Abstract
Introduction
Tobacco is still one of the single most important risk factors among the lifestyle habits that cause morbidity and mortality in humans. Furthermore, tobacco has a heavy social gradient, as the consequences are even worse among disadvantaged and vulnerable groups. To reduce tobacco-related inequity in health, those most in need should be offered the most effective tobacco cessation intervention. The aim of this study is to facilitate and improve the evaluation of already implemented national tobacco cessation efforts, focusing on 10 disadvantaged and vulnerable groups of tobacco users.
Methods and analysis
This is a prospective cohort study. Data will be collected by established tobacco cessation counsellors in Sweden. The study includes adult tobacco or e-cigarette users, including disadvantaged and vulnerable patients, receiving in-person interventions for tobacco or e-cigarette cessation (smoking, snus and/or e-cigarettes). Patient inclusion was initiated in April 2020. For data analyses patients will be sorted into vulnerable groups based on risk factors and compared with tobacco users without the risk factor in question.
The primary outcome is continuous successful quitting after 6 months, measured by self-reporting. Secondary outcomes include abstinence at the end of the treatment programme, which could be from minutes over days to weeks, 14-day point prevalence after 6 months, and patient satisfaction with the intervention. Effectiveness of successful quitting will be examined by comparing vulnerable with non-vulnerable patients using a mixed-effect logistic regression model adjusting for potential prognostic factors and known confounders.
Ethics and dissemination
The project will follow the guidelines from the Swedish Data Protection Authority and have been approved by the Swedish Ethical Review Authority before patient inclusion (Dnr: 2019-02221). Only patients providing written informed consent will be included. Both positive and negative results will be published in scientific peer-reviewed journals and presented at national and international conferences. Information will be provided through media available to the public, politicians, healthcare providers and planners as these are all important stakeholders.
Trial registration number
Keywords: epidemiology, quality in health care, public health
Strengths and limitations of this study.
This national project is the first of its kind in Sweden and will provide new knowledge about the effectiveness of tobacco cessation interventions in ‘real life’.
This study has the potential to identify the most effective interventions to assist different vulnerable and disadvantaged groups of tobacco users to successfully quit.
If the current cessation interventions show limited effect for specific vulnerable groups, the results of the systematically collected data can be used to tailor programmes to specific groups of tobacco users in the future.
Self-reported outcome measure.
Introduction
Tobacco causes the development of the most common chronic diseases such as cardiovascular disease, cancer and respiratory diseases, for example, chronic obstructive pulmonary disease (COPD),1 and smokers have about doubled incidence of surgical complications.2
Overall, smoking is an independent and preventable risk factor responsible for up to 60% of the inequity in health. In Sweden smoking is still one of the most important risk factors3 causing morbidity and mortality. Every year, 12 000 Swedish citizens die prematurely from smoking.4 In addition, a Danish study found that the quality of life is significantly reduced in the shorter life course of smokers.5 In 2002, more than SEK18 billion was lost in production due to tobacco-related illness.6 The overall societal costs has been estimated to SEK75 billion per year.7
Tobacco cessation interventions are among the most cost-effective treatments within the healthcare system. A smoker who successfully quits at the age of 30 will gain approximately 10 life-years compared with a continuous smoker. The benefits decrease with increased age at smoking cessation; however, an average 50-year-old smoker will still gain 5–6 life-years from quitting. Smoking has a heavy social gradient, as its severe influence on health strikes even harder among disadvantaged and vulnerable groups.8–11
To reduce the tobacco-related inequity in health, it is pivotal to reach out to those most in need with the most effective cessation interventions, and it is of the highest priority in the guidelines for healthy lifestyle by The National Board of Health and Welfare.12 In addition policies, strategies and campaigns should be used to prevent new users from initiating tobacco use.13
Though the smoking prevalence is relatively low in Sweden in an international context, specific groups have a very high prevalence; about 80% in people who abuse alcohol or drugs.14 A similar extreme level of daily snus users was not seen in the group of people who abuse alcohol or drugs, where the prevalence was 24%–25%.14 Sweden has a unique high prevalence of snus users, with 18% daily users among men and 4% among women in 2016.15 At that time the daily smoking prevalence in Sweden was 8% and 10% for men and women, respectively, resulting in a daily tobacco prevalence of 25% for men and 14% for women.15 Regarding the use of e-cigarettes the prevalence of daily users in 2020 was 0.4% for both men and women.15 In addition, products such as cigarettes, snus and e-cigarettes are often mixed, and the negative impact would increase, accordingly.
Cessation programmes are increasingly offered to users of snus and e-cigarettes, as well as heated tobacco products, though the use of the last is still very low in Sweden.16 However, the effectiveness in different groups of users remains unknown.
Effectiveness of tobacco cessation interventions
It is widely accepted that tobacco cessation interventions should build on strong evidence,12 but implementation is difficult17 and the effect in real life is seldom followed up. In Denmark, data on smoking cessation interventions and follow-up on effect are systematically collected through the national Danish Smoking Cessation Database.18 With approximately 150 000 participants registered since 2001, the Danish Smoking Cessation Database is one of a kind. A European survey and a comprehensive web search has revealed a few other databases,19 such as the UK National Health Service stop smoking services.20 Through collaboration we are familiar with national projects in Ireland and the Czech Republic inspired by the Danish model, implementing a similar data collection.
Tobacco cessation activities in Sweden
Despite the fact that about a thousand counsellors have been trained in manual-based person-centred tobacco cessation interventions, following the general Swedish guidelines12 (ie, the intervention is tailored to the individual tobacco user, regarding tobacco profile, health profile, needs and preferences according to the clinical guideline, allowing for variations in length as well as in content) in Sweden,21 it is unknown how effective the interventions are. There is namely no systematic follow-up in Sweden except for the activities performed by the national quitline.9 22 Therefore, as of today it is not possible, on a national level, to compare the effectiveness of variations of the in-person interventions, providers, or different groups of tobacco users including disadvantaged and vulnerable groups.
During the last decade there has been a common interest among tobacco researchers in Sweden, to document the effectiveness of tobacco cessation interventions across the country. This interest is supported by the independent think tank ‘Tobaksfakta’,23 and a network of approximately 700 Swedish counsellors declared their support for the project at their autumn-meeting 2016. In addition, tobacco cessation counsellors in Region Skåne and in Region Örebro län have evaluated the effect of smoking cessation interventions based on the Danish model with good results for example, the follow-up-rate was drastically improved compared with usual routine. The evaluation was done by collecting data on smokers undertaking a smoking cessation intervention, and after informed consent data were collected without any problems or barriers. Based on this it seems both possible and realistic to document the effectiveness of the tobacco cessation interventions in this new national project.
Study aim
The purpose of this study is to facilitate and improve the evaluation of the national tobacco cessation efforts, emphasising on which programmes are most effective for different groups throughout Sweden. This means that we will evaluate the effectiveness of already implemented cessation interventions targeting smoking, use of snus and/or e-cigarettes, focusing on disadvantaged and vulnerable groups of tobacco or e-cigarette users compared with non-vulnerable users. Furthermore, we want to identify important factors associated with a successful outcome after controlling for confounders (in relation to programme, patients and setting). This national project is the first of its kind in Sweden.
Disadvantaged and vulnerable groups include tobacco users, for example, without a job, with short or no education, without permanent housing, diagnosed with mental illness, diagnosed with COPD, undergoing surgery, adolescents, elderly, migrants, and pregnant women.
The groups prioritised were mainly defined by the National Board of Health and Welfare in Sweden and WHO (pregnant women, patients undergoing surgery, persons with severe mental illness, adolescents, migrants and the elderly).12 The remaining groups were chosen by the authors based on needs described in clinical guidelines.
Research questions
Among daily smokers what is the effectiveness of in-person tobacco cessation interventions measured as successful quitting after 6 months, among disadvantaged and vulnerable groups compared with other smokers.
What are the most important predictors for successful/unsuccessful quitting smoking when using an adjusted model?
What are (1) and (2) for daily users of snus and/or users of e-cigarettes?
Study design
This is a prospective cohort study, based on establishing a systematic collection of individual data to evaluate the effectiveness of already established tobacco cessation interventions organised throughout Sweden.
The data collection is built on the Danish data collection model,18 including relevant adaptions to Swedish conditions.
We aim to recruit a total of 8000 tobacco users and the patient inclusion was initiated in April 2020, and we have extended the patient recruitment period till the end of 2022.
Setting
This study builds on the involvement of trained tobacco cessation counsellors throughout Sweden. The counsellors will recruit patients and collect data for the project. We hope to collaborate with at least 200 certified counsellors (counsellors can be certified at Örebro, Karolinska and Sahlgrenska University Hospitals, Karolinska Institute, National Tobacco Quit-Line and Lund University among others) in the initial phase of the project.
All the officially certified counsellors working with in-person person-centred cessation programmes regarding smoking, snus and e-cigarette will be invited to participate in the project. The counsellors can work in primary or secondary care, public or private clinics or other settings.
Counsellors wanting to take part in the study will sign an agreement in accordance with the project. After signing up, information, consent forms and manuals/tutorials for data collection are distributed to the counsellors, and the patient inclusion can begin.
A list of the sites that have collected data to the project will be available at ClinicalTrials.gov.
Tobacco cessation interventions
In this study, we will include person-centred tobacco or e-cigarette cessation interventions aimed at smoking, snus and/or e-cigarettes with face-to-face sessions only. Face-to-face sessions can be conducted as online video calls as well as on-site meetings.
Any in-person tobacco cessation intervention already implemented into the daily clinical routine among the tobacco cessation counsellors throughout Sweden can be included, regardless of intensity, supportive medication and methods used. Information on the intervention given will be recorded through the standard questionnaires used in the study.
Participants
All adult tobacco users (of at least 18 years of age), including disadvantaged and vulnerable patients, receiving an in-person intervention for tobacco or e-cigarette cessation (smoking, snus and/or e-cigarettes) are eligible for inclusion in the project after giving informed consent. Both individual and group-based interventions can be included.
Exclusion criteria are withdrawing consent, or reduced ability to give informed consent, due to inadequate language skills, dementia and other conditions.
Recruitment
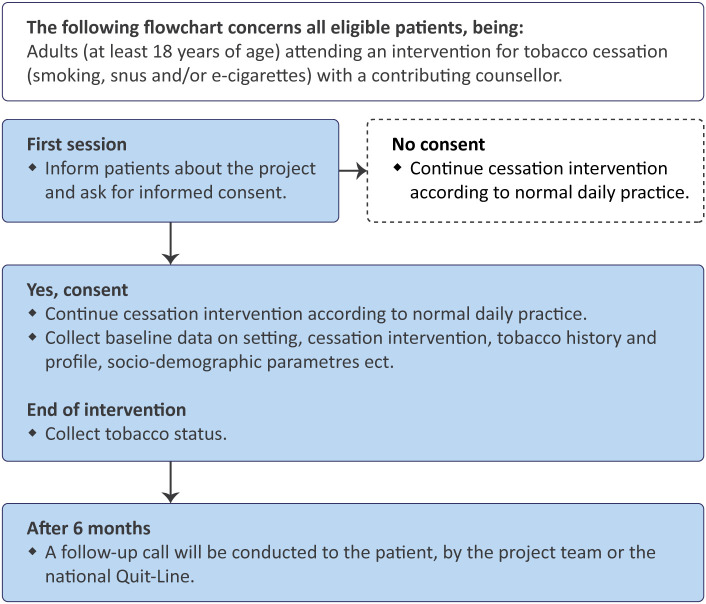
The contributing counsellors will inform all eligible patients about the project and ask for their informed consent to collect data on their cessation intervention (figure 1). If consent is not obtained, the treatment will continue according to the normal daily practice without further ado.
Figure 1.
Flow chart for the recruitment and data collection process.
After giving consent to be included in the project, the patient will likewise receive the treatment programme as planned. In addition, the counsellor will collect and document baseline information regarding the cessation activity and patient characteristics. At the end of the programme, the tobacco cessation status will be recorded. A manual-based follow-up call will be conducted after 6 months.
Data collection
Baseline date will be collected during the cessation intervention by the counsellors and the patients. Data questionnaires are filled in and mailed to the project data manager, who will enter the data into a Research Electronic Data Capture database, hosted at Lund University.24 25
All materials and questionnaires used are available on the project website (in Swedish).26
Baseline
After giving informed consent patients are included in the study and asked to fill in a questionnaire. The paper survey is filled in by the patient, with assistance from the counsellor. If necessary, the counsellor is allowed to read the questions to the patient and record the patient’s responses. All questions regarding tobacco use or quit attempts etc. are divided into three section (1) Smoking, (2) Use of snus and (3) Use of e-cigarettes. The baseline characteristics, include:
Years of smoking/snusing and using e‐cigarettes; current daily tobacco use (no/yes/not on a daily basis); previous quit attempts (none/1–3/>3/not using); cohabitating with a smoker (yes/no); healthcare personel who has encouraged the quitting (eg, general practitioner, hospital doctor, midwife, dentist); housing (eg, own house, rental, without permanent housing).
Social security number; level of education; employment; pregnancy (yes/no); planned surgery (yes/no); place of birth (Sweden/The Nordic countries/Europe/not Europe); mother tongue (Swedish/Nordic/European/not European).
Level of nicotine dependency (measured by Fagerström score (FTND)27 for smokers; and an adapted test used in the clinical setting for dependency among snus users, based on the Fagerström score for smokefree tobacco (FTND-ST)).28 29
The counsellors register details of the cessation intervention and process (both planned and performed), and follow-up at the end of the intervention, including:
Dates of initiating and ending the cessation intervention; date of quitting; setting.
Details of intervention method; individual/group format; group size; intensity of the intervention (number of meetings and duration); supplemental contacts); relapse prevention; user fees.
Compliance with the programme (treatment attendance); tobacco status at end of the programme.
Follow-up
Six months (±1 month) after the initial quit day a manual-based follow-up is conducted by calling each patient. To allow for a more objective evaluation the follow-up call will be conducted by a project team member (or personnel at the National Quit-Line) who had no contact with the patient before the follow-up call. This procedure will eliminate possible impact from the counsellor/patient interaction, as well as insure a unified follow-up procedure for all patients.
Follow-up data includes:
Continuous successful quitting since planned quit date (or alternatively since the end of the programme) and until the 6 months follow-up; 14 days point prevalence; user satisfaction; use and costs of pharmacologic support; present use of pharmacologic support (nicotine replacement therapy, bupropion, varenicline or other); interest in new cessation intervention.
For non-respondents: Reason for un-successful follow-up; for example, wrong telephone number, deceased, or not available.
If a patient does not want to participate in the follow-up or it is not possible to reach them by phone the reason for loss to follow-up is recorded. Before a patient is considered lost to follow-up at least four attempts to call on different times and days (at least one attempt must be after 17:00 hours) must be made.
Outcome
The primary outcome is self-reported continuous successful quitting after 6 months, measured from the planned quit day (or last day of the treatment if a specific quit date is not planned during the intervention) to the day of follow-up 6 months later. The planned quit day will be used as a time reference since the toxic effects of tobacco use should be terminated from that date. Continuous successful quitting is defined as smoking no more than one cigarette or similar concerning snus and/or e-cigarettes since the quit day.
We will be monitoring smoking, use of snus and use of e-cigarettes, as successfully quitting one of the above, may lead to an increased use of one or more of the others.
Secondary outcomes
Several secondary outcomes will be recorded, such as 14 days point prevalence (defined as not smoking/using at all (not even a puff) for the latest 14 days), tobacco abstinence at the end of the intervention and satisfaction with the intervention.
Comparators
The objective of this study is to facilitate and improve the evaluation of already implemented national tobacco cessation efforts, focusing on 10 disadvantaged and vulnerable groups of tobacco users. For data analyses, the patients will be sorted into ten different vulnerable groups based on risk factors and compared with tobacco users without the risk factor in question.
The vulnerable groups will be categorised according to the information collected by the tobacco cessation counsellor, and all patients will be cross-linked with additional data from the Swedish National Patient Register to extract relevant diagnoses to uncover, for example, COPD, severe mental illness or recently giving birth.30
Analytical strategy
Sample size
The sample size was calculated for the dichotomous main outcome (successful quitting (yes/no) after 6 months) and based on the following assumptions: a two-sided test, a 5% level of significance, a power of 80%, an estimated effect in the control group of 35%, and a minimum relevant difference of 5–10 percentage points.
The online calculator ‘Inference for Proportions: Comparing Two Independent Samples’ (www.stat.ubc.ca/%7Erollin/stats/ssize/) was used to estimate the necessary sample size of each group. Based on a minimal relevant difference on 10% and 5% each group should include at least 329 and 1377 tobacco users, respectively. As the study groups in this study are not equal-sized, the sample size gives the estimated size of the smallest group (the vulnerable group in question).
We expect to include 8000 patients. Based on the overall existing interest from the tobacco cessation counsellors, at least 200 of them are each expected to collect data from at least 20 patients/year. The large majority of potential patients are expected to accept inclusion and follow-up.31 To be able to manifest a difference in effect size of 10%, 4% for the included patients would have to belong to each of the given risk factors (vulnerable groups). To show a difference of 5%, this would be the case for 17% of the included patients.
Statistical analyses
Data will be analysed and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.32 After controlling for confounders, the effectiveness in the different groups of vulnerable patients is compared with the patients without the given risk factor. Differences between counsellors will be taken into consideration by deploying a mixed-effects model adjusted for hierarchical clustering using the different smoking cessation clinics reporting to the project. Each clinic is identified with its own unique ID-number, and the first level cluster will be composed of the group of patients registered in the same smoking cessation clinic.
Relevant univariable and multivariable analyses will be used to analyse differences in continuous successful quitting. The final multivariable logistic regression model will be fitted, based on initial univariable tests, and common knowledge, to include relevant variables. Potential predictors including confounders concerning patients, intervention and tobacco cessation clinic will be included, and as a minimum the following will be examined:
Patients: Sex, age, compliance with the intervention, tobacco/e-cigarette history, level of nicotine dependency, previous quit attempts, living with a smoker, level of education, job situation and belonging to more than one vulnerable group.
Intervention: Intensity, individual or group sessions, and treatment method.
Clinic: Setting and geographical location.
Statistically significant predictors of continuous successful quitting will be identified. Results will be presented as OR and corresponding 95% CI, and a two-sided p≤0.05 will be considered as statistically significant.
We expect to encounter both missing data and loss to follow-up. Depending on the size and nature of missing data they will be handled accordingly.20 33 If the proportion of missing data is small (<5%) missingness will be considered negligible and removed from the analysis. If possible multiple imputation will be used to deal with missing data. Otherwise, sensitivity analysis will be performed to explore the possible impact of the missing data.
Regarding the lost to follow-up we do not anticipate data to be missing at random but more likely lost to follow-up will be missing not at random. Hence a best-worst and worst-best case imputation will be carried out to investigate the theoretical uncertainty of the study results.20 33
All statistical calculations will be performed using STATA.IC V.16 or a later version.
Dissemination
Both positive and negative results of the project will be published in scientific peer-reviewed journals as well as being presented at national and international conferences. All authors must meet the Vancouver criteria.
Information about the project and results will be disseminated throughout the project time via a public homepage and other media available to the public, politicians, healthcare providers and planners as these are all important stakeholders.
Ethical considerations
Participants are included only after informed consent. The consent can be withdrawn at any time without explanation and without any influence on the treatment programme.
The project will follow the guidelines from the Swedish Data Protection Authority and have been approved by the Swedish Ethical Review Authority before the patient inclusion (Dnr: 2019-02221.)
All research data remains confidential, and it will never be possible to recognise individuals when data is presented and published. Financing of the project, institutional affiliations and potential conflicts of interest will also be published.
Data statement
After publication of study results technical appendix, statistical code and anonymised datasets will be available on reasonable request to the corresponding author.
Patient and public involvement
Patient and public have not been involved in the planning of the study, and there are no current plans of involvement.
Discussion
Updates on the global burden of diseases show that tobacco is still a major risk factor for physical illness in Sweden.34 Though cessation interventions are one of the most cost-effective interventions in the healthcare system, there is no national systematic registration of how many and which groups of tobacco users are treated or about the effect of the interventions in Sweden. However, focusing tobacco cessation services on disadvantaged and vulnerable tobacco users is a key to reduce tobacco related health inequity.11 This study will close a major knowledge gap regarding which programmes that work best for different groups of users in different settings, clinics and regions in Sweden.
Effect of tobacco cessation intervention in disadvantaged and vulnerable groups
The intensity of the cessation programmes seems to be of major importance for successful quitting.35 Already, in the year of 2000, the term ‘intensive smoking cessation intervention’ was defined internationally as a face-to-face programme with at least 4 meetings of at least 10 min.36 37
A non-intensive standard programme in the UK showed weak effect among low socioeconomic groups in real life setting.38 In contrast, the Danish standard intensive cessation intervention is effective in real life settings across socioeconomic groups, for heavy smokers, pregnant women, elderly smokers, smokers scheduled for surgery and mentally ill smokers.39–44 In addition, the Irish results also favour intensive programmes (unpublished data). The current project will add knowledge about the effect of the Swedish cessation interventions.
Snus and e-cigarettes cessation interventions
E-cigarettes are tested as a specific treatment for smoking cessation with contradictory results. A recent study showed that smokers also using e-cigarettes have a lower quit rate compared with smokers not using e-cigarettes simultaneously.45 46 A Swedish study has shown that it is possible to quit the use of snus by similar pharmacological support, traditionally used in the smoking cessation programmes.47 Still, research is lacking on quitting e-cigarettes, themselves.
What this study adds
This project provides new knowledge about the effectiveness of tobacco cessation interventions in the ‘real-life setting’.
Our study has potential to contribute to this research area, as it is highly relevant to identify how these specific groups of tobacco users can get the best possible help to successful quitting. If the current cessation interventions show limited effect for specific groups of smokers, the obtained results and knowledge can be used to tailor programmes to specific groups of smokers and tobacco users in the future. This will be of great importance for the individual patient, as it will be beneficial to public health and the socioeconomy in general, to offer the best programmes in the future. This will further contribute to evening out the inequality in health.
A positive side effect would be the possibility to consolidate the culture of systematic monitoring, follow-up and dissemination of effect after the project, which raises the awareness of effectiveness and exchange of knowledge among cessation provides across sectors. The project can also stimulate a rise in the interest in research and development of methods among the participating tobacco cessation providers. Furthermore, the systematic data collection can contribute to an administrative relief and be timesaving for the counsellors, time which can be spent treating tobacco addiction instead.
Supplementary Material
Footnotes
Twitter: @mettemyr
Contributors: HT, MR, ML, HG, JA, AP and ARH designed the study. SW and TB-H made contributions to the conception and design of the project. All authors contributed to the methodology of the study. MR and HT drafted the manuscript. All authors read, revised and approved the manuscript.
Funding: This research project is supported by FORTE, Swedish Research Council for Health, Working life and Welfare, grant number: 2017-01681 and 2021-01714.
Competing interests: HG: Doctors Against Tobacco (unpaid NGO chair). AP: Nurses against Tobacco (unpaid NGO vice chair); NGO Tobaksfakta-independent think tank (paid general secretary). SW: Received in total £4650 from Pfizer AB, and £490 from Sanofi, for lectures and education about smoking cessation. ML: Received in total £3500 from Phizer AB, £3500 from ASTRA Zenega AB, £1500 from MSD, and £1000 from Boehringer Ingelheim AB, all for lectures, speech, or education about smoking cessation and/or smoking and COVID-19.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Swedish Ethical Review Authority (Dnr: 2019–02221.)
References
- 1.U.S. Department of Health and Human Services . The health consequences of smoking: a report of the surgeon General 2004, 2012: 5–8. [Google Scholar]
- 2.Tønnesen H, Nielsen PR, Lauritzen JB, et al. Smoking and alcohol intervention before surgery: evidence for best practice. Br J Anaesth 2009;102:297–306. 10.1093/bja/aen401 [DOI] [PubMed] [Google Scholar]
- 3.Allebeck P, Agardh E. Den globala sjukdomsbördan har både minskat och ökat - Uppdateringen av det globala sjukdomsbördeprojektet är nu klar. Lakartidningen 2017;114:ED34. [PubMed] [Google Scholar]
- 4.Socialstyrelsen . Registeruppgifter Om tobaksrökningens skadeverkningar, 2014. [Google Scholar]
- 5.Brønnum-Hansen H, Juel K. [Health life years lost due to smoking]. Ugeskr Laeger 2002;164:3953–8. [PubMed] [Google Scholar]
- 6.Bolin K, Lindgren B. Rökning - produktionsbortfall och sjukvårdskostnader. Statens Folkhälsoinstitut, 2004. [Google Scholar]
- 7.Tobaksfakta.se . ”Skrämmande – men inget nytt att rökningen kostar” n.d. Available: http://www.tobaksfakta.se/skrammande-men-inget-nytt-att-rokningen-kostar-2/ [Accessed 25 Feb 2019].
- 8.Hiscock R, Bauld L, Amos A, et al. Socioeconomic status and smoking: a review. Ann N Y Acad Sci 2012;1248:107–23. 10.1111/j.1749-6632.2011.06202.x [DOI] [PubMed] [Google Scholar]
- 9.Nohlert E, Öhrvik J, Helgason AR. Effectiveness of proactive and reactive services at the Swedish national tobacco Quitline in a randomized trial. Tob Induc Dis 2014;12:9. 10.1186/1617-9625-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Tobacco and inequities, 2014. [Google Scholar]
- 11.Eikemo TA, Hoffmann R, Kulik MC, et al. How can inequalities in mortality be reduced? A quantitative analysis of 6 risk factors in 21 European populations. PLoS One 2014;9:e110952. 10.1371/journal.pone.0110952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Socialstyrrelsen . Nationella riktlinjer för prevention och behandling Vid ohälsosamma levnadsvanor Stöd för styrning och ledning, 2018. [Google Scholar]
- 13.Hill S, Amos A, Clifford D, et al. Impact of tobacco control interventions on socioeconomic inequalities in smoking: review of the evidence. Tob Control 2014;23:e89–97. 10.1136/tobaccocontrol-2013-051110 [DOI] [PubMed] [Google Scholar]
- 14.Hovhannisyan K, Rasmussen M, Adami J, et al. Evaluation of very integrated program: health promotion for patients with alcohol and drug Addiction-A randomized trial. Alcohol Clin Exp Res 2020;44:1456–67. 10.1111/acer.14364 [DOI] [PubMed] [Google Scholar]
- 15.Folkhälsomyndigheten. Nationella Folkhälsoenkäten: tobak n.d. Available: http://fohm-app.folkhalsomyndigheten.se/Folkhalsodata/pxweb/sv/B_HLV/B_HLV__aLevvanor__aagLevvanortobak/?rxid=19215807-23cd-44cf-8f63-b1eed980d297 [Accessed 31 Aug 2021].
- 16.Ebbert JO, Elrashidi MY, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database Syst Rev 2015:CD004306. 10.1002/14651858.CD004306.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svane JK. Fast-Track implementation of clinical health promotion. Clinhp 2018;8:1–58. 10.29102/clinhp.18002s [DOI] [Google Scholar]
- 18.Rasmussen M, Tønnesen H. The Danish smoking cessation database. Clinhp 2016;6:36–41. 10.29102/clinhp.16006 [DOI] [Google Scholar]
- 19.Rasmussen M. Intensive Smoking Cessation Interventions in Denmark - Based on data from the Danish Smoking Cessation Database. Clin Heal Promot 2018;8:1–43. [Google Scholar]
- 20.Stop smoking treatments - NHS n.d. Available: https://www.nhs.uk/conditions/stop-smoking-treatments/ [Accessed 27 Apr 2021].
- 21.Landgren AJ, Gilljam H. Barriers and supportive factors in certified tobacco cessation counselors in Sweden. Tob Prev Cessat 2019;5:4. 10.18332/tpc/102995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobaksfakta n.d. Available: http://www.tobaksfakta.se/ [Accessed 21 Jan 2017].
- 23.Tobaksfakta.se about the tobacco cessation project n.d. Available: https://www.rokstoppsprojektet.org/sagt-om-rökstoppsprojektet [Accessed 18 Mar 2021].
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rökstoppsprojektet. Blanketter | Rökstoppsprojektet n.d. Available: https://www.rokstoppsprojektet.org/blanketter [Accessed 23 Apr 2021].
- 27.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Addiction 1991;86:1119–27. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 28.Ebbert JO, Patten CA, Schroeder DR. The Fagerström test for nicotine Dependence-Smokeless tobacco (FTND-ST). Addict Behav 2006;31:1716–21. 10.1016/j.addbeh.2005.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snustest (in Swedish) n.d. Available: https://thl.fi/documents/10531/105429/thl_nuuskatesti_se.pdf [Accessed 26 Mar 2021].
- 30.Information available in the National patient register (NPr), 2019. Available: https://www.socialstyrelsen.se/register/halsodataregister/patientregistret/inenglish [Accessed 23 Jan 2019].
- 31.Tønnesen H, Ekfors H, Raffing R. Health promoting attitude from a patient and staff perspective: Eksperiences and preferences, 2014. [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 33.Little RJ, D'Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med Overseas Ed 2012;367:1355–60. 10.1056/NEJMsr1203730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbafati C, Machado DB, Cislaghi B, et al. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1223–49. 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Hajj MS, Kheir N, Al Mulla AM, et al. Effectiveness of a pharmacist-delivered smoking cessation program in the state of Qatar: a randomized controlled trial. BMC Public Health 2017;17:215. 10.1186/s12889-017-4103-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.A clinical practice guideline for treating tobacco use and dependence: a US public health service report. the tobacco use and dependence clinical practice guideline panel, staff, and Consortium representatives. JAMA 2000;283:3244–54. [PubMed] [Google Scholar]
- 37.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff . A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med 2008;35:158–76. 10.1016/j.amepre.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiscock R, Murray S, Brose LS, et al. Behavioural therapy for smoking cessation: the effectiveness of different intervention types for disadvantaged and affluent smokers. Addict Behav 2013;38:2787–96. 10.1016/j.addbeh.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kehlet M, Schroeder TV, Tønnesen H. The gold standard program for smoking cessation is effective for participants over 60 years of age. Int J Environ Res Public Health 2015;12:2574–87. 10.3390/ijerph120302574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neumann T, Rasmussen M, Ghith N, et al. The gold standard programme: smoking cessation interventions for disadvantaged smokers are effective in a real-life setting. Tob Control 2013;22:e9. 10.1136/tobaccocontrol-2011-050194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmussen M, Heitmann BL, Tønnesen H. Effectiveness of the gold standard programmes (Gsp) for smoking cessation in pregnant and non-pregnant women. Int J Environ Res Public Health 2013;10:3653–66. 10.3390/ijerph10083653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neumann T, Rasmussen M, Heitmann BL, et al. Gold standard program for heavy smokers in a real-life setting. Int J Environ Res Public Health 2013;10:4186–99. 10.3390/ijerph10094186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghith N, Ammari ABH, Rasmussen M. Impact of compliance on quit rates in a smoking cessation intervention: population study in Denmark. Clin Heal Promot 2012;2:111–9. 10.29102/clinhp.12016 [DOI] [Google Scholar]
- 44.Rasmussen M, Klinge M, Krogh J, et al. Effectiveness of the gold standard programme (Gsp) for smoking cessation on smokers with and without a severe mental disorder: a Danish cohort study. BMJ Open 2018;8:e021114. 10.1136/bmjopen-2017-021114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev 2014:CD010216. 10.1002/14651858.CD010216.pub2 [DOI] [PubMed] [Google Scholar]
- 46.Kalkhoran S, Glantz SA. E-Cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med 2016;4:116–28. 10.1016/S2213-2600(15)00521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fagerström K, Gilljam H, Metcalfe M, et al. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ 2010;341:c6549. 10.1136/bmj.c6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.