Abstract
The mature skeletons of hard corals, termed stony or scleractinian corals, are made of aragonite (CaCO3). During their formation, particles attaching to the skeleton’s growing surface are calcium carbonate, transiently amorphous. Here we show that amorphous particles are observed frequently and reproducibly just outside the skeleton, where a calicoblastic cell layer envelops and deposits the forming skeleton. The observation of particles in these locations, therefore, is consistent with nucleation and growth of particles in intracellular vesicles. The observed extraskeletal particles range in size between 0.2 and 1.0 μm and contain more of the amorphous precursor phases than the skeleton surface or bulk, where they gradually crystallize to aragonite. This observation was repeated in three diverse genera of corals, Acropora sp., Stylophora pistillata—differently sensitive to ocean acidification (OA)—and Turbinaria peltata, demonstrating that intracellular particles are a major source of material during the additive manufacturing of coral skeletons. Thus, particles are formed away from seawater, in a presumed intracellular calcifying fluid (ICF) in closed vesicles and not, as previously assumed, in the extracellular calcifying fluid (ECF), which, unlike ICF, is partly open to seawater. After particle attachment, the growing skeleton surface remains exposed to ECF, and, remarkably, its crystallization rate varies significantly across genera. The skeleton surface layers containing amorphous pixels vary in thickness across genera: ∼2.1 μm in Acropora, 1.1 μm in Stylophora, and 0.9 μm in Turbinaria. Thus, the slow-crystallizing Acropora skeleton surface remains amorphous and soluble longer, including overnight, when the pH in the ECF drops. Increased skeleton surface solubility is consistent with Acropora’s vulnerability to OA, whereas the Stylophora skeleton surface layer crystallizes faster, consistent with Stylophora’s resilience to OA. Turbinaria, whose response to OA has not yet been tested, is expected to be even more resilient than Stylophora, based on the present data.
Introduction
All scleractinian or stony corals form aragonite (CaCO3) skeletons, which provide the scaffolding for entire coral reef ecosystems. Will coral reefs survive ocean acidification (OA)? Current models and projections include predictions that corals will continue to calcify even when water chemistry in coral reefs switches from net precipitation to net dissolution, which is expected to happen in 2050.1 Why this is the case, however, is unclear. Importantly, different coral genera respond differently to OA.2Stylophora pistillata, one of the most studied coral species, exhibits the greatest resilience to OA.2S. pistillata was shown to form its aragonite skeleton by attachment of amorphous precursor particles, including hydrated and anhydrous amorphous calcium carbonate (ACC-H2O and ACC).3 Then, this result was reproduced in five other genera and expanded to include both particle attachment and ion attachment to achieve dense, space-filling skeletons at the nano- and microscales.4 Particles were assumed to nucleate and grow inside intracellular vesicles, within the calicoblastic epithelium—that is, the cell layer that deposits the coral skeleton and tightly envelops the forming surface. These vesicles were presumed to be filled with intracellular calcifying fluid (ICF), with closely biologically controlled chemical composition, favoring particle formation. Once formed, intracellular particles are presumed to be delivered, exocytosed, and attached to the forming surface of the coral skeleton.4 Between the calicoblastic epithelium and the coral skeleton is a small amount, 1–2 μm thick,5,6 of liquid or gel termed extracellular calcifying fluid (ECF). The ECF is known to acidify when seawater acidifies,2 because the ECF is partly open to seawater.7 In contrast, the ICF is intracellular; thus, it is expected to be precisely controlled by cells and not at all open to seawater. (Embryonic corals may differ.6)
To simulate OA in aquaria, Venn et al.2 bubbled CO2 in seawater and observed the effects on the coral calcification rate, on pH in the ECF, and in the cytoplasm of calicoblastic cells. With four different seawater pH values—8.1, 7.8, 7.4, and 7.2—and three different coral species—Stylophora pistillata, Pocillopora damicornis, and Acropora hyacinthus—they observed that the daytime calcification rate decreased significantly for Pocillopora and Acropora but remained constant for Stylophora. The ICF pH in intracellular vesicles was not measured, but the intracellular pH in the cytoplasm of calicoblastic cells decreased slightly and similarly (pH changed by ∼ –0.3 for all genera from control values ∼7.4), as did the ECF pH measured during the day (∼ –0.5 for all genera from control values ∼8.2). The parameter that changed most dramatically and differently across genera was the ECF pH measured at night: ∼ –0.4, ∼ –0.6, and ∼ –0.7 change for Stylophora, Pocillopora, and Acropora, respectively. The decrease of calcification and ECF night-time pH with OA for these three corals is shown in Figure 1.
Figure 1.
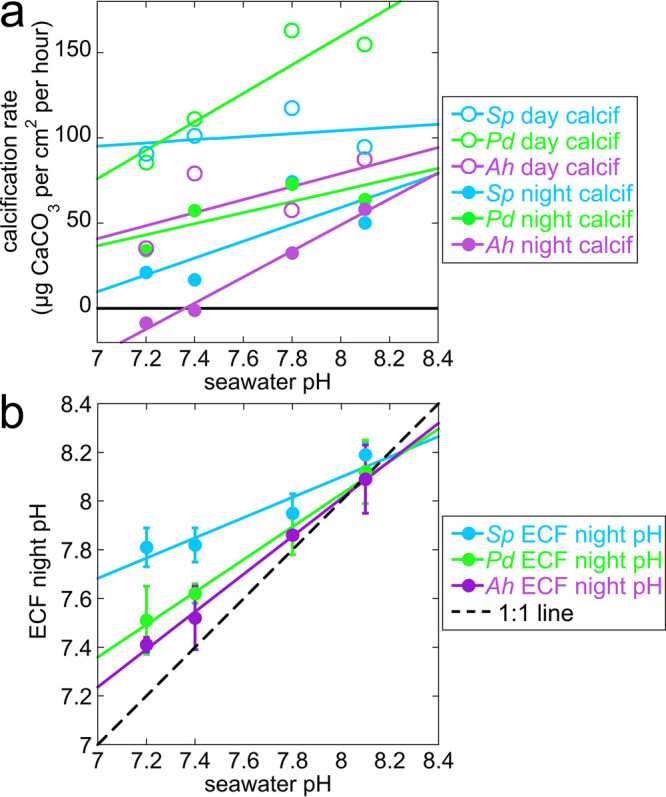
(a) Calcification rate and (b) night-time pH measured in extracellular calcifying fluid (ECF), between calicoblastic epithelium and forming skeleton surface, as seawater pH decreases in simulated ocean acidification (OA, pH 8.1 → 7.2) experiments in three coral genera and species: Stylophora pistillata (Sp, light blue), Pocillopora damicornis (Pd, green), and Acropora hyacinthus (Ah, purple). These are selected, replotted data from Venn et al., 2019.2 (a) During the day (open circles) the calcification rate is constant for Stylophora, but it decreases with OA for Pocillopora and Acropora. At night, calcification decreases with OA for all genera, but especially for Acropora, which goes below zero (black solid line); thus, the skeleton formed during the day dissolves at night. (b) The pH values in the ECF during the day, when photosynthesis is active, decrease to 7.8 identically for all three genera; thus, they are omitted here. Only night-time pH values in the ECF are shown, as they vary dramatically across the three genera. The solid lines are linear fits of the data; the 1:1 line (black dashed line) is where pHECF = pHseawater. Clearly, as the seawater pH decreases, the ECF night-time pH decreases, but at slower rates for all genera compared to seawater. Stylophora ECF pH is the slowest, Pocillopora intermediate, and Acropora the fastest, that is, closest to the seawater pH decrease with OA.
The ECF night-time pH accompanies the observed decrease in night-time calcification rate for the three genera, and best distinguishes them from one another in their resilience to OA. Clearly, for all genera, the ECF night-time pH is biologically controlled, as indicated by the different slopes for the three genera, all of them above the 1:1 line in Figure 1b. Is it possible that intracellular particle formation and ICF are less or not at all affected by OA, but once the particles are exocytosed and exposed to ECF they dissolve as the ECF pH decreases with OA? This would explain the difference across genera observed at night but not during the day.2 Biomineralization and respiration proceed day and night, whereas photosynthesis is only active during the day. Thus, it is not surprising that biomineralization conditions differ between day and night. Photosynthesis, done by the coral polyp animal’s symbiont dinoflagellates, changes the chemistry internal to the animal in two different ways: it removes aqueous CO2 and therefore increases the pH in the surrounding fluids. Furthermore, photosynthesis produces carbohydrates that feed the coral polyp animal, thus providing metabolic energy, which the animal can use to better control its internal chemistry, and specifically the ECF day-time pH.
If the nucleation and growth of nanoparticles occurs in intracellular vesicles, as hypothesized previously,4 it should be possible to observe particles in intracellular vesicles in the calicoblastic cells in all coral genera—those extremely sensitive to OA, such as Acropora, and those resilient to OA, such as Stylophora. To test this hypothesis, we searched for particles outside the forming skeleton surface and strived to analyze the mineral phases present in such particles, if they exist, in three diverse corals.
Where the particles nucleate and grow before they attach to the forming skeleton surface, in the ECF or in the hypothesized but never directly observed ICF, makes a significant difference. If they nucleate and grow in the ECF, OA will be inescapably fatal for the most sensitive corals, such as Acropora.
If instead nucleation and growth of particles occurs intracellularly, even the most sensitive genera may have a chance at surviving OA. As long as the pH of the oceans stays above the threshold of net dissolution, the animals will still be able to form new skeletons, even if ECF conditions prevent particle nucleation and growth.
The supersaturation with respect to aragonite is
where the solubility product is Ksp = 7.184, as obtained by Sevilgen et al.8 using the salinity values in Mucci.9 As shown by Cohen and Holcomb,10 nucleation of aragonite occurs at very high saturation states, Ωaragonite ≥ 20, whereas at lower supersaturation states, with 6 < Ωaragonite < 19, crystal growth is favored over nucleation.10 For other carbonates, amorphous and crystalline, similar supersaturation ranges are expected for nucleation and growth. In the ECF, the supersaturation measured by Sevilgen et al.8 in Stylophora is Ωaragonite = 12; thus, only crystal growth occurs, and this must be growth by ion attachment. If nanoparticles are observed in the tissue, they require that ICF conditions be appropriate for nucleation; thus, the supersaturation in ICF must be greater than in ECF, or else nucleation could not occur.10 Such intracellular vesicles, and the ICF they contain, in which particles nucleate and grow, have been observed in single cells of sea urchin embryos, where they contained ICF with more than 1 M calcium!11
If such high-concentration and supersaturated droplets of solution exist intracellularly in corals, sea urchin embryos,11 or adult regenerating sea urchin spines,12 they must be confined by vesicles, with phospholipid membranes that keep them well isolated from the cytoplasm. This is because the calcium concentrations necessary for nucleation (mM–M) are toxic for any eukaryotic cells, whose cytoplasm has orders of magnitude lower concentrations (nM).4
Results and Discussion
Extraskeletal Particles
Using photoemission electron microscopy (PEEM), we analyzed species that are representative of three different genera of corals: one extremely sensitive to OA, Acropora sp.; one resilient to OA, S. pistillata; and one with unknown response to OA, Turbinaria peltata. Mapping the spectral components at the surface of the fresh, forming coral skeleton, we observed that all genera form calcium carbonate particles just outside (<2 μm) the skeleton surface, indicated by a yellow line, and just inside the surface (1–2 μm), in the recently deposited skeleton (see Figures 2–4 and Figures S2–S4.
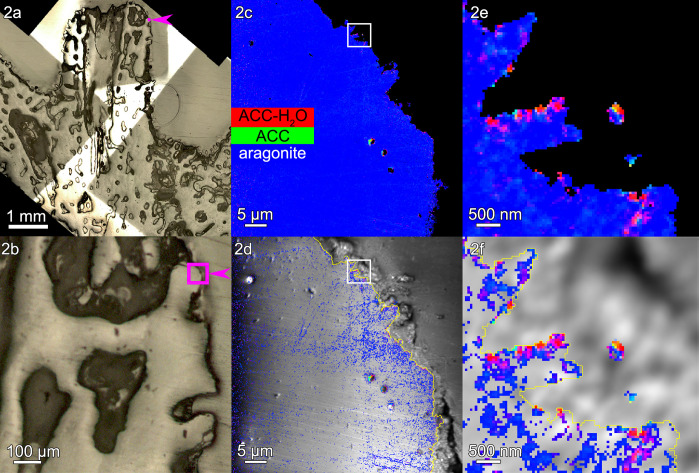
Figure 2.
Acropora sp.: (a, b) polarized light microscopy (PLM) images and (c) a component map with a black mask where the Ca signal is undetectable and pixels colored according to the mineral phase spectroscopically observed. In this and all other component maps in this work, red pixels are ACC-H2O, green pixels are ACC, and blue pixels are aragonite. The component spectra used to obtain all component maps are shown in Figure S1. (d) Average PEEM image overlaid with the component map, with both black mask and pure blue aragonite removed. (e, f) One region of interest, boxed in panels c and d, magnified here to show the precise locations of both intra- and extraskeletal amorphous pixels. Extraskeletal particles are no farther than 2 μm outside the skeleton’s surface (yellow line in panels d and f), are mostly amorphous, and are assumed to be inside the calicoblastic epithelium. Intraskeletal amorphous pixels in panel d extend several micrometers inside the yellow line. See Figure S2 for more images of this area.
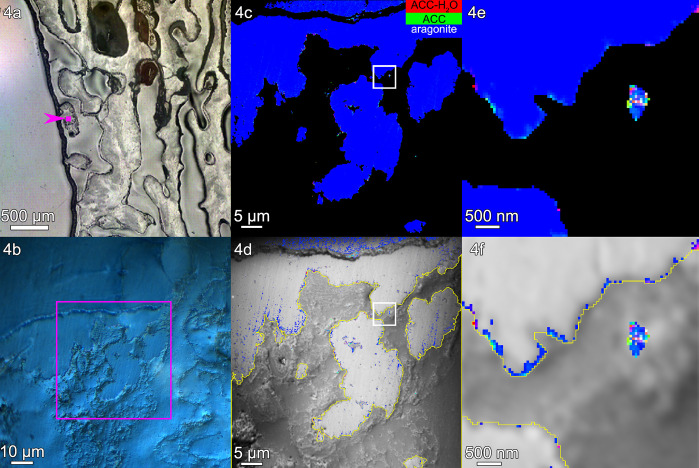
Figure 4.
Turbinaria peltata: (a) PLM image, (b) Differential Interference Contrast (DIC) image, (c) component map, and (d) average PEEM image overlaid with the component map, as described in Figure 2. (e, f) The regions boxed in panels c and d are magnified here to show amorphous particles where calicoblastic cells are expected. Amorphous pixels also appear along the edge of the skeleton. Extraskeletal particles are mostly amorphous within the calicoblastic epithelium, <2 μm outside the yellow line in panels d and f. Intraskeletal amorphous pixels in panel f extend <1 μm inside the yellow line. See Figure S4 for more images of this area.
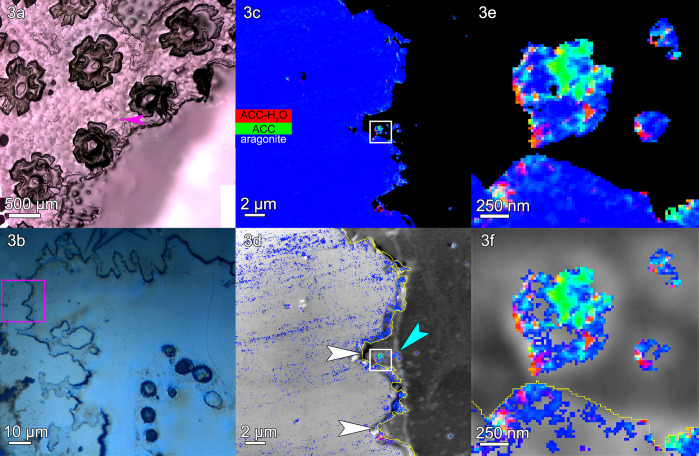
The extraskeletal particles observed vary in size between 200 nm and 1 μm, and spectromicroscopic analysis of their composition reveals a mix of ACC-H2O, ACC, and aragonite. The density of extraskeletal particles varies from area to area, ranging from ∼1 to 30 particles/μm3 in the probed volume, which ranges from ∼1 to 7 μm3 for each area, as shown in Table S1. The density of extraskeletal particles was expected to be small, as previously observed and calculated in regenerating sea urchin spines,12 because the probing depth of PEEM component mapping at the Ca L-edge is only 3 nm, as shown in refs (13 and 14); thus, even higher densities for particles appear sparser in such a thin slice.
The location of extraskeletal calcium carbonate particles is consistent with them being intracellular, inside calicoblastic cells. These particles must have formed where they were observed, and when the tissue was fixed they were interrupted in their transfer to the forming skeleton surface, where, in time, they would have crystallized. Several observations, explained below, support this deduction.
First, the grayscale PEEM images at the Ca L-edge in Figures 2–4, panels d, and all other areas in Figure S5 are not good enough to identify cells or cell structures. However, calicoblastic cells are well known to envelop the forming skeleton, and all of it,5 and are well preserved by the fixation method used here and previously.4 Thus, any Ca-rich extraskeletal nanoparticles observed within a 2 μm distance can safely be interpreted as intracellular to calicoblastic cells.5 The distances outside the skeleton surfaces (yellow lines in panels d and f) in Figures 2–4 and in four additional areas per genus are consistently within ∼2 μm (Figure S5). Representative single-pixel spectra for each mineral phase and each coral skeleton in Figures 2–4 are shown in Figure S6.
Second, given the supersaturation of the ECF, Ωaragonite = 12 measured in Stylophora, nucleation of particles in the ECF is extremely unlikely.8,10
Third, amorphous pixels, either ACC-H2O or ACC, are observed in greater percentage in extraskeletal particles than intraskeletally, even when comparing extraskeletal particles with only the skeleton surface, as shown in Tables S2 and S3; thus, intracellular particles are distinct, and not simply material dislodged from the skeleton during embedding or polishing. Figures 2–4, panels e and f, show such extraskeletal and intracellular particles and their amorphous phases just outside the surface of the forming skeleton (yellow line). Intraskeletal amorphous pixels are substantially less spatially dense than in extraskeletal particles; thus, there is a distinct chemical difference between amorphous phases inside or outside the skeleton.
Fourth, if extraskeletal particles were not present intracellularly but were in the ECF, they would have been washed away when the corals were fixed and then rinsed tens of times, so there must have been something holding the particles in place—likely the calicoblastic cells, as these are well known to envelop the forming skeleton.5 It is possible that more particles were originally present that were washed away during the tens of rinsing steps. No conclusions were made based on lost particles.
All extraskeletal particles observed here could also be localized within a network of organic fibrils15 or filopodia6 that were recently observed, using cryo-scanning electron microscopy, between cells and skeleton.6 These occur between the skeleton growing surface and calicoblastic cells or desmocytes, alike, and are attached to the skeleton surface;15 thus, even where cells were detached from the skeleton during sample preparation, fibrils could, in principle, remain attached. Since the fibrils are expected to contain Ca-rich particles, it is possible that some or all the extraskeletal particles observed here in all genera are within fibrils. It is important to note that these fibrils are still part of calicoblastic cells; thus, particles within fibrils should still be considered intracellular. Particles in either cell bodies or fibrils protruding from cell bodies are collectively referred to in this work as in-tissue or intracellular extraskeletal particles.
The Ca-rich biggest particle in Figure 3e,f is comparable in size and position to one of the vacuoles observed by Clode and Marshall in a desmocyte (labeled V in their Figure 1),15 although this similarity must be further investigated to be confirmed.
Figure 3.
Stylophora pistillata: (a) PLM image, (b) DIC image, (c) a component map, and (d) an average PEEM image overlaid with the component map, as described in Figure 2. (e, f) The regions boxed in panels c and d are magnified here to show amorphous particles where calicoblastic cells are expected. This region shows several scars left by desmocytes, the cells that bind the calicoblastic epithelium to the skeleton and form 3-μm-deep V-shaped scars, indicated by white arrows in panel d. Distinct particles are visible just outside the skeleton surface (e.g., cyan arrowhead in panel d, and three particles in panel f). These extraskeletal particles have both a greater percentage of amorphous pixels and a greater concentration of amorphous phases per pixel, compared to the skeleton surface or bulk (Tables S2 and S3). Extraskeletal particles are mostly amorphous within the calicoblastic epithelium, <2 μm outside the yellow line in panels d and f. Intraskeletal amorphous pixels in panel d extend ∼1 μm inside the yellow line. See Figure S3 for more images of this area.
Extraskeletal amorphous particles, consistent with intracellular vesicles, were also observed in regenerating sea urchin spines.12
Skeletal Surface Crystallization Rates Differ across Genera
The most striking difference across genera is the thickness of the surface layer containing intraskeletal amorphous pixels, measured from the yellow line at the surface of each skeleton. This thickness is 2.1 μm in Acropora, 1.1 μm in Stylophora, and 0.9 μm in Turbinaria. The average measurements of these thicknesses are shown in Figure 5, and all the precise data from 15 areas in three genera are presented in Table S4. The same trends are visible in Figures 2d, 3d, and 4d, where the pure aragonite blue pixels were removed: intraskeletal amorphous pixels are farther from the surface in Acropora, intermediate in Stylophora, and closer in Turbinaria. Thus, crystallization rates differ across the three genera, as shown in Figure 5.
Figure 5.
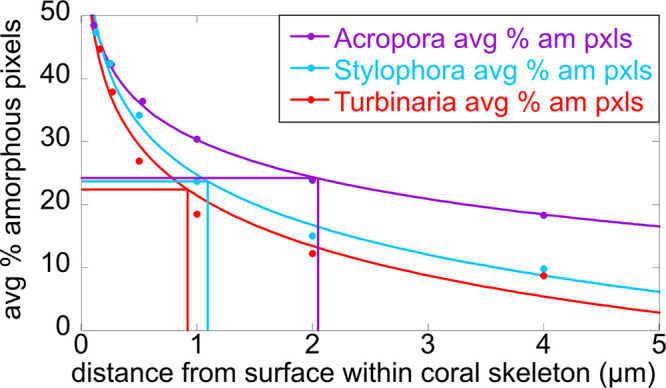
Amorphous, soluble thickness decays with distance from the skeleton surface. Comparison of the three genera analyzed here, for percentage of amorphous pixels (either ACC-H2O or ACC) as a function of distance from the surface, indicated by a yellow line in Figures 2–4. Each percentage is averaged over the five areas analyzed per genus (Table S4), the averages are displayed as circles, and the solid lines are logarithmic decays (among others tested, a logarithmic decay provided better fits, with R > 0.98). The half-lengths (vertical lines) are the distances at which the amorphous pixels have decayed to 50% of the surface value for that genus (horizontal lines). The half-lengths are 2.1 μm for Acropora, 1.1 μm for Stylophora, and 0.9 μm for Turbinaria.
There are also differences in the composition of these intraskeletal surface bands: Acropora has the greatest density of amorphous pixels, and its pixels are mostly ACC-H2O; Stylophora has intermediate amorphous density and mostly ACC pixels; and finally Turbinaria has the lowest density and fewest of both ACC-H2O and ACC pixels (Table S2).
In all genera, almost all pixels observed farther into the skeleton than 1–2 μm were pure aragonite. (In Figure 3d, some lines of pixels were not selected by the Magic Wand because they were non-pure aragonite—these are topographic artifacts caused by scratches on the sample’s imperfectly polished surface.)
The density of amorphous pixels was quantified in each genus by measuring the percentage of amorphous pixels within 2 μm of the skeleton surface. For Acropora, Stylophora, and Turbinaria, we found that approximately 24%, 15%, and 12% of the pixels contained amorphous phases. These values were obtained from 15 areas analyzed. The precise values from each area and genus are shown in Table S2. The areas in Figures 2–4 have slightly different percentages of amorphous pixels than the average, but they follow the same trend as the average, with Acropora having the most amorphous and Turbinaria the most crystalline 2-μm-thick surface layer (Table S2).
We note that the times for sample preparation and analysis varied between 15 and 45 h post-mortem. All of these times are immensely longer than the crystallization rate of ACC-H2O in laboratory conditions, which takes less than 1 min, especially in contact with water.16 Therefore, the observed amorphous phases are biologically stabilized, or they would not be observable in these experiments. Amorphous-phase stabilizing molecules are well known to exist in biominerals.17−23
The increased amorphous content observed in the surface layer of Acropora skeleton means that these amorphous phases last longer during the process of skeleton formation in Acropora. Thus, crystallization of amorphous phases is slow in Acropora, faster in Stylophora, and fastest in Turbinaria.
This observation directly explains why different genera vary in sensitivity to OA, as shown in Figure 1. The forming surface in Acropora skeletons crystallizes slowly from its amorphous precursors; thus, newly attached ACC-H2O and ACC particles are exposed to the ECF for a longer time.
ACC and ACC-H2O are well known to be more soluble than crystalline aragonite in water;24 thus, they can dissolve in ECF, as occurs for Acropora at night (Figure 1). The solubility product Ksp,ACC is ∼100× larger than Ksp,arag in deionized water,24 making ACC significantly more soluble than aragonite. The solubility of ACC in ECF or even in seawater conditions is not known, but it is certainly greater than that of aragonite in those conditions as well. In contrast, the faster-crystallizing skeleton surface in Stylophora exposes more insoluble aragonite than soluble ACC to night-time ECF, thus reducing dissolution. While this may not be the only factor contributing to OA resilience, these data suggest that Turbinaria should be even more resilient to OA than Stylophora, due to its fastest crystallization rate observed here. We stress that this is the crystallization rate from the amorphous precursors to crystalline aragonite, not the calcification rate, which is known to be slower in Turbinaria than in Stylophora.25,26 There are at present no data on Turbinaria’s resilience to OA.
Notice that the calcification rate in Acropora is lower than in Stylophora, which is in turn lower than in Pocillopora, as shown in Figure 1, for both day- and night-time calcification. The synthesis rate of precursors is equal to the calcification rate, independent of crystallization rate. Thus, the thickest amorphous layer observed in Acropora cannot be caused by greater production of amorphous precursor, because Acropora is the slowest calcifier. We also observe a lower density of extraskeletal particles in Acropora than in both Turbinaria and Stylophora (Table S1), consistent with the lower calcification rate (Figure 1).
The origin of differing crystallization rates is not explored in this study. We speculate that either organic molecules or elemental impurities stabilize amorphous phases for a longer time in Acropora. For instance, magnesium is known to stabilize ACC in sea urchin spines,16,27 and proteins are known to do the same in sea urchin spicules.17
Conclusion
Here, analyzing fresh corals inside and outside the surface of the forming skeletons, we observed particles consistent with intracellular localization, presumably inside calicoblastic cells.
Intraskeletal amorphous phases localized near the skeleton’s surface contain, respectively, a lower or greater percentage of the amorphous phases compared to the extraskeletal particles or the skeleton bulk, which is almost completely crystalline aragonite. These spectroscopically detectable localizations in space and phase transition trends are consistent with previous models of coral skeleton biomineralization.4,28,29 They are also consistent with a time sequence in which extraskeletal amorphous nanoparticle nucleation and growth in intracellular vesicles is followed by attachment to the growing surface of the skeleton,30,31 followed by gradual crystallization of the amorphous phases into crystalline aragonite.
The precise chemical environment (ICF) and cellular location in which the particles nucleate remain unknown. We observe directly extraskeletal particles, likely in intracellular vesicles within calicoblastic cells forming the epithelium that envelops the growing skeleton surface.
Once the particles are exocytosed into the ECF and attach to the growing skeleton surface, they are exposed to increasingly lower pH during OA. The partial control of ECF pH will slow down but not completely counter the effect of OA on the ECF, which is partly open to seawater,7 especially for sensitive corals such as Acropora, where with seawater pH 7.2, the ECF night-time pH is as low as 7.4, as shown in Figure 1.
The differential sensitivity of calcification to OA, shown in Figure 1A, is elegantly explained by faster or slower crystallization of the forming skeleton surface, which makes it less or more soluble, and thus more or less resilient to OA. We observed less-amorphous surfaces in resilient Stylophora than in vulnerable Acropora, and even less in Turbinaria, whose response to OA is unknown but is predicted, on the basis of these data, to be even more resilient than Stylophora.
Methods
Samples
All corals were obtained live from Albany Aquarium, Albany, CA, USA. First, 1-cm fragments of skeleton and tissue were fixed to preserve the tissue and all its nanoparticle content, and then they were dehydrated with increasing concentrations of ethanol as described by Sun et al. in 2020.4 TheStylophora and Turbinaria samples were then embedded into EpoFix (EMS, Hatfield, PA, USA), the Acropora sample was embedded in Solarez (Solarez, Vista, CA, USA) and UV-cured, and all three were polished, coated with 1 nm Pt in the regions to be analyzed, and 40 nm Pt elsewhere, as described in refs (32−35).
The three coral samples were analyzed with component mapping approximately 1–2 days after they were fixed. Precisely, the analysis lasted 13–24 h post-mortem for Acropora (14.5–15 for the data in Figure 2), 23–44.5 h post-mortem for Stylophora (44–44.5 for the data in Figure 3), and 24.5–36 h post-mortem for Turbinaria (25.5–26 for the data in Figure 4).
The post-mortem times of the corals differ, but this does not invalidate the deduced amorphous precursor crystallization rates. The rate of crystallization for ACC in the lab is on the order of seconds;16 thus, the post-mortem times used here are many orders of magnitude larger, making differences between them insignificant. Furthermore, the longest post-mortem sample, Stylophora, does not contain the fewest amorphous pixels, implying that these phases are stabilized biologically17−23 and last days post-mortem. Finally, all sample preparations greatly reduce the exposure of the amorphous phases to water and air, both of which induce ACC crystallization.36
Component Mapping
PEEM images, component maps, and spectra shown in all figures were acquired using the photoemission electron microscope (PEEM) at the Advanced Light Source on beamline 11.0.1.1. All data were acquired across the Ca L-edge. The intracellular particles observed in calicoblastic cells vary in size between 200 nm and 1 μm, and spectromicroscopic analysis of their composition reveals a mix of hydrated and anhydrous amorphous calcium carbonate (ACC-H2O and ACC) and aragonite.
PEEM images were taken with 54, 24, or 56 nm pixel resolution for Figures 2, 3 or 4, respectively, and 3 nm probing depth.13 Each stack of 121 PEEM images contained 106 pixels/image, and thus 106 complete Ca spectra. All Ca stacks of images were acquired between 340 and 360 eV, with 0.1 eV energy steps between 345 and 355 eV where the Ca peaks are, and 0.5 eV steps outside of this range. All Ca spectra were acquired with circular polarization to minimize crystal orientation effects.
Images were stacked to produce component maps, in which each pixel spectrum is best-fitted to a linear combination of known “component spectra”, using IGOR Pro Carbon 8 with open-source GG Macros, available free of charge from ref (37). The “Cni7” component spectra (Figure S1) are provided as Supporting Information.
Figures 2d, 3d, and 4d were all made by overlaying the component map from Figures 2c, 3c, and 4c on a PEEM average image in Adobe Photoshop and then using the Magic Wand tool to remove both black (the Ca poor mask) and pure blue (aragonite) from the component map, with a threshold of 26, which is 10% of 255.
The component maps in Figures 2–4 are “brightness enhanced” 37 to improve visibility of amorphous phases. Before brightness enhancement, the amounts of RGB add up to 255 and are entirely quantitative. Brightness enhancement is accomplished by making the color value of the largest percentage component 255 and then scaling up the other two components proportionally. For example, if a pixel has RGB amounts [25,25,205], thus, approximately 10% ACC-H2O, 10% ACC, and 80% aragonite, brightness enhancement changes its RGB values to [31,31,255]. All operations using the Magic Wand tool in Adobe Photoshop were performed on non-brightness-enhanced pMaps of the component of interest.37 In Figures 2–4, the epoxy black pixels and aragonite blue pixels deleted from panels d and f were selected using the Magic Wand on non-brightness-enhanced RGB maps, so they were quantitatively accurate.
The black masks in Figures 2c, 3c, and 4c that cover the irrelevant parts of component maps are all produced from a combination of three different masks termed “difference mask”, “χ2 mask”, and “manual mask”. The difference mask covers all pixels with a zero or near-zero Ca concentration. It is produced in the GG Macros37 by digital subtraction of two images, acquired above and below the Ca L-edge at 352.6 and 344 eV energies, respectively. More precisely, in order to eliminate noise, each image used in the subtraction is the average of multiple images (five on-peak, nine off-peak), acquired at and around the stated energies. The resulting Ca concentration map is shown in Figure S7. The Ca concentration map is then thresholded so all pixels in the image with zero or near-zero Ca concentration are masked off and displayed as black. The threshold value is determined accurately by zooming in on a skeleton edge region and adjusted numerically until all the spectra at the skeleton edge with acceptable signal/noise ratio and distinct and identifiable Ca spectral peaks are retained, and the others are discarded. This numeric adjustment of the threshold is repeated in several regions of skeleton edge to ensure that the difference mask is consistently retaining good- and discarding bad-spectra pixels. The χ2 mask is produced by first mapping all χ2 values obtained from the best fit during component analysis of each pixel. Then, a threshold is applied to exclude all χ2 values above a numeric value, typically χ2 = 0.01. The resulting χ2 mask, therefore, makes black all pixels where, for any reason, the fit was poor. Both masks, difference and χ2, are then layered on top of one another in Adobe Photoshop. The third “manual mask” is produced by hand to discard (display in black) any spurious single pixels that were not eliminated by the other two masks. The rare single pixels observed are unrealistic, noisy spectra and are clearly Ca-poor in the epoxy or tissue regions. Figure S7 shows PEEM single and average images, the Ca map, and the final black mask over the component map and over the Ca map.
The yellow line, indicating the skeleton surface, was obtained by outlining the black mask (resulting from overlapping difference, χ2, and manual masks) from each component map, ignoring particles outside the skeleton and anything masked inside the skeleton. This was achieved by selecting the mask and outlining it with a yellow line using the “Stroke” layer tool in Adobe Photoshop. This line was obtained with 1-pixel resolution (24 nm in Stylophora; 54 and 56 nm in Acropora and Turbinaria) and used throughout all distance and thickness measurements with 1-pixel resolution, leaving no ambiguity about what is inside and outside the skeleton. For visibility and display purposes only, in panels d and f, the yellow line was made 3-pixels wide, expanding outside the skeleton, not to overlap any parts of it. Figure S7 shows the yellow outline and the amorphous pixels overlaid on an average image, as done in Figures 2–4.
We stress that the colored pixels from component maps (panels c and e in all figures) and the grayscale average PEEM image (panels d and f) were obtained from precisely the same Ca stack, not repeated acquisitions. Thus, we made no assumptions on where the Ca signal was high or low, where the tissue was, or where the skeleton was in space. The yellow line position is based exclusively on where the Ca signal is as high as in the mature skeleton. This is a conservative choice that excludes many amorphous pixels, but it is a rigorous one.
Amorphous Pixel Counting
Quantitative measurements of how amorphous the different skeletons surfaces are were performed in Adobe Photoshop and are presented in Tables S2 and S4. To obtain the amorphous pixels vs depth data in Table S4 and Figure 5, first we used the black mask in each area, and then the “Expand” selection tool to include the outermost 4, 2, 1, 0.5, 0.25, and 0.125 μm of skeleton. Next, the black mask was removed from the selection area, and finally the selected band was placed over the ACC-H2O or ACC proportion maps, that is, the grayscale images that show the proportion, between 0 and 1 or between 0 and 255, of ACC-H2O or ACC phase in each pixel. Then, using the Magic Wand tool, we selected a pixel that contained 100% of a given phase and varied the tolerance of the wand. To select pixels with at least 10% amorphous phase, a tolerance of 230 was used, which is 90% of 255. Once selected, the number of pixels was measured in “Windows” and “Histogram” in Adobe Photoshop. For aragonite we used a threshold of 26, which is 10% of 255. All pixel counts from all areas were logged in Microsoft Excel, as presented in Table S4, at the depth specified. Figure 5 was obtained in Kaleidagraph 5.0 for Mac (Synergy Software), where the data were fitted by a logarithmic decay with excellent correlation coefficients: R = 0.999 for Acropora, R = 0.995 for Stylophora, and R = 0.984 for Turbinaria. Other decay functions were tested, including exponential and parabolic, but the correlation coefficients R were lower than for logarithmic decay; hence, the latter was used for Figure 5.
Parallel Component Mapping by the Cnidarians
The Cni7 component spectra used for component mapping were optimized to eliminate any spectral background contributions that are mistakenly interpreted as different phases during component mapping. This undesirable effect was recently discovered for this work, when we processed many stacks of images in parallel, with a group of talented undergraduate students we call “the Cnidarians”, 13 of whom are co-authors of this work. Since October of 2020, the Cnidarians have been processing component mapping spectromicroscopy of biominerals data, collected over the past 10 years. The results of this meta-analysis, done in parallel on the same data by different people, are then compared during biweekly meetings, thus optimizing every choice. There are 5–10 different human choices to make to process component maps, and different people make these choices differently; thus, direct comparison of the results benefits from a diversity of people and greatly improves the final data quality. One major problem was revealed by these comparisons: the energy range used for component analysis (between 345 and 355 eV) greatly affected the outcome of component maps. When using previous component spectra, such as 0608 in Gong et al.,17 0709 in DeVol et al.,38 0823 in Mass et al.,3 or CY1 in Sun et al.,4 uneven backgrounds of spectra at the edges of the energy range (around 345 eV or around 355 eV) led to phase assignment differences in component maps when the energy range selected for peak-fitting differed from person to person. This undesirable mistake was eliminated by improving the component spectra so they have identical spectral backgrounds (2 arctan, 1 polynomial), as shown in Figure S1. Using the new “Cni7” component spectra makes the final component map only dependent on real, spectroscopic differences occurring at Ca peaks’ energy positions, not away from Ca peaks, e.g., at 345 or 355 eV. Table S5 shows all the fit parameters for all component spectra.
All data presented in this manuscript have been analyzed by at least 5–10 people, multiple times each, using different component spectra. Once we converged on using the Cni7 component spectra, 5 people analyzed these data in parallel, making individual choices on multiple parameters, e.g., threshold for difference mask and χ2 mask, peak shift in energy acceptable interval, etc. The data presented are the best fits to the data, because, even after small changes in parameters, the results consistently provide the same locations for amorphous phases in the skeleton and extraskeletal particles. The precise percentage of each phase fluctuates slightly, by ∼10%.
The component spectra, “Cni7”, were generated by P.U.P.A.G. using spectra taken from several biominerals (Figure S1). Component spectra for ACC-H2O and ACC were generated from averaged spectra taken from single pixels in sea urchin spines which previous analysis showed to be at least 90% of the desired component. The same process was applied to coral skeletons for the aragonite component spectra. For each component, more than 1000 single-pixel spectra were aligned in energy and averaged, and then we subtracted the backgrounds using a pre-edge linear fit for each spectrum. We then peak-fitted each spectrum, using the background (third-order polynomial and two arctangents) obtained for aragonite for all spectra identically.
Acknowledgments
We thank Jun “Jay” Zhang for preparing the Acropora sample, and Andreas Scholl, Rajesh V. Chopdekar, and Chang-Yu Sun for technical help during beamtime.
Glossary
Abbreviations
- ACC
amorphous calcium carbonate anhydrous
- ACC-H2O
amorphous calcium carbonate hydrated
- OA
ocean acidification
- ECF
extracellular calcifying fluid
- ICF
intracellular calcifying fluid
- PEEM
photoemission electron microscopy
- RGB
red, green, and blue
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c11434.
Figures S1–S7, showing component spectra Cni7, polarized light micrographs, component maps of all 15 areas analyzed in this work, single-pixel spectra, single PEEM image, average image, and a Ca concentration map; Tables S1–S5, showing extraskeletal particle density, intra- and extra-skeletal percentage of amorphous pixels, percentage of amorphous pixels vs distance from surface within the skeletons, and component spectra peak-fitting parameters (PDF)
Cni7 files: component spectra for ACC-H2O, ACC, and aragonite (ZIP)
P.U.P.A.G. received 40% support from DOE–BES–Chemical Sciences, Geosciences, Biosciences–Geosciences Grant DE-FG02-07ER15899, 40% support from the Laboratory Directed Research and Development (LDRD) program at Berkeley Lab, through DOE–BES, under Award Number DE-AC02-05CH11231, and 20% support from NSF Biomaterials Grant DMR-1603192. All PEEM experiments were done at the Advanced Light Source (ALS), which is supported by the Director, Office of Science, Office of Basic Energy Sciences, U.S. Department of Energy under Contract No. DE-AC02-05CH11231. T.M. received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 755876).
The authors declare no competing financial interest.
Notes
Pupa U. P. A. Gilbert was previously publishing as Gelsomina De Stasio.
Supplementary Material
References
- Eyre B. D.; Cyronak T.; Drupp P.; De Carlo E. H.; Sachs J. P.; Andersson A. J. Coral reefs will transition to net dissolving before end of century. Science 2018, 359 (6378), 908–911. 10.1126/science.aao1118. [DOI] [PubMed] [Google Scholar]
- Venn A.; Tambutté E.; Caminiti-Segonds N.; Techer N.; Allemand D.; Tambutté S. Effects of light and darkness on pH regulation in three coral species exposed to seawater acidification. Sci. Rep 2019, 9 (1), 2201. 10.1038/s41598-018-38168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass T.; Giuffre A. J.; Sun C.-Y.; Stifler C. A.; Frazier M. J.; Neder M.; Tamura N.; Stan C. V.; Marcus M. A.; Gilbert P. U. P. A. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. 2017, 114 (37), E7670–E7678. 10.1073/pnas.1707890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.-Y.; Stifler C. A.; Chopdekar R. V.; Schmidt C. A.; Parida G.; Schoeppler V.; Fordyce B. I.; Brau J. H.; Mass T.; Tambutté S.; Gilbert P. U. P. A. From particle attachment to space-filling coral skeletons. Proc. Natl. Acad. Sci. 2020, 117 (48), 30159–30170. 10.1073/pnas.2012025117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambutté E.; Allemand D.; Zoccola D.; Meibom A.; Lotto S.; Caminiti N.; Tambutté S. Observations of the tissue-skeleton interface in the scleractinian coral Stylophora pistillata. Coral Reefs 2007, 26 (3), 517–529. 10.1007/s00338-007-0263-5. [DOI] [Google Scholar]
- Mor Khalifa G.; Levy S.; Mass T. The calcifying interface in a stony coral primary polyp: An interplay between seawater and an extracellular calcifying space. J. Struct Biol. 2021, 213, 107803. 10.1016/j.jsb.2021.107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn A. A.; Bernardet C.; Chabenat A.; Tambutte E.; Tambutte S. Paracellular transport to the coral calcifying medium: effects of environmental parameters. J. Exp. Biol. 2020, 223 (17), 227074. 10.1242/jeb.227074. [DOI] [PubMed] [Google Scholar]
- Sevilgen D. S.; Venn A. A.; Hu M. Y.; Tambutté E.; de Beer D.; Planas-Bielsa V.; Tambutté S. Full in vivo characterization of carbonate chemistry at the site of calcification in corals. Sci. Adv. 2019, 5 (1), eaau7447. 10.1126/sciadv.aau7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci A. The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure. Am. J. Sci. 1983, 283 (7), 780–799. 10.2475/ajs.283.7.780. [DOI] [Google Scholar]
- Cohen A. L.; Holcomb M. Why corals care about ocean acidification: uncovering the mechanism. Oceanography 2009, 22 (4), 118–127. 10.5670/oceanog.2009.102. [DOI] [Google Scholar]
- Kahil K.; Varsano N.; Sorrentino A.; Pereiro E.; Rez P.; Weiner S.; Addadi L. Cellular pathways of calcium transport and concentration toward mineral formation in sea urchin larvae. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (49), 30957–30965. 10.1073/pnas.1918195117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifler C. A.; Killian C. E.; Gilbert P. U. P. A. Evidence for a liquid precursor to biomineral formation. Cryst. Growth Des. 2021, 21 (12), 6635–6641. 10.1021/acs.cgd.1c00865. [DOI] [Google Scholar]
- Frazer B. H.; Gilbert B.; Sonderegger B. R.; De Stasio G. The probing depth of total electron yield in the sub keV range: TEY-XAS and X-PEEM. Surf. Sci. 2003, 537, 161–167. 10.1016/S0039-6028(03)00613-7. [DOI] [Google Scholar]
- Gilbert P. U. P. A., Photoemission spectromicroscopy for the biomineralogist. In Biomineralization Sourcebook, Characterization of Biominerals and Biomimetic Materials; DiMasi E., Gower L. B., Eds.; CRC Press: Boca Raton, FL, 2014; pp 135–151. [Google Scholar]
- Clode P. L.; Marshall A. Calcium associated with a fibrillar organic matrix in the scleractinian coral Galaxea fascicularis. Protoplasma 2003, 220 (3), 153–161. 10.1007/s00709-002-0046-3. [DOI] [PubMed] [Google Scholar]
- Radha A. V.; Forbes T. Z.; Killian C. E.; Gilbert P. U. P. A.; Navrotsky A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. 2010, 107, 16438–16443. 10.1073/pnas.1009959107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y. U.; Killian C. E.; Olson I. C.; Appathurai N. P.; Amasino A. L.; Martin M. C.; Holt L. J.; Wilt F. H.; Gilbert P. U. P. A. Phase transitions in biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. 2012, 109 (16), 6088–6093. 10.1073/pnas.1118085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiva-Tal A.; Kababya S.; Balazs Y. S.; Glazer L.; Berman A.; Sagi A.; Schmidt A. In situ molecular NMR picture of bioavailable calcium stabilized as amorphous CaCO3 biomineral in crayfish gastroliths. Proc. Natl. Acad. Sci. 2011, 108 (36), 14763–14768. 10.1073/pnas.1102608108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sawalmih A.; Li C.; Siegel S.; Fratzl P.; Paris O. On the stability of amorphous minerals in lobster cuticle. Adv. Mater. 2009, 21 (40), 4011–4015. 10.1002/adma.200900295. [DOI] [Google Scholar]
- Stephens C. J.; Ladden S. F.; Meldrum F. C.; Christenson H. K. Amorphous calcium carbonate is stabilized in confinement. Adv. Funct Mater. 2010, 20 (13), 2108–2115. 10.1002/adfm.201000248. [DOI] [Google Scholar]
- Gal A.; Habraken W.; Gur D.; Fratzl P.; Weiner S.; Addadi L. Calcite crystal growth by a solid-state transformation of stabilized amorphous calcium carbonate nanospheres in a hydrogel. Angew. Chem. Int. 2013, 52 (18), 4867–4870. 10.1002/anie.201210329. [DOI] [PubMed] [Google Scholar]
- Shaked H.; Polishchuk I.; Nagel A.; Bekenstein Y.; Pokroy B. Long-term Stabilized Amorphous Calcium Carbonate–an Ink for Bio-inspired 3D Printing. Mater. Today Bio 2021, 11, 100120. 10.1016/j.mtbio.2021.100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentov S.; Weil S.; Glazer L.; Sagi A.; Berman A. Stabilization of amorphous calcium carbonate by phosphate rich organic matrix proteins and by single phosphoamino acids. J. Struct Biol. 2010, 171 (2), 207–215. 10.1016/j.jsb.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Brečević L.; Nielsen A. E. Solubility of amorphous calcium carbonate. J. Cryst. Growth 1989, 98 (3), 504–510. 10.1016/0022-0248(89)90168-1. [DOI] [Google Scholar]
- Ross C. L.; DeCarlo T. M.; McCulloch M. T. Environmental and physiochemical controls on coral calcification along a latitudinal temperature gradient in Western Australia. Glob. Change Biol. 2019, 25 (2), 431–447. 10.1111/gcb.14488. [DOI] [PubMed] [Google Scholar]
- Ross C. L.; Schoepf V.; DeCarlo T. M.; McCulloch M. T. Mechanisms and seasonal drivers of calcification in the temperate coral Turbinaria reniformis at its latitudinal limits. Proc. R Soc. B: Biol. Sci. 2018, 285 (1879), 20180215. 10.1098/rspb.2018.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Blanco J.; Shaw S.; Bots P.; Roncal-Herrero T.; Benning L. G. The role of pH and Mg on the stability and crystallization of amorphous calcium carbonate. J. Alloys Compd. 2012, 536, S477–S479. 10.1016/j.jallcom.2011.11.057. [DOI] [Google Scholar]
- Gránásy L.; Rátkai L.; Tóth G. I.; Gilbert P. U. P. A.; Zlotnikov I.; Pusztai T. Phase-Field Modeling of Biomineralization in Mollusks and Corals: Microstructure vs Formation Mechanism. JACS Au 2021, 1 (7), 1014–1033. 10.1021/jacsau.1c00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.-Y.; Gránásy L.; Stifler C. A.; Zaquin T.; Chopdekar R. V.; Tamura N.; Weaver J. C.; Zhang J. A. Y.; Goffredo S.; Falini G.; Marcus M. A.; Pusztai T.; Schoeppler V.; Mass T.; Gilbert P. U. P. A. Crystal nucleation and growth of spherulites demonstrated by coral skeletons and phase-field simulations. Acta Biomater. 2021, 120, 277–292. 10.1016/j.actbio.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. U. P. A.; Porter S. M.; Sun C.-Y.; Xiao S.; Gibson B. M.; Shenkar N.; Knoll A. H. Biomineralization by particle attachment in early animals. Proc. Natl. Acad. Sci. 2019, 116, 17659–17665. 10.1073/pnas.1902273116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Yoreo J. J.; Gilbert P. U. P. A.; Sommerdijk N. A. J. M.; Penn R. L.; Whitelam S.; Joester D.; Zhang H.; Rimer J. D.; Navrotsky A.; Banfield J. F.; Wallace A. F.; Michel F. M.; Meldrum F. C.; Cölfen H.; Dove P. M. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349 (6247), eaaa6760. 10.1126/science.aaa6760. [DOI] [PubMed] [Google Scholar]
- De Stasio G.; Frazer B. H.; Gilbert B.; Richter K. L.; Valley J. W. Compensation of charging in X-PEEM: a successful test on mineral inclusions in 4.4 Ga old zircon. Ultramicroscopy 2003, 98 (1), 57–62. 10.1016/S0304-3991(03)00088-3. [DOI] [PubMed] [Google Scholar]
- DeVol R. T.; Metzler R. A.; Kabalah-Amitai L.; Pokroy B.; Politi Y.; Gal A.; Addadi L.; Weiner S.; Fernandez-Martinez A.; Demichelis R.; Gale J. D.; Ihli J.; Meldrum F. C.; Blonsky A. Z.; Killian C. E.; Salling C. B.; Young A. T.; Marcus M. A.; Scholl A.; Doran A.; Jenkins C.; Bechtel H. A.; Gilbert P. U. P. A. Oxygen spectroscopy and Polarization-dependent Imaging Contrast (PIC)-mapping of calcium carbonate minerals and biominerals. J. Phys. Chem. B 2014, 118 (28), 8449–8457. 10.1021/jp503700g. [DOI] [PubMed] [Google Scholar]
- Metzler R. A.; Zhou D.; Abrecht M.; Chiou J.-W.; Guo J.; Ariosa D.; Coppersmith S. N.; Gilbert P. U. P. A. Polarization-dependent imaging contrast in abalone shells. Phys. Rev. B 2008, 77, 064110. 10.1103/PhysRevB.77.064110. [DOI] [Google Scholar]
- Olson I. C.; Metzler R. A.; Tamura N.; Kunz M.; Killian C. E.; Gilbert P. U. P. A. Crystal lattice tilting in prismatic calcite. J. Struct Biol. 2013, 183, 180–190. 10.1016/j.jsb.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Ihli J.; Wong W. C.; Noel E. H.; Kim Y.-Y.; Kulak A. N.; Christenson H. K.; Duer M. J.; Meldrum F. C. Dehydration and crystallization of amorphous calcium carbonate in solution and in air. Nat. Commun. 2014, 5 (1), 3169. 10.1038/ncomms4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GG-Macros, http://home.physics.wisc.edu/gilbert/software.htm (accessed 12/17/2021).
- DeVol R. T.; Sun C.-Y.; Marcus M. A.; Coppersmith S. N.; Myneni S. C. B.; Gilbert P. U. P. A. Nanoscale transforming mineral phases in fresh nacre. J. Am. Chem. Soc. 2015, 137 (41), 13325–13333. 10.1021/jacs.5b07931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.