Abstract
Background & Aims:
Risk of nonalcoholic fatty liver disease (NAFLD) and its progression may differ between men and women. We conducted a systematic review and meta-analysis to determine the relationship between sex and NAFLD, nonalcoholic steatohepatitis (NASH), and advanced NAFLD fibrosis
Methods:
Studies reporting sex-stratified NAFLD prevalence among population-based samples and either NASH or advanced fibrosis among patients with biopsy-proven NAFLD were identified from MEDLINE, EMBASE, and Cochrane databases through December 2017. We calculated pooled relative risk ratios comparing women vs men for each outcome.
Results:
Our final analysis comprised 54 studies. Samples sizes were 62,239 for the NAFLD analysis, 5428 for the NASH analysis, and 6444 for the advanced fibrosis analysis. Women had a 19% lower risk of NAFLD than men in the general population (pooled risk ratio [RR], 0.81; 95% CI, 0.68–0.97; I2, 97.5%). Women had a similar risk of NASH (RR, 1.00; 95% CI, 0.88–1.14; I2, 85.1%) and 37% higher risk of advanced fibrosis (RR, 1.37; 95% CI, 1.12–1.68; I2, 74.0%) than men. Age modified the effect of sex on NAFLD severity. Risks of NASH (RR, 1.17; 95% CI, 1.01–1.36) and advanced fibrosis (RR, 1.56; 95% CI, 1.36–1.80; I2=0) were substantially higher in women in study populations with average ages of 50 year or more; sex differences in NASH and advanced fibrosis were attenuated in younger populations.
Conclusions:
In a systematic review and meta-analysis, we found women to have a lower risk of NAFLD than men. However, once NAFLD is established, women have a higher risk of advanced fibrosis than men—especially after age 50 years.
Keywords: gender disparities, chronic liver disease, metabolic, comparison
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) affects 25% of the U.S. population (1). It is now the second leading indication for liver transplant (2) and accounts for an increasing proportion of hepatocellular carcinoma (3). NAFLD prevalence is increasing most significantly among adolescents (4) and adults under 45 years of age (5). The younger age of disease onset coupled with the rising prevalence and potentially broad clinical consequences foretell a substantial public health burden related to NAFLD (6).
There is a substantial gender disparity in the prevalence and severity of most chronic liver diseases. Specifically, hepatitis B and hepatitis C related liver disease are more common in men than in women (7). Furthermore, among persons with established chronic liver disease due to these risk factors, men have a higher risk of progression to advanced fibrosis and cirrhosis than women (8–11). This gender effect has been attributed to behavioral patterns, such as alcohol and injection drug use, and the effects of sex hormones (7).
The effect of gender on the risk and clinical course of NAFLD is unclear and needs clarification. Studies to date have reported mixed results where some studies show greater NAFLD prevalence among men compared to women (12–14) while others report greater prevalence of nonalcoholic steatohepatitis (NASH) – the progressive subtype of NAFLD – among women (15). Knowing whether and how gender influences the risk and severity of NAFLD is important for risk stratification, risk modification as well as prognostication.
Therefore, to better understand the relationship between gender and risk of NAFLD, we conducted a systematic review and meta-analysis of all observational studies that investigated the prevalence of NAFLD in the general population. We also examined gender differences in the prevalence of NASH and advanced fibrosis among persons with biopsy proven NAFLD.
METHODS
This study was conducted according to the published Meta-analysis of Observational Studies in Epidemiology and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (16, 17).
Literature Search
We performed a comprehensive search of the literature using Medline (Ovid), Embase, and Cochrane Library from inception of each database to December 31, 2017 to identify publications addressing the prevalence of NAFLD, NASH, and NAFLD with advanced fibrosis.
We developed our initial search strategy for Medline using the following Medical Subject Heading (MeSH) terms: fatty liver, non-alcoholic fatty liver disease and incidence, prevalence, and risk. Appropriate synonym keywords and phrases for these terms were determined and searched for within article titles, abstracts, and keywords (Supplementary Figure 1). We also conducted an ancestry search using the reference lists of relevant articles to identify publications that may have been missed by search terms.
Study selection
Published English-language studies were eligible for inclusion if they met the following criteria (i) cross-sectional, cohort, or case control study design reporting original data characterizing NAFLD prevalence or its severity (defined as histologic NASH or advanced fibrosis) among adults (age18≥years); (ii) identified NAFLD using biochemical or imaging criteria per American Association for the Study of Liver Disease guidelines in the absence of significant alcohol use (defined as average weekly alcohol consumption >14 standard drinks among women and >21 standard drinks among men) and viral hepatitis (18); and (iii) characterized NAFLD severity as NASH or presence of advanced fibrosis based on accepted histologic criteria (18–21). To be considered for inclusion in the NAFLD prevalence analysis, studies had to include population-based samples; studies that did not include population-based samples were excluded. To be considered for the NASH and advanced fibrosis analyses, studies had to include study cohorts comprised of patients with NAFLD based on explicit histologic criteria. Studies were excluded if they (i) did not include original human data (i.e., review articles, editorials, or animal studies), (ii) excluded subjects with conditions commonly associated with NAFLD (i.e. excluded patients with impaired glucose tolerance [IGT], type 2 diabetes [T2DM], dyslipidemia, hypertension, obesity, body mass index [BMI] ≥ 25kg/m2, thyroid disease, abnormal liver enzymes, atherosclerotic disease, or renal disease), (iii) were limited to pediatric/adolescent or young adults (age<40y), geriatric, or HIV infected patients or included persons with excessive alcohol use (iv) did not report sufficient data to determine the denominator for NAFLD prevalence/severity or to allow stratification of results by gender. For studies with overlapping study populations, we selected the publication with the most contemporary or most comprehensive data set.
Two of four reviewers (PP, SDV, VK, HA) independently screened each title, abstract and then full text if the articles met the inclusion criteria. Full texts of these selected articles were obtained and evaluated by two reviewers to confirm eligibility for inclusion. Any discrepancy was resolved via discussion with a third reviewer (M.B.)
Data extraction
The following data were abstracted: study characteristics (study design, time period, country), method of NAFLD diagnosis or histologic assessment, study population characteristics (sampling method, total number of adults evaluated for NAFLD or total number of NAFLD patients who underwent biopsy), demographic characteristics (age, gender), clinical characteristics (BMI, T2DM or IGT), and liver disease severity when relevant (proportion of patients with NASH and each stage of fibrosis separately when available).
Quality Assessment
We used a modified version of the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-sectional Studies to evaluate study quality (22). The original scoring system was designed to evaluate study characteristics ranging from study population selection to exposure and outcome assessments using 14 specified criteria that are assigned a response of yes, no, not applicable, or cannot determine.
Data synthesis and statistical analyses
Study Outcomes
We examined three outcomes: NAFLD, NASH, and advanced fibrosis. NAFLD could have been diagnosed based on imaging or aminotransferase elevations, but studies needed to specify their definition. NASH had to be diagnosed based on liver biopsy and accepted histologic criteria (Matteoni, modified Brunt, NASH CRN criteria or SAF scoring system (19–21)). We defined advanced fibrosis as ≥F2 (perisinusoidal/pericellular fibrosis with focal or extensive periportal fibrosis, bridging fibrosis, or cirrhosis) OR ≥F3 (bridging fibrosis or cirrhosis) based on the classification scheme used by the individual study authors.
Statistical Analysis
We calculated a risk ratio for each study outcome by gender (woman vs man). We used a random-effects (DerSimonian and Laird) meta-analysis to calculate the pooled risk ratio for gender with corresponding 95% confidence intervals (CI) for the prevalence of NAFLD, NASH, and advanced fibrosis. We selected a random-effects meta-analysis a priori due to the anticipated heterogeneity of the included studies. We examined between-study heterogeneity using the Cochran’s Q-test and I2 statistic. I2 values of 25%, 50%, and 75% were considered to be low, moderate, and high degrees of heterogeneity, respectively.
We examined the robustness of pooled estimates using leave-one-out sensitivity analyses and considered a study to be influential if the pooled estimate without it was outside of the 95% CIs of the overall pooled estimate. We also performed stratified analyses using the following variables identified a-priori: study region (North America, Europe, South Asia, East Asia, Middle East), overall study population average age (<50 years v.s. ≥50years), whether the study data were collected retrospectively or prospectively (for NASH and advanced fibrosis prevalence analyses), method of diagnosis (imaging versus aminotransferase elevations; for NAFLD prevalence analysis), and study population type (consecutive bariatric surgery patients vs consecutive patients with clinically diagnosed NAFLD vs patient data retrieved from research registries/pathology databases; for NASH and advanced fibrosis analyses). We compared the differences between subgroups using a χ2 test.
For the advanced fibrosis analysis we also performed a priori sensitivity analyses to assess whether the results were sensitive to definitions used by examining papers that classified advanced fibrosis as F2–4 and as F3–4 separately.
We assessed publication bias using visual inspection of funnel plots and Egger linear regression. A funnel plot is a scatter plot of the effect estimates from individual studies against each study’s size or precision (23). The standard error of the effect is chosen as the measure of study size and is plotted on the vertical axis with a reversed scale that places larger studies towards the top. The Egger regression gives the degree of funnel plot asymmetry as measured by the intercept from regression of standard normal deviates against precision (24). All data analysis were performed using metafor package in R, version 3.6.1 and Stata software version 13 (Stata Corporation, College Station, TX).
RESULTS
Literature Search
Of 9,639 retrieved citations, we selected 1,019 studies for full text review for eligibility. After full text review, 73 studies met our inclusion and exclusion criteria. Of these, 27 studies had overlapping study populations; we chose 8 studies that had either the most contemporary or comprehensive data. A total of 54 studies were included in the final analysis (Supplementary Figure 2). Six of the included studies reported both NASH and advanced fibrosis among patients with biopsy-proven NAFLD. None of the studies were designed specifically to determine differences in NAFLD prevalence or severity between men and women.
Prevalence of NAFLD
Study and Patient Characteristics
Seventeen population-based studies reported data on NAFLD prevalence by gender (study characteristics and references listed in Supplementary Table 1). The pooled sample size was 62,239, consisting of 35,119 women and 27,120 men. NAFLD was detected by ultrasound in 13 studies, liver enzymes in 2 studies, MR spectography in one study, and by a combination of ultrasound and CT scan in one study. Three studies were conducted in the U.S., 7 in China, 2 in South Asia, 4 in the Middle East, and 1 in South America. Four studies included nationwide study samples, 4 included study samples derived from and surrounding a single city, and 9 from a specific region. Across the studies, the average age of enrolled subjects ranged from 35.5 to 58.5 years, average BMI ranged 19.6 to 31.0 kg/m2, and the IGT or T2DM prevalence ranged from 4% to 30%.
Association between gender and prevalence of NAFLD
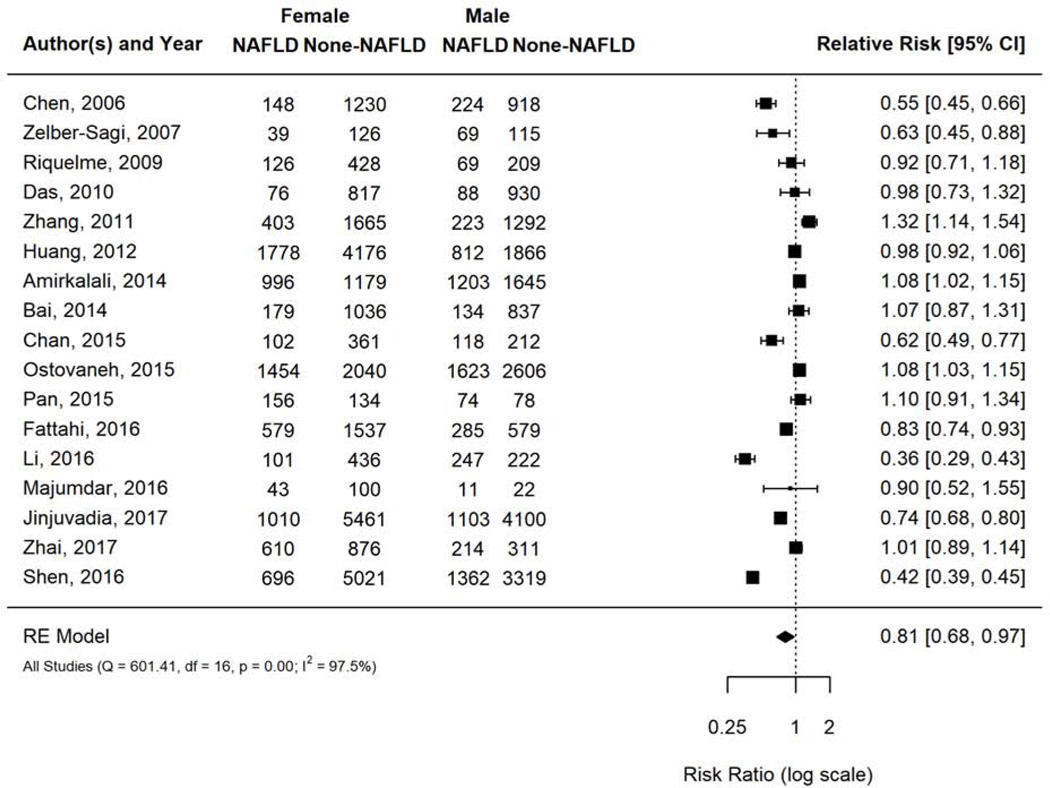
NAFLD prevalence ranged 8.6% to 52.0% across the included studies. Pooled overall NAFLD prevalence was 26.3% (95% CI 21.4–31.9%; I2=100%). Women had 19% lower prevalence of NAFLD compared to men with a (pooled risk ratio [RR] 0.81, 95% CI 0.68–0.97, I2 97.5%) (Figure 1).
Figure 1.
Risk of NAFLD in women versus men among population-based studies.
Forest plot visualization demonstrated that two studies - Li 2016 and Shen 2016 -were outliers. Li 2016 randomly sampled 1,006 people from an urban area of Northeast China, used ultrasound for NAFLD diagnosis, and had mean age <50 years and BMI of 24 kg/m2. Shen 2016 included 10,398 NHANES participants across the USA, used liver enzymes for NAFLD diagnosis, and had a mean age <50 years and BMI of 29–30kg/m2 (Supplementary Table 1). Both studies were kept in the primary analysis since neither possessed outlier study characteristics or methodologies justifying exclusion. A sensitivity analysis excluding one study at a time did not change the findings significantly.
Subgroup analyses by age (overall average age <50 years v ≥50 years) (Supplementary Figure 3a) and geographic location (Supplementary Figure 3b) did not significantly modify the effect of gender on risk of NAFLD. Most studies (13 of 17) used liver ultrasound to diagnose NAFLD; hence we did not conduct the a-priori subgroup analysis based on diagnostic modality.
Study Quality Assessment
All studies included in the NAFLD prevalence analysis were limited by a cross-sectional design. None had scores <5, 8 studies had scores 6–7 and 9 had scores ≥8 (Supplementary Table 4).
Publication Bias
No publication bias was found in the meta-analyses evaluating NAFLD prevalence in population-based studies (Egger test: t (df )= −0.86 (15), p=0.402). Funnel plot asymmetry was attributed to heterogeneity of the included studies (Supplementary Figure 3c).
Prevalence of NASH
Study and Patient Characteristics
In total, 22 studies reported NASH prevalence by gender among patients with biopsy proven NAFLD (study characteristics and references listed in Supplementary Table 2). The pooled sample size was 5,438 with 3,031 women and 2,407 men. NASH was diagnosed based on liver histology findings and exclusion of alternative diagnoses in all studies. Four studies evaluated NASH among consecutive morbidly obese patients who had liver biopsies obtained at the time of bariatric surgery, 7 among consecutive patients with clinically suspected NAFLD, and 11 among retrospectively reviewed NAFLD registry or pathology databases. Seventeen studies used retrospectively collected data and 3 studies used prospectively collected data. Eleven studies were conducted in North America, 6 in Europe, 2 in South Asia and 3 in East Asia. Average age ranged from 38.6 to 54.6 years, average BMI ranged from 26.2 kg/m2 to 55.7 kg/m2, and the prevalence of IGT or T2DM ranged from 9.3% to 100%.
Association between gender and prevalence of NASH
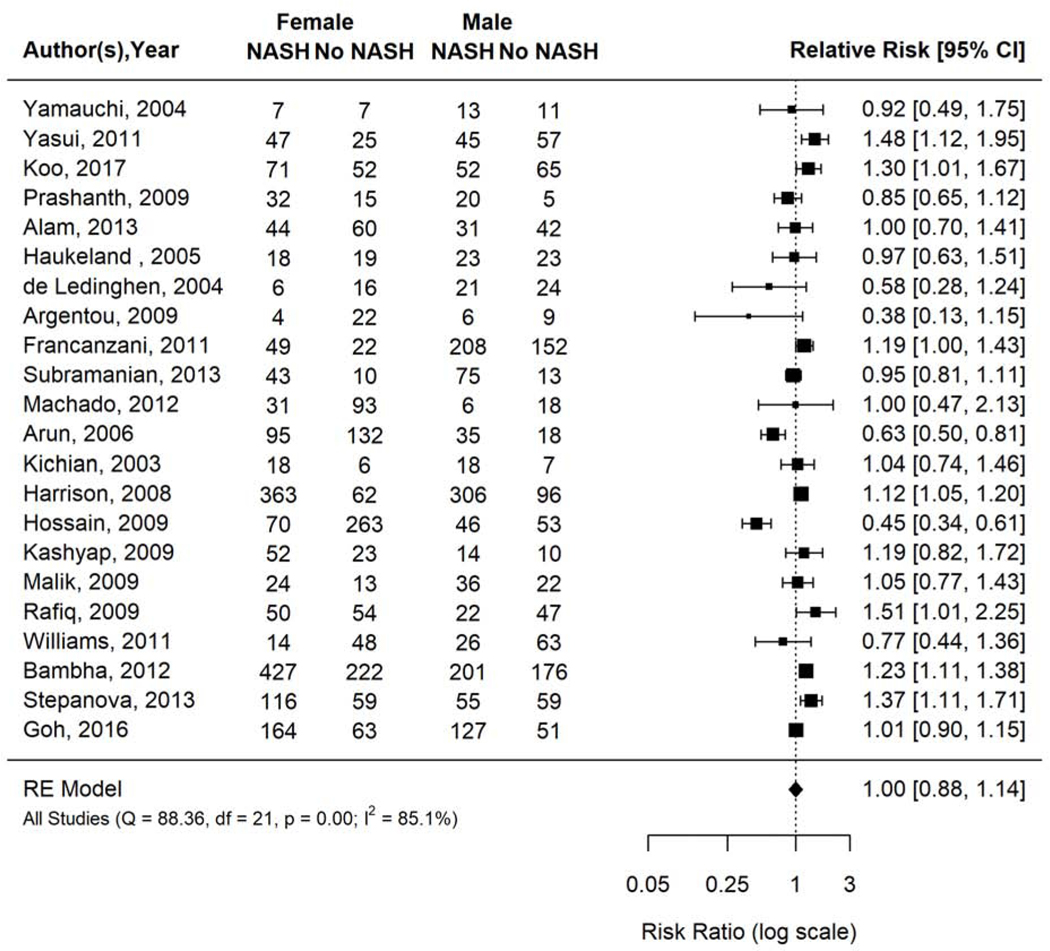
NASH prevalence ranged 24.4% to 83.7% across the included studies. Pooled NASH prevalence was 53.8% (95% CI 45.9–61.4%; I2=96.6%). Prevalence of NASH was not significantly different between women and men with biopsy-proven NAFLD (pooled RR 1.00, 95% CI 0.88–1.14, I2 85.1%) (Figure 2).
Figure 2.
Risk of NASH in women versus men with biopsy proven NAFLD.
Forest plot inspection demonstrated Hossain 2009 was an outlier. This study included a retrospective sample of 432 subjects with biopsy proven NAFLD from a single center in the U.S.A. with an average age of 43.6y, BMI 46.4kg/m2, and 25% prevalence of T2DM. It was kept in the primary analysis given there were no reasons justifying exclusion. Sensitivity analysis excluding one study at a time did not materially change risk ratios or heterogeneity.
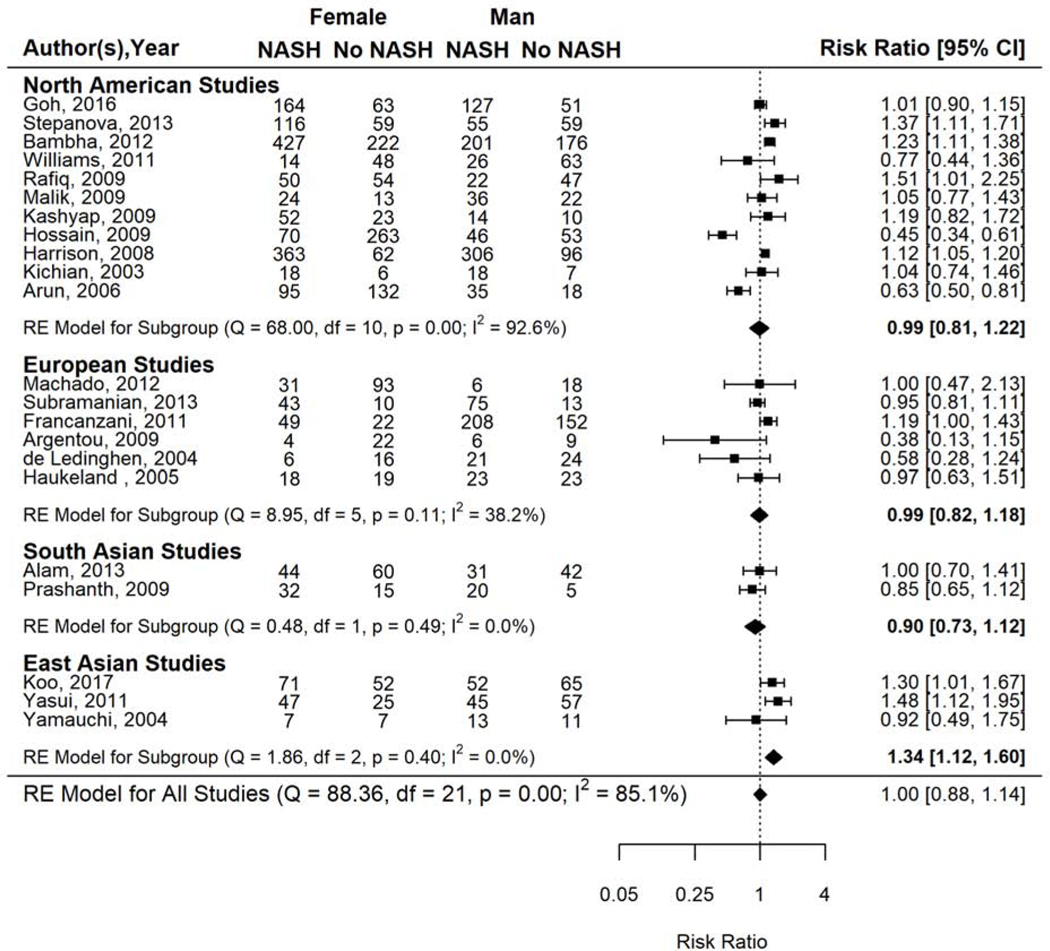
Subgroup analysis by age demonstrated differences in relative risk estimates (Supplementary Figure 4a). NASH prevalence was 17% higher among women compared to men in studies that included older patients (i.e., average study population age ≥50 years: RR 1.17, 95% CI 1.01–1.36) but not statistically significantly higher among women in studies of younger patients (i.e., average age <50 years: RR 0.90, 95% CI 0.76–1.07). Differences in relative risk estimates were also observed in subgroup analysis by geographic region (Figure 3). NASH prevalence was 34% higher in women than men (RR 1.34, 95% CI 1.12–1.60) in East Asian studies but not significantly different from men among studies conducted in North America (RR 0.99, 95% CI 0.81–1.22), South Asia (RR 0.90, 95% CI 0.73–1.12), or Europe (RR 0.99, 95% CI 0.82–1.18). Results from the analyses stratified by type of study population or study design were similar to those from the overall analysis (data not shown).
Figure 3.
Risk of NASH in women versus men with biopsy proven NAFLD, stratified by geography.
Study Quality Assessment
All studies were limited by cross sectional design and most (13 of 22 studies) used retrospective data. Five studies had scores of ≤5, 13 studies had scores 6–7 and 4 had a score ≥8 (Supplementary Table 4).
Publication Bias
We did not find any evidence of publication bias in the meta-analysis of NASH prevalence (Egger test: t(df)= −1.54 (20), p=0.141.) The mild asymmetry seen in the funnel plot was attributed to heterogeneity among the included studies (Supplementary Figure 4b).
Prevalence of Advanced Fibrosis
Study and Patient Characteristics
There were 21 studies that reported data on histologic fibrosis stage among women and men with biopsy proven NAFLD (study characteristics and references listed in Supplementary Table 3). The pooled sample size was 6,444 with 3,199 women and 3,245 men. Fibrosis stage was determined based on the classification scheme used by the individual study authors as categorized as ≥F2 (perisinusoidal/pericellular fibrosis with focal or extensive periportal fibrosis, bridging fibrosis, or cirrhosis) or ≥F3 (bridging fibrosis or cirrhosis). Most studies defined advanced NAFLD fibrosis as bridging fibrosis or cirrhosis (F3–4, n=16) while a smaller number of studies defined it as perisinusoidal/pericellular fibrosis with focal or extensive periportal fibrosis, bridging fibrosis, or cirrhosis (F2–4, n=5). Two studies examined NAFLD fibrosis stage among consecutive morbidly obese patients who had liver biopsies obtained at time of bariatric surgery, 5 among consecutive patients with clinically suspected NAFLD, and 14 were based on retrospectively reviewed NAFLD registries or pathology databases. Four were prospective and 17 were retrospective studies. Six studies were conducted in the U.S.A., 5 in Europe, 7 in East Asia, and 1 in the Middle East, 1 in Australia and 1 in Malaysia. Average age ranged from 32.9 to 56.3 years, BMI from 26.1 to 48.0 kg/m2, and IGT or DM2 prevalence ranged from 7% to 57% in these studies.
Association between Gender and Prevalence of Advanced Fibrosis
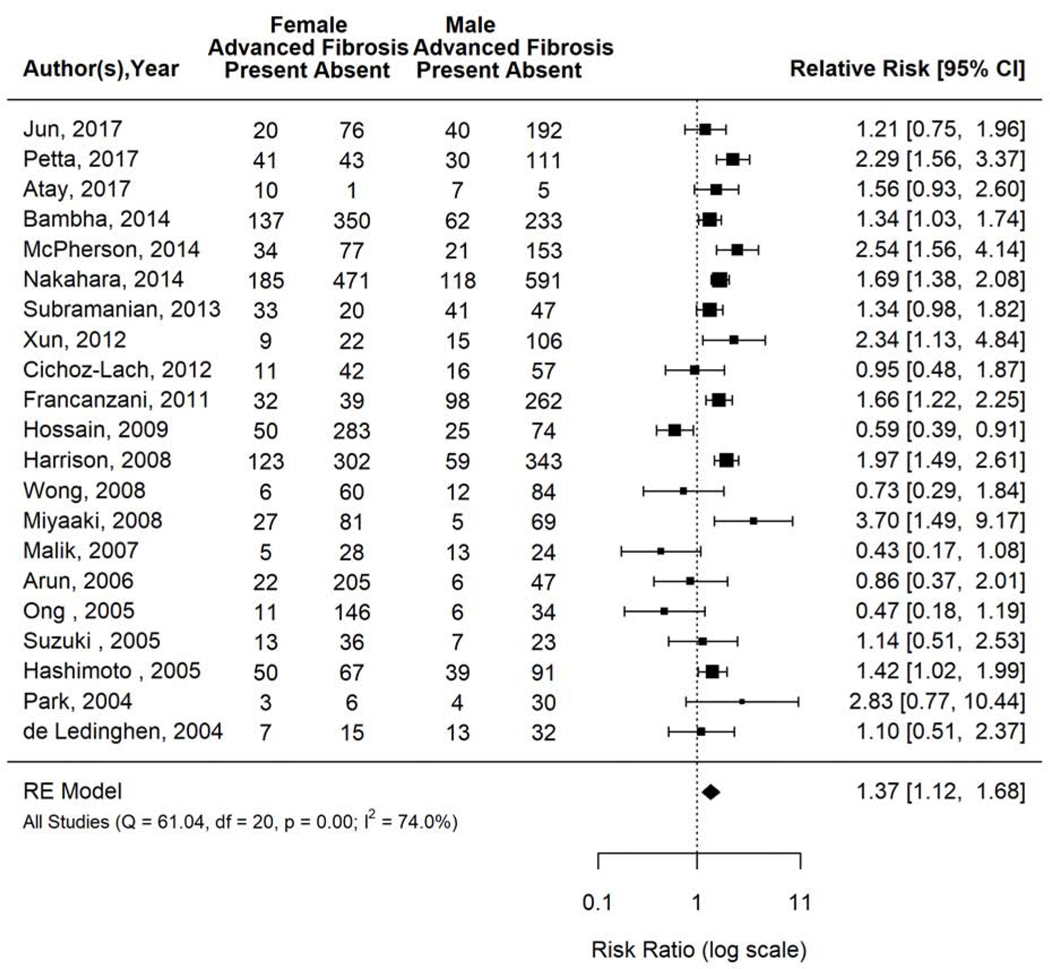
The prevalence of advanced fibrosis ranged from 9% to 74% across included studies. The pooled prevalence of advanced fibrosis was 22.9% (95% CI 19.4–26.8; I2=95.2%). Women had 37% higher pooled prevalence of advanced fibrosis than men in the 21 included studies (pooled RR 1.37, 95% CI 1.12–1.68, I2=74.0%) (Figure 4).
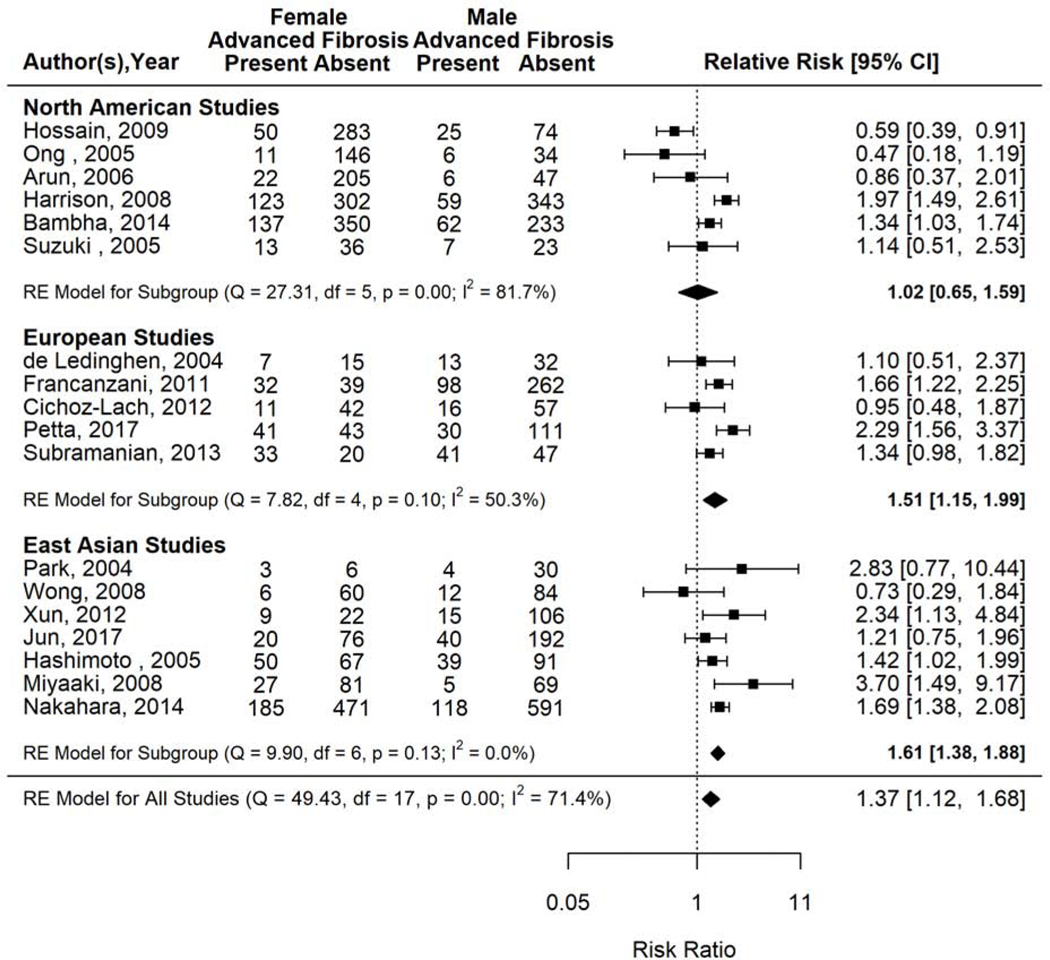
Figure 4.
Risk of advanced fibrosis in women versus men with biopsy proven NAFLD.
Forest plot inspection demonstrated that Ong 2005, Malik 2007, and Miyaaki 2008 were outliers. All three studies classified advanced fibrosis as F3–4 with clearly defined criteria. Ong 2005 was a prospective cross-sectional study that included a sample of 197 patients with histologic NAFLD determined at time of bariatric surgery in a single US center with an average age of 41.6years, BMI of 48kg/m2, and T2DM prevalence of 24%. Malik 2007 was a prospective cross-sectional study that evaluated for fibrosis among 70 consecutive patients referred to a single GI clinic in Malaysia with suspected NAFLD, with an average age of 47 years, BMI 28.1 kg/m2, and 29% type 2 diabetes prevalence. Miyaaki 2008 was a retrospective cross-sectional study that assessed for advanced fibrosis among a registry of patients with biopsy proven NAFLD. All three studies were kept in the primary analysis given there were no outlier study characteristics justifying exclusion. Sensitivity analysis removing one study at a time did not substantially change findings.
The gender specific prevalence of advanced fibrosis differed by age (Supplementary Figure 5a). Among older study populations with average age ≥50 years, advanced fibrosis prevalence was 56% higher among women than men (RR 1.56, 95% CI 1.36–1.80, I2=0) but was not significantly different between men and women among study populations with average age<50 years.
The gender specific prevalence of advanced fibrosis also differed by geographic location (Figure 5). The prevalence of advanced fibrosis among women was 51% higher than men among European studies (RR 1.51, 95% CI 1.15–1.99, I2=50%) and 61% higher than men among East Asian studies (RR 1.61, 95% CI 1.38–1.88, I2=0%) but not significantly different from men among studies conducted in North America (RR 1.02, 95% CI 0.65–1.59, I2=81.7%).
Figure 5.
Risk of advanced fibrosis in women versus men with biopsy proven NAFLD, stratified by geography.
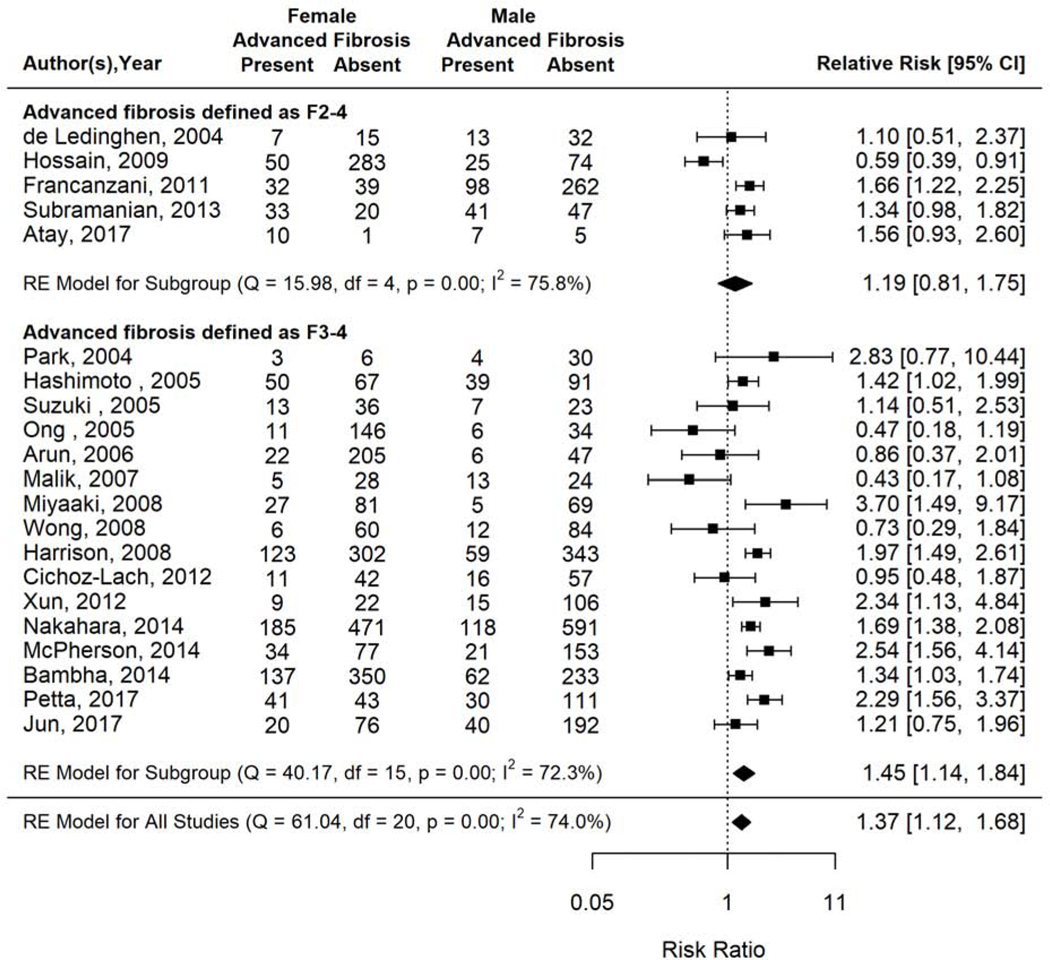
Sensitivity analysis by advanced fibrosis classification scheme demonstrated that women had 45% higher prevalence of advanced fibrosis compared to men among 16 studies that classified it as F3–4 (RR 1.45, 95% CI 1.14–1.84, I2=72.3%). This difference was attenuated though still higher among women compared to men in the 5 studies that classified advanced fibrosis as F2–4 (RR 1.19, 95% CI 0.81–1.75, I2=75.8%) (Figure 6).
Figure 6.
Risk of advanced fibrosis in women versus men with biopsy proven NAFLD, stratified by advanced fibrosis definition.
Study Quality Assessment
All studies were limited by cross-sectional study design and nearly all (17 out of 21 studies) used retrospective data. Ten studies had scores of ≤5, 11 studies had scores 6–7 and none had a score ≥8 (Supplementary Table 4.)
Publication Bias
We did not find evidence of publication bias (Egger test: t(df) = −1.36 (19), p=0.189). The mild asymmetry seen in the funnel plot was likely related to the heterogeneity of included studies (Supplementary Figure 5b).
DISCUSSION
In our meta-analysis of 17 population-based studies, the prevalence of NAFLD was 19% higher among men than women. However, among individuals with established NAFLD, women were as likely as men to have NASH and more likely to have advanced fibrosis than men. Among patients with NAFLD, women had a 37% higher prevalence of advanced fibrosis than men. These findings have important implications not only for disease stratification and counseling patients about risk of NAFLD, but also indicate that the future burden of NAFLD complications are likely to affect men and women equally.
Age modified the gender effect in our analysis. Among populations of older individuals with biopsy proven NAFLD, women had a substantially higher risk of NASH and advanced fibrosis compared to men. Our findings of an increased prevalence of severe phenotypes of NAFLD – NASH and advanced fibrosis – among older women fits well into the current understanding of disease pathogenesis. Central obesity, particularly visceral adiposity, is the main source of free fatty acids that drive NASH pathogenesis and progression (25). While men tend to accumulate adipose tissue centrally throughout their lifetime, adipose tissue distribution changes over the lifetime of women and is subject to estrogen exposure. Estrogen exposure during the premenopausal years promotes a gluteal femoral adipose pattern. Declines in estrogen during the menopausal transition, typically occurring around the age of 50 years, leads to a shift in adipose tissue deposition to a visceral distribution. Several metabolic sequelae of this change in body fat distribution, including increases in incidence of type 2 diabetes and atherogenic dyslipidemia beyond that expected by aging alone, are well documented among post-menopausal women (26, 27).
Our findings indicate that once NAFLD is established, the risk of progressive disease (i.e. NASH and advanced fibrosis) is not different and may be slightly higher among women than men. Estrogen is believed to exert a direct anti-fibrotic effect in the liver and may play a protective role against steatohepatitis. In studies of patients with chronic viral hepatitis, the slower progression of fibrosis as well as the later onset of cirrhosis observed in women has largely been attributed to the protective role of estrogen (8, 28). In contrast to the case of viral hepatitis, we found that among younger populations with NAFLD, women possess an equal risk of advanced fibrosis as men NAFLD; furthermore, our findings suggest that the loss of estrogen may in fact accelerate disease progression among postmenopausal women.
There were geographic variations in the association between gender and NAFLD severity. While the effect of gender on risk of NAFLD did not vary across geographic locations, a significantly higher prevalence of the severe histologic subtypes of NAFLD was observed among women compared to men among studies out of East Asia (for NASH and advanced fibrosis) and Europe (advanced fibrosis) but not North America. Age may partially explain the NASH and advanced fibrosis trends - as most of the large East Asian studies that examined NASH or advanced fibrosis included older patients – but not completely, as the majority of European studies (four of five) were comprised of younger study populations. These geographic differences may reflect cultural differences in gender behaviors (i.e., dietary habits) that influence NAFLD pathogenesis and risk of NASH and fibrotic progression. Alternatively, they may also be related to a differential prevalence of PNPLA3 genetic variants that interact with gender to drive NAFLD progression.
Our findings have implications for chronic liver disease epidemiology as well as women’s health. The presence of severe fibrosis (METAVIR stage F2–4 on liver biopsy) independently predicts an elevated liver-related mortality in NAFLD (29). Given the higher risk of advanced fibrosis observed among women compared to men with NAFLD in our meta-analysis, it is plausible that cirrhosis and its complications may occur with greater frequency among women than in men. Thus, women with NAFLD should be evaluated for NASH and fibrosis as vigorously as men. It is further possible that women with NAFLD, particularly those over 50 years of age, may require greater clinical vigilance to diagnose advanced fibrosis compared to men. Moreover, consideration of undiagnosed cirrhosis may be warranted among younger women with NAFLD risk factors who are considering pregnancy, as cirrhosis can be a serious health problem in women’s reproductive life. In addition, there are potential downstream effects of advanced maternal liver disease on fetal development and neonatal health. However, these hypotheses will need to be evaluated in future studies before firm recommendations can be made. Younger women with NASH may also be less likely to receive pharmacologic therapies given that nearly all existing and pipeline options are contraindicated during pregnancy or have unstudied fetal effects. Thus, more focused and intensified efforts may be warranted to target lifestyle modifications and weight loss among young women with NAFLD, particularly in the presence of NASH and/or advanced fibrosis.
Our study has limitations. First, there was significant heterogeneity among the included studies, as observed in previously published meta-analyses addressing NAFLD prevalence and severity (1, 30). It is unlikely that mode of NAFLD detection was a significant source of heterogeneity given that ultrasound was utilized among 13 out of 17 studies included in the NAFLD prevalence analysis. A large proportion of studies that were included in the NASH (15 out of 22 studies) and advanced fibrosis (17 out of 21 studies) analyses utilized data among patients with biopsy-proven NAFLD. It is plausible that the included study cohorts had undergone biopsies because more severe disease was suspected, however it is unlikely that gender influenced the likelihood of receiving a liver biopsy among the included patients. Thus, inclusion of these study cohorts may account for some of the observed heterogeneity but are unlikely to have introduced bias into the analyses. Gender, age, and body weight may interact to influence risk of advanced fibrosis among patients with NAFLD. However, none of the studies included in our analysis were designed specifically to address gender differences in NAFLD and granular data regarding metabolic comorbidities or body weight classification were not available by gender. Therefore, we could not explore potential interactions between body weight and gender in the current analysis. Finally, there are several unmeasured factors that may explain our findings. For example, polycystic ovarian syndrome, through high circulating androgens and insulin resistance, may account for the greater risk of NASH among women. The cumulative effects of hormonal therapies, pregnancies and related complications over women’s lifetime may contribute to NAFLD progression. Behavioral factors - that likely vary across cultural settings - may account for the increased risk of NAFLD severity among women.
In summary, we found that women have a lower risk of NAFLD than men; however, once NAFLD is established, women’s risk of progressive disease (NASH and advanced fibrosis) is higher than men. These finding have far-reaching implications for the future burden of liver disease, women’s health, and gender disparities in liver disease.
Supplementary Material
Supplementary Figure 1. Search Strategy
Supplementary Figure 2. Flow Diagram for Study Selection
Supplementary Figure 3a. NAFLD prevalence in population-based studies, stratified by average study population age.
Supplementary Figure 3b. NAFLD prevalence in population-based studies, stratified by geography
Supplementary Figure 3c. Funnel Plot for NAFLD risk analysis
Supplementary Figure 4a. Risk of NASH in women versus men with biopsy proven NAFLD, stratified by age.
Supplementary Figure 4b. Funnel Plot for NASH risk analysis
Supplementary Figure 5a. Risk of advanced fibrosis in women versus men with biopsy proven NAFLD, stratified by age.
Supplementary Figure 5b. Funnel Plot for advanced fibrosis risk analysis
Need to Know.
Background:
Risk and progression of nonalcoholic fatty liver disease (NAFLD) might differ between men and women.
Findings:
A systematic review and meta-analysis found that women have a lower risk of NAFLD than men. However, once NAFLD is established, women have a higher risk of advanced fibrosis than men—especially after age 50 years.
Implications for patient care:
Women with NAFLD, especially those older than 50 years, should be closely evaluated for advanced fibrosis.
Acknowledgments
Funding: This work was supported in part by National Institutes of Health grant P30 DK056338, which supports the Texas Medical Center Digestive Diseases Center.
Abbreviations:
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- F2
stage 2 fibrosis
- F3
stage 3 fibrosis
- IGT
Impaired glucose tolerance
- T2DM
Type 2 diabetes
Footnotes
Guarantor of the article: Fasiha Kanwal, MD
Conflict of Interest: The authors do not report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global Epidemiology of Non-Alcoholic Fatty Liver Disease–Meta-Analytic Assessment of Prevalence, Incidence and Outcomes. Hepatology 2015:n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 2.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723–1730. [DOI] [PubMed] [Google Scholar]
- 4.Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: The next frontier in the epidemic. Hepatology 2017;65:2100–2109. [DOI] [PubMed] [Google Scholar]
- 5.Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the Burden of Nonalcoholic Fatty Liver Disease in a United States Cohort of Veterans. Clin Gastroenterol Hepatol 2016;14:301–308.e301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, Carey W, et al. Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol 2017;112:581–587. [DOI] [PubMed] [Google Scholar]
- 7.Buzzetti E, Parikh PM, Gerussi A, Tsochatzis E. Gender differences in liver disease and the drug-dose gender gap. Pharmacol Res 2017;120:97–108. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu I, Kohno N, Tamaki K, Shono M, Huang HW, He JH, Yao DF. Female hepatology: favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol 2007;13:4295–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris HE, Ramsay ME, Andrews N, Eldridge KP. Clinical course of hepatitis C virus during the first decade of infection: cohort study. Bmj 2002;324:450–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol 2001;34:730–739. [DOI] [PubMed] [Google Scholar]
- 11.Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Croce L, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 1997;41:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–131. [DOI] [PubMed] [Google Scholar]
- 13.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395. [DOI] [PubMed] [Google Scholar]
- 14.Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect 2010;118:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, Unalp-Arida A, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology 2012;55:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269, w264. [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 19.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 20.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467–2474. [DOI] [PubMed] [Google Scholar]
- 21.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413–1419. [DOI] [PubMed] [Google Scholar]
- 22.NIH. Study Quality Assessment Tools. In. Bethesda, MD. [Google Scholar]
- 23.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–1055. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurka MJ, Vishnu A, Santen RJ, DeBoer MD. Progression of Metabolic Syndrome Severity During the Menopausal Transition. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallajzadeh J, Khoramdad M, Izadi N, Karamzad N, Almasi-Hashiani A, Ayubi E, Qorbani M, et al. Metabolic syndrome and its components in premenopausal and postmenopausal women: a comprehensive systematic review and meta-analysis on observational studies. Menopause 2018;25:1155–1164. [DOI] [PubMed] [Google Scholar]
- 28.Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, Moussalli J, et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology 2004;40:1426–1433. [DOI] [PubMed] [Google Scholar]
- 29.Hagstrom H, Nasr P, Ekstedt M, Hammar U, Stal P, Hultcrantz R, Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017. [DOI] [PubMed] [Google Scholar]
- 30.Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, Mayo H, et al. Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:198–210.e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Search Strategy
Supplementary Figure 2. Flow Diagram for Study Selection
Supplementary Figure 3a. NAFLD prevalence in population-based studies, stratified by average study population age.
Supplementary Figure 3b. NAFLD prevalence in population-based studies, stratified by geography
Supplementary Figure 3c. Funnel Plot for NAFLD risk analysis
Supplementary Figure 4a. Risk of NASH in women versus men with biopsy proven NAFLD, stratified by age.
Supplementary Figure 4b. Funnel Plot for NASH risk analysis
Supplementary Figure 5a. Risk of advanced fibrosis in women versus men with biopsy proven NAFLD, stratified by age.
Supplementary Figure 5b. Funnel Plot for advanced fibrosis risk analysis