Abstract
The prevalence rate of allergic diseases, such as asthma, atopic rhinitis (AR), and atopic dermatitis (AD), has been significantly increasing over the years because of environmental changes. Type 2 immunity is mediated by allergic inflammation initiated by an innate immune response. This response is orchestrated by type 2 cytokines (interleukin [IL]-4, IL-5, IL-9, and IL-13) together with other cells. The dendritic cell [DC]-T helper 2 (Th2) cell axis is the conventional type 2 immune pathway, and is currently known as the group 2 innate lymphoid cell (ILC2)-DC-Th2 axis that mediates type 2 inflammation. ILC2s strongly mediate type 2 inflammation in allergic diseases. ILC2s are activated by epithelial cell-derived cytokines, such as IL-25 and IL-33, and thymic stromal lymphopoietin. Additionally, ILC2s are activated by mast cell lipid inflammatory mediators, such as cysteinyl leukotrienes and prostaglandin D2. ILC2s produce a large amount of type 2 cytokines. The important role of ILC2s in the pathogenesis of type 2-mediated disease has resulted in ILC2-derived cytokines being a target for therapeutic development. In this review, we discuss type 2 immunity, mainly the ILC2-DC-Th2 axis, and other immune cells, the dominant role of ILC2s in asthma, AR, and AD, and therapeutic targets against type 2 cytokines.
Keywords: Group 2 innate lymphoid cell, type 2 immunity, asthma, atopic rhinitis, atopic dermatitis, interleukin, T helper 2 cell
Introduction
The prevalence rate of allergic diseases has increased exponentially in westernized, urbanized, and affluent societies. This increase is attributed to not only genetic factors, but also to a profound global transformation that has occurred in the past few decades. This increase in the prevalence of allergic diseases is probably a result of massive environmental changes, such as industrialization, housing design, family size, early-life microbial exposure, and exposure to significant amounts of pollutants.1,2
Type 2 immunity represents the typical adaptive immunity initiated by innate immunity in response to allergen exposure in atopic individuals 3 ; this results in a dysregulated immune response that leads to chronic inflammation in individuals with typical type 2-immune mediated diseases such as asthma, AR and AD, ensued from overexpression of type 2 inflammatory pathways. The type 2 immune reaction is characterized and orchestrated by a set of type 2 cytokines in conjunction with a repertoire of cellular mechanisms. These mechanisms involve T helper 2 (Th2) cells, dendritic cells (DCs), innate lymphoid cells (ILCs), epithelial cells, eosinophils, mast cells (MCs), and basophils.4,5
Activation of group 2 innate lymphoid cells (ILC2s) and Th2 pathways play a prominent part in type 2 inflammation, and both are capable of producing type 2 cytokines. 4 Remarkably, the recent discovery of ILCs, particularly ILC2, has broadened the understanding of the type 2 immune response. Unlike conventional Th2 cells, ILC2s produce copious amounts of type 2 cytokines. 6 ILC2s from naïve unchallenged mice express interleukin (IL)-5, while CD4 T cells require an activation signal to differentiate and secret effector cytokines. 7 ILC2s exist as lineage-negative innate cells producing type 2 cytokines. These cells arise from lymphoid progenitors in the bone marrow and this is facilitated by the transcriptional factors retinoic acid receptor-related orphan receptor α and GATA-binding factor 3. ILC2s lack antigen specificity and function in an antigen-independent manner. 8 ILC2s serve as a rapid, first line of defense against invading pathogens before the adaptive immune response (Th2 cells) becomes active. 9 Interestingly, over the past decade, many studies have confirmed the dominance of ILC2s in typical type 2-mediated diseases (asthma, atopic rhinitis [AR], and atopic dermatitis [AD]), which is attributed to the severity of the clinical conditions mentioned above. 10 These significant insights into ILC2 pathology have resulted in the consideration of ILC2 as a therapeutic target. 11 Recently, several biological drugs against type 2 cytokines were approved by the Food and Drug Administration (FDA), and they provide a favorable outcome. However, several drugs present with a therapeutic dilemma, and others are still in the developmental phase. 12 Therefore, the effect and role of ILC2s in the development of allergic diseases, especially asthma, AR, and AD, need to be determined. In this review, we analyze the relationship between ILC2s and allergic diseases, and provide a theoretical basis for the current treatment of allergic diseases.
Type 2 immunity
Type 2 immunity comprises the mechanisms through which an immune response is initiated following helminth infections, exposure to allergens, and other diverse environmental stimuli. 13 Type 2 immunity consists of innate immune response and an antigen-specific adaptive immune response. An innate immune system is the first line of defense against invading pathogens, while an adaptive immune response is slower-acting, longer-lasting, and requires antigen presentation to mount an effective counteractive response. 14 Epithelial cells are non-hematopoietic cells that are critical for initiating the type 2-immune response. Epithelial cell-derived cytokines (thymic stromal lymphopoietin [TSLP], IL-25, and IL-33) are crucial in the development and regulation of ILC2 and Th2 proliferation. 15 Type 2 immunity is characterized by the cytokines IL-4, IL-5, IL-9, and IL-13, and by associated cell types, including basophils, eosinophils, MCs, T helper cells, and TLC2s, which are crucial in orchestrating type 2 inflammation. 16 Traditionally, the type 2 immune response is recognized as the DC-Th2 cell axis, where Th2 cells take the center stage, although currently, the type 2 immune response is widely known as the ILC2-DC-Th2 axis. 6
DC-Th2 cell crosstalk is dependent on antigen-presenting cells, mainly DCs. Extracellular pathogens are recognized by DCs via specific pathogen-associated molecular patterns on the pathogen’s surface, resulting in destruction of pathogens through phagocytosis. DCs then move further to lymph nodes to present antigens to naive T-cells, resulting in differentiation of naive T-cells into Th2 cells. Furthermore, DCs release IL-6 and IL-4 to induce maturation and proliferation of Th2 cells, respectively. Mature Th2 cells produce type 2 cytokines to elicit the type 2 immune response and promote a humoral immune response. 17 Moreover, DCs’ expression of programed death-ligand 2 affects Th2 responses. 18
ILC2-Th2 cell crosstalk is more complex and has bidirectional communication. ILC2s affect Th2 cell responses through indirect effects (i.e., cytokines) or direct cell to cell contact. Although ILC2s mainly express IL-5 and IL-13, they can express IL-4 during helminth infection, which is involved in immune regulation to induce Th2 cell differentiation. 19 Therefore, Th2 cells are an abundant source of IL-4, which induces the conversion of the B cell isotype to immunoglobulin E (IgE) production. 20 In the indirect effect scenario, ILC2-derived IL-4 promotes early Th2 differentiation. Th2-derived IL-2 induces ILC2 proliferation and IL-13 secretion. However, in a direct cell to cell effect, ILC2s promote Th2 polarization in in vitro culture of ILC2s together with Th2 cells. Studies in IL-17 knockout mice have shown that contransfer of ILC2s and Th2 cells induces a potent antigen-specific type 2 immune response. 21 This finding suggests that coordination between ILC2s and Th2 cells is essential for the production of long-lasting type 2 immunity. Oliphant et al. showed that ILC2s expressed major histocompatibility complex II-mediated antigen presentation, which was able to process pathogens and present antigen peptide to activate Th2 cells. 22 Additionally, ILC2 expression of programed death-ligand 1 has a crucial role in inducing and maintaining Th2 responses via programmed death receptor 1 (PD-1) expression on Th2 cells. 18
In DC-ILC2 crosstalk, the OX40 ligand expressed by DCs is important for the effective response of Th2 cells to worm-derived antigens, which stimulate the activation of DCs. 23 Activated DC subsets (cDC1 and cDC2) can trigger the activation of ILC2 through the production of TSLP, IL-33, and tumor necrosis factor-like ligand 1A. 24 ILC2-derived IL-13 enhances migration of activated DCs into the lymph nodes where they promote the differentiation of naive T cells into Th2 cells. 25 The downstream repertoire of type 2 immunity includes eosinophils, basophils, and MCs. MCs are capable of producing epithelial-derived cytokines (TSLP, IL-25, and IL-33) to drive type 2 immune inflammation by further activation of ILC2s and Th2 cells. ILC2-derived IL-5 promotes the recruitment and production of eosinophils. ILC2s can produce IL-4 and express IL-4 receptor. IL-4-producing eosinophils cocultured with IL-33 greatly increase ILC2 proliferation and production of IL-5 and IL-13 to magnify the type 2 immune response. 26 Basophils directly modulate ILC2s, and basophil-derived IL-4 enhances the sensitivity of ILC2s to epithelial cell-derived cytokines and promotes the migration of ILC2s into the inflammation site. 27 MCs release IL-33, which leads to the expansion of ILC2s and the production of IL-13. Th9 cells secrete IL-9, which acts on MCs to release IL-2, and MC-derived IL-2 causes the proliferation of CD45 + ILC2 and cytokine production. Additionally, MCs produce lipid mediators, such as prostaglandin D2 (PGD2), which stimulates ILC2 migration into the lungs and facilitates the production of type 2 cytokines. 28 The recent discovery of ILC2s has increased the immunological understanding of ILC2s and the Th2 immunity nexus, along with other associated immune cells. Several studies have shown that the ILC2 and Th2 inflammation loop is a connected circuit and functions in a bidirectional manner, but many aspects of it remain complex and unclear. 29
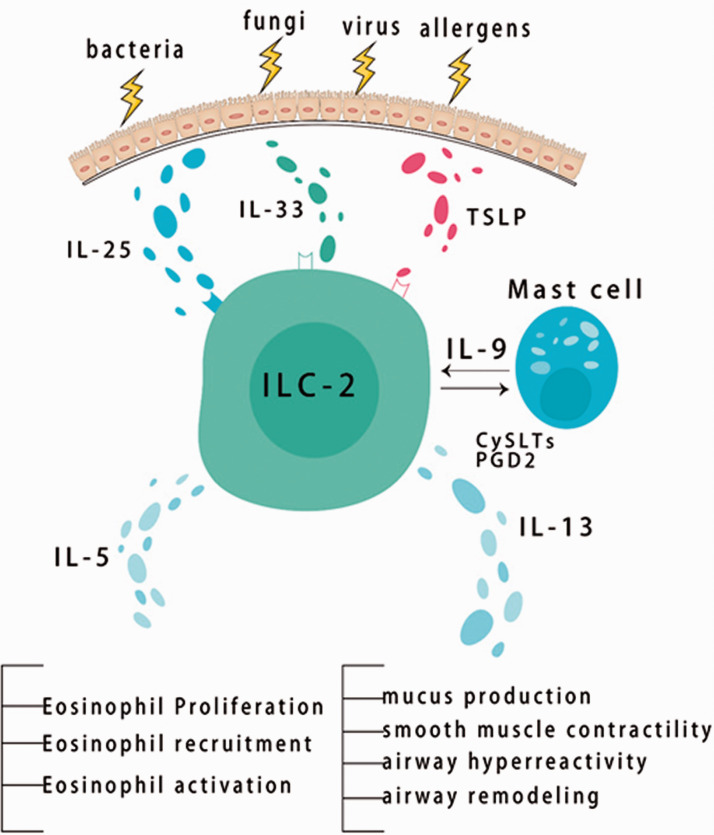
An overview of the ILC2-DC-Th2 axis associated with type 2 immunity is shown in Figure 1.
Figure 1.
Summary of type 2 immunity.
ILC2 activation and expansion
ILC2s are activated by epithelial cell-derived cytokines termed ILC2 alarmins, including IL-25, IL-33, and TSLP. ILC2 alarmins are expressed by epithelial cells and other immune cells owing to a pathogenic challenge resulting in ILC2 expansion and production of type-2 effector cytokines. 30 TSLP is a member of the IL-2 family, and is primarily present at epithelial cells lining the lungs, skin, and gastrointestinal tract. TSLP is also produced by DCs, basophils, and MCs. TSLP activates ILC2s by binding to the TSLP receptor that is coupled with IL-7α to promote the induction of type 2 cytokines. IL-25 (also known as IL-17E) is a member of the IL-17 family and is produced by epithelial cells, eosinophils, MCs, macrophages, and basophils. IL-25 is also more widely expressed by epithelial cells in the lungs and skin in patients with asthma or atopic disease compared with control subjects. IL-25 binds to IL-17RB in response to tissue injury to activate ILC2s downstream, and this mediates the production of type 2 cytokines, predominately IL-13. IL-33 belongs to the IL-1 family, and it activates ILC2s through binding to its receptor named suppression of tumorigenicity 2 (also referred to as IL-33R, IL-1RL1, or T1). IL-33 is largely expressed in barrier cells (epithelial or endothelial cells) and MCs. IL-33 proliferates into the extracellular space in response to cellular damage or tissue injury, resulting in enhanced production of type 2 cytokines, predominately IL-5 and IL-13. 31
Studies have shown that IL-33 is more potent than IL-25 or TSLP in ILC2 activation.32,33 However, ILC2s show a strong response to synergistic stimulation compared with a single cytokine effect. IL-33 along with IL-2 induce robust activation of ILC2s and increase expression of type 2 cytokines. IL-33 in combination with either IL-25 or TSLP doubles ILC2 proliferation with 5 days in vitro stimulation. 34 Furthermore, ILC2s not only respond to inducer cytokines, but also respond to MC lipid inflammatory mediators, such as cysteinyl leukotrienes (CysLTs) and PGD2. Doherty et al. showed that CysLT type 1 receptor (CysLT1R) was expressed on lung ILC2s in unaffected mice and remained expressed on lung ILC2s after an allergen attack, which was independent of B cells and T cells. 35 Human ILC2s express CysLT receptor. CysLTs, particularly leukotriene E4 (LTE4), trigger ILC2 survival, migration, and activation, and enhance type 2 cytokine (IL-4, IL-5, IL-13, and amphiregulin) production. The addition of IL-2 and epithelial cytokines to LTE4 causes considerable ILC2 activation. PGD2 alone or in combination with epithelial cytokines stimulates ILC2s and enhances production of type 2 cytokines. The efficacy of CysLTs in human ILC2s in vitro was significantly higher than that of endogenous cytokines (IL-33, IL-25, and TSLP), but lower than that of PGD2, when used individually. 36 Activated ILC2s produce type 2 cytokines, which orchestrate a type 2 immune response against allergens or pathogens. ILC2-derived IL-4 is required to modulate the recruitment and migration of ILC2 to the inflammation site in Heligmosomoides polygyrus and induce the differentiation of CD4+ Th2 cells. 37 ILC2-derived IL-5 helps activate and recruit inflammatory eosinophils, thereby promoting inflammation and tissue damage in type 2 immune-mediated diseases. 38 ILC2-derived IL-9 amplifies the role of ILC2s by promoting their survival and activation by stimulating MCs. This process promotes the function of ILC2 by releasing IL-2 growth factor, resulting in an effective ILC2 inflammatory response. 39 ILC2-derived IL-13 is crucial in facilitating the migration of activated lung DCs into draining lymph nodes, where naive T cells are initiated to Th2 cells, thereby activating an adaptive response. 40
An overview of the activation and extension of ILC2s is shown in Figure 2.
Figure 2.
Activation and expansion of group 2 innate lymphoid cells.
ILC2 dominance in different allergic diseases
ILC2 dominance in AD
AD is also referred to as atopic eczema in humans and presents with symptoms, such as dryness of the skin, pruritus, and chronic scratching. AD is also related to dysregulation of ILC2s, similar to other allergic diseases. ILC2s are significantly increased in the skin of patients with AD relative to skin samples obtained from healthy controls. Over the past decade, many studies have shown the dominance of ILC2s in skin AD-like inflammation. 41 Kim et al. isolated ILC2s from human and murine AD lesions and showed that the number of ILC2s was significantly higher compared with that in healthy skin. 42 These authors further reported that isolated ILC2s were independent of IL-33 and IL-25, but dependent on TSLP. Additionally, depletion of ILC2s in mice with AD was accompanied by a significant reduction in inflammation and AD-like disease. Imai et al. found that IL-33-induced AD depended on ILC2s. 43 They depleted the ILC2 population by transplanting retinoic acid receptor-related orphan receptor α-deficient bone marrow into IL33tg mice. In 15 weeks, ILC2s were effectively depleted, and AD-like inflammation was abolished in ILC2-lacking IL33tg mice. Mashiko et al. examined isolated skin lesions from patients with AD (n = 12) and patients with psoriasis (n = 11). 44 They found a remarkable elevation in ILC2s in patients with AD compared with those with psoriasis. Furthermore, they analyzed subtypes of ILCs (ILC2s and ILC3s) in AD and psoriasis, and they found a substantial increase in ILC2s in AD skin lesions and an increase in ILC3s in psoriatic skin. Salimi et al. showed that ILC2s are skin-resident lymphoid cells, but their frequency tended to increase in the presence of AD-like inflammation. 45 They examined AD skin biopsies to determine whether ILC2s contribute to the pathogenesis of AD, and found a significantly higher number of ILC2s in skin biopsies from patients with AD compared with that of ILC2s in healthy subjects. Moreover, they reported depletion of ILC2-inducing cytokines (IL-33, IL-25, and TSLP), which resulted in a decreased number of ILC2s and a decline in AD-like inflammation. Roediger et al. isolated skin ILC2s, and found that dermal ILC2s, which were abundant in the skin tissue, were dependent on IL-7 and essentially produced IL-13. 46 Additionally, upon IL-2 stimulation, IL-5 production was enhanced and this further activated other immune cells to cause spontaneous dermatitis. Such studies have contributed to proving that ILC2s reside in the skin tissue and that they are involved in the generation of type 2-associated skin inflammation and ILC2 interaction in a coordinated manner with other immune cells in the skin to regulate their function. However, further studies are required to assess the factors that promote and regulate ILC2-mediated skin inflammation. 47
ILC2 dominance in AR
AR is mediated by exposure to an allergen, and is caused by dysregulation of ILC2s and IgE in response to an invading allergen. Typical symptoms of AR include sneezing, nasal pruritus, rhinorrhea, and nasal congestion. Several studies have shown increased ILC2s, and increased concentrations of ILC2-inducing cytokines (IL-33, IL-25, and TSLP) and ILC2-derived cytokines (IL-4, IL-5, and IL-13) in patients with AR. 9 Liu et al. analyzed the presence of ILC2s from nasal mucosa in a murine model of AR. 48 They reported remarkably higher ILC2 infiltration in the nasal mucosa of AR mice compared with that in control mice. They also found that the nasal mucosa in AR mice showed elevated ILC2-derived cytokine and IgE concentrations compared with those in the control group. Doherty et al. studied patients with a cat allergy and reported a significant elevation in the percentage of ILC2-expressing prostaglandin D2 receptor (CRTH2) in the peripheral blood following an allergen challenge. 49 Similarly, Fan et al. analyzed ILC2s in the periphery in patients with house dust mite (HDM) sensitivity and AR, those with mugwort sensitivity and AR, and controls. 50 They reported a remarkably higher number of peripheral blood ILC2s in patients with HDM sensitivity and AR compared with that in controls. However, there was no notable difference in ILC2 levels between patients with mugwort sensitivity and AR and the control group. Furthermore, Fan et al. observed peripheral blood mononuclear cells (PBMCs) in patients with HDM sensitivity and AR when cocultured with IL-33 alone or in synergy with IL-25. However, coculture with IL-2 potently induced IL-13 and IL-5. 50 These findings suggested a dominant role of ILC2s in AR, particularly in patients with HDM sensitivity. In a murine model, Kato et al. induced local AR via nasal sensitization with phorbol 12-myristate 13-acetate and ionomycin, and they found that ILC2s in the nasal mucosa produced IL-5 and IL-13. 51 Lombardi et al. studied the number of ILC2s and ILC3s in the blood during and outside of the grass pollen season in a population of patients who were sensitized to grass pollen and an unsensitized group. 52 They observed a higher number of ILC2s and ILC3s in patients who were sensitized to grass pollen compared with that in the unsensitized group. Lombardi et al. further reported that the number of ILC1s in the blood remained unchanged during seasonal allergen exposure. 52 Zhong et al. reported an increase in ILC2 levels in patients with HDM responsiveness, and the severity of symptoms was associated with the number of peripheral blood ILC2s. 53 Similar to Salimi et al., 45 Qin et al. 54 examined the circulating ILC2s that expressed CysLT1R in patients with AR, in those with AD, and in controls. PBMCs were stimulated with LTE4, leukotriene C4, or leukotriene D4 (LTD4), and treated with a CysLT1R antagonist (montelukast). Interestingly, they found a higher frequency of ILC2s expressing CysLT1R in patients with AR compared with that in controls. However, treatment with montelukast in freshly isolated ILC2s resulted in a profound reduction in type 2 cytokines in the presence of leukotriene C4 and LTD4, but not LTE4. Qin et al. proposed that the activation of ILC2s in AR is more strongly associated with leukotriene C4 and LTD4, but not LTE4, and that treatment with montelukast in ILC2s expressing CysLT1R-related AR may be beneficial. 54
ILC2 dominance in asthma
Asthma is a chronic disease and heterogeneous, and is characterized by airway inflammation, reversible airflow obstruction, and airway hyperresponsiveness. The symptoms associated with asthma include shortness of breath, wheezing, and coughing. Previously, asthma was understood as a Th2 and eosinophilic dominant disease. However, since the discovery of ILC2s, many studies have suggested otherwise. Over the past decade, several studies have postulated the dominant role of ILC2s in the pathogenesis of asthma. 9 Chen et al. induced asthma attacks by an allergen challenge, and found that, 24 hours after sensitization, the number of ILC2s in the sputum was significantly higher than that before sensitization. 55 Additionally, the number of ILC2s expressing IL-5 or IL-13, and both IL-5 and IL-13 was significantly elevated in the sputum at 24 hours, but not at 48 hours, after sensitization. However, the number of blood and bone marrow ILC2s remained low and inactivated at 24 hours up until 48 hours after the allergen challenge. Their findings suggested early local activation of ILC2s before systemic activation of ILC2s in the presence of an allergen. Smith et al. reported higher numbers of blood and sputum ILC2s in patients with severe asthma in whom the rate of airway eosinophilia was >3% compared with those in patients with mild asthma. 56 However, sputum CD4+ lymphocytes were more numerous than ILC2s, but ILC2s were the major source of type 2 cytokines. Furthermore, the number of type 2 cytokines expressed by ILC2s in the airway and peripheral blood was significantly higher in patients with oral corticosteroid therapy and severe eosinophilic asthma compared with that in patients with steroid-naive mild asthma. This study suggests that a high number of ILC2s in individuals with severe eosinophilic asthma can be resistant to oral corticosteroid therapy. Similarly, Christianson et al. showed increased IL-33 concentrations in bronchoalveolar lavage fluid in patients with asthma. 57 This subsequently resulted in an increased number of ILC2s in bronchoalveolar lavage fluid, and IL-33 concentrations were correlated with asthma severity. Furthermore, depletion of T cells in a persistent asthma model resolved inflammation, but not airway hyperreactivity and airway remodeling. In contrast, depletion of ILC2s resulted in the resolution of all symptoms of asthma. These findings indicate the important role of ILC2s in airway remodeling. 57 Bartemes et al. investigated ILC2s in the peripheral blood of asthma in humans, and found that the frequency of ILC2s in PBMCs was significantly higher in patients with asthma compared with that in healthy controls. 58 Isolated ILC2s from human PBMCs when stimulated with IL-33 or IL-25 in the presence of IL-2, IL-7, or TSLP produced high IL-5 and IL-13 concentrations, but not IL-4 concentrations. 59 This finding suggests that IL-2, IL-7, and TSLP play an important role in the survival of ILC2s, and also indicates that ILC2s are the predominant source of IL-5 and IL-13, but not IL-4. The role of ILC2s in human and murine asthma has been extensively studied over the past years, but the role of ILC2s and their subsets in asthma are still not fully understood. Therefore, further studies are required to better understand the role of ILC2s in asthma.
Therapeutic targets against selected biologics
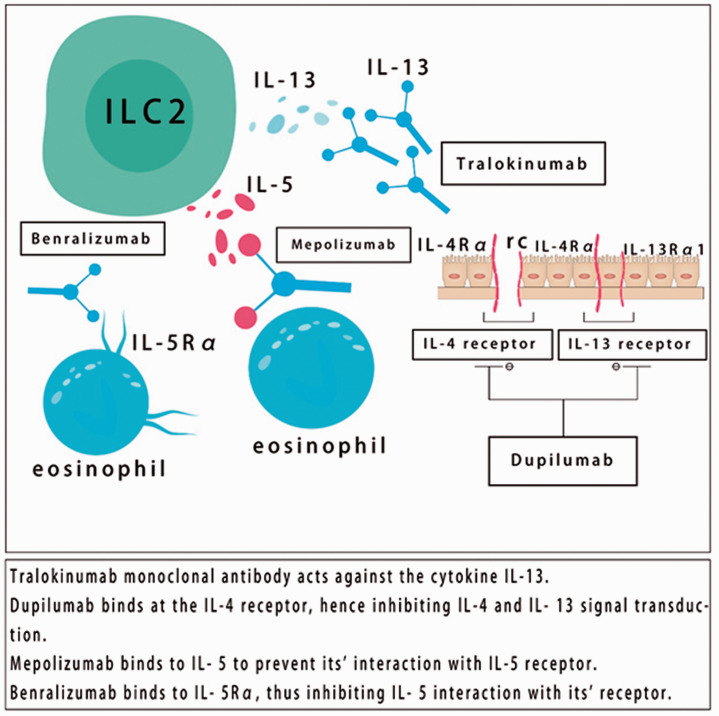
Dupilumab is a humanized IgG4 monoclonal antibody that acts against the alpha subunit of the IL-4 receptor (IL-4Rα). Dupilumab inhibits IL-4 and IL-13 signaling, which are important type 2 cytokines that promote atopic inflammation. IL-4Rα was recently approved by the US FDA for treating AD in adults and as add-on therapy in patients with asthma aged 12 years or older with eosinophilic asthma or with oral corticosteroid dependency. Dupilumab has a good efficacy and improves symptoms in atopic diseases. However, the safety and adverse effects of dupilumab remain unclear, and more data are required for further analysis. Moreover, advanced research is required to evaluate the efficacy of dupilumab in various atopic diseases and their effectiveness in pediatric patients. 60
Mepolizumab (anti-IL-5) is a humanized monoclonal IgG1 antibody, which selectively recognizes and blocks IL-5, and thus inhibit its ability to bind with IL-5Rα. IL-5 is a key cytokine secreted by ILC2s, Th2 cells, MCs, and eosinophils, and it plays a role in the activation, proliferation, maturation, and survival of eosinophils. Mepolizumab has been approved by the US FDA and the European Medicines Agency as an add-on therapy for treating severe eosinophilic asthma. Moreover, mepolizumab has shown effectiveness in treating individuals with difficult-to-treat asthma, decreases the number of eosinophils in the blood and sputum, and greatly lowers dependence on oral corticosteroids. Furthermore, treatment with mepolizumab decreases exacerbation of asthma, but has not been shown to improve overall asthma symptoms or lung function. Interestingly, mepolizumab has recently been included as an add-on therapy for severe eosinophilic asthma within the Global Initiative for Asthma Guidelines. 61
Benralizumab (anti-IL-5Rα) is a humanized monoclonal antibody that blocks IL-5Rα. Benralizumab has been approved by the FDA as a synergy for treating severe asthma in individuals aged 12 years or older with eosinophilia. This antibody has remarkable efficacy, improves asthma-related quality of life, decreases asthma exacerbation, and improves lung function in moderate to severe eosinophilic asthma. However, benralizumab’s adverse effects remain debatable. Therefore, benralizumab requires further comprehensive analysis. 62
Tralokinumab (anti-IL-13) is a humanized IgG4 monoclonal antibody that inhibits soluble IL-13 from binding with IL-4Rα/IL-13Rα1. IL-13 is responsible for goblet cell hyperplasia, airway hyperresponsiveness, and fibrosis. Tralokinumab improves lung function in moderate to severe asthma, but does not improve asthma symptoms or asthma exacerbation. However, tralokinumab reduces asthma exacerbation in patients with severe asthma and high fractional exhaled nitric oxide concentrations. The efficacy and safety of tralokinumab for treating asthma require further evaluation. 63 However, phase III clinical trials have demonstrated a significant role of tralokinumab in AD. 64 On 9 July 2020, LEO Pharma A/S announced that the Biologics License Application for tralokinumab for treating adults with moderate to severe AD had been accepted for review by the US FDA.
An overview of therapeutic targets against selected biologics and their mechanism is shown in Figure 3.
Figure 3.
Therapeutic targets against selected biologics.
There are some other therapies related to ILC2s and allergic diseases, especially asthma, such as PD-1 agonists and leukotriene receptor antagonists. PD-1, which is metabolic checkpoint in ILC2s, affects cellular activation and proliferation. Furthermore, PD-1 can limit the viability of ILC2s and downregulate the function of their effector. A human PD-1 agonist was developed, and it can ameliorate airway hyperreactivity and suppress lung inflammation in a humanized mouse model of allergic asthma. 65 CysLT1 has an extremely high affinity for LTD4, which is the most effective airway muscle constrictor. Montelukast, which is a CysLT1R antagonist, is commonly prescribed to improve asthma symptoms in humans and has been proven to have good results. 66
Conclusion
The incidence of asthma, AR, and AD is increasing in industrialized nations. Type 2 immunity consists of innate and adaptive immune responses that promote allergic inflammation. The relationship between the ILC2-DC-Th2 axis and other repertoires of immune cells is complex and intriguing, and some part of this circuit remains unclear and requires further investigation. Since the discovery of ILC2, many studies have confirmed the implications of ILC2s in various clinical conditions, such as asthma, AR, and AD. These important insights suggest that ILC2s could be an important therapeutic target. Recently, some drugs have been approved that function against type 2 cytokines and have shown a favorable outcome. However, some other drugs have shown no clinical benefits and some are still at the developmental stage.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was financially supported by the Natural Science Foundation of Jiangsu Province (No. BK20201226) and the Social Development Foundation of Zhenjiang, China (grant no. SH2020037).
ORCID iD: Jie Jin https://orcid.org/0000-0002-2414-6501
References
- 1.Custovic A. Middleton's Allergy Essentials ǁ Epidemiology of Allergic Diseases. 2017: 51–72.
- 2.Skaaby T, Husemoen LLN, Thuesen BH, et al. IgE sensitization to inhalant allergens and the risk of airway infection and disease: A population-based study. PLoS One 2017; 12: e0171525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marco C, Le PD, Diego B, et al. Type 2 immunity in asthma. World Allergy Organization Journal 2018; 11: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akdis CA, Arkwright PD, Brüggen MC, et al. Type 2 immunity in the skin and lungs. Allergy 2020; 75: 1582–1605. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd CM, Snelgrove RJ. Type 2 immunity: Expanding our view. Sci Immunol 2018; 3: eaat1604. [DOI] [PubMed] [Google Scholar]
- 6.Gurram RK, Zhu J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol Immunol 2019; 16: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Dyken SJ, Nussbaum JC, Lee J, et al. A tissue checkpoint regulates type 2 immunity. Nat Immunol 2016; 17: 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker JA, Mckenzie ANJ. Development and function of group 2 innate lymphoid cells. Curr Opin Immunol 2013; 25: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Cadena A, Romero P, Trabanelli S, et al. Detecting and analyzing murine innate lymphoid cells. Methods Enzymol 2020; 631: 329–342. [DOI] [PubMed] [Google Scholar]
- 10.Pasha MA, Patel G, Hopp R, et al. Role of innate lymphoid cells in allergic diseases. Allergy Asthma Proc 2019; 40: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ercolano G, Falquet M, Vanoni G, et al. ILC2s: New Actors in Tumor Immunity. Front Immunol 2019; 10: 2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erzurum S, et al. Asthma, An issue of clinics in chest medicine. 8th March 2019.
- 13.De Kouchkovsky DA, Ghosh S, Rothlin CV. Negative Regulation of Type 2 Immunity. Trends Immunol 2017; 38: 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezaei N. Physiology and Pathology of Innate Immune Response Against Pathogens. 2017, 10.5772/66541(Chapter 6).
- 15.Jing X, Yan W, Zeng H, et al. Qingfei oral liquid alleviates airway hyperresponsiveness and mucus hypersecretion via TRPV1 signaling in RSV-infected asthmatic mice. Biomed Pharmacother 2020; 128: 110340. [DOI] [PubMed] [Google Scholar]
- 16.Gieseck RL, 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 2017; 18: 62. [DOI] [PubMed] [Google Scholar]
- 17.Kim B, Kim TH. Fundamental role of dendritic cells in inducing Th2 responses. Korean J Intern Med 2018; 33: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batyrova B, Luwaert F, Maravelia P, et al. PD-1 expression affects cytokine production by ILC2 and is influenced by peroxisome proliferator-activated receptor-γ. Immun Inflamm Dis 2019; 8: 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25–dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 2006; 203: 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong H, Dolpady J, Wabl M, et al. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med 2012; 209: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4 + T cells cooperate to mediate type 2 immune response in mice. Allergy 2014; 69: 1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliphant CJ, Hwang YY, Walker JA, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014; 41: 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins SJ, Perona-Wright G, Worsley AGF, et al. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol 2007; 179: 3515–3523. [DOI] [PubMed] [Google Scholar]
- 24.Arthur M, Kyle B. Cytokine Networks between Innate Lymphoid Cells and Myeloid Cells. Front Immunol 2018; 9: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halim TYF, Steer CA, Mathä L, et al. Group 2 Innate Lymphoid Cells Are Critical for the Initiation of Adaptive T Helper 2 Cell-Mediated Allergic Lung Inflammation. Immunity 2014; 40: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Der Ploeg EK, Mascaro AC, Huylebroeck D, et al. Group 2 Innate Lymphoid Cells in Human Respiratory Disorders. J Innate Immun 2020; 12: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BS, Wang K, Siracusa MC, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 2014; 193: 3717–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortaz E, Amani S, Mumby S, et al. Role of mast cells and innate immune lymphoid (ILC2) cells in lung transplantation. J Immunol Res 2018; 2018: 2785971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain A, Pasare C. Innate Control of Adaptive Immunity: Beyond the Three-Signal Paradigm. J Immunol 2017; 198: 3791–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel NN, Kohanski MA, Maina IW, et al. Sentinels at the wall: epithelial‐derived cytokines serve as triggers of upper airway type 2 inflammation. Int Forum Allergy Rhinol 2019; 9: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell–derived cytokines: more than just signaling the alarm. J Clin Invest 2019; 129: 1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow JL, Peel S, Fox J, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol 2013; 132: 933–941. [DOI] [PubMed] [Google Scholar]
- 33.Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci 2019; 96: 2–7. [DOI] [PubMed] [Google Scholar]
- 34.Camelo A, Rosignoli G, Ohne Y, et al. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv 2017; 1: 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doherty TA, Khorram N, Lund S, et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol 2013; 132: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salimi M, Stöger L, Liu W, et al. Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol 2017; 140: 1090–1100.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelly VS, Kannan Y, Coomes SM, et al. IL-4-producing ILC2s are required for the differentiation of TH2 cells following Heligmosomoides polygyrus infection. Mucosal Immunol 2016; 9: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinmann S, Schoedsack M, Heinrich F, et al. Hepatic ILC2 activity is regulated by liver inflammation-induced cytokines and effector CD4+ T cells. Sci Rep 2020; 10: 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz-Kuhnt A, Greif V, Hildner K, et al. ILC2 Lung-Homing in Cystic Fibrosis Patients: Functional Involvement of CCR6 and Impact on Respiratory Failure. Front Immunol 2020; 11: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krempski JW, Iijima K, Kobayashi T, et al . ILC2-derived IL-13 likely promotes development of peanut allergy. J Immunol 2018; 200: 46.12. [Google Scholar]
- 41.Stier MT, Peebles RS., Jr. Innate lymphoid cells and allergic disease. Ann Allergy Asthma Immunol 2017; 119: 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim B, Siracusa M, Saenz SA, et al . TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 2013; 5: 170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai Y, Yasuda K, Nagai M, et al. IL-33-Induced Atopic Dermatitis-Like Inflammation in Mice Is Mediated by Group 2 Innate Lymphoid Cells in Concert with Basophils. J Invest Dermatol 2019; 139: 2185–2194.e3. [DOI] [PubMed] [Google Scholar]
- 44.Mashiko S, Mehta H, Bissonnette R, et al. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J Dermatol Sci 2017; 88: 167–174. [DOI] [PubMed] [Google Scholar]
- 45.Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med 2013; 210: 2939–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roediger B, Kyle R, Yip KH, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol 2013; 14: 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim BS. Innate Lymphoid Cells in the Skin. J Invest Dermatol 2015, 135: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, Yang X, Liu X, et al . Analysis of expression of ILC2 cells in nasal mucosa based on animal model of allergic bacterial infection rhinitis. J Infect Public Health 2021; 14: 77–83. [DOI] [PubMed] [Google Scholar]
- 49.Doherty TA, Scott D, Walford HH, et al. Allergen challenge in allergic rhinitis rapidly induces increased peripheral blood type 2 innate lymphoid cells that express CD84. J Allergy Clin Immunol 2014; 133: 1203–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan D, Wang X, Wang M, et al. Allergen-Dependent Differences in ILC2s Frequencies in Patients With Allergic Rhinitis. Allergy Asthma Immunol Res 2016; 8: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato Y, Akasaki S, Muto-Haenuki Y, et al. Nasal Sensitization with Ragweed Pollen Induces Local-Allergic-Rhinitis-Like Symptoms in Mice. PLoS One 2014; 9: e103540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lombardi V, Beuraud C, Neukirch C, et al. Circulating innate lymphoid cells are differentially regulated in allergic and nonallergic subjects. J Allergy Clin Immunol 2016; 138: 305–308. [DOI] [PubMed] [Google Scholar]
- 53.Zhong H, Fan XL, Yu QN, et al. Increased innate type 2 immune response in house dust mite-allergic patients with allergic rhinitis. Clin Immunol 2017; 183: 293–299. [DOI] [PubMed] [Google Scholar]
- 54.Qin ZL, Peng YQ, Fang SB, et al. CysLT1R expression on ILC2s and effects CysLT1R antagonist on ILC2 activity in patients with allergic rhinitis. Allergy 2020; 75: 977–981. [DOI] [PubMed] [Google Scholar]
- 55.Chen R, Smith SG, Salter B, et al. Allergen-induced increase in sputum levels of group 2 innate lymphoid cells in subjects with asthma. Am J Respir Crit Care Med 2017; 196: 700–712. [DOI] [PubMed] [Google Scholar]
- 56.Smith SG, Chen R, Kjarsgaard M, et al. Increase numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol 2016; 137: 75–86.e8. [DOI] [PubMed] [Google Scholar]
- 57.Christianson CA, Goplen NP, Zafar I, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol 2015; 136: 59–68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartemes KR, Kephart GM, Fox SJ, et al. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 2014; 134: 671–678.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Helfrich S, Mindt BC, Fritz JH, et al. Group 2 innate lymphoid cells in respiratory allergic inflammation. Front Immunol 2019; 10: 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thibodeaux Q, Smith MP, Ly K, et al. A review of Dupilumab in the treatment of atopic diseases. Hum Vaccin Immunother 2019; 15: 2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corrado P, Vatrella A, Busceti MT, et al . Severe eosinophilic asthma: From the pathogenic role of interleukin-5 to the therapeutic action of Mepolizumab. Drug Des Devel Ther 2017; 11: 3137–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W, Ma X, Zhou W. Adverse events of benralizumab in moderate to severe eosinophilic asthma. Medicine (Baltimore) 2019; 98: e15868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura Y, Sugano A, Ohta M, et al. Docking analysis and the possibility of production efficacy for an anti IL-13 biopharmaceutical treatment with tralokinumab and lebrikizumab for bronchial asthma. PLos One 2017; 129: e0188407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol 2021; 184: 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helou DG, Shafiei-Jahani P, Lo R, et al. PD-1 pathway regulates ILC2 metabolism and PD-1 agonist treatment ameliorates airway hyperreactivity. Nat Commun 2020; 11: 3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kittana N, Hattab S, Ziyadeh-Isleem A, et al. Montelukast, current indications and prospective future applications. Expert Rev Respir Med 2016; 10: 943–956. [DOI] [PubMed] [Google Scholar]