Abstract
Introduction:
Patient initiated, remote cardiac monitoring has proved to be a significant advance in the diagnosis and management of arrhythmias. Further improvements in ease of use and access to results will further improve health outcomes and cost-effectiveness. Here we describe a proof-of-concept evaluation to assess the feasibility of successfully implementing a cloud-based management system using KardiaPro (KP) for remote electrocardiogram (ECG) monitoring to interface into EPIC, an enterprise electronic health record (EHR) system.
Methods:
The KP management system was embedded using hypertext markup language (HTML) code directly into the EHR. Encrypted credentials and patient data were bundled with an application programming interface key allowing linkage of remote monitoring from patients’ smartphones. During the time of implementation, a total of 322 patients and 32 179 ECGs were recorded.
Results:
The KP-EHR interface provided full functionality, allowing detection, interpretation and documentation of atrial fibrillation (AF), flutter events, ventricular tachycardia, and complete heart block. Our study focused on KP’s detection of AF, and 16.7% of tracings were classified as probable AF with only 2.3% of tracings not analyzed by the KP algorithm because of tracings that were too noisy or truncated. Enhanced management was facilitated with clinical information immediately accessible. Blinded physician ECG review validated the KP proprietary algorithm interpretation and ECGs.
Conclusions:
Direct integration of KP into EHR was successful and practical. It allows for historical, point of care and immediate retrieval of remote ambulatory monitoring data and documentation into the electronic health record. KP EHR integration warrants further study as it has the potential to improve cost-effectiveness and clinical diagnostic value, leading to improvements in delivery of patient care.
Keywords: Remote monitoring, atrial fibrillation, wearables, ventricular tachycardia, electrocardiogram, AliveCor, KardiaPro, KardiaMobile, smartphone EKG, Kardia
Introduction
The KardiaMobile (KM; AliveCor, Mountain View, CA) device converts electrical signals from 2 metal electrodes to ultrasonic sound signals which are transmitted via the user’s smartphone microphone to display a single lead ECG. 1 One of the key strengths of the KM device lies in the ECG interpretation and user interface. The KM device’s algorithm first evaluates whether the ECG signal is of sufficient quality to evaluate, and if not, the patient is prompted to re-record or save the signal with no algorithm determination. ECGs may be rejected due to increased noise or truncated acquisitions. If the signal is of sufficient quality, the algorithm produces 1 of 5 results immediately visible to the patient: (1) Possible Atrial Fibrillation (AF), if AF is detected, (2) Normal, if the algorithm detects a normal rhythm, (3) Tachycardia, (4) Bradycardia, and (5) Unclassified, if the ECG is determined to be neither AF nor Normal. This information can be invaluable to a patient as it can alert them to seek healthcare professional advice sooner rather than later.
When a patient records a single-lead ECG using the KM device, they can easily share a PDF of the ECG with their physician via email. Alternatively, if the physician has access to KardiaPro (KP), a cloud-based data management system, ECGs taken by patients at home will automatically be uploaded to the KP platform. Although this removes the necessity for the patient to manually email the ECG to their physician, it still requires the physician to access a portal outside of their EHR system, requiring additional work and steps for physicians. This paper describes a proof-of-concept first use of KP interfaced into an enterprise HER system, EPIC (Epic System Corporation), with the goal of simplifying physician workflow to enhance ambulatory arrhythmia detection and management.
Methodology
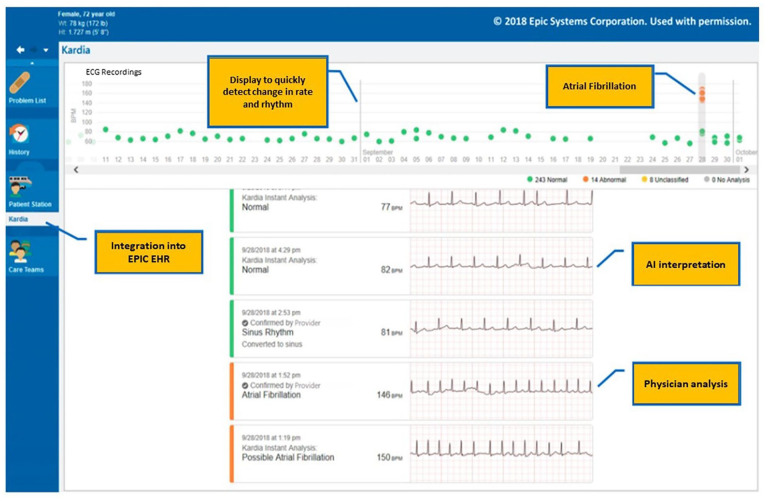
The research reported in this paper adhered to the CARE case report guidelines. Physicians offered patients the KP remote ECG monitoring system for those who qualified for, and who could clinically benefit from, ambulatory ECG monitoring. At the time of program enrollment, patients provided the investigators with general permission to use their ECG data for research purposes. Single lead ECGs were acquired by patients over 24 months on demand and stored on personal smartphones while simultaneously encrypted and transmitted to KP, which was further embedded into EPIC electronic health record using hypertext markup language code (HTML) (Figure 1). This system allowed for interpretation of ECGs, determination of patterns and enhanced documentation and treatment decisions. The Kardia dashboard is launched from the EMR’s graphical user interface via a standard HTML GET operation, passing a user and patient context, and the dashboard is displayed within a tab or pane inside the user window. Therefore, access was seamless with all clinical information available at the point of care in a single interface. Providers performed physician review of tracings generated by patient’s KP device within 48 hours of uploading into EPIC. All abnormal reads detected by the algorithm can be directed by the software for review and sent to adjustable locations; for example, to an individual physician or to a technician who can initially evaluate the strip and then refer on to the interpreting cardiologist. Workflows will be individually determined based on the need of the entity and technical capabilities of the entity’s platform. Our KP algorithm analysis focused on the detection of atrial fibrillation for the scope of this current study.
Figure 1.
Interface of KardioPro integrated with EPIC electronic health record.
Results
Of all tracings submitted, approximately 1068 ECGs (117 unique patients) were analyzed by the KM algorithm as well as by confirmed by blinded physician over-read. Based on this data, the KM atrial fibrillation detection performance was evaluated (Table 1). For this analysis, the algorithm results were considered correct unless changed by a physician, in which case, the physician’s interpretation was considered the true label. In our cohort, excluding those that were too short to analyze or were uninterpretable by either the algorithm or physician (ie, due to poor signal quality, n = 787). KM showed a sensitivity of 98% and specificity of 99% for AF detection (Table 1). This data is consistent with previous reports. 2
Table 1.
KardiaPro atrial fibrillation classification performance.
| Total ECGs | 32 179 | — |
| Normal | 19 865 | 61.70% |
| Arrhythmia detection by KardiaPro | ||
| Unclassified | 6203 | 19.70% |
| Probable AF | 5376 | 16.70% |
| Truncated/noisy signal | 735 | 2.30% |
| Number of patients with at least one episode of AF detected | 173 | 53% |
| Comparison of MD ECG read vs. KP ECG read | ||
| KP read AF | KP read non-AF | |
| MD read as AF | 5243 | 82 |
| MD read as non-AF | 101 | 25 966 |
Discussion
The KardiaPro remote monitoring system provides patients with on-demand single lead ECGs with a high degree of fidelity. Over 78% of recordings were adjudicated by the device algorithm, providing accurate, on-the-spot feedback to patients. The intuitive, wireless technology allows for ease of use even for patients with limited of technology savviness. EKG strips can be evaluated at the point of care with the patient’s entire record available in 1 platform for treatment and analysis allowing for better patient care and decision support. The present landscape of ECG monitoring includes Holter monitors which allow up to 14 days of consecutive recording, external or implanted loop recorders and pacemakers or implanted defibrillators. 3 The patient experience with these devices vary, with issues such as poor long-term compliance, skin-related problems from external electrodes and low motivation particularly in the absence of frequent or recurrent symptoms hindering diagnostic yield. 4 Results from the CRYSTAL-AF and EMBRACE trials have demonstrated the value of longer periods of ECG monitoring in detecting new episodes of atrial fibrillation in patients with prior cryptogenic stroke or transient ischemic attack.5,6 KardiaPro may supplement this approach by extending the monitoring period indefinitely for patients who have symptoms that can be captured at the point of care.
The KP system offers a reliable alternative or supplement for long term monitoring with ease for the end-user. Similarly, for the practitioner, the secure and real-time transmission of data from patient to the physician EHR allows for a broader range of care plans that can often bypass the office or emergency room. At a mean of nearly 100 ECGs per patient, processing such vast amounts of data and filtering out noise from clinical relevance can be a daunting task. The KP remote monitoring system offers logistics and artificial intelligence-based heuristics capable of handling large amounts of data while enabling the clinician to focus on care delivery. Among the participating physicians in this study, over 95% reported favorable experiences with the platform. Anecdotally, physicians expressed higher satisfaction with managing patients with atrial fibrillation and appreciated access to data in a user-friendly format. Better understanding of patients’ arrhythmia patterns allowed for medication optimization and enhanced control. Nevertheless, challenges such as managing patient expectations, reimbursement for time spent on data review and medico-legal risk mitigation will need to be considered.
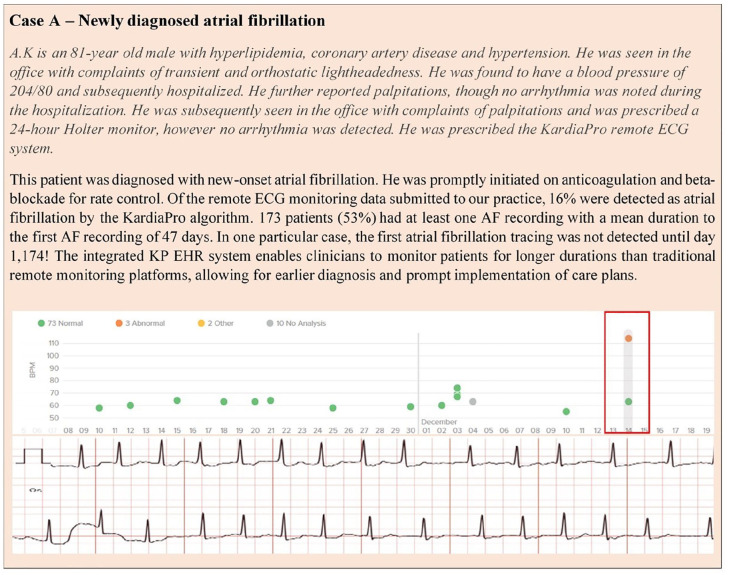
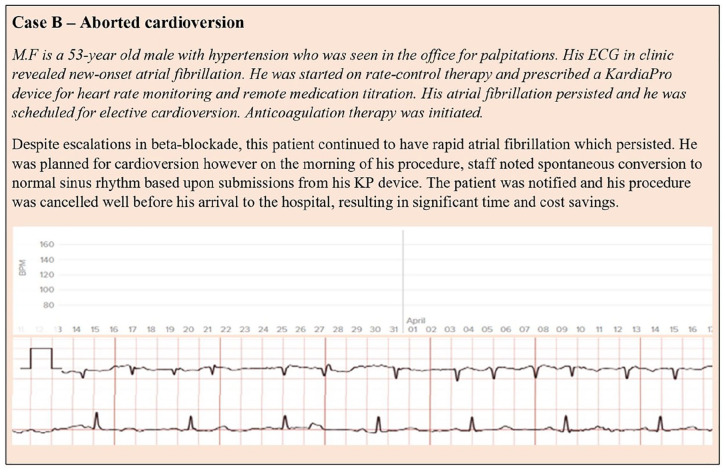
The clinical case studies included (Figure 2 and 3) demonstrate the versatility and value of a user-friendly dashboard, the KP system, displaying long-term ECG data by KardiaMobile in supporting clinical decision making including diagnosing, remote management, and guiding atrial fibrillation treatment decisions, including cardioversion. This reinforces the importance of integration of the cloud-based management system into the electronic health record enabling practices to cope with large volumes of clinical data.
Figure 2.
Case example—atrial fibrillation diagnosis.
Figure 3.
Case example—atrial fibrillation management.
Direct integration of KP management system into the EHR was demonstrated to be both practical and successful, allowing physicians to view remote ECG monitoring within their institution’s EHR and make informed clinical decisions in remote ambulatory monitoring. It further allowed for a historical point of care, signed documentation and physician authentication of algorithm-based ECG interpretation. The ability to titrate medication remotely or implement a new antiarrhythmic upon detection of new onset atrial fibrillation epitomizes a patient-centered care model. The clinical vignettes here highlight just a few examples of patient encounters. Remote monitoring can be an important component of patient-centered care delivery.
The KardiaPro integrated management system warrants a few considerations in future iterations. The pool of “Unclassified” ECGs approached 20%, suggesting a need for improved algorithm performance. Further patient training and hardware enhancements may reduce the number of uninterpretable or truncated acquisitions. Direct integration of the KP management system into the EHR compels: (a) monitoring access to and interpretation of ECGs to ensure continued high fidelity, (b) support staff to immediately evaluate all ECGs received and notify physicians when concerning rhythms are identified, and (c) closed-loop communication between patient and physician to provide reassurance or further medical therapy when appropriate. At the time of this article, KP performance classification has not been significantly reported, and additional studies such as the work presented here are needed to further refine its interface to allow for improved analysis and subsequent clinical patient care. Therefore, this KP EHR integration warrants further study as it has the potential to improve cost-effectiveness and clinical diagnostic value, leading to improvements in delivery of patient care.
Highlights
– The KardiaPro remote monitoring system provides high fidelity ECG recordings with artificial intelligence-based detection of atrial fibrillation.
– Integration of a cloud-based management system into the electronic health record using KardiaPro for remote ECG monitoring provides a clinically meaningful and user-friendly manner to interface with large amounts of data.
– The KardiaPro system displays long-term ECG data that aids clinicians in the diagnosis, management, and guiding of atrial fibrillation treatment decisions, including cardioversion.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Note: Ronald P Karlsberg is now affiliated to UCLA David Geffen School of Medicine, Los Angeles, CA, USA.
Author Contributions: All authors were significant involved in the research, data collection, manuscript composition, and review of this article.
ORCID iDs: Geoffrey W Cho  https://orcid.org/0000-0002-4438-2093
https://orcid.org/0000-0002-4438-2093
Ronald P Karlsberg  https://orcid.org/0000-0003-4695-9092
https://orcid.org/0000-0003-4695-9092
References
- 1. AliveCor Support [Internet]. Accessed January 7, 2019. https://alivecor.zendesk.com/hc/en-us
- 2. Williams J, Pearce K, Benett I. The effectiveness of a mobile ECG device in identifying AF: sensitivity, specificity and predictive value. Br J Cardiol. [Internet]. Accessed January 10, 2019. https://bjcardio.co.uk/2015/04/the-effectiveness-of-a-mobile-ecg-device-in-identifying-af-sensitivity-specificity-and-predictive-value/
- 3. Deftereos S, Papoutsidakis N, Giannopoulos G, Kossyvakis C, Lekakis J. Remote monitoring of the cardiac rhythm: where do we stand today? Wiley Online Library. 2016;2:168-175 [Google Scholar]
- 4. Subbiah R, Gula LJ, Klein GJ, Skanes AC, Yee R, Krahn AD. Syncope: review of monitoring modalities. Curr Cardiol Rev. 2008;4:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486. [DOI] [PubMed] [Google Scholar]
- 6. Gladstone DJ, Dorian P, Spring M, et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke. 2015;46:936-941. [DOI] [PubMed] [Google Scholar]