Abstract
Recent reports of natural paratuberculosis (or Johne's disease) in rabbits, foxes, and stoats has focused debate on the presence and importance of wildlife reservoirs in the epidemiology of this disease. This paper describes an extensive study investigating 18 nonruminant wildlife species for evidence of paratuberculosis. Using both culture and histopathological analysis, fox, stoat, weasel, crow, rook, jackdaw, rat, wood mouse, hare, and badger were found to harbor Mycobacterium avium subsp. paratuberculosis, the causative organism of paratuberculosis, suggesting that the epidemiology of this disease is more complex than previously realized.
Paratuberculosis, or Johne's disease, is a chronic enteritis of ruminants caused by Mycobacterium avium subsp. paratuberculosis. The disease is found worldwide and causes considerable economic loss to affected farms and industries. In one study it was estimated that paratuberculosis costs the U.S. dairy industry alone 200 to 250 million dollars annually (29). Paratuberculosis in Scotland is of growing importance, due to the increasing incidence of the disease in domestic livestock, especially cattle (1), and possible zoonotic links with Crohn's disease (36).
The bacterium is passed from animal to animal by the fecal-oral route. Young animals (less than 30 days old) are most susceptible to infection, although clinical disease is apparent only after a long incubation period of 2 to 3 years. There is no effective treatment for paratuberculosis, and current control measures rely upon prompt culling of infected animals and preventing the introduction of the disease into uninfected herds and flocks. Control and eradication programs for paratuberculosis are hampered by difficulties in the diagnosis of the condition, especially during the long subclinical stage of the disease, and the lack of an accurate understanding of the epidemiology of paratuberculosis.
The existence and importance of wildlife reservoirs of M. avium subsp. paratuberculosis is still undetermined. Natural paratuberculosis infection has been well documented in wild ruminants, including white-tailed deer (Odocoileus virginianus) (7), red (Cervus elaphus) and roe (Capreolus capreolus) deer (33), bighorn sheep (Ovis canadenis) (39), tule elk (Carvus nannodes) (17), and bison (Bison bison) (5), but there have been few investigations examining nonruminant wildlife for the presence of M. avium subsp. paratuberculosis. Until recently, it was accepted that the natural host range of M. avium subsp. paratuberculosis was restricted to ruminant species, with infection of nonruminant species possible only under experimental conditions, for example, in rabbits (25, 26), lemmings (20), hamsters (14), guinea pigs (10), and mice (13). However, recent investigations in Scotland have indicated that the host range is broader than this, with reports of natural M. avium subsp. paratuberculosis infection of free-living rabbits (11, 12), foxes and stoats (2). If present, sylvatic cycles of M. avium subsp. paratuberculosis may undermine control and eradication programs and facilitate farm-to-farm spread of this disease.
The aim of this study was to screen a wide range of wildlife species from a paratuberculosis-affected area in Scotland and examine them for evidence of M. avium subsp. paratuberculosis infection. Carnivores, including foxes, stoats, and weasels, as well as carrion-eating birds and rodents, were found to harbor the organism, suggesting that M. avium subsp. paratuberculosis has a much wider natural host range than previously suspected.
MATERIALS AND METHODS
Collection of samples.
A total of 591 samples, from 18 species, were collected from August 1998 to October 1999 from four farms and adjacent properties in eastern Scotland: two in Angus and two in the Perth and Kinross regions. All four farms had a history of moderate-to-high levels of paratuberculosis in cattle and rabbits, and one also had confirmed cases of paratuberculosis in sheep. All farms were greater than 20 km apart from each other and thus were considered independent. Most samples were collected as part of vermin control programs conducted by landowners. The hares, pheasants, and badgers were road casualties, while the buzzard died after it collided with electricity power lines. Post mortem examination was conducted within 12 h of collection, except with a small number of cases where a delay of up to 24 h was unavoidable. A small number of samples (mainly stoats) were stored frozen and thus excluded from histopathological analyses. The abdomen was the only body cavity opened on post mortem, and the following samples were taken for both culture and histopathology: duodenum, ileum, cecum, colon, and mesenteric lymph node (MLN). A sample of liver was also included for histopathological examination. Due to the sizes of MLNs in small mammals and birds, collection of this tissue was not always possible. A sample of feces was taken from 27 foxes, 12 crows, 2 wood mice, 3 hares, 6 stoats, 7 rats, 12 sparrows, 1 buzzard, and 1 rook for culture. Urine was collected and cultured from 13 foxes.
Histopathology.
Samples were fixed in 10% formal saline for a minimum of 24 h and then trimmed, dehydrated through graded alcohols, and embedded in paraffin wax. Five-micrometer-thick sections were cut and stained with hematoxylin and eosin for routine histopathological examination and to test for acid-fast bacilli (AFB) by the Ziehl-Neelsen (ZN) method. Samples from 24 stoats, 12 rats, 1 fox, and 1 badger were not processed for histopathology due to advanced autolysis of the tissues.
Culture and PCR.
Gut and MLN samples (where possible) and fecal samples were cultured as described earlier (11). After homogenization and decontamination, samples were inoculated onto two slants of Middlebrook 7H11 agar supplemented with 20% (vol/vol) heat-inactivated newborn calf serum, Selectatabs (amphotericin B, polymixin B, carbenicillin, and trimethoprim; code MS 24; MAST Laboratories, Ltd., Merseyside, United Kingdom), 10% Middlebrook oleic acid-albumin-dextrose-catalase enrichment medium (Difco, Surrey, United Kingdom), and 2 μg of mycobactin J (Allied Monitor, Fayette, Mo.) per ml. The cultures were incubated at 37°C for up to 16 weeks and examined regularly for bacterial growth. The majority of tissues were pooled for each animal, with the exception of those of 17 foxes and 25 stoats, for which MLN and intestine samples were cultured separately. Urine samples were centrifuged at 3,800 × g for 30 min at room temperature, and the deposit was resuspended in 10 ml of 0.75% hexadecyl pyridinium chloride. This suspension was left to stand at room temperature overnight, and then the supernatant was processed as for feces and tissue.
Using a PCR-based detection method, all mycobacterial isolates were screened for the presence of the species-specific IS900 insertion sequence. This DNA sequence is found only in M. avium subsp. paratuberculosis (37). Briefly, 200 μl of sterile distilled water was inoculated with a single bacterial colony from each positive culture. The mycobacteria were lysed using a Hybaid (Ashford, United Kingdom) ribolyzer at 5.5 m per second for 20 s, with cooling on ice before and after treatment. The DNA was extracted using guanidine hydrochloride as described previously (6). Five microliters of DNA was analyzed using a PCR-enzyme-linked immunosorbent microplate assay (K. Stevenson et al., unpublished data). Briefly, PCR was carried out using primers 90 and 91 as described previously (11), except that primer 91 was biotinylated. The PCR products were captured on streptavidin-coated microplates after heat denaturation and hybridization to a 2,4-dinitrophenyl-labeled oligonucleotide probe complementary to the amplified sequence. Amplified products were detected using peroxidase-conjugated rabbit anti-2,4-dinitrophenyl antibody and O-phenylenediamine dihydrochloride. Optical densities at 450 nm were obtained using a standard laboratory microplate reader.
RESULTS
A total of 591 animals, representing 18 different species, were collected and examined (Table 1). M. avium subsp. paratuberculosis was grown from the tissues of 90 animals, and histopathological lesions consistent with a diagnosis of M. avium subsp. paratuberculosis infection were noted in 19 animals.
TABLE 1.
Details of wildlife species collected
| Common name | Scientific name | No. collected |
|---|---|---|
| Fox | Vulpes vulpes | 27 |
| Stoat | Mustela erminea | 37 |
| Weasel | Mustela nivalis | 4 |
| Hare | Lepus europaeus | 6 |
| Badger | Meles meles | 2 |
| Rat | Rattus norvegicus | 35 |
| House mouse | Mus domesticus | 89 |
| Wood mouse | Apodemus sylvaticus | 88 |
| Field vole | Microtus agrestis | 7 |
| Bank vole | Clethrionomys glareolus | 19 |
| Crow | Corvus corone | 60 |
| Rook | Corvus frugilegus | 53 |
| Jackdaw | Corvus monedula | 38 |
| Pheasant | Phasianus colchicus | 4 |
| Buzzard | Buteo buteo | 1 |
| Feral pigeon | Columba livia | 59 |
| Wood pigeon | Columba palumbus | 15 |
| House sparrow | Passer domesticus | 47 |
| Total | 591 |
Culture.
Of a total of 591 animals submitted for culture, M. avium subsp. paratuberculosis was grown from the tissues of 90, representing 10 of the 18 species examined: fox, stoat, weasel, crow, rook, jackdaw, rat, wood mouse, hare, and badger (Table 2). Positive fecal cultures were obtained from fox, stoat, crow, rook, and wood mouse (Table 2). None of the 13 fox urine samples cultured were positive for M. avium subsp. paratuberculosis (Table 3).
TABLE 2.
Summary of culture and histopathology results from each species of wildlife studied
| Species | No. of positive cultures/total no. of cultures
|
No. of histopathology-positive tissue samples/total no. of tissue samples | |
|---|---|---|---|
| Tissue | Feces | ||
| Fox | 23/27 | 3/27 | 12/26 |
| Stoat | 17/37 | 1/6 | 1/13 |
| Weasel | 2/4 | NAa | 2/4 |
| Hare | 1/6 | 0/3 | 0/4 |
| Badger | 1/2b | NA | 0/1 |
| Rat | 3/35 | 0/7 | 0/23 |
| House mouse | 0/89 | NA | 0/89 |
| Wood mouse | 3/88 | 2/2 | 1/88 |
| Field vole | 0/7 | NA | 0/7 |
| Bank vole | 0/19 | NA | 1/19c |
| Crow | 36/60 | 4/12 | 1/60 |
| Rook | 3/53 | 1/1 | 0/53 |
| Jackdaw | 1/38 | NA | 0/38 |
| Pheasant | 0/4 | NA | 0/4 |
| Buzzard | 0/1 | 0/1 | 0/1 |
| Feral pigeon | 0/59 | NA | 0/59 |
| Wood pigeon | 0/15 | NA | 0/15 |
| House sparrow | 0/47 | 0/12 | 0/47 |
| Total | 90/591 | 11/71 | 18/551 |
NA, not available (procedure not carried out on samples).
One badger was collected from outside the sample area. This animal was positive on culture for M. avium subsp. paratuberculosis, but no lesions were noted on histopathological examination.
AFB were noted from one bank vole but were judged unlikely to be M. avium subsp. paratuberculosis.
TABLE 3.
Comparison of results from fox samples, with details of the correlation among the results of tissue culture, fecal culture, and pathology testing
| Tissue culture result | Total no. of samples | No. of samples with lesions in tissues/total no. of samples | No. of urine culture-positive samples/ total no.of samples | No. of fecal-culture-positive samples/ total no. of samples |
|---|---|---|---|---|
| Positivea | 23 | 11/22 | 0/12 | 3/23 |
| Negative | 4 | 1/4 | 0/1 | 0/4 |
| Total | 27 | 12/26 | 0/13 | 3/27 |
Comprised of animals positive on intestinal culture, MLN culture, or both.
Histology.
Tissues from 548 animals were examined histologically. Nineteen samples from the following five species showed lesions consistent with M. avium subsp. paratuberculosis infection: fox, stoat, weasel, crow, and wood mouse. There were similarities noted in the pathologies of the carnivores (foxes, stoat, and weasels). Small numbers of large macrophage-like cells were noted in the MLN and mucosa-associated lymphoid tissue (MALT) of the gut (Fig. 1). These cells were most commonly found around the periphery of the MALT or in the interfollicular area of the cortex of the MLN. They formed a small granuloma consisting of 10 or fewer cells or were identified as single cells interspersed among the lymphocytes. A small number of AFB were identified in the cytoplasms of some of these macrophage-like cells. As few as one AFB was identified in a section.
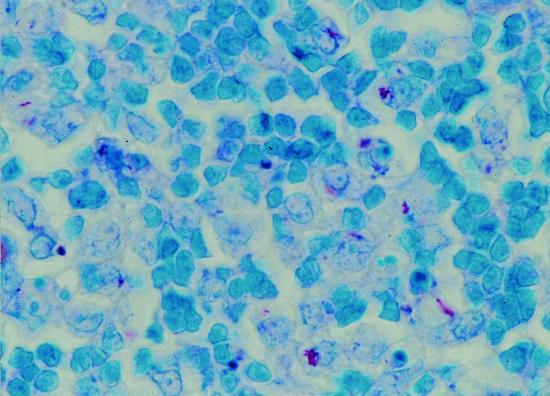
FIG. 1.
ZN-stained section of MALT from an infected fox, showing numerous intracellular AFB (magnification, ×1,000).
AFB were found in one crow, one wood mouse, and one bank vole. The lesions noted in the crow were slight: one section of gut contained AFB-positive cells scattered throughout the lamina propria. Only rarely were there more than five AFB seen in the cytoplasms of these cells. The liver of the crow contained numerous heterophilic granulomata which were invariably AFB negative. The lesions in the wood mouse consisted of numerous AFB-containing macrophage-like cells, both as single cells and as cells in granulomata, scattered throughout the cortex of the MLN. A small number of AFB-positive cells were also noted in the villi of the small intestine. In the bank vole there was extensive and widespread infiltration of the liver parenchyma with numerous granulomata containing macrophages. The cytoplasms of these macrophages contained many AFB. A small number of similar AFB-positive granulomata were noted at the base of the intestinal villi. The AFB were judged to be more elongated than those seen in the carnivores (Fig. 2).
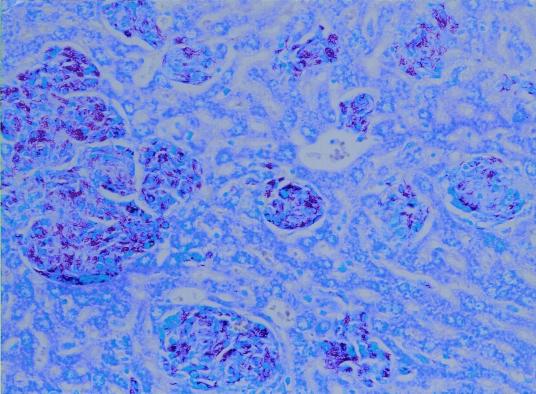
FIG. 2.
Section from the liver of a bank vole, with AFB-containing granulomata scattered throughout the parenchyma (ZN stain; magnification, ×200).
DISCUSSION
Evidence of natural M. avium subsp. paratuberculosis infection was demonstrated in a wide range of wildlife species on paratuberculosis-infected farms in eastern Scotland. M. avium subsp. paratuberculosis was isolated from the tissues of fox, stoat, weasel, crow, rook, jackdaw, rat, wood mouse, hare, and badger. Histopathological lesions consistent with M. avium subsp. paratuberculosis infection were noted in tissues from fox, stoat, weasel, crow, and wood mouse. This survey is the first report of natural M. avium subsp. paratuberculosis isolation from weasel, crow, rook, jackdaw, rat, wood mouse, and badger and provides further details of the initial fox and stoat M. avium subsp. paratuberculosis infections previously reported (2).
This investigation provides strong evidence of enzootic natural M. avium subsp. paratuberculosis infection of foxes. Eighty-five percent of foxes examined showed evidence of M. avium subsp. paratuberculosis infection. Histopathological lesions were consistent with infection by a slow-growing mycobacterial species, such as M. avium subsp. paratuberculosis. All positive histopathological results, except one, were accompanied by culture of M. avium subsp. paratuberculosis from corresponding tissues (Table 3), supporting the hypothesis that the AFB identified in the MALTs and MLNs of the foxes were M. avium subsp. paratuberculosis.
The isolation of M. avium subsp. paratuberculosis from the lymph nodes of the fox and stoat ruled out the possibility that the positive tissue cultures were due to recently ingested infected tissue in the lumen of the intestine. The positive lymph node cultures and associated histopathological changes suggest that carnivores may be chronically infected with M. avium subsp. paratuberculosis and that the organism resides in macrophage-like cells in the lymphoid tissue of the gut, characteristics similar to those of early, subclinical infections described for ruminants (30).
Reports of mycobacterial infection in free-living canid species are rare. Bruning-Fann and coworkers (4) cultured M. bovis from the lymph nodes of three free-living coyotes (Canis latrans) in Michigan. M. bovis has been cultured from tissues of 1.15% of 954 foxes examined in the United Kingdom (19), and natural infection of domestic dogs with mycobacteria has been reported (15, 23). There have been no previous reports of M. avium subsp. paratuberculosis infection of weasels and only one preliminary report of M. avium subsp. paratuberculosis infection of stoats (2). Natural M. bovis infection has been reported in feral ferrets (Mustela furo) (22) and stoats (9).
The pathology noted in the foxes, weasels, and stoats was subtle in comparison to the lesions seen either in advanced ruminant paratuberculosis (8) or in severely infected rabbits (2a), where much more extensive chronic inflammatory changes were seen with far greater numbers of AFB. It is not clear whether the carnivorous species examined in this report eventually develop similar extensive granulomatous lesions or whether they are able to control the multiplication of M. avium subsp. paratuberculosis and limit infection to small areas in the lymphoid compartments of the intestine.
The source of M. avium subsp. paratuberculosis infection in the carnivores in this survey is still a matter of conjecture. The natural diets of foxes, stoats, and weasels includes rabbits and small rodents (21) (18), suggesting that the infected rabbit population on the farms was the most likely source of M. avium subsp. paratuberculosis infection of carnivores, rather than infected ruminants. Foxes ingest only small amounts of soil in their diet (3), so environmental contamination is unlikely to be the major source of M. avium subsp. paratuberculosis organisms. Thus, it may be that M. avium subsp. paratuberculosis infection of carnivores is reliant on a sylvatic cycle of M. avium subsp. paratuberculosis in the rabbit population.
Badgers are known to eat mammals, including rabbits (28). Two badgers were examined in this survey, but only one was collected from within the sample area. This animal was negative on culture, and the tissues were too autolyzed for histopathological examination. The second badger was taken from an area approximately 80 miles northeast of the study area and within 5 miles of a farm whose animals were known to be infected with paratuberculosis. It was positive on culture for M. avium subsp. paratuberculosis but negative by histopathology. However, more extensive examination of the tissues was hampered by advanced autolysis. Badgers in the southwestern portion of the United Kingdom are naturally infected with M. bovis and may play a crucial role in the epidemiology of bovine tuberculosis (16, 19). In light of the suspected role of badgers in bovine tuberculosis, natural infection of badgers with M. avium subsp. paratuberculosis deserves further investigation.
M. avium subsp. paratuberculosis was isolated from tissues of a number of species which had few or no corresponding lesions noted on histopathological examination. These species included crow, rook, jackdaw, rat, wood mouse, and hare (Table 2). This discrepancy may indicate passive transmission and dissemination of ingested M. avium subsp. paratuberculosis-infected material through the gastrointestinal systems of these species. Large numbers of AFB were noted in the liver, gut, and lymph node of a bank vole, but no M. avium subsp. paratuberculosis organisms were grown from the tissues. The particularly extensive liver lesions were not consistent with a diagnosis of paratuberculosis. If we considered the negative culture result, it is probable that the AFB seen were not M. avium subsp. paratuberculosis but another acid-fast species.
While M. avium subsp. paratuberculosis infection of free-living avian species has not previously been reported, they are susceptible to slow-growing mycobacterial species. Naturally occurring avian M. avium complex infections have previously been reported (27, 31, 32, 34, 35). The M. avium subsp. paratuberculosis-positive birds in this survey (crows, rooks, and jackdaws) are carrion eaters (24, 38) and might be expected to ingest rabbit and ruminant tissue, possibly becoming infected by this route. The source of the M. avium subsp. paratuberculosis organisms isolated from the tissues of rats and wood mice is less clear. Scavenging on the floors of barns in which cattle and sheep are housed is a possible route of transmission to the rodents.
No evidence of M. avium subsp. paratuberculosis infection was noted from house mice, feral pigeons, wood pigeons, house sparrows, field voles, pheasants, or a buzzard. Only a few pheasants, field voles, and one buzzard were examined; therefore, it is not possible to make any firm conclusions about the role of these species in the epidemiology of paratuberculosis.
The discovery of M. avium subsp. paratuberculosis in the feces of a wide range of wildlife species suggests that the greatest risk of transmission to cattle and sheep comes from the fecal contamination of feedstuffs and drinking water. All species which were found to be infected with M. avium subsp. paratuberculosis are known to have the potential to contaminate either stored feed (rodents and birds) or pasture (carnivores).
Conclusion.
There is evidence that wildlife in Scotland are naturally infected with M. avium subsp. paratuberculosis and that the host range is much wider than previously thought. The positive fecal cultures from foxes, stoats, crows, rooks, rats, and wood mice suggest that environmental contamination with M. avium subsp. paratuberculosis can occur and can thereby pose a risk to grazing livestock and farms adjoining paratuberculosis-infected properties. Further investigations are required to clarify the role of wildlife in the epidemiology of this important disease.
ACKNOWLEDGMENTS
This work was funded by the Scottish Executive Rural Affairs Department (SERAD). Grateful thanks are extended to the landowners who allowed us access to their wildlife, Valerie Forbes and Alison Baird for technical assistance, and Alastair Wood (VLA Lasswade) for advice on avian histopathology.
REFERENCES
- 1.Anonymous. Scottish Agricultural College Veterinary Science Division summary. Vet Rec. 2000;146:5. [Google Scholar]
- 2.Beard P M, Henderson D, Daniels M J, Pirie A, Buxton D, Greig A, Hutchings M R, McKendrick I, Rhind S, Stevenson K, Sharp J M. Evidence of paratuberculosis in fox (Vulpes vulpes) and stoat (Mustela erminea) Vet Rec. 1999;145:612–613. [Google Scholar]
- 2a.Beard, P. M., S. Rhind, D. Buxton, M. J. Daniels, D. Henderson, A. Pirie, K. Rudge, A. Greig, M. R. Hutchings, K. Stevenson, and J. M. Sharp. Natural paratuberculosis infection in rabbits in Scotland. J. Comp. Pathol., in press. [DOI] [PubMed]
- 3.Beyer W N, Connor E E, Gerould S. Estimates of soil ingestion by wildlife. J Wildl Manag. 1994;58:375–382. [Google Scholar]
- 4.Bruning-Fann C S, Schmitt S M, Fitzgerald S D, Payeur J B, Whipple D L, Cooley T M, Carlson T, Friedrich P. Mycobacterium bovis in coyotes from Michigan. J Wildl Dis. 1998;34:632–636. doi: 10.7589/0090-3558-47.632.636. [DOI] [PubMed] [Google Scholar]
- 5.Buergelt C D, Ginn P. The histopathological diagnosis of early Johne's disease in North American bison (Bison bison) In: Manning E J B, Collins M T, editors. Proceedings of the Sixth International Colloquium on Paratuberculosis. Madison, Wis: International Association for Paratuberculosis; 1999. pp. 395–399. [Google Scholar]
- 6.Challans J A, Stevenson K, Reid H W, Sharp J M. A rapid method for the extraction and detection of Mycobacterium avium subspecies paratuberculosis from clinical specimens. Vet Rec. 1994;134:95–96. doi: 10.1136/vr.134.4.95. [DOI] [PubMed] [Google Scholar]
- 7.Chiodini R J, Vankruiningen H J. Eastern white-tailed deer as a reservoir of ruminant paratuberculosis. J Am Vet Med Assoc. 1983;182:168–169. [PubMed] [Google Scholar]
- 8.Clarke C J. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 9.Cooke M M, Jackson R, Coleman J D. Tuberculosis in a free-living brown hare (Lepus europaeus occidentalis) N Z Vet J. 1993;41:144–146. doi: 10.1080/00480169.1993.35755. [DOI] [PubMed] [Google Scholar]
- 10.Francis J. Infection of laboratory animals with Mycobacterium johnei. J Comp Pathol Theriogenol. 1943;53:140–150. [Google Scholar]
- 11.Greig A, Stevenson K, Henderson D, Perez V, Hughes V, Pavlik I, Hines M E, McKendrick I, Sharp J M. Epidemiological study of paratuberculosis in wild rabbits in Scotland. J Clin Microbiol. 1999;37:1746–1751. doi: 10.1128/jcm.37.6.1746-1751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greig A, Stevenson K, Perez V, Pirie A A, Grant J M, Sharp J M. Paratuberculosis in wild rabbits (Oryctolagus cuniculus) Vet Rec. 1997;140:141–143. doi: 10.1136/vr.140.6.141. [DOI] [PubMed] [Google Scholar]
- 13.Harding H. The histopathology of Mycobacterium johnei infection in small laboratory animals. J Pathol Bacteriol. 1959;78:157–169. doi: 10.1002/path.1700780117. [DOI] [PubMed] [Google Scholar]
- 14.Hirch A. Infection of hamsters and rabbits with Mycobacterium johnei. J Comp Pathol. 1956;66:260–269. doi: 10.1016/s0368-1742(56)80027-1. [DOI] [PubMed] [Google Scholar]
- 15.Hughes M S, James G, Ball N, Scally M, Malik R, Wigney D I, Martin P, Chen S, Mitchell D, Love D N. Identification by 16S rRNA gene analyses of a potential novel mycobacterial species as an etiological agent of canine leproid granuloma syndrome. J Clin Microbiol. 2000;38:953–959. doi: 10.1128/jcm.38.3.953-959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchings M R, Harris S. Quantifying the risks of TB infection to cattle posed by badger excreta. Epidemiol Infect. 1999;122:167–173. doi: 10.1017/s0950268898001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessup D A, Abbas B, Behymer D, Gogan P. Para-tuberculosis in tule elk in California. J Am Vet Med Assoc. 1981;179:1252–1254. [PubMed] [Google Scholar]
- 18.King C M. Stoat Mustela erminea. In: Corbet G B, Harris S, editors. A handbook of British mammals. Oxford, United Kingdom: Blackwell Scientific Press; 1991. pp. 351–387. [Google Scholar]
- 19.Krebs J R. Bovine tuberculosis in cattle and badgers. London, United Kingdom: Ministry of Agriculture; 1997. [Google Scholar]
- 20.Larsen A B, Miller J M. Susceptibility of the lemming (Dicrostonyx rubricatus) to Mycobacterium paratuberculosis. Am J Vet Res. 1979;40:1657–1658. [PubMed] [Google Scholar]
- 21.Leckie F M, Thirgood S J, May R, Redpath S M. Variation in the diet of red foxes on Scottish moorland in relation to prey abundance. Ecography. 1998;21:599–604. [Google Scholar]
- 22.Lugton I W, Wobeser G, Morris R S, Caley P. Epidemiology of Mycobacterium bovis infection in feral ferrets (Mustela furo) in New Zealand. 1. Pathology and diagnosis. N Z Vet J. 1997;45:140–150. doi: 10.1080/00480169.1997.36014. [DOI] [PubMed] [Google Scholar]
- 23.Malik, R., D. N. Love, D. I. Wigney, and P. Martin. 1998. Mycobacterial nodular granulomas affecting the subcutis and skin of dogs (canine leproid granuloma syndrome). Aust. Vet. J. 403–407. [DOI] [PubMed]
- 24.Mason, C. F., and S. M. Macdonald. 1995. Corvids feeding on carrion. 42:255–256.
- 25.Mokresh A H, Butler D G. Granulomatous enteritis following oral inoculation of newborn rabbits with Mycobacterium paratuberculosis of bovine origin. Can J Vet Res. 1990;54:313–319. [PMC free article] [PubMed] [Google Scholar]
- 26.Mokresh A H, Czuprynski C J, Butler D G. A rabbit model for study of Mycobacterium paratuberculosis infection. Infect Immun. 1989;57:3798–3807. doi: 10.1128/iai.57.12.3798-3807.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morishita T Y, Fullerton A T, Lowenstine L J, Gardner I A, Brooks D L. Morbidity and mortality in free-living raptorial birds of northern California: a retrospective study, 1983–1994. J Avian Med Surg. 1998;12:78–81. [Google Scholar]
- 28.Neal E G, Cheeseman C L. Badger Meles meles. In: Corbet G B, Harris S, editors. A handbook of British mammals. Oxford, United Kingdom: Blackwell Scientific Press; 1991. pp. 415–423. [Google Scholar]
- 29.Ott S L, Wells S J, Wagner B A. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev Vet Med. 1999;40:179–192. doi: 10.1016/s0167-5877(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 30.Perez V, Marin J F G, Badiola J J. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J Comp Pathol. 1996;114:107–122. doi: 10.1016/s0021-9975(96)80001-6. [DOI] [PubMed] [Google Scholar]
- 31.Saxegaard F. Serological investigation of Mycobacterium avium and M. avium-like bacteria isolated from domestic and wild animals. Acta Vet Scand. 1981;22:153–161. doi: 10.1186/BF03547504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxegaard F. Om Mykobakterier og mycobakterioser med spesielt henblikk pa nontuberkulose (atypiske) mykobakterier hos dyr i Morge. Nor Veterinaertidsskr. 1985;97:547–555. [Google Scholar]
- 33.Sharp J M, Stevenson K, Challans J A, Ramage C, Hitchcock D, Reid H W. Mycobacterial infections of free living deer in Scotland. In: Chiodini R J, Hines M E, Collins M T, editors. Proceedings of the 5th International Colloquium on Paratuberculosis. Madison, Wis: International Association for Paratuberculosis; 1995. pp. 180–182. [Google Scholar]
- 34.Smit T, Eger A, Haagsma J, Bakhuizen T. Avian tuberculosis in wild birds in the Netherlands. J Wild Dis. 1987;23:485–487. doi: 10.7589/0090-3558-23.3.485. [DOI] [PubMed] [Google Scholar]
- 35.Thoen C O, Himes E M, Barrett R E. Mycobacterium avium serotype 1 infection in a sandhill crane (Grus canadensis) J Wildl Dis. 1977;13:40–42. doi: 10.7589/0090-3558-13.1.40. [DOI] [PubMed] [Google Scholar]
- 36.Thompson D E. The role of mycobacteria in Crohn's disease. J Med Microbiol. 1994;41:74–94. doi: 10.1099/00222615-41-2-74. [DOI] [PubMed] [Google Scholar]
- 37.Vary P H, Andersen P R, Green E, Hermontaylor J, McFadden J J. Use of highly specific DNA probes and the polymerase chain reaction to detect Mycobacterium paratuberculosis in Johne's disease. J Clin Microbiol. 1990;28:933–937. doi: 10.1128/jcm.28.5.933-937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waite R K. Food caching and recovery by farmland corvids. Bird Study. 1985;32:45–49. [Google Scholar]
- 39.Williams E S, Spraker T R, Schoonveld G G. Paratuberculosis (Johne's disease) in bighorn sheep and a rocky mountain goat in Colorado. J Wild Dis. 1979;15:221–227. doi: 10.7589/0090-3558-15.2.221. [DOI] [PubMed] [Google Scholar]