Abstract
Enhanced intrapulmonary gas transport enables oscillatory ventilation modalities to support gas exchange using extremely low tidal volumes at high frequencies. However, it is unknown whether gas transport rates can be improved by combining multiple frequencies of oscillation simultaneously. The goal of this study was to investigate distributed gas transport in vivo during multi-frequency oscillatory ventilation (MFOV) as compared with conventional mechanical ventilation (CMV) or high-frequency oscillatory ventilation (HFOV). We hypothesized that MFOV would result in more uniform rates of gas transport compared to HFOV, measured using contrast-enhanced CT imaging during wash-in of xenon gas. In 13 pigs, xenon wash-in equilibration rates were comparable between CMV and MFOV, but 21 to 39% slower for HFOV. By contrast, the root-mean-square delivered volume was lowest for MFOV, increased by 70% during HFOV and 365% during CMV. Overall gas transport heterogeneity was similar across all modalities, but gravitational gradients and regional patchiness of specific ventilation contributed to regional ventilation heterogeneity, depending on ventilator modality. We conclude that MFOV combines benefits of low lung stretch, similar to HFOV, but with fast rates of gas transport, similar to CMV.
Keywords: Mechanical ventilation, High-frequency ventilation, Pulmonary gas exchange, Respiratory-gated imaging techniques
INTRODUCTION
Multi-frequency oscillatory ventilation (MFOV) has been proposed as an alternative modality of mechanical ventilation that delivers to the patient airway opening multiple sinusoidal pressure and flow oscillations at different frequencies delivered simultaneously 13,23. Compared to conventional mechanical ventilation (CMV) and high-frequency oscillatory ventilation (HFOV), which use a single breathing or oscillatory frequency, MFOV may provide enhanced gas transport, reduced strain heterogeneity, and lung protection 18,19. A previous study in premature surfactant-deficient lambs demonstrated a benefit of MFOV over HFOV for maintaining a lower oxygenation index and a lower ventilatory cost function 23. The theorized mechanism of benefit for MFOV is improved regional ventilation-to-perfusion matching, due to the frequency-dependence of distributed flows throughout the lung. Such features in a lung-protective, oscillatory waveform may be highly desirable in the acute respiratory distress syndrome (ARDS).
During CMV, respiratory flows at low frequencies are distributed primarily according to regional lung compliance, and gas transport is dominated by bulk advection of fresh gas directly into alveolar spaces via the conducting airways 42. Thus the alveolar concentration of any individual gas species would be expected to equilibrate faster in lung regions with relatively higher compliance and lower regional deadspace 7,30, at least for a specified blood solubility and cardiac output. This typically occurs in the dorsal lung regions of a supine subject, which exhibit lower end-expiratory inflation level and higher compliance due to the hydrostatic weight of the lung and mediastinal contents 12,20,22. During HFOV, the distribution of regional flow in the lung becomes increasingly dependent on airway resistance, gas inertia, and asymmetrical airway network branching 5,17. Gas transport is not dominated by bulk advection at high frequencies, but rather depends on a variety of mechanisms contributing to intrapulmonary mixing 4,32,37,41. The transport of gases from any particular lung region to the airway opening may depend on regional ventilation distribution more strongly during CMV compared to HFOV 28,39. Such regional differences in gas exchange will depend on the frequency of ventilation 17, making MFOV ideally suited for the mechanically heterogeneous lung.
The goal of this study was to investigate the effect of MFOV on distributed gas transport in vivo, as compared with CMV or HFOV. Xenon (Xe), a dense noble gas, has been used extensively as a tracer gas or contrast agent for x-ray computed tomography (CT) 21,26. Inspired xenon distributes throughout the lung and increases CT voxel value wherever xenon concentration is elevated, providing image contrast for quantitative analysis of regional ventilation distribution 27,28,30. In this study, we hypothesized that MFOV would result in more uniform rates of gas transport compared to HFOV, measured using xenon-enhanced CT imaging (XeCT). To test this hypothesis, we performed a randomized, crossover trial in pigs receiving CMV, single-frequency HFOV, and MFOV during XeCT. Some of the results of this study have been previously reported in the form of an abstract 16.
MATERIALS AND METHODS
Animal Preparation
All experimental procedures were approved by the University of Iowa Institutional Animal Care and Use Committee. Another study of respiratory mechanics and structural dynamics was previously published using data collected from a subset of the subjects used in this study 13. Thirteen porcine subjects were used in this study, weighing 9 to 13 kg. Following intramuscular injection of 2.2 mg kg−1 telazol, 1.1 mg kg−1 ketamine, and 1.1 mg kg−1 xylazine, deep sedation was maintained throughout surgical procedures using inhaled isoflurane. Each subject was intubated and mechanically ventilated using a Uni-Vent Eagle Model 754 (Impact Instrumentation, West Caldwell, NJ). End-tidal capnography, peripheral oxygen saturation, and electrocardiogram waveforms were obtained using a Philips patient monitor equipped with the M3001A measurement module (Philips Healthcare, Andover, MA). Surgical incision into the neck was performed to allow placement of central venous and arterial lines, as well as relocation of the endotracheal tube into an incision in the trachea inferior to the larynx. Endotracheal tubes were manually shortened prior to tracheostomy, to reduce apparatus deadspace. Following completion of surgical procedures, subjects were transported to the CT scanner. Inhaled isoflurane was discontinued, and sedation was maintained using an intravenous infusion of propofol at 7 to 9 mg kg−1 hr−1. Subjects were paralyzed during experimental ventilation and imaging procedures with intermittent doses of 1 mg kg−1 rocuronium.
Mechanical Ventilation
Subjects were mechanically ventilated using a FabianHFO hybrid oscillator/ventilator (Acutronic Medical Systems AG, Switzerland). A compressed gas cylinder containing a 20%-80% oxygen-xenon mixture was connected by a 3-way ball valve in parallel with the pressurized medical air supply. Figure 1 shows a schematic of the apparatus for xenon delivery during mechanical ventilation. Each subject was mechanically ventilated with CMV, HFOV, and MFOV in randomized order over 30-minute intervals. For all modalities, mean airway pressure () was set via the ventilator interface at 12 cmH2O, with fractional inspired oxygen (FiO2) at 40% for consistency with 40%-60% oxygen-xenon use during imaging. Control of during CMV was achieved via positive end-expiratory pressure (PEEP). CMV was delivered at rates between 20 to 32 min−1 (0.33 to 0.53 Hz), with driving pressure 13 cmH2O and inspiratory:expiratory time ratio 1:2. HFOV was delivered at 5 Hz, with peak-to-peak (or peak-to-trough) pressure amplitude approximately 50 cmH2O. MFOV was delivered using a combination of 5, 10, 15, and 20 Hz superimposed oscillations 23, with peak-to-peak pressure amplitude approximately 84 cmH2O. Ventilator driving pressure or pressure amplitudes were adjusted to maintain arterial CO2 tension (PaCO2) between 30 to 60 mmHg. Each 30-minute experimental ventilation interval was followed by arterial blood gas analysis and a XeCT scan sequence, without interrupting mechanical ventilation. Between each experimental ventilation interval, a 15-minute ventilation wash-out interval using CMV was conducted to restore baseline mechanical and physiological state.
Figure 1.

(A) Schematic of xenon delivery using a ventilator/oscillator with a three-way valve used to switch gas supplies between air and xenon-oxygen mixtures. After switching the valve without disrupting ventilation, xenon concentration [Xe] in the lung gradually increases (B). The rise in [Xe] is assumed to produce a proportional rise in CT density within lung voxels. CT scans are synchronized with the ventilator to acquire scans at the same phase of ventilation or oscillation, with irregular intervals throughout xenon wash-in. Specific ventilation is estimated by the reciprocal of the time constant of an exponential regression to [Xe] during xenon wash-in.
Ventilatory cost function (VC) was defined as the product of mean squared delivered volume () and arterial carbon dioxide tension (PaCO2) divided by body weight (Wt) 23:
| (1) |
with Vrms computed as:
| (2) |
where V(t) denotes the volume waveform measured at the airway opening, Δt is the duration of sampling (i.e., either 1 breath during CMV, or 1 second during HFOV and MFOV), and is the mean of the volume waveform. The use of Vrms enables comparison between single-frequency modalities, which are typically described by a single tidal volume, and MFOV, which is described by a distinct volume amplitude at each frequency 13,23.
Image Acquisition
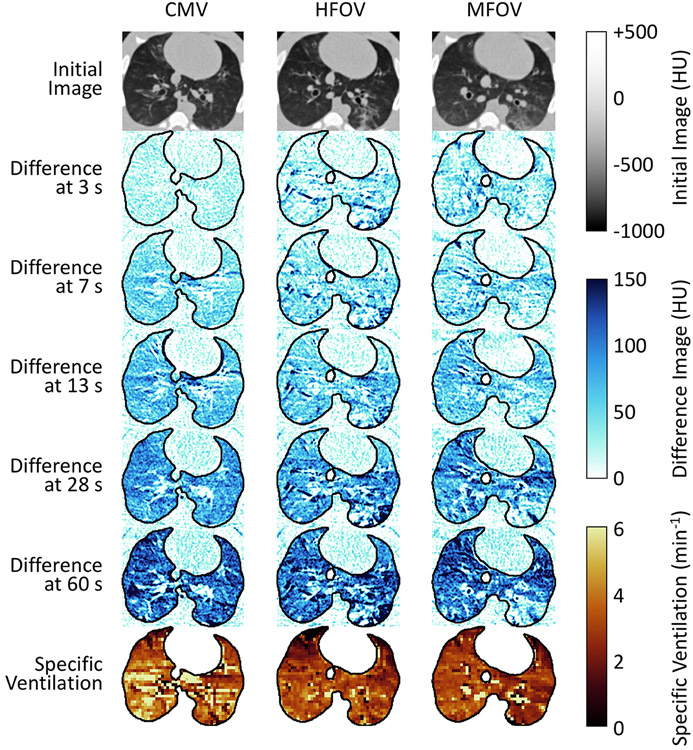
CT scans were acquired using a Siemens SOMATOM Force (Forchheim, Germany) in an axial scanning mode, with 5.76 cm of axial coverage (0.6 mm slice thickness, 96 slices) and 17 cm field of view (0.3 mm in-plane spatial resolution). Subjects were scanned at 70 kVp tube voltage and 200 mA tube current, with 250 ms scanner rotation period. Each scan sequence was initiated immediately following the changeover of gas supply from an approximately 40%-60% oxygen-nitrogen mixture to a 40%-60% oxygen-xenon mixture 28. XeCT scan sequences were timed to acquire a series of images at the same phase of the ventilatory cycle over a wash-in duration of approximately 60 seconds 28. Scans during conventional mechanical ventilation were acquired once per breath, i.e., at the same rate as mechanical ventilation. Scans during high-frequency and multi-frequency oscillatory ventilation were acquired at 1-second intervals over the first 40 seconds, followed by 2-second intervals over the last 20 seconds, for a total of 51 images. Figure 2 shows several images taken from a sequence acquired in one subject during xenon wash-in, as well as the resulting specific ventilation image. Supplemental Video 1 provides an animation of the changes in CT image intensity during the xenon wash-in.
Figure 2.
Representative subject exhibiting changes in CT voxel value during xenon wash-in (0.3 mm spatial resolution), with a few example scan timings indicated at left. Specific ventilation images characterize the rate constant of xenon gas equilibration obtained by exponential regression (2.4 mm spatial resolution). Ventilation modalities were conventional mechanical ventilation (CMV), high-frequency oscillatory ventilation (HFOV), and multi-frequency oscillatory ventilation (MFOV).
Image Processing
Each image in the XeCT image sequence was denoted In(x), where n indexes the number of images in the sequence 0 through N – 1, and x is a vector representing 3-dimensional spatial position. Each image was associated with a corresponding elasped time tn since initial image acquisition. The first image in the sequence was denoted I0(x) with t0 = 0 seconds. The acquisition times for each scan were selected to coincide consistently with the same phase of the mechanical ventilation cycle. However, asynchronous cardiogenic motion presented a source of structural misalignment throughout the XeCT image sequence 14. To compensate for this extraneous motion, each image In(x) was warped by pairwise deformable image registration using I0(x) as the reference or “fixed” image 24. Thus, each deformed image In→0(x) contained voxel intensities from In(x), but was structurally aligned with I0(x). Supplemental Figure 1 shows an example of nonrespiratory motion in an image sequence, which produced large fluctuations in CT density that were reduced following image registration. The resulting deformed images were then downsampled to 2.4 mm isotropic resolution to mitigate effects of imaging noise 28,30. The time-varying intensity within each voxel was fit to an exponential model using nonlinear regression:
| (3) |
where D0(x), D∞(x), and τ(x) are the regionally varying initial intensities, equilibrium intensities, and time constants of equilibration, respectively 35. Assuming that temporal variations in regional image intensity within co-registered images are due solely to temporal variations in regional xenon concentration, then τ(x) can also be considered the regional xenon equilibration time constant. Values for D0(x), D∞(x), and τ(x) were computed at each spatial location x using a nonlinear gradient descent technique (MATLAB, Natick, MA) to minimize the sum of squared differences between model-predicted intensities and measured intensities In→0(x). Regional specific ventilation was computed by the reciprocal of the regional time constant τ(x), such that also represents the regional rate constant of xenon equilibration 34. Supplemental Figure 2 shows an example of in transverse sections for one subject, with varying degrees of downsampling prior to τ(x) estimation. Voxels corresponding to spatial positions within the lungs were identified by an automated segmentation algorithm using a deep convolutional neural network 10,11. A single lung mask M(x) was generated for each XeCT image sequence based on the reference image I0(x). Voxels within 7.2 mm of the image boundaries along the rostral-caudal axis were excluded from the mask, due to potential registration artifacts caused by motion of lung tissue into or out of the axial field of view. Voxels with negligible aeration in the reference image (i.e., I0(x) ≥ −300 HU) were also excluded 30.
Distributed specific ventilation was assessed by the median and quartile coefficient of dispersion of within each subject. Coefficient of dispersion, defined as half the difference between the 75th and 25th percentiles divided by their average, was used as a measure of overall specific ventilation spatial heterogeneity 2. The mean and coefficient of variation were also considered. Spatial gradients of specific ventilation were assessed along three principal axes: ventral-dorsal , right-left , and rostral-caudal . The spatial gradients gVD, gRL, and gRC were determined as the slopes computed by linear regression of with respect to position along each principal axis. A least-median-of-squares regression was used for robustness against non-normal distributions of data about the regression line 33. Figure 3 shows an example of voxel-wise linear regressions for along the dorsal-ventral axis in one subject.
Figure 3.
Sagittal sections of a CT scan from one representative subject during conventional mechanical ventilation (CMV), high-frequency oscillatory ventilation (HFOV), and multi-frequency oscillatory ventilation (MFOV). Downsampled lung voxels are colored according to specific ventilation. Histograms show counts of voxels binned according to both specific ventilation and spatial position with respect to the dorsal-ventral axis. Solid lines represent least-median-of-squares linear regression of specific ventilation in the dorsal-ventral direction.
Regional patchiness of was assessed by two techniques relying on recursive subdivision of regions-of-interest within the lung mask: octree and supervoxel decomposition. Both methods were initiallized with a single region of interest (ROI) containing the entire lung mask. At each recursive step, an ROI was designated for subdivision into multiple new ROIs if the standard deviation of voxel values contained within was greater than a fixed threshold. The threshold value for standard deviation in this study was chosen empirically to be 1.3 min−1, to produce nontrivial distributions of ROI size (i.e., either failing to partition even once, or decomposing the entire region into the smallest possible size ROIs). Octree decomposition, a three-dimensional extension of quadtree decomposition, was used to partition each ROI into eight octants according to bisecting coronal, sagittal, and transverse planes 29. Supervoxel decomposition was used to partition each ROI into two new ROIs by a weighted k-means clustering of voxels according to intensity similarity and spatial proximity 6. Recursive subdivision was continued until all ROIs either contained below-threshold values of standard deviation, or were too small to be further partitioned. The threshold value for minimum ROI size in this study was chosen to be 0.2% of the lung mask to avoid decomposing to ROIs into individual voxels. Regional patchiness was then quantified by the mean ROI volume as a fraction of the lung mask.
Statistical Tests
A non-parametric Friedman test of ranks was performed for each imaging outcome (i.e., median, coefficient of dispersion, gVD, gRL, gRC, and ) to test for significant effects of ventilation modality and intersubject variability at the 0.05 significance level. A non-parametric Skillings-Mack test was performed (rather than the Friedman test) for each gas exchange outcome (i.e., Vrms·Wt−1, PaO2, PaCO2, pH, and VC), due to missing data for one subject 36. Wilcoxon signed-rank post hoc tests were used for all pairwise comparisons. Additionally, for each ventilation modality, a Wilcoxon signed-rank test was used to test for non-zero means of each spatial gradient (i.e., gVD, gRL, and gRC) at the 0.05 significance level.
RESULTS
Figure 2 shows an example of in transverse sections for one subject during CMV, HFOV, and MFOV (animated sequence of difference images during xenon wash-in is available in Supplemental Video 1). Supplemental Figure 3 shows pressure waveforms measured by the ventilator at the proximal end of the endotracheal tube throughout five cycles of periodic mechanical ventilation. Ventilator settings for mean pressure were constant across all modalities to maintain consistent lung volumes during ventilation and imaging. Note that pressure amplitudes at the endotracheal tube were greatly increased during HFOV and MFOV with some negative pressure excursions, typical features of oscillatory ventilation. Table 1 provides a summary of gas exchange parameters following 30 minutes of mechanical ventilation. Ventilatory cost function (VC) was defined as the product of mean squared delivered volume and arterial carbon dioxide tension divided by body weight 23. The use of root-mean-squared volume enables comparison between single-frequency modalities, which are typically described by a single tidal volume, and MFOV, which is described by a distinct volume amplitude at each frequency 13,23. Note that only 12 subjects are represented for CMV in Table 1 because arterial blood gas could not be collected for one subject following the 30-minute CMV period.
Table 1.
Gas exchange data from arterial blood gas samples after 30 minutes of mechanical ventilation with 40% inspired oxygen and 12 cmH2O mean airway pressure. Statistical comparisons using Skillings-Mack non-parametric tests and Wilcoxon signed-rank post hoc tests are shown. Bold text indicates significance at the 0.05 level.
| Modality | N sub |
Vrms·Wt−1 (mL kg−1) |
PaO2 (mmHg) |
PaCO2 (mmHg) |
pH |
VC (mL2 mmHg g−1) |
|---|---|---|---|---|---|---|
| CMV | 12 | 3.21 ± 0.65 | 192.8 ± 32.7 | 40.6 ± 5.3 | 7.49 ± 0.05 | 4.33 ± 1.32 |
| HFOV | 13 | 1.19 ± 0.27 | 201.7 ± 16.8 | 43.2 ± 9.5 | 7.46 ± 0.11 | 0.63 ± 0.20 |
| MFOV | 13 | 0.69 ± 0.16 | 190.2 ± 18.9 | 50.2 ± 14.5 | 7.40 ± 0.12 | 0.24 ± 0.07 |
| Statistics | Vrms·Wt−1 | PaO2 | PaCO2 | pH | V C | |
| Effect of Subject | 0.067 | 0.374 | 0.014 | 0.018 | 0.335 | |
| Effect of Modality | < 0.001 | 0.331 | 0.004 | 0.014 | < 0.001 | |
| CMV vs. HFOV | < 0.001 | 0.424 | 0.583 | < 0.001 | ||
| CMV vs. MFOV | < 0.001 | 0.009 | 0.016 | < 0.001 | ||
| MFOV vs. HFOV | < 0.001 | 0.022 | 0.033 | < 0.001 | ||
CMV = conventional mechanical ventilation; HFOV = high-frequency oscillatory ventilation; MFOV = multi-frequency oscillatory ventilation; Nsub = number of subjects; Vrms = root-mean-square volume (mL); Wt = body weight (kg); PaO2 = partial pressure of oxygen in arterial blood (mmHg); PaCO2 = partial pressure of carbon dioxide in arterial blood (mmHg); VC = ventilatory cost function (mL2 mmHg g−1).
Figure 4 shows a comparison of specific ventilation median and coefficient of dispersion across subjects during CMV, HFOV, and MFOV with a fixed isotropic image resolution of 2.4 mm. A non-parametric Friedman test reported a significant effect of ventilation modality for median (p < 0.05). The median during HFOV was 39% lower compared to CMV (p < 0.001) and 21% lower compared to MFOV (p < 0.05), but the median was not significantly different between CMV and MFOV (p = 0.09). No statistical differences were found for the cofficient of dispersion. For comparison across multiple isotropic image resolutions see Supplemental Figure 2 showing example transverse sections of specific ventilation, and Supplemental Figures 4 and 5 showing summaries of specific ventilation median and mean, respectively.
Figure 4.

Box-and-whisker plots showing the minimum, maximum, and quartiles across subjects of specific ventilation median (A) and quartile coefficient of dispersion (B) for each subject, grouped by ventilation modality: conventional mechanical ventilation (CMV), high-frequency oscillatory ventilation (HFOV), and multi-frequency oscillatory ventilation (MFOV). Higher specific ventilation implies faster turnover of alveolar gas with fresh gas; higher coefficient of dispersion implies more heterogeneity of specific ventilation throughout the lung. Voxel size was 2.4 mm isotropic. Significant differences between group medians are indicated by * for p < 0.05 and ** for p < 0.001.
Supplemental Figures 6 and 7 show comparisons of specific ventilation spatial gradients. At a fixed isotropic image resolution of 2.4 mm, a non-parametric Friedman test reported no significant effect of ventilation modality for differences in any of the three principal directions. Non-parametric signed-rank tests revealed significantly non-zero gradients in the ventral-dorsal direction for all ventilation modalities (p < 0.001). The median values across all subjects of ventral-dorsal gradient (and gradient normalized by median) were 0.34, 0.23, and 0.23 min−1 cm−1 (1.18, 1.04, 0.96 % cm−1) during CMV, HFOV, and MFOV, respectively. There were significantly non-zero gradients in the rostral-caudal direction for CMV only (p < 0.05) and in the left-right direction for HFOV only (p < 0.05), however the median gradient (and normalized gradient) values in these cases were only 0.09 and 0.02 min−1 cm−1 (0.29 and 0.10 % cm−1), respectively.
Supplemental Table 1 provides statistical comparisons at multiple isotropic image resolution levels from 0.6 to 4.8 mm. Most of the statistically significant effects reported in this study at the 2.4 mm resolution were consistent across the range of image resolutions examined, particularly median, mean, and ventral-dorsal gradient. Note that all image-based ventilation measures were estimated by exponential regression of voxel values (Equation 3) after downsampling each individual volumetric image, as opposed to simply downsampling the resulting itself.
Figure 5 shows a comparison of regional patchiness using recursive octree or supervoxel decomposition to obtain multiple ROIs with internally consistent voxel values. Each ROI represents a contiguous region of increased or reduced relative to the neighboring regions. The distribution of ROI sizes therefore provides quantification of the size scale of regional ventilation heterogeneity. Mean ROI volumes were consistently smaller using the octree technique compared to the supervoxel technique. A non-parametric Friedman test reported a significant effect of ventilation modality for mean ROI volume using either octree (p < 0.01) or supervoxel (p < 0.01) decomposition. HFOV resulted in larger mean ROI volume compared to CMV (p < 0.01) and MFOV (p < 0.001), but mean ROI volume was not significantly different between CMV and MFOV (octree: p = 0.893, supervoxel: p = 0.735).
Figure 5.
Box-and-whisker plots showing the minimum, maximum, and quartiles across subjects of specific ventilation mean cluster volume after recursive supervoxel (A) or octree (B) decomposition, grouped by ventilation modality. Voxel size was 2.4 mm isotropic. Significant differences between group medians are indicated by * for p < 0.01 and ** for p < 0.001. Two-dimensional transverse slices and three-dimensional perspective renderings of clusters are shown for a representative subject, with randomized colors.
DISCUSSION
This exploratory study of oscillatory ventilation and gas transport in porcine subjects revealed several key findings in comparisons between oscillatory modalities and conventional mechanical ventilation. First, xenon equilibration rates (assessed by median or mean) were comparable between CMV and MFOV, but slower for HFOV. Thus, with considerably smaller regional volume changes, MFOV was able to mimic gas exchange rates similar to CMV and significantly faster than HFOV. The median time duration required for xenon concentration to reach 99.3% of its equilibrium value (i.e., occurring after a duration of ) was 139 seconds during HFOV, compared to 109 seconds during MFOV or 84 seconds during CMV. Thus any sudden changes in gas composition, e.g., due to fractional inspired oxygen or bias flow, influence patient blood gas content more rapidly during MFOV and CMV compared to HFOV. Similarly to a previous study of gas exchange during MFOV in premature surfactant-deficient lambs 23, MFOV produced a substantial reduction in ventilatory cost function compared to HFOV, indicating greater ventilatory efficiency with lower lung stretch.
Second, overall gas transport heterogeneity (assessed by coefficient of dispersion) was not significantly different across ventilatory modality. By contrast, spatial gradients in the ventral-dorsal direction—aligned with the gravitational field—were significantly non-zero for all modalities. Thus the dependent regions of the lung exhibited the fastest rates of xenon equilibration regardless of modality. However, the ventral-dorsal gradient magnitude tended to be lower during oscillatory modalities compared to CMV, although this difference was not statistically significant. In addition, our analysis of regional patchiness demonstrated clustering of with larger sizes during HFOV compared to CMV and MFOV. This large-scale spatial clustering during HFOV likely reflects lower mean compared to CMV and MFOV, despite similar total coefficient of variation. Smaller mean cluster volumes during CMV and MFOV implies reduced large-scale patchiness, i.e., a reduced tendency to develop large, contiguous regions of relatively increased or reduced ventilation. During CMV, the tendency for higher ventral-dorsal gradient may have also contributed to smaller cluster sizes: large spatial gradients may be a source of increased variance within each cluster, requiring further decomposition into smaller clusters to achieve internally consistent voxel values within each cluster.
The major results of this study were consistent despite repeated analyses at multiple image resolutions. Image analysis without downsampling affords enhanced spatial resolution at the expense of increased susceptibility to noise. Variability in voxel value throughout the XeCT image sequence may arise from a variety of unintended sources, including misalignment of regional structures between images, imperfect synchronization between image acquisition and ventilation, and CT imaging noise within each image. Spatial averaging of voxel values at each time point mitigates the influence of noise sources on estimation of regional time constants and 35. Nevertheless, group-wise differences in the primary outcomes of this study ( median, coefficient of dispersion, and spatial gradients) were robust to the choice of spatial resolution. Measurements of regional patchiness using recursive octree and supervoxel decomposition were only performed at the 2.4 mm image resolution level due to susceptibility of these techniques to noise and regional variability.
These findings support the conclusions of previous imaging studies revealing reduced gravitational dependence of during HFOV compared to CMV 28,43, although the difference was not statistically significant in this study. Mulreany et al. suggested that the discrepancy in XeCT-derived gravitational dependence between CMV and HFOV indicates a decoupling of regional ventilation and mechanics during HFOV 28. By contrast, Reinhardt et al. demonstrated strong correlation between XeCT-derived measurements of and regional volumetric strain obtained by respiratory-gated image registration during CMV 30. However, this correlation may weaken during HFOV due to fundamental differences in determinants of flow distribution 1,39 and mechanisms of gas transport 17,32. Such differences may be exacerbated by heterogeneous mechanical properties throughout the lungs, depending on the oscillation frequency 1,38. Reduced tidal volume and enhanced intrapulmonary mixing during HFOV lead to reduced correlation between regional strain and ventilation, whereas ventilation during CMV occurs predominantly by bulk advection of fresh gas into the alveolar spaces 4,17,41. By comparison, the MFOV waveforms used in this study consisted of frequencies between 5 to 20 Hz, and therefore may rely on intrapulmonary mixing similarly to HFOV. However, MFOV was found to produce greater median than HFOV, indicating advantageous gas transport achieved by the use of the additional frequency components in the ventilator waveform.
Several studies using positron emission tomography with nitrogen-13 tracer gas found enhanced ventilation in the basal regions of canine lungs during HFOV at frequencies of 3 and 6 Hz compared to either CMV or 9 Hz HFOV 40,43. We found no evidence of rostral-caudal gradients in during HFOV or MFOV. There was a significantly non-zero gradient in the rostral-caudal direction observed during CMV yet with a relatively small magnitude. The lack of apical-basal gradients of observed in this study may be a consequence of the limited axial field of view. We report results over only 43.2 mm of axial coverage—compared to 80 mm in Yamada et al. 43—as a result of CT scanning in axial mode for high temporal resolution, and excluding 7.2 mm at the edges of the image following image registration. Slight differences in tidal volume and frequency may also contribute to variability in fresh gas ventilation of alveolar spaces, which dramatically alters the regional specific ventilation 40. There may also be differences in ventilation distribution across different species and subject sizes: 10.3 kg pigs in this study vs. 16.2 kg dogs in Yamada et al. 43. Mulreany et al. did not observe apical-basal gradients in 19.2 kg dogs using XeCT over 128 mm of axial coverage 28.
Limitations
An important limitation of this study is the single-frequency HFOV waveform against which MFOV was compared. Our HFOV waveform consisted of a single frequency at 5 Hz, whereas our MFOV waveform included frequencies up to 20 Hz. It is therefore possible that differences in measured outcomes during MFOV may be attributed to the use of higher frequencies above 5 Hz, the effect of combining multiple frequencies, or both factors together. Higher frequencies during HFOV are associated with reduced pressure amplitudes in the lung periphery but also increased regional heterogeneity 8,25,44, depending on characteristics of lung mechanical impedance such as the resonant and corner frequencies 15. Thus regional flow heterogeneity (and gas exchange heterogeneity) at a specific frequency may be disadvantageous during HFOV, yet potentially advantageous during MFOV when it is combined with other complementary frequencies 18. Healthy pigs of this size exhibit typical resonant frequencies of about 6 to 7 Hz 13, slightly higher than the 5 Hz frequency used in our HFOV waveform. However our MFOV waveform included additional higher frequencies up to 20 Hz, which may have resulted in increased frequency-dependent flow heterogeneity. Further investigation across a wider range of HFOV and MFOV frequencies may distinguish which waveform characteristics determine the most efficacious gas exchange and lung protective characteristics.
Dual-energy CT scanning was not used in this study, but may provide enhanced signal-to-noise ratio for quantification of regional xenon concentration 9. With conventional single-energy CT, changes in xenon concentration are assessed by changes in Hounsfield units, which may also be affected by changes in regional aeration due to inflation, deflation, atelectasis, cardiogenic motion, etc. These extraneous signals were compensated in this study by respiratory gating and image registration, but might be further mitigated using dual-energy CT.
The size and arrangement of dead space may affect regional gas transport rates and specific ventilation in different ways depending on the ventilation modality 4. In this study, the dead space was minimized by placing a shortened endotracheal tube via tracheostomy. It is difficult to predict whether the relative differences in gas transport rates observed in this study would persist given standard endotracheal tube lengths and positioning. Furthermore the effects of varying dead space on gas transport rates during MFOV are yet unknown.
Another important limitation of XeCT imaging is the over three-fold increased density of 60%-40% xenon-oxygen gas mixtures compared to air or air-oxygen mixtures. Dense gas mixtures may tend to distribute preferentially towards dependent lung regions at low flow rates, following the gravitational field 9. Furthermore, dense gas mixtures result in reduced lung resonant frequency (i.e., the frequency maximizing the ratio of volume to pressure amplitudes) 3,31. Since frequency-dependent flow heterogeneity increases above resonance 8,17, the use of xenon during HFOV may result in relatively more flow heterogeneity compared to ventilation with the same frequency and dry air. Therefore regional gas transport rates during xenon wash-in may be altered during ventilation with air-oxygen mixtures. The addition of helium to the gas mixture (e.g., 40%-40%-20% helium-xenon-oxygen) may partially compensate for the high-density xenon gas. However, such mixtures may still be two-fold more dense than dry air 9. Although these limitations warrant caution when extrapolating these results to ventilation using the same settings with dry air, the relative differences between each modality (i.e., CMV, HFOV, and MFOV) may still be preserved.
CONCLUSION
Xenon wash-in equilibration rates were comparable between conventional mechanical ventilation and multi-frequency oscillatory ventilation, but 21% to 39% slower for high-frequency oscillatory ventilation. By contrast, the root-mean-square delivered volume was lowest for MFOV, increased by 70% during HFOV and 365% during CMV. Overall gas transport heterogeneity was similar across all modalities, but gravitational gradients and regional patchiness of specific ventilation may contribute to regional ventilation heterogeneity depending on ventilator modality. Thus MFOV combines key benefits of low lung stretch, similar to high-frequency oscillatory ventilation, with fast rates of gas transport, similar to conventional mechanical ventilation. Such features may be highly desirable across many etiologies from acute respiratory failure presenting with heterogeneous changes in lung mechanics.
Supplementary Material
ACKNOWLEDGMENTS
Andrea Fonseca da Cruz, Bakir Hajdarevic, Eli Schmidt, Chelsea Sloan, and Kelly M. Stark assisted with performing the experimental protocol. Melissa Saylor, Jarron Atha, and Shayna Hogue assisted with computed tomographic imaging. ZOLL Medical Corporation generously loaned a portable ventilator for use in animal experiments. The authors are deeply grateful for their contributions.
Sources of Funding
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Medical Research Program under Award No. W81XWH-16-1-0434. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. This study was also supported by the Department of Anesthesia at the University of Iowa Hospital and Clinics, and by National Institutes of Health awards R41 HL140640 and R01 HL142625. This work utilized a CT scanner that was purchased with funding from National Institutes of Health Shared Instrumentation Grant 1S10OD018526. This research was supported in part through high-performance computing resources provided by The University of Iowa, Iowa City, Iowa.
ABBREVIATIONS
- CMV
Conventional mechanical ventilation
- CO2
Carbon dioxide
- CT
Computed tomography
- HFOV
High-frequency oscillatory ventilation
- HU
Hounsfield units
- MFOV
Multi-frequency oscillatory ventilation
- ROI
Region of interest
- XeCT
Xenon contrast-enhanced computed tomography
- [Xe]
Xenon concentration
GLOSSARY OF TERMS
- D 0
Model-predicted CT density at scan sequence initiation (HU)
- D ∞
Model-predicted CT density at final equilibrium state (HU)
- FiO2
Fraction of oxygen in inspired gas (%)
- g RC
Spatial gradient of specific ventilation, rostral-caudal direction (min−1 cm−1)
- g RL
Spatial gradient of specific ventilation, right-left direction (min−1 cm−1)
- g VD
Spatial gradient of specific ventilation, ventral-dorsal direction (min−1 cm−1)
- I
Volumetric CT density image (HU)
- I n→0
Result of deforming the nth image to align structures with the initial image (HU)
- ΔI
Difference in CT density relative to initial time point (HU)
Model-predicted CT density image (HU)
- M
Lung segmentation mask
- n
Image index in xenon wash-in sequence
- N
Number of images in xenon wash-in sequence
Mean airway pressure (cmH2O)
- PaCO2
Partial pressure of carbon dioxide in arterial blood (mmHg)
- P a O 2
Partial pressure of oxygen in arterial blood (mmHg)
Specific ventilation (min−1)
- t
Time (s)
- T
Time duration of the ventilatory cycle (s)
- V
Volume (mL)
Time-averaged volume over the ventilatory cycle (mL)
- V rms
Root-mean-square volume (mL)
Mean cluster size after recursive octree or supervoxel decomposition (mL)
- V C
Ventilatory cost function (mL2 mmHg g−1)
- Wt
Body weight (g)
- x
Vector of 3-dimensional spatial coordinates (cm)
Spatial axis corresponding to the rostral-caudal direction
Spatial axis corresponding to the right-left direction
Spatial axis corresponding to the ventral-dorsal direction
- τ
Model-predicted time constant of regional xenon equilibration (min)
Footnotes
Conflict of Interest
Authors JH and DWK are cofounders and shareholders of OscillaVent, Inc. Authors JH and DWK are consultants for ZOLL Medical Corporation. Author DWK was a Scientific Advisor to Monitor Mask, Inc. Authors EAH and JMR are cofounders and shareholders of VIDA Diagnostics, Inc. The remaining authors do not have any relevant conflicts of interest to disclose.
REFERENCES
- 1.Amini R, and Kaczka DW. Impact of ventilation frequency and parenchymal stiffness on flow and pressure distribution in a canine lung model. Ann. Biomed. Eng 41:2699–2711, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonett DG Confidence interval for a coefficient of quartile variation. Comput. Stat. Data Anal 50:2953–2957, 2006. [Google Scholar]
- 3.Calvet JH, Louis B, Giry P, Harf A, and Isabey D. Effect of gas density variations on respiratory input impedance in humans. Respir. Physiol 104:241–250, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Chang HK Mechanisms of gas transport during ventilation by high-frequency oscillation. J. Appl. Physiol 56:553–563, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Choi J, Xia G, Tawhai MH, Hoffman EA, and Lin C-L. Numerical study of high-frequency oscillatory air flow and convective mixing in a CT-based human airway model. Ann. Biomed. Eng 38:3550–71, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conze PH, Noblet V, Rousseau F, Heitz F, de Blasi V, Memeo R, and Pessaux P. Scale-adaptive supervoxel-based random forests for liver tumor segmentation in dynamic contrast-enhanced CT scans. Int. J. Comput. Assist. Radiol. Surg 12:223–233, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Fortune JB, and Wagner PD. Effects of common dead space on inert gas exchange in mathematical models of the lung. J. Appl. Physiol 47:896–906, 1979. [DOI] [PubMed] [Google Scholar]
- 8.Fredberg JJ, Keefe DH, Glass GM, Castile RG, and Frantz ID. Alveolar pressure nonhomogeneity during small-amplitude high-frequency oscillation. J. Appl. Physiol 57:788–800, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Fuld MK, Halaweish AF, Newell JD, Krauss B, and Hoffman EA. Optimization of dual-energy xenon-computed tomography for quantitative assessment of regional pulmonary ventilation. Invest. Radiol 48:629–37, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerard SE, Herrmann J, Kaczka DW, Musch G, Fernandez-Bustamante A, and Reinhardt JM. Multi-resolution convolutional neural networks for fully automated segmentation of acutely injured lungs in multiple species. Med. Image Anal 60:101592, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerard SE, Herrmann J, Kaczka DW, and Reinhardt JM. Transfer learning for segmentation of injured lungs using coarse-to-fine convolutional neural networks. In: Image Analysis for Moving Organ, Breast, and Thoracic Images. Springer International Publishing, 2018, pp. 191–201. [Google Scholar]
- 12.Glenny RW, and Robertson HT. Spatial distribution of ventilation and perfusion: mechanisms and regulation. Compr. Physiol 1:375–95, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann J, Gerard SE, Shao W, Hawley ML, Reinhardt JM, Christensen GE, Hoffman EA, and Kaczka DW. Quantifying regional lung deformation using four-dimensional computed tomography: A comparison of conventional and oscillatory ventilation. Front. Physiol 11:1–20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann J, Hoffman EA, and Kaczka DW. Frequency-selective computed tomography: Applications during periodic thoracic motion. IEEE Trans. Med. Imaging 36:1722–1732, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann J, Lilitwat W, Tawhai MH, and Kaczka DW. High-frequency oscillatory ventilation and ventilator-induced lung injury: Size does matter. Crit. Care Med 48:e66–e73, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann J, Reinhardt JM, Hoffman EA, and Kaczka DW. Xenon-enhanced CT for measurement of regional gas transport during oscillatory ventilation. Am. J. Respir. Crit. Care Med 197:A4477, 2018. [Google Scholar]
- 17.Herrmann J, Tawhai MH, and Kaczka DW. Regional gas transport in the heterogeneous lung during oscillatory ventilation. J. Appl. Physiol 121:1306–1318, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann J, Tawhai MH, and Kaczka DW. Parenchymal strain heterogeneity during oscillatory ventilation: Why two frequencies are better than one. J. Appl. Physiol 124:653–663, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrmann J, Tawhai MH, and Kaczka DW. Strain, strain rate, and mechanical power: An optimization comparison for oscillatory ventilation. Int. J. Numer. Method. Biomed. Eng 35:e3238, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman EA Effect of body orientation on regional lung expansion: A computed tomographic approach. J. Appl. Physiol 59:468–480, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman EA, and Chon D. Computed tomography studies of lung ventilation and perfusion. Proc. Am. Thorac. Soc 2:492–498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson NJ, Luks AM, and Glenny RW. Gas exchange in the prone posture. Respir. Care 62:1097–1110, 2017. [DOI] [PubMed] [Google Scholar]
- 23.Kaczka DW, Herrmann J, Zonneveld CEE, Tingay DG, Lavizzari A, Noble PB, and Pillow JJ. Multifrequency oscillatory ventilation in the premature lung: effects on gas exchange, mechanics, and ventilation distribution. Anesthesiology 123:1394–1403, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein S, Staring M, Murphy K, Viergever MA, and Pluim JPW. Elastix: A toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 29:196–205, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Yi Y, Wang M, Chen Q, Huang Y, Liu L, Xie J, Zhou D, and Qiu H. Higher frequency ventilation attenuates lung injury during high-frequency oscillatory ventilation in sheep models of acute respiratory distress syndrome. Anesthesiology 119:398–411, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Lusic H, and Grinstaff MW. X-ray computed tomography contrast agents. Chem. Rev 113:1641–1666, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcucci C, Nyhan D, and Simon BA. Distribution of pulmonary ventilation using Xe-enhanced computed tomography in prone and supine dogs. J. Appl. Physiol 90:421–430, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Mulreany DG, Simon BA, Murphy KJ, and Easley RB. Volumetric xenon-CT imaging of conventional and high-frequency oscillatory ventilation. Acad. Radiol 16:718–725, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perchiazzi G, Rylander C, Derosa S, Pellegrini M, Pitagora L, Polieri D, Vena A, Tannoia A, Fiore T, and Hedenstierna G. Regional distribution of lung compliance by image analysis of computed tomograms. Respir. Physiol. Neurobiol 201:60–70, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Reinhardt JM, Ding K, Cao K, Christensen GE, Hoffman EA, and V Bodas S. Registration-based estimates of local lung tissue expansion compared to xenon CT measures of specific ventilation. Med. Image Anal 12:752–763, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotger M, Farré R, Navajas D, and Peslin R. Respiratory input impedance up to 256 Hz in healthy humans breathing foreign gases. J. Appl. Physiol 75:307–320, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Roth CJ, Förster KM, Hilgendorff A, Ertl-Wagner B, Wall WA, and Flemmer AW. Gas exchange mechanisms in preterm infants on HFOV – a computational approach. Sci. Rep 8:1–8, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rousseeuw PJ Least median of squares regression. J. Am. Stat. Assoc 79:871–880, 1984. [Google Scholar]
- 34.Simon BA Non-invasive imaging of regional lung function using x-ray computed tomography. J. Clin. Monit. Comput 16:433–442, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Simon BA, Marcucci C, Fung M, and Lele SR. Parameter estimation and confidence intervals for Xe-CT ventilation studies: a Monte Carlo approach. J. Appl. Physiol 84:709–716, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Skillings JH, and Mack GA. On the use of a Friedman-type statistic in balanced and unbalanced block designs. Technometrics 23:171–177, 1981. [Google Scholar]
- 37.Sklar MC, Fan E, and Goligher EC. High-frequency oscillatory ventilation in adults with ARDS: Past, present, and future. Chest 152:1306–1317, 2017. [DOI] [PubMed] [Google Scholar]
- 38.Tsuzaki K, Hales CA, Strieder DJ, and Venegas JG. Regional lung mechanics and gas transport in lungs with inhomogeneous compliance. J. Appl. Physiol 75:206–16, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Venegas JG, Tsuzaki K, Fox BJ, Simon BA, and Hales CA. Regional coupling between chest wall and lung expansion during HFV: A positron imaging study. J. Appl. Physiol 74:2242–52, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Venegas JG, Yamada Y, Burnham C, and Hales CA. Local gas transport in eucapnic ventilation: Effects of gravity and breathing frequency. J. Appl. Physiol 68:2287–2295, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Venegas JG, Yamada Y, Custer J, and Hales CA. Effects of respiratory variables on regional gas transport during high-frequency ventilation. J. Appl. Physiol 64:2108–18, 1988. [DOI] [PubMed] [Google Scholar]
- 42.West JB Respiratory Physiology: The Essentials. Philadelphia, PA: Lippincott Williams & Wilkins, 2012, 200 pp. [Google Scholar]
- 43.Yamada Y, Burnham C, Hales CA, and Venegas JG. Regional mapping of gas transport during high-frequency and conventional ventilation. J. Appl. Physiol 66:1209–18, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Zannin E, Dellacà RL, Dognini G, Marconi L, Perego M, Pillow JJ, Tagliabue PE, and Ventura ML. Effect of frequency on pressure cost of ventilation and gas exchange in newborns receiving high-frequency oscillatory ventilation. Pediatr. Res 82:994–999, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.