Abstract
Mycotoxins are secondary metabolites of different species of fungi. Aflatoxin B1 (AFB1), deoxynivalenol (DON), zearalenone (ZEN) and fumonisin B1 (FB1) are the main mycotoxins contaminating animal feedstuffs. These mycotoxins can primarily induce hepatotoxicity, immunotoxicity, neurotoxicity and nephrotoxicity, consequently cause adverse effects on the health and performance of animals. Therefore, physical, chemical, biological and nutritional regulation approaches have been developed as primary strategies for the decontamination and detoxification of these mycotoxins in the feed industry. Meanwhile, each of these techniques has its drawbacks, including inefficient, costly, or impractically applied on large scale. This review summarized the advantages and disadvantages of the different remediation strategies, as well as updates of the research progress of these strategies for AFB1, DON, ZEN and FB1 control in the feed industry.
Keywords: Animal health, Feed, Mycotoxin, Performance, Remediation strategies
Introduction
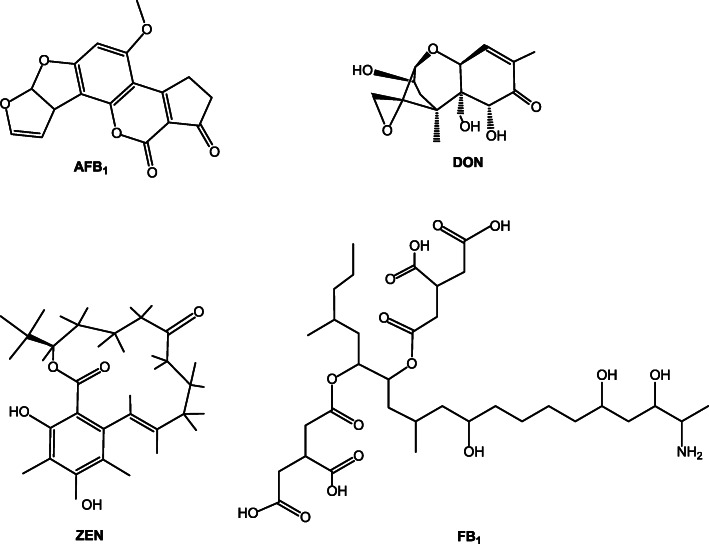
Mycotoxins are secondary metabolites of various species of fungi that can cause chronic or acute toxicity in animals. Although over 500 mycotoxins have been identified, those of importance in feed safety are primarily produced by the five fungal genera Aspergillus, Fusarium, Penicillium, Claviceps and Alternaria [1–5]. Aflatoxin B1 (AFB1), deoxynivalenol (DON), zearalenone (ZEN) and fumonisin B1 (FB1) are well-known as the main mycotoxins contaminating animal feedstuffs, such as corn, barley, wheat, peanuts, peas, nuts, millet, forage, and their by-products [3–6]. The toxicity of these mycotoxins varies depending on their chemical structure (Fig. 1). The most toxic mycotoxin is AFB1, mainly produced by Aspergillus, which is classified as a Group one carcinogen [7]. It displays hepatotoxic, immunotoxic, mutagenic, carcinogenic and teratogenic characteristics in many animal species [8–11]. Notably, all of DON, ZEN and FB1 are primarily produced by Fusarium molds [5, 12]. DON, a type B trichothecene, can induce anorexia, vomiting, and endanger intestinal and immune functions in different animals by inhibiting the synthesis of nucleic acids and proteins [13–16]. ZEN has a similar structure to estrogen and thus competing with 17 β-estradiol for estrogen receptor binding, consequently leading to fertility and reproductive disorders in livestock [16–19]. FB1 is the most plentiful fumonisins, which can cause hepatotoxicity, neurotoxicity, nephrotoxicity, immunotoxicity, developmental toxicity and cancer in humans and animals [20].
Fig. 1.
Structural diversity of AFB1, DON, ZEN and FB1. AFB1: Aflatoxin B1; DON: deoxynivalenol ; ZEN: zearalenone; FB1: fumonisin B1
Mycotoxins have been proven to have significant effects on animal health, performance, as well as quality and safety of products, this led to intensive studies over the past few decades on counteracting methods for mycotoxins control in feedstuffs and feed. Generally, physical, chemical, biological and nutritional regulation approaches have been developed as the main strategies for the detoxification of mycotoxins in the feed industry [21–26]. Nevertheless, many techniques have been proven to be inefficiency, costly, or impractically applied on large scale [21, 22]. The purpose of this review was to summarize the advantages and disadvantages of the various detoxification strategies, as well as update the research progress of these strategies for AFB1, DON, ZEN and FB1 control in the feed industry.
The strategies of mycotoxin reduction and detoxification
Physical methods
Decontamination of mycotoxin by physical techniques mainly includes sorting and separation, washing, solvent extraction, heating, irradiation, and adsorption [27, 28]. The commonly used methods of physical detoxification of mycotoxins are summarized in Table 1.
Table 1.
Summary of physical methods for mycotoxins decontaminationa
| Methods | Commonly used measures and reagents | Decontamination efficiency | References |
|---|---|---|---|
| Sorting and separation | Sieving, aspiration, gravity separation, photoelectric separation, image processing | Removed at least 51%, 63%, 93% of AFs, trichothecenes and fumonisins from the shelled white maize. | [27] |
| Washing and solvent extraction | Washing, solvent extraction (methanol, ethanol, hexane, acetonitrile, isopropanol and aqueous acetone etc.) | Removed aflatoxins, trichothecenes, ZEN and fumonisins by 51-72%, 64-69%, 2-61% and 73-74% from the grains through floating and washing with water. | [25, 27, 29] |
| Heating | High temperature, high voltage |
Decomposited 78-88% of AFB1 in rice by cooking with pressure (0.10 MPa) at 160 °C for 20 min. Destroyed 90% of DON or ZEN in barely power at 220 °C in 11 or 85min. Reduced 80% FB1 while cooking rice at 100 °C for 10 min. |
[30–32] |
| Irradiation |
X-rays, γ-rays and electron beam, ultraviolet rays, infrared and microwave |
Reduced 22.0-90.7% of AFB1 by irradiation. Decomposited 17.2-100% of DON by irradiation. Decontaminated 25.0-86.0% and 60.0-100% of ZEN by γ-rays and ultraviolet rays. FB1 was inactivated by 63.5-100%, 58.1% and 93.3% by γ-rays, electron beam and microwave in feedstuffs. |
[33–41] |
aAFs Aflatoxins, AFB1 Aflatoxin B1, DON deoxynivalenol, ZEN zearalenone, FB1 fumonisin B1
Sorting and separation
The mycotoxins are not uniformly distributed in grains, which mainly appeared in the moldy, broken and discolored parts [42, 43]. Meanwhile, the specific gravity of the mycotoxins-contaminated cereals is relatively lower than the normal ones. These characteristics enable sieving, aspiration, gravity separation, photoelectric separation, image processing techniques to be used to isolate the mycotoxins-contaminated feedstuffs [27, 44]. Specifically, Matumba et al. [27] reported that flotation, dehulling and hand sorting alone can remove at least 51%, 63%, 93% of aflatoxins (AFs), trichothecenes and fumonisins, respectively, from the shelled white maize, while 98% of these mycotoxins can be removed when combining three of these methods. However, these techniques are costly and only suitable for small-scale applications. Aspiration and gravity separation methods can reduce the DON in wheat, while it reduced the yield of harvested grain [21]. Additionally, near-infrared spectroscopy and optical visual sorting strategies can be used to detect the moldy maize and wheat kernels with more than 92% level of accuracy [22–28, 42–46].
Washing and solvent extraction
According to the water-soluble or fat-soluble properties of mycotoxin, it could be decontaminated by washing with water or extraction with organic solvent [47]. Floating and washing with water can remove AFs, trichothecenes, ZEN and fumonisins by 51-72%, 64-69%, 2-61% and 73-74%, respectively, from the grains [25, 27, 29]. Notably, floating and washing with a water solution consists of 10-30% NaCl, 30% sucrose, or 1 mol/L sodium carbonate can increase the removal rate of fumonisins from the corn and wheat [25, 48]. A combination of washing and hand sorting technologies together can reduce 84% of fumonisins [49]. The solvents, including methanol, ethanol, hexane, acetonitrile, isopropanol and aqueous acetone, are most commonly used for mycotoxin extraction. Previous studies showed that hexane-aqueous acetone-water (56%:42%:2%) and dimethyl ether can eliminate over 98% of AFs in oil crops [50, 51]. However, these methods have major disadvantages as they result in loss of nutrients, and costly due to drying and toxic extracts disposal, which limit their large-scale application.
Heating
Thermal treatment has been applied for the decontamination of mycotoxins in feed for many years. The efficiency of this method depends on the chemical structure and concentration of mycotoxins, temperature, duration, moisture content, pH and ionic concentration during the thermal treatment [52]. AFB1, DON, ZEN and FB1 are heat-stable compounds with decomposition temperatures more than 237, 175, 220, 150 °C, respectively [30, 53, 54], which makes it difficult to eliminate them by conventional thermal processing. Conventional hydrothermal treatment (cooking) with pressure (0.10 MPa) at 160 °C for 20 min can decompose AFB1 by 78-88% in rice [31], as well as pressure heating (0.10 MPa) at 120 °C for 4 h can degrade AFB1 by 95% in moist peanut powder [55]. Yumbe-Guevara et al. [30] reported that 90% of DON or ZEN in barley powder can be destroyed at 220 °C for 11 or 85 min. Frying chips at 190 °C for 15 min or drying rice from 150 to 200 °C for 40 min resulted in a loss of 67-70% of FB1, while cooking rice at 100 °C for 10 min reduced 80% of FB1 [32, 56]. Nevertheless, thermal treatments use an excessive amount of energy, also high temperature-induced Maillard reaction would reduce the nutritional values of feed ingredients. This led to a restriction in the application of heat treatments in the feed industry [33].
Irradiation
Irradiation might be a feasible technology for removing mycotoxins from the feed on an industrial scale. It can be classified into ionizing (x-rays, γ-rays and electron beam) and non-ionizing radiations (ultraviolet rays, infrared and microwave) [57, 58]. The action of irradiation on feedstuffs can induce physical, chemical and biological effects, which reduce or eliminate the mycotoxins [59, 60]. Specifically, AFB1 can be reduced by 43.0-87.8%, 65.7-71.5%, 22.0-100%, 90.7% by γ-rays, electron beam, ultraviolet rays and microwave, respectively, in different cereals [33–35]. DON can be decomposed by 37.0-82.4%, 17.2-56.3%, 83.4-100% by γ-rays, electron beam and ultraviolet rays, respectively, in feedstuffs [36–39]. ZEN can be decontaminated by 25.0-86.0% and 60.0-100% by γ-rays and ultraviolet rays, respectively, in grains [34, 36–38]. FB1 was inactivated by 63.5-100%, 58.1% and 93.3% by γ-rays, electron beam and microwave, respectively, in feedstuffs [35, 40, 41]. These different decomposition efficiencies of irradiation depend on the variation in the treatment condition, including doses and time of irradiation, the shape and composition of feedstuffs [61, 62]. Although irradiations can be considered as a potentially promising approach to decontaminate mycotoxins in feedstuffs, their safety issues such as mutagenesis that generates harmful microorganisms and damage the nutritional values of feedstuffs require a declaration and further studies.
Adsorption
Adsorption binders can form a complex with mycotoxins, thus prevent mycotoxins passage from the gastrointestinal tract into the blood and organs of animals. In the past decades, numerous binders from different origins have been investigated for their capacity to adsorb mycotoxins [52, 63]. Therefore, the adsorbent detoxification treatment is currently well understood and widely used to detoxify mycotoxins in the feed industry. In general, any ideal mycotoxin absorbent should possess these following properties, including high adsorption capacity against either range of mycotoxins (especially mycotoxins with low hydrophobicity), low non-specific binding to nutrients, as well as high safety, stability and palatability [52]. Table 2 shows lists of current patents related to adsorbing mycotoxin including AFB1, DON, ZEN and FB1 control in the feed.
Table 2.
Summary of adsorbents with mycotoxins mitigation effectsa
| Adsorbent | Mycotoxins | Binding efficiency | Reference |
|---|---|---|---|
| Zeolite | AFB1 | Decreased AFB1 residue in duck meat by 65% significantly and numerically decreased AFB1 residue in liver and egg. | [64] |
| Bentonite clay | AFB1 | Decreased liver AFB1 residue by 41-87% when broilers fed AFB1 in diet. | [65] |
| Sodium bentonite | AFB1 | Decreased liver AFB1 residue by 62.5% when broilers fed AFB1 in diet. | [66] |
| Modified maifanite | ZEN | Decreased ZEN residue in liver and muscle by 54.96% and 42.41% respectively at the dose of 1% when pig fed 1.11 mg/kg AFB1 in diet. | [67] |
| Bentonite or montmorillonite | AFB1, ZEN | Decreased rumen concentration of AFB1 and ZEN, decreased AFM1 in milk and ZEN in feces. | [68] |
| Organo-clay composites | AFB1 | Decreased AFB1 concentrations in liver, kidney and plasma significantly in chickens. | [69] |
| Tri-octahedral bentonite | DON, ZEN | Adsorbed more than 90% of ZEN and FB1 while the adsorption dose up to 0.20%, w/v. | [70] |
| Pillared montmorillonite | DON | Adsorbed 14.7-23.4% and 21.8-27.4% of DON at at pH 2.0 and pH 6.8. | [71] |
| Nonionic surfactant octylphenol polyoxyethylene ether modified montmorillonites | AFB1, ZEN | The adsorption capacities of modified montmorillonites to AFB1 and ZEN increased up to 2.78 and 8.54 mg/g respectively from 0.51 and 0.00 mg/g by the raw montmorillonite. | [72] |
| Hydrated sodium calcium alumino silicate | AFB1, FB1 | Adsorbed AFB1and FB1 in an aqueous solution, and the adsorption ratio ranged from 95.3% to 99.1% and 84.7% to 92.4%, respectively. | [73] |
| Modified Hydrated sodium calcium alumino silicate | DON | Reduced the toxicity of DON in weaning piglets. | [16] |
| Esterified glucomannan | AFs, ZEN, DON | Adsorbed 95%, 80% and 12% of aflatoxin, ZEN and DON. | [73, 74] |
| Inactivated yeast cell wall and low Yeast fermenting volatile organic compound | AFs, DON | Decreased AFs and DON synthesis by 82% and 93% respectively. | [75] |
| Distillers' wet grain, distillers' dried grains and distillers' dried grain with solubles | DON, ZEN | Adsorbed 48.9% and 67.9% of DON and ZEN (1 ppm each) using 5 g/L of micronized (20 mkm) yeast mass at 37 °C for 1h. | [76] |
| Yeast cell wall extract | ZEN | Adsorbed 40% of the total ZEN content in the intestines in monogastric animals. | [77, 78] |
| Activated charcoal | AFB1, ZEN | Reduced the toxicity of AFB1 on broilers and decreased the absorption rate of ZEN in small intestine from 32% to 5% when adding 2%. | [79, 80] |
| Cholestyramine | ZEN | Decreased the absorption rate of ZEN in small intestine from 32% to 16%. | [80] |
| Magnetic carbon nanocomposites | AFB1 | Adsorbed nearly 90% of AFB1 within 180 min at pH 7.0. | [81] |
| Cross-lined chitosan polymers | AFB1, ZEN, FB1, DON | Adsorbed 73% AFB1, 94% ZEN and 99% FB1, but the adsorption ratio of DON less than 30%. | [82] |
| Polyvinylpyrrolidone | ZEN | Adsorbed 2.1 mg/g of ZEN. | [83] |
| Lactobacillus casei | AFB1 | Reduced the absorption of aflatoxin in the intestinal tract significantly. | [84] |
| Lactobacillus plantarum F22 | AFB1 | Adsorbed 56.8% of AFB1. | [85] |
| Lactobacillus plantarum B7 | FB1 | Adsorbed 52.9% of FB1. | [86] |
| Lactobacillus pentosus X8 | FB1 | Adsorbed 58% of FB1. | [86] |
aAFs Aflatoxins, AFB1 Aflatoxin B1, DON deoxynivalenol, ZEN: zearalenone; FB1: fumonisin B1
Aluminosilicate minerals, as the largest class of mycotoxin adsorbents, are the most widely applied and studied minerals in the decontamination of mycotoxin. Such adsorption binders mainly include bentonite, montmorillonite, zeolite, hydrated sodium calcium aluminosilicate, kaolin, illite, etc. [63]. The binding efficacy of mineral adsorbents is associated with the structures of both the binders and the mycotoxins. The binding efficiency depends significantly on the surface area, charge distribution and pore size of adsorption binders and the charge distribution, polarity and shape of the mycotoxins [52]. Some mycotoxins such as AFs have an ionic charge, thus clay minerals such as bentonite, illite, zeolite and kaolin are effective at removing them from the feed with more than 90% efficiency [87]. Numerous studies reported that zeolite, bentonite clay and sodium bentonite decreased AFB1 residues in the liver by 41-87% and numerically decreased AFB1 residue in the meat and egg when broilers or ducks fed AFB1 contaminated diet [64–66]. Chen et al. [67] reported that ZEN residue in liver and muscle of pigs were decreased by 55.0% and 42.4%, respectively, when supplemented with 1.0% modified maifanite in diet included 1.11 mg/kg ZEN. In ruminant feed, bentonite or montmorillonite decreased rumen concentration of AFB1 and ZEN and also decreased AFM1 in the milk and ZEN in the feces in goats [68]. Tzou et al. [69] prepared organo-clay composites by mixing bentonite-enriched clay with nonionic surfactants (Brij 30 and Igepal CO-890) and added organo-clay composites to feed. After chickens had consumed amended feed for 11 weeks, AFB1 concentrations in the liver, kidney, and plasma were significantly lower than the AFB1 control dietary treatment. Although many aluminosilicate adsorbents can adsorb strongly polar toxins, such as AFB1, FB1, etc. as supported by many studies, they appear to be ineffective at absorbing other non-aflatoxin mycotoxins including DON and ZEN [88, 89]. Bentonites have been considered as promising adsorbents for high-efficient removal of mycotoxins from the animal feed as they are eco-friendly, low-cost and highly efficient in adsorption of mycotoxins, modifying clays also could help to increase their adsorptive ability to non-polar mycotoxins [90–92]. To date, only one di-octahedral bentonite (1m588) was authorized as an anti-aflatoxin additive by the EU Regulation in 2009 [93]. Vila-Donat et al. [70] reported that tri-octahedral bentonite could adsorb more than 90% of ZEN and FB1 while the adsorption dose up to 0.20% (w/v). Nonionic surfactant octylphenol polyoxyethylene ether and modified montmorillonites, as mycotoxins adsorbent, were used for adsorption of AFB1 and weak polar ZEN in both single and binary-contaminate systems by simulating the conditions of the gastrointestinal tract. Modified montmorillonites increased the adsorption capacities to AFB1 from 0.51 mg/g of raw montmorillonite to 2.78 mg/g and ZEN from 0.00 mg/g of raw montmorillonite to 8.54 mg/g [72]. Adsorption of DON by pillared montmorillonite modified with aluminum, iron and titanium was investigated using UPLC-MSMS (at pH 2.0 and 6.8) and the results demonstrated that the adsorption ratios were 14.7-23.4% at pH 2.0 and 21.8-27.4% at pH 6.8 [71]. The commercially hydrated sodium calcium aluminosilicate has an excellent capability of adsorbing AFB1 and FB1 in an aqueous solution, and the adsorption ratio ranged from 95.3-99.1% and 84.7-92.4% of the available AFB1 and FB1, respectively [73]. Mineral adsorbents have been modified with quaternary long-chain alkyl/aryl amines to improve the adsorption of non-aflatoxin mycotoxins [74]. The binder Amdetox™ is mainly comprised of hydrated sodium calcium aluminosilicate that has been modified by cetylpyridinium chloride and intercalation with β-glucan [94]; these modifications increase the surface area of hydrated sodium calcium aluminosilicate, which maximizes the binding of mycotoxins with minimal adsorption of nutrients. Zhang et al. [16] reported that a modified hydrated sodium calcium aluminosilicate adsorbent could reduce the toxicity of DON in weaning piglets [16]. Furthermore, it must be noted that these adsorbents can adsorb micronutrients and have negative effects on the bioavailability of trace minerals and vitamins.
Second generation adsorbents have been developed originating from the cell wall component of microorganisms. Glucomannan is a common adsorbent that cannot be used by gut microbes and strongly adsorbed toxic substances and harmful pathogenic bacteria in animals. Mycotoxins can be adsorbed by esterified glucomannan, which is a kind of broad-spectrum mycotoxin adsorbent with an effective binding ability for AFs, ZEN, FBs and DON by 95%, 75%, 59% and 12%, respectively [73, 74]. Esterified glucomannan has been proved to improve the adverse consequences of mycotoxins on the performance, immunity, blood haematological and biochemical indices of chickens [70, 76, 78, 94]. The β-D-glucan chains of yeast cell walls have been demonstrated to effectively inactivate ZEN [77, 95]. Zeidan et al. [75] reported that inactivated yeast cell walls and low yeast fermenting (L. thermotolerans) volatile organic compounds could decrease AFs and DON synthesis by 82% and 93%, respectively, in vitro. A combination of mineral clay and yeast cell walls showed a considerably enhanced binding capacity of AFs, ZEN and fumonisins in an in vitro study; however, the adsorption abilities toward DON, ochratoxin A and T-2 toxin were low (< 60%) [96]. The yeast biomass obtained from distillers’ wet grain, distillers’ dried grains and distillers’ dried grain with solubles have the ability to bind various mycotoxins and adsorbed 48.9% and 67.9% of DON and ZEN (1.0 mg/kg each), respectively, using 5.0 g/L micronized yeast mass at 37 °C for 1 h [76]. In addition, the yeast cell walls extract adsorbed ZEN in the gastrointestinal tracts of monogastrics [77] and was able to adsorb 40% of the total ZEN contents in the intestines [78].
Activated charcoal, as a general adsorbent, has a large surface area and excellent adsorption capabilities in aqueous environments. Activated charcoal has demonstrated the ability to reduce AFs, ZEN, DON due to its porous structure in several studies [97, 98]. The partial protection induced by activated charcoal in lowering mycotoxin residues in the liver of broilers has been observed previously [65, 99]. The addition of 0.1% activated carbon to feed containing 10 mg/kg AFB1 was able to reduce the detrimental effects of AFB1 on broilers [79]. Avantaggiato et al. [80] found that the absorption rate of ZEN in the small intestine decreased from 32% to 5% when activated carbon was added at 2.0% in an in vitro gastrointestinal model. Cholestyramine is an anion exchange resin. The addition of cholestyramine decreased the absorption rate of ZEN in the small intestine from 32% to 16% using a laboratory model that mimics the metabolic processes of the gastrointestinal tract of healthy pigs [80]. Polyvinylpyrrolidone has good adsorption and selectivity. In vitro adsorption experiments showed that polyvinylpyrrolidone could adsorb 0.3 mg/g of ZEN, and the adsorption capacity of modified polyvinylpyrrolidone could be increased to adsorb 2.1 mg/g of ZEN [83]. Durian peel is an agricultural waste that is widely used for organic and inorganic pollutant adsorption. Adunphatcharaphon et al. [100] reported that the acid-treated durian peel adsorbed 98.4% of AFB1, 98.4% of ZEN, 86.1% of FB1 and 2.0% of DON through its larger surface area and a surface charge modification.
Numerous studies have suggested that removal of mycotoxins with magnetic materials is effective and these are promising adsorbents in the feed industry. Magro et al. [101] reported that the adsorbent-mycotoxin complex was characterized and was structurally and magnetically well conserved [101]. Magnetic carbon nanocomposites produced by maize wastes were used for the removal of AFB1, and the adsorption ratio was nearly 90% within 180 min at pH 7.0 [81]. In addition, cross-linked chitosan polymers as generic adsorbents for simultaneous adsorption could adsorb multiple mycotoxins. Cross-linked chitosan-glutaraldehyde complex presented high adsorption capability for AFB1 (73%), ZEN (94%) and FB1 (99%), but no obvious adsorption for DON and T-2 toxin (< 30%) [82]. Some volatile bioactive compounds have proved to be effective in inhibiting mould growth and reducing mycotoxin accumulation.
The principle of microbial adsorbent detoxification is that the bacterium adsorbs mycotoxins to form a complex and then excretes it together with the toxins, thus reducing the hazard [102]. Lactic acid bacteria and yeast are the most studied microbial adsorbents. Lactobacillus casei can significantly reduce the absorption of aflatoxin in the intestinal tract [84]. Zeng et al. [85] reported that Lactobacillus plantarum F22 had a strong adsorption capacity on AFB1 and the adsorption rate could reach 56.8%. Lactobacillus plantarum B7 and Lactobacillus pentosus X8 can remove 52.9% and 58.0% of FB1 [86]. Halttunen et al. [103] compared the adsorption effect of multiple lactic acid bacteria on aflatoxin and they found that a composite agent consisting of multiple lactic acid bacteria was more effective than a single strain.
Chemical methods
Chemical techniques can destroy the structure of the mycotoxins, which generate mildly toxic or nontoxic products. Decontamination of mycotoxin by chemical techniques primarily includes alkaline and ozone treatments, as well as other chemical agent treatments [104, 105]. The commonly used methods of chemical detoxification of mycotoxins are summarized in Table 3.
Table 3.
Summary of physical methods for mycotoxins detoxificationa.
| Methods | Measures and reagents | Detoxification efficiency | Reference |
|---|---|---|---|
| Alkaline treatment | Ammonia, sodium hydroxide, potassium hydroxide and sodium carbonate etc. | Removed 95% of AFB1 in various cereals by ammoniation and hydroxide salts treatments. | [106–111] |
| Reduced DON by 83.9-100% in different feedstuffs through sodium carbonate and hydroxide salts treatments. | |||
| Ozone treatment | Ozone, hydrogen peroxide, chlorine, sodium and calcium hypochlorite etc. |
Reduced 92-95%, 91% and 78% of AFBs in corn, cottonseed and peanut meal respectively by ozone. DON can be reduced 70-90% in corn and 20-80% in wheat by ozone. The degradation of ZEN in corn can reach 90.7% through the ozone treatment with 100 mg/L ozone for 180 min. |
[112–119] |
aAFB1 Aflatoxin B1, DON deoxynivalenol, ZEN zearalenone, FB1 fumonisin B1
Alkaline treatment
Alkaline chemicals, including ammonia, sodium hydroxide, potassium hydroxide and sodium carbonate, etc., have been used for the destruction of various mycotoxins in the moldy feedstuffs [104, 105]. The lactone ring structure of AFB1 can be opened by base hydrolysis to produce coumarin sodium salt and then further be eliminated by washing with water [120]. Ammoniation and hydroxide salts treatments are the common approach that has been used to remove AFB1 from feed ingredients, with more than 95% removal rate in various cereals [107–110]. An epoxide at C-12 and C-13, essential for the toxicity of DON, can be destructed under alkaline conditions [28]. Sodium carbonate and hydroxide salts treatments can reduce DON by 83.9-100% in different feedstuffs [111, 112]. Although these treatments could nearly reduce the complete concentration of mycotoxins, the possible transformation of mycotoxins to other forms such as masked mycotoxins, along with the harmful side effects on the environment and food (changes in nutritional quality, texture, or flavor), the quality and safety assessments of chemically treated products are necessary [104, 105].
Ozone treatment
Mycotoxin oxidizing agent treatment is an effective detoxification method through changing the molecular structure of mycotoxins. The oxidizers commonly used are ozone, hydrogen peroxide, sodium and calcium hypochlorite, chlorine and other oxidizers [106, 121]. AFs, DON, ZEN and FB1 have been shown to be effectively degraded by ozone [122–124]. Agriopoulou et al. [125] has found that ozone has the ability to degrade AFs (AFB1, AFB2, AFG1 and AFG2). Trombete et al. [126] reported that ozone concentration, form and exposure time influenced positively the reduction of DON, AFs and fungal count. AFs can be reduced by 92-95% in corn and by 91% or 78% in cottonseed or peanut meal, respectively, by ozone [113, 127, 128]. DON can be reduced by 70-90% in corn and by 20-80% in wheat by ozone [112, 114–116]. The degradation of ZEN in corn can reach 90.7% through the ozone treatment with 100 mg/L ozone for 180 min [117]. Furthermore, there are other oxidizing agents such as sodium hypochlorite and hydrogen peroxide that can effectively degrade mycotoxins [118, 119, 129, 130].
Although the ozone treatment can result in a complete reduction in the mycotoxin concentration, it can cause changes in the physical and chemical composition of the feed, such as changes in starch structure, lipid oxidation, protein denaturation, color change and processing properties [106, 113, 126]. Moreover, these treatments may produce some harmful chemicals to the health of animals [106, 113, 126].
Biological methods
Although many physical and chemical decontamination strategies have been developed to reduce or eliminate mycotoxins in feed ingredients or complete feed, few techniques met the requirements of practical application owing to their limitation of binding efficiency, bio-safety or cost-effectiveness. Therefore, as a promising strategy, biodegradation of mycotoxin by microorganism or enzymes attracted the attention of scientists [131–133]. The biological strategies that have been developed for the biodegradation of AFB1, DON, ZEN and FB1 in the feed are presented in Table 4.
Table 4.
Biological biotransformation approaches by microorganisms for the detoxification of mycotoxinsa.
| Mycotoxins | Microorganisms | Biotransformation efficiency | Reference |
|---|---|---|---|
| AFB1 | Aspergillus niger FS10 | 98.65% | [133] |
| Aspergillus niger RAF106 | 88.59% | [132] | |
| Stenotrophomonas sp. CW117 | 100.00% | [134] | |
| S. cerevisiae ŁOCK 0119 | 69.00% | [131] | |
| Escherichia coli CG1061 | 93.70% | [135] | |
| Bacillus velezensis DY3108 | 91.50% | [136] | |
| Bacillus subtilis JSW-1 | 67.20% | [137] | |
| Bacillus shackletonii L7 | 92.10% | [138] | |
| Bacillus licheniformis CFR1 | 94.70% | [139] | |
| Pseudomonas putida | 90.00% | [140] | |
| Bacillus subtilis UTBSP1 | 95.00% | [141] | |
| DON | Bacterial consortium C20 | 74.29% | [142] |
| Bacillus subtilis ASAG 216 | 81.10% | [143] | |
| Devosia insulae A16 | 88.00% | [144] | |
| Pseudomonas sp. Y1 and Lysobacter sp. S1 | 100.00% | [145] | |
| Eggerthella sp. DII-9 | 100.00% | [146] | |
| Aspergillus (NJA-1) | 94.40% | [147] | |
| Bacterial isolates LS100 & SS3 | 100.00% | [148] | |
| Bacterial strain BBSH 797 | - | [149] | |
| Strain E3-39 | 100.00% | [150] | |
| ZEN | Bacillus subtilis | 100.00% | [151] |
| Bacillus natto | 87.00% | [151] | |
| Bacillus pumilus ES-21 | 95.70% | [152] | |
| Bacillus amyloliquefaciens ZDS-1 | 95.70% | [153] | |
| Bacillus subtilis ANSB01G | 88.65% | [154] | |
| FB1 | Bacterial consortium SAAS79 | 100.00% | [155] |
| Strain NCB 1492 | 100.00% | [156] | |
| Saccharomyces cerevisiae IS1/1 and SC82 | 22%-50% | [157] | |
| Bacillus spp. S9, S10 and S69 | 43%-83% | [158] |
aAFB1 Aflatoxin B1, DON deoxynivalenol, ZEN zearalenone, FB1 fumonisin B1
-means the biotransformation efficiency did not reported
Microorganisms with detoxification activities
Biology-based detoxification methods are widely recognized as specific, efficient and environment-friendly. The nutritive and sensory characteristics like color and flavor are reserved without involving harmful chemicals. Screening and isolating naturally existing microorganisms that show biotransformation capabilities against specific mycotoxins have been a popular strategy. Mycotoxin biodegradation technology is the process by which the toxic group of the mycotoxin molecules is broken down and destroyed by the secondary metabolites produced by microorganisms or their secreted intracellular and extracellular enzymes, while producing non-toxic or less toxic degradation products.
A number of different fungal have been shown to detoxify AFB1. Fungal strains such as S. cerevisiae ŁOCK 0119 has been shown to degrade AFB1 at levels of 69.0% [131]. Similarly, some studies reported that the ability of various Aspergillus strains such as A. niger FS10 and A. niger RAF106 have shown the ability to degrade AFB1 to levels between 88.6% and 98.7% [132, 133]. Bacteria degraded AFs mainly by secreting extracellular enzymes. Some strains of Nocardia corynebacterioides, Flavobacterium aurantiacum and Bacillus have been shown to degrade AFB1. Smiley and Draughon reported that the degradation efficiency of AFB1 by Nocardia corynebacterioides reached 74.5% in 24 h [159]. Flavobacterium aurantiacum could degrade AFB1 efficiently and its crude protein extract could degrade 74.5% of AFB1 [160, 161]. Bacillus is an important class of bacteria capable of degrading AFB1. Farzaneh et al. [141] isolated Bacillus subtilis UTBSP1 from Iranian pistachio nut and the degradation rate of AFB1 reached 78.4-95.0%. Bacillus subtilis ANSB060 isolated from the fish intestine could degrade 81.5% of AFB1 within 72 h [162]. In addition, other Bacillus such as Bacillus licheniformis CFR1, Bacillus velezensis DY3108, Bacillus subtilis JSW-1 and Bacillus shackletonii L7 have been able to degrade AFB1 to levels between 67.2-94.7% [136–139]. Other bacteria such as Pseudomonas putida, Escherichia coli CG1061 and Stenotrophomonas sp. CW117 also showed very efficient biodegradation rates up to 90% or more for AFB1 [134, 135, 140].
Devosia insulae A16, Strain E3-39, Bacterial consortium C20, Pseudomonas sp. Y1 and Lysobacter sp. S1 isolated from soil samples can convert DON to 3-keto-DON or 3-epi-DON, a less toxic derivative [142, 144, 145, 150]. Several studies have revealed that these strains resulted in 74-100% reduction of DON [142, 144, 145, 150]. From a different point of view, Bacterial isolates LS100 and SS3, Bacterial strain BBSH 797 and Eggerthella sp. DII-9 presented a high biotransformation activity of converting DON to diepoxy-deoxynivalenol [146, 148, 149]. Strains isolated from the intestine of donkeys and soil samples, namely Bacillus subtilis ASAG 216 and Aspergillus (NJA-1) have shown to decrease DON concentration by 81.1% and 94.4% [143, 147].
Microorganisms metabolize ZEN mainly through conversion or degradation to α-zearalenol, β-zearalenol, sulfate and other secondary metabolites with low or non-toxicity. Bacillus natto and Bacillus subtilis strains were shown to remove ZEN from the liquid medium: more than 75% ZEN could be biodegraded after incubation. In another study, up to 99% of ZEN was degraded by B. subtilis strain [151]. Lei et al. [154] isolated Bacillus subtilis ANSB01G from broiler intestinal chyme, and the degradation rate of ZEN by this strain in a liquid medium, natural mold corn, distillers' dried grain with solubles and a complete pig feed were 88.7%, 84.6%, 66.3% and 83.0%, respectively. Bacillus pumilus ES-21 and Bacillus amyloliquefaciens ZDS-1, isolated from soil samples, showed 95.7% reduction of ZEN [152, 153].
Some fungal and bacterial microorganisms have been reported to be able to degrade fumonisins. Styriak et al. [157] screened two strains of preserved yeast from the laboratory that were able to significantly degrade fumonisins in the culture medium. One is Saccharomyces cerevisiae IS1/1, which can degrade 45% of FB1 and 50% of the mixture FB1 and FB2 in the culture medium, the other one is Saccharomyces cerevisiae SC82, which also degrade FB1 and the mixture FB1 and FB2, the degradation rates were 22% and 25%, respectively [157]. Camilo et al. [158] screened three strains such as Bacillus spp. S9, S10 and S69, that degraded 43%, 48% and 83% FB1, respectively. Strain NCB 1492, isolated from soil samples, can completely degrade FB1 under 25°C, after 24 h [156]. Notably, another study reported that the degradation rate of FB1 by Bacterial consortium SAAS79 can reach 100% [155].
The usage of catabolizing enzymes
Although some microorganisms are highly active in biodegrading mycotoxins, some of them might secrete harmful metabolites or cannot survive in the gastrointestinal tract of the animals [163, 164]. Therefore, screening the enzymes from these microorganisms might be the promising strategy to solve the issues. Recently, there are many researches that have focused on the isolation of the enzymes that can biodegrade AFB1, DON, ZEN and FB1. The enzymes for the biodegradation of AFB1, DON, ZEN and FB1 in the feed are presented in Table 5.
Table 5.
The usage of degrading enzymes for the detoxification of mycotoxinsa
| Mycotoxins | Degrading enzyme | Origin | Reference |
|---|---|---|---|
| AFB1 | Bacillus aflatoxin-degrading enzyme | Bacillus shackletonii L7 | [138] |
| Manganese peroxidase | Pleurotus ostreatus | [165] | |
| Aflatoxin-Oxidase | Armillariella tabescens | [163] | |
| Myxobacteria aflatoxin degradation enzyme | Myxococcus fulvus ANSM068 | [166] | |
| Laccase | White rot fungi | [167] | |
| DON | Manganese peroxidase and Lignin peroxidase | Spent Mushroom Substrate | [168] |
| Quinone-dependent dehydrogenase, NADPH-dependent aldo/keto reductases | Devosia sp. D6-9 | [169] | |
| Aldo-keto reductase DepA/DepB | Devosia mutans 17-2-E-8 | [170] | |
| Peroxidase | Rice bran | [171] | |
| Cytochrome P450 system | Sphingomonas sp. strain KSM1 | [172] | |
| ZEN | ZEN-specific lactonohydrolase | Recombinant enzymes | [173] |
| A fusion enzyme by combining ZEN-specific lactonohydrolase and carboxypeptidase | Clonostachys rosea strain IFO7063 and Bacillus amyloliquefaciens strain ASAG1 | [174] | |
| FB1 | Fumonisin carboxylesterase FumD | Recombinant enzymes | [175] |
aAFB1 Aflatoxin B1, DON deoxynivalenol, ZEN zearalenone, FB1 fumonisin B1
The main fungal enzymes known to have degradation activity against AFB1 are laccase and oxidase [163]. The enzyme for AFB1 detoxification designated as aflatoxin-detoxifizyme was reported [164]. The gene was identified and cloned from an Armillariella tabescens. The recombinant aflatoxin-detoxifizyme was able to detoxify AFB1 and significantly reduce its mutagenic effects. Manganese peroxidase (1.5 U/mL) can degrade 90% AFB1 after 48 h of reaction [165]. Alberts et al. [167] recombinantly expressed the laccase gene by gene cloning and its degradation rate of AFB1 was 55%. Bacillus aflatoxin-degrading enzyme and myxobacteria aflatoxin degradation enzyme secreted by Bacillus shackletonii L7 and Myxococcus fulvus ANSM068 are also efficient in degrading AFB1 [138, 166].
Although there are early reports on an NADH-dependent bacterial cytochrome P450 system that transforms DON into 16-hydroxy-DON, no efficient DON biotransformation enzymes are patented yet [172]. Peroxidase such as manganese peroxidase and lignin peroxidase showed the potential for significant DON degradation [168, 171]. Aldo-keto reductase DepA and DepB can transfer DON to 3-keto-DON and 3-epi-DON which have lower toxicity than DON [170]. A quinone-dependent dehydrogenase and two NADPH-dependent aldo/keto reductases (AKR13B2 and AKR6D1) can detoxify deoxynivalenol in wheat via epimerization in a Devosia strain [169].
Laccases are copper-containing oxidases have high potential in degrading the heat-stable mycotoxin ZEN, which involved in many industrial application [176, 177]. A novel ZEN-specific lactonohydrolase was developed previously as a producer of different hydrolytic enzymes for feed biorefinery. The recombinant ZEN-specific lactonohydrolase secreted by the transformed fungal clones into the culture liquid was shown to remove ZEN [173]. A recombinant fusion enzyme by combining two single genes named ZEN-specific lactonohydrolase and carboxypeptidase have demonstrated that can completely degrade ZEN to the non-toxic product in 2 h at an optimum pH of 7 and a temperature of 35 °C [174].
The fumonisin carboxylesterase FumD can degrade FB1 to its less toxic metabolite the hydrolyzed FB1 in the gastrointestinal tract of turkeys and pigs [175]. Within 2 h of incubation with FumD, FB1 was completely degraded to hydrolyzed FB1 in the duodenum and jejunum in an ex vivo pig model [175].
Nutritional strategies
It is well accepted that none of the physical, chemical or biological strategies can totally decontaminate the mycotoxin in feed, considering that even a low consumption level of a mycotoxin can cause chronic toxicity including a reduction of the performance and immunosuppression in animals [45], therefore, development of nutritional strategies to help mitigation of the mycotoxicoses is also important. Some nutritional strategies that have been disclosed are presented in Table 6.
Table 6.
Nutritional strategies to mitigate mycotoxins toxicitya
| Mycotoxins | Nutritional strategies | Mechanisms | Reference |
|---|---|---|---|
| AFB1 | Selenium, vitamins C, vitamins E, vitamin B1, carotenoids, silymarin, curcumin, butylated hydroxytoluene, alpha lipoic acid, quercetin, resveratrol, rhamnoides oil | Mainly by improving antioxidant capacity and detoxification enzyme activities to alleviate the harm of AFB1 to livestock and poultry | [11, 178–188] |
| DON | Selenium, vitamins C, vitamins E, silymarin, curcumin, functional amino acid (methionine, glutamic acid, arginine, aspartate and lysine), antimicrobial peptide, astragalus | Primarily through enhancement of antioxidant capacity and immune functions to improve the resistance to DON in livestock and poultry. | [179, 180, 189–194] |
| ZEN | Retinol, as-corbic acid, alpha-tocopherol, silymarin, soybean isoflavone | Alleviated the toxic effects of ZEN by improving the antioxidant capacity and inhibiting the estrogenic toxicity of ZEN. | [17, 190, 195] |
| FB1 | Vitamin E, silymarin, curcumin, soybean isoflavone | Mainly via counteracting the oxidative stress caused by FB1 to livestock. | [180, 196, 197] |
aAFB1 Aflatoxin B1, DON deoxynivalenol, ZEN zearalenone, FB1 fumonisin B1
It is feasible to modulate the mycotoxin detoxification system through nutritional measures. On the one hand, detoxification systems in animals including CYP450s, ketoreductase, α-glutathione transferase, etc. can degrade mycotoxins [9, 10]. Therefore, any nutrient that can promote the normal functioning of one of the above detoxification enzyme systems can be used as a nutritional regulator. Glutamate, cysteine and glycine can be used as substrates for the synthesis of glutathione and participate in the detoxification process by forming glutathione. On the other hand, mycotoxins can reduce nutrient uptake, so adding critical nutrients is one of the ways to mitigate the harmful effects of mycotoxins [13–15].
Oxidative stress is an important mechanism of cytotoxicity caused by mycotoxins [9, 10]. Adding antioxidants to mycotoxin-contaminated feed can improve the antioxidant capacity of the organism and increase the resistance of livestock and poultry to mycotoxins. Selenium, some vitamins A, C and E, and their precursors have marked antioxidant properties that act as superoxide anion scavengers. For these reasons, these substances have been investigated as protecting agents against toxic effects of mycotoxins. Selenium is an essential trace element for humans and animals as it plays an important role in antioxidant defense, anticancer, immunity, and detoxification [181, 182]. Previous studies have shown that dietary selenium supplementation can help to protect against AFB1-induced hepatotoxicity, immunotoxicity, and genotoxicity in chicks, which is mainly associated with regulation of redox/inflammation/apoptotic signaling and CYP450 isozymes [11]. Selenium has the potential to counteract DON-induced immunosuppression in piglets by increased the expression levels of IL-2, IL-10, IFN-γ, IgG, and IgM mRNA and protein in piglet splenic lymphocyte [190]. Selenium, vitamins C and E could be used as antioxidants to protect the spleen and brain cell membranes from DON toxicity and against DNA damage in liver caused by DON [191]. Nagaraj et al. [183] reported that dietary supplemented vitamin B1 reduced the toxicity of fusarium in chicks. Vitamin E supplementation counteracts the adverse impacts of FB1 on reproductive hormones, gestation length and milk production in rabbits [196]. Grosse et al. [195] observed that retinol, as-corbic acid and alpha-tocopherol reduced DNA adducts in the kidney and liver of mice exposed to ochratoxin A and ZEN from 70-90%. Carotenoids (carotene and xanthophylls) are excellent antioxidants with antimutagenic and anticarcinogenic properties, which have been demonstrated can inhibit AFB1-induced liver DNA damage in rats [178].
Silymarin is a potent antihepatotoxic agent provide protection against the negative effects of AFB1 on performance of broiler chicks [184]. Curcumin alleviates AFB1 toxicity through downregulating CYP450 enzymes, promoting ATPase activities in chickens [185]. Pretreatment with silymarin, curcumin enhanced the viability of cells exposed to the mycotoxins and attenuated reactive oxygen species formation by DON, partially reduced ROS formation by FB1 [180]. Curcumin significantly decreased apoptosis in cells exposed to DON, whereas silymarin was able to prevent apoptosis exposed to FB1 and DON in PK-15 cells [180]. Gao et al. [17] reported that dietary silymarin supplementation protected rats from ZEN-induced hepatotoxicity and reproductive toxicity through improvement in the antioxidant capacity and regulation in the genes related to ZEN metabolism, hormone synthesis, protein synthesis, and ABC transporters in the tissues.
Butylated hydroxytoluene, a dietary antioxidant in mammals, has been shown to lessen the toxic effects of AFB1 by inducing the activity of glutathione sulfotransferase and inhibiting the activity of cytochrome P450 1A5 [198]. Li et al. [186] reported that alpha lipoic acid improved the growth performance and alleviated the liver damage associated with improved the antioxidant capacity in the broilers exposed to AFB1. Quercetin exerted its beneficial effects by depressing the bioactivation of AFB1 and counterbalancing its pro-oxidant effects in a bovine mammary epithelial cell line [187]. Resveratrol, a polyphenol derived from red grapes, berries and peanuts, exerted anti-inflammatory and antioxidant effects. Dietary supplementation of resveratrol helped in increasing the activities of the oxidative enzymes and in improving the plasma total antioxidant capacity and total protein in broilers fed with AFB1 [188]. Solcan et al. [199] reported that rhamnoides oil had a potent hepatoprotective activity, reduced the concentration of AFs in the liver and diminished their adverse effects in broilers.
Andretta et al. [192] suggested that methionine can alleviate the DON induced adverse effects in growing pigs. Supplementing glutamic acid, arginine, aspartate and lysine to a diet had positive effects on remission of visceral disease induced by DON, enhancement of antioxidant ability and improvement of blood physiological and biochemical indexes of fattening pigs [200]. Dietary supplementation of 2.0% glutamic acid could mitigate DON induced negative effects on the growth performance and intestinal injury in the weaned piglets [193]. Xiao et al. [179, 194] found that an antimicrobial peptide complex composed of lactoferrin peptide, plant defensin and active yeast effectively improved the adverse effects of DON on production performance, autoimmunity and intestinal functions of weaned piglets. Astragalus played an important role in the reduction of immunosuppression and organ damages of the liver and kidney induced by DON and can improve the immunofunction significantly in mice [189]. Wang et al. [190] suggested that soybean isoflavone added to diets at 600 mg/kg could reduce the harmful effects induced by 2.0 mg/kg ZEN on the reproductive organs in prepubertal gilts during the growth phase. In an in vivo study on rats, Lu [197] reported that soybean isoflavone extract has a marked protective action against FB1 hepatotoxicity by the suppression of FB1-stimulated prostaglandin production.
Conclusion and perspectives
The occurrence of mycotoxins in the feed is of a great concern and an unavoidable problem in the feed industry around the world. Mycotoxins also endanger human health through the cycle of the food chain. This review summarizes a number of strategies to reduce mycotoxin contamination in terms of physical detoxification (separation, washing, heating, irradiation and adsorption), chemical treatments (bases and oxidizing agents), biological detoxification methods (microorganisms and enzymes), and nutritional regulation strategies. Each of these approaches can be practically used while along with their own advantages and disadvantages. However, with the growing awareness of environmental protection as well as feed and food safety, there is a growing expectation for more green and innovative technologies to control mycotoxin contamination.
Acknowledgments
We apologize in advance to the investigators, whose studies were inadequately presented or their studies related to this review but were not described in this manuscript.
Abbreviations
- AFs
Aflatoxins
- AFB1
Aflatoxin B1
- DON
Deoxynivalenol
- ZEN
Zearalenone
- FB1
Fumonisin B1
Authors’ contributions
LHS conceptualized and designed this review. ML, LZ, GXG, LZ, LS, JFD, YMH, and YYW collected the data. ML and LHS wrote the manuscript. MMK and JFD have revised the grammar of the manuscript. All authors have read and approved the final manuscript.
Funding
This project was supported by the Chinese Natural Science Foundation projects (32072775 and 31772636), and Nutreco N.V. (Netherlands).
Availability of data and materials
The datasets used and/or analyzed during the current study are publicly available.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have approved the final manuscript.
Competing interests
The authors declare no conflict of interest.
References
- 1.Steyn PS. Mycotoxins, general view, chemistry and structure. Toxicol Lett. 1995;82–83:843–851. doi: 10.1016/0378-4274(95)03525-7. [DOI] [PubMed] [Google Scholar]
- 2.Haque MA, Wang Y, Shen Z, Li X, Saleemi MK, He C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb Pathog. 2020;142:104095. doi: 10.1016/j.micpath.2020.104095. [DOI] [PubMed] [Google Scholar]
- 3.Sun LH, Lei MY, Zhang NY, Zhao L, Krumm CS, Qi DS. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem Toxicol. 2014;74:289–293. doi: 10.1016/j.fct.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Ma R, Zhang L, Liu M, Su YT, Xie WM, Zhang NY, et al. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in china. Toxins (Basel) 2018;10(3):113. doi: 10.3390/toxins10030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao L, Zhang L, Xu ZJ, Liu XD, Chen LY, Dai JF, et al. Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018-2020. J Anim Sci Biotechnol. 2021;12:74. [DOI] [PMC free article] [PubMed]
- 6.Lee HJ, Ryu D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: public health perspectives of their co-occurrence. J Agric Food Chem. 2017;65(33):7034–7051. doi: 10.1021/acs.jafc.6b04847. [DOI] [PubMed] [Google Scholar]
- 7.Zhang LY, Zhao XJ, Liu S, Zhang YG. Biological detoxification of aflatoxin for food and feed: a review. Chin J Anim Nutr. 2019;31(2):521–529. [Google Scholar]
- 8.International Agency for Research on Cancer (IARC) IARC Monographs on the evaluation of carcinogenic risk of chemicals to humans-overall evaluation of carcinogenicity: an updating of IARC monographs. IARC Lyon France. 1987;7(1):106–116. [Google Scholar]
- 9.Sun LH, Zhang NY, Zhu MK, Zhao L, Zhou JC, Qi DS. Prevention of aflatoxin B1 hepatoxicity by dietary selenium is associated with inhibition of cytochrome P450 isozymes and up-regulation of 6 selenoprotein genes in chick liver. J Nutr. 2016;146(4):655–661. doi: 10.3945/jn.115.224626. [DOI] [PubMed] [Google Scholar]
- 10.Zhang NY, Qi M, Zhao L, Zhu MK, Guo J, Liu J, et al. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins (Basel) 2016;8(11):327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Feng Y, Deng J, Zhang NY, Zhang WP, Liu XL, et al. Selenium deficiency aggravates aflatoxin B1-induced immunotoxicity in chick spleen by regulating 6 selenoprotein genes and redox/inflammation/apoptotic signaling. J Nutr. 2019;149(6):894–901. doi: 10.1093/jn/nxz019. [DOI] [PubMed] [Google Scholar]
- 12.Kócsó DJ, Ali O, Kovács M, Mézes M, Balogh K, Kachlek ML, et al. A preliminary study on changes in heat shock protein 70 levels induced by Fusarium mycotoxins in rats: in vivo stud. Mycotoxin Res. 2021;37(2):141–148. doi: 10.1007/s12550-021-00425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji X, Zhang Q, Zheng WJ, Yao W. Morphological and molecular response of small intestine to lactulose and hydrogen-rich water in female piglets fed Fusarium mycotoxins contaminated diet. J Anim Sci Biotechnol. 2019;10:9. doi: 10.1186/s40104-019-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi D, Zhou J, Zhao L, Rong X, Fan Y, Hamid H, et al. Alleviation of mycotoxin biodegradation agent on zearalenone and deoxynivalenol toxicosis in immature gilts. J Anim Sci Biotechnol. 2018;9:42. doi: 10.1186/s40104-018-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Zhang L, Chu XH, Ma R, Wang YW, Liu Q, et al. Effects of deoxynivalenol on the porcine growth performance and intestinal microbiota and potential remediation by a modified HSCAS binder. Food Chem Toxicol. 2020;141:111373. doi: 10.1016/j.fct.2020.111373. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Ma R, Zhu MX, Zhang NY, Liu XL, Wang YW, et al. Effect of deoxynivalenol on the porcine acquired immune response and potential remediation by a novel modified HSCAS adsorbent. Food Chem Toxicol. 2020;138:111187. doi: 10.1016/j.fct.2020.111187. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Xiao ZH, Liu M, Zhang NY, Khalil MM, Gu CQ, et al. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J Nutr. 2018;148(8):1209–1216. doi: 10.1093/jn/nxy114. [DOI] [PubMed] [Google Scholar]
- 18.Takemura H, Shim JY, Sayama K, Tsubura A, Zhu BT, Shimoi K. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J Steroid Biochem Mol Biol. 2007;103(2):170–177. doi: 10.1016/j.jsbmb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Sun LH, Zhang NY, Li C, Zhang J, Xiao ZH, et al. Gestational zearalenone exposure causes reproductive and developmental toxicity in pregnant rats and female offspring. Toxins (Basel) 2017;9(1):21. doi: 10.3390/toxins9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Wei Z, Wang Y, Long M, Wu WD, Kuca K. Fumonisin B1: mechanisms of toxicity and biological detoxification progress in animals. Food Chem Toxicol. 2021;149(3):111977. doi: 10.1016/j.fct.2021.111977. [DOI] [PubMed] [Google Scholar]
- 21.Salgado JD, Wallhead M, Madden LV, Paul PA. Grain harvesting strategies to minimize grain quality losses due to fusarium head blight in wheat. Plant Dis. 2011;95(11):1448–1457. doi: 10.1094/PDIS-04-11-0309. [DOI] [PubMed] [Google Scholar]
- 22.Cui GJ. Research on photoelectric sorting technology of wheat grain with red mold. Henan Univ Technol. 2013.
- 23.Liu Y, Yamdeu JH, Gong YY, Orfila C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr Rev Food Sci Food Saf. 2020;19(4):1521–1560. doi: 10.1111/1541-4337.12562. [DOI] [PubMed] [Google Scholar]
- 24.Hu D, Wu A. Chemical and physical treatments for reducing mycotoxin contaminations. In: Wu A, editors. Food Safety & Mycotoxins. Singapore: Springer; 2019. p. 145–62.
- 25.Karlovsky P, Suman M, Berthiller F, De Meester J, Eisenbrand G, Perrin I, et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016;32(4):179–205. doi: 10.1007/s12550-016-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi HT, Li SL, Bai YY, Prates LL, Lei YG, Yu PQ. Mycotoxin contamination of food and feed in China: Occurrence, detection techniques, toxicological effects and advances in mitigation technologies. Food Control. 2018;91:202–215. [Google Scholar]
- 27.Matumba L, Poucke CV, Ediage EN, Jacobs B, Saeger SD. Effectiveness of hand sorting, flotation/washing, dehulling and combinations thereof on the decontamination of mycotoxin-contaminated white maize. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32(6):960–969. doi: 10.1080/19440049.2015.1029535. [DOI] [PubMed] [Google Scholar]
- 28.Shapira R, Paster N. Control of mycotoxins in storage and techniques for their decontamination. Mycotoxins Food Detect Control. 2004:190–223.
- 29.Fandohan P, Zoumenou D, Hounhouigan DJ, Marasas WFO, Wingfield MJ, Hell K. Fate of aflatoxins and fumonisins during the processing of maize into food products in Benin. Int J Food Microbiol. 2005;98(3):249–259. doi: 10.1016/j.ijfoodmicro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Yumbe-Guevara BE, Imoto T, Yoshizawa T. Effects of heating procedures on deoxynivalenol, nivalenol and zearalenone levels in naturally contaminated barley and wheat. Food Addit Contam. 2003;20(12):1132–1140. doi: 10.1080/02652030310001620432. [DOI] [PubMed] [Google Scholar]
- 31.Park JW, Kim YB. Effect of pressure cooking on aflatoxin B1 in rice. J Agric Food Chem. 2006;54(6):2431–2435. doi: 10.1021/jf053007e. [DOI] [PubMed] [Google Scholar]
- 32.Becker-Algeri TA, Heidtmann-Bemvenuti R, Hackbart HCS, Badiale-Furlong E. Thermal treatments and their effects on the fumonisin B1 level in rice. Food Control. 2013;34(2):488–493. [Google Scholar]
- 33.Pankaj SK, Shi H, Keenera KM. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci Tech. 2018;71:73–83. [Google Scholar]
- 34.Aziz NH, Moussa LAA, Far FM. Reduction of fungi and mycotoxins formation in seeds by gamma-radiation. J Food Safety. 2004;24(2):109–127. [Google Scholar]
- 35.Yang J. Research on irradiation-induced degradation of mycotoxins in agricultural products [D]. Chin Acad Agric Sci. 2009.
- 36.Hooshmand H, Klopfenstein CF. Effects of gamma irradiation on mycotoxin disappearance and amino acid contents of corn, wheat, and soybeans with different moisture contents. Plant Foods Hum Nutr. 1995;47(3):227–238. doi: 10.1007/BF01088331. [DOI] [PubMed] [Google Scholar]
- 37.Aziz NH, Attia ES, Farag SA. Effect of gamma-irradiation on the natural occurrence of Fusarium mycotoxins in wheat, flour and bread. Nahrung. 1997;41(1):34–37. doi: 10.1002/food.19970410109. [DOI] [PubMed] [Google Scholar]
- 38.Murata H, Mitsumatsu M, Shimada N. Reduction of feed-contaminating mycotoxins by ultraviolet irradiation: an in vitro study. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25(9):1107–1110. doi: 10.1080/02652030802057343. [DOI] [PubMed] [Google Scholar]
- 39.Zhang K. Study on the degradation effect of electron beam irradiation on deoxynivalenol of Fusarium oxysporum in wheat with red mold. Henan Univ Technol. 2014.
- 40.Aziz NH, El-Far FM, Shahin AA, Roushy SM. Control of Fusarium moulds and fumonisin B1 in seeds by gamma-irradiation. Food Control. 2007;18(11):1337–1342. [Google Scholar]
- 41.Xiao LX, Wang F, Yu HT, Zhao XY, Hu XS. Detoxication of aflatoxin B1 and fumonisin B1 in purple corn. Food Sci. 2011;32(9):114–117. [Google Scholar]
- 42.Mutungi C, Muthoni F, Bekunda M, Gaspar A, Kabula E, Abass A. Physical quality of maize grain harvested and stored by smallholder farmers in the Northern Highlands of Tanzania : Effects of harvesting and pre-storage handling practices in two marginally contrasting agro-locations. J Stored Prod Res. 2019;84:101517. [Google Scholar]
- 43.Reddy KR, Reddy CS, Abbas HK, Abel CA, Muralidharan K. Mycotoxigenic fungi, mycotoxins, and management of rice grains. Toxin Rev. 2008;27:287–317. [Google Scholar]
- 44.Tibola CS, Fernandes MC, Guarienti EM. Effect of cleaning, sorting and milling processes in wheat mycotoxin content. Food Control. 2016;60:174–179. [Google Scholar]
- 45.Delwiche SR, Pearson TC, Brabec DL. High-speed optical sorting of soft wheat for reduction of deoxynivalenol. Plant Dis. 2005;89(11):1214–1219. doi: 10.1094/PD-89-1214. [DOI] [PubMed] [Google Scholar]
- 46.Chu X, Tao Y, Wang W, Yuan Y, Xi MJ. Rapid detection method of moldy maize kernels based on color feature. Adv Mech Eng. 2014;6:625090. [Google Scholar]
- 47.Braunberg RC, Gantt OO, Friedman L. Toxicological evaluation of compounds found in food using rat renal explants. Food Chem Toxicol. 1982;20(5):541–546. doi: 10.1016/s0278-6915(82)80062-8. [DOI] [PubMed] [Google Scholar]
- 48.Shetty PH, Bhat RV. A physical method for segregation of fumonisin-contaminated maize. Food Chem. 1999;66:371–374. [Google Scholar]
- 49.Westhuizen L, Shephard GS, Rheeder JP, Burger HM, Gelderblom WC, Wild CP, et al. Optimising sorting and washing of home-grown maize to reduce fumonisin contamination under laboratory-controlled conditions. Food Control. 2011;22:396–400. [Google Scholar]
- 50.Wang CY. Measures to deal with aflatoxin in food. China New Technol New Prod. 2010;171(5):8. [Google Scholar]
- 51.Cui CP. Study on cultivation of Rhodococcus erythropolis and its degradation of afIatoxinB1. Ocean Univ China. 2012.
- 52.Kabak B, Dobson A, Var I. Strategies to prevent mycotoxin contamination of food and animal feed: a review. Crit Rev Food Sci Nutr. 2006;46(8):593–619. doi: 10.1080/10408390500436185. [DOI] [PubMed] [Google Scholar]
- 53.Jackson LS, Hlywka JJ, Senthil KR, Bullerman LB, Musser SM. Effects of time, temperature, and pH on the stability of fumonisin B1 in an aqueous model system. J Agric Food Chem. 1996;44(3):906–912. [Google Scholar]
- 54.Ryu D, Hanna AM, Eskridge MK, Bullerman BL. Heat stability of zearalenone in an aqueous buffered model system. J Agric Food Chem. 2003;51(6):1746–1748. doi: 10.1021/jf0210021. [DOI] [PubMed] [Google Scholar]
- 55.Fan HY. Integrated control of aflatoxin in feed. Hebei Anim Husb Vet. 2003;019(001):40–41. [Google Scholar]
- 56.Jackson LS, Katta SK, Fingerhut DD, DeVries JW, Bullerman LB. Effects of baking and frying on the fumonisin B1 content of corn-based foods. J Agric Food Chem. 1997;45(12):4800–4805. [Google Scholar]
- 57.Aziz NH, Moussa LA. Influence of gamma-radiation on mycotoxin producing moulds and mycotoxins in fruits. Food Control. 2002;13(4–5):281–288. [Google Scholar]
- 58.Molins RA, Motarjemi Y. K¨aferstein F K. Irradiation: a critical control point in ensuring the microbiological safety of raw foods. Food Control. 2001;12(6):347–356. [Google Scholar]
- 59.He JW, Zhou T. Patented techniques for detoxification of mycotoxins in feeds and food matrices. Recent Pat Food Nutr Agric. 2010;2(2):96–104. doi: 10.2174/2212798411002020096. [DOI] [PubMed] [Google Scholar]
- 60.Scott PM. Industrial and farm detoxification processes for mycotoxins. Rev Med Vet Fr. 1998;146(6):543–548. [Google Scholar]
- 61.Jubeen F, Bhatti IA, Khan MZ, Hassan ZU, Shahid M. Effect of UVC irradiation on aflatoxins in ground nut (arachis hypogea) and tree nuts (juglans regia, prunus duclus and pistachio vera) J Chem Soc Pakistan. 2012;34(6):1366–1374. [Google Scholar]
- 62.Suzuki T. Light-irradiation wavelength and intensity changes influence aflatoxin synthesis in fungi. Toxins (Basel) 2018;10(1):31. doi: 10.3390/toxins10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adamovic’ M, Stojanovic’ M, Grubišic’ M, Ileš D, Milojkovic’ J. Importance of aluminosilicate minerals in safe food production. Macedonian Journal of Animal Science. 2011;1:175–80.
- 64.Sumantri I, Herliani H, Yuliani M, Nuryono N. Effects of zeolite in aflatoxin B1 contaminated diet on aflatoxin residues and liver histopathology of laying duck. Conf Ser Earth Environ Sci. 2018;207:012017. [Google Scholar]
- 65.Bhatti SA, Khan MZ, Hassan ZU, Saleemi MK, Saqib M, Khatoon A, et al. Comparative efficacy of Bentonite clay, activated charcoal and trichosporon mycotoxinivorans in regulating the feed-to-tissue transfer of mycotoxins. J Sci Food Agric. 2017;98(3):884–890. doi: 10.1002/jsfa.8533. [DOI] [PubMed] [Google Scholar]
- 66.Magnoli AP, Texeira M, Rosa CA, Miazzo RD, Cavaglieri LR, Magnoli CE, et al. Sodium bentonite and monensin under chronic aflatoxicosis in broiler chickens. Poult Sci. 2011;90(2):352–357. doi: 10.3382/ps.2010-00834. [DOI] [PubMed] [Google Scholar]
- 67.Chen Q, Lu Z, Hou W, Shi B, Shan A. Effects of modified maifanite on zearalenone toxicity in female weaner pigs. Ital J Anim Sci. 2015;14(2):3597. [Google Scholar]
- 68.Gouda GA, Khattab HM, Abdel-Wahhab MA, El-Nor SA, El-Sayed HM, Kholif SM. Clay minerals as sorbents for mycotoxins in lactating goat’s diets: intake, digestibility, blood chemistry, ruminal fermentation, milk yield and composition, and milk aflatoxin M1 content. Small Rumin Res. 2019;175(6):15–22. [Google Scholar]
- 69.Tzou YM, Chan YT, Chen SE, Wang CC, Chiang PN, Teah HY, et al. Use 3-D tomography to reveal structural modification of bentonite-enriched clay by nonionic surfactants: Application of organo-clay composites to detoxify aflatoxin B1 in chickens. J Hazard Mater. 2019;375(5):312–319. doi: 10.1016/j.jhazmat.2019.04.084. [DOI] [PubMed] [Google Scholar]
- 70.Vila-Donat P, Marín S, Sanchis V, Ramos AJ. Tri-octahedral bentonites as potential technological feed additive for fusarium mycotoxin reduction. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2020;37(8):1374–1387. doi: 10.1080/19440049.2020.1766702. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Q, Zhang YL, Liu SS, Wu YZ, Zhou Q, Zhang YZ, et al. Adsorption of deoxynivalenol by pillared montmorillonite. Food Chem. 2020;343(1):128391. doi: 10.1016/j.foodchem.2020.128391. [DOI] [PubMed] [Google Scholar]
- 72.Wang G, Lian C, Xi YF, Sun ZM, Zheng SL. Evaluation of nonionic surfactant modified montmorillonite as mycotoxins adsorbent for aflatoxin B1 and zearalenone. J Colloid Interface Sci. 2018;518:48–56. doi: 10.1016/j.jcis.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 73.Aly SE, Abdel-Galil MM, Abdel-Wahhab MA. Application of adsorbent agents technology in the removal of aflatoxin B1 and fumonisin B1 from malt extract. Food Chem Toxicol. 2004;42(11):1825–1831. doi: 10.1016/j.fct.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 74.Daković A, Tomašević-Čanović M, Rottinghaus GE, Matijašević S, Sekulić Z. Fumonisin B1 adsorption to octadecyldimethylbenzyl ammonium-modified clinoptilolite-rich zeolitic tuff. Microporous Mesoporous Mater. 2007;105:285–290. [Google Scholar]
- 75.Zeidan R, Ul-Hassan Z, Al-Thani R, Balmas V, Jaoua S. Application of lowfermenting yeast Lachancea thermotolerans for the control of toxigenic fungi Aspergillus parasiticus, Penicillium verrucosum and Fusarium graminearum and their mycotoxins. Toxins (Basel) 2018;10(6):242. doi: 10.3390/toxins10060242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tranquil DT, Tranquil E. Compositions and methods for mycotoxin decontamination, nucleotide, protein and vitamin enrichment and palatability enhancement of food and animal feed using micronized yeast biomass. US Patent Appl Publ. 2012; No. 20120082759 A1.
- 77.Yiannikouris A, André G, Buléon A, Jeminet G, Canet I, François J, et al. Comprehensive conformational study of key interactions involved in zearalenone complexation with beta-D-glucans. Biomacromolecules. 2004;5(6):2176–2185. doi: 10.1021/bm049775g. [DOI] [PubMed] [Google Scholar]
- 78.Yiannikouris A, Kettunen H, Apajalahti J, Pennala E, Moran CA. Comparison of the sequestering properties of yeast cell wall extract and hydrated sodium calcium aluminosilicate in three in vitro models accounting for the animal physiological bioavailability of zearalenone. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30(9):1641–1650. doi: 10.1080/19440049.2013.809625. [DOI] [PubMed] [Google Scholar]
- 79.Dalvi RR, Mcgowan C. Experimental induction of chronic aflatoxicosis in chickens by purified aflatoxin B1 and its reversal by activated charcoal, phenobarbital, and reduced glutathione. Poult Sci. 1984;63(3):485–491. doi: 10.3382/ps.0630485. [DOI] [PubMed] [Google Scholar]
- 80.Avantaggiato G, Havenaar R, Visconti A. Assessing the zearalenone-binding activity of adsorbent materials during passage through a dynamic in vitro gastrointestinal model. Food Chem Toxicol. 2003;41(10):1283–1290. doi: 10.1016/s0278-6915(03)00113-3. [DOI] [PubMed] [Google Scholar]
- 81.Khan F, Zahoor M. In vivo detoxification of aflatoxinB1 by magnetic carbon nanostructures prepared from bagasse. BMC Vet Res. 2014;10:255. doi: 10.1186/s12917-014-0255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao ZY, Liu N, Yang LC, Wang JH, Song SQ, Nie DX, et al. Cross-linked chitosan polymers as generic adsorbents for simultaneous adsorption of multiple mycotoxins. Food Cont. 2015;57:362–369. [Google Scholar]
- 83.Ramos AJ, Hernández E. In vitro aflatoxin adsorption by means of a montmorillonite silicate. A study of adsorption isotherms. Anim Feed Sci Tech. 1996;62(2–4):263–269. [Google Scholar]
- 84.Hernandez-Mendoza A, Guzman-De-Peña D, González-Córdova AF, Vallejo-Córdoba B, Garcia HS. In vivo assessment of the potential protective effect of Lactobacillus casei Shirota against aflatoxin B1. Dairy Sci Technol. 2010;90(6):729–740. [Google Scholar]
- 85.Zeng D, Tang YR, Ni XQ, Zhang ZL, Ran J. Absorption characteristics of lactobacillus plantarum F22 to aflatoxin B1. Food Sci. 2009;30:23. [Google Scholar]
- 86.Wang X, Zheng JY, Peng XL, Qiao YF, Zhou LL, Zhang BL. Study on the removal of fumonisins by Lactobacillus strains. Sci Technol Food Ind. 2015;101(4):849–856. [Google Scholar]
- 87.Hart F. The use of naturally occurring minerals in animal feed. Ind Miner. 2014;565:22. [Google Scholar]
- 88.Kubena LF, Harvey RB, Huff WE, Elissalde MH, Yersin AG, Phillips TD, et al. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. Poult Sci. 1993;72(1):51–59. doi: 10.3382/ps.0720051. [DOI] [PubMed] [Google Scholar]
- 89.Mussaddeq Y, Begum I, Akhter S. Activity of aflatoxins adsorbents in poultry feed. Pak J Biol Sci. 2000;3(10):1697–1699. [Google Scholar]
- 90.Phillips TD, Wang M, Elmore SE, Hearon S, Wang JS. NovaSil clay for the protection of humans and animals from aflatoxins and other contaminants. Clays Clay Miner. 2019;67(1):99–110. doi: 10.1007/s42860-019-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robinson A, Johnson NM, Strey A, Taylor JF, Marroquin-Cardona A, Mitchell NJ, et al. Calcium montmorillonite clay reduces urinary biomarkers of FB1 exposure in rats and humans. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29(5):809–818. doi: 10.1080/19440049.2011.651628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Y, Tian GY, Dong GY, Bai SS, Han XY, Liang JS, et al. Research progress on the raw and modified montmorillonites as adsorbents for mycotoxins: a review. Appl Clay Sci. 2018;163:299–311. [Google Scholar]
- 93.European Commission. Commission Regulation (EC) No 1060/2013 of 29 October 2013 concerning the authorisation of bentonite as feed additive for all animal species. 2013;289:33–7.
- 94.Wei JT, Wu KT, Sun H, Khalil MM, Dai JF, Liu Y, et al. A novel modified hydrated sodium calcium aluminosilicate (HSCAS) adsorbent can effectively reduce T-2 toxin-induced toxicity in growth performance, nutrient digestibility, serum biochemistry, and small intestinal morphology in chicks. Toxins (Basel) 2019;11(4):199. doi: 10.3390/toxins11040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yiannikouris A, Kwiatkowski S, Kudupoje MB, Matney C. Synthetic mycotoxin adsorbents and methods of making and utilizing the same. US Patent Publ. 2014:RE45274.
- 96.Howes AD, Newman KE. Compositions and methods for removal of mycotoxins from animal feed. US Patent Publ. 1999; No.6045834 A.
- 97.Alexander H, Stefan F, Othmar K, Hans D. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol Lett. 2001;122(2):179–188. doi: 10.1016/s0378-4274(01)00360-5. [DOI] [PubMed] [Google Scholar]
- 98.Liu YL, Meng GQ, Wang HR, Zhu HL, Hou YQ, Wang WJ, et al. Effect of three mycotoxin adsorbents on growth performance: Nutrient retention and meat quality in broilers fed on mould-contaminated feed. Br Poult Sci. 2011;52(2):255–263. doi: 10.1080/00071668.2011.559453. [DOI] [PubMed] [Google Scholar]
- 99.Teleb HM, Hegazy AA, Hussein YA. Efficiency of kaolin and activated charcoal to reduce the toxicity of low level of aflatoxins in broilers. Sci J King Faisal Univ. 2004;5:145–160. [Google Scholar]
- 100.Adunphatcharaphon S, Petchkongkaew A, Greco D, D'Ascanio D, Visessanguan W, Avantaggiato G. The effectiveness of durian peel as a multi-mycotoxin adsorbent. Toxins (Basel) 2020;12(2):108. doi: 10.3390/toxins12020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Magro M, Moritz DE, Bonaiuto E, Baratella D, Terzo M, Jakubec P, et al. Citrinin mycotoxin recognition and removal by naked magnetic nanoparticles. Food Chem. 2016;203:505–512. doi: 10.1016/j.foodchem.2016.01.147. [DOI] [PubMed] [Google Scholar]
- 102.LuoY LX, Yuan L, Li J. Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends Food Sci Tech. 2020;96:127–134. [Google Scholar]
- 103.Halttunen T, Collado MC, El-Nezami H, Meriluoto J, Salminen S. Combining strains of lactic acid bacteria may reduce their toxin and heavy metal removal efficiency from aqueous solution. Lett Appl Microbiol. 2008;46(2):160–165. doi: 10.1111/j.1472-765X.2007.02276.x. [DOI] [PubMed] [Google Scholar]
- 104.Jalili M, Son S. The effect of chemical treatment on reduction of aflatoxins and ochratoxin A in black and white pepper during washing. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28(4):485–493. doi: 10.1080/19440049.2010.551300. [DOI] [PubMed] [Google Scholar]
- 105.Dvorák M. Possibilities of chemical detoxification of aflatoxin. Vet Med (Praha) 1990;35(1):37–42. [PubMed] [Google Scholar]
- 106.McKenzie KS, Sarr AB, Mayura K, Bailey RH, Miller DR, Rogers TD, et al. Oxidative degradation and detoxification using a novel source of ozone. Food Chem Toxicol. 1997;35(8):807–820. doi: 10.1016/s0278-6915(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 107.Masri MS, Vix HLE, Goldblatt LA. Process for de-toxifying substances contaminated with aflatoxin. US Patent. 1969: US3429709DA.
- 108.Gardner HK, Koltun SP, Dollear FG, Rayner ET. Inactivation of aflatoxins in peanut and cottonseed meals by ammoniation. J Am Oil Chem Soc. 1971;48(2):70–73. doi: 10.1007/BF02635688. [DOI] [PubMed] [Google Scholar]
- 109.Brekke OL, Stringfellow AC, Peplinski AJ. Aflatoxin inactivation in corn by aqua ammonia: laboratory trials. J Agric Food Chem. 1978;26(6):1383–1389. doi: 10.1021/jf60220a050. [DOI] [PubMed] [Google Scholar]
- 110.Park DL. Perspectives on mycotoxin decontamination procedures. Food Addit Contam. 1993;10(1):49–60. doi: 10.1080/02652039309374129. [DOI] [PubMed] [Google Scholar]
- 111.Bretz M, Beyer M, Cramer B, Knecht A, Humpf HU. Thermal degradation of the Fusarium mycotoxin deoxynivalenol. J Agric Food Chem. 2006;54(17):6445–6451. doi: 10.1021/jf061008g. [DOI] [PubMed] [Google Scholar]
- 112.Young JC, Subryan LM, Potts D, McLaren ME. Reduction in levels of deoxynivalenol in contaminated corn by chemical and physical treatment. J. Agric Food Chem. 1986;34(3):465–467. [Google Scholar]
- 113.Prudente AD, King JM. Efficacy and safety evaluation of ozonation to degrade aflatoxin in corn. J Food Sci. 2002;67(8):2866–2872. [Google Scholar]
- 114.Li R, Wang X, Zhou T, Yang D, Wang Q, Zhou Y. Occurrence of four mycotoxins in cereal and oil products in yangtze delta region of china and their food safety risks. Food Control. 2014;35(1):117–122. [Google Scholar]
- 115.Sun C, Ji J, Wu SL, Sun CP, Pi FW, Zhang YZ, et al. Saturated aqueous ozone degradation of deoxynivalenol and its application in contaminated grains. Food Control. 2016;69:185–190. [Google Scholar]
- 116.Wang L, Luo YP, Luo XH, Wang R, Li YF, Shao HL, et al. Effect of deoxynivalenol detoxification by ozone treatment in wheat grains. Food Control. 2016;66:137–144. [Google Scholar]
- 117.Qi LJ, Li YL, Luo XH, Wang R, Zheng RH, Wang L, et al. Detoxification of zearalenone and ochratoxin A by ozone and quality evaluation of ozonised corn. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016;33(11):1700–1710. doi: 10.1080/19440049.2016.1232863. [DOI] [PubMed] [Google Scholar]
- 118.Trager W, Stoloff L. Possible reactions for aflatoxin detoxification. J Agric Food Chem. 1967;15(4):679–681. [Google Scholar]
- 119.Natarajan KR, Rhee KC, Cater CM, Mattil KF. Destruction of aflatoxins in peanut protein isolates by sodium hypochlorite. J Am Oil Chem Soc. 1975;52(5):160–163. doi: 10.1007/BF02557949. [DOI] [PubMed] [Google Scholar]
- 120.Javanmardi F, Khodaei D, Sheidaei Z, Bashiry M, Nayebzadeh K, Vasseghian Y, et al. Decontamination of aflatoxins in edible oils: a comprehensive review. Food Rev Int. 2020;17:1–17. [Google Scholar]
- 121.Natarajan KR. Chemical inactivation of aflatoxins in peanut protein ingredients. J Environ Pathol Toxicol Oncol. 1992;11(4):217–227. [PubMed] [Google Scholar]
- 122.Alencar ER, Faroni LR, Soares NF, Silva WA, Carvalho MC. Efficacy of ozone as a fungicidal and detoxifying agent of aflatoxins in peanuts. J Sci Food Agric. 2012;92(4):899–905. doi: 10.1002/jsfa.4668. [DOI] [PubMed] [Google Scholar]
- 123.Piemontese L, Messia MC, Marconi E, Falasca L, Zivoli R, Gambacorta L, et al. Effect of gaseous ozone treatments on DON, microbial contaminants and technological parameters of wheat and semolina. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2018;35(4):760–771. doi: 10.1080/19440049.2017.1419285. [DOI] [PubMed] [Google Scholar]
- 124.Santos Alexandre AP, Vela-Paredes RS, Santos AS, Costa NS, Canniatti-Brazaca SG, Calori-Domingues MA, et al. Ozone treatment to reduce deoxynivalenol (DON) and zearalenone (ZEN) contamination in wheat bran and its impact on nutritional quality. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2018;35(6):1189–1199. doi: 10.1080/19440049.2018.1432899. [DOI] [PubMed] [Google Scholar]
- 125.Agriopoulou S, Koliadima A, Karaiskakis G, Kapolos J. Kinetic study of aflatoxins' degradation in the presence of ozone. Food Control. 2016;61:221–226. [Google Scholar]
- 126.Trombete FM, Porto YD, Freitas-silva O, Pereira RV, Direito GM, Saldanha T, et al. Efficacy of ozone treatment on mycotoxins and fungal reduction in artificially contaminated soft wheat grains. J Food Process Pres. 2016;41(3):e12927.1–e1292710. [Google Scholar]
- 127.Dwarakanath CT, Rayner ET, Mann GE, Dollear FG. Reduction of aflatoxin levels in cottonseed and peanut meals by ozonization. J Am Oil Chem Soc. 1968;45(2):93–95. doi: 10.1007/BF02890715. [DOI] [PubMed] [Google Scholar]
- 128.McKenzie KS, Kubena LF, Denvir AJ, Rogers TD, Hitchens GD, Bailey RH, et al. Aflatoxicosis in turkey poults is prevented by treatment of naturally contaminated corn with ozone generated by electrolysis. Poult Sci. 1998;77(8):1094–1102. doi: 10.1093/ps/77.8.1094. [DOI] [PubMed] [Google Scholar]
- 129.Altug T, Yousef AE, Marth EH. Degradation of aflatoxin B1 in dried figs by sodium bisulfite with or without heat, ultraviolet energy or hydrogen peroxide. J Food Prot. 1990;53(7):581–582. doi: 10.4315/0362-028X-53.7.581. [DOI] [PubMed] [Google Scholar]
- 130.Abd-Alla ES. Zearalenone: incidence, toxigenic fungi and chemical decontamination in Egyptian cereals. Nahrung. 1997;41(6):362–365. doi: 10.1002/food.19970410610. [DOI] [PubMed] [Google Scholar]
- 131.Chlebicz A, Śliżewska K. In vitro detoxification of aflatoxin B1, deoxynivalenol, fumonisins, T-2 Toxin and zearalenone by probiotic bacteria from genus lactobacillus and saccharomyces cerevisiae yeast. Probiotics Antimicrob Proteins. 2020;12(1):289–301. doi: 10.1007/s12602-018-9512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fang QA, Du MR, Chen JW, Liu T, Zheng Y, Liao ZL, et al. Degradation and detoxification of aflatoxin B1 by tea-derived aspergillus niger RAF106. Toxins (Basel) 2020;12(12):777. doi: 10.3390/toxins12120777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qiu TY, Wang HM, Yang Y, Yu J, Ji J, Sun JD, et al. Exploration of biodegradation mechanism by AFB1-degrading strain Aspergillus niger FS10 and its metabolic feedback. Food Control. 2021;121(2):107609. [Google Scholar]
- 134.Cai MY, Qian YY, Chen N, Ling TJ, Wang JJ, Jiang H, et al. Detoxification of aflatoxin B1 by Stenotrophomonas sp. CW117 and characterization the thermophilic degradation process. Environ Pollut. 2020;261:114178. doi: 10.1016/j.envpol.2020.114178. [DOI] [PubMed] [Google Scholar]
- 135.Wang LL, Wu J, Liu ZW, Shi YT, Liu JQ, Xu XF, et al. Aflatoxin B1 degradation and detoxification by Escherichia coli CG1061 isolated from chicken cecum. Front Pharmacol. 2019;9:1548. doi: 10.3389/fphar.2018.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shu X, Wang YT, Zhou Q, Li MH, Hu H, Ma YH, et al. Biological degradation of aflatoxin B1 by cell-free extracts of bacillus velezensis DY3108 with broad PH stability and excellent thermostability. Toxins (Basel) 2018;10(8):330. doi: 10.3390/toxins10080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xia XH, Zhang Y, Li MY, Garba B, Zhang Q, Wang Y, et al. Isolation and characterization of a bacillus subtilis strain with aflatoxin B1 biodegradation capability. Food Control. 2017;75:92–98. [Google Scholar]
- 138.Xu L, Ahmed MF, Sangare L, Zhao YJ, Selvaraj JN, Xing FG, et al. Novel aflatoxin-degrading enzyme from bacillus shackletonii L7. Toxins (Basel) 2017;9(1):36. doi: 10.3390/toxins9010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Raksha Rao K, Vipin AV. Hariprasad P, Anu Appaiah KA, Venkateswaran G. Biological detoxification of aflatoxin B1 by bacillus licheniformis CFR1. Food Control. 2016;71:234–41.
- 140.Samuel MS, Sivaramakrishna A, Mehta A. Degradation and detoxification of aflatoxin B1 by Pseudomonas putida. Int Biodeter Biodegr. 2014;86:202–209. [Google Scholar]
- 141.Farzaneh M, Shi ZQ, Ghassempour A, Sedaghat N, Ahmadzadeh M, Mirabolfathy M, et al. Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Control. 2012;23(1):100–106. [Google Scholar]
- 142.Wang YX, Wang G, Dai YJ, Wang Y, Lee YW, Shi JR, et al. Biodegradation of deoxynivalenol by a novel microbial consortium. Front Microbiol. 2020;10:2964. doi: 10.3389/fmicb.2019.02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jia R, Cao LR, Liu WB, Shen ZY. Detoxification of deoxynivalenol by Bacillus subtilis ASAG 216 and characterization the degradation process. Eur Food Res Technol. 2021;247:67–76. [Google Scholar]
- 144.Wang G, Wang YX, Ji F, Xu LM, Yu MZ, Shi JR, et al. Biodegradation of deoxynivalenol and its derivatives by Devosia insulae A16. Food Chem. 2019;276:436–442. doi: 10.1016/j.foodchem.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 145.Zhai YY, Zhong L, Gao H, Lu ZX, Bie XM, Zhao HZ, et al. Detoxification of deoxynivalenol by a mixed culture of soil bacteria with 3-epi-deoxynivalenol as the main intermediate. Front Microbiol. 2019;10:2172. doi: 10.3389/fmicb.2019.02172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gao XJ, Mu PQ, Wen JK, Sun Y, Chen QM, Deng YQ. Detoxification of trichothecene mycotoxins by a novel bacterium, Eggerthella sp DII-9. Food Chem Toxicol. 2018;112:310–319. doi: 10.1016/j.fct.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 147.He CH, Fan YH, Liu GF, Zhang HB. Isolation and identification of a strain of aspergillus tubingensis with deoxynivalenol biotransformation capability. Int J Mol Sci. 2008;9(12):2366–2375. doi: 10.3390/ijms9122366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Young JC, Zhou T, Yu H, Zhu HH, Gong JH. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem Toxicol. 2007;45(1):136–143. doi: 10.1016/j.fct.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 149.Fuchs E, Binder EM, Heidler D, Krska R. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit Contam. 2002;19(4):379–386. doi: 10.1080/02652030110091154. [DOI] [PubMed] [Google Scholar]
- 150.Shima J, Takase S, Takahashi Y, Fujimoto H, Yamazaki M, Ochi K. Novel detoxification of the trichothecene mycotoxin deoxynivalenol by a soil bacterium isolated by enrichment culture. Appl Environ Microbiol. 1997;63(10):3825–3830. doi: 10.1128/aem.63.10.3825-3830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ju J, Tinyiro SE, Yao WR, Yu H. The ability of Bacillus subtilis and Bacillus natto to degrade zearalenone and its application in food. J Food Process Pres. 2019;43(10):e14122. [Google Scholar]
- 152.Wang G, Yu MZ, Dong F, Shi JR, Xu JH. Esterase activity inspired selection and characterization of zearalenone degrading bacteria Bacillus pumilus ES-21. Food Control. 2017;77:57–64. [Google Scholar]
- 153.Xu JH, Wang HJ, Zhu ZW, Ji F, Yin XC, Hong Q, et al. Isolation and characterization of bacillus amyloliquefaciens ZDS-1: exploring the degradation of zearalenone by bacillus spp. Food Control. 2016;68:244–250. [Google Scholar]
- 154.Lei YP, Zhao LH, Ma QG, Zhang JY. Degradation of zearalenone in swine feed and feed ingredients by Bacillus subtilis ANSB01G. Duxbury Press. 2014;7(2):143–151. [Google Scholar]
- 155.Zhao ZY, Zhang YM, Gong AD, Liu N, Chen SS, Zhao XY, et al. Biodegradation of mycotoxin fumonisin B1 by a novel bacterial consortium SAAS79. Appl Microbiol Biotechnol. 2019;103(17):7129–7140. doi: 10.1007/s00253-019-09979-6. [DOI] [PubMed] [Google Scholar]
- 156.Benedetti R, Nazzi F, Locci R, Firrao G. Degradation of fumonisin B1 by a bacterial strain isolated from soil. Biodegradation. 2006;17(1):31–38. doi: 10.1007/s10532-005-2797-y. [DOI] [PubMed] [Google Scholar]
- 157.Styriak I, Conková E, Kmec V, Böhm J, Razzazi E. The use of yeast for microbial degradation of some selected mycotoxins. Mycotoxin Res. 2001;17(Suppl1):24–27. doi: 10.1007/BF03036705. [DOI] [PubMed] [Google Scholar]
- 158.Camilo SB, Ono CJ, Ueno Y, Hirooka EY. Anti-Fusarium moniliforme activity and fumonisin biodegradation by corn and silage microflora. Bra Arch Biol Techn. 2000;43(2):159–164. [Google Scholar]
- 159.Smiley RD, Draughon FA. Preliminary evidence that degradation of aflatoxin B1 by Flavobacterium aurantiacum is enzymatic. J Food Prot. 2000;63(3):415–418. doi: 10.4315/0362-028x-63.3.415. [DOI] [PubMed] [Google Scholar]
- 160.Lillehoj EB, Stubblefield RD, Shannon GM, Shotwell OL. Aflatoxin M1 removal from aqueous solutions by Flavobacterium aurantiacum. Mycopathol Mycol Appl. 1971;45(3):259–266. doi: 10.1007/BF02051973. [DOI] [PubMed] [Google Scholar]
- 161.Line JE, Brackett RE, Wilkinson RE. Evidence for degradation of aflatoxin B1 by Flavobacterium aurantiacum. J Food Prot. 1994;57(9):788–791. doi: 10.4315/0362-028X-57.9.788. [DOI] [PubMed] [Google Scholar]
- 162.Gao X, Ma QG, Zhao LH, Lei YP. Isolation of Bacillus subtilis: screening for aflatoxins B1, M1, and G1 detoxification. Eur Food Res Technol. 232(6):957–62.
- 163.Cao H, Liu DL, Mo XM, Xie CF, Yao DS. A fungal enzyme with the ability of aflatoxin B1 conversion: purification and ESI-MS/MS identification. Microbiol Res. 2011;166(6):475–483. doi: 10.1016/j.micres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 164.Yao D, Liu D, Guan M, Xie C. Detoxifizyme with activity of transforming aflatoxin and the gene encodes thereof. US Patent Publ. 2010; No.7695751B2.
- 165.Yehia SR. Aflatoxin detoxification by manganese peroxidase purified from Pleurotus ostreatus. Braz J Microbiol. 2014;45(1):127–133. doi: 10.1590/S1517-83822014005000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao LH, Guan S, Gao X, Ma QG, Lei YP, Bai XM, et al. Preparation, purification and characteristics of an aflatoxin degradation enzyme from myxococcus fulvus ANSM068. J Appl Microbiol. 2011;110(1):147–155. doi: 10.1111/j.1365-2672.2010.04867.x. [DOI] [PubMed] [Google Scholar]
- 167.Alberts JF, Gelderblom WC, Botha A, Zyl WH. Degradation of aflatoxin B (1) by fungal laccase enzymes. Int J Food Microbiol. 2009;135(1):47–52. doi: 10.1016/j.ijfoodmicro.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 168.Tso KH, Lumsangkul C, Ju JC, Fan YK, Chiang H. The potential of peroxidases extracted from the spent mushroom (flammulina velutipes) substrate significantly degrade mycotoxin deoxynivaleno. Toxins (Basel) 2021;13(1):72. doi: 10.3390/toxins13010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.He WJ, Shi MM, Yang P, Huang T, Zhao Y, Wu AB, et al. A quinone-dependent dehydrogenase and two NADPH-dependent aldo/keto reductases detoxify deoxynivalenol in wheat via epimerization in a Devosia strain. Food Chem. 2020;321:126703. doi: 10.1016/j.foodchem.2020.126703. [DOI] [PubMed] [Google Scholar]
- 170.Carere J, Hassan YI, Lepp D, Zhou T. The enzymatic detoxification of the mycotoxin deoxynivalenol: identification of DepA from the DON epimerization pathway. Microb Biotechnol. 2018;11(6):1106–1111. doi: 10.1111/1751-7915.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Feltrin AC, Garcia SD, Caldas SS, Primel EG, Badiale-Furlong E, Garda-Buffon J. Characterization and application of the enzyme peroxidase to the degradation of the mycotoxin DON. J Environ Sci Health B. 2017;52(10):777–783. doi: 10.1080/03601234.2017.1356672. [DOI] [PubMed] [Google Scholar]
- 172.Ito M, Sato I, Ishizaka M, Yoshida S, Koitabashi M. Bacterial cytochrome P450 system catabolizing the Fusarium toxin deoxynivalenol. Appl Environ Microbiol. 2013;79(5):1619–1628. doi: 10.1128/AEM.03227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shcherbakova L, Rozhkova A, Osipov D, Zorov I, Mikityuk O, Statsyuk N, et al. Effective zearalenone degradation in model solutions and infected wheat grain using a novel heterologous lactonohydrolase secreted by recombinant penicillium canescens. Toxins (Basel) 2020;12(8):475. doi: 10.3390/toxins12080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Azam MS, Yu DZ, Liu N, Wu AB. Degrading ochratoxin A and zearalenone mycotoxins using a multifunctional recombinant enzyme. Toxins (Basel). 2019;11(5):301. [DOI] [PMC free article] [PubMed]
- 175.Masching S, Naehrer K, Karin N, Schwartz-Zimmermann HE, Sărăndan M, Schaumberger S, et al. Gastrointestinal degradation of Fumonisin B1 by carboxylesterase FumD prevents fumonisin induced alteration of sphingolipid metabolism in turkey and swine. Toxins (Basel) 2016;8(3):84. doi: 10.3390/toxins8030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Karlovsky P, Crane EH, Gilliam JT, Maddox JR. Compositions and methods of zearalenone detoxification. US Patent:6812380.
- 177.Viksoe-Nielsen A, Birthe Hauerbach S. Process for degrading zearalenone in a feed product employing laccase. 2009; AU–A–2008337569.
- 178.Gradelet S, Le Bon AM, Bergès R, Suschetet M, Astorg P. Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in the rat: role of the modulation of aflatoxin B1 metabolism. Carcinogenesis. 1998;19(3):403–411. doi: 10.1093/carcin/19.3.403. [DOI] [PubMed] [Google Scholar]
- 179.Xiao H, Tan BE, Wu MM, Yin YL, Li TJ, Yuan DX, et al. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: II. Intestinal morphology and function. J Anim Sci. 2013;91(10):4750–4756. doi: 10.2527/jas.2013-6427. [DOI] [PubMed] [Google Scholar]
- 180.Ledur PC, Santurio JM. Cytoprotective effects of curcumin and silymarin on PK-15 cells exposed to ochratoxin A, fumonisin B1 and deoxynivalenol. Toxicon. 2020;185:97–103. doi: 10.1016/j.toxicon.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 181.Cao L, Zhang L, Zeng H, Wu RT, Wu TL, Cheng WH. Analyses of selenotranscriptomes and selenium concentrations in response to dietary selenium deficiency and age reveal common and distinct patterns by tissue and sex in telomere-dysfunctional mice. J Nutr. 2017;147(10):1858–1866. doi: 10.3945/jn.117.247775. [DOI] [PubMed] [Google Scholar]
- 182.Zhao L, Sun LH, Huang JQ, Briens M, Qi DS, Xu SW, et al. A novel organic selenium compound exerts unique regulation of selenium speciation, selenogenome, and selenoproteins in broiler chicks. J Nutr. 2017;147(5):789–797. doi: 10.3945/jn.116.247338. [DOI] [PubMed] [Google Scholar]
- 183.Nagaraj RY, Wu WD, Vesonder RF. Toxicity of corn culture material of fusarium proliferatum M-7176 and nutritional intervention in chicks. Poult Sci. 1994;73(5):617–626. doi: 10.3382/ps.0730617. [DOI] [PubMed] [Google Scholar]
- 184.Tedesco D, Steidler S, Galletti S, Tameni M, Sonzgnoi O, Ravarotto L. Efficacy of silymarin-phospholipid complex in reducing the toxicity of aflatoxin B1 in broiler chicks. Poult Sci. 2004;83(11):1839–1843. doi: 10.1093/ps/83.11.1839. [DOI] [PubMed] [Google Scholar]
- 185.Cheng P, Ishfaq M, Yu H, Yang YQ, Li XT, Fazlani SA, et al. Curcumin ameliorates duodenal toxicity of AFB1 in chicken through inducing P-glycoprotein and downregulating cytochrome P450 enzymes. Poult Sci. 2020;99(12):7035–7045. doi: 10.1016/j.psj.2020.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li Y, Ma QG, Zhao LH, Guo YQ, Duan GX, Zhang JY, et al. Protective efficacy of alpha-lipoic acid against aflatoxin B1-induced oxidative damage in the liver. Asian-Australas J Anim Sci. 2014;27(6):907–915. doi: 10.5713/ajas.2013.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ghadiri S, Spalenza V, Dellafiora L, Badino P, Barbarossa A, Dall'Asta C, et al. Modulation of aflatoxin B1 cytotoxicity and aflatoxin M1 synthesis by natural antioxidants in a bovine mammary epithelial cell line. Toxicol Vitro. 2019;57:174–183. doi: 10.1016/j.tiv.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 188.Sridhar M, Suganthi RU, Thammiaha V. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds. J Anim Physiol Anim Nutr (Berl) 2015;99(6):1094–1104. doi: 10.1111/jpn.12260. [DOI] [PubMed] [Google Scholar]
- 189.Liu D, Guo WB, Yang JH, Zhao ZH, Liu JG. Protective effect of astragalus granules on the immunosuppression of BALB/c mice caused by deoxynivalenol. Chin Vet Sci. 2018.
- 190.Wang XM, Zuo ZC, Deng JL, Zhang Z, Chen CH, Fan Y, et al. Protective role of selenium in immune-relevant cytokine and immunoglobulin production by piglet splenic lymphocytes exposed to deoxynivalenol. Biol Trace Elem Res. 2018;184(1):83–91. doi: 10.1007/s12011-017-1160-6. [DOI] [PubMed] [Google Scholar]
- 191.Atroshi F, Rizzo A, Biese I, Salonen M, Lindberg LA, Saloniem H. Effects of feeding T-2 toxin and deoxynivalenol on DNA and GSH contents of brain and spleen of rats supplemented with vitamin E and C and selenium combination. J Anim Phys Anim Nut. 2010;74(1–5):157–164. [Google Scholar]
- 192.Andretta I, Kipper M, Lehnen CR, Hauschild L, Vale MM, Lovatto PA. Meta-analytical study of productive and nutritional interactions of mycotoxins in growing pigs. Animal. 2012;6(9):1476–1482. doi: 10.1017/S1751731111002278. [DOI] [PubMed] [Google Scholar]
- 193.Wu MM, Xiao H. Yin YL, Li LL, Li TJ. Intervention effects of glutamic acid on the changes of growth performance, blood routine and serum biochemical indexes in deoxynivalenol stressed weaner piglets. Chin J Anim Nutr. 2013.
- 194.Xiao H, Wu MM, Tan BE, Yin YL, Li TJ, Xiao DF, et al. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J Anim Sci. 2013;91(10):4772–4780. doi: 10.2527/jas.2013-6426. [DOI] [PubMed] [Google Scholar]
- 195.Grosse Y, Chekir-Ghedira L, Huc A, Obrecht-Pflumio S, Dirheimer G, Bacha H, et al. Retinol, as-corbic acid and alpha-tocopherol prevent DNA adduct formation in mice treated with the mycotoxins ochratoxin A and zearalenone. Cancer Lett. 1997;114(1–2):225–229. doi: 10.1016/s0304-3835(97)04669-7. [DOI] [PubMed] [Google Scholar]
- 196.Gbore FA, Adu OA. Ameliorative potential of vitamin E on the impact of dietary fumonisin B1 on reproductive performance of female rabbits. J Agr Rural Dev Trop. 2017;118(2):161–169. [Google Scholar]
- 197.Lu ZB. Dose-dependent fumonisin B1 hepatotoxicity and hepatocarcinogenicity, detoxification of fumonisin B1, and suppression by isoflavones of fumonisin B1-promoted hepatocarcinogenesis in rats. Dissertations Theses. 1997.
- 198.Klein PJ, Vleet T, Hall JO, Coulombe RA., Jr Dietary butylated hydroxytoluene protects against aflatoxicosis in Turkeys. Toxicol Appl Pharmacol. 2002;182(1):11–19. doi: 10.1006/taap.2002.9433. [DOI] [PubMed] [Google Scholar]
- 199.Solcan W, Gogu M, Floristean V, Oprisan B, Solcan G. The hepatoprotective effect of sea buckthorn (Hippophae rhamnoides) berries on induced aflatoxin B1 poisoning in chickens. Poult Sci. 2013;92(4):966–974. doi: 10.3382/ps.2012-02572. [DOI] [PubMed] [Google Scholar]
- 200.Chen MH, Yin J, Deng JW, Wu MM, Xiao H, Duan JL, et al. Interventional effects of amino acids on blood physiological and biochemical damages of fattening pigs induced by deoxynivalenol. Hunan Agric Sci. 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are publicly available.