Abstract
Introduction:
Inguinal lymph node dissection (ILND) is an integral part in the management of carcinoma penis. The concerns about the postoperative morbidity associated with open ILND led to modification in the template of dissection and adoption of minimally invasive techniques such as video endoscopic inguinal lymphadenectomy (VEIL) and robotic-assisted VEIL (R-VEIL). In this review, we aim to describe the techniques, case selection, perioperative outcomes, and oncological outcomes of VEIL and R-VEIL and to compare it with open ILND.
Methods:
Databases of PubMed, Embase, and Google Scholar were searched to identify the articles for VEIL and R-VEIL. Using PRISMA guidelines, literature search yielded 3783 articles, of which 32 full-text articles relevant to the topic were selected and reviewed, after consensus from authors.
Results:
After the first description of VEIL, various modifications in port placements and approaches were described. Several studies have shown, VEIL and R VEIL are safe and feasible in both node-negative and node-positive Ca penis patients. Compared to open ILND, VEIL had fewer wound infections and skin necrosis, minimal blood loss, shorter mean hospital stays, and reduced duration of drain kept. There is no difference in mean lymph node yield and recurrence rates between open ILND, VEIL, R-VEIL.
Conclusion:
VEIL and R-VEIL are safe and have comparable oncological outcomes with open ILND.
INTRODUCTION
Penile cancer accounts for about 0.5% of the cancers occurring in men worldwide, with India, Brazil, and South Africa being the countries with the highest incidence.[1,2,3] Inguinal lymphadenectomy (ILND) is an integral part in the management of the disease as it serves as a diagnostic, therapeutic, and prognostic tool. ILND is recommended in patients with intermediate/high-risk Ca Penis with nonpalpable inguinal nodes and unilateral/bilateral palpable inguinal lymph nodes.[4] Since ILND is associated with significant complications,[5] various techniques such as modifying the template of dissection, video endoscopic inguinal dissection (VEIL), and robotic VEIL (R-VEIL) have been described. Despite the VEIL and Robotic VEIL gaining momentum in recent years, there is a paucity of data on their utility. We reviewed techniques, case selection, perioperative outcomes, and oncological outcomes of VEIL and R-VEIL in penile cancer.
METHODOLOGY
Study design
We performed a review of medical literature to identify the articles relevant to VEIL and R-VEIL. Preferred Reporting Items for Systematic reviews and Meta-Analysis[6] guidelines were followed while conducting this study.
Search strategy
Online search using MeSH terms “Video Endoscopic Inguinal Dissection or VEIL or Robot-assisted Inguinal dissection or R-VEIL or RAVEIL or Minimal invasive Inguinal dissection” using ENDNOTE X9 in PubMed, Goolge Scholar and Embase library on June 2021. Search was limited to articles published after the year 2000.
Selection criteria
Two authors reviewed all the articles confirming to search criteria. All non-English, conference poster presentations, letters to editor, and case reports were excluded. Articles remaining after exclusion were analyzed under one of these heads: technique, case selection, perioperative outcomes, and oncological outcomes.
DISCUSSION
Initial search in PUBMED and EMBASE using Endnote X9, yielded 2479 and 878 results respectively. Google scholar yielded 426 results. Citations of all these were loaded in citation manager and duplicates removed. Title and abstracts relevant to the topic were scrutinized by two authors and after filtering with inclusion criteria, yielded 92 full-text articles. After excluding the VEIL in malignancies other than Carcinoma Penis yielded 32 articles [Figure 1]. These articles will be discussed in this manuscript under the heads as described.
Figure 1.
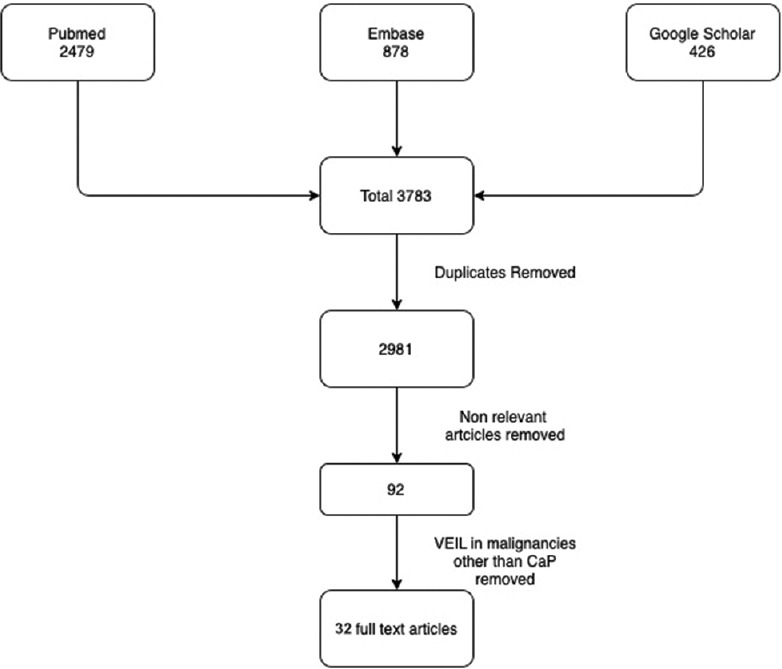
Literature Search
Technique
VEIL
After initial report of Bishoff et al.[7] in 2003 on few cadaveric dissections and one patient, Machado et al. in 2006 described this approach after a feasibility study in 7 patients.[8] The patient is positioned with knee flexed, hip externally rotated, and abducted (frog-leg position). An incision is made 2 cm caudal to the apex of femoral triangle. Finger dissection is used to create the working space deep to Scarpa's fascia through which the Camera is inserted. Two working ports are placed superolateral and superio-medial to the camera port, ensuring a distance of 6–8 cm between the ports to avoid clashing of the instruments [Figure 2]. Ports are fixed to the skin to prevent slipping out. The dissection is extended under vision using laparoscopic camera. During this step, transillumination of the skin helps in maintaining the adequate thickness of the camper's fascia in skin and to avoid a buttonhole [Figure 3]. The blood vessels in camper's fascia seen during transillumination should be preserved to prevent the necrosis of the skin flap. Template of dissection is similar to that of open ILND. The saphenous vein is sacrificed if it is surrounded by nodes.[9] After completing the superficial Inguinal dissection, the fascia lata is divided and deep inguinal lymph nodes including pectineus group and Cloquet nodes are removed after identifying and preserving femoral vessels[Figure 4]. The specimen is retrieved through the initial incision or in an endo bag [Figure 5]. Port sites are closed after placing a suction drain and a compressive bandage is applied over the limb and the operative area to avoid lymphorrhea.
Figure 2.
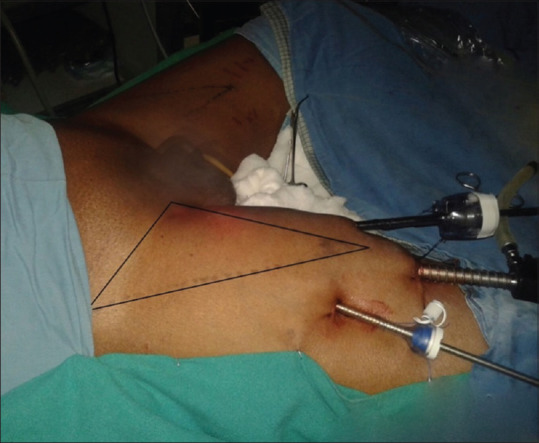
Patient position and port placement
Figure 3.
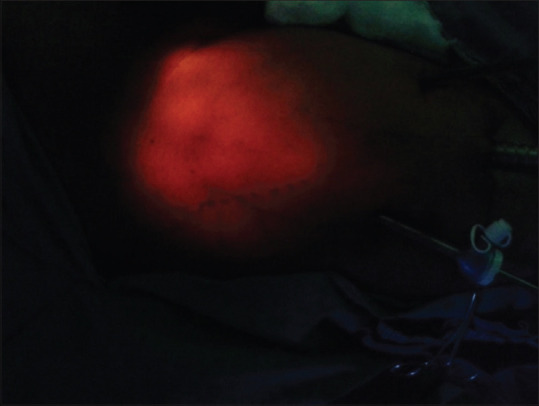
Transillumination of the skin flap
Figure 4.
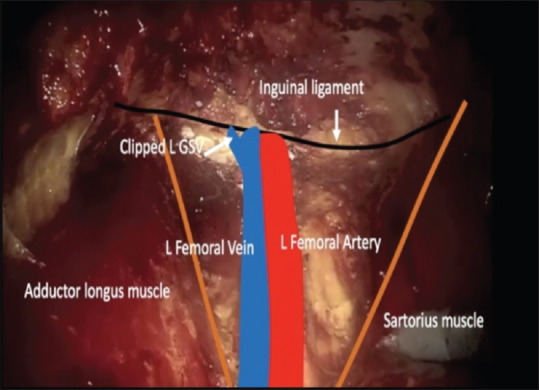
View after lymphadenectomy
Figure 5.
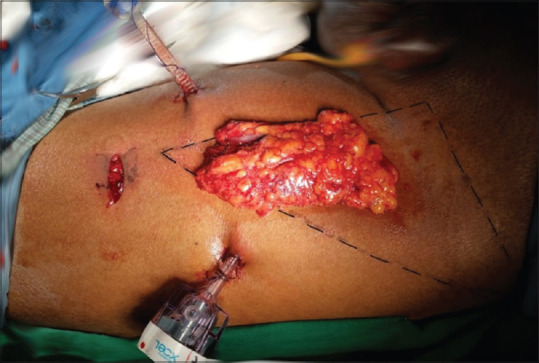
After specimen retrieval
Various modifications in port placements and approach have been described. Wang et al.[10] described hypogastric approach in VEIL for Vulvar cancer which was later adopted for penile cancer as well. Yuan et al.[11] compared hypogastric approach with standard approach and found no difference in outcomes. Nayak et al.[12] described lateral approach (L-VEIL) in which all the three ports are placed lateral to the sartorius muscle and the dissection carried out from lateral to medial in the femoral triangle. The short-term oncological outcomes of L-VEIL are comparable to standard VEIL.[13] Authors claim the shortcomings of the standard approach such as restriction of camera movement and difficulty for assistant to hold the camera could be avoided with L-VEIL. Tamhankar et al. described initial space creation using a balloon.[14] Pahwa et al. described cannula-assisted port placement, where the working ports are placed by using the primary port cannula as a guide.[15] Many authors describe initial plane creation deep to deep fascia followed by dropping the fibrofatty layer from the Camper's fascia.[16,17] Saphenous vein sparing and nonsparing are also described.[16,18] Machado et al.[19] described the laparo-endoscopic single-site VEIL (LESS VEIL or SS VEIL) technique in 2011, which requires specialized angulated instruments and is technically demanding. LESS Veil is claimed to be better cosmetically. Herrel et al. in 2012 and Pompeo et al. in 2013, came out with the feasibility of simultaneous bilateral VEIL in an effort to reduce the anesthesia and operative time.[20,21]
Robotic VEIL
Josephson et al. described R-VEIL in 2009.[22] DaVinci Si, X and Xi platforms are commonly used in R-VEIL. The first robotic port (Camera) is placed 25 cm caudal to the mid inguinal port. After creating the space, two robotic ports are placed 6–8 cm lateral and medial to the camera port. The fourth robotic port is usually omitted, due to small working space. A 12 mm port for assistant can be placed between and lateral to camera port and lateral port. Rest of the dissection is similar to the standard VEIL.
The advantages with R-VEIL are similar to any robotic procedures such as 3D vision, better dexterity for dissection, and ergonomics for the surgeon. This procedure needs a trained bedside assistant to monitor the skin flap thickness as the surgeon in not in the direct vision of the flap. Yu et al.[23] advocate a hypogastric approach for R-VEIL with utilizing skin incisions for the ports for pelvic lymphadenectomy if needed. Injecting indocyanine green at the base of the tumor during partial penectomy and dissecting the nodes using near infrared fluorescence (firefly) during R-VEIL has also been described.[24]
Case selection
Although VEIL is done in vulval cancers, urethral cancer, cutaneous malignancies of lower limb, and penile cancer, we have confined our search to VEIL in penile cancer. The first described VEIL procedure by Tobias et al.[8] was in node-negative groin. Carlos et al.[25] did a feasibility study and proved VEIL can be safely done in node-positive groin as well. Except for few studies,[26,27,28] most of the studies on VEIL are on mixed population of node-negative and positive patients with >70% the patients in former category. With the advent of robotic assistance, more node-positive patients were subjected to VEIL.[16,18,29] Ahlawat et al.[17] did robotic bilateral VEIL with bilateral pelvic lymphadenectomy in a single setting, expanding the feasibility.
Perioperative outcomes
Perioperative outcomes including the complications were evaluated in multiple studies [Table 1]. The mean operative duration of VEIL varies from 68 to 194.86 min. Compared to node-negative patients, the mean duration for VEIL was 30 min more for node-positive patients.[25] The reported mean operative duration in RVEIL was less than standard VEIL (68-151 min vs. 85-180 min).[16,18] Ahlawat et al. reported mean operative duration of 453 min, in the study where the feasibility of B/L VEIL and Pelvic lymphadenectomy was assessed.
Table 1.
Perioperative outcomes
| Author, year | Technique | Number of patients | Operative duration | Blood loss | Lymph node yield | Days drain kept | Hospital stay | Complications |
|---|---|---|---|---|---|---|---|---|
| Meneses et al., 2019[30] | VEIL | 11 | 85 (60-120) | <50 ml | 5.8 (1-12) | Mean 8 | 4 (2–11) | Skin complications 10%, lymphatic complications 25% |
| Carlos et al., 2013[25] | VEIL (NovsN1) | 40 | N0-95.5, N1-126.8 | N/A | 9 | N0-5.7, N1-6.7 | N0-3.1, N1-5.1 | Global complications 26% × 36%, cellulitis 0% × 4.5%, lymphocele 23% in both groups, skin necrosis 3% × 1%, myocutaneous necrosis 0% × 4.5% |
| Pahwa et al., 2013[31] | VEIL | 10 | 144 (120-180) | N/A | 5.6 (7-12) | 5.1 (5–8) | N/A | Subcutaneous emphysema (self-resolving) - all patients, skin complications - nil, lymphocele - 20% |
| Matin et al., 2013[32] | R VEIL | 10 | 100 (90–120) | 100 (10–200) | 9 (5-21) | N/A | N/a | Skin complications 10%, lymphocele 20%, cellulitis 10% |
| Chiapparrone et al., 2018[33] | VEIL | 1 | 180 min | N/A | 16 | 7 | 8 | Nil |
| Elsamra and Poch 2017[34] | R VEIL | 10 | 151.4 (99.0-224.0) | 30 (12.5-50.0) | 10.4 (6.0–16.0) | N/A | N/A | Skin complication: 10% |
| Sudhir et al., 2012[35] | VEIL | 22 | N/A | N/A | N/A | N/A | N/A | Skin necrosis: 2.53%, lymphocele 10.45, delayed bleed - 2.53% |
| Yuan et al., 2018[11] | VEIL | 72 | 107 (90–185) | 100 (85-120) | 20 (12–29) | 4 (3-12) | 5 (3-13) | Wound infection 8.42%, seroma - 6.91%, lymphorrhea - 5.66%, cellulitis - 2.66% |
| Elbalka et al., 2020[13] | VEIL | 15 | 128+/−28 | 20 (15-35) | 10.73+/−3.60 | 3 (3-21) | 3 (3-14) | Wound infection: 6.7, lymphocele 10%, flap necrosis: 3.3%, |
| Jindal and Meena 2021[26] | VEIL and R VEIL | 30 | 100 (80-140) | N/A | 7 (5-9) | 7 (5-12) | 3.1 (2-5) | Wound infection: 3.33%, lymphocele: 6.66% |
| Russell et al., 2017[27] | R VEIL and VEIL | 18 | 141 (120-162) | 50 (15-50) | 10 (6-12.5) | 2 (2-5) | N/A | Wound complication: 9%, lymphocele 6%, DVT 3% |
| Tobias-Machado et al., 2017[28] | VEIL | 10 | 126 (80-130) | N/A | 9.7 (6-14) | Mean 4.9 days | N/A | Lymphorrea - 10%, Hematoma - 10% |
| Yu et al., 2019[23] | R VEIL | 9 | 68+/−13 | N/A | 12 (5-21) | N/A | N/A | Nil |
| Singh et al., 2018[18] | R VEIL | 51 | 75 (70–85) | 75 (65-80) | 13 (11-14.5) | 12 (10-15) | 3 (3-3.75) | Wound complications: 11.8%, lymphocele - 49%, cellulitis 9.8% |
| Thyavihally et al., 2021[16] | RVEIL | 47 | 90 (50-140) | 30 (10-70) | 10 (7-14) | N/A | 6.1 (4-12) | Wound complications: 7.95%, lymphocele 20.45%, no flap necrosis |
| Yadav et al., 2018[29] | VEIL | 29 | 162.83 | N/A | 7.6 | N/A | 4.6 (4-8) | Skin necrosis: 2.8%, lymphocele 10.3%, lymphedema: 10.3%, no wound infection an d skin necrosis |
| Ahlawat et al., 2016[17] | R VEIL + ILND | 3 | 453 | N/A | 16.3 | 44 days | 4 | No skin complications, lymphocele in 33% |
| Kumar and Sethia 2017[36] | VEIL | 20 | 97 | N/A | 9.36 | N/A | 2.5 (0-14) | Wound complications 6%, prolonged lymphocele 27% |
| Romanelli et al., 2013[37] | VEIL | 20 | 119 (55-210) | N/A | 8 (3-16) | N/A | 5 (2-10) | Wound complications 6%, lymphatic complications: 27.2% |
| Chaudhari et al., 2016[38] | VEIL | 14 | 194.86 (178-210) | N/A | 7.68 (5-11) | N/A | N/A | Lymphocele: 27.2%, surgical emphysema: 13.63% |
VEIL=Video endoscopic inguinal lymph adenectomy, R VEIL=Robotic assisted VEIL, N/A=Not available, ILND=Inguinal lymph node dissection
Estimated blood loss in VEIL ranges from 20 to 100 ml. Singh et al.[18] reported comparable estimated blood loss (about 75 ml) for R-VEIL and open ILND. Thyavihally et al. reported less mean estimated blood loss in RVEIL than in Open ILND (30 ml vs. 100 ml, P = 0.005). Thus, the estimated blood loss in VEIL is comparable or less than that of open procedure.
Mean hospital stay was 3–5 days. This is in contrast to the mean hospital stay of about 10 days in Open ILND.[16,29] Singh et al.[18] reported mean hospital stay of 4 days in ILND group and 3 days on VEIL group (P = 0.008). Thus, VEIL offers quick recovery and earlier discharge from the hospital. This trend is also seen with mean duration of drain kept.
Similar to the open ILND, the complications encountered in minimal invasive surgery are wound infection including cellulitis, lymphocele requiring repeated aspirations, early and delayed lymphedema, and skin necrosis ranging from edge necrosis to flap necrosis. The incidence of the complications in VEIL differs widely between various studies [Table 2]. It can be as low as 10%[28] and as high as 78.4%.[18] Large variability between the complications in different studies could be partly due to the difference in reporting criteria used.
Table 2.
Comparison of complications between open and video endoscopic inguinal lymph adenectomy
| Author, year | Comparison | Wound infection (%) | Lymphocele (%) | Lymphedema (%) | Skin necrosis (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| Open | MIS | Open | MIS | Open | MIS | Open | MIS | ||
| Tobias-Machado 2017[28] | Open versus VEIL | 20 | 0 | 20 | 10 | 20 | 0 | 20 | 0 |
| Yu et al., 2019[23] | Open versus R VEIL | 45 | 0 | 20 | 12.50 | 0 | 0 | 20 | 0 |
| Singh et al., 2018[18] | Open versus R VEIL | 20 | 9.80 | 55 | 49 | 38 | 23.50 | 36 | 11.80 |
| Thyavihally et al., 2021[16] | Open versus R VEIL | 42.30 | 7.90 | 23.73 | 20.45 | 13.56 | 11.36 | 23.73 | 0.00 |
| Yadav et al., 2018[29] | Open versus VEIL | 13.70 | 0 | 24.10 | 10.30 | 13.70 | 10.30 | 27.58 | 6.89 |
| Kumar and Sethia 2017[36] | Open versus VEIL | 68 | 6 | 20 | 27 | 37 | 3 | N/A | N/A |
N/A=Not available, VEIL=Video endoscopic inguinal lymph adenectomy, R VEIL=Robotic assisted VEIL, MIS=Minimally Invasive Surgery
Wound infection and skin necrosis are rarely encountered in VEIL when compared to open surgery.[16,23,28] The incidence of lymphocele and lymphedema were similar in both the groups.[16,18,23] Complications reported with VEIL, were few in number and of less severity requiring conservative management.[18] More severe complications particularly wound infection and cellulitis were reported in groin node-positive patients.[18,25,29] In multivariate analysis of risk factors, nodal stage and open ILND had a significant association with complications after lymphadenectomy.[16]
Oncological outcomes
Lymph node yield and recurrence rates are the indicators for assessing the oncologic adequacy. Lymph node yield in VEIL varies from 5 to 16. Lymph node yield did not differ between the node-positive and node-negative patients.[25] Higher lymph node yield with VEIL was reported in the prospective study which compared open ILND and VEIL (7.11 vs. 9.36, P = 0.013).[36] In other studies, lymph node yield is comparable between VEIL and open surgery [Table 3]. The only study which directly compared VEIL (n = 7) and R-VEIL (n = 27) showed no difference in lymph node yield.[27] In node-negative patients, the open approach uses either DSNB or modified ILND but in the VEIL approach, a complete ILND is performed. Hence the lymph node yield may not be comparable.
Table 3.
Comparison of lymph node yield
| Article | Open | VEIL/R VEIL | P |
|---|---|---|---|
| Tobias-Machado 2017[28] | 9.7 (6-14) | 10 (6-16) | 0.52 |
| Yu et al., 2019[23] | 12 (5-21) | 11 (2-27) | 0.84 |
| Singh et al., 2018[18] | 12.5 (10.5-14.25) | 13 (11-14.5) | 0.44 |
| Thyavihally et al., 2021[16] | 11 (7-16) | 10 (7-14) | 0.325 |
| Yadav et al., 2018[29] | 8.3 | 7.6 | 0.68 |
| Kumar and Sethia 2017[36] | 7.11 | 9.36 | 0.013 |
VEIL=Video endoscopic inguinal lymph adenectomy, R VEIL=Robotic assisted VEIL
There is a paucity of data on the long-term outcome after VEIL. Meneses et al.[30] followed up 11 patients for a mean period of 28 months and 2 patients had recurrence. Carlos et al.[25] reported cancer-specific mortality of 5% at 35.3 months of follow-up in node-negative patients and 7% at 17.3 months follow-up in node-positive patients. Singh et al.[18] had the longest follow-up of 41 months for 51 patients after VEIL and 100 patients of open ILND, with no recurrence during the period. In the study by Yu et al., 2 out of 9 patients (18%) developed pelvic and abdominal metastasis, compared to 4 out of 10 patients in open ILND (P = 0.536). All the patients with recurrence had advanced pathological node status.[23]
With similar lymph node yield and recurrence rates, VEIL can be considered non inferior to open ILND. The efficacy of VEIL in high volume disease is not answered with current literature. VEIL has a shorter recovery period which enables early institution of adjuvant treatment in deserving patients with possible improvement of outcome.[39] All the papers reviewed here were of retrospective nature, carrying its inherent limitation in uniformity of case selection and reporting. Prospective large volume studies with long-term follow-up are required to fill the existing lacunae in evidence.
CONCLUSION
Inguinal lymphadenectomy is the cornerstone in the management of carcinoma of penis. Minimally invasive procedures such as VEIL and R VEIL are safe and feasible in both node-negative and node-positive patients. Compared to open ILND, VEIL is associated with low morbidity, less convalescence and similar oncological outcomes, making it a promising alternate to open ILND in selected cases. The safety of minimally invasive procedures like VEIL and R VEIL in high volume lymph node disease is yet to be proved with better evidence.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: Systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. doi: 10.26633/RPSP.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vieira CB, Feitoza L, Pinho J, Teixeira-Júnior A, Lages J, Calixto J, et al. Profile of patients with penile cancer in the region with the highest worldwide incidence. Sci Rep. 2020;10:2965. doi: 10.1038/s41598-020-59831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [Accessed Sept 11, 2020];Global Cancer Observatory. International Agency for Research on Cancer2020. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx . [Google Scholar]
- 4.Clark PE, Spiess PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, et al. Penile cancer: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2013;11:594–615. doi: 10.6004/jnccn.2013.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuiver MM, Djajadiningrat RS, Graafland NM, Vincent AD, Lucas C, Horenblas S. Early wound complications after inguinal lymphadenectomy in penile cancer: A historical cohort study and risk-factor analysis. Eur Urol. 2013;64:486–92. doi: 10.1016/j.eururo.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Hébert R, Raîche M, Dubois MF, Gueye NR, Dubuc N, Tousignant M, et al. Impact of PRISMA, a coordination-type integrated service delivery system for frail older people in Quebec (Canada): A quasi-experimental study. J Gerontol B Psychol Sci Soc Sci. 2010;65B:107–18. doi: 10.1093/geronb/gbp027. [DOI] [PubMed] [Google Scholar]
- 7.Bishoff JT. Endoscopic subcutaneous modified inguinal lymph node dissection for squamous cell carcinoma of the penis. In: Smith AD, Glenn GH, Preminger GM, Kavoussi LR, editors. Smith's Textbook of Endourology. 3rd ed. Chichester, UK: Blackwell Publishing Ltd; 2012. [Google Scholar]
- 8.Tobias-Machado M, Tavares A, Ornellas AA, Molina WR, Jr, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy: A new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J Urol. 2007;177:953–7. doi: 10.1016/j.juro.2006.10.075. discussion 958. [DOI] [PubMed] [Google Scholar]
- 9.Raghunath SK, Nagaraja H, Srivatsa N. VEIL surgical steps. Indian J Surg Oncol. 2017;8:64–6. doi: 10.1007/s13193-016-0596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Li L, Yao D, Li F, Zhang J, Yang Z. Preliminary experience of performing a video endoscopic inguinal lymphadenectomy using a hypogastric subcutaneous approach in patients with vulvar cancer. Oncol Lett. 2015;9:752–6. doi: 10.3892/ol.2014.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan P, Zhao C, Liu Z, Ou Z, He W, Cai Y, et al. Comparative study of video endoscopic inguinal lymphadenectomy through a hypogastric vs leg subcutaneous approach for penile cancer. J Endourol. 2018;32:66–72. doi: 10.1089/end.2017.0455. [DOI] [PubMed] [Google Scholar]
- 12.Nayak SP, Pokharkar H, Gurawalia J, Dev K, Chanduri S, Vijayakumar M. Efficacy and safety of lateral approach-video endoscopic inguinal lymphadenectomy (L-VEIL) over open inguinal block dissection: A retrospective study. Indian J Surg Oncol. 2019;10:555–62. doi: 10.1007/s13193-019-00951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbalka SS, Taha A, Srinivas C, Hegazy MA, Kotb SZ, Elnahas W, et al. Short-term surgical outcomes of standard and lateral video endoscopic inguinal lymphadenectomy: A multinational retrospective study. J Laparoendosc Adv Surg Tech A. 2020;30:373–7. doi: 10.1089/lap.2019.0733. [DOI] [PubMed] [Google Scholar]
- 14.Tamhankar AS, Ojha SP, Ahluwalia P, Gautam G. Technical caveats in robot assisted video endoscopic inguinal lymph node dissection-evolution of a modified technique. Int Braz J Urol. 2021;47:216–7. doi: 10.1590/S1677-5538.IBJU.2019.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pahwa HS, Pal AK, Kumar A, Misra S, Kaur G. Cannula-assisted port placement during video endoscopic inguinal lymphadenectomy (VEIL)-a novel and safe technique. Indian J Surg Oncol. 2019;10:570–3. doi: 10.1007/s13193-019-00902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thyavihally YB, Dev P, Waigankar SS, Pednekar A, Kulkarni B, Sharma A, et al. Comparative study of perioperative and survival outcomes after video endoscopic inguinal lymphadenectomy (VEIL) and open inguinal lymph node dissection (O-ILND) in the management of inguinal lymph nodes in carcinoma of the penis. J Robot Surg. 2021;15:905–14. doi: 10.1007/s11701-020-01189-x. [DOI] [PubMed] [Google Scholar]
- 17.Ahlawat R, Khera R, Gautam G, Kumar A. Robot-assisted simultaneous bilateral radical inguinal lymphadenectomy along with robotic bilateral pelvic lymphadenectomy: A feasibility study. J Laparoendosc Adv Surg Tech A. 2016;26:845–9. doi: 10.1089/lap.2015.0611. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Jaipuria J, Goel A, Shah S, Bhardwaj R, Baidya S, et al. Comparing outcomes of robotic and open inguinal lymph node dissection in patients with carcinoma of the penis. J Urol. 2018;199:1518–25. doi: 10.1016/j.juro.2017.12.061. [DOI] [PubMed] [Google Scholar]
- 19.Tobias-Machado M, Correa WF, Reis LO, Starling ES, de Castro Neves O, Juliano RV, et al. Single-site video endoscopic inguinal lymphadenectomy: Initial report. J Endourol. 2011;25:607–10. doi: 10.1089/end.2010.0269. [DOI] [PubMed] [Google Scholar]
- 20.Herrel LA, Butterworth RM, Jafri SM, Ying C, Delman KA, Kooby DA, et al. Bilateral endoscopic inguinofemoral lymphadenectomy using simultaneous carbon dioxide insufflation: An initial report of a novel approach. Can J Urol. 2012;19:6306–9. [PubMed] [Google Scholar]
- 21.Pompeo A, Tobias-Machado M, Molina WR, Lucio J, 2nd, Sehrt D, Pompeo AC, et al. Extending boundaries in minimally invasive procedures with simultaneous bilateral video endoscopic inguinal lymphadenectomy (veil) for penile cancer: Initial Denver health medical center and ABC school of medicine experience and surgical considerations. Int Braz J Urol. 2013;39:587–92. doi: 10.1590/S1677-5538.IBJU.2013.04.18. [DOI] [PubMed] [Google Scholar]
- 22.Josephson DY, Jacobsohn KM, Link BA, Wilson TG. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology. 2009;73:167–70. doi: 10.1016/j.urology.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Lu Y, Xiao Y, Guo J, Yin X, Yang Y, et al. Robot-assisted laparoscopic antegrade versus open inguinal lymphadenectomy: A retrospective controlled study. BMC Urol. 2019;19:135. doi: 10.1186/s12894-019-0571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sávio LF, Barboza MP, Alameddine M, Ahdoot M, Alonzo D, Ritch CR. Combined partial penectomy with bilateral robotic inguinal lymphadenectomy using near-infrared fluorescence guidance. Urology. 2018;113:251. doi: 10.1016/j.urology.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Carlos AS, Romanelli P, Nishimoto R, Montoya LM, Juliano CA, Costa RM, Jr, et al. Expanded criteria for video endoscopic inguinal lymphadenectomy (VEIL) in penile cancer: Palpable lymph nodes. Int Braz J Urol. 2013;39:893. doi: 10.1590/S1677-5538.IBJU.2013.06.17. [DOI] [PubMed] [Google Scholar]
- 26.Jindal T, Meena M. Laparoscopic and robotic video endoscopic inguinal lymphadenectomy by the lateral approach. Asian J Endosc Surg. 2021;14:464–9. doi: 10.1111/ases.12898. [DOI] [PubMed] [Google Scholar]
- 27.Russell CM, Salami SS, Niemann A, Weizer AZ, Tomlins SA, Morgan TM, et al. Minimally invasive inguinal lymphadenectomy in the management of penile carcinoma. Urology. 2017;106:113–8. doi: 10.1016/j.urology.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Tobias-Machado M. Video endoscopic inguinal lymphadenectomy (VEIL): Is a new standard ready to be accepted? BJU Int. 2017;119:504–5. doi: 10.1111/bju.13723. [DOI] [PubMed] [Google Scholar]
- 29.Yadav SS, Tomar V, Bhattar R, Jha AK, Priyadarshi S. Video endoscopic inguinal lymphadenectomy vs open inguinal lymphadenectomy for carcinoma penis: Expanding role and comparison of outcomes. Urology. 2018;113:79–84. doi: 10.1016/j.urology.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Meneses AD, Mattos PA, Filho WM, de Moura Fé TS, Rodrigues RM, Tobias-Machado M. Initial experience of video endoscopic inguinal lymphadenectomy in a center located at northeast Brazilian region. Int Braz J Urol. 2019;45:325–31. doi: 10.1590/S1677-5538.IBJU.2018.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pahwa HS, Misra S, Kumar A, Kumar V, Agarwal A, Srivastava R. Video Endoscopic Inguinal Lymphadenectomy (VEIL)-a prospective critical perioperative assessment of feasibility and morbidity with points of technique in penile carcinoma. World J Surg Oncol. 2013;11:42. doi: 10.1186/1477-7819-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matin SF, Cormier JN, Ward JF, Pisters LL, Wood CG, Dinney CP, et al. Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int. 2013;111:1068–74. doi: 10.1111/j.1464-410X.2012.11729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiapparrone G, Rapisarda S, de Concilio B, Zeccolini G, Antoniutti M, Celia A. Saphenous-sparing laparoscopic inguinal lymphadenectomy. Int Braz J Urol. 2018;44:645–6. doi: 10.1590/S1677-5538.IBJU.2017.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elsamra SE, Poch MA. Robotic inguinal lymphadenectomy for penile cancer: The why, how, and what. Transl Androl Urol. 2017;6:826–32. doi: 10.21037/tau.2017.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sudhir R, Krishnappa RS, Khanna S, Sekon R, Koul R. Video endoscopic inguinal lymphadenectomy (VEIL): Minimally invasive radical inguinal lymphadenectomy technique. Indian J Surg Oncol. 2012;3:257–61. doi: 10.1007/s13193-012-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V, Sethia KK. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU Int. 2017;119:530–4. doi: 10.1111/bju.13660. [DOI] [PubMed] [Google Scholar]
- 37.Romanelli P, Nishimoto R, Suarez R, Decia R, Abreu D, Machado M, et al. Video endoscopic inguinal lymphadenectomy: Surgical and oncological results. Actas Urol Esp. 2013;37:305–10. doi: 10.1016/j.acuro.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhari R, Khant SR, Patel D. Video endoscopic inguinal lymphadenectomy for radical management of inguinal nodes in patients with penile squamous cell carcinoma. Urol Ann. 2016;8:281–5. doi: 10.4103/0974-7796.184883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobroschke J, Groß T, Weitz J, Reißfelder C. Videoendoscopic minimally invasive inguinal lymphadenectomy: An alternative to open inguinal lymphadenectomy with fwer complications. Zentralbl Chir. 2018;143:348–50. doi: 10.1055/s-0044-102257. [DOI] [PubMed] [Google Scholar]


