Abstract
Background and Aims
European mesic meadows are semi-natural open habitats of high biodiversity and an essential part of European landscapes. These species-rich communities can be a source of seed mixes for ecological restoration, urban greening and rewilding. However, limited knowledge of species germination traits is a bottleneck to the development of a competitive native seed industry. Here, we synthesize the seed ecology of mesic meadows.
Methods
We combined our own experimental data with data obtained from databases to create a combined dataset containing 2005 germination records of 90 plant species from 31 European countries. We performed a Bayesian meta-analysis of this dataset to test the seed germination response to environmental cues including scarification, stratification, temperature, alternating temperature and light. We also used multivariate ordination to check the relationship between seed traits (germination and morphology) and species ecological preferences, and to compare the seed ecology of mesic meadows with that of other herbaceous plant communities from the same geographic area.
Key Results
The seed ecology of mesic meadows is characterized by (1) high seed germinability when compared with other herbaceous plant communities; (2) low correspondence between seed traits and species ecological preferences; and (3) a deep phylogenetic separation between the two major families, Poaceae and Fabaceae. Poaceae produce many light seeds that respond to gap-detecting germination cues (alternating temperatures and light); Fabaceae produce fewer heavy seeds, which need scarification to break their physical dormancy.
Conclusions
High germinability of meadow seeds will reduce their capacity to form persistent seed banks, resulting in dispersal limitations to passive regeneration. For centuries, human activities have shaped the regeneration of meadows, leading to a loss of seed dormancy and decoupling seeds from seasonal cycles, as has been found in many domesticated species. The same anthropic processes that have shaped semi-natural mesic meadows have left them dependent on continued human intervention for their regeneration, highlighting the importance of active restoration via seed supply.
Keywords: Arrhenatheretalia, Arrhenatherion, Asteraceae, Europe, Fabaceae, domestication, hay meadows, mesic grasslands, Poaceae, seed germination, seed morphology, semi-natural, species-rich meadows
INTRODUCTION
The mesic meadows of Europe are semi-natural open habitats that occupy clearings created by human intervention over a temperate wooded landscape (Poschlod et al., 2009; Hejcman et al., 2013), in sites with moderately fertile and well-drained soils (Mucina et al., 2016). These novel anthropogenic plant communities, with a composite flora made up of species from different ecological backgrounds, have constantly changed in response to shifts in human society and associated management practices (Chytrý, 2012; Hejcman et al., 2013), but nonetheless characterize Europe’s natural history (Finck et al., 2002).
Traditional classification of mesic grasslands emphasizes the separation between meadows used for hay making versus pastures maintained by grazing, but a recent revision at the European level showed that the main driver of variation in species composition is the intensity rather than the type of management (Rodríguez-Rojo et al., 2017). Indeed, changes in the intensity of management, including land abandonment and agricultural intensification, are threatening the maintenance of mesic meadows in large parts of Europe (Carboni et al., 2015). For this reason, the European Habitats Directive (92/43/EEC) lists as habitats of conservation interest those species-rich meadows that are traditionally managed by one or two annual cuts and light grazing.
One of the reasons for the high conservation interest of traditional mesic meadows is their high species richness, and for this same reason they have been highlighted as a valuable source of natural seed material to be used in ecological restoration, rewilding and urban greening (Krautzer et al., 2013; Haslgrübler et al., 2014; Golińska et al., 2017). However, a lack of knowledge about species germination traits has already been identified as a bottleneck (Ladouceur et al., 2018) hampering the development of a competitive native seed industry (De Vitis et al., 2017) in Europe. Understanding seed germination is part of the practical scientific framework needed to tackle large-scale ecological restoration challenges (Merritt and Dixon, 2011) and to maintain ex situ collections of plant genetic resources (Li and Pritchard, 2009). When such knowledge is available, ambitious regional schemes of seed-based landscape restoration can be designed (Jiménez-Alfaro et al., 2020).
Mesic meadows are dominated by mesophilous grasses of the family Poaceae, which make up most of the biomass in the community and define the structure of the vegetation. The dispersal unit in Poaceae is generally the floret (Fig. 1A), a composite structure made up of the caryopsis or grain (a dry, indehiscent and monospermic fruit) and its surrounding bracts (i.e. modified leaves): the lemma and the palea. Removal of the lemma and palea, as well as puncturing the pericarp, can aid in seed germination (Probert et al., 1985). The dispersal unit often carries awns or hairs (Fig. 1B) that are assumed to aid dispersal, although it is not always the case that they do (Schonfeld and Chancellor, 1983). Several genera of Poaceae that grow in European meadows show seed heteromorphism (e.g. Aegilops, Avena, Dasypirum, Poa) (Guzzon et al., 2018). They produce different seed morphs characterized by different seed traits in terms of dormancy, longevity, morphology, phenology, tolerance to abiotic stresses and susceptibility to predation. This is connected to bet-hedging ecological strategies, typical of ruderal/disturbed habitats. Regarding germination, meadow Poaceae seeds have been reported as having physiological seed dormancy in various degrees (Sprague, 1940; Dixon, 1995; Baskin and Baskin, 2014), but germinability is usually high even without treating the seeds with cold stratification (Grime et al., 1981; Schonfeld and Chancellor, 1983; Williams, 1983a; Bean et al., 1984; Froud-Williams et al., 1984, 1986; Froud-Williams and Ferris, 1987; Dixon, 1995; Pérez-Fernández and Rodríguez-Echeverría, 2003; Pérez-Fernández et al., 2006; Stanisavljevic et al., 2011, 2015; Oliveira et al., 2012; Wille et al., 2013). Freshly harvested seeds are comparatively more dormant than stored seeds, but dormancy tends to disappear quickly in dry storage (Sprague, 1940; Dixon, 1995). Germination has been reported to occur at temperatures ranging from 5–30 °C (Grime et al., 1981; Williams, 1983b; Pannangpetch and Bean, 1984; Probert et al., 1986; Froud-Williams et al., 1986; Dixon, 1995). Most Poaceae species have also been reported to germinate better in light than in darkness (Williams, 1983b, c; Froud-Williams et al., 1984; Probert et al., 1985, 1986; Probert and Smith, 1986; Thompson, 1989; Dixon, 1995) and even to be unable to germinate in darkness (Froud-Williams et al., 1986). Nonetheless, species of Bromus have been reported as germinating better in darkness (Thompson, 1989) and Cynosurus cristatus as being indifferent to light/darkness (Williams, 1983b, c). In Poa trivialis, germination is promoted by light but not by diurnal alternating temperatures (i.e. germination conditions where different temperatures are applied during the day and the night, in diurnal cycles) (Froud-Williams and Ferris, 1987), although alternating temperatures do encourage some germination in darkness (Froud-Williams et al., 1986). Wild Poaceae ecotypes usually have a germination response to alternating temperatures (Schonfeld and Chancellor, 1983; Williams, 1983b, c; Pannangpetch and Bean, 1984; Probert et al., 1985, 1986; Probert and Smith, 1986; Thompson, 1989), although this response is missing in some wild ecotypes and in the domesticated cultivars (Pannangpetch and Bean, 1984), and some species such as Lolium perenne have been reported as insensitive to temperature alternation (Thompson et al., 1977; Williams, 1983c).
Fig. 1.
Diversity of dispersal units in plant species from mesic meadows: (A) Floret of Cynosurus cristatus (Poaceae); (B) floret of Arrhenatherum elatius (Poaceae); (C) seed of Lathyrus pratensis (Fabaceae); (D) legume of Onobrychis viciifolia (Fabaceae); (E) loment fragment of Ornithopus perpusillus (Fabaceae); (F) achene with pappus of Centaurea scabiosa (Asteraceae); (G) achene of Ranunculus acris (Ranunculaceae); (H) nutlet of Prunella grandiflora (Lamiaceae); (I) achene of Knautia nevadensis (Caprifoliaceae); (J) perigynium of Carex binervis (Cyperaceae); (K) receptacle of Sanguisorba minor (Rosaceae); (L) mericarp of Carum verticillatum (Apiaceae); (M) seed of Cerastium fontanum (Caryophyllaceae); (N) seed of Plantago lanceolata (Plantaginaceae); (O) seed of Rhinanthus angustifolius (Orobanchaceae).
Next in abundance to Poaceae are the legumes of the family Fabaceae. Fabaceae species contribute to the nutritional value of meadow fodder, as thanks to their N-fixing capabilities they have high N contents (Reiné et al., 2020; Álvarez et al., 2021). The dispersal unit of most Fabaceae is the seed itself (Fig. 1C) but in some species dispersal units are more complex, including indehiscent monospermic fruits (e.g. Onobrychis, Fig. 1D) or indehiscent monospermic fruit fragments, i.e. loments (e.g. Ornithopus, Fig. 1E). Fabaceae are generally hard-seeded: they have a water-impermeable seed coat that needs to become permeable before germination can happen (i.e. physical seed dormancy) (Grime et al., 1981; Jones and Turkington, 1986; Ehrman and Cocks, 1996; Kupferschmid et al., 2000; Baskin and Baskin, 2014). In Medicago, seeds that have not reached full maturity can germinate before they become impermeable, but the completion of maturation imposes coat impermeability, and thereafter the seed must be scarified to allow water imbibition and germination (Gresta et al., 2007). Buried Fabaceae seeds can track the seasons, and in some species germination seems to be promoted by cold stratification and alternating temperatures (Van Assche et al., 2003); some of these species have been described as having combinational dormancy (i.e. physical + physiological) (Van Assche and Vandelook, 2010). However, Fabaceae seeds have also been reported to germinate without any previous treatment (Marchiol et al., 2000; Nikolic et al., 2007; Kabouw et al., 2010; Oliveira et al., 2012), and to lose dormancy during storage (Van Assche and Vandelook, 2010). As in Poaceae, seeds of Fabaceae have been reported to germinate in high numbers across a range of temperatures from 5 to 25 °C (Grime et al., 1981; Gresta et al., 2007). Fabaceae seeds have been described as not being responsive to light and as capable of germinating in darkness (Silvertown, 1980; Grime et al., 1981).
Poaceae and Fabaceae are accompanied by a diversity of other families which, even if present in lower abundances, contribute to the high biodiversity of the system and increase the aesthetic value of the meadows as perceived by people (Southon et al., 2017; Chollet et al., 2018). They also add nutritional scope for livestock, being richer than Poaceae and Fabaceae in specific elements (Reiné et al., 2020; Álvarez et al., 2021). In many of these plant families (e.g. Asteraceae, Cyperaceae, Caprifoliaceae, Lamiaceae, Polygonaceae, Ranunculaceae) the dispersal unit is the achene: dry, indehiscent and monospermic fruits. The morphology of these achenes is varied: cypselae with a hairy pappus in Centaurea (Asteraceae, Fig. 1F); beaked in Ranunculus (Ranunculaceae, Fig. 1G); hardened nutlets in Prunella (Lamiaceae, Fig. 1H); hairy and with an elaiosome in Knautia (Caprifoliaceae, Fig. 1I); surrounded by a perigynium which aids in dispersal by water in Carex (Cyperaceae, Fig. 1J). In the genus Sanguisorba (Rosaceae), the dispersal unit is the urn-shaped receptacle containing one to three achenes (Fig. 1K). In Apiaceae, it is the mericarp (Fig. 1L), an indehiscent monospermic fragment of the fruit. In some other minor families, the dispersal unit is the seed itself, such as in Caryophyllaceae (Fig. 1M), Plantaginaceae (Fig. 1N), Juncaceae or the hemiparasitic species of Rhinanthus (Orobanchaceae) (Fig. 1O). In Asteraceae, high germination without previous treatments has been reported in Taraxacum officinale (Mezynski and Cole, 1974; Washitani, 1984; Noronha, 1997; Benvenuti and Pardossi, 2016; Masin et al., 2017), Hypochaeris radicata (Oomes and Elberse, 1976; Benvenuti and Pardossi, 2016) and Achillea millefolium (Oomes and Elberse, 1976). Taraxacum officinale germinates between 5 and 30 °C (Mezynski and Cole, 1974; Washitani, 1984; Masin et al., 2017) and has higher germination in light (Thompson, 1989; Letchamo and Gosselin, 1996; Noronha, 1997) and in alternating temperatures (Mezynski and Cole, 1974). In Stachys officinalis (Lamiaceae), seeds need either cold stratification, light or alternating temperatures to germinate (Wagner et al., 2011; Kolodziejek et al., 2017). Underdeveloped embryos that need to grow inside the seed before germination (i.e. morphological dormancy) are widespread in Ranunculaceae and Apiaceae (Jauzein and Mansour, 1992; Baskin and Baskin, 2014). Ranunculus repens (Ranunculaceae) germinates between 10 and 25 °C, but the germination percentages have been reported to be low (Harris et al., 1998); the same species has been reported to respond to alternating temperatures, which can promote its germination even in darkness (Thompson and Grime, 1983). In Polygonaceae, Rumex acetosa can germinate immediately after dispersal and between 7 and 27 °C, while the congeneric Rumex acetosella does not: this difference is due to the former being able to germinate at constant temperatures in the darkness (Grime et al., 1981; Van Assche et al., 2002), while the latter has an absolute requirement for light (Van Assche et al., 2002). In Heracleum sphondylium (Apiaceae), growth of the embryo only occurs below 10 °C, in moist conditions (Jauzein and Mansour, 1992). Sanguisorba minor (Rosaceae) increases its germination after abrasion of the seeds with bleach (Tavşanoğlu et al., 2015; Benvenuti and Pardossi, 2016), although germination without previous treatment has also been reported (Ludewig et al., 2014; Tavşanoğlu et al., 2015). Seeds of the hemiparasitic species Rhinanthus angustifolius and Rhinanthus minor (Orobanchaceae) require relatively long periods of cold stratification to germinate (Ter Borg, 2005) and can germinate in the dark (Marin et al., 2019).
Although a wealth of studies has accumulated, a synthesis of the seed ecology of European mesic meadows is still missing. In this article, we review for the first time this topic to provide an overview of traits related to plant regeneration by seed, as a knowledge basis to expand our understanding of meadow communities and assist in their management by conservation and restoration practitioners. We combine newly generated data on seed morphology and germination with records from existing databases (Kleyer et al., 2008; Royal Botanic Gardens, Kew, 2017; Fernández-Pascual, 2021). The resulting dataset contains 2005 germination records of 90 plant species from 31 European countries. We use this dataset to test the seed germination response to environmental cues including scarification, stratification, temperature, alternating temperature and light, applying Bayesian meta-analysis (Pappalardo et al., 2020). Further, using well-preserved meadows of the Iberian Peninsula as a study system, we analyse the covariation between seed traits and species environmental preferences, and compare the germination ecology of mesic meadows with that of other herbaceous plant communities from the same geographic area.
MATERIALS AND METHODS
Data sources
Selection of mesic meadow species
To create a list of representative European mesic meadow species for inclusion in our analysis, we used the list of species provided by Chytrý et al. (2020) as dominant, constant and diagnostic species of the EUNIS habitats ‘low and medium altitude hay meadows’ and ‘mountain hay meadows.’ This list included 120 species and was used to test the response to germination cues at the European level (see below).
To conduct further analyses at the Iberian level, we constructed a second species list based on a dataset comprising 118 vegetation relevés (i.e. records of plant species co-occurring in sampling plots) from three Iberian regions with well-maintained mesic meadows: 43 relevés from Northern Portugal, 25 from the Cantabrian Mountains of Spain and 50 from the Pyrenees. These relevés were used to assess variation in seed responses along environmental gradients (i.e. the relationship between species seed traits and species ecological requirements, see below), as they were recorded along a major stress gradient related to summer drought (Rodríguez-Rojo et al., 2014): the Pyrenees and Cantabrian Mountains have a temperate macroclimate, whereas Northern Portugal is transitional between the temperate and Mediterranean macroclimates. Furthermore, meadows from the Pyrenees are closest to the European optimum of mesic meadow vegetation while the Portuguese ones are in suboptimal areas at the limit of the European distribution of temperate meadows (Rodríguez-Rojo et al., 2017). Finally, the traditional management of meadows (i.e. mowing for haymaking once or twice per year plus light grazing) is relatively well preserved in these three regions compared with their European context (Prince et al., 2012; Guadilla-Sáez et al., 2019).
The Iberian meadows were maintained by traditional agricultural practices: mowing for haymaking once or twice per year plus light grazing. Each vegetation sampling plot was placed in a square area (25–100 m2 area) situated in the central part of the meadow, avoiding the margins. Vegetation sampling took place in 2016–17, at the peak of plant development, just before mowing. All vascular plant species in the plots were recorded and given a cover value using the transformation of the Braun-Blanquet scale to coverage (+ = 0.1 %, 1 = 5 %, 2 = 17.5 %, 3 = 37.5 %, 4 = 62.5 % and 5 = 87.5 %). All plant names were assigned following the nomenclature of Euro+Med (2006), which is used throughout this article. As expected, the vegetation of the sampled meadows was dominated by Poaceae and Fabaceae: these two families represented 47 and 17 %, respectively, of the total plant cover recorded in all the plots. Other 20 families were recorded, the largest of which was Asteraceae; each of these other families represented <10 % of the total cover.
Using all the relevés, we calculated the cumulative cover of each species in the entire area. To perform the calculation, first we standardized the cover values of the plots by dividing the cover of each species in each plot by the total plant cover in that plot. Then, for each species, we calculated its total cover in the dataset, by summing its standardized cover values from all the plots. Finally, we rescaled the values of all species to a 1–100 scale to obtain the cumulative cover values. From the resulting list of species, we removed 208 species with cumulative cover values <2 %, considering them to be transient species (Mariotte, 2014) that might have been recorded by chance and may not represent the core mesic meadows flora. We used the remaining 116 species as the core list of Iberian meadow species. Most of these species were hemicryptophytes (78 %), with some therophytes (16 %) and a few chamaephytes and geophytes (3 % of each). The family with most species in the list was Poaceae (22 %), with another 17 % belonging to Fabaceae, 15 % to Asteraceae, 8 % to Apiaceae, and the rest of the families representing <5 % each.
Species ecological requirements
We also used the Iberian relevés as a basis to characterize the preferences of the selected species for three environmental factors (cold, summer drought and soil reaction) that have been found to be major ecological drivers of mesic meadow plant diversity (Rodríguez-Rojo et al., 2014). For cold and drought, we used the coordinates of the plots to retrieve from CHELSA (Karger et al., 2017) the bioclimatic variables bio06 (minimum temperature in the coldest month) and bio14 (precipitation in the driest month). For soil reaction (pH), we took from each plot five soil samples from between 0 and 20 cm depth with a Dutch auger and combined them to make a bulk soil horizon, which we subsequently air-dried, crumbled, finely crushed and sieved with a 2-mm screen, to finally measure the pH in H2O with a glass electrode in a suspension of soil:water (1:2.5). With each of these three environmental variables (bio06, bio14, pH) measured at the plot level we calculated the species niche centroids (SNCs). The SNC for any given species and variable is the mean of the environmental variable in all the plots where the species occurs, weighted by species cover in each plot (Zelený, 2018).
Seed morphology
From the vegetation plots described above, we collected dispersal units (hereafter called seeds) during the dispersal seasons of 2016, 2017 and 2018. Seed collection followed the methodology of ENSCONET (2009). To describe seed morphology, we acquired images of 100-seed samples of each species using a flatbed scanner (Brother LC985) with a resolution of 200 dpi and a scanning area of 1024 × 1024 pixels (Bacchetta et al., 2008). We distributed the seeds on the transparent glass of the scanner, in a 10 × 10 grid. For each sample, and without moving the seeds, we repeated the scan with black and white backgrounds. In the case of the black background, we covered samples with a black box to avoid interference from environmental light. For the white background, we used the scanner cover. We digitized the obtained images and stored them in JPEG format (Joint Photographic Experts Group). We processed the scanned images using ImageJ, an open-source image processing program designed for scientific multidimensional images (Schneider et al., 2012). The program calculates several biometric parameters for each seed on the sample, and among those we chose seed length and width. Additionally, we retrieved species values of seed mass from the Seed Information Database (Royal Botanic Gardens, Kew, 2017) and of seed number at the individual/ramet level from the LEDA database (Kleyer et al., 2008). The dataset with the length and width measures is available at GitHub (see Data Availability Statement).
Seed germination
We constructed a germination dataset by combining the results of our own germination experiments with Iberian seeds, and records of seed germination of meadow seeds from across Europe.
We germinated the collected Iberian seeds using three germination treatments to determine the germination response to temperatures that are representative of the study area: 14/4 °C representing the capacity of freshly-dispersed seeds to germinate at cool temperatures of spring and autumn, 22/12 °C as summer temperature, and 30/20 °C as sun-heated soil, e.g. soil exposed to sun after hay cutting. Additionally, we compared, for each of these temperature regimes, the germination of fresh seeds versus seeds subjected to a dormancy-breaking treatment. This treatment was adjusted to the most probable dormancy type per taxonomic group (Baskin and Baskin, 2014). In the case of Fabaceae and other families that might present physical dormancy (Baskin and Baskin, 2014), the treatment consisted in scarification by chipping the seed coat with a scalpel. For the rest of the families, we used gibberellic acid (GA3; 0.0645 mm) in darkness for 24 h, as a treatment to remove potential physiological seed dormancy (Blandino et al., 2019). For each species and treatment, we sowed four Petri dishes with 25 seeds each. The germination substrate was 1 % distilled water agar. We sealed dishes with Parafilm to prevent desiccation. Trials took place in a germination chamber (KBW 400, Binder, Tuttlingen, Germany) with a 12/12-h photoperiod (the light period corresponding to the higher temperature). Experiments lasted for 4 weeks, with germination scoring once per week. The germination criterion was 2-mm radicle emergence. After 4 weeks, we cut the seeds that failed to germinate and examined them under a magnifying glass. We classified them as normal when the embryo was visible and firm, empty when they lacked an embryo, and contaminated when they were mouldy. We only considered normal seeds when calculating germination proportions and conducting subsequent analyses.
In addition to these experimental germination data, we retrieved European seed germination records for the European list of study species from ENSCOBASE, the seed germination database of the European Native Seed Conservation Network (http://enscobase.maich.gr/index.tml); and the SylvanSeeds database of seed germination records for the nemoral biome (Fernández-Pascual, 2021). These new records included additional records of species in our experimental dataset, plus records of new species absent from our experimental dataset, and in all cases corresponded to seed lots originally collected within Europe. The combined dataset, including our own experimental data and the records from ENSCOBASE and SylvanSeeds, contained 90 species (i.e. 90 % of the core list of meadow species was covered) and 2005 germination records (i.e. germination proportions for a given seed lot of a species, recorded in a set of laboratory experimental conditions) from 31 European countries. Overall, 156 252 seeds had been used in the experiments. The range of experimental germination temperatures (weighted average of the daily thermoperiod) that had been used in the experiments spanned from 2 to 31 °C, with 1226 records of constant temperatures (i.e. experiments that used the same temperature during all their duration) and 779 of alternating temperatures (i.e. experiments where different temperatures were applied during the day and the night, in diurnal cycles). Seeds had been exposed to light during some part of the diurnal cycle in 1907 records or kept in total darkness in 98 records. Experiments had been performed with unstratified seeds (i.e. not subjected to a previous dormancy-breaking incubation) in 1726 records and with stratified seeds (i.e. subjected to previous incubation in dormancy-breaking conditions, including treatments of wet incubation under cold, warm and combinations of cold and warm conditions) in 279 records. There were 262 records where GA3 had been applied, and 369 records where seeds had been scarified.
Finally, to compare the germination of mesic meadow species with that of other herbaceous communities, we retrieved data from previous works on the seed germination ecology of bogs and fens (Fernández-Pascual et al., 2013; Fernández-Pascual, 2016), alpine and subalpine grasslands (Fernández-Pascual et al., 2017a), and coastal plant communities of rocky cliffs and sand dunes (Fernández-Pascual et al., 2017b). These additional germination records had been obtained using the same experimental methodology as the one employed for some of the germination experiments of this study: recently collected seeds, untreated for physiological dormancy (but scarified in the cases of families known for having physical dormancy), had been subjected to three germination thermoperiods (14/4, 22/12, 30/20 °C). All seeds had been collected in the Cantabrian Mountains of Spain or the neighbouring coast. We combined these records with the records with matching experimental conditions from the meadows germination dataset to create a Cantabrian dataset.
Statistical analysis
We conducted all analyses in R (R Core Team, 2020), and the code for analysis and creation of the figures and manuscript is available at GitHub (see Data Availability Statement).
Seed responses to germination cues
We used the European germination dataset (available at GitHub, see Data Availability Statement) to test the effect of germination treatments on seed germination proportions. The dataset used included 2005 germination records of 90 species; of these, 222 records came from our own experimental data and the rest from the databases. We performed a meta-analysis (Pappalardo et al., 2020) of the germination dataset by fitting binomial generalized mixed models with Bayesian estimation [Markov chain Monte Carlo generalized linear mixed models (MCMCglmms)] (Fernández-Pascual et al., 2021) using the R package MCMCglmm (Hadfield, 2010). We fitted four models: (1) to the entire dataset; and separately for each of the three botanical groups of mesic meadows: (2) Poaceae, (3) Fabaceae, and (4) a third group including the rest of the families. To account for the effect of a shared phylogenetic history in species traits, models included as a random effect a reconstructed phylogenetic tree of the study species. We created the phylogeny using the R package V.PhyloMaker (Jin and Qian, 2019), which contains an updated mega-tree of the seed plants based on Smith and Brown (2018). We placed taxa absent from the mega-tree at the genus-level basal node. The phylogenetic tree is available at GitHub (see Data Availability Statement). The response variable of the models was the proportion of germinated seeds. The fixed effects were the germination treatments (scarification, stratification, GA3, temperature, alternating temperature and light). Random effects included the phylogenetic tree, species identity, seed lot and source of the data. In all models, fixed effect variables were scaled so their contribution to the effect sizes could be compared. We used weakly informative priors in all models, with parameter-expanded priors for the random effects. Each model was run for 500 000 MCMC steps, with an initial burn-in phase of 50 000 and a thinning interval of 50 (De Villemereuil and Nakagawa, 2014), resulting, on average, in 9000 posterior distributions. From the resulting posterior distributions, we calculated mean parameter estimates and 95 % highest posterior density (HPD) and credible intervals (CIs). We estimated the significance of model parameters by examining CIs, considering parameters with CIs overlapping with zero as non-significant. To estimate the phylogenetic signal in the models we used Pagel’s λ (Pagel, 1999), estimated simultaneously with the models by calculating the mean of the posterior distribution and the 95 % CI of λ as indicated by De Villemereuil and Nakagawa (2014). When λ = 0, related taxa are no more similar than expected by chance, while when λ = 1, the trait is evolving following a constant variance random walk or Brownian motion model; intermediate values of λ indicate a phylogenetic correlation in trait evolution that does not fully follow a Brownian motion model (Pagel, 1999). The detailed output of the models is available at GitHub (see Data Availability Statement).
Seed traits versus species environmental preferences
We used the Iberian dataset (available at GitHub, see Data Availability Statement) to check whether seed traits and plant ecological preferences were related. The used dataset contained 76 species with complete trait data for seed germination, seed morphology and ecological requirements. We did a principal component analysis (PCA) of seed traits and species SNCs for cold, drought and pH. We performed the PCA ordination at the species level, i.e. calculating a series of continuous seed traits for each species. We transformed the final germination proportions to create a continuous variable for the germination cues (i.e. stratification, scarification, temperature, alternating temperatures and light). To do so, for each cue and species, we calculated a weighted average of the cue levels (in the case of temperature, cue levels were the temperature treatments; for the other cues, the levels were 0 = absence and 1 = presence), weighting by the germination proportion at each level. This approach underrepresents the importance of the levels that had not been tested for a given species, but can serve as a proxy of the response to the germination cues when visualized across the whole dataset. We also included seed mass and seed number in the ordination (species average values). We left GA3 out of the PCA because its ecological interpretation is subordinated to stratification (as both cues break physiological seed dormancy). We also left seed length and width out because these values were not available for enough species. We calculated the PCA with the package FactoMineR (Lê et al., 2008).
Germination in meadows versus other habitats
We used the Cantabrian dataset (available at GitHub, see Data Availability Statement) to compare the germination of mesic meadows with that of other herbaceous communities from the same region. The Cantabrian dataset included 131 plant species. We analysed this dataset by PCA.
RESULTS
Seed morphology
Poaceae had lower values of seed mass and higher values of seed number, while Fabaceae had heavier but fewer seeds (Fig. 2). The other families covered the range of values showed by Poaceae and Fabaceae, but their median values were high for both traits: their median seed mass was close to that of the Fabaceae, while their median seed number was higher than that of the Poaceae (Fig. 2). Seed shape also showed a divergence between Poaceae and Fabaceae, with seeds being elongated in the former and round in the latter (Fig. 2). The other families covered the full range of variation, with both elongated and round seeds (Fig. 2).
Fig. 2.
Morphology of mesic meadow seeds. The two panels on the left show the probability densities of species values for seed mass and seed number, log-transformed for ease of visualization. The three horizontal lines within the probability densities represent the first quartile, the median and the third quartile of the values. The panel on the right shows values of seed length and width obtained by image analysis, each point being a seed. In all cases, data are divided between the grasses (Poaceae), legumes (Fabaceae) and the other plant families.
Seed germination
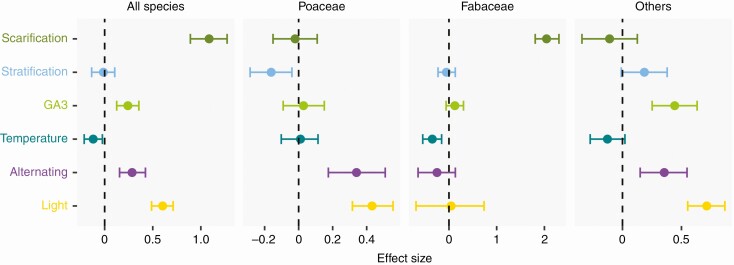
When considering the full set of mesic meadow species, all the studied germination cues except for stratification had a significant effect on final germination proportions (Fig. 3). The germination of meadow seeds was positively associated with scarification, GA3, alternating temperatures, and light. Average temperature had a negative effect, indicating a trend towards higher germination at lower temperatures. Averaging the whole dataset, the highest germination proportions were achieved at 20 °C. Between 0 and 20 °C, germination proportions increased steadily with increasing temperatures. Above 20 °C, germination declined more sharply, and meadow seeds rarely germinated at 30 °C.
Fig. 3.
Germination cues in mesic meadows. Effect of germination cues simulated in the laboratory over the final germination proportions of mesic meadow seeds. Dots indicate the posterior mean of the effect size for each cue, and whiskers the 95 % CI of the effect size. The line of zero effect is shown: when a credible interval overlaps with the zero-effect line, the effect can be regarded as non-significant. In separate panels, the figure shows the results of a general model including data for all species, plus specific models for the three main botanical groups of mesic meadows: grasses (Poaceae), legumes (Fabaceae) and the other families.
Some differences became apparent when dividing the dataset in the three floristic groups that compose mesic meadow vegetation. In Poaceae (Fig. 3), no effect was found for scarification, GA3 or temperature. Stratification had a negative effect on germination. The major drivers of Poaceae germination appeared to be alternating temperatures and light, with both having a positive effect. In Fabaceae (Fig. 3), the largest positive effect on germination was produced by scarification, with no effect of stratification, GA3, alternating temperatures or light. Temperature had a negative effect, and in fact Fabaceae species had higher germination proportions at temperatures under 20 °C. Finally, in the remaining families (Fig. 3), the main cues having a positive effect on germination were GA3, alternating temperatures and light. These species did not respond to scarification, stratification or average temperatures.
To describe the effects of the random factors, we will refer only to the full model that included all species in the dataset. The strongest effect was that of the phylogeny (mean = 5.42, CI = 2.91–8.2), followed by the source of the data (mean = 3.06, CI = 1.43–5.13) and the seed lot (mean = 1.28, CI = 0.94–1.64). The phylogenetic signal in the germination responses was relatively high (λ = 0.68, CI = 0.57–0.79).
Seed traits and species ecological preferences
Principal component analysis indicated a clear separation between environmental preferences and seed traits, with each set of variables contributing to different axes (Fig. 4). The first PCA axis explained 29 % of the variation and was related to environmental preferences. The variables with the largest contribution to this first axis were soil reaction (pH), winter cold (bio06) and summer rainfall (bio14). This horizontal axis separated (i, left) species with preferences for sites with warm winter temperatures from (ii, right) species with preferences for sites with high summer rainfall and less acidic soils. Axis 2 explained 17 % of the variability, mostly related to seed traits. The main contributing variables were seed mass, seed number and the germination response to scarification. This axis separated (iii, bottom) Poaceae species that produce many seeds with a positive germination response to alternating temperatures from (iv, top) Fabaceae species that produce heavy seeds with a positive response to scarification.
Fig. 4.
Environment and seed traits are separate axes of variation in mesic meadows. Principal component analysis ordination of mesic meadow species considering their environmental preferences and their seed traits. Each point is a species, coloured by the three main botanical groups of mesic meadows: grasses (Poaceae), legumes (Fabaceae) and the other families. Labels indicate the contribution of the variables to the axes: grey-background labels for environmental preferences and white-background labels for seed traits. Environmental preferences were calculated as species niche centroids (SNCs) for the minimal temperature of the coldest month (bio06), precipitation of the driest month (bio14) and soil pH. To calculate the SNCs, a vegetation dataset of mesic meadows of the Iberian Peninsula was used. The seed traits are seed mass and the germination relative indices for the response to scarification, stratification, average germination temperature (temperature), alternating temperature (alternating) and light. All environmental preferences aligned to the first axis, while seed traits aligned to the second axis, showing that environment and seed traits are separate axes of variation in mesic meadows. Stratification and average germination temperature showed very low variation, in accordance with their small effect in Fig. 2.
Comparison with other habitats
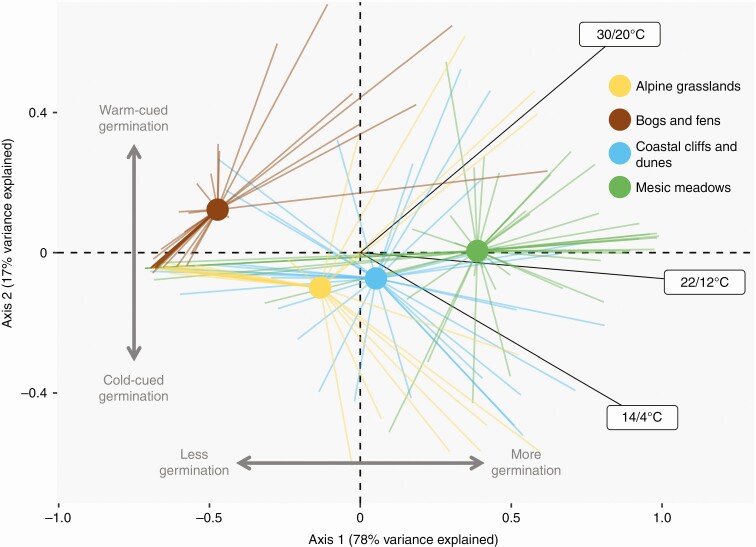
Ordination resulted in a separation of mesic meadow species from plant species belonging to natural herbaceous communities of the same region (Fig. 5). Axis 1 explained 78 % of the variance and was positively associated with high germination at all three temperature treatments. This horizontal axis separated (i, left) species with low germination across treatments from (ii, right) species with high germinability across treatments. The second axis explained 17 % of the variation and separated (iii, bottom) species that responded more to the cool germination temperature from (iv, top) species that responded more to the warm germination treatment. Meadow species tended to be situated in the right sector of the ordination, indicating high germinability, and at the centre of the vertical axis, indicating a neutral response to temperature. On the other hand, species from the other communities were positioned at the left of the horizontal axis, indicating a lower germinability; and were more separated along the vertical axis, indicating a preference for either warmer (bogs and fens) or cooler (alpine grasslands, coastal communities) germination treatments.
Fig. 5.
Higher germinability in mesic meadows compared with other herbaceous plant communities. Principal component analysis ordination of species responses to three seed germination treatments (fresh seeds germinated at 30/20, 22/12 and 14/4 °C in a 12/12-h light/darkness photoperiod). Species are grouped by their habitat, with points showing the centroid for each habitat group, and rays linking the centroid with the position of each species in the group. Labels indicate the contribution of the germination treatments to the axes. All species had been collected in herbaceous vegetation types of the Cantabrian Mountains of Spain and the neighbouring coast. All seeds were untreated, except for scarification, which was applied routinely to all botanical families presenting physical dormancy. Germination treatments consisted in 12/12-h periods with a warmer phase in light and a cooler phase in darkness. The position of mesic meadows compared with the other habitats indicates that their species tended to have higher germinability even if untreated (i.e. less seed dormancy) and were more neutral in their thermal requirements.
DISCUSSION
Our meta-analysis of germination records showed that the seed ecology of mesic meadows is characterized by (1) high germinability when compared with other herbaceous plant communities; (2) low correspondence between seed trait variability and the natural environmental drivers of mesic meadow diversity; and (3) a deep phylogenetic separation between the two major families, Poaceae and Fabaceae.
We found that alternating temperatures and light are the two most influential factors triggering germination in all taxonomic groups except for Fabaceae. Alternating temperatures and light are usually considered to be micro-environmental cues that indicate, at a fine scale, the existence of safe sites for regeneration (Jumpponen et al., 1999). The diurnal alternation of temperature decreases with burial depth in the soil, and by the presence of vegetation cover (Thompson et al., 1977; Van Assche and Vanlerberghe, 1989; Saatkamp et al., 2011). Thus, a positive germination response to alternating temperatures could detect the depth of seed burial and prevent the germination of seeds that are buried too deep for seedlings to survive before emerging from the ground (Bond et al., 1999). Perhaps more importantly in mesic meadows, alternating temperatures could also indicate that the vegetation cover has been diminished by either mowing or grazing, marking an appropriate time for seedling regeneration, when the competition by the established plants is lessened. Similar functions can be ascribed to the germination response to light, as physiologically active light in significant amounts only travels the uppermost millimetres of soil (Tester and Morris, 1987) and the quality of light will be affected by vegetation cover (Jankowska-Blaszczuk and Daws, 2007). Therefore, the germination response to alternating temperatures and light indicates conditions that are found immediately after mowing for haymaking, a predictable perturbation that occurs approximately at the same time every year, and which forces meadow plants to establish, grow and reproduce in a regular time frame (Grime, 2006; Klimešová et al., 2010). Theoretically, these germination traits would also promote the formation of a transient soil seed bank (Williams, 1983b; Venn and Morgan, 2010). But it is worth mentioning that Poaceae, which show the germination response to alternating temperatures and light, also have the more elongated seed shape, a trait that is thought to reduce the capacity of seeds to enter the soil seed bank in temperate meadows (Thompson et al., 1993; Funes et al., 1999).
The strong effect of scarification was specifically related to the hard-seeded Fabaceae. In this family, physical dormancy as a result of an impermeable seed coat works as a mechanism to detect seasonal cycles of temperature and humidity (Van Assche et al., 2003). It has been also proposed that physical dormancy can prevent seed predation, by preventing the emission of olfactory cues that are elicited by seed imbibition and that can be perceived by seed predators (Paulsen et al., 2013). In the case of this study, Fabaceae seeds are amongst the heaviest, and they clearly follow a separate regeneration strategy from that of the other families. While other groups rely on alternating temperatures and light as gap-detecting mechanisms, Fabaceae seeds are regulated instead by scarification, while they also respond to cooler temperatures than the rest of the families. This suggests that Fabaceae seeds would tend to germinate when temperatures are cooler, and thus before or after the summer haymaking season. Their larger size, and the related larger reserves, could allow Fabaceae seedlings to emerge from greater depths (Bond et al., 1999), e.g. when the meadow grass is still high before mowing. Furthermore, larger seed size also increases seed survival in cattle dung (Peco et al., 2006) and thus improves the capacity to be dispersed by animal depositions (Traba et al., 2003).
Seed germination responses to average temperatures and stratification are understood to be a mechanism to track seasonal climatic cycles (Finch-Savage and Leubner-Metzger, 2006). Apart from the response to cool temperatures in Fabaceae, the rest of the families did not show a response to average temperatures, further highlighting their reliance on micro-habitat and short-term cueing. Moreover, the dominant family, Poaceae, showed a negative response to cold stratification. The less frequent families, however, showed a divergence from the Poaceae strategy: their positive response to GA3. Gibberellic acid is a phytohormone that can work as a substitute for cold stratification to overcome physiological dormancy (Bewley et al., 2013). The response to GA3 indicates which, the non-dominant species have a degree of physiological dormancy, which could postpone their germination until overwintering has occurred (Baskin and Baskin, 2014).
The strong phylogenetic signal in germination responses highlights the phylogenetic clustering of regeneration strategies that we have described so far. (1) The dominant family Poaceae shows a lack of response to seasonal cues (average temperature and cold stratification). Instead, Poaceae rely on large amounts of propagules and on detecting micro-niche cues (alternating temperatures, light) that can be associated with the yearly perturbation of mowing. (2) The second most-dominant family, Fabaceae, does not respond to micro-niche cues, and regulates germination timing through scarification and cooler germination temperatures, possibly because their larger size and reserves allow their seeds to decouple their emergence timing from the mowing disturbance. (3) The minor families respond to micro-cues in a similar way to Poaceae, but are differentiated from them by showing a positive response to GA3, indicating that they rely on physiological seed dormancy to track seasonal cycles. This third strategy would make it possible to fine-tune their germination to the micro-environmental conditions plus the inter-annual climatic variation. Phylogenetic clustering is also apparent regarding seed shape, mass and number, with Poaceae producing many elongated and light seeds, and Fabaceae producing fewer, rounder and heavier seeds. The shape of Poaceae seeds could make them particularly successful in attaching to hay and dispersing with it, and Poaceae seeds tend to be overrepresented in seed mixes obtained via haymaking (Scotton et al., 2009; Haslgrübler et al., 2014). Asteraceae and Apiaceae, some of the most abundant among the minor families, also tend to have shapes resembling those of Poaceae.
As we have seen, the regeneration strategy of high germinability appears to be general in meadow species, across regional environmental gradients. Seed traits usually show variation along environmental gradients; some examples include warm-cued germination in species from colder sites (Rosbakh and Poschlod, 2015) or smaller seeds in species from higher elevations (Wang et al., 2014). Such relationships are missing in the hay meadows of the Iberian Peninsula, highlighting the semi-natural character of this vegetation, conditioned by human management as much as by environmental constraints. To some extent, the lower germinability that we found in coastal habitats, mires and alpine grasslands could be a result of the stressors imposed by salinity, waterlogging and temperature extremes. At the same time, the seed ecology of meadow species could be a response to the potential selective force that is the predictable perturbation by yearly mowing. Past research supports the relationship between mowing and germination timing. In an abandoned meadow of the Swiss Prealps, resuming mowing promoted germination and emergence, although natural regeneration was limited due to a lack of seed dispersal to the site (Kupferschmid et al., 2000). Mowing also promoted autumn germination in a dry grassland of northern Germany (Kahmen and Poschlod, 2008). Past studies also agree with the lack of dormancy and the high germinability of meadow seeds that we have found. For example, meadow seeds of Poa trivialis were less dormant than seeds of the same species collected in an arable field (Froud-Williams et al., 1986). Ten Brink et al. (2013), when comparing congeneric herbaceous species from open grasslands or forest habitats, found that species from the open grasslands needed less cold stratification. Similarly, seeds collected from a hay meadow germinated better when left untreated, rather than when being exposed to dormancy-breaking treatments (Haslgrübler et al., 2014). The high-germinability syndrome as a response to artificial selection by mowing is indeed an intriguing hypothesis, and future work should be designed to test it.
The high-germinability strategy, however, has the practical consequence of greatly limiting the long-term seed bank persistence of mesic meadow species: the soil seed bank has been reported as being transient in hay meadows and related grasslands (Milberg, 1992; McDonald, 1993; Hutchings and Booth, 1996). In Poland, the soil bank of a hay meadow was dominated by arable and weedy forbs, with low representation of Poaceae and Fabaceae (Janicka, 2017). The inability to form persistent seed banks leads to dispersal limitations to passive natural regeneration (Kupferschmid et al., 2000), making mesic meadows quite sensitive to land use change, and stressing the importance of active actions of meadow restoration via seed supply.
Understanding the germination requirements of the different plant groups that coexist in mesic meadows can help to manage, conserve and restore their biological diversity. For centuries, human activities have shaped the regeneration of hay meadows, and apparently this has led to a loss of seed dormancy, decoupling seeds from seasonal cycles, as has been found in many domesticated species (Dürr et al., 2015). The same anthropic processes that have shaped semi-natural mesic meadows may have left them dependent on continued human intervention for their regeneration. The high germinability of meadow seeds makes them relatively easy to use in restoration projects. But it also creates a fascinating dilemma to the restoration practitioner: instead of sourcing seed from existing – and seemingly domesticated – meadows, it could be advisable to also source seeds from related wild populations of meadow species, which may hold the genetic variability and phenotypic plasticity to cope with the threats posed by new environmental challenges (Parmesan and Hanley, 2015).
Supplementary Material
AUTHOR CONTRIBUTIONS
All authors contributed data. E.F.P. conceived the study and performed the analyses. E.F.P. wrote the manuscript with help from A.C. All authors revised the manuscript and approved the final version.
FUNDING
This work was supported by the European Regional Development Fund via Interreg Europe (SOS PRADERAS—SOE1/P5/E037); the Government of Asturias and the FP7—Marie Curie—COFUND programme of the European Commission (CLARÍN-ACB17-19 to E.F.P); and the Jardín Botánico Atlántico (SV-20-GIJON-JBA to E.F.P.).
OPEN DATA
The original data, R code for the analysis and creation of the manuscript can be accessed at the GitHub repository https://github.com/efernandezpascual/meadows. Upon publication, a version of record of the repository will be deposited in Zenodo.
LITERATURE CITED
- Álvarez J, Afif E, Díaz TE, García L, Oliveira JA. 2021. Effects of management practices on soil properties and plant nutrition in hay meadows in Picos de Europa. Environments 8: 38. [Google Scholar]
- Van Assche JA, Vanlerberghe KA. 1989. The role of temperature on the dormancy cycle of seeds of Rumex obtusifolius L. Functional Ecology 3: 107–115. [Google Scholar]
- Van Assche JA, Van Nerum D, Darius P. 2002. The comparative germination ecology of nine Rumex species. Plant Ecology 159: 131–142. [Google Scholar]
- Van Assche JA, Debucquoy KLA, Rommens WAF. 2003. Seasonal cycles in the germination capacity of buried seeds of some Leguminosae (Fabaceae). New Phytologist 158: 315–323. [Google Scholar]
- Van Assche JA, Vandelook FEA. 2010. Combinational dormancy in winter annual Fabaceae. Seed Science Research 20: 237–242. [Google Scholar]
- Bacchetta G, Grillo O, Mattana E, Venora G. 2008. Morpho-colorimetric characterization by image analysis to identify diaspores of wild plant species. Flora 203: 669–682. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds. Ecology, biogeography and evolution of dormancy and germination, 2nd edn. San Diego: Academic Press. [Google Scholar]
- Bean EW, Sengul S, Tyler BF. 1984. The germination of grass seeds after storage at different temperatures in aluminium foil and manilla paper packets. Annals of Applied Biology 105: 399–403. [Google Scholar]
- Benvenuti S, Pardossi A. 2016. Germination ecology of nutraceutical herbs for agronomic perspectives. European Journal of Agronomy 76: 118–129. [Google Scholar]
- Bewley JD, Bradford K, Hilhorst H, Nonogaki H. 2013. Seeds: physiology of development, germination and dormancy, 3rd edn. Berlin: Springer. [Google Scholar]
- Blandino C, Fernández-Pascual E, Marin M, Vernet A, Pritchard HW. 2019. Seed ecology of the geophyte Conopodium majus (Apiaceae), indicator species of ancient woodland understories and oligotrophic meadows. Plant Biology 21: 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond WJ, Honig M, Maze KE. 1999. Seed size and seedling emergence: an allometric relationship and some ecological implications. Oecologia 120: 132–136. [DOI] [PubMed] [Google Scholar]
- Ter Borg SJ. 2005. Dormancy and germination of six Rhinanthus species in relation to climate. Folia Geobotanica 40: 243–260. [Google Scholar]
- ten Brink D-J, Hendriksma HP, Bruun HH. 2013. Habitat specialization through germination cueing: a comparative study of herbs from forests and open habitats. Annals of Botany 111: 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni M, Dengler J, Mantilla-Contreras J, Venn S, Török P. 2015. Conservation value, management and restoration of Europe’s semi-natural open landscapes. Hacquetia 14: 5–17. [Google Scholar]
- Chollet S, Brabant C, Tessier S, Jung V. 2018. From urban lawns to urban meadows: reduction of mowing frequency increases plant taxonomic, functional and phylogenetic diversity. Landscape and Urban Planning 180: 121–124. [Google Scholar]
- Chytrý M. 2012. Vegetation of the Czech Republic: diversity, ecology, history and dynamics. Preslia 84: 427–504. [Google Scholar]
- Chytrý M, Tichý L, Hennekens SM, et al. 2020. EUNIS Habitat Classification: expert system, characteristic species combinations and distribution maps of European habitats. Applied Vegetation Science 23: 648–675. [Google Scholar]
- Dixon J. 1995. Trisetum flavescens (L.) Beauv. (T. pratense Pers., Avena flavescens L.). Journal of Ecology 83: 895–909. [Google Scholar]
- Dürr C, Dickie JB, Yang XY, Pritchard HW. 2015. Ranges of critical temperature and water potential values for the germination of species worldwide: contribution to a seed trait database. Agricultural and Forest Meteorology 200: 222–232. [Google Scholar]
- Ehrman T, Cocks PS. 1996. Reproductive patterns in annual legume species on an aridity gradient. Vegetatio 122: 47–59. [Google Scholar]
- ENSCONET. 2009. Seed collecting manual for wild species. Kew: Royal Botanic Gardens. [Google Scholar]
- Euro+Med. 2006. Euro+Med PlantBase – the information resource for Euro-Mediterranean plant diversity.http://ww2.bgbm.org/EuroPlusMed/ (January 2019, date last accessed).
- Fernández-Pascual E. 2016. Comparative seed germination traits in bog and fen mire wetlands. Aquatic Botany 130: 21–26. [Google Scholar]
- Fernández-Pascual E. 2021. SylvanSeeds, a seed germination database for temperate deciduous forests. Journal of Vegetation Science 21: e12960. [Google Scholar]
- Fernández-Pascual E, Jiménez-Alfaro B, Díaz TE. 2013. The temperature dimension of the seed germination niche in fen wetlands. Plant Ecology 214: 489–499. [Google Scholar]
- Fernández-Pascual E, Jiménez-Alfaro B, Bueno Á. 2017a. Comparative seed germination traits in alpine and subalpine grasslands: higher elevations are associated with warmer germination temperatures. Plant Biology 19: 32–40. [DOI] [PubMed] [Google Scholar]
- Fernández-Pascual E, Pérez-Arcoiza A, Prieto JA, Díaz TE. 2017b. Environmental filtering drives the shape and breadth of the seed germination niche in coastal plant communities. Annals of Botany 119: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Pascual E, Carta A, Mondoni A, et al. 2021. The seed germination spectrum of alpine plants: a global meta-analysis. New Phytologist 229: 3573–3586. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Finck P, Riecken U, Schröder E. 2002. Pasture landscapes and nature conservation – new strategies for the preservation of open landscapes in Europe. In: Redecker B, Härdtle W, Finck P, Riecken U, Schröder E, eds. Pasture landscapes and nature conservation. Berlin: Springer, 1–13. [Google Scholar]
- Froud-Williams RJ, Ferris R. 1987. Germination of proximal and distal seeds of Poa trivialis L. from contrasting habitats. Weed Research 27: 245–250. [Google Scholar]
- Froud-Williams RJ, Drennan DSH, Chancellor RJ. 1984. The influence of burial and dry-storage upon cyclic changes in dormancy, germination and response to light in seeds of various arable weeds. New Phytologist 96: 473–481. [Google Scholar]
- Froud-Williams RJ, Hilton JR, Dixon J. 1986. Evidence for an endogenous cycle of dormancy in dry stored seeds of Poa trivialis L. New Phytologist 102: 123–131. [DOI] [PubMed] [Google Scholar]
- Funes G, Basconcelo S, Díaz S, Cabido M. 1999. Seed size and shape are good predictors of seed persistence in soil in temperate mountain grasslands of Argentina. Seed Science Research 9: 341–345. [Google Scholar]
- Golińska B, Czerwiński M, Goliński P. 2017. Harvesting seeds of an Arrhenatherion meadow as a source of propagation material for grassland restoration. In: Porqueddu C, Franca A, Lombardi G. et al. , eds. Grassland resources for extensive farming systems in marginal lands: major drivers and future scenarios. Proceedings of the 19th Symposium of the European Grassland Federation . Sassari: CNR-ISPAAM, 485–487. [Google Scholar]
- Gresta F, Avola G, Anastasi U, Miano V. 2007. Effect of maturation stage, storage time and temperature on seed germination of Medicago species. Seed Science and Technology 35: 698–708. [Google Scholar]
- Grime JP. 2006. Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. Journal of Vegetation Science 17: 255–260. [Google Scholar]
- Grime JP, Mason G, Curtis AV, et al. 1981. A comparative study of germination characteristics in a local flora. Journal of Ecology 69: 1017–1059. [Google Scholar]
- Guadilla-Sáez S, Pardo-de-Santayana M, Reyes-García V. 2019. The role of traditional management practices in shaping a diverse habitat mosaic in a mountain region of northern Spain. Land Use Policy 89: 104235. [Google Scholar]
- Guzzon F, Orsenigo S, Gianella M, et al. 2018. Seed heteromorphy influences seed longevity in Aegilops. Seed Science Research 28: 277–285. [Google Scholar]
- Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. Journal of Statistical Software 33: 1–22. [PMC free article] [PubMed] [Google Scholar]
- Harris SM, Doohan DJ, Gordon RJ, Jensen KIN. 1998. The effect of thermal time and soil water on emergence of Ranunculus repens. Weed Research 38: 405–412. [Google Scholar]
- Haslgrübler P, Krautzer B, Blaschka A, Graiss W, Pötsch EM. 2014. Quality and germination capacity of seed material harvested from an Arrhenatherion meadow. Grass and Forage Science 69: 454–461. [Google Scholar]
- Hejcman M, Hejcmanová P, Pavlů V, Beneš J. 2013. Origin and history of grasslands in Central Europe – a review. Grass and Forage Science 68: 345–363. [Google Scholar]
- Hutchings MJ, Booth KD. 1996. Studies on the feasibility of re-creating chalk grassland vegetation on ex-arable land. I. The potential roles of the seed bank and the seed rain. Journal of Applied Ecology 33: 1171–1181. [Google Scholar]
- Janicka M. 2017. The evaluation of soil seed bank in two Arrhenatherion meadow habitats in central Poland. Acta Scientiarum Polonorum Agricultura 15: 25–38. [Google Scholar]
- Jankowska-Blaszczuk M, Daws MI. 2007. Impact of red:far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Functional Ecology 21: 1055–1062. [Google Scholar]
- Jauzein P, Mansour A. 1992. Principaux facteurs de la germination de Heracleum sphondylium L.: importance de l’oxygène. Agronomie 12: 85–96. [Google Scholar]
- Jiménez-Alfaro B, Frischie S, Stolz J, Gálvez-Ramírez C. 2020. Native plants for greening Mediterranean agroecosystems. Nature Plants 6: 209–214. [DOI] [PubMed] [Google Scholar]
- Jin Y, Qian H. 2019. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography 42: 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Turkington R. 1986. Lotus corniculatus L. Journal of Ecology 74: 1185–1212. [Google Scholar]
- Jumpponen A, Väre H, Mattson KG, Ohtonen R, Trappe JM. 1999. Characterization of ‘safe sites’ for pioneers in primary succession on recently deglaciated terrain. Journal of Ecology 87: 98–105. [Google Scholar]
- Kabouw P, Nab M, van Dam NM. 2010. Activated carbon addition affects substrate pH and germination of six plant species. Soil Biology and Biochemistry 42: 1165–1167. [Google Scholar]
- Kahmen S, Poschlod P. 2008. Does germination success differ with respect to seed mass and germination season? Experimental testing of plant functional trait responses to grassland management. Annals of Botany 101: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karger DN, Conrad O, Böhner J, et al. 2017. Climatologies at high resolution for the earth’s land surface areas. Scientific Data 4: 170122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyer M, Bekker RM, Knevel IC, et al. 2008. The LEDA Traitbase: a database of life-history traits of the Northwest European flora. Journal of Ecology 96: 1266–1274. [Google Scholar]
- Klimešová J, Janeček Š, Bartušková A, Lanta V, Doležal J. 2010. How is regeneration of plants after mowing affected by shoot size in two species-rich meadows with different water supply? Folia Geobotanica 45: 225–238. [Google Scholar]
- Kolodziejek J, Patykowski J, Wala M. 2017. Effect of light, gibberellic acid and nitrogen source on germination of eight taxa from disappearing European temperate forest, Potentillo albae-Quercetum. Scientific Reports 7: 13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautzer B, Graiss W, Haslgrübler P, Kirmer A, Tischew S, Pötsch E. 2013. Grassland science in Europe, Vol. 22. In: Helgadóttir Á, Hopkins A, eds. The role of grasslands in a green future. Threats and perspectives in less favoured areas. Proceedings of the 17th Symposium of the European Grassland Federation. Akureyri, Iceland, 23–26 June 2013. [Google Scholar]
- Kupferschmid AD, Stampfli A, Newbery DM. 2000. Dispersal and microsite limitation in an abandoned calcareous grassland of the southern Prealps. Folia Geobotanica 35: 125–141. [Google Scholar]
- Ladouceur E, Jiménez-Alfaro B, Marin M, et al. 2018. Native seed supply and the restoration species pool. Conservation Letters 11: e12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25: 1–18. [Google Scholar]
- Letchamo W, Gosselin A. 1996. Light, temperature and duration of storage govern the germination and emergence of Taraxacum officinale seed. Journal of Horticultural Science 71: 373–377. [Google Scholar]
- Li D- Z, Pritchard HW. 2009. The science and economics of ex situ plant conservation. Trends in Plant Science 14: 614–621. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Zelle B, Eckstein RL, Mosner E, Otte A, Donath TW. 2014. Differential effects of reduced water potential on the germination of floodplain grassland species indicative of wet and dry habitats. Seed Science Research 24: 49–61. [Google Scholar]
- Marchiol L, Cesco S, Pinton R, Zerbi G. 2000. Germination and initial root growth of four legumes as affected by landfill biogas atmosphere. Restoration Ecology 8: 93–98. [Google Scholar]
- Marin M, Laverack G, Matthews S, Powell AA. 2019. Germination characteristics of Rhinanthus minor influence field emergence, competitiveness and potential use in restoration projects. Plant Biology 21: 470–479. [DOI] [PubMed] [Google Scholar]
- Mariotte P. 2014. Do subordinate species punch above their weight? Evidence from above- and below-ground. New Phytologist 203: 16–21. [DOI] [PubMed] [Google Scholar]
- Masin R, Onofri A, Gasparini V, Zanin G, Gonzalez-Andujar J. 2017. Can alternating temperatures be used to estimate base temperature for seed germination? Weed Research 57: 390–398. [Google Scholar]
- McDonald AW. 1993. The role of seedbank and sown seeds in the restoration of an English flood-meadow. Journal of Vegetation Science 4: 395–400. [Google Scholar]
- Merritt DJ, Dixon KW. 2011. Restoration seed banks—a matter of scale. Science 332: 424–425. [DOI] [PubMed] [Google Scholar]
- Mezynski PR, Cole DF. 1974. Germination of dandelion seed on a thermogradient plate. Weed Science 22: 506–507. [Google Scholar]
- Milberg P. 1992. Seed bank in a 35-year-old experiment with different treatments of a semi-natural grassland. Acta Oecologica 13: 743–752. [Google Scholar]
- Mucina L, Bültmann H, Dierßen K, et al. 2016. Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Applied Vegetation Science 19: 3–264. [Google Scholar]
- Nikolic R, Mitic N, Zivkovic S, Grubisic D, Neskovic M. 2007. Cytokinins and urea derivatives stimulate seed germination in Lotus corniculatus L. Archives of Biological Sciences 59: 125–128. [Google Scholar]
- Noronha A. 1997. Rate of change in dormancy level and light requirement in weed seeds during stratification. Annals of Botany 80: 795–801. [Google Scholar]
- Oliveira G, Nunes A, Clemente A, Correia O. 2012. Testing germination of species for hydroseeding degraded Mediterranean areas. Restoration Ecology 20: 623–630. [Google Scholar]
- Oomes MJM, Elberse WT. 1976. Germination of six grassland herbs in microsites with different water contents. Journal of Ecology 64: 745–755. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Pannangpetch K, Bean EW. 1984. Effects of temperature on germination in populations of Dactylis glomerata from NW Spain and Central Italy. Annals of Botany 53: 633–639. [Google Scholar]
- Pappalardo P, Ogle K, Hamman EA, Bence JR, Hungate BA, Osenberg CW. 2020. Comparing traditional and Bayesian approaches to ecological meta-analysis. Methods in Ecology and Evolution 11: 1286–1295. [Google Scholar]
- Parmesan C, Hanley ME. 2015. Plants and climate change: complexities and surprises. Annals of Botany 116: 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen TR, Colville L, Kranner I, et al. 2013. Physical dormancy in seeds: a game of hide and seek? New Phytologist 198: 496–503. [DOI] [PubMed] [Google Scholar]
- Peco B, Lopez-Merino L, Alvir M. 2006. Survival and germination of Mediterranean grassland species after simulated sheep ingestion: ecological correlates with seed traits. Acta Oecologica 30: 269–275. [Google Scholar]
- Pérez-Fernández MA, Rodríguez-Echeverría S. 2003. Effect of smoke, charred wood, and nitrogenous compounds on seed germination of ten species from woodland in central-western Spain. Journal of Chemical Ecology 29: 237–251. [DOI] [PubMed] [Google Scholar]
- Pérez-Fernández MA, Calvo-Magro E, Montanero-Fernández J, Oyola-Velasco JA. 2006. Seed germination in response to chemicals: effect of nitrogen and pH in the media. Journal of Environmental Biology 27: 13–20. [PubMed] [Google Scholar]
- Poschlod P, Baumann A, Karlik P. 2009. Origin and development of grasslands in Central Europe. In: Veen P, Jefferson R, de Smidt J, van der Straaten J, eds. Grasslands in Europe. Zeist: KNNV Publishing, 15–25. [Google Scholar]
- Prince HE, Bunce RGH, Jongman RHG. 2012. Changes in the vegetation composition of hay meadows between 1993 and 2009 in the Picos de Europa and implications for nature conservation. Journal for Nature Conservation 20: 162–169. [Google Scholar]
- Probert RJ, Smith RO. 1986. The joint action of phytochrome and alternating temperatures in the control of seed germination in Dactylis glomerata. Physiologia Plantarum 67: 299–304. [Google Scholar]
- Probert RJ, Smith RD, Birch P. 1985. Germination responses to light and alternating temperatures in European populations of Dactylis glomerata L. New Phytologist 100: 447–455. [DOI] [PubMed] [Google Scholar]
- Probert RJ, Smith RD, Birch P. 1986. Germination responses to light and alternating temperatures in European populations of Dactylis glomerata L. V. the principle components of the alternating temperature requirement. New Phytologist 102: 133–142. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2020. R: a language and environment for statistical computing. Version 4.0.3. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Reiné R, Ascaso J, Barrantes O. 2020. Nutritional quality of plant species in Pyrenean hay meadows of high diversity. Agronomy 10: 883. [Google Scholar]
- Rodríguez-Rojo MP, Fernández-González F, Tichý L, Chytrý M. 2014. Vegetation diversity of mesic grasslands (Arrhenatheretalia) in the Iberian Peninsula. Applied Vegetation Science 17: 780–796. [Google Scholar]
- Rodríguez-Rojo MP, Jiménez-Alfaro B, Jandt U, et al. 2017. Diversity of lowland hay meadows and pastures in Western and Central Europe. Applied Vegetation Science 20: 702–719. [Google Scholar]
- Rosbakh S, Poschlod P. 2015. Initial temperature of seed germination as related to species occurrence along a temperature gradient. Functional Ecology 29: 5–14. [Google Scholar]
- Royal Botanic Gardens, Kew. 2017. Seed information database (SID). Version 7.1.https://data.kew.org/sid/ (October 2017).
- Saatkamp A, Affre L, Baumberger T, et al. 2011. Soil depth detection by seeds and diurnally fluctuating temperatures: different dynamics in 10 annual plants. Plant and Soil 349: 331–340. [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld MA, Chancellor RJ. 1983. Factors influencing seed movement and dormancy in grass seeds. Grass and Forage Science 38: 243–250. [Google Scholar]
- Scotton M, Piccinin L, Dainese M, Sancin F. 2009. Seed harvesting for ecological restoration: efficiency of haymaking and seed-stripping on different grassland types in the eastern Italian Alps. Ecological Restoration 27: 66–75. [Google Scholar]
- Silvertown J. 1980. Leaf-canopy-induced seed dormancy in a grassland flora. New Phytologist 85: 109–118. [Google Scholar]
- Smith SA, Brown JW. 2018. Constructing a broadly inclusive seed plant phylogeny. American Journal of Botany 105: 302–314. [DOI] [PubMed] [Google Scholar]
- Southon GE, Jorgensen A, Dunnett N, Hoyle H, Evans KL. 2017. Biodiverse perennial meadows have aesthetic value and increase residents’ perceptions of site quality in urban green-space. Landscape and Urban Planning 158: 105–118. [Google Scholar]
- Sprague V. 1940. Germination of freshly harvested seeds of several Poa species and of Dactylis glomerata. Journal of the American Society of Agronomy 32: 715–721. [Google Scholar]
- Stanisavljevic R, Ðjokic D, Milenkovic J, et al. 2011. Seed germination and seedling vigour of Italian ryegrass, cocksfoot and timothy following harvest and storage. Ciência e Agrotecnologia 35: 1141–1148. [Google Scholar]
- Stanisavljevic R, Vuckovic S, Strbanovic R, et al. 2015. Enhancement of seed germination in three grass species using chemical and temperature treatments. Range Management and Agroforestry 36: 115–121. [Google Scholar]
- Tavşanoğlu Ç, Çatav ŞS, Özüdoğru B. 2015. Fire-related germination and early seedling growth in 21 herbaceous species in Central Anatolian steppe. Journal of Arid Environments 122: 109–116. [Google Scholar]
- Tester M, Morris C. 1987. The penetration of light through soil. Plant, Cell & Environment 10: 281–286. [Google Scholar]
- Thompson K. 1989. A comparative study of germination responses to high irradiance light. Annals of Botany 63: 159–162. [Google Scholar]
- Thompson K, Grime JP. 1983. A comparative study of germination responses to diurnally-fluctuating temperatures. Journal of Applied Ecology 20: 141–146. [Google Scholar]
- Thompson K, Band SR, Hodgson JG. 1993. Seed size and shape predict persistence in soil. Functional Ecology 7: 236–241. [Google Scholar]
- Thompson K, Grime JP, Mason G. 1977. Seed germination in response to diurnal fluctuations of temperature. Nature 267: 147–149. [DOI] [PubMed] [Google Scholar]
- Traba J, Levassor C, Peco B. 2003. Restoration of species richness in abandoned Mediterranean grasslands: seeds in cattle dung. Restoration Ecology 11: 378–384. [Google Scholar]
- De Villemereuil P, Nakagawa S. 2014. General quantitative genetic methods for comparative biology. In: Garamszegi LZ, ed. Modern phylogenetic comparative methods and their application in evolutionary biology. Springer, 287–303. [Google Scholar]
- De Vitis M, Abbandonato H, Dixon KW, Laverack G, Bonomi C, Pedrini S. 2017. The European native seed industry: characterization and perspectives in grassland restoration. Sustainability 9: 1682. [Google Scholar]
- Venn SE, Morgan JW. 2010. Soil seedbank composition and dynamics across alpine summits in south-eastern Australia. Australian Journal of Botany 58: 349–362. [Google Scholar]
- Wagner M, Pywell RF, Knopp T, Bullock JM, Heard MS. 2011. The germination niches of grassland species targeted for restoration: effects of seed pre-treatments. Seed Science Research 21: 117–131. [Google Scholar]
- Wang Y, Wang J, Lai L, et al. 2014. Geographic variation in seed traits within and among forty-two species of Rhododendron (Ericaceae) on the Tibetan plateau: relationships with altitude, habitat, plant height, and phylogeny. Ecology and Evolution 4: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washitani I. 1984. Germination responses of a seed population of Taraxacum officinale Weber to constant temperatures including the supra-optimal range. Plant Cell and Environment 7: 655–659. [Google Scholar]
- Wille W, Thiele J, Walker EA, Kollmann J. 2013. Limited evidence for allelopathic effects of giant hogweed on germination of native herbs. Seed Science Research 23: 157–162. [Google Scholar]
- Williams ED. 1983a. Germinability and enforced dormancy in seeds of species of indigenous grassland. Annals of Applied Biology 102: 557–566. [Google Scholar]
- Williams ED. 1983b. Effects of temperature fluctuation, red and far-red light and nitrate on seed germination of five grasses. Journal of Applied Ecology 20: 923–935. [Google Scholar]
- Williams ED. 1983c. Effects of temperature, light, nitrate and pre chilling on seed germination of grassland plants. Annals of Applied Biology 103: 161–172. [Google Scholar]
- Zelený D. 2018. Which results of the standard test for community-weighted mean approach are too optimistic? Journal of Vegetation Science 29: 953–966. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.