Key Points
Question
Can plinabulin, a selective immunomodulating microtubule-binding agent, perform as well as pegfilgrastim to prevent chemotherapy-induced neutropenia and its clinical consequences?
Findings
In this randomized clinical trial of 105 patients, plinabulin had comparable efficacy to pegfilgrastim for the prevention of chemotherapy-induced neutropenia with better safety findings, less bone pain, and a lower immunosuppressive profile.
Meaning
The findings of this study suggest that plinabulin’s same-day dosing compared with pegfilgrastim’s next-day dosing offers distinct advantages including reducing use of health care services.
This randomized clinical trial compares the efficacy of plinabulin vs pegfilgrastim to prevent severe neutropenia in patients receiving docetaxel myelosuppressive chemotherapy.
Abstract
Importance
Prevention of chemotherapy-induced neutropenia (CIN) and its clinical consequences is an unmet need for which plinabulin, a selective immunomodulating microtubule-binding agent, is being tested.
Objective
To demonstrate noninferiority between plinabulin and pegfilgrastim for days of severe neutropenia in cycle 1 in patients with solid tumors treated with docetaxel.
Design, Setting, and Participants
The Plinabulin vs Pegfilgrastim for the Prevention of Docetaxel-Induced Neutropenia in Patients With Solid Tumors (PROTECTIVE-1) double-blind phase 3 randomized clinical trial was performed in multiple centers in China, Russia, Ukraine, and the US. Participants included patients with breast, prostate, or non–small cell lung cancer treated with single-agent docetaxel chemotherapy. Data were collected from June 1, 2018, to January 31, 2019. The database was locked on February 18, 2021. Data analysis was based on intention to treat and safety and performed from October 5, 2018, to February 23, 2021.
Interventions
Plinabulin, 40 mg, plus placebo or pegfilgrastim, 6 mg, plus placebo.
Main Outcomes and Measures
The primary end point was day of severe neutropenia in cycle 1. Additional end points included clinical consequences of CIN (febrile neutropenia, hospitalizations, infections, antibiotic use, and modifications of chemotherapy dose), patient-reported outcomes for bone pain score, markers for immune suppression (neutrophil-to-lymphocyte ratio [NLR] of >5), immature neutrophils (band, promyelocyte, and myelocyte counts >0), and safety.
Results
Among the 105 patients included in the analysis (65 [6.19%] women; median age, 59 [range, 31-81] years), the primary end point was met within a noninferiority margin of 0.65 days, with a mean difference of 0.52 days (98.52% CI, 0.40-0.65 days). Grade 4 neutropenia frequency in cycle 1 was not significantly different. Plinabulin had earlier onset of action with less grade 4 neutropenia in week 1 of cycle 1. Plinabulin had fewer adverse clinical consequences with rates of febrile neutropenia (0 of 52 vs 1 of 53 [1.9%]), infections (4 of 52 [7.7%] vs 8 of 53 [15.1%]), chemotherapy dose delay of more than 7 days (2 of 52 [3.8%] vs 3 of 53 [5.7%]), and permanent chemotherapy discontinuation (7 of 52 [13.5%] vs 14 of 53 [26.4%]). Patients receiving plinabulin had significantly less bone pain (difference, −0.67 [95% CI, −1.17 to −0.16]; P = .01) and a better immunosuppressive profile (NLR >5 at day 8, 2 of 52 [3.8%] vs 24 of 51 [46.0%]; P < .001). Plinabulin was well tolerated, with comparable safety to pegfilgrastim.
Conclusions and Relevance
Plinabulin has comparable efficacy to pegfilgrastim for the prevention of CIN, with better safety and a better immunosuppressive profile. Plinabulin’s same-day dosing compared with pegfilgrastim’s next-day dosing offers distinct advantages, including reducing use of health care services.
Trial Registration
ClinicalTrials.gov Identifier: NCT03102606
Introduction
Myelosuppression is the primary toxic effect of many chemotherapy regimens. Both the duration of severe neutropenia and the depth of the neutrophil level nadir have been correlated with severe and life-threatening infections and unplanned hospitalizations.1,2,3 Severe neutropenia often necessitates modification of the chemotherapy regimen and may compromise anticancer efficacy.4 Neutropenia prevention is a major patient benefit for safety, treatment efficacy, and cost efficiency reasons.2,5,6
The risk of developing febrile neutropenia and grades 3 to 4 neutropenia is mitigated by reducing chemotherapy dosages or prolonging the chemotherapy interval, which reduces survival rates because of a reduction in chemotherapy dose intensity.7 Granulocyte colony–stimulating factors (G-CSFs) such as filgrastim and pegfilgrastim constitute a standard of care to reduce chemotherapy-induced neutropenia (CIN) and to facilitate optimum chemotherapy administration. Febrile neutropenia risk–based National Comprehensive Cancer Network guidelines recommend prophylactic G-CSF administration8,9,10,11 for patients at significant risk of febrile neutropenia.12 However, prophylactic G-CSF use has several efficacy limitations, predominantly its reduced neutropenia protection in the first week of the cycle.
Plinabulin is a novel non–G-CSF selective immunomodulating microtubule-binding agent that has hematopoietic stem cell–protective properties and anticancer benefits. In animals, plinabulin ameliorates neutropenia induced by various chemotherapies, including docetaxel, doxorubicin hydrochloride, and cyclophosphamide, through a mechanism distinct from G-CSF.13 Plinabulin has beneficial effects in week 1 after chemotherapy, which is the area of severe unmet medical need.13 Furthermore, plinabulin acts at the bone marrow level, increasing numbers of peripheral CD34+ progenitor stem cells.14 This phase 3 study compared plinabulin vs pegfilgrastim to prevent severe neutropenia in patients receiving docetaxel myelosuppressive chemotherapy.
Methods
Participants
The phase 3 portion of the Plinabulin vs Pegfilgrastim for the Prevention of Docetaxel-Induced Neutropenia in Patients With Solid Tumors (PROTECTIVE-1) study was a multicenter, double-blind randomized clinical trial. The trial protocol is provided in Supplement 1. The trial was conducted in accordance with Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki.15 This report follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized studies and was approved by the relevant independent ethics committee or institutional review board at each site. Each participant provided written informed consent.
Key eligibility criteria included adults with breast, prostate, or lung cancer who could benefit from single-agent docetaxel chemotherapy and had 1 or more of the following risk factors for febrile neutropenia: prior chemotherapy or radiotherapy, bone marrow involvement by tumor, surgery and/or open wounds within 4 weeks of the first administration of study drug, being older than 65 years, and receiving a full-intensity dose of chemotherapy.16 Patients with advanced or metastatic breast cancer who underwent less than 5 prior lines of failed chemotherapy with non–small cell lung cancer after platinum therapy failure or with hormone-refractory prostate cancer were eligible. Adequate organ function and a negative pregnancy test result at screening were required. Key exclusion criteria consisted of active wound infections, other anticancer treatment, and the current use of strong cytochrome P450 3A4 inhibitors.
All patients received docetaxel, 75 mg/m2, on day 1 and were randomized 1:1 to receive either plinabulin, 40 mg, on day 1 and placebo-matching pegfilgrastim on day 2, or pegfilgrastim, 6 mg, on day 2 and placebo-matching plinabulin on day 2. Once either treatment group reached at least one-third of total patients with a cancer type, that group was closed to that cancer type, and enrollment continued for patients with other cancer types until the planned maximum number of patients was reached.
Docetaxel premedication with corticosteroids and dose reductions were specified for cycles 2 to 4. Samples for complete blood counts and absolute neutrophil counts (ANCs) were drawn at the same time each day and measured at a central laboratory at a pretreatment screening visit; on days 1, 2, 6 to 10, and 15 of cycle 1; on days 1 and 8 of cycles 2 to 4; at the end of treatment; and a day 30 end-of-treatment follow-up. Cycle 1, day 1 preinfusion and postinfusion blood pressure was measured with an automated device every 15 minutes for 4 hours. Blood pressure was measured before and after plinabulin infusion in each subsequent cycle. The planned treatment was 4 cycles.
Patients were randomized using an interactive web response system. Randomization schedule files were created by Statogen Consulting LLC and provided to Suvoda LLC, who assigned and maintained the randomization. An independent data safety monitoring board oversaw study conduct.
Study Objectives
The primary end point was days of severe neutropenia (DSN) during cycle 1. Days of severe neutropenia consists of the number of consecutive days (1, 2, 6, 7, 8, 9, 10, and 15) from the first day when a patient’s ANC was less than 500/μL until the patient reached an ANC of greater than 500/μL in cycle 1 (to convert to ×109/L, multiply by 0.001). For patients who did not experience any severe neutropenia in cycle 1, DSN was 0. Secondary end points included assessment of maximum platelet count decrease from baseline in cycle 1, the proportion of patients with a neutrophil-to-lymphocyte ratio (NLR) of greater than 5 (cycle 1, days 7-15), and change in estimated mean bone pain score. Exploratory end points included clinical consequences of CIN (febrile neutropenia, hospitalizations, rate of infections, antibiotic use, and chemotherapy dose modifications), and band, promyelocyte, and myelocyte counts of greater than 0 (cycle 1, days 7-15).
Safety and Compliance
All patients were included in both the intention to treat population and the safety population. Safety data are presented descriptively by study groups. All treatment-emergent adverse effects (TEAEs) and abnormal laboratory variables were summarized by the Medical Dictionary for Regulatory Activities System Organ Class and Preferred Term and assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Safety end points included incidence, occurrence, and severity of adverse events and serious adverse events (SAEs), physical examination results, and safety laboratory assessments.
Validated Questionnaires
Bone pain was evaluated with the Brief Pain Inventory before study drug infusion on day 1 and on days 2, 3, 5, 7, 9, and 21 of cycle 1.17,18 Health-related quality of life was evaluated using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and EQ-5D-5L questionnaires collected before docetaxel infusion on day 1 of each cycle.19,20,21,22
Statistical Analysis
The database was locked on February 18, 2021, and data analysis was performed from October 5, 2018, through February 23, 2021. A copy of the statistical analysis plan is found in Supplement 1. Data were tabulated by treatment group, with listings provided for all data captured in the electronic case report forms. Data during treatment were assessed descriptively as both observed values and changes from pretreatment. When tabulated, data were presented using descriptive statistics (eg, mean [SD], median [range], and percentage for categorically scaled parameters). The negative binomial regression model was used to analyze the DSN end point during the fixed time window of analysis, with the treatment group as the only covariate.
Inferential assessment of treatment effects was performed for efficacy outcomes. For continuously scaled parameters, methods of longitudinal assessment using mixed models were applied. Overall treatment effects were estimated, as were pairwise effects at individual points. For categorically scaled parameters, χ2 test was applied as appropriate.
Approximately 150 patients were planned to be enrolled. The null hypothesis would be rejected if the upper confidence limit was less than 0.65. With 75 patients in each treatment group, there was at least a 90% power to reject the null hypothesis of 0.65 days of inferiority in DSN between the treatment means with an SD of 0.75, at a 2-sided α = .05, using a 2-sample zero-inflated Poisson model and an O’Brien-Fleming spending function to account for the interim analysis at two-thirds (66.7%) of information. The study design was group sequential with 1 interim analysis (after approximately 50 patients in each treatment group completed at least 1 cycle in each of the treatment groups and 1 final analysis). Statistical analyses were conducted using SAS version 4 (SAS Institute Inc).
Results
Study Population
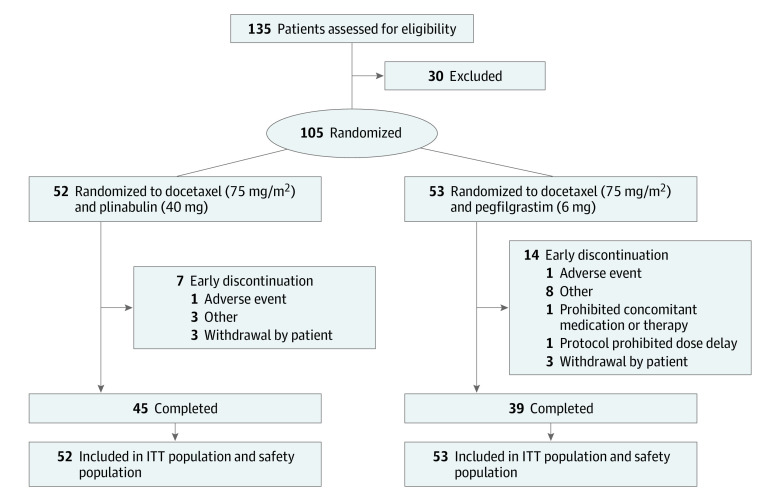
A total of 105 patients were enrolled, 52 in the plinabulin group and 53 in the pegfilgrastim group (Figure 1). This study was conducted in 17 study sites in 4 countries (China, Russia, Ukraine, and the US) from June 1, 2018, to January 31, 2019. Because Asian patients may respond differently to G-CSF agents,8 sites collected and scored race and ethnicity as Asian or non-Asian. The median age of study participants was 59 (range, 31-81) years, with 74 patients (70.5%) younger than 65 years, 65 (61.9%) women, and 40 (38.1%) men. Fifty-three patients (50.5%) had breast cancer, 33 (31.4%) had non–small cell lung cancer, and 19 (18.1%) had hormone-refractory prostate cancer. Further details on patient race and cancer type are given in eTable 1 in Supplement 2. All patients had 1 to 2 febrile neutropenia risk factors, and risk factor categories between treatment groups were comparable (eg, prior chemotherapy or radiotherapy, 49 of 53 [92.5%] for pegfilgrastim vs 51 of 52 [98.1%] for plinabulin) (eTable 1 in Supplement 2).
Figure 1. Disposition of Study Patients.
ITT indicates intention to treat.
All randomized patients received at least 1 dose of study medication and were in the intention to treat and safety populations. In the plinabulin group, 45 of 52 patients (86.5%) completed the study, compared with 39 of 53 (73.6%) in the pegfilgrastim group. Study treatment was discontinued in 1 patient in each group (1.9%) due to an adverse event.
Primary Clinical Outcome
The study met its prespecified noninferiority margin end point of DSN less than 0.65 days. The mean difference in DSN between the 2 treatment groups was 0.52 days (98.52% CI, 0.40-0.65 days) (Table 1). At the first primary analysis specified by the statistical analysis plan, the nominal significance level was 1.48% (ie, 100% − 98.52%), and the study met its noninferiority end point. The study was subsequently stopped owing to COVID-19–related logistical reasons, including patient and specimen transport to study sites and central laboratories.
Table 1. Summary and Analysis of DSN in Cycle 1.
| Treatment groupa | Mean DSN | Noninferiority metb |
|---|---|---|
| Pegfilgrastimc | 0.25 (0.21-0.29) | NA |
| Plinabulinc | 0.77 (0.68-0.86) | NA |
| Mean difference between treatment armsd | 0.52 (0.40-0.65) | Met |
Abbreviations: DSN, duration of severe neutropenia; NA, not applicable.
The pegfilgrastim group received docetaxel, 75 mg/m2, plus pegfilgrastim, 6 mg; the plinabulin group received docetaxel, 75 mg/m2, plus plinabulin, 40 mg.
Defined as an upper confidence limit of less than 0.65.
Using 2-sided 95% CI with group sequential adjustment.
Using 2-sided 98.52% CI with group sequential adjustment.
In cycle 1, patients in both treatment groups had mean ANC greater than grade 3 neutropenia (ANC >1000/μL). Patients in the pegfilgrastim group had mean ANC levels above the upper limit of ANC (ie, >8000/μL) on days 1 and 2 and from days 8 to 15 in cycle 1 (Figure 2A). Because results of the noninferiority testing were statistically significant, testing for superiority was performed, and the result was not statistically significant.
Figure 2. Outcomes by Time and Treatment Group in the Intention to Treat and Safety Analysis Sets.
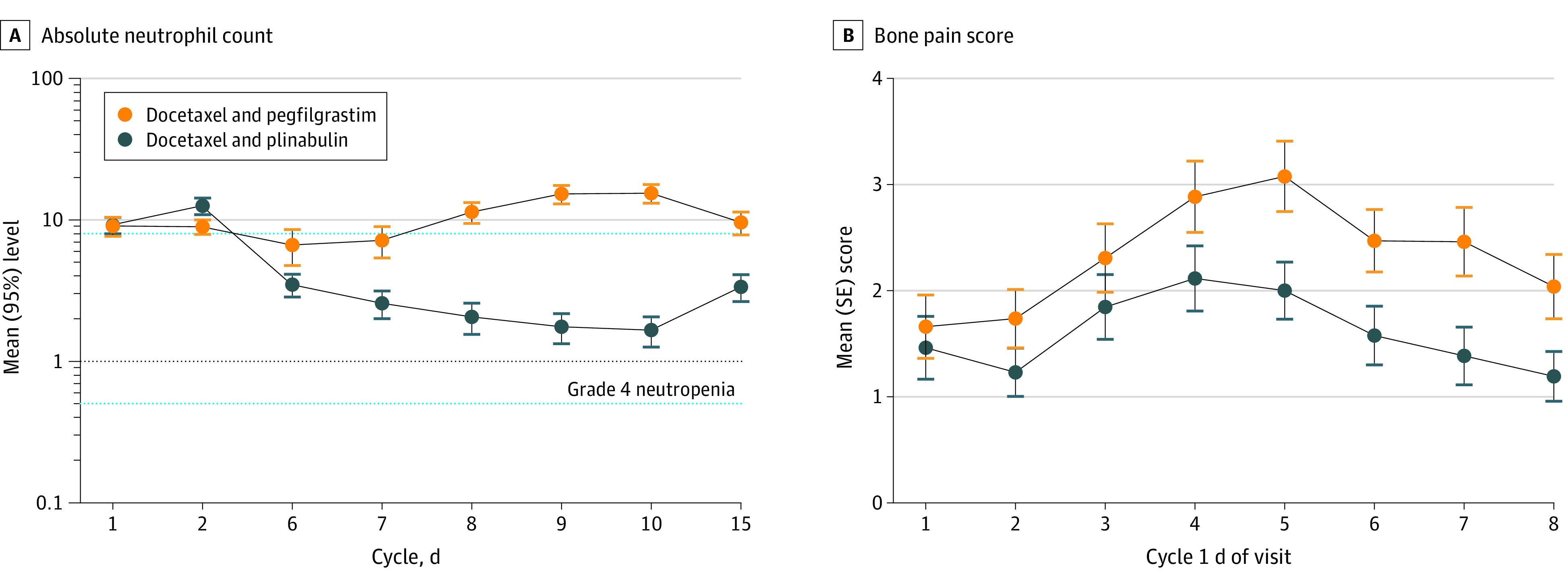
A, Semilog plot of the mean absolute neutrophil count in cycle 1 for the intention to treat analysis. B, Plot of the mean patient reported bone pain score in cycle 1 in the safety analysis.
Additional Clinical Outcomes
Additional end points included clinical consequences of CIN, bone pain score, and mean change in platelet count. In the plinabulin vs pegfilgrastim groups, febrile neutropenia (0 of 52 vs 1 of 53 [1.9%]), infection (4 of 52 [7.7%] vs 8 of 53 [15.1%]), antibiotic use (8 of 52 [15.4%] vs 7 of 53 [13.2%]), all-cause hospitalization (7 of 52 [13.5%] vs 5 of 53 [9.4%]), relative dose intensity of less than 85% (3 of 52 [5.8%] vs 2 of 53 [38%]), and docetaxel dose delay (2 of 52 [3.8%] vs 3 of 53 [5.7%]) or discontinuations (7 of 52 [13.5%] vs 14 of 53 [26.4%]) were mostly comparable between treatment groups, across cycles 1 to 4 (Table 2).
Table 2. Summary and Analysis of Clinical Consequences of Chemotherapy-Induced Neutropenia in Cycles 1 to 4.
| Clinical consequence | Treatment group, No. (%) of patientsa | |
|---|---|---|
| Pegfilgrastim (n = 53) | Plinabulin (n = 52) | |
| Febrile neutropenia | 1 (1.9) | 0 |
| Infection | 8 (15.1) | 4 (7.7) |
| Antibiotic use | 7 (13.2) | 8 (15.4) |
| Hospitalization (all cause) | 5 (9.4) | 7 (13.5) |
| Change in docetaxel use | ||
| Dose reduction to <85% | 2 (3.8) | 3 (5.8)b |
| Dose delay >7 d | 3 (5.7) | 2 (3.8)c |
| Discontinuation | 14 (26.4) | 7 (13.5)d |
The pegfilgrastim group received docetaxel, 75 mg/m2, plus pegfilgrastim, 6 mg; the plinabulin group received docetaxel, 75 mg/m2, plus plinabulin, 40 mg.
P = .68 between groups, Fisher exact test.
P = .66 for cycles 1 to 4 between groups, Cochran-Mantel-Haenszel test.
P = .10 for cycles 1 to 4 and P = .03 for cycle 4 between groups, Cochran-Mantel-Haenszel test.
Reasons for study discontinuation are provided in eTable 2 in Supplement 2. Hospitalizations and investigator-assessed infection details are provided in eTables 3 and 4 in Supplement 2.
Across cycle 1, patients in the plinabulin arm had significantly less bone pain compared with the pegfilgrastim group (least squares mean difference, –0.67 [95% CI, −1.17 to −0.16]; P = .01). The mean (SD) area under the curve for bone pain was 11.5 (11.8) for plinabulin and 16.6 (13.8) for pegfilgrastim (P = .07) (Figure 2B). All patients developed some degree of thrombocytopenia, but patients in the plinabulin group had uniformly higher platelet counts than the pegfilgrastim group (mean, 10% [SD, 19%] vs mean, −62% [SD, 23%] lower on cycle 1, day 15; P < .001) (eFigure 1 in Supplement 2).
Clinical outcomes also included NLR of greater than 5 and band, promyelocyte, and myelocyte counts of greater than 0. Significantly fewer patients in the plinabulin group had an NLR of greater than 5 in cycle 1 days 7 to 15 compared with the pegfilgrastim group (eg, cycle 1, day 8, 2 of 52 [3.8%] vs 23 of 50 [46.0%]; P < .001) (eTable 5 in Supplement 2).
The total ANC consists of mature neutrophils, which function in fighting infections, and immature neutrophils, including bands, promyelocytes, and myelocytes, which are less functional. Patients in the plinabulin group had fewer bands compared with the pegfilgrastim group in cycle 1 (days 7-15; 14 of 52 [26.9%] vs 30 of 53 [56.6%]; difference, 15 [95% CI, 0.65-29.22]; P = .05) (eFigure 2 in Supplement 2). Furthermore, patients in the plinabulin group had significantly fewer promyelocytes and myelocytes than patients in the pegfilgrastim group in cycle 1 (days 7-15) (eg, day 15, 1 of 52 [1.9%] vs 3 of 51 [5.9%]; P < .001) (eTable 6 in Supplement 2).
No patient in either group required a platelet transfusion. In cycle 1, fewer patients experienced thrombocytopenia in the plinabulin group compared with the pegfilgrastim group (10 of 52 [19.2%] vs 19 of 53 [35.8%]) (eTable 7 in Supplement 2). However, the difference was not statistically significant (P = .06).
Quality of Life
Health-related quality of life, assessed via EORTC QLQ-C30, was similar for all points and across all domains for both treatment groups. Similarly, EQ-5D-5L visual analog scale scores and health utility values were comparable across all time points for both treatment groups (eTable 8 in Supplement 2).
Adverse Events
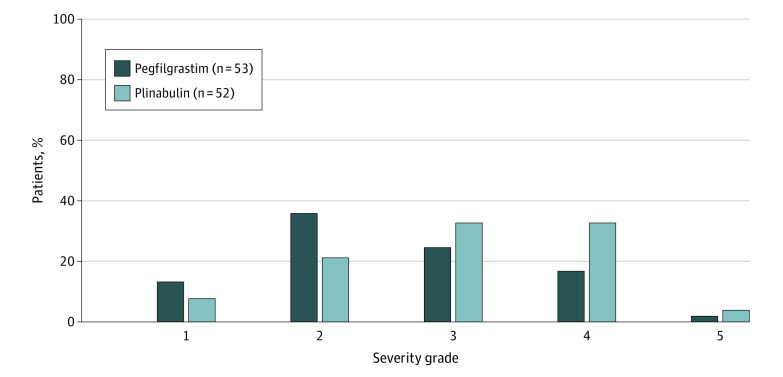
Treatment-emergent adverse events occurred in almost all patients receiving chemotherapy and study drug. In the plinabulin group, 624 adverse events occurred in 51 of 52 patients (98.1%). Of the patients who experienced adverse events, 50 (98.0%) experienced TEAEs, and 8 (15.7%) experienced SAEs. Study treatment was delayed or discontinued for 6 patients in the treatment group (11.8%), and 2 (3.9%) died because of a TEAE. In the pegfilgrastim group, 409 adverse events occurred in 49 of 53 patients (92.5%). Of the patients who experienced adverse events, 44 (89.8%) experienced TEAEs, and 6 (12.2%) experienced SAEs. Study treatment was delayed or discontinued for 7 patients in the treatment group (13.2%), and 1 (1.9%) died due to a TEAE. No major difference in the severity of TEAEs was noted between treatment groups (Figure 3). The most common TEAEs (≥10%) in the plinabulin group were decreased ANC, decreased white blood cell count, and bone pain. In addition, some patients who reported an adverse effect of bone pain had concomitant bisphosphonate use, most likely for bone metastases (eTable 9 in Supplement 2), which may confound interpretation of the adverse event data. Common grades 3 to 4 TEAEs (≥10%) were decreased neutrophil count, decreased white blood cell count, and neutropenia. In the pegfilgrastim group, the most common TEAEs were alopecia, bone pain, and anemia, with common grades 3 to 4 TEAEs being decreased neutrophil count.
Figure 3. Treatment Emergent Adverse Events by Severity Grade.
Grades are given in the Methods section.
For nonhematological TEAEs, there were no marked differences between treatments. For hematological TEAEs, investigators reported more grade 4 neutropenia and decreased white blood cell count events for plinabulin; however, treatment-emergent grade 4 neutropenia frequencies were comparable between the 2 treatment groups. The difference in hematological TEAEs was primarily due to the choice of safety blood draw timing (day 8) in cycles 2 to 4, which approximately coincided with plinabulin ANC nadir, but was several days after the pegfilgrastim ANC nadir. Anemia was comparable between treatment groups. No clinically significant trends were observed for chemistry, urinalysis, or vital signs.
Six patients (3 in the plinabulin group and 3 in the pegfilgrastim group) died during the study. Three deaths were due to SAEs (1 due to kidney failure and 1 due to status asthmaticus unrelated to the study treatment in the plinabulin group; 1 due to febrile neutropenia unrelated to the study treatment in the pegfilgrastim group), and 3 deaths were due to disease progression (1 in the plinabulin group and 2 in the pegfilgrastim group). The investigators attributed no deaths or SAEs to plinabulin.
Discussion
The phase 3 PROTECTIVE-1 randomized clinical trial used chemotherapy with an intermediate (10%-20%) risk of febrile neutropenia, for which the recent National Comprehensive Cancer Network guidelines recommend CIN prophylaxis. Plinabulin has a CIN prevention benefit that is noninferior to the current CIN prophylaxis standard of care, pegfilgrastim, and less grade 4 neutropenia in cycle 1, week 1. In addition, plinabulin-treated patients had less platelet decrease, less bone pain, and use on the same day as chemotherapy (compared with the day after chemotherapy dosing for pegfilgrastim and for filgrastim, which requires daily injection for 8-10 days after chemotherapy). Importantly, clinical consequences of CIN (febrile neutropenia rate, infection rate, and permanent chemotherapy discontinuations) were also reduced in plinabulin-treated patients. However, the study was underpowered to achieve statistical significance for these end points. In summary, single-agent plinabulin is at least as effective as pegfilgrastim for mitigating CIN and its clinical consequences, but with less bone pain and less decrease of platelet counts, and has the advantage of the same-day dosing as chemotherapy.
In our pegfilgrastim-treated patients, we observed high NLRs, which were not seen in the plinabulin-treated patients. Elevated NLR and circulating immature neutrophil forms have been associated with poor response to immune therapies.23,24 Because pegfilgrastim results in an overshoot of ANC (and a high NLR), avoidance of pegfilgrastim and substitution of plinabulin should be tested in chemoimmunotherapy combination anticancer regimens.
Quality of life was similar for all points and across all domains for both treatment groups. Safety profiles were overall comparable, and the number of deaths was equal (n = 3) in both groups.
Although the mean bone pain score was significantly lower with plinabulin compared with pegfilgrastim, with improvements in bone pain seen throughout treatment cycle 1, TEAEs of bone pain were slightly higher in the plinabulin group compared with the pegfilgrastim group for all cycles. This is likely due to an imbalance in the prevalence of bone metastases between the 2 groups, because more patients had concomitant bisphosphonate use, most likely for bone metastases, in the plinabulin group. The frequency of neutropenia and decreased white blood cell count across cycles 1 to 4 was higher with plinabulin vs pegfilgrastim, which is primarily due to the choice of the day of safety blood draw (day 8) in cycles 2, 3, and 4, which approximately coincides with ANC nadir with plinabulin, but is several days after the ANC nadir with pegfilgrastim (ANC nadir occurs on day 5 or 6). Overall, a comparable number of clinical consequences of neutropenia were observed with plinabulin vs pegfilgrastim.
Neutropenia is a frequent toxic effect of myelotoxic chemotherapy. US Food and Drug Administration–approved CIN-protective agents filgrastim and pegfilgrastim revolutionized CIN prevention.10,11,12,25 Use of these G-CSF–based compounds resulted in fewer CIN-related complications and allowed the development of dose-intense and dose-dense chemotherapy regimens. However, complications of neutropenia still occur, especially in the first week after chemotherapy, which corresponds to the G-CSF ANC nadir. Plinabulin’s early protection in week 1 can help with this unmet medical need or neutropenia vulnerability gap. Another approach to this unmet medical need, which we are testing, is combining plinabulin’s week 1 effectiveness with pegfilgrastim’s week 2 protection to enhance CIN protection.26 We are also investigating plinabulin’s effect in the treatment of nonsolid tumors. Furthermore, plinabulin may have a role in CIN protection in conjunction with weekly chemotherapeutic regimens, for which there are no data with pegfilgrastim.
Limitations
This study has several limitations. The sampling duration of postchemotherapy ANC and complete blood cell count was limited for patient convenience and may have missed events later in the chemotherapy cycle. We are reassured by our observation that all patients had ANC kinetics trending toward recovery at the last ANC measurement on cycle 1, day 15. Our study was underpowered to detect significant clinical consequences of neutropenia (febrile neutropenia, infections, and hospitalizations), but these were numerically fewer with plinabulin treatment. The use of DSN as an end point in studies comparing 2 agents has insufficient dynamic range (ie, variations of <1 day) and less clinical relevance than the 6- to 1-day DSN reduction associated with the use of filgrastim compared with no CIN-protective agent.27 Furthermore, pegfilgrastim-induced immature neutrophils may not have the full infection-protection function of more mature neutrophils in the plinabulin treatment. Therefore, pegfilgrastim may have higher ANC numbers and shorter DSN, which may not translate into better protection against the clinical consequences of neutropenia. Additionally, the blood draw schedule, which began on day 6 in cycle 1, may have missed the nadir in any patient who began the nadir period earlier than day 6 and artifactually favored pegfilgrastim.
Conclusions
This randomized clinical trial found that plinabulin, a novel selective immunomodulating microtubule-binding agent, could be a valuable addition to the therapeutic armamentarium for CIN protection. Plinabulin is noninferior to pegfilgrastim, the current standard of care, and has early onset of protection in week 1, with less bone pain, less platelet reduction, lower immunosuppressive potential, and the advantage of dosing on the same day as chemotherapy. In addition, plinabulin offers comparable protection to pegfilgrastim against febrile neutropenia, infection, hospitalization, and chemotherapy dose reduction and discontinuation. All these advantages should be explored in future studies.
Trial Protocol
eTable 1. Patient Disposition and Demographics
eTable 2. Reasons for Discontinuations Categorized as Other
eTable 3. Listing of All-Cause Hospitalizations
eTable 4. Summary of Infections
eTable 5. Summary and Analysis of Patients with Neutrophil-to-Lymphocyte Ratio (NLR) Greater Than 5 after Day 7 Through Day 15 in Cycle 1
eTable 6. Summary and Analysis of Patients With Promyelocyte Plus Myelocyte Counts Greater Than 0 After Days 7-15 in Cycle 1—ITT Analysis Set
eTable 7. Summary and Analysis of Patients With Thrombocytopenia (All Grades) in Cycles 1-4
eTable 8. Summary and Analysis of EQ-5D-5L Evaluation—Safety Analysis Set
eTable 9. Summary of Patients With Bone Metastasis
eFigure 1. Mean Change From Baseline for Platelets (Gi/L) by Study Day for Cycle 1—ITT Analysis Set
eFigure 2. Summary and Analysis of Patients With Band Counts Greater Than 0, Cycle 1, Days 1-15
Data Sharing Statement
References
- 1.Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328(18):1323-1332. doi: 10.1056/NEJM199305063281808 [DOI] [PubMed] [Google Scholar]
- 2.Dinan MA, Hirsch BR, Lyman GH. Management of chemotherapy-induced neutropenia: measuring quality, cost, and value. J Natl Compr Canc Netw. 2015;13(1):e1-e7. doi: 10.6004/jnccn.2015.0014 [DOI] [PubMed] [Google Scholar]
- 3.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non–small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589-1597. doi: 10.1200/JCO.2004.08.163 [DOI] [PubMed] [Google Scholar]
- 4.Aarts MJ, Peters FP, Mandigers CM, et al. Primary granulocyte colony-stimulating factor prophylaxis during the first two cycles only or throughout all chemotherapy cycles in patients with breast cancer at risk for febrile neutropenia. J Clin Oncol. 2013;31(34):4290-4296. doi: 10.1200/JCO.2012.44.6229 [DOI] [PubMed] [Google Scholar]
- 5.Burris HA, Belani CP, Kaufman PA, et al. Pegfilgrastim on the same day versus next day of chemotherapy in patients with breast cancer, non–small-cell lung cancer, ovarian cancer, and non-Hodgkin’s lymphoma: results of four multicenter, double-blind, randomized phase 2 studies. J Oncol Pract. 2010;6(3):133-140. doi: 10.1200/JOP.091094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martín M, Seguí MA, Antón A, et al. ; GEICAM 9805 Investigators . Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010;363(23):2200-2210. doi: 10.1056/NEJMoa0910320 [DOI] [PubMed] [Google Scholar]
- 7.Nabholtz JM, Riva A. Taxane/anthracycline combinations: setting a new standard in breast cancer? Oncologist. 2001;6(suppl 3):5-12. doi: 10.1634/theoncologist.6-suppl_3-5 [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Lee JE, Kim Z, et al. Pegfilgrastim for primary prophylaxis of febrile neutropenia in breast cancer patients undergoing TAC chemotherapy. Ann Surg Treat Res. 2018;94(5):223-228. doi: 10.4174/astr.2018.94.5.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda N, Tokuda Y, Nakamura S, Shimazaki R, Ito Y, Tamura K. Dose response of pegfilgrastim in Japanese breast cancer patients receiving six cycles of docetaxel, doxorubicin, and cyclophosphamide therapy: a randomized controlled trial. Support Care Cancer. 2015;23(10):2891-2898. doi: 10.1007/s00520-015-2654-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20(3):727-731. doi: 10.1200/JCO.2002.20.3.727 [DOI] [PubMed] [Google Scholar]
- 11.Holmes FA, Jones SE, O’Shaughnessy J, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13(6):903-909. doi: 10.1093/annonc/mdf130 [DOI] [PubMed] [Google Scholar]
- 12.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228-237. doi: 10.1002/cncr.11882 [DOI] [PubMed] [Google Scholar]
- 13.Tonra JR, Lloyd GK, Mohanlal R, Huang L. Plinabulin ameliorates neutropenia induced by multiple chemotherapies through a mechanism distinct from G-CSF therapies. Cancer Chemother Pharmacol. 2020;85(2):461-468. doi: 10.1007/s00280-019-03998-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blayney DW, Huang L, Mohanlal RW. A comparison of CD34+ mobilization effects of standard dose pegfilgrastim (Peg) versus low-dose peg combined with plinabulin. J Clin Oncol. 2020;38(suppl 15):e20000. doi: 10.1200/JCO.2020.38.15_suppl.e20000 [DOI] [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Aagaard T, Roen A, Reekie J, et al. Development and validation of a risk score for febrile neutropenia after chemotherapy in patients with cancer: the Fence score. JNCI Cancer Spectr. 2018;2(4):pky053. doi: 10.1093/jncics/pky053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129-138. [PubMed] [Google Scholar]
- 18.Cleeland CS. The Brief Pain Inventory User Guide. MD Anderson Cancer Center; 2009. [Google Scholar]
- 19.Bedard G, Zeng L, Zhang L, et al. Minimal important differences in the EORTC QLQ-C30 in patients with advanced cancer. Asia Pac J Clin Oncol. 2014;10(2):109-117. doi: 10.1111/ajco.12070 [DOI] [PubMed] [Google Scholar]
- 20.Phillips R, Gandhi M, Cheung YB, et al. Summary scores captured changes in subjects’ QoL as measured by the multiple scales of the EORTC QLQ-C30. J Clin Epidemiol. 2015;68(8):895-902. doi: 10.1016/j.jclinepi.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 21.EORTC Quality of Life Group : The EORTC QLQ-C30 Manuals, Reference Values and Bibliography Brussels. EORTC Quality of Life Unit; 2002. [Google Scholar]
- 22.van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708-715. doi: 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 23.Cassidy MR, Wolchok RE, Zheng J, et al. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine. 2017;18:56-61. doi: 10.1016/j.ebiom.2017.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lalani AA, Xie W, Martini DJ, et al. Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6(1):5. doi: 10.1186/s40425-018-0315-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashyap AS, Fernandez-Rodriguez L, Zhao Y, et al. GEF-H1 signaling upon microtubule destabilization is required for dendritic cell activation and specific anti-tumor responses. Cell Rep. 2019;28(13):3367-3380.e8. doi: 10.1016/j.celrep.2019.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blayney DW, Shi Y, Adamchuk H, et al. Clinical trial testing superiority of combination plinabulin (Plin) and pegfilgrastim (Peg) versus peg alone in breast cancer treated with high-risk febrile neutropenia risk chemotherapy (chemo): final results of the phase 3 protective-2 in chemo-induced neutropenia (CIN) prevention. J Clin Oncol. 2021;39(suppl 15):533. doi: 10.1200/JCO.2021.39.15_suppl.533 [DOI] [Google Scholar]
- 27.Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325(3):164-170. doi: 10.1056/NEJM199107183250305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Patient Disposition and Demographics
eTable 2. Reasons for Discontinuations Categorized as Other
eTable 3. Listing of All-Cause Hospitalizations
eTable 4. Summary of Infections
eTable 5. Summary and Analysis of Patients with Neutrophil-to-Lymphocyte Ratio (NLR) Greater Than 5 after Day 7 Through Day 15 in Cycle 1
eTable 6. Summary and Analysis of Patients With Promyelocyte Plus Myelocyte Counts Greater Than 0 After Days 7-15 in Cycle 1—ITT Analysis Set
eTable 7. Summary and Analysis of Patients With Thrombocytopenia (All Grades) in Cycles 1-4
eTable 8. Summary and Analysis of EQ-5D-5L Evaluation—Safety Analysis Set
eTable 9. Summary of Patients With Bone Metastasis
eFigure 1. Mean Change From Baseline for Platelets (Gi/L) by Study Day for Cycle 1—ITT Analysis Set
eFigure 2. Summary and Analysis of Patients With Band Counts Greater Than 0, Cycle 1, Days 1-15
Data Sharing Statement