Abstract
Background:
Antibody-drug conjugates have recently been introduced as a treatment for advanced urothelial carcinoma. The EV-301 study demonstrated that enfortumab vedotin (EV) improved overall survival compared with conventional chemotherapy. To assess the cost-effectiveness of EV for the treatment of advanced urothelial carcinoma (UC) from a payer perspective in middle- and high-income countries.
Methods:
A decision analysis model was developed to assess the efficacy and economic viability of EV as a subsequent-line treatment following disease progression in patients with advanced urothelial carcinoma already treated with PD-1 or PD-L1 inhibitors. Clinical and utility values were obtained from the published literature and available databases. Cost data were obtained from payer perspectives in the United States, United Kingdom, and China. Quality-adjusted life-years (QALYs) were used to measure health outcomes, and incremental cost-effectiveness ratios (ICERs) used to evaluate cost-effectiveness in comparison to willingness-to-pay in the United States, United Kingdom, and China. One-way sensitivity analysis and probabilistic sensitivity analysis were performed to assess the robustness of the model.
Results:
Compared with chemotherapy, EV increased the benefit by 0.16-0.17 QALYs, resulting in ICERs of $2,168,746.71, $2,164,494.38, and $1,775,576.56 per QALY in the United States, United Kingdom, and China, respectively. One-way sensitivity analysis indicated that the largest effect on outcome was the utility value for progression-free survival. Probabilistic sensitivity analysis demonstrated that the probability of EV being cost-effective was 0%.
Conclusions:
EV provides an additional health benefit over chemotherapy for patients with advanced urothelial carcinoma but is not cost-effective from a payer perspective in the United States, United Kingdom, or China.
Keywords: chemotherapy, cost effectiveness, enfortumab vedotin, Markov model, urothelial carcinoma
Introduction
According to National Cancer Institute estimates, over 79,000 new cases of UC were diagnosed in 2017, and more than 16,000 people died from the disease in the United States. 1 Statistics from the International Agency for Research on Cancer (IARC) indicate that urothelial carcinoma (UC) causes more than 165,000 deaths per year and is ranked the ninth most common cancer worldwide. Approximately 151,000 new cases of UC are diagnosed annually in Europe, resulting in 52,000 deaths. 2 Over 80,000 new cases are diagnosed and 33,000 deaths occur annually in China. 3
First and second-line treatments for advanced urothelial carcinoma are based on platinum-based chemotherapy and immune checkpoint inhibitors (ICIs) targeting programmed death 1/programmed death-ligand 1 (PD-1/PD-L1), respectively.4–6 However, the majority of patients suffer progression of the disease, and no therapies had been approved for use in patients with advanced UC after failure of immunotherapy until the advent of enfortumab vedotin (EV).
EV is a fully human antibody against nectin-4 linked via a cleavable drug linker to the payload microtubule-disrupting chemotherapy agent: monomethyl auristatin E (MMAE).7–9 It was granted breakthrough therapy designation approval by the Food and Drug Administration (FDA) in 2018 then approved in December 2019 to treat patients with locally advanced or metastatic urothelial carcinoma previously treated with platinum-based chemotherapy and ICI and in July 2021, expanded the label indication to the second line setting.10–12 The EV-301 study demonstrated that EV showed a survival benefit compared to chemotherapy in advanced urothelial carcinoma, with improvements in median overall survival (OS) and median progression-free survival (PFS) of 3.9 and 1.8 months, respectively. 13 As for safety, the EV group and the chemotherapy group had similar rates of all grades and grade ⩾ 3 of treatment-related adverse events. 13
Healthcare budgets are limited and under growing pressure, in part due to increased costs associated with drug and technology innovations in the field of health care. Cost-effectiveness analysis (CEA) is a standard and well-validated method for testing the value of a drug that considers both cost and efficacy in a given indication. Still, the transferability and generalizability of CEA findings are limited because the cost estimates are region-specific. 14 The current study investigated the economic outcomes of the incorporation of EV as a follow-up to failed platinum-based chemotherapy and immune checkpoint inhibitor therapy in patients with advanced urothelial carcinoma in the United States, United Kingdom, and China, with a high degree of portability and scalability, representing both developed and developing regions.
Material and methods
Model construction
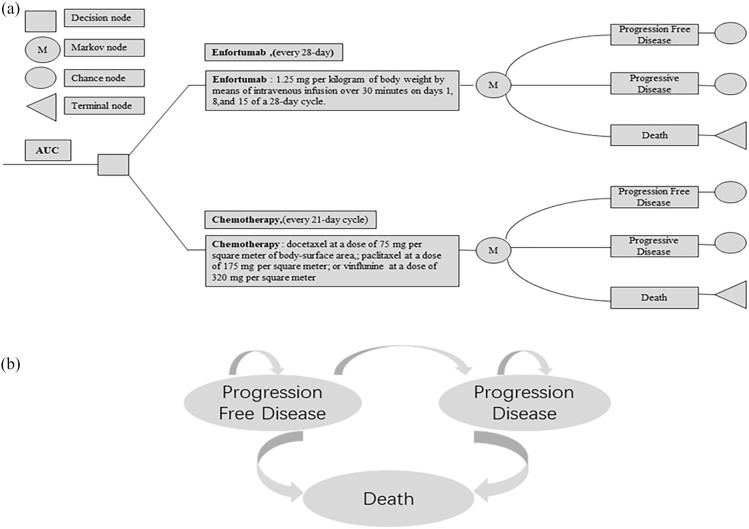
In the present study, a Markov model was constructed to simulate the cost and health benefits of EV or chemotherapy for advanced UC from the perspective of patients in the United States, United Kingdom, and China based on the EV-301 study. 13 Each patient was described as being in one of three mutually exclusive health states: progression-free survival (PFS), progressive disease, or death. All patients started in the PFS group, but could exhibit advanced disease and die or transfer to a progressive disease state at any time (Figure 1). The model was based on a 1-month cycle with a time horizon of 10 years in order that the patients’ medication regimen and its entire life cycle could be evaluated in the present study. TreeAge Pro statistical software version 2020 R1 (TreeAge Software, LLC) was used for analysis.
Figure 1.
(a) Abbreviated decision tree and markov model used to compare two strategies for treating patients with previously treated advanced urothelial carcinoma. (b) Influence diagram shows a network of three health states linked by transitional variables.
The primary endpoint of the model included total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). As recommended by relevant WHO guidelines and published literature, the willingness-to-pay thresholds (WTP) in this study were set at $150,000/QALY, $41,666.67/QALY, and $30,447.09/QALY for the United States, United Kingdom, and China, respectively.15–17
Mortality estimates
Mortality rates for any state in the model were estimated as the probability of death from advanced urothelial carcinoma and mortality from other causes. We obtained survival curves for EV and chemotherapy from the EV301 study which included 608 patients by capturing the survival probabilities at each time point through the Plot Digitizer (version 2.6.8; http://plotdigitizer.sourceforge.net/), 13 and then reconstructing the survival curves using an algorithm developed by Hoyle and Henley. 18 Using this process, we compared distributions including the Weibull, exponential, log-normal, and log-logistic distributions by the Bayesian information criterion (BIC) and Akaike information criterion (AIC) and found that the Weibull distribution was the best choice.
The risk of progression was estimated using the same methodology, with background mortality rates for each age group based on United States, United Kingdom, and China life tables.19–21
Cost and utilities
Only direct costs were considered in the present study, including drug costs, tests, administration, and management costs associated with adverse events (AEs). The cost assessment of EV was based on the administration of the drug by intravenous infusion at a dose of 1.25 mg per kilogram body weight on days 1, 8, and 15 of a 28-day cycle. Costs for chemotherapy were calculated for docetaxel at 75 mg/m2 and paclitaxel at 175 mg/m2, and vinflunine at 320 mg/m2 administered intravenously every 3 weeks until disease had progressed. The cost of vinflunine was not considered in United States or Chinese perspectives as it is not an approved indication by the FDA or National Medical Products Administration (NMPA).
We calculated the cost of treatment for grades 3–4 AEs with a significant difference in incidence between the two groups reported in the EV301 study, based on relevant treatment guidelines, expert opinion, and published studies. Prices for drugs and other administrative costs were those in the Red Book, the published literature, and other publicly available databases (Table 1). The costs in the model were converted to US dollars based on the following exchange rates: 1US $ = CNY 6.4391, 1US $ = GBP 0.72.
Table 1.
Model parameters and assumptions.
| Parameter | US value (Range) | UK value (Range) | China value (Range) |
|---|---|---|---|
| Price of Enfortumab Vedotin per 20 mg | 2630.4 act (1841.28–3419.52) a 22 | 2630.4 (1841.28–3419.52) a 22 | 2630.4 (1841.28–3419.52) a 22 |
| Price of Docetaxel per 20 mg | 690.70 (483.49–897.91) a 22 | 226.83 (158.78–294.88) a 23 | 201.89 (141.32–262.46) a 24 |
| Price of Paclitaxel per 30 mg | 16.61 (11.63–21.59) a 22 | 161.74 (113.22–210.26) a 23 | 105.60 (73.92–137.28) a 24 |
| Price of Vinflunine per 250 mg | NA | 1480.81 (1036.57–1925.05) a 23 | NA |
| BSC/cycle, $ | 1213 (987–1438) a 25 | 88.23 (70.53–105.9) 26 | 807 (564.9–1049.1) a 27 |
| Cost of drug administration per unit | 184.8 (129.39–240.29) a 28 | 405.3 (283.71–526.89) a 29 | 15.69 (10.99–20.40) a , b |
| Cost of managing adverse events, per unit, $ | |||
| Maculopapular rash | 15,709 (10,996.3–20,421.7) 30 | 143 (100.1–185.9) a 31 | 72 (40.0–104.0) 32 |
| Anemia | 4638 (3326–5949) 33 | 3242 (3097–3388) 34 | 999.21 (378.16–1621.03) 35 |
| Decreased neutrophil count | 17,181 (12,026.7–22,335.3) a 31 | 226 (158.2–293.8) a 31 | 446.86 (312.80–580.92) a 36 |
| Febrile neutropenia | 21,625 (15,137.5–28,112.5) a 37 | 2856.72 (1999.70–3713.74) a 38 | 953 (667.1–1238.9) a 35 |
| Discount rate, % | 3 39 | 3.5 29 | 5 29 |
| Utilities | |||
| PFS | 0.60 (0.427–0.793) a 40 | ||
| PD | 0.52 (0.364–0.676) a 40 | ||
| Disutility due to Grade 1–2 AEs | 0.01 (0.008–0.02) 41 | ||
| Disutility due to Grade ⩾3 AEs | 0.28 (0.21–0.35) 42 | ||
AE, adverse event; BSC: best supportive care; PD, progressed disease; PFS, progression-free survival;
Range indicates 30% change.
Local estimated.
Sensitivity analysis
One-way and probabilistic sensitivity analyses were used to further analyze the results in this study and assess model stability. One-way sensitivity analysis compared the effects of critical model input parameters on ICER by the model by adjusting the relevant parameters to their respective low and high values, with the results plotted using a Tornado diagram. The range of parameter variation was derived from published literature or varied by ± 30%. For probabilistic sensitivity analysis, the model was run 1000 times in accordance with a Monte Carlo simulation, the input parameters sampled from the cost parameters with a gamma distribution and utility parameters with a beta distribution to obtain ICER cost-effectiveness acceptability curves. In addition, an assumed 10% of the current price of EV was used for probabilistic sensitivity analyses to obtain cost-effectiveness acceptability curves.
Results
Base case results
EV provided a benefit of 0.16-0.17 QALYs compared with chemotherapy in the United States, United Kingdom, and China. However, the additional costs of this augmentation in the United States, United Kingdom, and China were as high as $368,686.94, $346,319.1, and $284,092.25, respectively, resulting in ICERs of $2,168,746.71, $2,164,494.38, and $1,775,576.56 per QALY gained, respectively. The results of these base cases are presented in Table 2.
Table 2.
Summary of cost and outcome results in the base-case analysis.
| Strategy | Cost | Effectiveness (QALY) | ICER, per QALY ($) a | Probability of cost-effectiveness (%) |
|---|---|---|---|---|
| United States | ||||
| Enfortumab vedotin | 426,771.64 | 0.69 | NA | NA |
| Chemotherapy | 58,084.70 | 0.52 | 2,168,746.71 | 0 |
| Enfortumab vedotin at 10% cost | 77,831.58 | 0.69 | 116,158.12 | 68.5 |
| United Kingdom | ||||
| Enfortumab vedotin | 37,1276.24 | 0.69 | NA | NA |
| Chemotherapy | 24,957.14 | 0.53 | 2,164,494.38 | 0 |
| Enfortumab vedotin at 10% cost | 44,407.61 | 0.69 | 121,565.44 | 7.3 |
| CHINA | ||||
| Enfortumab vedotin | 30,1263.11 | 0.68 | NA | NA |
| Chemotherapy | 17,170.86 | 0.52 | 1,775,576.56 | 0 |
| Enfortumab vedotin at 10% cost | 37,532.94 | 0.68 | 127,263.00 | 1.1 |
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Compared with Chemotherapy.
Sensitivity analysis
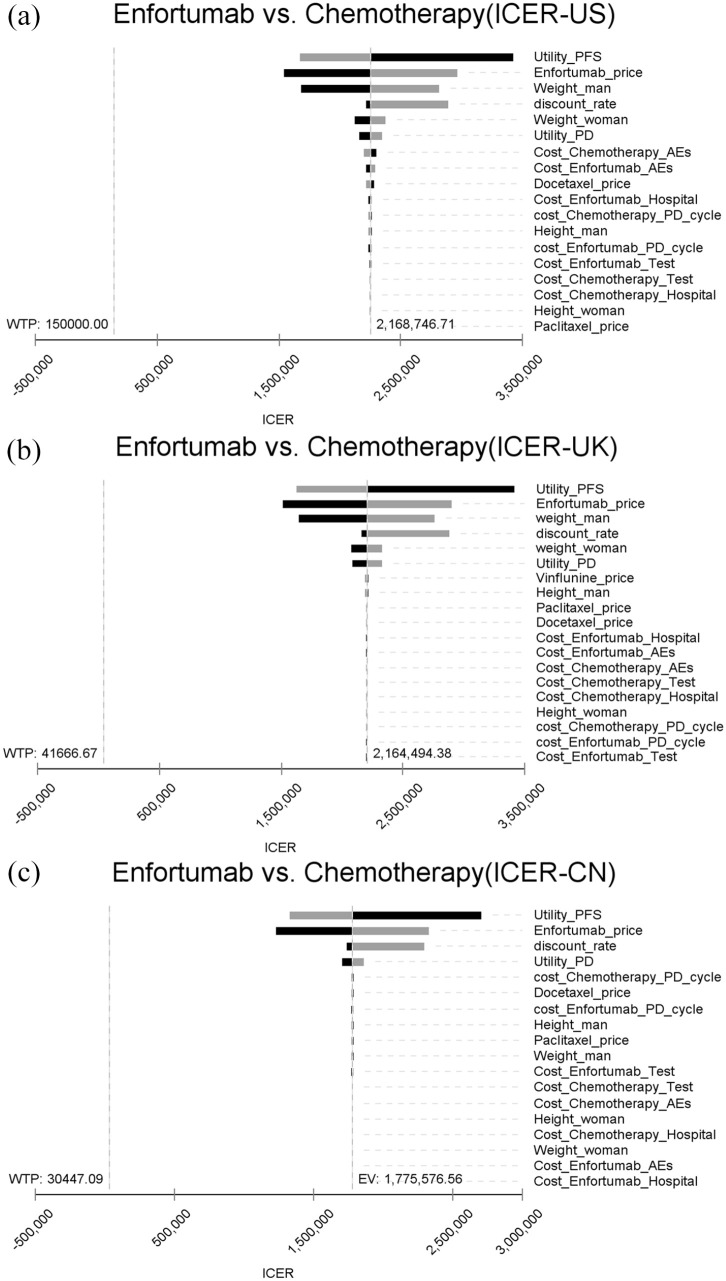
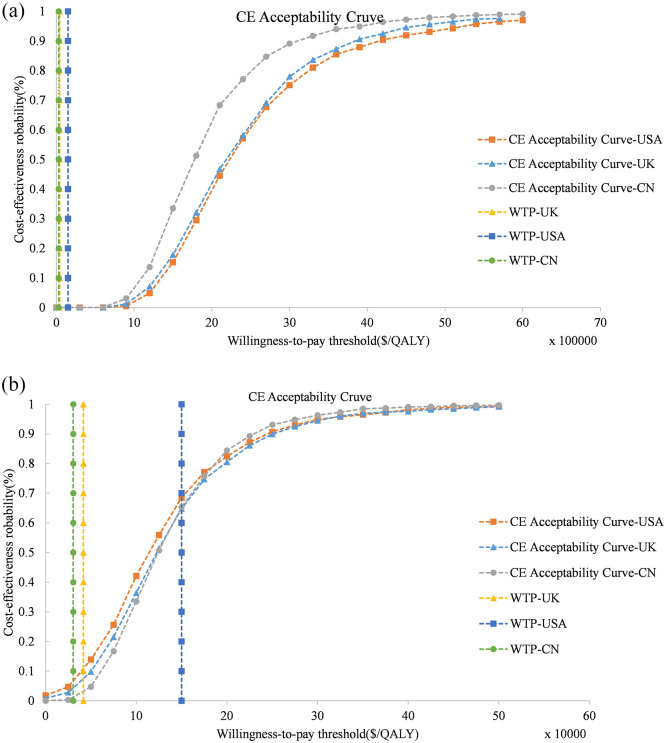
The one-way sensitivity analysis showed that the greatest influence on outcome was the utility values in PFS status, respectively, followed by the price of EV. The ICER was substantially above the WTP threshold for the United States, United Kingdom, and China when all parameters were within a ± 30% range (Figure 2). Similarly, probabilistic sensitivity analyses showed the probability of EV being cost-effective was 0% at the base case WTP and current price. Therefore, we conducted a probabilistic sensitivity analysis using 10% of the current price of EV. The cost-effectiveness acceptability curves for EV were approximately 68.5%, 7.4%, and 1.1% probabilities of cost-effectiveness for thresholds equal to $150,000, $41,666.67, and $30,447.09 in the United States, United Kingdom, and China, respectively (Figure 3).
Figure 2.
One-way sensitivity analysis. This diagram shows incremental cost effectiveness ratio (ICER) of Enfortumab Vedotin vs Chemotherapy for different model input parameters in United States (a), United Kingdom (b) and China (c). PFS, progression-free survival, PD, progression disease; AEs, adverse events.
Figure 3.
Probabilistic sensitivity analysis. Cost-effectiveness acceptable curves showing the cost-effective probability of Enfortumab Vedotin at different prices. (a): Enfortumab Vedotin at 100% cost; (b): Enfortumab Vedotin at 10% cost. The dotted vertical lines represent the willingness to pay thresholds.
Discussion
Advanced urothelial carcinoma lacks effective therapeutic options. Following the use of immune checkpoint inhibitors, the advent of the antibody-drug conjugates class drug EV offered an active treatment choice for such patients, as demonstrated in the EV301 study. The creation of innovative drugs is concomitant with significant research and development costs, which are passed through to the price of the drugs. Using a Markov model, this is the first analysis to evaluate the cost-effectiveness of EV as a follow-up treatment in developed and developing countries, finding that EV is unlikely to be cost-effective, with ICERs > $1,000,000 in one-way sensitivity analyses.
Meanwhile, Newer drugs are often not cost-effective when compared to existing standard protocols due to the undoubtedly increasingly enormous research and development cost. Many pharmacoeconomic studies of checkpoint inhibitor immunotherapy have found them to be not cost-effective in the context of the setting.39,40 Other biologics such as Cabozantinib, Osimertinib, and PARP inhibitors having also a harder time being cost-effective.43–45 It has become increasingly common to provide modest benefits at high cost for a wide range of advanced treatments and diseases, but this should not force patients to accept less effective treatment strategies. In the absence of further breakthroughs in efficacy at this time, a price reduction is required to ensure that EV is cost-effective and affordable. Compared with the United States, where private health care is dominant, countries with predominantly government-funded health services (China and the United Kingdom) may be able to negotiate better drug prices with pharmaceutical companies. As an example, the price of Pembrolizumab in China and the United Kingdom is $2782.69 and $3652.78, approximately 1/2 and 2/3 that of the United States, respectively.22–24 However, in our sensitivity analysis, the probability of EV being cost-effective is > 50% in the United States only at 10% of the current price, with a WTP of up to $150,000/QALY. Therefore, the potential for EV to be cost-effective in the real world, even after price reductions or other patient assistance programs, is cause for concern.
In addition, subgroup analysis of the EV301 study indicated a survival benefit in some populations. 13 Therefore, it is crucial to explore effective biomarkers for specific patients to maximize the efficacy of EV in an era where personalized medicine is becoming increasingly prevalent. It will undoubtedly improve the cost-effectiveness of this drug. Currently, relevant clinical trials about enfortumab vedotin (EV) given intravenously as monotherapy and in combination with other anticancer therapies as first-line and second-line treatment for patients with urothelial carcinoma are ongoing, including combination immunotherapy regimens (NCT03288545 and NCT04960709), or with cabozantinib (NCT04878029). EV would likely result in benefits and benefit-ratios which would be larger and even more significant if in earlier settings or combination therapy clinical studies provided longer PFS and OS. And, EV may be able to provide a more cost-effective option for patients with advanced urothelial carcinoma than the more expensive immunotherapy.
Conclusions about cost-effectiveness depend heavily on the WTP, which varies in different countries, with usually no precise maximum acceptable WTP threshold. For China, as the National Health Commission of the People’s Republic of China does not have an explicit WTP threshold, WHO recommendations of three times the GDP per capita were used as the maximum WTP threshold. 17 In the United States, the WTP threshold in most pharmacoeconomic analyses is considered $50,000-$150,000 per QALY, also close to three times the US GDP per capita. 15 In the United Kingdom, the WTP threshold is considered to be between £20,000 and £30,000. 16 For treatment programs or technologies that exceed £30,000, the National Institute for Health and Care Excellence assesses each case individually, including factors such as special criteria, innovation, etc. EV will be expected to meet the criteria of life-extending treatment at the end of life, and so its WTP threshold in the United Kingdom could be increased to £50,000 ($69,444.44). The probability of being cost-effective would therefore be further increased to17.9% if its price were accompanied by a reduction to 10% of the current price.
As with other models, this analysis is limited by model assumptions and data availability. First, there is an inevitable bias in the modeling of survival in the EV301 trial using the Weibull function. We examined the degree of fit of the model and found no significant differences between the survival data of simulated curves and the trial (Supplementary material). Second, the parameter with the greatest impact on ICER in all cases was the utility score of progression-free survival, highlighting the impact of quality of life on cost-effectiveness. Because utility scores have not been reported for EV301 at this time, we obtained utility values from the published literature. Therefore, we included a wide range of utility values in the sensitivity analysis, which did not change the results of the analysis. Third, because EV is not currently approved for marketing in China or the United Kingdom, the US drug price was used. This may overestimate the cost of EV in the United Kingdom and China, but sensitivity analyses suggested that differences in prices between countries would likely not be sufficient to change the economic outcomes. Fourth, we assumed in the model that all patients received the best supportive care after progression, which may differ from actual treatment choices. Fifth, we did not consider drug wastage or dose adjustment due to adverse reactions.
Conclusion
While many other factors should be considered when making treatment options and Medicare coverage decisions, the results of cost-effectiveness analyses can provide important insights into the relative value of new drugs, thereby providing information for government agencies, healthcare providers, and patients with which to make decisions. In conclusion, the current findings suggest that EV is unlikely to be cost-effective as a strategy for the subsequent treatment of advanced urothelial carcinoma from a patient perspective in the United States, United Kingdom, or China. However, considering the significant clinical effects that can result from the use of EV, broader discussions and negotiations on the pricing, optimal therapeutic population screening, and related medical support policies for this innovative drug are required.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211068733 for Cost-effectiveness of enfortumab vedotin in previously treated advanced urothelial carcinoma by Qiuji Wu, Yi Qin, Weiting Liao, Mengxi Zhang, Yang Yang, Pengfei Zhang and Qiu Li in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: Qiuji Wu: Conceptualization; Methodology; Supervision; Validation; Writing – review & editing.
Yi Qin: Methodology; Writing – review & editing.
Weiting Liao: Validation; Writing – review & editing.
Mengxi Zhang: Methodology; Writing – review & editing.
Yang Yang: Methodology; Writing – review & editing.
Pengfei Zhang: Supervision; Writing – review & editing.
Qiu Li: Conceptualization; Methodology; Resources; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYJC18010).
Ethics approval: This study was based on a literature review and modeling techniques; this study did not require approval by an institutional research ethics board.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Qiuji Wu, Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, ChinaWest China Biomedical Big Data Center, Sichuan University, Chengdu, China.
Yi Qin, Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, ChinaWest China Biomedical Big Data Center, Sichuan University, Chengdu, China.
Weiting Liao, Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, ChinaWest China Biomedical Big Data Center, Sichuan University, Chengdu, China.
Mengxi Zhang, Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, ChinaWest China Biomedical Big Data Center, Sichuan University, Chengdu, China.
Yang Yang, Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, ChinaWest China Biomedical Big Data Center, Sichuan University, Chengdu, China.
Pengfei Zhang, Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, Chengdu, ChinaWest China Biomedical Big Data Center, Sichuan University, Chengdu, China.
Qiu Li, Cancer Center, Department of Medical Oncology, West China Hospital, Sichuan University, No. 37 Guo Xue Xiang, Chengdu 610041, China West China Biomedical Big Data Center, Sichuan University, Chengdu, China.
References
- 1. SEER Cancer Stat Facts. https://seer.cancer.gov/statfacts/ (accessed 15 February 2021).
- 2. Wong MCS, Fung FDH, Leung C, et al. The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep 2018; 8: 1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang S, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2015. J Natl Cancer Center 2020; 1: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068–3077. [DOI] [PubMed] [Google Scholar]
- 5. Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 2001; 19: 2638–2646. [DOI] [PubMed] [Google Scholar]
- 6. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 2016; 76: 3003–3013. [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg J, Sridhar SS, Zhang J, et al. EV-101: a phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol 2020; 38: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 2019; 37: 2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Food and Drug Administration. Breakthrough therapy approvals, https://www.fda.gov/drugs/nda-and-bla-approvals/breakthrough-therapy-approvals (accessed 15 February 2021).
- 11. U.S. Food and Drug Administration. Novel drug approvals for 2019, https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019 (accessed 15 February 2021).
- 12. FDA grants regular approval to enfortumab vedotin-ejfv for locally advanced or metastatic urothelial cancer. U.S. Food and Drug Administration, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer (accessed 29 September 2021).
- 13. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 2021; 384: 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petrou P. A systematic review of economic evaluations of tyrosine kinase inhibitors of vascular endothelial growth factor receptors, mammalian target of rapamycin inhibitors and programmed death-1 inhibitors in metastatic renal cell cancer. Expert Rev Pharmacoecon Outcomes Res 2018; 18: 255–265. [DOI] [PubMed] [Google Scholar]
- 15. Vanness DJ, Lomas J, Ahn H. A health opportunity cost threshold for cost-effectiveness analysis in the United States. Ann Intern Med 2021; 174: 25–32. [DOI] [PubMed] [Google Scholar]
- 16. National Institution for Health and Care Excellence. Guide to the methods of technology appraisal, 2013, https://www.nice.org.uk/process/pmg9/chapter/the-appraisal-of-the-evidence-and-structured-decision-making (accessed 15 February 2021). [PubMed]
- 17. World Health Organization. The world health report 2002: Reducing risks, promoting healthy life. Geneva: World Health Organization, 2002. [Google Scholar]
- 18. Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Rese Methodol 2011; 11: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arias E. United States Life Tables, 2017. Natl Vital Stat Rep 2019; 68: 1–66. [PubMed] [Google Scholar]
- 20. National life tables: UK, https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 15 February 2021).
- 21. Global Health Observatory data repository life: tables: China, https://apps.who.int/gho/data/view.main.60340?lang=en, (accessed 15 February 2021). [DOI] [PubMed]
- 22. RedBook, https://www.micromedexsolutions.com/micromedex2/librarian/ssl/true (accessed 15 February 2021).
- 23. National Institute for Health Care Excellence, https://bnf.nice.org.uk/drug/ (accessed 15 February 2021).
- 24. DrugDataexpy, https://data.yaozh.com/ (accessed 15 February 2020).
- 25. Henk HJ, Chen C, Benedict A, et al. Retrospective claims analysis of best supportive care costs and survival in a US metastatic renal cell population. Clinicoecon Outcomes Res 2013; 5: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amdahl J, Diaz J, Sharma A, et al. Cost-effectiveness of pazopanib versus sunitinib for metastatic renal cell carcinoma in the United Kingdom. PLoS ONE 2017; 12: e0175920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Zeng X, Deng H, et al. Cost-effectiveness analysis of adding palbociclib as a second-line endocrine therapy for HR/HER2 metastatic breast cancer from the US and Chinese perspectives. Clin Ther 2019; 41: 1175–1185. [DOI] [PubMed] [Google Scholar]
- 28. Wan XM, Peng LB, Ma JA, et al. Economic evaluation of nivolumab as a second-line treatment for advanced renal cell carcinoma from US and Chinese perspectives. Cancer 2017; 123: 2634–2641. [DOI] [PubMed] [Google Scholar]
- 29. Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer 2018; 6: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong W, Yim Y, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PLoS ONE 2018; 13: e0196007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Georgieva M, da Silveira Nogueira Lima JP, Aguiar P, Jr, et al. Cost-effectiveness of pembrolizumab as first-line therapy for advanced non-small cell lung cancer. Lung Cancer 2018; 124: 248–254. [DOI] [PubMed] [Google Scholar]
- 32. Zhu J, He W, Ye M, et al. Cost-effectiveness of afatinib and erlotinib as second-line treatments for advanced squamous cell carcinoma of the lung. Future Oncol 2018; 14: 2833–2840. [DOI] [PubMed] [Google Scholar]
- 33. Liou SY, Stephens JM, Carpiuc KT, et al. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig 2007; 27: 381–396. [DOI] [PubMed] [Google Scholar]
- 34. Mickisch G, Gore M, Escudier B, et al. Costs of managing adverse events in the treatment of first-line metastatic renal cell carcinoma: bevacizumab in combination with interferon-alpha2a compared with sunitinib. Br J Cancer 2010; 102: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gu X, Zhang Q, Chu Y, et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer 2019; 127: 84–89. [DOI] [PubMed] [Google Scholar]
- 36. Shi G, Park SH, Ren H, et al. Cost analysis for different sequential treatment regimens for metastatic renal cell carcinoma in China. J Med Econ 2018; 21: 1150–1158. [DOI] [PubMed] [Google Scholar]
- 37. Hill G, Barron R, Fust K, et al. Primary vs secondary prophylaxis with pegfilgrastim for the reduction of febrile neutropenia risk in patients receiving chemotherapy for non-Hodgkin’s lymphoma: cost-effectiveness analyses. J Med Econ 2014; 17: 32–42. [DOI] [PubMed] [Google Scholar]
- 38. Younis T, Rayson D, Jovanovic S, et al. Cost-effectiveness of febrile neutropenia prevention with primary versus secondary G-CSF prophylaxis for adjuvant chemotherapy in breast cancer: a systematic review. Breast Cancer Res Treat 2016; 159: 425–432. [DOI] [PubMed] [Google Scholar]
- 39. Sarfaty M, Leshno M, Gordon N, et al. Cost effectiveness of nivolumab in advanced renal cell carcinoma. Eur Urol 2018; 73: 628–634. [DOI] [PubMed] [Google Scholar]
- 40. Sarfaty M, Hall PS, Chan KKW, et al. Cost-effectiveness of pembrolizumab in second-line advanced bladder cancer. Eur Urol 2018; 74: 57–62. [DOI] [PubMed] [Google Scholar]
- 41. Mistry R, May JR, Suri G, et al. Cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole and letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2- advanced or metastatic breast cancer: a U.S. payer perspective. J Manag Care Spec Pharm 2018; 24: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Durkee BY, Qian Y, Pollom EL, et al. Cost-effectiveness of pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2016; 34: 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu B, Gu X, Zhang Q. Cost-effectiveness of osimertinib for EGFR mutation-positive non-small cell lung cancer after progression following first-line EGFR TKI therapy. J Thorac Oncol 2018; 13: 184–193. [DOI] [PubMed] [Google Scholar]
- 44. Soto-Perez-de-Celis E, Aguiar PN, Cordón ML, et al. Cost-effectiveness of cabozantinib in the second-line treatment of advanced hepatocellular carcinoma. J Natl Compr Canc Netw 2019; 17: 669–675. [DOI] [PubMed] [Google Scholar]
- 45. Smith HJ, Walters Haygood CL, Arend RC, et al. PARP inhibitor maintenance therapy for patients with platinum-sensitive recurrent ovarian cancer: a cost-effectiveness analysis. Gynecol Oncol 2015; 139: 59–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211068733 for Cost-effectiveness of enfortumab vedotin in previously treated advanced urothelial carcinoma by Qiuji Wu, Yi Qin, Weiting Liao, Mengxi Zhang, Yang Yang, Pengfei Zhang and Qiu Li in Therapeutic Advances in Medical Oncology