Abstract
According to Article 12 of Regulation (EC) No 396/2005, EFSA has reviewed the maximum residue levels (MRLs) currently established at European level for the pesticide active substance tetraconazole. To assess the occurrence of tetraconazole residues in plants, processed commodities, rotational crops and livestock, EFSA considered the conclusions derived in the framework of Directive 91/414/EEC, as well as the import tolerances and European authorisations reported by Member States and the UK (including the supporting residues data). Based on the assessment of the available data, MRL proposals were derived and a consumer risk assessment was carried out. Although no apparent risk to consumers was identified, some information required by the regulatory framework was missing. Hence, the consumer risk assessment is considered indicative only and, with the exception of the MRL proposal for kaki, all MRL proposals derived by EFSA still require further consideration by risk managers. Regarding triazole derivative metabolites (TDMs), separate indicative exposure assessments were performed and no risk to consumers was identified for what regards these metabolites individually. However, TDMs may be generated by several pesticides belonging to the group of triazole fungicides, and a comprehensive risk assessment has thus to be performed that covers all existing European uses for all pesticides belonging to the class of triazole fungicides. EFSA recommended to elaborate together with risk managers a strategy to ensure that the required data are made available to finalise the overall risk assessment for triazole fungicides.
Keywords: tetraconazole, MRL review, Regulation (EC) No 396/2005, consumer risk assessment, fungicide, triazole, triazole derivative metabolites
Summary
Tetraconazole was included in Annex I to Council Directive 91/414/EEC on 1 January 2010 by Council Directive 2009/82/EC, and has been deemed to be approved under Regulation (EC) No 1107/2009, in accordance with Commission Implementing Regulation (EU) No 540/2011, as amended by Commission Implementing Regulation (EU) No 541/2011.
As the active substance was approved after the entry into force of Regulation (EC) No 396/2005 on 2 September 2008, the European Food Safety Authority (EFSA) is required to provide a reasoned opinion on the review of the existing maximum residue levels (MRLs) for that active substance in compliance with Article 12(1) of the aforementioned regulation.
As the basis for the MRL review, on 16 October 2020, EFSA initiated the collection of data for this active substance. In a first step, Member States and the UK were invited to submit by 13 November 2020 their national Good Agricultural Practices (GAPs) in a standardised way, in the format of specific GAP forms, allowing the designated rapporteur Member State (RMS), France, to identify the critical GAPs in the format of a specific GAP overview file. Subsequently, Member States and the UK were requested to provide residue data supporting the critical GAPs, within a period of 1 month, by 7 January 2021. On the basis of all the data submitted by Member States, the UK and the EU Reference Laboratories for Pesticides Residues (EURLs), EFSA asked the RMS to complete the Pesticide Residues Overview Files (PROFile) for tetraconazole and for each triazole derivative metabolite and to prepare a supporting evaluation report. The PROFiles and evaluation report, together with Pesticide Residues Intake Model (PRIMo) calculations and an updated GAP overview file were provided by the RMS to EFSA on 16 April 2021. Subsequently, EFSA performed the completeness check of these documents with the RMS. The outcome of this exercise including the clarifications provided by the RMS, if any, was compiled in the completeness check report.
Based on the information provided by the RMS, Member States, the UK and the EURLs, and taking into account the conclusions derived by EFSA in the framework of Directive 91/414/EEC, EFSA prepared in November 2021 a draft reasoned opinion, which was circulated to Member States and the EURLs for consultation via a written procedure. Comments received by 29 November 2021 were considered during the finalisation of this reasoned opinion. The following conclusions are derived.
The metabolism of tetraconazole in plant was investigated in primary and rotational crops. According to the results of the metabolism studies, the residue definition for enforcement can be proposed as tetraconazole. As regards risk assessment, four residue definitions are set separately, namely, RD‐RA1: tetraconazole; RD‐RA2: triazole alanine (TA) and triazole lactic acid (TLA), RD‐RA3: triazole acetic acid (TAA); RD‐RA4: 1,2,4‐triazole (1,2,4‐T). These residue definitions are applicable to raw (from primary and rotational crops) and processed commodities. Fully validated analytical methods are available for the enforcement of the proposed residue definition in plant matrices at the limit of quantification (LOQ) of 0.01 mg/kg. According to the EURLs, the LOQ of 0.01 mg/kg is achievable in the four main matrix groups of plant origin by using the QuEChERS method in routine analyses.
Available residue trials data were considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for sweet peppers, globe artichokes, barley and oat grain, buckwheat, maize, millet and sorghum grain and rice grain for which no data are available to derive MRLs for tetraconazole and risk assessment values for tetraconazole, the sum of TA and TLA, and TAA. Residues of parent tetraconazole are expected to be negligible in rotational crops. However, for TDMs, EFSA could not derive robust risk assessment values covering residues in rotational crops.
Tetraconazole is authorised for use on crops that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance. According to the proposed residue definitions for risk assessment, separate calculations were performed for tetraconazole (RD‐RA1), the sum of TA and TLA (RD‐RA2), TAA (RD‐RA3) and 1,2,4‐T (RD‐RA4). For RD‐RA1, RD‐RA2 and RD‐RA3, the dietary burdens calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM). Behaviour of residues of these compounds was therefore assessed in all commodities of animal origin. Although yet to be confirmed, the calculated dietary burdens for RD‐RA4 were found to be below the trigger value of 0.1 mg/kg DM for all groups of livestock, and further investigation of residues for 1,2,4‐T in commodities of animal origin is unnecessary.
The metabolism of tetraconazole residues in livestock was investigated in lactating goats and laying hens at dose rate covering the maximum dietary burdens calculated in this review. Metabolism studies on goats and hens were also available with TA directly fed to animals. According to the results of these studies, the residue definition for enforcement in livestock commodities was proposed as tetraconazole. As regards risk assessment, four residue definitions were set separately, namely, RD‐RA1: tetraconazole; RD‐RA2: TA and TLA; RD‐RA3: TAA; RD‐RA4: 1,2,4‐T. An analytical method for the enforcement of the proposed residue definition at the LOQ of 0.01 mg/kg in livestock matrices is available. According to EURLs, parent tetraconazole can be monitored in milk with an LOQ of 0.01 mg/kg, in routine analysis, and in eggs with a screening detection limit (SDL) of 0.005 mg/kg. In muscle, milk and honey, even lower SDLs down to 0.0025 mg/kg were achieved. Based on this, an LOQ of 0.01 mg/kg is supposed to be achievable for the other animal products (e.g. liver, kidney, fat).
Livestock feeding studies on dairy cows and laying hens fed with parent tetraconazole were used to derive MRL and risk assessment values in milk, eggs and tissues of ruminants and poultry for parent. Since extrapolation from ruminants to pigs is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values of parent tetraconazole in pigs. Considering that mangel fodder beet was the major contributor to the dietary burden of bovine and swine diets, and that input values for this commodity may be overestimated (based on trials performed according to a more critical GAP), MRLs for bovine tissues and milk, and swine tissues are considered tentative.
Regarding TDMs, poultry and ruminants feeding studies were both performed with TA and TAA. These feeding studies were used to derive risk assessment values for RD‐RA2 (TA and TLA) and RD‐RA3 (TAA). Considering that wheat/rye or canola products (which are not fully supported by residue trials) were found to be the major contributors to the dietary burden of livestock diets for RD‐RA2 and RD‐RA3, all risk assessment values derived in livestock for these two residue definitions should be considered as tentative.
As different toxicological reference values were derived, respectively, for tetraconazole and for the TDMs, EFSA performed separate consumer risk assessments for tetraconazole (RD‐RA1), for the sum of TA and TLA (RD‐RA2), for TAA (RD‐RA3) and for 1,2,4‐T (RD‐RA4). Chronic and acute consumer exposures resulting from the authorised uses reported in the framework of this review were calculated using revision 3.1 of the EFSA PRIMo. It is underlined that for the sum of TA and TLA (RD‐RA2) and for TAA (RD‐RA3), the contributions of commodities for which no residue data were available to derive at least tentative risk assessment values (sweet peppers, witloofs, barley, buckwheat, maize, millet, oat, rice and sorghum) were not included in the calculation. For tetraconazole, the highest chronic exposure was calculated for Dutch toddlers, representing 65% of the acceptable daily intake (ADI), and the highest acute exposure was calculated for pears, representing 45% of the acute reference dose (ARfD). For the sum of TA and TLA, the highest chronic exposure was calculated for Dutch toddlers representing 4% of the ADI, and the highest acute exposure was calculated for pears, representing 24% of the ARfD. Concerning TAA, the highest chronic exposure was calculated for Dutch toddlers representing 0.1% of the ADI, and the highest acute exposure was calculated for pears, representing 0.3% of the ARfD. For 1,2,4‐T, the highest chronic exposure was calculated for Dutch toddlers representing 2% of the ADI, and the highest acute exposure was calculated for melons, representing 2% of the ARfD.
EFSA emphasises that a comprehensive risk assessment including all crops and all pesticides belonging to the class of triazole fungicides has not been performed in the framework of the current review.
Background
Regulation (EC) No 396/2005 1 (hereinafter referred to as ‘the Regulation’) establishes the rules governing the setting and the review of pesticide maximum residue levels (MRLs) at European level. Article 12(1) of that Regulation stipulates that the European Food Safety Authority (EFSA) shall provide within 12 months from the date of the inclusion or non‐inclusion of an active substance in Annex I to Directive 91/414/EEC 2 a reasoned opinion on the review of the existing MRLs for that active substance.
Tetraconazole was included in Annex I to Council Directive 91/414/EEC on 1 January 2010 by means of Council Directive 2009/82/EC 3 which has been deemed to be approved under Regulation (EC) No 1107/2009 4 , in accordance with Commission Implementing Regulation (EU) No 540/2011 5 , as amended by Commission Implementing Regulation (EU) No 541/2011 6 . Therefore, EFSA initiated the review of all existing MRLs for that active substance.
By way of background information, tetraconazole was evaluated by Italy, designated as rapporteur Member State (RMS) in the framework of Directive 91/414/EEC. Subsequently, a peer review on the initial evaluation of the RMS was conducted by EFSA, leading to the conclusions as set out in the EFSA scientific output (EFSA, 2008). The approval of tetraconazole was restricted to uses as fungicide on field crops with a restricted rate and timing of application and uses in apples and grapes were entirely excluded. Following an application for the amendment of the conditions of approval, these restrictions were removed by Council Directive 2010/82/EC 7 . A peer review for the triazole derivative metabolites (TDMs) in light of confirmatory data was conducted by EFSA (EFSA, 2018b; European Commission, 2021).
According to the legal provisions, EFSA shall base its reasoned opinion in particular on the relevant assessment report prepared under Directive 91/414/EEC repealed by Regulation (EC) No 1107/2009. It should be noted, however, that, in the framework of Regulation (EC) No 1107/2009, only a few representative uses are evaluated, whereas MRLs set out in Regulation (EC) No 396/2005 should accommodate all uses authorised within the European Union (EU), and uses authorised in third countries that have a significant impact on international trade. The information included in the assessment report prepared under Regulation (EC) No 1107/2009 is therefore insufficient for the assessment of all existing MRLs for a given active substance.
To gain an overview of the pesticide residues data that have been considered for the setting of the existing MRLs, EFSA developed the Pesticide Residues Overview File (PROFile). The PROFile is an inventory of all pesticide residues data relevant to the risk assessment and MRL setting for a given active substance. This includes data on:
the nature and magnitude of residues in primary crops;
the nature and magnitude of residues in processed commodities;
the nature and magnitude of residues in rotational crops;
the nature and magnitude of residues in livestock commodities;
the analytical methods for enforcement of the proposed MRLs.
As the basis for the MRL review, on 16 October 2020, EFSA initiated the collection of data for this active substance. In a first step, Member States and the UK 8 were invited to submit by 13 November 2020 their Good Agricultural Practices (GAPs) that are authorised nationally, in a standardised way, in the format of specific GAP forms. In the framework of this consultation 16 Member States and the UK provided feedback on their national authorisations of tetraconazole. Based on the GAP data submitted, the designated RMS France was asked to identify the critical GAPs to be further considered in the assessment, in the format of a specific GAP overview file. Subsequently, in a second step, Member States and the UK were requested to provide residue data supporting the critical GAPs by 7 January 2021.
On the basis of all the data submitted by Member States, the UK and the EU Reference Laboratories for Pesticides Residues (EURLs), EFSA asked France to complete the PROFile for tetraconazole and for each triazole derivative metabolite and to prepare a supporting evaluation report. The PROFile and the supporting evaluation report, together with the Pesticide Residues Intake Model (PRIMo) calculations and an updated GAP overview file, were submitted to EFSA on 16 April 2021. Subsequently, EFSA performed the completeness check of these documents with the RMS. The outcome of this exercise including the clarifications provided by the RMS, if any, was compiled in the completeness check report.
Considering all the available information, EFSA prepared in November 2021 a draft reasoned opinion, which was circulated to Member States and the EURLs for commenting via a written procedure. All comments received by 29 November 2021 were considered by EFSA during the finalisation of the reasoned opinion.
The evaluation report submitted by the RMS (France, 2021), taking into account also the information provided by Member States and the UK during the collection of data, and the EURLs report on analytical methods (EURLs, 2021) are considered as main supporting documents to this reasoned opinion and, thus, made publicly available.
In addition, further supporting documents to this reasoned opinion are the completeness check report (EFSA, 2021a) and the Member States consultation report (EFSA, 2021b). These reports are developed to address all issues raised in the course of the review, from the initial completeness check to the reasoned opinion. Furthermore, the exposure calculations for all crops reported in the framework of this review performed using the EFSA Pesticide Residues Intake Model (PRIMo) and the PROFiles as well as the GAP overview file listing all authorised uses and import tolerances are key supporting documents and made publicly available as background documents to this reasoned opinion. A screenshot of the report sheet of the PRIMo is presented in Appendix C.
Terms of reference
According to Article 12 of Regulation (EC) No 396/2005, EFSA shall provide a reasoned opinion on:
the inclusion of the active substance in Annex IV to the Regulation, when appropriate;
the necessity of setting new MRLs for the active substance or deleting/modifying existing MRLs set out in Annex II or III of the Regulation;
the inclusion of the recommended MRLs in Annex II or III to the Regulation;
the setting of specific processing factors as referred to in Article 20(2) of the Regulation.
The active substance and its use pattern
Tetraconazole is the ISO common name for the racemic mixture (RS)‐2‐(2,4‐dichlorophenyl)‐3‐(1H‐1,2,4‐triazol‐1‐yl)propyl‐1,1,2,2‐tetrafluoroethyl ether (IUPAC).
The chemical structure of the active substance and its main metabolites are reported in Appendix F.
The EU MRLs for tetraconazole are established in Annex IIIA of Regulation (EC) No 396/2005. Codex maximum residue limits (CXLs) for tetraconazole are not available. An overview of the MRL changes that occurred since the entry into force of the Regulation mentioned above is provided below (Table 1).
Table 1.
Overview of the MRL changes since the entry into force of Regulation (EC) No 396/2005
| Procedure | Legal implementation | Remarks |
|---|---|---|
| MRL application | Regulation (EU) No 2019/1015 a | kaki/Japanese persimmon, linseeds and poppy seeds (EFSA, 2019a) |
| MRL application | Not implemented | Commodities of plant and animal origin (EFSA, 2013b) |
| MRL application | Regulation (EC) No 34/2013 b | Rapeseed (EFSA, 2012) |
| MRL application | Regulation (EC) No 822/2009 c | Apricots (EFSA, 2009) |
Commission Regulation (EU) 2019/1015 of 20 June 2019 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for aminopyralid, captan, cyazofamid, flutianil, kresoxim‐methyl, lambda‐cyhalothrin, mandipropamid, pyraclostrobin, spiromesifen, spirotetramat, teflubenzuron and tetraconazole in or on certain products. OJ L 165, 21.6.2019, p. 23–64.
Commission Regulation (EU) No 34/2013 of 16 January 2013 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for 2‐phenylphenol, ametoctradin, Aureobasidium pullulans strains DSM 14940 and DSM 14941, cyproconazole, difenoconazole, dithiocarbamates, folpet, propamocarb, spinosad, spirodiclofen, tebufenpyrad and tetraconazole in or on certain products. OJ L 25, 26.1.2013, p. 1–48.
Commission Regulation (EC) No 822/2009 of 27 August 2009 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for azoxystrobin, atrazine, chlormequat, cyprodinil, dithiocarbamates, fludioxonil, fluroxypyr, indoxacarb, mandipropamid, potassium tri‐iodide, spirotetramat, tetraconazole and thiram in or on certain products. OJ L 239, 10.9.2009, p. 5–45.
For the purpose of this MRL review, all the uses of tetraconazole currently authorised within the EU and in third countries as submitted by the Member States and the UK during the GAP collection have been reported by the RMS in the GAP overview file. The critical GAPs identified in the GAP overview file were then summarised in the PROFiles and considered in the assessment. The details of the authorised critical GAPs for tetraconazole are given in Appendix A.
Assessment
EFSA has based its assessment on the following documents:
the PROFiles submitted by the RMS;
the evaluation report accompanying the PROFile (France, 2021);
the draft assessment report (DAR) and its addenda prepared under Council Directive 91/414/EEC (Italy, 2005, 2007, 2008);
the conclusion on the peer review of the pesticide risk assessment of the active substance tetraconazole (EFSA, 2008);
the conclusion on the peer review of the pesticide risk assessment of the TDMs in light of confirmatory data submitted (EFSA, 2018b);
the previous reasoned opinions on tetraconazole (EFSA, 2009, 2012, 2013b, 2019a).
The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/2011 9 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1996, 1997a‐g, 2000, 2010a,b, 2020; OECD, 2008, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of tetraconazole was investigated after foliar application in cereals (wheat), roots (sugar beet) and fruits (grapes) and assessed in the framework of the peer review (Italy, 2005; EFSA, 2008). In all the studies, tetraconazole was radiolabelled in the phenyl and the triazole ring of the molecule. In addition, translocation studies with topical application in growth chamber were performed with triazole label only (Italy, 2005; EFSA, 2008).
After four foliar applications of 22.5–26.5 mg a.i./ha on grapes, the major component identified at maturity in the whole fruit was parent tetraconazole, representing 53% of the total radioactive residues (TRR) (0.088 mg eq./kg) in the triazole labelled study and 55% TRR (0.119 mg eq./kg) in the phenyl labelled study. Five non‐identified metabolites were quantified, individually representing less than 4% of the TRR (< 0.01 mg eq./kg) (Italy, 2005). To be noted that this metabolism study on grapes is underdosed (0.7N) compared with the most critical GAP on fruits under assessment (southern and indoor uses on strawberries). However, in view of the results of the study, where parent was the only significant component of the residue in both labels, the metabolism study is considered acceptable to elucidate the nature of residues in fruits (France, 2021).
After three foliar applications of 100 g a.i./ha on sugar beet (representing 1N compared with critical GAP (cGAP)), the major component identified in roots and leaves was parent tetraconazole for both labels. In the study conducted with the triazole label, parent tetraconazole was identified only in leaves and was metabolised from 97% TRR (1.536 mg eq./kg) immediately after treatment to 48% TRR at maturity (0.647 mg eq./kg). The triazole derivative metabolites (TDMs) triazole lactic acid (TLA), triazole acetic acid (TAA) and 1,2,4‐triazole (1,2,4‐T) were found in mature leaves at percentages below 10% TRR but at levels representing more than 0.05 mg eq./kg. With the phenyl label, parent tetraconazole was present in both root and leaves representing 33% TRR (0.002 mg eq./kg) and 71% TRR (3.57 mg eq./kg), respectively. An additional study with an exaggerated rate (5N) carried out with phenyl label confirmed that parent was the main component of the residue in roots representing 71% TRR (0.03 mg eq./kg) (Italy, 2005).
After two to three foliar applications of 125 g a.i./ha on wheat (1.5N), the major component identified in the studies performed with phenyl label was parent tetraconazole, representing up to 69% TRR (7.986 mg eq./kg) in straw and up to 52% TRR (0.047 mg eq./kg) in grain, at harvest. With triazole label, TDMs triazole alanine (TA) (50.1% TRR; 0.332 mg eq./kg) and TAA (24.9% TRR; 0.165 mg eq./kg) were present in significantly higher amounts than tetraconazole (6.3% TRR; 0.042 mg eq./kg) in cereal grain at harvest (Italy, 2005).
The peer review concluded that the metabolism of tetraconazole after foliar application was comparable in the three crop groups investigated (EFSA, 2008), and this conclusion is still considered valid under the present assessment.
1.1.2. Nature of residues in rotational crops
Tetraconazole is authorised on crops that may be grown in rotation. The field DT90 reported in the soil degradation studies evaluated in the framework of the peer review was > 5,000 days for parent tetraconazole (EFSA, 2008). Furthermore, field DT90 of metabolite 1,2,4‐T was reported to be 718 days (EFSA, 2013a). Therefore, an investigation of residues in rotational crops is required.
One confined rotational crop study with tetraconazole radiolabelled on the phenyl and triazole rings was available for this review (Italy, 2005; EFSA, 2008). Tetraconazole was applied at a rate of 500 g a.i./ha onto bare soil. Additional plots treated at 2,500 g a.i./ha for the phenyl‐labelled study and at 5000 g a.i./ha for the triazole‐labelled study were used to assist in the identification of metabolites. Crops were planted at nominal plant back intervals (PBI) of 30, 120, 223 and 365 days after treatment (DAT). Crops planted at each interval consisted of lettuce, carrots and wheat/sorghum, representatives of leafy vegetables, root/tuber vegetables and cereals.
The major compounds identified with triazole labelling were triazole derivative metabolites TAA, TLA and TA, while 1,2,4‐T was detected but usually at much lower levels. In lettuce, the main proportion of the residue was present as TLA (47–80% TRR; 0.206–0.668 mg eq./kg), exhibiting the highest levels at the longest PBI. In carrots, TLA was predominant in foliage, accounting for 60–69.5% TRR (0.358–0.387 mg eq./kg), while TA was the main component in roots (55.5–67% TRR; 0.236–0.138 mg eq./kg). In wheat straw, the three mentioned TDMs were detected, being TLA the most predominant one (29–38% TRR; 0.24–0.56 mg eq./kg). In grain, TA and TAA were the predominant metabolites, present in amounts one order of magnitude higher (up to 57% TRR; 1.484 mg eq./kg) than parent tetraconazole. With the phenyl label, the most relevant residue was parent tetraconazole in all three crops. In carrots, it decreased from 81% TRR (0.03 mg eq./kg) at PBI 30–72% TRR (0.03 mg eq./kg) at PBI 365. In lettuce, the observed trend was the opposite, i.e. tetraconazole levels increased from 40% TRR (0.01 mg eq./kg) at shortest PBI to 67% TRR (0.012 mg eq./kg) at longest PBI. Finally, in wheat grain, the highest levels of parent were found at 365 PBI (80% TRR; 0.004 mg eq./kg). At PBIs 30 and 120, both parent and metabolite M14360 acid were the main component of the residue, each one representing about 15–20% of the total residue (Italy, 2005; EFSA, 2008).
During the peer review, it was concluded that the metabolism and distribution of tetraconazole in rotational crops were similar to the metabolic pathway observed in primary crops (EFSA, 2008), and this conclusion is still considered valid under the present assessment.
1.1.3. Nature of residues in processed commodities
Studies investigating the nature of residues in processed commodities were assessed in the framework of the peer review (Italy, 2005; EFSA, 2008). Studies were conducted with radiolabelled tetraconazole on the phenyl and triazole ring of the molecule simulating representative hydrolytic conditions for pasteurisation (20 min at 90°C, pH 4), boiling, brewing and baking (60 min at 100°C, pH 5) and sterilisation (20 min at 120°C, pH 6). It was concluded that parent tetraconazole is hydrolytically stable under the representative conditions (EFSA, 2008).
Regarding TDMs, no studies were provided in the framework of this review. However, standard hydrolysis studies on TDMs are available and were assessed by EFSA as confirmatory data submitted for the recent risk assessment of the TDMs (EFSA, 2018b). These studies showed that TA, TAA, TLA and 1,2,4‐T remain stable under the standard hydrolysis conditions simulating processing of pasteurisation, baking, brewing and boiling and sterilisation.
Therefore, it can be concluded that the nature of the residues in processed commodities resulting from the authorised uses of tetraconazole is sufficiently addressed.
1.1.4. Methods of analysis in plants
In the framework of this review, a QuEChERS multiresidue method based on liquid chromatography (LC) coupled to tandem mass spectrometry (MS/MS) detection was validated for parent tetraconazole in high water (apple, sugar beet roots), high acid (grape), high oil (oilseed rape) and dry commodities (wheat) with a limit of quantification (LOQ) of 0.01 mg/kg for all commodities. Data on cereal straw was also reported but with low recoveries (< 70%) (France, 2021).
One additional multiple reaction monitoring (MRM) transition (in LC–MS/MS) was monitored for confirmation purposes. This method is supported for the same commodities by independent laboratory validation (ILV).
During the completeness check, EURLs reported a QuEChERS multiresidue method using gas and liquid chromatography coupled to tandem mass spectrometry (GC– and LC‐MS/MS) for the routine analysis of tetraconazole with an LOQ of 0.01 mg/kg in the four main plant matrices and tea (matrix difficult to be analysed) (EURL, 2021).
1.1.5. Stability of residues in plants
The storage stability of parent tetraconazole was investigated in the framework of the peer review (Italy, 2005; EFSA, 2008) in apple, cereal grain and straw, sugar beet root and wine grape, and in the framework of a previous MRL application (Italy, 2011; EFSA, 2012) in rapeseeds and refined oil.
In high water content, high acid content, high oil content, dry commodities and cereal straw, the available studies demonstrated storage stability for parent tetraconazole for a period of 36 months when stored at −20°C (EFSA, 2008, 2012). In refined oil (rapeseeds), tetraconazole was found to be stable for up to 24 months at −20°C (Italy, 2011; EFSA, 2012).
For the triazole derivative metabolites, additional data were assessed during the peer review of the risk assessment for the TDMs in the light of confirmatory data (EFSA, 2018b). Available data show that TLA was stable for 48 months in all matrix groups when stored at −20°C, except in cereal straw. At the same storage temperature, TA and TAA were found to be stable for 53 months in high water content and in dry/high starch content commodities. In dry/high protein commodities, TA was stable for 15 months and TAA for 25 months. In high oil content matrices, TAA was stable for 53 months. TA was found to be only stable in soya beans for 26 months while the study was inconclusive for rapeseeds (data gap), where the results suggested a rapid degradation during or immediately after fortification. No data were reported for the storage stability of TA and TAA in high acid content commodities, what represents a data gap for TA, while for TAA, this information is considered only desirable (see Section 1.2.1). Finally, 1,2,4‐T was demonstrated to be stable for 12 months in cereal grain (dry/high starch) and for 6 months in apple (high water) and sugar beet root (high starch) when stored at −20°C. In high oil content matrices, 1,2,4‐T was stable for 12 months in soya beans but found unstable (< 3 months) in rapeseeds (data gap). No data are available supporting storage stability of 1,2,4‐T in high acid content and dry/high protein content commodities, but no additional studies are required for these matrices since this metabolite is not expected to be present at significant levels following treatment with tetraconazole in high acid content commodities (fruit crops) and no uses are currently authorised for crops belonging to the dry/high protein group (see Sections 1.1.6 and 1.2.1).
1.1.6. Proposed residue definitions
The metabolism of tetraconazole was similar in all crops assessed. The metabolism in rotational crops is similar to the metabolism observed in primary crops and the processing of tetraconazole is not expected to modify the nature of residues.
As parent tetraconazole was found to be a sufficient marker in the three groups investigated, i.e. cereals, roots and fruits, the residue definition for enforcement in plant commodities is proposed as tetraconazole. This residue definition is identical to the one currently set under Regulation (EC) No 396/2005.
An analytical method for the enforcement of the proposed residue definition at the LOQ of 0.01 mg/kg in all four main plant matrices is available (France, 2021). According to the EURLs, this LOQ is achievable in the four main matrix groups of plant origin by using the QuEChERS method in routine analyses (EURLs, 2021).
In the recently published conclusion on TDMs (EFSA, 2018b), EFSA proposed the following residue definitions for risk assessment for the triazole active substances:
parent compound and any other relevant metabolite exclusively linked to the parent compound;
TA and TLA, since these compounds share the same toxicity;
TAA;
1,2,4‐T.
Considering the metabolism of tetraconazole, other metabolites than the TDMs were found only at very low absolute levels and were not considered relevant. Therefore, in this MRL review, residue definition for risk assessment 1 (RD‐RA1) includes the parent tetraconazole only. These above‐mentioned residue definitions were found appropriate for the current assessment.
To summarise, the proposed residue definition for monitoring for all plant groups is tetraconazole (EFSA, 2008), and the proposed residue definitions for risk assessment for all plant groups is: (1) tetraconazole; (2) TA and TLA; (3) TAA; (4) 1,2,4‐T.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
To assess the magnitude of parent tetraconazole and triazole derivative metabolites resulting from the reported GAPs, EFSA considered all residue trials reported by the RMS in its evaluation report (France, 2021) as well as the residue trials evaluated during the peer review for the approval of tetraconazole (Italy, 2005; EFSA, 2008) and in the confirmatory data on TDMs (United Kingdom, 2018; EFSA, 2018b), or in the framework of previous MRL applications (EFSA, 2012, 2013b, 2019a).
All residue trial samples considered in this framework were stored in compliance with the conditions for which storage stability of tetraconazole was demonstrated. Decline of residues during storage of the trial samples is therefore not expected.
For what concerns the residue trials analysing for the TDMs, all samples considered in this framework were stored in compliance with the conditions for which storage stability of TDMs was demonstrated, except the following cases: no storage stability studies are available for TA, TAA and 1,2,4‐T in high acid content commodities and for TLA in cereal straw. Furthermore, metabolite 1,2,4‐T was found to be unstable in rapeseeds while the storage stability study for TA in rapeseeds was inconclusive (see Section 1.1.5).
Based on the results from the available trials and from the metabolism studies on primary and rotational crops, metabolite 1,2,4‐T is not expected to be present at significant levels in high acid content commodities (fruit crops) treated with tetraconazole. Nevertheless, for rapeseeds, a decline of this metabolite during the storage of the samples from residue trials cannot be excluded. Hence, residue trials on rapeseed analysing for this metabolite within 30 days from sampling or a new storage stability study is still required to confirm the risk assessment values derived for 1,2,4‐T in this crop.
For what concerns TLA in straw and TAA in high acid content commodities, since these metabolites were, respectively, stable for at least 48 months and 26 months in all other matrices (see Section 1.1.5), and samples from trials were stored for a maximum of 24 months, additional studies investigating the storage stability are only desirable.
Finally, regarding TA in high acid content matrices and in rapeseeds, a decline of this metabolite during the storage of the samples from residue trials on wine grapes, strawberries and rapeseeds cannot be excluded. Hence, studies investigating the storage stability of metabolite TA in high acid content commodities and residue trials on rapeseed analysing for this metabolite within 30 days from sampling or a new storage stability study on rapeseed are still required to confirm the risk assessment values derived for these crops.
The number of residue trials and extrapolations was evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2020).
Residue trials are not available (or not sufficient) to support the authorisations on sweet peppers, globe artichokes, barley, buckwheat, maize, millet, oat, rice and sorghum. Therefore, MRLs for tetraconazole and risk assessment values for tetraconazole, the sum of TA and TLA, and TAA could not be derived for these crops and the following data gaps were identified:
Sweet peppers: eight trials compliant with the southern outdoor GAP and eight trials compliant with the indoor GAP, all analysing for tetraconazole, TAA, and the sum of TA and TLA are required. For what concerns 1,2,4‐T there is enough evidence from metabolism studies and available residue trials on other fruit crops to conclude that residues at harvest will remain below the LOQ in sweet peppers. Therefore, risk assessment values for 1,2,4‐T were proposed at the LOQ and no additional trials are required.
Globe artichokes: The number of residue trials supporting the southern outdoor GAP is not compliant with the data requirement for this crop and no trials are available to support the indoor GAP. Therefore, two additional trials compliant with the southern outdoor GAP and four trials compliant with the indoor GAP, all analysing for tetraconazole, TAA, and the sum of TA and TLA are still required. For what concerns 1,2,4‐T, there is enough evidence from metabolism studies and available residue trials on globe artichokes to conclude that residues at harvest will remain below the LOQ in this crop. Therefore, risk assessment values for 1,2,4‐T were proposed at the LOQ and no additional trials are required.
Barley and oats: Eight trials compliant with the northern outdoor GAP and eight trials compliant with the southern outdoor GAP, all analysing for tetraconazole, TAA, and the sum of TA and TLA are required. For what concerns 1,2,4‐T, based on the results of the metabolism studies performed at 1.5N and considering that in the crops where it was analysed, this metabolite was always below limit of detection (LOD) or below the LOQ, risk assessment values for 1,2,4‐T were tentatively proposed at the LOQ. Nevertheless, in line with the applicable guidance document on data requirements for MRL setting (European Commission, 2020), residue trials are still required to support the ‘< LOQ residue’ situation. Taking into account the results of all available trials and the large margin of safety in the consumer risk assessment (see Section 3), EFSA is of the opinion that a reduced number of trials (i.e. 2 trials per zone) will be sufficient to confirm that 1,2,4‐T will remain below the LOQ.
Buckwheat: Four trials compliant with the southern outdoor GAP analysing for tetraconazole, TAA, and the sum of TA and TLA are required. For what concerns 1,2,4‐T, the same considerations as reported above for barley and oats are applicable and two residue trials are still required to confirm that residues of 1,2,4‐T will remain below the LOQ.
Maize and sorghum: Eight trials compliant with the southern outdoor GAP analysing for tetraconazole, TAA, and the sum of TA and TLA are required. For what concerns 1,2,4‐T, the same considerations as reported above for barley and oats are applicable and two residue trials are still required to confirm that residues of 1,2,4‐T will remain below the LOQ.
Millet: Four trials compliant with the southern outdoor GAP analysing for tetraconazole, TAA, and the sum of TA and TLA are required. For what concerns 1,2,4‐T, the same considerations as reported above for barley and oats are applicable and two residue trials are still required to confirm that residues of 1,2,4‐T will remain below the LOQ.
Rice: Eight trials compliant with the southern outdoor GAP analysing for tetraconazole, TAA, and the sum of TA and TLA are required. For what concerns 1,2,4‐T, the same considerations as reported above for barley and oats are applicable and two residue trials are still required to confirm that residues of 1,2,4‐T will remain below the LOQ.
For all other crops, available residue trials are sufficient to derive (tentative) MRL for tetraconazole and risk assessment values for tetraconazole and the triazole derivative metabolites, taking note of the following considerations:
Pome fruits, except loquats: All available northern trials (7) on apples were underdosed compared to the critical GAP, but within the acceptable deviation of 25%. In line with the EFSA technical report on recommendations on the use of the proportionality approach in the framework of risk assessment for pesticide residues (EFSA, 2018c) and as proposed by the RMS, France (France, 2021), the proportionality approach was used to scale up the entire residue data set to the nominal application rate to avoid a systematic bias. It is noted however that, the proposed MRL and risk assessment values for tetraconazole shall be considered as tentative, and one additional trial compliant with the northern GAP and analysing for tetraconazole is still required.
Furthermore, the number of residue trials analysing for the TDMs and supporting the northern and the southern outdoor GAPs is not compliant with the data requirements for these crops. Therefore, six and two additional trials supporting the northern outdoor GAP for apples and pears, and medlars and quinces, respectively, and four additional trials supporting the southern outdoor GAP for apples and pears, all analysing for TAA and the sum of TA and TLA are still required. For what concerns 1,2,4‐T, there is enough evidence from metabolism studies and available residue trials on fruit crops to conclude that residues at harvest will remain below the LOQ in these crops. Therefore, risk assessment values for 1,2,4‐T were proposed at the LOQ and no additional trials are required.
Loquats: The number of residue trials analysing for the TDMs and supporting the northern outdoor GAP is not compliant with the data requirements for this crop. Therefore, 2 additional trials analysing for TAA, and the sum of TA and TLA, and compliant with the northern outdoor GAP are still required. For what concerns 1,2,4‐T, the same considerations as reported above for the other pome fruits are applicable and no additional trials are required.
Apricots and peaches: The number of residue trials analysing for tetraconazole and the TDMs and supporting the southern outdoor GAP is not compliant with the data requirements for these crops. Therefore, four additional trials on apricots (that will allow to have a combined data set of apricots and peaches) compliant with the southern outdoor GAP and analysing for tetraconazole, TAA and the sum of TA and TLA are still required to support uses on both crops. For what concerns 1,2,4‐T, the same considerations as reported above for pome fruits are applicable and no additional trials are required.
Table and wine grapes: Although MRL and risk assessment values for tetraconazole can be derived from the data supporting the northern and southern outdoor GAPs, eight residue trials compliant with the import tolerance GAP and analysing for tetraconazole, TAA and the sum of TA and TLA are still required.
Furthermore, the number of residue trials analysing for the TDMs and supporting the northern GAP for wine grapes and the southern outdoor GAP for table and wine grapes is not compliant with the data requirements for these crops. Therefore, four additional trials compliant with the northern outdoor GAP for wine grapes and four additional trials compliant with the southern outdoor GAP for table and wine grapes, all analysing for TAA, and the sum of TA and TLA are still required. For what concerns 1,2,4‐T, the same considerations as reported above for pome fruits are applicable and no additional trials are required.
Strawberries: The number of residue trials supporting the northern outdoor GAP analysing for tetraconazole is not compliant with the data requirement for this crop. However, considering that the indoor GAP is clearly more critical, no additional trials analysing for tetraconazole are required to support the northern outdoor GAP.
It is noted nonetheless that no residue trials analysing for tetraconazole, the sum of TA and TLA, and TAA are available to support the southern outdoor GAP. Moreover, no residue trials analysing for TA, TLA and TAA are available to support the indoor GAP, and only a reduced number of trials analysing for TA, TLA and TAA is available to support the northern outdoor GAP.
Therefore, the following data gaps are identified for strawberries: eight trials compliant with the southern outdoor GAP and analysing for tetraconazole, TAA and the sum of TA and TLA; eight trials compliant with the indoor GAP and analysing for TAA, and the sum of TA and TLA; six additional trials compliant with the northern outdoor GAP and analysing for TAA, and the sum of TA and TLA. For what concerns 1,2,4‐T, the same considerations as reported above for pome fruits are applicable and no additional trials are required.
Tomatoes and aubergines: Although MRL and risk assessment values for tetraconazole can be derived from the data supporting the southern outdoor GAP, eight residue trials compliant with the indoor GAP and analysing for tetraconazole, TAA, and the sum of TA and TLA are still required.
Furthermore, the number of residue trials analysing for the TDMs and supporting the southern GAP is not compliant with the data requirements for these crops. Therefore, six additional trials compliant with the southern outdoor GAP analysing for TAA, and the sum of TA and TLA are still required. For what concerns 1,2,4‐T, the same considerations as reported above for pome fruits are applicable and no additional trials are required.
Cucumbers and courgettes: The number of residue trials analysing for the TDMs and supporting the southern outdoor and the indoor GAPs is not compliant with the data requirements for these crops. Since TAA was always below the LOD, further trials analysing for TAA are not required. Nevertheless, four additional trials compliant with the southern outdoor GAP (relevant for courgettes) and two additional trials (relevant for both, cucumbers and courgettes) compliant with the indoor GAP, all analysing for the sum of TA and TLA, are still required. For what concerns 1,2,4‐T, the same considerations as reported above for pome fruits are applicable and no additional trials are required.
Cucurbits with inedible peel: Although MRL and risk assessment values for tetraconazole can be derived from the data supporting the southern outdoor GAP, eight residue trials compliant with the indoor GAP for melons and watermelons and analysing for tetraconazole, TAA and the sum of TA and TLA are still required. Furthermore, the number of residue trials analysing for the TDMs and supporting the southern GAP is not compliant with the data requirements for these crops. Therefore, six additional trials compliant with the southern outdoor GAP on melons, pumpkins and watermelon and analysing for TAA, TA and TLA are still required. For what concerns 1,2,4‐T, the same considerations as reported above for pome fruits are applicable and no additional trials are required.
Witloofs/Belgian endives: The number of residue trials supporting the northern outdoor GAP is not compliant with the data requirements for this crop. For tetraconazole, the reduced number of northern trials is considered acceptable as all residue results were below the LOQ of the method used in the trials (0.02 mg/kg), and no additional trials are required. For what concerns TDMs, no residue trials are available. Consequently, four trials compliant with northern GAP and analysing for TAA, TA and TLA are still required.
For what concerns 1,2,4‐T, considering that in the crops where it was analysed this metabolite was always below LOD or below the LOQ, risk assessment values for 1,2,4‐T were tentatively proposed at the LOQ. Nevertheless, in line with the applicable guidance document on data requirements for MRL setting (European Commission, 2020) residue trials are still required to support the ‘< LOQ residue’ situation. Taking into account the results of all available trials and the large margin of safety in the consumer risk assessment (see Section 3), EFSA is of the opinion that a reduced number of trials (i.e. 2 trials) will be sufficient to confirm that 1,2,4‐T will remain below the LOQ. Therefore, two trials compliant with the northern outdoor GAP are still required to confirm that 1,2,4‐T will remain below the LOQ.
Rapeseed and linseeds: All available trials (12) on rapeseeds were performed at a later growth stage (BBCH 69–75) compared with the critical GAP (BBCH 65) for rapeseed and linseed. Furthermore, available trials were overdosed (1.25N) when considering the GAP for linseeds. Therefore, MRL and risk assessment values derived for these two crops should be considered as tentative, and a minimum of eight and four trials compliant with the northern outdoor GAP for rapeseeds and linseeds, respectively, are still required. All required trials should analyse for tetraconazole, TAA and the sum of TA and TLA.
For what concerns 1,2,4‐T, although results from the trials showed residues below the LOD, these are not supported by the available storage stability study. Therefore, risk assessment values for this metabolite were tentatively proposed at the LOQ but should be confirmed by at least two trials with residues analysed within 30 days from sampling or by a new storage stability study.
Wheat and rye grains: the number of residue trials supporting the northern outdoor GAP is not compliant with the data requirements for these crops. For tetraconazole, the reduced number of northern trials is considered acceptable as all residue results were below the LOQ of the methods used in the trials (0.015 and 0.02 mg/kg), and no additional trials are required.
For what concerns TA and TLA, and TAA, in view of the metabolism studies and the results of the available southern trial, residues higher than LOQ cannot be disregarded for TA, TAA and TLA. Therefore, six additional trials compliant with the northern outdoor GAP and analysing for TA and TAA, eight trials compliant with the northern outdoor GAP and analysing for TLA and seven additional trials compliant with the southern outdoor GAP and analysing for TAA and for the sum of TA and TLA are still required.
For what concerns 1,2,4‐T, based on the results of the metabolism studies performed at 1.5 N and the available residue trial compliant with the southern use, risk assessment values for 1,2,4‐T were tentatively proposed at the LOQ. Nevertheless, in line with the applicable guidance document on data requirements for MRL setting (European Commission, 2020) residue trials are still required to support the ‘< LOQ residue’ situation. Taking into account the results of all available trials and the large margin of safety in the consumer risk assessment (see Section 3), EFSA is of the opinion that a reduced number of trials (i.e. 2 trials per zone) will be sufficient to confirm that 1,2,4‐T will remain below the LOQ. Therefore, at least one additional trial compliant with southern outdoor GAP, and two compliant with the northern one analysing for 1,2,4‐T are required to confirm that residues will remain below the LOQ.
Wheat and rye straw: Only one residue trial analysing for the TAA and the sum of TA and TLA is available to support the southern GAP, while no residue trials at all are available to support the northern use. Hence, four residue trials compliant with the northern outdoor GAP and three residue trials compliant with the southern outdoor GAP, all analysing for TAA, and the sum of TA and TLA might be still required to refine dietary burden calculations of RD‐RA2 and RD‐RA3.
For 1,2,4‐T, residues were found to be below the LOQ in the sole residue trial available supporting the southern outdoor GAP and thus, for the same reasoning reported for wheat and rye grain, one additional trial compliant with the southern GAP and two compliant with the northern one might be required to corroborate that residues will remain below the LOQ, thereby confirming the calculated dietary burden for 1,2,4‐T.
Sugar beet roots: Residue trials supporting the southern outdoor GAP for this crop and analysing for tetraconazole were performed according to a more critical GAP (PHI 14 instead of 21). Since in these trials the parent was always below the LOQ, no additional trials analysing for tetraconazole are required. It is noted that some trials were analysed with an analytical method having an LOQ of 0.02 mg/kg. According to the metabolism studies, residues higher than 0.01 mg/kg are not expected in roots, and therefore, MRL and risk assessment values are proposed at the level of the LOQ for enforcement (0.01* mg/kg).
No residue trials analysing for the TDMs are available. According to the metabolism studies performed at 1N, no translocation into the roots was observed with the triazole label and the risk assessment values for TDMs were tentatively proposed at the LOQ. Nevertheless, in line with the applicable guidance document on data requirements for MRL setting (European Commission, 2020), residue trials are still required to confirm the ‘< LOQ residue’ situation. Consequently, four trials compliant with the northern outdoor GAP and four compliant with the southern outdoor GAP, all analysing for TAA, the sum of TA and TLA are required to confirm that residues of these TDMs will remain below the LOQ. For metabolite 1,2,4‐T, considering the reasons explained above, only two trials are required.
Chicory roots: The number of residue trials supporting the northern outdoor GAP is not compliant with the data requirements for this crop. Hence, one additional trial analysing for tetraconazole is required.
No residue trials analysing for the TDMs are available. According to the metabolism studies performed at 3N, no translocation into the roots was observed with the triazole label a ‘zero residue’ situation can be anticipated for this crop. Therefore, risk assessment values for TDMs were proposed at the LOQ. Considering that chicory root is a minor crop and in line with the applicable guidance document on data requirements for MRL setting (European Commission, 2020), no additional trials are required.
Fodder beet roots: No residue trials analysing for the TDMs are available. According to the metabolism studies performed at 3N, no translocation into the roots was observed with the triazole label and risk assessment values for TDMs were tentatively proposed at the LOQ. Nevertheless, in line with the applicable guidance document on data requirements for MRL setting (European Commission, 2020), residue trials are still required in major crops to confirm the ‘zero residue’ situation. Therefore, three residue trials compliant with the northern outdoor GAP and analysing for TAA, and the sum of TA and TLA might still be required to confirm dietary burden calculations of RD‐RA2 and RD‐RA3. For metabolite 1,2,4‐T, considering the reasons explained above, only two trials might still be required to confirm the dietary burden calculations for 1,2,4‐T.
Fodder beet tops: All available trials on tetraconazole were overdosed (2N) compared to the targeted rate of the cGAP. Although no MRLs are currently set for feed items, risk assessment values for tetraconazole should be considered tentative, and eight GAP compliant trials might still be required (relevant to refine dietary burden calculations according to RD‐RA1).
Moreover, no residue trials analysing for the TDMs are available and the occurrence of significant residues cannot be excluded from metabolism studies. Therefore, eight residue trials compliant with the northern outdoor GAP and analysing for TAA, the sum of TA and TLA and 1,2,4‐T might still be required (relevant for the dietary burden calculations according to RD‐RA2, RD‐RA3 and RD‐RA4).
Sugar beet tops: All available southern trials on tetraconazole were performed according to a more critical GAP (PHI 14 instead of 21). Although no MRLs are currently set for feed items, eight GAP compliant trials might still be required to refine livestock dietary burden calculations (see also Section 2).
Furthermore, no residue trials analysing for the TDMs are available and the occurrence of significant residues cannot be excluded from metabolism studies. Thus, eight residue trials compliant with the northern outdoor GAP and eight residue trials compliant with the southern outdoor GAP, all analysing for TAA, the sum of TA and TLA and 1,2,4‐T might still be required (relevant for the dietary burden calculations according to RD‐RA2, RD‐RA3 and RD‐RA4).
1.2.2. Magnitude of residues in rotational crops
Four field rotational crop studies were available for this review. Two of them were evaluated in the framework of the peer review (Italy, 2005; EFSA, 2008), while the other two were submitted as confirmatory data in the frame of the peer review for TDMs (United Kingdom, 2018; EFSA, 2018b).
In the first study, tetraconazole was applied at 125, 250, 750 and 1,500 g a.i./ha onto bare soil. Within 7–9 days following the application of tetraconazole to the soil, rotational crops (spring wheat, peas, potatoes, oilseed rape and sugar beets) were sown into the treated soil and harvested at maturity. In the second study, tetraconazole was applied to winter cereal (wheat or barely) at the rate of 125 g a.i./ha during three growing seasons, either at two (resulting in 6 applications in 3 years), three (9 applications) or four applications (11 applications). The effective total dose after 11 applications was calculated to be 137.5 g a.i./ha, considering 90% crop interception, BBCH 40–69. Following the third year of continuous applications of tetraconazole, rotational crops (onions, oilseed rape, beans, peas, potatoes and sugar beets) were planted, at various plant back intervals (76–328 days), into the same soil where tetraconazole had been applied, then grown to maturity and harvested. Samples of both studies were analysed for parent tetraconazole and it was found that residues of parent were below LOQ (0.01 or 0.02 mg/kg, depending on the study) in all samples analysed (Italy, 2005; EFSA, 2008). The peer review concluded that these studies demonstrated that under practical conditions of use, residues of tetraconazole compounds above LOQ are unlikely to occur in rotational crops. The available field studies were, however, not considered to fully address the issue of residues in following crops as they did not include leafy vegetables and the studies did not analyse for residues of triazole metabolites.
Consequently, two additional field crop studies were submitted as confirmatory data regarding TDMs (United Kingdom, 2018). In the third study, two applications of 100 g a.i./ha (representing 0.7N rate of the authorised SEU cGAP on sugar beets) were performed on sugar beet (first application at BBCH 38–39 with 20 ± 2 days interval). Effective total dose applied to soil was calculated to be 40 g a.i./ha considering worst‐case foliar interception. Rotational crops (lettuce and wheat) were sown at PBIs of 22–286 for lettuce and 97–250 for wheat. Samples were analysed for parent and the four triazole derivative metabolites (TA, TLA, TAA and 1,2,4‐T). Tetraconazole and 1,2,4‐T residues levels were always below the LOQ. TA was the main metabolite found in all plant commodities with high levels in wheat grain (up to 0.244 mg/kg at PBI 90–110 days) and wheat green plant (up to 0.589 mg/kg at PBI 90–110 days). Lower levels were found for TAA (up to 0.079 mg/kg in wheat grain at PBI 90–110 days) and TLA (up to 0.202 in wheat straw at PBI 90–110 days). TAA was not quantified in lettuce. TA and TAA reached, respectively, maximum levels of 0.065 mg/kg and 0.032 mg/kg in lettuce heads (PBI 20–60 days). In the fourth study, one application of 125 g a.i./ha was performed on wheat at BBCH 51–60 (0.5N rate of the authorised NEU cGAP on cereals). The effective total dose applied was calculated to be 12.5 g/ha, considering 90% foliar crop interception. Sugar beet was sown as rotated crop at PBI 288–302 days. In roots, only TA was quantified (0.078 mg/kg). In leaves, TAA and 1,2,4‐T were not detected, while TA (up to 0.083 mg/kg) and TLA (up to 0.089 mg/kg) were observed as main metabolites. Tetraconazole residues were always below LOQ of 0.01 mg/kg, except in one trial where it was quantified at 0.011 mg/kg in leaves. In this trial, positive control was observed.
Given the high persistence of tetraconazole in soil (see Section 1.1.2), the potential occurrence of residues following multiannual applications should also be investigated. Considering the maximum application rate of 150 g a.s./ha per season assessed in this review (authorised SEU GAP on strawberries), a soil bulk density of 1.5 g/cm3, a soil mixing depth of 20 cm and crop interception (60% at BBCH 90), the predicted environmental concentration in soil (PECsoil), taking into account accumulation, is calculated to be of 0.144 mg a.i./kg soil, which corresponds to a soil application rate of 431 g a.i./ha, according to OECD guidance (OECD, 2018). Among the available field rotational crop studies, only the first one with direct soil application onto bare soil up to 1,500 g a.i./ha covers the calculated plateau for multiannual applications of tetraconazole. In this study only parent was analysed, and no residues were quantified in any rotated crop. Despite leafy vegetables not being included in the study, the peer review (EFSA, 2008) concluded that residues of parent tetraconazole are expected to be negligible in rotational crops. For this MRL review, EFSA concludes that significant residue levels of parent tetraconazole are not expected in succeeding crops, provided that the active substance is applied in compliance with the GAPs reported in Appendix A. For what concerns TDMs, EFSA concludes that residues of TA, TAA, TLA and 1,2,4‐T above 0.01 mg/kg cannot be excluded in rotational crops and that the present study is not expected to cover the concentrations of these metabolites in soil following annual and multiannual applications according to the most critical GAP currently authorised.
Consequently, field rotational crops studies analysing for TA, TAA, TLA and 1,2,4‐T and covering the most critical GAP currently authorised on crops that can be rotated and the calculated PEC soil are still required (data gap).
1.2.3. Magnitude of residues in processed commodities
The effect of industrial processing and household preparation was assessed on studies conducted on apples, grapes, tomato, melon and rapeseed (EFSA, 2008, 2012; France, 2021). An overview of all available processing studies is available in Appendix B.1.2.4. Robust processing factors (fully supported by data) could be derived for apple, and by extrapolation to pears (dry pomace and juice), grape (must, wine and raisins), melon, and by extrapolation to pumpkin and watermelon (peeled), rapeseeds, and by extrapolation to linseed (cake, crude oil and refined oil), while limited processing factors (not fully supported by data) were derived for apple (sauce), apple and by extrapolation to pears (wet pomace), grape (juice and wet pomace) and tomato (sauce, juice and preserve).
No new studies on TDMs were submitted in the framework of this review. However, two processing studies on wheat have been submitted as confirmatory data and evaluated by the United Kingdom (United Kingdom, 2018). Sufficient data are available to derive robust processing factors (fully supported by data) for wheat (bran and flour) for TAA and for the sum of TA and TLA. Limited processing factors (not fully supported by data) were derived for wheat (whole meal flour) for the same metabolites.
Further processing studies are not required as they are not expected to affect the outcome of the risk assessment. However, if more robust processing factors were to be required by risk managers, in particular for enforcement purposes, additional processing studies would be needed.
1.2.4. Proposed MRLs
The available data are considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for sweet peppers, globe artichokes, barley and oat grain, buckwheat, maize, millet and sorghum grain, and rice grain for which no data are available to derive MRLs for tetraconazole and risk assessment values for tetraconazole, the sum of TA and TLA, and TAA.
Tentative MRLs were also derived for feed crops (wheat and rye straw, fodder beet roots and tops and sugar beet tops) in view of the future need to set MRLs in feed items.
Residues of parent tetraconazole are expected to be negligible in rotational crops provided that the active substance is applied in compliance with the GAPs reported in Appendix A. However, for TDMs, EFSA could not derive robust risk assessment values covering the residues in rotational crops.
2. Residues in livestock
Tetraconazole is authorised for use on crops that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance (OECD, 2013), which has now also been agreed upon at European level. The input values for all relevant commodities are summarised in Appendix D.
Following the recently published EFSA conclusion on TDMs (EFSA, 2018b), the animal dietary burden should be calculated in accordance with the agreed residue definitions for risk assessment as derived for triazole pesticide active substances. In the framework of this review, dietary burden calculations were thus performed for parent tetraconazole (RD‐RA1), TA and TLA (RD‐RA2), TAA (RD‐RA3) and 1,2,4‐T (RD‐RA4).
For parent tetraconazole (RD‐RA1), the dietary burdens calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM). Behaviour of residues was therefore assessed in all commodities of animal origin. It is highlighted that for several feed items, i.e. cereals–except wheat and rye, no residue data were available. The animal intake of tetraconazole residues via these commodities has therefore not been assessed and may have been underestimated. Furthermore, it is noted that for rapeseeds/linseeds, fodder beet tops and sugar beet tops (SEU), the residue data available were coming from more critical GAPs than the ones currently authorised and the input values from the feed items derived from these crops might have been overestimated. This is particularly relevant for cattle (beef and dairy) and swine, where mangel fodder beet was found to be the major contributor.
For metabolites TA and TLA (RD‐RA2), the calculated dietary burdens were found to exceed the trigger value of 0.1 mg/kg DM for all groups of livestock. It is noted that for several feed items, i.e. cereals except wheat and rye, sugar and fodder beet tops, no residue data were available and the animal intake of TA and TLA via these commodities has not been assessed and may have been underestimated. Moreover, it is underlined that wheat or canola was found to be the major contributors to the different livestock diets and robust residue data sets for these two commodities were not available (see Appendix B.1.2.2). Finally, it is worth mentioning that in the available residue data on the main contributors to the livestock exposure, TLA levels were lower than the TA (up to one order of magnitude in rapeseeds/canola and in the only available residue trial on wheat grain). Therefore, the contribution of TLA to the overall dietary burden to the sum of TA and TLA is expected to be negligible in view of the current data. However, this may be reconsidered in the future, once the requested residue data on these metabolites will be made available. In this MRL review, risk assessment values for RD‐RA2 in livestock commodities were derived from livestock feeding studies performed with TA only.
For metabolite TAA (RD‐RA3), the calculated dietary burdens were found to exceed the trigger value of 0.1 mg/kg DM for all groups of livestock. No residue data were available for the same feed items as for RD‐RA2, and animal intake of TAA via these items (cereals – except wheat and rye, sugar and fodder beet tops has not been assessed and may have been underestimated. The major contributor to all livestock diets were products derived from wheat, for which a robust residue data set was not available.
Finally, for metabolite 1,2,4‐T (RD‐RA4), the calculated dietary burdens for all groups of livestock were found to be below the trigger value of 0.1 mg/kg DM, thus further investigation of residues in commodities of animal origin is unnecessary for this metabolite (1,2,4‐T). It is noted, however, that no residue data were available for sugar and fodder beet tops and the animal intake of 1,2,4‐T via these commodities has not been assessed and may have been underestimated. Moreover, the input value of 0.01* used for cereals (grain and straw) and sugar and fodder beet roots has to be confirmed by additional residue trials demonstrating that residues in these commodities remain < LOQ.
It should be noted that residues of TDMs from rotational crops were not considered in the dietary burden calculations.
2.1. Nature of residues and methods of analysis in livestock
The metabolism of tetraconazole residues in livestock was investigated in ruminants and poultry at dose rates covering the maximum dietary burdens calculated in this review (see Section B.2 ). These studies were assessed in the framework of the peer review (EFSA, 2008) and in a previous MRL application (EFSA, 2019a).
The metabolism of tetraconazole in ruminants was assessed in lactating goats (oral daily dose of 0.45 mg/kg body weight (bw) for five consecutive days) using the compound radiolabelled in the phenyl or the triazole ring (EFSA, 2008). Approximately 0.4–4% of the total dose was excreted in the milk, with 64–76% recovered in the faeces and urine. Liver and kidney contained the highest concentrations of residues. Tetraconazole and 1,2,4‐triazole were the major components of the residues. In the triazole‐ring labelled study, milk, muscle and kidney contained predominantly 1,2,4‐triazole, while liver and fat contained essentially unchanged tetraconazole. In the study conducted with phenyl labelling, unchanged tetraconazole was the main component in milk and tissues (EFSA, 2008).
Based on these data, it was concluded that unchanged tetraconazole was a sufficient marker in all edible ruminant commodities. The peer review concluded that the metabolism of tetraconazole in ruminants was comparable with the metabolism in rats and a metabolism study on pigs was not required. To be noted that tetraconazole is considered fat soluble.
In laying hens, a new metabolism study dosed with radiolabelled tetraconazole, in the triazole and phenyl rings, for 3 days (1.3–1.4 mg/kg bw per day) was assessed by EFSA in the framework of a previous MRL application (Italy, 2018; EFSA, 2019a). The major residue in all tissues and eggs was tetraconazole (from 84% to 105% of TRR) and it was concluded that the nature of residue was tetraconazole only (EFSA, 2019a).
In the recently published EFSA conclusion on TDMs (EFSA, 2018b), the available livestock metabolism studies for several triazole fungicides and for TA were re‐assessed in view of deriving common risk assessment residue definitions applicable for animal products, which would cover all triazole fungicides assessed. In these hen and goat studies, TA was radiolabelled in the triazole ring of the molecule. In laying hens, the dose rate was 11.20 mg a.s./kg dry feed/day (0.81 mg/kg bw per day), the total radioactivity in edible organs and tissues amounted to 2.6% of the dose. TA was the major residue in eggs and edible matrices. 1,2,4‐T and TLA were observed as minor metabolites in eggs, fat and liver; 1,2,4‐T was also detected in muscle. For goats, the dosing rate was 15.24 mg a.s./kg dry feed/day (0.7 mg/kg bw per day), and the overall recovery of radioactivity was 70.5%. The major component observed was the parent compound TA, which comprised most of the residues in all matrices except milk, where the 1,2,4‐T metabolite was more predominant at 85.9% of the TRR.
No metabolism studies are available to elucidate the potential transfer of the other TDMs (TAA, TLA and 1,2,4‐T) present in feed items to animal matrices. However, the available metabolism and feeding studies were found to be sufficient to agree on residue definitions (EFSA, 2018b).
The general residue definition for enforcement was derived as the triazole parent compound only in line with the residue definition derived in previous assessments (EFSA, 2008, 2019a).
For risk assessment, separate residue definitions for triazole pesticide active substances in animals have been agreed in the framework of the TDMs confirmatory data (EFSA, 2018b) and are proposed as follows:
parent compound and any other relevant metabolite exclusively linked to the parent compound;
TA and TLA, since they share the same toxicity;
TAA;
1,2,4‐T.
In this Article 12 review of tetraconazole, residue definition for risk assessment 1 (RD‐RA1) includes the parent only, since no other metabolites than the TDMs were found to be relevant. These residue definitions were found appropriate for the current assessment.
A QuEChERS (LC‐MS/MS) multiresidue analytical method was fully validated for the determination of the proposed residue definition for enforcement in muscle, fat, liver, milk and eggs, with an LOQ of 0.01 mg/kg. This primary method is also supported by an ILV (France, 2021). According to the EURLs, parent tetraconazole can be monitored in milk with an LOQ of 0.01 mg/kg, in routine analysis. Screening data generated by EURLs for commodities of animal origin showed that tetraconazole can be monitored in eggs with a screening detection limit (SDL) of 0.005 mg/kg. In muscle, milk and honey, even lower SDLs down to 0.0025 mg/kg were achieved. Based on the experience gained for muscle, honey, egg and milk, an LOQ of 0.01 mg/kg is supposed to be achievable for the other animal products (e.g. liver, kidney, fat) (EURL, 2021).
The storage stability of parent tetraconazole in animal tissues was previously assessed in the framework of an MRL application (EFSA, 2019a). This study showed that tetraconazole is stable for 42 days in muscle, fat, liver and kidney, and for 39 days in eggs. No studies on milk are available, but they are not required for this assessment since samples were analysed for parent within 1 month.
For what concerns TDMs, the available storage stability data demonstrated acceptable freezer storage stability of 1,2,4‐T in milk for 18 months and in eggs, liver, muscle and fat for 12 months. Storage stability data analysing for residues of TA and TAA in milk and eggs were provided but were not considered acceptable by the peer review of TDMs confirmatory data (EFSA, 2018b). No data were available for TLA. However, no additional studies on TA, TAA and TLA are required for the current assessment since samples were analysed for TDMs within 1 month.
2.2. Magnitude of residues in livestock
In the framework of the peer review and a previous MRL application, feeding studies were performed with dairy cows (Italy, 2005; EFSA, 2008) and laying hens (Italy, 2018; EFSA, 2019a), respectively. In the cow study, tetraconazole was administered daily for 28–30 consecutive days at dosing levels 0.012, 0.036 and 0.12 mg/kg bw per day. In the study conducted with laying hens, animals were dosed with tetraconazole for a minimum of 40 days with 0.0046, 0.0166 and 0.045 mg/kg bw per day.
The study performed on cows was used to derive MRL and risk assessment values in milk and tissues of ruminants. Since extrapolation from ruminants to pigs is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in pigs. In this study, samples of tissues and milk were analysed for tetraconazole and metabolite 1,2,4‐T. During the completeness check, the Rapporteur Member State – France confirmed that samples were stored in compliance with the conditions for which storage stability was demonstrated thus decline of residues during storage of the samples is not expected (France, 2021). Based on this study, MRL and risk assessment values were derived for all commodities of dairy and meat ruminants, and pigs, in compliance with the latest recommendations on this matter (FAO, 2009). It is noted that except for sheep and swine muscle, and sheep milk, where MRLs are proposed at the LOQ, for other tissues/milk of ruminants/pigs, significant levels of tetraconazole are expected. Considering that mangel fodder beet was the major contributor to the dietary burden of bovine and swine diets, and that input values for this commodity may be overestimated (available residue trials were performed according to a more critical GAP), MRLs for bovine tissues and milk, and swine tissues are considered tentative.
The study performed on laying hens was used to derive MRL and risk assessment values in birds’ eggs and tissues. In this study, samples were analysed for parent tetraconazole. The storage period of the samples was covered by the conditions for which storage stability was demonstrated thus decline of residues during storage of the trial samples is not expected. Based on this study, MRL and risk assessment values were derived for all commodities of poultry, in compliance with the latest recommendations on this matter (FAO, 2009). It is noted that significant levels of tetraconazole are expected in bird’s eggs and tissues.
Regarding TDMs, poultry and ruminants feeding studies were both performed with TA and TAA. These studies were assessed in the framework of the peer review of TDMs (EFSA, 2018b). In all four studies, samples of tissues, eggs and milk were analysed for the magnitude of TA, TLA, TAA and 1,2,4‐T. The storage period of the samples was below 30 days.
In the study on poultry performed with TA, laying hens were divided in five groups and were fed at actual dose levels ranging from 0.12 to 13.4 mg/kg DM (i.e. 0.008–0.85 mg/kg bw per day). In the study on ruminants, lactating cows were fed with TA administered at five dose levels from 0.21 to 25.7 mg/kg DM (i.e. 0.008–0.94 mg/kg bw per day). In poultry, TA was the main residue found in all matrices and a slight metabolisation of TA to 1,2,4‐T was observed in eggs, liver and muscles at the highest dose level. TAA and TLA were always below LOQ. In ruminants, TA was still the major compound in all tissues and its metabolisation to 1,2,4‐T was significant in milk. TAA was only observed at lower levels in kidney and TLA in fat (EFSA, 2018b).
In the feeding study conducted with TAA on poultry, the substance was administered to five groups of animals using different dosing levels ranging from 0 to 5.6 mg/kg in diet (equivalent to 0–0.35 mg/kg body weight (bw) per day). In the study on ruminant, TAA was administered to five groups of lactating cows at dosing levels ranging from 0 to 7.2 mg/kg in diet (equivalent to 0–0.26 mg/kg bw per day). The study performed on poultry demonstrated no significant residue transfer from the animal diet to eggs and tissues. TAA was detected only at the highest dose level in eggs, fat and liver, while TA was quantified at all dosing levels only in liver. In ruminants, TAA was detected only at the highest dose level in whole milk and in all tissues, whilst TA was identified in liver, muscle and kidney at all the dosing levels. Metabolites 1,2,4‐T and TLA were always below LOQ of the study (0.01 mg/kg) (EFSA, 2018b).
The feeding studies carried out with TA and TAA were used to derive risk assessment values. As aforementioned, risk assessment values for RD‐RA2 (TA and TLA) in livestock commodities were derived from livestock feeding studies performed with TA. Significant residues are expected in all commodities, except in eggs, where risk assessment values were set at the LOQ. Concerning TAA (RD‐RA3), no residues are expected in any livestock commodity and risk assessment values were set at the LOQ. Considering the above‐mentioned missing data on TDMs on feed items, all risk assessment values derived in livestock for RD‐RA2 and RD‐RA3 should be considered as tentative.
Ruminants and poultry are not expected to be exposed to significant levels of 1,2,4‐T when they are directly fed with it. However, this metabolite can be present in ruminants and swine tissues as a result of feeding with parent tetraconazole (see Section 2.1). Therefore, levels of this metabolite in ruminants/swine’s tissues and milk were estimated considering the feeding study performed on ruminants fed with parent and analysing for 1,2,4‐T (Italy, 2005; EFSA, 2008). According to the results of this study, at the calculated dietary burden, residues of 1,2,4‐T are expected to remain below the LOQ in milk, but significant residues cannot be excluded in liver and kidney. Nonetheless, considering the minor contribution of these two animal commodities in the European diets and the large margin of safety in the consumer risk assessment for this metabolite (see Section 3), this is not expected to have a significant impact in the assessment and was not considered further in the present review.
3. Consumer risk assessment
As different toxicological reference values were derived, respectively, for tetraconazole and for the TDMs, EFSA performed separate consumer risk assessments for tetraconazole (RD‐RA1), for the sum of TA and TLA (RD‐RA2), for TAA (RD‐RA3) and for 1,2,4‐T (RD‐RA4).
In the framework of this review, only the uses of tetraconazole reported by the RMS in Appendix A were considered.
For tetraconazole, for the sum of TA and TLA, for TAA and for 1,2,4‐T, chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 3.1 of the EFSA PRIMo (EFSA, 2018a, 2019b). Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix E. Hence, for those commodities where an MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies (FAO, 2009). All input values included in the exposure calculations are summarised in Appendix D. It is underlined that for the sum of TA and TLA (RD‐RA2) and for TAA (RD‐RA3), the contributions of commodities for which no residue data were available to derive at least tentative risk assessment values (sweet peppers, witloofs, barley, buckwheat, maize, millet, oat, rice and sorghum) were not included in the calculation. Residues of TDMs from rotational crops were not considered in the risk assessment calculations.
3.1. Consumer risk assessment for tetraconazole
The exposure values calculated were compared with the toxicological reference values for tetraconazole derived by EFSA (2008) and legally implemented by the European Commission under Directive 91/414/EEC (European Commission, 2021). The highest chronic exposure was calculated for Dutch toddlers, representing 65% of the acceptable daily intake (ADI), and the highest acute exposure was calculated for pears, representing 45% of the ARfD. These calculations indicate that the uses assessed under this review result in a consumer exposure lower than the toxicological reference values. Although uncertainties remain due to the data gaps identified in the previous sections, this indicative exposure calculation did not indicate a risk to consumer’s health.
3.2. Indicative consumer risk assessment for triazole derivative metabolites
Separate calculations were performed for the sum of TA and TLA, for TAA and for 1,2,4‐T, and the exposure values calculated were compared with their respective toxicological reference values derived by EFSA (2018b).
For the sum of TA and TLA, the highest chronic exposure was calculated for Dutch toddlers representing 4% of the ADI, and the highest acute exposure was calculated for pears, representing 24% of the ARfD.
Concerning TAA, the highest chronic exposure was calculated for Dutch toddlers representing 0.1% of the ADI, and the highest acute exposure was calculated for pears, representing 0.3% of the ARfD.
For 1,2,4‐T, the highest chronic exposure was calculated for Dutch toddlers representing 2% of the ADI, and the highest acute exposure was calculated for melons, representing 2% of the ARfD.
These calculations indicate that the uses assessed under this review result in a consumer exposure lower than the toxicological reference values. Although uncertainties remain due to the data gaps identified in the previous sections, this indicative exposure calculation did not indicate a risk to consumer’s health.
EFSA emphasises that a comprehensive risk assessment, including all crops and all pesticides belonging to the class of triazole fungicides, could not be performed in the framework of this MRL review. EFSA recommended to elaborate together with risk managers a strategy to ensure that all required data are made available to finalise the overall risk assessment for triazole fungicides.
Conclusions
The metabolism of tetraconazole in plant was investigated in primary and rotational crops. According to the results of the metabolism studies, the residue definition for enforcement can be proposed as tetraconazole. As regards risk assessment, four residue definitions are set separately, namely, RD‐RA1: tetraconazole; RD‐RA2: triazole alanine (TA) and triazole lactic acid (TLA), RD‐RA3: triazole acetic acid (TAA); RD‐RA4: 1,2,4‐triazole (1,2,4‐T). These residue definitions are applicable to raw (from primary and rotational crops) and processed commodities. Fully validated analytical methods are available for the enforcement of the proposed residue definition in plant matrices at the LOQ of 0.01 mg/kg. According to the EURLs, the LOQ of 0.01 mg/kg is achievable in the four main matrix groups of plant origin by using the QuEChERS method in routine analyses.
Available residue trials data were considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for sweet peppers, globe artichokes, barley and oat grain, buckwheat, maize, millet and sorghum grain, and rice grain for which no data are available to derive MRLs for tetraconazole and risk assessment values for tetraconazole, the sum of TA and TLA, and TAA. Residues of parent tetraconazole are expected to be negligible in rotational crops. However, for TDMs, EFSA could not derive robust risk assessment values covering residues in rotational crops.
Tetraconazole is authorised for use on crops that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance. According to the proposed residue definitions for risk assessment, separate calculations were performed for tetraconazole (RD‐RA1), the sum of TA and TLA (RD‐RA2), TAA (RD‐RA3) and 1,2,4‐T (RD‐RA4). For RD‐RA1, RD‐RA2 and RD‐RA3, the dietary burdens calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM). Behaviour of residues of these compounds was therefore assessed in all commodities of animal origin. Although yet to be confirmed, the calculated dietary burdens for RD‐RA4 were found to be below the trigger value of 0.1 mg/kg DM for all groups of livestock, and further investigation of residues for 1,2,4‐T in commodities of animal origin is unnecessary.
The metabolism of tetraconazole residues in livestock was investigated in lactating goats and laying hens at dose rate covering the maximum dietary burdens calculated in this review. Metabolism studies on goats and hens were also available with TA directly fed to animals. According to the results of these studies, the residue definition for enforcement in livestock commodities was proposed as tetraconazole. As regards risk assessment, four residue definitions were set separately, namely, RD‐RA1: tetraconazole; RD‐RA2: TA and TLA; RD‐RA3: TAA; RD‐RA4: 1,2,4‐T. An analytical method for the enforcement of the proposed residue definition at the LOQ of 0.01 mg/kg in livestock matrices is available. According to EURLs, parent tetraconazole can be monitored in milk with an LOQ of 0.01 mg/kg, in routine analysis, and in eggs with a screening detection limit (SDL) of 0.005 mg/kg. In muscle and milk, even lower SDLs down to 0.0025 mg/kg were achieved. Based on this, an LOQ of 0.01 mg/kg is supposed to be achievable for the other animal products (e.g. liver, kidney, fat).
Livestock feeding studies on dairy cows and laying hens fed with parent tetraconazole were used to derive MRL and risk assessment values in milk, eggs and tissues of ruminants and poultry for parent. Since extrapolation from ruminants to pigs is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values of parent tetraconazole in pigs. Considering that mangel fodder beet was the major contributor to the dietary burden of bovine and swine diets, and that input values for this commodity may be overestimated based on trials performed according to a more critical GAP, MRLs for bovine tissues and milk, and swine tissues are considered tentative.
Regarding TDMs, poultry and ruminants feeding studies were both performed with TA and TAA. These feeding studies were used to derive risk assessment values for RD‐RA2 (TA and TLA) and RD‐RA3 (TAA). Considering that wheat/rye or canola products (which are not fully supported by residue trials) were found to be the major contributors to the dietary burden of livestock diets for RD‐RA2 and RD‐RA3, all risk assessment values derived in livestock for these two residue definitions should be considered as tentative.
As different toxicological reference values were derived, respectively, for tetraconazole and for the TDMs, EFSA performed separate consumer risk assessments for tetraconazole (RD‐RA1), for the sum of TA and TLA (RD‐RA2), for TAA (RD‐RA3) and for 1,2,4‐T (RD‐RA4). Chronic and acute consumer exposures resulting from the authorised uses reported in the framework of this review were calculated using revision 3.1 of the EFSA PRIMo. It is underlined that for the sum of TA and TLA (RD‐RA2) and for TAA (RD‐RA3), the contributions of commodities for which no residue data were available to derive at least tentative risk assessment values (sweet peppers, witloofs, barley, buckwheat, maize, millet, oat, rice and sorghum) were not included in the calculation. For tetraconazole, the highest chronic exposure was calculated for Dutch toddlers, representing 65% of the acceptable daily intake (ADI), and the highest acute exposure was calculated for pears, representing 45% of the ARfD. For the sum of TA and TLA, the highest chronic exposure was calculated for Dutch toddlers representing 4% of the ADI, and the highest acute exposure was calculated for pears, representing 24% of the ARfD. Concerning TAA, the highest chronic exposure was calculated for Dutch toddlers representing 0.1% of the ADI, and the highest acute exposure was calculated for pears, representing 0.3% of the ARfD. For 1,2,4‐T, the highest chronic exposure was calculated for Dutch toddlers representing 2% of the ADI, and the highest acute exposure was calculated for melons, representing 2% of the ARfD.
EFSA emphasises that a comprehensive risk assessment including all crops and all pesticides belonging to the class of triazole fungicides has not been performed in the framework of the current review.
Recommendations
MRL recommendations were derived in compliance with the decision tree reported in Appendix E of the reasoned opinion (see Table 2). With the exception of the MRL proposal for kaki, none of the MRL values listed in the table are recommended for inclusion in Annex II to the Regulation as they are not sufficiently supported by data and require further consideration by risk managers (see Table 2 footnotes for details). The MRL value for kaki listed as ‘Recommended’ in the table is sufficiently supported by data and is therefore proposed for inclusion in Annex II to the Regulation.
Table 2.
Summary table
| Code number | Commodity | Existing EU MRL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|
| MRL (mg/kg) | Comment | |||
| Enforcement residue definition: tetraconazole (F) | ||||
| 130010 | Apples | 0.3 | 0.3 | Further consideration needed(a) data gaps #1, 3, 4 |
| 130020 | Pears | 0.3 | 0.3 | Further consideration needed(a) data gaps #1, 3, 4 |
| 130030 | Quinces | 0.3 | 0.3 | Further consideration needed(a) data gap #3 |
| 130040 | Medlar | 0.3 | 0.3 | Further consideration needed(a) data gap #3 |
| 130050 | Loquat | 0.3 | 0.2 | Further consideration needed(a) data gap #3 |
| 140010 | Apricots | 0.1 | 0.03 | Further consideration needed(a) data gaps #1, 3, 4 |
| 140030 | Peaches | 0.1 | 0.03 | Further consideration needed(a) data gaps #1, 3, 4 |
| 151010 | Table grapes | 0.5 | 0.07 | Further consideration needed(a) data gaps # 7 |
| 151020 | Wine grapes | 0.5 | 0.07 | Further consideration needed(a) data gaps #3, 4, 7 |
| 152000 | Strawberries | 0.2 | 0.15 | Further consideration needed(a) data gaps #3, 4, 7, 9 |
| 161060 | Kaki/Japanese persimmons | 0.09 | 0.09 | Recommended b |
| 231010 | Tomatoes | 0.1 | 0.15 | Further consideration needed(a) data gaps #3, 4, 9 |
| 231020 | Peppers | 0.1 | 0.1 | Further consideration needed(c) data gaps #1, 3, 4, 9 |
| 231030 | Aubergines (egg plants) | 0.02* | 0.15 | Further consideration needed(a) data gaps #3, 4, 9 |
| 232010 | Cucumbers | 0.2 | 0.15 | Further consideration needed(a) data gap #3, 9 |
| 232020 | Gherkins | 0.2 | 0.15 | Further consideration needed(a) data gap #9 |
| 232030 | Courgettes | 0.2 | 0.15 | Further consideration needed(a) data gap #3, 9 |
| 233010 | Melons | 0.05 | 0.08 | Further consideration needed(a) data gaps #3, 4, 9 |
| 233020 | Pumpkins | 0.05 | 0.08 | Further consideration needed(a) data gaps #3, 4, 9 |
| 233030 | Watermelons | 0.05 | 0.08 | Further consideration needed(a) data gaps #3, 4, 9 |
| 255000 | Witloofs | 0.02* | 0.02 | Further consideration needed(a) data gaps #3, 4, 5 |
| 270050 | Globe artichokes | 0.2 | 0.2 | Further consideration needed(c) data gaps #1, 3, 4, 9 |
| 401010 | Linseed | 0.15 | 0.15 | Further consideration needed(a) data gaps #1, 3, 4, 6, 9 |
| 401060 | Rapeseed | 0.15 | 0.15 | Further consideration needed(a) data gaps #1, 3, 4, 6, 9 |
| 500010 | Barley grain | 0.1 | 0.1 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500020 | Buckwheat grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500030 | Maize grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500040 | Millet grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500050 | Oat grain | 0.1 | 0.1 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500060 | Rice grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500070 | Rye grain | 0.05 | 0.02 | Further consideration needed(a) data gaps #3, 4, 5, 9 |
| 500080 | Sorghum grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500090 | Wheat grain | 0.1 | 0.02 | Further consideration needed(a) data gaps #3, 4, 5, 9 |
| 900010 | Sugar beet (root) | 0.05 | 0.01* | Further consideration needed(a) data gaps #3, 4, 5, 9 |
| 900030 | Chicory roots | 0.05 | 0.06 | Further consideration needed(a) data gap # 1 |
| 1011010 | Swine meat | 0.05 | 0.01* | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1011020 | Swine fat (free of lean meat) | 0.5 | 0.07 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1011030 | Swine liver | 1 | 0.7 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1011040 | Swine kidney | 0.2 | 0.04 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1012010 | Bovine meat | 0.05 | 0.015 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1012020 | Bovine fat | 0.5 | 0.2 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1012030 | Bovine liver | 1 | 1.5 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1012040 | Bovine kidney | 0.2 | 0.06 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1013010 | Sheep meat | 0.05 | 0.01* | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1013020 | Sheep fat | 0.5 | 0.09 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1013030 | Sheep liver | 1 | 0.9 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1013040 | Sheep kidney | 0.5 | 0.05 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1014010 | Goat meat | 0.5 | 0.01* | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1014020 | Goat fat | 0.5 | 0.09 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1014030 | Goat liver | 1 | 0.9 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1014040 | Goat kidney | 0.5 | 0.05 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1015010 | Horse meat | 0.5 | 0.015 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1015020 | Horse fat | 0.5 | 0.2 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1015030 | Horse liver | 1 | 1.5 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1015040 | Horse kidney | 0.5 | 0.06 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1016010 | Poultry meat | 0.02* | 0.015 | Further consideration needed(a) data gap #1, 3, 4, 5 |
| 1016020 | Poultry fat | 0.2 | 0.3 | Further consideration needed(a) data gap #1, 3, 4, 5 |
| 1016030 | Poultry liver | 1 | 0.05 | Further consideration needed(a) data gap #1, 3, 4, 5 |
| 1020010 | Cattle milk | 0.05 | 0.02 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1020020 | Sheep milk | 0.05 | 0.01* | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1020030 | Goat milk | 0.05 | 0.01* | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1020040 | Horse milk | 0.05 | 0.02 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1030000 | Birds' eggs | 0.05 | 0.05 | Further consideration needed(a) data gap #1, 3, 4, 5 |
| – | Other commodities of plant and/or animal origin | See Reg. (EU) 2019/1015 | – | Further consideration needed d |
MRL: maximum residue level.
*: Indicates that the MRL is set at the limit of quantification.
(F): The residue definition is fat soluble.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination F‐I in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination H‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination D‐I in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Tentative MRLs need to be confirmed by the following data:
Additional residue trials analysing for tetraconazole and supporting the authorised uses on apples, pears, apricots (also relevant for peaches), sweet peppers, globe artichokes, rapeseeds, linseeds, barley, oat, buckwheat, maize, millet, sorghum, rice and chicory roots. It is underlined that requested trials on cereals, rapeseeds and linseeds might also be relevant for all products of animal origin;
GAP compliant trials analysing for tetraconazole and supporting the authorised uses on sugar and fodder beet tops (data gap relevant for the MRLs derived for bovine/equine and swine tissues and for bovine/equine milk);
Additional trials analysing for the sum of TA and TLA and supporting the authorised uses on apples, pears, quinces, medlars, loquats, apricots (also relevant for peaches), wine grapes, strawberries, tomatoes, aubergines, sweet peppers, cucumbers, courgettes, cucurbits with inedible peel, witloofs, globe artichokes, rapeseeds, linseeds, wheat, rye, barley, oat, buckwheat, maize, millet, sorghum, rice and sugar beet. It is underlined that requested trials on cereals, rapeseeds, linseeds and sugar beet might also be relevant for all products of animal origin;
Additional trials analysing for TAA and supporting the authorised uses on apples, pears, apricots (also relevant for peaches), wine grapes, strawberries, tomatoes, aubergines, sweet peppers, cucurbits with inedible peel, witloofs, globe artichokes, rapeseeds, linseeds, wheat, rye, barley, oat, buckwheat, maize, millet, sorghum, rice and sugar beets. It is underlined that requested trials on cereals, rapeseeds, linseeds and sugar beet might also be relevant for all products of animal origin;
Additional trials analysing for 1,2,4‐T and supporting the authorised uses on witloofs, wheat, rye, barley, oat, buckwheat, maize, millet, sorghum, rice and sugar beets. It is underlined that requested trials on cereals, rapeseeds, linseeds and sugar beet might also be relevant for all products of animal origin;
Additional trials on rapeseeds (relevant also for linseeds) analysing for TA and 1,2,4‐T with samples analysed within 30 days from day of sampling or a new storage stability study on rapeseeds;
A study investigating the storage stability of TA in high acid content commodities (relevant for strawberries and grapes);
Additional trials analysing for the sum of TA and TLA, for TAA, and 1,2,4‐T and supporting the authorised use on fodder beets (data gap relevant for all products of animal origin, except poultry products and eggs);
Field rotational crop studies analysing for TA, TAA, TLA and 1,2,4‐T and covering the most critical GAP currently authorised for tetraconazole on crops that can be rotated (SEU GAP on strawberries) and the calculated PEC soil. Considering the large margin of safety of the risk assessment performed with TDMs resulting from the authorised uses of tetraconazole assessed in this MRL review, this data gap is not expected to be an issue of concern for tetraconazole. Nevertheless, this might be relevant for the comprehensive assessment of all triazole fungicides.
It is highlighted, however, that some of the MRLs derived result from a GAP in one climatic zone only, whereas other GAPs reported by the RMS were not fully supported by data. EFSA therefore identified the following data gaps which are not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
Additional residue trials analysing for tetraconazole and supporting the authorised uses on grapes (import tolerance), strawberries (southern outdoor), tomatoes and aubergines (indoor), melons and watermelons (indoor);
Additional residue trials analysing for the sum of TA and TLA and supporting the authorised southern outdoor and import tolerance use on table grapes;
Additional residue trials analysing for TAA and supporting the authorised northern uses on quinces, medlars and loquats, and southern outdoor and import tolerance use for table grapes.
If the above reported data gaps are not addressed in the future, Member States are recommended to withdraw or modify the relevant authorisations at national level.
Minor deficiencies were also identified in the assessment, but these deficiencies are not expected to impact either on the validity of the MRLs derived or on the national authorisations. The following data are therefore considered desirable but not essential:
Studies investigating the storage stability of TLA in straw and TAA in high acid content commodities.
EFSA emphasises that a comprehensive risk assessment that covers all existing EU uses for all pesticides belonging to the class of triazole fungicides has still to be performed. EFSA recommended to elaborate together with risk managers a strategy to ensure that the required data are made available to finalise the overall risk assessment for triazole fungicides.
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- cGAP
critical GAP
- CXL
codex maximum residue limit
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DT90
period required for 90% dissipation (define method of estimation)
- EC
emulsifiable concentrate
- ECD
electron capture detector
- eq
residue expressed as a.s. equivalent
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- GC‐MS/MS
gas chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- LC
liquid chromatography
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantification
- Mo
monitoring
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- MS/MS
tandem mass spectrometry detector
- NEDI
national estimated daily intake
- NESTI
national estimated short‐term intake
- NTMDI
national theoretical maximum daily intake
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
preharvest interval
- Ppm
parts per million (10‐6)
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged and Safe (analytical method)
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SDL
screening detection limit
- SEU
southern European Union
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- WHO
World Health Organization
Appendix A – Summary of authorised uses considered for the review of MRLs
A.1. Authorised outdoor uses in northern EU
| Crop and/or situation | MS or country | F G or I a | Preparation | Application | Application rate per treatment | PHI (days) d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type b |
Conc. a.s. |
Method kind |
Range of growth stages and season c |
Number min–max |
Interval Between application (min) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | |||||
| Apples | HU, PL, AT | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 3 | 7 | – | – | 40 g a.i./ha | 14 | |
| Pears | HU | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 3 | 7 | – | – | 40 g a.i./ha | 14 | |
| Quinces | HU | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 3 | 7 | – | – | 40 g a.i./ha | 14 | |
| Medlars | HU | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 3 | 7 | – | – | 40 g a.i./ha | 14 | |
| Loquats | FR | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 2–3 | 7 | – | – | 30 g a.i./ha | 14 | |
| Table grapes | DE, CZ, AT, HU | F | EC | 80 g/L | Foliar treatment – broadcast spraying | 55–79 | 3 | 10 | – | – | 32 g a.i./ha | 28 | Application rate from BBCH 55–61: 8 g a.i./ha; from BBCH 61–71: 16 g a.i./ha; from BBCH 71–75: 24 g a.i./ha; from BBCH 75–79: 32 g a.i./ha. |
| Wine grapes | DE, AT, CZ, FR, HU | F | EC | 80 g/L | Foliar treatment – broadcast spraying | 55–79 | 3 | 10 | – | – | 32 g a.i./ha | 28 | Application rate from BBCH 55–61: 8 g a.i./ha; from BBCH 61–71: 16 g a.i./ha; from BBCH 71–75: 24 g a.i./ha; from BBCH 75–79: 32 g a.i./ha. |
| Strawberries | PL, AT | F | EC | 100 g/L | Foliar treatment – general | 61–69 | 2 | 7 | – | – | 60 g a.i./ha | 7 | |
| Witloofs/Belgian endives | BE | F | Foliar treatment – general | 1 | 100 g a.i./ha | 30 | |||||||
| Linseeds | BE | F | ME | 125 g/L | Foliar treatment – general (see also comment field) | 51–65 | 1 | – | – | 100 g a.i./ha | 41 | ||
| Rapeseeds | CZ | F | SE | 70 g/L | Foliar treatment – broadcast spraying | 61–65 | 1 | – | – | 122.5 g a.i./ha | n.a. | ||
| Barley | HU | F | EC | 125 g/L | Foliar treatment – broadcast spraying | 30–61 | 2 | 14 | – | – | 125 g a.i./ha | 42 | |
| Oat | HU | F | EC | 125 g/L | Foliar treatment – broadcast spraying | 30–61 | 2 | 14 | – | – | 125 g a.i./ha | 42 | |
| Rye | HU | F | EC | 125 g/L | Foliar treatment – broadcast spraying | 30–61 | 2 | 14 | – | – | 125 g a.i./ha | 42 | |
| Wheat | HU, DE | F | EC | 125 g/L | Foliar treatment – broadcast spraying | 30–61 | 2 | 14 | – | – | 125 g a.i./ha | 42 | |
| Sugar beets | DE, AT | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 49 | 2 | 21 | – | – | 100 g a.i./ha | 28 | |
| Chicory roots | BE | F | ME | 125 g/L | Foliar treatment – general (see also comment field) | Foliar treatment – general (see also comment field) | 1 | – | – | 100 g a.i./ha | 30 | culture of plants for witloof roots production | |
| Fodder beets | FR | F | ME | 125 g/L | Foliar treatment – broadcast spraying | 40 to | 1 | – | – | 100 g a.i./ha | 14 | ||
MS: Member State; a.i: active ingredient.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
A.2. Authorised outdoor uses in southern EU
| Crop and/or situation | MS or country | F G or I a | Preparation | Application | Application rate per treatment | PHI (days) d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type b |
Conc. a.s. |
Method kind |
Range of growth stages and season c |
Number min–max |
Interval between application (min) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | |||||
| Apples | FR, IT, PT EL, ES | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 2–3 | 7 | – | – | 30 g a.i./ha | 14 | |
| Pears | FR, IT, PT, EL, ES | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 2–3 | 7 | – | – | 30 g a.i./ha | 14 | |
| Quinces | FR | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 2–3 | 7 | – | – | 30 g a.i./ha | 14 | |
| Medlars | FR | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 2–3 | 7 | – | – | 30 g a.i./ha | 14 | |
| Loquats | FR | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 57–83 | 2–3 | 7 | – | – | 30 g a.i./ha | 14 | |
| Apricots | EL, ES, IT | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 61–75 | 3 | 10 | – | – | 40 g a.i./ha | 14 | |
| Peaches | EL, ES, IT | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 61–75 | 3 | 10 | – | – | 40 g a.i./ha | 14 | |
| Table grapes | PT, FR, IT, EL | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 53–79 | 1–3 | 12 | – | – | 30 g a.i./ha | 28 | |
| Wine grapes | PT, FR, IT, EL | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 53–79 | 1–3 | 12 | – | – | 30 g a.i./ha | 28 | |
| Strawberries | IT, EL | F | ME | 125 g/L | Foliar treatment – broadcast spraying | 60–90 | 1–3 | 7 | – | – | 50 g a.i./ha | 1 | |
| Kaki/Japanese persimmons | ES | F | Foliar treatment – broadcast spraying (see also comment field) | 57–83 | 3 | 14 | – | – | 30 g a.i./ha | 14 | RMS (FR) 09072021: use requested by an applicant, not authorised at the time of completeness check. Extrapolation from apple. | ||
| Tomatoes | EL, ES, HR | F | ME | 40 g/L | Foliar treatment – broadcast spraying (see also comment field) | 60–89 | 3 | 8 | – | – | 76 g a.i./ha | 3 | Oidiopsis sp.: 0.08–0.125 L product/hL. leaf mould of tomato: 0.125–0.19 L product/hL. |
| Sweet peppers | EL | F | EW | 125 g/L | Foliar treatment – broadcast spraying | 60–89 | 2–3 | 8 | – | – | 75 g a.i./ha | 3 | |
| Aubergines | EL | F | EW | 125 g/L | Foliar treatment – broadcast spraying | 60–89 | 2–3 | 8 | – | – | 75 g a.i./ha | 3 | |
| Cucumbers | EL, ES, IT | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 60–90 | 3 | 7 | – | – | 50 g a.i./ha | 3 | |
| Gherkins | EL, ES | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 60–90 | 3 | 7 | – | – | 50 g a.i./ha | 3 | |
| Courgettes | EL, IT, ES | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 60–90 | 3 | 7 | – | – | 50 g a.i./ha | 3 | |
| Melons | ES | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 60–90 | 3 | 7 | – | – | 50 g a.i./ha | 7 | |
| Pumpkins | ES | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 60–90 | 3 | 7 | – | – | 50 g a.i./ha | 7 | |
| Watermelons | ES | F | EC | 100 g/L | Foliar treatment – general (see also comment field) | 60–90 | 3 | 7 | – | – | 50 g a.i./ha | 7 | |
| Globe artichokes | IT | F | EC | 100 g/L | Foliar treatment – broadcast spraying | 31–49 | 3 | 7 | – | – | 40 g a.i./ha | 7 | |
| Barley | EL, ES | F | SE | 70 g/L | Foliar treatment – broadcast spraying | 25–69 | 1 | – | – | 122.5 g a.i./ha | 42 | ||
| Buckwheat | HR | F | SE | 70 g/L | Foliar treatment – broadcast spraying | 25–69 | 1 | – | – | 122.5 g a.i./ha | 42 | ||
| Maize | HR | F | SE | 70 g/L | Foliar treatment – broadcast spraying | 25–69 | 1 | – | – | 122.5 g a.i./ha | 42 | ||
| Common millet | HR | F | SE | 70 g/L | Foliar treatment – broadcast spraying | 25–69 | 1 | – | – | 122.5 g a.i./ha | 42 | ||
| Oat | HR | F | SE | 70 g/L | Foliar treatment – broadcast spraying | 25–69 | 1 | – | – | 122.5 g a.i./ha | 42 | ||
| Rice | HR | F | SE | 70 g/L | Foliar treatment – broadcast spraying | 25–69 | 1 | – | – | 122.5 g a.i./ha | 42 | ||
| Rye | HR | F | SE | 70 g/L | Foliar treatment – broadcast spraying | 25–69 | 1 | – | – | 122.5 g a.i./ha | 42 | ||
| Sorghum | HR | F | SE | 70 g/L | Foliar treatment – broadcast spraying | 25–69 | 1 | – | – | 122.5 g a.i./ha | 42 | ||
| Wheat | HR | F | SE | 70 g/L | Foliar treatment – broadcast spraying (see also comment field) | 25–69 | 1 | – | – | 122.5 g a.i./ha | 42 | Last application end of flowering 112.5–125 g ai/ha | |
| Sugar beets | HR | F | ME | 40 g/L | Foliar treatment – broadcast spraying | – | 1–3 | 21 | – | 100 g a.i./ha | 21 | ||
MS: Member State; a.i: active ingredient.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152–4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
A.3. Authorised indoor uses in EU
| Crop and/or situation | MS or country | F G or I a | Preparation | Application | Application rate per treatment | PHI (days) d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type b |
Conc. a.s. |
Method kind |
Range of growth stages and season c |
Number min–max |
Interval Between application (min) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | |||||
| Strawberries | IT, EL, ES | I | ME | 125 g/L | Foliar treatment – broadcast spraying | 60–90 | 1–3 | 7 | – | – | 50 g a.i./ha | 1 | |
| Tomatoes | EL | I | EW | 125 g/L | Foliar treatment – broadcast spraying | 60–89 | 2–3 | 8 | – | – | 75 g a.i./ha | 3 | |
| Sweet peppers | EL | I | EW | 125 g/L | Foliar treatment – broadcast spraying | 60–89 | 2–3 | 8 | – | – | 75 g a.i./ha | 3 | |
| Aubergines | EL | I | EW | 125 g/L | Foliar treatment – broadcast spraying | 60–89 | 2–3 | 8 | – | – | 75 g a.i./ha | 3 | |
| Cucumbers | EL, ES, IT | I | EC | 100 g/L | Foliar treatment – general (see also comment field) | 60–90 | 3 | 7 | – | – | 50 g a.i./ha | 3 | |
| Gherkins | EL | I | EC | 100 g/L | Foliar treatment – general (see also comment field) | 60–90 | 3 | 7 | – | – | 50 g a.i./ha | 3 | |
| Courgettes | IT, EL | I | ME | 125 g/L | Foliar treatment – broadcast spraying | 60–90 | 1–3 | 10 | – | – | 50 g a.i./ha | 3 | |
| Melons | IT, EL | I | ME | 125 g/L | Foliar treatment – broadcast spraying | 60–90 | 1–3 | 10 | – | – | 50 g a.i./ha | 7 | |
| Watermelons | IT, EL | I | ME | 125 g/L | Foliar treatment – broadcast spraying | 60–90 | 1–3 | 10 | – | – | 50 g a.i./ha | 7 | |
| Globe artichokes | IT | I | ME | 125 g/L | Foliar treatment – broadcast spraying | 31–49 | 1–3 | 15 | – | – | 37.5 g a.i./ha | 3 | |
MS: Member State; a.i: active ingredient.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
A.4. Import tolerance
| Crop and/or situation | MS or country | F G or I a | Preparation | Application | Application rate per treatment | PHI (days) d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type b |
Conc. a.s. |
Method kind |
Range of growth stages & season c |
Number min–max |
Interval Between application (min) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | |||||
| Table grapes | RU | F | EC | 80 g/L | Foliar treatment – broadcast spraying | – | 4 | 14 | – | 32 g a.i./ha | 32 g a.i./ha | 28 | Spraying during the vegetation period |
| Wine grapes | RU | F | EC | 80 g/L | Foliar treatment – broadcast spraying | – | 4 | 14 | – | 32 g a.i./ha | 32 g a.i./ha | 28 | Spraying during the vegetation period |
MS: Member State; a.i: active ingredient.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | grapes | Foliar: 4 × 26.5 mg a.i./L (= 4 × 26.5 g a.i./ha; France, 2021) | 0, 14, 28, 42, 102 DAT1 | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005; EFSA, 2008) | |
| Foliar: 4 × 22.5 mg a.i./L (= 4 × 22.5 g a.i./ha; France, 2021) | 0, 14, 28, 42, 102 DAT1 | Radiolabelled active substance: [14C‐phenyl] tetraconazole (Italy, 2005; EFSA, 2008) | |||
| Topical in growth chamber (translocation study): 0.49 μCi/leaf | 2h, 3, 7, 10, 14, 28 DAT | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005) | |||
| Topical in growth chamber (translocation study): rate not clearly reported | 0, 1, 4, 8, 14, 20, 32 DAT | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005) | |||
| Root crops | Sugar beet | Foliar: 3 × 100 g a.i./ha | 0, 20, 41, 76 DAT1 | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005; EFSA, 2008) | |
| Foliar: 3 × 100 g a.i./ha and 3 × 500 g a.i./ha | 23 DAT3 | Radiolabelled active substance: [14C‐phenyl] tetraconazole (Italy, 2005; EFSA, 2008) | |||
| Topical in growth chamber (translocation study): 0.96 μCi/leaf | 2h, 3, 7, 14 and 21 DAT | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005) | |||
| Topical in growth chamber (translocation study): 0.45 μCi/leaf | 2h, 3, 7, and 14 DAT | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005) | |||
| Cereals/grass | wheat | Foliar: 2 × 125 g a.i./ha | Post‐1st application (0 DAT1), pre‐ and post‐ 2nd application (23 DAT1), harvest (64 DAT1) | Radiolabelled active substance: [14C‐phenyl] tetraconazole (Italy, 2005; EFSA, 2008) | |
| Foliar: 2 × 125 g a.i./ha | Post‐1st application (0 DAT1), pre‐ and post‐2nd application (23 DAT1), harvest (64 DAT1) | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005; EFSA, 2008) | |||
| Foliar: 3 × 125 g a.i./ha | Maturity (BBCH 89–90) | Radiolabelled active substance: [14C‐triazole] and [14C‐phenyl] tetraconazole (Italy, 2005; EFSA, 2008) | |||
| Topical in growth chamber (translocation study): rate not clearly reported | 0, 4, 7, 14, 21 DAT | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005) | |||
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
| Root/tuber crops | carrots |
Bare soil: 500 g a.i./ha Additional plots treated at 2,500 g a.i./ha to facilitate identification |
30, 120, 365 | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005; EFSA, 2008) | |
|
Bare soil: 500 g a.i./ha Additional plots treated at 5,000 g a.i./ha to facilitate identification (PBI 120) |
30, 120, 223, 365 | Radiolabelled active substance: [14C‐phenyl] tetraconazole (Italy, 2005; EFSA, 2008) | |||
| Leafy crops | lettuce |
Bare soil: 500 g a.i./ha Additional plots treated at 2,500 g a.i./ha to facilitate identification |
30, 120, 365 | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005; EFSA, 2008) | |
|
Bare soil: 500 g a.i./ha Additional plots treated at 5,000 g a.i./ha to facilitate identification (PBI 120) |
30, 120, 223, 365 | Radiolabelled active substance: [14C‐phenyl] tetraconazole (Italy, 2005; EFSA, 2008) | |||
| Cereal (small grain) | Wheat |
Bare soil: 500 g a.i./ha Additional plots treated at 2,500 g a.i./ha to facilitate identification |
30, 120, 365 | Radiolabelled active substance: [14C‐triazole] tetraconazole (Italy, 2005; EFSA, 2008) | |
| Wheat, sorghum |
Bare soil: 500 g a.i./ha Additional plots treated at 5,000 g a.i./ha to facilitate identification (PBI 120) |
30 (wheat), 120 (wheat), 223 (sorghum), 365 (wheat) | Radiolabelled active substance: [14C‐phenyl] tetraconazole (Italy, 2005; EFSA, 2008) | ||
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source | ||
| Pasteurisation (20 min, 90°C, pH 4) | Yes | Parent (Italy, 2005; EFSA, 2008) and TDMs (TA, TAA, TLA and 1,2,4‐T) (EFSA, 2018b) | |||
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | Parent (Italy, 2005; EFSA, 2008) and TDMs (TA, TAA, TLA and 1,2,4‐T) (EFSA, 2018b) | |||
| Sterilisation (20 min, 120°C, pH 6) | Yes | Parent (Italy, 2005; EFSA, 2008) and TDMs (TA, TAA, TLA and 1,2,4‐T) (EFSA, 2018b) | |||

B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Apple | –20 | 36 | Months | tetraconazole | Italy (2005), EFSA (2008) | |
| Apples, tomatoes, mustard leaves, wheat forage, radishes tops/roots, turnips roots, cabbages, lettuces | –20 | ≤ 6 | Months | 1,2,4‐T | EFSA (2018b) | ||
| –20 | 53 | Months | TA, TAA | EFSA (2018b) | |||
| –20 | 48 | Months | TLA | EFSA (2018b) | |||
| High oil content | rapeseeds | –20 | 36 | Months | tetraconazole | Italy (2011), EFSA (2012) | |
| –20 | < 3 | Months | 1,2,4‐T | Not stable, data gap (EFSA, 2018b). | |||
| –20 | Inconclusive | Inconclusive | TA | Not stable, data gap (EFSA, 2018b) | |||
| –20 | 53 | Months | TAA | EFSA (2018b) | |||
| –20 | 48 | Months | TLA | EFSA (2018b) | |||
| soya beans | –20 | 12 | Months | 1,2,4‐T | EFSA (2018b) | ||
| –20 | 26 | Months | TA | EFSA (2018b) | |||
| –20 | 53 | Months | TAA | EFSA (2018b) | |||
| –20 | 48 | Months | TLA | EFSA (2018b) | |||
| Dry/high starch content | barley, wheat grains | –20 | 36 | Months | Tetraconazole | Italy (2005), EFSA (2008) | |
| –20 | 12 | Months | 1,2,4‐T | EFSA (2018b) | |||
| –20 | 26 | Months | TA, TAA | EFSA (2018b) | |||
| –20 | 48 | Months | TLA | EFSA (2018b) | |||
| sugar beet root | –20 | 36 | Months | tetraconazole | Italy (2005), EFSA (2008) | ||
| –20 | 6 | Months | 1,2,4‐T | EFSA (2018b) | |||
| –20 | 53 | Months | TA, TAA | EFSA (2018b) | |||
| –20 | 48 | Months | TLA | EFSA (2018b) | |||
| Dry/high protein | Peas, dry; Navy beans | – | – | – | tetraconazole | Not relevant for the GAPs under assessment | |
| – | – | – | 1,2,4‐T | Not available and not required since no uses are currently authorised for crops belonging to this matrix group | |||
| –20 | 15 | Months | TA | EFSA (2018b) | |||
| –20 | 25 | Months | TAA | EFSA (2018b) | |||
| –20 | 48 | Months | TLA | EFSA (2018b) | |||
| High acid content | wine grape | –20 | 36 | Months | tetraconazole | Italy (2005), EFSA (2008) | |
| Orange | – | – | – | 1,2,4‐T | Not available and not required since this metabolite is not expected to be present at significant levels in crops belonging to this matrix group (fruit crops) following treatment with tetraconazole (EFSA, 2018b) | ||
| – | – | – | TA | Not available, data gap (EFSA, 2018b) | |||
| TAA | Not available but only desirable considering that in all other matrices TAA was stable for at least 26 months and samples were stored for a maximum of 24 months. | ||||||
| –20 | 48 | Months | TLA | EFSA (2018b) | |||
| Processed products | Refined oil | –20 | 24 | Months | Tetraconazole | Italy (2011), EFSA (2012) | |
| Others | barley, wheat straw | –20 | 36 | Months | Tetraconazole | Italy (2005), EFSA (2008) | |
| –20 | 12 | Months | 1,2,4‐T | EFSA (2018b) | |||
| –20 | 53 | Months | TA | EFSA (2018b) | |||
| –20 | 40 | Months | TAA | EFSA (2018b) | |||
| – | – | – | TLA | Not available but only desirable considering that in all other matrices TLA was stable for at least 48 months and samples were stored for a maximum of 15.5 months | |||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials – Primary crops – Tetraconazole (RD‐Mo and RD‐RA1)
| Commodity | Region a | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HR b (mg/kg) | STMR c (mg/kg) |
|---|---|---|---|---|---|---|
| RD‐Mo and RD‐RA1: tetraconazole | ||||||
|
Apples Pears Quinces Medlars |
NEU |
Unscaled: 0.018; 0.022; 0.041; 0.044; 0.046; 0.051; 0.123 Scaling factors: 1.29; 1.33; 1.33; 1.29; 1.33; 1.29; 1.33 Scaled: 0.023; 0.029; 0.055; 0.057; 0.061; 0.066; 0.164 |
Reduced data set of trials on apples underdosed but within acceptable 25% deviation (EFSA, 2013b). Proportionality applied to avoid systematic bias (France, 2021). Extrapolation to pears, quinces and medlars applicable. MRLOECD = 0.25 |
0.30 d (tentative for apples and pears) | 0.16 | 0.06 |
| SEU | 2 × < 0.005 e ; 2 × < 0.01; 0.013; 0.014; 2 × 0.021; 0.025; 2 × 0.035; 0.057 |
Trials on apples compliant with GAP (EFSA, 2013b; United Kingdom, 2018). Extrapolation to pears, quinces and medlars applicable. MRLOECD = 0.08 |
0.09 | 0.06 | 0.02 | |
| Loquats/Japanese medlars | NEU | < 0.010; 0.018; 0.022; 0.041; 0.044; 0.046; 0.051; 0.123 |
Extrapolated from trials on apples compliant with GAP on loquats (EFSA, 2013b). MRLOECD = 0.18 |
0.2 | 0.12 | 0.04 |
| SEU | 2 × < 0.005 e ; 2 × < 0.01; 0.013; 0.014; 2 × 0.021; 0.025; 2 × 0.035; 0.057 |
Extrapolated from trials on apples compliant with GAP on loquats (EFSA, 2013b; United Kingdom, 2018). MRLOECD = 0.08 |
0.09 | 0.06 | 0.02 | |
|
Apricots Peaches |
SEU | < 0.010; < 0.012; 0.013; 0.016 |
Reduced data set on peaches compliant with GAP (France, 2021), used on a tentative basis for apricots and peaches. MRLOECD = 0.03 |
0.03 d (tentative) | 0.02 | 0.01 |
|
Table grapes Wine grapes |
NEU | < 0.005 e ; < 0.01; 0.012; 0.014; 0.018; 0.019; 0.021; 0.025; 0.027; 0.034; 2 × 0.042 |
Trials on wine grape performed at PHI falling within 25% deviation (EFSA, 2013b; United Kingdom, 2018). Extrapolation to table grape is applicable. MRLOECD = 0.07 |
0.07 | 0.04 | 0.02 |
| SEU | 2 × < 0.005 e ; < 0.01; 0.012; 0.013; 2 × 0.014; 0.018; 0.030; 0.033; 0.034; 0.044 |
Trials on wine grape performed at PHI falling within 25% deviation (EFSA, 2013b; United Kingdom, 2018). Extrapolation to table grape is applicable. MRLOECD = 0.07 |
0.07 | 0.04 | 0.01 | |
| Import (RU) | – | No GAP compliant trials available. | – | – | – | |
| Strawberries | NEU | 0.016; 0.020; 0.021; 0.029; 0.040 |
Reduced data set of trials on strawberries compliant with GAP (France, 2021). No additional trials are required since the indoor use is clearly more critical. MRLOECD = 0.08 |
0.08 | 0.04 | 0.02 |
| SEU | – | No GAP compliant trials available. | – | – | – | |
| EU | 2 × 0.03; 3 × 0.04; 0.06; 0.07 f ; 0.08 |
Trials on strawberries compliant with GAP (France, 2021). MRLOECD = 0.15 |
0.15 | 0.08 | 0.04 | |
| Kaki/Japanese persimmons | SEU |
2 × < 0.005 e ; 2 × < 0.01; 0.013; 0.014; 2 × 0.021; 0.025; 2 × 0.035; 0.057 |
Extrapolation from trials on apples compliant with GAP on kaki (EFSA, 2013b; United Kingdom, 2018). MRLOECD = 0.08 |
0.09 | 0.06 | 0.02 |
|
Tomatoes Aubergines/eggplants |
SEU | < 0.02; 0.022; 0.033; 0.037; 0.041; 0.045; 0.048; 0.057 f ; 0.068 |
Trials on tomato compliant with GAP (France, 2021). Extrapolation to aubergines is applicable. MRLOECD = 0.11 |
0.15 | 0.07 | 0.04 |
| EU | – | No GAP compliant trials available. | – | – | – | |
| Sweet peppers/bell peppers | SEU | – | No GAP compliant trials available. | – | – | – |
| EU | – | No GAP compliant trials available. | – | – | – | |
|
Cucumber Gherkins Courgettes |
SEU | < 0.01; 0.011; 0.013; 0.019; 0.023; 0.032; 0.042; 0.093 |
Trials on cucumber compliant with GAP (France, 2021). Extrapolation to courgettes and gherkins applicable. MRLOECD = 0.14 |
0.15 | 0.09 | 0.02 |
| EU | < 0.01; 0.011; 0.012; 0.015; 0.023; 0.024; 0.049; 0.064 |
Trials on cucumber compliant with GAP (France, 2021). Extrapolation to courgettes and gherkins applicable. MRLOECD = 0.11 |
0.15 | 0.06 | 0.02 | |
|
Melons Pumpkins Watermelons |
SEU | < 0.005 e ; < 0.01; 2 × < 0.015; 0.015; 0.016; 0.020; 0.021; 0.023; 0.053 |
Trials on melons compliant with GAP (France, 2021). Extrapolation to watermelon and pumpkin is applicable. MRLOECD = 0.07 |
0.08 | 0.05 | 0.02 |
| EU | – | No GAP compliant trials available. Indoor GAP authorised on melons and watermelons only. | – | – | – | |
| Witloofs/Belgian endives | NEU | 3 × < 0.02 | Reduced data set of overdosed (1.3N) trials on witloofs (after forcery) deemed acceptable as all residues < LOQ of trials’ method (Belgium, 2021). | 0.02 | 0.02 | 0.02 |
| Globe artichokes | SEU | 0.069; 0.093 | Reduced dataset of trials on globe artichoke compliant with GAP (France, 2021), not sufficient to derive MRL. | – | 0.09 | 0.08 |
| EU | – | No GAP compliant trials available. | – | – | – | |
|
Rapeseeds/canola seeds Linseeds |
NEU | 2 × < 0.01; 0.010; 0.018; 0.019; < 0.020; 0.026; 0.027+; 0.036; 0.042; 0.061; 0.082 |
Trials on rapeseeds performed at more critical BBCH (69–75 instead of 65) and overdosed when compared to linseeds’ GAP (1.25N) used on a tentative basis for both crops (EFSA, 2012, 2019a; France, 2021). MRLOECD = 0.12 |
0.15 g (tentative) | 0.08 | 0.02 |
|
Barley grain and straw Oat grains and straw |
NEU | – | No GAP compliant trials available. | – | – | – |
| SEU | – | No GAP compliant trials available. | – | – | – | |
| Buckwheat and other pseudo‐cereal grains | SEU | – | No GAP compliant trials available. | – | – | – |
|
Maize/corn grains and stover Common millet/proso millet grains and straw Sorghum grains and stover |
SEU | – | No GAP compliant trials available. | – | – | – |
| Rice grains and straw | SEU | – | No GAP compliant trials available. | – | – | – |
|
Wheat grains Rye grains |
NEU | 2 × < 0.015; 3 × < 0.02 |
Reduced data set of trials on wheat compliant with GAP, deemed acceptable as all residues < LOQ of trials’ method (EFSA, 2008; France, 2021). Extrapolation to rye applicable. MRLOECD = 0.02 |
0.02 |
0.02 | 0.02 |
| SEU | 9 × < 0.005 e |
Residue trials on wheat performed with PHI within 25% deviation (EFSA, 2008; United Kingdom, 2018). Extrapolation to rye applicable. MRLOECD = 0.01 |
0.01* | 0.01 | 0.01 | |
| Sugar beet roots | NEU | 7 × < 0.01; < 0.02 |
Trials on sugar beet performed at PHI within 25% deviation (EFSA, 2008). No residues > 0.01* mg/kg expected according to metabolism studies. MRLOECD = 0.02 |
0.01* | 0.01 | 0.01 |
| SEU | 5 × < 0.01; 3 × < 0.02 |
Trials on sugar beet performed at more critical PHI (14 instead of 21), deemed acceptable since all residues were < LOQ (EFSA, 2008; France, 2021). No residues > 0.01* mg/kg expected according to metabolism studies. MRLOECD = 0.02 |
0.01* | 0.01 | 0.01 | |
| Chicory roots | NEU |
Unscaled: 0.02; 0.03; 0.03 Scaling factor: 0.78; 0.76; 0.83 Scaled: < 0.02; 0.022; 0.025 |
Reduced data set of overdosed trials on chicory roots (1.3N), used on a tentative basis proportionality applied (France, 2021). MRLOECD = 0.05 |
0.06 d (tentative) | 0.03 | 0.02 |
|
Wheat straw Rye straw |
NEU | 0.6; 0.695; 0.752; 1.3; 2.4 |
Residue trials on wheat compliant with GAP (EFSA, 2008; France, 2021). Extrapolation to rye applicable. MRLOECD = 4.15 |
5 h (tentative) | 2.40 | 0.75 |
| SEU | 0.381; 0.392; 0.416; 0.494; 0.670; 0.917; 1.089; 1.282; 1.374 |
Residue trials on wheat performed at PHI within 25% deviation (EFSA, 2008; United Kingdom, 2018). Extrapolation to rye applicable. MRLOECD = 2.36 |
3 h (tentative) | 1.37 | 0.67 | |
| Fodder beet roots | NEU | 4 × < 0.01 |
Overdosed (2 instead of 1 application) and reduced data set of trials on sugar beet deemed acceptable since all residues below LOQ and no residues expected according to metabolism studies (Italy, 2005; France, 2021). Values at PHI 14 selected to comply with GAP on fodder beet. MRLOECD = 0.01 |
0.01*, h (tentative) | 0.01 | 0.01 |
| Fodder beet tops | NEU | 0.638; 0.933; 1.371; 1.387 |
Reduced data set of overdosed trials (2N) on sugar beet (EFSA, 2008) used on a tentative basis. Values at PHI 14 selected to comply with GAP on fodder beet. MRLOECD = 3.25 |
4 g , h (tentative) | 1.39 | 1.15 |
| Sugar beet tops | NEU | 0.206; 0.304; 0.333; 0.452; 0.629; 0.649; 0.687; 0.767 |
Trials on sugar beet performed at PHI within 25% deviation (EFSA, 2008). MRLOECD = 1.51 |
1.50 h (tentative) | 0.77 | 0.54 |
| SEU | 0.381; 0.550; 0.559; 0.631; 0.669; 0.909; 1.006; 1.219 |
Trials on sugar beet performed at more critical PHI (14 instead of 21) (EFSA, 2008), used on a tentative basis. MRLOECD = 2.22 |
3 g , h (tentative) | 1.22 | 0.65 | |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level.
Mo: residue levels expressed according to the monitoring residue definition; RA: residue levels expressed according to risk assessment residue definition.
Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, EU: indoor EU trials, Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Tentative MRL in the absence of sufficient number of GAP‐compliant trials.
Residue value < LOD (0.005 mg/kg) of risk assessment method used in residue trials.
Selected value corresponds to higher residue levels observed at a longer PHI.
Tentative MRL in the absence of GAP‐compliant trials.
A tentative MRL is derived in view of the future need to set MRLs in livestock feed items.
B.1.2.2. Summary of residues data from the supervised residue trials – Primary crops – Triazole derivative metabolites (RD‐RA2, RD‐RA3 and RD‐RA4)
| Commodity | Region a | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HR b (mg/kg) | STMR c (mg/kg) |
|---|---|---|---|---|---|---|
| RD‐RA2: triazole alanine (TA) and triazole lactic acid (TLA) | ||||||
|
Apples Pears Quinces Medlars |
NEU |
Scaled TA: 0.263; 0.464 Scaled TLA: 0.032; 0.053 Summed: 0.295; 0.517 |
Trials on apples underdosed but within acceptable 25% deviation (United Kingdom, 2018). Proportionality applied to avoid systematic bias (France, 2021). Reduced data set considered to derive tentative risk assessment values for apples, pears, quinces and medlars. | – | 0.52 | 0.41 |
| SEU |
TA: 0.015; 0.030; 0.038; 0.039 TLA: 0.03; 0.026; 0.03; 0.028 Summed: 0.045; 0.056; 0.068; 0.067 |
Reduced data set on apples compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for apples and pears and robust risk assessment values for quinces and medlars. | – | 0.07 | 0.06 | |
| Loquats/Japanese medlars | NEU |
TA: 0.198; 0.349 TLA: 0.024; 0.04 Summed: 0.222; 0.389 |
Reduced data set on apples compliant with GAP on loquats (United Kingdom, 2018) considered to derive tentative risk assessment values. | – | 0.39 | 0.31 |
| SEU |
TA: 0.015; 0.030; 0.038; 0.039 TLA: 0.03; 0.026; 0.03; 0.028 Summed: 0.045; 0.056; 0.068; 0.067 |
Trials on apple compliant with GAP on loquats (EFSA, 2013b). | – | 0.07 | 0.06 | |
|
Apricots Peaches |
SEU |
TA: < 0.01; 0.03; 0.195; 0.581 TLA: < 0.005; < 0.005; < 0.005; 0.032 Summed: < 0.015; 0.035; 0.2; 0.613 |
Reduced data set on peaches compliant with GAP (France, 2021) considered to derive tentative risk assessment values for apricots and peaches | – | 0.61 | 0.12 |
|
Table grapes Wine grapes |
NEU |
TA: < 0.005; < 0.005; 0.029; 0.036 TLA: 0.022; 0.033; 0.016; 0.0591 Summed: 0.027; 0.038; 0.045; 0.0951 |
Trials on grapes compliant with GAP for table and wine grapes (United Kingdom, 2018). Derived risk assessment values are tentative for wine grapes (major crop in NEU). Results for TA should be confirmed by a storage stability study. | – | 0.10 | 0.04 |
| SEU |
TA: < 0.005; < 0.005; 0.025; 0.037, TLA: < 0.005; 0.012; 0.013; 0.023 Summed: 0.01; 0.017; 0.038; 0.06 |
Reduced data set on grapes compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for table and wine grapes. Results for TA should be confirmed by a storage stability study. | – | 0.06 | 0.03 | |
| Import (RU) | – | No GAP compliant trials available. | – | – | – | |
| Strawberries | NEU |
TA: 0.015; 0.045 TLA: < 0.01; 0.011 Summed: 0.025; 0.056 |
Reduced data set on strawberries compliant with GAP (France, 2021) considered to derive tentative risk assessment values. Results for TA should be confirmed by a storage stability study. | – | 0.06 | 0.04 |
| SEU | – | No GAP compliant trials available. | – | – | – | |
| EU | – | No GAP compliant trials available. | – | – | – | |
| Kaki | SEU |
TA: 0.015; 0.030; 0.038; 0.039 TLA: 0.03; 0.026; 0.03; 0.028 Summed: 0.045; 0.056; 0.068; 0.067 |
Trials on apples compliant with GAP (United Kingdom, 2018). Extrapolation to kaki applicable. | – | 0.07 | 0.06 |
|
Tomatoes Aubergines/eggplants |
SEU |
TA: 0.015; 0.079 TLA: < 0.005; 0.017 Summed: 0.02; 0.096 |
Reduced data set on tomatoes compliant with GAP (France, 2021) considered to derive tentative risk assessment values for tomatoes and aubergines. | – | 0.10 | 0.06 |
| EU | – | No GAP compliant trials available. | – | – | – | |
| Sweet peppers/bell peppers | SEU | – | No GAP compliant trials available. | – | – | – |
| EU | – | No GAP compliant trials available. | – | – | – | |
|
Cucumber Gherkins Courgettes |
SEU |
TA: 0.015; 0.025; 0.127; 0.411 TLA: < 0.005; < 0.005; < 0.005; < 0.01 Summed: 0.02; 0.03; 0.132; 0.421 |
Trials on cucumbers compliant with GAP for cucurbits with edible peel (France, 2021). Derived risk assessment values are tentative for courgettes (major crop in SEU). | – | 0.42 | 0.08 |
| EU |
TA: 0.022; 0.031; 0.083; 0.111; 0.112; 0.196 TLA: < 0.005; < 0.005; < 0.01; 0.012; < 0.005; < 0.005 Summed: 0.027; 0.036; 0.093; 0.123; 0.117; 0.201 |
Trials on cucumbers compliant with GAP for cucurbits with edible peel (France, 2021). Derived risk assessment values are tentative for cucumbers and courgettes (major crops in EU). | – | 0.20 | 0.11 | |
|
Melons Pumpkins Watermelons |
SEU |
TA: 0.052; 0.35 TLA: 0.049; < 0.01 Summed: 0.101; 0.355 |
Reduced data set of trials on melons compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for cucurbits with inedible peel. Reported results refer to residues in pulp. | – | 0.36 | 0.23 |
| EU | – | No GAP compliant trials available. Indoor GAP authorised on melons and watermelons only. | – | – | – | |
| Witloofs/Belgian endives | NEU | – | No GAP compliant trials available. | – | – | – |
|
Globe artichokes |
SEU |
TA: 0.027; 0.044 TLA: < 0.005; < 0.005 Summed: 0.032; 0.049 |
Reduced data set on globe artichokes compliant with GAP (France, 2021) considered to derive tentative risk assessment values. | – | 0.05 | 0.04 |
| EU | – | No GAP compliant trials available. | – | – | – | |
|
Rapeseeds/canola seeds Linseeds |
NEU |
TA: 0.259; 0.621; 0.988; 1.015; 1.033; 1.890 TLA: 0.024; 0.015; 0.059; 0.043; 0.03; 0.064 Summed: 0.283; 0.636; 1.047; 1.058; 1.063; 1.954 |
Reduced data set on rapeseeds performed according to a more critical GAP (BBCH 69–72 instead of BBCH 65) considered to derive tentative risk assessment values for rapeseeds and linseeds (United Kingdom, 2018). Results for TA should be confirmed by additional trials with samples analysed within 30 days from sampling or by a new storage stability study. |
– | 1.95 | 1.05 |
|
Barley grain and straw Oat grains and straw |
NEU | – | No GAP compliant trials available. | – | – | – |
| SEU | – | No GAP compliant trials available. | – | – | – | |
| Buckwheat and other pseudo‐cereal grains | SEU | – | No GAP compliant trials available. | – | – | – |
|
Maize/corn grains and stover Common millet/proso millet grains and straw Sorghum grains and stover |
SEU | – | No GAP compliant trials available. | – | – | – |
| Rice grains and straw | SEU | – | No GAP compliant trials available. | – | – | – |
|
Wheat grains Rye grains |
NEU |
TA: < 0.005; < 0.005 TLA: –; –; Summed: < 0.005; < 0.005 |
Reduced data set on wheat grains compliant with GAP (France, 2021) considered to derive tentative risk assessment values for wheat and rye. | – | 0.01 | 0.01 |
| SEU |
TA: 0.606 TLA: 0.021 Summed: 0.627 |
Reduced data set on wheat grains compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for wheat and rye. | – | 0.63 | 0.63 | |
| Sugar beet roots | NEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies. Nevertheless, reduced data set of four trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
| SEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies. Nevertheless, reduced data set of four trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 | |
| Sugar beet tops | NEU | – | No GAP compliant trials available. | – | – | – |
| SEU | – | No GAP compliant trials available. | – | – | – | |
| Chicory roots | NEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies performed at 3N. Since chicory roots is a minor crop, no residue trials are required. | – | 0.01 | 0.01 |
|
Wheat straw Rye straw |
NEU | – | No GAP compliant trials available. | – | – | – |
| SEU |
TA: 0.024 TLA: 0.039 Summed: 0.063 |
Reduced data set on wheat straw compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for wheat and rye. | – | 0.06 | 0.06 | |
| Fodder beet roots | NEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies performed at 3N. Nevertheless, reduced data set of three trials is still required to confirm that residues will remain < LOQ. | 0.01 | 0.01 | |
| Fodder beet tops | NEU | – | No GAP compliant trials available. | – | – | – |
| RD‐RA3: triazole acetic acid (TAA) | ||||||
|
Apples Pears Quinces Medlars |
NEU | 2 × < 0.005 | Trials on apples underdosed but within acceptable 25% deviation (United Kingdom, 2018). Reduced data set considered to derive tentative risk assessment values for apples, pears, quinces and medlars. | – | 0.01 | 0.01 |
| SEU | < 0.01; 0.013; 0.018; 0.020 | Reduced data set on apples compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for apples and pears and robust risk assessment values for quinces and medlars. | – | 0.02 | 0.02 | |
| Loquats/Japanese medlars | NEU | 2 × < 0.005 | Reduced data set on apples compliant with GAP on loquats (United Kingdom, 2018) considered to derive tentative risk assessment values. | – | 0.01 | 0.01 |
| SEU | < 0.01; 0.013; 0.018; 0.020 | Trials on apple compliant with GAP on loquats (United Kingdom, 2018). | – | 0.02 | 0.02 | |
|
Apricots Peaches |
SEU | 2 × < 0.005; < 0.01; 0.017 | Reduced dataset on peaches compliant with GAP (France, 2021) considered to derive tentative risk assessment values for apricots and peaches. | – | 0.02 | 0.01 |
|
Table grapes Wine grapes |
NEU | 2 × < 0.005; 0.015; 0.026 | Trials on grapes compliant with GAP for table and wine grapes (United Kingdom, 2018). Derived risk assessment values are tentative for wine grapes (major crop in NEU). | – | 0.03 | 0.01 |
| SEU | 3 × < 0.005; 0.017 | Reduced data set on grapes compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for table and wine grapes. | – | 0.02 | 0.01 | |
| Import (RU) | – | No GAP compliant trials available. | – | – | – | |
| Strawberries | NEU | 2 × < 0.005 | Reduced data set on strawberries compliant with GAP (France, 2021), considered to derive tentative risk assessment values. | – | 0.01 | 0.01 |
| SEU | – | No GAP compliant trials available. | – | – | – | |
| EU | – | No GAP compliant trials available. | – | – | – | |
| Kaki | SEU | < 0.01; 0.013; 0.018; 0.020 | Trials on apples compliant with GAP (United Kingdom, 2018). Extrapolation to kaki applicable. | – | 0.02 | 0.02 |
|
Tomatoes Aubergines/eggplants |
SEU | 2 × < 0.005 | Reduced data set on tomatoes compliant with GAP (France, 2021) considered to derive tentative risk assessment values for tomatoes and aubergines. | – | 0.01 | 0.01 |
| EU | – | No GAP compliant trials available. | – | – | – | |
| Sweet peppers/bell peppers | SEU | – | No GAP compliant trials available. | – | – | – |
| EU | – | No GAP compliant trials available. | – | – | – | |
|
Cucumber Gherkins Courgettes |
SEU | 4 × < 0.005 | Trials on cucumbers compliant with GAP for cucurbits with edible peel (France, 2021). Reduced number of trials acceptable since residues were always below the LOD. Extrapolation to courgettes and gherkins is applicable. | – | 0.01 | 0.01 |
| EU | 6 × < 0.005 | Trials on cucumbers compliant with GAP for cucurbits with edible peel (France, 2021). Reduced number of trials acceptable since residues were always below the LOD. Extrapolation to courgettes and gherkins is applicable. | – | 0.01 | 0.01 | |
|
Melons Pumpkins Watermelons |
SEU | 2 × < 0.01 | Reduced data set of trials on melons compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for cucurbits with inedible peel. Reported results refer to residues in pulp. | – | 0.01 | 0.01 |
| EU | – |
No GAP compliant trials available. Indoor GAP authorised on melons and watermelons only. |
– | – | – | |
| Witloofs/Belgian endives | NEU | – | No GAP compliant trials available. | – | – | – |
| Globe artichokes | SEU | 2 × < 0.005 | Reduced data set on globe artichokes compliant with GAP (France, 2021) considered to derive tentative risk assessment values. | – | 0.01 | 0.01 |
| EU | – | No GAP compliant trials available. | – | – | – | |
|
Rapeseeds/canola seeds Linseeds |
NEU | 0.015; 0.021; 0.024; 0.032; 0.043; 0.064 | Reduced data set on rapeseeds performed according to a more critical GAP (BBCH 69–72 instead of BBCH 65) considered to derive tentative risk assessment values for rapeseeds and linseeds (United Kingdom, 2018). | – | 0.06 | 0.03 |
|
Barley grain and straw Oat grains and straw |
NEU | – | No GAP compliant trials available. | – | – | – |
| SEU | – | No GAP compliant trials available. | – | – | – | |
| Buckwheat and other pseudo‐cereal grains | SEU | – | No GAP compliant trials available. | – | – | – |
|
Maize/corn grains and stover Common millet/proso millet grains and straw Sorghum grains and stover |
SEU | – | No GAP compliant trials available. | – | – | – |
| Rice grains and straw | SEU | – | No GAP compliant trials available. | – | – | – |
|
Wheat grains Rye grains |
NEU | 2 × < 0.005 | Reduced data set on wheat grains compliant with GAP (France, 2021) considered to derive tentative risk assessment values for wheat and rye. | – | 0.01 | 0.01 |
| SEU | 0.094 | Reduced data set on wheat grains compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for wheat and rye. | – | 0.09 | 0.09 | |
| Sugar beet roots | NEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies. Nevertheless, reduced data set of four trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
| SEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies. Nevertheless, reduced data set of four trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 | |
| Sugar beet tops | NEU | – | No GAP compliant trials available. | – | – | – |
| SEU | – | No GAP compliant trials available. | – | – | – | |
| Chicory roots | NEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies performed at 3N. Since chicory roots is a minor crop, no residue trials are required. | – | 0.01 | 0.01 |
|
Wheat straw Rye straw |
NEU | – | No GAP compliant trials available. | – | – | – |
| SEU | 0.093 | Reduced data set on wheat straw compliant with GAP (United Kingdom, 2018) considered to derive tentative risk assessment values for wheat and rye. | – | 0.09 | 0.09 | |
| Fodder beet roots | NEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies performed at 3N. Nevertheless, reduced data set of three trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
| Fodder beet tops | NEU | – | No GAP compliant trials available. | – | – | – |
| RD‐RA4: 1,2,4‐T (1,2,4‐triazole) | ||||||
|
Apples Pears Quinces Medlars |
NEU | 2 × < 0.005 | Trials on apples underdosed but within acceptable 25% deviation extrapolated to pears, quinces and medlars (United Kingdom, 2018). Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 |
| SEU | 4 × < 0.005 | Trials on apples compliant with GAP (United Kingdom, 2018) extrapolated to pears, quinces and medlars. Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 | |
| Loquats/Japanese medlars | NEU | 2 × < 0.005 | Reduced data set on apples compliant with GAP on loquats (United Kingdom, 2018). Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 |
| SEU | 4 × < 0.005 | Reduced data set on apples compliant with GAP for loquats (United Kingdom, 2018). Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 | |
|
Apricots Peaches |
SEU | 4 × < 0.005 | Trials on peaches compliant with GAP (France, 2021). Reduced number of trials acceptable for apricots and peaches since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 |
|
Table grapes Wine grapes |
NEU | 4 × < 0.005 | Trials on wine grapes compliant with GAP for table and wine grapes (United Kingdom, 2018). Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 |
| SEU | 4 × < 0.005 | Trials on wine grapes compliant with GAP for table and wine grapes (United Kingdom, 2018). Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 | |
| Import (RU) | – | No GAP compliant trials available and not required since according to the metabolism studies and trials available from SEU no residues are expected. | – | 0.01 | 0.01 | |
| Strawberries | NEU | 2 × < 0.005 | Reduced data set on strawberries compliant with GAP (France, 2021), acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 |
| SEU | – | No GAP compliant trials available and not required since according to the metabolism studies and trials available from NEU no residues are expected. | – | 0.01 | 0.01 | |
| EU | – | No GAP compliant trials available and not required since according to the metabolism studies and trials available from NEU no residues are expected. | – | 0.01 | 0.01 | |
| Kaki | SEU | 4 × < 0.005 | Trials on apples compliant with GAP (United Kingdom, 2018). Extrapolation to kaki applicable. | – | 0.01 | 0.01 |
|
Tomatoes Aubergines/eggplants |
SEU | 2 × < 0.005 | Trials on tomatoes compliant with GAP for tomatoes and aubergines (France, 2021). Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 |
| EU | – | No GAP compliant trials available and not required since according to the metabolism studies and trials available from SEU no residues are expected. | – | 0.01 | 0.01 | |
| Sweet peppers/bell peppers | SEU | – | No GAP compliant trials available and not required since according to the metabolism studies and trials available on other fruit crops no residues are expected. | – | 0.01 | 0.01 |
| EU | – | No GAP compliant trials available and not required since according to the metabolism studies and trials available on other fruit crops no residues are expected. | – | 0.01 | 0.01 | |
|
Cucumber Gherkins Courgettes |
SEU | 4 × < 0.005 | Trials on cucumbers compliant with GAP for cucurbits with edible peel (France, 2021). Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 |
| EU | 5 × < 0.005; < 0.01 | Trials on cucumbers compliant with GAP for cucurbits with edible peel (France, 2021). Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 | |
|
Melons Pumpkins Watermelons |
SEU | < 0.005; < 0.01 | Trials on melons compliant with GAP for cucurbits with inedible peel (United Kingdom, 2018). Reported results refer to residues in pulp. Reduced number of trials acceptable since according to metabolism studies and available trials no residues are expected. | – | 0.01 | 0.01 |
| EU | – | No GAP compliant trials available and not required since according to the metabolism studies and trials available from SEU no residues are expected. Indoor GAP authorised on melons and watermelons only. | ‐ | 0.01 | 0.01 | |
| Witloofs/Belgian endives | NEU | – | No GAP compliant trials available. No residues above the LOQ are expected in this crop according to the trials available on other leafy crops. Nevertheless, reduced data set of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
|
Globe artichokes |
SEU | 2 × < 0.005 | Reduced data set on globe artichokes compliant with GAP (France, 2021), deemed acceptable as residues were always below the LOD. | – | 0.01 | 0.01 |
| EU | – | No GAP compliant trials available and not required since according to the trials available from SEU no residues are expected. | – | 0.01 | 0.01 | |
|
Rapeseeds/canola seeds Linseeds |
NEU | 2 × < 0.005 | Trials on rapeseeds performed at more critical BBCH (69–72 instead of 65) (United Kingdom, 2018) and overdosed when compared to linseeds’ GAP, deemed acceptable as all results were below the LOD. Nevertheless, results should be confirmed by additional trials with samples analysed within 30 days from sampling or by a new storage stability study. | – | 0.01 | 0.01 |
|
Barley grain and straw Oat grains and straw |
NEU | – | No GAP complaint trials available. No residues above the LOQ are expected in this crop according to the trials available on other cereals and metabolism studies. Nevertheless, reduced data set of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
| SEU | – | No GAP compliant trials available. No residues above the LOQ are expected in this crop according to the trials available on other cereals and metabolism studies. Nevertheless, reduced data set of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 | |
| Buckwheat and other pseudo‐cereal grains | SEU | – | No GAP compliant trials available. No residues above the LOQ are expected in this crop according to the trials available on other cereals and metabolism studies. Nevertheless, reduced data set of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
|
Maize/corn grains and stover Common millet/proso millet grains and straw Sorghum grains and stover |
SEU | – | No GAP compliant trials available. No residues above the LOQ are expected in this crop according to the trials available on other cereals and metabolism studies. Nevertheless, reduced dataset of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
| Rice grains and straw | SEU | – | No GAP compliant trials available. No residues above the LOQ are expected in this crop according to the trials available on other cereals and metabolism studies. Nevertheless, reduced data set of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
|
Wheat grains Rye grains |
NEU | – | No GAP compliant trials available. No residues above LOQ are expected according to the available trial supporting the southern GAP and metabolism studies. Nevertheless, reduced data set of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
| SEU | < 0.005 | Trial on wheat grains compliant with GAP for wheat and rye (United Kingdom, 2018). One additional trial required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 | |
|
Sugar beet roots |
NEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies. Nevertheless, reduced data set of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
| SEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies. Nevertheless, reduced data set of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 | |
| Sugar beet tops | NEU | – | No GAP compliant trials available. | – | – | – |
| SEU | – | No GAP compliant trials available | – | – | – | |
| Chicory roots | NEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies performed at 3N. Since chicory roots is a minor crop, no residue trials are required. | – | 0.01 | 0.01 |
|
Wheat straw Rye straw |
NEU | – | No GAP compliant trials available. No residues above LOQ are expected according to the available trial supporting the southern GAP and metabolism studies. Nevertheless, reduced data set of two trials might still be required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
| SEU | < 0.005 | Trial on wheat straw compliant with GAP. One additional trial might be required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 | |
| Fodder beet roots | NEU | – | No GAP compliant trials available. No residues above LOQ are expected in this crop according to metabolism studies performed at 3N. Nevertheless, reduced data set of two trials is still required to confirm that residues will remain < LOQ. | – | 0.01 | 0.01 |
| Fodder beet tops | NEU | – | No GAP compliant trials available. | – | ||
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: Maximum residue level.
Mo: Residue levels expressed according to the monitoring residue definition; RA: Residue levels expressed according to risk assessment residue definition.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, EU: indoor EU trials, Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
B.1.2.3. Residues in rotational crops
Overall summary

B.1.2.4. Processing factors
| Processed commodity | Number of valid studies a | Processing Factor (PF) | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|
| Individual values | Median PF | ||||
| Apples and pears, dry pomace | 3 | 2.86; 4.7; 5.04; | 4.7 | tetraconazole | Extrapolated from apples (EFSA, 2008) |
| Apples and pears, juice | 5 | < 0.15; < 0.36; < 0.37;< 0.39; < 0.5 | < 0.37 | tetraconazole | Extrapolated from apples (EFSA, 2008) |
| Apples, sauce | 1 | < 0.30 | < 0.30 | tetraconazole | Tentative b (EFSA, 2008) |
| Apples and pears, wet pomace | 1 | < 0.77 | < 0.77 | tetraconazole | Tentative b , extrapolated from apples (EFSA, 2008) |
| Grapes, juice | 1 | < 0.45 | < 0.45 | tetraconazole | Tentative b (EFSA, 2008) |
| Grapes, must | 6 | < 0.28; 2 × < 0.34; < 0.40; < 0.45; 1.10 | < 0.37 | tetraconazole | (EFSA, 2008) |
| Grapes, wine | 7 | < 0.12; 0.12; < 0.28; 2 × < 0.34; < 0.40; < 0.45 | < 0.34 | tetraconazole | (EFSA, 2008) |
| Grapes, raisins | 3 | 1.42; 2.16; 3.26 | 2.16 | tetraconazole | (EFSA, 2008) |
| Grapes, wet pomace | 2 | 4.14; 6.57 | 5.36 | tetraconazole | Tentative b (EFSA, 2008) |
| Tomato, sauce | 1 | < 0.40 | < 0.40 | tetraconazole | Tentative b (France, 2021) |
| Tomato, juice | 1 | < 0.20 | < 0.20 | tetraconazole | Tentative b (France, 2021) |
| Tomato, preserve | 1 | < 0.20 | < 0.20 | tetraconazole | Tentative b (France, 2021) |
| Cucurbits with inedible peel, peeled | 6 | < 0.19; < 0.43; < 0.71; < 0.75; < 0.94; < 1 | < 0.73 | tetraconazole | Extrapolated from melons (France, 2021) |
| Rapeseed and linseed, cake | 4 | 0.41; 0.7; 1.06; 1.78 | 0.88 | tetraconazole | Extrapolated from rapeseed (EFSA, 2012) |
| Rapeseed and linseed, crude oil | 4 | 1.32; 1.7; 2.55; 4.89 | 2.13 | tetraconazole | Extrapolated from rapeseed (EFSA, 2012) |
| Rapeseed and linseed, refined oil | 3 | 1.27; 1.9; 5.67 | 1.9 | tetraconazole | Extrapolated from rapeseed (EFSA, 2012) |
| Wheat, bran | 3 | < 0.25; 0.93; 1.68 | < 0.93 | TAA | France (2021) |
| 3 | 1.68; 2.09; 2.13 | 2.09 | TA, TLA | Residues of TLA < 0.01 mg/kg in raw and processed commodity in two out of the three studies (France, 2021). In these studies, LOQ of 0.01 mg/kg was used for TLA in raw and processed commodities for the calculation of the PFs. | |
| Wheat, flour | 3 | 0.85; 1.55; 1.58 | 1.55 | TAA | France (2021) |
| 3 | 0.97; 0.98; 1.25 | 0.98 | TA, TLA | Residues of TLA < 0.01 mg/kg in raw and processed commodity in two out of the three studies (France, 2021). In these studies, LOQ of 0.01 mg/kg was used for TLA in raw and processed commodities for the calculation of the PFs. | |
| Wheat, whole meal flour | 1 | 0.80 | 0.80 | TAA | Tentative b (France, 2021) |
| 1 | 0.92 | 0.92 | TA, TLA | Tentative b , residues of TLA < 0.01 mg/kg in raw and processed commodity (France, 2021). LOQ of 0.01 mg/kg was used for TLA for the calculation of the PF. | |
PF: Processing factor (=Residue level in processed commodity expressed according to RD‐Mo/ Residue level in raw commodity expressed according to RD‐Mo).
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
A tentative PF is derived based on a limited data set.
B.2. Residues in livestock
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical subgroup a | Most critical commodity b | Trigger exceeded (Y/N) | Comments | |||
|---|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | |||||||
| Median | Maximum | Median | Maximum | |||||
| Risk assessment residue definition 1: tetraconazole | ||||||||
| Cattle (all) | 0.076 | 0.091 | 2.36 | 2.83 | Dairy cattle | Beet, mangel fodder | Yes | – |
| Cattle (dairy only) | 0.076 | 0.091 | 1.98 | 2.37 | Dairy cattle | Beet, mangel fodder | Yes | – |
| Sheep (all) | 0.036 | 0.050 | 0.86 | 1.17 | Lamb | Rye straw | Yes | – |
| Sheep (ewe only) | 0.028 | 0.039 | 0.84 | 1.16 | Ram/Ewe | Rye straw | Yes | – |
| Swine (all) | 0.029 | 0.034 | 1.24 | 1.47 | Swine (breeding) | Beet, mangel fodder | Yes | – |
| Poultry (all) | 0.013 | 0.022 | 0.19 | 0.32 | Poultry layer | Wheat straw | Yes | – |
| Poultry (layer only) | 0.013 | 0.022 | 0.19 | 0.32 | Poultry layer | Wheat straw | Yes | – |
| Fish | – | – | – | – | – | – | – | – |
| Risk assessment residue definition 2: triazole alanine (TA) and triazole lactic acid (TLA) | ||||||||
| Cattle (all) | 0.033 | 0.033 | 1.32 | 1.32 | Dairy cattle | Wheat gluten, meal | Yes | – |
| Cattle (dairy only) | 0.033 | 0.033 | 0.87 | 0.87 | Dairy cattle | Wheat gluten, meal | Yes | – |
| Sheep (all) | 0.054 | 0.054 | 1.28 | 1.28 | Lamb | Wheat gluten, meal | Yes | – |
| Sheep (ewe only) | 0.038 | 0.038 | 1.15 | 1.15 | Ram/Ewe | Wheat gluten, meal | Yes | – |
| Swine (all) | 0.033 | 0.033 | 1.10 | 1.10 | Swine (finishing) | Wheat, milled by‐pdts | Yes | – |
| Poultry (all) | 0.066 | 0.066 | 0.93 | 0.93 | Poultry broiler | Canola, meal | Yes | – |
| Poultry (layer only) | 0.055 | 0.055 | 0.80 | 0.80 | Poultry layer | Wheat, milled by‐pdts | Yes | – |
| Fish | – | – | – | – | – | – | – | – |
| Risk assessment residue definition 3: triazole acetic acid (TAA) | ||||||||
| Cattle (all) | 0.006 | 0.006 | 0.15 | 0.15 | Dairy cattle | Wheat gluten, meal | Yes | – |
| Cattle (dairy only) | 0.006 | 0.006 | 0.15 | 0.15 | Dairy cattle | Wheat gluten, meal | Yes | – |
| Sheep (all) | 0.009 | 0.009 | 0.20 | 0.20 | Lamb | Wheat gluten, meal | Yes | – |
| Sheep (ewe only) | 0.007 | 0.007 | 0.20 | 0.20 | Ram/Ewe | Wheat gluten, meal | Yes | – |
| Swine (all) | 0.004 | 0.004 | 0.13 | 0.13 | Swine (finishing) | Rye, grain | Yes | – |
| Poultry (all) | 0.009 | 0.009 | 0.13 | 0.13 | Poultry layer | Wheat gluten, meal | Yes | – |
| Poultry (layer only) | 0.009 | 0.009 | 0.13 | 0.13 | Poultry layer | Wheat gluten, meal | Yes | – |
| Fish | – | – | – | – | – | – | – | – |
| Risk assessment residue definition 4: 1,2,4‐triazole (1,2,4‐T) | ||||||||
| Cattle (all) | 0.002 | 0.002 | 0.05 | 0.05 | Dairy cattle | Beet, mangel fodder | No | – |
| Cattle (dairy only) | 0.002 | 0.002 | 0.05 | 0.05 | Dairy cattle | Beet, mangel fodder | No | – |
| Sheep (all) | 0.001 | 0.001 | 0.02 | 0.02 | Lamb | Corn, field, gluten feed | No | – |
| Sheep (ewe only) | 0.001 | 0.001 | 0.02 | 0.02 | Ram/Ewe | Corn, field, gluten feed | No | – |
| Swine (all) | 0.000 | 0.000 | 0.02 | 0.02 | Swine (breeding) | Beet, mangel fodder | No | – |
| Poultry (all) | 0.001 | 0.001 | 0.01 | 0.01 | Poultry layer | Corn, field, milled by‐pdts | No | – |
| Poultry (layer only) | 0.001 | 0.001 | 0.01 | 0.01 | Poultry layer | Corn, field, milled by‐pdts | No | – |
| Fish | – | – | – | – | – | – | – | – |
When one group of livestock includes several subgroups (e.g. poultry ‘all’ including broiler, layer and turkey), the result of the most critical subgroup is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
B.2.1. Nature of residues and methods of analysis in livestock
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock (available studies) | Animal | Dose (mg/kg bw/d) | Duration (days) | Comment/Source |
|---|---|---|---|---|
| Laying hen | 1.3–1.4 | 3 | 59N compared to the maximum dietary burden calculated for poultry layer. Radiolabelled active substance: 14C‐triazole‐tetraconazole and 14C‐phenyl‐tetraconazole (EFSA, 2019a). | |
| 0.81 | 14 | 12N compared to the maximum dietary burden calculated for poultry broiler. Radiolabelled active substance: 14C‐triazole‐UL‐triazole alanine (TA) (EFSA, 2018b). | ||
| Lactating ruminants | 0.45 | 5 | 5N compared to the maximum dietary burden calculated for cattle dairy. Radiolabelled active substance: 14C‐triazole‐tetraconazole and 14C‐phenyl‐tetraconazole (EFSA, 2008). | |
| 0.7 | 7 | 13N compared to the maximum dietary burden calculated for lamb. Radiolabelled active substance: 14C‐triazole‐UL‐triazole alanine (TA) (EFSA, 2018b). | ||
| Pig | – | – | Not available and not required (extrapolated from ruminants). | |
| Fish | – | – | – |
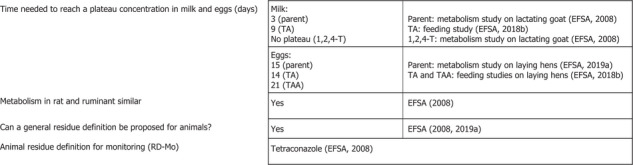

B.2.1.2. Stability of residues in livestock
| Animal products (available studies) | Animal | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| Poultry | Muscle | –20 | 42 | Days | Tetraconazole | EFSA (2019a) | |
| Bovine | –20 | 12 | Months | 1,2,4‐T | EFSA (2018b) | ||
| Poultry | Fat | –20 | 42 | Days | Tetraconazole | EFSA (2019a) | |
| Bovine | –20 | 12 | Months | 1,2,4‐T | EFSA (2018b) | ||
| Poultry | Liver | –20 | 42 | Days | Tetraconazole | EFSA (2019a) | |
| Bovine | –20 | 12 | Months | 1,2,4‐T | EFSA (2018b) | ||
| Poultry | Kidney | –20 | 42 | Days | Tetraconazole | EFSA (2019a) | |
| Bovine | Milk | – | – | – | Tetraconazole | Not available and not required (samples analysed within 1 month) | |
| –20 | 18 | Months | 1,2,4‐T | EFSA (2018b) | |||
| Poultry | Eggs | –20 | 39 | Days | Tetraconazole | EFSA (2019a) | |
| –20 | 12 | Months | 1,2,4‐T | EFSA (2018b) | |||
B.2.2. Magnitude of residues in livestock
B.2.2.1. Summary of the residue data from livestock feeding studies: tetraconazole (RD‐Mo and RD‐RA1)
Calculations performed with Animal model 2017 10
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | ||
|---|---|---|---|---|---|
| Mean | Highest | STMRMo a (mg/kg) | HRMo b (mg/kg) | ||
| Cattle (all) – Closest feeding level (0.12 mg/kg bw; 1.3 N rate) c | |||||
| Muscle | 0.02 | 0.02 | 0.01 | 0.01 | 0.015 (tentative) g |
| Fat | 0.20 | 0.20 | 0.08 | 0.15 | 0.2 (tentative) g |
| Liver | 1.64 | 1.64 | 0.87 | 1.30 | 1.5 (tentative) g |
| Kidney | 0.07 | 0.07 | 0.04 | 0.06 | 0.06 (tentative) g |
| Cattle (dairy only) – Closest feeding level (0.12 mg/kg bw; 1.3 N rate) c | |||||
| Milk d | 0.02 | n.a. | 0.01 | 0.02 | 0.02 (tentative) e |
| Sheep (all) e – Closest feeding level (0.036 mg/kg bw; 0.7 N rate) c | |||||
| Muscle | 0.01 | 0.01 | 0.01 | 0.01 | 0.01* |
| Fat | 0.07 | 0.07 | 0.05 | 0.09 | 0.09 |
| Liver | 0.66 | 0.66 | 0.46 | 0.82 | 0.9 |
| Kidney | 0.04 | 0.04 | 0.02 | 0.04 | 0.05 |
| Sheep (ewe only) e – Closest feeding level (0.036 mg/kg bw; 0.9 N rate) c | |||||
| Milk d | 0.01 | n.a. | 0.01 | 0.01 | 0.01* |
| Swine (all) e – Closest feeding level (0.036 mg/kg bw; 1.1 N rate) c | |||||
| Muscle | 0.01 | 0.01 | 0.00 | 0.01 | 0.01* (tentative)(g) |
| Fat | 0.07 | 0.07 | 0.04 | 0.07 | 0.07 (tentative) g |
| Liver | 0.66 | 0.66 | 0.38 | 0.64 | 0.7 (tentative) g |
| kidney | 0.04 | 0.04 | 0.02 | 0.04 | 0.04 (tentative) g |
| Poultry (all) – Closest feeding level (0.0166 mg/kg bw; 0.8 N rate) c | |||||
| Muscle | 0.01 | 0.01 | 0.01 | 0.01 | 0.015 |
| Fat | 0.12 | 0.14 | 0.10 | 0.21 | 0.3 |
| Liver | 0.03 | 0.03 | 0.03 | 0.04 | 0.05 |
| Poultry (layer only) – Closest feeding level (0.0166 mg/kg bw; 0.8 N rate) c | |||||
| Eggs f | 0.03 | 0.03 | 0.02 | 0.05 | 0.05 |
n.a.: not applicable.
*: Indicates that the MRL is proposed at the limit of quantification.
Median residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the median dietary burden.
Highest residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the maximum dietary burden.
Closest feeding level and N dose rate related to the maximum dietary burden.
For milk, mean was derived from samplings performed from day 7 to day 28 (daily mean of 3 cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in sheep and swine.
For eggs, mean and highest residues were derived from samplings performed from day 15 to day 39 (daily mean or daily highest of 3 laying hens).
Tentative MRL since dietary burden might be overestimated resulting from the major contribution of mangel fodder beet (trials performed according to a more critical GAP) to these diets.
B.2.2.2. Summary of the residue data from livestock feeding studies: triazole alanine (TA) and triazole lactic acid (TLA) (RD‐RA2)
Calculations performed with Animal model 201710
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | Comments | ||
|---|---|---|---|---|---|
| Mean | Highest | STMRMo a (mg/kg) | HRMo b (mg/kg) | ||
| Cattle (all) – Closest feeding level (0.0608 mg/kg bw; 1.8 N rate) c | |||||
| Muscle | 0.05 | 0.05 | 0.02 | 0.03 | Tentative g |
| Fat | 0.02 | 0.02 | 0.01 | 0.01 | Tentative g |
| Liver | 0.15 | 0.15 | 0.05 | 0.08 | Tentative g |
| Kidney | 0.04 | 0.04 | 0.02 | 0.02 | Tentative g |
| Cattle (dairy only) – Closest feeding level (0.0608 mg/kg bw; 1.8 N rate) c | |||||
| Milk d | 0.05 | n.a. | 0.02 | 0.03 | Tentative g |
| Sheep (all) e – Closest feeding level (0.0608 mg/kg bw; 1.1 N rate) c | |||||
| Muscle | 0.05 | 0.05 | 0.03 | 0.04 | Tentative g |
| Fat | 0.02 | 0.02 | 0.02 | 0.02 | Tentative g |
| Liver | 0.15 | 0.15 | 0.09 | 0.13 | Tentative g |
| Kidney | 0.04 | 0.04 | 0.03 | 0.03 | Tentative g |
| Sheep (ewe only) e – Closest feeding level (0.0608 mg/kg bw; 1.6 N rate) c | |||||
| Milk d | 0.05 | n.a. | 0.01 | 0.03 | Tentative g |
| Swine (all) e – Closest feeding level (0.0608 mg/kg bw; 1.8 N rate) c | |||||
| Muscle | 0.05 | 0.05 | 0.02 | 0.03 | Tentative g |
| Fat | 0.02 | 0.02 | 0.01 | 0.01 | Tentative g |
| Liver | 0.15 | 0.15 | 0.05 | 0.08 | Tentative g |
| kidney | 0.04 | 0.04 | 0.02 | 0.02 | Tentative g |
| Poultry (all) – Closest feeding level (0.1026 mg/kg bw; 1.6 N rate) c | |||||
| Muscle | 0.04 | 0.04 | 0.03 | 0.03 | Tentative h |
| Fat | 0.03 | 0.03 | 0.03 | 0.03 | Tentative h |
| Liver | 0.12 | 0.12 | 0.08 | 0.09 | Tentative h |
| Poultry (layer only) – Closest feeding level (0.1026 mg/kg bw; 1.9 N rate) c | |||||
| Eggs f | 0.02 | 0.02 | 0.01 | 0.01 | Tentative g |
n.a.: not applicable.
Median residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the median dietary burden.
Highest residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the maximum dietary burden.
Closest feeding level and N dose rate related to the maximum dietary burden.
For milk, mean was derived from samplings performed from day 17 to day 28 (daily mean of 3 cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in sheep and swine.
For eggs, mean and highest residues were derived from samplings performed from day 14 to day 27 (daily mean or daily highest of 12 laying hens).
Tentative risk assessment values derived since wheat products were found to be the major contributor to the relevant livestock diets, and no robust residues data set for this feed item were available.
Tentative risk assessment values derived since wheat and canola were found to be the major contributors to the relevant livestock diets, and no robust residues data set for these feed items were available.
B.2.2.3. Summary of the residue data from livestock feeding studies: triazole acetic acid (TAA) (RD‐RA3)
Calculations performed with Animal model 201710
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | Comments | ||
|---|---|---|---|---|---|
| Mean | Highest | STMRMo a (mg/kg) | HRMo b (mg/kg) | ||
| Cattle (all) – Closest feeding level (0.01462 mg/kg bw; 2.6 N rate) c | |||||
| Muscle | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Fat | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Liver | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Kidney | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Cattle (dairy only) – Closest feeding level (0.0142 mg/kg bw; 2.6 N rate) c | |||||
| Milk d | 0.01 | n.a. | 0.01 | 0.01 | Tentative g |
| Sheep (all) f – Closest feeding level (0.01462 mg/kg bw; 1.7 N rate) c | |||||
| Muscle | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Fat | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Liver | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Kidney | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Sheep (ewe only) e – Closest feeding level (0.01462 mg/kg bw; 2.2 N rate) c | |||||
| Milk d | 0.01 | n.a. | 0.01 | 0.01 | Tentative g |
| Swine (all) e – Closest feeding level (0.1462 mg/kg bw; 3.4 N rate) c | |||||
| Muscle | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Fat | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Liver | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| kidney | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Poultry (all) – Closest feeding level (0.0192 mg/kg bw; 2.2 N rate) c | |||||
| Muscle | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Fat | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Liver | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
| Poultry (layer only) – Closest feeding level (0.0192 mg/kg bw; 2.2 N rate) c | |||||
| Eggs f | 0.01 | 0.01 | 0.01 | 0.01 | Tentative g |
n.a.: not applicable.
Median residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the median dietary burden.
Highest residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the maximum dietary burden.
Closest feeding level and N dose rate related to the maximum dietary burden.
For milk, mean was derived from samplings performed from day 9 to day 22 (daily mean of 3 cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in sheep and swine.
For eggs, mean and highest residues were derived from samplings performed from day 21 to day 27 (daily mean or daily highest of 12 laying hens).
Tentative risk assessment values derived since wheat/rye products were found to be the major contributor to all livestock diets, and no robust residues data set for this feed item were available.
B.2.2.4. Summary of the residue data from livestock feeding studies: 1,2,4‐T (1,2,4‐triazole) (RD‐RA4)
Not relevant. For metabolite 1,2,4‐T, dietary burden calculations are below the trigger value. Therefore, no feeding studies are required.
B.3. Consumer risk assessment



Consumer exposure assessment through drinking water resulting from groundwater metabolite(s) according to SANCO/221/2000 rev.10 Final (25/02/2003)
B.4. Proposed MRLs
| Code number | Commodity | Existing EU MRL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|
| MRL (mg/kg) | Comment | |||
| Enforcement residue definition: tetraconazole (F) | ||||
| 130010 | Apples | 0.3 | 0.3 | Further consideration needed(a) data gaps #1, 3, 4 |
| 130020 | Pears | 0.3 | 0.3 | Further consideration needed(a) data gaps #1, 3, 4 |
| 130030 | Quinces | 0.3 | 0.3 | Further consideration needed(a) data gap #3 |
| 130040 | Medlar | 0.3 | 0.3 | Further consideration needed(a) data gap #3 |
| 130050 | Loquat | 0.3 | 0.2 | Further consideration needed(a) data gap #3 |
| 140010 | Apricots | 0.1 | 0.03 | Further consideration needed(a) data gaps #1, 3, 4 |
| 140030 | Peaches | 0.1 | 0.03 | Further consideration needed(a) data gaps #1, 3, 4 |
| 151010 | Table grapes | 0.5 | 0.07 | Further consideration needed(a) data gaps # 7 |
| 151020 | Wine grapes | 0.5 | 0.07 | Further consideration needed(a) data gaps #3, 4, 7 |
| 152000 | Strawberries | 0.2 | 0.15 | Further consideration needed(a) data gaps #3, 4, 7, 9 |
| 161060 | Kaki/Japanese persimmons | 0.09 | 0.09 | Recommended b |
| 231010 | Tomatoes | 0.1 | 0.15 | Further consideration needed(a) data gaps #3, 4, 9 |
| 231020 | Peppers | 0.1 | 0.1 | Further consideration needed(c) data gaps #1, 3, 4, 9 |
| 231030 | Aubergines (egg plants) | 0.02* | 0.15 | Further consideration needed(a) data gaps #3, 4, 9 |
| 232010 | Cucumbers | 0.2 | 0.15 | Further consideration needed(a) data gap #3, 9 |
| 232020 | Gherkins | 0.2 | 0.15 | Further consideration needed(a) data gap #9 |
| 232030 | Courgettes | 0.2 | 0.15 | Further consideration needed(a) data gap #3, 9 |
| 233010 | Melons | 0.05 | 0.08 | Further consideration needed(a) data gaps #3, 4, 9 |
| 233020 | Pumpkins | 0.05 | 0.08 | Further consideration needed(a) data gaps #3, 4, 9 |
| 233030 | Watermelons | 0.05 | 0.08 | Further consideration needed(a) data gaps #3, 4, 9 |
| 255000 | Witloofs | 0.02* | 0.02 | Further consideration needed a data gaps #3, 4, 5 |
| 270050 | Globe artichokes | 0.2 | 0.2 | Further consideration needed c data gaps #1, 3, 4, 9 |
| 401010 | Linseed | 0.15 | 0.15 | Further consideration needed(a) data gaps #1, 3, 4, 6, 9 |
| 401060 | Rapeseed | 0.15 | 0.15 | Further consideration needed(a) data gaps #1, 3, 4, 6, 9 |
| 500010 | Barley grain | 0.1 | 0.1 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500020 | Buckwheat grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500030 | Maize grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500040 | Millet grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500050 | Oat grain | 0.1 | 0.1 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500060 | Rice grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500070 | Rye grain | 0.05 | 0.02 | Further consideration needed(a) data gaps #3, 4, 5, 9 |
| 500080 | Sorghum grain | 0.05 | 0.05 | Further consideration needed(c) data gaps #1, 3, 4, 5, 9 |
| 500090 | Wheat grain | 0.1 | 0.02 | Further consideration needed(a) data gaps #3, 4, 5, 9 |
| 900010 | Sugar beet (root) | 0.05 | 0.01* | Further consideration needed(a) data gaps #3, 4, 5, 9 |
| 900030 | Chicory roots | 0.05 | 0.06 | Further consideration needed(a) data gap # 1 |
| 1011010 | Swine meat | 0.05 | 0.01* | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1011020 | Swine fat (free of lean meat) | 0.5 | 0.07 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1011030 | Swine liver | 1 | 0.7 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1011040 | Swine kidney | 0.2 | 0.04 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1012010 | Bovine meat | 0.05 | 0.015 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1012020 | Bovine fat | 0.5 | 0.2 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1012030 | Bovine liver | 1 | 1.5 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1012040 | Bovine kidney | 0.2 | 0.06 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1013010 | Sheep meat | 0.05 | 0.01* | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1013020 | Sheep fat | 0.5 | 0.09 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1013030 | Sheep liver | 1 | 0.9 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1013040 | Sheep kidney | 0.5 | 0.05 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1014010 | Goat meat | 0.5 | 0.01* | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1014020 | Goat fat | 0.5 | 0.09 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1014030 | Goat liver | 1 | 0.9 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1014040 | Goat kidney | 0.5 | 0.05 | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1015010 | Horse meat | 0.5 | 0.015 | Further consideration needed (a) data gap #1, 2, 3, 4, 5, 8 |
| 1015020 | Horse fat | 0.5 | 0.2 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1015030 | Horse liver | 1 | 1.5 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1015040 | Horse kidney | 0.5 | 0.06 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1016010 | Poultry meat | 0.02* | 0.015 | Further consideration needed(a) data gap #1, 3, 4, 5 |
| 1016020 | Poultry fat | 0.2 | 0.3 | Further consideration needed(a) data gap #1, 3, 4, 5 |
| 1016030 | Poultry liver | 1 | 0.05 | Further consideration needed(a) data gap #1, 3, 4, 5 |
| 1020010 | Cattle milk | 0.05 | 0.02 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1020020 | Sheep milk | 0.05 | 0.01* | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1020030 | Goat milk | 0.05 | 0.01* | Further consideration needed(a) data gap #1, 3, 4, 5, 8 |
| 1020040 | Horse milk | 0.05 | 0.02 | Further consideration needed(a) data gap #1, 2, 3, 4, 5, 8 |
| 1030000 | Birds' eggs | 0.05 | 0.05 | Further consideration needed(a) data gap #1, 3, 4, 5 |
| – | Other commodities of plant and/or animal origin | See Reg. (EU) 2019/1015 | – | Further consideration needed d |
MRL: maximum residue level; CXL: codex maximum residue limit.
Indicates that the MRL is set at the limit of quantification.
(F): The residue definition is fat soluble.
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination F‐I in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination H‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination D‐I in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Appendix C – Pesticide Residue Intake Model (PRIMo)
• PRIMo parent tetraconazole (RD‐RA1)

• PRIMo metabolites TA and TLA (RD‐RA2)

• PRIMo metabolite TAA (RD‐RA3)

• PRIMo metabolite 1,2,4‐T (RD‐RA4)

Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition 1: tetraconazole | ||||
| Beet, mangel fodder | 1.15 | STMR | 1.39 | HR |
| Beet, sugar tops | 0.65 | STMR | 1.22 | HR |
| Rye straw | 0.75 | STMR | 2.4 | HR |
| Triticale straw | 0.75 | STMR | 2.4 | HR |
| Wheat straw | 0.75 | STMR | 2.4 | HR |
| Rye grain | 0.02 | STMR | 0.02 | STMR |
| Triticale grain | 0.02 | STMR | 0.02 | STMR |
| Wheat grain | 0.02 | STMR | 0.02 | STMR |
| Apple pomace, wet | 0.04 | STMR × PF (0.8) | 0.04 | STMR × PF (0.8) |
| Beet, sugar dried pulp | 0.01* | STMR a | 0.01* | STMR a |
| Beet, sugar ensiled pulp | 0.01* | STMR a | 0.01* | STMR a |
| Beet, sugar molasses | 0.01* | STMR a | 0.01* | STMR a |
| Canola (Rapeseed) meal | 0.02 | STMR × PF (0.9) | 0.02 | STMR × PF (0.9) |
| Distiller's grain dried | 0.07 | STMR × default PF (3.3) b | 0.07 | STMR × default PF (3.3) b |
| Flaxseed/Linseed meal | 0.02 | STMR × PF (0.9) | 0.02 | STMR × PF (0.9) |
| Rape meal | 0.02 | STMR × PF (0.9) | 0.02 | STMR × PF (0.9) |
| Wheat gluten meal | 0.04 | STMR × default PF (1.8) b | 0.04 | STMR × default PF (1.8) b |
| Wheat milled by‐products | 0.14 | STMR × default PF (7) b | 0.14 | STMR × default PF (7) b |
| Risk assessment residue definition 2: triazole alanine (TA) and triazole lactic acid (TLA) | ||||
| Beet, mangel fodder | 0.01* | STMR | 0.01* | HR |
| Rye straw | 0.06 | STMR | 0.06 | HR |
| Triticale straw | 0.06 | STMR | 0.06 | HR |
| Wheat straw | 0.06 | STMR | 0.06 | HR |
| Rye grain | 0.63 | STMR | 0.63 | STMR |
| Triticale grain | 0.63 | STMR | 0.63 | STMR |
| Wheat grain | 0.63 | STMR | 0.63 | STMR |
| Apple pomace, wet | 2.03 | STMR × default PF (5) b | 2.03 | STMR × default PF (5) b |
| Beet, sugar dried pulp | 0.01* | STMR a | 0.01* | STMR a |
| Beet, sugar ensiled pulp | 0.01* | STMR a | 0.01* | STMR a |
| Beet, sugar molasses | 0.01* | STMR a | 0.01* | STMR a |
| Canola (Rapeseed) meal | 2.11 | STMR × default PF (2) b | 2.11 | STMR × default PF (2) b |
| Distiller's grain dried | 2.07 | STMR × default PF (3.3) b | 2.07 | STMR × default PF (3.3) b |
| Flaxseed/Linseed meal | 2.11 | STMR × default PF (2) b | 2.11 | STMR × default PF (2) b |
| Rape meal | 2.11 | STMR × default PF (2) b | 2.11 | STMR × default PF (2) b |
| Wheat gluten meal | 1.13 | STMR × default PF (1.8) b | 1.13 | STMR × default PF (1.8) b |
| Wheat milled by‐pdts | 1.31 | STMR × PF (2.1) | 1.31 | STMR × PF (2.1) |
| Risk assessment residue definition 3: triazole acetic acid (TAA) | ||||
| Beet, mangel fodder | 0.01* | STMR | 0.01* | HR |
| Rye straw | 0.09 | STMR | 0.09 | HR |
| Triticale straw | 0.09 | STMR | 0.09 | HR |
| Wheat straw | 0.09 | STMR | 0.09 | HR |
| Rye grain | 0.09 | STMR | 0.09 | STMR |
| Triticale grain | 0.09 | STMR | 0.09 | STMR |
| Wheat grain | 0.09 | STMR | 0.09 | STMR |
| Apple pomace, wet | 0.08 | STMR × default PF (5) b | 0.08 | STMR × default PF (5) b |
| Beet, sugar dried pulp | 0.01* | STMR a | 0.01* | STMR a |
| Beet, sugar ensiled pulp | 0.01* | STMR a | 0.01* | STMR a |
| Beet, sugar molasses | 0.01* | STMR a | 0.01* | STMR a |
| Canola (Rapeseed) meal | 0.06 | STMR × default PF (2) b | 0.06 | STMR × default PF (2) b |
| Distiller's grain dried | 0.31 | STMR × default PF (3.3) b | 0.31 | STMR × default PF (3.3) b |
| Flaxseed/Linseed meal | 0.06 | STMR × default PF (2) b | 0.06 | STMR × default PF (2) b |
| Rape meal | 0.06 | STMR × default PF (2) b | 0.06 | STMR × default PF (2) b |
| Wheat gluten meal | 0.17 | STMR × default PF (1.8) b | 0.17 | STMR × default PF (1.8) b |
| Wheat milled by‐pdts | 0.09 | STMR × PF (0.9) | 0.09 | STMR × PF (0.9) |
| Risk assessment residue definition 4: 1,2,4‐triazole (1,2,4‐T) | ||||
| Barley straw | 0.01* | STMR | 0.01* | HR |
| Beet, mangel fodder | 0.01* | STMR | 0.01* | HR |
| Corn, field stover (fodder) | 0.01* | STMR | 0.01* | HR |
| Corn, pop stover (fodder) | 0.01* | STMR | 0.01* | HR |
| Millet straw (fodder, dry) | 0.01* | STMR | 0.01* | HR |
| Oat straw | 0.01* | STMR | 0.01* | HR |
| Rice straw | 0.01* | STMR | 0.01* | HR |
| Rye straw | 0.01* | STMR | 0.01* | HR |
| Sorghum, grain stover | 0.01* | STMR | 0.01* | HR |
| Triticale straw | 0.01* | STMR | 0.01* | HR |
| Wheat straw | 0.01* | STMR | 0.01* | HR |
| Barley grain | 0.01* | STMR | 0.01* | STMR |
| Corn, field (Maize) grain | 0.01* | STMR | 0.01* | STMR |
| Corn, pop grain | 0.01* | STMR | 0.01* | STMR |
| Millet grain | 0.01* | STMR | 0.01* | STMR |
| Oat grain | 0.01* | STMR | 0.01* | STMR |
| Rye grain | 0.01* | STMR | 0.01* | STMR |
| Sorghum grain | 0.01* | STMR | 0.01* | STMR |
| Triticale grain | 0.01* | STMR | 0.01* | STMR |
| Wheat grain | 0.01* | STMR | 0.01* | STMR |
| Apple pomace, wet | 0.01* | STMR a | 0.01* | STMR a |
| Beet, sugar dried pulp | 0.01* | STMR a | 0.01* | STMR a |
| Beet, sugar ensiled pulp | 0.01* | STMR a | 0.01* | STMR a |
| Beet, sugar molasses | 0.01* | STMR a | 0.01* | STMR a |
| Brewer's grain dried | 0.01* | STMR a | 0.01* | STMR a |
| Canola (Rapeseed) meal | 0.01* | STMR a | 0.01* | STMR a |
| Corn, field milled by‐pdts | 0.01* | STMR a | 0.01* | STMR a |
| Corn, field hominy meal | 0.01* | STMR a | 0.01* | STMR a |
| Corn, field gluten feed | 0.01* | STMR a | 0.01* | STMR a |
| Corn, field gluten, meal | 0.01* | STMR a | 0.01* | STMR a |
| Distiller's grain dried | 0.01* | STMR a | 0.01* | STMR a |
| Flaxseed/Linseed meal | 0.01* | STMR a | 0.01* | STMR a |
| Rape meal | 0.01* | STMR a | 0.01* | STMR a |
| Rice bran/pollard | 0.01* | STMR a | 0.01* | STMR a |
| Wheat gluten meal | 0.01* | STMR a | 0.01* | STMR a |
| Wheat milled by‐pdts | 0.01* | STMR a | 0.01* | STMR a |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
*: Indicates that the input value is proposed at the limit of quantification.
For these feed items no default processing factor was applied because residues are expected to be below the LOQ according to residue trials and/or metabolism studies. Concentration of residues in these commodities is therefore not expected.
In the absence of processing factors supported by data, default the processing factor of was included in the calculation to consider the potential concentration of residues in these commodities.
D.2. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition 1: tetraconazole | ||||
| Apples, Pears | 0.06 | STMR (tentative) | 0.16 | HR (tentative) |
| Quinces, Medlars | 0.06 | STMR | 0.16 | HR |
| Loquats/Japanese medlars | 0.04 | STMR | 0.12 | HR |
| Apricots, Peaches | 0.01 | STMR (tentative) | 0.02 | HR (tentative) |
| Table and Wine grapes | 0.02 | STMR | 0.04 | HR |
| Strawberries | 0.04 | STMR | 0.08 | HR |
| Kaki/Japanese persimmons | 0.02 | STMR | 0.06 | HR |
| Tomatoes, Aubergines/eggplants | 0.04 | STMR | 0.07 | HR |
| Sweet peppers/bell peppers | 0.10 | EU MRL | 0.10 | EU MRL |
| Cucumbers, Gherkins, Courgettes | 0.02 | STMR | 0.09 | HR |
| Melons, Pumpkins, Watermelons | 0.01 | STMR × PF (0.73) | 0.04 | HR × PF (0.73) |
| Witloofs/Belgian endives | 0.02 | STMR | 0.02 | HR |
| Globe artichokes | 0.20 | EU MRL | 0.20 | EU MRL |
| Linseeds, Rapeseeds/canola seeds | 0.02 | STMR (tentative) | 0.02 | STMR (tentative) |
| Barley grains, Oat grains | 0.10 | EU MRL | 0.10 | EU MRL |
| Buckwheat and other pseudo‐cereal grains | 0.05 | EU MRL | 0.05 | EU MRL |
|
Maize/corn grains, Common millet/proso millet grains Sorghum grains |
0.05 | EU MRL | 0.05 | EU MRL |
| Rice grains | 0.05 | EU MRL | 0.05 | EU MRL |
| Rye grains, Wheat grains | 0.02 | STMR | 0.02 | STMR |
| Sugar beet roots | 0.01* | STMR | 0.01* | HR |
| Chicory roots | 0.02 | STMR (tentative) | 0.03 | HR (tentative) |
| Swine meat | 0.01 | 0.8 × STMR muscle + 0.2 × STMR fat (tentative) | 0.02 | 0.8 × HR muscle + 0.2 × HR fat (tentative) |
| Swine fat | 0.04 | STMR (tentative) | 0.07 | HR (tentative) |
| Swine liver | 0.38 | STMR (tentative) | 0.64 | HR (tentative) |
| Swine kidney | 0.02 | STMR (tentative) | 0.04 | HR (tentative) |
| Bovine and equine meat | 0.02 | 0.8 × STMR muscle + 0.2 × STMR fat (tentative) | 0.04 | 0.8 × HR muscle + 0.2 × HR fat (tentative) |
| Bovine and equine fat | 0.08 | STMR (tentative) | 0.15 | HR (tentative) |
| Bovine and equine liver | 0.87 | STMR (tentative) | 1.30 | HR (tentative) |
| Bovine and equine kidney | 0.04 | STMR (tentative) | 0.06 | HR (tentative) |
| Sheep and goat meat | 0.01 | 0.8 × STMR muscle + 0.2 × STMR fat | 0.02 | 0.8 × HR muscle + 0.2 × HR fat |
| Sheep and goat fat | 0.05 | STMR | 0.09 | HR |
| Sheep and goat liver | 0.46 | STMR | 0.82 | HR |
| Sheep and goat kidney | 0.02 | STMR | 0.04 | HR |
| Poultry meat | 0.02 | 0.9 × STMR muscle + 0.1 × STMR fat | 0.03 | 0.9 × HR muscle + 0.1 × HR fat |
| Poultry fat | 0.10 | STMR | 0.21 | HR |
| Poultry liver | 0.03 | STMR | 0.04 | HR |
| Cattle and horse milk | 0.01 | STMR (tentative) | 0.01 | STMR (tentative) |
| Sheep and goat milk | 0.01 | STMR | 0.01 | STMR |
| Birds eggs | 0.02 | STMR | 0.05 | HR |
| Risk assessment residue definition 2: triazole alanine (TA) and triazole lactic acid (TLA) | ||||
| Apples, Pears, Quinces, Medlars | 0.41 | STMR (tentative) | 0.52 | HR (tentative) |
| Loquats/Japanese medlars | 0.31 | STMR (tentative) | 0.39 | HR (tentative) |
| Apricots, Peaches | 0.12 | STMR (tentative) | 0.61 | HR (tentative) |
| Table and Wine grapes | 0.04 | STMR (tentative for wine grapes) | 0.10 | HR (tentative for wine grapes) |
| Strawberries | 0.04 | STMR (tentative) | 0.06 | HR (tentative) |
| Kaki/Japanese persimmons | 0.06 | STMR | 0.07 | HR |
| Tomatoes, Aubergines/eggplants | 0.06 | STMR (tentative) | 0.10 | HR (tentative) |
| Sweet peppers/bell peppers | – a | – | – a | – |
| Cucumbers and Courgettes | 0.11 | STMR (tentative) | 0.42 | HR (tentative for courgettes) |
| Gherkins | 0.11 | STMR | 0.42 | HR |
| Melons, Pumpkins, Watermelons | 0.23 | STMR (tentative) | 0.36 | HR (tentative) |
| Witloofs | – a | – | – a | – |
| Globe artichokes | 0.04 | STMR (tentative) | 0.05 | HR (tentative) |
| Linseeds, Rapeseeds/canola seeds | 1.05 | STMR (tentative) | 1.05 | STMR (tentative) |
| Barley grains, Oat grains | – a | – | – a | – |
|
Buckwheat and other pseudo‐cereal grains, Maize/corn grains, Common millet/proso millet grains Rice grains Sorghum grains |
– a | – | – a | – |
|
Rye grains, Wheat grains |
0.63 | STMR (tentative) | 0.63 | STMR (tentative) |
| Sugar beet roots | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Chicory roots | 0.01* | STMR | 0.01* | HR |
| Swine meat | 0.02 | STMR muscle (tentative) | 0.03 | HR muscle (tentative) |
| Swine fat | 0.01 | STMR (tentative) | 0.01 | HR (tentative) |
| Swine liver | 0.05 | STMR (tentative) | 0.08 | HR (tentative) |
| Swine kidney | 0.02 | STMR (tentative) | 0.02 | HR (tentative) |
| Bovine and equine meat | 0.02 | STMR muscle (tentative) | 0.03 | HR muscle (tentative) |
| Bovine and equine fat | 0.01 | STMR (tentative) | 0.01 | HR (tentative) |
| Bovine and equine liver | 0.05 | STMR (tentative) | 0.08 | HR (tentative) |
| Bovine and equine kidney | 0.02 | STMR (tentative) | 0.02 | HR (tentative) |
| Sheep and goat meat | 0.03 | STMR muscle (tentative) | 0.04 | HR muscle (tentative) |
| Sheep and goat fat | 0.02 | STMR (tentative) | 0.02 | HR (tentative) |
| Sheep and goat liver | 0.09 | STMR (tentative) | 0.13 | HR (tentative) |
| Sheep and goat kidney | 0.03 | STMR (tentative) | 0.03 | HR (tentative) |
| Poultry meat | 0.03 | STMR muscle (tentative) | 0.03 | HR muscle (tentative) |
| Poultry fat | 0.03 | STMR (tentative) | 0.03 | HR (tentative) |
| Poultry liver | 0.08 | STMR (tentative) | 0.09 | HR (tentative) |
| Cattle and horse milk | 0.02 | STMR (tentative) | 0.02 | STMR (tentative) |
| Sheep and goat milk | 0.01 | STMR (tentative) | 0.01 | STMR (tentative) |
| Birds eggs | 0.01 | STMR (tentative) | 0.01 | HR (tentative) |
| Risk assessment residue definition 3: triazole acetic acid (TAA) | ||||
| Apples, Pears | 0.02 | STMR (tentative) | 0.02 | HR (tentative) |
| Quinces, Medlars | 0.02 | STMR | 0.02 | HR |
| Loquats/Japanese medlars | 0.02 | STMR | 0.02 | HR |
| Apricots, Peaches | 0.01 | STMR (tentative) | 0.02 | HR (tentative) |
| Table grapes | 0.01 | STMR | 0.03 | HR |
| Wine grapes | 0.01 | STMR (tentative) | 0.03 | HR (tentative) |
| Strawberries | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Kaki/Japanese persimmons | 0.02 | STMR | 0.02 | HR |
| Tomatoes, Aubergines/eggplants | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Sweet peppers/bell peppers | – a | – | – a | – |
| Cucumbers, Gherkins, Courgettes | 0.01* | STMR | 0.01* | HR |
| Melons, Pumpkins, Watermelons | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Witloofs | – a | – | – a | – |
| Globe artichokes | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Linseeds, Rapeseeds/canola seeds | 0.03 | STMR (tentative) | 0.03 | STMR (tentative) |
| Barley grains, Oat grains | – a | – | – a | – |
|
Buckwheat and other pseudo‐cereal grains, Maize/corn grains, Common millet/proso millet grains Rice grains Sorghum grains |
– a | – | – a | – |
|
Rye grains, Wheat grains |
0.09 | STMR (tentative) | 0.09 | STMR (tentative) |
| Sugar beet roots | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Chicory roots | 0.01* | STMR | 0.01* | HR |
| Swine meat | 0.01* | STMR muscle (tentative) | 0.01* | HR muscle (tentative) |
| Swine fat | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Swine liver | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Swine kidney | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Bovine and equine meat | 0.01* | STMR muscle (tentative) | 0.01* | HR muscle (tentative) |
| Bovine and equine fat | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Bovine and equine liver | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Bovine and equine kidney | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Sheep and goat meat | 0.01* | STMR muscle (tentative) | 0.01* | HR muscle (tentative) |
| Sheep and goat fat | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Sheep and goat liver | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Sheep and goat kidney | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Poultry meat | 0.01* | STMR muscle (tentative) | 0.01* | HR muscle (tentative) |
| Poultry fat | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Poultry liver | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Cattle and horse milk | 0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
| Sheep and goat milk | 0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
| Birds eggs | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Risk assessment residue definition 4: 1,2,4‐triazole (1,2,4‐T) | ||||
| Apples, Pears, Quinces, Medlars | 0.01* | STMR | 0.01* | HR |
| Loquats/Japanese medlars | 0.01* | STMR | 0.01* | HR |
| Apricots, Peaches | 0.01* | STMR | 0.01* | HR |
| Table and Wine grapes | 0.01* | STMR | 0.01* | HR |
| Strawberries | 0.01* | STMR | 0.01* | HR |
| Kaki/Japanese persimmons | 0.01* | STMR | 0.01* | HR |
| Tomatoes, Aubergines/eggplants | 0.01* | STMR | 0.01* | HR |
| Sweet peppers/bell peppers | 0.01* | STMR | 0.01* | HR |
| Cucumbers, Gherkins, Courgettes | 0.01* | STMR | 0.01* | HR |
| Melons, Pumpkins, Watermelons | 0.01* | STMR | 0.01* | HR |
| Witloofs | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Globe artichokes | 0.01* | STMR | 0.01* | HR |
| Linseeds, Rapeseeds/canola seeds | 0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
| Barley grains, Oat grains | 0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
|
Buckwheat and other pseudo‐cereal grains, Maize/corn grains, Common millet/proso millet grains Rice grains Sorghum grains |
0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
|
Rye grains, Wheat grains |
0.01* | STMR (tentative) | 0.01* | STMR (tentative) |
| Sugar beet roots | 0.01* | STMR (tentative) | 0.01* | HR (tentative) |
| Chicory roots | 0.01* | STMR | 0.01* | HR |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor.
*: Indicates that the input value is proposed at the limit of quantification.
HR and STMR values of sweet peppers, witloofs, barley, buckwheat, maize, millet, oat and rice sorghum were not calculated as no residue data were available to derive tentative risk assessment values. Existing EU MRL was not applied in the calculations since they refer to parent tetraconazole and not to TAA, and the sum of TA and TLA.
Appendix E – Decision tree for deriving MRL recommendations

Appendix F – Used compound codes
| Code/trivial name a | IUPAC name/SMILES notation/InChiKey b | Structural formula c |
|---|---|---|
| Tetraconazole |
(RS)‐2‐(2,4‐dichlorophenyl)‐3‐(1H‐1,2,4‐triazol‐1‐yl)propyl‐1,1,2,2‐tetrafluoroethyl ether FC(F)C(F)(F)OCC(Cn1cncn1)c1ccc(Cl)cc1Cl LQDARGUHUSPFNL‐UHFFFAOYSA‐N |

|
| Metabolite M14360‐acid |
2‐(2,4‐dichlorophenyl)‐3‐(1H‐1,2,4‐triazol‐1‐yl)propanoic acid OC(=O)C(Cn1cncn1)c1ccc(Cl)cc1Cl MFGQUIFCNUUDBI‐UHFFFAOYSA‐N |

|
| Triazole derivative metabolites | ||
|
1,2,4‐triazole 1,2,4‐T |
1H‐1,2,4‐triazole c1ncnn1 NSPMIYGKQJPBQR‐UHFFFAOYSA‐N |

|
|
Triazole alanine TA |
3‐(1H‐1,2,4‐triazol‐1‐yl)‐D,L‐alanine NC(Cn1cncn1)C(=O)O XVWFTOJHOHJIMQ‐UHFFFAOYSA‐N |

|
|
Triazole acetic acid TAA |
1H‐1,2,4‐triazol‐1‐ylacetic acid O = C(O)Cn1cncn1 RXDBSQXFIWBJSR‐UHFFFAOYSA‐N |

|
|
Triazole lactic acid or Triazole hydroxy propionic acid TLA |
(2RS)‐2‐hydroxy‐3‐(1H‐1,2,4‐triazol‐1‐yl)propanoic acid OC(Cn1cncn1)C(=O)O KJRGHGWETVMENC‐UHFFFAOYSA‐N |

|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116563, 15 June 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121153, 22 March 2021).
Suggested citation: EFSA (European Food Safety Authority) , Bellisai G, Bernasconi G, Brancato A, Carrasco Cabrera L, Ferreira L, Giner G, Greco L, Jarrah S, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Ruocco S, Santos M, Scarlato AP, Theobald A, Vagenende B and Verani A, 2022. Reasoned opinion on the review of the existing maximum residue levels for tetraconazole according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2022;20(1):7111, 98 pp. 10.2903/j.efsa.2022.7111
Requestor: European Commission
Question number: EFSA‐Q‐2010‐00207
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgement: EFSA wishes to thank the rapporteur Member State, France, for the preparatory work and Stathis Anagnos, Laszlo Bura, Aija Kazocina, Andrea Mioč, Marta Szot, Aikaterini Vlachou for the support provided to this scientific output.
Adopted: 21 December 2021
Notes
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Council Directive 2009/82/EC of 13 July 2009 amending Directive 91/414/EEC to include tetraconazole as an active substance. OJ L 196, 28.7.2009, p. 10–13.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187–188.
Council Directive 2010/82/EC of 29 November 2010 amending Council Directive 91/414/EEC as regards an extension of the use of the active substance tetraconazole. OJ L 313, 30.11.2010, p. 10–11.
The United Kingdom withdrew from EU on 1 February 2020. In accordance with the Agreement on the withdrawal of the United Kingdom from the EU, and with the established transition period, the EU requirements on data reporting also apply to the United Kingdom data collected until 31 December 2020.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- EFSA (European Food Safety Authority) , 2008. Conclusion on the peer review of the pesticide risk assessment of the active substance tetraconazole. EFSA Journal 2008;6(10):152, 86 pp. 10.2903/j.efsa.2008.152r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Reasoned opinion on the modification of the existing MRLs for tetraconazole in apricots prepared by EFSA Pesticide Risk Assessment Peer Review (PRAPeR) Unit. EFSA Journal 2009;7(1):230r, 25 pp. 10.2903/j.efsa.2009.230r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2012. Reasoned opinion on the modification of the existing MRL for tetraconazole in rape seed. EFSA Journal 2012;10(7):2842, 31 pp. 10.2903/j.efsa.2012.2842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2013a. Conclusion on the peer review of the pesticide risk assessment of the active substance ipconazole. EFSA Journal 2013;11(4):3181, 76 pp. 10.2903/j.efsa.2013.3181 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2013b. Reasoned opinion on the modification of the existing MRLs for tetraconazole in commodities of plant and animal origin. EFSA Journal 2013;11(5):3223, 40 pp. 10.2903/j.efsa.2013.3223 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018a. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Carrasco Cabrera L, Chiusolo A, Civitella C, Court Marques D, Crivellente F, De Lentdecker C, Erdös Z, Ferreira L, Goumenou M, Greco L, Istace F, Jarrah S, Kardassi D, Leuschner R, Medina P, Mineo D, Miron I, Molnar T, Nave S, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Terron A, Theobald A, Vagenende B and Villamar‐Bouza L, 2018b. Conclusion on the peer review of the pesticide risk assessment for the triazole derivative metabolites in light of confirmatory data. EFSA Journal 2018;16(7):5376, 103 pp. 10.2903/j.efsa.2018.5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2018c. Recommendations on the use of the proportionality approach in the framework of risk assessment for pesticide residues. EFSA Supporting Publication 2018;EN‐1503, 17 pp. 10.2903/sp.efsa.2018.EN-1503 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Brocca D, Carrasco Cabrera L, De Lentdecker C, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Medina P, Miron I, Molnar T, Nave S, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B and Villamar‐Bouza L, 2019a. Reasoned opinion on the modification of the existing maximum residue levels for tetraconazole in kaki/Japanese persimmon, linseeds and poppy seeds. EFSA Journal 2019;17(1):5577, 34 pp. 10.2903/j.efsa.2019.5577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019b. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA Supporting Publication 2019;EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2021a. Completeness check report on the review of the existing MRLs of tetraconazole prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 4 November 2021. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority) , 2021b. Member States consultation report on the review of the existing MRLs of tetraconazole prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 16 December 2021. Available online: www.efsa.europa.eu
- EURLs (European Union Reference Laboratories for Pesticide Residues) , 2021. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Analytical methods validated by the EURLs and overall capability of official laboratories to be considered for the review of the existing MRLs for tetraconazole. February 2021. Available online: www.efsa.europa.eu
- European Commission , 1996. Appendix G. Livestock feeding studies. 7031/VI/95‐rev 4, 22 July 1996.
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/VI/95‐rev.3, 22 July 1997.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirements for Annex II (part A, section 4) and Annex III (part A, section 5) of Directive 91/414. SANCO/3029/99‐rev. 4. 11 July 2000.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2020. Appendix D. Technical guidelines on data requirements for setting maximum residue levels, comparability of residue trials and extrapolation on residue data on products from plant and animal origin. SANTE/2019/12752, 23 November 2020.
- European Commission , 2021. Review report for the active substance tetraconazole. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 26. February 2009 and updated on 20 July 2017 and 26 January 2021 in view of the inclusion of tetraconazole in Annex I of Directive 91/414/EEC. SANCO/144/08‐Final rev.4, 26 January 2021.
- FAO (Food and Agriculture Organization of the United Nations) , 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Edition. FAO Plant Production and Protection Paper 197, 264 pp.
- France , 2021. Evaluation report prepared under Article 12.1 of Regulation (EC) No 396/2005. Review of the existing MRLs for tetraconazole, 13 April 2021 revised on August 2021. Available online: www.efsa.europa.eu
- Italy , 2005. Draft assessment report on the active substance tetraconazole prepared by the rapporteur Member State Italy in the framework of Council Directive 91/414/EEC, May 2005. Available online: www.efsa.europa.eu
- Italy , 2007. Addendum to the draft assessment report on the active substance tetraconazole prepared by the rapporteur Member State Italy in the framework of Council Directive 91/414/EEC, November 2007.
- Italy , 2008. Final addendum to the draft assessment report on the active substance tetraconazole prepared by the rapporteur Member State Italy in the framework of Council Directive 91/414/EEC, compiled by EFSA, June 2008.
- Italy , 2011. Evaluation report on the setting/modification/deletion of MRLs for tetraconazole in commodities prepared by the evaluating Member State Italy under Article 8 of Regulation (EC) No 396/2005, 22 September 2011. 143 pp.
- Italy , 2018. Evaluation report on the modification of MRLs for tetraconazole in various commodities. January 2018, as revised in September 2018, 90 pp.
- OECD (Organisation for Economic Co‐operation and Development) , 2008. Guidance document on the magnitude of pesticide residues in processed commodities. In: Series of Testing and Assessment No 96ENV/JM/MONO(2008) 23, 29 July 2008.
- OECD (Organisation for Economic Co‐operation and Development) , 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: https://www.oecd.org [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73ENV/JM/MONO(2013) 8, 4 September 2013. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2018. Guidance Document on Residues in Rotational Crops. In: Series on Pesticides No 97. ENV/JM/MONO(2018)9, 22 May 2018.
- United Kingdom , 2018. Triazole Derivate Metabolites, addendum – confirmatory data prepared by the rapporteur Member State, the United Kingdom in the framework of Regulation (EC) No 1107/2009, revised version of February 2018. Available online: www.efsa.europa.eu


