Abstract
Background
Acute pancreatitis (AP) is a common acute abdominal disease. Rapid evaluation of the severity is important for AP prognosis and treatment. Free triiodothyronine (fT3) level is associated with the prognosis of AP patients. This study aimed to investigate the fT3 level in patients with acute pancreatitis; early warning signs of inflammation, including interleukin-6 (IL-6) and interleukin-10 (IL-10); and the correlation of fT3 level with illness severity.
Material/Methods
Enrolled AP patients (N=312) were divided into an SAP group (N=92) and a non-SAP group (N=220) according to the Revision of Atlanta classification. Blood or tissue samples and baseline clinical characteristics were recorded. The t test and chi-square test were used to evaluate differences between the 2 groups. Multivariate logistic regression analysis and receiver operating characteristic (ROC) curves were used to investigate protective factors. One-way repeated measures analysis of variance was used to evaluate the prognosis of SAP patients.
Results
In our study, compared with APACHII score (AUC 0.829 [95% CIs 0.769–0.889]) and Ranson score (AUC 0.629 [95% CIs 0.542–0.715]), our predictive model (AUC 0.918 [95% CIs 0.875–0.961]) showed better prognostic performance in predicting poor patient outcomes. In the SAP group, changes in fT3 level were significantly associated with prognosis (P<0.05).
Conclusions
The predictive model can improve the diagnostic accuracy and prediction of the severity of disease. FT3 level could be used as an independent risk factor to predict the mortality of SAP patients.
Keywords: 3′-bromo-3,5,5′-triiodothyronine; Interleukin-10; Interleukin-6; Pancreatitis
Background
Acute pancreatitis (AP) is an acute inflammatory process of the pancreas that results in local and systemic complications. It is the most common acute abdominal disease requiring hospital admission [1–5]. Severe AP (SAP) was redefined by the 2012 Atlanta classification for AP as AP with persistent organ failure (organ failure lasting for more than 48 h), whose mortality rate is from 15% to 20% [6–9]. The development of AP is not yet fully clear, with 2 phases defined during AP: the early phase involving resultant persistent organ failure, and a systemic inflammatory response. There are presently no effective medical treatments or surgeries available to cure SAP; therefore, most treatment is supportive [10]. Thus, rapid evaluation of disease severity and determining the development phase of AP are pivotal in selecting a treatment strategy, as starting appropriate therapy can significantly reduce mortality in patients who eventually develop SAP. Cumulative date suggest that metabolism of local organization play pivotal roles during the early phase of the systemic inflammatory response that driven by local pancreatic injury [11–13]. Thyroid hormone plays a pivotal role in the pathogenesis of numerous diseases, including heart disease, prognosis of cardiac surgery, and acute and chronic liver diseases.
Thyroid hormone is important for normal development, differentiation, growth, and maintenance of metabolic homeostasis [14] and can easily be measured by clinicians. Compared with other thyroid hormones, low triiodothyronine (T3) has been frequently and extensively used in the differential prognosis of disease. Recently, fT3 (free triiodothyronine) was shown to be associated with inflammatory reactions or organ failure [15]. FT3 was further demonstrated to be an independent predictor of intensive care unit mortality in early-stage patients [16]. For patients with AP, fT3 has been shown to be positively associated with AP, but it is not yet clear whether fT3 is associated with the prognosis of SAP patients or whether fT3 can be used as an independent risk factor for SAP patients. Recent studies support that IL-6, as a pro-inflammatory factor, plays an important role in the development of acute pancreatitis into severe acute pancreatitis [17]. IL-6 can strengthen the intensity of the systemic inflammatory response, leading to injury of various organs in the body, and promotes the production and release of IL-6 [18]. A study found that IL-10 levels were significantly elevated in the early stages of acute pancreatitis and were even higher in patients who eventually developed severe acute pancreatitis [19]. As an anti-inflammatory factor, IL-10 mainly plays a role in inhibiting cell necrosis during the development of acute pancreatitis to prevent its further development [20]. Illness severity of patients with acute pancreatitis can be quickly assessed by measuring fT3 and interleukin together. In the present study, we aimed to establish a new predictive model for assessing the prognosis and severity of acute pancreatitis.
Material and Methods
Patients
This consecutive cohort study enrolled 312 patients diagnosed AP admitted to the Affiliated Hospital of Qingdao University between December 2019 between December 2020. Three classifications were defined according to the Atlanta classification of AP.
Inclusion criteria: (1) First episode of acute pancreatitis as defined by the revised Atlanta classification [21]; (2) Age ≥18 years old, no previous history of mental disorders, and able to communicate freely; (3) All patients (or their immediate family members) signed the informed consent.
Exclusion criteria: (1) Preexisting history of hypothyroidism or hyperthyroidism diagnosed before admission; (2) Pancreatitis induced by surgery, trauma, chemotherapy; (3) Pregnancy; (4) Chronic pancreatitis; (5) Referral from other hospitals.
The study protocol was conducted according to the principles of the Declaration of Helsinki, and was approved by the Affiliated Hospital of Qingdao University. Written informed consent was obtained from all participants before enrolment (Figure 1).
Figure 1.
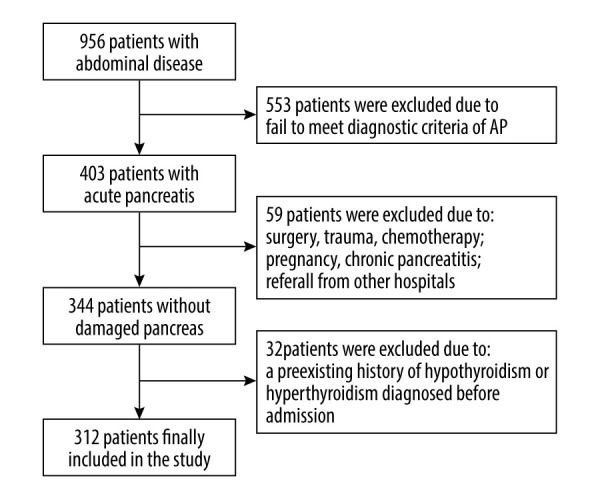
Flowchart of patient enrolment. (PS: Photoshop, Photoshop CC, Adobe systems). This illustration represents our standard procedure for patient inclusion.
Records and Assays
All laboratory data were obtained from patients during the first 24 h after emergency admission, including: thyroid hormone, blood routine, blood biochemistry, liver and kidney function, electrolyte, blood gas analysis, inflammation factors, physiological function, and acute and chronic health II (APACHE II) scores and Ranson score. Serum thyroid hormones were respectively collected at 1 day, 7 days, 14 days, and at the end-point (discharge or death), and were detected using chemiluminescence immunoassay (Immulite 2000; Siemens, Germany) in the Laboratory Department, Affiliated Hospital of Qingdao University (reference range, TG 1.4–78 ng/ml, fT3 3.1–6.8 pmol/L, fT4 12–22 pmol/L, TSH 0.27–4.2 mIU/L, TPO 0–34 IU/ml). The coefficient of variation was less than 5%. Clinical data were recorded in clinical big data from the Affiliated Hospital of Qingdao University. The prognosis at the end-point of all enrolled SAP patients was recorded. According to end-point results and previous studies [22], there is little difference in prognosis between patients with mild vs those with moderate acute pancreatitis difference, and there is little difference early after treatment; therefore, patients with mild and moderate acute pancreatitis were combined into a single group to be compared with severe acute pancreatitis. Thus, SAP with higher mortality was divided into a group of 92 cases with mild acute pancreatitis (MAP) and moderately severe acute pancreatitis (MSAP) with lower mortality was divided into a group of 220 cases. The thyroid value was compared between the 2 groups. We used receiver operator characteristic (ROC) curves to assess the prognostic value of thyroid for SAP patients.
Statistical Analysis
Statistical analysis and clinical data entry were performed using SPSS 26.0 (SPSS, Inc, Chicago IL, USA) and Microsoft Excel. Continuous data are reported as mean and standard (SD). Categorical data are presented as numbers (frequency). According to the experimental design, overall data sources were independent of each other. The outliers were analyzed according to the stem-leaf diagram. The Shapiro-Wilk test was used to test normality. Levene’s test was used to assess data homogeneity of variance. The t test and Mann-Whitney U test were used to assess the difference in baseline characteristics between the 2 groups. The chi-square test and Kruskal-Wallis test were used to evaluate differences in multiple group comparisons for categorical variables and continuous variables. The risk factors were determined using multivariate logistic regression analysis for predicting prognosis in AP patients. To further evaluate the prognostic value of different parameters in predicting prognosis, we used receiver operating characteristic (ROC) curves, and the area under the ROC curve (AUC) was estimated. The Shapiro-Wilk test and Mauchly’s test of sphericity were used to assess the data of serum thyroid levels in SAP patients at 1 day, 7 days, 14 days, and end-point. One-way repeated measure analysis of variance was used to evaluate prognosis of SAP patients. Hazard ratios and 95% confidence intervals are presented. P values <0.05 were considered significant.
The sample size was calculated according to our pre-experiment and the literature [23,24]. The mean, standard deviation, adjusted relative allowable error, absolute allowable error, and parameter α were 2.53, 0.05, 0.05, and 0.05, respectively, and the ratio of loss to follow-up was set to 0.1. The SAP group sample size of 88 patients was calculated. Similarly, the mean and standard deviation of the non-SAP group were 3.45 and 0.86, respectively, and the required sample size was 211 patients. For our study to reach meaningful conclusions, at least 299 patients were collected for serological indicators [25,26].
Results
Clinical Characteristics of the Study Population
In the non-SAP group, a total of 220 patients (64.55% male and 35.45% female) in the non-severe acute pancreatitis group were enrolled in this study, with an average age of 56.19±17.69 years. Each cause accounted for 27.27% of bile, 31.82% of lipid, 4.55% of bile and lipid coexisting, 0.91% of alcohol, and 35.45% of other possible causes (iatrogenic, drug-induced, autoimmune). The average length of hospitalization was 11.62±7.19 days, and the average hospitalization cost was $3785.98 ±2867.33 USD.
In the SAP group, 92 patients (66.30% male, 33.70% female) were included, with an average age of 56.83±14.79 years. The proportions of each cause were: gallbladder 60.87%, lipogenic 30.43%, the coexistence of gallbladder and lipogenic 8.70%, alcoholic 0%, and other possible causes (iatrogenic, drug-induced, autoimmune) 0%. Their average hospitalization time was 18.26±6.41 days, and the average hospitalization cost was $8553.44 ± 4238.37 USD.
In 2 groups of patients, age, sex, BMI, PLT, Cr, and smoking history were not significantly different (P>0.05) and with the increase of severity, patients with history of drinking, APACHE II score, and Ranson score difference was statistically significant (Table 1).
Table 1.
Comparison of general data of patients with between the SAP group and non-SAP group.
| Variable | SAP group (n=92) | Non-SAP group (n=220) | P value |
|---|---|---|---|
| Age, years | 56.82±14.79 | 56.19±17.69 | 0.745 |
| Sex, Male/Female | 61/31 | 142/78 | 0.766 |
| Etiology | * | ||
| BAP | 56 | 60 | |
| HTGAP | 28 | 70 | |
| BAP+HTGAP | 8 | 10 | |
| AAP | 0 | 2 | |
| Others | 0 | 78 | |
| BMI, kg/m2 | 25.53±6.26 | 24.09±3.03 | 0.649 |
| Alcohol, yes/no | 48/44 | 74/146 | 0.002 |
| Tobacco, yes/no | 40/52 | 78/142 | 0.183 |
| TSH, ng/ml | 0.81±1.40 | 0.89±1.26 | 0.651 |
| FT4, nmol/L | 12.58±3.49 | 14.76±2.38 | * |
| FT3, nmol/L | 2.53±1.03 | 3.45±0.86 | * |
| LDH, U/L | 1.84±1.19 | 1.29±0.66 | 0.002 |
| PLT, 10 9 /L | 226.2±46.03 | 203.04±66.48 | 0.139 |
| PLT(48), 109/L | 240.79±106.09 | 274.26±79.64 | 0.007 |
| Tbil, μmol/L | 37.82±29.44 | 24.29±28.25 | * |
| Tbil(48), μmol/L | 43.89±15.77 | 18.27±8.92 | * |
| Cr, μmol/L | 118.32±140.51 | 102.04±102.94 | 0.256 |
| Cr(48), μmol/L | 119.35±160.55 | 31.99±39.87 | * |
| P/F, mmHg | 250.57±85.62 | 443.48±37.32 | * |
| P/F (48), mmHg | 248.91±71.71 | 457.65±29.89 | * |
| SBP, mmHg | 137.48±20.87 | 132.95±18.05 | 0.071 |
| SBP(48), mmHg | 133.53±15.77 | 127.9±12.83 | 0.001 |
| CRP, mg/L | 127.70±68.48 | 62.80±60.29 | * |
| PCT, ng/ml | 9.19±13.24 | 0.22±0.17 | * |
| WBC, 10 9 /L | 21.15±8.20 | 13.91±2.80 | * |
| IL-10, pg/ml | 5.64±13.6 | 1.4±1.37 | * |
| IL-1β, pg/ml | 20.76±42.7 | 2.29±1.7 | * |
| IL-2, pg/ml | 99.05±634.66 | 1.95±2.92 | 0.024 |
| IL-4, pg/ml | 2.48±4.86 | 1.17±2.79 | 0.003 |
| IL-5, pg/ml | 2.16±2.41 | 0.85±0.45 | * |
| IL-6, pg/ml | 106.63±165.52 | 23.01±150.78 | * |
| IL-8, pg/ml | 262.22±1372.12 | 5.02±12.16 | 0.006 |
| GCS score | 12.38±1.43 | 13.70±0.86 | * |
| GCS score(48) | 12.52±1.2 | 14.36±0.85 | * |
| SOFA score | 5.21±1.87 | 1.74±1.18 | * |
| SOFA score(48) | 5.14±2.13 | 0.75±0.77 | * |
| Ranson score | 3.88±2.30 | 2.54±2.31 | * |
| APACHE II score | 18.36±8.37 | 6.49±4.51 | * |
Data are presented as the mean±SD
P value <0.001.
BAP – biliary acute pancreatitis; HTGAP – hypertriglycemic acute pancreatitis; AAP – alcohol-induced acute pancreatitis; BMI – body mass index; LDH – lactate; PLT – platelet; Tbil – total bilirubin; Cr – creatinine; TSH – thyroid-stimulating hormone; FT4 – free tetraiodothyronine; FT3 – free triiodothyronine.
ROC Curves of Thyroid and Other Parameters for Predicting AP Prognosis
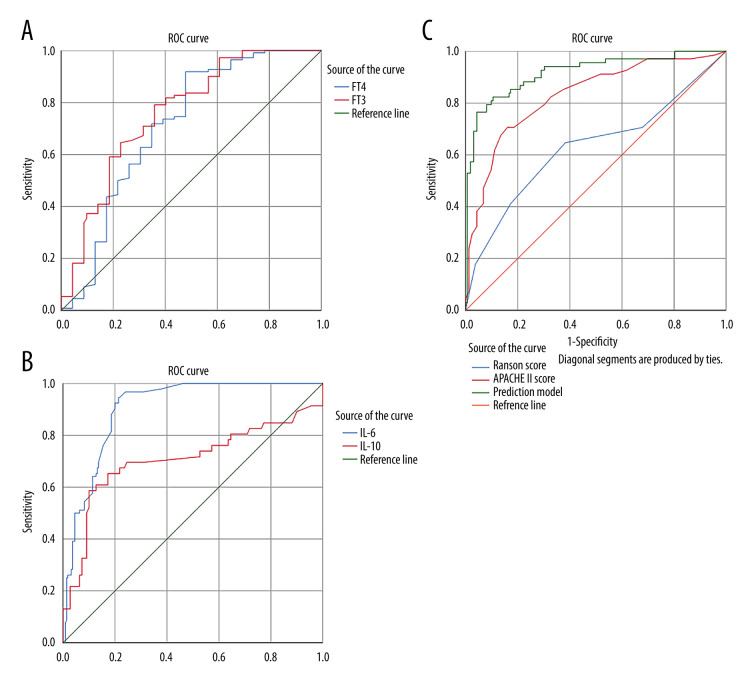
According to our pre-experiment and the literature [17], fT3, IL-10, and IL-6 were chosen to construct a prediction model. Binary logistic analysis was used to calculate probabilities. To calculate ROC of prediction model, we further considered the predictive value of these parameters detected upon presentation. Compared to fT3 (AUC 0.759 [95% CIs 0.698 to 0.821]), IL-6 (AUC 0.904 [95% CIs 0.872 to 0.936]), APACHE II score (AUC 0.829 [95% CIs 0.769 to 0.889]), the prediction model (AUC 0.918 [95% CIs 0.875 to 0.961]) showed superior prognostic performance for predicting severe outcomes. The predictive values of these parameters for SAP and prognosis were higher than fT4 (AUC 0.712 [95% CIs 0.641 to 0.783]), IL-10 (AUC 0.707 [95% CIs 0.632 to 0.781]), Ranson score (AUC 0.629 [95% CIs 0.542 to 0.715]) (Table 2.) The cut-off value of fT3, fT4, IL-10, IL-6, prediction model, APACHE II score, and Ranson score for the prediction of the outcomes respectively were 2.815 nmol/L, 11.9 nmol/L, 1.715 pg/ml, 6.875 pg/ml, 0.467, 13, and 3, respectively. The cut-off value for the prediction model was best, with 76.5% sensitivity and 95.7% specificity. The results showed that FT3 did not have an obvious advantage over FT4 in predicting the prognosis of SAP, and IL-6 had an obvious advantage over IL-10. Our prediction model had an obvious prediction advantage over APACHE II score and Ranson score (Figure 2).
Table 2.
Diagnostic capacity of various indicators values to discriminate of AP.
| Variable | Cut-off | AUC | 95% CI | SE | SP |
|---|---|---|---|---|---|
| fT4, nmol/L | 11.91 | 0.712 | 0.641–0.783 | 0.918 | 0.522 |
| fT3, nmol/L | 2.815 | 0.759 | 0.698–0.821 | 0.791 | 0.641 |
| IL-10, pg/ml | 1.715 | 0.707 | 0.632–0.781 | 0.587 | 0.9 |
| IL-6, pg/ml | 6.875 | 0.904 | 0.872–0.936 | 0.946 | 0.786 |
| Prediction model | 0.467 | 0.918 | 0.875–0.961 | 0.765 | 0.957 |
| APACHE II score | 13 | 0.829 | 0.769–0.889 | 0.706 | 0.840 |
| Ranson score | 3 | 0.629 | 0.542–0.715 | 0.647 | 0.617 |
AUC – area under curve; SE – sensitivity; SP – specificity.
Figure 2.
ROC curves of thyroid and other parameters for predicting AP prognosis. Panel A is the comparison between ROC curve of FT3 and that of FT4. Panel B is the ROC curve comparison of IL-6 and IL-10. Panel C is ROC curve analysis of the prediction model composed of Ranson score, APACHE II score. (SPSS: Statistical Product and Service Solutions, SPSS 26.0, IBM; PS: Photoshop, Photoshop CC, Adobe systems).
For comparison of serum free triiodothyronine in different time periods in SAP patients, FT3 was continuously measured on day 7, day 14, and at discharge in patients with severe acute pancreatitis. The serum concentrations of FT3 on day 1, day 7, day 14 and after the end-event (death or discharge) were 2.28±0.89 nmol/L, 3.31±1.48 nmol/L, 3.80±1.86 nmol/L, and 3.92±2.13 nmol/L, respectively. The difference of serum FT3 concentrations in the 4 groups was statistically significant, F (49, 891, 1.980)=25.2 after correction, P<0.001. The serum concentration of FT3 on day 7 was significantly increased by 1.025 nmol/L (95% CI: 0.543–1.508) compared with that on day 1 (P<0.001). The serum concentration of FT3 on day 14 was 1.511 (95% CI: 0.923–2.099) nmol/L higher than that on day 1 (P<0.001). The serum FT3 concentration of the final event was 1.634 (95% CI: 0.975–2.293) nmol/L higher than that of the first day (P<0.001). The serum FT3 concentration of the final event was 0.609 (95% CI: 0.002–1.216) nmol/L higher on day 7 (P=0.049). There was no significant difference among other groups (Table 3). With the prolongation of disease course and improvement of prognosis, FT3 level of SAP patients gradually recovered and gradually approached normal (Figure 3, 4).
Table 3.
Comparison of serum free triiodothyronine in different time periods in SAP patients.
| Group | Mean±SD | 95% CI |
|---|---|---|
| 1d | 2.28±0.89 | 2.08–2.49 |
| 7d | 3.31±1.48 | 2.97–3.64 |
| 14d | 3.80±1.86 | 3.37–4.22 |
| End-point | 3.92±2.13 | 3.44–4.40 |
End-point – dead or discharge.
Figure 3.
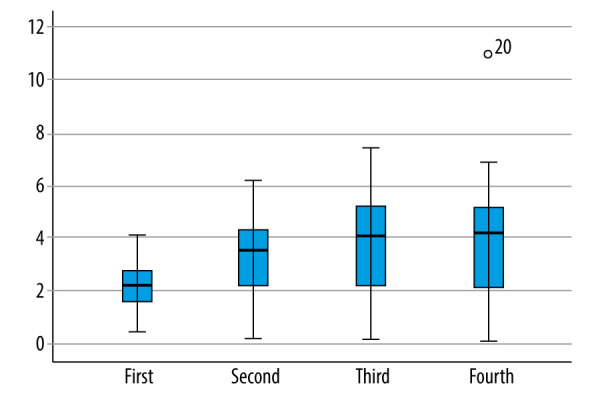
Basic distribution of continuous changes in FT3 in SAP patients. First: FT3 was collected on the first day of admission. Second: FT3 was collected on the 7th day of admission. Third: FT3 was collected on the 14th day of admission. Fourth: FT3 was collected on the day after the end-event (death or discharge). (SPSS: Statistical Product and Service Solutions, SPSS 26.0, IBM; Photoshop CC, Adobe systems).
Figure 4.
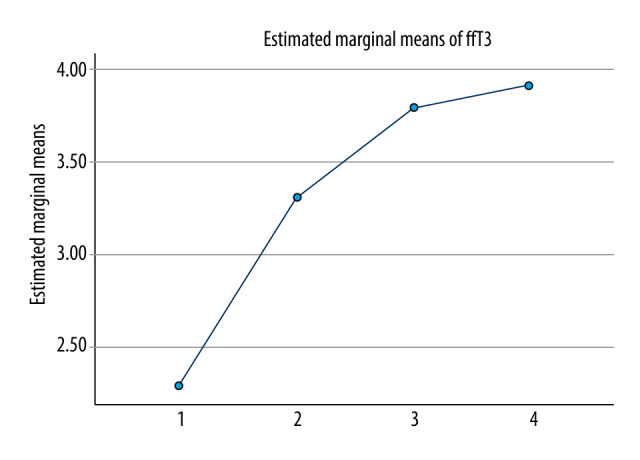
Continuous changes in FT3 in SAP patients after admission. 1: FT3 was collected on the first day of admission. 2: FT3 was collected on the 7th day of admission. 3: FT3 was collected on the 14th day of admission. 4: FT3 was collected on the day after the end-event (death or discharge) of admission. (SPSS: Statistical Product and Service Solutions, SPSS 26.0, IBM; Photoshop CC, Adobe systems).
On day 1 and day 7 day, with the increase of severity of acute pancreatitis, serum thyroid hormone decreased or remained the same, and did not return to normal levels (P<0.05). With the increase of length of hospital stay until discharged from the hospital, serum thyroid hormone levels in severe acute pancreatitis recovered (P<0.05). The serum thyroid hormone concentration of the dead patients continued to not recover, and the difference was statistically significant compared with that of the cured patients (P<0.05) (Table 4).
Table 4.
Comparison of mortality in SAP and serum free triiodothyronine (fT3) in different time periods.
| Group | Cases | 1 day | 7 days | 14 days | Termination |
|---|---|---|---|---|---|
| Survival | 74 | 2.67±1.01 | 3.88±1.25 | 4.54±1.32 | 4.98±1.30 |
| Dead | 18 | 1.94±0.94 | 1.74±1.21 | 1.35±1.10 | 0.74±0.52 |
Comparison of FT3 between survival and death of patients with severe acute pancreatitis: Comparison on day 1 of admission: P<0.05; Compared with the 7th day of admission: P<0.05; Compared with the 14th day of admission: P<0.05; P<0.05; P<0.05;
In an analysis of mortality in different modes of treatment for SAP, 8 patients received interventional therapy with a mortality rate of 50%; 8 patients received surgical therapy with a mortality rate of 75%; the mortality rate of SAP patients with necrosis on CT was 47.62%; and the mortality rate of SAP patients with septic complications was 37.93% (Table 5).
Table 5.
Comparison of mortality rates in SAP.
| Group | Cases | Survival | Dead | Death rate |
|---|---|---|---|---|
| Interventional therapy | 8 | 4 | 4 | 50.00% |
| Surgical therapy | 8 | 2 | 6 | 75.00% |
| Necrosis on CT | 21 | 11 | 10 | 47.62% |
| Septic complications | 29 | 18 | 11 | 37.93% |
Discussion
The pancreas is the mixed secretory gland in humans, affecting every organ in the body. The secretory system is often subject to damage by pancreatic disease [27]. AP is one of the most common pancreatic disease worldwide, and SAP has very high mortality. In the present study, rapidly recognizing SAP patients with high mortality risk and providing more active therapy can decrease mortality [28]. We found that fT3 contributes to early evaluation of the severity and prognosis for SAP patients, and the prediction model had a greater ROC AUC vs either APACHE II score or Ranson score in predicting the prognosis of SAP patients, suggesting that the prediction model may have batter prognostic value for SAP patients.
Thyroid hormone regulates the metabolic processes of body tissues [29]. The elevated inflammation levels can suppress thyroid hormones levels. In this prospective cohort study, we found that fT3 was significantly elevated in AP patients who progressed to SAP. A similar elevation was seen in both the APACHE II score and Ranson score. In our study, early evaluation of the severity was more important for fT3. The fT3 level can be obtained easily and cheaply, and has better sensitivity and is less affected by other factors [30]. The low fT3 state has been described as a predictor of cardiac mortality in patients with heart failure [16]. The downregulation of fT3 levels is considered to be an adaptive mechanism to disease by conserving energy and decreasing catabolism. While this status is considered to have a beneficial effect on the activation of adaptive mechanisms under stress during the long term, adverse effects are also found for various acute diseases. For the pathogenesis of acute pancreatitis, the disorder of pancreatic blood supply is one of the main initiation mechanisms, and acute pancreatitis secondary to hemorrhagic shock is a typical clinical example. The pancreatic arteriovenous system is a terminal artery structure [31], which is supplied by a vascular system in which it is difficult to establish effective collateral circulation, and does not form collateral circulation with the surrounding arteries. Therefore, pancreatic tissue necrosis easily occurs when the peripheral circulation is disrupted. The relationship between downregulation of fT3 levels and pancreatic microcirculation disturbance is a cycle in which impairment in pancreatic microcirculation leads to lower fT3 levels, and low levels of fT3 reduce the body’s ability to mobilize cellular metabolism in response to stress, which in turn further blocks pancreatic tissue recovery. Eventually, this status causes irreversible damage. Thus, fT3 can be used to predict SAP patient prognosis. Theoretically, early detection and intervention are very important. However, in clinical practice, the therapeutic effects of thyroid hormone in improving disease prognosis are limited. Some studies found that cells have difficulty efficiently taking up circulating thyroid hormones during the disease, and the thyroid hormone levels in tissues and cells remain unchanged. Thus, improvement of disease prognosis has been limited.
The present study also showed that fT3 continued to decline in the early stage of severe acute pancreatitis, and mortality increased in patients with no apparent recovery after 14 days. Further observation showed that the serum fT3 level of the patients who were discharged from hospital after improvement mostly returned to normal on the 7th day after admission, while the serum fT3 levels of patients who eventually died also did not recover as well, and even if the recovery was short, levels were still lower than normal. Thus, we can assess the prognosis by continuously observing serum fT3 levels in patients with severe acute pancreatitis and providing more aggressive treatment.
Inflammation is driven by interleukins [32], especially IL-6 and IL-10 [33]. Severe local tissue damage in SAP leads to severe ischemia and hypoxia. The balance of inflammatory factors in the body is completely broken, and further irreversible damage is caused. Our results further confirmed that IL-6 and IL-10 levels were also higher in SAP patients than in AP patients. Therefore, we are constructed a prediction model by combining fT3, IL-6, and IL-10 to further assess prognosis of AP patients.
The APACHE II score and Ranson score are both commonly used for prognosis prediction in critically ill patients. A recent article provides an excellent review of acute pancreatitis [34]. Clinicians refer to clinical scoring systems as “complex and cumbersome and not routinely used.” The strengths of the Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring and Ranson’s criteria are good sensitivity and high positive predictive value. However, an experienced clinician is needed for evaluation, but unfortunately, such experience is not uniformly available, and acute pancreatitis is commonly first encountered by inexperienced clinicians or trainees who are unfamiliar with managing the complications of severe disease. Thus, assessing levels of FT3, IL-10, and IL-6 can rapidly identify patients with potentially severe acute pancreatitis and can alert physicians to the need for more aggressive treatment. In our study, the AUC of the prediction model was better than those of the APACHE II score and Ranson score, and fT3, IL-6, and IL-10 were found to be independent risk factors for predicting the prognosis of AP patients. Continuously changing fT3 level is a superior predictor of prognosis of SAP patients. Therefore, fT3 is an easily obtained, inexpensive, and useful indicator that should be widely used in ICU practice.
There are some limitations to our study, in that it was a single-center study with a small sample size, and we only examined the prognostic value of changing interleukin levels in SAP patients. Larger trials are needed to verify our results.
Conclusions
We found that the prediction model performs significantly better than APACHE II score and Ranson score in evaluating the severity of AP. FT3, IL-6, and IL-10 were independent risk factors for predicting the prognosis of AP patients. The prognosis of SAP patients is associated with continuously changing fT3 levels.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: National Natural Science Foundation of China (no. 81870440)
References
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–87e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet (London, England) 2020;396(10252):726–34. doi: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JR. Acute pancreatitis. N Engl J Med. 2017;376(6):598. doi: 10.1056/NEJMc1616177. [DOI] [PubMed] [Google Scholar]
- 4.Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004;291(23):2865–68. doi: 10.1001/jama.291.23.2865. [DOI] [PubMed] [Google Scholar]
- 5.Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144(6):1272–81. doi: 10.1053/j.gastro.2013.01.075. [DOI] [PubMed] [Google Scholar]
- 6.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis – 2012: Revision of the Atlanta Classification and Definitions by international consensus. Gut. 2013;62(1):102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 7.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16(8):479–96. doi: 10.1038/s41575-019-0158-2. [DOI] [PubMed] [Google Scholar]
- 8.Dombernowsky T, Kristensen M, Rysgaard S, et al. Risk factors for and impact of respiratory failure on mortality in the early phase of acute pancreati tis. Pancreatology. 2016;16(5):756–60. doi: 10.1016/j.pan.2016.06.664. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Dong L, Zhang Y, et al. Prediction of severe acute pancreatitis using a decision tree model based on the revised Atlanta Classification of acute pancreatitis. PLoS One. 2015;10(11):e0143486. doi: 10.1371/journal.pone.0143486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vege SS, DiMagno MJ, Forsmark CE, et al. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology. 2018;154(4):1103–39. doi: 10.1053/j.gastro.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Landahl P, Ansari D, Andersson R. Severe acute pancreatitis: gut barrier failure, systemic inflammatory response, acute lung injury, an d the role of the mesenteric lymph. Surg Infect (Larchmt) 2015;16(6):651–56. doi: 10.1089/sur.2015.034. [DOI] [PubMed] [Google Scholar]
- 12.Barbeiro DF, Koike MK, Coelho AM, et al. Intestinal barrier dysfunction and increased COX-2 gene expression in the gut of elderly rats with ac ute pancreatitis. Pancreatology. 2016;16(1):52–56. doi: 10.1016/j.pan.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Schietroma M, Pessia B, Carlei F, et al. Intestinal permeability and systemic endotoxemia in patients with acute pancreatitis. Ann Ital Chir. 2016;87:138–44. [PubMed] [Google Scholar]
- 14.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81(3):1097–142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 15.Neves JS, Vale C, von Hafe M, et al. Thyroid hormones and modulation of diastolic function: A promising target for heart failure with pres erved ejection fraction. Ther Adv Endocrinol Metab. 2020;11:2042018820958331. doi: 10.1177/2042018820958331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang CP, Jong YS, Wu CY, Lo HM. Impact of triiodothyronine and N-terminal pro-B-type natriuretic peptide on the long-term survival of critically ill patients with acute heart failure. Am J Cardiol. 2014;113(5):845–50. doi: 10.1016/j.amjcard.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6, IL-8, TNF): Early and reliable predictors of severe acute pancreatitis. Journal of clinical gastroenterology. 2003;37(3):263–66. doi: 10.1097/00004836-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Soyalp M, Yalcin M, Oter V, Ozgonul A. Investigation of procalcitonin, IL-6, oxidative stress index (OSI) plasma and tissue levels in experi mental mild and severe pancreatitis in rats. Bratisl Lek Listy. 2017;118(3):137–41. doi: 10.4149/BLL_2017_027. [DOI] [PubMed] [Google Scholar]
- 19.Vasseur P, Devaure I, Sellier J, et al. High plasma levels of the pro-inflammatory cytokine IL-22 and the anti-inflammatory cytokines IL-10 a nd IL-1ra in acute pancreatitis. Pancreatology. 2014;14(6):465–69. doi: 10.1016/j.pan.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Pezzilli R, Billi P, Miniero R, Barakat B. Serum interleukin-10 in human acute pancreatitis. Dig Dis Sci. 1997;42(7):1469–72. doi: 10.1023/a:1018814710291. [DOI] [PubMed] [Google Scholar]
- 21.Sarr MG. 2012 revision of the Atlanta classification of acute pancreatitis. Pol Arch Med Wewn. 2013;123(3):118–24. doi: 10.20452/pamw.1627. [DOI] [PubMed] [Google Scholar]
- 22.Yang WQ, Yang Q, Chen WJ, et al. Low FT3 is a valuable predictor of severe acute pancreatitis in the emergency department. J Dig Dis. 2018;19(7):431–38. doi: 10.1111/1751-2980.12609. [DOI] [PubMed] [Google Scholar]
- 23.Obuchowski NA, Zhou XH. Prospective studies of diagnostic test accuracy when disease prevalence is low. Biostatistics. 2002;3(4):477–92. doi: 10.1093/biostatistics/3.4.477. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Fine J. On sample size for sensitivity and specificity in prospective diagnostic accuracy studies. Stat Med. 2004;23(16):2537–50. doi: 10.1002/sim.1836. [DOI] [PubMed] [Google Scholar]
- 25.Dose finding studies. Sample Size Tables for Clinical Studies. 2008:195–206. [Google Scholar]
- 26.Introduction to meta-analysis for diagnostic accuracy studies. Statistical Methods in Diagnostic Medicine. 2011:231–60. [Google Scholar]
- 27.Chen CY, Tsai MM, Chi HC, Lin KH. Biological significance of a thyroid hormone-regulated secretome. Biochim Biophys Acta. 2013;1834(11):2271–84. doi: 10.1016/j.bbapap.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Gibor U, Perry Z, Netz U, et al. Circulating cell-free DNA in patients with acute biliary pancreatitis: Association with disease markers and prolonged hospitalization time – a prospective cohort study. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004679. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Coscia F, Taler-Verčič A, Chang VT, et al. The structure of human thyroglobulin. Nature. 2020;578(7796):627–30. doi: 10.1038/s41586-020-1995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, Wang W, Zhang K, Tang YD. Association between low T3 syndrome and poor prognosis in adult patients with acute myocarditis. Front Endocrinol (Lausanne) 2021;12:571765. doi: 10.3389/fendo.2021.571765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almaça J, Caicedo A. Blood flow in the pancreatic islet: Not so isolated anymore. Diabetes. 2020;69(7):1336–38. doi: 10.2337/dbi20-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Gioia M, Spreafico R, Springstead JR, et al. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat Immunol. 2020;21(1):42–53. doi: 10.1038/s41590-019-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye M, Joosse ME, Liu L, et al. Deletion of IL-6 exacerbates colitis and induces systemic inflammation in IL-10-deficient mice. J Crohns Colitis. 2020;14(6):831–40. doi: 10.1093/ecco-jcc/jjz176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rijkers AP, van Eijck CH. Acute pancreatitis. N Engl J Med. 2017;376(6):596–97. doi: 10.1056/NEJMc1616177. [DOI] [PubMed] [Google Scholar]