Abstract
Introduction
Patient education serves an essential purpose in the long-term management of allergic diseases as a secondary prevention approach. However, evidence on using education for primary prevention is limited. This study aims to evaluate the effect of an educational intervention, that is, the Preventive Antenatal Educational Program on Allergic Diseases (PAEPAD), on infantile allergic disease incidences compared with the standard care.
Methods and analysis
This is a single-centre randomised controlled trial of expecting mother–children dyads in Daxing Teaching Hospital of Beijing, China. A total of 2266 expecting mothers will be recruited. Expecting mothers enlisted in the birth registry of Daxing Teaching Hospital of Capital Medical University and intend to give birth at this location will be screened for eligibility. Women aged≥18 years with less than 14+6 weeks of pregnancy who intends to remain resident in Daxing district for at least 2 years postpartum will be entered into the run-in phase. Randomisation will take place at 30 weeks of gestation. Women at high risk for miscarriage or intend to have abortions will be excluded. The participants will be allocated into two groups (ie, the PAEPAD and the standard care group) by random allocation (1:1). The PAEPAD group will receive a multidisciplinary education of neonatal care, including standard education as the control group and additional information on skincare of infants, sun protection, topical corticosteroids and an overview of atopic dermatitis (AD), whereas the standard care group will receive the standard neonatal care education carried out by obstetricians. Participants will be followed for 2 years. The primary outcome will be infantile AD cumulative incidence at 2 years postpartum. Secondary outcomes will include other AD outcomes, atopic march outcomes, knowledge outcomes and other maternal and neonatal outcomes. Data collection will be carried out using both electronic and paper questionnaires. Biological samples will also be collected longitudinally.
Ethics and dissemination
The study design was approved by the ethical committee of Capital Medical University Daxing Teaching Hospital, Beijing, China. The trial results will be published in peer-reviewed journals and at conferences.
Trial registration number
ChiCTR registry (Trial ID: ChiCTR2000040463).
Keywords: eczema, epidemiology, paediatrics, paediatric dermatology
Strengths and limitations of this study.
Large single-centre investigator-blinded randomised controlled trial.
First attempt to investigate education for primary prevention of incident cases of allergic diseases (atopic dermatitis for primary outcome).
Prone to potential contamination due to single-centre design and non-medication intervention.
Potential heterogeneity of intervention effect due to variation in how the intervention will be carried out by individual educator although provision of formal training for educators.
Introduction
Atopic disorders place a substantial burden on both individuals and the healthcare system.1–4 The sequential occurrence of atopic dermatitis (AD), followed by one or more disorders characterised by allergen-specific type 2 (including T helper 2 (Th2)) responses are designated as atopic march.5 The underlying mechanisms feature both a genetic susceptibility in which barrier dysfunction and immune dysregulation predispose atopic individuals to Th2 immunity, and a progression in which AD and its pathological changes function as instigating events to subsequent development of other atopic comorbidities.5 6 The human skin is a functional immune organ with an abundance of immunocompetent cells. In the resting state, an intact skin barrier and a balance of immune cell populations, cytokines and chemokines promote immune tolerance, which is otherwise disrupted in AD patients. Barrier dysfunction from both loss-of-function mutations in filaggrin and the itch-scratch cycle was postulated as the driving component for atopic march programming by promoting Th2 and Th17 differentiation.7–9 Subsequently, sensitisations to food allergens develop in the setting of Th2 skewing, which is otherwise Th1 skewed in the gastrointestinal-homing T cells in tolerant subjects.10 Similarly, in asthmatic airways, disrupted junctional adhesion, mucus plugging develops due to Th2 polarisation. While the severity of subsequent allergic airway inflammation was affected by AD, abrogation of AD by topical treatment prevents worsening of subsequent airway inflammation by counteracting the Th17 pathway.11 Granted that both the genetic predisposition and the atopic progression contribute to the development of atopic march, longitudinal studies are warranted to explore the impact of immune activation of AD on the development of other Th2 comorbidities. Meanwhile, the role of epithelial-immune crosstalk in the atopic march needs to be further elucidated.
To date, extensive research has been conducted on the therapeutic effect of barrier enhancement using emollients. Compelling evidence indicated that emollients render the skin less susceptible to irritants and reduce flares of AD (secondary prevention)12 and therefore was regarded as a cornerstone of AD therapy.13 However, evidence was inconsistent regarding the preventive effect of emollient application.12 14–17 The discordance from these behavioural intervention studies is probably a result of the difference in when the outcome assessment took place. In the Barrier Enhancement for Eczema Prevention (BEEP)12 and Preventing Atopic Dermatitis and ALLergies (PreventADALL)14 studies, the outcome was measured after a long washout period to ensure that any mild AD would not be concealed by the ongoing application of emollients, whereas in the small studies15 16 the immediate effect was evaluated. However, the null long-term result should not overshadow the strong efficacy signal displayed at the immediate outcome assessments. Moreover, as behaviour change is a process that unfolds through a continuum of stages from precontemplation, contemplation and preparation to action and maintenance, such straightforward behavioural intervention may not echo with the real-world scenario (the stages of change model), where behaviour changes are achieved through health promotion initiatives, including health education and health policies.18 19 Consequently, the generalisability of these studies might be affected due to the difference in how intervention was delivered and the setting under which outcome assessment took place.
In realising the tremendous burden inflicted by atopic disorders and the critical role of AD in atopic march, significant therapeutic discoveries have been made over the past century. Nonetheless, in AD and other chronic diseases alike, adherence to treatment can be strikingly poor.20 The underlying factors were postulated to be the complexity of treatment regimen, corticosteroid phobia and caregiver burden.20 Addressing these issues calls for consistent efforts in patient education that can hardly be accomplished in a typical clinical visit. Recent studies have shown that structured patient education can significantly reduce disease severity in AD patients.21 Given this, guidelines for AD have acknowledged education of patients and caregivers as an essential form of secondary prevention to reduce disease flares and improve quality of lives.22 However, little is known about how education performs as a primary prevention approach to reduce AD disease burden.
Antenatal education is a crucial component of antenatal care. Since its implementation from the 19th century, mortality in children has decreased tremendously. In 2005, the WHO called for ‘realising the potential of antenatal care’23 and thence started using it as a platform for primary prevention of malnutrition, HIV/AIDS, sexually transmitted infections, tuberculosis and prevention of postpartum and neonatal diseases.24–26 Its diversified usage has led us to hypothesise that educational intervention delivered through the antenatal education platform may yield informative data for assessing the primary prevention of allergic diseases. Thus, the Preventive Antenatal Educational Program on Allergic Diseases (PAEPAD) Study focuses on using the antenatal care platform as a more affordable and effective health education approach. In this investigator-blinded, randomised controlled trial (RCT), we aim to evaluate the effect of such intervention versus standard education on atopic disease outcomes. The primary objective is to evaluate the preventive effect of PAEPAD on AD and atopic comorbidity incidences. The secondary objectives are: (1) to explore the immune-barrier crosstalk between the immune system, commensal flora and skin barrier function that may explain the development of atopic march; (2) to identify biomarkers (metabolites, microRNAs) that can be used to characterise individuals at high risk of AD and atopic march and (3) to elucidate whether and to what extent the maternal immune milieu influences paediatric AD development through cord blood and breast milk.
Methods and analysis
Study design
The study is a prospective investigator-blinded RCT with two arms (PAEPAD vs standard antenatal education). All expectant mothers planning to give birth at the Daxing Teaching Hospital of Capital Medical University will be invited to participate. Daxing Teaching Hospital of Capital Medical University is a general hospital located in Daxing district of Beijing, China, with more than 6000 deliveries annually. Daxing district makes up approximately 6.3% of Beijing geographically. Daxing Teaching Hospital of Capital Medical University makes up approximately 2.7% of Beijing’s annual deliveries. This protocol was drafted (17 August 2020) before participant recruitment (30 October 2020).
Recruitment, inclusion and exclusion criteria
All expectant parents living in the catchment area of the Daxing Teaching Hospital of Capital Medical University will receive an information leaflet about the study at their first visit to the maternity clinic. Recruitment will take place in their first mandatory maternity class between gestation weeks 7 and 14+6. All expectant mothers will be given detailed information about the study. A trained research nurse will outline key information (ie, inclusion and exclusion criteria, scheduled research visits and instructions on biological sample collection) about the study in the form of a short lecture session. The parents who wish to participate will be requested to provide written informed consent. They will be inquired on five levels of consent: (1) consent to participate in the study; (2) consent to biological sample collections of the mother that are non-invasive, including but not limited to the recollection of blood from routine pregnancy workups; (3) consent to biological sample collections of the mother that are minimally invasive or could potentially cause discomfort, iethat is, additional blood draw and vaginal swabs; (4) consent to biological sample collections of the child that are non-invasive, including but not limited to the recollection of blood from routine checkups and (5) consent to biological sample collections of the child that are minimally invasive or could potentially cause discomfort, that is, additional blood draw and tape stripping. At birth, another consent form on the children’s biological sample collection will be signed to allow for any change of consent status on non-invasive and minimally invasive sample collections. Consented participants will subsequently receive a QR code through their cellphone after registration. By scanning the QR code, the recruitment staff will be able to confirm their participation status, at the end of which a study-specific ID number will be generated automatically.
Inclusion and exclusion criteria
Run-in phase inclusion criteria: (1) Enlisted in the birth registry of Daxing Teaching Hospital of Capital Medical University and intend to give birth at this location; (2) women aged≥18 years; (3) less than 14+6 gestational week when recruited as measured by last menstrual period; (4) residents of Daxing and intend to remain residing in Daxing for at least 2 years postpartum and (5) written consent form.
Randomisation phase inclusion criteria:
Pregnant women enlisted in the birth registry of Daxing Teaching Hospital of Capital Medical University and intend to give birth at this location and pass the run-in phase criteria.
Withdrawal criteria: (1) Stillbirth; (2) abortion (spontaneous or induced) and (3) rare comorbidities that present after inclusion into the study that may render the participant unsuitable for participation, including but not limited to malignancies, amniotic fluid embolism, eclampsia and major birth abnormalities of the child.
Exclusion criteria: (1) Planned abortion; (2) rare comorbidities of the mother that may cause miscarriage and congenital disabilities as determined by obstetrics (OB) departments, including but not limited to malignancies, congenital heart diseases and monogenetic diseases; (3) recurrent miscarriage and (4) mental, psychological or intellectual disabilities of either one of the expecting parents.
Treatment allocation
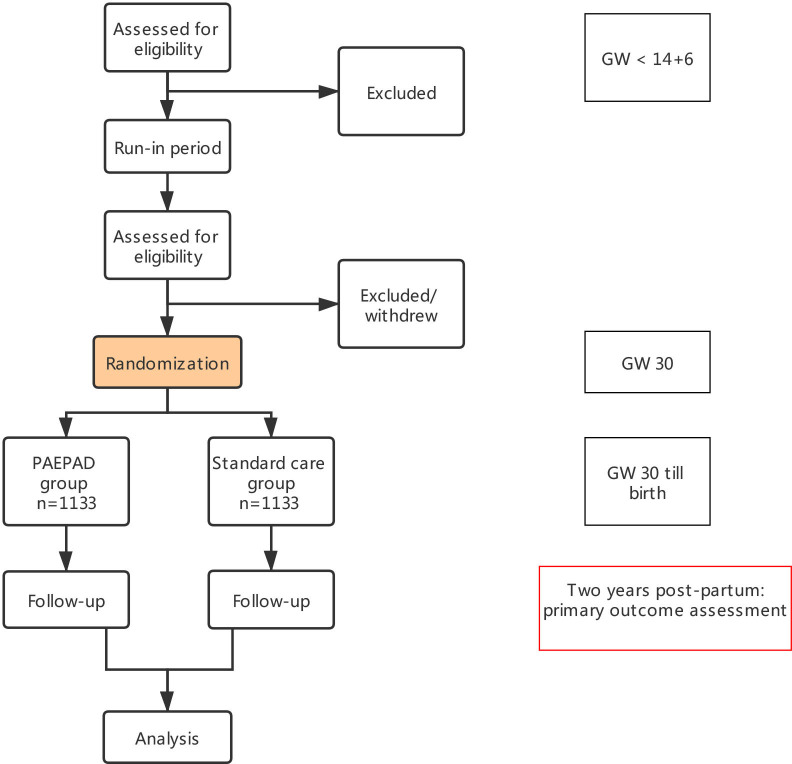
The study flow is as follows (figure 1): Participants will be randomly assigned to one of the two arms (ie, PAEPAD vs standard antenatal care). Randomisation will be conducted by an epidemiologist using a computer-generated list with the number of groups being two and the distribution ratio of the two groups being 1:1. The list will be generated using block randomisation with block sizes hidden from all investigators. Group allocation will be placed in sealed opaque envelopes, labelled by numbers only. The envelopes will be opened in consecutive order. Participants will then be informed about their allocated groups by a research nurse.
Figure 1.
Flow diagram of the Preventive Antenatal Educational Program on Allergic Diseases (PAEPAD) Study. GW, gestational week.
Blinding
This is a researcher-blinded study. Treatment allocation will be performed by an epidemiologist, and the researchers who evaluate the outcome matrices and analyse data will be blinded and work independently from the group of clinicians who will carry out the intervention. Data entry will be undertaken by trial administrators blinded to group allocation.
Intervention
After randomisation at gestational week 30, the participants will be informed on their allocation and members of the research team will send out weekly invitations through messages to participants who have not yet completed the intervention. For those who failed to complete the intervention prior to admission into the OB department, a prerecorded video will be played during their hospital stay. In the standard care group, patients will receive the standard neonatal care session from an experienced obstetrician, which will include breast feeding, newborn screening tests, infant physiology, immunisation, solid food introduction and belly and eye care (45 min). This session is one of the five mandatory sessions with participation of 83.7%–91.9% over the past 5 years (unpublished data). The treatment group will receive an educational programme designed by a multidisciplinary group of experienced obstetricians and paediatric dermatologists. The programme will be focused on neonatal care as the control group (45 min) and (1) skin care of the newborns with a practical demonstration on bathing and emollient application (20 min); (2) sun protection (3 min); (3) a brief introduction on commonly used topical agents during infancy, including topical corticosteroids, antibiotics and astringents (5 min) and (4) besides, the programme will also contain a 5 min presentation on AD disease burden, its precipitators, managements, disease courses and the atopic march. Specific recommendations are listed in the online supplemental table S1. At the neonatal care class, all participants will first receive the standard education, which will be held non-concurrently to minimise group contamination. Online courses will be held whenever gathering is restricted due to the COVID-19 pandemic. At the end of the sessions, participants of the PAEPAD cohort will be asked to participate in the PAEPAD session, which will last for less than 40 min. Participants of the standard neonatal care cohort will be asked to leave. The intervention will be entirely educational, no cleanser or emollient product will be provided or recommended. At follow-up visits, no further education will be implemented. Any crossover and non-compliance will be surveyed by a research nurse at the beginning and end of the antenatal sessions. The research nurses who collect data on compliance and who send out invitations will not be involved in outcome assessment.
bmjopen-2020-048083supp001.pdf (131.1KB, pdf)
Study outcomes and follow-up
Study outcomes
All outcome measures are summarised in table 1. There will be both fixed and disease prompted postnatal follow-up visits during which outcome assessments will take place.
Table 1.
Outcome assessments of the PAEPAD Study
| Outcomes | Instruments/diagnostic criteria | Immediately post intervention | On discharge from OB ward | On disease flares | 3 months postnatal | 1 year postnatal | 2 years postnatal |
| Primary outcomes | |||||||
| Cumulative incidence of AD at 2 years | Hanifin and Rajka | ✕ | |||||
| Secondary outcomes | |||||||
| AD outcomes | |||||||
| Time to first AD episode | Hanifin and Rajka | ✕ | |||||
| Time to first topical corticosteroid exposure | ✕ | ||||||
| Disease-related quality of life | IDQOL | ✕ | |||||
| Disease severity | SCORAD, EASI and IGA | ✕ | |||||
| Frequency of AD flares | |||||||
| AD disease-free days | IGA | ||||||
| Cumulative clinical visit duration | From the parents being seated within the consulting room to their exit thereafter | ✕ | |||||
| Atopic march outcomes | |||||||
| Asthma incidence | ✕ | ||||||
| Recurrent wheeze incidence | ✕ | ||||||
| Rhinitis incidence | ✕ | ||||||
| Food sensitisation incidence | Allergen-specific IgE | ✕ | |||||
| Obstetric outcomes | |||||||
| Mode of birth | Eutocic/dystocic/C-section | ✕ | |||||
| Pathological pregnancy | Gestational diabetes, pre-eclampsia, placenta previa, placental abruption, prelabor rupture of membranes, puerperal infection, postpartum haemorrhage | ✕ | |||||
| Neonatal outcomes: | |||||||
| Newborn’s weight | ✕ | ||||||
| Admission in neonatal care unit (yes/no) | ✕ | ||||||
| Apgar Score | ✕ | ||||||
| Fetal growth retardation | ✕ | ||||||
| Any morbidity of the newborn that results in hospitalisation in the first 3 months of life | ✕ | ||||||
| Emollient usage | Total volume, brand, frequency | ✕ | ✕ | ✕ | |||
| Bathing practice | Frequency, duration of bathing, bath temperature, cleanser usage, soap usage | ✕ | ✕ | ✕ | |||
| Patient disease knowledge and attitude changes | Questionnaire currently under validation | ✕ | ✕ | ✕ | ✕ | ✕ | |
AD, atopic dermatitis; EASI, Eczema Area and Severity Index; IDQOL, Infantile Dermatitis Quality of Life; IGA, Investigator Global Assessment; OB, obstetrics; PAEPAD, Preventive Antenatal Educational Program on Allergic Diseases; SCORAD, SCORing of Atopic Dermatitis.
Diagnostic criteria for AD will be based on Hanifin and Rajka criteria, which are regarded as the ‘gold standard’ for hospital-based research. The Infants’ Dermatitis Quality of Life Index is a questionnaire of 10 items that has been translated into 21 languages. This questionnaire is validated in infants aged 0–3 years27. Disease severity at disease flares will be measured by SCORing of Atopic Dermatitis (SCORAD) and Eczema Area and Severity Index (EASI) and through the course of the disease by Investigator’s Global Assessment (IGA). SCORAD and EASI are validated instruments for disease severity assessment.28 IGA provides the most straightforward assessment of disease severity and will thus be employed to assess AD disease-free days. An IGA of less than two is defined as clearance of disease. An episode of expiratory wheezing will be defined as bronchial obstruction lasting for at least 24 hours preceded by at least a 1-week non-wheezing healthy period, as defined by a physician. Recurrent wheezing will be defined as the occurrence of three or more episodes of expiratory wheezing diagnosed by a physician in a 12-month period.29 Rhinitis will be defined as symptoms of sneezing, a runny or blocked nose or itchy, red and watery eyes after exposure to furred pets or pollen the year before follow-up and/or doctor’s diagnosis of allergic rhinitis.30 Sensitisation will be defined as allergen-specific IgE≥0.35 kUA/L. Parental knowledge and attitude will be quantified using a questionnaire. The items of the questionnaire were developed following rigorous procedures, including a review of literature, a patient focus group and a panel discussion of experts. This questionnaire is currently under validation (unpublished data).
Follow-up
Participants will receive both fixed and disease prompted follow-up visits. Follow-up visits will be carried out at Daxing hospital and five health service centres during immunisation. At follow-ups, biological samples, surveys and physical examination data will be collected. Specific items are listed in table 2. In addition, children will be assessed on disease onsets and flares. During follow-ups, all cases will be treated according to the guidelines2 13 31–34 by specialists who are actively engaged in the care of paediatric AD patients. Participants are not allowed to participate in other clinical trials after inclusion into the study till the end of the last follow-up visit.
Table 2.
Biological sample collection at fixed follow-up visits
| Samples | GW 12 | GW 24–28 | GW 36 | Delivery | 24–72 hour postpartum | 42 days postpartum | 3 months postpartum | 6 months postpartum | 12 months postpartum | 24 months postpartum |
| Biological sample of the infant | ||||||||||
| Blood | ✕ | ✕ | ✕ | |||||||
| Stool | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ||||
| Skin swab | ✕ | ✕ | ✕ | ✕ | ✕ | |||||
| Tongue dorsum swab | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | |||
| Tape stripping | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ||||
| Biological sample of the mother | ||||||||||
| Blood | ✕ | ✕ | ✕ | ✕ | ||||||
| Stool | ✕ | ✕ | ✕ | ✕ | ✕ | |||||
| Urine | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ||
| Skin swab | ✕ | ✕ | ✕ | ✕ | ✕ | |||||
| Breast milk | ✕ | ✕ | ✕ | |||||||
| Placenta | ✕ | |||||||||
| Tongue dorsum swab | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ||||
| Vaginal swab | ✕ | ✕ | ✕ | |||||||
GW, gestational week.
Covariates
Relevant covariates will include age, sex, social-economic status, familial history of allergic diseases, administration of systemic medication and nutrient supplements and maternal psychological status was measured with Kessler-10 prenatally. Other variables, including the Edinburgh Postnatal Depression Scale, maternal comorbidities during pregnancy, postnatal nutrition status and indicators for feeding practices (ie, minimum dietary diversity, the introduction of solid, semisolid or soft foods, duration of breast feeding) will also be collected.
Data collection and management
Data collection at fixed time points will be conducted with an electronic database designed specifically for this project. At each visit, patients will be asked to present a patient-specific QR code, by scanning which two different questionnaires will be delivered to the participants and a research nurse separately. Less than 15 items will be surveyed in a standard questionnaire for the patient to minimise respondent fatigue caused by lengthy questionnaires. The rest of the relevant data will be collected by the research nurses during a face-to-face interview. Routine lab workups will be collected from the participants’ medical records. Data will be checked by the members of the research team and incorrect or missing questions will be sent back to the participants. All data recorded in this electronic database will be accessible only by the team members. Participants will receive text reminders prior to each follow-up visit and will be interviewed through phone calls for incomplete visits. Data collection at disease onsets and flares will be carried out with paper surveys, which will be encrypted and kept accessible only by team members with authorisation. Group allocation data are accessible through unique identifiers on a separate sheet by only the principal investigators. All data handling (data entry, storage and analysis) will be confidential. The principal investigators are responsible for ensuring data quality.
Biological sample collection
At each time point, biological samples from mothers and children (where applies) will be collected and stored (table 2). These samples include maternal blood, urine, faeces, skin, vaginal and oral swabs, breast milk, placenta, cord blood and meconium, and blood, faeces, skin, nasopharyngeal and external auditory canal swabs and tape stripping of skin lipids from the children. Procedures are detailed in the online supplemental material.
Sample size
The PAEPAD Study will be based on a sample of 2266 expecting mothers. We calculated that assuming 20%35 AD rate, 20% lost to follow-up, with a clinically significant estimate of a cumulative incidence ratio of 0.75, 80% power and two-sided 5% significance level, the estimated sample size of the primary outcome would be 2266. The cut-off value of clinical significance of such educational intervention was derived by a survey of expert opinion (n=7, unpublished data) and from previous RCTs with behavioural interventions.15 17
Analyses
Definition of population sets
Primary analysis population: The modified intention-to-treat population (MITTP), which will comprise of expectant women who undergo randomisation, with data of at least one postintervention follow-up.
Per-protocol (PP) population: All expectant women complying with the study protocol, with data of at least one postintervention follow-up.
As-treated population: All randomised participants who received the intervention (whether complied or not), with data of at least one postintervention follow-up.
Statistical analysis
Statistical analyses will be performed using STATA V.14.0, R V.1.0.44 and SAS V.9.2 statistical software.
The primary analysis will be based on the MITTP. Sensitivity analyses will be done with both the PP population and the as-treated population. For primary analyses, we will use χ2 tests to compare categorical outcomes. For continuous variables, normally distributed continuous variables will be compared using the t-test and the Wilcoxon rank-sum test will be used for skewed variables. For time to event data, for example, time to first AD episode and time to the first topical corticosteroid exposure, will be calculated using the Kaplan-Meier method. The HRs comparing PAEPAD and standard care will be estimated using Cox regression model. Multiple imputations will be conducted if loss to follow-up exceeds 30%. Subgroup analysis will be done for the relative risk of AD, asthma, rhinitis and food sensitisation stratified by familial history of atopic disorders and AD severity for allergic comorbidities (whenever applies). Regression models with interaction terms will be used to test for statistical significance among subgroups.
For sensitivity analysis that shall be done with the PP population, the analyses above will be conducted. For the sensitivity analysis that shall be done with the secondary analysis population, both traditional multivariate comparison and propensity score matching will be used to better balance the covariates and identify comparable groups. An additional sensitivity analysis will be conducted on the population that receives in-person education (as opposed to video recorded).
Data monitoring
An epidemiologist who is independent of the research team will be tasked to monitor the data. An interim analysis on knowledge will be performed when 50% of the patients complete the 1-year follow-up. The aim of this interim analysis is to give a better sense if the final primary outcome of incidence will be different between groups, as it is indicated by the stages of change model that knowledge change may lead to behavioural change.
Patient and public involvement
No patient was involved with study design, recruitment or conduct.
Ethics and dissemination
The PAEPAD Study is approved by the Ethics Committee of Capital Medical University Daxing Teaching Hospital, Beijing, China (No. 20190614LLKYLX-3-5). Written informed consent will be obtained from all participants. Participation in the project is voluntary and will not impact the medical care of the women regardless of their participation status throughout pregnancy. All participants have the right to withdraw from the study at any point and have their data removed from the study database. All patient data will be securely stored and kept accessible by the research members only, with previous authorisation from the Principal Investigator (PI). The results will be disseminated through peer-reviewed journals. Results will also be communicated at scientific conferences.
Discussion
PAEPAD Study is a large single-centre RCT of an antenatal educational intervention for prevention of AD and the atopic march. Previous studies on educational interventions have been therapeutic, aiming at reducing symptoms and improving quality of life. Therapeutic patient education (secondary prevention) in AD has been proved to be effective with a significant reduction in disease severity.21 However, little is known about the primary preventive effect of educational and lifestyle interventions.
A strength of this study is the large sample size. In a similar study that aims to evaluate the effect of prenatal education on knowledge and behavioural changes for allergic disease prevention, the sample size was determined based on the behavioural matrices, resulting in a total sample size of 120.36 As behavioural changes do not necessarily modify disease outcomes, a larger sample size is warranted to provide adequate power to detect disease outcome differences. Another strength is that we chose the topics of the PAEPAD sessions based on both experts’ opinion and a previous survey (online supplemental table S2), which indicated that new mothers needed help on infant skin care, safe practice of sun protection and building an unbiased understanding on common topical drugs, especially corticosteroids. We did not discuss about the treatment of atopic diseases further than emphasising the importance of following the guideline-oriented instructions of physicians2 13 31–34 in this paper. Recent pilot studies provided strong efficacy signals for the hypothesis that daily emollient use could prevent AD.15 17 While the subsequent large trials yielded null results,12 14 it is crucial to realise that the hypotheses in these trials differ significantly. In the pilot studies, emollients were continued until the outcome assessment, whereas in the larger pragmatic trials, a washout period was implemented. In the BEEP Study, the rates of continued emollient and wash product use extended beyond the intervention period until outcome assessment were as low as 4% and 5% for the intervention and the control group, respectively.12 Thus, the pilot studies assessed the immediate preventive effect, whereas the larger studies assessed whether this effect, if there is any, is sustainable. While it is reasonable to hypothesise that the protective effect of emollients may not sustain beyond a year after refraining from application, incorporating conceptual and behavioural changes into daily skincare routine can be substantially beneficial for short term preventive effects.
The current study will explore the preventive effect of an educational intervention, which bears a closer resemblance to the real-world scenario through which behavioural changes are achieved. In addition, we plan to longitudinally collect biological specimens to study the crosstalk of lifestyle changes and molecular biology.
The study has some limitations. First, this study is subject to contamination due to the nature of a non-medication intervention and the single-centre design. Consequently, the effect size to be detected will likely be a more conservative estimation of the real preventive effect. Therefore, we plan to collect cross-over data and conduct sensitivity analyses based on the PP population. Second, although the PAEPAD lectures will be led by experienced obstetricians and dermatologists, the heterogeneity may nonetheless constitute potential bias. We aim to reduce the heterogeneity by providing a training session and mock classrooms before the project launching during which lecturers will be evaluated on the organisation of the class, clarity, student engagement and consistency of performance.
In conclusion, the PAEPAD Study will add to our knowledge the preventive effect of antenatal education on allergic disease outcomes and identify cellular and molecular changes that will warrant future studies. We expect that results from the PAEPAD Study will expand our understanding of the primary prevention of allergic disorders.
Supplementary Material
Footnotes
Contributors: LinM and XM are the principal investigators of this trial and conceptualised the trial. MZ, YL, JT, FS, YangW, LiliM, YingW and WG are responsible for the execution of the project. MZ has written the protocol manuscript. CS, SW, LJ, YangW and XS are responsible for data registration. MZ and XY will carry out data analyses once the trial is completed. All authors critically reviewed the article and approved the final manuscript.
Funding: This study has received a public grant from the Capital’s Funds for Health Improvement and Research (CFH2020-2-7121). The funders had no role in the design of this study and will not have any role during its execution, analyses, interpretation of data or submission of outcomes.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Muraro A, Halken S, Arshad SH, et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 2014;69:590–601. 10.1111/all.12398 [DOI] [PubMed] [Google Scholar]
- 2.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J Eur Acad Dermatol Venereol 2018;32:657–82. 10.1111/jdv.14891 [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Schunemann HJ, Fonseca J, et al. MACVIA-ARIA sentinel network for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy 2015;70:1372–92. 10.1111/all.12686 [DOI] [PubMed] [Google Scholar]
- 4.Sasaki M, Koplin JJ, Dharmage SC, et al. Prevalence of clinic-defined food allergy in early adolescence: the SchoolNuts study. J Allergy Clin Immunol 2018;141:391–8. 10.1016/j.jaci.2017.05.041 [DOI] [PubMed] [Google Scholar]
- 5.Paller AS, Spergel JM, Mina-Osorio P, et al. The atopic March and atopic multimorbidity: many trajectories, many pathways. J Allergy Clin Immunol 2019;143:46–55. 10.1016/j.jaci.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 6.Liang Y, Chang C, Lu Q. The genetics and epigenetics of atopic Dermatitis-Filaggrin and other polymorphisms. Clin Rev Allergy Immunol 2016;51:315–28. 10.1007/s12016-015-8508-5 [DOI] [PubMed] [Google Scholar]
- 7.Chinthrajah RS, Hernandez JD, Boyd SD, et al. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol 2016;137:984–97. 10.1016/j.jaci.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimura S, Takai T, Iida H, et al. Epicutaneous allergic sensitization by cooperation between allergen protease activity and mechanical skin barrier damage in mice. J Invest Dermatol 2016;136:1408–17. 10.1016/j.jid.2016.02.810 [DOI] [PubMed] [Google Scholar]
- 9.Guttman-Yassky E, Zhou L, Krueger JG. The skin as an immune organ: tolerance versus effector responses and applications to food allergy and hypersensitivity reactions. J Allergy Clin Immunol 2019;144:362–74. 10.1016/j.jaci.2019.03.021 [DOI] [PubMed] [Google Scholar]
- 10.Chan SMH, Turcanu V, Stephens AC, et al. Cutaneous lymphocyte antigen and α4β7 T-lymphocyte responses are associated with peanut allergy and tolerance in children. Allergy 2012;67:336–42. 10.1111/j.1398-9995.2011.02765.x [DOI] [PubMed] [Google Scholar]
- 11.Deckers J, Bougarne N, Mylka V, et al. Co-activation of glucocorticoid receptor and peroxisome proliferator-activated receptor-γ in murine skin prevents worsening of atopic March. J Invest Dermatol 2018;138:1360–70. 10.1016/j.jid.2017.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalmers JR, Haines RH, Bradshaw LE, et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet 2020;395:962–72. 10.1016/S0140-6736(19)32984-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014;71:116–32. 10.1016/j.jaad.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skjerven HO, Rehbinder EM, Vettukattil R, et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster-randomised trial. Lancet 2020;395:951–61. 10.1016/S0140-6736(19)32983-6 [DOI] [PubMed] [Google Scholar]
- 15.Horimukai K, Morita K, Narita M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014;134:824–30. 10.1016/j.jaci.2014.07.060 [DOI] [PubMed] [Google Scholar]
- 16.McClanahan D, Wong A, Kezic S, et al. A randomized controlled trial of an emollient with ceramide and filaggrin-associated amino acids for the primary prevention of atopic dermatitis in high-risk infants. J Eur Acad Dermatol Venereol 2019;33:2087–94. 10.1111/jdv.15786 [DOI] [PubMed] [Google Scholar]
- 17.Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134:818–23. 10.1016/j.jaci.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health Behavior and Health Education . Theory, research, and practice. 4th edn. San Francisco, CA: Jossey-Bass, 2008. [Google Scholar]
- 19.World Health Organization ROftEM . Health education: theoretical concepts, effective strategies and core competencies: a foundation document to guide capacity development of health educators, 2012. [Google Scholar]
- 20.Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv 2012;9:1263–71. 10.1517/17425247.2012.711756 [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Liang Y, Shen C, et al. Patient education programs in pediatric atopic dermatitis: a systematic review of randomized controlled trials and meta-analysis. Dermatol Ther 2020;10:449–64. 10.1007/s13555-020-00365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol 2014;71:1218–33. 10.1016/j.jaad.2014.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Ateeq MA, Al-Rusaiess AA. Health education during antenatal care: the need for more. Int J Womens Health 2015;7:239–42. 10.2147/IJWH.S75164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parat S, Nègre V, Baptiste A, et al. Prenatal education of overweight or obese pregnant women to prevent childhood overweight (the ETOIG study): an open-label, randomized controlled trial. Int J Obes 2019;43:362–73. 10.1038/s41366-018-0205-z [DOI] [PubMed] [Google Scholar]
- 25.Suto M, Takehara K, Yamane Y, et al. Effects of prenatal childbirth education for partners of pregnant women on paternal postnatal mental health and couple relationship: a systematic review. J Affect Disord 2017;210:115–21. 10.1016/j.jad.2016.12.025 [DOI] [PubMed] [Google Scholar]
- 26.Nnam NM. Improving maternal nutrition for better pregnancy outcomes. Proc Nutr Soc 2015;74:454–9. 10.1017/S0029665115002396 [DOI] [PubMed] [Google Scholar]
- 27.Basra MKA, Gada V, Ungaro S, et al. Infants' dermatitis quality of life index: a decade of experience of validation and clinical application. Br J Dermatol 2013;169:760–8. 10.1111/bjd.12563 [DOI] [PubMed] [Google Scholar]
- 28.Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014;70:338–51. 10.1016/j.jaad.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mochizuki H, Kusuda S, Okada K, et al. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. six-year follow-up study. Am J Respir Crit Care Med 2017;196:29–38. 10.1164/rccm.201609-1812OC [DOI] [PubMed] [Google Scholar]
- 30.Ballardini N, Bergström A, Wahlgren C-F, et al. IgE antibodies in relation to prevalence and multimorbidity of eczema, asthma, and rhinitis from birth to adolescence. Allergy 2016;71:342–9. 10.1111/all.12798 [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Ma L, Tan Q, et al. Chinese expert consensus on the diagnosis and management of food allergy in children with atopic dermatitis. Int J Dermatol Venereol 2020;3:135–41. 10.1097/JD9.0000000000000091 [DOI] [Google Scholar]
- 32.Okubo K, Kurono Y, Ichimura K, et al. Japanese guidelines for allergic rhinitis 2017. Allergol Int 2017;66:205–19. 10.1016/j.alit.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 33.Scadding GK, Kariyawasam HH, Scadding G, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (revised edition 2017; first edition 2007). Clin Exp Allergy 2017;47:856–89. 10.1111/cea.12953 [DOI] [PubMed] [Google Scholar]
- 34.Arakawa H, Hamasaki Y, Kohno Y, et al. Japanese guidelines for childhood asthma 2017. Allergol Int 2017;66:190–204. 10.1016/j.alit.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Li P, Tang J, et al. Prevalence of atopic dermatitis in Chinese children aged 1-7 ys. Sci Rep 2016;6:29751. 10.1038/srep29751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura R, Ishiguro N, Naru E, et al. The effect of an educational program for pregnant women to prevent allergic diseases in infants: study protocol for a randomized controlled trial. Trials 2019;20:755. 10.1186/s13063-019-3797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-048083supp001.pdf (131.1KB, pdf)