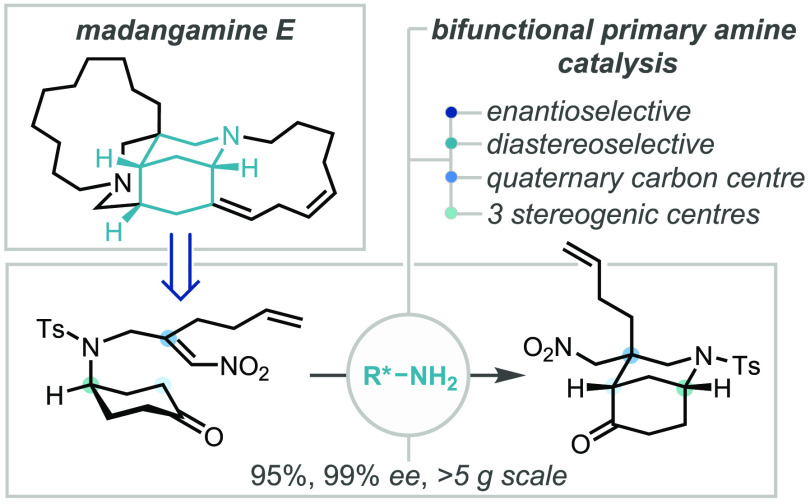
Abstract
The enantioselective total synthesis of madangamine E has been completed in 30 steps, enabled by a new catalytic and highly enantioselective desymmetrizing intramolecular Michael addition reaction of a prochiral ketone to a tethered β,β′-disubstituted nitroolefin. This key carbon–carbon bond forming reaction efficiently constructed a chiral bicyclic core in near-perfect enantio- and diastereo-selectivity, concurrently established three stereogenic centers, including a quaternary carbon, and proved highly scalable. Furthermore, the pathway and origins of enantioselectivity in this catalytic cyclization were probed using density functional theory (DFT) calculations, which revealed the crucial substrate/catalyst interactions in the enantio-determining step. Following construction of the bicyclic core, the total synthesis of madangamine E could be completed, with key steps including a mild one-pot oxidative lactamization of an amino alcohol, a two-step Z-selective olefination of a sterically hindered ketone, and ring-closing metatheses to install the two macrocyclic rings.
Introduction
New methods for the efficient elaboration of complex enantioenriched sp3-rich three-dimensional molecular scaffolds are of great value to synthetic chemistry and are highly coveted reactions. Enantioselective desymmetrization represents a powerful strategic framework within which to develop such reactions and enables the conversion of relatively simple achiral starting materials to high-value stereochemically rich products.1 Furthermore, given the prominent role that natural products are forecast to play in the future of medicinal chemistry,2 efficient routes that enable rapid generation of structural and stereochemical complexity are of particularly high importance. We therefore envisioned that the development of highly enantio- and diastereoselective organocatalytic desymmetrization reactions and their application in natural product synthesis would elegantly demonstrate the enabling power and scope of such a synthetic strategy.1d
The madangamine natural product family, isolated from marine sea sponges of the Xestospongia genus,3 are characterized by an architecturally complex pentacyclic fused-ring system possessing two nitrogen atoms (Scheme 1A). Three of the rings constitute a diazatricyclic core (ABC rings) that is common to all family members (madangamines A–F), as is the macrocyclic E ring (with the exception of madangamine F), while the D ring differs in each and every madangamine. We were attracted to this structurally unique family and reasoned that, via judicious application of an enantioselective desymmetrization reaction, we would be able to install all the ensuing stereochemical information from this single chirality inducing step.
Scheme 1. (a) The Madangamine Family and Divergence in the D Rings of Members A–E; (b) Prior Total Syntheses of Madangamine Natural Products; (c) Our Retrosynthetic Strategy to Madangamine E.

Since their isolation, the madangamines have attracted significant attention from the synthetic community with numerous reported strategies to polycyclic core fragments.4 However, there exist only two reports of total syntheses of members of the family and a single formal synthesis of madangamine A (Scheme 1B).5 In 2014, Amat reported the first asymmetric total synthesis of madangamine D, employing a stereoselective cyclocondensation of phenylglycinol to establish an enantio-enriched bicyclic (rings BC) scaffold. In 2017, Chida and Sato’s unified total synthesis of madangamine’s A, C and E demonstrated the divergent application of a late-stage tetracyclic intermediate. This intermediate was constructed via a key N-acyl-iminium cyclization of an enantioenriched octahydronaphthyridine (rings AB) derivative, and its elaboration hinged upon a highly stereoselective allene hydroboration to install the Z-configured double bond observed in the macrocyclic E ring.
Furthermore, the biological properties of these unique natural products are of growing interest; a recent study uncovered a novel lysosome inhibition pathway following treatment with madangamine A,6 resulting in antiproliferation of human cancer cell lines. This further supported Amat’s work which highlighted the cytotoxic activity of madangamine D against human cancer cell lines as well as the original isolation study from Andersen, investigating the cytotoxicity of madangamine A.3a,5a
In contrast to the previous synthetic strategies, we recognized that the AC bicyclic motif would be well-suited to construction from a catalytic enantioselective desymmetrization reaction while also providing a rigid scaffold onto which the remaining madangamine structural features could be elaborated with high stereochemical fidelity. Toward this end, we wish to report our findings, including the discovery of a novel, highly enantioselective and efficient organocatalytic desymmetrization reaction of trisubstituted nitroolefin-linked cyclohexanones and its application to the total synthesis of madangamine E.
Tackling the pentacyclic skeleton retrosynthetically (Scheme 1C), the end-game to madangamine E (1) would rely on the functionalization of tetracyclic ketone 3. Two promising strategies for this macrocycle construction included mirroring Amat’s stereoselective Wittig approach using a cis-configured octenoate phosphonium ylide or, alternatively, introducing two alkyl chains bearing terminal alkenes that could be connected via a ring-closing metathesis (RCM) reaction. Our aim was to access ketone 5 in isomerically pure form from prochiral cyclohexanone 6 using a highly enantio- and diastereo-selective desymmetrization reaction. Functional group manipulation, an oxidative lactamization, and ring-closing metathesis would complete the B and D rings (5 to 3, via 4).
Pivotal to our synthetic strategy was the development and deployment of an unprecedented enantioselective desymmetrizing Michael addition of a 4-aminocyclohexanone derivative possessing a tethered β,β′-disubstituted nitroolefin 6.
As inspiration for this key disconnection, we took the related enantioselective desymmetrising intramolecular Michael addition to α,β-unsaturated esters previously reported by our group using bifunctional primary amine thiourea organocatalysts (Scheme 2A).7 However, this work focused exclusively on unsaturated ester Michael acceptors and many potential synthetic challenges remained in expanding this transformation to challenging β,β′-disubstituted nitroolefins, while maintaining the high enantio- and diastereoselectivity previously observed. Furthermore, while enantioselective intermolecular Michael addition reactions with nitroolefins have been well-established, intramolecular desymmetrising variants that concurrently establish a quaternary carbon center are unreported.8−11
Scheme 2. (a) Prior Work on Related Enantioselective Desymmetrisation of Cyclohexanones; (b) Catalyst Screen for the Desymmetrizing Intramolecular Michael Addition of Nitroolefin 7 (n.d. = not detected, SCXRD = single crystal X-ray diffraction); (c) Proposed Catalytic Cycle of the Enantioselective Desymmetrization of Nitro-olefin-Tethered Cyclohexanones Catalyzed by a Primary-Amine Thiourea Organocatalyst, Including Off-Cycle Species Investigated Computationally.

Results and Discussion
Methyl-substituted nitroolefin 7 was selected as an ideal model substrate to develop the enantioselective intramolecular Michael addition reaction. An oxidative radical nitration was employed to efficiently construct 7 in three steps from commercially available 1,4-cyclohexanedione monoethylene acetal (see SI, Scheme S1).12 With 7 in hand, the performance of a range of chiral single enantiomer primary and cyclic secondary amine organocatalysts was investigated, including trans-cyclohexanediamine- and proline-derived scaffolds (Scheme 2B), in dichloromethane, at 20 mol % loading, in the presence of benzoic acid as a cocatalyst. Most of the tested catalysts (9a–9d, 9g–9k) did not provide cyclized product 8 but gave rise instead to complex product mixtures.
Despite this unfavorable and challenging reactivity, Jacobsen’s thiourea catalyst 9f, which previously gave one of the best results for the organocatalytic desymmetrization of α,β-unsaturated esters, provided an almost uniquely efficient reaction profile and gave the desired bicyclic Michael adduct 8 in 70% yield as a single diastereomer in 99% ee. The relative and absolute stereochemical configuration of 8 were established by single-crystal X-ray diffraction analysis (see SI).13 Furthermore, a simplified bifunctional primary amine catalyst 9e also yielded 8 in 51% yield as a single diastereoisomer in 96% ee.
To further understand the nature of this reaction, including such a narrow catalyst structure allowance, the intramolecular Michael addition of nitroolefin 7 was investigated computationally as a model substrate using DFT calculations (Scheme 2C, Scheme 3). The computed reaction pathway begins with an addition reaction of the primary amine of the catalyst to the ketone substrate to form a hemiaminal intermediate Int1, which, after elimination of water, then forms imine Int2.14 Despite the formation of this imine being endergonic, it is likely that the thiourea activates the ketone and promotes collapse of the hemiaminal. Enamine Int3 is then formed by tautomerization of the imine Int2. A conformational search for the transition structure of the key stereoselectivity-determining C–C bond forming intramolecular Michael addition identified 16 possible structures (see SI, Figure S2). TS1 emerged as the most energetically favorable transition structure by ΔΔG‡ = 3.0 kcal mol–1 compared to the second-lowest-energy transition structure, amounting to a computed 99% ee that is in excellent agreement with the experimental enantioselectivity (99% ee). This Michael addition is highly exergonic (ΔGrxn = −19.4 kcal mol–1) and furnishes an iminium-nitronate species Int4, which has been proposed as a reaction intermediate by several other groups in related, but distinct reactions.15 Therefore, this C–C bond formation through TS1 is an irreversible and stereoselectivity determining step, and the kinetically preferred TS is stabilized, compared to other possible TSs, because it adopts a low energy conformation that additionally benefits from several hydrogen bonding interactions with the thiourea, nitro group, and enamine moieties, consistent with a cooperative push/pull-type mechanism (see SI, Figure S3).16
Scheme 3. Computed Reaction Energy Profile (ΔG in kcal mol–1) for the Pathway through the Lowest-Energy Intramolecular Michael Addition Transition Structure Leading to the Morphan Core Computed at COSMO(DCM)-ZORA-M06-2X/TZ2P//COSMO(DCM)-ZORA-BLYP-D3(BJ)/DZP.

Following C–C bond formation, we considered several pathways from the iminium-nitronate species Int4 to obtain the cyclized product. The energy barrier for intermolecular protonation of Int4 by benzoic acid through TS2 is lower than the one for intramolecular protonation by the thiourea, and this process is faster than the reverse C–C bond cleavage reaction, indicating that the enantio- and diastereoselectivities are, indeed, determined by the kinetically controlled intramolecular Michael addition (see SI for more details). Interestingly, when the nitro group is computationally replaced with an α,β-unsaturated ester, the DFT calculations indicate that the intramolecular protonation reaction is preferred over the intermolecular protonation.7 It is expected that this difference in mechanism is the result of the pKa difference between the nitronate and enolate [pKa = 17 (nitro) < 20 (thiourea) < 30 (ester)]. In other words, the deprotonation of the thiourea by the enolate is favorable, whereas the deprotonation by the nitronate is unfavorable. The formation of dihydrooxazine oxide intermediate IV (Scheme 2C) and its reactivity were also studied, but this pathway is energetically unfavorable and goes with a higher energy barrier than TS2 (see SI, Figure S4). The cyclobutane intermediate V can be formed from Int4 prior to the intermolecular protonation process; however, the highly strained species is energetically unstable and can easily reopen with a low energy barrier. Lastly, O-protonation of the nitronate VI by a proton transfer process from the iminium is also facile; however, the formed aci-nitro species VII is less basic at the α-position than the corresponding nitronate VI. Therefore the α-protonation process from this species does not occur. After the protonation step, the resulting complex Int6 is hydrolyzed through the hemiaminal species Int7 to furnish the product with the experimentally observed stereochemical configuration, as confirmed by single crystal X-ray diffraction studies. This energetically most favorable pathway constitutes a catalytic cycle summarized in Scheme 2C.
Following the discovery of this highly efficient, enantio- and diastereoselective, organocatalytic desymmetrization reaction on the model nitroolefin, its application in the total synthesis of the madangamine natural products, in particular madangamine E, was investigated. To introduce the requisite functional handles, nitroolefin 17, possessing a β-butenyl substituent, was required; however, translation of the previously successful nitration chemistry failed and, accordingly, a new, high yielding and scalable route to access 17 was sought.
Our restructured route to access nitroolefin 17 (Scheme 4A) began with a reductive amination of 1,4-cyclohexanedione monoethylene acetal (10) with allylamine and subsequent N-tosylation, to give tosyl amide 11 over 2 steps. Oxidative cleavage of the terminal alkene, Henry reaction, and then dehydration, mediated by mesyl chloride and base, gave β-substituted nitroolefin 13 (72% yield over 3 steps). Installation of the butenyl chain was achieved by Michael addition reaction of an organo-zinc–copper complex, as reported by Denmark,17 to give the branched nitroalkane 14 in 90% yield and proved highly scalable (17 g scale). Selenoxide elimination chemistry proved most effective for the installation of the nitroolefin (see SI) and proceeded in 53% yield over 3 steps (E/Z = 3.8:1; see SI for confirmation of stereochemical configuration), followed by a final deprotection of the ketal, to reveal desymmetrization precursor 17E/Z. It was observed that the efficiency of the selenoxide elimination demonstrated strong dependence on the solvent mixture employed in the reaction, with Et2O/CH2Cl2 (2:1, 0.033 M) proving optimal.
Scheme 4. Preparation of Nitroolefin 17 and Key Enantioselective Desymmetrization Reaction.
The newly developed route provided both scalable and efficient access to multigram quantities of the desired β,β′-disubstituted nitroolefin, enabling investigation of the key organocatalytic enantioselective desymmetrization (Scheme 4B). Pleasingly, treatment of 17E with 20 mol % catalyst 9f in the presence of 20 mol % benzoic acid provided the desired bicyclic nitroalkane 18 in excellent yield (95%) and near-perfect enantio- and diastereoselectivity (>99% ee, single diastereomer, >5 g scale). The relative and absolute stereochemical configuration of 18 were determined through single crystal X-ray diffraction analysis of the corresponding enone S9 (see SI).13
With the successful realization of a catalytic, expedient and highly enantioselective synthesis of the bicyclic core, synthetic efforts could focus on advancing the synthesis toward madangamine E (Scheme 5). Elaboration of bicyclic ketone 18 toward the key intermediate 4 required reduction of the nitro group, and β-hydroxylation, with a subsequent one carbon homologation, at the ketone moiety. Attempts to reduce the nitro group to an amine in the presence of the unprotected ketone led to an undesired pyrrolidine-containing product. Therefore, we investigated ketone protection and observed that, upon treatment with catalytic p-toluenesulfonic acid in methanol, methyl enol ether 19 was observed in good yield. Given the unexpected stability of the enol ether—attributed to the sterically hindered bicyclic skeleton—the synthesis was advanced from 19. Reduction of the nitro group, with LiAlH4, provided a primary amine which could be protected as the Boc-PMB-amine and then trivially converted to ketone 20, with a total yield of 70% yield over 4 steps—notably, employing the methyl enol ether as a convenient protecting group for such a bicyclic ketone.
Scheme 5. Total Synthesis of Madangamine E.

The challenge of both homologating the ketone in 20 while introducing oxygenation at the β-position was succinctly achieved by adapting Garg’s three-step strategy developed in the synthesis of the akuammiline natural products.18 The dehydrogenation of ketone 20 was rigorously investigated, with Nicolaou’s IBX-NMO-mediated oxidation reaction proving optimal and affording enone 21 in moderate yield.19 Epoxidation of 21, with hydrogen peroxide and aqueous NaOH in MeOH, introduced the requisite oxygenation at C3 and proceeded in high yield. The Wittig homologation, using (methoxymethyl)triphenylphosphonium chloride and NaHMDS as base, was investigated to effect the simultaneous ketone homologation and epoxide ring opening. Under standard reaction conditions, complete consumption of the substrate was observed; however, the yield of aldehyde 23 was low (26%). By incorporating a rigorous reaction mixture quench using saturated aqueous NH4Cl, at 60 °C for 3 h, following treatment of 22 with the phosphonium ylide, the yield of desired aldehyde 23 could be improved to 86% (b.r.s.m 99%; see SI). Sequential aldehyde reduction, and partial amine deprotection steps were successfully carried out with sodium borohydride and ceric ammonium nitrate (CAN), giving diol 24 in 77% yield over 2 steps. Following Boc-deprotection with trifluoroacetic acid, a reductive amination with oct-7-en-1-al, in the presence of sodium triacetoxyborohydride and acetic acid, was employed to install the remaining carbon atoms of ring D and thus provide aminodiol 4 in 79% yield over 2 steps. Closure of the B ring was efficiently enabled by an oxidative lactamization employing Iwabuchi’s modification to Stahl’s oxidation conditions, accompanied by the desired concurrent oxidation of the secondary alcohol.20 In this way, treatment of 4 with catalytic quantities of AZADO, copper chloride, 2,2′-bipyridine, and DMAP, open to air, smoothly facilitated construction of the B ring system to afford the desired tricyclic enone 25 in good yield. This elegantly establishes the utility of such mild oxidative lactamization reactions in the synthesis of complex alkaloid skeletons. A RCM reaction of 25 was carried out with Grubbs first generation catalyst in CH2Cl2, under high dilution conditions, at 40 °C to close the D ring efficiently, giving tetracyclic enone 26 in 82% yield.5a Subsequent treatment of 26 with palladium on carbon under ∼1 bar hydrogen atmosphere gave saturated ketone 3. Overreduction product S6 accounted for the rest of the material and could be converted back to ketone 3, employing catalytic AZADO and PIDA as the oxidant, in high yield.
Elaboration of ketone 3 proved highly challenging, with it resisting almost all trialled conditions and deprotonation consistently out-competing the desired functionalization attempts. Notably, attempted application of Amat’s Wittig reaction of an octenoate phosphonium salt was largely unsuccessful despite extensive experimentation and invaluable advice from the original authors. We concluded that the subtly different bond geometries and flexibility of ketone 3, as compared to Amat’s related substrate, render it significantly more sensitive to a competing deleterious deprotonation pathway. Ultimately, following rigorous investigation, ketone 3 was found to react with nonbasic, yet nucleophilic organocerium reagents.21 Incorporation of a butenyl chain was possible in high yield, employing the butenyl cerium reagent derived from the corresponding Grignard reagent; however, the reaction proved highly capricious and was particularly sensitive to the quality of the organocerium reagent. In the search for a more reproducible and operationally straightforward transformation, triorganozincate reagents, reported by Ishihara as non-basic nucleophilic reagents for ketone alkylation, were investigated.22 To our delight, following minor modification to Ishihara’s procedure, tertiary alcohol 27 could be afforded in high yields (70–78%) in a highly reproducible manner.
In a single operation, the tosyl protecting group could be exchanged for a hexenoate side chain in good yield to afford RCM precursor 2. All attempts to form the macrocyclic E ring via RCM resulted in no desired cyclization–a result attributed to the distant spatial arrangement of the terminal alkenes. Opting instead for the early elimination of tertiary alcohol 27, preceding the RCM reaction, proceeded to give the desired Z-configured skipped diene 28 in excellent yield and good Z-selectivity (90%, 4.3:1), when using SOCl2 with 2,6-di-tert-butyl-4-methylpyridine (DTBMP). RCM precursor 29 could be efficiently reached in a single step as above, and treatment of 29 with Hoveyda–Grubbs second generation catalyst, in PhMe at 110 °C, employing cocatalytic p-benzoquinone (p-BQ) to minimize undesirable isomerization and at very high dilution to minimize dimerization, afforded diamide 30 in 71% yield.23 The profound effect that the spatial organization of reacting alkenes had on the outcome of the RCM reaction is noteworthy and should guide future macrocyclization campaigns employing this strategy.
A final double amide reduction was successfully carried out in 72% yield using excess LiAlH4 in Et2O to afford synthetic madangamine E (1). All analytical data were highly consistent with those reported by Chida and Sato and by the isolation team of Andersen.3b,5c
Conclusion
A 30-step enantioselective total synthesis of madangamine E has been achieved. Most notably, the early application of an organocatalytic enantioselective desymmetrization reaction enabled the construction of the bicyclic madangamine core with exquisite enantio- and diastereoselectivity and introduces a new method for the construction of stereogenic quaternary carbon centers from β,β′-dialkylsubstituted nitroolefins. This powerful transformation has been probed computationally, and its near-perfect selectivity profile has been rationalized by means of state-of-the-art density functional theory. Both the relative and absolute stereochemical configuration of the three newly formed stereogenic centers are set by the irreversible intramolecular Michael addition of an enamine to a thiourea-activated nitroolefin, and the lowest-energy conformation of the transition structure for this step benefits from stabilization from several hydrogen bonding interactions involving the thiourea, nitro group, and the enamine moieties. The subsequent construction of madangamine E hinged upon this early introduction of stereochemical information and featured a one-pot oxidative lactamization, a two-step Z-selective olefination of a sterically hindered ketone, and two complementary RCM reactions to close the two macrocyclic rings. These results demonstrate the utility of catalytic enantioselective desymmetrization reactions in the synthesis of high-value saturated molecular scaffolds and highlight their potential for the rapid and atom-economical generation of stereochemical complexity in target molecule synthesis.
Acknowledgments
S.S. acknowledges funding from the European Union’s Horizon 2020 research and innovation framework programme under the Marie Skłodowska-Curie Grant Agreement No. 797329 and thanks the Uehara memorial Foundation for a postdoctoral fellowship. B.D.A.S. is grateful to the Centre for Doctoral Training in Synthesis for Biology and Medicine for a studentship, generously supported by GlaxoSmithKline, MSD, Syngenta, and Vertex and thanks the Oxford-Leon E & Iris L Beghian Scholarship for funding. K.Y. thanks the Honjo International Scholarship Foundation for a postgraduate scholarship. T.A.H. thanks The Netherlands Organization for Scientific Research (NWO) for financial support. This work was carried out on the Dutch national e-infrastructure with the support of SURF Cooperative. D.V. and A.L.F.A. acknowledge funding from the European Union’s Horizon 2020 research and innovation framework programme under the Marie Skłodowska-Curie Grant Agreement Nos. 703789 and 660125. Kristina Aertker is thanked for early work on starting material preparation. Xiaoyu Xu is thanked for preliminary work. The authors thank Dr. Heyao Shi (University of Oxford) for X-ray data collection and Dr. Amber L. Thompson (Oxford Chemical Crystallography Service) for X-ray mentoring and structure determination.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c12040.
Additional optimization data, full synthetic methods, and characterization data; crystallographic data for 8 (CCDC 2113247) and S9 (CCDC 2113248) (PDF)
Accession Codes
CCDC 2113247–2113248 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
§ S.S. and B.D.A.S contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- a Borissov A.; Davies T. Q.; Ellis S. R.; Fleming T. A.; Richardson M. S. W.; Dixon D. J. Organocatalytic Enantioselective Desymmetrisation. Chem. Soc. Rev. 2016, 45, 5474–5540. 10.1039/C5CS00015G. [DOI] [PubMed] [Google Scholar]; b Zeng X. P.; Cao Z. Y.; Wang Y. H.; Zhou F.; Zhou J. Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev. 2016, 116, 7330–7396. 10.1021/acs.chemrev.6b00094. [DOI] [PubMed] [Google Scholar]; c Quasdorf K. W.; Overman L. E. Catalytic Enantioselective Synthesis of Quaternary Carbon Stereocentres. Nature 2014, 516, 181–191. 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Merad J.; Candy M.; Pons J. M.; Bressy C. Catalytic Enantioselective Desymmetrization of Meso Compounds in Total Synthesis of Natural Products: Towards an Economy of Chiral Reagents. Synthesis 2017, 49, 1938–1954. 10.1055/s-0036-1589493. [DOI] [Google Scholar]
- a Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]; b Harvey A. L.; Edrada-Ebel R.; Quinn R. J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]; c Méndez-Lucio O.; Medina-Franco J. L. The Many Roles of Molecular Complexity in Drug Discovery. Drug Discov. Today 2017, 22, 120–126. 10.1016/j.drudis.2016.08.009. [DOI] [PubMed] [Google Scholar]
- a Kong F.; Andersen R. J.; Allen T. M. Madangamine A, a Novel Cytotoxic Alkaloid from the Marine Sponge Xestospongia Ingens. J. Am. Chem. Soc. 1994, 116, 6007–6008. 10.1021/ja00092a077. [DOI] [Google Scholar]; b Kong F.; Graziani E. I.; Andersen R. J. Madangamines B-E, Pentacyclic Alkaloids from the Marine Sponge Xestospongia Ingens. J. Nat. Prod. 1998, 61, 267–271. 10.1021/np970377r. [DOI] [PubMed] [Google Scholar]; c De Oliveira J. H. H. L.; Nascimento A. M.; Kossuga M. H.; Cavalcanti B. C.; Pessoa C. O.; Moraes M. O.; Macedo M. L.; Ferreira A. G.; Hajdu E.; Pinheiro U. S.; Berlinck R. G. S. Cytotoxic Alkylpiperidine Alkaloids from the Brazilian Marine Sponge Pachychalina Alcaloidifera. J. Nat. Prod. 2007, 70, 538–543. 10.1021/np060450q. [DOI] [PubMed] [Google Scholar]
- a Amat M.; Pérez M.; Minaglia A. T.; Peretto B.; Bosch J. A General Synthetic Route to Enantiopure Cis-Fused Perhydrocycloalka[c]Pyridines from Phenylglycinol-Derived Lactams. Tetrahedron 2007, 63, 5839–5848. 10.1016/j.tet.2007.01.072. [DOI] [Google Scholar]; b Amat M.; Pérez M.; Proto S.; Gatti T.; Bosch J. First Enantioselective Synthesis of the Diazatricyclic Core of Madangamine Alkaloids. Chem. - Eur. J. 2010, 16, 9438–9441. 10.1002/chem.201000421. [DOI] [PubMed] [Google Scholar]; c Proto S.; Amat M.; Pérez M.; Ballette R.; Romagnoli F.; Mancinelli A.; Bosch J. Model Studies on the Synthesis of Madangamine Alkaloids. Assembly of the Macrocyclic Rings. Org. Lett. 2012, 14, 3916–3919. 10.1021/ol301672y. [DOI] [PubMed] [Google Scholar]; d Amat M.; Ballette R.; Proto S.; Pérez M.; Bosch J. First Enantioselective Synthesis of Tetracyclic Intermediates En Route to Madangamine D. Chem. Commun. 2013, 49, 3149–3151. 10.1039/c3cc41104d. [DOI] [PubMed] [Google Scholar]; e Are C.; Pérez M.; Ballette R.; Proto S.; Caso F.; Yayik N.; Bosch J.; Amat M. Access to Enantiopure Advanced Intermediates En Route to Madangamines. Chem. - Eur. J. 2019, 25, 15929–15933. 10.1002/chem.201904045. [DOI] [PubMed] [Google Scholar]; f Yamazaki N.; Kusanagi T.; Kibayashi C. Synthesis of the Diazatricyclic Core of the Marine Alkaloids Madangamines. Tetrahedron Lett. 2004, 45, 6509–6512. 10.1016/j.tetlet.2004.06.103. [DOI] [Google Scholar]; g Yoshimura Y.; Inoue J.; Yamazaki N.; Aoyagi S.; Kibayashi C. Synthesis of the 11-Membered Ring of the Marine Alkaloids, Madangamines. Tetrahedron Lett. 2006, 47, 3489–3492. 10.1016/j.tetlet.2006.02.160. [DOI] [Google Scholar]; h Yoshimura Y.; Kusanagi T.; Kibayashi C.; Yamazaki N.; Aoyagi S. Synthesis of the Diazatricyclic Core of Madangamines via Cyclic N,O-Acetalization-Bridgehead Reduction. Heterocycles 2008, 75, 1329–1354. 10.3987/COM-07-11276. [DOI] [Google Scholar]; i Yanagita Y.; Suto T.; Matsuo N.; Kurosu Y.; Sato T.; Chida N. Synthesis of Diazatricyclic Common Structure of Madangamine Alkaloids. Org. Lett. 2015, 17, 1946–1949. 10.1021/acs.orglett.5b00661. [DOI] [PubMed] [Google Scholar]; j Eastwood M. S.; Douglas C. J. Synthesis of the Madangamine Alkaloid Core by a C-C Bond Activation Cascade. Org. Lett. 2019, 21, 6149–6154. 10.1021/acs.orglett.9b02331. [DOI] [PubMed] [Google Scholar]; k Bhattacharjee A.; Gerasimov M. V.; Dejong S.; Wardrop D. J. Oxamidation of Unsaturated O-Alkyl Hydroxamates: Synthesis of the Madangamine Diazatricylic (ABC Rings) Skeleton. Org. Lett. 2017, 19, 6570–6573. 10.1021/acs.orglett.7b03283. [DOI] [PubMed] [Google Scholar]; l Matzanke N.; Gregg R. J.; Weinreb S. M.; Parvez M. A Concise Approach to the Tricyclic Core of the Cytotoxic Marine Alkaloid Madangamine A. J. Org. Chem. 1997, 62, 1920–1921. 10.1021/jo970208b. [DOI] [PubMed] [Google Scholar]; m Quirante J.; Paloma L.; Diaba F.; Vila X.; Bonjoch J. Synthesis of Diazatricyclic Core of Madangamines from Cis- Perhydroisoquinolines. J. Org. Chem. 2008, 73, 768–771. 10.1021/jo702340w. [DOI] [PubMed] [Google Scholar]; n Tong H. M.; Martin M. T.; Chiaroni A.; Bénéchie M.; Marazano C. An Approach to the Tricyclic Core of Madangamines Based upon a Biogenetic Scheme. Org. Lett. 2005, 7, 2437–2440. 10.1021/ol050740i. [DOI] [PubMed] [Google Scholar]; o Amat M.; Pérez M.; Ballette R.; Proto S.; Bosch J. The Alkaloids of the Madangamine Group. Alkaloids Chem. Biol. 2015, 74, 159–199. 10.1016/bs.alkal.2014.10.001. [DOI] [PubMed] [Google Scholar]; p Berlinck R. G. S.Polycyclic Diamine Alkaloids from Marine Sponges. Bioactive Heterocycles IV. Topics in Heterocyclic Chemistry, vol 10; Springer: Berlin, Heidelberg, 2007; pp 211–238. [Google Scholar]; q Cheng B.; Reyes J. Recent Progress on the Total Syntheses of Macrocyclic Diamine Alkaloids. Nat. Prod. Rep. 2020, 37, 322–337. 10.1039/C9NP00031C. [DOI] [PubMed] [Google Scholar]
- a Ballette R.; Pérez M.; Proto S.; Amat M.; Bosch J. Total Synthesis of (+)-Madangamine D. Angew. Chem., Int. Ed. 2014, 53, 6202–6205. 10.1002/anie.201402263. [DOI] [PubMed] [Google Scholar]; b Are C.; Pérez M.; Bosch J.; Amat M. Enantioselective Formal Synthesis of (+)-Madangamine A. Chem. Commun. 2019, 55, 7207–7210. 10.1039/C9CC03690C. [DOI] [PubMed] [Google Scholar]; c Suto T.; Yanagita Y.; Nagashima Y.; Takikawa S.; Kurosu Y.; Matsuo N.; Sato T.; Chida N. Unified Total Synthesis of Madangamines A, C, and E. J. Am. Chem. Soc. 2017, 139, 2952–2955. 10.1021/jacs.7b00807. [DOI] [PubMed] [Google Scholar]; d Suto T.; Yanagita Y.; Nagashima Y.; Takikawa S.; Kurosu Y.; Matsuo N.; Miura K.; Simizu S.; Sato T.; Chida N. Unified Total Synthesis of Madangamine Alkaloids. Bull. Chem. Soc. Jpn. 2019, 92, 545–571. 10.1246/bcsj.20180334. [DOI] [Google Scholar]
- Miura K.; Kawano S.; Suto T.; Sato T.; Chida N.; Simizu S. Identification of madangamine A as a novel lysosomotropic agent to inhibit autophagy. Biorg. Med. Chem. 2021, 34, 116041. 10.1016/j.bmc.2021.116041. [DOI] [PubMed] [Google Scholar]
- Gammack Yamagata A. D.; Datta S.; Jackson K. E.; Stegbauer L.; Paton R. S.; Dixon D. J. Enantioselective Desymmetrization of Prochiral Cyclohexanones by Organocatalytic Intramolecular Michael Additions to α,β-Unsaturated Esters. Angew. Chem., Int. Ed. 2015, 54, 4899–4903. 10.1002/anie.201411924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of enantioselective addition of nitroolefins:; a Huang H.; Jacobsen E. N. Highly Enantioselective Direct Conjugate Addition of Ketones to Nitroalkenes Promoted by a Chiral Primary Amine-Thiourea Catalyst. J. Am. Chem. Soc. 2006, 128, 7170–7171. 10.1021/ja0620890. [DOI] [PubMed] [Google Scholar]; b Bui T.; Syed S.; Carlos F.; Barbas I. Thiourea-Catalyzed Highly Enantio- and Diastereoselective Additions of Oxindoles to Nitroolefins: Application to the Formal Synthesis of (+)-Physostigmine. J. Am. Chem. Soc. 2009, 131, 8758–8759. 10.1021/ja903520c. [DOI] [PubMed] [Google Scholar]; c Schnitzer T.; Budinská A.; Wennemers H. Organocatalysed Conjugate Addition Reactions of Aldehydes to Nitroolefins with Anti Selectivity. Nat. Catal. 2020, 3, 143–147. 10.1038/s41929-019-0406-4. [DOI] [Google Scholar]; d Tsogoeva S. B.; Wei S. Highly Enantioselective Addition of Ketones to Nitroolefins Catalyzed by New Thiourea-Amine Bifunctional Organocatalysts. Chem. Commun. 2006, 1451–1453. 10.1039/b517937h. [DOI] [PubMed] [Google Scholar]; e Freund M.; Schenker S.; Tsogoeva S. B. Enantioselective Nitro-Michael Reactions Catalyzed by Short Peptides on Water. Org. Biomol. Chem. 2009, 7, 4279–4284. 10.1039/b910249c. [DOI] [PubMed] [Google Scholar]
- For intermolecular desymmetrization reactions of cycloalkanones with nitroolefins:; a Capitta F.; Frongia A.; Ollivier J.; Aitken D. J.; Secci F.; Piras P. P.; Guillot R. Enantioselective Organocatalyzed Desymmetrization of 3-Substituted Cyclobutanones through Michael Addition to Nitroalkenes. Synlett 2014, 26, 123–126. 10.1055/s-0034-1379489. [DOI] [Google Scholar]; b Nakashima K.; Hirashima S. I.; Kawada M.; Koseki Y.; Tada N.; Itoh A.; Miura T. Pyrrolidine-Diaminomethylenemalononitrile Organocatalyst for Michael Additions of Carbonyl Compounds to Nitroalkenes under Solvent-Free Conditions. Tetrahedron Lett. 2014, 55, 2703–2706. 10.1016/j.tetlet.2014.03.042. [DOI] [Google Scholar]; c Hirashima S.-I.; Narushima T.; Kawada M.; Nakashima K.; Hanai K.; Koseki Y.; Miura T. Asymmetric Conjugate Additions of Carbonyl Compounds to Nitroalkenes under Solvent-Free Conditions Using Fluorous Diaminomethylenemalononitrile Organocatalyst. Chem. Pharm. Bull. 2017, 65, 1185–1190. 10.1248/cpb.c17-00596. [DOI] [PubMed] [Google Scholar]
- For Michael additions to nitroolefins generating quaternary carbon centers:; a Kastl R.; Wennemers H. Peptide-Catalyzed Stereoselective Conjugate Addition Reactions Generating All-Carbon Quaternary Stereogenic Centers. Angew. Chem., Int. Ed. 2013, 52, 7228–7232. 10.1002/anie.201301583. [DOI] [PubMed] [Google Scholar]; b Sim J. H.; Song C. E. Water-Enabled Catalytic Asymmetric Michael Reactions of Unreactive Nitroalkenes: One-Pot Synthesis of Chiral GABA-Analogs with All-Carbon Quaternary Stereogenic Centers. Angew. Chem., Int. Ed. 2017, 56, 1835–1839. 10.1002/anie.201611466. [DOI] [PubMed] [Google Scholar]; c Urruzuno I.; Mugica O.; Oiarbide M.; Palomo C. Bifunctional Brønsted Base Catalyst Enables Regio-, Diastereo-, and Enantioselective Cα-Alkylation of β-Tetralones and Related Aromatic-Ring-Fused Cycloalkanones. Angew. Chem., Int. Ed. 2017, 56, 2059–2063. 10.1002/anie.201612332. [DOI] [PubMed] [Google Scholar]; d Chang H. H.; Chu K. T.; Chiang M. H.; Han J. L. Organocatalytic Enantioselective Michael Reaction of 1,3-Dicarbonyls with α-Substituted β-Nitroacrylates. Tetrahedron 2017, 73, 727–734. 10.1016/j.tet.2016.12.039. [DOI] [Google Scholar]; e Frias M.; Mas-Ballesté R.; Arias S.; Alvarado C.; Alemán J. Asymmetric Synthesis of Rauhut-Currier Type Products by a Regioselective Mukaiyama Reaction under Bifunctional Catalysis. J. Am. Chem. Soc. 2017, 139, 672–679. 10.1021/jacs.6b07851. [DOI] [PubMed] [Google Scholar]; f Gao J.-R.; Wu H.; Xiang B.; Yu W.-B.; Han L.; Jia Y.-X. Highly Enantioselective Construction of Trifluoromethylated All-Carbon Quaternary Stereocenters via Nickel-Catalyzed Friedel-Crafts Alkylation Reaction. J. Am. Chem. Soc. 2013, 135, 2983–2986. 10.1021/ja400650m. [DOI] [PubMed] [Google Scholar]; g Ma H.; Xie L.; Zhang Z.; Wu L. G.; Fu B.; Qin Z. Enantioselective Conjugate Addition of 2-Acetyl Azaarenes to β,β-Disubstituted Nitroalkene for the Construction of All-Carbon Quaternary Stereocenters. J. Org. Chem. 2017, 82, 7353–7362. 10.1021/acs.joc.7b01014. [DOI] [PubMed] [Google Scholar]; h Manzano R.; Andrés J. M.; Muruzábal M. D.; Pedrosa R. Stereocontrolled Construction of Quaternary Stereocenters by Inter- and Intramolecular Nitro-Michael Additions Catalyzed by Bifunctional Thioureas. Adv. Synth. Catal. 2010, 352, 3364–3372. 10.1002/adsc.201000612. [DOI] [Google Scholar]; i Chen S.; Lou Q.; Ding Y.; Zhang S.; Hu W.; Zhao J. Organocatalytic Enantioselective Michael Reaction of Malononitrile with β,β-Disubstituted Nitroalkenes. Adv. Synth. Catal. 2015, 357, 2437–2441. 10.1002/adsc.201500079. [DOI] [Google Scholar]; j Sekikawa T.; Kitaguchi T.; Kitaura H.; Minami T.; Hatanaka Y. Anti-Selective Asymmetric Nitro-Michael Reaction of Furanones: Diastereocontrol by Catalyst. Org. Lett. 2016, 18, 646–649. 10.1021/acs.orglett.5b03539. [DOI] [PubMed] [Google Scholar]; k Okino T.; Hoashi Y.; Takemoto Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. 10.1021/ja036972z. [DOI] [PubMed] [Google Scholar]; l Okino T.; Hoashi Y.; Furukawa T.; Xu X.; Takemoto Y. Enantio- and Diastereoselective Michael Reaction of 1,3-Dicarbonyl Compounds to Nitroolefins Catalyzed by a Bifunctional Thiourea. J. Am. Chem. Soc. 2005, 127, 119–125. 10.1021/ja044370p. [DOI] [PubMed] [Google Scholar]
- For review literature covering the addition to nitroolefins:; a Roux C.; Bressy C.. Addition to Nitroolefins and Vinyl Sulfones. In Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications; Dalko P. I., Ed.; John Wiley & Sons, Ltd: 2013; Vol. 3–3, pp 1013–1042. [Google Scholar]; b Alonso D. A.; Baeza A.; Chinchilla R.; Gómez C.; Guillena G.; Pastor I. M.; Ramón D. J. Recent Advances in Asymmetric Organocatalyzed Conjugate Additions to Nitroalkenes. Molecules 2017, 22, 895. 10.3390/molecules22060895. [DOI] [PMC free article] [PubMed] [Google Scholar]; KočovskÅ P.Addition Reactions. In Organic Reaction Mechanisms · 2017: An annual survey covering the literature dated January to December 2017; Knipe A. C., Moloney M. G., Eds.; John Wiley & Sons, Ltd: 2020; pp 377–500. [Google Scholar]
- Maity S.; Manna S.; Rana S.; Naveen T.; Mallick A.; Maiti D. Efficient and Stereoselective Nitration of Mono- and Disubstituted Olefins with AgNO2 and TEMPO. J. Am. Chem. Soc. 2013, 135, 3355–3358. 10.1021/ja311942e. [DOI] [PubMed] [Google Scholar]
- Single crystal X-ray diffraction data were collected using a Nonius Kappa CCD or a Rigaku/Oxford Diffraction Supernova A diffractometer. The structures were solved using SuperFlip [J. Appl. Crystallogr. 2007, 40, 786–790 10.1107/S0021889807029238] [DOI] [Google Scholar]; and refined using CRYSTALS; [J. Appl. Crystallogr. 2003, 36, 1487. 10.1107/S0021889803021800] as per the ESI. [DOI] [Google Scholar]
- Zou Y. Q.; Hörmann F. M.; Bach T. Iminium and Enamine Catalysis in Enantioselective Photochemical Reactions. Chem. Soc. Rev. 2018, 47, 278–290. 10.1039/C7CS00509A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yalalov D. A.; Tsogoeva S. B.; Schmatz S. Chiral Thiourea-Based Bifunctional Organocatalysts in the Asymmetric Nitro-Michael Addition: A Joint Experimental-Theoretical Study. Adv. Synth. Catal. 2006, 348, 826–832. 10.1002/adsc.200505443. [DOI] [Google Scholar]; b Burés J.; Armstrong A.; Blackmond D. G. Mechanistic Rationalization of Organocatalyzed Conjugate Addition of Linear Aldehydes to Nitro-Olefins. J. Am. Chem. Soc. 2011, 133, 8822–8825. 10.1021/ja203660r. [DOI] [PubMed] [Google Scholar]; c Ji Y.; Blackmond D. G. The Role of Reversibility in the Enantioselective Conjugate Addition of α,α-Disubstituted Aldehydes to Nitro-Olefins Catalyzed by Primary Amine Thioureas. Catal. Sci. Technol. 2014, 4, 3505–3509. 10.1039/C4CY00648H. [DOI] [Google Scholar]; d Sahoo G.; Rahaman H.; Madarász Á.; Pápai I.; Melarto M.; Valkonen A.; Pihko P. M. Dihydrooxazine Oxides as Key Intermediates in Organocatalytic Michael Additions of Aldehydes to Nitroalkenes. Angew. Chem., Int. Ed. 2012, 51, 13144–13148. 10.1002/anie.201204833. [DOI] [PubMed] [Google Scholar]; e Seebach D.; Sun X.; Ebert M. O.; Schweizer W. B.; Purkayastha N.; Beck A. K.; Duschmalé J.; Wennemers H.; Mukaiyama T.; Benohoud M.; Hayashi Y.; Reiher M. Stoichiometric Reactions of Enamines Derived from Diphenylprolinol Silyl Ethers with Nitro Olefins and Lessons for the Corresponding Organocatalytic Conversions - A Survey. Helv. Chim. Acta 2013, 96, 799–852. 10.1002/hlca.201300079. [DOI] [Google Scholar]
- Lalonde M. P.; Chen Y.; Jacobsen E. N. A Chiral Primary Amine Thiourea Catalyst for the Highly Enantioselective Direct Conjugate Addition of α,α-Disubstituted Aldehydes to Nitroalkenes. Angew. Chem., Int. Ed. 2006, 45, 6366–6370. 10.1002/anie.200602221. [DOI] [PubMed] [Google Scholar]
- Denmark S. E.; Marcin L. R. A General Method for the Preparation of 2,2-Disubstituted 1-Nitroalkenes. J. Org. Chem. 1993, 58, 3850–3856. 10.1021/jo00067a016. [DOI] [Google Scholar]
- Smith J. M.; Moreno J.; Boal B. W.; Garg N. K. Total Synthesis of the Akuammiline Alkaloid Picrinine. J. Am. Chem. Soc. 2014, 136, 4504–4507. 10.1021/ja501780w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou K. C.; Montagnon T.; Baran P. S. Modulation of the Reactivity Profile of IBX by Ligand Complexation: Ambient Temperature Dehydrogenation of Aldehydes and Ketones to α,β-Unsaturated Carbonyl Compounds. Angew. Chem., Int. Ed. 2002, 41, 993–996. . [DOI] [PubMed] [Google Scholar]
- a Sasano Y.; Nagasawa S.; Yamazaki M.; Shibuya M.; Park J.; Iwabuchi Y. Highly Chemoselective Aerobic Oxidation of Amino Alcohols into Amino Carbonyl Compounds. Angew. Chem., Int. Ed. 2014, 53, 3236–3240. 10.1002/anie.201309634. [DOI] [PubMed] [Google Scholar]; b Hoover J. M.; Stahl S. S. Highly Practical Copper(I)/TEMPO Catalyst System for Chemoselective Aerobic Oxidation of Primary Alcohols. J. Am. Chem. Soc. 2011, 133, 16901–16910. 10.1021/ja206230h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto T.; Takiyama N.; Nakamura K.; Hatajima T.; Kamiya Y. Reactions of Carbonyl Compounds with Grignard Reagents in the Presence of Cerium Chloride. J. Am. Chem. Soc. 1989, 111, 4392–4398. 10.1021/ja00194a037. [DOI] [Google Scholar]
- Hatano M.; Suzuki S.; Ishihara K. Highly Chemoselective Stoichiometric Alkylation of Ketones with Grignard Reagent Derived Zinc(II) Ate Complexes. Synlett 2010, 2010, 321–324. 10.1055/s-0029-1219220. [DOI] [Google Scholar]
- Hong S. H.; Sanders D. P.; Lee C. W.; Grubbs R. H. Prevention of Undesirable Isomerization during Olefin Metathesis. J. Am. Chem. Soc. 2005, 127, 17160–17161. 10.1021/ja052939w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.