Abstract
Gastric infection with Helicobacter heilmannii (previously known as Gastrospirillum hominis) is invariably linked with the presence of chronic gastritis and the risk of developing low-grade mucosa-associated lymphoid tissue lymphoma in humans. In contrast to Helicobacter pylori, various H. heilmannii species colonize the stomachs of domestic animals, which might be a reservoir for transmission to humans (zoonosis). To identify the number and prevalence of different H. heilmanni types in humans, we analyzed 89 gastric biopsy samples histologically identified as H. heilmannii positive by fluorescence in situ hybridization. Of these gastric specimens, 84 (94.4%) contained a single H. heilmannii type. In five samples, however, two different H. heilmannii types were detected. The most prevalent species in monoinfected samples is H. heilmannii type 1, found in 78.5% (66 of 84) of the specimens, followed by a novel H. heilmannii-like organism (HHLO), HHLO type 4, identified in 9.6% (8 of 84) of tissue sections. H. heilmannii type 2 and a further HHLO type not described before, type 3, were found in 8.3% (7 of 84) and 1.2% (1 of 84) of the monoinfected samples, respectively. Additionally, HHLO type 5 with a 16S ribosomal DNA sequence identical to that of Helicobacter salomonis was found with a prevalence of 2.4% (2 of 89). Thirteen of these biopsy samples were also investigated by a PCR approach developed for this study that allows a Helicobacter-specific amplification of a variable portion of the 16S rRNA gene and subsequent sequencing. In total, five different types of HHLOs could be identified within these samples. We conclude that humans can be infected by at least five different HHLO types, which presumably have their origin in animal species like dogs, cats, and pigs.
Chronic Helicobacter infections of the stomach are increasingly recognized as a major risk factor for development of gastroduodenal disease. Helicobacter pylori is the major stomach-colonizing bacterium of humans that causes gastritis and peptic ulcer disease and is considered a risk factor for gastric adenocarcinoma (15) and malignant mucosa-associated lymphoid tissue (MALT) lymphoma (20, 23). Helicobacter heilmannii (17), previously known as Gastrospirillum hominis, infects humans to a much lower extent than H. pylori, with frequencies ranging between 0.25 and 1.7% (10, 24). Most of those infected suffer from chronic active gastritis (24), but sporadic cases of gastric erosions (9) and gastric cancer have also been reported for such patients (13, 24). More important, H. heilmannii infection is associated with the development of primary gastric MALT lymphoma in humans as well as in experimental animal infections (11). Eradication of H. heilmannii by antibiotic treatment of patients resulted in complete remission of the MALT lymphoma (14), indicating a causal relationship between H. heilmannii infection and MALT lymphoma.
Spiral microorganisms have regularly been observed in gastric tissue samples obtained from humans and different animals. Nevertheless, it was not until 1987 that Dent and coworkers coined the epithet Gastrospirillum hominis for these corkscrew-shaped bacteria found in human gastric samples (5). Subsequently, sequencing of the 16S ribosomal DNA (rDNA) of these bacteria showed that Gastrospirillum hominis belongs to the genus Helicobacter, and the name Helicobacter heilmannii has been proposed (18).
Unlike H. pylori infections, gastric infections with H. heilmannii or Gastrospirillum-like organisms are not restricted to humans. A broad range of animals, including dogs, cats, pigs, and cattle, are naturally infected, with frequencies ranging from 80 to 100% (4, 6, 8). It has been suggested that H. heilmannii infection in humans is a zoonosis and that animals serve as a reservoir for transmission to humans (6, 12). In humans, at least two closely related but different H. heilmannii isolates (G. hominis 1 and G. hominis 2) were identified (17). One H. heilmannii isolate has been cultivated in vitro (2).
However, evidence is accumulating that suggests that H. heilmannii is an assembly of quite variable subtypes of one species or may even consist of different species. Whereas Andersen and coworkers successfully isolated H. heilmannii from human gastric tissue samples (2), other investigators failed to cultivate this species in vitro. Furthermore, two 16S rDNA sequences were retrieved from clinical samples of this species that differed significantly from each other (17).
Finally, although a correlation between animal contact and colonization with H. heilmannii exists, no particular animal species could be identified as a reservoir for human infection. The proposal of the present study was therefore to clarify whether or not different types of H. heilmannii exist in human gastric tissue samples and to sample data on their prevalence. Since cultivation of H. heilmannii is not possible for all subtypes yet, cultivation-independent techniques, i.e., rDNA-targeted PCR and fluorescent in situ hybridization (FISH), have been applied.
MATERIALS AND METHODS
Strains and cultivation conditions.
The following bacterial strains were used to evaluate specificity of PCR primers and hybridization probes developed for this study: H. pylori (NCTC 11637), Helicobacter acinonychis (CCUG11284), Helicobacter nemestrinae (ATCC 49396) Helicobacter salomonis (CCUG37845), Helicobacter felis CS1 (ATCC 49179), Helicobacter mustelae (NCTC 12032), Helicobacter bilis (ATCC 51630), Helicobacter canis (CCUG 32756), Helicobacter muridarum (CCUG29262), Helicobacter fennelliae (LMG1746), Helicobacter cinaedi (LMG13991), Helicobacter pullorum (NCTC 12824), Campylobacter rectus (DSM3266), Campylobacter jejuni (ATCC 33560), and Campylobacter coli (TU Munich). More distantly related species tested were Wolinella succinogenes (ATCC 29543), Arcobacter cryaerophilus (LMG6622), Arcobacter butzleri (LMG6620), and Proteus vulgaris (ATCC 13315). Helicobacter strains, C. jejuni, C. coli, and Arcobacter strains were grown on GC agar plates (Difco) supplemented with horse serum (8%), vancomycin (10 mg liter−1), trimethoprim (5 mg liter−1), and nystatin (1 mg liter−1) (serum plates) and incubated for 2 to 3 days in a microaerophilic atmosphere (85% N2, 10% CO2, 5% O2) at 37°C. Bacteria were transferred with an inoculation loop to a phosphate-buffered saline solution and were thoroughly suspended. Proteus vulgaris and Pseudomonas aeruginosa were grown aerobically in Luria-Bertani broth. Bacterial cell suspensions were fixed with 3 volumes of freshly prepared 4% paraformaldehyde solution as described by Amann et al. (1). W. succinogenes and C. rectus were grown by the Deutsche Sammlung von Mikroorganismen und Zellkulturen as active cultures and were directly fixed by the supplier with 1/10 volume of 37% paraformaldehyde before delivery.
Patient material.
Gastric biopsy specimens of nonulcer dyspepsia patients without cases of ulcers, carcinoma, or MALT lymphoma taken during diagnostic endoscopies from 1988 to 1998 in Germany were included in this study. In total, 543 H. heilmannii gastritis samples were collected in that time frame at the Institute for Pathology in Bayreuth, which corresponded to 0.2% of the H. pylori-induced gastritis cases diagnosed at the same time. From those, 89 biopsies were taken for this study. To guarantee optimal performance of the test, fixation of the biopsies should immediately follow sampling. Biopsies were fixed in 10% freshly prepared buffered formalin solution (incubation time in formalin should not exceed 48 h), paraffin embedded, and cut into 4-μm sections. Gastritis was histologically diagnosed with hematoxylin and eosin staining according to the updated Sydney System (7), and Warthin-Starry-stained sections (22) were used to detect H. heilmannii.
PCR primers and probes for in situ hybridization.
Comparative analysis of more than 10,000 16S rRNA sequences was performed with the probe design tool of the ARB program to develop specific probes for the different HHLO types. Furthermore, a heminested PCR system was developed suitable for a sensitive detection of all bacterial species assigned to the genus Helicobacter (forward primer HelF and two reverse primers, HelR1 and HelR2). Probe and primer names and specifications are listed in Table 1. All probe and primer sequences were subjected to a BLAST search and were compared to a comprehensive database comprising all published 16S rRNA sequences of the ɛ subdivision of the Proteobacteria (16). Probes and primers were provided by Metabion GmbH (Munich, Germany). Probes were 5′ labeled either with Cy3 or with 5 (and 6)-carboxyfluorescein by the supplier.
TABLE 1.
Sequences of specific primers and probes for FISH and PCR
| Name | Sequencea | Specificity | Probe targetb positions |
|---|---|---|---|
| Hhe-1 | 5′-CCC-ACA-CTC-CAG-AAG-RAT-AG-3′ | “H. heilmannii” type 1; H. suis | 642–661 |
| Hhe-2 | 5′-CCC-ACA-CTC-TAG-GGT-KGC-AG-3′ | “H. heilmannii” type 2 | 642–661 |
| Hhe-3 | 5′-CCC-ACA-CTC-TAG-AAA-GAT-AG-3′ | Novel HHLO type | 642–661 |
| Hhe-4 | 5′-CAC-ATC-TGA-CTT-GCC-ACT-CCG-3′ | Novel HHLO type | 586–606 |
| Hhe-5 | 5′-CCC-ACA-CTC-CAG-AGT-TGT-AG-3′ | H. felis, H. bizzozeroni, H. salomonis | 642–661 |
| HelF | 5′-CGT-GGA-GGA-TGA-AGG-TTT-TA-3′ | Helicobacter genus, PCR | 402–421 |
| HelR1 | 5′-TAC-ACC-AAG-AAT-TCC-ACC-TA-3′ | Helicobacter genus, PCR | 667–686 |
| HelR2 | 5′-AAT-TCC-ACC-TAC-CTC-TCC-C-3′ | Helicobacter genus, PCR | 659–677 |
| Hpy-1 | 5′-CAC-ACC-TGA-CTG-ACT-ATC-CCG-3′ | H. pylori | 585–605 |
| Eub-338 | 5′-GCT-GCC-TCC-CGT-3′ | Bacteria | 338–349 |
R corresponds to A or G; and K corresopnds to G or T (3).
The target in each case is 16S rRNA.
PCR amplification and sequencing of rDNA.
For preparation of DNA from biopsy specimens, six paraffin-embedded tissue sections of each of the 13 patient samples were chosen. The sections were placed in a microcentrifuge tube, and paraffin was extracted from the tissue sections by two 30-min incubations in hexane and two subsequent 15-min incubations in 96% ethanol. After air drying, DNA was extracted from the samples with the QIAamp DNA minikit (Qiagen GmbH, Hilden, Germany) according to the instructions of the manufacturer. Five microliters of the obtained DNA solution was applied to a 50-μl PCR solution. The first PCR was performed employing primers HelF and HelR1 (Table 1) with 2.5 U Taq Gold polymerase (Perkin Elmer) in a PCR buffer supplied by Perkin Elmer. After 10 min of denaturation at 94°C, 30 cycles (30 s at 94°C, 30 s at 58°C, and 30 s at 72°C) were performed on a 9700 thermocycler (Perkin Elmer). Five microliters of the obtained PCR product was transferred to a new Eppendorf tube, and a second PCR with primers HelF and HelR2 was performed under the same conditions as the first PCR. Five microliters of each PCR product was analyzed by electrophoresis on 2% agarose gels. Both strands of the amplified 16S rDNA portions were sequenced with primers HelF and HelR2 using the cycle sequencing protocol and an ABI Prism 373A automatic sequencer (Applied Biosystems, Weiterstadt, Germany).
Partial 16S rDNA sequences of 250 bp obtained from human gastric tissue samples during the present study were introduced into the ARB program package (O. Strunk and W. A. Ludwig, microbiologist's sequence database tools; public domain software available at http://www.biol.chemie.tu-muenchen.de) and aligned by the autoaligner tool. Alignments were corrected manually according to secondary structure data delivered by the program.
Whole-cell hybridization.
Five microliters of cell suspensions containing paraformaldehyde-fixed reference cells (17) were spotted onto six-well Teflon-coated microscopic slides. Air-dried samples were subjected to an ascending ethanol series (50, 80, and 96% ethanol, each) to permeabilize bacterial membranes and subsequently air dried again. Hybridization of the slides was performed according to a protocol published by Amann et al. (1). In brief, each well carrying bacterial cells was covered with 10 μl of hybridization buffer. Slides carrying deparaffinized gastric tissue sections were spotted with 50 μl of hybridization buffer (0.9 M NaCl; 0.02 M Tris-HCl, pH 8.0; 0.01% sodium dodecyl sulfate) containing 30% formamide and 5 ng of each probe μl−1 and covered with a coverslip to minimize evaporation. Slides were placed for 90 min at 46°C in a humid chamber. Subsequently, hybridized slides were briefly rinsed with washing buffer and inoculated for 15 min at 48°C in a washing buffer (0.112 M NaCl, 20 mM Tris-HCl [pH 8.0], and 0.01% sodium dodecyl sulfate). In some cases, slides were counterstained with a 1-μg ml−1 4′,6-diamidino-2-phenylindol (DAPI) solution for 10 min. Hybridized samples were shortly rinsed with phosphate-buffered saline, mounted in Citifluor (Citifluor Ltd., London, United Kingdom), and examined with a DMRBE epifluorescence microscope (Leica, Heerbrug, Switzerland) equipped with filters A for blue fluorescence, I3 for green fluorescence, and N 2.1 for red fluorescence. Photomicrographs were taken with the same fluorescence microscope. All gastric sections were hybridized with probe Hpy-1 (Table 1) (21), specific for H. pylori, to detect a possible presence of these bacteria in addition to H. heilmannii-like organisms (HHLOs).
RESULTS
In situ hybridization of 89 human gastric biopsy samples with probes Hhe-1 and Hhe-2.
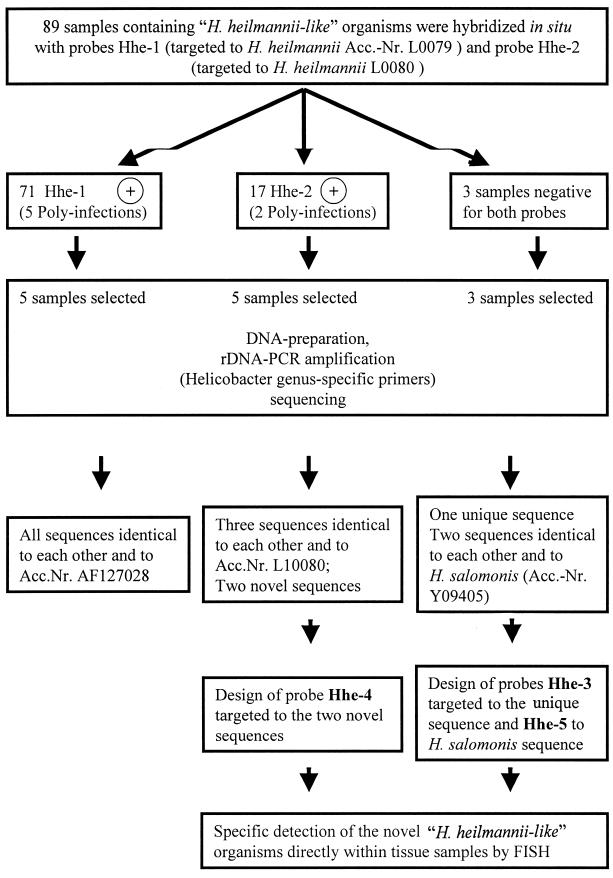
The primary goal of the present study was to evaluate the prevalence of two known H. heilmannii types in 89 human gastric biopsy samples. Therefore, hybridization probes for these two different types were developed (Table 1). In order to illustrate the strategy of oligonucleotide probe design, which included FISH, genus-specific PCR, and sequencing of the obtained partial 16S rDNA sequences, the complete procedure is summarized in a flow chart in Fig. 1. The oligonucleotide probe sequences were subjected to an extensive “in silico” analysis at the National Center for Biotechnology Information database, which contains all hitherto-published sequences of Proteobacteria from the ɛ subdivision, in order to identify putative cross-hybridizing gene sequences. In addition to their original 16S rRNA sequences, the Hhe-1 target sequence is one base moiety different from the 16S rRNA sequence from Candidatus Helicobacter suis (4), and Hhe-2, as expected, picked up H. heilmannii type 2.
FIG. 1.
Scheme illustrating the strategy used in this study to analyze 89 human gastric biopsy samples.
The specificity of the oligonucleotide probes was analyzed under experimental conditions by hybridizing 18 different species belonging to the ɛ subdivision of Proteobacteria to the developed H. heilmannii probes. Twelve Helicobacter species different from H. heilmannii were included (see Materials and Methods). Neither Hhe-1 nor Hhe-2 hybridized to any of the strains tested. In contrast, Hhe-1 hybridized to 66 monoinfected gastric tissue sections (71 with mixed infections) and Hhe-2 complementary sequences were detected within 15 monoinfected tissue samples (17 with mixed infections) (Table 2; Fig. 2). Two biopsy specimens harbored two distinct cell populations. One population hybridized with Hhe-1, whereas the other population bound to probe Hhe-2. Three gastric tissue samples did not hybridize to Hhe-1 or Hhe-2. However, cells within these specimens strongly hybridized to probe Eub-338 (1). This probe is complementary to a 16S rRNA region conserved in almost all bacteria but not in eukaryotes and Archaea, and it was used as a control in our FISH assays to demonstrate the presence of rRNA in the cells. Furthermore, all specimens hybridized to probe Hpy-1 (21), which specifically detects H. pylori, but we did not detect H. pylori in the gastric sections.
TABLE 2.
Distribution of different HHLOs in human gastric tissue sections as determined by FISH
| Gastric tissue sample type (n) | No. of samples hybridizing with probea
|
|||||
|---|---|---|---|---|---|---|
| Eub-338 | Hhe-1 (78.5) | Hhe-2 (8.3) | Hhe-3 (1.2) | Hhe-4 (9.6) | Hhe-5 (2.4) | |
| All samples (89) | 89 | 71 | 9 | 1 | 8 | 5 |
| Monoinfection (84) | 84 | 66 | 7 | 1 | 8 | 2 |
| Polyinfection (5) | 5 | |||||
Numbers in parentheses are prevalence of monoinfections with the corresponding organism (in percent). Two gastric tissue samples hybridized with probes Hhe-1 and Hhe-2, and three samples hybridized with probes Hhe-1 and Hhe-5.
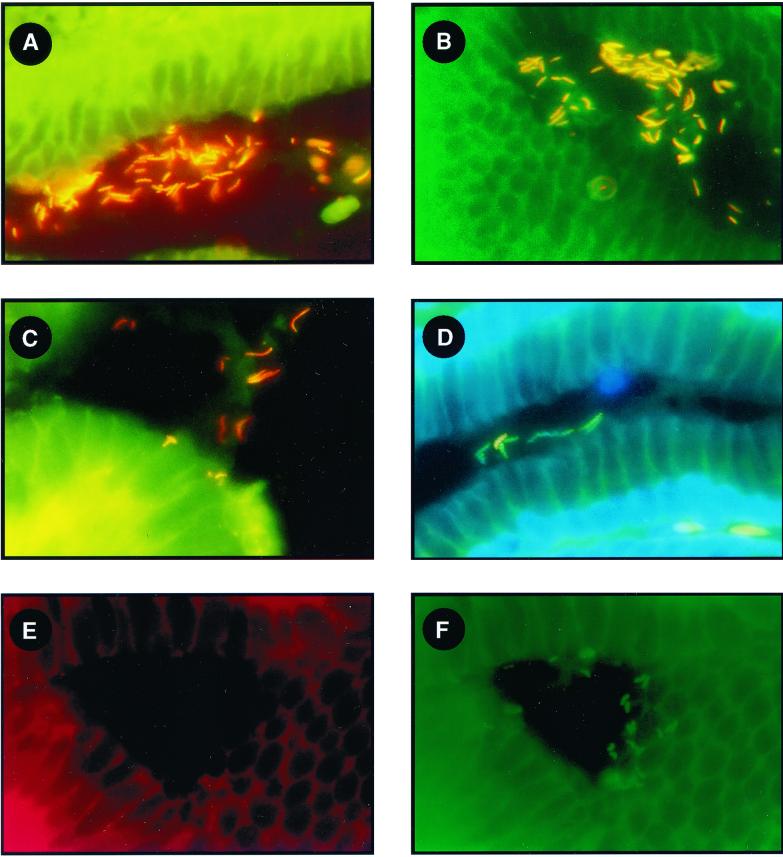
FIG. 2.
Formalin-fixed gastric sections of patients with “H. heilmannii” types 1 and 2 and HHLOs detected by FISH using the oligonucleotide probes summarized in Table 1. (A) “H. heilmannii” type 1; (B) “H. heilmannii ” type 2; (C) HHLO-4; (D) HHLO-5; (E and F) H. pylori. In panels A to D the Eub-338 probe labeled in green (Fluos) was used together with the corresponding HHLO type-specific probe, labeled in red (Cy-3). The mixed yellow and orange color indicates binding of both probes simultaneously. (D) A mixed infection with “H. heilmannii ” type 1 and HHLO-5 is demonstrated; one Helicobacter isolate (yellow-orange) binds the Eub-388 and HHLO-1 probes, and the other bacteria bind the Eub338 probe only and represent HHLO-5 organisms, as specified with the corresponding probe (not shown). DNA is labeled with DAPI (in blue). (E and F) Gastric sections with H. pylori were used to demonstrate the specificity of the probes. The Hhe-1 probe is labeled in red (Cy-3) (E), and the Hpy-1 probe is labeled in green (Fluos) (F).
PCR-based amplification and sequence analysis of partial 16S rDNA sequences retrieved from formalin-fixed biopsy samples.
Next, a Helicobacter genus-specific PCR approach was developed to specifically amplify a variable portion of Helicobacter rDNA from the human gastric tissue samples. Primers HelF, HelR1, and HelR2 were developed by comparing all available Helicobacter 16S rDNA gene sequences in the database (Table 1). A 250-bp PCR product was obtained from all Helicobacter species enrolled in this study (see Material and Methods). In contrast, no amplification was obtained with DNA preparations of five further ɛ subdivision Proteobacteria species belonging to the genera Campylobacter, Wolinella, and Arcobacter and from the remotely related genera Proteus and Pseudomonas (see Strains and cultivation conditions in Materials and Methods). As little as 50 fg of DNA (corresponding to 30 H. pylori genomes) prepared from H. pylori and H. mustelae could be successfully amplified.
This PCR approach was applied to substantiate the results obtained by in situ hybridization of human gastric samples with probes Hhe-1 and Hhe-2. Five biopsy samples hybridizing to Hhe-1 and five biopsy samples hybridizing to Hhe-2 were chosen. Total chromosomal DNA was extracted and Helicobacter 16S rDNA was amplified by the PCR approach mentioned above. The same procedure was applied to the biopsy samples negative for both probes (three samples) (Fig. 1). The resulting partial 16S rDNA sequences were aligned with the sequences in the 16S rRNA database of the ARB program package, and the most closely related 16S rRNA sequences were determined.
All five samples positive for Hhe-1 were completely identical to the sequence of Candidatus H. suis (accession no. AF127028). The published sequence of H. heilmannii type 1 showed one mismatch to the Hhe-1 sequences retrieved in this study. However, Hhe-2-positive sequences could be divided into two different rRNA groups. Two sequences were found to be identical to the H. heilmannii type 2 sequence, as expected. The three remaining Hhe-2-positive samples, however, showed the expected identity within the probe target region, but they were significantly different throughout the remaining part of the sequence (at least 2.8% difference from any rDNA sequence known) (Fig. 1).
Two of the sequences obtained from samples negative for probes Hhe-1 and -2 were identical and showed 100% identity to H. salomonis and H. felis (accession no. AF103880), both isolated from dogs. The third sequence retrieved from these samples was unique and most closely related (97.2% homology) to an H. heilmannii isolate cultured from human gastric samples (2) (Table 3).
TABLE 3.
16S rRNA similarity matrixa
| Organism | Similarity
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| 1. H. pylori NCTC 11637 | |||||||||||||||||||
| 2. H. acinonychis ATCC 49396 | 0.016 | ||||||||||||||||||
| 3. H. felis DS3 | 0.040 | 0.000 | |||||||||||||||||
| 4. G. hominis 2 G2A9 | 0.040 | 0.020 | 0.024 | ||||||||||||||||
| 5. G. hominis 1 G1A1 | 0.036 | 0.036 | 0.048 | 0.056 | |||||||||||||||
| 6. H. hepaticus Hh-2 | 0.060 | 0.039 | 0.051 | 0.060 | 0.056 | ||||||||||||||
| 7. H. cinaedi ATCC 35683 | 0.085 | 0.073 | 0.077 | 0.077 | 0.073 | 0.056 | |||||||||||||
| 8. H. bizzozeroni 865-2 | 0.040 | 0.000 | 0.000 | 0.024 | 0.048 | 0.052 | 0.077 | ||||||||||||
| 9. H. felis 390-9 | 0.040 | 0.008 | 0.008 | 0.016 | 0.048 | 0.051 | 0.077 | 0.008 | |||||||||||
| 10. H. felis 144-10 | 0.036 | 0.008 | 0.012 | 0.020 | 0.052 | 0.064 | 0.077 | 0.012 | 0.020 | ||||||||||
| 11. H. felis 1136-7 | 0.048 | 0.004 | 0.008 | 0.024 | 0.056 | 0.051 | 0.077 | 0.008 | 0.016 | 0.020 | |||||||||
| 12. H. heilmannii AF53 | 0.036 | 0.008 | 0.012 | 0.020 | 0.044 | 0.056 | 0.077 | 0.012 | 0.004 | 0.024 | 0.020 | ||||||||
| 13. H. salomonis, isolate 4 | 0.040 | 0.008 | 0.008 | 0.016 | 0.048 | 0.051 | 0.077 | 0.008 | 0.000 | 0.020 | 0.016 | 0.004 | |||||||
| 14. H. heilmannii type 2, isolate 1 | 0.040 | 0.008 | 0.008 | 0.024 | 0.048 | 0.056 | 0.081 | 0.008 | 0.016 | 0.020 | 0.016 | 0.020 | 0.016 | ||||||
| 15. H. felis 937-12 | 0.044 | 0.000 | 0.004 | 0.020 | 0.052 | 0.047 | 0.073 | 0.004 | 0.012 | 0.016 | 0.004 | 0.016 | 0.012 | 0.012 | |||||
| 16. “Pig C2” | 0.036 | 0.024 | 0.028 | 0.012 | 0.052 | 0.068 | 0.081 | 0.028 | 0.020 | 0.024 | 0.036 | 0.016 | 0.020 | 0.020 | 0.032 | ||||
| 17. H. suis V2BXA | 0.044 | 0.044 | 0.052 | 0.060 | 0.008 | 0.064 | 0.073 | 0.052 | 0.052 | 0.056 | 0.060 | 0.048 | 0.052 | 0.052 | 0.056 | 0.056 | |||
| 18. HHLO-3 | 0.040 | 0.032 | 0.040 | 0.040 | 0.036 | 0.068 | 0.056 | 0.040 | 0.032 | 0.036 | 0.048 | 0.028 | 0.032 | 0.048 | 0.044 | 0.036 | 0.036 | ||
| 19. HHLO-4 | 0.056 | 0.028 | 0.032 | 0.032 | 0.060 | 0.064 | 0.065 | 0.032 | 0.040 | 0.028 | 0.032 | 0.044 | 0.040 | 0.032 | 0.028 | 0.036 | 0.060 | 0.048 | |
| 20. HHLO-5 | 0.040 | 0.008 | 0.008 | 0.016 | 0.048 | 0.051 | 0.077 | 0.008 | 0.000 | 0.020 | 0.016 | 0.004 | 0.000 | 0.016 | 0.012 | 0.020 | 0.052 | 0.032 | 0.040 |
Values are differences between the corresponding rRNA sequences. The lowest differences between the novel H. heilmannii subtypes and the corresponding sequence of published 16S rRNA sequences are in bold. The accession numbers for sequences obtained from GenBank are as follows: H. pylori, Z25741; H. acinonychis, M88148; H. felis, M37643; G. hominis 2, L10080; G. hominis 1, L10079; H. hepaticus, U07574; H. cinaedi, M88150, H. bizzozeroni, AF103883; H. felis, AF103881; H. felis, AF103881; H. heilmannii, Y18028; H. salomonis, Y09405; H. heilmannii type 2, AF058768; H. felis, AF103879; “Pig C2,” AF142152; H. suis, AF127028; HHLO-3, AY014859; HHLO-4, AY014861; HHLO-5, AY014860.
Prevalence of three HHLOs newly discovered in human gastric tissue samples.
Based on the partial 16S rRNA sequences retrieved in the present study, specific oligonucleotide probes were developed for HHLO types 3, 4, and 5, named Hhe-3, Hhe-4, and Hhe-5, respectively. According to the procedures employed for probes Hhe-1 and Hhe-2, specificity of the probes was tested in vitro with the reference strains mentioned above and in silico in the respective databases. Whereas probe Hhe-3 and Hhe-4 did not hybridize to any of the bacterial species tested, probe Hhe-5 hybridized to H. felis. These results were confirmed by in silico analysis. No matching sequences were found for Hhe-3 and Hhe-4. In contrast, 12 matching sequences were found for Hhe-5. Within the probe target region nine sequences were identical to H. felis sequences, two were identical to Helicobacter bizzozeroni sequences, and one was identical to H. salomonis sequences. The newly developed probes Hhe-3 and Hhe-5 hybridized to their source biopsy samples, as expected, but not to any other tissue sample. As expected from the sequence data, Hhe-4 hybridized to a subset of Hhe-2-positive samples but not to any Hhe-1-, Hhe-3-, or Hhe-5-positive samples.
Since all probes hybridized specifically to their respective target organisms, prevalence studies were extended to the HHLOs newly discovered in human gastric biopsy samples. A single HHLO was observed in 84 biopsy samples (94.4%). H. heilmannii type 1, originally identified by Solnick et al. (17), was found in 78.5% (66 of 84) of samples containing a single HHLO type and thus corresponds to the predominant species in human H. heilmannii infections. The novel HHLO-4 was detected as the sole HHLO type in 9.6% (8 of 84) of biopsy specimens, followed in frequency by H. heilmannii type 2, with 8.3% (7 of 89), and HHLO-5, with 2.4% (2 of 84). HHLO-3 was detected only in a single sample (1.2%). The remaining five patient samples (5.6%) contained two different HHLOs (Table 2).
DISCUSSION
In contrast to H. pylori, which predominantly colonizes humans, H. heilmannii has often been found in animals, like cats, pigs, and cattle. It has been postulated that upon close contact with these animals, transmission to humans may occur. However, although a correlation between colonization with H. heilmannii and animal contact is obvious (6, 12), a distinct species could not be identified as a reservoir. Also, conflicting results were reported about the ability to grow H. heilmannii on artificial media. A probable explanation for these results may be the existence of morphologically similar subtypes of HHLO, differing in animal reservoir and growth requirements. rDNA sequencing performed by Solnick and coworkers provided the first evidence for such differences (17). They retrieved two different 16S rRNA sequences from human gastric tissue samples with less then 97% homology, which was considered to be the interspecies border (19). Despite this significant difference, Solnick et al. assigned both sequences to one species and proposed the epithet Helicobacter heilmannii for this morphologically defined species.
The primary goals of the present study were therefore to prove by FISH that the previously retrieved rRNA sequence data really do colocate with the corkscrew-shaped bacteria within human gastric tissue samples and to determine the prevalence of these two rRNA sequences in 89 human gastric biopsy samples known to harbor H. heilmannii. The results of this study clearly demonstrate that H. heilmannii type 1 is the predominant HHLO type colonizing human gastric tissue samples. In 78.1% of biopsy samples containing only one HHLO corkscrew-shaped bacterium could be assigned to H. heilmannii type 1. Interestingly, De Groote et al. retrieved 16S rRNA sequences from gastric samples of pigs with 99.5% homology to H. heilmannii type 1 (4). Since cultivation of this gastric spiral bacterium failed, they describe a Candidatus Helicobacter suis (4). Data presented by De Groote et al. indicate a close phylogenetic relationship between Candidatus H. suis and H. heilmannii type 1. These authors therefore postulate a possible zoonotic role of Candidatus H. suis. This assumption is further corroborated by the data presented in this study, since the sequence retrieved from gastric biopsy samples shows one mismatch to the H. heilmannii type 1 sequence but is 100% identical to the sequences available from De Groote and coworkers. Taking into account the prevalence data obtained here with human biopsy specimens, more than 75% of H. heilmannii infections found in humans may be transmitted from pigs. As reported by De Groote et al. and others, this particular HHLO type has never been obtained in culture (4).
A second hybridization probe, which was originally developed to detect H. heilmannii type 2, hybridized to two different HHLO types, as revealed by genus-specific PCR and subsequent partial 16S rDNA sequencing. One sequence obtained was identical to the H. heilmannii type 2 sequence reported by Solnick et al. (17). However, a novel, hitherto-unknown 16S rRNA sequence was also found by sequencing, showing 2.8% sequence difference to H. heilmannii type 2. A third probe was developed to allow specific detection of this novel H. heilmannii type within human tissue sections. Use of these probes in FISH not only allowed allocation of the retrieved 16S rRNA data to distinct morphotypes within gastric samples, it also showed for the first time that this new HHLO type was more frequent in human gastric samples (9.6%) than H. heilmannii type 2 (8.3%). Although similar 16S rRNA sequences have already been amplified from feline samples, successful cultivation of Helicobacter strains from these groups have not been described. In contrast, another partial 16S rRNA sequence retrieved during this study showed sequence identity to H. salomonis and to one particular H. felis sequence (Table 3). These species, predominantly isolated from dogs, have all been obtained in pure culture. Therefore, in contrast to H. heilmannii type 1 and type 2, cultivation of this particular HHLO type of H. heilmannii from human gastric tissue samples may be possible if appropriate cultivation conditions are applied. This assumption was supported by the 16S rRNA data obtained by Andersen and coworkers for a cultivable H. heilmannii isolate from human tissue samples (2). The closest relative of this isolate was H. salomonis (98.9% homology). The sequence difference between the 16S rRNA determined by Andersen et al. and the sequence of HHLO-5 retrieved from human gastric tissue during the present study is only 0.04% (Table 3). Another HHLO type (type 3) was only found in one patient sample. Therefore, further HHLO types may be present, however, with low frequencies.
In five biopsy samples more than one H. heilmannii subtype was detected. Different subtypes were found in these mixed colonizations. However, HHLO subtype 5 was found in three of them. Whether HHLO-5 needs H. heilmannii type 1 for efficient colonization of human gastric biopsy samples or whether cocolonized hosts or subsequent infections are responsible for this phenomenon has yet to be addressed. A remarkable fact is that no cocolonization of H. pylori and H. heilmannii has been detected; however, in two of the patients massive duodenal infections with Giardia lamblia were observed. Such a coinfection has also been reported by others (V. Grouls and C. Seidl, Letter, Dtsch. Med. Wochenschr. 124:611, 1999). However, whether this means that H. heilmannii colonization prevents H. pylori infection cannot be answered by the present study.
FISH is a powerful tool for the specific detection of noncultivable Helicobacter species in gastric biopsy specimens. Compared to PCR-based techniques, FISH detects its target in intact bacterial cells, i.e., no liberated DNA released after cell death is detected. Furthermore, H. heilmannii can be detected directly in intact tissue specimens, and information concerning histology can be directly linked to the location of the bacteria. In addition, no inhibition of detection was observed by formalin fixation of tissues routinely used as gastric specimens. Therefore, application of this technique in human and animal samples in future will, in combination with PCR, be an appropriate tool for answering three important questions. First, are distinct HHLO types restricted to specific animal hosts and are these hosts the reservoir for human infection? Second, is there any correlation between severity or type of infection, e.g., development of MALT lymphoma, in humans and a particular HHLO type? Finally, which H. heilmannii types are present in different animals and can these H. heilmannii types be correlated to distinct diseases in these animals?
In conclusion, this study clearly demonstrated that H. heilmannii found in human gastric specimen is a mixture of different subtypes or even different species. Unequivocally, the species H. heilmannii urgently awaits systematic investigation regarding its taxonomic status. H. heilmannii type 1 is by far the most prominent species in human gastric biopsy samples. At least four further subtypes of H. heilmannii with different prevalence rates can be detected, and mixed infections are rare, but they do occur.
ACKNOWLEDGMENTS
This work was supported by a grant from Creatogen GmbH, Augsburg, Germany.
We thank B. P. Burns for critical reading of the manuscript.
REFERENCES
- 1.Amann R I, Binder B, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen L P, Boye K, Blom J, Holck S, Norgaard A, Elsborg L. Characterization of a culturable “Gastrospirillum hominis ” (Helicobacter heilmannii) strain isolated from human gastric mucosa. J Clin Microbiol. 1999;37:1069–1076. doi: 10.1128/jcm.37.4.1069-1076.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosius J, Dull T J, Sleeter D D, Noller H F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 4.De Groote D, van Doorn L J, Ducatelle R, Verschuuren A, Haesebrouck F, Quint W G, Jalava K, Vandamme P. ‘Candidatus Helicobacter suis’, a gastric helicobacter from pigs, and its phylogenetic relatedness to other gastrospirilla. Int J Syst Bacteriol. 1999;49:1769–1777. doi: 10.1099/00207713-49-4-1769. [DOI] [PubMed] [Google Scholar]
- 5.Dent J C, McNulty C A, Uff J C, Wilkinson S P, Gear M W. Spiral organisms in the gastric antrum. Lancet. 1987;ii:96. doi: 10.1016/s0140-6736(87)92754-1. [DOI] [PubMed] [Google Scholar]
- 6.Dieterich C, Wiesel P, Neiger R, Blum A, Corthesy-Theulaz I. Presence of multiple “Helicobacter heilmannii ” strains in an individual suffering from ulcers and in his two cats. J Clin Microbiol. 1998;36:1366–1370. doi: 10.1128/jcm.36.5.1366-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon F M, Genta R M, Yardley J H, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Eaton K A, Dewhirst F E, Paster B J, Tzellas N, Coleman B E, Paola J, Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996;34:3165–3170. doi: 10.1128/jcm.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goddard A F, Logan R P H, Atherton J C, Jenkins D, Spiller R C. Healing of duodenal ulcer after eradication of Helicobacter heilmannii. Lancet. 1997;349:1815–1816. doi: 10.1016/S0140-6736(05)61696-0. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann K L, Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991;32:137–140. doi: 10.1136/gut.32.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee A, O'Rourke J, Enno A. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations—mouse models of gastric lymphoma. Recent Results Cancer Res. 2000;156:42–51. doi: 10.1007/978-3-642-57054-4_6. [DOI] [PubMed] [Google Scholar]
- 12.Meining A, Kroher G, Stolte M. Animal reservoirs in the transmission of Helicobacter heilmannii. Results of a questionnaire-based study. Scand J Gastroenterol. 1998;33:795–798. doi: 10.1080/00365529850171422. [DOI] [PubMed] [Google Scholar]
- 13.Morgner A, Bayerdorffer E, Meining A, Stolte M, Kroher G. Helicobacter heilmannii and gastric cancer. Lancet. 1995;346:511–512. doi: 10.1016/s0140-6736(95)91364-5. [DOI] [PubMed] [Google Scholar]
- 14.Morgner A, Lehn N, Andersen L P, Thiede C, Bennedsen M, Trebesius K, Neubauer B, Neubauer A, Stolte M, Bayerdorffer E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821–828. doi: 10.1016/s0016-5085(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 15.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 16.Paster B J, Dewhirst F E. Phylogeny of campylobacters, wolinellas, Bacteroides gracilis, and Bacteroides ureolyticus by 16S ribosomal ribonucleic acid sequencing. Int J Syst Bacteriol. 1988;38:56–62. [Google Scholar]
- 17.Solnick J V, O'Rourke J, Lee A, Paster B J, Dewhirst F E, Tompkins L S. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993;168:379–385. doi: 10.1093/infdis/168.2.379. [DOI] [PubMed] [Google Scholar]
- 18.Solnick J V, O'Rourke J, Lee A, Tompkins L. Molecular analysis of urease genes from a newly identified uncultured species of Helicobacter. Infect Immun. 1994;62:1631–1638. doi: 10.1128/iai.62.5.1631-1638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stackebrandt E, Goebel B M. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 20.Stolte M, Eidt S. Healing gastric MALT lymphomas by eradicating H. pylori. Lancet. 1993;342:568. doi: 10.1016/0140-6736(93)91404-a. [DOI] [PubMed] [Google Scholar]
- 21.Trebesius K, Panthel K, Strobel S, Vogt K, Faller G, Kirchner T, Kist M, Heesemann J, Haas R. Rapid and specific detection of Helicobacter pylori macrolide resistance in gastric tissue by fluorescent in situ hybridisation. Gut. 2000;46:608–614. doi: 10.1136/gut.46.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warthin A S, Starry A C. A more rapid and improved method of demonstrating spirochaetes in tissues. Am J Syph. 1920;4:97–99. [Google Scholar]
- 23.Wotherspoon A C, Doglioni C, Diss T C, Pan L, Moschini A, de Boni M, Isaacson P G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Li X, Xu Z, Zhou D. “Helicobacter heilmannii” infection in a patient with gastric cancer. Dig Dis Sci. 1995;40:1013–1014. doi: 10.1007/BF02064190. [DOI] [PubMed] [Google Scholar]