Abstract
Objectives
Vitamin D deficiency/insufficiency is a worldwide public health issue that has been linked to numerous inflammatory disorders, including periodontitis. There is increasing support for a role for adequate vitamin D levels in overall health. Populations with darker skin color have a higher prevalence of vitamin D deficiency/insufficiency and periodontitis. The purpose of this small pilot study was to investigate the influence of 12 weeks of 25(OH)D vitamin D supplementation on mediators of systemic inflammation in dark-skinned, periodontitis patients.
Materials and Methods
A total of 23 patients with moderate to severe periodontitis were randomly assigned to the vitamin D group or Placebo group and received intensive single visit scaling and root planing to elicit a systemic inflammatory response.
Results
Vitamin D supplementation (VDS) increased serum 25(OH)D levels approximately 2-fold over baseline levels; moreover, VDS group had reduced peripheral blood CD3 and CD3+CD8+ cytotoxic T lymphocyte (CTLs) counts and reduced pro-inflammatory salivary cytokines. In contrast, VDS group had higher levels of the autophagy-related proteins and other proteins crucial for anti-microbial autophagy in whole blood PBMCs.
Conclusion
In conclusion, vitamin D supplementation has multiple benefits for reducing systemic inflammation and promoting induction of autophagy related proteins related to anti-microbial functions.
Key Words (MeSH): Vitamin D, Periodontitis, Autophagy, Saliva, Immunity
1. INTRODUCTION
Vitamin D deficiency/insufficiency is widely prevalent, despite being a preventable condition in most people. The activated form of vitamin D is crucial to bone mineral regulation and immunomodulation (Baeke et al., 2010), and has been implicated as having a role in multiple chronic conditions such as cardiovascular disease, cancer, autoimmune disease, musculoskeletal disorders, infectious disease, kidney disease, Alzheimer’s, and periodontitis. Vitamin D deficiency is defined as having a 25-hydroxyvitamin D [25(OH)D] level equal to or less than 20 ng/ml, and insufficiency is a concentration of 21 to 29 ng/ml (Dawson-Hughes et al., 2005). This equates to roughly a billion of the global population to be vitamin D insufficient and/or deficient (Holick, 2006). Although it is difficult for many to attain adequate vitamin D levels from diet alone, most people achieve sufficient amounts by skin exposure to sunlight (Holick & Chen, 2008). However, it has recently been shown that even in a geographical region with a year-round sunny climate, people with dark skin pigmentation had significantly lower plasma [25(OH)D] levels in every season versus Caucasians (Dong et al., 2010a).
Periodontitis (PD), a widespread multifactorial chronic inflammatory disease that affects tissues surrounding the teeth, is a host-mediated disease characterized by local tissue destruction initiated by exposure to bacterial plaque and their metabolic byproducts. The 2009–10 data from the US Centers for Disease Control’s (CDC) National Health and Nutrition Examination Survey (NHANES) revealed that nearly 1 of every 2 individuals in the US aged 30 years or over has periodontitis, and the incidence went up to over 70% in those aged 65 or older (Eke et al., 2012, Holick, 2008). The ethnic groups with the highest prevalence were African-Americans and Mexican-Americans, who were twice as likely to have periodontal disease as Caucasian-Americans. The literature also suggests that serum vitamin D concentrations and periodontal disease are inversely proportional (Dietrich et al., 2005, Zhan et al., 2014a, Alshouibi et al., 2013, Antonoglou et al., 2014, Drury et al., 1996). Darker skinned patients and those that live in higher latitudes have a greater prevalence of inadequate vitamin D levels, as well as periodontitis (Grant & Boucher, 2010).
Autophagy is a homeostatic process through which the cell disposes of damaged intracellular organelles and protein aggregates via trafficking the cellular cargo to acidic compartments. The same molecular machinery is exploited by immune cells to fight against and degrade invading microbes (Yamaguchi et al., 2009, El-Awady et al., 2015). Importantly, vitamin D is involved in various signaling pathways controlling the autophagy process. Vitamin D has been shown to promote an anti-mycobacterial response by enhancing autophagy (Fabri & Modlin, 2009).
We have previously demonstrated that [25(OH)D] levels were associated with a variety of adverse cardiometabolic risk factors such as adiponectin, leptin, and fibrinogen, as well as fasting glucose, insulin resistance, HDL cholesterol, systolic and diastolic blood pressure (Parikh et al., 2012). In addition, we have shown that vitamin D supplementation (VDS) in African-Americans improved arterial stiffness, as measured by pulse wave velocity (Dong et al., 2010b). Vitamin D is a well-known regulator of immune and inflammatory responses; for example, vitamin D directly inhibits T cell proliferation, IFN-γ and IL-17 while inducing IL-4 and T-reg mediated responses, highlighting vitamin D’s significant anti-inflammatory pro-resolution properties.
The purpose of this study was to determine the influence of VDS on response of PD patients to intensive single visit initial therapy (Tonetti et al., 2007), including local and systemic inflammatory responses, levels of autophagy-related proteins in PBMCs and serum and salivary inflammatory cytokine profiles.
2. MATERIAL AND METHODS.
2.1. Experimental overview:
This double-blind pilot randomized clinical trial was evaluated and approved by Institutional Review Board Office at Augusta University [IRB 611824–20]. An initial screening appointment was held at the Georgia Prevention Institute Clinic (Augusta, GA) where informed consent was obtained, followed by an oral health screening to determine patient eligibility. Patients were invited to participate in this study if the following inclusion criteria was met:
Dark-skinned individuals who score 21 or higher and have skin type IV or above on Fitzpatrick skin type questionnaire.
Age of 18 years old and above.
Generalized chronic moderate to severe periodontitis (probing pocket depth (PPD) of >4 mm, attachment loss of >3 mm, bleeding on probing, and alveolar bone crest >3 mm from the cementoenamel junction), as described by Armitage 1999 (Armitage, 1999).
Exclusion criteria included:
Currently taking antibiotics, anti-inflammatory agents, vitamin, mineral or herbal medications and/or supplements.
Female patients who were pregnant or attempting to conceive.
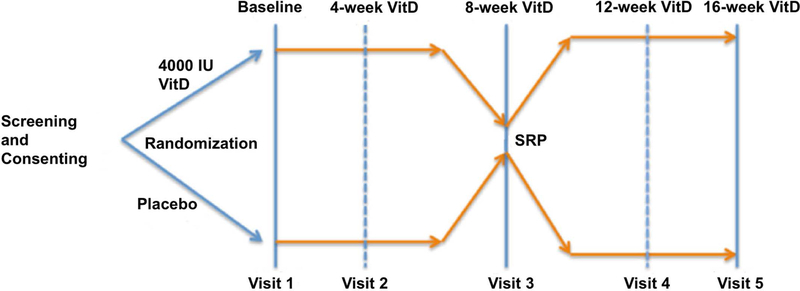
The study experimental design is summarized in (Figure 1) and (Table 1). Baseline visits consisted of a thorough medical history as well as a full mouth periodontal exam. Serum vitamin D levels were assessed by radioimmunoassay (Hollis et al., 1996) in the Dong laboratory as reported (Bhagatwala et al., 2015) The laboratory is certified by the vitamin D external quality assessment scheme (DEQAS), an international program monitoring accuracy of 25(OH)D measurements.
Figure 1:
Study experimental design.SRP: scaling and root planning.
Table 1.
Summary of treatment and sample collection schedule
| Event | Screening | Visit 1 Vit D or Placebo (Baseline) |
Visit 2 Compliance testing/Refill (4 wks from Baseline) |
Visit 3 Compliance testing/Refill (8 wks from Baseline) |
Visit 4 Compliance testing/Refill (12 wks from Baseline) |
Visit 5 (8 wks from SRP) (16 wks from Baseline) |
|---|---|---|---|---|---|---|
| Medical history | ☓ | |||||
| Informed consent | ☓ | ☓ | ||||
| Intensive SRP | ☓ | |||||
| 25(OH)D | ☓ | ☓ | ☓ | |||
| Peripheral blood, Whole saliva | ☓ | ☓ | ☓ | ☓ | ||
| Gingival biopsy | ☓ | ☓ | ☓ | |||
| Vit D supplementation | ☓ | ☓ | ☓ | ☓ | ||
| Sub-gingival plaque sample | ☓ | ☓ | ☓ | |||
| Check gingival healing | ☓ | ☓ |
Unstimulated whole saliva was also collected. Then study subjects were randomized for a 30-day supply of either an oral placebo or supplemental vitamin D. At 4 weeks, a follow up visit consisted of a compliance assessment (flask verification) and an additional 30-day supply of the corresponding placebo or supplemental vitamin D. At 8 weeks, a medical history and periodontal exam was completed again, including all measurements listed above, as well as another plasma sample for 25(OH)D blood testing and saliva testing. Then subjects received intensive single visit SRP, as previously reported to induce a systemic inflammatory response (Tonetti et al., 2007). At 12 weeks, a follow up appointment consisted of a compliance assessment and an additional 30-day continuous supply of either the placebo or supplemental Vitamin D. At 16 weeks, a final appointment for the study consisted of medical history and a final full periodontal re-evaluation exam.
2.2. Study Outcome Measures:
Mean changes from baseline values after 8 and 16 weeks were calculated for all dependent variables including serum vitamin D and for the salivary immune cytokines including CCL-20, TNF-α, EFABP (epidermal fatty acid-binding protein), GM-CSF (Granulocyte-macrophage colony-stimulating factor), IL-1β, IL-2, IL-4 IL-5, IL-6, IL-8 and IL-10. Furthermore, the autophagy-related protein levels were analyzed in PBMCs (peripheral blood mononuclear cells) in PD patients vs healthy subjects at baseline, and in PD patients before and after vitamin D supplementation by Western blotting analysis as described below.
2.3. Interventions:
Patients allocated to the vitamin D group received 4,000 IU/day oral Vitamin D supplementation for 16 weeks. All patients were treated for periodontitis by means of non-surgical periodontal therapy, which consisted of single visit intensive scaling and root planning (SRP) with ultrasonic and manual instrumentation under local anesthesia (Lidocaine~9mcg-18mcg, and Septocaine ~ 17mcg - 68mcg both with ~1:100k epinephrine in metered doses) where indicated to remove supra and subgingival calculus accumulation.
2.4. Study enrollment/withdrawal (consort statement):
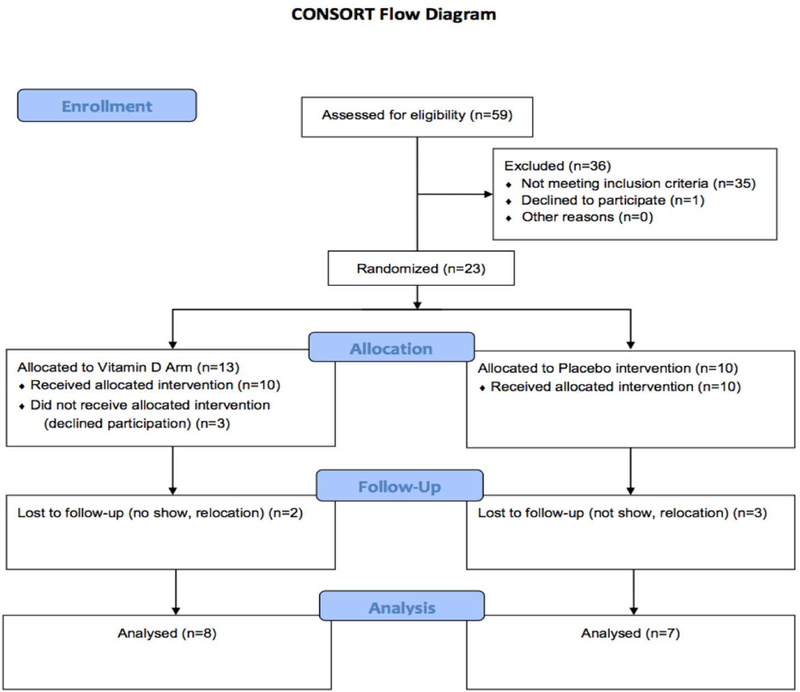
A CONSORT flow diagram is presented in (Figure 2). A total of 59 patients were screened at the Georgia Prevention Institute for inclusion criteria between January 2015 and August 2016. 35 patients (~59%) did not meet the inclusion criteria and 1 patient declined to participate. 23 subjects were randomized to the vitamin D (n=13) or the placebo (n=10) group. 3 Subjects in the vitamin D group did not receive intervention and 2 additional patients were lost to follow up. 3 patients were lost to follow up in the placebo control group.
Figure 2:
Consort diagram showing the study design.
2.5. Laboratory measurements
Fasting blood samples were obtained at baseline, 8-week pro- and post SRP, and 16-week, which were frozen and stored at −80 °C until assayed. Serum vitamin D level [25(OH)D] concentrations were measured using enzyme immunoassay (Immunodiagnostic Systems, Fountain Hills, AZ). The intra- and inter-assay coefficients of variation (CV) were 5.6 and 6.6 %, respectively. Our laboratory is certified by the vitamin D3 external quality assessment scheme (DEQAS), an international program monitoring accuracy of 25(OH)D measurements. Fresh peripheral blood was collected and sent to the clinical pathology core lab at the Medical College of Georgia within 2 hours for the complete blood count with differential, which included the total leukocyte count and percentages of peripheral blood cell types including neutrophils, lymphocytes, monocytes, eosinophils and basophils. Immune phenotype (CD3+, CD4+ and CD8+) was performed using flow cytometry.
2.6. Western Blot:
Whole-cell lysates were prepared from PBMCs by centrifuging and washing the pellet with ice-cold PBS. Proteins were extracted by adding ice-cold Radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich Inc., St. Louis, MO) supplemented by protease and phosphatase inhibitor cocktail (Cell Signaling Technology, Danvers, MA). The cell lysates were centrifuged at 12,000 × g for 10 min at 4°C. Samples were normalized to the amount of total protein in the supernatant using Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Carlsbad, CA). Protein aliquots (30μg) were separated by size on a 4–15% Mini-PROTEAN TGX stain-free precast gels (Bio-Rad Laboratories, Inc.) and transferred to Immobilon (PVDF) membrane (Sigma-Aldrich Inc., St. Louis, MO). Nonspecific binding sites were blocked by incubation in 1× TBS-T (0.2 m Tris, 0.14 m NaCl, 0.1% Tween 20) containing 5% non-fat milk for 1 hour at room temperature, followed by overnight incubation at 4°C with 1:1000 dilution of primary antibodies obtained from Cell Signaling Technology, Inc.: (ATG5–12 conjugate, ATG7, ATG16L1, Beclin 1, Bcl2, Raptor, mTOR, ULK1, p-ULK1 (Ser757) and β-actin) in 1× TBS-T containing 5% non-fat milk. After membranes were washed three times in 1× TBS-T, horseradish peroxidase-conjugated secondary antibody was added at a 1:2000 dilution and incubated for 1 h at room temperature. After three more washes with 1× TBS-T, the immunoreactive peptide was detected by Western Lightning ECL Pro Chemiluminescent reagent (PerkinElmer Inc., Waltham, MA) and imaged using ChemiDoc™ MP Imaging System (Bio-Rad Laboratories, Inc., Hercules, CA).
2.7. Real-Time Quantitative Reverse Transcription PCR (qRT-PCR):
Total RNA was extracted from gingival samples by homogenization of tissues in TRIzol™ Reagent (Thermo Fisher Scientific, Carlsbad, CA) and RNeasy kit (Qiagen, Germantown, MD) following the manufacturer’s protocol. Total RNA was reverse transcribed into cDNA. The cDNA was then amplified by PCR using the High-Capacity cDNA Reverse Transcription Kit® with random primers in total reaction of 20 μL. For qrt-PCR, TaqMan® gene expression primers obtained from Thermo Fisher Scientific were used: Atg5 (Assay ID: Hs00169468_m1), Atg16L1 (Assay ID: Hs01003142_m1), Beclin 1 (Assay ID: Hs01007018_m1) and GAPDH (Assay ID: Hs02758991-g1)
2.8. MAGPIX Multiplexing:
The level of the following cytokines in the saliva was measured by conducting MAGPIX multiplexing assay: CCL-20, TNF-α, EFABP, GM-CSF, IL-1β, IL-2, IL-4 IL-5, IL-6, IL-8 and IL-10 (Thermo Fisher Scientific, Carlsbad, CA) using Bio-Plex® MAGPIX™ Multiplex Reader with Bio-Plex Manager™ MP Software (Bio-Rad Laboratories, Inc., Hercules, CA).
2.9. Statistical Analysis:
Repeated measures analysis was used to examine the effect of the between factor (treatment group) and the within factor (time point) for each clinical parameter. Thus, we compared the vitamin D supplementation and placebo groups at each of the four time points: Baseline (BL), 24 hrs Pre-SRP, 24 hrs Post-SRP, and Re-Evaluation (Re-Eval). We also evaluated the change in each cytokine in the saliva across the four time points, separately for each group. The following clinical parameters were considered: CD3 Count, CD3 Per (percentage), CD4 Count, CD4 Per, CD8 Count and CD8 Per. Mixed-effects regression models (MRMs) were used to perform the repeated measures analysis; the advantage of MRMs is that they make use of all available data. The vitamin D and Placebo groups were compared at baseline in terms of patient characteristics using Fisher’s exact test for categorical variables (e.g., gender) and the two-sample t-test for continuous variables (e.g., age). Any characteristics that differed significantly between the vitamin D and Placebo groups at baseline were examined as possible confounders in each of the treatment group comparisons (Table 2). We used MRMs to adjust for the effect of any significant confounders. Because of the pilot and exploratory nature of the study, no adjustment was made for multiple testing. Descriptive statistics at each time point within each group were reported as least squares means (LSM) ± standard error (SE) obtained from the MRM analyses. All statistical tests were two-tailed and were performed at the 0.05 level of significance.
Table 2.
Demographics of study population
| Variable | Total (n = 23) |
Placebo (n=12) |
VitD (n=11) |
p-value |
|---|---|---|---|---|
| Age, yr (Mean ± SD) | 44.8 ± 9.4 | 42.5 ± 9.2 | 47.3 ± 9.2 | 0.230 |
|
| ||||
| Gender (%) | ||||
| Male | 12 (52%) | 9 (75%) | 3 (27%) | 0.039* |
| Female | 11 (48%) | 3 (25%) | 8 (73%) | |
|
| ||||
| Marital Status (%) | ||||
| Single - never married | 11 (48%) | 6 (50%) | 5 (45%) | 1.000 |
| All Others | 12 (52%) | 6 (50%) | 6 (55%) | |
|
| ||||
| Educational level (%) | ||||
| > high school | 12 (55%) | 6 (55%) | 6 (55%) | 0.461 |
| High school graduate | 6 (27%) | 4 (36%) | 2 (18%) | |
| < high school | 4 (18%) | 1 (9%) | 3 (27%) | |
|
| ||||
| Current Tobacco Use (%) | ||||
| Yes | 11 (48%) | 5 (42%) | 6 (55%) | 0.684 |
| No | 12 (52%) | 7 (58%) | 5 (45%) | |
|
| ||||
| Smoking Severity, number of packs/wk (Mean ± SD) | 1.1 ± 1.8 | 0.4 ± 0.6 | 1.9 ± 2.2 | 0.034* |
|
| ||||
| History of Smoking, yr (Mean ± SD) | 12.4 ± 11.1 (n = 9) | 16.3 ± 10.3 (n = 4) | 9.3 ± 11.9 (n = 5) | 0.387 |
|
| ||||
| HbA1c (Mean ± SD) | 6.0 ± 0.6 | 5.9 ± 0.6 | 6.0 ± 0.6 | 0.723 |
|
| ||||
| BMI (Mean ± SD) | 34.5 ± 12.9 | 31.3 ± 12.1 | 38.0 ± 13.4 | 0.221 |
|
| ||||
| Periodontitis Status (%) | ||||
| Moderate | 12 (52%) | 6 (50%) | 6 (55%) | 1.000 |
| Severe | 11 (48%) | 6 (50%) | 5 (45%) | |
3. RESULTS
3.1. Vitamin D supplementation (VDS) with periodontal therapy reduces CD3+CD8+ CTL-mediated immune response
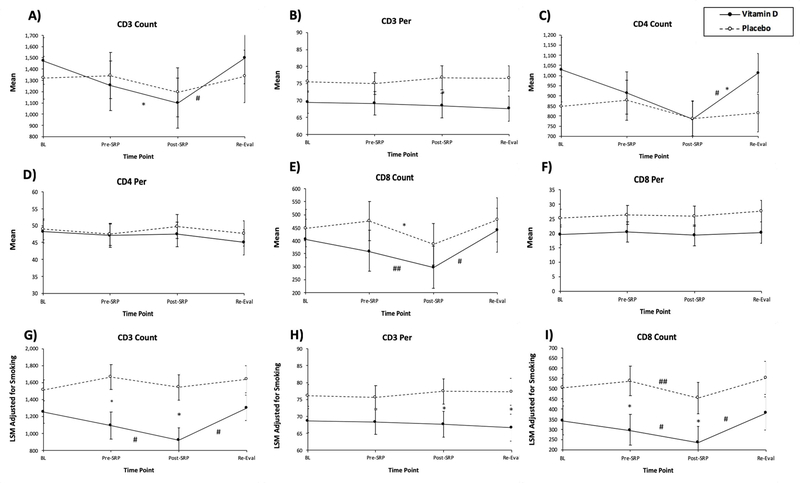
We tested the serum vitamin D level in all subjects in the vitamin D treated group and Placebo treat group. While not initially vitamin D deficient, vitamin D levels significantly increased in the vitamin D treated group from baseline to 8 weeks of supplementation and 6 weeks of re-evaluation (Supplementary Figure 1). To test the influence of VDS along with periodontal therapy on CD8+ T cell-mediated immune response, we investigated T lymphocytes mean cell counts (Count) and percentages (Per): CD3 Count, CD3 Per, CD4 Count, CD4 Per, CD8 count and CD8 Per in PBMCs of PD patients at all timepoints. The results showed that the mean CD3 Count (Figure 3A) in the Vitamin D group at Post-SRP was significantly lower than the means at Pre-SRP (p=0.026) and Re-Eval (p<0.001). For CD3 Per (Figure 3B), comparison of the placebo and Vitamin D groups at Post-SRP almost reached statistical significance (p=0.056), with the mean CD3 Per being lower in the vitamin D group. For CD4 Count (Figure 3C), in the Vitamin D group, the mean at Re-Eval was significantly less than the mean at BL (p=0.020) and significantly greater than the mean at Post-SRP (p<0.001). For CD8 Count (Figure 3E), in the Vitamin D group, the mean at Post-SRP was significantly lower than the means at Re-Eval (p<0.001) and Pre-SRP (p=0.014). There were no significant differences between treatment groups, or between time points in either group, for each of the following outcomes: CD4 Per (Figure 3D) and CD8 Per (Figure 3F). In the placebo group, the mean CD8 Count at Post-SRP was significantly less than the mean at Pre-SRP (p = 0.012) (Figure 3E). However, the vitamin D and placebo groups differed significantly in terms of two potential covariates at baseline: gender and smoking severity (Table 2). We used MRMs to adjust the results of each of the repeated measures analyses described above for any significant confounding effects of these two covariates. Smoking severity was a significant confounder for CD3 Count, CD3 Per and CD8 Count. For CD3 Count, after adjusting for smoking (Figure 3G), in the vitamin D group, the adjusted mean CD3 Count at Post-SRP was significantly lower than the adjusted mean at Pre-SRP (p=0.030) and at Re-Eval (p < 0.001). In addition, the adjusted mean CD3 Count in the vitamin D group was significantly lower than the adjusted mean in the Placebo group at Post-SRP (p=0.031) and the treatment group comparison almost reached significance at Pre-SRP (p=0.054). For CD3 Per, after adjusting for smoking (Figure 3H), the adjusted mean CD3 Per in the vitamin D group was significantly lower than the adjusted mean in the Placebo group at Post-SRP (p=0.031) and Pre-SRP (p=0.024). For CD8 Count, in the vitamin D group, after adjusting for smoking (Figure 3I), the adjusted mean CD8 Count at Post-SRP was significantly lower than the adjusted mean at Pre-SRP (p=0.015) and at Re-Eval (p < 0.001). In the Placebo group, after adjusting for smoking, the adjusted mean CD8 Count at Post-SRP was significantly lower than the adjusted mean at Pre-SRP (p=0.016). In addition, the comparison of the adjusted mean CD8 Count in the vitamin D group vs. that in the Placebo group almost reached significance at Pre-SRP (p=0.051) and Post-SRP (p=0.052) (Figure 3I).
Figure 3:
Influence of vitamin D supplementation with periodontal therapy on T lymphocytes count and percentage. Figures (a), (b), (c), (d), (e), (f), (g), (h), and (i) show CD3 Count, CD3 Per, CD4 Count, CD4 Per, CD8 Count, CD8 Per, CD3 Count adjusted for smoking, CD3 Per adjusted for smoking, and CD8 Count adjusted for smoking, respectively.
3.2. PD patients exhibit low autophagic profile at baseline
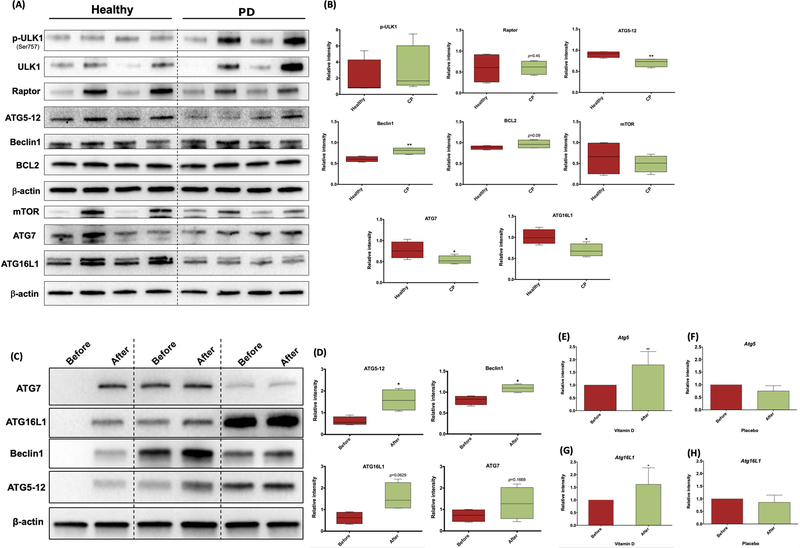
Due to reported links between vitamin D levels and autophagy (Fabri & Modlin, 2009), we analyzed the expression levels of the autophagy-related proteins and proteins pivotal for autophagy signaling in the total PBMCs isolated from PD patients to healthy subjects. Western blot analysis of PBMCs revealed an apparent decrease in the expression level of the autophagy related proteins ATG5–12 conjugate, ATG16L1 and ATG7 in PD patients relative to healthy subjects (Figure 4A and B). In addition, the expression level of the key autophagy regulators mTOR and Raptor were decreased and p-ULK1 was increased in PD patients. Furthermore, although Beclin 1 levels were significantly increased in PD subjects, it was also associated with increase in negative regulator of autophagy BCL2.
Figure 4:
(a) Western blotting analysis of the autophagy-related protein expression in total PBMCs from Healthy versus PD subjects. (b) Quantification of autophagy markers shown in (a) normalized to β-actin. (c) Autophagy-related protein expression analysis by Western blotting before and after vitamin D supplementation. (d) Quantification of the blots shown in (c) normalized to β-actin. (e) and (g) Atg5 and Atg16L1 mRNA expression, respectively, in gingival tissue from PD patients treated with vitamin D. (f) and (h) Atg5 and Atg16L1 mRNA expression, respectively, in gingival tissue from PD patients treated with Placebo. mRNA expression was normalized to housekeeping gene GAPDH. N = 3. *p ≤ 0.05, **p ≤ 0.01.
3.3. VDS rescues autophagy inhibition in PD patients
To determine the influence of VDS on the autophagy related proteins in PD patients, we compared the expression levels in PBMCs of PD patients at baseline (before VDS) and before SRP (8 weeks after VDS). Western blot analysis on total cell lysates isolated from PBMCs showed a significant increase in the expression of ATG5–12 conjugate and Beclin1, ATG16L1 and ATG7 with vitamin D treatment (Figure 4C and D). Furthermore, the mRNA expression of ATG5 and ATG16L1 was significantly increased in the gingival tissues isolated from PD patients before and after vitamin D supplementation (Figure 4E and G). No significant differences were observed in the placebo group at the same time points (Figure 4F and H), suggesting a beneficial effect of VDS in enhancing induction of autophagy related proteins in blood of PD patients.
3.4. Salivary cytokines profile influenced by periodontal therapy and VDS
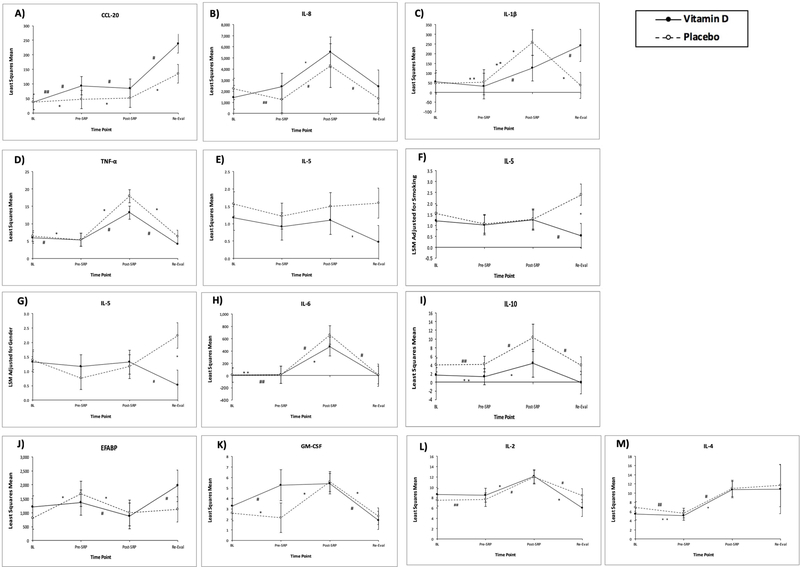
The change in a wide range of salivary cytokines across the four time points, separately for each group, were analyzed using MAGPIX Multiplexing system (Bio-Rad Laboratories, Inc., Hercules, CA). While there was extensive variability in salivary cytokines over time and in the Vit D and placebo group (Figure 5), several conclusions could be drawn from the data. In saliva of the vitamin D group, CCL-20, chemokine involved in attracting immune cells to the periphery (Yamazaki et al., 2008), consistently increased across all timepoints relative to baseline (Figure 5A), while IL-8 increased at Post-SRP (Figure 5B). In contrast, salivary IL-1β (Figure 5C), TNF-α (Figure 5D) IL-5 (Figure 5E–G) IL-6 Post-SRP (Figure 5H) and IL-10 (Figure 5I) levels were inhibited in the vitamin D group Post-SRP. Other cytokines measured, including EFABP, GM-CSF, IL-2 and IL-4 (Figures 5J–M) varied inconsistently based on timepoint and treatment group.
Figure 5:
Salivary cytokines profile influenced by periodontal therapy and vitamin D supplementation. Figures (a), (b), (c), (d), (e-g), (h), and (i) show salivary cytokines CCL-20, IL-8, IL-1B, TNF-, IL-5, IL-6, and IL-10 expression, respectively, in vitamin D and placebo groups analyzed by MAGPIX Multiplexing
3.5. The influence of vitamin D supplementation on the periodontal clinical parameters
VDS did not have a significant effect on the periodontal parameters tested in this study at the timepoints tested, probably because patients were not vitamin D deficient at onset, which we learned well into the study. CAL, PD, BOP, GI and furcation involvement were significantly decreased by initial therapy at the 16 weeks reevaluation in both treatment groups. Teeth mobility insignificantly decreased with vitamin D supplementation after 8 weeks and 16 weeks of supplementation. No significant differences were observed in gingival recession and PI in both treatment groups (Supplementary Figure 2). No significant differences in most of the periodontal parameters in patients involved in this study could be attributed to the fact that no surgical treatment was done.
4. DISCUSSION
Periodontal disease is the most common oral inflammatory disease, affecting around half of the population in the United States (Eke et al., 2015). It is a multifactorial disease with a still unclear pathophysiologic mechanism contributing to the development of the disease in susceptible hosts. Several studies indicate that sufficient vitamin D levels may improve periodontal health (Dietrich et al., 2004, Grant & Boucher, 2010, Bashutski et al., 2011, Alshouibi et al., 2013), though some recent reports give conflicting data (Millen et al., 2013, Millen et al., 2014). It has been shown that increased 25-hydroxyvitamin D reduces gingival inflammation (Dietrich et al., 2005), and that the anti-inflammatory effect of vitamin D is dose-dependent (Hiremath et al., 2013). Decreased tooth loss has also been demonstrated with increased [25(OH)D] levels (Jimenez et al., 2014, Zhan et al., 2014b). Prospective studies of vitamin D status in the treatment of periodontal disease are lacking, and it is this gap in knowledge where research should be focused. Causality has not been proven and randomized clinical trials examining vitamin D supplementation (placebo-controlled) with mechanical therapy are needed, especially in populations at much greater risk for vitamin D deficiency and periodontal disease. Given the high prevalence of periodontal disease and poor vitamin D status in the dark skin populace, this study was designed to shed light upon the influence of vitamin D supplementation in the treatment and of periodontal disease and regulation of the immune health status of PD patients.
Our study established, for the first time, using randomized controlled trial, a link in a periodontitis patient cohort, between vitamin D supplementation and CD3+CD8+ cytotoxic T-lymphocytes (CTLs). CTLs have long been implicated in protective and destructive immune responses. Furthermore, preventing CTL-mediated damage is suggested in previous studies as a therapeutic approach for the treatment of some inflammatory conditions (Neumann et al., 2002). In periodontal disease, elevation in CTL counts have been linked to the increased periodontal tissue damage (Cifcibasi et al., 2015). So, regulating the CTLs response during periodontal therapy via vitamin D supplementation would be a therapeutic approach to counteract hyperresponsiveness of CTLs.
Autophagy is a cellular process that is utilized to protect the cell by disposing its cellular content such as damaged proteins, produce cellular energy, fight invading microbes and enhancing the cellular immune response (Oh & Lee, 2014). Previous studies have shown that disruption in autophagy-related protein functions contributes to the susceptibility to infection, chronic inflammatory diseases and autoimmune diseases (Kimmey et al., 2015, Lopez et al., 2013). In this study, we showed that the expression level of the autophagy-related proteins and other proteins that regulate the autophagy process were significantly decreased in whole blood PBMCs from PD patients was comparing to healthy subjects, suggesting a perturbation in the process of the antimicrobial autophagy in PD patients. Although the results showed that Beclin-1 was significantly increased in PD patients, this was accompanied by an increase in the BCL2 expression level. BCL2 is a pro-survival member of the BCL2 family that inhibits autophagy by binding to the BH3 domain in Beclin-1 (Pattingre et al., 2005). Since autophagy plays an important role in degrading invading pathogens and shaping the immune response, this data suggest that PD patients may be more susceptible to infection in the absence of VDS. It is clear that susceptibility to periodontal disease is highly variable from host to host, in a manner that is not dependent on the ability of periodontal pathogens to colonize the host (Kinane & Hart, 2003, Chen et al., 2018). Periodontal disease is a disease that is initiated by the bacteria that colonizes the subgingival environment at the early stage and become more virulent to the host. The ability of the host’s immune response to efficiently counteract invading bacteria is probably the tipping point in whether or not the disease develops.
Several studies have reported the role of vitamin D in the induction of autophagy via regulating a multitude of signaling pathways essential for autophagy (Wang et al., 2008, Hoyer-Hansen et al., 2007, Yuk et al., 2009). It has been shown that vitamin D-associated autophagy plays a crucial role in innate immunity, infection and inflammatory diseases (Guillet et al., 2015). Interestingly, it is still unknown how vitamin D affects autophagic profile in PD patients. Our results show that the expression level of the autophagy related proteins was enhanced in PBMCs isolated from PD patients after vitamin D supplementation, relative to before vitamin D intake. Although the patients in this study were not initially vitamin D deficient, this study suggests that vitamin D supplementation can be beneficial for the immune system of PD susceptible hosts to fight periodontal pathogens, without disrupting immune homeostatic function.
Among the immunomodulatory functions of vitamin D are its ability to shift the immune response towards an anti-inflammatory response (Hoe et al., 2016). Albeit effective in treating periodontal disease, active periodontal therapy such as SRP results in transient bacteremia and entry of periodontal pathogens into the blood stream. This results in activation of the pro-inflammatory arms of the immune system (Forner et al., 2006, Waghmare et al., 2013). Our results on the levels of salivary cytokines with vitamin D treatment suggest a vitamin D-mediated immunoregulation that may prove to be locally beneficial in a larger clinical trial of patients who are vitamin D deficient.
In summary, considering the limitations of the current study, including the small sample size and the fact that none of the subjects were vitamin D deficient, our results showed an important role for vitamin D supplementation in inducing local and systemic anti-inflammatory response and enhancing the autophagic profile in PD patients after SRP.
Supplementary Material
Acknowledgments
Funding Information:
This study was supported by funds from the Carlos and Marguerite Mason trust to improve kidney transplant outcomes in Georgia and NIH-NIDCR (R01 DE014328 [to CWC])
REFERENCES
- Alshouibi EN, Kaye EK, Cabral HJ, Leone CW and Garcia RI (2013). Vitamin D and periodontal health in older men. Journal of dental research 92: 689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonoglou GN, Knuuttila M, Niemela O, Raunio T, Karttunen R, Vainio O, Hedberg P, Ylostalo P and Tervonen T (2014). Low serum level of 1,25(OH) D is associated with chronic periodontitis. J Periodontal Res [DOI] [PubMed]
- Armitage GC (1999). Development of a classification system for periodontal diseases and conditions. Annals of periodontology 4: 1–6. [DOI] [PubMed] [Google Scholar]
- Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, Gysemans C and Mathieu C (2010). Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol 121: 221–7. [DOI] [PubMed] [Google Scholar]
- Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, Giannobile WV and McCauley LK (2011). The impact of vitamin D status on periodontal surgery outcomes. Journal of dental research 90: 1007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagatwala J, Zhu H, Parikh SJ, Guo DH, Kotak I, Huang Y, Havens R, Pham M, Afari E, Kim S, Cutler C, Pollock NK, Dong Y, Raed A and Dong Y (2015). Dose and time responses of vitamin D biomarkers to monthly vitamin D3 supplementation in overweight/obese African Americans with suboptimal vitamin d status: a placebo controlled randomized clinical trial. BMC Obes 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Hemme C, Beleno J, Shi ZJ, Ning D, Qin Y, Tu Q, Jorgensen M, He Z, Wu L and Zhou J (2018). Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. The ISME journal 12: 1210–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifcibasi E, Ciblak M, Kiran B, Badur S, Firatli E, Issever H and Cintan S (2015). The role of activated cytotoxic T cells in etiopathogenesis of periodontal disease: does it harm or does it heal? Scientific reports 5: 9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ and Vieth R (2005). Estimates of optimal vitamin D status. Osteoporos Int 16: 713–6. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Joshipura KJ, Dawson-Hughes B and Bischoff-Ferrari HA (2004). Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 80: 108–13. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Nunn M, Dawson-Hughes B and Bischoff-Ferrari HA (2005). Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr 82: 575–80. [DOI] [PubMed] [Google Scholar]
- Dong Y, Pollock N, Stallmann-Jorgensen IS, Gutin B, Lan L, Chen TC, Keeton D, Petty K, Holick MF and Zhu H (2010a). Low 25-hydroxyvitamin D levels in adolescents: race, season, adiposity, physical activity, and fitness. Pediatrics 125: 1104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, Li K, Bassali R, Guo DH, Thomas J, Pierce GL, White J, Holick MF and Zhu H (2010b). A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. The Journal of clinical endocrinology and metabolism 95: 4584–91. [DOI] [PubMed] [Google Scholar]
- Drury TF, Winn DM, Snowden CB, Kingman A, Kleinman DV and Lewis B (1996). An overview of the oral health component of the 1988–1991 National Health and Nutrition Examination Survey (NHANES III-Phase 1). Journal of dental research 75 Spec No: 620–30. [DOI] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD and Genco RJ (2015). Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. Journal of periodontology 86: 611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJand Cdc Periodontal Disease Surveillance workgroup: James Beck GDRP (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of dental research 91: 914–20. [DOI] [PubMed] [Google Scholar]
- El-Awady AR, Miles B, Scisci E, Kurago ZB, Palani CD, Arce RM, Waller JL, Genco CA, Slocum C, Manning M, Schoenlein PV and Cutler CW (2015). Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS pathogens 10: e1004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri M and Modlin RL (2009). A vitamin for autophagy. Cell host & microbe 6: 201–3. [DOI] [PubMed] [Google Scholar]
- Forner L, Larsen T, Kilian M and Holmstrup P (2006). Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. Journal of clinical periodontology 33: 401–7. [DOI] [PubMed] [Google Scholar]
- Grant WB and Boucher BJ (2010). Are Hill’s criteria for causality satisfied for vitamin D and periodontal disease? Dermatoendocrinol 2: 30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet A, Brocard A, Bach Ngohou K, Graveline N, Leloup AG, Ali D, Nguyen JM, Loirat MJ, Chevalier C, Khammari A and Dreno B (2015). Verneuil’s disease, innate immunity and vitamin D: a pilot study. Journal of the European Academy of Dermatology and Venereology : JEADV 29: 1347–53. [DOI] [PubMed] [Google Scholar]
- Hiremath VP, Rao CB, Naik V and Prasad KV (2013). Anti-inflammatory effect of vitamin D on gingivitis: a dose-response randomised control trial. Oral health & preventive dentistry 11: 61–9. [DOI] [PubMed] [Google Scholar]
- Hoe E, Nathanielsz J, Toh ZQ, Spry L, Marimla R, Balloch A, Mulholland K and Licciardi PV (2016). Anti-Inflammatory Effects of Vitamin D on Human Immune Cells in the Context of Bacterial Infection. Nutrients 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF (2006). High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81: 353–73. [DOI] [PubMed] [Google Scholar]
- Holick MF (2008). The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med 29: 361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF and Chen TC (2008). Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87: 1080S–6S. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J and Napoli JL (1996). Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem 42: 586–92. [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS and Jaattela M (2007). Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Molecular cell 25: 193–205. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Giovannucci E, Krall Kaye E, Joshipura KJ and Dietrich T (2014). Predicted vitamin D status and incidence of tooth loss and periodontitis. Public health nutrition 17: 844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmey JM, Huynh JP, Weiss LA, Park S, Kambal A, Debnath J, Virgin HW and Stallings CL (2015). Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature 528: 565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane DF and Hart TC (2003). Genes and gene polymorphisms associated with periodontal disease. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists 14: 430–49. [DOI] [PubMed] [Google Scholar]
- Lopez P, Alonso-Perez E, Rodriguez-Carrio J and Suarez A (2013). Influence of Atg5 mutation in SLE depends on functional IL-10 genotype. PloS one 8: e78756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen AE, Andrews CA, LaMonte MJ, Hovey KM, Swanson M, Genco RJ and Wactawski-Wende J (2014). Vitamin D status and 5-year changes in periodontal disease measures among postmenopausal women: the Buffalo OsteoPerio Study. Journal of periodontology 85: 1321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen AE, Hovey KM, LaMonte MJ, Swanson M, Andrews CA, Kluczynski MA, Genco RJ and Wactawski-Wende J (2013). Plasma 25-hydroxyvitamin D concentrations and periodontal disease in postmenopausal women. Journal of periodontology 84: 1243–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Medana IM, Bauer J and Lassmann H (2002). Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends in neurosciences 25: 313–9. [DOI] [PubMed] [Google Scholar]
- Oh JE and Lee HK (2014). Pattern recognition receptors and autophagy. Frontiers in immunology 5: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh S, Guo DH, Pollock NK, Petty K, Bhagatwala J, Gutin B, Houk C, Zhu H and Dong Y (2012). Circulating 25-hydroxyvitamin D concentrations are correlated with cardiometabolic risk among American black and white adolescents living in a year-round sunny climate. Diabetes care 35: 1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD and Levine B (2005). Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–39. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P and Deanfield J (2007). Treatment of periodontitis and endothelial function. N Engl J Med 356: 911–20. [DOI] [PubMed] [Google Scholar]
- Waghmare AS, Vhanmane PB, Savitha B, Chawla RL and Bagde HS (2013). Bacteremia following scaling and root planing: A clinico-microbiological study. Journal of Indian Society of Periodontology 17: 725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lian H, Zhao Y, Kauss MA and Spindel S (2008). Vitamin D3 induces autophagy of human myeloid leukemia cells. The Journal of biological chemistry 283: 25596–605. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Nakagawa I, Yamamoto A, Amano A, Noda T and Yoshimori T (2009). An initial step of GAS-containing autophagosome-like vacuoles formation requires Rab7. PLoS pathogens 5: e1000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q and Dong C (2008). CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol 181: 8391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM and Jo EK (2009). Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell host & microbe 6: 231–43. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Samietz S, Holtfreter B, Hannemann A, Meisel P, Nauck M, Volzke H, Wallaschofski H, Dietrich T and Kocher T (2014a). Prospective Study of Serum 25-hydroxy Vitamin D and Tooth Loss. Journal of dental research 93: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Samietz S, Holtfreter B, Hannemann A, Meisel P, Nauck M, Volzke H, Wallaschofski H, Dietrich T and Kocher T (2014b). Prospective Study of Serum 25-hydroxy Vitamin D and Tooth Loss. Journal of dental research 93: 639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.