and aged mean (SD) 53 (9) years, with BMI mean (SD) 42.9 (6.6) and weight 130.0 (22.7) kg. Forty‐one percent had diabetes mellitus, 45% had metabolic syndrome without diabetes, 72% had hypertension, 51% had hyperlipidemia and 16% had coronary artery disease. Why? Severely obese subjects with a high prevalence of diabetes or the metabolic syndrome would have a greater weight loss, without detrimental effects on risk factors for atherosclerosis, while on a low‐carbohydrate diets. There are concerns that carbohydrate restriction might have adverse effects on serum lipids. The intervention was administered to determine weight loss and changes in cardiovascular risk factors resulting from a low‐carbohydrate diet. What (materials)? Diet overview handout, instructional nutrition labels, sample menus and recipes, and a book on counting calories and carbohydrates. No specific exercise programme was recommended What (procedures)? Participants were prescribed carbohydrate restriction of 30 g or less per day. They received two hours of group‐teaching sessions each week for four weeks, thereafter one hour per month for five additional months. All sessions were led by a an expert in nutritional counselling. Who provided? Experts in nutritional counselling How and where? Face‐to‐face group sessions, location NR When and how much? Four weekly sessions of two hours each, followed by five monthly sessions of one hour; over the course of six months Strategies to improve or maintain fidelity; tailoring and modification: Sessions were held in groups, weighing and waist measurement at six months and determination of dietary compliance using a validated instrument for 24‐hour recall of consumption Extent of intervention fidelity: Subjects decreased their caloric intake from 2153 (1060) to 1343 (731) kcal (P < 0.001). These subjects also significantly increased their intake of fat to 44 (16)% and protein to 25 (10)%, and decreased their carbohydrate intake to 31 (19)%. Concomitant interventions: Of intervention participants; 11% were treated with sulfonylurea, 17% with metformin, 2% with peroxisome proliferator‐activated receptor gamma antagonist and 9% with insulin. Sixty‐four percent received antihypertensive therapy, 42% received statins and 3% received gemfibrozil. Control diet: Name (as reported) and brief description: Low‐fat diet, subjects were instructed to follow diet according to the obesity‐management guidelines of the National Heart, Lung and Blood Institute, including caloric restriction sufficient to create a deficit of 500 calories per day, with 30% or less of total calories from fat. From the guideline: 55% or more of total calories; protein approximately 15% of total calories and 30% or less of total fat. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Subjects were 15% female and aged mean (SD) 54 (9) years, with BMI mean (SD) 42.9 (7.7) and weight 131.8 (27.3) kg. Thirty‐eight percent had diabetes mellitus, 41% had the metabolic syndrome without diabetes, 57% had hypertension, 50% had hyperlipidemia and 16% had coronary artery disease. Why? NR What (materials)? Diet overview handouts, instructional nutritional labels, sample menus and recipes as well as a book on how to count calories and carbohydrates (National Heart, Lung, and Blood Institute 1998). What (procedures)? Participants were prescribed a low‐fat diet. They received two hours of group‐teaching sessions each week for four weeks, thereafter one hour per month for five additional months. All sessions were led by a an expert in nutritional counselling. Who provided? Experts in nutritional counselling How and where? Face‐to‐face group sessions, location NR When and how much? Four weekly sessions of two hours each, followed by five monthly sessions of one hour; over the course of six months Strategies to improve or maintain fidelity; tailoring and modification: Sessions were held in groups, weighing and waist measurement at six months and determination of dietary compliance using a validated instrument for 24‐hour recall of consumption Extent of intervention fidelity: [From 25 Seshadri 2005] Subjects decreased their caloric intake from 1882 (820) to 1590 (679) kcal (P = 0.057). There was no significant change in macronutrient intake, with intake at 16 (5)% for protein, 51 (15)% for carbohydrate and 32 (15)% for fat at six months. Concomitant interventions: Of control participants; 16% were treated with sulfonylurea, 13% with metformin, 2% with peroxisome proliferator‐activated receptor gamma antagonist and 4% with insulin. Fifty‐seven percent received antihypertensive therapy, 37% received statins and 2% received niacin. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes, in participants with type 2 diabetes mellitus Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Veterans Affairs Healthcare Network Competitive Pilot Project Grant Declaration of interest: NR for primary reference. Stern et al 2004: "None disclosed." |
|
Saslow 2017a.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT01713764 Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient centre for integrative medicine in San Francisco Eligibility criteria: Participants 18 years or older with BMI ≥ 25 kg/m2 and HbA1c > 6.0%. Participants were excluded if they were currently using insulin or were taking more than three glucose‐lowering agents. Type 2 diabetes at baseline: Mixed; stratified with T2DM since participant eligibility criteria were diabetes or prediabetes (HbA1c ≥ 6.5 or > 6%, respectively), and at baseline 75% of treatment & 72% of control group were on diabetes medication. Impaired glucose tolerance at baseline: Mixed Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 34 Total attrition in trial: 5 Treatment diet Participants randomised: 16 Participants withdrawn (voluntary): NR Total attrition: 2 Control diet: Participants randomised: 18 Participants withdrawn (voluntary): NR Total attrition: 3 Baseline data treatment diet: Randomised participants not included: 1/16 Age (years): mean (SD) 64.8 (7.7) Gender distribution (as reported): female 9/16 (56.3%), male 7/16 (43.7%) Weight (kg): mean (SD) 100.1 (26.4) BMI (kg/m2): mean (SD) 36.2 (8.2) DBP (mmHg): mean (SD) 76.3 (6.8) SBP (mmHg): mean (SD) 130.7 (10.5) HbA1c (%): mean (SD) 6.6 (0.3) LDL (mmol/L): mean (SD) 2.31 (0.67) HDL (mmol/L): mean (SD) 1.30 (0.37) Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): mean (SD) 1.40 (0.69) Baseline data control diet: Randomised participants not included: 0/18 Age (years): mean (SD) 55.1 (13.5) Gender distribution (as reported): female 16/18 n/N (88.9%), male 2/18 n/N (11.1%) Weight (kg): mean (SD) 99.7 (24.2) BMI (kg/m2): mean (SD) 37.4 (6.4) DBP (mmHg): mean (SD) 79.9 (12.2) SBP (mmHg): mean (SD) 129.5 (13.0) HbA1c (%): mean (SD) 6.9 (0.7) LDL (mmol/L): mean (SD) 2.55 (0.64) HDL (mmol/L): mean (SD) 1.21 (0.28) Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): mean (SD) 1.95 (0.84) Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Participant flow in supplementary files is different to that reported in Saslow et al 2014. Baseline characteristics are estimated marginal means. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Low‐carbohydrate ketogenic (LCK) ad libitum diet, very low carbohydrate, high‐fat, non calorie‐restricted diet reducing carbohydrate intake to 20‐50 grams per day. Participants were instructed to eat a normal amount of protein and derive their remaining calories from fat. Treatment diet type (carbohydrate‐fat‐protein): Very low‐unclear‐unclear Exercise component? No Recipients: Diabetics or prediabetics, 56.3% female, aged mean (SD) 64.8 (7.7) years with 69% having a BMI above 30. A total of 81% had dyslipidemia and 63% had hypertension. Why? Uncertainty exists around the optimal diet for managing diabetics and prediabetics. Some research indicates that ad libitum low‐carbohydrate ketogenic diets may improve metabolic measures of these patients, and reduce the need for medication. What (materials)? "Abbott Precision Xtra Monitoring System and blood ketone test strips. Home glucose meter for participants on diabetes medication other than metformin. Audio CDs for meditation." What (procedures)? Participants attended twelve weekly classes followed by three classes every two weeks and four classes every two months related to eating a low‐carbohydrate ketogenic diet, which was presented by an author in the manuscript. On week 6 of the intervention participants were taught the importance of sleep and exercise while a third group leader instructed participants on increasing positive affect and mindfulness as well as meditation. To develop mindful eating skills, participants were asked to practice a guided meditation 10 minutes per day at least three times a week using audio CDs recorded for the intervention, and to use several mindful eating practices during meal times, such as focussing awareness on the taste and texture of foods while eating. Blood glucose was monitored in patients on diabetes medications, except metformin. Who provided? The group leader responsible for teaching classes was an author of the manuscript and had experience in the low‐carbohydrate dietary approach. Qualification of group leader providing information on supportive behavioural strategies NR How and where? Format and location of meetings NR. Ketosis and blood glucose were measured at home by participants. When and how much? Twelve weekly classes were for two hours, three classes every two weeks for two hours and four classes every two months were 1.5 hours over the course of the 12‐month intervention. Meditation for 10 minutes per day at least three days a week was encouraged. Ketosis was tested for twice a week and glucose monitoring done in the morning before breakfast and just before dinner. Strategies to improve or maintain fidelity; tailoring and modification: Teaching positive affect and mindfulness, meditation, recording of food using Automated Self‐Administered 24‐h Dietary Recall (ASA24), measurement of weight during visits Extent of intervention fidelity: Authors only reported 'fair' adherence rates. Concomitant interventions: Around 31% participants used metformin alone, 44% used metformin combination therapy, behavioural adherence strategies to increase positive affect and mindfulness. Control diet: Name (as reported) and brief description: Moderate‐carbohydrate, calorie‐restricted (MCCR) diet, consistent with guidelines from ADA, where participants were encouraged to derive 45 to 50% of calories from carbohdyrates through counting carbohydrates in 15 g units, and eating 500 fewer kilocalories (kcal) per day than their calculated maintenance needs. Control diet type (carbohydrate‐fat‐protein): Balanced‐unclear‐unclear Exercise component? No Recipients: Diabetics or prediabetics, 88.9% female, aged mean (SD) 55.1 (13.5) years with 83% having a BMI above 30. A total of 56% had dyslipidemia and 78% had hypertension. Why? Uncertainty exists around the optimal diet for managing diabetics and prediabetics. What (materials)? "American Diabetes Association guidelines (ADA 2009; Inzucchi 2012). Audio CDs for meditation." What (procedures)? Participants attended twelve weekly classes followed by three classes every two weeks and four classes every two months related to eating a moderate‐carbohydrate calorie‐restricted diet, which was presented by a registered dietitian. In week 6 of the intervention participants were taught the importance of sleep and exercise while a third group leader instructed participants on increasing positive affect and mindfulness as well as meditation. Topics were drawn from two prior behavioural intervention models for diet and health behaviour change. To develop mindful eating skills, participants were asked to practice a guided meditation 10 minutes per day at least three times a week using audio CDs recorded for the intervention, and to use several mindful eating practices during meal times, such as focussing awareness on the taste and texture of foods while eating. Blood glucose was monitored in patients on diabetes medications, except metformin. Physical symptoms and activity was also assessed. Who provided? The group leader responsible for teaching classes is a registered dietitian with several years of diabetes education experience. Qualification of group leader providing information on supportive behavioural strategies NR How and where? Format and location of meetings NR When and how much? Twelve weekly classes were for two hours, three classes every two weeks for two hours and four classes every two months were 1.5 hours over the course of the 12 month intervention. Meditation for 10 minutes per day at least three days a week was encouraged. Strategies to improve or maintain fidelity; tailoring and modification: Teaching positive affect and mindfulness, meditation, recording of food using Automated Self‐Administered 24‐h Dietary Recall (ASA24), measurement of weight during visits Extent of intervention fidelity: Authors only reported 'fair' adherence rates. Concomitant interventions: Around 31% participants used metformin alone, 44% used metformin combination therapy, behavioural adherence strategies to increase positive affect and mindfulness. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: Yes Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: SUCCEED Trial funded by: William K. Bowes, Jr. Foundation grant and the Mount Zion Health Fund Declaration of interest: "Stephen Phinney is a paid member of the Atkins Scientific Advisory Board, a founder of Virta Health, and has authored books on low‐carbohydrate, high fat diets: New Atkins and You, The Art and Science of Low Carbohydrate Living, and The Art and Science of Low Carbohydrate Performance. Frederick Hecht is on the Scientific Advisory Board for Virta Health. The other authors declare no competing financial interests." Author contacted, but requested information not provided. |
|
Sato 2017.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3, 4 Study design: RCT, parallel, single‐centre (1) Trial registry number: UMINID000010663 Total number of trial arms: 2 Year trial started: 2013 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in HbA1c Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: 12 months Other notes about methods: NA |
|
| Participants |
Country and setting: Japan, outpatient clinic of a university hospital in Tokyo Eligibility criteria: Participants with type 2 diabetes; aged between 20 and 75 years with HbA1c above 7.5% and fluctuations within ±0.5%for the past three months, who had a BMI more than 23 kg/m2 and had received two or more educational programmes on caloric restriction. Participants were excluded if they had been diagnosed with proliferative or severe retinopathy; serious kidney disease with specified range of serum creatinine (with or without microalbuminuria); serious liver disease with specified ranges of aspartate and alanine aminotransferase levels; acute heart failure in the past three months or chronic heart failure; active malignancies; serious pancreatic or infectious disease; were pregnant; suffered a traumatic injury; were alcohol dependent or ate less than 130 g carbohydrates per day. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 66 Total attrition in trial: 17 Treatment diet Participants randomised: 33 Participants withdrawn (voluntary): 5 Total attrition: 11 Control diet: Participants randomised: 33 Participants withdrawn (voluntary): 5 Total attrition: 6 Baseline data treatment diet: Randomised participants not included: 3/33 Age (years): mean (SD) 60.5 (10.5) Gender distribution (as reported): female 7/30, male 23/30 Weight (kg): median (IQR) 74.0 (66.2 to 86.4) BMI (kg/m2): median (IQR) 26.7 (25.0 to 30.0) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): median (IQR) 8.0 (7.6 to 8.9) LDL (mmol/L): median (IQR) 2.63 (2.19 to 3.03) HDL (mmol/L): median (IQR) 1.13 (1.01 to 1.36) Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): median (IQR) 1.62 (1.16 to 2.68) Baseline data control diet: Randomised participants not included: 1/33 Age (years): mean (SD) 58.4 (10.0) Gender distribution (as reported): female 8/32, male 24/32 Weight (kg): median (IQR) 73.6 (68.1 to 88.0) BMI (kg/m2): median (IQR) 26.5 (24.6 to 30.1) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): median IQR 8.3 (8.0 to 9.3) LDL (mmol/L): median (IQR) 2.51 (2.25 to 3.34) HDL (mmol/L): median (IQR) 1.22 (0.99 to 1.39) Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): median (IQR) 1.68 (1.01 to 2.27) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Participants were Japanese and BMI cut‐offs are lower for this population. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Low‐carbohydrate diet (LCD) consumed ad libitum, set target carbohydrate intake at 130 g/d with participants asked to consume equal amounts of carbohydrates per meal (43.3 g). No other restrictions were given with the exception of recommending the intake of unsaturated rather than saturated fat. Treatment diet type (carbohydrate‐fat‐protein): Low‐unclear‐unclear Exercise component? No Recipients: Participants were type 2 diabetics, 23.3% female and aged 60.5 (10.5) years, with median BMI 26.7 (IQR 25.0 to 30.0) and median weight 74.0 (IQR 66.2 to 86.4) with the disease for an average of 15.5 years. Why? Many patients with type 2 diabetes struggle to adhere to calorie‐restricted diets. In the United States and Europe there is much debate about the use of low‐carbohydrate diets in the treatment of type 2 diabetes. LCD in patients with uncontrolled T2DM is preferable for HbA1c and weight reduction. What (materials)? Recommendation as per Accurso 2008. Other written materials prepared by study physicians and dietitians, containing key points on nutrition therapy and food lists. What (procedures)? Participants were assigned to the low‐carbohydrate diet (< 130 g/day), with target carbohydrate contents per meal and preferential use of unsaturated over saturated fat prescribed. Who provided? Study physicians explained the protocol and supported with the preparation of written materials. Dietitians led regular patients meetings. Attending outpatient physicians were allowed to make changes to medications at each study visit. Participants kept three‐day weighed/measured food records. Physicians followed up during the weight maintenance phase. How and where? Individual face‐to‐face meetings at an outpatient clinic of Juntendo University Hospital When and how much? The intervention duration comprised six months of active weight loss and a 12‐month weight maintenance phase. The initial visit was a detailed explanation of the protocol by study physicians, followed by 30‐minute‐long nutrition meetings with dietitians at one, two, four and six months in the active weight‐loss phase; one meeting in the weight maintenance phase at 18 months. As part of these meetings, participant‐recorded three‐day weighed/measured food records were used to analyse food intake. In the weight maintenance phase, participants were followed up by physicians approximately every two months. Strategies to improve or maintain fidelity; tailoring and modification: Three‐day weighed/measured food records for each visit with dietitian, completion of compliance questionnaire at the end of the study, weigh‐ins at study visits Extent of intervention fidelity: The authors reported that more participants withdrew from the low‐carbohydrate group and interpreted this as difficulty with adherence. They did, however, report that adherence was good. Carbohydrate intake from baseline to six months decreased from 223.2 (52.4) to 148.8 (31.3) g/day. Concomitant interventions: Four participants (13.3%) were on basal supported oral therapy, and 23.3% on intensive insulin therapy (basal insulin at 17 (11) units/day and bolus insulin at 16 (18) units/day). A total of 36.7% were on metformin, 63.3% on thiazolidine, 13.3% on dipeptidyl peptidase‐4 inhibitor, 53.3% on alpha‐glucosidase inhibitor and 23.3% on glinide. Two participants (6.7%) were on GLP‐1 receptor antagonist. Furthermore, 36.7% were on antihypertensive agents, 66.7% were on lipid‐lowering agents and 33.3% were on other medications. Control diet: Name (as reported) and brief description: Calorie‐restricted diet (CRD), target carbohydrate content of 50 to 60% TE and 1.0 to 1.2 g protein per kg body weight, with the balance of calories from fat. Total calorie intake was calculated by multiplication of the ideal body weight by 28 kcal/kg. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Participants were type 2 diabetics, 25.0% female and aged 58.4 (10.0) years, with median BMI 26.5 (IQR 24.6 to 30.1) and median weight 73.6 (IQR 68.1 to 88.0) with the disease for an average of 13 years. Why? To assess whether calorie‐restricted diet was better than LCD for weight loss What (materials)? Recommendations of the Japan Diabetes Society (The Japan Diabetes Society 2016). Other written materials prepared by study physicians and dietitians, containing key points on nutrition therapy and food lists What (procedures)? Participants were assigned to caloric restriction. Who provided? Study physicians explained the protocol and supported with the preparation of written materials. Dietitians led patient regular patients meetings. Attending outpatient physicians were allowed to make changes to medications at each study visit. Participants kept three‐day weighed/measured food records. Physicians followed up during the weight maintenance phase. How and where? Individual face‐to‐face meetings at an outpatient clinic of Juntendo University Hospital When and how much? The intervention duration comprised six months of active weight loss and a 12‐month weight maintenance phase. The initial visit was a detailed explanation of the protocol by study physicians, followed by 30‐minute‐long nutrition meetings with dietitians at one, two, four and six months in the active weight‐loss phase; one meeting in the weight maintenance phase at 18 months. As part of these meetings, participant‐recorded three‐day weighed/measured food records were used to analyse food intake. In the weight maintenance phase, participants were followed up by physicians approximately every two months. Strategies to improve or maintain fidelity; tailoring and modification: Three‐day weighed/measured food records for each visit with dietitian, completion of compliance questionnaire at the end of the study, weigh‐ins at study visits Extent of intervention fidelity: The authors reported that adherence was good. Carbohydrate intake from baseline to six months changed from 214.4 (51.1) to 203.9 (56.7) g/day. Concomitant interventions: One participant (3.1%) was on basal supported oral therapy, and 40.6% on intensive insulin therapy (basal insulin at 16 (7) units/day and bolus insulin at 22 (9) units/day). A total of 43.8% were on metformin, 65.6% on thiazolidine, 15.6% on dipeptidyl peptidase‐4 inhibitor, 56.3% on alpha‐glucosidase inhibitor and 9.4% on glinide. One participant (3.1%) was on GLP‐1 receptor antagonist. Furthermore, 40.6% were on antihypertensive agents, 68.8% were on lipid‐lowering agents and 31.3% were on other medications. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: Yes Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Nordisk Pharma, Sanofi and Takeda Pharmaceutical Co. Declaration of interest: "JS has received lecture fees from Novartis Pharmaceuticals, Novo Nordisk Pharma, Sanofi, and Takeda Pharmaceutical Co. AK has received lecture fees from Kissei Pharma, Sanofi and Takeda Pharmaceutical Co. YT has received lecture fees from Takeda Pharmaceutical Co., MSD, Eli Lilly, Kissei Pharma and AstraZeneca. TM has received lecture fees from MSD, Takeda Pharmaceutical Co., and Eli Lilly. YF has received lecture fees from Novartis Pharmaceuticals and Eli Lilly, research funds from Novartis Pharmaceuticals, MSD and Takeda Pharmaceutical Co. HW has received lecture fees from Asteras, Astrazeneca, Boehringer Ingelheim, Daiichi Sankyo Inc., Eli Lilly and Company, Kissei Pharmaceutical Co., Kowa Pharmaceutical CO., Kyowa Hakko Kirin Co., MSD, Novartis Pharmaceuticals, Novo Nordisk Pharma, Ono Pharmaceutical Co., Mitsubishi Tanabe Pharma, Sanofi‐Aventis, Sanwakagaku Kenkyusho, and Takeda Pharmaceutical Co. and research funds from Asteras, Astrazeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo Inc., Dainippon Sumitomo Pharma, Eli Lilly, Johnson and Johnson, Kissei Pharmaceutical Co., Kowa Pharmaceutical Co., Kyowa Hakko Kirin Co. MSD, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical Co., Novartis Pharmaceuticals, Novo Nordisk Pharma, Pfizer, Sanwakagaku Kenkyusho, Sanofi, and Takeda Pharmaceutical Co. All the other authors report no conflict of interest." |
|
Stentz 2016.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT01642849 Total number of trial arms: 2 Year trial started: 2009 Sample size calculation: Yes Outcome(s) used for sample size calculation: NR Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: USA, outpatient clinical research centre at a university in Knoxville. All food required to complete the respective diets was provided, predominantly as frozen foods. Eligibility criteria: Participants were aged 20 to 50 years with a BMI 30 to 55 kg/m2 and pre‐diabetes; with fasting glucose < 126 mg/dL, 2‐hour oral glucose tolerance test level of 140 to 199 mg/dL and HbA1c of 5.7 to 6.4%. Participants were excluded if they had proteinuria or serum creatinine above 1.5 mg/dL; surgically‐induced or premature menopause; history of liver disease, abnormal liver function or diabetes; were on antidiabetic medication or insulin; had thyroid disease with abnormal thyroid‐stimulating hormone levels; weighed more than 350 pounds; had triglyceride level > 400 mg/dL, LDL cholesterol > 160 mg/dL, systolic blood pressure > 145 or diastolic blood pressure > 100 mmHg; used medication known to affect the metabolism of lipids or glucose; were pregnant or wanting to become pregnant in the next six months; had weight loss of more than 5% of body weight in the past six months; smoked or had a history of cancer which was actively treated. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Yes Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 38 Total attrition in trial: 14 Treatment diet Participants randomised: 18 Participants withdrawn (voluntary): 6 Total attrition: 6 Control diet: Participants randomised: 20 Participants withdrawn (voluntary): 8 Total attrition: 8 Baseline data treatment diet: Randomised participants not included: 6/18 Age (years): mean (SE) 43.1 (1.3) Gender distribution (as reported): female 9/12 (75%), male 3/12 (25%) Weight (kg): NR BMI (kg/m2): mean (SE) 40.5 (1.8) DBP (mmHg): mean (SE) 81 (2) SBP (mmHg): mean (SE) 130 (3) HbA1c (%): mean (SE) 6.0 (0.02) LDL (mmol/L): mean (SE) 2.74 (0.11) HDL (mmol/L): mean (SE) 1.16 (0.04) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 4.28 (0.15) TG (mmol/L): mean (SE) 1.21 (0.11) Baseline data control diet: Randomised participants not included: 8/20 Age (years): mean (SE) 41.1 (1.7) Gender distribution (as reported): female 10/12 (83.3%), male 2/12 (16.7%) Weight (kg): NR BMI (kg/m2): mean (SE) 37.4 (1.7) DBP (mmHg): mean (SE) 81 (2) SBP (mmHg): mean (SE) 126 (3) HbA1c (%): mean (SE) 5.93 (0.12) LDL (mmol/L): mean (SE) 2.75 (0.15) HDL (mmol/L): mean (SE) 1.19 (0.07) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 4.35 (0.16) TG (mmol/L): mean (SE) 1.24 (0.12) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High‐protein low‐carbohydrate (HP) diet, 40% carbohydrates, 30% fat and 30% protein. Energy reduced to 500 kcal/day less than caloric need. Caloric needs were established for each individual and 500 kcal/day were subtracted from the determined caloric needs for maintenance. Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: Sedentary adults (female 75%; male 25%) with a mean age of 43.1 (1.3) years, with a mean BMI of 40.5 (1.8) kg/m2, and with pre‐diabetes (fasting glucose of <126 mg/dL, 2‐hour glucose level of 140–199 mg/dL during a standard oral glucose tolerance test (OGTT), and glycated haemoglobin (HbA1c) of 5.7–6.4%) Why? A hypocaloric high‐protein diet may result in greater satiety and reduced energy intake, compared to high‐carbohydrate diet. It may also help preserve B‐cell function by increasing insulin sensitivity and thus decreasing the insulin load per meal. Therefore, such a diet may result in the remission of pre‐diabetes to normal glucose tolerance, in obese participants. What (materials)? Daily food menus ‐ three meals a day plus snacks between meals; provision of prepackaged frozen food (available at local grocery stores) for the duration of the study What (procedures)? Participants were asked to keep a food diary for a week. Those found to be non‐adherent and unable to keep diet diary or deemed unable to adhere to the protocol were excluded. Calorie maintenance needs were determined on an individual basis using the RMR (indirect calorimetry). After maintenance, caloric needs were established for each individual; 500 kcals/day were subtracted from the determined caloric needs to promote a 1–2 lbs weekly weight loss. Dietary fat sources (30% of total energy) in the diet focused on monounsaturated and polyunsaturated fats, i.e. plant oils, semi‐liquid margarine, and nuts; dietary carbohydrate sources (40% of total energy) emphasised whole grains, fruits, vegetables, and legumes; and dietary protein sources ( 30% of total energy) included lean meats, fish chicken, eggs, and nonfat dairy foods, i.e. fat‐free milk and low‐fat cheese, consistent with American Diabetes Association and Institute of Medicine guidelines. The diet met the recommended daily intake for minerals and vitamins for women aged 20 to 50 years. Who provided? Dietitian How and where? Face‐to‐face consultations at the General Clinical Research Centre, University of Tennessee; phone and email consultation provided when needed When and how much? Every week for 24 weeks. A one‐time calorie reduction of 200 kcal was made if a participant reached a plateau and did not lose weight for two consecutive weeks during the 6 months. Strategies to improve or maintain fidelity; tailoring and modification: Dietary compliance assessed by both subjective and objective parameters (not described in detail). Strategies to increase compliance included frequent interaction, behaviour modification, individualised diet with food variety and food record system. The meals were dispensed as prepackaged foods from stored frozen food by the dietitian affiliated with the UT GCRC dietary services on the site of the GCRC, maintaining the macronutrient and caloric requirements established at randomisation. The food records served as a motivational enforcement compliance. Extent of intervention fidelity: % compliance reported: 93 (1.6). Concomitant interventions: NR Control diet: Name (as reported) and brief description: High‐carbohydrate low‐protein (HC) diet, 55% carbohydrates, 30% fat and 15% protein. Energy reduced to 500 kcal/day less than caloric need. Caloric needs were established for each individual and 500 kcal/day were subtracted from the determined caloric needs for maintenance. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Adults ( female 83%; male 17%) with a mean age of 41.1 (1.7) years, with a mean BMI of 37.4 (1.7) kg/m2, and with pre‐diabetes (fasting glucose of < 126 mg/dL, 2‐hour glucose level of 140–199 mg/dL during a standard oral glucose tolerance test (OGTT), and glycated haemoglobin (HbA1c) of 5.7–6.4%) Why? A hypocaloric high‐carbohydrate diet may not result in greater satiety and reduced energy intake, compared to a high‐protein diet. It may not help preserve B‐cell function by increasing insulin sensitivity and thus decreasing the insulin load per meal. Therefore, such a diet may not result in the remission of pre‐diabetes to normal glocose tolerance, in obese participants. What (materials)? Daily food menus ‐ three meals a day plus snacks between meals; provision of prepackaged frozen food (available at local grocery stores) for the duration of the study What (procedures)? Participants were asked to keep a food diary for a week. Those found to be non‐adherent and unable to keep diet diary or deemed unable to adhere to the protocol were excluded. Calorie maintenance needs were determined on an individual basis using the RMR (indirect calorimetry). After maintenance, caloric needs were established for each individual; 500 kcals/day were subtracted from the determined caloric needs to promote a 1–2 lbs weekly weight loss. Dietary fat sources (30% of total energy) in the diet focussed on monounsaturated and polyunsaturated fats, i.e. plant oils, semi‐liquid margarine, and nuts; dietary carbohydrate sources (55% of total energy) emphasised whole grains, fruits, vegetables, and legumes; and dietary protein sources ( 15% of total energy) included lean meats, fish chicken, eggs, and nonfat dairy foods, i.e. fat‐free milk and low‐fat cheese, consistent with American Diabetes Association and Institute of Medicine guidelines. The diet met the recommended daily intake for minerals and vitamins for women aged 20 to 50 years. Who provided? Dietitian How and where? Face‐to‐face consultations at the General Clinical Research Centre, University of Tennessee; phone and email consultation provided when needed When and how much? Every week for 24 weeks. A one‐time calorie reduction of 200 kcal was made if a participant reached a plateau and did not lose weight for two consecutive weeks during the 6 months. Strategies to improve or maintain fidelity; tailoring and modification: Dietary compliance assessed by both subjective and objective parameters (not described in detail). Strategies to increase compliance included frequent interaction, behaviour modification, individualised diet with food variety and food record system. The meals were dispensed as prepackaged foods from stored frozen food by the dietitian affiliated with the UT GCRC dietary services on the site of the GCRC, maintaining the macronutrient and caloric requirements established at randomisation. The food records served as a motivational enforcement compliance. Extent of intervention fidelity: % compliance reported: 94 (2.1) Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: No Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: American Diabetes Association (grant 7‐12‐CT‐41) and the AD Baskin Research Fund Declaration of interest: "None declared." Author contacted, but requested information not provided. |
|
Tay 2008.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, number of centres NR Trial registry number: ACTRN12606000203550 Total number of trial arms: 2 Year trial started: 2006 Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 52 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely Australia based on authors and funders Eligibility criteria: Participants aged 18 to 65 years with abdominal obesity and the presence of at least one additional risk factor for metabolic syndrome. Participants were excluded if they had a history of liver, cardiovascular, peripheral vascular, respiratory or gastrointestinal diseases; diabetes; or malignancy. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Mixed Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 118 Total attrition in trial: 30 Treatment diet Participants randomised: 57 Participants withdrawn (voluntary): 12 Total attrition: 12 Control diet: Participants randomised: 61 Participants withdrawn (voluntary): 18 Total attrition: 18 Baseline data treatment diet: Randomised participants not included: 12/57 Age (years): mean (SD) 50.3 (8.4) Gender distribution (as reported): female 31/45 (68.9%), male 14/45 (31.1%) Weight (kg): mean (SD) 94.4 (15.5) BMI (kg/m2): mean (SD) 33.9 (4.3) DBP (mmHg): mean (SD) 73.6 (11.6) SBP (mmHg): mean (SD) 133.5 (14.5) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.2 (0.9) HDL (mmol/L): mean (SD) 1.4 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.4 (0.9) TG (mmol/L): mean (SD) 1.6 (0.7) Baseline data control diet: Randomised participants not included: 18/61 Age (years): mean (SD) 51.0 (7.5) Gender distribution (as reported): female 26/43 n/N (60.5%), male 17/43 n/N (39.5%) Weight (kg): mean (SD) 95.2 (12.6) BMI (kg/m2): mean (SD) 33.5 (4.1) DBP (mmHg): mean (SD) 77.8 (10.1) SBP (mmHg): mean (SD) 136.1 (12.6) HbA1c (%): NR LDL (mmol/L): mean (SD) 3.3 (0.7) HDL (mmol/L): mean (SD) 1.3 (0.4) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.4 (0.8) TG (mmol/L): mean (SD) 1.8 (0.8) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Very‐low‐carbohydrate high‐fat (VLCHF) diet. 4% of total energy as carbohydrate, 35% protein and 61% total fat (20% saturated). Carbohydrate intake was restricted to < 20 g/day during the first 8 weeks of the study whereafter subjects were given the option to increase to < 40 g/day for the remaining 16 weeks. Moderate energy restriction of 30% (prescription of 6000 kJ for women and 7000 kJ for men). Treatment diet type (carbohydrate‐fat‐protein): Very low‐high‐high Exercise component? No Recipients: Abdominally obese participants, 68.9% female, aged 50.3 (8.4) with BMI 33.9 (4.3) and weight 94.4 (15.5) Why? VLCHF diet produces greater reductions in triacylglycerols (TAG) and increases in high‐density lipoprotein cholesterol (HDL‐C) and at least comparable changes in blood pressure and insulin resistance, without detrimental effects on low‐density lipoprotein cholesterol (LDL‐C) for periods up to 1 year. What (materials)? Key foods for the macronutrient profile, comprising approximately 30% of energy, was provided for the first eight weeks. Thereafter $40 food vouchers were given to participants for the next 16 weeks. Scales for weighing food were provided. What (procedures)? Subjects were instructed to follow a very‐low‐carbohydrate diet and were given key foods for eight weeks and food vouchers for the following 16 weeks to assist with compliance. Semiquantitative food records were kept and food was weighed. A qualified dietitian provided individualised dietary advice, meal planning, and recipe information at each clinic visit. Three‐day food records were kept every two weeks and dietary composition was determined. Dietary intake was assessed by food frequency questionnaire. Who provided? Qualified dietitians provided individual consultations. How and where? Face‐to‐face consultations with dieticians at a clinic and food records kept by participants at home When and how much? During the course of the 24‐week intervention subjects attended the clinic fortnightly for eight weeks and monthly thereafter. Key foods were provided on a fortnightly basis for eight weeks. Food vouchers were provided for the remaining 16 weeks on a monthly basis. Food records were completed daily and individual consultations with dietitians occurred during clinic visits. Three‐day food records were done every two weeks and food frequency questionnaires were completed at baseline and 24 weeks. Strategies to improve or maintain fidelity; tailoring and modification: Keeping of food records and analysis thereof for dietary composition, weighing of food, regular determination of body weight and plasma ketones as study outcome, regular meetings with dietitian, provision of key foods and food vouchers. Dietary advice was individualised. Subjects in the HCLF diet were asked to restrict saturated fat intake to < 10 g/day for the study duration. Extent of intervention fidelity: High level of dietary compliance found according to dietary data and the level of plasma ketones Concomitant interventions: In the total group, 17.0% were taking estrogen (12.5% HRT and 4.5% oral contraceptive), 30.7% were on antihypertensive medications, 20.5% were taking lipid‐lowering medication and none were taking hypoglycemic medication; no significant differences between medication use in the two trial arms. Control diet: Name (as reported) and brief description: High‐carbohydrate low‐fat (HCLF) diet. 46% of total energy as carbohydrate, 24% as protein and 30% as total fat (< 8% saturated). Moderate energy restriction of 30% (prescription of 6000 kJ for women and 7000 kJ for men) Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐high Exercise component? No Recipients: Abdominally obese participants, 60.5% female, aged 51.0 (7.5) with BMI 33.5 (4.1) and weight 95.2 (12.6). Why? Dietary recommendations for the management of weight and treatment of obesity are to follow a high‐carbohydrate, low‐fat, energy‐restricted diet. What (materials)? Key foods for the macronutrient profile, comprising approximately 30% of energy, were provided for the first eight weeks. Thereafter $40 food vouchers were given to participants for the next 16 weeks. Scales for weighing food were provided. What (procedures)? Subjects were instructed to follow a high‐carbohydrate low‐fat diet and were given key foods for eight weeks and food vouchers for the following 16 weeks to assist with compliance. Semiquantitative food records were kept and food was weighed. Individual consultations with a dietitian comprised dietary advice and meal planning. Three‐day food records were kept every two weeks and dietary composition was determined. Dietary intake was assessed by food frequency questionnaire. Who provided? Qualified dietitians provided individual consultations. How and where? Face‐to‐face consultations with dieticians at a clinic and food records kept by participants at home When and how much? During the course of the 24‐week intervention subjects attended the clinic fortnightly for eight weeks and monthly thereafter. Key foods were provided on a fortnightly basis for eight weeks. Food vouchers were provided for the remaining 16 weeks on a monthly basis. Food records were completed daily and individual consultations with dietitians occurred during clinic visits. Three‐day food records were done every two weeks and food frequency questionnaires were completed at baseline and 24 weeks. Strategies to improve or maintain fidelity; tailoring and modification: Keeping of food records and analysis thereof for dietary composition, weighing of food, regular determination of body weight and plasma ketones as study outcome, regular meetings with dietitian, provision of key foods and food vouchers. Dietary advice was individualised. Subjects in the HCLF diet were asked to restrict saturated fat intake to < 10 g/day for the study duration. Extent of intervention fidelity: High level of dietary compliance found according to dietary data Concomitant interventions: In the total group, 17.0% were taking estrogen (12.5% HRT and 4.5% oral contraceptive), 30.7% were on antihypertensive medications, 20.5% were taking lipid‐lowering medication and none were taking hypoglycemic medication; no significant differences between medication use in the two trial arms |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: Yes Number of participants with 5% weight loss from baseline at ≥ 12 months: Yes Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: Yes Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmol/L) at ≥ 12 months: Yes Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: National Heart Foundation of Australia and National Health and Medical Research Council of Australia project grants Declaration of interest: "None of the funding agencies played a role in the conception, design, or conduct of the study collection, management, analysis, and interpretation of the data or in the preparation, review, and approval of the manuscript." Author contacted, but requested information not provided. |
|
Tay 2014.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, single‐centre (1) Trial registry number: ACTRN12612000369820 Total number of trial arms: 2 Year trial started: 2012 Sample size calculation: Yes Outcome(s) used for sample size calculation: Difference in HbA1c Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 2 years What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Australia, outpatient research organisation in Adelaide. Key food representative of the macronutrient composition, and totalling 30% of energy, were provided to participants for the first twelve weeks; and key foods or a 50 AUD voucher on alternate months following the 12 weeks. Eligibility criteria: Participants were aged 35 to 68 years and were overweight or obese (defined as BMI of 26 to 45 kg/m2) with type 2 diabetes, defined as HbA1c ≥ 7.0% or treated with diabetes medication. Participants were excluded if they had type 1 diabetes, impaired renal function or proteinuria; had abnormal liver function test or any overt endocrine problems (except treated and stable thyroid disease); history of malignancy; respiratory, gastrointestinal or cardiovascular disease; were pregnant or lactating; had clinical depression; had a history of or current eating disorder, or were an ex or current smoker. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 131 Total attrition in trial: 38 Treatment diet Participants randomised: 64 Participants withdrawn (voluntary): 13 Total attrition: 18 Control diet: Participants randomised: 67 Participants withdrawn (voluntary): 13 Total attrition: 20 Baseline data treatment diet: Randomised participants not included: 6/64 Age (years): mean (SD) 58 (7) Gender distribution (as reported): female 21/58 (36%), male 37/58 (64%) Weight (kg): mean (SD) 101.7 (14.4) BMI (kg/m2): mean (SD) 34.2 (4.5) DBP (mmHg): mean (SD) 80.0 (8.9) SBP (mmHg): mean (SD) 130.4 (13.1) HbA1c (%): mean (SD) 7.3 (1.1) LDL (mmol/L): mean (SD) 2.5 (0.9) HDL (mmol/L): mean (SD) 1.2 (0.2) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.5 (1.0) TG (mmol/L): mean (SD) 1.6 (0.7) Baseline data control diet: Randomised participants not included: 10/67 Age (years): mean (SD) 58 (7) Gender distribution (as reported): female 28/57 (49%), male 29/57 (51%) Weight (kg): mean (SD) 101.6 (15.8) BMI (kg/m2): mean (SD) 35.1 (4.1) DBP (mmHg): mean (SD) 80.8 (10.1) SBP (mmHg): mean (SD) 132.6 (13.2) HbA1c (%): mean (SD) 7.4 (1.1) LDL (mmol/L): mean (SD) 2.4 (0.9) HDL (mmol/L): mean (SD) 1.3 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.3 (1.0) TG (mmol/L): mean (SD) 1.4 (0.6) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Low‐carbohydrate (LC) diet. Planned macronutrient profiles of 14% carbohydrate (with the objective of restricting to < 50 g/d), 28% protein and 58% total fat (35% MUFA, 13% PUFA and < 10% saturated) plus structured exercise 60 min on three nonconsecutive days. Individualised energy prescription with moderate restriction (500–1000 kcal/day) Treatment diet type (carbohydrate‐fat‐protein): Very low‐high‐high Exercise component? Yes Recipients: Subjects were 51% female, aged mean (SD) 58 (7) years, with BMI mean (SD) 35.1 (4.1), and weight 101.6 (15.8)kgs Why? LC diet with high–unsaturated and low–saturated fat content may promote greater improvements in glycaemic control in T2DM without detrimental effects on LDL‐C. What (materials)? Participants were supplied key foods or AU$50 food vouchers on alternate months. What (procedures)? LC diet, 14% of total energy as carbohydrate (objective to restrict intake to 50 g/day), 28% protein, and 58% total fat. Under supervision of exercise professionals, participants undertook, free of charge, 60‐min structured exercise classes on 3 nonconsecutive days per week, incorporating moderate exercise. Dietitians provided dietary advice and instruction on the eating plan and reporting requirements. Who provided? Dietitian How and where? Face‐to‐face individual sessions, location NR When and how much? Biweekly sessions for 12 weeks then monthly sessions Strategies to improve or maintain fidelity; tailoring and modification: Attendance records were kept and participants were encouraged to make up any missed sessions. Dietary intake and adherence were assessed from 7 consecutive days (including 2 weekend days) of daily weighed food records for every 14‐day period. To facilitate compliance, participants met individually with a dietitian biweekly for 12 weeks and monthly thereafter. Participants undertook, free of charge, 60‐min structured exercise classes. Extent of intervention fidelity: Energy intake did not differ between groups (HC 1587 +/‐ 171 kcal; P = 0.56). Relative to the HC diet group, the LC diet group consumed less carbohydrate (LC 56.7 +/‐ 8.0 vs. HC 204.9 +/‐ 22.8 g; 14 +/‐ 2 vs. 50 +/‐ 2% total energy) and dietary fibre (24.7 +/‐ 3.5 vs. 31.1 +/‐ 3.2 g), more protein (102.8 +/‐ 14.7 vs. 73.6 +/‐ 8.3 g; 27 +/‐ 1 vs. 19 +/‐ 1% total energy), total fat (96.5 +/‐ 16.5 vs. 44.3 +/‐ 7.4 g; 54 +/‐ 3 vs. 25 +/‐ 3% total energy), saturated fat (10.0 +/‐ 0.9 vs. 7.5 +/‐ 1.1% total energy), monounsaturated fat (30.4 +/‐ 1.8 vs. 11.5 +/‐ 1.3% total energy), polyunsaturated fat (12.2 +/‐ 1.1 vs. 4.1 +/‐ 0.6% total energy), and cholesterol (243 +/‐ 42 vs. 138 +/‐ 25 mg); P < 0.001 for all. Concomitant interventions: After 24 weeks, the LC diet group experienced twofold greater reductions in the antiglycaemic MES, with more participants experiencing a reduction of 20% compared with HC diet group (P < 0.005). Six participants reduced (LC 4 and HC 2) and five increased (LC 3 and HC 2) lipid‐lowering medication. Eleven participants reduced (LC 10 and HC 1) and six increased (LC 3 and HC 3) antihypertensive medication. Control diet: Name (as reported) and brief description: High‐carbohydrate (HC) diet. Planned macronutrient profiles of 53% carbohydrate with emphasis on low GI foods, 17% protein and < 30% total fat (15% MUFA, 9% PUFA and < 10% saturated) plus structured exercise 60 min on three nonconsecutive days. Individualised energy prescription with moderate restriction (500–1000 kcal/day) Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? Yes Recipients: Subjects were 36% female, aged mean (SD) 58 (7) years, with BMI mean (SD) 34.2 (4.5), and weight 101.7 (14.4)kgs. Why? NR What (materials)? Participants were supplied key foods or AU$50 food vouchers on alternate months. What (procedures)? HC diet, 53% carbohydrate with emphasis on low–glycaemic index foods, 17% protein, and 30% total fat. Under supervision of exercise professionals, participants undertook, free of charge, 60‐min structured exercise classes on 3 nonconsecutive days per week, incorporating moderate exercise. Dietitians provided dietary advice and instruction on the eating plan and reporting requirements. Who provided? Dietitian How and where? Face‐to‐face individual sessions, location NR When and how much? Biweekly sessions for 12 weeks then monthly sessions Strategies to improve or maintain fidelity; tailoring and modification: Attendance records were kept and participants were encouraged to make up any missed sessions. Dietary intake and adherence were assessed from 7 consecutive days (including 2 weekend days) of daily weighed food records for every 14‐day period. To facilitate compliance, participants met individually with a dietitian biweekly for 12 weeks and monthly thereafter. Participants undertook, free of charge, 60‐min structured exercise classes. Extent of intervention fidelity: Energy intake did not differ between groups (HC 1587 +/‐ 171 kcal; P = 0.56) Relative to the HC diet group, the LC diet group consumed less carbohydrate (LC 56.7 +/‐ 8.0 vs. HC 204.9 +/‐ 22.8 g; 14 +/‐ 2 vs. 50 +/‐ 2% total energy) and dietary fibre (24.7 +/‐ 3.5 vs. 31.1 +/‐ 3.2 g), more protein (102.8 +/‐ 14.7 vs. 73.6 +/‐ 8.3 g; 27 +/‐ 1 vs. 19 +/‐ 1% total energy), total fat (96.5 +/‐ 16.5 vs. 44.3 +/‐ 7.4 g; 54 +/‐ 3 vs. 25 +/‐ 3% total energy), saturated fat (10.0 +/‐ 0.9 vs. 7.5 +/‐ 1.1% total energy), monounsaturated fat (30.4 +/‐ 1.8 vs. 11.5 +/‐ 1.3% total energy), polyunsaturated fat (12.2 +/‐ 1.1 vs. 4.1 +/‐ 0.6% total energy), and cholesterol (243 +/‐ 42 vs. 138 +/‐ 25 mg); P < 0.001 for all. Concomitant interventions: After 24 weeks, the LC diet group experienced twofold greater reductions in the antiglycaemic MES, with more participants experiencing a reduction of 20% compared with HC diet group (P < 0.005). Six participants reduced (LC 4 and HC 2) and five increased (LC 3 and HC 2) lipid‐lowering medication. Eleven participants reduced (LC 10 and HC 1) and six increased (LC 3 and HC 3) antihypertensive medication. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: National Health and Medical Research Council project grant 103415 Declaration of interest: "No potential conflicts of interest relevant to this article were reported." |
|
Veum 2017.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: NCT01750021 Total number of trial arms: 2 Year trial started: 2012 Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: Post hoc power analysis indicated ~40% power for per protocol analysis and type II errors could not be ruled out. |
|
| Participants |
Country and setting: Norway, outpatient university hospital in Bergen Eligibility criteria: Male subjects with abdominal obesity, normal fasting blood glucose < 7 mmol/L, waist circumference > 98 cm, and BMI > 29 kg/m2 or percentage body fat ≥ 25. Subjects were excluded if they had severe diseases, including inflammatory bowel diseases, known food allergies, were on regular medication (except for alkalising gastric buffers), made attempts at systematic weight reduction over the previous six months, and/or regularly consumed > 2 units of alcohol per week. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Male Total number randomised: 46 Total attrition in trial: 8 Treatment diet Participants randomised: 24 Participants withdrawn (voluntary): 2 Total attrition: 4 Control diet: Participants randomised: 22 Participants withdrawn (voluntary): 4 Total attrition: 4 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 40.3 (5.53) Gender distribution (as reported): female 0%, male 100% Weight (kg): mean (SD) 112 (10.1) BMI (kg/m2): mean (SD) 33.8 (2.5) DBP (mmHg): mean (SD) 84.0 (9.1) SBP (mmHg): mean (SD) 131 (14.2) HbA1c (%): mean (SD) 5.58 (0.5) LDL (mmol/L): mean (SD) 3.65 (1.14) HDL (mmol/L): mean (SD) 1.05 (0.30) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.35 (1.17) TG (mmol/L): mean (SD) 1.52 (0.60) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 40.2 (4.50) Gender distribution (as reported): female 0%, male 100% Weight (kg): mean (SD) 111 (12.9) BMI (kg/m2): mean (SD) 33.6 (3.4) DBP (mmHg): mean (SD) 84.2 (7.9) SBP (mmHg): mean (SD) 131 (16.2) HbA1c (%): mean (SD) 5.62 (0.7) LDL (mmol/L): mean (SD) 3.68 (1.07) HDL (mmol/L): mean (SD) 1.23 (0.24) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.42 (1.14) TG (mmol/L): mean (SD) 1.45 (0.53) Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Age, HbA1c, LDL, HDL, total cholesterol and triglycerides baseline values were per protocol (missing 4/24 intervention and 4/22 control participants). |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Very high‐fat low‐carbohydrate (VHFLC) diet, with macronutrient profile of 10% (50 g) energy from carbohydrates, 17% (90g) from protein and 73% (170 g) from fat. All participants were asked to consume a total of 8750 kJ/d. Treatment diet type (carbohydrate‐fat‐protein): Very low‐high‐balanced Exercise component? No Recipients: Abdominally obese men with normal fasting glucose aged 40.3 (5.53) years, with BMI 33.8 (2.5) kg/m2 and weight 112 (10.1) kg. Why? Low‐carbohydrate has gained popularity, in part due to the temporary and long‐term weight loss as well as improved insulin sensitivity. The health concerns surrounding this diet is possibly due to studies normally including energy‐dense diets with considerable amounts of processed carbohydrates. What (materials)? Food products donated by suppliers, i.e. butter, coconut fat, erythritol, stevia and mixes of almond flour and plant fibres. A comprehensive recipe booklet with > 225 recipes, with precalculated nutrient content and clear instructions for meal and snack preparation, participants could choose from. What (procedures)? Before baseline assessments, the participants completed a half‐day course at which they received detailed instructions on how to implement the recipe booklet system. Study visits at four, eight and 12 weeks with nutritionist and physician. Diet recording over five successive days each month for three months Who provided? Nutritionists and physicians How and where? Face‐to‐face meetings with nutritionist and physician at study visits. Diet recording online When and how much? Intervention duration was three months. Meetings with nutritionist and physician for 15 minutes at each study visit (four, eight and 12 weeks); dietary recording over five successive days each month for three months Strategies to improve or maintain fidelity; tailoring and modification: Participants were monitored closely and contacted regularly between study visits. They were also offered individual counselling if needed and completed diet recording each month. Recipe booklets were provided. Extent of intervention fidelity: Actual macronutrient intakes at the end of the intervention were 56.1 (5.1) g carbohydrates (from per protocol analysis). Concomitant interventions: NR Control diet: Name (as reported) and brief description: Low‐fat high‐carbohydrate (LFHC) diet, with macronutrient profile 53% (275 g) energy from carbohydrates, 17% (90 g) from protein and 30% (70 g) from fat. All participants were asked to consume a total of 8750 kJ/d. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Abdominally obese men with normal fasting glucose aged 40.2 (4.50) years, with BMI 33.6 (3.4) kg/m2 and weight 111 (12.9) kg Why? There is concern that low‐carbohydrate diets increase the intake of total and saturated fat, leading to potential increases in visceral fat and promotion of NAFLD. Saturated fat is also linked to stimulation of increased circulating cholesterol levels, in particular LDL and non‐HDL, as well as increased inflammation and interference with insulin signaling. What (materials)? Food products donated by suppliers, i.e. butter, coconut fat, erythritol, stevia and mixes of almond flour and plant fibres. A comprehensive recipe booklet with > 225 recipes, with precalculated nutrient content and clear instructions for meal and snack preparation, participants could choose from. What (procedures)? Study visits at four, eight and 12 weeks with nutritionist and physician. Diet recording over five successive days each month for three months Who provided? Nutritionists and physicians How and where? Face‐to‐face meetings with nutritionist and physician at study visits. Diet recording online When and how much? Intervention duration was three months. Meetings with nutritionist and physician for 15 minutes at each study visit (four, eight and 12 weeks); dietary recording over five successive days each months for three months Strategies to improve or maintain fidelity; tailoring and modification: Participants were monitored closely and contacted regularly between study visits. They were also offered individual counselling if needed and completed diet recording each month. Recipe booklets were provided. Extent of intervention fidelity: Actual macronutrient intakes at the end of the intervention were 281 (23.5) g carbohydrates (from per protocol analysis). Concomitant interventions: NR |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: FATFUNC Trial funded by: Western Norway Regional Health Authority, Meltzerfondet, Bergen Medical Research Foundation and the University of Bergen Declaration of interest: "None of the authors reported a conflict of interest related to the study." |
|
Volek 2009.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, number of centres NR Trial registry number: NR Total number of trial arms: 2 Year trial started: NR Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely USA based on authors and funders Eligibility criteria: Participants with atherogenic dyslipidemia, aged 18 to 55 years, with a BMI above 25 kg/m2. moderately elevated triglycerides and low HDL cholesterol. Participants were excluded if they had metabolic or endocrine disorders; used glucose‐lowering, lipid‐lowering or vasoactive medication or supplements, were on a carbohydrate‐restricted diet at baseline, or had lost more than 5 kg in the past three months. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 40 Total attrition in trial: NR Treatment diet Participants randomised: 20 Participants withdrawn (voluntary): NR Total attrition: NR Control diet: Participants randomised: 20 Participants withdrawn (voluntary): NR Total attrition: NR Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 32.6 (11.3) Gender distribution (as reported): NR Weight (kg): mean (SD) 96.5 (13.7) BMI (kg/m2): mean (SD) 33.5 (5.2) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.37 (0.57) HDL (mmol/L): mean (SD) 0.93 (0.18) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.39 (0.67) TG (mmol/L): mean (SD) 2.38 (0.66) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 36.9 (12.5) Gender distribution (as reported): NR Weight (kg): mean (SD) 94.4 (15.2) BMI (kg/m2): mean (SD) 32.1 (4.1) DBP (mmHg): NR SBP (mmHg): NR HbA1c (%): NR LDL (mmol/L): mean (SD) 3.32 (0.80) HDL (mmol/L): mean (SD) 1.01 (0.16) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.28 (0.83) TG (mmol/L): mean (SD) 2.11 (0.66) Group differences at baseline: NR Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Unrestricted/ad libitum prescription in both diets Treatment diet: Name (as reported) and brief description: Carbohydrate‐restricted diet (CRD) consumed ad libitum, with the main goal to restrict carbohydrates to a level that induced ketosis Treatment diet type (carbohydrate‐fat‐protein): Very low‐unclear‐unclear Exercise component? No Recipients: Men and women with atherogenic dyslipidemia aged 32.6 (11.3) years, with BMI 33.5 (5.2) and weight 96.5 (13.7) kg Why? It is hypothesised that carbohydrate restriction improves markers which define the metabolic syndrome through insulin regulation. What (materials)? Detailed booklets outlining dietary goals, listing appropriate food for the diet, recipes, meal plans and food record log sheets. A multivitamin and ‐mineral complex was provided. Urine reagent strips for testing ketosis What (procedures)? Subjects were instructed to follow a carbohydrate‐restricted diet by a registered dietitian. Two baseline visits were conducted prior to commencing intervention where participants' blood samples and assessments were taken following a 12‐h overnight fast and 24‐h abstinence from alcohol and strenuous exercise. These tests were repeated after 12 weeks of intervention. Dietary intake was assessed with detailed and weighed seven‐day food records collected at baseline to assess habitual intake. Weekly follow‐up counselling during in which body mass was measured, compliance was assessed, and further dietetic education was provided. Oral fat tolerance test and other blood ana;yses were also done. Seven‐day weighed food records were kept during weeks 1, 6, and 12 of the intervention and were analysed for energy and macro/micronutrient content. Who provided? Registered dietitians How and where? Face‐to‐face When and how much? Follow‐up counselling was done weekly and monitoring of ketosis occurred daily for the duration of the 12‐week intervention. In week one, six and 12 of the intervention seven‐day weighed food records were kept. Strategies to improve or maintain fidelity; tailoring and modification: Weighing before and after intervention, dietary intake assessed by detailed seven‐day weighed food records, compliance was assessed during weekly counselling, ketosis was monitored. Subjects received individualised and personalised counselling. Extent of intervention fidelity: High degree of compliance reported with urinary ketone levels above trace on 85% of days Concomitant interventions: Multivitamins at levels < 100% of the RDA (one pill every other day) Control diet: Name (as reported) and brief description: Low‐fat diet (LFD) consumed ad libitum, participants were instructed to consume < 10% of total calories from saturated fat and < 300 mg cholesterol daily. Macronutrient balanced of ~55% energy from carbohydrates, ~25% from fat and ~20% from protein Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Men and women with atherogenic dyslipidemia aged 36.9 (12.5) years, with BMI 32.1 (4.1) and weight 94.4 (15.2) kg Why? "Low fat diets may be effective for weight loss..." What (materials)? Detailed booklets outlining dietary goals, listing appropriate food for the diet, recipes, meal plans and food record log sheets. A multivitamin and ‐mineral complex was provided. What (procedures)? Subjects were instructed to follow a low‐fat diet by a registered dietitian. Two baseline visits were conducted prior to commencing the intervention where participants' assessments and blood samples were taken following a 12‐h overnight fast and 24‐h abstinence from alcohol and strenuous exercise. These tests were repeated after 12 weeks of intervention. weekly follow‐up counselling during which body mass was measured, compliance was assessed, and further dietetic education was provided. Oral fat tolerance test and other blood analyses were also done. Seven‐day weighed food records were kept during weeks 1, 6, and 12 of the intervention and were analysed for energy and macro/micronutrient content. Who provided? Registered dietitians How and where? Face‐to‐face When and how much? Follow‐up counselling was done weekly and monitoring of ketosis occurred daily for the duration of the 12‐week intervention. In week one, six and 12 of the intervention seven‐day weighed food records were kept. Strategies to improve or maintain fidelity; tailoring and modification: Weighing before and after the intervention, dietary intake assessed by detailed seven‐day weighed food records, compliance was assessed during weekly counselling. Subjects received individualised and personalised counselling. Extent of intervention fidelity: NR Concomitant interventions: Multivitamins at levels < 100% of the RDA (one pill every other day) |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: Yes Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: The Graduate School and the Health Disparity EXPORT Center at the University of Connecticut, USDA Hatch, the Dr. Robert C. Atkins Foundation, the Egg Nutrition Center and the Research Foundation of the State University of New York Declaration of interest: NR |
|
Watson 2016.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3, 4 Study design: RCT, parallel, number of centres NR Trial registry number: ANZCTR12613000008729 Total number of trial arms: 2 Year trial started: 2013 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in HbA1c Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 12 weeks What was the duration of the weight maintenance phase: 12 weeks Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely Australia based on authors and funders Eligibility criteria: Participants were aged 18 to 70 years and overweight or obese (defined as BMI ≥ 25 kg/m2) with type 2 diabetes (HbA1c 6.5 to 10.5%). Participants were excluded if they were diagnosed with or treated for any neurological or psychiatric condition (except for those on stable antidepressants for the past three months). Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 63 Total attrition in trial: 19 Treatment diet Participants randomised: 32 Participants withdrawn (voluntary): 9 Total attrition: 9 Control diet: Participants randomised: 31 Participants withdrawn (voluntary): 9 Total attrition: 10 Baseline data treatment diet: Randomised participants not included: 0/32 Age (years): mean (SD) 54 (8) Gender distribution (as reported): female 15/32 (47%), male 17/32 (53%) Weight (kg): mean (SD) 97.3 (17.1) BMI (kg/m2): mean (SD) 34.3 (5.4) DBP (mmHg): mean (SD) 78.4 (8.2) SBP (mmHg): mean (SD) 131.8 (13.2) HbA1c (%): mean (SD) 8.0 (1.3) LDL (mmol/L): mean (SD) 2.7 (0.9) HDL (mmol/L): mean (SD) 1.2 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.7 (0.9) TG (mmol/L): mean (SD) 2.0 (1.2) Baseline data control diet: Randomised participants not included: 2/31 Age (years): mean (SD) 54 (8) Gender distribution (as reported): female 13/29 (45%), male 16/29 (55%) Weight (kg): mean (SD) 101.5 (16.6) BMI (kg/m2): mean (SD) 34.4 (4.7) DBP (mmHg): mean (SD) 79.0 (7.1) SBP (mmHg): mean (SD) 135.1 (8.3) HbA1c (%): mean (SD) 8.1 (1.5) LDL (mmol/L): mean (SD) 2.5 (0.6) HDL (mmol/L): mean (SD) 1.2 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.5 (0.8) TG (mmol/L): mean (SD) 2.0 (1.0) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Higher‐protein diet. Planned macronutrient distribution of 32% protein, 33% carbohydrate and 30% fat with < 10% saturated. Physical activity 150 min/week. Moderate energy restriction of ~30% of the individual estimated energy requirement Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? Yes Recipients: Subjects were 47% female, aged mean (SD) 54 (8), BMI mean (SD) 34.3 (5.4) and weight 97.3 (17.1) kgs Why? The aim of this study was therefore to compare the effects of isocaloric HP and HC diets, combined with regular moderate intensity exercise, on glycaemic control and cardiometabolic risk factors in overweight and obese adults with T2DM initially following 12 weeks of active weight loss, and then to re‐evaluate the outcomes following 12 weeks of energy balance without the influence of weight change. By including an exercise component, we are able to determine the effects of the dietary patterns when administrated as part of a holistic lifestyle intervention as recommended in the management of T2DM. What (materials)? Core study foods corresponding to their assigned dietary pattern What (procedures)? HP diet being 32% of total energy as protein, 33% carbohydrate, and 30% total fat (< 10% as saturated fat). Participants received comprehensive dietary advice every 2 weeks. 30 mins of moderate intensity aerobic exercise, 5 times a week. Who provided? Dietitian How and where? Face‐to‐face session, location NR When and how much? Dietary intervention for 24 weeks; 12 weeks for weight loss and 12 weeks for maintenance. Participants attended clinic appointments at baseline and the end of each study phase (weeks 0, 12, and 24) for outcome assessments. Participants received comprehensive dietary advice from a qualified dietitian at baseline and every two weeks during the study. Daily semi‐quantitative food records were completed to guide dietary intake and to permit subsequent dietary analysis. Throughout the study, participants were asked to undertake a minimum of 30 min of moderate intensity aerobic exercise, five times per week. Strategies to improve or maintain fidelity; tailoring and modification: Daily semi‐quantitative food records were completed to guide dietary intake and to permit subsequent dietary analysis. Participants completed physical activity logs to monitor compliance. Extent of intervention fidelity: Total energy intake was similar between the groups for both intervention phases. Compared to the HC group, the HP group consumed significantly more protein and less carbohydrate and fibre (P < 0.05 for each nutrient) for each phase. As expected, the total fat intake in the HP diet was higher than the HC diet as a result of larger meat portions and the inclusion of reduced‐fat cheese and almonds, however both groups met the dietary recommendations for saturated fat intake (< 10% of energy intake). Concomitant interventions: 58% metformin; 19% insulin; 52% lipid‐lowering medication; 61% antihypertensive medication Control diet: Name (as reported) and brief description: Higher‐carbohydrate diet. Planned macronutrient distribution of 22% protein, 51% carbohydrate and 22% fat with < 10% saturated. Physical activity 150 min/week. Moderate energy restriction of ~30% of the individual estimated energy requirement Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐high Exercise component? Yes Recipients: Subjects were 45% female, aged mean (SD) 55 (8), BMI mean (SD) 34.4 (4.7) and weight 101.5 (16.6) kgs. Why? NR What (materials)? Core study foods corresponding to their assigned dietary pattern What (procedures)? HC diet was 22% protein, 51% carbohydrate, and 22% total fat (< 10% as saturated fat). Participants received comprehensive dietary advice every 2 weeks. 30 mins of moderate intensity aerobic exercise, 5 times a week Who provided? Dietitian How and where? Face‐to‐face session, location NR When and how much? Dietary intervention for 24 weeks; 12 weeks for weight loss and 12 weeks for maintenance. Participants attended clinic appointments at baseline and the end of each study phase (weeks 0, 12, and 24) for outcome assessments. Participants received comprehensive dietary advice from a qualified dietitian at baseline and every two weeks during the study. Daily semi‐quantitative food records were completed to guide dietary intake and to permit subsequent dietary analysis. Throughout the study, participants were asked to undertake a minimum of 30 min of moderate intensity aerobic exercise, five times per week. Strategies to improve or maintain fidelity; tailoring and modification: Daily semi‐quantitative food records were completed to guide dietary intake and to permit subsequent dietary analysis. Participants completed physical activity logs to monitor compliance. Extent of intervention fidelity: Total energy intake was similar between the groups for both intervention phases. Compared to the HC group, the HP group consumed significantly more protein and less carbohydrate and fibre (P < 0.05 for each nutrient) for each phase. As expected, the total fat intake in the HP diet was higher than the HC diet as a result of larger meat portions and the inclusion of reduced‐fat cheese and almonds, however both groups met the dietary recommendations for saturated fat intake (< 10% of energy intake). Concomitant interventions: 64 % meformin; 21% insulin; 64% lipid‐lowering medication; 43% antihypertensive medication |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: Yes Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Pork Co‐operative Research Centre (Pork CRC) grant, an initiative of the Australian government Declaration of interest: "This study was funded by a grant from the Pork Co‐operative Research Centre (Pork CRC), an Australian Government funding initiative. NAW is supported by a post‐graduate research scholarship from the Pork CRC." |
|
Westman 2008.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, number of centres NR Trial registry number: NR Total number of trial arms: 2 Year trial started: 2004 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in HbA1c Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 24 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: NR; likely USA based on authors and funders Eligibility criteria: Participants were aged 18 to 65 years with BMI 27 to 50 kg/m2 and type 2 diabetes for at least one year (defined as HbA1c > 6.0%) with onset after 15 years and no history of diabetic ketoacidosis; and with a desire to lose weight. Participants were excluded if they had an unstable or serious medical condition; significant comorbidities such as liver or kidney disease (defined as AST or ALT above 100 IU/L, or serum creatinine above 1.5 mg/dL); have cancer; were pregnant or nursing. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 97 Total attrition in trial: 47 Treatment diet Participants randomised: 48 Participants withdrawn (voluntary): 24 Total attrition: 27 Control diet: Participants randomised: 49 Participants withdrawn (voluntary): 17 Total attrition: 20 Baseline data treatment diet: Randomised participants not included: 10/48 for age, gender, weight and BMI; 27/48 for DBP, SBP, HbA1c, LDL, HDL, total cholesterol and triglycerides Age (years): mean (SD) 51.8 (7.3) Gender distribution (as reported): female 76.3%, male 23.7% Weight (kg): mean (SD) 105.5 (19.5) BMI (kg/m2): mean (SD) 37.7 (6.1) DBP (mmHg): mean (SD) 83.9 (10.3) SBP (mmHg): mean (SD) 144.4 (15.0) HbA1c (%): mean (SD) 8.8 (1.8) LDL (mmol/L): mean (SD) 2.74 (0.67) HDL (mmol/L): mean (SD) 1.14 (0.23) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.96 (0.83) TG (mmol/L): mean (SD) 2.38 (0.12) Baseline data control diet: Randomised participants not included: 3/49 for age, gender, weight and BMI; 20/49 for DBP, SBP, HbA1c, LDL, HDL, total cholesterol and triglycerides Age (years): mean (SD) 51.8 (7.8) Gender distribution (as reported): female 80.4%, male 19.6% Weight (kg): mean (SD) 106.3 (20.1) BMI (kg/m2): mean (SD) 38.5 (5.6) DBP (mmHg): mean (SD) 84.1 (11.0) SBP (mmHg): mean (SD) 140.8 (15.7) HbA1c (%): mean (SD) 8.3 (1.9) LDL (mmol/L): mean (SD) 2.95 (1.06) HDL (mmol/L): mean (SD) 1.26 (0.31) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.94 (1.13) TG (mmol/L): mean (SD) 1.89 (1.42) Group differences at baseline: "Another possible limitation is the baseline imbalance in the primary outcome, HbA1c, which occurred despite random allocation." Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Low‐carbohydrate, ketogenic diet group intervention (LCKD), participants instructed to restrict intake of dietary carbohydrates to fewer than 20 g per day without explicit caloric restriction. Exercise recommended for 30 min three times a week Treatment diet type (carbohydrate‐fat‐protein): Very low‐unclear‐unclear Exercise component? Yes Recipients: Subjects were 76.3% female, aged mean (SD) 51.8 (7.3), with BMI mean (SD) 37.7 (5.0), and weight 105.5 (19.5) kgs Why? Dietary carbohydrate is the major determinant of postprandial glucose levels, and several clinical studies have shown that low‐carbohydrate diets improve glycaemic control. In this study, we tested the hypothesis that a diet lower in carbohydrate would lead to greater improvement in glycaemic control over a 24‐week period in patients with obesity and type 2 diabetes mellitus. What (materials)? Lay‐press diet book and additional handouts What (procedures)? A registered dietitian instructed participants to restrict intake of dietary carbohydrate to fewer than 20 grams per day, without explicitly restricting caloric intake. The intervention for both groups included group sessions, diet instruction, nutritional supplements, and an exercise recommendation. Group meetings took place at an outpatient research clinic every week for 3 months, then every other week for 3 months. Participants were encouraged to exercise for 30 minutes at least 3 times per week, but no formal exercise programme was provided. Who provided? Dietitian How and where? Face‐to‐face, individual and group; research clinic When and how much? Diet intervention for 6 months, Group meetings took place every week for 3 months, then every other week for 3 months. Strategies to improve or maintain fidelity; tailoring and modification: Adherence with the diet and exercise recommendations was measured by self‐report, food records, and urinary ketones. Extent of intervention fidelity: Prior to the study intervention, the mean ± SD dietary intake for both groups was 2128 ± 993 kcal, 245 ± 136 g of carbohydrate (46% of daily energy intake), 86 ± 33 g of protein (18% of daily energy intake), 88 ± 57 g of fat (36% of daily energy intake). Over the 24‐week duration of the intervention, the LCKD group consumed 1550 ± 440 kcal per day, 49 ± 33 g of carbohydrate (13% of daily energy intake), 108 ± 33 g of protein (28% of daily energy intake), 101 ± 35 g of fat (59% of daily energy intake). There was no difference in self‐reported exercise between the groups: the mean number of exercise sessions per week increased from 2.0 ± 2.0 to 3.0 ± 2.0 for the LCKD group. Concomitant interventions: At baseline, 20 (95.2%) of the LCKD group were taking hypoglycemic medications (insulin + oral agents n = 4, insulin only n = 4, oral agents only n = 12). Twenty of 21 (95.2%) LCKD group participants had an elimination or reduction in medication. 4 in the LCKD group, who were taking over 20 units of insulin at baseline were no longer taking insulin at the end of the study. Control diet: Name (as reported) and brief description: Low‐glycaemic index diet group intervention (LGID), participants instructed to follow a low‐GI reduced calorie diet with approximately 55% of daily caloric intake from carbohydrate. Exercise recommended for 30 min three times a week. The energy prescription was individualised to be 2100 kJ less than the participant's calculated energy intake for weight maintenance. Control diet type (carbohydrate‐fat‐protein): Balanced‐unclear‐unclear Exercise component? Yes Recipients: Subjects were 80.4% female, aged mean (SD) 51.8 (7.8), with BMI mean (SD) 38.5 (5.6), and weight 106.3 (20.1) kgs. Why? NR What (materials)? Lay‐press diet book and additional handouts What (procedures)? A registered dietitian instructed participants to follow a low‐glycaemic index, reduced‐calorie diet with approximately 55% of daily caloric intake from carbohydrate. The intervention for both groups included group sessions, diet instruction, nutritional supplements, and an exercise recommendation. Group meetings took place at an outpatient research clinic every week for 3 months, then every other week for 3 months. Participants were encouraged to exercise for 30 minutes at least 3 times per week, but no formal exercise programme was provided. Who provided? Dietitian How and where? Face‐to‐face, individual and group; research clinic When and how much? Diet intervention for 6 months, group meetings took place every week for 3 months, then every other week for 3 months. Strategies to improve or maintain fidelity; tailoring and modification: Adherence with the diet and exercise recommendations was measured by self‐report, food records, and urinary ketones. Extent of intervention fidelity: Prior to the study intervention, the mean ± SD dietary intake for both groups was 2128 ± 993 kcal, 245 ± 136 g of carbohydrate (46% of daily energy intake), 86 ± 33 g of protein (18% of daily energy intake), 88 ± 57 g of fat (36% of daily energy intake). Over the 24‐week duration of the intervention, the LGID group consumed 1335 ± 372 kcal per day, 149 ± 46 g of carbohydrate (44% of daily energy intake), 67 ± 20 g of protein (20% of daily energy intake), 55 ± 23 g of fat (36% of daily energy intake). There was no difference in self‐reported exercise between the groups: the mean number of exercise sessions per week increased from 2.2 ± 2.2 to 3.8 ± 2.9 for the LGID group (P = 0.39 for comparison). Concomitant interventions: At baseline, 22 (75.9%) of the LGID group were taking hypoglycemic medications (insulin only n = 3, oral agents only n = 19), LCKD group participants had an elimination or reduction in medication, compared with 18 of 29 (62.1%) LGID group participants (P < 0.01). 1 in the LGID group who were taking over 20 units of insulin at baseline was no longer taking insulin at the end of the study. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Robert C. Atkins Foundation Declaration of interest: "The authors declare that they have no competing interests." |
|
Wycherley 2010.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, single‐centre (1) Trial registry number: ACTRN12608000206325 Total number of trial arms: 4 Year trial started: 2008 Sample size calculation: No Outcome(s) used for sample size calculation: NA Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 16 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: Post hoc power analysis indicated that the study was not powered for weight loss. |
|
| Participants |
Country and setting: Australia, outpatient research clinic in Adelaide. Key foods representing the macronutrient composition of each diet were provided every two weeks. Eligibility criteria: Participants who were overweight or obese, sedentary and had type 2 diabetes. Participants were excluded if they had proteinurea; malignancy; a history of liver, kidney, cardiovascular, respiratory or gastrointestinal disease; uncontrolled hypertension; musculoskeletal injury or joint/peripheral vascular disease impeding exercise; were pregnant or lactating, a smoker or using insulin; or participated in physical exercise in the six months prior to the study. Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: No Gender: Mixed Total number randomised: 83 Total attrition in trial: 24 Treatment diet Participants randomised: 21 Participants withdrawn (voluntary): 6 Total attrition: 9 Control diet: Participants randomised: 19 Participants withdrawn (voluntary): 2 Total attrition: 3 Baseline data treatment diet: Randomised participants not included: 9/21 Age (years): NR Gender distribution (as reported): NR Weight (kg): mean (SD) 102.7 (15.4) BMI (kg/m2): mean (SD) 35.6 (3.8) DBP (mmHg): mean (SD) 83 (9) SBP (mmHg): mean (SD) 141 (11) HbA1c (%): mean (SD) 8.0 (1.8) LDL (mmol/L): mean (SD) 2.7 (0.9) HDL (mmol/L): mean (SD) 1.2 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 5.0 (1.1) TG (mmol/L): mean (SD) 2.0 (1.1) Baseline data control diet: Randomised participants not included: 3/19 Age (years): NR Gender distribution (as reported): NR Weight (kg): mean (SD) 97.0 (10.6) BMI (kg/m2): mean (SD) 34.8 (4.9) DBP (mmHg): mean (SD) 79 (9) SBP (mmHg): mean (SD) 137 (12) HbA1c (%): mean (SD) 7.6 (1.0) LDL (mmol/L): mean (SD) 2.7 (0.9) HDL (mmol/L): mean (SD) 1.2 (0.3) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SD) 4.8 (1.0) TG (mmol/L): mean (SD) 2.3 (1.3) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: Higher‐protein moderate‐carbohydrate diet (HP). Target profile of 43% carbohydrate, 33% protein and 22% fat. Moderate energy restriction of 6000 kJ/day for women and 7000 kJ/day for men Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: Participants were diagnosed with impaired fasting glucose or T2DM according to guidelines with mean age 56.5 years, mean BMI 33.2 kg/m2 and weight 102.7 (15.4) kg. Why? Substitution of carbohydrate with protein in a energy‐restricted low‐fat (< 30%) diet may improve body composition and cardiovascular disease (CVD) risk factors including insulin sensitivity, glycaemic control, and blood lipids in study populations, such as those with type 2 diabetes. What (materials)? Key foods were supplied that were representative of each diet’s macronutrient profile‐ providing approx 50% total energy. Each participant received a scale to weight food quantities. What (procedures)? Participants were assigned to a high‐protein diet and provided with detailed dietary advice, meal planning and recipe information. Key foods representative of the macronutrient composition were supplied and participants were asked to weigh their food in order to complete a daily semi‐quantitative food record. Provision of dietary advice, meal planning and recipe information at every session. Diets structured to include specific quantities of key foods to ensure correct macronutrient and energy intake Who provided? Qualified dietitians provided meal planning, recipe information and detailed dietary advice; and also assessed dietary composition from food records. How and where? Face‐to‐face meetings with dietitians, location NR. Measurements were taken at research clinic. When and how much? The duration of the intervention was 16 weeks, with meetings with a qualified dietitian every two weeks, duration NR. Seven consecutive days of food records were analysed every two weeks. Key foods representative of the macronutrient composition were provided every two weeks. Strategies to improve or maintain fidelity; tailoring and modification: Weigh‐in at 16 weeks, meetings every two weeks providing dietary advice, meal planning and recipe information to improve compliance, key foods were provided, food had to be weighed and food records kept, food records were analysed to assess compliance. A dietitian analysed dietary intake data from each participant, using data from 7 consecutive days from the semi‐quantitative food record of each 2‐week follow‐up period. Extent of intervention fidelity: Three of the 21 participants were excluded for non‐compliance. The authors reported good compliance based on food records with significantly more protein and fewer carbohydrates consumed. A significant diet effect for urea‐to‐creatinine excretion ratio in urine was reported, with increase in high‐protein group (31.1 (9.8) to 33.6 (11.2)). Concomitant interventions: A total of 33.3% (n = 7) of participants were on hypoglycemic medication, 19.0% (n = 4) were on antihypertensive drugs and 23.8% (n = 5) were on lipid‐lowering medication. Control diet: Name (as reported) and brief description: Standard carbohydrate, low‐protein and low‐fat diet (CON). Target profile of 53% carbohydrate, 19% protein and 26% fat. Moderate energy restriction of 6000 kJ/day for women and 7000 kJ/day for men Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Participants were diagnosed with impaired fasting glucose or T2DM according to guidelines with mean age 54.6 years, mean BMI 32.3 kg/m2 and weight 97.0 (10.6) kg. Why? Isocaloric energy‐restricted low‐fat diet to facilitate weight loss and improve cardiovascular disease (CVD) risk factors including insulin sensitivity, glycaemic control, and blood lipids in study population What (materials)? Key foods were supplied that were representative of each diet’s macronutrient profile‐ providing approx 50% total energy. Each participant received a scale to weigh food quantities. What (procedures)? Participants were assigned to a standard carbohydrate, low‐protein, low‐fat diet and provided with detailed dietary advice, meal planning and recipe information. Key foods representative of the macronutrient composition were supplied and participants were asked to weigh their food and keep food records. Who provided? Qualified dietitians provided meal planning, recipe information and detailed dietary advice; and also assessed dietary composition from food records. How and where? Face‐to‐face meetings with dietitians, location NR. Measurements were taken at research clinic. When and how much? The duration of the intervention was 16 weeks, with meetings with a qualified dietitian every two weeks, duration NR. Seven consecutive days of food records were analysed every two weeks. Key foods representative of the macronutrient composition were provided every two weeks. Strategies to improve or maintain fidelity; tailoring and modification: Weigh‐in at 16 weeks, meetings every two weeks providing dietary advice, meal planning and recipe information to improve compliance, key foods were provided, food had to be weighed and food records kept, food records were analysed to assess compliance. A dietitian analysed dietary intake data from each participant, using data from 7 consecutive days from the semi‐quantitative food record of each 2‐week follow‐up period. Extent of intervention fidelity: One of the 19 participants were excluded for non‐compliance. The authors reported good compliance based on food records. A significant diet effect for urea‐to‐creatinine excretion ratio in urine was reported, with decrease in control group (30.8 (8.6) to 26.7 (3.8)). Concomitant interventions: A total of 57.9% (n = 11)of participants were on hypoglycemic medication, 47.4% (n = 9) were on antihypertensive drugs and 47.4% (n = 5) were on lipid‐lowering medication. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article, trial registry report Trial acronym/name: None Trial funded by: National Heart Foundation of Australia, Diabetes Australia Research Trust and the Pork Cooperative Research Centre project grants Declaration of interest: "No potential conflicts of interest relevant to this article were reported." |
|
Wycherley 2012.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 1 Study design: RCT, parallel, single‐centre (1) Trial registry number: ACTRN12606000002583 Total number of trial arms: 2 Year trial started: 2005 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in weight, change in fat mass (FM), change in fat‐free mass (FFM). Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 52 weeks What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Australia, outpatient research clinic in Adelaide. Key foods specific to each prescribed dietary pattern (accounting for approximately 60% of energy) were provided on a fortnightly basis until week 12. Eligibility criteria: Participants were overweight and obese males. Participants were excluded if they had BMI < 27 or > 40 kg/m2; were aged < 20 or > 65 years; had diabetes or uncontrolled hypertension, a history of renal, hepatic, coronary, gastrointestinal or metabolic disease or malignancy; were taking medication which might affect insulin sensitivity or hypoglycaemic drugs or were smokers. Type 2 diabetes at baseline: No Impaired glucose tolerance at baseline: Unclear Cardiovascular conditions/risk factors/events at baseline: No Gender: Male Total number randomised: 123 Total attrition in trial: 55 Treatment diet Participants randomised: 59 Participants withdrawn (voluntary): 5 Total attrition: 26 Control diet: Participants randomised: 64 Participants withdrawn (voluntary): 4 Total attrition: 29 Baseline data treatment diet: Randomised participants not included: 2/59 Age (years): mean (SE) 50.1 (1.2) Gender distribution (as reported): female 0%, male 100% Weight (kg): mean (SE) 105.2 (1.9) BMI (kg/m2): mean (SE) 33.8 (0.6) DBP (mmHg): mean (SE) 85.7 (1.4) SBP (mmHg): mean (SE) 134.5 (2.0) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.2 (0.1) HDL (mmol/L): mean (SE) 1.2 (0.05) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.2 (0.13) TG (mmol/L): mean (SE) 1.7 (0.12) Baseline data control diet: Randomised participants not included: 3/64 Age (years): mean (SE) 49.2 (1.2) Gender distribution (as reported): female 0%, male 100% Weight (kg): mean (SE) 102.6 (1.8) BMI (kg/m2): mean (SE) 32.7 (0.5) DBP (mmHg): mean (SE) 84.2 (1.3) SBP (mmHg): mean (SE) 135.9 (1.6) HbA1c (%): NR LDL (mmol/L): mean (SE) 3.2 (0.1) HDL (mmol/L): mean (SE) 1.3 (0.05) Non‐HDL (mmol/L): NR TC (mmol/L): mean (SE) 5.3 (0.1) TG (mmol/L): mean (SE) 1.9 (0.1) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: NA |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Similar energy prescription/approach to restrict energy intake in both diets Treatment diet: Name (as reported) and brief description: High protein (HP) diet. Macronutrient profile of 35% protein, 40% carbohydrate and 25% fat (total 53 g, saturated 14 g). Energy prescription of 7000 kJ per day Treatment diet type (carbohydrate‐fat‐protein): Low‐balanced‐high Exercise component? No Recipients: Men only N = 57, mean (SE) age 50.1(1.2) years, mean (SE) weight 105.2 (1.9) kg, mean (SE) 33.8 (0.6) kg/m2 Why? Sex differences in protein kinetics have been previously documented; hence, whether comparable effects of HP diet previously observed in females are also experienced by males remains largely unknown. This requires urgent investigation as males have a higher proportion of visceral adipose tissue, and a greater risk of cardiometabolic diseases. What (materials)? "Participants were supplied with a 2‐week provision of diet‐specific key foods, representing approximately 60% of the energy intake, to improve compliance and allow them to familiarize themselves with the food types and quantities utilized in the study. They were also supplied with digital weighing scales." What (procedures)? Participants were prescribed a HP diet made up of protein 35% (142 g, roughly 1.30 g per kg per day), carbohydrate 40% (135 g), fat 25% (total 53 g, saturated 14 g). Within the HP diet the prescribed daily protein distribution was approximately 20% (13 g) during the morning, 30% (39 g) at lunch time and 60% (78 g) of protein during the afternoon/evening period. Guided by dietitians every two weeks then monthly. Participants received detailed dietary prescription, meal planning advice and recipe information. To further facilitate dietary compliance, the dietary patterns were structured into quantities of daily foods and presented as a food checklist. Who provided? Dietitian How and where? Face‐to‐face session at the research centre When and how much? Dietary intervention for 52 weeks. At baseline, week 12 and at the end of the intervention (week 52), participants attended the clinic following an overnight fast for outcome assessment. Participants met individually with a qualified dietitian at baseline, and every 2 weeks during the first 12 weeks of the study and monthly thereafter. Strategies to improve or maintain fidelity; tailoring and modification: Participants were required to keep daily semi‐quantitative food records in which foods consumed with a variable weight were weighed using kitchen scales before recording and foods with a standard unit (i.e. slice of bread) were recorded without pre‐weighing. Dietary intake was assessed using a computerised database (Foodworks Professional Edition, version 4, 1998; Xyris Software, Highgate Hill, Australia) based on the analysis of 3 nonconsecutive days (1 weekend day and 2 weeks days) of each 2‐week period of diet‐record data throughout the study. The composite value for dietary intake for weeks 0–12 and 12–52 (Table 2) was calculated as an average of the 2 week diet‐record data blocks within each of the respective periods. To further facilitate dietary compliance, the dietary patterns were structured into quantities of daily foods and presented as a food checklist. Throughout weeks 0–12, participants were supplied with a 2‐week provision of diet‐specific key foods, representing approximately 60% of the energy intake, to improve compliance and allow them to familiarise themselves with the food types and quantities utilised in the study. Extent of intervention fidelity: On the basis of food record data, participants in both groups showed good compliance to the prescribed diets. The diets were similar in total energy (P > 0.05)but participants in the HP diet group consumed less carbohydrate, and more protein and fat than those consuming the HC diet. Concomitant interventions: Participants were excluded if they were taking hypoglycaemic medication or drugs which affect insulin sensitivity. Control diet: Name (as reported) and brief description: High‐carbohydrate (HC) diet. Macronutrient profile of 17% protein, 58% carbohydrate and 25% fat (total 51 g, saturated 14 g). Energy prescription of 7000 kJ per day Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Men only N = 61, mean (SE) age 49.2 (1.2) years, mean (SE) weight 102.6 (1.8) kg, mean (SE) 32.7 (0.5) kg/m2 Why? NR What (materials)? "Participants were supplied with a 2‐week provision of diet‐specific key foods, representing approximately 60% of the energy intake, to improve compliance and allow them to familiarize themselves with the food types and quantities utilized in the study. They were also supplied with digital weighing scales." What (procedures)? Participants were prescribed a HC diet made up of protein 17% (88 g, roughly 0.85 g per kg per day), carbohydrate 58% (198 g), fat 25% (total 51 g, saturated 14 g) that was designed to reflect current conventional dietary recommendations. Guided by dietitians every two weeks then monthly Who provided? Dietitian How and where? Face‐to‐face session at the research centre When and how much? Dietary intervention for 52 weeks. At baseline, week 12 and at the end of the intervention (week 52), participants attended the clinic following an overnight fast for outcome assessment. Participants met individually with a qualified dietitian at baseline, and every 2 weeks during the first 12 weeks of the study and monthly thereafter. Strategies to improve or maintain fidelity; tailoring and modification: Participants were required to keep daily semi‐quantitative food records in which foods consumed with a variable weight were weighed using kitchen scales before recording and foods with a standard unit (i.e. slice of bread) were recorded without pre‐weighing. Dietary intake was assessed using a computerised database (Foodworks Professional Edition, version 4, 1998; Xyris Software, Highgate Hill, Australia) based on the analysis of 3 nonconsecutive days (1 weekend day and 2 weeks days) of each 2‐week period of diet‐record data throughout the study. The composite value for dietary intake for weeks 0–12 and 12–52 (Table 2) was calculated as an average of the 2‐week diet‐record data blocks within each of the respective periods. To further facilitate dietary compliance, the dietary patterns were structured into quantities of daily foods and presented as a food checklist. Throughout weeks 0–12, participants were supplied with a 2‐week provision of diet‐specific key foods, representing approximately 60% of the energy intake, to improve compliance and allow them to familiarise themselves with the food types and quantities utilised in the study. Extent of intervention fidelity: On the basis of food record data, participants in both groups showed good compliance to the prescribed diets. The diets were similar in total energy (P > 0.05)but participants in the HP diet group consumed less carbohydrate, and more protein and fat than those consuming the HC diet. Concomitant interventions: Participants were excluded if they were taking hypoglycaemic medication or drugs which affect insulin sensitivity. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: Yes Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: Yes Change in BMI (kg/m2) at 3 to 12 months: No Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: Yes Change in SBP (mmHg) at ≥ 12 months: Yes All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: Yes Change in HDL (mmol/L) at ≥ 12 months: Yes Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: Yes Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: Yes Participant‐reported adverse effects: Yes |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: Meat and Livestock Australia through a project grant Declaration of interest: "The authors declare no conflict of interest." |
|
Yamada 2014.
| Study characteristics | ||
| Methods |
Review comparison(s) addressed by this study: 3 Study design: RCT, parallel, single‐centre (1) Trial registry number: NR Total number of trial arms: 2 Year trial started: 2011 Sample size calculation: Yes Outcome(s) used for sample size calculation: Change in HbA1c Duration of run‐in period (weeks): NA What was the duration of the weight loss phase: 6 months What was the duration of the weight maintenance phase: NA Other notes about methods: NA |
|
| Participants |
Country and setting: Japan, outpatient clinic at hospital diabetes centre in Tokyo Eligibility criteria: Participants with type 2 diabetes who had received guidance on caloric restriction at least once with an HbA1c level between 6.9 and 8.4%. Participants were excluded if they had specified ranges of proteinuria, serum creatinine, aspartate aminotransferase or alanine aminotransferase; had history of myocardial infarction or stroke in the past six months; experienced an absolute change in HbA1c of > 1.0% in the past six months or had ketosis (to avoid ketoacidosis as a complication). Type 2 diabetes at baseline: Yes Impaired glucose tolerance at baseline: No Cardiovascular conditions/risk factors/events at baseline: Unclear Gender: Mixed Total number randomised: 24 Total attrition in trial: 0 Treatment diet Participants randomised: 12 Participants withdrawn (voluntary): 0 Total attrition: 0 Control diet: Participants randomised: 12 Participants withdrawn (voluntary): 0 Total attrition: 0 Baseline data treatment diet: Randomised participants not included: None Age (years): mean (SD) 63.3 (13.5) Gender distribution (as reported): female 5/12, male 7/12 Weight (kg): mean (SD) 67.0 (15.9) BMI (kg/m2): mean (SD) 24.5 (4.3) DBP (mmHg): mean (SD) 72.6 (6.2) SBP (mmHg): mean (SD) 124.4 (10.8) HbA1c (%): mean (SD) 7.6 (0.4) LDL (mmol/L): mean (SD) 2.58 (0.73) HDL (mmol/L): mean (SD) 1.63 (0.44) Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): mean (SD) 1.60 (0.86) Baseline data control diet: Randomised participants not included: None Age (years): mean (SD) 63.2 (10.2) Gender distribution (as reported): female 7/12, male 5/12 Weight (kg): mean (SD) 68.1 (7.7) BMI (kg/m2): mean (SD) 27.0 (3.0) DBP (mmHg): mean (SD) 74.8 (10.6) SBP (mmHg): mean (SD) 124.9 (10.7) HbA1c (%): mean (SD) 7.7 (0.6) LDL (mmol/L): mean (SD) 2.91 (0.53) HDL (mmol/L): mean (SD) 1.55 (0.49) Non‐HDL (mmol/L): NR TC (mmol/L): NR TG (mmol/L): mean (SD) 1.75 (0.98) Group differences at baseline: No Characteristic(s) with significant group difference and relevant statistic: NA Other notes about participants: Patients are Japanese, BMI cutoff is different for overweight and obesity. |
|
| Interventions |
Energy (E) comparison of treatment vs control diets: Different ‐ ad libitum in treatment diet and restricted in control diet Treatment diet: Name (as reported) and brief description: Low‐carbohydrate diet consumed ad libitum, total carbohydrate intake set at < 130 g/day but with a lower limit of 70 g/day to prevent ketosis. The intake was distributed across meals with 20 to 40 g/meal and 5 g of carbohydrates from sweets twice daily. Treatment diet type (carbohydrate‐fat‐protein): Low‐unclear‐unclear Exercise component? No Recipients: Participants were type 2 diabetics, 41.7% female and aged 63.3 (13.5) years, with BMI 24.5 (4.3) and weight 67.0 (15.9) kg, mean HbA1c of 7.6%. Why? The American Diabetes Association has recommended that controlling carbohydrate intake should form part of treating diabetes. The ADA has also recognised low‐carbohydrate diets as an intervention which is effective for weight reduction. Dietary adherence to calorie‐restricted diets may be difficult to sustain over the long term. Low carbohydrate diets may be used as a treatment option in diabetics as well as assist with weight loss, blood glucose and lipid management. What (materials)? Recommendation as per Accurso et al (2008). Citation: Accurso A, Bernstein RK, Dahlgvist A, et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab (Lond) 5: 9, 2008. What (procedures)? Participants were assigned to the low‐carbohydrate diet (> 70 g/day and < 130 g/day), with target carbohydrate contents per meal prescribed. Consultations with diet instructions and assessments every 2 months were conducted. Who provided? Four registered dietitians instructed participants. How and where? Face‐to‐face, location at outpatient clinics When and how much? Intervention duration was six months with 2‐monthly follow‐up visits. Duration and frequency of instruction NR Strategies to improve or maintain fidelity; tailoring and modification: NR Extent of intervention fidelity: "The mean carbohydrate intake in the low carbohydrate group was < 130 g/day, suggesting that most patients were able to adhere to the meal instructions." Macronutrient intakes for carbohydrates, protein and fat were 29.8 (12.5)%, 25.3 (7.3)% and 45.4 (8.9)%. Concomitant interventions: Participants were on insulin (25.0%), metformin (41.7%), sulfonylurea (41.7%), glinide (8.3%), thiazolidinedione (33.3%), alpha‐glucosidase inhibitor (16.7%), DPP‐4 inhibitor (16.7%). All participants were on some form of glucose‐lowering drug; none were taking GLP‐1. Control diet: Name (as reported) and brief description: Calorie‐restricted diet as defined by the Japan Diabetes Society, with target macronutrient intakes of 50 to 60% carbohydrates, < 20% protein and < 25% fat. Target calorie intake was defined as total calorie intake (kcal) = ideal body weight × 25. Control diet type (carbohydrate‐fat‐protein): Balanced‐balanced‐balanced Exercise component? No Recipients: Participants were type 2 diabetics, 58.3% female and aged 63.2 (10.2) years, with BMI 27.0 (3.0) and weight 68.1 (7.7) kg, mean HbA1c of 7.7%. Why? The American Diabetes Association recognises caloric restriction as an effective strategy to reducing body weight. The Japan Diabetes Society also currently recommends caloric restriction for the management of blood glucose. What (materials)? Recommendations of the Japan Diabetes Society. Citation: The Japan Diabetes Society. Diet therapy. In Practice Guideline for the Treatment for Diabetes in Japan; Nankodo: Tokyo, Japan, 2016; pp. 37–66. (In Japanese). What (procedures)? Participants were assigned to caloric restriction and the calculation of calorie intake through the classification of macronutrients. Who provided? Four registered dietitians instructed participants. How and where? Face‐to‐face, location at outpatient clinics When and how much? Intervention duration was six months with 2‐monthly follow‐up visits. Duration and frequency of instruction NR Strategies to improve or maintain fidelity; tailoring and modification: NR Extent of intervention fidelity: Macronutrient intakes for carbohydrates, protein and fat were 51.0 (4.6)%, 16.6 (2.8)% and 32.3 (5.2)%. Concomitant interventions: Participants were on insulin (33.3%), metformin (8.3%), sulfonylurea (66.7%), thiazolidinedione (50.0%), DPP‐4 inhibitor (25.0%). All participants were on some form of glucose‐lowering drug; none were taking GLP‐1, glinide or alpha‐glucosidase inhibitor. |
|
| Outcomes |
Change in body weight (kg) at 3 to < 12 months: Yes Change in body weight (kg) at ≥ 12 months: No Number of participants with 5% weight loss from baseline at 3 to < 12 months: No Number of participants with 5% weight loss from baseline at ≥ 12 months: No Change in BMI (kg/m2) at 3 to 12 months: Yes Change in BMI (kg/m2) at ≥ 12 months: No Number of participants with 5% BMI reduction from baseline at 3 to 12 months: No Number of participants with 5% BMI reduction from baseline at ≥ 12 months: No Change in DBP (mmHg) at ≥ 12 months: No Change in SBP (mmHg) at ≥ 12 months: No All‐cause mortality at ≥ 12 months: No Cardiovascular mortality at ≥ 12 months: No Non‐fatal myocardial infarction at ≥ 12 months: No Non‐fatal stroke at ≥ 12 months: No Diagnosis of type 2 diabetes mellitus at ≥ 12 months: No Change in HbA1c (%) at ≥ 12 months: No Change in LDL (mmol/L) at ≥ 12 months: No Change in HDL (mmol/L) at ≥ 12 months: No Change in non‐HDL (mmom/L) at ≥ 12 months: No Change in total cholesterol (TC) (mmol/L) at ≥ 12 months: No Change in triglycerides (or triacylglycerides) (TG) (mmol/L) at ≥ 12 months: No Participant‐reported adverse effects: No |
|
| Notes |
Number and type of records(s): journal article Trial acronym/name: None Trial funded by: NR Declaration of interest: "The authors state that they have no Conflict of Interest (COI)." |
|
ACE: angiotensin‐converting enzyme ADA: American Dietetic Association ALT: alanine aminotransferase AMDR: Acceptable Macronutrient Distribution Range APMD: adequate protein medium dairy ASA24: Automated Self‐Administered 24‐h Dietary Recall AST: aspartate aminotransferase AUD: Australian dollar BCCA: branched‐chain amino acids BMD: bone mineral density BMI: body mass index BP: blood pressure Ca2+: calcium CARB: carbohydrate CD: compact disc CGMS: Continuous Glucose Measuring System CHO: carbohydrate CI: confidence interval CON: control CRC: Clinic Research Centre CRD: calorie‐restricted diet CSIRO: Commonwealth Scientific and Industrial Research Organisation CVD: cardiovascular disease C‐WL: control weight loss DBP: diastolic blood pressure DEXA: dual‐energy X‐ray absorptiometry DGE: Deutsche Gesellschaft für Ernährung (German Nutrition Society) DM: diabetes mellitus DRI: Dietary Reference Intake E: energy ER: energy restriction ESHA: Elizabeth Stewart Hands and Associates FFQ: food frequency questionnaire FM: fat mass FMD: flow‐mediated dilatation GCRC: General Clinical Research Centre GI: glycaemic index GL: glycaemic load GLP‐1: glucagon‐like peptide‐1 HbA1c: haemoglobin A1c/glycated haemoglobin HBV: high biological value HC: high‐carbohydrate HCD: high‐carbohydrate diet HDL(‐C): high‐density lipoprotein(‐cholesterol) HCLF: high‐carbohydrate low‐fat HDPMC: high‐dairy protein, moderate‐carbohydrate HF: high‐fat HFLC: high‐fat low‐carbohydrate HNP: high‐normal‐protein HOMA‐BCF: homeostatic model assessment of β‐cell function HOMA‐IR: homeostatic model assessment for insulin resistance HP: high‐protein HPHD: high‐protein high‐dairy HP‐WL: high‐protein weight‐loss HRT: hormone replacement therapy HUF: high‐unsaturated fat IFG: impaired fasting glucose iGFR: isotope glomerular filtration rate INS: insulin sensitivity IQR: interquartile range IR: insulin resistant/resistance IS: insulin sensitive/sensitivity ITT: intent(ion)‐to‐treat kcal: kilocalorie kg: kilogram LC: low‐carbohydrate LCD: low‐carbohydrate diet LCHP: low‐carbohydrate high‐protein LCK: low‐carbohydrate ketogenic LCKD: low‐carbohydrate ketogenic diet LCM: low‐carbohydrate Mediterranean LDL(‐C): low‐density lipoprotein(‐cholesterol) LF: low‐fat LFD: low‐fat diet LFHC: low‐fat high‐carbohydrate LGI: low‐glycaemic index LGID: low‐glycaemic index diet LGL: low‐glycaemic load LOGI: energy‐restricted low‐carbohydrate MCCR: moderate‐carbohydrate calorie‐restricted MLC: modified low‐carbohydrate MES: medication effect score MF: modified‐fat high‐carbohydrate MI: initial weight maintenance period MII: final weight maintenance period MN: micronucleus MUFA: monounsaturated fatty acid NA: not applicable NAFLD: non‐alcoholic fatty liver disease NCEP: (United States) National Cholesterol Education Program NIH: National Insitutes of Health NNR: Nordic Nutrition Recommendations NR: not reported NSAID: non‐steroidal anti‐inflammatory drug NU: nitrogen excretion in urine OGTT: oral glucose tolerance test PA: physical activity PABA: para‐aminobenzoic acid PD: Paleolithic diet PBL: peripheral blood lymphocytes PRO: protein PUFA: polyunsaturated fatty acid
1RM: one‐repetition maximum RCT: randomised controlled trial RD: registered dietitian RDA: Recommended Dietary Allowance REE: resting energy expenditure REX: resistance exercise RMR: resting metabolic rate SBP: systolic blood pressure SD: standard deviation SE: standard error SEM: standard error of the mean SFA: saturated fatty acid T2DM: type 2 diabetes mellitus TAG: triacylglyceride TC: total cholesterol TE: total energy TG: triglyceride TM: traditional Mediterranean UCR: urea/creatinine ratio VHFLC: very high‐fat low‐carbohydrate VLC: very low‐carbohydrate VLCHF: very low‐carbohydrate high‐fat WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ben Avraham 2009 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Bluher 2010 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Bluher 2012 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Brehm 2009 | Ineligible intervention: not low‐carbohydrate diet |
| Brun 2011 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Campbell 2012 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Daly 2006 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Davis 2009 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Davis 2012 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Djuric 2002 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Dorling 2020 | Record of an additional study using participants from an included trial |
| Fabricatore 2011 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Gardner 2018 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Golan 2012 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Greenberg 2009 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Guo 2019 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Iqbal 2010 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Itsiopoulos 2011 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Jönsson 2009 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Leichtle 2011 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Lindeberg 2007 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Locke 2020 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Luger 2013 | Ineligible indication: diets not for weight loss |
| Ma 2008 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| McIver 2010 | Ineligible intervention: not low‐carbohydrate diet |
| McIver 2011 | Ineligible intervention: not low‐carbohydrate diet |
| McLaughlin 2016 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Melanson 2012 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Millward 2014 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Morgan 2009 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| NCT00200720 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| NCT00990457 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Nielsen 2005 | Ineligible study design |
| Reid 2009 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Sakae 2015 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Saslow 2017b | Ineligible comparison: co‐interventions differed by group |
| Shai 2008 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Shai 2010 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Shih 2019 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Stewart 2013 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Toobert 2003 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Trepanowski 2017 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Vetter 2010 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Wood 2012 | Carbohydrate contents not adequately defined and could not be obtained from study authors |
| Wycherley 2013 | Record of an additional study using participants from an included trial |
| Yancy 2015 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
| Zelicha 2018 | No defined carbohydrate prescription used by study authors for intervention and/or control diets |
Characteristics of studies awaiting classification [ordered by study ID]
Aller 2019.
| Methods | |
| Participants | Men and women aged 20 to 65 years, with BMI > 30 |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
Cui 2006.
| Methods | |
| Participants | Obese subjects |
| Interventions | Low‐carbohydrate and low‐fat diets |
| Outcomes | Potential change in body weight |
| Notes | Placed under awaiting classification as no abstract or full text are available. Author correspondence yielded no response. |
De Luis 2007.
| Methods | |
| Participants | Obese men and women |
| Interventions | Low‐carboydrate and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2009a.
| Methods | |
| Participants | Men and women with BMI > 30 |
| Interventions | High‐fat/high‐protein and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2009b.
| Methods | |
| Participants | Obese men and women |
| Interventions | Low‐carbohydrate and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2010a.
| Methods | |
| Participants | Men and women with BMI ≥ 30 |
| Interventions | Low‐carbohydrate and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2010b.
| Methods | |
| Participants | Men and women with BMI ≥ 30 |
| Interventions | Low‐carbohydrate and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2012.
| Methods | |
| Participants | Obese men and women |
| Interventions | Low‐carbohydrate and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2015.
| Methods | |
| Participants | Men and women with BMI > 30 |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2015a.
| Methods | |
| Participants | Obese men and women |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2015b.
| Methods | |
| Participants | Men and women with BMI > 30 |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2015c.
| Methods | |
| Participants | Obese men and women |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2015d.
| Methods | |
| Participants | Men and women with BMI ≥ 30 |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2015e.
| Methods | |
| Participants | Men and women with BMI > 30 |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2016.
| Methods | |
| Participants | Obese men and women |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2016a.
| Methods | |
| Participants | Men and women with BMI > 30 |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2016b.
| Methods | |
| Participants | Obese subjects |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2017.
| Methods | |
| Participants | Men and women with BMI ≥ 30 |
| Interventions | Low‐carbohydrate and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2018.
| Methods | |
| Participants | Men and women aged 20 to 65 years, with BMI ≥ 30 |
| Interventions | Low‐carbohdyrate and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2018a.
| Methods | |
| Participants | Men and women aged 20 to 65 years, with BMI of 30 to 45 |
| Interventions | High‐fat and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2019.
| Methods | |
| Participants | Men and women aged 18 to 70 years, with BMI > 30 |
| Interventions | High‐protein and standard‐protein severe hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2019a.
| Methods | |
| Participants | Men and women aged 25 to 65, with BMI ≥ 30 |
| Interventions | High‐fat and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2020.
| Methods | |
| Participants | Adults aged 18 to 70 years, with BMI ≥ 30 |
| Interventions | High‐protein and standard‐protein severe hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2020a.
| Methods | |
| Participants | Men and women aged 20 to 60 years, with BMI ≥ 30 |
| Interventions | High‐fat and low‐fat diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
De Luis 2021.
| Methods | |
| Participants | Men and women aged between 25 and 65 years, with BMI ≥ 30 kg/m2 |
| Interventions | High‐protein/low‐carbohydrate and standard‐protein severe hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
Evangelista 2009.
| Methods | |
| Participants | Men and women aged 59 (± 10) years, with heart failure and BMI > 27 |
| Interventions | High‐protein and standard‐protein diets |
| Outcomes | Short‐term change in body weight |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on the same participants from another reference by the same author; reference shares considerable similarity with other reference by the same author also placed under awaiting classification. Attempts to contact the author yielded no response. No protocol or prospective trial registry could be found. |
Evangelista 2017.
| Methods | |
| Participants | Men and women aged 58.8 (± 9.7) years, with heart failure and mean BMI of 37 (± 6.2) |
| Interventions | High‐protein and standard‐protein diets |
| Outcomes | Short‐term change in body weight |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on the same participants from another reference by the same author; reference shares considerable similarity with other reference by the same author also placed under awaiting classification. Attempts to contact the author yielded no response. No protocol or prospective trial registry could be found. |
Fernandez 2020.
| Methods | |
| Participants | Men and women with mean (SD) age 52.3 (13.1) years, with BMI > 30 kg/m2 |
| Interventions | Optimal‐protein, carbohydrate‐restricted and standard low‐fat, low‐calorie, guideline‐based diets |
| Outcomes | Short‐term change in body weight |
| Notes | Placed under awaiting classification as dietary composition could not be assessed from this conference abstract. |
Fleming 2002.
| Methods | |
| Participants | Men and women |
| Interventions | High‐fat and moderate‐fat diets |
| Outcomes | Short‐term change in body weight; long‐term change in body weight; long‐term changes in LDL, HDL and total cholesterol; long‐term change in triglycerides |
| Notes | Placed under awaiting classification due to important inconsistencies in the reporting. Attempts to contact the author for clarity yielded no response. No protocol or prospective trial registry could be found. |
Greene 2004.
| Methods | |
| Participants | Men and women |
| Interventions | Very low‐carbohydrate and low‐fat diets |
| Outcomes | Short‐term change in body weight |
| Notes | Placed under awaiting assessment as the conference abstract was the only source of information, with data not in a usable format. Attempts to contact the author yielded no results, but a co‐author indicated that the study was never published in full. |
Izaola 2019.
| Methods | |
| Participants | Men and women aged 18 to 70 years, with BMI ≥ 30 |
| Interventions | High‐protein and standard‐protein hypocaloric diets |
| Outcomes | Short‐term change in body weight, short‐term change in BMI |
| Notes | Placed under awaiting classification to avoid potential double‐counting. We are uncertain if this is a distinct trial or reporting on a subsample of participants from other references by the same author; reference shares considerable similarity with other references by the same author also placed under awaiting classification. Author correspondence did not provide the required detail to preclude potential double‐counting. No protocol or prospective trial registry could be found. |
Mukhtar 2009.
| Methods | |
| Participants | Subjects with metabolic syndrome |
| Interventions | Low‐carbohydrate/high‐protein and low‐fat/high‐carbohydrate diets |
| Outcomes | Potential short‐term change in body weight; long‐term change in body weight, triglycerides, HDL cholesterol and HbA1c |
| Notes | Placed under awaiting assessment as the conference abstract was the only source of information, with no usable data. Attempts to locate the first author yielded no results, contact with a co‐author yielded no response. |
Sun 2019.
| Methods | |
| Participants | Overweight adults |
| Interventions | Low‐carbohydrate, calorie‐restricted and 'normal' diets |
| Outcomes | Short‐term change in body weight |
| Notes | Placed under awaiting assessment as the conference abstract was the only source of information, with no usable data. Attempts to locate the first author yielded no results. |
BMI: body mass index HbA1c: haemoglobin A1c/glycated haemoglobin HDL: high‐density lipoprotein(‐cholesterol) LDL: low‐density lipoprotein(‐cholesterol) SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
NCT03832933.
| Study name | Comparing high‐ and normal‐protein diets for the dietary remission of type 2 diabetes |
| Methods | Randomised trial (parallel assignment) |
| Participants | Males and females aged > 18 years, T2D diagnosis within previous 6 years (documented physician diagnosis, use of anti‐diabetic medication, fasting glucose ≥ 126 mg/dL, and/or HbA1c ≥ 6.5%), BMI of > 27 kg/m2, weight stable (+/‐ 3 kgs in the last 3 months), participants using GLP‐1 agonists or SGLT‐inhibitors must be on a stable (≥ 3 months) medication dosage and not be planning to change medication dosage, and willing and able to participate in a weekly group class for the first 16 weeks of the study, biweekly classes for the remainder of the study, and willing to participate in 4 study visits over the 52‐week study period. Exclusion criteria: HbA1c concentrations of > 12%, pregnant or lactating within the past 6 months or trying to become pregnant, individual following a vegetarian/vegan only diet, food allergies (to red meats or other common protein sources), using exogenous insulin for T2D management, taking other medication that could cause weight loss or weight gain (such as steroids, tricyclic antidepressants, chemotherapy, antipsychotics, prescribed or OTC weight‐loss agents). Oral contraceptives can be used as long as subject agrees to not change use of these during the study. Vitamins and minerals that do not have a weight effect are allowed as long as use is continued without change during the study. Current alcohol or drug abuse or dependence (subjects who have quit smoking in the last 6 months) will be excluded. Smokers whose smoking habits have been stable for the last 6 months and which remain stable during the study can be included), current eating disorder (anorexia or bulimia), any medical condition for which following a high‐protein diet and/or 70 minutes of exercise daily would be inadvisable and untreated or unstable hypothyroidism. Thyroid medications must be stable for at least 3 months. |
| Interventions | Interventions ‐ High‐protein diet: 16‐week weight‐loss and 36‐week weight‐loss maintenance intervention. Approximate macronutrient distributions (carbohydrate:protein:fat) will be 32%:40%:28%. Control ‐ Standard‐protein diet: 16‐week weight‐loss and 36‐week weight‐loss maintenance intervention. Approximate macronutrient distribution (carbohydrate:protein:fat) will be 53%:21%:25%. |
| Outcomes | Primary outcomes: 1) change in body weight (baseline to 16 and 52 weeks) Secondary outcomes: 1) change in type 2 diabetes mellitus diagnosis (baseline to 52 weeks), 2) change in percentage body fat (baseline to 16 and 52 weeks), 3) change in percentage fat‐free mass (baseline to 16 and 52 weeks) |
| Starting date | July 2019 |
| Contact information | Drew Sayer, PhD, 205‐354‐8950, sayerd@uab.edu |
| Notes | Prospectively registered |
NCT04014296.
| Study name | Weight loss in adults over 50 with obesity |
| Methods | Randomised trial (parallel assignment) |
| Participants | Females, ages > 50 years, post‐menopausal with a BMI of 35 to 50 kg/m2. Exclusion criteria included: untreated hyper or hypotension, cancer (except basal cell), gastrointestinal disorders affecting food intake, > 6 months of stable use of medications that affect metabolism, body weight, energy expenditure or appetite, current eating disorder (e.g. binge eating disorder, anorexia, or bulimia) and any medical condition for which following a HP diet, and/or 70 minutes of exercise daily would be inadvisable. |
| Interventions | Intervention ‐ High‐protein diet: 16‐week weight loss programme with high‐protein diet and supervised flexibility and balance training. Approximate macronutrient distributions (carbohydrate:protein:fat) will be 32%:40%:28%. Control ‐ Resistance training: 16‐week weight‐loss programme with normal protein diet and supervised resistance training. Approximate macronutrient distributions (carbohydrate:protein:fat) will be 53%:20%:26%. |
| Outcomes | Primary outcomes: 1) change in fat mass (baseline to 8 and 16 weeks), 2) change in fat free mass (baseline to 8 and 16 weeks) Secondary outcomes: 1) change in glucose, 2) change in insulin, 3) change in lipids, 4) change in disposition index (oral glucose tolerance testing), 5) change in aerobic fitness, 6) change in physcial activity, 7) change in muscle health and overall strength (all: baseline to 8, 16 and 52 weeks) 8) change in body composition (16 to 52 weeks) |
| Starting date | March 2020 |
| Contact information | Caroline Cohen, PhD, 2059349213, cwyoder@uab.edu |
| Notes | Prospectively registered |
NCT04023942.
| Study name | Personalised nutrition and e‐health: lifestyle intervention study for weight loss maintenance (LION) |
| Methods | Randomised trial (parallel assignment) |
| Participants | Men and women aged 18 to 65 years with a BMI of 30‐39.9 kg/m2, owner of a smartphone, Caucasian with no severe diseases and with the ability to give written informed consent. Exclusion criteria include: diabetes mellitus, severe cardiovascular and/or respiratory disease, untreated high blood pressure, severe kidney disease, active cancer (or in remission), inflammatory bowel disease, severe chronic infections and/or inflammations, severe metal‐health disorders, neurodegenerative disorders, endocrine diseases, lipoedema, pregnancy and lactation, vigorous weight fluctuations (> 5kgs) in the last 3 months, immobility, surgery in the last 3 months, participation in other intervention studies, carrier of pacemakers, blood donation or transfusions in the last 3 months |
| Interventions | Intervention ‐ Low‐carbohydrate (app‐based group): Low‐carbohydrate is defined as 30 per cent energy from carbohydrates and consists of 20 per cent energy from protein. The daily energy requirement is calculated for each participant individually, based on his/her resting metabolic rate and physical activity level. Daily energy intake should be 10% lower than the calculated daily energy requirement. Participants assigned to the app‐based group works together with a personal coach via app, providing nutritional guidance and support during the 12‐month weight‐maintenance step. Low‐carbohydrate (newsletter‐based group): Low carbohydrate is defined as 30 per cent energy from carbohydrates and consists of 20 per cent energy from protein. The daily energy requirement is calculated for each participant individually, based on his/her resting metabolic rate and physical activity level. Daily energy intake should be 10% lower than the calculated daily energy requirement. The newsletter intervention group gets regularly digital newsletters, in the same frequency as 'contacts' take place in the app‐based group. |
| Outcomes | Primary outcome: Weight maintenance 12 months after weight‐loss intervention (15 months) Secondary outcomes: 1) weight change after eight weeks of formula diet (3 months), 2) change in glucose, 3) change in insulin, 4) change in lipids, 5) change in leptin (all at 0, 3, 6, 9, 12, 15 and 27 months), 6) change in resting metabolic rate, 7) change in magnetic resonance imaging (both at 0, 3, 15 and 27 months), 8) change in postprandial insulin, glucose and lipid responses (all at 0 months) |
| Starting date | 17 July 2019 |
| Contact information | Christina Holzapfel, PhD, +49 89 28924923 christina.holzapfel@tum.de |
| Notes | Retrospectively registered |
NCT04136093.
| Study name | Diet for the maintenance of weight loss and metabolic health in obese postmenopausal women (WELCOME) |
| Methods | Randomised trial (parallel assignment) |
| Participants | Females aged 50‐65 years who are all postmenopausal, with the absence of menses of over 12 months or serum follicle stimulating hormone > 30 IU/mL, with central obesity waist circumference (WC) > 80 cm, with low physical activity, who wished to lose weight and weight‐loss maintenance, and have at least one other criterion of metabolic syndrome: increased systolic blood pressure ≥ 130 mm Hg or high blood pressure diastolic ≥ 85 mm Hg or ongoing treatment of previously diagnosed hypertension, increased serum triglyceride levels above > 150 mg/dL (1.7 mmol/L) or ongoing therapy hypertriglyceridemia, decreased HDL cholesterol < 50 mg/dL (1.3 mmol/L), and fasting blood glucose > 100 mg/dL (5.6 mmol/L) or ongoing treatment of previously diagnosed type 2 diabetes. Exclusion criteria: thyroid disease (hypothyroidism, hyperthyroidism, thyroiditis), hypercortisolism, Cushing's syndrome, kidney diseases, type 1 diabetes, asthma treated with oral and injectable steroids, cancers, mental disorders, New York Heart Association (NYHA) Class II heart failure, as well as any drug known to influence liver function, endocrine disorders, hormonal replacement therapy, significant weight change in the 6 months prior to the current study, impaired absorption of nutrients (celiac disease, inflammatory bowel disease), intolerance or food allergy to key components of the intervention diets, smoking and excessive alcohol consumption (consumption of more than 2 alcohol units per day ‐ one alcohol unit equals one bottle of beer (340 g) or one glass of wine (140 g) or one glass of spirits (42.5 g of 40% spirit) |
| Interventions | Intervention ‐ the MED diet: The MED diet will be composed of the basis food items traditional for the Mediterranean region i.e. olive oil, nuts, vegetables, fruits, and fish and this diet will be given an ad libitum approach. The MED diet will be giving a higher proportion of fat, at least 40% of the total energy, with 20% of the total energy from MUFAs, and less proportion of carbohydrates. To ensure the assumed supply of MUFAs and polyunsaturated fatty acids (PUFAs), the participants will be asked to consume a daily intake of 60 g (6 spoons) of extra virgin olive oil and 30 g (6 pieces) walnuts. Control ‐ the DASH diet: The DASH diet will be giving a higher proportion of carbohydrates, at least 60% of total energy and less fat. The DASH diet will be composed of wholegrain cereal products, fruit, vegetables, low‐fat dairy products, fish, seafood, poultry, beans, seeds and nuts and will be recommending eating of the traditional Polish food items, for example, oatmeal, rye bread, barley groats, apples, plums, etc. and reducing the supply of salt, sweets, sugars, fats especially saturated fats and red meat. In our study, the DASH diet will be given an ad libitum approach. The sufficient supply of carbohydrates and β glucans in the DASH diet will be ensured by daily consumption at least 50 g oatmeal and 50 g of barley groats. |
| Outcomes | Primary outcomes: 1) change in body weight, 2) change in waist circumference , 3) change in fat mass (all at 8, 12, 16, 20, 24, 28, 32 and 52 weeks), 4) change in HDL cholesterol, 5) change in triglycerides, 6) change in glucose (all at 8, 32 and 52 weeks), 7) changes in systolic and diastolic blood pressure (both at 8, 12, 16, 20, 24, 28, 32 and 52 weeks) Secondary outcomes: 1) change in physical activity level, 2) change in eating behaviour (both at 8, 32 and 52 weeks), 3) change in adherence to prescribed diet, 4) change in dietary intake (both at 8, 12, 16, 20, 24, 28, 32 and 52 weeks) |
| Starting date | February 2020 |
| Contact information | Lidia Małczak, +48787656850, lidia.malczak@up.poznan.pl and Joanna Bajerska, +48618466056, joanna.bajerska@up.poznan.pl |
| Notes | Prospectively registered |
NCT04192357.
| Study name | A randomised controlled trial of a weight loss maintenance program for adults with obesity |
| Methods | Randomised trial (parallel assignment) |
| Participants | Men and premenopausal women (self‐reported) aged 18 to 50 years with a BMI of > 30 kg/m2 and < 39.9kg/m2, interested in being enrolled in a weight‐loss programme and available to comply with study protocol and sign informed consent. Exclusion criteria included: pregnant, breastfeeding or planning to become pregnant within study period, menopausal women, subjects with chronic inflammatory bowel disease (clinical history of Crohn's disease, ulcerative colitis, irritable colon and diverticulitis), subjects with hormonal or thyroid pathology (hyper and hypothyroidism where TSH is not within normal range), subjects with renal impairment, subjects with chronic liver disease other than non‐alcoholic hepatic steatosis, subjects with autoimmune diseases and/or chronic use of corticosteroids, use of weight‐loss medications/other nutritional supplements, subjects with psychiatric illness, subjects sensitive to any component of supplements, subjects with surgery or hospitalisation in the last 30 days, subjects prescribed with more than 2 antidepressants or more than 2 drugs for hypercholesterolemia, subjects currently smoking, use of drugs with impact on lipid profile, previous attempt to lose weight in the last month and/or weight loss of more than 10 kg in the 3 months prior to the start of the study, and subjects with excessive alcohol consumption (self‐reported: drinking more than 3 glasses of wine/day ‐ or equivalent) |
| Interventions | Intervention ‐ M3F program: the M3F programme is divided into three phases, with the first two being weight loss and the third phase being weight maintenance. Control ‐ Low‐carb diet: The low‐carb diet programme is divided into two phases with the first being weight loss which follows a low‐carb diet, and a second phase being weight maintenance. |
| Outcomes | Primary outcome: change in weight (at 72 weeks) Secondary outcomes: 1) change in total fat mass, 2) change in waist circumference, 3) change in body mass index, 4) change in systolic and diastolic blood pressure, 5) change in intestinal microbiota (bacterial DNA 16SRNA gene) (all at 72 weeks) |
| Starting date | February 2020 |
| Contact information | Conceição Calhau, PhD, 00351 218803035 ext 20401, ccalhau@nms.unl.pt and André Rosário, PhD, 00351 225513622 ext 26926, andrerosario@med.up.pt |
| Notes | Prospectively registered |
NCT04230928.
| Study name | Giving a low carbohydrate diet to overcome hypertension (GLOH) |
| Methods | Prospective randomised 2‐group trial (parallel assignment) |
| Participants | Men and women aged > 18 years, willing to participate in a 10‐month study, systolic blood pressure > 120 mm/Hg +/‐ diastolic blood pressure > 80 mm/Hg and African‐American race. Exclusion criteria: diagnosed congestive heart failure, hypertension stage 4 or higher, received or needing more than 3 antihypertensive medications, pregnant or planning to become pregnant, receiving or needing a heart transplant, using injected long or short‐acting insulin for diabetes treatment, not African‐American race, unable to speak and read English with fluency |
| Interventions | Intervention ‐ Very low‐carbohydrate (VLC) diet: Individuals will receive a version of the Diabetes Prevention Program‐Group Lifestyle Balance (DPP‐GLB) programme in which 4 of the 12 modules will teach a very low‐carbohydrate diet. All other components of the DPP‐GLB will follow the standard programme. Control ‐ Standard diet: Individuals will receive a version of the Diabetes Prevention Program‐Group Lifestyle Balance (DPP‐GLB) program in which 4 of the 12 modules will teach a standard very low‐fat, calorie‐restricted diet. |
| Outcomes | Primary outcomes: 1) changes in systolic blood pressure, 2) dietary changes in carbohydrate intake (both at baseline, 12 weeks and 10 months) Secondary outcomes: 1) change in fasting blood glucose, 2) change in haemoglobin A1c, 3) change in cholesterol and lipoproteins, 4) change in percentage body weight, 5) change in body mass index, 6) dietary changes in fat and caloric intake, 7) change in diastolic blood pressure (all at baseline, 12 weeks and 10 months) |
| Starting date | 1 February 2020 |
| Contact information | Aisha H Montgomery, 2148653086, aisha.montgomery@bswhealth.org |
| Notes | Prospectively registered |
NCT04382183.
| Study name | Impact of ketogenic diets in preventing relapse in obesity management (Ketomaintain) |
| Methods | Prospective randomised 2‐group trial (parallel assignment) |
| Participants | Healthy male and female volunteers aged 18 to 65 years, stable weight over the last three months (< 2 kg fluctuation), not currently dieting with the intention to lose weight, inactive lifestyle (< 150 min physical activity per week). Women should be taking oral contraceptives, with regular menstrual cycles, or be postmenopausal; as appetite and resting metabolic rate have been shown to be affected by the phase of menstrual cycle in normally ovulating women. Exclusion criteria: pregnancy, breastfeeding, drug or alcohol abuse within the last two years, clinically significant illness (including diabetes; gastrointestinal, kidney (GFR < 60 mL/min) or liver disease; osteoporosis (T‐score < ‐2.5) or osteopenia (T‐score < ‐1.5)), treatment with anti‐osteoporotic drug, depression or other psychological disorders, eating disorders, milk intolerance, current medication known to affect appetite, metabolism or induce weight loss, planned surgery during the study period and participating in another research study |
| Interventions | Intervention ‐ Ketogenic weight‐loss maintenance group, following a ketogenic diet (50 g CHO/day) plant‐based for 1 year. Control ‐ Isocaloric balanced weight‐loss maintenance group, following the standard Norwegian Health Directorate recommendations for 1 year |
| Outcomes | Primary outcome: body weight regain (at baseline, 5 weeks, 6 months and 1 year) Secondary outcomes: 1) fasting lipid profile, 2) bone mineral density (both at baseline, 6 months and 1 year) |
| Starting date | 3 August 2020 |
| Contact information | Silvia Coutinho, +4798639859, silvia.coutinho@ntnu.no and Cátia Martins, +4772825358, catia.martins@ntnu.no |
| Notes | Prospectively registered |
NCT04699448.
| Study name | Gene‐diet interactions on body weight regulation and lifestyle parameters (iMPROVE) |
| Methods | Randomised trial (parallel assignment) |
| Participants | Men and women, aged 18 to 65 years, with a BMI of > 25 kg/m2. Exclusion criteria included: pregnancy or lactation; the presence of comorbidities such as type 1 or 2 diabetes, cardiovascular disease, gastrointestinal disorders, mental illness or dietary disorders; taking dietary supplements aimed at body weight loss; or parallel participation in a different research study. |
| Interventions | Intervention ‐ High‐protein hypocaloric diet: 6‐month diet designed for body weight loss. Approximate macronutrient distributions (carbohydrate: protein:fat) will be 30%:40%:30%. Control‐ High‐carbohydrate hypocaloric diet: 6‐month diet designed for body weight loss. Approximate macronutrient distributions (carbohydrate: protein:fat) will be 60%:18%:22%. |
| Outcomes | Primary outcomes: 1) change in body weight Secondary outcomes: 1) change in body fat percentage, 2) change in waist circumference, 3) change in visceral fat, 4) change in biochemical biomarkers related to metabolic syndrome and cardiovascular disease risk (i.e. total cholesterol, glucose, triglyceride and HDL levels), 5) change in sleep quality, 6) change in depression symptoms, 7) change in overall health status |
| Starting date | April 2020 |
| Contact information | Prof. Georgios Dedoussis, dedousi@hua.gr and Prof. Andriana Kaliora, akaliora@hua.gr |
| Notes | Retrospectively registered |
NCT04745572.
| Study name | Development of an adaptive treatment for weight loss in people with prediabetes |
| Methods | Randomised trial (sequential assignment) |
| Participants | Adults aged 18 to 75 years, with a BMI of ≥ 27 kg/m2 and at risk for developing type 2 diabetes mellitus (prediabetes). Exclusion criteria included: pregnancy, planned pregnancy or breastfeeding; or uncontrolled hypo‐ or hyperthyroidism. |
| Interventions | Intervention ‐ Reduced‐carbohydrate diet: response assessed at four weeks on a State of Slim (SOS) weight management programme combined with a reduced‐carbohdyrate (RC) diet; responders to continue and non‐responders re‐randomised to RC with time‐restricted eating (TRE) or RC with structured exercise counselling sessions. Control ‐ High‐carbohydrate diet: response assessed at four weeks on a State of Slim (SOS) weight management programme combined with a high‐carbohdyrate (HC) diet; responders to continue and non‐responders re‐randomised to HC with time‐restricted eating (TRE) or HC with structured exercise counselling sessions |
| Outcomes | Primary outcomes: 1) change in body weight, 2) change in glucose, 3) change in insulin, 4) change in lipids, 5) change in fat mass, 6) changes in QUICKI index (predictor of insulin resistance) Secondary outcomes: 1) change in overall strength, 2) change in aerobic fitness, 3) diet intake and physical activity monitoring |
| Starting date | May 2021 |
| Contact information | Chelsi Reynolds, cnreynolds@uabmc.edu and Drew Sayer, sayerd@uab.edu |
| Notes | Prospectively registered |
BMI: body mass index CHO: carbohydrate DASH: Dietary Approaches to Stop Hypertension DNA: deoxyribonucleic acid DPP‐GLB: Diabetes Prevention Program‐Group Lifestyle Balance GFR: glomerular filtration rate GLOH: Giving a low carbohydrate diet to overcome hypertension (trial acronym) GLP‐1: glucagon‐like peptide‐1 HbA1c: haemoglobin A1c/glycated haemoglobin HC: high‐carbohydrate HDL: high‐density lipoprotein(‐cholesterol) HP: high‐protein LDL: low‐density lipoprotein(‐cholesterol) LION: Lifestyle intervention study for weight loss maintenance (trial acronym) MED: Mediterranean MUFA: monounsaturated fatty acid NYHA: New York Heart Association OTC: over‐the‐counter PUFA: polyunsaturated fatty acid RC: reduced‐carbohydrate SD: standard deviation SGLT: sodium‐glucose cotransporter SOS: State of Slim T2D: type 2 diabetes TRE: time‐restricted eating TSH: thyroid stimulating hormone VLC: very low‐carbohydrate WC: waist circumference WELCOME: Diet for the maintenance of weight loss and metabolic health in obese postmenopausal women (trial acronym)
Differences between protocol and review
We added the outcome, change in weight at three to < 12 months, to the list of outcomes for inclusion in the summary of findings tables. After inputs from clinicians, recent studies and people wanting to follow weight‐reduction diets, we considered short‐term weight reduction to be a clinician‐ and patient‐important outcome.
We included four comparisons instead of two as stated in the protocol. This was done in order to separate findings where people implemented only an active weight‐reducing phase of the diets, from findings where people implemented a weight‐reducing phase followed by weight‐maintenance phases of the diets (periods where initial diet prescriptions or advice changed to indicate less focus on active weight reduction, for example, changes in energy prescriptions or no restrictions on carbohydrate intake).
We changed one of the sensitivity analyses from "attrition bias (i.e. first pool all relevant studies per outcome, and then pool only studies with < 15% missing data from the total initial sample)" to "first pool all relevant studies per outcome, and then pool only studies with low or some concerns for bias due to missing outcome data (domain 3 in the Cochrane Risk of Bias 2 tool)."
We excluded the adherence assessment detailed in our protocol as we did not deem it relevant for a review aiming to assess assignment to intervention.
Contributions of authors
CN and AB drafted the review and AS, KN, MR and JV provided inputs to finalise the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
Foreign, Commonwealth and Development Office, UK
Project number 300342‐104
Declarations of interest
CN: Celeste is partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies. No known conflicts of interest.
AB: Amanda is partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies. No known conflicts of interest.
AS: Anel is partly supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government's official policies. No known conflicts of interest.
KN: No known conflicts of interest.
MC: No known conflicts of interest.
JV: No known conflicts of interest.
New
References
References to studies included in this review
Aude 2004 {published data only}
- Aude Y, Agatston A, Lopez-Jimenez F, Lieberman E, Almon M, Hansen M, et al.The National Cholesterol Education Program Diets a diet lower in carbohydrates and higher in protein and monounsaturated fat. Archives of Internal Medicine 2004;164:2141-6. [DOI] [PubMed] [Google Scholar]
Bales 2017 {published data only}
- Bales C, Porter Starr K, Orenduff M, McDonald S, Molnar K, Jarman A, et al.Influence of protein intake, race and age on responses to a weight-reduction intervention in obese women. Current Developmens in Nutrition 2017;1(5):1-10. [NCT02033655] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bazzano 2014 {published data only}
- Bazzano L, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, et al.Effects of low-carbohydrate and low-fat diets: a randomized trial. Annals of Internal Medicine 2014;161(5):309-18. [NCT00609271] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benassi‐Evans 2009 {published data only}
- Benassi-Evans B, Clifton P, Noakes M, Keogh J, Fenech M.High protein–high red meat versus high carbohydrate weight loss diets do not differ in effect on genome stability and cell death in lymphocytes of overweight men. Mutagenesis 2009;24(3):271-7. [DOI: 10.1093/mutage/gep006] [DOI] [PubMed] [Google Scholar]
Brehm 2003 {published data only}
- Brehm B, Seeley R, Daniels S, D'Alessio D.A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. Journal of Clinical Endocrinology & Metabolism 2003;88(4):1617-23. [DOI: 10.1210/jc.2002-021480] [DOI] [PubMed] [Google Scholar]
- NCT00498394.Effects of a ketogenic diet on body weight and cardiovascular risk factors. https://clinicaltrials.gov/ct2/show/NCT00498394 (first received 10 July 2007).
- O’Brien K, Brehm B, Seeley R, Bean J, Wener M, Daniels S, et al.Diet-induced weight loss is associated with decreases in plasma serum amyloid A and C-reactive protein independent of dietary macronutrient composition in obese subjects. Journal of Clinical Endocrinology & Metabolism 2005;90(4):2244-9. [DOI: 10.1210/jc.2004-1011] [DOI] [PubMed] [Google Scholar]
- Summer SS, Brehm BJ, Benoit SC, D'Alessio DA.Adiponectin changes in relation to the macronutrient composition of a weight-loss diet. Obesity 2011;19:2198-204. [DOI: 10.1038/oby.2011.60] [DOI] [PubMed] [Google Scholar]
Brehm 2005 {published data only}
- Brehm B, Spang S, Lattin B, Seeley R, Daniels S, D'Alessio D.The role of energy expenditure in the differential weight loss in obese women on low-fat and low carbohydrate diets. Journal of Clinical Endocrinology and Metabolism 2005;90(3):1475-82. [DOI: 10.1210/jc.2004-1540] [DOI] [PubMed] [Google Scholar]
- Summer S, Brehm B, Benoit S, D'Alessio D.Adiponectin changes in relation to the macronutrient composition of a weight-loss diet. Obesity 2011;19(11):2198-204. [DOI: 10.1038/oby.2011.60] [DOI] [PubMed] [Google Scholar]
Calleja‐Fernández 2012 {published data only (unpublished sought but not used)}
- Ballesteros-Pomar MD, Calleja-Fernández AR, Vidal-Casariego A, Urioste-Fondo AM, Cano-Rodríguez I.Effectiveness of energy-restricted diets with different protein:carbohydrate ratios: the relationship to insulin sensitivity. Public Health Nutrition 2009;13(12):2119-26. [DOI: 10.1017/S1368980009991881] [DOI] [PubMed] [Google Scholar]
- Calleja-Fernández A, Vidal-Casariego A, Cano-Rodríguez I, Ballesteros-Pomar MD.One-year effectiveness of two hypocaloric diets with different protein/carbohydrate ratios in weight loss and insulin resistance. Nutricion Hospitalaria 2012;27:2093-101. [DOI: 10.3305/nh.2012.27.6.6133] [DOI] [PubMed] [Google Scholar]
Cornier 2005 {published data only}
- Cornier M-A, Donahoo W, Pereira R, Gurevich I, Westergren R, Enerback S, et al.Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obesity Research 2005;13(4):703-9. [DOI] [PubMed] [Google Scholar]
Dyson 2007 {published and unpublished data}
- Brand A.Request for additional information: Dyson et al 2010 [personal communication]. Email to: P Dyson 11 June 2020.
- Dyson P, Beatty S, Matthews D.A low-carbohydrate diet is more effective in reducing body weight than healthy eating in both diabetic and non-diabetic subjects. Diabetic Medicine 2007;24:1430-5. [DOI: 10.1111/j.1464-5491.2007.02290.x] [DOI] [PubMed] [Google Scholar]
- Dyson P, Beatty S, Matthews D.An assessment of low-carbohydrate or low-fat diets for weight loss at 2 year’s follow-up. Diabetic Medicine 2010;27:363-8. [DOI: 10.1111/j.1464-5491.2010.02926.x] [DOI] [PubMed] [Google Scholar]
Ebbeling 2007 {published and unpublished data}
- Brand A.Request for additional information: Ebbeling et al 2007 [personal communication]. Email to: D. Ludwig 18 February 2020.
- Ebbeling C, Leidig M, Feldman H, Lovesky M, Ludwig D.Effects of a low–glycemic load vs low-fat diet in obese young adults: a randomized trial. Journal of the American Medical Association 2007;297(19):2092-102. [DOI] [PubMed] [Google Scholar]
- NCT00130299.An 18-month trial of a low glycemic load diet [An 18-month randomized controlled trial of a low glycemic load diet]. https://clinicaltrials.gov/ct2/show/NCT00130299 (first received 15 August 2005).
Elhayany 2010 {published data only (unpublished sought but not used)}
- Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S.A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes, Obesity and Metabolism 2010;12(3):204-9. [DOI: 10.1111/j.1463-1326.2009.01151.x] [DOI] [PubMed] [Google Scholar]
Evangelista 2021 {published data only}
- Evangelista LS, Jose MM, Sallam H, Serag H, Golovko G, Khanipov K, et al.High-protein vs. standard-protein diets in overweight and obese patients with heart failure and diabetes mellitus: findings of the Pro-HEART trial. European Society of Cardiology Heart Failure 2021;8(2):1342-8. [DOI: 10.1002/ehf2.13213.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motie M, Evangelista LS, Horwich T, Hamilton M, Lombardo D, Cooper DM, et al.Pro-HEART - a randomized clinical trial to test the effectiveness of a high protein diet targeting obese individuals with heart failure: rationale, design and baseline characteristics. Contemporary Clinical Trials 2013;36(2):371-81. [DOI: 10.1016/j.cct.2013.08.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01423266.Effects of a high protein diet on clinical outcomes in heart failure [Examining the effects of a high protein diet on adiposity and cardiac structure in patients with heart failure, obesity, and diabetes mellitus]. https://clinicaltrials.gov/ct2/show/NCT01423266 (first received 25 August 2011).
Farnsworth 2003 {published and unpublished data}
- A Schoonees.Luscombe et al 2003 [personal communication]. Email to: N. Luscombe-Marsh N 19 January 2017.
- Brand A.Request for additional information: Farnsworth et al 2003 [personal communication]. Email to: P. Clifton 4 March 2020.
- Brinkworth G, Noakes M, Keogh J, Luscombe N, Wittert G, Clifton P.Long-term effects of a high-protein, low-carbohydrate diet on weight control and cardiovascular risk markers in obese hyperinsulinemic subjects. International Journal of Obesity 2004;28:661-70. [DOI: 10.1038/sj.ijo.0802617] [DOI] [PubMed] [Google Scholar]
- Farnsworth E, Luscombe N, Noakes M, Wittert G, Argyiou E, Clifton P.Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in 13 overweight and obese hyperinsulinemic men and women. American Journal of Clinical Nutrition 2003;78:31-9. [DOI] [PubMed] [Google Scholar]
Foraker 2014 {published and unpublished data}
- Brand A.Request for additional information: Foraker et al 2014 [personal communication]. Email to: M. Pennell 15 July 2020.
- Foraker RE, Pennell M, Sprangers P, Vitolins MZ, De Graffinreid C, Paskett ED.Effect of a low-fat or low-carbohydrate weight-loss diet on markers of cardiovascular risk among premenopausal women: a randomized trial. Journal of Women's Health 2014;23(8):675-80. [DOI: 10.1089/jwh.2013.4638] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos AA, Krok JL, Peng J, Pennell ML, Olivo-Marston S, Vitolins MZ, et al.Favorable effects of low-fat and low-carbohydrate dietary patterns on serum leptin, but not adiponectin, among overweight and obese premenopausal women: a randomized trial. Springer Plus 2014;3:175. [DOI: 10.1186/2193-1801-3-175] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01559194.Low fat versus protein sparing diet for weight loss & impact on biomarkers associated with breast cancer risk (LEAF) [A randomized comparison of a low fat or low carbohydrate dietary pattern for weight loss and impact on biomarkers associated with breast cancer risk in overweight and obese premenopausal women: lifestyle eating and fitness]. https://clinicaltrials.gov/ct2/show/NCT01559194 (first received 21 March 2012).
- Olivo-Marston S, Grainger E, Bittoni M, Pennell M, Vitolins MZ, De Graffinreid CR, et al.Biomarkers of breast cancer risk in a randomized trial of a low-fat or low-carbohydrate weight loss intervention and physical activity among overweight and obese premenopausal women. Cancer Research 2012;72(8 Supplement):4456. [DOI: 10.1158/1538-7445.AM2012-4456] [DOI] [Google Scholar]
Foster 2003 {published data only (unpublished sought but not used)}
- Foster G, Wyatt HO, Hill J, McGuckin B, Brill C, Mohammed S, et al.A randomized trial of a low-carbohydrate diet for obesity. New English Journal of Medicine 2003;348:2082-90. [DOI] [PubMed] [Google Scholar]
Foster 2010 {published data only (unpublished sought but not used)}
- Foster G, Wyatt H, Hill J, Makris A, Rosenbaum D, Brill C, et al.Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet. Annals of Internal Medicine 2010;153:147-57. [NCT00143936] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Rosenbaum D, Han H, Geiselman P, Wyatt H, Hill J, et al.Change in food cravings, food preferences, and appetite during a low-carbohydrate and low-fat diet. Obesity 2011;19(10):1963-70. [DOI: 10.1038/oby.2011.62] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frisch 2009 {published data only}
- Frisch S, Zittermann A, Berthold H, Götting C, Kuhn J, Kleesiek K, et al.A randomized controlled trial on the efficacy of carbohydrate-reduced or fat-reduced diets in patients attending a telemedically guided weight loss program. Cardiovascular Diabetology 2009;8:36. [DOI: 10.1186/1475-2840-8-36] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 2007 {published and unpublished data}
- Brand A.Request for additional information: Gardner et al 2007 [personal communication]. Email to: C. Gardner 12 February 2020.
- Gardner C, Kiazand A, Alhassan S, Kim S, Stafford R, Balise, R, et al.Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A to Z weight loss study: a randomized trial. Journal of the American Medical Association 2007;297(9):969-77. [DOI] [PubMed] [Google Scholar]
Goni 2018 {published data only}
- Goni L, Riezu-Boj J, Milagro F, Corrales F, Ortiz L, Cuervo M, et al.Interaction between an ADCY3 genetic variant and two weight-lowering diets affecting body fatness and body composition outcomes depending on macronutrient distribution: A randomized trial. Nutrients May 2018;10(789):1-10. [DOI: 10.3390/nu10060789] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Goni L, Cuervo M, Martinez JA.Differential lipid metabolism outcomes associated with ADRB2 gene polymorphisms in response to two dietary interventions in overweight/obese subjects. Nutrition, Metabolism & Cardiovascular Diseases 2018;28:165-72. [DOI: 10.1016/j.numecd.2017.11.006] [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lozada C, Cuervo M, Cuevas-Sierra A, Goni L, Riezu-Boj JI, Navas-Carretero S, et al.Changes in anxiety and depression traits induced by energy restriction: predictive value of the baseline status. Nutrients 2019;11(6):1206. [DOI: 10.3390/nu11061206] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 2013 {published data only}
- Cheng H, Griffin H, Claes B, Petocz P, Steinbeck K, Rooney K, et al.Influence of dietary macronutrient composition on eating behaviour and self-perception in young women undergoing weight management. Eating and Weight Disorders 2014;19:241–7. [10.1007/s40519-014-0110-y] [DOI] [PubMed] [Google Scholar]
- Griffin H, Cheng H, O’Connor H, Rooney K, Petocz P, Steinbeck K.Higher protein diet for weight management in young overweight women: a 12-month randomized controlled trial. Diabetes, Obesity and Metabolism 2013;15(6):572-5. [DOI: 10.1111/dom.12056] [DOI] [PubMed] [Google Scholar]
Guldbrand 2012 {published data only}
- Guldbrand H, Dizdar B, Bunjaku B, Lindström T, Bachrach-Lindström M, Fredrikson M.In type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss. Diabetologia 2012;55:2118-27. [DOI: 10.1007/s00125-012-2567-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L, Guldbrand H, Lundberg AK, Nystrom FH.Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Annals of Medicine 2014;46(3):182-7. [DOI: 10.3109/07853890.2014.894286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haufe 2013 {published and unpublished data}
- Brand A.Request for additional information: Haufe et al 2011 [personal communication]. Email to: S. Haufe 6 August 2019.
- Brand A.Request for additional information: Haufe et al 2013 [personal communication]. Email to: S. Haufe 17 February 2020.
- Haufe S, Engeli S, Kaminski J, Witt H, Rein D, Kamlage B, et al.Branched-chain amino acid catabolism rather than amino acids plasma concentrations is associated with diet-induced changes in insulin resistance in overweight to obese individuals. Nutrition, Metabolism & Cardiovascular Diseases 2017;27(10):858-64. [DOI: 10.1016/j.numecd.2017.07.001] [DOI] [PubMed] [Google Scholar]
- Haufe S, Engeli S, Kast P, Böhnke J, Utz W, Haas V, et al.Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 2011;53:1504-14. [DOI: 10.1002/hep.24242] [DOI] [PubMed] [Google Scholar]
- Haufe S, Mähler A, Haas V, Luft F, Utz W, Schultz-Menger J, et al.Long-lasting improvements in liver fat and metabolism despite body weight regain after dietary weight loss. Diabetes Care 2013;36:3786-92. [DOI: 10.2337/dc13-0102] [NCT00956566] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00956566.Comparison of low fat and low carbohydrate diets with respect to weight loss and metabolic effects (B-SMART). https://clinicaltrials.gov/ct2/show/NCT00956566 (first received 11 August 2009).
Hockaday 1978 {published data only}
- Hockaday T, Hockaday J, Mann J, Turner R.Prospective comparison of modified-fat-high-carbohydrate with standard low-carbohydrate dietary advice in the treatment of diabetes: one year follow-up study. British Journal of Nutrition 1978;39:357-62. [DOI] [PubMed] [Google Scholar]
Jesudason 2013 {published data only}
- Jesudason D, Nordin B, Keogh J, Clifton P.Comparison of 2 weight-loss diets of different protein content on bone health: a randomized trial. American Journal of Clinical Nutrition 2013;98:1343-52. [DOI: 10.3945/ajcn.113.058586] [ACTRN12608000229370.] [DOI] [PubMed] [Google Scholar]
Josse 2011 {published and unpublished data}
- Brand A.Request for additional information: Josse et al 2011 [personal communication]. Email to: A Josse 4 March 2020.
- Josse A, Atkinson S, Tarnopolsky M, Phillip S.Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. Journal of Nutrition 2011;141(9):1626-34. [DOI: 10.3945/jn.111.141028] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM.Diets higher in dairy foods and dietary protein support bone health during diet- and exercise-induced weight loss in overweight and obese premenopausal women. Journal of Clinical Endocrinology and Metabolism 2012;97(1):251-60. [DOI: 10.1210/jc.2011-2165] [DOI] [PubMed] [Google Scholar]
- NCT00710398."Improving diet, exercise and lifestyle (IDEAL) for women" study [The impact of higher dairy and dietary protein on the "quality" of hypoenergetic diet- and exercise-induced weight loss in pre-menopausal, overweight and obese young women]. https://clinicaltrials.gov/ct2/show/NCT00710398 (first received 4 July 2008).
Juanola‐Falgarona 2014 {published and unpublished data}
- Brand A.Request for additional information: Juanola-Falgarona et al 2014 [personal communication]. Email to: M. Bullo 17 April 2020.
- Juanola-Falgarona M, Ibarrola-Jurado N, Salas-Salvadó J, Rabassa-Soler A, Bulló M.Design and methods of the GLYNDIET study; assessing the role of glycemic index on weight loss and metabolic risk markers. Nutrición Hospitalaria 2013;28(2):382-90. [DOI] [PubMed] [Google Scholar]
- Juanola-Falgarona M, Salas-Salvadó J, Ibarrola-Jurado N, Rabassa-Soler A, Díaz-Lopez A, Guasch-Ferre ´ M, et al.Effect of the glycemic index of the diet on weight loss, modulation of satiety, inflammation, and other metabolic risk factors: a randomized controlled trial. American Journal of Clinical Nutrition 2014;100:27-35. [ISRCTN54971867] [DOI] [PubMed] [Google Scholar]
Keogh 2007 {published and unpublished data}
- Brand A.Request for additional information: Keogh et al 2007 [personal communication]. Email to: J. Keogh 29 April 2020.
- Keogh J, Brinkworth G, Clifton P.Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. British Journal of Nutrition 2007;98:852-9. [DOI: 10.1017/S0007114507747815] [DOI] [PubMed] [Google Scholar]
Kitabchi 2013 {published data only}
- Kitabchi A, McDaniel K, Wan J, Tylavsky F, Jacovino C, Sands C, et al.Effects of high-protein versus high-carbohydrate diets on markers of b-cell function, oxidative stress, lipid peroxidation, proinflammatory cytokines, and adipokines in obese, premenopausal women without diabetes. Diabetes Care 2013;36:1919-25. [DOI: 10.2337/dc12-1912] [NCT0164284] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klemsdal 2010 {published data only}
- Klemsdal T, Holme I, Nerland H, Pedersen T, Tonstad S.Effects of a low glycemic load diet versus a low-fat diet in subjects with and without the metabolic syndrome. Nutrition, Metabolism & Cardiovascular Diseases 2010;20:195-201. [DOI: 10.1016/j.numecd.2009.03.010] [DOI] [PubMed] [Google Scholar]
Krebs 2012 {published data only}
- Krebs J, Elley C, Parry-Strong A, Lunt H, Drury P, Bell D, et al.The Diabetes Excess Weight Loss (DEWL) trial: a randomised controlled trial of high-protein versus high-carbohydrate diets over 2 years in type 2 diabetes. Diabetologia 2012;55:905-14. [DOI: 10.1007/s00125-012-2461-0] [DOI] [PubMed] [Google Scholar]
Landers 2002 {published data only}
- Landers P, Wolfe M, Glore S, Guild R, Phillips L.Effetct of weight loss plans on body composition and diet duration. Journal of the Oklahoma State Medical Association 2002;95(5):329-31. [PubMed] [Google Scholar]
Larsen 2011 {published and unpublished data}
- Brand A.Request for additional information: Larsen et al 2011 [personal communication]. Email to: R. Larsen 2 March 2020.
- Larsen R, Mann N, Maclean E, Shaw J.The effect of high-protein, low-carbohydrate diets in the treatment of type 2 diabetes: a 12 month randomised controlled trial. Diabetologia 2011;54:731-40. [DOI: 10.1007/s00125-010-2027-y] [DOI] [PubMed] [Google Scholar]
Lasker 2008 {published data only}
- Lasker D, Evans E, Layman D.Moderate carbohydrate, moderate protein weight loss diet reduces cardiovascular disease risk compared to high carbohydrate, low protein diet in obese adults: a randomized clinical trial. Nutrition and Metabolism 2008;5:30. [DOI: 10.1186/1743-7075-5-30] [DOI] [PMC free article] [PubMed] [Google Scholar]
Layman 2005 {published data only}
- Layman D, Evans E, Baum J, Seyler J, Erickson D, Boileau R.Dietary protein and exercise have additive effects on body composition during weight loss in adult women. Journal of Nutrition 2005;135:1903-10. [DOI] [PubMed] [Google Scholar]
Layman 2009 {published data only (unpublished sought but not used)}
- Evans E, Mojtahedi M, Thorpe M, Valentine R, Kris-Etherton P, Layman D.Effects of protein intake and gender on body composition changes: a randomized clinical weight loss trial. Nutrition and Metabolism 2012;9:55. [DOI: 10.1186/1743-7075-9-55] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman D, Evans E, Erickson D, Seyler J, Weber J, Bagshaw D, et al.A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. Journal of Nutrition 2009;139:514-21. [DOI: 10.3945/jn.108.099440] [DOI] [PubMed] [Google Scholar]
Lean 1997 {published data only}
- Lean M, Han T, Prvan T, Richmond P, Avenell A.Weight loss with high and low carbohydrate 1200 kcal diets in free living women. European Journal of Clinical Nutrition 1997;51:243-8. [DOI] [PubMed] [Google Scholar]
Lim 2010 {published and unpublished data}
- Brand A.Request for additional information: Lim et al 2010 [personal communication]. Email to: S. Lim 3 August 2020.
- Lim S, Noakes M, Keogh J, Clifton P.Long-term effects of a low carbohydrate, low fat or high unsaturated fat diet compared to a no-intervention control. Nutrition, Metabolism & Cardiovascular Diseases 2010;20:599-607. [DOI: 10.1016/j.numecd.2009.05.003] [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only (unpublished sought but not used)}
- Liu X, Zhang G, Ye X, Li H, Chen X, Tang L, et al.Effects of a low-carbohydrate diet on weight loss and cardiometabolic profile in Chinese women: a randomised controlled feeding trial. British Journal of Nutrition 2013;110:1444-53. [DOI: 10.1017/S0007114513000640] [DOI] [PubMed] [Google Scholar]
- NCT01358890.Low-carbohydrate diet intervention on body weight [Effect of low-carbohydrate or restricted-calories diet on body weight]. https://clinicaltrials.gov/ct2/show/NCT01358890 (first posted 24 May 2011).
Marco‐Benedi 2019 {published and unpublished data}
- Brand A.Request for additional information: Marco-Benedi et al 2019 [personal communication]. Email to: R. Mateo-Gallego 8 April 2020.
- Marco-Benedí V, Perez-Calahorra S, Bea A, Lamiquiz-Moneo I, Baila-Rueda L, Cenarro A, et al.High-protein energy-restricted diets induce greater improvement in glucose homeostasis but not in adipokines comparing to standard-protein diets in early-onset diabetic adults with overweight or obesity. Clinical Nutrition 2020;39(5):1354-63. [DOI: 10.1016/j.clnu.2019.06.005] [DOI] [PubMed] [Google Scholar]
- NCT02559479.A study to assess the effect of a normal vs. high protein diets in carbohydrates metabolism in obese subjects with diabetes or prediabetes. https://clinicaltrials.gov/ct2/show/NCT02559479 (first posted 24 September 2015).
Mateo‐Gallego 2017 {published and unpublished data}
- Brand A.Request for additional information: Mateo-Gallego et al 2017 [personal communication]. Email to: R. Mateo-Gallego 3 March 2020.
- Mateo-Gallego R, Lamiquiz-Moneo I, Perez-Calahorra S, Marco-Benedí V, Bea A, Baila-Rueda L, et al.Different protein composition of low-calorie diet differently impacts adipokine profile irrespective of weight loss in overweight and obese women. Nutrition, Metabolism and Cardiovascular Diseases 2018;28:133-42. [DOI: 10.1016/j.numecd.2017.10.024] [DOI] [PubMed] [Google Scholar]
- Mateo-Gallego R, Marco-Benedi V́, Perez-Calahorra S, Bea A, Baila-Rueda L, Lamiquiz-Moneo I, et al.Energy-restricted, high-protein diets more effectively impact cardiometabolic profile in overweight and obese women than lower-protein diets. Clinical Nutrition 2017;36:371-9. [DOI: 10.1016/j.clnu.2016.01.018] [DOI] [PubMed] [Google Scholar]
Mellberg 2014 {published data only (unpublished sought but not used)}
- Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C, et al.Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a two-year randomized trial. European Journal of Clinical Nutrition 2014;68(3):350-7. [DOI: 10.1038/ejcn.2013.290.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ooi 2021 {published data only}
- Ooi DS, Ling JQ, Sadananthan SA, Velan SS, Ong FY, Khoo CM, et al.Branched-chain amino acid supplementation does not preserve lean mass or affect metabolic profile in adults with overweight or obesity in a randomized controlled weight loss intervention. Journal of Nutrition 2021;151(4):911-20. [DOI: 10.1093/jn/nxaa414.] [DOI] [PubMed] [Google Scholar]
Parr 2016 {published data only}
- Parr E, Coffey V, Cato L, Phillips S, Burke L, Hawley J.A randomized trial of high-dairy-protein, variable-carbohydrate diets and exercise on body composition in adults with obesity. Obesity 2016;24:1035-45. [DOI: 10.1002/oby.21451] [DOI] [PubMed] [Google Scholar]
Pedersen 2014 {published and unpublished data}
- Brand A.Request for additional information: Pedersen, Jesudason & Clifton 2014 [personal communication]. Email to: P. Clifton 26 February 2020.
- Pedersen E, Jesudason D, Clifton P.High protein weight loss diets in obese subjects with type 2 diabetes mellitus. Nutrition, Metabolism & Cardiovascular Diseases 2014;24:554-62. [DOI: 10.1016/j.numecd.2013.11.003] [DOI] [PubMed] [Google Scholar]
Pittas 2005 {published data only (unpublished sought but not used)}
- Pittas A, Das S, Hajduk C, Golden J, Saltzman E, Stark P, et al.A low–glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE trial. Diabetes Care 2005;28(12):2939-41. [DOI] [PubMed] [Google Scholar]
Racette 1995 {published data only (unpublished sought but not used)}
- Racette S, Schoeller D, Kushner R, Neil K, Herling-laffalda K.Effects of aerobic exercise and dietary carbohydrate on energy expenditure and body composition during weight reduction in obese women. American Journal of Clinical Nutrition 1995;61:486-94. [DOI] [PubMed] [Google Scholar]
Ruth 2013 {published data only (unpublished sought but not used)}
- Ruth M, Port A, Shah M, Bourland A, Istfan N, Nelson K, et al.Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C-reactive protein, and raises serum adiponectin and high density lipoprotein-cholesterol in obese subjects. Metabolism 2013;62(12):1-17. [DOI: 10.1016/j.metabol.2013.07.006.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sacks 2009 {published and unpublished data}
- Brand A.Request for additional information: Sacks et al 2009 [personal communication]. Email to: F. Sacks 28 July 2020.
- De Jonge L, Bray GA, Smith SR, Ryan DH, De Souza R, Loria CM, et al.Effect of diet composition and weight loss on resting energy expenditure in the POUNDS LOST study. Obesity 2012;20(12):2384-9. [DOI: 10.1038/oby.2012.127] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naude C.Data [personal communication]. Email to: F. Sacks 9 November 2012.
- Sacks F, Bray G, Carey V, Smith S, Ryan D, Anton S, et al.Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. New English Journal of Medicine 2009;360(9):859-73. [NCT00072995] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DA, Anton SD, Han H, Champagne CM, Allen R, Leblanc E, et al.Early behavioral adherence predicts short and long-term weight loss in the POUNDS LOST study. Journal of Behavioral Medicine 2010;33(4):305-14. [DOI: 10.1007/s10865-010-9253-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samaha 2003 {published data only}
- Samaha F, Iqbal N, Seshadri P, Chicano K, Daily D, McGrory J, et al.A low-carbohydrate as compared with a low-fat diet in severe obesity. New English Journal of Medicine 2003;348(21):2074-81. [DOI] [PubMed] [Google Scholar]
- Seshadri P, Samaha FF, Stern L, Ahima RS, Daily D, Iqbal N.Adipocytokine changes caused by low-carbohydrate compared to conventional diets in obesity. Metabolic Syndrome and Related Disorders 2005;3(1):66-74. [DOI: 10.1089/met.2005.3.66] [DOI] [PubMed] [Google Scholar]
- Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al.The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Annals of Internal Medicine 2004;140(10):778-85. [DOI: 10.7326/0003-4819-140-10-200405180-00007] [DOI] [PubMed] [Google Scholar]
Saslow 2017a {published data only (unpublished sought but not used)}
- Saslow L, Daubenmier J, Moskowitz J, Kim S, Murphy E, Phinney S, et al.Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutrition and Diabetes 2017;7(304):1-7. [DOI: 10.1038/s41387-017-0006-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslow L, Kim S, Daubenmier J, Moskowitz J, Phinney S, Goldman V, et al.A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLOS One 2014;9(4):e91027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 2017 {published and unpublished data}
- Brand A.Request for additional information: Sato et al 2017 [personal communication]. Email to: J. Sato 29 May 2020.
- Sato J, Kanazawa A, Hatae C, Makita S, Komiya K, Shimizu T, et al.One year follow-up after a randomized controlled trial of a 130 g/day low-carbohydrate diet in patients with type 2 diabetes mellitus and poor glycemic control. PLOS ONE 2017;12(12):e0188892. [DOI: 10.1371/ journal.pone.0188892] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J, Kanazawa A, Makita S, Hatae C, Komiya K, Shimizu T, et al.A randomized controlled trial of 130 g/day low-carbohydrate diet in type 2 diabetes with poor glycemic control. Clinical Nutrition 2017;36:992-1000. [DOI: 10.1016/j.clnu.2016.07.003] [DOI] [PubMed] [Google Scholar]
Stentz 2016 {published data only (unpublished sought but not used)}
- NCT01642849.Effect of diet composition on weight change and metabolic parameters. https://clinicaltrials.gov/ct2/show/NCT01642849 (first posted 17 July 2012).
- Stentz F, Brewer A, Wan J, Garber C, Daniels B, Sands C, et al.Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: randomized control trial. British Medical Journal 2016;4:1-9. [DOI: 10.1136/bmjdrc-2016- 000258] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tay 2008 {published data only (unpublished sought but not used)}
- Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ.Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Archives of Internal Medicine 2009;169(20):1873-80. [DOI: 10.1001/archinternmed.2009.329] [DOI] [PubMed] [Google Scholar]
- Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM.Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. American Journal of Clinical Nutrition 2009;90:23-32. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Wilson CJ, Buckley JD, Noakes M, Clifton PM, Brinkworth GD.Changes in endothelial function and depression scores are associated following long-term dietary intervention: a secondary analysis. Nutrition 2013;29(10):1271-4. [DOI: 10.1016/j.nut.2013.03.023] [DOI] [PubMed] [Google Scholar]
- Tay J, Brinkworth G, Noakes M, Keogh J, Clifton P.Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. Journal of the American College of Cardiology 2008;51(1):59-67. [DOI: 10.1016/j.jacc.2007.08.050] [DOI] [PubMed] [Google Scholar]
- Wycherley T, Brinkworth G, Keogh J, Noakes M, Buckley J, Clifton P.Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients. Journal of Internal Medicine 2010;267(5):452-61. [DOI] [PubMed] [Google Scholar]
Tay 2014 {published data only}
- Brinkworth GD, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert G, et al.Long-term effects of very low-carbohydrate and high-carbohydrate weight-loss diets on psychological health in obese adults with type 2 diabetes: randomized controlled trial. Journal of Internal Medicine 2016;280(4):388-97. [DOI: 10.1111/joim.12501] [DOI] [PubMed] [Google Scholar]
- Tay J, Luscombe-Marsh N, Thompson C, Noakes M, Buckley J, Wittert G, et al.A very low-carbohydrate, low–saturated fat diet for type 2 diabetes management: a randomized trial. Diabetes Care 2014;37:2909-18. [DOI: 10.2337/dc14-0845/-/DC1] [ACTRN12612000369820] [DOI] [PubMed] [Google Scholar]
- Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, et al.Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. American Journal of Clinical Nutrition 2015;102(4):780-90. [DOI: 10.3945/ajcn.115.112581] [DOI] [PubMed] [Google Scholar]
- Tay J, Thompson CH, Luscombe-Marsh ND, Noakes M, Buckley JD, Wittert GA, et al.Long-term effects of a very low carbohydrate compared with a high carbohydrate diet on renal function in individuals with type 2 diabetes. Medicine 2015;94(47):e2181. [DOI: 10.1097/MD.0000000000002181] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay J, Thompson CH, Luscombe-Marsh ND, Wycherley TP, Noakes M, Buckley JD, et al.Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: a 2-year randomized clinical trial. Diabetes, Obesity and Metabolism 2018;20(4):858-71. [DOI: 10.1111/dom.13164] [DOI] [PubMed] [Google Scholar]
- Tay J, Zajac I, Thompson C, Luscombe-Marsh N, Danthiir V, Noakes M, et al.A randomised-controlled trial of the effects of very low-carbohydrate and high-carbohydrate diets on cognitive performance in patients with type 2 diabetes. British Journal of Nutrition 2016;116:1745-53. [DOI] [PubMed] [Google Scholar]
- Wycherley TP, Thompson CH, Buckley JD, Luscombe-Marsh ND, Noakes M, Wittert GA, et al.Long-term effects of weight loss with a very-low carbohydrate, low saturated fat diet on flow mediated dilatation in patients with type 2 diabetes: a randomised controlled trial. Atherosclerosis 2016;252:28-31. [DOI: 10.1016/j.atherosclerosis.2016.07.908] [DOI] [PubMed] [Google Scholar]
Veum 2017 {published data only}
- Veum V, Laupsa-Borge J, Eng Ø, Rostrup E, Larsen T, Nordrehaug J, et al.Visceral adiposity and metabolic syndrome after very high-fat and low-fat isocaloric diets: a randomized controlled trial. American Journal of Clinical Nutrition 2017;105:85-99. [DOI: 10.3945/ajcn.115.123463] [NCT01750021] [DOI] [PubMed] [Google Scholar]
Volek 2009 {published data only}
- Volek J, Phinney S, Forsythe C, Quann E, Wood R, Puglisi M, et al.Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009;44:297-309. [DOI: 10.1007/s11745-008-3274-2] [DOI] [PubMed] [Google Scholar]
Watson 2016 {published data only}
- Watson N, Dyer K, Buckley J, Brinkworth G, Coates A, Parfitt G, et al.Effects of low-fat diets differing in protein and carbohydrate content on cardiometabolic risk factors during weight loss and weight maintenance in obese adults with type 2 diabetes. Nutrients 2016;8(289):1-15. [DOI: 10.3390/nu8050289] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NA, Dyer KA, Buckley JD, Brinkworth GD, Coates AM, Parfitt G, et al.Comparison of two low-fat diets, differing in protein and carbohydrate, on psychological wellbeing in adults with obesity and type 2 diabetes: a randomised clinical trial. Nutrition Journal 2018;17(62):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson NA, Dyer KA, Buckley JD, Brinkworth GD, Coates AM, Parfitt G, et al.Reductions in food cravings are similar with low-fat weight loss diets differing in protein and carbohydrate in overweight and obese adults with type 2 diabetes: a randomized clinical trial. Nutrition Research 2018;57:56-66. [DOI] [PubMed] [Google Scholar]
Westman 2008 {published data only}
- NCT00415688.Lifestyle modification for obesity-related type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT00415688 (first received 25 December 2006).
- Westman E, Yancy W, Mavropoulos J, Marquart M, McDuffie J.The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutrition and Metabolism 2008;5:36. [DOI: 10.1186/1743-7075-5-36] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wycherley 2010 {published data only}
- ACTRN12608000206325.2008 weight loss diets and resistance exercise training in type 2 diabetes study [A randomised study to evaluate high protein diets and resistance exercise training in type 2 diabetes study 2008]. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82626 (first received 11 April 2008).
- Wycherley T, Noakes M, Clifton P, Cleanthous X, Keogh J, Brinkworth G.A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care 2010;33(5):969-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wycherley 2012 {published and unpublished data}
- ACTRN12606000002583.Diet and weight loss in men [The effect of a high protein low carbohydrate compared to a high carbohydrate diet moderate protein diet on weight loss and wellbeing in obese men]. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=516 (first received 12 September 2005).
- Brand A.ACTR No: 126000002583 related to Wycherley et al 2013 [personal communication]. Email to: Australian NZ Clinical Trials Registry 11 August 2020.
- Brand A.Request for additional information: Wycherley et al 2012 and Wycherley et al 2013 [personal communication]. Email to: T. Wycherley 26 August 2020.
- Lutze J, Taylor P, Brinkworth GD, Wyld B, Syrette J, Wilson CJ, et al.Psychological well-being response to high protein and high carbohydrate weight loss diets in overweight and obese men: a randomised trial. e-SPEN Journal 2013;8(6):e232-40. [DOI: 10.1016/j.clnme.2013.08.002] [DOI] [Google Scholar]
- Moran LJ, Brinkworth GD, Martin S, Wycherley TP, Stuckey B, Lutze J, et al.Long-term effects of a randomised controlled trial comparing high protein or high carbohydrate weight loss diets on testosterone, SHBG, erectile and urinary function in overweight and obese men. PLOS ONE 2016;11(9):e0161297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycherley T, Brinkworth G, Clifton P, Noakes M.Comparison of the effects of 52 weeks weight loss with either a high-protein or high-carbohydrate diet on body composition and cardiometabolic risk factors in overweight and obese males. Nutrition and Diabetes 2012;2:1-8. [DOI: 10.1038/nutd.2012.11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycherley TP, Buckley JD, Noakes M, Clifton PM, Brinkworth GD.Comparison of the effects of weight loss from a high-protein versus standard-protein energy-restricted diet on strength and aerobic capacity in overweight and obese men. European Journal of Nutrition 2013;52:317-25. [DOI] [PubMed] [Google Scholar]
Yamada 2014 {published data only}
- Yamada Y, Uchida J, Izumi H, Tsukamoto Y, Inoue G, Watanabe Y, et al.A non-calorie-restricted low-carbohydrate diet is effective as an alternative therapy for patients with type 2 diabetes. Internal Medicine 2014;53:13-9. [DOI: 10.2169/internalmedicine.53.0861] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ben Avraham 2009 {published data only}
- Ben-Avraham S, Harman-Boehm I, Schwarzfuchs D, Shai I.Dietary strategies for patients with type 2 diabetes in the era of multi-approaches; review and results from the Dietary Intervention Randomized Controlled Trial (DIRECT). Diabetes Research and Clinical Practice 2009;86(Suppl 1):S41-8. [DOI: 10.1016/s0168-8227(09)70008-7] [DOI] [PubMed] [Google Scholar]
Bluher 2010 {published data only}
- Blüher M, Rudich A, Klöting N, Golan R, Rubin E, Schwarzfuchs D, et al.Two major patterns of dynamics in adipokine serum concentrations over 2 years of dietary weight-loss intervention. Diabetologia 2010;53(Suppl 1):S367. [DOI: 10.1007/s00125-010-1872-z] [DOI] [Google Scholar]
Bluher 2012 {published data only}
- Blüher M, Rudich A, Klöting N, Golan R, Henkin Y, Rubin E, et al.Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care 2012;35(2):342-9. [DOI: 10.2337/dc11-1267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brehm 2009 {published data only}
- Brehm BJ, Lattin BL, Summer SS, Boback JA, Gilchrist GM, Jandacek RJ, et al.One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care 2009;32(2):215-20. [DOI: 10.2337/dc08-0687] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brun 2011 {published and unpublished data}
- Brun J-F, Fédou C, Raynaud De Mauverger E.More sustained weight loss after a diet moderately enriched in purified protein compared to low fat diet in obese subjects. Clinical Nutrition Supplements 2011;6(1):103. [DOI: 10.1016/S1744-1161(11)70263-0] [DOI] [Google Scholar]
Campbell 2012 {published and unpublished data}
- Campbell DD, Meckling KA.Effect of the protein: carbohydrate ratio in hypoenergetic diets on metabolic syndrome risk factors in exercising overweight and obese women. British Journal of Nutrition 2012;108(9):1658-71. [DOI: 10.1017/s0007114511007215] [DOI] [PubMed] [Google Scholar]
Daly 2006 {published data only}
- Daly ME, Paisey R, Millward BA, Eccles C, Williams K, Hammersley S, et al.Short-term effects of severe dietary carbohydrate-restriction advice in type 2 diabetes - a randomized controlled trial. Diabetic Medicine 2006;23(1):15-20. [DOI: 10.1111/j.1464-5491.2005.01760.x] [DOI] [PubMed] [Google Scholar]
Davis 2009 {published and unpublished data}
- Davis NJ, Tomuta N, Schechter C, Isasi CR, Segal-Isaacson CJ, Stein D, et al.Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care 2009;32(7):1147-52. [DOI: 10.2337/dc08-2108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2012 {published and unpublished data}
- Davis NJ, Tomuta N, Isasi CR, Leung V, Wylie-Rosett J.Diabetes-specific quality of life after a low-carbohydrate and low-fat dietary intervention. Diabetes Educator 2012;38(2):250-5. [DOI: 10.1177/0145721711436132] [DOI] [PubMed] [Google Scholar]
Djuric 2002 {published data only}
- Djuric Z, Lababidi S, Heilbrun LK, Depper JB, Poore KM, Uhley VE.Effect of low-fat and/or low-energy diets on anthropometric measures in participants of the women's diet study. Journal of the American College of Nutrition 2002;21(1):38-46. [DOI: 10.1080/07315724.2002.10719192.] [DOI] [PubMed] [Google Scholar]
Dorling 2020 {published data only}
- Dorling JL, Das SK, Racette SB, Apolzan JW, Zhang D, Pieper CF, et al.Changes in body weight, adherence, and appetite during 2 years of calorie restriction: the CALERIE 2 randomized clinical trial. European Journal of Clinical Nutrition 2020;74(8):1210-20. [DOI: 10.1038/s41430-020-0593-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fabricatore 2011 {published data only}
- Fabricatore AN, Wadden TA, Ebbeling CB, Thomas JG, Stallings VA, Schwartz S, et al.Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Research and Clinical Practice 2011;92(1):37-45. [DOI: 10.1016/j.diabres.2010.12.016.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 2018 {published and unpublished data}
- Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JP, et al.Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA 2018;319(7):667-79. [DOI: 10.1001/jama.2018.0245.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Golan 2012 {published data only}
- Golan R, Tirosh A, Schwarzfuchs D, Harman-Boehm I, Thiery J, Fiedler GM, et al.Dietary intervention induces flow of changes within biomarkers of lipids, inflammation, liver enzymes, and glycemic control. Nutrition 2012;28(2):131-7. [DOI: 10.1016/j.nut.2011.04.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenberg 2009 {published data only}
- Greenberg I, Stampfer MJ, Schwarzfuchs D, Shai I, DIRECT Group.Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT). Journal of the American College of Nutrition 2009;28(2):159-68. [DOI: 10.1080/07315724.2009.10719767.] [DOI] [PubMed] [Google Scholar]
Guo 2019 {published and unpublished data}
- Guo J, Robinson JL, Gardner CD, Hall KD.Objective versus self-reported energy intake changes during low-carbohydrate and low-fat diets. Obesity 2019;27(3):420-6. [DOI: 10.1002/oby.22389] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iqbal 2010 {published data only}
- Iqbal N, Vetter ML, Moore RH, Chittams JL, Dalton-Bakes CV, Dowd M, et al.Effects of a low-intensity intervention that prescribed a low-carbohydrate vs. a low-fat diet in obese, diabetic participants. Obesity 2010;18(9):1733-8. [DOI: 10.1038/oby.2009.460] [DOI] [PubMed] [Google Scholar]
Itsiopoulos 2011 {published data only}
- Itsiopoulos C, Brazionis L, Kaimakamis M, Cameron M, Best JD, O'Dea K, et al.Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutrition, Metabolism & Cardiovascular Diseases 2011;21(9):740-7. [DOI: 10.1016/j.numecd.2010.03.005] [DOI] [PubMed] [Google Scholar]
Jönsson 2009 {published and unpublished data}
- Jönsson T, Granfeldt Y, Ahrén B, Branell U-C, Pålsson G, Hansson A, et al.Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovascular Diabetology 2009;8(35):1-14. [DOI: 10.1186/1475-2840-8-35] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leichtle 2011 {published data only}
- Leichtle AB, Helmschrodt C, Ceglarek U, Shai I, Henkin Y, Schwarzfuchs D, et al.Effects of a 2-y dietary weight-loss intervention on cholesterol metabolism in moderately obese men. American Journal of Clinical Nutrition 2011;94(5):1189‐95. [DOI: 10.3945/ajcn.111.018119] [DOI] [PubMed] [Google Scholar]
Lindeberg 2007 {published and unpublished data}
- Lindeberg S, Jönsson T, Granfeldt Y, Borgstrand E, Soffman J, Sjöström K, et al.A Palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia 2007;50(9):1795-807. [DOI: 10.1007/s00125-007-0716-y] [DOI] [PubMed] [Google Scholar]
Locke 2020 {published data only}
- Locke SR, Falkenhain K, Lowe DA, Lee T, Singer J, Weiss EJ, et al.Comparing the Keyto app and device with Weight Watchers' WW app for weight loss: protocol for a randomized trial. JMIR Research Protocols 2020;9(8):e19053. [DOI: 10.2196/19053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luger 2013 {published data only}
- Luger M, Holstein B, Schindler K, Kruschitz R, Ludvik B.Feasibility and efficacy of an isocaloric high-protein vs. standard diet on insulin requirement, body weight and metabolic parameters in patients with type 2 diabetes on insulin therapy. Experimental and Clinical Endocrinology & Diabetes 2013;121(5):286-94. [DOI: 10.1055/s-0033-1341472] [DOI] [PubMed] [Google Scholar]
Ma 2008 {published data only}
- Ma Y, Olendzki BC, Merriam PA, Chiriboga DE, Culver AL, Li W, et al.A randomized clinical trial comparing low-glycemic index versus ADA dietary education among individuals with type 2 diabetes. Nutrition 2008;24(1):45-56. [DOI: 10.1016/j.nut.2007.10.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIver 2010 {published and unpublished data}
- McIver CM, Clifton PM.High protein weight loss and gene expression in overweight/obese women. Obesity Reviews 2010;11(Suppl 1):97. [Google Scholar]
McIver 2011 {published and unpublished data}
- McIver CM, Clifton PM.High-protein, weight loss down-regulates gene expression involved in fatty acid and mitochondrial energy metabolism. Australasian Medical Journal 2011;4(12):732 . [Google Scholar]
McLaughlin 2016 {published and unpublished data}
- McLaughlin T, Liu L-F, Avery E, Shen W-J, Cohen C, Kraemer F, et al.Effect of low carbohydrate weight loss diet on adipose cell size. Diabetes 2016;65(Suppl 1):A443-4. [DOI: 10.2337/db16-1695-1770] [DOI] [Google Scholar]
Melanson 2012 {published data only}
- Melanson KJ, Summers A, Nguyen V, Brosnahan J, Lowndes J, Angelopoulos TJ, et al.Body composition, dietary composition, and components of metabolic syndrome in overweight and obese adults after a 12-week trial on dietary treatments focused on portion control, energy density, or glycemic index. Nutrition Journal 2012;11(57):1-9. [DOI: 10.1186/1475-2891-11-57] [DOI] [PMC free article] [PubMed] [Google Scholar]
Millward 2014 {published and unpublished data}
- Millward DJ, Truby H, Fox KR, Livingstone MB, Macdonald IA, Tothill P.Sex differences in the composition of weight gain and loss in overweight and obese adults. British Journal of Nutrition 2014;111(5):933-43. [DOI: 10.1017/S0007114513003103] [DOI] [PubMed] [Google Scholar]
Morgan 2009 {published and unpublished data}
- Morgan LM, Griffin BA, Millward DJ, DeLooy A, Fox KR, Baic S, et al.Comparison of the effects of four commercially available weight-loss programmes on lipid-based cardiovascular risk factors. Public Health Nutrition 2009;12(6):799‐807. [DOI: 10.1017/S1368980008003236] [DOI] [PubMed] [Google Scholar]
NCT00200720 {published data only}
- NCT00200720.Effectiveness of a low carbohydrate diet versus a high carbohydrate diet in promoting weight loss and improved health. https://clinicaltrials.gov/ct2/show/NCT00200720 (first received 20 September 2005).
NCT00990457 {published data only}
- NCT00990457.Evaluating the effects two diets combined with exercise in persons with abdominal obesity (the SHAPE5 study). https://clinicaltrials.gov/ct2/show/NCT00990457 (first received 6 October 2009).
Nielsen 2005 {published data only}
- Nielsen JV, Jönsson E, Nilsson A-K.Lasting improvement of hyperglycaemia and bodyweight: low-carbonhydrate diet in type 2 diabetes. A brief report. Upsala Journal of Medical Sciences 2005;110(1):69-74. [DOI: ] [DOI] [PubMed] [Google Scholar]
Reid 2009 {published data only}
- Reid J, Mukhtar R, Fishlock H, Taylor G, Reckless J.The effect of three dietary interventions on PAI-1 among metabolic syndrome subjects. Atherosclerosis Supplements 2009;10(2):e1459. [DOI: 10.1016/S1567-5688(09)71417-5] [DOI] [Google Scholar]
Sakae 2015 {published data only}
- Sakae PN, Bianco HT, Camargo LM, Carvalho JG, Izar MC, Ihara SS, et al.Effect of high protein/very low carbohydrate diet and standard hypocaloric diet in obese subjects: nutritional, biochemical and endothelial function evaluations. Biochimica et Biophysica Acta Clinical 2015;3(Suppl):S7. [DOI: 10.1016/j.bbacli.2015.05.020] [DOI] [Google Scholar]
Saslow 2017b {published data only}
- Saslow L, Mason E, Kim S, Goldman V, Ploutz-Snyder R, Bayandorian H, et al.An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. Journal of Medical Internet Research 2017;19(2):e36. [DOI: 10.2196/jmir.5806.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shai 2008 {published data only}
- Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al.Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. New England Journal of Medicine 2008;359(3):229‐41. [DOI: 10.1056/NEJMoa0708681] [DOI] [PubMed] [Google Scholar]
Shai 2010 {published data only}
- Shai I, Spence JD, Schwarzfuchs D, Henkin Y, Parraga G, Rudich A, et al.Dietary intervention to reverse carotid atherosclerosis. Circulation 2010;121(10):1200-8. [DOI: 10.1161/circulationaha.109.879254] [DOI] [PubMed] [Google Scholar]
Shih 2019 {published and unpublished data}
- Shih CW, Hauser ME, Aronica L, Rigdon J, Gardner CD.Changes in blood lipid concentrations associated with changes in intake of dietary saturated fat in the context of a healthy low-carbohydrate weight-loss diet: a secondary analysis of the Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) trial. American Journal of Clinical Nutrition 2019;109(2):433-41. [DOI: 10.1093/ajcn/nqy305] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2013 {published data only}
- Stewart KJ, Dobrosielski DA, Silber HA, Shapiro EP, Wang N-Y, Ouyang P.Six months of a low-carbohydrate versus low-fat weight loss diet plus exercise: effects on resting and postprandial endothelial function. Circulation 2013;128(Suppl 22):A18552. [DOI: 10.1161/circ.128.suppl_22.A18552] [DOI] [Google Scholar]
Toobert 2003 {published data only}
- Toobert DJ, Glasgow RE, Strycker LA, Barrera M Jnr, Radcliffe JL, Wander RC, et al.Biologic and quality-of-life outcomes from the Mediterranean Lifestyle Program: a randomized clinical trial. Diabetes Care 2003;26(8):2288-93. [DOI: 10.2337/diacare.26.8.2288] [DOI] [PubMed] [Google Scholar]
Trepanowski 2017 {published and unpublished data}
- Trepanowski JF, Gardner CD.Improved insulin sensitivity with a healthy low fat or a healthy low carbohydrate weight loss diet: a twelve month randomized trial. Circulation 2017;135(Suppl 1):AP226. [DOI: 10.1161/circ.135.suppl_1.p226] [DOI] [Google Scholar]
Vetter 2010 {published data only}
- Vetter ML, Wade A, Womble LG, Dalton-Bakes C, Wadden TA, Iqbal N.Effect of a low-carbohydrate diet versus a low-fat, calorie-restricted diet on adipokine levels in obese, diabetic participants. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2010;3:357-61. [DOI: 10.2147/dmsott.s13966] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2012 {published data only}
- Wood RJ, Gregory SM, Sawyer J, Milch CM, Matthews TD, Headley SA.Preservation of fat-free mass after two distinct weight loss diets with and without progressive resistance exercise. Metabolic Syndrome and Related Disorders 2012;10(3):167-74. [DOI: 10.1089/met.2011.0104] [DOI] [PubMed] [Google Scholar]
Wycherley 2013 {published data only}
- Wycherley TP, Buckley JD, Noakes M, Clifton PM, Brinkworth GD.Comparison of the effects of weight loss from a high-protein versus standard-protein energy-restricted diet on strength and aerobic capacity in overweight and obese men. European Journal of Nutrition 2013;52(1):317-25. [DOI: 10.1007/s00394-012-0338-0] [DOI] [PubMed] [Google Scholar]
Yancy 2015 {published and unpublished data}
- Yancy WS Jnr, Mayer SB, Coffman CJ, Smith VA, Kolotkin RL, Geiselman PJ, et al.Effect of allowing choice of diet on weight loss: a randomized trial. Annals of Internal Medicine 2015;162(12):805-14. [DOI: 10.7326/m14-2358] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelicha 2018 {published data only}
- Zelicha H, Schwarzfuchs D, Shelef I, Gepner Y, Tsaban G, Tene L, et al.Changes of renal sinus fat and renal parenchymal fat during an 18-month randomized weight loss trial. Clinical Nutrition 2018;37(4):1145-53. [DOI: 10.1016/j.clnu.2017.04.007] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aller 2019 {published data only}
- Aller R, Izaola O, Primo D, De Luis DA.The effect of single-nucleotide polymorphisms at the ADIPOQ gene locus rs1501299 on metabolic parameters after 9 mo of a high-protein/low-carbohydrate versus a standard hypocaloric diet. Nutrition 2019;65:44-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Cui 2006 {published data only}
- Cui MH, Tomuta V, Jayatillake H, Segal-Isaacson CJ, Hwang JH, Stein DT.Impact of low carbohydrate vs low fat weight loss diets on intramyocellular lipids and insulin sensitivity in obese subjects: a randomized cross over trial. Diabetes 2006;55:A332-3. [Google Scholar]
De Luis 2007 {published data only}
- De Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Bellioo D, Conde R.Effects of a low-fat versus a low-carbohydrate diet on adipocytokines in obese adults. Hormone Research 2007;67(6):296-300. [DOI: ] [DOI] [PubMed] [Google Scholar]
De Luis 2009a {published data only}
- De Luis DA, Aller R, Izaola O, Sagrado MG, Conde R.The effects of a low-fat versus a low carbohydrate diet in obese adults [Efectos de una dieta baja en grasas frente a una dieta rica en proteinas y grasa en pacientes obesos]. Medicina Clínica 2009;132(6):203-7. [DOI: DOI: 10.1016/j.medcli.2008.03.003] [DOI] [PubMed] [Google Scholar]
De Luis 2009b {published data only}
- De Luis DA, Sagrado MG, Conde R, Aller R, Izaola O.The effects of two different hypocaloric diets on glucagon-like peptide 1 in obese adults, relation with insulin response after weight loss. Journal of Diabetes and Its Complications 2009;23(4):239-43. [DOI: 10.1016/j.jdiacomp.2007.12.006] [DOI] [PubMed] [Google Scholar]
De Luis 2010a {published data only}
- De Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Conde R.Effect of two different hypocaloric diets in transaminases and insulin resistance in nonalcoholic fatty liver disease and obese patients. Nutrición Hospitalaria 2010;25(5):730-5. [DOI: 10.3305/nh.2010.25.5.4643] [DOI] [PubMed] [Google Scholar]
De Luis 2010b {published data only}
- De Luis DA, Sagrado MG, Aller R, Izaola O, Conde R.Effects of C358A missense polymorphism of the degrading enzyme fatty acid amide hydrolase on weight loss, adipocytokines, and insulin resistance after 2 hypocaloric diets. Metabolism: Clinical and Experimental 2010;59(9):1387-92. [DOI: 10.1016/j.metabol.2009.12.029] [DOI] [PubMed] [Google Scholar]
De Luis 2012 {published data only}
- De Luis DA, Sagrado MG, Aller R, Conde R, Izaola O, De la Fuente B, et al.Role of G1359A polymorphism of the cannabinoid receptor gene on weight loss and adipocytokines levels after two different hypocaloric diets. Journal of Nutritional Biochemistry 2012;23(3):287-91. [DOI: 10.1016/j.jnutbio.2010.12.006] [DOI] [PubMed] [Google Scholar]
De Luis 2015 {published data only}
- De Luis DA, Aller R, Izaola O, De la Fuente B, Romero E.Genetic variation in the beta-3-adrenoreceptor gene (Trp64arg polymorphism) and their influence on anthropometric parameters and insulin resistance after a high protein/low carbohydrate versus a standard hypocaloric diet [Variación genética en el gen beta-3-adrenorreceptor (Trp64arg polimorfismo) y su influencia en parámetros antropométricos y resistencia a la insulina después de una dieta con alto contenido en proteínas/baja en carbohidratos versus una dieta estándar hipocalórica]. Nutrición Hospitalaria 2015;32(2):487-93. [DOI: 10.3305/nh.2015.32.2.9293.] [DOI] [PubMed] [Google Scholar]
De Luis 2015a {published data only}
- De Luis DA, Aller R, Izaola O, Primo D, Urdiales S, Romero E.Effects of a high-protein/low-carbohydrate diet versus a standard hypocaloric diet on weight and cardiovascular risk factors: role of a genetic variation in the rs9939609 FTO gene variant. Journal of Nutrigenetics and Nutrigenomics 2015;8(3):128-36. [DOI: 10.1159/000441142] [DOI] [PubMed] [Google Scholar]
De Luis 2015b {published data only}
- De Luis DA, Aller R, Izaola O, Romero E.Effects of a high-protein/low-carbohydrate versus a standard hypocaloric diet on adipocytokine levels and cardiovascular risk factors during 9 months, role of rs6923761 gene variant of glucagon-like peptide 1 receptor. Journal of Endocrinological Investigation 2015;38(11):1183-9. [DOI: 10.1007/s40618-015-0304-9] [DOI] [PubMed] [Google Scholar]
De Luis 2015c {published data only}
- De Luis DA, Aller R, Izaola O, Díaz Soto G, López Gómez JJ, Gómez Hoyos E, et al.Effects of a high-protein/low-carbohydrate versus a standard hypocaloric diet on weight and cardiovascular risk factors during 9 months: role of a genetic variation in the cannabinoid receptor gene (CNR1) (G1359A polymorphism). Annals of Nutrition and Metabolism 2015;66(2-3):125-31. [DOI: 10.1159/000375412] [DOI] [PubMed] [Google Scholar]
De Luis 2015d {published data only}
- De Luis DA, Izaola O, Aller R, De la Fuente B, Bachiller R, Romero E.Effects of a high-protein/low carbohydrate versus a standard hypocaloric diet on adipocytokine levels and insulin resistance in obese patients along 9 months. Journal of Diabetes and Its Complications 2015;29(7):950-4. [DOI: 10.1016/j.jdiacomp.2015.06.002] [DOI] [PubMed] [Google Scholar]
De Luis 2015e {published data only}
- De Luis DA, Izaola O, De la Fuente B, Primo D, Romero E.Role of fatty acid-binding protein 2 Ala54Thr genotype on weight loss and cardiovascular risk factors after a high-protein/low-carbohydrate versus a standard hypocaloric diet during 9 months. Annals of Nutrition and Metabolism 2015;67(2):81-6. [DOI: 10.1159/000438947] [DOI] [PubMed] [Google Scholar]
De Luis 2016 {published data only}
- De Luis DA, Aller R, Izaola O, Romero E.Association of the TNF-alpha-308 G/A polymorphisms with metabolic responses secondary to a high protein/low carbohydrate versus a standard hypocaloric diet [Asociación del polimorfismo TNF-alpha -308 G/A con los cambios metabólicos secundarios a una dieta hipocalórica rica en proteínas/baja en hidratos de carbono versus una dieta hipocalórica estándar]. Nutrición Hospitalaria 2016;33(3):602-8. [DOI: 10.20960/nh.267] [DOI] [PubMed] [Google Scholar]
De Luis 2016a {published data only}
- De Luis DA, Aller R, Izaola O, Romero E.Effect of -55CT polymorphism of UCP3 on insulin resistance and cardiovascular risk factors after a high protein/low carbohydrate versus a standard hypocaloric diet. Annals of Nutrition and Metabolism 2016;68(3):157-63. [DOI: 10.1159/000444150] [DOI] [PubMed] [Google Scholar]
De Luis 2016b {published data only}
- De Luis D, Aller R, Izaola O, Romero E.Role of-55CT polymorphism of UCP3 on insulin resistance factors after 9 months of high protein/low carbohydrate vs. a standard hypocaloric diet. Diabetes 2016;65(Suppl 1):A560. [DOI: ] [DOI] [PubMed] [Google Scholar]
De Luis 2017 {published data only}
- De Luis DA, Izaola O, Primo D, Aller R.Polymorphism rs16147 of the neuropeptide Y gene modifies the response of cardiovascular risk biomarkers and adipokines to two hypocaloric diets. Journal of Nutrigenetics and Nutrigenomics 2017;10(1-2):63-72. [DOI: 10.1159/000478528] [DOI] [PubMed] [Google Scholar]
De Luis 2018 {published data only}
- De Luis DA, Mulero I, Primo D, Izaola O, Aller R.Effects of polymorphism rs3123554 in the cannabinoid receptor gene type 2 (CB2R) on metabolic and adiposity parameters after weight loss with two hypocaloric diets. Diabetes Research and Clinical Practice 2018;139:339-47. [DOI: 10.1016/j.diabres.2018.02.030] [DOI] [PubMed] [Google Scholar]
De Luis 2018a {published data only}
- De Luis DA, Izaola O, Primo D, Aller R.Impact of 2 different hypocaloric diets on serum omentin levels in obese subjects. Annals of Nutrition and Metabolism 2018;73(2):138-44. [DOI: 10.1159/000489130] [DOI] [PubMed] [Google Scholar]
De Luis 2019 {published data only}
- De Luis DA, Izaola O, Primo D, Aller R.Different effects of high-protein/low-carbohydrate versus standard hypocaloric diet on insulin resistance and lipid profile: role of rs16147 variant of neuropeptide Y. Diabetes Research and Clinical Practice 2019 Aug 23 [Epub];156:107825. [DOI: 10.1016/j.diabres.2019.107825] [DOI] [PubMed] [Google Scholar]
De Luis 2019a {published data only}
- De Luis D, Izaola O, Primo D, Aller R.Role of rs670 variant of APOA1 gene on metabolic response after a high fat vs. a low fat hypocaloric diets in obese human subjects. Journal of Diabetes and Its Complications 2019;33(3):249-54. [DOI: 10.1016/j.jdiacomp.2018.10.015] [DOI] [PubMed] [Google Scholar]
De Luis 2020 {published data only}
- De Luis DA, Izaola O, Primo D, Aller R.A circadian rhythm-related MTNR1B genetic variant (rs10830963) modulate body weight change and insulin resistance after 9 months of a high protein/low carbohydrate vs a standard hypocaloric diet. Journal of Diabetes and Its Complications 2020 Jan 13 [Epub];34(4):107534. [DOI: 10.1016/j.jdiacomp.2020.107534] [DOI] [PubMed] [Google Scholar]
De Luis 2020a {published data only}
- De Luis DA, Primo D, Izaola O, Aller R.Adiponectin gene variant rs266729 interacts with different macronutrient distribution of two different hypocaloric diets. Lifestyle Genomics 2020;13(1):20-7. [DOI: 10.1159/000503863] [DOI] [PubMed] [Google Scholar]
De Luis 2021 {published data only}
- De Luis D, Primo MD, Izaola O.Adiponectin gene variant rs266729 interacts with different macronutrient distributions of two different hypocaloric diets during nine months [La variante del gen de la adiponectina rs266729 interactúa con diferentes distribuciones de macronutrientes de dos dietas hipocalóricas durante nueve meses]. Nutrición Hospitalaria 2021;38(2):274-80. [DOI: 10.20960/nh.03423.] [DOI] [PubMed] [Google Scholar]
Evangelista 2009 {published data only}
- Evangelista LS, Heber D, Li Z, Bowerman S, Hamilton MA, Fonarow GC.Reduced body weight and adiposity with a high-protein diet improves functional status, lipid profiles, glycemic control, and quality of life in patients with heart failure: a feasibility study. Journal of Cardiovascular Nursing 2009;24(3):207-15. [DOI: 10.1097/JCN.0b013e31819846b9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evangelista 2017 {published data only}
- Evangelista LS, Lombardo D, Horwich T, Hamilton M, Fonarow GC.High vs. standard protein diets in obese patients with heart failure: effects on chronic disease risks. Circulation 2017;135(Suppl 1):AP167. [DOI: 10.1161/circ.135.suppl_1.p167] [DOI] [Google Scholar]
Fernandez 2020 {published data only}
- Fernandez C, Bazzano L.The effects of ideal protein versus low-fat/low-calorie weight loss diets: the RENEWAL trial. Obesity 2020;28(Suppl 2):167. [Google Scholar]
Fleming 2002 {published data only}
- Fleming RM.The effect of high-, moderate-, and low-fat diets on weight loss and cardiovascular disease risk factors. Preventive Cardiology 2002;5(3):110-8. [DOI: 10.1111/j.1520-037x.2002.01231.x.] [DOI] [PubMed] [Google Scholar]
Greene 2004 {published data only}
- Greene PJ, Devecis J, Willett WC.Effects of low-fat vs ultra-low-carbohydrate weight-loss diets: a 12-week pilot feeding study. Journal of Parenteral and Enteral Nutrition 2004;28(Suppl 1):S13-4. [DOI: 10.1177/01486071040280S101] [DOI] [Google Scholar]
Izaola 2019 {published data only}
- Izaola O, Primo D, Gomez Hoyos E, Lopez Gomez JJ, Ortola A, De Luis D.Association of rs670 variant of APOA1 gene with lipid profile and insulin resistance after 9 months of a high protein/low carbohydrate vs a standard hypocaloric diet. Clinical Nutrition 2020;39(4):988-93. [DOI: 10.1016/j.clnu.2019.04.030] [DOI] [PubMed] [Google Scholar]
Mukhtar 2009 {published data only}
- Mukhtar RY, Reid J, Fishlock HF, Taylor GJ, Reckless JP.The effect of three dietary regimens on insulin resistance in metabolic syndrome subjects. Diabetes 2009;58(Suppl 1):A. [Google Scholar]
Sun 2019 {published data only (unpublished sought but not used)}
- Sun J, Xu N, Lin N, Wu P, Yuan K, An S, et al.Optimal weight loss effect of short-term low carbohydrate diet with calorie restriction on overweight/obese subjects in South China - a multicenter randomized controlled trial. Diabetes 2019;68(Suppl 1):317-LB. [DOI: ] [Google Scholar]
References to ongoing studies
NCT03832933 {published data only}
- NCT03832933.Comparing high and normal protein diets for the dietary remission of type 2 diabetes. http://clinicaltrials.gov/ct2/show/NCT03832933 (first received 6 February 2019).
NCT04014296 {published data only}
- NCT04014296.Weight loss in adults over 50 with obesity. https://clinicaltrials.gov/ct2/show/NCT04014296 (first received 10 July 2019).
NCT04023942 {published data only}
- NCT04023942.Personalized nutrition and e-health: lifestyle intervention study for weight loss maintenance (LION). https://clinicaltrials.gov/ct2/show/NCT04023942 (first received 18 July 2019).
NCT04136093 {published data only}
- NCT04136093.Diet for the maintenance of weight loss and metabolic health in obese postmenopausal women (WELCOME). https://clinicaltrials.gov/ct2/show/NCT04136093 (first received 23 October 2019).
NCT04192357 {published data only}
- NCT04192357.A randomised controlled trial of a weight loss maintenance program for adults with obesity. https://clinicaltrials.gov/ct2/show/NCT04192357 (first received 10 December 2019).
NCT04230928 {published data only}
- NCT04230928.Giving a low carbohydrate diet to overcome hypertension (GLOH). https://clinicaltrials.gov/ct2/show/NCT04230928 (first received 18 January 2020).
NCT04382183 {published data only}
- NCT04382183.Impact of ketogenic diets in preventing relapse in obesity management (Ketomaintain). https://clinicaltrials.gov/ct2/show/NCT04382183 (first received 11 May 2020).
NCT04699448 {published data only}
- NCT04699448.Gene-diet interactions on body weight regulation and lifestyle parameters (iMPROVE) [Genetic predisposition and body weight regulation. Evaluation of target-genes in overweight and obese adults, under different dietary interventions]. https://clinicaltrials.gov/ct2/show/NCT04699448 (first received 7 January 2021).
NCT04745572 {published data only}
- NCT04745572.Development of an adaptive treatment for weight loss in people with prediabetes [Development of an adaptive treatment strategy for weight loss in people with prediabetes using a sequential multiple assignment randomized trial]. https://clinicaltrials.gov/ct2/show/NCT04745572 (first received 9 February 2021).
Additional references
Abdelhamid 2018
- Abdelhamid AS, Martin N, Bridges C, Brainard JS, Wang X, Brown TJ, et al.Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2018, Issue CD012345. Art. No: CD012345. [DOI: 10.1002/14651858.CD012345.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACC/AHA 2013 Full Report
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel.Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014;22 Suppl 2:S41-410. [DOI] [PubMed] [Google Scholar]
Accurso 2008
- Accurso A, Bernstein RK, Dahlqvist A, Draznin B, Feinman RD, Fine EJ, et al.Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutrition & Metabolism 2008;5:9. [DOI: 10.1186/1743-7075-5-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
ADA 2009
- American Diabetes Association.All about carbohydrate counting; 2009. Available at https://professional.diabetes.org/sites/professional.diabetes.org/files/media/All_About_Carbohydrate_Counting.pdf.
Agatston 2003
- Agatston A.The South Beach Diet: the Delicious, Doctor-Designed, Foolproof Plan for Fast and Healthy Weight Loss. New York: Random House, 2003. [Google Scholar]
Ahern 2017
- Ahern AL, Wheeler GM, Aveyard P, Boyland EJ, Halford JC, Mander AP, et al.Extended and standard duration weight-loss programme referrals for adults in primary care (WRAP): a randomised controlled trial. Lancet 2017;389(10085):2214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhassan 2008
- Alhassan S, Kim S, Bersamin A, King AC, Gardner CD.Dietary adherence and weight loss success among overweight women: results from the A TO Z weight loss study. International Journal of Obesity 2008;32(6):985-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhazmi 2014
- Alhazmi A, Stojanovski E, McEvoy M, Garg ML.The association between dietary patterns and type 2 diabetes: a systematic review and meta-analysis of cohort studies. Journal of Human Nutrition and Dietetics 2014;27(3):251-60. [DOI] [PubMed] [Google Scholar]
Astrup 2004
- Astrup A, Meinert Larsen T, Harper A.Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet 2004;364(9437):897-9. [DOI] [PubMed] [Google Scholar]
Atkins 1998
- Atkins RC.Dr. Atkins' New Diet Revolution. New York: Avon Books, 1998. [Google Scholar]
Atkins 1999
- Atkins RC.Dr Atkins New Diet Revolution. London: Vermillion, 1999. [Google Scholar]
Atkins 2002
- Atkins RC.Dr Atkins' New Diet Revolution. New York: Harper Collins, 2002. [Google Scholar]
Atkins 2011
- Atkins Nutritionals.Atkins. www.sa.atkins.com (accessed prior to 19 April 2019).
Barber 2021
- Barber TM, Hanson P, Kabisch S, Pfeiffer AFH, Weickert MO.The low-carbohydrate diet: short-term metabolic efficacy versus longer-term limitations. Nutrients 2021;13(4):1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bazzano 2012
- Bazzano LA, Reynolds K, Hu T, Yao L, Bunol C, Liu Y, et al.Effect of a low carbohydrate diet on weight and cardiovascular risk factors: a randomized controlled trial. Circulation 2012;125(10):AP306. [Google Scholar]
Becker 2005
- Becker W.New Nordic nutrition recommendations 2004. Physical activity as important as good nourishing food. Lakartidningen 2005;102(39):2757-62. [PubMed] [Google Scholar]
Berrington 2010
- Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnes RJ, et al.Body-mass index and mortality among 1.46 million white adults. New England Journal of Medicine 2010;363(23):2211–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhardwaj 2017
- Bhardwaj S, Misra A, Gulati S, Anoop S, Kamal VK, Pandey RM, et al.A randomized controlled trial to evaluate the effects of high Protein Complete (lActo) vEgetaRian (PACER) diet in non-diabetic obese Asian Indians in North India. Heliyon 2017;3(12):e00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boden 2005
- Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP.Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Annals of Internal Medicine 2005;142:403–11. [DOI] [PubMed] [Google Scholar]
Brehm 2003
- Brehm BJ, Seeley RJ, Daniels SR, D'Alessio DA.A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. Journal of Clinical Endocrinology and Metabolism 2003;88(4):1617-23. [DOI] [PubMed] [Google Scholar]
Brinkworth 2009a
- Brinkworth GD, Noakes M, Clifton PM, Bird AR.Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. British Journal of Nutrition 2009;101(10):1493-502. [DOI] [PubMed] [Google Scholar]
Brinkworth 2009b
- Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM.Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. American Journal of Clinical Nutrition 2009;90(1):23-32. [DOI] [PubMed] [Google Scholar]
Brinkworth 2009c
- Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ.Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Archives of Internal Medicine 2009;169(20):1873-80. [DOI] [PubMed] [Google Scholar]
British Dietetic Association 2013
- British Dietetic Association.Weight wise eating well: Your weight wise plan. www.bdaweightwise.com/eating/eating_plan.html (accessed prior to 13 December 2021).
Brown 2016
- Brown JD, Buscemi J, Milsom V, Malcolm R, O'Neil PM.Effects on cardiovascular risk factors of weight losses limited to 5-10. Translational Behavioral Medicine 2016;6(3):339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2012
- Campbell DD, Meckling KA.Effect of the protein:carbohydrate ratio in hypoenergetic diets on metabolic syndrome risk factors in exercising overweight and obese women. British Journal of Nutrition 2012;108(9):1658-71. [DOI] [PubMed] [Google Scholar]
Carreiro 2016
- Carreiro AL, Dhillon J, Gordon S, Higgins KA, Jacobs AG, McArthur BM, et al.The macronutrients, appetite, and energy intake. Annual Review of Nutrition 2016;36:73–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chawla 2020
- Chawla S, Silva FT, Medeiros SA, Mekary RA, Radenkovic D.The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients 2021;12(12):3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Churuangsuk 2018
- Churuangsuk C, Kherouf M, Combet E, Lean M.Low-carbohydrate diets for overweight and obesity: a systematic review of the systematic reviews. Obesity Reviews 2018;19(12):1700-18. [DOI] [PubMed] [Google Scholar]
Collins 2013
- Collins C, Neve M, Morgan P, Fletcher K, Williams R, Young M, et al.Effectiveness of interventions with a dietary component on weight loss maintenance: a systematic review. JBI Database of Systematic Reviews and Implementation Reports 2013;11(8):317–414. [Google Scholar]
Covidence [Computer program]
- Covidence.Version accessed prior to 19 April 2019. Melbourne, Australia: Veritas Health Innovation, 2017. Available at covidence.org.
Dansinger 2005
- Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ.Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005;293(1):43-53. [DOI] [PubMed] [Google Scholar]
Denke 2001
- Denke MA.Metabolic effects of high-protein, low-carbohydrate diets. American Journal of Cardiology 2001;88(1):59-61. [DOI] [PubMed] [Google Scholar]
Development Initiatives 2017
- Development Initiatives.Global Nutrition Report 2017: Nourishing the SDGs. Bristol, UK: Development Initiatives, 2017. [Google Scholar]
DGAC 2015
- United States Department of Health and Human Services, Dietary Guidelines Advisory Committee 2015.Scientific Report of the 2015 Dietary Guidelines Advisory Committee. United States Department of Health and Human Services, United States Department of Agriculture, 2015. [Google Scholar]
Dietz 2015
- Dietz WH, Baur LA, Hall K, Puhl RM, Taveras EM, Uauy R, et al.Management of obesity: improvement of health-care training and systems for prevention and care. Lancet 2015;385:2521-33. [DOI] [PubMed] [Google Scholar]
DistillerSR [Computer program]
- DistillerSR.Version accessed prior to 13 December 2021. Ottawa (Canada): Evidence Partners, 2020. available at https://www.evidencepartners.com/products/distillersr-systematic-review-software/.
Dong 2020
- Dong T, Guo M, Zhang P, Sun G, Chen B .The effects of low-carbohydrate diets on cardiovascular risk factors: a meta-analysis. PLOS One 2020;15(1):e0225348. [DOI] [PMC free article] [PubMed] [Google Scholar]
EFSA 2017
- European Food Safety Authority.Dietary reference values for nutrients: Summary report. EFSA supporting publication. www.efsa.europa.eu/sites/default/files/2017_09_DRVs_summary_report.pdf (accessed prior to 13 December 2021).
ERFC 2011
- Emerging Risk Factors Collaboration.Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 2011;377:1085-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 2014
- Esposito K, Chiodini P, Maiorino MI, Bellastella G, Panagiotakos D, Giugliano D.Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine 2014;47(1):107-16. [DOI] [PubMed] [Google Scholar]
Fernandez‐Friera 2017
- Fernandez-Friera L, Fuster V, Lopez-Melgar B, Oliva B, Garcia-Ruiz JM, Mendiguren J, et al .Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors . Journal of the American College of Cardiology 2017;70(24):2979-91. [DOI: 10.1016/j.jacc.2017.10.024] [DOI] [PubMed] [Google Scholar]
Forouzanfar 2015
- Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al, GBD 2013 Risk Factors Collaborators.Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386(10010):2287-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2010
- Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, et al.Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Annals of Internal Medicine 2010;153(3):147-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franz 2015
- Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ.Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. Journal of the Academy of Nutrition and Dietetics 2015;115(9):1447-63. [DOI] [PubMed] [Google Scholar]
Frigolet 2011
- Frigolet M, Ramos Barragán V, Tamez GM.Low-carbohydrate diets: a matter of love or hate. Annals of Nutrition and Metabolism 2011;58:320–34. [DOI] [PubMed] [Google Scholar]
GBD MCDC 2016
- GBD 2015 Mortality and Causes of Death Collaborators.Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
GBMRF for CD Collaboration 2014
- The Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects).Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014;383:970–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghoch 2016
- Ghoch MEI, Calugi S, Dalle Grave R.The effects of low-carbohydrate diets on psychosocial outcomes in obesity/overweight: a systematic review of randomized, controlled studies. Nutrients 2016;8(7):402. [DOI: 10.3390/nu8070402] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2017
- Gibson AA, Sainsbury A.Strategies to improve adherence to dietary weight loss interventions in research and real-world settings. Behavioral Sciences 2017;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2007
- Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al.European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. European Heart Journal 2007;17:2375-414. [DOI] [PubMed] [Google Scholar]
Hall 2011
- Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al.Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378(9793):826-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2012
- Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR.Energy balance and its components: implications for body weight regulation. American Journal of Clinical Nutrition 2012;95:989-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2015
- Hall KD, Bemis T, Brychta R, Chen KY, Courville A, Crayner EJ, et al.Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metabolism 2015;22(3):427-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halyburton 2007
- Halyburton AK, Brinkworth GD, Wilson CJ, Noakes M, Buckley JD, Keogh JB, et al.Low- and high-carbohydrate weight-loss diets have similar effects on mood but not cognitive performance. American Journal of Clinical Nutrition 2007;86(3):580-7. [DOI] [PubMed] [Google Scholar]
Hession 2009
- Hession M, Rolland C, Kulkarni U, Wise A, Broom J.Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obesity Reviews 2009;10(1):36-50. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2021
- Higgins JP, Tianjing L, Deeks JJ.Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from https://training.cochrane.org/handbook/current.
Hoffman 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al.Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;7:g1687. [DOI] [PubMed] [Google Scholar]
Hooper 2020
- Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS.Reduction in saturated fat intake for cardiovascular disease. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD011737. [DOI: 10.1002/14651858.CD011737.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2000
- Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, et al.LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The Strong Heart Study. Arteriosclerosis, thrombosis, and vascular biology 2000;20(3):830-5. [DOI: 10.1161/01.atv.20.3.830] [DOI] [PubMed] [Google Scholar]
Inzucchi 2012
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al.Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35(6):1364-79. [DOI: 10.2337/dc12-0413] [DOI] [PMC free article] [PubMed] [Google Scholar]
IOM 2005
- Institute of Medicine Food and Nutrition Board.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Washington DC: National Academies Press, 2005. [Google Scholar]
Jensen 2014
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al.2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2013;129(25 Suppl 2):S102-38. [DOI: 10.1161/01.cir.0000437739.71477.ee] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2014
- Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk RA, et al.Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 2014;312(9):923-33. [DOI] [PubMed] [Google Scholar]
Koloverou 2014
- Koloverou E, Esposito K, Giugliano D, Panagiotakos D.The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014;63(7):903-11. [DOI] [PubMed] [Google Scholar]
Lichtenstein 2005
- Lichtenstein AH, Russell RM.Essential nutrients: food or supplements? Where should the emphasis be? JAMA 2005;294(3):351–8. [DOI] [PubMed] [Google Scholar]
Liska 2019
- Liska D, Mah E, Brisbois T, Barrios PL, Baker LB, Spriet LL.Narrative review of hydration and selected health outcomes in the general population. Nutrients 2019;11(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ludwig 2014
- Ludwig DS, Friedman MI.Increasing adiposity: consequence or cause of overeating? JAMA 2014;311:2167-8. [DOI] [PubMed] [Google Scholar]
Meng 2017
- Meng Y, Bai H, Wang S, Li Z, Wang Q, Chen L .Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Research and Clinical Practice 2017;131:124-31. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moubarac 2014
- Moubarac JC, Parra DC, Cannon G, Monteiro CA.Food classification systems based on food processing: significance and implications for policies and actions: a systematic literature review and assessment. Current Obesity Reports 2014;3:256-72. [DOI] [PubMed] [Google Scholar]
National Heart, Lung, and Blood Institute 1998
- NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US).Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Bethesda, MD: National Heart, Lung, and Blood Institute, 1998. [Google Scholar]
Naude 2014
- Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J.Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLOS One 2014;9(7):e100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCD‐RisC 2017
- NCD Risk Factor Collaboration (NCD-RisC) – Africa Working Group.Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population-based studies. International Journal of Epidemiology 2017;46(5):1421-32. [DOI: 10.1093/ije/dyx078] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ndanuko 2016
- Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ.Dietary patterns and blood pressure in adults: a systematic review and meta-analysis of randomized controlled trials. Advances in Nutrition 2016;15:76-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
NHMRC 2006
- Australian National Health and Medical Research Council and the New Zealand Ministry of Health.Nutrient Reference Values for Australia and New Zealand: Including Recommended Dietary Intakes. Canberra: Australian National Health and Medical Research Council and the New Zealand Ministry of Health, 2006. [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence.NICE clinical guideline [CG189]. Obesity: identification, assessment and management. www.nice.org.uk/guidance/cg189/ifp/chapter/obesity (accessed prior to 14 December 2021).
NICE 2015
- National Institute for Health and Care Excellence (NICE).Type 2 diabetes in adults: management Clinical Guideline [NG28]. Available at https://www.nice.org.uk/guidance/ng28; 2015. [PubMed]
NNR 2012
- Nordic Nutrition Recommendations 2012 Working Group.Nordic Nutrition Recommendations NNR 2012. Nordic Council of Ministers, Nordic Committee of Senior Officials for Food Issues, 2012. [Google Scholar]
Noakes 2006
- Noakes M, Foster PR, Keogh JB, James AP, Mamo JC, Clifton PM.Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutrition & Metabolism 2006;3(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nordmann 2006
- Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS Jr, Brehm BJ, et al.Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors. Archives of Internal Medicine 2006;166:285–93. [DOI] [PubMed] [Google Scholar]
Ornish 2001
- Ornish D.Eat More, Weigh Less: Dr. Dean Ornish's Life Choice Program for Losing Weight Safely While Eating Abundantly. 1st edition. New York: NY, 2001. [Google Scholar]
Paoli 2013
- Paoli A, Rubini A, Volek JS, Grimaldi KA.Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. European Journal of Clinical Nutrition 2013;67(8):789-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raffensperger 2008
- Raffensperger JF.The least-cost low-carbohydrate diet is expensive. Nutrition Research 2008;28:6-12. [DOI] [PubMed] [Google Scholar]
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web).Version 2.2.1. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Reynolds 2019
- Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L .Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 2019;393:434-45. [DOI] [PubMed] [Google Scholar]
Rio 2001
- Rios VG.Complications of treatment of epilepsy by a ketogenic diet. Revista de Neurologia 2001;33(10):909-15. [PubMed] [Google Scholar]
Ross 2021
- Ross LJ, Byrnes A, Hay RL, Cawte A, Musial JE .Exploring the highs and lows of very low carbohydrate high fat diets on weight loss and diabetes- and cardiovascular disease-related risk markers: a systematic review. Nutrition & Dietetics 2021;78:41-56. [DOI] [PubMed] [Google Scholar]
Sabate 2014
- Sabate J, Soret S.Sustainability of plant-based diets: back to the future. American Journal of Clinical Nutrition 2014;100(Suppl 1):476S-82S. [DOI] [PubMed] [Google Scholar]
Sacks 2009
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al.Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. New England Journal of Medicine 2009;360:859-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
SACN 2015
- Scientific Advisory Committee on Nutrition.Carbohydrates and Health Report. www.gov.uk/government/publications/sacn-carbohydrates-and-health-report (accessed prior to 14 December 2021).
Saslow 2014
- Saslow LR, Kim S, Daubenmier JJ, Moskowitz JT, Phinney SD, Goldman V, et al.Randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLOS One 2014;9(4):e91027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seidelmann 2018
- Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, et al.Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 2018;3(9):e419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shim 2014
- Shim J, Oh K, Kim HC.Dietary assessment methods in epidemiologic studies. Epidemiology and Health 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sievenpiper 2020
- Sievenpiper JL.Low-carbohydrate diets and cardiometabolic health: the importance of carbohydrate quality over quantity. Nutrition Reviews 2020;78(Supplement 1):69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverii 2020
- Silverii GA, Botarelli L, Dicembrini I, Girolamo V, Santagiuliana F, Monami M, et al.Low‑carbohydrate diets and type 2 diabetes treatment: a meta‑analysis of randomized controlled trials. Acta Diabetologica 2020;57(11):1375-82. [DOI] [PubMed] [Google Scholar]
Simons‐Morton 2006
- Simons-Morton DG, Obarzanek E, Cutler JA .Obesity research – limitations of methods, measurements, and medications. Journal of the American Medical Association 2006;295:826-8. [DOI] [PubMed] [Google Scholar]
Singh 2013
- Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al.The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLOS One 2013;8:e65174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2020
- Smith ES, Smith HA, Betts JA, Gonzalez JT, Atkinson G.A systematic review and meta-analysis comparing heterogeneity in body mass responses between low-carbohydrate and low-fat diets. Obesity (Silver Spring, Md) 2020;28(10):1833-42. [DOI] [PubMed] [Google Scholar]
Snorgaard 2017
- Snorgaard O, Poulsen GM, Andersen HK, Astrup A.Systematic review and meta-analysisof dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Research and Care 2017;5:e000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sondike 2003
- Sondike SB, Copperman N, Jacobson MS.Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. Journal of Pediatrics 2003;142(3):253-8. [DOI] [PubMed] [Google Scholar]
Soret 2014
- Soret S, Mejia A, Batech M, Jaceldo-Siegl K, Harwatt H, Sabate J.Climate change mitigation and health effects of varied dietary patterns in real-life settings throughout North America. American Journal of Clinical Nutrition 2014;100(Suppl 1):490S-5S. [DOI] [PubMed] [Google Scholar]
Stern 2004
- Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al.The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Annals of Internal Medicine 2004;140(10):778-85. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al.RoB 2: a revised tool for assessing risk of bias in randomised trials. British Medical Journal 2019;366:l4898. [DOI: ] [DOI] [PubMed] [Google Scholar]
Swinburn 2011
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al.The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011;378(9793):804-14. [DOI] [PubMed] [Google Scholar]
Syed‐Abdul 2018
- Syed-Abdul MM, Hu Q, Jacome-Sosa M, Padilla J, Manrique-Acevedo C, Heimowitz C, et al.Effect of carbohydrate restriction-induced weight loss on aortic pulse wave velocity in overweight men and women. Applied Physiology Nutrition and Metabolism 2018;43:1247-56. [DOI] [PubMed] [Google Scholar]
Taubes 2007
- Taubes G.Good calories, Bad Calories: Challenging the Conventional Wisdom on Diet, Weight Control, and Disease. New York: Alfred A Knopf, 2007. [Google Scholar]
Tay 2015
- Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, et al.Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. American Journal of Clinical Nutrition 2015;102:780-90. [DOI: 10.3945/ajcn.115.112581.] [DOI] [PubMed] [Google Scholar]
The Japan Diabetes Society 2016
- The Japan Diabetes Society.Diet therapy. In: Practice guideline for the treatment for diabetes in Japan [In Japanese]. Tokyo, Japan: Nankodo, 2016:37-66. [Google Scholar]
Truby 2006
- Truby H, Baic S, deLooy A, Fox KR, Livingstone MB, Logan CM, et al.Randomised controlled trial of four commercial weight loss programmes in the UK: initial findings from the BBC "diet trials". BMJ 2006;332(7553):1309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
USDA 2014
- United States Department of Agriculture.A Series of Systematic Reviews on the Relationship Between Dietary Patterns and Health Outcomes. United States Department of Agriculture, Center for Nutrition Policy and Promotion, Evidence Analysis Library Division, 2014. [Google Scholar]
Van Zuuren 2018
- Van Zuuren EJ, Fedorowicz Z, Kuijpers T, Pijl H.Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments. American Journal of Clinical Nutrition 2018;108(2):300-31. [DOI] [PubMed] [Google Scholar]
Vuorinen 2021
- Vuorinen A, Helander E, Pietilä J, Korhonen I.Frequency of self-weighing and weight change: cohort study with 10,000 smart scale users. Journal of Medical Internet Research 2021;23(6):e25529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wald 2012
- Wald NJ, Bestwick JP, Morris JK.Body weight reduction to avoid the excess risk of type 2 diabetes. British Journal of General Practice 2012;62(599):e411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2017
- Wan Y, Wang F, Yuan J, Li J, Jiang D, Zhang J, et al.Effects of macronutrient distribution on weight and related cardiometabolic profile in healthy non-obese Chinese: a 6-month, randomized controlled-feeding trial. EBioMedicine 2017;22:200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westman 2007
- Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, et al.Low-carbohydrate nutrition and metabolism. American Journal of Clinical Nutrition 2007;86(2):276–84. [DOI] [PubMed] [Google Scholar]
Whelton 2002
- Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, et al.Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. Journal of the American Medical Association 2002;288(15):1882-8. [DOI: 10.1001/jama.288.15.1882] [DOI] [PubMed] [Google Scholar]
Whitlock 2009
- Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al, Prospective Studies Collaboration.Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373(9669):1083-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wing 2010
- Wing RR, Look AHEAD Research Group.Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Archives of Internal Medicine 2010;170(17):1566-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wylie‐Rosett 2013
- Wylie-Rosett J, Aebersold K, Conlon B, Isasi CR, Ostrovsky NW.Health effects of low-carbohydrate diets: where should new research go? Current Diabetes Reports 2013;13:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yancy 2004
- Yancy WS Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC.A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Annals of Internal Medicine 2004;140(10):769-77. [DOI] [PubMed] [Google Scholar]
Yang 1976
- Yang MU, Van Itallie TB.Composition of weight lost during short-term weight reduction. Metabolic responses of obese subjects to starvation and low-calorie ketogenic and nonketogenic diets. Journal of Clinical Investigation 1976;58:722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2021
- Yang Q, Lang X, Li W, Liang Y.The effects of low-fat, high-carbohydrate diets vs. low-carbohydrate, high-fat diets on weight, blood pressure, serum liquids and blood glucose: a systematic review and meta-analysis. European Journal of Clinical Nutrition 2021 [Epub ahead of print];Online ahead of print:Online ahead of print. [DOI: 10.1038/s41430-021-00927-0] [DOI] [PubMed] [Google Scholar]
Younge 2015
- Younge JO, Kouwenhoven-Pasmooij TA, Freak-Poli R, Roos-Hesselink JW, Hunink MM.Randomized study designs for lifestyle interventions: a tutorial. International Journal of Epidemiology 2015;44(6):2006-19. [DOI] [PubMed] [Google Scholar]
Zheng 2011
- Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, et al.Association between body-mass index and risk of death in more than 1 million Asians. New England Journal of Medicine 2011;364(8):719-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Naude 2019
- Naude CE, Schoonees A, Nguyen KA, Senekal M, Young T, Garner P, et al.Low carbohydrate versus balanced carbohydrate diets for reducing weight and cardiovascular risk. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD013334. [DOI: 10.1002/14651858.CD013334] [DOI] [PMC free article] [PubMed] [Google Scholar]


