Abstract
Animal models are crucial tools for evaluating the biological progress of human cancers and for the preclinical investigation of anticancer drugs and cancer prevention. Various animals are widely used in hepatopancreatobiliary cancer research, and mouse models are the most popular. Generally, genetic tools, graft transplantation, and chemical and physical measures are adopted to generate sundry mouse models of hepatopancreatobiliary cancer. Graft transplantation is commonly used to study tumour progression. Over the past few decades, subcutaneous or orthotopic cell-derived tumour xenograft models (CDX models) have been developed to simulate distinct tumours in patients. However, two major limitations exist in CDX models. One model poorly simulates the microenvironment of tumours in humans, such as the vascular, lymphatic and immune environments. The other model loses genetic heterogeneity compared with the corresponding primary tumour. Increased efforts have focused on developing better models for hepatopancreatobiliary cancer research. Hepatopancreatobiliary cancer is considered a tumour with high molecular heterogeneity, making precision medicine challenging in cancer treatment. Developing a new animal model that can better mimic tumour tissue and more accurately predict the efficacy of anticancer treatments is urgent. For the past several years, the patient-derived xenograft model (PDX model) has emerged as a promising tool for translational research. It can retain the genetic and histological stability of their originating tumour at limited passages and shed light on precision cancer medicine. In this review, we summarize the methodology, advantages/disadvantages and applications of PDX models in hepatopancreatobiliary cancer research.
Keywords: PDX model, Hepatopancreatobiliary cancer, Humanized
Introduction
Mouse models are critical tools in preclinical and translational research in hepatopancreatobiliary cancer [1], including drug screening, assessment of therapeutic efficacy, identification of biomarkers, and molecular subtyping. Traditionally, immortalized cancer cell lines derived from patient tumours are used to construct mouse models to simulate tumour growth in the human body [2]. In these models, immortalized cancer cell lines are injected into immunodeficient mice subcutaneously or orthotopically [3]. Comparatively speaking, subcutaneous tumour models cost little and are easy to construct and measure when the tumour grows; however, they cannot simulate the tumour environment in vivo [4]. Orthotopic tumour models can mimic the tumour environment to some degree but are challenging to construct and measuring the tumour size [4]. Both models have contributed substantially to the development of hepatopancreatobiliary cancer research in recent years. However, many investigators have shown that cell-derived xenograft models (CDX models) cannot accurately mimic the tumour condition in human genetic heterogeneity and the tumour microenvironment. Thus, more realistic and clinically relevant models are required to meet the higher remands of preclinical research.
In this context, patient-derived xenograft models (PDX models) have been developed to overcome these disadvantages of CDX models [5]. The PDX model was first reported in 1969. Rygaard and Povlsen implanted a piece of tumour from a 71-year-old patient with sigmoid cancer in nude mice that was maintained for 76 passages [6]. In 1996, the first PDX model of hepatocellular carcinoma was reported [7].
In recent years, PDX models have been verified to maintain the hereditary stability of primary tumours, at least at early passages [8]. Similar results have been reported in various cancers, such as diffuse large B-cell lymphoma (DLBCL) [9], breast cancer (BC) [10], non-small cell lung cancer (NSCLC) [11], neuroblastoma [12] and others [13].
In hepatocellular carcinoma (HCC) and pancreatic ductal adenocarcinoma (PDAC), Raquel et al. compared the transcription profile of PDX models in the 5th and 10th passages and found no major functional changes in the later passages [14]. Additionally, because the proportion of liver cancer in children is different from that in adults, orthotopic PDX models for paediatric liver cancer were also established [15]. The results indicated that the gene expression profiles for five generations (F0, F1, F2, F13, F20) appeared remarkably similar, and PDX models recaptured the metastatic ability of the primary tumour.
Therefore, PDX models are currently considered promising tools for clinical translational research in hepatopancreatobiliary cancer.
Methodology of establishing PDX models
To develop a PDX model, tumour tissues from a patient were collected and transplanted into immunodeficient mice [8]. The passage harbouring tumour tissue is called F0 (or G0), and successful passages are named consecutively (F1, F2, F3, etc.) [8]. Usually, tumours obtained in surgical operations or biopsy are used to develop PDX models. Additionally, tumour cells acquired from ascites [16] or pleural effusion [17] are reported to be valid in developing PDX models in some tumour types. For tumour fragments, two different pretreatment methods are used before implantation. Investigators cut the tumour tissue into approximately 20–50 mm3 pieces [15, 18], followed by subcutaneous or orthotopic implantation. Others prepare tumour tissues into a single-cell suspension for subsequent implantation [19–21]. Both methods have advantages and disadvantages. Tissue fragments can retain cell–cell interactions, which mimic the tumour microenvironment, and single-cell suspensions can avoid heterogeneity inside the tumour to some degree but have a lower success rate because of chemical or physical damage during pretreatment [22]. In most studies, fresh tumour tissue is selected for subsequent implantation. Frozen tumour tissue, however, is also reported in some studies to be an efficient source for implantation [10].
The success rate of developing PDX models is also associated with the mouse strain used to implant tumour tissue. Nude, NOD-SCID and NSG mice are commonly used to develop PDX models. The nude mouse is characterized by the absence of a thymus, resulting in a substantially reduced number of T cells. Additionally, body hair is lacking, making it easy to measure the tumour size. However, functional B cells and NK cells are retained in the body of nude mice, making it challenging for primary human tumour tissue to grow. Thus, the success rate of PDX models developed in nude mice is undesirable. NOD-SCID mice and NSG mice both have more severe immunodeficiency than nude mice because they have no functional T cells or B cells. Additionally, NSG mice have a complete deficiency of NK cells compared with NOD-SCID mice. Therefore, the NSG mouse was recently regarded as the most effective mouse strain for developing PDX models. Theoretically, the optimal transplantation site is exactly where the primary tumour grows, termed orthotopic implantation. Compared with subcutaneous implantation, it offers the most similar anatomic environment, retains spontaneous metastatic capacity [23] and has a higher engraftment rate in some tumour types [24]. However, only a small percentage of studies [15, 25] are based on orthotopic implantation because of its higher requirement for surgical technology and steep cost. Additionally, unlike subcutaneous implantation, imaging technology is needed to monitor tumour growth. The most common implantation site selected is the subcutaneous region in the flanks. This site is not complicated to evaluate, tumour growth can be easily monitored, and the procedure is inexpensive. More than 1000 subcutaneous PDX models of different tumour types, 195 of which were hepatopancreatobiliary cancer, were built by Novartis Institutes, contributing considerably to drug screening [26]. The renal capsule is also a good choice for implantation; it was reported to have a high engraftment rate (36 succeeded in 36) [27] in prostate cancer and to shorten the time for implantation. However, no similar studies have been performed in liver cancer thus far. At our centre, more than 100 HCC tissues have been implanted, and the uptake rate is approximately 39.47% (30/76) [28].
Applications of PDX models in hepatopancreatobiliary cancer
Hepatopancreatobiliary cancer, including liver cancer, pancreatic cancer, and biliary cancer, is characterized by high lethality and poor survival. Surgical resection is the most effective treatment for hepatopancreatobiliary cancer. However, many patients do not have an opportunity for surgery when the tumour is detected [29]. Because of its high heterogeneity, patient responses to other treatments vary widely [30]. Therefore, strategies to guide precision medicine. The most widely used animal models for hepatopancreatobiliary cancer are established using cell lines, which cannot simulate the heterogeneity of tumour tissues. PDX models may overcome this drawback. In hepatopancreatobiliary cancer, PDX models maintained gene expression similarities with primary tumours, and no major functional changes occurred between F5 and F10 PDX passages [14]. Recently, many studies have shown that the response rates to treatment in PDX models are highly consistent with those in patients [4, 31–35]. Therefore, PDX models are better preclinical for precision medicine in hepatopancreatobiliary cancer.
Precision selection of antitumour drugs
Cancer cell lines have been essential tools in drug screening for more than 25 years. The US National Cancer Institute (NCI) has developed 60 human cancer cell lines (NCI-60) to meet the demands [36]. However, in the spring of 2016, the institute retired the NCI-60 and launched a new cancer model derived from fresh patient tumour tissues instead. Cell lines were retired because they have been cultured for thousands of generations, making them adapt to survive on plastic culture disks and differ greatly from primary tumour tissues in genetic make-up and behaviour. Compared with cell lines, fresh tumour tissue shares the same genetic profile as the human body, and investigators have shown that PDX models maintain most genetic features compared to primary human tumour tissue.
For pancreatic ductal adenocarcinoma (PDAC), the response to gemcitabine in PDX models showed a strong correlation with that in clinical patients [37]. In hepatocellular carcinoma, sorafenib (HCC), an oral multikinase inhibitor, is the only US Food and Drug Administration (FDA)-approved first-line systemic therapy until September 2018, when lenvatinib was also approved by the FDA. The efficacy of sorafenib has been tested in the HCC PDX model, which was revealed as a promising tool to predict the efficacy of sorafenib because it shows a response to treatment similar to that in the primary patient [18, 38].
Additionally, PDX models were used to develop new therapeutic strategies in sorafenib-resistant patients. Mark Kin found that stearoyl-CoA desaturase-1 (SCD1) regulated sorafenib sensitivity through endoplasmic reticulum (ER) stress and that using an SCD1 inhibitor combined with sorafenib showed a suppressive effect in a sorafenib-resistant PDX model [39–41]. They also revealed that CD47 upregulation was associated with sorafenib resistance and that the combined use of an anti-CD47 antibody with sorafenib reversed drug resistance [42]. Other drug combination therapy strategies, such as MEK inhibitors combined with sorafenib [43] and mTOR inhibitors combined with bevacizumab [44], have been tested in HCC PDX models and have shown a significant suppressive effect on tumours [45–47].
Novartis [26] established 195 PDX models of hepatopancreatobiliary cancer containing different driver mutations to assess the population responses to diverse treatments. The response of PDX models to different treatments was similar to data collected from clinical trials. Thus, the PDX model has potential application value in the preclinical evaluation of cancer treatment drugs or methods and can predict the clinical trial response to some extent.
However, one major limitation of the PDX model is the loss of human tumour stroma, which is completely replaced by murine stroma in the second generation [48]. Because the interaction between tumour cells and the microenvironment plays a critical role in tumour progression, it likely affects the response of PDX models to a certain treatment. To overcome this problem, coimplantation of human stromal cells with primary tumour tissue may optimize traditional PDX models.
PDX models in identifying tumour biomarkers for molecular subtyping
Clarifying the consistency between the PDX model and primary tumour has not only helped to predict the effectiveness of drugs but has also helped to identify molecular biomarkers related to drug sensitivity or resistance [4] and patient prognosis [49, 50].
In HCC patients, frequent mutations of tuberous sclerosis complex (TSC1) and TSC2 were detected (16.2%, 18/111) [51]. Compared with normal PDX models, TSC2-mutated PDX models appeared more sensitive to rapamycin, an mTOR inhibitor, suggesting that individuals with different TSC expression levels had different susceptibilities to rapamycin. Intriguingly, another research group found that TSC2 was negatively correlated with the efficacy of sorafenib. In PDX models TSC2 upregulation, sorafenib treatment aggravated HCC progression [52], indicating that the PDX model is a promising tool to guide clinical medication. Additionally, polymeric immunoglobulin receptor (pIgR) was recently reported to promote cell transformation and proliferation, contributing to tumour growth. In PDX models, pIgR was shown to be an effective biomarker to predict the efficacy of dasatinib and MEK inhibitors [53].
In another study with 60 PDX models, whole-exome sequencing (WES) was performed on third-generation tumours [54]. Four PDX models were confirmed to contain JAK1 mutations. Compared with other normal models, these four PDX models were sensitive to ruxolitinib, an inhibitor of JAK-STAT, suggesting that PDX models effectively promote the development of HCC therapies. Other biomarkers, such as acetyl-coenzyme A carboxylase alpha (ACCα) [55], CD133 and CD44 [56], were also reported to be associated with tumour growth and a worse prognosis in PDX models.
In conclusion, the PDX model is biologically stable and maintains the gene profile of the primary tumour. Predicting sensitivity or resistance by identifying biomarkers is critical to help develop precision medicine strategies for patients with similar gene expression.
PDX models and Coclinical trials
Tumours are heterogeneous diseases, and individuals’ responses vary with the same treatment. Therefore, a valid model that better links basic research and the clinic is required to overcome this obstacle. The major problems of the traditional mouse model are the lack of tumour heterogeneity and genetic diversity between the models and primary tumours [57]. Medicine proved to be efficient in a traditional animal model [mainly cell-line-derived xenograft models (CDX) models] and had a low response rate when used in clinical trials. More than 95% percent of novel therapies fail in clinical trials [58]. Additionally, when a drug enters clinical trials, analysing and integrating information that may help to prioritize drug use are challenging [4].
Based on this background, the “coclinical trial” project was initiated by Caterina Nardella et al. [59]. They exploited mouse models that faithfully simulate the mutations observed in human bodies to perform preclinical trials parallel to ongoing human clinical trials. The mouse model they used was genetically engineered mice (GEM), and the PDX model was also used by investigators in other parallel studies called “Mouse Avatar” [57, 60]. In coclinical trials, anticancer reagents were administered to patients with a defined genetic makeup and mouse models with similar genetic mutations [60]. Generally, the aim of this project was to optimize treatment strategies in clinical trials to identify the best treatment strategy for patients.
The project was supported by several studies in pancreatic cancer and other tumour types [61–63]. Several clinical trials are ongoing to validate the efficiency of Mouse Avatar. In NCT02795650 [64], patients with metastatic pancreatic adenocarcinoma (PDAC) were recruited, and PDX models were developed to evaluate personalized treatments. Unfortunately, no similar coclinical trials are in progress in HCC that remain to be performed.
In summary, although more evidence must be collected, a coclinical trial is a promising model to optimize therapeutic strategies for patients with tumours. With these Avatar models, therapeutic regimens could be adjusted in a timely manner for a better response instead of waiting for clinical outcomes in patients.
Mini-PDX models in cancer therapy
Although PDX models are cracking tools to evaluate anticancer drug responses, the shortcomings of PDX models are also very obvious. In addition to its high cost, the largest obstacle is that it usually takes 2–6 months for the tumour to grow. This delayed feedback from PDX models limits their application in guiding the treatment of the original patients. To overcome this obstacle, a rapid in vivo drug sensitivity assay called mini-PDX was developed that can test the response of antitumour cancer drugs within 7 days [65].
To establish a mini-PDX model, tumour tissue is washed with Hank’s balanced salt solution to wipe off nontumour tissue followed by digestion with collagenase. After the preparation of the cancer cell suspension, it is filled into a modified microencapsulation and hollow fibre culture system (OncoVee capsules). Next, the capsules are transplanted subcutaneously into immunodeficient mice, and the mice are subsequently treated with antitumour drugs. Therapeutic responses are evaluated by measuring tumour cell proliferation in the capsules.
In a recent study, 26 tumour tissues acquired from patients with pancreatic cancer and other tumour types were collected to establish mini-PDX models. The efficiency of S-1, docetaxel, oxaliplatin, irinotecan and other drugs was assessed in mini-PDX models. Afterwards, the therapeutic response was compared with known clinical outcomes in patients. The mini-PDX model had a sensitivity of 80% and a specificity of 93%, indicating that the mini-PDX model is a powerful tool to evaluate efficiency [65]. In a case of pancreatic cancer, the sensitivity of chemotherapy drugs was consistent with the clinical response in the original patient. The patient, treated with the therapeutic regimen evaluated by the mini-PDX model, had a good reaction [66]. In another study, 12 gallbladder carcinoma tumour tissues were used to establish mini-PDX models to examine the sensitivity of five chemotherapy drugs. The results were adopted to guide the treatment of patients after surgery. Compared with patients treated with traditional chemotherapy, patients in the mini-PDX guided therapy group had a longer overall survival (OS) (18.6 months vs. 13.9 months) and longer disease-free survival (DFS) (17.6 months vs. 12.0 months) [67].
In conclusion, mini-PDX models are suitable for selecting effective or elucidating noneffective therapeutic strategies and show promise in precision medicine.
Humanized PDX models in cancer immunotherapy
The immune system plays a crucial role in both promoting and inhibiting tumour growth [68], and immunotherapy is a promising tool in cancer treatment. However, xenograft models are built on immunocompromised mice to avoid rejection of transplanted human tumour tissue or cells. Thus, these models are invalid in investigating the tumour microenvironment (TME), including the infiltration of immune cells and crosstalk between the tumour and immune system. To overcome these challenges, several humanized mice were developed, including genetically engineered humanized mice and immunologically humanized mice [69]. Genetically engineered humanized mice were developed by replacing the murine gene with an equivalent human transgene [70], and immunologically humanized mice were generated by engraftment of human immune cells. Thus, immunologically humanized mice can be used to construct PDX models.
CD34+ human haematopoietic stem and progenitor cells (HPSCs) are engrafted into immunodeficient mice to reconstruct the human immune system. HPSCs are isolated from human foetal liver samples or from cord blood by density gradient centrifugation [71]. The efficiency of reconstitution was evaluated by [%hCD45 + /(%hCD45+ + %mCD45+)], and recipient mice with 20–50% human CD45+ cells were used for subsequent implantation (HPSC-PDX) [72]. In this model, the human immune system, including functional T cells, natural killer cells and monocytes, can be developed [73].
In other studies, immunodeficient mice were transplanted with human peripheral blood mononuclear cells (PBMCs) followed by engraftment of tumour tissue to develop a humanized PDX model (PBMC-PDX) [74, 75]. Compared with HPSC-PDX, PBMC-PDX has a lower time cost (4 weeks vs. 10–14 weeks) and is more accurate in evaluating PD-L1/PD-1 targeted immunotherapies [75]. However, the time window for research in PBMC-PDX is only 3–4 weeks because of severe graft-versus-host disease (GVHD) [70, 76].
Conclusions
Patient-derived xenograft models (PDX models) have attracted increased attention in preclinical cancer research over the past several years. They play crucial roles in evaluating the efficiency of antineoplastic drugs and screening for biomarkers of drug sensitivity and resistance. To overcome the challenges of conventional PDX models, such as the lack of a tumour microenvironment (TME) and long-term cost, humanized PDX models have been developed to mimic the human immune system, and mini-PDX models have been developed to evaluate drug efficiency in a faster approach. A new project called “coclinical trials” was proposed to use PDX models as mouse avatars to optimize antitumour treatment strategies. These efforts were made to improve the survival rate of patients with tumours. Although PDX models have many advantages compared with traditional CDX models and are increasingly widely used in different stages of cancer research, shortcomings remain to be solved. One major limitation of the PDX model is the loss of human tumour stroma. Another is that the pharmacokinetic properties of drugs differ in diverse species, making it difficult to predict the efficacy of drugs in patients by animal models.
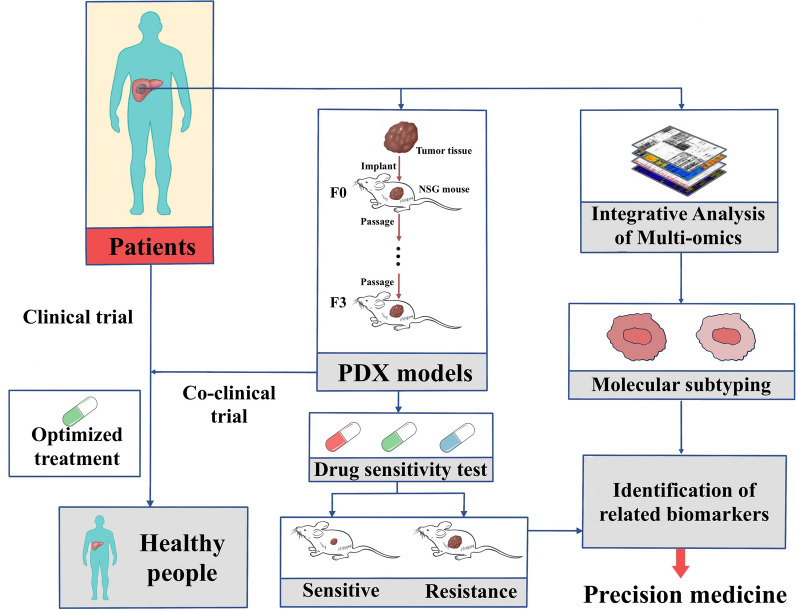
In conclusion, the PDX model will play an increasingly important role in the individual treatment of patients with cancer (Figs. 1, 2).
Fig. 1.
Methodology of establishing PDX model and applications. Tumour tissues collected from patients were transplanted into immunodeficient mice, 3rd passage PDX model was usually used for testing drug efficacies and co-clinical trials
Fig. 2.
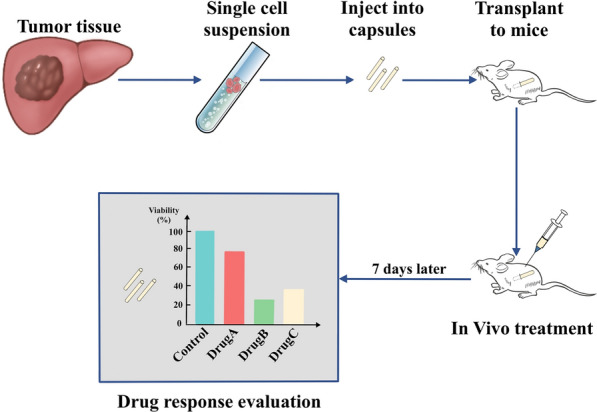
Methods to establish a Mini-PDX model. Single tumour cell suspension were prepared before injected into capsules, the capsules were then transplanted to mice for in vivo treatment and subsequent evaluation
Acknowledgements
We thank Zhengxing Lian for technical assistance.
Authors' contributions
XX designed the study and did the revision; BP drafted the manuscript and prepared the figures. XW edited the manuscript and partly prepared the figures. All authors read and approved the final manuscript.
Funding
This work was supported by Key Program, National Natural Science Foundation of China (No. 81930016), Key Research & Development Plan of Zhejiang Province (No. 2019C3050) and Youth Program of National Natural Science Foundation of China (No. 81702858).
Availability of data and materials
The material supporting the conclusion of this review has been included within the article.
Declarations
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gengenbacher N, Singhal M, Augustin HG. Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat Rev Cancer. 2017;17(12):751–765. doi: 10.1038/nrc.2017.92. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Liang T, Wang D, Li L, Cheng Y, Guo Q, Zhang G. IFNalpha-expressing amniotic fluid-derived mesenchymal stem cells migrate to and suppress hela cell-derived tumors in a mouse model. Stem cells international. 2018;2018:1241323. doi: 10.1155/2018/1241323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown ZJ, Heinrich B, Greten TF. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol 2018;89:67. [DOI] [PubMed]
- 4.Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Maelandsmo GM, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, Sailer V, Augello M, Puca L, Rosati R, et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017;7(5):462–477. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rygaard J, Povlsen CO. Heterotransplantation of a human malignant tumour to "Nude" mice. Acta Pathol Microbiol Scand. 1969;77(4):758–760. doi: 10.1111/j.1699-0463.1969.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun FX, Tang ZY, Lui KD, Ye SL, Xue Q, Gao DM, Ma ZC. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer. 1996;66(2):239–243. doi: 10.1002/(SICI)1097-0215(19960410)66:2<239::AID-IJC17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, Arcaroli JJ, Messersmith WA, Eckhardt SG. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapuy B, Cheng H, Watahiki A, Ducar MD, Tan Y, Chen L, Roemer MG, Ouyang J, Christie AL, Zhang L, et al. Diffuse large B-cell lymphoma patient-derived xenograft models capture the molecular and biological heterogeneity of the disease. Blood. 2016;127(18):2203–2213. doi: 10.1182/blood-2015-09-672352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17(11):1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Pham NA, Tong J, Sakashita S, Allo G, Kim L, Yanagawa N, Raghavan V, Wei Y, To C, et al. Molecular heterogeneity of non-small cell lung carcinoma patient-derived xenografts closely reflect their primary tumors. Int J Cancer. 2017;140(3):662–673. doi: 10.1002/ijc.30472. [DOI] [PubMed] [Google Scholar]
- 12.Braekeveldt N, de Stedingk K, Fransson S, Martinez-Monleon A, Lindgren D, Axelson H, Levander F, Willforss J, Hansson K, Ora I et al: Patient-derived xenograft models reveal intratumor heterogeneity and temporal stability in neuroblastoma. Cancer Res 2018;23:7. [DOI] [PubMed]
- 13.Hamid AA, Kaushal T, Ashraf R, Singh A, Chand Gupta A, Prakash O, Sarkar J, Chanda D, Bawankule DU, Khan F, et al. (22beta,25R)-3beta-Hydroxy-spirost-5-en-7-iminoxy-heptanoic acid exhibits anti-prostate cancer activity through caspase pathway. Steroids. 2017;119:43–52. doi: 10.1016/j.steroids.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Garcia R, Juan D, Rausell A, Munoz M, Banos N, Menendez C, Lopez-Casas PP, Rico D, Valencia A, Hidalgo M. Transcriptional dissection of pancreatic tumors engrafted in mice. Genome Med. 2014;6(4):27. doi: 10.1186/gm544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bissig-Choisat B, Kettlun-Leyton C, Legras XD, Zorman B, Barzi M, Chen LL, Amin MD, Huang YH, Pautler RG, Hampton OA, et al. Novel patient-derived xenograft and cell line models for therapeutic testing of pediatric liver cancer. J Hepatol. 2016;65(2):325–333. doi: 10.1016/j.jhep.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Zhu D, Li N, Yang H, Zhao Z, Li M. Characterization of ascites-derived tumor cells from an endometrial cancer patient. Cancer Sci. 2017;108(12):2352–2357. doi: 10.1111/cas.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roscilli G, De Vitis C, Ferrara FF, Noto A, Cherubini E, Ricci A, Mariotta S, Giarnieri E, Giovagnoli MR, Torrisi MR, et al. Human lung adenocarcinoma cell cultures derived from malignant pleural effusions as model system to predict patients chemosensitivity. J Transl Med. 2016;14:61. doi: 10.1186/s12967-016-0816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu Q, Zhang B, Sun H, Xu Q, Tan Y, Wang G, Luo Q, Xu W, Yang S, Li J, et al. Genomic characterization of a large panel of patient-derived hepatocellular carcinoma xenograft tumor models for preclinical development. Oncotarget. 2015;6(24):20160–20176. doi: 10.18632/oncotarget.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moiola CP, Lopez-Gil C, Cabrera S, Garcia A, Nyen T, Annibali D, Fonnes T, Vidal A, Villanueva A, Matias-Guiu X et al: Patient-Derived Xenograft Models for Endometrial Cancer Research. Int J Mol Sci 2018, 19:8. [DOI] [PMC free article] [PubMed]
- 20.Sathish Kumar B, Kumar A, Singh J, Hasanain M, Singh A, Fatima K, Yadav DK, Shukla V, Luqman S, Khan F, et al. Synthesis of 2-alkoxy and 2-benzyloxy analogues of estradiol as anti-breast cancer agents through microtubule stabilization. Eur J Med Chem. 2014;86:740–751. doi: 10.1016/j.ejmech.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Singh A, Fatima K, Singh A, Behl A, Mintoo MJ, Hasanain M, Ashraf R, Luqman S, Shanker K, Mondhe DM, et al. Anticancer activity and toxicity profiles of 2-benzylidene indanone lead molecule. Eur J Pharm Sci. 2015;76:57–67. doi: 10.1016/j.ejps.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Jin K, Teng L, Shen Y, He K, Xu Z, Li G. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol. 2010;12(7):473–480. doi: 10.1007/s12094-010-0540-6. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman RM. Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15(8):451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51(6):992–995. doi: 10.1002/ijc.2910510626. [DOI] [PubMed] [Google Scholar]
- 25.Stewart E, Federico SM, Chen X, Shelat AA, Bradley C, Gordon B, Karlstrom A, Twarog NR, Clay MR, Bahrami A, et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature. 2017;549(7670):96–100. doi: 10.1038/nature23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, Zhang C, Schnell C, Yang G, Zhang Y, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318–1325. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 27.Qu S, Wang K, Xue H, Wang Y, Wu R, Liu C, Gao AC, Gout PW, Collins CC, Wang Y. Enhanced anticancer activity of a combination of docetaxel and Aneustat (OMN54) in a patient-derived, advanced prostate cancer tissue xenograft model. Mol Oncol. 2014;8(2):311–322. doi: 10.1016/j.molonc.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuo J, Lu D, Wang J, Lian Z, Zhang J, Li H, Cen B, Wei X, Wei Q, Xie H, et al. Molecular phenotypes reveal heterogeneous engraftments of patient-derived hepatocellular carcinoma xenografts. Chin J Cancer Res. 2021;33(4):470–479. doi: 10.21147/j.issn.1000-9604.2021.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei L, Lee D, Law CT, Zhang MS, Shen J, Chin DW, Zhang A, Tsang FH, Wong CL, Ng IO, et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat Commun. 2019;10(1):4681. doi: 10.1038/s41467-019-12606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu Z, Li H, Zhang Z, Zhu Z, He S, Wang X, Wang P, Qin J, Zhuang L, Wang W et al: A Pharmacogenomic Landscape in Human Liver Cancers. Cancer Cell 2019, 36(2):179–193 e111. [DOI] [PMC free article] [PubMed]
- 31.Cheung PF, Yip CW, Ng LW, Lo KW, Chow C, Chan KF, Cheung TT, Cheung ST. Comprehensive characterization of the patient-derived xenograft and the paralleled primary hepatocellular carcinoma cell line. Cancer Cell Int. 2016;16:41. doi: 10.1186/s12935-016-0322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosfjord E, Lucas J, Li G, Gerber HP. Advances in patient-derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 2014;91(2):135–143. doi: 10.1016/j.bcp.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Xu S, Ling S, Shan Q, Ye Q, Zhan Q, Jiang G, Zhuo J, Pan B, Wen X, Feng T, et al. Self-Activated Cascade-Responsive Sorafenib and USP22 shRNA Co-Delivery System for Synergetic Hepatocellular Carcinoma Therapy. Adv Sci (Weinh) 2021;8(5):2003042. doi: 10.1002/advs.202003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh A, Fatima K, Srivastava A, Khwaja S, Priya D, Singh A, Mahajan G, Alam S, Saxena AK, Mondhe DM, et al. Anticancer activity of gallic acid template-based benzylidene indanone derivative as microtubule destabilizer. Chem Biol Drug Des. 2016;88(5):625–634. doi: 10.1111/cbdd.12805. [DOI] [PubMed] [Google Scholar]
- 35.Singh A, Mohanty I, Singh J, Rattan S: BDNF augments rat internal anal sphincter smooth muscle tone via RhoA/ROCK signaling and nonadrenergic noncholinergic relaxation via increased NO release. American Journal of Physiology-Gastrointestinal and Liver Physiology 2020, 318(1):G23-G33. [DOI] [PMC free article] [PubMed]
- 36.Ledford H. US cancer institute to overhaul tumour cell lines. Nature. 2016;530(7591):391. doi: 10.1038/nature.2016.19364. [DOI] [PubMed] [Google Scholar]
- 37.Garrido-Laguna I, Uson M, Rajeshkumar NV, Tan AC, de Oliveira E, Karikari C, Villaroel MC, Salomon A, Taylor G, Sharma R, et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(17):5793–5800. doi: 10.1158/1078-0432.CCR-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiao G, Geller J, Timchenko NA. Generation of pediatric liver cancer patient-derived xenograft platforms for pediatric liver cancer: A critical stage in the development of anticancer treatments. Hepatology (Baltimore, MD) 2016;64(4):1017–1019. doi: 10.1002/hep.28711. [DOI] [PubMed] [Google Scholar]
- 39.Huynh H, Ngo VC, Koong HN, Poon D, Choo SP, Thng CH, Chow P, Ong HS, Chung A, Soo KC. Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Cell Mol Med. 2009;13(8b):2673–2683. doi: 10.1111/j.1582-4934.2009.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sathish Kumar B, Singh A, Kumar A, Singh J, Hasanain M, Singh A, Masood N, Yadav DK, Konwar R, Mitra K, et al. Synthesis of neolignans as microtubule stabilisers. Bioorg Med Chem. 2014;22(4):1342–1354. doi: 10.1016/j.bmc.2013.12.067. [DOI] [PubMed] [Google Scholar]
- 41.Jain S, Singh A, Khare P, Chanda D, Mishra D, Shanker K, Karak T. Toxicity assessment of Bacopa monnieri L grown in biochar amended extremely acidic coal mine spoils. Ecol Eng 2017, 108:211–219.
- 42.Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, Lee TK. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology (Baltimore, MD) 2015;62(2):534–545. doi: 10.1002/hep.27859. [DOI] [PubMed] [Google Scholar]
- 43.Huynh H, Ngo VC, Koong HN, Poon D, Choo SP, Toh HC, Thng CH, Chow P, Ong HS, Chung A, et al. AZD6244 enhances the anti-tumor activity of sorafenib in ectopic and orthotopic models of human hepatocellular carcinoma (HCC) J Hepatol. 2010;52(1):79–87. doi: 10.1016/j.jhep.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Huynh H, Chow PK, Palanisamy N, Salto-Tellez M, Goh BC, Lee CK, Somani A, Lee HS, Kalpana R, Yu K, et al. Bevacizumab and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Hepatol. 2008;49(1):52–60. doi: 10.1016/j.jhep.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Srivastava A, Fatima K, Fatima E, Singh A, Singh A, Shukla A, Luqman S, Shanker K, Chanda D, Khan F, et al. Fluorinated benzylidene indanone exhibits antiproliferative activity through modulation of microtubule dynamics and antiangiogenic activity. Eur J Pharm Sci. 2020;154:105513. doi: 10.1016/j.ejps.2020.105513. [DOI] [PubMed] [Google Scholar]
- 46.Khwaja S, Fatima K, Hasanain M, Behera C, Kour A, Singh A, Luqman S, Sarkar J, Chanda D, Shanker K, et al. Antiproliferative efficacy of curcumin mimics through microtubule destabilization. Eur J Med Chem. 2018;151:51–61. doi: 10.1016/j.ejmech.2018.03.063. [DOI] [PubMed] [Google Scholar]
- 47.Singh A, Singh J, Rattan S: Evidence for the presence and release of BDNF in the neuronal and non-neuronal structures of the internal anal sphincter. Neurogastroenterol Motil 2021:e14099. [DOI] [PMC free article] [PubMed]
- 48.Blomme A, Van Simaeys G, Doumont G, Costanza B, Bellier J, Otaka Y, Sherer F, Lovinfosse P, Boutry S, Palacios AP, et al. Murine stroma adopts a human-like metabolic phenotype in the PDX model of colorectal cancer and liver metastases. Oncogene. 2018;37(9):1237–1250. doi: 10.1038/s41388-017-0018-x. [DOI] [PubMed] [Google Scholar]
- 49.Mo F, Lin D, Takhar M, Ramnarine VR, Dong X, Bell RH, Volik SV, Wang K, Xue H, Wang Y, et al. Stromal gene expression is predictive for metastatic primary prostate cancer. Eur Urol. 2018;73(4):524–532. doi: 10.1016/j.eururo.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamid AA, Hasanain M, Singh A, Bhukya B, Omprakash, Vasudev PG, Sarkar J, Chanda D, Khan F, Aiyelaagbe OO et al. Synthesis of novel anticancer agents through opening of spiroacetal ring of diosgenin. Steroids 2014; 87:108–118. [DOI] [PubMed]
- 51.Ho DWH, Chan LK, Chiu YT, Xu IMJ, Poon RTP, Cheung TT, Tang CN, Tang VWL, Lo ILO, Lam PWY, et al. TSC1/2 mutations define a molecular subset of HCC with aggressive behaviour and treatment implication. Gut. 2017;66(8):1496–1506. doi: 10.1136/gutjnl-2016-312734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guan DX, Shi J, Zhang Y, Zhao JS, Long LY, Chen TW, Zhang EB, Feng YY, Bao WD, Deng YZ, et al. Sorafenib enriches epithelial cell adhesion molecule-positive tumor initiating cells and exacerbates a subtype of hepatocellular carcinoma through TSC2-AKT cascade. Hepatology (Baltimore, MD) 2015;62(6):1791–1803. doi: 10.1002/hep.28117. [DOI] [PubMed] [Google Scholar]
- 53.Yue X, Ai J, Xu Y, Chen Y, Huang M, Yang X, Hu B, Zhang H, He C, Yang X, et al. Polymeric immunoglobulin receptor promotes tumor growth in hepatocellular carcinoma. Hepatology (Baltimore, MD) 2017;65(6):1948–1962. doi: 10.1002/hep.29036. [DOI] [PubMed] [Google Scholar]
- 54.Yang S, Luo C, Gu Q, Xu Q, Wang G, Sun H, Qian Z, Tan Y, Qin Y, Shen Y, et al. Activating JAK1 mutation may predict the sensitivity of JAK-STAT inhibition in hepatocellular carcinoma. Oncotarget. 2016;7(5):5461–5469. doi: 10.18632/oncotarget.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang MD, Wu H, Fu GB, Zhang HL, Zhou X, Tang L, Dong LW, Qin CJ, Huang S, Zhao LH, et al. Acetyl-coenzyme A carboxylase alpha promotion of glucose-mediated fatty acid synthesis enhances survival of hepatocellular carcinoma in mice and patients. Hepatology (Baltimore, MD) 2016;63(4):1272–1286. doi: 10.1002/hep.28415. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Q, Zhou H, Liu Q, Cao Y, Wang G, Hu A, Ruan L, Wang S, Bo Q, Chen W, et al. Prognostic value of the expression of cancer stem cell-related markers CD133 and CD44 in hepatocellular carcinoma: from patients to patient-derived tumor xenograft models. Oncotarget. 2016;7(30):47431–47443. doi: 10.18632/oncotarget.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malaney P, Nicosia SV, Dave V. One mouse, one patient paradigm: new avatars of personalized cancer therapy. Cancer Lett. 2014;344(1):1–12. doi: 10.1016/j.canlet.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pompili L, Porru M, Caruso C, Biroccio A, Leonetti C. Patient-derived xenografts: a relevant preclinical model for drug development. J Exp Clin Cancer Res. 2016;35(1):189. doi: 10.1186/s13046-016-0462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nardella C, Lunardi A, Patnaik A, Cantley LC, Pandolfi PP. The APL paradigm and the "co-clinical trial" project. Cancer Discov. 2011;1(2):108–116. doi: 10.1158/2159-8290.CD-11-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Byrne AT, Alferez DG, Amant F, Annibali D, Arribas J, Biankin AV, Bruna A, Budinska E, Caldas C, Chang DK, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17(4):254–268. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
- 61.Morelli MP, Calvo E, Ordonez E, Wick MJ, Viqueira BR, Lopez-Casas PP, Bruckheimer E, Calles-Blanco A, Sidransky D, Hidalgo M. Prioritizing phase I treatment options through preclinical testing on personalized tumorgraft. J Clin Oncol. 2012;30(4):e45–48. doi: 10.1200/JCO.2011.36.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, Ebbesen SH, Ainscough BJ, Ramu A, Iyer G, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature. 2015;518(7538):240–244. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azaro A, Rodon J, Calles A, Brana I, Hidalgo M, Lopez-Casas PP, Munoz M, Westwood P, Miller J, Moser BA, et al. A first-in-human phase I trial of LY2780301, a dual p70 S6 kinase and Akt Inhibitor, in patients with advanced or metastatic cancer. Invest New Drugs. 2015;33(3):710–719. doi: 10.1007/s10637-015-0241-7. [DOI] [PubMed] [Google Scholar]
- 64.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02795650
- 65.Zhang F, Wang W, Long Y, Liu H, Cheng J, Guo L, Li R, Meng C, Yu S, Zhao Q, et al. Characterization of drug responses of mini patient-derived xenografts in mice for predicting cancer patient clinical therapeutic response. Cancer Commun (London, England) 2018;38(1):60. doi: 10.1186/s40880-018-0329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao P, Chen H, Wen D, Mou S, Zhang F, Zheng S. Personalized treatment based on mini patient-derived xenografts and WES/RNA sequencing in a patient with metastatic duodenal adenocarcinoma. Cancer Commun (London, England) 2018;38(1):54. doi: 10.1186/s40880-018-0323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhan M, Yang RM, Wang H, He M, Chen W, Xu SW, Yang LH, Liu Q, Long MM, Wang J. Guided chemotherapy based on patient-derived mini-xenograft models improves survival of gallbladder carcinoma patients. Cancer Commun (London, England) 2018;38(1):48. doi: 10.1186/s40880-018-0318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164(6):1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown ZJ, Heinrich B, Greten TF. Mouse models of hepatocellular carcinoma: an overview and highlights for immunotherapy research. Nat Rev Gastroenterol Hepatol. 2018;15(9):536–554. doi: 10.1038/s41575-018-0033-6. [DOI] [PubMed] [Google Scholar]
- 70.Zitvogel L, Pitt JM, Daillere R, Smyth MJ, Kroemer G. Mouse models in oncoimmunology. Nat Rev Cancer. 2016;16(12):759–773. doi: 10.1038/nrc.2016.91. [DOI] [PubMed] [Google Scholar]
- 71.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32(4):364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao Y, Shuen TWH, Toh TB, Chan XY, Liu M, Tan SY, Fan Y, Yang H, Lyer SG, Bonney GK, et al. Development of a new patient-derived xenograft humanised mouse model to study human-specific tumour microenvironment and immunotherapy. Gut. 2018;67(10):1845–1854. doi: 10.1136/gutjnl-2017-315201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanmamed MF, Rodriguez I, Schalper KA, Onate C, Azpilikueta A, Rodriguez-Ruiz ME, Morales-Kastresana A, Labiano S, Perez-Gracia JL, Martin-Algarra S, et al. Nivolumab and urelumab enhance antitumor activity of human t lymphocytes engrafted in Rag2-/-IL2Rgammanull Immunodeficient Mice. Can Res. 2015;75(17):3466–3478. doi: 10.1158/0008-5472.CAN-14-3510. [DOI] [PubMed] [Google Scholar]
- 75.Lin S, Huang G, Cheng L, Li Z, Xiao Y, Deng Q, Jiang Y, Li B, Lin S, Wang S et al: Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy. Mabs 2018;98:1–11. [DOI] [PMC free article] [PubMed]
- 76.Nervi B, Rettig MP, Ritchey JK, Wang HL, Bauer G, Walker J, Bonyhadi ML, Berenson RJ, Prior JL, Piwnica-Worms D, et al. Factors affecting human T cell engraftment, trafficking, and associated xenogeneic graft-vs-host disease in NOD/SCID beta2mnull mice. Exp Hematol. 2007;35(12):1823–1838. doi: 10.1016/j.exphem.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The material supporting the conclusion of this review has been included within the article.