Abstract
Purpose:
Graft-versus-host disease (GVHD) is an important complication after human leukocyte antigen (HLA) haploidentical donor (HID) hematopoietic stem cell transplantation (HSCT), which may lead to poor prognosis. Our study intends to identify the efficacy and safety of mesenchymal stem cells (MSCs) for multidrug-resistant (MDR)-GVHD after HID HSCT.
Methods:
MDR-GVHD was referring to GVHD remaining no response to at least two types of therapy, and hUCB-MSCs were given at the dose of (1.0–2.0) × 106/kg once a week.
Results:
A total of 21 patients were enrolled in this retrospective study (acute GVHD (aGVHD): n = 14, chronic GVHD (cGVHD): n = 7). The median dose of MSCs was 1.2 × 106 cells/kg (range, 0.8–1.8 × 106) cells/kg, and the median numbers of infusion were 2 (range, 1–7) and 3 (range, 2–12) for MDR-aGVHD and MDR-cGVHD patients, respectively. In MDR-aGVHD patients, the overall response rate (ORR) was 57.1%, including 50.0% complete response (CR) and 7.1% partial response (PR), and the median time to response was 49.5 days (range, 16–118) days. The 2-year probability of overall survival after MSCs was 64.3%. Five patients (35.7%) developed infections after MSCs, and no obvious hematologic toxicities were observed. Five MDR-aGVHD patients died after MSCs treatments because of GVHD progression (n = 1), severe infection (bacterial central nervous system infection: n = 1; fungal pneumonia: n = 2), and poor graft function (n = 1). In MDR-cGVHD patients, three patients (42.9%) achieved PR after MSCs and the median time to response was 56 days (22–84) days. The ORRs for moderate and severe cGVHD were 50.0% and 33.3%, respectively. Four MDR-cGVHD patients died after MSCs treatments because of GVHD progression (n = 2), severe fungal pneumonia (n = 1), and relapse (n = 1).
Conclusion:
MSCs treatment may be safe and effective for MDR-GVHD after HID HSCT.
Keywords: graft-versus-host disease, haploidentical, hematopoietic stem cell transplantation, mesenchymal stem cells, multidrug resistant
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the most effective treatment for malignant hematologic diseases. 1 Graft-versus-host disease (GVHD) is a major complication and an important cause of non-relapse mortality after allo-HSCT. Although several methods had been used to induce immune tolerance, 2 there are still 40–50% and 30–70% of allo-HSCT recipients suffering from grade II to IV acute GVHD (aGVHD) and chronic GVHD (cGVHD), respectively.3–5
Corticosteroid is the first-line treatment for GVHD, but its response rate can only reach about 50% of patients with severe GVHD.6,7 There is no clear consensus on the best options for second- and third-line treatments of aGVHD and cGVHD yet. 8 In patients with steroid-refractory GVHD (SR-GVHD), 17–72% of patients remain unresponsive despite the availability of various second-line agents.7,9–11 There are few studies on salvage therapy for second-line treatment resistance (i.e. multidrug-resistant (MDR)-GVHD).
Mesenchymal stromal cells (MSCs) are a group of non-hematopoietic progenitor cells derived from the mesoderm of embryonic development with multispectral differentiation potential, which can be isolated from bone marrow, umbilical cord, adipose tissue, and so on. 12 MSCs have anti-inflammatory and tissue repair capabilities. In addition, MSCs can regulate T and B lymphocyte proliferation, activation and maturation, induce regulatory T lymphocyte production, and have regulatory functions on both the intrinsic and adaptive immune responses. Tissue repair and immunomodulatory functions of MSCs are the critical theoretical basis for their use in the treatment of GVHD. 13
There is a large amount of clinical data on MSCs in the treatment of GVHD in patients receiving identical sibling donors (ISDs) or unrelated donors (URDs) HSCT.14–19 With the development of HSCT, the haploidentical donors (HIDs) have become one of the most important alternative donors which could achieve similar clinical outcomes to ISDs and URDs.20–22 However, it is well-known that the incidence and severity of GVHD is higher23–25 and some post-transplant complications, such as viral reactivation and poor graft function (PGF), are more common in HID HSCT compared with those receiving ISD and URD HSCT.26–28 Although the study of Le Blanc et al. 29 included 18 HID HSCT recipients and the study of Cetin et al. 30 included 4 HID HSCT recipients, they did not identify the value of MSCs treatment in the specific population of HID HSCT recipients. Thus far, few studies had identified the efficacy and safety of MSCs treatment for GVHD in HID HSCT recipients, and the efficacy of MSCs in patients receiving HID HSCT remains unclear, particularly for those with MDR-GVHD.
Therefore, in this study, we aimed to identify the efficacy and safety of MSCs for the treatment of MDR-GVHD in HID HSCT recipients.
Method
Patients
Consecutive patients who received MSCs as salvage therapy for MDR-GVHD after HID HSCT 31 at the Peking University Institute of Hematology (PUIH) from 2016 to 2020 were enrolled in this retrospective study. The last follow-up visit was on October 30, 2020. The study protocol was approved by the institutional review board of Peking University People’s Hospital (Beijing, November 2016) and was conducted in accordance with the Declaration of Helsinki. The reporting of this study conformed to the STROBE statement. 32 Informed consent was obtained by all patients or their guardians.
Transplant regimen
For patients with hematologic malignancies, the preconditioning regimen mainly included cytarabine, busulfex, cyclophosphamide, simustine, and rabbit anti-thymocyte globulin (ATG).2,22,24 For patients with severe aplastic anemia, the preconditioning regimen mainly included busulfex, cyclophosphamide, fludarabine, and ATG. 33 We used cyclosporine A (CSA), mycophenolate mofetil (MMF), and methotrexate (MTX) to prevent GVHD. 34 As it was reported by Chen et al. 35 that the co-infusion of an unrelated cord blood unit may potentially improve the engraftment of HID HSCT, two patients with relatively higher donor-specific anti-human leukocyte antigen (HLA) antibodies levels (2000 ⩽ median fluorescent intensity ⩽ 10,000) received umbilical cord blood in their graft in this study. Particularly, for the patients receiving second transplantation for PGF, the mainly conditioning regimen included fludarabine and cyclophosphamide, 36 and GVHD prevention consisted of basixilimab 20 mg on days −1 and +4, plus CSA and MMF (Supplementary Methods). For the patients who had minimal residual disease or experienced relapse after allo-HSCT, modified donor lymphocyte infusion (DLI) was given according to our protocols (Supplementary Methods). 37
The diagnosis and treatment of GVHD
The diagnosis and grading of aGVHD was based on the international consensus criteria.38,39 When aGVHD was identified, corticosteroid and the optimized level of CSA applied first. 40 If the patients with SR-aGVHD, second-line treatments included basiliximab, MMF, tacrolimus, and ruxolitinib. The use of these drugs was mostly based on the competence and experience of each physician.
The diagnosis and grading of cGVHD was based on the consensus criteria of the National Institutes of Health (NIH).41,42 For patients with moderate or severe cGVHD, we first used the corticosteroid and/or CSA. 43 Second-line treatments include mercaptopurine (6-MP), MMF, ruxolitinib, basiliximab, and imatinib. The use of these drugs was also mostly based on the competence and experience of each physician.
Definition of MDR-GVHD
MDR-GVHD was defined as no improvement in GVHD after more than 2 types of treatments, regardless of GVHD prophylaxis. Patients with MDR-GVHD were eligible for MSCs treatment if they met the following criteria: (1) aGVHD progressed within 1 week or did not respond by 2 weeks after the start of second-line therapies or (2) cGVHD progressed within 4 weeks or did not respond by 8 weeks after the start of second-line therapies. 44
MSCs treatment
The human umbilical cord blood MSCs (hUCB-MSCs) were obtained from Beijing Engineering Lab for Cell Therapy (Beijing, China; aGVHD: n = 12; cGVHD: n = 5) and Beijing Cord Blood Bank (Beijing, China; aGVHD: n = 2; cGVHD: n = 2). The dose of MSCs was chosen based on the previous researches.29,45–47 Moreover, the hUCB-MSCs were mainly isolated from Wharton’s Jelly and perivascular tissues in the umbilical cord with informed consent of the mothers. The umbilical cord segments (about 10 cm) were cut longitudinally and minced into 3 cm sections. They were cultured in the serum-free culture system after dissecting into 1–3 mm3 pieces. The serum-free culture system included the UltraGRO™-Advanced (HPCFDCRL50, Helios Bioscience, America), which used platelet lysate. The cells were expanded until they reached subconfluence (80%) with changing the medium every week. The culture medium was poured out and the culture flask was washed with 0.9% normal saline twice, 1.5 mL of trypsin (0.125%) was added into the flask for digestion until the cells became round and suspended then 3 mL of medium was added to stop digestion. The cell suspension was centrifuged and cleaned then replated in the flask in the same culture conditions for passages. Cells were passaged until P5 and then the supernatant was tested for sterility and a small number of cells were taken for phenotypic testing. If cells passed all the tests, they would be conserved for clinical use. 48 It required to meet all the following criteria for clinical use: (1) all hUCB-MSCs were required to be within the 5th generation or less with the cell count of 5 × 107 (100 mL) and were spindle-shaped or fibroblast-like in vitro culture; (2) molecular marker detection of hUCB-MSCs requires CD34+/HLA-DR+/CD45+ ⩽ 2%; and CD44+/CD73+/CD90+/CD105+ ⩾ 95% (Beijing Cord Blood Bank) or CD73+/CD90+/CD105+ ⩾ 95% (Beijing Engineering Lab for Cell Therapy), (3) no viral, fungal, bacterial, or mycoplasma contamination, endotoxin ⩽ 0.5 EU/mL; (4) time from discharge to completion of infusion < 18 h, live cell ratio ⩾ 90% before infusion. Patients with a clinical diagnosis of MDR-GVHD were given hUCB-MSCs at the dose of (1.0–2.0) × 106/kg, once a week for 2–6 weeks.
The response evaluation of MDR-GVHD
Responses were evaluated every day after the first MSCs infusion. The definitions of response to GVHD treatment were based on the consensus criteria.49–51 The overall response rate (ORR) was defined as complete response (CR) plus partial response (PR).
Statistical analysis
Continuous variables were compared using the Mann–Whitney U-test; categorical variables were compared using χ 2 and Fisher’s exact tests. Survival was estimated by the Kaplan–Meier method. Univariable and multivariable Cox regression analysis were used to estimate hazard ratios (HRs) for clinical outcomes. The factors associated with the clinical outcomes with p < 0.1 by univariable analysis were included in the multivariable analysis (Supplementary Methods). The p values were two-sided, and p < 0.05 was considered to be statistically significant. The SPSS 20 (SPSS Inc./IBM, Armonk, NY, USA) and the R software package (version 4.0.0; http://www.r-project.org) were used for data analysis.
Result
Patient characteristics
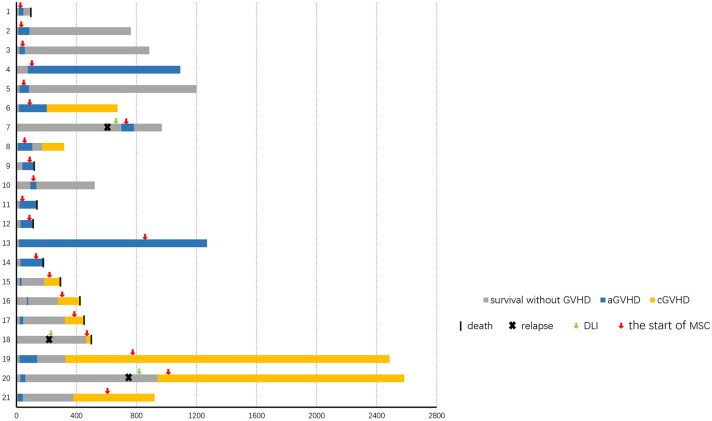
A total of 21 patients were included in this study (Table 1, Figure 1, Supplementary Table 1 and 2). The median time of aGVHD (n = 14) and cGVHD (n = 7) diagnosis was at 21 days (range, 11–699) days and at 326 days (range, 185–940) days after HSCT, respectively.
Table 1.
Patient characteristics of aGVHD and cGVHD.
| Characteristics | aGVHD (n = 14) | cGVHD (n = 7) |
|---|---|---|
| Age at HSCT, median (range) | 19 (6–54) | 24 (9–59) |
| Age at HSCT, n (%) | ||
| <18 years | 6 (42.9) | 2 (28.6) |
| ⩾18 years | 8 (57.1) | 5 (71.4) |
| Male sex, n (%) | 6 (42.9) | 2 (28.6) |
| Underlying disease, n (%) | ||
| Acute myeloid leukemia | 4 (28.6) | 5 (71.4) |
| Acute lymphoblastic leukemia | 4 (28.6) | 0 (0) |
| Myelodysplastic syndrome | 1 (7.1) | 1 (14.3) |
| Aplastic anemia | 3 (21.4) | 1 (14.3) |
| Fanconi anemia | 2 (14.3) | 0 (0) |
| Graft type, n (%) | ||
| Bone marrow and peripheral blood stem cells | 13 (92.9) | 7 (100) |
| Peripheral blood stem cells | 1 (7.1) | 0 (0) |
| Donor–recipient relationship, n (%) | ||
| Father–child | 8 (57.1) | 4 (57.1) |
| Mother–child | 3 (21.4) | 1 (14.3) |
| Sibling–sibling | 2 (14.3) | 1 (14.3) |
| Child–parent | 1 (7.1) | 1 (14.3) |
| Donor–recipient sex matched, n (%) | ||
| Male to male | 5 (35.7) | 2 (28.6) |
| Male to female | 6 (42.9) | 3 (42.8) |
| Female to male | 1 (7.1) | 0 (0) |
| Female to female | 2 (14.3) | 2 (28.6) |
| Number of HLA-A, HLA-B, and HLA-DR mismatches, n (%) | ||
| 1 | 0 (0) | 0 (0) |
| 2 | 2 (14.3) | 0 (0) |
| 3 | 12 (85.7) | 7 (100) |
| Donor–recipient blood type matched, n (%) a | ||
| Matched | 5 (35.7) | 6 (85.7) |
| Major mismatched | 5 (35.7) | 1 (14.3) |
| Minor mismatched | 4 (28.6) | 0 (0) |
| Major and minor mismatched | 0 (0) | 0 (0) |
aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation.
Minor ABO mismatched indicated that donor possessed isohemagglutinins against recipient red cells, including the following blood group combinations: O (donor) into A, B, or AB (recipient), and A or B (donor) into AB (recipient). Major ABO mismatched indicated that recipient possessed isohemagglutinins against donor red cells, including the following blood group combinations: A, B, or AB (donor) into O (recipient), and AB (donor) into A or B (recipient). Major–minor mismatched indicated that both donor and recipient possessed isohemagglutinins to each other; A into B and vice versa.
Figure 1.
Response. Swimmer plot displaying all patients who received MSCs treatments for MDR-GVHD.
The characteristics of aGVHD before MSCs treatment were shown in Table 2, and the median time from aGVHD occurrence to MSCs treatment was 22.5 days (range, 7–142 days). Two patients and 12 patients received MSCs treatments because of aGVHD progression and resistance to other second-line therapy, respectively. The characteristics of cGVHD before MSCs treatment were shown in Table 3, and the median time from cGVHD occurrence to MSCs treatment was 102 days (range, 19–456 days). Two patients and 5 patients received MSCs treatments because of cGVHD progression and resistance to other second-line therapies, respectively. One and 2 patients experienced aGVHD and cGVHD, respectively, after modified DLI.
Table 2.
Characteristics of aGVHD before MSC treatment.
| Characteristics of aGVHD | aGVHD (n = 14) |
|---|---|
| Severity of aGVHD, n (%) | |
| Grade II | 4 (28.6) |
| Grade III | 6 (42.8) |
| Grade IV | 4 (28.6) |
| Site of aGVHD, n (%) | |
| Skin | 9 (64.3) |
| GI | 13 (92.9) |
| Liver | 1 (7.1) |
| Number of sites, n (%) | |
| 1 | 6 (42.9) |
| 2 | 7 (50.0) |
| 3 | 1 (7.1) |
| Total aGVHD treatments before MSC, median (range) | 4 (3–5) |
| Second-line aGVHD treatments before MSC, n (%) | |
| 1 | 5 (35.7) |
| 2 | 6 (42.9) |
| ⩾3 | 3 (21.4) |
aGVHD, acute graft-versus-host disease; GI, gastrointestinal tract; MSC, mesenchymal stromal cell.
Table 3.
Characteristics of cGVHD before MSC treatment.
| Characteristics of cGVHD | cGHVD group (n = 7) |
|---|---|
| Severity of cGVHD, n (%) | |
| Moderate | 4 (57.1) |
| Severe | 3 (42.9) |
| Site of cGVHD, n (%) | |
| Skin | 5 (71.4) |
| Liver | 5 (71.4) |
| Eye | 2 (28.6) |
| Lung | 2 (28.6) |
| GI | 2 (28.6) |
| Number of sites, n (%) | |
| 1 | 1 (14.3) |
| ⩾1 | 6 (85.7) |
| Total cGVHD treatments before MSC, median (range) | 3 (2–5) |
| Second-line cGVHD treatments before MSC, n (%) | |
| 2 | 1 (14.3) |
| ⩾3 | 6 (85.7) |
cGVHD, chronic graft-versus-host disease; GI, gastrointestinal tract; MSC, mesenchymal stromal cell.
The median dose of MSCs was 1.2 × 106 cells/kg (range, 0.8–1.8 × 106) cells/kg, and the median numbers of infusion were 2 (range, 1–7) and 3 (range, 2–12) for MDR-aGVHD and MDR-cGVHD patients, respectively.
Response
aGVHD
The ORRs at 28 days and at any time were 21.4% and 57.1%, respectively. The median time of response was 49.5 days (range,16–118) days. The ORRs at any time were 100% (4/4) and 40% (4/10) for grades II and III–IV aGVHD, respectively (Figure 2(a)). The ORRs at any time were 66.7% (6/9), 61.5% (8/13), and 100% (1/1) for skin, gastrointestinal (GI), and liver aGVHD, respectively (Figure 2(b)). The ORRs were 40.0% (2/5) and 66.7% (6/9) for patients with 1 and 2–3 organs involvement, respectively (Figure 2(c)). The ORRs were 80.0% (4/5) and 44.4% (4/9) for patients with 2 and ⩾3 treatments prior or in concomitance to MSCs treatment, respectively (Figure 2(d)). Three patients experienced cGVHD occurrence after MSCs treatment and were all controlled without reuse of MSCs infusion.
Figure 2.
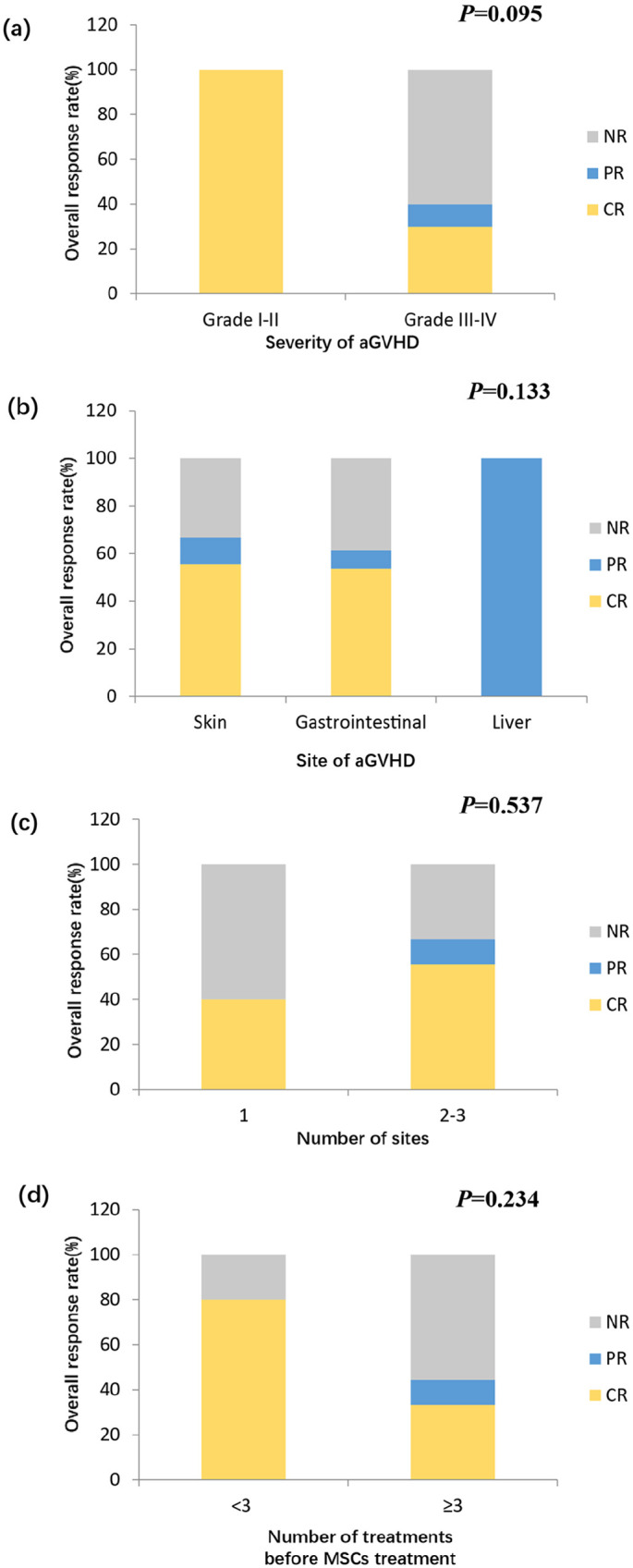
Response rate of MDR-aGVHD patients after MSCs treatment. Patients are grouped by (a) severity of aGVHD; (b) site of aGVHD; (c) number of involved sites; (d) number of treatments before MSCs treatment.
cGVHD
The ORRs at 28 days and at the time of final follow-up were 14.3% (1/7) and 42.9% (3/7), respectively. The median time of response was 56 days (range, 22–84) days. The ORRs of moderate and severe cGVHD were 50.0% (2/4) and 33.3% (1/3), respectively (Figure 3(a)). The ORRs for involved skin, GI, eyes, liver, and lung cGVHD were 40% (2/5), 50% (1/2), 50% (1/2), 40% (2/5), and 0% (0/2), respectively (Figure 3(b)). The ORR of 1–2 involved organs (75%, 3/4) seemed to be higher than that of more than two involved organs (0%, 0/3; Figure 3(c)). The ORRs were 0.0% (0/1) and 50.0% (3/6) for patients with 2 and ⩾3 treatments prior or in concomitance to MSCs treatment, respectively (Figure 3(d)).
Figure 3.
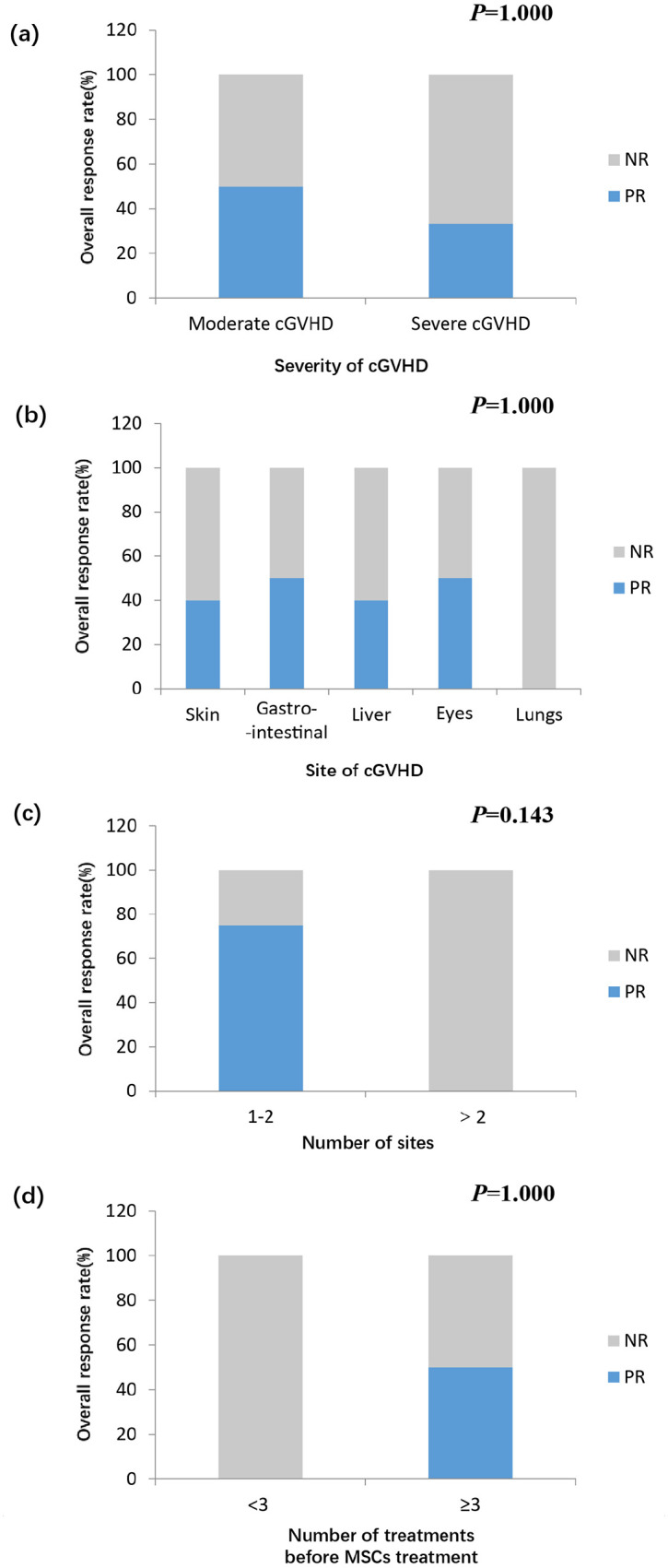
Response rate of MDR-cGVHD patients after MSCs treatment. Patients are grouped by (a) severity of cGVHD; (b) site of cGVHD; (c) number of involved sites; and (d) number of treatments before MSCs treatment.
Toxicities
No acute allergic reactions such as fever, rash, and acute laryngeal edema, occurred during or shortly after the infusion of hUCB-MSCs. Neither did infusion-related liver or kidney injury happen. No hematological toxicity was observed after MSCs treatment. No hUCB-MSCs-related tumors were observed. The infections events were showed as follows.
Infections events of aGVHD patients
Infection occurred in 5 (35.7%) patients, three had one type of infection and two had two types of infections (cytomegaloviremia plus central bacterial infection: 1; cytomegaloviremia plus Epstein-Barr viremia: 1). The incidence of cytomegaloviremia was 28.6% (4/14) with a median time of 32.5 days (range, 14–105) days after MSCs treatment. None of them experienced cytomegalovirus (CMV) disease. The rates of Epstein-Barr viremia, bacterial sepsis, and bacterial central infection after MSCs treatment were 7.1% (1/14), 7.1% (1/14), and 7.1% (1/14), respectively.
Infections events of cGVHD patients
One case (14.3%) of bacterial sepsis and bacterial pneumonia occurred at 16 days after MSCs treatment, and one case (14.3%) experienced fungal pneumonia at 49 days after MSCs treatment.
Long-term outcomes
Survival of aGVHD patients
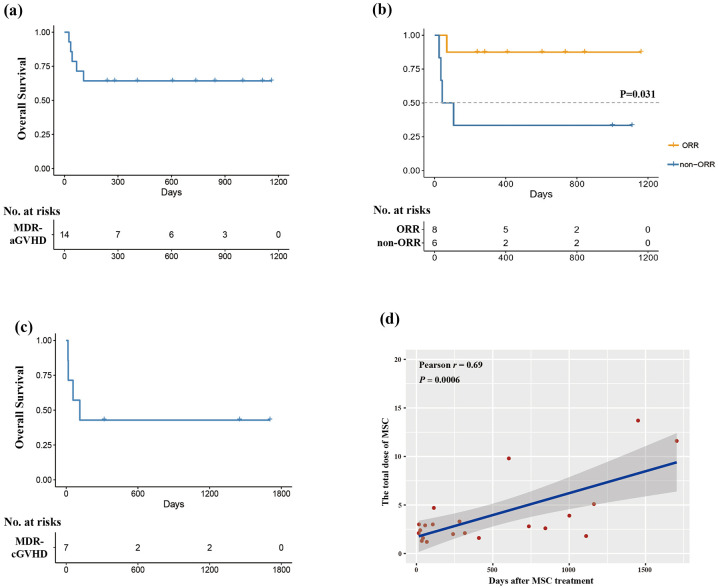
Causes of death after MSCs treatment included GVHD progression (n = 1), severe infection (bacterial central nervous system infection: n = 1; fungal pneumonia: n = 2), and PGF (n = 1). The median follow-up time after HSCT and MSCs treatment was 598 days (range, 93–1270) days and 345.5 days (range, 25–1162) days, respectively. The 2-year probability of OS after MSCs treatment was 64.3%, which was 87.5% and 33.3%, respectively for ORR and non-ORR groups (p = 0.031; Figure 4(a) and (b)).
Figure 4.
Overall survival of MDR-aGVHD and MDR-cGVHD patients after MSCs treatment. (a) the OS of MDR-aGVHD patients; (b) the OS of the ORR and non-ORR groups of MDR-aGVHD patients; (c) the OS of MDR-cGVHD patients; (d) the correlation analysis of the total MSCs dose and OS.
Survival of cGVHD patients
The median follow-up time after HSCT and MSCs treatment was 501 days (range, 302–2584) days and 114 days (range, 15–1704) days, respectively. The 2-year probability of OS was 42.9% (Figure 4(c)), and causes of death after MSCs treatment included GVHD progression (n = 2), severe fungal pneumonia (n = 1), and relapse (n = 1).
The correlation analysis for the total dose of MSCs and OS
The correlation analysis of the total dose of MSCs treatment and OS was conducted, and we observed that the total dose of MSCs was positively correlated with OS (r = 0.69, p = 0.0006, Figure 4(d)).
Univariable and multivariable analysis for ORR and OS
None of variables was significantly associated with ORR in the univariable analysis (data not shown). In the univariable analysis, the donor/recipient gender matched and GVHD severity were associated with poorer OS (Supplementary Table 3). However, none of variables was significantly associated with OS in multivariable analysis.
Discussion
According to our results, the ORRs at any time were 57.1% and 42.9%, respectively, for MDR-aGVHD and MDR-cGVHD patients after HID HSCT. This was the first study to identify the efficacy and safety of MSCs treatment in MDR-GVHD patients after HID HSCT.
In the studies enrolled patients most of who received ISD or URD HSCT, the meta-analysis of Hashmi et al. 52 showed that the ORR of MSCs treatment for SR-aGVHD was 72% (241/336), and the CR rate was 28% (25/336). Another meta-analysis included 13 studies with 301 patients showed that the ORR of SR-aGVHD patients receiving MSCs treatment was 68.1%. 53 In this study, the ORR at any time of MSCs treatment was 57.1% for MDR-aGVHD, which was similar to the previous studies.
Weng et al. 54 used MSCs to treat 19 ISD HSCT recipients with SR-cGVHD. Four and 10 patients achieved CR and PR, respectively, and the ORR was 73.3%. Similarly, Introna et al. 55 used MSCs to treat 40 ISD and URD HSCT recipients with SR-cGVHD and the ORR and CR rate was 67.5% and 27.5%, respectively. The meta-analysis of MSCs treatment for SR-cGVHD patients showed that the ORR and CR rate was 67% and 47%, respectively. 56 In this study, seven cGVHD patients received MSCs treatment and the ORRs at any time was 42.9% (3/7). The ORR was relatively low, which suggested that the MDR-cGVHD after HID HSCT might be more difficult to control than that of patients receiving ISD or URD HSCT.23–25
Infection events were common in other second-line treatments for SR-GVHD, for example, the infection rates of patients receiving basiliximab, ATG, MMF, infliximab, rituximab, and alemtuzumab were 59.6–74%, 67%, 23–67%, 81%, 15–66%, and 77.8–100%, respectively.9–11,57–64 In our study, we also observed that infection was the most common toxicity after MSCs treatment. Among 14 patients with MDR-aGVHD, 5 (35.7%) developed infections and the incidence of cytomegaloviremia was 28.6%. Among seven patients with MDR-cGVHD, two (28.6%) developed bacterial infections. In addition, we observed patients died of severe fungal infection (n = 3) or bacterial infection (n = 1) after MSCs treatments. Nevertheless, there was a high background rate of infectious events in SR-GVHD patients, 65 and infection events might not be attributed solely to MSCs treatment. Thus, the risk of infection of HID HSCT recipients receiving MSCs treatments was acceptable.
Compared with hUCB-MSCs, MSCs isolated from bone marrow are used more commonly.66,67 The ORR and OS of BM-MSCs treatment for SR-aGVHD patients were 60–70.9% and 62–80%, respectively.29,68–71 The ORR and OS of BM-MSCs treatment for SR-cGVHD patients were 87–100% and 78.3–100%, respectively.72,73 In this study, the ORR and OS of hUCB-MSCs treatment for MDR-aGVHD patients were 57% and 64.3%, respectively. The ORR and OS of hUCB-MSCs treatment for MDR-cGVHD patients were 42.9% and 42.1%, respectively. Thus, the results between hUCB-MSCs and BM-MSCs seemed to be similar in aGVHD patients; however, our results of hUCB-MSCs treatment seemed to be worse than BM-MSCs treatment in cGVHD patients. Considering that few patients with SR-cGVHD following HID HSCT were enrolled in the trials using BM-MSCs, the efficacy between hUCB-MSCs and BM-MSCs should be further identified in these patients.
Our study has several limitations. First, this was a retrospective study with a small sample size, which might affect the accuracy of our results. Second, the number of particularly involved organs (e.g. lung cGVHD) was small, and the efficacy of MSCs treatment on these organs needs to be further investigated. Third, our study used MSCs as an add-on immunosuppressive treatment for MDR-GVHD patients; however, most of the studies identifying MSCs treatment in GVHD used MSCs in concomitance to other second-line treatments.29,68–73 The protocols for SR-GVHD treatments prior to MSCs treatment were not uniform, but we observed that the former treatments seemed not to influence the ORR of MSCs treatment. Fourth, although ruxolitinib had been proved to be significantly better than best available therapy in SR-aGVHD and SR-cGVHD, only 13 patients receiving MSCs treatment were enrolled in REACH2 trial, 74 and MSCs were not included in the control group in REACH3 trial. 75 Thus, we could further compare the efficacy and safety between ruxolitinib and MSCs treatment in patients with MDR-GVHD in future. Finally, the median follow-up time after MSCs treatment was relatively short, and its long-term efficacy in HID HSCT needed to be further determined.
Conclusion
In conclusion, MSC may be a safe and effective therapy for MDR-GVHD patients after HID HSCT. It still needs to be confirmed by prospective large-scale registry trials in future.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207211072838 for Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation by Meng-Zhu Shen, Xin-Xin Liu, Zhi-Yuan Qiu, Lan-Ping Xu, Xiao-Hui Zhang, Yu Wang, Chen-Hua Yan, Huan Chen, Yu-Hong Chen, Wei Han, Feng-Rong Wang, Jing-Zhi Wang, Si-Ning Liu, Kai-Yan Liu, Xiao-Jun Huang and Xiao-Dong Mo in Therapeutic Advances in Hematology
Supplemental material, sj-docx-2-tah-10.1177_20406207211072838 for Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation by Meng-Zhu Shen, Xin-Xin Liu, Zhi-Yuan Qiu, Lan-Ping Xu, Xiao-Hui Zhang, Yu Wang, Chen-Hua Yan, Huan Chen, Yu-Hong Chen, Wei Han, Feng-Rong Wang, Jing-Zhi Wang, Si-Ning Liu, Kai-Yan Liu, Xiao-Jun Huang and Xiao-Dong Mo in Therapeutic Advances in Hematology
Supplemental material, sj-docx-3-tah-10.1177_20406207211072838 for Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation by Meng-Zhu Shen, Xin-Xin Liu, Zhi-Yuan Qiu, Lan-Ping Xu, Xiao-Hui Zhang, Yu Wang, Chen-Hua Yan, Huan Chen, Yu-Hong Chen, Wei Han, Feng-Rong Wang, Jing-Zhi Wang, Si-Ning Liu, Kai-Yan Liu, Xiao-Jun Huang and Xiao-Dong Mo in Therapeutic Advances in Hematology
Supplemental material, sj-docx-4-tah-10.1177_20406207211072838 for Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation by Meng-Zhu Shen, Xin-Xin Liu, Zhi-Yuan Qiu, Lan-Ping Xu, Xiao-Hui Zhang, Yu Wang, Chen-Hua Yan, Huan Chen, Yu-Hong Chen, Wei Han, Feng-Rong Wang, Jing-Zhi Wang, Si-Ning Liu, Kai-Yan Liu, Xiao-Jun Huang and Xiao-Dong Mo in Therapeutic Advances in Hematology
Footnotes
Author contributions: Meng-Zhu Shen: Investigation; Software; Visualization; Writing – original draft; Writing – review & editing.
Xin-Xin Liu: Data curation; Resources; Formal analysis.
Zhi-Yuan Qiu: Data curation; Resources; Formal analysis.
Lan-Ping Xu: Data curation; Resources.
Xiao-Hui Zhang: Data curation; Resources.
Yu Wang: Data curation; Resources.
Chen-Hua Yan: Data curation; Resources.
Huan Chen: Data curation; Resources.
Yu-Hong Chen: Data curation; Resources.
Wei Han: Data curation; Resources.
Feng-Rong Wang: Data curation; Resources.
Jing-Zhi Wang: Data curation; Resources.
Si-Ning Liu: Data curation; Resources.
Kai-Yan Liu: Data curation; Resources.
Xiao-Jun Huang: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Data curation; Resources; Supervision; Validation; Visualization.
Xiao-Dong Mo: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Program of the National Natural Science Foundation of China (grant no. 82170208), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant no. 81621001), the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant no. 2019-I2M-5-034), the Key Program of the National Natural Science Foundation of China (grant no.81930004), and the Fundamental Research Funds for the Central Universities.
ORCID iD: Xiao-Dong Mo  https://orcid.org/0000-0002-9881-7945
https://orcid.org/0000-0002-9881-7945
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Meng-Zhu Shen, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Xin-Xin Liu, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China; Department of Hematology, The Second Affiliated Hospital of Shandong First Medical University, Taian, China.
Zhi-Yuan Qiu, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China; Department of Hematology, Weifang People’s Hospital, Weifang, China.
Lan-Ping Xu, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Xiao-Hui Zhang, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Yu Wang, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Chen-Hua Yan, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Huan Chen, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Yu-Hong Chen, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Wei Han, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Feng-Rong Wang, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Jing-Zhi Wang, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Si-Ning Liu, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Kai-Yan Liu, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China.
Xiao-Jun Huang, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, China; Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China; Research Unit of Key Technique for Diagnosis and Treatments of Hematologic Malignancies, Chinese Academy of Medical Sciences, Beijing, China.
Xiao-Dong Mo, Peking University People’s Hospital, Peking University Institute of Hematology, No. 11 Xizhimen South Street, Xicheng District, Beijing 100044, China; Research Unit of Key Technique for Diagnosis and Treatments of Hematologic Malignancies, Chinese Academy of Medical Sciences, Beijing 2019RU029, China.
References
- 1. Zhang XH, Chen J, Han MZ, et al. The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol 2021; 14: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Liu Q-F, Lin R, et al. Optimizing antithymocyte globulin dosing in haploidentical hematopoietic cell transplantation: long-term follow-up of a multicenter, randomized controlled trial. Sci Bull 2021; 66: 2498–2505. [DOI] [PubMed] [Google Scholar]
- 3. Jamil MO, Mineishi S. State-of-the-art acute and chronic GVHD treatment. Int J Hematol 2015; 101: 452–466. [DOI] [PubMed] [Google Scholar]
- 4. Zeiser R, Blazar BR. Acute graft-versus-host disease – biologic process, prevention, and therapy. N Engl J Med 2017; 377: 2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gratwohl A, Brand R, Frassoni F, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant 2005; 36: 757–769. [DOI] [PubMed] [Google Scholar]
- 6. Garnett C, Apperley JF, Pavlů J. Treatment and management of graft-versus-host disease: improving response and survival. Ther Adv Hematol 2013; 4: 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2012; 18: 1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol 2020; 7: e157–e167. [DOI] [PubMed] [Google Scholar]
- 9. Khoury H, Kashyap A, Adkins DR, et al. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant 2001; 27: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 10. Liu SN, Zhang XH, Xu LP, et al. Prognostic factors and long-term follow-up of basiliximab for steroid-refractory acute graft-versus-host disease: updated experience from a large-scale study. Am J Hematol 2020; 95: 927–936. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt-Hieber M, Fietz T, Knauf W, et al. Efficacy of the interleukin-2 receptor antagonist basiliximab in steroid-refractory acute graft-versus-host disease. Br J Haematol 2005; 130: 568–574. [DOI] [PubMed] [Google Scholar]
- 12. Caplan AI. Mesenchymal stem cells. J Orthop Res 1991; 9: 641–650. [DOI] [PubMed] [Google Scholar]
- 13. Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 2018; 22: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang B, Song Y, Liao L, et al. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant Proc 2007; 39: 3358–3362. [DOI] [PubMed] [Google Scholar]
- 15. von Bonin M, Stolzel F, Goedecke A, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant 2009; 43: 245–251. [DOI] [PubMed] [Google Scholar]
- 16. Muller I, Kordowich S, Holzwarth C, et al. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis 2008; 40: 25–32. [DOI] [PubMed] [Google Scholar]
- 17. Prasad VK, Lucas KG, Kleiner GI, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant 2011; 17: 534–541. [DOI] [PubMed] [Google Scholar]
- 18. Perez-Simon JA, Lopez-Villar O, Andreu EJ, et al. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica 2011; 96: 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrmann R, Sturm M, Shaw K, et al. Mesenchymal stromal cell therapy for steroid-refractory acute and chronic graft versus host disease: a phase 1 study. Int J Hematol 2012; 95: 182–188. [DOI] [PubMed] [Google Scholar]
- 20. Mo X-D, Zhang X-H, Xu L-P, et al. Disease risk comorbidity index for patients receiving haploidentical allogeneic hematopoietic transplantation. Engineering 2021; 7: 162–169. [Google Scholar]
- 21. Guo H, Chang YJ, Hong Y, et al. Dynamic immune profiling identifies the stronger graft-versus-leukemia (GVL) effects with haploidentical allografts compared to HLA-matched stem cell transplantation. Cell Mol Immunol 2021; 18: 1172–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao-Jun H, Lan-Ping X, Kai-Yan L, et al. Partially matched related donor transplantation can achieve outcomes comparable with unrelated donor transplantation for patients with hematologic malignancies. Clin Cancer Res 2009; 15: 4777–4783. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood 2015; 125: 3956–3962. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Wang HX, Lai YR, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia 2016; 30: 2055–2063. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Wu DP, Liu QF, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol 2019; 12: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol 2013; 31: 1310–1316. [DOI] [PubMed] [Google Scholar]
- 27. Lu DP, Dong L, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107: 3065–3073. [DOI] [PubMed] [Google Scholar]
- 28. Sun YQ, He GL, Chang YJ, et al. The incidence, risk factors, and outcomes of primary poor graft function after unmanipulated haploidentical stem cell transplantation. Ann Hematol 2015; 94: 1699–1705. [DOI] [PubMed] [Google Scholar]
- 29. Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371: 1579–1586. [DOI] [PubMed] [Google Scholar]
- 30. Cetin M, Akyol G, Gonen ZB, et al. Additional infusions of mesenchymal stem cells improve response rate in multidrug-resistant GvHD patients. Bone Marrow Transplant 2017; 52: 783–785. [DOI] [PubMed] [Google Scholar]
- 31. Mo X-D, Zhang Y-Y, Zhang X-H, et al. The role of collateral related donors in haploidentical hematopoietic stem cell transplantation. Sci Bull 2018; 63: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 32. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin F, Zhang Y, Han T, et al. A modified conditioning regimen based on low-dose cyclophosphamide and fludarabine for haploidentical hematopoietic stem cell transplant in severe aplastic anemia patients at risk of severe cardiotoxicity. Clin Transplant. Epub ahead of print 16 October 2021. DOI: 10.1111/ctr.14514. [DOI] [PubMed] [Google Scholar]
- 34. Huang XJ, Liu DH, Liu KY, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006; 38: 291–297. [DOI] [PubMed] [Google Scholar]
- 35. Chen J, Wang RX, Chen F, et al. Combination of a haploidentical SCT with an unrelated cord blood unit: a single-arm prospective study. Bone Marrow Transplant 2014; 49: 206–211. [DOI] [PubMed] [Google Scholar]
- 36. Sun YQ, Wang Y, Wang FR, et al. Graft failure in patients with hematological malignancies: a successful salvage with a second transplantation from a different haploidentical donor. Front Med 2021; 8: 604085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yan CH, Liu DH, Liu KY, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012; 119: 3256–3262. [DOI] [PubMed] [Google Scholar]
- 38. Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant 2016; 22: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828. [PubMed] [Google Scholar]
- 40. Dignan FL, Clark A, Amrolia P, et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol 2012; 158: 30–45. [DOI] [PubMed] [Google Scholar]
- 41. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015; 21: 389–401.e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2005; 11: 945–956. [DOI] [PubMed] [Google Scholar]
- 43. Dignan FL, Amrolia P, Clark A, et al. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol 2012; 158: 46–61. [DOI] [PubMed] [Google Scholar]
- 44. Zhao JY, Liu SN, Xu LP, et al. Ruxolitinib is an effective salvage treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation without posttransplant cyclophosphamide. Ann Hematol 2021; 100: 169–180. [DOI] [PubMed] [Google Scholar]
- 45. Martin PJ, Uberti JP, Soiffer RJ, et al. Prochymal improves response rates in patients with steroid-refractory acute graft versus host disease (SR-GVHD) involving the liver and gut: results of a randomized, placebo-controlled, multicenter phase III trial in GVHD. Biol Blood Marrow Transplant 2010; 16: S169–S170. [Google Scholar]
- 46. Szabolcs P, Visani G, Locatelli F, et al. Treatment of steroid-refractory acute GVHD with mesenchymal stem cells improves outcomes in pediatric patients; results of the pediatric subset in a phase III randomized, placebo-controlled study. Biol Blood Marrow Transplant 2010; 16: S298. [Google Scholar]
- 47. Fisher SA, Cutler A, Doree C, et al. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst Rev 2019; 1: CD009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Bruyn C, Najar M, Raicevic G, et al. A rapid, simple, and reproducible method for the isolation of mesenchymal stromal cells from Wharton’s jelly without enzymatic treatment. Stem Cells Dev 2011; 20: 547–557. [DOI] [PubMed] [Google Scholar]
- 49. Gao L, Zhang Y, Hu B, et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol 2016; 34: 2843–2850. [DOI] [PubMed] [Google Scholar]
- 50. Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant 2015; 21: 984–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martin PJ, Bachier CR, Klingemann HG, et al. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: a joint statement. Biol Blood Marrow Transplant 2009; 15: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hashmi S, Ahmed M, Murad MH, et al. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol 2016; 3: e45–e52. [DOI] [PubMed] [Google Scholar]
- 53. Chen X, Wang C, Yin J, et al. Efficacy of mesenchymal stem cell therapy for steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. PLoS ONE 2015; 10: e0136991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weng JY, Du X, Geng SX, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant 2010; 45: 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Introna M, Lucchini G, Dander E, et al. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant 2014; 20: 375–381. [DOI] [PubMed] [Google Scholar]
- 56. Yim HW, Jeong H, Cho S-G, et al. Therapeutic evaluation of mesenchymal stem cell injection for graft-versus-host disease: a single arm meta-analysis. Cytotherapy 2015; 17: S40. [Google Scholar]
- 57. Couriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood 2004; 104: 649–654. [DOI] [PubMed] [Google Scholar]
- 58. Gómez-Almaguer D, Ruiz-Argüelles GJ, del Carmen Tarín-Arzaga L, et al. Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant 2008; 14: 10–15. [DOI] [PubMed] [Google Scholar]
- 59. Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, et al. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant 2009; 15: 1005–1013. [DOI] [PubMed] [Google Scholar]
- 60. Kim JG, Sohn SK, Kim DH, et al. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur J Haematol 2004; 73: 56–61. [DOI] [PubMed] [Google Scholar]
- 61. Krejci M, Doubek M, Buchler T, et al. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol 2005; 84: 681–685. [DOI] [PubMed] [Google Scholar]
- 62. Martínez C, Solano C, Ferrá C, et al. Alemtuzumab as treatment of steroid-refractory acute graft-versus-host disease: results of a phase II study. Biol Blood Marrow Transplant 2009; 15: 639–642. [DOI] [PubMed] [Google Scholar]
- 63. Schub N, Günther A, Schrauder A, et al. Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant 2011; 46: 143–147. [DOI] [PubMed] [Google Scholar]
- 64. Wang JZ, Liu KY, Xu LP, et al. Basiliximab for the treatment of steroid-refractory acute graft-versus-host disease after unmanipulated HLA-mismatched/haploidentical hematopoietic stem cell transplantation. Transplant Proc 2011; 43: 1928–1933. [DOI] [PubMed] [Google Scholar]
- 65. García-Cadenas I, Rivera I, Martino R, et al. Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease. Bone Marrow Transplant 2017; 52: 107–113. [DOI] [PubMed] [Google Scholar]
- 66. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells – current trends and future prospective. Biosci Rep 2015; 35: e00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Burnham AJ, Daley-Bauer LP, Horwitz EM. Mesenchymal stromal cells in hematopoietic cell transplantation. Blood Adv 2020; 4: 5877–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Salmenniemi U, Itala-Remes M, Nystedt J, et al. Good responses but high TRM in adult patients after MSC therapy for GvHD. Bone Marrow Transplant 2017; 52: 606–608. [DOI] [PubMed] [Google Scholar]
- 69. Muroi K, Miyamura K, Okada M, et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol 2016; 103: 243–250. [DOI] [PubMed] [Google Scholar]
- 70. Kebriaei P, Hayes J, Daly A, et al. A phase 3 randomized study of remestemcel-L versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant 2020; 26: 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kurtzberg J, Abdel-Azim H, Carpenter P, et al. A phase 3, single-arm, prospective study of remestemcel-L, ex vivo culture-expanded adult human mesenchymal stromal cells for the treatment of pediatric patients who failed to respond to steroid treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant 2020; 26: 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhou H, Guo M, Bian C, et al. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant 2010; 16: 403–412. [DOI] [PubMed] [Google Scholar]
- 73. Peng Y, Chen X, Liu Q, et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia 2015; 29: 636–646. [DOI] [PubMed] [Google Scholar]
- 74. Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med 2020; 382: 1800–1810. [DOI] [PubMed] [Google Scholar]
- 75. Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med 2021; 385: 228–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207211072838 for Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation by Meng-Zhu Shen, Xin-Xin Liu, Zhi-Yuan Qiu, Lan-Ping Xu, Xiao-Hui Zhang, Yu Wang, Chen-Hua Yan, Huan Chen, Yu-Hong Chen, Wei Han, Feng-Rong Wang, Jing-Zhi Wang, Si-Ning Liu, Kai-Yan Liu, Xiao-Jun Huang and Xiao-Dong Mo in Therapeutic Advances in Hematology
Supplemental material, sj-docx-2-tah-10.1177_20406207211072838 for Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation by Meng-Zhu Shen, Xin-Xin Liu, Zhi-Yuan Qiu, Lan-Ping Xu, Xiao-Hui Zhang, Yu Wang, Chen-Hua Yan, Huan Chen, Yu-Hong Chen, Wei Han, Feng-Rong Wang, Jing-Zhi Wang, Si-Ning Liu, Kai-Yan Liu, Xiao-Jun Huang and Xiao-Dong Mo in Therapeutic Advances in Hematology
Supplemental material, sj-docx-3-tah-10.1177_20406207211072838 for Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation by Meng-Zhu Shen, Xin-Xin Liu, Zhi-Yuan Qiu, Lan-Ping Xu, Xiao-Hui Zhang, Yu Wang, Chen-Hua Yan, Huan Chen, Yu-Hong Chen, Wei Han, Feng-Rong Wang, Jing-Zhi Wang, Si-Ning Liu, Kai-Yan Liu, Xiao-Jun Huang and Xiao-Dong Mo in Therapeutic Advances in Hematology
Supplemental material, sj-docx-4-tah-10.1177_20406207211072838 for Efficacy and safety of mesenchymal stem cells treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation by Meng-Zhu Shen, Xin-Xin Liu, Zhi-Yuan Qiu, Lan-Ping Xu, Xiao-Hui Zhang, Yu Wang, Chen-Hua Yan, Huan Chen, Yu-Hong Chen, Wei Han, Feng-Rong Wang, Jing-Zhi Wang, Si-Ning Liu, Kai-Yan Liu, Xiao-Jun Huang and Xiao-Dong Mo in Therapeutic Advances in Hematology