Key Points
Question
What were the clinical outcomes of patients who underwent a lung transplant after developing COVID-19–associated acute respiratory distress syndrome (ARDS) at a single center in the US?
Findings
In this retrospective case series involving 102 consecutive patients who underwent a lung transplant between January 21, 2020, and September 30, 2021, at a single center in Chicago, Illinois, patient survival was 100% for the 30 patients who had COVID-19–associated ARDS and 83% for the 72 patients without COVID-19, as of November 15, 2021.
Meaning
In this case series of patients who underwent a lung transplant, survival was 100% in patients who had COVID-19–associated ARDS as of November 15, 2021.
Abstract
Importance
Lung transplantation is a potentially lifesaving treatment for patients who are critically ill due to COVID-19–associated acute respiratory distress syndrome (ARDS), but there is limited information about the long-term outcome.
Objective
To report the clinical characteristics and outcomes of patients who had COVID-19–associated ARDS and underwent a lung transplant at a single US hospital.
Design, Setting, and Participants
Retrospective case series of 102 consecutive patients who underwent a lung transplant at Northwestern University Medical Center in Chicago, Illinois, between January 21, 2020, and September 30, 2021, including 30 patients who had COVID-19–associated ARDS. The date of final follow-up was November 15, 2021.
Exposures
Lung transplant.
Main Outcomes and Measures
Demographic, clinical, laboratory, and treatment data were collected and analyzed. Outcomes of lung transplant, including postoperative complications, intensive care unit and hospital length of stay, and survival, were recorded.
Results
Among the 102 lung transplant recipients, 30 patients (median age, 53 years [range, 27 to 62]; 13 women [43%]) had COVID-19–associated ARDS and 72 patients (median age, 62 years [range, 22 to 74]; 32 women [44%]) had chronic end-stage lung disease without COVID-19. For lung transplant recipients with COVID-19 compared with those without COVID-19, the median lung allocation scores were 85.8 vs 46.7, the median time on the lung transplant waitlist was 11.5 vs 15 days, and preoperative venovenous extracorporeal membrane oxygenation (ECMO) was used in 56.7% vs 1.4%, respectively. During transplant, patients who had COVID-19–associated ARDS received transfusion of a median of 6.5 units of packed red blood cells vs 0 in those without COVID-19, 96.7% vs 62.5% underwent intraoperative venoarterial ECMO, and the median operative time was 8.5 vs 7.4 hours, respectively. Postoperatively, the rates of primary graft dysfunction (grades 1 to 3) within 72 hours were 70% in the COVID-19 cohort vs 20.8% in those without COVID-19, the median time receiving invasive mechanical ventilation was 6.5 vs 2.0 days, the median duration of intensive care unit stay was 18 vs 9 days, the median post–lung transplant hospitalization duration was 28.5 vs 16 days, and 13.3% vs 5.5% required permanent hemodialysis, respectively. None of the lung transplant recipients who had COVID-19–associated ARDS demonstrated antibody-mediated rejection compared with 12.5% in those without COVID-19. At follow-up, all 30 lung transplant recipients who had COVID-19–associated ARDS were alive (median follow-up, 351 days [IQR, 176-555] after transplant) vs 60 patients (83%) who were alive in the non–COVID-19 cohort (median follow-up, 488 days [IQR, 368-570] after lung transplant).
Conclusions and Relevance
In this single-center case series of 102 consecutive patients who underwent a lung transplant between January 21, 2020, and September 30, 2021, survival was 100% in the 30 patients who had COVID-19–associated ARDS as of November 15, 2021.
This case series reports the clinical characteristics and outcomes of patients who had COVID-19–associated acute respiratory distress syndrome (ARDS) and underwent a lung transplant at a single US hospital.
Introduction
Patients with COVID-19 infection who become critically ill typically develop hypoxemia and respiratory failure, along with other potential extrapulmonary complications, including shock, acute kidney injury, and increased risk for thromboembolism. Between 6% and 10% of patients with COVID-19 progress to acute respiratory distress syndrome (ARDS) and require mechanical ventilation.1,2 The mortality rate of patients with COVID-19–associated ARDS may exceed 20% to 40%.3,4 COVID-19 can cause severe and irreversible lung parenchymal damage that is molecularly and structurally similar to end-stage idiopathic pulmonary fibrosis, for which lung transplant may be the only treatment option.5
Lung transplantation is a well-established treatment for several chronic end-stage lung diseases, including idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, cystic fibrosis, and pulmonary hypertension. However, prior to the COVID-19 pandemic, patients with ARDS were rarely considered for lung transplant.6 On May 9, 2021, a multinational consortium composed of transplant centers from the US, Austria, Italy, and India proposed guidelines for consideration of lung transplant in patients with COVID-19–associated ARDS.7,8 Adoption of these guidelines by international lung transplant programs has been inconsistent in part because the long-term outcome of lung transplants for patients with COVID-19–associated ARDS is unknown.
The objective of this case series was to report the outcomes of patients who had COVID-19–associated ARDS and underwent a lung transplant at a single US hospital. For context, the outcomes of patients without COVID-19 who received a lung transplant for chronic end-stage lung disease are also reported.
Methods
Study Design and Participants
This retrospective case series was performed at Northwestern University Medical Center in Chicago, Illinois. All consecutive patients who underwent a lung transplant between January 21, 2020, and September 30, 2021, were enrolled. All patients without COVID-19 signed a consent form for a lung transplant, and 29 of the 30 patients with COVID-19–associated ARDS consented to the surgery. For the 1 patient with COVID-19–associated ARDS who did not have medical decision-making capacity, a medical power of attorney provided consent. The institutional review board of Northwestern University approved this study (STU00207250 and STU00213616) and waived the need for additional consent from individual patients. Information about 4 of the 30 lung transplant recipients with COVID-19 was published in a prior article.7
Lung Transplantation
All patients with COVID-19–associated ARDS at Northwestern University/Northwestern Memorial Hospital were treated by a multidisciplinary team that included surgeons, infectious disease physicians, pulmonary and critical care physicians, and cardiologists over the entire duration of illness before being considered for a transplant. Referral for lung transplant was made when this multidisciplinary team concluded there was no longitudinal evidence of lung recovery after at least 4 to 6 weeks had elapsed from the onset of COVID-19–associated ARDS. The patients who underwent a lung transplant for COVID-19–associated ARDS were not required to ambulate. However, excluding the first patient in the COVID-19 cohort who lacked decisional capacity and was unable to participate, all patients with COVID-19–associated ARDS received pretransplant rehabilitation during their hospitalization, achieving enough truncal strength to sit upright and move all 4 limbs against gravity.
Similarly, all lung transplant recipients without COVID-19 were treated by a multidisciplinary team at Northwestern University Medical Center over the entire duration of illness before being considered for a transplant. Referral for lung transplant was made by a transplant pulmonologist based on a patient’s oxygen requirement or need for extracorporeal life support; the decision to place a patient on the lung transplant list was made by the multidisciplinary team after lung transplant evaluation.
Lung transplant evaluation was performed according to the International Society for Heart and Lung Transplantation guidelines. The broad transplant criteria for patients with COVID-19–associated ARDS included age 70 years or younger, 2 consecutive lower respiratory fluid polymerase chain reaction tests negative for SARS-CoV-2 on specimens obtained by endotracheal aspirate or bronchoscopy 24 hours apart, single organ failure, no evidence of irrecoverable brain damage, and body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) less than or equal to 35, which was the same BMI cutoff for lung transplant recipients without COVID-19. Patients being evaluated for multiorgan transplants were excluded from this study.
Details of transplant management for patients with COVID-19–associated ARDS in this patient series have been previously published.7 All patients who had COVID-19–associated ARDS underwent double lung transplant using central venoarterial extracorporeal membrane oxygenation (ECMO). Lung transplant recipients with and without COVID-19 received similar maintenance immunosuppression medications, which included an antimetabolite, calcineurin inhibitor, and steroid. All patients received standard protocolized treatment to prevent infection after lung transplant. Following acute hospitalization, all lung transplant recipients were considered for inpatient rehabilitation prior to being discharged from the hospital. The approach to lung transplant is described in the eMethods in the Supplement.
Ventilator and ECMO Management
Prior to lung transplant, all intubated patients were treated by a multidisciplinary team in accordance with guidelines from the National Heart, Lung, and Blood Institute’s ARDS Network.9 Indications for ECMO evaluation included refractory hypoxemia with Pao2 less than 55 mm Hg, pulse oximetry oxygen saturation less than 88%, and pH level less than 7.2, despite lung-protective mechanical ventilation with a plateau pressure less than 35 mm Hg, neuromuscular blockade, and prone positioning as indicated, in accordance with recommendations from the Extracorporeal Life Support Organization.10
The decision to initiate ECMO was made by a multidisciplinary ECMO team, which included pulmonologists, thoracic surgeons, ECMO specialists, and intensivists, using a teleconference line. Continuous anticoagulation was not used during ECMO unless there was clinical or radiological evidence of thrombosis (such as deep vein thrombosis, pulmonary embolism, and arterial thrombosis), consistent with our prior reports of the feasibility of using venovenous ECMO without anticoagulation.11,12,13 Patients treated with venovenous ECMO received unfractionated heparin at a dose of 5000 U given subcutaneously every 8 hours for deep venous thrombosis prophylaxis and ECMO circuit flow was maintained at least 3.0 to 3.5 L/min. Thresholds for packed red blood cell transfusion included a hemoglobin level less than 7 g/dL or hemodynamic instability in the setting of active blood loss. Platelet transfusion was recommended for platelet counts less than 50 000/mL. Coagulopathy (international normalized ratio >2) in the setting of active bleeding was treated with fresh frozen plasma with or without procoagulant factors such as cryoprecipitate.
Data Collection
Clinical and laboratory characteristics as well as treatment and outcomes data were obtained from electronic medical records by the investigative team and reviewed by trained physicians. Information recorded included demographic data, medical history, underlying comorbidities, laboratory findings, medical course, and treatments administered. Intraoperative procedures and postoperative complications were recorded. ARDS was defined according to the Berlin Definition.14 Primary graft dysfunction (PGD) was graded according to the criteria of the International Society of Heart and Lung Transplantation.15 Patients with normal Pao2 to fraction of inspired oxygen (Fio2) ratio and without diffuse pulmonary edema on chest radiograph were graded 0. Patients with diffuse pulmonary edema on chest radiograph were graded as follows: grade 1, Pao2:Fio2 greater than 300; grade 2, Pao2:Fio2 between 200 and 300; and grade 3, Pao2:Fio2 less than 200. Patients for whom ECMO was continued after lung transplant were classified as having PGD grade 3 if they demonstrated pulmonary infiltrates. Cumulated incidence of all grades of PGD at 72 hours was recorded. Acute kidney injury was assessed using the Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease classification.16 Hospital and intensive care unit (ICU) lengths of stay were also recorded. Survival after lung transplant was documented throughout the duration of the study using both in-person clinic visits and telemedicine.
Statistical Analysis
The sample size was equal to the number of patients treated during the study period and no statistical sample size was calculated. Continuous variables were presented as medians and IQRs. Categorical variables were expressed as frequency rates and percentages. There were no missing data in the institutional cohorts. The Kaplan-Meier method was used to estimate survival. Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University).
Results
Screened Population
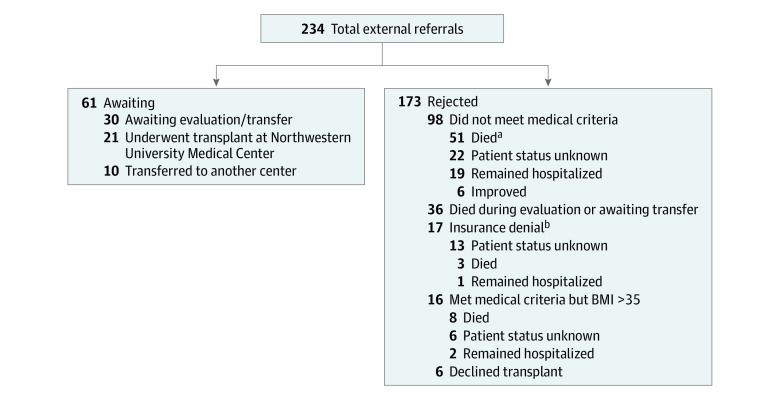
Between November 1, 2020, and December 21, 2021, a total of 234 external lung transplant referrals for patients who had COVID-19–associated ARDS were received by Northwestern University Medical Center (Figure 1). Of these, 21 (9%) underwent a lung transplant at this medical center and are included in this study, and 30 (12.8%) were completing the evaluation or awaiting transfer as of December 21, 2021. Ten patients (4.3%) accepted to Northwestern University Medical Center were transferred to another transplant center due to lack of available hospital beds. The remaining 173 (73.9%) of the external referrals for lung transplant for patients with COVID-19–associated ARDS were not accepted as candidates for lung transplant. Of all the referrals for patients with COVID-19–associated ARDS, 36 patients (15.4%) died while being evaluated for transplant or while awaiting transfer. Additionally, 12 patients (5.1%) who were deemed to have potential for lung recovery died while being supported by ECMO (Figure 1).
Figure 1. Outcomes of External Referrals for Lung Transplant Among Patients With COVID-19–Associated Acute Respiratory Distress Syndrome as of December 21, 2021.
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared).
aTwelve of these patients were deemed to have potential for lung recovery and were declined lung transplant.
bPayor refused to authorize lung transplant or declined the transplant if not performed at preferred centers.
During the study period, 49 patients with COVID-19–associated ARDS were treated with ECMO for more than 28 days. Of these, 17 (34.7%) underwent lung transplant at our medical center and were included in this study, and 32 (65.3%) were deemed not to be suitable candidates for lung transplant. Comparing the 49 patients receiving prolonged ECMO, the patients who did not receive a transplant were of similar age (median, 52 years) as the patients who did receive a transplant (median, 53 years) but had a higher mean BMI (32.1 vs 27.1) and were more likely to have severe hypoxemia (Pao2, 91.6 vs 153.2 mm Hg; eTable 1 in the Supplement).
Study Population
The study included 102 consecutive patients who underwent lung transplant between January 21, 2020, and September 30, 2021, at Northwestern University Medical Center in Chicago, Illinois. Clinical characteristics of these patients are shown in Table 1; eTable 2 in the Supplement provides information about the 30 lung transplant recipients who had COVID-19–associated ARDS, including details about their operative and postoperative course. During their ICU stay prior to lung transplant, 29 patients (97%) with COVID-19–associated ARDS underwent invasive mechanical ventilation and 1 (3%) was supported with noninvasive mechanical ventilation (eTable 2 in the Supplement). Of the 72 patients without COVID-19, interstitial lung disease and chronic obstructive pulmonary disease were the most common causes of chronic end-stage lung disease (Table 1). The median age of lung transplant recipients was 53 years (range, 27-62) in the COVID-19 lung transplant group and 62 years (range, 22-74) among those without COVID-19 but the sex distribution was similar between groups (43% vs 44% women, respectively). None of the patients who had COVID-19–associated ARDS was vaccinated for COVID-19 vs 1 patient (3.3%) in the non–COVID-19 lung transplant group (Table 1).
Table 1. Characteristics of Lung Transplant Recipients.
| Variable | Patients, No. (%) | |
|---|---|---|
| COVID-19 (n = 30) | Non–COVID-19 (n = 72) | |
| Demographics | ||
| Age, y | ||
| Median (IQR) | 53 (46-59) | 62 (52-69) |
| ≤40 | 5 (16.7) | 9 (12.5) |
| 41-55 | 14 (46.7) | 11 (15.3) |
| 56-65 | 9 (30) | 26 (36.1) |
| >65 | 2 (6.7) | 25 (34.7) |
| Sex | ||
| Women | 13 (43.3) | 32 (44.4) |
| Men | 17 (56.7) | 40 (55.6) |
| Race and ethnicity | ||
| Asian | 3 (10) | 4 (5.6) |
| Hispanic | 11 (36.7) | 7 (9.7) |
| Non-Hispanic | ||
| Black | 3 (10) | 7 (9.7) |
| White | 11 (36.7) | 46 (63.9) |
| Othera | 2 (6.7) | 8 (11.1) |
| Body mass index, median (IQR)b | 26.7 (24.4-28.9) | 27 (22.5-29.4) |
| Medical history | ||
| History of smoking | 3 (10) | 31 (43.1) |
| Hypertension | 15 (50) | 37 (51.4) |
| Diabetes | 9 (30) | 19 (26.4) |
| Chronic kidney diseasec | 0 | 4 (5.6) |
| Etiology of chronic lung disease | ||
| Interstitial lung disease | 0 | 35 (48.6) |
| Chronic obstructive pulmonary disease | 0 | 23 (31.9) |
| Pulmonary hypertension | 0 | 8 (11.1) |
| Othersd | 0 | 4 (5.5) |
| Cystic fibrosis | 0 | 2 (2.8) |
| Laboratory, median (IQR) | ||
| Hemoglobin, g/dL | 8.9 (7.3-10.2) | 12.2 (10.2-14) |
| White blood cell count, 1000/μL | 9.6 (7.2-11.7) | 9.1 (7.7-11.9) |
| Platelets, 1000/μL | 223 (186-277.8) | 236.5 (180-291) |
| Sodium, mEq/L | 140 (139-145.5) | 139.5 (138-141) |
| Blood urea nitrogen, mg/dL | 16 (14-29) | 13.5 (11.8-16) |
| Creatinine, mg/dL | 0.6 (0.4-0.8) | 0.7 (0.6-0.9) |
| Alanine aminotransferase, U/L | 21.5 (12.5-29.3) | 16.5 (11-24.3) |
| Aspartate aminotransferase, U/L | 21.5 (16.3-30) | 24.5 (18-33.8) |
| Albumin, g/dL | 3.7 (3.2-4) | 3.9 (3.5-4.3) |
| Total bilirubin, mg/dL | 0.5 (0.4-0.8) | 0.6 (0.4-1) |
| International normalized ratio | 1.1 (1-1.2) | 1.1 (1-1.1) |
| Arterial blood gas from the day of transplant | ||
| pH, median (IQR) | 7.41 (7.34-7.45) | 7.37 (7.34-7.41) |
| Paco2, median (IQR), mm Hg | 50.5 (41-58) | 49 (42-53.5) |
| Pao2, median (IQR), mm Hg | 173 (126.3-230.5) | 337 (194.5-390) |
| Panel-reactive antibodiese | 16 (53.3) | 29 (40.3) |
| Donor-specific antibodiesf | 7 (23.3) | 4 (5.6) |
| Pretransplant status | ||
| Karnofsky Performance Status, median (IQR)g | 30 (20-40) | 50 (40-60) |
| Respiratory support | ||
| Venovenous ECMO | 17 (56.7) | 1 (1.4) |
| Nasal cannula oxygen | 7 (23.3) | 65 (90.3) |
| Invasive mechanical ventilation | 4 (13.3) | 2 (2.8) |
| High-flow nasal cannula oxygen | 2 (6.7) | 3 (4.2) |
| Thrombotic events while receiving ECMO | ||
| Deep vein thrombosis | 6/17 (35.2) | 0/1 |
| Pulmonary embolism | 0/17 | 0/1 |
| Circuit thrombosis | 0/17 | 0/1 |
| Lung allocation score, median (IQR)h | 85.8 (69.4-87.5) | 46.7 (38.9-54.3) |
| Time on waitlist, median (IQR), d | 11.5 (5.0-26) | 15 (6.0-60) |
| COVID-19 vaccination prior to transplant | 0 | 1 (3.3) |
| Donor information | ||
| Age, median (IQR), y | 29 (21-39) | 31 (23.3-39.5) |
| Sex | ||
| Female | 9 (30) | 22 (30.6) |
| Male | 21 (70) | 50 (69.4) |
| Cause of donor death | ||
| Head trauma | 15 (50) | 29 (40.3) |
| Drug overdose | 12 (40) | 27 (37.5) |
| Other | 3 (10) | 16 (22.2) |
Abbreviation: ECMO, extracorporeal membrane oxygenation.
SI conversion factors: To convert alanine aminotransferase and aspartate aminotransferase to μkat/L, multiply by 0.0167; bilirubin to μmol/L, multiply by 17.104; blood urea nitrogen to mmol/L, multiply by 0.357; and creatinine to μmol/L, multiply by 88.4.
Other included American Indian, Middle Eastern, and unknown.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Chronic kidney disease is defined using estimated glomerular filtration rate less than 60 mL/min.
Includes α1-antitrypsin deficiency, primary ciliary dyskinesia, and mucinous adenocarcinoma.
Panel-reactive antibodies are a group of antibodies in a recipient test serum that are reactive against known specific antigens in a panel of test cells or purified HLA antigens from cells.
Donor-specific antibodies are antibodies specific to the donor’s HLA typing and checked before lung transplant.
Karnofsky Performance Status is used to determine functional status based on patient’s ability to carry out daily activities with or without assistance. The Karnofsky Performance Status score ranges from 0 to 100, with scores greater than 80 indicating normal; 50 to 70, unable to work and needs varying amounts of assistance; and less than 40, needs institutional or hospital care.
Lung allocation score is calculated using age, body mass index, etiology of lung failure, functional status, assisted ventilation, oxygen status, pulmonary artery systolic pressure, mean pulmonary artery pressure, cardiac index, 6-minute walk distance, total bilirubin level, and serum creatinine level. This score has a range from 0 to 100. Higher lung allocation scores indicate the patient is severely ill. The score calculator can be found at https://optn.transplant.hrsa.gov/data/allocation-calculators/las-calculator/.
Immediately prior to lung transplant, 17 patients (56.7%) with COVID-19–associated ARDS were being treated with ECMO and 4 (13.3%) were receiving invasive mechanical ventilation vs 1 (1.4%) receiving ECMO and 2 (2.8%) receiving invasive mechanical ventilation in the non–COVID-19 group. The median preoperative Karnofsky Performance Status score was 30 in the COVID-19 cohort and 50 in the non–COVID-19 cohort, and the median lung allocation scores were 85.8 and 46.7, respectively. The median lung transplant wait time was 11.5 days (IQR, 5.2-26.0) for the COVID-19 cohort vs 15 days (IQR, 6.0-60.0) for patients without COVID-19. The waitlist mortality was 18.9% among patients who had COVID-19–associated ARDS and 7.6% in patients without COVID-19 awaiting lung transplant.
Intraoperative and Postoperative Lung Transplant Outcomes
Comparing the patients who had COVID-19–associated ARDS with the non–COVID-19 cohort, the median duration of surgery was 8.5 vs 7.4 hours, the median allograft ischemic time (calculated from termination of cardiac circulation in the donor to reperfusion of the allograft following implantation) was 5.6 vs 4.7 hours, intraoperative venoarterial ECMO use was 96.7% vs 62.5%, and the median units of blood products transfused were 6.5 vs 0 units packed red blood cells, 1 vs 0 units of fresh frozen plasma, and 0.5 vs 0 units of platelets (Table 2). Postoperatively, the incidence of PGD (grades 1 to 3) at 72 hours was 70.0% vs 20.8%, the median number of ventilator days was 6.5 vs 2, the median ICU stay was 18 vs 9 days, the median hospital stay was 28.5 vs 16 days, and permanent hemodialysis was used by 13.3% vs 5.5% of patients who had COVID-19–associated ARDS compared with the non–COVID-19 cohort, respectively (Table 2). The rate of improvement in the Karnofsky Performance Status after lung transplant was 84.8% in the COVID-19 group vs 11% in the non–COVID-19 cohort (eFigure 1 in the Supplement). Antibody-mediated rejection occurred in none of the lung transplant recipients who had COVID-19–associated ARDS vs 12.5% in the non–COVID-19 cohort.
Table 2. Intra- and Postoperative Features of Lung Transplant Recipients.
| Variable | Patients, No. (%) | |
|---|---|---|
| COVID-19 (n = 30) | Non–COVID-19 (n = 72) | |
| Consent obtained from transplant recipientsa | 29 (96.7) | 72 (100) |
| Intraoperative | ||
| Operating time, median (IQR), h | 8.5 (8-9.7) | 7.4 (5.8-8.3) |
| Blood transfusion, median (IQR), units | ||
| Red blood cell | 6.5 (2-10) | 0 (0-2) |
| Fresh frozen plasma | 1 (0-5) | 0 (0-0) |
| Platelet | 0.5 (0-2) | 0 (0-0) |
| Venoarterial ECMO use | 29 (96.7) | 45 (62.5) |
| Venoarterial ECMO time, median (IQR), h | 3.1 (2.6-3.4) | 2.5 (0-3.3) |
| Allograft ischemic time, median (IQR), hb | 5.6 (5-6) | 4.7 (3.9-5.5) |
| Postoperative | ||
| Venovenous ECMO use | 13 (30) | 3 (4.1) |
| Venovenous ECMO duration, median (IQR), d | 0 (0-4) | 0 (0-0) |
| Acute kidney injuryc | 16 (53.3) | 26 (36.1) |
| Dialysis | 7 (23.3) | 8 (11.1) |
| Temporary | 3 (10.0) | 4 (5.5) |
| Permanent | 4 (13.3) | 4 (5.5) |
| Primary graft dysfunction grade 1-3 within 72 h | 21 (70.0) | 15 (20.8) |
| Post–lung transplant ICU stay, median (IQR), d | 18 (12-24.5) | 9 (6-15) |
| Posttransplant ventilator use, median (IQR), d | 6.5 (2-17) | 2 (1-4) |
| Pleural drainage, d | 19 (14.3-23.8) | 11 (8.8-18.8) |
| Posttransplant hospital stay, median (IQR), d | 28.5 (18.3-37.8) | 16 (11-28.5) |
| Improvement in Karnofsky Performance Status, %d | 84.8 | 11 |
| Histological acute rejection during study periode | 0 | 9 (12.5) |
| Creatinine level 1 mo after lung transplant, median (IQR) | 0.9 (0.6-1.2) | 0.9 (0.8-1.2) |
| Follow-up, median (IQR), d | 351 (176-555) | 488 (368-570) |
| No. of patients alivef | 30 (100) | 60 (83.3) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Medical power of attorney was used to obtain consent for lung transplant in 1 patient who was unable to provide consent.
Allograft ischemic time is defined as time from termination of cardiac circulation in the donor to reperfusion of the allograft.
Acute kidney injury is defined using the Risk, Failure, Loss of kidney function, and End-stage kidney disease classification as an increase in serum creatinine to 1.5 times baseline or higher or urine output less than 0.5 mL/kg/h for 6 hours or longer.
Karnofsky Performance Status is assessed prior to and then at 30 days after lung transplant and the percentage improvement was calculated. The Karnofsky Performance Status score ranges from 0 to 100, with scores greater than 80 indicating normal; 50 to 70, unable to work and needs varying amounts of assistance; and less than 40, needs institutional or hospital care.
Acute rejection was determined using bronchoscopic transbronchial biopsy and standard transplant rejection criteria on histology.
As of November 15, 2021.
Post–Lung Transplant Survival
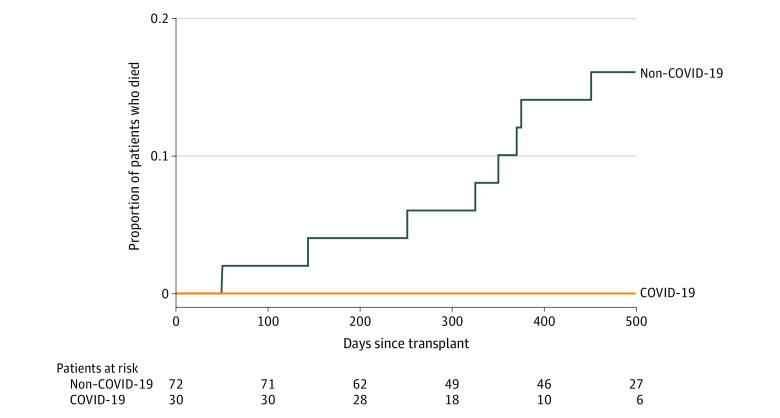
The median post–lung transplant follow-up was 351 days (IQR, 176-555) for patients who had COVID-19–associated ARDS vs 488 days (IQR, 368-570) in the non–COVID-19 cohort (eFigure 2 in the Supplement).
As of November 15, 2021, survival of lung transplant recipients who had COVID-19–associated ARDS was 100% (30/30) vs 83.3% (60/72) in the non–COVID-19 lung transplant cohort (Figure 2).
Figure 2. Posttransplant Survival of Study Cohorts.
Survival of concurrent transplants performed for COVID-19–associated acute respiratory distress syndrome and chronic end-stage lung diseases (non–COVID-19). The median post–lung transplant observation time was 351 days (95% CI, 318-397) for patients with COVID-19–associated acute respiratory distress syndrome and 488 days (95% CI, 429-552) for patients in the non–COVID-19 cohort.
Discussion
This single-center case series describes the clinical characteristics, intraoperative procedures, postoperative complications, and survival of a cohort of lung transplant recipients who had COVID-19–associated ARDS, with data about non–COVID-19–related lung transplant recipients presented for context.
As the COVID-19 pandemic evolves across the globe, lung transplantation will continue to be considered as a salvage therapy for patients with COVID-19–associated ARDS who do not recover despite maximum ventilatory support, use of ECMO, and optimal medical care.17,18,19 To our knowledge, this single-center case series represents the largest series to date reporting the posttransplant survival of patients undergoing lung transplant for COVID-19–associated ARDS.
Prior to lung transplant, patients with COVID-19–associated ARDS have prolonged respiratory failure when compared with patients with pneumonia secondary to other respiratory pathogens,20,21 and as a result, they commonly develop complications of prolonged critical illness including malnutrition and neuromuscular deconditioning.22 Many patients with COVID-19–associated ARDS also develop severe pleural adhesions and secondary pneumonias from invasive nosocomial pathogens.23,24 These complications raise concerns about the technical feasibility of lung transplant as well as posttransplant outcomes in this critically ill cohort. The findings of this study suggest that despite these concerns, successful outcomes can be achieved in these patients following lung transplant.
All patients in this study with COVID-19–associated ARDS underwent double lung transplant due to the severity of lung damage and the high incidence of pulmonary necrosis, bronchiectasis, ventilator-associated pneumonia caused by multidrug-resistant pathogens, and pulmonary hypertension with concomitant right ventricular dysfunction observed among these patients.7,25 Use of cardiopulmonary bypass may be associated with worse outcomes following lung transplant.26 However, due to the high incidence of pulmonary hypertension and right ventricular dysfunction observed in patients with COVID-19–associated ARDS,7 and concerns about the severity of pleural adhesions, intraoperative cardiopulmonary support was necessary. In an attempt to reduce total blood loss and decrease the need for blood transfusion anticipated with cardiopulmonary bypass,27,28,29 venoarterial ECMO was routinely used for the patients with COVID-19 in this study.
Patients with COVID-19–associated ARDS can develop ventilator-associated pneumonia, often with resistant pathogens.24 However, during surveillance bronchoscopies performed at 1, 3, 6, 9, and 12 months after lung transplant, none of the patients with COVID-19 had evidence of nosocomial pathogens. In addition, none demonstrated recurrence of SARS-CoV-2 on bronchoscopy following lung transplant, suggesting that the preoperative approach of assessing for clearance of SARS-CoV-2 using polymerase chain reaction testing of 2 consecutive lower respiratory fluid samples 24 hours apart was appropriate.30,31
Patients with COVID-19–associated ARDS had high intraoperative blood loss that required transfusion of a large number of blood products, likely reflecting the increased technical complexity of the procedure in the COVID-19 cohort.27 Despite high rates of primary graft dysfunction, patients with COVID-19–associated ARDS had a substantial improvement in their Karnofsky Performance Status after lung transplant. This improvement in Karnofsky Performance Status may have resulted from their relatively young age and relatively healthy baseline medical condition prior to the onset of COVID-19 infection.
None of the COVID-19–associated ARDS lung transplant recipients demonstrated acute rejection or development of de novo donor-specific antibodies, known risk factors for chronic lung allograft dysfunction.32 Possible explanations for the lack of rejection and donor-specific antibodies may include development of immune “accommodation”33 resulting from dilution of HLA antibody titers due to high intraoperative blood loss and increased blood transfusion, which are mechanisms thought to protect cardiac allografts from acute rejection.34 However, follow-up of longer duration is necessary to better determine the incidence of allograft rejection in patients who have undergone lung transplant for COVID-19–associated ARDS. Additionally, patients with COVID-19 may develop long-term secondary effects from their critical illness such as kidney failure.35 In this study, a higher percentage of patients with COVID-19–associated ARDS required long-term hemodialysis (13.3%) after lung transplant compared with the non–COVID-19 cohort (5.5%). Follow-up is needed to determine the long-term effects of kidney failure on patients in this study who required hemodialysis after lung transplant.
The median time from onset of COVID-19–associated ARDS to lung transplant in this study was 104 days. Several factors explain this long duration, including allowance of time for possible lung recovery, delays in the transplant referral and evaluation process, and limited ICU bed availability during COVID-19 surges. As discussed in the data presented regarding the screening process for transplant, 36 patients died while being evaluated for transplant or awaiting transfer after completion of lung transplant evaluation. Additionally, 12 patients deemed to have potential for lung recovery who were receiving ECMO died. It cannot be determined whether those patients would have benefited from earlier consideration for lung transplant. This experience underscores the importance of future studies to better inform decision-making to maximize the benefit of lung transplant while avoiding premature consideration of lung transplant.7
While offering double lung transplants to patients with COVID-19–associated ARDS has the potential to increase demand-supply discordance, this effect has not been documented in the medical literature to date.7 During this study, no increase in waitlist mortality was observed in the non–COVID-19 cohort at our center with the introduction of lung transplant for patients with COVID-19–associated ARDS; however, this needs to be studied in other centers with a different supply of donor lungs.
Recommendations for consideration for lung transplant evaluation in patients with COVID-19–associated ARDS have been proposed but consensus guidelines are lacking.7,8,17,36 Given the lack of clinical tests or biomarkers that indicate irreversible lung damage, the decision to proceed with lung transplant evaluation should be made by a multidisciplinary care team that specializes in ARDS management and lung transplantation. Due to the inherent uncertainty in prognosis, the progress of each patient needs to be determined longitudinally over time. With advanced ventilatory and extracorporeal support, more than 50% to 60% of patients with COVID-19–associated ARDS can demonstrate lung recovery.37 Because both the pre- and posttransplant care of patients with COVID-19–associated ARDS is highly complex, expensive, and resource intensive, these patients should preferably undergo lung transplant at high-volume centers with experienced multidisciplinary teams to provide optimal care.
Limitations
This study has several limitations. First, it was performed at a single center and the COVID-19 lung transplant cohort had a sample size of only 30 patients. Second, the patients who had COVID-19–associated ARDS were carefully selected for lung transplant; a less stringent approach to lung transplant in patients with this condition would likely not yield similar results. Third, posttransplant outcomes in this critically ill cohort are likely to vary depending on patient heterogeneity and center experience. Therefore, outcomes reported in this study may differ from national registries.
Conclusions
In this single-center case series of 102 consecutive patients who underwent a lung transplant between January 21, 2020, and September 30, 2021, survival was 100% in the 30 patients who had COVID-19–associated ARDS as of November 15, 2021.
eMethods
eFigure 1. Karnofsky Performance Status for COVID-19 and non-COVID-19 Patients Pre- and Post- Lung Transplant
eFigure 2. Cumulative Curve Showing Dates of All Lung Transplants Performed From January 2020 Through September 2021
eTable 1. Clinical Profile of Patients With COVID-19-Associated ARDS on ECMO Support for Greater Than 28 Days
eTable 2. Patient Characteristics, Intra-operative and Post-operative Findings of COVID-19-Associated ARDS Lung Transplant Recipients
eReferences
References
- 1.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;382(25):2411-2418. doi: 10.1056/NEJMoa2012410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of COVID-19: preliminary report: reply. N Engl J Med. 2020;383(10):994. doi: 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 5.Bharat A, Querrey M, Markov NS, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12(574):eabe4282. doi: 10.1126/scitranslmed.abe4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Mark SC, Hoek RAS, Hellemons ME. Developments in lung transplantation over the past decade. Eur Respir Rev. 2020;29(157):190132. doi: 10.1183/16000617.0132-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharat A, Machuca TN, Querrey M, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9(5):487-497. doi: 10.1016/S2213-2600(21)00077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machuca TN, Cypel M, Bharat A. Comment on “Let’s build bridges to recovery in COVID-19 ARDS, not burn them!” Ann Surg. 2021;274(6):e870-e871. doi: 10.1097/SLA.0000000000004623 [DOI] [PubMed] [Google Scholar]
- 9.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine . An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253-1263. doi: 10.1164/rccm.201703-0548ST [DOI] [PubMed] [Google Scholar]
- 10.Badulak J, Antonini MV, Stead CM, et al. ; ELSO COVID-19 Working Group Members . Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67(5):485-495. doi: 10.1097/MAT.0000000000001422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurihara C, Walter JM, Karim A, et al. Feasibility of venovenous extracorporeal membrane oxygenation without systemic anticoagulation. Ann Thorac Surg. 2020;110(4):1209-1215. doi: 10.1016/j.athoracsur.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurihara C, Manerikar A, Gao CA, et al. Outcomes after extracorporeal membrane oxygenation support in COVID-19 and non–COVID-19 patients. Artif Organs. 2021. doi: 10.1111/aor.14090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manerikar A, Watanabe S, Kandula V, et al. Indwelling central venous catheters drive bloodstream infection during veno-venous extracorporeal membrane oxygenation support. ASAIO J. 2021. doi: 10.1097/MAT.0000000000001575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 15.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: definition and grading: a 2016 consensus group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1097-1103. doi: 10.1016/j.healun.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 16.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup . Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-R212. doi: 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharat A, Hoetzenecker K, Machuca TN. Lung transplantation for COVID-19-associated ARDS: authors’ reply. Lancet Respir Med. 2021;9(9):e90. doi: 10.1016/S2213-2600(21)00288-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669-677. doi: 10.1016/S1473-3099(20)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362-367. doi: 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dmytriw AA, Chibbar R, Chen PPY, et al. Outcomes of acute respiratory distress syndrome in COVID-19 patients compared to the general population: a systematic review and meta-analysis. Expert Rev Respir Med. 2021;15(10):1347-1354. doi: 10.1080/17476348.2021.1920927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe COVID-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372(n436):n436. doi: 10.1136/bmj.n436 [DOI] [PubMed] [Google Scholar]
- 22.Barbaro RP, MacLaren G, Boonstra PS, et al. ; Extracorporeal Life Support Organization . Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071-1078. doi: 10.1016/S0140-6736(20)32008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickens CO, Gao CA, Cuttica MJ, et al. ; NU COVID Investigators . Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am J Respir Crit Care Med. 2021;204(8):921-932. doi: 10.1164/rccm.202106-1354OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budinger GRS, Misharin AV, Ridge KM, Singer BD, Wunderink RG. Distinctive features of severe SARS-CoV-2 pneumonia. J Clin Invest. 2021;131(14):149412. doi: 10.1172/JCI149412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weingarten N, Schraufnagel D, Plitt G, Zaki A, Ayyat KS, Elgharably H. Comparison of mechanical cardiopulmonary support strategies during lung transplantation. Expert Rev Med Devices. 2020;17(10):1075-1093. doi: 10.1080/17434440.2020.1841630 [DOI] [PubMed] [Google Scholar]
- 27.Bermudez CA, Shiose A, Esper SA, et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann Thorac Surg. 2014;98(6):1936-1942. doi: 10.1016/j.athoracsur.2014.06.072 [DOI] [PubMed] [Google Scholar]
- 28.Moreno Garijo J, Cypel M, McRae K, Machuca T, Cunningham V, Slinger P. The evolving role of extracorporeal membrane oxygenation in lung transplantation: implications for anesthetic management. J Cardiothorac Vasc Anesth. 2019;33(7):1995-2006. doi: 10.1053/j.jvca.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto K, Hoetzenecker K, Yeung JC, et al. Intraoperative extracorporeal support during lung transplantation in patients bridged with venovenous extracorporeal membrane oxygenation. J Heart Lung Transplant. 2018;37(12):1418-1424. doi: 10.1016/j.healun.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 30.Querrey M, Kurihara C, Manerikar A, et al. Lung donation following SARS-CoV-2 infection. Am J Transplant. 2021;21(12):4073-4078. doi: 10.1111/ajt.16777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis. 2020;71(10):2663-2666. doi: 10.1093/cid/ciaa638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd JL, Neely ML, Kopetskie H, et al. Risk factors for acute rejection in the first year after lung transplant: a multicenter study. Am J Respir Crit Care Med. 2020;202(4):576-585. doi: 10.1164/rccm.201910-1915OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bharat A, Saini D, Benshoff N, et al. Role of intra-islet endothelial cells in islet allo-immunity. Transplantation. 2007;84(10):1316-1323. doi: 10.1097/01.tp.0000288192.11396.70 [DOI] [PubMed] [Google Scholar]
- 34.Fernandez FG, Jaramillo A, Ewald G, et al. Blood transfusions decrease the incidence of acute rejection in cardiac allograft recipients. J Heart Lung Transplant. 2005;24(7 Suppl):S255-S261. doi: 10.1016/j.healun.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 35.Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in long COVID. J Am Soc Nephrol. 2021;32(11):2851-2862. doi: 10.1681/ASN.2021060734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cypel M, Keshavjee S. When to consider lung transplantation for COVID-19. Lancet Respir Med. 2020;8(10):944-946. doi: 10.1016/S2213-2600(20)30393-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbaro RP, MacLaren G, Boonstra PS, et al. ; Extracorporeal Life Support Organization . Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021;398(10307):1230-1238. doi: 10.1016/S0140-6736(21)01960-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Karnofsky Performance Status for COVID-19 and non-COVID-19 Patients Pre- and Post- Lung Transplant
eFigure 2. Cumulative Curve Showing Dates of All Lung Transplants Performed From January 2020 Through September 2021
eTable 1. Clinical Profile of Patients With COVID-19-Associated ARDS on ECMO Support for Greater Than 28 Days
eTable 2. Patient Characteristics, Intra-operative and Post-operative Findings of COVID-19-Associated ARDS Lung Transplant Recipients
eReferences