Figure 1. Fluoropyrimidine metabolism.
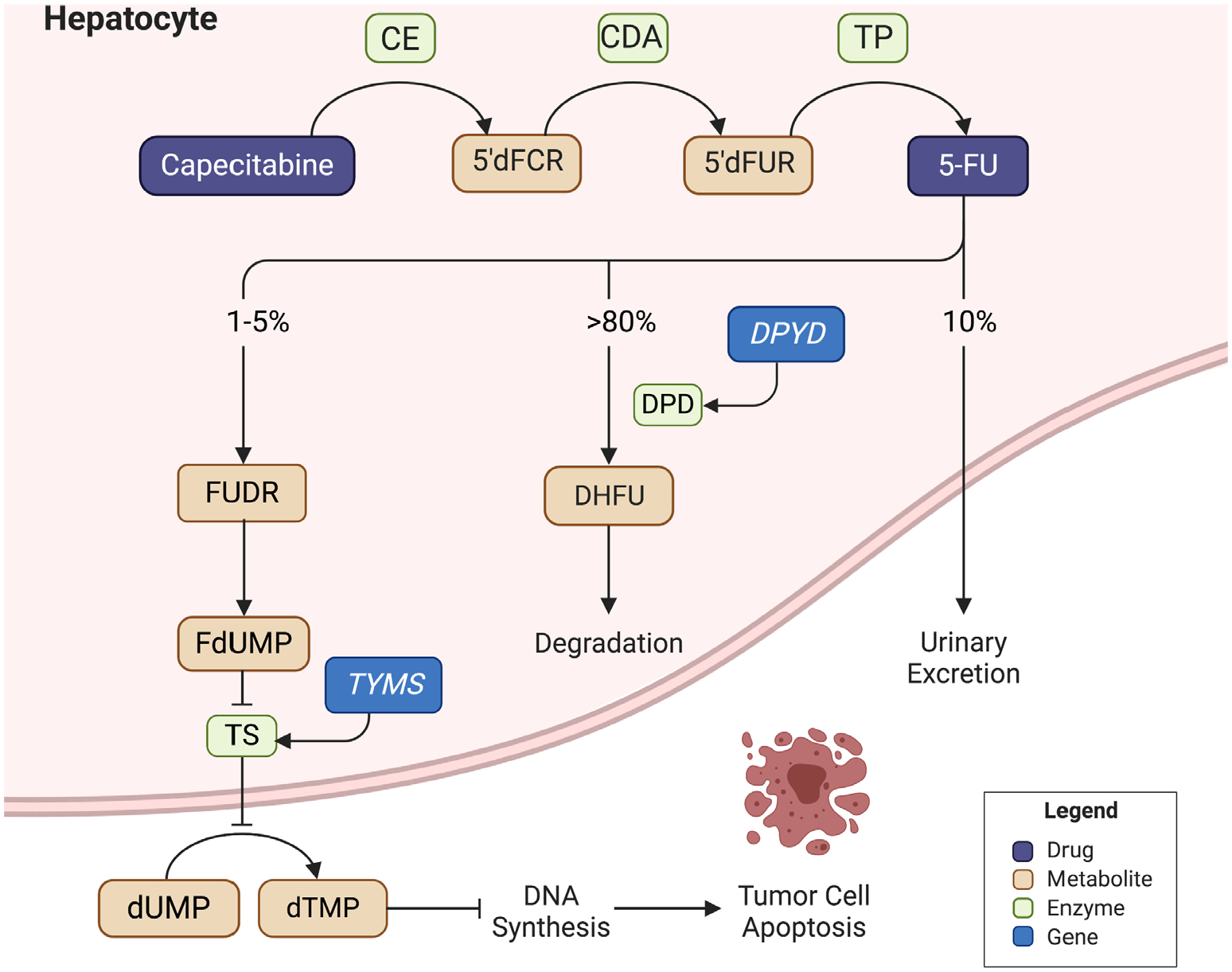
Capecitabine is an oral prodrug that undergoes conversion to 5-fluorouracil via a three-step enzymatic cascade. After metabolism to fluorodeoxyuridine and fluorodeoxyuridine monophosphate, a stable complex with thymidylate synthase is formed to inhibit deoxythymidine monophosphate production. A downstream depletion of deoxyribonucleic acid (DNA) synthesis occurs, leading to cytotoxicity. Catabolism is mediated by dihydrofluorouracil via dihydropyrimidine dehydrogenase. 5′dFCR: 5′-deoxy-5-fluorocytidine; 5-FU = fluorouracil; CDA = cytidine deaminase; CES = carboxylesterase; DHFU = dihydrofluorouracil; DPD = dihydropyrimidine dehydrogenase (encoded by DPYD); dTMP = deoxythymidine monophosphate; dUMP = deoxyuridine monophosphate; FdUMP = fluorodeoxyuridine monophosphate; FUDR = fluorodeoxyuridine; TP = thymidine phosphorylase; TS = thymidylate synthase (encoded by TYMS).
