Abstract
Mimicry of peripheral nerve gangliosides by Campylobacter jejuni lipopolysaccharides (LPSs) has been proposed to induce cross-reacting antiganglioside antibodies in Guillain-Barré syndrome (GBS). Because current methods for LPS characterization are labor-intensive and inhibit the screening of large numbers of strains, a rapid GM1 epitope screening assay was developed. Biomass from two agar plates of confluent growth yielded sufficient LPS using a novel phenol-water and ether extraction procedure. Extracts of LPS were reacted with cholera toxin (GM1 ligand), peanut agglutinin (Galβ1→3GalNAc ligand), and anti-GM1 antibodies. After the assay was validated, 12 of 59 (20%) C. jejuni serostrains, including four serotypes that have not previously been associated with GBS, reacted with two or more anti-GM1 ganglioside reagents. Subsequently, LPS extracts from 5 of 7 (71%) C. jejuni isolates and 2 of 3 (67%) C. jejuni culture collection strains bore GM1 structures. Overall, the assay system was reliable, efficient, and reproducible and may be adapted for large-scale epidemiological studies.
There is mounting evidence that Campylobacter jejuni, a causative agent of enteritis, plays a significant role in the development of Guillain-Barré syndrome (GBS), a demyelinating disease of the peripheral nervous system (26, 33, 49, 51). Several variants of GBS occur and include the demyelinating form called acute inflammatory demyelinating polyneuropathy (AIDP), the axonal form represented by acute motor axonal neuropathy (AMAN), and an ocular variant termed Miller Fisher syndrome (MFS) (18, 34). Characteristically, 76% of AMAN and 42% of AIDP patients have serologic evidence consistent with recent C. jejuni infection (18, 27).
O (Penner) serotyping distinguishes between C. jejuni strains on the basis of differences in the saccharide structure (O side chain and core oligosaccharide [OS]) of the lipopolysaccharide (LPS) of the bacterium (28, 38, 41). Some reports suggest that only specific C. jejuni serotypes are associated with GBS (30, 45). In a Japanese study, 81% of C. jejuni isolates from GBS patients belonged to serotype O:19 (20), and, other studies have shown an association with other serotypes (19, 31, 33, 37, 45, 48, 58). Autoreactive antibodies to gangliosides, especially GM1, are found in 30% of GBS patient sera, particularly after C. jejuni infection (15, 19, 26, 35, 36, 49, 58, 59, 63). Thus, it is currently hypothesized that antiganglioside antibodies may be induced as a result of molecular mimicry of peripheral nerve gangliosides by structurally similar C. jejuni LPSs (49, 59).
Furthermore, since anti-GM1 antibodies in human sera are likely to be a contributory factor in GBS development, an important step in elucidating the pathogenesis of the disease is determining the structure of the immunogenic epitopes in ganglioside-mimicking C. jejuni LPS. However, the LPSs from only a few C. jejuni GBS or MFS isolates have been studied at the chemical level to determine the precise nature of the ganglioside-like structures (3, 5, 7, 8, 10, 29, 39, 48, 61). Methods used for detecting and analyzing LPS are both labor-intensive and time-consuming. The major difficulty is that large amounts of LPS are required for chemical characterization, and this does not allow for the screening of large numbers of strains. However, serological analysis using antiganglioside antibodies and ligands has proven a useful approach for analysis of mimicry in C. jejuni LPS (39, 40, 49). Importantly, although GBS-associated strains can express high-molecular-weight (high-Mr) LPS (5, 6, 7), serological analysis using thin-layer chromatography (TLC) can detect ganglioside mimicry in the core OS of LPS (39, 40, 49).
The aim of this study was to develop a rapid screening test to detect strains that have a GM1-like epitope in their LPSs. The assay combined a rapid miniphenol-water extraction procedure with TLC and immunostaining. The conformation of the carbohydrate moiety of glycolipids is best preserved in TLC, which is thus an appropriate technique for an assay examining reactions of antibodies with LPS. The novel assay system was validated by comparing the data from binding studies using purified LPS with results obtained using LPSs extracted by the rapid method from the same C. jejuni strains. Only a limited number of serotypes have been found in association with GBS, and to answer the question whether ganglioside-like epitopes are limited to a few C. jejuni serotypes, a collection of C. jejuni serostrains was screened for the GM1 epitope using the new assay system. Finally, the technique was applied to the rapid screening of clinical isolates from GBS and enteritis patients.
(A preliminary report of this research was presented at the 10th International Workshop on Campylobacter, Helicobacter and Related Organisms, Baltimore, Md., 12 to 16 September 1999.)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Details of the C. jejuni culture collection strains and clinical isolates, as well as strains of Helicobacter pylori and Escherichia coli used in this study, are given in Table 1. In addition, 59 C. jejuni serostrains were also included in the study. C. jejuni and H. pylori strains were routinely grown on blood agar (Columbia Agar Base [Oxoid Ltd., London, England] with 10% unlysed horse blood) at 37°C for 48 h in a H2-enriched microaerobic atmosphere (GasPak BR38 [Oxoid] without a catalyst) according to an established protocol (22). E. coli strain J5 was grown in an aerobic atmosphere on tryptone soya agar (Oxoid) at 37°C for 24 h. C. jejuni strains used for validation purposes (Table 2) were grown on blood agar in a manner identical to that described above. Bacterial biomass was harvested, and bulk extraction of LPS was performed by the hot phenol-water extraction procedure as described previously (32, 55).
TABLE 1.
C. jejuni strains used in this study
| Bacterial species | Penner serotype | Strain designation | Source | Reference(s) |
|---|---|---|---|---|
| C. jejuni | O:2 | NCTC 11168 | National Collection of Type Cultures, London, England | 13 |
| O:2 | ATCC 43440 | American Type Culture Collection, Manassas, Va. | 8 | |
| O:3 | ATCC 43441 | American Type Culture Collection | 4 | |
| O:4 | ATCC 43442 | American Type Culture Collection | 10 | |
| O:19 | ATCC 43446 | American Type Culture Collection | 7 | |
| O:13 | CCUG 8680 | Culture Collection of the University of Göteborg, Göteborg, Sweden | 23 | |
| O:1 | CCUG 6951 | Culture Collection of the University of Göteborg | 23 | |
| O:18 | CCUG 6968 | Culture Collection of the University of Göteborg | 23 | |
| O:23 | AZR6491 | B. C. Jacobs, Rotterdam, The Netherlands | 5, 19 | |
| O:41 strains | —a | A. J. Lastovica, Cape Town, South Africa | 22, 39 | |
| H. pylori | NCTC 11637 | National Collection of Type Cultures | 11 | |
| E. coli | J5 (UK)+ | B. J. Appelmelk, Vrijee University, Amsterdam, The Netherlands | 2 |
Designations of C. jejuni serotype O:41 strains are as follows: 16971.94GSH, 260.94RXH, 28134.94GSH, 176.83, 212.95, 238.95, 299.95, 308.95, 319.95, 367.95, and 370.95.
TABLE 2.
Comparison of the reactions of ligands and anti-GM1 antibodies to pure and miniphenol-water-extracted LPSs for validation purposes
| C. jejuni strain designation (serotype) | Strength of reaction with LPSa:
|
|||||
|---|---|---|---|---|---|---|
| CT
|
PNA
|
Anti-GM1 antibodies
|
||||
| Pure LPS | Mini- LPSd | Pure LPS | Mini- LPSb | Pure LPS | Mini- LPS | |
| NCTC 11168 (O:2) | ++ | +++ | +++ | ++ | + | ++ |
| ATCC 43441 (O:3) | − | − | − | − | − | − |
| ATCC 43442 (O:4) | ++ | + | − | − | − | − |
| ATCC 43446 (O:19) | ++++ | ++ | + | + | ++ | + |
| AZR6491 (O:23) | +++ | +++ | − | − | ++ | ++ |
| 16971.94GSH (O:41) | ++++ | ++++ | ++ | + | ++ | ++ |
| 260.94RXH (O:41) | ++++ | +++ | ++ | + | ++ | ++ |
| 28134.94GSH (O:41) | +++ | ++ | +++ | + | ++ | ++ |
| 176.83 (O:41) | ++++ | +++ | ++ | + | ++ | +++ |
++++, very strong reaction; +++, strong reaction; ++, moderate reaction; +, weak reaction; (+), barely visible reaction; −, no reaction.
Mini-LPS, LPS extracted by the miniphenol-water extraction procedure.
Biotyping and serotyping.
Bacterial identification was carried out by established procedures (28, 41, 52). Serotyping on the basis of thermostable somatic O antigens was performed with the 66 antisera of the Penner scheme (41) and an additional 30 antisera to new serotypes not included in the Penner scheme.
Extraction of LPS using a miniphenol-water extraction procedure.
Biomass harvested from two agar plates with confluent growth was washed three times in phosphate-buffered saline (PBS; pH 7.4; Oxoid) by centrifugation (5,000 × g for 5 min) and resuspended in 3.0 ml of sterile PBS (25). An aliquot of 0.75 ml was removed, centrifuged as before, and resuspended in 0.75 ml of water. An equivalent volume of 90% phenol (preheated to 65°C) was added, and samples were mixed for 1 min using an autovortex mixer and then incubated for 10 min at 65°C. At regular intervals the samples were mixed and, after cooling on ice, the samples were centrifuged (12,000 × g for 3 min). At this stage, separated layers were visible in the suspension. Residual phenol was removed from the aqueous phase by extracting three times with diethyl ether. The diethyl ether phase was discarded, and the water phase (containing the LPS) was placed in a fume cupboard for 1 h to allow the remaining diethyl ether to evaporate.
Comparison of LPS extraction techniques.
To rule out the possibility that LPS extraction by the miniphenol-water procedure and LPS extraction by the hot phenol-water technique result in the purification of different subpopulations of LPS, materials extracted by the two methods were compared. Preparations of LPS were examined by polyacrylamide gel electrophoresis (PAGE) with silver staining, by immunoblotting, and by TLC with immunostaining with the ligands cholera toxin (CT), peanut agglutinin (PNA), and anti-GM1 antibodies. Furthermore, modifications of the miniphenol-water extraction procedure were performed with four C. jejuni strains, and the resulting material was included in the comparative studies. First, purified LPS (1.5 mg) was added to harvested C. jejuni biomass and a miniphenol-water extraction was performed on the resulting material. Second, a miniphenol-water extraction was performed on a pure LPS solution (2 mg/ml). Third, miniphenol-water-extracted LPS was subjected to enzymatic digestion with 80 μg of proteinase K (Sigma Chemical Co., St. Louis, Mo.) for 1 h at 60°C (17). Fourth, 0.2 mg each of DNase II (Sigma) and RNase A (Sigma) were incubated at 37°C overnight with the proteinase K-digested LPS extracts, and samples were then treated with proteinase K (0.8 mg). In addition, proteinase K-treated whole-cell (PKWC) extracts of C. jejuni strains were prepared as described by Hitchcock and Brown (17). Finally, boiled lysates were prepared by diluting harvested bacteria in PBS (pH 7.4) to an A600 of 0.3, followed by centrifugation (5,000 × g) and solubilization of the resulting pellet in 200 μl of PBS (for TLC) or in 200 μl of electrophoresis lysing buffer at 100°C for 1 h.
Additionally, we compared the LPS staining patterns of C. jejuni miniphenol-water-extracted LPS, pure LPS, and LPS prepared as described by Blake and Russell (12) by using the extraction procedure of Al-Hendy et al. (1).
SDS-PAGE and immunoblotting.
The discontinuous buffer system of Laemmli (21) was used to fractionate LPS extracts by sodium dodecyl sulfate (SDS)-PAGE using a stacking gel of 5% acrylamide and a separation gel of 15% acrylamide containing 3.2 M urea (BDH Laboratory Supplies, Poole, England) (39). After SDS-PAGE, the gels were fixed and the LPS was visualized by silver staining as described previously (54). Alternatively, LPSs fractionated by SDS-PAGE were electrotransferred from gels to nitrocellulose membranes (pore size, 0.45 μm; Bio-Rad Laboratories, Hercules, Calif.) (53). H. pylori LPS on nitrocellulose blots was visualized with an anti-Lewis Y monoclonal antibody (Signet Laboratories, Inc., Dedham, Mass.) against the O side chain (11) as the first antibody and horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin M (IgM) (Sigma) as the second antibody. Alternatively, for detection of E. coli LPS reactions, a monoclonal antibody to E. coli core OS (anti-R3) was used as the first antibody (2) and an HRP-conjugated anti-mouse IgG (Sigma) was used as the second antibody.
TLC.
Gangliosides (1-μg aliquots; Sigma) and LPS extracts (5-μl aliquots) were analyzed by TLC on precoated silica gel 60 glass plates (Merck, Darmstadt, Germany). Solvent systems consisting of chloroform–methanol–0.22% CaCl2 · 2H2O (50:45:10 [vol/vol/vol]) (47) and n-propanol–water–25% NH4OH (60:30:10 [vol/vol/vol]) (49, 59) were used as developers for gangliosides and LPSs, respectively. Gangliosides and LPS were visualized by spraying plates with resorcinol-HCl reagent (50).
Immunostaining.
TLC with immunostaining was performed using the procedure of Saito et al. (47) as modified by Schwerer et al. (49). Briefly, developed TLC plates were dried for 30 min in a vacuum desiccator, fixed in 0.2% polyisobutylmethacrylate (Aldrich, Steinheim, Germany) in n-hexane (Merck) for 1.5 min, and dried as before. Nonspecific binding was reduced by submerging the plates for 1 h in a solution of PBS containing 0.3% gelatin (gelatin-PBS). Subsequently, lanes were overlaid with rabbit antiserum to ganglioside GM1 (Matreya Inc., Pleasant Gap, Pa.), diluted 1:100 in gelatin-PBS. Plates were incubated at 4°C overnight, washed three times with cold PBS, overlaid with peroxidase-conjugated anti-rabbit IgG (Sigma) diluted 1:500 in gelatin-PBS, and incubated at room temperature for 1 h with gentle rocking. The plates were washed with cold PBS, and the immunoreactants were visualized by use of an HRP development system (Bio-Rad Laboratories). Control experiments for antibody binding were performed whereby (i) preimmune rabbit serum was used instead of anti-GM1 antiserum and (ii) TLC plates were overlaid with the second antibody but not with the first antibody. Binding studies with CT-peroxidase conjugate (Sigma) and PNA-peroxidase conjugate (Kem-En-Tec, Copenhagen, Denmark) were performed under the same conditions as those described for immunostaining. However, only one overlay step with peroxidase-conjugated CT (1 μg/ml) or PNA (20 μg/ml) was necessary. Control experiments for CT and PNA ligand binding were performed using tetanus toxin C (TTC, which binds to disialosyl, or B series, gangliosides), which does not react with ganglioside GM1, instead of CT or PNA.
RESULTS
Assay validation.
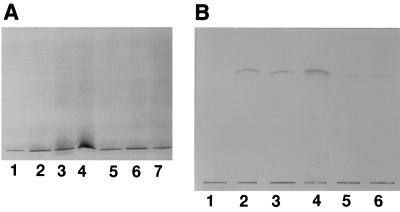
Silver-stained SDS-PAGE gels comparing miniphenol-water-extracted LPSs, LPSs extracted by a modification of that procedure, and pure (hot phenol-water-extracted) LPSs from C. jejuni serostrains O:19 and O:2, and from two serotype O:41 strains (16971.94GSH and 28134.94GSH), exhibited a pattern of bands migrating near the bottom of the gel. These bands corresponded to low-Mr rough-form LPS composed of core OS and lipid A (Fig. 1A). As C. jejuni high-Mr LPS is not visualized by the silver-staining procedure of Tsai and Frasch (54), immunoblotting was performed with C. jejuni typing antisera (41). High-Mr LPS was visualized for C. jejuni serostrain O:19, but as with silver staining, only low-Mr LPS was apparent for C. jejuni O:2 and serotype O:41 strains (data not shown). Within the same strain, the miniphenol-water-extracted LPS, LPSs extracted by a modification of that procedure, and pure LPS had identical banding profiles, demonstrating that the different extraction procedures and modifications used do not select for different subpopulations of LPS. In addition, the pattern of staining in the low-Mr region of the gel of the LPS extracted by the method of Blake and Russell (12) was identical to the profiles of both miniphenol-water-extracted LPS and purified LPS from the same strain (data not shown). Similarly, for each individual strain of the four C. jejuni strains described above, there were no differences in ligand or antibody affinities between LPS extracts regardless of the extraction procedure used. As shown in Fig. 1B, CT showed the same reactivity for each LPS extract, with the exception of a weaker reaction with the boiled extract and PKWC lysate. The weaker reaction of boiled and PKWC lysates was a consistent finding with all the C. jejuni strains and ligands used; it potentially reflects the presence of contaminating proteins. Supporting this, Coomassie blue-stained SDS-PAGE gels of each preparation demonstrated the presence of proteins in boiled extracts and PKWC lysates, but not in the miniphenol-water LPS extracts (data not shown). In addition, no reactions were observed on control TLC plates incubated with preimmune rabbit serum or on plates where the second antibody was incubated in the absence of the first antibody. Moreover, immunoblots of purified LPS and miniphenol-water-extracted LPS from H. pylori NCTC 11637 and E. coli J5 (UK) showed identical patterns of binding to corresponding antibodies, regardless of the LPS extraction procedure used (data not shown).
FIG. 1.
Silver stained SDS-PAGE gels (10-μl aliquots) (A) and binding of CT (5-μl aliquots) (B) to LPS extracts of C. jejuni NCTC 11168. (A) Lanes: 1, miniphenol-water-extracted LPS with added purified LPS; 2, miniphenol-water-extracted and DNase-RNase-proteinase K-treated LPS; 3, boiled extract; 4, PKWC extract; 5, pure LPS with miniphenol-water extraction; 6, pure LPS (1 μg); 7, miniphenol-water LPS. (B) Lanes: 1, C. jejuni O:3 pure LPS; 2, miniphenol-water- and proteinase K-treated LPS; 3, miniphenol-water-extracted LPS; 4, pure LPS (1 μg); 5, boiled extract; 6, PKWC extract.
Purified LPS and miniphenol-water-extracted LPS from nine C. jejuni strains were available (Table 2), and by comparing the reactions of these LPSs with CT, PNA, and anti-GM1 antibodies, it was possible to further validate the assay system. For each individual strain, LPSs from the miniphenol-water extraction method gave the same results for CT binding as purified LPSs, to within one degree of positivity (Table 2). Therefore, based on CT binding, good correlation was observed for pure LPS and LPS extracted by the miniphenol-water, or rapid, method. In addition, with respect to the reactions of pure LPS and miniphenol-water-extracted LPS with PNA, the results correlated well for 6 of 9 (67%) strains. However, purified LPSs from serostrain O:19 and from two serotype O:41 strains (260.94RXH and 28134.94GSH) reacted with PNA (Table 2), but LPS extracted by the rapid method did not reproducibly exhibit a positive reaction with PNA. However, proteinase K treatment of miniphenol-water-extracted LPS from these three strains yielded reproducible positive reactions. Polyclonal anti-GM1 antibodies, which weakly cross-react with asialo-GM1, GM2, and GD1b gangliosides (39), reacted with 7 of the 9 (78%) C. jejuni strains tested (Table 2). Moreover, a very good correlation for binding was observed with LPS of those strains which had been extracted by both methods. All of the LPSs that reacted with CT also reacted with anti-GM1 antibodies, with the exception of serostrain O:4 LPS, which mimics ganglioside GD1a and reacted with CT only. Although CT is described as a GM1 ligand, it also cross-reacts with gangliosides which have a GM1-related structure, e.g., asialo-GM1 and GM2 gangliosides (39, 40, 49). Therefore, the use of antibodies to ganglioside GM1 in conjunction with CT appears to be the most efficient way to screen for GM1 mimicry in C. jejuni strains.
To ensure that the rapid assay for screening GM1 epitopes was reproducible, six strains chosen at random were grown, extracted, and reexamined in the same manner, and identical binding results were observed on retesting. Overall, the assay system was reliable, efficient, and reproducible, and its use was validated when results of binding experiments with LPS extracted by the rapid method were compared to results using purified LPS.
Screening of the collection of C. jejuni serostrains for the GM1 epitope.
To answer the question whether ganglioside-like epitopes are carried only by a limited number of Penner serotypes, a collection of C. jejuni serostrains was screened for the GM1 epitope using the rapid assay system.
As shown in Table 3, LPSs from five different serostrains reacted with all three ligands (O:4, O:5, O:13, O:36, and O:44), and isolates of each of these serotypes have been associated with GBS (7, 9, 14, 31, 33, 39). The LPSs of two of these C. jejuni serostrains have been chemically characterized and reported to mimic ganglioside GD1a (O:4) and ganglioside GM2 (O:36) (3, 10). In our assay, GM1 mimicry was detected in LPS from serostrain O:13. An isolate of this serotype has recently been associated for the first time with GBS (14). Furthermore, strong binding of each of the ligands tested was observed with LPSs of serostrains O:5 and O:44, suggesting for the first time the presence of a GM1 structure in the LPSs of these strains. Serostrain O:2 LPS was negative for reaction with each of the ligands tested, although LPS of C. jejuni NCTC 11168, an O:2 serotype, was found to bear GM1 mimicry (Table 2), consistent with the differences observed between these strains (24). Furthermore, serostrain O:23 LPS did not react with CT, PNA, or anti-GM1 antibodies, in contrast to serostrain O:36, despite sharing an identical core OS structure. This indicates a difference in GM2 ganglioside epitope expression in the two serostrains, and it can be proposed that the O side chain may have an effect on the expression of core OS in serostrains O:23 and O:36 (6). Also, the C. jejuni AZR6491 isolate (serotype O:23) reacted with CT in the validation study (Table 2). This suggests that the core OS of serostrain O:23 LPS may be different from that of LPSs from C. jejuni isolates of the same serotype, which is consistent with our preliminary chemical studies (5) and which has also been observed previously with C. jejuni O:19 isolates (7).
TABLE 3.
Binding of ligands and anti-GM1 antibodies to serostrain C. jejuni LPSs extracted using the miniphenol-water procedurea
| C. jejuni serostrain | Strength of reaction with LPSb:
|
||
|---|---|---|---|
| CT | PNA | Anti-GM1 antibodies | |
| O:1 | + | − | − |
| O:4 | ++ | ++ | + |
| O:5 | + | ++ | ++ |
| O:10 | − | ++ | − |
| O:13 | + | ++ | + |
| O:14 | − | ++++ | ++ |
| O:19 | ++ | − | + |
| O:20 | − | − | (+) |
| O:25 | − | ++ | +++ |
| O:34 | − | + | + |
| O:36 | ++ | ++ | ++ |
| O:41 | +++ | − | ++ |
| O:42 | + | − | + |
| O:43 | − | + | ++ |
| O:44 | ++++ | ++++ | ++++ |
| O:45 | + | − | − |
| O:47 | (+) | − | − |
| O:48 | (+) | − | − |
| O:50 | (+) | − | − |
Results are shown only for C. jejuni serostrains whose LPSs were positive for binding one or more ligands.
++++, very strong reaction; +++, strong reaction; ++, moderate reaction; +, weak reaction; (+), barely visible reaction; −, no reaction.
Overall, the LPSs of seven serostrains reacted with anti-GM1 antibodies and one other ligand: three with CT (O:19, O:41, and O:42) and four with PNA (O:14, O:25, O:34, and O:43). The LPSs from serotype O:19 and O:41 strains are known to exhibit mimicry of ganglioside GM1 (7, 39), whereas serostrain O:42 LPS has not been chemically characterized. The results of this assay strongly suggest that the LPS of this serostrain bears a GM1-related structure. Anti-GM1 antibodies and PNA both cross-react with asialo-GM1 ganglioside, and thus it was deduced that LPS from serostrains O:14, O:25, O:34, and O:43 may have LPSs which mimic asialo-GM1 ganglioside.
As shown in Table 3, LPSs from seven serostrains reacted with only one ligand, and six of these reacted only weakly with that particular ligand. Thus, it was considered that LPS from serostrains O:1, O:20, O:45, O:47, O:48, and O:50 do not mimic ganglioside GM1. Thus, strains that were weakly positive for only one ligand were considered not to bear GM1-mimicking structures in their LPSs, and this criterion was assigned as the cutoff value for absence of GM1 ganglioside mimicry. A strain was considered to exhibit GM1 mimicry if the LPS reacted with two or more of the ligands tested. However, LPS from serostrain O:10 showed moderate binding with PNA, and while it was unlikely that this strain had GM1-bearing LPS, it was considered that the core OS of this strain had a Gal-GalNAc or Gal-Gal disaccharide.
In our assay, all strains with established GM1 ganglioside mimicry, such as serotypes O:4, O:19, and O:41, reacted with at least two of the three ligands tested. Also, the assay detected GM1 mimicry in the LPSs of some serostrains which have not yet been structurally characterized, such as O:5, O:13, O:14, O:25, O:34, O:42, O:43, and O:44. Overall, 46 serostrains (78%) did not react with any of the three ligands used or were weakly positive with one ligand, suggesting that GM1 ganglioside-like epitopes are carried only by some Penner serotypes.
Screening for GM1 mimicry in C. jejuni enteritis and GBS isolates.
The rapid assay technique was applied to the testing of the C. jejuni isolates shown in Table 4. Based on reactions with CT, PNA, and anti-GM1 antibodies, 3 of 5 (60%) serotype O:41 GBS isolates gave reactions that would be expected for the presence of GM1-like mimicry. However, LPS preparations, including pure LPSs, from two C. jejuni O:41 GBS isolates (319.95 and 367.95) did not appear to exhibit a GM1 ganglioside structure. Therefore, LPSs from these strains were tested for reaction with anti-asialo-GM1, anti-GD2, anti-GD3, and anti-GM2 antibodies and with a ligand that binds to B series, or disialosyl, gangliosides. However, LPSs from both strains failed to react with any of the ligands tested, indicating that LPSs from these strains do not resemble gangliosides such as GM1, GM2, GD2, GD3, and asialo-GM1. The possibility that LPSs from these two serotype O:41 strains could mimic ganglioside GD1a cannot be ruled out. Although CT, PNA, and anti-GM1 antibodies do not react with ganglioside GD1a (39), all three ligands recognized LPS from serostrain O:4, which exhibits mimicry of ganglioside GD1a (Table 3). However, microheterogeneity is present in this LPS, with ∼10% of the core OS molecules exhibiting GM1 mimicry (3, 10), and thus the presence of a GM1 epitope in serostrain O:4 LPS, rather than an ability of the reagents to recognize GD1a epitopes, accounts for the recognition by these three assay reagents. As ligands for detecting mimicry of ganglioside GD1a are not commercially available, whether the LPSs from the two serotype O:41 strains mimic ganglioside GD1a remains unanswered.
TABLE 4.
Binding of ligands and anti-GM1 antibodies to LPSs of C. jejuni isolates extracted using the miniphenol-water technique
| C. jejuni strain designation (serotype) | Associated disease | Strength of reaction with LPSa:
|
||
|---|---|---|---|---|
| CT | PNA | Anti-GM1 antibodies | ||
| 299.95 (O:41) | GBS | +++ | + | +++ |
| 308.95 (O:41) | GBS | ++++ | − | + |
| 319.95 (O:41) | GBS | + | − | − |
| 367.95 (O:41) | GBS | − | − | − |
| 370.95 (O:41) | GBS | ++++ | − | +++ |
| 238.95 (O:41) | Enteritis | +++ | − | +++ |
| 212.95 (O:41) | Kwashiorkorb | +++ | − | +++ |
| CCUG 6968 (O:18) | Enteritis | + | − | − |
| CCUG 6951 (O:1) | Enteritis | +++ | +++ | − |
| CCUG 8680 (O:13) | Enteritis | ++ | + | ++ |
++++, very strong reaction; +++, strong reaction; ++, moderate reaction; +, weak reaction; (+), barely visible reaction; −, no reaction.
Malnutrition disease caused by malabsorption of protein.
As shown in Table 4, the presence of GM1 mimicry in the LPSs of the two serotype O:41 non-GBS isolates (212.95 and 238.95) indicates the occurrence of ganglioside mimicry without the development of GBS. Similarly, the serotype O:13 enteritis isolate, C. jejuni CCUG 8680, reacted with all three ligands, and thus the LPS from this strain has GM1 ganglioside mimicry. Interestingly, serostrain O:13 LPS (Table 3) also exhibited a GM1-related structure. It is proposed that C. jejuni CCUG 6951 (O:1), also an enteritis isolate, mimics asialo-GM1 ganglioside, as the LPS reacted only with CT and PNA, but not with anti-GM1 antibodies. Thus, some uncomplicated enteritis isolates have LPSs bearing ganglioside-like structures, suggesting that host responses to the ganglioside molecules are important in determining the outcome of Campylobacter infection.
DISCUSSION
The association of GBS with preceding infection has led to a search for candidate bacterial antigens which may precipitate autoimmune responses in the host (16, 42, 56, 57). Gangliosides have been extensively studied as possible host antigens for autoimmune disease, since serum antibodies against gangliosides, especially GM1, are found in GBS sera, particularly when preceded by C. jejuni infection (15, 35, 44, 59, 60, 63). Molecular mimicry of gangliosides by core OSs of certain C. jejuni serotypes associated with GBS has been established (5, 7, 8, 10, 29, 31, 32, 56, 61), but LPSs from only a few C. jejuni GBS or MFS isolates have been studied at the molecular level. The main difficulty is that large amounts of biomass are required for LPS isolation, and this has not allowed for the screening of large numbers of strains. To overcome this problem, a laboratory-based rapid GM1 screening method that can be used to screen for cross-reactive epitopes in C. jejuni isolates was developed in the present study. Once the assay was validated, it was used to screen for GM1-bearing strains in a collection of 59 C. jejuni serostrains and was applied to the testing of a number of C. jejuni clinical isolates.
In the validation experiments, miniphenol-water-extracted LPS, LPSs from modifications of the miniphenol-water procedure, and pure LPS from the same strain had comparable banding patterns in SDS-PAGE, demonstrating that miniphenol-water extraction is a suitable LPS extraction procedure for use in the present study. In addition, LPS prepared as described by Blake and Russell (12), according to the procedure of Al-Hendy et al. (1), displayed low-Mr bands similar to those of miniphenol-water-extracted LPS and pure LPS when loaded at normal loading concentrations. However, higher loading concentrations (10 μg), as used in the original study of Al-Hendy et al., yielded bands in the high-Mr region which corresponded to aggregrates of LPS. Within the same strain, no differences in ligand or antibody affinities were observed among miniphenol-water-extracted LPS, LPSs from the miniphenol-water modified procedures, and pure LPS, again justifying the use of miniphenol-water extraction in our assay system. Additionally, ligand binding to ganglioside-mimicking pure LPSs was compared to ligand interactions with miniphenol-water-extracted LPSs from the same nine strains. In general, the ganglioside detection reagents had the same specificities for miniphenol-water-extracted LPSs and pure LPSs. The observation that PNA binding was reproducible only after proteinase K treatment of miniphenol-water extracts suggests that the use of this ligand may be justified only in tests using purer LPS. However, when LPS extraction was repeated using some of the strains, identical binding results were observed upon retesting, thus confirming the reproducibility of the assay. The reliability of the rapid assay was confirmed when miniphenol-water LPS extracts from strains with known ganglioside-like structures were tested (5, 39, 40, 49, 59, 61, 62).
Once the assay was validated and shown to be reliable and reproducible, 59 C. jejuni serostrains were screened for GM1-bearing LPS. Some serostrains with known ganglioside mimicry reacted with the ligands as expected, e.g., serostrains O:4, O:19, and O:36, which is consistent with GM1 or GM2 mimicry in these strains (7, 10, 29, 59, 62). C. jejuni serotypes that have been isolated from neuropathy patients and for which no LPS structural data is available include serotypes O:5, O:13, and O:44 and, in this study, were found to bear GM1-like epitopes. Based on recognition of PNA and anti-GM1 antibodies, LPSs from serostrains O:14, O:25, O:34, and O:43 were considered to exhibit asialo-GM1 mimicry. Interestingly, none of these serotypes have yet been found in association with GBS, although, to date, all neuropathy-associated strains bear LPSs which are sialylated.
Overall, 46 of 59 serostrains (78%) either failed to react with any of the three ligands used or were weakly positive with one ligand, suggesting that GM1 ganglioside-like epitopes are carried only by some Penner serotypes, which may account for the limited number of serotypes found in association with GBS. However, of the 46 serostrains that failed to react with any of the ligands tested, 11 have been found in association with GBS: serostrains O:1, O:2, O:15, O:16, O:18, O:20, O:23, O:24, O:30, O:37, and O:53 (31, 33). It is thought that the LPSs from these serotypes mimic more complex gangliosides that could not be detected with the reagents used in the present study. On the other hand, no serostrain with known GM1 mimicry failed to react with CT in combination with anti-GM1 antibodies, thus justifying the use of the rapid assay for GM1 screening of C. jejuni strains.
Subsequently, the assay was used for the screening of C. jejuni GBS and enteritis isolates for GM1 mimicry. The majority of the GBS-associated serotype O:41 strains had GM1-like structures in their LPSs, which is consistent with the GM1 mimicry previously reported for serotype O:41 GBS-associated strains (39, 40). Of the enteritis-associated strains, half had LPSs which had GM1 epitopes, and thus mimicry of ganglioside GM1 by core OS of C. jejuni strains is not limited to strains associated with GBS. This phenomenon has previously been reported by a number of groups (29, 34, 39, 46, 51). A study by Nachamkin et al. (34) showed that 26% of enteritis isolates were positive for the GM1-like epitope. Patients who develop enteritis and have isolates with ganglioside-mimicking LPS do not develop antiganglioside antibodies (37, 43, 51). The humoral immune response to neural cross-reactive epitopes in the LPSs of C. jejuni appears to be different in GBS than in uncomplicated enteritis (51). These factors suggest that other attributes of the host and/or bacterium in addition to ganglioside mimicry, contribute to the development of GBS or MFS.
The rapid screening assay described here has advantages over other systems reported previously (34, 46, 51). One assay involved spotting boiled cultures directly onto a nitrocellulose membrane and probing for GM1 epitopes with CT and PNA (34). First, in our rapid assay system, crude LPSs rather than boiled lysates are used, and thus there is no interference from non-LPS constituents. Second, the LPS is separated by TLC using silica as an adsorbent, a system whereby the conformation of the antigenic structure is unaltered, and this is considered not to be the case with the attachment of LPS to nitrocellulose membranes. Moreover, TLC separates LPS from contaminating components during the development process. In screening for GM1-bearing strains, Sheikh et al. (51) used purified LPS extracted by the hot phenol-water extraction procedure (55) and probed immunoblotted material with CT, PNA, and TTC. The main limitation of this assay was efficiency, as it was necessary to produce pure LPS and immunoblotting was required. Another assay, described by Sack et al. (46), was based on an inhibitory enzyme-labeled immunosorbent assay (ELISA) whereby strains with a GM1-like LPS bind to CT and inhibit the binding of control CT to ganglioside GM1. However, the assay had the disadvantage that crude boiled extracts were used, and the assay detected CT binding only, which can be misleading, and did not detect GM1 epitopes directly. Moreover, CT is a GM1 ligand, but it also cross-reacts with GM2 and asialo-GM1 gangliosides, and thus some of the strains detected by Sack et al. (46) may have possessed asialo-GM1 or GM2 epitopes. Nevertheless, the inhibition assay had the advantage that large numbers of strains could be tested quickly. In summary, the rapid screening assay described in the present study has the advantages of being reliable, reproducible, and able to screen large numbers of strains quickly; thus, the assay has attributes attractive for large-scale epidemiology studies.
ACKNOWLEDGMENTS
This study was supported by grants from the Irish Health Research Board (grant 86-95 to A.P.M. and grant 12/99 to M.M.P.).
We thank A. J. Lastovica (Cape Town, South Africa) for providing the C. jejuni O:41 strains and B. C. Jacobs (Rotterdam, The Netherlands) for providing the C. jejuni AZR 6491 isolate.
REFERENCES
- 1.Al-Hendy A, Toivanen P, Skurnik M. Rapid method for isolation and staining of bacterial lipopolysaccharide. Microbiol Immunol. 1991;35:331–333. doi: 10.1111/j.1348-0421.1991.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 2.Appelmelk B J, Maaskant J J, Verweij van Vught A M, van der Meer N M, Thijs B G, McLaren D M. Antigenic and immunogenic differences in lipopolysaccharides of Escherichia coli J5 vaccine strains of different origins. J Gen Microbiol. 1993;139:2641–2647. doi: 10.1099/00221287-139-11-2641. [DOI] [PubMed] [Google Scholar]
- 3.Aspinall G O, Fujimoto S, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharide from Campylobacter jejuni associated with Guillain-Barré syndrome patients mimic human gangliosides in structure. Infect Immun. 1994;62:2122–2125. doi: 10.1128/iai.62.5.2122-2125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aspinall G O, Lynch C M, Pang H, Shaver R T, Moran A P. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur J Biochem. 1995;231:570–578. [PubMed] [Google Scholar]
- 5.Aspinall G O, Mainkar A, Prendergast M M, Jacobs B C, Moran A P. Lipopolysaccharides from a neuropathy-associated infection from Campylobacter jejuni serotype O:23. In: Lastovica A J, Newell D G, Lastovica E E, editors. Campylobacter, Helicobacter and related organisms. Cape Town, South Africa: Institute of Child Health and University of Cape Town; 1997. pp. 93–96. [Google Scholar]
- 6.Aspinall G O, McDonald A G, Pang H. Structures of the O chains from lipopolysaccharides from Campylobacter jejuni serotypes O:23 and O:36. Carbohydr Res. 1992;231:13–20. doi: 10.1016/0008-6215(92)84003-b. [DOI] [PubMed] [Google Scholar]
- 7.Aspinall G O, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharides of Campylobacter jejuni serotype O:19. Structures of the core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry. 1994;33:241–249. doi: 10.1021/bi00167a032. [DOI] [PubMed] [Google Scholar]
- 8.Aspinall G O, McDonald A G, Raju T S, Pang H, Kurjanczyk L A, Penner J L. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharide. Eur J Biochem. 1993;213:1029–1037. doi: 10.1111/j.1432-1033.1993.tb17850.x. [DOI] [PubMed] [Google Scholar]
- 9.Aspinall G O, McDonald A G, Raju T S, Pang H, Mills S D, Kurjanczyk L A, Penner J L. Serological diversity and chemical structures of Campylobacter jejuni low-molecular-weight lipopolysaccharides. J Bacteriol. 1992;174:1324–1332. doi: 10.1128/jb.174.4.1324-1332.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aspinall G O, McDonald A G, Raju T S, Pang H, Moran A P, Penner J L. Chemical structure of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur J Biochem. 1993;213:1017–1027. doi: 10.1111/j.1432-1033.1993.tb17849.x. [DOI] [PubMed] [Google Scholar]
- 11.Aspinall G O, Monteiro M O, Pang H, Walsh E J, Moran A P. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): structure of the O antigen and core oligosaccharide regions. Biochemistry. 1996;35:2489–2497. doi: 10.1021/bi951852s. [DOI] [PubMed] [Google Scholar]
- 12.Blake D C, Russell R G. Demonstration of lipopolysaccharide with O-polysaccharide chains among different heat-stable serotypes of Campylobacter jejuni by silver staining of polyacrylamide gels. Infect Immun. 1993;61:5384–5387. doi: 10.1128/iai.61.12.5384-5387.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butzler J P, Dekeyser P, Detrain M, Dehaen F. Related vibrio in stools. J Pediatr. 1973;82:493–495. doi: 10.1016/s0022-3476(73)80131-3. [DOI] [PubMed] [Google Scholar]
- 14.Endtz H P, Ang C W, van den Braak N, Duim B, Rigter A, Price L J, Woodward D L, Rodgers F G, Johnson W M, Wegenaar J A, Jacobs B C, Verbrugh H A, Belkum A. Molecular characterization of Campylobacter jejuni from patients with Guillain-Barré and Miller Fisher syndromes. J Clin Microbiol. 2000;38:2297–2301. doi: 10.1128/jcm.38.6.2297-2301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregson N A, Koblar S, Hughes R A C. Antibodies to gangliosides in Guillain-Barré syndrome: specificity and relationship to clinical features. Q J Med. 1993;86:111–117. [PubMed] [Google Scholar]
- 16.Griffin J W, Ho T W H. The Guillain-Barré syndrome at 75: the Campylobacter connection. Ann Neurol. 1993;34:125–127. doi: 10.1002/ana.410340204. [DOI] [PubMed] [Google Scholar]
- 17.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho T W, Mishu B, Li C Y, Gao C Y, Cornblath D R, Griffin J W, Asbury A K, Blaser M J, McKhann G M. Guillain-Barré syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118:597–605. doi: 10.1093/brain/118.3.597. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs B, Endtz H, van der Meché F G A, Hazenberg M P, Achtereekte H A M, van Doorn P A. Serum anti-GQ1b IgG antibodies recognize surface epitopes on Campylobacter jejuni from patients with Miller Fisher syndrome. Ann Neurol. 1995;37:260–264. doi: 10.1002/ana.410370218. [DOI] [PubMed] [Google Scholar]
- 20.Kuroki S, Saida T, Nukina M, Haruta T, Yoshiota M, Kobayashi Y, Nakanishi H. Campylobacter jejuni strains from patients with Guillain-Barré syndrome belong mostly to Penner serogroup 19 and contain β-N-acetylglucosamine residues. Ann Neurol. 1993;33:243–247. doi: 10.1002/ana.410330304. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Lastovica A J, Goddard E A, Argent A C. Guillain-Barré syndrome in South Africa associated with Campylobacter jejuni O:41 strains. J Infect Dis. 1997;176(Suppl. 2):139–143. doi: 10.1086/513796. [DOI] [PubMed] [Google Scholar]
- 23.Lindblom G B, Kaijser B, Sjogren E. Enterotoxin production and serogroups of Campylobacter jejuni and Campylobacter coli from patients with diarrhea and from healthy laying hens. J Clin Microbiol. 1989;27:1272–1276. doi: 10.1128/jcm.27.6.1272-1276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linton D, Karlyshev A V, Hitchen P G, Morris H R, Dell A, Gregson N A, Wren B W. Multiple N-acetylneuraminic acid synthetase (neuB) genes in Campylobacter jejuni: identification and characterization of the gene involved in sialylation of lipo-oligosaccharide. Mol Microbiol. 2000;35:1120–1134. doi: 10.1046/j.1365-2958.2000.01780.x. [DOI] [PubMed] [Google Scholar]
- 25.Mills S D, Kurjanczyk L A, Penner J L. Antigenicity of Helicobacter pylori lipopolysaccharides. J Clin Microbiol. 1992;30:3175–3180. doi: 10.1128/jcm.30.12.3175-3180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishu B, Blaser M J. Role of infection due to Campylobacter jejuni in the initiation of Guillain-Barré syndrome. Clin Infect Dis. 1993;17:104–108. doi: 10.1093/clinids/17.1.104. [DOI] [PubMed] [Google Scholar]
- 27.Monos D S, Papaioakim M, Ho T W, Li C Y, McKhann G M. Differential distribution of HLA alleles in two forms of Guillain-Barré syndrome. J Infect Dis. 1997;176(Suppl. 2):180–182. doi: 10.1086/513786. [DOI] [PubMed] [Google Scholar]
- 28.Moran A P, Kosunen T U. Serological analysis of the heat-stable antigens involved in serotyping Campylobacter jejuni and Campylobacter coli. APMIS. 1989;97:253–260. [PubMed] [Google Scholar]
- 29.Moran A P, O'Malley D T. Potential role of lipopolysaccharides of Campylobacter jejuni in the development of Guillain-Barré syndrome. J Endotoxin Res. 1995;2:233–235. [Google Scholar]
- 30.Moran A P, Penner J L. Serotyping of Campylobacter jejuni based on heat-stable antigens: relevance, molecular basis and implications in pathogenesis. J Appl Microbiol. 1999;86:361–377. doi: 10.1046/j.1365-2672.1999.00713.x. [DOI] [PubMed] [Google Scholar]
- 31.Moran A P, Prendergast M M, Appelmelk B J. Molecular mimicry of host structures by bacterial lipopolysaccharide and its contribution to disease. FEMS Immunol Med Microbiol. 1996;16:105–115. doi: 10.1111/j.1574-695X.1996.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 32.Moran A P, Rietschel E T, Kosunen T U, Zahringer U. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-d-glucose. J Bacteriol. 1991;173:618–626. doi: 10.1128/jb.173.2.618-626.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nachamkin I, Allos B M, Ho T. Campylobacter species and Guillain-Barré syndrome. Clin Microbiol Rev. 1998;11:555–567. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nachamkin I, Ung H, Moran A P, Yoo D, Prendergast M M, Nicholson M A, Sheikh K A, Ho T W, Asbury A K, McKhann G M, Griffin J W. Ganglioside GM1 mimicry in Campylobacter strains from sporadic infections in the United States. J Infect Dis. 1999;179:1183–1189. doi: 10.1086/314725. [DOI] [PubMed] [Google Scholar]
- 35.Nobile-Orazio E, Carpo M, Meucci N, Grassi M P, Capitani E, Sciacco M, Mangoni A, Scarlato G. Guillain-Barré syndrome associated with high titres of anti-GM1 antibodies. J Neurol Sci. 1992;109:200–206. doi: 10.1016/0022-510x(92)90169-l. [DOI] [PubMed] [Google Scholar]
- 36.Obayashi H, Saida T, Kuroki S, Nukina M, Nishitani Y. Guillain-Barré syndrome and Campylobacter jejuni infection. Shinkei Naika. 1993;38:431–438. [Google Scholar]
- 37.Oomes P G, Jacobs B C, Hazenberg M P H, Bänffer J R J, van der Meché F G A. Anti-GM1 IgG antibodies and Campylobacter bacteria in Guillain-Barré syndrome: evidence of molecular mimicry. Ann Neurol. 1995;38:170–175. doi: 10.1002/ana.410380208. [DOI] [PubMed] [Google Scholar]
- 38.Penner J L, Hennessy J N, Congi R V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983;2:78–83. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- 39.Prendergast M M, Lastovica A J, Moran A P. Lipopolysaccharides from Campylobacter jejuni O:41 strains associated with Guillain-Barré syndrome exhibit mimicry of GM1 ganglioside. Infect Immun. 1998;66:3649–3655. doi: 10.1128/iai.66.8.3649-3655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prendergast M M, Willison H J, Moran A P. Human monoclonal IgM antibodies to GM1 ganglioside show diverse cross-reactivity with lipopolysaccharides of Campylobacter jejuni strains associated with Guillain-Barré syndrome. Infect Immun. 1999;67:3698–3701. doi: 10.1128/iai.67.7.3698-3701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston M A, Penner J L. Structural and antigenic properties of lipopolysaccharide from serotype reference strains of Campylobacter jejuni. Infect Immun. 1987;55:1806–1812. doi: 10.1128/iai.55.8.1806-1812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rees J H, Gregson N A, Griffiths P L, Hughes R A C. Campylobacter jejuni and Guillain-Barré syndrome. Q J Med. 1993;86:623–634. doi: 10.1093/qjmed/86.10.623. [DOI] [PubMed] [Google Scholar]
- 43.Rees J H, Gregson N A, Hughes R A C. Anti-ganglioside GM1 antibodies in Guillain-Barré syndrome and their relationship to Campylobacter jejuni infection. Ann Neurol. 1995;38:809–816. doi: 10.1002/ana.410380516. [DOI] [PubMed] [Google Scholar]
- 44.Rees J H, Hughes R A C. Campyobacter jejuni and Guillain-Barré syndrome. Ann Neurol. 1994;35:248–249. doi: 10.1002/ana.410350228. [DOI] [PubMed] [Google Scholar]
- 45.Rees J H, Soudain S E, Gregson N A, Hughes R A C. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 46.Sack D A, Lastovica A J, Chang S H, Pazzaglia G. Microtiter assay for detecting Campylobacter spp. and Helicobacter pylori with surface gangliosides which bind cholera toxin. J Clin Microbiol. 1998;36:2043–2045. doi: 10.1128/jcm.36.7.2043-2045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saito M, Kasai N, Yu R K. In situ immunological determination of basic carbohydrate structures of gangliosides on thin-layer plates. Anal Biochem. 1985;148:54–58. doi: 10.1016/0003-2697(85)90627-x. [DOI] [PubMed] [Google Scholar]
- 48.Salloway S, Mermel L A, Seamens M, Aspinall G O, Nam Shin J E, Kurjanczyk L A, Penner J L. Miller Fisher syndrome associated with Campylobacter jejuni bearing lipopolysaccharide molecules that mimic human ganglioside GD3. Infect Immun. 1996;64:2945–2949. doi: 10.1128/iai.64.8.2945-2949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwerer B, Neisser A, Polt R J, Bernheimer H, Moran A P. Antibody cross-reactivities between gangliosides and lipopolysaccharides of Campylobacter jejuni serotypes associated with Guillain-Barré syndrome. J Endotoxin Res. 1995;2:395–403. [Google Scholar]
- 50.Schwimmer S, Bevenue A. Reagent for differentiation of 1,4- and 1,6-linked glucosaccharides. Science. 1956;123:543–544. doi: 10.1126/science.123.3196.543. [DOI] [PubMed] [Google Scholar]
- 51.Sheikh K A, Nachamkin I, Ho T W, Willison H J, Veitch J, Ung H, Nicholson M, Li C Y, Wu H S, Shen B Q, Cornblath D R, Asbury A K, McKhann G M, Griffin J W. Campylobacter jejuni lipopolysaccharide in Guillain-Barré syndrome: molecular mimicry and host susceptibility. Neurology. 1998;51:371–378. doi: 10.1212/wnl.51.2.371. [DOI] [PubMed] [Google Scholar]
- 52.Skirrow M B, Benjamin J. Differentiation of enteropathogenic Campylobacter. J Clin Pathol. 1980;33:1122. doi: 10.1136/jcp.33.11.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 55.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–92. [Google Scholar]
- 56.Willison H J, Kennedy P G E. Gangliosides and bacterial toxins in Guillain-Barré syndrome. J Neuroimmunol. 1993;46:105–112. doi: 10.1016/0165-5728(93)90239-u. [DOI] [PubMed] [Google Scholar]
- 57.Yuki N. Pathogenesis of axonal Guillain-Barré syndrome: hypothesis. Muscle Nerve. 1994;17:680–682. doi: 10.1002/mus.880170619. [DOI] [PubMed] [Google Scholar]
- 58.Yuki N, Handa S, Tai T, Takahashi M, Saito K, Tsujino Y, Taki T. Ganglioside-like epitopes of lipopolysaccharide from Campylobacter jejuni (Pen 19) in three isolates from patients with Guillain-Barré syndrome. J Neurol Sci. 1995;130:112–116. doi: 10.1016/0022-510x(95)00045-4. [DOI] [PubMed] [Google Scholar]
- 59.Yuki N, Handa S, Taki T, Kasama T, Takihashi M, Saito K, Miyatake T. Cross-reactive antigen between nervous tissue and a bacterium elicits Guillain-Barré syndrome: molecular mimicry between ganglioside GM1 and lipopolysaccharide from Penner's serotype 19 of Campylobacter jejuni. Biomed Res. 1992;13:451–453. [Google Scholar]
- 60.Yuki N, Sato S, Itoh T, Miyatake T. HLA-B35 and acute axonal polyneuropathy following Campylobacter infection. Neurology. 1991;41:1561–1563. doi: 10.1212/wnl.41.10.1561. [DOI] [PubMed] [Google Scholar]
- 61.Yuki N, Taki T, Inagaki F, Kasama T, Takahashi T M, Saito M K, Handa S, Miyatake T. A bacterium lipopolysaccharide that elicits Guillain-Barré syndrome has a GM1 ganglioside-like structure. J Exp Med. 1993;178:1771–1775. doi: 10.1084/jem.178.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuki N, Taki T, Takahashi M, Saito K, Tai T, Miyatake T, Handa S. Penner's serogroup 4 of Campylobacter jejuni has a lipopolysaccharide that bears a GM1 ganglioside epitope as well as one that bears a GD1a epitope. Infect Immun. 1994;62:2101–2103. doi: 10.1128/iai.62.5.2101-2103.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuki N, Yoshino H, Sato S, Miyatake T. Acute axonal polyneuropathy associated with anti-GM1 antibodies following Campylobacter enteritis. Neurology. 1990;40:1900–1902. doi: 10.1212/wnl.40.12.1900. [DOI] [PubMed] [Google Scholar]